Abstract
Background
Nonsteroidal anti‐inflammatory drugs (NSAIDs) provide effective analgesia during the post‐operative period but can cause acute kidney injury (AKI) when used peri‐operatively (at or around the time of surgery). This is an update of a Cochrane review published in 2007.
Objectives
This review looked at the effect of NSAIDs used in the peri‐operative period on post‐operative kidney function in patients with normal kidney function.
Search methods
We searched Cochrane Kidney and Transplant's Specialised Register to 4 January 2018 through contact with the Information Specialist using search terms relevant to this review. Studies in the Specialised Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the use of NSAIDs versus placebo for the treatment of post‐operative pain in patients with normal kidney function were included.
Data collection and analysis
Data extraction was carried out independently by two authors as was assessment of risk of bias. Disagreements were resolved by a third author. Dichotomous outcomes are reported as relative risk (RR) and continuous outcomes as mean difference (MD) together with their 95% confidence intervals (CI). Meta‐analyses were used to assess the outcomes of AKI, change in serum creatinine (SCr), urine output, renal replacement therapy (RRT), death (all causes) and length of hospital stay.
Main results
We identified 26 studies (8835 participants). Risk of bias was high in 17, unclear in 6and low in three studies. There was high risk of attrition bias in six studies.
Only two studies measured AKI. The use of NSAIDs had uncertain effects on the incidence of AKI compared to placebo (7066 participants: RR 1.79, 95% CI 0.40 to 7.96; I2 = 59%; very low certainty evidence). One study was stopped early by the data monitoring committee due to increased rates of AKI in the NSAID group. Moreover, both of these studies were examining NSAIDs for indications other than analgesia and therefore utilised relatively low doses.
Compared to placebo, NSAIDs may slightly increase serum SCr (15 studies, 794 participants: MD 3.23 μmol/L, 95% CI ‐0.80 to 7.26; I2 = 63%; low certainty evidence). Studies displayed moderate to high heterogeneity and had multiple exclusion criteria including age and so were not representative of patients undergoing surgery. Three of these studies excluded patients if their creatinine rose post‐operatively.
NSAIDs may make little or no difference to post‐operative urine output compared to placebo (6 studies, 149 participants: SMD ‐0.02, 95% CI ‐0.31 to 0.27). No reliable conclusions could be drawn from these studies due to the differing units of measurements and measurement time points.
It is uncertain whether NSAIDs leads to the need for RRT because the certainty of this evidence is very low (2 studies, 7056 participants: RR 1.57, 95% CI 0.49 to 5.07; I2 = 26%); there were few events and the results were inconsistent.
It is uncertain whether NSAIDs lead to more deaths (2 studies, 312 participants: RR 1.44, 95% CI 0.19 to 11.12; I2 = 38%) or increased the length of hospital stay (3 studies, 410 participants: MD 0.12 days, 95% CI ‐0.48 to 0.72; I2 = 24%).
Authors' conclusions
Overall NSAIDs had uncertain effects on the risk of post‐operative AKI, may slightly increase post‐operative SCr, and it is uncertain whether NSAIDs lead to the need for RRT, death or increases the length of hospital stay. The available data therefore does not confirm the safety of NSAIDs in patients undergoing surgery. Further larger studies using the Kidney Disease Improving Global Outcomes definition for AKI including patients with co‐morbidities are required to confirm these findings. .
Plain language summary
Effect of nonsteroidal anti‐inflammatory medicines on kidney function in patients with normal kidney function undergoing surgery
What is the issue?
Nonsteroidal anti‐inflammatory drugs (NSAIDs) offer effective pain relief following surgery. Acute kidney injury (AKI) is the rapid loss of kidney function. It is associated with high death rate. NSAIDs can lead to AKI in up to 5% of patients using them. This is increased when there are other stresses placed on the kidney such as surgery. It is therefore important to establish whether these drugs are safe to use as pain relief in patients undergoing surgery. The aim of the review was to examine whether NSAIDs lead to increased rates of AKI in patients with normal kidney function undergoing surgery. We also aimed to examine whether NSAIDs were associated with higher death rates, increased length of hospital stay and need for dialysis.
What did we do?
We updated a previous review searching the Cochrane Kidney and Transplant Specialised Register until 4 January 2018 for randomised controlled trials (RCTs) comparing NSAIDs with placebo in patients with normal kidney function undergoing surgery.
What did we find?
We identified 26 studies studying 8835 participants. Risk of bias was high in 17, unclear in six studies and low in three studies. The use NSAIDs had uncertain effects on the incidence of AKI compared to placebo. Quality of evidence was very low due to inconsistencies between the two studies. One study was stopped early by the data monitoring committee due to increased rates of AKI in the NSAID group and both of these studies examined much lower doses of NSAIDs than would usually be used for pain relief. NSAIDs may slightly increase serum creatinine (a marker of kidney function which rises in kidney failure) compared with placebo. Quality of evidence was low. These studies only included fit, healthy patients. No reliable conclusions could be drawn from the studies examining urine output due to the different methods of measuring this. It is uncertain whether the use of NSAIDs leads to an increased need for renal replacement therapy (dialysis), more deaths, or increased length of hospital stay.
Conclusions
NSAIDs have uncertain effects on the rates of AKI when used in patients with normal kidney function following surgery. It is uncertain whether NSAIDs increase the need for dialysis. The available data therefore does not confirm the safety of NSAIDs in patients undergoing surgery. Further studies including patients with other health problems are required.
Summary of findings
Background
Description of the condition
There is increasing evidence that acute kidney injury (AKI) is associated with both short‐ and long‐term adverse consequences. These include increased length of hospital stay, death and future development of chronic kidney disease (CKD) even with small transient rises in serum creatinine (SCr) (Bucaloiu 2012; Chertow 2005; Coca 2012; Lassnigg 2004). Surgery is a leading cause of AKI in hospitalised patients (Carmichael 2003). There was previously significant variation in defining AKI. These included changes in SCr, urine output and creatinine clearance (CrCl). The Kidney Disease Improving Global Outcomes (KDIGO) definition has been universally accepted since 2012 KDIGO 2012.
Effective management of post‐operative pain is extremely important. It facilitates early mobilisation thereby reducing hospital costs through shortened duration of hospital in‐patient stay, reduces pulmonary and cardiovascular complications and risk of deep vein thrombosis. In addition, it impacts on quality of patient care by relieving suffering and distress and improving satisfaction. The major aim of post‐operative pain management is providing adequate pain relief using the minimal possible dose thereby minimising adverse effects. Clinical guidelines for managing perioperative pain by the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council were published in 2016 (Chou 2016). They recommend a multimodal approach to post‐operative pain including the use of both nonselective nonsteroidal anti‐inflammatory drugs; (NSAIDs) as well as selective NSAIDs (Cox‐2 inhibitors). NSAIDs can affect the kidneys in a number of ways. This includes haemodynamically mediated AKI, electrolyte and acid‐base disorders and acute interstitial nephritis. These adverse effects are thought to occur in 1% to 5% of all patients using NSAIDs (Whelton 1999).
Description of the intervention
NSAIDs inhibit prostaglandin synthesis by inhibiting cyclooxygenase‐1 (Cox‐1) and cyclooxygenase ‐2 (Cox‐2). Cox‐1 is expressed in most tissues regulating normal cellular processes such as gastric cytoprotection, vascular homeostasis, platelet aggregation and kidney function. Cox‐2 is expressed in brain, kidney and bone. Most traditional NSAIDs are non‐selective inhibitors of both Cox‐1 and Cox‐2. Selective Cox‐2 inhibitors include celecoxib, rofecoxib and valdecoxib.
Cyclooxygenases are produced at multiple sites within the kidney including glomerular and vascular endothelium, medullary and cortical collecting tubules and medullary interstitial cells. Cox‐1 is expressed in most tissues and Cox‐2 is expressed at low levels increasing with stimulation such as inflammation. Renal prostaglandins are primarily vasodilators in the kidneys. Under normal circumstances, renal prostaglandins do not contribute to regulation of kidney perfusion but in the setting of hypotension and reduced kidney perfusion from vasoconstriction prostaglandin synthesis is increased to maintain kidney perfusion and minimize ischaemia. Other kidney effects of prostaglandins include increased renin secretion, antagonism of anti‐diuretic hormone effects and increased sodium excretion.
How the intervention might work
The use of both non‐selective and selective NSAIDs for post‐operative pain has been evaluated in a number of Cochrane reviews. A single dose of ibuprofen lead to at least 50% pain relief in approximately half of patients with moderate to severe post‐operative pain. Adverse effects were similar to placebo (Derry 2009). Aspirin was found to confer a 50% or greater reduction in pain in 39% of those with moderate to severe pain, compared with 15% of those in the placebo group. Adverse events were similar for those taking a lower dose aspirin (600 mg or 650 mg). However, higher dose aspirin (900 mg to 1000 mg) experienced adverse events at more than twice the rate of patients receiving placebo (26% versus 12%) (Derry 2012a). The use of a single dose of the Cox‐2 inhibitor celecoxib in the treatment of acute post‐operative pain showed that 33% of patients receiving celecoxib 200 mg, and 44% receiving 400 mg, experienced at least 50% pain relief, compared with between 1% and 11% of patients receiving placebo. Adverse events were similar in the celecoxib and placebo groups (Derry 2012b).
Furthermore, there is evidence supporting the efficacy of NSAIDs for post‐operative pain with studies demonstrating opioid sparing effects (McDaid 2010).
NSAIDs have the potential to adversely affect kidney function in the peri‐operative setting. Pre‐renal insults such as hypovolaemia or hypotension peri‐operatively cause NSAID‐induced inhibition of prostaglandin mediated afferent arteriolar dilatation leading to reduced glomerular perfusion. The risk of AKI with NSAIDs has led the Medicines and Healthcare Products Regulatory Agency to issue drug safety advice recommending that NSAIDs be avoided in patients with hypovolaemia (MHRA 2009). Other adverse events associated with NSAIDs include gastrointestinal bleeding and cardiovascular events. These were not examined in this review.
Why it is important to do this review
This is an update of a Cochrane review last published in 2007 (Lee 2007). This review showed that NSAIDs caused a clinically unimportant transient reduction in kidney function in the early post‐operative period in patients with normal kidney function. Since its publication, a universal definition for AKI has been developed allowing a better understanding of its epidemiology and clinical significance (KDIGO 2012). Since the advent of the KDIGO definition for AKI, there is increasing evidence of the adverse clinical and economic consequences of AKI. In addition, National Institute for Clinical Excellence (NICE) AKI guidance recommends the avoidance of NSAIDs in the post‐operative period (Ftouh 2013).
It is therefore important to re‐assess the renal safety of NSAIDs in the peri‐operative period.
Objectives
This review looked at the effect of NSAIDs used in the peri‐operative period on post‐operative kidney function in patients with normal kidney function.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the use of NSAIDs versus placebo in the post‐operative phase in adults with normal kidney function were included.
Types of participants
People of at least 18 years of age undergoing surgical procedures who were treated with NSAIDs or Cox‐2 inhibitors with normal kidney function were included. Normal kidney function was defined as estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73 mm2 without other evidence of kidney disease (proteinuria, haematuria, genetic kidney disease or structural kidney abnormalities).
Types of interventions
All interventions comparing NSAID treatments including Cox‐2 inhibitors versus placebo were considered. Variable doses, all routes of administration and variable indications for NSAID use were considered.
Types of outcome measures
The primary endpoint was AKI. Studies measuring SCr and urine output in the post‐operative phase were also considered. The secondary outcomes of length of hospital stay, death and requirement of renal replacement therapy (RRT) were documented when available.
Primary outcomes
The primary outcome was AKI as defined by KDIGO which is based on SCr or urine output (KDIGO 2012). Change in SCr and urine output were also considered using the highest post‐operative creatinine or lowest post‐operative urine volume. These were analysed separately.
Secondary outcomes
Need for RRT
Death (all causes)
Length of hospital stay
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 4 January 2018 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable, however studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data were used in the analyses. Data extracted included study design, inclusion and exclusion criteria, patient numbers and characteristics and treatment regimen. For outcomes of interest (AKI, serum, creatinine, urine output, death, need for RRT and length of hospital stay), the raw data were extracted using mean, median and standard deviations for continuous outcomes, and event rate for dichotomous outcomes. Where data were collected at more than one time‐point, these were all extracted. Peak SCr and lowest urine volume were used for the analyses.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
In each domain, studies were labelled as low, high or unclear risk of bias with consideration given to the presence or absence of sufficient information to make a determination. Reasons for assessment were documented (See Characteristics of included studies), and a risk of bias summary is presented.
Measures of treatment effect
For the dichotomous outcomes (presence of AKI, need for RRT and death), results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Continuous scales of measurement such as mean difference (MD) and 95% CI was used to assess the effects of treatment for change in SCr and length of hospital stay. SCr was converted to standardised units (µmol/L) and peak post‐operative creatinine was used when more than one post‐operative creatinine was reported. Mean change in SCr was not given in studies and so the correlation coefficient between pre and post‐operative measures were not known. We therefore assumed a correlation coefficient of 0.50 (Follmann 1992). A sensitivity analysis was carried out assuming zero correlation. The standard deviation between pre and post‐operative measures for each treatment group was estimated using a method outlined in the Cochrane Collaboration Handbook. When the median and interquartile range were reported, we assumed that the mean was equivalent to the median and estimated the standard deviation to be the interquartile range/1.35 (O'Rourke 2002). Standardised mean difference (SMD) was used for urine output as different scales were used. Lowest post‐operative urine output was used when more than one time point was measured.
In studies comparing multiple different NSAIDs or varying dosing regimens, the dose or dug with the greatest adverse effect on kidney function was included in the analysis.
Unit of analysis issues
Studies with non‐standard designs, such as cross‐over studies and cluster‐randomised studies were not included in this review.
Dealing with missing data
We did not contact any authors as the required information was present in the studies. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Funnel plots were used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also be used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Meta‐regression was carried out to investigate possible explanations for heterogeneity of effects, including type of surgery (cardiac versus other), duration of NSAID therapy (>24h versus <24h) and whether age was an exclusion criterion (no versus yes) as potential explanatory variables. Meta‐regression used standard weighted (by standard error of estimate) linear regression in IBM SPSS Statistics 22.
Sensitivity analysis
We planned to perform sensitivity analyses if there were sufficient studies identified, in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
We were only able to investigate the influence of risk of bias on effect size due to the number of studies identified. The analysis was repeated excluding studies with high risk of bias, attrition bias or high risk of bias or attrition bias.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in Table 1.
Summary of findings for the main comparison. Nonsteroidal anti‐inflammatory drugs (NSAIDs) versus placebo or no treatment in the peri‐operative period.
| NSAIDs versus placebo or no treatment in the peri‐operative period | ||||||
|
Patient or population: adults with normal kidney function undergoing surgery Settings: hospitals, mainly high‐income countries (North America or Western Europe) Intervention: administration of NSAIDs in the peri‐operative period Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Intervention (NSAID) | |||||
| AKI within 30 days of surgery | 12 per 100 | 13 per 100 (12 to 14) | RR 1.79 (0.40 to 7.96) | 7066 (2) | ⊕⊝⊝⊝ very low1 | NAFARM 2011 was stopped by study monitoring committee because of increased risk of AKI. Both studies used NSAID doses that were much lower than would be used for analgesia in usual care. The results raise serious concerns about the safety of post‐operative analgesia with NSAIDs in unselected patients |
| SCr increase within 30 days of surgery | The mean difference in SCr in control group was decreased by ‐2.60 µmol/L | The mean difference in SCr in the intervention group was increased by 1.52 µmol/L (‐7.4 to 10.2) | Difference in post‐operative SCr increased by 3.23 µmol/L (‐0.8 to 7.26) | 794 (15) | ⊕⊕⊝⊝ low2 | Heterogeneity was not explained by pre‐specified effect modifiers (Table 2, Figure 1) |
| RRT within 30 days of surgery | 2 per 1000 | 5 per 1000 (2 to 11) | RR 1.57 (0.49 to 5.07) | 7056 (2) | ⊕⊝⊝⊝ very low3 | ‐‐ |
| Death (all causes) | 2 per 100 |
3 per 100 (0 to 6) |
RR 1.44 (0.19 to 11.12) | 312 (2) | ⊕⊝⊝⊝ very low3 | ‐‐ |
| Length of hospital stay | The mean length of hospital stay in control group was 10.0 days | The mean length of hospital stay in the intervention group was 10.6 days (range 5.3 to 18.33) | MD 0.12 (‐0.48 to 0.72) | 410 (3) | ⊕⊝⊝⊝ very low3 | ‐‐ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; MD: mean difference; AKI: acute kidney injury; SCr: serum creatinine; RRT: renal replacement therapy | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
The assumed risk is the median or mean across the control groups for each intervention
1 1 downgrade for study limitations, one for imprecision, and one for heterogeneity (Appendix 3).
2 We downgraded the certainty of evidence to low because of inconsistency and indirectness (Appendix 3).
3 We downgraded the certainty of evidence to very low because of risk of bias, imprecision, inconsistency and indirectness (Appendix 3).
AKI within 30 days of surgery
Mean difference in SCr increase in µmol/L within 30 days of surgery
RRT within 30 days of surgery
Death (all causes)
Length of hospital stay (days)
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies
Results of the search
In total 659 records were identified through database searches, and two records were added through other means. Forty‐five records were duplicates and were excluded. The titles and abstracts of the remaining 616 records were reviewed by two independent assessors and 52 records were deemed eligible for full text review. Nineteen records (21 studies) were excluded; details about the reason for exclusion can be found in the table Characteristics of excluded studies. The remaining 31 records (26 studies) were included (see Figure 2).
2.
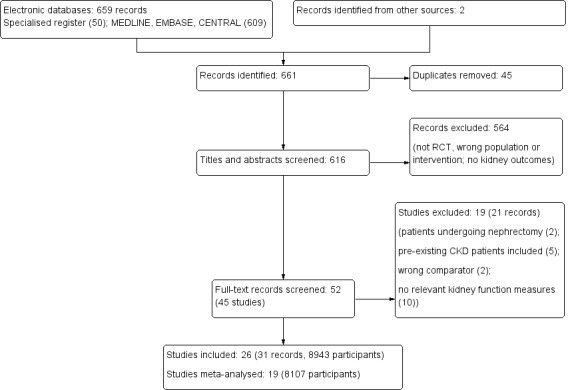
Study flow diagram.
Included studies
Twenty‐six studies (8943 patients) met our inclusion criteria. A detailed overview of all the included studies can be found in the Characteristics of included studies table. The studies were conducted between 1992 and 2017 and included adults with preserved kidney function prior to surgery. Of these, 19 (8107 participants) could be meta‐analysed.
Six studies (Chow 2001; Castiglione 1997; Nuutinen 1991; Parker 1994; Ready 1994; Rao 2000) included in the previous version of this review (Lee 2007), were not included in our review. One study enrolled patients who underwent a nephrectomy (Chow 2001); one study used NSAID in both groups (Castiglione 1997); and four studies were excluded due to the absence of concise post‐operative kidney outcomes in these studies (Nuutinen 1991; Parker 1994; Ready 1994; Rao 2000) – in line with the KDIGO diagnostic AKI criteria (KDIGO 2012).
Nine new studies (Eljezi 2017; Fayaz 2004; Koppert 2006; McCrory 2002; NAFARM 2011; Ott 2003; POISE‐2 2013; Puolakka 2009; Rafiq 2014) were added to this review.
Types of surgery
Patients underwent various types of surgery: 13 studies reviewed patients undergoing open cardiac surgery (Eljezi 2017; Fayaz 2004; Hynninen 2000; Immer 2003; Khalil 2006a; Kulik 2004; McCrory 2002; NAFARM 2011; Ott 2003; Perttunen 1992; Perttunen 1999; Rafiq 2014; Rapanos 1999) and six studies reviewed abdominal or pelvic surgeries (Aitken 1992; Jones 2000; Power 1992; Puolakka 2009; Turner 1994; Varrassi 1994). The remainder of the studies reviewed patients undergoing orthopaedic, breast, and various other non‐cardiac surgeries.
Interventions
NSAIDs included in the study were ketorolac (Aitken 1992; Laisalmi 2001a; Perttunen 1992; Perttunen 1999; Rafiq 2014; Varrassi 1994), indomethacin (Hynninen 2000; Rapanos 1999; Turner 1994), diclofenac (Fayaz 2004; Hynninen 2000; Immer 2003; Irwin 1995; Perttunen 1992; Perttunen 1999; Power 1992), aspirin (POISE‐2 2013), ibuprofen (Brinkmann 1998; McCrory 2002; Rafiq 2014), naproxen (Kulik 2004), tenoxicam (Jones 2000; Slaven 1998), etodolac (Immer 2003), and ketoprofen (Eljezi 2017). Selective COX‐2 inhibitors were used in four studies (Khalil 2006a; Koppert 2006; Ott 2003; Puolakka 2009).
Mode of delivery was via intravenous (IV) or intramuscular (IM) injection in 15 studies (Aitken 1992; Brinkmann 1998; Eljezi 2017; Jones 2000; Khalil 2006a; Koppert 2006; Kostamovaara 1996; Laisalmi 2001a; Perttunen 1992; Perttunen 1999; Power 1992; Puolakka 2009; Slaven 1998; Varrassi 1994). A combination of an IV bolus and oral maintenance dose was used by Ott 2003 and Rafiq 2014. The remaining studies used either oral, epidural or per rectum administration methods. Seven studies prescribed NSAIDs for the first post‐operative day only (Fayaz 2004; Hynninen 2000; Irwin 1995; Laisalmi 2001a; Puolakka 2009; Rapanos 1999; Varrassi 1994). The median duration of post‐operative NSAID exposure was 2 days (range 1 to 30 days).
Measurement of primary outcomes
One study defined AKI using the KDIGO definition (POISE‐2 2013) and one using an elevation in SCr of 150% times the baseline (NAFARM 2011). Creatinine was measured in 15 studies (Immer 2003; Koppert 2006; Kostamovaara 1996; Kulik 2004; Laisalmi 2001a; Ott 2003; Perttunen 1992; Perttunen 1999; POISE‐2 2013; Power 1992; Puolakka 2009; Rafiq 2014; Rapanos 1999; Turner 1994; Varrassi 1994). A percentage change in creatinine from baseline was reported by Eljezi 2017. Change in urine output post‐operatively was reported by seven studies (Aitken 1992; Eljezi 2017; Irwin 1995; Jones 2000; Laisalmi 2001a; Perttunen 1992; Perttunen 1999). Serum creatinine clearance was measured by one study (Brinkmann 1998) and urinary creatinine clearance by four studies (Khalil 2006a; Koppert 2006; McCrory 2002; Slaven 1998).
Measurement of secondary outcomes
Death (all causes) was reported by two studies (NAFARM 2011; Rafiq 2014); hospital stay by three studies (NAFARM 2011; Kulik 2004; Rafiq 2014), and two studies reported the need for RRT post‐operatively (Rafiq 2014; POISE‐2 2013).
Excluded studies
Nineteen studies (21 records) were excluded from this review. Details of the reason for exclusion for all of these are found in the Characteristics of excluded studies table.
Four studies were excluded due to the inclusion of patients with pre‐existing CKD into their cohort (Cheruku 2004; Merry 2002; Nussmeier 2005; Nussmeier 2006). Two studies published results about kidney function after nephrectomy (Chow 2001; Grimsby 2012) and were deemed unsuitable for analysis in our review. Eleven studies were excluded due to lack of concise post‐operative kidney outcomes (Daniels 2014; Fredman 1999; Hynes 2006; Leeson 2007; Ma 2015; Nuutinen 1991; Parker 1994; Rao 2000; Ready 1994; Southworth 2009; Varrassi 1999). Two studies were not suitable for inclusion in this review due to the lack of a suitable placebo group (Castiglione 1997; Doyle 1998).
Risk of bias in included studies
See Figure 3.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Nine studies were assessed as low risk, with sufficient information about the sequence generation process (Fayaz 2004; Jones 2000; Khalil 2006a; Kulik 2004; NAFARM 2011; Perttunen 1999; POISE‐2 2013; Puolakka 2009; Rapanos 1999). Most commonly described sequence generation method was computer software. In the remaining 17 studies randomisation was stated but no information was given about the method of sequence generation.
Allocation concealment
Fourteen studies described a randomisation method that was deemed adequate; investigators or participants did not know or have influence on the intervention group before entering the study (Eljezi 2017; Fayaz 2004; Hynninen 2000; Jones 2000; Khalil 2006a; Koppert 2006; Kulik 2004; NAFARM 2011; Perttunen 1999; POISE‐2 2013; Puolakka 2009; Rafiq 2014; Rapanos 1999; Turner 1994). Most commonly used method was sequentially numbered opaque, sealed envelopes. The remaining 12 studies did not provide enough information to determine the method of allocation concealment.
Blinding
Performance bias
All studies mentioned blinding of participants, investigators or both in their methods section, however in nine studies insufficient information was provided regarding the methods by which this was achieved. For that reason those studies were assessed as unclear risk of performance bias due to the knowledge of the allocated interventions by participants and personnel during the study (Aitken 1992; Brinkmann 1998; Immer 2003; Irwin 1995; Kostamovaara 1996; Laisalmi 2001a; Ott 2003; Power 1992; Varrassi 1994). Two studies were open‐label and were judged to be at high risk of bias (McCrory 2002; Rafiq 2014). The remaining 15 studies were judged to be a low risk of bias.
Detection bias
Seven studies were judged to be at low risk bias (Jones 2000; Kulik 2004; NAFARM 2011; Perttunen 1992; Perttunen 1999; POISE‐2 2013; Turner 1994). One study was classed as high risk of detection bias (Rafiq 2014), and for the remaining 18 studies risk of detection bias was judged to be unclear.
Incomplete outcome data
Patient drop‐out was reported in 16 studies.
Protocol violation or equipment failure was the cause of the drop‐out in seven studies (Aitken 1992; Irwin 1995; Koppert 2006; Ott 2003; Rafiq 2014; Rapanos 1999; Turner 1994).
Side effects of the treatment or complications of the surgery was the cause for drop‐out in five studies (Eljezi 2017: 3 patients dropped out due to complications of the surgery, reintubation and/or re‐surgery; Kostamovaara 1996: 1 patient dropped out due to side effect of fentanyl administration, no side effects of the study drug ketorolac were identified; Kulik 2004: 2 patients in the naproxen group and 4 patients in the placebo group suffered from complications after surgery; Rafiq 2014: 21 patients had a prolonged stay in intensive care post surgery ‐ equally distributed between groups; Varrassi 1994: 5 patients withdrew due to re‐laparotomy or inadequate pain control, equally distributed over the two groups).
NAFARM 2011 reported death due to cardiac surgery as the cause of drop‐out in five patients.
Oliguria or rise in creatinine post operatively was identified as cause for withdrawal in six studies (Fayaz 2004; Hynninen 2000; Immer 2003; Kulik 2004; Power 1992; Rafiq 2014) affecting eight patients in total. This is a significant cause for concern as only three of these studies (Hynninen 2000; Immer 2003; Rafiq 2014) acknowledged the potential effect of the study medication on kidney function in their conclusion.
Six studies were deemed high risk of attrition bias due to missing data which potentially has a significant effect on the overall outcome of the study. Aitken 1992 failed to present the reader with a reason for missing urine output data in 15 of 61 patients, while 26% of patients were withdrawn from Ott 2003; mainly due adverse events. Oliguria and/or rise in creatinine post‐operatively was identified as cause for withdrawal from the study in six studies. Four of these studies (Fayaz 2004; Immer 2003; Kulik 2004; Power 1992) were deemed high risk of bias since these patients who were particularly at risk of developing AKI (the primary outcome of this review) were withdrawn from the studies. Hynninen 2000 also withdrew one patient after one dose of indomethacin because of SCr increase > 20% post‐operatively. This patient did not receive further NSAIDs as per protocol. This study was classed as low risk as the patient was not included in the post‐operative outcome table and the plausible effect size of this one event is probably not enough to have a clinically relevant impact on observed effect size.
Fifteen studies were judged to be a low risk of attrition bias (Eljezi 2017; Hynninen 2000; Irwin 1995; Jones 2000; Khalil 2006a; Koppert 2006; Kostamovaara 1996; Laisalmi 2001a; NAFARM 2011; Perttunen 1999; POISE‐2 2013; Puolakka 2009; Rafiq 2014; Rapanos 1999; Varrassi 1994). The risk of attrition bias was unclear in the remaining five studies.
Selective reporting
Twenty‐five studies reported the outcomes that were prespecified in their methods. Eljezi 2017 failed to report the frequency of urinary output (4‐hourly for 48 hours) and creatinine measurements (baseline, post‐operative day 1 and 2) as they had set out to do in the methods section. Urinary output was documented at 48 hours only and a percentage change in SCr at 48 hours from baseline was reported. This study was classed as high risk for reporting bias as it is unclear whether a potential transient fall in urine output and rise in creatinine during the first post‐operative day has occurred, which would significantly change the conclusion drawn from this report.
Other potential sources of bias
Eleven studies reported to have received either no funding or funding from a non‐profit organisation and were therefore deemed at low risk of publication bias (Eljezi 2017; Kostamovaara 1996; Kulik 2004; Laisalmi 2001a; McCrory 2002; NAFARM 2011; Perttunen 1992; Perttunen 1999; Puolakka 2009; Rafiq 2014; Rapanos 1999). POISE‐2 2013 used several sources of funding; firstly two large governmental non‐profit organisations from Spain and Australia. Secondly an undefined amount of financial support as well as study drugs from a commercial body were disclosed. The authors state that the sponsors had no role in the design and conduct of the study, collection, management, analysis, review or approval of the manuscript; and decision to submit the manuscript for publication. Due to the combination of commercially as well as non commercially accrued funding sources used in this study, in combination with the extensive disclosure of the use of the commercially acquired funding, this study was classed as low risk. Slaven 1998 received the study drug and placebo as a gift from the manufacturer, however the study design and analysis of the results was independent of any company involvement and was judged to be at low risk of bias.
Six studies were classed as high risk of bias due to the use of commercial funding. An unknown quantity of financial support from a commercial body was received by three studies (Jones 2000; Khalil 2006a; Koppert 2006). Aitken 1992 received financial support as well as study drugs from a pharmaceutical company. Commercially provided study drugs were used by Immer 2003. Other potential biases were unclear in the remaining eight studies.
Effects of interventions
See: Table 1
See Table 1 for main comparisons.
Post‐operative acute kidney injury
One large study (6905 participants) POISE‐2 2013 reported AKI defined by the KDIGO criteria and one smaller study (161 participants) NAFARM 2011 defined AKI as a rise of SCr of 150% times the baseline. The use of NSAIDs had uncertain effects on the incidence of AKI compared to placebo (Analysis 1.1 (2 studies, 7066 participants): RR 1.79, 95% CI 0.40 to 7.96; I2 = 59%; very low certainty evidence). The analysis was dominated by POISE‐2 2013 with 70.4% of the weighting and medium level of heterogeneity. NAFARM 2011 was terminated early by the trial monitoring committee because of increased risk of AKI.
1.1. Analysis.
Comparison 1 Acute kidney injury, Outcome 1 AKI.
Post‐operative serum creatinine
Change in SCr was reported in 15 studies. AKI is defined as peak post‐operative SCr and so where creatinine was measured over several time points, peak SCr was used for the analysis. In addition, where several different NSAIDs or dosing regimens were compared in a study, the regime or drug with the greatest adverse effect on kidney function was included within the analysis. Ott 2003 defined kidney dysfunction as a creatinine value > 177 μmol/L and an increase of 62 μmol/L with an incidence of 2.6% in both groups. This study was not included in the pooled analysis due to the lack of absolute values.
Compared to placebo, NSAIDs may slightly increase serum SCr (Analysis 2.1 (15 studies, 794 participants): MD 3.23 µmol/L, 95% CI ‐0.80 to 7.26; I2 = 66%; low certainty evidence). Heterogeneity was medium to high.
2.1. Analysis.
Comparison 2 Serum creatinine, Outcome 1 Serum creatinine (all studies).
Meta‐regression of change in post‐operative serum creatinine by pre‐specified effect modifiers
Of the 15 studies in the meta‐analysis of change in post‐operative SCr (Analysis 2.1), seven studies were in cardiac surgery, eight with > 24 hours of NSAID use, and nine with no stated age exclusion (Table 2). As expected cardiac surgery and > 24 hours of NSAID use were associated with a positive beta (greater effect size) in the meta‐regression (Figure 1). In contrast, we expected that RCTs with no stated age exclusion would have greater effect size but beta was negative (lower effect size) for these RCTs in the univariate meta‐regression (Figure 1). Multivariate analysis did not identify significant effect modifiers (Figure 1).
1. Effect modifiers for meta‐regression of change in post‐operative serum creatinine.
| Study ID | Cardiac surgery | NSAIDs > 24 hours | Age exclusion |
| Eljezi 2017 | Yes | Yes | > 75 years |
| Fayaz 2004 | Yes | No | None reported |
| Hynninen 2000 | Yes | No | > 75 years |
| Immer 2003 | Yes | Yes, 3 days | > 70 years |
| Kostamovaara 1996 | No, hip or knee replacement | No | None reported |
| Kulik 2004 | Yes | Yes, 5 days | None reported |
| Laisalmi 2001a | No, breast surgery | No | None reported |
| Perttunen 1992 | No, thoracoscopy | Yes, 2 days | > 75 years |
| Perttunen 1999 | No, thoracoscopy | Yes, 2 days | > 75 years |
| Power 1992 | No, oesophagogastrectomy | Yes, 2 days | None reported |
| Puolakka 2009 | No, laparoscopic hysterectomy | No | None reported |
| Rafiq 2014 | Yes | Yes, 4 days | None reported |
| Rapanos 1999 | Yes | No | None reported |
| Turner 1994 | No, cholecystectomy | Yes, 3 days | None reported |
| Varrassi 1994 | No, cholecystectomy | No | > 65 years |
NSAIDs ‐ nonsteroidal anti‐inflammatory drugs
1.
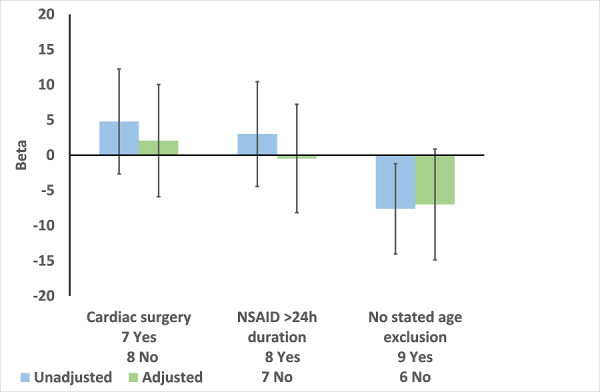
Meta‐regression of change in post‐operative serum creatinine (Analysis 2.1) by type of surgery, duration of NSAID use, and exclusion by age. Results are Beta with 95% CI. A positive value for Beta indicates that a variable is associated with increased effect size.
Sensitivity analyses
Exclusion of studies with an overall high risk of bias (Analysis 2.2), high risk of attrition bias (Analysis 2.3) and either high overall risk of bias or high risk of attrition bias (Analysis 2.4) reduced the effect estimate.
2.2. Analysis.
Comparison 2 Serum creatinine, Outcome 2 Serum creatinine (excluding high risk of bias).
2.3. Analysis.
Comparison 2 Serum creatinine, Outcome 3 Serum creatinine (excluding high attrition bias).
2.4. Analysis.
Comparison 2 Serum creatinine, Outcome 4 Serum creatinine (excluding high risk of bias or high attrition bias).
Post‐operative urine output
Change in urine output was measured in seven studies. Eljezi 2017 measured urine output at 48 hours but did not report baseline urine output. Where urine output was measured over several time points, lowest post‐operative urine output was used for the analysis. Urine output was measured as total volume, mL/min and mL/kg/h therefore standardised mean difference (SMD) was used for pooling the data.
NSAIDs may make little or no difference to post‐operative urine output compared to placebo (Analysis 3.1 (6 studies, 149 participants): SMD ‐0.49, 95% CI ‐1.21 to 0.24; I2 = 77%; low certainty evidence) Heterogeneity was high. The differences in units of measurements and time points when urine output was measured in the different studies rendered interpretation of these results difficult.
3.1. Analysis.
Comparison 3 Urine output, Outcome 1 Urine output.
Need for renal replacement therapy
Two studies reported the need for RRT (POISE‐2 2013; Rafiq 2014). It is uncertain whether NSAIDs leads to the need for RRT because the certainty of this evidence is very low (Analysis 4.1 (2 studies, 7056 participants): RR 1.57, 95% CI 0.49 to 5.07; I2 = 26%). Heterogeneity was low.
4.1. Analysis.
Comparison 4 Need for renal replacement therapy, Outcome 1 RRT.
Death (all causes)
Two studies reported death (NAFARM 2011; Rafiq 2014). It is uncertain whether NSAIDs leads to more deaths because the certainty of this evidence is very low. These were two small studies with a small number of events (Analysis 5.1 (2 studies, 312 participants): RR 1.44, 95% CI 0.19 to 11.12; I2 = 38%) Heterogeneity was low to medium.
5.1. Analysis.
Comparison 5 Death due to any cause, Outcome 1 Death due to any cause.
Length of hospital stay
Three studies examined length of hospital stay (NAFARM 2011; Kulik 2004; Rafiq 2014). It is uncertain whether NSAIDs result in a longer hospital stay because the certainty of this evidence is very low (Analysis 6.1 (3 studies, 410 participants): MD 0.12 days, 95% CI ‐0.48 to 0.72; I2 = 24%). Heterogeneity was low.
6.1. Analysis.
Comparison 6 Length of hospital stay, Outcome 1 Length of hospital stay.
Discussion
Summary of main results
We included 26 eligible studies (8943 participants) examining the use of NSAIDs in the perioperative period in patients with normal kidney function. The primary outcome of AKI, defined by KDIGO creatinine‐based criteria, was used in only two studies. Change in SCr was measured in 14 studies and urine output in seven. For the secondary outcomes, two studies examined RRT, two examined death, and two length of hospital stay. Type of surgery, duration of treatment and dosage varied among the studies. Kidney outcomes were secondary outcomes in 13 studies. Two studies examined the use of NSAIDs for indications other than analgesia (NAFARM 2011; POISE‐2 2013) and the NSAID doses were lower than would be used as analgesia. Overall risk of bias was high in 17, unclear in six studies and low in three studies. Overall NSAIDs had uncertain effects on the risk of post‐operative AKI, may slightly increase post‐operative SCr, and it is uncertain whether NSAIDs leads to the need for RRT, death or increases the length of hospital stay (Table 1)
The two studies with AKI as a primary outcome were the largest studies in the review and had few exclusions (NAFARM 2011; POISE‐2 2013). One study was stopped by the data monitoring committee because of increased risk of post‐operative AKI in the NSAID group (NAFARM 2011). The indication for NSAID use was to reduce risk of post‐operative atrial fibrillation. The dose of naproxen (550 mg/d) was below the lowest daily dose recommended for analgesia for osteoarthritis (750 mg/d; Chou 2011) and substantially lower than the dose of 1000 mg/d used for post‐operative analgesia in another study in our review (Kulik 2004). The contrast between the results of NAFARM 2011 and Kulik 2004 is striking. Both studies used the same NSAID (naproxen) for the same duration (four days) in the same patient group (coronary artery bypass graft surgery). However, despite using a much lower dose of naproxen, NAFARM 2011 was stopped because of increased risk of post‐operative AKI whereas Kulik 2004 reported that naproxen use was associated with a mean decrease in post‐operative SCr. POISE‐2 2013 aimed to reduce the risk of post‐operative AKI in patients undergoing elective or emergency surgery and included 6905 patients from 22 countries. The aspirin group received 200 mg on the day of surgery and then 100 mg/d for seven days, whereas the maximum recommended daily dose of aspirin is 4000 mg (NICE 2017).
Compared to placebo, NSAIDs may slightly increase serum SCr (3.23 μmol/L, 95% CI ‐0.80 to 7.26). Studies displayed moderate to high heterogeneity with multiple different exclusion criteria (e.g. age, diabetes, heart failure, use of diuretics) and so were not representative of patients undergoing surgery. Three of these studies excluded patients if their creatinine rose post‐operatively.
No reliable conclusions could be drawn from the studies examining urine output due to the differing units of measurements and measurement time points.
It is uncertain whether the use of NSAIDs leads to an increased need for RRT, more deaths, or increased length of hospital stay.
Overall completeness and applicability of evidence
There are significant limitations to this review. Most of the studies excluded patients with co‐morbidities such as diabetes, heart, liver, or respiratory failure. The population studied was therefore highly selected and non‐representative of the population of patients undergoing surgery in most hospitals. With the exception of one study (POISE‐2 2013), the studies were small and heterogeneous examining various types of NSAIDs, various doses and different types of surgery. A further important limitation was that three studies (Fayaz 2004; Hynninen 2000; Immer 2003) excluded patients if their SCr rose post‐operatively and one study (Power 1992) administered furosemide to patients if their post‐operative urine output fell. This impacts on the outcomes of these studies as they included both SCr and urine output.
The largest study (POISE‐2 2013) examined the kidney effects of aspirin for an indication other than analgesia in 6905 patients undergoing surgery. Types of surgery included major vascular, thoracic, urological, and gynaecological. Patients with co‐morbidities such as diabetes and cardiovascular disease were included. There were also patients with CKD included. Patients received aspirin at very low dose (100 mg/d; NICE 2017) and was associated with an uncertain effect on post‐operative AKI (Analysis 1.1) and RRT (Analysis 4.1). The risk difference for RRT was 3 patients per 1000 treated (95% CI 0 to 6). Inclusion of this large study impacted significantly on the findings of this review.
Quality of the evidence
We identified 26 eligible RCTs with 8835 participants examining the use of NSAIDs in the perioperative period in patients with normal kidney function. Risk of bias was high in 17, unclear in 6studies and low in 3 studies with high risk of attrition bias in 6 studies.
We have graded the evidence that NSAIDs increase the risk of post‐operative AKI as very low certainty (Table 1). These were the largest RCTs in the review and both were low risk of bias (NAFARM 2011, POISE‐2 2013). Importantly, both trials included patients with co‐morbidities and both studies used NSAIDs at relatively low doses for non‐analgesic indications. Our main concern was about the inconsistencies between the two studies. Risk of AKI in the control groups were completely different (1.2% in NAFARM 2011 versus 12.3% in POISE‐2 2013).
We graded the evidence about increase in post‐operative SCr as low certainty. The certainty of evidence was downgraded because of inconsistency (heterogeneity was not adequately explained by pre‐specified effect modifiers) and indirectness (studies had multiple exclusion criteria with the patients included in the RCTs likely to be different from those in routine care). The results of Kulik 2004 (decrease in SCr associated with naproxen for 5 days after cardiac surgery) are in stark contrast to the results of NAFARM 2011 (study stopped because of excess risk of AKI associated with a lower dose of naproxen for 5 days after cardiac surgery). There was potential for publication bias as studies were small and commercially funded. However, the Funnel Plot was symmetrical (Figure 4). For the outcome of RRT, certainty of evidence was very low. This was downgraded because of imprecision, inconsistency and publication bias. There was imprecision due to low number of events, inconsistency as the risk of RRT was completely different in the two control groups (0.3% in NAFARM 2011versus 2.7% in Rafiq 2014) and publication bias as one of the two studies was small and commercially funded.
4.

Funnel plot of comparison: 2.1 Mean difference in serum creatinine
We were concerned that studies with high risk of bias would underestimate the effect of NSAIDs on post‐operative kidney function. However, sensitivity analysis of post‐operative SCr increase showed that exclusion of studies with high risk of overall bias or attrition bias reduced the study effects (Analysis 2.2; Analysis 2.3; Analysis 2.4).
Potential biases in the review process
The review was conducted with standard Cochrane methodology. The review was completed independently by two authors, who participated in all steps of the review. This limited the risk of errors in determining study eligibility, data extraction, risk of bias assessment and data synthesis. We did not include the results of unpublished studies. Studies with both positive and negative results were identified, making the possibility of publication bias less likely. A strength of this review is that we included studies defining AKI using the KDIGO definition KDIGO 2012.
Agreements and disagreements with other studies or reviews
Lee 2007 concluded that NSAIDs caused a clinically unimportant reduction in kidney function on the first post‐operative day in patients with normal kidney function. They examined several surrogate measures for kidney function including urinary sodium and CrCl. CrCl estimations are based on steady state measurements and so are inaccurate in AKI with fluctuating creatinine levels. Lee 2007 found a reduction in CrCl of 16 mL/min (95% CI 5 to 28) in patients treated with NSAIDs. Since this review, there is now a universally agreed definition for AKI based on SCr or urine output which has been adopted by KDIGO (KDIGO 2012). The studies included in the previous review also excluded patients with co‐morbidities and so these results cannot be applied to the general population undergoing surgery as many these will be older patients with co‐morbidities.
Authors' conclusions
Implications for practice.
There is a lack of evidence about the safety of NSAIDs used in the peri‐operative period in all patients; patients with co‐morbidities were excluded and NSAIDs had uncertain effects on AKI and the need for RRT. Whilst, NSAIDs may be safely used in fit, healthy patients, care should be employed in high risk patients. We were unable to identify which patients are at risk based on the results of this review and so clinical judgement should be employed based on the individual and alternative analgesic strategies may need to be employed in selected cases.
Implications for research.
Our analysis was limited to small studies excluding patients with co‐morbidities. Several of the studies were designed to investigate AKI as a secondary outcome and used varying definitions for AKI. The indication for NSAID was not analgesia in all of the studies and the doses varied. Several studies excluded patients if their creatinine rose post‐operatively or their urine output fell. Further larger studies using the KDIGO definition for AKI including patients with co‐morbidities are required to confirm our findings.
Acknowledgements
We would like to thank the referees for their feedback and advice during the preparation of this review. We would like to thank Cochrane and Kidney Transplant's Information Specialist for their help.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 3. Decisions based on five GRADE criteria about certainty of evidence from RCTs in Summary of Findings Table
Outcome: post‐operative acute kidney injury
| Criterion | Evidence | Decision |
| Risk of bias | NAFARM 2011 had unclear selection bias but the POISE‐2 2013 was at low risk of bias | Not serious |
| Imprecision | 7066 participants and 897 events | Serious: POISE‐2 2013 had few events while NAFARM 2011 was stopped early |
| Inconsistency | Chi2 = 2.43, df = 1 (P = 0.12); I2 = 59% | Serious: the risk of AKI in the control groups was completely different: 1.2% in NAFARM 2011versus 12.3% in POISE‐2 2013 |
| Indirectness | The indications for NSAID were prevention of AKI (POISE‐2 2013) or atrial fibrillation (NAFARM 2011) rather than analgesia | Serious because the doses for these indications were lower than the doses that would be used for analgesia in routine care |
| Publication bias | Large studies, not commercially sponsored other than supply of intervention drugs and placebo | Not serious |
Outcome: difference in increase in post‐operative serum creatinine
| Criterion | Evidence | Decision |
| Risk of bias | Eight studies had high risk of bias overall or high risk of attrition bias | Not serious: the mean difference in SCr was higher in the six studies with low or unclear risk of bias (3.45, 0.12 to 6.78) than in all 15 studies (3.23, ‐0.80 to 7.26) |
| Imprecision | 794 participants | Not serious |
| Inconsistency | Chi2 = 40.98, df = 14 (P = 0.0002); I2 = 66% | Serious: the inconsistency was not adequately explained by pre‐specified effect modifiers (Table 2) |
| Indirectness | All of the studies had multiple exclusion criteria, including age in 6 (Table 2) | Serious: the patients in these RCTs are likely to be different from those in routine care. The results of Kulik 2004 (decrease in SCr associated with naproxen for 5 days after cardiac surgery) are in stark contrast to the results of NAFARM 2011 (trial stopped because of excess risk of acute kidney injury associated a lower dose of naproxen for 5 days after cardiac surgery) |
| Publication bias | None of the studies were commercially sponsored | Not serious |
Outcome: renal replacement therapy
| Criterion | Evidence | Decision |
| Risk of bias | Rafiq 2014 was at high risk of performance and detection bias | Serious |
| Imprecision | 7056 participants, 31 events | Serious, few events |
| Inconsistency | Chi2 = 1.35, df = 1 (P = 0.25); I2 = 26% | Serious: the risk of RRT in the control groups was completely different: 0.3% in NAFARM 2011 versus 2.7% in Rafiq 2014 |
| Indirectness | The indications for NSAID was prevention of atrial fibrillation in the largest study (NAFARM 2011) rather than analgesia | Serious because the dose of naproxen in NAFARM 2011 was much lower than would be used for analgesia |
| Publication bias | None of the studies were commercially sponsored | Not serious |
Outcome: death
| Criterion | Evidence | Decision |
| Risk of bias | Rafiq 2014 was at high risk of performance and detection bias | Serious |
| Imprecision | 312 participants, 8 events | Serious: few events |
| Inconsistency | Chi2 = 1.61, df = 1 (P = 0.20); I2 = 39% | Serious: the relative risk of death was in opposite directions: 3.85 in NAFARM 2011 versus 0.48 in Rafiq 2014 |
| Indirectness | The indications for NSAID was prevention of atrial fibrillation in the largest study (NAFARM 2011) rather than analgesia | Serious: the dose of naproxen in NAFARM 2011 was much lower than would be used for analgesia |
| Publication bias | None of the studies were commercially sponsored | Not serious |
Outcome: length of hospital stay
| Criterion | Evidence | Decision |
| Risk of bias | Rafiq 2014 was at high risk of performance and detection bias; Kulik 2004 was at high risk of attrition bias | Serious |
| Imprecision | 410 participants | Not serious: length of stay measured in all participants |
| Inconsistency | Chi2 = 2.63, df = 1 (P = 0.27); I2 = 24% | Serious: mean length of stay in control groups varied from 5.4 (Kulik 2004) to 17.2 days (NAFARM 2011) |
| Indirectness | The indications for NSAID was prevention of atrial fibrillation in the largest study (NAFARM 2011) rather than analgesia | Serious: the dose of naproxen in NAFARM 2011 was much lower than would be used for analgesia |
| Publication bias | None of the studies were commercially sponsored | Not serious |
Data and analyses
Comparison 1. Acute kidney injury.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 AKI | 2 | 7066 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.40, 7.96] |
Comparison 2. Serum creatinine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum creatinine (all studies) | 15 | 794 | Mean Difference (IV, Random, 95% CI) | 3.23 [‐0.80, 7.26] |
| 2 Serum creatinine (excluding high risk of bias) | 7 | 429 | Mean Difference (IV, Random, 95% CI) | 2.64 [‐1.28, 6.55] |
| 3 Serum creatinine (excluding high attrition bias) | 11 | 601 | Mean Difference (IV, Random, 95% CI) | 2.96 [‐1.57, 7.49] |
| 4 Serum creatinine (excluding high risk of bias or high attrition bias) | 6 | 331 | Mean Difference (IV, Random, 95% CI) | 3.57 [‐1.35, 8.48] |
Comparison 3. Urine output.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Urine output | 6 | 149 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.21, 0.24] |
Comparison 4. Need for renal replacement therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 RRT | 2 | 7056 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.49, 5.07] |
Comparison 5. Death due to any cause.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death due to any cause | 2 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.19, 11.12] |
Comparison 6. Length of hospital stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | 3 | 410 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.48, 0.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aitken 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as "double‐blind"; insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 67 patients were randomised, of which 63 patients were included in the patient data table. Six patients were withdrawn from the study after 24 or 48 h of treatment due to equipment failure or on patient's request. No data on randomisation of the withdrawn patients. Of remaining 61 patients there is missing data from 15 patients, probably equally distributed amongst the intervention and placebo groups. |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | High risk | A commercial funding source was used for this study |
Brinkmann 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | All intended measurements were reported at baseline, 1 and 6 hours after cross‐clamping, and on the first POD |
| Other bias | Unclear risk | The study was conducted by the anaesthetics department of the University of Ulm. There is no mention of funding sources |
Eljezi 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in an envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study drug was prepared by an anaesthetist nurse not involved of post‐operative care, under the control of the anaesthetist in charge of the patient, who opened the allocation envelope |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no incomplete data |
| Selective reporting (reporting bias) | High risk | Methods state 4 hourly urinary output measurements until 48 h post‐operatively and SCr measurement for POD 1 and POD 2. No urinary output results documented for 0 – 44 h. SCr documented at baseline and a percentage rise at 48 h reported. No results for POD 1 reported |
| Other bias | Low risk | The study was conducted by the Department of Anesthesiology (Medecine Peri‐Operatoire) and the Clinical Pharmacology centre (CPC‐CIC) of the University Hospital of Clermont‐Ferrand (CHU Clermont‐Ferrand), France. The sponsorship was limited to supplies and expenses. The sponsorship included payment for employees for study design, patient’s inclusion, data entry, and analysis of the data. They also provided the study drugs at no cost. They had no influence or interference after the protocol was designed |
Fayaz 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control Group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization code was computer generated by Lab View version 2" |
| Allocation concealment (selection bias) | Low risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drugs made up by pharmacist |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/60 patients withdrawn. Equally distributed across study groups and similar reasons for withdrawal given. 2 patients were withdrawn before entering the study due to oliguria and an early post‐operative SCr rise (> 20% from baseline) |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Hynninen 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Randomisation and preparation of study drug in identically shaped suppositories was done by hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation and preparation of study drug in identically shaped suppositories was done by hospital pharmacy |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/114 patients withdrawn. Of these six patients, 1 patient was withdrawn after one dose of indomethacin because of SCr increase > 20% post‐operatively. This patient did not receive further NSAIDs as per protocol and was not included in the post‐operative outcome table. This event was mentioned in the discussion of the paper. The plausible effect size of this one event is probably not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Immer 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 out of 69 patients were excluded post‐operatively, prior to randomisation. One of these patients was withdrawn due to a post‐operative SCr rise (> 150 µmol/L) |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | High risk | A commercial funding source was used for this study |
Irwin 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient withdrew from study; reason for missing outcome data unlikely to be related to outcome |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Jones 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Roche pharmaceuticals coded and allocated 30 patients using random number tables |
| Allocation concealment (selection bias) | Low risk | Study drugs made up by Roche pharmaceuticals |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The allocation was not released until the end of clinical data collection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The allocation was not released until the end of clinical data collection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | High risk | A commercial funding source was used for this study |
Khalil 2006a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation using a number generator |
| Allocation concealment (selection bias) | Low risk | A third party placed the results of the randomisation in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Envelopes were opened at close of the surgery and a third party prepared the study medication (placebo or treatment) which looked identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Initial power calculations resulted in an intended study population size of 60 patients. Following a global announcement of Pfizer that parecoxib was ‘contraindicated in patients with ischaemic heart disease’ further inclusion in the study was terminated at 40. Data of all 40 patients is presented |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | High risk | Commercial funding source Pharmacia, which is the manufacturer of Parecoxib |
Koppert 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | All study medication solutions were prepared by a hospital pharmacist who was not involved in the data collection |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The anaesthesiologist, nursing staff, and the investigators were all blinded to the treatment. At the surgical ward, patients and nursing staff were unblinded to the medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight of 83 patients withdrew from study. Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | High risk | A commercial funding source was used for this study |
Kostamovaara 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three of 76 patients withdrawn from study; reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | Non‐profit organisation funding received |
Kulik 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Medication was prepared by hospital pharmacy and appeared identical |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication administration and data collection were done in a double blinded fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Medication administration and data collection were done in a double blinded fashion |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 of 98 patients withdrawn from the study, of these one patient had a baseline creatinine of 115 µmol/L pre‐operatively. Remainder of the reasons for missing outcome data unlikely to be related to true outcome Despite 16 patients did not receive the intervention as allocated on randomisation ‐ post‐operative results of all 98 patients presented. The plausible effect size among missing outcomes enough to induce clinically relevant bias in observed effect size |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Laisalmi 2001a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | Non‐profit organisation funding received |
McCrory 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Two types of administration ‐ spinal and epidural. Each were assigned to following 3 groups Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Anaesthetists and patients did have knowledge of the study and allocated treatment group. This knowledge is unlikely to influence the primary renal outcome; 24 hour urinary creatinine Nursing staff was unaware of patients participating in the study and will therefore not impact on the pain score outcomes presented |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | Study conducted in a university teaching hospital, non‐profit academic research grand received |
NAFARM 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The pharmacist made the randomization list and allocated the placebo and naproxen pills without the knowledge of any other person" |
| Allocation concealment (selection bias) | Low risk | Sealed envelope, medication appears identical, nursing staff giving out drugs are not part of the investigation team |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sealed envelope, medication appears identical, nursing staff giving out drugs are not part of the investigation team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sealed envelope, medication appears identical, nursing staff giving out drugs are not part of the investigation team |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results of all 161 randomised patients reported for primary and secondary outcomes |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ott 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After randomisation and the administration of at least one dose of the study drug, 26% of the 462 patients (equally distributed between groups) were withdrawn from the study. Most frequent reason for withdrawal was an adverse event (15.6%) of which 1.3% in the control group and 1.9% in the NSAID group were due to rise in creatinine. Plausible effect size among missing outcomes enough to induce clinically relevant bias in observed effect size |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Perttunen 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medication looks identical; double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurse who made up the infusions was not involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing secondary outcome data from 4/30 patients; insufficient reporting of reason behind missing data to permit judgement. |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | Non‐profit organisation funding received |
Perttunen 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Envelopes were sealed and opened by nurse who was not involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Envelopes were sealed and opened by recovery nurse who made up the infusions. This nurse was not involved in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to allocation of infusions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | Non‐profit organisation funding received |
POISE‐2 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2 (not included in meta‐analyses)
Control group 1
Control group 2 (not included in meta‐analyses)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, clinicians, data collectors, and outcome adjudicators were blinded to the allocation of each intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients, clinicians, data collectors, and outcome adjudicators were blinded to the allocation of each intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 5% missing creatinine values were reported. Multiple imputation models were used to handle missing data, which all yielded similar results |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | Contribution of funding sources unclear, however financial support provided by two large governmental non‐profit organisations. The authors state that the sponsors had no role in the design and conduct of the study, collection, management, analysis, review or approval of the manuscript; and decision to submit the manuscript for publication |
Power 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was reported as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Standardised management for intervention and anaesthetic technique and fluid therapy, however unclear how patients and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 out of 20 patients, randomised to the active study drug group, was withdrawn after 18 h due to oliguria and severe AKI. It is plausible that the effect is enough to induce clinically relevant bias in observed effect size |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Puolakka 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation |
| Allocation concealment (selection bias) | Low risk | Random numbers in opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Nurse not involved in the study made up the study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients withdrawn. Missing data from 2 patients at variable time points. Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | Non‐profit organisation funding received |
Rafiq 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 180 randomised patients, 29 patients were withdrawn prior to administration of the study drug. Missing outcome data balanced in numbers across intervention group with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Maximum SCr reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rapanos 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation carried out by pharmacy department |
| Allocation concealment (selection bias) | Low risk | Sequential selection of previously randomised envelopes; envelopes containing study drug or placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs and placebo suppositories in envelopes appearing similar |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study drugs and placebo suppositories in envelopes appearing similar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Slaven 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed by pharmacist, randomisation technique unknown |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active agent and placebo drugs were delivered to the theatre room in prefilled syringes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Low risk | The tenocixam‐placebo was a gift from Roche Products New Zealand (the manufacturer). The study design and analysis of the results were independent of any pharmaceutical company involvement. |
Turner 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Sequential selection of previously randomised envelopes, study drugs appearing identical |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, nursing staff and medical staff were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients, nursing staff and medical staff were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 50 patients were included in the study, 2 patients were withdrawn due to protocol violation. Of the remaining 48 patients kidney outcome data was available from 38 patients (11% missing data). No reasons for missing data was provided |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Varrassi 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 out of 100 patients were withdrawn from the study after randomisation and administration of the study drug. Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Study protocol matches outcomes presented |
| Other bias | Unclear risk | Insufficient information to permit judgement |
AKI ‐ acute kidney injury; ASA ‐ American Society of Anesthesiologists; CrCl ‐ creatinine clearance; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; GI ‐ gastrointestinal; ICU ‐ intensive care unit; IM ‐ intramuscular; IV ‐ intravenous; MI ‐ myocardial infarction; NSAIDs ‐ nonsteroidal anti‐inflammatory drugs; POD ‐ post‐operative day/s; RRT ‐ renal replacement therapy; SCr ‐ serum creatinine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Castiglione 1997 | Wrong control group: control group also received ketorolac |
| Cheruku 2004 | Wrong population: patients included with a SCr up to 2.0 mg/dL Insufficient post‐operative outcome measures reported; kidney function given for 3/100 patients only; all of those had a SCr above 2.0 mg/dL |
| Chow 2001 | Wrong population: one third of patients underwent a nephrectomy; patients were excluded when a significant SCr rise was noted |
| Daniels 2014 | Abstract‐only publication; no kidney function outcome measures documented |
| Doyle 1998 | Wrong control group: patients randomised to 2 analgesic regimens |
| Fredman 1999 | No relevant post‐operative kidney outcome measures |
| Grimsby 2012 | Wrong population: patients with CKD were included and 111/128 patients underwent a nephrectomy |
| Hynes 2006 | No relevant post‐operative kidney outcome measures |
| Leeson 2007 | Kidney function parameters not clearly defined |
| Ma 2015 | No kidney function parameters documented |
| Merry 2002 | Wrong population: patients with CKD included |
| Nussmeier 2005 | Included 6 (1%) of patients with kidney insufficiency. Post‐operative kidney failure or dysfunction reported at any time during the 30 days after surgery. No data given for the first 2 days after surgery |
| Nussmeier 2006 | Patients were included when kidney disease was deemed significant by the investigator; 6 patients had kidney insufficiency (unknown eGFR) on randomisation. Adverse events were recorded. No SCr or urine output individually reported |
| Nuutinen 1991 | No concise kidney outcome measures reported |
| Parker 1994 | No concise kidney outcome measures reported |
| Rao 2000 | Ambiguity regarding inclusion criteria. Patients excluded when ‘significant renal disease’ was present. No concise kidney outcomes documented; 1 patient developed transient kidney failure |
| Ready 1994 | No concise kidney outcome measures reported |
| Southworth 2009 | No concise kidney outcome measures reported |
| Varrassi 1999 | No concise kidney outcomes measures reported; comment made that there was not statistically significant difference between treatment groups |
CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; eGFR ‐ estimated glomerular filtration rate; SCr ‐ serum creatinine
Differences between protocol and review
We did not perform a search for observational studies as outlined in the protocol as we found sufficient RCTs to address the aim of the review. We included two studies that had an objective other than pain relief in this review. NAFARM 2011 studied the effects of naproxen on prevention of atrial fibrillation after coronary artery bypass grafting and the POISE‐2 2013 studied adverse effects of low dose aspirin following non‐cardiac surgery.
Contributions of authors
Draft the protocol: SB
Study selection: SB, TR
Extract data from studies: SB, TR
Enter data into RevMan: SB
Carry out the analysis: SB, CM, PD
Interpret the analysis: SB, CM,PD
Draft the final review: SB, TR, CM, PD
Disagreement resolution: PD
Update the review: SB
Sources of support
Internal sources
University of Dundee and National Health Service Tayside, UK.
External sources
No sources of support supplied
Declarations of interest
Samira Bell: none known
Charis A Marwick: none known
Trijntje Rennie: none known
Peter Davey: none known
New
References
References to studies included in this review
Aitken 1992 {published data only}
- Aitken HA, Burns JW, McArdle CS, Kenny GN. Effects of ketorolac trometamol on renal function. British Journal of Anaesthesia 1992;68(5):481‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brinkmann 1998 {published data only}
- Brinkmann A, Seeling W, Wolf CF, Kneitinger E, Vogt N, Steinbach G, et al. Ibuprofen does not impair renal function in patients undergoing infrarenal aortic surgery with epidural anaesthesia. Intensive Care Medicine 1998;24(4):322‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eljezi 2017 {published data only}
- Eljezi V, Biboulet C, Boby H, Schoeffler P, Pereira B, Duale C. The dose‐dependent effects of ketoprofen on dynamic pain after open heart surgery. Pain Physician 2017;20(6):509‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Fayaz 2004 {published data only}
- Fayaz MK, Abel RJ, Pugh SC, Hall JE, Djaiani G, Mecklenburgh JS. Opioid‐sparing effects of diclofenac and paracetamol lead to improved outcomes after cardiac surgery. Journal of Cardiothoracic & Vascular Anaesthesia 2004;18(6):742‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hynninen 2000 {published data only}
- Hynninen MS, Cheng DC, Hossain I, Carroll J, Aumbhagavan SS, Yue R, et al. Non‐steroidal anti‐inflammatory drugs in treatment of postoperative pain after cardiac surgery. Canadian Journal of Anaesthesia 2000;47(12):1182‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Immer 2003 {published data only}
- Immer FF, Immer‐Bansi AS, Trachsel N, Berdat PA, Eigenmann V, Curatolo M, et al. Pain treatment with a COX‐2 inhibitor after coronary artery bypass operation: a randomized trial. Annals of Thoracic Surgery 2003;75(2):490‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Irwin 1995 {published data only}
- Irwin MG, Roulson CJ, Jones RD, Cheng IK, Visram AR, Chan YM. Peri‐operative administration of rectal diclofenac sodium. The effect on renal function in patients undergoing minor orthopaedic surgery. European Journal of Anaesthesiology 1995;12(4):403‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Jones 2000 {published data only}
- Jones RD, Endre Z, Miles W, Prankerd R, Chilvers M, Willgoss D. Tenoxicam i.v. for major gynaecological surgery‐‐effects on renal function. Anaesthesia & Intensive Care 2000;28(5):501‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jones RD, Miles W, Prankerd R, Lang C, Chilvers M, Lo SK. Tenoxicam i.v. in major gynaecological surgery‐‐pharmacokinetic, pain relief and haematological effects. Anaesthesia & Intensive Care 2000;28(5):491‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khalil 2006a {published data only}
- Khalil MW, Chaterjee A, MacBryde G, Sarkar PK, Marks RR. Single dose parecoxib significantly improves ventilatory function in early extubation coronary artery bypass surgery: a prospective randomized double blind placebo controlled trial. British Journal of Anaesthesia 2006;96(2):171‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koppert 2006 {published data only}
- Koppert W, Frotsch K, Huzurudin N, Boswald W, Griessinger N, Weisbach V, et al. The effects of paracetamol and parecoxib on kidney function in elderly patients undergoing orthopedic surgery. Anesthesia & Analgesia 2006;103(5):1170‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kostamovaara 1996 {published data only}
- Kostamovaara PA, Laitinen JO, Nuutinen LS, Koivuranta MK. Intravenous ketoprofen for pain relief after total hip or knee replacement. Acta Anaesthesiologica Scandinavica 1996;40(6):697‐703. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kulik 2004 {published and unpublished data}
- Kulik A, Ruel M, Bourke ME, Sawyer L, Penning J, Nathan HJ, et al. Postoperative naproxen after coronary artery bypass surgery: a double‐blind randomized controlled trial. European Journal of Cardio‐Thoracic Surgery 2004;26(4):694‐700. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laisalmi 2001a {published data only}
- Laisalmi M, Eriksson H, Koivusalo AM, Pere P, Rosenberg P, Lindgren L. Ketorolac is not nephrotoxic in connection with sevoflurane anesthesia in patients undergoing breast surgery. Anesthesia & Analgesia 2001;92(4):1058‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Laisalmi M, Teppo AM, Koivusalo AM, Honkanen E, Valta P, Lindgren L. The effect of ketorolac and sevoflurane anesthesia on renal glomerular and tubular function. Anesthesia & Analgesia 2001;93(5):1210‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCrory 2002 {published data only}
- McCrory C, Diviney D, Moriarty J, Luke D, Fitzgerald D. Comparison between repeat bolus intrathecal morphine and an epidurally delivered bupivacaine and fentanyl combination in the management of post‐thoracotomy pain with or without cyclooxygenase inhibition. Journal of Cardiothoracic & Vascular Anesthesia 2002;16(5):607‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NAFARM 2011 {published data only}
- Horbach SJ, Lopes RD, C Guaragna JC, Martini F, Mehta RH, Petracco JB, et al. Naproxen as prophylaxis against atrial fibrillation after cardiac surgery: the NAFARM randomized trial. American Journal of Medicine 2011;124(11):1036‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ott 2003 {published data only}
- Ott E, Nussmeier NA, Duke PC, Feneck RO, Alston RP, Snabes MC, et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. Journal of Thoracic & Cardiovascular Surgery 2003;125(6):1481‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perttunen 1992 {published data only}
- Perttunen K, Kalson E, Heinonen J, Salo J. IV diclofenac in post‐thoracotomy pain. British Journal of Anaesthesia 1992;68(5):474‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perttunen 1999 {published data only}
- Perttunen K, Nilsson E, Kalso E. I.V. diclofenac and ketorolac for pain after thoracoscopic surgery. British Journal of Anaesthesia 1999;82(2):221‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
POISE‐2 2013 {published data only}
- Chludzinski A, Irani C, Mascha EJ, Kurz A, Devereaux PJ, Sessler DI. Protocol understanding and anxiety in perioperative clinical trial patients approached for consent on the day of surgery. Mayo Clinic Proceedings 2013;88(5):446‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Devereaux PJ, POISE‐2 Investigators. Rationale and design of the PeriOperative ISchemic Evaluation‐2 (POISE‐2) trial: an international 2 x 2 factorial randomized controlled trial of acetyl‐salicylic acid vs. placebo and clonidine vs. placebo in patients undergoing noncardiac surgery. American Heart Journal 2014;167(6):804‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Garg AX, Kurz A, Sessler DI, Cuerden M, Robinson A, Mrkobrada M, et al. Aspirin and clonidine in non‐cardiac surgery: acute kidney injury substudy protocol of the Perioperative Ischaemic Evaluation (POISE) 2 randomised controlled trial. BMJ Open 2014;4(2):e004886. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AX, Kurz A, Sessler DI, Cuerden M, Robinson A, Mrkobrada M, et al. Perioperative aspirin and clonidine and risk of acute kidney injury: a randomized clinical trial. JAMA 2014;312(21):2254‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Power 1992 {published data only}
- Power I, Cumming AD, Pugh GC. Effect of diclofenac on renal function and prostacyclin generation after surgery. British Journal of Anaesthesia 1992;69(5):451‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Puolakka 2009 {published data only}
- Puolakka PA, Rintala S, Yli‐Hankala A, Luukkaala T, Harmoinen A, Lindgren L, et al. The effect of parecoxib on kidney function at laparoscopic hysterectomy. Renal Failure 2009;31(4):284‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rafiq 2014 {published data only}
- Rafiq S, Steinbruchel DA, Wanscher MJ, Andersen LW, Navne A, Lilleoer NB, et al. Multimodal analgesia versus traditional opiate based analgesia after cardiac surgery, a randomized controlled trial. Journal of Cardiothoracic Surgery 2014;9:52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rapanos 1999 {published data only}
- Rapanos T, Murphy P, Szalai JP, Burlacoff L, Lam‐McCulloch J, Kay J. Rectal indomethacin reduces postoperative pain and morphine use after cardiac surgery. Canadian Journal of Anaesthesia 1999;46(8):725‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Slaven 1998 {published data only}
- Slaven GM, Walker RJ, Zacharias M, Fawcett JP, Hodgson BF. Tenoxicam does not alter renal function during anaesthesia in normal individuals. Australian & New Zealand Journal of Medicine 1998;28(6):772‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turner 1994 {published data only}
- Turner GA, Gorringe J. Indomethacin as adjunct analgesia following open cholecystectomy. Anaesthesia & Intensive Care 1994;22(1):25‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Varrassi 1994 {published data only}
- Varrassi G, Panella L, Piroli A, Marinangeli F, Varrassi S, Wolman I, et al. The effects of perioperative ketorolac infusion on postoperative pain and endocrine‐metabolic response. Anesthesia & Analgesia 1994;78(3):514‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Castiglione 1997 {published data only}
- Castiglione G, Hauf ME, Panascia E, Scuderi C, Crimi G. Does the preoperative administration of ketorolac improve postoperative analgesia? [La somministrazione preoperatoria di ketorolac migliora l'analgesia postoperatoria?]. Minerva Anestesiologica 1997;63(7‐8):237‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Cheruku 2004 {published data only}
- Cheruku KK, Ghani A, Ahmad F, Pappas P, Silverman PR, Zelinger A, et al. Efficacy of nonsteroidal anti‐inflammatory medications for prevention of atrial fibrillation following coronary artery bypass graft surgery. Preventive Cardiology 2004;7(1):13‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chow 2001 {published data only}
- Chow GK, Fabrizio MD, Steer T, Potter SR, Jarrett TW, Gelman S, et al. Prospective double‐blind study of effect of ketorolac administration after laparoscopic urologic surgery. Journal of Endourology 2001;15(2):171‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Daniels 2014 {published data only}
- Daniels S, Solorio D, Young C. Lower‐dose diclofenac capsules using SoluMatrix fine particle technology provide effective pain relief in a phase 3 study of patients with acute pain following bunionectomy [abstract]. Journal of Pain 2014;15(4 Suppl 1):S77. [EMBASE: 71404540] [Google Scholar]
Doyle 1998 {published data only}
- Doyle E, Bowler GM. Pre‐emptive effect of multimodal analgesia in thoracic surgery. British Journal of Anaesthesia 1998;80(2):147‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fredman 1999 {published data only}
- Fredman B, Zohar E, Golan E, Tillinger M, Bernheim J, Jedeikin R. Diclofenac does not decrease renal blood flow or glomerular filtration in elderly patients undergoing orthopedic surgery. Anesthesia & Analgesia 1999;88(1):149‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Golan E, Fredman B, Tillinger M, Jedeikin R, Bernheim J. Diclofenac(d) does not decrease renal function in elderly patients undergoing surgery under general anasthesia [abstract]. 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998 Jun 6‐9; Rimini, Italy. 1998:184. [CENTRAL: CN‐00484115]
Grimsby 2012 {published data only}
- Grimsby GM, Andrews PE, Castle EP, Nunez R, Mihalik LA, Chang YH, et al. Long‐term renal function after donor nephrectomy: secondary follow‐up analysis of the randomized trial of ketorolac vs placebo. Urology 2014;84(1):78‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grimsby GM, Conley SP, Trentman TL, Castle EP, Andrews PE, Mihalik LA, et al. A double‐blind randomized controlled trial of continuous intravenous Ketorolac vs placebo for adjuvant pain control after renal surgery. Mayo Clinic Proceedings 2012;87(11):1089‐97. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hynes 2006 {published data only}
- Hynes D, McCarroll M, Hiesse‐Provost O. Analgesic efficacy of parenteral paracetamol (propacetamol) and diclofenac in post‐operative orthopaedic pain. Acta Anaesthesiologica Scandinavica 2006;50(3):374‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leeson 2007 {published data only}
- Leeson RM, Harrison S, Ernst CC, Hamilton DA, Mermelstein FH, Gawarecki DG, et al. Dyloject, a novel injectable diclofenac formulation, offers greater safety and efficacy than voltarol for postoperative dental pain. Regional Anesthesia & Pain Medicine 2007;32(4):303‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ma 2015 {published data only}
- Ma W, Wang K, Du J, Luan J, Lou G. Multi‐dose parecoxib provides an immunoprotective effect by balancing T helper 1 (Th1), Th2, Th17 and regulatory T cytokines following laparoscopy in patients with cervical cancer. Molecular Medicine Reports 2015;11(4):2999‐3008. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Merry 2002 {published data only}
- Merry AF, Sidebotham DA, Middleton NG, Calder MV, Webster CS. Tenoxicam 20 mg or 40 mg after thoracotomy: a prospective, randomized, double‐blind, placebo‐controlled study. Anaesthesia & Intensive Care 2002;30(2):160‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nussmeier 2005 {published data only}
- Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. New England Journal of Medicine 2005;352(11):1081‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nussmeier 2006 {published data only}
- Nussmeier NA, Whelton AA, Brown MT, Joshi GP, Langford RM, Singla NK, et al. Safety and efficacy of the cyclooxygenase‐2 inhibitors parecoxib and valdecoxib after noncardiac surgery. Anesthesiology 2006;104(3):518‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nuutinen 1991 {published data only}
- Nuutinen L, Laitinen J. The effect of a nonsteroidal anti‐inflammatory drug, diclofenac, on renal function in patients undergoing total hip replacement operation [abstract]. Acta Anaesthesiologica Scandinavica 1991;35(Suppl 96):31. [DOI] [PubMed] [Google Scholar]
Parker 1994 {published data only}
- Parker RK, Holtmann B, Smith I, White PF. Use of ketorolac after lower abdominal surgery. Effect on analgesic requirement and surgical outcome. Anesthesiology 1994;80(1):6‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rao 2000 {published data only}
- Rao AS, Cardosa M, Inbasegaran K. Morphine‐sparing effect of ketoprofen after abdominal surgery. Anaesthesia & Intensive Care 2000;28(1):22‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ready 1994 {published data only}
- Ready LB, Brown CR, Stahlgren LH, Egan KJ, Ross B, Wild L, et al. Evaluation of intravenous ketorolac administered by bolus or infusion for treatment of postoperative pain. A double‐blind, placebo‐controlled, multicenter study. Anesthesiology 1994;80(6):1277‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Southworth 2009 {published data only}
- Southworth S, Peters J, Rock A, Pavliv L. A multicenter, randomized, double‐blind, placebo‐controlled trial of intravenous ibuprofen 400 and 800 mg every 6 hours in the management of postoperative pain. Clinical Therapeutics 2009;31(9):1922‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Varrassi 1999 {published data only}
- Varrassi G, Marinangeli F, Agro F, Aloe L, Cillis P, Nicola A, et al. A double‐blinded evaluation of propacetamol versus ketorolac in combination with patient‐controlled analgesia morphine: analgesic efficacy and tolerability after gynecologic surgery. Anesthesia & Analgesia 1999;88(3):611‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Bucaloiu 2012
- Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney International 2012;81(5):477‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carmichael 2003
- Carmichael P, Carmichael AR. Acute renal failure in the surgical setting. ANZ Journal of Surgery 2003;73(3):144‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chertow 2005
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology 2005;16(11):3365‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chou 2011
- Chou R, McDonagh MS, Nakamoto E, Griffin J. Analgesics for osteoarthritis: an update of the 2006 Comparative Effectiveness Review. Comparative Effectiveness Reviews, No. 38. Rockville (MD): Agency for Healthcare Research and Quality, 2011. [PUBMED: 22091473] [PubMed] [Google Scholar]
Chou 2016
- Chou, R, Gordon, DB, de Leon‐Casasola, OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council.[Erratum appears in J Pain. 2016 Apr;17(4):508‐10 Note: Dosage error in article text; PMID: 27036536]. Journal of Pain 2016;17(2):131‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coca 2012
- Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta‐analysis. Kidney International 2012;81(5):442‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2009
- Derry CJ, Derry S, Moore RA, McQuay HJ. Single dose oral ibuprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001548.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012a
- Derry S, Moore RA. Single dose oral aspirin for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD002067.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012b
- Derry S, Moore RA. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD004233.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ftouh 2013
- Ftouh S, Thomas M, Acute Kidney Injury Guideline Development Group. Acute kidney injury: summary of NICE guidance. BMJ 2013;347:f4930. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
KDIGO 2012
- KDIGO (Kidney Disease: Improving Global Outcomes) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney International ‐ Supplement 2012;2(1):1‐138. [Google Scholar]
Lassnigg 2004
- Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. Journal of the American Society of Nephrology 2004;15(6):1597‐605. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2007
- Lee A, Cooper MG, Craig JC, Knight JF, Keneally JP. Effects of nonsteroidal anti‐inflammatory drugs on postoperative renal function in adults with normal renal function. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD002765.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDaid 2010
- McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non‐selective non‐steroidal anti‐inflammatory drugs (NSAIDs) for the reduction of morphine‐related side effects after major surgery: a systematic review. Health Technology Assessment (Winchester, England) 2010;14(17):1‐153, iii‐iv. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MHRA 2009
- Medicines and Healthcare Products Regulatory Agency. Drug Safety Update: Volume 2 Issue 10, May 2009. http://webarchive.nationalarchives.gov.uk/20141206024920/http://www.mhra.gov.uk/Publications/Safetyguidance/DrugSafetyUpdate/CON046451 Vol. (accessed 19 April 2018).
NICE 2017
- NICE National Institute for Health and Care Excellence. British National Formulary, Aspirin. https://www.evidence.nhs.uk/formulary/bnf/current/4‐central‐nervous‐system/47‐analgesics/471‐non‐opioid‐analgesics‐and‐compound‐analgesic‐preparations/aspirin 2017.
O'Rourke 2002
- O'Rourke K. Mixed means and medians: a unified approach to deal with disparate outcome summaries. Symposium on systematic reviews: pushing the boundaries. Oxford, 2002; Vol. 49.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Whelton 1999
- Whelton A. Nephrotoxicity of nonsteroidal anti‐inflammatory drugs: physiologic foundations and clinical implications. American Journal of Medicine 1999;106(5B):13S‐24S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bell 2014a
- Bell S, Marwick CA, Rennie T, Davey P. Effects of peri‐operative nonsteroidal anti‐inflammatory drugs on postoperative kidney function for adults with normal kidney function. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011274] [DOI] [PMC free article] [PubMed] [Google Scholar]


