ABSTRACT
Epigenetic regulation of gene expression has a crucial role allowing for the self-renewal and differentiation of stem and progenitor populations during organogenesis. The mammalian kidney maintains a population of self-renewing stem cells that differentiate to give rise to thousands of nephrons, which are the functional units that carry out filtration to maintain physiological homeostasis. The polycomb repressive complex 2 (PRC2) epigenetically represses gene expression during development by placing the H3K27me3 mark on histone H3 at promoter and enhancer sites, resulting in gene silencing. To understand the role of PRC2 in nephron differentiation, we conditionally inactivated the Eed gene, which encodes a nonredundant component of the PRC2 complex, in nephron progenitor cells. Resultant kidneys were smaller and showed premature loss of progenitor cells. The progenitors in Eed mutant mice that were induced to differentiate did not develop into properly formed nephrons. Lhx1, normally expressed in the renal vesicle, was overexpressed in kidneys of Eed mutant mice. Thus, PRC2 has a crucial role in suppressing the expression of genes that maintain the progenitor state, allowing nephron differentiation to proceed.
KEY WORDS: Epigenetics, Histone methylation, Kidney development, Nephron, Stem cell
Summary: Conditional inactivation of the polycomb gene Eed in nephron progenitor cells results in their premature loss and prevents normal nephron differentiation beyond the S-shaped tubule stage.
INTRODUCTION
The nephrons of the metanephric kidney contain many distinct types of highly differentiated epithelial cells. Nephrons are derived from groups of mesenchymal progenitor stem-like cells (nephron progenitor cells or NPCs) that both self-renew and are induced to differentiate into aggregates (pretubular aggregates, PTAs). Subsequent mesenchymal to epithelial transformation (MET) of PTAs results in formation of a simple tubule (the renal vesicle, RV), which then undergoes segmentation to ultimately form the nephron (reviewed in McMahon, 2016). It is increasingly recognized that the number of nephrons in a kidney is a major determinant of kidney function and overall cardiovascular health in humans (Hoy et al., 2008; Puelles et al., 2014). Nephron number is determined by the successful induction of sufficient numbers of NPCs during the development of the kidney. Development of the metanephric kidney depends on the interaction of the ureteric bud and the metanephric mesenchyme at the inception of kidney development, and thereafter by the maintenance of appropriate numbers of NPCs, also referred to as the ‘cap mesenchyme’ (Short et al., 2014). If appropriate numbers of NPCs are maintained, human kidneys each harbor several hundred thousand to nearly a million nephrons, whereas mouse kidneys contain ∼15,000 nephrons (Zimanyi et al., 2009).
Factors such as BMP7, WNT9B, FGF9 and FGF20 are expressed by the ureteric bud derivatives and the cap mesenchyme itself and regulate the balance between self-renewal and differentiation of kidney progenitors (McMahon, 2016). Additionally, a set of transcription factors expressed by NPCs in the cap mesenchyme, including WT1, PAX2, EYA1, and SIX1, are required to maintain viability of the metanephric mesenchyme (Kreidberg et al., 1993; Torres et al., 1995; Xu et al., 1999, 2003; Li et al., 2003). By contrast, the transcription factor SIX2 is involved in maintaining the balance between self-renewal and differentiation (Self et al., 2006). The absence of SIX2 results in the failure of self-renewal after the initial interaction of the ureteric bud and metanephric mesenchyme, resulting in the premature differentiation of a small number of nephrons, concomitant loss of the progenitor population, and a failure to develop normal kidneys (Self et al., 2006). Importantly, the successful induction of a nephron requires not only expression of these factors in NPCs, but also their subsequent repression as cells differentiate.
Unlike some organs that maintain a population of stem cells throughout life, and use these cells to regularly replenish populations of differentiated epithelial cells, the mammalian kidney does not maintain adult stem cells, according to our present understanding (McMahon, 2016). Rather, once a sufficient number of nephrons are induced, the cap mesenchyme disappears, and no additional nephrons can be formed once these NPCs are gone (Short et al., 2014). The molecular events that regulate the disappearance of this progenitor population are not well understood, although it might result, in part, from a steady decrease in their number during kidney development (Short et al., 2014). Based on our knowledge of how stem cell populations are specified in other organs, as well as recent studies on histone modification in the developing kidney (Adli et al., 2015; Hilliard and El-Dahr, 2016), it is likely that epigenetic regulation of gene expression has an important role in regulating the expression of transcription factors that determine progenitor self-renewal and/or nephron differentiation. Here, we investigate the role of the polycomb repressive complex (PRC) in the maintenance of NPCs in the kidney and in nephron differentiation. PRC refers to two complexes, PRC1 and PRC2 (Entrevan et al., 2016). PRC2 places the H3K27me3 mark at promoter and enhancer regions, resulting in transcriptional repression (Conway et al., 2015). Repression of gene expression by PRC1 and PRC2 is believed to be a major mechanism whereby gene expression is negatively regulated during development, the other major mechanism being histone deacetylation (Margueron et al., 2005). We found that nephron progenitors were prematurely lost from the developing kidney in the absence of functional PRC2, even though low-level SIX2 expression was detected beyond the time point at which it should cease. Additionally, we found that nephrons were unable to form properly in the absence of PRC2 function during nephron differentiation. Lhx1 (previously Lim1), a transcription factor involved in nephron differentiation (Kobayashi et al., 2005), is overexpressed in the absence of PRC2 function, suggesting that PRC2 is required to repress a progenitor program of gene expression and allow nephron differentiation to proceed.
RESULTS
Analysis of kidneys in Eed mutant mice
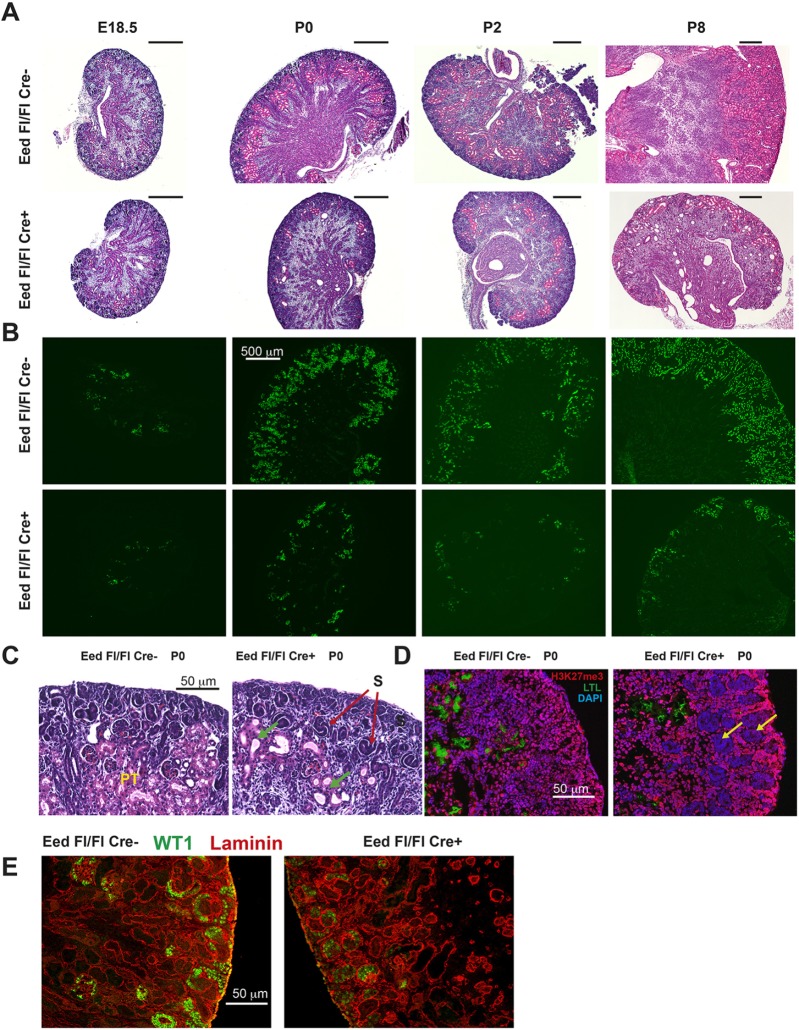
To determine the role of PRC2 in the developing kidney, we inactivated expression of Eed in NPCs using a previously reported bacterial artificial chromosome (BAC) Six2-GFP/Cre transgene (hereafter referred to as Six2-TGC) (Kobayashi et al., 2008), mated to mice carrying a floxed allele of Eed (Xie et al., 2014). EED is a nonredundant component of PRC2 (Cao et al., 2014), such that its inactivation should prevent deposition of H3K27me3. Eed fl/fl, Six2-TGC mice (referred to herein as ‘Eed mutants’) were runted and rarely survived more than 10 days. Kidneys of postnatal Eed mutant mice were smaller than those of control mice (Fig. 1A and Fig. S1). We used fluorescence-activated cell sorting (FACS) to assess the overall numbers of cells derived from SIX2-expressing progenitors, by combining an enhanced yellow fluorescent protein (eYFP) reporter allele from B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J mice (henceforth referred to as R26R-EYFP mice) with Eed mutants or control (Eed fl/+, Six2-TGC) mice (Fig. 2B). At embryonic day (E)18.5, approximately similar proportions of sorted cells expressed the eYFP reporter. However, by postnatal day (P)0 the percentage of eYFP-expressing cells was diminished in Eed mutants and was greatly decreased (10% versus 30%) by P8. Glomerular counts (as a measure of nephron number) also indicated that the numbers of induced and differentiated nephrons leveled off after E18.5 in Eed mutants, whereas these increased nearly sixfold in control Six2-TGC, Eed fl/+ mice between E18.5 and P8 (P8) (Fig. 2A), as previously described for mouse kidneys (Cebrián et al., 2004; Short et al., 2014).
Fig. 1.
Histology and characterization of Eed mutant kidneys. (A) Hematoxylin and Eosin stains of E18.5, P0, P2 and P8 kidneys as indicated at the top of each column. Genotypes: top row, Eed fl/fl; bottom row, Six2-TGC, Eed fl/fl. (B) LTL lectin staining at equivalent time points as in (A). Top and bottom row genotypes as in (A). (C) Higher-power histology of P0 kidneys showing S-shaped tubules in Eed mutants (red arrows) and proximal tubules (PT) abundant in controls and largely absent in Eed mutants. Dysgenic tubules are present in Eed mutants (green arrows). (D) H3K27me3 staining (pink) showing areas of absent staining in Eed mutants (yellow arrows). (E) WT1 (green) and laminin (red) immunostaining of P0 kidneys. All images are representative of at least three kidneys analyzed. Scale bars in A: 500 µm.
Fig. 2.
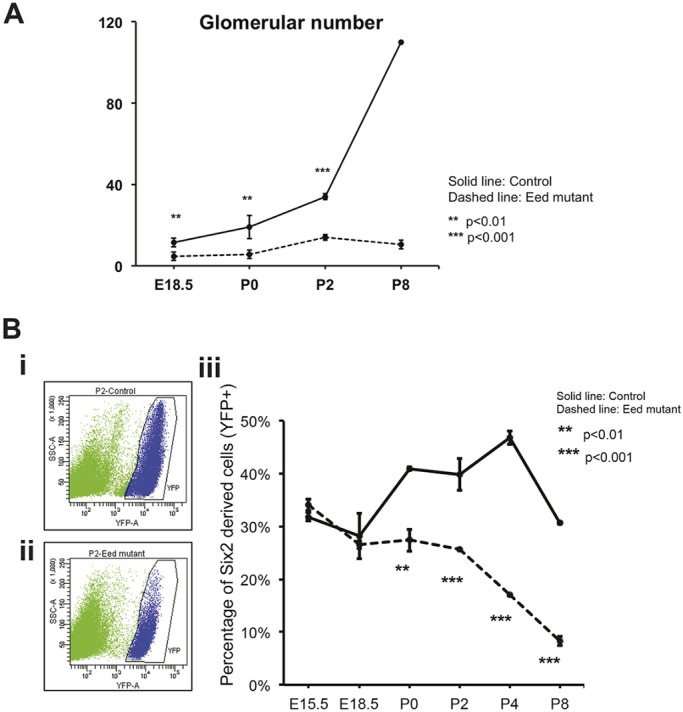
Quantification of kidney growth. (A) Glomerular counts at E18.5, P0, P2 and P8 kidneys. See Materials and Methods for details of counting. Data are presented as mean±s.d. **P<0.05, ***P<0.01, Student's t-test. (B) FACS analysis of cells derived from P2 (i) Six2-TGC; Eed fl/+ R26R-EYFP+ kidneys; (ii) Six2-TGC; Eed fl/fl R26R-EYFP+ kidneys. (iii) Cell counts from time points indicated on the x-axis are plotted as YFP-expressing cells as a percentage of the total cells sorted. The YFP+ cells were designated as shown in blue for the P2 time point in (i,ii). P-values in A and Biii refer to differences between controls and mutants at the specific time points. At least four kidneys per time point/genotype were used. Data are presented as mean±s.d. **P<0.01, ***P<0.001, Student's t-test.
Nephron differentiation in Eed mutant mice
Few differentiated tubules resembling mature nephrons were present based on the histological appearance of kidneys of Eed mutant mice. The tubules [defined by the presence of a laminin-containing basement membrane (Fig. 1E)] that were present did not have the histological appearance of normally differentiated nephrons, such as the presence of abundant eosinophilic proximal tubules (Fig. 1C and Fig. S2, for a larger version of Fig. 1C). Immunostaining for H3K27me3 revealed that areas containing undifferentiated tubules corresponded to the absence of H3K27me3 deposition (Fig. 1D). The growth of the kidney results from both the continual induction of new nephrons (between E12.5 and P2 in mouse) and the expansion of each individual nephron from a few hundred cells into an extensive epithelial unit (Short et al., 2014). We first examined nephron differentiation in Eed mutant mice. After MET, newly induced PTAs develop into an immature form known as the S-shaped tubule, where segmentation into the various parts of the nephron begins to become apparent. S-shaped tubules were present in Eed mutants (Fig. 1C and Fig. S2), but the reduced number of differentiated tubules indicated that PRC2 activity is required to form mature nephrons. Consistent with this appearance, Lotus tetragonolobus lectin (LTL)-lectin staining showed reduced numbers of differentiated proximal tubules in these kidneys at all time points examined, including E18.5, P0, P2 and P8 (Fig. 1B). Distal tubules, assessed by staining for SLC12A3, were present in proportion to the number of proximal tubules in Eed mutants (Fig. 3A,B). Staining for SLC12A1 was also used to assess the presence of loops of Henle (Fig. 3C,D). Although a few SLC12A1-containing tubules were present in Eed mutants, these tubules did not have the morphological appearance of loops of Henle, suggesting that this nephron segment involves additional differentiation steps requiring polycomb activity.
Fig. 3.
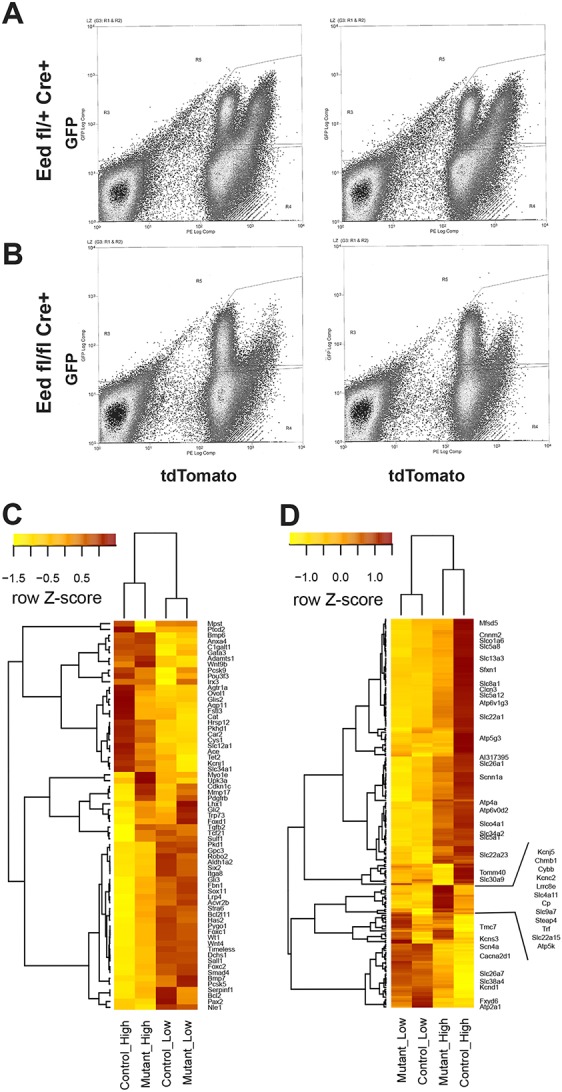
RNA-seq analysis of tdTomato-expressing cells. (A) FACS of kidney cells from Six2-TGC, Eed fl/+, R26R-tdTomato reporter mice. The results from two individual mice are shown, representative of 10 biological replicates. x-axis: tdTomato expression level; y-axis: GFP expression level. (B) FACS of Six2-TGC, Eed fl/fl, R26R-tdTomato reporter mice. (C,D) Heatmaps summarizing the expression of (C) kidney development (GO: 0001822) and (D) ion transport (GO:0006811)-specific gene sets identified as being differential. Expression values are normalized along the rows using Z-score transformation. Only selected genes are labeled in (D) for clarity.
In our further analyses of Eed mutants, we used the B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J reporter allele (henceforth referred to as R26R-tdTomato reporter mice) activated by the Six2-TGC transgene to define cell populations co-expressing GFP and tdTomato by FACS from kidneys of control R26R-tdTomato, Eed fl/+ and Eed mutant, R26R-tdTomato P0 mice. In these reporter mice, expression of the R26R-tdTomato reporter is directed by a CAG enhancer-promoter construct (Niwa et al., 1991). We made the fortuitous observation that two distinct populations of cells were present in control P0 kidneys based on lower or higher tdTomato expression along with high green fluorescent protein (GFP) expression from the Six2-TGC transgene (Fig. 3A, henceforth these are referred to as ‘tdTomato low’ and ‘tdTomato high’ populations). Most interestingly, in kidneys of P0 Eed mutant, R26R-tdTomato mice, the population expressing high levels of tdTomato was almost missing (Fig. 3B). The physiological basis for the differential expression of tdTomato from this transgene is unknown, but might relate to SRF/MRTF-binding sites in the chick ACTB promoter within the CAG construct and the differential activity of these actin level-sensitive transcription factors (Knöll, 2010; Xu et al., 2016) in various cell types of the developing kidney. RNA sequencing (RNA-seq) was used to define these populations obtained from control and Eed mutant mice by FACS (Table S1, Fig. S4 for reproducibility of samples). Two comparisons are shown, one emphasizing a kidney development gene set [with Gene Ontology (GO) annotation GO:0001822; Fig. 3C] and the other an ion transport gene set (with GO annotation GO:0006811) (Fig. 3D). GO analysis (Supek et al., 2011) of control tdTomato populations demonstrated that the lower tdTomato population mainly represented NPC and PTA cells, whereas the high tdTomato population was derived from more differentiated nephrons (Fig. 3C,D and Fig. S5A,B). As expected, the GO analysis identified components, including various ‘membrane transport’ and ‘metabolic’ processes, in the control high tdTomato-expressing population, and progenitor-related components, such as ‘urogenital system development’ and ‘tube development’, in the control low tdTomato-expressing population (Fig. S5A,B).
Comparisons of gene expression in four cell populations (control and Eed mutant tdTomato low, and control and Eed mutant, tdTomato high) revealed interesting observations suggestive of the role of polycomb in regulating gene expression during organogenesis. Using the kidney development gene set, the control and Eed mutant tdTomato low populations displayed a small but significant number of differences (Fig. 3C). Most notably Lhx1, which encodes a Lim-homeodomain transcription factor expressed in the renal vesicle (Kobayashi et al., 2005), was overexpressed in mutant kidneys (Figs 3C and 4C). Given that pretubular aggregates appear to undergo MET in Eed mutant kidneys, but fail to complete differentiation into mature nephrons, this overexpression of Lhx1 suggests that PRC2-mediated repression of Lhx1 is a crucial step in nephron differentiation beyond the renal vesicle stage. Additionally, a gene normally expressed in the stromal as opposed to the nephron lineage, Foxd1, was overexpressed in Eed mutant tdTomato-low cells, suggesting a role for PRC2 in maintaining lineage-specific patterns of gene expression. In contrast to genes that are involved in MET and nephron differentiation, such as Lhx1, genes that affect the proliferation and self-renewal of NPCs, such as Six2, Wt1, Pax2 and Sall1, were either expressed at slightly higher levels in control tdTomato-low cells or not differentially expressed, suggesting that PRC2 has a less crucial role in directly repressing the expression of genes required for the self-renewal of NPCs. A heat map summarizing our overall results is shown in Fig. S6. Consistent with the patterns emerging from the heat maps, GO analysis showed developmental and renal physiological processes to be over-represented in control tdTomato-low cells (Fig. S5D), whereas relatively few developmental processes were overexpressed in mutant control-low cells (Fig. S5C), and those present were representative of nonkidney-specific processes. Furthermore, consistent with the overall appearance of Eed mutant versus control kidneys, the ion channel heat map showed that most genes were overexpressed in control compared with Eed mutant kidneys (Fig. 3D) and GO analysis revealed that many kidney-specific physiological processes were over-represented in control tdTomato-high cells (Fig. S5F). A small number of ion channel and transport-related genes were overexpressed in Eed mutant tdTomato-high cells, including those encoding transferrin, ceruloplasmin and several others (Fig. 3D). At present, there is no apparent commonality or GO terms represented among these genes that distinguishes them from the larger group that is more highly expressed in control kidneys. Accordingly, fewer GO terms were over-represented in Eed mutant tdTomato-high cells (Fig. S5E). Nevertheless, several developmental GO terms were represented in the Eed mutant tdTomato-high cells, suggesting a general failure to repress developmental pathways in these cells.
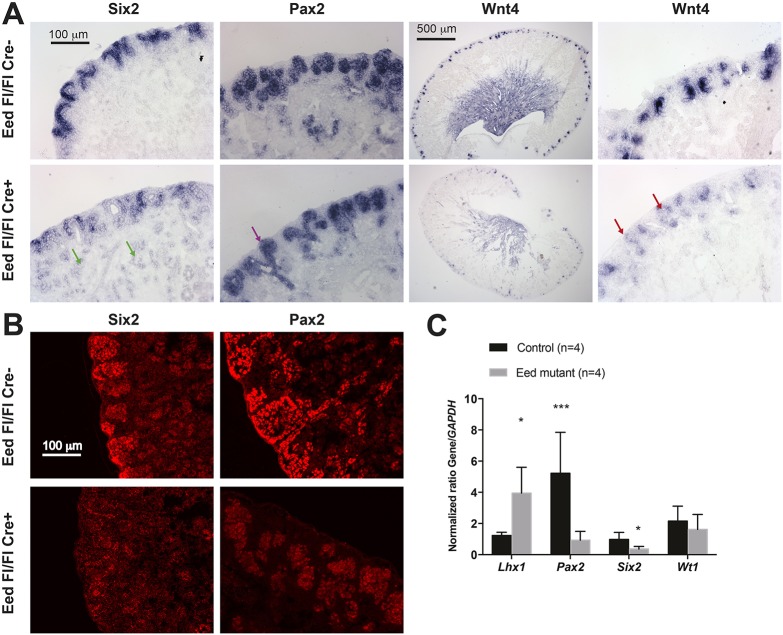
Fig. 4.
Gene expression analysis of Eed mutant kidneys. (A) In situ hybridization analysis of P0 kidneys. Top row: Eed fl/fl; bottom row: Six2-TGC, Eed fl/fl. The probe used is noted at the top of each column. Wnt4 is shown as both a low-power image and a higher-power image, whereas all others are at higher power. Green arrows indicate ectopic Six2 expression; purple arrow indicates Pax2-expressing ureteric bud without differentiating nephron structures; and red arrows indicate ectopic Wnt4 expression in the usual location of the cap mesenchyme adjacent to ureteric bud. The images are representative of three kidneys for each genotype. (B) Immunostaining of SIX2 and PAX2 in P2 kidneys. Top row: Six2-TGC, Eed fl/+; bottom row: Six2-TGC, Eed fl/fl. The images are representative of three kidneys for each genotype. (C) qPCR analysis of gene expression. *P<0.05, ***P<0.01.
Abnormal NPC gene expression in kidneys of Eed mutant mice
The smaller size of kidneys in Eed mutant mice suggested a premature disappearance of Six2-expressing NPCs. Therefore, we sought to determine the molecular basis for decreased progenitor cell endowment in kidneys of Eed mutant mice. Six2 mRNA is tightly restricted to the cap mesenchyme in the nephrogenic zone of developing kidneys (Self et al., 2006). In Eed mutant kidneys, Six2-expression cap mesenchyme cells were reduced in number, and Six2 mRNA levels were decreased by quantitative RT-PCR (qPCR) in tdTomato-low cells from Six2-TGC, R26R-tdTomato P2 kidneys (Fig. 4). Despite the reduced Six2 mRNA in the nephrogenic zone, low-level expression of Six2 mRNA could be detected by in situ hybridization in the dysgenic tubules that were present in Eed mutant kidneys (Fig. 4A,C). Antibody staining could not detect SIX2 protein in Eed mutant kidneys, suggesting that SIX2 protein was either below the limits of detection or not expressed in Eed mutants. Together, these results suggest that repression of gene expression by PRC2 is not only required to maintain self-renewal of kidney progenitor cells, particularly during the later stages of kidney development (post E18.5), but might also have a role in repressing Six2 expression to allow proper nephron differentiation. Furthermore, even though we inactivated a repressive complex, no patterns of increased gene expression were detected that would obviously account for the premature loss of NPCs.
In situ hybridization and antibody staining for PAX2 protein indicated that PTAs and renal vesicles were also decreased by P0 in Eed mutants, such that, in some places, only Pax2-expressing ureteric bud derivatives were observed without adjacent PTAs or comma and S-shaped tubules (Fig. 4A,C). Pax2 mRNA was also greatly reduced, as revealed by qPCR (Fig. 4C).
Expression of WT1, a transcription factor crucial for the maintenance of kidney NPCs, was also decreased in Eed mutant kidneys (Fig. 1E), although Wt1 mRNA levels in tdTomato-low cells did not differ significantly in Eed mutants compared with tdTomato-low cells from control kidneys (Fig. 4C). Wt1 is expressed in differentiating cells and in mature podocytes of the glomerulus. Accordingly, Wt1 staining showed fewer maturing glomeruli in mutant kidneys (Fig. 1E).
Wnt4 is expressed by PTAs (Stark et al., 1994), which are aggregates of NPCs and the formation of which is induced by Wnt9b expressed at the tips of the ureteric bud and its derivative branches (Carroll et al., 2005). Wnt4 acts in an autocrine fashion to induce MET of the PTA to form the renal vesicle. Wnt4 staining was less intense in PTAs of Eed mutant kidneys (Fig. 4A). In some locations along the nephrogenic zone of Eed mutant kidneys, Wnt4 appeared to be prematurely expressed in small numbers of NPCs adjacent to the ureteric bud derivatives (Fig. 4A), suggesting that PRC2 is involved in repressing Wnt4 expression in NPCs until they are induced to form PTAs.
Six2-expressing cells persist in kidneys of Eed mutant mice
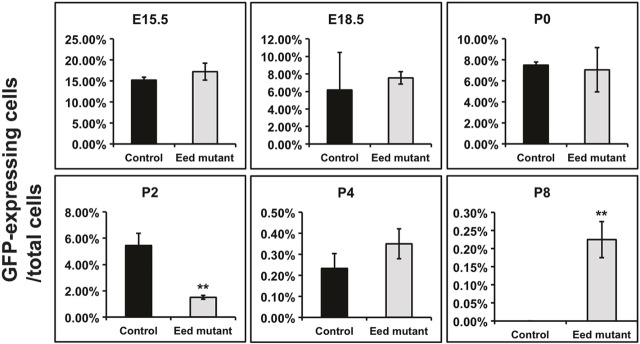
To further determine how PRC2 inactivation affects the maintenance of NPCs during development, FACS was used to detect eGFP-expressing cells using eGFP from the Six2-TGC transgene as a reporter of ongoing Six2 expression (Fig. 5 and Fig. S7). At P2, GFP-expressing cells represented nearly 6% of the total cells in control kidneys, whereas kidneys from Eed mutants had <2% GFP-expressing cells. By contrast, a small but significant number of GFP-expressing cells could be detected as late as P8 in Eed mutants, a time point at which GFP-expressing cells were absent in control kidneys (0.2% versus undetectable). Thus, it appears that the absence of PRC2-mediated transcriptional repression allows the persistence of low-level Six2 mRNA expression in the immature dysgenic tubules in kidneys of Eed mutant mice.
Fig. 5.
FACS analysis of Six2-expressing cells. The percentage of eGFP-expressing cells as a proportion of total cells is shown. Control: Six2-TGC, Eed fl/+, R26R-EYFP +/−; and Eed mutant: Six2-TGC, Eed fl/fl, R26R-EYFP +/−. The scales of the y-axis vary at different time points. Data are presented as mean±s.d. **P<0.01, Student's t-test. Time points without asterisks were not significantly different. N=at least three for each time point and/or genotype.
Aiden et al. analyzed the genome-wide chromatin state comparing Wilms' tumors (a tumor of the kidney found in infants and young children) to fetal and mature human kidneys (Aiden et al., 2010). Their study found bivalent chromatin (Bernstein et al., 2006) in fetal kidneys at genes known to be important developmental regulators of kidney development. Several of these bivalent sites converted to repressed chromatin, and were associated with the H3K27me3 mark, in mature kidneys. Among the genes noted by Aiden et al. to express bivalent chromatin was Six2, which displayed bivalent chromatin in fetal kidneys and the H3K27me3 mark in mature kidneys (Table S2). Thus, these results are consistent with our own observation that repression of Six2 expression requires PRC2 function and, consequently, Six2 is not completely repressed in kidneys of Eed mutant mice.
Elevated and abnormal HeyL expression in Eed mutant kidneys
Notch signaling is required to suppress Six2 expression and allow nephron differentiation (Chung et al., 2016). The expression of Notch1 and Notch2 were not significantly different in Eed mutants (Fig. 6A). HeyL, Hey1, Hey2 and Hes1 are basic helix-loop-helix (bHLH) transcriptional repressors that act downstream of Notch signaling (Maier and Gessler, 2000; Fischer et al., 2002). The expression of HeyL and Hey1 (and Hey2 to a lesser extent) were significantly increased in Eed mutants (Fig. 6A). In situ identification of HeyL mRNA in P2 kidneys demonstrated its expression in renal vesicles and segments of S-shaped tubules (Fig. 6B). By contrast, whereas all HeyL expression in controls was in differentiating structures, HeyL expression in Eed mutants appeared not only in structures resembling poorly formed S-shaped tubules, but also in mature, albeit dysgenic tubules not characteristic of typical nephrons (Fig. 6B). It is not known how Notch signaling represses Six2 expression (Chung et al., 2016), although HeyL and the other Notch-responsive transcriptional repressors are candidates to mediate this effect. Furthermore, persistence of Six2 expression despite the high expression of HeyL and Hey1 suggests that PRC2 is required for Notch-mediated repression of Six2 expression. Additionally, the increased expression of HeyL and Hey1 in Eed mutants suggests that PRC2 is also required to suppress what is usually a transient wave of Notch signaling during the induction of nephron differentiation.
Fig. 6.
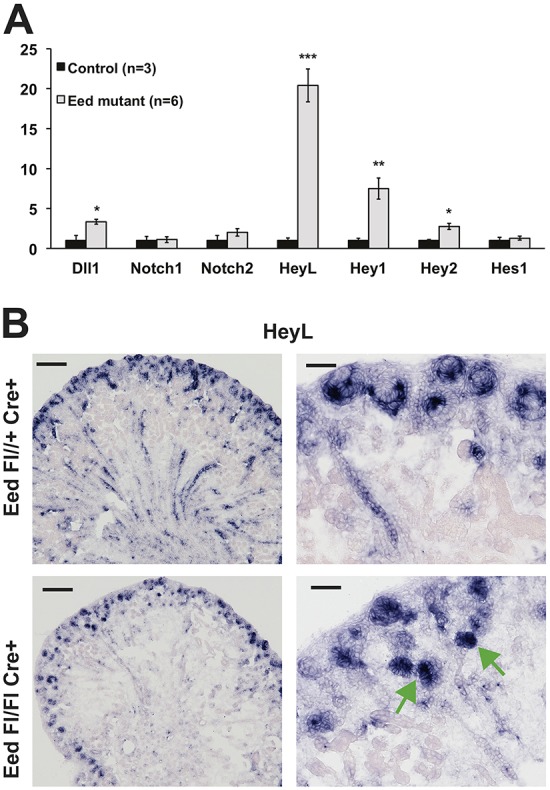
Notch signaling in Eed mutant kidneys. (A) qPCR analysis of gene expression of eYFP-FACS-sorted cells from Control: Six2-TGC, Eed fl/+, R26R-EYFP +/−; and Eed mutant: Six2-TGC, Eed fl/fl, R26R-EYFP +/−. N=3 controls and 6 mutants. Controls for each gene w set at ‘1’. Data are presented as mean±s.d. *P<0.05, **P<0.01, ***P<0.001, Student's t-test. All results were first normalized to GAPDH. (B) HeyL in situ hybridization (middle panels), merged images (right panels). HeyL is not present in tubules of maturing nephrons but is present in dysgenic tubules of Eed mutant (green arrows, lower right panel). Scale bars: 500 µm, left panels; 50 µm, right panels. Images are representative of three experiments.
DISCUSSION
We report here the phenotype of mice carrying a targeted inactivation of the polycomb gene Eed in kidney NPCs. We observed the earlier than usual disappearance of NPCs, along with a failure of NPCs to properly differentiate into nephrons. A small number of Six2-expressing cells (based on GFP detection from Six2-eGFP/Cre reporter transgenes) persisted in Eed mutant mice, suggesting that PRC2 is required to maintain NPCs. Lhx1, a transcription factor present in the renal vesicle and S-shaped tubules and required for proper nephron differentiation, was overexpressed in Eed mutant kidneys, indicating the PRC2 function is also required for nephron differentiation to proceed past the S-shaped tubule stage, although not specifically for MET.
Similar to other organs that arise from populations of stem-like progenitors, the nephrons of the kidney arise from a population of stem cells. This stem-like population disappears after a sufficient number of epithelial units, or nephrons, has been induced (McMahon, 2016). Six2 was originally characterized as a ‘gatekeeper’ gene that maintains the self-renewal of NPCs (Self et al., 2006). Recent studies indicate that Six2 activates the expression of target genes required for self-renewal, including Six2 itself, in NPCs, while at the same time in these NPCs, a complex that includes Six2, Osr1 and TCF factors might attract the Groucho-related co-repressor to repress Wnt4 and other differentiation-related genes (Park et al., 2012; Xu et al., 2014). Once canonical Wnt signaling is activated by Wnt9b, β-catenin might replace Groucho-related proteins on T cell factor (TCF) factors and convert this repressive complex into an activating complex that turns on the expression of genes involved in forming the PTA and initiating formation of the nephron (Park et al., 2012; Xu et al., 2014). Notably, in response to β-catenin, Wnt4 expression is turned on in the pretubular aggregate and is crucial to inducing MET. Wnt4 expression is then turned off after the renal vesicle and/or early S-shaped tubule stage.
Therefore, it is of particular interest that we observed premature Wnt4 expression in kidney NPCs (at least defined by their location in aggregates at the periphery of the kidney and in contact with the ureteric bud). We speculate that Wnt4 expression is turned on prematurely not only because the SIX2-associated repressive complex is unable to repress Wnt4 in the absence of PRC2, but also because WT1, the transcription factor that would typically activate Wnt4 expression in pretubular aggregates (Essafi et al., 2011), is already present in kidney NPCs. Thus, during normal development, when PRC2 is present, SIX2-mediated repression of Wnt4 in self-renewing NPCs predominates over WT1 activation of Wnt4. However, in the absence of PRC2, WT1 activation of Wnt4 might predominate, leading to an imbalance with decreased self-renewal and increased differentiation of NPCs. By contrast, Wnt4 spatial expression in PTAs and the renal vesicles themselves is reduced in Eed mutant kidneys, possibly because the premature depletion of NPCs results in smaller numbers of cells contributing to PTAs and subsequent renal vesicles and S-shaped tubules.
In contrast to Six2, we did not observe continued Wnt4 expression in the dysgenic tubules in Eed mutant kidneys. This might reflect that WT1 itself does not continue to be expressed, such that there is not a transcriptional activating complex at the Wnt4 gene. In addition, these observations might provide an indication that some genes, including Six2, require the H3K27me3 mark to terminate their expression, whereas other genes, such as Wnt4, do not strictly require the H3K27me3 mark for repression. Consistent with this possibility, relatively few genes in the kidney development set were elevated in Eed mutant tdTomato-low cells compared with controls (Fig. 3C), Lhx1 being a notable exception. This is also consistent with our analysis of the data of Aiden et al. (2010), showing that Six2 was one of only a few genes transiting from a bivalent to H3K27me3 state in fetal compared with mature kidneys. In this regard, it is also notable that overall, our RNA-seq analyses revealed most genes to be unchanged in all control-Eed mutant comparisons (Fig. S8). Therefore, we suggest that the PRC2 complex has specific targets during organogenesis and that there are other pathways for inactivating gene expression in the case of non-PRC2 target genes.
Our observations also lead us to question why, in the presence of ectopic Six2 mRNA expression, we observed the early demise of the progenitor population, especially given the inability to turn off Six2 expression. Furthermore, the inability to turn off Six2 expression could lead not only to persistent progenitors, but also to excessive nephrogenic blastema or even to Wilms' tumors, a blastemal tumor of the kidney found in young children. Even though there appeared to be very low-level persistence of Six2 mRNA in the cortex of Eed mutant kidneys, the Six2 mRNA expression present in NPCs of those kidneys appeared to be lower than normal and the SIX2 protein level was below the limits of detection. These observations suggest that, although PRC2 is required to completely turn off Six2 expression, there are factors required to maintain high levels of Six2 expression, likely to be in addition to Six2 itself, the expression of which is not maintained in Eed mutant kidneys. These factors are unknown [although we previously demonstrated that SIX2 is not regulated by WT1 (Hartwig et al., 2010)].
An additional answer to this question might be found in previous studies where defective nephron differentiation is accompanied by early loss of progenitors, an example being when we previously inactivated the Bmp7 gene in podocytes (Kazama et al., 2008), or when FGF8, expressed by PTAs, was inactivated in embryonic kidneys (Grieshammer et al., 2005; Perantoni et al., 2005). This, and other observations, led us to suggest that the self-renewal of NPCs requires ongoing nephrogenesis. Indeed, there are few, if any, examples of phenotypes where self-renewal and proliferation of NPCs is maintained in the absence of ongoing nephrogenesis.
As mentioned above, Six2 is thought to positively regulate its own expression (Park et al., 2012). FGF9 and -20 (Barak et al., 2012), as well as BMP7 (Dudley et al., 1995; Luo et al., 1995; Blank et al., 2009), promote the self-renewal and proliferation of NPCs, suggesting that Six2 transcription is also downstream of receptor tyrosine kinase signaling and BMP receptor signaling, the latter through JNK MAP kinase (Blank et al., 2009). It remains unclear why these signals might be attenuated in the absence of a functional PRC2 complex. We recently demonstrated that the WT1 target gene Gas1, which encodes a GPI-linked membrane protein, amplifies FGF signaling in NPCs (Kann et al., 2015). In Eed mutants, WT1 protein levels were significantly reduced in NPCs, suggesting that FGF signaling mediated by GAS1 was reduced in Eed mutants, providing an additional explanation for reduced levels of Six2, and consequently decreased self-renewal of NPCs.
Recent studies demonstrate that Notch-mediated cessation of Six2 expression is required for the differentiation of renal vesicles into S-shaped tubules and more differentiated nephrons (Chung et al., 2016). Therefore, it is possible that the persistent Six2 expression we observed in Eed mutants was partially responsible for the failure of nephron differentiation. Arguing against this explanation is our failure to detect SIX2 by immunostaining in Eed mutants, suggesting that, if SIX2 is present, it is likely at low levels. If ectopic expression of Six2 is partially responsible for the defect in nephron differentiation, the block is probably post MET and at the point of the appearance of the renal vesicle or early S-shaped tubule. Given that Notch signaling is required to stop the expression of Six2 (Chung et al., 2016), the increased expression of HeyL and Hey1 in Eed mutant kidneys was surprising, because this suggests that Notch signaling is hyperactivated in Eed mutants. Together, these observations suggest either that HeyL or Hey1 are not the relevant mediators of Notch signaling involved in repressing Six2 expression, or that HeyL or Hey1 utilize PRC2 to repress Six2, and that this complex is absent in induced NPCs of Eed mutant kidneys. Further studies will require anti-HeyL or anti-Hey1 antibodies suitable for chromatin immunoprecipitation (ChIP) to test these hypotheses.
MATERIALS AND METHODS
Mice
All animal studies were carried out in accordance with the guidance of the Institutional Animal Care and Use Committee at Boston Children's Hospital. Eed floxed allele mice (Xie et al., 2014) and R26R-EYFP mice (Jackson Labs 006148) were obtained from Drs Huafeng Xie and Stuart Orkin (Boston Children's Hospital). R26R-tdTomato mice (Jackson Labs 007909) were obtained from Dr Joseph Majzoub (Boston Children's Hospital). BAC transgenic Six2-TGC mice (Kobayashi et al., 2008) were obtained from Andrew McMahon (formerly Harvard University).
Antibodies and reagents
The antibodies and reagents used were as follows: anti-Pax2, obtained from Greg Dressler (University of Michigan); anti-Six2 (ProteinTech 11562); anti-WT1 C-19 (Santa Cruz sc-192); anti-H3K27me3 (Abcam #6002); anti-laminin (Millipore 4E10); LTL-fluorescein (Vector Laboratories FL-1321); anti-SLC12A1 (Proteintech #18970-1-AP); and anti-SLC12A3 (Sigma #HPA028748).
Glomerular counts
Numbers of glomeruli were quantified by counting total glomeruli in a single histological section from the widest diameter of the kidney. For each time point, at least three kidneys from littermate individual mice were analyzed.
Immunohistochemistry
Kidneys were fixed with 4% paraformaldehyde/PBS overnight at 4°C, cyroprotected in 12% sucrose for 12 h and 30% sucrose overnight at 4°C, and then embedded/frozen in optimal cutting temperature (OCT) compound. Immunofluorescence was carried out on kidney cryosections (5 μm, Leica CM3050S) for either native fluorescence of fluorescent proteins or immunostaining of antibodies. For direct imaging of tdTomato red fluorescent protein, sections were coverslipped with Prolong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific P36931). For antibody immunostaining, sections were permeabilized with 0.25% Triton X-100 in PBS for 10 min at room temperature (RT) and blocked with 10% goat serum and 2% BSA for 1 h at RT. Sections were incubated with primary antibodies with 1:200-1:1000 dilution in 5% goat serum and 1% BSA and 0.1 Triton X-100 in PBS at 4°C overnight, then incubated in secondary antibody (Alexa Fluor) with 1:1000 dilution in 5% goat serum and 1% BSA and 0.1 Triton X-100 in PBS at RT for 45 min and mounted with an anti-fade reagent as described above.
RNA in situ hybridization
Kidneys were fixed in 4% paraformaldehyde overnight, cryoprotected in 30% sucrose for 24 h, and then embedded in OCT. 10 μm cryosections were cut by using a Leica CM3050S for tdTomato pre-imaging and then RNA in situ hybridization [as previously described (Kann et al., 2015)]. For pre-imaging of native tdTomato fluorescent proteins, sections were removed from −80°C, dried at RT for 20 min, fixed with 4% paraformaldehyde for 10 min, and then covered by RNase-free PBS and a coverslip. Fluorescent images were acquired, and the coverslip then was carefully removed from the section with forceps. Sections were then treated with H2O2 (Fischer Scientific, BP2633500), proteinase K (Ambion, AM2542) and acetic acid, prehybridized and subjected to RNA in situ hybridization using a standard protocol. Images were captured by the same microscope with bright-field function and merged with pre-imaged fluorescent images at the identical location. The probes were as previously described (Hartwig et al., 2010; Kann et al., 2015).
Fluorescence-activated cell sorting
Kidneys from embryos or mice of different ages (E15.5, E18.5, P0, P2 and P8) were cut into small pieces, trypsinized and dissociated by repetitive pipetting. The trypsinization was terminated by adding complete medium with 10% FBS (Gemini Bio-Products, 100-500). The cells were collected by centrifugation, resuspended in PBS, and filtered three times through 100 µm BD Falcon Cell-Strainer Cap (Falcon, 352235). The single cell population was transferred into a polypropylene tube (Falcon, 352063) and kept on ice until FACS. The GFP+, YFP+ or tdTomato+ (single-positive or double-positive) cells were isolated using a MoFlo (Beckman Coulter) for Figs 3 and 4 or BD FACSAria (BD Biosciences) for Fig. 5 and Fig. S3.
RT-qPCR
qPCR was done using a SuperScript III First strand synthesis system (Thermo Fisher Scientific #18080051) and SYBR Green PCR Master Mix (Thermo Fisher Scientific #4364346) and run on a StepOne Plus RT-PCR system (Applied Biosystems). Primer pairs were as previously described (Hartwig et al., 2010; Kann et al., 2015).
RNA-seq analysis
In total, four replicates, each corresponding to the tdTomato-high and tdTomato-low population, respectively, were sequenced for Control and Eed mutant P0 kidneys. However, we excluded four libraries corresponding to two Control High and two Mutant High samples because of the failure of sequencing libraries. Sequenced reads were mapped to the mouse genome (mm9) using bowtie2 with default parameters. HTSeq, DESeq2 and CuffDiff2 packages were used for generating counts and robustly identifying over- and underexpressed genes in High and Low progeny (Langmead and Salzberg, 2012; Trapnell et al., 2013; Love et al., 2014; Anders et al., 2015). GO analysis was summarized using the REVIGO package (revigo.irb.hr). Subsequent customized analyses were done in R. The raw and processed RNA-seq files have been deposited in GEO under accession number GSE102690.
Statistics
All data are presented as mean±s.d. and calculations were performed using Microsoft Excel. Unpaired one-tailed Student's t-test was used for glomerular counts and unpaired two-tailed Student's t-test was used for all other data to determine statistical significance. P-values <0.05 were considered to be statistically significant.
Acknowledgements
We thank Drs Huafeng Xie and Stuart Orkin for sharing the Eed floxed mice, Dr Joseph Majzoub for the R26R-tdTomato mice, members of the Kreidberg laboratory for valuable discussions, and Dr Valerie Schumacher for comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: L.Z., J.A.K.; Formal analysis: L.Z., S.E., D.J., J.A.K.; Investigation: L.Z., S.E., M.K., D.J., L.Y., C.S., Y.L., K.N., M.E.T., P.J.P., J.A.K.; Resources: J.A.K.; Data curation: D.J., L.Y., P.J.P., J.A.K.; Writing - original draft: L.Z., J.A.K.; Writing - review & editing: L.Z., J.A.K.; Supervision: J.A.K.; Project administration: J.A.K.; Funding acquisition: J.A.K.
Funding
These studies were supported by a grant from the March of Dimes Foundation (1-FY13-481).
Data availability
The raw and processed RNA-seq files have been deposited in GEO under accession number GSE102690.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.157149.supplemental
References
- Adli M., Parlak M., Li Y. and El-Dahr S. S. (2015). Epigenetic States of nephron progenitors and epithelial differentiation. J. Cell. Biochem. 116, 893-902. 10.1002/jcb.25048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiden A. P., Rivera M. N., Rheinbay E., Ku M., Coffman E. J., Truong T. T., Vargas S. O., Lander E. S., Haber D. A. and Bernstein B. E. (2010). Wilms tumor chromatin profiles highlight stem cell properties and a renal developmental network. Cell Stem Cell 6, 591-602. 10.1016/j.stem.2010.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P. T. and Huber W. (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166-169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak H., Huh S.-H., Chen S., Jeanpierre C., Martinovic J., Parisot M., Bole-Feysot C., Nitschké P., Salomon R., Antignac C. et al. (2012). FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev. Cell 22, 1191-1207. 10.1016/j.devcel.2012.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B. E., Mikkelsen T. S., Xie X., Kamal M., Huebert D. J., Cuff J., Fry B., Meissner A., Wernig M., Plath K. et al. (2006). A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315-326. 10.1016/j.cell.2006.02.041 [DOI] [PubMed] [Google Scholar]
- Blank U., Brown A., Adams D. C., Karolak M. J. and Oxburgh L. (2009). BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development 136, 3557-3566. 10.1242/dev.036335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q., Wang X., Zhao M., Yang R., Malik R., Qiao Y., Poliakov A., Yocum A. K., Li Y., Chen W. et al. (2014). The central role of EED in the orchestration of polycomb group complexes. Nat. Commun. 5, 3127 10.1038/ncomms4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll T. J., Park J.-S., Hayashi S., Majumdar A. and McMahon A. P. (2005). Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283-292. 10.1016/j.devcel.2005.05.016 [DOI] [PubMed] [Google Scholar]
- Cebrián C., Borodo K., Charles N. and Herzlinger D. A. (2004). Morphometric index of the developing murine kidney. Dev. Dyn. 231, 601-608. 10.1002/dvdy.20143 [DOI] [PubMed] [Google Scholar]
- Chung E., Deacon P., Marable S., Shin J. and Park J.-S. (2016). Notch signaling promotes nephrogenesis by downregulating Six2. Development 143, 3907-3913. 10.1242/dev.143503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E., Healy E. and Bracken A. P. (2015). PRC2 mediated H3K27 methylations in cellular identity and cancer. Curr. Opin. Cell Biol. 37, 42-48. 10.1016/j.ceb.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Dudley A. T., Lyons K. M. and Robertson E. J. (1995). A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 9, 2795-2807. 10.1101/gad.9.22.2795 [DOI] [PubMed] [Google Scholar]
- Entrevan M., Schuettengruber B. and Cavalli G. (2016). Regulation of genome architecture and function by polycomb proteins. Trends Cell Biol. 26, 511-525. 10.1016/j.tcb.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Essafi A., Webb A., Berry R. L., Slight J., Burn S. F., Spraggon L., Velecela V., Martinez-Estrada O. M., Wiltshire J. H., Roberts S. G. E. et al. (2011). A wt1-controlled chromatin switching mechanism underpins tissue-specific wnt4 activation and repression. Dev. Cell 21, 559-574. 10.1016/j.devcel.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Leimeister C., Winkler C., Schumacher N., Klamt B., Elmasri H., Steidl C., Maier M., Knobeloch K.-P., Amann K. et al. (2002). Hey bHLH factors in cardiovascular development. Cold Spring Harb. Symp. Quant. Biol. 67, 63-70. 10.1101/sqb.2002.67.63 [DOI] [PubMed] [Google Scholar]
- Grieshammer U., Cebrian C., Ilagan R., Meyers E., Herzlinger D. and Martin G. R. (2005). FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132, 3847-3857. 10.1242/dev.01944 [DOI] [PubMed] [Google Scholar]
- Hartwig S., Ho J., Pandey P., MacIsaac K., Taglienti M., Xiang M., Alterovitz G., Ramoni M., Fraenkel E. and Kreidberg J. A. (2010). Genomic characterization of Wilms’ tumor suppressor 1 targets in nephron progenitor cells during kidney development. Development 137, 1189-1203. 10.1242/dev.045732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard S. A. and El-Dahr S. S. (2016). Epigenetics of renal development and disease. Yale J. Biol. Med. 89, 565-573. [PMC free article] [PubMed] [Google Scholar]
- Hoy W. E., Bertram J. F., Denton R. D., Zimanyi M., Samuel T. and Hughson M. D. (2008). Nephron number, glomerular volume, renal disease and hypertension. Curr. Opin Nephrol. Hypertens. 17, 258-265. 10.1097/MNH.0b013e3282f9b1a5 [DOI] [PubMed] [Google Scholar]
- Kann M., Bae E., Lenz M. O., Li L., Trannguyen B., Schumacher V. A., Taglienti M. E., Bordeianou L., Hartwig S., Rinschen M. M. et al. (2015). WT1 targets Gas1 to maintain nephron progenitor cells by modulating FGF signals. Development 142, 1254-1266. 10.1242/dev.119735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama I., Mahoney Z., Miner J. H., Graf D., Economides A. N. and Kreidberg J. A. (2008). Podocyte-derived BMP7 is critical for nephron development. J. Am. Soc. Nephrol. 19, 2181-2191. 10.1681/ASN.2007111212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöll B. (2010). Actin-mediated gene expression in neurons: the MRTF-SRF connection. Biol. Chem. 391, 591-597. 10.1515/bc.2010.061 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kwan K. M., Carroll T. J., McMahon A. P., Mendelsohn C. L. and Behringer R. R. (2005). Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132, 2809-2823. 10.1242/dev.01858 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Valerius M. T., Mugford J. W., Carroll T. J., Self M., Oliver G. and McMahon A. P. (2008). Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169-181. 10.1016/j.stem.2008.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D. and Jaenisch R. (1993). WT-1 is required for early kidney development. Cell 74, 679-691. 10.1016/0092-8674(93)90515-R [DOI] [PubMed] [Google Scholar]
- Langmead B. and Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357-359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Oghi K. A., Zhang J., Krones A., Bush K. T., Glass C. K., Nigam S. K., Aggarwal A. K., Maas R., Rose D. W. et al. (2003). Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature 426, 247-254. 10.1038/nature02083 [DOI] [PubMed] [Google Scholar]
- Love M. I., Huber W. and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Hofmann C., Bronckers A. L., Sohocki M., Bradley A. and Karsenty G. (1995). BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 9, 2808-2820. 10.1101/gad.9.22.2808 [DOI] [PubMed] [Google Scholar]
- Maier M. M. and Gessler M. (2000). Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genes. Biochem. Biophys. Res. Commun. 275, 652-660. 10.1006/bbrc.2000.3354 [DOI] [PubMed] [Google Scholar]
- Margueron R., Trojer P. and Reinberg D. (2005). The key to development: interpreting the histone code? Curr. Opin. Genet. Dev. 15, 163-176. 10.1016/j.gde.2005.01.005 [DOI] [PubMed] [Google Scholar]
- McMahon A. P. (2016). Development of the mammalian kidney. Curr. Top. Dev. Biol. 117, 31-64. 10.1016/bs.ctdb.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Yamamura K. and Miyazaki J. (1991). Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193-199. 10.1016/0378-1119(91)90434-D [DOI] [PubMed] [Google Scholar]
- Park J.-S., Ma W., O'Brien L. L., Chung E., Guo J.-J., Cheng J.-G., Valerius M. T., McMahon J. A., Wong W. H. and McMahon A. P. (2012). Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev. Cell 23, 637-651. 10.1016/j.devcel.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perantoni A. O., Timofeeva O., Naillat F., Richman C., Pajni-Underwood S., Wilson C., Vainio S., Dove L. F. and Lewandoski M. (2005). Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132, 3859-3871. 10.1242/dev.01945 [DOI] [PubMed] [Google Scholar]
- Puelles V. G., Douglas-Denton R. N., Zimanyi M. A., Armitage J. A., Hughson M. D., Kerr P. G. and Bertram J. F. (2014). Glomerular hypertrophy in subjects with low nephron number: contributions of sex, body size and race. Nephrol. Dial. Transplant. 29, 1686-1695. 10.1093/ndt/gfu088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self M., Lagutin O. V., Bowling B., Hendrix J., Cai Y., Dressler G. R. and Oliver G. (2006). Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 25, 5214-5228. 10.1038/sj.emboj.7601381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K. M., Combes A. N., Lefevre J., Ju A. L., Georgas K. M., Lamberton T., Cairncross O., Rumballe B. A., McMahon A. P., Hamilton N. A. et al. (2014). Global quantification of tissue dynamics in the developing mouse kidney. Dev. Cell 29, 188-202. 10.1016/j.devcel.2014.02.017 [DOI] [PubMed] [Google Scholar]
- Stark K., Vainio S., Vassileva G. and McMahon A. P. (1994). Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679-683. 10.1038/372679a0 [DOI] [PubMed] [Google Scholar]
- Supek F., Bošnjak M., Škunca N. and Šmuc T. (2011). REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6, e21800 10.1371/journal.pone.0021800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M., Gomez P. E., Dressler G. R. and Gruss P. (1995). Pax-2 controls multiple steps of urogenital development. Development 121, 4057-4065. [DOI] [PubMed] [Google Scholar]
- Trapnell C., Hendrickson D. G., Sauvageau M., Goff L., Rinn J. L. and Pachter L. (2013). Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31, 46-53. 10.1038/nbt.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Xu J., Hsu J. H., Nguyen M., Fujiwara Y., Peng C. and Orkin S. H. (2014). Polycomb repressive complex 2 regulates normal hematopoietic stem cell function in a developmental-stage-specific manner. Cell Stem Cell 14, 68-80. 10.1016/j.stem.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P.-X., Adams J., Peters H., Brown M. C., Heaney S. and Maas R. (1999). Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 23, 113-117. 10.1038/12722 [DOI] [PubMed] [Google Scholar]
- Xu P.-X., Zheng W., Huang L., Maire P., Laclef C. and Silvius D. (2003). Six1 is required for the early organogenesis of mammalian kidney. Development 130, 3085-3094. 10.1242/dev.00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Liu H., Park J.-S., Lan Y. and Jiang R. (2014). Osr1 acts downstream of and interacts synergistically with Six2 to maintain nephron progenitor cells during kidney organogenesis. Development 141, 1442-1452. 10.1242/dev.103283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Gonzalez-Hurtado E. and Martinez E. (2016). Core promoter-specific gene regulation: TATA box selectivity and Initiator-dependent bi-directionality of serum response factor-activated transcription. Biochim. Biophys. Acta 1859, 553-563. 10.1016/j.bbagrm.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimanyi M. A., Hoy W. E., Douglas-Denton R. N., Hughson M. D., Holden L. M. and Bertram J. F. (2009). Nephron number and individual glomerular volumes in male Caucasian and African American subjects. Nephrol. Dial. Transplant. 24, 2428-2433. 10.1093/ndt/gfp116 [DOI] [PMC free article] [PubMed] [Google Scholar]