Abstract
The production of l-leucine was improved by the disruption of ltbR encoding transcriptional regulator and overexpression of the key genes (leuAilvBNCE) of the l-leucine biosynthesis pathway in Corynebacterium glutamicum XQ-9. In order to improve l-leucine production, we rationally engineered C. glutamicum to enhance l-leucine production, by improving the redox flux. On the basis of this, we manipulated the redox state of the cells by mutating the coenzyme-binding domains of acetohydroxyacid isomeroreductase encoded by ilvC, inserting NAD-specific leucine dehydrogenase, encoded by leuDH from Lysinibacillus sphaericus, and glutamate dehydrogenase encoded by rocG from Bacillus subtilis, instead of endogenous branched-chain amino acid transaminase and glutamate dehydrogenase, respectively. The yield of l-leucine reached 22.62 ± 0.17 g·L−1 by strain ΔLtbR-acetohydroxyacid isomeroreductase (AHAIR)M/ABNCME, and the concentrations of the by-products (l-valine and l-alanine) increased, compared to the strain ΔLtbR/ABNCE. Strain ΔLtbR-AHAIRMLeuDH/ABNCMLDH accumulated 22.87±0.31 g·L−1 l-leucine, but showed a drastically low l-valine accumulation (from 8.06 ± 0.35 g·L−1 to 2.72 ± 0.11 g·L−1), in comparison to strain ΔLtbR-AHAIRM/ABNCME, which indicated that LeuDH has much specificity for l-leucine synthesis but not for l-valine synthesis. Subsequently, the resultant strain ΔLtbR-AHAIRMLeuDHRocG/ABNCMLDH accumulated 23.31 ± 0.24 g·L−1 l-leucine with a glucose conversion efficiency of 0.191 g·g−1.
Keywords: Corynebacterium glutamicum, l-leucine, acetohydroxyacid isomeroreductase, leucine dehydrogenase, NAD-dependent glutamate dehydrogenase
1. Introduction
l-leucine, one of three branched-chain amino acids (BCAAs), is an essential amino acid that is not synthesized in mammals, and is used in food and animal feed additives, pharmaceuticals, and cosmetics [1,2,3,4]. l-leucine acts as potent nutritional signaling molecules for regulating the rate of protein synthesis and glucose homeostasis [2,5]. Therefore, l-leucine has also been widely used as additives of infusion solutions, together with the other BCAAs for patients with hepatic diseases, to improve the nutritional status [2].
Corynebacterium glutamicum is widely used in industrial fermentation to produce several million tons of amino acids annually, in particular the flavor enhancer l-glutamate and the feed additive l-lysine [6,7]. Besides l-glutamate and l-lysine, C. glutamicum can also be used for production of a variety of other amino acids, including the BCAAs [8,9,10]. To date, C. glutamicum mutant strains remain the dominant industrial producers of l-leucine [11]. At present, microbial fermentation is the major method for producing l-leucine because of the advantages of suitable reaction conditions, environmental friendliness, and a stable product quality [12]. Nowadays, the global production capacity of l-leucine is about 1000 tons per year, though the market is substantially growing [13]. There is a need for developing more effective production methods, such as giving high yields, to meet growing market demands [14,15].
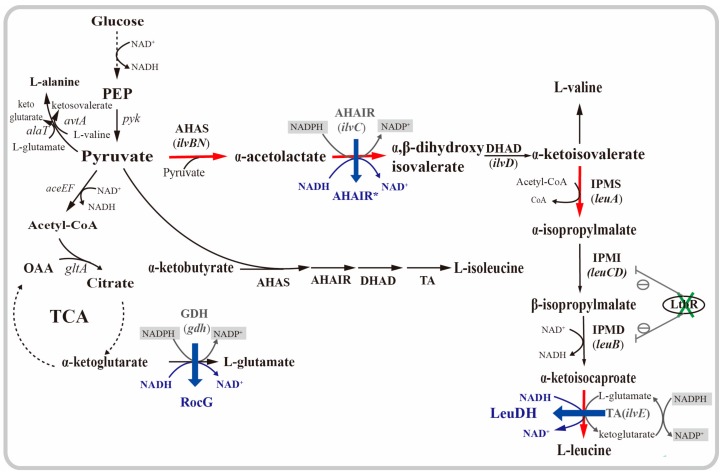
l-leucine is synthesized from pyruvate in a pathway comprising seven reactions (Figure 1), catalyzed by acetohydroxyacid synthase (AHAS), acetohydroxyacid isomeroreductase (AHAIR), dihydroxyacid dehydratase (DHAD), α-isopropylmalate synthase (IPMS), α-isopropylmalate isomerase, β-isopropylmalate dehydrogenase (IPMD), and branched-chain amino acid transaminase (TA) [16]. The l-leucine biosynthetic pathway is complex and tightly regulated because the overlapping biosynthesis pathways of the three BCAAs partly share the same precursors and enzymes [8]. l-valine and l-isoleucine are synthesized in parallel pathways in which the reaction steps are catalyzed by the same enzymes, whereas l-leucine is formed in a specific series of four reactions, starting with the last intermediate of l-valine biosynthesis [17]. Two genes, alaT or avtA, are responsible for the conversion of pyruvate to l-alanine. Additionally, the gene leuB together with the genes leuCD is repressed by the transcriptional regulator LtbR. Therefore, the regular strategies for the construction of l-leucine producing strains are, removing the l-leucine inhibition and transcriptional attenuation (leuA, ilvBNC) [3,18], enhancing the metabolic influx (leuACDB, ilvBNCE) to precursor [8], altering substrate and enzyme specificity [19], and so on. However, improvement of the redox cofactor requirement for the l-leucine production has not been investigated.
Figure 1.
The l-leucine biosynthesis pathway of Corynebacterium glutamicum and the metabolic engineering steps performed in this study. Enzymes and corresponding genes are shown. An encircled minus indicates repression of gene expression (solid lines). Deletion of the gene encoding LtbR is indicated by green “×”. Red thick arrows indicate increased metabolic fluxes. Introduction of enzymatic reactions are highlighted in blue. NADPH and NADP+ are highlighted in grey shaded boxes. Abbreviations: AHAS—acetotydroxyacid synthetase; AHAIR—acetohydroxyacid isomeroreductase; DHAD—dihydroxyacid dehydratase; TA—branched-chain amino acid transaminase; IPMS—α-isopropylmalate synthase; IPMI—α-isopropylmalate isomerase; IPMD—β-isopropylmalate dehydrogenase; LeuDH—leucine dehydrogenase; PEP—phosphoenolpyruvate; OAA—oxaloacetic acid; TCA—tricarboxylic acid cycle; GDH and RocG—glutamate dehydrogenase.
As shown in Figure 1, there is a net consumption of reducing equivalents from NADPH, during the l-leucine biosynthesis. The synthesis of one mole of l-leucine consumes a total of two moles of NADPH. The consumption of NADPH is at the AHAIR reaction and at the regeneration of l-glutamate as an amino-group donor for the TA reaction [6]. The importance of NADPH supply for amino acid production is well-known [20,21]. A limited supply of reducing equivalents might be a suspected rate-limiting factor in l-leucine production by C. glutamicum [7]. In this study, we report the rational design of l-leucine producer C. glutamicum XQ-9, with a double l-isoleucine and l-methionine auxotrophy. Recombinant strains were constructed by deleting ltbR and the overexpressing leuAilvBNCE. Then, we improved the l-leucine production in C. glutamicum strains by improving the redox balance. These strategies were via the modification of the coenzyme specificity of AHAIR, introduction of NAD-specific glutamate dehydrogenase (RocG) (GenBank: NC_000964.3), derived from Bacillus subtilisI and NAD-specific leucine dehydrogenase (LeuDH) (GenBank: AB103119.1) derived from Lysinibacillus sphaerius, instead of glutamate dehydrogenase (GDH) and TA [22,23,24]. All mutant AHAIR, RocG, and LeuDH consumed NADH in their reactions, and regulated cofactor production and consumption, during l-leucine biosynthesis (Figure 1). As a result, the l-leucine production and NADPH/NADP+ ratio were significantly increased.
2. Results
2.1. Effect of Deletion of ltbR and Expression of Genes Involved in Leucine Biosynthesis
The strain C. glutamicum XQ-9 is auxotrophic for l-isoleucine and l-methionine (see Section 4). It can produce 14.12 g·L−1 l-leucine, and the productivity was 0.196 g·L−1·h−1. We sequenced the key genes in C. glutamicum XQ-9, and sequences of the key genes can be found in the supplementary materials. There are many amino acid exchanges in C. glutamicum XQ-9 with respect to the corresponding sequence from the wild-type strain ATCC 13032, some of which must be potentially responsible for the fact that C. glutamicum XQ-9 accumulated 14.12 g·L−1 l-leucine, while ATCC 13032 typically did not [3]. Especially, there were six amino acid exchanges (G92D, I162V, N210D, E216K, R494H, and G526D) encoded by the leuA gene. Since the transcriptional regulator LtbR was shown to repress the expression of leuCD and leuB [3], ltbR was deleted in C. glutamicum XQ-9. In order to increase the metabolic carbon flux and improve the redox balance, the leuAilvBNCE genes from C. glutamicum XQ-9 were cloned into the expression vector pEC-XK99E, resulting in plasmid pEC-ABNCE. The plasmid was transferred into strain ΔLtbR, and the enzyme activities of crude extracts were determined. The leuCD gene encoded isopropylmalate isomerase (IPMI). In strain ΔLtbR, an IPMI activity of 167 ± 13 mU·mgprotein−1 was measured, which corresponded to a 4.5-fold increase in comparison to the C. glutamicum XQ-9 (37 ± 9 mU·mgprotein−1) (Table 1). As shown in Table 1, all enzyme activities intended to be overexpressed in ΔLtbR/ABNCE were indeed increased to a certain extent, and the intracellular NADPH/NADP+ ratio of ΔLtbR/ABNCE reached 0.54 ± 0.03 (Figure 2D). Both, the increased glucose consumption and the increased NADPH/NADP+ ratio resulted in an increased leucine biosynthesis, based on reducing equivalents from NADH.
Table 1.
Enzyme activities for the l-leucine biosynthesis by C. glutamicum strains.
| Strains | Enzyme Activity (mU·mgprotein−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| IPMS | IPMI | AHAS | AHAIR | TA | LeuDH | GDH | RocG | |
| XQ-9 | 262 ± 6 | 37 ± 5 | 143 ± 9 | 31 ± 8 | 17 ± 3 | - | 131 ± 8 | - |
| ΔLtbR | 289 ± 6 | 167 ± 8 | 139 ± 8 | 32 ± 5 | 19 ± 2 | 136 ± 5 | ||
| ΔLtbR/ABNCE | 660 ± 13 | 153 ± 6 | 312 ± 14 | 74 ± 10 | 43 ± 5 | - | 134 ± 10 | - |
| ΔLtbR-AHAIRM/ABNCME | 697 ± 23 | 151 ± 4 | 264 ± 10 | 63 ± 12 | 37 ± 4 | - | 135 ± 12 | - |
| ΔLtbR-AHAIRMLeuDH/ABNCMLDH | 528 ± 18 | 149 ± 5 | 196 ± 8 | 49 ± 9 | - | 446 ± 43 | 126 ± 5 | - |
| ΔLtbR-AHAIRMLeuDHRocG/ABNCMLDH | 489 ± 13 | 136 ± 4 | 185 ± 12 | 97 ± 11 | - | 414 ± 16 | - | 107 ± 8 |
All data represent values of three determinations of triplicate independent experiments with ±SEM.
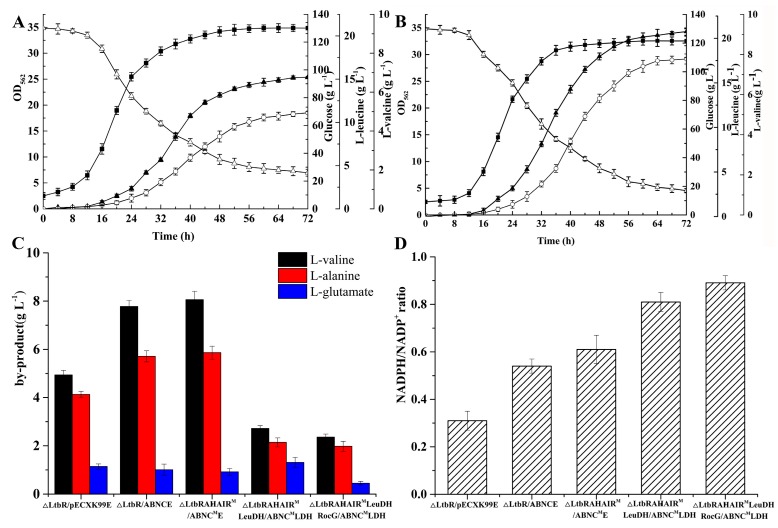
Figure 2.
Comparison of the different C. glutamicum strains during cultivation in shake-flasks with the fermentation medium, to test the effect of inserting leuAilvBNCE. (A) ΔLtbR /pECXK99E (pECXK99E was used here as a control plasmid), (B) ΔLtbR/ABNCE, (C) by-products, and (D) NADPH/NADP+ ratio. Solid squares—OD562, hollow triangles—glucose, solid triangles—l-leucine: hollow circles—l-valine. The data represent the mean values and standard deviations obtained from three independent cultivations.
Strain ΔLtbR /pECXK99E, as a control group, only accumulated 15.22 g·L−1 l-Leucine (Figure 2A). Nevertheless, l-leucine production and glucose conversion efficiency reached 20.75 g·L−1 and 0.183 g·g−1 by ΔLtbR/ABNCE (Figure 2B). However, it cannot be ignored that the concentration of l-valine was 7.78 ± 0.25 g·L−1 by ΔLtbR/ABNCE, i.e., higher than 4.94 ± 0.19 g·L−1 by ΔLtbR/pECXK99E (Figure 2C). We see this as a consequence of shared intermediates and the enzymes in the l-leucine and l-valine biosynthesis pathway [25]. The l-valine production was yet increased by the overexpressing l-leucine biosynthesis genes. Additionally, the concentration of l-alanine as the other main by-product was increased by 27.67% (from 4.13 ± 0.13 g·L−1 to 5.71 ± 0.23 g·L−1) (Figure 2C), suggesting that l-alanine synthesis might have accumulated through the reaction catalyzed by avtA (valine-pyruvate aminotransferases) when l-valine biosynthesis was enhanced [26]. However, the concentrations of l-glutamate did not significantly change (Figure 2D).
2.2. Effect of ilvCM on L-Leucine Production
On the basis of the Rossmann fold in the coenzyme-binding domains, NAD-specific enzymes commonly possess the β-α-β motif and exhibit a highly conserved GXGXXGXXXG sequence (where X is any amino acid) [27,28,29]. Negatively charged glutamate or aspartate residues form hydrogen bonds to the 2′-and/or 3′-hydroxyl groups of NAD, while positively charged arginine residue corresponds to the negatively charged 2′-phosphate group of NADP [24]. AHAIR reaction requires NADPH as a coenzyme [30]. In order to further improve the formation of l-leucine, mutagenesis of AHAIR was carried out in the strain ΔLtbR, to improve the redox flux on l-leucine production. Thus, the mutation S34G corresponds to the fourth glycine residue on the NAD-specific conserved sequence, and the mutation L48E and R49F causes better association with the NAD. Accordingly, based on these observations, the mutant AHAIR with substitution of three amino acid residues (S34G, L48E, and R49F) by site-directed mutagenesis, can mainly utilize NADH as coenzyme [23]. Firstly, wild-type AHAIR and mutant AHAIR were purified for kinetic analysis from crude extracts of strains BL21/pET28a-ilvC and BL21/pET28a-ilvCM. Table 2 shows kinetic constants determined for α-acetolactate and coenzyme of the wild-type AHAIR and mutant AHAIR. Wild-type AHAIR had a higher affinity for NADPH, because of the 27-fold lower Km for NADPH than NADH (Table 2). However, kcat/Km of mutant AHAIR with NADPH, decreased significantly, and kcat/Km of mutant AHAIR using NADH, was six-fold higher than that using NADPH (Table 2). These results suggested that the coenzyme specificity of mutant AHAIR was reversed from NADPH to NADH, even though mutant AHAIR activity using NADH as a coenzyme was not significantly improved.
Table 2.
Kinetic parameters of the wild-type and mutant AHAIRs for NADH or NADPH a.
| AHAIR | NADH | NADPH | ||||
|---|---|---|---|---|---|---|
| Km (μmol L−1) | kcat (s−1) | kcat/Km (s−1/mmol L−1) | Km (μmol L−1) | kcat (s−1) | kcat/Km (s−1/mmol L−1) | |
| Wild-type | 256 ± 13 | 0.93 ± 0.05 | 3.63 | 6.16 ± 1.34 | 0.6 ± 0.03 | 97.40 |
| Mutant | 276 ± 16 | 0.74 ± 0.03 | 2.68 | 67 ± 7 | 0.03 ± 0.0012 | 0.45 |
a The α-acetolactate concentrations were 10 mmol L−1 and variable amounts of NAD(P)H were present. All data represent values of three determinations of triplicate independent experiments with ±SD.
Then, mutagenesis was carried out in strain C. glutamicum ΔLtbR with pK18mobsacB-ilvCM via two-step homologous recombination, and the correct integration was verified by sequencing. With the leuAilvBNCME genes over-expressed, the l-leucine production and conversion efficiency were enhanced to 22.62 ± 0.17 g·L−1 and 0.193 g·g−1 by ΔLtbR-AHAIRM/ABNCME, compared with ΔLtbR/ABNCE (Figure 2B and Figure 3A). The productivity reached 0.314 g·L−1.h−1 by ΔLtbR-AHAIRM/ABNCME. Meanwhile, the intracellular NADPH/NADP+ ratio of ΔLtbR-AHAIRM/ABNCME was 0.61 ± 0.06, a little higher than that of ΔLtbR/ABNCE (Figure 2D). Additionally, strain ΔLtbR-AHAIRM/ABNCME with the mutated ilvC showed a notably slower growth (Figure 3A), compared to ΔLtbR-AHAIRM/ABNCME (Figure 2B and Figure 3A). Already this could account for the 9% increase in the l-leucine formation. The concentrations of the two byproducts (8.06 ± 0.35 g·L−1 l-valine and 5.86 ± 0.27 g·L−1 l-alanine) were not significantly increased and were very much similar to the byproduct formation of ΔLtbR/ABNCE, while l-leucine production was enhanced in ΔLtbR-AHAIRM/ABNCME (Figure 2C and Figure 3A). Consequently, ΔLtbR-AHAIRM was used for further studies.
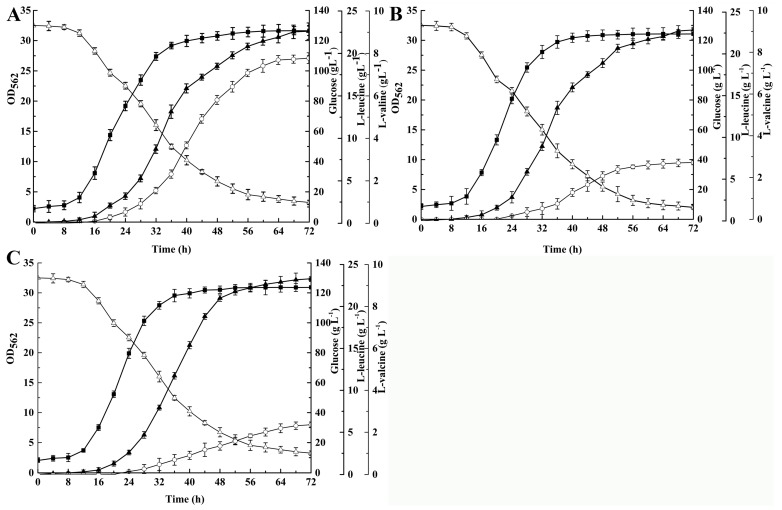
Figure 3.
Comparison of the different C. glutamicum strains during cultivation in shake-flasks with fermentation medium to test the effect of additional reducing equivalents delivered by NADH. (A) ΔLtbR-AHAIRM/ABNCME, (B) ΔLtbR-AHAIRMLeuDH/ABNCMLDH, and (C) ΔLtbR-AHAIRMLeuDHRocG/ABNCMLDH. Solid squares—OD562, hollow triangles—glucose, solid triangles—l-leucine, hollow circles—l-valine. The data represent the mean values and standard deviations obtained from three independent cultivation.
2.3. Effect of leuDH to Reduce l-Valine Production
Leucine dehydrogenase LeuDH, encoded by leuDH, is a NAD(H)-dependent amino acid dehydrogenase, catalyzing the reductive amination of a variety of aliphatic keto-acids to the corresponding l-amino acids [31]. The reaction is reversible using NAD+ as a factor (Figure 1). LeuDH was mainly found in several Bacillus strains, such as Bacillus cereus, B. stearothermophilus, Lysinibacillus sphaericus [32,33,34]. However, this NAD-specific amino acid dehydrogenase did not occur in C. glutamicum. Hence, in order to introduce LeuDH and abolish the aminotransferase reaction, we first deleted ilvE in ΔLtbR-AHAIRM, to generate the leucine-auxotrophic strain ΔLtbR-AHAIRMΔTA. As expected, the growth of this strain was severely restricted, and the consumption of glucose was at a low level because the pathway of glucose to BCAAs was blocked. The similar results were reported by LiYan Feng [19]. Subsequently, this strain was transformed with pk18mobsacB-ΔilvE::leuDH, carrying the leuDH gene from L. sphaericus, under the control of the Ptac promoter, and the correct integration was verified by sequencing.
With the leuAilvBNCMleuDH genes being over-expressed, the resulting strain, ΔLtbR-AHAIRMLeuDH/ABNCMLDH, accumulated approximately 22.87 ± 0.31 g·L−1 l-leucine, and the intracellular NADPH/NADP+ ratio was slightly increased to 0.81 ± 0.04 (Figure 2C and Figure 3B). Additionally, glucose conversion efficiency and productivity reached 0.187 g·g−1 and 0.318 g·L−1·h−1, respectively. Surprisingly, the concentrations of the by-products l-valine (2.72 ± 0.11 g·L−1) and l-alanine (2.14 ± 0.19 g·L−1) were at a lower level. Perhaps branched-chain aminotransferase mainly catalyzed the BCAAs biosynthesis and LeuDH had a higher preference for l-leucine biosynthesis than for l-valine biosynthesis. In addition, l-glutamate production reached 1.31 ± 0.21 g·L−1, which was higher than other C. glutamicum strains. l-glutamate served as the amino group donor for several amino acids, except in the ΔLtbR-AHAIRMLeuDH strains [35]. This is because LeuDH uses NH3 as the amino-group donor and NADH as a cofactor [32]. In C. glutamicum strains, to produce l-leucine, adding additional l-glutamate in the fermentation medium might increase the l-leucine production. This result indicated that the fermentation progress of ΔLtbR-AHAIRMLeuDH/ABNCMLDH did not require any additional l-glutamate supply. Although the l-leucine production was lower than expected, it had a significant positive influence on product composition. Strain ΔLtbR-AHAIRMLeuDH was used for further improvement of the l-leucine production.
2.4. Effect of rocG in L-Leucine Production
B. subtilis glutamate dehydrogenase RocG, encoded by rocG, converts the formation of glutamate, through the reductive amination of 2-ketoglutarate [36]. The reaction requires NADH as a cofactor [22]. The expression of rocG to replace endogenous glutamate dehydrogenase might improve the redox balance for l-leucine biosynthesis [22]. Hence, in order to introduce rocG and abolish the endogenous glutamate dehydrogenase reaction, ΔLtbR-AHAIRMLeuDHRocG was constructed with pk18mobsacB-Δgdh::rocG, carrying the rocG gene under the control of the Ptac promoter, and the correct integration was verified by sequencing. As shown in Table 1 and Figure 3C, with the leuAilvBNCMleuDH genes over-expressed, RocG activity of 107 ± 13 mU·mgprotein−1 was measured, and the concentration of l-leucine was increased to 23.31 ± 0.24 g·L−1, with a glucose conversion efficiency of 0.191 g·g−1. The productivity reached 0.324 g·L−1·h−1. Additionally, the intracellular NADPH/NADP+ ratio reached 0.89 ± 0.03, possibly reducing more equivalents from NADH for the l-leucine accumulation. However, the l-glutamate production dramatically decreased to 0.45 ± 0.08 g·L−1 by ΔLtbR-AHAIRMLeuDHRocG/ABNCMLDH. Perhaps the RocG catalyzed reaction was reversible, and l-glutamate was degraded by RocG in ΔLtbR-AHAIRMLeuDHRocG/ABNCMLDH [37]. Under this condition, glucose consumption and cell growth showed no dramatic difference compared to ΔLtbR-AHAIRMLeuDH/ABNCMLDH (Figure 3B,C).
3. Discussion
NADPH plays a crucial role in the biochemical reactions, and has several physiological functions in the amino acid-producing strains, acting as a key cofactor in metabolism, especially anabolism. As shown in Figure 1, there is a net consumption of the reducing equivalents from NADPH, during the l-leucine biosynthesis. In order to make use of the additional reducing equivalents delivered by NADH, the coenzyme specificity of AHAIR was reversed, and NAD-specific LeuDH and RocG were introduced, instead of the endogenous TA and GDH, respectively.
While kcat/Km of the mutant AHAIR using NADH was slightly lower than that of the wild-type AHAIR (Table 1), the mutant AHAIR preferred NADH as a cofactor over NADPH. The intracellular NADPH concentration in ΔLtbR-AHAIRM/ABNCME was enhanced in comparison to ΔLtbR/ABNCE, while the AHAIR activity in ΔLtbR-AHAIRM/ABNCME was decreased (in the supplementary materials). The intracellular NADPH concentration determined during l-leucine production reached 3.92 ± 0.33 μmol g−1, compared to 3.48 ± 0.21 μmol g−1 of △LtbR/ABNCE (in the supplementary materials). Thus, it appears that the mutant AHAIR (S34G, L48E, and R49F) might indeed redirect redox fluxes and be able to work efficiently for L-leucine production, using NADH [24,27,28,29].
LeuDH, which is an amino acid dehydrogenase using NADH as a cofactor, catalyzes the reversible oxidative deamination and reductive amination between l-leucine or other branched chain amino acids and their corresponding α-keto acids [38]. Although various active or artificial amino acids have been synthesized by leucine dehydrogenases in vitro [6,31], the effect of leucine dehydrogenases in biological l-leucine fermentation has not yet been recognized in detail. Nevertheless, the NAD-dependent LeuDH was expected to be effective for l-leucine biosynthesis. In fact, LeuDH improved intracellular redox balance and had much more specificity for the biosynthesis of l-leucine than for the biosynthesis of l-valine [26]. It could be seen that the concentration of the by-product l-valine (2.72 ± 0.11 g·L−1) was at a low level for ΔLtbR-AHAIRMLeuDH/ABNCMLDH. As expected, the NAD-preferring LeuDH significantly increased the NADPH/NADP+ ratio. As known, LeuDH uses NH3 as an amino-group donor, whereas TA has a major preference for l-glutamate, and NADPH is consumed for the regeneration of l-glutamate [39]. Thus, the demand of an amino-group donor for l-leucine synthesis was changed from l-glutamate to NH3 in C. glutamicum XQ-9ΔltbRilvCMΔilvE::leuDH strains. Indeed the demand of l-glutamate in ΔLtbR-AHAIRMLeuDH strains was decreased, and l-glutamate was accumulated (Figure 1 and Figure 2C). On the other hand, in order to ensure sufficient amounts of NH3 for LeuDH, adding NH3 to maintain the pH of the fermentation solution could be easily provided.
In order to further improve the balance of the redox fluxes, the NAD-dependent glutamate dehydrogenase rocG was used for the l-leucine production. As a result, ΔLtbR-AHAIRMLeuDHRocG/ABNCMLDH produced 23.31±0.24 g·L−1 l-leucine. Additionally, the NADPH/NADP+ ratio reached 0.89 ± 0.03, slightly higher than 0.81 ± 0.04 by ΔLtbR-AHAIRMLeuDH/ABNCMLDH. However, l-glutamate only accumulated to a concentration of 0.45 ± 0.08 g·L−1 by ΔLtbR-AHAIRMLeuDHRocG/ABNCMLDH. Depending on the cofactor and metabolite levels, B. subtilis RocG converts ketoglutarate to l-glutamate, in the presence of NADH or catalyzes the reverse reaction. RocG can catalyze the reductive deamination of glutamate to form ketoglutarate, and thus, enable a direct utilization of glutamate as a carbon and nitrogen source [36]. We interpret the lower l-glutamate production of this strain to be a consequence of the intracellular levels of NAD+, NADH, ketoglutarate, and l-glutamate, so that RocG catalyzes the interconversion in the direction of l-glutamate consumption. Presumably, this process makes part of a futile cycle with l-glutamate production through the GS/GOGAT system, which has a high capacity when GDH is absent [37].
The regular strategies to improve the l-leucine yield have largely focused on the metabolic engineering of removing feedback inhibition [18,40,41], upstream central carbon flux [3], and downstream by-product synthesis pathways [8]. This is the first report on improvement in redox flux, to enhance the production of l-leucine by C. glutamicum. The mutant AHAIR (S34G, L48E, and R49F) not only increased the NADPH/NADP+ ratio but also increased the product l-leucine and the by-product production. LeuDH has much specificity for l-leucine biosynthesis and not for l-valine biosynthesis; thereby, it will significantly ease or improve product purification. RocG may well have catalyzed conversion of l-glutamate into ketoglutarate.
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Culture Conditions
All strains and plasmids used in this study as well as their relevant characteristics are listed in Table 3. C. glutamicum XQ-9 was engineered by repeated random mutagenesis and through directed selection from wild type C. glutamicum ATCC 13032. C. glutamicum XQ-9 was resistant to α-thiazolealanine, α-aminobutyic acid, sulfaguanidine, and l-Leucin-hydroxyamid; was auxotrophic for l-isoleucine and l-methionine; could produce 14.12 g·L−1 l-leucine; and was used as the working and parent strain. All DNA oligonucleotides were synthesized by the General Biosystems Co. Ltd. (Anhui, China) and are listed in Table S2 (in the supplementary materials). Site-directed mutagenesis was performed using Mut ExpressR II Fast Mutagenesis Kit V2 (Vazyme, Nanjing, China). Using this kit, the target plasmid amplification product was digested by Dpn I, and directly transformed after the recombinant cyclization, using recombinase Exnase II. The leuDH gene from L. sphaericus was synthesized by the General Biosystems Co. Ltd. (Anhui, China), with the addition of an SD sequence (GAAAGGAGATATACC) before ATG. We decided to not change any codons in leuDH, according to the different codon usage statistics in L. sphaericus and C. glutamicum. Promoter and terminator elements could be used from pDXW-8. Thus, leuDH fragment was cloned into the vector pDXW-8. The expression vector pET28a carrying His tag was for purification and kinetic analysis of AHAIR. The shuttle vectors pDXW-8 carrying an IPTG-inducible tac promoter and pEC-XK99E, were for the gene transfer between E. coli and C. glutamicum, and were used for gene overexpression in C. glutamicum [42]. The vector pk18mobsacB was for gene deletions and integrations via a two-step homologous recombination in C. glutamicum [19]. The plasmids constructed in this study can be found in the supplementary materials. For strain construction, plasmids were transformed into C. glutamicum by electroporation. All constructed plasmids including chromosomal deletions and integrations in engineered strains were finally verified by DNA sequencing.
Table 3.
Bacterial strains and plasmids used in this study.
| Strain or Plasmid | Relevant Characteristics | Source or Reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM109 | recA1 end1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F’(traD36 proAB+ lacq lacZ ΔM15) | Lab stock |
| BL21(DE3) | F- ompT gal dcm lon hsdSB (rB- mB-) λ(DE3) | Lab stock |
| Bacillus subtilis 168 | Wild type | ATCC |
| C. glutamicum | ||
| Wild type | Wild type ATCC 13032, biotin auxotrophic | ATCC |
| XQ-9 | l-leucine producing C. glutamicum strain created by random mutagenesis; resistant to α-thiazolealanine and α-aminobutyic acid; auxotrophic for l-isoleucine and l-methionine; can produce 14.12 g·L-1 l-leucine | Lab stock |
| ΔLtbR | C. glutamicum XQ-9 derivative with in-frame deletion of ltbR | This study |
| ΔLtbR/pECXK99E | C. glutamicum ΔLtbR harboring pECXK99E | This study |
| ΔLtbR/ABNCE | C. glutamicum ΔLtbR harboring pEC- ABNCE | This study |
| ΔLtbRAHAIRM | C. glutamicum ΔLtbR derivative with chromosomally integrated mutations into ilvCM coding for amino acid exchanges S34G, L48E, and R49F | This study |
| ΔLtbRAHAIRM/ABNCME | C. glutamicum ΔLtbRAHAIRM harboring pEC-ABNCME | This study |
| ΔLtbRAHAIRMLeuDH | C. glutamicum ΔLtbRAHAIRM derivative with chromosomal integration of leuDH under control of the tac promoter integrated into the intergenic region between upstream and downstream of ilvE | This study |
| ΔLtbRAHAIRMLeuDH/ABNCMELDH | C. glutamicum ΔLtbRAHAIRMLeuDH harboring pEC- ABNCMLDH | This study |
| ΔLtbRAHAIRMLeuDHRocG | C. glutamicum ΔLtbRAHAIRMLeuDH derivative with chromosomal integration of rocG under control of the tac promoter integrated into the intergenic region between upstream and downstream of gdh | This study |
| ΔLtbRAHAIRMLeuDHRocG/ABNCMLDH | C. glutamicum ΔLtbRAHAIRMLeuDHRocG harboring pEC- ABNCMLDH | This study |
| Plasmids | ||
| pDXW-8 | Kanr, E. coli-C. glutamicum shuttle vector for inducible gene expression (Ptac, lacl,) | Lab stock |
| pET28a | Kanr, E. coli expression vector, PT7, pBR322orivector for inducible gene expression | Lab stock |
| pECXK99E | Kanr, E. coli-C. glutamicum shuttle vector for inducible gene expression | Lab stock |
| pK18mobsacB | Kanr, integration vector; oriVEc oriT sacB, allows for selection of double crossover C. glutamicum | Lab stock |
| pET28a-ilvC | Kanr, pET28a derivative containing gene ilvC (C. glutamicum) | |
| pET28a-ilvCM | Kanr, pET28a derivative with integrated mutations into ilvC coding for amino acid exchanges S34G, L48E, and R49F | |
| pEC-ABNCE | Kanr, pECXK99E derivative containing gene leuA (C. glutamicum), ilvBNC (C. glutamicum) and ilvE (C. glutamicum) | This study |
| pEC-ABNCME | Kanr, pEC-ABNCE derivative integrated mutations into ilvC coding for amino acid exchanges S34G, L48E, and R49F | This study |
| pEC-ABNCMLDH | Kanr, pEC-ABNCLDH derivative with integrated mutations into ilvC coding for amino acid exchanges S34G, L48E, and R49F | This study |
| pDXW-8-leuDH | Kanr, pDXW-8 derivative containing gene leuDH (Lysinibacillus sphaericus) | This study |
| pDXW-8-rocG | Kanr, pDXW-8 derivative containing gene rocG (Bacillus subtilis) | This study |
| pK18mobsacB-ΔltbR | Kanr, pK18mobsacB derivative for in-frame deletion of gene ltbR | This study |
| pK18mobsacB-ilvCM | Kanr, pK18mobsacB derivative with chromosomally integrated mutations into ilvC coding for amino acid exchanges S34G, L48E, and R49F | This study |
| pK18mobsacB-ΔilvE::leuDH | Kanr, pK18mobsacB-ΔilvE derivative containing leuDH gene from pDXW-8-leuDH under control of the tac promoter | This study |
| pK18mobsacB-Δgdh::rocG | Kanr, pK18mobsacB-Δgdh derivative containing rocG gene from pDXW-8-rocG under control of the tac promoter | This study |
For the recombinant DNA work, E. coli JM109 and BL21(DE3) were used and cultivated in a Luria–Bertani (LB) medium (5 g·L−1 yeast extract, 10 g·L−1 tryptone, and 10 g·L−1 NaCl), at 37 °C. Where appropriate, 50 mg·L−1 kanamycin or 0.1 mmol·L−1 isopropyl β-D-thiogalactoside (IPTG) were added to the medium. C. glutamicum and its recombinant derivatives were routinely cultivated aerobically at 30 °C in the LBG medium (LB medium supplemented with 5 g·L−1 glucose). LBHIS medium (5 g·L−1 tryptone, 5 g·L−1 NaCl, 2.5 g·L−1 yeast extract, 18.5 g·L−1 Brain Heart Infusion powder, and 91 g·L−1 sorbitol) was used for the transformation of the mutant gene into C. glutamicum cells. When necessary, 25 mg·L−1 kanamycin was added to the medium. Bacterial growth was followed by measuring the optical density at 562 nm (OD562).
The medium used for seed culture consisted of (per liter) 30 g glucose, 35 g corn steep liquor, 5 g (NH4)2SO4, 1.3 g KH2PO4·3H2O, 0.4 g MgSO4·7H2O, 0.01 g MnSO4·H2O, 10 g sodium citrate ·2H2O, 10 g yeast extract, 2 g urea, 0.4 g l-methionine, 200 μg biotin, 300 μg thiamine, and 20 g CaCO3. The fermentation medium contained (per liter) 130 g glucose, 25 g corn steep liquor, 15 g (NH4)2SO4, 15 g CH3COONH4, 1.3 g KH2PO4·3H2O, 0.5 g MgSO4·7H2O, 0.01 g MnSO4·H2O, 2 g sodium citrate ·2H2O, 2 g urea, 0.8 g l-methionine, 0.06 g l-isoleucine, 0.5 g l-glutamate, 100 μg biotin, 200 μg thiamine, and 30 g CaCO3. Both media were adjusted to pH 7.3 with NaOH.
4.2. Preparation of Crude Extracts and Enzyme Assays
Crude cell extracts were prepared for the determination of AHAS, AHAIR, IPMS, IPMD, TA, LeuDH, and GDH activity. Cells were grown in the LBG medium. Cultivated cells were harvested by centrifugation (8000× g, 20 min) at 4 °C, and washed twice with 50 mmol L−1 Tris-HCl. Then, they were treated with lysozyme (20 mg mL−1) at 37 °C for 1 h [43]. The resulting cell suspensions were sonicated, using an ultrasonic homogenizer in an ice water bath, for 15 min. Cell debris was removed by centrifugation (8000× g, 10 min) at 4 °C. Protein concentrations were determined using the Bradford Protein Quantification Kit (Sangon, Shanghai, China) with bovine serum albumin as standard. The analyses of enzyme activities and protein concentrations were done in triplicates.
AHAS activity was determined at 30 °C as the decrease of pyruvate absorbance at 333 nm (the extinction coefficient was 17.5 M−1cm−1) by using a Microplate reader (Molecular Devices, UAS). The reaction mixture contained 100 mmol L−1 potassium phosphate (pH 7.5), 50 mmol L−1 sodium pyruvate, 10 mmol L−1 MgCl2, 0.1 mmol L−1 thiamine pyrophosphate, 0.1 mmol L−1 flavin adenine dinucleotide, and crude extract [23]. One unit of AHAS activity was defined as the activity needed to form 1 μmol of α-acetolactate per min.
AHAIR activity was determined at 30 °C, by monitoring NADPH decrease at 340 nm. The reaction mixture contained 100 mmol L−1 potassium phosphate (pH 7.5), 10 mmol L−1 α-acetolactate, 5 mmol L−1 MgCl2, 0.2 mmol L−1 NADPH, and crude extract [23]. One unit of AHAIR activity was defined as the activity necessary to oxidize 1 μmol of NADPH per min.
IPMS activity was determined at 30 °C, by monitoring coenzyme A formation at 412 nm (the extinction coefficient was 13.6 M−1cm−1) [44]. The reaction mixture contained 50 mmol L−1 Tris-HCl buffer (pH 7.5) with acetyl-CoA solution (3 mmol L−1 in 50 mmol L−1 Tris-HCl pH 7.5), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) solution (1 mmol L−1 in 50 mmol L−1 Tris-HCl pH 7.5), 400 mmol L−1 potassium glutamate, deionized water, and crude extract [2,3,4]. The reaction was started by adding α-ketoisovalerate solution (40 mmol L−1 in 50 mmol L−1 Tris-HCl, pH 7.5). One unit of IPMS activity was defined as the activity needed to form 1 μmol of α-isopropylmalate per min.
IPMI activity was determined at 30 °C, by monitoring the reaction intermediate α-isopropylmaleate formation at 235 nm (the extinction coefficient was 4530 M−1cm−1) [45]. The reaction mixture contained 200 mmol L−1 potassium phosphate (pH 7.0), deionized water, and crude extract. The reaction was started by adding 40 μL β-isopropylmalate solution (40 mmol L−1 in deionized water). One unit of TA activity was defined as the activity necessary to form 1μmol of α-isopropylmaleate per min.
TA activity was determined at 30 °C by monitoring leucine formation by amination of α-ketoisocaproate using l-glutamate as an amino-group donor. The reaction mixture contained 100 mmol L−1 Tris-HCl (pH 9.0), 5 mmol L−1 sodium α-ketoisocaproate, 10 mmol L−1 sodium glutamate, 0.25 mmol L−1 pyridoxal-5′-phosphate, and crude extract [23]. The reaction was terminated by adding 21% perchloric acid. l-leucine formation was quantified by high-pressure liquid chromatography (HPLC) after neutralization with 5 mol L−1 KOH and centrifugation (8000× g, 10 min) at 4 °C. One unit of TA activity was defined as the activity necessary to form 1μmol of l-leucine per min.
LeuDH activity was measured at 30 °C by monitoring the oxidation of NADPH at 340 nm. The reaction mixture contained 100 mmol L−1 glycine-NaOH (pH 9.5), 10 mmol L−1 sodium α-ketoisocaproate, 0.2 mmol L−1 NADH, 200 mmol L−1 NH4Cl, and crude extract [23]. One unit of LeuDH activity was defined as the activity required to oxidize 1 μmol of NADH per min.
GDH activity was determined at 25 °C, by monitoring the oxidation of NADPH (or NADH) at 340 nm. The reaction mixture contained 55 mmol L−1 Tris-HCl (pH 7.5) with 2% glycerol, 10 mmol L−1 α-ketoglutarate, 10 mmol L−1 L NaCl, 100 mmol L−1 NH4Cl, 0.2 mmol L−1 NADPH (or NADH), and crude extract [23]. One unit of glutamate dehydrogenase activity was defined as the amount of enzyme that catalyzed the oxidation of 1 mmol NADPH (or NADH) per min.
4.3. Purification and Kinetic Analysis of AHAIR
Wild-type and mutant AHAIR were purified from BL21/pET28a-ilvC and BL21/pET28a-ilvCTM, and all purification steps were performed at 4 °C. Cultivated cells were disrupted by sonication and centrifuged to remove cell debris. Then, extract was purified by a protein purifier (AKTA Explorer 10, GE Healthcare, Uppsala, Sweden). Purified wild-type AHAIR and mutant AHAIR were used to perform enzyme activity for kinetic analysis. The decrease of NAD(P)H was measured at 340 nm. The α-acetolactate and NAD(P)H concentrations were from 0 to 20 mmol L−1 and 0 to 1 mmol L−1, respectively.
4.4. NADPH/NADP+ Assay
The cultivated cells were harvested by centrifugation (8000× g, 10 min) at 4 °C, and then cells were washed three times, to remove the residual extracellular metabolites, with ice-cold quenching solution (60% MeOH and 70 mmol L−1 HEPES). Intracellular NADPH and NADP+ were extracted and quantified using the Coenzyme II NADP(H) Content Assay kit (Solarbio, Beijing, China), respectively, following the manufacturer’s instructions. Then, the NADPH/NADP+ ratio was calculated.
4.5. Analytical Methods
Samples were taken every 4 h to determine some parameters. Cell growth was monitored by measuring the optical density of the culture at 562 nm (OD562), using a spectrophotometer (721N, shanghai, China), after diluting 0.2 mL of the sample with 5 mL 0.25 mol L−1 HCl, to dissolve the CaCO3 [46]. Glucose concentration was determined by SBA-40E immobilized enzyme biosensor (Biology Institute of Shandong Academy of Sciences, Jinan, China). Organic acid concentrations were determined by high-pressure liquid chromatography (HPLC) on an Agilent 1200 system (Agilent Technologies, Santa Clara, CA, USA) with UV detection (215 nm). Amino acid concentrations were determined by HPLC (Agilent Technologies, Santa Clara, CA, USA) with DAD detection (338 nm), after automatic precolumn derivatization with ortho-phthaldialdehyde [17].
Acknowledgments
We thank P.-D.C. for his careful work in revising this manuscript.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/8/2020/s1.
Author Contributions
W.-G.Z. and J.-Z.X. conceived and designed the experiments. F.Z., Y.-Y.W. performed the experiments and wrote the paper. Y.-Y.W. and J.-Z.X. analyzed the data. X.-L.C. and L.-M.L. critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Top-Notch Academic Programs Project of Jiangsu Higher Education Institutions, the National Natural Science Foundation of China (Grant number 31601459), China Postdoctoral Science Foundation Grant (Grant number 2016M590410). The APC was funded by National First-class Discipline Program of Light Industry Technology and Engineering (Grant number LITE2018-07).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yamamoto K., Tsuchisaka A., Yukawa H. Branched-Chain Amino Acids. Adv. Biochem. Eng. Biotechnol. 2016 doi: 10.1007/10_2016_28. [DOI] [PubMed] [Google Scholar]
- 2.Columbus D.A., Fiorotto M.L., Davis T.A. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids. 2015;47:259–270. doi: 10.1007/s00726-014-1866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogt M., Haas S., Klaffl S., Polen T., Eggeling L., van Ooyen J., Bott M. Pushing product formation to its limit: Metabolic engineering of Corynebacterium glutamicum for l-leucine overproduction. Metab. Eng. 2014;22:40–52. doi: 10.1016/j.ymben.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Wang C., Guo F. Branched chain amino acids and metabolic regulation. Chin. Sci. Bull. 2013;58:1228–1235. doi: 10.1007/s11434-013-5681-x. [DOI] [Google Scholar]
- 5.Umbarger H.E. Amino acid biosynthesis and its regulation. Annu. Rev. Biochem. 1978;47:533–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Zhang H., Quinn P.J. Production of l-valine from metabolically engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2018 doi: 10.1007/s00253-018-8952-2. [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Cong H., Liu B., Song J., Sun X., Zhang J., Yang Q. Metabolic engineering of Corynebacterium glutamicum for methionine production by removing feedback inhibition and increasing NADPH level. Antonie Van Leeuwenhoek. 2016;109:1185–1197. doi: 10.1007/s10482-016-0719-0. [DOI] [PubMed] [Google Scholar]
- 8.Huang Q., Liang L., Wu W., Wu S., Huang J. Metabolic engineering of Corynebacterium glutamicum to enhance l-leucine production. Afr. J. Biotechnol. 2017;16:1048–1060. doi: 10.5897/AJB2017.15911. [DOI] [Google Scholar]
- 9.Yin L., Zhao J., Chen C., Hu X., Wang X. Enhancing the carbon flux and NADPH supply to increase l-isoleucine production in Corynebacterium glutamicum. Biotechnol. Bioprocess Eng. 2014;19:132–142. doi: 10.1007/s12257-013-0416-z. [DOI] [Google Scholar]
- 10.Chen C., Li Y., Hu J., Dong X., Wang X. Metabolic engineering of Corynebacterium glutamicum ATCC13869 for l-valine production. Metab. Eng. 2015;29:66–75. doi: 10.1016/j.ymben.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Gerstmeir R., Ramos-Vera H., Marin K. Method for Producing l-Leucine, l-Valine, l-Isoleucine, Alpha-Ketoisovalerate, Alpha-Keto-Beta-Methylvalerate, or Alpha-Ketoisocaproate Using Recombinant Corynebacteria that Contain the Ilvbn Operon Which Can Be Induced by Propionate. Application No. 14/894,513. U.S. Patent. 2016 Apr 28;
- 12.Becker J., Wittmann C. Systems and synthetic metabolic engineering for amino acid production—The heartbeat of industrial strain development. Curr. Opin. Biotechnol. 2012;23:718–726. doi: 10.1016/j.copbio.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Tao H., Guo D., Zhang Y., Deng Z., Liu T. Metabolic engineering of microbes for branched-chain biodiesel production with low-temperature property. Biotechnol. Biofuels. 2015;8:92. doi: 10.1186/s13068-015-0270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michael V., Haas S., Polen T., van Ooyen J., Bott M. Production of 2-ketoisocaproate with Corynebacterium glutamicum strains devoid of plasmids and heterologous genes. Microb. Biotechnol. 2015;8:351–360. doi: 10.1111/1751-7915.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bückle-Vallant V., Krause F.S., Messerschmidt S., Eikmanns B.J. Metabolic engineering of Corynebacterium glutamicum for 2-ketoisocaproate production. Appl. Microbiol. Biotechnol. 2013;98:297–311. doi: 10.1007/s00253-013-5310-2. [DOI] [PubMed] [Google Scholar]
- 16.Park J.H., Lee S.Y. Fermentative production of branched chain amino acids: A focus on metabolic engineering. Appl. Microbiol. Biotechnol. 2010;85:491–506. doi: 10.1007/s00253-009-2307-y. [DOI] [PubMed] [Google Scholar]
- 17.Xu J., Han M., Zhang J., Guo Y., Zhang W. Metabolic engineering Corynebacterium glutamicum for the l-lysine production by increasing the flux into l-lysine biosynthetic pathway. Amino Acids. 2014;46:2165–2175. doi: 10.1007/s00726-014-1768-1. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchida T., Momose H. Improvement of an l-leucine-producing mutant of Brevibacterium lactofermentum 2256 by genetically desensitizing it to α-acetohydroxy acid synthetase. Appl. Environ. Microbiol. 1986;51:1024–1027. doi: 10.1128/aem.51.5.1024-1027.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LY F., JZ X., WG Z. Improved l-Leucine Production in Corynebacterium glutamicum by optimizing the Aminotransferases. Molecules. 2018;23:2102. doi: 10.3390/molecules23092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J.Z., Yang H.K., Zhang W.G. NADPH metabolism: A survey of its theoretical characteristics and manipulation strategies in amino acid biosynthesis. Crit. Rev. Biotechnol. 2018;38:1061–1076. doi: 10.1080/07388551.2018.1437387. [DOI] [PubMed] [Google Scholar]
- 21.Zhan M., Kan B., Dong J., Xu G., Han R., Ni Y. Metabolic engineering of Corynebacterium glutamicum for improved l-arginine synthesis by enhancing NADPH supply. J. Ind. Microbiol. Biotechnol. 2019;46:45–54. doi: 10.1007/s10295-018-2103-8. [DOI] [PubMed] [Google Scholar]
- 22.Jiang L.Y., Zhang Y.Y., Li Z., Liu J.Z. Metabolic engineering of Corynebacterium glutamicum for increasing the production of l-ornithine by increasing NADPH availability. J. Ind. Microbiol. Biotechnol. 2013;40:1143–1151. doi: 10.1007/s10295-013-1306-2. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa S., Suda M., Uematsu K., Natsuma Y., Hiraga K., Jojima T., Inui M., Yukawa H. Engineering of Corynebacterium glutamicum for high-yield l-valine production under oxygen deprivation conditions. Appl. Environ. Microbiol. 2013;79:1250–1257. doi: 10.1128/AEM.02806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa S., Uematsu K., Natsuma Y., Suda M., Hiraga K., Jojima T., Inui M., Yukawa H. Improvement of the Redox Balance Increases l-Valine Production by Corynebacterium glutamicum under Oxygen Deprivation Conditions. Appl. Environ. Microbiol. 2012;78:865–875. doi: 10.1128/AEM.07056-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwentner A., Feith A., Münch E., Busche T., Rückert C., Kalinowski J., Takors R., Blombach B. Metabolic engineering to guide evolution—Creating a novel mode for l-valine production with Corynebacterium glutamicum. Metab. Eng. 2018;47:31–41. doi: 10.1016/j.ymben.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Marienhagen J., Eggeling L. Metabolic function of Corynebacterium glutamicum aminotransferases AlaT and AvtA and impact on l-valine production. Appl. Environ. Microbiol. 2008;74:7457–7462. doi: 10.1128/AEM.01025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rane M.J., Calvo K.C. Reversal of the Nucleotide Specificity of Ketol Acid Reductoisomerase by Site-Directed Mutagenesis Identifies the NADPH Binding Site1. Arch. Biochem. Biophys. 1997;338:83–89. doi: 10.1006/abbi.1996.9802. [DOI] [PubMed] [Google Scholar]
- 28.Scrutton N.S., Berry A., Perham R. Redesign of the coenzyme specificity of a dehydrogenase by protein engineering. Nature. 1990;343:38–43. doi: 10.1038/343038a0. [DOI] [PubMed] [Google Scholar]
- 29.Ahn H.J., Eom S.J., Yoon H.J., Lee B.I., Cho H., Suh S.W. Crystal structure of class I acetohydroxy acid isomeroreductase from Pseudomonas aeruginosa. J. Mol. Biol. 2003;328:505–515. doi: 10.1016/S0022-2836(03)00264-X. [DOI] [PubMed] [Google Scholar]
- 30.Monica R., Takashi A., Arnaud M.B., Berlyn M.K.B., Blattner F.R., Chaudhuri R.R., Glasner J.D., Takashi H., Keseler I.M., Takehide K. Escherichia coli K-12: A cooperatively developed annotation snapshot—2005. Nucleic Acids Res. 2006;34:1–9. doi: 10.1093/nar/gkj405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J.P., Wang Y.L., Chen J.J., Xu M.J., Yang T.W., Zheng J.X., Zhang X., Rao Z.M. Rational Engineering of Bacillus cereus Leucine Dehydrogenase towards α-keto Acid Reduction for Improving Unnatural Amino Acid Production. Biotechnol. J. 2018 doi: 10.1002/biot.201800253. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L., Wu Z., Jin J.M., Tang S.Y. Directed evolution of leucine dehydrogenase for improved efficiency of l-tert-leucine synthesis. Appl. Microbiol. Biotechnol. 2016;100:5805–5813. doi: 10.1007/s00253-016-7371-5. [DOI] [PubMed] [Google Scholar]
- 33.Kataoka K., Tanizawa K. Alteration of substrate specificity of leucine dehydrogenase by site-directed mutagenesis. J. Mol. Catal. B Enzym. 2003;23:299–309. doi: 10.1016/S1381-1177(03)00093-6. [DOI] [Google Scholar]
- 34.Liu W., Ma H., Luo J., Shen W., Xu X., Li S., Hu Y., Huang H. Efficient synthesis of l-tert-leucine through reductive amination using leucine dehydrogenase and formate dehydrogenase coexpressed in recombinant E. coli. Biochem. Eng. J. 2014;91:204–209. doi: 10.1016/j.bej.2014.08.003. [DOI] [Google Scholar]
- 35.Commichau F.M., Gunka K., Landmann J.J., Stülke J. Glutamate Metabolism in Bacillus subtilis: Gene Expression and Enzyme Activities Evolved to Avoid Futile Cycles and to Allow Rapid Responses to Perturbations of the System. J. Bacteriol. 2008;190:3557–3564. doi: 10.1128/JB.00099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noda-Garcia L., Romero Romero M.L., Longo L.M., Kolodkin-Gal I., Tawfik D.S. Bacilli glutamate dehydrogenases diverged via coevolution of transcription and enzyme regulation. EMBO Rep. 2017;18:1139–1149. doi: 10.15252/embr.201743990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stannek L., Thiele M.J., Ischebeck T., Gunka K., Hammer E., Volker U., Commichau F.M. Evidence for synergistic control of glutamate biosynthesis by glutamate dehydrogenases and glutamate in Bacillus subtilis. Environ. Microbiol. 2015;17:3379–3390. doi: 10.1111/1462-2920.12813. [DOI] [PubMed] [Google Scholar]
- 38.Ohshima T., Misono H., Soda K. Properties of crystalline leucine dehydrogenase from Bacillus sphaericus. J. Biol. Chem. 1978;253:5719–5725. [PubMed] [Google Scholar]
- 39.Börmann E.R., Eikmanns B.J., Sahm H. Molecular analysis of the Corynebacterium glutamicum gdh gene encoding glutamate dehydrogenase. Mol. Microbiol. 1992;6:317–326. doi: 10.1111/j.1365-2958.1992.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 40.Gusyatiner M.M., Lunts M.G., Kozlov Y.I., Ivanovskaya L.V., Voroshilova E.B. DNA Coding for Mutant Isopropylmalate Synthase l-Leucine-Producing Microorganism and Method for Producing l-Leucine. No. 6,403,342. U.S. Patent. 2002 Jun 11;
- 41.TsuCHIDA T., Yoshinaga F., Kubota K., Momose H., Okumura S. Production of l-Leucine by a Mutant of Brevibacterium lactofermentum 2256. Agric. Biol. Chem. 1974;38:1907–1911. [Google Scholar]
- 42.Hou X., Chen X., Zhang Y., Qian H., Zhang W. (l)-Valine production with minimization of by-products’ synthesis in Corynebacterium glutamicum and Brevibacterium flavum. Amino Acids. 2012;43:2301–2311. doi: 10.1007/s00726-012-1308-9. [DOI] [PubMed] [Google Scholar]
- 43.Artymiuk P.J., Blake C.C., Grace D.E., Oatley S.J., Phillips D.C., Sternberg M.J. Crystallographic studies of the dynamic properties of lysozyme. Nature. 1979;280:563–568. doi: 10.1038/280563a0. [DOI] [PubMed] [Google Scholar]
- 44.Kohlhaw G.B. Methods in Enzymology. Volume 166. Academic Press; Cambridge, MA, USA: 1988. [52] α-Isopropylmalate synthase from yeast; pp. 414–423. [DOI] [PubMed] [Google Scholar]
- 45.Kohlhaw G.B. Isopropylmalate dehydratase from yeast. Methods Enzymol. 1988;166:423–429. doi: 10.1016/s0076-6879(88)66055-1. [DOI] [PubMed] [Google Scholar]
- 46.Hou X., Ge X., Wu D., Qian H., Zhang W. Improvement of l-valine production at high temperature in Brevibacterium flavum by overexpressing ilvEBNrC genes. J. Ind. Microbiol. Biotechnol. 2012;39:63–72. doi: 10.1007/s10295-011-1000-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.