Abstract
Objective
To develop consensus recommendations on urine drug monitoring (UDM) in patients with chronic pain who are prescribed opioids.
Methods
An interdisciplinary group of clinicians with expertise in pain, substance use disorders, and primary care conducted virtual meetings to review relevant literature and existing guidelines and share their clinical experience in UDM before reaching consensus recommendations.
Results
Definitive (e.g., chromatography-based) testing is recommended as most clinically appropriate for UDM because of its accuracy; however, institutional or payer policies may require initial use of presumptive testing (i.e., immunoassay). The rational choice of substances to analyze for UDM involves considerations that are specific to each patient and related to illicit drug availability. Appropriate opioid risk stratification is based on patient history (especially psychiatric conditions or history of opioid or substance use disorder), prescription drug monitoring program data, results from validated risk assessment tools, and previous UDM. Urine drug monitoring is suggested to be performed at baseline for most patients prescribed opioids for chronic pain and at least annually for those at low risk, two or more times per year for those at moderate risk, and three or more times per year for those at high risk. Additional UDM should be performed as needed on the basis of clinical judgment.
Conclusions
Although evidence on the efficacy of UDM in preventing opioid use disorder, overdose, and diversion is limited, UDM is recommended by the panel as part of ongoing comprehensive risk monitoring in patients prescribed opioids for chronic pain.
Keywords: Urine Drug Monitoring, Pain Management, Chronic Pain, Substance Use Disorders, Opioids, Screening Tools
Introduction
Key Issues Regarding Drug Monitoring for Patients Prescribed Opioids for Chronic Pain
Rationale for Drug Monitoring
Opioid analgesics are prescribed for up to one-third of patients with chronic pain treated in primary care clinics [1] but may be associated with unintended consequences of misuse, opioid use disorder, overdose, and diversion. Morbidity and mortality, presumably due to respiratory depression, increase with concomitant use of opioids and other central nervous system depressants (e.g., benzodiazepines, nonbenzodiazepine sleep medications, muscle relaxants, tricyclic antidepressants, and alcohol) [2–5]. Prescription opioid use has reportedly decreased from 2010 to 2014 [6], and opioid-related deaths also decreased from 2010 to 2012 [7]. However, deaths from illicitly produced fentanyl and related compounds [8–12] and heroin [7,13] have increased across various time periods in the past decade.
Patients often do not voluntarily report prescription drug misuse or illicit substance use [14,15], and some may feign symptoms to obtain opioids for diversion [16], which necessitates objective assessments such as drug monitoring [17]. Although opioid use is monitored primarily for patient and public safety, drug monitoring has important implications for compliance with regulatory mandates and standards for responsible opioid prescribing [18–22]. Urine drug monitoring (UDM) has the added benefit of improving patient adherence to opioid therapy [23,24], potentially leading to better patient outcomes and greater trust between provider and patient.
Types of Drug Monitoring and Terminology
This consensus report focuses on UDM, although drug use can be detected via multiple biological samples (e.g., oral fluid, blood, hair [25]). Use of nonurine matrices may prevent sample alteration and avoid privacy concerns; however, urine testing has been widely adopted in clinical practice for monitoring because of adequate drug concentration in the urine, accuracy of developed tests, and clinically relevant detection windows (i.e., three to five days) [25–27].
In this consensus report, the term urine drug monitoring (UDM) is used instead of urine drug screening, urine toxicology screening, or urine drug testing. The UDM term conveys an ongoing process rather than a single testing event, and UDM has a nonpunitive, patient-centric connotation, as variations on the term drug testing may be associated with punitive intent in lay language. Diversion is used in this consensus report to mean a transfer (e.g., giving, selling) of prescription drugs for unlawful distribution or use [20]. Misuse is defined as taking medications in a manner other than prescribed, even when treating a medical condition [20,28]. Substance use disorders are a cluster of cognitive, behavioral, and physiological symptoms indicating continued use of a psychoactive substance (e.g., to get “high”) despite negative consequences and harm [29,30]. Opioid use disorder is characterized by signs and symptoms of compulsive, prolonged self-administration of opioids in doses exceeding a medically appropriate amount or for no legitimate medical purpose despite clinically and functionally significant impairments, such as health problems and failure to meet major social responsibilities [30]. Because the colloquial labels “dirty” and “clean” to describe UDM results can stigmatize patients and reduce their likelihood of seeking and accepting recommended help [31], “inconsistent with therapy,” “unexpected results,” “consistent with therapy,” and “expected results” are used in this report.
Description of UDM Technologies
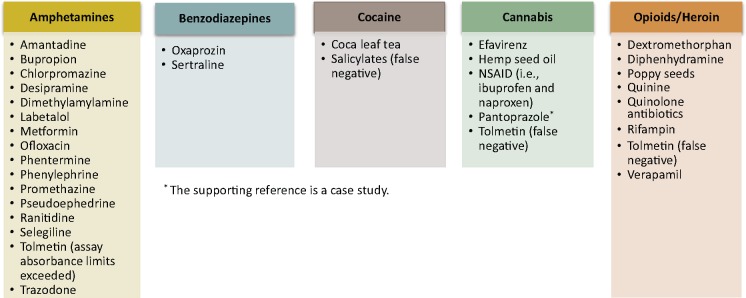
A presumptive UDM test is a screening immunoassay that is relatively inexpensive, can be used in the office at point of care (POC), and produces a rapid result (e.g., within minutes) [28]. Clinicians may be unfamiliar with the characteristics of immunoassays, which have variable sensitivity and specificity (e.g., 0%–50% missed positive results and 11%–100% incorrectly identified positive results across drug classes) [32], and may therefore miss substances that can lead to inaccurate immunoassay results (Figure 1) [33–40]. The classic “urine screen tests” are often enzyme immunoassays that target amphetamines/methamphetamines, cannabis, cocaine, phencyclidine, and opioids (i.e., the “federal five” [41]) and are based on a specific antidrug antibody reaction [42]. Opiate immunoassays can more accurately detect naturally occurring opiate alkaloids (i.e., morphine, codeine) than commonly prescribed synthetic (e.g., fentanyl, methadone) and semisynthetic (e.g., buprenorphine, oxycodone, oxymorphone, hydromorphone) opioids [42]. Immunoassays are at best semiquantitative (i.e., an estimate of levels only) because of cross-reaction across multiple drugs [43]. Reasonably sensitive options are now available for testing many common drug classes [43].
Figure 1.
Agents that may interfere (false positive unless otherwise specified) with urine drug monitoring results for various classes of immunoassays [33–40]. NSAID = nonsteroidal anti-inflammatory drug.
Definitive UDM can be used for initial or confirmatory testing (i.e., to verify the results of a presumptive test that are contested by the patient) and includes qualitative or quantitative gas chromatography (GC)–mass spectrometry (MS), liquid chromatography (LC)–MS, and LC–tandem MS (LC-MS/MS) technologies [28,44]. Definitive testing is often more specific and usually more sensitive than immunoassay for the substances tested, but it is also more costly [28]. Although some immunoassays can detect chemical adulterants (i.e., any substance that lessens validity of testing) [45], definitive testing is less susceptible to adulterants and decreases the likelihood of inaccurate or false results [28]. Definitive testing accurately identifies metabolites to confirm that the parent drug was indeed ingested [28] and can also detect potentially abnormal opioid metabolism [46]. Metabolite results may be confusing to clinicians who are not aware that some prescribed opioids are metabolites of others (e.g., oxymorphone is a metabolite of oxycodone, and hydromorphone is a metabolite of hydrocodone [18]).
UDM Challenges and Unmet Needs
Although the effectiveness of UDM as a risk mitigation tool or strategy against overdose, opioid use disorder, and diversion has been inadequately studied, national guidelines from the last decade [18–22,40] have recommended UDM as best practice in patients prescribed opioids for chronic pain. These guidelines usually suggest initial immunoassay before (confirmatory) definitive UDM because of cost concerns [18,20,40].
Potential conflicts of interest (e.g., clinic owners financially benefiting from frequent POC testing [47] and commercial laboratories promoting the use of definitive UDM beyond clinically appropriate thresholds) have led payers to increase scrutiny of UDM [48,49]. Current restrictive payer policies can limit use of and reimbursement for UDM. Reimbursement changes regularly, and authors recommend that clinicians refer to current Centers for Medicare and Medicaid Services (CMS) reimbursement policies (Table 1) [44] and commercial insurance coverage benefits [50], as well as consider variations in costs across practice settings, to stay up to date on changes. Of note, costs of appropriate UDM may be offset by savings in overall health care (i.e., via improvement in care and reductions in drug misuse, opioid use disorder, and diversion) [51], but this relationship requires further study.
Table 1.
| Test Type | Description | AMA CPT Code | CMS HCPCS G Code |
|---|---|---|---|
| Presumptive | |||
| Read by direct optical observation only | 80305 | G0477 | |
| Instrument-assisted direct optical observation | 80306 | G0478 | |
| Performed by instrument chemistry analyzers | 80307 | G0479 | |
| Definitive | |||
| 1–7 drug classes | Individual CPT codes for each drug | G0480 | |
| 8–14 drug classes | G0481 | ||
| 15–21 drug classes | G0482 | ||
| ≥22 drug classes | G0483 | ||
AMA = American Medical Association; CMS = Centers for Medicare and Medicaid Services; CPT = Current Procedural Terminology; HCPCS = Healthcare Common Procedure Coding System; UDM = urine drug monitoring.
Clinicians receive minimal education on UDM and often lack adequate knowledge of how to both choose an appropriate UDM test [52] and interpret complex results [53]. Lack of understanding can lead to misinterpretation of UDM results, failure to identify patterns of harmful drug use, and inappropriate management of patients. Clinicians may compromise patient care by denying appropriate treatment or discharging patients from their practice after inaccurately concluding that they are misusing or diverting opioids owing to a false-positive or false-negative UDM result [52,53].
With UDM as a current best practice and given its inherent complexities in clinical practice, updated guidance is needed. The purpose of this consensus report is to provide clinicians with a framework for practical and rational (i.e., high-value and individualized) UDM in patients receiving opioids for chronic pain. This report presents the following information:
discussions and views of a multidisciplinary consensus panel regarding recent UDM guidelines and literature;
best practices for UDM in patients prescribed opioid therapy for chronic pain;
areas for further UDM research and evaluation.
These consensus recommendations are intended for a broad range of physicians and other health care professionals (e.g., pharmacists, physician assistants [PAs], nurse practitioners, and certified registered nurse anesthetists) involved in the management of patients with chronic pain.
Methods
Phase 1: Prioritizing Issues of Greatest Importance to UDM on the Basis of Research, Published Guidelines, and Panel Member Experiences
A diverse group of panelists from various clinical settings (e.g., pain medicine, addiction medicine, internal medicine, primary care, pharmacotherapeutics, and toxicology) were recruited to serve as experts in UDM as well as to provide a payer perspective when possible. Before the UDM consensus panel meeting, the panelists were asked to provide topics for consensus recommendation followed by feedback on a preliminary list of questions on these topics; the co-chairs (CEA and LRW, chosen for their in-depth experience and long-standing association with the American Academy of Pain Medicine [AAPM]) adjudicated any discrepancies to develop the final list of questions:
Which UDM test(s) should be used and in which patients prescribed opioids for chronic pain should the tests be used according to a medical literature review, clinical experience, clinical chemistry of drug testing, and practical considerations?
How should patients undergoing UDM be stratified for opioid misuse risk?
How often should UDM occur in patients with low, medium, and high risk for opioid misuse or opioid use disorder?
For background, the panel members identified existing guidelines that have been most influential in UDM in chronic pain practice. Articles were obtained from PubMed using the search terms urine drug testing and chronic pain. Studies published from June 13, 2012 (i.e., the date of the previous guidelines specific to UDM [40]), to April 6, 2016 (the date of the search), were included. Search results were filtered to exclude narrative reviews, case reports, studies involving nonurine matrices (e.g., blood, oral fluid, hair) laboratory tests, and any studies with findings not relevant to the three selected questions on UDM. Studies in patients with cancer pain were excluded to narrow the patient population, similar to other guidelines [18–20]; however, this should not be construed as a recommendation to not perform UDM in patients prescribed opioids for cancer-related chronic pain. To address potential literature gaps, additional references (e.g., key landmark studies) were identified through reference lists and by panelist recommendations.
Using the process followed by the Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain [18,54], an abbreviated Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was performed. Studies were evaluated and graded as type 1 through 4, which generally corresponded to the following study categories [54]:
randomized controlled trials (RCTs) or observational studies with overwhelming evidence;
RCTs with important limitations or observational studies with exceptionally strong evidence;
observational studies or RCTs with notable limitations;
clinical experience and observations, observational studies with important limitations, or RCTs with several major limitations.
Phase 2: Convening Expert Panel Members for Interactive Discussions and Voting to Reach Consensus
Consensus panel meetings for UDM occurred via web-based teleconferences on August 11 and 18, 2016, for a total of five hours. Section leaders (JF, JAG, RCP, and DPA; selected by co-chairs to lead discussions) reviewed the literature and led interactive discussions about the main questions related to UDM before consensus on recommendations was reached with a modified nominal group technique [55,56]. This consensus method was selected because of its demonstrated validity, long history of use, and time efficiency, as well as the ability for all panelists to provide input [57]. After every panelist provided an answer to a question, panelists voted for their preferred answer if multiple options were proposed. Additional discussion cycles and voting were performed until consensus was reached.
Phase 3: Preparation of the Consensus Report
The content of this report reflects an extensive review of existing UDM research and guidelines, discussion from several meetings and communications among the expert panelists, and consensus recommendations. Panelists reviewed and revised content in multiple stages of manuscript development before finalization.
Results
Six recent guidelines that address UDM in patients with chronic pain were identified by panelists as most relevant to the three key questions. The literature search found 85 studies pertaining to UDM; 21 additional references were added to address gaps in the existing literature on topics requested by panelists. After filtering for relevance to the three main questions (performed by an editorial service, directed by the authors), 41 references from the expanded literature search were included, all of which were graded for scientific merit as type 3 (low quality) or 4 (lowest quality) by the authors. Validation studies, even when well designed, were not considered equivalent to RCTs and were rated as lower quality.
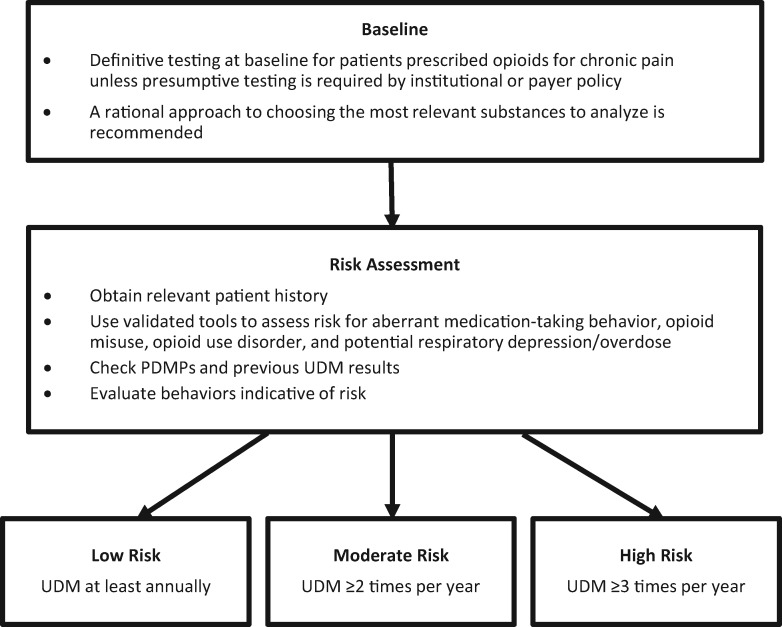
All graded references are included in the Relevant Literature sections for each question. Because of the lack of high-quality evidence addressing the priority issues for this report, recommendations were not assigned a strength category. Recommendations (Figure 2) should be considered consensus opinions based on evolving evidence.
Figure 2.
Consensus recommendations. PDMP = prescription drug monitoring program; UDM = urine drug monitoring.
Discussion and Recommendations
Question 1: Which UDM Test(s) Should Be Used and in Which Patients Prescribed Opioids for Chronic Pain Should the Tests Be Used According to a Medical Literature Review, Clinical Experience, Clinical Chemistry of Drug Testing, and Practical Considerations?
Expert Panel Recommendations
Use definitive UDM testing (e.g., with GC-MS, LC-MS, or LC-MS/MS) as the most accurate method for assessing baseline opioid use and opioid misuse in almost all patients with chronic pain being considered for opioids as well as for ongoing monitoring of patients receiving opioids for chronic pain, unless presumptive testing is required by institutional or payer policies. A rational approach to choosing the most relevant analytes (i.e., substances to be tested) for UDM testing is proposed (in the Expert Panel Discussion section for question 1) and should be documented by the clinician.
Existing Guidelines
In contrast to this expert panel’s recommendation, previously published guidelines suggest that patients undergo presumptive testing with immunoassay when they are prescribed opioids for chronic pain [18,20,22,40]; some guidelines specify that the test should be POC [20,40]. Confirmatory definitive tests are generally reserved for resolving unexpected results from immunoassays [18,20,22,40] or for detecting specific opioids that cannot be identified with standard immunoassays [18]. According to the CDC [18], CMS [58], and other payer policies [50,59], definitive testing is appropriate only when it affects clinical decision-making and patient management. The Washington State guidelines suggest considering the following drugs for a UDM panel in addition to medications prescribed: alcohol, amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, fentanyl, methadone, opiates, and oxycodone [22].
Relevant Literature
Surveys indicate that UDM in patients prescribed opioids for chronic pain varies widely (i.e., 19%–63.6% of patients receive UDM at some point during treatment [60–63], and 6.9%–100% of clinicians administer UDM [29,52,63–66]), which demonstrates a need for guidance. Traditionally, POC immunoassays have been used for initial screening in UDM because they provide rapid results at relatively low cost [28]. However, false-positive results (e.g., 22% for opiate immunoassays [32]) can be caused by cross-reactivity [28], and false-negative results (e.g., 30% for opiate assays [32]) may occur because of drug concentration cutoffs and cross-reactivity, in particular among drugs in similar chemical classes [67]. Some opioids and benzodiazepines are not well detected by immunoassays [67].
Compared with immunoassays, definitive testing can detect a greater number of compounds (e.g., various benzodiazepines [67–69]) and demonstrates higher sensitivity and specificity [70]. The gold standard of definitive testing was considered to be GC-MS [71], but LC-MS/MS has become a favored method [28] because it is associated with less drug interference and can be performed with smaller urine volumes than for GC-MS [71]. Validation of two new LC-MS/MS methods was identified through our literature search [72,73]. As a caveat, detection of metabolites of some prescription opioids (e.g., buprenorphine by certain routes of administration) or illicit substances (e.g., heroin) with LC-MS/MS is challenging, in part because of insufficient sensitivity of tests and individual variation in drug metabolism [74,75].
Although (confirmatory) definitive testing is often used to verify unexpected immunoassay results, recent evidence suggests that it is more accurate than immunoassay at assessing patients’ substance use at initial screening [76–78]. A hybrid UDM approach, in which each drug was measured with either immunoassay or LC-MS (depending on which platform has better accuracy and speed for each drug), reduced the need for confirmation testing and time to results [79]. In a study at a private pain practice, clinicians and patients discussed unexpected results from initial immunoassay UDM, and open nonjudgmental communication was emphasized; this approach led to only 3% to 5% of cases requiring confirmatory GC-MS testing [80]. The clinical utility of a hybrid immunoassay/LC-MS approach and of effective patient communication to prevent the need for confirmatory GC-MS testing will need to be further established.
Expert Panel Discussion
The panelists expressed concerns about limited sensitivity and specificity as well as misinterpretation errors with class-specific immunoassays, especially in the primary care setting. The initial low cost of immunoassays may be negated by their potential inaccuracy, which can increase the downstream financial burden to confirm conflicting or unexpected results and potentially increase costs for additional treatments and office visits when a patient is inappropriately continued or discontinued from opioids because of misinterpretation of results. Definitive testing, although often more expensive than immunoassay initially, includes a more comprehensive panel of substances and is more sensitive and specific for detecting adherence to prescribed opioids and use of other substances. For these reasons, several panelists consider definitive testing to be the most rational choice for UDM and elect to use it exclusively for both preliminary testing and follow-up testing when results are contested by the patient.
The expert panel recognizes that not all clinicians have reliable access to definitive testing laboratories, and some payers reimburse for definitive testing only after an immunoassay result is inconsistent with therapy. The recommendations in this consensus are intended to be considered together with practical clinical and payer concerns. When required by payers and institutions, immunoassays may be sufficient for monitoring low-risk patients, particularly when clinicians and patients engage in open communication.
Given the potentially high cost of definitive testing, rational selection of analytes is recommended. Appropriate substances to analyze for UDM include all controlled substances (and selected noncontrolled coanalgesics, such as antidepressants and anticonvulsants) that a patient is prescribed, as well as substances unexpectedly found at previous UDM. Inclusion of additional medications and/or alcohol is driven by their potential for harm (i.e., risk-relevant testing). For identification of substances most likely to be used and diverted, consult recent national survey results [81] and relevant geographic data [82]. Greater numbers of analytes will likely need to be tested for patients at higher risk for substance use disorders.
Complexities regarding UDM test properties necessitate that clinicians have access to an expert (e.g., toxicologist, knowledgeable pain or addiction specialist, pathologist) when choosing the most appropriate test and interpreting unexpected results. Clinicians should also be aware of relevant state mandates, regulations, and guidelines [83]; descriptions of UDM requirements by state are available from state medical boards and the AAPM website (http://www.painmed.org/advocacy/state-updates/).
Question 2: How Should Patients Undergoing UDM Be Stratified for Opioid Misuse Risk?
Expert Panel Recommendations
To guide UDM frequency, assess and stratify patients who are prescribed opioid therapy for chronic pain with the following strategies:
Perform a physical examination and obtain relevant patient history for events/diagnoses and behaviors that have been shown to predict opioid misuse and opioid use disorder (e.g., history of substance use disorders, psychiatric conditions, sexual abuse), including information from previous providers for patients transferring care.
Use validated tools to assess the risk for aberrant medication-taking behavior, opioid misuse, opioid use disorder, and the potential for respiratory depression/overdose.
Check prescription drug monitoring programs (PDMPs) and previous UDM results when available.
Existing Guidelines
Some guidelines [18,20,22] recommend the use of risk assessment tools to stratify patients receiving opioids; the Opioid Risk Tool (ORT) and Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP®-R) are frequently mentioned in other guidelines [18,22,40,84]. Tools deemed most relevant by panelists on the basis of validation studies and use in practice are described in Table 2 [18,19,22,84–94]. There is a need for further clinical validation of existing tools [19] and for development of newer and more accurate tools that incorporate additional risk factors [18,95]. Risk tools are just one part of a comprehensive patient evaluation [20,40].
Table 2.
Summary of tools to assess risks with opioids
| Tool | Number of Guidelines Mentioning | Description | Time to Complete | Validation | Summary of Diagnostic Accuracy | Additional Notes |
|---|---|---|---|---|---|---|
| Opioid Risk Tool (ORT) | 5 | 10-item patient self-report tool [84] that assesses risk of aberrant drug-related behaviors [19] | 1 min [22] | Yes [22] | With a cutoff score of > 4 or unspecified, sensitivity from 20% to 99% and specificity from 16% to 88% were reported for detecting risk of opioid overdose, addiction, abuse, or misuse (5 studies) [18] | – |
| Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) | 5 | 24-item patient self-report tool [22] that assesses risk of drug-related behaviors [19] | <10 min [22] | Yes [22] | With a cutoff score of > 3 or unspecified, sensitivity was from 25% to 53% and specificity was from 62% to 73% for detecting risk of opioid overdose, addiction, abuse, or misuse for likelihood ratios close to 1 (2 studies) [18] | • Designed to prevent patient deception [84] • Requires licensing agreement [22] but no fee for individual clinical use |
| Current Opioid Misuse Measure (COMM) | 4 | 17-item patient self-report tool to identify patients receiving long-term opioid therapy who are exhibiting aberrant behaviors [19] | <10 min [22] | Yes [22] | With a cutoff score of ≥ 10, sensitivity was 74% and specificity was 73%, and with a cutoff score of ≥ 9, sensitivity was 77% and specificity was 66% for the detection of aberrant drug-related behavior (1 study) [84] | Requires licensing agreement [22] but no fee for individual clinical use |
| Diagnosis, Intractability, Risk, Efficacy (DIRE) | 3 | 7-item clinician interview to predict efficacy of analgesia and patient adherence with long-term opioid treatment [85] | <2 min [22] | Yes [22] | At a cutoff point of 13, sensitivity was 94% and specificity was 87% for poor vs good/fair adherence (1 study) [85] | – |
| Pain Assessment and Documentation Tool (PADT) | 2 | Clinician-directed interview with 4 domains to document potentially aberrant drug-related behavior during treatment of pain [19] | <10 min [86] | Yes [19] | No studies identified | Addresses abuse risk in only a small component of the tool [87] |
| Cut down/Annoyed/Guilty/Eye-Opener Adapted to Include Drugs (CAGE-AID) | 1 | 4-question patient self-report, parent-report, or clinician-report tool to screen for substance use disorders [88] | <5 min [22] | Yes [22] | A cutoff of 2 led to sensitivity of 91% and specificity of 98% in adolescents; a cutoff of 1 led to sensitivity of 88% and specificity of 55% in adults [88]; a cutoff of 1 led to sensitivity of 79% and specificity of 77%, and a cutoff of 2 led to sensitivity of 70% and specificity of 85% in adults (2 studies total) [89] | – |
| Addiction Behaviors Checklist (ABC) | 1 | 20-item clinician-administered tool to track behaviors characteristic of addiction to opioids in patients with chronic pain [90] | Described as “brief” [90] | Yes [90] | At a cutoff of ≥ 3 (using ABC data from initial visit only), sensitivity was 87.50% and specificity was 86.14% (1 study) [90] | – |
| Single-Item Form of the Coping Strategies Question-naire | 0 | 1 question (“It’s terrible and I feel it is never going to get better”) to predict opioid misuse risk [91] | Very brief; only 1 question [91] | Yes [91] | Is highly predictive vs SOAPP-R (1 study) [91] | Study published as an abstract |
| Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) | 0 | 17-item [92] and 16-item [94] clinician-administered tool to assess risk of overdose or serous opioid-induced respiratory depression | Not specified | Yes (17-item version in a Veterans Health Administration population [92] and 16-item version in a commercial health plan claims database [94]) | Excellent agreement between predicted and observed incidences across risk classes; 85% (17-item) to 90% accuracy (16-item) in discriminating between patients with and without an event [92,94] | – |
| Single-item screening question for current drug use | 0 | 1 question (“How many times in the past year have you used an illegal drug or used a prescription medication for nonmedical reasons?”) to assess current drug use [93] | Very brief; only 1 question [93] | Yes [93] | Sensitivity for substance use disorders, self-reported current drug use, and drug use detected by oral fluid testing or self-report: 100%, 92.9%, and 84.7%, respectively; specificity for these measures: 73.5%, 94.1%, and 96.2%, respectively [93] | – |
Relevant Literature
Several tools for predicting and determining current risk of aberrant medication-taking behaviors (e.g., lost prescription, request for early refill), opioid misuse, and opioid use disorder are reported in the literature [96–99]. In a pain clinic–based study, a semistructured clinical interview by an addiction psychologist was more sensitive than the ORT, Pain Medication Questionnaire, and SOAPP-R at predicting aberrant drug-related behavior [98]. The SOAPP-R showed the highest sensitivity of these self-report measures but may overestimate risk [98].
The use of external information (e.g., PDMPs) can also improve patient management [100]. In a university-based multidisciplinary pain management center, misuse and diversion were detected more frequently by adding UDM and/or a PDMP check to a baseline strategy of comparing patient reports and review of medical records [101]. However, a systematic review of primary care, pain clinic, and Veterans Administration (VA) Medical Center settings concluded that no single procedure or set of variables was sufficient to identify patients with chronic pain who are at risk for opioid misuse or harmful substance use [97].
Expert Panel Discussion
The expert panelists suggest that patients be assessed for risk of aberrant medication-taking behavior, misuse, and opioid use disorder to determine the frequency of UDM. A high-dose cutoff alone (e.g., 120-mg morphine equivalent dose per day [22]) was perceived as being inadequate for identifying high-risk patients because high doses may be appropriate to accommodate development of tolerance in specific patients [19,21,87]. In addition, patients predisposed to misuse may be at risk of opioid use disorder or unsafe use even with low to moderate doses.
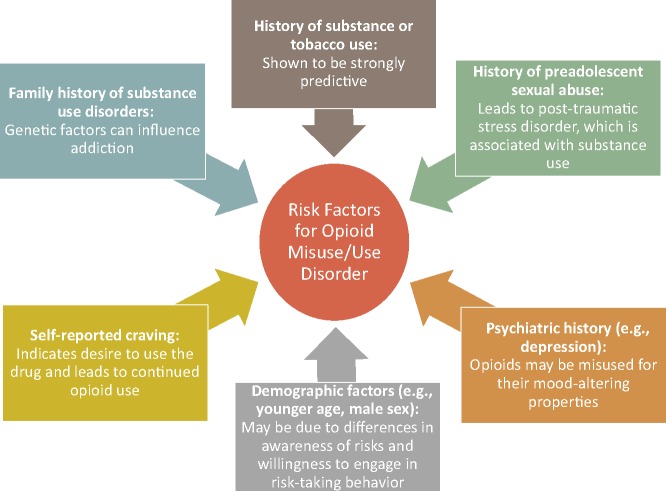
No specific risk assessment tool or behavioral assessment is recommended by panelists. Clinicians are encouraged to choose one or more of the many available tools that match their preferences and can be incorporated into their electronic medical records and work flow. Understanding why specific factors predict risk is more important than knowledge of the risk assessment tools themselves (Figure 3 [19,97,102–107]). Risk stratification is not static, and regular reevaluation of patient circumstances (e.g., loss of a job, divorce) is recommended. An unexpected UDM result increases a patient’s risk of misuse and opioid use disorder, necessitates more frequent testing, and prompts reconsideration of whether to modify opioid therapy or refer the patient to a pain or addiction specialist.
Figure 3.
Explanations for risk factors of opioid misuse and opioid use disorder [19,97,102–107].
A comprehensive evaluation to assess the presence or risk of misuse and opioid use disorder includes questions about patients’ history of substance use disorders and current clinical characteristics (e.g., from a physical examination), input from patients’ family members and other health care providers (e.g., psychiatrists, previous pain specialists), and pill counts. Behaviors that may indicate increased likelihood of misuse or opioid use disorder include requests for early refills [108], unauthorized dose escalations [109], and use of another individual’s medication [109]. Substance use disorder is generally associated with one or more of the four “Cs” (i.e., impaired Control over use, Compulsive use, Continued use despite harm, and Craving) [110].
A few simple questions (e.g., single-item screening questions [SISQs] for alcohol and drug use [93,111]) followed by a discussion with patients is an efficient way for clinicians to incorporate risk assessment into their practice. Reviewing a patient’s history of controlled substance prescriptions in the PDMP is another method for assessing potential opioid misuse or substance use disorder. Clinicians should be aware of their state’s regulations, recommendations, and resources regarding PDMPs [112]. All states have or are planning to implement a PDMP, but reporting times vary and not every provider is required to participate in PDMPs in all states [112–114]. The Comprehensive Addiction and Recovery Act of 2016 is intended to improve the efficiency of these programs to track prescription drug use and help prevent inappropriate use [115]. Despite their inconsistencies, PDMPs (when available) have become standard of care as part of patient risk evaluation. Whether PDMP data will predict aberrant behaviors or other adverse outcomes is not yet known and represents a gap in the science and an unmet need.
Question 3: How Often Should UDM Occur in Patients with Low, Medium, and High Risk for Opioid Misuse or Opioid Use Disorder?
Expert Panel Recommendation
Perform UDM at baseline in patients prescribed opioids for chronic pain. During ongoing monitoring, perform UDM at least annually for low-risk patients, two or more times per year for moderate-risk patients, and three or more times per year for high-risk patients. Additional monitoring can be performed at any risk level as frequently as necessary according to clinical judgment.
Existing Guidelines
Recent guidelines recommend UDM at least annually for all patients regardless of risk [18], every six months to two years for patients at low risk for opioid misuse or opioid use disorder [20,22,40], one to three times per year for moderate-risk patients [20,22], and at least two to four times per year for high-risk patients [20,22,40]. The CDC Guideline for Prescribing Opioids for Chronic Pain does not recommend tailoring the monitoring schedule according to risk because tools that predict harmful use lack sufficient accuracy [18]. Several guidelines [18,21,22] suggest periodic UDM testing during scheduled visits; truly random testing outside of scheduled clinic visits may not be feasible in many settings (e.g., primary care) or appropriate for all patients [18].
Relevant Literature
The identified studies related to UDM frequency do not differentiate strategies for low-, moderate-, and high-risk levels. Nevertheless, clinical trials assessing the effects of UDM on outcomes are particularly relevant to decisions regarding frequency of monitoring (Supplementary Table 1). Adherence to prescribed opioids is higher with more frequent UDM, according to a retrospective analysis of patients with chronic pain in private practices [116]. In two prospective trials at an interventional pain management practice, adherence monitoring that included random UDM was associated with reductions in indicators of opioid misuse (determined via periodic chart review, UDM, pill counts, and verification of information with treating clinicians and pharmacies) [24] and illicit drug use (determined via UDM) [117]. In another study, a comprehensive risk reduction strategy, including UDM, led to decreased pill confiscations by law enforcement agents and improved primary care providers’ perceptions of the overall quality of pain management [118]. A structured program designed to support primary care providers with chronic pain management led to a greater-than-two-fold increase in UDM 22 months after initiation of the program, which was associated with a 72.7% relative decrease in total emergency department (ED) visits and a 59.6% relative decrease in unscheduled primary care provider visits per patient on average [119].
The main negative clinical consequence of UDM reported in the literature was a lower likelihood of patients attending a second visit at an urban academic pain clinic after urine testing was used in the first office visit [120]. Individuals with positive test results for an illicit substance were less likely to attend the second visit than those with a negative result [120]. Although this study suggests that UDM at first visit may hinder patient-clinician trust, patient response to UDM may vary by clinical setting, by how the rationale for UDM is explained to the patient, and by the degree to which UDM becomes routine in clinical practice.
Many studies showed significant benefits of UDM; however, a few did not. No association between UDM and all-cause mortality was found in VA patients [121], although an association was not necessarily expected in this sample of patients with a high burden of comorbidities. Another study conducted in a large health care system showed that an opioid risk reduction initiative (including recommendations on UDM) increased use of UDM without affecting rates of unexpected results (e.g., negative for prescribed opioids or positive for cannabis) [122].
Several studies and surveys of physicians (e.g., pain and addiction specialists), nurses, PAs, and other health care professionals, mainly from pain practices and academic centers, have assessed the frequency of UDM during opioid therapy for chronic pain [29,65,80,123,124], which provides context for recommendations on frequency. Patients with chronic pain received an average of 3.4 UDM tests per year, according to a 2007 study in a Kentucky private pain practice [80]. The frequency of UDM testing increased by an average of 34% yearly from 2005 to 2008 in a clinical laboratory serving a large academic center [123]. Surveys indicated that individual pain and addiction experts differed in their frequency of UDM use, from testing at every office visit to yearly [29,65], although most preferred random testing [65,124]. As a caveat, these surveys did not focus on primary care settings.
Expert Panel Discussion
The expert panel did not specify how the relative risk categories should be determined, aside from suggesting several potential risk assessment tools and strategies (see the Question 2 section). Baseline UDM testing is to be performed either before initiation of opioid therapy or, for those continuing opioid therapy from another provider, within three months of the first office visit. If the patient received UDM testing from another provider within the previous year and the results were consistent with prescribed opioid therapy, no immediate retesting may be needed to continue therapy.
Regular UDM is recommended even in low-risk patients because their circumstances/behaviors can change over the course of therapy. Patients at especially low risk (e.g., receiving low-dose, as-needed opioids) may require infrequent UDM (e.g., every two years). Moderate-risk patients may become higher or lower risk depending on their environment and stressors; intense monitoring is usually not required in these patients, but periodic reevaluation of their situations/risk factors is appropriate.
High-risk patients require more frequent individualized UDM testing than those at low or moderate risk, although the evidence supporting the effectiveness of intense monitoring is weak. Most primary care providers can best care for high-risk patients by referring them to a pain and addiction specialist when available. Primary care clinicians who are trained in chronic pain management, utilize UDM, are experienced in interpreting UDM results, and have access to consultant toxicologists may be able to appropriately care for high-risk patients and/or comanage them with a pain specialist. Current guidelines for UDM in patients with substance use disorder recommend use of personalized testing regimens [28].
Additional Expert Panel Recommendations and Discussion
UDM Rationale
Clinicians are encouraged to include information related to UDM in patient-provider agreements and explain to patients that the primary goals of UDM are to improve the safety and effectiveness of therapy by monitoring adherence. Furthermore, UDM is strongly recommended as part of a universal precautions approach [125]. Consistent UDM results provide objective data to support decision-making during chronic opioid therapy.
UDM Process
The panelists recommend that clinicians discuss the UDM process openly with patients, as they do with all other clinical testing, and document the time of last medication use before urine collection. Unexpected results may be caused by laboratory errors (e.g., mislabeling) or by sample adulteration, including substitution of synthetic or another individual’s urine. Signs of tampering include temperature outside the normal range of 90 °F to 100 °F within four minutes of sample collection, pH outside the normal range of 4.5 to 8.0, low creatinine concentration (i.e., ≤20 mg/dL), low specific gravity (i.e., ≤1.003), presence of adulterants, or detection of parent drug without metabolites [28]. Variation in metabolite levels may also result from pharmacogenetic anomalies or drug-drug interactions affecting drug metabolism [28,126].
Strategies to prevent sample tampering depend on the clinical setting and can include observed collection (viewed as overly invasive of a patient’s privacy), a chain-of-custody protocol (i.e., documentation for handling of the specimen), and a truly random collection (i.e., a protocol to notify the patients of required testing within a set period) [28]. Although observed urine sample collection at pain clinics has been recommended [83], this practice and chain-of-custody protocols are typically not followed. Despite risk-mitigation strategies, dedicated individuals can still manipulate their UDM samples or consume prescribed medication before office visits to conceal misuse or diversion. Patients with an opioid use disorder may not be able to control their drug use to avoid unexpected UDM results despite scheduled collection.
Alcohol Screening
For patients with a substance use disorder, alcohol may be problematic and prohibited in any amount; for other patients with chronic pain, only high-risk alcohol use (e.g., binge drinking) is a concern. Many opioids include black box warnings about the concurrent use of alcohol because of the potential for fatal overdose [127]. One reviewed guideline recommends screening for alcohol with UDM in patients with chronic pain [22]; ethyl glucuronide, ethyl sulfate, or ethanol in UDM indicates alcohol use [128]. As a caveat, immunoassay and definitive UDM may not differentiate between alcoholic beverages and alcohol-containing medications, mouthwashes, and hand sanitizers [28]. Instead of UDM, the panelists recommend the SISQ “How many times in the past year have you had (five or more drinks [men], four or more drinks [women]) in a day?” from the National Institute on Alcohol Abuse and Alcoholism [111] to identify unhealthy alcohol use, as this question is simple to administer and is validated [129]. In primary care settings, the sensitivity of the alcohol SISQ for identifying alcohol use disorder is 88%, and the specificity is 84% [130].
Cannabis Screening
Practices for cannabis screening vary [131]; individual prescribers can decide whether detecting cannabis by UDM is appropriate by consulting local laws [132,133] and common practice, as well as by considering the advantages/disadvantages discussed below. At the time of this publication, cannabis is federally illegal (i.e., classified by the US Drug Enforcement Administration as Schedule 1 under the Controlled Substances Act), but several states have passed legislation to decriminalize it for medical and/or recreational use [131–134]. The CDC Guideline for Prescribing Opioids for Chronic Pain indicates that the clinical implications of a positive UDM result for cannabis are uncertain [18]; the VA/US Department of Defense guidelines estimate the length of time it can be detected in urine [21]; and the Washington State guidelines suggest implementing an office policy for patients who use cannabis [22]. These guidelines [18,21,22] and this consensus report do not provide formal graded recommendations for or against UDM screening for cannabis or for interpretation of the results.
Clinicians who avoid cannabis testing may miss critical information that could inform patient monitoring and improve safety. Data from Colorado indicate increased ED visits, hospitalizations, and proportions of fatal motor vehicle accidents related to cannabis intoxication after decriminalization [134–136]. Illegal use of cannabis is a marker for opioid misuse and substance use disorders [137] and a rationale to classify a patient as high risk. Another concern is that patients may divert prescription opioids to purchase cannabis.
If clinicians choose to assess patients’ nonprescription cannabinoid use, there is a validated SISQ for drugs including cannabis (i.e., “How many times in the past year have you used an illegal drug or used a prescription medication for nonmedical reasons?”) [93], with a sensitivity of 97% and a specificity of 79% for substance use disorders in primary care settings [130]. Although some metabolites of synthetic cannabinoids (e.g., spice) can be analyzed with specialized immunoassays, chromatography/spectrometry-based assays are better for assessing the many emerging synthetic cannabinoids and their metabolites [138].
A subject of ongoing debate is whether co-administration of opioids and cannabis (used recreationally or medically) is safe or reasonable for clinicians to allow. Cannabis is increasingly accepted for medical purposes, especially for cancer pain syndromes. However, allowing patients with chronic pain to use cannabis is a potential liability for clinicians (including pharmacists) regardless of the drug’s legal status in their state [139]. The safety of cannabis for patients with chronic pain is not clearly established [140], and little is known regarding the safety of concurrent use with opioids apart from the potential opioid-sparing effects of cannabis [141]. The composition and dose of the many active components in cannabis vary widely [133,142], which leads to variable toxicity [143] and complicates safety studies. Cannabinoids may inhibit cytochrome P450 2D6 [144] (involved in opioid metabolism [145]) and therefore affect both the efficacy of opioids and the ability to detect metabolites in UDM. The panelists proposed that a single clinician be responsible for managing both opioid and cannabis use in states where the drug has been decriminalized and the benefits of concurrent use outweigh the risks.
Interpreting UDM Results and Implications for Changing Clinical Practice
The panelists believe that clinicians should follow manufacturer instructions for specific POC UDM tests and direct any questions about interpreting results to an expert in toxicology or clinical pathology. Laboratories performing UDM have a responsibility to provide clear test results, answer questions, and offer support on clinical decisions. When clinicians are confronted with unexpected results, potential causes for false-positive and false-negative results (e.g., quinolone antibiotics, tolmetin; Figure 1) are important to investigate. A summary of communications and discussions about results with the laboratory and other experts can be included in the medical record to document the medical necessity of testing and related clinical decision-making.
Communications with patients about the purpose and results of UDM should be nonjudgmental and nonpunitive and should focus on safety and risks associated with misuse and opioid use disorder. To avoid unexpected results, open discussion with patients is recommended and may include asking questions, such as “If we test you today, what will we find in your urine? Will there be any surprises?” Development of a plan to address UDM results that are inconsistent with therapy is suggested to mitigate safety risks for patients and potential regulatory board sanctions for clinicians. Of note, UDM results that are consistent with prescribed opioids cannot differentiate among appropriate use, occasional use or misuse, and opioid use disorder; negative UDM results for prescribed opioids cannot distinguish between infrequent need for medicine, overuse and running out, falsification of the urine sample, and diversion. Results of UDM are only part of the clinical information (e.g., including patient history) that must be considered before a treatment plan is changed.
Detailed recommendations on proper opioid prescribing and appropriate changes to patient care after unexpected UDM results are outside the scope of this consensus report but are discussed in other guidelines [18,20,22,40]. In brief, options to address unexpected UDM results include open and nonjudgmental discussions with patients, additional confirmation testing, increased monitoring, a switch to a nonopioid pain medication, or referral for treatment of an opioid use disorder [18,20,22,40].
Three studies from the Philadelphia VA Medical Center investigated how UDM results affected provider behavior [119,146,147]; additional research to determine how UDM results should influence patients’ therapy is warranted. In the absence of this information, a follow-up consensus meeting to evaluate expert opinion was suggested.
Conclusions
In summary, the expert panel made the following recommendations regarding UDM (Figure 2):
Use definitive UDM testing (e.g., with GC-MS, LC-MS, or LC-MS/MS) as the most clinically appropriate method for assessing baseline opioid use and opioid misuse in most patients with chronic pain being considered for opioids, as well as for ongoing monitoring of patients receiving opioids for chronic pain, unless presumptive testing is required by institutional or payer policies. A rational approach to choosing the most relevant analytes for UDM testing is proposed and should be documented by the clinician.
-
To guide UDM frequency, assess and stratify patients prescribed opioid therapy for chronic pain with the following strategies:
perform a physical examination and obtain relevant patient history for events/diagnoses and behaviors that have been shown to predict opioid misuse and opioid use disorder (e.g., history of substance use disorders, psychiatric conditions, sexual abuse) including information from previous providers for patients transferring care;
use validated tools to assess risk for aberrant medication-taking behavior, opioid misuse, opioid use disorder, and the potential for respiratory depression/overdose;
check PDMPs and previous UDM results when available.
Perform UDM at baseline in patients receiving opioids for chronic pain. During ongoing monitoring, perform UDM at least annually for low-risk patients, two or more times per year for moderate-risk patients, and three or more times per year for high-risk patients. Additional monitoring can be performed as frequently as necessary according to clinical judgment (e.g., worrisome patient behavior related to the prescribed medication).
The recommendations in this consensus report are meant to provide practical advice on implementing rational UDM across a broad range of clinical practices in a patient-centric manner. Some clinicians may not have access to appropriate resources to perform routine definitive UDM or to refer patients to pain specialists; alternatives are provided in the expert panel discussion sections. Higher-quality studies on patient outcomes with UDM are needed. As a next step, a consensus recommendation initiative similar to this one that discusses appropriate clinician actions in response to test results that are inconsistent with prescribed therapy is warranted.
Authors’ Contributions
All authors contributed to the comprehensive review of the published literature, consensus meeting discussions, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for scientific soundness and intellectual content, and approval of the final manuscript. JF, JAG, RCP, and DPA contributed to presentation of the literature at the consensus meeting. CEA and LRW provided general supervision.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
Supplementary Material
References
- 1. Prunuske JP, St Hill CA, Hager KD, et al. Opioid prescribing patterns for non-malignant chronic pain for rural versus non-rural US adults: A population-based study using 2010 NAMCS data. BMC Health Serv Res 2014;141:563.. 10.1186/s12913-014-0563-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gudin JA, Mogali S, Jones JD, Comer SD.. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med 2013;1254:115–30. 10.3810/pgm.2013.07.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dasgupta N, Funk MJ, Proescholdbell S, et al. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016;171:85–98. [DOI] [PubMed] [Google Scholar]
- 4. Larochelle MR, Zhang F, Ross-Degnan D, Wharam JF.. Trends in opioid prescribing and co-prescribing of sedative hypnotics for acute and chronic musculoskeletal pain: 2001-2010. Pharmacoepidemiol Drug Saf 2015;248:885–92. [DOI] [PubMed] [Google Scholar]
- 5. Jarzyna D, Jungquist CR, Pasero C, et al. American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Manag Nurs 2011;123:118–45.e10. [DOI] [PubMed] [Google Scholar]
- 6. Cicero TJ, Ellis MS, Harney J.. Shifting patterns of prescription opioid and heroin abuse in the United States. N Engl J Med 2015;37318:1789–90. 10.1056/NEJMc1505541 [DOI] [PubMed] [Google Scholar]
- 7. Rudd RA, Paulozzi LJ, Bauer MJ, et al. Increases in heroin overdose deaths—28 states, 2010 to 2012. MMWR Morb Mortal Wkly Rep 2014;6339:849–54. [PMC free article] [PubMed] [Google Scholar]
- 8. Office of the Chief Medical Examiner. Connecticut accidental drug intoxication deaths. 2017. Available at: http://www.ct.gov/ocme/lib/ocme/AccidentalDrugIntoxication2012-2016.pdf (accessed March 2017).
- 9. New Hampshire Information and Analysis Center. New Hampshire drug monitoring initiative. 2016. Available at: https://www.dhhs.nh.gov/dcbcs/bdas/documents/dmi-june-16.pdf (accessed March 2017).
- 10. Office of the Maine Attorney General. Overdose deaths claim more than one person per day in Maine during 2016. 2017. Available at: http://www.maine.gov/ag/news/article.shtml? id=729779 (accessed March 2017).
- 11. Casale JF, Mallette JR, Guest EM.. Analysis of illicit carfentanil: Emergence of the death dragon. Forensic Chem 2017;3:74–80. 10.1016/j.forc.2017.02.003 [DOI] [Google Scholar]
- 12. Somerville NJ, O’Donnell J, Gladden RM, et al. Characteristics of fentanyl overdose—Massachusetts, 2014-2016. MMWR Morb Mortal Wkly Rep 2017;6614:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frank RG, Pollack HA.. Addressing the fentanyl threat to public health. N Engl J Med 2017;3767:605.. 10.1056/NEJMp1615145 [DOI] [PubMed] [Google Scholar]
- 14. Matteliano D, Chang YP.. Describing prescription opioid adherence among individuals with chronic pain using urine drug testing. Pain Manag Nurs 2015;161:51–9. 10.1016/j.pmn.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 15. Zgierska A, Wallace ML, Burzinski CA, Cox J, Backonja M.. Pharmacological and toxicological profile of opioid-treated, chronic low back pain patients entering a mindfulness intervention randomized controlled trial. J Opioid Manag 2014;105:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frannis FW., Jr Musings of a cynical curmudgeon: Pain or feign? Oncology (Williston Park) 2013;27:769–72. [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. 2016. Available at: http://www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf (accessed August 2016).
- 18. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;651:1–49. [DOI] [PubMed] [Google Scholar]
- 19. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;102:113–30. 10.1016/j.jpain.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manchikanti L, Kaye AM, Knezevic NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician 2017;20:S3–92. [PubMed] [Google Scholar]
- 21. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for opioid therapy for chronic pain. 2017. Available at: https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf (accessed October 2017).
- 22. Washington State Agency Medical Directors’ Group. Interagency guideline on prescribing opioids for pain. 2015. Available at: http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf (accessed April 2016).
- 23. Pesce A, West C, Rosenthal M, et al. Illicit drug use in the pain patient population decreases with continued drug testing. Pain Physician 2011;142:189–93. [PubMed] [Google Scholar]
- 24. Manchikanti L, Manchukonda R, Damron KS, et al. Does adherence monitoring reduce controlled substance abuse in chronic pain patients? Pain Physician 2006;9:57–60. [PubMed] [Google Scholar]
- 25. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol 2012;84:408–16. 10.1007/s13181-012-0274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bush DM. The U.S. mandatory guidelines for federal workplace drug testing programs: Current status and future considerations. Forensic Sci Int 2008;174(2–3):111–9. [DOI] [PubMed] [Google Scholar]
- 27. The National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines: Using clinical laboratory tests to monitor drug therapy in pain management patients. 2016. Available at: https://www.aacc.org/∼/media/practice-guidelines/pain-management/rough-draft-pain-management-lmpg-v6aacc.pdf? la=en (accessed March 2017).
- 28. American Society of Addiction Medicine. Drug testing: A white paper of the American Society of Addiction Medicine (ASAM). 2013. Available at: http://www.asam.org/docs/default-source/public-policy-statements/drug-testing-a-white-paper-by-asam.pdf (accessed July 2016).
- 29. Kirsh KL, Baxter LE, Rzetelny A, Mazuk M, Passik SD.. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med 2015;95:399–404. [DOI] [PubMed] [Google Scholar]
- 30. American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders DSM-5. Washington, DC: American Psychiatric Association; 2013. Available at: http://HZ9PJ6FE4T.search.serialssolutions.com/? V=1.0&L=HZ9PJ6FE4T&S=JCs&C=TC0000893411&T=marc&tab=BOOKS (accessed May 2017).
- 31. Kelly JF, Wakeman SE, Saitz R.. Stop talking ‘dirty’: Clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med 2015;1281:8–9. [DOI] [PubMed] [Google Scholar]
- 32. Kirsh KL, Heit HA, Huskey A, et al. Trends in drug use from urine drug testing of addiction treatment clients. J Opioid Manag 2015;111:61–8. 10.5055/jom.2015.0253 [DOI] [PubMed] [Google Scholar]
- 33. Moeller KE, Lee KC, Kissack JC.. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc 2008;831:66–76. 10.4065/83.1.66 [DOI] [PubMed] [Google Scholar]
- 34. Saitman A, Park HD, Fitzgerald RL.. False-positive interferences of common urine drug screen immunoassays: A review. J Anal Toxicol 2014;387:387–96. 10.1093/jat/bku075 [DOI] [PubMed] [Google Scholar]
- 35. Joseph R, Dickerson S, Willis R, et al. Interference by nonsteroidal anti-inflammatory drugs in EMIT and TDx assays for drugs of abuse. J Anal Toxicol 1995;191:13–7. 10.1093/jat/19.1.13 [DOI] [PubMed] [Google Scholar]
- 36. Wagener RE, Linder MW, Valdes R Jr.. Decreased signal in Emit assays of drugs of abuse in urine after ingestion of aspirin: Potential for false-negative results. Clin Chem 1994;40:608–12. [PubMed] [Google Scholar]
- 37. Lehmann T, Sager F, Brenneisen R.. Excretion of cannabinoids in urine after ingestion of cannabis seed oil. J Anal Toxicol 1997;215:373–5. 10.1093/jat/21.5.373 [DOI] [PubMed] [Google Scholar]
- 38. Brahm NC, Yeager LL, Fox MD, Farmer KC, Palmer TA.. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm 2010;6716:1344–50. [DOI] [PubMed] [Google Scholar]
- 39. Felton D, Zitomersky N, Manzi S, Lightdale JR.. 13-year-old girl with recurrent, episodic, persistent vomiting: Out of the pot and into the fire. Pediatrics 2015;1354:e1060–3. [DOI] [PubMed] [Google Scholar]
- 40. Peppin JF, Passik SD, Couto JE, et al. Recommendations for urine drug monitoring as a component of opioid therapy in the treatment of chronic pain. Pain Med 2012;137:886–96. 10.1111/j.1526-4637.2012.01414.x [DOI] [PubMed] [Google Scholar]
- 41. Florete OG., Jr. Urinary drug testing in pain management. Pract Pain Manag 2017. Available at: https://www.practicalpainmanagement.com/treatments/pharmacological/opioids/urinary-drug-testing-pain-management (accessed March 31, 2017). [Google Scholar]
- 42. Tenore PL. Advanced urine toxicology testing. J Addict Dis 2010;294:436–48. 10.1080/10550887.2010.509277 [DOI] [PubMed] [Google Scholar]
- 43. DePriest AZ, Black DL, Robert TA.. Immunoassay in healthcare testing applications. J Opioid Manag 2015;111:13–25. 10.5055/jom.2015.0248 [DOI] [PubMed] [Google Scholar]
- 44. Centers for Medicare and Medicaid Services. Calendar year (CY) 2017 clinical laboratory fee schedule (CLFS) final determinations. 2017. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/CY2017-CLFS-Codes-Final-Determinations.pdf (accessed April 2017).
- 45. Dasgupta A, Chughtai O, Hannah C, Davis B, Wells A.. Comparison of spot tests with AdultaCheck 6 and Intect 7 urine test strips for detecting the presence of adulterants in urine specimens. Clin Chim Acta 2004;348(1–2):19–25. [DOI] [PubMed] [Google Scholar]
- 46. Trescot AM, Faynboym S.. A review of the role of genetic testing in pain medicine. Pain Physician 2014;175:425–45. [PubMed] [Google Scholar]
- 47. Gourlay DL, Helt HA, Caplan YH. Urine drug testing in clinical practice: The art and science of patient care, edition 6. 2016. Available at: http://www.remitigate.com/wp-content/uploads/2015/11/Urine-Drug-Testing-in-Clinical-Practice-Ed6_2015-08.pdf (accessed October 2017).
- 48. Weaver C, Mathews AW. Doctors cash in on drug tests for seniors, and Medicare pays the bill. The Wall Street Journal. 2014. Available at: http://www.wsj.com/articles/doctors-cash-in-on-drug-tests-for-seniors-and-medicare-pays-the-bill-1415676782 (accessed September 2016).
- 49. Lipman AG. The controversy over urine drug testing in pain management patient monitoring. J Pain Palliat Care Pharmacother 2013;274:320–1. 10.3109/15360288.2013.849453 [DOI] [PubMed] [Google Scholar]
- 50. UHA Health Insurance. Urine drug screening. 2016. Available at: https://uhahealth.com/uploads/forms/form_dia_Urine-Drug-Screening-UDS.pdf (accessed April 2017).
- 51. Laffler A, Murphy R, Winegarden W, et al. An economic analysis of the costs and benefits associated with regular urine drug testing for chronic pain patients in the United States. 2011. Available at: https://www.researchgate.net/profile/Charles_Mikel/publication/268175852_An_Economic_Analysis_of_the_Costs_and_Benefits_Associated_with_Regular_Urine_Drug_Testing_for_Chronic_Pain_Patients_in_the_United_States_Laffer_Associates_An_Economic_Analysis_of_the_Costs_and_Benefit/links/5571bfeb08ae7536374c5d4e.pdf (accessed April 2016).
- 52. Reisfield GM, Webb FJ, Bertholf RL, Sloan PA, Wilson GR.. Family physicians’ proficiency in urine drug test interpretation. J Opioid Manag 2007;36:333–7. [DOI] [PubMed] [Google Scholar]
- 53. Starrels JL, Fox AD, Kunins HV, Cunningham CO.. They don’t know what they don’t know: Internal medicine residents’ knowledge and confidence in urine drug test interpretation for patients with chronic pain. J Gen Intern Med 2012;2711:1521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ahmed F. Advisory Committee on Immunization Practices Handbook for Developing Evidence-Based Recommendations. Version 1.2. Atlanta, GA: US Department of Health and Human Services, CDC; 2013. Available at: http://www.cdc.gov/vaccines/acip/recs/GRADE/about-grade.html#resources (accessed November 2016).
- 55. Gallagher M, Hares T, Spencer J, Bradshaw C, Webb I.. The nominal group technique: A research tool for general practice? Fam Pract 1993;101:76–81. [DOI] [PubMed] [Google Scholar]
- 56. Jones J, Hunter D.. Consensus methods for medical and health services research. BMJ 1995;3117001:376–80. 10.1136/bmj.311.7001.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Harvey N, Holmes CA.. Nominal group technique: An effective method for obtaining group consensus. Int J Nurs Pract 2012;182:188–94. 10.1111/j.1440-172X.2012.02017.x [DOI] [PubMed] [Google Scholar]
- 58. Palmetto GBA. Controlled substance monitoring and drugs of abuse coding and billing guidelines (M00109, V9). 2015. Available at: http://www.palmettogba.com/palmetto/providers.nsf/DocsCat/Providers∼JM%20Part%20B∼Browse%20by%20Topic∼Lab∼9SDPFR2173 (accessed September 2016).
- 59. Moda Health Plan, Inc. Therapeutic drug monitoring. 2016. Available at: https://www.modahealth.com/pdfs/med_criteria/TherapeuticDrugMonitoring.pdf (accessed September 2016).
- 60. Bauer SR, Hitchner L, Harrison H, Gerstenberger J, Steiger S.. Predictors of higher-risk chronic opioid prescriptions in an academic primary care setting. Subst Abus 2016;371:110–7. [DOI] [PubMed] [Google Scholar]
- 61. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med 2015;163:480–7. 10.1111/pme.12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morasco BJ, Peters D, Krebs EE, et al. Predictors of urine drug testing for patients with chronic pain: Results from a national cohort of U.S. veterans. Subst Abus 2016;371:82–7. [DOI] [PubMed] [Google Scholar]
- 63. Pergolizzi J, Pappagallo M, Stauffer J, et al. The role of urine drug testing for patients on opioid therapy. Pain Pract 2010;106:497–507. 10.1111/j.1533-2500.2010.00375.x [DOI] [PubMed] [Google Scholar]
- 64. Levy S, Harris SK, Sherritt L, Angulo M, Knight JR.. Drug testing of adolescents in ambulatory medicine: Physician practices and knowledge. Arch Pediatr Adolesc Med 2006;1602:146–50. [DOI] [PubMed] [Google Scholar]
- 65. Owen GT, Burton AW, Schade CM, Passik S.. Urine drug testing: Current recommendations and best practices. Pain Physician 2012;15(suppl 3):ES119–33. [PubMed] [Google Scholar]
- 66. Bhamb B, Brown D, Hariharan J, et al. Survey of select practice behaviors by primary care physicians on the use of opioids for chronic pain. Curr Med Res Opin 2006;229:1859–65. 10.1185/030079906X132398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectrometry versus immunoassay drug testing in pain patients. Pain Physician 2010;13:273–81. [PubMed] [Google Scholar]
- 68. Manchikanti L, Malla Y, Wargo BW, Fellows B.. Comparative evaluation of the accuracy of benzodiazepine testing in chronic pain patients utilizing immunoassay with liquid chromatography tandem mass spectrometry (LC/MS/MS) of urine drug testing. Pain Physician 2011;14:259–70. [PubMed] [Google Scholar]
- 69. Melanson SE, Ptolemy AS, Wasan AD.. Optimizing urine drug testing for monitoring medication compliance in pain management. Pain Med 2013;1412:1813–20. 10.1111/pme.12207 [DOI] [PubMed] [Google Scholar]
- 70. Dickerson JA, Laha TJ, Pagano MB, O’Donnell BR, Hoofnagle AN.. Improved detection of opioid use in chronic pain patients through monitoring of opioid glucuronides in urine. J Anal Toxicol 2012;368:541–7. [DOI] [PubMed] [Google Scholar]
- 71. Mikel C, Pesce A, West C.. A tale of two drug testing technologies: GC-MS and LC-MS/MS. Pain Physician 2010;131:91–2. [PubMed] [Google Scholar]
- 72. Cao Z, Kaleta E, Wang P.. Simultaneous quantitation of 78 drugs and metabolites in urine with a dilute-and-shoot LC-MS-MS assay. J Anal Toxicol 2015;395:335–46. 10.1093/jat/bkv024 [DOI] [PubMed] [Google Scholar]
- 73. Wang J, Yang Z, Lechago J.. Rapid and simultaneous determination of multiple classes of abused drugs and metabolites in human urine by a robust LC-MS/MS method—application to urine drug testing in pain clinics. Biomed Chromatogr 2013;2711:1463–80. [DOI] [PubMed] [Google Scholar]
- 74. Markman JD, Barbosa WA, Gewandter JS, et al. Interpretation of urine drug testing results in patients using transdermal buprenorphine preparations for the treatment of chronic noncancer pain. Pain Med 2015;166:1132–6. 10.1111/pme.12740 [DOI] [PubMed] [Google Scholar]
- 75. Knight J, Puet BL, DePriest A, et al. Prevalence of heroin markers in urine for pain management patients. Forensic Sci Int 2014;243:79–83. 10.1016/j.forsciint.2014.04.037 [DOI] [PubMed] [Google Scholar]
- 76. Manchikanti L, Malla Y, Wargo BW, Fellows B.. Comparative evaluation of the accuracy of immunoassay with liquid chromatography tandem mass spectrometry (LC/MS/MS) of urine drug testing (UDT) opioids and illicit drugs in chronic pain patients. Pain Physician 2011;14:175–87. [PubMed] [Google Scholar]
- 77. Darragh A, Snyder ML, Ptolemy AS, Melanson S.. KIMS, CEDIA, and HS-CEDIA immunoassays are inadequately sensitive for detection of benzodiazepines in urine from patients treated for chronic pain. Pain Physician 2014;174:359–66. [PubMed] [Google Scholar]
- 78. Smith ML, Hughes RO, Levine B, et al. Forensic drug testing for opiates. VI. Urine testing for hydromorphone, hydrocodone, oxymorphone, and oxycodone with commercial opiate immunoassays and gas chromatography-mass spectrometry. J Anal Toxicol 1995;191:18–26. 10.1093/jat/19.1.18 [DOI] [PubMed] [Google Scholar]
- 79. McMillin GA, Marin SJ, Johnson-Davis KL, Lawlor BG, Strathmann FG.. A hybrid approach to urine drug testing using high-resolution mass spectrometry and select immunoassays. Am J Clin Pathol 2015;1432:234–40. [DOI] [PubMed] [Google Scholar]
- 80. Gilbert JW, Wheeler GR, Mick GE, et al. Urine drug testing in the treatment of chronic noncancer pain in a Kentucky private neuroscience practice: The potential effect of Medicare benefit changes in Kentucky. Pain Physician 2010;13:187–94. [PubMed] [Google Scholar]
- 81. Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). 2014. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf (accessed March 2017).
- 82. Hughes A, Williams MR, Lipari RN, et al. Prescription drug use and misuse in the United States: Results from the 2015 National Survey on Drug Use and Health. NSDUH Data Review. 2016. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR2-2015/NSDUH-FFR2-2015.htm (accessed March 2017).
- 83. Federation of State Medical Boards. Guidelines for the chronic use of opioid analgesics. 2017. Available at: https://www.fsmb.org/Media/Default/PDF/Advocacy/Opioid%20Guidelines%20As%20Adopted%20April%202017_FINAL.pdf (accessed June 2017).
- 84. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: Prediction and identification of aberrant drug-related behaviors: A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009;102:131–46. 10.1016/j.jpain.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 85. Belgrade MJ, Schamber CD, Lindgren BR.. The DIRE score: Predicting outcomes of opioid prescribing for chronic pain. J Pain 2006;79:671–81. 10.1016/j.jpain.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 86. Passik SD, Kirsh KL, Whitcomb L, et al. A new tool to assess and document pain outcomes in chronic pain patients receiving opioid therapy. Clin Ther 2004;264:552–61. 10.1016/S0149-2918(04)90057-4 [DOI] [PubMed] [Google Scholar]
- 87. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part I–evidence assessment. Pain Physician 2012;15:S1–65. [PubMed] [Google Scholar]
- 88. Couwenbergh C, Van Der Gaag RJ, Koeter M, De Ruiter C, Van den Brink W.. Screening for substance abuse among adolescents validity of the CAGE-AID in youth mental health care. Subst Use Misuse 2009;446:823–34. [DOI] [PubMed] [Google Scholar]
- 89. Brown RL, Rounds LA.. Conjoint screening questionnaires for alcohol and other drug abuse: Criterion validity in a primary care practice. Wis Med J 1995;94:135–40. [PubMed] [Google Scholar]
- 90. Wu SM, Compton P, Bolus R, et al. The addiction behaviors checklist: Validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage 2006;324:342–51. 10.1016/j.jpainsymman.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 91. Gross R, Long S, Cox S.. Predicting opioid misuse with a brief screener of catastrophizing (abstract 197). J Pain 2016;174:S25. [Google Scholar]
- 92. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med 2015;168:1566–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R.. A single-question screening test for drug use in primary care. Arch Intern Med 2010;17013:1155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL.. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med 2017;191:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Volkow ND, McLellan AT.. Opioid abuse in chronic pain–misconceptions and mitigation strategies. N Engl J Med 2016;37413:1253–63. 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- 96. Jamison RN, Martel MO, Edwards RR, et al. Validation of a brief Opioid Compliance Checklist for patients with chronic pain. J Pain 2014;1511:1092–101. 10.1016/j.jpain.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Turk DC, Swanson KS, Gatchel RJ.. Predicting opioid misuse by chronic pain patients: A systematic review and literature synthesis. Clin J Pain 2008;246:497–508. 10.1097/AJP.0b013e31816b1070 [DOI] [PubMed] [Google Scholar]
- 98. Jones T, Moore T, Levy JL, et al. A comparison of various risk screening methods in predicting discharge from opioid treatment. Clin J Pain 2012;282:93–100. 10.1097/AJP.0b013e318225da9e [DOI] [PubMed] [Google Scholar]
- 99. Atluri S, Akbik H, Sudarshan G.. Prevention of opioid abuse in chronic non-cancer pain: An algorithmic, evidence based approach. Pain Physician 2012;15(suppl 3):ES177–89. [PubMed] [Google Scholar]
- 100. Katz N, Fanciullo GJ.. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain 2002;18(suppl 4):S76–82. [DOI] [PubMed] [Google Scholar]
- 101. Hamill-Ruth RJ, Larriviere K, McMasters MG.. Addition of objective data to identify risk for medication misuse and abuse: The inconsistency score. Pain Med 2013;1412:1900–7. 10.1111/pme.12221 [DOI] [PubMed] [Google Scholar]
- 102. Cheatle MD, O’Brien CP, Mathai K, et al. Aberrant behaviors in a primary care-based cohort of patients with chronic pain identified as misusing prescription opioids. J Opioid Manag 2013;95:315–24. [DOI] [PubMed] [Google Scholar]
- 103. Rice JB, White AG, Birnbaum HG, et al. A model to identify patients at risk for prescription opioid abuse, dependence, and misuse. Pain Med 2012;139:1162–73. 10.1111/j.1526-4637.2012.01450.x [DOI] [PubMed] [Google Scholar]
- 104. Webster LR, Webster RM.. Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the opioid risk tool. Pain Med 2005;66:432–42. 10.1111/j.1526-4637.2005.00072.x [DOI] [PubMed] [Google Scholar]
- 105. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR.. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med 2012;104:304–11. 10.1370/afm.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Minutillo A, Pacifici R, Scaravelli G, et al. Gender disparity in addiction: An Italian epidemiological sketch. Ann Ist Super Sanita 2016;522:176–83. [DOI] [PubMed] [Google Scholar]
- 107. Tsui JI, Anderson BJ, Strong DR, Stein MD.. Craving predicts opioid use in opioid-dependent patients initiating buprenorphine treatment: A longitudinal study. Am J Drug Alcohol Abuse 2014;402:163–9. 10.3109/00952990.2013.848875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Meltzer EC, Rybin D, Meshesha LZ, et al. Aberrant drug-related behaviors: Unsystematic documentation does not identify prescription drug use disorder. Pain Med 2012;1311:1436–43. 10.1111/j.1526-4637.2012.01497.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Passik SD, Kirsh KL, Donaghy KB, Portenoy RK.. Pain and aberrant drug-related behaviors in medically ill patients with and without histories of substance abuse. Clin J Pain 2006;222:173–81. 10.1097/01.ajp.0000161525.48245.aa [DOI] [PubMed] [Google Scholar]
- 110. Savage SR, Joranson DE, Covington EC, et al. Definitions related to the medical use of opioids: Evolution towards universal agreement. J Pain Symptom Manage 2003;261:655–67. 10.1016/S0885-3924(03)00219-7 [DOI] [PubMed] [Google Scholar]
- 111. Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R.. Primary care validation of a single-question alcohol screening test. J Gen Intern Med 2009;247:783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. National Alliance for Model State Drug Laws. 2015 annual review of prescription monitoring programs. 2015. Available at: http://www.namsdl.org/library/1810E284-A0D7-D440-C3A9A0560A1115D7/ (accessed October 2017).
- 113. Prescription Drug Monitoring Program Training and Technical Assistance Center. State profiles. Available at: http://www.pdmpassist.org/content/state-profiles (accessed October 2017).
- 114. Missouri Governor. Executive order 17-18. 2017. Available at: https://www.sos.mo.gov/library/reference/orders/2017/eo18 (accessed October 2017).
- 115. GovTrack.us. S. 524 (114th): Comprehensive addiction and recovery Act of 2016. Summary. 2016. Available at: https://www.govtrack.us/congress/bills/114/s524/summary (accessed September 2016).
- 116. Yee DA, Hughes MM, Guo AY, et al. Observation of improved adherence with frequent urine drug testing in patients with pain. J Opioid Manag 2014;102:111–8. 10.5055/jom.2014.0200 [DOI] [PubMed] [Google Scholar]
- 117. Manchikanti L, Manchukonda R, Pampati V, et al. Does random urine drug testing reduce illicit drug use in chronic pain patients receiving opioids? Pain Physician 2006;9:123–9. [PubMed] [Google Scholar]
- 118. Bujold E, Huff J, Staton EW, Pace WD.. Improving use of narcotics for nonmalignant chronic pain: A lesson from Community Care of North Carolina. J Opioid Manag 2012;86:363–7. 10.5055/jom.2012.0136 [DOI] [PubMed] [Google Scholar]
- 119. Wiedemer NL, Harden PS, Arndt IO, Gallagher RM.. The opioid renewal clinic: A primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med 2007;87:573–84. [DOI] [PubMed] [Google Scholar]
- 120. Krishnamurthy P, Ranganathan G, Williams C, Doulatram G.. Impact of urine drug screening on no shows and dropouts among chronic pain patients: A propensity-matched cohort study. Pain Physician 2016;19:89–100. [PubMed] [Google Scholar]
- 121. Gaither JR, Goulet JL, Becker WC, et al. The association between receipt of guideline-concordant long-term opioid therapy and all-cause mortality. J Gen Intern Med 2016;315:492–501. 10.1007/s11606-015-3571-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Turner JA, Saunders K, Shortreed SM, et al. Chronic opioid therapy risk reduction initiative: Impact on urine drug testing rates and results. J Gen Intern Med 2014;292:305–11. 10.1007/s11606-013-2651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Melanson SE, Kredlow MI, Jarolim P.. Analysis and interpretation of drug testing results from patients on chronic pain therapy: A clinical laboratory perspective. Clin Chem Lab Med 2009;47:971–6. [DOI] [PubMed] [Google Scholar]
- 124. Benzon HT, Kendall MC, Katz JA, et al. Prescription patterns of pain medicine physicians. Pain Pract 2013;136:440–50. 10.1111/papr.12011 [DOI] [PubMed] [Google Scholar]
- 125. Gourlay DL, Heit HA, Almahrezi A.. Universal precautions in pain medicine: A rational approach to the treatment of chronic pain. Pain Med 2005;62:107–12. 10.1111/j.1526-4637.2005.05031.x [DOI] [PubMed] [Google Scholar]
- 126. Smith HS. The metabolism of opioid agents and the clinical impact of their active metabolites. Clin J Pain 2011;279:824–38. 10.1097/AJP.0b013e31821d8ac1 [DOI] [PubMed] [Google Scholar]
- 127. FDA. New safety measures announced for opioid analgesics, prescription opioid cough products, and benzodiazepines. 2016. Available at: https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm518110.htm (accessed May 2017).
- 128. Reisfield GM, Goldberger BA, Pesce AJ, et al. Ethyl glucuronide, ethyl sulfate, and ethanol in urine after intensive exposure to high ethanol content mouthwash. J Anal Toxicol 2011;355:264–8. 10.1093/anatox/35.5.264 [DOI] [PubMed] [Google Scholar]
- 129. McNeely J, Cleland CM, Strauss SM, et al. Validation of self-administered single-item screening questions (SISQs) for unhealthy alcohol and drug use in primary care patients. J Gen Intern Med 2015;3012:1757–64. 10.1007/s11606-015-3391-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Saitz R, Cheng DM, Allensworth-Davies D, Winter MR, Smith PC.. The ability of single screening questions for unhealthy alcohol and other drug use to identify substance dependence in primary care. J Stud Alcohol Drugs 2014;751:153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2–guidance. Pain Physician 2012;15:S67–116. [PubMed] [Google Scholar]
- 132. Hood G. Regulatorily mandated urine drug testing: Marijuana, other consequences. 2012. Available at: http://boards.medscape.com/forums/? 128@@.2a355be7! comment=1 (accessed August 2016).
- 133. Wallace M, Furnish T.. What steps should be taken to integrate marijuana into pain regimens? Pain Manag 2015;54:225–7. [DOI] [PubMed] [Google Scholar]
- 134. Davis JM, Mendelson B, Berkes JJ, et al. Public health effects of medical marijuana legalization in Colorado. Am J Prev Med 2016;503:373–9. 10.1016/j.amepre.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Barker L, Hall K, Van Dyke M; Retail Marijuana Public Health Advisory Committee. Monitoring possible marijuana related health effects: Summary and key findings. 2015. Available at: https://drive.google.com/drive/folders/0BxqXhstk92DbV2VxZzVhWjJCd00 (accessed September 2016).
- 136. Salomonsen-Sautel S, Min SJ, Sakai JT, Thurstone C, Hopfer C.. Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend 2014;140:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Reisfield GM, Wasan AD, Jamison RN.. The prevalence and significance of cannabis use in patients prescribed chronic opioid therapy: A review of the extant literature. Pain Med 2009;108:1434–41. 10.1111/j.1526-4637.2009.00726.x [DOI] [PubMed] [Google Scholar]
- 138. Kronstrand R, Brinkhagen L, Birath-Karlsson C, Roman M, Josefsson M.. LC-QTOF-MS as a superior strategy to immunoassay for the comprehensive analysis of synthetic cannabinoids in urine. Anal Bioanal Chem 2014;40615:3599–609. [DOI] [PubMed] [Google Scholar]
- 139. Marcoux RM, Larrat EP, Vogenberg FR.. Medical marijuana and related legal aspects. P T 2013;3810:612–9. [PMC free article] [PubMed] [Google Scholar]
- 140. Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: A systematic review. Ann Intern Med 2017;1675:319–31. [DOI] [PubMed] [Google Scholar]
- 141. Leung L. Cannabis and its derivatives: Review of medical use. J Am Board Fam Med 2011;244:452–62. 10.3122/jabfm.2011.04.100280 [DOI] [PubMed] [Google Scholar]
- 142. Borgelt LM, Franson KL, Nussbaum AM, Wang GS.. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 2013;332:195–209. 10.1002/phar.1187 [DOI] [PubMed] [Google Scholar]
- 143. Cooper ZD. Adverse effects of synthetic cannabinoids: Management of acute toxicity and withdrawal. Curr Psychiatry Rep 2016;185:52.. 10.1007/s11920-016-0694-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yamaori S, Okamoto Y, Yamamoto I, Watanabe K.. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos 2011;3911:2049–56. 10.1124/dmd.111.041384 [DOI] [PubMed] [Google Scholar]
- 145. Monte AA, Heard KJ, Campbell J, et al. The effect of CYP2D6 drug-drug interactions on hydrocodone effectiveness. Acad Emerg Med 2014;218:879–85. 10.1111/acem.12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Barth KS, Becker WC, Wiedemer NL, et al. Association between urine drug test results and treatment outcome in high-risk chronic pain patients on opioids. J Addict Med 2010;43:167–73. 10.1097/ADM.0b013e3181c379ed [DOI] [PubMed] [Google Scholar]
- 147. Meghani SH, Wiedemer NL, Becker WC, Gracely EJ, Gallagher RM.. Predictors of resolution of aberrant drug behavior in chronic pain patients treated in a structured opioid risk management program. Pain Med 2009;105:858–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.