Abstract
PDZ domain‐containing proteins (PDZ proteins) act as scaffolds for protein–protein interactions and are crucial for a variety of signal transduction processes. However, the role of PDZ proteins in organismal lifespan and aging remains poorly understood. Here, we demonstrate that KIN‐4, a PDZ domain‐containing microtubule‐associated serine‐threonine (MAST) protein kinase, is a key longevity factor acting through binding PTEN phosphatase in Caenorhabditis elegans. Through a targeted genetic screen for PDZ proteins, we find that kin‐4 is required for the long lifespan of daf‐2/insulin/IGF‐1 receptor mutants. We then show that neurons are crucial tissues for the longevity‐promoting role of kin‐4. We find that the PDZ domain of KIN‐4 binds PTEN, a key factor for the longevity of daf‐2 mutants. Moreover, the interaction between KIN‐4 and PTEN is essential for the extended lifespan of daf‐2 mutants. As many aspects of lifespan regulation in C. elegans are evolutionarily conserved, MAST family kinases may regulate aging and/or age‐related diseases in mammals through their interaction with PTEN.
Keywords: aging, DAF‐18/PTEN, insulin/IGF‐1 signaling, KIN‐4/MAST kinase, lifespan, PDZ
1. INTRODUCTION
Insulin/IGF‐1 signaling (IIS) is an evolutionarily conserved signaling pathway that regulates organismal lifespan. In Caenorhabditis elegans, mutations in the insulin/IGF‐1 receptor, daf‐2, substantially increase lifespan (Kenyon, 2010). Longevity conferred by daf‐2 mutations requires various proteins, including DAF‐18/PTEN and transcription factors such as DAF‐16/FOXO, heat‐shock factor‐1 (HSF‐1), and SKN‐1/NRF2. The DAF‐18/PTEN protein dephosphorylates phosphatidylinositol‐3,4,5‐trisphosphate (PI(3,4,5)P3) to phosphatidylinositol‐4,5‐bisphosphate (PI(4,5)P2) (Solari et al., 2005). This leads to the inactivation of AKT kinases and subsequent activation of longevity‐promoting DAF‐16/FOXO (Reviewed in Altintas, Park, & Lee, 2016; Kenyon, 2010).
The PDZ (PSD‐95/Dlg‐1/ZO‐1) domain‐containing proteins (hereafter referred to as PDZ proteins) act as scaffolds for protein–protein interactions and mediate various cellular signaling processes (Reviewed in Kim & Sheng, 2004). PDZ domains are composed of six β‐sheets and two α‐helices and comprise ~90 amino acids. PDZ proteins typically bind the PDZ‐binding motifs that are located at the C‐terminal regions of the partner proteins. Roles of various PDZ proteins in cellular processes, such as signal transduction in neurons, are relatively well established; however, their function in aging and lifespan regulation is underexplored.
KIN‐4 is the sole homolog of the human microtubule‐associated serine/threonine kinase 1/2/3 (MAST1/2/3) in C. elegans, which contains a PDZ domain and a protein kinase domain (Manning, 2005). Human MAST family kinases are implicated in the inhibition of neurite outgrowth and regeneration in cultured cells (Loh, Francescut, Lingor, Bahr, & Nicotera, 2008). The sole Drosophila homolog of the human MAST kinase, Drop out, regulates dynein‐dependent transport during embryonic development (Hain et al., 2014). MAST kinases also bind to various proteins, including microtubules (Walden & Cowan, 1993), β2‐syntrophin (Lumeng et al., 1999), TNF receptor‐associated factor 6 (TRAF6) (Xiong, Li, Chen, Zhao, & Unkeless, 2004), cAMP‐regulated phospho‐protein (ARPP‐16) (Andrade et al., 2017), and PTEN (Valiente et al., 2005). Despite these findings, the role of MAST kinases in organismal aging remains unknown.
In the current study, we investigated the role of KIN‐4 in lifespan regulation conferred by reduced IIS in C. elegans. We used RNA interference (RNAi) to screen for genes encoding PDZ proteins that affected the lifespan of C. elegans. Our results showed that KIN‐4 was required for the long lifespan of daf‐2 mutants. kin‐4 was partly required for dauer formation and oxidative stress resistance in daf‐2 mutants. Moreover, kin‐4 in neurons was crucial for the extension of lifespan by daf‐2 mutations. Through a large‐scale yeast two‐hybrid screen and subsequent protein–protein binding assays, we found that DAF‐18/PTEN bound to the PDZ domain of KIN‐4 through its C‐terminus. More importantly, the interaction between KIN‐4 and DAF‐18/PTEN was required to extend the lifespan of daf‐2 mutants. Our findings suggest that MAST family kinases exert physiological effects on lifespan regulation via modulating IIS pathways through direct interaction with PTEN.
2. RESULTS
2.1. KIN‐4 is required for longevity conferred by reduced IIS
We aimed at identifying PDZ proteins that contribute to C. elegans longevity. We first identified 80 PDZ protein‐encoding genes by using Pfam and WormBase and by performing literature searches (Supporting information Table S1). We then examined the effect of each of the commercially available RNAi clones, targeting 49 candidate genes, on the lifespan of control worms and daf‐2/insulin/IGF‐1 receptor mutants (Figure 1a and Supporting information Table S2); we used daf‐2 mutants that display a prominent longevity phenotype (Kenyon, 2010), as the effect of RNAi on the lifespan of these mutants would be more pronounced than on the wild‐type (Seo et al., 2015). Many PDZ proteins play roles in neurons (Reviewed in E. Kim & Sheng, 2004); because C. elegans neurons are refractory to RNAi (Timmons, Court, & Fire, 2001), we used rrf‐3 mutant animals that display enhanced sensitivity to RNAi (Asikainen, Vartiainen, Lakso, Nass, & Wong, 2005) (Figure 1a; Supporting information Table S2). We found that RNAi targeting kin‐4, which encodes a MAST family kinase (Manning, 2005), or gipc‐1 and gipc‐2, which encode GAIP‐interacting protein C proteins (Vartiainen, Pehkonen, Lakso, Nass, & Wong, 2006), had greater lifespan‐shortening effects on daf‐2 mutants than on control animals (Figure 1a–c). We then determined the effects of genetic inhibition of kin‐4 or gipc‐1/‐2 on daf‐2(–) longevity using available loss of function mutants. Two independent kin‐4 deletion mutations substantially suppressed the long lifespan of daf‐2 mutants (Figure 2a, b; Supporting information Figure S1a, b), whereas gipc‐1; gipc‐2 double mutations did not (Supporting information Figure S1c). These data suggest that kin‐4 contributes to longevity caused by daf‐2 mutations.
Figure 1.
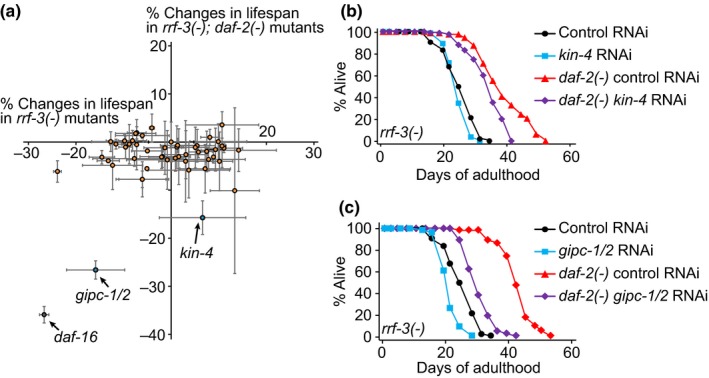
KIN‐4 is a PDZ protein that is required for the longevity of daf‐2/insulin/IGF‐1 receptor mutants. (a) A plot of percent changes in mean lifespan of rrf‐3(pk1426) [rrf‐3(−)] and rrf‐3(pk1426); daf‐2(e1370) [rrf‐3(−); daf‐2(−)] mutants upon knocking down each candidate PDZ protein‐encoding gene. Orange circles indicate the lifespan results after knocking down each targeted gene. Blue circles indicate the effects of kin‐4 RNAi and gipc‐1/‐2 RNAi on lifespan. A gray circle indicates the lifespan decrease by daf‐16 RNAi that was used as a positive control. Error bars represent standard error of mean (SEM) of two independent lifespan experiments for each RNAi clone. (b, c) Knock‐down of kin‐4 (b) or gipc‐1/‐2 (c) had a larger lifespan‐decreasing effect on rrf‐3(−); daf‐2(−) mutants than on rrf‐3(−) animals. See Supporting information Table S2 for statistics and additional repeats
Figure 2.
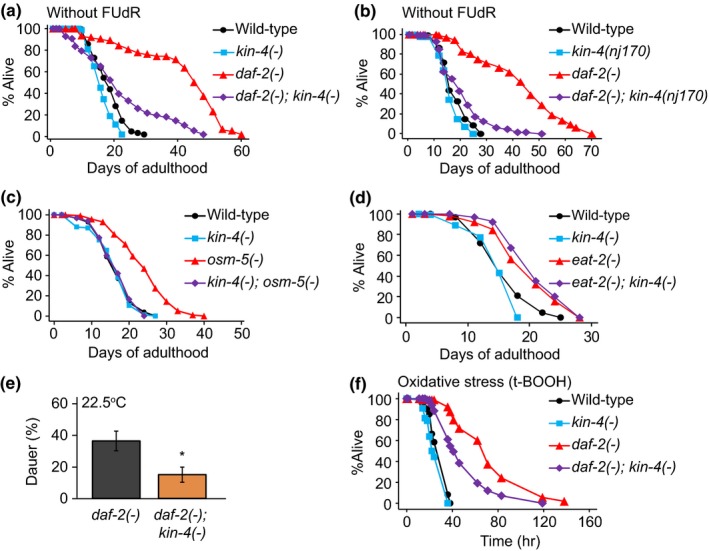
kin‐4 is required for various phenotypes conferred by reduced insulin/IGF‐1 signaling. (a, b) kin‐4(tm1049) [kin‐4(−)] (a) and kin‐4(nj170) (b) mutation decreased the long lifespan of daf‐2(e1370) [daf‐2(−)] mutants without FUdR treatment. This lifespan‐shortening effect of kin‐4(−) mutation was confirmed by using daf‐2(RNAi) worms with or without FUdR treatment (Supporting information Figure S1d, e). (c) kin‐4(−) fully suppressed the lifespan extension by osm‐5(p813) [osm‐5(−)]. (d) kin‐4(−) mutation did not affect the lifespan increase by eat‐2(ad1116) [eat‐2(−)]. See Supporting information Table S3 for experimental repeats and statistics. (e) kin‐4(−) mutation reduced the dauer formation of daf‐2(−) mutants at 22.5°C (10 independent experiments with ≥33 worms for each trial. Error bars represent SEM. two‐tailed Student's t test. ∗ p < 0.05). See Supporting information Table S4 for statistical analysis. (f) The enhanced oxidative stress resistance of daf‐2(−) mutants was partially suppressed by kin‐4(−) mutation upon treating with 7.5 mM of tert‐Butyl hydroperoxide (t‐BOOH). See Supporting information Table S5 for statistics and experimental repeats
Next, we determined the effect of kin‐4 mutation on longevity conferred by other gene mutations. The kin‐4 mutation fully suppressed the longevity of the sensory neuron‐defective osm‐5 mutants (Figure 2c), in which IIS is decreased (Apfeld & Kenyon, 1999), but did not affect the longevity of dietary restriction‐mimetic eat‐2 mutants (Figure 2d). These data are consistent with the possibility that KIN‐4 functions in IIS for C. elegans longevity.
2.2. kin‐4 is partly required for enhanced dauer formation and oxidative stress resistance caused by daf‐2 mutations
We sought to determine the role of KIN‐4 in other daf‐2(−)‐mediated physiological processes, including development and stress resistance. kin‐4 mutations significantly reduced the formation of daf‐2 mutation‐induced dauer, a hibernation‐like alternative larva (Hu, 2007), at 22.5°C but not at 25°C (Figure 2e; Supporting information Figure S2a). Additionally, the kin‐4 mutation partially suppressed the oxidative stress resistance of daf‐2 mutants (Figure 2f) but further increased the enhanced pathogen resistance conferred by daf‐2 mutations (Figure S2b). The thermotolerance of wild‐type and daf‐2 mutant worms was not significantly affected by the kin‐4 mutation (Supporting information Figure S2c). Thus, KIN‐4 appears to play roles in various IIS‐mediated physiological processes in a context‐dependent manner.
2.3. Neuronal kin‐4 contributes to longevity conferred by daf‐2 mutation
KIN‐4 contains a protein kinase domain and a PDZ domain (Figure 3a) and is highly conserved in multiple species (Supporting information Figure S3a, b). To determine the expression pattern of KIN‐4 and the effect of kin‐4 overexpression, we characterized transgenic animals expressing KIN‐4 tagged with the green fluorescent protein (GFP). We found that fusions of both the genomic kin‐4 and kin‐4 isoform a (kin‐4a) with GFP (kin‐4::gfp and kin‐4a::gfp, respectively) were expressed mainly in neurons and also in intestinal cells (Figure 3b, c; Supporting information Figure S4a). These results were confirmed using transgenic animals expressing kin‐4 promoter‐driven GFP (kin‐4p::gfp) (Supporting information Figure S4b). The kin‐4a::gfp transgene largely restored longevity in daf‐2; kin‐4 double mutants (Figure 3d) but did not affect the lifespan of wild‐type animals (Figure 3e; Supporting information Figure S4c).
Figure 3.
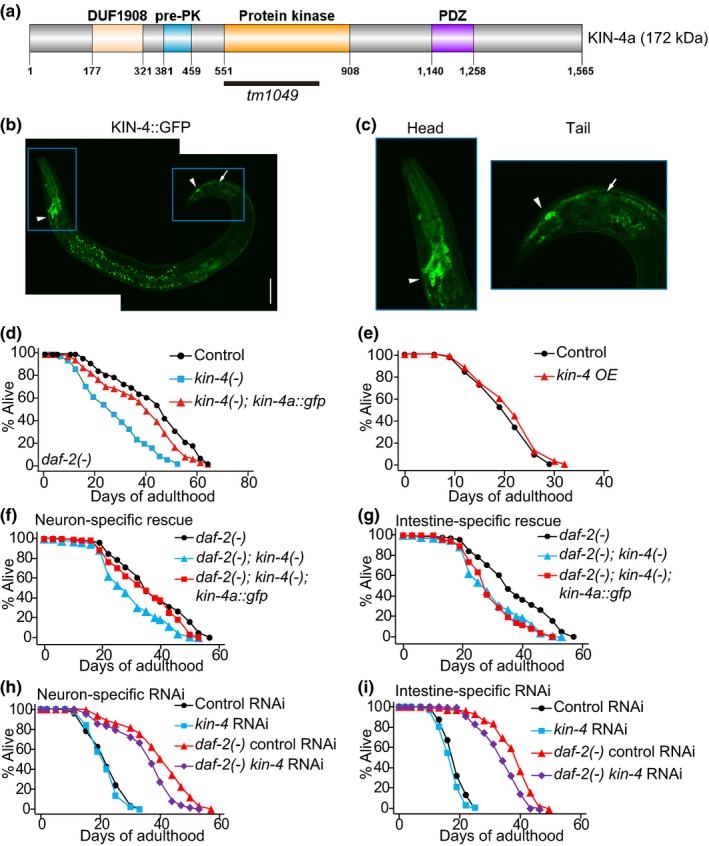
Neurons are crucial tissues for lifespan regulation by KIN‐4. (a) KIN‐4a is predicted to have 4 domains; DUF1908 whose function is not known, pre‐PK that is mainly found in MAST family kinases, protein kinase, and PDZ domains. The deleted part by kin‐4(tm1049) mutation is marked with a black solid line. (b, c) kin‐4::gfp transgene‐encoded protein (KIN‐4::GFP) was mainly expressed in head and tail (c) neurons (arrowheads) and dimly in the intestine (arrow). Scale bar indicates 50 μm. (d) KIN‐4a::GFP increased the shortened lifespan of daf‐2(e1370); kin‐4(tm1049) [daf‐2(−); kin‐4(−)] mutants. (e) kin‐4 transgenic worms did not display longevity. We also found that daf‐2 RNAi did not alter KIN‐4::GFP levels (Supporting information Figure S4d). See Supporting information Table S3 for experimental repeats and statistics. (f) Tissue‐specific promoter‐driven kin‐4a expression [daf‐2(−); kin‐4(−); kin‐4a::gfp] in neurons (daf‐2(e1370); kin‐4(tm1049); rgef‐1p::kin‐4a::gfp) prolonged the shortened lifespan of daf‐2(e1370); kin‐4(tm1049) [daf‐2(−); kin‐4(−)] mutants. (g) Transgenic expression of kin‐4a in the intestine (daf‐2(−); kin‐4(−); ges‐1p::kin‐4a::gfp) did not affect the decreased lifespan of daf‐2(−); kin‐4(−) mutants. (h) The long lifespan of daf‐2(−) mutants was decreased by neuron‐specific (sid‐1(pk3321); uIs69[myo‐2p::mCherry; unc‐119p::sid‐1]) kin‐4 RNAi treatment. (i) Knock‐down of kin‐4 in the intestine (rde‐1(ne213); kbIs7[nhx‐2p::rde‐1; rol‐6(su1006)]) significantly suppressed the long lifespan of daf‐2 mutants. See Supporting information Table S3 for experimental repeats and statistics
To further test the tissue‐specific roles of KIN‐4 in longevity, we performed lifespan assays with both tissue‐specific kin‐4 transgene and RNAi techniques. Neuronal expression of kin‐4 (Figure S5a) rescued the shortened lifespan of daf‐2; kin‐4 double mutants (Figure 3f). In contrast, kin‐4 expression in the intestine or hypodermis (Figure S5b, c) had no effect on the lifespan of daf‐2; kin‐4 mutants (Figure 3g; Supporting information Figure S5d). We then performed converse tissue‐specific kin‐4 RNAi knock‐down experiments. We first confirmed that treatment with kin‐4 RNAi decreased kin‐4 mRNA levels (Supporting information Figure S5e). Neuron‐specific knock‐down of kin‐4 shortened the lifespan of daf‐2 mutants but had minimal effects on the lifespan of control animals (Figure 3h). In addition, kin‐4 knock‐down in the intestine significantly suppressed the longevity of daf‐2 mutants (Figure 3i); however, kin‐4 RNAi in other tissues had no effect on longevity (Supporting information Figure S5f–j). Altogether, these data indicate that KIN‐4 expression in neurons is necessary for long lifespan and sufficient to restore the longevity in daf‐2 mutants.
2.4. KIN‐4 does not seem to affect canonical DAF‐16/FOXO transcription factor activity in daf‐2 mutants
DAF‐16/FOXO is an essential transcription factor that mediates the longevity of daf‐2 mutants (Reviewed in Altintas et al., 2016; Kenyon, 2010). We therefore asked whether kin‐4 influenced the activity of DAF‐16/FOXO for increasing lifespan in daf‐2 mutants. We found that kin‐4 mutations did not affect the nuclear localization of DAF‐16/FOXO in daf‐2 mutants (Supporting information Figure S6a, b). Similarly, kin‐4 mutations in daf‐2 mutants did not reduce the mRNA levels of three selected DAF‐16 targets, sod‐3, dod‐11, and mtl‐1 (Supporting information Figure S6c–e). We confirmed these results using sod‐3p::gfp transgenic animals (Supporting information Figure S6f). These data suggest that kin‐4 influences reduced IIS‐mediated longevity by acting through factors other than DAF‐16/FOXO.
2.5. Identification of KIN‐4‐interacting proteins that contribute to the longevity of daf‐2 mutants
Because KIN‐4 has a PDZ domain, we sought to identify proteins that bound to the PDZ domain, as these may contribute to longevity together with KIN‐4. We conducted an exhaustive yeast two‐hybrid screen using the PDZ domain of KIN‐4 as bait against C. elegans cDNA library and identified 41 prey proteins (Supporting information Figure S7). Of these, 23 proteins contained putative PDZ‐binding motifs at their C‐termini (Supporting information Table S6). To determine whether any of these 23 proteins were required for the longevity of daf‐2 mutants, we performed an RNAi lifespan screen targeting 21 genes (Figure 4a; Supporting information Table S7). Knock‐down of daf‐18/PTEN, mel‐11/myosin‐associated phosphatase regulatory subunit, or mig‐6/papilin and lacunin had greater lifespan‐shortening effects on daf‐2 mutants than on control animals (Figure 4b–d). In addition, RNAi targeting rps‐0, which encodes a small ribosomal subunit SA protein, further extended the lifespan of daf‐2 mutants (Figure 4e). Among these candidates, the expression pattern of MEL‐11 (Wissmann, Ingles, & Mains, 1999) or MIG‐6 (Jafari et al., 2010; Kawano et al., 2009) is not predicted to overlap with that of KIN‐4, and the effect of rps‐0 RNAi on lifespan was opposite of that caused by the genetic inhibition of kin‐4. Taking these results into consideration, we further analyzed the functional role of DAF‐18/PTEN in the longevity regulation by KIN‐4.
Figure 4.
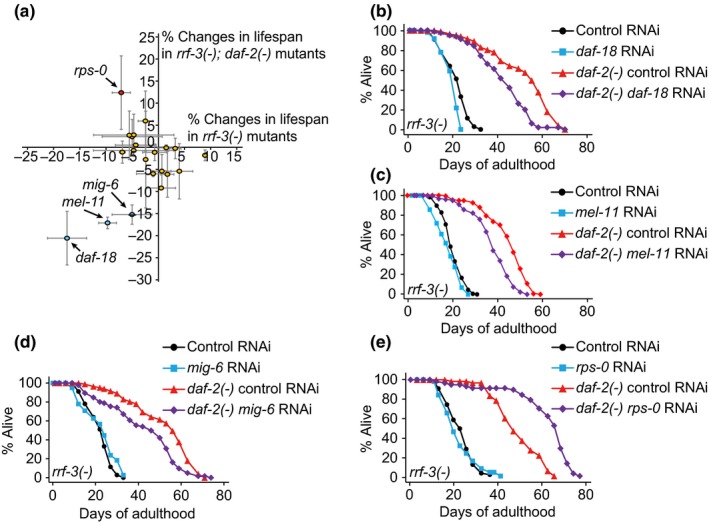
DAF‐18/PTEN binds KIN‐4 to affect the longevity of daf‐2 mutants. (a) Percent lifespan changes by knocking down each of 21 genes that encode proteins that bound the PDZ domain of KIN‐4 in rrf‐3(pk1426) [rrf‐3(−)] and rrf‐3(pk1426); daf‐2(e1370) [rrf‐3(−); daf‐2(−)] mutants. Orange circles indicate the effects of RNAi clones on lifespan. Blue circles indicate RNAi clones that displayed bigger lifespan‐decreasing effects on rrf‐3(−); daf‐2(−) mutants than on rrf‐3(−) worms. A red circle indicates an RNAi clone that further increased the long lifespan of rrf‐3(−); daf‐2(−) mutants. Error bars represent SEM of mean survival times from two or three independent RNAi lifespan experiments. (b−d) daf‐18 (b), mel‐11 (c), and mig‐6 (d) RNAi clones had bigger lifespan‐shortening effects on rrf‐3(−); daf‐2(−) mutant than on rrf‐3(−) animals. (e) rps‐0 RNAi treatment further increased the long lifespan induced by daf‐2(−) mutation in an rrf‐3(−) background. See Supporting information Table S7 for experimental repeats and statistical analyses
2.6. DAF‐18/PTEN binds to KIN‐4
DAF‐18/PTEN contributes to the longevity of daf‐2 mutants by acting as a downstream phosphoinositide phosphatase (Reviewed in Altintas et al., 2016; Kenyon, 2010). Human PTEN interacts with MAST2, a homolog of KIN‐4, in cultured cells (Valiente et al., 2005), although the physiological role of this interaction has not been investigated. We determined the subcellular localization of KIN‐4 and DAF‐18 in vivo and found that KIN‐4::GFP and mCherry‐tagged DAF‐18 (mCherry::DAF‐18) co‐localized to the cytoplasm of neurons (Figures 5a–c). Next, we performed in silico analysis of the PDZ domain of KIN‐4 (Figure 5d), based on the crystal structure of the PDZ domain of human MAST2 (Terrien et al., 2012). Our structural modeling indicated potential interaction between the PDZ domain of KIN‐4 and the C‐terminal region of DAF‐18/PTEN (Figure 5e). Specifically, the C‐terminal PDZ‐binding motif of DAF‐18/PTEN was located in the groove of the PDZ domain of KIN‐4 (Figure 5e). Additionally, key residues in the human MAST2 that interact with human PTEN, including isoleucine (I1121), valine (V1123), methionine (M1136), and leucine (L1191) (Terrien et al., 2012), were highly conserved as V1186, V1188, I1202, and L1257, respectively, in KIN‐4 (Figure 5e). Moreover, amino acid residues including tyrosine (T401) and valine (V403) in the PDZ‐binding motif and phenylalanine (F392) at the C‐terminal region of human PTEN, which are important for the interaction with human MAST2 (Terrien et al., 2012), were generally conserved in C. elegans DAF‐18/PTEN as I960, L962, and F951, respectively (Figure 5e; Supporting information Figure S8).
Figure 5.
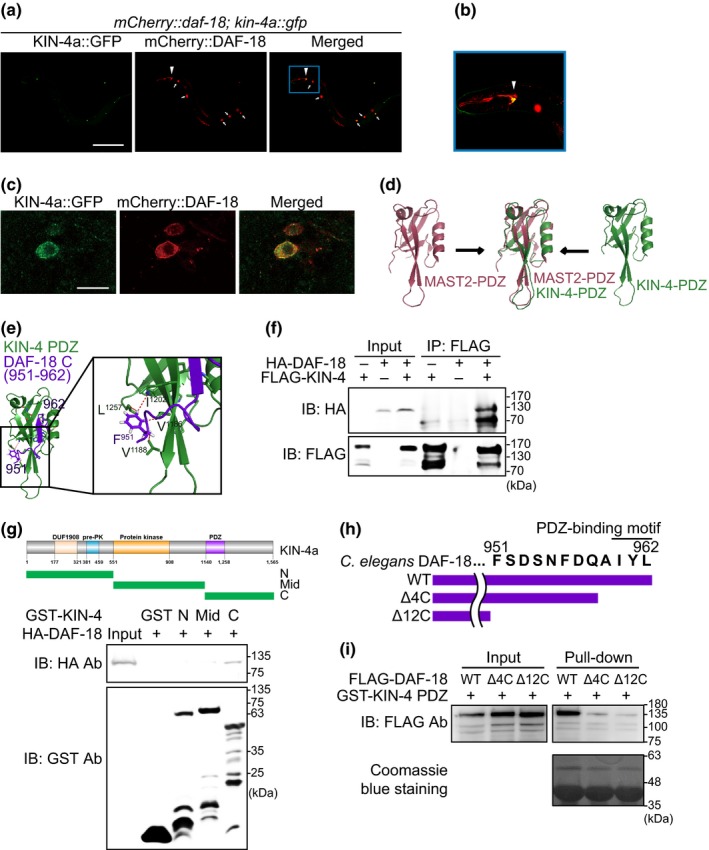
KIN‐4 physically interacts with DAF‐18/PTEN. (a‐c) Co‐localization of KIN‐4a::GFP and mCherry::DAF‐18. (a) DAF‐18 was mainly expressed in several head neurons (arrowhead). Arrows indicate red fluorescent signals of injection marker. Scale bar is 200 μm. (b) Shown in a blue rectangle is an enlarged picture of the head part in panel A. (c) Deconvolution images of a neuron. Subcellular localization of KIN‐4a::GFP and mCherry::DAF‐18 in a head neuron of mCherry::daf‐18; kin‐4a::gfp; ofm‐1p::rfp animals. Scale bar is 10 μm. (d) The PDZ domain of KIN‐4 (green) overlaps with that of MAST2 (magenta), one of human MAST family kinases. (e) The marked resides (V1186, V1188, I1202, and L1257) are conserved in the predicted structure of the PDZ domain of KIN‐4 (KIN‐4 PDZ, green) with that of human MAST2. F951 is a conserved residue in the C‐terminal region of DAF‐18 (DAF‐18 C, purple, See Supporting information Figure S8). DAF‐18 C from the 951st to the 962nd residues was predicted by substituting each residue of PTEN (2KYL) with a Coot program. (f) HA‐DAF‐18 was co‐immunoprecipitated by immunoprecipitating FLAG‐KIN‐4. IP: immunoprecipitation. IB: immunoblotting (g) A GST pull‐down assay of HA tag‐fused DAF‐18 [HA‐DAF‐18] with GST‐fused KIN‐4 fragment proteins. HA‐DAF‐18 was pulled down by using a PDZ domain‐containing KIN‐4 C‐terminal fragment [C], but not by an N‐terminal KIN‐4 fragment [N] or by a kinase domain‐containing KIN‐4 middle fragment [Mid]. See the upper illustration that depicts the domain regions in KIN‐4. (h) Illustration of wild‐type and deletion mutant DAF‐18 that were used for GST pull‐down assays. Wild‐type DAF‐18 [WT] has the intact DAF‐18 C‐terminal PDZ‐binding motif. DAF‐18 deletion mutant proteins, [Δ4C] and [Δ12C], do not contain the last 4 and 12 amino acids from its C‐terminal ends, respectively. (i) GST pull‐down assays using wild‐type or mutant FLAG‐tagged full‐length DAF‐18 [FLAG‐DAF‐18] with GST‐fused KIN‐4 PDZ domain [GST‐KIN‐4 PDZ] proteins. GST KIN‐4 PDZ strongly bound to FLAG‐DAF‐18 WT [WT] but weakly to FLAG‐DAF‐18 Δ4C [Δ4C] and FLAG‐DAF‐18 Δ12C [Δ12C]
We then experimentally tested the importance of the C‐terminal region of DAF‐18/PTEN for the interaction between KIN‐4 and DAF‐18/PTEN. Co‐immunoprecipitation assays using HEK293T cells confirmed that KIN‐4 bound to DAF‐18/PTEN (Figure 5f). Using glutathione S‐transferse (GST) pull‐down assays, we showed that the PDZ domain‐containing C‐terminal (C) region of KIN‐4 interacted with DAF‐18/PTEN, whereas the N‐terminal (N) or middle (Mid) region of KIN‐4 did not (Figure 5g). We then asked which regions and the residues of DAF‐18/PTEN were important for its interaction with KIN‐4 by generating FLAG‐tagged wild‐type and mutant DAF‐18/PTEN proteins and performing GST pull‐down assays using a GST‐fused KIN‐4 PDZ domain (Figure 5h–i). We found that 4‐amino acid and 12‐amino acid deletions at the C‐terminus (∆4C and ∆12C, respectively) substantially decreased the interaction between the PDZ domain of KIN‐4 and DAF‐18/PTEN (Figure 5i). These data indicate that the PDZ domain of KIN‐4 binds to the C‐terminal end of DAF‐18/PTEN.
2.7. Interaction between KIN‐4 and DAF‐18/PTEN is crucial for the longevity of daf‐2 mutants
Next, we examined whether the interaction between KIN‐4 and DAF‐18/PTEN contributed to reduced IIS‐mediated longevity. We generated transgenic animals expressing mutant forms of DAF‐18/PTEN proteins carrying ∆4C and ∆12C fused with mCherry (daf‐18 Δ4C and daf‐18 Δ12C, respectively) and those expressing mCherry‐tagged wild‐type (WT) DAF‐18 (daf‐18 WT) as a control. Expression of all these three mCherry‐fused DAF‐18/PTEN proteins was detected, and their subcellular localization was enriched at the plasma membrane (Figure 6a). We found that daf‐18 WT fully restored the longevity of daf‐2; daf‐18 double mutants (Figure 6b). In contrast, daf‐18 Δ4C or daf‐18 Δ12C only partially restored longevity in daf‐2; daf‐18 double mutants (Figure 6c, d). These data suggest that the C‐terminal end of DAF‐18/PTEN, which mediates the physical interaction with KIN‐4, is required for the full longevity of daf‐2 mutants. We also found that kin‐4 mutation further shortened the lifespan of daf‐18 Δ12C‐expressing daf‐2; daf‐18 mutants (Supporting information Figure S9). These data indicate that KIN‐4 promotes longevity in daf‐2 mutants via other factors in addition to DAF‐18, and/or that DAF‐18 C‐terminal region promotes daf-2(-) longevity via KIN‐4‐independent targets. Altogether, our data suggest that KIN‐4 contributes to longevity in daf‐2 mutants partly through binding to DAF‐18/PTEN at the C‐terminus.
Figure 6.
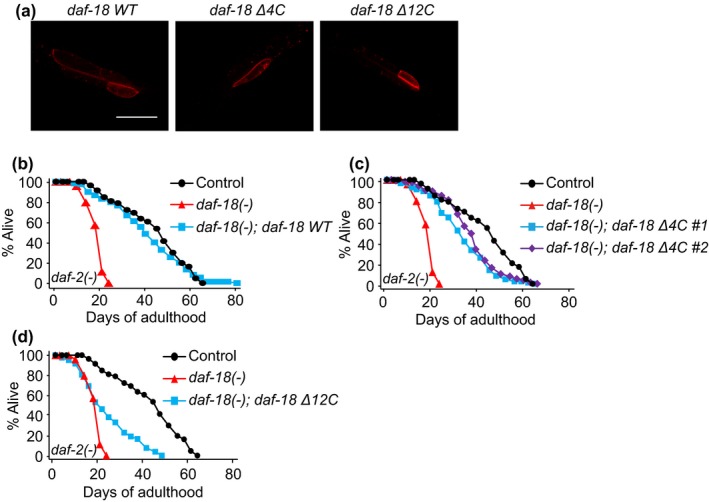
The interaction between KIN‐4 and DAF‐18 is crucial for longevity caused by IIS reduction. (a) Expression pattern of wild‐type [daf‐18 WT], the last four amino acid deletion mutant DAF‐18 transgene [daf‐18 Δ4C] and the last 12 amino acid deletion mutant daf‐18 transgene [daf‐18 Δ12C], fused with mCherry in the posterior intestine cells. (b) daf‐18 WT fully suppressed lifespan reduction by daf‐18(nr2037) [daf‐18(−)] mutation in daf‐2(e1370) [daf‐2(−)] animals. (c) Two independent lines of daf‐18 Δ4C only partially increased the shortened lifespan of daf‐2(−); daf‐18(−) mutants. (d) daf‐18 Δ12C had small lifespan‐increasing effects on the shortened lifespan of daf‐2(−); daf‐18(−) animals. See Supporting information Table S3 for statistics and additional repeats
3. DISCUSSION
3.1. C. elegans MAST family kinase KIN‐4 promotes longevity via binding DAF‐18/PTEN
PDZ proteins function as scaffolds that organize proteins for transducing various intracellular signals. However, the role of PDZ proteins in IIS, an important evolutionarily conserved lifespan‐regulatory signaling, has been poorly understood. In this study, we showed that KIN‐4/MAST kinase was a lifespan‐regulatory PDZ protein acting through DAF‐18/PTEN. KIN‐4, mainly in neurons, influenced the longevity conferred by reduced IIS. We also identified DAF‐18/PTEN as a key interacting protein of KIN‐4. The interaction between KIN‐4 and DAF‐18/PTEN was important for the lifespan regulation by IIS. Our study suggests that KIN‐4/MAST family kinase is a novel longevity‐promoting protein that acts in IIS via binding DAF‐18/PTEN protein.
3.2. KIN‐4 affects lifespan differently from previously reported PDZ proteins, MICS‐1 and MPZ‐1
Previous studies have reported lifespan‐influencing PDZ proteins, including MICS‐1/synaptojanin 2 binding protein (SYNJ2BP) and multi‐PDZ domain scaffold protein (MPZ‐1), in C. elegans (Hoffmann et al., 2012; Palmitessa & Benovic, 2010). The genetic inhibition of mics‐1 promotes longevity (Hoffmann et al., 2012), and mpz‐1 knock‐down increases lifespan, perhaps by activating DAF‐18/PTEN (Palmitessa & Benovic, 2010). Both mics‐1 and mpz‐1 genes were included in our RNAi lifespan screen (Supporting information Table S2); however, RNAi targeting of mics‐1 or mpz‐1 had small or no effect on lifespan in wild‐type or daf‐2 mutant animals (Figure 1a; Supporting information Table S2). In contrast, kin‐4 mutation or RNAi substantially suppressed the extended lifespan of daf‐2 mutants. This difference may have arisen from different genetic backgrounds and/or experimental conditions; for example, mics‐1 RNAi lifespan assay was performed at 25°C (Hoffmann et al., 2012), and mpz‐1 RNAi was applied for two generations to wild‐type worms (Palmitessa & Benovic, 2010). Here, we performed lifespan assays using RNAi‐sensitive rrf‐3 mutants treated with each of these RNAi clones only during adulthood at 20°C. Despite the difference, previous reports do not conflict with our data showing that KIN‐4 is required for the extended lifespan of C. elegans conferred by reduced IIS. We speculate that KIN‐4 contributes to IIS‐mediated longevity in a manner distinct from MPZ‐1 or MICS‐1.
3.3. kin‐4 mutation appears to elicit a compensatory activation of DAF‐16/FOXO in daf‐2 mutants
Although a major protein acting downstream of DAF‐18/PTEN lipid phosphatase is DAF‐16/FOXO, we did not find evidence supporting the possibility that KIN‐4 acts with DAF‐18/PTEN for increasing DAF‐16/FOXO activity. In contrast, DAF‐16/FOXO activity in daf‐2 mutant animals appears to be further increased by kin‐4(−) mutations (Supporting information Figure S6). We speculate that kin‐4(−) mutations decrease lifespan in daf‐2 mutants via DAF‐18/PTEN while acting through factors other than DAF‐16/FOXO and that this in turn enhances DAF‐16/FOXO activity as a compensatory response. Genetic inhibition of several DAF‐16/FOXO‐independent factors that contribute to reduced IIS‐mediated longevity has been shown to elicit compensatory activation of DAF‐16/FOXO. For example, we previously reported that mutations in smg‐2/UPF1, a key factor for nonsense‐mediated mRNA decay, decrease the longevity of daf‐2 mutants, while further increasing DAF‐16/FOXO activity (Son et al., 2017). In addition, PQM‐1, paraquat (methyl viologen)‐responsive transcription factor, is required for longevity in daf‐2 mutants, but genetic inhibition of pqm‐1 increases the nuclear localization of DAF‐16 (Tepper et al., 2013). It will be interesting to determine whether KIN‐4 and DAF‐18/PTEN act with DAF‐16/FOXO‐independent longevity factors for contributing to long lifespan in C. elegans with reduced IIS.
3.4. KIN‐4 may increase DAF‐18/PTEN activity via phosphorylation
Post‐translational modifications of PTEN, including phosphorylation, are important for its regulation (Fragoso & Barata, 2015). In many cases, phosphorylation at the C‐terminal region of PTEN leads to its conformational change that inhibits the lipid phosphatase activity of PTEN (Fragoso & Barata, 2015). On the other hand, Rak tyrosine kinase and polo‐like kinase 3 (Plk3) activate PTEN by phosphorylating the C2 domain and C‐terminal part of PTEN, respectively (Xu, Yao, Jiang, Lu, & Dai, 2010; Yim et al., 2009). In addition, RhoA‐associated kinase (ROCK) phosphorylates PTEN and increases its activity (Li et al., 2005). Mammalian MAST2 also phosphorylates PTEN in vitro (Valiente et al., 2005), although its effect on the activity of PTEN remains unexplored. Given the similar lifespan phenotypes caused by mutations in daf‐18/PTEN and kin‐4, we speculate that C. elegans KIN‐4 may increase the activity of DAF‐18/PTEN through binding and phosphorylation.
3.5. KIN‐4 contributes to longevity possibly through altering the protein phosphatase activity of DAF‐18/PTEN
Taking into consideration that DAF‐18/PTEN has dual phosphatase activity for both proteins and lipids (Fragoso & Barata, 2015), it seems likely that the binding of KIN‐4 may affect the phosphatase activity of DAF‐18/PTEN for as yet unidentified substrate proteins. In mammals, substrate proteins of PTEN phosphatase include protein kinase B/Akt and cyclic AMP response element‐binding protein (CREB; Gu et al., 2011; Phadngam et al., 2016). Therefore, homologs of these proteins may mediate the effects of the interaction between KIN‐4 and DAF‐18/PTEN on the longevity of C. elegans. One limitation regarding this scenario is that tyrosine 138, which is essential for the protein phosphatase activity of PTEN in mammals (Davidson et al., 2010), is replaced by leucine in C. elegans DAF‐18/PTEN. Nevertheless, C. elegans DAF‐18/PTEN can act as a protein phosphatase for VAB‐1/ephrin receptor (Brisbin et al., 2009). Therefore, it will be important to identify and functionally characterize protein substrates of DAF‐18/PTEN in C. elegans in future research, with respect to the role of KIN‐4 in protein–protein interaction and longevity.
3.6. KIN‐4 appears to contribute to longevity in daf‐2 mutants by acting with other factors as well as with DAF‐18/PTEN
In this paper, we showed that physical interaction between KIN‐4 and DAF‐18/PTEN is required for full longevity of daf‐2 mutants. However, KIN‐4 also seems to contribute to reduced IIS‐mediated longevity through additional factors. The mammalian MAST kinases bind various proteins, including microtubules (Walden & Cowan, 1993), β2‐syntrophin (Lumeng et al., 1999), TRAF6 (Xiong et al., 2004), and ARPP‐16 (Andrade et al., 2017). We also identified various factors that bound the PDZ domain of C. elegans KIN‐4 through a yeast two‐hybrid screen (Supporting information Table S6). Thus, KIN‐4 binding to some of these proteins may contribute to longevity in daf‐2 mutants. In addition, MAST2 and MAST3 kinases phosphorylate Na+/H+ exchanger 3 (NHE3; Wang et al., 2006) and ARPP‐16 (Andrade et al., 2017), respectively, and therefore, phosphorylation of C. elegans homologs of these proteins by KIN‐4 may affect reduced IIS‐mediated longevity. It will be important to test these possibilities by performing biochemical and molecular genetic experiments in future studies.
3.7. Interaction between MAST kinases and PTEN may play important roles in mammalian physiology
Several lines of evidence indicate that MAST kinases are crucial for the physiology of cancer cells (Eissmann et al., 2013; Robinson et al., 2011; Tomoshige et al., 2015). In some breast cancer cells, MAST1 and MAST2 are fused with other proteins through recurrent gene rearrangements; these fusion proteins act as putative tumorigenic drivers (Robinson et al., 2011). A pedigree study reported that MAST1 variants are associated with lung cancer (Tomoshige et al., 2015). Furthermore, MAST2 influences glioblastoma tumor growth, potentially as an apoptosis suppressor (Eissmann et al., 2013). In this study, we characterized the physiological role of the physical interaction between KIN‐4 and DAF‐18/PTEN in C. elegans. Considering evolutionarily conserved amino acid sequences of these proteins, our study may provide insights into the role of MAST kinases and PTEN in tumorigenesis and aging, thus contributing toward the development of therapeutic strategies for anti‐cancer and anti‐aging medicine.
4. EXPERIMENTAL PROCEDURES
4.1. Strains
All strains used in this study are described in the Supporting Information.
4.2. Identification of PDZ proteins
Identification of potential C. elegans PDZ proteins was performed by using combination of bioinformatic methods similarly to a previous paper (Seo et al., 2015). See the supporting information for details.
4.3. Lifespan assays
Lifespan assays were conducted at 20°C as previously described, with some modifications (Lee et al., 2015). See the Supporting Information for details.
4.4. Dauer formation
Dauer assays were performed as previously described, with some modifications (Gaglia et al., 2012). Gravid adult worms were placed on OP50‐seeded NGM plates and were allowed to lay eggs for 3 hr at 22.5°C and 25°C. The number of dauers and total worms was counted when non‐dauer worms reached L4 or young adult stage. Error bars indicate SEM, and statistics were calculated by using Student's t test.
4.5. Stress resistance assays
Stress resistance assays were performed by following a previous report with some modifications (Seo et al., 2015). See the Supporting Information for details.
4.6. Generation of transgenic worms
Transgenic worms were generated as previously described, with some modification (Artan et al., 2016). See the Supporting Information for details.
4.7. Generation of mutant strain using CRISPR
To generate kin‐4(nj170), the homologous recombination was performed essentially by following a previous report (Dickinson, Ward, Reiner, & Goldstein, 2013). First, the kin‐4 locus was replaced by a repair template (pSN588) that carries the loxP‐unc‐119(+)‐loxP sequence flanked by the left and right arms corresponding to the 1st intron and the 3′ UTR genomic sequence of kin‐4, respectively. This repair template was injected into unc‐119(ed3) animals with peft‐3::Cas9 and an sgRNA plasmid (pSN598) to generate a double‐stranded break at the kin‐4 locus. Progeny were screened for animals in which the kin‐4 locus was replaced by the repair template. The loxP‐unc‐119(+)‐loxP cassette was subsequently removed by injecting peft‐3::Cre plasmid (pDD104). The animals in which the unc‐119(+) gene was excised were isolated and were confirmed by DNA sequence analysis. The resulting strain carries a 17 kb deletion in the kin‐4 locus and removes the sequence between 24,629 of the cosmid F22B3 and 11,644 of the cosmid C10C6. The unc‐119(ed3) mutation was then crossed out to obtain IK2019.
4.8. Yeast two‐hybrid screen
Yeast two‐hybrid screen was performed by Panbionet (http://panbionet.com) following a previous report (Kim et al., 2008). See the Supporting information for details.
4.9. Prediction of protein domains, structure modeling and sequence alignment, and generation of the phylogenic tree
Prediction of protein domains, structure modeling and sequence alignment, and generation of the phylogenic tree were performed similarly to a previous paper (Hwang et al., 2015), with modifications. See the Supporting Information for details.
4.10. Co‐immunoprecipitation and immunoblotting
Co‐immunoprecipitation and immunoblotting were performed following a previous report (Seo et al., 2015) with some modifications. See the Supporting Information for details.
4.11. GST pull‐down assay
GST pull‐down assays were performed following methods described in a previous report (Dev, Nishimune, Henley, & Nakanishi, 1999) with some modifications. See the Supporting Information for details.
4.12. Microscopy
Microscopy experiments were executed as previously described with some modifications (Son et al., 2017). See the Supporting Information for details.
4.13. Quantitative RT–PCR analysis
Quantitative RT–PCR experiments were performed as previously described with some modifications (Son et al., 2017). See the Supporting information for details.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
S.W.A.A. contributed to designing and performing the majority of experimental assays in this manuscript, data analysis, and writing manuscript; E.‐S.C. initiated the project and performed survival assay experiments and data analysis; W.H., H.G.S., K.S., and E.J.E.K. contributed to survival assay experiments; J.‐S.Y., H.‐J.N., and S.K. contributed to bioinformatics analysis; N.T.H.N. and J.‐Y.Y. contributed to performing biochemistry experiments; B.K.S., S.K.P., Y.R., and C.M.H. contributed to confocal microscopy imaging; Y.K. contributed to prediction of protein structure modeling; S.N. and I.M. contributed to generating crucial mutants and transgenic worms for this manuscript; S.‐J.V.L. contributed to designing all experiments, performing the initial RNAi screen, some survival assay experiments, data analysis, and writing manuscript.
Supporting information
ACKNOWLEDGMENTS
Some C. elegans strains were provided by Dr. Cynthia Kenyon laboratory and Caenorhabditis Genetics Center. We thank all Lee laboratory members for discussion and help. These authors have no competing interests to declare. This study is supported by the Korean Government (MSIP) through the National Research Foundation of Korea (NRF) (NRF‐2016R1E1A1A01941152) and NRF (NRF‐2017R1A5A1015366) to S‐J.V.L., and by KBRI basic research program through Korea Brain Research Institute funded by Ministry of Science and ICT (18‐BR‐03‐03) to H.C.M.
An SWA, Choi E‐S, Hwang W, et al. KIN‐4/MAST kinase promotes PTEN‐mediated longevity of Caenorhabditis elegans via binding through a PDZ domain. Aging Cell. 2019;18:e12906 10.1111/acel.12906
REFERENCES
- Altintas, O. , Park, S. , & Lee, S. J. (2016). The role of insulin/IGF‐1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster . BMB Rep, 49(2), 81–92. 10.5483/BMBRep.2016.49.2.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade, E. C. , Musante, V. , Horiuchi, A. , Matsuzaki, H. , Brody, A. H. , Wu, T. , … Nairn, A. C. (2017). ARPP‐16 Is a striatal‐enriched inhibitor of protein phosphatase 2A regulated by microtubule‐associated serine/threonine kinase 3 (Mast 3 Kinase). Journal of Neuroscience, 37(10), 2709–2722. 10.1523/jneurosci.4559-15.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld, J. , & Kenyon, C. (1999). Regulation of lifespan by sensory perception in Caenorhabditis elegans . Nature, 402(6763), 804–809. 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Artan, M. , Jeong, D. E. , Lee, D. , Kim, Y. I. , Son, H. G. , Husain, Z. , … Lee, S. J. (2016). Food‐derived sensory cues modulate longevity via distinct neuroendocrine insulin‐like peptides. Genes & Development, 30(9), 1047–1057. 10.1101/gad.279448.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asikainen, S. , Vartiainen, S. , Lakso, M. , Nass, R. , & Wong, G. (2005). Selective sensitivity of Caenorhabditis elegans neurons to RNA interference. NeuroReport, 16(18), 1995–1999. 10.1097/00001756-200512190-00005 [DOI] [PubMed] [Google Scholar]
- Brisbin, S. , Liu, J. , Boudreau, J. , Peng, J. , Evangelista, M. , & Chin‐Sang, I. (2009). A role for C. elegans Eph RTK signaling in PTEN regulation. Developmental Cell, 17(4), 459–469. 10.1016/j.devcel.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Davidson, L. , Maccario, H. , Perera, N. M. , Yang, X. , Spinelli, L. , Tibarewal, P. , … Leslie, N. R. (2010). Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene, 29(5), 687–697. 10.1038/onc.2009.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev, K. K. , Nishimune, A. , Henley, J. M. , & Nakanishi, S. (1999). The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology, 38(5), 635–644. [DOI] [PubMed] [Google Scholar]
- Dickinson, D. J. , Ward, J. D. , Reiner, D. J. , & Goldstein, B. (2013). Engineering the Caenorhabditis elegans genome using Cas9‐triggered homologous recombination. Nature Methods, 10(10), 1028–1034. 10.1038/nmeth.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissmann, M. , Schwamb, B. , Melzer, I. M. , Moser, J. , Siele, D. , Kohl, U. , … Zornig, M. (2013). A functional yeast survival screen of tumor‐derived cDNA libraries designed to identify anti‐apoptotic mammalian oncogenes. PLoS ONE, 8(5), e64873 10.1371/journal.pone.0064873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso, R. , & Barata, J. T. (2015). Kinases, tails and more: Regulation of PTEN function by phosphorylation. Methods, 77–78, 75–81. 10.1016/j.ymeth.2014.10.015 [DOI] [PubMed] [Google Scholar]
- Gaglia, M. M. , Jeong, D. E. , Ryu, E. A. , Lee, D. , Kenyon, C. , & Lee, S. J. (2012). Genes that act downstream of sensory neurons to influence longevity, dauer formation, and pathogen responses in Caenorhabditis elegans . PLoS Genetics, 8(12), e1003133 10.1371/journal.pgen.1003133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, T. , Zhang, Z. , Wang, J. , Guo, J. , Shen, W. H. , & Yin, Y. (2011). CREB is a novel nuclear target of PTEN phosphatase. Cancer Research, 71(8), 2821–2825. 10.1158/0008-5472.can-10-3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain, D. , Langlands, A. , Sonnenberg, H. C. , Bailey, C. , Bullock, S. L. , & Muller, H. A. (2014). The Drosophila MAST kinase Drop out is required to initiate membrane compartmentalisation during cellularisation and regulates dynein‐based transport. Development, 141(10), 2119–2130. 10.1242/dev.104711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Honnen, S. , Mayatepek, E. , Watjen, W. , Koopman, W. J. , Bossinger, O. , & Distelmaier, F. (2012). MICS‐1 interacts with mitochondrial ATAD‐3 and modulates lifespan in C. elegans . Experimental Gerontology, 47(3), 270–275. 10.1016/j.exger.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Hu, P. J. (2007). Dauer. WormBook, 1–19, 10.1895/wormbook.1.144.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, W. , Artan, M. , Seo, M. , Lee, D. , Nam, H. G. , & Lee, S. J. (2015). Inhibition of elongin C promotes longevity and protein homeostasis via HIF‐1 in C. elegans . Aging Cell, 14(6), 995–1002. 10.1111/acel.12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari, G. , Burghoorn, J. , Kawano, T. , Mathew, M. , Morck, C. , Axang, C. , … Pilon, M. (2010). Genetics of extracellular matrix remodeling during organ growth using the Caenorhabditis elegans pharynx model. Genetics, 186(3), 969–982. 10.1534/genetics.110.120519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, T. , Zheng, H. , Merz, D. C. , Kohara, Y. , Tamai, K. K. , Nishiwaki, K. , & Culotti, J. G. (2009). C. elegans mig‐6 encodes papilin isoforms that affect distinct aspects of DTC migration, and interacts genetically with mig‐17 and collagen IV. Development, 136(9), 1433–1442. 10.1242/dev.028472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon, C. J. (2010). The genetics of ageing. Nature, 464(7288), 504–512. 10.1038/nature08980 [DOI] [PubMed] [Google Scholar]
- Kim, E. , & Sheng, M. (2004). PDZ domain proteins of synapses. Nature Reviews Neuroscience, 5(10), 771–781. 10.1038/nrn1517 [DOI] [PubMed] [Google Scholar]
- Kim, J. E. , Ryu, I. , Kim, W. J. , Song, O. K. , Ryu, J. , Kwon, M. Y. , … Jang, S. K. (2008). Proline‐rich transcript in brain protein induces stress granule formation. Molecular and Cellular Biology, 28(2), 803–813. 10.1128/mcb.01226-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. , Jeong, D. E. , Son, H. G. , Yamaoka, Y. , Kim, H. , Seo, K. , … Lee, S. J. (2015). SREBP and MDT‐15 protect C. elegans from glucose‐induced accelerated aging by preventing accumulation of saturated fat. Genes & Development, 29(23), 2490–2503. 10.1101/gad.266304.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Dong, X. , Wang, Z. , Liu, W. , Deng, N. , Ding, Y. , … Wu, D. (2005). Regulation of PTEN by Rho small GTPases. Nature Cell Biology, 7(4), 399–404. 10.1038/ncb1236 [DOI] [PubMed] [Google Scholar]
- Loh, S. H. , Francescut, L. , Lingor, P. , Bahr, M. , & Nicotera, P. (2008). Identification of new kinase clusters required for neurite outgrowth and retraction by a loss‐of‐function RNA interference screen. Cell Death and Differentiation, 15(2), 283–298. 10.1038/sj.cdd.4402258 [DOI] [PubMed] [Google Scholar]
- Lumeng, C. , Phelps, S. , Crawford, G. E. , Walden, P. D. , Barald, K. , & Chamberlain, J. S. (1999). Interactions between beta 2‐syntrophin and a family of microtubule‐associated serine/threonine kinases. Nature Neuroscience, 2(7), 611–617. 10.1038/10165 [DOI] [PubMed] [Google Scholar]
- Manning, G. (2005). Genomic overview of protein kinases. WormBook, 1–19, 10.1895/wormbook.1.60.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmitessa, A. , & Benovic, J. L. (2010). Arrestin and the multi‐PDZ domain‐containing protein MPZ‐1 interact with phosphatase and tensin homolog (PTEN) and regulate Caenorhabditis elegans longevity. Journal of Biological Chemistry, 285(20), 15187–15200. 10.1074/jbc.M110.104612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadngam, S. , Castiglioni, A. , Ferraresi, A. , Morani, F. , Follo, C. , & Isidoro, C. (2016). PTEN dephosphorylates AKT to prevent the expression of GLUT1 on plasmamembrane and to limit glucose consumption in cancer cells. Oncotarget, 7(51), 84999–85020. 10.18632/oncotarget.13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D. R. , Kalyana‐Sundaram, S. , Wu, Y. M. , Shankar, S. , Cao, X. , Ateeq, B. , … Chinnaiyan, A. M. (2011). Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nature Medicine, 17(12), 1646–1651. 10.1038/nm.2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, M. , Seo, K. , Hwang, W. , Koo, H. J. , Hahm, J. H. , Yang, J. S. , … Lee, S. J. (2015). RNA helicase HEL‐1 promotes longevity by specifically activating DAF‐16/FOXO transcription factor signaling in Caenorhabditis elegans . Proceedings of the National Academy of Sciences of the United States of America, 112(31), E4246–4255. 10.1073/pnas.1505451112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari, F. , Bourbon‐Piffaut, A. , Masse, I. , Payrastre, B. , Chan, A. M. , & Billaud, M. (2005). The human tumour suppressor PTEN regulates longevity and dauer formation in Caenorhabditis elegans . Oncogene, 24(1), 20–27. 10.1038/sj.onc.1207978 [DOI] [PubMed] [Google Scholar]
- Son, H. G. , Seo, M. , Ham, S. , Hwang, W. , Lee, D. , An, S. W. , … Roh, T. Y. (2017). RNA surveillance via nonsense‐mediated mRNA decay is crucial for longevity in daf‐2/insulin/IGF‐1 mutant C. elegans . Nature Communications, 8, 14749 10.1038/ncomms14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper, R. G. , Ashraf, J. , Kaletsky, R. , Kleemann, G. , Murphy, C. T. , & Bussemaker, H. J. (2013). PQM‐1 complements DAF‐16 as a key transcriptional regulator of DAF‐2‐mediated development and longevity. Cell, 154(3), 676–690. 10.1016/j.cell.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrien, E. , Chaffotte, A. , Lafage, M. , Khan, Z. , Prehaud, C. , Cordier, F. , … Wolff, N. (2012). Interference with the PTEN‐MAST2 interaction by a viral protein leads to cellular relocalization of PTEN. Science Signalling, 5(237), ra58 10.1126/scisignal.2002941 [DOI] [PubMed] [Google Scholar]
- Timmons, L. , Court, D. L. , & Fire, A. (2001). Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans . Gene, 263(1–2), 103–112. 10.1016/S0378-1119(00)00579-5 [DOI] [PubMed] [Google Scholar]
- Tomoshige, K. , Matsumoto, K. , Tsuchiya, T. , Oikawa, M. , Miyazaki, T. , Yamasaki, N. , & Mishima, H. (2015). Germline mutations causing familial lung cancer. Journal of Human Genetics, 60(10), 597–603. 10.1038/jhg.2015.75 [DOI] [PubMed] [Google Scholar]
- Valiente, M. , Andres‐Pons, A. , Gomar, B. , Torres, J. , Gil, A. , Tapparel, C. , … Pulido, R. (2005). Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule‐associated serine/threonine kinases. Journal of Biological Chemistry, 280(32), 28936–28943. 10.1074/jbc.M504761200. [DOI] [PubMed] [Google Scholar]
- Vartiainen, S. , Pehkonen, P. , Lakso, M. , Nass, R. , & Wong, G. (2006). Identification of gene expression changes in transgenic C. elegans overexpressing human alpha‐synuclein. Neurobiology of Diseases, 22(3), 477–486. 10.1016/j.nbd.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Walden, P. D. , & Cowan, N. J. (1993). A novel 205‐kilodalton testis‐specific serine/threonine protein kinase associated with microtubules of the spermatid manchette. Molecular and Cellular Biology, 13(12), 7625–7635. 10.1128/MCB.13.12.7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Lee, H. J. , Cooper, D. S. , Cebotaro, L. , Walden, P. D. , Choi, I. , & Yun, C. C. (2006). Coexpression of MAST205 inhibits the activity of Na+/H+ exchanger NHE3. American Journal of Physiology. Renal Physiology, 290(2), F428–437. 10.1152/ajprenal.00161.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmann, A. , Ingles, J. , & Mains, P. E. (1999). The Caenorhabditis elegans mel‐11 myosin phosphatase regulatory subunit affects tissue contraction in the somatic gonad and the embryonic epidermis and genetically interacts with the Rac signaling pathway. Developmental Biology, 209(1), 111–127. 10.1006/dbio.1999.9242 [DOI] [PubMed] [Google Scholar]
- Xiong, H. , Li, H. , Chen, Y. , Zhao, J. , & Unkeless, J. C. (2004). Interaction of TRAF6 with MAST205 regulates NF‐kappaB activation and MAST205 stability. Journal of Biological Chemistry, 279(42), 43675–43683. 10.1074/jbc.M404328200 [DOI] [PubMed] [Google Scholar]
- Xu, D. , Yao, Y. , Jiang, X. , Lu, L. , & Dai, W. (2010). Regulation of PTEN stability and activity by Plk3. Journal of Biological Chemistry, 285(51), 39935–39942. 10.1074/jbc.M110.166462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim, E. K. , Peng, G. , Dai, H. , Hu, R. , Li, K. , Lu, Y. , … Lin, S. Y. (2009). Rak functions as a tumor suppressor by regulating PTEN protein stability and function. Cancer Cell, 15(4), 304–314. 10.1016/j.ccr.2009.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


