Abstract
BACKGROUND: Declining liver function is a concerning side effect associated with radiation therapy. Biomarkers of liver toxicity would be useful in personalizing therapy. METHODS: As part of two prospective clinical trials examining adaptive radiation therapy, we collected serum samples from patients receiving liver radiation. We performed a screen of 22 cytokines using a multiplex assay then used ELISA to quantify the cytokines of greatest interest. Subjects were split into screening and validation cohorts. Toxicity was defined as an increase in Child-Pugh score of 2 points or greater within 6 months. Logistic regression models were used to estimate the relationship between our toxicity endpoint and serum cytokine concentrations. RESULTS: Our initial screen (46 subjects, 11 events) identified hepatocyte growth factor (HGF), CD40L (CD154), and eotaxin (CCL11) as potentially predictive of toxicity. We then tested these markers in an expanded patient cohort (104 subjects, 18 events) with a batch correction due to varying age of the samples which confirmed that high HGF and low CD40L were associated with a subsequent decline in liver function following radiation therapy. Multivariate analysis factoring in baseline Child-Pugh score and mean liver radiation dose demonstrated that HGF and CD40L were potentially predictive of toxicity (HGF OR 4.3, P = .009; CD40L OR 0.5 P = .06). Additionally, higher than median baseline HGF levels (1.4 ng/ml) were significantly associated with decreased survival following liver radiation (27.1 vs 14.5 months, P = .03). CONCLUSIONS: Our study identifies high HGF and low CD40L as potential markers of liver toxicity following radiation therapy.
Introduction
The management of patients with hepatocellular carcinoma or liver metastases is a challenge given the sensitivity of the liver to locoregional therapies. Patients often develop new lesions over time and receive multiple treatment courses. As their disease progresses, it is common to develop worsening liver function due to tumor burden and/or worsening of their chronic liver disease. Radiation therapy is effective at controlling liver cancer locally and regionally; however, its use is limited in patients with large tumors or poor liver function due to concern for toxicity [1], [2]. We have previously developed normal tissue complication probability models to predict the risk of radiation-induced liver disease over a population of patients with either primary or secondary liver cancer [3], [4]. The limitations of a population-based model are that patients who have a natural tolerance to radiation will be undertreated and patients with increased sensitivity are at a higher risk of complications than the model may predict. Therefore, biomarkers of liver sensitivity to radiation would be useful to individualize treatment.
The techniques for liver radiation have rapidly evolved over the past decade. Historically, fractionated courses, sometimes to the whole liver, have been studied and a number of predictors of radiation induced liver disease (RILD) have been established [5], [6], [7]. Currently, stereotactic body radiation therapy is the predominant external beam method used to treat liver tumors. Cases of RILD from stereotactic body radiation therapy (SBRT) are rare for patients with small lesions and good liver function; however, in patients with tumors greater than 4 cm or with poor baseline liver function, injury following liver irradiation is commonly seen [8], [9].
Serum cytokines and related blood based biomarkers have the potential to predict toxicity prior to clinical manifestations [10], [11]. Following radiation therapy, a complex series of changes occurs in the liver which, over several weeks to months, may lead to a decline in liver function. In this study, we analyzed serial serum samples from patients undergoing liver radiation for primary and metastatic liver cancer to determine markers of radiation-induced liver toxicity.
Methods
Clinical Trial Design
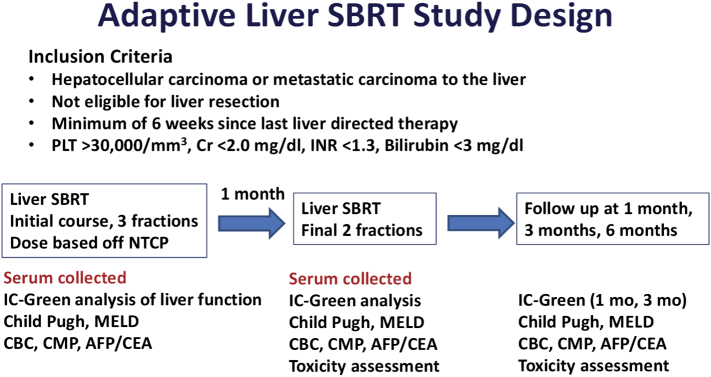
Samples for the study were collected from two prospective phase II trials of adaptive liver radiation (NCT01522937, NCT02460835). The clinical trial schema is shown in Figure 1. In the first clinical trial, patients with primary liver cancer or liver metastases received a split course of adaptive liver radiation therapy. In the first part of their course, the dose was determined using an NTCP model, with most patients receiving 10-12 Gy per fraction [12]. Baseline and midtreatment liver function assessments were used to individualize the second part of treatment [13]. In the second clinical trial, patients were also treated using an adaptive split course of radiation therapy; however, only patients with hepatocellular carcinoma were eligible, and patients with tumors larger than 5 cm received treatment over 20 fractions.
Figure 1.
Clinical trial schema. Patients included on this study were treated on two prospective phase 2 studies. Two-thirds of the planned treatment was administered initially; then 1 month later, the remaining third of treatment was delivered with a dose adjustment based off IC-green retention. Serum samples were collected at baseline and 1 month. Toxicity was assessed at 1, 3, and 6 months.
Radiation Therapy
Patients were simulated with contrast-enhanced CT and MRI scans. Controlled breath hold techniques were used if possible. For free-breathing patients, a 4DCT was acquired to generate an internal target volume. Expansion of the GTV to PTV was typically 5 mm in the axial dimension and 8 mm superior and inferior. Most patients were treated to 28-55 Gy in three to five fractions. A range of doses was used as subjects were enrolled on an adaptive SBRT protocol. Adaptation was performed after 60% of the course was delivered based off indocyanine (IC)-green clearance. A small percentage of patients were treated with fractionated image guided radiation therapy. Fractionated patients received up to 60 Gy total over 20 treatments using stereotactic treatment techniques with a 1-month break after the 12th fraction as described above.
Specimen Collection
All specimens were prospectively collected under an institutional review board–approved protocol after informed consent was obtained. Blood was obtained from each subject at baseline and 1 month after the initial course of radiation as shown in Figure 1. Serum and plasma were isolated from whole blood using a centrifuge and stored at −80 °C until analysis.
Toxicity Endpoints
For this analysis, we defined liver toxicity as an increase in Child-Pugh score of 2 points or greater within 6 months of receiving liver radiation. Child-Pugh score includes a combination of albumin, bilirubin, INR, ascites, and encephalopathy. Scores were prospectively acquired for each patient at baseline and 1, 3, and 6 months after the start of radiation.
Cytokine Analysis
Serum cytokine levels were analyzed using a Luminex Screening Assay (R&D Systems, Minneapolis, MN). Twenty-two cytokines were initially selected based on their potential role in liver disease and radiation injury. Forty-six of the 84 patients from the first clinical trial were selected for the initial screen including all of the patients who had a decline in Child-Pugh score (n = 11) and randomly selected control patients who did not experience a decline in Child-Pugh score. The cytokines that were found to be predictive of toxicity were then tested in a validation set including the remaining patients from our first clinical trial (n = 38) and all patients on the second clinical trial (n = 20).
Statistical Methods
Cytokine values were log-transformed for all analyses, unless otherwise stated. Logistic regression models were used to estimate the relationship between the binary toxicity endpoint and serum cytokine concentrations. Primary analyses were based on separate screening and validation sets. We also performed overall analyses of the combined dataset. In this larger dataset, we were able to assess whether the cytokines significantly improved toxicity prediction beyond what is possible using baseline Child-Pugh score and mean liver dose. Additional clinical factors including baseline IC-green clearance, portal vein thrombosis, age, sex, number of prior liver therapies, and local progression were considered, but Child-Pugh score and mean liver dose were identified as the most statistically significant predictors of toxicity using stepwise variable selection. In combined analyses, we also adjusted for possible “batch effects” and sample age (the time from sample collection to processing) by constructing linear regression models of baseline and 1-month cytokine values as a function of sample age and batch. Residuals from these models were taken to be the new cytokine values, as they represented the remainder of the cytokine that could not be explained by batch and sample age. Using the batch and sample age corrected cytokine values, the change from baseline to 1 month was calculated.
Overall survival was calculated from the time of starting radiation to death or last follow-up. All subjects were split into two cohorts for each cytokine marker based on the median value for a reference. The Kaplan-Meier method was used to determine overall survival based on this reference value. The log-rank test was used to compare overall survival curves between patients with low versus high cytokine expression. Additionally, we used a Cox proportional-hazards model for overall survival that incorporated baseline Child-Pugh score with batch and sample age adjusted cytokines as covariates. All analysis was performed using SAS version 9.4 software.
Results
Patient Characteristics
The characteristics of the patients included in the screening and validation cohorts are shown in Table 1. Overall, the two cohorts were balanced in respect to baseline patient characteristics. The screening and validation cohorts had a similar median age of 64 and 62, respectively. Approximately three-quarters of patients in both cohorts had cirrhosis. Seventy-six percent of the screening cohort and 74% of the validation cohort had a Child-Pugh score of 6 or less at baseline. Seventy-six percent of the screening cohort and 72% of the validation cohort had received prior liver-directed therapy. Over 95% of the patients on this study received SBRT.
Table 1.
Patient Characteristics
| Screening | Validation | Combined | |
|---|---|---|---|
| N | 46 | 58 | 104 |
| Age (median) | 64 | 62 | 63 |
| Cirrhosis | |||
| Yes | 34 (74%) | 42 (72%) | 76 (73%) |
| No | 12 (26%) | 16 (28%) | 28 (27%) |
| Child-Pugh score | |||
| <7 | 35 (76%) | 43 (74%) | 78 (75%) |
| 7-8 | 10 (22%) | 13 (22%) | 23 (22%) |
| >8 | 1 (2%) | 2 (3%) | 3 (3%) |
| Diagnosis | |||
| HCC | 40 (87%) | 47 (81%) | 87 (84%) |
| Other | 6 (13%) | 11 (19%) | 17 (16%) |
| Treatment | |||
| SBRT (3-5 fractions) | 46 (100%) | 53 (91%) | 99 (95%) |
| Fractionated | 0 | 5 (9%) | 5 (5%) |
| # Prior therapies | |||
| 0 | 11 (24%) | 16 (28%) | 27 (26%) |
| 1 | 9 (20%) | 11 (20%) | 20 (19%) |
| > 1 | 26 (57%) | 31 (53%) | 57 (55%) |
Initial Cytokine Screen with Luminex Multiplex Assay
An initial screen of 22 cytokines potentially associated with radiation and/or liver toxicity was performed on 46 patients (11 toxicity events) using the Luminex Multiplex Assay (R&D Biosystems). Results from this screen including areas under the curve (AUC) and P values are shown in Table 2. Baseline, midtreatment (1 month), and the change between the two time points were analyzed for each cytokine. Univariate analysis found that baseline HGF (AUC 0.70, P = .09), CD40L (AUC 0.83, P = .02), and Eotaxin (CCL11) (AUC 0.87, P = .03) were potentially associated with toxicity, defined as an increase in Child-Pugh score of 2 or more points within 6 months. Midtreatment HGF (AUC 0.84, P = .02) and CD40 ligand (CD40L) (AUC 0.86, P = .08) were also potentially predictive of toxicity. IL-8, IP-10, MCP-1, VEGF, CCL-22, TRAILR2, IL-2Ra, CXCL5, IL-1RI, IL1-RII, FGF-2, GCSF, GM-CSF, Fractalkine, GROα, IL-10, IL-1a, TNFα, and TGFβ were not associated with a two-point or greater change in Child-Pugh score.
Table 2.
Results from Initial Cytokine Multiplex Screen
| Baseline |
Midtreatment |
Change |
||||
|---|---|---|---|---|---|---|
| P | AUC | P | AUC | P | AUC | |
| IL8 | .48 | 0.54 | .67 | 0.55 | .61 | 0.55 |
| IP10 | .27 | 0.62 | .31 | 0.59 | .54 | 0.56 |
| MCP1 | .95 | 0.51 | .51 | 0.58 | .70 | 0.53 |
| VEGF | .86 | 0.58 | .71 | 0.51 | .43 | 0.56 |
| CCL22 | .54 | 0.50 | .62 | 0.52 | .56 | 0.68 |
| TRAILR2 | .61 | 0.52 | .55 | 0.61 | .10 | 0.63 |
| IL2Ra | .66 | 0.53 | .29 | 0.61 | .63 | 0.52 |
| CXCL5 | .64 | 0.54 | .60 | 0.55 | .20 | 0.63 |
| IL1RI | .91 | 0.55 | .31 | 0.68 | .17 | 0.65 |
| HGF | .09 | 0.70 | .02 | 0.84 | .59 | 0.65 |
| CD40L | .02 | 0.83 | .08 | 0.86 | .56 | 0.65 |
| CCL11 | .03 | 0.87 | .25 | 0.67 | .12 | 0.65 |
| IL1RII | .34 | 0.60 | .12 | 0.65 | .34 | 0.63 |
| FGF2 | .44 | 0.48 | .35 | 0.52 | .89 | 0.52 |
| GCSF | .93 | 0.55 | .88 | 0.65 | .90 | 0.55 |
| GMCSF | .41 | 0.56 | .29 | 0.62 | .67 | 0.52 |
| Fractalkine | .81 | 0.54 | .78 | 0.51 | .31 | 0.53 |
| Groa | .48 | 0.56 | .14 | 0.65 | .27 | 0.58 |
| IL10 | .44 | 0.58 | .33 | 0.50 | .33 | 0.66 |
| IL1a | .40 | 0.58 | .66 | 0.55 | .11 | 0.75 |
| TNFa | .17 | 0.63 | .20 | 0.60 | .65 | 0.52 |
Screening Set ELISA for HGF, CD40L, and Eotaxin
After identifying HGF, CD40L, and Eotaxin (CCL11) as potentially predictive of liver toxicity, we next performed ELISA to more accurately quantify serum levels of these markers in all subjects. A univariate analysis of our original screening set using the ELISA data was consistent with the results from our multiplex assay. Low levels of CD40L and high levels of HGF were associated with a subsequent worsening in Child-Pugh score in our screening cohort (Table 3). The odds ratio for baseline CD40L was 0.47 (95% CI 0.201-1.098, P value = .081), and the odds ratio for 1-month CD40L was 0.28 (95% CI 0.086-0.897, P value = .032). The odds ratio for baseline HGF was 6.97 (95% CI 1.05-46.36, P value = .045), and for 1-month HGF, it was 7.82 (95% CI 1.14-53.6, P value .036).
Table 3.
Results Screening ELISA Assay for CD40L, HGF, and Eotaxin
| Univariate Predictor | OR | 95% CI | P Value |
|---|---|---|---|
| CD40L baseline | 0.470 | (0.201-1.098) | .081 |
| CD40L 1 month | 0.278 | (0.086-0.897) | .032 |
| Change CD40L | 0.625 | (0.244-1.597) | .33 |
| HGF baseline | 6.970 | (1.048-46.363) | .045 |
| HGF 1 month | 7.817 | (1.140-53.596) | .036 |
| Change HGF | 2.297 | (0.031-168.244) | .70 |
| Eotaxin baseline | 0.570 | (0.135-2.407) | .44 |
| Eotaxin 1 month | 0.999 | (0.172-5.806) | 1.00 |
| Change eotaxin | 3.526 | (0.403-30.827) | .26 |
Validation Set ELISA for HGF, CD40L, and Eotaxin
We next analyzed HGF, CD40L, and Eotaxin in our validation cohort consisting of the original 46 subjects and an additional 58 subjects. Our initial intention was to analyze the 58 unique subjects alone; however, due to an age-related batch effect (Supplemental Figures 1 and 2), we incorporated a batch correction factor. Additionally, we sought to assess whether the cytokines added to the predictive ability of the standard clinical factors: baseline Child-Pugh score and mean liver dose. We therefore combined all patients (104 subjects, 18 events) for the validation set, as each individual set was too small to perform this analysis on. For the combined cohort, median HGF at baseline and 1 month was 1.4 ng/ml and 1.7 ng/ml, respectively. Median CD40L at baseline and 1 month was 1500 pg/ml and 1608 pg/ml.
On univariate analysis of the combined dataset with batch correction, 1-month (midtreatment) HGF was found to be predictive of toxicity with an odds ratio of 4.49 (95% CI 1.54-13.1, P = .006). Low baseline CD40L and 1-month CD40L were also borderline associated with liver toxicity with odds ratios of 0.57 (CI 0.32-1.04, P value = .07) and 0.50 (CI 0.25-1.01, P value = .05; Table 4A). The box plots for CD40L in the combined dataset at baseline and 1 month are shown in Supplemental Figure 3.
Table 4A.
Univariate Results for All Patients Using Adjustment for Batch Effect
| OR | 95% CI | P Value | |
|---|---|---|---|
| CD40L baseline | 0.573 | (0.316-1.041) | .068 |
| CD40L 1 month | 0.503 | (0.250-1.014) | .055 |
| Change CD40L | 0.947 | (0.504-1.780) | .87 |
| HGF baseline | 1.742 | (0.820-3.701) | .15 |
| HGF 1 month | 4.490 | (1.541-13.080) | .006 |
| Change HGF | 1.131 | (0.604-2.117) | .70 |
| Eotaxin baseline | 1.259 | (0.396-4.000) | .70 |
| Eotaxin 1 month | 1.910 | (0.594-6.137) | .28 |
| Change eotaxin | 2.391 | (0.593-9.638) | .22 |
Our multivariate analysis included the baseline variables Child-Pugh score and mean liver radiation dose with an adjustment for the batch effect. In this analysis, 1-month CD40L and HGF were associated with toxicity. One-month HGF was the strongest predictor, with an odds ratio of 4.28 (95% CI 1.44-12.78, P value = .009). One-month CD40L also was borderline predictive, with an odds ratio of 0.50 (CI 0.24-1.0, P value = .06, Table 4B). Baseline Child-Pugh score and mean liver dose were not predictive of toxicity in these models, suggesting that cytokines may be better predictors of radiation-associated liver injury than the standard clinical factors of dose or baseline liver function. Eotaxin was not associated with toxicity in our multivariate model.
Table 4B.
Multivariate Analysis of Combined Dataset Using Batch Adjustment.
| OR | 95% CI | P Value | |
|---|---|---|---|
| CD40L baseline | 0.629 | (0.337-1.175) | .15 |
| Baseline CP | 1.391 | (0.931-2.078) | .11 |
| MLD | 1.004 | (0.931-1.083) | .92 |
| HGF baseline | 1.732 | (0.812-3.691) | .16 |
| Baseline CP | 1.336 | (0.795-2.244) | .27 |
| MLD | 1.012 | (0.930-1.103) | .78 |
| Eotaxin baseline | 1.244 | (0.405-3.827) | .70 |
| Baseline CP | 1.450 | (0.976-2.155) | .066 |
| MLD | 1.008 | (0.938-1.085) | .82 |
| CD40L 1 month | 0.499 | (0.241-1.035) | .062 |
| Baseline CP | 1.293 | (0.793-2.108) | .30 |
| MLD | 1.029 | (0.951-1.113) | .48 |
| HGF 1 month | 4.282 | (1.435-12.775) | .009 |
| Baseline CP | 1.257 | (0.752-2.101) | .38 |
| MLD | 1.011 | (0.935-1.093) | .79 |
| Eotaxin 1 month | 1.666 | (0.496-5.594) | .41 |
| Baseline CP | 1.514 | (1.001-2.290) | .049 |
| MLD | 1.015 | (0.941-1.095) | .70 |
CP, Child-Pugh; MLD, mean liver dose.
Relationship Between Cytokines and Survival
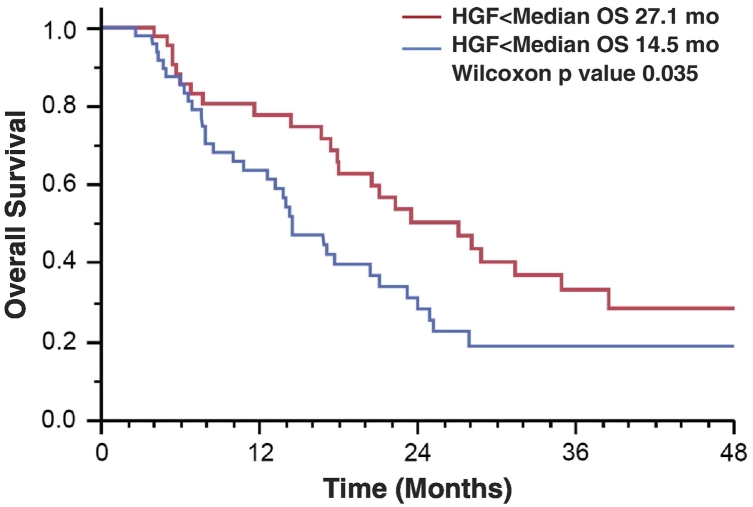
We next analyzed the ability of cytokines to predict survival in patients receiving liver radiation. The median value for the entire cohort (1.4 ng/ml) was used to stratify patients into a low– or high–serum HGF group for Kaplan-Meier analysis. For all patients, high baseline HGF was associated with shorter survival after liver SBRT, with a median overall survival of 14.5 months in patients with high HGF versus 27.1 months in patients with low HGF (P = .035, Figure 2). We then used a Cox model with baseline Child-Pugh and batch/age-adjusted cytokines for survival analysis. In this model, 1-month HGF values were associated with decreased overall survival with a hazard ratio of 1.75 (per log pg/ml, P = .032). Additionally, baseline and 1-month values of eotaxin were also correlated with overall survival in the Cox analysis (Table 5).
Figure 2.
Kaplan-Meier survival analysis of all patients included in the study. Patients were split into two cohorts based off whether or not they had a serum HGF level greater than or less than the median value for the cohort.
Table 5.
Cox Proportional Models for Overall Survival
| Variable | Hazard Ratio | P Value |
|---|---|---|
| CD40L baseline | 0.876 | .122 |
| HGF baseline | 1.370 | .125 |
| Eotaxin baseline | 1.808 | .018 |
| CD40L 1 month | 0.907 | .568 |
| HGF 1 month | 1.748 | .032 |
| Eotaxin 1 month | 1.887 | .010 |
| Delta CD40L | 1.128 | .458 |
| Delta HGF | 1.074 | .790 |
| Delta eotaxin | 1.204 | .555 |
Results displayed in table are from Cox proportional models fit to both screening and validation subjects where all cytokines were batch-corrected. All models adjusted for baseline Child-Pugh and were fit separately for each cytokine at each time point.
Discussion
In this study, we identified serum cytokines associated with toxicity and survival in patients receiving liver radiation. We found that high HGF and low CD40L were potentially associated with an increase in Child-Pugh score following treatment. We initially selected HGF and CD40L from a dataset enriched for patients who had toxicity and then performed a confirmatory analysis in a validation cohort. The results from the confirmatory analysis were consistent with our initial findings. We also carried out a multivariate analysis using a combined dataset and found that high HGF was associated with toxicity and decreased survival. These models were adjusted for baseline Child-Pugh score and mean liver radiation dose. These findings suggest that high HGF and low CD40L are associated with declining liver function following liver radiation therapy.
The major limitation of our study was the batch effect present in our screening and validation cohorts. The screening samples were older than the validation samples, which likely explained this finding. To account for this, we applied a batch correction in our multivariate analysis of all samples. This batch correction was not applied to each individual set, and both sets independently showed a relationship between HGF and CD40L on toxicity.
We previously presented results on a preliminary analysis of HGF and CD40L using our screening cohort [14]. More recently, independent results using samples from a phase II study including 41 patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma have been presented supporting our findings [15]. In this study, high baseline HGF was associated with an increase in Child-Pugh score and decreased overall survival, consistent with the findings in our screening and validation cohorts. These studies taken together support the use of HGF as a biomarker of liver toxicity and overall survival.
HGF is the primary ligand for the receptor tyrosine kinase c-MET [16]. As the name infers, HGF plays an important role in liver regeneration [17], [18], [19], [20]. Following surgery, HGF levels increase in order to promote regeneration [21], [22]. Additionally, HGF-MET is known to be associated with tumor invasion and metastasis [23]. What is not clear from our study is if patients with high serum HGF are more prone to liver toxicity given the aggressiveness of their underlying disease or if this is a marker of poor liver function.
CD40L (also known as CD154) is a member of the tumor necrosis factor family of cytokines. It can be platelet derived or present on a subset of T cells [24], [25]. Prior studies show 95% of circulating CD40L is platelet derived; however, these studies did not specifically examine patients with chronic liver disease. In our study, low levels of CD40L were predictive of toxicity. Low platelet counts are associated with poor liver function in patients with advanced cirrhosis. There was no association between platelet count and serum CD40L concentration in our study.
CD40L potentially has a protective role in the liver. This hypothesis is supported by the natural history of patients with X-linked immunodeficiency with hyperimmunoglobulin M (XLIHM), a rare genetic disorder where patients are deficient in CD40L. In an observational study, 12 out of 16 patients with XLIHM who lived beyond 20 years developed liver disease [26]. This study suggests that CD40L plays a protective role in the liver. Additionally, studies using genetically engineered mouse models of CD40L deficiency have suggested a protective role of this cytokine in the liver. Villeneuve et al. fed CD40L-deficient mice a fatty diet and observed that these mice developed fatty liver disease significantly earlier than wild-type mice [27].
Prior studies have identified an association between cytokine levels and radiation toxicity. A study by Anscher et al. in breast cancer patients receiving bone marrow transplants revealed that TGFβ levels strongly correlated with veno-occlusive disease and idiopathic interstitial pneumonitis [28]. In our study, we did not find an association between TGFβ and liver injury following SBRT; however, our toxicity endpoint was change in Child-Pugh score and not veno-occlusive disease, which is rare following liver SBRT given the small target volume. Preclinical studies have also focused on the role of TNFα and liver injury [29]. Mouse models show that blocking TNFα protects the liver from radiation-induced apoptosis. We did not see a relationship between TNFα and liver injury, likely because we examined cytokine markers 1 month following treatment and TNFα is upregulated much earlier than this. Furthermore, TNFα is very labile and maybe difficult to detect in peripheral blood. Future studies focusing on more stable TNFα-related proteins will be needed to better understand the role of this cytokine and its related pathways in liver injury.
A biomarker of liver toxicity would be very useful clinically. Patients identified as high risk for radiation-induced liver toxicity may benefit from alternative liver-directed therapies or have their radiation dose reduced accordingly. Adapting therapy would likely be most useful in patients with a high tumor burden and/or poor underlying liver function. Additionally, the potential prognostic value of HGF may be useful for selecting patients for liver directed therapy and for stratifying patients in future clinical trials.
The patients included in our analysis were treated on two adaptive liver radiation trials (NCT01522937, NCT02460835). These studies used IC-green clearance, a dynamic functional blood test, to determine and adjust radiation dose during a split course of liver radiation. Although, IC-green is an effective marker of liver function [30], the resources required to perform this assay limit its use in many institutions, whereas quantifying HGF and other cytokines from serum and plasma samples uses straightforward assays that centers can perform with standard equipment and blood collection protocols. In our future studies, we plan to incorporate HGF and other blood-based biomarkers into our predictive models of radiation associated liver toxicity.
Footnotes
Presented at the American Society for Radiation Oncology Annual Meeting 2015, San Antonio, TX.
Funding: Research reported in this publication was supported by the National Institutes of Health (USA) under award numbers P01CA59827 and P30CA046592. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no potential conflicts of interest.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2019.04.003.
Appendix A. Supplementary data
Supplemental Figure 1. Age related batch effect of HGF. Scatter plot showing HGF levels as a function of sample age at the time of processing for subjects treated on study 1 (blue) and study 2 (red). Given the batch effect, sample age was built into our multivariate model.
Supplemental Figure 2. Box plot of 1 month post-treatment HGF values for patients who did or did not have an increase in Child-Pugh score of 2 points or greater for the first (top) and second (bottom) studies. High levels of HGF were associated with increased risk of toxicity in patients treated on either study.
Supplemental Figure 3. Box plot of CD40L values at baseline (top) and 1 month (bottom) for all patients who did or did not have an increase in Child-Pugh score of 2 points or greater. Low CD40L values at baseline and 1 month were associated with increased risk of toxicity.
References
- 1.Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87:22–32. doi: 10.1016/j.ijrobp.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 2.McPartlin AJ, Dawson LA. Stereotactic body radiotherapy for hepatocellular carcinoma. Cancer J. 2016;22:296–301. doi: 10.1097/PPO.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 3.McGinn CJ, Ten Haken RK, WD Ensminger S, Walker S., Wang S., Lawrence T.S. Wang and TS Lawrence, Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol. 1998;16:2246–2252. doi: 10.1200/JCO.1998.16.6.2246. [DOI] [PubMed] [Google Scholar]
- 4.Jackson A, Ten Haken RK, Robertson JM, Kessler ML, Kutcher GJ, Lawrence TS. Analysis of clinical complication data for radiation hepatitis using a parallel architecture model. Int J Radiat Oncol Biol Phys. 1995;31:883–891. doi: 10.1016/0360-3016(94)00471-4. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Platt JF, Francis IR, Balter JM, Pan C, Normolle D, Ben-Josef E, Haken RK, Lawrence TS. The prediction of radiation-induced liver dysfunction using a local dose and regional venous perfusion model. Med Phys. 2007;34:604–612. doi: 10.1118/1.2431081. [DOI] [PubMed] [Google Scholar]
- 6.Ten Haken RK, Lawrence T.S., Dawson L.A. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:189–195. doi: 10.1016/j.ijrobp.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 8.Feng M, Ben-Josef E. Radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2011;21:271–277. doi: 10.1016/j.semradonc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, Ringash J, Dawson LA. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111:412–417. doi: 10.1016/j.radonc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178:505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ao X, Zhao L, Davis MA, Lubman DM, Lawrence TS, Kong FM. Radiation produces differential changes in cytokine profiles in radiation lung fibrosis sensitive and resistant mice. J Hematol Oncol. 2009;2:6. doi: 10.1186/1756-8722-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng M, Suresh K, Schipper MJ, Bazzi L, Ben-Josef E, Matuszak MM, Parikh ND, Welling TH, Normolle D, Ten Haken RK. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. 2018;4:40–47. doi: 10.1001/jamaoncol.2017.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenmark MH, Cao Y, Wang H, Jackson A, Ben-Josef E, Ten Haken RK, Lawrence TS, Feng M. Estimating functional liver reserve following hepatic irradiation: adaptive normal tissue response models. Radiother Oncol. 2014;111:418–423. doi: 10.1016/j.radonc.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuneo KC, Davis MA, Schipper MJ, Lawrence TS, Feng M. High-serum HGF and low-serum CD40L are associated with liver toxicity after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93:S114. [Google Scholar]
- 15.Hong TS, Grassberger C, Yeap BY, Jiang W, Wo JY, Goyal L, Clark JW, Crane CH, Koay EJ, Dima S. Pretreatment plasma HGF as potential biomarker for susceptibility to radiation-induced liver dysfunction after radiotherapy. NPJ Precis Oncol. 2018;2:22. doi: 10.1038/s41698-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiki Y, Ohnishi H, Muto Y, Matsumoto K, Nakamura T. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology. 1992;16:1227–1235. [PubMed] [Google Scholar]
- 18.Roos F, Terrell TG, Godowski PJ, Chamow SM, Schwall RH. Reduction of alpha-naphthylisothiocyanate-induced hepatotoxicity by recombinant human hepatocyte growth factor. Endocrinology. 1992;131:2540–2544. doi: 10.1210/endo.131.6.1446596. [DOI] [PubMed] [Google Scholar]
- 19.Morita M, Watanabe Y, Akaike TT. Protective effect of hepatocyte growth factor on interferon-gamma-induced cytotoxicity in mouse hepatocytes. Hepatology. 1995;21:1585–1593. [PubMed] [Google Scholar]
- 20.Tsubouchi H. Hepatocyte growth factor for liver disease. Hepatology. 1999;30:333–334. doi: 10.1002/hep.510300149. [DOI] [PubMed] [Google Scholar]
- 21.Efimova EA, Glanemann M, Nussler AK, Schumacher G, Settmacher U, Jonas S, Nussler N, Neuhaus P. Changes in serum levels of growth factors in healthy individuals after living related liver donation. Transplant Proc. 2005;37:1074–1075. doi: 10.1016/j.transproceed.2004.12.170. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog. 1992;3:27–54. [PubMed] [Google Scholar]
- 23.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 24.Kritharides L, Lau GT, Freedman B. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:2575–2577. doi: 10.1056/NEJM200306193482516. [DOI] [PubMed] [Google Scholar]
- 25.Freedman JE. CD40-CD40L and platelet function: beyond hemostasis. Circ Res. 2003;92:944–946. doi: 10.1161/01.RES.0000074030.98009.FF. [DOI] [PubMed] [Google Scholar]
- 26.Hayward A.R., Levy J., Facchetti F., Notarangelo L., Ochs H.D., Etzioni A., Bonnefoy J.Y., Cosyns M., Weinberg A. Cosyns and A Weinberg, Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J Immunol. 1997;158:977–983. [PubMed] [Google Scholar]
- 27.Villeneuve J., Lepreux S., Mulot A., BerardAM Higa-Nishiyama A., Costet P., De Ledinghen V., Bioulac-Sage P., Balabaud C., Nurden A.T. A protective role for CD154 in hepatic steatosis in mice. Hepatology. 2010;52:1968–1979. doi: 10.1002/hep.23935. [DOI] [PubMed] [Google Scholar]
- 28.Anscher MS, Crocker IR, Jirtle RL. Transforming growth factor-beta 1 expression in irradiated liver. Radiat Res. 1990;122:77–85. [PubMed] [Google Scholar]
- 29.Huang XW, Yang J, Dragovic AF, Zhang H, Lawrence TS, Zhang M. Antisense oligonucleotide inhibition of tumor necrosis factor receptor 1 protects the liver from radiation-induced apoptosis. Clin Cancer Res. 2006;12:2849–2855. doi: 10.1158/1078-0432.CCR-06-0360. [DOI] [PubMed] [Google Scholar]
- 30.Suresh K, Owen D, Bazzi L, Jackson W, Ten Haken RK, Cuneo K, Feng M, Lawrence TS, Schipper MJ. Using indocyanine green extraction to predict liver function after stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018;100:131–137. doi: 10.1016/j.ijrobp.2017.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Age related batch effect of HGF. Scatter plot showing HGF levels as a function of sample age at the time of processing for subjects treated on study 1 (blue) and study 2 (red). Given the batch effect, sample age was built into our multivariate model.
Supplemental Figure 2. Box plot of 1 month post-treatment HGF values for patients who did or did not have an increase in Child-Pugh score of 2 points or greater for the first (top) and second (bottom) studies. High levels of HGF were associated with increased risk of toxicity in patients treated on either study.
Supplemental Figure 3. Box plot of CD40L values at baseline (top) and 1 month (bottom) for all patients who did or did not have an increase in Child-Pugh score of 2 points or greater. Low CD40L values at baseline and 1 month were associated with increased risk of toxicity.