Abstract
Background
Autism spectrum disorder (ASD) has an estimated prevalence of around 1.7% of the population. People with ASD often also have language difficulties, and about 25% to 30% of children with ASD either fail to develop functional language or are minimally verbal. The ability to communicate effectively is an essential life skill, and difficulties with communication can have a range of adverse outcomes, including poorer academic achievement, behavioural difficulties and reduced quality of life. Historically, most studies have investigated communication interventions for ASD in verbal children. We cannot assume the same interventions will work for minimally verbal children with ASD.
Objectives
To assess the effects of communication interventions for ASD in minimally verbal children.
Search methods
We searched CENTRAL, MEDLINE and Embase as well as 12 other databases and three trials registers in November 2017. We also checked the reference lists of all included studies and relevant reviews, contacting experts in the field as well as authors of identified studies about other potentially relevant ongoing and unpublished studies.
Selection criteria
Randomised controlled trials (RCTs) of communication‐focused interventions for children (under 12 years of age) diagnosed with ASD and who are minimally verbal (fewer than 30 functional words or unable to use speech alone to communicate), compared with no treatment, wait‐list control or treatment as usual.
Data collection and analysis
We used standard Cochrane methodological procedures.
Main results
This review includes two RCTs (154 children aged 32 months to 11 years) of communication interventions for ASD in minimally verbal children compared with a control group (treatment as usual). One RCT used a verbally based intervention (focused playtime intervention; FPI) administered by parents in the home, whereas the other used an alternative and augmentative communication (AAC) intervention (Picture Exchange Communication System; PECS) administered by teachers in a school setting.
The FPI study took place in the USA and included 70 participants (64 boys) aged 32 to 82 months who were minimally verbal and had received a diagnosis of ASD. This intervention focused on developing coordinated toy play between child and parent. Participants received 12 in‐home parent training sessions for 90 minutes per session for 12 weeks, and they were also invited to attend parent advocacy coaching sessions. This study was funded by the National Institute of Child Health and Human Development, the MIND Institute Research Program and a Professional Staff Congress‐City University of New York grant. The PECS study included 84 minimally verbal participants (73 boys) aged 4 to 11 years who had a formal diagnosis of ASD and who were not using PECS beyond phase 1 at baseline. All children attended autism‐specific classes or units, and most classes had a child to adult ratio of 2:1. Teachers and parents received PECS training (two‐day workshop). PECS consultants also conducted six half‐day consultations with each class once per month over five months. This study took place in the UK and was funded by the Three Guineas Trust.
Both included studies had high or unclear risk of bias in at least four of the seven 'Risk of bias' categories, with a lack of blinding for participants and personnel being the most problematic area. Using the GRADE approach, we rated the overall quality of the evidence as very low due to risk of bias, imprecision (small sample sizes and wide confidence intervals) and because there was only one trial identified per type of intervention (i.e. verbally based or AAC).
Both studies focused primarily on communication outcomes (verbal and non‐verbal). One of the studies also collected information on social communication. The FPI study found no significant improvement in spoken communication, measured using the expressive language domain of the Mullen Scale of Early Learning expressive language, at postintervention. However, this study found that children with lower expressive language at baseline (less than 11.3 months age‐equivalent) improved more than children with better expressive language and that the intervention produced expressive language gains in some children. The PECS study found that children enrolled in the AAC intervention were significantly more likely to use verbal initiations and PECS symbols immediately postintervention; however, gains were not maintained 10 months later. There was no evidence that AAC improved frequency of speech, verbal expressive vocabulary or children's social communication or pragmatic language immediately postintervention. Overall, neither of the interventions (PECS or FPI) resulted in maintained improvements in spoken or non‐verbal communication in most children.
Neither study collected information on adverse events, other communication skills, quality of life or behavioural outcomes.
Authors' conclusions
There is limited evidence that verbally based and ACC interventions improve spoken and non‐verbal communication in minimally verbal children with ASD. A substantial number of studies have investigated communication interventions for minimally verbal children with ASD, yet only two studies met inclusion criteria for this review, and we considered the overall quality of the evidence to be very low. In the study that used an AAC intervention, there were significant gains in frequency of PECS use and verbal and non‐verbal initiations, but not in expressive vocabulary or social communication immediately postintervention. In the study that investigated a verbally based intervention, there were no significant gains in expressive language postintervention, but children with lower expressive language at the beginning of the study improved more than those with better expressive language at baseline. Neither study investigated adverse events, other communication skills, quality of life or behavioural outcomes. Future RCTs that compare two interventions and include a control group will allow us to better understand treatment effects in the context of spontaneous maturation and will allow further comparison of different interventions as well as the investigation of moderating factors.
Plain language summary
Are communication interventions effective for minimally verbal children with autism spectrum disorder?
Background
Autism spectrum disorder (ASD) is a condition that is characterised by difficulties with the social aspects of communication, and repetitive and restricted interests and behaviours (e.g. repetitive body movements such as hand flapping, sensory sensitivities and circumscribed interests). People with ASD commonly also have language difficulties, and around 25% to 30% of children are unable to use verbal language to communicate or are minimally verbal (use fewer than 30 words). The ability to communicate is a crucial life skill, and difficulties with communication can have a range of negative consequences such as poorer academic performance, poorer quality of life and behavioural difficulties. Communication interventions generally aim to improve children's ability to communicate either through speech or by supplementing speech with other means (e.g. sign language or pictures).
What did we look at?
We searched 18 databases and trials registers in November 2016 and updated the search in November 2017.
What did our study find?
We identified two trials involving 154 minimally verbal children who had ASD (aged 32 months to 11 years). The studies randomly divided participants into those that received a communication intervention and a control group that did not receive the intervention but received treatment as usual in the community. Both studies focused primarily on communication outcomes (verbal and non‐verbal). One of the studies also collected information on social communication. Neither study collected information on adverse events, other communication skills, quality of life or behavioural outcomes.
One study looked at an alternative and augmentative communication (ACC) intervention (Picture Exchange Communication System; PECS), which teachers gave the children in school. This intervention was conducted over five months and involved teacher training and consultation. PECS is a staged approach where children are taught to exchange a single picture of a desired item or action to another person who then responds to the request. The system progresses toward putting pictures together in sentences and using these sentences in a variety of ways such as commenting and answering questions. This study included 84 participants (73 boys) aged 4 to 11 years and was funded by the Three Guineas Trust. The other study looked at a verbally based intervention (focused playtime intervention; FPI), which is a home‐based parent education programme that aims to promote coordinated play with toys between parents and their children. This study included 70 participants (64 boys) aged 32 months to 82 months and was funded by a Clinical and Patient Educators Association grant (HD35470) from the National Institute of Child Health and Human Development, the MIND Institute Research Program, and a Professional Staff Congress‐City University of New York grant.
Main results
There is limited evidence that verbally based and AAC interventions improve spoken and non‐verbal communication in minimally verbal children with ASD. Both studies included in this review reported gains in aspects of verbal or non‐verbal communication (or both) for some children immediately after the intervention. Neither of the interventions resulted in improvements in verbal or non‐verbal communication that were maintained over time for most children. We rated the overall quality of the evidence as very low because we only found two eligible studies, and they involved few participants. Furthermore, both studies had some methodological limitations that increased their risk of bias.
Recommendations
There is currently limited evidence that verbally based and ACC interventions improve expressive communication skills in minimally verbal children with ASD aged 32 months to 11 years. Additional trials that use communication interventions and compare the effects of these interventions to a control group are urgently required to build the evidence base.
Summary of findings
Background
Description of the condition
Autism spectrum disorder (ASD) is one of the most common neurodevelopmental disabilities. Data from the Autism and Developmental Disabilities Monitoring (ADDM) Network, an active surveillance system in the USA, has reported an increase in prevalence from 6.7 per 1000 in the year 2000 to 16.9 per 1000 in 2014 (Baio 2018). Similar increasing trends have also been observed worldwide (Croen 2002; Gillberg 1999; Lai 2014; May 2017).
ASD is characterised by social communication difficulties and repetitive, restricted behaviours and routines. A clinical diagnosis of ASD is based on observed behavioural criteria, defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM), currently in its fifth edition (APA 2013). Genetic causes for autism are increasingly being identified (Stessman 2017), and the environment is also thought to play a role (Chaste 2012; Modabbernia 2017).
The most recent edition of the DSM removed language difficulties as a core feature of ASD (APA 2013). However, a significant proportion of children with ASD experience difficulties acquiring spoken language. The severity of these difficulties varies considerably. Most children with ASD acquire language during the preschool years (Anderson 2007; Howlin 2009), typically by five years of age (Tager‐Flusberg 2005). However, 25% to 30% of children with ASD fail to develop any functional spoken language or remain minimally verbal (Anderson 2007; Norrelgen 2015; Rose 2016; Tager‐Flusberg 2013). Language difficulties in children with ASD can result in a number of adverse sequelae, including behavioural difficulties (Bott 1997; McClintock 2003; Sigafoos 2000), poor adaptive functioning skills and poor social skills (Anderson 2007; Baghdadli 2007; Hudry 2010). This can result in reduced quality of life and fewer opportunities to participate in the community. Specifically, in some studies of individuals with ASD who are minimally verbal, communication impairment has been found to predict higher levels of aggression (Hartley 2008; Matson 2008), and in one study, up to 25% of minimally verbal children with ASD were found to show an increase in aberrant behaviours such as social withdrawal during adolescence (Lord 2010). Furthermore, self‐injurious behaviour has also been negatively associated with expressive language in children with ASD (Baghdadli 2003).
There has been a lack of consensus regarding the definition of the term 'minimally verbal'. For example, Tager‐Flusberg 2013 proposed benchmark criteria to identify this group; the first stage is 'pre‐verbal', and the next stage is 'first words' where the child is required to have an age‐equivalence of greater than 15 months for vocabulary and pragmatic abilities. Kasari 2013 defines minimally verbal children as those with "a very small repertoire of spoken words or fixed phrases that are used communicatively" (p 480). Others describe this group as children who use no words or single words (Thurm 2015). A number of studies have used definitions provided by diagnostic tools. For example, the Autism Diagnostic Interview ‐ Revised (ADI‐R) is a structured parent interview for diagnosing ASD. Based on parent report about the child's language abilities on some of the interview questions, the ADI‐R can group individuals into different levels. For example, "no phrase speech and greater than or equal to three words but single words used on a daily basis" would be coded as a one, and "no speech used on a daily basis and less than a 5 word vocabulary" would be coded as a two (Rutter 2003). For the purposes of this review, we define minimally verbal children as those having fewer than 30 functional words and/or being unable to use speech alone to communicate, despite being of an age where one would expect them to use language (i.e. mental age of greater than two years). This working definition encompasses many specific definitions used across studies.
To date, research has not been able to identify a consistent reason why some children with ASD fail to acquire verbal language, although several hypotheses have been proposed. Further to the underlying genetic and environmental markers of ASD and language, researchers have applied structural and functional imaging or neurophysiological techniques to examine potential abnormalities in the brain structures of children with ASD to explain language outcomes (De Fossé 2004; Freitag 2009; Just 2004; Kumar 2010; Stanfield 2008). It remains unclear, however, how these structural and functional changes directly explain the language difficulties that occur in children with ASD.
Further to the neural underpinnings of language and ASD, some researchers have focused on cognitive mechanisms impacting verbal development in these children. Cognitive ability (IQ) and difficulties with social communication skills seem to be influential contributors (Norrelgen 2015). For example, one study found non‐verbal cognitive ability, gestures and imitation to be the strongest predictors of later expressive language ability in children with ASD (Luyster 2008). Joint attention skills may also have an impact on the development of language in children with ASD. Joint attention has been defined as the ability to respond to social interaction bids from others and the ability to initiate social interaction with others, as well as the co‐ordination of these two skills (Alessandri 2005; Mundy 2007). A number of studies have found joint attention to be predictive of later language abilities in both children with ASD and in typically developing children (e.g. Charman 2003; Mundy 1990; Mundy 2007). Consequently, a number of intervention programmes have considered joint attention (Dawson 2010; Kasari 2012; Lawton 2012). Another study found that vocal and motor imitation, along with joint attention, were more impaired in children with ASD who had not developed language by five years of age (Luyster 2008; Thurm 2007). It has been proposed that childhood apraxia of speech may cause some children with ASD to fail to develop verbal communication; however, to date, there has been limited evidence to support this hypothesis (Pickett 2009; Schoen 2011; Shriberg 2011).
Whilst aetiological mechanisms are poorly understood, arguably more work has been conducted on prognostication of outcomes in these children. Studies suggest that early acquisition of speech and language (by five years of age) is predictive of more favourable outcomes, such as adaptive and social functioning, in later years (Anderson 2007). There is some evidence that communication interventions are less effective if applied after five years of age (Pickett 2009). Some children develop spoken language during adolescence (12 years of age and above) (Wodka 2013); the chance of this happening is less likely than at younger ages (Tager‐Flusberg 2013). These differential responses to intervention based on a child's age warrant further research, stratified by different age groups (preschool age or school age).
Description of the intervention
To date, there is no consensus regarding the most appropriate and effective communication intervention for children with ASD who are minimally verbal. This Cochrane Review will focus on interventions that target the acquisition and development of communication skills delivered directly during social‐communicative interactions between the child with ASD and another person (usually a therapist). As such, the review will not include pharmaceutical interventions, dietary interventions, or interventions delivered to children through other means without another person facilitating this intervention (e.g. through computers, other forms of technology or animals). In brief, we will include the following four categories of communication interventions in this review: verbally based communication interventions; augmentative and alternative communication (AAC) interventions; combined communication interventions (verbally based intervention plus ACC); and comprehensive interventions with a communication focus. The first three are language‐focused interventions that address specific communication skills, whereas the final approach is a comprehensive treatment model. We categorised the interventions based on our expertise in the area, clinical reasoning (typically interventions are described as verbal, AAC or both/multimodal), and what is known about the mechanisms around interventions for minimally verbal children with ASD.
Verbally based communication interventions
Verbally based interventions use verbal strategies to improve the use of sounds, words and sentences to express oneself. They range from naturalistic, child‐centred and developmental‐pragmatic approaches (e.g. Gutstein 2002), to structured and more didactic methods based on discrete trial training (DTT; e.g. Delprato 2001; Paul 2013); for an overview, see Paul 2008 and Prizant 1998. Responsive Education Prelinguistic Milieu Teaching (RPMT) is an approach that uses modelling of communicative behaviour and correction of child responses, time delay (waiting for the child to initiate or respond) and incidental teaching in natural environments. This approach capitalises on the child's natural interests (Yoder 2006a). Some novel approaches are also being evaluated to see if these may address the specific difficulties experienced by minimally verbal children with ASD (e.g. Rapid Motor Imitation Antecedent, a programme that has been adapted from the DTT model) (Paul 2013).
AAC interventions
AAC interventions refer to a variety of non‐verbal communication methods to help minimally verbal children with ASD acquire and develop speech and language skills (Ganz 2004; Kasari 2014; Merinda 2009). AAC also provides children with an alternative means of communicating if they are unable to do so through speech. There are two main types of AAC: aided and unaided. Aided systems use supplementary materials, including graphic symbols such as picture books, texture‐based systems such as Braille, and speech‐generating devices (SGD) that produce digitalised speech. Unaided systems use manual signs and graphic gestures; these may be formal such as sign language and key word signs, or informal such as idiosyncratic movements. Some AAC interventions incorporate structured and hierarchical behavioural approaches. The Picture Exchange Communication System (PECS; Bondy 1998), for example, includes six phases of teaching; the child moves up the hierarchy as they make progress. In the first phase the child is physically prompted to make specific requests for items they want using pictures, and in the final, most advanced phase, the child uses the pictures to communicate independently. In recent years, the use of new technologies, such as smartphones, iPads and tablets, has burgeoned. A systematic review of tablet computers and portable media devices that had been adapted to serve as SGD found that the devices usually facilitated verbal ability and that language acquisition was faster for individuals using SGDs compared to manual signs or low‐technology AAC (Lorah 2015).
Combined communication interventions (verbally based intervention plus AAC)
Combined programmes, sometimes referred to as 'total communication' interventions, use components from both verbally based communication interventions and AAC interventions. The Hanen More than Words programme (Sussman 2001), for example, is a parent training programme that teaches parents to use strategies (e.g. comment on the child's interests, use AAC, use cues to encourage turn‐taking) in their everyday routines to help their child to communicate. The Means, Opportunities, Reasons and Expectations (MORE) programme is another approach that uses both verbally based communication interventions and AAC interventions (Emerson 2013).
Comprehensive interventions with a communication focus
A broad range of comprehensive programmes for ASD have been developed. These target a range of developmental skills in addition to communication, such as cognition, behaviour, play, emotional regulation and social skills. Pivotal Response Training is an example of a naturalistic behavioural intervention, which facilitates stimulus and response generalisation, increases spontaneity, reduces prompt dependency and increases motivation (Koegel 2006). Other examples of comprehensive interventions include the Denver Model (and Early Start Denver Model) (Rogers 2000; Rogers 2009), the Relationship Development Intervention (Gutstein 2002), the Learning Experience and Alternative Program (LEAP; Strain 1998), the Treatment and Education of Autistic and Related Communication Handicapped Children (TEACCH) programme (Mesibov 2005), the Social Communication, Emotional Regulation Transactional Support (SCERTS) model (Prizant 2006), and applied behaviour analysis (Lovaas 1987; Reichow 2009).
Comprehensive programmes, most of which have not been adapted for use in children who are minimally verbal, go beyond the scope of the current review. We will only include such programmes if they have been adapted, so that the focus is on communication and the primary aim of the study is to improve communication skills. An example of the type of intervention that may be included is that used in a recently published trial by Kasari 2014. This trial combined Joint Attention, Symbolic Play, Engagement and Regulation intervention with Enhanced Milieu Teaching (JASP + EMT) to improve communicative spoken language in minimally verbal children. Similarly, we will only include parent‐mediated interventions, such as the Parent‐Mediated Communication Focused Treatment (PACT; Green 2010) and the Hanen More than Words programme (Sussman 2001), if the intervention targets communication and the aims of the study are communication specific.
Each of the approaches above use different mechanisms to improve speech acquisition and development in minimally verbal children (see How the intervention might work section). Consequently, we had planned to conduct separate subgroup analyses to explore these different types of interventions further (classified in the manner stated above, or even more precisely, depending on the number of studies included in each subgroup).
How the intervention might work
Verbally based communication interventions
The underlying theory behind many verbally based interventions is that the lack of verbal communication originates from other inherent areas of difficulty in ASD, including reduced levels of social motivation, reduced attention to child‐directed speech, immaturity of speech motor development and generally poor imitation skills. Limitations in all of these domains, if serious enough, may lead to severe language impairment. If this theory is correct, an intervention that focuses specifically on speech production together with more intensive and orientated guidance from caregivers, may be enough to trigger the speech learning process. Similarly, efforts that seek out approaches for reciprocal interaction mediated by word exchanges might also work through 'tuning on' or 'tuning up' the expressive language system (Schoen 2011; Shriberg 2011).
AAC interventions
There are a number of theories as to why AAC systems may facilitate vocal production. First, based on the principle of automatic reinforcement, AAC interventions may form an interactive reinforcement system that increases the effectiveness of speech production (Millar 2006). Essentially, if the spoken word and its symbol are presented simultaneously along with a reinforcer, minimally verbal children might begin to produce approximations of the word. Second, for those children with deficits in motor skills or cognitive function, mastering other skills for establishing basic communication may help them to conquer the difficulties encountered during vocal production (Romski 1996). Third, it has been proposed that AAC interventions may reduce the pressure for children to communicate verbally, and, in doing so, reduce demands on auditory‐vocal channels and indirectly increase the chances of spontaneous vocal production (Kasari 2014).
Comprehensive interventions with a communication focus
Some comprehensive programmes have been adapted to specifically target communication. For instance, PRT was designed to target 'pivotal' areas of a child's development (including motivation, response to multiple cues, self‐management, and the initiation of social interactions) (Koegel 2006). Pivotal behaviours are central to a broad range of areas of a child's functioning and, when promoted, may lead to improvements in verbal communication. In addition, parent‐mediated communication interventions aim to enhance parent‐child interactions by increasing parental sensitivity and responsiveness to the child's communication needs. Through a range of interaction strategies, such as routines and familiar, repetitive language and pauses, the child's prelinguistic and early language skills may improve (Green 2010; Sussman 2001). Finally, JASPER interventions may help develop the child's verbal skills by promoting the child's play skills and attention to social interaction (Kasari 2013).
Why it is important to do this review
The ability to communicate is an essential life skill. Communication is key to forming and maintaining relationships, performing academically and enabling people to participate and function in their community. Difficulties communicating can also have an impact on family quality of life and stress. The evidence suggests that 25% to 30% of children with ASD will remain minimally verbal when they reach school age (Anderson 2007; Norrelgen 2015; Tager‐Flusberg 2013). Historically, most studies that have investigated communication interventions for children with ASD have focused on the language development of verbal children. Little attention has been given to children who are minimally verbal (Kasari 2013; Paul 2013; Tager‐Flusberg 2013), with the exception of a workshop on the topic of minimally verbal children with ASD organised by the National Institutes of Health (NIH) in 2010, which signalled the critical need for greater research focus in this area (NIH 2010). At present there is no consensus on what the most effective intervention approach for minimally verbal children with ASD may be. We cannot assume that interventions that work for verbal children will also work for children who are minimally verbal, so a systematic review to evaluate the existing evidence on interventions for this population is needed.
A number of reviews have investigated communication interventions for children with ASD (e.g. Goldstein 2002; Kim 2009; Thunberg 2013). None of these reviews have focused specifically on children with ASD who are minimally verbal. The existing reviews have not systematically reviewed the quality of included studies, making it difficult to judge risk of bias for each included study. This Cochrane Review will use a more comprehensive range of databases to search the literature, apply different inclusion criteria compared to the previous reviews, and provide the most up‐to‐date information on the available evidence on interventions for minimally verbal children with ASD.
In this review, we aim to address two main questions. First, are communication interventions beneficial for minimally verbal children with ASD and, if so, which type of intervention is the most effective? Second, do the outcomes of preschool and school‐age children with ASD differ when such interventions are applied? This review will provide a summary of the available evidence on interventions for children with ASD who are minimally verbal. This will assist decision‐making around the types and amount of intervention for this group of children as well as inform the planning of resources to support them. This information is highly relevant for clinicians, service‐providers, families and policymakers.
Objectives
To assess the effects of communication interventions for ASD in minimally verbal children.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
We included participants that met the following four criteria.
They had received a diagnosis of ASD, autism, autistic disorder, Asperger's syndrome, pervasive developmental disorder (PDD) and PDD ‐ not otherwise specified (PDD‐NOS). The diagnosis must have been made using standard diagnostic criteria, such as the Childhood Autism Rating Scale (CARS; Schopler 1986), Gilliam Autism Rating Scale (GARS; Gilliam 1995), Autism Diagnostic Interview ‐ Revised (ADI‐R; Lord 1994), Autism Diagnostic Observation Schedule (ADOS; Lord 2000), or the Diagnostic Interview for Social and Communication Disorders (DISCO; Wing 2002), or by using established diagnostic criteria such as the International Classification of Diseases (ICD; WHO 1992) or the DSM (APA 2013).
They were under 12 years of age.
They were minimally verbal, defined in any of the following ways: having fewer than 30 functional words (Kasari 2013), being unable to use speech alone to communicate (described as being non‐verbal, having little or no speech, complex communication needs, severe communication impairment), or both.
They were at a cognitive level where one would expect them to use words (i.e. mental age of greater than 12 months, as measured by non‐verbal developmental quotient or IQ). This was to ensure they were not pre‐verbal.
We did not exclude participants if they had comorbidities (e.g. attention deficit hyperactivity disorder (ADHD), epilepsy) in addition to ASD.
Types of interventions
Language‐focused interventions that primarily aimed to improve spoken communication (expressive language or speech, or both) or use of non‐verbal communication (e.g. AAC) compared with no intervention, wait‐list control or treatment as usual. We excluded studies that had other treatment controls (i.e. where one intervention is directly compared to another in the RCT) because there is still no standard or established communication intervention for minimally verbal children with ASD and therefore no reference intervention. Eligible interventions included the following.
Verbally based communication interventions (such as Prelinguistic Milieu Teaching (PMT; Yoder 2006a), Discrete Trial Training (DTT; Lovaas 1987), Prompts for Restructuring Oral Muscular Phonetic Targets (PROMPT; Chumpelik 1984)).
AAC interventions (such as Picture Exchange Communication System (PECS; Bondy 1998), SGDs, sign language).
Combined communication interventions (verbally based communication and AAC interventions).
Comprehensive (multi‐modal) interventions that aim to improve spoken communication or AAC ability, or both.
We excluded studies that used comprehensive interventions for ASD that targeted a range of developmental skills (such as fine motor skills) unless the aims of the study were specifically focused on spoken communication or the use of AAC, or both. Equally, we only included parent training programmes if they had a specific focus on spoken communication, use of AAC or both.
We excluded interventions that focused on improving social skills as a primary aim, although social communication may have been a secondary outcome. We excluded interventions that required physical support from a third party for the child to communicate; for example, Facilitated Communication, described by Biklen 1990, and Rapid Prompting Method (HALO 2016). We only included interventions that involved the child communicating independently.
Types of outcome measures
Primary outcomes
Spoken communication (expressive language or speech, or both), measured using formal standardised assessments, standardised parent‐report checklists and tools, novel instruments (newly designed scales specific to a study), language samples and vocabulary counts. Spoken communication could have been in the form of sounds, words and phrases or sentences, and used in a variety of ways (e.g. to request, comment).
Non‐verbal communication or AAC, measured by, for example, the phase of PECS (Bondy 1998), frequency of use of vocabulary on a speech generating device, number of initiations using PECS or the number of key word signs a child uses.
Combined spoken and non‐verbal communication. This refers to measures that do not distinguish whether they are spoken or non‐verbal. For example, the outcome 'frequency of initiations' may include both spoken and unspoken initiations.
Adverse events (e.g. increased stress in parents or increased anxiety in the child in response to completing a particular intervention), measured by tools such as the Parenting Stress Index (Abidin 1995) or the Spence Children's Anxiety Scale (Spence 1998).
Secondary outcomes
Social communication and pragmatic language skills, measured using tools such as the ADOS social interaction domain or the Early Social Communication Scales (Lord 2000; Mundy 2003).
Other communication skills (e.g. adaptive communication), measured by, for example, the Vineland Adaptive Behavior Scales ‐ Second Edition (VABS‐II; Sparrow 2005).
Quality of life for the individual or their family (e.g. emotional well‐being and support) and parent satisfaction, measured by either standardised instruments, such as the Parenting Stress Index (Abidin 1995), Beach Family Quality of Life Scale (Beach Center on Disabilities 2006), tools such as Focus on the Outcomes of Communication Under Six (Thomas‐Stonell 2013), or by novel instruments invented by the study designers.
Non‐core aspects of behaviour and function (e.g. non‐verbal cognition, challenging behaviours, self‐mutilation and aggression), measured either by standardised instruments or by novel instruments invented by the study designers.
Search methods for identification of studies
Electronic searches
We searched the electronic databases and trials registers listed below in November 2016 and updated the searches in November 2017. We did not use any date or language restrictions, and we sought translations of non‐English language papers and assessed them for potential inclusion in the review, as necessary.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 10), in the Cochrane Library, and which includes the Cochrane Developmental, Psychosocial and Learning Problems Group Specialized Register (searched 8 November 2017).
MEDLINE Ovid (1946 to October week 4 2017).
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (searched 7 November 2017).
MEDLINE Epub Ahead of Print Ovid (searched 7 November 2017).
Embase Ovid (1974 to 7 November 2017).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 8 November 2017).
PsycINFO Ovid (1967 to December week 1 2017).
ERIC EBSCOhost (Education Resources Information Center; 1966 to 8 November 2017).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 7 November 2017).
Conference Proceedings Citation Index ‐ Social Sciences & Humanities Web of Science (CPCI‐SS&H; 1990 to 7 November 2017).
SpeechBITE (speechbite.com; all available years; searched 8 November 2017).
Epistemonikos (epistemonikos.org; all available years; searched 8 November 2017).
Cochrane Database of Systematic Reviews (CDSR; 2017, Issue 11), part of the Cochrane Library (searched 8 November 2017).
Database of Abstracts of Reviews of Effect (DARE; 2015, Issue 2), part of the Cochrane Library (searched 17 November 2016, which was the final issue of DARE; new records are no longer being added).
WorldCat (worldcat.org; all available years; searched 8 November 2017).
ClinicalTrials.gov (clinicaltrials.gov; all available years; searched 8 November 2017).
ISRCTN Registry (www.isrctn.com; all available years; searched 8 November 2017).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; who.int/ictrp/en; all available years; searched 8 November 2017).
The search strategies are in Appendix 1.
Searching other resources
We checked the reference lists of all included studies and relevant reviews for additional references. In addition, we asked experts in the field to provide details of ongoing clinical trials and any relevant unpublished material not captured by our Electronic searches. We also contacted authors of identified trials to ask if they knew of any other published or unpublished studies that our searches missed.
Data collection and analysis
Table 3 summarises the methods we had planned to use, as per our published protocol (Brignell 2016), but which did not employ or were not relevant to this review. We may use these preplanned methods in subsequent updates of this review.
1. Unused methods.
| Method | Approach |
| Types of outcomes | We will synthesise results for the following time points: at the end of intervention, one year after the end of intervention, and after more than one year of follow‐up. |
| Measures of treatment effects |
Dichotomous data If a study only presents data for the change from baseline to follow‐up in the published report, we will contact the corresponding author of the study to obtain data at each time point. Multiple outcomes If included studies provide multiple, interchangeable measures of the same construct at the same time point, we will calculate the average SMD across the outcomes and the average estimated variances for continuous variables; for dichotomous measures, we will choose only the most reliable measure based on the authors' statement or our judgement (e.g. measures from the most commonly used scales). If included studies report the same outcomes (measured by the same scale/tool) differently (e.g. as a dichotomous variable in one study but as a continuous variable in another), we will attempt to transform them to uniform variables using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). In case a well‐established cut‐off point exists, we will transform continuous data to dichotomous data. Otherwise, we will require detailed information from the study authors when they reported dichotomous results. Alternatively, we will use the SMD (or log odds ratios) and their standard errors to combine dichotomous and continuous data, when possible, using the generic inverse variance method in Review Manager 5 (RevMan 5; ReMan 2014). If we are unable to transform the variables (e.g. the study authors do not reply to our request) or to combine them appropriately, we will conduct separate analyses on the variables with different formats. |
| Unit of analysis issues |
Cluster‐randomised trials We will include cluster‐RCTs along with individual‐RCTs in the analysis. We will assess cluster‐RCTs carefully (in terms of recruitment bias, baseline imbalance, loss of clusters and comparability with individually RCTs) for potential unit‐of‐analysis errors. If it is unclear whether or not an included study applied proper controls for clustering, we will contact the corresponding author for further details. If the study does not use appropriate controls, we will request individual participant data from the study authors and reanalyse the data using appropriate multilevel models. We will perform the analyses according to the approach described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will analyse effect sizes and standard errors using the generic inverse method in RevMan 5 (ReMan 2014). To adjust for clustering (reducing the size of effect of each clustered trial to its 'effective sample size'), we will use an estimate of the intracluster correlation coefficient (ICC) extracted from the trial, as described in Higgins 2011. Where we can derive ICCs from other sources, we will state this clearly in the Results section and we will conduct a sensitivity analysis to investigate the effects of variation in the ICC (see 'Sensitivity analysis' section below). Studies with multiple intervention arms If a single included trial reports multiple intervention arms, we will only include the relevant arms. If more than one intervention arm is relevant to our review, we will first estimate if they are sufficiently similar to be combined. For instance, arms with the same intervention but different frequency of application, or arms with essentially the same intervention but with minor modifications in each group, can be treated as a whole intervention group. If so, we will combine all eligible intervention groups and compare them with the combined results of eligible control groups, thus making single, pair‐wise comparisons. Where two comparisons (e.g. intervention A versus control and intervention B versus control) are required to be entered into the same meta‐analysis separately, we will halve the number of participants in the control group to avoid double counting the participants. |
| Dealing with missing data | For studies with missing data due to loss of follow‐up/attrition, we will conduct analyses using the intention‐to‐treat (ITT) approach. We will impute the outcomes for the missing participants using both a 'best‐case' and 'worst case' scenario for dichotomous data. In the case that the missing data are continuous variables (i.e. no mean or standard deviation (SD) reported), we will attempt to calculate them using the standard errors, confidence intervals (CI) and t values, according to the methods described in Higgins 2011. If we are unable to retrieve or derive the missing data, we will describe the missing data for each trial included in the review in the 'Risk of bias' tables (beneath the 'Characteristics of included studies' tables); and if the proportion is large (greater than 20%), we will consider downgrading the quality level of the body of evidence. We will discuss the extent to which missing data can affect the results and mention it in the Authors' conclusions section. We will conduct a sensitivity analysis to explore the impact of including studies with high levels of missing data in the overall assessment of effect (see 'Sensitivity analysis' section below), using the strategy described in Deeks 2017. |
| Assessment of heterogeneity | We will perform tests for heterogeneity using the Chi2 test, to assess whether observed differences in results are compatible with chance alone. Furthermore, we will use the I2 statistic to quantify inconsistency across studies. We will define the presence of heterogeneity by a P value of less than 0.10 from the Chi2 test and an I2 statistic value of greater than 50%, as described in Deeks 2017. We are aware that, in the case of small sample size or few included studies, a non‐significant result of heterogeneity analyses must not be taken as evidence of no heterogeneity. We will explore possible sources of heterogeneity by subgroup analysis and investigation of heterogeneity (see 'Subgroup analysis and investigation of heterogeneity' section below) and sensitivity analysis (see 'Sensitivity analysis' section below). |
| Assessment of reporting bias | Where we are able to pool outcome data from 10 trials or more, we will draw a funnel plot (intervention effect estimate versus standard error of intervention effect estimate), to examine the possibility of reporting bias. If we find funnel plot asymmetry, we will further investigate clinical heterogeneity of studies as a possible explanation. We will use the 'contour‐enhanced' funnel plot (Peters 2008), to distinguish asymmetry due to publication bias from that due to other factors; asymmetry is more likely caused by factors other than by publication bias when the supposed missing studies are in areas of higher statistical significance. |
| Data synthesis | We will use RevMan 5 to pool all eligible trials that apply communication interventions on minimally verbal children compared to no intervention or usual treatment (ReMan 2014). In the primary analyses, we will pool data from all types of interventions together. Given that we expected to find substantial clinical heterogeneity — the included interventions will have been designed according to different theories and approaches — we will pool the available data using a random‐effects model, weighted by the inverse of the variance estimate, as described in Deeks 2017. We will report the estimate of the between‐study variance in a random‐effects meta‐analysis (known as Tau2). We will conduct separate analyses for different types of interventions using subgroup analyses (see 'Subgroup analysis and investigation of heterogeneity' section directly below). |
| Subgroup analysis and investigation of heterogeneity | We will use the approach developed by Borenstein 2008, to formally investigate differences between two or more subgroups. This method conducts a standard test for heterogeneity across subgroup results rather than across individual study results, and has been implemented in RevMan 5 (ReMan 2014). If we identify a small number of studies or small sample sizes (or both), we will use caution when we interpret the subgroup analyses and will discuss the limitations of the findings (e.g. potential for confounding) to avoid over‐interpretation of the results. For ethical reasons, most intervention studies in the field of ASD do not conduct RCTs using a 'true' control group (i.e. one group that receives an intervention and the other receives no intervention at all). Most studies use a treatment as usual (TAU) control group. TAU means the children may be receiving a range of interventions in the community (e.g. one session of speech pathology per week), but these interventions are not an intervention arm in the randomised controlled trial. We will discuss the limitations of interpreting data when a study has used TAU control groups. We will examine the following data presented in the included studies by conducting subgroup analyses.
|
| Sensitivity analysis | We will perform sensitivity analyses to assess the impact of each of the following on the effect estimate.
|
AAC: augmentative and alternative communication interventions; ASD: autism spectrum disorder; RCT: randomised controlled trial; SMD: standardised mean difference; TAU: treatment as usual.
Selection of studies
Two review authors (AB, KC) independently screened titles and abstracts identified by our searches for potentially relevant studies. Of those deemed potentially relevant, the same review authors obtained and independently assessed the full text against the inclusion criteria (Criteria for considering studies for this review). We resolved any disagreements through discussion or, if required, by consulting a third review author (AM).
We identified and excluded duplicates, and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We listed all excluded studies and the reasons for their exclusion in Characteristics of excluded studies tables.
We recorded the selection process in sufficient detail to produce a PRISMA flow diagram (Liberati 2009).
Data extraction and management
We extracted data on each of the following criteria from each included study.
Methods (study design, total duration, number of study centres and location, study setting, withdrawals, date of study).
Participants (number (N), mean age, age range, sex, severity of condition, diagnostic criteria, inclusion criteria, exclusion criteria).
Interventions (intervention, comparison, concomitant intervention, excluded interventions).
Outcomes (primary and secondary outcomes specified and collected, time points reported).
Notes (funding for trial, or any notable conflicts of interest of trial authors).
Two review authors (AB, KC) independently extracted data from the included studies and recorded them in the Characteristics of included studies tables. We resolved disagreements by consensus or by involving a third review author (AM).
One review author (AB) manually inputted the data from the data collection form into Review Manager 5 (RevMan 5) (ReMan 2014). A second review author (KC) spot‐checked study characteristics for accuracy against the trial report. Once completed, both reviewers (AB, KC) double checked that they had entered the data correctly, by comparing the study reports with how the data were presented in the systematic review.
Assessment of risk of bias in included studies
Two review authors (AB, KC) independently assessed the risk of bias of each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions and set out in Appendix 2 (Higgins 2017). The same two review authors consulted a third assessor (AM) to resolve any differences of opinion. Both reviewers assessed the risk of bias for each included study across the following seven domains and assigned ratings of low, high or unclear risk of bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias.
Where available, we have provided a quote from the study report together with a justification for the judgement in the 'Risk of bias' table (beneath the Characteristics of included studies table). We summarised the 'Risk of bias' judgements across different studies for each of the domains listed above by graph and by text in the Risk of bias in included studies section of the review. Where information on risk of bias related to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
When considering the effects of interventions, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
Dichotomous outcomes
We calculated odds ratios (OR) for dichotomous variables (e.g. clinical improvement or no clinical improvement) and presented these with 95% confidence intervals (CI).
Ordinal outcomes
We presented ordinal outcomes directly as ordinal data, and presented assumed (control) and corresponding (intervention) risk compared for each ordinal value separately. We presented data as corresponding risk per 1000. We did not transform ordinal data into continuous or dichotomous data due to the skew of the data and the nature of the ordinal scales (an arbitrary cut‐point would not be as clinically meaningful as the full ordinal scale).
Continuous outcomes
For continuous data, we calculated mean differences (MD) as long as studies used the same measurement, or standardised mean differences (SMDs) when studies use different scales, together with their corresponding 95% CI. We ensured that higher scores for continuous outcomes had the same meaning for the particular outcome, explained the direction to the reader, and reported where we reversed the directions if this was necessary. If a study did not report standard deviations (SD) or standard errors, we contacted the corresponding author of the study to obtain this information. If necessary, we sought to calculate effect estimates from t statistics, analysis of variance (ANOVA) tables or other statistics, as appropriate.
Unit of analysis issues
We encountered no unit of analysis problems. Howlin 2007 was a cluster‐randomised trial (with 17 clusters), and the analysis took clustering into account. See Table 3 and Brignell 2016 for the methods reported in our published protocol for managing unit of analysis issues should they arise in subsequent updates of this review.
Dealing with missing data
For studies without complete reports (studies identified by abstract only), or without complete information in full reports (critical data could not be found in report), we contacted investigators or study sponsors to obtain the missing data, where possible. We documented any details provided by the study authors and used for further analysis. Table 3 summarises the methods we had planned to use, as per our published protocol (Brignell 2016), but which did not employ or were not relevant to this review. We may use these preplanned methods in subsequent updates of this review.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining differences between the two included studies. We examined participant characteristics, timing and type of interventions or controls, as well as types of outcomes measured (see Description of studies in the Results section below).
Assessment of reporting biases
We were unable to assess reporting bias as planned (Brignell 2016), as there were too few studies.
Data synthesis
We were unable to conduct our analyses as planned because of the small number of studies that met inclusion criteria for this review (see Table 3 for unused methods and Brignell 2016). Given that no comparison included more than one study, it was not appropriate to synthesise the data into one meta‐analysis. Instead, we have presented the results of each included study separately and conducted 'Risk of bias' assessment on each study.
Subgroup analysis and investigation of heterogeneity
When an individual study provided information on our prespecified subgroups, we examined differences visually, by inspecting their CI; non‐overlapping CIs indicate a statistically significant difference in effect between subgroups.
We were not able to conduct our preplanned subgroup analyses (Brignell 2016), as there were too few studies for meta‐analyses. See Table 3 for details on these planned subgroup analyses.
Sensitivity analysis
We were unable to conduct our preplanned sensitivity analyses (Brignell 2016), as there were too few studies for meta‐analyses. See Table 3 for details on these planned sensitivity analyses.
'Summary of findings' tables
We created 'Summary of findings' tables using the software developed by the GRADE working group for our two main comparisons (GRADEpro 2015): PECS or FPI versus treatment as usual. We included the following outcomes, assessed immediately postintervention, in the tables: spoken communication; non‐verbal communication or AAC; combined verbal and non‐verbal communication or AAC; adverse events; social communication and pragmatic language skills; other communication skills; quality of life for the individual or their family and parent satisfaction; and non‐core aspects of behaviour and function.
Two review authors (HS, AB) independently assessed the overall quality of the body of evidence using the GRADE approach (GRADE 2004; Guyatt 2008); AM arbitrated any disagreements. Using this approach, the same two authors graded the quality of the evidence for each outcome as high, moderate, low or very low, according to the presence of the following criteria: limitations in the design and implementation of studies; indirectness of evidence; unexplained heterogeneity or inconsistency of results; imprecision of results; and high probability of publication bias. We presented these ratings in the 'Summary of findings' tables and provided our reasons for downgrading the quality of the evidence in the footnotes.
Results
Description of studies
Results of the search
The search yielded 8248 records. Of these, 1915 records were duplicates, and we judged 6101 to be irrelevant based on their titles and abstracts, mostly because they were not RCTs. We retrieved and assessed the full‐text reports of the remaining 232 records. Of these, 69 appeared to be eligible for inclusion (Criteria for considering studies for this review). However, on closer inspection and discussion between three review authors (AB, KC, AM), we decided that only two studies (from four reports) met our inclusion criteria. Additionally, one study (from one report) is awaiting classification, and two trials are ongoing (i.e. they have been registered but do not yet have results). See Figure 1.
1.

Study flow diagram.
Included studies
Two studies met our inclusion criteria for this review (Howlin 2007; Siller 2013). See the Characteristics of included studies for full details of each study.
Study design
Howlin 2007 was an open‐label, cluster‐RCT (17 clusters) in which the unit of randomisation was the school classroom (average class size of five children). Siller 2013 was a standard, single‐blinded RCT (blind to assessor).
Participants
Both included studies aimed to include children with ASD who had limited or no use of spoken language. However, their inclusion criteria had differences in their definition of spoken language, as well as the age range of participants: Howlin 2007 included children aged 4 to 11 years, whereas Siller 2013 included children aged six years or younger.
Verbally based intervention
Siller 2013 included 70 children (64 boys, 6 girls), with a mean age of 58.3 months (SD 12.7) in the intervention group and 55.9 months (SD 11.9) in the control group. The study included children with limited or no use of spoken language (generally fewer than 25 words and no phrases), and whose mothers were fluent in English and who lived within a 90‐minute driving distance to the lab. All children had a previous diagnosis of ASD, which was confirmed in the study using both the ADI‐R and ADOS‐G. All children met the criteria for autistic disorder on the ADI‐R, with 64 meeting the criteria for autistic disorder on the ADOS. The remaining five children were classified as having ASD. One child did not complete the ADOS.
AAC intervention
Howlin 2007 included 84 children (73 boys, 11 girls), with a mean age of 73.1 months (SD 15.8) in the immediate treatment group, 86.6 months (SD 12.7) in the delayed treatment group, and 85.6 months (SD 3.6) in the no treatment group. The study recruited children from 17 classes (clusters) and required that each class had a minimum of three children meeting the following criteria: children had little or no functional language (i.e. not exceeding single words/productions), no evidence of sensory impairment, and were not using PECS beyond phase 1 (i.e. could only exchange symbols with prompting). All children had a formal clinical diagnosis of autism (i.e. they met the criteria for ASD on the ADOS). Notably, Howlin 2007 used multiple intervention groups (i.e. one immediate treatment group and one delayed treatment group, compared to one control (no treatment) group).
Interventions
The two included studies examined different interventions: Howlin 2007 utilised an AAC intervention and Siller 2013 utilised a verbally based intervention. No studies that utilised combined or comprehensive interventions met our inclusion criteria (see Criteria for considering studies for this review section).
Verbally based intervention
Siller 2013 compared focused playtime intervention (FPI) to a control group. FPI is capacity‐building approach that promotes coordinated toy play between the parent and child. There is an ordered sequence of eight topics. Both the parent and clinician were involved in first half of the session, but only the parent conducted therapy in the second half of the session. Both intervention and control groups were invited to a parent advocacy group. Information was collected on the non‐project services and school programmes attended by both groups 12 months before the study, between intake and exit, and between exit and follow‐up.
AAC intervention
Howlin 2007 compared the PECS intervention to a control group, and administered the intervention within a school context. PECS is an intervention that involves teaching an individual to exchange pictures with other people for desired objects or activities, thereby facilitating the child's ability to initiate communication. There are a series of phases that are arranged in a hierarchy of increasing difficulty. In this study teachers and parents received PECS training (two‐day workshop or 13 hours) followed by consultation. The active treatment period began about one week after training. PECS consultants conducted six half‐day consultations with each class once per month over five months. The consultants recommended and demonstrated strategies to improve children's use of PECS in the classroom, monitored teachers' progress, and provided systematic feedback on implementation of PECS. The classroom teachers were not completely naïve to PECS, but generally their prior use of PECS had been minimal and limited to the first phase of PECS, which included supporting (scaffolding) the child to make requests (Howlin 2007).
Outcome measures
Both included studies used different tools to measure our primary outcomes of spoken and non‐verbal communication, and neither study mentioned adverse events in their reports (Howlin 2007; Siller 2013).
Verbally based intervention
Siller 2013 used the expressive language subtest of the Mullen Scale of Early Learning (MSEL) to assess language change over time. The MSEL is a developmental assessment tool that measures a range of developmental areas. It contains two language subtests: expressive and receptive language. Siller 2013 used age‐equivalent scores and collected measures at baseline, after the 12‐week intervention, and 12 months after the completion of the intervention.
Siller 2013 did not measure any of our secondary outcomes.
AAC intervention
Howlin 2007 videotaped daily snack sessions for a maximum of 15 minutes and coded three variables: frequency of child communicative initiations; frequency of use of PECS symbols; and frequency of speech (including non‐word vocalisations). Frequencies were expressed as rates per minute. The trial also included standardised measures based on the Expressive One Word Picture Vocabulary Test and the British Picture Vocabulary Scales. These scales were completed three times throughout the study; at baseline after randomisation, after two school terms (7.5 to 10.7 months after baseline assessments), and at the follow‐up assessment (10.4 months after the end of intervention for the immediate treatment group, 7.1 months after the end of intervention for control group, and 4.6 months after the end of intervention for the delayed treatment group).
Regarding the secondary outcomes, Howlin 2007 evaluated change in social communication over time using two scores from the ADOS‐G: the communication domain score and the reciprocal social interaction (RSI) domain score. Howlin 2007 did not measure any of our other secondary outcomes.
Funding
The Three Guineas Trust provided funding support for one study (Howlin 2007). The other study, Siller 2013, was supported by a Clinical and Patient Educators Association grant (HD35470) from the National Institute of Child Health and Human Development, the MIND Institute Research Program, and a Professional Staff Congress‐City University of New York grant.
Excluded studies
Of the 69 full‐text reports that appeared to meet our inclusion criteria (Criteria for considering studies for this review), 11 had two or more publications (as noted in the reference list of Excluded studies). After grouping together multiple reports of the same study, we identified 52 unique studies. We excluded all 52 studies for the following reasons.
Study design: we excluded 11 studies because they were not RCTs (D'Elia 2014; Jalili 2014; Mandell 2013; Rogers 2006; Sallows 2005; Schroder 2015; Serret 2017; Stock 2013; Sweeney 2016; Vernay 2017; Zeina 2015).
Population: we excluded 25 studies due to ineligible participants; in 19 studies participants did not meet the prespecified definition of 'minimally verbal' (Aldred 2004; Casenhiser 2013; Chang 2016; Elder 2011; Fletcher‐Watson 2013; Flores 2014; Fteiha 2017; Hardan 2015; Ingersol 2012; Jemison Pollard 2010; Kaale 2014; Kaiser 2013; Kasari 2006; Oosterling 2010; Pickles 2016; Reitzel 2013; Roberts 2011; Solomon 2014; Venker 2012); 2 studies included participants with low language levels, but the inclusion criteria and descriptive characteristics did not specify that the children were 'minimally verbal' (Landa 2011; Kasari 2015); 3 studies did not use prespecified ASD diagnostic criteria (Martins 2013; Simpson 2013; Smith 2000); and 1 study included participants without ASD (Romski 2010).
Intervention: we excluded six studies due to ineligible interventions; four studies used a broader intervention programme without a specific communication focus (Drew 2002; Tonge 2014; Wetherby 2014; Whelan 2010), and two studies did not include communication‐focused interventions (Field 2001; Wong 2010).
Comparator: we excluded 10 studies due to lack of control comparator (Goods 2013; Gould 2015; Kasari 2014; Paul 2013; Sandiford 2013; Schriebman 2014; Yoder 1988; Yoder 2006b; NCT01018407; NCT01751698). Although all 10 RCTs specifically focused on communication interventions for minimally verbal children with ASD, they did not have a control group for comparison. Rather, they compared one type of intervention to another. These studies compared a range of interventions, including one type of verbally based intervention versus another type of verbally based intervention, and an AAC intervention versus a verbally based intervention. These studies are listed below.
Verbally based intervention versus another type of verbally based intervention
Six studies compared a verbally based intervention with another type of verbally based intervention. Paul 2013 compared Milieu Communication Training versus a Rapid Motor Imitation Antecedent intervention. Sandiford 2013 compared melodic based communication therapy with traditional speech and language therapy. Gould 2015 compared a Joint Attention, Symbolic Play, Engagement and Regulation (JASPER) intervention versus Discrete Trial Training (DTT); the primary outcomes were based around play, but investigators also collected language outcomes. In a pilot study by Goods 2013, all participants received behaviour‐based interventions at school 30 hours per week. Fifteen children who were minimally verbal were randomised into a control or intervention group, which consisted of substitution of JASPER intervention for the behaviour‐based intervention for 30 minutes, twice weekly for three months. We also excluded two ongoing studies: NCT01751698 and NCT01018407. One of these is investigating DTT versus an interpersonal developmental approach (NCT01018407), and the other is comparing DTT versus JASP + EMT (NCT01751698).
AAC intervention versus verbally based intervention
Four studies compared an AAC intervention to a verbally based intervention. Two studies compared the PECS to a different intervention; one compared PECS to Responsive Education and Prelinguistic Milieu Teaching (Yoder 2006b), and the other compared PECS to Pivotal Response Training (Schriebman 2014). Kasari 2014 administered a JASP + EMT intervention for all participants but added an SGD intervention to one of the groups. One study compared four groups who received signing alone, speech therapy alone, simultaneous signing plus speech therapy, and alternating signing plus speech therapy (Yoder 1988).
See Table 4 for further details on studies comparing two different interventions. Note, we have not included ongoing studies in the table, as they do not yet have results.
2. Characteristics of excluded randomised comparison trials.
| Author | Study Design | Participants | Intervention type | Intervention dose/duration | Outcome measures | Outcomes | Methodological issues |
| Goods 2013 | Pilot randomised comparison trial | 15 children with autism. Mean age 4.56 years months (SD 0.85); developmental quotient 31.81. All had < 10 spontaneous functional words (parent report). All attending non‐public school and receiving minimum of 30 h ABA/week | ABA only group versus JASPER + ABA | 12 weeks. Both had 30 h of ABA but 1 group had substitution of JASPER for 30 min, twice a week | MSEL (development). RDLS (receptive/expressive language). Classroom observation measure (engagement states, spontaneous use of gesture in 20‐min free play). ESCS (joint attention, requesting gestures). Structured Play Assessment (play types and diversity) | Significant differences and large effect sizes between groups, with the intervention group having: more play diversity (d = 0.81); less time unengaged (d = 1.63); more initiating, more requesting gestures (d = 1.51). No significant difference in initiating joint attention and requesting gestures. No significant group difference in receptive/expressive language baseline to exit | Small sample and 3 children discontinued. Measured expressive language with RDLS, which assesses a range of language skills so hard to know if vocabulary specifically improved. No traditional control group (all children were receiving intensive ABA intervention) so hard to know if maturation or true treatment effects |
| Gould 2015 | Treatment comparison trial | 65 minimally verbal (< 30 spontaneous, non‐echoed words heard during entry assessments) children with ASD. Age 33–54 months. Cognitive level ≥ 12 months | DTT versus JASPER | 6 months total. 4 months of sessions × 5 days a week, 1 month of sessions × 3 days a week, one month of sessions ×2 days a week. Each session was 60 min. Parent training also included | MSEL (receptive and expressive language). Main outcome measure was frequency and type of play (structured play assessment) | Changes in symbolic play types predicted improvement in receptive (b = 0.89, t (df = 60) = 2.50, P = 0.015) and expressive language (b = 0.51, t (df = 60) = 3.02, P = 0.04). | Study mainly focused on play behaviours as outcomes, although language outcomes were also collected |
| Kasari 2014 | Randomised trial: SMART design (sequential multiple assignment randomised trial) | 61 minimally verbal (< 20 spontaneous different words in 20 min) children with autism. 51 boys; 10 girls. Mean age 6.31 years. Receptive language > 24 months. Excluded those with major medical conditions, sensory or motor disability, uncontrolled seizures, proficient use of SGD. Mean Brief IQ 68.18 (SD 18.96) | JASP + EMT versus JASP +EMT+SGD | First phase: 12 weeks (24 sessions × 1 h each) for each group. SGD was used to model speech 50% of the time in the JASP + EMT + SGD group. Second phase: parents were included in the 24 sessions. For second phase, slow responders had 1 of 3 adapted interventions: intensity of JASP + EMT increased (3 h per week for 12 weeks); addition of SGD to the JASP + SGD (1 h per week for 12 weeks); or intensity of JASP + EMT + SGD increased (3 h per week for 12 weeks). Early responders continued with same phase‐1 intervention | 20‐min natural language sample (both verbal utterances and SGD‐produced utterances were counted). Primary outcome = total number spontaneous communicative utterances (TSCU), including comments, requests and protests. Secondary outcome = total number of different word roots (TDWR) and total number of comments (TCOM) | JASP + EMT + SGD intervention group (versus starting with JASP + EMT alone) had greater TSCU at week 24 (P < 0.01). Average TSCU at week 24 for JASP + EMT + SGD = 61.9 utterances (95% CI 52.8 to 71.0) and JASP + EMT = 40.3 utterances (95% CI 32.7 to 48.0). Approximately double the rate of communicative utterance per minute at baseline. Moderate‐to‐large effect size (0.62). JASP + EMT + SGD group had greater TDWR (P = 0.04) and TCOM (P < 0.01) at week 24. Small‐to‐moderate effect sizes (0.29 for TDWR and 0.44 for TCOM). For all outcomes JASP + EMT + SGD were superior at week 12 in adaptive interventions. For adapted interventions for slow responders, beginning with JASP + EMT and adding the SGD component and intensifying the JASP + EMT + SGD intervention led to greater TSCU (42.7, 95% CI 33.2 to 52.3) than the intensifying JASP + EMT (39.6, 95% CI 28.5 to 50.7), but there was no significant difference between the groups. Overall response rate was 70% by week 12 (77.7% in JASP + EMT + SGD versus 62.2% in JASP + EMT group), which was not statistically significant. Approximately 6.5 children needed to be treated initially with the JASP + EMT + SGD rather than the JASP + EMT for 1 additional child to respond by 12 weeks. Adding in the SGD later for slow responders to JASP + EMT alone was not as effective as having the SGD at the beginning. | Only enrolled 2/3 of intervention targets. The smaller sample size may impact statistical power. No control group so hard to know if maturation or true treatment effects |
| Paul 2013 | Treatment comparison trial (quasi‐randomised) | 22 children with autistic disorder or PDD‐NOS (module 1 ADOS), with < 15 spontaneous words reported by parents measured on CSBS Caregiver Questionnaire and noted during 20‐min play observation and on CSBS behavioural observation. Expressive language < 18 months age‐equivalent on VABS. Non‐verbal mental age of at least 12 months (Mullen Visual Reception Scale). Generalised motor imitation. Excluded those with uncorrected vision or hearing disability. Mean age 4.3 years (SD 1.2) in RMIA group (6 boys, 4 girls), and 3.5 years (SD0.8) years in MCT group (11 boys, 1 girl) | RMIA versus MCT | 36 x 45‐min sessions over 12 weeks. Maintenance assessment occurred 3–6 months postintervention | Tager‐Flusberg 2009 criteria for making progress with intervention from 1 stage to another | No significant differences between the two intervention groups on any of the outcomes at postintervention or maintenance assessments. On average, the RMIA group produced significantly more words and used more language in everyday situations postintervention than before, and maintained these gains 3 to 6 months postintervention. Similar findings for MCT group; however, significant improvement in VABS expressive language scales was not seen. RMIA group: 5/10 met Tager‐Flusberg et al benchmark ‐ VABS EL age‐equivalent of > 15 months, parent report > 30 word on CDI, more than 7 different words types on CSBS play session, expression of at least 2 different communicative intentions with words and 4 different consonants used in CV syllables. All 5 children retained or exceeded these gains at maintenance assessment. MCT group: 5/12 met Tager‐Flusberg et al benchmark ‐ VABS expressive age‐equivalent of > 15 months, parent report of > 20 words on CDI, more than 5 different word types on CSBS play session, expression of 2 different communicative intentions in words and 4 different consonants used in CV syllables. All 5 children retained or exceeded these levels at maintenance assessment. In a moderator analysis, children with higher joint attention scores pre‐intervention improved more than children with lower joint attention scores, regardless of intervention group. Receptive language age equivalent of around 18 months was an important cut point, with those below 18 months more likely to do better with RMIA, and those scoring above 18 months more likely to do better with MCT. |
No control group so hard to know if maturation or true treatment effects. Small sample size limits statistical power and strict inclusion criteria means cannot generalise to all minimally verbal children with ASD. Design not fully randomised as child allocated non‐randomly to MCT group if unable to master the preintervention motor imitation skills required for the RMIA group. This may have biased findings in favour of RMIA group. |
| Sandiford 2013 | Randomised comparison trial | 12 children with autism (11 boys, 1 girl). Non‐verbal (≤ 10 words used on a daily basis and no functional speech). Excluded if receiving speech therapy externally, or has severe hearing or vision impairment |
MBCT versus TSLT | Both groups received 5 weeks of intervention. 4 × 45‐min individual sessions were provided each week | Number of verbal attempts, number of correct words, number of words reported by the parent, and number of imitative attempts. To measure number of verbal attempts and number of correct words over time, a criterion‐referenced vocabulary test was developed by the study. This was given at baseline, the beginning of each intervention week and finally, at the end of intervention | Both groups made significant progress with intervention in number of verbal attempts (P < 0.001), number of correct words (P = 0.04) and number of imitative attempts (P = 0.02) following intervention. The MBCT group progressed significantly in number of verbal attempts after weeks 1–4 and number of correct words after weeks 1 and 3. The TSLT group progressed significantly after weeks 4 and 5. No significant differences in number of verbal attempts or number of correct words between groups postintervention. A significant number of new words were heard at home for the MBCT group (P = 0.04). Children in the MBCT group had more imitative attempts (P = 0.03) relative to the TSLT group. |
Small sample size, especially after some lost to attrition. Not all children completed all sessions. No follow‐up assessment. No control group so hard to know if maturation or true treatment effects |
| Schriebman 2014 | Randomised comparison trial | 39 children with autism (34 boys, 5 girls). Mean age 29.7 months (SD 5.67). Had < 9 intelligible words. No prior PRT or PECS training. No evidence of primary mental retardation or major neurological or sensory impairment | PRT versus PECS | 258 h of intervention (for both the PRT or PECS group). For the first 15 weeks parents participated in 2 × weekly, 2‐h parent education sessions. Child received five × 2‐h sessions/week at home | Spoken language (MSEL: Expressive Language Scale). Spoken vocabulary (EOWVT and the MacArthur Bates Communicative Developmental Inventories). Adaptive communication (Vineland Adaptive Behaviour Scales). AAC (phase of PECS; score of 1–6). Parent satisfaction (survey) | For each measure, a main effect of time indicated improvement in spoken language, adaptive communication, and spoken vocabulary. When collapsed across conditions, gains on the measures from pre‐ to postintervention to follow‐up were statistically significant. In many cases, effect sizes were quite large, especially for vocabulary. There was no main effect of intervention type found on any measure. 12/19 children in the PECS group reached stage 6 PECS, 2 reached stage 5, 2 reached stage 3, and 1 reached stage 2. | No control group so hard to know if maturation or true treatment effects. No maintenance/follow‐up assessment. A significant proportion of children were at basal on EOWVT tool. Some of the coders were not blinded to child's intervention assignment |
| Yoder 1988 | Randomised comparison trial with 4 different interventions | 60 children with (moderate to severe) autism. ≤ 25 word vocabulary (established by parent questionnaire). Expressive/receptive ages ≤ 28 months on SICD tool, hearing and vision WNL. Mean age 5.0–5.6 years. Mean NVIQ 40.5–44.4 | 4 interventions were compared: speech alone, sign alone, simultaneous presentation of sign and speech, and alternating presentation of sign and speech |
Language training: 90 individual sessions daily for 40 min | Total number of different child‐initiated spoken words observed during the 40‐min training sessions. Words had to be initiated by child and used for an intentional purpose. Could not be echolalia or imitated directly from adult | There was a significant effect after controlling for verbal imitation level (P < 0.05). Children in the sign‐alone group (mean 0.78 (SD 0.62)) used significantly fewer spontaneous words than the other 3 groups (Scheffd's D = 0.39, P = 0.01). The effects of speech alone (mean 1.25 (SD 0.67)), simultaneous presentation of sign and speech (mean 1.01 (SD 0.78)) and alternating presentation of sign and speech (mean 0.97 (SD 0.64)) were not significantly different to each other. Children with higher verbal imitation scores used more spontaneous words regardless of intervention group. Verbal imitation ability and vocabulary size predicted later spontaneous words in the sign alone group. Age (P < 0.001), IQ (P < 0.01), and verbal imitation level (P < 0.001) predicted use of spontaneous words (P < 0.001) in the other 3 groups. | Relatively small samples once divided into 4 groups (n = 15 each). Implications for statistical analyses. No control group so hard to know if maturation or true treatment effects |
| Yoder 2006b | Randomised comparison trial | 36 children with ASD (33 autism and 3 PDD‐NOS). Mean age 33.6 (SD 8.4, range 21–54). Mean NVMA 18.6 (SD 3.7, range 11.5–26.5). 31 boys, 5 girls. Minimally verbal ≤ 20 different words used cumulatively during 3 communication samples. Excluded if severe sensory or motor impairments or if primary language at home was not English |
RPMT versus PECS | 3 × 20‐min sessions per week for 6 months. Parents also offered up to 15 h training | 15‐min, semi‐structured, free‐play with examiner where spoken communication measures (frequency of non‐imitative spoken acts and number of different non‐imitative words) were collected. The free‐play measures were repeated at the end of intervention and 6 months after the end of intervention | PECS was more effective than RPMT in increasing the number of non‐imitative spoken communication acts (Cohen's d = 1.15) and number of different non‐imitative words used (d = 1.12) postintervention. Moderate effect sizes. At 6 months follow‐up, the growth rate of the number of different non‐imitative words was faster in the PECS than the RPMT group for children who had relatively higher object exploration at baseline. Children with relatively low object exploration at baseline improved faster when in the RPMT group than in the PECS group. Effects were not maintained 6 months after the end of intervention | No control group so hard to know if maturation or true treatment effects. Examiners and coders not blind to intervention assignment |
ABA: applied behaviour analysis; ADOS: Autism Diagnostic Observation Schedule; CARS: Childhood Autism Rating Scale; CDI: MacArthur Bates communicative development inventories; CSBS: Communication and Symbolic Behavior Scales; DTT: discrete trial training; EL: expressive language; EMT: Enhanced Milieu Teaching; ESI: Early Social Interaction project; EOWVT: Expressive One Word Picture Vocabulary Test;ESCS: Early Social Communication Scales; FPI: focused playtime intervention;IS: Interpersonal Synchrony intervention;JA: joint attention intervention; JASP: joint attention and symbolic play intervention; JASPER: joint attention, symbolic play, engagement and regulation intervention; MBCT: melodic based communication therapy; MCT: Milieu Communication Training; MIT: Melodic Intonation Therapy; MLU: mean length of utterance; MSEL: Mullen Scales of Early Learning; NVIQ: non‐verbal intelligence quotient; NVMA: non‐verbal mental age;PDD‐NOS: pervasive developmental disorder ‐ not otherwise specified;PEAC: Parent Education and Counselling intervention; PEBM: Parent Education and Behaviour Management Program;PECS: Picture Exchange Communication System; PEI: Psychoeducational Intervention; PLS: Preschool Language Scales; PRT: Pivotal Response Training; RDLS: Reynell Developmental Language Scales; RL: receptive language; RMIA: Rapid Motor Imitation Antecedent intervention; RPMT: Responsive Education and Prelinguistic Milieu Teaching; SCERTS: Social Communication, Emotional Regulation, and Transactional Supports; SD: standard deviation; SGD: Speech Generating Device; SP: Symbolic Play intervention; STAR: Strategies for Teaching based on Autism Research; TS(L)T: traditional speech (and language) therapy; VABS: Vineland Adaptive Behaviour Scales; WNL: within normal limits (age appropriate).
Studies awaiting classification
Based on title and abstract, one conference paper, Gilbert 2012, appeared to meet our inclusion criteria (Criteria for considering studies for this review). However, the study authors did not confirm if it did, and we were not able to obtain further detail or data. The study authors reported the participants were also part of a larger, ongoing study (see the Characteristics of studies awaiting classification table for details). This study compared a verbally based intervention to a control group.
Ongoing studies
Two trials met our inclusion criteria but are not yet completed (NCT02291172; NCT02464527). See the Characteristics of ongoing studies tables for details on these studies.
Risk of bias in included studies
Please see the 'Risk of Bias' tables (under Characteristics of included studies tables) for details of the risk of bias in each included study, and Figure 2 for a tabular summary. We judged one study, Howlin 2007, to be at low risk of bias in three domains (selection (random sequence generation), attrition and reporting bias); high risk of bias in two domains (performance and detection bias); and unclear risk of bias in two domains (selection (allocation concealment) and other potential sources of bias). We judged the other study, Siller 2013, to be at low risk of bias in three domains (detection, attrition and reporting bias); high risk of bias in one domain (performance bias); and unclear risk of bias in three domains (selection (random sequence generation and allocation concealment) and other potential sources of bias).
2.
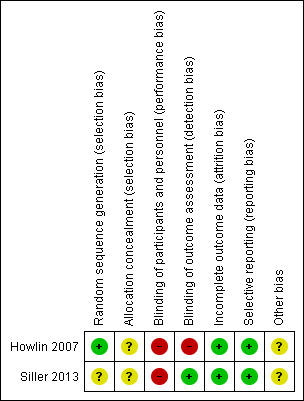
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We considered one study, Howlin 2007, to be at low risk of bias in this domain. In this school‐based study, classes were stratified according to size. In each stratum, classes were randomly allocated to one of the three conditions using an online randomisation programme (www.random.org). We judged the other study, Siller 2013, to be at unclear risk of bias in this domain as they did not report details of how randomisation was completed. In this study, to ensure that two out of every four consecutive children were assigned to the control group, the children were randomised in blocks of four.
Allocation concealment
Neither study reported on how the individuals were informed of their allocation to intervention or control groups; for example, by using sealed envelopes (Howlin 2007; Siller 2013). The nature of these included studies makes it impossible to blind the participants or people administering the intervention to their group allocation. Consequently, we rated both studies at unclear risk of bias in this domain.
Blinding
Blinding of participants and personnel (performance bias)
We considered both studies to be at high risk of performance bias (Howlin 2007; Siller 2013). Participants and personnel were aware of allocation to intervention or control groups in both studies.
Blinding of outcome assessment (detection bias)
We judged one study to be at low risk of detection bias (Siller 2013). Staff conducting assessments or coding were blinded to group assignment, and prior to all outcome assessment sessions, parents were reminded not to reveal their group assignment to the assessment staff. We judged the other study, Howlin 2007, to be at high risk of detection bias, as assessors were not blinded to group assignment. The study authors stated in the manuscript that this was because of financial and personnel limitations.
Incomplete outcome data
We rated both included studies to be at low risk of attrition bias (Howlin 2007; Siller 2013). In Siller 2013, three participants dropped out from the intervention group and two from the control group at the one year follow‐up point (Siller 2013). Analyses were conducted on an intention‐to‐treat basis using multiple imputations to deal with the missing data. Statistical analysis comparing those lost to follow‐up and those who remained found that participants who dropped out of the study took longer to complete the intervention period and received fewer autism‐specific, non‐project services in the community, on average. These differences were then statistically corrected. In Howlin 2007, one child from the immediate treatment group moved away after the trial started, and seven children in the delayed treatment group moved out of classes prior to the start of the intervention. These children did complete assessments, and participants were analysed on an intention‐to‐treat basis (Howlin 2007).
Selective reporting
We rated both studies to be at low risk of reporting bias as all outcomes reported in the study had been prespecified in the prospective trial registration/protocol (Howlin 2007; Siller 2013). Neither study, however, reported data on adverse events.
Other potential sources of bias
We considered both trials to be at unclear risk of other bias (Howlin 2007; Siller 2013). It is difficult in autism spectrum research to control for interventions being obtained in the local community, and neither trial collected detailed information on any additional interventions received by the control groups. In addition, Howlin 2007 reported that some classes in the control arm may have been using some PECS. Treatment fidelity may also have been an issue, although the study authors argue it was intended as a pragmatic trial. The cluster‐randomised controlled design has the potential to introduce biases through differential recruitment of participants within each cluster and methodological difficulties in applying the intention‐to‐treat approach. In the Howlin 2007 study, differential recruitment was unlikely to have taken place, as all children enrolled in each class received the intervention allocated to the class, and there was no opt‐out of any children. However, the Howlin 2007 study had withdrawal of both one whole cluster as well as individual participants within a cluster, along with the addition of one child to the cluster part way through the trial. It is unclear how to analyse these participants from an intention‐to‐treat perspective and the effect of any bias introduced.
Effects of interventions
Summary of findings for the main comparison. Summary of findings: focused playtime intervention versus treatment as usual for minimally verbal children with autism spectrum disorder.
| Focused playtime intervention versus treatment as usual for minimally verbal children with autism spectrum disorder | |||
|
Patient or population: minimally verbal children with autism spectrum disorder Settings: child's home (study set in California, USA) Intervention: focused playtime intervention Comparison: treatment as usual | |||
| Outcomes | Impact | Number of participants (studies) | Quality of the evidence (GRADE) |
|
Spoken communication Measured by: Mullen Scale of Early Learning: Expressive Language Index (MSEL: log (base 2) transformed age equivalent scores) Follow‐up: 20 to 21 weeks |
No significant main effect of functional playtime intervention on expressive language outcomes (t (df = 57) = 1.21, P = 0.23) | 70 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b |
| Non‐verbal communication/AAC | No data were reported for this outcome | ||
| Combined spoken and non‐verbal communication/AAC | No data were reported for this outcome | ||
| Adverse events | No data were reported for this outcome | ||
| Social communication and pragmatic language skills | No data were reported for this outcome | ||
| Other communication skills | No data were reported for this outcome | ||
| Quality of life for the individual or their family and parent satisfaction | No data were reported for this outcome | ||
| Non‐core aspects of behaviour and function | No data were reported for this outcome | ||
| CI: confidence interval. | |||
| GRADE Working Group grades of evidence
High quality: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||
aDowngraded one level for risk of bias (rated as unclear or high risk of bias on 4/7 domains). bDowngraded two levels for imprecision due to small sample size and only one trial identified for comparison.
Summary of findings 2. Summary of findings: Picture Exchange Communication System (PECS) versus treatment as usual for minimally verbal children with autism spectrum disorder.
| Picture Exchange Communication System (PECS) versus treatment as usual for minimally verbal children with autism spectrum disorder | |||
|
Patient or population: minimally verbal children with autism spectrum disorder Settings: school (autism‐specific schools and units in South East London, UK) Intervention: PECS (teacher training and consultation; 2 groups: immediate treatment group and delayed treatment group) Comparison: control (classes where teachers had not received any active direct, in class training/consultancy with PECS consultants) | |||
| Outcomes | Relative effect* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) |
|
Spoken communication Measured by: frequency of speech, including non‐word vocalisations (expressed as rates per minute)a Follow‐up: 2 school termsb |
There was no significant main effect of the PECS intervention on frequency of speech (OR 1.10, 95% CI 0.46 to 2.62, P = 0.83) | 84 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d |
|
Non‐verbal communication/AAC Measured by: frequency of use of PECS symbols (expressed as rates per minute)a Follow‐up: 2 school termsb |
Children in the PECS group were 3.90 times more likely to be in a higher PECS‐use category than children in the control group (OR 3.90, 95% CI 1.75 to 8.68, P < 0.001) | 84 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d |
|
Combined spoken and non‐verbal communication/AAC Measured by: verbal and non‐verbal initiations (expressed as rates per minute)a Follow‐up: 2 school termsb |
Children in the PECS group were 2.73 times (OR 2.73, 95% CI 1.22 to 6.08, P < 0.05 (specific P value not reported in paper)) more likely to to be in the higher initiation‐rate category than the control group | 84 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d |
| Adverse events | No data were reported for this outcome | ||
|
Social communication or pragmatic language Measured by: reciprocal social interaction (ADOS‐G domain scores)a Follow‐up: 2 school termsb |
There was no significant main effect of the PECS intervention on reciprocal social interaction OR 0.55 (95% CI 0.25 to 1.19, P = 0.13) | 84 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d |
| Other communication skills | No data were reported for this outcome | ||
| Quality of life for the individual or their family and parent satisfaction | No data were reported for this outcome | ||
| Non‐core aspects of behaviour and function | No data were reported for this outcome | ||
| The relative risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| AACADOS‐G: Autism Diagnostic Observation Schedule ‐ Generic; CI: Confidence interval; OR: odds ratio; PECS: Picture Exchange Communication System; RCT: Randomised controlled trial. | |||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||
aOnly one outcome measure is reported for each outcome in this table (although further outcome measures were collected by the studies). Outcomes Measures were chosen based on how clinically meaningful they were. b7.5 to 10.7 months after the baseline assessment. cDowngraded one level for risk of bias (rated as unclear or high risk of bias on 4/7 domains). dDowngraded two levels for imprecision due to small sample size, wide CI and only one trial identified for comparison.
We included two studies in this review, both of which utilised different types of intervention: Howlin 2007 utilised an AAC (PECS) intervention, whereas Siller 2013 utilised a verbally based, play‐based (FPI) intervention. No studies met our inclusion criteria for the remaining two intervention types (combined interventions and comprehensive interventions). There was substantial clinical heterogeneity between the included studies, including varied age ranges and inclusion criteria, and different outcome measures (standardised language scores versus coded verbal and non‐verbal behaviours). There was also methodological heterogeneity in the trial designs (one cluster RCT and one standard RCT). Thus, we decided that it was not appropriate to pool the data in meta‐analysis, and consequently have provided a narrative description of the results below.
FPI versus treatment as usual
The primary outcome described below is taken from the same study with 70 children (Siller 2013).
Primary outcomes
Spoken communication
Siller 2013 measured expressive language using the expressive language domain of the Mullen Scale of Early Learning developmental assessment tool. This study reported that the main effect for FPI was not significant (t (df = 57) = 1.21, P = 0.23; very low‐quality evidence, Table 1). Exploratory analysis found that baseline measures of expressive language (specifically 24 children who entered the study with expressive language ability under the 11.3‐month level) moderated a significant, medium‐to‐large effect, accounting for approximately 25% of the variance in the children's subsequent language gains.
Adverse events
Siller 2013 reported no data on adverse events.
Siller 2013 did not measure our other primary (non‐verbal communication or AAC, or combined verbal and non‐verbal communication or AAC) or secondary outcomes (social communication and pragmatic language skills; other communication skills; quality of life for the individual or their family and parent satisfaction; non‐core aspects of behaviour and function).
PECS versus treatment as usual
All primary and secondary outcomes described below come from the same, single study with 84 children (Howlin 2007).
Primary outcomes
Spoken communication
Frequency of speech (including non‐word vocalisations)
Howlin 2007 found no significant main effect of PECS on frequency of speech (OR 1.10, 95% CI 0.46 to 2.62, P = 0.83; very low‐quality evidence, Table 2).
Expressive vocabulary
Howlin 2007 found no significant main effect of PECS on expressive vocabulary (OR 1.01, 95% CI 0.89 to 1.15, P = 0.87).
Non‐verbal communication or AAC: frequency of use of PECS symbols
Howlin 2007 reported a significant main effect of PECS on frequency of use of PECS symbols immediately postintervention. Children in the PECS group were 3.90 times (OR 3.90, 95% CI 1.75 to 8.68, P < 0.001; very low‐quality evidence, Table 2) more likely to to be in the higher PECS‐use category than the control group from time point 1 (baseline) to time point 2 (7.5 to 10.7 months after baseline). However, the main effect was not maintained at the 10‐month follow‐up (time point 3) for the group who had the intervention earlier, with these children being no more likely than the control group to be in a higher PECS‐rate category (OR 1.56, 95% CI 0.46 to 2.62, P = 0.48).
Combined verbal and non‐verbal communication or AAC: frequency of child communicative initiations (included both verbal and non‐verbal initiations)
Howlin 2007 reported a significant main effect of PECS on the frequency of communicative initiations immediately postintervention. Children in the PECS group were 2.73 times more likely to to be in the higher initiation‐rate category than the control group from time point 1 to time point 2 (OR 2.73 95% CI 1.22 to 6.08, P < 0.05 (specific P value not reported in paper); very low‐quality evidence, Table 2). However, the main effect was not maintained at the 10‐month follow‐up for the group that had the intervention earlier, with these children being no more likely than the control group to have a higher initiation rate (OR 1.08, 95% CI 0.30 to 3.90, P = 0.91).
Adverse events
Howlin 2007 reported no data on adverse events.
Secondary outcomes: social communication and pragmatic language skills
Howlin 2007 found no evidence of a significant main effect of PECS immediately postintervention on ADOS communication domain scores (OR 0.52, 95% CI 0.24 to 1.12, P = 0.10) or on the RSI domain scores (OR 0.55, 95% CI 0.25 to 1.19, P = 0.13). However, at the 10‐month follow‐up of the immediate treatment group, there was a significant main effect for RSI scores on the ADOS (OR 0.28, 95% CI 0.09 to 0.89, P < 0.05 (specific P value not reported in paper)). PECS was associated with a decrease in severity scores on this domain, with children being 3.57 times (OR) more likely to be in the lower ordinal category (less severe difficulties) on the RSI scale.
Subgroup analysis and investigation of heterogeneity
We included only two studies in this review (Howlin 2007; Siller 2013), investigating different types of interventions. Thus, we were not able to conduct subgroup analyses or assess heterogeneity as we had planned (Brignell 2016); see Table 3 for methods archived for use in future updates. Both included studies provided limited information on subgroups (Howlin 2007; Siller 2013). Neither study provided the specific subgroup information prespecified in our protocol (Brignell 2016); however, one study provided information that was related to baseline language capability (Siller 2013). In this study, authors conducted exploratory moderator analyses to assess whether the baseline characteristics of the children modified treatment gains. They found that baseline measures of expressive language moderated effects on residual gain scores in expressive language (t (df = 57) = −2.47, P < 0.05). For 24 children with expressive language below a 11.3‐month level at baseline, effect size estimates indicated a medium‐to‐large effect: ʄ2 = 0.25 (range 0.09 to 0.36). We have reported analyses conducted by the study authors as we did not have the data required to see if the CI overlapped.
Quality of the evidence
Overall, based on the GRADE criteria (GRADE 2004), we rated the quality of the evidence for all reported outcomes as very low. For one study, Siller 2013, we downgraded the quality of the evidence by one level for risk of bias (rated at unclear or high risk of bias on 4/7 domains) and by two levels for imprecision due to small sample size and because there was only one trial for comparison (see Table 1). For the other study, Howlin 2007, we downgraded the quality of the evidence by one level for risk of bias (rated at unclear or high risk of bias on 4/7 domains) and by two levels for imprecision due to small sample size, wide CIs, and because there was only one trial for comparison (see Table 2).
Discussion
Summary of main results
We identified and included in this review two studies (N = 154) that met our inclusion criteria (Howlin 2007; Siller 2013). We were not able to pool the data from these studies in a meta‐analysis because they used different types of communication interventions. One study assessed a verbally based intervention (FPI) versus a control group that involved parent training (Siller 2013), whereas the other study assessed an AAC intervention (PECS) administered by teachers versus a control group (Howlin 2007).
The study that examined a verbally based intervention (FPI) versus a control group, Siller 2013, found no significant effect for the intervention group on spoken communication (assessed with expressive language age‐equivalent scores). However, a further exploratory analysis found that children with relatively lower expressive language ability at baseline (specifically, less than 11.3 months age‐equivalent) benefited more from the intervention than children with better expressive language at baseline. The authors concluded that parents of children with relatively better language may require additional strategies to FPI.
The other study that assessed an AAC intervention (PECS) versus a control group, Howlin 2007, found that children in the PECS group were significantly more likely to use verbal communicative initiations and PECS symbols more frequently immediately postintervention. However, this effect was not maintained for either of these two outcomes at the follow‐up assessment 10 months later. This study did not find a significant effect of PECS for frequency of speech or for expressive and receptive vocabulary based on standardised tools (Howlin 2007). Of interest, the study found no effect for the secondary outcomes of communication and RSI ADOS scores immediately postintervention; however, PECS was associated with a decrease in RSI severity score (i.e. less severe deficits in RSI skills) at the 10‐month follow‐up point.
Neither study found an effect on the primary outcome that was maintained over time, and neither study provided information on adverse effects of interventions. It was possible there was some contamination in one of the included studies (Howlin 2007), as some teachers designated to the control group may still have been using some PECS, as it was not a requirement that PECS use be ceased for the control group. Furthermore, children in the control group in the Siller 2013 study may also have been accessing treatments in the community. This may have impacted the strength of the findings in each study and should be considered when interpreting our results.
The findings from Siller 2013 on moderators of interventions raises important questions around variation in treatment response. Given the substantial heterogeneity in ASD and wide range of underpinning factors that may be at play in preventing a child from talking, it is not surprising that children respond differently to different interventions (see Hudry 2017, Trembath 2014 and Vivanti 2014 for further discussion around predictors of treatment response). One approach that may facilitate a better understanding of who responds better to which intervention is to compare two interventions and investigate child factors that may moderate outcomes from each one. This approach has been used in several studies of children with ASD who are minimally verbal (e.g. Paul 2013; Yoder 2006b), and these studies have identified factors that may be important in predicting a child's response to different interventions. For example, Paul 2013 found that children with a receptive language age‐equivalent below 18 months were more likely to do better with the Rapid Motor Imitation Antecedent training, whereas those scoring above 18 months were more likely to do better with Milieu Communication Training. Furthermore, Yoder 2006b found that Responsive Education and Prelinguistic Milieu Teaching increased the frequency of generalised turn‐taking and generalised initiating joint attention more than PECS, but that the increase in generalising joint attention only occurred for children who had at least some initiating joint attention at baseline. PECS, however, increased the children's ability to make generalised requests more than Responsive Education and Prelinguistic Milieu Teaching for those children who had limited ability to initiate joint attention at baseline. Our understanding of who responds to what type of intervention is still in its infancy, but studies such as these have the potential to help us to move further toward personalising currently available interventions.
We identified two studies that compared interventions to control groups; however, it is important to note that there are 10 other trials focused on communication interventions for minimally verbal children with ASD that compared two different interventions (rather than using a control group). These studies make contributions to the literature, but we excluded them based on our study protocol (Brignell 2016), in which we specified that we would only include studies that compared an intervention to a control. We could not include these studies in the data analysis for our review, but several showed positive results (e.g. Goods 2013; Kasari 2014; Paul 2013; Yoder 1988), providing some indirect evidence for interventions in minimally verbal children with ASD. Such studies help us to parse the heterogeneity in the minimally verbal population with ASD by showing that, while different treatments may not be effective for all children in this population, they may improve expressive language in certain subgroups. At the highest level, however, these studies suggest that expressive language can at least be improved in some minimally verbal children with ASD and point the way to future work to more carefully identify the baseline characteristics of children who may respond to one or another therapy. We have provided detail on these studies in this review because we recognise their value to the field of interventions in minimally verbal children with ASD (see Table 4).
Overall completeness and applicability of evidence
This review provides up‐to‐date evidence on communication interventions for children with ASD who are minimally verbal. We identified only two studies with a total of 154 participants. One study was conducted at the children's homes, the other at school, and both studies had good applicability. We found data extraction and analysis difficult because of the absence of a uniform tool for outcome measurement, as well as the non‐standardised design in one study (cluster‐RCT with multiple groups). Moreover, we were unable to examine some important outcomes, such as adverse events and functional communication skills. No studies that employed comprehensive or combined interventions met our inclusion criteria, so we were unable to draw any conclusions about the evidence for these interventions. Overall, we conclude that the completeness of current evidence is low. Across all outcomes, available data are insufficient to show whether verbally based or AAC interventions (specifically PECS and FPI) are effective at increasing verbal or augmentative communication skills in minimally verbal children with ASD.
Quality of the evidence
Using the GRADE approach (GRADE 2004), we considered the overall quality of the evidence to be very low. We downgraded all outcomes by at least two points due to imprecision (only two trials identified, both of which had small sample sizes) and inconsistency (large CIs). The studies did not always report sufficient detail to allow assessment of risk bias.
Potential biases in the review process
We did not note any obvious biases in this review. To minimise bias, two review authors independently screened the records, extracted data, and assessed the risk of bias and quality of evidence. With the exception of one study that could not be classified due to lack of detail around inclusion criteria (Characteristics of studies awaiting classification), we included all studies that met our inclusion criteria (Criteria for considering studies for this review). A limitation of this review is that we did not include studies that included treatment‐to‐treatment comparisons. These studies make up most intervention trials for children who are minimally verbal. However, we followed our prior, published protocol of this review (Brignell 2016), and we have reported details of the treatment‐to‐treatment comparison trials in a separate table (see Table 4).
Agreements and disagreements with other studies or reviews
We did not identify any other studies or reviews.
Authors' conclusions
Implications for practice.
This review identified two RCTs that used a communication intervention in minimally verbal children with ASD and included a control group. There was limited evidence that communication interventions (AAC and verbally based) improve aspects of communication for minimally verbal children with ASD. In the study that used an AAC intervention (PECS), children in the intervention group were more likely to use verbal or non‐verbal communicative initiations and PECS symbols than the control group immediately after the intervention. In the study that used a verbally based intervention, children did not make significant improvements in their expressive language as a group; however, a subgroup of children did improve. Both studies had methodological limitations, and we rated the overall quality of the evidence as very low. The search used in this review also identified 10 RCTs that compared one or more interventions in minimally verbal children with ASD but did not have a control or treatment‐as‐usual comparison group (Goods 2013; Gould 2015; Kasari 2014; NCT01018407; NCT01751698; Paul 2013; Sandiford 2013; Schriebman 2014; Yoder 1988; Yoder 2006b). These studies did not meet our inclusion criteria (Criteria for considering studies for this review), but they make a contribution to the literature in the field of ASD in minimally verbal children. Clinicians, families and consumers should be aware of the lack of evidence for the effectiveness of communication interventions for minimally verbal children with ASD. If they choose to use these interventions, they should carefully monitor the child's progress and adapt as indicated.
Implications for research.
Our review may be viewed as having inclusion criteria that were too strict to capture the aforementioned 10 studies (Goods 2013; Gould 2015; Kasari 2014; NCT01018407; NCT01751698; Paul 2013; Sandiford 2013; Schriebman 2014; Yoder 1988; Yoder 2006b), as well as other studies that used non‐RCT designs (e.g. single‐case design studies). While the inclusion of these studies was beyond the scope of the current review, a systematic review of such studies that employs appropriate statistical methods for treatment‐to‐treatment comparisons, along with an assessment of study quality, would be desirable. Future studies that compare two interventions but also include a control group will allow us to better understand effects in the context of spontaneous maturation as well allow comparison of two interventions. Control groups can include treatment as usual or wait‐list controls, and these types of groups avoid the ethical challenges around withholding intervention for such a high‐needs group. To increase sample sizes and improve the evidence base, intervention studies that include children with a range of language abilities could potentially present the results of the minimally verbal children as a separate subgroup, or studies could use subsamples of children who have genetic diagnoses (and are at greater risk for being minimally verbal).
Appropriate outcome measures that are sensitive to change are required because currently available standardised tools are not well suited to study outcomes in minimally verbal children. In addition, studies should investigate other important outcomes, such as function and participation, social communication, adaptive communication skills, quality of life and behaviour, along with primary measures of communication. Economic implications should also be evaluated. Studies will need to be creative in developing novel approaches to interventions that are closely linked to our developing understanding of the aetiology and neurobiology of ASD and related disorders, the ultimate goal being tailored interventions based on child and family characteristics, needs and strengths. This review found that most studies of minimally verbal children have focused on the preschool and early primary school years, and there is an urgent need for studies in older children who remain minimally verbal. While the literature has emphasised the importance of being able to speak before five years of age on later outcomes (e.g. Pickett 2009), we cannot assume that children over the age of five years do not make the same progress with intervention, or that interventions for younger children are appropriate and effective for older children.
Acknowledgements
We thank Joanne Duffield, Managing Editor, and Geraldine Macdonald, Co‐ordinating Editor, of Cochrane Developmental, Psychosocial and Learning Problems, for providing advice and administrative and logistical support for the conduct of the current review. We wish to thank Margaret Anderson for assistance with the searches. We also thank Sarah Arnup for her statistical advice around data from the cluster‐RCT.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, and which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register
Searched: 17 November 2016 (340 records); 8 November 2017 (41 records)
#1[mh "child development disorders,pervasive"] #2[mh ^"Developmental Disabilities"] #3pervasive next development* next disorder* #4(pervasive near/3 child*) #5(PDD or PDDs or PDD‐NOS or ASD or ASDs) #6autis* #7asperger* #8kanner* #9"childhood schizophrenia" #10Rett* #11{or #1‐#10} #12[mh Communication] #13[mh "communication disorders"] #14[mh "language development disorders"] #15[mh "Language Development"] #16[mh "nonverbal communication"] #17[mh "Verbal Behavior"] #18[mh "Verbal learning"] #19((communicat* or speech or language) near/5 (need* or dysfunction* or impair* or disabil* or disabl* or delay*)) #20(minimal* near/1 (speech* or verbal*)) #21(limited near/1 (speech* or verbal*)) #22(nonverbal or non‐verbal or "no speech") #23(pre NEXT linguistic or prelinguistic) #24[mh vocabulary] #25(vocabular* or lexicon*) #26functional next word* #27[mh Mutism] #28(mute or mutism) #29{or #12‐#28} #30#11 and #29 in Trials [Note: Final line of search 2016] #31#11 and #29 Publication Year from 2016 to 2017, in Trials [Note: Final line of search 2017]
MEDLINE Ovid
Searched: 17 November 2016 (2118 records); 8 November 2017 (41 records)
1 exp child development disorders, pervasive/ 2 Developmental Disabilities/ 3 pervasive development$ disorder$.tw,kf. 4 (pervasive adj3 child$).tw,kf. 5 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 6 autis$.tw,kf. 7 asperger$.tw,kf. 8 kanner$.tw,kf. 9 childhood schizophrenia.tw,kf. 10 Rett$.tw,kf. 11 or/1‐10 12 exp Communication/ 13 exp communication disorders/ 14 language development disorders/ 15 exp Language Development/ 16 nonverbal communication/ 17 Verbal Behavior/ 18 exp Verbal learning/ 19 ((communicat$ or speech or language) adj4 (need$ or dysfunction$ or impair$ or disabil$ or disabl$ or delay$)).tw,kf. 20 (minimal$ adj1 (speech$ or verbal$)).tw,kf. 21 (limited adj1 (speech$ or verbal$)).tw,kf. 22 (nonverbal or non‐verbal or "no speech").tw,kf. 23 (pre‐linguistic or prelinguistic).tw,kf. 24 vocabulary/ 25 (vocabular$ or lexicon$).tw,kf. 26 functional word$.tw,kf. 27 Mutism/ 28 (mute or mutism).tw,kf. 29 or/12‐28 30 randomized controlled trial.pt. 31 controlled clinical trial.pt. 32 randomi#ed.ab. 33 placebo$.ab. 34 drug therapy.fs. 35 randomly.ab. 36 trial.ab. 37 groups.ab. 38 or/30‐37 39 exp animals/ not humans.sh. 40 38 not 39 41 11 and 29 and 40 42 remove duplicates from 41 [Note: Final line of search 2016] 43 Limit 42 to ed=20161101‐20171026 [Note:Final line of search 2017]
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid
Searched: 17 November 2016 (240 records); 8 November 2017 (128 records after deduplication with previous set)
1 pervasive development$ disorder$.tw,kf. 2 (pervasive adj3 child$).tw,kf. 3 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 4 autis$.tw,kf. 5 asperger$.tw,kf. 6 kanner$.tw,kf. 7 childhood schizophrenia.tw,kf. 8 Rett$.tw,kf. 9 or/1‐8 10 ((communicat$ or speech or language) adj4 (need$ or dysfunction$ or impair$ or disabil$ or disabl$ or delay$)).tw,kf. 11 (minimal$ adj1 (speech$ or verbal$)).tw,kf. 12 (limited adj1 (speech$ or verbal$)).tw,kf. 13 (nonverbal or non‐verbal or "no speech").tw,kf. 14 (pre‐linguistic or prelinguistic).tw,kf. 15 (vocabular$ or lexicon$).tw,kf. 16 functional word$.tw,kf. 17 (mute or mutism).tw,kf. 18 or/10‐17 19 9 and 18 20 (random$ or trial$ or control$ or group$ or blind$ or placebo$ or prospective or meta‐analysis or systematic review or RCT).mp. 21 19 and 20
MEDLINE Epub Ahead of Print Ovid
Searched: 17 November 2016 (62 records); 8 November 2017 (51 records after deduplication with previous set)
1 pervasive development$ disorder$.tw,kf. 2 (pervasive adj3 child$).tw,kf. 3 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 4 autis$.tw,kf. 5 asperger$.tw,kf. 6 kanner$.tw,kf. 7 childhood schizophrenia.tw,kf. 8 Rett$.tw,kf. 9 or/1‐8 10 ((communicat$ or speech or language) adj4 (need$ or dysfunction$ or impair$ or disabil$ or disabl$ or delay$)).tw,kf. 11 (minimal$ adj1 (speech$ or verbal$)).tw,kf. 12 (limited adj1 (speech$ or verbal$)).tw,kf. 13 (nonverbal or non‐verbal or "no speech").tw,kf. 14 (pre‐linguistic or prelinguistic).tw,kf. 15 (vocabular$ or lexicon$).tw,kf. 16 functional word$.tw,kf. 17 (mute or mutism).tw,kf. 18 or/10‐17 19 9 and 18 20 (random$ or trial$ or control$ or group$ or blind$ or placebo$ or prospective or meta‐analysis or systematic review or RCT).mp. 21 19 and 20
Embase Ovid
Searched: 17 November 2016 (1532 records); 8 November 2017 (157 records after deduplication with previous set)
1 exp autism/ 2 (PDD or PDDs or ASD or ASDs).tw. 3 autis$.tw. 4 asperger$.tw. 5 kanner$.tw. 6 childhood schizophreni$.tw. 7 Rett$.tw. 8 developmental disorder/ 9 pervasive development$ disorder$.tw. 10 (pervasive adj3 child$).tw. 11 or/1‐10 12 interpersonal communication/ 13 communication skill/ 14 language ability/ 15 exp communication disorder/ 16 language development/ 17 speech disorder/ 18 exp verbal behavior/ 19 nonverbal communication/ 20 developmental language disorder/ 21 ((communicat$ or speech or language) adj4 (need$ or dysfunction$ or impair$ or disabil$ or disabl$ or delay$)).tw,kw. 22 (limited adj1 (speech$ or verbal$)).tw,kw. 23 (nonverbal or non‐verbal or "no speech").tw,kw. 24 (pre‐linguistic or prelinguistic).tw,kw. 25 (vocabular$ or lexicon$).tw,kw. 26 functional word$.tw,kw. 27 mutism/ 28 (mute or mutism).tw,kw. 29 or/12‐28 30 11 and 29 31 Randomized controlled trial/ 32 controlled clinical trial/ 33 Single blind procedure/ 34 Double blind procedure/ 35 triple blind procedure/ 36 Crossover procedure/ 37 (crossover or cross‐over).tw. 38 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 39 Placebo/ 40 placebo.tw. 41 prospective.tw. 42 factorial$.tw. 43 random$.tw. 44 assign$.ab. 45 allocat$.tw. 46 volunteer$.ab. 47 or/31‐46 48 30 and 47 49 remove duplicates from 48
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
Searched: 17 November 2016 (924 records); 8 November 2017 (98 records after deduplication with previous set)
S1 (MH "Child Development Disorders, Pervasive+") S2 pervasive development* disorder* S3 pervasive n3 child* S4 (PDD or PDDs or PDD‐NOS or ASD or ASDs) S5 autis* S6 asperger* S7 kanner* S8 childhood schizophrenia S9 Rett* S10 (MH "Developmental Disabilities") S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 S12 (MH "Communication+") S13 (MH "Communicative Disorders") S14 (MH "Language Disorders") S15 (MH "Language Development") S16 (MH "Nonverbal Communication+") S17 (MH "Verbal Behavior") S18 ((communicat* or speech or language) n5 (need* or dysfunction* or impair* or disabil* or disabl* or delay*)) S19 (minimal* n1 (speech* or verbal*)) S20 (limited n1 (speech* or verbal*)) S21 (nonverbal or non‐verbal or "no speech") S22 (pre‐linguistic or prelinguistic) S23 (MH "Vocabulary") S24 (vocabular* or lexicon*) S25 functional word* S26 (MH "Mutism") S27 (mute or mutism) S28 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 S29 S11 AND S28 S30 (MH "Clinical Trials+") S31 MH random assignment S32 (MH "Meta Analysis") S33 (MH "Crossover Design") S34 (MH "Quantitative Studies") S35 PT randomized controlled trial S36 PT Clinical trial S37 (clinical trial*) or (control* N2 trial*) S38 ("follow‐up study" or "follow‐up research") S39 (prospectiv* study or prospectiv* research) S40 (evaluat* N2 study or evaluat* N2 research) S41 (MH "Program Evaluation") S42 (MH "Treatment Outcomes") S43 TI(single N2 mask* or single N2 blind*) OR AB(single N2 mask* or single N2 blind*) S44 TI((doubl* N2 mask*) or (doubl* N2 blind*)) OR AB((doubl* N2 mask*) or (doubl* N2 blind*)) S45 TI ((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*)) OR AB((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*) S46 random* S47 S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 S48 S29 AND S47
PsycINFO Ovid
Searched: without limits 18 December 2017 (884 records)
1 exp pervasive developmental disorders/ 2 Developmental disabilities/ 3 pervasive development$ disorder$.tw. 4 (pervasive adj3 child$).tw. 5 autis$.tw. 6 asperger$.tw. 7 (autis$ or ASD or ASDs).tw. 8 Rett$.tw. 9 Kanner$.tw. 10 (PDD or PDDs or PDD‐NOS).tw. 11 childhood schizophren$.tw. 12 or/1‐11 13 exp Communication Disorders/ 14 exp Language Disorders/ 15 Language Development/ 16 Language Delay/ 17 nonverbal communication/ 18 oral communication/ 19 verbal communication/ 20 verbal ability/ 21 ((communicat$ or speech or language) adj3 (need$ or dysfunction$ or impair$ or disabil$ or disabl$ or delay$)).tw,id. 22 (minimal$ adj1 (speech$ or verbal$)).tw,id. 23 (limited adj1 (speech$ or verbal$)).tw,id. 24 (nonverbal or non‐verbal or "no speech").tw,id. 25 (pre‐linguistic or prelinguistic).tw,id. 26 vocabulary/ 27 (vocabular$ or lexicon$).tw,id. 28 (mute or mutism).tw,id. 29 or/13‐28 30 12 and 29 31 clinical trials/ 32 random sampling/ 33 placebo/ 34 Experiment controls/ 35 ((clinic$ or control$) adj (study or trial$ or experiment$)).tw,id. 36 ((compar$ or control$ or experiment$ or treat$) adj3 (subjects or group$)).tw,id. 37 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw,id. 38 exp program evaluation/ 39 exp treatment outcomes/ 40 exp treatment effectiveness evaluation/ 41 (random$ or RCT).tw,id. 42 or/31‐41 43 30 and 42
ERIC EBSCOhost (Education Resources Information Center)
Searched: 17 November 2016 (1205 records); 8 November 2017 (50 records after deduplication with previous set)
S1 DE "Developmental Disabilities" S2 DE "Pervasive Developmental Disorders" OR DE "Asperger Syndrome" OR DE "Autism" S3 (pervasive development* disorder* or PDD or PDDs) S4 (autis* or ASD or ASDs) S5 Asperger* S6 Rett* S7 Kanner* S8 childhood schizophren* S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10 DE "Communication Disorders" S11 DE "Language Impairments" S12 DE "Language Acquisition" S13 DE "Interpersonal Communication" S14 DE "Augmentative and Alternative Communication" S15 DE "Nonverbal Communication" S16 DE "Communication Problems" S17 ((communicat* or speech or language) n5 (need* or dysfunction* or impair* or disabil* or disabl* or delay*)) S18 (minimal* n1 (speech* or verbal*)) S19 (limited n1 (speech* or verbal*)) S20 (nonverbal or non‐verbal or "no speech") S21 (pre‐linguistic or prelinguistic) S22 DE "Verbal Ability" S23 DE "Speech Skills" S24 (pre‐linguistic or prelinguistic) S25 DE "Communication Skills" S26 DE "Communication Strategies" S27 DE "Vocabulary Development" S28 DE "Vocabulary Skills" S29 (vocabular* or lexicon*) S30 functional word* S31 (mute or mutism) S32 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 S33 S9 AND S32 S34 DE "Meta Analysis" OR DE "Evaluation Research" OR DE "Control Groups" OR DE "Experimental Groups" OR DE "Longitudinal Studies" OR DE "Followup Studies" OR DE "Program Effectiveness" OR DE "Program Evaluation" S35 TI (random* or trial* or EXPERIMENT* OR PROSPECTIVE* OR longitudinal or BLIND* or CONTROL*) OR AB (random* or trial* or PROSPECTIVE* OR EXPERIMENT*or longitudinal or BLIND* or CONTROL*) S36 S34 OR S35 S37 S33 AND S36
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S)
Searched: 17 November 2016 (58 records); 8 November 2017 (12 records after deduplication with previous set)
#21 #20 AND #19 #20 TS=(random* or trial* or control* or group* or placebo* or prospectiv* or assign* or "meta‐analysis" or "systematic review" or RCT) #19 #18 AND #9 #18 #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 #17 TS=(mute or mutism) #16 TS=("functional word*") #15 TS=(vocabular* or lexicon*) #14 TS=(pre‐linguistic or prelinguistic) #13 TS=(nonverbal or non‐verbal or "no speech") #12 TS=(limited near/1 (speech* or verbal*)) #11 TS=(minimal* near/1 (speech* or verbal*)) #10 TS=((communicat* or speech or language) near/5 (need* or dysfunction* or impair* or disabil* or disabl* or delay*)) #9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #8 TS=(Rett* ) #7 TS=("childhood schizophrenia" ) #6 TS=(Kanner*) #5 TS=(asperger*) #4 TS=(autis*) #3 TS=(PDD or PDDs or PDD‐NOS or ASD or ASDs) #2 TS=(pervasive near/3 child*) #1 TS= ("pervasive development* disorder* ")
Conference Proceedings Citation Index ‐ Social Sciences & Humanities Web of Science (CPCI‐SS&H)
Searched: 17 November 2016 (51 records); 8 November 2017 (7 records after deduplication with previous set)
#21 #20 AND #19 #20 TS=(random* or trial* or control* or group* or placebo* or prospectiv* or assign* or "meta‐analysis" or "systematic review" or RCT) #19 #18 AND #9 #18 #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 #17 TS=(mute or mutism) #16 TS=("functional word*") #15 TS=(vocabular* or lexicon*) #14 TS=(pre‐linguistic or prelinguistic) #13 TS=(nonverbal or non‐verbal or "no speech") #12 TS=(limited near/1 (speech* or verbal*)) #11 TS=(minimal* near/1 (speech* or verbal*)) #10 TS=((communicat* or speech or language) near/5 (need* or dysfunction* or impair* or disabil* or disabl* or delay*)) #9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #8 TS=(Rett* ) #7 TS=("childhood schizophrenia" ) #6 TS=(Kanner*) #5 TS=(asperger*) #4 TS=(autis*) #3 TS=(PDD or PDDs or PDD‐NOS or ASD or ASDs) #2 TS=(pervasive near/3 child*) #1 TS= ("pervasive development* disorder* ")
speechBITE (speechbite.com)
Searched: 18 November 2016 (17 records); 8 November 2017 (3 records after deduplication with previous set)
Advanced search:
keywords minimally verbal AND Population = autistic spectrum disorders AND Research design = RCT keywords non verbal AND Population = autistic AND Research design = RCT keywords nonverbal AND Population = autistic AND Research design = RCT keywords pre‐verbal AND Population = autistic AND Research design = RCT keywords preverbal AND Population = autistic AND Research design = RCT keywords prelinguistic AND Population = autistic AND Research design = RCT keywords pre linguistic AND Population = autistic AND Research design = RCT
Epistemonikos (epistemonikos.org)
Searched: 18 November 2016 (23 records); 8 November 2017 (3 records after deduplication with previous set)
(title:(autis* OR Asperg* OR ASD OR pervasive) OR abstract:(autis* OR Asperg* OR ASD OR pervasive)) AND (title:("minimally verbal" OR "non verbal" OR "nonverbal" OR mute OR mutism) OR abstract:("minimally verbal" OR "non verbal" OR "nonverbal" OR mute OR mutism))
Cochrane Database of Systematic Reviews (CDSR), part of the Cochrane Library
Searched: 18 November 2016 (5 records); 8 November 2017 (2 records after deduplication with previous set)
#1[mh "child development disorders, pervasive"] #2[mh ^"Developmental Disabilities"] #3(pervasive next development* next disorder*):ti,ab #4(pervasive near/3 child*):ti,ab #5(PDD or PDDs or PDD‐NOS or ASD or ASDs):ti,ab #6autis*:ti,ab #7asperger*:ti,ab #8kanner*:ti,ab #9"childhood schizophrenia":ti,ab #10Rett*:ti,ab #11{or #1‐#10} #12[mh Communication] #13[mh "communication disorders"] #14[mh "language development disorders"] #15[mh "Language Development"] #16[mh "nonverbal communication"] #17[mh "Verbal Behavior"] #18[mh "Verbal learning"] #19((communicat* or speech or language) near/5 (need* or dysfunction* or impair* or disabil* or disabl* or delay*)):ti,ab #20(minimal* near/1 (speech* or verbal*)):ti,ab #21(limited near/1 (speech* or verbal*)):ti,ab #22(nonverbal or non‐verbal or "no speech"):ti,ab #23(pre‐linguistic or prelinguistic):ti,ab #24 [mh vocabulary] #25(vocabular* or lexicon*):ti,ab #26 (functional next word*):ti,ab #27[mh Mutism] #28(mute or mutism):ti,ab #29{or #12‐#28} #30 #11 and #29 in Cochrane Reviews (Reviews and Protocols)
Database of Abstracts of Reviews of Effect (DARE), part of the Cochrane Library
Searched: 18 November 2016 (27 records). Not searched in 2017 as this was DARE's final issue.
#1[mh "child development disorders, pervasive"] #2[mh ^"Developmental Disabilities"] #3(pervasive next development* next disorder*):ti,ab #4(pervasive near/3 child*):ti,ab #5(PDD or PDDs or PDD‐NOS or ASD or ASDs):ti,ab #6autis*:ti,ab #7asperger*:ti,ab #8kanner*:ti,ab #9"childhood schizophrenia":ti,ab #10Rett*:ti,ab #11{or #1‐#10} #12[mh Communication] #13[mh "communication disorders"] #14[mh "language development disorders"] #15[mh "Language Development"] #16[mh "nonverbal communication"] #17[mh "Verbal Behavior"] #18[mh "Verbal learning"] #19((communicat* or speech or language) near/5 (need* or dysfunction* or impair* or disabil* or disabl* or delay*)):ti,ab #20(minimal* near/1 (speech* or verbal*)):ti,ab #21(limited near/1 (speech* or verbal*)):ti,ab #22(nonverbal or non‐verbal or "no speech"):ti,ab #23(pre‐linguistic or prelinguistic):ti,ab #24 [mh vocabulary] #25(vocabular* or lexicon*):ti,ab #26 (functional next word*):ti,ab #27[mh Mutism] #28(mute or mutism):ti,ab #29{or #12‐#28} #30#11 and #29 in Other Reviews
WorldCat (www.worldcat.org)
Searched: 18 November 2016 (0 records); 8 November 2017 (1 record)
kw:((MINIMALLY VERBAL or NONVERBAL or NON VERBAL or mute) AND (autis* or asperg*)) AND format=thesis/dissertation
ClinicalTrials.gov (clinicaltrials.gov)
Searched: 18 November 2016 (17 records); 8 November 2017 (3 records first posted from 18 November 2016 to 8 November 2017)
Advanced search: Search terms | non verbal OR nonverbal OR minimally verbal OR mute | Interventional Studies | Autism OR ASD OR Asperger OR PDD OR " pervasive developmental " | Child
ISRCTN Registry (www.isrctn.com)
Searched: 18 November 2016 (1 records); 8 November 2017 (2 records)
VERBAL within Condition: AUTISM AND Participant age range: Child
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; who.int/ictrp/en)
Searched: 18 November 2016 (22 records); 8 November 2017 (2 records registered since previous search)
TITLE verbal OR nonverbal OR mute OR speech AND CONDITION autistic OR autism OR asd
Appendix 2. 'Risk of bias' criteria
Random sequence generation
Low risk of bias: the sequence generation process was truly random; for example, a random number table or computer random number generator was used
High risk of bias: the sequence generation process was non‐random; for example, allocation by judgement of the clinician or preference of the participant
Unclear risk of bias: there was insufficient information about the sequence generation process to permit a judgement of low or high risk of bias
Allocation concealment
Low risk of bias: allocation of participants was done using central allocation or sequentially numbered, opaque, sealed envelopes
High risk of bias: the allocation sequence was known to the investigators or participants
Unclear risk of bias: the trial was described as randomised, but the method used to conceal the allocation was not described
Blinding of participants and personnel
Low risk of bias: blinding of participants and key study personnel was ensured and it was unlikely that blinding was broken, or unlikely a lack of blinding would influence the outcome (or both)
High risk of bias: blinding of participants and key study personnel was not done or was broken, and outcomes were likely to be influenced by the lack of blinding
Unclear risk of bias: the term 'blinding' was mentioned but no details were given for who was blinded and how the blinding was ensured to permit a judgement of low or high risk of bias
Blinding of outcome assessment
Low risk of bias: the outcome assessors were blinded to the intervention received by the participants, or the outcome was unlikely to be influenced by lack of blinding
High risk of bias: no blinding of outcome assessment was mentioned but measurement was likely to be influenced by lack of blinding, or blinding could have been broken
Unclear risk of bias: the term 'double‐blinded' was mentioned but no details were given with regards to how the outcome assessors were blinded to the intervention received by the participants
Incomplete outcome data
Low risk of bias: there were no missing outcome data, or the reasons for missing data were unlikely to be related to the true study outcome, or the numbers and reasons were balanced across intervention groups
High risk of bias: there were missing outcome data and the reasons were likely to be related to the true study outcome with either imbalance in numbers or reasons for missing data across intervention groups
Unclear risk of bias: there was insufficient reporting of attrition or exclusion, or both, to permit a judgement of low or high risk of bias
Selective outcome reporting
We assessed the possibility of selective outcome reporting by checking study protocols, if available, and comparing the outcomes listed in the protocol with the published study report.
Low risk of bias: it is clear that all of the study's prespecified and expected outcomes of interest were reported in the prespecified way
High risk of bias: not all the study's prespecified outcomes were reported, or one or more primary outcomes were reported in a way that was not prespecified, or one or more reported primary outcomes were not prespecified, or one or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis, or the study failed to include the results of a key outcome that was expected to have been reported
Unclear risk of bias: there was insufficient information to permit a judgement of low or high risk of bias
Other bias
Low risk of bias: the study appeared to be free of other sources of bias
High risk of bias: there was at least one problem in the study that could put it at risk of bias; for example, the study was claimed to be fraudulent, or there was extreme baseline imbalance
Unclear risk of bias: there was a lack of information to permit a judgement of low or high risk of bias
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Howlin 2007.
| Methods |
Design: cluster‐randomised‐controlled trial with school as the randomisation unit (schools randomised into 3 groups: immediate treatment group (ITG), delayed treatment group (DTG), and no treatment group (NTG)) Date of study: not stated but study published in 2007 Duration of study:
Setting: greater London and South East England, UK. A total of 17 classes with > 4 children in each class were enrolled in the study. All children were attending autism‐specific classes or units. Most had child:adult ratio of 2:1. All classes followed the national curriculum. Teaching approaches differed but most took an eclectic approach utilising pictures and visuals and structured teaching. |
|
| Participants |
Sample size: 84 (note, n = 88 after randomisation but one ITG class (n = 4) withdrew before baseline assessment)
Withdrawals: 1 class withdrew after randomisation. 1 child withdrawn from NTG (failed to meet diagnostic criteria after baseline assessments done), and 1 child joined the DTG. 1 child form ITG moved away following intervention and before final assessment. 7 children moved out of DTG pre‐intervention but completed final assessments. Sex:
Age:
Inclusion criteria*:
*Each class required minimum of 3 children meeting the above criteria Exclusion criteria:
|
|
| Interventions |
Intervention: PECS
Control: NTG; 15.3 months with no PECS training Administration: intervention and assessments occurred in the school setting. Teachers and parents received PECS training (2‐day workshop or 13 h) followed by consultation. The active intervention period began about 1 week after training. PECS consultants conducted 6 half‐day consultations with each class once per month over 5 months. The consultants recommended and demonstrated strategies to improve children's use of PECS in the classroom, monitored teachers' progress and provided systematic feedback on implementation of PECS. Note, classroom teachers were not completely naïve to PECS, but generally the use of this was minimal and limited to phase I scaffolded requesting. |
|
| Outcomes |
Primary outcomes Researchers videotaped daily snack session for maximum of 15 min. Three variables were coded:
Secondary outcomes These included standardised measures: Expressive One Word Picture Vocabulary Test, British Picture Vocabulary Scales completed 3 times through the study. The Communication and Reciprocal Social Interaction Domain scores of the ADOS‐G were also used. Timing of outcome measurement: children were filmed/assessed at baseline, after the end of the first intervention period, and after the end of the second intervention period |
|
| Notes |
Comment: none Funding source: supported by the Three Guineas Trust. Pyramid UK also supported the project (by providing the PECS consultants). It is unclear if these consultants were paid. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: 15/18 class groups excluded due to fewer than 3 eligible children in the same classroom. Classes were stratified according to size. In each stratum, classes were randomly allocated to 1 of the 3 conditions using an online randomisation programme (www.random.org). |
| Allocation concealment (selection bias) | Unclear risk | Comment: study does not mention how allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Comment: participants and personnel were aware of allocation. It is not possible to perform blinding as placebo not possible for this type of trial Quote: "Because of financial and personnel limitations… assessors (and videotape coders, see below) were not blind to group assignment" (p 476) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: staff conducting assessments or coding (or both) were not blinded to group assignment. See directly above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 child from the immediate treatment group moved away after the trial started, and 7 children in the delayed treatment group moved out of classes prior to the intervention starting. Analyses were conducted on an intention‐to‐treat basis. Did not compare those who dropped out to those who remained in terms of characteristics |
| Selective reporting (reporting bias) | Low risk | Comment: all measures reported in the published trial protocol were reported in the Results section. Does not state whether adverse outcomes were collected in protocol or paper |
| Other bias | Unclear risk |
Comment: PECS may have been used to some extent in the control group. Also, there may be bias due to the cluster‐randomised control design of the study; however, the extent of this bias is unclear (see Risk of bias in included studies for more details). Quote: "it was not possible to collect ongoing measures of treatment fidelity… [but] this is of less importance for pragmatic effectiveness studies than for efficacy studies." (p 479) |
Siller 2013.
| Methods |
Design: randomised controlled/clinical trial Date of study: data collected between 2004 and 2007 Duration of study:
Follow‐up assessment scheduled approximately 12 months after exit (mean 13.9 months (SD 4.7, range 9–32)) Setting: Los Angeles, California |
|
| Participants |
Sample size: 70 (36 intervention, 34 control) Withdrawals:
Sex: 64 boys, 6 girls Age:
Inclusion criteria:
Exclusion criteria: not stated |
|
| Interventions |
Intervention: focused playtime intervention (FPI). FPI is capacity building approach that promotes co‐ordinated toy play between the parent and child. There is an ordered sequence of 8 topics. Both parent and clinician are involved in the first half of the session, then only parent in second half (further details are provided in the report). Participants received 12 in‐home training sessions lasting 90 min per session for 12 weeks. They were also invited to attend parent advocacy coaching sessions. Control group: invited to attend parent advocacy coaching sessions Administration: 3 assessments conducted; 2 in UCLA lab and 1 at home. Information was collected on the non‐project services and school programmes attended by intervention and control groups: 12 months before study, between intake and exit, and between exit and follow‐up. No significant differences were found between groups. |
|
| Outcomes |
Primary outcomes
Timing of outcome measurement: measures collected at baseline, after 12‐week intervention and 12 months after completion of intervention |
|
| Notes |
Comment: none Funding source: study was supported by CPEA grant (HD35470) from the National Institute of Child Health and Human Development, the MIND Institute Research Program, and a PSC‐CUNY grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: children were randomised in clusters of 4 to ensure groups had equal numbers; however, the method is not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: study does not mention how allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel were aware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: staff conducting assessments or coding (or both) were blinded to group assignment, and prior to all outcome assessment sessions, parents were reminded not to reveal their group assignment to the assessment staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: some participants dropped out (3 intervention, 2 control). Analyses were conducted on an intention‐to‐treat basis. Multiple imputation analysis done to take into account missing data or those lost to follow‐up. Statistical analysis comparing those lost to follow‐up to those who remained found that participants who dropped out of the study took longer to complete the intervention period and received fewer autism‐specific, non‐project services in the community, on average. These differences were then corrected for statistically. |
| Selective reporting (reporting bias) | Low risk | Comment: all measures reported in the published trial protocol were reported in the Results section; however, it does not state whether adverse outcomes were collected in protocol or paper |
| Other bias | Unclear risk | Comment: did not report on any other possible interventions that may have been received by control group (e.g. through the community) |
ADI‐R: Autism Diagnostic Interview‐Revised; ADOS: Autism Diagnostic Observtion Schedule; ADOS‐G: Autism Diagnostic Observtion Schedule‐ Generic; ASD: autism spectrum disorder; CPEA: Clinical and Patient Educators Association; PSC‐CUNY: Professional Staff Congress‐City University of New York; PECS: Picture Exchange Communication System; SD: standard deviation; UCLA: University of California, Los Angeles.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aldred 2004 | Not minimally verbal. Most children > 50 words on CDI. 8 children in each group described as "high‐functioning" |
| Casenhiser 2013 | Not minimally verbal. Mean PLS expressive language age = 24.5 months (SD = 12.7) in intervention group and 24.7 months (SD = 12.6) in control group. Mean length of utterance = 1.3 (SD = 0.5) |
| Chang 2016 | Not minimally verbal |
| D'Elia 2014 | Not an RCT |
| Drew 2002 | Not a communication‐focused intervention. Broader training programme. Young cohort. Age: 21–23 months. 22/24 were non‐verbal, others used single words. CDI mean number of words produced = 6.8 (SD 20.9) in intervention group and 6.6 (SD 13.7) in control group |
| Elder 2011 | Not minimally verbal. Mean number of intelligible words = 40.9 at baseline |
| Field 2001 | Focused specifically on imitation rather than being a communication intervention |
| Fletcher‐Watson 2013 | Not minimally verbal. Mean number of words = 142 at baseline |
| Flores 2014 | Not minimally verbal. Some children spoke in sentences |
| Fteiha 2017 | Not minimally verbal: "mild degree of autism". A proportion of sample have > 19 words |
| Goods 2013 | No control group. Treatment comparison (30 hours ABA ± JASPER) |
| Gould 2015 | No control group. Treatment comparison (JASPER vs DTT) |
| Hardan 2015 | Not minimally verbal. Minimally verbal was not a required inclusion criteria. Mean number words produced on CDI at baseline = 137.1 (SD 118.1) and 169.5 (SD 134.2) |
| Ingersol 2012 | Not a language outcome. JA and social‐emotional functioning outcomes. Not minimally verbal. Mean PLS expressive language age = 17.3 months (SD 5.5, range 9–23 months); NVMA = 20.8 months (SD 6.6, range 8–30 months) |
| Jalili 2014 | Not an RCT. Groups homogenised based on CARS scores |
| Jemison Pollard 2010 | Intervention was not language focused. Study used a music intervention that aimed to improve social skills |
| Kaale 2014 | Not minimally verbal. Mean RDLS expressive language age = 18.8 months (SD 10.5) in intervention group and 24.9 months (SD 12.8) in control group. Minimally verbal was not an inclusion criteria |
| Kaiser 2013 | Not minimally verbal. Mean length of utterance = 1.4 units (units include the total number of words and morphemes per utterance) |
| Kasari 2006 | Not minimally verbal. Mean MSEL expressive language age = 20.6 months (SD 6.51) in JA group, 21.43 months (SD 7.59) in SP group, and 19.41 months (SD = 7.70) in control group |
| Kasari 2014 | No control group. Treatment comparison (JASPER ± SGD) |
| Kasari 2015 | No control group. Treatment comparison (JASPER vs PEI). Mean MSEL expressive language age = 14.09 months (SD 6.84) in JASPER group and 14.98 months (SD 7.02) in PEI group. Minimally verbal was not an inclusion criteria |
| Landa 2011 | No control group. Treatment comparison (intervention given to both groups ± IS intervention). Mean MSEL expressive language T score = 23.92 (SD 5.50) for IS group and 25.92 (SD = 8.12) in non‐IS group. Expressive language change was a secondary outcome. Minimally verbal was not an inclusion criteria |
| Mandell 2013 | Not an RCT. Treatment comparison (STAR vs structured teaching) |
| Martins 2013 | Did not use required ASD diagnostic criteria |
| NCT01018407 | No control group. Treatment comparison (DTT vs interpersonal developmental approach) |
| NCT01751698 | No control group. Treatment comparison (DTT vs JASP‐EMT) |
| Oosterling 2010 | Not minimally verbal. CDI mean number of "words said" = 106.8 (SD 122) in intervention group and 101.7 (SD 110) in control group |
| Paul 2013 | No control group. Treatment comparison (RMIA vs MCT) |
| Pickles 2016 | Not minimally verbal. 64% were assessed using ADOS modules 2 & 3 (i.e. using > single words) |
| Reitzel 2013 | Not minimally verbal. Mean MSEL expressive language age = 16 months (SD 8) in intervention group and 21.5 (SD 9.2) in control group. Mean visual reception score on MSEL = 29.0 (SD 9.2) in intervention group and 22.4 (SD 8.2) in control group. Mean ratio IQ = 42.5 (SD 12.0) in intervention group and 31.1 (SD 10.6) in control group. Used standard scores on communication subscale of VABS for outcomes |
| Roberts 2011 | Not minimally verbal |
| Rogers 2006 | Not an RCT. Single‐subject design. Treatment comparison (Denver Model vs PROMPT) |
| Romski 2010 | Not all ASD. Sample included minimally verbal children without ASD (e.g. Down Syndrome and cerebral palsy) |
| Sallows 2005 | Not an RCT |
| Sandiford 2013 | No control group. Treatment comparison (melodic intonation therapy vs traditional speech therapy) |
| Schriebman 2014 | No control group. Treatment comparison (PECS vs pivotal response training) |
| Schroder 2015 | Not an RCT |
| Serret 2017 | Not an RCT |
| Simpson 2013 | Did not use required ASD diagnostic criteria |
| Smith 2000 | Did not use required ASD diagnostic criteria |
| Solomon 2014 | Not minimally verbal. CDI mean number words produced = 76 (SD 54) for intervention group and 78 (SD 70) for control group |
| Stock 2013 | Not an RCT |
| Sweeney 2016 | Not an RCT |
| Tonge 2014 | Not a communication‐focused intervention. Broader parent education programs. PEAC, PEBM, control group |
| Venker 2012 | Not minimally verbal. CDI mean number words produced = 108.2 (SD 151, range 0–385) |
| Vernay 2017 | Not an RCT |
| Wetherby 2014 | Not a communication‐focused intervention but targeted a range of skills, including autism symptoms. Does not specify minimally verbal. Very young (16–20 months old) children diagnosed with ASD. MSEL T scores = 29.61 (SD 11.22) for individual and 28.68 (SD 10.95) for group intervention. No control group. Compared 2 modes of intervention: individual vs group ESI using the SCERTS curriculum |
| Whelan 2010 | Not a communication‐focused intervention. Broader intervention. NVMA < 24 months and child using computer programme for a significant proportion of intervention |
| Wong 2010 | Not a language‐focused intervention. Aim of study was focused on preverbal skills. Lower range for NVMA < 12 months. Minimally verbal not an inclusion criteria although children do appear to be minimally verbal based on text |
| Yoder 1988 | No control group. Treatment comparison (4 interventions: sign alone, speech alone, simultaneous, alternating) |
| Yoder 2006b | No control group. Treatment comparison (RPMT vs PECS) |
| Zeina 2015 | Not an RCT. Three comparison groups based on IQ level |
ABA: applied behaviour analysis; ADOS: Autism Diagnostic Observation Schedule; ASD: autism spectrum disorder; CARS: Childhood Autism Rating Scale; CDI: Communicative Development Inventory; DTT: Discrete Trial Training; ESI: Early Social Interation project; IQ: intelligence quotient; IS: interpersonal synchrony; JA: joint attention; JASPER: Joint Attention, Symbolic Play, Engagement and Regulation; JASP‐EMT: Joint Attention, Symbolic Play and Enhanced Milieu Teaching; MCT: Milieu Communication Training; MLU: mean length of utterance; MSEL: Mullen Scales of Early Learning; NVMA: nonverbal mental age; PEAC: Partnerships between Education and Autism Communities; PEBM: Parent Education and Behaviour Management; PECS: Picture Exchange Communication System; PEI: psychoeducational intervention; PLS: Preschool Language Scale; PROMPT: Prompts for Restructuring Oral Muscular Phonetics Targets; RCT: randomised controlled trial; RDLS: Reynell Developmental Language Scales; RMIA: Rapid Motor Imitation Antecedent training; RPMT: Responsive Education and Prelinguistic Mileu Teaching; SCERTS: Social Communication, Emotional Regulation Transactional Support; SD: standard deviation; SGD: speech generating device; SP: symbolic play; STAR: Strategies for Teaching based on Autism Research; VABS: Vineland Adaptive Behaviour Scales.
Characteristics of studies awaiting assessment [ordered by study ID]
Gilbert 2012.
| Methods |
Design: group design with randomised treatment and wait‐list control Date of study: 2012 Duration of study: not stated. 32 weeks of intervention then follow‐up Setting: Hofstra University, Hempstead, USA |
| Participants |
Sample size: not stated Withdrawals: not stated Sex: not stated Mean age: 6 years 10 months Inclusion criteria
Exclusion criteria: not stated |
| Interventions |
Intervention: Milieu Communication Treatment Control: age‐ and ability‐matched wait‐list control group Administration: not stated Duration: 32 weeks |
| Outcomes | Outcomes: detailed outcome measures not provided. Authors state they measured individual differences on specified pre‐linguistic behaviours |
| Notes |
Comment: This is an abstract from conference proceedings. The study appears to be eligible for inclusion but we could only extract limited detail from the abstract. We contacted the authors but were unable to obtain the necessary detail to classify the study as included or excluded. Funding source: not stated |
ASD: autism spectrum disorder.
Characteristics of ongoing studies [ordered by study ID]
NCT02291172.
| Trial name or title |
Public title: Comprehensive communication intervention for minimally verbal children with autism Official title: Comprehensive communication intervention for minimally verbal children with autism |
| Methods |
Design: randomised, single‐group assignment Duration of study: 4 months Setting: Nashville, Tennessee, USA |
| Participants |
Sample size: 74 enrolled Sex: male and female children eligible for inclusion Age: range 36 months to 54 months Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: a blend of JASP + EMT using SGDs with parent training intervention with the addition of individualised DTT to teach receptive language, imitation, and joint attention when children lack these skills at entry Control: business as usual Administration: this is a 3‐month (42 session) intervention. Children will be assessed at 4 time points: preintervention, postintervention, 2 months postintervention, and 4 months postintervention. |
| Outcomes | Primary outcome: spontaneous communicative utterances (time frame: 12 weeks), measured from a naturalistic language sample |
| Starting date | September 2014 Status: recruitment completed |
| Contact information |
Name: Ann Kaiser Position: Susan W Gray Professor of Education and Human Development Address: Department of Special Education, Vanderbilt University |
| Notes |
Comment: further detail available at clinicaltrials.gov/show/NCT02291172 Funding source: Vanderbilt University |
NCT02464527.
| Trial name or title |
Public title: Stimulus‐stimulus pairing study (SSP) Official title: A pilot feasibility study to increase vocal language in minimally verbal children diagnosed with autism spectrum disorders |
| Methods |
Design: randomised, parallel assignment Duration of study: 6–12 weeks (intervention 6 weeks, control (wait‐list) wait 6 weeks and then receive the 6‐week intervention) Setting: Emory University |
| Participants |
Sample size: 16 enrolled Sex: male and female children eligible for inclusion Age: range 24 months to 47 months Inclusion criteria:
Exclusion criteria: children with significant problem behaviour that interferes with structured intervention |
| Interventions |
Intervention: SSP, consisting of sounds and words being systematically paired with delivery of a preferred item Control: wait‐list control Administration: participants in the SSP l attend clinic‐based sessions for 1 h per day, 5 days per week, for 6 weeks. The SSP procedure will consist of sounds and words being systematically paired with delivery of a preferred item. The participants will be recorded by a vocal recorder by the parent/guardian at home or in the community.The wait‐list control group will wait for 6 weeks; children will not receive any treatment during this period. The wait‐list control group will then receive the SSP procedure (delayed procedure) after the 6‐week wait. |
| Outcomes |
Primary outcomes
|
| Starting date | June 2014 Status: recruitment completed |
| Contact information |
Name: Alice Shillingsburg Position: Assistant Professor Address: Emory University |
| Notes |
Comment: further detail available at clinicaltrials.gov/show/NCT02464527 Funding source: Emory University |
ADOS: Autism Diagnostic Observation Schedule; ASD: autism spectrum disorder; DTT: discrete trial training; JASP + EMT: Joint Attention, Symbolic Play, Engagement and Enhanced Milieu Teaching; SGD: speech generating device; SSP: stimulus‐stimulus pairing.
Differences between protocol and review
-
Authorship
Amanda Brignell now works for Murdoch Children's Research Institute.
Huan Song now works for the University of Iceland.
DongHao Lu was an author on the protocol (Brignell 2016), but not the review.
-
We reduced the minimum non‐verbal mental age from at least 24 months to at least 12 months. This was based on clinical reasoning, previous studies, and experts in minimally verbal children with ASD who had used 12 months as the non‐verbal mental‐age cut‐off point (e.g. Paul 2013).
-
We added 'combined spoken and non‐verbal communication/AAC' as an outcome because some studies do not collect or report on spoken and non‐verbal outcomes separately. For example, one of the outcomes measured in one study, Howlin 2007, was 'frequency of initiations', and this could include both spoken or non‐verbal initiations.
-
We added information on how we would present ordinal outcomes in the 'Summary of findings' tables. We had not previously described the methods for dealing with ordinal data, so it was necessary to explain this.
We also present the OR rather than the RR in the review, as we were not able to extract the RR from the data presented in the trial report.
-
We searched two additional Ovid MEDLINE segments which are updated daily: MEDLINE In‐Process & Other Non‐Indexed Citations, and MEDLINE EPub Ahead of Print. We also searched Database of Abstracts of Reviews of Effect (DARE), and the ISRCTN Registry.
We report methods that we had planned in our protocol, Brignell 2016, but did not use in the review in Table 3.
Contributions of authors
Protocol
HS conceived the protocol. AB, HS, JZ, DL and CS designed the protocol. AB and HS co‐ordinated the protocol. AB, HS and JZ designed the search strategies. AB, HS, JZ, CS and AM wrote the protocol. AB, DL and AM provided general advice on the protocol. AB, HS, JZ and DL performed previous work that was the foundation of the current study.
Review
AB and KC screened all records and identified included and excluded studies. AM arbitrated any disagreements. AB, HS and JZ retrieved missing abstracts and full‐text reports. AB and KC compiled studies and extracted data on excluded studies. AB and KC extracted data for the 'Characteristics of included studies' tables and assessed risk of bias of included studies. AM arbitrated any disagreements. AB and HS extracted data for the 'Summary of findings' tables and conducted the GRADE assessment. AM arbitrated any disagreements. AB wrote the first draft of the review. AM, KC, HS and CS provided feedback on the draft manuscript. AB drafted feedback in response to reviewers.
Amanda Brignell is the guarantor for this review.
Sources of support
Internal sources
-
Karolinska Institutet, Sweden.
Provided fellowship to Huan Song
External sources
-
National Health and Medical Research Council, Australia.
NHMRC Centre of Research Excellence in Speech and Language Neurobiology (CRE‐SLANG) (APP1116976) awarded to Angela T Morgan
-
National Health and Medical Research Council, Australia.
NHMRC Practitioner Fellowship (APP1105008) awarded to Angela T Morgan
-
National Health and Medical Research Council, Australia.
NHMRC Project Grant (APP1127144) awarded to Angela T Morgan
-
National Health and Medical Research Council, Australia.
Provided income support for Amanda Brignell
Declarations of interest
Amanda Brignell (AB) has completed a systematic review on language outcomes for ASD and has conducted two studies looking at communication trajectories in children with ASD. Her publications have not included children with ASD who are minimally verbal. Karen Chenausky (KC) has published papers on children with ASD who are minimally verbal. KC did not assess the eligibility, extract data or assess the risk of bias or the quality of the evidence for any of the studies she contributed to, where they appeared in the searches for this review. Huan Song ‐ none known. JianWei Zhu ‐ none known. Chen Suo ‐ none known. Anagela Tamsin Morgan (AM) receives royalties for books that she has edited on Dysphagia Post Trauma and Case Study Interventions in Child Speech Impairment, neither of which are specific to communication in autism.
New
References
References to studies included in this review
Howlin 2007 {published data only}
- Gordon K, Pasco G, McElduff F, Wade A, Howlin P, Charman T. A communication‐based intervention for nonverbal children with autism: what changes? Who benefits?. Journal of Consulting and Clinical Psychology 2011;79(4):447‐57. [DOI: 10.1037/a0024379; PUBMED: 21787048] [DOI] [PubMed] [Google Scholar]
- Howlin P, Gordon RK, Pasco G, Wade A, Charman T. The effectiveness of Picture Exchange Communication System (PECS) training for teachers of children with autism: a pragmatic, group randomised controlled trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2007;48(5):473‐81. [DOI: 10.1111/j.1469-7610.2006.01707.x; PUBMED: 17501728] [DOI] [PubMed] [Google Scholar]
Siller 2013 {published data only}
- Siller M, Hutman T, Sigman M. A parent‐mediated intervention to increase responsive parental behaviors and child communication in children with ASD: a randomized clinical trial. Journal of Autism and Developmental Disorders 2013;43(3):540‐55. [DOI: 10.1007/s10803-012-1584-y; PMC3511916; PUBMED: 22825926] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller M, Swanson M, Gerber A, Hutman T, Sigman M. A parent‐mediated intervention that targets responsive parental behaviors increases attachment behaviors in children with ASD: results from a randomized clinical trial. Journal of Autism and Developmental Disorders 2014;44(7):1720‐32. [DOI: 10.1007/s10803-014-2049-2; PMC4371529; PUBMED: 24488157] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aldred 2004 {published data only}
- Aldred C, Green J, Adams C. A new social communication intervention for children with autism: pilot randomised controlled treatment study suggesting effectiveness. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2004;45(8):1420‐30. [DOI: 10.1111/j.1469-7610.2004.00848.x; PUBMED: 15482502] [DOI] [PubMed] [Google Scholar]
Casenhiser 2013 {published data only}
- Casenhiser DM, Binns A, McGill F, Morderer O, Shanker SG. Measuring and supporting language function for children with autism: evidence from a randomized control trial of a social‐interaction‐based therapy. Journal of Autism and Developmental Disorders 2015;45(3):846‐57. [DOI: 10.1007/s10803-014-2242-3; PUBMED: 25234481] [DOI] [PubMed] [Google Scholar]
- Casenhiser DM, Shanker SG, Stieben J. Learning through interaction in children with autism: preliminary data from asocial‐communication‐based intervention. Autism 2013;17(2):220‐41. [DOI: 10.1177/1362361311422052; PUBMED: 21949005] [DOI] [PubMed] [Google Scholar]
Chang 2016 {published data only}
- Chang YC, Shire SY, Shih W, Gelfand C, Kasari C. Preschool deployment of evidence‐based social communication intervention: JASPER in the classroom. Journal of Autism and Developmental Disorders 2016;46(6):2211‐23. [DOI: 10.1007/s10803-016-2752-2; PUBMED: 26936161] [DOI] [PubMed] [Google Scholar]
D'Elia 2014 {published data only}
- D'Elia L, Valeri G, Sonnino F, Fontana I, Mammone A, Vicari S. A longitudinal study of the Teacch program in different settings: the potential benefits of low intensity intervention in preschool children with autism spectrum disorder. Journal of Autism and Developmental Disorders 2014;44(3):615‐26. [DOI: 10.1007/s10803-013-1911-y; PUBMED: 23949000] [DOI] [PubMed] [Google Scholar]
Drew 2002 {published data only}
- Drew A, Baird G, Baron‐Cohen S, Cox A, Slonims V, Wheelwright S, et al. A pilot randomised control trial of a parent training intervention for pre‐school children with autism. Preliminary findings and methodological challenges. European Child & Adolescent Psychiatry 2002;11(6):266‐72. [DOI: 10.1007/s00787-002-0299-6; PUBMED: 12541005] [DOI] [PubMed] [Google Scholar]
Elder 2011 {published data only}
- Elder JH, Donaldson SO, Kairalla J, Valcante G, Bendixen R, Ferdig R, et al. In‐home training for fathers of children with autism: a follow up study and evaluation of four individual training components. Journal of Child and Family Studies 2011;20(3):263‐71. [DOI: 10.1007/s10826-010-9387-2; PMC3087101; PUBMED: 21654918] [DOI] [PMC free article] [PubMed] [Google Scholar]
Field 2001 {published data only}
- Ezell S. Imitation Effects on Intentional Communication Behaviors of Children with Autism [PhD thesis]. Santa Barbara (CA): Fielding Graduate University, 2011. [Google Scholar]
- Ezell S, Field T, Nadel J, Newton R, Murrey G, Siddalingappa V, et al. Imitation effects on joint attention behaviors of children with autism. Psychology 2012;3(9):681‐5. [DOI: 10.4236/psych.2012.39103] [DOI] [Google Scholar]
- Field T, Field T, Sanders C, Nadel J. Children with autism display more social behaviors after repeated imitation sessions. Autism 2001;5(3):317‐23. [DOI: 10.1177/1362361301005003008; PUBMED: 11708590] [DOI] [PubMed] [Google Scholar]
Fletcher‐Watson 2013 {published data only}
- Fletcher‐Watson S, O'Hare A, Pain H, Petrou A, McConachie H. Click‐East: a randomised controlled trial of a new iPad‐based social attention intervention for toddlers and pre‐schoolers with autism. Developmental Medicine & Child Neurology 2013;55(Suppl 2):17. [PS 9.3] [Google Scholar]
- Fletcher‐Watson S, Petrou A, Scott‐Barrett J, Dicks P, Graham C, O'Hare A, et al. A trial of an iPadTM intervention targeting social communication skills in children with autism. Autism 2016;20(7):771‐82. [DOI: 10.1177/1362361315605624; PMC5015758; PUBMED: 26503990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flores 2014 {published data only}
- Flores MM, Ganz JB. Comparison of direct instruction and discrete trial teaching on the curriculum‐based assessment of language performance of students with autism. Exceptionality 2014;22(4):191‐204. [DOI: 10.1080/09362835.2013.865533] [DOI] [Google Scholar]
Fteiha 2017 {published data only}
- Fteiha MA. Effectiveness of assistive technology in enhancing language skills for children with autism. International Journal of Developmental Disabilities 2017;63(1):36‐44. [DOI: 10.1080/20473869.2015.1136129] [DOI] [Google Scholar]
Goods 2013 {published data only}
- Goods KS, Ishijima E, Chang Y‐C, Kasari C. Preschool based JASPER intervention in minimally verbal children with autism: pilot RCT. Journal of Autism and Developmental Disorders 2013;43(5):1050‐6. [DOI: 10.1007/s10803-012-1644-3; PMC4222903; PUBMED: 22965298] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gould 2015 {published data only}
- Gould HM. Teaching to Play or Playing to Teach: An Examination of Play Targets and Generalization in Two Interventions for Children with Autism [PhD thesis]. Los Angeles (CA): University of California, 2015. [Google Scholar]
Hardan 2015 {published data only}
- Hardan A. The LENA system in clinical trials: evidence from pivotal response treatment studies. Journal of the American Academy of Child & Adolescent Psychiatry 2016;55(Suppl 10):S302. [DOI: 10.1016/j.jaac.2016.07.281; 28.3] [DOI] [Google Scholar]
- Hardan AY, Gengoux GW, Berquist KL, Libove RA, Ardel CM, Phillips J, et al. A randomized controlled trial of Pivotal Response Treatment group for parents of children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2015;56(8):884‐92. [DOI: 10.1111/jcpp.12354; PUBMED: 25346345] [DOI] [PubMed] [Google Scholar]
Ingersol 2012 {published data only}
- Ingersoll B. Brief report: effect of a focused imitation intervention on social functioning in children with autism. Journal of Autism & Developmental Disorders 2012;42(8):1768‐73. [DOI: 10.1007/s10803-011-1423-6; PMC3667953; PUBMED: 22146934] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jalili 2014 {published data only}
- Jalili M, Jahangiri N, Yazdi AA, Ashrafzadeh F. The effects of imitative vs cognitive methods on the speech development of children with autism. Iranian Journal of Child Neurology 2014;8(1):37‐46. [PMC3943057; PUBMED: 24665326] [PMC free article] [PubMed] [Google Scholar]
Jemison Pollard 2010 {published data only}
- Jeminson Pollard D. The Use of Music to Improve Social Skills Development in Children Diagnosed with Autism [PhD thesis]. Houston (TX): Texas Southern University, 2010. [Google Scholar]
Kaale 2014 {published data only}
- Kaale A, Fagerland MW, Martinsen EW, Smith L. Preschool‐based social communication treatment for children with autism: 12‐month follow‐up of a randomized trial. Journal of the American Academy of Child & Adolescent Psychiatry 2014;53(2):188‐98. [DOI: 10.1016/j.jaac.2013.09.019; PUBMED: 24472253] [DOI] [PubMed] [Google Scholar]
Kaiser 2013 {published data only}
- Kaiser AP, Roberts MY. Parent‐implemented enhanced milieu teaching with preschool children who have intellectual disabilities. Journal of Speech, Language, and Hearing Research 2013;56(1):295‐309. [DOI: 10.1044/1092-4388(2012/11-0231); PMC3740334; PUBMED: 22744141] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasari 2006 {published data only}
- Gulsrud AC, Kasari C, Freeman S, Paparella T. Children with autism's response to novel stimuli while participating in interventions targeting joint attention or symbolic play skills. Autism 2007;11(6):535‐46. [DOI: 10.1177/1362361307083255; PUBMED: 17947289] [DOI] [PubMed] [Google Scholar]
- Kasari C, Freeman S, Paparella T. Joint attention and symbolic play in young children with autism: a randomized controlled intervention study. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2006;47(6):611‐20. [DOI: 10.1111/j.1469-7610.2005.01567.x; PUBMED: 16712638] [DOI] [PubMed] [Google Scholar]
- Kasari C, Gulsrud A, Freeman S, Paparella T, Hellemann G. Longitudinal follow‐up of children with autism receiving targeted interventions on joint attention and play. Journal of the American Academy of Child & Adolescent Psychiatry 2012;51(5):487‐95. [DOI: 10.1016/j.jaac.2012.02.019; PMC3338205; PUBMED: 22525955] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton K, Kasari C. Teacher‐implemented joint attention intervention: pilot randomized controlled study for preschoolers with autism. Journal of Consulting and Clinical Psychology 2012;80(4):687‐93. [DOI: 10.1037/a0028506; PUBMED: 22582764] [DOI] [PubMed] [Google Scholar]
Kasari 2014 {published data only}
- Almirall D, DiStefano C, Chang YC, Shire S, Kaiser A, Lu X, et al. Longitudinal effects of adaptive interventions with a speech‐generating device in minimally verbal children with ASD. Journal of Clinical Child and Adolescent Psychology 2016;45(4):442‐56. [DOI: 10.1080/15374416.2016.1138407; PMC4930379; PUBMED: 26954267] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano C, Shih W, Kaiser A, Landa R, Kasari C. Communication growth in minimally verbal children with ASD: the importance of interaction. Autism Research 2016;9(10):1093‐102. [DOI: 10.1002/aur.1594; PUBMED: 26824676] [DOI] [PubMed] [Google Scholar]
- Kasari C, Kaiser A, Goods K, Nietfeld J, Mathy P, Landa R, et al. Communication interventions for minimally verbal children with autism: a sequential multiple assignment randomized trial. Journal of the American Academy of Child & Adolescent Psychiatry 2014;53(6):635‐46. [DOI: 10.1016/j.jaac.2014.01.019; PMC4030683; PUBMED: 24839882] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shire SY, Goods K, Shih W, Distefano C, Kaiser A, Wright C, et al. Parents' adoption of social communication intervention strategies: families including children with autism spectrum disorder who are minimally verbal. Journal of Autism and Developmental Disorders 2015;45(6):1712‐24. [DOI: 10.1007/s10803-014-2329-x; PMC4442706; PUBMED: 25475363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasari 2015 {published data only}
- Kasari C, Gulsrud A, Paparella T, Hellemann G, Berry K. Randomized comparative efficacy study of parent‐mediated interventions for toddlers with autism. Journal of Consulting and Clinical Psychology 2015;83(3):554‐63. [DOI: 10.1037/a0039080; PMC4755315; PUBMED: 25822242] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shire SY, Gulsrud A, Kasari C. Increasing responsive parent‐child interactions and joint engagement: comparing the influence of parent‐mediated intervention and parent psychoeducation. Journal of Autism and Developmental Disorders 2016;46(5):1737‐47. [DOI: 10.1007/s10803-016-2702-z; PMC4826805; PUBMED: 26797940] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landa 2011 {published data only}
- Landa RJ, Holman KC, O'Neill AH, Stuart EA. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2011;52(1):13‐21. [DOI: 10.1111/j.1469-7610.2010.02288.x; PMC3059234; PUBMED: 21126245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandell 2013 {published data only}
- Mandell DS, Stahmer AC, Shin S, Xie M, Reisinger E, Marcus SC. The role of treatment fidelity on outcomes during a randomized field trial of an autism intervention. Autism 2013;17(3):281‐95. [DOI: 10.1177/1362361312473666; PMC3738205; PUBMED: 23592849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martins 2013 {published data only}
- Martins LZ, Fernandes FD. Short‐term speech‐language intervention for children with disorders of the autism spectrum. Codas 2013;25(6):542‐7. [DOI: 10.1590/S2317-17822014000100007; PUBMED: 24626980] [DOI] [PubMed] [Google Scholar]
NCT01018407 {unpublished data only}
- NCT01018407. Interventions for communication in autism network (ICAN) [Multisite randomized control treatment of early intervention for spoken communication in autism]. clinicaltrials.gov/show/NCT01018407 (first received 20 November 2009).
NCT01751698 {unpublished data only}
- NCT01751698. Adaptive interventions for minimally verbal children with ASD in the community [Adaptive interventions for minimally verbal children with ASD in the community]. clinicaltrials.gov/show/NCT01751698 (first received 14 December 2012).
Oosterling 2010 {published data only}
- Oosterling I, Visser J, Swinkels S, Rommelse N, Donders R, Woudenberg T, et al. Randomized controlled trial of the focus parent training for toddlers with autism: 1‐year outcome. Journal of Autism and Developmental Disorders 2010;40(12):1447‐58. [DOI: 10.1007/s10803-010-1004-0; PMC2980624; PUBMED: 20440639] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2013 {published data only}
- Paul R, Campbell D, Gilbert K, Tsiouri I. Comparing spoken language treatments for minimally verbal preschoolers with autism spectrum disorders. Journal of Autism and Developmental Disorders 2013;43(2):418‐31. [DOI: 10.1007/s10803-012-1583-z; PUBMED: 22733301] [DOI] [PubMed] [Google Scholar]
- Paul R, Tsiouri I, Gilbert K. Results of a randomized controlled trial of speech interventions for nonverbal preschoolers with ASD. Neuropsychiatrie de l'Enfance et de l'Adolescence 2012;60(Suppl 5):S219. [DOI: 10.1016/j.neurenf.2012.04.488] [DOI] [Google Scholar]
Pickles 2016 {published data only}
- Pickles A, Couteur A, Leadbitter K, Salomone E, Cole‐Fletcher R, Tobin H, et al. Parent‐mediated social communication therapy for young children with autism (PACT): long‐term follow‐up of a randomised controlled trial. Lancet 2016;388(10059):2501‐9. [DOI: 10.1016/S0140-6736(16)31229-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitzel 2013 {published data only}
- Reitzel J, Summers J, Lorv B, Szatmari P, Zwaigenbaum L, Georgiades S, et al. Pilot randomized controlled trial of a functional behavior skills training program for young children with autism spectrum disorder who have significant early learning skill impairments and their families. Research in Autism Spectrum Disorders 2013;7(11):1418‐32. [DOI: 10.1016/j.rasd.2013.07.025] [DOI] [Google Scholar]
Roberts 2011 {published data only}
- Roberts J, Williams K, Carter M, Evans D, Parmenter T, Silove N, et al. A randomised controlled trial of two early intervention programs for young children with autism: centre‐based with parent program and home‐based. Research in Autism Spectrum Disorders 2011;5(4):1553‐66. [DOI: 10.1016/j.rasd.2011.03.001] [DOI] [Google Scholar]
Rogers 2006 {published data only}
- Rogers SJ, Hayden D, Hepburn S, Charlifue‐Smith R, Hall T, Hayes A. Teaching young nonverbal children with autism useful speech: a pilot study of the Denver Model and PROMPT interventions. Journal of Autism and Developmental Disorders 2006;36(8):1007‐24. [DOI: 10.1007/s10803-006-0142-x; PUBMED: 16845576] [DOI] [PubMed] [Google Scholar]
Romski 2010 {published data only}
- Romski M, Sevcik RA, Adamson LB, Cheslock M, Smith A, Barker RM, et al. Randomized comparison of augmented and nonaugmented language interventions for toddlers with developmental delays and their parents. Journal of Speech, Language, and Hearing Research 2010;53(2):350‐64. [DOI: 10.1044/1092-4388(2009/08-0156); PUBMED: 20360461] [DOI] [PubMed] [Google Scholar]
Sallows 2005 {published data only}
- Sallows GO, Graupner TD. Intensive behavioral treatment for children with autism: four‐year outcome and predictors. American Journal of Mental Retardation 2005;110(6):417‐38. [DOI: 10.1352/0895-8017(2005)110[417:IBTFCW]2.0.CO;2; PUBMED: 16212446] [DOI] [PubMed] [Google Scholar]
Sandiford 2013 {published data only}
- Sandiford GA, Mainess KJ, Daher NS. A pilot study on the efficacy of melodic based communication therapy for eliciting speech in nonverbal children with autism. Journal of Autism and Developmental Disorders 2013;43(6):1298‐307. [DOI: 10.1007/s10803-012-1672-z; PUBMED: 23065117] [DOI] [PubMed] [Google Scholar]
Schriebman 2014 {published data only}
- Schreibman L, Stahmer AC. A randomized trial comparison of the effects of verbal and pictorial naturalistic communication strategies on spoken language for young children with autism. Journal of Autism and Developmental Disorders 2014;44(5):1244‐51. [DOI: 10.1007/s10803-013-1972-y; PMC4005390; PUBMED: 24272416] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroder 2015 {published data only}
- Schröder CM, Florence E, Dubrovskaya A, Lambs B, Stritmatter P, Vecchionacci V, et al. The Early Start Denver Model. An early treatment approach for children with autism spectrum disorder [Le modèle de Denver (Early Start Denver Model). Une approche d'intervention précoce pour les troubles du spectre autistique]. Neuropsychiatrie de l'Enfance et de l'Adolescence 2015;63(5):279‐87. [DOI: 10.1016/j.neurenf.2015.04.001] [DOI] [Google Scholar]
Serret 2017 {published data only}
- Serret S, Hun S, Thümmler S, Peirron P, Santos A, Bourgeois J, et al. Teaching literacy skills to French minimally verbal school‐aged children with autism spectrum disorders with the serious game SEMA‐TIC: an exploratory study. Frontiers in Psychology 2017;8:1523. [DOI: 10.3389/fpsyg.2017.01523; PMC5591836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2013 {published data only}
- Simpson K, Keen D, Lamb J. Teaching receptive labelling to children with autism spectrum disorder: a comparative study using infant‐directed song and infant‐directed speech. Journal of Intellectual & Developmental Disability 2015;40(2):126‐36. [DOI: 10.3109/13668250.2015.1014026] [DOI] [Google Scholar]
- Simpson K, Keen D, Lamb J. The use of music to engage children with autism in a receptive labelling task. Research in Autism Spectrum Disorders 2013;7(12):1489‐96. [DOI: 10.1016/j.rasd.2013.08.013] [DOI] [Google Scholar]
Smith 2000 {published data only}
- Smith T, Groen AD, Wynn JW. Randomized trial of intensive early intervention for children with pervasive developmental disorder. American Journal on Mental Retardation 2000;105(4):269‐85. [DOI: 10.1352/0895-8017(2000)105%3C0269:RTOIEI%3E2.0.CO;2; PUBMED: 10934569 ] [DOI] [PubMed] [Google Scholar]
Solomon 2014 {published data only}
- Solomon R, Egeren LA, Mahoney G, Quon Huber MS, Zimmerman P. PLAY Project Home Consultation intervention program for young children with autism spectrum disorders: a randomized controlled trial. Journal of Developmental and Behavioral Pediatrics 2014;35(8):475‐85. [DOI: 10.1097/DBP.0000000000000096; NCT01768806.; NIHMS619258; PMC4181375] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stock 2013 {published data only}
- Stock R, Mirenda P, Smith IM. Comparison of community‐based verbal behavior and pivotal response treatment programs for young children with autism spectrum disorder. Research in Autism Spectrum Disorders 2013;7(9):1168‐81. [DOI: 10.1016/j.rasd.2013.06.002] [DOI] [Google Scholar]
Sweeney 2016 {published data only}
- Sweeney M, Lebersfeld JB. First words for nonverbal children and adults with autism. Archives of Physical Medicine and Rehabilitation 2016;97(12):e31‐2. [DOI: 10.1016/j.apmr.2016.09.086] [DOI] [Google Scholar]
Tonge 2014 {published data only}
- Tonge B, Brereton A, Kiomall M, Mackinnon A, Rinehart NJ. A randomised group comparison controlled trial of 'preschoolers with autism': a parent education and skills training intervention for young children with autistic disorder. Autism 2014;18(2):166‐77. [DOI: 10.1177/1362361312458186; PUBMED: 22987897] [DOI] [PubMed] [Google Scholar]
Venker 2012 {published data only}
- Venker CE, McDuffie A, Ellis Weismer S, Abbeduto L. Increasing verbal responsiveness in parents of children with autism: a pilot study. Autism 2012;16(6):568‐85. [DOI: 10.1177/1362361311413396; PMC3389583; PUBMED: 21846665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernay 2017 {published data only}
- Vernay F, Kahina H, Thierry M, Jean‐Yves R. Self‐paced segmentation of written words on a touchscreen tablet promotes the oral production of nonverbal and minimally verbal children with autism. Journal of Research in Special Educational Needs 2017;17(4):265‐73. [DOI: 10.1111/1471-3802.12384] [DOI] [Google Scholar]
Wetherby 2014 {published data only}
- Wetherby AM, Guthrie W, Woods J, Schatschneider C, Holland RD, Morgan L, et al. Parent‐implemented social intervention for toddlers with autism: an RCT. Pediatrics 2014;134(6):1084‐93. [DOI: 10.1542/peds.2014-0757; NCT00760812; PMC4243066; PUBMED: 25367544] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whelan 2010 {published data only}
- Whalen C, Moss D, Ilan AB, Vaupel M, Fielding P, Macdonald K, et al. Efficacy of TeachTown: basics computer‐assisted intervention for the Intensive Comprehensive Autism Program in Los Angeles Unified School District. Autism 2010;14(3):179‐97. [DOI: 10.1177/1362361310363282; PUBMED: 20484002] [DOI] [PubMed] [Google Scholar]
Wong 2010 {published data only}
- Wong VC, Kwan QK. Randomized controlled trial for early intervention for autism: a pilot study of the Autism 1‐2‐3 Project. Journal of Autism and Developmental Disorders 2010;40(6):677‐88. [DOI: 10.1007/s10803-009-0916-z; PUBMED: 20020319] [DOI] [PubMed] [Google Scholar]
Yoder 1988 {published data only}
- Layton TL. Language training with autistic children using four different modes of presentation. Journal of Communication Disorders 1988;21(4):333‐50. [PUBMED: 3170784] [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Layton TL. Speech following sign language training in autistic children with minimal verbal language. Journal of Autism and Developmental Disorders 1988;18(2):217‐29. [PUBMED: 3410812] [DOI] [PubMed] [Google Scholar]
Yoder 2006b {published data only}
- Yoder P, Stone WL. A randomized comparison of the effect of two prelinguistic communication interventions on the acquisition of spoken communication in preschoolers with ASD. Journal of Speech, Language, and Hearing Research 2006;49(4):698‐711. [DOI: 10.1044/1092-4388(2006/051); PUBMED: 16908870] [DOI] [PubMed] [Google Scholar]
- Yoder P, Stone WL. Randomized comparison of two communication interventions for preschoolers with autism spectrum disorders. Journal of Consulting and Clinical Psychology 2006;74(3):426‐35. [DOI: 10.1037/0022-006X.74.3.426; PUBMED: 16822100] [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Lieberman RG. Brief report: randomized test of the efficacy of picture exchange communication system on highly generalized picture exchanges in children with ASD. Journal of Autism and Developmental Disorders 2010;40(5):629‐32. [DOI: 10.1007/s10803-009-0897-y; PMC4136745; PUBMED: 19904596] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeina 2015 {published data only}
- Zeina R, Al‐Ayadhi L, Bashir S. The impact of IQ on using high‐tech augmentative alternative communication AAC in children with autism spectrum disorder ASD. Procedia ‐ Social and Behavioral Sciences 2015;171(6):366‐73. [DOI: 10.1016/j.sbspro.2015.01.134] [DOI] [Google Scholar]
References to studies awaiting assessment
Gilbert 2012 {unpublished data only}
- Gilbert KA, Paul R. Efficacy of milieu communication treatment for late talkers with autism spectrum disorders. Neuropsychiatrie de l'Enfance et de l'Adolescence 2012;60(5S):S212‐3. [DOI: 10.1016/j.neurenf.2012.04.456] [DOI] [Google Scholar]
References to ongoing studies
NCT02291172 {unpublished data only}
- NCT02291172. Comprehensive communication intervention for minimally verbal children with autism [Comprehensive communication intervention for minimally verbal children with autism]. clinicaltrials.gov/show/NCT02291172 (first received 6 November 2014). [NCT02291172]
NCT02464527 {unpublished data only}
- NCT02464527. Stimulus‐stimulus pairing study [A pilot feasibility study to increase vocal language in minimally verbal children diagnosed with autism spectrum disorders]. clinicaltrials.gov/show/NCT02464527 (first received 13 May 2015). [NCT02464527]
Additional references
Abidin 1995
- Abidin RR. Parenting Stress Index: Professional Manual. 3rd Edition. Odessa, FL: Psychological Assessment Resources, 1995. [Google Scholar]
Alessandri 2005
- Alessandri M, Mundy P, Tuchman RF. The social deficit in autism: focus on joint attention [Déficit social en el autismo: un enfoque en la atención conjunta]. Revista de Neurología 2005;40(Suppl 1):S137‐41. [PUBMED: 15736077] [PubMed] [Google Scholar]
Anderson 2007
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, et al. Patterns of growth in verbal abilities among children with autism spectrum disorder. Journal of Consulting and Clinical Psychology 2007;75(4):594‐604. [DOI: 10.1037/0022-006X.75.4.594] [DOI] [PubMed] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
Baghdadli 2003
- Baghdadli A, Pascal C, Grisi S, Aussilloux C. Risk factors for self‐injurious behaviors among 222 young children with autistic disorders. Journal of Intellectual Disability Research 2003;47(Pt 8):622–7. [PUBMED: 14641810] [DOI] [PubMed] [Google Scholar]
Baghdadli 2007
- Baghdadli A, Picot M‐C, Michelon C, Bodet J, Pernon E, Burstezjn C, et al. What happens to children with PDD when they grow up? Prospective follow‐up of 219 children from preschool age to mid‐childhood. Acta Psychiatrica Scandinavica 2007;115(5):403–12. [DOI: 10.1111/j.1600-0447.2006.00898.x] [DOI] [PubMed] [Google Scholar]
Baio 2018
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morbidity and Mortality Weekly Report, Surveillance Summary 2018;67(6):1‐23. [DOI: 10.15585/mmwr.ss6706a1; PMC5919599; PUBMED: 29701730] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beach Center on Disabilities 2006
- Beach Center on Disabilities. Family Quality of Life Scale. Lawrence, KS: Beach Center on Disabilities, 2006. [Google Scholar]
Biklen 1990
- Biklen D. Communication unbound: autism and praxis. Harvard Educational Review 1990;60(3):291‐314. [Google Scholar]
Bondy 1998
- Bondy AS, Frost LA. The Picture Exchange Communication System. Seminars in Speech and Language 1998;19(4):373‐88. [PUBMED: 9857393] [DOI] [PubMed] [Google Scholar]
Borenstein 2008
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Bott 1997
- Bott C, Farmer R, Rhode J. Behaviour problems associated with lack of speech in people with learning disabilities. Journal of Intellectual Disability Research 1997;41(1):3‐7. [DOI: 10.1111/j.1365-2788.1997.tb00671.x] [DOI] [PubMed] [Google Scholar]
Charman 2003
- Charman T, Baron‐Cohen S, Swettenham J, Baird G, Drew A, Cox A. Predicting language outcome in infants with autism and pervasive developmental disorder. International Journal of Language & Communication Disorders 2003;38(3):265‐85. [PUBMED: 12851079] [DOI] [PubMed] [Google Scholar]
Chaste 2012
- Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene‐environment interactions. Dialogues in Clinical Neuroscience 2012;14(3):281‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chumpelik 1984
- Chumpelik D. The PROMPT system of therapy: theoretical framework and application for developmental apraxia of speech. Seminars in Speech and Language 1984;5:139‐56. [Google Scholar]
Croen 2002
- Croen LA, Grether JK, Hoogstrate J, Selvin S. The changing prevalence of autism in California. Journal of Autism and Developmental Disorders 2002;32(3):207‐15. [PUBMED: 12108622] [DOI] [PubMed] [Google Scholar]
Dawson 2010
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics 2010;125(1):e17‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Fossé 2004
- Fossé L, Hodge SM, Makris N, Kennedy DN, Caviness VS Jr, McGrath L, et al. Language‐association cortex asymmetry in autism and specific language impairment. Annals of Neurology 2004;56(6):757‐66. [PUBMED: 15478219 ] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Delprato 2001
- Delprato DJ. Comparisons of discrete‐trial and normalized behavioral language intervention for young children with autism. Journal of Autism and Developmental Disorders 2001;31(3):315–25. [PUBMED: 11518484] [DOI] [PubMed] [Google Scholar]
Emerson 2013
- Emerson A, Dearden J. Accommodating to motor difficulties and communication impairments in people with autism: the MORE intervention model. Frontiers in Integrative Neuroscience 2013;7:45. [DOI: 10.3389/fnint.2013.00045] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freitag 2009
- Freitag CM, Luders E, Hulst HE, Narr KL, Thompson PM, Toga AW, et al. Total brain volume and corpus callosum size in medication‐naïve adolescents and young adults with autism spectrum disorder. Biological Psychiatry 2009;66(4):316‐9. [PUBMED: 19409535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganz 2004
- Ganz JB, Simpson RL. Effects on communicative requesting and speech development of the Picture Exchange Communication System in children with characteristics of autism. Journal of Autism and Developmental Disorders 2004;34(4):395‐409. [DOI] [PubMed] [Google Scholar]
Gillberg 1999
- Gillberg C, Wing L. Autism: not an extremely rare disorder. Acta Psychiatrica Scandinavica 1999;99(6):399–406. [PUBMED: 10408260 ] [DOI] [PubMed] [Google Scholar]
Gilliam 1995
- Gilliam JE. Gilliam Autism Rating Scale. Austin, TX: Pro‐Ed, 1995. [Google Scholar]
Goldstein 2002
- Goldstein H. Communication intervention for children with autism: a review of treatment efficacy. Journal of Autism and Developmental Disorders 2002;32(5):373‐96. [PUBMED: 12463516] [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐8. [DOI: 10.1136/bmj.328.7454.1490; PMC428525; PUBMED: 15205295] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 10 October 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Green 2010
- Green J, Charman T, McConachie H, Aldred C, Slonims V, Howlin P, et al. Parent‐mediated communication‐focused treatment in children with autism (PACT): a randomised controlled trial. Lancet 2010;375(9732):2152–60. [DOI: 10.1016/S0140-6736(10)60587-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gutstein 2002
- Gutstein S, Sheely R. Relationship Development Intervention with Children, Adolescents, and Adults. London: Jessica Kingsley Publishers, 2002. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
HALO 2016
- HALO. Helping autism through learning and outreach. www.halo‐soma.org (accessed 12 July 2016).
Hartley 2008
- Hartley SL, Sikora DM, McCoy R. Prevalence and risk factors of maladaptive behaviour in young children with autistic disorder. Journal of Intellectual Disability Research 2008;52(10):819–29. [DOI: 10.1111/j.1365-2788.2008.01065.x; NIHMS178273; PMC2838711] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook..
Howlin 2009
- Howlin P, Magiati I, Charman T. Systematic review of early intensive behavioral interventions for children with autism. American Journal of Intellectual and Developmental Disabilities 2009;114(1):23‐41. [DOI: 10.1352/2009.114:23-41] [DOI] [PubMed] [Google Scholar]
Hudry 2010
- Hudry K, Leadbitter K, Temple K, Slonims V, McConachie H, Aldred C, et al. Preschoolers with autism show greater impairment in receptive compared with expressive language abilities. International Journal of Language & Communication Disorders 2010;45(6):681‐90. [PUBMED: 20102259] [DOI] [PubMed] [Google Scholar]
Hudry 2017
- Hudrey K, Dimov S. Spoken language shows some improvement following intervention for children with autism: but for which children and why?. Evidence‐Based Mental Health 2017;20(3):e16. [DOI: 10.1136/eb-2016-102435; PUBMED: 28663170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Just 2004
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high‐functioning autism: evidence of under connectivity. Brain 2004;127(Pt 8):1811–21. [DOI: 10.1093/brain/awh199] [DOI] [PubMed] [Google Scholar]
Kasari 2012
- Kasari C, Gulsrud A, Freeman S, Paparella T, Hellemann G. Longitudinal follow‐up of children with autism receiving targeted interventions on joint attention and play. Journal of the American Academy of Child & Adolescent Psychiatry 2012;51(5):487‐95. [DOI: 10.1016/j.jaac.2012.02.019; PMC3338205; PUBMED: 22525955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasari 2013
- Kasari C, Brady N, Lord C, Tager‐Flusberg H. Assessing the minimally verbal school‐aged child with autism spectrum disorder. Autism Research 2013;6(6):479‐93. [DOI: 10.1002/aur.1334] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2009
- Kim J‐M, Utley A. A review of communication intervention for young children with autism spectrum disorder. Journal on Developmental Disabilities 2009;15(3):23‐47. [Google Scholar]
Koegel 2006
- Koegel RL, Koegel LK. Pivotal Response Treatments for Autism. Baltimore: Brookes Publishing Company, 2006. [Google Scholar]
Kumar 2010
- Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, et al. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cerebral Cortex 2010;20(9):2103‐13. [PUBMED: 20019145 ] [DOI] [PubMed] [Google Scholar]
Lai 2014
- Lai MC, Lombardo MV, Baron‐Cohen S. Autism. Lancet 2014;383(9920):896‐910. [DOI: 10.1016/S0140-6736(13)61539-1] [DOI] [PubMed] [Google Scholar]
Lawton 2012
- Lawton K, Kasari C. Brief report: longitudinal improvements in the quality of joint attention in preschool children with autism. Journal of Autism and Developmental Disorders 2012;42(2):307‐12. [DOI: 10.1007/s10803-011-1231-z; PMC4194068; PUBMED: 22187107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. British Medical Journal 2009;339:b2700. [DOI: 10.1136/bmj.b2700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorah 2015
- Lorah E, Parnell A, Whitby P, Hantula D. A systematic review of tablet computers and portable media players as speech generating devices for individuals with autism spectrum disorder. Journal of Autism and Developmental Disorders 2015;45(12):3792‐804. [PUBMED: 25413144] [DOI] [PubMed] [Google Scholar]
Lord 1994
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview ‐ Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24(5):659‐85. [PUBMED: 23785315] [DOI] [PubMed] [Google Scholar]
Lord 2000
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule‐generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders 2000;30(3):205‐23. [PUBMED: 11055457] [PubMed] [Google Scholar]
Lord 2010
- Lord C. Who remains non‐verbal? What are the characteristics of this population?. NIH Workshop on Non‐verbal School Age Children with Autism; 2010 April 13‐14; Rockville, Maryland. Rockville, Maryland, 2010.
Lovaas 1987
- Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. Journal of Consulting and Clinical Psychology 1987;55(1):3‐9. [PUBMED: 3571656] [DOI] [PubMed] [Google Scholar]
Luyster 2008
- Luyster RJ, Kadlec MB, Carter A, Tager‐Flusberg H. Language assessment and development in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders 2008;38(8):1426‐38. [DOI: 10.1007/s10803-007-0510-1] [DOI] [PubMed] [Google Scholar]
Matson 2008
- Matson JL, Rivet TT. The effects of severity of autism and PDD‐NOS symptoms on challenging behaviors in adults with intellectual disabilities. Journal of Developmental and Physical Disabilities 2008;20(1):41–51. [DOI: 10.1007/s10882-007-9078-0] [DOI] [Google Scholar]
May 2017
- May T, Sciberras E, Brignell A, Williams K. Autism spectrum disorder: updated prevalence and comparison of two birth cohorts in a nationally representative Australian sample. BMJ Open 2017;7(5):e015549. [DOI: 10.1136/bmjopen-2016-015549.; PMC5623420; PUBMED: 28490562] [DOI] [PMC free article] [PubMed] [Google Scholar]
McClintock 2003
- McClintock K, Hall S, Oliver C. Risk markers associated with challenging behaviours in people with developmental disabilities: a meta‐analytic study. Journal of Intellectual Disability Research 2003;47(Pt 6):405‐16. [DOI] [PubMed] [Google Scholar]
Merinda 2009
- Merinda P, Iacono T. Autism Spectrum Disorders and AAC. Baltimore: Brookes Publishing Co, 2009. [Google Scholar]
Mesibov 2005
- Mesibov GB, Shea V, Schopler E, Adams L, Burgess S, Chapman SM, et al. The TEACCH Approach to Autism Spectrum Disorders. New York: Springer, 2005. [Google Scholar]
Millar 2006
- Millar DC, Light JC, Schlosser RW. The impact of augmentative and alternative communication intervention on the speech production of individuals with developmental disabilities: a research review. Journal of Speech, Language, and Hearing Research 2006;49(2):248‐64. [DOI: 10.1044/1092-4388(2006/021)] [DOI] [PubMed] [Google Scholar]
Modabbernia 2017
- Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence‐based review of systematic reviews and meta‐analyses. Molecular Autism 2017;8:13. [DOI: 10.1186/s13229-017-0121-4; PMC5356236; PUBMED: 28331572] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mundy 1990
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders 1990;20(1):115‐28. [PUBMED: 2324051] [DOI] [PubMed] [Google Scholar]
Mundy 2003
- Mundy P, Delgado C, Block J, Venezia M, Hogan A, Seibert, J. A manual for the abridged early social communication scales. Coral Gables, FL: University of Miami, 2003. [Google Scholar]
Mundy 2007
- Mundy P, Newell L. Attention, joint attention, and social cognition. Current Directions in Psychological Sciences 2007;16(5):269‐74. [DOI: 10.1111/j.1467-8721.2007.00518.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
NIH 2010
- National Institute of Health. NIH workshop on nonverbal school‐aged children with autism. www.nidcd.nih.gov/funding/programs/10autism/pages/default.aspx (accessed 25 August 2014).
Norrelgen 2015
- Norrelgen F, Fernell E, Eriksson M, Hedvall Å, Persson C, Sjölin M, et al. Children with autism spectrum disorders who do not develop phrase speech in the preschool years. Autism 2015;19(8):934‐43. [DOI: 10.1177/1362361314556782] [DOI] [PubMed] [Google Scholar]
Paul 2008
- Paul R. Intervention to improve communication in autism. Child and Adolescent Psychiatric Clinics of North America 2008;17(4):835‐56. [PUBMED: 18775373] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991‐6. [PUBMED: 18538991] [DOI] [PubMed] [Google Scholar]
Pickett 2009
- Pickett E, Pullara O, O'Grady J, Gordon B. Speech acquisition in older nonverbal individuals with autism: a review of features, methods, and prognosis. Cognitive and Behavioral Neurology 2009;22(1):1‐21. [PUBMED: 19372766] [DOI] [PubMed] [Google Scholar]
Prizant 1998
- Prizant BM, Wetherby AM. Understanding the continuum of discrete trial traditional behavioral to social‐pragmatic developmental approaches to communication enhancement for young children with autism/PDD. Seminars in Speech and Language 1998;19(4):329‐52. [PUBMED: 9857391] [DOI] [PubMed] [Google Scholar]
Prizant 2006
- Prizant B, Wetherby A, Rubin E, Laurent A, Rydell P. The SCERTS Model: A Comprehensive Educational Approach for Children with Autism Spectrum Disorders. Baltimore, MD: Brookes Publishing Co, 2006. [Google Scholar]
Reichow 2009
- Reichow B, Wolery M. Comprehensive synthesis of early intensive behavioral interventions for young children with autism based on the UCLA young autism project model. Journal of Autism and Developmental Disorders 2009;39(1):23‐41. [DOI: 10.1007/s10803-008-0596-0] [DOI] [PubMed] [Google Scholar]
ReMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogers 2000
- Rogers SJ. Interventions that facilitate socialization in children with autism. Journal of Autism and Developmental Disorders 2000;30(5):399‐409. [DOI] [PubMed] [Google Scholar]
Rogers 2009
- Rogers SJ, Dawson G. Early Start Denver Model for Young Children with Autism: Promoting Language, Learning, and Engagement. New York: The Guildford Press, 2009. [Google Scholar]
Romski 1996
- Romski MA, Sevcik RA. Breaking the Speech Barrier: Language Development through Augmented Means. Baltimore: Brookes Publishing Co, 1996. [Google Scholar]
Rose 2016
- Rose V, Trembath D, Keen D, Paynter J. The proportion of minimally verbal children with autism spectrum disorder in a community‐based early intervention programme. Journal of Intellectual Disability Research 2016;60(5):464–77. [DOI: 10.1111/jir.12284; PUBMED: 27120989] [DOI] [PubMed] [Google Scholar]
Rutter 2003
- Rutter M, Le Couteur, Lord C. The Autism Diagnostic Interview‐Revised. Los Angeles: Western Psychological Services, 2003. [Google Scholar]
Schoen 2011
- Schoen E, Paul R, Chawarska K. Phonology and vocal behavior in toddlers with autism spectrum disorders. Autism Research 2011;4(3):177‐88. [DOI: 10.1002/aur.183] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schopler 1986
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale (CARS): For Diagnostic Screening and Classification of Autism. New York, NY: Irvington, 1986. [Google Scholar]
Shriberg 2011
- Shriberg LD, Paul R, Black LM, Santen JP. The hypothesis of apraxia of speech in children with autism spectrum disorder. Journal of Autism Developmental Disorders 2011;41(4):405‐26. [DOI: 10.1007/s10803-010-1117-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sigafoos 2000
- Sigafoos J. Communication development and aberrant behaviour in children with developmental disabilities. Education and Training in Mental Retardation and Developmental Disabilities 2000;35(2):168‐76. [Google Scholar]
Sparrow 2005
- Sparrow S, Cicchetti D, Balla DA. Vineland Adaptive Behavior Scales—II. 2nd Edition. San Antonio, TX: Pearson Assessment, 2005. [Google Scholar]
Spence 1998
- Spence SH. A measure of anxiety symptoms among children. Behaviour Research and Therapy 1998;36(5):545‐566.. [DOI] [PubMed] [Google Scholar]
Stanfield 2008
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: a systematic review and meta‐analysis of structural magnetic resonance imaging studies. European Psychiatry 2008;23(4):289–99. [DOI: 10.1016/j.eurpsy.2007.05.006] [DOI] [PubMed] [Google Scholar]
Stessman 2017
- Stessman HA, Xiong B, Coe BP, Wang T, Hoekzema K, Fenckova M, et al. Targeted sequencing identifies 91 neurodevelopmental‐disorder risk genes with autism and developmental‐disability biases. Nature Genetics 2017;49(4):515‐26. [DOI: 10.1038/ng.3792; PMC5374041; PUBMED: 28191889] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strain 1998
- Strain PS, Kohler FW. Peer‐mediated social intervention for young children with autism. Seminars in Speech and Language 1998;19(4):391‐405. [DOI] [PubMed] [Google Scholar]
Sussman 2001
- Sussman F. More than Words. Toronto: The Hanen Centre, 2001. [Google Scholar]
Tager‐Flusberg 2005
- Tager‐Flusberg H, Paul R, Lord CE. Language and communication in autism. In: Volkmar F, Paul R, Klin A, Cohen DJ editor(s). Handbook of Autism and Pervasive Developmental Disorder. 3rd Edition. Vol. 1, New York: John Wiley & Sons, 2005:335–64. [Google Scholar]
Tager‐Flusberg 2009
- Tager‐Flusberg H, Rogers S, Cooper J, Landa R, Lord C, Paul R, et al. Defining spoken language benchmarks and selecting measures of expressive language development for young children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research 2009;52(3):643–52. [DOI: 10.1044/1092-4388(2009/08-0136); PMC2819321; PUBMED: 19380608] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tager‐Flusberg 2013
- Tager‐Flusberg H, Kasari C. Minimally verbal school‐aged children with autism spectrum disorder: the neglected end of the spectrum. Autism Research 2013;6(6):468‐78. [PUBMED: 24124067] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas‐Stonell 2013
- Thomas‐Stonell N, Washington K, Oddson B, Robertson B, Rosenbaum P. Measuring communicative participation using the FOCUS©: Focus on the Outcomes of Communication Under Six. Child: Care, Health and Development 2013;39(4):474‐80. [DOI: 10.1111/cch.12049] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thunberg 2013
- Thunberg G. Early communication intervention for children with autism spectrum disorder. In: Fitzgerald M editor(s). Recent Advances in Autism Spectrum Disorders. Vol. 1, Rijeka: InTech, 2013:717‐44. [DOI: 10.5772/54881] [DOI] [Google Scholar]
Thurm 2007
- Thurm A, Lord C, Lee LC, Newschaffer C. Predictors of language acquisition in preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders 2007;37(9):1721‐34. [DOI: 10.1007/s10803-006-0300-1] [DOI] [PubMed] [Google Scholar]
Thurm 2015
- Thurm A, Manwaring S, Swineford L, Farmer C. Longitudinal study of symptom severity and language in minimally verbal children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2015;56(1):97‐104. [PUBMED: 24961159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trembath 2014
- Trembath D, Vivanti G. Problematic but predictive: individual differences in children with autism spectrum disorders. International Journal of Speech‐Language Pathology 2014;16(1):57‐60. [DOI: 10.3109/17549507.2013.859300] [DOI] [PubMed] [Google Scholar]
Vivanti 2014
- Vivanti G, Prior M, Williams K, Dissanayake C. Predictors of outcomes in autism early intervention: why don't we know more?. Frontiers in Pediatrics 2014;2:58. [DOI: 10.3389/fped.2014.00058; PMC4064565] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. [Google Scholar]
Wing 2002
- Wing L, Leekam SR, Libby SJ, Gould J, Larcombe M. The Diagnostic Interview for Social and Communciation Disorders: background, inter‐rater reliability and clinical use. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2002;43(3):307‐25. [PUBMED: 11944874] [DOI] [PubMed] [Google Scholar]
Wodka 2013
- Wodka EL, Mathy P, Kalb L. Predictors of phrase and fluent speech in children with autism and severe language delay. Pediatrics 2013;131(4):e1128‐34. [PUBMED: 23460690] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoder 2006a
- Yoder P, Stone WL. Randomized comparison of two communication interventions for preschoolers with autism spectrum disorders. Journal of Consulting and Clinical Psychology 2006;74(3):426‐35. [DOI: 10.1037/0022-006X.74.3.426; PUBMED: 16822100] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brignell 2016
- Brignell A, Song H, Zhu J, Suo C, Lu D, Morgan AT. Communication intervention for autism spectrum disorders in minimally verbal children. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012324] [DOI] [PMC free article] [PubMed] [Google Scholar]


