Abstract
Background
Cognitive behavioural therapy (CBT) is a psychosocial treatment that aims to re‐mediate distressing emotional experiences or dysfunctional behaviour by changing the way in which a person interprets and evaluates the experience or cognates on its consequence and meaning. This approach helps to link the person's feelings and patterns of thinking which underpin distress. CBT is now recommended by the National Institute for Health and Care Excellence (NICE) as an add‐on treatment for people with a diagnosis of schizophrenia. This review is also part of a family of Cochrane CBT reviews for people with schizophrenia.
Objectives
To assess the effects of cognitive behavioural therapy added to standard care compared with standard care alone for people with schizophrenia.
Search methods
We searched the Cochrane Schizophrenia Group's Trials Register (up to March 6, 2017). This register is compiled by systematic searches of major resources (including AMED, BIOSIS CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings, with no language, date, document type, or publication status limitations for inclusion of records into the register.
Selection criteria
We selected all randomised controlled clinical trials (RCTs) involving people diagnosed with schizophrenia or related disorders, which compared adding CBT to standard care with standard care given alone. Outcomes of interest included relapse, rehospitalisation, mental state, adverse events, social functioning, quality of life, and satisfaction with treatment.We included studies fulfilling the predefined inclusion criteria and reporting useable data.
Data collection and analysis
We complied with the Cochrane recommended standard of conduct for data screening and collection. Where possible, we calculated relative risk (RR) and its 95% confidence interval (CI) for binary data and mean difference (MD) and its 95% confidence interval for continuous data. We assessed risk of bias for included studies and created a 'Summary of findings' table using GRADE.
Main results
This review now includes 60 trials with 5,992 participants, all comparing CBT added to standard care with standard care alone. Results for the main outcomes of interest (all long term) showed no clear difference between CBT and standard care for relapse (RR 0.78, 95% CI 0.61 to 1.00; participants = 1538; studies = 13, low‐quality evidence). Two trials reported global state improvement. More participants in the CBT groups showed clinically important improvement in global state (RR 0.57, 95% CI 0.39 to 0.84; participants = 82; studies = 2 , very low‐quality evidence). Five trials reported mental state improvement. No differences in mental state improvement were observed (RR 0.81, 95% CI 0.65 to 1.02; participants = 501; studies = 5, very low‐quality evidence). In terms of safety, adding CBT to standard care may reduce the risk of having an adverse event (RR 0.44, 95% CI 0.27 to 0.72; participants = 146; studies = 2, very low‐quality evidence) but appears to have no effect on long‐term social functioning (MD 0.56, 95% CI ‐2.64 to 3.76; participants = 295; studies = 2, very low‐quality evidence, nor on long‐term quality of life (MD ‐3.60, 95% CI ‐11.32 to 4.12; participants = 71; study = 1, very low‐quality evidence). It also has no effect on long‐term satisfaction with treatment (measured as 'leaving the study early') (RR 0.93, 95% CI 0.77 to 1.12; participants = 1945; studies = 19, moderate‐quality evidence).
Authors' conclusions
Relative to standard care alone, adding CBT to standard care appears to have no effect on long‐term risk of relapse. A very small proportion of the available evidence indicated CBT plus standard care may improve long term global state and may reduce the risk of adverse events. Whether adding CBT to standard care leads to clinically important improvement in patients' long‐term mental state, quality of life, and social function remains unclear. Satisfaction with care (measured as number of people leaving the study early) was no higher for participants receiving CBT compared to participants receiving standard care. It should be noted that although much research has been carried out in this area, the quality of evidence available is poor ‐ mostly low or very low quality and we still cannot make firm conclusions until more high quality data are available.
Plain language summary
Is Cognitive behavioural therapy as effective as standard care for people with schizophrenia
Background
People with serious mental illnesses such as schizophrenia can experience severe disturbances in their thought processes, which may lead to delusions (beliefs that are not based on reality) and hallucinations (seeing and hearing things that are not really there). The mainstay (provides most support for the condition) treatment for schizophrenia is antipsychotic medication, but these medications are not always successful on their own and additional treatments such as psychosocial therapies (including cognitive behavioural therapy (CBT)) are recommended for people with schizophrenia. CBT aims to help people re‐evaluate their views of their symptoms. This process is thought to help reduce distress and change behaviours. It is often used to help people with illnesses such as anxiety and depression. However, CBT is expensive and the evidence for its effectiveness is not clear, particularly for people with schizophrenia.
Searches
The Information Specialist of Cochrane Schizophrenia searched the specialised register for trials that allocated people with schizophrenia to receive either CBT or standard care (the care the participant would normally receive for their condition, in the area the trial was conducted), up to March 2017. These searches found 1730 records. The review authors inspected and screened these records.
Main results
After screening search results we were able to include 60 trials with 5992 participants. These studies randomly allocated people with schizophrenia to receive either CBT as an add‐on treatment to their standard care or standard care alone. The quality of evidence for our main outcomes of interest was mainly very low, or at best, low. Results showed that adding CBT to standard care did not appear to affect the long‐term risk of relapse. Only two trials (82 participants) provided useful data for long‐term global state; these data showed CBT could be better for long‐term improvement in global state than standard care alone. Adding CBT to standard care may reduce the risk of adverse events but appears to have no advantage over standard care for improving long‐term mental state. Whether adding CBT to standard care improves patient quality of life or social function also remains unclear.
Conclusions
Currently, the evidence available is unclear and not robust enough to make firm conclusions about the effectiveness of adding CBT to standard care for people with schizophrenia compared to standard care alone.
Summary of findings
Summary of findings for the main comparison. COMPARISON 1: CBT+ STANDARD CARE compared to STANDARD CARE ALONE for people with schizophrenia.
| COMPARISON 1: CBT+ STANDARD CARE compared to STANDARD CARE ALONE for people with schizophrenia | ||||||
| Patient or population: people with schizophrenia Setting: inpatient and outpatient Intervention: CBT+ standard care Comparison: Standard care alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with STANDARD CARE ALONE | Risk with COMPARISON 1: CBT+ STANDARD CARE | |||||
| Global state: 1a. Relapse ‐ long term | Study population | RR 0.78 (0.61 to 1.00) | 1538 (13 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 333 per 1,000 | 260 per 1,000 (203 to 333) | |||||
| Global state: 2. Clinically important change (no improvement) ‐ long term | Study population | RR 0.57 (0.39 to 0.84) | 82 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | ||
| 750 per 1,000 | 428 per 1,000 (293 to 630) | |||||
| Mental state: General ‐ clinically important change (no improvement) ‐ long term | Study population | RR 0.81 (0.65 to 1.02) | 501 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 5 | ||
| 423 per 1,000 | 343 per 1,000 (275 to 431) | |||||
| Adverse events: General: any adverse event | Study population | RR 0.44 (0.27 to 0.72) | 146 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | ||
| 432 per 1,000 | 190 per 1,000 (117 to 311) | |||||
| Functioning: Social (average endpoint score SOFAS, high = good) ‐ long term | MD 0.56 higher (2.64 lower to 3.76 higher) | ‐ | 295 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 6 | The predefined outcome: 'Functioning: clinically important change in social functioning' was not reported. | |
| Quality of life: General (average endpoint score QLS, high = good) ‐ long term * | MD 3.6 lower (11.32 lower to 4.12 higher) | ‐ | 71 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 4 6 | * The predefined outcome of importance: 'Quality of life: clinically important change was not reported. | |
| Satisfaction with treatment: 1. Leaving the study early ‐ long term | Study population | RR 0.93 (0.77 to 1.12) | 1945 (19 RCTs) | ⊕⊕⊕⊝ MODERATE 7 | ||
| 184 per 1,000 | 171 per 1,000 (141 to 206) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to heterogeneity.
2 Downgraded one level due to imprecision: confidence interval of the effect estimation includes both appreciable benefit and harm.
3 Downgraded one level due to study limitations: several bias domains were of high or unclear risk, including blinding of trialists and participants (high risk) and random sequence generation and allocation concealment (unclear risk).
4 Downgraded two levels due to imprecision: very small sample size and very low number of events
5 Downgraded one level due to imprecision: low number of events.
6 Downgraded one level due to indirectness: average scale scores used to measure outcome, not clinically important change.
7 Downgraded one level due to indirectness: leaving the study early used to predict satisfaction with treatment.
Background
Description of the condition
Schizophrenia is a serious mental illness affecting one per cent of the population, irrespective of culture, class, or race. It varies in its severity and in range of symptoms. Every year, one person per 10,000 falls ill with schizophrenia, making it about twice as common as epilepsy (APA 1995). The first episode of schizophrenia often occurs when a person is in their early twenties (WHO 1973) and the course of the illness is variable. Many people experience considerable disability and there is a substantial increase in mortality (Drake 1986). Some people have difficulties with their thoughts, making illogical associations and developing false and sometimes bizarre explanations for their feelings (delusions). Hallucinations may occur, for example, hearing voices or seeing visions. Difficulties with concentration, attention, and motivation may also lead to poor social and occupational functioning. The range of emotional expression, capacity to think and behave appropriately may be reduced, together with a reduced ability to experience pleasure. It is customary to view the symptoms of schizophrenia as falling into two broad categories: (i) 'positive' symptoms, which are unusual by their presence (for example, hearing voices); and (ii) 'negative' symptoms, which are unusual by their absence (for example, restricted range and intensity of emotional expression).
Description of the intervention
Medication is the mainstay of treatment for schizophrenia, but 5% to 25% of people continue to experience symptoms in spite of medication (Christison 1991; Davis 1977; Meltzer 1992) and may experience side effects that are unwanted and unpleasant.
Talking therapies are often used in addition to medication. In cognitive behaviour therapy (CBT), links are made between the person's feelings and patterns of thinking which underpin their distress. The participant is encouraged to take an active part by using the following techniques.People are encouraged to establish links between their thoughts, feelings or actions and their current or past symptoms, and/or functioning. It should promote re‐evaluation of people's perceptions, beliefs or reasoning related to the target symptoms and include at least one of the following: people monitoring their own thoughts, feelings or behaviours with respect to their symptoms or recurrence of symptoms promoting alternative ways of coping with the target symptom reducing distress improving functioning.Examining and disputing the evidence for and against the problematic and/or distressing beliefs and reasons for maintaining problematic behaviours.
Examining and disputing the evidence for and against the problematic and/or distressing beliefs and reasons for maintaining problematic behaviours.
Using reasoning abilities and personal experience to develop rational, useful and personally acceptable alternative explanations and interpretations (Alford 1994); and to test these alternative explanations and abandon associated safety behaviours in real‐world situations. Tarrier 1993 has also stressed the beneficial effects of enhancing coping strategies and general problem‐solving skills.
We note that the above description of CBT is consistent with that within the National Institute for Health and Care Excellence (NICE) guidance for CBT‐P (NICE 2014). NICE guidance proposes that CBT should be delivered on a one‐to‐one basis over at least 16 planned sessions (where typically each session lasts between 30 minutes to 60 minutes and occurs weekly or fortnightly) and follow a treatment manual.
During the evolution of CBT for schizophrenia, a variety of interventions have been labelled as CBT. We note that not all of these interventions specifically target beliefs (e.g. psychoeducation, relapse prevention, symptom‐focused coping strategies, etc.), and it is difficult to provide a single, unambiguous definition of the interventions which can be included under the rubric of CBT. Many of the trials of CBT for psychosis have incorporated additional active therapeutic elements (e.g. psychoeducation and relapse prevention, etc.) that would be considered adjunctive to techniques which are specifically targeted at eliciting beliefs and behavioural changes (e.g. guided discovery or behavioural experiments). In recognition, the review authors have constructed criteria that are felt to be workable and to capture the elements of good practice in CBT. These criteria are described below.
How the intervention might work
CBT aims to re‐mediate distressing emotional experiences or dysfunctional behaviour by changing the way in which the individual interprets and evaluates the experience or cognates on its consequence and meaning. CBT encourages the person to identify and challenge biased interpretations of experiences that may be maintaining symptoms.
Why it is important to do this review
Despite national treatment guidelines recommending CBT as an adjunct therapy for serious mental illness (NICE 2014), CBT is still not as widely available for people with schizophrenia as it is for people with other disorders (for example, depression and panic disorder).
The first case report of CBT for delusional beliefs in 1952, reported by Beck 2005, did not lead to widespread development of CBT for schizophrenia or its symptoms. Psychological interventions have become more widely accepted over the past two decades and are now seen as part of a comprehensive set of routine interventions in the treatment and management of schizophrenia (NICE 2014). However, the availability of CBT and other evidence‐based therapies in the NHS is extremely limited. The 2012 National Audit reveals that 34% had not been offered psychological therapy, with 20% waiting over a year (Royal College of Psychiatrists 2012). The delivery of CBT to people with schizophrenia also depends upon having a commitment from health service managers to support and facilitate training and supervision (Turkington 2004).
Since the publication of the original Cochrane Review entitled Cognitive behavioural therapy for schizophrenia (Jones 2004), there has been a substantial increase in the number of published and relevant randomised controlled trials (RCTs), and a refinement in the definition and working models of CBT. In addition, there has also been a diversification of research, with trials not only assessing overall effectiveness of CBT but investigating more specific aspects of CBT. It was necessary to update and split the original review on CBT to create a family of CBT reviews (Jones 2009a and Jones 2018) to incorporate and address these new more diverse data. This particular review provides information about CBT's relative effectiveness compared with standard care.
Objectives
To assess the effects of adding cognitive behavioural therapy to standard care compared with standard care alone for people with schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. We excluded quasi‐randomised trials, such as those where allocation was undertaken on surname. If a trial had been described as double‐blind, but it was implied it had been randomised, we would have included these trials in a Sensitivity analysis. We would have included randomised cross‐over trials but only used data up to the point of first cross‐over because of the instability of the problem behaviours and the likely carry‐over effects of all treatments (Elbourne 2002).
As CBT requires the person to actively engage and participate in the therapy, it may not be possible to blind the participant to treatment condition (that is, it may not be possible to provide a placebo control condition to reduce the effects of anticipated outcome on behalf of the participant). However, it is both possible and desirable to blind the researcher to condition (that is, the person collecting outcome data is unaware of the allocation of the individual participant). Accordingly, single‐blind trials were considered of appropriate methodological quality for the assessment of this type of intervention.
We compared the outcomes of trials that described a single‐blind procedure with trials that did not describe any blinding procedure in a Sensitivity analysis. If there was no substantive difference within primary outcomes (see Types of outcome measures) when these non‐blinded studies were added, then we included them in the final analysis. If there was a substantive difference, we used only single‐blinded randomised trials. The results of the sensitivity analysis are also described in the text.
Types of participants
Participants were people with a current diagnosis of schizophrenia or closely related illness such as schizoaffective disorder, diagnosed by any criteria, irrespective of gender or race.
We did not include trials where participants had a very late onset of illness (onset after the age of 60 years) or those where the majority of participants had disorders such as bipolar affective disorder, substance‐induced psychosis. If studies randomised people with a range of diagnoses, we only included trials where more than 50% of the participants had a diagnosis of schizophrenia or similar illness.
This review did not include trials that reported outcomes from participants deemed to be 'at‐risk' of developing schizophrenia in the future.
We are interested in making sure that information is as relevant as possible to the current care of people with schizophrenia, so aimed to highlight the current clinical state clearly (acute, early post‐acute, partial remission, remission), as well as the stage (prodromal, first episode, early illness, persistent), and whether the studies primarily focused on people with particular problems (for example, negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Cognitive behavioural therapy (CBT)
The label cognitive behavioural therapy has been applied to a variety of interventions and it is difficult to provide a single, unambiguous definition. Recognising this, the review authors constructed criteria that were felt to be both workable and to capture the elements of good practice in CBT.
In order to be classified as 'well‐defined', the intervention must clearly demonstrate the following components:
a discrete psychological intervention, which is in addition to, and separate from, other therapeutic interventions (for example, behavioural family therapy); and
recipients establish links between their symptoms, thoughts, and beliefs, and consequent distress or problem behaviour; and
the re‐evaluation of their perceptions, beliefs, or reasoning relating to the target symptoms; this may include the re‐evaluation of situation specific 'inferential' beliefs or more global 'evaluative' beliefs.
All therapies that did not meet these criteria (or that provided insufficient information) but were labelled as 'CBT' or 'Cognitive Therapy' were included as 'less‐well‐defined CBT'. We conducted a sensitivity analysis on the primary outcomes of this review (see Types of outcome measures) in order to investigate whether a 'well‐defined' implementation of this therapy presented with differential outcomes.
In addition, for primary outcomes, we undertook sensitivity analyses between studies that employed experienced CBT therapists compared with relatively inexperienced CBT therapists. Experienced CBT therapists were defined as:
persons possessing appropriate professional qualifications for the provision of CBT (e.g. British Association of Behavioural and Cognitive Psychotherapy (BABCP) accreditation, Diploma in CBT, or other professionally accredited qualifications involving CBT as major part of training (e.g. Clinical or Counselling Psychologist)); or
persons where their qualifications were unclear but they appeared to have received training in CBT or specific training for the trial and there was clear evidence of the use a thorough adherence protocol.
2. Standard care
We defined this as the care a person with schizophrenia would normally receive had they not been involved in the trial. This could, in some areas, just involve treatment with antipsychotics, but normally included a biological, psychological, and social approach to care, including antipsychotic medication, and utilisation of services including hospital stay, day hospital attendance, and community psychiatric nursing involvement.
Types of outcome measures
Outcomes could be categorised as being short‐, medium‐ or long‐term. A short‐term outcome was defined as occurring within the period typically associated with active treatment. The National Institute for Health and Care Excellence (NICE) asserts that "for it to make a difference, [the patient] should have CBT treatment for more than 16 planned sessions" (NICE 2014). Accordingly, in this review, we have grouped outcomes into those measured in the short term (within 24 weeks of the onset of therapy), medium term (within 24 to 52 weeks of the onset of therapy) and long term (over 52 weeks since the onset of therapy).
We aimed to report binary outcomes recording clear and clinically meaningful degrees of change (e.g. global impression of much improved, or more than 50% improvement on a rating scale ‐ as defined within the trials) before any others. Thereafter, we listed other binary outcomes and then those that were continuous.
* see Differences between protocol and review.
Primary outcomes
1. Global state
1.1 Relapse 1.2 Clinically important change ‐ as defined by the individual studies (for example, global impression much improved, or less than 50% reduction on a specified rating scale) ‐ short‐, medium‐ and long‐term.
2. Mental state
2.1 Clinically important change ‐ as defined by the individual studies (for example, mental state much improved, or less than 50% reduction on a specified rating scale) ‐ short‐, medium‐ and long‐term.
Secondary outcomes
1. Global state
1.1 Hospitalisation 1.2 Healthy days 1.3 Average endpoint/change score global state scale
2. Mental state
2.2 Any change in general mental state 2.3 Average endpoint general mental state score 2.4 Average change in general mental state scores 2.5 Clinically important change in specific symptoms 2.6 Any change in specific symptoms 2.7 Average endpoint specific symptom score 2.8 Average change in specific symptom scores
3. Adverse effects
3.1 Any adverse effect/event(s) 3.2 Average endpoint general adverse effect score 3.3 Average change in general adverse effect scores 3.4 Clinically important specific adverse effect ‐ as defined by individual studies 3.5 Any specific adverse effects 3.6 Average endpoint specific adverse effects 3.7 Average change in specific adverse effects
4. Functioning
4.1 Average endpoint general functioning score 4.2 Average change in general functioning scores 4.3 Clinically important change in specific aspects of functioning, such as social or life skills 4.4 Any change in specific aspects of functioning, such as social or life skills 4.5 Average endpoint specific aspects of functioning, such as social or life skills 4.6 Average change in specific aspects of functioning, such as social or life skills
5. Quality of life
5.1 Clinically important change in quality of life ‐ as defined by individual studies 5.2 Any change in quality of life 5.3 Average endpoint quality of life score 5.4 Average change in quality of life scores 5.5 Clinically important change in specific aspects of quality of life ‐ as defined by individual studies 5.6 Any change in specific aspects of quality of life 5.7 Average endpoint specific aspects of quality of life 5.8 Average change in specific aspects of quality of life
6. Satisfaction with treatment
6.1 Leaving the study early: specific reason 6.2 Recipient of care satisfied with treatment 6.3 Recipient of care average satisfaction score 6.4 Recipient of care average change in satisfaction scores 6.6 Carer satisfied with treatment 6.7 Carer average satisfaction score 6.8 Carer average change in satisfaction scores
7. Engagement with services
7.1 Clinically important engagement ‐ as defined by individual studies 7.2 Any engagement 7.3 Average endpoint engagement score 7.4 Average change in engagement scores 7.5 Compliance with medication/treatment
8. Economic
8.1 Direct costs 8.2 Indirect costs
'Summary of findings' tables
We used the GRADE approach to interpret findings (Schünemann 2011); and used GRADEpro GDT to export data from our review to create a 'Summary of findings' table. These tables provide outcome‐specific information concerning the overall certainty of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' table.
Global state: relapse
Global state: clinically important change
Mental state: general ‐ clinically important change ‐ as defined by individual studies
Adverse effect: clinically important adverse event ‐ as defined by individual studies
Functioning: clinically important change in social functioning
Quality of life: clinically important change
Satisfaction with treatment ‐ leaving the study early for any reason
If data were not available for these prespecified outcomes but were available for ones that were similar, we presented the closest outcome to the prespecified one in the table but took this into account when grading the finding.
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group's Study‐Based Register of Trials
On 6 March 2017, the information specialist searched the register using the following search strategy:
*Cognit* in Intervention Field of STUDY
In such a study‐based register, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics (Shokraneh 2017; Shokraneh 2018).
This register is compiled by systematic searches of major resources (AMED, BIOSIS, CENTRAL, CINAHL, ClinicalTrials.Gov, Embase, MEDLINE, PsycINFO, PubMed, WHO ICTRP) and their monthly updates, ProQuest Dissertations and Theses A&I and its quarterly update, Chinese databases (CBM, CNKI, and Wanfang) and their annual updates, handsearches, grey literature, and conference proceedings (see Group's website). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
For previous search strategy see Appendix 1.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
We did not contact the first author of each included study for information regarding unpublished trials.
Data collection and analysis
The methods employed below have been updated to reflect changes to Cochrane methods since publication of the protocol in 2009.
Selection of studies
Review authors (SZ and CS) independently inspected citations from the searches and identified relevant abstracts. A random 20% sample was independently re‐inspected by JX and CJ to ensure reliability. Where disputes arose, we acquired the full report for more detailed scrutiny. SZ and CS inspected the full reports of the abstracts meeting the review criteria. JX and CJ inspected a random 20% of full reports in order to ensure reliable selection. We resolved disagreement by discussion and did not need to contact the authors of original studies for clarification on selection.
Data extraction and management
1. Extraction
Review authors (SZ and CS) extracted data from all included studies. In addition, to ensure reliability, JX independently extracted data from a random sample of these studies, comprising 10% of the total. We resolved any disagreement by discussion, and documented decisions. We intended, where necessary, to contact authors of original studies for more data. We we would have presented data presented only in graphs and figures only if SZ and CS independently extracted the same result. Where multicentre studies reported outcomes separately for each component centre, we would have extracted data relevant to each component centre and would have reported these separately. Review author JC helped with data extraction for Chinese trials.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000);
the measuring instrument had not been written or modified by one of the trialists for that particular trial; and
the instrument was a global assessment of an area of functioning and not subscores which are not, in themselves, validated or shown to be reliable.
It should be noted that some subscale scores were included in this review (for instance, we did include subscores from mental state scales measuring specific mental state symptoms of schizophrenia), however, in all cases the subscale scores were well‐validated and were in common use within the empirical literature.
Ideally, the measuring instrument would either be i. a self‐report, or ii. completed by an independent rater or relative (not the therapist). We realised that this is not often reported clearly; in Description of studies, where possible, we noted if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data: change data can remove a component of between‐person variability from the analysis; however, calculation of change needs two assessments (baseline and endpoint) that can be difficult to obtain in unstable and difficult‐to‐measure conditions such as schizophrenia. We preferred to use endpoint data throughout.
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant continuous endpoint data before inclusion.
For endpoint data from studies including fewer than 200 participants:
a) when a scale started from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than one, it strongly suggests that the data are skewed and we excluded these data. If this ratio was higher than one but less than two, there is a suggestion that the data are skewed: we entered these data and tested whether their inclusion or exclusion would change the results substantially. If the data changed results, we presented them as 'other' data. Finally, if the ratio was larger than two, we included these data, because it is less likely they are skewed (Altman 1996; Higgins 2011a).
b) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210 (Kay 1986)), we modified the calculation described above to take the scale starting point into account. In these cases, it was considered that skewed data were present if 2 SD > (S − S min), where S was the mean score and 'S min' was the minimum score.
Please note: we entered all relevant data from studies of more than 200 participants in the analysis irrespective of the above rules, because skewed data posed less of a problem in large studies.
2.5 Common measure
To facilitate comparison between trials, where possible, we converted variables that were reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we converted continuous outcome measures to dichotomous data. This could be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there had been a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the PANSS (Kay 1987), this can be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
We aimed to enter data in such a way that the area to the left of the line of no effect indicated a favourable outcome for CBT.
Assessment of risk of bias in included studies
Again, review authors (SZ and CS) assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). This tool encourages consideration of how the randomisation sequence was generated, how allocation was concealed, the integrity of blinding at outcome measurement, the completeness of outcome data, selective reporting, and other biases. We excluded studies where sequence generation was at a high risk of bias or where allocation was clearly not concealed. If disputes arose as to the correct category for a trial, this was resolved through discussion and, if necessary, adjudication by the other review authors (AM and CI). If this was not possible because further information was necessary, we intended not to enter the data but to allocate the trial to the list of those awaiting assessment. Review authors were not blinded to the names of the authors, institutions, journal of publication, or results of the trials.
Measures of treatment effect
We adopted P = 0.05 as the conventional level of a clear difference (statistically significant) but we were especially cautious where results were only slightly below this, and, in these situations, we reported 95% confidence intervals (CI) in preference to P values.
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI) as it has been shown that RR is more intuitive than odds ratios (Boissel 1999) and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). Although the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), with their CIs, are intuitively attractive to clinicians, they are problematic to calculate and interpret in meta‐analyses (Hutton 2009). For binary data presented in the 'Summary of findings' table(s), where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated mean differences (MD) and the 95% confidence interval between groups. We preferred not to calculate standardised effect size measures (SMD). However if scales that were very similar had been used, we would have presumed there was a small difference in measurement, and we would have calculated the effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, CIs unduly narrow, and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Where clustering was not accounted for in primary studies, we had planned to present data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, if such data are reported, we will seek to contact first authors of studies to obtain intra‐class correlation coefficients for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non‐cluster randomised study, but adjust for the clustering effect.
We have sought statistical advice and been advised that the binary data presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intra‐class correlation coefficient (ICC) [Design effect = 1 + (m ‐ 1) * ICC] (Donner 2002). If the ICC was not reported, it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological, or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase, the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we planned to use data from only the first phase of the study.
3. Trials with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary we simply added and combined within the two‐by‐two table. If data were continuous, we combined data following the formula in the Cochrane Handbook for Systemic reviews of InterventionsHiggins 2011a. Where the additional treatment arms were not relevant, we did not use these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss to follow‐up, the findings of a trial must lose credibility (Xia 2009). We were forced to make a judgment where the level of loss to follow‐up was too great for short‐term trials to be included in this review. If more than 40% of data were unaccounted for at eight weeks, we did not use these data within the analyses.
2. Binary
If attrition for a binary outcome was between 0% and 40% and if the outcomes of these participants were described, we included these data as reported. Where these data were not clearly described for the primary outcome, we assumed the worst for each person who was lost to follow‐up, and for adverse effects, we assumed rates similar to those among participants who did continue to have their data recorded.
3. Continuous
3.1 Attrition
We have reported data where attrition for a continuous outcome was between 0% and 40% and completer‐only data were reported in the study.
3.2 Missing standard deviations
We first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data but an exact standard error (SE) and CI were available for group means, and either 'P' value or 't' value were available for differences in the mean, we noted these, and calculated them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). When only the SE is reported, standard deviations (SDs) can be calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) present detailed formula for estimating SDs from P values, t or F values, CIs, ranges, or other statistics. If these formula do not apply, in the future we will calculate SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Some of these imputation strategies can introduce error. The alternative would be to exclude a given study’s outcome and thus to lose information. We will examine the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers; others use the method of last‐observation‐carried‐forward (LOCF); while more recently, methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences between groups in their reasons for doing so is often the core problem in randomised schizophrenia trials. Therefore, we did not exclude studies based on the statistical approach used. However, by preference we used the more sophisticated approaches, i.e. we preferred to use MMRM or multiple‐imputation to LOCF, and we only presented completer analyses if some kind of ITT data were not available at all. Moreover, we addressed this issue in the item 'Incomplete outcome data' of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, we fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, we fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
Heterogeneity between studies was investigated by considering the I2 method alongside the Chi2 'P' value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects, and ii. strength of evidence for heterogeneity (e.g. 'P' value from Chi2 test, or a CI for I2). We interpreted an I2 estimate greater than or equal to 75% accompanied by a statistically significant Chi2 statistic as evidence of substantial levels of heterogeneity (Deeks 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10.1 of the Cochrane Handbook for Systemic reviews of Interventions (Sterne 2011).
1. Protocol versus full study
We attempted to locate protocols of included randomised trials. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was not available, we compared outcomes listed in the methods section of the trial report with actual reported results.
2. Funnel plot
We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were ten or fewer studies, or where all studies were of similar size. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect these studies can either inflate or deflate the effect size. We used a fixed‐effect model for analyses, except if there was a statistically significant heterogeneity where the source of heterogeneity could not be identified.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
We anticipated subgroup analyses to test the hypothesis that CBT may be highlighted to have different effects when compared with:
1.1 Standard care including antipsychotics as opposed to standard care not including antipsychotics
We aimed to undertake the analysis for only the primary outcomes of this review or the nearest we could find to them (see Types of outcome measures), and, if data were available, we would have discussed the findings.
Sensitivity analysis
If there were substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed below, we would not have added data from the lower‐quality studies to the results of the higher‐quality trials, but would have presented these data within a subcategory. If their inclusion did not result in a substantive difference, they remained in the analyses.
1. Implication of randomisation
We planned to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcomes, if there was no substantive difference when the implied randomised studies were added to those studies with better description of randomisation, we would have included these studies.
2. Blinding
We compared the outcomes of trials that described a single‐blind procedure with trials that did not describe any blinding procedure. If there was no substantive difference within primary outcomes (see Types of outcome measures) when these non‐blinded studies were added, then we included them in the final analysis. If there was a substantive difference, we only used only single‐blinded randomised trials.
3. Well‐defined CBT versus less‐well‐defined CBT
For the primary outcomes, we compared findings for trials meeting our criteria for 'well‐defined' CBT as opposed to those studies that labelled the therapy as CBT but either did not contain the 'inferential' and 'evaluative' component or who did not provide enough information for this discrimination to be made (see Types of interventions).
4. Therapist experience
For the primary outcomes, we compared findings for trials meeting the criteria for experienced CBT therapists compared with trials using relatively inexperienced CBT therapists or who did not provide enough information for this discrimination to be made (see Types of interventions).
5. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption and where we made the comparison with completer data only. If there was a substantial difference, we reported these results and discussed them, but continued to employ our assumption.
6. Risk of bias
For the primary outcomes, we analysed the effects of excluding trials that had a high risk of bias across one or more of the domains (see Assessment of risk of bias in included studies).
7. Imputed values
We undertook a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐randomised trials.
8. Fixed‐ and random‐effects
For the primary outcomes, we synthesised data using a random‐effects model to evaluate whether this altered the significance of the results.
Results
Description of studies
Results of the search
The electronic search yielded 1730 citations and, additionally, we identified 72 references through cross‐reference check of relevant papers. After duplicates were removed, 1802 unique records remained for screening. We excluded 1571 references through inspection of titles and abstracts, and obtained full texts for the remaining 231 articles to further assess eligibility. We excluded 103 trials with 116 references; the reasons for exclusion are described in Excluded studies. Eight studies with nine references are in the Studies awaiting classification list as we had no access to the full‐text article (Chen 2015c; Fohlmann 2010; Hardy 2015; Hassan 2014; Moun 2015; Nagui 2016; Tang 2015; Tecic 2012). Fifteen trials with 15 references meeting our inclusion criteria are ongoing trials (Edwards 2008; ISRCTN06022197; ISRCTN12668007; ISRCTN33695128; ISRCTN61621571; NCT00484302; NCT00495911; NCT02134418; NCT02380885; NCT02408198; NCT02427542; NCT02653729; NCT02787122; NCT02787135; Waller 2014). In all, 60 trials with 91 references were included in this review. Figure 1 presents the study screening flow diagram.
1.
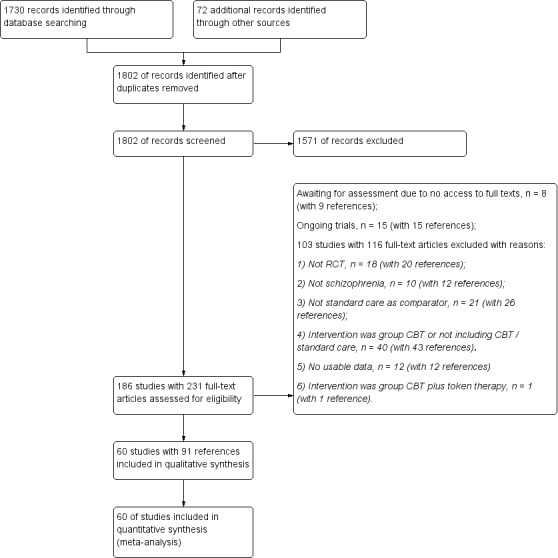
Study flow diagram for 2017 search
Included studies
We included a total of 60 trials with 5992 participants.
1. Size
The sample size of included trials ranged from 30 participants to 422 participants, and the treatment duration ranged from 28 days (He 2012) to two years (Cao 2014; Grawe 2006).
2. Duration
In 27 trials, the treatment duration was no longer than 12 weeks (Chen 2014; Edwards 2011; Freeman 2014; Freeman 2015; Gumley 2003; Guo 2015; Habib 2015; He 2012; Hu 2014; Jia 2005; Jiao 2014; Lewis 2002; Li 2013a; Pan 2012; Qin 2014a; Qiu 2014b; Sun 2014; Tarrier 1999; Tarrier 2014; Wang 2005; Wang 2008; Yao 2015; Zhang 2014; Zhang 2015; Zhao 2013; Zhao 2014; Zou 2013).
In 19 trials, the treatment duration ranged from 13 weeks to 26 weeks (Birchwood 2014; Chen 2015; England 2007; Granholm 2005; Hu 2013; Jackson 2009; Li 2014; Li 2015; Liu 2012; Lu 2014; Ma 2016; Naeem 2015; Naeem 2016; Rector 2003; Startup 2004; Trower 2004; Tuikington 2002; Wang 2012; Wang 2015).
In 13 trials, the treatment duration was longer than 26 weeks (Barrowclough 2010; Barrowclough 2014; Cao 2014; Durham 2003; Farhall 2009; Fowler 2009; Garety 2008; Gleeson 2009; Grawe 2006; Kuipers 1997; Li 2015a; Qian 2012; Velligan 2014).
The remaining trial did not report the treatment duration (Barrowclough 2001).
3. Setting
Participants were recruited from inpatient, outpatient, or community settings.
Thirty‐one trials with 2812 participants were conducted in China (Cao 2014; Chen 2014; Chen 2015; Guo 2015; He 2012; Hu 2013; Hu 2014; Jia 2005; Jiao 2014; Li 2013a; Li 2014; Li 2015; Li 2015a; Liu 2012; Lu 2014; Ma 2016; Pan 2012; Qian 2012; Qin 2014a; Qiu 2014b; Sun 2014; Wang 2005; Wang 2012; Wang 2015; Wang 2008; Yao 2015; Zhang 2014; Zhang 2015; Zhao 2013; Zhao 2014; Zou 2013).
Eighteen trials with 2440 participants were conducted in the United Kingdom (Barrowclough 2001; Barrowclough 2010; Barrowclough 2014; Birchwood 2014; Durham 2003; Fowler 2009; Freeman 2014; Freeman 2015; Garety 2008; Gumley 2003; Jackson 2009; Kuipers 1997; Lewis 2002; Startup 2004; Tarrier 1999; Tarrier 2014; Trower 2004; Tuikington 2002;).
Of the remaining 11 trials, three were conducted in Australia (n = 223) (Edwards 2011; Farhall 2009; Gleeson 2009), two in America (n = 161) (Granholm 2005; Velligan 2014); three in Canada (n = 148) (England 2007; Naeem 2016; Rector 2003), one in Norway (n = 50; Grawe 2006) and two in Parkistan (n = 158) (Habib 2015; Naeem 2015).
4. Participants
4.1 Diagnosis
Forty‐five trials with 4119 participants were diagnosed with schizophrenia, schizoaffective disorder, schizophreniform disorder, or paranoid schizophrenia (DSM‐IV, CCMD‐3 or ICD‐10) (Barrowclough 2001; Cao 2014; Chen 2014; Chen 2015; England 2007; Granholm 2005; Grawe 2006; Gumley 2003; Guo 2015; Habib 2015; He 2012; Hu 2013; Hu 2014; Jia 2005; Jiao 2014; Kuipers 1997; Li 2013a; Li 2014; Li 2015; Li 2015a; Liu 2012; Lu 2014; Ma 2016; Naeem 2015; Naeem 2016; Pan 2012; Qian 2012; Qin 2014a; Qiu 2014b; Rector 2003; Startup 2004; Sun 2014; Trower 2004; Tuikington 2002; Velligan 2014; Wang 2005; Wang 2008; Wang 2012; Wang 2015; Yao 2015; Zhang 2014; Zhang 2015; Zhao 2013; Zhao 2014; Zou 2013). Participants were reported to have comorbid symptoms, such as depression (Chen 2014; Pan 2012) or hallucination (Chen 2015; Trower 2004).
The other fifteen trials with 1873 participants were diagnosed with schizophrenia and other psychosis such as delusional disorders, mood disorders, bipolar disorder, major depressive disorder, substance‐induced psychotic disorder, and others; however, only a small proportion of people within in each study was diagnosed with other psychotic disorders (less than 50%).
Most trials excluded participants with comorbid substance abuse or dependency; however, four trials did include such participants (Barrowclough 2001; Barrowclough 2010; Barrowclough 2014; Gleeson 2009).
No included studies clearly described the severity of illness.
Only nine studies (n = 801) reported participants with first episode schizophrenia (Barrowclough 2014; Cao 2014; Edwards 2011; Fowler 2009; He 2012; Jackson 2009; Jiao 2014; Sun 2014; Zhang 2015). Forty out of 60 included studies reported the average length of illness, which ranged from more than one month (Sun 2014) to 30.1 years (Granholm 2005); the other studies did not report this information. Most of the trials excluded people with comorbid substance misuse, evidence of organic brain disorder, learning disability, or marked thought disorder and/or conceptual disorganisation.
4.1 Age and gender
The age of all included participants ranged from 16 years to 78 years old. The included participants involved 3228 males and 2023 females. It should be noted that these numbers are a good representation of the proportional sex distribution, but they are not exact, as six trials did not report the accurate number or distribution by sex (Barrowclough 2010; England 2007; Grawe 2006; Qian 2012; Wang 2008; Zhang 2014).
5. Interventions
Details of the cognitive behavioural therapy arms of each included trial can be seen in Characteristics of included studies. In addition, Table 2 gives further details.
1. More detailed description of interventions in the included studies.
| Cognitive Behavioural Therapy | ||||
| Study ID | No other active therapies | Experienced therapists | Well‐defined CBT | Details about CBT |
| Barrowclough 2001 | √ | √ | √ | Content: The interventions began with the motivational interviewing phase and five initial weekly sessions designed to assess and then enhance the patient’s motivation to change. If the patient’s commitment was obtained, changes in substance use were negotiated on an individual basis. With the introduction of the individual cognitive behaviour therapy at week 6 (or earlier, if appropriate), the motivational interviewing style was integrated into subsequent cognitive behaviour therapy sessions. The individual cognitive behaviour therapy took place over approximately 18 weekly sessions, followed by six biweekly sessions (a total of 29 individual sessions, including the motivational interviewing). Six clinicians (five clinical psychologists and one nurse therapist) conducted the cognitive behaviour therapies (individual and family). All had experience in cognitive behaviour therapy work with psychotic patients and were eligible for accreditation as cognitive behaviour therapists with the British Association for Behavioural and Cognitive Psychotherapy. Therapy was detailed in a comprehensive treatment manual (available from CB), and the therapists received weekly supervision based on audiotape sessions to ensure treatment fidelity. |
| Barrowclough 2010 | √ | ? | √ | Content: psychological therapy consisted of 26 individual sessions delivered over 12 months. Treatment was built around two phases. The first phase used motivational interviewing to reinforce motivation to change. In phase two of the intervention, CBT from both the psychosis and substance misuse evidence base was used to formulate a change plan to help the patients to implement and maintain changes (e.g. strategies for dealing with distressing voices and depressed mood, responding to relapses, and coping with cravings and urges). |
| Barrowclough 2014 | √ | √ | ? | Content: motivation building which is to elicit and understand patients' perspective in relation to life goals, explore and resolve ambivalence so as to facilitate motivation for change; CBT techniques from both the psychosis and substance use evidence base were used to help the patient implement and maintain changes; Delivered by: The trial therapists all had experience in conducting CBT with people with first‐episode psychosis. |
| Birchwood 2014 | √ | √ | √ | Content: cognitive behaviour therapy techniques are used to assess and modify conviction in four beliefs linked to the construct of voice power. Protocol for cognitive therapy for command hallucinations was developed by MB and details are provided in our casebook manuals. Delivered by: cognitive therapists who were supervised in each centre by a lead clinician with expertise in cognitive behaviour therapy for psychosis. |
| Cao 2014 | √ | √ | ✗ | Content: The intervention included health education to help patients recognize and correct their wrong beliefs or cognition; behavioural therapy included relaxation training. Delivered by: unclear. |
| Chen 2014 | √ | ? | √ | Content: psychoeducation: help for participants to figure out their inappropriate beliefs and attitude; help participants recognize their cognition problems, and rebuild their personality and behaviour. Psychoeducation was given to families. Delivered by: unclear. |
| Chen 2015 | √ | ? | ? | Content: the content of CBT was not stated. Delivered by: not stated. |
| Durham 2003 | √ | √ | ? | Content: An initial emphasis on engagement, education and building a therapeutic alliance; functional analysis of key symptoms, leading to a formulation and problem list; development of a normalising rationale for the patient's psychotic experiences; exploration and enhancement of current coping strategies; acquisition of additional coping strategies for hallucinations and delusions; and focus on accompanying affective symptomatology using relaxation training, personal effectiveness training and problem‐solving, as appropriate. Delivered by: five clinical nurse specialists with extensive professional experience of severe mental disorder. The therapists received training mainly focused on CBT. |
| Edwards 2011 | ? | ? | √ | Content: A manualised CBT program, the systematic treatment of persistent psychosis (STOPP; Hermann‐Doig 2003). Delivered by: not reported. |
| England 2007 | √ | √ | ✗ | Content: CBT was applied by delivery of 12, 90‐min sessions of individualised counselling to voice hearers over a period of 4 months. CBT consisted of reasoning and decision support, counselling strategies tied to the techniques of Socratic learning, the verbal challenge, or empirical reality trial, homework assignments, and summarisation of the counselling sessions. The counselling sessions were audio‐taped to allow for audit of the nurse's counselling strategies. Delivered by: an experienced psychiatric clinical nurse specialist. |
| Farhall 2009 | √ | ✗ | √ | Content: The CBT intervention is based on efficacy trials conducted in the UK (Kuipers 1998). It is similar in scope and content to the therapy outlined by Fowler 1995. Therapists work with patients for 12‐24 sessions on agreed recovery goals using one or more of the following recovery therapy components:
Delivered by: 12 clinical psychologists. |
| Fowler 2009 | √ | √ | √ | Content: An initial emphasis on engagement, education, and building a therapeutic alliance; functional analysis of key symptoms, leading to a formulation and problem list; development of a normalising rationale for the patient's psychotic experiences; exploration and enhancement of current coping strategies; acquisition of additional coping strategies for hallucinations and delusions; and focus on accompanying affective symptomatology using relaxation training, personal effectiveness training, and problem‐solving, as appropriate. Delivered by: five clinical nurse specialists with extensive professional experience of severe mental disorder. The therapists received training mainly focused on CBT. |
| Freeman 2014 | √ | ✗ | ✗ | Content: 1. negative thoughts about the self, 2. positive activities, and 3. positive thoughts about the self. Delivered by: clinical psychologists. |
| Freeman 2015 | √ | ? | ? | Content: The main techniques were psychoeducation about worry, identification and reviewing of positive and negative beliefs about worry, increasing awareness of the initiation of worry and individual triggers, use of worry periods, planning activity at times of worry (whichcould include relaxation), and learning to let go of worry. Delivered by: not reported. |
| Garety 2008 | √ | ✗ | ? | Content: CBT targeted at relapse prevention, done by exploring people's understanding of triggers and risks of relapse and by developing a new model of disorder emphasising alternatives to delusional thinking; targets often included persistent negative beliefs about self and others, characteristic reasoning styles such as jumping to conclusions and distressing emotional reactions to events and anomalous experiences; administered by skilled practitioners (doctorial level clinical psychologists) and treatment fidelity assessed using the Cognitive Therapy for Psychosis Adherence Scale. Delivered by: five clinical nurse specialists with extensive professional experience of severe mental disorder. |
| Gleeson 2009 | ✗ | ✗ | ? | Content: CBT focused upon relapse prevention although nonadherence to treatment, substance abuse, coping with stress, and comorbid anxiety and depression were also targeted. There were parallel individual CBT sessions and family therapy sessions (based upon cognitive behavioural family therapy for schizophrenia (Falloon, 1988; Mueser & Glynn, 1999). The family therapy focused upon communication skills, psychoeducation regarding relapse risk, and a review of early warning signs and documentation of a relapse prevention plan. Delivered by: individual research therapist, who additionally adopted the role of outpatient cases. |
| Granholm 2005 | √ | √ | √ | Content: The treatment manual included a patient workbook that contained homework forms. The CBT was developed specifically for patients with schizophrenia; the age‐relevant content modifications were added. To simplify learning and to help patients remember to use cognitive techniques in everyday life, mnemonic aids were provided; there were also behavioural role‐playing exercises and problem‐solving skills. Delivered by: psychologists or senior graduate students who had 2 years of clinical experience. |
| Grawe 2006 | ✗ | ? | ? | Content: integrated treatment provided by multidisciplinary team, including pharmacotherapy and case management. Structured family psychoeducation, cognitive behavioural family education, problem‐solving skills training, individual cognitive behavioural strategies for residue symptoms. |
| Gumley 2003 | √ | √ | √ | Content: CBT was divided into two phases. Targeted CBT included identifying and targeting beliefs and behaviours, which increased risk to self or others, identifying and targeting beliefs and behaviours accelerating relapse and developing alternative beliefs and reinforcing those through behaviour change. During the study period, the CBT group received a median(range) of 6 (0–14) outpatient medical consultations and 28.5 (0 – 86) community mental health team contacts. Delivered by: a clinical psychologist. |
| Guo 2015 | √ | ✗ | √ | Content: CBT procedure was edited according to previous study and guideline (Li 2015 and Wright 2010). Delivered by: rehabilitation therapists. |
| Habib 2015 | √ | √ | √ | Content: Therapy was provided according to a manualised treatment protocol (Kingdon and Turkington, 1994), and was culturally adapted. Delivered by: psychologist who had received training in CBTp. |
| He 2012 | √ | ? | ? | Content:The intervention was based on a cognitive behavioural therapy handbook developed by the investigators. The therapeutic milieu and content was applied according to the handbook. Delivered by: unclear. |
| Hu 2013 | ? | √ | ? | Content: CBT and risperidone. Delivered by: six experienced psychologists. |
| Hu 2014 | √ | ? | ✗ | Content: The cognitive behavioural therapy included wrong behaviour correction, relaxation, etc. Delivered by: unclear. |
| Jackson 2009 | √ | √ | √ | Content: The cognitive therapy based recovery intervention (CRI) was designed to be delivered on a weekly basis over a 6‐month period (i.e. it was limited to a maximum of 26 sessions) and followed a protocol‐based modular approach. There were three key components: (a) engagement and formulation; (b) trauma processing; and (c) appraisals of psychotic illness (shame, loss, and entrapment). The intervention, therefore, is not just designed for those who could be described as 'traumatised' by their experiences of psychosis. It is intended to be helpful for all first‐episode patients adjusting to and recovering from a first episode of psychosis. Delivered by: four clinical psychologists and a cognitive behavioural psychotherapist. All clinicians had over 4 years experience in the practice of cognitive therapy for early psychosis and received regular case supervision. |
| Jia 2005 | √ | ? | ✗ | Content: Rational thinking training, helping the participant realise his or her inappropriate cognition, behavioural training, diary and health education. Delivered by: unclear. |
| Jiao 2014 | √ | ? | ✗ | Content: to help participants understand their symptoms and strategies to prevent the symptoms, cognitive rebuild, communication with therapists. The dosage of risperidone was 3.8 ± 0.7 mg/day. Delivered by: unclear. |
| Kuipers 1997 | ✗ | √ | ✗ | Content: Initial sessions were focused on facilitating engagement in treatment. Considerable effort was spent on building and maintaining a good basic therapeutic relationship, and this relationship was characterised by considerable flexibility on the part of the therapist. When necessary, treatment was arranged in locations convenient to the client, including home visits and proactive outreach. Behavioural therapy techniques, including activity scheduling, relaxation and skills training. Delivered by: experienced clinical psychologists. |
| Lewis 2002 | √ | √ | √ | The CBT was manual‐based with four stages. Stage 1: a cognitive behavioural analysis of how symptoms might relate to cognitions, behaviour and coping strategies. Education about the nature and treatment of psychosis, using a stress vulnerability model to link biological and psychological mechanisms, was used to help engagement. Stage 2: a problem list was generated collaboratively with the patient. This was then prioritised according to the degree of distress attached, feasibility and, where relevant, clinical risk involved. Prioritised problems were assessed in detail and a formulation was agreed which included such issues as trigger situations and cognitions. Stage 3: Interventions particularly addressed positive psychotic symptoms of delusions and hallucinations, generating alternative hypotheses for abnormal beliefs and hallucinations, identifying precipitating and alleviating factors and reducing associated distress. Stage 4: monitoring positive psychotic symptoms of delusions and hallucinations. Delivered by:one of five therapists trained in CBT in psychosis, supervised by experienced cognitive therapists. |
| Li 2013a | √ | ? | ✗ | Content: Cognitive therapy was conducted to help participant correct their wrong beliefs or thinking process; establish and intensify the right cognition. Delivered by: not reported. |
| Li 2014 | √ | ? | ✗ | Content: psychoeducation about voice; discuss the content of hallucinations; introduction of the ABC model; discuss the link between voice and behaviour; coping strategies. Delivered by: not stated. |
| Li 2015 | √ | √ | √ | Content: building of a therapeutic alliance; functional analysis of key symptoms, leading to a formulation and problem list; scheduling of activity; simulated scene training and case explanation; exploration and enhancement of current coping strategies; homework assignments. Delivered by: therapists. |
| Li 2015a | √ | √ | √ | Content: functional analysis of symptoms and negative behaviour, providing treatment therapy, help patients to develop positive attitude, improve cognitive abilities, reduce conflicts with social interactions, improve clinical compliance, reduce negative mood, improve the way of thinking. Delivered by: specially trained therapists. |
| Liu 2012 | ✗ | ? | √ | Content: rehabilitation training, cognitive and behaviour modification, life skill training, rebuild the link between cognition, behaviour, and psychology. Delivered by: not stated. |
| Lu 2014 | √ | ? | ✗ | Content: cognitive coping strategies, behavioural therapy, etc. Delivered by: unclear. |
| Ma 2016 | √ | √ | ✗ | Content: CBT therapy included a therapeutic alliance building with patients, help to develop personal behaviour control ability, help to correct cognitions in thought, beliefs and attitudes, help patients to aware of the importance of medications. Delivered by: therapists. |
| Naeem 2015 | √ | ✗ | ✗ | Content: A spiritual dimension was included in formulation, understanding and in therapy plan; Urdu equivalents of CBT jargons were used in the therapy; culturally appropriate homework assignments were selected and participants were encouraged to attend even if they were unable to complete their homework; folk stories and examples relevant to the religious beliefs of the local population were used to clarify issues. Delivered by: psychology graduates with more than 5 years experience of working in mental health. |
| Naeem 2016 | ✗ | √ | √ | Content: CBTp consisted of a total of 17 handouts and eight worksheets, that could be flexibly given by a health professional over 12‐16 sessions. The handouts focused on psychoeducation, dealing with hallucinations, paranoia, changing negative thinking, behavioural activation, problem‐solving, improving relationships and communication skills. Health professionals were trained in formulating and devising a plan to suit the individuals' needs. The intervention was then delivered according to this plan. Frequency: a 15‐30 minutes CBT was conducted in each session. Delivered by: frontline mental health professionals. |
| Pan 2012 | ? | ? | ? | Content: not stated. Delivered by: not stated. |
| Qian 2012 | √ | ? | √ | Content: CBT combined with antipsychotics. CBT involves: 1) establish the consultant connection between participants and investigator; 2) help the participants recognise their wrong beliefs and thinking process; 3) help the participants realize their wrong recognition based on their problematic beliefs and guiding them to the correct recognition style; 4) help the participants realise and correct the inappropriate points in their thinking process. 5) encourage the participant to express his/her own viewpoints and promote his/her introspectiveness. 6) help the participants inspect their external misconceptions and correct the deep cause of misconceptions by demonstration, imitation, or didactic suggestion; 7) help participants consolidate their reestablished conceptions and beliefs. Delivered by: unclear. |
| Qin 2014a | ✗ | ✗ | ✗ | Content: cognition correction and group psychoeducation, training exercise. Delivered by: psychologists or nurse. |
| Qiu 2014b | √ | ? | ✗ | Content: coping strategies and relapse prevention. Delivered by: unclear. |
| Rector 2003 | √ | √ | √ | Content: The CBT approach in this study was guided by the principles and strategies developed by Beck et al. (1979, 1985). The first phase of therapy focused on engagement and assessment. The second phase of therapy aimed to socialise the patient to the cognitive model and to impart cognitive and behavioural coping skills, including self‐monitoring with a thought record and the completion of homework tasks. Overlapping with the first two phases of treatment, a third aspect of treatment focused on providing psychoeducation with a normalising rationale. Delivered by: two doctoral level psychologists and one psychiatrist, all with formal training and practice in cognitive behavioural interventions. |
| Startup 2004 | √ | √ | √ | Content: This is a highly individualised, needs‐based form of CBT for psychotic disorders and is based on collaborative empiricism and (evolving) cognitive‐behavioural formulations. Delivered by: clinical psychologists who were employed as specialists in serious mental illness and conducted CBT for schizophrenia on a routine basis. |
| Sun 2014 | ✗ | ? | ✗ | Content: CBT included the building of a therapeutic alliance with patients, functional analysis of symptoms, help to deal with hallucinations and delusions, relaxation training, personal effectiveness training and problem‐solving, as appropriate. Delivered by: not stated. |
| Tarrier 1999 | √ | √ | √ | Content: coping strategy enhancement, training in problem‐solving, strategies to reduce relapse plus standard care. Delivered by: three experienced clinical psychologists and followed a protocol manual. |
| Tarrier 2014 | √ | √ | √ | Content: CBSPp was based on a treatment manual and was derived from an explanatory model of suicide behaviour; the intervention consisted of three phases: 1) Information processing biases; 2) appraisals of defeat, entrapment, social isolation, emotional dysregulation, and interpersonal problem‐solving. 3) suicide schema. Delivered by: clinical psychologists (JK, JM) who had extensive experience in delivering CBT for psychosis. |
| Trower 2004 | √ | ? | √ | Content: four core dysfunctional beliefs (and their functional relation to behaviour and emotion) that define the client ‐ voice (social rank) power relationship. Using the methods of collaborative empiricism and Socratic dialogue, the therapist seeks to engage the client to question, challenge and undermine the power beliefs, then to use behavioural tests to help the client gain disconfirming evidence against the beliefs. These strategies are also used to build clients' alternative beliefs in their own power and status, and finally, where appropriate, to explore the origins of the schema so clients have an explanation for why they developed those beliefs about the voice in the first place. Delivered by: not stated. |
| Tuikington 2002 | ✗ | √ | √ | Content: based on same manual used in Turkington 2000, including assessment and engaging, developing explanations, case formulation, symptom management, adherence, working with core beliefs and relapse prevention. Delivered by: nurses receiving 10 days of intensive training. |
| Velligan 2014 | √ | √ | ✗ | Content: The focus of the sessions was on patient‐identified problems, particularly those that interfered with daily functioning or were distressing, normalising symptoms, and using CBT techniques to develop alternative explanations. Delivered by: master's and doctoral level professionals with > 2 years' experience in assessment and treatment of serious mental illness. |
| Wang 2005 | √ | ? | √ | Content: help patients to understand their symptoms and the impact of symptoms to emotion, realise the relationship between behaviour and disease; strengthened behaviour therapy; cognitive behavioural therapy. Delivered by: unclear. |
| Wang 2012 | √ | ? | ✗ | Content: psychoeducation about symptoms and relapse, coping strategies to hallucination and delusions; cognitive modification. Delivered by: 6 psychologists. |
| Wang 2015 | √ | √ | √ | Content: The intervention was based on two published cognitive behavioural therapy handbooks. Delivered by: psychologist who had been trained to conduct CBT. |
| Wang 2008 | √ | √ | ? | Content: establishing therapeutic relationship and collating comprehensive illness history of individual patients. Treatment is divided into psychological and behaviour aspects. Participants were give psychoeducation about schizophrenia symptoms in order to improve treatment compliance, and meanwhile, behavioural intervention was given to reinforce symptom self‐monitoring, relapse prevention, and ways of managing thoughts and actions. Standard care is Risperidol, 0.5 mg/day, increased to 4 mg/day by the second week of intervention and maximum dosage is 6 mg/day. Delivered by: psychologist who had been trained to conduct CBT. |
| Yao 2015 | √ | √ | ✗ | Content: CBT included: 1) active promotion of social activity; 2) help to deal with hallucinations, paranoia, changing negative thinking; 3) help to self‐regulate psychotic symptoms and improve social recovery from psychosis; 4) psychoeducation; 5) relax training with a duration of 30 minutes; 6) promoting of patients' and guardians' confidence; 7) activity scheduling. Delivered by: qualified doctors and senior nurse. |
| Zhang 2014 | √ | ✗ | ✗ | Content: psychoeducation and cognition modification. Delivered by: three psychologists. |
| Zhang 2015 | √ | √ | √ | Content: CBT included cognitive therapy and rational‐emotive therapy. Cognitive therapy helped patients to change negative thinking by providing psychoeducation. In rational‐emotive therapy, doctors planned therapy for each patient individually depending on patients' background and symptoms, to help patients to build up confidence and solve emotional problems. The therapies included psycho‐diagnosis, helping patients to understand, analysis of patients' background, implementation and strengthening of therapies. Delivered by: qualified doctors. |
| Zhao 2013 | √ | ✗ | ✗ | Content: psychoeducation about symptoms and coping strategies to symptoms; cognition modification, and encouragement of social intercourse. Delivered by: five psychologists. |
| Zhao 2014 | ✗ | ? | ✗ | Content: practicing daily life activity, recreation therapy, and cognition modification. Delivered by: not stated. |
| Zou 2013 | ✗ | √ | ✗ | Content: cognition modification, psychoeducation about disease, and physical exercise. Delivered by: nurses who had five years experience of CBT. |
√ = criteria fulfilled; ✗ = criteria not fulfilled; ? = unclear.
5.1 Cognitive behavioural therapy
In 47 trials, the CBT intervention was not mixed with other contemporaneous active psychological therapies which would not normally be a standard component of CBT. However, three trials (Edwards 2011; Hu 2013; Pan 2012) did not describe enough detail in the report, therefore, it is not clear whether the CBT included other active therapies. Ten trials included other active therapies in the CBT arm. Gleeson 2009 used family intervention in the CBT arm, and the family therapy focused upon communication skills, psychoeducation regarding relapse risk, and a review of early warning signs and documentation of a relapse prevention plan. The differential effects of the CBT and the family intervention were not evaluated. Likewise, four trials (Grawe 2006; Liu 2012; Naeem 2016; Sun 2014) incorporated life skill training or social skill training with CBT the intervention. Kuipers 1997 described a CBT intervention which included skills training, however, did not provide more detail about the skills. Two trials (Qin 2014a; Zou 2013) combined physical exercise with CBT therapy. Tuikington 2002 used case formulation which is not a standard component of CBT. Zhao 2014 engaged the participants in the CBT group to receive recreation therapy such as watching television, listening to music, dancing, or other physical activity.
The CBT interventions varied with regards to both the target of the therapy and the degree of specificity of the focus of the intervention. For example, Durham 2003 and Fowler 2009 used a CBT intervention focused on engagement in treatment, medication compliance, and enhancement of coping strategies, whereas Garety 2008 used a CBT only focused on relapse prevention. Farhall 2009 had a wider focus, incorporating schizophrenia relevant symptoms, relapse prevention, personal/emotional issues or comorbid disorders, and family or social reintegration.
Some trials focused on using CBT for specific symptoms. For example, Birchwood 2014 and Li 2014 assessed CBT's effect on the command hallucination. Freeman 2015 assessed the effect of CBT for worry. The CBT described by Gleeson 2009 focused on coping with stress, anxiety, and depression.
5.1.1 Well‐defined CBT
Only 27 trials met our criteria for 'well‐defined CBT' (see Types of interventions), in that they clearly reported a therapeutic focus on belief change or re‐evaluating the subjective meaning of symptoms. We assessed 22 trials as not 'well‐defined' CBT as the CBT intervention did not explicitly establish links between participant's thoughts and symptoms and there was a lack of re‐evaluation regarding perceptions, beliefs, or reasoning related to the target symptoms. It is difficult to judge whether the CBT is well defined in 11 trials as the description about CBT was unclear. Table 2 gives more details.
5.1.2 CBT provided by qualified therapists
Twenty‐nine trials met the criteria for qualified CBT therapists (see Types of interventions). The therapists in nine trials did not meet our criteria. The remaining 22 trials did not provide sufficient information to assess the experience of the CBT therapists. Table 2 gives more details.
5.2 Standard care
Standard care in the included trials typically involved antipsychotics treatment, nursing care, community‐based healthcare such as community follow‐up, community‐based rehabilitative activities, early intervention, medication monitoring by their psychiatrists, case management, psychoeducation, as well as family support. For 12 trials, standard care involved only antipsychotic treatment (Edwards 2011; Garety 2008; He 2012; Hu 2013; Hu 2014; Jiao 2014; Li 2013a; Li 2014; Qiu 2014b; Wang 2005; Wang 2012; Zhao 2013).
6. Outcomes
We grouped the symptoms into categories such as global state, mental state and others (Table 3 presents further details of this categorisation).
2. Outcome categories.
| Category | Description |
| Global state | These relate to meaningful changes in symptomatology and general clinical condition, recovery and well‐being. These outcomes include relapse, rehospitalisation, healthy days, or other clinical important change in global state. |
| Mental state | These refer to presence or absence of symptoms of psychosis as well as continuous measures relating to characteristics of such symptoms (e.g. preoccupation; conviction; frequency; duration; intensity, loudness; perceived interference with daily living) and insight. Measures of general affect (e.g. anxiety, depression, shame, hopelessness, anger; self‐esteem) and symptom‐related affect measures (e.g. voice‐related distress; delusional distress) are also considered. The presence or frequency of problematic behaviours (suicide attempts; deliberate self‐harm; violence to others, etc.) and functional and adaptive behaviours (e.g. increased coping strategies) are included. |
| Adverse effects | All health interventions have the capacity for unintended and unwanted side effects. To date, there has been a paucity of studies that have attempted to identify adverse effects of psychological therapies. Such outcomes might include dependency, increased distress, increased family dysfunction, and disengagement from mental health services. |
| Functioning | These outcomes might include changes in employment, occupational and educational status, level of received benefits or social welfare, perceived quality of life, and level of social functioning. |
| Quality of life | These outcomes might include changes in the general quality of life or specific aspects relevant to quality of life. |
| Satisfaction with treatment | These outcomes might include both recipients of care satisfied with treatment and carers' satisfaction with treatment. |
| Engagement with service | The measurement of service utilisation and functional outcomes may convey important information regarding health economic benefits, as well as provide indirect markers of personal independence. Such outcomes might include number of acute hospital/inpatient respite days, number of acute hospital admissions or equivalent (e.g. home treatment/crisis team intervention; respite admissions), changes in legal status (MHA 1983), changes in level of care (including accommodation type and intensity of service (Assertive Outreach Team versus Community Mental Health Team)). These outcomes would also include alterations in the degree of compliance with the prescribed medication regimen, as well as alterations to the prescribed medication including changes in type of medication and prescribed dosage. |
| Economic | Direct costs of care and Indirect costs of care. |
6.1 Relapse
Relapse data were reported in 17 trials (Barrowclough 2001; Barrowclough 2010; Barrowclough 2014; Cao 2014; Garety 2008; Gleeson 2009; Grawe 2006; Gumley 2003; Guo 2015; Lewis 2002; Pan 2012; Qian 2012; Qiu 2014b; Tarrier 1999; Tuikington 2002; Wang 2015; Zou 2013). However, different trials used varied criteria for relapse. For instance, Barrowclough 2014 defined relapse as "an exacerbation of psychotic symptoms that lasted for longer than 2 weeks and resulted in a change in participant management (increased observation by the clinical team, increase in antipsychotic medication, or both)". Garety 2008 defined relapse as "the re‐emergence of, or significant deterioration in, positive psychotic symptoms of at least moderate degree persisting for at least 2 weeks" whereas Gleeson 2009 adopted the following criteria for relapse: "3 (mild) or below to ratings of 6 or 7 (severe and very severe) on any one of the three items: (a) unusual thought content, (b) hallucinations, and (c) conceptual disorganization, with a duration criterion of 1 week added for the purpose of differentiating relapses from brief flurries of symptoms." Gumley and colleagues used two sets of criteria for relapse: "For participants with residual symptoms, a 50% increase in the positive scale score, sustained for at least 1 week, was defined as relapse; for those without residual symptoms, an increase in positive symptoms (rated ≥ 3), sustained for at least I week, was defined as relapse" (Gumley 2003). Lewis and colleagues defined relapse as "an exacerbation of psychotic symptoms lasting longer than one week and leading to a change in patient management as recorded by hospital charts such as increases in medication, admission and so on" (Lewis 2002).
6.2 Rehospitalisation
Seven studies reported this outcome (Barrowclough 2014; Fowler 2009; Freeman 2014; Grawe 2006; Gumley 2003; Guo 2015; Lewis 2002).
6.3 Global state
6.3.1 Clinically important change
Three trials reported clinically important change in global state (Edwards 2011; Grawe 2006; Wang 2015). Edwards 2011 used scores on the BPRS positive with a Clinical Global Impression (CGI) severity rating. In both these scales, low scores indicate less severity of symptoms. Participants with a score higher than 3 on the BPRS or a rating of moderate or higher on the CGI were considered to have 'no improvement'. Wang 2015 used scores on the CGI‐GI of less than 2 to indicate 'no improvement'. Grawe 2006 did not describe how they defined 'no improvement'.
6.3.2 Global state scales reporting useful data
Clinical Global Impression scale ‐ CGI (Guy 1976)
Three trials (Chen 2015; Edwards 2011; Wang 2015) also reported continuous measurements of global state by using the Clinical Global Impression (CGI) scale. CGI is a three‐item scale used to measure the global severity of illness. Higher scores indicate greater severity of clinical condition.
6.3 Mental state
6.3.1 Clinically important change
Eleven trials reported clinically important change in mental health (Durham 2003; Garety 2008; Guo 2015; Jia 2005; Jiao 2014; Kuipers 1997; Ma 2016; Qiu 2014b; Tarrier 1999; Wang 2008; Zhao 2013). The definitions of important or reliable change varied between the trials; some used measures for 'improvement', others used measures for 'no improvement. For example, Durham 2003 used the criteria of 50% decrease in symptom severity on the PANSS as clinically important change of mental state. Garety 2008 defined important or reliable change as partial or full remission of symptoms without further episode. Four trials (Guo 2015; Jia 2005; Qiu 2014b; Wang 2008) defined no clinical improvement as a decrease rate of PANSS score < 25%. Jiao 2014 and Zhao 2013 defined no clinical improvement as a decrease rate of BPRS score < 30%, and Ma 2016 defined that as a decreased rate of BPRS score < 25% . Kuipers 1997 used a change of less than five points on the BPRS as indicating no reliable clinical change. Tarrier 1999 defined this outcome as less than 50% improvement in psychotic symptoms in both severity and number of symptoms.
England 2007 also reported data for clinically important change in hallucination, which was defined as "a less than 3‐point improvement in hallucination severity scores measured as a voice hearer's score on item 12 of the BPRS". Pan 2012 reported data for clinically important change in depression.
Trialists also reported a continuous measure of mental health outcomes.
6.3.2 Mental state scales reporting useful data
Auditory Hallucinations Rating Scale ‐ AHRS (Hoffman 2003)
AHRS is a 7‐item questionnaire measuring the severity of hallucination including "hallucination frequency, number of distinct speaking voices, perceived loudness, vividness, attentional salience, length of hallucinations, and degree of distress". Higher score indicates severe hallucination. Two trials reported outcomes on this scale (Chen 2015; Li 2014).
Beck Anxiety Inventory ‐ BAI/BAS (Fydrich 1992)
BAI is a self‐reported 4‐point inventory for measuring the severity of anxiety. The scale includes 21 items describing common symptoms of anxiety. The total score ranges from 0 to 63, and a higher score indicates severe anxiety. Five trials reported outcomes on this scale (Barrowclough 2014; Fowler 2009; Freeman 2014; Garety 2008; Tarrier 2014).
Beliefs About Voices Questionnaire ‐ BAVQ (Chadwick 2000)
BAVQ is a self‐reported scale measuring key beliefs about auditory hallucinations. Higher scores represent more severe symptoms. One study used this scale (Trower 2004).
Beck Cognitive Insight Scale ‐ BCIS (Beck 2004)
BCIS (29) is a 15‐item self‐report inventory for measuring insight. The scale includes two subscales, self‐reflectiveness and self‐certainty, with higher scores indicating better cognitive insight. One trial reported outcomes on this scale (Granholm 2005).
Beck Depression Inventory ‐ BDI/BDS (Beck 1961)
BDI is a self‐report questionnaire measuring the intensity of depressive symptoms. The 4‐point scale (0‐3) includes 21 items with total score ranging from 0 to 61. Higher scores indicate severe depression. Five trials reported outcomes on this scale (Edwards 2011; Fowler 2009; Freeman 2014; Garety 2008; Rector 2003).
Beck Hopelessness Scale ‐ BHS (Beck 1974)
BHS is a 20‐item scale for assessment of hopelessness for the future. The possible score ranges from 0 to 20 with higher scores indicating poor hope for the future. Three trials reported outcomes on this scale (Birchwood 2014; Fowler 2009; Tarrier 2014).
Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962)
The BPRS is an 18‐item scale measuring positive symptoms, general psychopathology, and affective symptoms. Each item is rated on a 7‐point scale varying from 'not present' to 'extremely severe'. The possible score ranges from 0 to 126 with high scores indicating more severe symptoms. Nine trials reported outcomes on this scale (England 2007; Edwards 2011; Gleeson 2009; Jiao 2014; Kuipers 1997; Ma 2016; Pan 2012; Startup 2004; Zhao 2013).
Brief Core Schema Scales (BCSS) (Fowler 2006)
The self‐report BCSS is a five‐point scale assessing negative and positive beliefs about the self and others with 24 items. Higher scores reflect greater endorsement of items. One study used this scale (Freeman 2014).
Comphrehensive Schizophrenia Change Scale ‐ CPRS (Asberg 1978)
CPRS is a scale for rating the severity of psychiatric symptoms and observed behaviour. It consists of 65 items covering symptoms commonly reported by participants with higher scores indicating more severe symptoms. One trial reported outcomes on this scale (Tuikington 2002).
Choice of Outcome In Cbt for psychosEs ‐ CHOICE (Greenwood 2010)
CHOICE is a self‐reported 24‐item scale used to measure the severity of diease and satisfaction of participants. A higher score indicates better patient outcomes. One study used this scale (Freeman 2015).
Calgary Depression Scale ‐ CDS (Addington 1993)
CDS is a 9‐item scale designed to measure depression in schizophrenia patients without negative symptoms. The possible score ranges from 0 to 27 with higher scores indicating poor depression state. Four trials reported outcomes on this scale (Barrowclough 2014; Jackson 2009; Tarrier 2014; Trower 2004).
Green Paranoid Thoughts Scale ‐ GPTS (Green 2008)
GPTS is a 32‐item self‐reported scale measuring paranoid thinking. Each item is rated on a 5‐point scale. A high score means greater level of paranoid thinking. Two trials reported outcomes on this scale (Freeman 2014; Freeman 2015).
General Self‐Efficacy Scale ‐ GSES (Wang 1998)
GSES is a 10‐item psychometric scale that is designed to assess optimistic self‐beliefs to cope with a variety of difficult demands in life. A higher score indicates better self‐efficacy. Two trials reported outcomes on this scale (Lu 2014; Ma 2016).
Hospital Anxiety and Depression Scale ‐ HADS (Zigmond 1983)
The Hospital Anxiety and Depression Scale consists of 14 items assessing the severity of anxiety and depression during hospitalisation. Each item is rated on 4‐point scale (0‐3). The possible score ranges from 0 to 52 with higher scores indicating poor depression/anxiety states. One trial reported data on this scale (Farhall 2009).
Hamilton Rating Scale for Depression/Hamilton Depression Rating Scale ‐ HAMD (Hamilton 1967)
HAMD is 17‐item scale used to assess the severity of depression. Each item is rated on 3‐ or 5‐ point scale. A higher score indicates severe depression. Three trials reported data on this scale (Chen 2014; Granholm 2005; Pan 2012).
Hamilton Anxiety Rating Scale ‐ HAMA (Hamilton 1976)
HAMA is a 14‐item psychological questionnaire to assess the severity of anxiety. Each item is rated on 5‐point scale, with a possible total score varying from 0 to 56. A higher score indicates severe anxiety. One trial reported data on this scale (He 2012).
Insight Treatment Attitude Questionnaire ‐ ITAQ (McEvoy 1989)
ITAQ is a 11‐item questionnaire for measuring awareness of illness and attitude to medication and services, as well as follow‐up evaluation. The possible total score ranges from 0 to 22, with high scores indicating better insight. Two trials reported data on this scale (Startup 2004; Zhang 2014).
Montgomery–Åsberg Depression Rating Scale ‐ MADRS (Montgomery 1979)
MADRS is a 10‐item scale for measuring severity of depressive episodes in people with mood disorders. The total possible score ranges from 0 to 54 points, with higher scores indicating worse outcome. Two trials reported data on this scale (Gleeson 2009; Tuikington 2002).
Negative Symptom Rating scale ‐ NSRS (Iager 1985)
NSRS is a valid instrument assessing negative symptoms in schizophrenia, with higher scores indicating worse outcomes. One trial reported data on this scale (Tuikington 2002).
Positive and Negative Syndrome Scale ‐ PANSS (Kay 1987)
PANSS is a 30‐item scale including three subscales for measuring the severity of general psychopathology, positive symptoms, and negative symptoms. Each item is rated on a 7‐point scale, with higher scores indicating worse outcome. Thirty trials reported data on this outcome (Chen 2015; Barrowclough 2001; Barrowclough 2010; Birchwood 2014; Barrowclough 2014; Gumley 2003; Durham 2003; Farhall 2009; Fowler 2009; Freeman 2015; Garety 2008; Granholm 2005; Guo 2015; Habib 2015; Hu 2013; Jia 2005; Lewis 2002; Li 2013a; Li 2014; Farhall 2009; Naeem 2015; Naeem 2016; Rector 2003; Qian 2012; Qiu 2014b; Sun 2014; Tarrier 2014; Wang 2008; Wang 2015; Zhao 2014).
Penn State Worry Questionnaire ‐ PSWQ (Meyer 1990)
PSWQ is a 16‐item questionnaire that aims to measure the trait of worry, using Likert rating from 1 (not at all typical of me) to 5 (very typical of me). Higher PSWQ scores reflect greater levels of pathological worry. One trial reported outcomes on this scale (Freeman 2015).
Psychotic Symptom Rating Scales ‐ PsyRATs (Hassock 1999)
PsyRATs aims to assess dimensions of hallucination and delusions reliably and validly. Higher scores indicate severer symptoms. Ten trials reported outcomes on this scale (Birchwood 2014; Durham 2003; Freeman 2014; Freeman 2015; Naeem 2015; Naeem 2016; Tarrier 2014; Tuikington 2002; Habib 2015; Trower 2004).
Perseverative Thinking Questionnaire ‐ PTQ (Ehring 2011)
PTQ is a self‐administered, 15‐item, Likert‐type scale designed to measure the broad idea of repetitive negative thought. One trial reported outcomes on this scale (Freeman 2015).
Robson Self Concept Questionnaire ‐ RSCQ (Robson 1989)
RSCQ is a psychological scale used to measure self‐esteem. A score of 1 standard deviation below the mean (total score of <= 120) indicates low self‐esteem. The scale comprises 30 items, with responses rated on an 8‐point scale (0 to 8) that range from "completely disagree" to "completely agree". Three trials reported outcomes on this scale (England 2007; Freeman 2014; Jackson 2009).
Rosenberg Self‐Esteem Scale ‐ RSES/SES (Rosenberg 1965)
RESR is a 10‐item self‐reported scale for measuring self‐esteem. Each item is rated on a 4‐point scale (from 1 to 4). Higher scores indicate higher self‐esteem. Two trials reported data on this scale (Farhall 2009; Gumley 2003;).
Schedule of Assessment of Insight ‐ SAI (David 1990)
SAI measures the dimensions of relabelling of unusual mental events as abnormal, awareness of illness, and recognition of the need for treatment. Four trials reported outcomes on this scale (Guo 2015; Habib 2015; Naeem 2015; Tuikington 2002).
Scale for the Assessment of Negative Symptoms ‐ SANS (Andreasen 1984)
The SANS is a valid instrument to assess the negative symptoms of schizophrenia. Each item is based on 6‐point scale. Higher scores indicate more symptoms. Four trials reported data on this outcome (Edwards 2011; Pan 2012; Tarrier 1999; Wang 2005).
Scale for the Assessment of Positive Symptoms ‐ SAPS (Anderasen 2004)
SAPS is a rating scale to measure positive symptoms in schizophrenia. The scale is split into 4 domains, and within each domain separate symptoms are rated from 0 (absent) to 5 (severe). One trial reported outcomes on this scale (Wang 2015).
Symptom Assessment Scale ‐ SAS (Aoun 2011)
SAS describes the participant's level of distress relating to individual physical symptoms on a scale of 0 to 10. Two trials reported outcomes on this scale (Qin 2014a; Yao 2015).
Symptom Checklist 90 ‐ SCL‐90 (Derogatis 1975)
SCL‐90 is a relatively brief self‐report psychometric instrument (questionnaire). It consists of 90 items, yielding nine scores along primary symptom dimensions and three scores among global distress indices. The primary symptom dimensions that are assessed are somatisation, obsessive‐compulsive disorder, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, psychoticism, and a category of 'additional items' which helps clinicians assess other aspect of the clients symptoms. Two trials reported outcomes on this scale (Li 2015a; Zhang 2015).
Severity of Dependence Scale ‐ SDS (Gossop 1995)
SDS is a 5‐item questionnaire that provides a score indicating the severity of dependence on opioids. Each of the five items is scored on a 4‐point scale (0‐3). The total score is obtained through the addition of the 5‐item ratings. Two trials reported outcomes on this scale (Qin 2014a; Yao 2015).
Self‐Esteem Rating Scale‐Short form ‐ SERS‐SF (Lecomte 2006)
SERS‐SF is a 20‐item scale measuring self‐esteem. It consists of two subscales: 10 items for positive symptoms and 10 items for negative symptoms. Each item is rated on a 7‐point scale with higher scores indicating better self‐esteem. The possible scores for positive symptoms ranges from 10 to 70 and for negative symptoms ranges from ‐10 to ‐70. One trial reported this outcome (Tarrier 2014).
Self‐Reflection and Insight Scale ‐ SRIS (Grant 2002)
SRIS is a 20‐item scale, which delineates two different factors: self‐reflection and insight. One trial reported outcomes on this scale (Farhall 2009).
Lecomte 2008a also reported specific affective symptoms (insight) by the insight scale.
The Voice Power Differential scale ‐ VPD (Birchwood 2000)
The VPD is a five‐point rating scale to measure the perceived relative power differential between voice and voice hearer. Higher scores indicate severe power of the dominant voice. One study reported this outcome (Trower 2004)
The Voice Compliance Scale ‐ VCS (Sander 1997)
The VCS is an observer‐rated scale to measure the frequency of command hallucinations and the level of compliance/hallucinations. Higher scores indicated severe hallucinations. One study reported this outcome (Trower 2004).
6.4 Adverse effect(s)/event(s)
Two trials reported rates for any adverse effects (Li 2014; Pan 2012). Three trials (Grawe 2006; Garety 2008; Li 2014) reported rates for specific adverse events such as suicide attempts, violent incidence, and others. Two trials (Chen 2015; Qiu 2014b) reported data on the Treatment Emergent Symptom Scale (TESS). Mortality was only reported in nine trials (Barrowclough 2001; Barrowclough 2010; Birchwood 2014; Farhall 2009; Garety 2008; Kuipers 1997; Lewis 2002; Startup 2004; Tarrier 1999), with Lewis 2002 specifically reporting suicides.
6.4 Functioning
6.4.1 Scales reporting useful data
Children's Memory Scale ‐ CMS (Cohen 1997)
CMS measures learning in a variety of memory dimensions including attention and working memory, verbal and visual memory, short‐ and long‐delay memory, recall and recognition, and learning characteristics. One trial (Li 2015) reported data on this scale.
Global Assessment of Functioning ‐ GAF (DSM (IV) 1994)
GAF is a 90‐point rating scale that assesses psychological, social, and occupational functioning. A high score indicates a better outcome. Six trials reported data on this scale (Barrowclough 2001; Barrowclough 2010; Barrowclough 2014; Durham 2003; Startup 2004; Tarrier 2014).
Independent Living Skills Survey ‐ ILSS (Wallace 2000)
ILSS is a detailed assessment of a client’s social and independent living skills and has 103 items which assess 12 areas of skills. One trial (Granholm 2005) reported data on this scale.
Life Skills Progression ‐ LSP (Parker 1991)
LSP describes individual parent and infant/toddler progress using 43 items of life skills, which are grouped into 5 scales (relationships, education, mental health/substance abuse and other risks, basic essentials, infant/toddler development). One trial (Farhall 2009) reported data on this scale.
Personal and Social Performance Scale ‐ PSP (Si 2009)
PSP scale is a validated clinician‐related scale that measures personal and social functioning in the domains of: socially useful activities (e.g. work and study), personal and social relationships, self‐care, and disturbing and aggressive behaviours. Two trials (Guo 2015; Wang 2015) reported data on this scale.
Social Functioning Scale ‐ SFS (Birchwood 1990)
SFS measures social role and behavioural functioning across seven basic areas of community functioning: social engagement, interpersonal behaviour, prosocial activities, recreation, independence, employment. Two trials reported data on this scale (Barrowclough 2001; Startup 2004).
Social and Occupational Functioning Assessment Scale ‐ SOFAS (Goldman 1992)
SOFAS is a measure of social and occupational functioning on a continuum from excellent to grossly impaired functioning. Four trials reported data on this scale (Edwards 2011; Fowler 2009; Garety 2008; Gleeson 2009).
University of California, San Diego, Performance‐Based Skills Assessment ‐ UPSA (Patterson 2001)
UPSA assesses the skills necessary for functioning in the community by asking participants to perform relevant tasks and rating their performance. Skills are assessed in the following five areas: household chores, communication, finance, transportation, and planning recreational activities. One trial (Granholm 2005) reported data on this scale.
Wechsler Adult Intelligence Scale ‐ WAIS (Mittenberg 1995)
WAIS is an intelligence test that is used quite commonly, and it measures the verbal and nonverbal abilities of adults. Two trials (Hu 2014; Sun 2014) reported data on this scale.
Wisconsin Card Sorting Test ‐ WCST (Eling 2008)
WCST is a neuropsychological test of 'set‐shifting', i.e. the ability to display flexibility in the face of changing schedules of reinforcement. One trial (Li 2015) reported data on this scale.
World Health Organization Disability Assessment Schedule ‐ WHODAS (Ustun 2010)
WHODAS is a participant self‐report assessment tool that evaluates the participant's ability to perform activities in six domains of functioning over the previous 30 days, and uses these to calculate a score representing global disability. It comes in 36‐ and 12‐item questionnaires. One trial (Naeem 2016) reported data on this scale.
Wechsler Memory Scale ‐ WMS (Wechsler 2009)
WMS is a neuropsychological test designed to measure different memory functions in a person. One trial (Sun 2014) reported data on this scale.
MATRICS Consensus Cognitive Battery ‐ MCCB (Green 2004)
MCCB is a scale to assess cognitive change in patients with schizophrenia. It consists of 10 items. Higher scores indicate poor cognitive function. One study reported this outcome (Hu 2013).
6.5 Quality of life
Ten trials reported on this important outcome (Chen 2015; Cao 2014; Edwards 2011; Fowler 2009; Garety 2008; Gleeson 2009; Liu 2012; Lu 2014; Naeem 2015; Wang 2015).
6.5.1 Quality of life scales reporting useful data
European Quality of Life Questionnaire ‐ EuroQOL. (Dolan 1997)
This is also known as the EuroQoL or the EQ‐5D. This is a self‐rated measure of five dimensions of health‐related quality of life (mobility, self care, usual activities, pain/discomfort, anxiety/depression). One trial reported data from this scale (Garety 2008).
General Quality of Life Inventory‐74 ‐ GQOLI‐74 (Wang 1999)
GQOLI‐74 is a 74‐item quality of life assessment scale. It contains four subscales that assess physical functioning, psychological functioning, social functioning, and standard of living. High scores indicate better quality of life. One trial reported data from this scale (Cao 2014).
Quality of Life Scale ‐ QLS (Heinrichs 1984)
QLS consists of 10 items. High scores indicate better quality of life. Two trials (Edwards 2011; Fowler 2009) reported data from this scale.
Social Disability Screening Schedule ‐ SDSS (Wu 1998)
SDSS consists of 10 items. High scores indicate poor quality of life. One trial (Qiu 2014b) reported data from this scale.
Short Form (36) Health Survey ‐ SF‐36 (McHorney 1993)
SF‐36 is a 36‐item, patient‐reported survey of patient health. High scores indicate better quality of life. One trial (Liu 2012) reported data from this scale.
Schizophrenia Quality of Life Scale ‐ SQLS (Wilkinson 2000)
SQLS consists of three subscales ('psychosocial', 'motivation and energy', and 'symptoms and side‐effects') with 30 items. High scores indicate poor quality of life. Two trials (Chen 2015; Lu 2014) reported data from this scale.
World Health Organization Quality of Life ‐ Brief ‐ WHOQOL‐BREF (WHOQOL Group 1998)
WHOQOL‐BREF consists of 26 items measuring the following domains of quality of life: physical health, psychological health, social relationships, and environment. Higher scores indicate better quality of life. Three trials reported data on this scale (Garety 2008; Gleeson 2009; Wang 2015).
6.6 Satisfaction with treatment
None of the trials directly measured satisfaction with treatment. Leaving the study early was used as an indirect measure for this important outcome.
6.6.1 Leaving the study early.
Thirty trials (Barrowclough 2001; Barrowclough 2010; Barrowclough 2014; Birchwood 2014; Durham 2003; England 2007; Farhall 2009; Fowler 2009; Garety 2008; Gleeson 2009; Grawe 2006; Gumley 2003; Guo 2015; Jackson 2009; Kuipers 1997; Rector 2003; Trower 2004; Tuikington 2002; Velligan 2014; Wang 2005; Chen 2014; Freeman 2015; Granholm 2005; Lewis 2002; Naeem 2015; Naeem 2016; Tarrier 1999; Tarrier 2014; Wang 2008; Zhao 2013) reported attrition data.
6.7 Engagement with services
6.7.1 Compliance to treatment/medication
Six trials reported binary data for participant compliance to medication (Cao 2014; Chen 2014; Pan 2012; Qiu 2014b; Zhang 2014; Zou 2013).
Two trials reported binary data for participants refusing treatment (Li 2013a; Wang 2012).
Two trials used scales to measure compliance, reporting continuous data for this outcome (Gleeson 2009; Qian 2012).
6.7.2 Compliance scales reporting useful data
Medication Adherence Rating Scale ‐ MARS (Thompson 2000)
MARS is a 10‐item scale measuring medication adherence. Higher scores indicate better medication adherence. Two trials reported data on this scale (Gleeson 2009; Qian 2012).
6.8 Economic outcomes
No study reported data for economic outcomes such as direct and indirect costs of care.
Excluded studies
We excluded 103 trials (116 references) from this review.
1. Issues relating to methods
A total of 19 trials with 20 references were not randomised controlled trials (Agius 2007; Bechdolf 2005b; Byerly 2005; Chen 2012; Feng 2013; Hang 2014; Huang 2014; Ibranhim 2012; Jackson 1998; Kong 2015; Li 2015c; Mo 2015; O'Driscoll 2015; Owen 2015; Qi 2012; Xie 2013; Xu 2014; Zhang 2005).
2. Issues relating to participants
Participants in 10 trials with 12 references did not meet the diagnostic criteria of schizophrenia. Morrison 2014, Phillips 2002 and ACTRN12606000 included participants who were at high risk of psychosis or schizophrenia. ISRCTN77762753 and Jenner 2004 included participants with auditory hallucinations. ISRCTN47998710 included participants with psychological difficulties. Eack 2014 included participants with substance misuse and schizophrenia. O'Connor 2007 included participants with delusional disorders. Cai 2014c and ISRCTN11889976 included participants with psychosis and did not state whether they included schizophrenia or not.
3. Issues relating to comparison
A total of 21 trials with 26 references did not use standard care as comparator.
These trials compared CBT with other psychosocial therapies such as supportive counselling (Haddock 1998; Johnson 2008; Lu 2014a; NCT02751632; O'Donnell 2003; Penn 2009; Pinto 1999; Valmaggia 2005), befriending (Jackson 2008; Sensky 2000; Turkington 2008), psychoeducation (Bechdolf 2004; Cather 2005; Rector 2005), cognitive remediation therapy (Klingberg 2009; Penades 2006; ), recreation and support (Drury 1996), psychological nursing care (Wu 2013), and problem‐solving (Bradshaw 1996). One trial (ISRCTN34966555) compared CBT delivered though mobile application (app) with a symptoms monitoring app where the control group was not standard care. The groups in NCT02420015 received cognitive‐behavioural smoking cessation counselling.
4. Issues relating to intervention
A total of 41 trials with 44 references were excluded because the intervention group did not meet our inclusion criteria.
Four trials reported CBT interventions as an element of a treatment package where it was not possible to identify the effect of CBT (Granholm 2007; ; Lincoln 2012; NCT00810355; Richmond 2005).
The remaining 37 trials employed therapeutic strategies which did not meet our criteria for adjunct CBT (Bach 2002; Barrowclough 2006; Cella 2014; Deng 2014; NCT00960375; Farreny 2012; Favrod 2014; Gaudiano 2006; Hert 2000; Hogarty 1997; Hogarty 2004; Kidd 2014; Kuipers 2004; Leclerc 2000; Lecomte 2008; Li 2013; Li 2013b; Li 2014b; Liu 2013; Lu 2012; Lysaker 2009; NCT02535923; NCT02105779; Nordentoff 2005; Reeder 2014; Sellwood 2000; Song 2012; Song 2014; Wang 2003; Wang 2013; Wang 2013a; Wang 2014; Wei 2012; Wykes 2005; Yang 2012; Zhao 2012; Zhou 2015b).
In particular, Bach 2002 employed a new wave of CBT involving radical acceptance without cognitive or behavioural modifications. The same applied to acceptance and commitment therapy (Gaudiano 2006), which, similarly to CBT, had a focus on cognitions. It, however, aimed to help participants respond differently to their thoughts rather than directly challenge or test out their validity. Participants were encouraged to accept and experience their internal events without judgement and this treatment did not meet our criteria for CBT. Personal therapy (Hogarty 1997), like CBT, aims to prevent relapse and promote personal and social adjustment. However, personal therapy differs from CBT in that it consists of psychoeducation awareness of early signs, supportive therapy techniques, social skills training, the teaching of coping strategies, without an explicit focus on beliefs and cognitive restructuring. Thus, this treatment did not meet our criteria for CBT. Nine studies investigated a group‐based CBT intervention which did not meet our criteria (Barrowclough 2006; Deng 2014; Lecomte 2008; Li 2013; Li 2013b; Song 2012; Song 2014; Wykes 2005; Zhou 2015b). Hogarty 2004 reported the use of therapeutic strategies designed to overcome intellectual and memory deficits associated with schizophrenia rather than psychotic symptoms, beliefs, or cognitive distortions. Liu 2013 investigated a group CBT plus token therapy intervention which did not meet our criteria.
5. Issues relating to outcomes
The remaining 12 trials with 12 references did not have usable data for meta‐analysis (Bradshaw 2000; Dong 2015; Du 2016; Jiang 2014; Lang 2014; Lin 2014; Liu 2015; McLeod 2007; Shao 2013; Shi 2015; Wu 2012; Zeng 2014).
Studies awaiting classification
Eight trials (Chen 2015c; Fohlmann 2010; Hardy 2015; Hassan 2014; Moun 2015; Nagui 2016; Tang 2015; Tecic 2012) are awaiting assessment as we currently are unable to access the full‐text report.
Ongoing studies
Please refer to Characteristics of ongoing studies for more details.
We identified 15 ongoing trials that started between 2003 and 2016, but results had not been published. Three of these trials (ISRCTN12668007; NCT02787122; NCT02787135) were still in the recruiting phase.
Risk of bias in included studies
The summary of risk of bias in included trials was presented in Figure 2 and Figure 3
2.
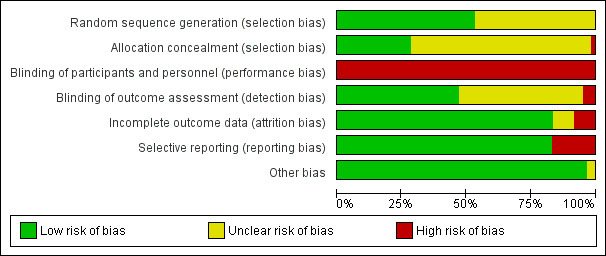
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
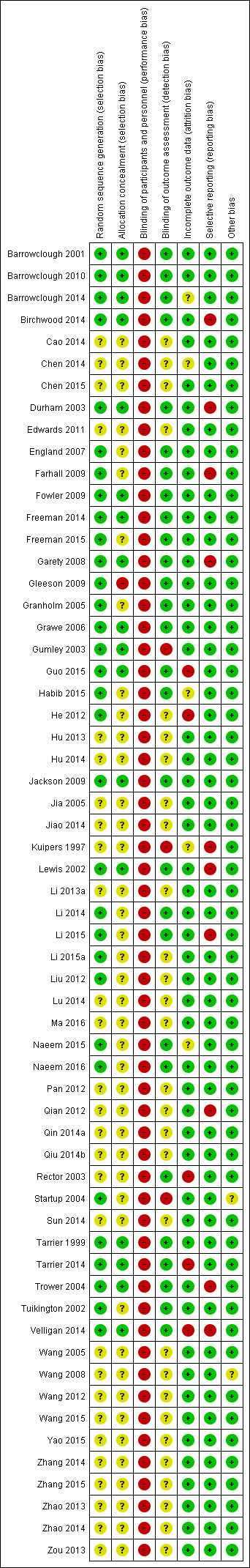
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirty‐two included trials (32/60, 53.3%) were rated as having low risk of bias from randomisation as they described adequate random sequence generation. The methods used for sequence generation were computer‐generated random numbers, programme or web‐based randomisation system, block randomisation, random number table, and tossing coins. The remaining trials were rated as having unclear risk for selection bias as they did not describe the method of sequence generation.
Seventeen trials (17/60, 28.3%) were rated as having low risk of bias from allocation concealment as they reported that the randomisation was conducted by staff independent of the research team or was conducted centrally, therefore, these trials had good allocation concealment. However, most of the included trials did not state the methods used to keep allocation concealed, and they were rated as having unclear risk of bias. One trial clearly stated that it did not conceal the group assignment regimen (Gleeson 2009) and this was rated as having a high risk of this selection bias.
Blinding
All trials were all rated as having high risk of performance bias as CBT therapy cannot be 'masked' and is very different to standard care. It is very likely that all staff providing the care during the trial would know the group assignment.
As for the blinding of outcome assessors, 31 trials (28/60, 46.7%) were rated as having low risk of detection bias as they blinded the outcomes assessors fromr group assignment. Twenty‐two trials did not state whether they keep the outcome assessors blinded, therefore, they were rated as having unclear risk of detection bias. The remaining three trials (Gumley 2003; Kuipers 1997; Startup 2004) revealed that the outcomes assessors were not blinded, hence they were rated as having high risk of detection bias.
Incomplete outcome data
Nearly 83% (50/60) of the included trials were rated as having low risk of attrition bias, as they had no missing data, a low proportion of participants left the trial early, or an intention‐to‐treat (ITT) analysis was used to deal with the missing data. Four trials (Barrowclough 2014; Chen 2014; Kuipers 1997; Naeem 2015) had a relatively low proportion of missing data and the authors did not report the cause of withdrawal, so we rated these trials as having unclear risk for attrition bias. Another trial did not report the number of withdrawals, but reported an intention‐to‐treat analysis was used to deal with the missing data (Habib 2015). Therefore, this trial was also rated as having unclear risk of attrition bias. Five trials (Guo 2015; He 2012; Rector 2003; Tarrier 2014; Velligan 2014) were rated as having high risk of attrition bias due to a high proportion of missing data.
Selective reporting
A majority of included trials (50/60, 83%) were rated as having low risk of selective reporting as they reported data for all measured outcomes. Only ten trials (Birchwood 2014; Durham 2003; Kuipers 1997;Garety 2008; Lewis 2002; Li 2015; Qian 2012; Velligan 2014; Trower 2004; Farhall 2009) were rated as having high risk of selective reporting for failure to report results of several prespecified outcomes.
Other potential sources of bias
We did not think there was a high risk of other potential sources of bias within the included trials.
Effects of interventions
See: Table 1
COMPARISON 1: CBT plus standard care versus standard care alone
1.1 Global state: 1a. Relapse
Eighteen trials reported data on relapse (Analysis 1.1).
1.1. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 1 Global state: 1a. Relapse.
1.1.1 Short term
Two trials reported short‐term relapse data. There was not a clear difference between CBT plus standard care and standard care alone groups (RR 0.22, 95% CI 0.04 to 1.24; participants = 92; studies = 2).
1.1.2 Medium term
Five trials reported medium‐term data. There was a clear difference, favouring CBT plus standard care (RR 0.53, 95% CI 0.39 to 0.72; participants = 667; studies = 5).
1.1.3 Long term
Thirteen trials reported long‐term data. There was no clear difference between CBT plus standard care and standard care alone groups (RR 0.78, 95% CI 0.61 to 1.00; participants = 1538; studies = 13, low‐quality evidence). This result was presented in the SOF table as an outcome of importance. The quality of evidence was low due to significant heterogeneity (Chi2 = 25.21; df = 12; P = 0.01; I2 = 52%) and the wide confidence interval which included both appreciable benefit and harm. Removing outliers from this analysis did not alter the result.
1.2 Global state: 1b. Relapse ‐ number (skewed data)
Barrowclough 2010 reported relapse data, however, these data were presented as 'Other data' because of marked skew, which makes it difficult to interpret (Analysis 1.2)
1.2. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 2 Global state: 1b. Relapse (skewed data).
| Global state: 1b. Relapse (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| Medium term | ||||
| Barrowclough 2010 | CBT + standard care | 0.3 | 0.58 | 161 |
| Barrowclough 2010 | Standard care | 0.27 | 0.54 | 161 |
| Long term | ||||
| Barrowclough 2010 | CBT + standard care | 0.27 | 0.55 | 161 |
| Barrowclough 2010 | Standard care | 0.23 | 0.45 | 159 |
1.3 Global state: 2. Clinically important change (no improvement) ‐ defined by individual studies
Three trials provided data at the medium and long term (Analysis 1.3).
1.3. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 3 Global state: 2. Clinically important change (no improvement) ‐ defined by individual studies.
1.3.1 Short term
Edwards 2011 found no clear difference between CBT plus standard care and standard care alone groups (RR 1.01, 95% CI 0.61 to 1.66; participants = 48; study = 1).
1.3.2 Long term
Two trials provided useful long‐term data. There was a favourable effect for CBT plus standard care compared to standard care alone (RR 0.57, 95% CI 0.39 to 0.84; participants = 82; studies = 2; very low‐quality evidence). This result was presented in the SOF table as an outcome of importance. The quality of the evidence for this result was very low due to high risk of bias across several domains and the small number of trials with small sample size and very low number of events contributing data to this result.
1.4 Global state: 3a. Rehospitalisation
Eight trials reported data on rehospitalisation (Analysis 1.4).
1.4. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 4 Global state: 3a. Rehospitalisation.
1.4.1 Short term
Freeman 2014 reported short‐term data. There was no clear difference between CBT plus standard care and standard care alone groups (RR 1.00, 95% CI 0.07 to 14.55; participants = 30; study = 1).
1.4.2 Long term
Six trials reported long‐term data. There was no clear difference between CBT plus standard care and standard care alone groups (RR 0.79, 95% CI 0.60 to 1.04; participants = 648; studies = 6).
1.5 Global state: 3b. Hospitalisation ‐ number of admissions (skewed data)
Barrowclough 2010 reported 'number of admissions', however these data were presented in Other data tables because of marked skew which makes it difficult to interpret (Analysis 1.5).
1.5. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 5 Global state: 3b. Hospitalisation ‐ number of admissions (skewed data).
| Global state: 3b. Hospitalisation ‐ number of admissions (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| Medium term | ||||
| Barrowclough 2010 | CBT + standard care | 0.22 | 0.58 | 163 |
| Barrowclough 2010 | Standard care | 0.22 | 0.63 | 162 |
| Long term | ||||
| Barrowclough 2010 | CBT + standard care | 0.27 | 0.65 | 162 |
| Barrowclough 2010 | Standard care | 0.19 | 0.49 | 159 |
1.6 Global state: 4. Average endpoint total score CGI, high = poor
Three trials (Chen 2014, Edwards 2011; Wang 2015) reported global state data as endpoint scores on the CGI. They reported short‐, medium‐ and long‐term follow‐up (Analysis 1.6).
1.6. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 6 Global state: 4. Average endpoint total score CGI, high = poor.
1.6.1 Short term
Short‐term data showed a clear difference, favouring CBT plus standard care (MD ‐0.32, 95% CI ‐0.63 to ‐0.01; participants = 128; studies = 3).
1.6.2 Medium term
Medium‐term data showed a clear difference, favouring CBT plus standard care (MD ‐0.52, 95% CI ‐0.89 to ‐0.15; participants = 80; studies = 2).
1.6.3 Long term
Only Wang 2015 reported useful long‐term data. Data showed a clear difference, favouring CBT plus standard care (MD ‐0.67, 95% CI ‐1.07 to ‐0.27; participants = 32; study = 1).
1.7 Mental state: 1. General ‐ clinically important change (no improvement)
Eleven trials reported clinically important change in general mental state as 'no improvement'. Seven trials reported short‐term follow‐up, and five at the long term (Analysis 1.7).
1.7. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 7 Mental state: 1. General ‐ clinically important change (no improvement).
1.7.1 Short term
Short‐term data showed a favourable effect for CBT plus standard care compared to standard care alone (RR 0.44, 95% CI 0.21 to 0.92; participants = 680; studies = 7). This subgroup had important levels of heterogeneity (Chi2 = 20.26, df = 6, P = 0.006, I2 = 70%). The statistical heterogeneity was not significant after removing Ma 2016 from the meta‐analysis. With Ma 2016 removed, a clear effect favouring CBT group was still observed (RR 0.70 95% CI 0.55 to 0.90).
1.7.2 Long term
Long‐term data showed no difference between the treatment groups (RR 0.81, 95% CI 0.65 to 1.02; participants = 501; studies = 5, very low‐quality of evidence). This subgroup had important levels of heterogeneity (Chi2 = 7.26, df = 3, P = 0.06, I2 = 59%, very low‐quality evidence). The quality of evidence was very low due to serious risk of bias of the included studies, significant heterogeneity, and low number of events.The statistical heterogeneity was not significant after removing Garety 2008 from the meta‐analysis. With Garety 2008 removed, no clear difference between the compared groups was still observed (RR 0.87 95% CI 0.73 to 1.03).
1.8 Mental state: 2a. General (average total endpoint score BPRS, high = poor)
Eight trials reported general mental state using BPRS total scores (Analysis 1.8).
1.8. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 8 Mental state: 2a. General (average total endpoint score BPRS, high = poor).
1.8.1 Short term
Five trials reported short‐term data. There was a clear difference between the treatment groups, favouring CBT plus standard care (MD ‐5.09 CI ‐8.44 to ‐1.74; participants = 541; studies = 5). This subgroup had important levels of heterogeneity (Chi2 = 26.08; df = 4; P < 0.0001; I2 = 85%).The statistical heterogeneity was still significant after successively removing outliers. We could not find the source of the heterogeneity.
1.8.2 Medium‐term
Three trials reported medium‐term data. There was no clear difference between CBT plus standard care and standard care alone groups (MD ‐2.57, 95% CI ‐5.73 to 0.60; participants = 199; studies = 3). This result remained the same when using a fixed‐effect model.
1.8.3 Long term
Three trials reported long term data . There was a clear difference between the treatment groups, favouring CBT plus standard care ((MD ‐8.77, 95% CI ‐14.08 to ‐3.46, Analysis 1.8). This subgroup had important levels of heterogeneity (Chi2 = 16.17; df = 2; P = 0.02; I2 = 73%). The statistical heterogeneity was reduced to I2 = 0% after removing England 2007 from the meta‐analysis with the positive effect for CBT remaining (MD ‐6.14, 95% CI ‐9.47 to ‐2.80; participants = 110; studies = 2).
1.9 Mental state: 2b.General (average total endpoint score PANSS, high = poor)
Twenty‐two trials reported general mental stated using PANSS total scores (Analysis 1.9) .
1.9. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 9 Mental state: 2b. General (average total endpoint score PANSS, high = poor).
1.9.1 Short term
Eleven trials reported short‐term data. There was a clear difference between the treatment groups, favouring CBT plus standard care (MD ‐7.21, 95% CI ‐10.12 to ‐4.30, participants = 962; studies = 11). This subgroup had important levels of heterogeneity (Chi2 = 51.61; df = 11; P < 0.00001; I2 = 81%). The statistical heterogeneity was still significant after successively removing outliers. We could not find the source of this heterogeneity.
1.9.2 Medium term
Eleven relevant trials reported medium‐term data. There was a clear difference between the treatment groups, favouring CBT plus standard care (MD ‐3.68, 95% CI ‐6.12 to ‐1.24; participants = 963; studies = 11). This subgroup had important levels of heterogeneity (Chi2 = 30.35; df = 14; P = 0.0008; I2 = 67%). The statistical heterogeneity was not significant after removing Qiu 2014b from the meta‐analysis and the positive effect for CBT remained (MD ‐2.64, 95% CI ‐4.71 to ‐ 0.58). Accordingly, the treatment effect was robust to heterogeneity amongst the studies.
1.9.3 Long term
Twelve trials provided reported long‐term data. There was a clear difference between the treatment groups, favouring CBT plus standard care (MD ‐3.74, 95% CI ‐6.46 to ‐1.02; participants = 1284; studies = 12). This subgroup also had important levels of heterogeneity (Chi2 = 34.88; df = 11; P = 0.0003; I2 = 68%). The statistical heterogeneity was still significant after successively removing outliers. We could not find the source of this heterogeneity.
1.10 Mental state: 2c. General (average total endpoint score PSYRATS, high = poor)
One trial (Birchwood 2014) reported medium‐ and long‐term data for this outcome (Analysis 1.10).
1.10. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 10 Mental state: 2c. General (average total endpoint score PsyRAT, high = poor).
1.10.1 Medium term
There was no clear difference between CBT plus standard care and standard care groups at the medium term (MD 1.05, 95% CI ‐1.20 to 3.30; participants = 197; studies = 1).
1.10.2 Long term
There was no clear difference between CBT plus standard care and standard care groups at the long term (MD 0.63, 95% CI ‐1.48 to 2.74; participants = 197; studies = 1).
1.11 Mental State: 2d. General (average total change score, various scales)
Two trials reported change scores from mental state scales (Analysis 1.11)
1.11. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 11 Mental state: 2d. General (average total change score, various scales).
1.11.1 CHOICE ‐ short term
Freeman 2015 reported change score from CHOICE at the short term. There was a favourable effect for CBT plus standard care compared to standard care alone (MD 9.10, 95% CI 1.74 to 16.46, participants = 136, study = 1).
1.11.2 CPRS ‐ medium term
Tuikington 2002 reported change score from the CPRS at the medium term. There was no clear difference between CBT plus standard care and standard care alone groups (MD 1.32, 95% CI ‐0.97 to 3.61, participants = 336, study = 1).
1.12 Mental State: 2e. General (average total change score SCL‐90, high = poor)
Li 2015a reported average change scores from the SCL‐90. As these data showed significant skew, they were presented as 'Other data' (Analysis 1.12).
1.12. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 12 Mental state: 2e. General (average total endpoint score SCL‐90, high = poor) ‐ long term.
| Mental state: 2e. General (average total endpoint score SCL‐90, high = poor) ‐ long term | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| Li 2015a | CBT + standard care | 48.00 | 18.04 | 48 |
| Li 2015a | Standard care | 53.91 | 34.31 | 44 |
1.13 Mental state: 3a. Specific ‐ positive symptoms (average endpoint score PANSS sub scale, high = poor)
Seventeen trials reported PANSS subscale scores for positive symptoms (Table 4).
3. Data added into generic inverse variance outcome (1.14).
| CBT group | Standard care group | |||||
| Short term | Mean | SD | N | Mean | SD | N |
| Barrowclough 2014 | 14.01 | 3.92 | 48 | 14.2 | 5.2 | 24 |
| Chen 2015 | 10.6 | 3.7 | 25 | 11.5 | 4.1 | 25 |
| Guo 2015 | 11.8 | 3.9 | 32 | 13.0 | 4.4 | 32 |
| Jia 2005 | 9.82 | 2.13 | 22 | 12.18 | 1.84 | 38 |
| Lewis 2002 | 13.3 | 5.06 | 78 | 13.67 | 5.33 | 60 |
| Li 2013a | 12.11 | 3.08 | 60 | 17.76 | 4.39 | 58 |
| Naeem 2015 | 13.1 | 4.7 | 53 | 16.9 | 5.5 | 49 |
| Qiu 2014b | 13.07 | 5.3 | 30 | 17.28 | 4.3 | 29 |
| Wang 2008 | 9.8 | 3.4 | 43 | 11.2 | 7.3 | 41 |
| Wang 2015 | 10.8 | 3.51 | 15 | 13.33 | 4.32 | 15 |
| Total | 406 | 371 | ||||
| Medium term | Mean | SD | N | Mean | SD | N |
| Barrowclough 2001 | 13.35 | 4.57 | 17 | 16.07 | 5.54 | 15 |
| Barrowclough 2010 | 14.62 | 4.85 | 137 | 14.59 | 5.35 | 137 |
| Barrowclough 2014 | 13.36 | 3.92 | 47 | 14.6 | 5.1 | 23 |
| Birchwood 2014 | 16.06 | 4.53 | 98 | 17.85 | 5.51 | 99 |
| Chen 2015 | 10.2 | 3.7 | 25 | 11.3 | 3.9 | 25 |
| Granholm 2005 | 13.2 | 5.4 | 32 | 12.9 | 4.6 | 33 |
| Guo 2015 | 11.3 | 4.0 | 32 | 11.9 | 4.6 | 32 |
| Hu 2013 | 10.2 | 4.8 | 40 | 10.1 | 4.9 | 39 |
| Qiu 2014b | 8.17 | 3.7 | 30 | 11.97 | 4.4 | 29 |
| Rector 2003 | 12.0 | 4.0 | 24 | 13.9 | 6.7 | 18 |
| Tarrier 2014 | 12.4 | 4.9 | 17 | 14.9 | 4.0 | 18 |
| Wang 2015 | 9.53 | 1.68 | 15 | 11.8 | 2.98 | 15 |
| Total | 514 | 483 | ||||
| Long term | Mean | SD | N | Mean | SD | N |
| Barrowclough 2001 | 13.87 | 4.27 | 17 | 12.93 | 4.23 | 17 |
| Barrowclough 2010 | 14.07 | 5.38 | 129 | 13.55 | 5.22 | 118 |
| Barrowclough 2014 | 13.62 | 4.34 | 50 | 12.7 | 5.1 | 21 |
| Birchwood 2014 | 16.96 | 5.32 | 98 | 17.3 | 5.78 | 99 |
| Farhall 2009 | 11.78 | 5.13 | 45 | 13.0 | 4.67 | 47 |
| Garety 2008 | 15.12 | 6.25 | 111 | 16.21 | 6.24 | 113 |
| Granholm 2005 | 12.8 | 6.6 | 31 | 14.1 | 5.0 | 33 |
| Gumley 2003 | 8.85 | 2.09 | 72 | 9.88 | 3.61 | 72 |
| Guo 2015 | 10.8 | 3.8 | 32 | 12.8 | 4.3 | 32 |
| Lewis 2002 | 12.45 | 4.1001 | 75 | 15.25 | 6.44 | 71 |
| Rector 2003 | 10.9 | 3.4 | 21 | 11.5 | 4.7 | 13 |
| Wang 2015 | 8.93 | 1.53 | 15 | 11.13 | 3.66 | 15 |
| Total | 696 | 651 | ||||
One trial reported short‐term data for this outcome as SEs. We used the RevMan calculator to convert data presented in Table 4 into SE for use in Analysis 1.13.
1.13. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 13 Mental state: 3a. Specific ‐ positive symptoms (average endpoint score PANSS subscale, high = poor).
1.13.1 Short term
Short term data showed an effect favouring CBT (MD ‐3.11, 95% CI ‐4.97 to ‐1.24; studies = 11). For this outcome, heterogeneity was high (Chi2 = 10.4; df = 1.0; P = 0.0; I2 = 90%). The statistical heterogeneity was not significant after removing the outliers Habib 2015 and Li 2013a from the meta‐analysis and the positive effect for CBT remained. Accordingly, the treatment effect was robust to heterogeneity amongst the studies. We could not identify a cause for this heterogeneity.
1.13.2 Medium term
Medium‐term data showed an effect favouring CBT (MD ‐1.23, 95% CI ‐1.90 to ‐0.55; studies = 12).
1.13.3 Long term
Long‐term data showed an effect favouring CBT (MD ‐0.98, 95% CI ‐1.63 to ‐0.34; studies = 12).
1.14 Mental state: 3b. Specific ‐ positive symptoms (average endpoint score BPSR/SAPS, high = poor)
Two trials reported scores from various other scales that measured positive symptoms (Analysis 1.14).
1.14. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 14 Mental state: 3b. Specific ‐ positive symptoms (average endpoint score BPRS/SAPS, high = poor) ‐ short term.
1.14.1 BPRS ‐ short term
Edwards 2011 reported BPRS scores. An effect favouring CBT plus standard care was observed (MD ‐1.84 95% CI ‐3.4 to ‐0.27; participants = 48; study = 1).
1.14.2 SAPS ‐ short term
Wang 2015 reported SAPS scores. An effect favouring CBT plus standard care was observed (MD ‐1.83 95% CI ‐3.61 to ‐0.05; participants = 64; study = 1).
1.15 Mental state: 4a. Specific ‐ hallucination ‐ clinically important change ‐ no improvement (< 3 point improvement BPRS ‐ hallucination severity)
England 2007 reported clinically important change as 'no improvement' which was measured as 'less than 3‐point improvement in hallucination severity scores' on the BPRS (Analysis 1.15).
1.15. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 15 Mental state: 4a. Specific ‐ hallucination ‐ clinically important change (no improvement ‐ < 3 point improvement BPRS (hallucination severity score)).
1.15.1 Short term
An effect favouring CBT plus standard care was observed at the short term (RR 0.09 95% CI 0.03 to 0.27; participants = 65; study = 1).
1.15.2 Long term
An effect favouring CBT plus standard care was observed at the long term (RR 0.08 95% CI 0.03 to 0.26; participants = 65; study = 1).
1.16 Mental state: 4b. Specific ‐ hallucination (average endpoint score various scales, high = poor)
Six trials reported hallucination scores from various scales at the short, medium and long term (Analysis 1.16).
1.16. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 16 Mental state: 4b. Specific ‐ hallucination (average endpoint score various scales, high = poor).
1.16.1 AHRS ‐ short term
Chen 2015 used the AHRS to measure 'hallucination'. At short‐term follow‐up, there was a clear difference between treatment groups, favouring CBT plus standard care (MD ‐3.60, 95% CI ‐6.74 to ‐0.46; participants = 50; study = 1). This result remained the same when using a fixed‐effect model.
1.16.2 PANSS ‐ short term
Jia 2005 used the PANSS to measure 'hallucination'. At short‐term follow‐up, there was a clear difference between treatment groups, favouring CBT plus standard care (MD ‐0.48, 95% CI ‐0.92 to ‐0.04; participants = 60; study = 1). This result remained the same when using a fixed‐effect model.
1.16.3 AHRS ‐ medium term
Medium‐term data from the AHRS did not show a clear difference between CBT plus standard care and standard care alone groups (MD ‐2.57, 95% CI ‐7.07 to 1.93; participants = 128; studies = 2). This subgroup had important levels of heterogeneity (Chi2 = 4.75; df = 1; P = 0.03; I2 = 79%) and the source of this heterogeneity remained unidentified.
1.16.4 PANSS ‐ medium term
Medium‐term data from PANSS showed no clear difference between CBT plus standard care and standard care alone groups (MD ‐0.33, 95% CI ‐0.79 to 0.13; participants = 197; study = 1). This result remained the same when using a fixed‐effect model.
1.16.5 Malevolence ‐ BAVQ ‐ medium term
Medium‐term data from BAVQ also showed no clear difference between CBT plus standard care and standard care alone groups (MD 1.00, 95% CI ‐5.26 to 7.26; participants = 29; study = 1).
1.16.6 Omniscience ‐ BAVQ ‐ medium term
Medium‐term data from BAVQ also showed no clear difference between CBT plus standard care and standard care alone groups (MD ‐0.90, 95% CI ‐3.47 to 1.67; participants = 29; study = 1).
1.16.7 VPD ‐ medium term
Medium‐term data from the VPD scale showed a clear difference, between treatment groups, favouring CBT plus standard care (MD ‐11.10, 95% CI ‐15.73 to ‐6.47; participants = 29; study = 1).
1.16.8 AHRS ‐ long term
Long‐term data from the AHRS did not show a clear difference between CBT plus standard care and standard care alone groups (MD ‐4.40, 95% CI ‐6.60 to ‐2.20; participants = 78; study = 1).
1.16.9 PANSS ‐ long term
Long‐term data from the PANSS did not show a clear difference between CBT plus standard care and standard care alone groups (MD 0.14, 95% CI ‐0.3 to 0.58; participants = 197; study = 1).
1.16.10 BPRS ‐ long term
Long‐term data from the BPRS showed a clear difference between treatment groups, favouring CBT plus standard care (MD ‐2.82, 95% CI ‐3.74 to ‐1.90; participants = 65; study = 1).
1.17 Mental state: 4c. Specific ‐ hallucinations (average endpoint score PsyRATs, high = poor)
1.17.1 Short term
Habib 2015 and Naeem 2015 reported SE data for hallucinations using PsyRATs. However, significant heterogeneity was observed (Chi2 = 24.87, df = 1 P < 0.00001, I2 = 96%). As there were only two studies contributing to this outcome, it was not possible to reduce heterogeneity and we decided not to combine these data within the meta‐analysis (Analysis 1.17).
1.17. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 17 Mental state: 4c. Specific ‐ hallucinations (average endpoint score, PsyRATs, high = poor).
1.17.2 Medium term
Only Tuikington 2002 reported medium‐term data. There was no clear difference between CBT plus standard care and standard care alone groups (MD ‐1.17, 95% CI ‐3.26 to 0.92; participants = 422; study = 1). Results were unaltered when applying the fixed‐effect model (Analysis 1.17).
1.18 Mental state: 4d. Specific ‐ hallucinations (average endpoint score, various scales, high = poor) (skewed data)
Naeem 2016; Durham 2003; England 2007; and Trower 2004 also presented scale scores for 'hallucination'. These data were presented as 'Other data' because of the presence of excessive skew (Analysis 1.18).
1.18. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 18 Mental state: 4d. Specific ‐ hallucinations (average endpoint score various scales, high = poor) (skewed data).
| Mental state: 4d. Specific ‐ hallucinations (average endpoint score various scales, high = poor) (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| PsyRATs ‐ short term | ||||
| Naeem 2016 | CBT + standard care | 9.78 | 12.56 | 15 |
| Naeem 2016 | Standard care | 22.87 | 8.03 | 13 |
| PsyRATs ‐ long term | ||||
| Durham 2003 | CBT + standard care | 18.5 | 12.8 | 20 |
| Durham 2003 | Standard care | 17.2 | 11.7 | 17 |
| BPRS ‐ short term | ||||
| England 2007 | CBT + standard care | 1.52 | 1.48 | 44 |
| England 2007 | Standard care | 4.81 | 1.66 | 21 |
| VCS ‐ short term | ||||
| Trower 2004 | CBT + standard care | 1.8 | 1.2 | 14 |
| Trower 2004 | Standard care | 3.1 | 1.4 | 15 |
| VCS ‐ medium term | ||||
| Trower 2004 | CBT + standard care | 1.7 | 1.1 | 14 |
| Trower 2004 | Standard care | 3.4 | 1.6 | 15 |
1.19 Mental state: 5a. Specific ‐ delusions (average endpoint score PsyRATs, high = poor)
Five studies reported short‐term data for delusions using PsyRATs. Habib 2015; Naeem 2015; and Naeem 2016 reported data for this outcome as SEs. We used the RevMan calculator to convert data reported by Freeman 2014 and Freeman 2015 (presented in Table 5) into SE for use in Analysis 1.19 .
4. Data added into generic inverse variance outcome (1.17).
| CBT group | Standard care group | |||||
| Short term | Mean | SD | N | Mean | SD | N |
| Freeman 2014 | 15.1 | 4.9 | 15 | 13.7 | 5.4 | 15 |
| Freeman 2015 | 13.6 | 5.6 | 68 | 16.4 | 4.8 | 72 |
| Total | 83 | 87 | ||||
1.19.1 Short term
At the short term, a positive effect for CBT was observed (MD ‐4.33, 95% CI ‐7.58 to ‐1.08; studies = 5)
This outcome had important levels of heterogeneity (Chi2 = 16.35; df = 2.0; P = 0.0003; I2 = 90%), which were reduced to I2 < 50 % when the outliers Habib 2015 and Freeman 2014 were excluded, retaining a positive effect for CBT.
1.20 Mental state: 5b. Specific ‐ delusions (average endpoint score PANSS, high = poor)
Jia 2005 reported delusion scores using the PANSS (Analysis 1.20).
1.20. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 20 Mental state: 5b. Specific ‐ delusions (average endpoint score PANSS, high = poor).
1.20.1 Short term
There was a single trial in this subgroup. For this outcome, within this subgroup, we did find evidence that CBT was clearly different in its effects compared with 'standard care alone' (MD ‐0.64 95% CI ‐1.16 to ‐0.12; participants = 60; study = 1).
1.20.2 Medium term
There was a single trial in this subgroup. For this outcome, within this subgroup, we did not find evidence that CBT was clearly different in its effects compared with 'standard care alone' (MD ‐0.30 95% CI ‐0.71 to 0.11; participants = 197; study = 1).
1.20.3 Long term
We found one trial to be relevant to this subgroup. For this outcome, within this subgroup, we found no evidence that CBT was different in its effects compared with 'standard care alone' (MD ‐0.10 95% CI ‐0.53 to 0.33; participants = 197; study = 1).
1.21. Mental state: 5c. Specific ‐ delusions (average change score PsyRATs, high = poor)
1.21.1 Medium term
Tuikington 2002 reported change scores. There was no evidence of a clear difference between CBT and standard care within this subgroup (MD ‐0.43 95% CI ‐1.82 to 0.96; participants = 336; study = 1) (Analysis 1.21).
1.21. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 21 Mental state: 5c. Specific ‐ delusions (average change score PsyRATs, high = poor).
1.22 Mental state: 5d. Specific ‐ delusions (average endpoint score PSYRATs high = poor) (skewed data)
These data were presented in Other data tables because of excessive skew in the data (Analysis 1.22).
1.22. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 22 Mental state: 5d. Specific ‐ delusions (average endpoint score PsyRATS, high = poor) (skewed data).
| Mental state: 5d. Specific ‐ delusions (average endpoint score PsyRATS, high = poor) (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| Medium term | ||||
| Tarrier 2014 | CBT + standard care | 7.19 | 7.09 | 17 |
| Tarrier 2014 | Standard care | 11.29 | 8.12 | 18 |
| Long term | ||||
| Durham 2003 | CBT + standard care | 11.1 | 5.8 | 21 |
| Durham 2003 | Standard care | 11.2 | 6.5 | 18 |
| Lewis 2002 | CBT + standard care (Liverpool) | 3.5 | 6.0 | 24 |
| Lewis 2002 | Standard care | 8.6 | 8.6 | 19 |
1.23 Mental state: 6a. Specific ‐ negative symptoms (average endpoint score PANSS sub scale, high = poor)
Twenty‐one studies reported negative symptom scores using PANSS subscale at the short, medium and long term. Two trials reported short‐term data for this outcome as SEs. We used the RevMan calculator to convert data reported by the other trials (see Table 6) into SE to enable use in (Analysis 1.23) (Analysis 1.23).
5. Data added into generic inverse variance outcome (1.23).
| CBT group | Standard care group | |||||
| Short term | Mean | SD | N | Mean | SD | N |
| Barrowclough 2014 | 13.93 | 4.94 | 48 | 12.7 | 5.5 | 24 |
| Chen 2015 | 16.8 | 6.1 | 25 | 19.3 | 5.7 | 25 |
| Guo 2015 | 12.1 | 4.3 | 32 | 14.3 | 5.2 | 32 |
| Jia 2005 | 17.18 | 3.46 | 22 | 20.66 | 3.36 | 38 |
| Li 2013a | 11.8 | 3.61 | 60 | 16.87 | 4.03 | 58 |
| Naeem 2015 | 11.2 | 3.5 | 53 | 14.8 | 4.9 | 49 |
| Qian 2012 | 17.23 | 4.02 | 45 | 19.37 | 4.51 | 45 |
| Qiu 2014b | 12.6 | 3.6 | 30 | 14.62 | 3.4 | 29 |
| Wang 2008 | 9.3 | 3.3 | 43 | 11.3 | 4.2 | 41 |
| Wang 2015 | 13.6 | 2.97 | 15 | 15.26 | 2.15 | 15 |
| Total | 373 | 356 | ||||
| Medium term | Mean | SD | N | Mean | SD | N |
| Barrowclough 2001 | 12.65 | 4.97 | 17 | 14.67 | 6.02 | 15 |
| Barrowclough 2010 | 13.36 | 4.65 | 137 | 12.97 | 4.08 | 137 |
| Barrowclough 2014 | 13.47 | 4.41 | 47 | 12.7 | 4.4 | 23 |
| Birchwood 2014 | 12.94 | 5.22 | 98 | 13.45 | 4.97 | 99 |
| Chen 2015 | 16.1 | 4.9 | 25 | 18.1 | 6.0 | 25 |
| Granholm 2005 | 12.9 | 3.8 | 32 | 13.7 | 5.2 | 33 |
| Guo 2015 | 12.1 | 4.3 | 32 | 14.4 | 5.1 | 32 |
| Hu 2013 | 10.8 | 2.5 | 40 | 13.9 | 4.4 | 39 |
| Qian 2012 | 9.43 | 4.09 | 45 | 17.25 | 4.34 | 45 |
| Qiu 2014b | 8.63 | 3.6 | 30 | 10.72 | 3.9 | 29 |
| Rector 2003 | 13.1 | 4.5 | 24 | 16.0 | 7.2 | 18 |
| Tarrier 2014 | 11.1 | 2.3 | 17 | 11.9 | 3.1 | 18 |
| Wang 2015 | 12.73 | 3.03 | 15 | 13.13 | 1.88 | 15 |
| Total | 559 | 528 | ||||
| Long term | Mean | SD | N | Mean | SD | N |
| Barrowclough 2001 | 10.27 | 2.25 | 17 | 15.5 | 5.71 | 17 |
| Barrowclough 2010 | 12.62 | 4.24 | 129 | 12.48 | 3.78 | 118 |
| Barrowclough 2014 | 14.02 | 4.14 | 49 | 15.2 | 6.5 | 21 |
| Birchwood 2014 | 13.33 | 5.47 | 98 | 12.96 | 4.48 | 99 |
| Farhall 2009 | 13.69 | 4.84 | 45 | 12.34 | 4.12 | 47 |
| Garety 2008 | 12.11 | 4.90 | 111 | 12.75 | 6.16 | 113 |
| Granholm 2005 | 14.6 | 6.8 | 31 | 13.8 | 4.8 | 33 |
| Gumley 2003 | 10.55 | 4.07 | 72 | 12.22 | 5.36 | 72 |
| Guo 2015 | 11.2 | 4.4 | 32 | 14.3 | 5.1 | 32 |
| Lewis 2002 | 14.34 | 5.20 | 75 | 15.99 | 6.16 | 71 |
| Qian 2012 | 8.21 | 3.03 | 45 | 15.47 | 3.67 | 45 |
| Rector 2003 | 10.9 | 4.0 | 21 | 16.5 | 6.0 | 13 |
| Wang 2015 | 11.73 | 2.31 | 15 | 12.87 | 2.42 | 15 |
| Total | 740 | 696 | ||||
1.23. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 23 Mental state: 6a. Specific ‐ negative symptoms (average endpoint score, PANSS subscale, high = poor).
1.23.1 Short term
Short‐term data from 12 trials showed a positive effect for the CBT group (MD ‐3.35, 95% CI ‐3.84 to ‐2.85; studies = 12).
1.23.2 Medium term
Medium‐term data from 13 trials showed a positive effect for the CBT group (MD ‐3.35, 95% CI ‐3.84 to ‐2.85; studies = 12)
1.23.3 Long term
Long‐term data from 13 trials showed a positive effect for the CBT group (MD ‐1.47, 95% CI ‐1.94 to ‐0.99; studies = 13).
All three subgroups had very high heterogeneity (I2 > 75%), and removing outliers did not alter the result. We could not identify a cause for the heterogeneity.
1.24 Mental state: 6b. Specific ‐ negative symptoms (average endpoint score SANS, high = poor)
Four trials reported negative symptoms using SANS (Analysis 1.24).
1.24. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 24 Mental state: 6b. Specific ‐ negative symptoms (average endpoint score SANS, high = poor).
1.24.1 Short term
Four trials reported short‐term data. There was no clear difference between CBT plus standard care and standard care groups (MD ‐4.11 95% CI ‐10.40 to 2.17; participants = 231; studies = 4). This subgroup had important levels of heterogeneity (Chi2 = 61.03; df = 3; P < 0.00001; I2 = 95%). The statistical heterogeneity was not significant after removing Wang 2005 from the meta‐analysis and there was still no clear difference between the treatment groups (MD ‐0.59, 95% CI ‐1.99 to 0.80). Accordingly, the treatment effect was robust to heterogeneity amongst the studies.
1.24.2 Long term
One trial reported long‐term data. There was no clear difference between CBT plus standard care and standard care groups (MD ‐1.07 95% CI ‐3.29 to 1.15; participants = 49; study = 1). This result was the same when using a fixed‐effect model.
1.25 Mental state: 6c. Specific ‐ negative symptoms (average endpoint score NSRS, high = poor) ‐ medium term
Tuikington 2002 reported data. There was no clear difference between the two treatments groups (MD 0.60, 95% CI ‐0.05 to 1.25; participants = 336; study = 1) (Analysis 1.25).
1.25. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 25 Mental state: 6c. Specific ‐ negative symptoms (average endpoint score NSRS, high = poor) ‐ medium term.
1.26 Mental state: 7a. Specific ‐ affective symptoms (average endpoint score PANSS sub scale, high = poor)
Seventeen studies reported affective symptoms using the PANSS subscale. Two trials reported short‐term data for this outcome as SEs. We used the RevMan calculator to convert data reported by the other trials (Table 7 ) into SEs to enable use in Analysis 1.26 (Analysis 1.26).
6. Data added into generic inverse variance outcome (1.26).
| CBT group | Standard care group | |||||
| Short term | Mean | SD | N | Mean | SD | N |
| Barrowclough 2014 | 31.81 | 7.88 | 48 | 32.0 | 8.8 | 24 |
| Chen 2015 | 26.7 | 5.4 | 25 | 27.4 | 6.2 | 25 |
| Guo 2015 | 30.4 | 6.0 | 32 | 30.4 | 6.8 | 32 |
| Li 2013a | 21.17 | 5.37 | 60 | 28.29 | 6.9 | 58 |
| Naeem 2015 | 23.7 | 6.2 | 53 | 30.0 | 8.4 | 49 |
| Qiu 2014b | 22.4 | 4.2 | 30 | 25.82 | 5.2 | 29 |
| Wang 2008 | 25.1 | 4.1 | 43 | 26.9 | 4.7 | 41 |
| Wang 2015 | 32.67 | 8.23 | 15 | 37.07 | 6.27 | 15 |
| Total | 306 | 273 | ||||
| Medium term | Mean | SD | N | Mean | SD | N |
| Barrowclough 2010 | 29.85 | 7.52 | 137 | 27.74 | 6.94 | 137 |
| Barrowclough 2014 | 32.59 | 7.68 | 47 | 32.1 | 9.1 | 23 |
| Birchwood 2014 | 30.85 | 8.36 | 98 | 32.64 | 9.1 | 99 |
| Chen 2015 | 25.3 | 4.8 | 25 | 26.3 | 5.7 | 25 |
| Guo 2015 | 27.4 | 5.7 | 32 | 29.1 | 6.3 | 32 |
| Hu 2013 | 25.3 | 5.1 | 40 | 25.3 | 6.1 | 39 |
| Qiu 2014b | 17.47 | 4.5 | 30 | 21.14 | 5.3 | 29 |
| Rector 2003 | 25.6 | 6.1 | 24 | 31.6 | 12.2 | 18 |
| Tarrier 2014 | 24.4 | 6.6 | 17 | 27.1 | 7.8 | 18 |
| Wang 2015 | 27.27 | 5.59 | 15 | 31.4 | 5.18 | 15 |
| Total | 465 | 435 | ||||
| Long term | Mean | SD | N | Mean | SD | N |
| Barrowclough 2010 | 27.87 | 7.8 | 129 | 25.84 | 6.44 | 118 |
| Barrowclough 2014 | 34.14 | 8.59 | 49 | 31.6 | 8.5 | 21 |
| Birchwood 2014 | 31.22 | 8.4 | 98 | 32.73 | 9.36 | 99 |
| Farhall 2009 | 24.93 | 5.03 | 45 | 25.19 | 6.01 | 47 |
| Garety 2008 | 29.74 | 7.63 | 111 | 30.43 | 8.00 | 113 |
| Gumley 2003 | 24.73 | 6.5 | 72 | 26.9 | 8.1 | 72 |
| Guo 2015 | 25.6 | 5.3 | 32 | 28.7 | 6.8 | 32 |
| Lewis 2002 | 32.00 | 8.86 | 75 | 35.45 | 8.89 | 71 |
| Rector 2003 | 24.2 | 6.9 | 21 | 29.4 | 10.0 | 13 |
| Wang 2015 | 25.47 | 3.96 | 15 | 31.26 | 5.18 | 15 |
| Total | 647 | 601 | ||||
1.26. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 26 Mental state: 7a. Specific ‐ affective symptoms (average enpoint score, PANSS subscale, high = poor).
1.26.1 Short term
Short‐term data from ten trials showed a positive effect for CBT (MD ‐4.86, 95% CI ‐5.75 to ‐3.96; studies = 10).
1.26.2 Medium term
There was no clear difference between treatment groups at medium term (MD ‐0.80, 95% CI ‐1.70 to 0.09; studies = 10).
1.26.3 Long term
Long‐term data from ten trials showed a positive effect for CBT(MD ‐1.00, 95% CI ‐1.82 to ‐0.18; studies = 10)
Heterogeneity was high for all three subgroups (I2 > 60%). The positive effect for CBT remained when outliers were removed. We could not identify the cause of heterogeneity.
1.27 Mental state: 8. Specific ‐ distress (average endpoint score PsyRATs/SADS, high = poor)
Three trials reported stress scores (Analysis 1.27).
1.27. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 27 Mental state: 8. Specific ‐ distress (average endpoint score PsyRATs/SADS, high = poor).
1.27.1 Short term
Freeman 2015 reported short‐term data. There was a clear difference, favouring CBT plus standard care (MD ‐1.10 95% CI ‐1.77 to ‐0.43; participants = 140; study = 1). These results remained the same when using the fixed‐effect model.
1.27.2 Medium term
Birchwood 2014 and Trower 2004 reported medium‐term data. There was no clear difference between CBT plus standard care and standard care alone (MD 0.08, 95% CI ‐0.50 to 0.66; participants = 226; studies = 2). For this outcome, heterogeneity was high (Chi2 = 2.26; df = 1.0; P = 0.13; I2 = 56%). We failed to find the source of the heterogeneity.
1.27.3 Long term
Birchwood 2014 found no clear difference between treatment groups in the long term (MD ‐0.10, 95% CI ‐0.47 to 0.27; participants = 197; study = 1). This result remained the same when using the fixed‐effect model.
1.28 Mental state: 9. Specific ‐ anxiety (average endpoint score various scales, high = poor)
Ten trials used a variety of measuring scales to report anxiety scores (Analysis 1.28).
1.28. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 28 Mental state: 9. Specific ‐ anxiety (average endpoint score various scales, high = poor).
1.28.1 BAI ‐ short term
Barrowclough 2014 and Freeman 2014 reported short‐term scores from the BAI. There was no clear difference between the two treatments groups (MD ‐0.32, 95% CI ‐5.40 to 4.77; participants = 105; studies = 2).
1.28.2 SAS ‐ short term
Qin 2014a and Yao 2015 reported short‐term scores from the SAS. There was a clear difference between groups with a favourable effect for CBT plus standard care (MD ‐6.21, 95% CI ‐7.36 to ‐5.05; participants = 188; studies = 2).
1.28.3 HAMA ‐ short term
He 2012 reported short‐term scores from the HAMA. There was a clear difference between groups, favouring CBT plus standard care (MD ‐1.79, 95% CI ‐2.29 to ‐1.29; participants = 75; study = 1).
1.28.4 SCL‐90 ‐ short term
Zhang 2015 reported short‐term scores from the SCL‐90. There was a clear difference between groups, favouring CBT plus standard care (MD ‐1.43, 95% CI ‐1.53 to ‐1.33; participants = 90; study = 1).
1.28.5 BAI ‐ medium term
Barrowclough 2014 and Tarrier 2014 reported medium‐term scores from the BAI. There was no clear difference between CBT plus standard care and standard care groups (MD ‐1.34, 95% CI ‐6.55 to 3.87; participants = 108; studies = 2).
1.28.6 BAI ‐ long term
Barrowclough 2014; Fowler 2009 and Garety 2008 reported long‐term scores from the BAI. There was no clear difference between CBT plus standard care and standard care groups (MD 1.5, 95% CI ‐1.19 to 4.19; participants = 335; studies = 3).
1.28.7 HADS ‐ long term
Farhall 2009 reported long‐term scores from the HADS. There was no clear difference between CBT plus standard care and standard care groups (MD 0.66, 95% CI ‐1.22 to 2.54; participants = 92; study = 1).
1.29 Mental state: 10a. Specific ‐ depression ‐ clinically important change (no improvement = reduction HAMD score < 25%) ‐ short term
Pan 2012 measured clinically important change in depression, defining 'no improvement' as less than 25% reduction of the HAMD score. Similar numbers of participants from both groups showed 'no improvement' ‐ i.e. no advantage for CBT was observed (RR 0.67, 95% CI 0.21 to 2.15; participants = 68; study = 1) (Analysis 1.29).
1.29. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 29 Mental state: 10a. Specific ‐ depression ‐ clinically important change (no improvement = reduction HAMD score < 25%) ‐ short term.
1.30 Mental state: 10b. Specific ‐ depression (average endpoint score various scales, high = poor) ‐ short term
Eight trials reported depression scores at the short term from various scales (Analysis 1.30).
1.30. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 30 Mental state: 10b. Specific ‐ depression (average endpoint score various scales, high = poor) ‐ short term.
1.30.1 BDI
Edwards 2011 and Freeman 2014 reported BDI scores. There was no clear difference between CBT plus standard care and standard care groups (MD ‐1.11, 95% CI ‐4.25 to 2.03; participants = 78; studies = 2).
1.30.2 SDS
Qin 2014a and Yao 2015 reported SDS scores. There was a clear difference between treatment groups with a favourable effect for CBT plus standard care (MD ‐3.29, 95% CI ‐4.4 to ‐2.19; participants = 188; studies = 2).
1.30.3 HAMD ‐ short term
Chen 2014 and Pan 2012 reported HAMD scores. There was a clear difference between treatment groups with a favourable effect for CBT plus standard care (MD ‐4.95, 95% CI ‐6.69 to ‐3.2; participants = 143; studies = 2).
1.30.4 SCL‐90
Zhang 2015 reported SCL‐90 scores. At the short term, there was a clear difference between treatment groups with a favourable effect for CBT plus standard care (MD ‐0.58, 95% CI ‐0.65 to ‐0.51; participants = 90; study = 1).
1.31 Mental state: 10c. Specific ‐ depression (average change score MADRS, high = poor) ‐ medium term
Tuikington 2002 reported MADRS change scores. At the medium term, there was no clear difference between the two treatments (MD 0.15, 95% CI ‐1.26 to 1.56; participants = 336; studies = 1) (Analysis 1.31).
1.31. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 31 Mental state: 10c. Specific ‐ depression (average change score MADRS, high = poor).
1.32 Mental state: 10d. Specific ‐ depression (average endpoint score various scales, high = poor)
Several other trials presented scores from various scales for depression. They were presented as 'Other data' due to excess skew (Analysis 1.32).
1.32. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 32 Mental state: 10d. Specific ‐ depression (average endpoint score various scales, high = poor) (skewed data).
| Mental state: 10d. Specific ‐ depression (average endpoint score various scales, high = poor) (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| CDS ‐ short term | ||||
| Barrowclough 2014 | CBT + standard care | 6.3667 | 4.3131 | 48 |
| Barrowclough 2014 | Standard care | 4.8 | 4.0 | 23 |
| CDS ‐ medium term | ||||
| Barrowclough 2014 | CBT + standard care | 6.0 | 4.338 | 48 |
| Barrowclough 2014 | Standard care | 6.1 | 5.3 | 23 |
| Birchwood 2014 | CBT + standard care | 8.77 | 6.04 | 98 |
| Birchwood 2014 | Standard care | 8.76 | 6.24 | 99 |
| Jackson 2009 | CBT + standard care | 3.9 | 3.5 | 36 |
| Jackson 2009 | Standard care | 5.9 | 4.8 | 30 |
| Tarrier 2014 | CBT + standard care | 4.0 | 3.8 | 17 |
| Tarrier 2014 | Standard care | 7.2 | 5.2 | 18 |
| Trower 2004 | CBT + standard care | 8.1 | 7.4 | 14 |
| Trower 2004 | Standard care | 12.6 | 6.7 | 15 |
| BDI ‐ medium term | ||||
| Rector 2003 | CBT + standard care | 11.7 | 7.9 | 24 |
| Rector 2003 | Standard care | 11.8 | 11.5 | 18 |
| HAMD ‐ medium term | ||||
| Granholm 2005 | CBT + standard care | 11.4 | 6.3 | 32 |
| Granholm 2005 | Standard care | 10.6 | 6.3 | 33 |
| MADRS ‐ medium term | ||||
| Gleeson 2009 | CBT + standard care | 8.3 | 9.0 | 41 |
| Gleeson 2009 | Standard care | 10.8 | 11.5 | 40 |
| BDI ‐ long term | ||||
| Fowler 2009 | CBT + standard care | 14.4 | 12.7 | 33 |
| Fowler 2009 | Standard care | 13.6 | 10.6 | 38 |
| Garety 2008 | CBT + standard care | 16.26 | 13.3075 | 103 |
| Garety 2008 | Standard care | 16.4155 | 13.4444 | 104 |
| Rector 2003 | CBT + standard care | 12.0 | 7.8 | 21 |
| Rector 2003 | Standard care | 12.7 | 11.8 | 13 |
| CDS ‐ long term | ||||
| Barrowclough 2014 | CBT + standard care | 7.0918 | 5.4566 | 49 |
| Barrowclough 2014 | Standard care | 5.9 | 5.0 | 21 |
| Birchwood 2014 | CBT + standard care | 12.44 | 6.33 | 98 |
| Birchwood 2014 | Standard care | 11.75 | 5.68 | 99 |
| Jackson 2009 | CBT + standard care | 3.7 | 3.9 | 36 |
| Jackson 2009 | Standard care | 3.9 | 3.3 | 30 |
| HADS ‐ long term | ||||
| Farhall 2009 | CBT + standard care | 6.71 | 4.54 | 45 |
| Farhall 2009 | Standard care | 6.57 | 4.81 | 47 |
| HAMD ‐ long term | ||||
| Granholm 2005 | CBT + standard care | 9.7 | 5.5 | 31 |
| Granholm 2005 | Standard care | 11.3 | 6.8 | 33 |
1.33 Mental state: 11a. Specific ‐ self‐esteem (average endpoint score ‐ various scales, high = good)
Ten trials reported self‐esteem scores from various scales (Analysis 1.33).
1.33. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 33 Mental state: 11a. Specific ‐ self esteem (average endpoint score various scales, high = good).
1.33.1 RSES ‐ short term
Gumley 2003 used the RSES scale to measure self‐esteem. At short‐term follow‐up, there was no clear difference between the treatment groups (MD 0.40, 95% CI ‐1.43 to 2.23; participants = 144; study = 1). This result remained the same when using a fixed‐effect model.
1.33.2 SES ‐ short term
Qin 2014a and Yao 2015 used the SES to measure self‐esteem. At short‐term follow‐up, there was a clear difference between treatment groups, favouring CBT plus standard care (MD 3.29, 95% CI 2.43 to 4.16; participants = 188; studies = 2). This result remained the same when using a fixed‐effect model.
1.33.3 RSCQ ‐ short term
England 2007 and Freeman 2014 used the RSCQ to measure self‐esteem. At short‐term follow‐up, there was a clear difference between the two treatment groups (MD 8.29, 95% CI ‐0.08 to 16.66; participants = 95; studies = 2). This result remained the same when using a fixed‐effect model.
1.33.4 GSES ‐ short term
Ma 2016 used the GSES to measure self‐esteem. At short‐term follow‐up, there was a clear difference between treatment groups, favouring CBT plus standard care (MD 1.48 95% CI 0.05 to 2.91; participants = 190; study = 1). This result remained the same when using a fixed‐effect model.
1.33.5 RSES ‐ medium term
Gumley 2003 reported medium‐term data from the RSES. There was not a clear difference between treatment groups (MD 0.90, 95% CI ‐0.79 to 2.59; participants = 144; study = 1). This result remained the same when using a fixed‐effect model.
1.33.6 RSCQ ‐ medium term
Jackson 2009 reported medium‐term data from the RSCQ. There was not a clear difference between treatment groups (MD 0.40, 95% CI ‐7.46 to 8.26; participants = 66; study = 1). This result remained the same when using a fixed‐effect model.
1.33.7 SERS ‐ medium term
Tarrier 2014 used the SERS to measure self‐esteem. At medium‐term follow‐up, there was a clear difference between treatment groups, favouring CBT plus standard care (MD 16.9, 95% CI 1.25 to 32.55; participants = 35; study = 1). This result remained the same when using a fixed‐effect model.
1.33.8 GSES ‐ medium term
Lu 2014 reported medium‐term data from the GSES. There was a clear difference between treatment groups, favouring CBT plus standard care (MD 0.76, 95% CI 0.53 to 0.99; participants = 104; study = 1). This result remained the same when using a fixed‐effect model.
1.33.9 RSES ‐ long term
Farhall 2009 and Gumley 2003 reported long‐term data from the RSES. There was no clear difference between treatment groups (MD ‐0.33, 95% CI ‐1.79 to 1.14; participants = 236; studies = 2). This result remained the same when using a fixed‐effect model.
1.33.10 RSCQ ‐ long term
England 2007 and Jackson 2009 reported long‐term data from the RSCQ. There was no clear difference between treatment groups (MD 6.23, 95% CI ‐8.56 to 21.03; participants = 131; studies = 2). For this outcome, heterogeneity was high (Chi2 = 6.05; df = 1; P = 0.01; I2 = 83%). We failed to find the source of the heterogeneity.
1.34 Mental state: 11b. Specific ‐ self‐esteem (average endpoint score various scales) ‐ short term (skewed data)
These data are presented in Other data tables because of a marked skew in the data which makes it difficult to interpret the findings (Analysis 1.34).
1.34. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 34 Mental state: 11b. Specific ‐ self esteem (average endpoint score various scales) ‐ short term (skewed data).
| Mental state: 11b. Specific ‐ self esteem (average endpoint score various scales) ‐ short term (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| SCS (high = good) | ||||
| Freeman 2014 | CBT + standard care | 754.8 | 406.6 | 15 |
| Freeman 2014 | Standard care | 636.0 | 311.9 | 15 |
| positive self ‐ BCSS (high = good) | ||||
| Freeman 2014 | CBT + standard care | 7.3 | 5.4 | 15 |
| Freeman 2014 | Standard care | 6.3 | 4.8 | 15 |
| negative self ‐ BCSS (high = poor) | ||||
| Freeman 2014 | CBT + standard care | 7.6 | 5.3 | 15 |
| Freeman 2014 | Standard care | 8.1 | 5.6 | 15 |
1.35 Mental state: 12a. Specific ‐ insight (average endpoint score various scales, high = good)
Four trials reported insight scores using various scales (Analysis 1.35).
1.35. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 35 Mental state: 12a. Specific ‐ insight (average endpoint score various scales, high = good).
1.35.1 ITAQ ‐ short term
Zhang 2014 used the ITAQ to measure insight. At the short term, there was a clear difference between treatment groups, favouring CBT plus standard care (MD 4.92, 95% CI 3.19 to 6.65; participants = 75; study = 1).
1.35.2 BCIS ‐ medium term
Granholm 2005 used the BCIS to measure insight. At medium‐term follow‐up, there was no clear difference between treatment groups (MD 1.33, 95% CI ‐1.24 to 3.90; participants = 65; study = 1).
1.35.3 ITAQ ‐ medium term
Startup 2004 used the ITAQ to measure insight. At medium‐term follow‐up, there was no clear difference between treatment groups (MD ‐0.10, 95% CI ‐2.97 to 2.77; participants = 74; study = 1).
1.35.4 SRIS ‐ long term
Farhall 2009 used the SRIS to measure insight. At the long term, there was not a clear difference between treatment groups (MD 0.02, 95% CI ‐1.3 to 1.34; participants = 92; study = 1).
1.35.5 BCIS ‐ long term
Granholm 2005 reported long‐term scores from the BCIS. There was no clear difference between the two treatments (MD ‐0.54, 95% CI ‐3.7 to 2.62; participants = 64; study = 1).
1.35.6 ITAQ ‐ long term
Startup 2004 reported long‐term scores from the ITAQ.. There was not a clear difference between the treatment groups (MD 0.40, 95% CI ‐2.2 to 3.0; participants = 74; study = 1).
1.36 Mental state: 12b. Specific ‐ insight (average endpoint score SAI, high = good)
One trial reported short‐term SEs for this outcome and two other trials reported mean and SD. We used the RevMan calculator to convert relevant data presented in Table 8 into SE for use in Analysis 1.36.
7. Data added into generic inverse variance outcome (1.36).
| CBT group | Standard care group | |||||
| Short term | Mean | SD | N | Mean | SD | N |
| Guo 2015 | 9.4 | 3.0 | 32 | 7.0 | 3.7 | 32 |
| Naeem 2015 | 11.2 | 4.1 | 53 | 9.2 | 4.1 | 49 |
| Total | 85 | 81 | ||||
| Medium term | Mean | SD | N | Mean | SD | N |
| Guo 2015 | 9.4 | 3.5 | 32 | 7.8 | 3.9 | 32 |
| Total | 32 | 32 | ||||
| Long term | Mean | SD | N | Mean | SD | N |
| Guo 2015 | 10.4 | 3.6 | 32 | 7.5 | 4.3 | 32 |
| Total | 32 | 32 | ||||
1.36. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 36 Mental state: 12b. Specific ‐ insight (average endpoint score SAI, high = good) ‐ short term.
1.36.1 Short term
A favourable effect for CBT was found at the short term (MD 6.50, 95% CI 5.84 to 7.16; studies = 3).
1.36.2 Medium term
No clear difference between treatment groups was observed at the medium term (MD 1.60, 95% CI ‐0.19 to 3.39; studies = 1).
1.36.3 Long term
A favourable effect for CBT was found at the long term (MD 2.90, 95% CI 0.96 to 4.84; studies = 1)
1.37 Mental state: 12c. Specific ‐ insight (average change score SAI, high = good) ‐ medium term
Tuikington 2002 reported SAI change scores for insight. At medium‐term follow‐up, there was no clear difference between treatment groups (MD ‐0.69, 95% CI ‐1.41 to 0.03; participants = 336; study = 1) (Analysis 1.37).
1.37. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 37 Mental state: 12c. Specific ‐ insight (average change score SAI, high = good).
1.38 Mental state: 13. Specific ‐ well‐being (average endpoint WEMW score, high = good) ‐ short term
Freeman 2014 and Freeman 2015 reported short‐term scores for well being. There was a favourable effect for CBT plus standard care (MD 4.08, 95% CI 0.90 to 7.26; participants = 170; studies = 2) (Analysis 1.38).
1.38. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 38 Mental state: 13. Specific ‐ well‐being (average endpoint score WEMWS, high = good) ‐ short term.
1.39 Mental state: 14a. Specific ‐ various other symptoms (average endpoint score, high = poor)
Six trials reported on a variety of other specific mental state symptoms, using various scales (Analysis 1.39).
1.39. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 39 Mental state: 14a. Specific ‐ various other symptoms (average endpoint score various scales high = poor).
1.39.1 Psychotic symptoms ‐ SCL‐90 ‐ short term
Zhang 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐0.58, 95% CI ‐0.72 to ‐0.44; participants = 90; study = 1).
1.39.2 Somatisation ‐ SCL‐90 ‐ short term
Zhang 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐1.86, 95% CI ‐1.98 to ‐1.74; participants = 90; study = 1).
1.39.3 Sensitivity of interpersonal relationship ‐ SCL‐90 ‐ short term
Zhang 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐1.1, 95% CI ‐1.19 to ‐1.01; participants = 90; study = 1).
1.39.4 Obsessive‐compulsive disorder ‐ SCL‐90 ‐ short term
Zhang 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐1.29, 95% CI ‐1.36 to ‐1.22; participants = 90; study = 1).
1.39.5 Hostility ‐ SCL‐90 ‐ short term
Zhang 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐0.84, 95% CI ‐1.0 to ‐0.68; participants = 90; study = 1).
1.39.6 Phobia ‐ SCL‐90 ‐ short term
Zhang 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐0.51, 95% CI ‐0.61 to ‐0.41; participants = 90; study = 1).
1.39.7 Paranoia ‐ SCL‐90 ‐ short term
Zhang 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐0.7, 95% CI ‐0.8 to ‐0.6; participants = 90; study = 1).
1.39.8 Paranoia ‐ GPTS ‐ short term
Freeman 2014 and Freeman 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐13.32, 95% CI ‐22.97 to ‐3.68; participants = 170; studies = 2). For this outcome, heterogeneity was low (Chi2 = 0.01; df = 1; P = 0.93; I2 = 0%).
1.39.9 Worry ‐ PSWQ ‐ short term
Freeman 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐3.70, 95% CI ‐7.12 to ‐0.28; participants = 141; study = 1).
1.39.10 Rumination ‐ PTQ ‐ short term
Freeman 2015 reported data for this outcome. There was a favourable effect for CBT plus standard care (MD ‐5.40, 95% CI ‐8.96 to ‐1.84; participants = 135; study = 1).
1.39.11 Hopelessness ‐ BHS ‐ medium term
Birchwood 2014 and Tarrier 2014 reported data for this outcome. There was no clear difference between treatment groups (MD ‐0.56, 95% CI ‐1.93 to 0.82; participants = 232; studies = 2).
1.39.12 Hopelessness ‐ BHS ‐ long term
Birchwood 2014 and Fowler 2009 reported data for this outcome. There was no clear difference between treatment groups (MD 0.74, 95% CI ‐0.54 to 2.01; participants = 268; studies = 2).
1.40 Mental state: 14b. Specific ‐ various other symptoms (average endpoint score SCL‐90, high = poor) ‐ long term
Li 2015a reported on a variety of other specific mental state symptoms, using the SCL‐90, however these data were skewed and were presented as 'Other data' (Analysis 1.40).
1.40. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 40 Mental state: 14b. Specific ‐ various other symptoms (average endpoint score SCL‐90, high = poor) ‐ long term (skewed data).
| Mental state: 14b. Specific ‐ various other symptoms (average endpoint score SCL‐90, high = poor) ‐ long term (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| anxiety | ||||
| Li 2015a | CBT + standard care | 0.57 | 0.24 | 48 |
| Li 2015a | Standard care | 0.69 | 0.56 | 44 |
| depression | ||||
| Li 2015a | CBT + standard care | 0.66 | 0.40 | 48 |
| Li 2015a | Standard care | 0.66 | 0.69 | 44 |
| psychotic symptom | ||||
| Li 2015a | CBT + standard care | 0.38 | 0.24 | 48 |
| Li 2015a | Standard care | 0.46 | 0.25 | 44 |
| somatization | ||||
| Li 2015a | CBT + standard care | 0.73 | 0.40 | 48 |
| Li 2015a | Standard care | 0.70 | 0.45 | 44 |
| sensitivity of interpersonal relationship | ||||
| Li 2015a | CBT + standard care | 0.47 | 0.28 | 48 |
| Li 2015a | Standard care | 0.62 | 0.43 | 44 |
| obsessive‐compulsiive | ||||
| Li 2015a | CBT + standard care | 0.50 | 0.19 | 48 |
| Li 2015a | Standard care | 0.59 | 0.41 | 44 |
| hostility | ||||
| Li 2015a | CBT + standard care | 0.60 | 0.33 | 48 |
| Li 2015a | Standard care | 0.63 | 0.51 | 44 |
| paranoid | ||||
| Li 2015a | CBT + standard care | 0.30 | 0.20 | 48 |
| Li 2015a | Standard care | 0.34 | 0.30 | 44 |
| phobia | ||||
| Li 2015a | CBT + standard care | 0.23 | 0.27 | 48 |
| Li 2015a | Standard care | 0.56 | 0.57 | 44 |
1.41 Adverse effect/event(s): 1a. General ‐ any adverse event
Li 2014 and Pan 2012 reported incidence of 'any adverse event'. There was a clear difference between treatment groups with fewer reports of adverse events in the CBT plus standard care groups (RR 0.44, 95% CI 0.27 to 0.72; participants = 146; studies = 2; very low‐quality evidence). This result was presented in the SOF table as an outcome of importance. The quality of evidence was very low due to serious risk of bias of the included studies, very small sample size, and a very low number of events (Analysis 1.41).
1.41. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 41 Adverse effect/event(s): 1a. General ‐ any adverse event.
1.42 Adverse effect/event(s): 1b. General (average total endpoint score TESS, high = poor) ‐ medium term
Chen 2015 and Qiu 2014b reported TESS scores. At medium‐term follow‐up, there was no clear difference between treatment groups (MD 0.24, 95% CI ‐1.43 to 1.9; participants = 109; studies = 2, Analysis 1.42).
1.42. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 42 Adverse effect/event(s): 1b. General (average total endpoint score TESS, high = poor) ‐ medium term.
1.43 Adverse effect/event(s): 2a. Specific ‐ various effects
Li 2014 reported incidence of various specific adverse effects (Analysis 1.43).
1.43. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 43 Adverse effect/event(s): 2a. Specific ‐ various effects.
1.43.1 Drowsiness
There was no clear difference between treatment groups for this outcome (RR 0.53, 95% CI 0.05 to 5.57; participants = 78; study = 1).
1.43.2 Headache
There was no clear difference between treatment groups for this outcome (RR 1.05, 95% CI 0.07 to 16.24; participants = 78; study = 1).
1.43.3 Mild lactation
There was no clear difference between treatment groups for this outcome (RR 0.15, 95% CI 0.01 to 2.81; participants = 78; study = 1).
1.43.4 Opsomenorrhea
There was no a clear difference between treatment groups for this outcome (RR 0.21, 95% CI 0.01 to 4.24; participants = 78; study = 1).
1.44. Adverse effect/event(s): 2b. Specific ‐ suicide attempt
Garety 2008 and Grawe 2006 reported suicide attempts. There was no clear difference between the CBT plus standard care and standard care groups (RR 1.84, 95% CI 0.84 to 4.04; participants = 323; studies = 2) (Analysis 1.44).
1.44. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 44 Adverse effect/event(s): 2b. Specific ‐ suicide attempt.
1.45 Adverse effect/event(s): 2c. Specific ‐ death
1.45.1 Any cause
Nine trials reported deaths (any cause). There was no clear difference between the CBT plus standard care and standard care groups (RR 0.78, 95% CI 0.38 to 1.58; participants = 1341; studies = 9) (Analysis 1.45).
1.45. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 45 Adverse effect/event(s): 2c. Specific ‐ death.
1.46 Functioning: 1. General (average endpoint score GAF, high = good)
Six trials reported GAF general functioning scores (Analysis 1.46).
1.46. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 46 Functioning: 1. General (average endpoint score GAF, high = good).
1.46.1 Short term
Barrowclough 2014 reported short‐term data. There was no clear difference between treatment groups (MD ‐0.68, 95% CI ‐5.82 to 4.47; participants = 72; study = 1). The result remained the same when using a fixed‐effect model.
1.46.2 Medium term
Five trials reported medium‐term data. There was no clear difference between treatment groups (MD 3.37, 95% CI ‐1.66 to 8.41; participants = 482; studies = 5). For this outcome heterogeneity was high (Chi2 = 11.12; df = 4; P = 0.03; I2 = 64%). The statistical heterogeneity was not significant after removing Startup 2004 from the meta‐analysis (MD 1.19, 95% CI ‐3.02 to 5.40). Accordingly, the treatment effect was robust to heterogeneity amongst the studies.
1.46.3 Long term
Five trials reported long‐term data. There was no clear difference between treatment groups (MD 1.79, 95% CI ‐1.95 to 5.53; participants = 446; studies = 5). For this subgroup, heterogeneity was moderately high (Chi2 = 8.13; df = 4.0; P = 0.09; I2 = 51%). The statistical heterogeneity was not significant after removing Startup 2004 from the meta‐analysis (MD ‐0.15, 95% CI ‐2.38 to 2.07). Accordingly, the treatment effect was robust to heterogeneity amongst the studies.
1.47 Functioning: 2a. Social (average endpoint ILSS, high = good)
Granholm 2005 reported data for social functioning using the ILSS (Analysis 1.47).
1.47. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 47 Functioning: 2a. Social (average endpoint score ILSS, high = good).
1.47.1 Medium term
No clear difference between treatment groups was observed at the medium term (MD 0.04, 95% CI ‐0.02 to 0.09; participants = 61; studies = 1).
1.47.2 Long term
No clear difference between treatment groups was observed at the long term (MD 0.05, 95% CI 0.00 to 0.11; participants = 63; studies = 1).
1.48 Functioning: 2b. Social (average endpoint SFS, high = good)
Two trials (Barrowclough 2001 and Startup 2004) reported social functioning data from the SFS (Analysis 1.48).
1.48. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 48 Functioning: 2b. Social (average endpoint score SFS, high = good).
1.48.1 Medium term
A clear difference between treatment groups, favouring CBT plus standard care, was observed at the medium term (MD 7.23, 95% CI 2.91 to 11.55; participants = 92; studies = 2).
1.48.2 Long term
A clear difference between treatment groups, favouring CBT plus standard care, was observed at the long term (MD 6.88, 95% CI 1.99 to 11.76; participants = 103; studies = 2).
1.49 Functioning: 2c. Social (average endpoint SOFAS, high = good)
Four trials reported social functioning data using the SOFAS (Analysis 1.49).
1.49. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 49 Functioning: 2c. Social (average endpoint score SOFAS, high = good).
1.49.1 Short term
Edwards 2011 reported short‐term data. No clear difference between treatment groups was observed (MD 0.98, 95% CI ‐4.40 to 6.36; participants = 48; studies = 1).
1.49.1 Medium term
Gleeson 2009 reported medium‐term data. No clear difference between treatment groups was observed (MD ‐1.00, 95% CI ‐8.02 to 6.02; participants = 81; studies = 1).
1.49.2 Long term
Fowler 2009 and Garety 2008 reported long‐term data. No clear difference between treatment groups was observed (MD 0.56, 95% CI ‐2.64 to 3.76; participants = 295; studies = 2; very low‐quality evidence). This result was presented as an 'important outcome' in the SOF table. The quality of evidence for this result was very low due to high risk of bias across several domains, a low number of studies with a low sample size, and low number of events contributing data. These data are also scale data and did not measure a clinically important change in social functioning.
1.50 Functioning: 2d. Social (average endpoint PSP, high = good)
Two trials (Guo 2015; Wang 2015) reported social functioning data from PSP at the short, medium and long term (Analysis 1.50).
1.50. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 50 Functioning: 2d. Social (average endpoint score PSP, high = good).
1.50.1 Short term
A clear difference between treatment groups, favouring CBT plus standard care, was observed at the short term (MD 7.96, 95% CI 3.15 to 12.78; participants = 92; studies = 2).
1.50.2 Medium term
A clear difference between treatment groups, favouring CBT plus standard care, was observed at the medium term (MD 7.23, 95% CI 2.91 to 11.55; participants = 92; studies = 2).
1.50.3 Long term
A clear difference between treatment groups, favouring CBT plus standard care, was observed at the long term (MD 12.66, 95% CI 8.65 to 16.67; participants = 92; studies = 2).
1.51 Functioning: 2e. Social (average endpoint UPSA, high = good)
Granholm 2005 reported social functioning data using the UPSA (Analysis 1.51).
1.51. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 51 Functioning: 2e. Social (average endpoint score UPSA, high = good).
1.51.1 Medium term
No clear difference between treatment groups was observed at the medium term (MD ‐0.01, 95% CI ‐0.10 to 0.08; participants = 64; studies = 1).
1.51.2 Long term
No clear difference between treatment groups was observed at the long term (MD 0.02, 95% CI ‐0.07 to 0.11; participants = 58; studies = 1).
1.52 Functioning: 3. Life skills (average endpoint score LSP, high = poor)
1.52.1 Long term
Farhall 2009 reported endpoint scores at the long term for life skills using the LSP. No clear difference between treatment groups was observed (MD ‐3.32, 95% CI ‐8.40 to 1.76; participants = 92; studies = 1) (Analysis 1.52).
1.52. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 52 Functioning: 3. Life skills (average endpoint score LSP, high = poor).
1.53 Functioning: 4a. Cognitive ‐ overall (average total endpoint score WCST, high = poor)
Li 2015 reported cognitive functioning data from the WCST (Analysis 1.53).
1.53. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 53 Functioning: 4a. Cognitive ‐ overall (average total endpoint score WCST, high = poor).
1.53.1 Medium term
No clear difference between treatment groups was observed at the medium term (MD ‐0.30, 95% CI ‐8.89 to 8.29; participants = 100; studies = 1).
1.53.2 Long term
A clear difference between treatment groups, favouring CBT plus standard care, was observed at the long term (MD ‐9.80, 95% CI ‐17.76 to ‐1.84; participants = 100; studies = 1).
1.54 Functioning: 4b. Cognitive ‐ memory (average endpoint score WMS, high = good)
1.54.1 Short term
Sun 2014 reported short‐term data from the WMS, and a favourable effect for CBT plus standard care was observed (MD 9.33, 95% CI 1.54 to 17.12; participants = 100; studies = 1).
1.55 Functioning: 4c. Cognitive ‐ memory (average endpoint score CMS, high = good)
Li 2015 reported scores from the CMS (Analysis 1.55).
1.55. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 55 Functioning: 4c. Cognitive ‐ memory (average endpoint score CMS, high = good).
1.55.1 Medium term
No clear difference between treatment groups was observed at the medium term (MD 0.40, 95% CI ‐7.42 to 8.22; participants = 100; studies = 1).
1.55.2 Long term
No clear difference between treatment groups was observed at the long term (MD 0.90, 95% CI ‐6.24 to 8.04; participants = 100; studies = 1).
1.56 Functioning: 4d. Congitive ‐ various (average endpoint score MCCB, high = poor) ‐ medium term
Hu 2013 reported medium‐term MCCB scores for various aspects of cognitive functioning (Analysis 1.56).
1.56. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 56 Functioning: 4d. Cognitive ‐ various (average endpoint score MCCB, high = poor) ‐ medium term.
1.56.1 Continuous performance
A favourable effect for CBT plus standard care was observed (MD ‐44.10, 95% CI ‐52.40 to ‐35.80; participants = 79; studies = 1).
1.56.2 Mood management
A favourable effect for CBT plus standard care was observed (MD ‐1.60, 95% CI ‐2.15 to ‐1.05; participants = 79; studies = 1).
1.56.3 Sematic influencing
A favourable effect for CBT plus standard care was observed (MD ‐2.40, 95% CI ‐4.63 to ‐0.17; participants = 79; studies = 1).
1.56.4 Verbal memory
A favourable effect for CBT plus standard care was observed (MD ‐2.80, 95% CI ‐5.06 to ‐0.54; participants = 79; studies = 1).
1.56.5 Visual memory
No clear difference between treatment groups was observed (MD ‐2.60, 95% CI ‐5.64 to 0.44; participants = 79; studies = 1).
1.57 Functioning: 5. Intelligence (average endpoint score WAIS, high = good)
Sun 2014 reported WAIS 'intelligence' scores (Analysis 1.57).
1.57. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 57 Functioning: 5. Intelligence (average endpoint score WAIS, high = good).
1.57.1 Short term
At the short term, no clear difference between treatment groups was observed (MD 4.89, 95% CI ‐2.43 to 12.21; participants = 100; studies = 1).
1.57.2 Medium term
At the medium term, a clear effect, favouring CBT plus standard care was observed (MD 11.83, 95% CI 9.27 to 14.39; participants = 80; studies = 1).
1.58 Functioning: 6. Disability (SE data, WHODAS, high = poor)
Naeem 2016 reported SE from the WHODAS and found a clear difference between treatment groups, favouring CBT plus standard care ( (MD ‐10.52, 95% CI ‐14.65 to ‐6.39; studies = 1) (Analysis 1.58).
1.58. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 58 Functioning: 6. Disability (average endpoint score WHODAS, high = poor).
1.59 Quality of life: 1a. General (average total endpoint score various scales, high = good) ‐ short term
Two trials reported scores for general quality of life at short‐term follow‐up (Analysis 1.59).
1.59. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 59 Quality of life: 1a. General (average total endpoint score various scales, high = good) ‐ short term.
1.59.1 QLS
Edwards 2011 reported scores from QLS. No clear difference between treatment groups was observed (MD ‐1.90, 95% CI ‐10.63 to 6.83; participants = 48; studies = 1).
1.59.2 WHOQOL‐BREF
Wang 2015 reported scores from WHOQOL‐BREF. No clear difference between treatment groups was observed (MD 6.64, 95% CI ‐1.36 to 14.64; participants = 28; studies = 1).
1.60 Quality of life: 1b. General (average total endpoint score various scales, high = good) ‐ medium term
1.60.1 WHOQOL‐BREF
Wang 2015 also reported medium term scores from WHOQOL‐BREF. A clear difference between treatment groups, favouring CBT plus standard care, was observed (MD 8.20, 95% CI 0.66 to 15.74; participants = 28; studies = 1).
1.61 Quality of life: 1c. General (average total endpoint score various scales, high = good) ‐ long term
Four trials reported long‐term scores from various scales for general quality of life (Analysis 1.61).
1.61. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 61 Quality of life: 1c. General (average total endpoint score various scales, high = good) ‐ long term.
1.61.1 QLS
Fowler 2009 used the QLS. No clear difference between treatment groups was observed (MD ‐3.60, 95% CI ‐11.32 to 4.12; participants = 71; studies = 1; very low‐quality evidence).This result was used in the SOF table but given a grading of very low‐quality evidence due to very small sample size and the data reported were not clinically important changes in quality of life.
1.61.2 GQOLI‐74
Cao 2014 reported GQOLI‐74 scores. A favourable effect for CBT plus standard care was observed (MD 2.82, 95% CI 1.62 to 4.02; participants = 80; studies = 1).
1.61.3 WHOQOL‐BREF
Wang 2015 reported WHOQOL‐BREF scores. A favourable effect for CBT plus standard care was observed (MD 8.85, 95% CI 1.01 to 16.69; participants = 28; studies = 1).
1.61.4 EuroQOL
Garety 2008 reported EuroQOL scores. No clear difference between treatment groups was observed (MD ‐4.50, 95% CI ‐10.65 to 1.65; participants = 190; studies = 1).
1.62 Quality of life: 1d. General (average total endpoint score SQLS, high = poor)
1.62.1 Medium term
Lu 2014 reported SQLS scores. A favourable effect for CBT plus standard care was observed (MD ‐29.50, 95% CI ‐40.28 to ‐18.72; participants = 104; studies = 1) (Analysis 1.62).
1.62. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 62 Quality of life: 1d. General (average total endpoint score SQLS, high = poor).
1.63 Quality of life: 2a. Specific ‐ physical (average endpoint score WHQOL‐BREF, high = good)
Two trials reported data for physical quality of life using WHQOL‐BREF (Analysis 1.63).
1.63. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 63 Quality of life: 2a. Specific ‐ physical (average endpoint score WHOQOL‐BREF, high = good).
1.63.1 Short term
Wang 2015 reported short‐term scores. No clear difference between treatment groups was observed (MD 1.71, 95% CI ‐1.01 to 4.43; participants = 28; studies = 1).
1.63.2 Medium term
Gleeson 2009 and Wang 2015 reported medium‐term scores. A favourable effect for CBT plus standard care was observed (MD 2.60, 95% CI 0.20 to 5.00; participants = 109; studies = 2).
1.63.3 Long term
Wang 2015 reported long‐term scores. A favourable effect for CBT plus standard care was observed (MD 2.71, 95% CI 0.11 to 5.31; participants = 28; studies = 1).
1.64 Quality of life: 2b. Specific ‐ physical (average endpoint score GQOLI‐74, high = good)
1.64.1 Long term
Cao 2014 reported GQOLI‐74 scores and observed a favourable effect for CBT plus standard care at the long term (MD 13.69, 95% CI 9.62 to 17.76; participants = 80; studies = 1).
1.65 Quality of life: 3a. Specific ‐ psychological (average endpoint score WHQOL‐BREF, high = good)
Two trials reported psychological scores from the WHQOL‐BREF (Analysis 1.65).
1.65. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 65 Quality of life: 3a. Specific ‐ psychological (average endpoint score WHOQOL‐BREF, high = good).
1.65.1 Short term
Wang 2015 reported short‐term scores. A favourable effect for CBT plus standard care was observed (MD 2.22, 95% CI 0.28 to 4.16; participants = 28; studies = 1).
1.65.2 Medium term
Gleeson 2009 and Wang 2015 reported medium‐term scores. A favourable effect for CBT plus standard care was observed (MD 2.52, 95% CI 0.71 to 4.33; participants = 109; studies = 2).
1.65.3 Long term
Wang 2015 reported long‐term scores. A favourable effect for CBT plus standard care was observed (MD 2.37, 95% CI 0.56 to 4.18; participants = 28; studies = 1).
1.66 Quality of life: 3b. Specific ‐ psychological (average endpoint score GQOL‐74, high = good)
1.66.1 Long term
Cao 2014 reported long‐term psychological scores from the GQOL‐74. A favourable effect for CBT plus standard care was observed (MD 17.03, 95% CI 13.07 to 20.99; participants = 80; studies = 1) (Analysis 1.66).
1.66. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 66 Quality of life: 3b. Specific ‐ psychological (average endpoint score GQOL‐74, high = good).
1.67 Quality of life: 3c. Specific ‐ psychological (average endpoint score SQLS, high = poor)
1.67.1 Medium term
Chen 2015 used the SQLS and reported medium‐term psychological scores. No clear difference between treatment groups was observed (MD ‐1.26, 95% CI ‐5.19 to 2.67; participants = 50; studies = 1) (Analysis 1.67).
1.67. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 67 Quality of life: 3c. Specific ‐ psychological (average endpoint score SQLS, high = poor).
1.68 Quality of life: 4a. Specific ‐ various other aspects (average endpoint score WHQOL‐BREF, high = good) ‐ short term
Wang 2015 reported short‐term WHQOL‐BREF scores for various other aspects of quality of life (Analysis 1.68).
1.68. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 68 Quality of life: 4a. Specific ‐ various other aspects (average endpoint score WHQOL‐BREF, high = good) ‐ short term.
1.68.1 Environment
No clear difference between treatment groups was observed (MD 1.82, 95% CI ‐1.71 to 5.35; participants = 28; studies = 1).
1.68.2 Social relationship
No clear difference between treatment groups was observed (MD 0.87, 95% CI ‐0.62 to 2.36; participants = 28; studies = 1).
1.69 Quality of life: 4b. Specific ‐ various other aspects (average endpoint score various scales, high = good) ‐ medium term
Three trials reported medium‐term scores from WHOQOL‐BREF and SF‐36 for various other aspects of quality of life (Analysis 1.69).
1.69. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 69 Quality of life: 4b. Specific ‐ various other aspects (average endpoint score various scales, high = good) ‐ medium term.
1.69.1 Environment (WHOQOL‐BREF)
Gleeson 2009 and Wang 2015 reported WHOQOL‐BREF environment scores. No clear difference between treatment groups was observed (MD 2.56, 95% CI ‐0.21 to 5.34; participants = 109; studies = 2).
1.69.2 Physical functioning (SF‐36)
Liu 2012 reported SF‐36 physical functioning scores. A favourable effect for CBT plus standard care was observed (MD 22.30, 95% CI 17.65 to 26.95; participants = 89; studies = 1).
1.69.3 Role emotional (SF‐36)
Liu 2012 reported SF‐36 role emotional scores. A favourable effect for CBT plus standard care was observed (MD 26.90, 95% CI 19.74 to 34.06; participants = 89; studies = 1).
1.69.4 Role Physcial (SF‐36)
Liu 2012 reported SF‐36 role physical scores. A favourable effect for CBT plus standard care was observed (MD 31.20, 95% CI 25.94 to 36.46; participants = 89; studies = 1).
1.69.5 Social Relationship (WHOQOL‐BREF)
Gleeson 2009 and Wang 2015 reported WHOQOL‐BREF social relationship scores. No clear difference between treatment groups was observed (MD 0.90, 95% CI ‐0.60 to 2.39; participants = 109; studies = 2).
1.70 Quality of life: 4c. Specific ‐ various other aspects (average endpoint score various scales, high = good) ‐ long term
Two trials reported long‐term data on various other aspects of quality of life (Analysis 1.70).
1.70. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 70 Quality of life: 4c. Specific ‐ various other aspects (average endpoint score various scales, high = good) ‐ long term.
1.70.1 Environment (WHOQOL‐BREF)
Wang 2015 reported WHOQOL‐BREF environment scores. No clear difference between treatment groups was observed (MD 2.76, 95% CI ‐0.31 to 5.83; participants = 28; studies = 1).
1.70.2 Social function (GQOLI‐74)
Cao 2014 reported GQOLI‐74 social function scores. A favourable effect for CBT plus standard care was observed (MD 16.19, 95% CI 11.72 to 20.66; participants = 80; studies = 1).
1.70.3 Social relationship (WHOQOL‐BREF)
Wang 2015 reported WHOQOL‐BREF social relationship scores. No clear difference between treatment groups was observed (MD 1.02, 95% CI ‐0.55 to 2.59; participants = 28; studies = 1).
1.71 Quality of life: 4d. Specific ‐ various other aspects (average endpoint score various scales, high = poor) ‐ long term
Three trials used the SQLS and SDSS to report various other aspects of quality of life at long‐term follow‐up (Analysis 1.71).
1.71. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 71 Quality of life: 4d. Specific ‐ various aspects (average endpoint score various scales, high = poor) ‐ medium term.
1.71.1 Insight/treatment attitude (SQLS)
Chen 2015 reported SQLS insight/treatment attitude scores. A favourable effect for standard care was observed (MD 3.14, 95% CI 1.96 to 4.32; participants = 50; studies = 1).
1.71.2 Motivation/vitality (SQLS)
Chen 2015 and Lu 2014 reported SQLS motivation/vitality scores. A favourable effect for CBT plus standard care was observed (MD ‐3.43, 95% CI ‐5.45 to ‐1.40; participants = 154; studies = 2).
1.71.3 Social function (SDSS)
Qiu 2014b reported SDSS social function scores. A favourable effect for CBT plus standard care was observed (MD ‐1.51, 95% CI ‐2.34 to ‐0.68; participants = 59; studies = 1).
1.71.4 Symptoms/side effects (SQLS)
Chen 2015 reported SQLS symptoms/side effects scores. No difference between treatment groups was observed (MD ‐0.25, 95% CI ‐2.76 to 2.26; participants = 50; studies = 1).
1.72 Quality of life: 5a. Specific ‐ psychological (average endpoint score SQLS, high = poor) ‐ medium term data (skewed data)
Data for this outcome were presented as Other data because of marked skew (Analysis 1.72), which makes it difficult to interpret the findings.
1.72. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 72 Quality of life: 5a. Specific ‐ psychological (average endpoint score SQLS, high = poor) ‐ medium term (skewed data).
| Quality of life: 5a. Specific ‐ psychological (average endpoint score SQLS, high = poor) ‐ medium term (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| Lu 2014 | CBT + standard care | 17.5 | 13.2 | 52 |
| Lu 2014 | Standard care | 27.4 | 16.3 | 52 |
1.73 Quality of life: 5b. Specific ‐ role functioning (average endpoint score QLS, high = good) ‐ long term (skewed data)
Data for this outcome were presented as Other data because of marked skew (Analysis 1.73), which makes it difficult to interpret the findings.
1.73. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 73 Quality of life: 5b. Specific ‐ role functioning (average endpoint score QLS, high = good) ‐ long term (skewed data).
| Quality of life: 5b. Specific ‐ role functioning (average endpoint score QLS, high = good) ‐ long term (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| Fowler 2009 | CBT + standard care | 7.2 | 5.7 | 33 |
| Fowler 2009 | Standard care | 9 | 5.6 | 38 |
1.74 Quality of life: 5c. Specific ‐ symptoms/side effects (average endpoint score SQLS, high = poor) ‐ medium term (skewed data)
Data for this outcome were presented as Other data because of marked skew (Analysis 1.74), which makes it difficult to interpret the findings.
1.74. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 74 Quality of life: 5c. Specific ‐ symptoms/side effects (average endpoint score SQLS, high = poor) ‐ medium term (skewed data).
| Quality of life: 5c. Specific ‐ symptoms/side effects (average endpoint score SQLS, high = poor) ‐ medium term (skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Mean | SD | N |
| Lu 2014 | CBT + standard care | 13.5 | 10.8 | 52 |
| Lu 2014 | Standard care | 24.8 | 16.6 | 52 |
1.75 Satisfaction with treatment: 1. Leaving the study early ‐ for any reason
We were able to collect leaving the study data from 35 trials (Analysis 1.75).
1.75. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 75 Satisfaction with treatment: 1. Leaving the study early ‐ for any reason.
1.75.1 Short term
Short‐term data from 12 trials showed no clear difference between treatment groups (RR 1.02, 95% CI 0.77 to 1.35; participants = 1214; study = 12).
1.75.2 Medium term
Medium‐term data from 11 trials showed no clear difference between treatment groups (RR 0.91, 95% CI 0.74 to 1.11; participants = 1402; studies = 11).
1.75.3 Long term
Long‐term data from 19 trials showed no clear difference between treatment groups (RR 0.93, 95% CI 0.77 to 1.12; participants = 1945; studies = 19; moderate‐quality evidence). This result was presented in the SOF table and was graded as moderate quality as leaving the study early data are a 'proxy measure' for predicting satisfaction with treatment.
1.76 Engagement with services: 1a. Compliance to medication
Six trials reported numbers of participants compliant with medication (Analysis 1.76).
1.76. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 76 Engagement with services: 1a. Compliance to medication.
1.76.1 Short term
Cao 2014; Chen 2014; Zhang 2014; and Zou 2013 reported short‐term data. No clear difference between treatment groups was observed (RR 1.45, 95% CI 0.81 to 2.60; participants = 261; studies = 4). For this subgroup, heterogeneity was high (Chi2 = 38.64; df = 3.0; P = 0.0003; I2 = 81%). The statistical heterogeneity was still significant after leaving out trials one by one. We failed to find the source of the heterogeneity.
1.76.2 Medium term
Pan 2012 and Qiu 2014b reported medium‐term data. A favourable effect for CBT plus standard care was observed (RR 1.23, 95% CI 1.02 to 1.49; participants = 128; studies = 2). The result remained the same when using a fixed‐effect model.
1.76.3 Long term
Cao 2014 and Pan 2012 reported long‐term data. A favourable effect for CBT plus standard care was observed (RR 1.35, 95% CI 1.1 to 1.65; participants = 148; studies = 2). The result remained the same when using a fixed‐effect model (Chi2 = 0.63; df = 1.0; P = 0.43; I2 = 0%).
1.77 Engagment with services: 1b. Refusing treatment
Li 2013a and Wang 2012 reported the number of participants refusing treatment at the short term. No clear difference between treatment groups was observed (RR 0.49, 95% CI 0.18 to 1.38; participants = 190; studies = 2) (Analysis 1.77).
1.77. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 77 Engagement with services: 1b. Refusing treatment.
1.78 Engagement with services: 1c. Compliance with medication (average endpoint score MARS, high = good)
Two trials measured compliance with medication using MARS (Analysis 1.78).
1.78. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 78 Engagement with services: 1c. Compliance with medication (average endpoint score MARS, high = good).
1.78.1 Medium term
Gleeson 2009 reported medium‐term data. No clear difference between treatment groups was observed (MD ‐0.60, 95% CI ‐1.41 to 0.21; participants = 81; study = 1).
1.78.2 Long term
Qian 2012 reported long‐term data. A favourable effect for CBT plus standard care was observed (MD 38.02, 95% CI 33.48 to 42.56; participants = 90; study = 1).
2. Sensitivity analyses
Where relevant, we carried out sensitivity analyses.
2.1. Blinding
Sensitivity analysis showed that there was no substantial difference between results including trials with non‐blind outcome assessment and results excluding trials with non‐blind outcome assessment (Analysis 2.1; Analysis 2.2).
2.1. Analysis.
Comparison 2 SENSITIVITY ANALYSIS 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE (NON‐BLIND OUTCOME ASSESSMENT), Outcome 1 Global state: 1. Relapse ‐ long term.
2.2. Analysis.
Comparison 2 SENSITIVITY ANALYSIS 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE (NON‐BLIND OUTCOME ASSESSMENT), Outcome 2 Mental state: 1. General ‐ clinically important change (no improvement) ‐ long term.
2.3 Well‐defined CBT versus less‐well‐defined CBT
After removing trials with less‐well‐defined CBT, there was not a clear difference between CBT and standard care for relapse rate at the medium term (Analysis 3.1) and global state improvement at the long term (Analysis 3.3). No clear difference in results was found when trials with less‐well‐defined CBT were excluded from the meta‐analyses of the other primary outcomes (Analysis 3.2; Analysis 3.4).
3.1. Analysis.
Comparison 3 SENSITIVITY ANALYSIS 2: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS‐WELL DEFINED CBT), Outcome 1 Global state: 1. Relapse ‐ medium term.
3.3. Analysis.
Comparison 3 SENSITIVITY ANALYSIS 2: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS‐WELL DEFINED CBT), Outcome 3 Global state: 2. Clinically important change (no improvement) ‐ long term.
3.2. Analysis.
Comparison 3 SENSITIVITY ANALYSIS 2: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS‐WELL DEFINED CBT), Outcome 2 Global state: 1. Relapse ‐ long term.
3.4. Analysis.
Comparison 3 SENSITIVITY ANALYSIS 2: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS‐WELL DEFINED CBT), Outcome 4 Mental state: 1. General ‐ clinically important change (no improvement) ‐ long term.
2.4 Therapist experience
After removing trials with less experienced therapists of CBT, there was not a clear difference between CBT and standard care for global state improvement at the long term (Analysis 4.3) and mental state improvement at the short term (Analysis 4.4). No clear difference in results were found when trials with less experienced therapists are excluded from the meta‐analyses of the primary outcome (Analysis 4.1; Analysis 4.2; Analysis 4.5).
4.3. Analysis.
Comparison 4 SENSITIVITY ANALYSIS 3: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS EXPERIENCED THERAPIST), Outcome 3 Global state: 2. Clinically important change (no improvement) ‐ long term.
4.4. Analysis.
Comparison 4 SENSITIVITY ANALYSIS 3: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS EXPERIENCED THERAPIST), Outcome 4 Mental state: 1. General ‐ clinically important change (no improvement) ‐ short term.
4.1. Analysis.
Comparison 4 SENSITIVITY ANALYSIS 3: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS EXPERIENCED THERAPIST), Outcome 1 Global state: 1. Relapse ‐ medium term.
4.2. Analysis.
Comparison 4 SENSITIVITY ANALYSIS 3: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS EXPERIENCED THERAPIST), Outcome 2 Global state: 1. Relapse ‐ long term.
4.5. Analysis.
Comparison 4 SENSITIVITY ANALYSIS 3: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS EXPERIENCED THERAPIST), Outcome 5 Mental state: 1. General ‐ clinically important change (no improvement) ‐ long term.
2.5 Assumptions for lost binary data
No clear difference in results was found between results derived from completers only and results with an assumption for missing data (Analysis 5.1; Analysis 5.2).
5.1. Analysis.
Comparison 5 SENSITIVITY ANALYSIS 4: CBT+ STANDARD CARE versus STANDARD CARE ALONE (ASSUMATPION FOR MISSING DATA), Outcome 1 Global state: 1. Relapse ‐ long term.
5.2. Analysis.
Comparison 5 SENSITIVITY ANALYSIS 4: CBT+ STANDARD CARE versus STANDARD CARE ALONE (ASSUMATPION FOR MISSING DATA), Outcome 2 Mental state: 1. General ‐ clinically important change (no improvement) ‐ short term.
2.6. Imputed values
Only one trial was a cluster‐randomised controlled trial (Zou 2013), which contributed data for relapse at the short term. No clear difference in results was found when the trial with imputed values was excluded from the meta‐analyses of the primary outcome (Analysis 6.1).
6.1. Analysis.
Comparison 6 SENSITIVITY ANALYSIS 5: CBT+ STANDARD CARE versus STANDARD CARE ALONE (IMPUTED VALUES), Outcome 1 Global state: 1a. Relapse ‐ short term.
2.7. Fixed‐ and random‐effects
No substantive difference in results was found when we combined data using a random‐effects model for the primary outcome (Analysis 7.1).
7.1. Analysis.
Comparison 7 SENSITIVITY ANALYSIS 6: CBT+ STANDARD CARE versus STANDARD CARE ALONE (RANDOM EFFECT MODEL), Outcome 1 Global state: 2. Clinically important change (no improvement).
3. Subgroup analysis
3.1 Standard care including antipsychotics as opposed to standard care not including antipsychotics
In 60 trials, CBT was added to standard care where it was clear that standard care included prescription antipsychotics. For six trials (Fowler 2009; Gleeson 2009; Trower 2004; Tuikington 2002; Zhang 2015), it was not clear if antipsychotics were part of standard care during the trial period. No trial clearly stated that standard care did not include antipsychotics, so we were unable to conduct a subgroup analysis.
Discussion
Summary of main results
This review now includes 60 trials comparing CBT plus standard care with standard care alone. Prespecified outcomes were included in the Table 1 and, where those outcomes were not available, the finding that we judged closest to the first pre‐stipulated one was used.
1. Global state: Relapse ‐ long term; clinically important change
We chose to measure this outcome at the long term as we thought this was likely to be the most clinically important. Considerable investment is made within CBT therapy and any benefit, therefore, should be expected not to be transient. The trialists also seemed to concur with this by reporting data for the important outcome of 'relapse' over various but protracted periods that fell into our category of 'long term' (13 RCTs, 1538 people). We could only grade the evidence as 'low quality' and there is not a clear difference between groups (RR 0.78, 95% CI 0.61 to 1.00). However, no clear evidence of effect is not the same as evidence of no effect and these data are compatible with an important degree of relapse reduction across a year. However, taking the point estimate of these figures as correct, assuming more data would only increase the precision of the result, around 12 people would need to be treated in order to avoid one relapse. This could suggest that CBT may have something to offer over and above standard care, in unblinded trials, largely undertaken by those who were very skilled CBT practitioners ‐ but not that much to offer, and at considerable expense. The second global state outcome (clinically important change) was rarely reported (2 RCTs, 82 participants), and although this was more favourable for the CBT group, the quality of data had to be graded as 'very low'.
2. Mental state: General ‐ clinically important change
Although not statistically significant, this finding does concur with the global findings in that there is some support for CBT having some positive effect. The quality of evidence had to be graded as 'very low' for the reasons specified in the Table 1, and any effect, even if it should really exist, is modest. By now, with the maturity of this question and the trials being undertaken addressing this question, findings should really be of higher quality, and greater precision. Despite every effort to bring mental state measures together, the trials in this review reported on this outcome in 38 different ways. Many results based on endpoint or change scores did favour the CBT group. With a 'glass‐half‐full' approach, this could be seen as encouraging; with a 'glass‐half‐empty' approach, it could be seen as a wasteful chaos of measurement of outcomes of unclear clinical meaning, and opening opportunities for inclusion of bias.
3. Adverse events: General: any adverse event
Adverse effects of the talking therapies are rarely reported and talking therapy approaches can have adverse consequences (McMurran 2016); the studies included in this review are no exception to the poor reporting trend in this area of care. However, there are some data on adverse events and these very‐low quality data do favour the CBT groups (RR 0.44, 95% CI 0.27 to 0.72). This is important, albeit from very few data. If CBT does help people avoid adverse events ‐ such as self‐harm ‐ this might be further investigated but probably in qualitative work. Replicating this finding within more trials could be prohibitively expensive as the sample size would have be large, and the eventual investment for self‐harm saved painfully high.
4. Functioning: Social ‐ clinically important change in social functioning
We were surprised that no trial reported this outcome so we had to employ a proxy measure for the Table 1. Future trials could just ask a binary question to clarify this important issue. The average endpoint score on the relevant scale (SOFAS) was not clearly different between the groups. We are unclear of the clinical meaning of changes on the SOFAS, but scales tend to measure fine‐grained changes ‐ and even with that, no real effect was identified. Although all data were of very low quality, the fact that this important aspect of functioning is seemingly unaffected by CBT is a clinical disappointment. It would seem that this is a highly important outcome for the families of people with schizophrenia.
5. Quality of life: General ‐ clinically important change
The same issue applies to this outcome as it did for the functioning measure above. Surprisingly, we had to employ a proxy measure, this fine‐grained measure provided equivocal very low‐quality data, and again with no suggestion of any effect. It would seem likely that this is an outcome of major importance to people with schizophrenia.
6. Satisfaction with treatment: Leaving the study early ‐ long term
These studies were good at retaining people across the long term with < 20% leaving by around one year. There was no clear difference between the two groups (19 RCTs, 1945 people, moderate‐quality evidence). CBT seems not to be off‐putting to people ‐ but nor does it keep any more in care.
Overall completeness and applicability of evidence
1. Completeness
For the completeness of the evidence, the completeness of participants and interventions was good, but the outcomes were not. Reporting on outcomes of key importance was patchy, and only 28% of the included studies reported our primary outcomes. In addition, very few studies reported clinically important data on adverse events, function, and quality of life. There were no good economic data at all. Most data we do have was of low quality and we remain unsure of the effects of CBT for things so fundamental as people's quality of life. It is easy to say in hindsight but there appeared to be a chaos of reporting in these studies, leading to production of low‐quality evidence. This could be reduced by trialists agreeing together on the measures and timings that are clinically meaningful and relevant to clinicians and patients. The research waste evidenced in these studies is difficult to justify.
2. Applicability
There was a relatively complete representation of the population. The participants within the trials did seem 'recognisable' for everyday care. There was a wide age range, with a good gender balance and from inpatient and community settings. Ages ranged from 16 years to 78 years and a majority of the trials involved working age adults, which is also the most prevalent age of schizophrenia. There was a good gender balance and setting (inpatients and community patients) across all the included trials. The length of illness ranged from one month to 30.1 years, and most trials involved people who had the condition for over 12 months, hence the current evidence is more applicable to people with chronic schizophrenia. For most trials, the CBT intervention was not mixed with other active psychotherapies and for all trials, CBT was given in combination with the standard care intervention of the trial which typically included antipsychotic medication and psychiatric care.
However, about half of the trials (48%) employed qualified CBT therapists and only 15% supported less specialised staff delivering the CBT (other trials did not specify). We are therefore unclear about the effect of CBT delivered by 'usual' healthcare professionals and this is important in terms of applicability. Few mental healthcare services can afford an experienced and trained CBT therapist in addition to other staff.
Quality of the evidence
The current body of evidence available does not allow for robust conclusions regarding the effects of adding CBT to standard care for people with schizophrenia. This is mainly due to the risk of bias among included trials, imprecision of the effect estimate, and heterogeneity. Although the review included 60 trials, not all trials reported data on all prespecified outcomes, hence, often there are only a handful of small trials contributing data to any given outcome. Consequently, the pooled summary effects of a majority of outcomes is either with wide confidence intervals or below the threshold of optimal information size. The heterogeneity was substantial and we failed to explain the source of heterogeneity by sensitivity analysis.
Considering the maturity of trials in this area, the quality of evidence available is embarrassingly low. The veracity of the findings of the trials is threatened by biases, uncollaborative working, and poor reporting. These issues will have led to much research waste ‐ of funding, and opportunity for researchers, carers, and recipients of care.
Potential biases in the review process
We performed comprehensive searches of all relevant databases with no language, date, or publication status restrictions. Although every effort was made to minimise bias in the review process, the potential risk of missing trials cannot be completely eliminated.
The data screening and extraction process was strictly adhered to the Cochrane recommended procedures and standards. Nevertheless, unlike pharmaceutical therapies, CBT is comparatively more difficult to identify due to its nature as a talking therapy. We used strict measures to improve screening accuracy and consulted content experts, where necessary, however, the risk of missing identified trials is not entirely unlikely.
Several review authors for this review are authors of the original Cochrane review (Jones 2004) and are familiar with many of the trials in this review from past work. This could have biased us ‐ but we trust the rigorous Cochrane methods and replication we have undertaken in this review protect data from that potential.
Agreements and disagreements with other studies or reviews
There are many reviews of CBT for people with schizophrenia and they do differ. Fully providing possible reasons for this would be an interesting but impossibly time‐consuming addition to this review. We feel a key difference across reviews is the openness and clarity of method. Cochrane's methods have been robust to criticism and can be again. Methods for Cochrane reviews do evolve and become even more rigorous across time. However, this review has ‐ slowly ‐ moved across time from the original version (Jones 2004) and split to become one of a family of CBT reviews for people with schizophrenia (Jones 2018; Jones 2009a). Essentially, the findings of these comprehensive updates do not differ from the original findings of Jones 2004.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
Becoming involved in an additional CBT programme is a big commitment for people with schizophrenia but, the moderate‐quality evidence for people leaving the study early in this review suggests that this is acceptable. Having CBT might help people with schizophrenia to avoid (the often damaging) relapses, and could help mental state.There is no convincing evidence that there will be any effect on social functioning or quality of life.
In any event, people with schizophrenia, if offered this therapy, should know that any effects are likely to be modest in degree. If offered inclusion in a trial, people with schizophrenia could insist in ensuring study outcomes truly reflect their needs and that their data are accessible to all once the trial is completed.
2. For clinicians
If a skilled CBT therapist is available, adding this approach to standard care might reduce negative outcomes such as relapse, even in the long term, without putting off the person attending care. However, the investment for any modest benefit is considerable. We think it unlikely that the findings of this review would encourage many to instigate programmes of CBT for people with schizophrenia.
3. For policy makers
All important evidence in this review is of low‐ or very low‐quality ‐ or non‐existent. These data are not strong enough to support encouragement of wide use of additional CBT of the sort reviewed in this work ‐ even if resources are infinite.
Implications for research.
1. General
There could have been more information to report should there have been some sort of concordance on outcomes and generosity in sharing data. This review alone provides strong argument for the work of the COMET and ALLTRIALS initiatives.
2. Specific to cognitive behavioural therapy trials
We are genuinely unsure if more trials are really needed. Although data are not good, it would seem unlikely that large better studies would fully overturn the findings of this review. However, should that argument be being made, we have given thought to the design for future trials (Table 9).
8. Suggested design of study.
| Methods | Allocation: randomised, fully explicit description of methods of randomisation and allocation concealment. Blinding: single, tested. Setting: community rather than hospital. Duration: 12 weeks treatment, and then follow‐up to at least 52 weeks. |
| Participants | Diagnosis: schizophrenia (ICD). N = 300.* Age: adults. Sex: both. |
| Interventions | 1. Cognitive behaviour therapy plus standard care. N = 150. Content:
Delivered by: experienced therapists. 2. Other psychosocial therapy plus standard care . N = 150. |
| Outcomes | General: time to all‐cause treatment failure marked by its discontinuation, relapse/rehospitalisation, general impression of clinician (CGI), carer/other, compliance with treatment. Mental state: BPRS and PANSS. Global state: CGI (Clinical Global Impression). Quality of life. QOL (Quality of Life Questionnaire). Social functioning: return to everyday living for 80% of time.* Economic outcomes. |
| Notes | * Powered to be able to identify a difference of ˜ 20% between groups for primary outcome with adequate degree of certainty. |
BPRS = Brief Psychiatric Rating Scale CGI = ICD = International Classification of Diseases PANSS = Positive and Negative Syndrome Scale QOL =
Acknowledgements
We would like to thank Clive Adams, Rebecca Syed, Claire Irving, and Farhad Shokraneh at the CSG editorial base for their continued advice and support. We would also like to thank Colin Campbell and Irene Cormac for helping to write and develop the protocol.
This review is one of three sibling reviews (see also Jones 2009a; Jones 2018), replacing the original Cochrane CBT review published in 2004 (Jones 2004). Background and methods text in these reviews have been used across all three to create a set of 'harmonised' reviews using similar participants and interventions and assessing, where possible, similar outcomes for the effects of CBT for people with schizophrenia.
We also would like to thank Cochrane for awarding an incentive grant to help us complete this review (see Sources of support)
Appendices
Appendix 1. Previous searches
We searched The Cochrane Schizophrenia Group Trials Register (February 2009) using the phrase:
{[(*cogniti* AND (*behavio* or therap*)) OR (*cogniti* and (*technique* or *restructur* or *challeng*)) OR (*self* and (*instruct* or *management* or *attribution*)) OR (*rational* and *emotiv*) in title, abstract, index terms of REFERENCE] or [Cognitive* in interventions of STUDY]}
This register is compiled by systematic searches of major databases, handsearches, and conference proceedings (see Group Module).
Data and analyses
Comparison 1. COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. Relapse | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short term | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.04, 1.24] |
| 1.2 Medium term | 5 | 667 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.39, 0.72] |
| 1.3 Long term | 13 | 1538 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.61, 1.00] |
| 2 Global state: 1b. Relapse (skewed data) | Other data | No numeric data | ||
| 2.1 Medium term | Other data | No numeric data | ||
| 2.2 Long term | Other data | No numeric data | ||
| 3 Global state: 2. Clinically important change (no improvement) ‐ defined by individual studies | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.61, 1.66] |
| 3.2 Long term | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| 4 Global state: 3a. Rehospitalisation | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short term | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.55] |
| 4.2 Long term | 6 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.04] |
| 5 Global state: 3b. Hospitalisation ‐ number of admissions (skewed data) | Other data | No numeric data | ||
| 5.1 Medium term | Other data | No numeric data | ||
| 5.2 Long term | Other data | No numeric data | ||
| 6 Global state: 4. Average endpoint total score CGI, high = poor | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Short term | 3 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.63, ‐0.01] |
| 6.2 Medium term | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.89, ‐0.15] |
| 6.3 Long term | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.07, ‐0.27] |
| 7 Mental state: 1. General ‐ clinically important change (no improvement) | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Short term | 7 | 680 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.92] |
| 7.2 Long term | 5 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.02] |
| 8 Mental state: 2a. General (average total endpoint score BPRS, high = poor) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Short term | 5 | 541 | Mean Difference (IV, Random, 95% CI) | ‐5.09 [‐8.44, ‐1.74] |
| 8.2 Medium term | 3 | 199 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐5.73, 0.60] |
| 8.3 Long term | 3 | 175 | Mean Difference (IV, Random, 95% CI) | ‐8.77 [‐14.08, ‐3.46] |
| 9 Mental state: 2b. General (average total endpoint score PANSS, high = poor) | 22 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Short term | 11 | 962 | Mean Difference (IV, Random, 95% CI) | ‐7.21 [‐10.12, ‐4.30] |
| 9.2 Medium term | 11 | 963 | Mean Difference (IV, Random, 95% CI) | ‐3.68 [‐6.12, ‐1.24] |
| 9.3 Long term | 12 | 1284 | Mean Difference (IV, Random, 95% CI) | ‐3.74 [‐6.46, ‐1.02] |
| 10 Mental state: 2c. General (average total endpoint score PsyRAT, high = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Medium term | 1 | 197 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [‐1.20, 3.30] |
| 10.2 Long term | 1 | 197 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐1.48, 2.74] |
| 11 Mental state: 2d. General (average total change score, various scales) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 CHOICE ‐ short term | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 9.10 [1.74, 16.46] |
| 11.2 CPRS ‐ medium term | 1 | 336 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [‐0.97, 3.61] |
| 12 Mental state: 2e. General (average total endpoint score SCL‐90, high = poor) ‐ long term | Other data | No numeric data | ||
| 13 Mental state: 3a. Specific ‐ positive symptoms (average endpoint score PANSS subscale, high = poor) | 22 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 13.1 Short term | 11 | Mean Difference (Random, 95% CI) | ‐3.11 [‐4.97, ‐1.24] | |
| 13.2 Medium term | 12 | Mean Difference (Random, 95% CI) | ‐1.23 [‐1.90, ‐0.55] | |
| 13.3 Long term | 12 | Mean Difference (Random, 95% CI) | ‐0.98 [‐1.63, ‐0.34] | |
| 14 Mental state: 3b. Specific ‐ positive symptoms (average endpoint score BPRS/SAPS, high = poor) ‐ short term | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 BPRS | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐3.40, ‐0.27] |
| 14.2 SAPS | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐3.61, ‐0.05] |
| 15 Mental state: 4a. Specific ‐ hallucination ‐ clinically important change (no improvement ‐ < 3 point improvement BPRS (hallucination severity score)) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Short term | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.27] |
| 15.2 Long term | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.26] |
| 16 Mental state: 4b. Specific ‐ hallucination (average endpoint score various scales, high = poor) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 AHRS ‐ short term | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐6.74, ‐0.46] |
| 16.2 PANSS ‐ short term | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.92, ‐0.04] |
| 16.3 AHRS ‐ medium term | 2 | 128 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐7.07, 1.93] |
| 16.4 PANSS ‐ medium term | 1 | 197 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.79, 0.13] |
| 16.5 Malevolence ‐ BAVQ ‐ medium term | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐5.26, 7.26] |
| 16.6 Omniscience ‐ BAVQ ‐ medium term | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐3.47, 1.67] |
| 16.7 VPD ‐ medium term | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐11.10 [‐15.73, ‐6.47] |
| 16.8 AHRS ‐ long term | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐4.40 [‐6.60, ‐2.20] |
| 16.9 PANSS ‐ long term | 1 | 197 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.30, 0.58] |
| 16.10 BPRS ‐ long term | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐2.82 [‐3.74, ‐1.90] |
| 17 Mental state: 4c. Specific ‐ hallucinations (average endpoint score, PsyRATs, high = poor) | 3 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 17.1 Short term | 2 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Medium term | 1 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Mental state: 4d. Specific ‐ hallucinations (average endpoint score various scales, high = poor) (skewed data) | Other data | No numeric data | ||
| 18.1 PsyRATs ‐ short term | Other data | No numeric data | ||
| 18.2 PsyRATs ‐ long term | Other data | No numeric data | ||
| 18.3 BPRS ‐ short term | Other data | No numeric data | ||
| 18.4 VCS ‐ short term | Other data | No numeric data | ||
| 18.5 VCS ‐ medium term | Other data | No numeric data | ||
| 19 Mental state: 5a. Specific ‐ delusions (average endpoint score, PsyRATs , high = poor)) ‐ short term | 5 | Mean Difference (Random, 95% CI) | ‐4.33 [‐7.58, ‐1.08] | |
| 20 Mental state: 5b. Specific ‐ delusions (average endpoint score PANSS, high = poor) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 Short term | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.16, ‐0.12] |
| 20.2 Medium term | 1 | 197 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.71, 0.11] |
| 20.3 Long term | 1 | 197 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.53, 0.33] |
| 21 Mental state: 5c. Specific ‐ delusions (average change score PsyRATs, high = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 Medium term | 1 | 336 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.82, 0.96] |
| 22 Mental state: 5d. Specific ‐ delusions (average endpoint score PsyRATS, high = poor) (skewed data) | Other data | No numeric data | ||
| 22.1 Medium term | Other data | No numeric data | ||
| 22.2 Long term | Other data | No numeric data | ||
| 23 Mental state: 6a. Specific ‐ negative symptoms (average endpoint score, PANSS subscale, high = poor) | 24 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 23.1 Short term | 12 | Mean Difference (Fixed, 95% CI) | ‐3.35 [‐3.84, ‐2.85] | |
| 23.2 Medium term | 13 | Mean Difference (Fixed, 95% CI) | ‐1.43 [‐1.94, ‐0.93] | |
| 23.3 Long term | 13 | Mean Difference (Fixed, 95% CI) | ‐1.47 [‐1.94, ‐0.99] | |
| 24 Mental state: 6b. Specific ‐ negative symptoms (average endpoint score SANS, high = poor) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 24.1 Short term | 4 | 231 | Mean Difference (IV, Random, 95% CI) | ‐4.11 [‐10.40, 2.17] |
| 24.2 Long term | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐3.29, 1.15] |
| 25 Mental state: 6c. Specific ‐ negative symptoms (average endpoint score NSRS, high = poor) ‐ medium term | 1 | 336 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.05, 1.25] |
| 26 Mental state: 7a. Specific ‐ affective symptoms (average enpoint score, PANSS subscale, high = poor) | 19 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 26.1 Short term | 10 | Mean Difference (Fixed, 95% CI) | ‐4.86 [‐5.75, ‐3.96] | |
| 26.2 Medium term | 10 | Mean Difference (Fixed, 95% CI) | ‐0.80 [‐1.70, 0.09] | |
| 26.3 Long term | 10 | Mean Difference (Fixed, 95% CI) | ‐1.00 [‐1.82, ‐0.18] | |
| 27 Mental state: 8. Specific ‐ distress (average endpoint score PsyRATs/SADS, high = poor) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 27.1 Short term | 1 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.77, ‐0.43] |
| 27.2 Medium term | 2 | 226 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.50, 0.66] |
| 27.3 Long term | 1 | 197 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.47, 0.27] |
| 28 Mental state: 9. Specific ‐ anxiety (average endpoint score various scales, high = poor) | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 28.1 BAI ‐ short term | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐5.40, 4.77] |
| 28.2 SAS ‐ short term | 2 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐6.21 [‐7.36, ‐5.05] |
| 28.3 HAMA ‐ short term | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐1.79 [‐2.29, ‐1.29] |
| 28.4 SCL‐90 ‐ short term | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐1.53, ‐1.33] |
| 28.5 BAI ‐ medium term | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐6.55, 3.87] |
| 28.6 BAI ‐ long term | 3 | 335 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [‐1.19, 4.19] |
| 28.7 HADS ‐ long term | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [‐1.22, 2.54] |
| 29 Mental state: 10a. Specific ‐ depression ‐ clinically important change (no improvement = reduction HAMD score < 25%) ‐ short term | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.21, 2.15] |
| 30 Mental state: 10b. Specific ‐ depression (average endpoint score various scales, high = poor) ‐ short term | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 30.1 BDI | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.11 [‐4.25, 2.03] |
| 30.2 SDS | 2 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐3.29 [‐4.40, ‐2.19] |
| 30.3 HAMD | 2 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐4.95 [‐6.69, ‐3.20] |
| 30.4 SCL‐90 | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.65, ‐0.51] |
| 31 Mental state: 10c. Specific ‐ depression (average change score MADRS, high = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 31.1 Medium term | 1 | 336 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐1.26, 1.56] |
| 32 Mental state: 10d. Specific ‐ depression (average endpoint score various scales, high = poor) (skewed data) | Other data | No numeric data | ||
| 32.1 CDS ‐ short term | Other data | No numeric data | ||
| 32.2 CDS ‐ medium term | Other data | No numeric data | ||
| 32.3 BDI ‐ medium term | Other data | No numeric data | ||
| 32.4 HAMD ‐ medium term | Other data | No numeric data | ||
| 32.5 MADRS ‐ medium term | Other data | No numeric data | ||
| 32.6 BDI ‐ long term | Other data | No numeric data | ||
| 32.7 CDS ‐ long term | Other data | No numeric data | ||
| 32.8 HADS ‐ long term | Other data | No numeric data | ||
| 32.9 HAMD ‐ long term | Other data | No numeric data | ||
| 33 Mental state: 11a. Specific ‐ self esteem (average endpoint score various scales, high = good) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 33.1 RSES ‐ short term | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐1.43, 2.23] |
| 33.2 SES ‐ short term | 2 | 188 | Mean Difference (IV, Random, 95% CI) | 3.29 [2.43, 4.16] |
| 33.3 RSCQ ‐ short term | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 8.29 [‐0.08, 16.66] |
| 33.4 GSES ‐ short term | 1 | 190 | Mean Difference (IV, Random, 95% CI) | 1.48 [0.05, 2.91] |
| 33.5 RSES ‐ medium term | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.79, 2.59] |
| 33.6 RSCQ ‐ medium term | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐7.46, 8.26] |
| 33.7 SERS ‐ medium term | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 16.90 [1.25, 32.55] |
| 33.8 GSES ‐ medium term | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.53, 0.99] |
| 33.9 RSES ‐ long term | 2 | 236 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐1.79, 1.14] |
| 33.10 RSCQ ‐ long term | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 6.23 [‐8.56, 21.03] |
| 34 Mental state: 11b. Specific ‐ self esteem (average endpoint score various scales) ‐ short term (skewed data) | Other data | No numeric data | ||
| 34.1 SCS (high = good) | Other data | No numeric data | ||
| 34.2 positive self ‐ BCSS (high = good) | Other data | No numeric data | ||
| 34.3 negative self ‐ BCSS (high = poor) | Other data | No numeric data | ||
| 35 Mental state: 12a. Specific ‐ insight (average endpoint score various scales, high = good) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 35.1 ITAQ ‐ short term | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 4.92 [3.19, 6.65] |
| 35.2 BCIS ‐ medium term | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 1.33 [‐1.24, 3.90] |
| 35.3 ITAQ ‐ medium term | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.97, 2.77] |
| 35.4 SRIS ‐ long term | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐1.30, 1.34] |
| 35.5 BCIS ‐ long term | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐3.70, 2.62] |
| 35.6 ITAQ ‐ long term | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.20, 3.00] |
| 36 Mental state: 12b. Specific ‐ insight (average endpoint score SAI, high = good) ‐ short term | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 36.1 Short term | 3 | Mean Difference (Fixed, 95% CI) | 6.50 [5.84, 7.16] | |
| 36.2 Medium term | 1 | Mean Difference (Fixed, 95% CI) | 1.6 [‐0.19, 3.39] | |
| 36.3 Long term | 1 | Mean Difference (Fixed, 95% CI) | 2.9 [0.96, 4.84] | |
| 37 Mental state: 12c. Specific ‐ insight (average change score SAI, high = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 37.1 Medium term | 1 | 336 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.41, 0.03] |
| 38 Mental state: 13. Specific ‐ well‐being (average endpoint score WEMWS, high = good) ‐ short term | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | 4.08 [0.90, 7.26] |
| 39 Mental state: 14a. Specific ‐ various other symptoms (average endpoint score various scales high = poor) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 39.1 Psychotic symptom ‐ SCL‐90 ‐ short term | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.72, ‐0.44] |
| 39.2 Somatization ‐ SCL‐90 ‐ short term | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.86 [‐1.98, ‐1.74] |
| 39.3 Sensitivity of interpersonal relationship ‐ SCL‐90 ‐ short term | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.19, ‐1.01] |
| 39.4 Obsessive‐compulsive ‐ SCL‐90 ‐ short term | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐1.36, ‐1.22] |
| 39.5 Hostility ‐ SCL‐90 ‐ short term | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [1.00, ‐0.68] |
| 39.6 Phobia ‐ SCL‐90 ‐ short term | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.61, ‐0.41] |
| 39.7 Paranoia ‐ SCL‐90 ‐ short term | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.80, ‐0.60] |
| 39.8 Paranoia ‐ GPTS ‐ short term | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐13.32 [‐22.97, ‐3.68] |
| 39.9 Worry ‐ PSWQ ‐ short term | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐7.12, ‐0.28] |
| 39.10 Rumination ‐ PTQ ‐ short term | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐5.40 [‐8.96, ‐1.84] |
| 39.11 Hopelessness ‐ BHS ‐ medium term | 2 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.93, 0.82] |
| 39.12 Hopelessness ‐ BHS ‐ long term | 2 | 268 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.54, 2.01] |
| 40 Mental state: 14b. Specific ‐ various other symptoms (average endpoint score SCL‐90, high = poor) ‐ long term (skewed data) | Other data | No numeric data | ||
| 40.1 anxiety | Other data | No numeric data | ||
| 40.2 depression | Other data | No numeric data | ||
| 40.3 psychotic symptom | Other data | No numeric data | ||
| 40.4 somatization | Other data | No numeric data | ||
| 40.5 sensitivity of interpersonal relationship | Other data | No numeric data | ||
| 40.6 obsessive‐compulsiive | Other data | No numeric data | ||
| 40.7 hostility | Other data | No numeric data | ||
| 40.8 paranoid | Other data | No numeric data | ||
| 40.9 phobia | Other data | No numeric data | ||
| 41 Adverse effect/event(s): 1a. General ‐ any adverse event | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.27, 0.72] |
| 42 Adverse effect/event(s): 1b. General (average total endpoint score TESS, high = poor) ‐ medium term | 2 | 109 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐1.43, 1.90] |
| 43 Adverse effect/event(s): 2a. Specific ‐ various effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 43.1 Drowsiness | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.57] |
| 43.2 Headache | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.24] |
| 43.3 Mild lactation | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.81] |
| 43.4 Opsomenorrhea | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.24] |
| 44 Adverse effect/event(s): 2b. Specific ‐ suicide attempt | 2 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.84, 4.04] |
| 45 Adverse effect/event(s): 2c. Specific ‐ death | 9 | 1341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.38, 1.58] |
| 45.1 Any cause | 9 | 1341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.38, 1.58] |
| 46 Functioning: 1. General (average endpoint score GAF, high = good) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 46.1 Short term | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐5.82, 4.47] |
| 46.2 Medium term | 5 | 482 | Mean Difference (IV, Random, 95% CI) | 3.37 [‐1.66, 8.41] |
| 46.3 Long term | 5 | 446 | Mean Difference (IV, Random, 95% CI) | 1.79 [‐1.95, 5.53] |
| 47 Functioning: 2a. Social (average endpoint score ILSS, high = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 47.1 Medium term | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.02, 0.09] |
| 47.2 Long term | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.00, 0.11] |
| 48 Functioning: 2b. Social (average endpoint score SFS, high = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 Medium term | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [1.85, 9.76] |
| 48.2 Long term | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | 6.88 [1.99, 11.76] |
| 49 Functioning: 2c. Social (average endpoint score SOFAS, high = good) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 49.1 Short term | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐4.40, 6.36] |
| 49.2 Medium term | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐8.02, 6.02] |
| 49.3 Long term | 2 | 295 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [‐2.64, 3.76] |
| 50 Functioning: 2d. Social (average endpoint score PSP, high = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 50.1 Short term | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | 7.96 [3.15, 12.78] |
| 50.2 Medium term | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | 7.23 [2.91, 11.55] |
| 50.3 Long term | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | 12.66 [8.65, 16.67] |
| 51 Functioning: 2e. Social (average endpoint score UPSA, high = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 51.1 Medium term | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.10, 0.08] |
| 51.2 Long term | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.07, 0.11] |
| 52 Functioning: 3. Life skills (average endpoint score LSP, high = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 52.1 Long term | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐3.32 [‐8.40, 1.76] |
| 53 Functioning: 4a. Cognitive ‐ overall (average total endpoint score WCST, high = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 53.1 Medium term | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐8.89, 8.29] |
| 53.2 Long term | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐9.80 [‐17.76, ‐1.84] |
| 54 Functioning: 4b. Cognitive ‐ memory (average endpoint score WMS, high = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 54.1 Short term | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 9.33 [1.54, 17.12] |
| 55 Functioning: 4c. Cognitive ‐ memory (average endpoint score CMS, high = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 55.1 Medium term | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐7.42, 8.22] |
| 55.2 Long term | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐6.24, 8.04] |
| 56 Functioning: 4d. Cognitive ‐ various (average endpoint score MCCB, high = poor) ‐ medium term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 56.1 Continuous performance | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐44.10 [‐52.40, ‐35.80] |
| 56.2 Mood management | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.15, ‐1.05] |
| 56.3 Sematic influencing | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐4.63, ‐0.17] |
| 56.4 Verbal memory | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐5.06, ‐0.54] |
| 56.5 Visual memory | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐5.64, 0.44] |
| 57 Functioning: 5. Intelligence (average endpoint score WAIS, high = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 57.1 Short term | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.89 [‐2.43, 12.21] |
| 57.2 Medium term | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 11.83 [9.27, 14.39] |
| 58 Functioning: 6. Disability (average endpoint score WHODAS, high = poor) | 1 | Mean Difference (Fixed, 95% CI) | ‐10.52 [‐14.65, ‐6.39] | |
| 59 Quality of life: 1a. General (average total endpoint score various scales, high = good) ‐ short term | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 59.1 QLS | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐10.63, 6.83] |
| 59.2 WHOQOL‐BREF | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 6.64 [‐1.36, 14.64] |
| 60 Quality of life: 1b. General (average total endpoint score various scales, high = good) ‐ medium term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 60.1 WHOQOL‐BREF | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 8.20 [0.66, 15.74] |
| 61 Quality of life: 1c. General (average total endpoint score various scales, high = good) ‐ long term | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 61.1 QLS | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐11.32, 4.12] |
| 61.2 GQOLI‐74 | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.82 [1.62, 4.02] |
| 61.3 WHOQOL‐BREF | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 8.85 [1.01, 16.69] |
| 61.4 EuroQOL | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐10.65, 1.65] |
| 62 Quality of life: 1d. General (average total endpoint score SQLS, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 62.1 Medium term | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐29.5 [‐40.28, ‐18.72] |
| 63 Quality of life: 2a. Specific ‐ physical (average endpoint score WHOQOL‐BREF, high = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 63.1 Short term | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.71 [‐1.01, 4.43] |
| 63.2 Medium term | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [0.20, 5.00] |
| 63.3 Long term | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 2.71 [0.11, 5.31] |
| 64 Quality of life: 2b. Specific ‐ physical (average endpoint score GQOLI‐74, high = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 64.1 Long term | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 13.69 [9.62, 17.76] |
| 65 Quality of life: 3a. Specific ‐ psychological (average endpoint score WHOQOL‐BREF, high = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 65.1 Short term | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 2.22 [0.28, 4.16] |
| 65.2 Medium term | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | 2.52 [0.71, 4.33] |
| 65.3 Long term | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 2.37 [0.56, 4.18] |
| 66 Quality of life: 3b. Specific ‐ psychological (average endpoint score GQOL‐74, high = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 66.1 Long term | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 17.03 [13.07, 20.99] |
| 67 Quality of life: 3c. Specific ‐ psychological (average endpoint score SQLS, high = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 67.1 Medium term | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.26 [‐5.19, 2.67] |
| 68 Quality of life: 4a. Specific ‐ various other aspects (average endpoint score WHQOL‐BREF, high = good) ‐ short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 68.1 Environment | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.82 [‐1.71, 5.35] |
| 68.2 Social relationship | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.87 [‐0.62, 2.36] |
| 69 Quality of life: 4b. Specific ‐ various other aspects (average endpoint score various scales, high = good) ‐ medium term | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 69.1 Environment (WHOQOL‐BREF) | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | 2.56 [‐0.21, 5.34] |
| 69.2 Physical functioning (SF‐36) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 22.30 [17.65, 26.95] |
| 69.3 Role emotional (SF‐36) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 26.9 [19.74, 34.06] |
| 69.4 Role physical (SF‐36) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 31.20 [25.94, 36.46] |
| 69.5 Social relationship (WHOQOL‐BREF) | 2 | 109 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.60, 2.39] |
| 70 Quality of life: 4c. Specific ‐ various other aspects (average endpoint score various scales, high = good) ‐ long term | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 70.1 Environment (WHOQOL‐BREF) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 2.76 [‐0.31, 5.83] |
| 70.2 Social function (GQOLI‐74) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 16.19 [11.72, 20.66] |
| 70.3 Social relationship (WHOQOL‐BREF) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [‐0.55, 2.59] |
| 71 Quality of life: 4d. Specific ‐ various aspects (average endpoint score various scales, high = poor) ‐ medium term | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 71.1 Insight / treatment attitude (SQLS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.14 [1.96, 4.32] |
| 71.2 Motivation / vitality (SQLS) | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐3.43 [‐5.45, ‐1.40] |
| 71.3 Social function (SDSS) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐1.51 [‐2.34, ‐0.68] |
| 71.4 Symptoms / side effects (SQLS) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐2.76, 2.26] |
| 72 Quality of life: 5a. Specific ‐ psychological (average endpoint score SQLS, high = poor) ‐ medium term (skewed data) | Other data | No numeric data | ||
| 73 Quality of life: 5b. Specific ‐ role functioning (average endpoint score QLS, high = good) ‐ long term (skewed data) | Other data | No numeric data | ||
| 74 Quality of life: 5c. Specific ‐ symptoms/side effects (average endpoint score SQLS, high = poor) ‐ medium term (skewed data) | Other data | No numeric data | ||
| 75 Satisfaction with treatment: 1. Leaving the study early ‐ for any reason | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 75.1 Short term | 12 | 1214 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.77, 1.35] |
| 75.2 Medium term | 11 | 1402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.11] |
| 75.3 Long term | 19 | 1945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.77, 1.12] |
| 76 Engagement with services: 1a. Compliance to medication | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 76.1 Short term | 4 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.81, 2.60] |
| 76.2 Medium term | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.02, 1.49] |
| 76.3 Long term | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.10, 1.65] |
| 77 Engagement with services: 1b. Refusing treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 77.1 Short term | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.38] |
| 78 Engagement with services: 1c. Compliance with medication (average endpoint score MARS, high = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 78.1 Medium term | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.41, 0.21] |
| 78.2 Long term | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 38.02 [33.48, 42.56] |
1.19. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 19 Mental state: 5a. Specific ‐ delusions (average endpoint score, PsyRATs , high = poor)) ‐ short term.
1.54. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 54 Functioning: 4b. Cognitive ‐ memory (average endpoint score WMS, high = good).
1.60. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 60 Quality of life: 1b. General (average total endpoint score various scales, high = good) ‐ medium term.
1.64. Analysis.
Comparison 1 COMPARISON 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE, Outcome 64 Quality of life: 2b. Specific ‐ physical (average endpoint score GQOLI‐74, high = good).
Comparison 2. SENSITIVITY ANALYSIS 1: CBT+ STANDARD CARE versus STANDARD CARE ALONE (NON‐BLIND OUTCOME ASSESSMENT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Relapse ‐ long term | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 With non‐blind outcome assessment studies | 13 | 1538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 1.2 Without non‐blinded outcome assessment studies | 12 | 1394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.04] |
| 2 Mental state: 1. General ‐ clinically important change (no improvement) ‐ long term | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 With non‐blind outcome assessment studies | 5 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.02] |
| 2.2 Without non‐blind outcome assessment studies | 4 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.08] |
Comparison 3. SENSITIVITY ANALYSIS 2: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS‐WELL DEFINED CBT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Relapse ‐ medium term | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 With less‐well defined CBT | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.71] |
| 1.2 Without less‐well defined CBT | 2 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.84] |
| 2 Global state: 1. Relapse ‐ long term | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 With less‐well defined CBT | 13 | 1538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 2.2 Without less‐well defined CBT | 8 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.06] |
| 3 Global state: 2. Clinically important change (no improvement) ‐ long term | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 With less‐well defined CBT | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| 3.2 Without less‐well defined CBT | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.25, 1.00] |
| 4 Mental state: 1. General ‐ clinically important change (no improvement) ‐ long term | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 With less‐well defined CBT | 5 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.02] |
| 4.2 Without less‐well defined CBT | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.50, 1.33] |
Comparison 4. SENSITIVITY ANALYSIS 3: CBT+ STANDARD CARE versus STANDARD CARE ALONE (LESS EXPERIENCED THERAPIST).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Relapse ‐ medium term | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 With less‐experienced therapist | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.71] |
| 1.2 Without less‐experienced therapist | 2 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.84] |
| 2 Global state: 1. Relapse ‐ long term | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 With less‐experienced therapist | 13 | 1538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 2.2 Without less‐experienced therapist | 7 | 666 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.95] |
| 3 Global state: 2. Clinically important change (no improvement) ‐ long term | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 With less‐experienced therapist | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| 3.2 Without less‐experienced therapist | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.25, 1.00] |
| 4 Mental state: 1. General ‐ clinically important change (no improvement) ‐ short term | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 With less‐experienced therapist | 7 | 680 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.92] |
| 4.2 Without less‐experienced therapist | 3 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.11, 1.96] |
| 5 Mental state: 1. General ‐ clinically important change (no improvement) ‐ long term | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 With less‐experienced therapist | 5 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.02] |
| 5.2 Without less‐experienced therapist | 3 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.03] |
Comparison 5. SENSITIVITY ANALYSIS 4: CBT+ STANDARD CARE versus STANDARD CARE ALONE (ASSUMATPION FOR MISSING DATA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Relapse ‐ long term | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 With assumption | 13 | 1538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 1.2 Without assumption | 13 | 1523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.97] |
| 2 Mental state: 1. General ‐ clinically important change (no improvement) ‐ short term | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 With assumption | 7 | 680 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.92] |
| 2.2 Without assumption | 7 | 675 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.19, 0.99] |
Comparison 6. SENSITIVITY ANALYSIS 5: CBT+ STANDARD CARE versus STANDARD CARE ALONE (IMPUTED VALUES).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. Relapse ‐ short term | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 With imputed values | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 1.02] |
| 1.2 Without imputed values | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.69] |
Comparison 7. SENSITIVITY ANALYSIS 6: CBT+ STANDARD CARE versus STANDARD CARE ALONE (RANDOM EFFECT MODEL).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 2. Clinically important change (no improvement) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short term | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.61, 1.66] |
| 1.2 Long term | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.40, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrowclough 2001.
| Methods | Allocation: randomised Blinding: assessor blind Location: Tameside and Glossop, Stockport and Oldham, UK Length of follow‐up: 18 months |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (ICD‐10 and DSM‐IV criteria) N = 36 Sex: 33 M, 3 F Age: 18 ‐ 65 years, mean ˜ 31 years, SD ˜10 years Included: length of illness: unclear, meeting DSM‐IV criteria for substance abuse or dependence, in current contact with mental health services, a minimum of 10 hours of face‐to face contact with the caregiver per week Excluded: organic brain disease, clinically significant concurrent medical illness, or learning disability |
|
| Interventions | 1. CBT group*: N = 18 Content: "The interventions began with the motivational interviewing phase and five initial weekly sessions designed to assess and then enhance the patient’s motivation to change. If the patient's commitment was obtained, changes in substance use were negotiated on an individual basis. With the introduction of the individual cognitive behavior therapy at week 6 (or earlier if appropriate), the motivational interviewing style was integrated into subsequent cognitive behavior therapy sessions." (page 1707) Delivered by: Six clinicians (five clinical psychologists and one nurse therapist) conducted the cognitive behaviour therapies (individual and family). All had experience in cognitive behaviour therapy work with psychotic patients and were eligible for accreditation as cognitive behaviour therapists with the British Association for Behavioural and Cognitive Psychotherapy. Therapy was detailed in a comprehensive treatment manual (available from CB), and the therapists received weekly supervision based on audio‐taped sessions to ensure treatment fidelity. Frequency: 18 weekly sessions, followed by six biweekly sessions 2. Standard care group: N = 18 Content: Routine care in the context of the National Health Service of Great Britain consists of psychiatric management by the clinical team, coordinated through case management and including maintenance antipsychotic medication, monitoring through outpatient and community follow‐up, and access to community‐based rehabilitative activities, such as day centres and drop‐in clinics. All of the patients in the integrated treatment program also received routine care. Delivered by: the clinical team Frequency: not reported Treatment duration: 29 weeks |
|
| Outcomes | Global state: relapse Mental state: general, positive symptoms, negative symptoms (PANSS scores) Adverse events: death Functioning: general (GAF scores), social (SFS scores) Satisfaction with treatment: leaving the study early |
|
| Notes | Pilot study for Barrowclough 2010 * Participants in CBT group also received the standard care intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Individual patients were allocated to each condition by a third party with no affiliation to the study who used a computer‐generated randomization list stratified for sex and three types of substance use (alcohol alone, drugs alone, or drugs and alcohol) to ensure equal male‐female and substance use representation in each arm of the trial." (p.1707) Comment: Computer‐generated randomisation list was used. |
| Allocation concealment (selection bias) | Low risk | Quote: "allocated to each condition by a third party with no affiliation to the study." (p.1707) Comment: Allocation was concealed sufficiently. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants were not blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The assessors were blind to treatment allocation; attempts to maintain their blindness included use of separate rooms and administrative procedures for project staff, multiple coding of treatment allocations, and requesting subjects not to disclose information about the treatment." (p.1707) Comment: Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 8 of 36 participants left the trial early, which was a relatively acceptable amount of dropout. |
| Selective reporting (reporting bias) | Low risk | Comment: all data reported |
| Other bias | Low risk | Comment: no other risks identified |
Barrowclough 2010.
| Methods | Allocation: randomised Blinding: assessor blind Location: London and Manchester, UK Length of follow‐up: 24 months: trial (12 months) and follow‐up (24 months) |
|
| Participants | Diagnosis: non‐affective psychotic disorder (DSM‐IV) (diagnoses were established on the basis of case note review) N = 327 Sex: male and female (numbers not reported) Age: ˜ 39.5 years of age Length of illness: ˜12 years Included: in contact with catchment area‐based adult mental health services in the target localities; alcohol use exceeding 28 units for males, 21 units for females on at least half the weeks in the previous 3 months and/or use of illicit drugs on at least two days per week in at least half the weeks in the 3 months prior to assessment; DSM‐IV diagnosis of drug and/or alcohol dependence or abuse; no significant history of organic factors implicated in the aetiology of psychotic symptoms; English speaking; informed patient consent; and having a fixed abode. Having a fixed abode is operationalised as having a current address (including B & B or open access hostel) and evidence (e.g. from care coordinator) indicating that the person is more likely than not to have a reliable address throughout the 2 years. Excluded: not reported |
|
| Interventions | 1: CBT group*: N = 164 Content: Psychological therapy consisted of 26 individual sessions delivered over 12 months. Treatment was built around two phases. The first phase used motivational interviewing to reinforce motivation to change. In phase two of the intervention, cognitive behavioural technique from both the psychosis and substance misuse evidence base was used to formulate a change plan to help the participants to implement and maintain changes (e.g. strategies for dealing with distressing voices and depressed mood, responding to relapses, and coping with cravings and urges). Delivered by: not reported Frequency: 26 individual sessions delivered over 12 months Treatment duration: 12 months 2. Standard care group: N = 163 Content: antipsychotic medication, outpatients and community follow‐up and access to community rehabilitation activities Delivered by: not reported Frequency: not reported Treatment duration: throughout trial |
|
| Outcomes | Global state: relapse Mental state: general, positive symptoms, negative symptoms, affective symptoms (PANSS scores) Adverse events: death Functioning: general (GAF scores) Satisfaction with treatment: leaving the study early Unable to use: Global state: mean relapse, mean hospitalisation (skewed data) |
|
| Notes |
Barrowclough 2001 provided pilot data for this full trial. * Participants in CBT group also received the standard care intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...Randomisation was performed independently of the research team within each of the localities to the two groups (MiCBT plus standard care and standard care alone) after stratifying by variables which could be predictive of treatment participation or outcome: substance type (alcohol alone, drugs alone, alcohol and drugs) and main drug of use (cannabis, amphetamines; opiates; other). Other variables potentially predictive of participation or outcome, including chronicity of illness and gender, were recorded for use as covariates in the analyses of outcome. Allocation was done via a telephone link to a remote randomisation service using randomised permuted blocks with randomly varying block size". Comments: Reliable method of random sequence generation was used. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was done via a telephone link to a remote randomisation service using randomised permuted blocks with randomly varying block size." Comment: implied that allocation was concealed via the telephone randomisation service, even though the concealment was not explicitly described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: No details of the blinding of participants and personnel were provided. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Independent and blind assessment of the groups was carried out at a subsequent 4 assessment points over a 24 month follow‐up period....All potential unblinding of assessors was recorded and in cases where a researcher did become unblinded, a second assessor was allocated to continue with the follow up." Comment: Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: By the end of the study, at 24 months, 38% of the participants were lost to follow‐up; however, intention‐to‐treat analysis and robust treatment effect estimate was used. |
| Selective reporting (reporting bias) | Low risk | Comment: no selective reporting identified |
| Other bias | Low risk | Comment: no other bias identified |
Barrowclough 2014.
| Methods | Allocation: randomised Blinding: assessor blind Location: five participating National Health Service mental health trust sites, UK Length of follow‐up: 18 months |
|
| Participants | Diagnosis: schizophrenia (n = 54); schizophreniform (n = 9); schizoaffective (n = 13); other psychosis (n = 34) (DSM‐IV) N = 110 Sex: 98 M, 12 F Age: 16 ‐ 35 years, mean ˜ 23.4 years, SD ˜ 3.8 years. Included: first episode (length of illness 1.4 ‐ 62.8 months), DSM‐IV diagnosis of cannabis dependence or abuse; cannabis use of at least 1 day per week in at least half the weeks in the 3 months prior to assessment Excluded: history of organic factors implicated in the aetiology of psychotic symptoms |
|
| Interventions | 1. CBT (12 sessions) group*: N = 38 Content: motivation building which is to elicit and understand participants' perspective in relation to life goals, explore and resolve ambivalence so as to facilitate motivation for change; CBT techniques from both the psychosis and substance use evidence base were used to help the participant implement and maintain changes.. Delivered by: The trial therapists all had experience in conducting CBT with people with first‐episode psychosis. Frequency: 12 sessions Treatment duration: 4.5 months 2. CBT (24 sessions)*: N = 37 Content: motivation building which is to elicit and understand participants' perspective in relation to life goals, explore and resolve ambivalence so as to facilitate motivation for change; CBT techniques from both the psychosis and substance use evidence base were used to help the participant implement and maintain changes. Delivered by: The trial therapists all had experience in conducting CBT with people with first‐episode psychosis. Frequency: 24 sessions Treatment duration: 9 months 3. Standard care*, N = 35 Content: early Intervention services plus intensive case management and crisis response Treatment duration: 9 months |
|
| Outcomes | Global state: relapse**, rehospitalisation Mental state: positive, negative, affective (PANSS scores), anxiety (BAI scores), depression (CDS scores) Functioning: general (GAF scores) Satisfaction with treatment: leaving the study early Unable to use: Substance use: number of days absent from cannabis; motivation to change substance use (not predefined outcome in protocol) |
|
| Notes | * We combined data from Group 1 and Group 2 into a single group. The term "Treatment‐as‐usual (TAU)" was used in this paper for standard care. CBT group also received standard care intervention. **Defined as an exacerbation of psychotic symptoms that lasted for longer than 2 weeks and resulted in a change in patient management (increased observation by the clinical team, increase in antipsychotic medication, or both) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...was performed using an independent remote service." (p.2750) Comments: adequate randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "...was performed using an independent remote service." (p.2750) Comments: Participants or personnel could not foresee the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Research and therapy staff members were housed in different locations, assessment and therapy data were stored separately and participants and care coordinators were reminded not to divulge information that might lead to 'unbinding'..." (p.2751) Comments: Above descriptions indicated that the participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...rater‐blind RCT...using computer generated randomised permuted blocks." (p.2750) Comments: Outcome assessor could not foresee the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comments: 21 out of 75 participants from the CBT group and 13 out of 35 participants from the TAU group left the study early. No reasons were reported. |
| Selective reporting (reporting bias) | Low risk | Comments: PANSS total score was not reported. |
| Other bias | Low risk | Comments: none obvious. This article represented research commissioned by the UK' s National Institute for Health Research (NIHR) under its Programme Grants for Applied Research scheme (RP‐PG‐0606‐1302). |
Birchwood 2014.
| Methods | Allocation: randomised Blinding: assessor blind Location: three UK centres, UK Length of follow‐up: 18 months |
|
| Participants | Diagnosis: schizophrenia, schizoaffective or mood disorders (schizophrenia (n = 98); schizoaffective disorder (n = 29); paranoid schizophrenia (n = 17); psychosis (n = 50); bipolar disorder (n = 3) (ICD‐10)) N = 197 Sex: 113 M, 84 F Age: > 16 years, mean ˜ 37.4 years, SD ˜ 12.1 years Included: length of illness: not stated, had a history of harmful command hallucinations for at least 6 months with recent (< 9 months) history of harm to self or others, or major social transgressions as a result of the commands (full or incomplete compliance); or had harmful command hallucinations whereby the individual was distressed and appeasing the powerful voice Excluded: organic impairment or addictive disorder considered to be the primary diagnosis and insufficient command of the English language |
|
| Interventions | 1. CBT group*: N = 98 Content: Cognitive behavioural therapy for command hallucinations (CTCH): behavioural therapy techniques were used to assess and modify conviction in four beliefs linked to the construct of voice power. Protocol for cognitive therapy for command hallucinations were developed by the author and details were provided in our casebook manuals. Delivered by: cognitive therapists who were supervised in each centre by a lead clinician with expertise in cognitive behaviour therapy for psychosis Frequency: a maximum of 9 months (about 25 sessions of therapy) Treatment duration: 6 months 2. Standard care* group: N = 99 Content: treatment‐as‐usual was provided by community mental health and assertive outreach and early intervention teams. Treatment‐as‐usual included antipsychotic medication. Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Mental state: general, positive symptoms, negative symptoms, hallucinations, delusions, affective symptoms (PANSS scores); general, distress, (PSYRATS scores); hopelessness (BHS scores) Adverse events: death Satisfaction with treatment: leaving the study early Unable to use: Mental state: depression (CDS scores) ‐ skewed data Behavioural responses to voices (not predefined in protocol) |
|
| Notes | *The term "Treatment‐as‐usual (TAU)" was used in this paper. Participants in CBT group also received the standard care intervention. This trial shared the same intervention protocol as Trower 2004 but reported data from different participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using an allocation sequence generated with OpenCDMS.25 and were stratified by the centre with permuted blocks with a randomly varying block size after stratification by centre." (p.24) Comments:The investigators described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "...using an allocation sequence generated with OpenCDMS.25 and were stratified by the centre." (p.24) Comments:The outcome assessor could not foresee assignment, however the participants and therapists were informed of the allocation assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "After randomisation, an email notification about group allocation was sent to the trial manager, trial administrator, and therapists. An email notification confirming that the participant had been randomly assigned to treatment (with no information about group allocation) was sent to the centre research assistant. The trial administrator then sent a letter to the participant and the care coordinator informing them about the outcome of the randomisation." (p.25) Comments: The participants and therapists were informed of the allocation assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Randomization was masked from the assessors." "When masking was broken, another rater, masked to group assignment, assessed and rated the participant for all subsequent assessments; accordingly, all final ratings were masked..." (p.25) Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analysis was by intention to treat." (p.26) Comments: All randomised participants were analysed. |
| Selective reporting (reporting bias) | High risk | Comments: We looked through the protocol of this trial and found that the author did not report the childhood trauma, the quality of life, and costs of the interventions. |
| Other bias | Low risk | Comments: This trial was registered, number ISRCTN62304114. The funder did not play a role in data collection, analysis, or interpretation. Other bias was not obvious. |
Cao 2014.
| Methods | Allocation: randomised Blinding: no information Location: inpatients, China Length of follow‐up: 2 years |
|
| Participants | Diagnosis: first‐episode schizophrenia (CCMD‐3) N = 80 Sex: 48 M, 32 F Age: 15 ‐ 50 years (mean ˜ 26.35 years, SD ˜ 12.8 years) Included: length of illness (mean ˜1.80 years, SD ˜ 1.20 years) Excluded: pregnancy, chronic physical disorder, brain organic disease, affective disorder, personality disorder, alcohol or drug abuse |
|
| Interventions | 1. CBT group*: N = 40 Content: The intervention included health education to help participants recognise and correct their wrong beliefs or cognition; behavioural therapy included relaxation training. Delivered by: not reported Frequency: The intervention was conducted during hospitalisations and once per month after discharge. Treatment duration: 2 years 2. Standard care group: N = 40 Content: antipsychotics and nursing care Delivered by: not reported Frequency: not reported Treatment duration: 2 years |
|
| Outcomes | Global state: relapse Quality of life: general, social, physical, psychological (GQOLI‐74 scores) Engagement with services: compliance with medication Unable to use: Insight: ITAQ (ranked ordinal data) |
|
| Notes | *Participants in CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned..." (p.297). Comments: No details of the randomisation procedure were provided. Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The study did not address the allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The study did not address the blindness, however, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The study did not address the blindness of outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious. This study is funded by the science and technology project of Jiang Men, Zhejiang Province. |
Chen 2014.
| Methods | Allocation: randomised Blinding: not reported Location: inpatients, China Length of follow‐up: 8 weeks |
|
| Participants | Diagnosis: schizophrenia with depression (CCMD‐3) N = 90 Sex: 44 M, 46 F Age: 24 ‐ 51 years Included: length of illness: not stated; achieved clinical response (the decrease rate of PANSS total score ≥ 50% or PANSS total score ≤ 60) after drug therapy Excluded: participants combined with mental retardation or drug abuse; participants with bipolar disorder; participants with severe physical disorder or severe brain organic disorder; suicidal attempts |
|
| Interventions | 1. CBT group*: N = 45 Content: psychoeducation; help for participants to figure out their inappropriate beliefs and attitude; help for participants to recognise their cognitive problems and rebuild their personality and behaviour; psychoeducation to families Delivered by: not reported Frequency: 30 minutes each session; once per week for 8 weeks Treatment duration: 8 weeks 2. Standard care group: N = 45 Content: received usual nursing care and antipsychotics Treatment duration: 8 weeks |
|
| Outcomes | Mental state: depression (HAMD score) Satisfaction with treatment: leaving the study early Engagement with services: compliance to medication |
|
| Notes | * Participants in CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.85). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The method of blindness was not described. It was likely that the blinding could have been broken, because participants in the treatment group received CBT. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comments: Eight participant from the CBT group and seven participants from the antipsychotic and nursing group failed to complete the trial. No reasons were reported. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Chen 2015.
| Methods | Allocation: randomised Blinding: not reported Location: inpatients, China Length of follow‐up: 6 months |
|
| Participants | Diagnosis: schizophrenia with auditory hallucination N = 50 Sex: 30 M, 20 F Age: mean ˜ 36.7 years, SD ˜ 8.5 years Included: length of illness: mean ˜ 5.32 years, SD ˜ 4.63 years; hallucinations not relieved after receiving medication for at least 2 months; the total score of PANSS ≥ 3; be able to understand and cooperate with the clinicians; give informed consent to proposed treatment Excluded: not reported |
|
| Interventions | 1. CBT group*, N = 25 Content: The content of CBT was not stated. The dosage of risperidone in the CBT group was 1/3 amount of which was used in antipsychotics control group; benzodiazepines and antan could be used when necessary. Delivered by: not reported Frequency: A 40‐minute CBT was conducted weekly in the first month, twice per month in the third and fourth months and once per month in the fifth and sixth months. Treatment duration: 6 months 2. Standard care group: N = 25 Content: Risperidone was titrated from 1 mg/day to 4 ‐ 6 mg/day, the dose of risperidone was adjusted by the participants' response. Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Global state: CGI score (severity scale) Mental state: general , positive symptoms score, negative symptoms score, affective symptoms (PANSS scores); hallucinations (AHRS score) Adverse events: general (TESS score) Quality of life: various specific aspects (SQLS scores) |
|
| Notes | *Participants in CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.2063). Comments: No details of the randomisation procedure were provided. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not describe allocation concealment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The author did not describe the blinding of outcome assessment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious. The study funded by scientific project funding from Department of Science and Technology of Shandong province (112). |
Durham 2003.
| Methods | Allocation: randomised Blinding: assessor blind Location: two adjacent mental health services in Tayside and Fifemental health services in Tayside and Fife, Scotland Length of follow‐up: 6 years |
|
| Participants | Diagnosis: schizophrenia (n = 36); schizoaffective disorder (n = 5); delusional disorder (n = 2); (ICD‐10 and DSM‐IV) N = 43* Sex: 30 M, 13 F Age: mean ˜ 36 years, SD ˜ 10 years Included: length of illness: 2 ‐ 31 years; aged 16 ‐ 65 years who are known to the psychiatric services as experiencing positive symptoms, symptoms of persistent and distressing hallucinations or delusions, or both, and who have been stabilised on anti‐psychotic medication for at least a 6‐month period with medication under the care of a consultant psychiatrist Excluded: primary diagnosis of alcoholism or drug misuse, evidence of alcoholism or drug misuse, evidence of organic brain disease and history of violence |
|
| Interventions | 1. CBT** group: N = 22 Content: an initial emphasis on engagement, education and building a therapeutic alliance; functional analysis of key symptoms, leading to a formulation and problem list; development of a normalising rationale for the participant's psychotic experiences; exploration and enhancement of current coping strategies; acquisition of additional coping strategies for hallucinations and delusions; and focus on accompanying affective symptomatology using relaxation training, personal effectiveness training and problem‐solving, as appropriate Delivered by: five clinical nurse specialists with extensive professional experience of severe mental disorder. The therapists received training mainly focused on CBT. Frequency: 20 therapy sessions of approximately half an hour in length over a 9‐month period Treatment duration: 9 months 2. Standard care** group: N = 21 Content: participants received the usual care provided by the psychiatric services in Tayside and Fife. Services are well developed in these two areas, with a focus on community care delivered by community mental health teams. Services include regular psychiatric consultation and contact with a key worker (typically a trained community psychiatric nurse), with emergency assessment and hospital admission available as required. Facilities in the community include day care, sheltered work, supported accommodation and volunteer befriending. Delivered by: not reported Frequency: not reported Treatment duration: 9 months |
|
| Outcomes | Mental state: clinically important change (no improvement, defined as 50% decrease in symptom severity on PANSS scale), general (PANSS total scores); hallucinations, delusions (PSYRATS score) Functioning: general (GAF scores) Satisfaction with treatment: leaving the study early Unable to use: Participants attitude to treatment (not validated scale, but the participants' response to a series of questions) Self‐report measures of symptom severity, self‐esteem, and attitude to illness (data not reported) |
|
| Notes | * We only used data from two arms: CBT plus standard care and standard care. ** The term "TAU" was used in this paper. * Participants in CBT group also received the standard care intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation procedure (sealed envelope technique) was devised by the project statistician and administered centrally by the non‐clinical project coordinator. It was carried out separately within each treatment centre using randomised permuted blocking." (p.303) Comments: The author described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation procedure (sealed envelope technique) was devised by the project statistician and administered centrally by the non‐clinical project coordinator. It was carried out separately within each treatment centre using randomised permuted blocking." (p.304) Comments: Participants and investigators enrolling participants could not foresee assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients also were asked not to mention any details of their treatment during post‐treatment assessment..." (p.304). Comments: Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome evaluation by an independent assessor, an experienced psychiatrist, blind to treatment allocation at post‐treatment and 3‐month follow‐up." (p.304) Comments: The outcome assessor could not foresee the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There was a relatively small amount of missing data at post‐treatment (9%) and follow‐up (14%). The analyses were repeated with the missing values replaced either with previous values carried forward or with group means, and the same pattern of significance was found." (p.307) Comments: It seemed unlikely to cause attrition bias. |
| Selective reporting (reporting bias) | High risk | Quote: "Self‐report measures were administered to assess symptom severity, self‐esteem and attitude to illness, but these are not reported." (p.304) Comments: Self‐report measures were not reported. |
| Other bias | Low risk | Comments: none obvious |
Edwards 2011.
| Methods | Allocation: randomised Blinding: single‐blind Location: the Early Psychosis Prevention and Intervention Centre, Australia Length of follow‐up: 24 weeks |
|
| Participants | Diagnosis: first treated episode of a psychotic disorder (schizophrenia (n = 39); schizophreniform (n = 8); delusional disorder (n = 1)) N = 48 Sex: 34 M, 14 F Age: mean ˜ 21.4 years, SD ˜ 3.5 years Included: length of illness: not reported; registered with EPPIC for 12 to 26 weeks; and continuing to experience moderate to severe positive symptoms, defined as a score ≥ 4 on at least one of the hallucinations, unusual thought content, and conceptual disorganisation items of the expanded version of the brief psychiatric rating scale (BPRS), with a score of not less than 3 on these items for a period of 14 consecutive days or more during the preceding 12 weeks; treated with at least one atypical antipsychotic (usually risperidone, olanzapine or quetiapine) at doses up to 500 mg chlorpromazine equivalence (if tolerated), with demonstrated medication compliance for at least the past 4 weeks Excluded: an organic mental disorder, pregnancy or lactation, requiring antidepressant medication, a mood stabiliser or ECT, and a history of drug‐induced granulocytopenia |
|
| Interventions | 1. CBT plus clozapine group*: N = 11 Content: a manualised CBT program, the systematic treatment of persistent psychosis (STOPP, Hermann‐Doig 2003) Delivered by: not reported Frequency: twice weekly Treatment duration: 12 weeks 2. CBT plus thioridazine group*: N = 12 Content: a manualised CBT program, the systematic treatment of persistent psychosis Delivered by: not reported Frequency: twice weekly Treatment duration: 12 weeks 3. Clozapine group*: N = 14 Content: Participants commenced treatment at a dose of 12.5 mg/day which was titrated upwards in 25 mg/day increments up to a maximum dose of 300 mg/day, depending on clinical response. Delivered by: not reported Treatment duration: 12 weeks 4. Thioridazine group*: N = 11 Content: Participants commenced treatment at a dose of 12.5 mg/day which was titrated upwards in 25 mg/day increments up to a maximum dose of 300 mg/day, depending on clinical response. Delivered by: not reported Frequency: every day Treatment duration: 12 weeks |
|
| Outcomes | Global state: clinically important change (no improvement)**, general (CGI scores) Mental state: positive symptoms (BPRS scores), negative symptoms (SANS scores), depression (BDI scores) Functioning: social (SOFAS scores) Quality of life: general (QLS scores) |
|
| Notes | *All participants received routine clinical care, which included access to a 24‐hour mobile assessment and treatment team, inpatient service, case management and psychiatric care. **Defined as a score of more than 3 on each item of the BPRS positive subscale (unusual thought content, hallucinations, and conceptual disorganisation) and a CGI severity rating of moderate or higher. We combined data from the two CBT groups as the single intervention group (CBT plus standard care); and combined data from the 'clozapine' and 'thioridazine' groups as the single control group (standard care). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study was conducted as a single‐blind randomised controlled trial..." (p.2). Comments: No details of the randomisation procedure were provided. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not describe allocation concealment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "...a single‐blind..." (p.2). Comments: no details of the object of blinding. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: The author did not state the number of participants leaving the study early, however, data were analysed according to the intention‐to‐treat (ITT) principle. Missing data were handled by using multiple imputation. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: The research was supported by the Victorian Government's Health Promotion Foundation and NOVARTIS. The funder did not play role in data collection, analysis, or interpretation. |
England 2007.
| Methods | Allocation: randomised Blinding: assessor blind Location: community‐dwelling, Canada Length of follow‐up: 12 months |
|
| Participants | Diagnosis: voice hearers assigned a DSM‐IV diagnosis of schizophrenia or schizoaffective disorder N = 65 Sex: not reported Age: mean ˜ 41 years Included: length of illness: not reported; able to understand and speak English; negative voices in the previous 6 months; adherence to a prescribed, antipsychotic medication regimen at least 80% of the time; and competence to give informed consent to a proposed treatment Excluded: not reported |
|
| Interventions | 1. CBT group*: N = 44 Content: CBT was applied by delivery of 12 90‐min sessions of individualised counselling to voice hearers over a period of 4 months. CBT consisted of reasoning and decision support, counselling strategies tied to the techniques of Socratic learning, the verbal challenge, or empirical reality trial, homework assignments, and summarisation of the counselling sessions. The counselling sessions were audio‐taped to allow for audit of the nurse's counselling strategies. Delivered by: an experienced psychiatric clinical nurse specialist Frequency: 12 90‐min sessions of CBT over a period of 4 months Treatment duration: 4 months 2. Standard care group: N = 21 Content: Standard care comprised healthcare or service provider's routine use of communication strategies while providing psychiatric or primary care services including medication to voice hearers. Standard care was delivered over a period of 4 months at the discretion of their providers, and designed to promote comfort, health, and functional well‐being. Delivered by: not reported Frequency: not reported Treatment duration: 4 months |
|
| Outcomes | Mental state: general (BPRS scores), clinically important change in hallucination (BPRS)**, hallucination (BPRS long‐term scores), self‐esteem (RSCQ scores). Satisfaction with treatment: leaving the study early Unable to use: Mental state: hallucination (BPRS short‐term scores) ‐ skewed data |
|
| Notes | *Participants in CBT group also received the standard care intervention. **Defined as a less than 3‐point improvement in hallucination severity scores measured as a voice hearer's score on item 12 of the BPRS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was randomised. Study participants were assigned randomly to treatment using a table of random numbers." (p.73) Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not state the information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The personnel delivering CBT were also blind..." (p.72). Comments: Tt was likely that the blinding could have been broken, because participants in treatment group received CBT. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A research associate blinded to the random assignment and type of treatment provided to patients obtained data from participants 18 weeks and 54 weeks following initiation of their treatment." (p.73). Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Sixty‐five candidates met the criteria and took part in all phases of the study." (p.71) Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Farhall 2009.
| Methods | Allocation: randomised Blinding: assessor blind Location: outpatients, in Melbourne, Australia Length of follow‐up: 18 months |
|
| Participants | Diagnosis: schizophrenia (n = 51); schizoaffective disorder (n = 7); schizophreniform disorder (n = 8); delusional disorder (n = 6); mood disorder with hallucinations/delusions (n = 13); others with positive symptoms (n = 7). (DSM‐IV) N = 94 (92 participants completed the trial) Sex: 55 M, 37 F Age: mean ˜ 32 years, SD ˜ 9.6 years Included: length of illness: not reported; in the opinion of their case manager, one or more recovery needs that could potentially be addressed by a component of the local version of CBTp (see CBT group below) Excluded: participants with a diagnosis of any DSM‐IV non‐psychotic disorder, brief psychotic disorder, drug‐induced psychosis, mood disorder without hallucinations or delusions, or participants with a comorbid intellectual disability or without conversational English |
|
| Interventions | 1. CBT group*: N = 45 Content: The CBT intervention is based on efficacy trials conducted in the UK (Kuipers 1998). It is similar in scope and content to the therapy outlined by Fowler 1995. Therapists work with participants for 12 ‐ 24 sessions on agreed recovery goals using one or more of the following recovery therapy components: everyday coping, working with symptoms, understanding the experience of psychosis, strengthening adaptive view of self, personal/emotional issues or comorbid disorders, relapse prevention, and family or social reintegration. Delivered by: 12 clinical psychologists Frequency: 12 ‐ 24 sessions Treatment duration: 9 ‐ 12 months after baseline 2. Standard care group: N = 49 Content: Standard care was delivered within a case management framework and comprised medication and one or more of a range of services as required including: information, support, illness education, linkage to other services, assistance with benefits, crisis intervention, and family support. Delivered by: not reported Frequency: not reported Treatment duration: 9 ‐ 12 months after baseline |
|
| Outcomes | Mental state: general, positive symptoms, negative symptoms, affective symptoms (PANSS scores); anxiety, depression (HADS scores), self‐esteem (RSES scores), insight (SRIS scores) Adverse events: death Functioning: life skills (LSP scores) Satisfaction with treatment: leaving the study early Unable to use: Functioning: general (GAF, WRAT, MMSE scores) ‐ data not reported Satisfaction with treatment: CSQ‐8 (scores) ‐ data not reported |
|
| Notes | *Participants in CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was randomly assigned on the basis of a tossed coin. The allocation was witnessed by an independent observer. " (p.50) Comments: The author described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not state the allocation concealment method. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not state the method of blinding here. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A research psychologist who was not involved in the therapy intervention administered all assessments but was not blind to participants' group assignments, apart from at baseline. A research assistant who was blind to group assignment scored and analysed the instruments." (p.50) Comments: The outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Intention‐to‐treat analyses were conducted. |
| Selective reporting (reporting bias) | High risk | Comments: Several measured outcomes were not reported. |
| Other bias | Low risk | Comments: none obvious |
Fowler 2009.
| Methods | Allocation: randomised Blinding: assessor blind Location: secondary mental health services in the East Anglia region of the UK Length of follow‐up: 9 months |
|
| Participants | Diagnosis: a diagnosis of affective or non‐affective psychosis (including schizophrenia, schizoaffective disorder, bipolar disorder, and psychotic depression) but not first episode. 65% of participants had non‐affective psychosis. N = 77 Sex: 55 M, 22 F Age: mean ˜ 27.8 years, SD ˜ 6.1 years Included: length of illness: mean ˜ 4.9 years, SD ˜ 2 years; illness duration less than 8 years; positive psychotic symptoms (hallucinations and delusions) in relative remission; unemployed status or currently engaged in < 16 hours paid employment or education Excluded: if psychotic disorder was thought to have an organic basis; acute psychosis present; primary diagnosis was drug dependency on opiates or cocaine |
|
| Interventions | 1. CBT group*: N = 35 Content: consisted of three stages and combined techniques of CBT with vocational case management Stage 1 involved developing a formulation of the person in social recovery. The focus was on identifying meaningful personal goals that could be linked with achievable day‐to‐day activity targets and thus address motivation and hopelessness. Stage 2 involved identifying and working towards medium‐ to long‐term goals. Where relevant, this included referral to relevant vocational agencies, or alternatively direct liaison with employers or education providers. Cognitive work at this stage involved promoting a sense of agency and addressing hopelessness, feelings of stigma, and negative beliefs about self and others. Stage 3 involved the active promotion of social activity, work, education, and leisure linked to meaningful goals. This involved promotion of activity by behavioural experiments, while managing symptoms of anxiety and low‐level psychotic symptoms. Specific therapeutic procedures used in the study were drawn from existing CBT manuals, especially procedures to focus on self‐regulation of psychotic symptoms and improve social recovery from psychosis. Therapists were also encouraged to use techniques of activity scheduling and reviewing mastery and pleasure and behavioural experiment approaches to manage social anxiety. Delivered by: Therapy in Norfolk was carried out by case managers who had no previous formal training in CBT. Therapy in the Cambridge‐based centre was carried out by CBT therapists. Frequency: not reported Treatment duration: 9 months 2. Standard care group: N = 42 Content: involved active case management by multi‐disciplinary secondary care mental health teams Delivered by: not reported Frequency: nor reported Treatment duration: 9 months |
|
| Outcomes | Global state: rehospitalisation Mental state: general (PANSS scores), anxiety (BAI scores), depression (BDI scores), hopelessness (BHS scores) Functioning: social (SOFAS scores) Quality of life: general (QLS scores) Satisfaction with treatment: leaving the study early Unable to use: Qualtiy of life: role functioning (QLS scores) ‐ skewed data Service use: Time Use Survey (scale not validated) |
|
| Notes | *Participants in the CBT group also received the standard care intervention.. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified for diagnosis (affective/non‐affective psychosis was considered a prognostic factor) and administrative centre (Norfolk/Cambridgeshire)." (p.1628) Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was stratified for diagnosis (affective/non‐affective psychosis was considered a prognostic factor) and administrative centre (Norfolk/Cambridgeshire)." (p.1628) Comments: Randomisation was administrated by centre. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Baseline and post‐treatment assessments were conducted by research assistants who were blind to group allocation." (p.1628) "Where blindness was broken, another research assistant conducted the post‐treatment assessment." (p.1631). "The research assistants made allocation guesses after post‐treatment CBT for improving social recovery in psychosis assessments. The result was within the levels that would be expected by chance." (p.1632) Comments: Blinding of the outcome assessor was well conducted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Primary analyses and significance testing were conducted on an intention‐to‐treat basis." (p.1632) Comments: Missing data have been imputed using appropriate methods. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Freeman 2014.
| Methods | Allocation: randomised Blinding: assessor blind Location: Oxford Health NHS Foundation Trust, UK Length of follow‐up: 12 weeks |
|
| Participants | Diagnosis: schizophrenia (n = 22); schizoaffective disorder (n = 6); other (n = 2) N = 30 Sex: 20 M, 10 F Age: mean ˜ 41.9 years, SD ˜ 11.5 years Included: length of illness: not reported; a current persecutory delusion; scoring at least 3 on the conviction scale of the PSYRATS; the delusion had persisted for at least three months; negative beliefs about the self as indicated by endorsing at least one negative schematic belief on the Brief Core Schema Scale (BCSS); aged between 18 and 70; and where major changes in medication are being made, entry to the study would not occur until at least a month after stabilisation of dosage Excluded: a primary diagnosis of alcohol or substance dependency; organic syndrome or learning disability; a command of spoken English inadequate for engaging in therapy or the assessments; and currently having individual CBT (though previous experience of CBT was not an exclusion criterion) |
|
| Interventions | 1. CBT group*: N = 15 Content: 1) negative thoughts about the self, 2) positive activities, and 3) positive thoughts about the self Delivered by: clinical psychologists Frequency: six sessions to each individual over eight weeks Treatment duration: 8 weeks 2. Standard care group: N = 15 Content: Standard care was delivered according to national and local service protocols and guidelines. It usually consisted of prescription of anti‐psychotic medication, visits from a community mental health worker, and regular outpatient appointments with a psychiatrist. Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks |
|
| Outcomes | Global state: rehospitalisation Mental state: delusions (PsyRATS scores), anxiety (BAI scores), depression (BDI scores), self‐esteem (RSCQ, SCS, BCSS scores), paranoia (GPTS scores), well‐being (WEMWS scores) |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomisation was carried out by using varying randomised permuted blocks via a sequence obtained from web site." (p.2) Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation was conducted by a researcher independent of recruitment and assessment process." (p.2) Comments: Allocation was well concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Informing patients of allocation was carried out by a therapist." (p.2) Comments: Participants and personnel knew the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessments were carried out by a rater, a graduate psychologist, blind to allocation." (p.2) Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Freeman 2015.
| Methods | Allocation: randomised Blinding: assessor blind Location: two UK centres Length of follow‐up: 24 weeks |
|
| Participants | Diagnosis: schizophrenia (n = 111); schizoaffective disorder (n = 11); other (n = 28) N = 150 Sex: 86 M, 64 F Age: mean ˜ 40.9 years, SD ˜ 10.5 years Included: length of illness: not reported; a current persecutory delusion; scoring at least 3 on the conviction scale of the PSYRATS; the delusion had persisted for at least three months; negative beliefs about the self as indicated by endorsing at least one negative schematic belief on the Brief Core Schema Scale (BCSS); aged between 18 and 70 years; and where major changes in medication are being made, entry to the study would not occur until at least a month after stabilisation of dosage Excluded: a primary diagnosis of alcohol or substance dependency or personality disorder; an organic syndrome or learning disability; a command of spoken English that was inadequate for engaging in therapy; and currently having individual CBT |
|
| Interventions | 1. CBT group*: N = 73 Content: The main techniques were psychoeducation about worry, identification, and reviewing of positive and negative beliefs about worry, increasing awareness of the initiation of worry and individual triggers, use of worry periods, planning activity at times of worry (which could include relaxation), and learning to let go of worry. Delivered by: not stated Frequency: six sessions over 8 weeks Treatment duration: 8 weeks 2. Standard care group: N = 77 Content: Standard care was delivered according to national and local service protocols and guidelines. This usually consists of prescription antipsychotic drugs, visits from a community mental health worker, and regular outpatient appointments with a psychiatrist. Delivered by: not reported Frequency: nor reported Treatment duration: 8 weeks |
|
| Outcomes | Mental state: general (PANSS, CHOICE scores), delusions (PsyRATS scores), distress (PsyRATs scores) , paranoia (GPTS scores), worry, (PSWQ scores), rumination (PTQ scores), well‐being (WEMWS scores) Satisfaction with treatment: leaving the study early |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a web based randomisation system, written by the Oxford Clinical Trials Unit for Mental Illness." (p.306) Comments: Randomisation was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not address this information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The assessors were masked to patients' treatment allocations, but all patients were informed of their allocation by a trial therapist." (p.306) Comments: blinding of participants not ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The assessors were masked to patients' treatment allocations, but all patients were informed of their allocation by a trial therapist." (p.306) Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: 5 participants from the intervention group and 4 from the control group left the study early, however, an intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Garety 2008.
| Methods | Allocation: randomised
Blinding: assessor blind
Location: five local mental health services in London Length of follow‐up: 24 months |
|
| Participants | Diagnosis: non‐affective psychosis* (ICD‐10 and DSM‐IV) with at least one positive symptom of moderate severity on the PANSS
Total: N = 301** Sex: 211 M, 90 F Age: mean ˜ 37.1 years, SD ˜ 10.9 years Included: length of illness: mean ˜ 9.9 years, SD ˜ 8.7 years; second or subsequent episode which started not more than three months before entry; rated at least four (moderate severity) on the Positive and Negative Syndrome Scale (PANSS) on at least one positive psychotic symptom Excluded: primary diagnosis of alcoholism or drug misuse, evidence of organic brain disease, and history of organic brain disease and history of violence |
|
| Interventions |
Pathway 1 (for participants without carers) 1. CBT group*: N = 106 Content: targeted at relapse prevention, done by exploring people's understanding of triggers and risks of relapse and by developing new model of disorder emphasising alternatives to delusional thinking, targets often including persistent negative beliefs about self and others, characteristic reasoning styles such as jumping to conclusions and distressing emotional reactions to events and anomalous experiences; administered by skilled practitioners (doctorial level clinical psychologists) and treatment fidelity assessed using the Cognitive Therapy for Psychosis Adherence Scale Delivered by: five clinical nurse specialists with extensive professional experience of severe mental disorder Frequency: 12 to 20 sessions within 9 months Treatment duration: 9 months 2. Standard care group: N = 112 Content: good standard care delivered according to national and local service protocols and guidelines, including the prescription of antipsychotic medication Delivered by: not reported Frequency: nor reported Treatment duration: 9 months Pathway 2 (for participants with carers) 1. CBT group*: N = 27 Content: targeted at relapse prevention, done by exploring people's understanding of triggers and risks of relapse and by developing new model of disorder emphasising alternatives to delusional thinking, targets often including persistent negative beliefs about self and others, characteristic reasoning styles such as jumping to conclusions and distressing emotional reactions to events and anomalous experiences; administered by skilled practitioners (doctorial level clinical psychologists) and treatment fidelity assessed using the Cognitive Therapy for Psychosis Adherence Scale Delivered by: five clinical nurse specialists with extensive professional experience of severe mental disorder Frequency: 12 to 20 sessions within 9 months Treatment duration: 9 months 2. Family intervention ** group: N = 28 Content: emphasis on improving communication, offering discussion of up‐to‐date information about psychosis, problem‐solving, reducing criticism and conflict, improving activity, and emotional processing of grief, loss and anger Delivered by: 16 mental health professionals Frequency: not stated Treatment duration: 9 months 3. Standard care** group: N = 28 Content: good standard care delivered according to national and local service protocols and guidelines, including the prescription of antipsychotic medication Delivered by: not reported Frequency: not reported Treatment duration: 9 months |
|
| Outcomes | Global state: relapse Mental state: clinically important change (no improvement); general, positive symptoms, negative symptoms, affective symptoms (PANSS scores), anxiety (BAI scores) Adverse events: suicide attempts, death Functioning: social (SOFAS scores) Quality of life: general (EuroQOL scores) Satisfaction with treatment: leaving the study early Unable to use: Mental state: depression (BDI scores) ‐ skewed data Mental state: delusion, hallucination (PSYRATS) ‐ data not reported Violent incidents ‐ not predefined outcome for this review |
|
| Notes | *In this trial 'treatment‐as‐usual' was the term used to describe standard care. Participants in CBT and family therapy groups also received the standard care intervention. **We did not use data from the family therapy group and only used data from participants receiving CBT plus standard care or standard care alone (N = 273). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using randomised permuted blocks with a block size randomly varying between two and ten for the no carer pathway and three and nine for the carer pathway..." (p.413). Comments: adequate randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation schedules were independently generated by a trial randomisation service in a separate location from all trial centres..." (p.413). Comments: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Trial research assessors were independent of treatment delivery and every effort was made to ensure they were kept masked to allocation." (p.415) Comments: Participants and therapists knew the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Trial research assessors were independent of treatment delivery and every effort was made to ensure they were kept masked to allocation." (p.415) Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Intention‐to‐treat analysis was undertaken. |
| Selective reporting (reporting bias) | High risk | Comments: Delusion, hallucination were not reported. |
| Other bias | Low risk | Comments: no clear indication of other bias |
Gleeson 2009.
| Methods | Allocation: randomised
Blinding: assessor blind Location: early Psychosis Prevention and Intervention Centre (EPPIC) in Melbourne and from Barwon Health, Victoria, Australia Length of follow‐up: 30 months |
|
| Participants | Diagnosis: schizophrenia (n = 27); schizophreniform (n = 9); schizoaffective disorder (n = 4); major depressive disorder with psychotic features (n = 5); bipolar disorder (n = 4); delusional disorder (n = 1); substance‐induced psychotic disorder (n = 3); psychotic disorder (n = 24) N = 81 Sex: 51 M, 30 F Age: mean ˜ 20.1 years, SD ˜ 2.9 years Included: length of illness: not reported; less than 6 months of prior treatment with antipsychotic medications, age 15 to 25 years inclusive, and remission on positive symptoms of psychosis. Remission was defined as 4 weeks or more of scores of 3 (mild) or below on the subscale items hallucinations, unusual thought disorder, conceptual disorganisation, and suspiciousness on the expanded version of the Brief Psychiatric Rating Scale (BPRS). Excluded: ongoing active positive symptoms of psychosis, severe intellectual disability, inability to converse in or read English, and participation in previous CBT trials |
|
| Interventions | 1. CBT group*: N = 41 Content: CBT focused upon relapse prevention although non‐adherence to treatment, substance abuse, coping with stress, and comorbid anxiety and depression were also targeted. There were parallel individual CBT sessions and family therapy sessions (based upon cognitive behavioural family therapy for schizophrenia (Falloon, 1988; Mueser & Glynn, 1999) where the family therapy focused upon communication skills, psychoeducation regarding relapse risk, and a review of early warning signs and documentation of a relapse prevention plan. Delivered by: individual research therapist, who additionally adopted the role of outpatient case manager for the duration of their treatment at EPPIC Frequency: 7‐month therapy window, approximately fortnightly Treatment duration: 7 months 2. Standard care group: N = 40 Content: standard care was coordinated via an outpatient case manager and outpatient consultant psychiatrist, with access to home‐based treatment and a group programme as indicated. Standard care was manualised for case managers with specific guidelines regarding the frequency of follow‐up and an outline of the treatments that should be covered in relation to phases of recovery. Delivered by: not reported Frequency: nor reported Treatment duration: 7 months |
|
| Outcomes | Global state: relapse Mental state: general (BPRS scores) Functioning: social (SOFAS scores) Quality of life: physical, psychological, social relationship, environment (WHOQOL‐BREF scores) Satisfaction with treatment: leaving the study early Engagement with services: compliance with medication/treatment (MARS scores) Unable to use: Mental state: negative symptom (SANS scores) ‐ no total endpoint score Mental state: depression (MADRS scores) ‐ skewed data Premorbid IQ: the Wechsler Test of Adult Reading (not predefined outcome for this review) Substance use (clinician alcohol use scale, clinician drug use scale, Alcochol Use Disorders Identification Test, World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test) (not predefined outcome for this review). |
|
| Notes | *Participants in the CBT group also received the standard care intervention.. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random allocation was managed by the study statistician (SC) using computer generated random numbers." (p.478) Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | High risk | Quote: "The trial coordinator (DW), who was informed of the outcome of randomisation via email and telephone, informed the treating team and, in relevant cases, the research therapists of the outcome." (p.478) Comments: Participants or investigators could possibly foresee assignments. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The trial coordinator (DW), who was informed of the outcome of randomisation via email and telephone, informed the treating team and, in relevant cases, the research therapists of the outcome." (p.478) Comments: Therapists knew the allocation assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome raters were kept blind to treatment allocation via (1) regular and frequent reminders were sent to all clinical staff regarding the importance of the blind; (2) the research assistant reminded participants. of the importance of the blind at the commencement of each research interview; (3) the research assistant was excluded from all clinical discussions regarding participants; and (4) the research assistant was forbidden from reading participants' medical records." (p.478) Comments: Blinding of the outcome assessor was well conducted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Intention‐to‐treat analyses were provided. ITT employed a last‐observation‐carried‐forward procedure. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Granholm 2005.
| Methods | Allocation: randomised
Blinding: assessor blind Location: treatment and residential settings, San Diego, America Length of follow‐up: 12 months after the end of treatment |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV) N = 76 Sex: 56 M, 20 F Age: mean ˜ 54.5 years, SD ˜ 7 years Included: length of illness: mean ˜ 30.1 years, SD ˜ 11.3 years; age from 42 to 74 years old Excluded: disabling medical problems that would interfere with testing, absence of medical records to inform diagnosis, and diagnosis of dependence on substances other than nicotine or caffeine within the past 6 months |
|
| Interventions | 1. CBT group*: N = 39 Content: The treatment manual included a participant workbook that contained homework forms. The CBT was developed specifically for patients with schizophrenia; the age‐relevant content modifications were added. To simplify learning and to help participants remember to use cognitive techniques in everyday life, mnemonic aids were provided; behavioural role‐playing exercises and problem‐solving skills. Delivered by: Psychologists or senior graduate students who had 2 years of clinical experience delivered CBT. Frequency: 24 weekly 2‐hour group psychotherapy sessions Treatment duration: 6 months 2. Standard care group: N = 37 Content: Participants continued in whatever ongoing care they were receiving including antipsychotics. Delivered by: not reported Frequency: nor reported Treatment duration: 6 months |
|
| Outcomes | Mental state: general, positive symptoms, negative symptoms (PANSS scores); depression (HAMD scores), insight (BCSI scores) Functioning: social (UPSA, ILSS scores) Satisfaction with treatment: leaving the study early Unable to use: Knowledge of specific skills and information (The Comprehensive Module Test) (not predefined outcome for this review) Medication dose (not predefined outcome for this review) |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A stratified randomisation procedure was used to assign participants to treatments... sequential list of random numbers." (p.522) Comments: adequate randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not state the information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...the coordinator was the only staff person other than therapists with knowledge of group membership... The assessors were blind to treatment group." (p. 522) Comments: As the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The assessors were blind to treatment group and measures were taken to assure the blinding." (p.522) Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Six participants from the CBT group and five participants from the control group left the study early, however, ITT analysis was used to deal with the missing data. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Grawe 2006.
| Methods | Allocation: randomised Blinding: Assessor was blinded, however, it was unclear if the trialists and the participants were also blinded. Location: Norway Length of follow‐up: 2 years |
|
| Participants | Diagnosis: schizophrenia (DSM‐IV) N = 50 Sex: male and female (numbers not reported) Age: ˜ 25.4 years, SD ˜4.6 years Included: length of illness: mean ˜ 2 years; recent onset, no substance abuse, no mental retardation, has shown period of recovery from an initial psychotic episode Excluded: not reported |
|
| Interventions | 1. CBT group*: N = 30 Content: integrated treatment provided by multi‐disciplinary team, including pharmacotherapy and case management. Structured family psychoeducation, cognitive behavioural family education, problem‐solving skills training, individual cognitive behavioural strategies for residual symptoms Delivered by: not reported Frequency: one hour per week for the first two months; and then every third week for an hour for the first year; monthly for the second year Treatment duration: 2 years 2. Standard care group: N = 20 Content: antipsychotic medication, supportive house and day care, crisis inpatient treatment, rehabilitation, brief psychoeducation and supportive psychotherapy Delivered by: not reported Frequency: not reported Treatment duration: not reported |
|
| Outcomes | Global state: relapse, clinically important change (no improvement)**, rehospitalisation Adverse event: suicidal attempts Satisfaction with treatment: leaving the study early Unable to use: Mental state: general (BPRS scores) ‐ unable to extract data from graph Functioning: general (GAF scores) ‐ data not reported by groups |
|
| Notes | *Participants in the CBT group also received the standard care intervention. ** |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...random numbers provided by the central Optimal Treatment project administration. Blocks were of variable size (8 ‐ 12), stratified according to sex and with a ratio of IT to ST of 3:2 to ensure that the majority of cases received the experimental treatment." Comment: Block and stratified randomisation was used. |
| Allocation concealment (selection bias) | Low risk | Quote: ''...random numbers provided by the central Optimal Treatment project administration. Blocks were of variable size (8 ‐ 12), stratified according to sex and with a ratio of IT to ST of 3:2 to ensure that the majority of cases received the experimental treatment..." Comment: Allocation was concealed via Optimal Treatment Project administration. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Neither participants, nor therapists were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Ratings were made by an independent rater who was blind to treatment conditions..." Comment: Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: no selective reporting identified |
| Other bias | Low risk | Comment: no other bias identified |
Gumley 2003.
| Methods | Allocation: randomised Blinding: no blinding Location: local community mental health teams in the West of Scotland (six CMHTs in Ayrshire and two CMHTs in Glasgow) Length of follow‐up: 52 weeks |
|
| Participants | Diagnosis: schizophrenia or a related disorder (DSM‐IV) N = 144 Sex: 105 M, 39 F Age: mean ˜ 35.8 years, SD ˜ 9.6 years Included: length of illness: mean ˜ 113 months, SD ˜ 81 months; receiving antipsychotic medication, and considered relapse‐prone Excluded: non‐English speaker, organic brain disorder, presence of significant learning disability, severe positive psychotic symptoms (rating of 5 or more on any one item of the positive scale of the Positive and Negative Syndrome Scale; PANSS), a primary drug or alcohol dependence disorder (based on the opinion of the key worker), or being in receipt of a concurrent psychotherapy (outside the study) |
|
| Interventions | 1. CBT group*: N = 72 Content: CBT was divided into two phases. Targeted CBT included identifying and targeting beliefs and behaviours, which increased risk to self or others, identifying and targeting beliefs and behaviours accelerating relapse and developing alternative beliefs and reinforcing those through behaviour change. During the study period, the CBT group received a median of 6 (range 0 ‐ 14) outpatient medical consultations and 28.5 (0 ‐ 86) community mental health team contacts. Delivered by: a clinical psychologist Frequency: A five‐session engagement phase was delivered between entry and 12 weeks. An intensive targeted phase (2 ‐ 3 sessions per week) was delivered at the appearance of early signs of relapse. Treatment duration: 12 weeks 2. Standard care group*: N = 72 Content: all participants continued to receive their ongoing usual treatment, overseen by the participants' consultant psychiatrist. TAU entailed ongoing medication, regular psychiatric review and regular follow‐up from a key worker, usually a community mental health nurse. In addition, all participants had access to a wider multidisciplinary community mental health team. Delivered by: not reported Frequency: not reported Treatment duration: 12 weeks |
|
| Outcomes | Global state: relapse, rehospitalisation Mental state: general, positive symptoms, negative symptoms, affective symptoms (PANSS scores); self‐esteem (RSES scores) Satisfaction with treatment: leaving the study early Unable to use: Brief Symptom Inventory (BSI scores) ‐ data were not reported Behaviour (PBIQ scores) ‐ not predefined outcomes for this review |
|
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper; participants in the CBT group also received standard care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patient was randomised according to predetermined envelopes containing the treatment group to which participants would be allocated (TAU or CBT) devised by one of the authors, which was unbeknown to the assessors, therapist or participants." (p.421) Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | Low risk | Quote: "The group allocation result which was unbeknown to the assessors, therapist or participants." (p.421). Comments: Participants and investigators enrolling participants could not foresee assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A member of the research team opened an envelope that informed as to which group individual participants were: to be allocated." (p.421) Comments: The blinding was broken as the envelope was opened after randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Two research nurses, who were not blind to the treatment allocation of participants, conducted follow‐up assessments." (p.421) Comments: The outcome assessor was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: All analyses were according to the intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Low risk | Comments: Psychological distress was measured using the Brief Symptom Inventory (BSI); the author did not report this outcome. |
| Other bias | Low risk | Comments: none obvious |
Guo 2015.
| Methods | Allocation: randomised Blinding: researchers were blinded to randomisation results Location: community care, China Length of follow‐up: 64 weeks |
|
| Participants | Diagnosis: schizophrenia (ICD‐10) N = 64 Sex: 27 M, 37 F Age: mean ˜ 30.1 years, SD ˜ 7.6 years Included: length of illness: mean ˜ 102.5 months, SD ˜ 76.0 months; participants with age 18 ‐ 60 years old, at least one of the PANSS scores ≥ 3, had been stabilised on antipsychotic medication for at least a 4‐week period, give informed consent to a proposed treatment Excluded: being seriously ill and in hospital or need to be hospitalised, with seriously physical disease or with combination of other psychotic disorders, had not been able to communicate effectively, previous experience of electroconvulsive therapy inside of one month period |
|
| Interventions | 1. CBT group*: N = 32 Content: CBT procedure was edited according to previous study and guideline (Li 2015 and Wright 2010). Delivered by: rehabilitation therapists Frequency: A 50 ‐ 60 minute CBT was conducted with the first 3 sessions at the first two weeks and the next 5 sessions at the next 10 weeks; in total, there were 8 sessions in 12 weeks. Treatment duration: 12 weeks 2. Standard care group*: N = 32 Content: antipsychotics (first generation and second generation) and nursing Delivered by: not reported Frequency: not reported Treatment duration: 12 weeks |
|
| Outcomes | Global state: relapse**, rehospitalisation Mental state: clinically important change (no improvement) ***; general, positive symptoms, negative symptoms, affective symptoms (PANSS scores), insight (SAI scores) Functioning: social (PSP scores) Satisfaction with treatment: leaving the study early Unable to use: Type and dosage of antipsychotic drugs received, number of staff on work, duration of work ‐ not predefined outcomes for this review |
|
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper; participants in CBT group also received standard care. **Defined as PANSS score of any core symptom > 5 or PANSS score of two symptom > 4 ***Defined as the reducing rate of PANSS score < 25% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''...computer generated random number...'' (p.333). Comments: The investigators described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent researcher organized and assigned the random number." (p.333). Comments: Allocation could not be foreseen. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: No details of the blinding of participants and personnel were provided. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...participants were not allowed to give group information to researchers..." (p.334). Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ''ITT analysis was used to deal with drop‐outs.'' (p.335) Comments: 2 participants (6.25%) in CBT group and 9 participants (28.1%) in control group left the study early or were lost to follow‐up. The number was not balanced in the two compared groups and no reasons reported, although ITT analysis was used. The dropout rate of the control group was high. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious. The study was funded by Beijing Medicine Research and Development Fund (009—1050); China and WHO cooperation: WPCHN1003566. |
Habib 2015.
| Methods | Allocation: randomised Blinding: assessor blind Location: inpatient psychiatry units, Pakistan Length of follow‐up: 24 weeks |
|
| Participants | Diagnosis: schizophrenia (DSM‐IV‐TR) N = 42 Sex: 25 M, 17 F Age: mean ˜ 33.5 years, SD ˜ 10.5 years Included: length of illness: mean ˜ 8.8, SD ˜ 5.7, however, the unit was not stated; being able to engage with a therapist, age 18 to 65 years, and with at least 5 years of education of the participant or a carer at school level Excluded: comorbid alcohol or substance dependence, organic brain syndrome or learning disability, and high levels of disturbed behaviour, or high risk of suicide or homicide based on clinical impression |
|
| Interventions | 1. CBT group*: N = 21 Content: Therapy was provided according to a manualised treatment protocol (Kingdon and Turkington, 1994), and was culturally adapted. Delivered by: psychologist who had received training in CBTp Frequency: 16 sessions lasting approximately one hour, twice weekly session Treatment duration: 12 weeks 2. Standard care group*: N = 21 Content: antipsychotic medication and nursing care Delivered by: not reported Frequency: not reported Treatment duration: 4 ‐ 6 months |
|
| Outcomes | Mental state: positive symptoms, negative symptoms, affective symptoms, hallucination, delusions (PANSS scores); insight (SAI scores) | |
| Notes | *Participants in the CBT group also received standard care intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using (www.randomization.com)." (p. 202) Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not state the information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After taking informed consent participants were assessed by blind assessors." (p.202) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comments: The author did not address the number of dropouts. Intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
He 2012.
| Methods | Allocation: randomised Blinding: no blinding Location: inpatients, China Length of follow‐up: 12 weeks |
|
| Participants | Diagnosis: first‐episode paranoia schizophrenia (ICD‐10) N = 100 Sex: 100 M, 0 F Age: mean ˜ 37.8 years, SD ˜ 6.5 years Included: length of illness: mean ˜ 9.82 months, SD ˜ 3.78 months; achieved clinical response (the decreased rate of PANSS total score ≥ 50% or PANSS total score ≤ 60) after acute management; the score of item 'G12' in PANSS scale ≤ 3; 16 ‐ 40 years old; female; participants signed the informed consent; stable condition with current antipsychotics use and no plan to change the medication in future Excluded: participants combined with mental retardation or brain organic disease; participants with severe recession or agitation who are unavailable to cooperate; participants with severe depression, anxiety or drug abuse; participants with severe physical disorder or severe medication; relevant adverse events; lack of insight; the length of hospitalisation more than 1 year |
|
| Interventions | 1. CBT group*: N = 50 Content: The intervention was based on a cognitive behavioural therapy handbook developed by the investigators. The therapeutic milieu and content was applied according to the handbook. Delivered by: not stated Frequency: a one‐hour CBT intervention every day for 28 days Treatment duration: 28 days 2. Standard care group: N = 50 Content: 15 participants received risperidone, 11 received perphenazine, and 14 participants received quetiapine. Delivered by: not reported Frequency: not reported Treatment duration: 28 days |
|
| Outcomes | Mental state: anxiety (HAMA scores) Satisfaction with treatment: leaving the study early |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned based on random number table." (p.652) Comments: The investigators described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The study did not address blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comments: high rate of dropouts. Fifteen of fifty in the CBT group and 10 of 50 in the antipsychotics group left the study early. The author did not state reasons for the dropouts. |
| Selective reporting (reporting bias) | Low risk | Comments: Ameasured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Hu 2013.
| Methods | Allocation: randomised Blinding: no blinding Location: inpatients, China Length of follow‐up: 24 weeks |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 79 Sex: 44 M, 35 F Age: mean ˜ 26.5 years, SD ˜ 1.3 years Included: length of illness: mean ˜ 5.3 years, SD ˜ 1.5 years Excluded: not reported |
|
| Interventions | 1. CBT group*: N = 40 Content: The author did not state details on CBT. Delivered by: six experienced psychologists Frequency: The length of CBT was 60 minutes per time, once per week for 24 weeks. Treatment duration: 24 weeks 2. Standard care group: N = 39 Content: Participants received risperidone with a dosage of 3 ‐ 6 mg/time. A length of four weeks was considered as one course of treatment. The intervention involves 6 courses of treatment in total. Delivered by: not reported Frequency: not reported Treatment duration: 24 weeks |
|
| Outcomes | Mental state: general, positive symptoms, negative symptoms, affective symptoms (PANSS scores) Functioning: semantic influencing, mood management, continuous performance, visual memory and verbal memory (MCCB scores) |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.17). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Hu 2014.
| Methods | Allocation: randomised Blinding: no blinding Location: inpatients, China Length of follow‐up: 6 months |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 80 Sex: 40 M, 40 F Age: mean ˜ 33.98 years, SD ˜ 8.13 years Included: length of illness: mean ˜ 2.73 years, SD ˜ 1.06 years; length of illness less than 5 years; length of hospitalisation more than 6 months, age 20‐50 years old Excluded: severe physical disorder |
|
| Interventions | 1. CBT group*: N = 40 Content: The cognitive behavioural therapy included wrong behaviour correction, relaxation, etc. Delivered by: not reported Frequency: one‐hour CBT, once per week Treatment duration: not reported 2. Standard care group: N= 40 Content: antipsychotics Delivered by: not reported Frequency: not reported Treatment duration: not reported |
|
| Outcomes | Functioning: intelligence (WAIS‐RC scores) Unable to use: Verbal intelligence and performance intelligence measured by WAIS‐RC ‐ item scores within a subscale |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.8). Comments: insufficient information to make judgement |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: Ameasured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Jackson 2009.
| Methods | Allocation: randomised Blinding: assessor blind Location: four Mental Health Services throughout the West Midlands in the UK Length of follow‐up: 12 months |
|
| Participants | Diagnosis: first episode of non‐affective* psychosis (ICD‐10) N = 76 Sex: 49 M, 17 F Age: 16 ‐ 35 years, mean ˜ 24.1 years, SD ˜4.7 years Included: length of illness: mean ˜ 17.4 weeks, SD ˜ 25.4 weeks; experienced a first episode of psychosis within the previous 6 ‐ 18 months Excluded: non English speakers; unable to give informed consent |
|
| Interventions | 1. CBT group**: N = 36 Content: The cognitive therapy‐based recovery intervention (CRI) was designed to be delivered on a weekly basis over a 6 month period (i.e. it was limited to a maximum of 26 sessions) and followed a protocol‐based modular approach. There were three key components: (a) engagement and formulation; (b) trauma processing; and (c) appraisals of psychotic illness (shame, loss, and entrapment). The intervention, therefore, is not just designed for those who could be described as 'traumatised' by their experiences of psychosis. It is intended to be helpful for all first episode patients adjusting to and recovering from a first episode of psychosis. Delivered by: four clinical psychologists and a cognitive behavioural psychotherapist Freqency: a weekly basis over a 6‐month period Treatment duration: 6 months 2. Standard care group: N = 30 Content: Those assigned to the standard care group received treatment‐as‐usual from their local mental health services. Standard care consisted of a combination of case management and antipsychotic medication. Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Mental state: depression (CDS scores), self‐esteem (RSCQ scores) Satisfaction with treatment: leaving the study early Unable to use: Post‐traumatic phenomena: IES score ‐ not predefined outcome for this review Attraction, worth, auto self‐regulation, comp, value of existence: RSCQ scores ‐ not predefined outcomes for this review |
|
| Notes | *We think 'non‐affective' could be schizophrenia, but not necessarily 100%. In this case, we have given this trial the benefit of the doubt and decided to include it. **The term 'Treatment‐as‐usual (TAU)' was used in this paper. Participants in the CBT group also received the standard care intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to CBT or Standard care by means of a computerised random number generator administered by the Birmingham University Clinical Trials Unit independent of the research team." (p.455) Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | Low risk | Quote: "...random number generator administered by the Birmingham University Clinical Trials Unit independent of the research team." (p.455) Comments: Participants and investigators enrolling participants could not foresee assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In addition, to maintain blindness, therapists and clients were asked not to discuss with the research associates which group they were allocated to and research staff did not attend treatment meetings or access case notes following randomisation. Assessors were asked to record any loss of masking to treatment allocation. " (p.455) Comments: The outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: intention‐to‐treat analysis undertaken |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Jia 2005.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 8 weeks |
|
| Participants | Diagnosis: schizophrenia (CCMD ‐ 3/DSM ‐ IV) N = 60 Sex: 44 M, 16 F Age: mean ˜ 22.1 years, SD ˜ 3.98 years Included: length of illness: mean ˜ 2.04 years, SD ˜ 1.16 years; 15 ‐ 35 years old; participants able to give signed, informed consent; stable condition with current antipsychotics use; no aggressive action, participants with consistent hallucination, delusion, and volitional behaviour disturbance Excluded: participants who are with organic brain disease or had received CBT therapy; participants who were addicted to alcohol or had drug abuse |
|
| Interventions | 1. CBT group*: N = 22 Content: rational thinking training, help for the participants to realise their inappropriate cognition, behavioural training, diary and health education Delivered by: not stated Frequency: once or twice per week for 8 weeks Treatment duration: 8 weeks 2. Standard care group: N = 38 Content: standard psychiatry nursing care, emotional support and health education Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks |
|
| Outcomes | Mental state: clinically important change (no improvement)**; general, positive symptoms, negative symptoms, hallucinations, delusions (PANSS) Unable to use: Mental state: excitement, cognition, passive/apathetic social withdrawal, disturbance of volition, lack of judgement & insight (PANSS item scores) ‐ not validated item scores |
|
| Notes | *Participants in the CBT group also received the standard care intervention. **Defined as reducing rate of PANSS score < 25% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.10) Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Jiao 2014.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 8 weeks |
|
| Participants | Diagnosis: first‐episode paranoia schizophrenia (ICD‐10) N = 120 Sex: 43 M, 77 F Age: mean ˜ 34.7 years, SD ˜ 9.2 years Included: length of illness: mean ˜ 6.42 years, SD ˜ 4.6 years Excluded: not reported |
|
| Interventions | 1. CBT group*: N = 60 Content: to help participants understand their symptoms and strategies to prevent the symptoms, cognitive rebuild, communication with therapists. The dosage of risperidone was 3.8 ± 0.7 mg/day. Delivered by: not reported Frequency: a one‐hour CBT intervention every day for 28 days Treatment duration: 8 weeks 2.Standard care group: N = 60 Content: The dosage of risperidone was 3.8 ± 0.6 mg/day. Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks |
|
| Outcomes | Mental state: clinically important change (no improvement) *, general (BPRS scores) | |
| Notes | *Participants in the CBT group also received the standard care intervention. **Defined as decrease rate of BPRS score < 30% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.10). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Kuipers 1997.
| Methods | Allocation: randomised
Blinding: no blinding Location: the Maudsley Trust, London; Addenbrooke's Hospital Trust, Cambridge and Norfolk Mental Health Trust, Norwich, UK Length of follow‐up: 18 months |
|
| Participants | Diagnosis: schizophrenia (n = 39); delusion disorder (n = 13); schizoaffective disorder (n = 2) N = 60 Sex: 38 M, 22 F Age:19 ‐ 65 years old Included: length of illness: 1 ‐ 26 years; at least one current positive psychotic symptom (such as delusions or hallucinations) that was distressing, unremitting (at least the past six months) and medication‐resistant, that is, had not responded to a previous trial of at least six months of appropriate antipsychotic medication. Clients prescribed clozapine needed to have been stable on this for at least one year (to allow time for all benefit to occur). Excluded: drug, alcohol or organic problems as primary features |
|
| Interventions | 1. CBT group*: N = 28 Content: Initial sessions were focused on facilitating engagement in treatment. Considerable effort was spent on building and maintaining a good basic therapeutic relationship, and this relationship was characterised by considerable flexibility on the part of the therapist. When necessary, treatment was arranged in locations convenient to the client, including home visits and proactive outreach. Behavioural therapy techniques, including activity scheduling, relaxation, and skills training. Delivered by: experienced clinical psychologists Frequency: one‐hour session conducted weekly then fortnightly Treatment duration: 9 months 2. Standard care group: N = 32 Content: case management and medication Delivered by: not reported Frequency: not reported Treatment duration: 9 months |
|
| Outcomes | Mental state: clinically important change (no improvement) *, general (BPRS scores) Adverse events: death Satisfaction with treatment: leaving the study early Unable to use: Mental state: changes in key psychotic symptoms (PSE‐10, MADS, BAI, BDI, BHS, SCQ scores ‐ data not reported Functioning: social (SFS scores) ‐ data not reported |
|
| Notes | *Participants in the CBT group also received the standard care intervention. **A change of less than five points on the BPRS was taken as indicating no reliable clinical change. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomised permuted blocking and a block size of six." (p.319) Comments: adequate randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "...it is extremely difficult to make assessments that are totally blind to the treatment condition and this was not attempted." (p.319) Comments: blinding of outcome assessment not ensured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comments: Four participants from the CBT group and seven participants from the control group left the study early. However, no reasons were reported. |
| Selective reporting (reporting bias) | High risk | Comments: Many measured outcomes were not reported. |
| Other bias | Low risk | Comments: none obvious |
Lewis 2002.
| Methods | Allocation: randomised Blinding: assessor blind Location: 11 mental health units serving 3 geographically defined catchment areas, Manchester/Salford, Liverpool, and north Nortinghamshire Length of follow‐up: 18 months | |
| Participants | Diagnosis: schizophrenia, schizophreniform disorder, schizoaffective disorder or delusional disorder (DSM‐IV) N = 203* Sex: 141 M, 62 F Age: median ˜ 27 years Included: length of illness: not reported; either first or second admission (within 2 years of a first admission) to inpatient or day patient unit for treatment of psychosis; positive psychotic symptoms for 4 weeks or more; score of 4 or more (moderate or severe) on the PANSS target item either for delusions (Pl) or hallucinations (P3); neither substance misuse nor organic disorder judged to be the major cause of psychotic symptoms Excluded: not reported |
|
| Interventions | 1. CBT group**: N = 101 Content: The CBT was manual‐based with four stages: Stage 1: a cognitive–behavioural analysis of how symptoms might relate to cognitions, behaviour, and coping strategies. Education about the nature and treatment of psychosis, using a stress vulnerability model to link biological and psychological mechanisms, was used to help engagement. Stage 2: A problem list was generated collaboratively with the participant. This was then prioritised according to the degree of distress attached, feasibility and, where relevant, clinical risk involved. Prioritised problems were assessed in detail and a formulation was agreed which included such issues as trigger situations and cognitions. Stage 3: Interventions particularly addressed positive psychotic symptoms of delusions and hallucinations, generating alternative hypotheses for abnormal beliefs and hallucinations, identifying precipitating and alleviating factors, and reducing associated distress. Stage 4: Monitoring positive psychotic symptoms of delusions and hallucinations. Delivered by:one of five therapists trained in CBT in psychosis, supervised by experienced cognitive therapists. Freqency: 15 ‐ 20 hours within a 5‐week treatment envelope, plus 'booster' sessions at a further 2 weeks and 1, 2, and 3 months Treatment duration: 70 days 2. Standard care group: N = 102 Content: There was no attempt to standardise 'routine care'. This means that the content of 'routine care' was not specifiable, except that it always included day or inpatient treatment and included antipsychotic drugs Delivered by: not reported Frequency: not reported Treatment duration: 70 days |
|
| Outcomes | Global state: relapse, rehospitalisation Mental state: general, positive symptoms, negative symptoms, affective symptoms (PANSS scores); delusions (PsyRATs scores) Adverse effects: death of any cause Satisfaction with treatment: leaving the study early Unable to use: Mental state: hallucination ‐ not able to use as data only derived from a subgroup of population) Mental state: (BIS, RSES scores) ‐ data for each group not reported Functioning: social (SFS scores) ‐ data for each group not reported Engagement with treatment: compliance with medication ‐ data for each group not reported Substance misuse ‐ not a predefined outcome for this review |
|
| Notes | *This is a triple‐arm study, and 305 participants were included in this study, six people excluded after randomisation. We did not use data from the participants in the supportive therapy arm (n = 106). **Participants in the CBT group also received the standard care intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Independent, concealed randomisation of individuals with minimisation was then performed by a trial administrator at each centre." (p.s92) Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | Low risk | Quote: "Independent, concealed randomisation of individuals with minimisation was then performed by a trial administrator at each centre." (p.s92) Comments: Participants and investigators enrolling participants could not foresee assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The interventions were carried out independently of clinical staff, who were kept unaware of treatment allocation." "Clinical staff were instructed not to divulge details of therapist contacts to the raters." (p.s92) Comments: As the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All outcome assessments were made blind to treatment allocation. Extensive steps were taken to maintain blindness of raters." (p.s92) Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Intention‐to‐treat analysis was conducted. |
| Selective reporting (reporting bias) | High risk | Comments: The author did not report data for four outcomes. |
| Other bias | Low risk | Comments: none obvious |
Li 2013a.
| Methods | Allocation: randomised Blinding: not addressed Location: China Length of follow‐up: 8 weeks |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 118 Sex: 62 M, 56 F Age: 19 ‐ 60 years Included: Further description of illness not reported. Excluded: Participants with cerebrovascular disease or severe physical disorder were excluded. Pregnant or breast feeding females were also excluded. |
|
| Interventions | 1. CBT group*: N = 60 Content: Ziprasidone was titrated from 20 ‐ 40 mg/d to 80 ‐ 120mg/d, taken orally Cognitive therapy was conducted to help the participant correct his or her wrong beliefs or thinking process; establish and intensify the right cognition. Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks 2. Standard care group: N = 58 Content: ziprasidone, no more details Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks |
|
| Outcomes | Mental state: general, positive symptoms, negative symptoms, affective symptoms (PANSS scores) Engagement with services: refusing treatment |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.111). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: The author did not describe the dropouts. However from the reported data, there was no attrition. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Li 2014.
| Methods | Allocation: randomised Blinding: assessor blind Location: inpatients, China Length of follow‐up: 12 months |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 80 Sex: 50 M, 30 F Age: mean ˜ 36.7 years, SD ˜ 8.5 years Included: length of illness: mean ˜ 5.32 years, SD ˜ 4.63 years; the item score of hallucination in PANSS ≥ 3; age 20 ‐ 50 years; without receiving antipsychotics, antidepressive drugs, antimanic drugs, and anti‐epileptic drugs four weeks before randomisation Excluded: participants combined with brain organic disease and severe physical disorder; participants who has a history of electric shock; alcohol or drug abuse; other mental disorder; have received CBT before randomisation |
|
| Interventions | 1. CBT group*: N = 40 Content: psychoeducation about voice; discuss the content of hallucinations; introduction of the ABC model; discuss the link between voice and behaviour; coping strategies Delivered by: not reported Frequency: forty minutes per time; 12 sessions among 6 months Treatment duration: 6 months 2. Standard care group: N = 40 Content: Participants received risperidone titrated upwards from 1 mg/d to 6 mg/d. Benzodiazepine or artane can be used when necessary. Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Mental state: general (PANSS scores), hallucinations (AHRS scores) Adverse events: any adverse event, various specific events Satisfaction with treatment: leaving the study early |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned based on random number table." (p.503) Comments: adequate randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comments: blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Two participants in the treatment group left the study early. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Li 2015.
| Methods | Allocation: randomised Blinding: Researchers were blinded to randomisation results. Location: inpatients, China Length of follow‐up: 6 months |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 100 Sex: 64 M, 36 F Age: mean ˜ 36.5 years, SD ˜ 8.7 years Included: length of illness: mean ˜ 5.23 years, SD ˜ 4.37 years; the sum of positive and negative syndrome of PANSS scores ≥ 60; be able to understand and cooperate with the clinicians; give informed consent to proposed treatment Excluded: with or a history of brain disease; serious physical disease; previous experience of electroconvulsive therapy; previous experience of CBT |
|
| Interventions | 1. CBT group*: N = 50 Content: building of a therapeutic alliance; functional analysis of key symptoms, leading to a formulation and problem list; scheduling of activity; simulated scene training and case explanation; exploration and enhancement of current coping strategies; homework assignments. The dosage of risperidone in the CBT group was 1/3 amount of that used in the antipsychotics group. Delivered by: therapists Frequency: A 40‐minute CBT was conducted weekly in the first 4 weeks, twice per week during 5 ‐ 16 weeks, and weekly during 17 ‐ 24 weeks. Treatment duration: 6 months 2. Standard care group: N = 50 Content: Risperidone was titrated from 1 mg/day to 4 ‐ 6 mg/day; the dosage of risperidone was adjusted by the participants' response; benzodiazepines and antan can be used when necessary. Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Functioning: cognitive (WCST), memory (CMS) Unable to use: Mental state: general PANSS (data not reported) Executive function of the frontal lobe: subscales of WCST (not predefined for this review) Left temporal lobe function: subscales of AAT (not predefined for this review) |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was carried out by using random number table." (p.211) Comments: The investigators described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comments: Researchers were blinded to randomisation results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | High risk | Comments: The PANSS score were not reported. |
| Other bias | Low risk | Comments: none obvious. The study was funded by scientific project funding from the Department of Science and Technology of Shandong province (2013) no.137. |
Li 2015a.
| Methods | Allocation: randomised Blinding: not addressed Location: community care, China Length of follow‐up: 1 year |
|
| Participants | Diagnosis: chronic schizophrenia (CCMD‐3) N = 100 Sex: 48 M, 44 F Age: 18 ‐ 60 years, mean ˜ 37.3 years, SD ˜ 10 years Included: length of illness: mean ˜ 14 years, SD ˜ 10.9 years; length of illness > 5 years, state of the illness was stabilised and medication was continued, living in community and taken care by at least one of the direct relatives Excluded: mental retardation, serious physical disease, pregnancy or lactation |
|
| Interventions | 1. CBT group*: N = 48 Content: functional analysis of symptoms and negative behaviour, providing treatment therapy, to help participants to develop positive attitudes, improve cognitive abilities, reduce conflicts with social interaction, improve clinical compliance, reduce negative mood, improve the way of thinking Delivered by: specially trained therapists Frequency: A 50‐minute CBT was conducted twice weekly in the first 6 months, once per week in the next 6 months, with a specialist coming weekly in assistance with the therapies. Treatment duration: 1 year 2. Standard care group**: N = 44 Content: not reported Delivered by: not reported Frequency: not reported Treatment duration: 1 year |
|
| Outcomes | Mental state: general (SCL‐90 scores) Satisfaction with treatment: leaving the study early Unable to use: Mental state: depression, anxiety, psychotic symptoms, somatisation, sensitivity of interpersonal relationships, obsessive‐compulsive disorder, hostility, paranoia, phobia (SCL‐90 scores) ‐ skewed data Burden: family burden scale score for relatives of the participant (not predefined outcome for this review) |
|
| Notes | *Participants in the CBT group also received the standard care intervention. **The term 'Control' was used for the comparator group with no further details given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was carried out by using random number table." (p.2) Comments: The investigators described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not describe allocation concealment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The author did not describe the blinding of outcome assessment. Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: 2 participants in the CBT group and 6 participants in the standard care group dropped out during the study, due to the participants or relatives refusing treatment. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious. The study was funded by Chang Zhou scientific project funding from the Department of Science and Technology of Jiangsu province, no. CS20102013. |
Liu 2012.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 6 months |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 112 Sex: 68 M, 44 F Age: mean ˜ 41.6 years, SD ˜ 3.5 years Included: length of illness: mean ˜ 4.9 years, SD ˜ 0.5 years; achieved clinical response after one hospitalisation; at the stage of rehabilitation Excluded: not reported |
|
| Interventions | 1. CBT group*: N = 56 Content: antipsychotics, rehabilitation training, cognitive and behaviour modification, life skill training, rebuilding the link between cognition, behaviour, and psychology Delivered by: not reported Frequency: not reported Treatment duration: 6 months 2. Standard care group: N = 56 Content: antipsychotics, psychoeducation, coping strategies, problem‐solving training Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Quality of life: physical, role physical, role emotional (SF‐36 scores) Satisfaction with treatment: leaving the study early Unable to use: The feeling of stigma ‐ not predefined outcome for this review |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned based on random number table..." (p.72). Comment: adequate randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: 9 participants and 14 participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Lu 2014.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 6 months |
|
| Participants | Diagnosis: chronic schizophrenia (ICD‐10) N = 104 Sex: 56 M, 48 F Age: mean ˜ 46.5 years, SD ˜ 5.44 years Included: length of illness: mean ˜ 43.3 months, SD ˜ 1.31 months; age 22 ‐ 55 years Excluded: mental retardation or brain organic disease; severe recession or agitation; severe depression, anxiety or drug abuse; severe physical disorder or severe medication; relevant adverse events; lack of insight; length of hospitalisation more than one year |
|
| Interventions | 1. CBT group*: N = 52 Content: cognitive coping strategies, behavioural therapy, etc. Delivered by: not reported Frequency: twice weekly sessions, forty‐five minutes per session Treatment duration: 6 months 2. Standard care group: N = 52 Content: Participants received antipsychotics plus psychoeducation. Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Mental state: self‐esteem (GSES scores) Quality of life: general, psychological, various aspects (SQLS scores) |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.348). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Ma 2016.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatient, China Length of follow‐up: 3 months |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 190 Sex: 98 M, 92 F Age: 22 ‐ 78 years; mean ˜ 45.67 years, SD ˜ 6.58 years Included: length of illness: mean ˜ 32.4 months, SD ˜ 22.7 months; finished at least the secondary school, state of an illness stabilised under medication at least one week; able to give informed consent to proposed treatment Excluded:serious physical disease and other psychotic disorders; difficulty with communication; experience of other psychological therapy; experience with electroconvulsive therapy |
|
| Interventions | 1. CBT group*: N = 95 Content: CBT therapy included a therapeutic alliance building with participants, help to develop personal behaviour control ability, help to correct cognitions in thought, beliefs and attitudes, help for participants to be aware of the importance of medications. Delivered by: therapists Frequency: A one‐hour CBT was conducted weekly in three months. Treatment duration: 3 months 2. Standard care group: N = 95 Content: Participants received conventional drug treatment. Delivered by: not reported Frequency: not reported Treatment duration: 3 months |
|
| Outcomes | Mental state: clinically important change (no improvement)*, general (BPRS scores), self‐esteem (GSES scores) | |
| Notes | *Participants in the CBT group also received the standard care intervention. **Defined as reduction in BPRS score < 25% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned..." (p.1). Comments: No details of the randomisation procedure were provided. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not describe allocation concealment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The author did not describe the blinding of outcome assessment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Naeem 2015.
| Methods | Allocation: randomised Blinding: Assessors were blind to allocation and were based in a separate location. Location: 2 hospitals, Pakistan Length of follow‐up: 4 months |
|
| Participants | Diagnosis: schizophrenia or a related disorder (ICD‐10, RDC) N = 116 Sex: 70 M, 46 F Age: 18 ‐ 65 years, mean ˜ 31.1 years, SD ˜ 7.4 years Included: length of illness: mean ˜ 5.8 years, SD ˜ 3.7 years; living within travelling distance of the hospital; having at least 5 years of education or living with a carer with at least 5 years of education Excluded: comorbid alcohol or substance dependence; severe learning impairment; problems due to an organic condition; high levels of disturbed behaviour, or high risk of suicide or homicide |
|
| Interventions | 1. CBT group*: N = 59 Content: A spiritual dimension was included in formulation, understanding and in therapy plan; equivalents of CBT jargons were used in the therapy; culturally appropriate home work assignments were selected and participants were encouraged to attend even if they were unable to complete their homework; folk stories and examples relevant to the religious beliefs of the local population were used to clarify issues. Delivered by: psychology graduates with more than 5 years experience of working in mental health Frequency: 6 to 10 sessions Treatment duration: 4 months 2. Standard care group: N = 57 Content: This normally consists of prescribing antipsychotic medication as considered suitable by the treating psychiatrist and nursing care. Delivered by: not reported Frequency: not reported Treatment duration: 4 months |
|
| Outcomes | Mental state: positive symptoms, negative symptoms, affective symptoms (PANSS scores); delusion, hallucination (PSYRATs scores), insight (SAI scores) Satisfaction with treatment: leaving the study early |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned, allocation lists were generated by a web‐based automated randomisation system..." (p.145). Comments: The investigators described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessors were blind to allocation and were based in a separate location." (p.144) Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comments: Six participants from CBT group and eight participants from control group left the study early. No reason was reported. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Naeem 2016.
| Methods | Allocation: randomised Blinding: assessor blind Location: community mental health services, Canada Length of follow‐up: 16 weeks |
|
| Participants | Diagnosis: schizophrenia (DSM‐IV) N = 33 Sex: 17 M, 16 F Age: ≥ 18 years, mean ˜ 40.5 years, SD ˜ 11.7 years Included: length of illness not reported; finished at least high school; engaged with mental health services; considered stable for at least six months and has a case manager Excluded: substance dependence, organic brain syndrome or intellectual disability, high levels of disturbed behaviour, high risk of suicide or homicide based on clinical impression |
|
| Interventions | 1. CBT group*: N = 18 Content: CBT for psychosis (CBTp) based Guided Self‐help (CBTp‐GSH) consisted of a total of 17 handouts and eight worksheets, that could be flexibly given by a health professional over 12 ‐ 16 sessions. The handouts focused on psychoeducation, dealing with hallucinations, paranoia, changing negative thinking, behavioural activation, problem‐solving, improving relationships, and communication skills. Health professionals were trained in formulating and devising a plan to suit the individuals' needs. The intervention was then delivered according to this plan. Delivered by: frontline mental health professionals Frequency: A 15 ‐ 30 minutes CBT was conducted in each session. Treatment duration: 16 weeks 2. Standard care group: N = 15 Content: conventional drug treatment Delivered by: not reported Frequency: not reported Treatment duration: 16 weeks |
|
| Outcomes | Mental state: positive symptoms, negative symptoms, affective symptoms (PANSS scores); hallucination, delusion (PsyRATs scores) General functioning: disability (WHODAS scores) Satisfaction with treatment: leaving the study early Unable to use: Satisfaction with treatment ‐ data not reported for standard care group |
|
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using computer‐generated numbers..." "Block randomisation with randomly permuted block size was used to ensure similar numbers of participants were allocated..." (p.70). Comments: The investigators described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not describe allocation concealment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comments: The staff to conduct outcome assessments was blinded with the randomisation results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: ITT analysis was applied in this study. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Pan 2012.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 1 year |
|
| Participants | Diagnosis: schizophrenia with depression (CCMD‐3) N = 68 Sex: 39 M, 29 F Age: mean ˜ 31.36 years, SD ˜ 10.78 years Included: length of illness not reported; total score of HAMD ≥ 17 Excluded: participants with severe physical disorder, epilepsy or depression induced by other reasons; drug abuse, allergic to antipsychotics or suicidal attempts; abnormal laboratory tests; extrapyramidal side effects induced by antipsychotics |
|
| Interventions | 1. CBT group*: N = 34 Content: not reported Delivered by: not reported Frequency: not reported Treatment duration: 6 weeks 2. Standard care group: N = 34 Content: not reported Delivered by: not reported Frequency: not reported Treatment duration: 6 weeks All participants received antipsychotics. |
|
| Outcomes | Global state: relapse Mental state: general (BPRS scores), negative symptoms (SANS scores), depression (clinically important change (no improvement)**, depression (HAMD scores) Adverse events: any adverse event Engagement with services: compliance to medication |
|
| Notes | *Participants in the CBT group also received the standard care intervention. **Defined as reduction in HAMD score < 25% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.206). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Qian 2012.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 1 year |
|
| Participants | Diagnosis: stable schizophrenia (CCMD‐3) N = 90 Sex: not reported Age: not reported Included: length of illness not reported Excluded: severe physical disorder |
|
| Interventions | 1. CBT group*: N = 45 Content: CBT combined with antipsychotics. CBT involves: 1) establish the consultant connection between participants and investigator; 2) help the participants recognise their wrong beliefs and thinking process; 3) help the participants realise their wrong recognition based on their problematic beliefs and guiding them to the correct recognition style; 4) help the participants realise and correct the inappropriate points in their thinking process; 5) encourage the participant to express his or her own viewpoint and promote introspectiveness; 6) help the participants inspect their external misconceptions and correct the deep cause of misconceptions by demonstration, imitation, or didactic suggestion; 7) help participants consolidate their reestablished conceptions and beliefs. Delivered by: not reported Frequency: not reported Treatment duration: 1 year 2. Standard care group: N = 45 Content: antipsychotics and health education Delivered by: not reported Frequency: not reported Treatment duration: 1 year |
|
| Outcomes | Global state: relapse Mental state: negative symptoms (PANSS scores) Engagement with services: compliance with medication (MARS scores) Unable to use: Mental state: general (PANSS scores) (data not reported) Functioning: social (SDSS scores) (data not reported) |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.294). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: No participants left the study early. |
| Selective reporting (reporting bias) | High risk | Comments: The author did not report PANSS total score and SDSS. |
| Other bias | Low risk | Comments: none obvious |
Qin 2014a.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 2 months |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 100 Sex: 61 M, 39 F Age: mean ˜ 38.73 years, SD ˜ 9.47 years Included: length of illness: 2 ‐ 4 years; stable condition with current antipsychotics use; total score of BPRS < 28 Excluded: participants with severe depression, anxiety |
|
| Interventions | 1. CBT group*: N = 50 Content: cognition correction and group psychoeducation, training exercise Delivered by: psychologists or nurse Frequency: three 30‐minute sessions per month for 2 months Treatment duration: 2 months 2. Standard care group: N = 50 Content: standard psychological treatment and nursing care Delivered by: not reported Frequency: not reported Treatment duration: 2 months |
|
| Outcomes | Mental state: anxiety (SAS scores), depression (SDS scores), self‐esteem (SES scores) | |
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.41). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Qiu 2014b.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 6 months |
|
| Participants | Diagnosis: schizophrenia (ICD‐10) N = 60 Sex: 0 M, 60 F Age: mean ˜ 28.3 years, SD ˜ 7.2 years Included: length of illness: mean ˜ 12.3 months, SD ˜ 6.4 months; PANSS total score ≥ 60; female; first episode; length of illness less than 2 years; 16 ‐ 45 years old Excluded: participants with severe physical disorder; participants with severe agitation; participants combined with mental retardation; pregnancy or lactating; alcohol or drug abuse |
|
| Interventions | 1. CBT group*: N = 30 Content: coping strategies and relapse prevention Delivered by: not reported Frequency: 12 sessions, 45 ‐ 60 minutes per session Treatment duration: 12 weeks 2.Standard care group: N = 30 Content: 5 ‐ 20 mg/day olanzapine; benzhexol or benzodiazepine can be used when necessary. Delivered by: not reported Frequency: not reported Treatment duration: 12 weeks |
|
| Outcomes | Global state: relapse Mental state: clinically important change (no improvement)**; general, negative symptoms, positive symptoms, affective symptoms (PANSS scores) Adverse events: general (TESS scores) Quality of life: various aspects (SDSS scores) Engagement with service: compliance to medication |
|
| Notes | *Participants in the CBT group also received standard care intervention. *Defined as reduction in PANSS score < 25%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.10). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Rector 2003.
| Methods | Allocation: randomised
Blinding: assessor blind Location: two large psychiatric facilities in Canada Length of follow‐up: 12 months |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV) N = 50 Sex: 20 M, 22 F Age: mean ˜ 37.5 years, SD ˜ 8.3 years Included: length of illness: not reported; the presence of persistent positive and negative psychotic symptoms in the past 6 months as determined by the SCID‐I interview; stable treatment with antipsychotic medications; age 18 ‐ 65 years old Excluded: suspected organic brain pathology; concurrent substance abuse or dependence; and past treatment with either behavioural or cognitive behavioural therapy in either individual or family format |
|
| Interventions | 1. CBT group*: N= 24 Content: Cognitive behavioural therapy was delivered on an individual basis for 6 months. The CBT approach in this study was guided by the principles and strategies developed by Beck (1979, 1985). The first phase of therapy focused on engagement and assessment. The second phase of therapy aimed to socialise the participant to the cognitive model and to impart cognitive and behavioural coping skills, including self‐monitoring with a thought record and the completion of homework tasks. Overlapping with the first two phases of treatment, a third aspect of treatment focused on providing psychoeducation with a normalising rationale. Delivered by: two doctoral level psychologists and one psychiatrist, all with formal training and practice in cognitive behavioural interventions Frequency: weekly conducted for 20 sessions Treatment duration: 6 months 2. Standard care group*: N = 18 Content: enriched treatment‐as‐usual comprised comprehensive psychiatric management with medication optimisation and clinical case management Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Mental state: positive symptoms, negative symptoms, affective symptoms (PANSS scores); depression (BDI scores) Satisfaction with treatment: leaving the study early Unable to use: Mean dosage of antipsychotic use (not predefined outcome for this review) |
|
| Notes | *The term 'Enhanced treatment‐as‐usual' was used in this paper. Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomised controlled..." (p.2). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...blind raters..." (p.2). Comments: blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comments: Eight participants from each group left the study early. High attrition rate. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Startup 2004.
| Methods | Allocation: randomised
Blinding: assessor blind Location: psychiatric hospitals, UK Length of follow‐up: 24 months |
|
| Participants | Diagnosis: schizophrenia, schizophreniform or schizoaffective disorder N = 90 Sex: 68 M, 22 F Age: 18 ‐ 65 years, mean ˜ 30.5 years, SD ˜ 8.7 years Included: resident within the catchment area, currently experiencing an acute psychotic episode, not already receiving psychological treatment, showing no evidence of organic mental disorder Excluded: not reported |
|
| Interventions | 1. CBT group*: N = 47 Content: This is a highly individualised, needs‐based form of CBT for psychotic disorders and is based on collaborative empiricism and (evolving) cognitive‐behavioural formulations. Delivered by: clinical psychologists who were employed as specialists in serious mental illness and conducted CBT for schizophrenia on a routine basis Frequency: 90‐minute session, up to a maximum of 25 sessions, were provided at weekly intervals where possible. Treatment duration: 6 months 2. Standard care group: N = 43 Content: Treatment‐as‐usual comprised pharmacotherapy, nursing care during hospitalisation, and community care after discharge. Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Mental state: general (BPRS scores), insight (ITAQ scores) Adverse events: death (any cause) Functioning: general (GAF scores), social (SFS scores) Unable to use: Mental state: psychotic and disorganisation (SAPS subscale scores) ‐ not validated scale Satisfaction with treatment: leaving the study early ‐ data not reported for standard care group |
|
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were randomized to groups by inviting the patients themselves to toss a coin and let it fall to the ground in front of the assessor." (p.420) Comment: adequate randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "...the follow‐up assessments were not conducted blind to group allocation." (p.420) Comments: The outcome assessor could foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: ITT analysis was used to deal with the missing data. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Unclear risk | Quote: "The baseline general function in TAU group is higher than that in standard care group." (p.420) |
Sun 2014.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatient, China Length of follow‐up: 12 weeks |
|
| Participants | Diagnosis: first‐episode schizophrenia (CCMD‐3) N = 100 Sex: 49 M, 51 F Age: 20 ‐ 42 years; mean ˜ 28.8 years, SD ˜ 6.1 years Included: length of illness: 1 ‐ 6 months; mean ˜ 2.3 months, SD ˜ 1.8 months; no experience of medication treatment, able to give informed consent to proposed treatment Excluded: serious physical illness or epilepsy, substance dependence, allergy to drug, pregnancy or lactation |
|
| Interventions | 1. CBT group*: N = 50 Content: CBT included the building of a therapeutic alliance with participants, functional analysis of symptoms, help to deal with hallucinations and delusions, relaxation training, personal effectiveness training and problem‐solving, as appropriate. Ziprasidone dose range 80 ‐ 160 mg/day, benzodiazepines and benzhexol can be used when necessary. Delivered by: not reported Frequency: a 40‐minute CBT was conducted weekly Treatment duration: 12 weeks 2. Standard care group: N = 50 Content: ziprasidone dose range 80 ‐ 160 mg/day, benzodiazepines and benzhexol can be used when necessary Delivered by: not reported Frequency: not reported Treatment duration: 12 weeks |
|
| Outcomes | Mental state: general (PANSS scores) Functioning: intelligence (WAIS‐R scores), memory (WMS scores) Unable to use: Functioning: executive functioning (WAIS‐R) ‐ item score rather than subscale score |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned..." (p.54). Comments: No details of the randomisation procedure were provided. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not describe allocation concealment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The author did not describe the blinding of outcome assessment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Tarrier 1999.
| Methods | Allocation: randomised
Blinding: assessor Location: National Health Service trusts in Greater Manchester Lenght of follow‐up: 24 months |
|
| Participants | Diagnosis: schizophrenia, schizoaffective psychosis, delusional disorder (DSM‐III R)
N = 87 Sex: 69 M, 18 F Age: mean ˜ 39 years, SD ˜ 11 years Included: length of illness: median ˜ 11 years; experiencing psychotic symptoms (i.e. hallucinations or delusions) for at least six months which did not appear to be responding further to medication; no evidence of organic pathology which could have explained the psychopathology; ages 16 ‐ 65 years; receiving regular and stable antipsychotic medication Excluded: not reported |
|
| Interventions | 1. CBT group*: N = 33 Content: coping strategy enhancement, training in problem‐solving, strategies to reduce relapse Delivered by: three experienced clinical psychologists and followed a protocol manual Frequency: six hourly sessions, each of which were followed by two summary sessions. Sessions were carried out twice a week and 20 sessions of treatment were carried out over ten weeks. Four booster sessions were given once a month for four months. Treatment duration: 10 weeks 2. Standard care group: N = 28 Content: standard psychiatric management with medication, monitoring outpatient follow‐up and care programme approach Delivered by: not reported Frequency: not reported Treatment duration: 10 weeks |
|
| Outcomes | Global state: relapse Mental state: clinically important change (no improvement)**, negative symptoms (SANS) Adverse event: death (any cause) Satisfaction with treatment: leaving the study early Unable to use: Mental state: positive symptoms ‐ (log transformed data) calculated by combining PSE and BPRS scores (data not in the format suitable for analysis and we were unable to convert it) |
|
| Notes | *Participants in the CBT group also received the standard care intervention. **defined as not achieved 50% improvement in psychotic symptoms in both severity and number of symptoms We did not use the data from a third arm where the intervention was supportive counselling plus standard care (n = 26). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...generated by the Institute for Medical Biometry using a computerized algorithm and was stored by CenTrial." (p.S102) Comments: The investigators described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "...generated by the Institute for Medical Biometry using a computerized algorithm and was stored by CenTrial." (p.S102) Comments: The allocation assignment were conducted centrally. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The therapist then gives the information about treatment allocation to the patient." (p.S102) Comments: Participants and therapists knew the allocation assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...assessor blind regarding the study condition..." "...the result of the randomisation only to the therapist in order to keep the assessor blind regarding the study condition." (p.S102) Comments: blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: 26 participants dropped out from the trial at 2‐year follow‐up, however, the intention‐to‐treat sample included all randomised participants. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcome were reported. |
| Other bias | Low risk | Funding source: This study was funded publicly by the German Research Foundation (Deutsche Forschungsgemeinschaft, grants Kl 1179/2‐1 and Kl 1179/3‐1). Comments: none obvious |
Tarrier 2014.
| Methods | Allocation: randomised Blinding: Research assistant and assessors were blinded. Location: Community Mental Health Teams (CMHT), Early Intervention (EI) teams, and Assertive Outreach (AO) teams across four National Mental Health Service trusts including, Greater Manchester West, Manchester Mental Health and Social Care, Pennine Care, and Five Boroughs in the North West of England, UK Length of follow‐up: 6 months |
|
| Participants | Diagnosis: schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder or psychotic disorder not otherwise specified (DSM‐IV) N = 49 Sex: 31 M, 18 F Age: 18 ‐ 65 years, mean ˜ 34.9 years, SD ˜ 13.1 years Included: previous suicide attempts or experiencing current suicidal ideation; under the care of an appropriate clinical team and currently in contact with mental health services; receiving appropriate antipsychotic medication; not currently receiving CBT or other empirically validated psychological treatments Excluded: serious suicidal intent and currently considered a danger to themselves; primary diagnosis of bipolar depression or substance induced psychosis; organic brain disease |
|
| Interventions | 1. CBT group*: N = 25 Content: CBT was based on a treatment manual and was derived from an explanatory model of suicide behaviour. The intervention consisted of three phases: 1) information processing biases; 2) appraisals of defeat, entrapment, social isolation, emotional dysregulation, and interpersonal problem‐solving; 3) suicide schema. Delivered by: clinical psychologists who had extensive experience in delivering CBT for psychosis Frequency: up to 24 individual therapy sessions delivered twice a week across 12 weeks Treatment duration:12 weeks 2. Standard care group*: N = 24 Content: treatment‐as‐usual Delivered by: not reported Frequency: not reported Treatment duration: 12 weeks |
|
| Outcomes | Mental state: general, positive symptoms, negative symptoms, affective symptoms (PANSS scores); hallucination, delusion (PSYRATs scores), depression (CDS scores), anxiety (BAS scores), self‐esteem (SERS scores), hopelessness (BHS scores) Functioning: general (GAF scores) Satisfaction with treatment: leaving the study early Unable to use: Suicidual probability: subscales of The Suicide Probability Scale (SPS) including suicidal ideation, suicidal hopelessness, suicidal negative self‐evaluation, hostility (not predefined for this review) |
|
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. Participants in the CBT group also received the standard care intervention, | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...participants were randomised using a clinical data management system and allocated to..." (p.205). Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was controlled by staff not directly linked to the trial to ensure independence and blindness to the trial allocation arms..." (p.205). Comments: Participants and investigators enrolling participants could not foresee assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was a single blind randomised control trial, the research assistant and assessors were blinded..." (p.205). Comments :The participants and therapists were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This was a single blind randomised control trial, the research assistant and assessors were blinded..." (p.205). Comments: The outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comments: Eight out of 25 participants in the treatment group and six out of 24 participants in the control group dropped out of the study. High attrition rate. |
| Selective reporting (reporting bias) | Low risk | Comments: The author reported all measured outcomes. |
| Other bias | Low risk | Quote: "This report/article presents independent research commissioned by the National Institute for Health Research (NIHR) UK under its Programme Grants for Applied Research scheme (RP‐PG‐0606‐1086)." Comments: none obvious |
Trower 2004.
| Methods | Allocation: randomised
Blinding: assessor blind Location: local mental health services in Birmingham and Solihull, Sandwell, and West Midlands Length of follow‐up: 12 months |
|
| Participants | Diagnosis: schizophrenia or related disorder with command hallucinations (ICD‐10) N = 38 Sex: 24 M, 14 F Age: mean ˜ 36.6 years, SD ˜ 10.3 years Included: command hallucinations for at least 6 months, recent history of compliance, appeasement of voices with severe commands, including harm to self, others or major social transgressions Excluded: primary organic or addictive disorder |
|
| Interventions | 1. CBT group*: N = 18 Content: four core dysfunctional beliefs (and their functional relation to behaviour and emotion) that define the client‐voice (social rank) power relationship. Using the methods of collaborative empiricism and Socratic dialogue, the therapist seeks to engage the client to question, challenge, and undermine the power beliefs, then to use behavioural tests to help the client gain disconfirming evidence against the beliefs. These strategies are also used to build clients' alternative beliefs in their own power and status, and finally, where appropriate, to explore the origins of the schema so clients have an explanation for why they developed those beliefs about the voice in the first place. Delivered by: not reported Frequency: not reported Treatment duration: 6 months 2. Standard care group*: N = 20 Content: This was delivered by community mental health teams. Delivered by: not reported Frequency: not reported Treatment duration: 6 months |
|
| Outcomes | Mental state: hallucination (BAVQ, VPD, VCS scores); distress (PsyRATs); depression (CDS scores)
Satisfaction with treatment: leaving the study early Unable to use: Mental state: PANSS scores ‐ data not reported Individual's feelings and behaviour in relation to the voice ‐ The Cognitive Assessment Schedule ‐ data not reported Hearer's beliefs about the knowledge of their voice ‐ The Omniscience Scale data not reported |
|
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. Participants in the CBT group also received the standard care intervention. This trial shares the same intervention protocol as Birchwood 2014 but reported data from different participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned to CTCH or TAU by means of a computerised random number generator administered by the Birmingham Clinical Trials Unit independent of the research team." (p.313) Comments: adequate randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned to CTCH or TAU by means of a computerised random number generator administered by the Birmingham Clinical Trials Unit independent of the research team." (p.313) Comments: Allocation concealment was adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The research associate responsible for outcome evaluation was blind to group allocation." (p.313) Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Four participants from the CBT group and five participants from the TAU group left the study early. Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | High risk | Comments: The data for PANSS, CAS, the Omniscience scale was not reported. |
| Other bias | Low risk | Comments: none obvious |
Tuikington 2002.
| Methods | Allocation: randomised Blinding: assessors blind Location: six sites in UK (Belfast, Glasgow, Hackney, Newcastle, Southampton, and Swansea) Length of follow‐up: 1 year |
|
| Participants | Diagnosis: schizophrenia (ICD‐10) N = 422 Sex: 325 M, 97 F Age: mean ˜ 40.47 years Included: not reported Exclusion criteria: participants who were deteriorating and who needed inpatient care or intensive home treatment, primary diagnosis of drug or alcohol dependence, organic brain disease or severe learning disability |
|
| Interventions | 1. CBT group*: N = 257 Content: assessment and engaging, developing explanations, case formulation, symptom management, adherence, working with core beliefs, and relapse prevention Delivered by: nurses receiving 10 days of intensive training Frequency: six‐hour sessions over a period of two or three months Treatment duration: 5 months 2. Standard care group: N = 165 Content: treatment‐as‐usual Delivered by: not reported Frequency: not reported Treatment duration: 5 months |
|
| Outcomes | Global state: relapse Mental state: general (CPRS scores); delusions, hallucination (PSYRATs scores) negative symptoms (NSRS scores), insight (SAI scores), depression (MADRS scores) Satisfaction with treatment: leaving the study early Unable to use: Satisfaction with treatment: participant and carer satisfaction (no usable data) Burden of carer (Burden of Care Questionnaire) ‐ not predefined outcome for this review. Health of Nation Outcome Scale ‐ not predefined outcome for this review |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A geographically separate worker randomised patients on the basis of computer‐generated numbers in blocks of six." (p.213) Comments: adequate randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comments: Assessors were blinded to randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: The author conducted intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Velligan 2014.
| Methods | Allocation: randomised Blinding: assessor blind Location: public mental health clinics in 2 counties in Texas Length of follow‐up: 15 months |
|
| Participants | Diagnosis: schizophrenia (DSM‐IV) N = 166* Sex: 37 M, 37 F Age: 18 ‐ 60 years, mean ˜ 39.2 years, SD ˜ 12.5 years Included: fluent English speakers; receiving ongoing treatment with an oral antipsychotic; persisting positive symptoms as evidenced by a score of ≥ 4 on BPRS expanded version, ratings of delusions, hallucinations, and/or suspiciousness; functional impairment as evidenced by a score of < 70 on the social and occupational functioning scale; stable residence; able to understand and complete assessments Excluded: a documented history of significant head trauma, seizure disorder, or mental retardation; a history of substance abuse or dependence in the past month; or a history of violence in the past 6 months (as a safety measure for staff making home visits) |
|
| Interventions | 1. CBT group*: N = 43 Content: The focus of the sessions was on participant‐identified problems, particularly those that interfered with daily functioning or were distressing, normalising symptoms, and using CBT techniques to develop alternative explanations. Delivered by: masters and doctoral level professionals with > 2 years' experience in assessment and treatment of serious mental illness Frequency: not reported Treatment duration: 9 months 2. Standard care* group: N = 42 Content: consisted of case management and medication follow‐up appointments provided by the local community mental health centre. Medication follow‐up visits occurred approximately every 3 months. Delivered by: not reported Frequency: not reported Treatment duration: 9 months 3. Cognitive Adaptation Training (CAT)**: N = 41 Content: manual‐driven compensatory strategies and environmental supports (signs, checklists, electronic cueing devices) established by a CAT therapist/trainer Delivered by: experienced therapists and non‐experienced therapists Frequency: not reported Treamtent duration: 9 months 4. Cognitive Adaptation Training (CAT) + CBT**: N = 40 Content: CAT and CBT Delivered by: not reported Frequency: not reported Treatment duration: 9 months |
|
| Outcomes | Satisfaction with treatment: leaving the study early Unable to use: Global state: MCAS ‐ post‐treatment data not reported Mental state: BPRS, AHR, DRS ‐ post‐treatment data not reported |
|
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. Participants in the CBT group also received the standard care intervention. **We did not use data from the Cognitive Adaptation Training (CAT) and MCog (CAT + CBT) groups Study was registered with ClinicalTrials.gov (identifier #NCT01915017). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified by gender and age using a computer generated algorithm created by the study statistician who had no patient contact." (p.2) Comments: Randomisation was well conducted. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was stratified by gender and age using a computer generated algorithm created by the study statistician who had no patient contact." (p.2) Comments: Participants and investigators enrolling participants could not foresee assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All raters were blind to treatment condition." (p.4) Comments: The outcome assessor could not foresee assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comments: 19 participants in the treatment group and 15 participants in the control group dropped out from the study. High attrition rate. |
| Selective reporting (reporting bias) | High risk | Comments: The post‐treatment data were not reported. |
| Other bias | Low risk | Comments: none obvious. This study was funded by National Institute of Mental Health (5R01MH082793). |
Wang 2005.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 8 weeks |
|
| Participants | Diagnosis: chronic schizophrenia (CCMD‐2‐R) N = 64 Sex: 48 M, 16 F Age: 16 ‐ 40 years, mean ˜ 31 years, SD ˜ 6.4 years Included: length of illness: mean ˜ 12 years, SD ˜ 3 years; length of illness ≥ 2 years Excluded: obvious clinical response after receiving antipsychotics; with severe physical disorder |
|
| Interventions | 1. CBT group*: N = 32 Content: help for participants to understand their symptoms and the impact of symptoms on emotion, realise the relationship between behaviour and disease; strengthened behaviour therapy; cognitive behavioural therapy Delivered by: not reported Frequency: four 30‐minute sessions per week for 8 weeks Treatment duration: 8 weeks 2. Standard care group: N = 32 Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks |
|
| Outcomes | Mental state: positive symptoms (SAPS scores), negative symptoms (SANS scores) Unable to use: Behaviour: NOSIE ‐ not predefined outcome for this review |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.548). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Wang 2008.
| Methods | Allocation: randomised Blinding: not reported Location: inpatients, China Length of follow‐up: 8 weeks |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 89 Sex: male and female Age: 18 to 60 years of age Included: length of illness: mean ˜ 12.5 months, SD ˜ 7.9 months; overall PANSS score > 60; 18 ‐ 60 years old Excluded: admitted to hospital due to severe physical impairment, or with severe heart, liver or kidney dysfunction |
|
| Interventions | 1. CBT group*: N = 45 Content: establishing therapeutic relationship and collating comprehensive illness history of individual participants. Treatment was divided into psychological and behaviour aspects. Participants were give psychoeducation about schizophrenia symptoms in order to improve treatment compliance, and meanwhile, behavioural intervention was given to reinforce symptoms of self‐monitoring, relapse prevention and ways of managing thoughts and actions. Standard care was risperidol, 0.5 mg/day, increased to 4 mg/day by the second week of intervention and maximum dosage was 6 mg/day. Delivered by: psychologist who had been trained to conduct CBT Frequency: twice per week, 45 to 60 minutes each session Treatment duration: 8 weeks 2. Standard care group: N = 44 Content: risperidol, 0.5 mg/day, increased to 4 mg/day by the second week of intervention and maximum dosage was 6 mg/day Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks |
|
| Outcomes | Mental state: clinically important change (no improvement)**; general, positive symptoms, negative symptoms, affective symptoms (PANSS) Satisfaction with treatment: leaving the study early |
|
| Notes | *Participants in the CBT group also received the standard care intervention. **PANSS score reduction of > 75% was regarded as full recovery; 50% ‐ 74% was markedly improved; 24% ‐ 49% was improved; < 25% was no clinical improvement. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.17). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: A small number of participants were lost to follow‐up (CBT group n = 2; control group n = 3), |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Unclear risk | Comments: none obvious |
Wang 2012.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 24 weeks |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 72 Sex: 25 M, 47 F Age: mean ˜ 45.8 years, SD ˜ 14.3 years Included: length of illness: mean ˜ 8.04 years, SD ˜ 9.3 years; schizophrenia without severe physical disorder Excluded: not reported |
|
| Interventions | 1. CBT group*: N = 36 Content: psychodeucation about symptoms and relapse. coping strategies to hallucination and delusions; cognitive modification Delivered by: 6 psychologists Frequency: 50 minutes for each session; once per week Treatment duration: 24 weeks 2. Standard care group: N = 36 Content: not reported Delivered: not reported Frequency: not reported Treatment duration: 24 weeks |
|
| Outcomes | Engagement with services: refusing treatment Unable to use: The score of health relevant knowledge test ‐ not predefined outcome for this review |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.651). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Wang 2015.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 64 weeks |
|
| Participants | Diagnosis: schizophrenia (ICD‐10) N = 32 Sex: 17 M, 15 F Age: mean ˜ 39.9 years, SD ˜ 11.4 years Included: length of illness: mean ˜ 175.6 months, SD ˜ 96.9 months; at least one item score of PANSS scale ≤ 3; 18 ‐ 60 years old Excluded: admitted to hospital due to severe condition; participants combined with severe physical disorder or other mental disorder; participants received modified electroconvulsive therapy |
|
| Interventions | 1. CBT group*: N = 16 Content: The intervention was based on two published cognitive behavioural therapy handbooks. Delivered by: psychologist who had been trained to conduct CBT Frequency: 8 sessions for 12 weeks, 45 to 60 minutes each session Treatment duration: 3 months 2. Standard care group: N = 16 Content: antipsychotics, case management, entertainment therapy, social support, and psychoeducation Delivered by: not reported Frequency: not reported Treatment duration: 3 months |
|
| Outcomes | Global state: relapse, clinically important change (no improvement)**, (CGI scores) Mental state: general, positive symptoms, negative symptoms, affective symptoms (PANSS scores) Functioning: social function (PSP scores) Quality of life: general (WHOQOL‐BREF scores) Satisfaction with treatment: leaving the study early |
|
| Notes | *Participants in the CBT group also received the standard care intervention. **Defined as the score of CGI‐GI more than 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.17). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Two participants left the study early; one from each group before intervention. Low proportion of dropouts. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Yao 2015.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatient, China Length of follow‐up: 2 months |
|
| Participants | Diagnosis: schizophrenia in recovery N = 88 Sex: 56 M, 32 F Age: 16 ‐ 67 years; mean ˜ 37.31 years, SD ˜ 4.31 years Included: not reported Excluded: not reported |
|
| Interventions | 1. CBT group*: N = 44 Content: CBT included: 1) active promotion of social activity; 2) help to deal with hallucinations, paranoia, changing negative thinking; 3) help to self‐regulate psychotic symptoms and improve social recovery from psychosis; 4) psychoeducation; 5) relaxation training with a duration of 30 minutes; 6) promoting of participants' and guardians' confidences; 7) activity scheduling. Delivered by: qualified doctors and senior nurse Frequency: A 3‐minute CBT was conducted three times weekly. Treatment duration: 2 months 2. Standard care group: N = 44 Content: regular medication treatments and nursing Delivered by: not reported Frequency: not reported Treatment duration: 2 months |
|
| Outcomes | Mental state: anxiety (SAS scores), depression (SDS scores), self‐esteem (SES scores) | |
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to..." (p.251). Comments: No details of the randomisation procedure were provided. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not describe allocation concealment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The author did not describe the blinding of outcome assessment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Zhang 2014.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 6 weeks |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 79 Sex: not reported Age: not reported Included: not reported Excluded: not reported |
|
| Interventions | 1. CBT group: N = 39 Content: psychoeducation and cognition modification Delivered by: three psychologists Frequency: not reported Treatment duration: 6 weeks 2. Standard care group: N = 40 Content: antipsychotics and nursing care Delivered by: not reported Frequency: not reported Treatment duration: 6 weeks |
|
| Outcomes | Mental state: insight (ITAQ scores) Satisfaction with treatment: leaving the study early Engagement with services: compliance with medication |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.117). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Four cases withdrew from the study early. Two cases in each group. Low proportion of dropouts. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Zhang 2015.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatient, China Length of follow‐up: 8 weeks |
|
| Participants | Diagnosis: first‐episode schizophrenia in recovery phase (CCMD‐3) N = 90 Sex: 42 M, 48 F Age: 23 ‐ 59 years; mean ˜ 35.18 years, SD ˜ 2.39 years Included: not reported Excluded: psychotic symptoms after medication treatments; other complications |
|
| Interventions | 1. CBT group*: N = 45 Content: CBT included cognitive therapy and rational‐emotive therapy. Cognitive therapy helped participants to change negative thinking by providing psychoeducation. In rational‐emotive therapy, doctors planned therapy for each participant individually depending on participants' background and symptoms, to help participants to build up confidence and solve emotional problems. The therapies included psycho‐diagnosis, help for participants to understand, analysis of participants' background, implementation and strengthening of therapies. Delivered by: qualified doctors Frequency: not stated Treatment duration: 8 weeks 2. Standard care group*: N = 45 Content: routine nursing Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks |
|
| Outcomes | Mental state: somatisation, obsessive‐compulsive disorder, sensitivity of interpersonal relationship, depression, anxiety, hostility, phobia, paranoia, psychotic symptoms (SCL‐90 scores) | |
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to..." (p.191). Comments: No details of the randomisation procedure were provided. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The author did not describe allocation concealment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not describe the blinding of participants and personnel. However, as the CBT was based on standard care, participants and personnel were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The author did not describe the blinding of outcome assessment. Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Zhao 2013.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 8 weeks |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 100 Sex: 33 M, 65 F Age: 18 ‐ 60 years, mean ˜ 36 years, SD ˜ 7 years Included: length of illness: mean ˜ 6.2 years, SD ˜ 3.5 years; schizophrenia (CCMD‐3) Excluded: mental retardation; dementia or other brain organic disease; severe physical disorder |
|
| Interventions | 1. CBT group*: N = 50 Content: psychoeducation about symptoms and coping strategies for symptoms; cognition modification, and encouragement of social intercourse Delivered by: five psychologists Frequency: 45‐minute session, one or two sessions per week for 4 weeks, three sessions or four sessions per month after 4 weeks Treatment duration: 8 weeks 2. Standard care group: N = 50 Content: antipsychotics Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks |
|
| Outcomes | Mental state: clinically important change (no improvement)**, general (BPRS scores) Satisfaction with treatment: leaving the study early Unable to use: Social support (not predefined outcome for this review) |
|
| Notes | *Participants in the CBT group also received the standard care intervention. **Defined as reduction in BPRS score < 30% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.117). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: Two participants in the control group dropped out from the study, with no reasons reported. It was not possible that the low proportion of missing dataaffected the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Zhao 2014.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 8 weeks |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) N = 120 Sex: 57 M, 63 F Age: 30 ‐ 50 years old, mean ˜ 35.26 years, SD ˜ 2.24 years Included: length of illness: mean ˜ 42.3 months, SD ˜ 1.21 months Excluded: brain organic disease; severe physical disorder; personality disorder; alcohol or drug abuse |
|
| Interventions | 1. CBT group*: N = 60 Content: practicing daily life activity, entertainment therapy, and cognition modification Delivered by: not stated Frequency: not stated Treatment duration: 8 weeks 2. Standard care group: N = 60 Content: antipsychotics and nursing care Delivered by: not reported Frequency: not reported Treatment duration: 8 weeks |
|
| Outcomes | Mental state: general (PANSS scores) Unable to use: Behaviour: NOSIE ‐ not predefined outcome for this review |
|
| Notes | *Participants in the CBT group also received the standard care intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.209). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
Zou 2013.
| Methods | Allocation: randomised Blinding: not addressed Location: inpatients, China Length of follow‐up: 3 months |
|
| Participants | Diagnosis: schizophrenia (ICD‐10) N = 133 Sex: 74 M, 59 F Age: 18 ‐ 65 years, mean ˜ 32.65 years, SD ˜ 12.4 years Included: length of illness: mean ˜ 7.23 years, SD ˜ 3.32 years; the total score of PANSS ≥ 60 Excluded: with severe physical disease |
|
| Interventions | 1. CBT group*: N = 65 Content: cognition modification, psychoeducation about disease, and physical exercise Delivered by: nurses who had five years experience of CBT Frequency: 40 minutes each session for 10 sessions Treatment duration: 12 weeks 2. Standard care group: N = 68 Content: antipsychotics, psychoeducation and nursing care Delivered by: not reported Frequency: not reported Treatment duration: 12 weeks |
|
| Outcomes | Global state: relapse** Engagement with services: compliance with medication** Unable to use: Adverse events: weight gain ‐ SD not reported |
|
| Notes | *Participants in the CBT group also received the standard care intervention. **Trial authors did not report ICC; we assumed ICC = 0.1, as stated in the methods. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned..." (p.33). Comments: insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Comments: The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: The author did not address this information. However, participants and personnel were not likely to be blinded because participants in the treatment group received CBT, and the control group only received standard care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comments: The method of blindness was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: no attrition |
| Selective reporting (reporting bias) | Low risk | Comments: All measured outcomes were reported. |
| Other bias | Low risk | Comments: none obvious |
AAT: ABC = ADL = Activity of Daily Living Scale AHR = AHRS = Auditory Hallucinations Rating Scale AO = ASI = The Addiction Severity Index ASIQ = Adult Suicidal Ideation Questionnaire B & B = BAVQ/BAVQ‐R = Beliefs About Voices Questionnaire BDI = Beck Depression Inventory BAI = Beck Anxiety Inventory BAVQ = Belief about Voices Questionnaire BCSI = Beck Cognitive Insight Scale BCSS = Brief Core Schema Scales BDI = Beck Depression Inventory‐II BHS = The Beck Hopelessness Scale BIS = Birchwood Insight Scale BPRS = Brief Psychiatric Rating Scale BSS = Beck Scale for Suicidal ideation CAN = The Camberwell Assessment of Needs CAS = CAT = CBT = CBTp = CCMD(‐2‐R), also (‐3) = CCS = Cybernetic Coping Scale CDS = CDSS/CDS = Calgary Depression Scale CGI = CHOICE = CMHT = CMS = Clinical Memory Scale CPRS = Comphrehensive Schizophrenia Change Scale CRI = CSQ = the Client Satisfaction Questionnaire CGI‐GI = Clinical Global Impression‐global improvement CGI‐SI = Clinical Global Impression‐severity of illness CTCH = DRS = Delusion Rating Scale DSM‐IV(‐TR) or DSM‐III(‐R) = ECT = EI = EPPIC = EuroQOL = F = GAF = Global Assessment of Functioning GPTS = Green et al Paranoid Thoughts Scale GQOLI‐74 = GSH = GSES = General Self‐Efficacy Scale HADS = Hospital Anxiety and Depression Scale HAMD = MATRICS Consensus Cognitive Battery ICD‐10 = IES = Impact of Events Scale ILSS = Independent Living Skills Survey IPROS = Inpatient psychiatric rehabilitation outcomes scale IQ = IS = The Insight Scale IT = ITAQ = Insight and Treatment Attitudes Questionnaire ITT = LSP = Life Skills Profile M = MADS = Maudsley Assessment of Delusions Schedule MADRS = Montgomery‐Asberg Depression Rating Scale MARS = Medication Adherence Rating Scale MCAS = Multnomah Community Ability Scale MCCB = MATRICS Consensus Cognitive Battery MICBT = MMSE = Mini‐Mental State Examination NOSIE = Nurses' Observation Scale for Inpatient Evaluation NSRS = Negative Symptom Rating scale P1 = P3 = PANSS = The Positive and Negative Syndrome Scale PBIQ = Personal Beliefs about Illness Questionnaire PSE ‐ 10 = Present State Examination PSP = Personal Social Performance Scale PsyRATS = Psychotic Symptom Rating Scales PSWQ = Penn State Worry Questionnaire PTQ = Perseverative Thinking Questionnaire QLS = Quality of Life Scale RDC = RSCQ = Robson Self Concept Questionnaire RSES = Rosenberg Self‐Esteem scale RSQ = Robson Self‐Concept Questionnaire SAI = the Schedule for Assessment of Insight SADS = Social Avoidance and Distress Scale SANS = The Scale for the Assessment of Negative Symptoms SAPS = The Scale for the Assessment of Positive Symptoms SAS = Self‐rating Anxiety Scale SBS = Social Behaviour Schedule SCID‐1 = SCL‐90 = SCQ = Self Concept Questionnaire SCS = Social Comparison Scale SDS = Self‐rating Depression Scale SDSS = Social Disability Screening Schedule SERS = Self‐Esteem Rating Scale SES = The Self‐Esteem Scale SF‐36 = The Short Form‐36 SFS = The Social Functioning Scale SOFAS = The Social and Occupational Functioning Assessment Scale SPS = The Social Provision Scale SPS = The Suicide Probability Scale SQLS = Schizophrenia Quality of Life Scale SRIS = Self‐Report Insight Scale SSPI = Scale of Social‐Skills for Psychiatric Inpatients ST = TAU = Treatment‐as‐usual TESS = Treatment Emergent Symptom Scale UPSA = UCSD Performance‐Based Skills Assessment VCS = VPD = WAIS ‐ RC = Wechsler adult intelligence scale ‐ revised WCST = The Wisconsin Card Sorting Test WEMWS = WHODAS = WHOQOL‐BREF = World Health Organization Quality of Life Assessment abbreviated version WMS = WRAT = Wide Range Achievement Test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12606000 | Allocation: randomised Participants: not schizophrenia. Study undertaken with persons who were at ultrahigh risk of transition into psychosis. |
| Agius 2007 | Allocation: an 'open‐label' cohort study |
| Bach 2002 | Allocation: randomised Participants: nonaffective psychotic disorder (schizophrenia or schizoaffective disorder). Interventions: the intervention was not a traditional CBT, but about radical acceptance without cognitive or behavioural modifications. |
| Barrowclough 2006 | Allocation: randomised Participants: people with schizophrenia Interventions: group CBT + treatment‐as‐usual versus treatment‐as‐usual |
| Bechdolf 2004 | Allocation: randomised controlled trial Participants: people with schizophrenia Interventions: CBT versus psychoeducation |
| Bechdolf 2005b | Allocation: an uncontrolled prospective study |
| Bradshaw 1996 | Allocation: randomised controlled trial Participants: people with schizophrenia Interventions: CBT versus problem‐solving group |
| Bradshaw 2000 | Allocation: randomised Participants: people with schizophrenia Intervention: CBT plus standard care versus standard care Outcome: no usable data, the author did not report the number of participants in each group. |
| Byerly 2005 | Allocation: not randomised |
| Cai 2014c | Allocation: randomised Participants: people with psychosis (type not stated) |
| Cather 2005 | Allocation: randomised Participants: schizophrenia or schizoaffective disorder, depressed type with residual psychotic symptoms Interventions: CBT versus psychoeducation |
| Cella 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: cognitive remediation plus treatment‐as‐usual versus treatment‐as‐usual |
| Chen 2012 | Allocation: retrospective study |
| ChiCTR‐TRC‐14004187 2014 | Allocation: randomised Participants: schizophrenia, schizoaffective, or delusional disorder according to the criteria of DSM‐V (APA, 2013) Interventions: group CBT versus control intervention (unclear intervention) |
| Deng 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: group CBT plus entertainment therapy versus entertainment therapy |
| Dong 2015 | Allocation: randomised Participants: schizophrenia Interventions: CBT plus risperidone versus risperidone Outcomes: no usable data and the author did not reported the tools used for measurements |
| Drury 1996 | Allocation: randomised Participants: schizophrenia or schizoaffective disorder, depressed type with residual psychotic symptoms Interventions: CBT versus recreation and support |
| Du 2016 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT plus fluoxetine and other conventional medicine versus fluoxetine and other conventional medicine Outcomes: no usable data and the author did not state the duration of treatment |
| Eack 2014 | Allocation: randomised Participants: people with substance misuse and schizophrenia |
| Farreny 2012 | Allocation: randomised Participants: people with schizophrenia Interventions: cognitive remediation (not CBT) versus leisure control |
| Favrod 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: meta‐cognitive training plus treatment‐as‐usual versus treatment‐as‐usual |
| Feng 2013 | Allocation: not randomised |
| Gaudiano 2006 | Allocation: randomised
Participants: people with psychotic disorder Interventions: not CBT, active intervention based on Acceptance and Commitment Therapy |
| Granholm 2007 | Allocation: randomised
Participants: people with chronic schizophrenia Interventions: combination of CBT and other active therapies (social skills training plus cognitive remediation/rehabilitation) |
| Haddock 1998 | Allocation: randomised
Participants: people with schizophrenia or schizoaffective disorder (DSM‐IV) Intervention: CBT versus supportive counselling |
| Hang 2014 | Allocation: quasi‐randomised |
| Hert 2000 | Allocation: randomised
Participants: people with schizophrenia Interventions: not CBT, active intervention was a relapse prevention program |
| Hogarty 1997 | Allocation: randomised
Participants: people with schizophrenia Interventions: not CBT, active intervention was personal therapy |
| Hogarty 2004 | Allocation: randomised
Participants: people with schizophrenia or schizoaffective disorder Interventions: not CBT, active intervention focused upon cognitive neurorehabilitation and retraining |
| Huang 2014 | Allocation: quasi‐randomised |
| Ibranhim 2012 | Allocation: quasi‐randomised |
| ISRCTN11889976 | Allocation: randomised Participants: people with first episode psychosis |
| ISRCTN34966555 | Allocation: randomised Participants: people with psychosis with positive symptoms Interventions: CBT delivered through mobile application (app) versus symptoms monitoring app |
| ISRCTN47998710 | Allocation: randomised Participants: people with psychological difficulties, not schizophrenia |
| ISRCTN77762753 | Allocation: randomised Participants: auditory hallucinations (people who heard distress voices) |
| Jackson 1998 | Allocation: not randomised |
| Jackson 2008 | Allocation: randomised
Participants: people with schizophrenia or schizoaffective disorder Interventions: CBT versus Befriending (not treatment‐as‐usual) |
| Jenner 2004 | Allocation: randomised Participants: PenllteDt (> 10 years), drug‐refractory auditory hallucinations |
| Jiang 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT plus standard care plus psychoeducation versus standard care plus psychoeducation Outcomes: No usable data; the reported outcomes were not predefined for this review (Activity of Daily Living score, Fast Blood Glucose, Post prandial glucose after 2 hours, and HbA1c) |
| Johnson 2008 | Allocation: randomised Participants: outpatients with schizophrenia Interventions: group CBT versus group supportive therapy |
| Kidd 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: cognitive remediation plus supported education versus supported education |
| Klingberg 2009 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT versus cognitive remediation |
| Kong 2015 | Allocation: quasi‐randomised study |
| Kuipers 2004 | Allocation: randomised Participants: people with any functional psychosis Interventions: Croydon Outreach and Assertive Support Team versus treatment‐as‐usual |
| Lang 2014 | Allocation: randomised Participants: children with schizophrenia Interventions: CBT plus standard care versus standard care Outcomes: no usable data, the reported outcomes were not predefined for this review (subscale scores of Wechsler Memory Scale) |
| Leclerc 2000 | Allocation: randomised Participants: people with schizophrenia Interventions: coping skill (not CBT) versus control |
| Lecomte 2008 | Allocation: randomised Participants: people with schizophrenia Interventions: group CBT plus standard care versus standard care versus social skill training |
| Li 2013 | Allocation: randomised Participants: people with schizophrenia Interventions: group CBT plus antipsychotics versus antipsychotics |
| Li 2013b | Allocation: randomised Participants: people with schizophrenia Interventions: group CBT plus antipsychotics versus antipsychotics |
| Li 2014b | Allocation: randomised Participants: people with schizophrenia Interventions: CBT versus traditional health education (not standard care) |
| Li 2015c | Allocation: quasi‐randomised study |
| Lin 2014 | Allocation: randomised Participants: schizophrenia with depression Interventions: CBT citalopram plus antipsychotics versus citalopram plus antipsychotics Outcomes: no usable data, the author did not report data for predefined outcomes |
| Lincoln 2012 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT with elements of meta‐cognitive techniques |
| Liu 2013 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT and token therapy plus standard care versus standard care |
| Liu 2015 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT plus standard care versus standard care Outcomes: no usable data, the reported outcomes were not predefined for this review |
| Lu 2012 | Allocation: randomised Participants: people with schizophrenia Interventions: cognitive remediation versus treatment‐as‐usual |
| Lu 2014a | Allocation: randomised Participants: schizophrenia Interventions: CBT versus general supportive psychotherapy |
| Lysaker 2009 | Allocation: randomised Participants: people with schizophrenia spectrum disorders Interventions: Indianapolis Vocational Intervention Program versus support services |
| McLeod 2007 | Allocation: randomised Participants: people with schizophrenia who were experiencing auditory hallucinations Interventions: CBT plus standard care versus standard care Outcomes: no usable data, author did not report any predefined outcome data relevant to this review |
| Mo 2015 | Allocation: quasi‐randomised |
| Morrison 2014 | Allocation: randomised Participants: people at risk of schizophrenia ‐ not people with schizophrenia |
| NCT00810355 | Allocation: randomised Participants: people with schizophrenia Interventions: Cognitive Behaviour Therapy (CBT) combined with Cognitive Remediation (CR) versus CBT alone versus Support Services (SS) alone |
| NCT00960375 | Allocation: randomised Participants: people with schizoaffective disorder or schizophrenia mood disorders, or both, with psychotic features Interventions: behavioural treatment of smoking cessation versus a manualised smoking cessation program |
| NCT02105779 | Allocation: randomised Participants: people with schizophrenia Interventions: cognitive remediation treatment versus control |
| NCT02420015 | Allocation: randomised Participants: people with schizophrenia Intervention: the two groups received cognitive‐behavioural smoking cessation counselling |
| NCT02535923 | Allocation: randomised Participants: people with psychosis Intervention: CBT versus 'health and wellness' |
| NCT02751632 | Allocation: randomised Participants: people with psychosis Intervention: CBT versus 'support and problem‐solving' |
| Nordentoff 2005 | Allocation: randomised Participants: first episode of psychosis Interventions: not CBT, assertive community treatment, family involvement, and social skills training |
| O'Connor 2007 | Allocation: randomised Participants: people with delusional disorders (criteria for schizophrenia had never been met) |
| O'Donnell 2003 | Allocation: randomised Participants: people with schizophrenia Interventions: compliance therapy versus supportive counselling |
| O'Driscoll 2015 | Allocation: only one arm from an RCT |
| Owen 2015 | Allocation: quasi‐randomised |
| Penades 2006 | Allocation: randomised Participants: people with schizophrenia disorder Interventions: CBT versus cognitive remediation therapy |
| Penn 2009 | Allocation: randomised Participants: people with schizophrenia spectrum disorders Interventions: group CBT versus Supportive Therapy |
| Phillips 2002 | Allocation: not randomised Participants: people at risk of developing psychosis |
| Pinto 1999 | Allocation: randomised Participants: people with schizophrenia disorder Interventions: CBT combined with social skill training versus supportive therapy |
| Qi 2012 | Allocation: quasi‐randomised |
| Rector 2005 | Allocation: randomised Participants: people with schizophrenia Intervention: CBT versus psychoeducation (psychoeducation was not standard care) |
| Reeder 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: cognitive remediation versus treatment‐as‐usual |
| Richmond 2005 | Allocation: randomised Participants: schizophrenia with tobacco dependence Interventions: Cognitive Behaviour Therapy (CBT) and nicotine replacement therapy (NRT) versus treatment‐as‐usual |
| Sellwood 2000 | Allocation: randomised Participants: schizophrenia Interventions: family‐based intervention versus treatment‐as‐usual |
| Sensky 2000 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT versus befriending |
| Shao 2013 | Allocation: randomised Participants: people with psychosis, with 59% schizophrenia Interventions: CBT plus antipsychotics versus antipsychotics Outcomes: no usable data, the author did not report the treatment duration and length of follow‐up |
| Shi 2015 | Allocation: cluster‐randomised Participants: people with chronic schizophrenia Interventions: CBT plus medication versus medication Outcomes: no usable data, the author did not report the number of clusters |
| Song 2012 | Allocation: randomised Participants: people with schizophrenia Interventions: group CBT versus control group (unclear intervention) |
| Song 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: group CBT plus standard care versus standard care |
| Turkington 2008 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT versus befriending |
| Valmaggia 2005 | Allocation: randomised Participants: people with schizophrenia Intervention: CBT versus supportive counselling |
| Wang 2003 | Allocation: randomised Participants: first‐episode schizophrenia Intervention: cognitive therapy plus antipsychotics versus antipsychotics. The cognitive therapy focused on acceptance without cognitive or behavioural modifications. |
| Wang 2013 | Allocation: randomised Participants: people with schizophrenia Interventions: antipsychotics plus CBT versus antipsychotics plus health education |
| Wang 2013a | Allocation: randomised Participants: people with schizophrenia Interventions: cognitive existence intervention versus community follow‐up |
| Wang 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: not CBT but cognitive rehabilitation nursing |
| Wei 2012 | Allocation: randomised Participants: people with schizophrenia Interventions: cognitive therapy (not CBT) plus antipsychotics versus antipsychotics |
| Wu 2012 | Allocation: randomised Participants: first‐episode paranoid schizophrenia Interventions: CBT plus antipsychotics versus antipsychotics Outcomes: no usable data, the author did not report the number of participants in each group |
| Wu 2013 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT versus routine nursing psychological care (the nursing psychological care was not standard care and the CBT group did not receive this psychological care) |
| Wykes 2005 | Allocation: randomised Participants: people with schizophrenia Interventions: group CBT plus standard care versus standard care |
| Xie 2013 | Allocation: quasi‐randomised |
| Xu 2014 | Allocation: quasi‐randomised |
| Yang 2012 | Allocation: randomised Participants: people with schizophrenia Interventions: antipsychotics plus CBT versus antipsychotics plus health education (the health education was not standard care and the CBT group did not receive health education) |
| Zeng 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: CBT plus standard care versus standard care Outcomes: no usable data, the author did not report the treatment duration |
| Zhang 2005 | Allocation: quasi‐randomised |
| Zhao 2012 | Allocation: randomised Participants: people with schizophrenia Interventions: cognitive insight therapy plus medication versus medication |
| Zhou 2015b | Allocation: randomised Participants: people with schizophrenia Interventions: group CBT versus standard care |
CBT = CR = DSM‐IV (or ‐V) = NRT = PenliteD = SS =
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2015c.
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: schizophrenia Total: N = 73 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. CBT plus regular medication plus psychiatric nursing group 2. Regular medication plus psychiatric nursing group |
| Outcomes | Quality of life |
| Notes | Awaiting full text |
Fohlmann 2010.
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: a diagnosis with schizophrenic spectrum disorder (F2, ICD‐10) and co‐occurring cannabis abuse (F12, ICD‐10) Total: N = 103 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Motivational Interviewing (MI) and Cognitive Behavioural Therapy (CBT) group 2. Standard care (treatment‐as‐usual) group |
| Outcomes | Awaiting full text |
| Notes | Awaiting full text |
Hardy 2015.
| Methods | Allocation: randomised Blinding: single‐blind Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: PTSD (post‐traumatic stress disorder) in schizophrenia (DSM‐IV) Total: N = 61 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. CBT group 2.Treatment‐as‐usual group |
| Outcomes | PTSD symptoms Positive and negative symptoms |
| Notes | Awaiting full text |
Hassan 2014.
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: psychotic illness Total: N = 14 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Cognitive‐behavioural intervention for weight loss group 2. Standard care (treatment‐as‐usual) group |
| Outcomes | Awaiting full text |
| Notes | Awaiting full text |
Moun 2015.
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: patients with schizophrenia Total: N = 74 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Homebased cognitive training along with standard care (treatment‐as‐usual) group 2. Standard care (treatment‐as‐usual) alone group |
| Outcomes | Awaiting full text |
| Notes | Awaiting full text |
Nagui 2016.
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: schizophrenia Total: N = 40 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. CBT plus standard care group 2. Standard care (treatment‐as‐usual) group |
| Outcomes | Mental state: PANSS score General functioning: the Arabic version of Beliefs About Voices Questionnaire (BAVQ) and the General Assessment of Functioning scale (GAF) |
| Notes | Awaiting full text |
Tang 2015.
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: first‐episode schizophrenia Total: N = 120 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. CBT plus palperidone palmitate group 2. Paliperidone palmitate group |
| Outcomes | Mental state: no clinical response, PANSS score Adverse events: TESS score General functioning: relapse, rehospitalisation, individual/social function ‐ PSP score Satisfaction with treatment: MSQ score |
| Notes | Awaiting full text |
Tecic 2012.
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: patients with schizophrenia Total: N = 49 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Modified cognitive behavioural treatment (mCBT) plus standard care (treatment‐as‐usual) group 2. Standard care (treatment‐as‐usual) alone group |
| Outcomes | PANSS Positive‐Scale The Global Functioning Scale (GAF) Quality of Life (MSLQ) |
| Notes | Awaiting full text |
Characteristics of ongoing studies [ordered by study ID]
Edwards 2008.
| Trial name or title | A clinical audit of the first six months of care of first‐episode psychosis patients in seven European sites |
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: Total: N = 235 Sex: not reported Age: 15 ‐ 35 years old Length of illness: not reported Inclusion criteria: aged 15 – 35 years at service entry; first episode of schizophrenia or related psychotic disorder; and entering the service during a designated period Exclusion criteria: not reported |
| Interventions | 1. Cognitive behavioural case management plus standard care group: N = N/A Content: not reported Delivered by: not reported Frequency: not reported Treatment duration: not reported 2. Standard care group: N = N/A Content: not reported Delivered by: not reported Frequency: not reported Treatment duration: not reported |
| Outcomes | Outcomes: not reported |
| Starting date | 2003 |
| Contact information | PD McGorry, ORYGEN Youth Health, Melbourne Health and University of Melbourne |
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. |
ISRCTN06022197.
| Trial name or title | A pilot study of a randomised controlled trial of antipsychotic medication in comparison to cognitive behaviour therapy and a combined treatment in adults with psychosis |
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, or delusional disorder (ICD‐10) Total: N = 60 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Cognitive behaviour therapy plus antipsychotics group 2. Antipsychotics medication group |
| Outcomes | PANSS. Clinical global impression scales (CGI) Hospital Anxiety and Depression scale (HADS) Personal and social performance scale (PSP) Questionaire about the process of recovery (QPR) WHOQOL |
| Starting date | March 1, 2104 |
| Contact information | Miss Heather Law Psychology Department Prestwich Hospital Bury New Road Pretwich Manchester M25 3BL United Kingdom heather.law@gmw.nhs.uk |
| Notes | None |
ISRCTN12668007.
| Trial name or title | The Nightmare Intervention Study: a pilot randomised controlled trial of a brief cognitive behavioural therapy for nightmares for patients with persecutory delusions |
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: not reported Total: N = N/A Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: participants experiencing regular nightmares, persistent persecutory delusions, and having a diagnosis of non‐affective* (not related to disturbance of mood) psychosis (e.g. schizophrenia) Exclusion criteria: not reported |
| Interventions | 1. CBT plus standard care group 2. Standard care group |
| Outcomes | Acceptability and feasibility of the intervention and recruitment and retention rates Nightmare severity ‐ Distressing Dreams and Nightmare Severity Index Psychological well‐being ‐ Warwick Edinburgh Mental Wellbeing Scale Persecutory beliefs ‐ Green Paranoid Thoughts Scale Hallucinatory experiences ‐ Cardiff Anomalous Perceptions Scale Affect ‐ Depression Anxiety and Stress Scale ‐ 21‐item version Symptoms of insomnia ‐ Sleep Condition Indicator Sleep quality ‐ Pittsburgh Sleep Quality Index Dissociative symptoms ‐ Brief Dissociative Experiences Scale |
| Starting date | November 16, 2015 |
| Contact information | Dr Bryony Sheaves Department of Psychiatry Warneford Hospital Warneford Lane Headington Oxford OX3 7JX United Kingdom +44 1865 226486 bryony.sheaves@psych.ox.ac.uk [mail to:bryony.sheaves@psych.ox.ac.uk] |
| Notes | Not yet recruiting *We think 'non‐affective' could be schizophrenia, but not necessarily 100%. In this case, we would give this trial the benefit of the doubt and include it. |
ISRCTN33695128.
| Trial name or title | The effects of using cognitive behavioural therapy to improve sleep for patients with delusions and hallucinations (the BEST study): study protocol for a randomised controlled trial |
| Methods | Allocation: randomised Blinding: assessor blinded Location: outpatient and inpatient clinical teams, UK Length of follow‐up: 6 months |
| Participants | Diagnosis: clinical diagnosis of schizophrenia, schizoaffective disorder, or delusional disorder N = 60 Sex: not reported Age: 18 ‐ 65 years Length of illness: not reported Inclusion criteria: a current delusion or hallucination that has persisted for at least three months; a score of at least 2 on the distress scale of the Psychotic Symptom Rating Scales (PSYRATS) for either a delusion or hallucination; a clinical diagnosis of schizophrenia, schizoaffective disorder, or delusional disorder (that is, diagnosis of non‐affective psychosis (F2) in the International Classification of Diseases and Diagnostic and Statistical Manual IV); sleep difficulties lasting one month or longer with an ISI score of 15 or above (that is, above subthreshold insomnia). Participants must be aged between 18 and 65, and, where changes in medication are being made, entry to the study would not occur until at least a month after stabilisation of dosage. It should be noted that we will be seeing participants. Exclusion criteria: a primary diagnosis of sleep apnoea, alcohol or substance dependency, an organic syndrome or learning disability, a command of spoken English inadequate for engaging in therapy; and current individual CBT |
| Interventions | 1. CBT plus standard care group: N = 30 Content: 1) psychoeducation about sleep difficulties, assessment of the triggering and maintenance of sleep difficulties, and goal setting, 2) active therapeutic techniques that are used included sleep hygiene, stimulus control therapy, 3) relaxation, and, less often, cognitive techniques to address unhelpful beliefs and attitudes about sleep, attentional bias, monitoring, and safety behaviours. The intervention is deliberately simplified, with the principal therapeutic technique being stimulus control; that is, learning to associate bed with sleep. Delivered by: carried out by a qualified clinical psychologist Frequency: up to 8 sessions over 12 weeks; follow‐up at 6 months Treatment duration: 12 weeks 2. Standard care group: N = 30 Content: standard care delivered according to national and local service protocols and guidelines Delivered by: not reported Frequency: up to 8 sessions over 12 weeks; follow‐up at 6 months Treatment duration: 12 weeks |
| Outcomes | The Positive and Negative Symptom Scale Insomnia: ISI (Insomnia Severity Index) Hallucinations: PSYRATS The Beck Anxiety Inventory The Beck Depression Inventory Insomnia (self‐reported): the Pittsburgh Sleep Quality Index Quality of life: EQ‐5D‐5 levels Well‐being: the Warwick‐Edinburgh Mental Well‐being Scale The priorities, such as self‐confidence, peace of mind, and a sense of being in control: CHOICE Fatigue: Multidimensional Fatigue Inventory Suspicious thoughts: the Green Paranoid Thoughts Scale Service use including medication consumption (type, dose, and time taken), use of alcohol, illicit drugs, and nicotine, physical health history, adverse events, and hospital admission data (including use of the Client Service Receipt Inventory. A night‐time worry scale and an activity diary |
| Starting date | November 1, 2012 |
| Contact information | Prof Daniel Freeman, University Department of Psychiatry Warneford Lane, Headington, City/town Oxford, Zip/Postcode OX3 7JX Country United Kingdom. Email Daniel.Freeman@psych.ox.ac.uk |
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. |
ISRCTN61621571.
| Trial name or title | Sustaining Positive Engagement and Recovery (SUPEREDEN) ‐ Improving social recovery in young people with emerging severe social disability |
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: patients with non‐affective psychosis Total: N = 150 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Social Recovery Orientated Cognitive Behavioural Therapy plus standard care group 2. Standard care group. |
| Outcomes | Time Use Survey The Positive and Negative Syndrome Scale (PANSS) |
| Starting date | July 1st, 2012 |
| Contact information | Prof Max Birchwood School of Psychology Edgbaston Birmingham B15 2TT United Kingdom m.j.birchwood.20@birmingham.ac.uk |
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. |
NCT00484302.
| Trial name or title | Specialized addiction treatment versus treatment as usual for young patients with cannabis abuse and psychosis (CapOpus) |
| Methods | Allocation: randomised Blinding: single‐blind Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: not reported Total: N = 140 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: young people with psychosis Exclusion criteria: not reported |
| Interventions | 1. all part of the cognitive therapeutical framework, using psychoeducation, cognitive behavioural therapy, and social skills training treatment‐as‐usual group 2. non‐specialised individual treatment group |
| Outcomes | Severity of abuse Influence and severity of other substance use PANSS Cognitive function Social functioning Quality of life User satisfaction Expenses for the experimental intervention |
| Starting date | June 2007 |
| Contact information | Merete Nordentoft, MD, PhD, MPH Tel: plus4520607552 Email:merete.nordentoft@dadlnet.dk |
| Notes | None |
NCT00495911.
| Trial name or title | A randomized controlled trial of individual therapy for first episode psychosis |
| Methods | Allocation: randomised Blinding: single‐blind Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: meet DSM‐IV criteria for: schizophrenia, schizophreniform disorder, brief psychotic disorder, delusional disorder, schizoaffective disorder, substance induced psychotic disorder, or psychotic disorder NOS Total: N = 309 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Cognitive behavioural therapy group 2. Befriending group 3. Routine care group |
| Outcomes | Social Functioning Scale (SFS) Positive and Negative Syndrome Scale (PANSS) Psychotic Symptom Rating Scales (PSYRATS) Calgary Depression SCale for Schizophrenia (CDSS) The Time‐Line Follow Back (TLFB) Alcohol and Drug Use Scale (AUS; DUS) Medication Event Monitoring System (MEMS) Rosenberg Self‐Esteem Scale Maastrich Assessment of Coping Skills (MACS) |
| Starting date | June 2007 |
| Contact information | Jean Addington, PhD Email: Jean_Addington@camh.net |
| Notes | None |
NCT02134418.
| Trial name or title | Evaluation of social skills intervention on cognitive function in schizophrenia |
| Methods | Allocation: randomised Blinding: open‐label Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: clinical diagnosis of schizophrenia Total: N = 35 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. CBT of irony comprehension group 2. No Intervention group |
| Outcomes | Irony behavioural hemispheric results Improved theory of mind abilities |
| Starting date | March 2015 |
| Contact information | Dror Dolfin Tel: 972505406993 Email: zdolfin@gmail.com |
| Notes | None |
NCT02380885.
| Trial name or title | RCT Social cognition training and therapeutic alliance focused therapy for persons with severe mental illness (RCT SCIT) |
| Methods | Allocation: randomised Blinding: double‐blind Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: severe mental disorders (schizophrenia, schizoaffective, bi‐polar, depression) Total: N = 120 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Social cognition and interaction training: psychosocial group intervention group 2. Therapeutic alliance focused therapy group 3. Standard care group |
| Outcomes | Wisconsin Social Quality of Life Scale
The Face Emotion Identification Task
Faux‐Pas task Ambiguous Intentions Hostility Questionnaire Social Skill Performance Assessment The Working Alliance Inventory Narrative evaluation of intervention interview |
| Starting date | January 2015 |
| Contact information | Ilanit Hasson‐Ohayon, Prof. Tel: plus97225318477 Email: ilanithasson@gmail.com |
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. |
NCT02408198.
| Trial name or title | The street smart group: a feasibility trial of a group intervention targeting anxiety processes in paranoia |
| Methods | Allocation: randomised Blinding: open‐label Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: non‐affective* psychosis (ICD‐10, F20‐F29) Total: N = 18 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Anxiety intervention, based on a brief CBT for psychosis (CBTp) programme (therapy will be delivered for a period of 6 weeks immediately after randomisation) group 2. Delayed therapy (therapy will be delayed until 10 weeks following randomisation, and then delivered over a 6 week period) group |
| Outcomes | Green Paranoid Thoughts Scale |
| Starting date | February 2015 |
| Contact information | Dr Amy Hardy, King's College London |
| Notes | The trial completed in May 2016, but no results were reported *We think 'non‐affective' could be schizophrenia, but not necessarily 100%. In this case, we would give this trial the benefit of the doubt and include it. |
NCT02427542.
| Trial name or title | Feasibility trial of CBT for depersonalisation in psychosis |
| Methods | Allocation: randomised Blinding: single‐blind Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: psychotic disorders Total: N = 30 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. CBT for depersonalisation/derealisation group 2. Standard care group |
| Outcomes | Feasibility of intervention
Feasibility estimates of delivering the intervention including recruitment rates, acceptance rates, dropouts
Depersonalisation score (Score on the Cambridge Depersonalisation Scale)
Acceptability of intervention Depression (Score on Beck Depression Inventory) Anxiety (Score on Beck Anxiety Inventory) Psychosis (Score on the Psychotic Symptom Rating Scale (PSYRATS) |
| Starting date | March 2015 |
| Contact information | Simone Farrelly Tel: plus447960431781 Email: simone.farrelly@kcl.ac.uk |
| Notes | *The term 'Treatment‐as‐usual (TAU)' was used in this paper. |
NCT02653729.
| Trial name or title | Cognitive behaviour therapy for psychosis in first episode patient and the outcome of cognitive behaviour therapy on psychotic symptoms |
| Methods | Allocation: randomised Blinding: double‐blind (participant, investigator) Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: schizophrenia Total: N = 50 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. CBT plus medications group (espidone, olepra, and donu) 2. Medications group (espidone, olepra, and donu) |
| Outcomes | PANSS, SAI, and PSRS score |
| Starting date | September 2015 |
| Contact information | Aisha Andleeb, Bahauddin Zakariya University |
| Notes | The trial was completed in January 2016, but no results were reported. |
NCT02787122.
| Trial name or title | Comparison of emotion‐focused cognitive behaviour therapy for patients with schizophrenia with standard treatment: effects on psychological parameters and rehospitalisation |
| Methods | Allocation: randomised Blinding: single‐blind (participant) Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, delusional disorder, or brief psychotic disorder Total: N = 80 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Emotion‐focused Cognitive behaviour therapy group 2. Treatment‐as‐usual group |
| Outcomes | Change in Psychotic Rating Scale (PSYRATS) delusions scale Change in Positive and Negative Syndrome Scale (PANSS) Change in Calgary Depression Rating Scale for Schizophrenia (CDSS) Change in Role Functioning Scale (RFS) Change in Paranoia Checklist (PCL) Change in Beck Depression Inventory‐II Change in Peters et al. Delusions Inventory Change in Reactions to Paranoid Thoughts Scale (REPT) Change in Symptom Checklist 9 (SCL‐9) Change in Satisfaction With Life Scale (SWLS) Change in Pittsburg Sleep Quality Inventory (PSQI) Change in number of social contacts (SozE) Change in Perseverative Thinking Questionnaire (PTQ) Change in Scale of Emotion Regulation Competencies (SEK‐27) Change in Self‐Compassion Scale (SCS) Change in Brief Core Schema Scale (BCSS) |
| Starting date | January 2014 |
| Contact information | Prof. Dr. Stephanie Mehl, Philipps University Marburg Medical Center |
| Notes | Not recruiting |
NCT02787135.
| Trial name or title | Efficacy and mechanisms of change of an emotion‐oriented version of cognitive‐behavioural therapy for psychosis (CBTp‐E) in reducing delusions. A randomized‐controlled treatment Study (CBTd‐E) |
| Methods | Allocation: randomised Blinding: single‐blind (participant) Duration: not reported Location: not reported Length of follow‐up: not reported |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, delusional disorder (DSM‐V) Total: N = 102 Sex: not reported Age: not reported Length of illness: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 1. Emotion‐oriented cognitive behaviour therapy group 2. Treatment‐as‐usual group |
| Outcomes | Change in Psychotic Rating Scale (PSYRATS) delusions scale Change in Positive and Negative Syndrome Scale (PANSS) Change in Calgary Depression Rating Scale for Schizophrenia (CDSS) Change in Role Functioning Scale (RFS) Change in Peters et al. Delusions Inventory (PDI‐R) Change in Emotion‐regulation Questionnaire (ERQ) Change in Emotion regulation Inventory Change in paranoia assessed with electronical mobile assessment Change in sleep quality as assessed with an Actiwatch Change in emotion regulation quality as assessed experimentally using International Affect Picture System paradigm for the assessment of emotion regulation (IAPS) Change in heart rate variability Change in Brief Core Schema Scale (BCSS) Change in Insomnia Severity Index (ISI) Change in Self‐Compassion Scale (SCS) Change in Self‐perception of emotional competencies (SEK‐27) |
| Starting date | May 2016 |
| Contact information | Stephanie Mehl, PhD Tel: +491631879762 Email: stephanie.mehl@staff.uni‐marburg.de |
| Notes | This study is currently recruiting participants. |
Waller 2014.
| Trial name or title | The effects of a brief CBT intervention, delivered by frontline mental health staff, to promote recovery in people with psychosis and comorbid anxiety or depression (the GOALS study): study protocol for a randomised controlled trial |
| Methods | Allocation: randomised
Blinding: All study members (including the statistician and assistant psychologists conducting the assessments) will be blind to treatment allocation. Duration: not reported Location: community psychosis teams, UK Length of follow‐up:18 weeks |
| Participants | Diagnosis: diagnosis of a schizophrenia spectrum disorder or currently experiencing psychotic symptoms Total: N = 66 Sex: not reported Age:18 to 65 years old Length of illness: not reported Inclusion criteria: diagnosis of a schizophrenia spectrum disorder or currently experiencing psychotic symptoms (for example, with diagnoses of personality disorder, bipolar disorder, or psychotic depression); 18 to 65 years old (or accessing adult services); clinical levels of anxiety‐related avoidance or depression on outcome measures; and a desire to increase the current level of activities Exclusion criteria: participants not meeting the above criteria, or who are currently refusing all medication; or who are currently or recently (previous 3 months) in receipt of CBT; or who have a primary diagnosis of an organic mental health problem; or who have a primary substance dependency |
| Interventions | Group 1: CBT plus standard care* group, n = 33 Content: Participants received eight weekly CBT sessions with a trained member of staff, each for around one hour. After one month, all participants will receive two brief CBT interventions: graded exposure for anxious avoidance and behavioural activation for depression. Delivered by: psychiatrists Frequency: 8 weekly sessions, 1 hour/session Treatment duration:8 weeks Group 2: standard care* group, n = 33 Content: All the treatment and support the participants were received before the start of the trial, including input from their general practitioner and psychiatrist, and they will be seen by their care coordinator at least monthly. Treatment duration:8 weeks |
| Outcomes | Activity: Time Budget Measure Psychotic symptoms: PANSS (the Positive and Negative Syndromes Scale); Psychotic Symptom Rating Scales Anxiety and Depression: HADS (Hospital Anxiety and Depression Scale); Mobility Inventory Well‐being, quality of life: the Warwick‐Edinburgh Mental Well‐being Scale; the Manchester Short Assessment of Quality of Life Clinical Outcomes in Routine Evaluation – 10 (CORE‐10) Cost‐effectiveness: the Client Service Receipt Inventory |
| Starting date | April 2013 |
| Contact information | Author's name: Helen Waller Institute: Department of Psychology, Institute of Psychiatry, King's College London Address: Department of Psychology, Institute of Psychiatry, King's College London, London, UK Email: helen.waller@kcl.ac.uk |
| Notes | * The term 'Treatment‐as‐usual (TAU)' was used in this paper. This is a protocol of an ongoing study. Trial registration: Current Controlled Trials ISRCTN: 73188383. http://public.ukcrn.org.uk/search/StudyDetail.aspx? StudyID=13538 Fund: The study is funded by the National Institute for Health Research: Research for Patient Benefit (reference: PB‐PG‐0711‐25010). The authors declare that they have no competing interests. |
Differences between protocol and review
1. Secondary objectives
We previous stated some additional objectives of the review were to assess if the following differences in participants or interventions had any effects:
people in their first episode of illness with those who have a longer history of illness;
level of therapist experience and qualification;
length of treatment/number of sessions.
These are now presented in the methods as subgroup analyses. It was, however, not possible to collect data for some of these subgroups (see below).
2. Outcomes
The outcomes have been reordered to reflect the order of outcomes reported in the 'Summary of findings' table. We have separated global state and general functioning outcomes for clarity. We have also made a post hoc decision regarding the importance of 'death' as a primary outcome for psychological interventions. We feel evidence regarding the global state of participants and their satisfaction with treatment are better outcomes by which to evaluate the effectiveness of CBT, rather than an event that rarely occurs with psychological therapies. Death is now part of the adverse events outcome. We have also changed outcomes from 'no' clinically important change to clinically important change to avoid the use of confusing double negatives. We have also used the longest follow‐up time point available for presenting in the SOF.
3. Methods update
We have updated the methods to reflect the latest changes in Cochrane Schizophrenia's template and harmonise the three sibling reviews in this suite of CBT reviews. This includes updates to sensitivity analyses.
4. Subgroup analyses
Due to lack of data, we have decided not to anticipate subgroup analysis for people in a first episode of illness versus those at a later stage of illness. The length of treatment is also now addressed in another Cochrane Review Naeem 2015a.
Contributions of authors
Chris Jones: ‐ protocol development, trial selection, report writing Dave Hacker ‐ protocol development, writing Jun Xia ‐ random check of the study screening and data extraction ‐ management of SRS authors Alan Meaden ‐ protocol development, 'risk of bias' assessment, checking of final report Claire Irving ‐ protocol development, 'risk of bias' assessment, substantial editorial checks, and rewriting of full report Sai Zhao: study selection, data extraction, 'risk of bias' assessment, draft write‐up of results Chunhu Shi ‐ screened and extracted data, 'risk of bias' assessment Jue Chen ‐ clinical input on the extraction and analysis of Chinese trial data, report writing
Sources of support
Internal sources
-
University of Birmingham, UK.
Employs lead author Chris Jones
-
Rampton Hospital, UK.
Employs review author Irene Cormac
-
Birmingham and Solihull Mental Health Foundation NHS Trust,, UK.
Employs review authors David Hacker and Alan Meaden
-
University of Nottingham, UK.
Host institution for Cochrane Schizophrenia
-
Shanghai Jiao Tong University School of Medicine, China.
Employs review author Jue Chen
-
University of Manchester, UK.
Employs review author Chunhu Shi
External sources
-
Systematic Review Solutions Ltd, UK.
Company receiving grant to complete trial selection and data extraction for this review. Employs review authors Jun Xia, Sai Zhao, Jue Chen and Chunhu Shi
Declarations of interest
Chris Jones: clinical psychologist who uses cognitive behavioural therapy for those with serious mental illnesses, employed by Birmingham and Solihull Mental Health Foundation Trust and the University of Birmingham.
David Hacker: clinical psychologist who uses cognitive behavioural therapy for those with serious mental illnesses, employed by Birmingham and Solihull Mental Health Foundation Trust.
Jun Xia: Owner of SRS ‐ Systematic Review Solutions ‐ professional review writing company.
Alan Meaden: none known.
Claire Irving: Managing Editor of Cochrane Schizophrenia.
Sai Zhao: Employed by SRS as an author to complete systematic reviews.
Chunhu Shi: Employed by SRS as an author to complete systematic reviews.
Jue Chen: Employed by SRS as an author to complete systematic reviews.
New
References
References to studies included in this review
Barrowclough 2001 {published data only}
- Barrowclough C, Haddock G, Tarrier N, Lewis SW, Moring J, O'Brien R, et al. Randomized controlled trial of motivational interviewing, cognitive behavior therapy, and family intervention for patients with comorbid schizophrenia and substance use disorders. American Journal of Psychiatry 2001;158(10):1706‐13. [PUBMED: 11579006] [DOI] [PubMed] [Google Scholar]
- Haddock G, Barrowclough C, Tarrier N, Moring J, O'Brien R, Schofield N, et al. Cognitive‐behavioural therapy and motivational intervention for schizophrenia and substance misuse. 18‐month outcomes of a randomised controlled trial. British Journal of Psychiatry 2003;183:418‐26. [PUBMED: 14594917] [DOI] [PubMed] [Google Scholar]
Barrowclough 2010 {published data only}
- Barrowclough C, Haddock G, Beardmore R, Conrod P, Craig T, Davies L, et al. Evaluating integrated MI and CBT for people with psychosis and substance misuse: recruitment, retention and sample characteristics of the MIDAS trial. Addictive Behaviours 2009;34(10):859‐66. [PUBMED: 19362429] [DOI] [PubMed] [Google Scholar]
- Barrowclough C, Haddock G, Wykes T, Beardmore R, Conrod P, Craig T, et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial. BMJ (Clinical research ed.) 2010;341:c6325. [PUBMED: 21106618] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry K, Allott R, Emsley R, Ennion S, Barrowclough C. Perceived empowerment in people with a dual diagnosis of schizophrenia spectrum disorder and substance misuse. Social Psychiatry and Psychiatric Epidemiology 2014;49(3):377‐84. [PUBMED: 24141697] [DOI] [PubMed] [Google Scholar]
- Berry K, Gregg L, Hartwell R, Haddock G, Fitzsimmons M, Barrowclough C. Therapist‐client relationships in a psychological therapy trial for psychosis and substance misuse. Drug and Alcohol Dependence 2015;152:170‐6. [PUBMED: 25962788] [DOI] [PubMed] [Google Scholar]
- Berry K, Gregg L, Vasconcelos e Sa D, Haddock G, Barrowclough C. Staff‐patient relationships and outcomes in schizophrenia: the role of staff attributions. Behaviour Research and Therapy 2012;50(3):210‐4. [PUBMED: 22325807] [DOI] [PubMed] [Google Scholar]
- Hartley S, Haddock G, Barrowclough C. Anxiety and depression and their links with delusions and hallucinations in people with a dual diagnosis of psychosis and substance misuse: a study using data from a randomised controlled trial. Behaviour Research and Therapy 2012;50(1):65‐71. [PUBMED: 22088611] [DOI] [PubMed] [Google Scholar]
Barrowclough 2014 {published data only}
- Barrowclough C, Marshall M, Gregg L, Fitzsimmons M, Tomenson B, Warburton J, et al. A phase‐specific psychological therapy for people with problematic cannabis use following a first episode of psychosis: a randomized controlled trial. Psychological Medicine 2014;44(13):2749‐61. [DOI] [PubMed] [Google Scholar]
Birchwood 2014 {published data only}
- Birchwood M, Michail M, Meaden A, Lewis S, Davies L, Dunn G, et al. The MRC command trial: results of a multi‐centre, randomised controlled trial of cognitive therapy to prevent harmful compliance with command hallucinations. Schizophrenia Research 2014;153:S74. [Google Scholar]
- Birchwood M, Michail M, Meaden A, Tarrier N, Lewis S, Wykes T, et al. Cognitive behaviour therapy to prevent harmful compliance with command hallucinations (COMMAND): a randomised controlled trial. Lancet Psychiatry 2014;1(1):23‐33. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Peters E, Wykes T, Tarrier N, Lewis S, Dunn G, et al. A multi‐centre randomised controlled trial of cognitive therapy to prevent harmful compliance with command hallucinations. BMC Psychiatry 2011, (11):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN62304114. A multicentre, randomised controlled trial of cognitive therapy to reduce harmful compliance with command hallucinations. isrctn.com/ISRCTN62304114 (accessed 27 March 2008).
Cao 2014 {published data only}
- Cao J, Zhu J, Xu L, Lin L, Qiao S, Wu S. Study on the effects of cognitive‐behavioral intervention on the rehabilitation of first‐episode schizophrenia [认知行为干预对首发精神分裂症康复效果的追踪调查研究]. Journal of Psychiatry [精神医学杂志] 2014;27:297‐9. [Google Scholar]
Chen 2014 {published data only}
- Chen S, Wang H. The effect of cognitive behavioural therapy for depression in patients with schizophrenia [认知疗法对精神分裂症后抑郁的效果观察]. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease [实用心脑肺血管病杂志] 2014;22:85‐6. [Google Scholar]
Chen 2015 {published data only}
- Chen L, Yao H, Li X. CBT in the treatment of refractory auditory hallucinations of schizophrenia: a comparative study [CBT治疗精神分裂症顽固性幻听疗效的对照研究]. Journal of Qiqihar Medical College [齐齐哈尔医学院学报] 2015;36(14):2063‐5. [Google Scholar]
Durham 2003 {published data only}
- Durham RC, Guthrie M, Morton RV, Reid DA, Treliving LR, Fowler D, et al. Tayside‐Fife clinical trial of cognitive‐behavioural therapy for medication‐resistant psychotic symptoms. Results to 3‐month follow‐up. British Journal of Psychiatry 2003;182:303–11. [DOI] [PubMed] [Google Scholar]
Edwards 2011 {published data only}
- Edwards J, Cocks J, Burnett P, Maud D, Wong L, Yuen HP, et al. Randomized controlled trial of clozapine and CBT for first‐episode psychosis with enduring positive symptoms: a pilot study. Schizophrenia Research and Treatment 2011:394896. [DOI] [PMC free article] [PubMed] [Google Scholar]
England 2007 {published data only}
- England M. Efficacy of cognitive nursing intervention for voice hearing. Perspectives in Psychiatric Care 2007;43:69‐76. [DOI] [PubMed] [Google Scholar]
- England M. Significance of cognitive intervention for voice hearers. Perspectives in Psychiatric Care 2008;44:40‐7. [DOI] [PubMed] [Google Scholar]
Farhall 2009 {published data only}
- Farhall J, Freeman NC, Shawyer F, Trauer T. An effectiveness trial of cognitive behaviour therapy in a representative sample of outpatients with psychosis. British Journal of Clinical Psychology 2009;48:47‐62. [DOI] [PubMed] [Google Scholar]
Fowler 2009 {published data only}
- Barton GR, Hodgekins J, Mugford M, Jones PB, Croudace T, Fowler D. Cognitive behaviour therapy for improving social recovery in psychosis: cost‐effectiveness analysis. Schizophrenia Research 2009;112:158‐63. [DOI] [PubMed] [Google Scholar]
- Fowler D, Hodgekins J, Painter M, Reilly T, Crane C, Macmillan I, et al. Cognitive behaviour therapy for improving social recovery in psychosis: a report from the ISREP MRC Trial Platform study (Improving Social Recovery in Early Psychosis). Psychological Medicine 2009;39:1627‐36. [DOI] [PubMed] [Google Scholar]
Freeman 2014 {published data only}
- Freeman D, Pugh K, Dunn G, Evans N, Sheaves B, Waite F, et al. An early Phase II randomised controlled trial testing the effect on persecutory delusions of using CBT to reduce negative cognitions about the self: The potential benefits of enhancing self confidence. Schizophrenia Research 2014;160(1‐3):186‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 2015 {published data only}
- Freeman D, Dunn G, Startup H, Pugh K, Cordwell J, Mander H, et al. Effects of cognitive behaviour therapy for worry on persecutory delusions in patients with psychosis (WIT): a parallel, single‐blind, randomised controlled trial with a mediation analysis. Lancet Psychiatry 2015;2:305‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garety 2008 {published data only}
- Garety PA, Fowler DG, Freeman D, Bebbington P, Dunn G, Kuipers E. A randomised controlled trial of cognitive behavioural therapy and family intervention for the prevention of relapse and reduction of symptoms in psychosis. British Journal of Psychiatry 2008;192:412‐23. [DOI] [PubMed] [Google Scholar]
- Kuipers E, Kuipers. The role of CBT in relapse prevention of schizophrenia. Schizophrenia Research 2012;136(Suppl.1):S58‐9. [Google Scholar]
Gleeson 2009 {published data only}
- Gleeson J, Wade D, Castle D, Gee D, Crisp K, Pearce T, et al. The EPISODE II trial of cognitive and family therapy for relapse prevention in early psychosis: rationale and sample characteristics. Journal of Mental Health 2008;17:19‐32. [Google Scholar]
- Gleeson JFM, Cotton SM, Alvarez‐Jimenez M, Wade D, Gee D, Crisp K, et al. A randomized controlled trial of relapse prevention therapy for first‐episode psychosis patients. Journal of Clinical Psychiatry 2009;70:477‐86. [DOI] [PubMed] [Google Scholar]
Granholm 2005 {published data only}
- Granholm E, McQuaid JR, Auslander LA, McClure FS. Group cognitive‐behavioral social skills training for older outpatients with chronic schizophrenia. Journal of Cognitive Psychotherapy 2004;18:265‐79. [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Auslander LA, Perivoliotis D, Pedrelli P, et al. A randomized, controlled trial of cognitive behavioral social skills training for middle‐aged and older outpatients with chronic schizophrenia. American Journal of Psychiatry 2005;162:520‐9. [DOI] [PubMed] [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Link PC, Perivoliotis D, Gottlieb JD, et al. Randomized controlled trial of cognitive behavioral social skills training for older people with schizophrenia: 12‐month follow‐up. Journal of Clinical Psychiatry 2007;68:730‐7. [DOI] [PubMed] [Google Scholar]
- McQuaid JR, Granholm E, McClure FS, Roepke S, Pedrelli P, Patterson TL, et al. Development of an integrated cognitive‐behavioral and social skills training intervention for older patients with schizophrenia. Journal of Psychotherapy Practice and Research 2000;9(3):149‐56. [PMC free article] [PubMed] [Google Scholar]
Grawe 2006 {published data only}
- Grawe RW, Falloon IR, Widen JH, Skogvoll E. Two years of continued early treatment for recent‐onset schizophrenia: a randomised controlled study. Acta Psychiatrica Scandinavica 2006;114(5):328‐36. [PUBMED: 17022792] [DOI] [PubMed] [Google Scholar]
Gumley 2003 {published data only}
- Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al. Long‐term outcome of cognitive behaviour therapy clinical trials in central Scotland. Health Technology Assessment 2005;9(42):1–174. [DOI] [PubMed] [Google Scholar]
- Gumley A, Karatzias A, Power K, Reilly J, McNay L, O'Grady M. Early intervention for relapse in schizophrenia: impact of cognitive behavioural therapy on negative beliefs about psychosis and self‐esteem. British Journal of Clinical Psychology 2006;45:247‐60. [DOI] [PubMed] [Google Scholar]
- Gumley A, O'Grady M, McNay L, Reilly J, Power K, Norrie J. Early intervention for relapse in schizophrenia: results of a 12‐month randomized controlled trial of cognitive behavioural therapy. Psychological Medicine 2003;33:419‐31. [DOI] [PubMed] [Google Scholar]
Guo 2015 {published data only}
- Guo Z, Li Z, Ma Y, Zhou Y, Chao W, Guo J, et al. Short‐term cognitive‐behavioral treatment combined with regular therapy on community schizophrenia patients: preliminary observation of efficacy [短程认知行为治疗联合常规治疗对社区精神分裂症患者疗效的初步观察]. Chinese Journal of Psychiatry [中华精神科杂志] 2015;48(6):331‐8. [Google Scholar]
Habib 2015 {published data only}
- Habib N, Dawood S, Kingdon D, Naeem F. Preliminary evaluation of Culturally Adapted CBT for psychosis (CA‐CBTp): findings from Developing Culturally‐sensitive CBT Project (DCCP). Behavioural and Cognitive Psychotherapy 2015;43(2):200‐8. [DOI] [PubMed] [Google Scholar]
He 2012 {published data only}
- He X, Li Z, Chen Y. Efficacy of cognitive behavioral therapy for anxiety in females with first‐episode paranoid schizophrenia [认知行为疗法对首发女性偏执型精神分裂症康复期焦虑情绪的疗效观察]. Medical Journal of Chinese People's Health [中国民康医学] 2012;24(6):651‐3. [Google Scholar]
- Li Z, He X, Chen Y, Li Z. Control study of the effect of cognitive behavioral therapy combined with on the depression syndromes patients who had been in the recovery status of paranoid schizophrenia [认知行为疗法对首发女性偏执型精神分裂症康复期抑郁情绪疗效观察]. Medical Journal of Chinese People's Health [中国民康医学] 2012;5:518‐20. [Google Scholar]
Hu 2013 {published data only}
- Hu C. Clinical studies of cognitive behavioral therapy combined with medication for residual schizophrenia [认知行为联合药物治疗精神分裂症残留型40例临床研究]. Journal of Community Medicine [社区医学杂志] 2013;8:17‐9. [Google Scholar]
Hu 2014 {published data only}
- Hu X, Pan X. Effects of cognitive behavioral therapy on cognitive function in schizophrenia patients [认知行为治疗对精神分裂症患者认知功能的影响]. Medical Journal of Chinese People's Health [中国民康医学] 2014;26(7):8‐9. [Google Scholar]
Jackson 2009 {published data only}
- Jackson C, Trower P, Reid I, Smith J, Hall M, Townend M, et al. Improving psychological adjustment following a first episode of psychosis: a randomised controlled trial of cognitive therapy to reduce post psychotic trauma symptoms. Behaviour Research and Therapy 2009;47(6):454‐62. [DOI] [PubMed] [Google Scholar]
Jia 2005 {published data only}
- Jia Y, Lou F, Feng M. Cognitive behavioral nursing therapy for patients with schizophrenia. Journal of Nursing Science 2005;20:10‐2. [Google Scholar]
Jiao 2014 {published data only}
- Jiao C, Wu B, Zhang Z, Tang S, Sheng C, Li J, et al. Efficacy of alliance therapy of risperidone and cognitive‐behavioral treatment in schizophrenia patients during recovery stage [联合应用利培酮和认知行为疗法治疗康复期精神分裂症的疗效观察]. Contemporary Medicine Forum [当代医药论丛] 2014;12(12):238‐9. [Google Scholar]
Kuipers 1997 {published data only}
- Garety P, Kuipers E, Fowler D, Chamberlain F, Dunn G. Cognitive behavioural therapy for drug resistant psychosis. British Journal of Psychiatry 1994;67:259‐71. [DOI] [PubMed] [Google Scholar]
- Kuipers E, Fowler D, Garety P, Chisholm D, Freeman D, Dunn G, et al. London East‐Anglia randomised controlled trial of cognitive behavioural therapy for psychosis. British Journal of Psychiatry 1998;173:61‐8. [DOI] [PubMed] [Google Scholar]
- Kuipers E, Garety P, Fowler D, Dunn G, Freeman D, Bebbington P, et al. London East‐Anglia randomised controlled trial of cognitive behavioural therapy for psychosis. I: Effects of treatment phase. British Journal of Psychiatry 1997;171:319‐27. [DOI] [PubMed] [Google Scholar]
Lewis 2002 {published data only}
- Lewis S, Tarrier N, Haddock G, Bentall R, Kinderman P, Kingdon D, et al. Randomised controlled trial of cognitive‐behavioural therapy in early schizophrenia: acute‐phase outcomes. British Journal of Psychiatry 2002;181(S43):S91‐7. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Haddock G, Lewis S, Drake R, Gregg L. Suicide behaviour over 18 months in recent onset schizophrenic patients: the effects of CBT. Schizophrenia Research 2006;83(1):15‐27. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Lewis S, Haddock G, Bentall R, Drake R, Kinderman P, et al. Cognitive‐behavioural therapy in first‐episode and early schizophrenia. British Journal of Psychiatry 2004;184:231‐9. [DOI] [PubMed] [Google Scholar]
Li 2013a {published data only}
- 李杰. Cognitive therapy combined with clinical efficacy of ziprasidone treatment of schizophrenia and its impact on treatment compliance [认知疗法联合齐拉西酮治疗精神分裂症的临床疗效及对治疗依从性的影响]. Seek Medical and Ask the Medicine 2013;11(6):111‐2. [Google Scholar]
Li 2014 {published data only}
- Li X, Guo Y, Fu C, Chen L. Clinical efficiency and safety of CBT combined with low‐dose risperidone alliance treatment on auditory hallucination in schizophrenia patients [CBT联合小剂量利培酮治疗精神分裂症患者幻听的临床效果及安全性研究]. Sichuan Mental Health [四川精神卫生] 2014;27(6):502‐4. [Google Scholar]
Li 2015 {published data only}
- Li X, Guo Y, Fu C, Chen L. Effects of CBT combined with low‐dose risperidone alliance treatment on cognitive function in schizophrenia patients: neuropsychological assessment [CBT联合小剂量利培酮对精神分裂症患者认知功能影响的神经心理学评估]. Sichuan Mental Health [四川精神卫生] 2015;23(03):211‐4. [Google Scholar]
Li 2015a {published data only}
- Li H, Shu J, Huang C, Sun H, Zhu Y, Wang S. Comparative study of the effect of integrated community rehabilitation intervention for the mental health of patients with chronic schizophrenia and their caregivers [社区综合康复对慢性精神分裂症患者的家庭负担及照料者心理健康的对比研究]. Medical Journal of Chinese Peoples Health [中国民康医学] 2015;27(9):1‐4+7. [Google Scholar]
Liu 2012 {published data only}
- Liu G. Cognitive behavioural therapy for the quality of life and stigma of patients with schizophrenia [认知心理治疗对精神分裂症康复期患者生活质量及病耻感的影响]. Xinli Yisheng [心理医生] 2012;8:71. [Google Scholar]
Lu 2014 {published data only}
- Lu J, Mao Z. Effects of cognitive‐behavioral therapy on quality of life and self‐efficacy in schizophrenia patients [认知行为治疗对慢性精神分裂症患者生活质量和自我效能感的影响]. Journal of Psychiatry [精神医学杂志] 2014;27(5):347‐9. [Google Scholar]
Ma 2016 {published data only}
- Ma W, Shi S, Yu H, Zhao Y. Efficacy of cognitive‐behavioral therapy on quality of life in 190 schizophrenia patients [认知行为治疗对190例精神分裂症患者生活质量的疗效观察]. Medical Journal of Chinese People's Health [中国民康医学] 2016;28(02):1‐2+9. [Google Scholar]
Naeem 2015 {published data only}
- Naeem F, Saeed S, Irfan M, Kiran T, Mehmood N, Gul M, et al. Brief culturally adapted CBT for psychosis (CaCBTp): a randomized controlled trial from a low income country. Schizophrenia Research 2015;164(1‐3):143‐8. [DOI] [PubMed] [Google Scholar]
Naeem 2016 {published data only}
- Naeem F, Johal R, McKenna C, Rathod S, Ayub M, Lecomte T, et al. Cognitive Behavior Therapy for psychosis based Guided Self‐Help (CBTp‐GSH) delivered by frontline mental health professionals: results of a feasibility study. Schizophrenia Research 2016;173(1‐2):69‐74. [DOI] [PubMed] [Google Scholar]
Pan 2012 {published data only}
- Pan S. Cognitive therapy in the care of schizophrenic depression [认知疗法在精神分裂症伴抑郁障碍护理中的应用]. Xin Li Yisheng [心理医生] 2012;7:206‐7. [Google Scholar]
Qian 2012 {published data only}
- Qian D. Efficacy of cognitive behavioral interventions for the rehabilitation of patients with schizophrenia [认知行为干预对精神分裂症患者康复期的效果观察]. Journal of Medical Theory and Practice [医学理论与实践] 2012;25(3):294‐5. [Google Scholar]
Qin 2014a {published data only}
- Qin C. Effects of cognitive‐behavioral intervention on anxiety and depression in schizophrenia patients during recovery stage [认知行为干预对康复期精神分裂症患者焦虑抑郁症状的影响]. Journal of Clinical Nursing [临床护理杂志] 2014;13(2):40‐2. [Google Scholar]
Qiu 2014b {published data only}
- Qiu L, Wang J, Wu Y, Liu D, Zhu D. Short‐term efficacy of cognitive‐behavioral therapy in female first‐episode paranoid schizophrenia [认知行为治疗对女性首发偏执型精神分裂症的早期疗效观察]. Journal of Chinese People's Health [中国民康医学] 2014;26(5):15‐7. [Google Scholar]
Rector 2003 {published data only}
- Rector NA, Seeman MV, Segal ZV. Cognitive therapy for schizophrenia: a preliminary randomized controlled trial. Schizophrenia Research 2003;63:1‐11. [DOI] [PubMed] [Google Scholar]
Startup 2004 {published data only}
- Startup M, Jackson MC, Startup S. Insight and recovery from acute psychotic episodes: the effects of cognitive behavior therapy and premature termination of treatment. Journal of Nervous and Mental Disease 2004;194(10):740‐50. [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Sun J, Feng N, Sun Q, Guo H. Effects of cognitive therapy on cognitive function in first‐episode schizophrenia patients [认知疗法对首发精神分裂症患者认知功能的影响]. Journal of Clinical Psychosomatic Diseases [临床身心疾病杂志] 2014;20(1):54‐6, 83. [Google Scholar]
Tarrier 1999 {published data only}
- Tarrier N, Beckett R, Harwood S, Baker A, Yusupoff L, Ugarteburu I. A trial of two cognitive‐behavioural methods of treating drug‐resistant residual psychotic symptoms in schizophrenic patients: 1. outcome. British Journal of Psychiatry 1993;162:524‐32. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Kinney C, McCarthy E, Humphreys L, Wittkowski A, Morris J. Two‐year follow‐up of cognitive‐behavioral therapy and supportive counselling in the treatment of persistent symptoms in chronic schizophrenia. Journal of Consulting and Clinical Psychology 2000;68(5):917‐22. [PubMed] [Google Scholar]
- Tarrier N, Wittkowski A, Kinney C, McCarthy E, Morris J, Humpherys L. Durability of the effects of cognitive‐behavioural therapy in the treatment of chronic schizophrenia: 12‐month follow‐up. British Journal of Psychiatry 1999;174:500‐4. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Yusupoff L, Kinney C, McCarthy E, Gledhill A, Haddock G, et al. Randomised controlled trial of intensive cognitive behavioural therapy for patients with chronic schizophrenia. BMJ 1998;317:303‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarrier 2014 {published data only}
- Tarrier N, Kelly J, Maqsood S, Snelson N, Maxwell J, Law H, et al. The cognitive behavioural prevention of suicide in psychosis: a clinical trial. Schizophrenia Research 2014;156(2‐3):204‐10. [DOI] [PubMed] [Google Scholar]
Trower 2004 {published data only}
- Trower P, Birchwood M, Meaden A, Byrne S, Nelson A, Ross K. Cognitive therapy for command hallucinations: randomised controlled trial. British Journal of Psychiatry 2004;184:312‐20. [DOI] [PubMed] [Google Scholar]
Tuikington 2002 {published data only}
- Rathod S, Kingdon D, Smith P, Turkington D. Insight into schizophrenia: the effects of cognitive behavioural therapy on the components of insight and association with sociodemographics ‐ data on a previously published randomised controlled trial. Schizophrenia Research 2005;74(2‐3):211‐9. [DOI] [PubMed] [Google Scholar]
- Turkington D, Kingdon D, Rathod S, Hammond K, Pelton J, Mehta R. Outcomes of an effectiveness trial of cognitive‐behavioural intervention by mental health nurses in schizophrenia. British Journal of Psychiatry 2006;189:36‐40. [DOI] [PubMed] [Google Scholar]
- Turkington D, Kingdon D, Turner T. Effectiveness of a brief cognitive‐behavioural therapy intervention in the treatment of schizophrenia. British Journal of Psychiatry 2002;180:523‐7. [DOI] [PubMed] [Google Scholar]
Velligan 2014 {published data only}
- Velligan DI, Tai S, Roberts DL, Maples‐Aguilar N, Brown M, Mintz J, et al. A randomized controlled trial comparing cognitive behavior therapy, cognitive adaptation training, their combination and treatment as usual in chronic schizophrenia. Schizophrenia Bulletin 2015;41(3):597‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang L, Du X, Li X, Guo Y. Control study of cognitive behavioral nursing therapy for chronic schizophrenic [认知行为护理疗法对慢性精神分裂症作用的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2005;17(9):548‐9. [Google Scholar]
Wang 2008 {published data only}
- Wang D, Li X, Xu J, Yin G, Chen S. A comparison of clinical study of schizophrenia with and without cognitive behavior therapy [认知行为治疗精神分裂症患者临床对照研究]. China Medical Herald [中国医药导报] 2008;5(28):39‐40. [Google Scholar]
Wang 2012 {published data only}
- Wang C, An Q. The effect of cognitive behavioural therapy for the compliance behaviour in patients with schizophrenia [认知行为治疗对精神分裂症患者遵医行为的影响]. Seek Medical and Ask the Medicine [求医问药] 2012;7:651. [Google Scholar]
Wang 2015 {published data only}
- Wang Z, Guo Z, Han G, Wang J, Li Z. Efficacy of cognitive‐behavioral therapy in community schizophrenia patients [认知行为治疗对社区精神分裂症患者的疗效]. China Journal of Health Psychology [中国健康心理学杂志] 2015;23(1):16‐9. [Google Scholar]
- Wang Z, Guo Z, Wang J, Yan G, Han G, Li Z. Effects of normalized cognitive‐behavioral therapy on quality of life and social function in community residual schizophrenia patients [规范化认知行为治疗对社区残留型精神分裂症患者生活质量和社会功能的影响]. Journal of Neuroscience and Mental Health [神经疾病与精神卫生] 2014;14(5):494‐6. [Google Scholar]
Yao 2015 {published data only}
- Yao L. Nursing interventions on anxiety and depression in schizophrenics during recovery stage [康复期精神分裂症患者焦虑抑郁情绪的护理措施]. World Latest Medicine Information [世界最新医学信息文摘] 2015;15(41):251‐2. [Google Scholar]
Zhang 2014 {published data only}
- Zhang Y. Effects of cognitive behavioral therapy on medication adherence in homeless psychotic patients during recovery stage [认知行为干预对恢复期住院流浪精神病患者服药依从性的影响]. Medical Journal of Chinese People's Health [中国民康医学] 2014;26(22):117‐8. [Google Scholar]
Zhang 2015 {published data only}
- Zhang H. The effect of cognitive psychology therapy for patients with psychosis [康复期精神病患者认知心理治疗的护理观察]. World Latest Medicine Information [世界最新医学信息文摘] 2015;15(41):191, 19. [Google Scholar]
Zhao 2013 {published data only}
- Zhao D, Zhao X, Guo J, Wu L, Xu N, Huo X, et al. Effects of cognitive behavioral therapy on social support system in schizophrenia patients [认知行为疗法改善精神分裂症患者社会支持系统的临床研究]. 中外健康文摘 2014;5(22):66‐7. [Google Scholar]
- Zhao X, Zhao D, Guo J, Wu L. Clinical study of cognitive behavioral therapy combined with risperidone for social support system in patients with schizophrenia [认知行为疗法联合利培酮对精神分裂症患者社会支持系统进行干预的临床研究]. Gansu Science and Technology [甘肃科技] 2013, (12):117‐20. [Google Scholar]
Zhao 2014 {published data only}
- Zhao S, Wang J. Rehabilitation efficacy of cognitive behavioral therapy on chronic schizophrenia patients [认知行为治疗对慢性精神分裂症患者康复疗效的对照研究]. Chinese Journal of Trauma and Disability Medicine [中国伤残医学] 2014;22(8):209‐10. [Google Scholar]
Zou 2013 {published data only}
- Zou H, Wang F, Li Z, Yao X, Zhang L, Feng Y, et al. Cognitive behavioral therapy and exercise intervention for patients taking antipsychotic [认知行为治疗和运动干预对服用抗精神病药物患者影响的效果评价]. Chinese Nursing Management [中国护理管理] 2013, (4):33‐6. [Google Scholar]
References to studies excluded from this review
ACTRN12606000 {unpublished data only}
- ACTRN12606000101583. Randomised controlled trial of cognitive behaviour therapy to prevent psychosis among people with at‐risk mental states. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=1167 (first received.
Agius 2007 {published data only}
- Agius M, Shah S, Ramkisson R, Murphy S, Zaman R. Three year outcomes of an early intervention for psychosis service as compared with treatment as usual for first psychotic episodes in a standard community mental health team. Final results. Psychiatria Danubina 2007;19(3):130‐8. [PubMed] [Google Scholar]
Bach 2002 {published data only}
- Bach P, Hayes SC. The use of acceptance and commitment therapy to prevent the rehospitalization of psychotic patients: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2002;70(5):1129‐39. [DOI] [PubMed] [Google Scholar]
Barrowclough 2006 {published data only}
- Barrowclough C, Haddock G, Lobban F, Jones S, Siddle R, Roberts C, et al. Group cognitive‐behavioural therapy for schizophrenia. Randomised controlled trial. British Journal of Psychiatry 2006;189:527‐32. [DOI] [PubMed] [Google Scholar]
Bechdolf 2004 {published data only}
- Bechdolf A, Knost B, Kuntermann C, Schiller S, Klosterkötter J, Hambrecht M, et al. A randomized comparison of group cognitive‐behavioural therapy and group psychoeducation in patients with schizophrenia. Acta Psychiatrica Scandinavica 2004;110:21‐8. [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Kçhn D, Knost B, Pukrop R, Klosterkçtter J. A randomized comparison of group cognitive‐behavioural therapy and group psychoeducation in acute patients with schizophrenia: outcome at 24 months. Acta Psychiatrica Scandinavica 2005;112:173‐9. [DOI] [PubMed] [Google Scholar]
Bechdolf 2005b {published data only}
- Bechdolf A, Veitha V, Schwarzer D, Schormann M, Stamm E, Janssen B, et al. Cognitive‐behavioral therapy in the pre‐psychotic phase: an exploratory study. Psychiatry Research 2005;136:251–5. [DOI] [PubMed] [Google Scholar]
Bradshaw 1996 {published data only}
- Bradshaw W. Structured group work for individuals with schizophrenia: a coping skills approach. Research on Social Work Practice 1996;6(2):139‐54. [Google Scholar]
Bradshaw 2000 {published data only}
- Bradshaw W. Integrating cognitive behavioural psychotherapy for persons with schizophrenia into a psychiatric rehabilitation programme: results of a three‐year trial. Community Mental Health Journal 2000;36(5):491‐500. [DOI] [PubMed] [Google Scholar]
Byerly 2005 {published data only}
- Byerly MJ, Fisher R, Cramody T, Rush AJ. A trial of compliance therapy in outpatients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry 2005;66(8):997‐1001. [DOI] [PubMed] [Google Scholar]
Cai 2014c {published data only}
- Cai S. Effects of cognitive psychotherapy in schizophrenia patients during recovery stage [康复期精神病患者认知心理治疗的效果观察]. World Latest Medicine Information [世界最新医学信息文摘] 2014;14:161‐4. [Google Scholar]
Cather 2005 {published data only}
- Cathera C, Penn D, Ottoa MW, Yovela I, Mueserc KT, Goff DC. A pilot study of functional Cognitive Behavioral Therapy (fCBT) for schizophrenia. Schizophrenia Research 2005;75:201–9. [DOI] [PubMed] [Google Scholar]
Cella 2014 {published data only}
- Cella M, Reeder C, Wykes T. It is all in the factors: effects of cognitive remediation on symptom dimensions. Schizophrenia Research 2014;156(1):60‐2. [DOI] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen R. The analysis of cognitive‐behavioral psychological intervention on the rehabilitation of chronic schizophrenia [认知行为心理干预对慢性精神分裂症康复作用分析]. Guide of China Medicine [中国医药指南] 2012, (11):57‐8. [Google Scholar]
ChiCTR‐TRC‐14004187 2014 {published data only}
- ChiCTR‐TRC‐14004187. Group cognitive behavioral therapy for psychosis. chictr.org.cn/com/25/showprojen.aspx?proj=5381. China, (accessed: 21 January 2014).
- HKUCTR‐1829. Randomized controlled trial of group cognitive‐behavioral therapy for psychosis. hkuctr.com (accessed: 22 April 2014).
Deng 2014 {published data only}
- Deng M, Ding N. Effects of group CBT on schizophrenia patients during recovery stage [对精神分裂症康复期患者实施团体CBT的效果观察]. Today Nurse [当代护士] 2014;10:116‐8. [Google Scholar]
Dong 2015 {published data only}
- Dong X. Cognitive behavioural therapy in combination with antipsychotic medication for schizophrenia. Medical Information 2015;28(19):324. [Google Scholar]
Drury 1996 {published data only}
- Drury V, Birchwood M, Cochrane R. Cognitive therapy and recovery from acute psychosis: a controlled trial. III. Five‐year follow‐up. British Journal of Psychiatry 2000;177:8‐14. [DOI] [PubMed] [Google Scholar]
- Drury V, Birchwood M, Cochrane R, Macmillan F. Cognitive therapy and recovery from acute psychosis: a controlled trial. I. Impact on psychotic symptoms. British Journal of Psychiatry 1996;169:593‐601. [DOI] [PubMed] [Google Scholar]
- Drury V, Birchwood M, Cochrane R, Macmillan F. Cognitive therapy and recovery from acute psychosis: a controlled trial. II. Impact on recovery time. British Journal of Psychiatry 1996;169:602‐7. [DOI] [PubMed] [Google Scholar]
Du 2016 {published data only}
- Du LN, Pei JZ, Liu QQ. Cognitive behavioural therapy for the rehabilitation of schizophrenia [认知行为疗法治疗精神分裂症的康复效果观察]. 临床合理用药杂志 2015;8(16):140. [Google Scholar]
Eack 2014 {published data only}
- Eack SM, Hogarty SS, Greenwald DP, Litschge MY, McKnight SA, Bangalore SS, et al. Cognitive enhancement therapy in substance misusing schizophrenia: results of an 18‐month feasibility trial. Schizophrenia Research 2016;161(2‐3):478‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farreny 2012 {published data only}
- Farreny A, Aguado J, Ochoa S, Huerta‐Ramos E, Marsà F, López‐Carrilero R, et al. REPYFLEC cognitive remediation group training in schizophrenia: looking for an integrative approach. Schizophrenia Research 2012;142(1‐3):137‐44. [DOI] [PubMed] [Google Scholar]
Favrod 2014 {published data only}
- Favrod J, Rexhaj S, Bardy S, Ferrari P, Hayoz C, Moritz S, et al. Sustained antipsychotic effect of metacognitive training in psychosis: a randomized‐controlled study. European Psychiatry 2014;29(5):275‐81. [DOI] [PubMed] [Google Scholar]
Feng 2013 {published data only}
- Feng Y, Song H, Wang X. The effect of venlafaxine plus cognitive therapy for depression after the treatment of schizophrenia: a controlled study [文拉法辛合并认知疗法治疗精神分裂症后抑郁的对照研究]. Yi Xue Li Lun Yu Shi Jian [医学理论与实践] 2013, (15):2014‐5. [Google Scholar]
Gaudiano 2006 {published data only}
- Gaudiano BA, Herbert JD. Acute treatment of inpatients with psychotic symptoms using Acceptance and Commitment Therapy: pilot results. Behavioural Research and Therapy 2006;44(3):415‐37. [DOI] [PubMed] [Google Scholar]
Granholm 2007 {published data only}
- Granholm E, McQuaid JR, McClure FS, Auslander LA, Perivoliotis D, Pedrelli P, et al. A randomized, controlled trial of cognitive behavioral social skills training for middle‐aged and older outpatients with chronic schizophrenia. American Journal of Psychiatry 2005;162:520‐9. [DOI] [PubMed] [Google Scholar]
Haddock 1998 {published data only}
- Haddock G, Tarrier N, Morrison AP, Hopkins R, Drake R, Lewis S. A pilot study evaluating the effectiveness of individual inpatient cognitive‐behavioural therapy in early psychosis. Social Psychiatry and Psychiatric Epidemiology 1999;34:254‐8. [DOI] [PubMed] [Google Scholar]
Hang 2014 {published data only}
- Hang RH, Cheng WL, Wu MF, Feng LP. Cognitive training for geriatric chronic schizophrenia patients [认知训练对老年慢性精神分裂症患者认知功能的影响]. Chinese Journal of Gerontology [中国老年学杂志] 2014;34:2678‐80. [Google Scholar]
Hert 2000 {published data only}
- Herz MI, Lamberti JS, Mintz J, Scott R, O'Dell SP, McCartan L, et al. A program for relapse prevention in schizophrenia: a controlled study. Archives General Psychiatry 2000;57(3):277‐83. [DOI] [PubMed] [Google Scholar]
Hogarty 1997 {published data only}
- Hogarty GE, Greenwald D, Ulrich RF, Kornblith SJ, DiBarry AL, Cooley S. Three‐year trials of personal therapy among schizophrenic patients living with or independent of family, II: Effects on adjustment of patients. American Journal of Psychiatry 1997;154(11):1514–24. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Kornblith SJ, Greenwald D, DiBarry AL, Cooley S, Ulrich RF, et al. Three‐year trials of personal therapy among schizophrenic patients living with or independent of family, I: Description of study and effects on relapse rates. American Journal of Psychiatry 1997;154(11):1504–13. [DOI] [PubMed] [Google Scholar]
Hogarty 2004 {published data only}
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue‐Geile M. Cognitive enhancement therapy for schizophrenia: effects of a 2‐year randomized trial on cognition and behavior. Archives General Psychiatry 2004;61(9):866–76. [DOI] [PubMed] [Google Scholar]
Huang 2014 {published data only}
- Huang S, Wang J. The application of cognitive therapy in 120 cases of patients with schizophrenia [认知治疗在120例精神分裂症缓解期患者中的应用分析]. Guide of China Medicine [中国医药指南] 2014;12:115‐6. [Google Scholar]
Ibranhim 2012 {published data only}
- Ibrahim WE, El‐Bilsha MA, Abeldayem SM. Effect of cognitive behavior therapy (CBT) on depression among schizophrenic patients with low self esteem. Middle East Journal of Psychiatry and Alzheimers 2012;3(4):3‐10. [Google Scholar]
ISRCTN11889976 {unpublished data only}
- ISRCTN11889976. Comparison between extension of specialised early intervention for first episode psychosis and regular care: a randomised controlled trial. isrctn.com/ISRCTN11889976.
ISRCTN34966555 {published data only}
- ISRCTN34966555. Active assistance for psychological therapy (Actissist): using mobile technology to deliver cognitive behaviour therapy for psychosis ‐ RCT. isrctn.com/ISRCTN34966555.
ISRCTN47998710 {published data only}
- ISRCTN47998710. PRODIGY: Prevention of long term social disability amongst young people with emerging psychological difficulties ‐ a pilot randomised controlled trial of social recovery cognitive behavioural therapy. isrctn.com/ISRCTN47998710 [Date Accessed: 29 November 2012].
ISRCTN77762753 {published data only}
- Hazell CM, Hayward M, Cavanagh K, Jones AM, Strauss C. Guided self‐help cognitive behavioral intervention for VoicEs (GiVE): study protocol for a pilot randomized controlled trial. Trials 2016;17(1):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN77762753. A pilot randomised controlled trial of guided self‐help cognitive behaviour therapy for distressing voices. isrctn.com/search?q=ISRCTN77762753 (first received 23 July 2015).
Jackson 1998 {published data only}
- Jackson H, McGorry P, Edwards J, Hulbert C, Henry L, Francey S, et al. Cognitively‐oriented psychotherapy for early psychosis (COPE). Preliminary results. British Journal Psychiatry 1998;172(33 suppl):93‐100. [PubMed] [Google Scholar]
- Jackson H, McGorry P, Edwards J, Hulbert C, Henry L, Harrigan S, et al. A controlled trial of cognitively oriented psychotherapy for early psychosis (COPE) with four‐year follow‐up readmission data. Psychological Medicine 2005;35(9):1295‐306. [DOI] [PubMed] [Google Scholar]
- Jackson H, McGorry P, Henry L, Edwards J, Hulbert C, Harrigan S, et al. Cognitively oriented psychotherapy for early psychosis (COPE): a 1‐year follow‐up. British Journal Clinical Psychology 2001;40(Pt 1):57‐70. [DOI] [PubMed] [Google Scholar]
Jackson 2008 {published data only}
- Bendall S, Jackson HJ, Killackey E, Allott K, Johnson T, Harrigan S, et al. The credibility and acceptability of befriending as a control therapy in a randomized controlled trial of cognitive behaviour therapy for acute first episode psychosis. Behavioural and Cognitive Psychotherapy 2006;34:277‐91. [Google Scholar]
- Jackson HJ, McGorry PD, Killackey E, Bendall S, Allott K, Dudgeon P, et al. Acute‐phase and 1‐year follow‐up results of a randomized controlled trial of CBT versus befriending for first‐episode psychosis: the ACE project. Psychological Medicine 2008;38(5):725‐35. [DOI] [PubMed] [Google Scholar]
Jenner 2004 {published data only}
- Jenner JA, Nienhuis FJ, Wiersma D, Willige G. Hallucination focused integrative treatment: a randomized controlled trial. Schizophrenia Bulletin 2004;30(1):133–45. [DOI] [PubMed] [Google Scholar]
Jiang 2014 {published data only}
- Jiang M, Chen H, Sun X. Application of cognitive‐behavioral therapy on schizophrenia patients comorbid with diabetes mellitus [认知行为疗法在精神分裂症伴糖尿病患者中的应用]. Journal of Nursing Science [护理学杂志] 2014;29(17):69‐70. [Google Scholar]
Johnson 2008 {published data only}
- Johnson DP, Penn DL, Bauer DJ, Meyer P, Evans E. Predictors of the therapeutic alliance in group therapy for individuals with treatment‐resistant auditory hallucinations. British Journal of Clinical Psychology 2008;47(Pt. 2):171‐83. [DOI] [PubMed] [Google Scholar]
Kidd 2014 {published data only}
- Kidd SA, Kaur J, Virdee G, George TP, McKenzie K, Herman Y, et al. Cognitive remediation for individuals with psychosis in a supported education setting: a randomized controlled trial. Schizophrenia Research 2014;157(1‐3):90‐8. [DOI] [PubMed] [Google Scholar]
Klingberg 2009 {published data only}
- Klingberg S1, Wittorf A, Herrlich J, Wiedemann G, Meisner C, Buchkremer G, et al. Cognitive behavioural treatment of negative symptoms in schizophrenia patients: study design of the TONES study, feasibility and safety of treatment. European Archives of Psychiatry and Clinical Neuroscience 2009;259(2):149‐54. [DOI] [PubMed] [Google Scholar]
Kong 2015 {published data only}
- Kong LJ, Zuo XY, Zhou YB, Jiao F. The effect of cognitive behavioural therapy for quality of life in patients with schizophrenia. Journal of Yangtze University (Natural Science Edition) 2015;12(30):70‐2. [Google Scholar]
Kuipers 2004 {published data only}
- Kuipers E, Holloway F, Rabe‐Hesketh S, Tennakoon L, Croydon Outreach and Assertive Support Team (COAST). An RCT of early intervention in psychosis. Social Psychiatry and Psychiatric Epidemiology 2004;39(5):358–63. [DOI] [PubMed] [Google Scholar]
Lang 2014 {published data only}
- Lang Y, Li W, Wang F, Xu X. The effect of cognitive nursing intervention on the rehabilitation in young schizophrenia patients with impaired memory [认知护理干预在儿童精神分裂症记忆损害患儿恢复中的应用]. Nursing Practice and Research [护理实践与研究] 2014;11:104‐5. [Google Scholar]
Leclerc 2000 {published data only}
- Leclerc C, Lesage AD, Ricard N, Lecomte T, Cyr M. Assessment of a new rehabilitative coping skills module for persons with schizophrenia. American Journal of Orthopsychiatry 2000;70(3):380‐8. [DOI] [PubMed] [Google Scholar]
Lecomte 2008 {published data only}
- Lecomte T, Leclerc C, Corbiere M, Wykes T, Wallace CJ, Spidel A. Group cognitive behavior therapy or social skills training for individuals with a recent onset of psychosis? Results of a randomized controlled trial. Journal of Nervous Mental Disorders 2008;196:866‐75. [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li J, Deng X, Xie Y, Liang L. Effects of cognitive behavioral therapy on cognitive function in patients with chronic schizophrenia [认知行为治疗对慢性精神分裂症患者认知功能的影响]. Chinese and Foreign Medical Research [中外医学研究] 2013, (24):130‐2. [Google Scholar]
Li 2013b {published data only}
- Li X, Guo Y, Fu C, Chen L, Zhang Z. The effect of group cognitive behavioral therapy for refractory auditory hallucinations in schizophrenia [小组认知行为治疗对精神分裂症顽固性幻听的疗效观察 ]. Chinese Journal of Rehabilitation [中国康复] 2013, (4):313‐5. [Google Scholar]
Li 2014b {published data only}
- Li L, Hu L, Liang Z, Wen Z. Impact of cognitive intervention on quality of living and related factor of patients with chronic schizophrenia [认知干预对慢性精神分裂症患者生活质量及相关因素的影响]. Clinical Medical and Engineering [临床医学工程] 2014;21(8):1073‐4. [Google Scholar]
Li 2015c {published data only}
- Li Y, Yang Y, Wei Z, Yin Y. The influence of cognitive behavior intervention on olanzapine induced weight gain in schizophrenia [认知行为干预对奥氮平致精神分裂症患者体质量增加的影响]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2015;21:29‐31. [Google Scholar]
Lin 2014 {published data only}
- Lin J. Effects of cognitive‐behavioral therapy for post‐schizophrenic depression [认知行为疗法在精神分裂症后抑郁中的应用效果分析]. Guide of China Medicine [中国医药指南] 2014;12(33):174. [Google Scholar]
Lincoln 2012 {published data only}
- Lincoln TM, Ziegler M, Mehl S, Kesting ML, Lullmann E, Westermann S, et al. Moving from efficacy to effectiveness in cognitive behavioral therapy for psychosis: a randomized clinical practice trial. Journal of Consulting and Clinical Psychology 2012;80:674‐86. [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu D, Liu J, Li S, Feng A. Effects of token therapy and cognitive therapy on quality of life in schizophrenia patients [代币疗法与认知治疗对精神分裂症患者生活质量的影响]. China Health Industry [中国卫生产业] 2014, (2):107+109. [Google Scholar]
Liu 2015 {published data only}
- Liu J, Yong S, Xu X, Zhang J, Xie P, Xu C, et al. Influence of CBT on rehabilitation outcome of hospitalized schizophrenics [认知行为治疗对住院精神分裂症患者康复效果的影响]. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2015;21:64‐6. [Google Scholar]
Lu 2012 {published data only}
- Lu H, Li Y, Li F, Jiao X, Shi W, Guo K, et al. Randomized controlled trial on adjunctive cognitive remediation therapy for chronically hospitalised patients with schizophrenia [慢性精神分裂症住院患者辅以认知矫正治疗的随机对照研究]. Shanghai Archives of Psychiatry [上海精神医学] 2012;24:149‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2014a {published data only}
- Lu HM, Wang XL, Tang Y, Fei P. The application of cognitive therapy in patients with schizophrenia. Medical Information 2014;27:463. [Google Scholar]
Lysaker 2009 {published data only}
- Davis LW, Lysaker PH, Lancaster RS, Bryson GJ, Bell MD. The Indianapolis Vocational Intervention Program: a cognitive behavioral approach to addressing rehabilitation issues in schizophrenia. Journal of Rehabilitation Research and Development 2005;42(1):35. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Davis LW, Bryson GJ, Bell MD. Effects of cognitive behavioral therapy on work outcomes in vocational rehabilitation for participants with schizophrenia spectrum disorders. Schizophrenia Research 2009;107:186‐91. [DOI] [PubMed] [Google Scholar]
McLeod 2007 {published data only}
- McLeod T, Morris M, Birchwood M, Dovey A. Cognitive behavioural therapy group work with voice hearers. Part 1. British Journal of Nursing 2007;16:248‐52. [DOI] [PubMed] [Google Scholar]
Mo 2015 {published data only}
- Mo Y, Li X, Fu C, He R, Cai Y, Mo T. Effects of cognitive‐behavioral intervention on mental status in homeless schizophrenia patients [认知行为干预对流浪精神疾病患者心理状态的影响]. China Modern Medicine [中国现代药物应用] 2015; Vol. 9, issue 1:201‐2.
Morrison 2014 {published data only}
NCT00810355 {unpublished data only}
- NCT00810355. Cognitive Behavior Therapy and Work Outcome. clinicaltrials.gov/ct2/show/NCT00810355 [Date Accessed: 18 December 2008].
NCT00960375 {unpublished data only}
- NCT00960375. Smoking Cessation for Veterans With Severe and Persistent Mental Illness. clinicaltrials.gov/ct2/show/NCT00960375 [Date Accessed: 17 August 2009].
NCT02105779 {published data only}
- NCT02105779. Optimizing Cognitive Remediation Outcomes in Schizophrenia. clinicaltrials.gov/ct2/show/NCT02105779 [Date Accessed: 7 April 2014].
NCT02420015 {published data only}
- NCT02420015. Mobile Health Technology to Enhance Abstinence in Smokers With Schizophrenia. clinicaltrials.gov/ct2/show/NCT02420015 [Date Accessed: 17 April 2015].
NCT02535923 {published data only}
- NCT02535923. CBT‐I for Psychosis: Guidelines, Preliminary Efficacy, and Functional Outcomes. clinicaltrials.gov/ct2/show/NCT02535923 [Date Accessed: 31 August 2015].
NCT02751632 {published data only}
- ACTRN12616000098437. Staged Treatment in Early Psychosis (STEP): a sequential multistage randomized clinical trial (SMART) of interventions for Ultra High Risk (UHR) of psychosis patients. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369499 [Date Accessed: 1 February 2016].
- NCT02751632. The Staged Treatment in Early Psychosis Study. clinicaltrials.gov/ct2/show/NCT02751632 [Date Accessed: 26 April 2016].
Nordentoff 2005 {published data only}
- Bertelsen M, Jeppesen P, Petersen L, Thorup A, Øhlenschlaeger J, Quach P, et al. Five‐year follow‐up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Archives of General Psychiatry 2008;65(7):762‐71. [DOI] [PubMed] [Google Scholar]
O'Connor 2007 {published data only}
- O'Connor K, Stip E, Pelissier MC, Aardema F, Guay S, Gaudette G, et al. Treating delusional disorder: a comparison of cognitive‐behavioural therapy and attention placebo control. Canadian Journal of Psychiatry 2007;52(3):182‐90. [DOI] [PubMed] [Google Scholar]
O'Donnell 2003 {published data only}
- O'Donnell C, Donohoe G, Sharkey L, Owens N, Migone M, Harries R, et al. Compliance therapy: a randomised controlled trial in schizophrenia. BMJ 2003;327(7419):834. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Driscoll 2015 {published data only}
- O'Driscoll C, Mason O, Brady F, Smith B, Steel C. Process analysis of trauma‐focused cognitive behavioural therapy for individuals with schizophrenia. Psychology and Psychotherapy 2016;89(2):117‐32. [DOI] [PubMed] [Google Scholar]
Owen 2015 {published data only}
- Owen M, Sellwood W, Kan S, Murray J, Sarsam M. Group CBT for psychosis: a longitudinal, controlled trial with inpatients. Behaviour Research and Therapy 2015;65:76‐85. [DOI] [PubMed] [Google Scholar]
Penades 2006 {published data only}
- Penades R, Catalen R, Salamero M, Boget T, Puig O, Guarch J, et al. Cognitive remediation therapy for outpatients with chronic schizophrenia: a controlled and randomized study. Schizophrenia Research 2006;87:323–31. [DOI] [PubMed] [Google Scholar]
Penn 2009 {published data only}
- Penn DL, Meyer PS, Evans E, Wirth RJ, Cai K, Burchinal M. A randomized controlled trial of group cognitive‐behavioral therapy vs. enhanced supportive therapy for auditory hallucinations. Schizophrenia Research 2009;109:52–9. [DOI] [PubMed] [Google Scholar]
Phillips 2002 {published data only}
- Phillips LJ, Leicester SB, O’Dwyer LE, Francey SM, Koutsogiannis J, Abdel‐Baki A, et al. The PACE Clinic: identification and management of young people at "ultra" high risk of psychosis. Journal of Psychiatric Practice 2002;8(5):255‐9. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Yung AR, Yuen HP, Pantelis C, McGorry PD. Prediction and prevention of transition to psychosis in young people at incipient risk for schizophrenia. American Journal of Medical Genetics 2002;114(8):929–37. [DOI] [PubMed] [Google Scholar]
Pinto 1999 {published data only}
- Pinto A, Pia SL, Mennella R, Giorgio D, DeSimone L. Cognitive‐behavioral therapy and clozapine for clients with treatment‐refractory schizophrenia. Psychiatry Service 1999;50(7):901–4. [DOI] [PubMed] [Google Scholar]
Qi 2012 {published data only}
- Qi HM, Liu HY. Cognitive behavioral therapy for schizophrenia. Hebei Medical Journal 2012;34(21):3229‐31. [Google Scholar]
Rector 2005 {published data only}
- Rector NA. Cognitive behavioural therapy reduces short term rehospitalisation compared with psychoeducation in inpatients with schizophrenia. Evidence Based Mental Health 2005;8(1):8. [DOI] [PubMed] [Google Scholar]
Reeder 2014 {published data only}
- Reeder C, Harris V, Pickles A, Patel A, Cella M, Wykes T, et al. Does change in cognitive function predict change in costs of care for people with a schizophrenia diagnosis following cognitive remediation therapy?. Schizophrenia Bulletin 2014;40(6):1472‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richmond 2005 {unpublished data only}
- Richmond RL, Baker A, Haile M, Carr V, Lewin T, Wilhelm K, et al. Intervention for tobacco dependence among people with a psychotic illness: randomized controlled trial with one‐year outcome. Nicotine and Tobacco Research 2005;7:681. [Google Scholar]
Sellwood 2000 {published data only}
- Sellwood W, Barrowclough C, Tarrier N, Quinn J, Mainwaring J, Lewis S. Needs‐based cognitive‐behavioural family intervention for carers of patients suffering from schizophrenia: 12‐month follow‐up. Acta Psychiatrica Scandinavica 2001;104(5):346‐55. [DOI] [PubMed] [Google Scholar]
- Sellwood W, Wittkowski A, Tarrier N, Barrowclough C. Needs‐based cognitive‐behavioural family intervention for patients suffering from schizophrenia: 5‐year follow‐up of a randomized controlled effectiveness trial. Acta Psychiatrica Scandinavica 2007;116(6):447–52. [DOI] [PubMed] [Google Scholar]
Sensky 2000 {published data only}
- Sensky T, Turkington D, Kingdon D, Scott JL, Scott J, Siddle R, et al. A randomized controlled trial of cognitive‐behavioral therapy for persistent symptoms in schizophrenia resistant to medication. Archives of General Psychiatry 2000;57(2):165‐72. [DOI] [PubMed] [Google Scholar]
Shao 2013 {published data only}
- Shao G. Cognitive behavioral therapy used in the treatment of mentally ill [认知行为疗法在精神病康复治疗中的应用]. Guide of China Medicine [中国医药指南] 2013, (11):567‐8. [Google Scholar]
Shi 2015 {published data only}
- Shi Y, Jiao Y, Jiang L, Sun Y, Bo Z, Wu Q, et al. Effects of cognitive‐behavioral therapy on executive function in chronic schizophrenia patients [认知行为治疗对慢性精神分裂症患者执行功能的影响]. Journal of Neuroscience and Mental Health [神经疾病与精神卫生] 2015;15(5):497‐9. [Google Scholar]
Song 2012 {published data only}
- Song X, Liu Y, Zhang Y, Zhou L. The effective of cognitive behavioral therapy on negative symptoms of schizophrenia [认知行为治疗对改善精神分裂症病人阴性症状的效果研究]. XinLi YiSheng [心理医生] 2012;7:30‐1. [Google Scholar]
Song 2014 {published data only}
- Song X, Li J, Zhang Y, Wang X, Wang E. Effects of group CBT on quality of life in schizophrenia patients with auditory hallucination [团体CBT对精神分裂症幻听患者生存质量的影响]. International Medicine and Health Guidance News [国际医药卫生导报] 2014;20(16):2572‐4. [Google Scholar]
Turkington 2008 {published data only}
- Turkington D, Sensky T, Scott J, Barnes TRE, Nur U, Siddle R, et al. A randomized controlled trial of cognitive‐behavior therapy for persistent symptoms in schizophrenia: a five‐year follow‐up. Schizophrenia Research 2008;98:1‐7. [DOI] [PubMed] [Google Scholar]
Valmaggia 2005 {published data only}
- Valmaggia LR, Gaag M, Tarrier N, Pijnenborg M, Slooff CJ. Cognitive‐behavioural therapy for refractory psychotic symptoms of schizophrenia resistant to atypical antipsychotic medication. Randomised controlled trial. British Journal of Psychiatry 2005;186:324‐30. [DOI] [PubMed] [Google Scholar]
Wang 2003 {published data only}
- Wang C, Li Y, Zhao Z, Pan M, Feng Y, Sun F, et al. Controlled study on long‐term effect of cognitive behavior intervention on first episode schizophrenia. Chinese Mental Health Journal 2003;7466:200‐2. [Google Scholar]
Wang 2013 {published data only}
- Wang H, Chen Y, Xie W, Xiao B, Qiu C, Xie S. The effect of cognitive treatment on the compliance for treatment of outpatient with schizophrenia [认知治疗对门诊精神分裂症患者治疗依从性的影响]. Medical Journal of Chinese People's Health [中国民康医学] 2013;25(7):16‐8. [Google Scholar]
Wang 2013a {published data only}
- Wang C, Cheng M, Sheng M. Cognitive ‐ the existence group therapy for patients with chronic schizophrenia in community [认知‐存在团体对社区慢性精神分裂症患者的干预效果]. China Journal of Health Psychology [中国健康心理学杂志] 2013;21:974‐7. [Google Scholar]
Wang 2014 {published data only}
- Wang E. Effects of cognitive rehabilitation care on cognitive function impairment in 40 schizophrenia patients [40例认知康复护理对精神分裂症患者认知功能障碍的影响]. Contemporary Medicine [当代医学] 2014;20(19):129‐30. [Google Scholar]
Wei 2012 {published data only}
- Wei Q, Li J, Wen C. Clinical efficacy of cognitive therapy for schizophrenia and its impact on the social function of patients [认知疗法治疗精神分裂症的临床疗效及对患者社会功能的影响]. Chinese Journal of Medicinal Guide [中国医药导刊] 2012;14(11):1910‐1. [Google Scholar]
Wu 2012 {published data only}
- Wu S. Cognitive psychotic therapy for depression in patients with schizophrenia [认知心理治疗改善精神分裂症后抑郁的疗效分析]. Journal of Modern Medicine and Health [现代医药卫生] 2012;14:2155‐6. [Google Scholar]
Wu 2013 {published data only}
- Wu L. Application of psychological intervention in nursing care of patients with chronic schizophrenia with negative symptoms [心理干预在慢性精神分裂症阴性症状患者护理中的应用]. Medical Journal of Chinese People's Health [中国民康医学] 2013;25(9):81‐2. [Google Scholar]
Wykes 2005 {published data only}
- Wykes T, Hayward P, Thomas N, Green N, Surguladze S, Fannon D, et al. What are the effects of group cognitive behaviour therapy for voices? A randomised control trial. Schizophrenia Research 2005;77(2):201‐10. [DOI] [PubMed] [Google Scholar]
Xie 2013 {published data only}
- Xie W, Xie Y, Xiao Y, Wang H, Xiao B. The application of multimedia in cognitive intervention to enhance its effect in patients with schizophrenia [精神分裂症患者应用多媒体加强认知干预的效果研究]. Journal of Nursing [护理学报] 2013;20(4B):57‐9. [Google Scholar]
Xu 2014 {published data only}
- Xu M. Effects of cognitive behavioral social work team on anxiety in schizophrenia inpatients [认知行为社工小组对住院精神分裂症患者焦虑情绪的影响]. Medical Journal of Chinese People's Health [中国民康医学] 2014;26(1):23‐4. [Google Scholar]
Yang 2012 {published data only}
- Yang X, Xiong Y. Cognitive psychotherapy efficacy for the prevention of relapse of schizophrenia research [认知心理治疗对预防精神分裂症复发的疗效研究]. China Foreign Medical Treatment [中外医疗] 2012, (5):18+20. [Google Scholar]
Zeng 2014 {published data only}
- Zeng Y, Lin S, Dai C. Effects of different nursing models on negative symptoms and social function in psychotic patients [不同护理模式干预对精神病患者阴性症状和社会功能的影响]. China Modern Medicine [中国当代医药] 2014;21(18):108‐9+112. [Google Scholar]
Zhang 2005 {published data only}
- Zhang J. Cognitive behavioral therapy for rehabilitation of schizophrenic patients with anxiety [认知行为疗法对康复期精神分裂患者焦虑情绪的影响]. Journal of Qiqihar Medical College [齐齐哈尔医学院学报] 2005;26(9):1010‐1. [Google Scholar]
Zhao 2012 {published data only}
- Zhao Y, Qin F. Risperidone combined with cognitive insight therapy efficacy study of first‐episode schizophrenia [利培酮合并认知领悟治疗对首发精神分裂症疗效研究]. Journal of Mathematical Medicine [数理医药学杂志] 2012;25(2):211‐3. [Google Scholar]
Zhou 2015b {published data only}
- Zhou J. Effects of cognitive behavioral therapy on social function and quality of life in schizophrenia patients [认知行为疗法对精神分裂症患者社会功能和生活质量的影响]. Health Research [健康研究] 2015;35(05):539‐41. [Google Scholar]
References to studies awaiting assessment
Chen 2015c {published data only}
- Chen G. Efficacy of cognitive‐behavioral therapy on schizophrenia patients during recovery stage [认知行为疗法治疗精神分裂症患者康复期的疗效观察]. 14th academic annual meeting of the Committee on Mental Illness of China Association of Traditional Chinese and Western Medicine [中国中西医结合学会精神疾病专业委员会第十四届学术年会暨首届国际中西医结合精神病学研究进展培训班]. 2015:4.
Fohlmann 2010 {published data only}
- Fohlmann AH, Hjorthoej C, Larsen A, Nordentoft M. Capopus. Randomized clinical trial: specialized addiction treatment (MI & CBT) versus treatment as usual for young patients with cannabis abuse and psychosis. Early Intervention in Psychiatry 2010;4(Suppl. 1):160. [Google Scholar]
- Hjorthaj CR. Validity of self‐reported cannabis use as measured by the timeline follow‐back instrument in a trial randomizing people with comorbid cannabis use disorder and schizophrenia spectrum disorder. Early Intervention in Psychiatry 2010;4(Suppl. 1):160. [Google Scholar]
Hardy 2015 {published data only}
- Hardy A, Steel C, Smith B, Wykes T, Rose S, Enright S, et al. Cognitive behavioural therapy for the treatment of posttraumatic stress disorder in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 2015;1:S31‐2. [Google Scholar]
Hassan 2014 {published data only}
- Hassan S, Ganguli R, Flett G, Suleiman A, Hewitt P. Perfectionism and working alliance in a cognitive‐behavioral intervention for weight loss in psychotic illness. Schizophrenia Research 2014;153:S166. [Google Scholar]
Moun 2015 {published data only}
- Moun V, Tripathi A, Dalal PK, Sinha PK. Home based cognitive training in patients of schizophrenia. Indian Journal of Psychiatry 2015;57:S78. [Google Scholar]
Nagui 2016 {published data only}
- Rizk DN, Salama H, Molokhiya T, Kassem L. Effectiveness of brief individual cognitive behavioral therapy for auditory hallucinations in a sample of Egyptian patients with schizophrenia. European Psychiatry 2016;33(Suppl.):S259‐60. [Google Scholar]
Tang 2015 {published data only}
- Tang W, Zhang J, Wen N, Pan J, Yang F, Jiang S. Effects on prognosis recurrence and efficacy in schizophrenia patients treated with palpiperone palmitate long‐acting injection and cognitive‐behavioral intervention [棕榈酸帕利哌酮长效针剂联合认知行为干预对精神分裂症患者疗效及预后复发的影响]. 30th anniversary of the establishment of China Mental Health Association and the eighth National Academic Conference on Mental Health [中国心理卫生协会成立30周年纪念活动暨第八次全国心理卫生学术大会]. 2015:192.
Tecic 2012 {published data only}
- Tecic T, Guttgemanns J, Lehmkuhl G, Mueller K, Stoesser D, Wiedemann G, et al. Modified cognitive behavioral therapy in adolescents with persistent psychotic symptoms ‐ results of a randomized controlled trial. Schizophrenia Research 2012;136:S302. [Google Scholar]
References to ongoing studies
Edwards 2008 {published data only}
- Edwards J, Harris M, Harrigan S, Merritt A, Amminger G, Lurdes Santos M, et al. A clinical audit of the first six months of care of first‐episode psychosis patients in seven European sites. Early Intervention in Psychiatry 2008;2:A25. [Google Scholar]
ISRCTN06022197 {published data only}
- ISRCTN06022197. A pilot study of a randomised controlled trial of antipsychotic medication in comparison to cognitive behaviour therapy and a combined treatment in adults with psychosis. www.isrctn.com/ISRCTN06022197. Uk, [Date Accessed: 20 March 2014].
ISRCTN12668007 {published data only}
- ISRCTN12668007. The nightmare intervention study. www.isrctn.com/ISRCTN12668007 [Date Accessed: 20 November 2015].
ISRCTN33695128 {published data only}
- ISRCTN33695128. Better Sleep Trial: a pilot randomised controlled trial for patients with delusions and/or hallucinations. www.isrctn.com/ISRCTN33695128 [Date Accessed: 26 September 2012].
ISRCTN61621571 {published data only}
- ISRCTN61621571. Sustaining positive engagement and recovery (supereden) ‐ the next step after early intervention for psychosis. Study 3: Improving social recovery in young people with emerging severe social disability: a proof of principle randomised controlled trial. www.isrctn.com/ISRCTN61621571 [Date Accessed: 26 June 2012].
NCT00484302 {published data only}
- NCT00484302. Cannabis and psychosis randomized clinical trial: specialized addiction treatment versus treatment as usual for young patients with cannabis abuse and psychosis. clinicaltrials.gov/ct2/show/NCT00484302 [Date Accessed: 8 June 2007].
NCT00495911 {published data only}
- NCT00495911. A randomized controlled trial of individual therapy for first episode psychosis. clinicaltrials.gov/ct2/show/NCT00495911 [Date Accessed: 25 July 2008].
NCT02134418 {published data only}
- NCT02134418. Evaluation of an intervention program designed to improve understanding of Irony on the hemispheric processing of ambiguous figurative language in adults with schizophrenia. clinicaltrials.gov/ct2/show/NCT02134418 [Date Accessed: 9 May 2014].
NCT02380885 {published data only}
- NCT02380885. RCT social cognition training and therapeutic alliance focused therapy for persons with severe mental illness. clinicaltrials.gov/ct2/show/NCT02380885 [Date Accessed: 5 March 2015].
NCT02408198 {published data only}
- NCT02408198. The Street Smart Group: a feasibility trial of a group intervention targeting anxiety processes in paranoia. clinicaltrials.gov/ct2/show/NCT02408198 [Date Accessed: 3 April 2015].
NCT02427542 {published data only}
- NCT02427542. Feasibility trial of CBT for depersonalisation in psychosis. clinicaltrials.gov/ct2/show/NCT02427542 [Date Accessed: 28 April 2015].
NCT02653729 {published data only}
- NCT02653729. CBT for psychosis and affect on psychosis symptoms. clinicaltrials.gov/ct2/show/NCT02653729 [Date Accessed: 12 January 2016].
NCT02787122 {published data only}
- NCT02787122. Pilot‐trial of emotion‐focused cognitive behavior therapy for patients with schizophrenia. clinicaltrials.gov/ct2/show/NCT02787122 [Date Accessed: 1 June 2016].
NCT02787135 {published data only}
- NCT02787135. Efficacy and mechanisms of change of an emotion‐oriented version of cognitive‐behavioral therapy for psychosis. clinicaltrials.gov/ct2/show/NCT02787135 [Date Accessed: 1 June 2016].
Waller 2014 {published data only}
- Waller H, Craig T, Landau S, Fornells‐Ambrojo M, Hassanali N, Iredale C, et al. The effects of a brief CBT intervention, delivered by frontline mental health staff, to promote recovery in people with psychosis and comorbid anxiety or depression (the GOALS study): study protocol for a randomized controlled trial. Trials 2014;15:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Addington 1993
- Addington D, Addington J, Matickatyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. British Journal of Psychiatry 1993;163(Suppl):39‐44. [PubMed] [Google Scholar]
Alford 1994
- Alford BA, Beck AT. Cognitive therapy of delusional beliefs. Behaviour Research and Therapy 1994;32:369‐80. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderasen 2004
- Anderasen NC. Scale for the Assessment of Positive Symptoms (SAPS). 2004. [Google Scholar]
Andreasen 1984
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa, 1984. [Google Scholar]
Aoun 2011
- Aoun SM, Monterosso L, Kristjanson LJ, et al. Measuring symptom distress in palliative care: psychometric properties of the Symptom Assessment Scale (SAS). Journal of Palliative Medicine 2011;14:315‐21. [DOI] [PubMed] [Google Scholar]
APA 1995
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. American Psychiatric Association, 1995. [Google Scholar]
Asberg 1978
- Asberg M, Montgomery SA, Perris C, Schalling D, Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatrica Scandinavica 1978;271:5‐27. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Beck 1974
- Beck AT, Weissman A, Lester D, et al. The measurement of pessimism: the Hopelessness Scale. Journal of Consulting and Clinical Psychology 1974;42:861‐5. [DOI] [PubMed] [Google Scholar]
Beck 2004
- Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophrenia Research 2004;68:319‐29. [DOI] [PubMed] [Google Scholar]
Beck 2005
- Beck AT. The current state of cognitive therapy. Archives of General Psychiatry 2005;62:953‐9. [DOI] [PubMed] [Google Scholar]
Birchwood 1990
- Birchwood M, Smith J, Cochrane R, et al. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programme with schizophrenic patients. British Journal of Psychiatry 1990;157:853‐9. [DOI] [PubMed] [Google Scholar]
Birchwood 2000
- Birchwood M, Meaden A, Trower P, Birchwood M, Meaden A, Trower P, et al. The power and omnipotence of voices: subordination and entrapment by voices and significant others. Psychological Medicine 2000;30:337‐44. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315(7108):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Aperçu sur la problématique des indices d'efficacité thérapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Chadwick 2000
- Chadwick P, Lees S, Birchwood M. The revised Beliefs About Voices Questionnaire (BAVQ‐R). British Journal of Psychiatry 2000;177:229‐32. [DOI] [PubMed] [Google Scholar]
Christison 1991
- Christison GW, Kirch DG, Wyatt RJ. When symptoms persist: choosing among alternative somatic symptoms for schizophrenia. Schizophrenia Bulletin 1991;17:217‐45. [DOI] [PubMed] [Google Scholar]
Cohen 1997
- Cohen M. Children's Memory Scale Manual. San Antonio (TX): The Psychological Corporation, 1997. [Google Scholar]
David 1990
- David AS. Insight and psychosis. British Journal of Psychiatry 1990;156:789‐808. [DOI] [PubMed] [Google Scholar]
Davis 1977
- Davis JM, Casper R. Antipsychotic drugs: clinical pharmacology and therapeutic use. Drugs 1977;14:260‐82. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Derogatis 1975
- Derogatis L R. SCL‐90‐R: Symptom Checklist‐90‐R: Administration, scoring, and procedures manual. NCS Pearson, 1975. [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Dolan 1997
- Dolan P. Modeling valuations for EuroQol health states. Medical Care 1997;35:1095‐108. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21(19):2971‐80. [DOI] [PubMed] [Google Scholar]
Drake 1986
- Drake RE, Cotton PG. Depression, hopelessness and suicide in chronic schizophrenia. British Journal of Psychiatry 1986;148:554. [DOI] [PubMed] [Google Scholar]
DSM (IV) 1994
- DSM (IV). Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehring 2011
- Ehring T, Zetsche U, Weidacker K, Wahl K, Schönfeld S, Ehlers A. The Perseverative Thinking Questionnaire (PTQ): validation of a content‐independent measure of repetitive negative thinking. Journal of Behavior Therapy and Experimental Psychiatry 2011;42:225‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JP, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Eling 2008
- Eling P, Derckx K, Maes R. On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain and Cognition 2008;67:247‐53. [DOI] [PubMed] [Google Scholar]
Fowler 2006
- Fowler D, Freeman D, Smith B, Kuipers E, Bebbington P, Bashforth H. The Brief Core Schema Scales (BCSS): psychometric properties and associations with paranoia and grandiosity in non‐clinical and psychosis samples. Psychological Medicine 2006;36:749–59. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Fydrich 1992
- Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the Beck Anxiety Inventory. Journal of Anxiety Disorders 1992;6:55‐61. [Google Scholar]
Goldman 1992
- Goldman HH, Skodol AE, Lave TR. Revising axisV for DSM‐IV: a review of measures of social functioning. American Journal of Psychiatry 1992;149:1148‐56. [DOI] [PubMed] [Google Scholar]
Gossop 1995
- Gossop M, Darke S, Griffiths P, et al. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 1995;90:607‐14. [DOI] [PubMed] [Google Scholar]
Grant 2002
- Grant AM, Franklin J, Langford P. The self‐reflection and insight scale: a new measure of private self‐consciousness. Social Behavior and Personality 2002;30:821‐35. [Google Scholar]
Green 2004
- Green MF. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH‐MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry 2004;56:301‐7. [DOI] [PubMed] [Google Scholar]
Green 2008
- Green CE, Freeman D, Kuipers E, et al. Measuring ideas of persecution and social reference: the Green et al. Paranoid Thought Scales (GPTS). Psychological Medicine 2008;38:101‐11. [DOI] [PubMed] [Google Scholar]
Greenwood 2010
- Greenwood KE, Sweeney A, Williams S, Garety P, Kuipers E, Scott J, et al. CHoice of Outcome In Cbt for psychosEs (CHOICE): the development of a new service user‐led outcome measure of CBT for psychosis. Schizophrenia Bulletin 2010;36(1):126‐35. [PUBMED: 19880823] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W, Rockville, MD, editor(s). Clinical global impression. ECDEU Assessment Manual for Psychopharmacology. Washington DC (USA): US Department of Health, Education and Welfare, 1976. [Google Scholar]
Hamilton 1967
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal Social Clinical Psychology 1967;6:278‐96. [DOI] [PubMed] [Google Scholar]
Hamilton 1976
- Hamilton M. HAMA Hamilton Anxiety Scale. ECDEU Assessment Manual for Psychopharmacology 193‐8.
Hassock 1999
- Haddock G, Mccarron J, Tarrier N, Faragher E. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychological Medicine 1999;29:879‐89. [DOI] [PubMed] [Google Scholar]
Heinrichs 1984
- Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin 1984;10:388‐98. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 (updated September 2008). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
- Higgins JP, Green S. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2003
- Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, Krystal JH. Transcranial magnetic stimulation of left temporoparietal cortex and medication‐resistant auditory hallucinations. Archives General Psychiatry 2003;60:49‐56. [DOI] [PubMed] [Google Scholar]
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [PUBMED: 19438480] [DOI] [PubMed] [Google Scholar]
Iager 1985
- Iager AC, Kirch DG, Wyatt RJ. A negative symptom rating scale. Psychiatry Research 1985;16:27‐36. [DOI] [PubMed] [Google Scholar]
Jones 2004
- Jones C, Cormac I, Mota J, Campbell C. Cognitive behaviour therapy for schizophrenia. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000524.pub2] [DOI] [PubMed] [Google Scholar]
Jones 2009a
- Jones C, Cormac I, Campbell C, Meaden A, Hacker D, Irving CB. Cognitive behaviour therapy versus specific pharmacological treatments for schizophrenia. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007965] [DOI] [Google Scholar]
Jones 2018
- Jones C, Hacker D, Meaden A, Cormac I, Irving CB, Xia J, et al. Cognitive behavioural therapy plus standard care versus standard care plus other psychosocial treatments for people with schizophrenia. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.CD008712.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda (NY): Multi‐Health Systems, 1986. [Google Scholar]
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13:261‐76. [DOI] [PubMed] [Google Scholar]
Lecomte 2006
- Lecomte T, Corbière M, Laisné F. Investigating self‐esteem in individuals with schizophrenia: relevance of the Self‐Esteem Rating Scale‐Short Form. Psychiatry Research 2006;143:99‐108. [DOI] [PubMed] [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang‐Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001‐5. [PUBMED: 16905632] [DOI] [PubMed] [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
McEvoy 1989
- McEvoy JP, et al. Insight in schizophrenia. Its relationship to acute psychopathology. Journal of Nervous Mental Disorders 1989;177:43‐7. [DOI] [PubMed] [Google Scholar]
McHorney 1993
- McHorney CA, Ware Jr. JE, et al. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 1993;31:247‐63. [DOI] [PubMed] [Google Scholar]
Meltzer 1992
- Meltzer HY. Treatment of the neuroleptic‐nonresponsive schizophrenic patient. Schizophrenia Bulletin 1992;18:515‐42. [DOI] [PubMed] [Google Scholar]
Meyer 1990
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn state worry questionnaire. Behaviour Research and Therapy 1990;28:487‐95. [DOI] [PubMed] [Google Scholar]
Mittenberg 1995
- Mittenberg W, Theroux‐Fichera S, Zielinski RE, Heilbronner RL. Identification of malingered head injury on the Wechsler Adult Intelligence Scale‐Revised. Professional Psychology: Research and Practice 1995;26:491‐8. [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Naeem 2015a
- Naeem F, Farooq S, Kingdon D. Cognitive behavioural therapy (brief versus standard duration) for schizophrenia. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD010646.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Clinical Excellence. Psychosis and schizophrenia in adults: prevention and management. nice.org.uk/guidance/cg178/chapter/1‐Recommendations (accessed on 01 January 2018).
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Parker 1991
- Parker G, Rosen A, Emdur N, Hadzi‐Pavlovic D. The life skills profile: psychometric properties of a measure assessing function and disability in schizophrenia. Acta Psychiatrica Scandinavica 1991;83:145‐52. [DOI] [PubMed] [Google Scholar]
Patterson 2001
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste D. UCSD performance‐based skills assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophrenia Bulletin 2001;27:235‐45. [DOI] [PubMed] [Google Scholar]
Robson 1989
- Robson P. Development of a new self report questionnaire to measure self‐esteem. Psychological Medicine 1989;19:513‐8. [DOI] [PubMed] [Google Scholar]
Rosenberg 1965
- Rosenberg. Society and the Adolescent Self‐image. Princeton, NJ: Princeton University Press, 1965. [Google Scholar]
Royal College of Psychiatrists 2012
- Royal College of Psychiatrists. Report of the National Audit of Schizophrenia (NAS) 2012. London: Healthcare Quality Improvement Partnership.
Sander 1997
- Beck‐Sander A, Birchwood M, Chadwick P. Acting on command hallucinations: a cognitive approach. British Journal of Clinical Psychology 1997;36:139‐48. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shokraneh 2017
- Shokraneh F, Adams CE. Study‐based registers of randomized controlled trials: starting a systematic review with data extraction or meta‐analysis. BioImpacts 2017;7(4):209‐17. [DOI: 10.15171/bi.2017.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2018
- Shokraneh F, Adams CE. Gallstone, snake venom and witchcraft for schizophrenia: the challenges of classifying [schizophrenia] trials. Evidence‐Based Medicine 2018;23(Suppl. 1):A18. [DOI: 10.1136/bmjebm-2018-111024.36] [DOI] [Google Scholar]
Si 2009
- Si TM, Shu L, Tian CH, Su YA, Yan J, Cheng J, et al. Reliability and validity of the Chinese version of the Personaland Social Performancescale (PSP) among patients with schizophrenia. Chinese Mental Health Journal 2009;23:790‐4. [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tarrier 1993
- Tarrier N, Beckett R, Harwood S, Baker A, Yusupoff L, Ugarteburu I. A trial of two cognitive‐behavioural methods of treating drug‐resistant residual psychotic symptoms in schizophrenic patients: I. Outcome. British Journal of Psychiatry 1993;162:524‐32. [DOI] [PubMed] [Google Scholar]
Thompson 2000
- Thompson K, Kulkami J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophrenia Research 2000;42:241‐47. [DOI] [PubMed] [Google Scholar]
Turkington 2004
- Turkington D, Dudley R, Warman DM, Beck AT. Cognitive‐behavioral therapy for schizophrenia: a review. Journal of Psychiatric Practice 2004;10(1):5‐16. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PubMed] [Google Scholar]
Ustun 2010
- Üstün T, Bedirhan, Chatterji, Somnath, Kostanjsek, Nenad, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bulletin of the World Health Organization 2010;88:815‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 2000
- Wallace CJ, Liberman RP, Tauber R, Wallace J. The Independent Living Skill Survey: a comprehensive measure of the community functioning of severely and persistently mentally ill individuals. Schizophrenia Bulletin 2000;26:631‐58. [DOI] [PubMed] [Google Scholar]
Wang 1998
- Wang X. Manual of Mental Health Rating Scales. Beijing: Journal of China Mental Health, 1998. [Google Scholar]
Wang 1999
- Wang XD, Wang XL, Ma H. Handbook of Rating Scales for Mental Health. Chinese Mental Health Journal, 1999. [Google Scholar]
Wechsler 2009
- Wechsler D. Wechsler Memory Scale ‐ Fourth Edition (WMS‐IV). San Antonio (TX): Pearson Assessment, 2009. [Google Scholar]
WHO 1973
- World Health Organization. Report of the International Pilot Study of Schizophrenia. WHO.
WHOQOL Group 1998
- WHOQOL Group. Development of the World Health Organization WHOQOL‐BREF quality of life assessment.. Psychological Medicine 1998;28:551‐8. [DOI] [PubMed] [Google Scholar]
Wilkinson 2000
- Wilkinson G, Hesdon B, Wild D, Cookson R, Farina C, Sharma V. Self‐report quality of life measure for people with schizophrenia: the SQLS. British Journal of Psychiatry 2000;177:42‐6. [DOI] [PubMed] [Google Scholar]
Wu 1998
- Wu WY. Manual of Psychiatric Rating Scales. 2nd Edition. Hunan Changsa: Hunan Science and Technology Press, 1998. [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavia 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jones 2009c
- Jones C, Campbell C, Cormac I, Hacker D, Meaden A, Irving CB. Cognitive behaviour therapy versus standard care for schizophrenia. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007964] [DOI] [Google Scholar]


