Abstract
Background
Postoperative hypocalcemia after thyroid surgery has a high prevalence ( 16-55% in different series). Incidental parathyroidectomy (IP) is a less discussed complication of thyroidectomy with consequences not properly defined. The aim of our study was to find incidence, risk factors and how to prevent IP.
Methods
Extensive search of English literature publications via PubMed was performed and 73 papers from 1980 to 2017 were analysed using the GRADE system/classification, quality of evidence was classified as “strong” when the result is highly unlikely to change existing recommendation and “weak” when opposite.
Results
Incidence of IP is 3.7-24.9%, while prevalence of permanent hypoparathyroidism is less frequent 6-12%. Direct relation between IP and hypoparathyroidism/hypocalcemia remains controversial. Female patients, ectopic parathyroids, small thyroids, Graves’, malignancy, redo surgeries and total thyroidectomy favour IP. Routine visualization of parathyroids, new hemostatic devices, magnifying instruments and fluorescence can prevent incidental removal of parathyroids. Incidence of IP during videoassisted or robotic thyroidectomies was similar to open procedures. High volume, experienced and younger surgeons have lower complication rates (including hypoparathyroidism).
Conclusions
Incidental parathyroidectomy is more frequent than we might have expected. It should be avoided and parathyroid glands should be kept in situ. Majority of studies are retrospective (low degree of evidence according to previous mentioned GRADE classification) and further meta-analysis or randomized control studies are welcome in order to define the impact of incidental removal of parathyroids on postoperative outcome.
Keywords: incidental parathyroidectomy, risk, prevention, thyroidectomy
INTRODUCTION
Thyroid surgery improved significantly over the last decades. It is now safer, with lower complication rate and can be performed for selected cases as ‘day surgery’. Postoperative hypocalcemia is the most frequent complication with a prevalence of 16-55% (according to the lowest total serum calcium level) (1, 2) and is defined as a serum calcium level <8 mg/dL (<2 mmol/L) within 24 hours after surgery requiring calcium/vitamin D replacement therapy at the time of hospital discharge (3). Postoperative hypocalcemia can be related to operative stress, calcitonin secretion due to surgery, vitamin D deficiency or hemodilution (4) but also due to intraoperative injury of the parathyroid glands (PG), i.e. mechanical, thermal trauma, gland devascularization or accidental excision of parathyroid tissue (5, 6). Hypocalcemia is usually transient (temporary) and subclinical. Protracted hypoparathyroidism is defined as an abnormal PTH (parathyroid hormone) concentration (<13 pg/mL) and/or need for calcium/vitamin D replacement at 4-6 weeks after surgery while clinically permanent hypoparathyroidism is rare but severe complication with PTH below 13 pg/mL and/or need for calcium/vitamin D replacement 1 year after total thyroidectomy (3). Permanent hypoparathyroidism interferes with quality of life and may lead to serious complications, i.e. infections or neurocognitive symptoms (7).
Incidental, inadvertent, unintentional or accidental parathyroidectomy (IP) is a less discussed complication of thyroid surgery with consequences not explored yet properly. Incidental removal of parathyroid gland(s) (PGs) is not mandatory followed by biochemical or clinical hypocalcemia; however, some authors do report some degree of hypocalcemia if the thyroidectomy histology contains one or more incidentally removed PGs (2, 3, 8). Surgeons should be aware of this iatrogenic condition during thyroid surgeries (with all the consequences).
This paper tries to review the existing controversies and risk factors involved with inadvertent removal of parathyroid glands.
Evidence acquisition
We searched English literature via PubMed. Our aim was to review risk factors, prophylactic methods and controversies of incidental removal of PGs. Keywords used: “inadvertent parathyroidectomy”, “incidental parathyroidectomy”, “unintentional parathyroidectomy” or “accidental parathyroidectomy”. Using these keywords we found an initial number of 154 publications. Papers referring to IP (not strictly as a postthyroidectomy complication) and those not analysing risk factors or prophylactic methods of postoperative hypocalcemia caused by removal of parathyroid glands were excluded from this study. We also excluded case reports and studies with less than 100 analyzed cases. 73 papers were finally analysed, searching for risk factors or how to prevent incidental removal of parathyroid glands during thyroid surgery. Relevant information regarding incidence, type of the study, relation between IP and hypocalcemia was obtained from 35 papers and included in Table 1. Data regarding risk factors and prevention extracted from the other 38 papers were not included in the table, but mentioned in references. Heterogeneity of the data did not facilitate a statistical analysis of risk factors for IP. We used the GRADE system/classification in order to analyse the grade of evidence for these publications (9). The Grading Recommendations Assessment, Development and Evaluation (GRADE) approach provides a system for rating quality of evidence and strength of recommendations that is clear, comprehensive, transparent, and pragmatic and is increasingly being adopted by organizations worldwide. According to this system, quality of evidence for different studies can be classified as high, moderate, low or very low. A randomized controlled trial (RCT) usually offers high quality evidence which of course might be challenged by limitations, inconsistency of results or different biases. An observational study on the other hand starts with a low quality rating, but the latter could be upgraded if the magnitude of the treatment effect is very large for example (9). Strength of recommendation according to the same system can be either “strong” when the result is highly unlikely to change further existing recommendation or “weak” when opposite.
Table 1.
Incidence of IP in different studies
| First author, year, (ref) | Type of study | Sample size | Incidental Ptx (%) | Relation with postoperative hypocalcemia |
| Du et al. 2017 (5) | prospective | 341 | 10.3 | nr |
| Mc Goldrick et al. 2017, (10) | retrospective | 230 | 17.3 | nr |
| Lin et al, 2017, (11) | retrospective | 3186 | 6.4 | r |
| Manatakis et al. 2016, (12) | retrospective | 281 | 24.9 | nr* |
| Ozemir et al. 2016, (13) | retrospective | 417 | 13.4 | r |
| Applewhite et al. 2016, (14) | retrospective | 1767 | 16.2 | r |
| Sitges - Serra et al, 2016, (15) | prospective | 170 | 28 | r |
| Zhou et al. 2016, (16) | retrospective | 386 | 20 | r |
| Hone et al. 2016, (17) | prospective | 266 | 16 | ND |
| Praženica et al. 2014, (18) | retrospective | 1068 | 5.4 | r |
| Yazici et al. 2014, (19) | retrospective | 245 | 13.8 | nr |
| Ozogul et al. 2014, (20) | retrospective | 801 | 2.3 | r |
| Del Rio et al. 2014, (21) | retrospective | 538 | 4.8 | nr |
| Song et al. 2014, (22) | retrospective | 454 | 19.8 | r |
| Sheahan et al. 2013, (23) | prospective | 126 | 9.5 | nr |
| Nair et al. 2013, (24) | prospective | 806 | ND | r |
| Campos et al. 2012, (25) | retrospective | 442 | 2.93 | ND |
| Qasaimeh et al. 2011, (26) | retrospective | 233 | 8.6 | r |
| Khairy, Al-Saif. 2011, (27) | retrospective | 287 | 16.4 | r |
| Youssef et al. 2010, (28) | prospective | 207 | 12.6 | nr |
| Spiliotis et al. 2010, (29) | retrospective | 315 | 10.2 | r |
| Erbil et al. 2009, (30) | prospective | 440 | 10.9 | nr |
| Sorgato et al. 2009 (7) | retrospective | 882 | 7.9 | nr |
| Rajinikanth J et al. 2009, (31) | retrospective | 365 | 12.95 | r |
| Manouras et al. 2008, (32) | retrospective | 508 | 19.7 | nr |
| Page, Strunski, 2007, (33) | retrospective | 351 | 5.2 | nr |
| Abboud et al. 2007, (34) | retrospective | 307 | 12 | nr |
| Irkorucu et al. 2007, (35) | prospective | 273 | 3.7 | nr |
| Sippel et al. 2007, (36) | prospective | 513 | 6.4 | nr* |
| Gourgiotis et al. 2006, (37) | retrospective | 315 | 21.6 | nr |
| Sciumè et al. 2006, (38) | retrospective | 313 | 0.95 | nr |
| Rix, Sinha, 2006, (39) | retrospective | 126 | 17.4 | nr |
| Sakorafas et al. 2005, (40) | retrospective | 158 | 17.7 | nr |
| Lin et al. 2002, (41) | retrospective | 220 | 9.1 | nr |
| Sasson et al. 2001, (42) | retrospective | 144 | 15 | nr |
| Lee et al. 1999, (43) | retrospective | 414 | 11 | nr |
| Berghamaschi et al. 1998, (44) | retrospective | 1163 | 9 | nr |
*only biochemical hypoglycemia; ND= no data; r= related; nr= not related
Evidence synthesis
There are few papers in English literature with a high degree of evidence, i.e. metaanalysis or randomized trials. Majority of papers were retrospective studies with a few nonrandomised prospective analysis; according to GRADE classification these studies will offer low or moderate levels of evidence with results and conclusions to be changed by further research.
a. Incidental parathyroidectomy: incidence and relation with hypoparathyroidism
Incidence of IP in literature is reported widely different with intervals between 3.7-24.9% (Table 1 - 2, 5-7, 10-44). A large retrospective analysis of two academic centers including 1767 total thyroidectomies reported an incidence of 16.2% (14); in 19.2% of cases this was related to an intrathyroidal parathyroid. Another recent study involving 170 total thyroidectomies and central compartment neck dissection (CCLND) for differentiated thyroid cancer over 1 cm performed by Sitges-Serra et al. reported an incidence of IP of 28% (15); in 14 cases the pathology report included fragments rather then entire parathyroid gland. Direct relation between IP and hypoparathyroidism /hypocalcemia is controversial. Majority of authors admit that the main cause of postoperative hypocalcemia is acute parathyroid insufficiency due to parathyroid ‘trauma’ during surgery by means of mechanic, thermal, blood supply injury, inadvertent resection or autotransplantion (3, 15, 18, 23), while others concluded that hypocalcemia is not related to IP (37, 41, 42).
b. Risk Factors for inadvertent parathyroidectomy
Demographics
Inadvertent parathyroidectomy is more frequent in female patients probably due to higher incidence of thyroid diseases and thyroidectomies (32,40). A retrospective analysis of 2576 patients concluded that IP is not age related (2) confirmed by others (5). In other studies IP is more frequent in older patients (45) while other data suggest the opposite (7, 36).
Parathyroid localisation
Intrathyroidal or subcapsular parathyroids are predictive factors for IP (30, 42). Superior parathyroids are rare ectopic and usually located on the posterior aspect of the thyroid lobe, at the level of the cricoid cartilage; easier to identify and less exposed to be incidentally resected during a thyroidectomy. Inferior PGs can migrate along with the thymus and are more often ectopic and therefore more often resected during thyroidectomy (43). For Lee et al. (43) PGs are located extracapsular in just 58% while in 42% these are intrathyroidal or beneath the thyroid capsule (intra- or subcapsular). A study based on 503 autopsies identified 17% of parathroids to be subcapsular while 26% are within the thymus (48); another study mentioned that 50% of incidentally removed parathyroids were located within the thyroid lobe (42). Furthermore, Erbil et al. (30) in a prospective clinical study including 440 consecutive patients found intrathyroidal parathyroids in 68.8% of the cases. These data is in opposite to anatomy studies (ectopic and or intrathyroidal parathyroids could be less frequent than reported in surgical studies); Lappas et al. (48) in a large study based on autopsies concluded that 90% of parathyroids are in an eutopic position while only 0.2% are in an ectopic, intrathyroidal position. This contradiction between anatomy and surgical studies questions the modality of reporting of the ectopic parathyroids found incidentally on the thyroid specimen. Number of parathyroids removed incidentally is a risk factor for postoperative hypocalcemia; majority of authors reported one excised gland (15, 18, 20) while others found more than one PG on histology (31, 41, 43); Lin et al. (41) examining 220 thyroid specimens with 20 cases of IP and one patient had three incidentally removed PGs . Similarly, Lee et al. (43) in 414 thyroidectomies and 45 IP, reported three cases with three foci of excised parathyroids. Lorente-Poch et al. (3) showed that less parathyroid glands identified during total thyroidectomy increased the risk of gland injury and accidental parathyroidectomy; same authors concluded that few PGs kept in situ due to IP or autotransplantation can lead to acute parathyroid insufficiency. Sorgato et al. (7) in a review of 882 thyroidectomies reported one incidental removal of 4 parathyroid glands followed by a severe transient hypocalcemia. Number of excised parathyroids was usually calculated by findings on histology specimen rather than identifying PGs during operation. It is difficult to advice for routine identifying 4 parathyroid glands to minimize the risk of IP due to significant high rate of ectopic PGs.
Size and diseases of thyroid
Small size of thyroid glands could be a risk factor (31), while in other studies enlarged glands seem to increase incidence of IP (15, 32, 36, 41). Some thyroid conditions could favor IP during thyroidectomy but no definitive data is available for either benign or malignant pathology. In a retrospective study of over 1000 thyroidectomies performed by a single surgeon, Prazenica found Graves’ disease to be a predictive factor for IP (18). Erbil et al. (30) concluded that surgery for high risk endocrine cases (Graves’ disease, retrosternal and recurrent goiters due to difficult dissection) increases the risk of IP. Thyroiditis is not a clear risk factor for IP; some authors describe a significant relation between Hashimoto and IP (27, 30), while others found no correlation (36, 39, 41, 43).
Malignancy and extension of surgery
Majority of studies found a significant association between malignancy, extension of operation and IP (7, 20, 25, 26, 28, 31, 36). Not all authors found a definitive relation between extrathyroidal extension and IP (31, 40, 41, 43) but IP and postoperative hypocalcemia are more frequent when CCLND is performed (28, 41, 42). Size of tumour, multifocality, T and N categories, CCLND, number of excised lymph nodes during neck dissection are risk factors for Song et al. (22) and Bergenfelz et al. (8). There is no relation described between different histolgy of thyroid cancer and IP (20, 28, 31). Surprisingly Sakorafas et al. (40) noted a lower rate of IP in thyroidectomy for cancer (probably due to more careful dissection) same results reported by other research studies (37).
Surgical technique and redo-surgery
Total thyroidectomies are followed by a higher incidence of postoperative hypoparathyroidism and IP comparing to near-total or subtotal resections (30). With few exceptions (5, 30, 32) the majority of authors do not mention the type and extent of thyroid surgery when reporting IP (42, 43). Erbil et al. (30) found for example a 13-fold increased risk of IP for TT when comparing with subtotal resections (OR 13.7;95% CI:4.08-46.05). Completion thyroidectomy and other redo thyroid operations have a higher rate of complications (including higher incidence of IP) when comparing with first time surgery due to difficult dissection in scar tissue (28, 39, 41, 43, 49). Use of new hemostatic devices and technology i.e. Harmonic scalpel (HS) might reduce postoperative complications and improve outcome during thyroid surgery (50) including lower incidence of temporary hypoparathyroidism (51), while other studies showed no difference when comparing to “classic” instruments (monopolar or bipolar cautery) (52). Materazzi et al. (53) comparing harmonic focus to clamp-and-tie hemostasis showed a significantly reduced risk of transient hypocalcemia with Harmonic device while Zarebczan et al. (54) retrospectively compared Harmonic scalpel to Ligasure device and found no significant differences between both regarding transient and permanent hypocalcemia rate.
How to prevent IP?
Routine visual identification of parathyroid glands
Identifying PGs during operation could be logical and sufficient to avoid postoperative hypoparathyroidism (7, 12, 33). In some cases PGs are beneath the thyroid capsule or “hidden” within the central compartment fat and lymph nodes; identifying true intrathyroidal PGs during operation or on the thyroid specimen is usually impossible. Du et al. (5) showed in a study with 341 patients who underwent TT and CCLND that 42.1% of incidental excised parathyroids are intrathyroidal, debated in different other studies (18, 32, 41-43); same author (5) described a similar incidence of PGs within the thymus, pointing the risk of IP when performing CCLND. Multiple studies found a direct relation between incidence of postoperative acute hypoparathyroidism and number of incidental excised PGs (55, 56). Pattou et al. (55) showed that incidence of postoperative hypoparathyroidism is higher if less than 3 glands have been identified while Tomusch et al. (56) reported same results in analysis of 5846 cases if there were less than 2 identified PGs. However, in a prospective study including 1256 patients, Sheahan et al. (23) found that parathyroid identification is not mandatory if dissection is performed close to the thyroid capsule; surprisingly even with capsular dissection the reported incidence of IP in this study was as high as 9.5%. Some authors found that incidence of IP is influenced by counting the parathyroids on the thyroid specimen, considering that most patients have four parathyroids (18, 22, 55, 56). A study based on 942 autopsies demonstrated that a 5th parathyroid could be identified in 5% while 2% of the population may have 3 parathyroid glands (48). We believe that the most reliable method of identifying PGs is visualization during surgery and not ‘presuming’ number of incidental ‘harvested’ PGs on histology specimen. Reliability of the histopathology result is another question-mark; is the report mentioning an entire of only a fragment of the PGs? (5, 42, 43). For these authors the incidental resected PG is 4-5 mm while others (18) found a mean size of 6 mm; differences due to resection of a “smaller” gland or described as a fragment on histology report. Sitges - Serra et al. (15) in a recent study involving 170 patients with total thyroidectomy and CCLND reported an incidence of IP to be 28% (only 14 cases reported to encounter just fragments of PGs). Dissection of subcapsular PGs could leave small fragments of tissue on specimen (found on histology). Identifying parathyroid glands during surgery and maintaining their blood supply could be even more difficult to achieve when performing CCLND. PGs take their blood supply from both superior and inferior thyroid vessels which can be easily compromised during surgery. Incidental removed parathyroid glands identified during operation should be sent for frozen section (a small part to confirm aetiology) and then autotransplanted in sternocleidomastoid muscle. In a retrospective review of 454 patients with total thyroidectomy with or without neck dissection IP occurred in 19.8% of the patients; one PG was identified in 17.6%, two in 1.5%, and three in 0.7% of these cases (22).
Figure 1.
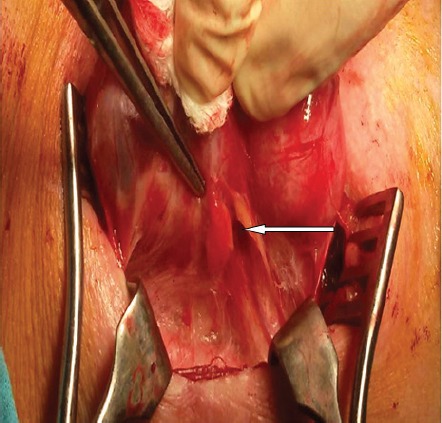
Parathyroid gland located subcapsular on the thyroid lobe (white arrow).
Perioperative adjuncts to identify parathyroid glands
Identifying and preserving parathyroids is provocative for the operating surgeon, with a lot of perioperative adjuncts available to facilitate this. Ultrasound is a well established imaging method for preoperative evaluation of patients undergoing thyroid surgery, which offers the advantages of being less expensive, widely available, noninvasive but may not identify normal parathyroid glands. SPECT-CT parathyroids is an imaging study with a sensitivity and specificity of 92.8% for parathyroid adenomas but not used to identify normal parathyroid glands (57). Other methods are either controversial, i.e. methylene blue which can induce toxic encephalopathy (58) or have not proven reliability, i.e. gamma-probe, optical coherence tomography (59).
Recent studies described the use of carbon nanoparticles in order to identify PGs during surgery (60). Wang et al. (61) confirmed that this method is useful to identify lymphnodes during CCLND, avoiding incidentally removal of PGs; in situ preservation along with their blood supply offers a better recovery of parathyroid function after extensive neck surgery for thyroid cancer. The method is based on the capacity of carbon pellets suspension to concentrate in thyroid and lymph nodes and not in PGs (coloring these structures in black and facilitating the dissection).
Indocyanine green enhanced fluorescence has also been tested for the identification of the PGs based on the principle that the glands had a significantly higher fluorescence intensity than surrounding tissues (thyroid, lymph nodes and fat) which facilitates finding and preserving them during surgery (62, 63). Autofluorescence imaging enables surgeons to capture and review high-quality fluorescence images of circulation, including lymphatics, blood vessels, and related tissue perfusion during thyroid surgical procedures (64). However, further large randomized prospective trials are needed to validate this new technique.
Intra-operative parathyroid hormone assay can be used to monitor parathyroid function during thyroidectomy and helps to identify patients at risk of clinically significant hypocalcemia and to assist in selecting patients requiring parathyroid autotransplantation (avoid unnecessary dissection for parathyroid glands in the presence of normal PTH) (65). It can also guide intraoperative decisions such as a further look at thyroid specimen for incidentally removed parathyroid glands and can predict a safe discharge or the need of calcium/vitamin D supplementation (66).
Autotransplantation of parathyroid tissue
Parathyroid autotransplantation is used when PGs lost their blood supply but left in situ or when incidentally removed at the end of a thyroid operation. Comparative studies confirmed the efficiency of this method to prevent permanent hypoparathyroidism after thyroid surgery (44). Wei et al. (67) in a retrospective study followed the incidence of hypoparathyroidism in 2 subgroups of patients, i.e. with autotransplant or in situ preservation; patients with autotransplantation seem to have better outcome. Others did not found any significant differences between transient or permanent hypocalcemia rates between the 2 subgroups (autotransplant due to IP and control group), even though there is a more significant drop of intact PTH (iPTH) in the autotransplant group (68). Barczynski et al. (69) performed a RCT to compare intra-operative PTH guided autotransplantation versus elective auto-transplantation of at least 1 PG with 170 patients in each group. They found that ioPTH-guided autotransplantation significantly reduced the rates of transient hypocalcemia with no effect on permanent hypocalcemia. The preferred site for autotransplantation is the sternocleidomastoid muscle due to proximity and easy technique; Cavallaro et al. (70) in a prospective study showed that autotransplantation in the forearm muscle might have better results (classic technique used by Wells in surgery for renal hyperparathyroidism). Others found that incidental parathyroidectomy and parathyroid autotransplantation increased the incidence of all parathyroid failure syndromes (protracted and permanent hypoparathyroidism) resulting in less than four parathyroid glands preserved in situ (22, 53). Lorente-Poch et al. (3) concluded there is no evidence that normal transplanted parathyroid tissue fragments will prevent permanent hypoparathyroidism. Prevalence of parathyroid failure was similar whether the fourth gland was autografted or accidentally resected.
Minimally invasive surgery and intraoperative magnification of the surgical field
Minimally invasive thyroidectomy (MIVAT) or video assisted thyroid surgery (VAT) are the most popular minimally invasive techniques worldwide, first described by Miccoli et al. (71). They reported in 2008 a series of 1320 MIVAT and found, apart from cosmetic reasons, shorter hospital stay, a decreased rate of complications (including hypoparathyroidism). Del Rio et al. (72) showed that MIVAT can help surgeon identify and preserve parathyroid glands. Robotic thyroid procedures, i.e. transaxillary, retroauricular, transoral, bilateral axillo breast approach (BABA) have developed over the last decade, hence data regarding incidence, management and prophylaxis of IP is very scarce. One study involving 218 patients undergoing robotic vs. open thyroidectomy for papillary thyroid carcinoma showed IP to have the same incidence 38.5% vs. 37.6% while the number of parathyroid glands saved during thyroidectomy are similar between the two groups (73). Another prospective randomized trial regarding outcomes of total thyroidectomy with CCLND showed that parathyroid gland autotransplantation because of IP was 20% for the robotic and 32% for the open approach (74). Recent studies showed the role of magnification in thyroid surgery in order to facilitate identification of the RLN and PGs. Testini et al. (75) carried out a RCT to assess the benefit of using loupe magnification in 317 patients undergoing TT. They found that the incidence of transient hypocalcemia was 4.3% with loupe magnification compared to 14% without. In a similar retrospective study D’Orazi et al. (76) analysing 782 thyroidectomies with magnification loupes have also reported very low rates of postoperative complications, with only 3 cases of prolonged postoperative hypoparathyroidism. Using of microscope during thyroid surgery is a reliable method to improve outcome for some authors, but with an increased operating time and theatre facilities (77).
Surgical workload
Surgical experience and volume of surgeries for each surgeon have shown to have a significant impact on postoperative outcome for most of the surgical subspecialities. New technologies increased the safety of surgeries; even with improving technologies or robotic surgeries it will be almost impossible to reduce the risk to zero, surgeons expertise remaining a crucial element of treatment outcome. Endocrine surgery is not an exception, volume of surgeries per year is associated with a lower complication rate and with shorter hospital stay (78, 79). Definition of high volume surgeons is differently quantified in literature, some authors believe that surgical experience over 5 year reduces the risk of complications, including postoperative hypocalcemia (79). Emre et al. (80) in a study of 144 patients shows that post-thyroidectomy complications were similar when surgery was performed by trainees and senior surgeons. Duclos et al. (78) in a prospective study involving 3574 thyroid surgeries performed by 28 surgeons in 5 academic French centres followed the surgical expertise in gaining less postoperative complications. For these authors the rate of major complications after thyroidectomy (RLN injury, hypoparathyroidism) was significantly more frequent in the group of surgeons having more than 20 years of experience; best results were achieved by the group aged 35-50 years with 5-19 years of experience.
In conclusion, incidental parathyroidectomy is not as rare as we might imagine. In a short paper published in 1961, Murley and Peters (81) pathologists based in London were trying to find a relationship between IP and postoperative tetania analysing the thyroidectomy specimens. No conclusions were found and no other recent studies could find a direct relation. Hypocalcemia and RLN injury remain the most frequent and fearful complications of thyroid surgery with significant clinical consequences (4-6). Majority of analysed studies are retrospective, with a low degree of evidence according to previously mentioned GRADE classification and might be changed by future research. Meta-analysis or RCT are welcome to obtain further information of impact of IP on postoperative outcome of thyroid operations. Our current view is that inadvertent parathyroidectomy should be avoided and all efforts be made to preserve the parathyroid glands in situ.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian S P. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg. 2014;101(4):307–320. doi: 10.1002/bjs.9384. [DOI] [PubMed] [Google Scholar]
- 2.Sitges-Serra A, Ruiz S, Girvent M, Manjón H, Dueñas JP, Sancho JJ. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg. 2010;97(11):1687–1695. doi: 10.1002/bjs.7219. [DOI] [PubMed] [Google Scholar]
- 3.Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg. 2015;4(1):82–90. doi: 10.3978/j.issn.2227-684X.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Harrison B. Hypocalcemia after Thyroidectomy: The Need for Improved Definitions. World J End Surg. 2010;2(1):17–20. [Google Scholar]
- 5.Du W, Fang Q, Zhang X, Cui M, Zhao M, Lou W. Unintentional parathyroidectomy during total thyroidectomy surgery: A single surgeon’s experience. Medicine (Baltimore) 2017;96(11):e6411. doi: 10.1097/MD.0000000000006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorente-Poch L, Sancho J, Muñoz JL, Gallego-Otaegui L, Martínez-Ruiz C, Sitges-Serra A. Failure of fragmented parathyroid gland autotransplantation to prevent permanent hypoparathyroidism after total thyroidectomy. Langenbecks Arch Surg. 2017;402(2):281–287. doi: 10.1007/s00423-016-1548-3. [DOI] [PubMed] [Google Scholar]
- 7.Sorgato N, Pennelli G, Boschin IM, Ide EC, Pagetta C, Piotto A, Toniato A, De Salvo GL, Hindié E, Al-Nahhas A, Rubello D, Pelizzo MR. Can we avoid inadvertent parathyroidectomy during thyroid surgery? In Vivo. 2009;23(3):433–439. [PubMed] [Google Scholar]
- 8.Bergenfelz A, Jansson S, Kristoffersson A, Martensson H, Reihner E, Wallin G, Lausen I. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393(5):667–673. doi: 10.1007/s00423-008-0366-7. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mc Goldrick DM, Majeed M, Achakzai AA, Redmond HP. Inadvertent parathyroidectomy during thyroid surgery. Ir J Med Sci. doi: 10.1007/s11845-017-1560-9. [DOI] [PubMed] [Google Scholar]
- 11.Lin YS, Hsueh C, Wu HY, Yu MC, Chao TC. Incidental parathyroidectomy during thyroidectomy increases the risk of postoperative hypocalcemia. Laryngoscope. 2017 doi: 10.1002/lary.26448. [DOI] [PubMed] [Google Scholar]
- 12.Manatakis DK, Balalis D, Soulu VN, Korkolis DP, Plataniotis G, Gontikakis E. Incidental parathyroidectomy during total thyroidectomy: risk factors and consequences. Int J Endocrinol. 2016 doi: 10.1155/2016/7825305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozemir IA, Buldanli MZ, Yener O, Leblebici M, Eren T, Baysal H, Alimoglu O. Factors affecting postoperative hypocalcemia after thyroid surgery: Importance of incidental parathyroidectomy. North Clin Istanbul. 2016;3(1):9–14. doi: 10.14744/nci.2016.48802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Applewhite MK, White MG, Xiong M, Pasternak JD, Abdulrasool L, Ogawa L, Suh I, Gosnell JE, Kaplan EL, Duh QY, Angelos P, Shen WT, Grogan RH. Incidence, Risk Factors, and Clinical Outcomes of Incidental Parathyroidectomy During Thyroid Surgery. Ann Surg Oncol. 2016;23(13):4310–4315. doi: 10.1245/s10434-016-5439-1. [DOI] [PubMed] [Google Scholar]
- 15.Sitges-Serra A, Gallego-Otaegui L, Suárez S, Lorente-Poch L, Munné A, Sancho JJ. Inadvertent parathyroidectomy during total thyroidectomy and central neck dissection for papillary thyroid carcinoma. Surgery. 2016;161(3):712–719. doi: 10.1016/j.surg.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Zhou HY, He JC, McHenry CR. Inadvertent parathyroidectomy: incidence, risk factors, and outcomes. J Surg Res. 2016;205(1):70–75. doi: 10.1016/j.jss.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Hone RW, Tikka T, Kaleva AI, Hoey A, Alexander V, Balfour A, Nixon IJ. Analysis of the incidence and factors predictive of inadvertent parathyroidectomy during thyroid surgery. J Laryngol Otol. 2016;130(7):669–673. doi: 10.1017/S0022215116008136. [DOI] [PubMed] [Google Scholar]
- 18.Praženica P, O’Driscoll K, Holy R. Incidental parathyroidectomy during thyroid surgery using capsular dissection technique. Otolaryngol Head Neck Surg. 2014;150(5):754–761. doi: 10.1177/0194599814521365. [DOI] [PubMed] [Google Scholar]
- 19.Yazici P, Bozkurt E, Citgez B, Kaya C, Mihmanli M, Uludag M. Incidental parathyroidectomy as a cause of postoperative hypocalcemia after thyroid surgery: reality or illusion? Minerva Chir. 2014;69(6):315–320. [PubMed] [Google Scholar]
- 20.Özoğul B, Akçay MN, Kısaoğlu A, Atamanalp SŞ, Öztürk G, Aydınlı B. Incidental parathyroidectomy during thyroid surgery: risk factors, incidence, and outcomes. Turk J Med Sci. 2014;44(1):84–88. [PubMed] [Google Scholar]
- 21.Del Rio P, De Simone B, Viani L, Arcuri MF, Sianesi M. Unintentional parathyroidectomy and postoperative hypocalcaemia. Conventional thyroidectomy versus miniinvasive thyroidectomy. Ann Ital Chir. 2014;85(5):470–473. [PubMed] [Google Scholar]
- 22.Song CM, Jung JH, Ji YB, Min HJ, Ahn YH, Tae K. Relationship between hypoparathyroidism and the number of parathyroid glands preserved during thyroidectomy. Surgical Oncology. 2014;12:200. doi: 10.1186/1477-7819-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheahan P, Mehanna R, Basheeth N, Murphy MS. Is systematic identification of all four parathyroid glands necessary during total thyroidectomy?: a prospective study. Laryngoscope. 2013;123(9):2324–2328. doi: 10.1002/lary.23954. [DOI] [PubMed] [Google Scholar]
- 24.Nair CG, Babu MJ, Menon R, Jacob P. Hypocalcaemia following total thyroidectomy: An analysis of 806 patients. Indian J Endocrinol Metab. 2013;17(2):298–303. doi: 10.4103/2230-8210.109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campos NS, Cardoso LP, Tanios RT, Oliveira BC, Guimarães AV, Dedivitis RA, Marcopito LF. Risk factors for incidental parathyroidectomy during thyroidectomy. Braz J Otorhinolaryngol. 2012;78(1):57–61. doi: 10.1590/S1808-86942012000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qasaimeh GR, Al Nemri S, Al Omari AK. Incidental extirpation of the parathyroid glands at thyroid surgery: risk factors and post-operative hypocalcemia. Eur Arch Otorhinolaryngol. 2011;268(7):1047–1051. doi: 10.1007/s00405-010-1413-x. [DOI] [PubMed] [Google Scholar]
- 27.Khairy GA, Al-Saif A. Incidental parathyroidectomy during thyroid resection: incidence, risk factors, and outcome. Ann Saudi Med. 2011;31(3):274–278. doi: 10.4103/0256-4947.81545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youssef T, Gaballah G, Abd-Elaal E, El-Dosoky E. Assessment of risk factors of incidental parathyroidectomy during thyroid surgery: a prospective study. Int J Surg. 2010;8(3):207–211. doi: 10.1016/j.ijsu.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Spiliotis J, Vaxevanidou A, Sergouniotis F, Tsiveriotis K, Datsis A, Rogdakis A, Kekelos S. Risk factors and consequences of incidental parathyroidectomy during thyroidectomy. Am Surg. 2010;76(4):436–441. [PubMed] [Google Scholar]
- 30.Erbil Y, Barbaros U, Ozbey N, Aral F, Ozarmağan S. Risk factors of incidental parathyroidectomy after thyroidectomy for benign thyroid disorders. Int J Surg. 2009;7(1):58–61. doi: 10.1016/j.ijsu.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Rajinikanth J, Paul MJ, Abraham DT, Ben Selvan CK, Nair A. Surgical Audit of Inadvertent Parathyroidectomy During Total Thyroidectomy: Incidence, Risk Factors, and Outcome. The Medscape Journal of Medicine. 2009;11(1):29. [PMC free article] [PubMed] [Google Scholar]
- 32.Manouras A, Markogiannakis H, Lagoudianakis E, Antonakis P, Genetzakis M, Papadima A, Konstantoulaki E, Papanikolaou D, Kekis P. Unintentional parathyroidectomy during total thyroidectomy. Head Neck. 2008;30(4):497–502. doi: 10.1002/hed.20728. [DOI] [PubMed] [Google Scholar]
- 33.Page C, Strunski V. Parathyroid risk in total thyroidectomy for bilateral, benign multinodular goiter: report of 351 surgical cases. J Laryngol Otol. 2007;121(3):237–241. doi: 10.1017/S0022215106003501. [DOI] [PubMed] [Google Scholar]
- 34.Abboud B, Sleilaty G, Braidy C, Zeineddine S, Ghorra C, Abadjian G, Tabchy B. Careful examination of thyroid specimen intraoperatively to reduce incidence of inadvertent parathyroidectomy during thyroid surgery. Arch Otolaryngol Head Neck Surg. 2007;133(11):1105–1110. doi: 10.1001/archotol.133.11.1105. [DOI] [PubMed] [Google Scholar]
- 35.Irkorucu O, Tascilar O, Cakmak GK, Emre AU, Ucan HB, Karakaya K, Comert M. Inadvertent parathyroidectomy and temporary hypocalcemia: an adverse natural outcome or a true complication during thyroidectomy? Endocr Regul. 2007;41(4):143–148. [PubMed] [Google Scholar]
- 36.Sippel RS, Ozgul O, Hartig GK, Mack EA, Chen H. Risks and consequences of incidental parathyroidectomy during thyroid resection. Aust N Z J Surg. 2007;77(1-2):33–36. doi: 10.1111/j.1445-2197.2006.03972.x. [DOI] [PubMed] [Google Scholar]
- 37.Gourgiotis S, Moustafellos P, Dimopoulos N, Papaxoinis G, Baratsis S, Hadjiyannakis E. Inadvertent parathyroidectomy during thyroid surgery: the incidence of a complication of thyroidectomy. Langenbecks Arch Surg. 2006;391(6):557–560. doi: 10.1007/s00423-006-0079-8. [DOI] [PubMed] [Google Scholar]
- 38.Sciumè C, Geraci G, Pisello F, Facella T, Li Volsi F, Licata A, Modica G. Complications in thyroid surgery: symptomatic post-operative hypoparathyroidism incidence, surgical technique, and treatment. Ann Ital Chir. 2006;77(2):115–122. [PubMed] [Google Scholar]
- 39.Rix TE, Sinha P. Inadvertent parathyroid excision during thyroid surgery. Surgeon. 2006;4(6):339–342. doi: 10.1016/s1479-666x(06)80108-3. [DOI] [PubMed] [Google Scholar]
- 40.Sakorafas GH, Stafyla V, Bramis C, Kotsifopoulos N, Kolettis T, Kassaras G. Incidental parathyroidecomy during thyroid surgery: an underappreciated complication of thyroidectomy. World J Surg. 2005;29(12):1539–1543. doi: 10.1007/s00268-005-0032-y. [DOI] [PubMed] [Google Scholar]
- 41.Lin DT, Patel SG, Shaha AR, Singh B, Shah JP. Incidence of inadvertent parathyroid removal during thyroidectomy. Laryngoscope. 2002;112(4):608–611. doi: 10.1097/00005537-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Sasson AR, Pingpank JF, Jr, Wetherington RW, Hanlon AL, Ridge JA. Incidental parathyroidectomy during thyroid surgery does not cause transient symptomatic hypocalcemia. Arch Otolaryngol Head Neck Surg. 2001;127(3):304–308. doi: 10.1001/archotol.127.3.304. [DOI] [PubMed] [Google Scholar]
- 43.Lee NJ, Blakey JD, Bhuta S, Calcaterra TC. Unintentional parathyroidectomy during thyroidectomy. Laryngoscope. 1999;109(8):1238–1240. doi: 10.1097/00005537-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Bergamaschi R, Becouarn G, Roceray J, Arnaud JP. Morbidity of thyroid surgery. Am J Surg. 1998;176(1):71–75. doi: 10.1016/s0002-9610(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 45.Kamer E, Unalp HR, Erbil Y, Akguner T, Issever H, Tarcan E. Early prediction of hypocalcemia after thyroidectomy by parathormone measurement in surgical site irrigation fluid. Int J Surg. 2009;7(5):466–471. doi: 10.1016/j.ijsu.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Lang BH, Yih PC, Ng KK. A prospective evaluation of quick intraoperative parathyroid hormone assay at the time of skin closure in predicting clinically relevant hypocalcemia after thyroidectomy. World J Surg. 2012;36(6):1300–1306. doi: 10.1007/s00268-012-1561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akrerstrom G, Malmaeus J, Bergstrom R. Surgical anatomy of parathyroid glands. Surgery. 1984;95(1):14–21. [PubMed] [Google Scholar]
- 48.Lappas D, Noussios G, Anagnostis P, Adamidou F, Chatzigeorgiou A, Skandalakis P. Location, number and morphology of parathyroid glands:results from a large anatomical series. Anat Sci Int. 2012;87(3):160–164. doi: 10.1007/s12565-012-0142-1. [DOI] [PubMed] [Google Scholar]
- 49.Ondik MP, McGinn J, Ruggiero F, Goldenberg D. Unintentional parathyroidectomy and hypoparathyroidism in secondary central compartment surgery for thyroid cancer. Head Neck. 2010;32(4):462–466. doi: 10.1002/hed.21205. [DOI] [PubMed] [Google Scholar]
- 50.Miccoli P, Berti P, Dionigi G, D’Agostino J, Orlandini C, Donatini G. Randomized controlled trial of harmonic scalpel use during thyroidectomy. Arch Otolaryngol Head Neck Surg. 2006;132(10):1069–1073. doi: 10.1001/archotol.132.10.1069. [DOI] [PubMed] [Google Scholar]
- 51.Ferri E, Armato E, Spinato G, Spinato R. Focus harmonic scalpel compared to conventional hemostasis in open total thyroidectomy: a prospective randomized trial. Int J Otolaryngol. 2011;(2011):357195. doi: 10.1155/2011/357195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antakia R, Edafe O, Uttley L, Balasubramanian SP. Effectiveness of preventative and other surgical measures on hypocalcemia following bilateral thyroid surgery: a systematic review and metaanalysis. Thyroid. 2015;25(1):95–106. doi: 10.1089/thy.2014.0101. [DOI] [PubMed] [Google Scholar]
- 53.Materazzi G, Caravaglios G, Matteucci V, Aghababyan A, Miccoli M, Miccoli P. The impact of 607 the Harmonic FOCUS on complications in thyroid surgery: a prospective multicenter study. Updates Surg. 2013;65(4):295–299. doi: 10.1007/s13304-013-0223-2. [DOI] [PubMed] [Google Scholar]
- 54.Zarebczan B, Mohanty D, Chen H. A Comparison of the LigaSure and harmonic scalpel in thyroid surgery: a single institution review. Ann Surg Oncol. 2011;18(1):214–218. doi: 10.1245/s10434-010-1334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pattou F, Combermale F, Fabre S, Carnaille B, Decoulx M, Wemeau JL, Racadot A, Proye C. Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg. 1998;22(7):718–724. doi: 10.1007/s002689900459. [DOI] [PubMed] [Google Scholar]
- 56.Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery. 2003;133(2):180–185. doi: 10.1067/msy.2003.61. [DOI] [PubMed] [Google Scholar]
- 57.Hara N, Takayama T, Onoguchi M, Obane N, Miyati T, Yoshioka T, Sakaguchi K, Honda M. Subtraction SPECT for parathyroid scintigraphy based on maximization of mutual information. J Nucl Med Technol. 2007;35(2):84–90. doi: 10.2967/jnmt.106.033480. [DOI] [PubMed] [Google Scholar]
- 58.Patel HP, Chadwick DR, Harrison BJ, Balasubramanian SP. Systematic review of intravenous methylene blue in parathyroid surgery. Br J Surg. 2012;99(10):1345–1351. doi: 10.1002/bjs.8814. [DOI] [PubMed] [Google Scholar]
- 59.Prosst RL, Weiss J, Hupp L, Willeke F, Post S. Fluorescence-guided minimally invasive parathyroidectomy: clinical experience with a novel intraoperative detection technique for parathyroid glands. World J Surg. 2010;34(9):2217–2222. doi: 10.1007/s00268-010-0621-2. [DOI] [PubMed] [Google Scholar]
- 60.Zhu Y, Chen X, Zhang H, Chen L, Zhou S, Wu K, Wang K, Kong L, Zhuang H. Carbon nanoparticle-guided central lymph node dissection in clinically node-negative patients with papillary thyroid carcinoma. Head Neck. 2016;38(6):840–845. doi: 10.1002/hed.24060. [DOI] [PubMed] [Google Scholar]
- 61.Wang B, Du ZP, Qiu NC, Liu ME, Liu S, Jiang DZ, Zhang W, Qiu M. Application of carbon nanoparticles accelerates the rapid recovery of parathyroid function during thyroid carcinoma surgery with central lymph node dissection: A retrospective cohort study. Int J Surg. 2016;36(Pt A):164–169. doi: 10.1016/j.ijsu.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 62.Lavazza M, Liu X, Wu C, Anuwong A, Kim HY, Liu R, Randolph GW, Inversini D, Boni D, Rausei S, Frattini F, Dionigi G. Indocyanine green enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland Surg. 2016;5(15):512–521. doi: 10.21037/gs.2016.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fortuny JW, Karenovics W, Triponez F, Sadowski S M. Intra-Operative Indocyanine Green Angiographyof the Parathyroid Gland. World J Surg. 2016;40:2378–2381. doi: 10.1007/s00268-016-3493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ladurner R, Sommerey S, Arabi NA, Hallfeldt KK, Stepp H, Gallwas JK. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc. 2017;31(8):3140–3145. doi: 10.1007/s00464-016-5338-3. [DOI] [PubMed] [Google Scholar]
- 65.Lo CY, Luk JM, Tam SC. Applicability of Intraoperative Parathyroid Hormone Assay During Thyroidectomy. Ann Surgery. 2002;236(5):564–569. doi: 10.1097/00000658-200211000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Higgins KM, Mandell DL, Govindaraj S, Genden EM, Mechanick JI, Bergman DA, Diamond EJ, Urken ML. The Role of Intraoperative Rapid Parathyroid Hormone Monitoring for Predicting Thyroidectomy-Related Hypocalcemia. Arch Otolaryngol Head Neck Surg. 2004;130(1):63–67. doi: 10.1001/archotol.130.1.63. [DOI] [PubMed] [Google Scholar]
- 67.Wei T, Li Z, Jin J, Chen R, Gong Y, Du Z, Gong R, Zhu J. Autotransplantation of Inferior Parathyroid glands during central neck dissection for papillary thyroid carcinoma: a retrospective cohort study. Int J Surg. 2014;12(12):1286–1290. doi: 10.1016/j.ijsu.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Kirdak T, Dundar HZ, Uysal E, Ocakoglu G, Korun N. Outcomes of Parathyroid Autotransplantation During Total Thyroidectomy: A Comparison with Age- and Sex-Matched Controls. J Invest Surg. 2017;30(3):201–209. doi: 10.1080/08941939.2016.1232768. [DOI] [PubMed] [Google Scholar]
- 69.Barczynski M, Cichon S, Konturek A, Cichon W. Applicability of intraoperative parathyroid hormone assay during total thyroidectomy as a guide for the surgeon to selective parathyroid tissue autotransplantation. World J Surg. 2008;32(5):822–288. doi: 10.1007/s00268-007-9405-8. [DOI] [PubMed] [Google Scholar]
- 70.Cavallaro G, Iorio O, Centanni M, Porta N, Iossa A, Gargano L, Del Duca S, Gurrado A, Testini M, Petrozza V, Silecchia G. Parathyroid Reimplantation in Forearm Subcutaneous Tissue During Thyroidectomy: A Simple and Effective Way to Avoid Hypoparathyroidism. World J Surg. 2015;39(8):1936–1942. doi: 10.1007/s00268-015-3070-0. [DOI] [PubMed] [Google Scholar]
- 71.Miccoli P, Berti P, Ambrosini CE. Perspectives and lessons learned after a decade of minimally invasive video-assisted thyroidectomy. ORL J Otorhinolaryngol Relat Spec. 2008;70(5):282–286. doi: 10.1159/000149829. [DOI] [PubMed] [Google Scholar]
- 72.Del Rio P, De Simone B, Viani L, Arcuri MF, Sianesi M. Unintentional parathyroidectomy and postoperative hypocalcaemia. Conventional thyroidectomy versus miniinvasive thyroidectomy. Ann Ital Chir. 2014;85(5):470–473. [PubMed] [Google Scholar]
- 73.Cho JN, Park WS, Min SY, Han SA, Song JY. Surgical outcomes of robotic thyroidectomy vs. conventional open thyroidectomy for papillary thyroid carcinoma. World J Surg Oncol. 2016 Jul 9;14(1):181. doi: 10.1186/s12957-016-0929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He QQ, Zhuang DY, Fan ZY, Zheng LM, Zhou P, Hou L, Yu F, Li YN, Xiao L, Dong XF, Ni GF. Comparative study between robotic total thyroidectomy with central lymph node dissection via bilateral axillo-breast approach and conventional open procedure for papillary thyroid microcarcinoma. Chin Med J. 2016;129(18):2160–2166. doi: 10.4103/0366-6999.189911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Testini M, Nacchiero M, Piccinni G, Portincasa P, Di Venere B, Lissidini G, Bonomo GM. Total thyroidectomy is improved by loupe magnification. Microsurgery. 2004;24(1):39–42. doi: 10.1002/micr.10195. [DOI] [PubMed] [Google Scholar]
- 76.D’Orazi V, Panunzi A, Di Lorenzo E, Ortensi Al, Cialini M, Anichini S, Ortensi A. Use of loupes magnification and microsurgical technique in thyroid surgery: ten years experience in a single center. G Chir. 2016;37(3):101–107. doi: 10.11138/gchir/2016.37.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams SP, Wilkie MD, Tahery J. Microscope-assisted thyroidectomy: Our experience in one hundred and twenty-one consecutive cases. Clin Otolaryngol. 2014;39(5):289–315. doi: 10.1111/coa.12284. [DOI] [PubMed] [Google Scholar]
- 78.Duclos A, Peix JL, Colin C, Kraimps JL, Menegaux F, Pattou F, Sebag F, Touzet S, Bourdy S, Voirin N, Lifante JC, CATHY Study Group Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ. 2012;344:d8041. doi: 10.1136/bmj.d8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Qurayshi Z, Robins R, Hauch A, Randolph GW, Kandil E. Association of Surgeon Volume With Outcomes and Cost Savings Following Thyroidectomy: A National Forecast. JAMA Otolaryngol Head Neck Surg. 2016;142(1):32–39. doi: 10.1001/jamaoto.2015.2503. [DOI] [PubMed] [Google Scholar]
- 80.Emre AU, Cakmak GK, Tascilar O, Ucan BH, Irkorucu O, Karakaya K, Balbaloglu H, Dibekliloglu S, Gul M, Ankarali H, Comert M. Complications of total thyroidectomy performed by surgical residents versus specialist surgeons. Surg Today. 2008;38(10):879–885. doi: 10.1007/s00595-008-3760-4. [DOI] [PubMed] [Google Scholar]
- 81.Murley RS, Peters PM. Inadvertent parathyroidectomy. Proc R Soc Med. 1961;54:487–489. doi: 10.1177/003591576105400608. [DOI] [PMC free article] [PubMed] [Google Scholar]


