Abstract
Background
Automated systems use closed‐loop control to enable ventilators to perform basic and advanced functions while supporting respiration. Selected automated systems can now not only measure selected respiratory variables and adapt ventilator output to individual patient needs by operationalizing predetermined algorithms but also automate the conduct of spontaneous breathing trials (SBTs).
Objectives
To summarize the evidence comparing automated weaning and SBT systems to non‐automated mechanical ventilation strategies on time to mechanical ventilation discontinuation in adult postoperative patients. In secondary objectives we ascertained differences between automated weaning and SBT systems and non‐automated mechanical ventilation discontinuation strategies on clinical outcomes (time to successful extubation, time to first SBT and first successful SBT, mortality, total duration of ventilation, intensive care unit (ICU) and hospital lengths of stay, use of non‐invasive ventilation (NIV) following extubation, and adverse events).
Search methods
We searched CENTRAL (The Cochrane Library 2013, Issue 5); MEDLINE (OvidSP) (1966 to May 2013); EMBASE (OvidSP) (1988 to May 2013); CINAHL (EBSCOhost) (1982 to May 2013), Evidence Based Medicine Reviews and Ovid Health Star (1999 to May 2013), conference proceedings, trial registration websites, and contacted authors and content experts to identify potentially eligible trials.
Selection criteria
Randomized and quasi‐randomized trials comparing automated weaning and SBT systems to non‐automated mechanical ventilation discontinuation strategies in intubated adults in the postoperative setting.
Data collection and analysis
Two review authors independently assessed trial quality and abstracted data according to prespecified criteria. Sensitivity and subgroup analyses were planned to assess the impact of the type of (i) clinician primarily involved in implementing the automated weaning and SBT systems, (ii) intensive care unit (ICU), and (iii) non‐automated discontinuation (control) strategy utilized on selected outcomes.
Main results
We identified one randomized controlled trial of high quality, involving 300 patients , comparing SmartCare™ to a written protocol. In this trial, SmartCare™ had no effect on discontinuation time. While SmartCare™ significantly reduced the time to the first SBT (mean difference (MD) ‐0.34 days, 95% CI ‐0.60 to ‐0.08; P = 0.01) it did not reduce the time to the first successful SBT (MD ‐0.25 days, 95% CI ‐0.55 to 0.05; P = 0.10) and other clinically important outcomes. SmartCare™ did not demonstrate beneficial effects on most clinically important outcomes including time to successful extubation, total duration of mechanical ventilation, ICU and hospital lengths of stay, and the requirement for tracheostomy. Moreover, SmartCare™ did not favourably impact reintubation, mortality, self‐extubation, and the proportion of patients undergoing protracted mechanical ventilation, with a small numbers of events in this single trial.
Authors' conclusions
There is a paucity of evidence from randomized controlled trials to support or refute use of automated weaning and SBT systems in discontinuing invasive mechanical ventilation in adult postoperative patients. In a single large trial of high methodologic quality, while the use of SmartCare™ to adjust ventilator settings and conduct SBTs shortened the time to undergoing the first SBT, it did not reduce the time to the first successful SBT or the rate of tracheostomy compared to a written protocol implemented by physicians. SmartCare™ did not demonstrate beneficial effects on clinically important outcomes including time to mechanical ventilation discontinuation, time to successful discontinuation, total duration of mechanical ventilation, and ICU and hospital lengths of stay. Additional well‐designed, adequately powered randomized controlled trials are needed to clarify the role for SmartCare™ on important outcomes in patients who predominantly require short term ventilation and in specific postoperative patient populations.
Plain language summary
Use of SmartCare™ to aid discontinuing mechanical ventilation of adults in the postoperative period
We searched the medical literature databases to May 2013. In a single large trial of high methodologic quality that involved 300 patients, use of a system (SmartCare™) that automatically adjusted ventilator settings and conducted tests of patients' ability to breathe spontaneously there was no clear benefit of SmartCare™. While it reduced the time to undergo the first test of spontaneous breathing, it did not reduce the time to the first successful spontaneous breathing test compared to a written weaning protocol applied by physicians. There was no clear benefit of SmartCare™ on other clinically important outcomes including the time to successful discontinuation of artificial ventilation , the total duration of mechanical ventilation, the time spent in the intensive care unit and hospital, and the requirement for tracheostomy (an airway inserted into the trachea). Further studies are needed to clarify the role of SmartCare™ in the postoperative setting, in general, and in specific patient populations.
Background
Description of the condition
Postoperative patients consist of several different populations including those who are otherwise healthy, the acutely ill and the chronically ill, and each population has different needs. Otherwise healthy patients are typically extubated following surgery unless an unexpected event occurs (Price 1999). These events include difficult intubation, a prolonged operation, excessive fluid administration, a protracted intraoperative course, requirement for administration of large amounts of sedative or paralytic agents, concerns regarding airway oedema, and unanticipated intraoperative events. Under these circumstances, otherwise healthy patients may require postoperative ventilation. Acutely ill patients may enter the operating room from the emergency department, a hospital ward, or an intensive care unit (ICU). Similar to acutely ill patients, chronically ill patients may be intubated and mechanically ventilated at the time of presentation to the operating room, or may undergo intubation in the operating room. However, chronically ill patients frequently have underlying conditions that may render extubation after surgery challenging or inadvisable.
Several conditions and predisposing factors are recognized to complicate successful extubation following surgery. These conditions include chronic lung disease (Barisione 1997), cardiovascular disease, neuromuscular disorders (Witt 1991), age (Mircea 1982), sex (Barisione 1997), and obesity (Mircea 1982). Surgical procedures in the thorax and upper abdomen are more likely to cause splinting of respiration, resulting in atelectasis and predisposition to postoperative pulmonary complications. Surgery has been shown to reduce peak flows, forced vital capacity by 50%, and functional residual volume by up to 70% (Barisione 1997; Dureuil 1986). Moreover, upper abdominal surgery impairs diaphragmatic function due to reflexes from the peritoneum and chest wall that impede phrenic nerve function (Dureuil 1986). Factors including postoperative use of nasogastric intubation and a longer duration of the surgery have been shown to significantly increase postoperative pulmonary complications (acute bronchitis, bronchospasm, atelectasis, pneumonia, adult respiratory distress syndrome, pleural effusion, pneumothorax, prolonged mechanical ventilation, or death secondary to acute respiratory failure) (Mitchell 1998). Finally, metabolic derangements or medications may result in respiratory depression by altering serum levels of paralytic agents, up regulating acetylcholine receptors at motor end‐plates (Lee 1995), blocking sodium channels on acetylcholine receptors (Bowman 1993), potentiating the action of neuromuscular blockers (Viby‐Mogensen 1981), and interfering with muscle conductance (Argov 1979) or synaptic function (Price 1999).
Description of the intervention
If patients cannot be extubated postoperatively, a mode of ventilation must be chosen to support respiration until the factors precipitating respiratory compromise can be addressed. Several modes of mechanical ventilation are available and their choice, either as an initial mode of support or to transition patients to extubation, depends upon the patient's ability to breath spontaneously, underlying comorbid illnesses, and the clinical circumstances. Not all patients require formal weaning, or transitioning the work of breathing from the ventilator back to the patient. In elective surgical populations the majority of patients have no lung pathology prior to surgery and may only require a strategy to discontinue support once the effects of the anaesthetic agents have abated.
With volume controlled ventilation (VCV), clinicians set the tidal volume (VT), respiratory rate, peak flow rate, flow pattern, fractional concentration of oxygen (FiO2), and the positive end‐expiratory pressure (PEEP) delivered; inspiration terminates after delivery of the preset VT. Synchronized intermittent mechanical ventilation (SIMV) and assist control (AC) are two commonly used modes of volume‐limited ventilation. Patients can increase their minute ventilation (VE) by initiating spontaneous breaths with variable VT (SIMV) or by triggering additional breaths delivered at a preset VT (AC).
With pressure‐controlled ventilation (PCV), clinicians set an inspiratory time (or inspiratory to expiratory ratio), inspiratory pressure level, respiratory rate, FiO2 and PEEP. Inspiration ends after a set inspiratory pressure is delivered for a set time inspiratory time. With PCV, tidal volumes vary according to airway resistance, compliance, endotracheal tube resistance, set inspiratory pressure and end‐expiratory alveolar pressure. Compared to VCV, PCV limits maximal inspiratory pressure.
With pressure support (PS), breaths triggered by patients are supported up to a predetermined inspiratory pressure level. Unlike PCV, the ventilator cycles into expiration after inspiratory flow has decreased to a predetermined level. PS is thus a spontaneous mode of ventilation where all breaths are initiated by the patient and supported by a preset pressure. This preset pressure can be titrated up or down by the clinician according to the respiratory status of the patient. PS can be used in combination with SIMV (SIMV + PS) such that breaths triggered in the spontaneous period are supported by a preselected PS level (Banner 1997). With SIMV + PS the end of the inspiratory period occurs either after a set time for an SIMV breath or following a predetermined decrease in flow after a PS breath. SIMV can provide a range of ventilatory supports. With SIMV, patients can increase their minute ventilation by triggering a mandatory breath (in the SIMV period) or a spontaneous breath (if triggering occurs earlier in a spontaneous period) prior to the next mandatory breath.
Early attempts were made to enable interaction between patients and the ventilator adapted SIMV and PS (Strickland 1991; Strickland 1993). More recently investigators have conducted pilot trials (Bouadma 2005) and retrospective studies (Kataoka 2007) of automated systems that adapt PS alone. Automated systems use closed‐loop control to enable ventilators to perform basic and advanced functions while supporting respiration. Closed‐loop systems adapt the ventilator output by comparing measured and targeted values of selected respiratory variables and either minimizing, equilibrating (negative feedback) or amplifying (positive feedback) the differences between these values (Burns 2008). Automated modes of mechanical ventilation use more sophisticated closed‐loop systems to enable interaction between patients and the ventilator.
How the intervention might work
Several closed‐loop, automated systems are currently marketed. Mandatory minute ventilation (MMV) (Evita 4, Draeger Medical Inc, Lübeck, Germany) combines features of controlled ventilation with mandatory and spontaneous breaths as does VCV + PS or SIMV + PS. Clinicians can set VT, the mandatory breath rate, the level of PS provided during spontaneous breaths and a target VE. After considering the patient's spontaneous respiratory rate, MMV adapts the mandatory respiratory rate to achieve the target VE. Adaptive support ventilation (ASV) (Galileo, Raphael and Hamilton‐G5, Hamilton Medical AG, Rhaezuens, Switzerland) is an automated system that adapts inspiratory pressure in PCV or PS mode to achieve a target VT . ASV targets a desired VE, set as a percentage of normal ventilation, and seeks the optimal VT and respiratory rate (least energy expenditure) to achieve this VE using the Otis equation. Neither MMV nor ASV automate the conduct of SBTs. Conversely, SmartCare™ (Draeger Medical Inc, Lübeck, Germany) measures selected respiratory variables, adapts ventilator output by operationalizing predetermined algorithms, and automates the conduct of spontaneous breathing trials (SBTs) (Burns 2008). To initiate SmartCare™, end‐users enter the patient's weight, the presence or absence of chronic obstructive pulmonary disease (COPD) or a central neurologic disorder, the type of airway prosthesis (tracheostomy or oro‐nasal endotracheal tube), and the type of humidification (heated humidification (HH) or heat and moisture exchanger (HME)) in use. The first three variables establish limits for respiratory rate, VT, and partial pressure of end‐tidal carbon dioxide (PETCO2), and the latter two items determine the threshold to cycle into a SBT (ranging from 5 to 12 cm H2O). SmartCare™ categorizes patients into one of eight diagnostic categories based on average measurements of these variables that are made every two to five minutes (Burns 2008). With SmartCare™, patients may have a respiratory rate ranging from 15 to 30 breaths/min (RR min), alternatively 34 breaths/min for patients with neurologic disease (RR max), a VT above a minimum threshold (VT Min = 250 mL if weight < 55 kg, or VT Min = 300 mL if weight > 55 kg) and a PETCO2 below a maximum threshold (max PETCO2 = 55 mmHg or max PETCO2 = 65 mm Hg for COPD patients). SmartCare™ ascribes a state of normal ventilation when a patient's ventilatory measurements fall within these constraints. If the patient's measured values fall outside of the constraints, an alternate diagnosis is made and the system adjusts the level of PS provided, up or down, to achieve these targets.
SmartCare™ automatically initiates a SBT (observation period) when predetermined PS thresholds are reached, provided that the patient is in a state of normal ventilation and their PEEP is < 5 cm H2O. The 'observation period' varies from 30 minutes to two hours in duration. Upon successful completion of an SBT, the ventilator issues a directive stating that the patient is 'ready for separation from ventilator'. Clinicians must ensure that patients meet criteria to proceed with extubation. In the SmartCare™ system, clinicians control titration of the FiO2 and PEEP. Consequently, if PEEP is not titrated to < 5 cm H2O an SBT will not be conducted. Clinicians can specify whether the automated algorithms are applied during the day only or continuously.
Why it is important to do this review
Regardless of the mode of ventilation selected to support patients in the postoperative period, limiting the duration of invasive ventilation and identifying the optimal time for discontinuation in order to limit development of postoperative complications, especially pulmonary complications, is an important goal in providing care for postoperative patients. Systems that automate weaning and SBTs obviate the need for clinicians to recognize and manually adjust ventilator settings to wean and conduct SBTs. Consequently, with these systems liberation is unencumbered by limited clinician availability in the busy intensive care unit (ICU) setting. In this review, we will identify, critically appraise and synthesize the best current evidence comparing automated weaning and SBT systems to non‐automated systems to discontinue mechanical support in invasively ventilated adults in the postoperative setting.
Objectives
To summarize the evidence comparing automated weaning and SBT systems to non‐automated mechanical ventilation strategies on time to mechanical ventilation discontinuation in adult postoperative patients. In secondary objectives we ascertained differences between automated weaning and SBT systems and non‐automated mechanical ventilation discontinuation strategies on clinical outcomes (time to successful extubation, time to first SBT and first successful SBT, mortality, total duration of ventilation, intensive care unit (ICU) and hospital length of stay, use of non‐invasive (NIV) ventilation following extubation, and adverse events).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐randomized trials comparing automated weaning and SBT systems to non‐automated mechanical ventilation discontinuation strategies. Whereas an RCT was defined as a study that generates an unpredictable sequence to allocate participants to study groups (for example, a random number table, computer‐generated random numbers, shuffling of envelopes or throwing dice) (Higgins 2011), a quasi‐randomized trial was defined as trials where participants are allocated to treatment arms by alternate or predictable assignment.
Types of participants
We included trials investigating alternative discontinuation strategies in adults in the postoperative setting. We used authors' definitions of adults as criteria for admission to adult ICUs may vary internationally. We did not restrict the participants to specific population characteristics, including sex, age, race or the presence of selected risk factors. We excluded trials wherein the majority of patients required more protracted ventilation (that is, weaning as opposed to planned short term ventilation) or that involved exclusively tracheostomized patients.
Types of interventions
We included RCTs and quasi‐randomized trials that compared automated weaning and SBT systems to non‐automated discontinuation strategies. Non‐automated strategies included usual care, standard care, protocolized care or other strategies (as defined by the study authors) but did not involve the use of a nearly fully automated system. We excluded modes that are not usually used for discontinuation of mechanical ventilation (for example, AutoFlow (Draeger Medical Incorporated)) and pressure‐regulated volume control (Maquet Dynamed, Tyco), nearly fully automated systems (for example, ASV (Hamilton Medical)) and modes that switch from pressure control (PC) to pressure support (PS), that is, Automode (Maquet Dynamed, Tyco Healthcare) and strategies in which modifications of PS were linked to inspiratory flow (automatic tube compensation). Non‐automated discontinuation strategies included usual care, standard care, protocolized care or other strategies (as defined by the study authors) but did not involve the use of a closed‐loop system. We excluded studies that: (i) compared the alternative strategies as weaning strategies (that is, weaning as opposed to planned short term ventilation for the majority of patients), (ii) explored the use of non‐invasive ventilation (NIV) in this regard (that is, extubation to NIV), (iii) evaluated exclusively tracheostomized patients, or (iv) explored the use of a nearly fully automated closed‐loop system (applied invasively or non‐invasively) in the control arm.
Types of outcome measures
Primary outcomes
The primary outcome was time to mechanical ventilation discontinuation (from randomization to extubation) as defined by the study authors.
Secondary outcomes
Our secondary outcomes included:
time to successful extubation (time from randomization to successful extubation, as defined by study authors);
time to first SBT and first successful SBT (times from randomization to first SBT and first successful SBT, as defined by study authors);
mortality (the most protracted and at times reported by study authors);
total duration of ventilation (time from invasive ventilation initiation to extubation, as defined by the study authors);
ICU length of stay;
use of non‐invasive ventilation (NIV) following extubation;
adverse events (including reintubation, self‐extubation, requirement for tracheostomy and prolonged ventilation, as defined by the study authors);
hospital length of stay.
To be included, studies had to report at least one of the aforementioned primary or secondary outcomes.
Search methods for identification of studies
Electronic searches
We used database specific search strategies to search the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 5); MEDLINE (OvidSP) (1966 to May 2013); EMBASE (OvidSP) (1988 to May 2013); CINAHL (EBSCOhost) (1982 to May 2013), Evidence Based Medicine Reviews and Ovid Health Star (1999 to May 2013) to identify potentially eligible trials. We based our search strategies on the optimally sensitive search strategies of The Cochrane Collaboration in order to identify randomized trials in MEDLINE and EMBASE (Dickerson 1994; Lefebvre 2001; Robinson 2002). We combined our subject search terms in MEDLINE with the Cochrane highly sensitive search strategy for identifying RCTs as contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted our MEDLINE search strategy to other databases (see Appendix 1: CENTRAL; Appendix 2: MEDLINE; Appendix 3: EMBASE; Appendix 4: CINAHL; Appendix 5: Evidence based Medicine Reviews; and Appendix 6: Ovid Healthstar). We did not limit our search by language or publication status.
Searching other resources
We contacted the first authors of all included studies and content experts to obtain additional information on unpublished trials or trials in progress. We searched the bibliographies of all retrieved trials and review papers for potentially relevant trials. Additionally, we handsearched conference proceedings from five scientific meetings (Annual Congress of the European Society of Intensive Care Medicine (2001 to 2011), College of Chest Physicians (2003 to 2011), American Thoracic Society (2004 to 2011), the International Symposium of Intensive Care and Emergency Medicine (2004 to 2011), and Critical Care Medicine (2004 to 2012)) to identify abstracts of randomized trials meeting our inclusion criteria. Finally, we searched for ongoing trials on the following websites: www.controlled‐trials.com, www.clinicalstudyresults.org and http://clinicaltrials.gov.
Data collection and analysis
Trial identification
We utilized the methods of the Cochrane Anaesthesia Review Group. Two authors (KB, FL) independently screened titles and abstracts identified by electronic and manual searches. Two authors (KB, JF) retrieved and evaluated the full text versions of potentially relevant trials.
Selection of studies
Two authors (KB, JF) independently selected trials meeting the study inclusion criteria using a checklist developed for this purpose (Appendix 7). We resolved disagreements first through discussion then in consultation with a third review author (ML) if agreement could not be achieved. We recorded reasons for study exclusion in the table 'Characteristics of excluded studies'. One author (JF) handsearched conference proceedings.
Data extraction and management
Two authors (KB, JF) independently extracted data using a standardized data collection form (Appendix 7) that included information regarding the name of the first author, year of publication, study design, study population and study setting. In addition to information pertaining to patient characteristics, study inclusion and exclusion criteria, details of the interventions compared, clinicians involved in implementing the discontinuation strategies and study outcomes, we extracted information regarding study methodology. This included the method of randomization, allocation concealment, frequency and handling of withdrawals, selective outcomes reporting, stopping early for benefit, and adherence to the intention‐to‐treat principle. We attempted to contact the first authors of included trials to obtain missing data or to clarify study design features, where necessary. We resolved disagreements through discussion and in consultation with a third review author (ML) as required. We did not blind reviewers to the names of the study authors, investigators, institutions, nor the study results.
Assessment of risk of bias in included studies
The quality of all included trials was assessed by two authors (KB, JF), independently and in duplicate. We judged study quality on the basis of the following (Higgins 2011).
1. Was sequence generation truly random?
Adequate sequence generation included reference to a random number table, use of a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing lots, or minimization.
2. Was allocation adequately concealed? Adequate allocation concealment included central randomization (for example, allocation by a central office unaware of participant characteristics unless based on stratification); an on‐site computer system combined with the allocation sequence being kept in a locked unreadable computer file accessed only after the characteristics of an enrolled participant were entered; sequentially numbered, sealed, opaque envelopes; or other similar approaches that ensured the person generating the allocation sequence did not administer it.
3. Was knowledge of the allocated interventions adequately prevented during the study? Blinding of study participants and personnel from study intervention allocation after inclusion of participants is not feasible, however, we judged whether outcome assessors were separate from the individuals administering or supervising the assigned interventions.
4. Were withdrawals described and did they occur with similar frequency between the intervention and control groups?
5. Were reports of the study free of suggestion of selective outcome reporting?
6. Did the trial stop early for benefit? What was the impact of stopping of the trial early, if applicable?
7. Were participants analysed according to the intervention to which they were allocated, whether they received it or not?
Within studies, we described what was reported for each domain and contacted study authors for further information.
We assigned a judgement related to the risk of bias for each domain as follows: 'Yes', criteria appropriately applied and described in the report or acknowledged from the primary author of the study; 'Unclear', criteria not described or impossible to acquire from the author; 'No', criteria inappropriately applied.
A judgement of 'Yes' indicated a low risk of bias, 'No' indicated a high risk of bias, and 'Unclear' indicated an unknown or unclear risk of bias. For example, low risk of bias was assigned when allocation concealment was adequate (including central randomization such as allocation by a central office unaware of participant characteristics; on‐site computer system combined with allocation kept in a locked unreadable computer file that could be accessed only after the characteristics of an enrolled participant had been entered; sequentially numbered, sealed, opaque envelopes or other similar approaches that ensured the person who generated the allocation scheme did not administer it). We assigned an unclear risk of bias when allocation concealment was unclear or when the authors did not clearly report their approach; and a high risk of bias when allocation concealment was not applied. We evaluated the impact of methodologic quality (low or unclear versus high risk of bias) on discontinuation time. We planned to construct a 'Risk of bias' (RoB) table to depict the results. Two authors (KB, JF) entered data into Review Manager (RevMan 5.1) for statistical analysis.
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence in our review associated with specific outcomes (discontinuation time, time to successful extubation, time to first SBT and first successful SBT, mortality, total duration of mechanical ventilation, ICU length of stay, and reintubation) and constructed a 'Summary of findings' (SoF) table using the GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considered: within study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Measures of treatment effect
We summarized the treatment effect using risk ratio (RR) and the mean difference (MD) for binary and continuous outcomes, respectively.
Unit of analysis issues
We used proportions for binary outcomes and preferentially used mean and standard deviation, where reported or available through correspondence with authors, in pooled analyses. Summary estimates constitute the units of analysis in this review.
Dealing with missing data
For published reports with insufficient or ambiguous information, we contacted investigators, where feasible, to clarify study methods and inquire about missing data.
Assessment of heterogeneity
We assessed clinical heterogeneity by judging, qualitatively, the differences between studies with regard to the populations enrolled, implementation of the discontinuation strategies, and outcomes reporting. We conducted statistical tests of heterogeneity and assessed the impact of heterogeneity for each outcome using the I2 statistic. This statistic describes the percentage of total variance across studies that can be attributed to heterogeneity rather than chance (Higgins 2003). We considered I2 statistic thresholds of 0% to 40%, 30% to 60%, 50% to 90% and > 75% to represent between study heterogeneity that might not be important, moderate, substantial or considerable, respectively (Higgins 2011). In the absence of appreciable heterogeneity that precluded pooling, we performed meta‐analyses using random‐effects models (RE) and reported summary estimates with their associated 95% confidence intervals (CI).
Assessment of reporting biases
Publication bias occurs when published trials are not fully representative of all completed trials, as positive trials (large and small) tend to be published more often than negative trials, especially small, negative trials. We examined funnel plots (a graphical display) for asymmetry and the size of the treatment effect (primary outcome) against trial precision (1/standard error) to assess for publication bias, if a sufficient number (at least 10) of studies were identified (Egger 1997).
In the included studies, interventions were continuously applied and outcomes were reported at multiple time points. We recognized that the conduct of multiple analyses increases the chance of spurious positive findings. While many statistical approaches have been developed to adjust for multiple testing, there is no consensus regarding when multiplicity should be taken into consideration. Further, adjustments for multiple testing are not routinely conducted in systematic reviews. Consequently, a priori, we proposed to highlight the primary outcome and the first five secondary outcomes in this protocol as key outcomes featured in the SoF table. Additionally, we emphasized estimation of intervention effects rather than tests for them and considered subgroup analyses as exploratory in nature.
Data synthesis
We used RE models to pool data quantitatively, using Review Manager 5.1 software (RevMan 5.1), when studies were overall clinically similar.
Subgroup analysis and investigation of heterogeneity
A priori, we planned to perform subgroup analyses to assess the impact of the following study design features on discontinuation time, ICU length of stay, mortality, use of NIV, and reintubation:
the type of clinician principally involved in implementing the automated discontinuation strategy (i.e., registered respiratory therapist (RRT) versus other, including mixed clinicians), as defined by the study authors;
the type of ICU (i.e., medical and surgical, and purely surgical versus purely medical, including coronary care units), as defined by the study authors;
the type of non‐automated (control) discontinuation strategy utilized (predominantly protocolized versus predominantly non‐protocolized care, or other), as defined by the study authors.
We anticipated that subgroup analyses would be underpowered. We viewed subgroup analyses as exploratory given their tendency to generate misleading conclusions (Oxman 1992; Yusuf 1991).
Sensitivity analysis
A sensitivity analysis was planned to assess the impact on discontinuation time of excluding studies with a high risk of bias.
Results
Description of studies
We identified 12 randomized trials (Beale 2007; Bifulco 2008; Burns 2012; Jiang 2006; Lellouche 2006; Ma 2010; Papirov 2007; Reardon 2011; Rose 2008; Schadler 2012; Stahl 2009; Wong 2008) comparing SmartCare™ to a non‐automated strategy and potentially meeting our study inclusion criteria, including one quasi‐randomized trial (Jiang 2006). We did not identify other automated weaning and SBT systems other than SmartCare™. Through correspondence, one author confirmed that the trial never started (Beale 2007); another acknowledged that their trial was stopped due to slow recruitment after enrolment of three patients (Wong 2008); and a final author confirmed that his trial included exclusively tracheostomized patients and stopped prematurely due to a need to return the study ventilators (Papirov 2007). All three trials (Beale 2007; Papirov 2007; Wong 2008) were identified on trial registration websites.
Results of the search
We screened 2560 citations to identify 18 articles that potentially met our inclusion criteria (Figure 1).
1.
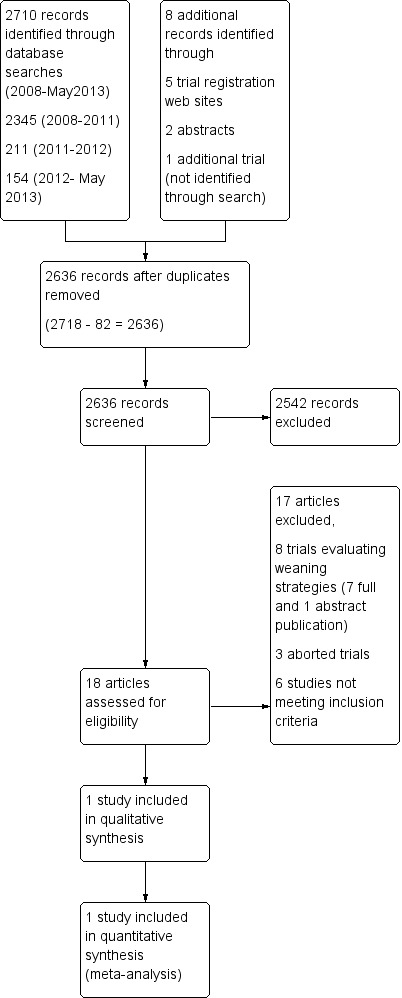
Study flow diagram.
Included studies
Only one trial (Schadler 2012), published in full, evaluated SmartCare™ as a discontinuation strategy in a postoperative population requiring short term ventilation. Full details of the participants, interventions and outcomes for this trial (Schadler 2012) are provided in the Characteristics of included studies table.
Excluded studies
Eight trials (Bifulco 2008; Burns 2012; Jiang 2006; Lellouche 2006; Ma 2010; Reardon 2011; Rose 2008; Stahl 2009) evaluated patients who received mechanical ventilation after approximately 24 hours of invasive ventilation and were deemed weaning trials and were excluded. These trials are summarized in a separate review (Burns 2010b). Additionally, we excluded three aborted trials (Beale 2007; Papirov 2007; Wong 2008) and six studies (Chen 2008; Donglemans 2007; Jolliet 2006; Jouvet 2007; Kataoka 2007; Taniguchi 2009) not meeting the study inclusion criteria (Characteristics of excluded studies). The two review authors (KB, JF) achieved complete agreement on study selection.
In a randomized, open label study conducted at three ICUs in a university medical centre in Germany, Schadler and coworkers (Schadler 2012) included, under deferred consent, all patients who were mechanically ventilated in the postoperative period for longer than nine hours after ICU admission and who did not meet any of the following exclusion criteria: (i) cerebral surgery or trauma, (ii) < 18 years of age, (iii) had a do not resuscitate order, (iv) duration of mechanical ventilation (MV) > 24 hours, or (v) already a study participant. Patients or their legal representatives were approached for written consent before or during the 36 hours following study inclusion and randomization.
In this trial (Schadler 2012), patients from three surgical ICUs (cardiovascular, interdisciplinary and surgical) serving all surgical disciplines were randomly assigned, using an electronically generated system, to either the SmartCare™ or control groups (standardized, written protocol implemented by physicians). Allocation was concealed but not stratified. All patients were ventilated with Evita XL respirators (software version 6.0, Draeger Medical, Lübeck, Germany) equipped with SmartCare (version 1.1).
Following study inclusion, mechanical ventilation was continued as set prior to randomization and: (i) FiO2 and PEEP were set to achieve a peripheral oxygen saturation (SpO2) of > 95%, (ii) inspiratory pressure and the level of PS were set to achieve a VT of 6 to 8 ml/kg predicted body weight, and (iii) the partial pressure of carbon dioxide (CO2) was kept between 35 and 50 mm Hg. During the study, the flow trigger was adjusted to 2 L/min and a heat and moisture exchanger (HME) was recommended. In addition, an end tidal CO2 sensor was used and automatic tube compensation (ATC) was not permitted. Analgesia was maintained using a continuous infusion of sufentanil (range 0.1 to 0.4 μg/kg/hour) according to individual patient requirements and sedation was achieved using a continuous infusion of propofol (maximum 4 mg/kg/hour) in the 24 hour period after study inclusion. Thereafter, patients received boluses of midazolam titrated to achieve a Ramsay Score of R2 (cooperative, oriented, tolerant of mechanical ventilation).
If patients were haemodynamically stable, with (up to a maximum of 0.01 mg/kg/hour) or without epinephrine and norepinephrine, a post‐randomization PS trial (on PS of 15 to 30 cm H2O with identical FiO2 and PEEP) was conducted for 30 minutes by the responsible ICU physician or during study visits when clinically indicated. The PS test was considered successful when the respiratory rate was < 35 breaths/min, VT> 6 cc/kg predicted body weight within the permitted PS range, SpO2> 90% and the patient remained clinically stable. Once the patient successfully completed the PS test, the allocated study group assignment was commenced.
Control discontinuation strategy
Ventilator adjustments were made to the level of PS to keep the respiratory rate < 35 breaths/minute with good clinical tolerance. The investigators aimed to decrease PS at least three times daily by 2 or 3 cm H2O and in response to tachypnoea (respiratory rate > 35 breaths/minute for longer than three minutes). An SBT of 30 minutes duration was initiated when PS < 12 cm H2O, PEEP < 5 cm H2O and FiO2< 0.50. The SBT was deemed successful if the respiratory rate was < 35 breaths/min, SpO2> 90% and the patient remained clinically stable. Following a failed SBT, the SBT was re‐initiated at least once during the next 24 hour period when the starting criteria were met.
SmartCare™ discontinuation strategy
The investigators deactivated the night rest and ATC settings and reported use of HME. An SBT was automatically initiated once the applied PS level was equal to or lower than the target PS level, the patient remained stable in the 'respiratory comfort zone' and PEEP was < 5 cm H20.
In both treatment groups, controlled ventilation was permitted if respiratory rate < 6 breaths/min, induction of general anaesthesia, and when a maximum PS > 35 cm H2O and respiratory rate > 35 breaths/min for longer than three minutes occurred in the absence of another cause (pain, anxiety, endotracheal tube obstruction). FiO2 and PEEP were set based on the ratio of the arterial partial pressure of oxygen to FiO2. FiO2 was preferentially reduced stepwise to 0.4 and subsequently PEEP was decreased by steps not exceeding 3 cm H2O to a final target value of 5 cm H2O. Readiness for extubation was indicated by either a proposal of separation by the SmartCare™ system or successful SBT completion in the control group. Extubation or disconnection (tracheostomized patients) was performed when PaO2/FiO2 > 200, GCS > 8 or the patient was awake or deemed able to protect their airway, the patient coughed effectively and there was no surgical contraindication.
A priori, the authors (Schadler 2012) planned to examine: (i) cardiac surgery, (ii) septic and (iii) COPD patients in subgroup analyses. The primary endpoint was ventilation time during the ICU stay defined as the requirement for invasive or non‐invasive support over the 28 day study period. Secondary endpoints included time in the respiratory comfort zone, the number of ventilator manipulations and alarms during invasive ventilation, ICU and hospital lengths of stay, 28 and 90 day mortality.
Risk of bias in included studies
The included trial was at low risk of bias (Figure 2) (Characteristics of included studies). The study was, by necessity, not blinded.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
The single trial (Schadler 2012) involving 300 adult postoperative patients evaluated the following outcomes.
1.1 Discontinuation time (randomization to extubation)
The results of this trial showed a non‐significant effect of SmartCare™ versus a standardized written protocol implemented by physicians (MD ‐0.09 days, 95% CI ‐0.35 to 0.17; P = 0.50) (Analysis 1.1).
1.1. Analysis.

Comparison 1 Discontinuation time (randomization to extubation), Outcome 1 Discontinuation time (randomization to extubation).
1.2 Time to successful extubation
We did not find evidence of an effect of SmartCare™ compared to a control discontinuation strategy on time to successful extubation (MD ‐0.29 days, 95% CI ‐0.79 to 0.21; P = 0.25) in this trial (Schadler 2012) (Analysis 2.1).
2.1. Analysis.

Comparison 2 Time to successful extubation, Outcome 1 Time to successful extubation.
1.3 and 1.4 Time to first SBT and first successful SBT
SmartCare™ significantly decreased the time to first SBT (MD ‐0.34 days, 95% CI ‐0.60 to ‐0.08; P = 0.01) (Analysis 3.1) but did not reduce the time to first successful SBT (MD ‐0.25 days, 95% CI ‐0.55 to 0.05; P = 0.10) (Analysis 4.1) compared to the control discontinuation strategy in this trial.
3.1. Analysis.

Comparison 3 Time to first spontaneous breathing trial, Outcome 1 Time to first spontaneous breathing trial.
4.1. Analysis.

Comparison 4 Time to first successful spontaneous breathing trial, Outcome 1 Time to first successful spontaneous breathing trial.
1.5 Most protracted measure of mortality
At 90 days, there was no difference in mortality with SmartCare™ compared to a written, standardized control discontinuation strategy implemented by physicians (RR 1.13, 95% CI 0.74 to 1.71; P = 0.58) in 300 patients with 36 and 32 events in the SmartCare™ and control arms, respectively (Analysis 5.1).
5.1. Analysis.
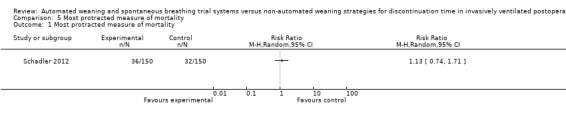
Comparison 5 Most protracted measure of mortality, Outcome 1 Most protracted measure of mortality.
1.6 Total duration of mechanical ventilation
This trial showed a non‐significant difference between SmartCare™ and the control discontinuation strategy (MD ‐1.00 days, 95% CI ‐2.46 to 0.46; P = 0.18) on total duration of mechanical ventilation (Analysis 6.1).
6.1. Analysis.

Comparison 6 Total duration of mechanical ventilation, Outcome 1 Total duration of mechanical ventilation.
1.7 Intensive care unit (ICU) length of stay
We did not find evidence of an effect of SmartCare™ compared to a standardized control discontinuation strategy on ICU length of stay (MD 0.40 days, 95% CI ‐1.08 to 1.88; P = 0.60) (Analysis 7.1).
7.1. Analysis.

Comparison 7 Intensive care unit length of stay, Outcome 1 Intensive care unit length of stay.
1.8 Use of non‐invasive ventilation following extubation
Following extubation, SmartCare™ did not significantly reduce the proportion of patients who received non‐invasive ventilation (RR 0.89, 95% CI 0.47 to 1.68; P = 0.72) (Analysis 8.1) with 16 and 18 events in the SmartCare™ and control arms, respectively.
8.1. Analysis.
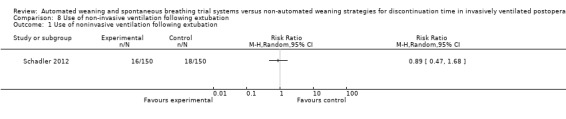
Comparison 8 Use of non‐invasive ventilation following extubation, Outcome 1 Use of noninvasive ventilation following extubation.
1.9 to 1.13 Adverse events (reintubation, self‐extubation, tracheostomy, protracted ventilation for more than 14 and 21 days)
SmartCare™ compared to the control discontinuation strategy did not reduce the proportion of patients requiring reintubation (RR 0.88, 95% CI 0.59 to 1.30; P = 0.51) (Analysis 9.1) or undergoing prolonged ventilation for more than 14 (RR 0.76, 95% CI 0.39 to 1.52; P = 0.44) (Analysis 10.1) and 21 days (RR 0.88, 95% CI 0.33 to 2.35; P = 0.79) (Analysis 11.1). SmartCare™ did not reduce the proportion of patients undergoing tracheostomy (RR 0.61, 95% CI 0.35 to 1.06; P = 0.08) with 17 and 28 events in the SmartCare™ and control arms, respectively (Analysis 12.1). With few events in the SmartCare™ (n = 4) and control arms (n = 1) and wide confidence intervals, the rate of self‐extubation was not different between SmartCare™ and the control discontinuation strategy (RR 4.00, 95% CI 0.45 to 35.37; P = 0.21) (Analysis 13.1).
9.1. Analysis.
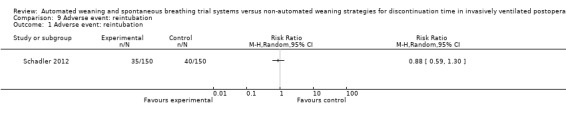
Comparison 9 Adverse event: reintubation, Outcome 1 Adverse event: reintubation.
10.1. Analysis.
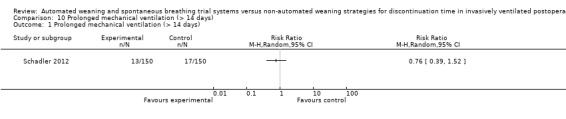
Comparison 10 Prolonged mechanical ventilation (> 14 days), Outcome 1 Prolonged mechanical ventilation (> 14 days).
11.1. Analysis.
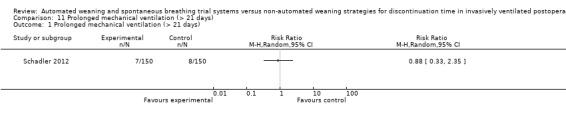
Comparison 11 Prolonged mechanical ventilation (> 21 days), Outcome 1 Prolonged mechanical ventilation (> 21 days).
12.1. Analysis.
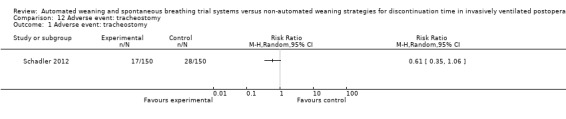
Comparison 12 Adverse event: tracheostomy, Outcome 1 Adverse event: tracheostomy.
13.1. Analysis.
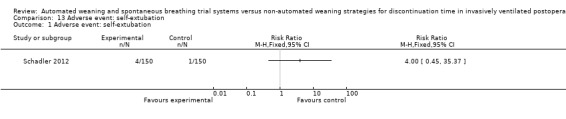
Comparison 13 Adverse event: self‐extubation, Outcome 1 Adverse event: self‐extubation.
1.14 Hospital length of stay
We did not find evidence of an effect of SmartCare™ compared to a standardized control discontinuation strategy on hospital length of stay (MD ‐1.10 days, 95% CI ‐5.49 to 3.29; P = 0.62) (Analysis 14.1).
14.1. Analysis.

Comparison 14 Hospital length of stay, Outcome 1 Hospital length of stay.
Heterogeneity assessments and subgroup and sensitivity analyses
Assessments of heterogeneity and planned subgroup and sensitivity analyses were not possible given that only one trial met our inclusion criteria.
Publication bias
We did not assess for publication bias.
Discussion
Summary of main results
We identified a single, large, high quality, randomized controlled trial involving 300 patients that compared SmartCare™ to a written discontinuation protocol implemented by physicians in the postoperative period. In this trial, SmartCare™ had no effect on discontinuation time (Analysis 1.1). While SmartCare™ significantly reduced the time to the first SBT (Analysis 3.1), it did not reduce the time to the first successful SBT (Analysis 4.1), time to successful extubation (Analysis 2.1), total duration of mechanical ventilation (Analysis 6.1), ICU and hospital lengths of stay (Analysis 7.1; Analysis 14.1) and the requirement for tracheostomy (Analysis 12.1). Moreover, there was no benefit of SmartCare™ on reintubation, mortality, rates of non‐invasive ventilation (NIV) use and self‐extubation, or the proportion of patients undergoing protracted mechanical ventilation (Analysis 9.1; Analysis 5.1; Analysis 8.1; Analysis 13.1; Analysis 10.1; Analysis 11.1) amidst the small numbers of events in this trial.
Overall completeness and applicability of evidence
We found significant differences in one large trial of high methodologic quality demonstrating an effect of SmartCare™ compared to a written discontinuation protocol directed by physicians in reducing the time to first SBT. However, SmartCare™ did not reduce the time to successful completion of a first SBT or the proportion of patients receiving a tracheostomy. Moreover, we did not find evidence of a beneficial effect of SmartCare™ on other clinically important outcomes including discontinuation time, the total duration of mechanical ventilation, ICU and hospital lengths of stay, and rates of NIV use following extubation or self‐extubation. Despite reducing the time to first SBT, SmartCare™ had no effect on ICU and hospital mortality or the incidence of ventilator associated pneumonia (VAP). Unlike the non‐invasive approach to weaning (Burns 2010a), which has been shown to significantly reduce mortality and VAP compared to continued invasive weaning, SmartCare™, despite reducing the time to first SBT, did not favourably impact upon mortality and VAP rates. This may be related to the fact that unlike NIV, SmartCare™ requires an indwelling airway. In addition, the duration of discontinuation was shorter, measured in hours in postoperative patients, leaving a small time window for SmartCare™ to impact upon measures related to the duration of ventilation and to influence intubation and mechanical ventilation related complications. In a parallel SmartCare™ weaning systematic review (Burns 2010b), wherein we included studies of patients mechanically ventilated for at least 24 hours or studies with an average duration of ventilation prior to randomization of at least 24 hours, we found that SmartCare™ significantly reduced weaning time and the total duration of mechanical ventilation. Consequently, the benefits of SmartCare™ may be best realized in circumstances where the time trajectory for mechanical support is more protracted or in specific patient populations (that is, the cardiac surgery subgroup in the Schadler trial) (Burns 2010b; Schadler 2012).
Quality of the evidence
The risk of bias in this trial was low given the use of an electronic randomization system, concealed allocation, absence of selective outcome reporting, adequacy of follow‐up, absence of stopping early for benefit, and adherence to the intention‐to‐treat principle. The analysis was based on 300 randomized patients for which consent was obtained. The authors used a hybrid approach to obtaining consent including a priori patient and substitute decision maker (SDM) consent as well as deferred consent wherein consent from patients or SDMs could be sought following randomization. Consequently, in 17 randomized patients deferred or after‐the‐fact consent was not obtained. Data could therefore only be reported in 300 of the 317 randomized patients. While intended to facilitate enrolment, use of deferred consent and the inability to secure patient and SDM assent following randomization did not allow the authors to report outcomes for these patients. We do not know whether the patients for whom consent could not be obtained differed between groups or differed systematically from included patients in some manner (that is, patients were sicker). While the impact of these patients on continuous outcomes (where each patient contributes an outcome) remains unknown, it is unlikely that inclusion of these patients would have influenced binary event rates in an important manner.
Potential biases in the review process
This review was strengthened by an extensive search for relevant trials. We conducted duplicate, independent citation screening and data abstraction and corresponded with the principal study investigator to clarify study methods, where needed. To distinguish the impact of SmartCare™ in patients receiving invasive ventilation for shorter and longer time periods, we considered this trial separately because most, but not all, patients underwent extubation following short term ventilation. Notwithstanding, we acknowledge that 26% of SmartCare™ and 31% of control patients received ventilation for more than four days, with approximately 10% of the study population ventilated for more than 14 days. An individual patient meta‐analysis would be necessary to assess the effect of the alternative strategies based on actual duration of ventilation and was beyond the scope of this systematic review.
Agreements and disagreements with other studies or reviews
This is the first systematic review and meta‐analysis comparing the effect of SmartCare™ to non‐automated control discontinuation strategies on important clinical outcomes in the postoperative period. The rationale for using SmartCare™ in the comparison, as opposed to all automated (closed‐loop) weaning strategies, was the recognition that unlike other closed‐loop systems SmartCare™ integrates several strategies (use of a weaning protocol, conduct of SBTs, and use of PS mode) that have been demonstrated in randomized trials to be of benefit in weaning. We excluded trials involving patients who required more formal weaning (following more protracted invasive ventilation) in an effort to evaluate the effect of SmartCare™ in a homogeneous patient population. Notwithstanding, the strength of the conclusions that can be made from our review are limited by the identification of only one large, well‐conducted trial evaluating SmartCare™ as a discontinuation strategy in a heterogenous cohort of surgical patients. Summary estimates from this trial suggest that SmartCare™ reduces the time to first SBT but did not favorably impact upon other clinically important outcomes, likely due to the small time trajectory in which it can act in patients who predominantly require discontinuation.
Authors' conclusions
Implications for practice.
There is a paucity of evidence from randomized trials to support or refute use of SmartCare™ in discontinuing invasive mechanical ventilation in adult postoperative patients. In a single large trial of high methodologic quality, use of an automated system to adjust ventilator settings and conduct SBTs did not favorably influence discontinuation time. While SmartCare™ significantly reduced the time to undergoing a first SBT compared to a written protocol implemented by physicians, there was no clear evidence of benefit on clinically important outcomes including the time to first successful SBT, successful extubation, total duration of mechanical ventilation, ICU and hospital lengths of stay, and the requirement for a tracheostomy.
Implications for research.
Additional well‐designed, adequately powered randomized controlled trials are needed to assess the impact of SmartCare™ on beneficial and clinically important outcomes in patients who require short term ventilation and in specific postoperative populations (for example, cardiac surgical).
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 8, 2010 Review first published: Issue 2, 2014
| Date | Event | Description |
|---|---|---|
| 7 March 2011 | Amended | Contact details updated. |
Notes
In future iterations of the review, we will consider including other nearly fully automated (closed‐loop) systems that automate both alterations in the level of support provided and the conduct of SBTs.
Acknowledgements
We would like to thank David Lightfoot for his help in preparing the search strategies and Jane Cracknell for her editorial advice in preparing the systematic review.
We would like to thank Harald Herkner (content editor), Nathan Pace (Statistical editor), Janet Wale (consumer editor), Michael Davies, Marjolein de Wit, and Morten Vester‐Andersen (peer reviewers) for their help and editorial advice during the preparation of this systematic review.
We also thank Mathew Zacharias, content editor of protocol. We are grateful to Bronagh Blackwood for her willingness to assist with data abstraction, however, her collaboration was precluded by identification of a single trial abstracted by Dr Friedrich.
Appendices
Appendix 1. CENTRAL search strategy
#1MeSH descriptor Therapy, Computer‐Assisted explode all trees #2automat* near system* #3smartcare or (smart near care) #4computer near assist* #5(#1 OR #2 OR #3 OR #4) #6MeSH descriptor Postoperative Period explode all trees #7MeSH descriptor Ventilator Weaning explode all trees #8MeSH descriptor Ventilators, Negative‐Pressure explode all trees #9(ventilat* or wean*):ti,ab #10(invasive near ventil*) or (artificial near respirat*) #11MeSH descriptor Ventilators, Mechanical explode all trees #12postoperative near (setting* or period) #13(#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14(#5 AND #13)
Appendix 2. MEDLINE (OvidSP) search strategy
1. exp ventilators, mechanical/or exp ventilator weaning/ or exp ventilators, negative‐pressure/ or Postoperative Complications/ or exp Postoperative Period/ or (postoperative adj3 (setting* or period)).mp. or ventilat$.mp. or (invasive adj3 ventil*).mp. or wean*.mp. or (artificial adj3 respirat*).mp. 2.exp "Therapy, Computer‐Assisted"/ or (automat* adj3 system*).mp. or (smartcare or (smart adj3 care)).mp. or (computer adj3 assist*).mp. 3. 1 and 2 4. (randomised controlled trial.pt. or controlled clinical trial.pt.or randomised.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals.sh not (humans.sh and animals.sh)) 5. 3 and 4 6. smartcare.mp. 7. 6 or 5
Appendix 3. EMBASE (OvidSP) search strategy
1. exp Ventilator/ or (ventilat$ or wean$).mp 2. (artificial adj3 respirat*).mp. or exp Artificial Ventilation/ or exp postoperative period/ or postoperative care/ 3. ((mechanical or invasive) adj3 ventil*).mp. or (postoperative adj3 (setting* or period)).mp. 4. 1 or 2 or 3 5. exp Computer System/ or (computer adj3 assist*).mp. or (automat* adj3 system*).mp. 6. SmartCare.mp. or (Smart adj3 care).mp. 7. 4 and (or/5‐6) 8. ((((singl* or doubl* or tripl*) adj3 blind) or crossover).ti,ab. or multicenter.ab. or placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. 9. 8 and 7 10. SmartCare.mp. 11. 9 or 10
Appendix 4. CINAHL (via EBSCOhost) search strategy
S1. TX ventilator or (MM "Ventilators, Mechanical") or (MM "Pressure Support Ventilation") or (MH "Ventilation, High Frequency+") or (MH "Postoperative Complications") or (MM "Postoperative Period") or (Postoperative N3 period) or (Postoperative N3 setting*) S2. TX computer assisted or (MH "Decision Making, Computer Assisted+") or (MH "Computers and Computerization+") S3. S1 and S2 S4. TX smartcare or TX smart care S5. (MM "Random Assignment" or MH "Clinical Trials+" or MM "Placebos" or ( (MM "Single‐Blind Studies") or (MM "Triple‐Blind Studies") ) or MM "Multi center Studies" ) or ( MM "Crossover Design" or TI ( random* or placebo* or multi?center or crossover ) or AB ( random* or placebo* or multi?center or crossover ) or TI trial* or AB ( controlled and study )) S6. S5 and (S4 or S3)
Appendix 5. All EBM reviews
We will use the same strategy as per the Cochrane Database of Systematic Reviews to search other Evidence Based Medicine Reviews including ACP Journal Club, DARE, CCTR, CMR, HTA and NHSEED.
1. ventilator$.mp. or ventilation.mp.
2. Artificial respirat$.mp. [mp=ti, ot, ab, tx, kw, ct, sh, hw]
3. 1 or 2
4. computer assisted.mp.
5. SmartCare.mp.
6. 4 or 5
7. 3 and 6
Appendix 6. Ovid Healthstar search strategy
1999 to date
1. exp ventilator, mechanical/ or exp ventilator weaning/ or exp ventilators, negative‐pressure/ or ventilat$.mp.
2.*"Therapy, Computer‐Assisted"/ and ventilat$.mp.
3. (smartcare or (smart adj1 care)).mp.
4. 1 and (2 or 3)
Appendix 7. Data extraction form
Smartcare Discontinuation Systematic Review and Meta‐analysis
Name of data abstractor (first, last) ______________ __________________
1. Study ID
First Author Surname, Year of Publication _______________ ___________________
Is this a duplicate publication?
□ No
□ Yes, please provide details _____________________________________________
2. Study Eligibility
a. Study design
Is the study clearly randomized? □ Yes □ Unclear □ No
Is the study pseudo‐randomized? □ Yes □ Unclear □ No
b. Study Participants
Are the participants adults? □ Yes □ Unclear □ No
Are the participants invasively ventilated? □ Yes □ Unclear □ No
c. Study Interventions
Did one group undergo discontinuation □ Yes □ Unclear □ No
using Smartcare?
Did another group undergo discontinuation □ Yes □ Unclear □ No
using a non‐automated weaning strategy (i.e., not involving
a closed‐loop system)?
d. Study Outcomes
Did the study report any of the following outcomes?
Time from randomization to extubation □ Yes □ Unclear □ No
Time to successful extubation □ Yes □ Unclear □ No
Time to first spontaneous breathing trial □ Yes □ Unclear □ No
Time to first successful SBT □ Yes □ Unclear □ No
Mortality, specify time point(s)_________ □ Yes □ Unclear □ No
Mortality, specify time point(s)_________ □ Yes □ Unclear □ No
Ventilator associated pneumonia □ Yes □ Unclear □ No
Total duration of mechanical ventilation □ Yes □ Unclear □ No
Intensive care unit length of stay □ Yes □ Unclear □ No
Hospital length of stay □ Yes □ Unclear □ No
Use of NIV following extubation □ Yes □ Unclear □ No
Adverse events (including but not limited to □ Yes □ Unclear □ No
reintubation, self‐extubation, tracheostomy,
prolonged ventilation, or other adverse event)
Exclusion Criteria
Did the author report on a study in which the:
Majority of patients require long term ventilation □ Yes □ Unclear □ No
Study explores use of NIV in discontin/weaning □ Yes □ Unclear □ No
Study evaluates exclusively tracheostomized □ Yes □ Unclear □ No
patients
Does the study meet all of the above criteria and meet none of the exclusion criteria? □ Yes □ No
If yes, please proceed to page 2.
Decision □ Include □ Exclude, reason__________________________________
□ Additional information is required before a decision can be made.
3. Information source
How was the article/abstract identified?
Search of electronic databases? □ Yes □ No
Search of trials registries? □ Yes □ No
Manual searches of conference proceedings? □ Yes □ No
Unpublished data? □ Yes □ No
4. Potential Sources of Bias
Adequate/Yes (criteria appropriately applied and described in the report or acknowledged from the primary author of the study); Unclear (criteria not described or impossible to acquire from the author); Inadequate/No (criteria inappropriately applied)
Selection bias
Method of randomization?
Describe the method used to generate the allocation sequence. Specify______________________________________
Check grade. □ Adequate □ Unclear □ Inadequate
Time of randomization
(e.g., admission, upon meeting criteria) Specify_______________________________________
Allocation concealment
Describe the method used to conceal the random allocation Specify_______________________________________
sequence. Check grade. □ Adequate □ Unclear □ Inadequate □ Not used
Detection bias
Outcome assessor blinding?
Were outcomes assessors separate from individuals
administering or supervising the assigned interventions? Specify_______________________________________
Check ONE. □ Yes □ Unclear □ No
Attrition bias
Drop outs/withdrawals?
Were any withdrawals/drop outs described? (Check ONE) □ Yes □ Unclear □ No
Did they occur with similar frequency between study groups? (Check ONE) □ Yes □ No
Intention to treat analysis?
Were all patients analysed according to the group they were
initially assigned to whether they received it or not?
Check ONE.
□ All participants entered into trial (indicate 1 of 2 below)
1. □ 15% or fewer excluded
2. □ more than 15% excluded
□ Unclear
□ Not analysed as intention to treat
Overall quality classification
Overall summary (assign one category) □ All criteria met □ One or more criteria unclear □ One or more criteria not applied
5. Setting
Country/countries ____________________________________________________________
Number of participating ICUs ______________________________________________________________
Type of ICU(s) □ Medical □ Surgical □ Medical Surgical □ Cardiac surgical
(check all that apply) □ Coronary care unit □ Other, specify___________________________
6. Participants
| Criterion |
SmartCare group (n= ) |
Control group 1 (n= ) |
Control group 2 (n= ) |
|
No. randomized |
|||
| No analysed | |||
|
Reasons for differences (if any) |
|
||
|
Inclusion criteria |
______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ |
||
|
Exclusion criteria |
______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ |
||
7. Study interventions
|
Did the study include readiness to discontinue MV criteria? (If yes, please list) Did the study screen daily for these criteria? |
□ Yes □ Unclear □ No _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ □ Yes □ Unclear □ No |
|
Did the study include an SBT? If yes, what technique was used for the SBT? (e.g. PS, T‐tube, CPAP, other, not specified) If yes, what was the duration of SBT? If yes, criteria for SBT failure provided? |
□ Yes □ Unclear □ No _________________________________________________________ _________________________________________________________ _________________________________________________________ □ Yes □ Unclear □ No If yes, please list criteria: _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ |
|
Control arm discontinuation strategy Control strategy described? If yes, how was discontinuation guided in the control arm? If yes, what mode or technique was used in the control arm? Type of clinician responsible for implementing the control strategy? (check ALL that apply) |
□ Yes □ Unclear □ No □ Protocol □ Usual practice (clinician discretion) □ Other, please specify______________________________________ _________________________________________________________ _________________________________________________________ □ SIMV □ PS □ Daily T‐piece □ Intermittent (multiple daily) T‐piece □ Combination of the above, please specify ________________________________________________________ □ Other, please specify ________________________________________________________ □ Physician □ Nurse □ Respiratory Therapist □ Kinesiotherapist □ Other, specify___________________________________________ □ Mixed, specify___________________________________________ |
|
SmartCare discontinuation arm Was SmartCare used in the intervention arm? Type of clinician responsible for implementing SmartCare strategy? (check ALL that apply) |
□ Yes □ Unclear □ No □ Physician □ Nurse □ Respiratory Therapist □ Kinesiotherapist □ Other, specify___________________________________________ □ Mixed, specify___________________________________________ |
8. Study outcomes
| Discontinuation time (time from randomization to extubation) | □ Yes □ Unclear □ No |
| Time to successful extubation | □ Yes □ Unclear □ No |
| Time to first SBT | □ Yes □ Unclear □ No |
| Time to first successful SBT | □ Yes □ Unclear □ No |
| Mortality time point #1 __________ time point #2__________ time point #3__________ |
□ Yes □ Unclear □ No □ Yes □ Unclear □ No |
| Total duration of mechanical ventilation (initiation to extubation) | □ Yes □ Unclear □ No |
| ICU length of stay | □ Yes □ Unclear □ No |
| Hospital length of stay | □ Yes □ Unclear □ No |
| Use of non‐invasive ventilation following extubation | □ Yes □ Unclear □ No |
| Adverse events: (please check) reintubation self‐extubation requirement for tracheostomy prolonged mechanical ventilation _________days other (specify) ____________________________ |
□ Yes □ Unclear □ No □ Yes □ Unclear □ No □ Yes □ Unclear □ No □ Yes □ Unclear □ No □ Yes □ Unclear □ No |
Continuous outcomes
| Outcomes | Unit of measurement | Intervention Group | Control Group | 95% CI or additional information | |||||
| n |
Mean (SD) |
Median (IQR) | n | Mean (SD) | Median (IQR) | P‐value | |||
| Discontinuation time (time from randomization to extubation) | |||||||||
| Time to successful extubation | |||||||||
| Time to first SBT | |||||||||
| Time to first successful SBT | |
||||||||
| Total duration of mechanical ventilation (initiation to extubation) | |||||||||
| ICU length of stay | |||||||||
| Hospital length of stay | |||||||||
| Other, please specify_____________________________ |
|||||||||
Dichotomous outcomes
| Outcomes |
Intervention Group (n = ) |
Control Group (n = ) |
P‐value | Additional information |
| Mortality time point #1 |
||||
| Mortality time point #2 |
||||
| Mortality time point #3 |
||||
| Use of non‐invasive ventilation following extubation |
||||
| Adverse events: ‐ reintubation ‐ self‐extubation ‐ requirement for tracheostomy ‐ prolonged mechanical ventilation _________days other (specify) _________________________ |
||||
| Other outcome, please specify |
Please specify the numerator and denominator for each outcome.
Other information which you feel is relevant to the results:
| Please provide data obtained from the primary author, additional results extrapolated from graphs, figures etc. in the space provided below |
| Additional concerns/points to be clarified? |
Data and analyses
Comparison 1. Discontinuation time (randomization to extubation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation time (randomization to extubation) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Time to successful extubation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to successful extubation | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Time to first spontaneous breathing trial.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first spontaneous breathing trial | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Time to first successful spontaneous breathing trial.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first successful spontaneous breathing trial | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Most protracted measure of mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Most protracted measure of mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Total duration of mechanical ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total duration of mechanical ventilation | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Intensive care unit length of stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intensive care unit length of stay | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Use of non‐invasive ventilation following extubation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Use of noninvasive ventilation following extubation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 9. Adverse event: reintubation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event: reintubation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Prolonged mechanical ventilation (> 14 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged mechanical ventilation (> 14 days) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 11. Prolonged mechanical ventilation (> 21 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged mechanical ventilation (> 21 days) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 12. Adverse event: tracheostomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event: tracheostomy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 13. Adverse event: self‐extubation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event: self‐extubation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 14. Hospital length of stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital length of stay | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Schadler 2012.
| Methods | ||
| Participants | Patients ventilated for > 9 hours at 9:00 am in three ICUs (cardiovascular, interdisciplinary, surgical) serving all surgical disciplines in an academic tertiary hospital. Most patients were included following elective or emergency surgery with small numbers of patients included preoperatively or for non‐surgical reasons. In subgroup analysis, they examined patients who underwent cardiac surgery (n=132), and with sepsis (n=44) or chronic obstructive pulmonary disease (n=41) | |
| Interventions | SmartCare™ versus weaning based on standardized written protocol | |
| Outcomes | Discontinuation time (time from randomization to extubation) Time to successful extubation Time to first SBT Time to first successful SBT Total duration of mechanical ventilation (initiation to extubation) ICU length of stay Hospital length of stay Mortality ‐ 28 day Mortality ‐ 90 day Use of non‐invasive ventilation following extubation Adverse event ‐ reintubation Adverse event ‐ self‐extubation Adverse event ‐ tracheostomy Prolonged mechanical ventilation > 14 days Prolonged mechanical ventilation > 21 days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An electronic randomization system was used to generate randomization lists |
| Allocation concealment (selection bias) | Low risk | Allocation reported to be concealed and allocation was disclosed to investigators by sealed envelopes containing patient numbers such that patient one received study envelope one |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors randomized 317 patients for which consent could not be obtained in 17 patients. Reported analyses included all 300 randomized patients for which consent was obtained. Withdrawals by treatment group were not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Did the trial stop early for benefit? | Low risk | Trial did not stop early for benefit |
| Participants analysed according to the group allocated to? | Low risk | Yes |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beale 2007 | Through correspondence, the principal investigator confirmed that this trial was never initiated |
| Bifulco 2008 | This randomized trial comparing SmartCare™ to conventional weaning (used in their ICU) enrolled patients that were ventilated for at least 24 hours |
| Burns 2012 | This randomized trial comparing SmartCare™ to a paper‐based weaning protocol enrolled patients that were ventilated for more than 24 hours |
| Chen 2008 | This non‐randomized trial compared 109 patients who were treated with adaptive support ventilation to 110 patients managed by a respiratory therapist driven protocol |
| Donglemans 2007 | This trial compared adaptive support ventilation with pressure control/pressure support in 122 fast track coronary artery bypass surgery patients. The trial did not evaluate SmartCare |
| Jiang 2006 | This pseudo‐randomized trial compared SmartCare™ weaning to weaning with SBTs. Although no time period was specified among their inclusion criteria, to be included COPD patients had to be haemodynamically stable with a PaO2 to FiO2 ratio above 200 with PEEP < 5 cm H2O and capable of spontaneous breathing. Their average results indicate that the mean times to extubation were 8.54 and 13.32 days in the SmartCare™ and SBT groups, respectively. We adjudicated this to represent a weaning trial, as opposed to a discontinuation trial, based on this information |
| Jolliet 2006 | This feasibility study was non‐randomized and reported on the use of SmartCare™ during non‐invasive ventilation in patients with acute respiratory failure |
| Jouvet 2007 | This randomized single centre trial evaluated SmartCare™ in a paediatric population |
| Kataoka 2007 | This retrospective study reported on a single centre's experience using SmartCare™ after off‐pump coronary artery bypass, for early extubation |
| Lellouche 2006 | This randomized trial comparing SmartCare™ to usual care and enrolled patients that were ventilated for at least 24 hours using an assisted mode |
| Lim 2012 | This single centre trial included patients between the ages of 21 and 85 in a coronary care unit on an assisted mode of mechanical ventilation for > 24 hours and compared SmartCare™ to usual care |
| Liu 2013 | This randomized trial compared computer‐driven weaning with SmartCare™ to physician‐controlled local practice in 48 patients who failed an initial SBT (identified using daily screening) and on average included patients ventilated for 3 to 5 days prior to randomization |
| Ma 2010 | This randomized trial comparing SmartCare™ to a strategy including SIMV, PS and T‐piece trials enrolled patients that were ventilated for 48 hours |
| Papirov 2007 | This 60 patient pilot RCT was designed to compare computer driven weaning with SmartCare™ to physician directed weaning in elderly patients at a geriatric rehabilitation hospital and regional weaning centre (non‐ICU setting). To be included patients had to have stabilization of the acute health problems that prompted admission to the referral hospital. Through correspondence, the principal investigator confirmed that his trial included exclusively tracheostomized patients and stopped prematurely due to a need to return the study ventilators (Papirov 2007) |
| Reardon 2011 | This trial, comparing SmartCare to an evidence based standard of care for mechanical ventilation discontinuation, was excluded because participants were ventilated for more than 48 hours at inclusion |
| Rose 2008 | This randomized trial comparing SmartCare™ to usual care included patients on mechanical ventilation with volume or pressure targeted mandatory modes for more than 24 hours |
| Stahl 2009 | We excluded this randomized trial comparing SmartCare™ to physician directed weaning as one of the criteria for inclusion was invasive ventilation for at least 24 hours through an endotracheal tube or tracheostomy tube |
| Taniguchi 2009 | This trial compared manual versus automatic reduction in pressure support in a randomized trail of 106 postoperative patients. The automated system used mandatory rate ventilation with a Taema‐Horus Ventialtor (Air Liquid, France) |
| Wong 2008 | Through e‐mail correspondence, we clarified that this trial was stopped due to slow recruitment, after enrolment of three patients |
Differences between protocol and review
The title of the review has changed. It was formerly "SmartCare™ versus non‐automated mechanical ventilation strategies on discontinuation time for adults in the postoperative period" (Burns 2010c).
Dr Bronagh Blackwood is not an author on the review. Her active participation in the review was precluded by identification of only one trial.
Contributions of authors
Conceiving the review: KB
Co‐ordinating the review: KB
Undertaking manual searches: JF
Screening search results: KB, FL
Organizing retrieval of papers: KB
Screening retrieved papers against inclusion criteria: KB, FL
Adjudicating disagreements regarding trials for inclusion: ML
Appraising quality of papers: KB, JF
Abstracting data from papers: KB, JF
Adjudicating disagreements on study quality and methods: ML
Writing to authors of papers for additional information: KB
Providing additional data about papers: KB
Obtaining and screening data on unpublished studies: KB, FL
Data management for the review: KB
Entering data into Review Manager (RevMan 5.1): KB
RevMan statistical data: KB, RN
Other statistical analysis not using RevMan: RN
Double entry of data: KB, JF
Interpretation of data: KB,
Statistical inferences: KB, RN
Writing the review: KB, FL, ML, JF
Securing funding for the review: not applicable
Performing previous work that was the foundation of the present study: not applicable
Guarantor for the review (one author): KB
Person responsible for reading and checking review before submission: KB, FL, RN, ML, JF
Sources of support
Internal sources
No sources of support supplied
External sources
-
Canadian Institutes of Health Research, Canada.
Drs. Burns and Friedrich hold a CIHR Clinician Scientist ‐ Phase 2 Award
Declarations of interest
Drs Burns and Lellouche hold a $5000 CDN travel bursary from Draeger Medical Inc (Canada) for the purpose of conducting site visits to participating centres in the WEAN Study. The WEAN Study is an investigator‐initiated trial comparing SmartCare™ and protocolized weaning for which the co‐principal investigators (Drs Burns and Lellouche) obtained peer‐review funding to implement. Draeger Medical Inc provided ventilators and ventilator upgrades for the WEAN Study and a central randomization system using electronic mail correspondence (Draeger Medical, Germany). Draeger Medical was not involved in any aspects of the study design and oversight, data management or data analysis.
Drs Burns, Lellouche and Lessard have self‐identified as investigators of trials that apply the intervention in question. However, the methods used in conducting this review do not allow bias in the selection, data extraction or risk of bias assessment of any included or excluded studies from these authors.
Drs Friedrich and Nisenbaum have no conflicts of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Schadler 2012 {published and unpublished data}
- Schadler D, Engel C, Gunnar E, Pulletz S, Haake N, Frerichs I, et al. Automatic control of pressure support for ventilator weaning in surgical intensive care patients. American Journal of Respiratory and Critical Care Medicine 2012;185(6):637‐44. [PUBMED: 22268137] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beale 2007 {unpublished data only}
- Beale R. Comparison of an automated weaning programme and a standard clinical weaning protocol for weaning critically ill patients. www.controlled‐trials.com 2007. [ISRCTN82559457]
Bifulco 2008 {published data only}
- Bifulco F, Donato L, Giuricin F, Narni Mancinelli V, Vicchio C, Servillo G. Weaning with SmartCare: Our experience. 21st ESICM Annual Congress. 2008:S79.
Burns 2012 {unpublished data only}
- Burns KEA, Meade MO, Lessard M, Hand L, Keenan SP, Zhou Q, et al. Wean Earlier and Automatically with New Technology: The WEAN Study. American Journal of Respiratory and Critical Care Medicine 2011;183:A6248. [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen CW, Wu CP, Dai YL, Perng WC, Huang YC. Adaptive support ventilation (ASV) facilitates ventilator weaning in a medical ICU with limited respiratory therapist support. American Journal of Respiratory and Critical Care Medicine 2008;177:A379. [Google Scholar]
Donglemans 2007 {published data only}
- Dongelmans DA, Veelo DP, Binnekade JM, Vroom MB, Schultz MJ. Pressure controlled/pressure support (PC/PS) versus adaptive support ventilation (ASV) in post‐cardiac surgery patients ‐ A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2007;175:A433. [Google Scholar]
Jiang 2006 {published data only}
- Jiang H, Yu S, Wang L. Comparison of SmartCare and spontaneous breathing trials for weaning old patients with chronic obstructive pulmonary disease. Chinese Journal of Tuberculosis and Respiratory Diseases 2006;29:545‐8. [PUBMED: 17074269] [PubMed] [Google Scholar]
Jolliet 2006 {published data only}
- Jolliet P, Battisti A, Roeseler J, Tasseaux D. Automatic adjustment of pressure support (PS) with a computer‐driven knowledge‐based system (SmartCare) during noninvasive ventilation (NIV) in acute respiratory failure. Proceedings of the American Thoracic Society 2006;3:A472. [DOI] [PubMed] [Google Scholar]
Jouvet 2007 {published data only}
- Jouvet P, Farges C, Hatzakis G, Monir A, Lesage F, Dupic L, et al. Weaning children from mechanical ventilation with a computer‐driven system (closed‐loop protocol): a pilot study. Pediatric Critical Care Medicine 2007;8:425‐32. [PUBMED: 17693913] [DOI] [PubMed] [Google Scholar]
Kataoka 2007 {published data only}
- Katoako G, Murai N, Kodera K, Sasaki A, Asano R, Ikeda M, et al. Clinical experience with Smart Care after off‐pump coronary artery bypass for early extubation. Journal of Artificial Organs 2007;10:218‐222. [PUBMED: 18071851] [DOI] [PubMed] [Google Scholar]
Lellouche 2006 {published data only}
- Lellouche F, Mancebo J, Jolliet P, Roeselar J, Schortgen F, Dojat M, et al. A multicentre randomized trial of computer‐driven protocolized weaning from mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 2006;174:894‐900. [PUBMED: 16840741] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2012 {published and unpublished data}
- Lim ETS, Hamid N, Beh NC, Phua GC, Tan J. Comparison of knowledge‐based weaning (KBW) and physician‐driven weaning of mechanically ventilated patients in the coronary care unit. European Heart Journal 2012;33:714:P4188. [Google Scholar]
Liu 2013 {published and unpublished data}
- Liu L, Xu X, Yang Y, Huang Y, Liu S, Qiu H. Computer‐driven automated weaning reduces weaning duration in difficult‐to‐wean patients. Chinese Medical Journal 2013;126(10):1814‐8. [PubMed] [Google Scholar]
Ma 2010 {published data only}
- Ma YJ, Yang XJ, Cao XY, Ma XG. Comparison of computer‐driven weaning and physician‐directed weaning from mechanical ventilation: a randomized prospective study. Zhonghua Jie He He Hu Xi Za Zhi 2010;33:174‐8. [PUBMED: 20450634] [PubMed] [Google Scholar]
Papirov 2007 {unpublished data only}
- Papirov G. Computer driven management of weaning following prolonged mechanical ventilation: a pilot study. http://clinicaltrials.gov/show/NCT00502489. [NCT00502489]
Reardon 2011 {published data only}
- Reardon C. Clinical trial of a computer‐driven weaning system for patients requiring mechanical ventilation. ClinicalTrials.gov 2011. [NCT00606554]
Rose 2008 {published data only}
- Rose L, Presneill JJ, Johnston L, Cade JF. A randomized, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCare/PS. Intensive Care Medicine 2008;34:1788‐95. [DOI] [PubMed] [Google Scholar]
Stahl 2009 {published and unpublished data}
- Stahl C, Dahmen G, Ziegler A, Muhl E. Comparison of automated protocol ‐based versus non‐protocol‐based physician‐directed weaning from mechanical ventilation: A controlled clinical trial. Intensivmed 2009;46:441‐6. [PUBMED: 21274740]21274740 [Google Scholar]
Taniguchi 2009 {published data only}
- Taniguchi C, Eid RC, Saghabi C, Souza R, Silva E, Knobel E, Paes AT, et al. Automatic versus manual pressure support reduction in the weaning of post‐operative patients: a randomized controlled trial. Critical Care 2009;13:R6. [DOI: 10.1186/cc7695] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2008 {unpublished data only}
- Wong J. Automated ventilator controlled weaning vs. daily spontaneous breathing trial in difficult to wean ICU patients. www.clinicaltrials.gov 2008; Vol. December. [NCT00813839]
Additional references
Argov 1979
- Argov Z, Mastaglia FL. Disorders of neuromuscular transmission caused by drugs. New England Journal of Medicine 1979;301:4090‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Banner 1997
- Banner MJ, Lampotang S, Blanch PB. Mechanical ventilation. In: Civetta JM editor(s). Critical Care. Philadelphia, PA: Lippincott‐Raven, 1997. [Google Scholar]
Barisione 1997
- Barisione G, Rovida S, Gazzaniga GM. Upper abdominal surgery: does a lung function test exist to predict early severe postoperative respiratory complications?. The European Respiratory Journal 1997;10:1301‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bouadma 2005
- Bouadma L, Lellouche F, Cabello B, Taille S, Mancebo J, Dojat M, et al. Computer‐driven management of prolonged mechanical ventilation and weaning: A pilot study. Intensive Care Medicine 2005;10:1446‐50. [PUBMED: 16132889] [DOI] [PubMed] [Google Scholar]
Bowman 1993
- Bowman W. Physiology and pharmacology of neuromuscular transmission with special reference to the possible consequences of prolonged blockade. Intensive Care Medicine 1993;19:545‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burns 2008
- Burns KEA, Lellouche F, Lessard M. Automating the weaning process with advanced closed loop systems. Intensive Care Medicine 2008;34:1757‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burns 2010a
- Burns KEA, Adhikari NKJ, Keenan SP, Meade MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. The Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD004127.pub2] [DOI] [PubMed] [Google Scholar]
Burns 2010b
- Burns KEA, Lellouche F, Nisenbaum R, Lessard MR, Friedrich JO. SmartCare™ versus non‐automated weaning strategies for weaning time in invasively ventilated critically ill adults. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD008638] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickerson 1994
- Dickerson K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dureuil 1986
- Dureuil B, Viires N, Cantineau JP. Diaphragmatic contractility after upper abdominal surgery. Journal of Applied Physiology 1986;61:1775‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith D, Philips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327.(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011].The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Lee 1995
- Lee C. Intensive care unit neuromuscular syndrome?. Anesthesiology 1995;83:237‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2001
- Lefebvre C, Clarke M. Identifying randomized trials. Egger M, Davey‐Smith G, Altman DG (editors). Systematic reviews in health care: meta‐analysis in context. 2nd Edition. London: BMJ Books, 2001:69‐86. [Google Scholar]
Mircea 1982
- Mircea N, Constantinescu C, Jianu E. Risk of pulmonary complications in surgical patients. Resuscitation 1982;10:33‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitchell 1998
- Mitchell CK, Smoger SH, Pfeifer MP. Multivariate analysis of factors associated with pulmonary complications following elective general surgery. Archives of Surgery 1998;133:194‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116:78‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Price 1999
- Price JA, Rizk NW. Postoperative ventilatory management. Chest 1999;115 Suppl:130‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strickland 1991
- Strickland JH, Hasson JH. A computer‐controlled ventilator weaning system. Chest 1991;100:1914564. [PUBMED: 1914564] [DOI] [PubMed] [Google Scholar]
Strickland 1993
- Strickland JH, Hasson JH. A computer‐controlled ventilator weaning system. Chest 1993;103:1220‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Viby‐Mogensen 1981
- Viby‐Mogensen J. Succinylcholine neuromuscular blockade in subjects homozygous for atypical plasma cholinesterase. Anesthesiology 1981;55:429‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Witt 1991
- Witt NJ, Zochodne DW, Bolton CF, Grand'Maison F, Wells G, Young GB, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest 1991;99:176‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yusuf 1991
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266:93‐8. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Burns 2010c
- Burns KEA, Lellouche F, Nisenbaum R, Lessard MR, Friedrich JO. SmartCare™ versus non‐automated mechanical ventilation strategies on discontinuation time for adults in the postoperative period. Cochrane Database of Systematic Reviews 2010, Issue 8. [CENTRAL: CD008639; DOI: 10.1002/14651858.CD008639] [DOI] [Google Scholar]


