Abstract
Background
Current treatment modalities for cancer have been successful in achieving improved survivorship; however, they come with a number of long‐term adverse effects. Accidental falls are a common and clinically significant adverse event in people living with and beyond cancer and rates are higher than in the rest of the population.
Objectives
To assess the effects of prescribed or provided exercise for reducing accidental falls, and falls risk factors of strength, flexibility and balance, in people living with and beyond cancer.
Search methods
We searched the following electronic databases from inception to 10 July 2018, with no restrictions: CENTRAL, MEDLINE, Embase, and seven other databases. We searched clinicaltrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP) for ongoing trials, and reference lists of reviews and retrieved articles for additional studies.
Selection criteria
We included all randomised controlled trials investigating exercise interventions versus no treatment, usual care or non‐exercise interventions on falls incidence or falls risk factors in adults living with and beyond cancer (18 years of age or older at diagnosis). We excluded cross‐over studies and studies in acute or inpatient hospice care.
Data collection and analysis
At least two review authors independently completed data extraction for included papers. We used Covidence software to manage screening, data collection and extraction. We assessed evidence using GRADE and presented results in a 'Summary of findings' table.
Main results
Eleven studies (835 participants) compared exercise to usual care. No studies compared exercise with no treatment or non‐exercise interventions. The quality of the evidence was very low for the primary outcome rates of falls, and very low to low for the secondary outcomes. We downgraded the evidence due to study limitations (risk of bias), and issues of imprecision due to small sample sizes, inconsistency and indirectness. All studies were at high risk of bias for blinding of participants and personnel due to inability to blind participants to an exercise intervention. Risk of bias was generally low or unclear for other categories.
There was generally little information on the important outcomes comparing exercise to usual care.
Rates of falls and number of fallers: one study (223 participants) measured accidental falls, but reported neither the rate of falls or the number of fallers; there was no difference in the number of falls between exercise and usual care (very low‐quality evidence).
Strength: 10 studies (813 participants) reported on strength outcomes. Two analyses favoured exercise over usual care: quadriceps strength (2 studies, 72 participants; mean difference (MD) 8.99 kg, 95% confidence interval (CI) 1.29 to 16.70; low‐quality evidence), and leg press (4 studies, 388 participants; MD 21.1 kg, 95% CI 8.47 to 33.74; low‐quality evidence). In one analysis of the Sit‐to‐Stand Test, there was no difference between exercise and usual care (4 studies, 214 participants; standardised mean difference (SMD) –0.45, 95% CI –1.05 to 0.14; very low‐quality evidence).
Flexibility: one study (21 participants) reported on flexibility for Sit‐and‐Reach Distance (MD 2.05 cm, 95% CI 0.59 to 3.51; very low‐quality evidence).
Balance: five studies (350 participants) measured three different balance outcomes. Two analyses favoured exercise over usual care: postural balance (4 studies, 127 participants; standardised mean difference (SMD) 0.44, 95% CI 0.08 to 0.79; very low‐quality evidence), and Backward Walk Test (2 studies, 280 participants; SMD –0.24, 95% CI –0.48 to –0.01; low‐quality evidence). There was no difference between exercise and usual care for the Timed Up‐and‐Go Test (1 study, 15 participants; MD –0.35 seconds, 95% CI –1.47 to 0.77; low‐quality evidence).
Number of people sustaining a fall‐related fracture: the quality of the evidence for exercise reducing fall‐related fractures was very low.
Adverse events: a single study (223 participants) noted some temporary muscle soreness on initiation of exercise or when there was an increase in the weight lifted. As no occurrence data were reported, we could not assess this variable further. No studies reported musculoskeletal injury. Analysis indicated that there was very low‐quality evidence that exercise did not increase fatigue.
Authors' conclusions
There is a paucity of evidence for exercise training to reduce fall rates in people living with and beyond cancer. Exercise training may improve strength, flexibility and balance for people in this population, but the evidence is very low quality.
Plain language summary
Exercise for reducing falls in people living with and beyond cancer
Background
People living with and beyond cancer are at risk of long‐term problems including an increased risk of accidental falls. This is a result of the effect that the disease and the treatment can have on their body. Exercise reduces the rate and risk of falls in older people and is known to improve quality of life, tiredness and pain in people who have had cancer. It is not clear whether exercise can reduce the risk of falls in people living with and beyond cancer. This review was designed to determine the effect of exercise in reducing falls in people living with and beyond cancer.
Study characteristics
In July 2018, we searched for clinical trials about exercise to reduce falls in adults living with and beyond cancer. We found 11 studies of variable quality and size, including a total of 835 people, that compared exercise to usual care. Most of the studies were very small, four with fewer than 30 people. Only one study reported on accidental falls. All 11 studies reported on one or more measures that are risk factors for falling (e.g. strength, flexibility and balance).
Quality of the evidence
We rated the quality of the evidence from the studies using four levels: very low, low, moderate or high. Very low‐quality evidence means that we are very uncertain about the results. High‐quality evidence means that we are very confident in the results. The quality of the evidence was very low to low across all of the measures of interest. There were several weaknesses identified in the design of all studies including small numbers of participants. No study could prevent participants knowing their treatment and so there could have been bias.
Key findings
Only one study looked at the effect of exercise on accidental falls and found no difference in number of falls between people who exercised and people who did not (very low‐quality evidence). Therefore, there were insufficient data for conclusions to be drawn regarding the effects of exercise on reducing accidental falls for people living with and beyond cancer. There was improvement in some factors that are known to affect falls; we found improvement in some measures of strength, flexibility and balance, although the overall quality of this evidence was very low to low.
Summary of findings
Summary of findings 1. Exercise compared with usual care for people living with and beyond cancer.
| Effect of exercise compared with usual care for people living with and beyond cancer | ||||||
|
Patient or population: adults (18 years of age or older at diagnosis)
living with and beyond cancer Settings: outpatients Intervention: exercise Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Exercise | |||||
| Rates of falls per person‐years | — | — | Not estimable | 223 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Twiss 2009 reported number of falls per group only. 107 falls with exercise vs 117 falls with usual care. No data for rate of falls. |
|
Strength through equipment‐based measures: leg press (kg) strength Follow‐up: range 12–26 weeks |
The mean leg press strength was 79 kg | MD 21.1 higher (8.47 higher to 33.74 higher) | — | 388 (4 RCTs) | ⊕⊕⊝⊝ Lowa,d | Galvao 2014 also measured outcome in a subgroup but did not report sample sizes. |
|
Strength through functional measures; Sit‐to‐Stand Test Follow‐up: range 8–26 weeks |
— | SMD 0.45 lower (1.05 lower to 0.14 higher) | — | 214 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,d,e | Galvao 2010; Galvao 2014; Monga 2007 measured Five‐Times Sit‐to‐Stand time (seconds; lower result was better); SMD –0.45 representing an approximate decrease of 1.21 seconds (2.83 seconds lower to 0.38 seconds higher). Vollmers 2018 measured 30‐Second Sit‐to‐Stand (repetitions; higher result is better; data inverted for analysis). SMD –0.45 represents an approximate increase of 0.87 repetitions (0.27 repetitions lower to 2.03 repetitions higher). |
|
Flexibility: Sit‐and‐Reach Distance Test (cm) Follow‐up: 8 weeks |
The mean Sit‐and‐Reach Distance was 11 cm | MD 2.05 higher (0.59 higher to 3.51 higher) | — | 21 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,f | |
| Balance: postural stability | — | SMD 0.44 higher (0.08 higher to 0.79 higher) | — | 127 (4 RCTs) | ⊕⊕⊝⊝ Very lowa,c,g | Cormie 2013; Galvao 2010 measured Sensory Organisation Test (0–100 units higher better); SMD 0.44 representing an approximate increase of 3.20 units (0.58 higher to 5.75 higher). Schwenk 2015; Vollmers 2018 measured medio‐lateral sway – eyes open (cm, lower better – data inverted for analysis). SMD 0.44 represented an approximate decrease of 0.84 cm (0.15 cm lower to 1.50 cm lower). |
|
Balance: Backward Walk Test (seconds) Follow‐up: range 12 weeks to 24 months |
The mean backward walk was 16.4 seconds | SMD 0.24 lower (0.48 lower to 0.01 lower) | — | 280 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | SMD 0.24 representing an approximate decrease of 2.87 seconds (5.74 lower to 0.12 lower). |
| Adverse event: fatigue | — | SMD 0.81 higher (0.34 higher to 1.29 higher) | — | 78 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,d,e | Fatigue was measured using different scales with different scoring and consequently a mean result for the usual care group is not meaningful. Galvao 2010 used the 36‐item Short Form Vitality subscale (0–100 units, higher better); SMD 0.81 representing an increase of 17.1 units (7.2 higher to 27.3 higher). Monga 2007 used the Piper Fatigue Scale (arbitrary units lower better; data inverted for analysis). SMD 0.81 represents an approximate decrease of 1.77 arbitrary units (2.81 lower to 0.74 lower). Due to a difference in values at baseline a control mean was considered. |
| *The basis for the assumed risk (e.g. the median control group risk
across studies) is provided in footnotes. The corresponding risk (and its
95% confidence interval) is based on the assumed risk in the comparison group
and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; SMD: standard mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for study limitations due to high risk of bias due to lack of blinding of participants and assessors.
bDowngraded one level due to imprecision with fewer than 300 falls reported.
cDowngraded one level due to imprecision related to small sample size (fewer than 400 participants).
dDowngraded one level due to inconsistency related to high heterogeneity.
eDowngraded one level due to indirectness due to variability in proximity to acute treatment between studies.
fDowngraded one level for study limitations due to differences in baseline results in this measure between treatment groups in the sole study considered.
gDowngraded one level for indirectness due to differences between studies in the exercise intervention delivered.
Background
Description of the condition
Cancer is a generic term for a group of diseases characterised by the rapid creation of abnormal cells that metastasise into adjoining parts of the body and spread into other organs. Both the disease process and treatments make people with cancer vulnerable and at risk of adverse effects. In 2012, there were 14.1 million new cancer cases, 8.2 million cancer deaths and 32.6 million people living with and beyond cancer (within five years of diagnosis) worldwide (Ferlay 2015). Cancer is more prevalent in older people, where endurance and physiological capacity are diminished (Australian Institute of Health and Welfare 2012).
Current treatment modalities including surgery, radiation and chemotherapies have been successful in improving cancer survivorship (Australian Institute of Health and Welfare 2012). However, with this improved survivorship comes several long‐term adverse effects that place survivors at elevated risk of ongoing morbidity (Australian Institute of Health and Welfare 2012). Generalised effects of cancer and its treatments include fatigue, pain, sedentary behaviour contributing to exercise intolerance and reduced neuromuscular function (Schmitz 2005). Cancer treatment has also been linked to muscle atrophy and bone mineral loss (Freedman 2004; Kumar 2005), peripheral neuropathy (Kuroi 2004), and vestibular ototoxic injury (Bokemeyer 1998; Slattery 2014).
For the purposes of this review, 'beyond cancer' refers to people with a variety of statuses after cancer including people in remission and people who have been cured. One of the most common and clinically significant adverse events in older adults, including people living with and beyond cancer, is falls. Rates of accidental falls in the older general population are high, with 30% to 40% of people falling each year (Czerwinski 2008). In people living with and beyond cancer, rates of accidental falls are higher than in other community dwellers (Bird 2016; Mohile 2011). Over 55% of older adults who fall will sustain an injury (Nevitt 1991), and although most of these injuries are minor, fracture rates are between 6% (Nevitt 1991) and 10% (Tinetti 2003). In the event of a fall there may be an increased risk of fracture in people living with and beyond cancer because of an increased prevalence of osteoporosis due to the adverse effects of treatment and reduced physical activity levels (Rizzoli 2013). Indeed, fracture risks are increased after diagnosis of cancer, with an annualised rate of hip fractures up to 0.4% higher after cancer diagnosis with a concurrent increase in falls (Chen 2009). At one‐year posthip fracture, the resultant mortality is 23% for women and 31% for men in the community‐dwelling population; however, no specific data exist for cancer survivors (Wehren 2003).
Description of the intervention
This review included any exercise intervention of any modality, frequency, duration and intensity that was prescribed or supervised, or both. For the purposes of this review, we considered exercise to be a type of physical activity consisting of planned, structured and repetitive bodily movements done to maintain or improve one or more components of physical fitness (Caspersen 1985). Exercise modalities may include strength or resistance training; endurance, flexibility, balance and gait training; and functional or body awareness activities.
How the intervention might work
The major falls risk factors in the general community that are modifiable through exercise‐based interventions are reduced strength, reduced muscle mass, altered gait patterns and reduced balance control (Rubenstein 2006). In addition, low levels of physical activity and depressive states have been recognised independently as contributing factors. Targeted exercise for an individual has the capacity to reduce falls risk factors by improving exercise tolerance, muscle strength and muscle mass, balance control, gait performance, ankle flexibility and mental health (Rubenstein 2006). Poor performances in each of these factors have been associated with an increased risk of falling (Gillespie 2012).
Different exercise modalities can address each of these areas differently. Aerobic (endurance) exercise preferentially induces adaptations that improve maximum rate of oxygen consumption measured during incremental exercise (VO2max) (Hepple 1997), while resistance training improves muscle strength and mass and ability to perform activities of daily living (Beltran Valls 2014). Balance control is positively impacted by resistance training, flexibility training and balance training programmes (Bird 2009). Mental health is likewise improved by a range of exercise modalities (Morgan 2013).
People living with and beyond cancer are at particularly high risk of falls due to the disease, treatments they receive and related periods of inactivity. In addition, they are more vulnerable to injuries associated with such falls. Structured exercise has been demonstrated to reduce falls rates in community‐dwelling older adults (Gillespie 2012). Therefore, exercise has the potential to reduce falls rates and risk in people living with and beyond cancer.
Why it is important to do this review
Exercise is effective in improving factors such as quality of life, fatigue and pain in people living with and beyond cancer (Cramp 2012; Mishra 2012). While it is intuitive that exercise would be of benefit to people living with and beyond cancer to reduce falls rates, it is not standard practice to promote exercise as a method for falls prevention in this cohort. Furthermore, due to the demanding nature, difficulty obtaining participants and high cost of exercise studies, as well as the tendency for them to be locally driven, they tend to be performed on small sample groups. This reduces the ability of single studies to provide a strong evidence base to inform clinical practice. This review will strengthen the evidence base surrounding the effectiveness of exercise for falls risk in this population and may assist in identifying optimal exercise modalities to reduce falls risk and associated adverse outcomes.
Objectives
To assess the effects of prescribed or provided exercise for reducing accidental falls, and falls risk factors of strength, flexibility and balance, in people living with and beyond cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials with the exception of cross‐over studies, which we excluded due to the potential long‐term learning effects of exercise and the difficulty in determining suitable washout periods.
Types of participants
We included studies involving adults living with and beyond cancer (18 years of age or older at diagnosis). We excluded studies involving participants residing in acute or inpatient hospice care.
Types of interventions
We included any supervised and non‐supervised exercise modality that met the criteria outlined in the ProFaNE taxonomy (Lamb 2011). We accepted studies where the comparison group was provided with no treatment, routine (usual) care or non‐exercise interventions such as relaxation classes, or social group meetings that were considered unlikely to impact on risk factors for falls. We excluded multicomponent interventions where exercise was combined with another intervention and the effect of exercise could not be isolated from the other intervention (e.g. exercise and diet versus usual care). Where the effects of multiple interventions could not be isolated (e.g. exercise and diet and education versus education), we excluded the study.
Types of outcome measures
We constructed a 'Summary of findings' table as set out in the Cochrane Pain, Palliative and Supportive Care Group author guide (AUREF 2012), and recommended in Section 4.6.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), to present our outcome measures that included our primary outcome of number of falls and secondary outcomes which were defined as potential risk factors for falls.
We assessed number of falls as they were provided by the study authors, recorded by falls diaries, prospective and retrospective questionnaires, and telephone interviews.
Primary outcomes
Rates of falls and number of fallers measured prospectively or reported (retrospectively).
Secondary outcomes
We included the following outcomes known to be associated with accidental falls risk using validated instruments as utilised in each study:
strength through equipment‐based (e.g. handheld or isokinetic dynamometer) or functional measures (e.g. Five‐Times Sit‐to‐Stand Test);
flexibility through measurement of active or passive range of motion;
balance and co‐ordination measured through laboratory‐based measures (e.g. force platform indicators including centre of pressure behaviour or position, sway, anterior‐posterior or medio‐lateral stability (Winter 1995)), or functional measures (e.g. functional reach test (Duncan 1990), Timed Up‐and‐Go Test (Podsiadlo 1991), or Berg Balance Scale (Berg 1992);
number of people sustaining a fall‐related fracture;
incidence and severity of potential adverse events (e.g. fatigue, muscle pain, musculoskeletal injury or cardiovascular events).
Whilst cognitive function and mental health are potential risk factors for falls, their more complicated modalities of treatment include medication, psychological interventions and exercise. For this reason, we did not consider these risk factors in this review.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases in July 2018:
Cochrane Central Register of Controlled Trials (CENTRAL) the Cochrane Library, 2018, Issue 6;
MEDLINE (Ovid), 1946 to 10 July 2018;
Embase (Ovid) 1974 to 10 July 2018;
CINAHL (EBSCO) 1982 to 10 July 2018;
SPORTDiscus, to 11 July 2018;
PEDro (Physiotherapy Evidence Database), to 12 July 2018;
Web of Science (SCI‐EXPANDED, CPCI‐S, SSCI, CPCI‐SSH) to 10 July 2018;
SCOPUS, to 13 July 2018;
LILACS to 12 July 2018;
Health Technology Assessment Database, the Cochrane Library, 2016, Issue 4. This database is no longer updated.
We presented the search strategies used in Appendix 1.
Searching other resources
We searched clinicaltrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) for ongoing trials up to July 2018. In addition, we checked reference lists of reviews and retrieved articles for additional studies and performed citation searches on key articles. We contacted experts in the field for unpublished and ongoing trials.
Data collection and analysis
Selection of studies
Two review authors (AW and MB) independently performed independent title and abstract searches and reviewed all manuscripts identified as requiring full‐text review. Reasons for exclusion of manuscripts identified for full‐text review are outlined in Figure 1. We included a PRISMA flow chart in the full review that showed the status of identified studies (Moher 2009), as recommended in Section 11.2.1 of the Cochrane Handbookfor Systematic Reviews of Interventions (Higgins 2011a). We included studies in the review irrespective of whether measured outcome data were reported in a 'usable' way. We planned to seek further breakdowns of data from the study authors in the event that a study contained a heterogenous population and data were not reported separately for the cancer population. We planned to omit data if we were unable to determine the effects of cancer separately to other conditions.
1.
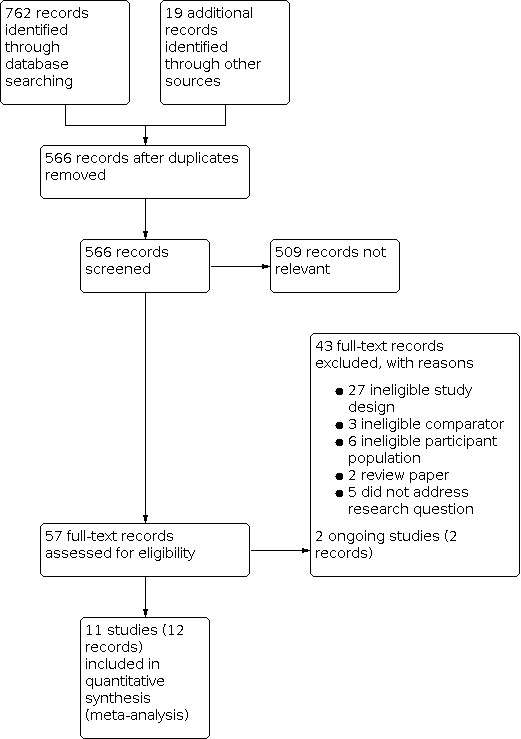
Study flow diagram.
Data extraction and management
Pairs of review authors (AW, MB, MK, SK, KO) completed data extraction independently for each included paper using a standardised data extraction form trialled on the first two papers. Each pair of review authors resolved any discrepancies by discussion. We collected data on the following criteria.
Study details: title, author names, publication status and year.
Study eligibility and characteristics: study type, participant characteristics, type and length of exercise and control interventions and follow‐up periods, outcomes and methods by which these were measured.
Methodological quality: method of sequence generation, allocation concealment, blinding of participants and assessors, incomplete outcome data, selective reporting, intention‐to‐treat analysis and compliance with previously stated methods.
Outcomes: for continuous outcomes, mean, standard deviation and number of participants per group; for dichotomous variables, total number of participants per group and number of participants experiencing the event.
Assessment of risk of bias in included studies
Pairs of review authors also independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion (Higgins 2011b). We completed a 'Risk of bias' table for each included study using the 'Risk of bias' tool in Review Manager 5 (Review Manager 2014).
For each study we assessed the following.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table, computer random number generator) or unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth, hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen, in advance of or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes) or unclear risk of bias (method not clearly stated).
Blinding of participants and personnel (checking for possible performance bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding), unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how this was achieved) or high risk of bias (no blinding was performed).
Blinding of outcome assessors (checking for possible detection bias). We assessed the methods used to blind outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding) or unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how this was achieved).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (less than 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both) or high risk of bias (used 'last observation carried forward' analysis or 'completer' analysis).
Selective outcome reporting (checking all stated outcomes are reported). We compared the results of included studies against protocols where available to determine if all planned variables were reported. We assessed the studies as low risk if it was clear that all results were included when compared against a published or registered protocol or unclear risk where no protocol was available.
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (200 participants or fewer per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm) or high risk of bias (fewer than 50 participants per treatment arm).
Other sources of bias (any methodological issues not covered elsewhere).
Measures of treatment effect
Outcome measures were reported as count data (number of falls), continuous data (measures of strength and flexibility), ordinal data (balance) or dichotomous data (falls/no falls).
If count data occurred commonly, we planned to treat it as continuous outcome data. If it occurred rarely, we planned to analyse it using rates/person‐years of follow‐up and calculate a rate ratio to compare the rates of events between the two groups.
Though unlikely, it was considered possible that falls could be measured as time‐to‐event data. In such cases, we planned to analyse it as dichotomous data, that is whether the event (fall) occurred or not over the time frame of the study, and express as risk ratios (RR; Deeks 2011). We did not feel that hazards ratios were relevant to the outcome of falls as it implied a time to a single event outcome. Given falls can occur multiple times in a single participant it was not seen as the appropriate measure.
Unit of analysis issues
If a cluster‐randomisation method was used, we planned to either analyse the data at the level of allocation using a summary measurement for each group, or if the study reported individual level data, determine whether the study used an appropriate method of adjustment for clustering (e.g. multilevel model, variance components analysis or generalised estimating equations). If an appropriate method was used, we planned to analyse the data using the generic inverse‐variance method. If an appropriate method was not used or it was unclear whether an appropriate method was used, we planned to give further consideration to the most appropriate method of analysis depending on what information was available (Higgins 2011a).
If more than one pair‐wise comparison from a multiarm study was relevant to the same meta‐analysis this may result in double counting of participants within the meta‐analysis and lead to an unaddressed correlation between the estimated intervention effects. To address this, we planned that in the case where there was more than one intervention group, we would combine the intervention groups and treat them as one arm, comparing them with the control group as a pair‐wise comparison.
Dealing with missing data
If an intended outcome was not reported, we endeavoured to contact the trial authors to request additional data. If summary data were not available, we endeavoured to contact the authors to obtain the relevant summary statistics. We examined each study to determine how the authors dealt with missing data and, where appropriate, reported this in the 'Risk of bias' assessment.
If we were unable to obtain data from researchers, we determined the likelihood that missing data were missing at random or if there was likely to be associated bias. We determined this for individual level data by examining for disparity in the numbers of missing data in each arm of the trial and the reasons for participant dropout. In the case that data were missing at random, we planned to perform the analysis by ignoring the missing data. However, if we decided that there was likely to be bias associated with the missing data, we planned to impute the data with an assumed value. This value depended on the data point in question and the perceived potential for bias. The options available to us were to: 1. use the last measure of the same outcome brought forward; 2. impute an assumed outcome, such as the mean of other values; 3. assume a worst‐possible outcome or 4. predict values based on regression. Which method we used would have depended on the outcome measure and have been determined in order to minimise bias (e.g. if we made an assumption that missing data may be related to poor outcome, we would impute a worst‐possible value). If we excluded or imputed data, we planned to discuss the implications of exclusion on the analysis.
If intention‐to‐treat analyses were not performed and the data were available to do so, we planned to perform them (Higgins 2011a). If there were large numbers of missing or incomplete data, we planned to conduct sensitivity analyses of best‐case and worst‐case scenarios (Higgins 2011a).
Assessment of heterogeneity
For outcomes where more than one study was available, we assessed heterogeneity for each outcome using the Chi² test in Review Manager 5 to test deviation of effect sizes from the overall effect (Review Manager 2014). To allow for studies with small sample sizes or a potential low number of studies, we used a P value of 0.10 to indicate significant heterogeneity (Review Manager 2014). We also used the I² statistic to assess the impact of heterogeneity in all meta‐analyses we conducted, interpreting the magnitude according to the recommendations made by Deeks and coworkers (Deeks 2011), with anything over 30% considered to represent possible heterogeneity and requiring further attention.
We used a fixed‐effect meta‐analysis for outcomes with low heterogeneity (P values greater than 0.1 and I² statistic less than 30% or not available). Where there was significant heterogeneity, we used a random‐effects model to account for the heterogeneity.
Assessment of reporting biases
We planned to use funnel plot symmetry to detect publication bias if sufficient studies were available (Sterne 2011). However, given the number of studies available was fewer than 10, we did not perform this analysis.
Data synthesis
Continuous variables were synthesised by calculating the mean difference (MD) as an estimate of effect size, using fixed‐effect or random‐effects MD (depending on heterogeneity) with 95% confidence intervals (CI). Where data for an outcome of interest were not reported in a consistent manner or involved different units of measurement, standardised mean difference (SMD) was used to estimate effect size.
Where studies used rating scales, we ensured that the measurement instrument was validated and that there was no variability or adaptation of the instrument between studies to ensure the validity of conducting a meta‐analysis of results.
For ordinal data, if the number of categories was large, we planned to treat the data as continuous data and analyse accordingly. For shorter ordinal scales, we planned to give consideration to dichotomising the outcomes, if valid, and treating as categorical data. Proportional odds ratios would have been used if either of the above two methods were not valid; however, these were not available in Review Manager 5 (Review Manager 2014).
We planned to synthesise treatment effects from multiple studies using dichotomous variables employing fixed‐effect or random‐effects models, depending on heterogeneity, calculated using RRs with 95% CIs. If the event rate was below 1%, we planned to use Peto odds ratio.
Quality of the evidence
Two review authors (AW, MK) independently rated the quality of each outcome. We used the GRADE system to rank the quality of the evidence using the GRADEprofiler Guideline Development Tool software (GRADEpro GDT 2015), and the guidelines provided in Chapter 12.2 of the CochraneHandbook for Systematic Reviews of Interventions (Higgins 2011b).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate; we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The grade of evidence was:
downgraded once if more than 25% of included studies were at high risk of bias in any criteria (study limitations);
downgraded once if heterogeneity was statistically significant and the I² value was more than 40% (inconsistency);
downgraded once if there were differences between included studies in methodological factors such as modalities of exercise used, differences in stages of treatment or differences in assessment tools used (indirectness);
downgraded once if there were fewer than 400 participants for continuous data or fewer than 300 events for dichotomous data (imprecision) (Guyatt 2011);
downgraded once where there was direct evidence of publication bias.
'Summary of findings' table
We included a 'Summary of findings' table to present the main findings in a transparent and simple tabular format for the comparison exercise compared with usual care. The search identified no studies that compared exercise with no treatment or non‐exercise interventions (i.e. no 'Summary of findings' tables possible). In particular, we included key information concerning the quality of evidence; the magnitude of effect of the interventions examined; and the sum of available data on the outcomes rates of falls, strength, flexibility, balance, and adverse events (fatigue).
Subgroup analysis and investigation of heterogeneity
If there were sufficient data, we intended to perform the following subgroup analyses.
Type of cancer.
Type of cancer treatment (e.g. types of chemotherapy, radiotherapy and surgical treatment).
Intervention characteristics (e.g. exercise type, duration and intensity).
Age of participants (less than 65 years versus 65 years or greater) in line with previous work (Gillespie 2012).
Demographic (gender).
Sensitivity analysis
We planned to conduct sensitivity analyses to determine the impact of including and excluding studies of high risk of bias and large amounts of missing data. Given the limited data available, and generally similar risk of bias across included studies, we did not see the benefit in conducting sensitivity analyses according to risk of bias. Similarly, there was not deemed to be any benefit of conducting sensitivity analyses according to missing data. Where there was significant heterogeneity, we used a random‐effects model for meta‐analysis.
Results
Description of studies
For a description of included and excluded studies, see Characteristics of included studies and Characteristics of excluded studies tables.
We used Covidence software to manage screening, data collection and extraction (Covidence 2018).
Results of the search
We identified 762 potential records through a title and abstract screen from 13 databases (see Search methods for identification of studies) with an additional 19 records identified through other sources. After removal of duplicates, 566 records remained. On the basis of title and abstract, we excluded 509 records as being clearly irrelevant, which resulted in 57 records that underwent full‐text review by two review authors (AW, MB). We contacted one study author to determine whether the study met the inclusion criteria (Winters‐Stone 2012a). We excluded 43 records as they did not meet the inclusion criteria (Characteristics of excluded studies table). We identified 11 studies (12 records) as appropriate for inclusion in this review (Characteristics of included studies table) and two ongoing studies (Characteristics of ongoing studies table). We contacted the corresponding author of two included papers for additional information; one related to sample size for a single variable (Galvao 2014), and the other for additional information regarding reported data (Vollmers 2018). Neither author group responded. The detailed PRISMA flow chart is in Figure 1.
Included studies
Eleven studies met the inclusion criteria (see Criteria for considering studies for this review) and underwent data extraction (Arbane 2011; Brown 2015; Cormie 2013; De Luca 2016; Galvao 2010; Galvao 2014; Monga 2007; Schwenk 2015; Twiss 2009; Vollmers 2018; Winters‐Stone 2016). These studies were all published in English.
Design
All 11 studies included in this review were randomised controlled trials with an exercise intervention for people living with and beyond cancer.
Modes of exercise differed across trials. Three trials prescribed strength training alone (Brown 2015; Twiss 2009; Winters‐Stone 2016), four prescribed strength training in combination with cardiovascular exercises (Arbane 2011; De Luca 2016; Galvao 2010; Galvao 2014), one prescribed a combination of strength and mobility training (Cormie 2013), one prescribed aerobic walking (Monga 2007), one used a combination of unspecified physical exercise training and sensorimotor exercises (Vollmers 2018), and one study used balance training (Schwenk 2015).
The control interventions in all identified studies were considered to be usual care. We found no studies that compared exercise with no treatment or alternative treatments. In nine studies, the control condition received standard treatments with no instruction about exercise or instruction to maintain physical activity (Arbane 2011; Brown 2015; Cormie 2013; De Luca 2016; Galvao 2010; Monga 2007; Schwenk 2015; Twiss 2009; Winters‐Stone 2016). These studies met the definition of usual care as other standard treatments were provided. In one study, the control group received an instruction sheet informing them about the current state of science concerning physical activity in malignant diseases (Vollmers 2018). The final included study provided an exercise booklet and pedometer to the control group (Galvao 2014); however, as an exercise booklet and pedometer were also provided to the intervention group, the comparator was considered to be usual care rather than an alternative treatment. The control condition did not receive an exercise intervention in any trial. Control group participants were asked not to alter their physical activity during the study (Brown 2015; Cormie 2013; De Luca 2016; Schwenk 2015; Winters‐Stone 2016), not to exercise (Monga 2007), provided with an instruction sheet (Vollmers 2018), provided with an education booklet about physical activity guidelines and a pedometer to monitor compliance with the guidelines (Galvao 2014), or provided arm mobilisation in the postsurgery period (Arbane 2011). Two studies did not explicitly identify the control condition except to state that no structured exercise was provided (Galvao 2010; Twiss 2009).
The frequency and duration of individual exercise sessions was variable between the trials. Frequency of the exercise programme ranged from twice daily during the early stages (Arbane 2011), to twice weekly (Brown 2015; Cormie 2013; De Luca 2016; Galvao 2010; Galvao 2014; Schwenk 2015; Twiss 2009; Vollmers 2018; Winters‐Stone 2016), with one study three times a week (Monga 2007). Durations of exercise sessions were not well reported but ranged from as little as 20 minutes to as much as 90 minutes per session. All trials implemented elements of the exercise training programme within an exercise facility at a hospital, university or community fitness centre with three studies also including home‐based components (Arbane 2011; Galvao 2014; Twiss 2009).
The length of the exercise intervention varied greatly between trials ranging from four weeks (Schwenk 2015), to 24 months (Twiss 2009), with a modal exercise intervention of 12 weeks (two trials). Four trials included a supervised exercise period followed by an unsupervised exercise period (Arbane 2011; Brown 2015; Galvao 2014; Twiss 2009). All trials conducted a postexercise assessment immediately following the prescribed exercise intervention period. Two studies provided longer‐term follow‐up, one at 12 months (Galvao 2014), and one at 36 months (Twiss 2009).
Seven studies reported adherence with the intervention based on attendance or self‐reported compliance (or both) with the exercise programme (Brown 2015; Cormie 2013; Galvao 2010; Galvao 2014; Schwenk 2015; Twiss 2009; Winters‐Stone 2016). Six of the seven studies found what would be considered reasonable adherence (Brown 2015; Cormie 2013; Galvao 2010; Galvao 2014; Schwenk 2015; Winters‐Stone 2016), with one reporting weaker adherence (Twiss 2009); however, over a longer time period. Brown and Schmitz reported a median adherence which reduced from 96% of sessions to 65% of sessions across the course of the study (Brown 2015), Cormie found that 83% of participants attended 20/24 sessions (Cormie 2013), Galvao reported high attendance with a mean of 23 out of 24 sessions in one study (Galvao 2010), while in the second study, the authors reported 77% attendance by the exercise group (Galvao 2014). Schwenk and colleagues reported that three of 11 participants randomised to the intervention withdrew from the study but that the remaining eight participants completed all exercise sessions (Schwenk 2015), and Winters‐Stone and colleagues reported that median attendance to exercise sessions was 78% (Winters‐Stone 2016). In contrast, Twiss and colleagues reported a ratio of reported to desired sessions of 24% across the 24 months of the study (Twiss 2009). Four studies did not report adherence to the intervention (Arbane 2011; De Luca 2016; Monga 2007; Vollmers 2018).
Sample sizes
Numbers of participants in the included studies ranged from 19 to 295. There was a total of 835 participants whose data were analysed.
Setting
The 11 included studies were conducted in a variety of settings. One study occurred in an acute care setting in the UK for five days and then the rest of the intervention was carried out in the community (Arbane 2011). Five studies were from the US, one in a community fitness centre (Brown 2015), one in either a fitness centre or exercises delivered at home (Twiss 2009), and three in Academic Medical Centres (Monga 2007; Schwenk 2015; Winters‐Stone 2016). One study was delivered in a university gymnasium in Italy (De Luca 2016). One study was delivered in a university hospital in Germany (Vollmers 2018). One study was a multicentre trial delivered in an outpatient setting across 13 university‐affiliated exercise clinics in Australia and New Zealand (Galvao 2014). The other two came from Perth, Western Australia, one in an exercise clinic (Cormie 2013), and the other in an outpatient setting (Galvao 2010).
Participants
The 11 studies recruited 953 participants with 835 completing the respective intervention and being included in analyses. Thirty two per cent of recruited participants were men (308/953) and 68% were women (645/953). The mean ages for participants in the included studies ranged from 46 to 73 years. Of the 11 studies, five with 271 participants included participants with prostate cancer (Cormie 2013; Galvao 2010; Galvao 2014; Monga 2007; Winters‐Stone 2016). Four studies recruited only women with breast cancer (607; Brown 2015; De Luca 2016; Twiss 2009; Vollmers 2018). Two studies recruited both men and women, one recruited participants with non‐small cell lung cancer (28 men and 25 women; Arbane 2011), and one recruited participants with multiple different cancer diagnoses (9 men and 13 women; Schwenk 2015).
Five studies reported on comorbidities of participants with four reporting incidence of non‐specified comorbidities (Cormie 2013; Galvao 2010; Galvao 2014; Winters‐Stone 2016), and one reporting incidence of hypertension, diabetes mellitus, chronic obstructive pulmonary disease and coronary artery disease in their participant population (Monga 2007). One study reported excluding participants with any other major disease (De Luca 2016); one reported excluding participants with existing cardiopulmonary, metabolic, renal or neurological diseases (Vollmers 2018); while the remaining four studies did not provide any information on other conditions (Arbane 2011; Brown 2015; Schwenk 2015; Twiss 2009).
Excluded studies
Following the title and abstract screen, we assessed 57 records for suitability by full‐text screening and we excluded 43. The basis for exclusion of these records were: ineligible study design (27; Bayego 2012; Betker 2006; Bender 2015; Bylow 2008; Bylow 2011; Curran 2013; Fong 2018; Galantino 2012; Galvao 2006; Grabenbauer 2016; Grote 2017; Hansen 2009; Hanson 2013; Hojan 2013; Holick 2008; Holmes 2005; Huang 2016; Irwin 2008; Kwan 2012; Martin 2016; Overcash 2013; Serdà 2010; Shahinian 2005; Silver 2011; Spoelstra 2010; Sternfeld 2009; Wampler 2007), ineligible comparator (three; Litterini 2013; Winters‐Stone 2011; Winters‐Stone 2012a), ineligible participant population (six; Delecluse 2004; Islam 2004; Pollock 2012; Stineman 2011; Verschueren 2004; Wright 2005), review article (two; Hanson 2011; Keogh 2012), or because it did not address the research question (five; Courneya 2002; Devin 2016; Lee 2014; Rossi 2016; Shobeiri 2016) (Characteristics of excluded studies table).
Ongoing studies
Two studies were protocols and we have added them to Ongoing studies (Bjerre 2016; Winters‐Stone 2012b). We contacted the authors of both studies (July 2017) who confirmed that the studies are not yet complete or are still undergoing data analyses.
Risk of bias in included studies
Our assessment on risk of bias is provided in the Characteristics of included studies tables. In addition, Figure 2 and Figure 3 provide a summary of the risk of bias assessment. All studies were at high risk of bias for blinding of participants and personnel due to the inability to blind participants to an exercise intervention. In contrast, risk of bias was generally low or unclear for other categories.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We planned to perform sensitivity analyses where studies of high and low risk of bias were included in the same analysis. Due to similar risk of bias across all studies and small numbers of studies included in individual analyses, we did not perform this analysis.
Allocation
Random sequence generation
Eight studies demonstrated a low risk of bias by the use of computer‐generated random sequence generation (Arbane 2011; Brown 2015; Cormie 2013; De Luca 2016; Galvao 2010; Galvao 2014; Schwenk 2015; Winters‐Stone 2016). As there was no description of the method of randomisation for the three remaining studies, we judged them at unclear risk of bias (Monga 2007; Twiss 2009; Vollmers 2018).
Allocation concealment
Five studies adequately described how the allocation of sequence was concealed and we judged them at low risk of bias for this domain (Arbane 2011; Cormie 2013; Galvao 2010; Schwenk 2015; Winters‐Stone 2016). The remaining six studies did not adequately describe this process and we judged their risk of bias as unclear (Brown 2015; De Luca 2016; Galvao 2014; Monga 2007; Twiss 2009; Vollmers 2018).
Blinding
Performance bias
We judged all 11 studies at high risk of bias regarding blinding of participants and personnel. The nature of the interventions and the non‐exercise control conditions meant that blinding of the participants was not possible.
Detection bias
We judged three studies at high risk of bias for blinding of outcome assessors (Arbane 2011; Monga 2007; Twiss 2009). Of these three, the assessors also delivered the intervention in Monga 2007, while Twiss 2009 did not blind the outcome assessors. A subset of the assessors were also involved in delivering the intervention in Arbane 2011. Five studies did not indicate whether assessors were blinded and were allocated an unclear risk of bias (Cormie 2013; De Luca 2016; Galvao 2010; Galvao 2014; Vollmers 2018).
Three studies identified that the outcome assessors were blinded and we allocated a low risk of bias (Brown 2015; Schwenk 2015; Winters‐Stone 2016).
Incomplete outcome data
We rated the majority of the studies at low risk as they reported all the data outcomes as stated in their methods (Brown 2015; De Luca 2016; Galvao 2010; Galvao 2014; Schwenk 2015; Twiss 2009; Winters‐Stone 2016).
We allocated a high risk of bias for this domain for the remaining four studies (Arbane 2011; Cormie 2013; Monga 2007; Vollmers 2018). Two studies had more than 15% of participants withdraw after allocation with no reasons for withdrawal provided (Monga 2007; Vollmers 2018). We were unsuccessful in contacting the authors for additional information by which to determine the likelihood of bias associated with these withdrawals. These studies contributed data to two meta‐analyses (strength outcome Sit‐to‐Stand Test: Monga 2007; Vollmers 2018; and postural balance outcome: Vollmers 2018), but data imputation was not possible and given other risks of bias in this and other studies in these meta‐analyses, we took no further action. Arbane 2011 had a high withdrawal rate (15 out of 45 for the variable of muscle strength). While reports described reasons for withdrawal, results may have been biased in a particular direction due to the number of withdrawals. However, because the method for measuring muscle strength in this study (magnetic stimulation) was fundamentally different from other measures of strength, we did not use data from this study in the meta‐analysis. No sensitivity analyses were required, neither did we attempt to impute data for this outcome. Cormie 2013 did not collect data for 25% of participants for the leg extension outcome due to femur bone metastases. Data imputation was not possible and given other risks of bias in this and other studies in this meta‐analysis, we took no further action.
Selective reporting
We assessed two studies at low risk of selective reporting bias, as the reported outcomes were consistent with those reported in the protocols (Galvao 2014; Schwenk 2015). One study was at high risk of bias due to primary outcome measures identified in the published protocol (fatigue, symptoms, depression and strain) not being included in the manuscript (Winters‐Stone 2016). The remaining eight studies were at unclear risk of reporting bias due to an inability to confirm that all the planned measures were reported (Arbane 2011; Brown 2015; Cormie 2013; De Luca 2016; Galvao 2010; Monga 2007; Twiss 2009; Vollmers 2018).
Size of studies
We assessed eight studies at high risk of bias due to having fewer than 50 participants per treatment arm (Arbane 2011; Cormie 2013; De Luca 2016; Galvao 2010; Monga 2007; Schwenk 2015; Vollmers 2018; Winters‐Stone 2016). The three remaining studies were at unclear risk of bias due to including between 50 and 199 participants per treatment group (Brown 2015; Galvao 2014; Twiss 2009).
Other potential sources of bias
We judged two studies at high risk of bias due to baseline differences in variables of interest to this review (Monga 2007; Vollmers 2018). The remaining nine studies were at low risk of other bias (Arbane 2011; Brown 2015; Cormie 2013; De Luca 2016; Galvao 2010; Galvao 2014; Schwenk 2015; Twiss 2009; Winters‐Stone 2016). There were too few studies for any given variable (fewer than 10) to conduct a funnel plot test to look for publication bias.
Effects of interventions
See: Table 1
To be included, studies must have reported on falls or on variables known to affect falls risk including muscle strength, flexibility or balance. We determined that it was valid to perform a meta‐analysis of the following outcomes: quadriceps strength, leg press, Sit‐to‐Stand Test, sensory organisation test, Backward Walk Test and fatigue (random‐effects meta‐analysis due to heterogeneity). For these outcomes, more than one study reported comparable measures and all, except leg press, Sit‐to‐Stand Test and fatigue, demonstrated low heterogeneity. One study reported three further outcome measures that we have reported: falls in person‐years, Timed Up‐and‐go Test and flexibility. As there were insufficient data we did not perform any of the intended subgroup analyses.
A summary of treatment effects using Review Manager 5 is presented in the 'Summary of findings' table (Schünemann 2011).
Exercise versus no treatment
We found no studies comparing exercise versus no treatment.
Exercise versus usual care
Primary outcomes
Rates of falls
Only one study (223 participants) examined the effect of exercise training on falls (Twiss 2009). This study reported the total number of falls in each group over the study period. They reported no significant effect of the exercise intervention on the number of falls between the two groups (107 falls with exercise versus 117 falls with usual care); however, the statistical analysis method they used was unclear. Furthermore, there was not enough information available for us to conduct our own statistical analysis. These data could not be analysed as rates/person‐years or as continuous data (mean number of falls/participant), as the authors did not provide enough information to allow this analysis (i.e. actual number of participants who experienced a fall). We judged the quality of evidence for exercise reducing accidental falls as very low (Table 1). We downgraded the quality of evidence once for study limitations due to the high risk of bias due to lack of blinding for participants and assessors, once for imprecision with fewer than 300 falls reported and once due to imprecision related to small sample size (fewer than 400 participants).
Number of fallers
No study reported on the number of participants who had one or more falls.
Secondary outcomes
Strength through equipment‐based (e.g. handheld or isokinetic dynamometer) or functional measures
Ten of the 11 included studies measured strength although the muscle groups investigated and the type of measure (equipment based or functional measures) varied between studies. All studies reported improvements in muscle strength relative to the control condition.
Four studies measured the strength of the quadriceps muscle group (Arbane 2011; Cormie 2013; Galvao 2010; Twiss 2009); two measured using voluntary one repetition maximum (Cormie 2013; Galvao 2010), one study measured maximal torque achieved as an index of bodyweight (Twiss 2009), and one study used direct magnetic stimulation of the motor nerve (Arbane 2011). We included two studies (72 participants) in a meta‐analysis that measured quadriceps strength using one repetition maximum. The result revealed a significant increase in strength following the exercise intervention versus usual care (MD 8.99 kg, 95% CI 1.29 to 16.70; Chi² = 0.08, degrees of freedom (df) = 1 (P = 0.77); I² = 0%; no heterogeneity; Analysis 1.1).
1.1. Analysis.

Comparison 1: Exercise versus usual care, Outcome 1: Strength: quadriceps
Four studies measured strength using the leg press exercise (Brown 2015; De Luca 2016; Galvao 2010; Winters‐Stone 2016). A meta‐analysis of their results (388 participants) also indicated significant increases in leg press strength in the intervention versus the control groups (MD 21.1 kg, 95% CI 8.47 to 33.74; Tau² = 111.25; Chi² = 19.62, df = 3 (P = 0.0002); I² = 85%; substantial heterogeneity; Analysis 1.2; Figure 4). An additional study presented data on this outcome measure in a subgroup; however, we were unable to include the data in the meta‐analysis due to failure to report the sample size for each arm of the trial for this subgroup (Galvao 2014).
1.2. Analysis.

Comparison 1: Exercise versus usual care, Outcome 2: Strength: leg press
4.

Forest plot of comparison: 1 Exercise versus Usual Care, outcome: 1.2 Leg Press (kg).
Four studies measured functional strength through variations of the Sit‐to‐Stand Test. Three studies measured Five‐times Sit‐to‐Stand (Galvao 2010; Galvao 2014; Monga 2007), and one study measured 20‐Second Sit‐to‐Stand (Vollmers 2018). The meta‐analysis conducted of these results (214 participants) indicated no evidence of a difference (SMD –0.45, 95% CI –1.05 to 0.14; Tau² = 0.26; Chi² = 11.74, df = 3 (P = 0.008); I² = 74%; substantial heterogeneity; Analysis 1.3; Figure 5).
1.3. Analysis.

Comparison 1: Exercise versus usual care, Outcome 3: Strength; Sit‐to‐Stand Test
5.

Forest plot of comparison: 1 Exercise versus usual care, outcome: 1.3 Sit‐to‐Stand Test (strength).
We judged the quality of evidence for exercise increasing strength as very low to low (Table 1). We downgraded the quality of the evidence once for study limitations across all strength measures due to the lack of blinding of participants and assessors. We downgraded the quality once for the quadriceps strength measure due to imprecision related to small sample sizes (fewer than 400 participants). We downgraded the quality for the measure of leg press once due to inconsistency related to high heterogeneity. We downgraded quality once for the Sit‐to‐Stand measure due to inconsistency due to high heterogeneity, and once for indirectness due to variability in proximity to acute treatment between studies.
Flexibility through measurement of active or passive range of motion
One study (21 participants) included flexibility as an outcome measure (Monga 2007). There was a small yet statistically significant increase in whole body flexibility (Sit‐and‐Reach Distance Test) following an exercise intervention versus usual care (MD 2.05 cm, 95% CI 0.59 to 3.51; Analysis 1.4).
1.4. Analysis.

Comparison 1: Exercise versus usual care, Outcome 4: Flexibility: Sit‐and‐Reach Distance Test
We judged the quality of the evidence for exercise increasing flexibility as very low (Table 1). We downgraded the quality of the evidence twice for study limitations due to a high risk of bias as a result of lack of blinding of participants and personnel and for differences in baseline results in this measure between treatment groups in the sole study considered, and once due to imprecision related to the small sample size (fewer than 400 participants).
Balance and co‐ordination measured through laboratory‐based measures
Five studies measured balance (Cormie 2013; Galvao 2010; Schwenk 2015; Twiss 2009; Vollmers 2018). Across the five studies there were three different domains of balance measured, postural stability (Sensory Organisation Test: 91 participants; Cormie 2013; Galvao 2010; and medio‐lateral sway: 55 participants; Schwenk 2015; Vollmers 2018); dynamic balance (Backward Walk Test: 280 participants; Galvao 2010; Twiss 2009); and functional balance (Timed Up‐and‐Go Test; 15 participants; Cormie 2013). The Sensory Organisation Test provides a score out of 100 for balance with a higher score indicating greater balance while medio‐lateral sway is a continuous variable measured in centimetres with a lower value indicating higher stability. Following the exercise intervention, there was a significant improvement in postural stability between participants who received an exercise intervention compared to participants who received usual care (SMD 0.44, 95% CI 0.08 to 0.79; participants = 127; studies = 4; Chi² = 1.89, df = 3 (P = 0.60); I² = 0%; no heterogeneity; Analysis 1.5). The Backward Walk Test measures the time required for the participant to walk backwards for 6 metres (m) or 18 feet. Following the exercise, intervention participants were able to complete this task significantly faster than control participants (SMD –0.24, 95% CI –0.48 to –0.01; participants = 280; studies = 2; Chi² = 0.08, df = 1 (P = 0.77); I² = 0%; no heterogeneity; Analysis 1.6; Figure 6). Timed Up‐and‐Go Test measures the time for the participant to rise from a chair, walk 3 m, turn around and walk back to the chair and sit down. There was no significant change in this measure following an exercise intervention (MD –0.35 seconds, 95% CI –1.47 to 0.77; participants = 15; studies = 1; Analysis 1.7). There was no heterogeneity for all preceding analyses (I² = 0%), so exploration for heterogeneity through subgroup analyses was not required.
1.5. Analysis.

Comparison 1: Exercise versus usual care, Outcome 5: Balance: postural stability
1.6. Analysis.

Comparison 1: Exercise versus usual care, Outcome 6: Balance: Backward Walk Test
6.

Forest plot of comparison: 1 Exercise versus usual care, outcome: 1.6 Backward Walk Test (functional balance in seconds).
1.7. Analysis.

Comparison 1: Exercise versus usual care, Outcome 7: Balance: Timed Up‐and‐Go Test
We judged the quality of the evidence for exercise increasing balance from very low to low (Table 1). We downgraded the quality of the evidence across all balance measures once due to study limitations related to the lack of blinding of participants and assessors and once due to imprecision related to small sample sizes (fewer than 400 participants). We downgraded quality once more for the postural stability for indirectness as a result of differences between studies in the exercise intervention delivered.
Subgroup analyses relating to participant characteristics were not possible because raw data were not available.
Number of people sustaining a fall‐related fracture
One study reported fall‐related fractures (Twiss 2009). The study authors reported 15 fractures (7/110 in the intervention group and 8/113 in the control group). Of these, 13/15 fractures were fall related with none occurring during the prescribed exercise. For the 13 fall‐related fractures, the authors did not identify the incidence in each group or the number of people sustaining a fall‐related fracture. Consequently these data are not included in the review.
We judged the quality of evidence for exercise reducing fall‐related fractures as very low. We downgraded the quality of evidence once for study limitations due to the high risk of bias due to lack of blinding for participants and assessors, once for imprecision due to fewer than 300 fractures reported and once for imprecision due to the small sample size (fewer than 400 participants).
Incidence and severity of potential adverse events
One study reported on the adverse event of pain (Twiss 2009). The authors noted some temporary muscle soreness for up to two days following the initiation of exercise or when there was an increase in the weight lifted. While the incidence was not reported the occurrence of temporary muscle pain when commencing exercise is not uncommon even in healthy populations. The same study also observed discomfort during arm exercises in three women with lymphedema. This issue was solved with adapted exercises for these women.
Two studies (78 participants) assessed the effect of exercise on fatigue (Galvao 2010; Monga 2007). As measurement of this variable in each study used different scales, the meta‐analysis of the results used SMD. The meta‐analysis indicated a statistically significant reduction in fatigue in participants who exercised compared to participants who completed the control interventions, indicating that fatigue was not an adverse event in this context and could potentially be considered a positive outcome (SMD 0.81, 95% CI 0.34 to 1.29; Chi² = 5.43, df = 1 (P = 0.02); I² = 82%; with substantial heterogeneity; Analysis 1.8). We judged the quality of the evidence related to fatigue as very low (Table 1).
1.8. Analysis.

Comparison 1: Exercise versus usual care, Outcome 8: Adverse event: fatigue
We downgraded the quality of the evidence once due to study limitations related to potential bias due to the lack of blinding of participants and assessors; once due to inconsistency due to high heterogeneity within the meta‐analysis (I² = 82%) and once due to indirectness as a results of variability in proximity to acute treatment between studies. Both studies included participants with prostate cancer and a similar intervention. As they both reported decreases in fatigue, we did not explore reasons for heterogeneity further.
Exercise versus non‐exercise interventions
We found no studies comparing exercise versus non‐exercise interventions.
Discussion
Summary of main results
We identified 11 studies comparing the role of exercise of various modalities versus usual care in people living with and beyond cancer for this review. Only one study included accidental falls as an outcome variable and there was limited evidence suggesting a lack of effect on accidental falls in people living with and beyond cancer (Twiss 2009). Exercise training was noted to result in improvements in lower limb strength, flexibility and balance, although only one study measured flexibility (Monga 2007), and five studies measured balance (Cormie 2013; Galvao 2010; Schwenk 2015; Twiss 2009; Vollmers 2018). While there were mean improvements in secondary variables in all studies that investigated that variable, the majority of studies were small and of low methodological quality. No studies were powered specifically to detect a reduction in falls risk. One study conducted a power calculation for secondary outcomes of interest to this review (muscle strength and balance) (Twiss 2009). Of the remaining studies, three conducted a power calculation aimed at detecting change in quality‐of‐life scales (Arbane 2011; Brown 2015; Cormie 2013), one aimed at detecting a change in bodyweight (Galvao 2010), one aimed at detecting a change in body composition (Winters‐Stone 2016), one aimed at detecting a change in 400‐m walk distance (Galvao 2014), one described a sample size calculation without describing what variable was considered (De Luca 2016), and three had no formal sample size calculation (Monga 2007; Schwenk 2015; Vollmers 2018). Therefore, data are insufficient for conclusions to be drawn regarding the effects of exercise training on reducing accidental falls. Only one study reported pain as an adverse event, with minimal discomfort observed.
Overall completeness and applicability of evidence
Only one study investigated the effect of exercise training on accidental falls incidence in cancer survivors (Twiss 2009). While this study did not detect a difference between groups, it was potentially underpowered and only reported the number of falls. It did not indicate whether these were all single falls or if there were repeat fallers. Attempts to gain this information from the authors were unsuccessful. Consequently, we recommend that more evidence is required before any conclusions can be made regarding the role of exercise in reducing falls in people living with and beyond cancer.
The review included measures of lower limb strength that are considered to be potential fall risk factors. These include knee extension as measured by quadriceps strength, combined hip and knee extension as measured by leg press, and functional strength as measured by the Five‐Times Sit‐to‐Stand and 30‐Second Sit‐to‐Stand Tests. One study included a measure of leg strength that relied on muscle stimulation force, which does not appear in the literature as a fall risk factor, therefore strength data from that study were not included in the meta‐analysis. The magnitude of improvement for all of the meta‐analyses related to strength were such that they may be considered clinically relevant for the reduction of falls risk in at‐risk groups (Rubenstein 2006).
Flexibility improved with one intervention; however, it was measured by the Sit‐and‐Reach Distance Test. While ankle range of motion limitation is associated with an increased likelihood of accidental falls, global flexibility has not been shown to influence fall rates in community‐dwelling people, limiting the applicability of this finding.
Dynamic balance (measured by Backward Walk Test) was responsive to exercise in this group, showing positive changes (shorter time to complete the tasks) after the interventions. This construct of balance is an applicable fall risk factor. The magnitude of improvement for the Backward Walk Test was between 2.6 seconds and 5.2 seconds, which is likely to be clinically meaningful (Podsiadlo 1991). Balance, as measured by postural stability was also responsive to exercise. Two of the studies contributing data to this variable recruited participants who had neurotoxic exposure which is known to reduce proprioceptive input and this may explain the improvements seen with balance training in this review. Functional balance, as measured by the Timed Up‐and‐Go Test, was not influenced by exercise in the studies examined in this review.
In healthy community‐dwelling older adults, high‐level evidence for improvements in fall rates with exercise interventions exist and the effective components of the exercise interventions have been identified. Exercise programmes should include balance training and be of sufficient dose. A single intervention included in this review identified a balance component to their training programme (Schwenk 2015), and this may limit the effectiveness of these programmes for improving fall rates (Sherrington 2011).
Quality of the evidence
The quality of evidence for the outcomes in this review using GRADE was low to very low (GRADEpro GDT 2015). The main reason for downgrading results across all studies was due to the failure to blind participants, personnel and assessors to the intervention groups. In the case of participants and personnel, this is unavoidable due to the nature of studies that compare exercise to a non‐exercising control. However, the failure of many studies to blind assessors to the intervention was also a major issue that was avoidable. Only one study reported on falls incidence, and, while the quality of the evidence provided was very low, it should be acknowledged that interpretation of results from this study reflect the limited evidence available (Twiss 2009). There is a larger volume of evidence to support the findings of this review for the secondary outcomes of muscle strength and balance, which are often used as surrogate outcomes of falls risk; however, the quality of the evidence for these outcomes varied from very low to low. Only one study examined the secondary outcome of flexibility and the quality of evidence for this outcome was very low (Monga 2007). In our protocol, we identified that we would perform sensitivity analyses where studies of high and low risk of bias were included in the same analysis (Williams 2015). Due to similar risk of bias across all studies and the small numbers of studies included in individual analyses, we did not perform this sensitivity analysis.
While there is evidence for using the secondary outcome variables as surrogate outcomes for falls risk in older non‐cancer populations (Ambrose 2013; Rubenstein 2006; Spink 2011), there is currently limited evidence that these outcomes translate to the population of people living with and beyond cancer who were investigated in this review.
Potential biases in the review process
In our protocol, we indicated that funnel plot analyses would be utilised to detect publication bias if we found sufficient studies for this analysis (Williams 2015). Unfortunately, there were insufficient studies eligible for inclusion in the review to allow this form of analysis and consequently there was no information regarding the likelihood of publication bias. Heterogeneity as measured by the I² statistic was low (0%) for all primary and secondary outcomes where sufficient studies allowed calculation of this measure with the exception of the strength measures of leg press (I² = 85%) and Sit‐to‐Stand (I² = 74%), which might be due to including both endpoint data and change data in our analyses for these variables for different studies.
Agreements and disagreements with other studies or reviews
This is the first review to assess the effects of prescribed or provided exercise for reducing accidental falls incidence and as such comparisons to other literature is not possible.
Studies awaiting assessment
While there is only one trial that to date has investigated the effect of exercise training on preventing falls in people living with and beyond cancer, we are aware of a study currently underway which has been powered to detect a reduction in falls of 47% or greater over one year (Winters‐Stone 2012b). We expect that this study when completed will provide important additional information regarding the effectiveness of regular exercise in preventing accidental falls in this population and we recommend that this review is updated at that point in time.
Authors' conclusions
Implications for practice.
There is a paucity of evidence for exercise training to reduce falls in people living with and beyond cancer in this review. Exercise training may improve strength, flexibility and balance for people in this population but the evidence is very low quality. This may mitigate potential falls risk and may represent clinically meaningful improvements.
Implications for research.
More high‐quality randomised controlled trials are required to increase certainty around the effects of exercise training on falls incidence in people living with and beyond cancer. Future trials should be adequately powered and of sufficient duration to measure the long‐term effects of exercise on falls outcomes. Consistent reporting of falls so that the rate of falls can be compared will enable a more sound evidence base to be built. Potential adverse events should also be specifically measured. The inability to blind participants to group allocation in controlled exercise interventions will be an ongoing issue for assessment of study quality.
There is evidence in healthy community‐dwelling older adults that for balance training to be effective it should be of at least two hours a week regardless of setting (Sherrington 2017), thus interventions to address falls in people living with and beyond cancer should take into account existing transferable evidence.
What's new
| Date | Event | Description |
|---|---|---|
| 27 October 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 5, 2015 Review first published: Issue 10, 2018
Notes
In September 2020 we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We acknowledge the guidance from Dr Kim Rooney (haemato‐oncologist, Launceston General Hospital, Tasmania, Australia). We are also grateful to Joanne Abbott for her assistance with the development of the search strategies and to Louise Earwaker for running the searches.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL and HTA
#1MeSH descriptor: [Neoplasms] explode all trees #2(cancer* or neoplas* or tumo* or carcinoma* or hodgkin* or nonhodgkin* or adenocarcinoma* or leuk?emia* or metasta* or malignan* or lymphoma* or sarcoma* or melanoma* or myeloma* or oncolog*):ti,ab,kw (Word variations have been searched) #3#1 or #2 #4MeSH descriptor: [Accidental Falls] this term only #5(fall or falls):ti,ab,kw (Word variations have been searched) #6#4 or #5 #7MeSH descriptor: [Exercise] this term only #8MeSH descriptor: [Muscle Stretching Exercises] this term only #9MeSH descriptor: [Running] this term only #10MeSH descriptor: [Swimming] this term only #11MeSH descriptor: [Walking] this term only #12MeSH descriptor: [Warm‐Up Exercise] this term only #13MeSH descriptor: [Exercise Therapy] this term only #14MeSH descriptor: [Exercise Movement Techniques] this term only #15MeSH descriptor: [Postural Balance] this term only #16MeSH descriptor: [Resistance Training] this term only #17MeSH descriptor: [Tai Ji] this term only #18MeSH descriptor: [Breathing Exercises] this term only #19MeSH descriptor: [Dance Therapy] this term only #20(exercis* or training):ti,ab,kw (Word variations have been searched) #21(balance near/3 (retraining or re‐training or reeducation or re‐education)):ti,ab,kw (Word variations have been searched) #22(aerobic next exercise*):ti,ab,kw (Word variations have been searched) #23pilates:ti,ab,kw (Word variations have been searched) #24MeSH descriptor: [Yoga] this term only #25(tai ji or yoga or tai‐chi or tai‐ji or tai chi or taiji*):ti,ab,kw (Word variations have been searched) #26#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 #27#3 and #6 and #26
MEDLINE (Ovid)
1. exp Neoplasms/
2. (cancer$ or neoplas$ or tumo$ or carcinoma$ or hodgkin$ or nonhodgkin$ or adenocarcinoma$ or leuk?emia$1 or metasta$ or malignan$ or lymphoma$ or sarcoma$ or melanoma$ or myeloma$ or oncolog$).tw.
3. Accidental Falls/
4. (fall or falls).tw.
5. 3 or 4
6. 1 or 2
7. exercise/ or muscle stretching exercises/ or running/ or swimming/ or walking/ or warm‐up exercise/
8. Exercise therapy/
9. Exercise Movement Techniques/
10. Postural Balance/
11. Resistance Training/
12. Tai Ji/
13. Breathing Exercises/
14. Dance Therapy/
15. (exercis$ or training).tw.
16. (balance adj3 (retraining or re‐training or reeducation or re‐e ducation)).tw.
17. (aerobic adj exercise$).tw.
18. pilates.tw.
19. Yoga/
20. (tai ji or yoga or tai‐chi or tai‐ji or tai chi or taiji*).tw.
21. or/7‐20
22. randomized controlled trial.pt.
23. controlled clinical trial.pt.
24. randomized.ab.
25. placebo.ab.
26. drug therapy.fs.
27. randomly.ab.
28. trial.ab.
29. groups.ab.
30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31. exp animals/ not humans.sh.
32. 30 not 31
33. 5 and 6 and 21 and 32
Embase Ovid
1. exp Neoplasms/ 2. (cancer$ or neoplas$ or tumo$ or carcinoma$ or hodgkin$ or nonhodgkin$ or adenocarcinoma$ or leuk?emia$1 or metasta$ or malignan$ or lymphoma$ or sarcoma$ or melanoma$ or myeloma$ or oncolog$).tw. 3. Accidental Falls/ 4. (fall or falls).tw. 5. 3 or 4 6. 1 or 2 7. exercise/ or muscle stretching exercises/ or running/ or swimming/ or walking/ or warm‐up exercise/ 8. Exercise therapy/ 9. Exercise Movement Techniques/ 10. Postural Balance/ 11. Resistance Training/ 12. Tai Ji/ 13. Breathing Exercises/ 14. Dance Therapy/ 15. (exercis$ or training).tw. 16. (balance adj3 (retraining or re‐training or reeducation or re‐e ducation)).tw. 17. (aerobic adj exercise$).tw. 18. pilates.tw. 19. Yoga/ 20. (tai ji or yoga or tai‐chi or tai‐ji or tai chi or taiji*).tw. 21. or/7‐20 22. random$.tw. 23. factorial$.tw. 24. crossover$.tw. 25. cross over$.tw. 26. cross‐over$.tw. 27. placebo$.tw. 28. (doubl$ adj blind$).tw. 29. (singl$ adj blind$).tw. 30. assign$.tw. 31. allocat$.tw. 32. volunteer$.tw. 33. Crossover Procedure/ 34. double‐blind procedure.tw. 35. Randomized Controlled Trial/ 36. Single Blind Procedure/ 37. or/22‐36 38. (animal/ or nonhuman/) not human/ 39. 37 not 38 40. 5 and 6 and 21 and 39
CINAHL (EBSCO)
S35 S25 AND S34 S34 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 S33 (allocat* random*) S32 (MH "Quantitative Studies") S31 (MH "Placebos") S30 placebo* S29 (random* allocat*) S28 (MH "Random Assignment") S27 (Randomi?ed control* trial*) S26 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* ) S25 S3 AND S6 AND S24 S24 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 S23 (tai ji or yoga or tai‐chi or tai‐ji or tai chi or taiji*) S22 (MH "Yoga") S21 pilates S20 (aerobic exercise*) S19 (balance N3 (retraining or re‐training or reeducation or re‐ education)) S18 (exercis* or training) S17 (MH "Dance Therapy") S16 (MH "Breathing Exercises") S15 (MH "Tai Chi") S14 (MH "Resistance Training") S13 (MH "Balance, Postural") S12 (MH "Therapeutic Exercise") S11 (MH "Warm‐Up Exercise") S10 (MH "Walking") S9 (MH "Swimming") S8 (MH "Running") S7 (MH "Exercise") S6 S4 OR S5 S5 (fall or falls) S4 (MH "Accidental Falls") S3 S1 OR S2 S2 (cancer* or neoplas* or tumo* or carcinoma* or hodgkin* or nonhodgkin* or adenocarcinoma* or leuk?emia* or metasta* or malignan* or lymphoma* or sarcoma* or melanoma* or myeloma* or oncolog*) S1 (MH "Neoplasms+")
Web of Science
#22 #21 AND #9 #21 #20 OR #17 OR #16 OR #15 OR #14 OR #11 OR #10 #20 #19 AND #18 #19 TS=random* OR TI=random* #18 TS=(allocate* OR assign*) OR TI=(allocate* OR assign*) #17 TS=crossover* OR TI=crossover* #16 TS=(mask* OR blind*) OR TI=(mask* OR blind*) #15 TS=(singl* OR Doubl* OR Tripl* OR Trebl*) OR TI=(singl* OR Doubl* OR Tripl* OR Trebl*) #14 #13 AND #12 #13 TS=trial* OR TI=trial* #12 TI=clin* OR TS=clin* #11 TI=randomi* OR TS=randomi* #10 TS=Randomized clinical trial* OR TI=Randomized clinical trial* #9 #8 AND #2 AND #1 #8 #7 OR #6 OR #5 OR #4 OR #3 #7 TOPIC: ((tai ji or yoga or tai‐chi or tai‐ji or tai chi or taiji*)) #6 TOPIC: (pilates) #5 TOPIC: (("aerobic exercise*")) #4 TOPIC: ((balance Near/3 (retraining or re‐training or reeducation or re‐education))) #3 TOPIC: ((exercis* or training)) #2 TOPIC: ((fall or falls)) #1 TOPIC: ((cancer* or neoplas* or tumo* or carcinoma* or hodgkin* or nonhodgkin* or adenocarcinoma* or leuk?emia* or metasta* or malignan* or lymphoma* or sarcoma* or melanoma* or myeloma* or oncolog*))
SCOPUS
TITLE‐ABS‐KEY ( melanoma* ) OR TITLE‐ABS‐KEY ( myeloma* ) OR TITLE‐ABS‐KEY ( oncolog* ) OR TITLE‐ABS‐KEY ( leuk?emia* ) OR TITLE‐ABS‐KEY ( leukemia* ) OR TITLE‐ABS‐KEY ( leukaemia* ) OR TITLE‐ABS‐KEY ( metasta* ) OR TITLE‐ABS‐KEY ( malignan* ) OR TITLE‐ABS‐KEY ( lymphoma* ) OR TITLE‐ABS‐KEY ( sarcoma* ) OR TITLE‐ABS‐KEY ( neoplasms ) OR TITLE‐ABS‐KEY ( cancer* ) OR TITLE‐ABS‐KEY ( neoplas* ) OR TITLE‐ABS‐KEY ( tumo* ) OR TITLE‐ABS‐KEY ( carcinoma* ) OR TITLE‐ABS‐KEY ( hodgkin* ) OR TITLE‐ABS‐KEY ( nonhodgkin* ) OR TITLE‐ABS‐KEY ( adenocarcinoma* ) AND TITLE‐ABS‐KEY ( exercise ) OR TITLE‐ABS‐KEY ( muscle stretching ) OR TITLE‐ABS‐KEY ( running ) OR TITLE‐ABS‐KEY ( swimming ) OR TITLE‐ABS‐KEY ( walking ) OR TITLE‐ABS‐KEY ( warm‐up exercise ) OR TITLE‐ABS‐KEY ( exercise therapy ) OR TITLE‐ABS‐KEY ( exercise movement ) OR TITLE‐ABS‐KEY ( postural balance ) OR TITLE‐ABS‐KEY ( resistance training ) OR TITLE‐ABS‐KEY ( tai ji ) OR TITLE‐ABS‐KEY ( breathing exercises ) OR TITLE‐ABS‐KEY ( dance therapy ) OR TITLE‐ABS‐KEY ( exercis* ) OR TITLE‐ABS‐KEY ( training ) OR TITLE‐ABS‐KEY ( balance ) OR TITLE‐ABS‐KEY ( balance retraining ) OR TITLE‐ABS‐KEY ( balance re‐training ) OR TITLE‐ABS‐KEY ( balance reeducation ) OR TITLE‐ABS‐KEY ( balance re‐education ) AND TITLE‐ABS‐KEY ( cross over* ) OR TITLE‐ABS‐KEY ( cross‐over* ) OR TITLE‐ABS‐KEY ( randomi?ed control* trial* ) OR TITLE‐ABS‐KEY ( placebo* ) OR TITLE‐ABS‐KEY ( random* allocat* ) OR TITLE‐ABS‐KEY ( singl* blind* ) OR TITLE‐ABS‐KEY ( doubl* blind* ) OR TITLE‐ABS‐KEY ( tripl* blind* ) OR TITLE‐ABS‐KEY ( trebl* blind* ) OR TITLE‐ABS‐KEY ( crossover* ) AND TITLE‐ABS‐KEY ( fall* ) OR TITLE‐ABS‐KEY ( accidental fall* )
SPORTDiscus
Running or muscle stretching exercises or exercise or Swimming or Walking or Warm‐Up Exercise or Exercise therapy or Exercise movement techniques or Postural Balance or Resistance Training or tai ji or Breathing Exercises or Dance Therapy or Exercis* or training or balance or balance training or balance re* or aerobic exercise* or aerobic* or pilates or yoga or tai chi or taiji or taichi AND fall* or Accidental Fall* AND cancer* or neoplas* or tumo* or carcinoma* or hodgkin* or nonhodgkin* or nonhodgkin or non?hodgkin* or non Hodgkin or non Hodgkin* or adenocarcinoma* or leuk?emia* or metasta* or malignan* or lymphoma* or sarcoma* or melanoma* or myeloma* or oncolog*
PEDro
#1. cancer* *fall* exercis*
#2. tumo* *fall* exercis*
#3. neoplas* *fall* exercis*
#4. cancer* *fall* *training
#5. tumo* *fall* *training
#6. Neoplas* *fall* *training #7. cancer* *fall* pilates
#8. tumo* *fall* pilates #9. neoplas* *fall* pilates
#10. cancer* *fall* balance*
#11. tumo* *fall* balance*
#12. neoplas* *fall* balance*
#13. neoplas* *fall* tai*
#14. cancer* *fall* tai*
#15. tumo* *fall* tai*
#16. cancer* *fall* yoga*
#17. tumo* *fall* yoga*
#18. neoplas* *fall* yoga*
#19. neoplas* *fall* stretch*
#20. cancer* *fall* stretch*
#21. tumo* *fall* stretch*
#22. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
LILACS
Advanced search Cancer$ or neoplas$ or tumo$ or carcinoma$ or hodgkin$ or nonhodgkin$ or adenocarcinoma$ or metasta$ or sarcoma$ or lymphoma$ or melanoma$ or myeloma$ or oncolog$ or leukemia$ or leukaemia$ AND accidental falls or fall or falls AND exercise or running or walking or swimming or muscle stretching or resistance training or exercise therapy or yoga or pilates or tai ji or tai chi or dance therapy or breathing exercises or warm‐up exercise or postural balance or exercise movement techniques or balance training or balance exercise
ClinicalTrials.gov
Basic search cancer AND exercise AND falls
WHO (ICRTP) apps.who.int/trialsearch/
Simple search cancer* AND fall* AND exercise*
Data and analyses
Comparison 1. Exercise versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Strength: quadriceps | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | 8.99 [1.29, 16.70] |
| 1.2 Strength: leg press | 4 | 388 | Mean Difference (IV, Random, 95% CI) | 21.10 [8.47, 33.74] |
| 1.3 Strength; Sit‐to‐Stand Test | 4 | 214 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.05, 0.14] |
| 1.4 Flexibility: Sit‐and‐Reach Distance Test | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5 Balance: postural stability | 4 | 127 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.08, 0.79] |
| 1.6 Balance: Backward Walk Test | 2 | 280 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.48, ‐0.01] |
| 1.7 Balance: Timed Up‐and‐Go Test | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.8 Adverse event: fatigue | 2 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.81 [0.34, 1.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arbane 2011.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics 53% men Usual care
Exercise
Inclusion criteria: people with non‐small cell lung cancer referred for lung resection via open‐thoracotomy or visual assisted thoracotomy Exclusion criteria: people undergoing thoracotomy procedure where no lung resection was carried out, people undergoing pneumonectomy, admission > 48 hours to intensive care unit postsurgery Group differences: none |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Quadriceps strength (via magnetic stimulation of the femoral
nerve)
|
|
| Identification |
Sponsorship source: St Georges Hospital Therapies Charitable
Finding, Faculty of Health and Social Care Sciences, St Georges and Kingston
University Country: UK Setting: acute care Comments: no comment Author's Name: Gill Arbane Institution: School of Physiotherapy, Faculty of Health and Social Services, St George's and Kingston University E‐mail: ku402345@sgul.kingston.co.uk Address: Cranmer Terrace, Tooting, London SW17 ORE, UK |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was performed using computer‐generated tables |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were kept by an independent member of the team and released after consent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not able to be blinded and this may have led to bias |
| Blinding of outcome assessment (detection bias) | High risk | Staff who delivered the intervention was also an outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The reasons for withdrawals were explained; however, the high proportion of withdrawals raised the risk that results might be biased in a particular direction and no intention‐to‐treat analysis was performed. The attrition rates, which varied for the different outcome measures were: quality of life: 5/26 with control and 4/27 with intervention; 6‐minute walk test: 9/26 with control and 5/27 with intervention; and quadriceps strength: 13/26 with control and 10/27 with intervention |
| Selective outcome reporting (reporting bias) | Unclear risk | Protocol not available, insufficient evidence to make a decision |
| Size of study | High risk | Fewer than 50 participants per treatment arm |
| Other sources of bias | Low risk | Study appeared free of other sources of bias |
Brown 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics 100% women Usual care
Exercise
Inclusion criteria: women survivors 1–15 yr after diagnosis; free from cancer at study entry; ≥ 1 lymph node(s) removed; no medical conditions or contraindicated medications that would prohibit participation in an exercise programme; body mass index ≤ 50 kg/m²; no plans for surgery during study; no history of bilateral lymph node removal; no weight lifting in previous year; and stable bodyweight and not attempting to lose weight Exclusion criteria: not reported Group differences: none |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Leg press
|
|
| Identification |
Sponsorship source: Kathryn H Schmitz Country: USA Setting: Community Fitness Centre Comments: no sponsorship details were disclosed. Authors indicated there were no conflicts of interest but it was unclear whether this meant the study was unfunded Author's name: Kathryn Schmitz Institution: Perelman School of Medicine, University of Pennsylvania E‐mail: schmitz@mail.med.upenn.edu Address: 423 Guardian Dr, 8th Floor, Blockley Hall, Philadelphia, PA, 19104 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study participants were randomly assigned to 1 of 2 study groups using minimisation |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described; however, there was no reason to believe that participants or investigators could foresee assignments |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was indicated to be single blind. Participants were aware which group they were allocated to. Although the blinding of participants is not possible for this type of research, it introduces potential bias |
| Blinding of outcome assessment (detection bias) | Low risk | Measurements were obtained by trained staff who followed a standardised protocol and were blinded to study group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Data on participants who were missing physical function measures at 12 months were imputed through the use of a multiple imputation procedure that included baseline physical function as well as demographic, clinical, and anthropometric variables." |
| Selective outcome reporting (reporting bias) | Unclear risk | Protocol not available, insufficient evidence to make a decision |
| Size of study | Unclear risk | Between 50 and 199 participants per treatment arm |
| Other sources of bias | Low risk | Study appeared free of other sources of bias |
Cormie 2013.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics 100% men Usual care
Exercise
Inclusion criteria: men with a histological diagnosis of prostate cancer, established bone metastatic disease. Participants required clearance from their treating physician to participate Exclusion criteria: moderate‐to‐severe pain that limited activities of daily living or had musculoskeletal, cardiovascular, neurological (or a combination of these) disorders that could prevent them from exercising Group differences: none |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Knee extension
Timed Up‐and‐Go Test
Balance (Sensory Organisation Test)
|
|
| Identification |
Sponsorship source: Cancer Council of Western Australia Country: Australia Setting: Exercise Clinic Comments: no comment Author's name: P Cormie Institution: Edith Cowan University Health and Wellness Institute E‐mail: p.cormie@ecu.edu.au Address: 270 Joondalup Drive, Joondalup, Western Australia, 6027 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following familiarisation and baseline testing sessions, participants were randomised into 2 arms: exercise or usual care. Stratification for age was carried out and participants were randomised in an allocation ratio of 1:1 using a random assignment computer program |
| Allocation concealment (selection bias) | Low risk | Quote: "The project coordinator and exercise physiologists involved in assigning participants to groups were blinded to the allocation sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded as it is obvious if intervention was applied. This confers high risk of bias, despite attempts to blind the assessors |
| Blinding of outcome assessment (detection bias) | Unclear risk | There was no mention of whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were not collected for 25% of participants for 2 outcomes (400‐m walk and leg extension) due to femur bone metastases |
| Selective outcome reporting (reporting bias) | Unclear risk | Protocol not available, insufficient evidence to make a decision |
| Size of study | High risk | Fewer than 50 participants per treatment arm |
| Other sources of bias | Low risk | Study appeared free of other sources of bias |
De Luca 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics 100% women Usual care
Exercise
Inclusion criteria: aged 40–60 years; undergone mastectomy; conclusion of all cancer‐related treatments ≥ 6 months previously; no engagement in any formal exercise programmes for ≥ 6 months; medical clearance to physical activity; absence of musculoskeletal disturbances that could limit participation in the exercise training programme Exclusion criteria: diagnosis of any other major illness or disease; other contraindications to exercise Group differences: not reported |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Leg press
|
|
| Identification |
Sponsorship source: the research did not receive any specific grant
from funding agencies in the public, commercial or not‐for‐profit
sectors Country: Italy Setting: university gymnasium Comments: none Author's Name: Carlo Minganti Institution: Department of Movement, Human and Health Sciences, University of Rome E‐mail: carlo.mingati@uniroma4.it Address: Lauro de Bosis 15, 00135 Rome, Italy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After completion of all baseline measures, each participant was randomly assigned to either the intervention (n=10) or control (n=10) group using a random number generator." |
| Allocation concealment (selection bias) | Unclear risk | The timing of the random number generation and method of concealment (if applicable) was not described; however, there was no reason to believe that participants or investigators could foresee assignments |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not able to be blinded and this may have led to bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors stated that no participants withdrew from the study and there was no indication that data were incomplete for any variable |
| Selective outcome reporting (reporting bias) | Unclear risk | Protocol not available, insufficient evidence to make a decision |
| Size of study | High risk | Fewer than 50 participants per treatment arm |
| Other sources of bias | Low risk | Study appeared free of other sources of bias |
Galvao 2010.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics 100% men Usual care
Exercise
Inclusion criteria: histologically documented prostate cancer, minimum prior exposure to androgen deprivation therapy > 2 months, without prostate‐specific antigen evidence of disease activity and anticipated to remain hypogonadal for the subsequent 6 months Exclusion criteria: bone metastatic disease; musculoskeletal, cardiovascular or neurological disorders that could inhibit them from exercising; inability to walk 400 m or undertake upper and lower limb exercise; and resistance training in the previous 3 months Group differences: none |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Leg press
Knee extension
6‐m Backward Walk Test
Sensory Organisation Test
Chair rises
|
|
| Identification |
Sponsorship source: Robert U Newton, The Cancer Council Western
Australia Country: Australia Setting: outpatient setting, participants recruited from Sir Charles Gairdner Hospital (Perth, Western Australia) Comments: none Author's name: Daniel A Galvao Institution: School of Exercise, Biomedical and Health Sciences, Edith Cowan University E‐mail: d.galvao@ecu.edu.au Address: 100 Joondalup Drive, Joondalup, Western Australia, 6027, Australia |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After completion of the baseline assessment, participants were randomly assigned to 2 arms: exercise or usual care in a ratio of 1:1 using a computer random assignment program |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was concealed from the project co‐ordinator and the exercise physiologist involved in assigning participants to groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of any attempt to blind either participants or personnel to whether they were in the intervention or control group. Neither was there any comment from the authors judging that the lack of blinding is not likely to influence outcomes. Blinding of participants is unavoidable given the intervention, so an assumption is made that participants were not blinded, which may have led to bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data for all but 2 outcomes; small amount of incomplete data only. No evidence that incomplete data were more likely in 1 group or the other. Intention‐to‐treat analysis. Imputation |
| Selective outcome reporting (reporting bias) | Unclear risk | Protocol not available, insufficient evidence to make a decision |
| Size of study | High risk | Fewer than 50 participants per treatment arm |
| Other sources of bias | Low risk | Study appeared free of other sources of bias |
Galvao 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics 100% men Usual care
Exercise
Inclusion criteria: participants enrolled in the Randomised Androgen Deprivation and Radiotherapy (RADAR) trial with no structured exercise within the past 6 months. Participants had previously been treated with androgen deprivation therapy, were able to walk 400 m and had medical clearance from their physician Exclusion criteria: bone metastases; acute illness; or any musculoskeletal, cardiovascular or neurological disorder that could inhibit or put them at risk from exercising Group differences: none |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Five‐Times Sit‐to‐Stand Test
Leg strength
|
|
| Identification |
Sponsorship source: Prostate Cancer Foundation of Australia Country: Australia and New Zealand Setting: University affiliated Exercise Clinics Comments: the sponsors did not participate in the design or conduct of the study, collection, management, analysis or interpretation of the data; or in preparation, review or approval of the manuscript. Author's name: Daniel Galvao Institution: Edith Cowan University Health and Wellness Institute E‐mail: d.galvao@ecu.edu.au Address: 270 Joondalup Drive, Joondalup, Western Australia, 6027, Australia |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program randomisation in 1:1 ratio using minimisation technique |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawals were described and were similar between groups. Missing data were accounted for through intention‐to‐treat analysis |
| Selective outcome reporting (reporting bias) | Low risk | All relevant outcomes described in the registered protocol (ACTRN) were reported |
| Size of study | Unclear risk | Between 50 and 199 participants per treatment arm |
| Other sources of bias | Low risk | Study appeared free of other sources of bias |
Monga 2007.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics 100% men Usual care
Exercise
Inclusion criteria: localised prostate cancer requiring radiotherapy treatment. First‐time diagnosis of cancer. Ambulatory. Able to complete self‐report measures Exclusion criteria: concurrently receiving chemotherapy; major health problems; recent history of dizziness, blurred vision or fainting spells; recent history of unstable angina; bone, back or neck pain of recent origin or inability to exercise Group differences: none |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Five‐Times Sit‐to‐Stand Test
Sit‐and‐Reach Distance Test
|
|
| Identification |
Sponsorship source: none declared Country: USA Setting: Oncology/Radiotherapy service at Academic Medical Centre Comments: none Author's name: Kuno P Zimmerman Institution: VA Medical Center, Houston, TX E‐mail: zimmerman.kunop@med.va.gov Address: Holcombe Blvd, Houston, TX, USA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information regarding randomisation process. Stated participants were randomised but did not specify how this occurred |
| Allocation concealment (selection bias) | Unclear risk | No reporting on process of allocation (i.e. no information about who or how) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No attempt to blind either participants or assessors |
| Blinding of outcome assessment (detection bias) | High risk | No attempt to blind outcome assessors. Potentially subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Relatively large proportion withdrawn after randomisation (5/30) without any information regarding whether they were in control or intervention group, or both. 4/30 withdrew after enrolment; not accounted for in the analysis. No intention‐to‐treat analysis. No data collected (or presented) on participants who dropped out |
| Selective outcome reporting (reporting bias) | Unclear risk | Protocol not available, insufficient evidence to make a decision |
| Size of study | High risk | Fewer than 50 participants per treatment arm |
| Other sources of bias | High risk | Baseline imbalance in the 2 variables of interest to this review; Stand‐to‐Sit Test difference 2.08 (P = 0.06) and flexibility difference 1.55 (P = 0.14) |
Schwenk 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Usual care
Exercise
Inclusion criteria: aged ≥ 55 years; able to provide written consent; diagnosis of current or previous cancer; ability to walk to 10 m without assistive device; presence of chemotherapy‐induced peripheral neuropathy as confirmed by symptoms (numbness, tingling or pain) and signs (reduced vibration perception threshold > 25 V) Excluded criteria: diabetes, foot ulcers or infection; neurological issues (e.g. Parkinson's disease, stroke or multiple sclerosis); severe visual impairment Group differences: none |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Medio‐lateral sway – eyes open
|
|
| Identification |
Sponsorship source: Flinn Foundation, and National Institute on
Aging Country: USA Setting: University of Arizona Cancer Center Comments: none Author's name: Michael Schwenk Institution: Interdisciplinary Consortium on Advanced Motion Performance, University of Arizona E‐mail: schwenk.michael@gmail.com Address: 1501 N Campbell Ave, AHSC 4303D, Tucson, AZ, USA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to groups after completion of baseline measurements by a person unrelated to the study using the urn design |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted by a person not involved in the study after baseline measurements were completed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to their allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators were unaware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data described |
| Selective outcome reporting (reporting bias) | Low risk | Outcome data checked against protocol registered with ClinicalTrials.gov |
| Size of study | High risk | Fewer than 50 participants per treatment arm |
| Other sources of bias | Low risk | Study appeared free of other sources of bias |
Twiss 2009.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics
Inclusion criteria: ≥ 12 months postmenopausal, aged 35–75 years, history (≥ 6 months' post‐treatment) of stage 0, I or II breast cancer, osteopenia or osteoporosis as defined by a bone mineral density T score of –1.0 or less at hip, lumbar spine or forearm, reside within 100 miles of research sites at Omaha, Lincoln, Kearney, and Scottsbluff Nebraska, physicians permission to participate Exclusion criteria: recurrence of breast cancer; currently taking hormone therapy, bisphosphonates, glucocorticosteroids, or other drugs affecting bone density; currently engaging in strength exercises; body mass index ≥ 35 kg/m², serum calcium, creatinine or thyroid‐stimulating hormone outside normal; active gastrointestinal problems or other conditions that prohibited strength exercises; intake of vitamin D or risedronate Group differences: not reported |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Tandem balance
Falls
|
|
| Identification |
Sponsorship source: funded by "NINR" Country: USA Setting: exercises delivered at home then at fitness centre. Testing a hospitals or rehabilitation centres at 4 sites. Comments: none Author's name: Nancy Waltman Institution: University of Nebraska Medical Center College of Nursing, Omaha E‐mail: nwaltman@unmc.edu Address: University of Nebraska Medical Center, College of Nursing, Commerce. Court PO Box 880220, Lincoln, NE, USA |
|
| Notes | All participants were grouped together at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated that participants were randomly allocated; however, did not detail the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | No information provided regarding allocation concealment to allow a judgement to be made |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No attempt to blind either the participants (understandably) or the personnel assessing outcome. Those involved in the intervention appeared to be making outcome assessments. It was possible that knowledge of the intervention group could have biased outcome assessments |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors appeared to be involved in delivery of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26/249 women randomised into the study did not complete the 24‐month testing. The dropout rate of the intervention group was comparable to that of the control group (14 vs 12) and reasons for withdrawal did not indicate potential for bias |
| Selective outcome reporting (reporting bias) | Unclear risk | Protocol not available, insufficient evidence to make a decision |
| Size of study | Unclear risk | Between 50 and 199 participants per treatment arm |
| Other sources of bias | Low risk | Study appeared free of other sources of bias |
Vollmers 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics 100% women Usual care
Exercise
Inclusion criteria: primary breast cancer, aged 18–75 years and primary paclitaxel treatment for 12 weeks. Exclusion criteria: existing cardiopulmonary diseases (e.g. New York Heart Association class III, myocardial infarction < 3 months), renal insufficiency (glomerular filtration rate < 30 mL/minute), neurological diseases (e.g. multiple sclerosis, other neuropathies), metabolic diseases (e.g. diabetes mellitus; severe obesity (body mass index > 35 kg/m²) and the extensive consumption of alcohol either currently or in the past Group differences: none |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Postural sway
Chair rising test
|
|
| Identification |
Sponsorship source: none declared Country: Germany Setting: University Hospital Comments: none Author's name: Paul Lennart Vollmers Institution: University Hospital for Women, Kiel Address: Arnold‐Heller‐Strabe 3, Haus 14, 24105 Kiel, Germany |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information regarding randomisation process. Stated participants were randomised (via 1:1 randomisation) but did not specify how this occurred |
| Allocation concealment (selection bias) | Unclear risk | No reporting on process of allocation (i.e. no information about who or how) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to their allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Relatively large proportion withdrawn after enrolment (7/43); not accounted for in the analysis. No intention‐to‐treat analysis. No data presented on participants who dropped out |
| Selective outcome reporting (reporting bias) | Unclear risk | Protocol not available, insufficient evidence to make a decision |
| Size of study | High risk | Fewer than 50 participants per treatment arm |
| Other sources of bias | High risk | Baseline imbalance between intervention and control groups for the balance variable |
Winters‐Stone 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics 100% men Usual care
Exercise
Inclusion criteria: received treatment for prostate cancer; not currently undergoing radiotherapy or chemotherapy; aged ≥ 60 years; residing with a spouse willing to participate; not currently resistance training ≥ 2 times/week; physician clearance to exercise Exclusion criteria: not reported Group differences: none |
|
| Interventions |
Usual care
Exercise
|
|
| Outcomes |
Leg press
|
|
| Identification |
Sponsorship source: National Institutes of Health Country: USA Setting: Knight Cancer Institute Comments: none Author's name: Kerri M Winters‐Stone Institution: Oregon Health and Science University E‐mail: wintersk@ohsu.edu Address: Knight Cancer Institute, Oregon Health and Science University, Portland, OR 97239, USA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following completion of baseline testing, participants received their group assignment. Sequence number generated by a statistician using MS Excel |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence was kept in sealed sequentially numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal were described. Missing data were accounted for through intention‐to‐treat analysis |
| Selective outcome reporting (reporting bias) | High risk | Multiple primary outcome measures identified in the published protocol (fatigue, symptoms, depression and strain) are not included in this manuscript |
| Size of study | High risk | Fewer than 50 participants per treatment arm |
| Other sources of bias | Low risk | Study appeared free of other sources of bias |
kg/m²: kilogram per metre square; m: metre; mL/minute: millilitre per minute; n: number of participants per arm; SD: standard deviation; V: volts; vs: versus; yr: year
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bayego 2012 | Not an RCT |
| Bender 2015 | Not an RCT |
| Betker 2006 | Not an RCT |
| Bylow 2008 | Not an RCT |
| Bylow 2011 | Not an RCT |
| Courneya 2002 | Did not address the research question |
| Curran 2013 | Not an RCT |
| Delecluse 2004 | Ineligible participant population |
| Devin 2016 | Did not address the research question |
| Fong 2018 | Not an RCT |
| Galantino 2012 | Not an RCT |
| Galvao 2006 | Not an RCT |
| Grabenbauer 2016 | Not an RCT |
| Grote 2017 | Not an RCT |
| Hansen 2009 | Not an RCT |
| Hanson 2011 | Review article |
| Hanson 2013 | Not an RCT |
| Hojan 2013 | Not an RCT |
| Holick 2008 | Not an RCT |
| Holmes 2005 | Not an RCT |
| Huang 2016 | Not an RCT |
| Irwin 2008 | Not an RCT |
| Islam 2004 | Ineligible participant population |
| Keogh 2012 | Review article |
| Kwan 2012 | Not an RCT |
| Lee 2014 | Did not address the research question |
| Litterini 2013 | Ineligible comparator (control group received an exercise‐based intervention) |
| Martin 2016 | Not an RCT |
| Overcash 2013 | Not an RCT |
| Pollock 2012 | Ineligible participant population |
| Rossi 2016 | Did not address the research question |
| Serdà 2010 | Not an RCT |
| Shahinian 2005 | Not an RCT |
| Shobeiri 2016 | Did not address the research question |
| Silver 2011 | Not an RCT |
| Spoelstra 2010 | Not an RCT |
| Sternfeld 2009 | Not an RCT |
| Stineman 2011 | Ineligible participant population |
| Verschueren 2004 | Ineligible participant population |
| Wampler 2007 | Not an RCT |
| Winters‐Stone 2011 | Ineligible comparator (control group received an exercise‐based intervention) |
| Winters‐Stone 2012a | Ineligible comparator (control group received an exercise‐based intervention) |
| Wright 2005 | Ineligible participant population |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Bjerre 2016.
| Study name | The FC Prostate Community Trial |
| Methods | 2‐group, parallel design, randomised controlled trial |
| Participants | Men, aged ≥ 18 years, diagnosed with prostate cancer |
| Interventions | Participation in community‐based recreational football vs usual care |
| Outcomes | Falls resulting in medical assessment, bone fractures, quality of life, self‐reported physical activity, muscle and fat mass, bone strength |
| Starting date | May 2015 |
| Contact information | eb@ucsf.dk |
| Notes | Contacted authors in July 2017, data collection is still underway, final outcome measurements will take place at the end of August. |
Winters‐Stone 2012b.
| Study name | The GET FIT Trial |
| Methods | 3‐group, single‐blind, parallel design, randomised controlled trial |
| Participants | Women, aged 50–75 years, who have completed chemotherapy for cancer |
| Interventions | Tai chi vs strength training vs placebo control group of seated stretching exercise |
| Outcomes | Falls incidence, leg muscle strength, postural stability and physical function |
| Starting date | January 2013 |
| Contact information | wintersk@ohsu.edu |
| Notes | Contacted authors in March 2017, data collection was completed in 2016 and data are currently being analysed. |
Differences between protocol and review
The title was changed from "Exercise for preventing falls in people with cancer living in the community" in the protocol to its current title: "Exercise for reducing falls in people living with and beyond cancer." It should be noted that the intended population did not change, rather the description changed to make the intended population for the systematic review clearer (Williams 2015).
In the protocol, we identified the objectives in this way: to assess the effects of prescribed or provided exercise for reducing accidental falls incidence and to affect strength, flexibility, balance, and aerobic endurance, as these are major factors known to affect falls risk in cancer survivors living in the community. Cancer survivors living in the community has been changed to people living with and beyond cancer in the community to make the language clearer for readers and to ensure consistency throughout the text. The protocol also identified that we would include aerobic endurance as a secondary outcome. During the preparation of the review we chose to omit this outcome variable due to a broad lack of relationship to falls risk. In the protocol, we identified adults as aged greater than 18 years; however, we classified adults as anyone aged 18 years or older at diagnosis in the review.
The protocol did not identify how results with significant heterogeneity would be accounted for. Where significant heterogeneity was observed, we used a random‐effects model.
Additional assessments of bias that were not included in the protocol description were added to the review. These were selective outcome reporting (checking all stated outcomes are reported) and other bias. Quality assessment of the evidence was performed using the GRADE framework.
We added adverse events as an extra outcome to be measured in the 'Summary of findings' table.
We identified that we would search the metaRegister of Controlled Trials (mRCT). Between publishing the protocol and the completion of the searches this database was incorporated into the World Health Organization trials portal and consequently was not searched separately.
Contributions of authors
AW wrote the protocol, screened and selected relevant trials, assessed trial quality, extracted data and wrote the review. AW will be responsible for updates.
MB wrote the protocol, screened and selected relevant trials, extracted data and wrote the review.
SK contributed to the protocol, extracted data and contributed to the text of the review.
MK contributed to the protocol, extracted data, assessed trial quality and contributed to the text of the review.
KO contributed to the protocol, extracted data, was consulted where disagreements occurred and wrote the review.
JW checked data for accuracy and assisted with data analysis, was consulted where disagreements occurred and contributed to the text of the review.
Sources of support
Internal sources
No external support provided, Other
External sources
No Support provided, Other
Declarations of interest
AW: none known; AW is a former director of Exercise & Sports Science Australia and a current Steering Group member for Tasmania Medicare Local. Both groups run projects utilising exercise as a treatment for a range of chronic conditions, including cancer.
MB: none known; MB is a practising physiotherapist who uses Pilates exercises as part of rehabilitation for women with breast cancer.
SK: none known.
MK: none known.
KO: none known.
JW: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Arbane 2011 {published data only}
- Arbane G, Tropman D, Jackson D, Garrod R. Evaluation of an early exercise intervention after thoracotomy for non-small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: randomised controlled trial. Lung Cancer 2011;71(2):229-34. [DOI: 10.1016/j.lungcan.2010.04.025] [DOI] [PubMed] [Google Scholar]
Brown 2015 {published data only}
- Brown JC, Schmitz KH. Weight lifting and physical function among survivors of breast cancer: a post hoc analysis of a randomized controlled trial. Journal of Clinical Oncology 2015;33(19):2184-9. [DOI: 10.1200/JCO.2014.57.7395] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cormie 2013 {published data only}
- Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer and Prostatic Diseases 2013;16(4):328-35. [DOI: 10.1038/pcan.2013.22] [DOI] [PubMed] [Google Scholar]
De Luca 2016 {published data only}
- De Luca V, Minganti C, Borrione P, Grazioli E, Cerulli C, Guerra E, et al. Effects of concurrent aerobic and strength training on breast cancer survivors: a pilot study. Public Health 2016;136:126-32. [DOI: 10.1016/j.puhe.2016.03.028] [DOI] [PubMed] [Google Scholar]
Galvao 2010 {published data only}
- Galvao DA, Taaffe DR, Spry N, Joseph D, Newton, RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. Journal of Clinical Oncology 2010;28(2):340-7. [DOI: 10.1200/JCO.2009.23.2488] [DOI] [PubMed] [Google Scholar]
Galvao 2014 {published data only}
- Buffart LM, Newton RU, Chinapaw MJ, Taaffe DR, Spry NA, Denham JW, et al. The effect, moderators, and mediators of resistance and aerobic exercise on health-related quality of life in older long-term survivors of prostate cancer. Cancer 2015;121(16):2821-30. [DOI: 10.1002/cncr.29406 ] [DOI] [PubMed] [Google Scholar]
- Galvao DA, Spry N, Denham J, Taaffe DR, Cormie P, Joseph D, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. European Urology 2014;65:856-64. [DOI: 10.1016/j.eururo.2013.09.041] [DOI] [PubMed] [Google Scholar]
Monga 2007 {published data only}
- Monga U, Garber SL, Thornby J, Valbona C, Kerrigan AJ, Monga TN, et al. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Archives of Physical Medicine and Rehabilitation 2007;88(11):1416-22. [DOI: 10.1016/j.apmr.2007.08.110] [DOI] [PubMed] [Google Scholar]
Schwenk 2015 {published data only}
- Schwenk M, Grewal GS, Holloway D, Muchna A, Garland L, Najafi B. Interactive sensor-based balance training in older cancer patients with chemotherapy-induced neuropathy: a randomized controlled trial. Gerontology 2015;62(5):553-63. [DOI: 10.1159/000442253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Twiss 2009 {published data only}
- Twiss JJ, Waltman NL, Berg K, Ott CD, Gross GJ, Lindsey AM. An exercise intervention for breast cancer survivors with bone loss. Journal of Nursing Scholarship 2009;41(1):20-8. [DOI: 10.1111/j.1547-5069.2009.01247.x] [DOI] [PubMed] [Google Scholar]
Vollmers 2018 {published data only}
- Vollmers PL, Mundhenke C, Maass N, Bauerschlag D, Kratzenstein S, Röcken C, et al. Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy. Journal of Cancer Research and Clinical Oncology 2018;144(9):1785-92. [DOI: 10.1007/s00432-018-2686-5] [DOI] [PubMed] [Google Scholar]
Winters‐Stone 2016 {published data only}
- Winters-Stone KM, Lyons KS, Dobek J, Dieckmann NF, Bennett JA, Nail L, et al. Benefits of partnered strength training for prostate cancer survivors and spouses: results from a randomized controlled trial of the Exercising Together project. Journal of Cancer Survivorship 2016;10:633-44. [DOI: 10.1007/s11764-015-0509-0] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bayego 2012 {published data only}
- Bayego ES, Vila GS, Martinez IS. Exercise prescription: indications, dosage and side effects. Medicina Clinica 2012;138(1):18-24. [DOI: 10.1016/j.medcli.2010.12.008] [DOI] [PubMed] [Google Scholar]
Bender 2015 {published data only}
- Bender A, Braun L, Franklin K, Kidd M, Rendler N, Wiggins M, et al. Implementing a 4 week balance protocol to impact quality of life in cancer patients. International Journal of Exercise Science 2015;8(2):145-53. [digitalcommons.wku.edu/ijes/vol8/iss2/5] [Google Scholar]
Betker 2006 {published data only}
- Betker AL, Szturm T, Moussavi ZK, Nett C. Video game-based exercises for balance rehabilitation: a single-subject design. Archives of Physical Medicine and Rehabilitation 2006;87(8):1141-9. [DOI: 10.1016/j.apmr.2006.04.010] [DOI] [PubMed] [Google Scholar]
Bylow 2008 {published data only}
- Bylow K, Dale W, Mustian K, Stadler WM, Rodin M, Hall W, et al. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology 2008;72(2):422-7. [DOI: 10.1016/j.urology.2008.03.032] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bylow 2011 {published data only}
- Bylow K, Hemmerich J, Mohile SG, Stadler WM, Sajid S, Dale W. Obese frailty, physical performance deficits, and falls in older men with biochemical recurrence of prostate cancer on androgen deprivation therapy: a case-control study. Urology 2011;77(4):934-40. [DOI: 10.1016/j.urology.2010.11.024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Courneya 2002 {published data only}
- Courneya KS, Friedenreich CM, Sela RA, Quinney HA, Rhodes RE. Correlates of adherence and contamination in a randomized controlled trial of exercise in cancer survivors: an application of the theory of planned behavior and the five factor model of personality. Annals of Behavioral Medicine 2002;24(4):257-68. [DOI: 10.1207/S15324796ABM2404_02] [DOI] [PubMed] [Google Scholar]
Curran 2013 {published data only}
- Curran MS, Gubin A, Saylor E, Scruggs L. Cancer to 5K. Psycho-Oncology 2013;22:120. [DOI: 10.10002/pon.3245] [DOI] [Google Scholar]
Delecluse 2004 {published data only}
- Delecluse C, Colman V, Roelants M, Verschueren S, Derave W, Ceux T, et al. Exercise programs for older men: mode and intensity to induce the highest possible health-related benefits. Preventive Medicine 2004;39(4):823-33. [DOI: 10.1016/j.ypmed.2004.03.023] [DOI] [PubMed] [Google Scholar]
Devin 2016 {published data only}
- Devin JL, Sax AT, Hughes GI, Jenkins DG, Aitken JF, Chambers SK, et al. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. Journal of Cancer Survivorship 2016;10:467-79. [DOI: 10.1007/s11764-015-0490-7] [DOI] [PubMed] [Google Scholar]
Fong 2018 {published data only}
- Fong SS, Choi AW, Luk WS, Yam TT, Leung JC, Chung JW. Bone mineral density, balance performance, balance self-efficacy, and falls with and without Qigong training: an observational study. Integrative Cancer Therapies 2018;17(1):124-30. [DOI: 10.1177/1534735416686687] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galantino 2012 {published data only}
- Galantino ML, Desali K, Greene L, DeMichele A, Stricker C, Mao JJ. Impact of yoga on functional outcomes in breast cancer survivors with aromatase inhibitor-associated arthralgias. Integrative Cancer Therapies 2012;11(4):313-20. [DOI: 10.1177/1534735411413270] [DOI] [PubMed] [Google Scholar]
Galvao 2006 {published data only}
- Galvao DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Medicine & Science in Sports and Exercise 2006;38(12):2045-52. [DOI: 10.1249/01.mss.0000233803.48691.8b] [DOI] [PubMed] [Google Scholar]
Grabenbauer 2016 {published data only}
- Grabenbauer A, Grabenbauer AJ, Lengenfelder R, Grabenbauer GG, Distel LV. Feasibility of a 12-month-exercise intervention during and after radiation and chemotherapy in cancer patients: impact on quality of life, peak oxygen consumption, and body composition. Radiation Oncology 2016;11(42):1-7. [DOI: 10.1186/s13014-016-0619-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grote 2017 {published data only}
- Grote S, Modeste NN, Sealy D, Dehom S, Tarleton HP. Fall-related comorbidity and health beliefs among cancer survivors participating in a community-based exercise intervention. American Journal of Health Behavior 2017;41(5):630-41. [DOI: 10.5993/AJHB.41.5.12] [DOI] [PubMed] [Google Scholar]
Hansen 2009 {published data only}
- Hansen PA, Dechet CB, Porucznik CA, LaStayo PC. Comparing eccentric resistance exercise in prostate cancer survivors on and off hormone therapy: a pilot study. PM & R : the Journal of Injury, Function, and Rehabilitation 2009;1(11):1019-24. [DOI: 10.1016/j.pmrj.2009.09.016] [DOI] [PubMed] [Google Scholar]
Hanson 2011 {published data only}
- Hanson ED, Hurley BF. Intervening on the side effects of hormone-dependent cancer treatment: the role of strength training. Journal of Aging Research 2011;2011:903291. [DOI: 10.4061/2011/903291] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanson 2013 {published data only}
- Hanson ED, Sheaff AK, Sood S, Ma L, Francis JD, Goldberg AP, et al. Strength training induces muscle hypertrophy and functional gains in black prostate cancer patients despite androgen deprivation therapy. Journals of Gerontology Series A: Biological Sciences & Medical Sciences 2013;68(4):490-8. [DOI: 10.1093/gerona/gls206] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hojan 2013 {published data only}
- Hojan K, Milecki P, Molinska-Glura M, Roszak A, Leszczynski P. Effect of physical activity on bone strength and body composition in breast cancer premenopausal women during endocrine therapy. European Journal of Physical and Rehabilitation Medicine 2013;49(3):331-9. [PMID: 23438652 ] [PubMed] [Google Scholar]
Holick 2008 {published data only}
- Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiology Biomarkers & Prevention 2008;17(2):379-86. [DOI: 10.1158/1055-9965.epi-07-0771] [DOI] [PubMed] [Google Scholar]
Holmes 2005 {published data only}
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama 2005;293(20):2479-86. [DOI: 10.1001/jama.293.20.2479] [DOI] [PubMed] [Google Scholar]
Huang 2016 {published data only}
- Huang SM, Tseng LM, Chien LY, Tai CJ, Chen PH, Hung CT, et al. Effects of non-sporting and sporting qigong on frailty and quality of life among breast cancer patients receiving chemotherapy. European Journal of Oncology Nursing 2016;21:257-65. [DOI: 10.1016/j.ejon.2015.10.012 ] [DOI] [PubMed] [Google Scholar]
Irwin 2008 {published data only}
- Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre-and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. Journal of Clinical Oncology 2008;26(24):3958-64. [DOI: 10.1200/jco.2007.15.9822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 2004 {published data only}
- Islam MM, Nasu E, Rogers ME, Koizumi D, Rogers NL, Takeshima N. Effects of combined sensory and muscular training on balance in Japanese older adults. Preventive Medicine 2004;39(6):1148-55. [DOI: 10.1016/j.ypmed.2004.04.048] [DOI] [PubMed] [Google Scholar]
Keogh 2012 {published data only}
- Keogh JWL, MacLeod RD. Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. Journal of Pain and Symptom Management 2012;43(1):96-110. [DOI: 10.1016/j.jpainsymman.2011.03.006] [DOI] [PubMed] [Google Scholar]
Kwan 2012 {published data only}
- Kwan ML, Sternfeld B, Ergas IJ, Timperi AW, Roh JM, Hong C-C, et al. Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Research and Treatment 2012;131(2):679-90. [DOI: 10.1007/s10549-011-1788-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee DS, Keay J, Lee M, Winter-Stone K, Goeres LM. Statins and lean body mass in breast cancer survivors. Journal of the American Geriatrics Society 2014;62:S20. [DOI: 10.1111/jgs.12870] [DOI] [Google Scholar]
Litterini 2013 {published data only}
- Litterini AL, Lee J. Exercise for individuals with metastatic cancer. Rehabilitation Oncology 2013;31(1):47-8. [Issn Print: 2168-3808] [Google Scholar]
Martin 2016 {published data only}
- Martin E, Battaglini C, Hands B, Naumann FL. Higher-intensity exercise helps cancer survivors remain motivated. Journal of Cancer Survivorship 2016;10:524-33. [DOI: 10.1007/s11764-015-0498-z] [DOI] [PubMed] [Google Scholar]
Overcash 2013 {published data only}
- Overcash J, Will KM, Lipetz DW. The benefits of medical Qigong in patients with cancer: a descriptive pilot study. Clinical Journal of Oncology Nursing 2013;17(6):654-8. [DOI: 10.1188/13.cjon.654-658] [DOI] [PubMed] [Google Scholar]
Pollock 2012 {published data only}
- Pollock RD, Martin FC, Newham DJ. Whole-body vibration in addition to strength and balance exercise for falls-related functional mobility of frail older adults: a single-blind randomized controlled trial. Clinical Rehabilitation 2012;26(10):915-23. [DOI: 10.1177/0269215511435688] [DOI] [PubMed] [Google Scholar]
Rossi 2016 {published data only}
- Rossi A, Garber CE, Ortiz M, Shankar V, Goldberg GL, Nevadunsky NS. Feasibility of a physical activity intervention for obese, socioculturally diverse endometrial cancer survivors. Gynecologic Oncology 2016;142(2):304-10. [DOI: 10.1016/j.ygyno.2016.05.034] [DOI] [PubMed] [Google Scholar]
Serdà 2010 {published data only}
- Serdà B-C, Vesa J, Valle A, Monreal P. Urinary incontinence and prostate cancer: a rehabilitation program design. Actas Urologicas Espanolas 2010;34(6):522-30. [PMID: ] [PubMed] [Google Scholar]
Shahinian 2005 {published data only}
- Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. New England Journal of Medicine 2005;352(2):154-64. [DOI: 10.1056/NEJMoa041943] [DOI] [PubMed] [Google Scholar]
Shobeiri 2016 {published data only}
- Shobeiri F, Masoumi SZ, Nikravesh A, Moghadam RH, Karami M. The impact of aerobic exercise on quality of life in women with breast cancer: a randomized controlled trial. Journal of Research in Health Sciences 2016;16(3):127-32. [PMID: 27840340 ] [PMC free article] [PubMed] [Google Scholar]
Silver 2011 {published data only}
- Silver JK, Gilchrist LS. Cancer rehabilitation with a focus on evidence-based outpatient physical and occupational therapy interventions. American Journal of Physical Medicine & Rehabilitation 2011;90:S5-15. [DOI: 10.1097/PHM.0b013e31820be4ae] [DOI] [PubMed] [Google Scholar]
Spoelstra 2010 {published data only}
- Spoelstra S, Given B, Eye A, Given C. Falls in the community-dwelling elderly with a history of cancer. Cancer Nursing 2010;33(2):149-55. [DOI: 10.1097/NCC.0b013e3181bbbe8a] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sternfeld 2009 {published data only}
- Sternfeld B, Weltzien E, Quesenberry Jr CP, Castillo AL, Kwan M, Slattery ML, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2009;18(1):87-95. [DOI: 10.1158/1055-9965.EPI-08-0595] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stineman 2011 {published data only}
- Stineman MG, Strumpf N, Kurichi JE, Charles J, Grisso JA, Jayadevappa R. Attempts to reach the oldest and frailest: recruitment, adherence, and retention of urban elderly persons to a falls reduction exercise program. Gerontologist 2011;51(Suppl 1):S59-72. [DOI: 10.1093/geront/gnr012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verschueren 2004 {published data only}
- Verschueren SMP, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. Journal of Bone and Mineral Research 2004;19(3):352-9. [DOI: 10.1359/JBMR.0301245] [DOI] [PubMed] [Google Scholar]
Wampler 2007 {published data only}
- Wampler MA, Topp KS, Miaskowski C, Byl NN, Rugo HS, Hamel K. Quantitative and clinical description of postural instability in women with breast cancer treated with taxane chemotherapy. Archives of Physical Medicine and Rehabilitation 2007;88(8):1002-8. [DOI: 10.1016/j.apmr.2007.05.007] [DOI] [PubMed] [Google Scholar]
Winters‐Stone 2011 {published data only}
- Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Research & Treatment 2011;127(2):447-56. [DOI: 10.1007/s10549-011-1444-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winters‐Stone 2012a {published data only}
- Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. Journal of Cancer Survivorship 2012;6(2):189-99. [DOI: 10.1007/s11764-011-0210-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2005 {published data only}
- Wright MJ, Galea V, Barr RD. Proficiency of balance in children and youth who have had acute lymphoblastic leukemia. Physical Therapy 2005;85(8):782-90. [DOI: 10.1093/ptj/85.8.782] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Bjerre 2016 {published data only}
- Bjerre E, Bruun DM, Tolver A, Brasso K, Krustrup P, Johansen C, et al. Effectiveness of community-based football compared to usual care in men with prostate cancer: protocol for a randomised, controlled, parallel group, multicenter superiority trial (The FC Prostate Community Trial). BMC Cancer 2016;16(767):1-10. [DOI: 10.1186/s12885-016-2805-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winters‐Stone 2012b {published data only}
- Winters-Stone KM, Li F, Horak F, Luoh S-W, Bennett JA, Nail L, et al. Comparison of tai chi vs. strength training for fall prevention among female cancer survivors: study protocol for the GET FIT trial. BMC Cancer 2012;12:577. [DOI: 10.1016/j.apmr.2007.05.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ambrose 2013
- Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas 2013;75(1):51-61. [DOI: 10.1016/j.maturitas.2013.02.009 ] [DOI] [PubMed] [Google Scholar]
AUREF 2012
- Cochrane Pain, Palliative and Supportive Care Review Group. PaPaS author and referee guidance (AUREF), 2012. papas.cochrane.org/papas-documents (accessed 17 December 2014).
Australian Institute of Health and Welfare 2012
- Australian Institute of Health and Welfare. Cancer Survival and Prevalence in Australia: Period Estimates From 1982 to 2010. Cancer Series No. 69 Cat. No. CAN 65. Canberra: AIHW, 2012. [DOI] [PubMed] [Google Scholar]
Beltran Valls 2014
- Beltran Valls MR, Dimauro I, Brunelli A, Tranchita E, Ciminelli E, Caserotti P, et al. Explosive type of moderate-resistance training induces functional, cardiovascular, and molecular adaptations in the elderly. Age (Dordrecht, Netherlands) 2014;36(2):759-72. [DOI: 10.1007/s11357-013-9584-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 1992
- Berg KO, Maki BE, Williams JI, Holliday PJ, Wood-Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Archives of Physical Medicine and Rehabilitation 1992;73(11):1073-80. [PMID: ] [PubMed] [Google Scholar]
Bird 2009
- Bird ML, Hill K, Ball M, Williams AD. Effects of resistance- and flexibility-exercise interventions on balance and related measures in older adults. Journal of Aging and Physical Activity 2009;17(4):444-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bird 2016
- Bird ML, Cheney MJ, Williams AD. Accidental fall rates in community-dwelling adults compared to cancer survivors during and post-treatment: a systematic review with meta-analysis. Oncology Nursing Forum 2016;43(2):E64-72. [DOI: 10.1188/16.ONF.E64-E72] [DOI] [PubMed] [Google Scholar]
Bokemeyer 1998
- Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. British Journal of Cancer 1998;77(8):1355-62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caspersen 1985
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports 1985;100(2):126-31. [PMID: PMC1424733] [PMC free article] [PubMed] [Google Scholar]
Chen 2009
- Chen Z, Maricic M, Aragaki AK, Mouton C, Arendell L, Lopez AM, et al. Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women's Health Initiative. Osteoporosis International 2009;20(4):527-36. [DOI: 10.1007/s00198-008-0721-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2018 [Computer program]
- Veritas Health Innovation Covidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation, 2018 (available at www.covidence.org).
Cramp 2012
- Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD006145. [DOI: 10.1002/14651858.CD006145.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Czerwinski 2008
- Czerwinski E, Kumorek A, Milert A, Borowy P. Causes of falls in women in Krakow population. Ortopedia Traumatologica Rehabilitacja 2008;10(5):429-40. [PMID: ] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Duncan 1990
- Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. Journal of Gerontology 1990;45(6):M192-7. [PMID: 2229941 ] [DOI] [PubMed] [Google Scholar]
Ferlay 2015
Freedman 2004
- Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, Hill S, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. Journal of Clinical Endocrinology and Metabolism 2004;89(5):2248-53. [DOI: 10.1210/jc.2003-031874] [DOI] [PubMed] [Google Scholar]
Gillespie 2012
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD007146. [DOI: 10.1002/14651858.CD007146.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI: 10.1016/j.jclinepi.2011.01.012] [DOI] [PubMed] [Google Scholar]
Hepple 1997
- Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. Journal of Applied Physiology 1997;82(4):1305-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Higgins 2011b
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Kumar 2005
- Kumar RJ, Barqawi A, Crawford ED. Adverse events associated with hormonal therapy for prostate cancer. Reviews in Urology 2005;7 Suppl 5:S37-43. [PMID: PMC1477613] [PMC free article] [PubMed] [Google Scholar]
Kuroi 2004
- Kuroi K, Shimozuma K. Neurotoxicity of taxanes: symptoms and quality of life assessment. Breast Cancer 2004;11(1):92-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lamb 2011
- Lamb SE, Becker C, Gillespie LD, Smith JL, Finnegan S, Potter R, et al. Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall-prevention interventions. Trials 2011;12:125. [DOI: 10.1186/1745-6215-12-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2012
- Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD008465. [DOI: 10.1002/14651858.CD008465.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohile 2011
- Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. Journal of Clinical Oncology 2011;29(11):1458-64. [DOI: 10.1200/JCO.2010.31.6695] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2013
- Morgan AJ, Parker AG, Alvarez-Jimenez M, Jorm AF. Exercise and mental health: an Exercise and Sports Science Australia commissioned review. Journal of Exercise Physiology Online / American Society of Exercise Physiologists 2013;16(4):64-73. [Google Scholar]
Nevitt 1991
- Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. Journal of Gerontology 1991;46(5):M164-70. [DOI: 10.1093/geronj/46.5.M164] [DOI] [PubMed] [Google Scholar]
Podsiadlo 1991
- Podsiadlo D, Richardson S. The Timed "Up & Go": a test of basic functional mobility for frail elderly persons. Journal of the American Geriatric Society 1991;39(2):142-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre; The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre; The Cochrane Collaboration, 2014.
Rizzoli 2013
- Rizzoli R, Body JJ, Brandi ML, Cannata-Andia J, Chappard D, El Maghraoui A, et al. Cancer-associated bone disease. Osteoporosis International 2013;24(12):2929-53. [DOI: 10.1007/s00198-013-2530-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubenstein 2006
- Rubenstein L. Falls in older people: epidemiology, risk factors and strategies for prevention. Age and Ageing 2006;35-S2:ii37-41. [DOI: 10.1093/ageing/afl084] [DOI] [PubMed] [Google Scholar]
Schmitz 2005
- Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiology Biomarkers and Prevention 2005;14(7):1588-95. [DOI: 10.1158/1055-9965.EPI-04-0703] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Sherrington 2011
- Sherrington C, Tiedemann A, Fairhall N, Close JC, Lord SR. Exercise to prevent falls in older adults: an updated meta-analysis and best practice recommendations. New South Wales Public Health Bulletin 2011;22(4):78-83. [DOI: 10.1071/NB10056] [DOI] [PubMed] [Google Scholar]
Sherrington 2017
- Sherrington C, Michaleff Z, Fairhall N, Paul S, Tiedemann A, Whitney J, et al. Exercise to prevent falls in older adults: an updated systematic review and meta analysis. British Journal of Sports Medicine 2017;51(24):1750-58. [DOI: 10.1136/bjsports-2016-096547] [DOI] [PubMed] [Google Scholar]
Slattery 2014
- Slattery EL, Oshima K, Heller S, Warchol ME. Cisplatin exposure damages resident stem cells of the mammalian inner ear. Developmental Dynamics 2014;243(10):1328-37. [DOI: 10.1002/dvdy.24150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spink 2011
- Spink MJ, Fotoohabadi MR, Wee E, Hill KD, Lord SR, Menz HB. Foot and ankle strength, range of motion, posture, and deformity are associated with balance and functional ability in older adults. Archives of Physical Medicine and Rehabilitation 2011;92(1):68-75. [DOI: 10.1016/j.apmr.2010.09.024] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Tinetti 2003
- Tinetti ME. Clinical practice. Preventing falls in elderly persons. New England Journal of Medicine 2003;348(1):42-9. [DOI: 10.1056/NEJMcp020719] [DOI] [PubMed] [Google Scholar]
Wehren 2003
- Wehren L, Hawkes W, Orwig D, Hebel J, Zimmerman S, Magaziner J. Gender differences in mortality after hip fracture; the role of infection. Journal of Bone and Mineral Research 2003;18(12):2231-7. [DOI: 10.1359/jbmr.2003.18.12.2231] [DOI] [PubMed] [Google Scholar]
Winter 1995
- Winter DA. ABC: Anatomy, Biomechanics and Control of Balance During Standing and Walking. Waterloo, ON: Waterloo Biomechanics, 1995. [ISBN: 0-9699420-0-1] [Google Scholar]
References to other published versions of this review
Williams 2015
- Williams AD, Bird ML, King SG, Kirschbaum M, Ogden KJ. Exercise for preventing falls in people with cancer living in the community. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD011687. [DOI: 10.1002/14651858.CD011687] [DOI] [Google Scholar]


