Abstract
Background
Sub-Saharan Africa suffers from a dual burden of infectious and non-communicable diseases. There is limited data on causes and trends of admission and death among patients on the medical wards. Understanding the major drivers of morbidity and mortality would help inform health systems improvements. We determined the causes and trends of admission and mortality among patients admitted to Mulago Hospital, Kampala, Uganda.
Methods and results
The medical record data base of patients admitted to Mulago Hospital adult medical wards from January 2011 to December 2014 were queried. A detailed history, physical examination and investigations were completed to confirm the diagnosis and identify comorbidities. Any histopathologic diagnoses were made by hematoxylin and eosin tissue staining. We identified the 10 commonest causes of hospitalization, and used Poisson regression to generate annual percentage change to describe the trends in causes of hospitalization. Survival was calculated from the date of admission to the date of death or date of discharge. Cox survival analysis was used to identify factors associate with in-hospital mortality. We used a statistical significance level of p<0.05. A total of 50,624 patients were hospitalized with a median age of 38 (range 13–122) years and 51.7% females. Majority of patients (72%) had an NCD condition as the primary reason for admission. Specific leading causes of morbidity were HIV/AIDS in 30% patients, hypertension in 14%, tuberculosis (TB) in 12%), non-TB pneumonia in11%) and heart failure in 9.3%. There was decline in the proportion of hospitalization due to malaria, TB and pneumonia with an annual percentage change (apc) of -20% to -6% (all p<0.03) with an increase in proportions of admissions due to chronic kidney disease, hypertension, stroke and cancer, with apc 13.4% to 24%(p<0.001). Overall, 8,637(17.1%) died during hospitalization with the highest case fatality rates from non-TB pneumonia (28.8%), TB (27.1%), stroke (26.8%), cancer (26.1%) and HIV/AIDS (25%). HIV-status, age above 50yrs and being male were associated with increased risk of death among patients with infections.
Conclusion
Admissions and case fatality rates for both infectious and non-infectious diseases were high, with declining trends in infectious diseases and a rising trend in NCDs. Health care systems in sub-Saharan region need to prepare to deal with dual burden of disease.
Introduction
The disease burden in developing countries is continuing to grow faster than the budget allocation for healthcare. In low income countries (LIC) medical admissions account for about 40% of total hospital admissions compared to 12–30% in high income countries[1,2]. This may be a reflection of disparities in socioeconomic conditions and healthcare systems, or differences in biological and/or environmental factors[3–5]. One factor which exacerbated the healthcare crisis in sub-Saharan Africa (SSA) was the advent of the HIV/AIDS epidemic which disproportionately affects the region [2,6,7]. Interventions, such as antiretroviral therapy for the treatment of HIV/AIDS, health education, access to clean water, and mass vaccinations, however, have decreased morbidity and mortality from communicable diseases[8]. The improved life expectancy and adoption of western lifestyles in LICs have led to the emergence of non-communicable diseases (NCDs), a new threat to public health[9,10].
In Uganda as with other sub-Saharan African countries, there is an emerging increase in NCDs such as hypertension and diabetes mellitus, impacting the health of patients with and without HIV[11–14]. However, as modeling studies show, malaria and lower respiratory infections are the leading causes of years of life lost[15]. Despite the growing evidence of declining trends of HIV in the community[16], anecdotal observations in Mulago hospital indicate that HIV-AIDS is still the leading cause of morbidity and mortality on medical wards in Uganda[17]. Few studies have examined the major causes of morbidity and mortality in Uganda and most of them have focused on individual diseases[17–20]. An autopsy study in a predominantly HIV-infected population on Mulago hospital carried out on 2 medical ward units only (Infectious diseases and Gastro-intestinal) noted that the leading cause of death among HIV-infected patients were tuberculosis and cryptococcal meningitis[21]. In contrast, in patients without HIV infection, the leading causes of death were non-infectious, with upper gastrointestinal bleeding secondary to liver cirrhosis being the most common[21]. Since 2007, Mulago Hospital has been operating specialty-based in-patient services run by sub-specialist physicians. There is however, limited data on trends in the incidence of specific diseases, as well as morbidity and mortality in these specialized units. Understanding the major drivers of morbidity and mortality in one of the largest hospitals in the region would help inform health systems improvements for Uganda and the East African region[22].
We therefore sought to describe the leading causes of hospitalization and death on the medical wards among adult patients in Mulago National Referral Hospital, Kampala, Uganda.
Materials and methods
We conducted a retrospective cohort study of patients admitted to Mulago National Referral Hospital adult medical wards from January 2011 to December 2014 using an electronic patient data registry. Mulago National Referral Hospital is one of the two national referral hospitals in a country with a population close to 40 million. It has 1500 bed-capacity and provides specialized care for patients referred from district and regional referral hospitals. In 2007, the Directorate of Medicine established sub-specialist run units to cater to the increasing specialized care needs. Consultants, physicians and senior house officers run these units. Patients undergo preliminary evaluation and investigations in the accident and emergency unit before admission to the appropriate specialized unit. On average, 15,000 emergency medical visits are made to Mulago National Referral Hospital annually and between 900–1200 patients are admitted to the medical wards per month[17].
Each patient receives a detailed history, physical examination and investigations are completed to confirm the diagnosis and identify comorbidities. Investigations performed include a complete blood count, HIV testing, chemistry, abdominal ultrasound, chest x-ray, sputum gene-expert, bone marrow biopsy, lumbar puncture, echocardiogram, and electrocardiogram as clinically indicated. Any histopathologic diagnoses were made by hematoxylin and eosin tissue staining.
In 2010, a patient registry was established in the Department of Medicine through the Rainer Arnhold Senior House Officers’ Teaching Support (RASHOTS) project. The purpose of the database was to support evaluation and improvements in quality of patient care and training for postgraduate students of Makerere University College of Health Sciences.
Three trained medical records clerks capture information on patient’s unique number, age, gender, district of residence, diagnosis, and vital status from the patient files at discharge. They enter this information directly into a password protected access registry. The diagnosis captured by the data clerks is the final diagnosis in the chart at the time of discharge or death. Diagnosis is coded based on the tenth revised International Classification of Diseases (ICD-10) [23]. Age groups were defined based on the World Health Organization "Provisional guidelines on standard international age classification” available at https://unstats.un.org/unsd/publications/. We used the ward/ service of admission as proxy for the reason for admission. Patients on cardiology, nephrology, neurology, and hematology were considered to have non-communicable disease (NCD) as the primary reason for admission whereas patients on infectious diseases wards and pulmonology (admits primarily pneumonia and TB patients) were considered to have an infectious disease (ID) as the primary reason for admission.
For this study, de-identified patient information was exported into excel using a standardized approach. We excluded cases with missing information on diagnosis and date of admission. Summary statistics were reported using medians and ranges for continuous variables and frequency counts and percentages for categorical variables. We identified the 10 commonest causes of hospitalization, and used Poisson regression to generate annual percentage change to describe the trends in causes of hospitalization. We used Pearson chi-square test for categorical variable and Wilcoxon rank-sum test for continuous variables to assess association between variables. Survival was calculated from the date of admission to the date of death or date of discharge. Cox survival analysis was used to identify factors associate with 30-day mortality. We used a statistical significance level of p<0.05. All analyses were performed using STATA version 13 (STATA Corp, College Station, TX).
The study was approved and received a waiver of consent from the Makerere University School of Medicine Research Ethics Committee and Uganda National Council of Science and Technology (UNCST).
Results
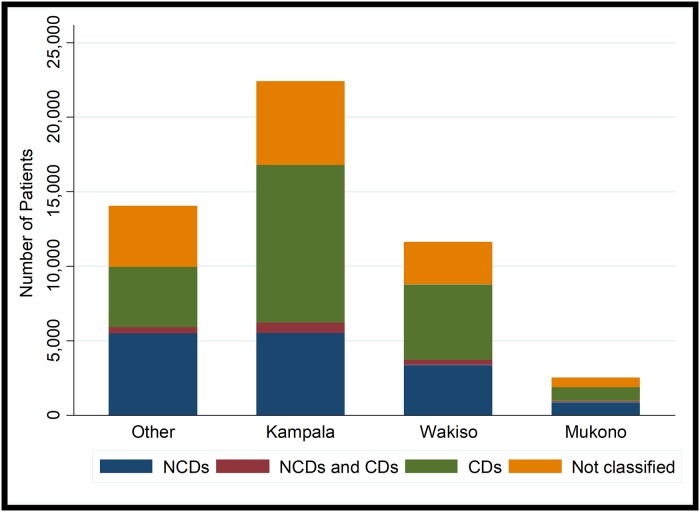
During the study period, there were 50,716 patient hospitalizations. We excluded 92 for lack of complete admission date. Of the remaining 50,624 patients, 23% were admitted in 2011, 27.2% in 2012, 25.7% in 2013, and 24.1% in 2014, 51.7% of these patients were female. The median age was 38 years (range13-122) About two thirds of patients were residents of urban and periurban areas (Kampala Capital City and its suburbs) (Fig 1 and Table 1).
Fig 1. Distribution of diagnoses by district of residence among patients admitted to Mulago Hospital.
Abbreviations: CDs-Communicable disease; NCDs-Non-communicable disease.
Table 1. Patient characteristics overall and by year of hospitalization at Mulago Hospital.
| Characteristic | Overall (N = 50,624) |
2011 (N = 11,637) |
2012 (N = 13,761) |
2013 (N = 13,028) |
2014 (N = 12,200) |
P value |
|---|---|---|---|---|---|---|
| Age, median(range) | 38(13–122) | 38(13–122) | 37(13–122) | 38(13–110) | 40(133–120) | 0.001 |
| Age group, n (%) | ||||||
| 13–24 | 9,135(18.0) | 2,126(18.3) | 2,567(18.7) | 2,393(18.4) | 2,049(16.8) | 0.001 |
| 25–54 | 28,866(57.0) | 6,619(56.9) | 8,055(58.5) | 7,322(56.2) | 6.870(56.3) | |
| 55–74 | 8,574(16.9) | 2,037(17.5) | 2,141(15.6) | 2,230(17.1) | 2,166(17.8) | |
| >74 | 3,716(7.3) | 771(6.6) | 925(6.7) | 985(7.6) | 1,035(8.5) | |
| Female, n (%) | 26,175(51.7) | 6008(51.7) | 7,134(51.9) | 6,709(51.5) | 6,324(51.9) | 0.9 |
| Urban residence* | 36,469(72.0%) | 7,978(68.6) | 10,100(73.4) | 9,497(72.9) | 8,894(72.9) | 0.001 |
*Urban–included Kampala City and its suburbs (Wakiso and Mukono districts)
Patients in the age bracket of 25–54 years accounted for over half of the admissions overall and across the years studied. Patients admitted in the later years of the study were significantly older compared to the earlier years of the study (p = 0.001). There was no difference in gender distribution of patients across the study period (0.11).
The primary reason for admission was an NCD condition in 62% of patients. Patients on the NCD wards were significantly older than patients on the ID wards (median age 42 vs 35 years, p<0.001), and more likely to be female (53% vs 49%, p<0.001). The leading NCDs included hypertension in 14.2% of the patient population, diabetes in 7%, heart failure in 9.3%, stroke in 4.9%, anemia in 7%, and cancer in 3.9%. Similarly, the leading infectious disease conditions included HIV in 30.4% of patients, tuberculosis (TB) in 13.1%, non-TB pneumonia in 11.1%, malaria in 6.9%, gastroenteritis in 4 and sepsis in 4.26%. HIV co-infection was 14.2% on the NCD wards compared to 57.6% on the ID wards. The commonest AIDS defining conditions included tuberculosis in 31.6% of patients with HIV, cryptococcal meningitis in 7.7%, KS in 2.1%, and PCP in 1.5%.
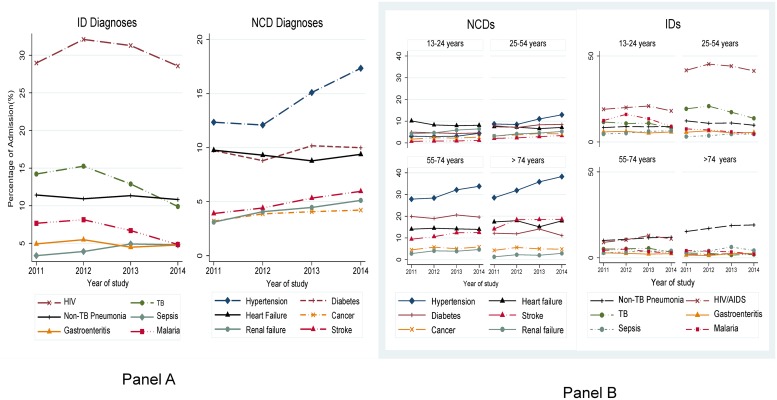
There was a general downward trend in communicable disease diagnoses and an upward trend in in NCD diagnoses over the study period (Fig 2 panel A). There was a significant decline in the proportion of patients with a final discharge diagnosis of malaria with an apc of -20% (95% CI: 0.71–0.91, p = 0.001), TB with an apc -12% (95% CI: 0.78–0.98, p = 0.03), and pneumonia with an apc of -6% (95% CI: 0.89–0.99, p = 0.02). In contrast, there was a significant increase in the proportion of patients with a final discharge diagnosis of CKD (apc = 24%, 95% CI:1.18–1.31, p<0.001), hypertension (apc = 14% (95% CI:1.11–1.16, p <0.001), stroke (apc = 13.4%(95% CI: 1.09–1.18), cancer (apc = 10%, 95% CI: 1.06–1.15, p<0.001), and heart failure (apc = 6%, 95% CI:1.04–1.09, p<0.001). However, there was no significant change in the number of patients with admission diagnosis of HIV/AIDS (p = 0.8) and diabetes mellitus (p = 0.09) (Fig 2 panel B).
Fig 2. Trends in major causes of morbidity by age group and calendar year at Mulago Hospital, 2011–2014.
Panel A shows trends of communicable and non-communicable diseases through the four years of study. Panel B shows the major causes of death by age group through the four years of study. Abbreviations: CDs-Communicable disease; NCDs-Non-communicable disease.
The distribution of these common conditions was markedly different among the four age groups is shown in Fig 2. NCDs predominated in the older age groups (above 54 years) compared to IDs in younger age groups (54 and below). Among those aged 25–54 years the prevalence of HIV was 43.2% compared to 10.9% in those 55–74 years of age; Tb was at 17.9% compared to 4.8%. On the contrary hypertension was commoner in those above 54 years of age (30% in 55–74 years and 34% in those 75 years and above) compared to 10.2% among those 25–54 years of age. The highest median age was 64 years (IQR: 50–75) among stroke patients and 57 years (IQR: 43–70) for hypertension, and the lowest median age was 30 years (IQR: 21–42) for malaria. Across the entire population, HIV infected patients were younger than those who were HIV negative (median age 41 years [IQR: 27–60] vs 35years [28–42], p <0.001).
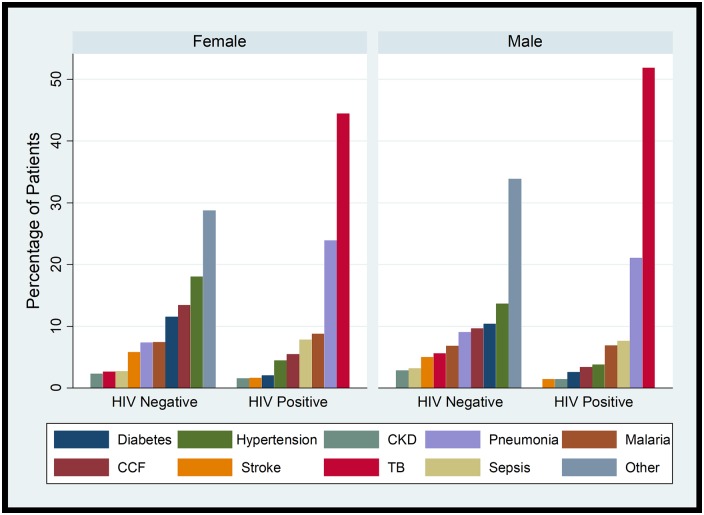
Women constituted the majority of patients for all diagnoses except for TB (44%) and cancer (43.1%) and constituted about half for non-TB pneumonia (50%), and CKD (52.2%). Additionally, there was a greater proportion of HIV-infected women than men (50.1% vs 54%).
HIV comorbidity was highest among patients with discharge diagnoses of TB (72.9%) and non-TB pneumonia (38.4%), and lowest among patients with discharge diagnoses of diabetes (4.5%) and hypertension (5.5%) (Fig 3).
Fig 3. Distribution of discharge diagnoses by gender and HIV status among patients in Mulago Hospital.
Abbreviations: CCF: Congestive cardiac failure; CKD-chronic kidney disease; TB-tuberculosis.
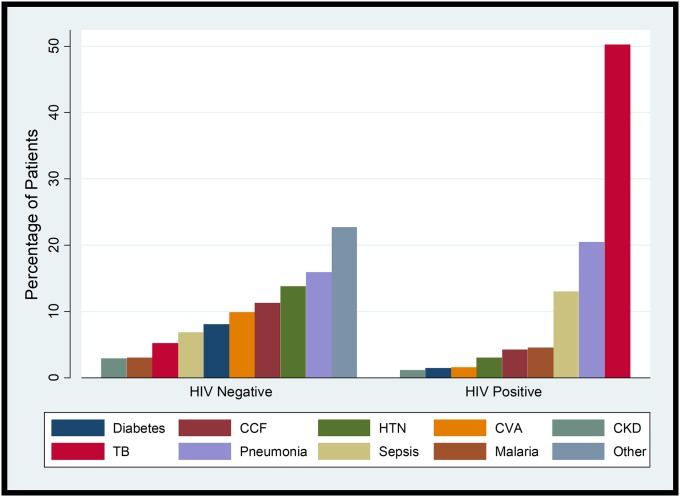
Overall 8,637(17.1%) died during hospitalization; the proportion of patients who died during hospitalization increased over the period from 13.1% in 2011, 18% in 2012, 18.7% in 2013 and 18.3% in 2014. Among the patients who died during their hospitalization, the final diagnoses listed in the chart included HIV/AIDS in 44.5%, TB in 19.7%, non-TB pneumonia in 17.9%, sepsis in 9%, malaria in 3.6%, hypertensions in 10.8%, diabetes in 6.2%, heart failure in 9.4%, CVA in 7.6%, CKD in 2.45%, and cancer in 6.4% (Fig 4).
Fig 4. Distribution by HIV Serostatus of discharge diagnoses among patients who died during hospitalization.
Abbreviations: CCF-Congestive cardiac failure; CKD-chronic kidney disease; CVA-cardiovascular accident; HTN-Hypertension; TB-tuberculosis.
In multivariate analysis, age, gender, HIV status, and district of residence were all associated with in-hospital mortality. Increasing age was significantly associated with increased risk of in-hospital mortality, with an increase of 7% among patients 31–60 years of age and 46% among patients with >60 years of age, compared to those less than 30 years of age(p = 0.01). Similarly, males had 23% increased risk of death compared to females (p <0.001). Patients with HIV had a 68% increased risk of death compared to their HIV negative counterparts (p<0.001). Compared to the baseline year (2011), the risk of death was significantly higher in all subsequent years–increased by 24% in 2012, 28% in 2013, and 30% in 2014 (Table 2). Patients with HIV where more likely to have non-TB pneumonia than their none HIV-infected counterparts irrespective of sex.
Table 2. Factors associated with. in-hospital mortality among patients admitted to Mulago Hospital.
| Characteristic | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR(95% CI) | p | HR(95% CI) | p | |
| Age category | ||||
| 11–30 | ref | ref | ||
| 31–50 | 1.12(1.07–1.18) | < 0.001 | 1.07(1.02–1.23) | 0.001 |
| >50 | 1.21(1.13–1.29) | < 0.001 | 1.46(1.37–1.56) | < 0.001 |
| Gender, n (%) | ||||
| Female | ref | ref | ||
| Male | 1.21(1.15–1.26) | < 0.001 | 1.23 (1.17–1.28) | < 0.001 |
| District of residence | ||||
| Others | ref | ref | ||
| Kampala | 1.10(1.04–1.16) | 0.001 | 1.05(0.99–1.11) | 0.08 |
| Wakiso | 1.19(1.12–1.27) | <0.001 | 1.13(1.06–1.20) | <0.001 |
| Mukono | 1.35(1.23–1.49) | <0.001 | 1.30(1.17–1.43) | <0.01 |
| Year of hospitalization | ||||
| 2011 | ref | ref | ||
| 2012 | 1.10(1.04–1.16) | < 0.001 | 1.24(1.16–1.33) | < 0.001 |
| 2013 | 1.19(1.12–1.27) | < 0.001 | 1.29(1.20–1.38) | < 0.001 |
| 2014 | 1.35(1.23–1.49) | P < 0.001 | 1.30(1.22–1.40) | P < 0.001 |
| HIV-coinfection, n (%) | ||||
| Negative | Ref | ref | ||
| Positive | 1.55(1.48–1.62) | < 0.001 | 1.68(1.60–1.76) | < 0.001 |
Discussion
In this retrospective cohort study of patients admitted to Mulago Hospital adult medical wards we found the leading diagnoses among hospitalized patients to be infections with HIV-AIDS, Tuberculosis and Malaria. The leading NCD diagnoses among hospitalized patients were hypertension, heart failure and diabetes mellitus.
Overall, the leading final diagnoses among patients who died in the hospital were HIV-AIDS and Tuberculosis for infections, and hypertension and heart failure for NCDs. There was a decline in percentage of hospitalization from infectious diseases over the four years with proportional increase in non-communicable diseases. The case fatality rate for communicable diseases was highest in patients with final diagnoses of non-TB pneumonia, TB, and HIV-AIDS while among patients with NCD final diagnoses, the case fatality rates were highest in those with stroke, cancer and chronic kidney disease. In-hospital mortality rates increased over the four years of the study.
These findings are similar to what has been reported in other parts of Africa. A study from Cameroon found the leading cause of death among hospitalized patients was related to HIV-AIDS[24]. In Malawi, among 2,911 of patients admitted in Kamuzu Central Hospital medical wards, up to 81% were HIV positive. Being HIV-positive, antiretroviral therapy (ART)-naïve or being a new ART-initiator were associated with high risk of mortality compared to HIV-negative patients[25].
Another study from South Africa found a high mortality rate in patients admitted to the medical wards with an inpatient mortality of 11% with a 12-month mortality of 41%[26]. Age above 40 years and a urea level above 7.0mmol/l, a diagnosis of HIV, TB or sepsis were associated with poor outcomes at 12 months. These findings serve to show the large contribution of infections to mortality, particularly in LICs, as has been outlined in the global burden of disease mapping[15]. It is quite surprising that ‘the big three’ diseases that have received most attention across the globe still remain a major cause of morbidity in Uganda while global trends are showing a decline for HIV and malaria[27,28].
Though we found declining trends of admissions from infectious diseases compared to NCDs, this has not been the case in other countries. As an example, in one South African study, the rates of admission increased from 228 to 628 over a similar 2-month period in 10 years (1991 and 2002) with tuberculosis and lower respiratory tract infections being the leading causes of admissions[29]. The reduction in rates of admission could be mirroring the improved care and survival of patients with HIV and its associated complications due to increased access to care and presence of robust ambulatory health systems for care of these patients[22,30].
On the flipside, few studies have described morbidity and mortality from NCDs as a whole. In a systematic review and meta-analysis of studies of patients with heart failure in LIC, the major cause of heart failure was hypertension among patients from African countries compared to their counterparts from other LICs where the leading cause of heart failure was ischemic heart disease[31]. The current literature largely focuses on specific NCDs among admitted patients. In one study in central Ghana, NCDs were the leading cause of death among patients hospitalized with neurological disorders[32]. In that study, the commonest cause of admission was cardiovascular accidents found in 54% of patients, increasing age was associated with higher mortality with a hazard ratio of 1.11(CI95% 1.06–1.17) for each 20-year increase in age. In the sub-Saharan Africa Survey of Heart Failure (THESUS-HF) study looking at acute heart failure from 12 hospitals in 9 SSA countries, the main predictors of 180-day mortality were malignancy, severe lung disease, smoking history, systolic blood pressure, kidney dysfunction, anaemia, and being HIV-positive[33]. In Mbarara Hospital in Eastern Uganda, patients who were hospitalized with heart failure had a doubled risk of death compared to their counterparts with heart failure who were not hospitalized for heart failure exacerbations after 6 months of follow up[34]. Another study demonstrated that Glasgow coma score of less than 14 and renal failure were major predictors of mortality among admitted patients[35]. There are few studies looking at trends of admissions among patients admitted with NCDs. A study from Nigeria showed that in the medical wards, the number of admissions from NCDs was 2 times that of infectious diseases[36]. A systematic review of 86,307 admissions to medical wards in Africa suggests that although infections are still the leading cause of admissions, cardiovascular diseases increased by fivefold from 3.9% (1950–59) to 19.9% (2000–2010) RR 5.1 (95% CI 4.5–5.8, test for trend p<0.00005)[37]. This trend seems to be similar to our findings.
There is an increasing interest in the overlap between infections and non-communicable diseases with particular attention to HIV. Though the patients with final diagnoses of DM in our study had very low rates of HIV co-infection, there has been a great overlap between HIV, TB and diabetes from studies done in other parts of Africa[38]. Diabetes mellitus increases the risk of active TB regardless of study design and population[39]. Patients who have diabetes with TB have increased risk of adverse outcomes such as treatment failure, TB relapse as well as death[39,40]. This calls for combined efforts in fighting both NCDs and infections rather than dealing with them as separate diseases beginning with the ambulatory clinics[13,41–44].
In our population, non-TB pneumonia, TB, HIV-infection, stroke, cancer and chronic kidney diseases were the diagnoses with the highest case fatality rates. It is hard to explain why non-TB pneumonia has the highest case fatality rate among our patients. This may be due to the fact that patients report late to hospital and may need oxygen and respiratory support which is not readily available, coupled with high rates of antibiotic resistance and frequent drug stock-outs at the hospital[20,37,45,46]. That said, patients with stroke, cancer or kidney disease often report with advanced disease to the hospital and there is lack of advanced healthcare services available for their care and support[47–50]. Another reason may be HIV-associated infection. Non-TB pneumonia was higher in HIV-infected patients compared to HIV negative patients regardless of gender.
The in-hospital mortality rates increased over the four years in comparison to 2011. This is rather surprising and may need further exploration to look at the driving factors of mortality which may be beyond the scope of this study.
Our study had several strengths. First, we looked at all the patients admitted within the study period with only less than 0.1% failing to meet the inclusion criteria. Additionally, to our knowledge, this is the first study looking at admissions, discharge diagnoses, and trends in mortality over a large category of diseases in the largest hospital in Uganda. As a result, we have been able to demonstrate that, though infectious diseases are still the leading cause of morbidity and mortality on the medical wards, NCDs are rising as a major cause of admissions and death. The healthcare system needs to adopt to this new change and provide resources to ensure that the health care system as a whole is able to deal with this rising tide[51,52].
Our study also had several weaknesses. The retrospective nature of the study means limited access to other factors that may predict outcomes, such as vital and other clinical signs at the time of presentation to the hospital, as well as laboratory results. We used the primary diagnosis as was made by the treating team at discharge and did not capture deaths on arrival as well as re-admissions. We also acknowledge the limitation of confirming a diagnosis in our patient population. These factors may have underestimated the rate and contributions of other co-morbidities to illness and mortality. Co-morbidities were only identified if they were included on the discharge form. The diagnosis that occurred in lower proportions were not reflected as specific diagnoses. We were also not able to capture data on surgical causes of morbidity and mortality because these are admitted to the surgical wards independent of the medical wards of our study focus. Additionally, there are two private subspecialized units on the Mulago campus (the Uganda Heart Institute and the Uganda Cancer Institute) where patients with cardiovascular disease and cancer can be referred or directly admitted. As these units were not included in our data collection, this may have led to an under-estimation of these two diseases in our study. However, the RASHOTS team undertakes due diligence to ensure that all patients admitted to the hospital had their data captured to the greatest detail available.
Conclusion
We found the leading discharge diagnoses in a national referral hospital to be HIV-infection, hypertension, tuberculosis and heart failure. Among all of the characterized diagnoses, non-TB pneumonia, TB, stroke, cancer and chronic kidney disease had the highest case fatality rates. There is an increasing trend of NCDs as a major cause of admissions over the 4-year period of study. There is an urgent need for Ugandan health care system to increase the focus on early detection and management of NCDs, while still maintaining current efforts to manage infectious diseases.
Supporting information
(XLSB)
Acknowledgments
We would like to thank the patients who contributed data for the study. The research assistants of RASHOTS, Cissy Namakula, Mulago Hospital administration and the Department of Medicine for their support in conducting the study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
RK is a Rainer Arnhold Teaching Fellow sponsored by The Mulago Foundation-https://mulagofoundation.org. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ali E, Woldie M. Reasons and outcomes of admissions to the medical wards of jimma university specialized hospital, southwest ethiopia. Ethiopian journal of health sciences. 2010;20(2):113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marszalek J, Villiers DP. Morbidity profile of admissions to GF Jooste Hospital, Manenberg, Cape Town. South African Family Practice. 2006;48(6). [Google Scholar]
- 3.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences. 2010;1186:69–101. 10.1111/j.1749-6632.2009.05339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public health reports. 2014;129 Suppl 2:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braveman P. Health disparities and health equity: concepts and measurement. Annual review of public health. 2006;27:167–94. 10.1146/annurev.publhealth.27.021405.102103 [DOI] [PubMed] [Google Scholar]
- 6.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granich R, Gupta S, Hersh B, Williams B, Montaner J, Young B, et al. Trends in AIDS Deaths, New Infections and ART Coverage in the Top 30 Countries with the Highest AIDS Mortality Burden; 1990–2013. PloS one. 2015;10(7):e0131353 10.1371/journal.pone.0131353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009;374(9693):934–47. 10.1016/S0140-6736(09)61087-4 [DOI] [PubMed] [Google Scholar]
- 10.Alwan A, Maclean DR, Riley LM, d’Espaignet ET, Mathers CD, Stevens GA, et al. Monitoring and surveillance of chronic non-communicable diseases: progress and capacity in high-burden countries. Lancet. 2010;376(9755):1861–8. 10.1016/S0140-6736(10)61853-3 [DOI] [PubMed] [Google Scholar]
- 11.Guwatudde D, Mutungi G, Wesonga R, Kajjura R, Kasule H, Muwonge J, et al. The Epidemiology of Hypertension in Uganda: Findings from the National Non-Communicable Diseases Risk Factor Survey. PloS one. 2015;10(9):e0138991 10.1371/journal.pone.0138991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiwanga FS, Njelekela MA, Diamond MB, Bajunirwe F, Guwatudde D, Nankya-Mutyoba J, et al. Urban and rural prevalence of diabetes and pre-diabetes and risk factors associated with diabetes in Tanzania and Uganda. Global health action. 2016;9:31440 10.3402/gha.v9.31440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyesubula R, Kayongo A, Semitala FC, Muhanguzi A, Katantazi N, Ayers D, et al. Trends and level of control of hypertension among adults attending an ambulatory HIV clinic in Kampala, Uganda: a retrospective study. BMJ global health. 2016;1(3):e000055 10.1136/bmjgh-2016-000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes MD, Dalal S, Volmink J, Adebamowo CA, Njelekela M, Fawzi WW, et al. Non-communicable diseases in sub-Saharan Africa: the case for cohort studies. PLoS medicine. 2010;7(5):e1000244 10.1371/journal.pmed.1000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DALYs GBD, Collaborators H, Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–91. 10.1016/S0140-6736(15)61340-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabowski MK, Serwadda DM, Gray RH, Nakigozi G, Kigozi G, Kagaayi J, et al. HIV Prevention Efforts and Incidence of HIV in Uganda. The New England journal of medicine. 2017;377(22):2154–66. 10.1056/NEJMoa1702150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Namutebi AM, Kamya MR, Byakika-Kibwika P. Causes and outcome of hospitalization among HIV-infected adults receiving antiretroviral therapy in Mulago hospital, Uganda. African health sciences. 2013;13(4):977–85. 10.4314/ahs.v13i4.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okello E, Longenecker CT, Beaton A, Kamya MR, Lwabi P. Rheumatic heart disease in Uganda: predictors of morbidity and mortality one year after presentation. BMC cardiovascular disorders. 2017;17(1):20 10.1186/s12872-016-0451-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagasha P, Nakwagala F, Kwizera A, Ssekasanvu E, Kalyesubula R. Acute kidney injury among adult patients with sepsis in a low-income country: clinical patterns and short-term outcomes. BMC nephrology. 2015;16:4 10.1186/1471-2369-16-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob ST, Moore CC, Banura P, Pinkerton R, Meya D, Opendi P, et al. Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PloS one. 2009;4(11):e7782 10.1371/journal.pone.0007782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox JA, Lukande RL, Nelson AM, Mayanja-Kizza H, Colebunders R, Van Marck E, et al. An autopsy study describing causes of death and comparing clinico-pathological findings among hospitalized patients in Kampala, Uganda. PloS one. 2012;7(3):e33685 10.1371/journal.pone.0033685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durrani H. Healthcare and healthcare systems: inspiring progress and future prospects. mHealth. 2016;2:3 10.3978/j.issn.2306-9740.2016.01.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngene NC, Moodley J. Assigning appropriate and comprehensive diagnosis for scientific report. Medical hypotheses. 2014;83(6):681–4. 10.1016/j.mehy.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 24.Mbuagbaw J, Jingi AM, Noubiap JJ, Kaze AD, Nansseu JR, Bigna JJ, et al. Patterns and trends in mortality among HIV-infected and HIV-uninfected patients in a major Internal Medicine Unit in Yaounde, Cameroon: a retrospective cohort study. JRSM open. 2016;7(9):2054270416654859 10.1177/2054270416654859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma S, Huang J, Song X. Integrative analysis and variable selection with multiple high-dimensional data sets. Biostatistics. 2011;12(4):763–75. 10.1093/biostatistics/kxr004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart-Clark H, Vorajee N, Zuma S, Van Niekerk L, Burch V, Raubenheimer P, et al. Twelve-month outcomes of patients admitted to the acute general medical service at Groote Schuur Hospital. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2012;102(6):549–53. [DOI] [PubMed] [Google Scholar]
- 27.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–70. 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luboga SA, Stover B, Lim TW, Makumbi F, Kiwanuka N, Lubega F, et al. Did PEPFAR investments result in health system strengthening? A retrospective longitudinal study measuring non-HIV health service utilization at the district level. Health policy and planning. 2016;31(7):897–909. 10.1093/heapol/czw009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid A, Dedicoat M, Lalloo D, Gilks CF. Trends in adult medical admissions in a rural South African hospital between 1991 and 2002. Journal of acquired immune deficiency syndromes. 2005;40(1):53–6. [DOI] [PubMed] [Google Scholar]
- 30.Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Annals of internal medicine. 2011;155(4):209–16. 10.7326/0003-4819-155-4-201108160-00358 [DOI] [PubMed] [Google Scholar]
- 31.Callender T, Woodward M, Roth G, Farzadfar F, Lemarie JC, Gicquel S, et al. Heart failure care in low- and middle-income countries: a systematic review and meta-analysis. PLoS medicine. 2014;11(8):e1001699 10.1371/journal.pmed.1001699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarfo FS, Awuah DO, Nkyi C, Akassi J, Opare-Sem OK, Ovbiagele B. Recent patterns and predictors of neurological mortality among hospitalized patients in Central Ghana. Journal of the neurological sciences. 2016;363:217–24. 10.1016/j.jns.2016.02.041 [DOI] [PubMed] [Google Scholar]
- 33.Sliwa K, Davison BA, Mayosi BM, Damasceno A, Sani M, Ogah OS, et al. Readmission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry. European heart journal. 2013;34(40):3151–9. 10.1093/eurheartj/eht393 [DOI] [PubMed] [Google Scholar]
- 34.Abeya FC, Lumori BAE, Akello SJ, Annex BH, Buda AJ, Okello S. Incidence and predictors of 6months mortality after an acute heart failure event in rural Uganda: The Mbarara Heart Failure Registry (MAHFER). International journal of cardiology. 2018;264:113–7. 10.1016/j.ijcard.2018.03.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbutiwi Ikwa Ndol F, Dramaix-Wilmet M, Meert P, Lepira Bompeka F, Nseka Mangani N, Malengreau M, et al. [Predictive factors of all-cause mortality in patients attending the medical emergency unit of Kinshasa University Hospital]. Revue d’epidemiologie et de sante publique. 2014;62(1):15–25. 10.1016/j.respe.2013.11.071 [DOI] [PubMed] [Google Scholar]
- 36.Ogunmola OJ, Oladosu OY. Pattern and outcome of admissions in the medical wards of a tertiary health center in a rural community of Ekiti State, Nigeria. Annals of African medicine. 2014;13(4):195–203. 10.4103/1596-3519.142291 [DOI] [PubMed] [Google Scholar]
- 37.Etyang AO, Scott JA. Medical causes of admissions to hospital among adults in Africa: a systematic review. Global health action. 2013;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oni T, Berkowitz N, Kubjane M, Goliath R, Levitt NS, Wilkinson RJ. Trilateral overlap of tuberculosis, diabetes and HIV-1 in a high-burden African setting: implications for TB control. The European respiratory journal. 2017;50(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS medicine. 2008;5(7):e152 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC medicine. 2011;9:81 10.1186/1741-7015-9-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabkin M, de Pinho H, Michaels-Strasser S, Naitore D, Rawat A, Topp SM. Strengthening the health workforce to support integration of HIV and noncommunicable disease services in sub-Saharan Africa. Aids. 2018;32 Suppl 1:S47–S54. [DOI] [PubMed] [Google Scholar]
- 42.Nugent R, Barnabas RV, Golovaty I, Osetinsky B, Roberts DA, Bisson C, et al. Costs and cost-effectiveness of HIV/noncommunicable disease integration in Africa: from theory to practice. Aids. 2018;32 Suppl 1:S83–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PloS one. 2012;7(8):e43400 10.1371/journal.pone.0043400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mateen FJ, Kanters S, Kalyesubula R, Mukasa B, Kawuma E, Kengne AP, et al. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. Journal of hypertension. 2013;31(7):1372–8; discussion 8. [DOI] [PubMed] [Google Scholar]
- 45.Koss CA, Jarlsberg LG, den Boon S, Cattamanchi A, Davis JL, Worodria W, et al. A Clinical Predictor Score for 30-Day Mortality among HIV-Infected Adults Hospitalized with Pneumonia in Uganda. PloS one. 2015;10(5):e0126591 10.1371/journal.pone.0126591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kateete DP, Nakanjako R, Okee M, Joloba ML, Najjuka CF. Genotypic diversity among multidrug resistant Pseudomonas aeruginosa and Acinetobacter species at Mulago Hospital in Kampala, Uganda. BMC research notes. 2017;10(1):284 10.1186/s13104-017-2612-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakibuuka J, Sajatovic M, Nankabirwa J, Ssendikadiwa C, Furlan AJ, Katabira E, et al. Early mortality and functional outcome after acute stroke in Uganda: prospective study with 30 day follow-up. SpringerPlus. 2015;4:450 10.1186/s40064-015-1252-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalyesubula R, Nankabirwa JI, Ssinabulya I, Siddharthan T, Kayima J, Nakibuuka J, et al. Kidney disease in Uganda: a community based study. BMC nephrology. 2017;18(1):116 10.1186/s12882-017-0521-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babua C, Kalyesubula R, Okello E, Kakande B, Sebatta E, Mungoma M, et al. Pattern and presentation of cardiac diseases among patients with chronic kidney disease attending a national referral hospital in Uganda: a cross sectional study. BMC nephrology. 2015;16:126 10.1186/s12882-015-0128-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orem J, Otieno MW, Remick SC. Challenges and opportunities for treatment and research of AIDS-related malignancies in Africa. Current opinion in oncology. 2006;18(5):479–86. 10.1097/01.cco.0000239887.90665.10 [DOI] [PubMed] [Google Scholar]
- 51.Katende D, Mutungi G, Baisley K, Biraro S, Ikoona E, Peck R, et al. Readiness of Ugandan health services for the management of outpatients with chronic diseases. Tropical medicine & international health: TM & IH. 2015;20(10):1385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers HE, Akiteng AR, Mutungi G, Ettinger AS, Schwartz JI. Capacity of Ugandan public sector health facilities to prevent and control non-communicable diseases: an assessment based upon WHO-PEN standards. BMC health services research. 2018;18(1):606 10.1186/s12913-018-3426-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSB)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.