Abstract
Background
Cannabis and cannabinoids are often promoted as treatment for many illnesses and are widely used among patients with ulcerative colitis (UC). Few studies have evaluated the use of these agents in UC. Further, cannabis has potential for adverse events and the long‐term consequences of cannabis and cannabinoid use in UC are unknown.
Objectives
To assess the efficacy and safety of cannabis and cannabinoids for the treatment of patients with UC.
Search methods
We searched MEDLINE, Embase, WHO ICTRP, AMED, PsychINFO, the Cochrane IBD Group Specialized Register, CENTRAL, ClinicalTrials.Gov and the European Clinical Trials Register from inception to 2 January 2018. Conference abstracts and references were searched to identify additional studies.
Selection criteria
Randomized controlled trials (RCTs) comparing any form or dose of cannabis or its cannabinoid derivatives (natural or synthetic) to placebo or an active therapy for adults (> 18 years) with UC were included.
Data collection and analysis
Two authors independently screened search results, extracted data and assessed bias using the Cochrane risk of bias tool. The primary outcomes were clinical remission and relapse (as defined by the primary studies). Secondary outcomes included clinical response, endoscopic remission, endoscopic response, histological response, quality of life, C‐reactive protein (CRP) and fecal calprotectin measurements, symptom improvement, adverse events, serious adverse events, withdrawal due to adverse events, psychotropic adverse events, and cannabis dependence and withdrawal effects. We calculated the risk ratio (RR) and corresponding 95% confidence interval for dichotomous outcomes. For continuous outcomes, we calculated the mean difference (MD) and corresponding 95% CI. Data were pooled for analysis when the interventions, patient groups and outcomes were sufficiently similar (determined by consensus). Data were analyzed on an intention‐to‐treat basis. GRADE was used to evaluate the overall certainty of evidence.
Main results
Two RCTs (92 participants) met the inclusion criteria. One study (N = 60) compared 10 weeks of cannabidiol capsules with up to 4.7% D9‐tetrahydrocannabinol (THC) with placebo capsules in participants with mild to moderate UC. The starting dose of cannabidiol was 50 mg twice daily increasing to 250 mg twice daily if tolerated. Another study (N = 32) compared 8 weeks of therapy with two cannabis cigarettes per day containing 0.5 g of cannabis, corresponding to 23 mg THC/day to placebo cigarettes in participants with UC who did not respond to conventional medical treatment. No studies were identified that assessed cannabis therapy in quiescent UC. The first study was rated as low risk of bias and the second study (published as an abstract) was rated as high risk of bias for blinding of participants and personnel. The studies were not pooled due to differences in the interventional drug.
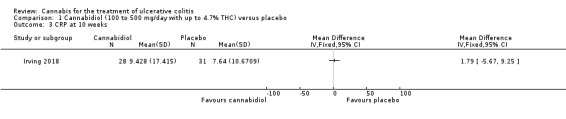
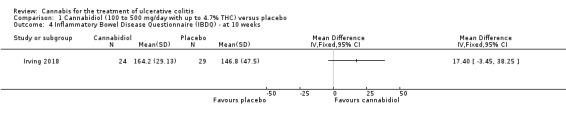
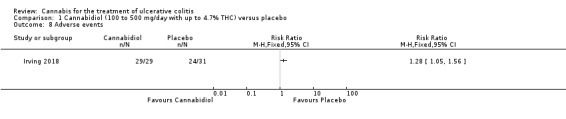
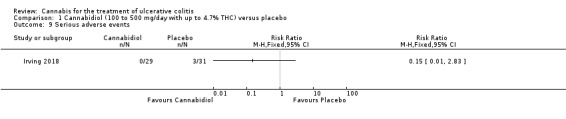
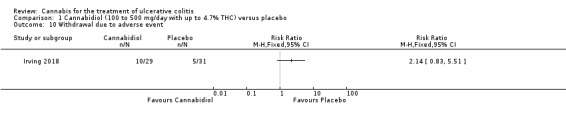
The effect of cannabidiol capsules (100 mg to 500 mg daily) compared to placebo on clinical remission and response is uncertain. Clinical remission at 10 weeks was achieved by 24% (7/29) of the cannabidiol group compared to 26% (8/31) in the placebo group (RR 0.94, 95% CI 0.39 to 2.25; low certainty evidence). Clinical response at 10 weeks was achieved in 31% (9/29) of cannabidiol participants compared to 22% (7/31) of placebo patients (RR 1.37, 95% CI 0.59 to 3.21; low certainty evidence). Serum CRP levels were similar in both groups after 10 weeks of therapy. The mean CRP in the cannabidiol group was 9.428 mg/L compared to 7.638 mg/L in the placebo group (MD 1.79, 95% CI ‐5.67 to 9.25; moderate certainty evidence). There may be a clinically meaningful improvement in quality of life at 10 weeks, measured with the IBDQ scale (MD 17.4, 95% CI ‐3.45 to 38.25; moderate certainty evidence). Adverse events were more frequent in cannabidiol participants compared to placebo. One hundred per cent (29/29) of cannabidiol participants had an adverse event, compared to 77% (24/31) of placebo participants (RR 1.28, 95% CI 1.05 to1.56; moderate certainty evidence). However, these adverse events were considered to be mild or moderate in severity. Common adverse events included dizziness, disturbance in attention, headache, nausea and fatigue. None (0/29) of the cannabidiol participants had a serious adverse event compared to 10% (3/31) of placebo participants (RR 0.15, 95% CI 0.01 to 2.83; low certainty evidence). Serious adverse events in the placebo group included worsening of UC and one complicated pregnancy. These serious adverse events were thought to be unrelated to the study drug. More participants in the cannabidiol group withdrew due to an adverse event than placebo participants. Thirty‐four per cent (10/29) of cannabidiol participants withdrew due to an adverse event compared to 16% (5/31) of placebo participants (RR 2.14, 95% CI 0.83 to 5.51; low certainty evidence). Withdrawls in the cannabidiol group were mostly due to dizziness. Withdrawals in the placebo group were due to worsening UC.
The effect of cannabis cigarettes (23 mg THC/day) compared to placebo on mean disease activity, CRP levels and mean fecal calprotectin levels is uncertain. After 8 weeks, the mean disease activity index score in cannabis participants was 4 compared with 8 in placebo participants (MD ‐4.00, 95% CI ‐5.98 to ‐2.02). After 8 weeks, the mean change in CRP levels was similar in both groups (MD ‐0.30, 95% CI ‐1.35 to 0.75; low certainty evidence). The mean fecal calprotectin level in cannabis participants was 115 mg/dl compared to 229 mg/dl in placebo participants (MD ‐114.00, 95% CI ‐246.01 to 18.01). No serious adverse events were observed. This study did not report on clinical remission, clinical response, quality of life, adverse events or withdrawal due to adverse events.
Authors' conclusions
The effects of cannabis and cannabidiol on UC are uncertain, thus no firm conclusions regarding the efficacy and safety of cannabis or cannabidiol in adults with active UC can be drawn.There is no evidence for cannabis or cannabinoid use for maintenance of remission in UC. Further studies with a larger number of patients are required to assess the effects of cannabis in UC patients with active and quiescent disease. Different doses of cannabis and routes of administration should be investigated. Lastly, follow‐up is needed to assess the long term safety outcomes of frequent cannabis use.
Plain language summary
Cannabis and cannabis oil for the treatment of ulcerative colitis
What is ulcerative colitis?
Ulcerative colitis is a chronic, long‐term illness that causes inflammation of the colon and rectum. Symptoms may include diarrhea, rectal bleeding, passage of mucus, and abdominal pain. It is characterized by periods of acute flares when people experience symptoms as well as periods of remission when symptoms stop.
What are cannabis and cannabinoids?
Cannabis is a widely used recreational drug that has multiple effects on the body via the endocannabinoid system. Cannabis contains multiple sub‐ingredients called cannabinoids. Cannabis and cannabis oil containing specific cannabinoids can cause cognitive changes such as feelings of euphoria and altered sensory perception. However, some cannabinoids, such as cannabidiol, do not have a psychoactive effect. Cannabis and some cannabinoids have been shown to decrease inflammation in animal and laboratory models which suggests it may help people with ulcerative colitis. For example, cannabidiol is one such cannabinoid that has shown anti‐inflammatory activity in mice.
What did the researchers investigate?
The researchers evaluated whether cannabis or cannabis oil (cannabidiol) was better than placebo (e.g. fake drug) for treating adults with active ulcerative colitis or ulcerative colitis that is in remission. The researchers searched the medical literature extensively up to 2 January 2018.
What did the researchers find?
Two studies including 92 adult participants with ulcerative colitis were included. Both studies assessed cannabis therapy in participants who had active ulcerative colitis. No studies that assessed cannabis therapy in participants with ulcerative colitis in remission were identified. One study (60 participants) compared 10 weeks of treatment with capsules containing cannabis oil with up to 4.7% D9‐tetrahydrocannabinol (THC) to placebo in participants with mild to moderately active ulcerative colitis. The starting dose of cannabidiol was 50 mg twice daily which was increased, if tolerated, to a target of 250 mg twice daily. The other study (32 participants) compared 8 weeks of treatment with two cannabis cigarettes per day containing 0.5 g of cannabis, corresponding to 11.5 mg THC to placebo cigarettes in participants with ulcerative colitis who did not respond to conventional medical treatment.
The study comparing cannabis oil capsules to placebo found no difference in remission rates at 10 weeks. Twenty four (7/29) percent of cannabidiol participants achieved clinical remission compared to 26% (8/31) of placebo participants. The study also showed higher self reported quality of life scores in cannabis oil participants compared to placebo participants. More side‐effects were observed in the cannabis oil participants compared to the placebo participants. These side effects were considered to be mild or moderate in severity. Common reported side effects include dizziness, disturbance in attention, headache, nausea and fatigue. No patients in the cannabis oil group had any serious side effects. Ten per cent (3/31) of the placebo group had a serious side effect. Serious side effects in the placebo group included worsening ulcerative colitis and one complicated pregnancy.
The second study comparing two cannabis cigarettes (23 mg THC/day) to placebo cigarettes showed lower disease activity index scores in the cannabis group compared to the placebo group. C‐reactive protein and fecal calprotectin levels (both measures of inflammation in the body) were similar in both groups. No serious side effects were reported. This study did not report on remission rates.
Conclusions
The effects of cannabis and cannabis oil on ulcerative colitis are uncertain, thus no firm conclusions regarding the effectiveness and safety of cannabis or cannabis oil in adults with active ulcerative colitis can be drawn. There is no evidence for cannabis or cannabis oil use for maintenance of remission in ulcerative colitis. Further studies with a larger number of participants are required to assess the effects of cannabis in people with active and inactive ulcerative colitis. Different doses of cannabis and routes of administration should be investigated. Lastly, follow‐up is needed to assess the long term safety outcomes of frequent cannabis use.
Summary of findings
Summary of findings for the main comparison. Cannabidiol compared to placebo for the treatment of ulcerative colitis.
| Cannabidiol compared to placebo for the treatment of ulcerative colitis | ||||||
| Patient or population: participants with active ulcerative colitis Setting: outpatient Intervention: cannabidiol (100 mg to 500 mg/day). The cannabidiol also contained up to 4.7% THC Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Cannabidiol with up to 4.7% THC | |||||
| Clinical remission at 10 weeks | 258 per 1,000 | 243 per 1,000 (101 to 581) | RR 0.94 (0.39 to 2.25) | 60 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Remisison was defined as a Mayo score of < 2 (with no sub‐score > 1) |
| Clinical response at 10 weeks | 226 per 1,000 | 309 per 1,000 (133 to 725) | RR 1.37 (0.59 to 3.21) | 60 (1 RCT) | ⊕⊕⊝⊝ LOW 2 | Response defined as decrease in Mayo score of ≥3 points compared to baseline, with a reduction of at least 1 point in endoscopy findings sub‐score |
| CRP at 10 weeks | The mean CRP at 10 weeks was 9.4 mg/L | MD 1.79 mg/L higher (5.67 lower to 9.25 higher) | ‐ | 59 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | |
| Quality of life Inflammatory Bowel Disease Questionnaire (IBDQ) at 10 weeks |
The mean IBDQ score at 10 weeks was 146.8 | MD 17.4 higher (3.45 lower to 38.25 higher) | ‐ | 53 (1 RCT) | ⊕⊕⊕⊝ MODERATE 4 | IBDQ scores range from 32 to 224 with a higher score indicating better quality of life |
| Adverse events | 774 per 1,000 | 991 per 1,000 (813 to 1,000) | RR 1.28 (1.05 to 1.56) | 60 (1 RCT) | ⊕⊕⊕⊝ MODERATE 5 | Common adverse events included dizziness, disturbance in attention, headache, nausea and fatigue |
| Serious adverse events | 97 per 1,000 | 15 per 1,000 (1 to 274) | RR 0.15 (0.01 to 2.83) | 60 (1 RCT) | ⊕⊕⊝⊝ LOW 6 | There were no serious adverse events in the cannabidiol group Serious adverse events in the placebo group included worsening of ulcerative colitis and one complicated pregnancy |
| Withdrawal due to adverse event | 161 per 1,000 | 345 per 1,000 (134 to 889) | RR 2.14 (0.83 to 5.51) | 60 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Withdrawls in the cannabidiol group were mostly due to dizziness Withdrawals in the placebo group were due to worsening ulcerative colitis |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: mean difference; THC: D9‐tetrahydrocannabinol | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to very sparse data (15 events).
2 Downgraded two levels due to very sparse data (16 events).
3 Downgraded one level due to sparse data (59 participants).
4 Downgraded one level due to sparse data (53 participants).
5 Downgraded one level due to sparse data (53 events).
6 Downgraded two levels due to very sparse data (4 events).
Summary of findings 2. Cannabis cigarettes compared to placebo for the treatment of ulcerative colitis.
| Cannabis cigarettes (23 mg THC/day) compared to placebo for the treatment of ulcerative colitis | ||||||
| Patient or population: participants with active ulcerative colitis Setting: outpatient Intervention: two cannabis cigarettes at a total dose of 23 mg THC daily Comparison: placebo cigarettes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with cannabis cigarettes (11.5 mg THC) | |||||
| Clinical remission | Not reported | This outcome was not reported | ||||
| Clinical response | Not reported | This outcome was not reported | ||||
| CRP at 8 weeks | The mean CRP at 8 weeks was 1.0 mg/L | MD 0.3 mg/L lower (1.35 lower to 0.75 higher) | ‐ | 28 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | |
| Quality of life Inflammatory Bowel Disease Questionnaire (IBDQ) |
Not reported | This outcome was not reported | ||||
| Adverse events | Not reported | This outcome was not reported | ||||
| Serious adverse events | 0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | No serious adverse events were observed | ||
| Withdrawal due to adverse events | Not reported | This outcome was not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; THC: D9‐tetrahydrocannabinol | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to very sparse data (28 participants).
Background
Cannabis or marijuana is often promoted as a treatment for various illnesses including cancer and autoimmune disorders (Hill 2015). It is a common recreational drug that alters sensory perception and elicits feelings of euphoria (Tibirica 2010). Cannabis is known to affect pain and discomfort via psychotropic effects of it's ingredient D9‐tetrahydrocannabinol (THC) (Tibirica 2010). However, cannabis also modulates the endocannabinoid system which acts on the nervous system and immune cell function (Klein 2006). It is hypothesized that cannabis and its derivatives may work through this pathway to exert a therapeutic effect on ulcerative colitis (UC) (Schicho 2014; Tibirica 2010). Cannabidiol is one such derivative of cannabis that lacks a psychotropic effect, but has shown anti‐inflammatory effects in experimental animal models (Schicho 2014).
There is a higher prevalence of cannabis use among patients with inflammatory bowel disease compared to the general population (Weiss 2015). Cannabis may relieve symptoms of UC such as abdominal pain, reduced appetite, and diarrhea (Lal 2011; Weiss 2015). However, it is not known if these potential benefits are related to centrally acting psychotropic effects or to anti‐inflammatory properties as suggested by animal studies (Hasenoehrl 2016; Klein 2006; Singh 2012). Studies looking at UC in animal and laboratory models have found benefit in attenuating inflammation (Borrelli 2009; Leinwand 2017). However, in humans, there is evidence that cannabis may be associated with harm and adverse effects such as dizziness and diarrhea (Whiting 2015).
Preliminary results from the first randomized, double‐blind, placebo‐controlled study in humans looking at the use of a cannabinoid in UC were published in 2015 (Irving 2015). This study posed important questions regarding whether cannabis and its derivatives can ameliorate symptoms of UC. For example, can cannabis objectively reduce inflammation in UC? If so, is this benefit clinically significant in the absence of psychotropic effects? Further, what is the safety and side‐effect profile associated with these agents?
Description of the condition
UC is a chronic immune‐mediated disorder that causes mucosal inflammation in the colon and rectum, and is associated with significant morbidity and a decreased quality of life (Lahat 2012). In North America, the prevalence of UC is estimated to be 37 to 246 cases per 100,000 person‐years (Friedman 2012). In Europe, the prevalence of UC ranges from 21.4 to 243 cases per 100,000 person‐years (Friedman 2012). Outside of these areas, UC is less common with the exception of Israel, Australia, and South Africa (Friedman 2012). However, the incidence of UC is rapidly rising in places such as Hong Kong, Japan, South Korea, Singapore, northern India, and Latin America (Friedman 2012). Mortality in UC is highest during the initial years of disease activity (Friedman 2012). However, in the long‐term, mortality in UC is due to an increased risk of colon cancer (Friedman 2012).
Patients with UC may have a genetic predisposition to this disease and the pathophysiology of this condition is multifactorial (Friedman 2012). The pathophysiology involves a dysregulated immune response towards commensal microbiota and dietary contents in the gastrointestinal tract (Friedman 2012). This immune response leads to an inflammatory cascade of activated T cells secreting excessive pro‐inflammatory cytokines including interleukin‐1 (IL‐1), interleukin‐6 (IL‐6), and tumour necrosis factor‐alpha (TNF‐α) antibodies resulting in inflammation and damage to previously healthy tissues (Friedman 2012). UC is a relapsing remitting disorder and symptoms may include diarrhea, rectal bleeding, tenesmus, passage of mucus, and abdominal pain (Friedman 2012). Treatment options include anti‐inflammatory and immunosuppressant agents (Friedman 2012). Common first line agents include 5‐aminosalicylates, corticosteroids, thiopurines (e.g. azathioprine or 6‐mercaptopurine), and biologic therapies including TNF‐α antagonists and vedolizumab (Friedman 2012).
Description of the intervention
Cannabis is derived from the leaves and flowering tops of the plant and is prepared in various forms such as cigarettes, hash oil and edible formulations (Mello 2012). Cannabis contains over 400 compounds and has numerous derivatives called cannabinoids (Mello 2012). Although THC is the main psychotropic derivative of cannabis, there are other derivatives such as cannabidiol that show anti‐inflammatory effects in animal models without psychotropic effects (Klein 2006). Cannabis is rapidly absorbed into the body due to its lipophilic nature, but then it is sequestered into tissues and very slowly cleared from the body through faeces (Mello 2012). While there are data identifying an increase in associated short‐term adverse effects of cannabinoids such as dizziness and diarrhea, there is a paucity of studies and data regarding the long‐term benefits and harms of cannabinoids (Whiting 2015).
How the intervention might work
Cannabis is hypothesized to affect disease activity in UC via the endocannabinoid system (Hasenoehrl 2016). The endocannabinoid system affects the nervous system, peripheral tissues, and the immune system (Tibirica 2010). It is composed of cannabinoid (CB) receptors 1 and 2, endogenous endocannabinoids, and associated enzymes (Hasenoehrl 2016; Klein 2006). It is hypothesized that modulating this system may therapeutically decrease inflammation in the gut (Hasenoehrl 2016).
CB1 receptors are found in the central nervous system, peripheral tissues and gastrointestinal system (Hasenoehrl 2016; Klein 2006). Activation of these receptors may help reduce intestinal transit time and reduce colon propulsion, and enhance epithelial wound closure in the colon (Pinto 2002; Wright 2005). There is evidence that these receptors play a physiologic role in protecting the colon during excessive inflammation (Massa 2004). In the central nervous system, CB1 receptors are associated with effects such as reduction in pain and nausea (Klein 2006; Tibirica 2010).
CB2 receptors are found in the myenteric plexus, immune cells and in epithelial cells in ulcerative colitis (Hasenoehrl 2016; Klein 2006; Marquez 2009). Cannabis is thought to influence immune cells through various pathways. For example, CB2 receptor activation may lead to T‐cell apoptosis, decreased T‐cell proliferation in colitis, decreased recruitment of leukocytes to the inflamed colon, and may also help reduce the release of cytokines ( Klein 2006; Lahat 2012; Singh 2012).
The endocannabinoid system also has other pathways that may be activated by cannabis and cannabinoids. For example, non‐psychotropic cannabinoids such as cannabidiol may reduce inflammation through the peroxisome proliferator‐activated receptors and transient receptor potential cation channels subfamily V receptor pathways (Hasenoehrl 2016).
Why it is important to do this review
This review was performed to evaluate the evidence supporting the use of cannabis and cannabinoids for the treatment of UC. Cannabis and cannabinoid use is becoming more common and physicians are faced with increased demands from their patients to add cannabis to their treatment. However, there is very little evidence regarding the benefits and harms of cannabis and cannabinoids, hence there is a need to review the current knowledge of its use. We assessed the efficacy and safety of cannabis and its derivatives for the treatment of UC. This systematic review helped distinguish objective markers of improvement from subjective scores and also helped identify adverse events associated with cannabis and cannabinoids.
Objectives
Our objective was to assess the efficacy and safety of cannabis and cannabinoids for the treatment of patients with UC.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials were considered for inclusion. Studies published as abstracts were only included if the authors could be reached for further information to allow for evaluation of quality and main outcomes. Any study duration was included.
Types of participants
Adult patients (> 18 years of age) with UC (diagnosed by conventional methods) were considered for inclusion. Patients with clinically active or quiescent UC were considered for inclusion. Clinical remission or quiescent disease was often defined by the Mayo Score or Disease Activity Index (DAI) for UC. Patients with active (e.g. DAI >2) or quiescent disease (defined as mild or absent symptoms prior to entering the study or by a DAI total score < 2 with no sub score > 1) were included. We included patients on all therapies for UC including those with a history of biologic therapy.
Types of interventions
Studies comparing any form of cannabis or cannabinoid derivatives to placebo or an active therapy for UC were included. We included studies that utilized any dosage and method of administration
Types of outcome measures
Primary outcomes
The primary outcome for induction of remission studies was clinical remission at study endpoint (as defined by the primary studies). The primary outcome for maintenance of remission studies was relapse at study endpoint (as defined by the primary studies). We included any validated scoring system. We included all short‐term and long‐term outcome time points.
Secondary outcomes
Secondary outcomes included:
1. Clinical response (as defined by the included studies);
2. Endoscopic remission (as defined by the included studies);
3. Endoscopic response (as defined by the included studies);
4. Histological response (as defined by the included studies);
5. Quality of life (as defined by validated instrument or primary study, e.g. the Inflammatory Bowel Disease Questionnaire or IBDQ);
6. C‐reactive protein (CRP) and fecal calprotectin measurements;
7. Symptom improvement (e.g. improvement in pain, nausea, or anorexia);
8. Adverse events (i.e. psychological effects, cognitive impairment, personal safety accidents, and GI upset);
9. Serious adverse events;
10. Withdrawal due to adverse events;
11. Psychotropic adverse events (including mental health effects such as psychosis and schizophrenia); and
12. Cannabis dependence and withdrawal effects (as defined and measured by primary studies).
Search methods for identification of studies
Electronic searches
We searched the following databases from inception to 2 January 2018:
1. MEDLINE (Ovid);
2. Embase (Ovid);
3. WHO ICTRP;
4. AMED (Allied & Alternative Medicine);
5. Psych INFO;
4. The Cochrane IBD Group Specialized Register;
5. CENTRAL;
6. ClinicalTrials.Gov; and
7. European Clinical Trials Register.
Conference proceedings were also searched to identify additional studies. We also contacted authors in this field for more information and upcoming abstracts or studies. See Appendix 1 for search strategy.
Searching other resources
We searched conference proceedings to identify studies only published in abstract form. We searched ClinicalTrials.gov and EU Clinical Trials Register to identify ongoing studies. We also searched the references sections of applicable studies and systematic reviews to identify additional studies that may meet the inclusion criteria.
Data collection and analysis
Selection of studies
We reviewed studies and abstracts identified by the literature search. Two authors (TK and NC) independently screened the search results to identify potentially relevant studies for full text evaluation. The studies selected for full text review were independently assessed by two authors (TK and NC) and consensus for study inclusion and exclusion was reached through discussion. Any conflicts regarding inclusion or exclusion were resolved by consultation with a third author (JKM) as necessary. Studies published in abstract form were only included if the authors could be reached for further information.
Data extraction and management
Two authors (TK and NC) independently extracted the outcome data of interest from each study. Any conflicts were resolved by discussion and consensus or by consultation with a third author (JKM) as necessary. If data were missing or unclear, the study authors were contacted for clarification.
Other information extracted from the studies included:
a. Study characteristics and design;
b. Characteristics of patients;
c. Inclusion and exclusion criteria;
d. Interventions (if available, we extracted specific information per the Herbal CONSORT statement) (Gagnier 2006); and
e. Outcomes scoring methods.
Assessment of risk of bias in included studies
Two authors (TK and NC) independently assessed bias using the Cochrane risk of bias tool (Higgins 2011). Any conflicts were resolved by discussion and consensus or by consultation with a third author (JKM) as required. Items assessed included: 1. Random sequence generation;
2. Allocation sequence concealment;
3. Blinding of participants, personnel and outcome assessors;
4. Incomplete outcome data;
5. Selective outcome reporting; and
6. Other potential sources of bias.
Each category was evaluated as low, high or unclear risk of bias and justification for judgement was provided in the characteristics of included studies section of the review.
GRADE Analysis
The overall certainty of the evidence supporting the primary outcome and selected secondary outcomes was evaluated using the GRADE criteria (Guyatt 2008; Schünemann 2011). Using this approach outcome data were rated as high, moderate, low or very low certainty. Outcome data from randomized controlled trials began as high certainty, but could be downgraded based on several criteria. These criteria included:
1. Risk of bias from the studies;
2. Indirect evidence (by comparison, population, setting);
3. Inconsistency (i.e. unexplained heterogeneity);
4. Imprecision (i.e. few events and wide confidence intervals); and
5. Likelihood of publication bias.
Measures of treatment effect
For dichotomous outcomes, we calculated the risk ratio (RR) and corresponding 95% confidence interval (CI). For continuous outcomes, we calculated the mean difference (MD) and corresponding 95% CI.
Unit of analysis issues
For multi‐arm trials (e.g. with two or more dose groups) with a single placebo group, we planned to split the placebo group across the treatment groups to avoid a unit of analysis error (Higgins 2011b). In order to avoid potential carry‐over effects, we would only include the first part of the study (i.e. before the cross‐over) for any cross‐over studies (Higgins 2011b). For studies where events may re‐occur we would only include the first event. When there were repeated observations on participants, we used the primary endpoint defined by the study. It was unlikely that we would find study designs applicable to cannabis in UC where multiple treatment attempts were used. We did not anticipate encountering any available cluster‐randomized studies.
Dealing with missing data
Data were analysed on an intention‐to‐treat (ITT) basis, whereby missing data with no explanations were assumed to be treatment failures. We counted treatment failures as a relapse for maintenance studies and as a failure to enter remission for induction studies. We conducted a sensitivity analysis to assess the impact of this assumption on the effect estimate. If needed, we imputed missing standard deviations. We conducted an available case analysis for missing continuous outcomes.
Assessment of heterogeneity
We planned to use the Chi2 test and the I2 statistic to assess heterogeneity. For the Chi2 test, we considered a P value of 0.10 to be statistically significant. We planned to use the I2 statistic to quantify the proportion of variation that is due to heterogeneity rather than to chance. An I2 value of 25% indicates low heterogeneity, >50% indicates moderate heterogeneity and >75% indicates high heterogeneity. We planned to visually inspected the forest plots to identify any outliers. If outliers are identified, a sensitivity analysis would be conducted to explore potential explanations for the heterogeneity.
Assessment of reporting biases
Reporting bias was assessed by comparing the outcomes pre‐specified in study protocols to the outcomes reported in the study manuscripts. However, if the protocols were not available, we assessed reporting bias by comparing the outcomes specified in the methods section of the manuscript to those reported in the results section. If a sufficient number of studies were included in the pooled analysis (i.e. > 10), we would have constructed a funnel plot to assess the potential for publication bias (Egger 1997).
Data synthesis
We planned to pool data from individual studies for meta‐analysis when the outcomes, patient groups and interventions were similar enough to justify pooling (determined by consensus). When pooling studies was not possible, we narratively summarized the results of individual trials. For dichotomous outcomes, we planned to calculate the pooled RR and 95% CI using a fixed‐effect model. For continuous outcomes, we planned to calculate the pooled MD and corresponding 95% CI. However, if the continuous outcomes utilize different scales to measure the same underlying construct (e.g. for quality of life), we planned to calculate the standardized mean difference (SMD) and corresponding 95% CI. If significant heterogeneity was identified, a random‐effects model would be used to pool data. We would not pool data for meta‐analysis if a high degree of heterogeneity was detected (e.g. I2 > 75%).
Subgroup analysis and investigation of heterogeneity
Subgroup analysis based on dose of cannabis or cannabinoid would have been performed if the data allowed for such comparisons. Other subgroup analyses of interest included the form of cannabis consumed, UC disease location, cigarette smoking status, history of prior biologic therapy and failure of biologic therapy.
Sensitivity analysis
We planned to perform a sensitivity analysis of study quality by excluding studies with a high risk of bias to see if there is an impact on the effect estimate.
Results
Description of studies
Results of the search
A literature search conducted on 2 January 2018 identified 116 studies. Two studies were identified through other sources. After duplicates were removed, a total of 66 studies were identified. Fifty‐seven studies were not applicable and were excluded. Nine studies remained for full text review. Two studies were excluded with reasons. Seven reports of two studies met the inclusion criteria and were included in the review (See Figure 1). Both of the included studies assessed cannabis therapy in participants with active UC. No studies were identified that assessed cannabis therapy in participants with quiescent UC.
1.
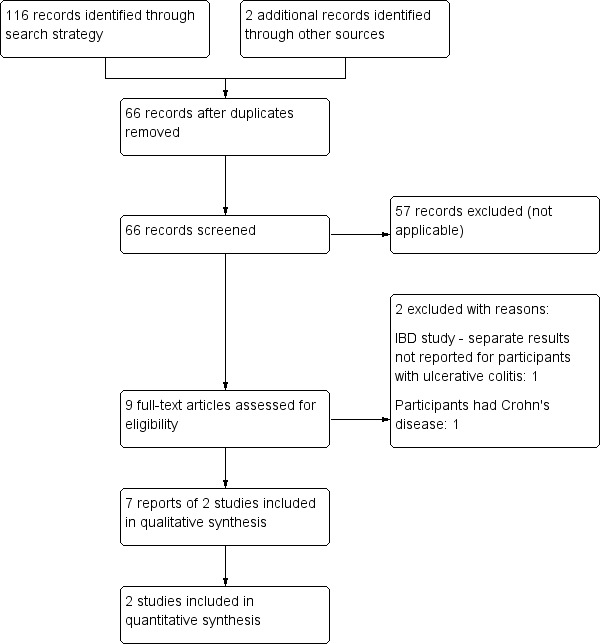
Flow diagram.
Included studies
Irving 2018 was a randomized, multi‐centre, double‐blind, placebo‐controlled study that compared cannabidiol capsules with up to 4.7% THC (n = 29) to placebo (n = 31) over a ten week period. The starting dose of cannabidiol was 50 mg twice daily which was increased, if tolerated, to target of 250 mg twice daily. Patients who were previously diagnosed with mild to moderate UC and were on stable doses of 5‐aminosalicylates for at least two weeks prior to screening for study entry were eligible for inclusion. Patients with severe UC or proctitis were excluded. The goal of the study was to determine whether cannabidiol had a positive benefit for treating UC symptoms and other markers such as CRP. The primary outcome was clinical remission (Mayo score of ≤2 with no subscore of >1) after 10 weeks treatment. The secondary outcomes included inflammatory marker levels (CRP, plasma interleukin and fecal calprotectin), inflammatory bowel disease questionnaire (IBDQ) score, physician global assessment of illness severity (PGAS) score, stool frequency and rectal bleeding. The original study protocol only planned an ITT analysis, but one year after completion of the study they added a per protocol (PP) analysis set.
Naftali 2018 is an abstract publication reporting a randomized placebo controlled trial. The study enrolled patients with UC who did not respond to conventional medical treatment (N = 32). Patients were given either two cannabis cigarettes (n = 17; 0.5 g of cannabis, corresponding to 11.5 mg of THC for a total of 23 mg THC/day) or placebo cigarettes (n = 15; cannabis leaves from which THC was extracted) daily for eight weeks. Outcomes reported in the abstract included disease activity index (DAI), Mayo endoscopic score, endoscopic findings and laboratory tests (CRP, fecal calprotectin).
Excluded studies
Two records were excluded. One study assessed the use of cannabis for the treatment of Crohn's disease (NCT01037322). Naftali 2013a assessed the use of cannabis for the treatment of inflammatory bowel disease. This study was excluded because separate data for patients with UC could not be obtained.
Risk of bias in included studies
See Figure 2 for a summary of the risk of bias results.
2.
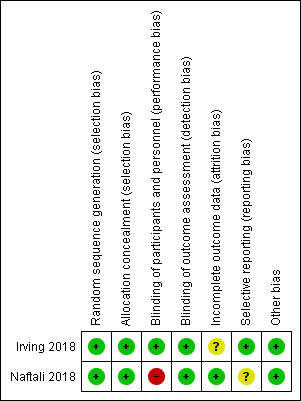
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was rated as low risk for Irving 2018. An independent statistician produced a central randomization schedule. Allocation concealment was rated as low risk of bias, as centralized randomization was used. Random sequence generation was rated as low risk for Naftali 2018, where block randomization was used to assign participants to receive either medical cannabis or placebo. Allocation concealment was rated as low risk of bias as sequentially numbered drug containers of identical appearance were used to conceal treatment allocation.
Blinding
Irving 2018 was double blinded and had quadruple masking "participant, care provider, investigator, outcomes assessor". Identical gelatin capsules were formulated to maintain blinding throughout the study. The maximum number of dose units was identical in treatment and placebo groups. This study was rated as low risk of bias for blinding of participants and personnel and for blinding of outcome assessment. The Naftali 2018 study was rated as high risk of bias for blinding of participants and personnel. Although placebo cigarettes were used, blinding was likely to be broken due to the psychotropic effects of cannabis that contained high doses of THC. Naftali 2018 was rated as low risk of bias because outcome assessors were blind to treatment assignment.
Incomplete outcome data
Irving 2018 was rated as unclear risk of bias for incomplete outcome data. There was a higher rate of withdrawal in the treatment group (13/29) compared to the placebo group (8/31). There were no withdrawals in the Naftali 2018 study. We rated this study as low risk of bias for incomplete outcome data.
Selective reporting
Irving 2018 was rated as low risk of bias for selective reporting. The Naftali 2018 study was rated as unclear risk of bias for selective reporting. The primary outcome reported in the study protocol was for Crohn's disease and was a reduction in the Crohn's Disease Activity Index of 70 points. The study enrolled participants with Crohn's disease and ulcerative colitis and the results for participants with Crohn's disease are reported elsewhere (Naftali 2013b). The secondary outcomes reported in the study protocol included adverse events due to cannabis smoking, change in quality of life, change in IL‐10, IL‐2, and TGF beta, None of these outcomes were reported in the abstract publication but could potentially be reported in a full manuscript.
Other potential sources of bias
The Irving 2018 and Naftali 2018 studies were rated as low risk of bias for other potential sources of bias.
Effects of interventions
Data from the two included studies were not pooled as the routes and formulas were different. Naftali 2018 studied cannabis cigarettes with 11.5 mg THC and Irving 2018 studied cannabidiol capsules with a dose range of 50 to 250 mg twice daily. There was up to 4.7% THC in each capsule.
Cannabidiol capsules (100 mg to 500 mg/day with up to 4.7% THC) versus placebo capsules at 10 weeks:
There was no difference between the cannabidiol group and the placebo group in clinical remission rates at 10 weeks (Irving 2018). Clinical remission was reported in 24% (7/29) of patients in the treatment group compared to 26% (8/31) of patients in the placebo group (RR 0.94, 95% CI 0.39 to 2.25). The GRADE analysis indicated that the overall certainty of evidence for this outcome was low due to very sparse data (See Table 1). There was no difference in clinical response rates at 10 weeks. Clinical response defined by a decrease in Mayo total score of ≥3 points, compared to baseline, with a reduction of at least 1 point in endoscopy findings sub‐score was reported in 31% (9/29) of patients in the cannabidiol group compared to 22% (7/31) of patients in the placebo group (RR 1.37, 95% CI 0.59 to 3.21). The GRADE analysis indicated that the overall certainty of evidence for this outcome was low due to very sparse data (See Table 1).
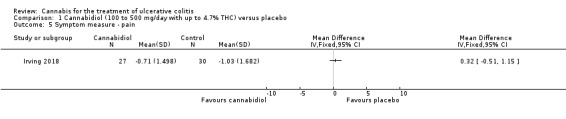
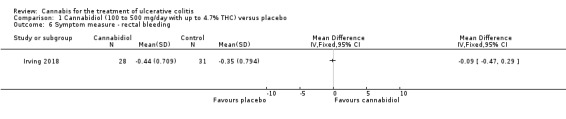
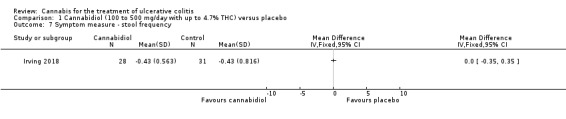
There was no difference in mean CRP levels at 10 weeks. The mean CRP in the cannabidiol group at 10 weeks was 9.428 mg/L + 17.4 compared to 7.638 mg/L + 10.7 in the placebo group (MD 1.79, 95% CI ‐5.67 to 9.25). The GRADE analysis indicated that the overall certainty of evidence for this outcome was moderate due to sparse data (See Table 1). There was no difference in mean IBDQ scores at 10 weeks. The mean IBDQ score at 10 weeks was 164.2 + 29.1 in the cannabidiol group compared to 146.8 + 47.5 in the placebo group (MD 17.40, 95% CI ‐3.45 to 38.25). The GRADE analysis indicated that the overall certainty of evidence for this outcome was moderate due to sparse data (See Table 1). There were no differences in pain (MD 0.32, 95% CI ‐0.51 to 1.15), stool frequency (MD 0.00, 95% CI ‐0.35 to 0.35), or rectal bleeding (MD ‐0.09, 95% CI ‐0.47 to 0.29) at 10 weeks. Adverse events were more frequent in the cannabidiol group compared to the placebo group. All the patients in the cannabis group (29/29) experienced an adverse event compared to 77% (24/31) of patients in the placebo group (RR 1.28, 95% CI 1.05 to 1.56). The GRADE analysis indicated that the overall certainty of evidence for this outcome was moderate due to sparse data (See Table 1). However, these adverse events were considered to be mild or moderate in severity. Common adverse events reported in the cannabidiol group included dizziness, somnolence, disturbance in attention, headache, memory impairment, nausea, dry mouth, vomiting, lower respiratory tract infection, disorientation and fatigue. Common adverse events reported in the placebo group include dizziness, headache, nausea, abdominal pain, worsening ulcerative colitis, abdominal distention, constipation, fatigue, back pain and rash. There was no difference in the proportion of participants who developed serious adverse events. None of the patients (0/29) in the cannabidiol group had a serious adverse event compared to 10% (3/31) of patients in the placebo group (RR 0.15, 95% CI 0.01 to 2.83). The GRADE analysis indicated that the overall certainty of evidence for this outcome was low due to very sparse data (See Table 1). Serious adverse events in the placebo group were related to worsening of disease and one complicated pregnancy. None of the serious adverse events were thought to be treatment‐related. Withdrawal due to adverse events was more frequent in the cannabidiol group. Thirty‐four per cent (10/29) of cannabidiol participants withdrew due to an adverse event (mostly dizziness) compared to 16% (5/31) of placebo participants (RR 2.14, 95% CI 0.83 to 5.51). The GRADE analysis indicated that the overall certainty of evidence for this outcome was low due to very sparse data (See Table 1). Withdrawls in the cannabidiol group were mostly due to dizziness. Withdrawls in the placebo group were due to worsening ulcerative colitis. The outcomes relapse, endoscopic remission, endoscopic response, histological response and cannabis withdrawal effects were not reported in this study.
Cannabis cigarettes (23 mg THC/day) versus placebo cigarettes at 8 weeks:
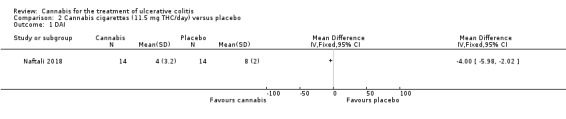
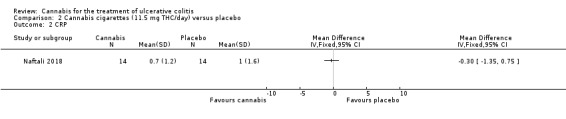
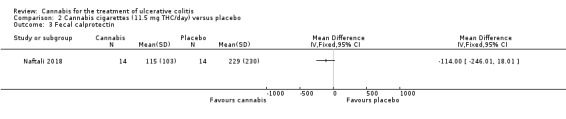
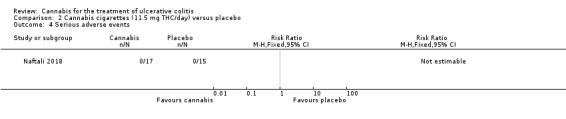
A small study (N = 32) compared cannabis cigarettes to placebo (Naftali 2018). Greater improvements were reported in DAI scores and the Mayo endoscopic score in the cannabis group compared to placebo. After eight weeks of therapy, the DAI in the cannabis group was 4 + 3.2 compared to 8 + 2 in the placebo group (MD ‐4.00, 95% CI ‐5.98 to ‐2.02; 28 participants). After eight weeks of treatment, the Mayo endoscopic score decreased from a median of 2 (IQR 2 to 2.5) to 1 (IQR 0 to 2) in the cannabis group and from 2 (IQR 2 to 2) to 2 (IQR 1.25 to 2) in placebo group. Mean serum CRP concentrations were similar at eight weeks. The mean CRP in the cannabis group was 0.7 mg/L + 1.2 compared to 1 mg/L + 1.6 in the placebo group (MD ‐0.30, 95% CI ‐1.35 to 0.75). The GRADE analysis indicated that the overall certainty of evidence for this outcome was low due to very sparse data (28 participants) (See Table 2). After eight weeks of treatment fecal calprotectin levels were lower in the cannabis group than the placebo group. The mean fecal calprotectin concentration was 115 μg/g ±103 in cannabis group compared to 229 μg/g ± 230 in the placebo group (MD ‐114.00, 95% CI ‐246.01 to 18.01). The authors reported that no serious adverse effects were observed. The outcomes relapse, clinical response, endoscopic remission, endoscopic response, histological response, quality of life, symptom improvement, adverse events, withdrawal due to adverse events and cannabis withdrawal effects were not reported in this study.
Discussion
Summary of main results
This systematic review included two randomized controlled trials (92 participants) that evaluated the efficacy and safety of cannabidiol or cannabis in active ulcerative colitis. The Naftali 2018 study was published as an abstract. However, we were able to obtain further information from the principal investigator to inform our risk of bias assessment. Overall, the studies included small numbers of participants and each study used different doses and formulas of cannabis or cannabinoids and different routes of administration. Irving 2018 used cannabidiol with up to 4.7% THC. Naftali 2018 used cannabis cigarettes containing 11.5 mg of THC (total dose 23 mg THC/day). The studies were not pooled due to differences in routes, formulas, dosages of interventions and patients. The Irving 2018 study enrolled participants with previously diagnosed mild to moderate UC. The Naftali 2018 study enrolled participants who did not respond to conventional medical treatment.
In the Irving 2018 study, the ITT analysis showed that cannabidiol (daily dose 100 mg to 500 mg with up to 4.7% THC) did not appear to provide a benefit over placebo in terms of induction of remission or clinical response. Clinical remission and response rates in the cannabidiol group were 24% and 31% respectively compared to 26% and 22% in the placebo group. GRADE analyses indicated that the overall certainty of the evidence supporting these outcomes was low due to very serious imprecision. Cannibidiol participants were more likely than placebo patients to report an adverse event. All of the patients (29/29) in the cannabidiol group had an adverse event compared to 77% (24/31) of placebo participants. A GRADE analysis indicated that the overall certainty of evidence for this outcome was moderate due to imprecision. These adverse events were considered to be mild or moderate in severity. Common adverse events reported in the cannabidiol group included dizziness, somnolence, disturbance in attention, headache, memory impairment, nausea, dry mouth, vomiting, lower respiratory tract infection, disorientation and fatigue. Common adverse events reported in the placebo group include dizziness, headache, nausea, abdominal pain, worsening ulcerative colitis, abdominal distention, constipation, fatigue, back pain and rash. More cannabidiol participants withdrew from the study due to adverse events compared to placebo. A GRADE analysis indicated that the overall certainty of the evidence supporting this outcome was low due to very serious imprecision. Withdrawls in the cannabidiol group were mostly due to dizziness. Withdrawls in the placebo group were due to worsening ulcerative colitis. There were no serious adverse events in the cannabidiol group (0/29) compared to a 13% (4/31) serious adverse event rate in the placebo group. A GRADE analysis indicated that the overall certainty of the evidence supporting this outcome was low due to very serious imprecision. The serious adverse events in the placebo group were due to worsening ulcerative colitis and one complicated pregnancy with fetal growth restriction and subsequent stillbirth.
Quality of life at eight weeks as measured by the IBDQ was higher in cannabidiol participants (mean 164.2) than in placebo patients (mean 146.8). The mean difference between the cannabidiol and placebo groups was 17.4 points on the IBDQ. An increase in IBDQ score of 16 to 32 points constitutes the upper and lower bounds of a clinically meaningful improvement in health‐related quality of life in patients with ulcerative colitis or Crohn's disease (Irvine 1994; Irvine 2008). However, the 95% confidence interval for this outcome also included no benefit. A GRADE analysis indicated that the overall certainty of the evidence supporting this outcome was moderate due to sparse data. Further research is required to confirm the possible benefit of cannabidiol on health‐related quality of life in people with ulcerative colitis. There was no difference in serum CRP levels at eight weeks. After eight weeks of treatment, there were no differences in pain, stool frequency or rectal bleeding numerical rating scales.
Of note, the intervention group was quite heterogenous with regards to dosage of cannabidiol. Patients received anywhere from 50 mg to 250 mg twice daily of cannabidiol with up to 4.7% THC. This was based on maximally tolerated dose and may have affected outcomes. The authors described a study in rats that found 10 mg/kg was an optimal dose, but did not describe how they selected their own dosing regimen (Jamontt 2010).
The Naftali 2018 study enrolled participants with UC who did not respond to conventional therapy. The primary outcome was not specified in the abstract publication. Participants in the cannabis cigarette group (23 mg of THC/day) had lower DAI scores (4 + 3.2) after eight weeks of treatment than participants in the placebo cigarette group (8 + 2). Treatment with cannabis cigarettes also appeared to impact on the Mayo endoscopic score which was significantly lower in participants who received active treatment. Mean serum CRP and mean fecal calprotectin levels were lower in cannabis cigarette participants compared to placebo cigarette participants. GRADE analyses indicated that the overall certainty of the evidence supporting the CRP outcome was low due to very serious imprecision. The authors reported that no serious adverse effects were observed. The outcomes relapse, clinical response, endoscopic remission, endoscopic response, histological response, quality of life, symptom improvement, adverse events, withdrawal due to adverse events and cannabis withdrawal effects were not reported in this study.
Overall completeness and applicability of evidence
The overall completeness of the evidence is a concern given this review only found two small studies with a total of 92 participants. Larger studies with higher methodological quality are needed to allow for more definite conclusions about the efficacy and safety of cannabis and cannabinoids in UC. Although this review may be applicable to patients with UC, there are concerns with the exclusion criteria of the included studies. Irving 2018 excluded patients with a history of cannabis use in the month prior to study entry and patients with a history of psychiatric disorders other than reactive depression. This is a concern given mental illness and cannabis use is prevalent amongst North American patients with IBD (Hauser 2014; Weiss 2015). Naftali 2018 enrolled patients with treatment‐resistant UC. Other inclusion and exclusion criteria were not described in this abstract publication.
Quality of the evidence
The overall risk of bias for the Irving 2018 study is low. Although the Naftali 2018 study used placebo cannabis cigarettes, we rated this study as high risk of bias for blinding of participants and personnel because unmasking of treatment assignment was very likely given the psychotropic nature of cannabis. GRADE analyses suggest that the overall certainty of evidence supporting the outcomes in this review ranges from low to moderate. For cannabidiol, we rated the overall quality of the evidence supporting the outcomes clinical remission, clinical response, serious adverse events and withdrawal due to adverse events as low quality. The overall certainty of the evidence supporting the outcomes quality of life, CRP and adverse events was rated as moderate. More research is needed before firm conclusions can be drawn regarding the efficacy and safety of cannabidiol in UC. For cannabis cigarettes, we rated the overall certainty of the evidence supporting the outcome CRP as low. Overall, we are uncertain about the benefits and harms of cannabis cigarettes in people with active UC. More research is needed before firm conclusions can be drawn about the use of cannabis cigarettes in UC.
Potential biases in the review process
A comprehensive literature search helped minimize bias related to study selection. Non‐traditional and humanities databases were also searched to capture relevant studies. Two authors (TK and NC) independently screened the studies, extracted data, and assessed the risk of bias. Limitations of this systematic review include the small number of included studies and sparse data. Both included studies were small in size and may have been underpowered to detect a benefit for cannabis in UC should one exist.
Agreements and disagreements with other studies or reviews
We were unable to identify any systematic reviews that assessed the efficacy and safety of cannabis therapy in UC.
Authors' conclusions
Implications for practice.
The effects of cannabis and cannabidiol on UC are uncertain. Thus no firm conclusions regarding the efficacy and safety of cannabis or cannabidiol in adults with active UC can be drawn. There is no evidence for cannabis or cannabinoid use for maintenance of remission in UC.
Implications for research.
Further studies with larger numbers of patients are required to assess the potential benefits and harms of cannabis in UC. Future studies should assess the effects of cannabis in UC patients with active and quiescent disease. Different doses of cannabis and cannabinoids as well as routes of administration should be investigated. Future RCTs should more clearly assess adverse events. Long‐term follow‐up is needed to assess both self‐reported and objective measurements of withdrawal, safety outcomes, consequences in terms of cognitive function, and capacity to function in activities of daily living while using cannabis.
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2019 | Amended | Correction to article metadata; no impact on article content |
History
Protocol first published: Issue 2, 2018 Review first published: Issue 11, 2018
| Date | Event | Description |
|---|---|---|
| 24 May 2019 | Amended | Correction of minor error in reporting of adverse event results |
Acknowledgements
Funding for the Cochrane IBD Group (May 1, 2017 ‐ April 30, 2022) has been provided by Crohn's and Colitis Canada (CCC).
We thank Dr. Peter Irving and Dr. Timna Naftali for providing additional information on their studies.
Appendices
Appendix 1. Appendix 1 ‐ Search Strategy
MEDLINE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. randomized controlled trial/
14. or/1‐13
15. Exp Ulcerative colitis/
16. UC.mp.
17. Ulcerative Colitis*.mp.
18. IBD.mp.
19. Inflammatory bowel disease*.mp.
20. Or/15‐19
21. Exp Marijuana/
22. Cannabis*.mp.
23. Weed*.mp.
24. Marijuana*.mp.
25. Cannabi*.mp.
26. Dronabinol.mp.
27. Cannabichromene.mp.
28. 8‐THC.mp.
29. Nabilone.mp.
30. Tetrahydrocannabivarin.mp.
31. Sativ*.mp.
32. Indica.mp.
33. THC.mp.
34. CBD.mp.
35. Tetrahydrocannabinol.mp.
36. Marinol.mp.
37. Syndros.mp.
38. Or/21‐37
39. 14 and 20 and 38
Embase
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. Exp Ulcerative colitis/
20. UC.mp.
21. Ulcerative colitis*.mp
22. IBD.mp.
23. Inflammatory bowel disease*.mp.
24. Or/19‐23
25. Exp Marijuana/
26. Cannabis*.mp.
27. Weed*.mp.
28. Marijuana*.mp.
29. Cannabi*.mp.
30. Dronabinol.mp.
31. Cannabichromene.mp.
32. 8‐THC.mp.
33. Nabilone.mp.
34. Tetrahydrocannabivarin.mp.
35. Sativ*.mp.
36. Indica.mp.
37. THC.mp.
38. CBD.mp.
39. Tetrahydrocannabinol.mp.
40. Marinol.mp.
41. Syndros.mp.
42. Or/25‐41
43. 18 and 24 and 42
AMED (Allied & Alternative Medicine)
1. Exp Inflammatory bowel disease/
2. UC.mp.
3. Ulcerative colitis*.mp
4. IBD.mp.
5. Or/1‐4
6. Exp Marijuana/
7. Cannabis*.mp.
8. Weed*.mp.
9. Marijuana*.mp.
10. Cannabi*.mp.
11. Dronabinol.mp.
12. Cannabichromene.mp.
13. 8‐THC.mp.
14. Nabilone.mp.
15. Tetrahydrocannabivarin.mp.
16. Sativ*.mp.
17. Indica.mp.
18. THC.mp.
19. CBD.mp.
20. Tetrahydrocannabinol.mp.
21. Marinol.mp.
22. Syndros.mp.
23. Or/6‐22
24. 5 and 23
Psych INFO
ti((Cannabi* OR marijuana OR weed* OR droning OR Cannabichromene OR 8‐tic OR Nabilone OR Tetrahydrocannabivarin OR Sativ* OR Indica OR THC OR CBD OR Tetrahydrocannabinol OR Marinol OR Syndros)) AND ti(( Ulcerative Colitis OR Inflammatory Bowel Disease OR UC))
CENTRAL
#1 MeSH: [Ulcerative colitis] explode all trees
#2 UC
#3 Inflammatory bowel disease
#4 IBD
#5 #1 or #2 or #3 or #4
#6 MeSH: [Cannabis] explode all trees
#7 Cannabis
#8 Marijuana
#9 Weed
#10 Cannabinoid
#11 cannabidiol
#12 cannabigerol
#13 dronabinol
#14 cannabichromene
#15 8 THC
#16 Nabilone
#17 Tetrahydrocannabivarin
#18 Sativ*
#19 Indica
#20 THC
#21 CBD
#22 Tetrahydrocannabinol
#23 Marinol
#24 Syndros
#25 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24
#26 #5 and #25
Clinical trials.gov
1. Ulcerative colitis and Cannabis
2. Ulcerative colitis and Marijuana
3. UC and cannabinoids
4. UC and Cannabis
5. IBD and Cannabis
6. THC and inflammatory bowel disease
European clinical trials register
1. Cannabis and Ulcerative colitis
2. Marijuana and Ulcerative colitis
3. Cannabis and inflammatory bowel disease
4. THC and inflammatory bowel disease
ICTRP
1. Cannabis and Ulcerative colitis
2. Marijuana and Ulcerative colitis
3. Cannabis and inflammatory bowel disease
4. THC and inflammatory bowel disease
Data and analyses
Comparison 1. Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical remission at 10 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical response at 10 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 CRP at 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Inflammatory Bowel Disease Questionnaire (IBDQ) ‐ at 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Symptom measure ‐ pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Symptom measure ‐ rectal bleeding | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Symptom measure ‐ stool frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Withdrawal due to adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
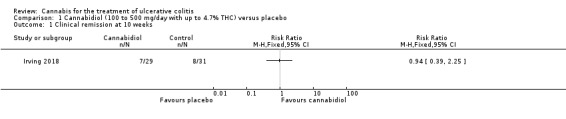
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 1 Clinical remission at 10 weeks.
1.2. Analysis.
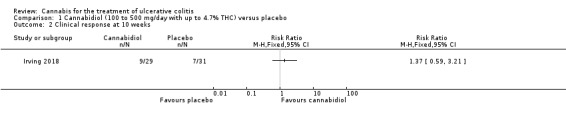
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 2 Clinical response at 10 weeks.
1.3. Analysis.
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 3 CRP at 10 weeks.
1.4. Analysis.
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 4 Inflammatory Bowel Disease Questionnaire (IBDQ) ‐ at 10 weeks.
1.5. Analysis.
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 5 Symptom measure ‐ pain.
1.6. Analysis.
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 6 Symptom measure ‐ rectal bleeding.
1.7. Analysis.
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 7 Symptom measure ‐ stool frequency.
1.8. Analysis.
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 8 Adverse events.
1.9. Analysis.
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 9 Serious adverse events.
1.10. Analysis.
Comparison 1 Cannabidiol (100 to 500 mg/day with up to 4.7% THC) versus placebo, Outcome 10 Withdrawal due to adverse event.
Comparison 2. Cannabis cigarettes (11.5 mg THC/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DAI | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 CRP | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Fecal calprotectin | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 1 DAI.
2.2. Analysis.
Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 2 CRP.
2.3. Analysis.
Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 3 Fecal calprotectin.
2.4. Analysis.
Comparison 2 Cannabis cigarettes (11.5 mg THC/day) versus placebo, Outcome 4 Serious adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Irving 2018.
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group pilot study | |
| Participants | Male or female participants (N = 60) aged 18 years or above (18‐65 years in the Czech Republic) Inclusion criteria included: (1) diagnosed with mild to moderate ulcerative colitis and on a fixed dose of 5‐ASA treatment and have been on a stable dose for at least 2 weeks prior to screening (0 mg dose of 5‐ASA is acceptable) (2) had at screening (Visit 1) and baseline (Visit 2) with a Mayo assessment score of greater than or equal to 4 (≥ 4) but less than or equal to 10 (≤ 10) and with an endoscopy score of at least 1 (≥ 1) , following an adequate exposure to oral or topical 5‐ASA, in the opinion of the investigator (3) In the opinion of the investigator, capable of complying with the study requirements and completing the study (4) Willing and able to give informed consent (5) Willing for his or her name to be notified to the responsible authorities for participation in this study, as applicable (6) Willing to allow his or her primary care practitioner and consultant, if appropriate, to be notified of participation in the study |
|
| Interventions | Patients received either cannabidiol with up to 4.7% THC (n = 29) or placebo (n = 31) gelatin capsules The cannabidiol dose ranged from 50 mg to 250 mg twice daily(GWP42003 is purified from a proprietary Cannabis sativa L. chemotype containing predominantly CBD, up to 4.7 % THC and other compounds) The placebo capsules had excipients alone The cannabidiol with 4.7% THC dose was titrated up to maximal tolerated dose over two‐weeks with maximum dose of 250 mg twice daily (i.e. intervention ranged from 1‐5 capsules taken twice daily) Treatment duration was 10 weeks. |
|
| Outcomes | Note: The original outcome measures submitted in March 2012 were changed in July 2015, one year after completion of the study 2012: Primary outcome was percentage of participants achieving remission, defined as a Mayo Score of < 2 with no sub‐score > 1 Secondary outcomes included serum C‐reactive protein (CRP), serum cytokines (IL‐6, IL‐2 & TNF‐α), fecal calprotectin, body weight measurement, clinical assessments with IBDQ, SGIC, PGAS, MAYO score; and daily diary of stool frequency (0‐4 numerical rate scale), rectal bleeding (0‐4 numerical rate scale), and pain (0‐10 numerical rate scale) 2015: Primary outcome was same, but included PP analysis as well as ITT Secondary outcomes included CRP, IL‐2, IL‐6, TNF‐alpha, stool calprotectin, Inflammatory Bowel Disease Questionnaire (IBDQ) total score, Subject Global Impression of Change (SGIC), Physician's Global Assessment of Illness Severity (PGAS), pain scores using a 0‐10 numerical rate scale, stool frequency scores using a 0‐4 numerical rate scale and PP analysis, rectal bleeding scores using a 0‐4 numerical rate scale and PP analysis, plasma endocannabinoid levels (2‐arachidonoyl glycerol (2‐AG), anandamide (AEA), oleoylethanolamide (OEA), and PEA, Mayo Total Score, Mayo Partial Score, Mayo responder analysis (responder defined as participant with a decrease in their Mayo total score of ≥ 3 points, compared to baseline, with a reduction of at least 1 point in endoscopy findings sub‐score, and body weight. |
|
| Notes | Industry funded by GW Research Limited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician produced a randomization schedule which was held centrally A unique number was then assigned to either the treatment or placebo group according to the randomization schedule |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported double blinding, but also "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" Used identical gelatin capsules for the treatment and placebo groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported double blinding, but also "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" The review of the data was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was a higher rate of withdrawals in the treatment group (13/29) compared to the placebo group (8/31) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the manuscript or on the clinicaltrials.gov web site |
| Other bias | Low risk | Although a higher proportion of the intervention group had previously used cannabis compared to the placebo group and the time since last cannabis use was greater in intervention group compared to the placebo group, these differences were not statistically significant The study appears to be free of other sources of bias |
Naftali 2018.
| Methods | Randomized placebo controlled trial | |
| Participants | Patients with UC who did not respond to conventional medical treatment (N = 32) | |
| Interventions | Either two cigarettes of cannabis (0.5 g of cannabis, corresponding to 11.5 mg THC, n = 17) or placebo (cannabis leaves from which THC was extracted, n = 15) daily for eight weeks | |
| Outcomes | General outcomes reported as Disease activity (DAI), Mayo endoscopic score, endoscopic findings and laboratory tests (CRP, fecal calprotectin) Abstract does not specify primary outcome |
|
| Notes | Additional information was supplied by the principal investigator Timna Naftali which informed our risk of bias assessment NCT01040910 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using block method in a 1:1 ratio to receive either medical cannabis or placebo |
| Allocation concealment (selection bias) | Low risk | The investigators used sequentially numbered drug containers of identical appearance, which were given to the patients outside of the hospital by the pharmacy staff so the medical team did not see them |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although placebo cigarettes were used, blinding was likely to be broken due to the psychotropic effects of cannabis |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded Blinding was open only at the end of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | The primary outcome reported in the study protocol was for Crohn's disease ‐ a reduction in CDAI of 70 points (the study enrolled participants with Crohn's disease and ulcerative colitis) The secondary outcomes reported in the study protocol included adverse events due to cannabis smoking, change in quality of life, change in IL‐10, IL‐2, and TGF beta None of these outcomes were reported in the abstract publication but could potentially be reported in a full manuscript |
| Other bias | Low risk | The study appears to be free of other sources of bias |
5‐ASA: 5‐aminosalicylates
THC: D9‐tetrahydrocannabinol
CDAI: Crohn's disease activity index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Naftali 2013a | Unable to acquire separate data for participants with ulcerative colitis There were 10 participants with ulcerative colitis and 22 participants patients with Crohn's disease Combined results for both groups reported |
| NCT01037322 | This randomized trial assessed the use of cannabis in participants with Crohn's disease |
Contributions of authors
The protocol was drafted by Tahir S Kafil, Tran M Nguyen, John K MacDonald and Nilesh Chande. All authors contributed to writing the final manuscript.
Declarations of interest
Tahir S Kafil: None known
Tran M Nguyen: None known
John K MacDonald: None known
Nilesh Chande has received funds from AbbVie, Ferring, Takeda, Pfizer, and Lupin for consulting; and payment for lectures from AbbVie, Allergan, Takeda, and Shire.
Edited (no change to conclusions)
References
References to studies included in this review
Irving 2018 {published and unpublished data}
- Irving P, Iqbal T, Nwokolo C, Subramanian S, Bloom S, Prasad N, et al. Trial to assess cannabidiol in the symptomatic treatment of ulcerative colitis. Gut 2015;64:A430. [DOI] [PubMed] [Google Scholar]
- Irving P, Iqbal T, Nwokolo C, Subramanian S, Bloom S, Prasar N, et al. Cannabidiol for symptomatic treatment of ulcerative colitis: Results from a randomised, double‐blind, placebo‐controlled, parallel group, multi‐centred pilot study. Journal of Crohn's and Colitis 2015;9:S287. [DOI] [PubMed] [Google Scholar]
- Irving P, et al. A randomised, double‐blind, placebo‐controlled, parallel group, multi‐centred pilot study to assess the symptomatic treatment of ulcerative colitis with cannabidiol. Gastroenterology 2015;148:S275. [Google Scholar]
- Irving PM, Iqbal T, Nwokolo C, Subramanian S, Bloom S, Prasad N, et al. A randomized, double‐blind, placebo‐controlled, parallel‐group, pilot study of cannabidiol‐rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflammatory Bowel Diseases 2018;24(4):714‐24. [DOI] [PubMed] [Google Scholar]
- NCT01562314. A pilot study of GWP42003 in the symptomatic treatment of ulcerative colitis [A randomised, double‐blind, placebo‐controlled parallel group, pilot study of GWP42003 in the symptomatic treatment of ulcerative colitis]. clinicaltrials.gov/ct2/show/NCT01562314 (accessed 1 February 2018).
Naftali 2018 {unpublished data only}
- NCT01040910. Cannabis for inflammatory bowel disease [A double blind placebo controlled study of cannabis smoking in inflammatory bowel disease]. clinicaltrials.gov/ct2/show/study/NCT01040910 (accessed 31 January 2018).
- Naftali T, Lev LB, Benjaminov F, Lish I, Hirsh J, Konikoff FM. Cannabis induces clinical and endoscopic improvement In moderately active ulcerative colitis. ECCO ‐ European Crohn's and Colitis Organisation. 2018.
References to studies excluded from this review
Naftali 2013a {published data only}
- Naftali T, et al. Tetrahydrocannabinol (THC) induces clinical and biochemical improvement with a steroid sparing effect in active inflammatory bowel disease. Journal of Crohn's and Colitis 2013;7:S153. [Google Scholar]
NCT01037322 {unpublished data only}
- NCT01037322. Cannabidiol for inflammatory bowel disease [Use of cannabidiol for the treatment of inflammatory bowel disease]. clinicaltrials.gov/ct2/show/NCT01037322 (accessed 31 January 2018).
Additional references
Borrelli 2009
- Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, et al. Cannabidiol, a safe and non‐psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. Journal of Molecular Medicine 2009;87(11):1111–21. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 2012
- Friedman S, Blumberg RS. Chapter 295: Inflammatory Bowel Disease. Longo et al (editors). Harrison’s Principles of Internal Medicine, 18th Edition. The McGraw‐Hill Companies, 2012. [Google Scholar]
Gagnier 2006
- Gagnier JJ, Boon H, Rochon P, Moher D, Joanne Barnes J, Bombardier C. Recommendations for reporting randomized controlled trials of herbal interventions: explanation and elaboration. Journal of Clinical Epidemiology 2006;59(11):1134‐49. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasenoehrl 2016
- Hasenoehrl C, Taschler U, Storr M, Schicho R. The gastrointestinal tract ‐ a central organ of cannabinoid signaling in health and disease. Journal of Neurogastroenterology and Motility 2016;28(12):1765–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauser 2014
- Hauser W, Moser G, Klose P, Mikocka‐Walus A. Psychosocial issues in evidence‐based guidelines on inflammatory bowel diseases: A review. World Journal of Gastroenterology 2014;20(13):3663‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. Higgins JPT, GreenS editor(s). Cochrane Handbook for Systematic Reviewsof Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
Hill 2015
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems a clinical review. JAMA 2015;313(24):2474‐83. [DOI] [PubMed] [Google Scholar]
Irvine 1994
- Irvine EJ, Feagan B, Rochon J, Archambault A, Fedorak RN, Groll A, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology 1994;106(2):287‐96. [DOI] [PubMed] [Google Scholar]
Irvine 2008
- Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflammatory Bowel Diseases 2008;14(4):554‐65. [DOI] [PubMed] [Google Scholar]
Irving 2015
- Irving P, Iqbal T, Nwokolo C, Subramanian S, Bloom S, Prasad N, et al. Cannabidiol for symptomatic treatment of ulcerative colitis: Results from a randomised, double‐blind, placebo‐controlled, parallel group, multi‐centred pilot study. Journal of Crohn's and Colitis 2015;9:S287. [DOI] [PubMed] [Google Scholar]
Jamontt 2010
- Jamontt JM, Molleman A, Pertwee RG, Parsons ME. The effects of delta‐tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br J Pharmacol 2010;160:712‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2006
- Klein TW, Carbral GA. Cannabinoid‐induced immune suppression and modulation of antigen‐presenting cells. Journal of Neuroimmune Pharmacology 2006;1(1):50–64. [DOI] [PubMed] [Google Scholar]
Lahat 2012
- Lahat A, Lang A, Ben‐Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel Ddisease patients: A pilot prospective study. Digestion 2012;85(1):1‐8. [DOI] [PubMed] [Google Scholar]
Lal 2011
- Lal S, Prasad N, Ryan M, Tangri S, Silverberg MS, Gordon A, et al. Cannabis use amongst patients with inflammatory bowel disease. European Journal of Gastroenterology & Hepatology 2011;23(10):891‐6. [DOI] [PubMed] [Google Scholar]
Leinwand 2017
- Leinwand KL, Gerich ME, Hoffenberg EJ, Collins CB. Manipulation of the endocannabinoid system in colitis: A comprehensive review. Inflammatory Bowel Diseases 2017;23(2):192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marquez 2009
- Marquez L, Suarez J, Iglesias M, Bermudez‐Silva FJ, Rodriguez deFonseca F, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS ONE 2009;4(9):e6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Massa 2004
- Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt B, et al. The endogenous cannabinoid system protects against colonic inflammation. Journal of Clinical Investigation 2004;113(8):1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mello 2012
- Mello NK, Mendelson JH. Chapter 394: Cocaine and Other Commonly Abused Drugs. Harrison’s Principles of Internal Medicine, 18th Edition. The McGraw‐Hill Companies, 2012. [Google Scholar]
Naftali 2013b
- Naftali T, Bar‐Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo‐controlled study. Clinical Gastroenterology and Hepatology 2013;11(10):1276‐80. [DOI] [PubMed] [Google Scholar]
Pinto 2002
- Pinto L, Izzo AA, Mascolo N, Capasso F, Cascio MG, Bisogno T, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 2002;123(1):227‐34. [DOI] [PubMed] [Google Scholar]
Schicho 2014
- Schicho R, Storr M. Cannabis Finds Its Way into Treatment of Crohn’s Disease. Pharmacology 2014;93(1‐2):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. Higgins JPT, GreenS editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
Singh 2012
- Singh UP, Singh NP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Cannabinoids receptor‐2 (CB2) agonist ameliorates colitis inIL‐10−/− mice by attenuating the activation of T cells and promoting their apoptosis. Toxicology and Applied Pharmacology 2012;258(2):256‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tibirica 2010
- Tibirica E. The multiple functions of the endocannabinoid system: a focus on the regulation of food intake. Diabetology & Metabolic Syndrome 2010;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2015
- Weiss A, Friedenberg F. Patterns of cannabis use in patients with inflammatory bowel disease: A population based analysis. Drug and Alcohol Dependence 2015;156:84–9. [DOI] [PubMed] [Google Scholar]
Whiting 2015
- Whiting PF, Wolff RF, Deshpande S, Nisio M, DuffyS, Hernandez A, et al. Cannabinoids for medical use a systematic review and meta‐analysis. JAMA 2015;313(24):2456–73. [DOI] [PubMed] [Google Scholar]
Wright 2005
- Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, et al. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology 2005;129(2):437–53. [DOI] [PubMed] [Google Scholar]


