Abstract
Current thought holds that factor Xa (FXa) bound in the prothrombinase complex is resistant to regulation by protein protease inhibitors during prothrombin activation. Here we provide evidence that, contrary to this view, the FXa-specific serpin inhibitor, protein Z-dependent protease inhibitor (ZPI), complexed with its cofactor, protein Z (PZ), functions as a physiologically significant inhibitor of prothrombinase-bound FXa during prothrombin activation. Kinetics studies showed that the rapid rate of inhibition of FXa by the ZPI-PZ complex on procoagulant membrane vesicles (ka(app) ∼107 m−1 s−1) was decreased ∼10-fold when FXa was bound to FVa in prothrombinase and a further ∼3–4–fold when plasma levels of S195A prothrombin were present (ka(app) 2 × 105 m−1 s−1). Nevertheless, the ZPI-PZ complex produced a major inhibition of thrombin generation during prothrombinase-catalyzed activation of prothrombin under physiologically relevant conditions. The importance of ZPI-PZ complex anticoagulant regulation of FXa both before and after incorporation into prothrombinase was supported by thrombin generation assays in plasma. These showed enhanced thrombin generation when the inhibitor was neutralized with a PZ-specific antibody and decreased thrombin generation when exogenous ZPI-PZ complex was added whether prothrombin was activated directly by FXa or through extrinsic or intrinsic pathway activators. Moreover, the PZ antibody enhanced thrombin generation both in the absence and presence of activated protein C (APC) anticoagulant activity. Taken together, these results suggest an important anticoagulant role for the ZPI-PZ complex in regulating both free FXa generated in the initiation phase of coagulation as well as prothrombinase-bound FXa in the propagation phase that complement prothrombinase regulation by APC.
Keywords: coagulation factor, serpin, prothrombin, protease inhibitor, protease, factor Va, factor Xa, protein Z, protein Z-dependent protease inhibitor, prothrombinase
Introduction
Protein Z-dependent protease inhibitor (ZPI)2 is a serpin family protein protease inhibitor that in conjunction with its cofactor, protein Z (PZ), functions to specifically and rapidly inhibit blood coagulation factor Xa (FXa) on a procoagulant membrane surface (ka(app) ∼107 m−1 s−1) (1–4). ZPI also inhibits factor XIa but this inhibition does not require PZ or procoagulant membrane cofactors (3). ZPI binds tightly to PZ and circulates in plasma mostly complexed with the cofactor (5). PZ is a vitamin K-dependent protein with an N-terminal Gla domain that mediates calcium-dependent binding to procoagulant membranes (6). PZ binding to ZPI is thought to enable ZPI to bind to a membrane surface and rapidly inhibit membrane-bound FXa (3, 4). The physiologic importance of the ZPI-PZ complex as an anticoagulant regulator of FXa is supported by the mild thrombotic risk associated with ZPI or PZ deficiency in mice or humans and severe thrombotic risk in ZPI- or PZ-deficient mice or PZ-deficient humans carrying the factor V (FV) Leiden mutation (7–10).
Previous studies have shown that membrane-associated FXa is rapidly inhibited by the ZPI-PZ complex in the presence of plasma levels of FV and prothrombin, but have suggested that the protease is fully protected from the serpin after FV is activated and binds FXa to form prothrombinase (3). Such findings have implied that the ZPI-PZ complex functions primarily to dampen the coagulation response by inhibiting FXa generated from the extrinsic or intrinsic FXase complexes prior to its incorporation into prothrombinase and to minimally regulate FXa during prothrombinase activation of prothrombin. However, no quantitative studies have been performed to determine the extent to which FXa is protected from inhibition by the ZPI-PZ complex when bound in prothrombinase and activating prothrombin and thus to assess the importance of ZPI-PZ complex in regulating prothrombinase activity during prothrombin activation. In the present study we have performed kinetic studies of ZPI-PZ complex inhibition of membrane-associated FXa in the absence and presence of FVa and prothrombin under physiologically relevant conditions. These studies establish a ∼40-fold reduced ka(app) for ZPI-PZ complex inhibition of FXa when the protease is incorporated into prothrombinase during prothrombin activation that is sufficient to cause a physiologically significant suppression of thrombin generation. A PZ antibody that disrupts the ZPI-PZ complex and neutralizes ZPI-PZ anti-FXa activity is further shown to markedly enhance thrombin generation and the exogenous ZPI-PZ complex is shown to decrease thrombin generation in plasma activated by FXa or by extrinsic or intrinsic pathway activators, consistent with an important anticoagulant function of ZPI-PZ complex in regulating both free and prothrombinase-bound FXa on a procoagulant membrane surface. We finally demonstrate that the ZPI-PZ complex suppresses thrombin generation in plasma independent of the suppression produced by activated protein C (APC), consistent with complementary roles for these two anticoagulants in regulating prothrombinase function.
Results
FVa and prothrombin attenuate ZPI-PZ complex anti-FXa activity
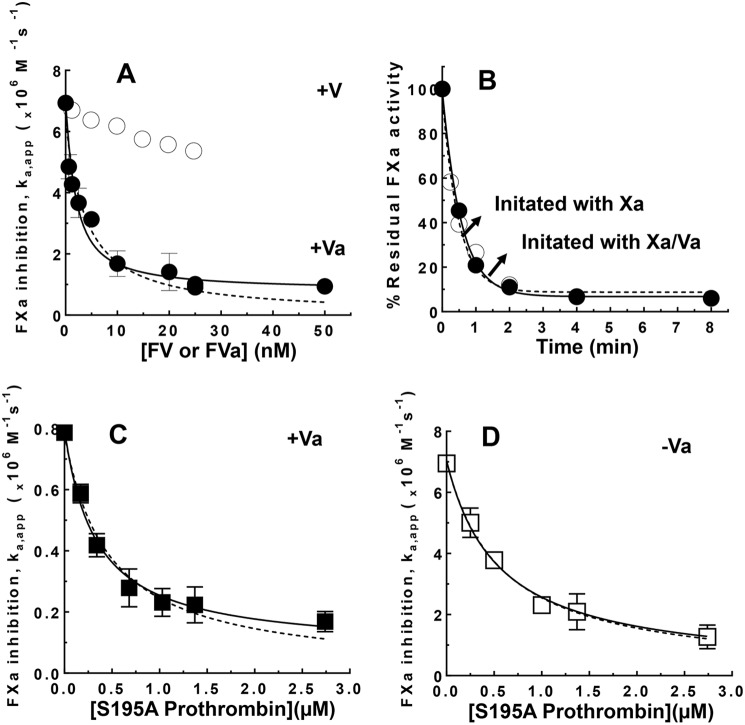
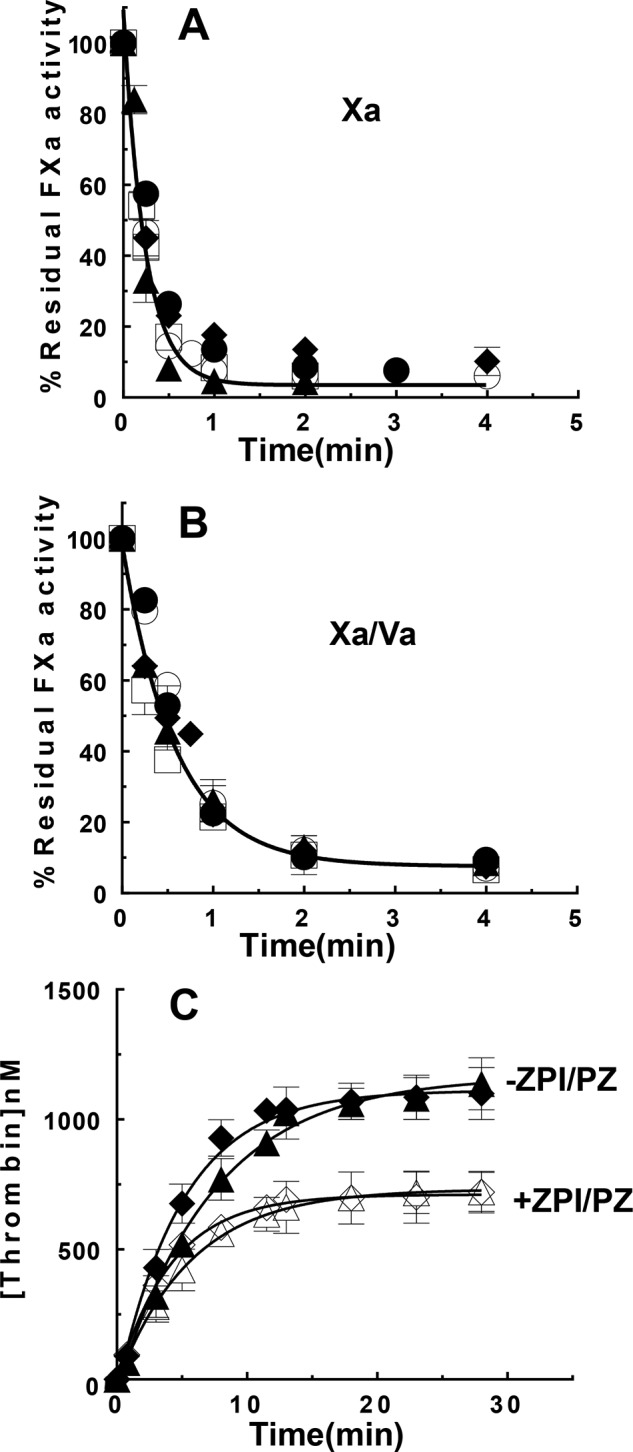
The susceptibility of FXa bound in the prothrombinase complex to inactivation by the ZPI-PZ complex was assessed by kinetic studies of FXa inhibition by the serpin-cofactor complex in the absence and presence of FVa and in the presence of procoagulant phospholipid membrane vesicles and calcium. The additional effect of prothrombin on the kinetics of inhibition of prothrombinase-bound FXa was also investigated by employing a variant S195A prothrombin that produces inactive thrombin upon activation (11). This enabled measurements of FXa inhibition by the ZPI-PZ complex with a fluorogenic FXa substrate without interference from the concomitant generation of active thrombin. ZPI and PZ at 25 nm each rapidly inhibited membrane-associated FXa in the absence of FVa with an apparent association rate constant (ka(app)) of ∼7 × 106 m−1 s−1 and actual ka of ∼107 m−1 s−1 after correction for the Km of 50 nm for the intermediate FXa-ZPI-PZ Michaelis complex, in agreement with past studies (4). Addition of increasing concentrations of factor Va progressively slowed the pseudo-first order inhibition rate constant in a saturable manner to reach a limiting ka(app) of ∼1 × 106 m−1 s−1 at factor Va concentrations of 25–50 nm (Fig. 1A). Progress curves were in all cases best fit by a single exponential process, consistent with factor Xa binding to and equilibrating with factor Va to form prothrombinase significantly faster than it was inactivated by the ZPI-PZ complex. Indeed, initiating reactions with FXa or with preincubated FXa-FVa resulted in indistinguishable progress curves at saturating FVa concentrations (Fig. 1B), consistent with an extremely rapid rate of prothrombinase assembly on a procoagulant lipid surface (12). The factor Va concentration dependence of the ZPI-PZ complex inhibition rate constant was fit best by a noncompetitive inhibition model in which factor Va binding to membrane-associated factor Xa with a KI of 1.5 ± 0.3 nm reduced ka(app) for ZPI-PZ complex inhibition of the protease to 0.8 ± 0.2 × 106 m−1 s−1. Indistinguishable limiting ka(app) values of 0.8–0.9 × 106 m−1 s−1 were measured at plasma concentrations of ZPI (60 nm) and PZ (50 nm) and FVa concentrations yielding >95% saturation of FXa (25–50 nm), verifying a marked susceptibility of prothrombinase-bound FXa to inhibition by the ZPI-PZ complex under physiologically relevant conditions. By contrast, addition of the procofactor, FV, reduced the inhibition rate only slightly over the same range of concentrations (∼1.3-fold) (Fig. 1A), consistent with the much weaker affinity of FV for FXa on a membrane surface and possibly reflecting some activation of FV by FXa during the reaction.
Figure 1.
FVa and S195A prothrombin attenuate ZPI-PZ complex inhibition of membrane-bound FXa. A, ka(app) for ZPI-PZ complex inhibition of 0.5 nm FXa as a function of increasing concentrations of FVa or FV in the presence of 25 μm SUVs (30% PS/70% PC) and 5 mm Ca2+ in Tris buffer, pH 7.4, at 25 °C. Reactions were initiated with FXa/Va/lipid/Ca2+ or FXa/lipid/Ca2+ when FV was present. B, full progress curves of ZPI-PZ complex inhibition of FXa in the presence of 16 nm FVa, lipid, and calcium as in A for reactions initiated with FXa/FVa/lipid/Ca2+ (●) or FXa/lipid/Ca2+ (○). Solid/dashed lines are fits by a single exponential function. C and D, ka(app) for ZPI-PZ complex inhibition of FXa in the presence of 26 nm FVa or the absence of FVa as indicated and in the presence of lipid/Ca2+ as a function of increasing concentrations of S195A prothrombin. Reaction conditions were as in A. ZPI and PZ concentrations were 25 nm each in A and B, 60 and 50 nm in C, and 20 nm each in D. ka(app) was measured by reacting FXa with ZPI-PZ complex in the absence or presence of FV, FVa, or S195A prothrombin plus lipid/Ca2+ for different times and assaying residual FXa activity with a fluorogenic FXa substrate, as described under “Experimental procedures.” Solid lines in A, C, and D are fits of data by a noncompetitive model and dashed lines are fits by a competitive model. The noncompetitive fit is significantly better than the competitive fit for A (p < 0.01) and C (p < 0.05) but not for D. Data represent the average of 3–4 independent measurements, mean ± S.D.
The reduced rate of ZPI-PZ complex inhibition of prothrombinase-bound FXa was progressively lowered even further when increasing concentrations of S195A prothrombin were added in the presence of saturating levels of FVa (Fig. 1C). The data were fit in this case marginally better by a noncompetitive versus a competitive model, yielding a KI of 0.46 ± 0.07 μm for S195A prothrombin, comparable with reported Km values for prothrombin activation by prothrombinase (13). At plasma prothrombin concentrations (1.4 μm), ka(app) was reduced an additional 3–4-fold from the value measured for prothrombinase-bound FXa in the absence of prothrombin. The ka(app) for ZPI-PZ complex inhibition of FXa is thus maximally reduced ∼40-fold to ∼2 × 105 m−1 s−1 at physiologic levels of FVa and prothrombin. ka(app) for ZPI-PZ complex inhibition of membrane-associated FXa in the absence of FVa was also reduced by S195A prothrombin in accordance with a competitive model, with a ∼3–4-fold reduced ka(app) at plasma prothrombin concentrations and apparent KI of 0.57 ± 0.07 μm for S195A prothrombin (Fig. 1D), similar to the KI measured for S195A prothrombin when FXa was bound to FVa in prothrombinase (Fig. 1C). The similar KI values was consistent with FVa binding to FXa affecting kcat and not Km for FXa activation of prothrombin (13). SDS-PAGE analyses confirmed normal activation of S195A prothrombin by prothrombinase in the absence of the ZPI-PZ complex, in accordance with previous studies (11).
The reduced rates of ZPI-PZ complex inhibition of prothrombinase-bound FXa did not result from significant alterations in the efficiency of inhibition, as was evident from the modest increases in inhibition stoichiometries from 2.8 ± 0.2 in the absence of FVa to 3.4 ± 0.1 and 4.7 ± 0.1 when saturating FVa (nm) alone or with plasma levels of S195A prothrombin was present, respectively. Moreover, SDS-PAGE immunoblotting analyses with anti-FXa and anti-ZPI antibodies showed that ZPI-PZ complex inhibition of membrane-associated free or prothrombinase-bound FXa produced identical covalent ZPI-FXa product complexes indicative of inhibition by the standard serpin mechanism (Fig. S1). These results indicate that FXa retains a significant susceptibility to ZPI-PZ complex inhibition when FXa is complexed with FVa in prothrombinase and physiologic concentrations of prothrombin are present.
ZPI-PZ complex inhibits prothrombinase activation of prothrombin
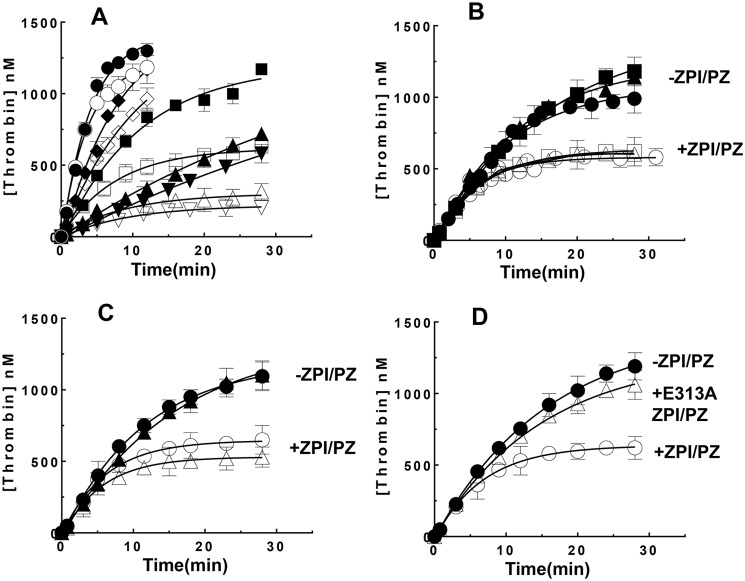
To show that the ZPI-PZ complex effectively inhibits prothrombinase-bound FXa during the activation of native prothrombin, we assessed the ability of the ZPI-PZ complex to inhibit thrombin generation, assayed with a thrombin chromogenic substrate, during prothrombinase-catalyzed activation of prothrombin. This was evaluated at plasma levels of ZPI (60 nm), PZ (50 nm), and prothrombin (1.4 μm), and prothrombinase assembled with 0.015–0.2 nm FXa, a large molar excess of FVa (2–16 nm) and procoagulant phospholipid vesicles, and calcium in reactions initiated with preassembled prothrombinase. The ZPI-PZ complex completely blocked thrombin generation in reactions in which FVa was replaced with FV, consistent with rapid inhibition of FXa by the ZPI-PZ complex before FV is activated to form prothrombinase (not shown). Thrombin generation from prothrombinase-catalyzed activation of prothrombin was significantly inhibited by plasma levels of ZPI and PZ (14, 15) under all conditions examined, with the extent of inhibition depending on the prothrombinase concentration (Fig. 2, A and B). Reactions in which prothrombinase was varied by fixing the FVa concentration at 9 nm and increasing the FXa concentration from 0.015 to 0.2 nm resulted in a complete inhibition of thrombin generation within 30 min in contrast to the full activation of prothrombin observed in the absence of the ZPI-PZ complex. The extent of inhibition progressively decreased as the prothrombinase concentration was increased, with maximal thrombin generation plateauing at ∼200–1200 nm as the prothrombinase concentration increased from 0.015 to 0.2 nm. Fixing the FXa concentration (0.06 nm) and increasing FVa over concentrations sufficient to mostly or fully saturate prothrombinase (2–16 nm) resulted in similar extents of inhibition of thrombin generation, with a maximal generation of ∼600 nm thrombin in 30 min. The extent of inhibition was minimally dependent on whether the reaction was initiated with FXa or with preincubated prothrombinase when FVa concentrations were saturating (Fig. 2C), consistent with saturating FVa favoring the incorporation of FXa into prothrombinase before FXa was inhibited by the ZPI-PZ complex. These results indicate that ZPI/PZ effectively competes with prothrombin for binding to and inhibiting FXa bound to FVa in prothrombinase during prothrombin activation at levels of prothrombinase generated during coagulation (<0.1 nm) (26), but is less effective once prothrombinase levels exceed 0.1 nm and favor prothrombin activation.
Figure 2.
ZPI/PZ inhibits prothrombinase-catalyzed activation of prothrombin. Time course of prothrombin activation (1.4 μm) by prothrombinase assembled with FVa and FXa at the concentrations indicated below, 25 μm 30% PS/70 %PC vesicles, and 5 mm Ca2+ in the absence (solid symbols) or presence (open symbols) of 60 nm ZPI and 50 nm PZ in Tris buffer, pH 7.4, at 25 °C. A, 9 nm FVa and 0.015 nm (▾, ▿), 0.03 nm (▴, ▵), 0.06 nm (■, □), 0.1 nm (♦, ♢), or 0.2 nm (●, ○) FXa; B, 0.06 nm FXa and 2 nm (●, ○), 9 nm (▴, ▵), or 16 nm FVa (■, □); C and D, 16 nm FVa and 0.06 nm FXa. Reactions were initiated with FXa/FVa/lipid/Ca2+ in all cases except in C, where initiation was with either FXa/FVa/lipid/Ca2+ (●, ○) or FXa/lipid/Ca2+ (▴, ▵). Panel D compares the effect of WT ZPI (○) with an E313A exosite mutant ZPI (▵). Reactions were quenched at varying times with a chromogenic thrombin substrate plus 10 mm EDTA in reaction buffer and residual thrombin activity was determined as described under “Experimental procedures.” Data represent the average of 3–4 independent measurements, mean ± S.D. Solid lines are empirical fits of data.
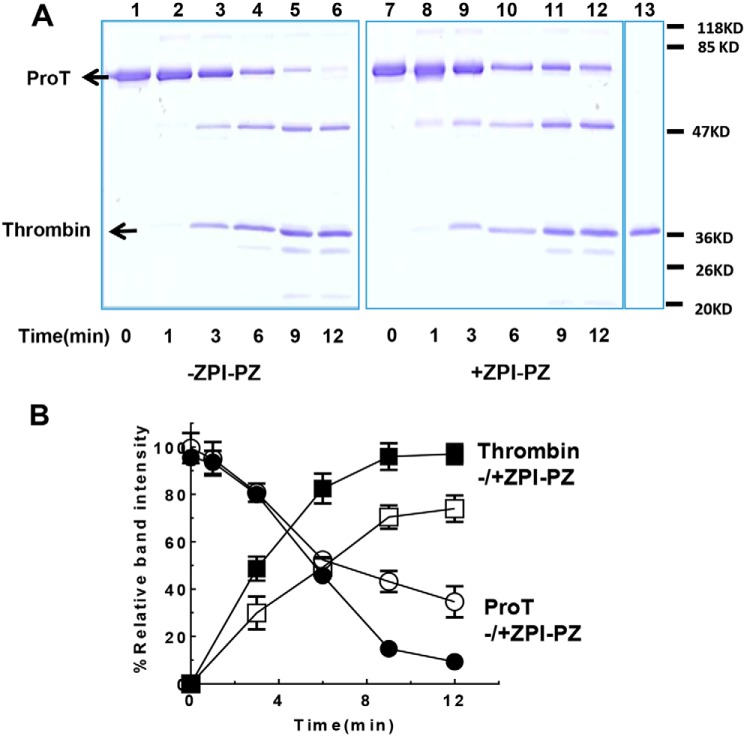
A mutant E313A ZPI that binds PZ normally but inhibits membrane-associated FXa with a ∼10-fold reduced ka(app) when complexed with PZ due to the loss of a ZPI exosite interaction with the autolysis loop of FXa (16, 17) showed a greatly reduced ability compared with WT ZPI to inhibit thrombin generation during prothrombinase-catalyzed activation of prothrombin (Fig. 2D). SDS-PAGE analyses confirmed that prothrombin activation by prothrombinase was slowed in the presence of ZPI/PZ and correlated well with the observed rates of thrombin generation (Fig. 3). These results demonstrate that FXa retains a marked susceptibility to ZPI-PZ complex inhibition when bound in prothrombinase during prothrombin activation at prothrombinase concentrations expected to be generated during coagulation, consistent with the reduced rate of ZPI-PZ complex inhibition of prothrombinase-bound FXa in the presence of prothrombin being physiologically relevant.
Figure 3.
SDS-PAGE analysis of ZPI-PZ complex inhibition of prothrombinase activation of prothrombin. A, prothrombin (ProT, 1. 4 μm) was activated by prothrombinase assembled with ∼0.1 nm FXa, 25 μm PS/PC lipid, 5 mm Ca2+, and 30 nm FVa in the absence or presence of 60 nm ZPI and 50 nm PZ in Tris buffer, pH 7.4, at 25 °C. Reactions were quenched at varying times with EGR-chloromethyl ketone (∼400 μm) and FPR-chloromethyl ketone (∼400 μm) in buffer and analyzed by 10% reducing SDS-PAGE with detection of proteins by Coomassie Blue staining. Prothrombin activation in the absence (lanes 1–6) and presence (lanes 7–12) of ZPI-PZ complex is shown. Lane 13 is a pure thrombin control (in the same gel of lanes 7–12). FVa, ZPI, PZ, and FXa in reactions were only weakly detected or undetectable. B, prothrombin disappearance (circles) and thrombin appearance (B Chain) (squares) in the absence (solid symbols) or presence (open symbols) of ZPI-PZ complex was quantitated with NIH ImageJ software and plotted against time. Data represent the average of 3 independent SDS-PAGE analyses, mean ± S.D.
Effect of lipid composition on ZPI-PZ complex inhibition of prothrombinase-bound FXa
The procoagulant lipid employed to characterize ZPI-PZ complex inhibition of free and prothrombinase-bound FXa in these and past studies contained a supraphysiologic phosphatidylserine (PS) composition. To ensure that ZPI-PZ complex inhibition of prothrombinase-bound FXa was also significant with procoagulant lipids more representative of the physiologically relevant membrane, we analyzed ZPI-PZ complex inhibition of free and prothrombinase-bound FXa with lipids of varying PS composition and containing both phosphatidylcholine (PC) and phosphatidylethanolamine (PE). Reducing the fraction of PS from 30 to 2.5% had minimal effect on the rapid rate of ZPI-PZ complex inhibition of free FXa in the absence of FVa (Fig. 4A) or on the slower rate of inhibition of prothrombinase-bound FXa in the presence of saturating FVa concentrations (Fig. 4B), as long as PE was present at 25–40% and PC comprised the remaining phospholipid fraction. Reducing the fraction of PS with only PC comprising the remaining lipid fraction caused the rates of ZPI/PZ inhibition of both free FXa and prothrombinase-bound FXa to be reduced (not shown). Thrombin generation from prothrombinase-catalyzed activation of prothrombin was similarly inhibited by plasma levels of ZPI and PZ in the presence of procoagulant lipid vesicles containing 10% PS/25% PE/65% PC or 30% PS/70% PC (Fig. 4C). These results indicate that lipid vesicles with compositions similar to those found in plasma membranes and activated platelet membranes, i.e. with low fractional PS along with PE and PC, are as effective as synthetic high PS with just PC containing vesicles in supporting ZPI-PZ inhibition of thrombin generation from prothrombinase-catalyzed activation of prothrombin under physiologically relevant conditions.
Figure 4.
ZPI/PZ inhibits free or prothrombinase-bound FXa or prothrombinase activation of prothrombin on SUVs that mimic activated platelet membranes. Progress curves of ZPI-PZ complex inhibition of A, free FXa, or B, prothrombinase-bound FXa in the presence of 25 μm SUVs comprised of 2.5% PS/25% PE/72.5% PC (●); 5% PS/25% PE/70% PC (○), 10% PS/25% PE/65% PC (♦); 20% PS/40% PE/40% PC (□); 30% PS/70% PC (▴) in Tris buffer, pH 7.4, containing 5 mm Ca2+ at 25 °C. Reactions contained ∼0.5 nm FXa with either no FVa (A) or 25 nm FVa (B), and ZPI and PZ at 25 (A) or 50 (B) nm each. C, ZPI/PZ (60/50 nm) inhibition of thrombin generation from prothrombin (1.4 μm) catalyzed by prothrombinase assembled with ∼0.08 nm FXa, 16 nm FVa, and 25 μm SUVs containing 10% PS/25% PE/65% PC (♦, ♢) or 30% PS/70% PC (▴, ▵) plus 5 mm Ca2+. Open and closed symbols indicate reactions in the presence or absence of ZPI/PZ. Reactions were initiated with FXa/lipid/Ca2+ in A, or with FXa/Va/lipid/Ca2+ in B and C. At varying time points, reactions were quenched with the fluorogenic FXa substrate containing 5 mm EDTA (A and B), or with the thrombin chromogenic substrate containing 10 mm EDTA (C), and residual FXa or thrombin activity was determined as described under “Experimental procedures.” Progress curves were fit by a single exponential decay with a nonzero end point for A and B (solid lines show fits of reaction with 30% PS/70% PC), or empirical fits of data for C. Data were derived from at least three independent measurements, mean ± S.D.
ZPI-PZ versus antithrombin regulation of membrane-associated-FXa
Antithrombin is thought to be an important physiologic inhibitor of membrane-associated FXa, but a poor inhibitor of prothrombinase-bound FXa (18, 19). To determine the relative importance of ZPI-PZ complex and antithrombin as inhibitors of membrane-associated FXa either free or bound with FVa in prothrombinase, we measured ka(app) for antithrombin inhibition of FXa in the absence and presence of saturating FVa and in the presence of phospholipid vesicles and calcium. ka(app) measured in the absence of FVa (1.6 × 103 m−1 s−1) was reduced ∼10-fold in the presence of saturating FVa (1.4 × 102 m−1 s−1) and reduced a further 2–3–fold in the presence of plasma levels of S195A prothrombin (∼70 m−1 s−1) (Fig. 5A). Comparison with ka(app) values for ZPI-PZ complex inhibition of unbound and prothrombinase-bound FXa suggests that at plasma concentrations of antithrombin (2.3 μm) (20) and ZPI/PZ (60 nm/50 nm), ZPI-PZ complex is predicted to inhibit membrane-associated FXa minimally ∼100-fold faster than antithrombin whether unbound or bound in the prothrombinase complex. Consistent with this expectation, antithrombin had minimal effect on the rate of ZPI-PZ complex inhibition of membrane-bound FXa in the absence or presence of saturating FVa and plasma levels of S195A prothrombin when the two serpins were present at their plasma concentrations (Fig. 5B). The overriding importance of the ZPI-PZ complex in regulating prothrombinase-bound FXa in the presence of antithrombin was further shown in thrombin generation assays of prothrombinase-catalyzed activation of prothrombin. In the absence of ZPI-PZ complex but presence of plasma levels of antithrombin, thrombin generation showed the expected bell-shaped profile with thrombin generation reaching a peak followed by a decline due to the significant rate of antithrombin inhibition of thrombin (Fig. 5C). Addition of plasma levels of ZPI-PZ complex caused a reduction in thrombin generation at the peak and subsequent descending part of the bell-shaped curve, consistent with the ZPI-PZ complex reducing the concentration of prothrombinase-bound FXa catalyst capable of activating prothrombin.
Figure 5.
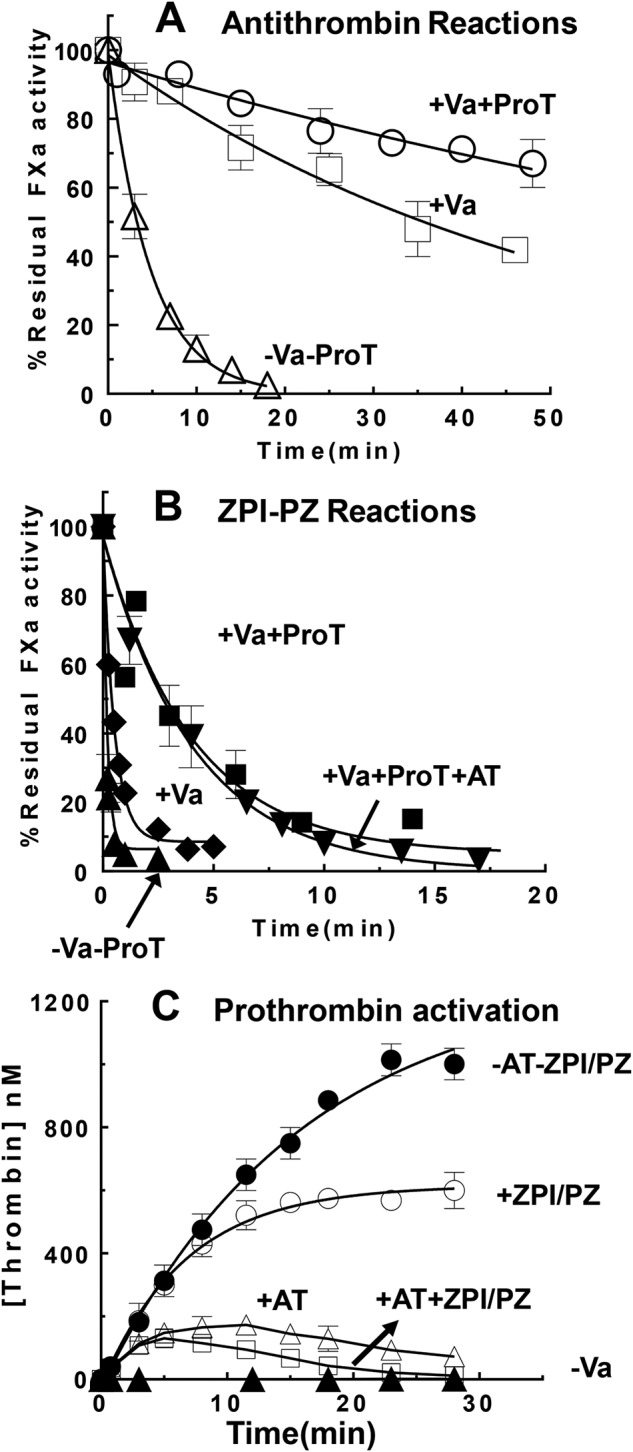
ZPI-PZ versus antithrombin (AT) inhibition of free and prothrombinase-bound FXa. Progress curves of FXa (∼0. 5 nm) inhibition by antithrombin (2.3 μm) (panel A) or by ZPI (60 nm)/PZ (50 nm) (panel B) in the presence of 25 μm PS/PC lipid, 5 mm Ca2+ was measured in the absence or presence of 30 nm FVa alone or 30 nm FVa plus 1.4 μm S195A prothrombin in Tris buffer, pH 7.4, at 25 °C. Reactions were initiated with FXa and samples withdrawn at different time points and diluted into FXa fluorogenic substrate containing 5 mm EDTA to assay residual FXa activity as described under “Experimental procedures.” Progress curves were fit by a single exponential decay with a zero end point for antithrombin reactions or a nonzero end point for ZPI/PZ reactions. C, thrombin generation curves resulting from the activation of 1.4 μm prothrombin by prothrombinase assembled with ∼0.06 nm FXa, 30 nm FVa, 25 μm PS/PC lipid, and 5 mm Ca2+ in the absence or presence of 60 nm ZPI/50 nm PZ alone, 2.3 μm antithrombin alone, or both ZPI/PZ and antithrombin in Tris buffer, pH 7.4, at 25 °C. Reactions were initiated with preincubated FXa/FVa/lipid/Ca2+. Thrombin generation was measured by quenching samples at different time points into thrombin chromogenic substrate plus 10 mm EDTA as described under “Experimental procedures.” Data were derived from at least three independent measurements, mean ± S.D.
PZ antibody neutralization of the ZPI-PZ complex
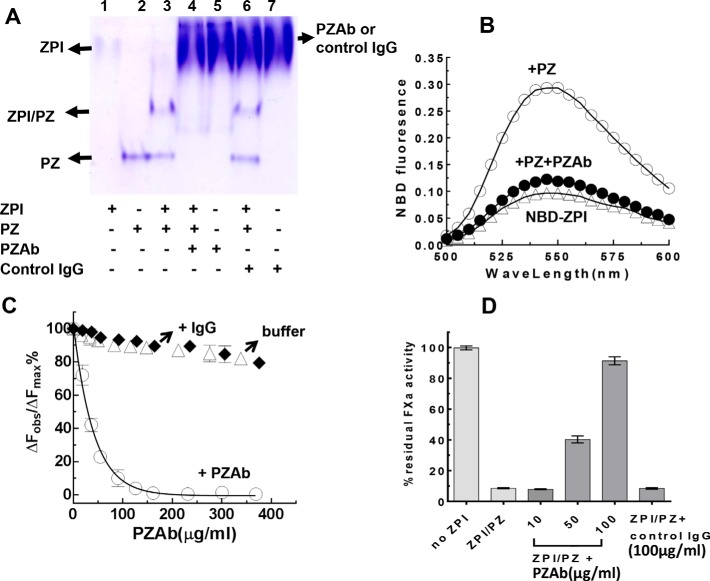
To further evaluate the anticoagulant effect of the ZPI-PZ complex on thrombin generation in plasma, we employed a neutralizing PZAb. The specificity of this antibody for binding PZ and for disrupting the ZPI-PZ complex was first assessed by native PAGE analysis. Complexation of ZPI with PZ is reflected on native PAGE by the appearance of a new complex band at a mobility intermediate between that of free ZPI and free PZ (4). Addition of the PZAb, but not a control IgG antibody, eliminated the ZPI-PZ complex band as well as the free PZ band, demonstrating that the PZAb specifically binds PZ and dissociates the complex (Fig. 6A). This finding was confirmed by examining the effect of the PZAb on the binding of PZ to a site-specific NBD fluorophore-labeled ZPI. The NBD fluorophore is covalently linked to ZPI through an engineered Cys-239 on the periphery of the PZ-binding site and acts as a reporter of PZ binding by undergoing a ∼300% fluorescence enhancement when PZ is bound (21). Addition of a molar excess of the PZAb to the NBD-labeled ZPI-PZ complex caused an almost complete quenching of the PZ-induced fluorescence enhancement, consistent with the antibody inducing the dissociation of the ZPI-PZ complex (Fig. 6B). Titration of the PZAb into the NBD-labeled ZPI-PZ complex caused a dose-dependent decrease in the NBD fluorescence to an end point corresponding to the fluorescence of free labeled ZPI, whereas adding an IgG control antibody or buffer caused minimal fluorescence changes (Fig. 6C). Fitting of the titration data to a competitive binding curve indicated that the PZAb bound to and displaced PZ from the labeled ZPI with a KD of 14.3 ± 0.2 nm. The PZAb but not a control IgG was further able to dose-dependently neutralize the anti-FXa activity of the ZPI-PZ complex measured in the presence of procoagulant phospholipid vesicles and calcium (Fig. 6D). The PZAb thus specifically binds PZ with high affinity and effectively displaces PZ from the ZPI-PZ complex to result in neutralization of ZPI-PZ complex anti-FXa activity.
Figure 6.
PZAb disrupts the ZPI-PZ complex and neutralizes ZPI/PZ anti-FXa activity. A, native PAGE (5. 5% gel) analysis of ZPI (2 μg) and PZ (2.5 μg) alone or together as indicated in the absence or presence of an anti-human PZAb (20 μg) or a control IgG (Sigma) (20 μg). B, fluorescence emission spectra of NBD-labeled K239C ZPI (50 nm) alone, after adding a molar excess of PZ (63 nm) and after adding both PZ and 100 μg/ml of PZAb were measured in Tris buffer, pH 7.4, at 25 °C with excitation at 480 nm. Triplicate spectra were averaged after correction for buffer background and dilution. C, titrations of NBD-labeled K239C ZPI (50 nm) plus PZ (63 nm) with PZAb (○), control IgG (♦), or buffer (▵). Observed relative fluorescence changes at the emission maximum (ΔFobs/ΔFmax) were fit by the cubic equation for competitive binding to obtain the KD for the PZAb-PZ interaction (solid line). D, reactions of 55 nm ZPI and 46 nm PZ with 4 nm FXa, 25 μm PS/PC, and 5 mm Ca2+ in Tris buffer, pH 7.4, at 25 °C after a 2-min preincubation of PZ alone with 0–100 μg/ml of PZAb or control IgG (100 μg/ml) in calcium containing buffer. Reactions were quenched into FXa chromogenic substrate plus 10 mm EDTA after 5 min and residual FXa activity was assayed. Data represent the average of 3–4 independent measurements, mean ± S.D., for C and D.
ZPI-PZ complex inhibits thrombin generation in plasma
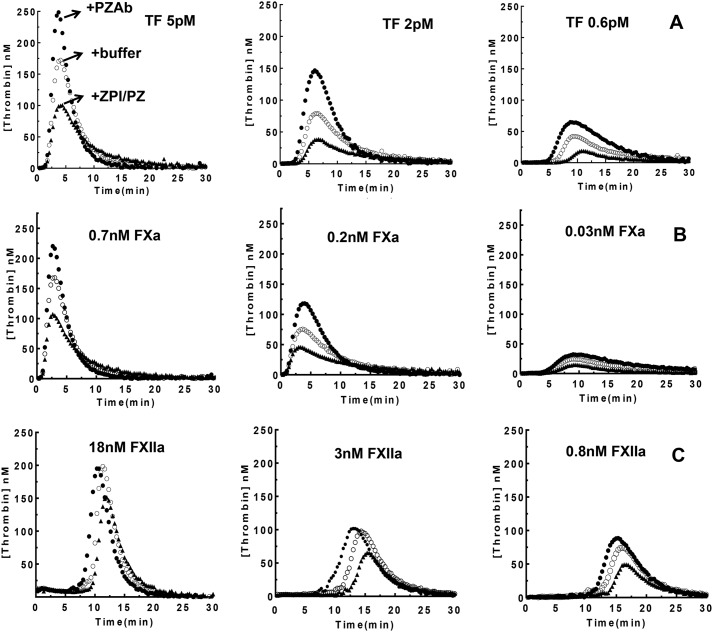
We next examined whether the ZPI-PZ complex effectively inhibited thrombin generation in normal human plasma when coagulation was activated by intrinsic (factor XIIa (FXIIa)) or extrinsic (tissue factor (TF)) pathway activators or by the prothrombin activator FXa, using calibrated automated thrombography (CAT) assays. Three doses of each activator were employed and thrombin generation was monitored in the absence or presence of the PZAb to neutralize endogenous ZPI-PZ complex or in the presence of added ZPI-PZ complex to increase the level of the anticoagulant complex. Addition of the PZAb significantly enhanced thrombin generation, whereas addition of the ZPI-PZ complex reduced thrombin generation for all three activators (Fig. 7), consistent with a potent dose-dependent anticoagulant effect of the ZPI-PZ complex on thrombin generation for all modes of activation. Control experiments showed that the control IgG had no effect on thrombin generation profiles in these assays (not shown). Moreover, the PZAb did not enhance thrombin generation in PZ-deficient plasma but did reverse a decrease in thrombin generation produced by exogenous ZPI-PZ complex addition to the PZ-deficient plasma (Fig. S2). The enhanced thrombin generation caused by the PZAb was evident from parallel changes in both the peak level of thrombin generated (TP) and the total thrombin generated (endogenous thrombin potential, ETP) except for the intermediate and highest doses of the intrinsic pathway FXIIa activator, which showed less significant increases (Table S1). The latter insensitivity of the PZAb may reflect the 50–100–fold faster activation of FX by intrinsic FXase compared with extrinsic FXase (22–25) and consequent increased prothrombinase levels generated at the higher FXIIa doses that are less efficiently inhibited by ZPI/PZ (Fig. 2A). Notably, the lag time before the onset of thrombin generation as well as the time to reach the thrombin peak progressively increased with increasing levels of ZPI-PZ complex for extrinsic and intrinsic activators, whereas these parameters showed no significant change with increasing doses of ZPI-PZ when FXa was the activator (Table S1). These findings are consistent with the importance of ZPI-PZ in regulating FXa prior to its incorporation into prothrombinase in the initiation phase of coagulation as well as after FXa is bound in prothrombinase in the propagation phase of coagulation.
Figure 7.
ZPI-PZ dose-dependently inhibits thrombin generation in plasma. Thrombin generation in normal human plasma after activating coagulation with A, TF; B, FXa; or C, factor XIIa was measured by CAT at pH 7. 4, 37 °C as described under “Experimental procedures.” To 80 μl of plasma samples containing fluorogenic substrate, CTI (only for TF and FXa activators), and lipid (20% PS/20% PE/60% PC) was added to 20 μl of activator (only for TF), PZAb, or ZPI-PZ as indicated. Thrombin generation was initiated by adding 20 μl of CaCl2 plus either FXa or FXIIa activator. Final concentrations were 420 μm fluorogenic substrate, 30 μg/ml of CTI, 25 μm lipid, 16 mm calcium, and the indicated concentrations of activators. Curves represent control plasma (open circles), addition of 160 μg/ml of PZAb (closed circles), or 25 nm each of ZPI and PZ (solid triangles). ThrombinoscopeTM software was used to analyze fluorescence data to obtain the lag time, the thrombin peak height (TP), time to thrombin peak (TTP), and the ETP (integrated area under the thrombogram) (Table S1). Data represent the average of triplicate measurements.
ZPI-PZ complex and APC independently reduce thrombin generation in plasma
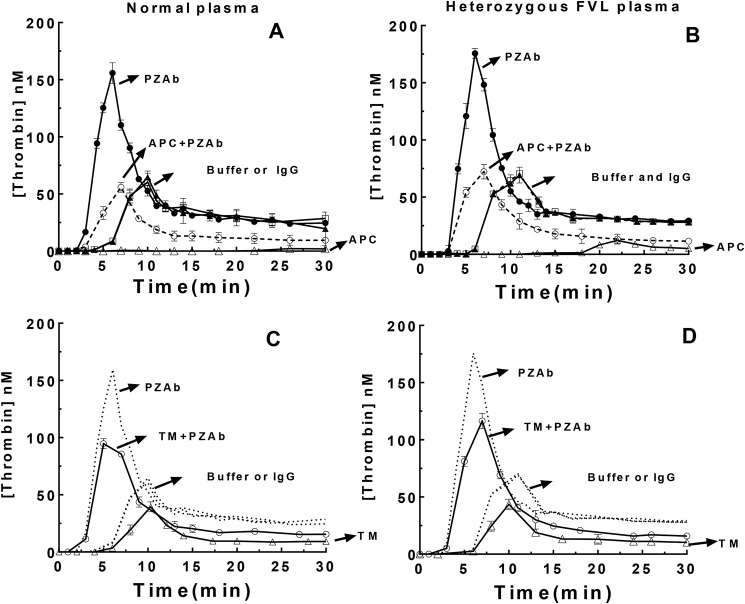
We further compared thrombin generation in normal and heterozygous FVL plasmas activated with TF in the absence and presence of exogenous APC or soluble thrombomodulin (TM) to activate endogenous protein C (Fig. 8). Thrombin generation in these assays was monitored by discontinuous assays of thrombin activity with a chromogenic substrate. Exogenous APC or TM decreased thrombin generation in normal and heterozygous FVL plasmas and this effect was dose-dependent (not shown) and marginally less in the FVL plasma, consistent with differential APC inhibition of normal and mutant FVas in these plasmas. Addition of the PZAb still significantly enhanced thrombin generation in these plasmas in the presence of exogenous APC or TM, consistent with complementary anticoagulant effects of endogenous ZPI/PZ and APC in inhibiting prothrombinase function. Control assays in the absence of exogenous APC or TM with or without addition of PZAb or control IgG/buffer to the plasmas confirmed that the PZAb but not the control IgG enhanced thrombin generation in both plasmas by blocking endogenous ZPI-PZ anti-FXa activity. Minor differences in thrombin generation profiles observed in normal and FVL plasmas in these control assays were abolished by addition of an APC antibody (not shown), consistent with traces of endogenous APC in these plasmas as reported previously (26). These findings suggest that ZPI-PZ and APC act independently to inhibit prothrombinase function and reduce thrombin generation.
Figure 8.
ZPI/PZ and APC anticoagulant activities are complementary in reducing thrombin generation. Thrombin generation in normal (A and C) or heterozygous FV Leiden (B and D) plasmas was measured by activating coagulation with TF, lipid, and CaCl2 in the presence of CTI as described in the legend to Fig. 7 and with or without addition of PZAb/control IgG, TM, or APC as indicated at pH 7.4, 37 °C. Final concentrations were 30 μg/ml of CTI, 25 μm lipid, 2 pm TF, 16 mm calcium, 160 μg/ml of PZAb or control IgG, 3.3 nm APC, 1.5 nm TM. Thrombin generation was assayed by quenching aliquots (10 μl) at different times into 1 ml of thrombin chromogenic substrate plus 10 mm EDTA in reaction buffer and residual thrombin activity was determined as described under “Experimental procedures.” The four curves in each panel represent additions of: (i) buffer (▴) or control IgG (□) to assess endogenous ZPI/PZ activity in the absence of APC activity, (ii) PZAb to block endogenous ZPI/PZ activity in the absence of APC activity (●), (iii) APC (A and B) or soluble thrombomodulin (TM) (C and D) to assess exogenous/endogenous APC activity and endogenous ZPI/PZ activity (▵), and (iv) APC or TM plus PZAb to assess exogenous/endogenous APC activity in the absence of endogenous ZPI/PZ activity (○). The dotted lines in C and D reproduce the corresponding data of A and B. Data were derived from three independent measurements, mean ± S.D.
Discussion
Our results demonstrate that the ZPI-PZ complex is a reasonably efficient inhibitor of prothrombinase-bound FXa on the membrane surface and effectively competes with prothrombin for binding to and inactivating FXa in prothrombinase under physiologically relevant conditions. The observed ∼40-fold reduction in the rate constant for ZPI-PZ complex inhibition of FXa when bound in prothrombinase in the presence of plasma levels of prothrombin compared with the near diffusion-limited inhibition of unbound FXa on a procoagulant membrane (107 m−1 s−1) thus indicates that at plasma concentrations of ZPI and PZ, prothrombinase-bound FXa is markedly susceptible to ZPI-PZ complex inhibition during prothrombin activation (half-life ∼90 s). Notably, our finding that FVa reduces ka(app) for ZPI-PZ complex inhibition of FXa to a limiting value of 8–9 × 105 m−1 s−1 at concentrations of the cofactor sufficient to saturate FXa, and independent of whether prothrombinase is preassembled or assembled in the presence of ZPI-PZ indicates that the ZPI-PZ complex directly inhibits prothrombinase-bound FXa in a noncompetitive manner and does not require FXa to dissociate from prothrombinase to render the protease susceptible to inhibition. Direct inhibition of prothrombinase-bound FXa by the ZPI-PZ complex is consistent with structural alignments of FXa in the homologous FXa-FVa prothrombinase complex from snake venom (27) with FXa in the antithrombin-FXa Michaelis complex (28) and with alignments of antithrombin in the latter complex with ZPI in the ZPI-PZ complex (16) (Fig. S3). These alignments suggest that FXa bound to FVa in prothrombinase interacts with the serpin reactive loop in a manner that minimally interferes with FVa by placing ZPI and bound PZ in an orthogonal orientation relative to that of FVa (Fig. S3). However, an interaction of the A2 domain C-terminal peptide of FVa with the FXa heparin-binding exosite may sterically interfere with the ZPI reactive center loop and exosite interactions with the FXa active-site and account for the reduced rate of ZPI-PZ complex inhibition of FXa in prothrombinase. FVa binding to FXa could also impede interactions between the PZ and FXa Gla domains on the membrane that promote FXa inhibition (3, 4, 17). The additional effect of prothrombin in slowing ZPI-PZ complex inhibition of prothrombinase-bound FXa may result from prothrombin binding to prothrombinase, further interfering with or excluding ZPI-PZ complex from binding FXa in prothrombinase (29). The observed 3–4-fold reduction in ka(app) is consistent with noncompetitive or competitive binding of the prothrombin substrate with a KI within the range of reported Km values (13). Noncompetitive binding is possible because the prothrombin Km is dominated by exosite rather than active site interactions (29) and may thus not exclude the ZPI-PZ complex from binding at the FXa active site.
That the observed rates of inhibition of prothrombinase-bound FXa by the ZPI-PZ complex are physiologically relevant was demonstrated by showing that plasma levels of the ZPI-PZ complex markedly inhibit thrombin generation in prothrombin activation assays at saturating FVa levels and FXa levels expected to be generated during the activation of coagulation (30). Moreover, our finding that PS/PE/PC vesicles with low PS content that mimic the composition of plasma membranes and activated platelet membranes (31, 32) are as effective as PS/PC vesicles with a high PS fraction in promoting ZPI-PZ complex inhibition of prothrombinase supports the physiologic significance of our findings and is in agreement with the report that low PS content membranes containing both PE and PC bind PZ as well as high PS content membranes containing PC but not PE (33). In TF-initiated clotting of whole blood, prothrombinase levels reach a maximum of ∼0.1 nm and are then inhibited despite residual prothrombin, and an excess of FVa and platelet prothrombinase sites, implying that inhibition of FXa bound in prothrombinase is responsible for this inhibition (34). Our results suggest that the ZPI-PZ complex may account for this inhibition. Notably, ZPI-PZ complex inhibition of thrombin generation was abrogated with a ZPI exosite mutant with a ∼10-fold reduced reactivity with FXa, implying that the rate of ZPI-PZ complex inhibition of FXa in prothrombinase is poised to limit the amount of thrombin produced during coagulation.
Our findings conflict with an earlier report that found complete protection of prothrombinase-bound FXa from ZPI-PZ complex inhibition when plasma levels of prothrombin were present (3). Two factors may explain this discordant finding. First, the previous report measured thrombin generation only over the early phase of the reaction (120 s) where more modest reductions in the rate of prothrombinase activation of prothrombin are evident compared with later stages (600 s), where a pronounced reduction in rate and extent of thrombin generation is clearly observed. Second, our finding that ZPI-PZ complex inhibition of thrombin generation is most effective when prothrombinase levels are less than 0.1 nm is in keeping with a lesser inhibition by the ZPI-PZ complex at the single prothrombinase concentration examined in this study of 0.1 nm.
The importance of ZPI-PZ complex regulation of prothrombinase-bound and unbound FXa was supported by thrombin generation assays in plasma. A PZ antibody provided a key reagent to block ZPI-PZ anticoagulant activity in plasma based on its demonstrated ability to specifically bind PZ, disrupt the ZPI-PZ complex, and neutralize ZPI-PZ complex anti-FXa activity. The PZ antibody significantly enhanced thrombin generation in plasma, whereas addition of exogenous ZPI-PZ complex suppressed thrombin generation when coagulation was activated with extrinsic or intrinsic pathway activators or by direct activation of prothrombin with FXa, indicating a marked dose-dependent anticoagulant effect of ZPI-PZ complex when coagulation was activated through either extrinsic or intrinsic pathways. The finding that altering the endogenous levels of the ZPI-PZ complex produces major effects on thrombin generation in plasma when prothrombin is directly activated with FXa is consistent with prothrombinase-bound FXa being a key anticoagulant target of the ZPI-PZ complex. This is supported by the insignificant effects on the lag time before the onset of thrombin generation or the time to reach the peak thrombin concentration, both of which reflect the level of prothrombinase formed in the initiation phase, but prominent effects on the peak thrombin concentration (TP) and total thrombin produced (ETP) that reflect prothrombinase levels in the propagation phase (23). By contrast, activation of coagulation in plasma by extrinsic or intrinsic activators results in significant effects of the ZPI-PZ complex on the lag time as well as the TP and ETP, implying that FXa generated by extrinsic or intrinsic activators before FV activation is an additional target of ZPI-PZ complex regulation. An earlier study similarly found dramatic effects of the ZPI-PZ complex on FXa generation and on the lag time and peak thrombin generation when barium-adsorbed plasma was supplemented with factor X or with both factor X and prothrombin, respectively, and activated with factor IXa (8). In keeping with the importance of the ZPI-PZ complex as a key anticoagulant regulator of FXa, Girard et al. (35) recently reported that knocking out ZPI or PZ in hemophilia A mice enhanced coagulation in hemophilia mice, therapeutically equivalent to 15% FVIII replacement. Our finding that FXa in prothrombinase is susceptible to inhibition by the ZPI-PZ complex contrasts with the resistance of prothrombinase-bound FXa to antithrombin inhibition at plasma concentrations of this serpin (18, 36). Such protection is consistent with our finding that ka(app) for antithrombin inhibition of prothrombinase-bound FXa is >1,000-fold slower than ka(app) for ZPI-PZ complex inhibition. Antithrombin inhibition of free FXa on a membrane was similarly found to be much slower (104-fold) than ZPI-PZ complex inhibition. Although past studies have suggested that FXa generated by extrinsic and intrinsic FXase activation of FX in the presence of TFPI and antithrombin is significantly regulated by antithrombin (19), the rate constants for ZPI-PZ complex and antithrombin determined in the present study predict that the ZPI-PZ complex rather than antithrombin will be the dominant regulator of FXa even after accounting for the 50-fold higher plasma concentration of antithrombin. Under in vivo conditions, antithrombin regulation of FXa may become important when the serpin binds and is activated by endogenous heparin sulfate or heparin molecules containing a pentasaccharide receptor sequence for the serpin. Heparin activation thus enhances the rate constant for antithrombin inhibition of unbound or prothrombinase-bound FXa ∼103-fold based on our measurements and published data (36). However, such active heparin molecules may be limiting in vivo (37) and result in a dependence on the ZPI-PZ complex for effective regulation of FXa activity.
Prothrombinase-bound FXa has been thought to be resistant to plasma protease inhibitors including TFPI (38) and to be regulated solely by the inactivation of FVa by APC in conjunction with the cofactor, protein S (39–42). The results of thrombin generation assays in normal and heterozygous FVL plasmas showed that blocking endogenous ZPI-PZ anti-FXa activity with the PZAb produced significant enhancements in thrombin generation that were independent of APC anticoagulant activity. Such findings suggest a new view in which the ZPI-PZ complex complements APC in dynamically regulating prothrombinase activity. Whereas FVa bound to FXa in prothrombinase and to prothrombin is largely protected from APC inactivation, this protective effect is reversed as prothrombin is activated by allowing protein S to displace FXa from FVa (39–42). By contrast, FXa bound in prothrombinase is directly inhibited by the ZPI-PZ complex at a reduced but physiologically significant rate during prothrombin activation that accelerates as prothrombin is consumed. Important complementary roles for ZPI-PZ and APC regulation of prothrombinase are suggested by clinical and animal model studies that have found a mild prothrombotic phenotype associated with ZPI-PZ deficiency or the FV Leiden mutation, and a severe thrombosis phenotype when ZPI or PZ deficiency is combined with the FV Leiden mutation (7–10, 43, 44). Such findings imply that loss of APC or ZPI-PZ anticoagulant activity alone cause a modest overactivity of prothrombinase and mild thrombotic risk, whereas the combined deficiency of both anticoagulants causes a marked overactivity of prothrombinase and consequent heightened risk of thrombosis. Whether this overactivity results from synergistic effects of combining ZPI/PZ deficiency with the factor V Leiden mutation remains to be determined by future studies of prothrombinase regulation by ZPI/PZ and APC in which prothrombinase assembled with normal and mutant factor V Leiden are compared. Our findings suggest that the resistance of FVa Leiden to APC inactivation could increase prothrombinase levels to an extent that reduces the anticoagulant effect of ZPI-PZ and therefore compounds the effects of ZPI or PZ deficiency.
Experimental procedures
Proteins and lipids
Plasma-derived human FXa, FXIIa, PZ, and prothrombin were from Enzyme Research Laboratories and human FV and FVa, sheep anti-human PZAb (polyclonal antibody, lot number P0831), APC, sheep anti-human protein C polyclonal antibody, and corn trypsin inhibitor (CTI) were from Hematologic Technologies. Recombinant human TM extracellular domain and normal sheep IgG were from Sigma. Recombinant human WT and mutant ZPIs were expressed in baculovirus-infected insect cells and purified and NBD-labeled K239C ZPI was prepared as described (4, 21). Recombinant S195A prothrombin was expressed and purified as described (11). Human antithrombin was purified from plasma as described (45). Protein concentrations were determined from the absorbance at 280 nm based on published extinction coefficients (4, 11). Small unilamellar phospholipid vesicles (SUVs) were prepared by sonication on ice under nitrogen of mixtures of 30% PS/70% PC; 2.5% PS/25% PE/72.5% PC; 5% PS/25% PE/70% PC; 10% PS/25% PE/65% PC; or 20% PS/40% PE/40% PC for 60–90 min as described (46, 47). SUVs for thrombogram assays contained 20% PS/20% PE/60% PC and were prepared as described (11, 47). All lipids were synthetic and contained dioleyl fatty acids, except for PE and PC lipids in vesicles with PS varied from 2.5 to 20%, which were natural lipids from bovine heart. All lipids were purchased from Avanti Polar Lipids.
Experimental conditions
All experiments were conducted in 50 mm Tris or Hepes buffer, pH 7.4, containing 0.1 m NaCl, 0.1% PEG 8000, 0.1 mg/ml of BSA at 25 °C, unless specified otherwise.
Kinetics of ZPI-PZ inhibition of free and prothrombinase-bound FXa
Reactions of ZPI-PZ complex with membrane-associated free and prothrombinase-bound FXa were done in the presence of 25 μm SUVs and 5 mm CaCl2, with or without FVa, FV, and S195A prothrombin under pseudo-first order conditions in which the inhibitor was in large molar excess over FXa (>20-fold). Reactions (50–100 μl) were initiated with FXa/FVa or FXa preincubated with lipid and calcium and after varying times quenched with 1 ml of Pefafluor FXa substrate (40 μm) containing 5 mm EDTA to measure residual FXa activity from the initial rate of substrate hydrolysis monitored fluorimetrically (4). The observed pseudo-first order inhibition rate constant (kobs) was obtained by fitting full progress curves to a single exponential with a nonzero end point or calculated from single reaction time points assuming end points obtained from such fits (37). Apparent second order association rate constants (ka(app)) were obtained by dividing kobs by the ZPI-PZ complex concentration calculated from a measured KD of 10 nm (21). Stoichiometries of ZPI-PZ inhibition of FXa in the absence and presence of FVa or prothrombin were measured by end point titrations of 100–200 nm FXa with the inhibitor as described (4).
Thrombin generation assays
Mixtures containing ZPI, PZ, 25 μm SUVs, 5 mm Ca2+, and prothrombin were incubated for 2 min at 25 °C, and then FXa/FVa or FXa were preincubated with lipid and calcium was added to initiate the reaction. At different time points, samples were withdrawn and diluted into buffer containing 200 μm S2238 and 10 mm EDTA and the thrombin generated was measured from the initial rate of absorbance change at 405 nm. Initial rates were converted to thrombin concentration by comparing with a standard curve constructed with known thrombin concentrations.
The effect of the PZAb or exogenous ZPI-PZ complex on thrombin generation in normal human plasma (George King Bio-medical) activated with TF (Innovin), FXIIa, or FXa was measured by CAT (48) assays at 37 °C, essentially as described in Ref. 49. TF activator, PZAb, or exogenous ZPI-PZ when present (20 μl) were added to 80 μl of plasma samples containing fluorogenic substrate, CTI (only for TF and FXa activators), and SUVs followed by initiation with 20 μl of buffered CaCl2. FXa and FXIIa activators were added with calcium. Final concentrations are given in Fig. 7. Plasma levels of ZPI and PZ were confirmed to be in the reported range (40–60 nm) (14, 15) by Western blotting with specific ZPI and PZ antibodies after calibration with known ZPI/PZ concentrations.
The effect of PZAb or exogenous APC and TM on thrombin generation in normal human and heterozygous factor V Leiden plasma (Precison Biologic) activated with TF was similarly performed. TF activator, PZAb, APC, TM, CTI, lipids, buffer, or control IgG (40 μl) were added to 100-μl plasma samples and after 2 min incubation at 37 °C, 10 μl of buffered CaCl2 was added to initiate coagulation. Final concentrations of added components are given in Fig. 8. At different time points, samples were withdrawn and diluted into buffer containing 200 μm S2238 and 10 mm EDTA and the thrombin generated was measured from the rate of substrate hydrolysis as described above.
Miscellaneous methods
Native PAGE and SDS-PAGE were performed as described (4). Immunoblotting analyses of ZPI-FXa complex formation in ZPI reactions with FXa in the presence of PZ, 25 μm PS/PC, and 5 mm Ca2+ and the absence or presence of saturating FVa were performed by acid denaturation of samples as previously reported (4). Fluorescence emission spectra and fixed wavelength titrations of NBD-labeled ZPI-PZ complex were performed with an SLM8000 spectrofluorometer as described (21). Binding of PZAb to PZ was measured by competitive binding titrations in which PZAb was titrated into a solution of NBD-labeled ZPI-PZ complex and the fluorescence decrease due to PZAb displacement of PZ from the labeled ZPI-PZ complex was measured. Observed relative fluorescence changes were fit by the cubic equation for competitive binding to obtain the KD for the PZAb-PZ interaction assuming a stoichiometry of 1.0 and independently determined KD for the labeled ZPI-PZ interaction as described (21). PZAb concentrations were calculated based on a molecular weight of 150 kDa. PZAb effects on ZPI-PZ anti-FXa activity were analyzed by quenching reactions of ZPI-PZ complex and FXa in the presence of 25 μm PS/PC and 5 mm Ca2+ with 100 μm Spectrozyme FXa substrate plus 10 mm EDTA after a fixed time and measuring residual FXa activity from the initial rate of absorbance change at 405 nm.
Author contributions
X. H. and P. E. B. conceptualization; X. H., R. S., and H. K. K. data curation; X. H. formal analysis; X. H. supervision; X. H. funding acquisition; X. H. validation; X. H. investigation; X. H., H. K. K., and P. E. B. methodology; X. H. writing-original draft; X. H. project administration; X. H. writing-review and editing.
Supplementary Material
Acknowledgments
We thank Dr. Steven Olson, University of Illinois at Chicago, for helpful advice and discussions during this study and for critical evaluation and editing of the manuscript. We thank Dr. Marie-Christine Bouton, Dr. Benjamin Richard, and Laurence Venisse, INSERM U698, Universite Paris, France, for assistance in reproducing some of our thrombogram data.
This work was supported by American Heart Association Scientist Development Grant SDG 48880022 (to X. H.) and National Institutes of Health Grants R37 HL39888 (to Drs. Steven Olson and X. H.) and R01 HL38779 (to P. E. B.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S3 and Table S1.
- ZPI
- protein Z-dependent protease inhibitor
- PZ
- protein Z
- ka(app)
- apparent second order association rate constant
- Gla
- γ-carboxyglutamic acid
- NBD
- N,N′-dimethyl-N-(acetyl)-N′-(7-nitrobenz-3-oxa-1,3-diazol-4-yl)ethylenediamine
- FV
- factor V
- FVa
- activated factor V
- FXa
- activated factor X
- FXIIa
- activated factor XII
- TFPI
- tissue factor pathway inhibitor
- APC
- activated protein C
- TM
- thrombomodulin
- CTI
- corn trypsin inhibitor
- SUV
- small unilamellar vesicles
- PZAb
- protein Z antibody
- CAT
- calibrated automated thrombography
- TP
- peak thrombin concentration
- ETP
- endogenous thrombin potential
- FVL
- factor V Leiden
- PS
- phosphatidylserine
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine.
References
- 1. Han X., Fiehler R., and Broze G. J. Jr. (1998) Isolation of a protein Z-dependent plasma protease inhibitor. Proc. Natl. Acad. Sci. U.S.A. 95, 9250–9255 10.1073/pnas.95.16.9250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han X., Huang Z. F., Fiehler R., and Broze G. J. Jr. (1999) The protein Z-dependent protease inhibitor is a serpin. Biochemistry 38, 11073–11078 10.1021/bi990641a [DOI] [PubMed] [Google Scholar]
- 3. Han X., Fiehler R., and Broze G. J. Jr. (2000) Characterization of the protein Z-dependent protease inhibitor. Blood 1, 3049–3055 [PubMed] [Google Scholar]
- 4. Huang X., Swanson R., Broze G. J. Jr., and Olson S. T. (2008) Kinetic characterization of the protein Z-dependent protease inhibitor reaction with blood coagulation factor Xa. J. Biol. Chem. 283, 29770–29783 10.1074/jbc.M805214200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tabatabai A., Fiehler R., and Broze G. J. Jr. (2001) Protein Z circulates in plasma in a complex with protein Z-dependent protease inhibitor. Thromb. Haemost. 85, 655–660 10.1055/s-0037-1615649 [DOI] [PubMed] [Google Scholar]
- 6. Broze G. J. Jr., and Miletich J. P. (1984) Human protein Z. J. Clin. Invest. 73, 933–938 10.1172/JCI111317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bafunno V., Santacroce R., and Margaglione M. (2011) The risk of occurrence of venous thrombosis: focus on protein Z. Thromb. Res. 128, 508–515 10.1016/j.thromres.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 8. Yin Z. F., Huang Z. F., Cui J., Fiehler R., Lasky N., Ginsburg D., and Broze G. J. Jr. (2000) Prothrombotic phenotype of protein Z deficiency. Proc. Natl. Acad. Sci., U.S.A. 97, 6734–6738 10.1073/pnas.120081897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang J., Tu Y., Lu L., Lasky N., and Broze G. J. Jr. (2008) Protein Z dependent protease inhibitor deficiency produces a more severe murine phenotype than protein Z deficiency. Blood 111, 4973–4978 10.1182/blood-2007-12-126391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kemkes-Matthes B., Nees M., Kühnel G., Matzdorff A., and Matthes K. J. (2002) Protein Z influences the prothrombotic phenotype in factor V Leiden patients. Thromb. Res. 106, 183–185 10.1016/S0049-3848(02)00181-0 [DOI] [PubMed] [Google Scholar]
- 11. Kroh H. K., Panizzi P., Tchaikovski S., Baird T. R., Wei N., Krishnaswamy S., Tans G., Rosing J., Furie B., Furie B. C., and Bock P. E. (2011) Active site-labeled prothrombin inhibits prothrombinase in vitro and thrombosis in vivo. J. Biol. Chem. 286, 23345–23356 10.1074/jbc.M111.230292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krishnaswamy S., Jone K. C, and Mann K. G. (1988) Prothrombinase complex assembly kinetic mechanism of enzyme assembly on phospholipid vesicles. J. Biol. Chem. 263, 3823–3834 [PubMed] [Google Scholar]
- 13. Krishnaswamy S., Church W. R., Nesheim M. E., and Mann K. G. (1987) Activation of human prothrombin by human prothrombinase influence of factor Va on the reaction mechanism. J. Biol. Chem. 262, 3291–3299 [PubMed] [Google Scholar]
- 14. Miletich J. P., and Broze G. J. Jr. (1987) Human plasma protein Z antigen: range in normal subjects and the effect of warfarin therapy. Blood 69, 1580–1586 [PubMed] [Google Scholar]
- 15. Bolkun L., Galar M., Piszcz J., Lemancewicz D., and Kloczko J. (2013) Plasma concentration of protein Z and protein Z-dependent protease inhibitor in patients with haemophilia A. Thromb. Res. 131, e110–113 10.1016/j.thromres.2012.11.031 [DOI] [PubMed] [Google Scholar]
- 16. Huang X., Dementiev A., Olson S. T., and Gettins P. G. (2010) Basis for the specificity and activation of the serpin protein Z-dependent proteinase inhibitor (ZPI) as an inhibitor of membrane-associated factor Xa. J. Biol. Chem. 285, 20399–20409 10.1074/jbc.M110.112748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rezaie A. R., Manithody C., and Yang L. (2005) Identification of factor Xa residues critical for interaction with protein Z-dependent protease inhibitor. J. Biol. Chem. 280, 32722–32728 10.1074/jbc.M505517200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindhout T., Baruch D., Schoen P., Franssen J., and Hemker H. C. (1986) Thrombin generation and inactivation in the presence of antithrombin III and heparin. Biochemistry 25, 5962–5969 10.1021/bi00368a019 [DOI] [PubMed] [Google Scholar]
- 19. Lu G., Broze G. J. Jr., and Krishnaswamy S. (2004) Formation of factors IXa and Xa by the extrinsic pathway. J. Biol. Chem. 279, 17241–17249 10.1074/jbc.M312827200 [DOI] [PubMed] [Google Scholar]
- 20. Conard J., Brosstad F., Lie Larsen M., Samama M., and Abildgaard U. (1983) Molar antithrombin concentration in normal human plasma. Haemostasis 13, 363–368 [DOI] [PubMed] [Google Scholar]
- 21. Huang X., Zhou J., Zhou A., and Olson S. T. (2015) Thermodynamic and kinetic characterization of the protein Z-dependent protease inhibitor (ZPI)-protein Z interaction reveals an unexpected role for ZPI Lys-239. J. Biol. Chem. 290, 9906–9918 10.1074/jbc.M114.633479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silverberg S. A., Nemerson Y., and Zur M. (1977) Kinetics of the activation of bovine coagulation factor X by components of the extrinsic pathway: kinetic behavior of two-chain factor VII in the presence and absence of tissue factor. J. Biol. Chem. 252, 8481–8488 [PubMed] [Google Scholar]
- 23. van Dieijen G., Tans G., Rosing J., and Hemker H. C. (1981) The role of phospholiplid and factor VIIIa in the activation of bovine factor X. J. Biol. Chem. 256, 3433–3442 [PubMed] [Google Scholar]
- 24. Lawson J. H., and Mann K. G. (1991) Cooperative activation of human factor IX by the human extrinsic pathway of blood coagulation. J. Biol. Chem. 266, 11317–11327 [PubMed] [Google Scholar]
- 25. Mann K. G., Krishnaswamy S., and Lawson J. H. (1992) Surface-dependent hemostasis. Semin. Hematol. 29, 213–226 [PubMed] [Google Scholar]
- 26. Gruber A., and Griffin J. H. (1992) Direct detection of activated protein C in blood from human subjects. Blood 79, 2340–2348 [PubMed] [Google Scholar]
- 27. Lechtenberg B. C., Murray-Rust T. A., Johnson D. J., Adams T. E., Krishnaswamy S., Camire R. M., and Huntington J. A. (2013) Crystal structure of the prothrombinase complex from the venom of Pseudonaja textilis. Blood 122, 2777–2783 10.1182/blood-2013-06-511733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson D. J., Li W., Adams T. E., and Huntington J. A. (2006) Antithrombin-S195A factor Xa-heparin structure reveals the mechanism of antithrombin activation. EMBO J. 25, 2029–2037 10.1038/sj.emboj.7601089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krishnaswamy S., and Betz A. (1997) Exosites determine macromolecular substrate recognition by prothrombinase. Biochemistry 36, 12080–12086 10.1021/bi970979+ [DOI] [PubMed] [Google Scholar]
- 30. Hockin M. F., Jones K. C., Everse S. J., and Mann K. G. (2002) A model for the stoichiometric regulation of blood coagulation. J. Biol. Chem. 277, 18322–18333 10.1074/jbc.M201173200 [DOI] [PubMed] [Google Scholar]
- 31. Clark S. R., Thomas C. P., Hammond V. J., Aldrovandi M., Wilkinson G. W., Hart K. W., Murphy R. C., Collins P. W., and O'Donnell V. B. (2013) Characterization of platelet aminophospholipid externalization reveals fatty acids as molecular determinants that regulate coagulation. Proc. Natl. Acad. Sci. U.S.A. 110, 5875–5880 10.1073/pnas.1222419110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Escribá P. V., González-Ros J. M., Goñi F. M., Kinnunen P. K., Vigh L., Sánchez-Magraner L., Fernández A. M., Busquets X., Horváth I., and Barceló-Coblijn G. (2008) Membranes: a meeting point for lipids, proteins and therapies. J. Cell Mol. Med. 12, 829–875 10.1111/j.1582-4934.2008.00281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sengupta T., and Manoj N. (2016) Phosphatidylserine and phosphatidylethanolamine bind to protein Z cooperatively and with equal affinity. PLoS ONE 11, e0161896 10.1371/journal.pone.0161896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rand M. D., Lock J. B., van't Veer C., Gaffney D. P., and Mann K. G. (1996) Blood clotting in minimally altered whole blood. Blood 88, 3432–3445 [PubMed] [Google Scholar]
- 35. Girard T. J., Lasky N. M., Grunz K., and Broze G. J. Jr. (2019) Suppressing protein Z-dependent inhibition of factor Xa improves coagulation in hemophilia A. J. Thromb. Haemost. 17, 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brufatto N., and Nesheim M. (2001) The use of prothrombin(S525C) labeled with fluorescein to directly study the inhibition of prothrombinase by antithrombin during prothrombin activation. J. Biol. Chem. 276, 17663–17671 10.1074/jbc.M011586200 [DOI] [PubMed] [Google Scholar]
- 37. Marcum J. A., Atha D. H., Fritze L. M., Nawroth P., Stern D., and Rosenberg R. D. (1986) Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparin sulfate proteoglycan. J. Biol. Chem. 261, 7507–7517 [PubMed] [Google Scholar]
- 38. Mast A. E., and Broze G. J. Jr. (1996) Physiological concentrations of tissue factor pathway inhibitor do not inhibit prothrombinase. Blood 87, 1845–1850 [PubMed] [Google Scholar]
- 39. Smirnov M. D., Safa O., Esmon N. L., and Esmon C. T. (1999) Inhibition of activated protein C anticoagulant activity by prothrombin. Blood 94, 3839–3846 [PubMed] [Google Scholar]
- 40. Tran S., Norstrøm E., and Dahlbäck B. (2008) Effects of prothrombin on the individual activated protein C-mediated cleavages of coagulation factor Va. J. Biol. Chem. 283, 6648–6655 10.1074/jbc.M708036200 [DOI] [PubMed] [Google Scholar]
- 41. Rosing J., Hoekema L., Nicolaes G. A., Thomassen M. C., Hemker H. C., Varadi K., Schwarz H. P., and Tans G. (1995) Effects of protein S and factor Xa on peptide bond cleavages during inactivation of factor Va and factor VaR506Q by activated protein C. J. Biol. Chem. 270, 27852–27888 10.1074/jbc.270.46.27852 [DOI] [PubMed] [Google Scholar]
- 42. Norstrøm E. A., Tran S., Steen M., and Dahlbäck B. (2006) Effects of factor Xa and protein S on the individual activated protein C-mediated cleavages of coagulation factor Va. J. Biol. Chem. 281, 31486–31494 10.1074/jbc.M606441200 [DOI] [PubMed] [Google Scholar]
- 43. Hille E. T., Westendorp R. G., Vandenbroucke J. P., and Rosendaal F. R. (1997) Mortality and causes of death in families with the factor V Leiden mutation (resistance to activated protein C). Blood 89, 1963–1967 [PubMed] [Google Scholar]
- 44. Cui J., Eitzman D. T., Westrick R. J., Christie P. D., Xu Z. J., Yang A. Y., Purkayastha A. A., Yang T. L., Metz A. L., Gallagher K. P., Tyson J. A., Rosenberg R. D., and Ginsburg D. (2000) Spontaneous thrombosis in mice carrying the factor V Leiden mutation. Blood 96, 4222–4226 [PubMed] [Google Scholar]
- 45. Olson S. T., Björk I., and Shore J. D. (1993) Kinetic characterization of heparin-catalyzed and uncatalyzed inhibition of blood coagulation proteinases by antithrombin. Methods Enzymol. 222, 525–559 10.1016/0076-6879(93)22033-C [DOI] [PubMed] [Google Scholar]
- 46. Huang X., Liu B., Wei Y., Beyea R., Yan H., and Olson S. T. (2017) Lipid oxidation inactivates the anticoagulant function of protein Z-dependent protease inhibitor (ZPI). J. Biol. Chem. 292, 14625–14635 10.1074/jbc.M117.793901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anderson P. J., Nesset A., Dharmawardana K. R., and Bock P. E. (2000) Role of proexosite I in factor Va-dependent substrate interactions of prothrombin activation. J. Biol. Chem. 275, 16435–16442 10.1074/jbc.M001255200 [DOI] [PubMed] [Google Scholar]
- 48. Dentali F., Gianni M., Lussana F., Squizzato A., Cattaneo M., and Ageno W. (2008) Polymorphisms of the Z protein protease inhibitor and risk of venous thromboembolism: a meta-analysis. Br. J. Haematol. 143, 284–287 10.1111/j.1365-2141.2008.07331.x [DOI] [PubMed] [Google Scholar]
- 49. Newell-Caito J. L., Laha M., Tharp A. C., Creamer J. I., Xu H., Maddur A. A., Tans G., and Bock P. E. (2011) Notecarin D binds human factor V and factor Va with high affinity in the absence of membranes. J. Biol. Chem. 286, 38286–38297 10.1074/jbc.M111.247122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.