Abstract
Background
Inflammation may contribute to preterm birth and to morbidity of preterm infants. Preterm infants are at risk for alterations in the normal protective microbiome. Oral probiotics administered directly to preterm infants have been shown to decrease the risk for severe necrotizing enterocolitis (NEC) as well as the risk of death, but there are safety concerns about administration of probiotics directly to preterm infants. Through decreasing maternal inflammation, probiotics may play a role in preventing preterm birth and/or decreasing the inflammatory milieu surrounding delivery of preterm infants, and may alter the microbiome of the preterm infant when given to mothers during pregnancy. Probiotics given to mothers after birth of preterm infants may effect infant bacterial colonization, which could potentially reduce the incidence of NEC.
Objectives
1. To compare the efficacy of maternal probiotic administration versus placebo or no intervention in mothers during pregnancy for the prevention of preterm birth and the prevention of morbidity and mortality of infants born preterm.
2. To compare the efficacy of maternal probiotic administration versus placebo, no intervention, or neonatal probiotic administration in mothers of preterm infants after birth on the prevention of mortality and preterm infant morbidities such as NEC.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 2), MEDLINE via PubMed (1966 to 21 March 2017), Embase (1980 to 21 March 2017), and CINAHL (1982 to 21 March 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials and quasi‐randomized trials.
Selection criteria
We included randomized controlled trials in the review if they administered oral probiotics to pregnant mothers at risk for preterm birth, or to mothers of preterm infants after birth. Quasi‐randomized trials were eligible for inclusion, but none were identified. Studies enrolling pregnant women needed to administer probiotics at < 36 weeks' gestation until the trimester of birth. Probiotics considered were of the genera Lactobacillus, Bifidobacterium or Saccharomyces.
Data collection and analysis
We used the standard methods of the Cochrane Collaboration and Cochrane Neonatal to determine the methodologic quality of studies, and for data collection and analysis.
Main results
We included 12 eligible trials with a total of 1450 mothers and 1204 known infants. Eleven trials administered probiotics to mothers during pregnancy and one trial administered probiotics to mothers after birth of their preterm infants. No studies compared maternal probiotic administration directly with neonatal administration. Included prenatal trials were highly variable in the indication for the trial, the gestational age and duration of administration of probiotics, as well as the dose and formulation of the probiotics. The pregnant women included in these trials were overall at low risk for preterm birth. In a meta‐analysis of trial data, oral probiotic administration to pregnant women did not reduce the incidence of preterm birth < 37 weeks (typical risk ratio (RR) 0.92, 95% confidence interval (CI) 0.32 to 2.67; 4 studies, 518 mothers and 506 infants), < 34 weeks (typical risk difference (RD) 0.00, 95% CI ‐0.02 to 0.02; 2 studies, 287 mothers and infants), the incidence of infant mortality (typical RD 0.00, 95% CI ‐0.02 to 0.02; 2 studies, 309 mothers and 298 infants), or the gestational age at birth (mean difference (MD) 0.15, 95% CI ‐0.33 to 0.63; 2 studies, 209 mothers with 207 infants).
One trial studied administration of probiotics to mothers after preterm birth and included 49 mothers and 58 infants. There were no significant differences in the risk of any NEC (RR 0.44, 95% CI 0.13 to 1.46; 1 study, 58 infants), surgery for NEC (RR 0.15, 95% CI 0.01 to 2.58; 1 study, 58 infants), death (RR 0.66, 95% CI 0.06 to 6.88; 1 study, 58 infants), and death or NEC (RR 0.53, 95% CI 0.19 to 1.49; 1 study, 58 infants). There was an improvement in time to reach 50% enteral feeds in infants whose mothers received probiotics, but the estimate is imprecise (MD ‐9.60 days, 95% CI ‐19.04 to ‐0.16 days; 58 infants). No other improvement in any neonatal outcomes were reported. The estimates were imprecise and do not exclude the possibility of meaningful harms or benefits from maternal probiotic administration. There were no cases of culture‐proven sepsis with the probiotic organism. The GRADE quality of evidence was judged to be low to very low due to inconsistency and imprecision.
Authors' conclusions
There is insufficient evidence to conclude whether there is appreciable benefit or harm to neonates of either oral supplementation of probiotics administered to pregnant women at low risk for preterm birth or oral supplementation of probiotics to mothers of preterm infants after birth. Oral supplementation of probiotics to mothers of preterm infants after birth may decrease time to 50% enteral feeds, however, this estimate is extremely imprecise. More research is needed for post‐natal administration of probiotics to mothers of preterm infants, as well as to pregnant mothers at high risk for preterm birth.
Plain language summary
Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants
Review question Do probiotics given to pregnant women prior to delivery and/or probiotics given to mothers of premature babies after birth compared with administration of placebo, no intervention or postnatal administration of probiotics to premature babies themselves reduce the risk of morbidity and mortality in premature babies?
Background Inflammation may contribute to premature birth and to morbidity of premature babies. Premature babies are at risk for alterations in the growth of normal "good" bacteria in their intestinal tract. Probiotics are supplements of "good bacteria." Giving these bacteria directly to premature babies may decrease inflammation and decrease the risk for severe gastrointestinal disease (necrotizing enterocolitis) as well as the risk of death. However, there are safety concerns about administration of probiotics directly to premature babies.
Study characteristics Twelve eligible trials with a total of 1450 mothers and 1204 known infants were included from searches, up to date as of March 2017. Eleven trials gave probiotics to mothers during pregnancy and one trial gave probiotics to mothers after the birth of their preterm infants. No studies compared maternal probiotic administration directly versus neonatal administration.The studies of pregnant women focused on various aspects of the studies with different probiotics given at different times. The pregnant women included in these trials were overall at low risk for preterm birth. The one trial with mothers given probiotics after birth was to mothers of infants who weighed less than 1500 g at birth.
Key results There is insufficient evidence to conclude whether there is appreciable benefit or harm to neonates of either oral supplementation of probiotics administered to pregnant women at low risk for preterm birth or oral supplementation of probiotics to mothers of preterm infants after birth. There were no trials that gave mothers at high risk for having a premature infant probiotics, so the effects of probiotics given to those mothers is unknown. More studies are needed to know if probiotics given to mothers of preterm infants decreases death, necrotizing enterocolitis, or other problems related to prematurity.
Quality of evidence In general, the evidence was of low to very low quality due to imprecision (small sample sizes) and indirectness (enrolled mothers were not necessarily at high risk for delivering their babies early).
Summary of findings
Background
Description of the condition
The microbiome is defined as the genomes and gene products of microbes that live within and on humans (Johnson 2012). Under normal conditions the microbiome of an infant is established through exposure with bacteria both prenatally and postnatally. The placenta, amniotic fluid and meconium have their own microbiota that influences the microbiome of the infant. Postnatal influences of the microbiome come from exposures mainly from the maternal microbiome which includes exposure to vaginal, oral, fecal, skin and breast milk bacteria, with a typical predominance of protective Lactobacillus and Bifidobacterium in the neonatal period.
Preterm infants are at risk for alterations in the normal protective microbiome due their increased cesarean delivery rate, exposure to antibiotics, nosocomial exposures to pathogens, lack of typical skin contact with maternal flora, as well as alterations in typical exposure to breast milk (Cilieborg 2012). Cesarean delivery alters colonization of neonates so their stool has lower bifidobacteria and more skin flora such as Staphylococcus, Corynebacterium and Propionibacterium (Faa 2013). Maternal intrapartum antibiotics and antibiotics given to mothers with prolonged rupture of membranes are associated with lower transfer of Lactobacillus to the infant, and even antibiotics given days before birth have been shown to alter the premature infant microbiome with less bacterial diversity of the first stool samples (Faa 2013; Keski‐Nisula 2013). Antibiotics given in the neonatal period have also been associated with decreased stool bifidobacteria (Faa 2013). This may lead to delayed colonization with low diversity of bacteria as well as colonization with pathogens and bacterial overgrowth which put infants at risk for necrotizing enterocolitis (NEC), sepsis and death in the neonatal period (Cilieborg 2012).
The development of NEC is typically associated with a dysregulation of inflammation in the gut that results in gas‐producing bacteria translocating through the bowel mucosa and is not usually caused by one single organism. It is a disorder that affects approximately 7% of infants with a birth weight of less than 1500 g, with a death rate of approximately 20% to 30% (Neu 2011). Strategies to prevent NEC have been employed including exclusive human milk diet, standardized feeding protocols, human milk fortifiers and postnatal probiotics, but prematurity and alterations of gut flora still put premature infants at risk.
Modifications of the neonatal microbiome may have long‐term effects on health that may last until adulthood. Altered microbiomes have been associated with the development of atopy (hereditary tendency to be hypersensitive to certain allergens), inflammatory bowel disease, obesity and impaired glucose regulation. It is unknown to what extent early preterm infant alterations in the microbiome may influence their lifelong risk for these diseases.
Description of the intervention
Probiotics are defined as living microorganisms that confer a health benefit to the host (Othman 2007); they are often given enterally to colonize the gastrointestinal tract. Typical probiotic bacteria administered are bifidobacteria, Lactobacillus or Saccharomyces (Dugoua 2009; Thomas 2010). They have been given directly to preterm infants with the intention to decrease the rate of NEC (AlFaleh 2014; Costeloe 2016; Deshpande 2010; Neu 2011), and there is emerging interest in promoting gastrointestinal colonization in preterm infants through administration of probiotics to their mothers instead of directly to infants. Probiotics have already been studied during pregnancy for other indications with the intention to treat genitourinary infections, prevent infant atopy, enhance metabolism and prevent preterm labor (Dugoua 2009; Gomez Arango 2015; Lahtinen 2009; Lindsay 2013; Reid 2003; VandeVusse 2013).
The focus of this investigation is the maternal oral administration of probiotics. Maternal probiotics can be administered to pregnant women at risk for preterm birth or to mothers of preterm infants after birth. Pregnant women administered probiotics in pregnancy will be compared to pregnant women receiving placebo or no intervention. Mothers of preterm infants administered probiotics after birth will be compared to either mothers of preterm infants administered placebo or no intervention, or to preterm neonates administered probiotics directly.
How the intervention might work
The microbiome of the pregnant and postpartum woman is influenced by the established microbiome, diet, probiotic exposures, and pregnancy itself as there are alterations of the microbiome that occur during pregnancy that are likely hormonally mediated. During pregnancy probiotics have been generally regarded as safe (Elias 2011; Gomez Arango 2015; Lindsay 2013), and beneficial for mothers in preventing and treating bacterial vaginitis, and reducing the risk of gestational diabetes, with hypotheses but no definitive evidence of their efficacy in preventing preterm birth (Gomez Arango 2015; Othman 2007). While studies have shown that probiotics taken by adults only change their microbiome while they are being taken, these short‐term alterations in maternal microbiome may have long‐term effects on establishment of the fetal and neonatal microbiome (Matamaros 2013).
Normal microbiome colonization occurs prenatally with bacteria found in the placenta, amniotic fluid and fetal meconium (Matamaros 2013), and postnatal colonization from the mother and environment, including vaginal Lactobacillus obtained at birth, maternal skin flora and bacteria in breast milk (Cilieborg 2012; Faa 2013). Especially predominant and beneficial to the healthy neonatal microbiome are lactobacilli and Bifidobacterium. Lactobacilli are facultative anaerobes that create an environment in which bifidobacteria, anaerobic gram‐positive bacilli, can colonize and predominate in the colon (Bergmann 2014; Dugoua 2009). These bacteria help maintain the mucosa of the intestines, prevent pathogens from colonizing the colon, modulate inflammation along the mucosa and activate the immune system (Hickey 2012), which may help to decrease the risk of NEC and subsequent development of sepsis.
Breast milk consumption greatly contributes to colonization of newborn infants. Studies have shown that there are more than 200 species of bacteria in human milk with a predominance of Lactobacillus and bifidobacteria in addition to Staphylococcus, Streptococcus and corynebacteria (Bergmann 2014). There is evidence that the neonatal gut microbiome reflects the bacteria found in breast milk and the mother’s stool (Bergmann 2014; Cilieborg 2012; Jost 2014). According to the theory of the entero‐mammary pathway, bacteria are deposited into the mammary milk ducts from the maternal gastrointestinal tract via active transport through the blood (Bergmann 2014; Jost 2014). Dendritic cells in the maternal gut lumen are thought to trap bacteria, and with the help of mononuclear cells, transport them through the blood to the breast (Bergmann 2014). This is thought to be hormonally mediated, occurring late in the third trimester, with the most bacteria present in the breast in the peripartum period (Bergmann 2014).
Studies have shown that lactobacilli taken orally by the mother are present in breast milk when taken prenatally and postnatally (Bergmann 2014). Supplementation with Lactobacillus reuteri (L. reuteri) during the third trimester prior to delivery resulted in a statistically significant increase in L reuteri in maternal colostrum (Abrahamsson 2009), and Lactobacillus GG taken by mothers for one month prior to delivery increased the diversity of fecal Bifidobacterium in neonates (Gueimonde 2006).
Why it is important to do this review
Probiotic administration in high‐risk very low birth weight (VLBW) infants has been shown to reduce the incidence of NEC as well as mortality (AlFaleh 2014; Deshpande 2010). Postnatal probiotic administration decreased the risk of developing stage II‐III NEC by more than half (AlFaleh 2014). In their analysis, Deshpande and coworkers suggest that routine use be implemented without the need for more placebo‐controlled trials (Deshpande 2010).
However, the studies included in these meta‐analyses are quite heterogeneous in regards to the specie(s), dose, timing and duration of administration, and there is concern about safety and quality assurance of probiotics which lie outside the purview of the Food and Drug Administration (FDA) regulation. A recent systematic review cautions universal use of probiotics in VLBW infants citing the lack of evidence of efficacy for specific strains (Mihatsch 2012), while others in the neonatal community cite both methodologic and safety concerns as cautions against universal adoption (Garland 2010; Soll 2010). The American Academy of Pediatrics 2010 Clinical Report written by the Committee on Nutrition states that there is insufficient data to recommend probiotics in infants weighing less than 1000 g, and that since not all probiotics have been studied, they cannot all be generally recommended (Thomas 2010). The European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition counsels caution in introducing potentially infectious agents to VLBW infants due to their immunologic immaturity, and states that there are not enough data to conclude that probiotic administration to preterm neonates is safe (Agostoni 2010). While there are many studies that suggest the safety of probiotics (AlFaleh 2014; Costeloe 2016), there have been cases of sepsis with the strains of probiotics given to preterm infants (Bertelli 2015), as well as a death due to gastrointestinal mucormycosis from Rhizopus oryzae‐contaminated probiotic directly administered to a preterm infant (CDC HAN 2014). With these emerging safety concerns, the FDA has issued a warning against the use of probiotics in immunocompromised patients (CDC 2015; FDA 2014).
These same safety concerns may not be universal where there are regulatory organizations responsible for overseeing the safety of probiotics, such as the Natural Health Products Directorate in Canada (Janvier 2013).
Currently, probiotic use in the VLBW population is not routine in many neonatal intensive care units (NICUs) in the USA. A recent survey of US NICUs that are members of Vermont Oxford Network showed that 14% of these NICUs give VLBW infants probiotics (Viswanathan 2016). Of these 14%, 8.8% of NICUs routinely give all VLBWs probiotics, while another 5.2% of NICUs give probiotics to select VLBW infants (Viswanathan 2016).
With these safety concerns regarding the direct administration of probiotics to preterm infants and evidence showing that probiotics given to preterm infants decreases the rate of NEC and mortality (AlFaleh 2014; Deshpande 2010), there is interest in alternate methods for inducing a normal microbiome in a safe manner. Since maternal flora and breast milk bacteria are known to be transmitted naturally to fetuses and neonates, an alternative method of exposure to probiotics that avoids direct administration to preterm infants, and therefore direct administration of possible contaminants, is maternal probiotic administration to mothers of preterm infants or pregnant women at risk for preterm birth. While not all species of probiotics or timing of administration within pregnancy have been studied thoroughly enough to make definitive statements about safety for all probiotics (Dugoua 2009), probiotics in pregnancy have been generally regarded as safe (Elias 2011; Gomez Arango 2015; Lindsay 2013).
Maternal probiotic administration in pregnancy for prevention of preterm labor and preterm birth has been addressed in one systematic Cochrane review focusing on probiotics specifically as a treatment or prevention of urogenital infections (Othman 2007). Our review will also address probiotic administration to pregnant women at risk of preterm birth, but its focus is on the preterm infant and includes pregnant women at risk for preterm birth for any reason. To our knowledge, this will be the first systematic review of maternal probiotic administration with a focus on mothers of preterm infants given probiotics after birth, the first to address the comparison of probiotics administered to mothers of preterm infants versus administration to the preterm infants themselves, and the first to address prenatal and postnatal probiotic administration to mothers at risk for preterm birth.
Objectives
To determine whether maternal probiotic administration to pregnant women and/or probiotic administration to mothers after preterm birth compared to administration of placebo, no intervention or postnatal administration to preterm infants reduces the risk of morbidity and mortality in preterm infants.
Comparisons
-
Probiotics administered to pregnant women (< 36 weeks' gestation) versus placebo or no intervention:
In pregnant women (< 36 weeks' gestation), maternal probiotics only prior to birth versus maternal placebo or no intervention prior to birth;
In pregnant women (< 36 weeks' gestation), maternal probiotics both prior to and after birth versus maternal placebo or no intervention prior to and after birth.
Probiotics administered exclusively after birth in mothers of preterm infants (< 37 weeks' gestation) versus maternal placebo or no intervention.
Probiotics administered exclusively after birth in mothers of preterm infants (< 37 weeks' gestation) versus neonatal probiotic administration.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials. Quasi‐randomized controlled trials, and cluster trials were eligible for inclusion but none were identified. Cross‐over trials were not eligible for inclusion.
Types of participants
Comparison 1: probiotics administered to pregnant women versus placebo or no intervention
Pregnant women at risk for preterm birth (birth at < 36 weeks' gestation).
Comparison 2: probiotics administered exclusively after birth in mothers of preterm infants versus maternal placebo or no intervention
Postpartum mothers who have given birth to a preterm infant born < 37 weeks' gestation.
Comparison 3: probiotics administered exclusively after birth in mothers of preterm infants versus neonatal probiotic administration
Postpartum mothers who have given birth to a preterm infant born < 37 weeks' gestation.
Newborn infants born < 37 weeks' gestation.
Types of interventions
Included probiotics included Lactobacillus species, Bifidobacterium species, or Saccharomyces, and mixed preparations of these probiotics at any dose with the intention to treat for a minimum of seven days.
For antenatal probiotic administration, probiotics were taken at some point during the trimester in which the mother gives birth, or within one week of birth if born early in the third trimester.
Types of outcome measures
Data on the mothers and the preterm infants of these mothers was collected.
Primary outcomes
Secondary outcomes
Gestational age at birth (limited to infants in Comparison 1).*
Early onset bacterial sepsis, defined by positive blood culture on 'day of life 3' or earlier (limited to infants in Comparison 1).
Late‐onset sepsis, defined by positive blood culture on 'day of life 3' or later.
Culture‐proven sepsis with supplemented probiotic(s) before hospital discharge.
Neonatal sepsis (at any time)*
Surgical NEC (Bell Stage III) (Bell 1978).*
For infants born < 37 weeks
Duration of hospital stay (days).
Duration of parenteral nutrition (days).
Days to full enteral feeds.
Days to 50% enteral feeds*
Growth (g/kg/day) during hospitalization (prior to discharge).
Growth Z score at 36 to 40 weeks' postmenstrual age (PMA).
Retinopathy of prematurity (ROP) (any stage, severe stage 3 or greater) (ICCROP 2005).
Intraventricular hemorrhage (IVH) (any grade and severe (Grade III‐IV)) (Papile 1978).
Cystic periventricular leukomalacia (PVL) (diagnosed by cranial imaging).
Patent ductus arteriosus (PDA) (treated either medically or surgically).
Bronchopulmonary dysplasia (BPD) assessed at 28 days and at 36 weeks' PMA.
Long‐term major neurodevelopmental disability assessed at 18 to 24 months in survivors (cerebral palsy (CP)), developmental delay (Bayley or Griffith assessment more than two standard deviations (SD) below the mean) or intellectual impairment (intelligence quotient (IQ) more than two SD below mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification) (Jacobs 2013).
Maternal secondary outcomes
Maternal chorioamnionitis: suspected or confirmed intrauterine inflammation or infection or both ("Triple I") based upon the criteria from the 2016 chorioamnionitis workshop Higgins 2016). Suspected Triple I is defined by a fever without a clear source plus baseline fetal tachycardia, maternal white blood count greater than 15,000/mm³ in the absence of corticosteroids or definite purulent fluid from the cervical os. Confirmed Triple I is defined as meeting criteria for suspected Triple I plus amniocentesis‐proven infection through a positive Gram stain, low glucose or positive amniotic fluid culture or placental pathology revealing diagnostic features of infection. For comparison 1 only.
Maternal endometritis: clinical diagnosis by obstetric provider consisting of an oral temperature of ≥ 38.0 °C on any two of the first 10 postpartum days or a temperature of ≥ 38.7 °C during the first 24 hours postpartum based upon the American Committee of Maternal Welfare's standard definition) (Mackeen 2015).
Maternal mastitis diagnosed clinically by obstetrics provider at any time during the study.
Maternal sepsis as defined by positive maternal blood culture and assessed at any time during the study.
Maternal Group B Streptococcus colonization (based upon most recent vaginal/rectal swab results prior to birth).
* outcome added post‐hoc after review of available study outcomes
Search methods for identification of studies
We used the criteria and standard methods of the Cochrane Neonatal (see the Cochrane Neonatal Group search strategy for specialized register).
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 2) in the Cochrane Library; MEDLINE via PubMed (1966 to 21 March 2017); Embase (1980 to 21 March 2017); and CINAHL (1982 to 21 March 2017) using the following search terms: (probiotic OR lactobacillus OR bifidobacter* OR saccharomyces), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry).
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Information Scientist (see Appendix 2).
Searching other resources
We searched the abstracts of the Society for Pediatric Research (US) (published in Pediatric Research) for the years 1985 to 1999 using the following key words: (probiotic OR lactobacillus OR bifidobacteria OR saccharomyces) AND (neonates OR infants).
We also searched for conference abstracts from Pediatric Academic Societies (PAS) and European Society for Paediatric Research (ESPR). We carried out searches in Abstracts to View (2000 to present) (www.abstracts2view.com/pasall/) and Pediatric Research as well as the Cochrane Pregnancy and Childbirth Group's Trials Register.
Data collection and analysis
We collected information regarding the method of randomization, blinding, drug intervention, stratification, and whether the trial was single or multicenter for each included study. We noted the information regarding trial participants including gestational age criteria, birth weight criteria, and other inclusion or exclusion criteria. We analyzed the information on clinical outcomes including death, severe NEC (Bell stage II or more), early onset sepsis (if probiotics were administered before delivery), late onset sepsis, prematurity (gestational age) (if probiotics were administered prior to delivery), culture‐proven sepsis with supplemented probiotic(s), duration of hospital stay (days), duration of parenteral nutrition (days), days to full enteral feeds, growth (g/kg/day), ROP (any, severe), IVH (Grade III‐IV), cystic PVL and PDA (treated either medically or surgically), chronic lung disease (CLD), and long‐term major neurodevelopmental disability. Maternal data collected included maternal chorioamnionitis, endometritis, mastitis and sepsis. We assessed the quality of evidence at the outcome level using the Grading of Recommendations Assessment Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013).
Selection of studies
We assessed the titles and abstracts resulting from the electronic searches. The full copy of all relevant or potentially relevant trials was obtained and assessed according to the 'Criteria for considering studies for this review'.
We included randomized controlled trials fulfilling the selection criteria described in the previous section. We planned to include cluster‐randomized and quasi‐randomized controlled trials but none were identified. Cross‐over studies were not eligible for inclusion. Both superiority trials and non‐inferiority trials were eligible for inclusion. All review authors reviewed the results of the search and separately selected the studies for inclusion. Disagreements about whether a trial should be included were resolved by discussion and consensus was reached. In cases where additional information was needed before a decision could be made as to whether to include a trial, we planned to obtain this information from the study investigator.
Data extraction and management
Two review authors extracted, assessed, and coded all data for each study, using a form designed specifically for this review. Any standard error (SE) of the mean was replaced by the corresponding standard deviation (SD). We resolved any disagreement by discussion. For each study, final data were entered into Review Manager 5 (RevMan) by one review author (JG) and then checked by the other review author (RS) (Review Manager 2014). If needed, we planned to contact authors of trials to obtain missing data.
Assessment of risk of bias in included studies
Two review authors (JG, RS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
Any disagreements were resolved by discussion or by a third assessor. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed the statistical analyses using Review Manager 5 software (Review Manager 2014). We analyzed categorical data using risk ratio (RR), and risk difference (RD). For statistically significant outcomes, we calculated the number needed to treat for an additional participant with a beneficial outcome (NNTB) or number needed to treat for an additional participant with a harmful outcome (NNTH). We analyzed continuous data using mean difference (MD) and the standardized mean difference (SMD). We reported the 95% confidence interval (CI) on all estimates.
Unit of analysis issues
Had we included any cluster‐randomized trials, we planned to use the intracluster correlation coefficient (ICC) to calculate effective sample sizes in an approximate analysis (Cochrane Handbook for Systematic Reviews of Interventions, 15.3.4). Once trials had been changed to their effective sample size, we planned to enter the data into RevMan in a manner similar to how data from randomized control trials are entered.
Dealing with missing data
The review was analyzed using an intention‐to‐treat paradigm. For studies with missing data, we attempted to contact the authors to obtain this data.
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. We graded the degree of heterogeneity as: less than 25% — no heterogeneity; 25% to 49% — low heterogeneity; 50% to 75% — moderate heterogeneity; more than 75% — substantial heterogeneity. If statistical heterogeneity (an I² > 50%) was noted, the possible causes were explored (for example, differences in study quality, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
We planned to create a funnel plot if there were at least 10 studies meeting our inclusion criteria.
Data synthesis
If multiple studies were identified and thought to be sufficiently similar in participant population, intervention and outcomes, meta‐analysis was carried out using Review Manager 2014. For categorical outcomes, we calculated the typical estimates of RR and RD, each with its 95% CI; and for continuous outcomes the MD or a summary estimate for the MD, each with its 95% CI, was calculated. We used a fixed‐effect model for meta‐analysis. If meta‐analysis was judged to be inappropriate, we analyzed individual trials and interpreted them separately.
Quality of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: preterm birth (any preterm birth (< 37 weeks) and preterm birth < 34 weeks) (limited to infants in Comparison 1); severe NEC (Bell stage II or more) (Bell 1978); death (mortality before discharge); death or severe NEC; culture‐proven sepsis with supplemented probiotic(s) before hospital discharge; late‐onset sepsis, defined by positive blood culture on 'day of life 3' or later.
We added several clinically important outcomes to the 'Summary of findings' tables based on review of available studies post hoc: for Comparison 1: gestational age at birth (weeks); miscarriage/stillbirth; for Comparison 2: any NEC, surgery for NEC, feeding with more than 50% breast milk (days).
Two review authors independently assessed the quality of the evidence for each of the outcomes listed above. We considered evidence from randomized controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT GDT Guideline Development Tool to create ‘Summary of findings’ tables to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We examined the effect of differences in the definitions and measurement of outcomes, such as diagnosis of NEC and infections and differences in length of follow‐up using methods for addressing clinical heterogeneity: subgroup analysis and meta‐regression. If any other significant heterogeneity was detected between studies, we planned to carry out subgroup analysis and avoid pooling of results.
Subgroups for analysis
The following pre‐planned subgroup analyses were performed.
Probiotic type: Lactobacillus sp ecies, Bifidobacterium species, Saccharomyces, or mixed preparations of these probiotics
Economic country setting (low‐income, lower‐middle income, upper‐middle income and high‐income economies as defined by the World Bank (The World Bank 2016)
In addition, a post hoc subgroup analysis was performed based on maternal gestational age at enrollment.
There were insufficient data to perform subgroup analyses based upon birth weight, race, ethnicity, sex, provision of breast milk, and maternal risk for preterm birth.
Sensitivity analysis
Excluding studies with high risk of bias (performed).
Excluding unpublished studies (planned but not performed due to lack of data).
Results
Description of studies
See the Characteristics of included studies.
See the Figure 1 study flow diagram.
1.
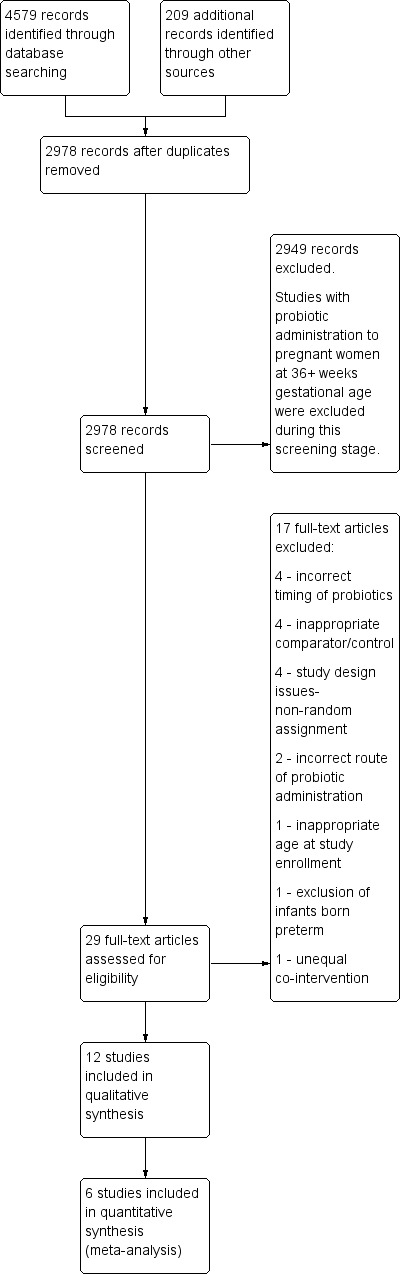
1 Study flow diagram.
Results of the search
Twelve studies were found that met the inclusion criteria; 11 for the comparison of probiotics administered to pregnant women (< 36 weeks' gestation) versus placebo or no intervention, (Comparison 1) (Badehnoosh 2018; Dolatkhah 2015; Fernández 2016; Jacobsson 2016; Kopp 2008; Laitinen 2009; Lindsay 2014; Lindsay 2015; Mantaring 2016; Ou 2012; Rautava 2012), and one for the comparison of probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation versus maternal placebo or no intervention, (Comparison 2) (Benor 2014). The studies in Comparison 1 and 2 combined included 1450 mothers and 1204 known infants (as not all studies reported the number of infants born). No studies were found meeting the criteria for the comparison of probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation versus neonatal probiotic administration (Comparison 3).
Nine studies reported on at least one outcome of interest and included 1199 mothers and 1048 known infants (as not all studies reported the number of infants born) (Badehnoosh 2018; Benor 2014; Fernández 2016; Kopp 2008; Laitinen 2009; Lindsay 2014; Lindsay 2015; Ou 2012; Rautava 2012), while three studies (Dolatkhah 2015; Jacobsson 2016; Mantaring 2016) with 260 mothers with 156 known infants did not report on outcomes of interest. Three studies conducted in pregnant women with atopy or with a fetus at risk for atopy that included 524 mothers and 484 infants (Kopp 2008; Ou 2012; Rautava 2012), only had gestational age at birth as an outcome of interest, and did not report the data in a format suitable for meta‐analysis. The remaining six studies reported on at least one outcome of interest for a total of 675 mothers and 564 infants. Five of these studies were in Comparison 1 and included 626 mothers and 506 infants; four administered the intervention only to mothers during pregnancy (Badehnoosh 2018; Fernández 2016; Lindsay 2014; Lindsay 2015), and one administered the intervention during pregnancy and after birth (Laitinen 2009). One study met the criteria for Comparison 2, administering the intervention to mothers of preterm infants after birth, for a total of 49 mothers with 58 infants (Benor 2014). See the 'Rrisk of bias' tables for data on risk of bias (Figure 2; Figure 3).
2.
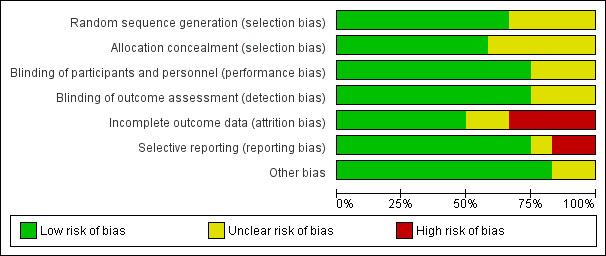
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
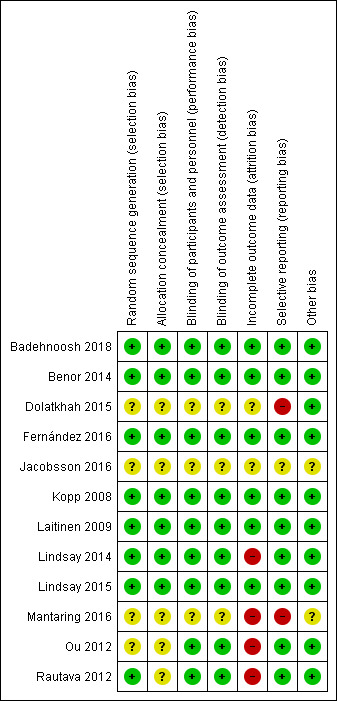
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Included studies
Comparison 1: Probiotics administered to pregnant women (< 36 weeks' gestation) versus placebo or no intervention
Before delivery (probiotics given only prior to delivery)
Summary: six studies were identified (Badehnoosh 2018; Dolatkhah 2015; Fernández 2016; Jacobsson 2016; Lindsay 2014; Lindsay 2015).
Participants: there were six studies that administered probiotics to women only during pregnancy: three to pregnant women with gestational diabetes (Badehnoosh 2018;Dolatkhah 2015; Lindsay 2015), one to obese women at risk for metabolic derangements (Lindsay 2014), one to low‐risk women to assess immunological or inflammatory responses (Jacobsson 2016), and one to pregnant women with a history of mastitis during lactation (Fernández 2016).
Interventions: two studies used combination probiotics with various species of lLctobacillus, Bifidobacterium and/or Streptococcus (Badehnoosh 2018; Dolatkhah 2015), while four studies used single probiotic agents, three administering Lactobacillus salivarius (L. salivarius) (Fernández 2016; Lindsay 2014; Lindsay 2015) and one using Lactobacillus rhamnosus (L. rhamnosus) (Jacobsson 2016). There was a degree of heterogeneity in terms of the timing and duration of the intervention, ranging from eight to 10 weeks until delivery (Jacobsson 2016), starting at 24 to 28 weeks and lasting four to eight weeks (Badehnoosh 2018; Dolatkhah 2015; Lindsay 2014), or starting at 30 weeks or < 34 weeks until delivery (Fernández 2016; Lindsay 2015). All control group participants received a placebo in these studies.
Outcomes of interest reported upon were preterm birth < 37 weeks (Badehnoosh 2018; Lindsay 2014; Lindsay 2015), preterm birth < 34 weeks (Lindsay 2014), gestational age at birth (Badehnoosh 2018; Lindsay 2014; Lindsay 2015), neonatal mortality (Lindsay 2014), miscarriage/stillbirth (Lindsay 2015), and mastitis (Fernández 2016).
Review of individual studies (based on study aims)
Treatment of maternal gestational diabetes
Badehnoosh 2018 conducted a randomized, double‐blind, placebo‐controlled clinical trial at the Akbarabadi Clinic in Tehran, Iran (April 2016 to September 2016), evaluating the effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes among participants with gestational diabetes (GDM). Sixty pregnant women diagnosed between 24 to 28 weeks with GDM who were not on oral hypoglycemic agents were randomly allocated to intake either probiotic capsule containing Lactobacillus acidophilus (L.acidophilus), Lactobacillus casei (L. casei) and Bifidobacterium bifidum (B. bifidum) ( 2 x 10^9 colony‐forming units (CFU)/g each) (n = 30) or placebo (n = 30) for six weeks. Primary outcomes were inflammatory markers, while secondary outcomes were biomarkers of oxidative stress and pregnancy outcomes, including preterm birth, gestational age at birth, Apgar scores, hospitalization, hypoglycemia, macrosomia, and cesarean delivery rates.
Dolatkhah 2015 conducted a double‐blind placebo‐controlled randomized clinical trial at Alzahra University Hospital in Tabriz, Iran (Spring and Summer 2014), to assess the effect of a probiotic supplementation on glucose metabolism and weight gain in pregnant women with newly diagnosed GDM. Sixty‐four pregnant women diagnosed between 24 to 28 weeks with GDM were randomly assigned to receive either aL.acidophilus LA‐5, Bifidobacterium BB‐12, Streptococcus thermophilus (S. thermophilus) STY‐31 and Lactobacillus delbrueckii bulgaricus (L. delbrueckii bulgaricus) LBY‐27 (4 biocap > 4 × 10^9 CFU) probiotic (n = 32) or placebo (n = 32) capsule along with dietary advice for eight consecutive weeks. Primary outcomes assessed were trend in weight gain and glucose metabolism indices in the mother. No outcomes of interest for this systematic review were reported.
Lindsay 2015 conducted a double‐blind placebo‐controlled randomized trial at the National Maternity Hospital in Dublin, Ireland (March 2012 to May 2014) to investigate the effects of a probiotic capsule intervention on maternal metabolic parameters and pregnancy outcome among pregnant women with gestational diabetes. One‐hundred forty‐nine singleton pregnant women with a new diagnosis of GDM in this pregnancy and who were < 34 weeks' gestation were randomly assigned to receive a capsule of probiotic (n = 74 ) with 100 mg of L. salivarius UCC118 at a target dose of 10^9 CFUs, or placebo (n = 75) until delivery. The primary outcome was the maternal fasting glucose and secondary outcomes were need for pharmacologic treatment of GDM, neonatal birth weight, stillbirths or miscarriages, gestational age at birth, preterm birth, cord blood metabolic parameters, Apgar score, small for gestational age (SGA) or large for gestational age (LGA) birth weight percentiles, and need for admission to the neonatal intensive care unit (NICU).
Treatment of maternal obesity
Lindsay 2014 (Probiotics in Pregnancy Study (ProP Study) conducted a placebo‐controlled, double‐blind, randomized trial at the National Maternity Hospital, Dublin, Ireland (March 2012 to March 2013) that investigated the effect of a probiotic capsule on maternal fasting glucose in obese pregnant women. One‐hundred seventy‐five pregnant obese women were assigned to probiotic administration with 100 mg L. salivarius UCC118 at 10^9 CFU (n = 83) or placebo capsule (n = 82) from 24 to 28 weeks' gestation. The primary outcome was the change in maternal fasting glucose and secondary outcomes included differences in the incidence of impaired glycemic control, neonatal anthropometric measures, delivery complications, cord blood metabolic variables, 5‐minute Apgar score, preterm birth rate < 37 weeks or < 34 weeks, gestational age at birth, perinatal mortality and admission to the NICU.
Inflammation/lipids
Jacobsson 2016 conducted a randomized, placebo‐controlled trial to assess the effects of supplementation with L. rhamnosus (LGG) in low‐risk pregnant women on maternal immunological or inflammatory responses. Forty low‐risk pregnant women at eight to 10 weeks of pregnancy were randomized to receive L. rhamnosus (10^8 CFU) (n = 20) or placebo (n = 20) until delivery. Main outcomes assessed were inflammatory and immunologic assays at recruitment, 25 weeks and 35 weeks of gestation. No outcomes of interest for this systematic review were reported.
Prevention of mastitis
Fernández 2016 conducted a double‐blind, randomized controlled trial at the Hospital Clínico San Carlos in Madrid, Spain that investigated the effect of an oral probiotic given to pregnant women to prevent infectious mastitis. One‐hundred‐eight pregnant women with a history of infectious mastitis after a prior pregnancy were assigned to probiotic capsule administration with L. salivarius PS2 at 9 x 10^10 CFU (n = 55) or placebo capsule (n = 53) from approximately week 30 of pregnancy until delivery. The primary outcome was the diagnosis of mastitis in the first three months after giving birth. Secondary outcomes included acute mastitis, subacute mastitis, breast pain scores, and breast milk bacterial counts.
Before and after delivery (probiotics given both prior to and after delivery)
Summary: five studies were identified that administered probiotics to women both prior to and after delivery (Kopp 2008, Laitinen 2009; Mantaring 2016;Ou 2012, Rautava 2012).
Participants: of the five studies, three studies enrolled pregnant women with a fetus at risk for atopy (Kopp 2008; Ou 2012; Rautava 2012), while two studies focused on healthy women with a focus on nutrition (Laitinen 2009; Mantaring 2016).
Interventions: two studies used a single agent probiotic of Lactobacillus GG (Kopp 2008; Ou 2012), while three used combination probiotics, one using L. rhamnosus and Bifidobacterium longum (B. longum) versus Lactobacillus paracasei (L. paracasei) and B. longum (Rautava 2012), and the other two using L. rhamnosus and Bifidobacterium lactis (B. lactis) (Laitinen 2009; Mantaring 2016). The beginning gestational age at first administration and the duration varied between studies from 14 weeks until end of exclusive breastfeeding (Laitinen 2009), four to six weeks before delivery until six months (Kopp 2008), 24 weeks until six months (Ou 2012), two months before delivery until two months after delivery (Rautava 2012), and the beginning of the third trimester until two months postpartum (Mantaring 2016). The studies that focused on atopic risk all had placebo capsules as the control (Kopp 2008, Ou 2012, Rautava 2012), while the other two studies had either nutritional counseling (Laitinen 2009), or nutritional beverage supplement (Mantaring 2016) that were administered in both the probiotic and control group.
Outcomes of interest reported upon were preterm birth < 37 weeks (Laitinen 2009), gestational age at birth (Kopp 2008; Ou 2012; Rautava 2012), although these studies did not report average and standard deviation (SD) and thus cannot be included quantitative analysis, neonatal mortality (Laitinen 2009), miscarriage/stillbirth (Laitinen 2009), and neonatal and maternal sepsis (Laitinen 2009).
Review of individual studies (based on study aims)
Multiple aims
Laitinen 2009 conducted a randomized controlled trial in maternal welfare clinics in Turku and South‐West Finland (April 2002 to November 2005) that investigated the impact of dietary counseling and/or probiotics on pregnant women with an aim to optimize maternal dietary intake and metabolism, to advance maternal health and reduce the risk of disease in the child. After final recruitment, 256 healthy pregnant women were recruited, 85 were in the control + placebo group, and 171 were in the nutritional intervention group. These 171 pregnant women were further randomized in a double‐blind fashion to receive probiotics (L. rhamnosus GG, and B. lactis Bb12, 10^9 CFU/day) and the nutritional intervention (n = 85), or placebo and the nutritional intervention (n = 86) from enrollment to the study around 14 weeks' gestation until the end of exclusive breastfeeding. Fourteen articles have been published from the original trial (Characteristics of included studies), with one only including a subset of enrolled patients as it was published before final recruitment. Primary and secondary outcomes vary between the articles, and include outcomes focused on maternal dietary compliance, adiposity, pregnancy weight gain, anthropometric measurements during and after pregnancy, maternal diagnosis of gestational diabetes, maternal laboratory indices for lipids, leptin, and glucose metabolism, as well as placental fatty acids, breast milk fatty acids and inflammatory markers, maternal sepsis and infant sensitization. Outcomes pertinent to the neonate include birth weight, birth length, birth head circumference, gestational age at birth, miscarriage rate, preterm birth < 37 weeks, preterm birth < 32 weeks, preterm birth 32 to 36 weeks, cesarean delivery rate, perinatal mortality, infant sepsis, and 5‐minute Apgar score. There are also reports of illness in mother and illness in child without further description. For this review, the two arms that received the nutritional intervention and either probiotic or placebo were included and compared.
Atopy
Kopp 2008 conducted a double‐blind, placebo‐controlled prospective trial at outpatient gynecology offices co‐ordinated with the University of Freiburg in Freiburg, Germany (July 2002 to June 2004), to investigate the immunologic proliferative response and cytokine release in cultures of isolated mononuclear cells from pregnant women and their neonates supplemented withLactobacillus GG (LGG) or placebo. Ninety‐four pregnant women with at least one first‐degree relative or a partner with an atopic disease were randomly assigned to receive either the probiotic LGG (ATCC 53103; 5 x 10^9 CFUs LGG twice daily) or placebo four to six weeks before expected delivery. This was followed by a post‐natal period of six months in which breastfeeding mothers took the randomized interventions for three months, but formula‐fed infants (n = 2 in the LGG group, n = 3 in the placebo group) were given the agents directly. After three months, the capsules were given only directly to infants until six months. Primary outcomes were proliferative response of cord blood mononuclear cells and peripheral blood mononuclear cells from the corresponding mother. Secondary outcomes included gestational age at birth. Only outcomes pertaining to the prenatal period of probiotic administration were included since formula‐fed infants were directly given the probiotic or placebo.
Ou 2012 conducted a prospective, double‐blind, placebo‐controlled clinical trial at Kaohsiung Chang Gung Memorial Hospital in Taiwan (August 2002 and January 2006), to evaluate the effectiveness of prenatal and postnatal probiotics in the prevention of early childhood and maternal allergic diseases. One‐hundred ninety‐one pregnant women with atopic diseases were randomly assigned to receive either probiotics (Lactobacillus GG; 1x10^ 10 CFUs daily) (n = 95) or placebo (n= 96) from 24 weeks of pregnancy until delivery. After delivery, the interventions were given exclusively to breastfeeding mothers or to non‐breastfeeding neonates until six months of age. Outcomes assessed were the point and cumulative prevalence of sensitization and development of allergic diseases, and improvement of maternal allergic symptom score and plasma immune parameters before and after intervention. Mother‐infant pairs were followed until 36 months. Secondary outcomes include gestational age at birth. Only outcomes pertaining to the prenatal period of probiotic administration were included in this review since formula‐fed infants were directly given probiotics or placebo.
Rautava 2012 conducted a parallel, double‐blind placebo‐controlled trial at a single tertiary center in Turku, Finland (August 2005 and April 2009), to investigate whether maternal probiotic supplementation during pregnancy and breast‐feeding reduces the risk of developing eczema in high‐risk infants. Two‐hundred forty‐one mothers with allergic disease and atopic sensitization were randomized to receive (1) L. rhamnosus 1 x 10^9 CFIU and B. longum 1 x 10^9 CFUs (n = 81), (2) L paracasei 1 x1 0^9 CFUs and B longum 1 x1 0^9 CFUs (n = 82), or (3) placebo (n = 78), beginning two months before delivery and during the first two months of breast‐feeding. The primary outcome was cumulative incidence of eczema in the infant up to two years old. Secondary outcomes were atopic sensitization, gestational age at birth, birth weight, and duration of exclusive breast‐feeding.
Nutrition
Mantaring 2016 conducted a double‐blind, randomized controlled study at the Community Hospital of Muntinlupa City, Philippines to assess the effects of maternal nutritional supplement beverages formulated both with and without a probiotic mixture on maternal health, fetal/infant growth and health when given to pregnant women in the third trimester and during lactation. Two‐hundred thirty‐three healthy women in the beginning of the third trimester of pregnancy who were willing to exclusively breast feed for at least two months were assigned to receive a daily nutritional supplement (S, n = 78); the same supplement with probiotics L. rhamnosus (CGMCC 1.3724) 7 x 10^8 CFU and B. lactis (CNCC I‐3446) 7 x 10^8 CFU per serving (S pro, n = 78), or no supplement (no‐S, n = 77) for the duration of their pregnancy and eight weeks' postpartum. Outcomes included maternal health outcomes at gestational months six, seven, eight, and delivery, as well as neonatal health outcomes, including fetal ultrasound at 24 to 28 weeks, birth weight, Apgar scores, and infant growth over the first year of life. No outcomes of interest for this systematic review were reported.
Comparison 2: probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation versus maternal placebo or no intervention
Summary: there was one study that administered probiotics to postpartum mothers who have given birth to a preterm infant born < 37 weeks' gestation identified (Benor 2014).
Participants: mothers of infants born with birth weight of 1500 g or less who intended to breast feed and were enrolled within 48 hours of birth, and their infants were included in Benor 2014.
Interventions:Benor 2014 administered L. acidophilus and B. lactis. Placebo capsules were used in the control group.
Outcomes of interest:Benor 2014 reported on the following outcomes of interest: any necrotizing enterocolitis (NEC), death prior to hospital discharge, death or NEC, surgery for NEC, bacterial sepsis, culture‐proven sepsis with probiotic organism, bronchopulmonary dysplasia (BPD), patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), days to feeding with more than 50% breast milk.
Benor 2014 conducted a prospective, randomized, double‐blind, placebo‐controlled trial at the Tel Aviv Medical Center in Israel (June 2007 to November 2009) which examined the effects of maternal oral probiotic supplementation on the incidence of NEC, death, and sepsis in very low birth weight (VLBW) infants fed with maternal breast milk. Mothers were assigned to supplementation with L. acidophilus and B. lactis 2 × 10(E) CFU/day or to placebo starting from one to three days postpartum. The primary outcome measures were NEC, sepsis, and death. A total 49 mothers of 58 VLBW infants were recruited (25 infants were in the probiotic group and 33 in the placebo group).
Comparison 3: probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation versus neonatal probiotic administration
Postpartum mothers who have given birth to a preterm infant born < 37 weeks' gestation; and
Newborn infants born < 37 weeks' gestation.
Summary: no studies identified.
Excluded studies
We excluded 17 studies from this review. Four studies were excluded due to timing of administration of probiotics that did not align with the criteria that the intervention be given in the trimester of birth, or intention to administer within one week of birth if stopped in the second trimester. Three studies were excluded due to probiotics administered only until the second trimester (Jain 2017; Jamilian 2016; Krauss‐Silva 2011), and one was excluded due to post‐natal administration of probiotics to mothers of term infants (Ortiz‐Andrellucchi 2008). Two studies administered the intervention vaginally, not orally, and were excluded on the grounds of incorrect route of administration (Daskalakis 2014; Karampelas 2013). As a placebo or no intervention was a requirement for the control group, four studies were excluded due to inappropriate comparator, with three studies using either yogurt or fermented product which presumably contain probiotics in both arms (Asemi 2011; Asemi 2012; Nishijima 2005), and one study was excluded due to a comparison of probiotic treatment versus antibiotic treatment (Hantoushzadeh 2012). Bisanz 2014 was excluded due to a co‐intervention only given to the probiotic group and not the control group. Gonai 2014 was excluded due to unclear gestational age at study entry, and Gronlund 2011; Grzeskowiak 2012; Hanson 2014; and Vitali 2012 were excluded due to issues with study design, including non‐random assignment and non‐random assessment of a larger randomized trial. Wickens 2008 was excluded due to multiple factors including recruitment at 35 weeks, mothers who gave birth prior to 37 weeks or had infants with a NICU admission were explicitly excluded, and probiotics were administered directly to the infant after birth starting on day two. See the table of Characteristics of excluded studies for more details about individual studies.
Post hoc the decision was made to exclude studies that administered probiotics starting at 36 weeks' gestation to pregnant women, as the majority of infants of these mothers would be born at term, especially with the inclusion criteria that the probiotics were intended to be administered for at least one week. The population of interest in this review is preterm infants, and the outcomes of interest relate to prematurity. Including these studies would not fit with the overall goal of this review. There is a large body of literature that fits this criteria and listing those studies is beyond the scope of this review.
Risk of bias in included studies
Allocation
Sequence generation: four studies did not describe how participants were randomized into groups (Dolatkhah 2015; Jacobsson 2016; Mantaring 2016; Ou 2012) and therefore have an unclear risk of selection bias in this domain. Eight studies used computer‐generated random numbers (Badehnoosh 2018; Benor 2014; Fernández 2016; Kopp 2008; Laitinen 2009; Lindsay 2014; Lindsay 2015; Rautava 2012,) with three of these studies having this sequence generation done by independent researchers or statisticians removed from the rest of the study (Laitinen 2009; Lindsay 2014; Lindsay 2015). Overall, there is a low risk of allocation bias due to issues with sequence generation, as those studies with unclear risk of bias did not contribute data to the outcomes included.
Allocation concealment: five studies did not describe their allocation concealment and were deemed to have an unclear risk (Dolatkhah 2015; Jacobsson 2016; Mantaring 2016; Ou 2012; Rautava 2012), while two studies did not specifically describe their allocation concealment but were deemed to be low risk due to the use of placebo capsules that resembled the probiotic capsules (Fernández 2016; Kopp 2008). In one study, allocation was said to be concealed from the researcher and participants until final analyses but not fully described (Badehnoosh 2018), and in another study sealed envelopes were opened in the room with the patient (Laitinen 2009). Sequentially‐numbered, sealed, opaque envelopes were used in two studies (Lindsay 2014; Lindsay 2015), and opaque medication boxes with identical capsules was used in one study (Benor 2014) as methods for allocation concealment. Overall, the risk of bias due to allocation concealment is low.
Blinding
Blinding of participants and personnel: nine studies were deemed to be low risk for performance bias with eight studies that were double blinded (Benor 2014 ;Fernández 2016; Kopp 2008; Laitinen 2009; Lindsay 2014; Lindsay 2015; Ou 2012; Rautava 2012), and one study (Badehnoosh 2018) in which it was unclear if the research nurse who randomized participants was blinded, but other personnel and participants were blinded. Three studies did not describe blinding of participants or personnel and are of unclear risk of performance bias (Dolatkhah 2015; Jacobsson 2016; Mantaring 2016).
Blinding of outcome assessors: four studies described that groups were concealed from the researchers at least until after outcomes were assessed (Badehnoosh 2018; Benor 2014; Ou 2012; Rautava 2012), three studies had no clear description of blinding but the outcomes assessed were thought to be straightforward enough to have low risk of detection bias (Fernández 2016; Kopp 2008; Laitinen 2009), while two studies had adequate blinding of assessors for most outcomes (Lindsay 2014; Lindsay 2015), totaling nine studies that are deemed to be low risk for detection bias. Three studies had no description of blinding of outcome assessors and are thus of unclear risk (Dolatkhah 2015; Jacobsson 2016; Mantaring 2016).
Incomplete outcome data
Six studies were deemed to have low attrition bias due to < 10% of participants lost to follow up in five studies (Badehnoosh 2018; Fernández 2016; Kopp 2008; Laitinen 2009; Lindsay 2015), and in one study that had 89% full outcome data, with the other 11% only being excluded due to a lack of ability to provide > 50% breast milk by one week postpartum (Benor 2014). Four studies were at high risk for attrition bias due to > 10% participants lost to follow‐up (Lindsay 2014; Mantaring 2016; Ou 2012; Rautava 2012), while two studies had unclear risk due to no description of their completeness of data (Dolatkhah 2015; Jacobsson 2016). Of note, the number of infants was lower than the number of mothers due to miscarriage and dropouts from the studies.
Selective reporting
Nine studies were deemed to be low risk, five since outcomes that were described in the study protocols were reported (Badehnoosh 2018; Benor 2014; Laitinen 2009; Lindsay 2014; Lindsay 2015), and four that did not have published study protocols but reported data for outcomes that were stated (Fernández 2016; Kopp 2008; Ou 2012; Rautava 2012). One study was deemed to have unclear risk (Jacobsson 2016) due to lack of information in the abstract, and two studies that did not report data for all planned outcomes were considered high risk of reporting bias (Dolatkhah 2015; Mantaring 2016).
Other potential sources of bias
Ten of the studies have low risk of other sources of bias (Badehnoosh 2018; Benor 2014; Dolatkhah 2015; Fernández 2016; Kopp 2008; Laitinen 2009; Lindsay 2014; Lindsay 2015; Ou 2012; Rautava 2012), while two have an unclear risk due to their lack of description in their abstracts (Jacobsson 2016; Mantaring 2016).
Effects of interventions
Summary of findings for the main comparison. Probiotics administered to pregnant women at risk of preterm birth compared to placebo or no intervention for prevention of morbidity and mortality in preterm infants.
| Probiotics administered to pregnant women compared to placebo or no intervention for prevention of morbidity and mortality in preterm infants | ||||||
| Patient or population: prevention of morbidity and mortality in preterm infants Setting: Intervention: probiotics administered to pregnant women < 36 weeks' gestation Comparison: placebo or no intervention. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no intervention. | Risk with Probiotics administered to pregnant women < 37 weeks' gestation | |||||
| Preterm birth < 37 weeks' gestation | Study population | Typical RR 0.92 (0.32 to 2.67) | 518 (4 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | ||
| 26 per 1,000 | 24 per 1,000 (8 to 70) | |||||
| Preterm birth < 34 weeks' gestation | Study population | ‐ | 287 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 3 | No preterm birth < 34 weeks' gestation in either group. | |
| see comment | see comment | |||||
| Gestational age at birth (weeks) | The mean gestational age at birth (weeks) was 0 | MD 0.15 higher (0.33 lower to 0.63 higher) | ‐ | 207 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 | Outcome added post hoc |
| Death | Study population | ‐ | 298 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 | No death reported in either group. | |
| see comment | see comment | |||||
| Miscarriage/stillbirth | Study population | Typical RR 0.79 (0.20 to 3.14) | 320 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | Outcome added post hoc | |
| 25 per 1,000 | 20 per 1,000 (5 to 78) | |||||
| Neonatal sepsis ≤ 3 days | Study population | not estimable | 162 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 3, 4 | ||
| 0 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Severe necrotizing enterocolitis (NEC) (Bell stage II or more) |
Not reported | |||||
| Death or severe NEC | Not reported | |||||
| Late‐onset sepsis | Not reported | |||||
| Culture‐proven sepsis with supplemented probiotic | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate, The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Indirectness: mothers enrolled not at high risk for preterm birth
2 Imprecision: wide confidence interval
3 Imprecision: below the optimal information size
4 Imprecision: only one study contributes to the evidence
Summary of findings 2. Probiotics administered exclusively after birth in mothers of preterm infants compared to maternal placebo or no intervention for prevention of morbidity and mortality in preterm infants.
| Probiotics administered exclusively after birth in mothers of preterm infants compared to maternal placebo or no intervention for prevention of morbidity and mortality in preterm infants | ||||||
| Patient or population: prevention of morbidity and mortality in preterm infants Setting: Intervention: probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation Comparison: maternal placebo or no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with maternal placebo or no intervention | Risk with Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation | |||||
| Any necrotizing Enterocolitis (NEC) | Study population | RR 0.44 (0.13 to 1.46) | 58 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Outcome added post hoc | |
| 273 per 1,000 | 120 per 1,000 (35 to 398) | |||||
| Death prior to hospital discharge | Study population | RR 0.66 (0.06 to 6.88) | 58 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 61 per 1,000 | 40 per 1,000 (4 to 417) | |||||
| Death or NEC | Study population | RR 0.53 (0.19 to 1.49) | 58 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 303 per 1,000 | 161 per 1,000 (58 to 452) | |||||
| Surgery for NEC | Study population | RR 0.15 (0.01 to 2.58) | 58 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Outcome added post hoc | |
| 121 per 1,000 | 18 per 1,000 (1 to 313) | |||||
| Neonatal sepsis | Study population | RR 1.32 (0.48 to 3.61) | 58 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 182 per 1,000 | 240 per 1,000 (87 to 656) | |||||
| Culture‐proven sepsis with probiotic organism | Study population | not estimable | 58 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 0 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Feeding with more than 50% breast milk (days) | The mean feeding with more than 50% breast milk (days) was 0 | MD 9.6 lower (19.04 lower to 0.16 lower) | ‐ | 58 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Outcome added post hoc Although statistically significant, the result is imprecise and only derived from the one included study |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Impression: wide confidence interval suggesting extreme imprecision. GRADE assessment downgraded two levels
Comparison 1: probiotics administered to pregnant women (< 36 weeks' gestation) versus placebo or no intervention
Preterm birth < 37 weeks' gestation (outcome 1.1 and outcome 1.2)
Four studies reported on preterm birth at < 37 weeks, three of which administered the intervention during pregnancy only (Badehnoosh 2018; Lindsay 2014; Lindsay 2015), and one in which the intervention was given to mothers during pregnancy and after birth (Laitinen 2009). A total of 518 mothers and 505 infants are included in this outcome. Of note, the number of infants was lower than the number of mothers due to miscarriage and dropouts from the study. There were no individual studies that reached statistical significance. The studies are relatively small and give very imprecise estimates of effect (Laitinen 2009: risk ratio (RR) 0.25, 95% confidence interval (CI) 0.03 to 2.22; Badehnoosh 2018: RR 2.00, 95% CI 0.19 to 20.90; Lindsay 2014: RR 1.79, 95% CI 0.31 to 10.35; and Lindsay 2015: RR not estimable, risk difference (RD) 0.0, 95% CI ‐0.03 to 0.03).
Overall, 2.5% (13/518) of mothers enrolled in these four studies delivered preterm. When combined in the meta‐analysis, there is no proven effect on preventing preterm birth at < 37 weeks' gestation and the results remain very imprecise (typical RR 0.92, 95% CI 0.32 to 2.67; 4 studies, 518 mothers). There was no significant heterogeneity for this meta‐analysis ( I2 of 14%).
Further subgroup analysis was undertaken, for preterm birth < 37 weeks as this was the only outcome with greater than two included studies.
Probiotic preparation: two studies (287 mothers) administered Lactobacillus preparations (Lindsay 2014; Lindsay 2015) and two studies (231 mothers) administered mixed probiotic preparations (Badehnoosh 2018; Laitinen 2009). No differences emerge regarding the impact on preterm birth at < 37 weeks' gestation based on probiotic preparation (Lactobacillus preparations: typical RR 1.79, 95% CI 0.31 to 10.35; mixed probiotic preparations: typical RR of 0.60, 95% CI 0.15 to 2.48) (Analysis 1.2).
1.2. Analysis.
Comparison 1 Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention., Outcome 2 Preterm birth < 37 weeks' gestation (probiotic preparation).
Gestational age at which probiotics were initiated: a subgroup analysis based upon the gestational age at which probiotics were initiated < 20 weeks' gestation or > 20 weeks' gestation was undertaken as a post‐hoc analysis.
Two studies administered probiotics to 309 mothers < 20 weeks' gestation (Laitinen 2009; Lindsay 2014) and two studies administered probiotics to 209 mothers > 20 weeks' gestation (Lindsay 2015: Badehnoosh 2018). When only studies that enrolled pregnant women < 20 weeks were included the typical RR was 0.74 (95% CI 0.22 to 2.51), and for those with enrollment > 20 weeks, the typical RR was 2.00 (95% CI 0.19 to 20.90) (Analysis 1.1). These results are non‐significant and highly imprecise.
1.1. Analysis.
Comparison 1 Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention., Outcome 1 Preterm birth < 37 weeks' gestation (age at enrollment).
Economic country setting: the subgroup analysis of economic country setting was also undertaken, with Badehnoosh 2018 taking place in a lower middle‐income country and Laitinen 2009, Lindsay 2014, and Lindsay 2015 taking place in high‐income countries. Excluding the results from Badehnoosh 2018 did not change the significance of the results with a typical RR of 0.74 (95% CI 0.22 to 2.51).
Methodologic quality: a sensitivity analysis was also performed to include only studies with high‐methodological quality. All of the included studies overall had high‐methodological quality, except for Lindsay 2014, where there was a concern for attrition bias. Excluding Lindsay 2014 does not significantly alter the results or significance of results with a typical RR of 0.60 (95% CI 0.5 to 2.48).
Other planned analyses: Planned subgroup analyses of mothers who provided breast milk, and subgroup analyses based upon infant weight and gestational age at birth were unable to be performed as studies performed during pregnancy did not classify infants into these categories. There were no studies of mothers with the risk of preterm birth of preterm premature rupture of the membranes (PPROM), preterm labor, genital infection or history of preterm birth, so no subgroup analysis could be performed based upon risk for preterm labor as planned.
Preterm birth < 34 weeks' gestation (outcome 1.3)
Preterm birth < 34 weeks was reported by two studies that included 287 mothers and infants: Lindsay 2014 and Lindsay 2015 both of which administered probiotics during pregnancy only. No infants were born less than 34 weeks in either of these studies, and thus the risk ratio is not estimable and the typical RD is 0.00 (95% CI ‐0.02 to 0.02; 2 studies, 287 mothers).
Gestational age at birth (weeks) (outcome 1.4)
The gestational age at birth was reported in five studies including 735 mothers and 733 infants (Badehnoosh 2018; Kopp 2008; Lindsay 2015; Ou 2012; Rautava 2012). Two studies with 209 mothers with 207 infants reported data that was in a form suitable for inclusion in the meta‐analysis (Badehnoosh 2018; Lindsay 2015), with both studies administering probiotics only before pregnancy. Neither study showed an individually statistically significant difference in gestational age, and when combined, the MD in gestational age at birth was 0.15, (95% CI ‐0.33 to 0.63; 2 studies, 207 mothers). The three other studies that reported gestational age at birth (Kopp 2008; Ou 2012; Rautava 2012) had similar median values and ranges or similar mean and SD range in the probiotic and control groups. Overall, there was not a significant difference in gestational age seen in these studies.
Death (outcome 1.5)
Neonatal mortality was reported in two studies (Laitinen 2009; Lindsay 2014), reporting on a total of 309 mothers and 298 infants. There was no infant mortality in either study out of 298 infants for a typical RD of 0.00 (95% CI‐0.02 to 0.02; 2 studies, 298 infants) and a non‐estimable relative risk.
Miscarriage/stillbirth (outcome 1.6)
Two studies (Laitinen 2009; Lindsay 2015) reported upon miscarriage or stillbirth reporting on a total of 320 mothers and 297 infants. Neither was statistically significant, and the data combined were not statistically significant with a typical RR of 0.79 (95% CI 0.20 to 3.14; 2 studies, 320 mothers). This yielded no significant heterogeneity in the meta‐analysis with an I2 of 24%.
Maternal sepsis (outcome 1.7)
One study with 171 mothers reported on maternal sepsis as an outcome (Laitinen 2009), with no reports of sepsis in either group. This yielded a non‐estimable RR and a RD of 0.00 (95% CI ‐0.02 to 0.02; 1 study, 171 mothers).
Maternal mastitis (outcome 1.8)
One study reported on maternal mastitis with a total of 108 mothers (Fernández 2016) and showed a statistically significant decrease in maternal mastitis with a RR of 0.45 (95% CI 0.27 to 0.75; 1 study, 108 mothers). Of note, this was in an enriched study population, eligible for study entry due to their previous diagnosis of mastitis during lactation after a previous pregnancy.
Neonatal sepsis ≤ three days (Outcome 1.9)
Neonatal sepsis was reported upon in one study (Laitinen 2009) that included 171 mothers with 159 infants. There were no reported cases of neonatal sepsis so the RR was not estimable, and the RD was 0.00, (95% CI ‐0.02 to 0.02; 1 study, 159 infants).
Comparison 2: probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation versus maternal placebo or no intervention
Postpartum mothers who gave birth to a preterm infant born < 37 weeks' gestation.
There was one study with 58 infants of 49 mothers (Benor 2014), 25 infants in the probiotic group and 33 in the control group, that reported on the following outcomes.
Any necrotizing enterocolitis (NEC) (outcome 2.1)
Benor 2014, in a study including 58 infants, reported on any NEC, and there was no significant difference (RR, 0.44, 95% CI 0.13 to 1.46).
Death prior to hospital discharge (outcome 2.2)
Death prior to hospital discharge was reported in one study with 58 infants (Benor 2014) (RR 0.66, 95% CI 0.06 to 6.88) for the probiotic group. There were three infants who died, one in the probiotic group, and two in the control group.
Death or NEC (outcome 2.3)
Benor 2014 reported upon death or NEC with a 16% rate in the probiotic group and a 30% rate in the control group, yielding a RR of 0.53. (95% CI 0.19 to 1.49).
Surgery for NEC (Bell's Stage 3) (outcome 2.4)
Surgery for NEC was reported in one study with 58 infants (Benor 2014) with a 0% rate in the probiotic group and a 12% rate of NEC in the control group. The probiotic group and had a RR of 0.15, (95% CI 0.01 to 2.58).
Neonatal sepsis (outcome 2.5)
Benor 2014 with 58 infants reported upon culture‐positive sepsis with a RR of 1.32, (95% CI 0.48 to 3.61). The rate in the probiotic group was 24% and in the control group 18% with no significant difference. To be noted, there was no distinction in this study between early onset and late onset sepsis.
Culture‐proven sepsis with probiotic organism (outcome 2.6)
One study with 58 infants (Benor 2014) reported upon culture‐proven sepsis with probiotic agent, with no cases reported for the probiotic or placebo group, yielding a RD of 0.00, (95% CI ‐0.07 to 0.07).
Bronchopulmonary dysplasia (BPD) (outcome 2.7)
Benor 2014 with 58 infants reported upon BPD with a 20% rate in the probiotic group and a 27% rate in the control group for a risk ratio of 0.73 (95% CI 0.28 to 1.92).
Patent ductus arteriosus (PDA) (outcome 2.8)
PDA was reported by Benor 2014 in a study with 58 infants with a rate of 44% in the probiotic group and 36% in the placebo group for a risk ratio of 1.21 (95% CI 0.64 to 2.28).
Intraventricular hemorrhage (IVH) any grade (outcome 2.9)
Benor 2014 with 58 infants reported on the presence of any IVH (RR 1.58, 95% CI 0.54 to 4.60) for the probiotic group.
Periventricular leukomalacia (PVL) (outcome 2.10)
Benor 2014 in a study with 58 infants reported upon the incidence of PVL, but not specifically cystic PVL with no clear definition of PVL reported in the study. The risk ratio for PVL in the probiotic group was 5.28, (95% CI 0.63 to 44.38).
Retinopathy of Prematurity (ROP) (outcome 2.11)
The risk ratio of ROP in the probiotic group was 1.98, (95% CI 0.36 to 10.97) in Benor 2014, which included 58 infants, with three cases in the probiotic group and two in the control group.
Days to achieve 50% enteral feeds (outcome 2.12)
Days to 50% enteral feeds was reported in one study with 58 infants, Benor 2014, (MD ‐9.60 days, 95% CI ‐19.04 to ‐0.16) in the probiotic group versus the control group, meaning the infants in the probiotic group reached 50% enteral feeds on average, sooner than those in the control group.
Comparison 3: Probiotics administered exclusively after birth in mothers of preterm infants (< 37 weeks' gestation) versus neonatal probiotic administration.
No studies identified.
Discussion
Summary of main results
Comparison 1: probiotics administered to pregnant women versus placebo or no intervention
There were data from 11 randomized controlled trials included in this review to determine how prenatal probiotics given to mothers affects neonatal outcome. There is insufficient evidence to conclude whether there is appreciable benefit or harm of maternal probiotics given prior to birth or both prior to and after birth regarding preterm birth < 37 weeks, preterm birth < 34 weeks, gestational age at birth, miscarriage, death, maternal sepsis, or neonatal sepsis. The quality of evidence for these outcomes is very low to low, due mostly to small sample size, clinical heterogeneity of the studies and indirectness. With evidence from one study (Fernández 2016), with an enriched cohort of pregnant women with a history of mastitis, there was a significant reduction in the incidence of maternal mastitis in the probiotic group compared to the placebo group, with a low GRADE of evidence.
Comparison 2: probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation versus maternal placebo or no intervention
There is insufficient evidence to conclude whether there is appreciable benefit or harm of probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation versus maternal placebo or no intervention. One study addressed this question with 58 very low birth weigh (VLBW) infants. Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation was associated with an improvement in time to reach 50% enteral feeds (mean difference (MD) ‐9.60 days, 95% confidence interval (CI) ‐19.04, ‐0.16 days). No other improvements in any neonatal outcomes were reported. The estimates were broad and consistent with meaningful harms or benefits to the infants from probiotic administration to these women. There were no cases of culture‐proven sepsis with the probiotic organism. The GRADE evaluation for all of this evidence is low due to imprecision.
Comparison 3: probiotics administered exclusively after birth in mothers of preterm infants (< 37 weeks' gestation) versus neonatal probiotic administration
No studies identified.
Overall completeness and applicability of evidence
Comparison 1: probiotics administered to pregnant women (< 36 weeks' gestation) versus placebo or no intervention
This review included 1150 pregnant women who were given probiotics for a variety of reasons from trying to treat gestational diabetes to intending to reduce the fetal risk of atopy. In the included studies, there was an overall low rate of preterm birth of 2.5%, with no infants born < 34 weeks' gestation and no prematurity‐related outcomes reported. Infants born in this late preterm period with access to medical care in developed countries are unlikely to die or suffer most major morbidities associated with prematurity. As such, the evidence from these studies can only be generalized to pregnant women at low risk for preterm birth and not broadly to all pregnant women.
These findings should be interpreted very cautiously when applied to pregnant women with increased risk for preterm birth. None of the studies included in this review focused on mothers at high risk of preterm birth, who are the mothers of the greatest interest for this review. Therefore, the question of whether probiotics given to pregnant women at high risk for preterm birth, especially for those at high risk of birth < 34 weeks, would alter the rate of preterm birth, the gestational age at birth, or affect subsequent premature infant morbidity or mortality has not been fully addressed by this review due to a paucity of studies on this topic.
The population that would be the greatest interest to this review are those pregnant women whose obstetrician or care provider deems at risk for delivery before 34 weeks, such as those with preterm premature rupture of the membranes (PPROM), cervical shortening, preterm labor, pre‐eclampsia, a history of preterm birth, and mothers whom their obstetrician or care provider deems to have a high likelihood to likely to deliver before 34 weeks. Currently, there are no studies that specifically examine the use of probiotics in this population with the intention of determining if probiotics change the mortality and morbidity of preterm infants, or affect the gestational age at which they are born.
Comparison 2: probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation versus maternal placebo or no intervention
We identified one small study of maternal probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation. With the small size of the study, this study is likely under‐powered to show any significant differences with precision and accuracy. None of the outcomes reached statistical significance for effect, except for a improvement in time to reach 50% enteral feeds with probiotics versus placebo. The evidence is incomplete and definite conclusions cannot be made without further study including more infants.
Comparison 3: probiotics administered exclusively after birth in mothers of preterm infants (< 37 weeks' gestation) versus neonatal probiotic administration
Although a Cochrane Review (AlFaleh 2014) reported on over 24 studies of neonatal administration of probiotics involving over 5500 infants, there are no studies that directly compare neonatal probiotic administration to postnatal maternal probiotic administration.
Quality of the evidence
Comparison 1: probiotics administered to pregnant women (< 36 weeks' gestation) versus placebo or no intervention
Overall, there is a low to very low quality of evidence in this review, owing mostly to small numbers of infants and mothers included in the outcomes, as well as the indirectness of the evidence, since the majority of mothers in this review were not at particularly high risk for preterm birth. The outcomes of very low GRADE were preterm birth < 34 weeks, and neonatal sepsis, while those of low GRADE were preterm birth < 37 weeks, gestational age at birth, death, and miscarriage/stillbirth. Maternal mastitis had low quality evidence.
Comparison 2: probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation versus maternal placebo or no intervention
Overall, there is low quality of evidence in this review for all outcomes due to only one study available that addresses this question with an overall small sample size of 58 infants leading to wide confidence intervals and significant imprecision.
Potential biases in the review process
It is possible that there was publication bias that we were not able to detect. There were insufficient trials in the meta‐analysis to effectively use funnel plots to evaluate for reporting bias.
Additionally, the actual inclusion criteria for this review was subject to bias in the broad definition of "at risk for preterm birth." The applicability of many of these studies that focused on women at low risk for preterm birth could bias the results, which is why subgroup analyses are important. However, since there were no studies identified that gave pregnant mothers probiotics during pregnancy that fit our inclusion criteria and were at especially high risk of preterm birth, we were unable to perform a subgroup analysis to counteract this possible bias.
Our post hoc addition of outcomes are possibly influenced by reporting bias (see Differences between protocol and review).
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review to investigate the effects of maternal probiotics on the morbidity or mortality of preterm infants.
Authors' conclusions
Implications for practice.
This review provides overall very low‐ to low‐quality evidence regarding the use of oral probiotics in mothers with preterm infants or in a broad group of mothers during pregnancy to prevent morbidity or mortality in preterm infants.
There is insufficient evidence to conclude whether there is appreciable benefit or harm to neonates of either oral supplementation of probiotics administered to pregnant women at low risk for preterm birth or oral supplementation of probiotics to mothers of preterm infants after birth.
Oral supplementation of probiotics administered to pregnant women at low risk for preterm birth has not been proven to decrease the risk of morbidity or mortality in preterm infants, nor has it been proven to decrease the incidence of preterm birth. Oral supplementation of probiotics to mothers of preterm infants after birth has not been proven to significantly decrease necrotizing enterocolitis (NEC), surgical NEC, death, or death and NEC, but may decrease time to 50% enteral feeds, based upon one small study.
This review does not provide any evidence regarding the use of probiotics in pregnant mothers at high risk of preterm birth as this cohort does not exist in the current literature. The small number of preterm infants born to mothers who received probiotics after birth yields imprecise estimates of effect on important outcomes including outcomes related to safety. There is currently no evidence to support or refute the use of probiotics in this population.
There are no studies that directly compare neonatal probiotic administration with postnatal maternal probiotic administration.
Implications for research.
Further high‐quality randomized controlled trials are needed in two areas to assess the role of maternal probiotics in preventing preterm birth and morbidity and mortality in infants born preterm.
First, high‐quality studies would need to be undertaken in pregnant women at high risk for preterm birth, and would need to administer the probiotics ideally from the time of recognition of risk for preterm birth at least until the time of birth. Some of the maternal risks for preterm birth that would be of interest would include preterm premature rupture of membranes (PPROM), cervical shortening, preterm labor, pre‐eclampsia, and a history of preterm birth. These studies would ideally be large enough to be powered to have an effect on an important outcome like preterm birth < 34 weeks.
Currently, there are three ongoing studies of maternal probiotics administered to pregnant women at high risk for preterm birth proposing to enroll 854 infants.
Second, high‐quality randomized studies are needed to address the effect of probiotic administration postnatally to breastfeeding mothers of preterm infants. These studies could include treatment or non treatment of the preterm infant. Safety concerns that exist due to quality control measures of probiotics in the United States regarding direct probiotic administration to preterm infants could be mitigated by administering these probiotics to the mother, whose own immune system might be able to neutralize potential harmful contaminants and deliver the probiotic to the infants via breast milk and skin flora. Studies designed to answer this question should aim to enroll infants at the highest risk for mortality and morbidities such as NEC, especially very low birth weight (VLBW) infants.
Currently, there is one proposed study of postnatal administration of probiotics to either mothers of infants and/or infants (NCT01454661). This study is of relatively small sample size (anticipated enrollment of 100 participants) and will primarily be evaluating impact on the microbiome.
In addition, these studies should also address the specific type of probiotic agent, dose and duration of therapy.
History
Protocol first published: Issue 1, 2017 Review first published: Issue 12, 2018
| Date | Event | Description |
|---|---|---|
| 25 January 2018 | New search has been performed | Performed the search, included results, discussion and conclusion. |
| 6 February 2017 | Amended | Added external source of support |
Appendices
Appendix 1. Search Strategies
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Pregnancy and Childbirth Group's Trials Register
The Register is a database containing over 20,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the PCG Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link for information about the ‘Specialized Register' of the Cochrane Pregnancy and Childbirth Group.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Information Specialist and contains trials identified from: 1. monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL); 2. weekly searches of MEDLINE (Ovid); 3. weekly searches of Embase (Ovid); 4. monthly searches of CINAHL (EBSCO); 5. handsearches of 30 journals and the proceedings of major conferences; 6. weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts. Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that will be fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
Appendix 3. 'Risk of bias' tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomization, and the blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as low, high, or unclear risk. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the table of Characteristics of included studies. We evaluated the following issues and entered the findings into the 'Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth < 37 weeks' gestation (age at enrollment) | 4 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.32, 2.67] |
| 1.1 Enrollment at < 20 weeks | 2 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.22, 2.51] |
| 1.2 Enrollment at > 20 weeks | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| 2 Preterm birth < 37 weeks' gestation (probiotic preparation) | 4 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.32, 2.67] |
| 2.1 Lactobaccillus preparation | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.31, 10.35] |
| 2.2 Mixed probiotic preparation | 2 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.15, 2.48] |
| 3 Preterm birth < 34 weeks' gestation | 2 | 287 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 4 Gestational age at birth (weeks) | 2 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.33, 0.63] |
| 5 Death prior to hospital discharge | 2 | 298 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 6 Miscarriage/stillbirth | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.20, 3.14] |
| 7 Maternal sepsis | 1 | 171 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 8 Maternal mastitis | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.75] |
| 9 Neonatal sepsis ≤ 3 days | 1 | 159 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
1.3. Analysis.
Comparison 1 Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention., Outcome 3 Preterm birth < 34 weeks' gestation.
1.4. Analysis.
Comparison 1 Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention., Outcome 4 Gestational age at birth (weeks).
1.5. Analysis.
Comparison 1 Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention., Outcome 5 Death prior to hospital discharge.
1.6. Analysis.
Comparison 1 Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention., Outcome 6 Miscarriage/stillbirth.
1.7. Analysis.
Comparison 1 Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention., Outcome 7 Maternal sepsis.
1.8. Analysis.
Comparison 1 Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention., Outcome 8 Maternal mastitis.
1.9. Analysis.
Comparison 1 Probiotics administered to pregnant women at risk of preterm birth (< 37 weeks' gestation) vs. placebo or no intervention., Outcome 9 Neonatal sepsis ≤ 3 days.
Comparison 2. Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any necrotizing enterocolitis (NEC) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.13, 1.46] |
| 2 Death prior to hospital discharge | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.06, 6.88] |
| 3 Death or NEC | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.19, 1.49] |
| 4 Surgery for NEC | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.58] |
| 5 Neonatal sepsis | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.48, 3.61] |
| 6 Culture‐proven sepsis with probiotic organism | 1 | 58 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 7 Bronchopulmonary dysplasia (BPD) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.28, 1.92] |
| 8 Patent ductus arteriosus | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.64, 2.28] |
| 9 Intraventricular hemorrhage (any grade) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.54, 4.60] |
| 10 Periventricular leukomalacia (PVL) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.63, 44.38] |
| 11 Retinopathy of prematurity | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.36, 10.97] |
| 12 Days to achieve 50% enteral feeds (days) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐9.60 [‐19.04, ‐0.16] |
2.1. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 1 Any necrotizing enterocolitis (NEC).
2.2. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 2 Death prior to hospital discharge.
2.3. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 3 Death or NEC.
2.4. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 4 Surgery for NEC.
2.5. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 5 Neonatal sepsis.
2.6. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 6 Culture‐proven sepsis with probiotic organism.
2.7. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 7 Bronchopulmonary dysplasia (BPD).
2.8. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 8 Patent ductus arteriosus.
2.9. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 9 Intraventricular hemorrhage (any grade).
2.10. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 10 Periventricular leukomalacia (PVL).
2.11. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 11 Retinopathy of prematurity.
2.12. Analysis.
Comparison 2 Probiotics administered exclusively after birth in mothers of preterm infants < 37 weeks' gestation vs. maternal placebo or no intervention, Outcome 12 Days to achieve 50% enteral feeds (days).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Badehnoosh 2018.
| Methods | The aim of the study was to evaluate the effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes among participants with GDM. Randomized, double‐blind, placebo‐controlled, parallel clinical trial Method of generating randomization sequence: computer‐generated random numbers Allocation concealment: allocation was concealed from the researchers and participants until the final analyses were completed Blinding of intervention: patients and researchers were blinded to the intervention. It is unclear if the trained midwife at the Gynecology Clinic who created the randomized allocation sequence by computer, enrolled patients and allocated patients was blinded. Blinding of outcome measurement: patients and researchers were blinded to the intervention until final analyses were completed Complete follow‐up: yes, 100% |
|
| Participants | 60 pregnant women diagnosed between 24 to 28 weeks with GDM who were not on oral hypoglycemic agents, and their 60 infants Exclusion criteria: placental abruption, pre‐eclampsia, eclampsia, hypo‐ and hyperthyroidism, urinary tract infection, smokers, those with kidney or liver diseases and required commencing insulin therapy during intervention, taking any probiotic products including probiotic yogurt and kefir during the trial. Demographic data (maternal) ‐ Maternal age: probiotics (N = 30): 28.8 ± 5.4 years; placebo (N = 30): 27.8 ± 3.7 years ‐ Gestational age: probiotics (N = 30): gestational age (prior to intervention): 25.7 ± 1.0 weeks; placebo (N = 30): gestational age (prior to intervention): 25.6 ± 1.2 weeks |
|
| Interventions | Probiotics group (N = 30) received a daily probiotic capsule with Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum ( 2 x 10^9 CFU/g each) Placebo group (N = 30) received a daily placebo capsule The intervention or placebo were administered for 6 weeks during the second and/or third trimester, starting at 24 to 28 weeks' gestation. |
|
| Outcomes | Primary outcomes were inflammatory markers, while secondary outcomes were biomarkers of oxidative stress and pregnancy outcomes, including preterm birth, newborn weight, length, and head circumference, Apgar scores, hyperbilirubinemia, newborn hospitalization, newborn hypoglycemia, macrosomia, cesarean delivery rates, maternal pre‐eclampsia, polyhydramnios, maternal hospitalization, and need for maternal insulin therapy. Outcomes of interest were preterm birth < 37 weeks and gestational age at birth. |
|
| Notes | Period of study: April 2016 to September 2016 Published: 2017 Source of funding: grant from the Vice chancellor for Research, IUMS, Tehran, Iran Sponsorship Source: Iran University of Medical Sciences (IUMS) Country: Iran Setting: single‐center, Akbarabadi Clinic in Tehran, Iran Author's contact details: Zatollah Asemi asemi_r@yahoo.com, jamilian.mehri@gmail.com TRIAL REGISTRATION: IRCT201611115623N91 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from the researchers and subjects until the final analyses were completed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Group was concealed from the researchers and participants until the final analyses were completed. It is unclear if the trained midwife at the gynecology clinic who created the randomized allocation sequence by computer, enrolled patients and allocated patients was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Group was concealed from the researchers and participants until the final analyses were completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the study protocol were reported in the study. |
| Other bias | Low risk | |
Benor 2014.
| Methods | The aim of the study was to determine the effects of maternal oral probiotic supplementation on the incidence of NEC, death, and sepsis in very low birth weight (VLBW) infants fed with maternal breast milk. Prospective, randomized, double‐blind, placebo‐controlled trial at the Tel Aviv Medical Center (June 2007‐November 2009). Method of generating randomization sequence: computer‐generated random numbers Allocation concealment: opaque medication boxes Blinding of intervention: nursing staff, physicians, researchers and participants were masked to group assignment. Blinding of outcome measurement: NICU physicians who determined clinical outcomes were masked to study assignment. Complete follow‐up: yes |
|
| Participants | Infants with a birth weight of 1500 g or less. Entry into the study during the first 48 hours after delivery. Mothers were recruited at 0 to 72 hours after birth. Exclusion criteria for the mothers: Quote: "Chronic disease of the heart, lung, gastrointestinal tract or kidneys, chronic use of antibiotics, chronic use of probiotic products, or inability to breast feed or express breast milk". Exclusion criteria for infants: Quote: "Too ill to receive enteral nutrition, the presence of congenital chromosomal, neurological, gastrointestinal or major cardiac anomalies, and/or hypoxic brain injury". Demographic data (infant) In total, 49 mothers with 58 VLBW infants were recruited. A total of 25 infants were in the probiotic group and 33 in the placebo group. Gestational age of enrolled infants: probiotics (N = 25) 29.49 ± 2.69 weeks; placebo (N = 33) 29.47 ± 2.57 weeks Birth weight of enrolled infants: probiotics (N = 25) 1105 ± 267 g; placebo (N = 33) 1080 ± 237 g Gestational age, birth weight and exposure to antenatal corticosteroids were similar between groups. There were more infants in the control group that were delivered by cesarean section than in the probiotic group (94% versus 72%, P = 0.03). |
|
| Interventions | Intervention was started as soon as the mother was recruited at 0 to 72 hours of life of their infant, and continued until the infant was discharged. Probiotic capsules that contained a mixture of Lactobacillus acidophilus (L. acidophilus NCFM) and Bifidobacteria lactis (Bi‐07) at a concentration of 2 x 10^10 CFU manufactured by Danisco (Copenhagen, Denmark)., Placebo capsule containing cellulose manufactured by Danisco (Copenhagen, Denmark). |
|
| Outcomes | Neonatal outcomes of interest: severe NEC, death, death or NEC, retinopathy of prematurity, Intraventricular hemorrhage, any grade, culture‐proven sepsis with probiotic, mean duration to reach full feeds | |
| Notes | Sponsorship Source: Department of Pediatrics, Dana Children’s Hospital, Tel Aviv Sourasky Medical Center Department of Neonatology, Lis Maternity Hospital, Tel Aviv Sourasky Medical Center Country: Israel Setting: Tel Aviv Medical Center NICU Author's contact details; Shira Benor, MD, Department of Pediatrics, Tel Aviv Sourasky Medical Center Address: 6 Weizman Street, Tel Aviv 64239, Israel Email: shirabenor@yahoo.com TRIAL REGISTRATION: ClinicalTrials.gov NCT00835874. (Dolberg 2007). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participating mothers were randomized by computer‐generated random numbers with opaque medication boxes without any marking on the box. |
| Allocation concealment (selection bias) | Low risk | Opaque medication boxes containing identical capsules (without any marking on the box) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Nursing staff, physicians, researchers and participants were masked to group assignment. Only the study pharmacist was aware of treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | NICU physicians who determined clinical outcomes were masked to study assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 49 of 55 mothers (89%), and 58 of 65 infants (89%) were included in analysis. The exclusion of 6 mothers to 7 infants was based upon the inability to provide at least 50% of infant milk at 1 week postpartum. |
| Selective reporting (reporting bias) | Low risk | Reported on outcomes described in the protocol |
| Other bias | Low risk | |
Dolatkhah 2015.
| Methods | The aim of the study was to assess the effect of a probiotic supplementation on glucose metabolism and weight gain in pregnant women with newly diagnosed GDM. Double‐blind placebo‐controlled randomized clinical trial Method of generating randomization sequence: not described Allocation concealment: not described Blinding of intervention: not described Blinding of outcome measurement: not described Complete follow‐up: 100% |
|
| Participants | 64 nulliparous pregnant women diagnosed between 24 to 28 weeks of gestation with GDM and their infants Exclusion criteria for mothers: age < 18 years or > 45 years, BMI < 18.5, history of type 2 diabetes mellitus, history of chronic diseases, smoking or alcohol consumption, probiotic food consumption 2 weeks before the intervention, antibiotics 1 month before intervention, acute gastrointestinal issues one month before the trial, use of glucocorticoids or immunosuppressive drugs, need for insulin or other diabetic drugs during study period Demographic data (maternal) Maternal age: probiotics (N = 29) 28.14 ± 6.24 years, placebo (N = 27) 26.48 ± 5.23 years; originally 32 mothers enrolled in each group. |
|
| Interventions | Probiotics group (N = 32) received a daily probiotic capsule containing Lactobacillus acidophilus LA‐5, Bifidobacterium BB‐12, Streptococcus thermophilus STY‐31 and Lactobacillus delbrueckii bulgaricus LBY‐27 (4 biocap > 4 × 109 CFU) Placebo group (N = 32) received a daily placebo capsule Participants were allocated to receive either probiotic supplement or placebo capsules once daily for 8 weeks at or after 24 to 28 weeks' gestation. |
|
| Outcomes | Primary outcomes assessed were trend in weight gain and glucose metabolism indices in the mother. No infant outcomes of interest were reported. |
|
| Notes | Period of study: spring and summer 2014 Published: 2015 Source of funding: Tehran Darou Pharmaceuticals provided the probiotic supplement Sponsorship Source: Department of Nutrition Sciences, Shahid Beheshti University of Medical Sciences (SBUMS), International Branch, Tehran, Iran Country: Iran Setting: Alzahra University Hospital in Tabriz, Northwest of Iran Author's contact details: Majid Hajifaraji; m.hajifaraji@nnftri.ac.ir TRIAL REGISTRATION: IRCT201405181597N3. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | Did not report the following planned laboratory outcomes from the study protocol: hs‐CRP, TNF‐α, Interleukine‐6, total antioxidant capacity/TAC, malondialdehyde/MDA, superoxide dismutase/SOD, Glutathione Reductase/GSH‐Red, Glutathione Peroxidase/GSH‐Px, Uric Acid |
| Other bias | Low risk | |
Fernández 2016.
| Methods | The aim of the trial was to evaluate the potential of probiotics to prevent mastitis when orally administered during late pregnancy to women who had experienced infectious mastitis after previous pregnancies. Double‐blind randomized controlled trial Method of generating randomization sequence: computer‐generated allocation sequence Allocation concealment: yes Blinding of intervention: control group received capsules with powdered milk Blinding of outcome measurement: yes Complete follow‐up: two women dropped out of study for Quote: "reasons not related to the study" |
|
| Participants | A total of 108 pregnant women, chronological age 24 to 35 and gestational age 27 to 32 weeks participated in this study. All met the following criteria: normal pregnancy, healthy status, and a history of lactational mastitis after at least one previous pregnancy. Demographic data Maternal age: Probiotics group N = 55: 31 years (95% CI 31 to 32) Placebo group N = 53: 31 years (95% CI 30 to 31) Gestational age at study enrollment (mean weeks (95% CI)) Probiotics group N = 55: 29.82 weeks (29 to 30) Placebo group N = 53: 30.06 weeks (30 to 30) |
|
| Interventions | Probiotic group (n = 55) ingested daily 9 log10 CFU of L. salivarius PS2, whereas those in the placebo group (n = 53) received a placebo. The intervention was given from approximately week 30 of pregnancy until delivery. |
|
| Outcomes | Mastitis, breast pain score, bacterial counts in milk sample, probiotic in milk sample. Outcome of interest reported: maternal mastitis. |
|
| Notes | Period of study: unknown Published: 2016 Source of funding: supported by AGL2013‐41980‐P project from the Ministerio de Economía y Competitividad (Spain). Sponsorship Source: approved by the Ethical Committee of Clinical Research of Hospital Clínico San Carlos Madrid. Country: Spain Setting: Hospital Clínico San Carlos Madrid, Spain Author's contact details: Department of Nutrition, Food Science, andFood Technology, Universidad Complutense de Madrid, Spain TRIAL REGISTRATION: Clinical Trials Registration. NCT01505361. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Control group received placebo capsules with powdered milk |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two women (out of 108 enrolled) dropped out of study for Quote: "reasons not related to the study" |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Jacobsson 2016.
| Methods |
Jacobsson 2016 studied the immunological and inflammatory response after antenatal supplementation with Lactobacillus rhamnosus in low‐risk pregnant women. The objective was to evaluate whether supplementation with Lactobacillus rhamnosus (LGG) in low‐risk pregnant women interfere on maternal immunological or inflammatory response. Randomized controlled trial Method of generating randomization sequence: unknown Allocation concealment: unknown Blinding of intervention: unknown Blinding of outcome measurement: unknown Complete follow‐up: unknown |
|
| Participants | Low‐risk pregnant women at 8 to 10 weeks' gestation (risk factors undefined) Exclusion criteria: unknown Demographic data Probiotics group N = 20 Placebo group N = 20 |
|
| Interventions | Low‐risk women randomized at week 8 to 10 weeks' gestation to supplementation with LGG (108 CFU) or placebo until delivery. | |
| Outcomes | Maternal blood was sampled at recruitment, at weeks 25 and 35. Fluorescence activated cell‐scanning analysis; FOXP3 coloring and Toll‐like receptor stimulation with Lactobacillus plantarum, Pseudomonas aeruginosa and lipopolysaccharides (LPS) was used to evaluate the response of 26 parameters. Variables were log transformed and data presented with geometric mean and geometric standard deviation. Baseline values and changes from baseline were compared with two sample t‐tests. No outcomes of interest reported. |
|
| Notes | Period of study: unknown Published: 2016 (abstract) Source of funding: unknown Sponsorship Source: unknown Country: Sweden and Norway Setting: conducted by investigators at Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Chalmers University, Gothenburg, Sweden; Institute of Public Health, Oslo, Norway. Author's contact details: not stated TRIAL REGISTRATION: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Published as abstract only. Little detail available on which to judge risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Published as abstract only. Little detail available on which to judge risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Published as abstract only. Little detail available on which to judge risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Published as abstract only. Little detail available on which to judge risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Published as abstract only. Little detail available on which to judge risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Published as abstract only. Little detail available on which to judge risk of bias. |
| Other bias | Unclear risk | Published as abstract only. Little detail available on which to judge risk of bias. |
Kopp 2008.
| Methods | The aim of the study was to investigate the immunologic proliferative response and cytokine release in cultures of isolated mononuclear cells in pregnant women with a first degree relative or partner with atopic disease and their neonates supplemented with probiotics during pregnancy. Double‐blind, randomized placebo‐controlled trial Method of generating randomization sequence: random computer generation in blocks of 4 Allocation concealment: placebo controlled Blinding of intervention: identical appearance, taste, smell and packaging of capsules Blinding of outcome measurement: yes Complete follow‐up: total enrolled 94 (cord blood obtained on 68 infants) |
|
| Participants | 94 pregnant women who had uneventful pregnancies and a family history of atopic disease (eczema, allergic rhinitis or asthma) and their 94 Infants probiotics N = 50, Placebo N = 44). Exclusion criteria: underlying chronic disease (i.e. diabetes mellitus, rheumatoid arthritis, chronic infectious disease) Maternal Demographics Maternal age at study enrollment: unknown |
|
| Interventions | Two capsules of placebo (microcrystalline cellulose) or L. rhamnosus GG (ATCC 53103) containing 5x10^9 CFU of LGG (Infectopharm, Heppenheim, Germany) daily for 4 to 6 weeks before expected delivery. After birth, breastfeeding mothers took the randomized interventions for 3 months, but formula‐fed infants (n = 2 in the LGG group, n = 3 in the placebo group) were given the agents directly. After 3 months, the capsules were given only directly to infants until 6 months . |
|
| Outcomes | Primary outcomes were proliferative response of cord blood mononuclear cells and peripheral blood mononuclear cells from the corresponding mother. Secondary outcomes related to the neonate were gestational age and birth weight. Outcome of interest: gestational age at birth. |
|
| Notes | Five formula‐fed infants were given the interventions directly after birth. Thus, only data pertaining to the time period of prenatal supplementation was included in this review. Period of study: July 2002 to June 2004 Published: 2007 Source of funding: University of Freiburg and by Infectopharm, Heppenheim, Germany Sponsorship Source: University of Freiburg Country: Germany Setting: outpatient Gynecology offices coordinated with the University of Freiburg Author's contact details: Dr Matthias Kopp, University Children’s Hospital, Mathildenstraße 1, D‐79106 Freiburg, Germany. E‐mail: Matthias.kopp@ uniklinik‐freiburg.de TRIAL REGISTRATION: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number assignment |
| Allocation concealment (selection bias) | Low risk | No description of how investigators received and distributed the sequence. Placebo‐controlled (capsules of placebo (microcrystalline cellulose) or L. rhamnosus GG (ATCC 53103) containing 5 x 10^9 CFU of LGG daily for 4 to 6 weeks before expected delivery). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No clear description of blinding, but numbered identical appearing interventions were given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No specific description of blinding of assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants are accounted for in an ITT analysis. Cord blood values only reported on 68/94 enrolled infants. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Laitinen 2009.
| Methods |
Laitinen 2009 investigated the impact of dietary counseling and/or probiotics on pregnant women with an aim to optimize maternal dietary intake and metabolism, to advance maternal health,and reduce the risk of disease in the child. Randomized, prospective, parallel‐group, combined dietary counseling and probiotics intervention study at a single center. Mothers were randomized to either 1. probiotics and dietary intervention, 2. placebo and dietary intervention, or 3. placebo and no dietary intervention. The data from the placebo group and no dietary intervention group were not included in this review as there is no appropriate comparator. Multiple reports were generated by this trial and are briefly noted below in the "Notes" section. Method of generating randomization sequence: computer‐generated block randomization of six women done by a statistician not involved in recruitment or study visits. Allocation concealment: sealed envelopes with participant group assignments Blinding of intervention: probiotic versus placebo intervention was double‐blinded and adequate. Nutritional counseling component was unblinded. For the included comparisons both groups received identical nutritional counseling. Blinding of outcome measurement: yes. Complete follow‐up: unclear (depending on outcome assessed). Greater than 10% loss to follow‐up for reasons varying from "moved" to "illness in child". |
|
| Participants | 256 pregnant women who were less than 17 weeks' gestation at enrollment. Exclusion criteria: metabolic or chronic diseases such as diabetes, rheumatoid arthritis, inflammatory bowel disease, thyroid diseases, malignancies, > 17 weeks' gestation pregnant, Demographic data Probiotics and dietary intervention group N = 85 pregnant women, N = 82 infants; maternal age 29·7 ± 4·1 years Placebo and dietary intervention group N = 86, N = 80 infants; maternal age 30·1 ± 5·2 years Placebo and control N = 85 (data were not included in review due to no probiotic and control group for comparison) |
|
| Interventions | Probiotics and dietary intervention group (N = 85) received Lactobacillus rhamnosus GG, and Bifidobacterium lactis 10^10 CFUs/day each. Placebo and dietary intervention group (N = 86) received identical‐looking capsules that contained cellulose and dextrose anhydrate. The intervention was given from the first study visit around 14 weeks until the end of exclusive breastfeeding. Dietary counseling intervention occurred at each study visit and aimed to modify dietary intake to conform to current dietary recommendations with a specific focus on dietary fat intake. |
|
| Outcomes | Primary and secondary outcomes vary between the articles, and include outcomes focused on maternal dietary compliance, adiposity, pregnancy weight gain, anthropometric measurements during and after pregnancy, maternal diagnosis of gestational diabetes, maternal laboratory indices for lipids, leptin, and glucose metabolism, as well as placental fatty acids, breast milk fatty acids and inflammatory markers, and infant sensitization. Outcomes pertinent to the neonate include birth weight, birth length, birth head circumference, gestational age at birth, miscarriage rate, preterm birth < 32 weeks, preterm birth 32 to 36 weeks, cesarean delivery rate, 5‐minute Apgar score. Outcomes of interest include preterm birth < 37 weeks, maternal miscarriage, maternal sepsis, neonatal mortality and neonatal early onset sepsis. |
|
| Notes | Period of study: April 2002 to November 2005 Published: Main study 2009; various publications from 2006 to 2014 Source of funding:grants from the Social Insurance Institution of Finland, the Sigrid Juselius Foundation and the Academy of Finland. Provision of food products was by Raisio plc (Raisio, Finland), B. lactis Bb12 by Chr. Hansen (Hoersholm, Denmark) and L. rhamnosus GG by Valio Ltd (Helsinki). Sponsorship Source: University of Turku Country: Finland Setting: study clinic in Turku University Central Hospital Author's contact details: Dr Kirsi Laitinen, fax þ358 2 333 6862, email kirsi.laitinen@utu.fi TRIAL REGISTRATION: ClinicalTrials.gov, NCT00167700 Study reports from the Laitinen 2009 cohort with descriptions of primary outcomes of each study: Piirainen 2006: focused on the outcomes of maternal dietary intake, compliance with diet, maternal weight gain. This study was published with only a subset of participants from the final study (n = 231; control n = 69, intervention n = 140 without description of probiotic versus placebo). Aaltonen 2008: primary outcome of interest: Infant blood pressure at 6 months of age. Secondary outcomes: maternal weight gain and blood pressure, dietary intake, 6‐month old weight, head circumference, length. Huurre 2008: primary outcome of interest: Infant sensitization at 6 and 12 months (Only including the mother‐infant pairs assigned to nutritional counseling); other outcomes included rates of exclusive breastfeeding, cesarean section rate, allergy testing of mothers, inflammatory markers in colostrum and breast milk. Hoppu 2012: breast milk fatty acid and cytokine composition (n= 125 due to breast milk sample availability). Other outcomes reported were maternal dietary intake. Hoppu 2014: blood lipid concentrations during and after pregnancy. Ilmonen 2011: maternal anthropometric measurements during and after pregnancy including BMI, adiposity, proportion of body fat and waist circumference. Secondary outcomes were dietary intakes of foods and nutrients and a healthy eating index during the postpartum period. Kaplas 2007: placental fatty acids (only looked at placenta and umbilical cord serum samples of 30 healthy women from the study) Laitinen 2009: maternal glucose metabolism characterized by plasma glucose concentration, blood glycated Hb A1C, serum insulin and HOMA and QUICKI indices. Other outcomes measured included dietary energy‐yielding nutrients assessed from food diaries, which were analyzed to explain changes in glucose metabolism. Laitinen 2011: maternal central adiposity (abstract only for European nutrition conference) Luoto 2009: gestational diabetes diagnosis (PAS abstract only). Secondary outcomes included adverse effects, prenatal growth, proportion of large for gestational age infants. Luoto 2010: gestational diabetes diagnosis. Secondary outcomes included miscarriage rate, preterm birth < 32 weeks, preterm birth 32 to 36 weeks, cesarean delivery rate, 5‐minute Apgar score, birth weight, birth length, birth head circumference. Luoto 2012: colostrum adiponectin concentration. Other outcomes reported include diagnosis of gestational diabetes, weight gain during pregnancy Vahamiko 2013: serum leptin concentrations in women, cord blood and infants at 1 month of age. Other outcomes include weight and rate of exclusive breastfeeding at 1 and 6 months of age. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization done by a statistician not involved in recruitment or study visits. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with participant group assignments that were opened in the room with the patient. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Nutritional counseling component was single‐blinded but both groups being compared in this review received the counseling, and the probiotic versus placebo intervention was double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No specific description of blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 92% of patients have birth data included |
| Selective reporting (reporting bias) | Low risk | Registered clinical trial outcomes are reported |
| Other bias | Low risk | |
Lindsay 2014.
| Methods | The aim of the Probiotics in Pregnancy Study was to investigate the effect of a probiotic capsule on maternal fasting glucose in obese pregnant women. Randomized controlled double‐blind placebo‐controlled trial Method of generating randomization sequence: computer‐generated randomization process by independent researcher Allocation concealment: allocation concealed from the research dietician in sealed sequentially‐numbered opaque envelopes Blinding of intervention: the identity of the capsules unknown to either the participants, researchers or the primary investigators. Blinding of outcome measurement: adequate for most outcomes Complete follow‐up: 138/175 completed and reported (mostly due to assessment of poor compliance) |
|
| Participants | 175 pregnant women less than 20 weeks' gestation with an early pregnancy body mass index (BMI; in kg/m2) from 30.0 to 39.9 were recruited from antenatal clinics at the National Maternity Hospital, Dublin, Ireland. Exclusion criteria were BMI < 30.0 or > 39.9, prepregnancy or gestational diabetes, age < 18 years, multiple pregnancy, and fetal anomaly. 138 women who completed the study (63 women in the probiotic group; 75 women in the placebo group) Demographic data Maternal age: Probiotics group: 31.4 ± 5.0 years (N = 63) Placebo group: 31.0 ± 5.2 (N = 75) |
|
| Interventions | Women were randomly assigned to receive either a daily probiotic (Lactobacillus salivarius at 109 CFUs) or a placebo capsule from 24 to 28 weeks of gestation in addition to routine antenatal care. | |
| Outcomes | The primary outcome was the change in fasting glucose between groups from pre‐intervention to post‐intervention. Secondary outcomes were the incidence of gestational diabetes and neonatal anthropometric measures. Outcomes of interest: preterm birth < 37 weeks, preterm birth < 34 weeks, neonatal mortality. |
|
| Notes | Period of study: recruitment from March 2012 to March 2013 Published: 2014 Source of funding: funded by the National Maternity Hospital Medical Fund with support from Ivo Drury Award Sponsorship Source: Alimentary Health Ltd. provided probiotic and placebo capsules free of charge Country: Ireland Setting: recruited from antenatal clinics at the National Maternity Hospital, Dublin, Ireland. Author's contact details: F. M. McAuliffe, University College Dublin, Obstetrics and Gynaecology, School of Medicine and Medical Science, National Maternity Hospital, Dublin Ireland. Fionnuala.mcauliffe@ucd.ie TRIAL REGISTRATION: This trial was registered at Current Controlled Trials as ISRCTN97241163 (part A). Am J Clin Nutr 2014;99:1432–9. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized controlled double‐blind, placebo‐controlled trial Method of generating randomization sequence: computer‐generated randomization process by independent researcher. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: Allocation concealed from the research dietician in sealed sequentially‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: the identity of the capsules unknown to either the participants, researchers or the primary investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: adequate for most outcomes (unclear which outcomes determined by the research nurse). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: 138/175 completed and reported (mostly due to assessment of poor compliance) |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Lindsay 2015.
| Methods | The aim of this study was to investigate the effects of a probiotic capsule intervention on maternal metabolic parameters and pregnancy outcome among women with gestational diabetes. Single center, double‐blind, placebo‐controlled randomized trial Method of generating randomization sequence: computer‐generated randomization process by independent researcher Allocation concealment: allocation concealed from the research dietician in sealed sequentially‐numbered opaque envelopes Blinding of intervention: the identity of the capsules unknown to either the participants, researchers or the primary investigators. Blinding of outcome measurement: adequate for most outcomes Complete follow‐up: 115/149 (77%) completed and reported (mostly due to assessment of poor compliance) |
|
| Participants | Recruited pregnant women < 34 weeks' gestation with a new diagnosis of gestational diabetes or impaired glucose tolerance following a 3‐hour 100 g glucose tolerance test. Exclusion criteria: prepregnancy diabetes, age < 18 years, gestational age ≥ 34 weeks' gestation, multiple pregnancy, and fetal anomaly. Excluded if planned medical treatment with insulin or oral hypoglycemic agents immediately after diagnosis. Demographic data Probiotics group N = 74 (48 in secondary per protocol analysis), maternal age 33.5 ± 5.0 years Placebo group N = 75 (52 in secondary per protocol analysis) maternal age 32.6 ±4.5 years |
|
| Interventions | Women were randomized to a daily probiotic (Lactobacillus salivarius UCC118 10^9 CFUs) or placebo capsule from diagnosis until delivery. | |
| Outcomes | Fasting blood samples were collected at baseline and 4 to 6 weeks after capsule commencement for analysis of glucose, insulin, C‐peptide, and lipids. The primary outcome was difference in fasting glucose postintervention, first analyzed on an ITT basis and followed by per‐protocol analysis that excluded women commenced on pharmacological therapy (insulin or metformin). Secondary outcomes were changes in insulin, C‐peptide, homeostasis model assessment and lipids, requirement for pharmacological therapy, and neonatal anthropometry. Outcomes of interest: preterm birth < 37 weeks, preterm birth < 34 weeks, gestational age at birth and maternal miscarriage. |
|
| Notes | Period of study: recruitment from March 2012 to March 2014 Published: 2015 Source of funding: funded by the National Maternity Hospital Medical Fund with support from Ivo Drury Award and the European Unions Seventh Framework Program, Project EarlyNutrition Sponsorship Source: Alimentary Health Ltd. provided probiotic and placebo capsules free of charge Country: Ireland Setting: recruited from antenatal clinics at the National Maternity Hospital, Dublin, Ireland. Author's contact details: F. M. McAuliffe, University College Dublin, Obstetrics and Gynaecology, School of Medicine and Medical Science, National Maternity Hospital, Dublin Ireland. Fionnuala.mcauliffe@ucd.ie TRIAL REGISTRATION: This trial was registered at Current Controlled Trials as ISRCTN97241163 (part B). Am J Clin Nutr 2014;99:1432–9. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized controlled, double‐blind, placebo‐controlled trial Method of generating randomization sequence: computer‐generated randomization process by independent researcher. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: allocation concealed from the research dietician in sealed sequentially‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: the identity of the capsules unknown to either the participants, researchers or the primary investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: adequate for most outcomes (unclear which outcomes determined by th research nurse). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up Probiotics group N = 74 (48 in secondary per protocol analysis) Placebo group N = 75 (52 in secondary per protocol analysis) |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Mantaring 2016.
| Methods | Mantaring 2016 conducted a double‐blind, randomized controlled study at the Community Hospital of Muntinlupa City, Philippines to assess the effects of maternal nutritional supplement beverages formulated both with and without a probiotic mixture on maternal health, fetal/infant growth and health when given to pregnant women in the third trimester and during lactation. Double‐blind, randomized controlled study Method of generating randomization sequence: unclear Allocation concealment: unclear Blinding of intervention: unclear Blinding of outcome measurement: unclear Complete follow‐up: 89% completed follow‐up |
|
| Participants | 233 healthy pregnant women at the beginning of their third trimester who were willing to exclusively breast feed for at least 2 months postpartum, and their infants Exclusion criteria: known allergy to cow's milk, participants previously diagnosed HIV(+) and Hepatitis B (+), multiple pregnancy, high‐risk pregnancy (pre‐eclampsia, diabetes, etc), Currently participating or having participated in another clinical trial during the last 3 months, Particpants who consumed pro‐ and /or prebiotics‐containing food/supplement in the month before inclusion Demographic data Probiotics + nutritional supplement group N = 78 Placebo (nutritional supplement) group N = 78 Control group (no probiotic or nutritional supplement) N = 77 |
|
| Interventions | Probiotics group + nutritional supplement (N = 78) received Lactobacillus rhamnosus (CGMCC 1.3724) 7 x1 0^8 CFU and Bifidobacterium lactis (CNCC I‐3446) 7 x 10^8 CFU per serving with a nutritional supplement (200 ml serving per day (140 kcal, 8 g protein, 21 g carbohydrate, 3.5 g fat including DHA)) Placebo group received nutritional supplement without probiotics (N = 78) (200 ml serving per day (140 kcal, 8 g protein, 21 g carbohydrate, 3.5 g fat including DHA)) Control group: no supplement and no probiotics (N = 77) Interventions were given from the beginning of the third trimester of pregnancy until 8 weeks of lactation |
|
| Outcomes | Outcomes included maternal health outcomes at gestational months 6, 7, 8, and delivery, as well as neonatal health outcomes, including fetal ultrasound at 24 to 28 weeks, birth weight, Apgar scores, and infant growth over the first year of life Outcomes of interest: none reported |
|
| Notes | Period of study: unclear Published: 2016 Source of funding: Nestlé Nutrition Sponsorship Source: Nestlé Nutrition Country: Phillipines Setting: Community Hospital of Muntinlupa City, Philippines. Author's contact details: Jojo Mantaring; Philippines General Hospital, Manilla City, Philippines TRIAL REGISTRATION: NCT01073033. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description in abstract |
| Allocation concealment (selection bias) | Unclear risk | No description in abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description in abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description in abstract |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 10% attrition rate |
| Selective reporting (reporting bias) | High risk | Outcomes in the registration of the trial were not reported in the abstract. |
| Other bias | Unclear risk | No description in abstract |
Ou 2012.
| Methods | This study evaluated the effectiveness of prenatal and postnatal probiotics in the prevention of early childhood and maternal allergic diseases. Prospective, double‐blind, randomized, placebo‐controlled trial Method of generating randomization sequence: unclear Allocation concealment: unclear Blinding of intervention: treatment codes were kept by the supplier until all data had been collected and analyzed Blinding of outcome measurement: treatment codes were kept by the supplier until all data had been collected and analyzed Complete follow‐up: 78% of participants randomized were analyzed in the newborn period |
|
| Participants | 191 pregnant women with any allergic diseases (asthma, eczema, food allergy or allergic rhinitis) AND elevated IgE (elevated total IgE and/or positive allergen specific IgE meeting specific thresholds) Exclusion criteria: maternal diabetes mellitus, rheumatoid arthritis, or infectious disease; no family history of allergic diseases Demographic data: Probiotics group N = 95; maternal age 31 years, range 23–40 years Placebo group N = 96; maternal age 30 years, range 25–42 years |
|
| Interventions | Probiotics group (N = 95) received Lactobacillus rhamnosus GG; 1 x 10^10 CFU daily Placebo group (N = 96) received placebo daily During pregnancy, the interventions began at 24 weeks' gestation and continued until delivery. After delivery, the interventions were given to exclusively to breastfeeding mothers or directly administered to non‐breastfeeding neonates until 6 months of age. |
|
| Outcomes |
Outcome of interest reported: gestational age at birth Other outcomes reported: point and cumulative prevalence of sensitization and development of allergic diseases, and improvement of maternal allergic symptom score and plasma immune parameters before and after intervention at 6, 18 and 36 months. |
|
| Notes | Only outcomes at birth regarding the probiotics being given during pregnancy were included, as postnatally, some infants received the probiotics directly. Period of study: August 2002 and January 2006 Published: 2012 Source of funding: unclear Sponsorship Source: Chang Gung Memorial Hospital Country: Taiwan Setting: Single center, Kaohsiung Chang Gung Memorial Hospital Author's contact details: Kuender D. Yang, Department of Medical Research, Show Chwan Memorial Hospital in Chang Bing, No. 6‐1 Lu‐Kung Road, Chang Bing Industrial Center, Lu‐Kang, Changhua 505, Taiwan. E‐mail: yangkd.yeh@hotmail.com TRIAL REGISTRATION: ClinicalTrials.gov NCT00325273. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment codes were kept by the supplier until all data had been collected and analyzed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment codes were kept by the supplier until all data had been collected and analyzed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 78% of randomized participants were analyzed at the newborn period. |
| Selective reporting (reporting bias) | Low risk | Changed the timing of post‐natal follow up laboratory tests of allergic sensitization from protocol to study |
| Other bias | Low risk | |
Rautava 2012.
| Methods | This study investigated whether maternal probiotic supplementation during pregnancy and breast‐feeding reduces the risk of developing eczema in high‐risk infants. Parallel, randomized, double‐blind, placebo‐controlled trial Method of generating randomization sequence: computer‐generated by the manufacturer of the study products, independent of the researchers Allocation concealment: study group code only known by manufacturer Blinding of intervention: double‐blind with group only known to researchers after all follow‐up and outcomes assessed Blinding of outcome measurement: double‐blind with group only known to researchers after all follow‐up and outcomes assessed Complete follow‐up: 12/241 infants were lost to follow‐up completely (5%), while 205/241 (85%) had outcomes assessed. |
|
| Participants | 241 pregnant women mothers with allergic disease and atopic sensitization, and their 241 infants Exclusion criteria: immune‐mediated disease other than atopic or allergic disease, and infants born of multiple pregnancies Demographic data Probiotics group #1, N = 81, maternal age 31 years, range 22‐43 years Probiotics group #2, N = 82, maternal age 30 years, range 22‐42 years Placebo group N = 78, maternal age 30 years, range 22‐40 years |
|
| Interventions | Probiotics group #1 receiving Lactobacillus rhamnosus 1 x 10^9 CFU and Bifidobacterium longum 1 x 10^9 CFU with a dietary food supplement of vitamins and minerals, N = 81 Probiotics group #2 receiving L paracasei 1 x 10^9 CFU and B longum 1 x10^9 CFU with a dietary food supplement of vitamins and minerals, N = 82 Placebo consisted of the same dietary food supplement of vitamins and minerals without added probiotics N = 78 Intervention or placebo began 2 months before delivery and continued until 2 months of breast feeding. |
|
| Outcomes | The primary outcome was cumulative incidence of eczema in the infant up to 2 years old. Secondary outcomes were atopic sensitization, gestational age at birth, birth weight, and duration of exclusive breast‐feeding. Outcome of interest: gestational age at birth. |
|
| Notes | Period of study: August 2005 to April 2009 Published: 2012 Source of funding: Sigrid Juselius Foundation and Turku University Hospital EVO research funding. Sponsorship Source: Turku University Hospital Country: Finland Setting: single tertiary center in Turku, Finland Author's contact details: Samuli Rautava, MD, PhD, Department of Paediatrics, Turku University Central Hospital, Kiinamyllynkatu 4‐8, 20520, Turku, Finland. E‐mail: samrau@utu.fi. TRIAL REGISTRATION: ClinicalTrials.gov NCT00167700. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described, but since only the drug manufacturer knew the group until after the study was complete, it is likely to be low. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Researchers and participants blinded until after all follow‐up and outcomes assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers and participants blinded until after all follow‐ up and outcomes assessed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15% with incomplete outcome |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
BMI: body mass index; CFU: colony‐forming unit; CI: confidence interval; DHA: Docosahexaenoic aciD; GDM: gestational diabetes mellitus; HOMA: homeostasis model assessment; IgE: Immunoglobulin E; ITT: intention‐to‐treat; LPS: lipopolysaccharides; NEC: necrotizing enterocolitis; NICU: neonatal intensive care unit; QUICKI: quantitative insulin‐sensitivity check index;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asemi 2011 | Asemi 2011 conducted a single‐blinded randomized clinical trial in Iran to assess the effects of daily consumption of probiotic yogurt versus conventional yogurt on inflammatory factors in pregnant women. This was excluded since yogurt, which inherently contains probiotic bacteria, was given to both the control and intervention group. |
| Asemi 2012 | Asemi 2012 conducted a randomized, single‐blind controlled trial in Iran to determine the effects of daily consumption of probiotic yogurt versus conventional yogurt on lipid profiles of pregnant women. This study was excluded due to both the intervention and the control receiving probiotics since the conventional yogurt contained S. thermophilus and L. bulgaricus. |
| Bisanz 2014 |
Bisanz 2014 conducted a randomized, clinical trial in the Mwanza Region of Tanzania evaluating the effects of probiotic and Moringa plant matter supplementation on maternal on maternal blood metal levels. Excluded due to co‐intervention (Moringa, a micronutrient‐rich plant) given only to probiotic group. Trial registration: ClinicalTrials.gov NCT02021799 |
| Daskalakis 2014 | Daskalakis 2014 reported preliminary results from their ongoing double‐blind clinical trial of pregnant women between 24 to 34 weeks of gestation with premature rupture of membranes designed to evaluate the effects of vaginal probiotic suppositories on time to delivery and neonatal outcomes. This study was excluded from this review since the route of administration of the probiotics was vaginal, not oral. |
| Gonai 2014 | Gonai 2014 conducted a randomized control trial in Japan of pregnant women with gestational diabetes to examine the effects of probiotic yogurt versus regular diet on glucose metabolism indices. This study was excluded due to the unclear gestational age at study entry. |
| Gronlund 2011 | Gronlund 2011 analyzed a subset of mother‐infant dyads from a larger randomized controlled trial in high risk allergic families in Finland comparing two different probiotic groups to each other and a placebo arm, with probiotics given 2 months before and after birth. Only a subset of dyads were included in this study, based upon the duration of breast‐feeding, thus, these comparison groups were not allocated via randomization. |
| Grzeskowiak 2012 | Grzeskowiak 2012 evaluated the impact of probiotic administration to mother or infant on gut microbiota composition in 6‐month‐old Finnish and German infants. This study included patients from the Gronlund 2011 trial, a trial of post‐natal administration of probiotic formula to infants, and a few non‐randomized infants who were exclusively breast fed. Reasons for exclusion include probiotics directly administered to infants post‐natally, and non‐randomization in the exclusively breast fed infants in the third arm of this study. |
| Hanson 2014 | Hanson 2014 conducted a non‐randomized pilot feasibility trial in the USA in mid‐gestation pregnant women to determine the effects of probiotic administration on Group B strep colonization. This study was excluded because the study groups were not randomized. |
| Hantoushzadeh 2012 | Hantoushzadeh 2012 conducted a randomized, placebo‐controlled clinical trial in Iran investigating the efficacy of oral probiotic versus clindamycin for one week to treat bacterial vaginosis. Excluded due to an antibiotic as the comparator. |
| Jain 2017 | Jain 2017 conducted a randomized, placebo‐controlled, double‐blind study in the USA in pregnant women ≤ 18 weeks to assess the safety and efficacy of probiotics versus placebo for 60 days. This study was excluded since the intervention not continued into the third trimester when presumably the majority, if not all, of infants were born. |
| Jamilian 2016 | Jamilian 2016 conducted a randomized clinical trial in Iran in pregnant women to determine the effects of probiotics on metabolic profiles by administering probiotic vs placebo from 9 to 21 weeks' gestation. This study was excluded since the intervention was not continue into the third trimester. |
| Karampelas 2013 | Karampelas 2013 conducted a double‐blind study in Greece in pregnant patients with confirmed PPROM between 24th and 34th week of gestation to determine the effects of probiotics on latency period. This study was excluded due to vaginal, not oral, probiotic administration. |
| Krauss‐Silva 2011 | Krauss‐Silva 2011 conducted a randomized, placebo‐controlled trial in Brazil to evaluate the efficacy of the early administration of probiotic lactobacilli to pregnant women with asymptomatic bacterial vaginosis to prevent spontaneous premature delivery and associated neonatal morbidity. While the intervention began at less than 20 weeks, this study was excluded since the intervention was stopped during the second trimester at 24 to 26 weeks. |
| Nishijima 2005 | Nishijima 2005 conducted a randomized, controlled study in Japan to evaluate the effect of fermented milk with probiotics on vaginal colonization with probiotics and potentially pathogenic bacteria in pregnant women enrolled 24 healthy Japanese women ≥ 35 weeks' gestation. Excluded due to the potential for probiotics in fermented milk products given to the control group. |
| Ortiz‐Andrellucchi 2008 | Ortiz‐Andrellucchi 2008 conducted a randomized controlled trial in postpartum, lactating women to determine if six weeks of probiotic intake would modulate the immune system. This study was excluded since probiotics were only administered after birth to mothers of infants born at term. |
| Vitali 2012 | Vitali 2012 conducted a pilot, non‐randomized trial in Italy to investigate the impact of probiotic supplementation on the vaginal microbiota and immunological profiles of healthy women during late pregnancy. This study was excluded since study groups were based upon ability to consume probiotic product and were not randomized. |
| Wickens 2008 | Wickens 2008 conducted a randomized, controlled trial in New Zealand to determine the efficacy of two different probiotics given to pregnant and postpartum women on the development of allergic sensitization and eczema in their offspring. Excluded since probiotics begun late in the third trimester at 35 weeks, mothers who gave birth prior to 37 weeks or had infants with a NICU admission were explicitly excluded, and probiotics were administered directly to the infant after birth starting on day 2. |
NICU: neonatal intensive care unit; PPROM: preterm premature rupture of the membranes
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611001208998.
| Trial name or title | SPRING: an RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women |
| Methods | Prospective, double‐blind, randomized controlled trial |
| Participants | Inclusion criteria: overweight and obese pregnant women less than 16 weeks' gestation at recruitment, singleton pregnancy, BMI > 25, >18 years old, able to read and understand English and provide informed consent. Exclusion criteria: pregnancy at > 16 weeks' gestation at recruitment; multiple pregnancy; known pre‐existing diabetes, impaired fasting glucose or impaired glucose tolerance; GDM prior to recruitment as diagnosed by early pregnancy glucose testing, taking medications likely to influence glucose metabolism, medical conditions associated with altered glucose metabolism, known major fetal abnormality noted on 12‐week ultrasound examination; and known ingestion of probiotics via capsules or sachets. |
| Interventions | Once daily oral probiotic combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB‐12 (Chr. Hansen A/S, Horsholm, Denmark) at a dose of >1 x 10^9 CFUs each per day or placebo (microcrystalline cellulose and dextrose anhydrate capsules –Chr. Hansen). Intervention or placebo will occur from enrollment prior to 16 weeks' gestation until birth. |
| Outcomes | Primary outcome: frequency of diagnosis of GDM at 28 weeks' gestation by a 75 g oral glucose tolerance test (OGTT). Secondary outcomes Maternal: gestational weight gain, pre‐eclampsia, induction of labor, cesarean delivery, change in gut microbiome, change in lipids and inflammatory profile, change in dietary indices and physical activity levels between baseline and 28 weeks Neonatal: body composition, anthropometry, preterm delivery, shoulder dystocia, hypoglycemia, treatment with supplemental fluids or feeds, nerve palsy, admission to the NICU, jaundice requiring phototherapy, bone fracture, neonatal death or stillbirth. |
| Starting date | November 2012 |
| Contact information | Prof Leonie Callaway Level 9 Health Sciences Building Royal Brisbane and Women's Hospital Buttereld Street HERSTON QLD 4029 Country Australia Phone +61 7 3346 5273 Email l.callaway@uq.edu.au |
| Notes | ACTRN12611001208998 DOI:10.1186/1471‐2393‐13‐50 |
ACTRN12612000196842.
| Trial name or title | The Probiotics in Pregnancy Study (PiP Study): rationale and design of a double blind randomized controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy |
| Methods | Two‐center, randomized, double‐blind, placebo‐controlled trial |
| Participants | Inclusion criteria: English‐speaking pregnant women who intend to breast feed their infant are eligible to be enrolled in the study between 14 and 16 weeks' gestation if either they or the unborn child’s biological father have a history of asthma or eczema treated by a doctor, or allergic rhinitis treated by a doctor or pharmacist. Exclusion criteria: under age 16, does not intend to stay in either of the study centers for the 18 months following enrollment, has a serious immunological disorder that suppresses immune function or is taking immune suppressant drugs, has known cardiac valve disease for which antibiotic prophylaxis is required when undergoing dental procedures, has a history of a transplant or human immunodeficiency virus, were on long‐term of continuous antibiotic therapy, required in vitro fertilisation to establish the current pregnancy, has a pre‐enrollment scan showing major fetal abnormalities, at the time of enrollment is using or intended to use probiotic drinks or supplements themselves or in their child, is participating in another randomized controlled trial, has a severe allergy to cow’s milk (as probiotic capsules may contain traces of milk), has previously participated in the study with an older child or is deemed unsuitable for study inclusion for any other medical reason. Any pregnant woman with pre‐existing type 1 or 2 diabetes is eligible for the study but is excluded from the OGTT and gestational diabetes outcomes. Infant inclusion/exclusion: all infants born in the study are eligible for inclusion in study outcomes. In the case of multiple births, only the first‐born infant will be included in the study. |
| Interventions | Probiotic supplementation by Lactobacillus rhamnosus HN001 (6 × 10^9 CFU/day) administered to mothers from 14 to 16 weeks' gestation until delivery and continuing until 6 months postpartum, if breastfeeding, versus placebo capsules with con‐derived maltodextrin with an identical appearance. |
| Outcomes | Primary Outcomes
Secondary Outcomes Maternal outcomes: maternal gestational diabetes mellitus, maternal BV, maternal vaginal GBS colonization, maternal postpartum depression and anxiety, gestation at delivery, maternal anthropometric measures at birth, and 6 and 12 months postpartum, caesarean section, fasting lipids and bile acids from fasting maternal blood taken at time of OGTT, gut microbiota and faecal SCFA from maternal faecal samples taken in second trimester, maternal postpartum depression and anxiety, maternal bacteremia/septicemia, hospitalizations, gastrointestinal symptoms. Infant related outcomes: anthropometric measures at birth, and 6 and 12 months, NICU admission, PROM, preterm birth (< 37 weeks), infant GBS, bacteremia/septicemia, hospitalizations (excluding birth admissions), 5‐minute Apgar scores, infant symptoms |
| Starting date | Not stated |
| Contact information | Christine Barthow |
| Notes | Wellington and Auckland, New Zealand DOI 10.1186/s12884‐016‐0923‐y Australian New Zealand Clinical Trials Registration: ACTRN12612000196842. Date Registered: 15/02/12. |
ACTRN12615000400561.
| Trial name or title | A randomized controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial |
| Methods | Two by two factorial randomized controlled demonstration trial |
| Participants | Inclusion criteria: women with a singleton pregnancy, BMI ≥ 30 kg/m2, between 12 and 17 6/7 weeks of gestation and able to provide informed written consent. Exclusion criteria: maternal pre‐existing diabetes or HbA1c at booking ≥ 50 mmol/mol, taking probiotic supplements, known congenital abnormality, medications or medical conditions which alter glucose metabolism, multiple pregnancy, bariatric surgery, and severe hyperemesis. |
| Interventions | Oral probiotic capsule consisting of Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12 at a dose of 7 × 10^9 CFUs per day each, or placebo from enrollment between 12 to 17 6/7 weeks until birth. Dietary intervention versus routine dietary advice There are four groups: dietary intervention with probiotic capsule, dietary intervention with placebo capsules, routine dietary advice with probiotic capsule and routine dietary advice with placebo capsules. |
| Outcomes | Primary outcomes Maternal: excessive gestational weight gain between enrollment and 36 weeks' gestation Infant: infant birth weight Secondary outcomes Maternal: maternal pregnancy glucose metabolism as assessed by OGTT parameters at 26 to 28 weeks and HbA1c at 28 and 36 weeks and 5 months postpartum, changes in diet quality and dietary patterns between recruitment, 28 to 30 weeks and 5 months postpartum, functional health and well‐being at 36 weeks and 5 months postpartum, depression and anxiety scores at 36 weeks’ and 5 months postpartum, maternal adiposity at 5 months postpartum; GDM, pregnancy‐induced hypertension (pre‐eclampsia and gestational hypertension), mode of birth, blood lipid concentrations at 28 to 30 and 36 weeks’ gestation and 5 months postpartum, maternal feedback about participation in the study Infant: neonatal anthropometry: head circumference and length, and associated Z‐scores, birth weight adjusted for length, girths (chest, arm and abdominal) adjusted for length, subscapular, triceps and supra iliac skin fold thickness adjusted for length, and arm muscle area adjusted for arm length, neonatal body composition (via PEA POD®) including fat mass and lean mass adjusted for length and fat mass adjusted for lean mass, gestational age at birth, LGA, small for gestational age (SGA, < 10th centile), admission to neonatal care unit (and reason), neonatal composite morbidity, including birth trauma (fracture, brachial plexus injury, cephalohematoma, subgaleal hematoma), hypoxic ischemic encephalopathy, sepsis, respiratory distress requiring continuous positive airway pressure support, hypoglycemia requiring dextrose treatment (buccal or intravenous), Initiation and establishment of breast feeding, including feeding in first two postnatal weeks (collected by phone call at 6 weeks and questionnaires), infant anthropometry and body composition at 5 months of age, infant feeding over first 5 months (breast feeding, formula use, complementary feeding with solids), feeding behavior as assessed by BEBQ scores, infant nutritional intake at 5 months Other secondary outcomes include: attendance at study visits, adherence to probiotic/placebo regimen, cost‐effectiveness of the intervention |
| Starting date | Unknown |
| Contact information | Lesley M. E. McCowan l.mccowan@auckland.ac.nz Department of Obstetrics and Gynaecology, Faculty of Medical and Health Science, University of Auckland, Private Bag 92019, Auckland, New Zealand |
| Notes | DOI 10.1186/s12884‐016‐1149‐8 ACTRN12615000400561 |
CTRI/2013/04/003577.
| Trial name or title | Effect of oral probiotic supplementation during pregnancy and lactation in modulating immune response in breast milk, fetus and neonate |
| Methods | Randomized, parallel group, placebo‐controlled trial |
| Participants | Pregnant and lactating women starting at 34 weeks' gestation who will exclusively breast feed for 14 weeks. |
| Interventions | Probiotic capsule contains a mix of 8 different strains of freeze‐dried lactic acid bacteria and bifidobacteria; each capsule containing not less than 112.5 billion CFU. Study Drug will be given orally 3 times a day starting from 34 weeks of gestation till 14 weeks postpartum. Placebo identical looking in size, color and appearance. The dose will be 3 times a day orally starting from 34 weeks of gestation till 14 weeks' postpartum. |
| Outcomes | Primary outcomes: proportion of mothers who will have detectable levels of immune markers in mature breast milk including cytokines‐IFN‐γ, IL‐6, IL‐10 antimicrobial agents‐ Total IgA, Lactoferrin and growth factors –IGF‐1. Secondary outcomes: proportion of fetus and neonates who will have detectable levels of immune markers in cord blood, including cytokines ‐IFN‐γ, IL‐6, IL‐10, and growth factors ‐ IGF‐1. Proportion of neonates with protective levels of antibody against Hepatitis B surface antigen (anti‐HbsAg) 6 weeks after vaccination with hepatitis B vaccination. |
| Starting date | 12/18/2014 |
| Contact information | Dr Neena Malhotra Department of Obstetrics & Gynaecology All India Institute of Medical Sciences, New Delhi‐110029 South 011‐26593361 malhotraneena@yahoo.com |
| Notes |
ISRCTN53023014.
| Trial name or title | To study the transit of bifidobacteria from mother to infant: a randomized controlled trial |
| Methods | Single‐centre, double‐blind, interventional, randomized controlled trial |
| Participants | Pregnant and postpartum women |
| Interventions | Bifidobacteria probiotic capsule or daily placebo capsule from 16 weeks of pregnancy until the baby is 3 months old |
| Outcomes | Primary outcome measures: presence of the supplemented bifidobacterial strain in the infant’s stool in the 1st week postpartum, at 1 month postpartum and at 3 months postpartum. To measure this, the infant's stool will be analyzed for the presence of the supplemented bifidobacterial strain. Secondary outcome measures Differences in the following between the probiotic and placebo groups:
Maternal bloods will be taken in early (approximately 16 weeks' gestation) and late (approx. 34 weeks' gestation) pregnancy and will be analyzed for these metabolic parameters. |
| Starting date | December 2015 |
| Contact information | Prof. Fionnuala McAuliffe The National Maternity Hospital (Ireland) UCD Department of Obstetrics & Gynaecology 65‐66 Lower Mount Street Dublin 2 |
| Notes |
Mehta 2016.
| Trial name or title | Randomized, double‐blind, placebo‐controlled trial of probiotics in pregnancy (PIP) and its effect on Group‐B streptococcal colonization‐ study protocol. |
| Methods | Single‐centre, double‐blinded, randomized controlled trial |
| Participants | Inclusion criteria: pregnant women who are 24 weeks' pregnant will be enrolled |
| Interventions | Probiotics or placebo starting at 24 weeks for 12 weeks. |
| Outcomes | Primary outcome: GBS colonization at 35 to 37 weeks' gestation |
| Starting date | Unknown |
| Contact information | Mehta S
Fiona Stanley Hospital, Perth, WA, Australia Email: shailender.mehta@health.wa.gov.au |
| Notes | Sample size of 460 is expected Funded by Telethon |
NCT00217308.
| Trial name or title | Effect of probiotic Lactobacilli on vaginal flora of pregnant women at high risk for preterm delivery |
| Methods | Double‐blind, randomized, placebo‐controlled trial |
| Participants | One hundred and sixty (160) women at high risk for PTL, based on a prior history of preterm birth, will be approached at their first antenatal visit to participate. Recruitment of 54 patients with symptomatic or asymptomatic BV (based on Nugent Scoring). Inclusion criteria: pregnant women with previous incidence of preterm labour, otherwise healthy, over 18 years of age, able to provide informed consent, less than or equal to 16 weeks' gestation, singleton pregnancy, and normal uterine cavity. Exclusion criteria: significant medical complications (pre‐eclampsia, thrombophilia, hypertension), multiple pregnancy, less than 18 years of age, patients receiving antibiotics or other antimicrobial therapies at time of recruitment, fetal complications such as intrauterine growth restriction or other abnormalities, diabetes, documented need for cervical cerclage, patient enrolled in other clinical trials |
| Interventions | Women with documented BV will be randomized to either treatment with lactobacilli preparation (n = 27) or placebo (n = 27). Women with symptomatic BV will be treated with oral metronidazole prior to starting the lactobacilli or placebo. |
| Outcomes | Vaginal flora and cytokine profile and the incidence of preterm labour. |
| Starting date | February 2005 |
| Contact information | Alan Bocking, MD, FRCSC Chief, Department of Obstetrics and Gynaecology, Mount Sinai Hospital Mount Sinai Hospital Toronto, Ontario, Canada, M5G 1X5 |
| Notes |
NCT00217308 This study has suspended participant recruitment. (Tightly defined inclusion criteria were making recruitment very slow.) |
NCT01436448.
| Trial name or title | Probiotics (Lactobacillus rhamnosus) in reducing glucose intolerance during and after pregnancy (GRIP) |
| Methods | Double‐blind, placebo‐controlled, randomized trial |
| Participants | Inclusion criteria: pregnant women who are 35 years old or older, have a family history of diabetes, or are overweight (BMI > 23) and have a singleton pregnancy with delivery planned at the study hospital at 12 to 14 weeks of gestation Exclusion criteria: history of gestational diabetes, known diabetes mellitus, known chronic diseases (hypothyroidism ,cardiac, renal, rheumatoid arthritis, carcinoma), women maintained on medications such as: corticosteroids, azathioprin, antiepileptic epileptic drugs, known Polycystic Ovarian Syndrome, and non‐residents of Karachi |
| Interventions | Lactobacillus rhamnosus 1 x 10^10 CFUs orally once daily or placebo from 12 to 14 weeks of gestation until delivery |
| Outcomes | Glucose intolerance at 24 to 28 weeks of pregnancy and at 6 to 8 weeks' postpartum. Feasibility‐ process of recruitment rate, recruitment rate and reasons for non‐participation Compliance at 36 weeks including maternal side effects Maternal safety: maternal mortality, weight gain, pre‐eclampsia, mode of delivery, induction of labor Fetal/neonatal safety from time of birth until 6 to 8 weeks of life: death, still births, preterm birth, birth trauma, macrosomia, SGA, polyhydramnios, hypoglycemia, large for gestational age, shoulder dystocia, 5‐minute Apgar < 7, hyperbilirubinemia, respiratory distress |
| Starting date | October 2011 |
| Contact information | Bilal Ahmed, Senior Instructor Research, Aga Khan University Karachi‐Pakistan |
| Notes |
NCT01436448 The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years. |
NCT01454661.
| Trial name or title | Probiotics and early microbial contact in preterm neonates (ProPre) |
| Methods | Randomized, placebo‐controlled, double‐blind, controlled trial |
| Participants | Inclusion criteria
Exclusion Criteria
|
| Interventions | Probiotics Lactobacillus rhamnosus GG 10E9 CFU / day (LGG) Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb‐12 administered 10E9 CFU/ day each (LGG+Bb‐12) Placebo: Microcrystalline cellulose Group 1: lactating mother gets placebo, infant gets LGG Group 2: lactating mother gets placebo, infant gets placebo Group 3: lactating mother gets LGG, infant gets placebo Group 4: lactating mother gets LGG+Bb‐12, infant gets placebo Group 5: lactating mother gets placebo, infant gets LGG+Bb‐12 |
| Outcomes | Primary outcomes: assessment of indigenous intestinal microbiota composition in premature neonates during the first month of life, neonatal intestinal immune gene expression from fecal samples during the first month of life, breast milk composition at one month. Secondary outcomes: intestinal immunity, breast milk composition |
| Starting date | April 2014 |
| Contact information | Samuli Rautava, Turku University Hospital Collaborators: University of Turku Massachusetts General Hospital |
| Notes | The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years. |
NCT01479478.
| Trial name or title | Effects of oral probiotic supplementation on Group B Strep (GBS) rectovaginal colonization in pregnancy |
| Methods | Double‐blind, randomized, placebo‐controlled trial |
| Participants | Inclusion criteria: pregnant women between 20 to 28 weeks' gestation who are 18 years of age or older and have a singleton gestation. Exclusion criteria
|
| Interventions | Beginning at 20 to 28 weeks' gestation until delivery |
| Outcomes | Primary outcome measure Group B Streptococcus rectovaginal colonization at 35 to 37 weeks' gestational age. Secondary outcome measures (assessed at 6 weeks' postpartum) Maternal antepartum, intrapartum, and postpartum outcomes: urinary tract infections, chorioamnionitis, endometritis, cellulitis, bacteremia, sepsis, and other infectious morbidity. Neonatal outcomes: gestational age at delivery, Apgar scores, bilirubin levels, C‐reactive protein, rule out sepsis evaluation, sepsis, pneumonia, meningitis, NICU admission, and length of hospital stay |
| Starting date | November 2011 |
| Contact information | Natali Aziz, MD; 6507248222; naziz@stanford.edu Aptos Women's Health Center Aptos, California, USA, 95003 Contact: Karen Erlich khemidwife@earthlink.net Dominican Hospital Santa Cruz, California, USA, 95065 Contact: Karen Erlich khemidwife@earthlink.net Stanford University School of Medicine/Lucile Packard Children's Hospital Stanford, California, USA, 94305 Contact: Anna Girsen, PhD; 6507255720; agirsen@stanford.edu |
| Notes | This study is currently recruiting participants NCT01479478 Estimated Enrollment: 372 Estimated Study Completion Date: November 2019 |
NCT01577108.
| Trial name or title | Oral probiotics Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 reduce Group B Streptococci colonization in pregnant women |
| Methods | Prospective, double‐blind, randomized clinical trial |
| Participants | Pregnant women with positive GBS screening culture at 35 to 37 weeks of gestation with a singleton gestation. |
| Interventions | U‐relax oral capsule, Lactobacillus GR‐1 and RC‐14 or Placebo daily for 14 days |
| Outcomes | Primary outcome measures: number of GBS‐positive pregnant women who became GBS‐negative at childbirth (time frame: 2 weeks after taking probiotic) |
| Starting date | April 2011 |
| Contact information | Ming Ho, MD China Medical University Hospital Taichung, Taiwan, 403 |
| Notes |
NCT01697683.
| Trial name or title | Probiotic therapy for the reversal of bacterial vaginosis in pregnancy (ProVIP) |
| Methods | Randomized controlled trial |
| Participants | Pregnant women who test positive for bacterial vaginosis prior to 17 weeks' gestation |
| Interventions | Probiotic lactobacilli vs. placebo for 12 weeks |
| Outcomes | Primary outcome measures: microbial DNA profile at 28 and at 35 weeks' gestation; a change in the vaginal microbial DNA profile. Secondary outcome measures: microbial function at 28 and at 35 weeks' gestation; a change in microbial function as measured by RNA transcriptomics. |
| Starting date | May 2012 |
| Contact information | Alan Bocking, Mount Sinai Hospital Mount Sinai Hospital Toronto, Ontario, Canada |
| Notes |
NCT01779193.
| Trial name or title | Probiotics capsule as supplemental therapy for Group B Streptococci infection and vaginitis during pregnancy |
| Methods | Double‐blind, randomized, placebo‐controlled trial |
| Participants | Pregnant women at 35 weeks' gestation |
| Interventions | Lactobacillus + cranberry vs. cranberry vs. placebo |
| Outcomes | Quote: "all cases vaginal group B streptococcus colonies" |
| Starting date | June 2012 |
| Contact information | Meng‐Hsing Wu, M.D., Ph.D. Department of Obstetrics and Gynecology, College of Medicine, National Cheng Kung University, Tainan, Taiwan +88662353535 ext 5222 mhwu68@mail.ncku.edu.tw |
| Notes | The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years. |
NCT01922791.
| Trial name or title | Nutrition and pregnancy intervention study |
| Methods | Randomized double blind placebo‐controlled trial with 2 interventions and 4 arms |
| Participants | Inclusion Criteria: pregnant, less than 16 weeks' gestation, overweight, healthy Exclusion Criteria: diabetes (type 1 or 2), celiac disease, Increased bleeding tendency |
| Interventions | The intervention will involve consumption of fish oil and/or probiotic capsules from early pregnancy until 6 months after delivery. Lactobacillus and Bifidobacterium Fish oil Placebo: microcrystalline cellulose Placebo: medium chain triglycerides |
| Outcomes | Primary outcome measures
Secondary outcome measures
Other outcome measures:
|
| Starting date | September 2013 |
| Contact information | Kirsi Laitinen Adjunct professor + 358 02 333 6063 kirsi.laitinen@utu.fi Turku University Hospital Turku, Finland, 20521 University of Turku Turku, Finland, 20521 |
| Notes | Estimated Enrollment: 440
Estimated Study Completion Date: December 2019
Estimated Primary Completion Date: March 2017 ClinicalTrials.gov Identifier: NCT01922791 |
NCT02377544.
| Trial name or title | The role of Bifidobacterium animalis Ssp Lactis DR10 supplementation in women during pregnancy and lactation on breast milk IL‐8 and gut mucosa integrity in Infant |
| Methods | Randomized controlled trial |
| Participants | Pregnant women with a gestational age of 32 to 34 weeks, with a normal pregnancy, a plan to deliver spontaneously, and no antibiotics |
| Interventions | ProbioticBifidobacterium animalis lactis vs. placebo |
| Outcomes | Primary outcome measures
|
| Starting date | December 2014 |
| Contact information | Naomi Esthernita Budi Kemuliaan Women and Children Hospital Jakarta, Central Jakarta, Indonesia, 10430 |
| Notes |
NCT02430246.
| Trial name or title | The association between the transfer of lactobacilli from the gastrointestinal tract to the vagina and the prevention/eradication of abnormal vaginal flora in high risk pregnancies |
| Methods | Double‐blind, randomized controlled trial |
| Participants | Pregnant women at high risk for preterm labor from at least 13 weeks of gestation |
| Interventions | Pregnant women at high risk for preterm labor from at least 13 weeks of gestation will be tested to detect AVF/BV by taking vaginal smear. Diagnosis will be according to the Nugent score criteria. Treatment will be given according to the results: patient with a positive smear for AVF‐ patients tested positive will be treated with either clindamycin or metronidazole. Following treatment, another smear will be taken according to which the patients will be divided into 2 research groups: (1) Assessing the effectiveness of probiotic formula to prevent AVF re‐infection (secondary infection) ‐ this group includes patients with normal vaginal flora following antibiotic administration. (2) Assessing the effectiveness of probiotic formula to eradicate AVF ‐ this group includes patients with persistent AVF following antibiotic administration (first and second line). In each group the patients will be divided into a research group which will receive the probiotic formula UREX PLUS (containing L. rhamnosus GR‐1and L. reuteri RC‐14) and a control group which will receive a placebo twice a day until 36.6 weeks of gestation. |
| Outcomes | Primary outcome measures: the amount of lactobacilli in the vaginal once a month until week 36.6 of labor. Lactobacilli culture will be made from a vaginal specimen. The pattern of bacterial growth will be used for a semi‐quantitative interpretation in a scale of 0 (no vaginal colonization) to 4 (substantial colonization).The rate of women with normal vaginal flora at enrollment, who developed AVF/BV during the study period. The rate of women with AVF/BV at enrollment whose infection was eradicated following antibiotics, who developed AVF/BV during the study period. The rate of women with AVF/BV at enrollment whose infection was not eradicated following antibiotics, who restored the normal vaginal flora during the study period. Secondary outcome measures
|
| Starting date | January 2016 |
| Contact information | Enav Yefet, MD/PhD HaEmek Medical Center, Israel +972‐4‐6494516 enavy1@gmail.com |
| Notes |
NCT02508844.
| Trial name or title | Effects of probiotics (Vivomixx®) in obese pregnant women and their newborn: study protocol for a randomized controlled trial |
| Methods | Single‐center, double‐blind, randomized, placebo‐controlled pilot study |
| Participants | Inclusion criteria: nulliparous pregnant women over 18 years old with a pre‐pregnancy BMI ≥ 30 and < 35 kg/m2, able to speak and read Danish, normal fetal ultrasound at 12 to 14 weeks, and oral glucose tolerance test at 14 to 20 weeks' gestation Exclusion criteria: pregnancy at over 20 weeks’ gestation at recruitment, pregestational diabetes or other serious diseases, Multiple pregnancy, Previous bariatric surgery, Ingestion of probiotics more than 1 month before, the inclusion or ingestion of probiotics other than the study probiotics, Alcohol or drug abuse |
| Interventions | Vivomixx® probiotic mixture (Streptococcus thermophilus DSM 24731, Bifidobacterium breve DSM 24732, Bifidobacterium longum DSM 24736, Bifidobacterium infantis DSM 24737, Lactobacillus acidophilus, DSM 24735, Lactobacillus plantarum DSM 24730, Lactobacillus paracasei DSM 24733, Lactobacillus delbrueckii subsp. bulgaricus DSM 24734 in vegetable capsules containing 112 billion lyophilized bacteria) two capsules per day or placebo twice daily from gestational weeks 14 to 20 until delivery. |
| Outcomes | Primary outcome: gestational weight gain, change in maternal fasting glucose from weeks 14 to 20 (pre‐intervention) to weeks 27 to 30, gestational diabetes diagnosis. Secondary outcomes Maternal: dietary intake, physical activity, fecal samples for microbial diversity, vaginal samples for colonization, maternal lipids, inflammatory markers, HbA1c, bone mineral density, diagnosis of pre‐eclampsia or gestational hypertension, mode of delivery, macrosomia or SGA infants. Neonatal: Infant body composition from birth to 9 months, abdominal circumference and birth anthropometric measurements, birth weight, Apgar score at 5 minutes, umbilical cord pH, neonatal admission to the NICU. |
| Starting date | March 2015 |
| Contact information | Andreas Munk Petersen, MD, Ph.D andreas.munk.petersen@regionh.dk Department of Gastroenterology, Copenhagen University Hospital Hvidovre, Copenhagen, Denmark |
| Notes | DOI 10.1186/s13063‐016‐1617‐5 NCT02508844 |
NCT02509988.
| Trial name or title | Nutritional intervention preconception and during pregnancy to maintain healthy glucose metabolism and offspring health (NiPPeR) |
| Methods | The study aims to assess whether a nutritional drink taken before conception and continuing through pregnancy, assists in the maintenance of healthy glucose metabolism in the mother and promotes offspring health. |
| Participants | Women aged 18 to 38 years planning to conceive within 6 months |
| Interventions | Study: nutritional drink containing a mix of micronutrients, probiotics and myo‐inositol. Control: standard nutritional drink Standard nutritional drink containing a mix of micronutrients. |
| Outcomes | Primary outcome measures: glucose tolerance during pregnancy: OGTT)measurement at 28 weeks' gestation Secondary outcome measures: duration of gestation, pregnancy weight gain, gestational diabetes frequency, nausea and vomiting frequency, antenatal maternal well being/mood, postnatal maternal well being/mood, mode of delivery, preconception maternal micronutrient status, antenatal maternal micronutrient status, preconception maternal gut microbiota composition profile, antenatal maternal gut microbiota composition profile, maternal preconception metabolomic profile, maternal antenatal metabolomic profile, maternal postnatal metabolomic profile, maternal preconception epigenetic profile, maternal antenatal epigenetic profile, maternal postnatal epigenetic profile, breast milk micronutrient profile, breast milk immune factor profile, breast milk epigenetic profile, breast milk metabolomic profile, healthy lactogenesis, offspring birth weight 2,500 g to 4,000 g, offspring size for gestational age size at birth, offspring adiposity, adiposity gain during infancy, cord blood C‐peptide, offspring healthy cardio‐metabolic risk factor profile, offspring allergic well‐being, offspring metabolomic profile related to metabolic health, offspring metabolomic profile related to allergy, offspring epigenetic profile related to metabolic health, offspring epigenetic profile related to allergy, Infant gut microbiota composition, health economics analysis, offspring neurocognitive development (relational binding analysis), offspring respiratory development, offspring allergic development |
| Starting date | July 2015 |
| Contact information | Sponsor: University of Southampton Wayne Cutfield PI at the University of Aukland Yap Seng Chong, PI at National University Hospital, Singapore Keith Godfrey, PI at the University of Southhampton National University Hospital, Singapore; Auckland UniServices Limited |
| Notes | This study is ongoing, but not recruiting participants. Expected to complete in December 2020. Expected enrollment of 1731 |
NCT02528981.
| Trial name or title | Effect of probiotics on GBS colonization status during pregnancy: a pilot randomized controlled trial |
| Methods | Double‐blind, randomized controlled trial |
| Participants | Pregnant women enrolled prior to 23 weeks' gestation |
| Interventions | Probiotic Lactobacillus GR‐1 and RC‐14 vs. placebo |
| Outcomes | Primary outcome measures: the GBS colonization status as determined by a vaginal/rectal swab after 12 weeks of taking capsules |
| Starting date | August 2015 |
| Contact information | Mary Sharpe Ryerson University |
| Notes |
NCT02637986.
| Trial name or title | The efficacy of orally administrated probiotic formula in preventing a recurrence of a urinary tract infection during pregnancy |
| Methods | Double‐blind, randomized, placebo‐controlled trial |
| Participants | Pregnant women who suffered from at least one episode of UTI and are less than 34th week of gestation at the time of the enrollment |
| Interventions | Dietary Supplement: Urex Plus ‐ containing L. rhamnosus GR‐1 and L. reuteri RC‐14 Placebo: capsule with no active ingredient |
| Outcomes | Primary outcome measure
Secondary outcome measures
|
| Starting date | December 2017 |
| Contact information | HaEmek Medical Center, Israel Enav Yefet, MD/PhD, enavy1@gmail.com, +972‐52‐3862160 ext +972‐52 Zohar Nachum, MD, nachum.zo@gmail.com, +972‐54‐7696562 ext +972‐54 |
| Notes |
NCT02692820.
| Trial name or title | Preventing preterm birth with probiotics (PrePro) |
| Methods | Double‐blind, randomized, pilot trial |
| Participants | Inclusion criteria: women aged 16 years and over at the time of the booking appointment and are between 9 to 14 weeks' gestation at the time of the dating scan. Exclusion criteria: lack of informed, written consent |
| Interventions | Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 (each at 2.5 x 10^9 CFUs) in a gelatin capsule once daily vs. placebo in a gelatin capsule. |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2016 |
| Contact information | Khalid S Khan, Phd; Queen Mary University of London Rehan Khan, MD; Barts Health NHS |
| Notes | Estimated Study Completion Date: June 2017 NCT02692820 |
NCT02693028.
| Trial name or title | Lactobacillus reuteri feasibility study on probiotic lozenges, probiotic chewing gum and probiotic capsules and perinatal microbiome seeding during pregnancy |
| Methods | Double‐blind, randomized controlled trial |
| Participants | Pregnant women enrolled at 28 to 36 weeks' gestation. |
| Interventions |
Lactobacillus reuteri lozenges, capsules or chewing gum vs. placebo lozenges, capsules or chewing gum. Enrollment will be done in gestational week 28 to 36. The treatment will last for the rest of pregnancy and six weeks' postpartum |
| Outcomes | Primary outcome measures Presence of Lactobacillus reuteri in different compartments in the mother and the child. In the mother from weeks 28 to 36 of pregnancy until 6 weeks' postpartum and in the child from birth till 4 years old. Both cultivation techniques for the specific bacteria and metagenomic techniques will be used to be able to study both cultivable and non‐cultivable bacteria. Secondary outcome measures Levels of Toll‐like receptor stimulated with Lactobacillus plantarum, Pseudomonas aeruginosa and LPS after probiotic treatment and after placebo in different maternal and neonatal compartments. Time frame in mothers from weeks 28 to 36 of pregnancy until 6 weeks' postpartum and in the child from birth till 4 years old. Levels of IL‐6, IL‐10, IL‐1beta, MCP‐1, IL‐17, TNF‐alfa after probiotic treatment and after placebo in different maternal and neonatal compartments. |
| Starting date | November 2015 |
| Contact information | Bo Jacobsson, bo.jacobsson@obgyn.gu.se, +46313436719 Maria Hallingstrom, midwife, maria.hallingstrom@vgregion.se, +46 31 343 67 31 Sahlgrenska University Hospital Gothenburg, Sweden |
| Notes | Estimated Study Completion Date: November 2018 |
NCT02693041.
| Trial name or title | Pregnancy complications ‐ a probiotic interventional study |
| Methods | Randomized, placebo‐controlled trial |
| Participants | Pregnant women at least 18 years old, pregnant with one fetus, generally healthy between pregnancies, or in general if nulliparous. Three types of participants: pregnant women without a history of preterm birth or pre‐eclampsia, pregnant women with a history of spontaneous preterm birth, or pregnant women with a history of pre‐eclampsia |
| Interventions | Probiotic capsules with Lactobacillus rhamnosus (LGG) (> 10 x 8 CFU), maltodextrin, and vegetal magnesium stearate. Placebo capsule: maltodextrin, and vegetal magnesium stearate. Protocol seems to imply this intervention is started before 18 weeks, but this is not explicitly stated. |
| Outcomes | Primary outcomes Number of women who deliver before 37.0 weeks Number of women who develop pre‐eclampsia Secondary outcomes Levels of Toll‐like receptors at 25 and 35 weeks' gestation |
| Starting date | June 2012 |
| Contact information | Bo Jacobsson, professor Gothenburg, Sweden, 416 85 +46313436719 bo.jacobsson@obgyn.gu.se Sahlgrenska University Hospital, Sweden |
| Notes | Gothenburg, Sweden, 416 85 |
NCT02768818.
| Trial name or title | Modulation of the intestinal flora With the probiotic VIVOMIXX™ in pregnant women at risk of metabolic complications |
| Methods | Prospective, multicenter, randomized, double‐blind, placebo‐controlled trial. |
| Participants | Pregnant women aged between 20 and 40 with singleton pregnancies and BMI at recruitment > 30 kg/m^2 or a BMI > 25 kg/m^2 and the simultaneous presence of at least 1 of the following risk factors: age > 35 years, previous fetal macrosomia (> 4500 g), family history of diabetes (first‐degree relative with type 2 diabetes mellitus), previous GDM. |
| Interventions | Probiotic VIVOMIXX™ vs. probiotic |
| Outcomes | Primary outcome measures Maternal and newborn fecal microbiota changes (bifidobacteria and lactobacilli) and related enzymatic activity (alkaline sphingomyelinase and alkaline phosphatase) modifications (Time frame: at baseline, at 24 to 26 weeks, at 36 to 38 weeks, 2 to 3 days after delivery) NMR‐based metabolomics, microbiota and high performance liquid chromatography‐mass spectrometry ‐HPLC/MS‐ of vitamins analysis Glucose metabolism changes (Time frame: at 24 to 26 weeks), positive OGTT. Secondary outcome measures Measured at baseline: at 24 to 26 weeks, at 36 to 38 weeks, 2 to 3 days after delivery. Weight changes measured in kg, maternal BMI measured in kg/m^2, waist/hip circumference ratio, gestational weight gain measured in kg, HOMA Index, HbA1c, glycemia, insulinemia, homocysteine plasmatic levels. Requirement for insulin therapy with recording of week of onset, requirement for insulin therapy, recording the dose of insulin, quality of sleep (hours of deep sleep measured through the armband), duration of sleep, measured in hours through the armband Measured at delivery: onset of hypertension/pre‐eclampsia, time of delivery, mode of delivery, complications during delivery including surgery and/or hemorrhage > 500 mL and/or shoulder dystocia, Apgar scores, newborn's weight, newborn's sex. Other measures Newborn's abdomen/head ratio, newborn's skinfold thickness at birth, neonatal hypoglycemia the first day of life, neonatal hypoglycemia (measured in mg/dL) that requires therapy, neonatal bilirubinemia, neonatal complications, RDS, NEC, ROP, bronchopulmonary dysplasia, neonatal death, admission to NICU, duration of stay in the NICU. |
| Starting date | January 2016 |
| Contact information | Fabio Fachhinetti, MD facchi@unimore.it 0039 0594222512 Mother‐Infant Department, University of Modena and Reggio Emilia, Italy Modena, Italy, 41124 |
| Notes | The investigators aimed at evaluating the effectiveness of probiotics ingestion in changing maternal microbiota and preventing gestational diabetes in overweight and obese women. |
NCT02795845.
| Trial name or title | Oral probiotics for the treatment and prevention of vulvovaginal infections in pregnancy ‐ double‐blind, randomized, placebo‐controlled study |
| Methods | Double‐blind, randomized, placebo‐controlled study |
| Participants | Pregnant women prior to 30 weeks' gestation |
| Interventions | Probiotic capsule containing L. acidophilus, L. Paracasei, L. Rhamnosus, S. thermophilus and B. bifidum vs. placebo. |
| Outcomes | Primary outcome measures The degree of vaginal lactobacilli colonization in the probiotic formula group versus placebo once a month until week 36.6 of labor. Lactobacilli culture will be made from a vaginal specimen. The pattern of bacterial growth will be used for a semi‐quantitative interpretation in a scale of 0 (no vaginal colonization) to 4 (substantial colonization). The rate of women with normal vaginal flora at enrollment, who developed any vaginal infection during the study period until delivery in the probiotic formula group versus placebo from the date of randomization until the date of first documented episode or until delivery (around 4 months). The rate of women with normal vaginal flora at enrollment, who developed AVF/BV during the study period until delivery in the probiotic formula group versus placebo. The rate of women with AVF/BV at enrollment whose infection was eradicated following antibiotics, who developed any vaginal infection during the study period until delivery in the probiotic formula group versus placebo. The rate of women with AVF/BV at enrollment whose infection was eradicated following antibiotics, who developed AVF/BV during the study period until delivery in the probiotic formula group versus placebo. The rate of women with AVF/BV at enrollment whose infection was not eradicated following antibiotics, who restored the normal vaginal flora during the study period until delivery in the probiotic formula group versus placebo. The rate of women with VVC at enrollment whose infection was eradicated following antimycotic treatment, who developed any vaginal infection during the study period until delivery in the probiotic formula group versus placebo. The rate of women with VVC at enrollment whose infection was eradicated following antimycotic treatment, who developed VVC during the study period until delivery in the probiotic formula group versus placebo. Secondary outcome measures Duration of time from the beginning of the study until an episode of vaginal infection (either AVF/BV or VVC). The number of episodes of vaginal infections during pregnancy (either AVF/BV or VVC). The rate of women, who suffer from obstetrical complications including preterm labor, intrauterine growth restriction (IUGR), PPROM, chorioamnionitis, postpartum fever, postpartum endometritis. The rate and type of adverse effects in the probiotic versus placebo groups (e.g. gastrointestinal symptoms). Number of UTIs during the study period. The rate of neonatal complications including neonatal acute RDS, IVH, neonatal sepsis, admission to the NICU. |
| Starting date | November 2016 |
| Contact information | Enav Yefet, MD/PhD HaEmek Medical Center, Israel +972‐4‐6494516 enavy1@gmail.com |
| Notes | Anticipated end date: September 2018 |
NCT02912416.
| Trial name or title | Efficacy of probiotics on iron status during pregnancy |
| Methods | Randomized, double‐blind, controlled trial |
| Participants | Healthy, pregnant women, aged 18 to 42 years, with a singleton gestation, enrolled at 10 to 12 weeks of gestation, and with a BMI ≥ 18 and ≤ 30 |
| Interventions | Probiotic vs. placebo |
| Outcomes | Primary outcome measures: serum ferritin level at 28 weeks of gestation |
| Starting date | September 2016 |
| Contact information | Lena Hulthén, Prof lena.hulthén@medfak.gu.se +46 31 786 3714 |
| Notes | Anticipated end date: June 2018 |
NCT03008421.
| Trial name or title | Oral probiotics to reduce vaginal Group B streptococcal colonization in late pregnancy |
| Methods | Randomized, placebo‐controlled. double‐blinded trial |
| Participants | Pregnant women between. 34 + 0 and 36 + 0 gestational weeks with a positive GBS screen and no current vaginal infection |
| Interventions | Astarte® vs. placebo |
| Outcomes | Primary outcome measures GBS status (Time frame: 2 weeks) Eradication of vaginal colonization with group B Streptococcus (GBS) at study visit 2 Secondary outcome measures Neonatal sepsis (Time frame: < 7 days vs. ≥ 7 days postpartum (early vs. late‐onset)). Gestational age at delivery. Term delivery at or after 37 + 0 gestational weeks vs. preterm delivery at or before 36 + 6 gestational weeks Neonatal birth weight Live birth Delivery mode |
| Starting date | February 2017 |
| Contact information | Alex Farr, MD PhD, Assistant Professor, Medical University of Vienna alex.farr@meduniwien.ac.at; +43140400 ext 28220 |
| Notes | Anticipated end date: January 2018 |
NCT03215784.
| Trial name or title | Gestational Oobesity and interventions with Probiotics or Fish Oil Trial (GOPROFIT) |
| Methods | Randomized controlled trial |
| Participants | Pregnant women ages 19 to 40 with a gestational age of 13 weeks and BMI prepregnancy greater than 29.9 kg/m² and less than 40 kg/m², or pre‐pregnancy BMI between 18.5 kg/m² and 24 9 kg/m² |
| Interventions | Probiotic, placebo or fish oil from 28 to 36 weeks' gestation |
| Outcomes | Primary outcome measures Association between maternal obesity and systemic Inflammation in the Mother and the Activation of Placental Inflammatory Pathways at 28 weeks. Association between fish oil intake in obese mothers and systemic Inflammation in the Mother and the Activation of Placental Inflammatory Pathways at 28 weeks. Investigation of the effects of fish oil supplementation on the metabolome of maternal serum, umbilical cord serum and placenta in obese pregnant women at 28 weeks. The metabolome of maternal serum, umbilical cord serum and placenta of obese and healthy pregnant women was analyzed by nuclear magnetic resonance. Association between probiotic supplementation and bacterial colonization in the maternal intestinal and vaginal microbiota at 36 weeks. Evaluation of the maternal intestinal and vaginal microbiota through the quantification of bacteria. Secondary outcome measures Association between maternal obesity and placental fatty acid transporter expression at 28 weeks. Association between fish oil intake in obese mothers and placental fatty acid transporter at 28 weeks. Association between maternal inflammatory status and pregnancy outcomes at 28 weeks. |
| Starting date | March 2015 |
| Contact information | Study Director: Fátima Lúcia C Sardinha, PhD, Instituto de Nutrição Josué de Castro/ UFRJ sardinhaflc@nutricao.ufrj.br; (55) 21 996355046 Contact: Lívia B Almeida, Ms (55) 21 991689325 livia.belcastro@gmail.com Maternidade Escola da Universidade Federal do Rio de Janeiro Rio de Janeiro, RJ, Brazil, 22240‐000 |
| Notes | Anticipated end date: December 2017 |
BMI: body mass index; BV: bacterial vaginosis; CFU: colony‐forming unit; GBS: Group B Streptococci; GDM: gestational diabetes mellitus; HOMA: homeostasis model assessment; IUGR: intrauterine growth restriction; LGA: large for gestational age; NEC: necrotizing enterocolitis; NICU: neonatal intensive care unit; OGTT: oral glucose tolerance test; LPS: lipopolysaccharides; PPROM: preterm premature rupture of membranes; PROM: premature rupture of membranes; RCT: randomized controlled trial; RDS: respiratory distress syndrome; ROP: retinopathy of prematurity; SGA: small for gestational age; UTI: urinary tract infection
Differences between protocol and review
The protocol stated that for comparison 1, studies must include mothers at risk for preterm birth to be included in this review. Since there could be a wide array of reasons for mothers to be at risk for preterm birth, this criteria was intentionally left open‐ended so as not to curtail inclusion of studies in which pregnant women were at risk for preterm birth, but with conditions not on a defined list. However, in a post‐hoc discussion, it was felt that to some extent, every pregnant woman is at risk for preterm birth, and that studies that fit the other criteria but did not include a higher risk for preterm birth should be included, and that those at specifically higher risk for preterm birth should be examined in subgroup analyses. This decision to include this broader population yields the most data about the effect of probiotics on the risk for preterm birth, while the subgroup analysis allowed for refined analysis by risk category.
Post hoc the authors decided to do a subgroup analysis of when probiotics were started in pregnant women, at < 20 weeks' gestation or > 20 weeks' gestation.
The specified probiotics did not included Streptococcus, but one study (Dolatkhah 2015) had a mixed probiotic that included Streptococcus, which was included due to the mixed probiotic formulation that overall fit the goal of this review. Incidentally, this study did not report on outcomes of interest and contributed no data to the review or meta‐analysis.
The post‐hoc decision was made to exclude studies that administered probiotics starting at 36 weeks' gestation to pregnant women, as the majority of infants of these mothers would be born at term. Thus, the inclusion was changed from probiotics versus placebo or no intervention given to mothers < 37 weeks' gestation, to < 36 weeks' gestation. The population of interest in this review is preterm infants, and the outcomes of interest relate to prematurity. Including these studies would not fit with the overall goal of this review and with the large body of literature that fits this criteria, listing the studies is beyond the scope of this review.
In a review of the literature, several secondary outcomes were added, specifically, any necrotizing enterocolitis (NEC), surgery for NEC, neonatal sepsis, death or NEC, and days to 50% enteral feeds (because these are the outcomes that the one study of interest in comparison 2 reported) and the outcome of miscarriage/stillbirth (for comparison 1).
In addition, in the protocol, the intention for comparison 1 was to make a distinction between studies in which pregnant women receive probiotics during pregnancy only, and those in which the mother receives probiotics during pregnancy and after birth of their preterm infant. Two of the five studies in which the mother receives probiotics after birth, certain subsets of infants also receive probiotics directly, so the decision was made to only analyze the outcomes that pertain to administration in the pregnancy period.
We added several clinically important outcomes to the 'Summary of findings' tables based on review of available studies post hoc: For Comparison 1: Gestational age at birth (weeks); Miscarriage/stillbirth; For Comparison 2: Any NEC, Surgery for NEC, Feeding with more than 50% breast milk (days).
Our plan for subgroup analysis was refined during the review process. As per our original plan, the following subgroup analyses were performed: 1. Probiotic type: Lactobacillus sp., Bifidobacterium sp., Saccharomyces, or mixed preparations of these probiotics; 2. Economic country setting (low‐income, lower‐middle income, upper‐middle income and high‐income economies as defined by the World Bank (The World Bank 2016). In addition, a post hoc subgroup analysis was performed based on maternal gestational age at enrollment. There were insufficient data to perform subgroup analyses based upon birth weight, race, ethnicity, sex, provision of breast milk, and maternal risk for preterm birth. We incorrectly stated that we would perform sensitivity analyses based on " quality of evidence based on GRADE" when in fact we performed our sensitivity analyses based on risk of bias.
Contributions of authors
Jacquelyn Grev is the primary author, designed the protocol, assessed the titles and abstracts, extracted, assessed, and coded all data for each study as well as wrote and edited the text.
Marie Berg edited and reviewed the protocol and the review.
Roger Soll edited and reviewed the protocol and assessed the titles and abstracts, extracted, assessed, and coded all data for each study and edited the text.
Sources of support
Internal sources
University of Vermont Medical Center, USA.
External sources
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
JG has no interests to declare.
MB has no interests to declare.
RS has no interests to declare.
New
References
References to studies included in this review
Badehnoosh 2018 {published data only}
- Badehnoosh B, Karamali M, Zarrati M, Jamilian M, Bahmani F, Tajabadi‐Ebrahimi M, et al. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. Journal of Maternal‐fetal & Neonatal Medicine 2018;31(9):1128‐36. [DOI: 10.1080/14767058.2017.1310193; PUBMED: 28326881] [DOI] [PubMed] [Google Scholar]
Benor 2014 {published data only}
- Benor S, Marom R, Ben Tov A, Armoni Domany K, Zaidenberg‐Israeli G, Dollberg S. Probiotic supplementation in mothers of very low birth weight infants. American Journal of Perinatology 2014;31(6):497‐504; Erratum in: American Journal of Perinatology: 2014: 31(6):e1. [DOI: 10.1055/s-0033-1353490; NCT00835874; PUBMED: 23934538] [DOI] [PubMed] [Google Scholar]
- Benor S, Marom R, Ben Tov A, Yarkoni I, Domany KA, Zaidenberg‐Israeli GA, et al. Probiotic supplementation in mothers of preterm infants: a randomized double blind, placebo controlled trial. Pediatric Academic Societies Annual Meeting; 2011 April 30‐ May 03; Denver, Colorado USA. 2011.
- NCT00835874. Probiotic administration to mothers of preterm infants to prevent necrotizing enterocolitis and sepsis. clinicaltrials.gov/show/NCT00835874 (first received 04 February 2009). [NCT00835874]
Dolatkhah 2015 {published data only}
- Dolatkhah N, Hajifaraji M, Abbasalizadeh F, Aghamohammadzadeh N, Mehrabi Y, Mesgari Abbasi M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. Journal of Health, Population, and Nutrition 2015;33(25):1‐8. [DOI: 10.1186/s41043-015-0034-9; PUBMED: 26825666] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández 2016 {published data only}
- Fernández L, Cárdenas N, Arroyo R, Manzano S, Jiménez E, Martín V, et al. Prevention of infectious mastitis by oral administration of lactobacillus salivarius PS2 during late pregnancy. Clinical Infectious Diseases 2016;62(5):568‐73. [DOI: 10.1093/cid/civ974; NCT01505361; PUBMED: 26611780] [DOI] [PubMed] [Google Scholar]
Jacobsson 2016 {published data only}
- Jacobsson B, Sengpiel V, Nilsson S, Hallingstrom M, Elfvin A, Brantsaeter AL, et al. Immunological and inflammatory response after antenatal supplementation with lactobacillus rhamnosus in low‐risk pregnant women. Reproductive Sciences 2016;23(Suppl 1):293A. [Google Scholar]
Kopp 2008 {published data only}
- Kopp MV, Goldstein M, Dietschek A, Sofke J, Heinzmann A, Urbanek R. Lactobacillus GG has in vitro effects on enhanced interleukin‐10 and interferon‐gamma release of mononuclear cells but no in vivo effects in supplemented mothers and their neonates. Clinical and Experimental Allergy 2008;38(4):602‐10. [DOI: 10.1111/j.1365-2222.2007.02911.x; PUBMED: 18167121] [DOI] [PubMed] [Google Scholar]
Laitinen 2009 {published data only}
- Aaltonen J, Ojala T, Laitinen K, Piirainen TJ, Poussa TA, Isolauri E. Evidence of infant blood pressure programming by maternal nutrition during pregnancy: a prospective randomized controlled intervention study. Journal of Pediatrics 2008;152(1):79‐84. [DOI: 10.1016/j.jpeds.2007.05.048; PUBMED: 18154905] [DOI] [PubMed] [Google Scholar]
- Aaltonen J, Ojala T, Laitinen K, Poussa T, Ozanne S, Isolauri E. Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming:a prospective randomized controlled study. European Journal of Clinical Nutrition 2011;65(1):10‐9. [DOI: 10.1038/ejcn.2010.225; PUBMED: 20948557] [DOI] [PubMed] [Google Scholar]
- Hoppu U, Isolauri E, Koskinen P, Laitinen K. Maternal dietary counseling reduces total and LDL cholesterol postpartum. Nutrition 2014;30(2):159‐64. [DOI: 10.1016/j.nut.2013.07.009; PUBMED: 24176529] [DOI] [PubMed] [Google Scholar]
- Hoppu U, Isolauri E, Laakso P, Matomäki J, Laitinen K. Probiotics and dietary counselling targeting maternal dietary fat intake modifies breast milk fatty acids and cytokines. European Journal of Nutrition 2012;51(2):211‐9. [DOI 10.1007/s00394‐011‐0209‐0; PUBMED: 21626296] [DOI] [PubMed] [Google Scholar]
- Huurre A, Laitinen K, Rautava S, Korkeamäki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double‐blind placebo‐controlled study. Clinical and Experimental Allergy 2008;38(8):1342‐8. [DOI: 10.1111/j.1365-2222.2008.03008.x; PUBMED: 18477013] [DOI] [PubMed] [Google Scholar]
- Ilmonen J, Isolauri E, Poussa T, Laitinen K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo‐controlled trial. Clinical Nutrition (Edinburgh, Scotland) 2011;30(2):156‐64. [DOI: 10.1016/j.clnu.2010.09.009; PUBMED: 20970896] [DOI] [PubMed] [Google Scholar]
- Kaplas N, Isolauri E, Lampi AM, Ojala T, Laitinen K. Dietary counseling and probiotic supplementation during pregnancy modify placental phospholipid fatty acids. Lipids 2007;42(9):865‐70. [DOI: 10.1007/s11745-007-3094-9] [DOI] [PubMed] [Google Scholar]
- Laitinen K, Ilmonen J, Isolauri E. Dietary counselling and probiotic intervention during pregnancy modify postpartum adiposity. Annals of Nutrition and Metabolism 2011;58(suppl 3):87. [Google Scholar]
- Laitinen K, Poussa T, Isolauri E, Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. British Journal of Nutrition 2009;101(11):1679–87. [DOI: 10.1017/S0007114508111461; PUBMED: 19017418] [DOI] [PubMed] [Google Scholar]
- Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic‐supplemented dietary counselling during pregnancy oncolostrum adiponectin concentration: a prospective, randomized,placebo‐controlled study. Early Human Development 2012;88(6):339‐44. [DOI: 10.1016/j.earlhumdev.2011.09.006; NCT00167700; PUBMED: 21945174] [DOI] [PubMed] [Google Scholar]
- Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic‐supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double‐blind, placebo‐controlled study. British Journal of Nutrition 2010;103(12):1792‐9. [DOI: 10.1017/S0007114509993898; PUBMED: 20128938] [DOI] [PubMed] [Google Scholar]
- Luoto R, Nermes M, Laitinen K, Isolauri E. Impact of maternal probiotic supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double blind, placebo‐controlled study. Pediatric Academic Societies' Annual Meeting; 2009 May 2‐5; Baltimore MD, United States. 2009. [2515.3] [DOI] [PubMed]
- Piirainen T, Isolauri E, Lagstrom H, Laitinen K. Impact of dietary counselling on nutrient intake during pregnancy: a prospective cohort study. British Journal of Nutrition 2006;96(6):1095‐104. [PUBMED: 17181885] [DOI] [PubMed] [Google Scholar]
- Vähämiko S, Isolauri E, Laitinen K. Weight status and dietary intake determine serum leptin concentrations in pregnant and lactating women and their infants. British Journal of Nutrition 2013;110(6):1098‐106. [DOI: 10.1017/S0007114513000214; PUBMED: 23432806] [DOI] [PubMed] [Google Scholar]
Lindsay 2014 {published data only}
- ISRCTN97241163. Probiotics in pregnancy study (ProP Study). isrctn.com/ISRCTN97241163 (first received 15 February 2012). [DOI: 10.1186/ISRCTN97241163] [DOI]
- Lindsay K, Brennan L, Kennelly M, Curran S, Coffey M, Smith T, et al. Influence of maternal metabolic response to gestational diabetes treatment on neonatal outcomes. American Journal of Obstetrics and Gynecology 2015;212(1):S247‐8. [DOI] [PubMed] [Google Scholar]
- Lindsay K, Maguire O, Smith T, Brennan L, McAuliffe F. A randomized controlled trial of probiotics to reduce maternal glycaemia in obese pregnancy. American Journal of Obstetrics & Gynecology 2014;210(1):S342. [Google Scholar]
- Lindsay KL, Brennan L, McAuliffe FM. Acceptability of and compliance with a probiotic capsule intervention in pregnancy. International Journal of Gynecology and Obstetrics 2014;125:279‐84. [DOI] [PubMed] [Google Scholar]
- Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, Shanahan F, et al. Probiotics in obese pregnancy do not reduce maternal fasting glucose:a double‐blind, placebo‐controlled, randomized trial (Probiotics in Pregnancy Study). American Journal of Clinical Nutrition 2014;99(6):1432‐9. [DOI: 10.3945/ajcn.113.079723; PUBMED: 24646819] [DOI] [PubMed] [Google Scholar]
- Lindsay KL, Kennelly M, Smith T, Maguire OC, Shanahan F, Brennan L, et al. Probiotics in obese pregnancy to reduce maternal fasting glucose: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(Suppl 1):A156. [DOI: 10.1136/archdischild-2014-306576.459] [DOI] [PubMed] [Google Scholar]
Lindsay 2015 {published data only}
- Lindsay KL, Brennan L, Kennelly MA, Maguire OC, Smith T, Curran S, et al. Impact of probiotics in women with gestationaldiabetes mellitus on metabolic health: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2015;212(496):e1‐11. [DOI: 10.1016/j.ajog.2015.02.008; PUBMED: 25687568] [DOI] [PubMed] [Google Scholar]
Mantaring 2016 {published data only}
- Guinto VT, Destura R, Volger S, Vidal K, Pecquet S, Mantaring III JV. Randomized controlled study of nutritional supplement beverages with and without probiotics taken during the third trimester of pregnancy: effects on maternal and fetal outcomes and fetal immune status. International Journal of Gynaecology and Obstetrics 2015;131(Suppl 5):E314. [Google Scholar]
- Mantaring J, Benyacoub J, Destura R, Pecquet S, Vidal K, Volger S, et al. A nutritional supplement beverage with and without probiotics during the third trimester of pregnancy and lactation show benefit on infant growth up to 12 months of age in the Philippines. Journal of Pediatric Gastroenterology and Nutrition 2016;62(Suppl 1):706. [Google Scholar]
Ou 2012 {published data only}
- Kuo H, Yang K, Ou C. Antenatal probiotics reduces maternal but not childhood atopic diseases: a randomised, double‐blind, placebo‐controlled trial. Allergy 2012;67(Suppl 96):66‐7. [DOI] [PubMed] [Google Scholar]
- Ou C, Kuo HC, Wang L, Hsu TY, Chuang H, Liu CA, et al. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: a randomized, double‐blind, placebo‐controlled trial. Clinical and Experimental Allergy 2012;42(9):1386‐96. [DOI: 10.1111/j.1365-2222.2012.04037.x; NCT00325273; PUBMED: 22925325] [DOI] [PubMed] [Google Scholar]
Rautava 2012 {published data only}
- Rautava S, Kainonen E, Salminen S, Isolauri E. Maternal probiotic supplementation during pregnancy and breast‐feeding reduces the risk of eczema in the infant. Journal of Allergy and Clinical Immunology 2012;130(6):1355‐60. [DOI: 10.1016/j.jaci.2012.09.003; PUBMED: 23083673] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asemi 2011 {published data only}
- Asemi A, Jazayeri S, Najafi M, Samimi M, Mofid V, Shidfar F, et al. Effects of daily consumption of probiotic yoghurt on inflammatory factors in pregnant women: a randomized controlled trial. Pakistan Journal of Biological Sciences: PJBS 2011;14(8):476‐82. [PUBMED: 21936251] [DOI] [PubMed] [Google Scholar]
Asemi 2012 {published data only}
- Asemi Z, Samimi M, Tabasi Z, Talebian P, Azarbad Z, Hydarzadeh Z, et al. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. Journal of Maternal‐fetal & Neonatal Medicine 2012;25(9):1552‐6. [DOI: 10.3109/14767058.2011.640372; PUBMED: 22098090] [DOI] [PubMed] [Google Scholar]
Bisanz 2014 {published data only}
- Bisanz JE, Enos MK, Mwanga JR, Changalucha J, Burton JP, Gloor GB, et al. Randomized open‐label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 2014;5(5):e01580‐14. [DOI: 10.1128/mBio.01580-14; PUBMED: 25293764 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daskalakis 2014 {published data only}
- Daskalakis G, Karampelas A, Gavrili V, Papantoniou N, Angelopoulos P, Ntomali A, et al. Probiotics for preterm premature rupture of membranes. Journal of Maternal‐fetal & Neonatal Medicine 2014;27(Suppl 1):370. [DOI: 10.3109/14767058.2014.924236] [DOI] [Google Scholar]
Gonai 2014 {published data only}
- Gonai M, Nakadaira I, Kurasaki K, Hamano K. The effects of lactobacilli on glycemic control and the secretion of glucagon‐like peptide‐1 in Japanese gestational diabetes mellitus patients. Diabetes 2014;63(Suppl 1):A3333. [Google Scholar]
Gronlund 2011 {published data only}
- Grönlund MM, Grześkowiak L, Isolauri E, Salminen S. Influence of mother's intestinal microbiota on gut colonization in the infant. Gut Microbes 2011;2(4):227‐33. [DOI: 10.4161/gmic.2.4.16799; PUBMED: 21983067] [DOI] [PubMed] [Google Scholar]
Grzeskowiak 2012 {published data only}
- Grzeskowiak L, Grönlund MM, Beckmann C, Salminen S, Berg A, Isolauri E. The impact of perinatal probiotic intervention on gut microbiota: double‐blind placebo‐controlled trials in Finland and Germany. Anaerobe 2012;18(1):7‐13. [DOI: 10.1016/j.anaerobe.2011.09.006; NCT00167700; PUBMED: 21979491] [DOI] [PubMed] [Google Scholar]
Hanson 2014 {published data only}
- Hanson L, VandeVusse L, Duster M, Warrack S, Safdar N. Feasibility of oral prenatal probiotics against maternal group B streptococcus vaginal and rectal colonization. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2014;43(3):294‐304. [DOI: 10.1111/1552-6909.12308; PUBMED: 24754328] [DOI] [PubMed] [Google Scholar]
- Hanson L, VandeVusse L, Safdar N, Duster M, Warrack S, Panjikar P. Effects of probiotic use during pregnancy on lactobacillus and Group B streptococcus vaginal colonization: pilot results. Journal of Midwifery & Women's Health 2012;57(5):537. [Google Scholar]
- Warrack SR, Hanson L, Vusse LV, Duster M, Panjikar P, Safdar N. The impact of prenatal probiotics on group B streptococcus colonization. American Journal of Infection Control 2013;41(6 Suppl 1):S142. [Google Scholar]
Hantoushzadeh 2012 {published data only}
- Hantoushzadeh S, Golshahi F, Javadian P, Khazardoost S, Aram S, Hashemi S, et al. Comparative efficacy of probiotic yoghurt and clindamycin in treatment of bacterial vaginosis in pregnant women: a randomized clinical trial. Journal of Maternal‐fetal & Neonatal Medicine 2012;25(7):1021‐4. [DOI: 10.3109/14767058.2011.614654; PUBMED: 21854132] [DOI] [PubMed] [Google Scholar]
Jain 2017 {published data only}
- Jain SK, Jain S, Saade G. Safety and efficacy of oral probiotics in pregnant women. American Journal of Obstetrics and Gynecology 2017;216(1 Supple 1):S523‐4. [Google Scholar]
Jamilian 2016 {published data only}
- Jamilian M, Bahmani F, Vahedpoor Z, Salmani A, Tajabadi‐Ebrahimi M, Jafari P. Effects of probiotic supplementation on metabolic status in pregnant women: a randomized, double‐blind, placebo‐controlled trial. Archives of Iranian Medicine 2016;19(10):687‐92. [DOI: ; PUBMED: 27743432] [PubMed] [Google Scholar]
Karampelas 2013 {published data only}
- Karampelas A, Daskalakis G, Papantoniou N, Gavrili V, Mesogitis S, Angelopoulos P, et al. Probiotics for preterm premature rupture of membranes. Journal of Perinatal Medicine 2013;41(Suppl 1):205. [Google Scholar]
Krauss‐Silva 2011 {published data only}
- Krauss‐Silva L, Moreira ME, Alves MB, Braga A, Camacho KG, Batista MR. A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: preliminary results. Trials 2011;12:239. [DOI: 10.1186/1745-6215-12-239; PUBMED: 22059409] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss‐Silva L, Moreira MEL, Alves MB, Rezende MR, Braga A, Camacho KG, et al. Randomized controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with intrauterine infection: study protocol. Reproductive Health 2010;7:14. [DOI: 10.1186/1742-4755-7-14; PUBMED: 20591191] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00303082. Probiotics for the prevention of premature birth and neonatal related morbidity. clinicaltrials.gov/show/NCT00303082 (first received 15 March 2006). [NCT00303082]
Nishijima 2005 {published data only}
- Nishijima K, Shukunami K, Kotsuji F. Probiotics affects vaginal flora in pregnant women, suggesting the possibility of preventing preterm labor. Journal of Clinical Gastroenterology 2005;39(5):447‐8. [PUBMED: 15815217] [DOI] [PubMed] [Google Scholar]
Ortiz‐Andrellucchi 2008 {published data only}
- Ortiz‐Andrellucchi A, Sánchez‐Villegas A, Rodríguez‐Gallego C, Lemes A, Molero T, Soria A, et al. Immunomodulatory effects of the intake of fermented milk with Lactobacillus casei DN114001 in lactating mothers and their children. British Journal of Nutrition 2008;100(4):834‐45. [DOI: 10.1017/S0007114508959183; PUBMED: 18341756] [DOI] [PubMed] [Google Scholar]
Vitali 2012 {published data only}
- Vitali B, Cruciani F, Baldassarre ME, Capursi T, Spisni E, Valerii MC. Dietary supplementation with probiotics during late pregnancy: outcome on vaginal microbiota and cytokine secretion. BMC Microbiology 2012;12:12. [DOI: 10.1186/1471-2180-12-236; NCT01367470; PUBMED: 23078375] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wickens 2008 {published data only}
- Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. Probiotic Study Group. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double‐blind, randomized, placebo controlled trial. Journal of Allergy and Clinical Immunology 2008;122(4):788‐94. [DOI: 10.1016/j.jaci.2008.07.011; PUBMED: 18762327] [DOI] [PubMed] [Google Scholar]
- Wickens K, Stanley TV, Mitchell EA, Barthow C, Fitzharris P, Purdie G. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitization?. Clinical and Experimental Allergy 2013;43(9):1048‐57. [DOI: 10.1111/cea.12154; PUBMED: 23957340] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12611001208998 {published data only}
- ACTRN12611001208998. Probiotics for the prevention of gestational diabetes in overweight and obese women [Randomized placebo controlled trial of probiotics in overweight and obese women to assess the prevention of gestational diabetes]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=347738&isReview=true (first received 21 November 2011).
- Nitert MD, Barrett HL, Foxcroft K, Tremellen A, Wilkinson S, Lingwood B, et al. SPRING: an RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy and Childbirth 2013;13(50):1‐7. [DOI: 10.1186/1471-2393-13-50; PUBMED: 23442391] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12612000196842 {published data only}
- ACTRN12612000196842. A randomized placebo controlled trial of the effects of the probiotic Lactobacillus rhamnosus HN001taken from the 1st trimester of pregnancy till 6 months post partum, if breastfeeding, on the development of eczema and atopic sensitization in infants by age 12 months. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362049&isReview=true (first received 07 Feburary 2012).
- Barthow C, Wickens K, Stanley T, Mitchell EA, Robyn Maude R, Abels P, et al. The Probiotics in Pregnancy Study (PiPStudy): rationale and design of a double blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy and Childbirth 2016; Vol. 16, issue 1:133. [DOI: 10.1186/s12884-016-0923-y; PUBMED: 27255079] [DOI] [PMC free article] [PubMed]
ACTRN12615000400561 {published data only}
- ACTRN12615000400561. A randomised controlled trial of nutritional interventions in obese pregnant women to optimise maternal pregnancy weight gain and infant birthweight: a demonstration study. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368302&isReview=true (first received 10 April 2015).
- Okesene‐Gafa K, LI M, Taylor RS, Thompson JM, Crowther CA, McKinlay CJ, et al. A randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial. BMC Pregnancy and Childbirth 2016;16(1):373. [DOI: 10.1186/s12884-016-1149-8; PUBMED: 27884128] [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2013/04/003577 {published data only}
- CTRI/2013/04/003577. Effects of oral probiotic supplementation during pregnancy and lactation in modulating immune response in breast milk, fetus, and neonate‐ a randomised double blind controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/04/003577 (first received 23 April 2013).
ISRCTN53023014 {published data only}
- ISRCTN53023014. To study the transit of beneficial bacteria from mother to infant. isrctn.com/ISRCTN53023014 (first received 16 August 2016).
Mehta 2016 {published data only}
- Mehta S, DuPlessis J, Sunanda G, Darragh H. Randomized double‐blind placebo‐controlled trial of probiotics in pregnancy (PIP) and its effect on Group‐B streptococcal colonization‐ study protocol. Journal of Paediatrics and Child Health 2016;52(Supple 2):68. [Google Scholar]
NCT00217308 {published data only}
- NCT00217308. Effect of probiotic lactobacilli on vaginal flora of pregnant women at high risk for preterm delivery. clinicaltrials.gov/show/NCT00217308 (first received 22 September 2005).
NCT01436448 {published data only}
- NCT01436448. Probiotics (Lactobacillus Rhamnosus) in reducing glucose intolerance during and after pregnancy(GRIP). clinicaltrials.gov/show/NCT01436448 (first received 19 September 2011).
NCT01454661 {published data only}
- NCT01454661. Probiotics and early microbial contact in preterm neonates (ProPre). clinicaltrials.gov/show/NCT01454661 (first received 19 October 2011).
NCT01479478 {published data only}
- NCT01479478. Effects of oral probiotic supplementation on group B strep (GBS) rectovaginal colonization in pregnancy. clinicaltrials.gov/show/NCT01479478 (first received 24 November 2011).
NCT01577108 {published data only}
- NCT01577108. Oral probiotics reduce group B streptococci colonization in pregnant women. clinicaltrials.gov/show/NCT01577108 (first received 13 April 2012).
NCT01697683 {published data only}
- NCT01697683. Probiotic therapy for the reversal of bacterial vaginosis in pregnancy. clinicaltrials.gov/show/NCT01697683 (first received 02 October 2014).
NCT01779193 {published data only}
- NCT01779193. Probiotics capsule as supplemental therapy for group B Streptococci infection and vaginitis during pregnancy. Clinicaltrials.gov/show/ NCT01779193 (first received 30 January 2013).
NCT01922791 {published data only}
- NCT01922791. Nutrition and pregnancy intervention study. Clinicaltrials.gov/show/NCT01922791 (first received 14 August 2013).
NCT02377544 {published data only}
- NCT02377544. The role of Bifidobacterium Animalis ssp Lactis DR10 supplementation in women during pregnancy and lactation on breast milk IL‐8 and gut mucosa integrity in infant. Clinicaltrials.gov/show/NCT02377544 (first received 03 March 2015).
NCT02430246 {published data only}
- NCT02430246. The association between the transfer of lactobacilli from the gastrointestinal tract to the vagina and the prevention/ eradication of abnormal vaginal flora in high risk pregnancies. Clinicaltrials.gov/show/NCT02430246 (first received 30 April 2015).
NCT02508844 {published data only}
- Halkjaer SI, Nilas L, Carlsen EM, Cortes D, Halldórsson TI, Olsen SF, et al. Effects of probiotics (Vivomixx®) in obese pregnant women and their newborn: study protocol for a randomized controlled trial. Trials 2016;17(1):491. [DOI: 10.1186/s13063-016-1617-5; PUBMED: 27724923] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02508844. Effect of probiotics (Vivomixx®) on weight, microbiota and glucose tolerance in obese pregnant women and their newborn (POP). Clinicaltrials.gov/show/NCT02508844 (first received 27 July 2015).
NCT02509988 {published data only}
- NCT02509988. Nutritional intervention preconception and during pregnancy to maintain healthy glucose metabolism and offspring health (NiPPeR). clinicaltrials.gov/show/NCT02509988 (first received 10 August 2005).
NCT02528981 {published data only}
- NCT02528981. Effect of probiotics on GBS colonization status during pregnancy: a pilot randomized controlled trial. Clinicaltrials.gov/show/NCT02528981 (first received 19 August 2015).
NCT02637986 {published data only}
- NCT02637986. The efficacy of orally administrated probiotic formula in preventing a recurrence of a urinary tract infection during pregnancy. Clinicaltrials.gov/show/NCT02637986 (first received 22 December 2015).
NCT02692820 {unpublished data only}
- NCT02692820. Preventing preterm birth with probiotics (PrePro). Clinicaltrials.gov/show/NCT02692820 (first received 26 February 2016).
NCT02693028 {published data only}
- NCT02693028. Lactobacillus Reuteri feasibility study on probiotic treatment and perinatal microbiome. Clinicaltrials.gov/show/NCT02693028 (first received 26 February 2016).
NCT02693041 {published data only}
- NCT02693041. Pregnancy complications ‐ a probiotic interventional study. Clinicaltrials.gov/show/NCT02693041 (first received 26 February 2016).
NCT02768818 {published data only}
- NCT02768818. Modulation of the intestinal flora with the probiotic VIVOMIXX™ in pregnant women at risk of metabolic complications. Clinicaltrials.gov/show/NCT02768818 (first received 11 May 2016).
NCT02795845 {published data only}
- NCT02795845. Oral probiotics for the treatment and prevention of vulvovaginal infections in pregnancy ‐ double‐blind, randomized, placebo‐controlled study. Clinicaltrials.gov/show/NCT02795845 (first received 10 June 2016).
NCT02912416 {published data only}
- NCT02912416. Efficacy of probiotics on iron status during pregnancy. Clinicaltrials.gov/show/NCT02912416 (first received 23 September 2016).
NCT03008421 {published data only}
- NCT03008421. Oral probiotics to reduce vaginal group B streptococcal colonization in late pregnancy. Clinicaltrials.gov/show/NCT03008421 (first received 02 January 2017).
NCT03215784 {published data only}
- NCT03215784. Gestational obesity and interventions with probiotics or fish oil trial. Clinicaltrials.gov/show/NCT03215784 (first received 12 July 2017).
Additional references
Abrahamsson 2009
- Abrahamsson TR, Sinkiewicz G, Jakobsson T, Fredrikson M, Bjorksten B. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. Journal of Pediatric Gastroenterology and Nutrition 2009;49(3):349–54. [DOI: 10.1097/MPG.0b013e31818f091b; PUBMED: 19525871] [DOI] [PubMed] [Google Scholar]
Agostoni 2010
- Agostoni C, Buonocore G, Carnielli VP, Curtis M, Darmaun D, Decsi T, et al. ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of PaediatricGastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85‐91. [DOI: 10.1097/MPG.0b013e3181adaee0; PUBMED: 19881390] [DOI] [PubMed] [Google Scholar]
AlFaleh 2014
- AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD005496.pub4] [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergmann 2014
- Bergmann H, Rodriguez JM, Salminen S, Szajewska H. Probiotics in human milk and probiotic supplementation in infant nutrition: a workshop report. British Journal of Nutrition 2014;112(7):1119–28. [DOI: 10.1017/S0007114514001949; PUBMED: 25160058] [DOI] [PubMed] [Google Scholar]
Bertelli 2015
- Bertelli C, Pillonel T, Torregrossa A, Prod'hom G, Fischer CJ, Greub G, et al. Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clinical Infectious Diseases 2015;60(6):924‐7. [DOI: 10.1093/cid/ciu946; PUBMED: 25472946] [DOI] [PubMed] [Google Scholar]
CDC 2015
- Vallabhaneni S, Walker TA, Lockhart SR, Ng D, Chiller T, Melchreit R, et al. Centers for Disease Control and Prevention (CDC). Notes from the Field: Fatal Gastrointestinal Mucormycosis in a Premature Infant Associated with a Contaminated Dietary Supplement — Connecticut, 2014. Centers for Disease Control and Prevention; Morbidity and Mortality Weekly Report (MMWR) February 20, 2015:https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6406a6.htm Accessed 6/15/18. [PMC free article] [PubMed]
CDC HAN 2014
- Center for Disease Control and Prevention Health Alert Network. Fatal gastrointestinal mucormycosis in an infant following ingestion of contaminated dietary supplement – Connecticut, 2014. emergency.cdc.gov/han/ (accessed 27 March 2018); Vol. CDCHAN‐00373.
Cilieborg 2012
- Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Human Development 2012;88(Suppl 1):S41‐9. [DOI: 10.1016/j.earlhumdev.2011.12.027; PUBMED: 22284985] [DOI] [PubMed] [Google Scholar]
Costeloe 2016
- Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MW, Probiotics in Preterm Infants Study Collaborative Group. Bifidobacterium breve BBG‐001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 2016;387(10019):649‐60. [DOI: 10.1016/S0140-6736(15)01027-2; PUBMED: 26628328] [DOI] [PubMed] [Google Scholar]
Deshpande 2010
- Deshpande G, Rao S, Patole S, Bulsara M. Updated meta‐analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010;125(5):921‐30. [DOI: 10.1542/peds.2009-1301; PUBMED: 20403939] [DOI] [PubMed] [Google Scholar]
Dugoua 2009
- Dugoua JJ, Machado M, Zhu X, Chen X, Koren G, Einarson TR. Probiotic safety in pregnancy: a systematic review and meta‐analysis of randomized controlled trials of Lactobacillus, Bifidobacterium, and Saccharomyces spp. Journal of Obstetrics and Gynaecology Canada 2009;31(6):542‐52. [DOI: 10.1016/S1701-2163(16)34218-9; PUBMED: 19646321] [DOI] [PubMed] [Google Scholar]
Elias 2011
- Elias J, Bozzo P, Einarson A. Are probiotics safe for use during pregnancy and lactation?. Canadian Family Physician Medecin de Famille Canadien 2011;57(3):299‐301. [PUBMED: 21402964] [PMC free article] [PubMed] [Google Scholar]
Faa 2013
- Faa G, Gerosa C, Fanni D, Nemolato S, Eyken P, Fanos V. Factors influencing the development of a personal tailored microbiota in the neonate, with particular emphasis on antibiotic therapy. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(Suppl 2):35‐43. [DOI: 10.3109/14767058.2013.829700; PUBMED: 24059551] [DOI] [PubMed] [Google Scholar]
FDA 2014
- U.S. Food, Drug Administration. Dietary Supplements Containing Live Bacteria or Yeast in Immunocompromised Persons: Warning ‐ Risk of Invasive Fungal Disease. www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm426331.htm 12/9/2014; Accessed in 2016.
Garland 2010
- Garland SM, Jacobs SE, Tobin JM, Opie GF, Donath S, ProPrems study group. A cautionary note on instituting probiotics into routine clinical care for premature infants. Pediatrics 2010;126(3):e741‐2; Erratum in: Pediatrics: 2010;126(5):1052. [DOI: 10.1542/peds.2010-1949B; PUBMED: 20810714] [DOI] [PubMed] [Google Scholar]
Gomez Arango 2015
- Gomez Arango LF, Barrett HL, Callaway LK, Nitert MD. Probiotics and pregnancy. Current Diabetes Reports 2015;15(1):567. [DOI: 10.1007/s11892-014-0567-0; PUBMED: 25398206] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 27 March 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gueimonde 2006
- Gueimonde M, Sakata S, Kalliomaki M, Isolauri E, Benno Y, Salminen S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. Journal of Pediatric Gastroenterology and Nutrition 2006;42(2):166–70. [DOI: 10.1097/01.mpg.0000189346.25172.fd; PUBMED: 16456409] [DOI] [PubMed] [Google Scholar]
Hickey 2012
- Hickey L, Jacobs SE, Garland SM, ProPrems Study Group. Probiotics in neonatology. Journal of Paediatrics and Child Health 2012;48(9):777‐83. [DOI: 10.1111/j.1440-1754.2012.02508.x; PUBMED: 22862675] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2016
- Higgins RD, Saade G, Polin RA, Grobman WA, Buhimschi IR, Watterberg K, et al. Chorioamnionitis Workshop Participants. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstetrics & Gynecology 2016;127(3):426‐36. [DOI: 10.1097/AOG.0000000000001246; PUBMED: 26855098] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [DOI: 10.1001/archopht.123.7.991; PUBMED: 16009843] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow‐Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janvier 2013
- Janvier A, Lantos J, Barrington K. The politics of probiotics: probiotics, necrotizing enterocolitis and the ethics of neonatal research. Acta Paediatrica 2013;102(2):116‐8. [DOI: 10.1111/apa.12083; PUBMED: 23146123] [DOI] [PubMed] [Google Scholar]
Johnson 2012
- Johnson CL, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics 2012;129(5):950‐60. [DOI: 10.1542/peds.2011-2736; PUBMED: 22473366] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jost 2014
- Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother–neonate transfer of maternal gut bacteria via breastfeeding. Environmental Microbiology 2014;16(9):2891‐904. [DOI: 10.1111/1462-2920.12238; PUBMED: 24033881] [DOI] [PubMed] [Google Scholar]
Keski‐Nisula 2013
- Keski‐Nisula L, Kyynarainen HR, Karkkainen U, Karhukorpi J, Heinonen S, Pekkanen J. Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatrica 2013;102(5):480‐5. [DOI: 10.1111/apa.12186; PUBMED: 23398392] [DOI] [PubMed] [Google Scholar]
Lahtinen 2009
- Lahtinen SJ, Boyle RJ, Kivivuori S, Oppedisano F, Smith KR, Robins‐Browne R, et al. Prenatal probiotic administration can influence Bifidobacterium microbiota development in infants at high risk of allergy. Journal of Allergy and Clinical Immunology 2009;123(2):499‐501.e8. [DOI: 10.1016/j.jaci.2008.11.034; PUBMED: 19135234] [DOI] [PubMed] [Google Scholar]
Lindsay 2013
- Lindsay KL, Walsh CA, Brennan L, McAuliffe FM. Probiotics in pregnancy and maternal outcomes: a systematic review. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(8):772‐8. [PUBMED: 10.3109/14767058.2012.755166; PUBMED: 23205866] [DOI] [PubMed] [Google Scholar]
Mackeen 2015
- Mackeen AD, Packard RE, Ota E, Speer L. Antibiotic regimens for postpartum endometritis. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD001067.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matamaros 2013
- Matamoros S, Gras‐Leguen C, Vacon F, Potel G, Cochetiere MF. Development of intestinal microbiota in infants and its impact on health. Human Microbiome 2013;21(4):167‐73. [DOI: 10.1016/j.tim.2012.12.001; PUBMED: 23332725] [DOI] [PubMed] [Google Scholar]
Mihatsch 2012
- Mihatscha WA, Braeggerb CP, Decsic T, Kolacekd S, Lanzingere H, Mayerf B, et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clinical Nutrition 2012;31(1):6‐15. [DOI: 10.1016/j.clnu.2011.09.004; PUBMED: 21996513] [DOI] [PubMed] [Google Scholar]
Neu 2011
- Neu J, Walker A. Necrotizing enterocolitis. New England Journal of Medicine 2011;364(3):255‐64. [DOI: 10.1056/NEJMra1005408; PUBMED: 21247316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Othman 2007
- Othman M, Alfirevic Z, Neilson JP. Probiotics for preventing preterm labour. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
Reid 2003
- Reid G, Bocking A. The potential for probiotics to prevent bacterial vaginosis and preterm labor. American Journal of Obstetrics and Gynecology 2003;189(4):1202‐8. [PUBMED: 14586379] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Soll 2010
- Soll RF. Probiotics: are we ready for routine use?. Pediatrics 2010;125(5):1071‐2. [DOI: 10.1542/peds.2010-0643; PUBMED: 20421256] [DOI] [PubMed] [Google Scholar]
The World Bank 2016
- The World Bank. World Bank Country and Lending Groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed 27 March 2018).
Thomas 2010
- Thomas DW, Greer FR, American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Section on Gastroenterology, Hepatology, Nutrition. Probiotics and prebiotics in pediatrics. Pediatrics 2010;126(6):1217‐31. [DOI: 10.1542/peds.2010-2548; PUBMED: 21115585] [DOI] [PubMed] [Google Scholar]
VandeVusse 2013
- VandeVusse L, Hanson L, Safdar N. Perinatal outcomes of prenatal probiotic and prebiotic administration: an integrative review. Journal of Perinatal & Neonatal Nursing 2013;27(4):288‐301. [DOI: 10.1097/JPN.0b013e3182a1e15d; PUBMED: 24164813] [DOI] [PubMed] [Google Scholar]
Viswanathan 2016
- Viswanathan S, Lau C, Akbari H, Hoyen C, Walsh MC. Survey and evidence based review of probiotics used in very low birth weight preterm infants within the United States. Journal of Perinatology 2016;36(12):1106‐11. [DOI: 10.1038/jp.2016.144; PUBMED: 27583387] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Grev 2017
- Grev J, Berg M, Soll R. Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012519] [DOI] [PMC free article] [PubMed] [Google Scholar]


