Abstract
Background
Nausea and vomiting is a common and distressing presenting complaint in emergency departments (ED). The aetiology of nausea and vomiting in EDs is diverse and drugs are commonly prescribed. There is currently no consensus as to the optimum drug treatment of nausea and vomiting in the adult ED setting.
Objectives
To provide evidence of the efficacy and safety of antiemetic medications in the management of nausea and vomiting in the adult ED setting.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 8), MEDLINE (OvidSP) (January 1966 to August 2014), EMBASE (OvidSP) (January 1980 to August 2014) and ISI Web of Science (January 1955 to August 2014). We also searched relevant clinical trial registries and conference proceedings.
Selection criteria
We included randomized controlled trials (RCTs) of any drug in the treatment of nausea and vomiting in the treatment of adults in the ED. Study eligibility was not restricted by language or publication status.
Data collection and analysis
Two review authors independently performed study selection, data extraction and assessment of risk of bias in included studies. We contacted authors of studies to obtain missing information if required.
Main results
We included eight trials, involving 952 participants, of which 64% were women. Included trials were generally of adequate quality, with six trials at low risk of bias, and two trials at high risk of bias. Three trials with 518 participants compared five different drugs with placebo; all reported the primary outcome as mean change in visual analogue scale (VAS) (0 to 100) for nausea severity from baseline to 30 minutes. Trials did not routinely report other primary outcomes of the change in nausea VAS at 60 minutes or number of vomiting episodes. Differences in mean VAS change from baseline to 30 minutes between placebo and the drugs evaluated were: metoclopramide (three trials, 301 participants; mean difference (MD) ‐5.27, 95% confidence interval (CI) ‐11.33 to 0.80), ondansetron (two trials, 250 participants; MD ‐4.32, 95% CI ‐11.20 to 2.56), prochlorperazine (one trial, 50 participants; MD ‐1.80, 95% CI ‐14.40 to 10.80), promethazine (one trial, 82 participants; MD ‐8.47, 95% CI ‐19.79 to 2.85) and droperidol (one trial, 48 participants; MD ‐15.8, 95% CI ‐26.98 to ‐4.62). The only statistically significant change in baseline VAS to 30 minutes was for droperidol, in a single trial of 48 participants. No other drug was statistically significantly superior to placebo. Other included trials evaluated a drug compared to "active controls" (alternative antiemetic). There was no convincing evidence of superiority of any particular drug compared to active control. All trials included in this review reported adverse events, but they were variably reported precluding meaningful pooling of results. Adverse events were generally mild, there were no reported serious adverse events. Overall, the quality of the evidence was low, mainly because there were not enough data.
Authors' conclusions
In an ED population, there is no definite evidence to support the superiority of any one drug over any other drug, or the superiority of any drug over placebo. Participants receiving placebo often reported clinically significant improvement in nausea, implying general supportive treatment such as intravenous fluids may be sufficient for the majority of people. If a drug is considered necessary, choice of drug may be dictated by other considerations such as a person's preference, adverse‐effect profile and cost. The review was limited by the paucity of clinical trials in this setting. Future research should include the use of placebo and consider focusing on specific diagnostic groups and controlling for factors such as intravenous fluid administered.
Plain language summary
Medicines in the treatment of emergency department nausea and vomiting
Review question
We reviewed the effects of medicines in the treatment of nausea and vomiting in adults in the emergency department.
Background
Nausea (feeling sick) and vomiting (being sick) is a common symptom in people in emergency departments, and can result from a number of different causes. In addition to being distressing, it can lead to other problems such as dehydration (where the body is losing more fluid than it is taking in). Medicines to treat nausea have been useful in other settings, such as after operations, although it is not known what is the best medicine for people in emergency departments.
Study characteristics
The evidence is current to August 2014. We included eight clinical trials of 952 participants. The trials assessed many different medicines at different doses, but only three trials included a placebo group (dummy medication). Six of these trials were of high quality, with low risk of error (i.e. bias, where the true effect is exaggerated). For this review, we included the effects of the medicines on nausea and vomiting up to one hour after the medicine was given.
Key results and quality of the evidence
The main results of interest were the effect on nausea between zero and 60 minutes after the medicine was given, number of vomits and side effects to medicines. Of these, only nausea at 30 minutes and side effects were reported by all trials. From all trials, only one medicine was reported to be better than placebo and other medicines. That was droperidol, which was included in one small trial of 97 participants. No other single medicine was definitely better than any other medicine, and none of the other trials that included a placebo group showed that the active medicines definitely worked better than the placebo. Side effects were mild.
Our results suggest that in people in the emergency department, nausea will generally improve, whether they are treated with specific medicines or placebo. Therefore, supportive treatment, such as intravenous fluids (where fluid is given directly into a blood vessel) may be sufficient for many people. Overall, the quality of the evidence was low, mainly because there was not enough data.
Summary of findings
Summary of findings for the main comparison. Metoclopramide for nausea and vomiting in the emergency department.
| Metoclopramide for nausea and vomiting in the emergency department | ||||||
|
Patient or population: people with nausea and vomiting Settings: emergency department Intervention: metoclopramide Comparisons: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Metoclopramide | |||||
| Change in nausea severity at 30 minutes Visual analogue scale Scale from: 0 to 100 Follow‐up: 30 minutes | The mean nausea severity decrease ‐ metoclopramide vs. placebo ranged across control groups from 23 to 38 mm | The mean nausea severity decrease ‐ metoclopramide vs. placebo in the intervention groups was 5.27 lower (11.33 lower to 0.8 higher) | ‐ | 301 (3 studies) | ⊕⊕⊝⊝ low1 | A larger decrease in nausea severity score indicates better control of symptoms. A difference of > 15 mm is thought to be the 'minimum clinically significant difference' |
| Number of vomiting episodes | See comment | See comment | Not estimable | 301 (3 studies) |
See comment | This outcome was not reported in any of the included studies |
| Adverse reactions | See comment | See comment | Not estimable | 301 (3 studies) |
See comment | No pooling of results was possible, due to variations in reporting. No studies reported any serious adverse reactions or significant difference in adverse reactions |
| Proportion of participants requiring rescue medication Physician's discretion Follow‐up: 60 minutes | Study population | OR 0.3 (0.17 to 0.53) | 299 (3 studies) | ⊕⊕⊝⊝ low2 | An OR < 1 means less need for the medication with metoclopramide | |
| 381 per 1000 | 156 per 1000 (95 to 246) | |||||
| Moderate | ||||||
| 363 per 1000 | 146 per 1000 (88 to 232) | |||||
| Participant satisfaction with intervention Self report | Study population | OR 1.07 (0.6 to 1.91) | 216 (2 studies) | ⊕⊕⊝⊝ low1 | An OR < 1 implies better satisfaction with metoclopramide | |
| 657 per 1000 | 672 per 1000 (535 to 785) | |||||
| Moderate | ||||||
| 721 per 1000 | 734 per 1000 (608 to 832) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded for imprecision (wide confidence interval and not achieving optimal information size). 2 Downgraded as this outcome was poorly described and variable in the included trials and imprecision.
Background
Description of the condition
Nausea and vomiting is a common and distressing presenting complaint in emergency departments (ED) with more than eight million presentations annually in the US (LaValley 2003). Nausea describes the unpleasant sensation of the imminent need to vomit, whereas vomiting refers to the forceful oral expulsion of gastric contents associated with contraction of the abdominal and chest wall musculature (Quigley 2001). Therefore, whereas nausea is a subjective experience, vomiting represents a physical event.
Nausea and vomiting can be extremely distressing and complications can range from trivial to serious, for example, dehydration, electrolyte disturbance, aspiration, Mallory‐Weiss syndrome (tears of gastric and oesophageal mucosa) and oesophageal rupture (Bork 2011; Zun 2010). The most common cause of nausea and vomiting in the ED is acute gastroenteritis (inflammation of the gastrointestinal tract); however, the aetiology of nausea and vomiting in the ED setting is diverse and may include physiological and pathological responses of the gastrointestinal tract, central nervous system disorders, endocrine or metabolic problems and toxins or medications, among others (Zun 2010). Nausea and vomiting in the ED may co‐exist with other medical conditions (e.g. myocardial infarction or small bowel obstruction) or result from other treatments prescribed in the ED (e.g. opiate analgesia)
Description of the intervention
ED management of a patient often involves identification of the cause of nausea and vomiting as well as recognition and correction of consequences and complications (AGA 2001; Quigley 2001). Antiemetics are commonly prescribed for undifferentiated nausea and vomiting in the ED setting, although there is little consensus as to the optimum management. Therapy is often directed at the presumed pathophysiological cause or extrapolated from evidence in other settings.
How the intervention might work
The pathophysiology of nausea and vomiting is a complex process. The physical aspect of vomiting is co‐ordinated by the vomiting centre of the brain, functionally located in the lateral reticular formation of the medulla. Efferent pathways from the vomiting centre are mainly through the vagus, phrenic and spinal nerves (Zun 2010). The vomiting centre receives afferent input from various sources including the chemoreceptor trigger zone (CTZ) located in the area postrema in the floor of the fourth ventricle, the vagus and sympathetic nerves, as well as impulses directly from the gastrointestinal tract and other sources (Bork 2011; Carpenter 1990). The CTZ is also activated by mediators in the circulation, which may include hormones, peptides, medications or toxins (Zun 2010).
Reflecting the complex nature of the process of nausea and vomiting, antiemetics consist of a diverse group of chemicals with varying mechanisms and sites of action. Targets of action include the CTZ through dopamine receptors, serotonin receptors in the area postrema and nucleus tractus solitarius, and cholinergic and histamine receptors. Other agents have their action peripherally on the gastrointestinal tract, and for others the mechanism of action is incompletely understood.
Why it is important to do this review
High‐level evidence supports the use of antiemetics in the management of nausea and vomiting in many settings and populations; however, there is little guidance or consensus in recommendations for the management of nausea and vomiting in the adult ED setting. Recommendations are inconsistent and rarely evidence based. Preferred pharmacological agents differ significantly between countries and regions (LaValley 2003; Mee 2011). Postoperative nausea and vomiting (Carlisle 2006), chemotherapy (Billio 2010; Jordan 2007), and radiotherapy (Kris 2006; Maranzano 2005) induced nausea and vomiting, in particular, have been extensively researched with systematic reviews and guidelines published. Cochrane systematic reviews have also been published on antiemetic use in people receiving palliative care (Dorman 2010; Perkins 2009), paediatric and adolescent gastroenteritis (Fedorowicz 2011), nausea and vomiting associated with early pregnancy (Mathews 2010), and the use of acupuncture pressure points (Ezzo 2006; Lee 2009). However, extrapolation of the evidence from these settings to the ED population is not straightforward because of differences in aetiologies, patient populations and other factors. This review is important to help establish the current evidence for management of nausea and vomiting in this clinically diverse setting, and to help determine future research priorities.
Objectives
To provide evidence of the efficacy and safety of antiemetic medications in the management of nausea and vomiting in the adult ED setting.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) of any drug in the treatment of nausea and vomiting in the ED setting. We did not restrict the study eligibility by language or publication status. We excluded prospective cohort studies and quasi‐randomized studies.
Types of participants
We included adult ED participants aged 16 years and older with nausea and vomiting. We only included trials if the study participants were identified as an 'adult', or if over 80% of the participants were aged over 16 years. We contacted study authors if age data were not available, and we did not include studies in this review if ages of the participants were not clear. We clearly identified the setting as ED.
Types of interventions
Interventions included any pharmacological agent prescribed for the treatment of nausea and vomiting. We considered any dose, formulation or route of administration. Appropriate comparators included placebo, no treatment or "active control" (alternative antiemetic).
Types of outcome measures
Severity of nausea, as assessed by use of any scale or score, and number of vomiting episodes.
Primary outcomes
Severity of nausea. Nausea was assessed as measured on any scale or score used by study authors, and transformed if required to a score between 0 and 100. It was recorded as complete resolution of nausea (e.g. including score 0 on a visual analogue scale (VAS)) and change from baseline value, with a minimum clinically significant difference (MCSD) from baseline defined as 15 mm on the VAS (Hendey 2005). We included time points between zero and 60 minutes as relevant to the practice of emergency medicine.
Number of vomiting episodes, both self reported and clinician‐reported outcomes.
Any adverse reactions.
Secondary outcomes
Proportion of participants requiring rescue medication.
Proportion of participants who required hospital admission.
Mean or median ED length of stay.
Participant satisfaction with intervention.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 8), MEDLINE (OvidSP) (January 1966 to August 2014), EMBASE (OvidSP) (January 1980 to August 2014) and ISI Web of Science (January 1955 to August 2014). The search used a combination of text words and MeSH, with no language restriction. We developed a specific strategy for each database. The search strategy for MEDLINE is outlined in Appendix 1, which contains the Cochrane highly sensitive search strategy (Higgins 2011). Search strategies for EMBASE (Appendix 2) and CENTRAL (Appendix 3) are also included.
Searching other resources
We handsearched the reference lists of identified papers to identify further relevant trials. We examined clinical trial registries for unpublished trials on the International Clinical Trials Registry Platform (www.who.int/ictrp/en/), USA Clinical Trials registry (clinicaltrials.gov/), and Controlled Trials metaRegister of Controlled Trials (www.controlled‐trials.com/mRCT and www.controlled‐trials.com/mrct/archived), and contacted study authors. We handsearched key journals (Annals of Emergency Medicine, Academic Emergency Medicine, Emergency Medicine, Journal of Emergency Medicine and Emergency Medicine Australasia) from January 2009 to August 2014.
We also handsearched the published abstracts from relevant conference proceedings for additional unpublished trials. The conferences searched included:
Society for Academic Emergency Medicine (SAEM) Annual Meeting ‐ Academic Emergency Medicine (1996 to August 2014);
American College of Emergency Physicians (ACEP) Scientific Assembly/Research Forum (1996 to August 2014);
Canadian Association of Emergency Medicine (CAEM) Annual Conference ‐ Canadian Journal of Emergency Medicine;
Australasian College of Emergency Medicine (ACEM) ‐ Annual Scientific Meeting ‐ Emergency Medicine Australasia (2004 to August 2014);
College of Emergency Medicine (UK) Scientific conference (2006 to August 2014) ‐ Emergency Medicine Journal ‐ supplements;
European Society for Emergency Medicine (EuSEM) Mediterranean Emergency Medicine Congress ‐ European Journal of Emergency Medicine.
We contacted other experts in the field to provide details of any ongoing clinical trials or unpublished materials.
Data collection and analysis
Selection of studies
We merged the search results with reference management software and removed duplicates. Two review authors (JF and RM) independently assessed titles and abstracts from studies identified by the search. We obtained full copies of all relevant or potentially relevant studies identified by either review author. We planned to obtain translations, if necessary, and contacted study authors for clarification, if necessary.
Two review authors (JF and RM) independently applied inclusion and exclusion criteria, and confirmed eligibility using a checklist in the data collection form (Appendix 5), which we developed for this review. We resolved disagreements by consensus or by consulting the third review author (DEW). We listed the characteristics of key excluded studies in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (JF and RM) independently extracted data using a specifically designed, piloted data collection form. We resolved discrepancies by consensus and by consulting the third review author (DEW). One review author (JF) entered data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (JF and RM) independently assessed methodological quality of the eligible trials. We resolved disagreements by discussion and, if we could not reach a consensus, a third review author (DEW) arbitrated.
We performed risk of bias assessment using the 'Risk of bias' tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed each trial according to the quality domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and any other potential threats to validity (Appendix 5).
We considered a trial as having a low risk of bias if we assessed all domains as adequate. We considered a trial as having a high risk of bias if we assessed two or more domains as inadequate or unclear, or if we considered any one of the domains of allocation concealment, blinding participants or personnel, or blinding outcome assessors inadequate or unclear. We performed sensitivity analysis to determine whether excluding studies at high risk of bias affected the results of the meta‐analysis.
We reported the 'Risk of bias' table as part of the Characteristics of included studies table and presented a 'Risk of bias' summary figure, which detailed all of the judgements made for all included studies in the review.
Measures of treatment effect
We reported the primary outcomes of included studies as either a dichotomous or continuous variable, for example vomiting yes or no; or nausea VAS score. We reported dichotomous outcomes as number and proportions and present continuous outcomes as mean change.
Unit of analysis issues
We used only individual level data.
Dealing with missing data
We contacted study authors by e‐mail with requests to provide missing data. We intended to use imputation methods for missing data using 'worst‐case', 'best‐case' and 'average‐case' scenarios for the primary outcome of change in nausea severity, and perform sensitivity analyses to assess how sensitive results were to assumptions made.
Assessment of heterogeneity
We assessed for statistical heterogeneity by visual inspection of the confidence intervals (CI) of forest plot results, P value < 0.05 for Chi2 test and I2 statistic with a value > 50% indicating significant heterogeneity (Higgins 2011). In addition, we assessed for clinical heterogeneity with consideration of the characteristics of included studies regarding participants, interventions and outcome measures. We presented the primary analysis using the random‐effects model to account for clinical heterogeneity.
Assessment of reporting biases
We planned to test for funnel plot asymmetry using weighted linear regression of effect estimates on their standard error (Egger 1997), if we included more than 10 trials. However, we included only eight studies in this review.
Data synthesis
We based outcome data on intention‐to‐treat analysis results. We combined data from dichotomous and continuous outcomes and performed meta‐analysis using Review Manager 5 when data from two or more RCTs were sufficient (RevMan 2014). For trials with multiple intervention groups, we combined groups to create single pair‐wise comparisons as outlined in Chapter 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For dichotomous outcomes, we summed both the sample sizes and the numbers of people with events across groups, and for continuous outcomes, we combined means and standard deviations (SD) using the methods described in Section 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used odds ratio (OR) to measure the treatment effect of dichotomous outcomes and the mean difference (MD) for continuous data using the inverse variance method. We used random‐effects model for analyses due to clinical heterogeneity of interventions and outcomes. When it was not appropriate to combine results, we presented them in narrative form.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis on nausea and vomiting associated with pregnancy, opiate administration and chemotherapy. This analysis was not possible as no data were available.
Sensitivity analysis
We performed sensitivity analyses using both the fixed‐effect and random‐effects models, and the effect on the overall primary results by excluding studies at high risk of bias (as defined above).
'Summary of findings' tables
We used the principles of the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system (Guyatt 2008) to assess the quality of the body of evidence associated with the specific outcomes change in nausea severity number of vomiting episodes, adverse reactions, proportion of participants requiring rescue medication and participant satisfaction with intervention, in our review and constructed Table 1 using GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
The electronic search identified 6799 studies with duplicates removed, consisting of 1389 from EMBASE, 3630 from MEDLINE, 320 from CENTRAL and 2507 from Web of Science. After screening titles and abstracts, we identified 13 studies for examination of the full text. We identified eight relevant studies from searching conference proceedings and clinical trial registries. Four of the studies appeared to report unique studies of relevance to our review, whereas four of the studies reported data subsequently published in journals and identified by the electronic database search. We contacted authors of the four other studies, but received no data from investigators, meaning information was only available in abstract form. Two of the authors of this Cochrane review were co‐authors of the Egerton‐Warburton 2014 study. Therefore, the search yielded 17 studies for consideration for inclusion. After evaluation of the full‐text articles, we included eight studies in the review (see Characteristics of included studies table), we excluded five studies (see Characteristics of excluded studies table), and we identified four studies that were available in abstract form, and had insufficient information to assess (see Characteristics of studies awaiting classification table). For a PRISMA flow diagram of search strategy, see Figure 1.
1.
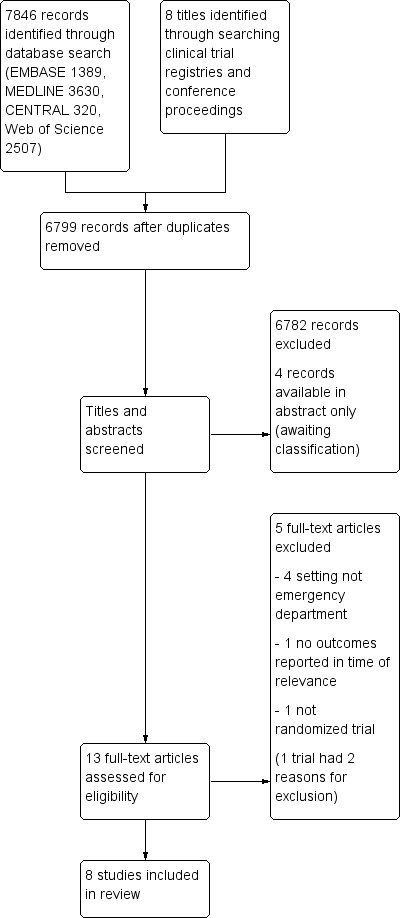
Study flow diagram.
Included studies
We included eight trials in this review (Barrett 2011; Braude 2006; Braude 2008; Chae 2011; Cham 2004; Egerton‐Warburton 2014; Ernst 2000; Patka 2011). Further details are available in the Characteristics of included studies table.
Design
The eight included trials were all parallel group, randomized trials. One trial was described as single blind (Cham 2004), the remainder were described as double blind. The included trials had two to four treatment arms, with only three trials including a placebo arm (Barrett 2011; Braude 2006; Egerton‐Warburton 2014), the other trials using an active control. One trial was described as a non‐inferiority trial (Braude 2008). The total sample size was 952 participants, consisting of 338 men and 614 women.
Participants and setting
The included trials were conducted in EDs in the US and Australia, mostly identified as university affiliated or teaching hospitals. All only included adults aged over 18 years. Most trials included nausea and vomiting from a variety of aetiologies, three trials excluded participants if their initial nausea VAS score was less than 40 mm (Barrett 2011; Braude 2006; Braude 2008). One trial specified the requirement for "uncomplicated gastritis and gastroenteritis" for eligibility (Ernst 2000). Women outnumbered men in all trials.
Intervention
The trials evaluated six different antiemetics. All trials included only intravenous antiemetics. Two trials had four arms (Barrett 2011; Braude 2006), and one trial had three arms (Egerton‐Warburton 2014). Only three trials included a placebo arm (Barrett 2011; Braude 2006; Egerton‐Warburton 2014). Five trials evaluated metoclopramide in doses of 10 mg, 20 mg and 0.4 mg/kg up to 32 mg. One trial compared two different doses of metoclopramide (Cham 2004). Five trials evaluated 5‐hydroxytryptamine‐3 (5‐HT3) blockers (Barrett 2011; Braude 2008; Chae 2011; Egerton‐Warburton 2014; Patka 2011), four using ondansetron 4 mg (Barrett 2011; Braude 2008; Egerton‐Warburton 2014; Patka 2011), and one using tropisetron 0.5 mg (Chae 2011). Three trials included prochlorperazine 10 mg (Braude 2006; Ernst 2000; Patka 2011); three trials evaluated promethazine, one trial at 12.5 mg (Barrett 2011), and two trials at 25 mg (Braude 2008; Ernst 2000). All trials involved administration of a single stat dose as a bolus or over two to five minutes. Most trials included administration of varying amounts of intravenous fluid during the study period.
Outcomes
All included studies reported the primary outcome of severity of nausea reported on any scale or score. Seven of the studies reported nausea on a 100‐mm VAS (Barrett 2011; Braude 2006; Braude 2008; Chae 2011; Egerton‐Warburton 2014; Ernst 2000; Patka 2011), and one study used a 0 to 10 numerical rating scale (NRS) (Cham 2004). All studies included the time point of 30 minutes; three trials also reported data at 60 minutes (Chae 2011; Ernst 2000; Patka 2011); two trials reported outcomes beyond 60 minutes that we did not consider relevant to this review (Chae 2011; Patka 2011).
Three trials reported the number of vomiting episodes (Chae 2011; Egerton‐Warburton 2014; Patka 2011). All trials reported adverse events, but the trials classified and reported them differently. All trials reported the outcome of requirement for rescue medication, but this was variably defined, or not defined in trials. Three trials reported the proportion of participants requiring hospital admission (Braude 2008; Ernst 2000; Patka 2011). No trials reported on ED length of stay, while three reported participant satisfaction (Braude 2006; Braude 2008; Egerton‐Warburton 2014).
Excluded studies
We excluded five studies; see Characteristics of excluded studies table for details. The study by Roy 1991 compared oral doses of metoclopramide and domperidone, three times a day over one week. The setting appeared to be in general practice and outcomes were measured beyond the time frame of relevance to this review. Another excluded trial, which evaluated one or two doses of intramuscular domperidone 10 mg versus placebo, measured outcomes beyond the relevant time frame and was not clearly identified as ED (Agorastos 1981). We excluded one report as it was not an RCT, but an uncontrolled prospective design with no appropriate comparator group (Ordog 1984). We excluded one large multicentre trial evaluating two different doses of ondansetron (8 mg and 16 mg) versus placebo for opiate‐associated nausea and vomiting (Sussman 1999). The setting was not clearly an ED, although it was stated that "many" participants were managed in EDs, and the primary outcome was resolution of symptoms at 24 hours, which was not relevant to this review. Finally, we excluded one single‐centre study from Israel because the setting was not an ED, but rather an outpatient setting, participants requiring intravenous treatment were excluded and time points of the outcome assessments were not of relevance to this review (Cohen 1999).
Awaiting classification
There are four trials awaiting classification (Friedland 2008; Haensel 2007; Thacker 2003; Thacker 2004; see Characteristics of studies awaiting classification table). These trials were available in abstract form only, with insufficient detail to allow inclusion in the review. We were unable to obtain further information from authors of these trials.
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
We assessed the risk of bias of each trial; see Characteristics of included studies table, Assessment of risk of bias in included studies and 'Risk of bias' summary (Figure 2; Figure 3).
2.
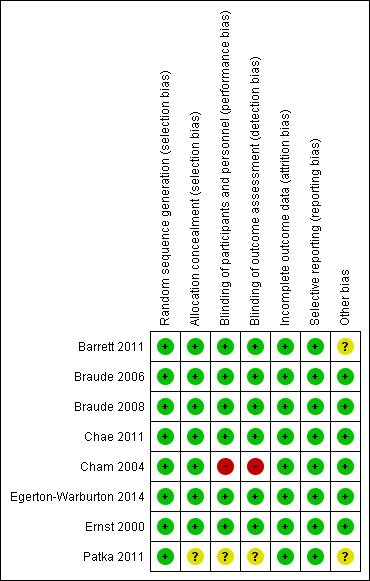
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
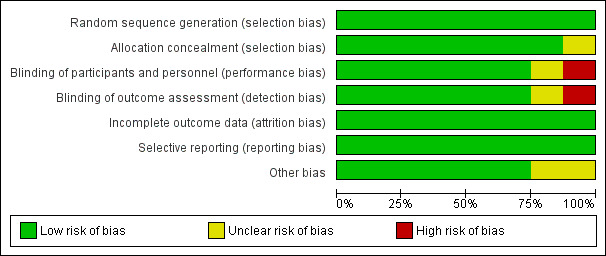
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All included trials reported acceptable methods of random sequence generation. The report by Braude 2008 reported only that drugs were "randomized in blocks of 10" and the method of sequence generation was not explicitly stated. This was clarified with the authors as being generated by computer program and recorded as low risk of bias for the review. Allocation concealment was adequate in seven out of eight studies. One study did not elucidate any mechanism of allocation concealment in the report, and was, therefore, considered unclear risk of bias (Patka 2011).
Blinding
Blinding of participants and personnel was adequate in six trials (Barrett 2011; Braude 2006; Braude 2008; Chae 2011; Egerton‐Warburton 2014; Ernst 2000), unclear in one trial (Patka 2011), and judged as high risk of bias in one trial (Cham 2004). Outcomes were self reported in all included trials. The trial judged to be high risk of bias was reported as single blind, with no further details provided, but made no attempt to blind clinical staff (Cham 2004). One other trial described preparation of the study drug by independent nurses from the usual ward stock, keeping the drug allocation concealed from the participant and treating doctor (Chae 2011). While we considered that this procedure could potentially compromise blinding, we thought this would have been unlikely to have occurred sufficiently to affect the outcome, and we, therefore, judged the trial to be low risk of bias for this domain. We assessed one trial as unclear risk of bias as the authors did not report any mechanism for blinding (Patka 2011).
With regards to detection bias, outcomes were all self reported, consequently the same trial was rated as high risk of bias (Cham 2004), and the same trial was reported as unclear risk of bias (Patka 2011) due to similar reasons as described above.
Incomplete outcome data
All trials were at low risk of attrition bias. Although two trials had some unexplained missing data (Chae 2011; Patka 2011), these appeared balanced between intervention groups, outside the time points considered most relevant to this review, and unlikely to have a significant impact on the intervention effect estimates.
Selective reporting
There was no evidence of selective reporting in any of the trials. Outcomes listed in methods sections were reflected in results reported. One trial listed two primary outcomes on a clinical trial registry, and reported the non‐significant outcome as a secondary outcome in the published report (Chae 2011). However, as all results were reported, we considered this to be low risk of bias.
Other potential sources of bias
We assessed two trials as 'unclear' with regards to other potential sources of bias. The Patka 2011 trial was generally poorly reported, with inconsistencies throughout the report, and no reason given for non‐recruitment of substantial numbers of potentially eligible participants. The trial by Barrett 2011 reported an unplanned interim analysis and post hoc power calculation. The trial was then stopped at just over one‐third of their planned recruitment target, because the likelihood of achieving a statistically significant result was remote, hence introducing the possibility of a type 2 error, which was acknowledged in the report. We judged that this may have introduced some bias.
Effects of interventions
See: Table 1
See Table 1 for the comparison of metoclopramide, the drug most commonly evaluated, versus placebo.
This section included results from all eight trials. The three trials that included a placebo arm evaluated five different drugs: metoclopramide, ondansetron, prochlorperazine, promethazine and droperidol (Barrett 2011; Braude 2006; Egerton‐Warburton 2014). The five non‐placebo trials evaluated the same five drugs (Braude 2008; Chae 2011; Cham 2004; Ernst 2000; Patka 2011), with one trial including the 5‐HT3 blocker tropisetron (Chae 2011).
To address the aims of this review, we combined the trials to allow comparisons of drugs versus placebo and each drug versus active control. Despite a degree of heterogeneity, this did allow for some pooling of results. We also presented the results of each drug studied versus each other drug. Some of these comparisons involved small numbers from one or two trials only, so caution is advised in interpretation of these findings.
Comparison of drug versus placebo
Three trials, with 518 participants, compared five different drugs with placebo (Barrett 2011; Braude 2006; Egerton‐Warburton 2014).
Primary outcomes
Severity of nausea
All three trials reported the primary outcome of mean VAS rating change for nausea severity from baseline to 30 minutes (Barrett 2011; Braude 2006; Egerton‐Warburton 2014).
All three trials evaluated metoclopramide and involved 301 participants. From pooled results, the MD in VAS rating change at 30 minutes between metoclopramide and placebo was ‐5.27 (95% CI ‐11.33 to 0.80) (Figure 4).
4.
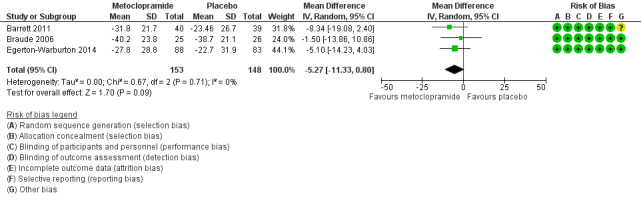
Forest plot of comparison: 1 Metoclopramide versus placebo, outcome: 1.1 Change in nausea severity at 30 minutes.
Two trials evaluated ondansetron and involved 250 participants (Barrett 2011; Egerton‐Warburton 2014). From pooled results, the MD in nausea VAS rating change at 30 minutes between ondansetron and placebo was ‐4.32 (95% CI ‐11.20 to 2.56) (Analysis 2.1).
2.1. Analysis.
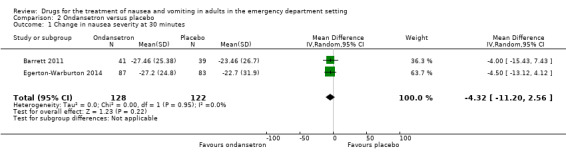
Comparison 2 Ondansetron versus placebo, Outcome 1 Change in nausea severity at 30 minutes.
One trial each evaluated prochlorperazine (50 participants; Braude 2006), promethazine (82 participants; Barrett 2011), and droperidol (48 participants; Braude 2006). The MD in VAS rating change at 30 minutes between prochlorperazine and placebo was ‐1.80 (95% CI ‐14.40 to 10.80) (Table 2). Between promethazine and placebo the MD was ‐8.47 (95% CI ‐19.79 to 2.85) (Table 3), and between droperidol and placebo the MD was ‐15.80 (95% CI ‐26.98 to ‐4.62) (Table 4).
1. Prochlorperazine versus placebo.
| Outcome | Participants | Statistical methods | Effect estimate |
| Change in nausea severity at 30 minutes | 50 | Mean Difference (IV, Random, 95% CI [mm]) | ‐1.80 [‐14.40, 10.80] |
| Proportion of participants requiring rescue medication | 50 | Odds Ratio (M‐H, Random, 95% CI) | 1.83 [0.45, 7.51] |
| Participant satisfaction | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.20, 4.13] |
Data from single trial comparing prochlorperazine versus placebo (Braude 2006).
CI: confidence interval.
2. Promethazine versus placebo.
| Outcome | Participants | Statistical methods | Effect estimate |
| Change in nausea severity at 30 minutes | 82 | Mean Difference (IV, Random, 95% CI [mm]) | ‐8.47 [‐19.79, 2.85] |
| Proportion of participants requiring rescue medication | 86 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.34] |
Data from single trial comparing promethazine versus placebo (Barrett 2011).
CI: confidence interval.
3. Droperidol versus placebo.
| Outcome | Participants | Statistical methods | Effect estimate |
| Change in nausea severity at 30 minutes | 48 | Mean Difference (IV, Random, 95% CI [mm]) | ‐15.80 [‐26.98, ‐4.62] |
| Proportion of participants requiring rescue medication | 48 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.54] |
| Participant satisfaction | 48 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.30, 11.02] |
Data from single trial comparing droperidol versus placebo (Braude 2006).
CI: confidence interval.
Only the result for droperidol favoured drug over placebo (Braude 2006; Table 4).
Number of vomiting episodes
One trial reported the reduction in number of vomiting episodes, which were similar for ondansetron (median 0, interquartile range (IQR) 0 to 1), metoclopramide (median 0, IQR 0 to 2) and placebo (median 0, IQR 0 to 1) (Egerton‐Warburton 2014). The other two trials did not report the number of vomiting episodes (Barrett 2011; Braude 2006).
Adverse reactions
All three trials reported adverse reactions (Barrett 2011; Braude 2006; Egerton‐Warburton 2014); however, differences in reporting precluded pooling of results. None of the trials reported any serious adverse events.
Barrett 2011, evaluating ondansetron, metoclopramide and promethazine versus placebo, separately reported the proportion of participants with akathisia, headache and sedation at baseline and 30 minutes (characterized as none, mild, moderate and severe). These symptoms were commonly reported at baseline making interpretation of 30‐minute data problematic. At 30 minutes, akathisia was more common with each drug compared with placebo (4/38 (11%) with ondansetron, 11/41 (27%) with metoclopramide, 2/43 (5%) with promethazine, 1/38 with placebo). Headache was reported by 11/39 (28%) participants with ondansetron, 8/41 (20%) with metoclopramide and 12/43 (28%) with promethazine, compared with 6/38 (16%) with placebo. Sedation was reported by 16/39 (41%) participants with ondansetron, 21/40 (53%) with metoclopramide and 25/43 (58%) with promethazine, compared with 13/38 (34%) with placebo.
Braude 2006 reported mean and SD change in anxiety and sedation on a VAS from baseline to 30 minutes. For anxiety, the mean change for droperidol was ‐25.9 (SD 30.2), metoclopramide ‐25.4 (SD 24.3), prochlorperazine ‐21.9 (SD 38.8) and placebo ‐31.7 (SD 31.6); these differences were not significant (P value = 0.79). For sedation, the mean change for droperidol was 13.5 (SD 32.2), metoclopramide 0.4 (SD 30.1), prochlorperazine 5.1 (SD 26.5) and placebo ‐4.8 (SD 25.0); these differences were not significant (P value = 0.75).
Egerton‐Warburton 2014 reported adverse events in 9/258 (3.5%) participants: six in participants who received metoclopramide (two akathisia, two restlessness, one sweatiness and one muscle twitching), two in participants who received ondansetron (one dizziness and one stinging at injection site) and one in a participant who received placebo (shaking and restlessness).
The only significant result was a higher rate of akathisia for the "any drug" group compared with placebo (Barrett 2011).
Secondary outcomes
Proportion of participants requiring rescue medication
All three trials reported the proportion the participants requiring rescue medication, with 510 participants (Barrett 2011; Braude 2006; Egerton‐Warburton 2014).
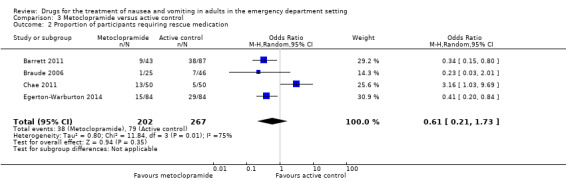
All three trials evaluated metoclopramide and included 299 participants (Barrett 2011; Braude 2006; Egerton‐Warburton 2014). The pooled outcome versus placebo favoured metoclopramide (OR 0.3, 95% CI 0.17 to 0.53) (Analysis 1.2).
1.2. Analysis.
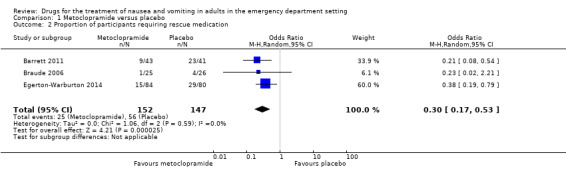
Comparison 1 Metoclopramide versus placebo, Outcome 2 Proportion of participants requiring rescue medication.
Two trials evaluated ondansetron and included 247 participants (Barrett 2011; Egerton‐Warburton 2014). There was no difference in pooled outcome versus placebo for this outcome (OR 0.82, 95% CI 0.49 to 1.37) (Analysis 2.2).
2.2. Analysis.
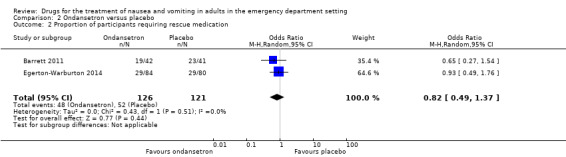
Comparison 2 Ondansetron versus placebo, Outcome 2 Proportion of participants requiring rescue medication.
One trial each evaluated prochlorperazine (50 participants; Braude 2006), promethazine (82 participants; Barrett 2011), and droperidol (48 participants; Braude 2006). There was no difference in outcome between any drug versus placebo (prochlorperazine: OR 1.83, 95% CI 0.45 to 7.51; Table 2; promethazine: OR 0.57, 95% CI 0.24 to 1.34; Table 3 droperidol: OR 0.26, 95% CI 0.03 to 2.54; Table 4).
The only result favouring a drug over placebo was for metoclopramide (Analysis 1.2).
Proportion of participants who required hospital admission
None of the three trials including a placebo arm reported the proportion of participants who required hospital admission (Barrett 2011; Braude 2006; Egerton‐Warburton 2014).
Mean or median emergency department length of stay
None of the three trials including a placebo arm reported the mean or median ED length of stay (Barrett 2011; Braude 2006; Egerton‐Warburton 2014).
Participant satisfaction with intervention
Two trials reported participant satisfaction with intervention (Braude 2006; Egerton‐Warburton 2014). Both trials evaluated metoclopramide, involving 216 participants. From pooled results, there was no difference in participant satisfaction between metoclopramide and placebo (OR 1.07, 95% CI 0.60 to 1.91) (Analysis 1.3).
1.3. Analysis.
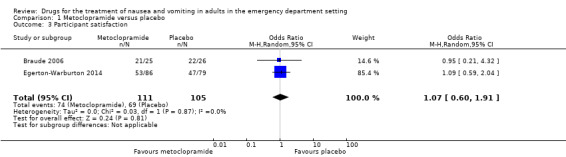
Comparison 1 Metoclopramide versus placebo, Outcome 3 Participant satisfaction.
One trial each evaluated ondansetron (164 participants; Egerton‐Warburton 2014), droperidol (48 participants; Braude 2006) and prochlorperazine (50 participants; Braude 2006). There was no difference in satisfaction for ondansetron, droperidol or prochlorperazine versus placebo (ondansetron: OR 0.80, 95% CI 0.43 to 1.49; Table 5 droperidol: OR 1.82, 95% CI 0.30 to 11.02; Table 4 prochlorperazine: OR 0.91, 95% CI 0.20 to 4.13; Table 2).
4. Ondansetron versus placebo.
| Outcome | Participants | Statistical methods | Effect estimate |
| Participant satisfaction | 164 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.43, 1.49] |
Data from single trials comparing drug versus placebo.
CI: confidence interval.
Comparison of metoclopramide versus active control
Five trials with 528 participants evaluated metoclopramide against an active control (Barrett 2011; Braude 2006; Chae 2011; Cham 2004; Egerton‐Warburton 2014).
Primary outcomes
Severity of nausea
One trial, involving 58 participants, compared metoclopramide 0.4 mg/kg (up to 32 mg) with a standard 10‐mg dose (Cham 2004). The outcome was reported as change in severity on a NRS of nausea 0 to 10. The median reduction in nausea was 5 (95% CI 4 to 6) in the 0.4‐mg/kg group compared with 4 (95% CI 3 to 5) in the 10‐mg group. This difference was not statistically significant (P value = 0.63).
The other four trials, involving 470 participants, included comparisons of metoclopramide with other active control, all reporting change in nausea severity on the VAS (mm) at 30 minutes (Barrett 2011; Braude 2006; Chae 2011; Egerton‐Warburton 2014). From pooled results, the MD in VAS rating at 30 minutes between metoclopramide and any active control was ‐0.00 (95% CI ‐4.50 to 4.49) (Analysis 3.1).
3.1. Analysis.
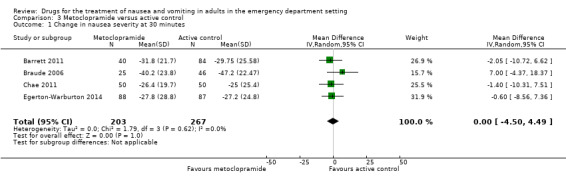
Comparison 3 Metoclopramide versus active control, Outcome 1 Change in nausea severity at 30 minutes.
Three trials, involving 356 participants, compared metoclopramide with a 5‐HT3 antagonist (Barrett 2011; Chae 2011; Egerton‐Warburton 2014). From pooled results, the MD in VAS rating at 30 minutes between metoclopramide and 5‐HT3 antagonist was ‐1.74 (95% CI ‐6.88 to 3.40) (Analysis 4.1). Two of these trials, involving 256 participants, compared metoclopramide with ondansetron (Barrett 2011; Egerton‐Warburton 2014), while the other trial, involving 100 participants, compared metoclopramide with tropisetron (Chae 2011). Separately for this outcome, the MDs were ‐2.00 (95% CI ‐8.30 to 4.29) (Analysis 5.1) for metoclopramide versus ondansetron and ‐1.20 (95% CI ‐10.11 to 7.71) (Table 6) for metoclopramide versus tropisetron. One trial, involving 83 participants, compared metoclopramide with promethazine (Barrett 2011). The change in VAS rating at 30 minutes between metoclopramide and promethazine was 0.10 (95% CI ‐10.06 to 10.26) (Table 7). One trial compared metoclopramide with prochlorperazine (49 participants) and droperidol (47 participants) (Braude 2006). The change in VAS rating at 30 minutes (MD) between metoclopramide and prochlorperazine was 0.30 (95% CI ‐13.12 to 13.72) (Table 8), and between metoclopramide and droperidol was 14.30 (95% CI 2.21 to 26.39) (Table 9).
4.1. Analysis.
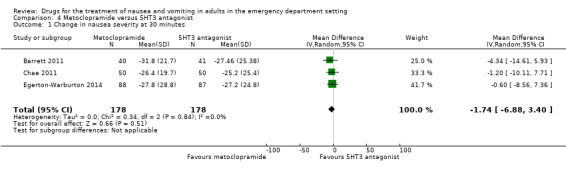
Comparison 4 Metoclopramide versus 5HT3 antagonist, Outcome 1 Change in nausea severity at 30 minutes.
5.1. Analysis.
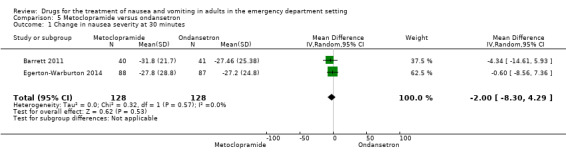
Comparison 5 Metoclopramide versus ondansetron, Outcome 1 Change in nausea severity at 30 minutes.
5. Metoclopramide versus tropisetron.
| Outcome | Participants | Statistical method | Effect estimate |
| Change in nausea severity at 30 minutes | 100 | Mean Difference (IV, Random, 95% CI [mm]) | ‐1.20 [‐10.11, 7.71] |
| Proportion of participants requiring rescue medication | 100 | Odds Ratio (M‐H, Random, 95% CI) | 3.16 [1.03, 9.69] |
Data from single trials comparing metoclopramide versus active control.
CI: confidence interval.
6. Metoclopramide versus promethazine.
| Outcome | Participants | Statistical method | Effect estimate |
| Change in nausea severity at 30 minutes | 83 | Mean Difference (IV, Random, 95% CI [mm]) | 0.10 [‐10.06, 10.26] |
| Proportion of participants requiring rescue medication | 88 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.93] |
Data from single trials comparing metoclopramide versus active control.
CI: confidence interval.
7. Metoclopramide versus prochlorperazine.
| Outcome | Participants | Statistical method | Effect estimate |
| Change in nausea severity at 30 minutes | 49 | Mean Difference (IV, Random, 95% CI [mm]) | 0.30 [‐13.12, 13.72] |
| Proportion of participants requiring rescue medication | 49 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 1.13] |
| Participant satisfaction | 49 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.23, 4.78] |
Data from single trials comparing metoclopramide versus active control.
CI: confidence interval.
8. Metoclopramide versus droperidol.
| Outcome | Participants | Statistical method | Effect estimate |
| Change in nausea severity at 30 minutes | 47 | Mean Difference (IV, Random, 95% CI [mm]) | 14.30 [2.21, 26.39] |
| Proportion of participants requiring rescue medication | 47 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.05, 14.87] |
| Participant satisfaction | 47 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.09, 3.19] |
Data from single trials comparing metoclopramide versus active control.
CI: confidence interval.
The only statistically significant result between metoclopramide and any active control was that favouring droperidol over metoclopramide (Table 9) (Braude 2006).
Number of vomiting episodes
Four of the trials did not report the number of vomiting episodes within the time frame of interest to this review (Barrett 2011; Braude 2006; Chae 2011; Cham 2004). The related findings of Egerton‐Warburton 2014 have been previously described (see 'Comparison of drug versus placebo: Primary outcomes: Number of vomiting episodes').
Adverse reactions
All five trials reported adverse events; however, differences in reporting precluded pooling of results. None of the trials reported any serious adverse events. The most commonly reported adverse events were akathisia and headache.
Cham 2004, evaluating a weight‐based dose of metoclopramide with standard dose, reported similar adverse event rates (weight‐based dose: 2/24 (8%); standard dose: 0/34 (0%); P value = 0.33).
Adverse events in three of the five trials have previously been described (see 'Comparison of drug versus placebo: Primary outcomes: Adverse reactions'), and there were no differences between metoclopramide and active control (Barrett 2011; Braude 2006; Egerton‐Warburton 2014).
Chae 2011 comparing metoclopramide with tropisetron reported higher rates of akathisia (scored from 0 to 17) in the metoclopramide group at both 30 and 60 minutes (at 30 minutes: MD 1.1, 95% CI 0.1 to 22; at 60 minutes: 1.2, 95% CI 1.01 to 2.5). Baseline akathisia scores were also higher in the metoclopramide group (MD 0.3, 95% CI ‐0.22 to 0.8). Headache was reported by 5/50 (10%) participants in the metoclopramide group and 11/50 (22%) participants in the tropisetron group (difference 12%, 95% CI ‐4.2% to 28.2%, P value = 0.17). Dizziness was reported by 3/50 (6%) participants in the metoclopramide group and 5/50 (10%) participants in the tropisetron group (difference 4.0%, 95% CI ‐8.6% to 16.6%, P value = 0.71).
The only significant result was of more frequent akathisia for metoclopramide in comparison with tropisetron (Chae 2011).
Secondary outcomes
Proportion of participants requiring rescue medication
All five trials reported proportion of participants requiring rescue medication (Barrett 2011; Braude 2006; Chae 2011; Cham 2004; Egerton‐Warburton 2014).
Cham 2004, comparing the different doses of metoclopramide, reported no difference in proportions requiring rescue medication (OR 0.83, 95% CI 0.18 to 3.86).
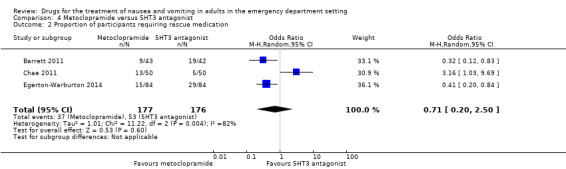
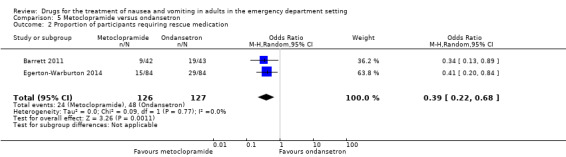
Four trials compared metoclopramide with any other active control on the outcome of rescue medication requirement in 469 participants (Barrett 2011; Braude 2006; Chae 2011; Egerton‐Warburton 2014). The pooled results showed no difference in requirement for rescue medication between metoclopramide and any active control (OR 0.61, 95% CI 0.21 to 1.73) (Analysis 3.2). Three trials, involving 353 participants, compared metoclopramide with 5‐HT3 blockers (Barrett 2011; Chae 2011; Egerton‐Warburton 2014). The pooled results showed no difference in the requirement for rescue medication (OR 0.71, 95% CI 0.20 to 2.50) (Analysis 4.2). However, pooled results from the two trials comparing metoclopramide and ondansetron, involving 253 participants, found that fewer participants receiving metoclopramide required rescue medication (OR 0.39, 95% CI 0.22 to 0.68) (Analysis 5.2) (Barrett 2011; Egerton‐Warburton 2014). One study comparing metoclopramide with tropisetron, involving 100 participants, found that more participants receiving metoclopramide required rescue medication (OR 3.16, 95% CI 1.03 to 9.69) (Table 6) (Chae 2011).
3.2. Analysis.
Comparison 3 Metoclopramide versus active control, Outcome 2 Proportion of participants requiring rescue medication.
4.2. Analysis.
Comparison 4 Metoclopramide versus 5HT3 antagonist, Outcome 2 Proportion of participants requiring rescue medication.
5.2. Analysis.
Comparison 5 Metoclopramide versus ondansetron, Outcome 2 Proportion of participants requiring rescue medication.
One trial compared metoclopramide with promethazine with fewer participants requiring rescue medication for metoclopramide (9/43 (22%) with metoclopramide versus 19/45 (44%) with promethazine; OR 0.36, 95% CI 0.14 to 0.93) (Table 7) (Barrett 2011). One trial compared metoclopramide with prochlorperazine or droperidol (Braude 2006). It found no difference in requirement for rescue medication (1/25 (4%) with metoclopramide versus 6/24 (25%) prochlorperazine; OR 0.13, 95% CI 0.01 to 1.13) (Table 8); 1/25 (4%) with metoclopramide versus 1/22 (4.5%) with droperidol; OR 0.88, 95% CI 0.05 to 14.87) (Table 9).
Proportion of participants who required hospital admission
None of the five trials evaluating metoclopramide reported proportion of participants who required hospital admission (Barrett 2011; Braude 2006; Chae 2011; Cham 2004; Egerton‐Warburton 2014).
Mean or median emergency department length of stay
None of the five trials evaluating metoclopramide reported mean or median ED length of stay (Barrett 2011; Braude 2006; Chae 2011; Cham 2004; Egerton‐Warburton 2014).
Participant satisfaction with intervention
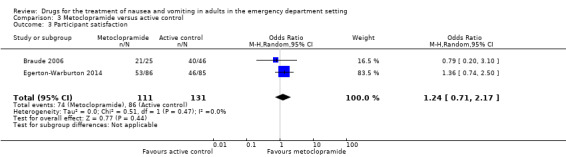
Two trials, involving 242 participants, reported participant satisfaction (Braude 2006; Egerton‐Warburton 2014). From pooled results, there was no difference in participant satisfaction between metoclopramide and active control (OR 1.24, 95% CI 0.71 to 2.17) (Analysis 3.3).
3.3. Analysis.
Comparison 3 Metoclopramide versus active control, Outcome 3 Participant satisfaction.
Braude 2006 reported satisfaction as 21/25 (84%) with metoclopramide and 20/24 (83%) with prochlorperazine (OR 1.05, 95% CI 0.23 to 4.78) (Table 8) and 20/22 (95%) with droperidol (OR 0.53, 95% CI 0.09 to 3.19) (Table 9).
Egerton‐Warburton 2014 reported satisfaction as 53/86 (61%) with metoclopramide and 46/85 (54.1%) with ondansetron (OR 1.36, 95% CI 0.74 to 2.50) (Table 10).
9. Metoclopramide versus ondansetron.
| Outcome | Participants | Statistical method | Effect estimate |
| Participant satisfaction | 171 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [0.74, 2.50] |
Data from single trials comparing metoclopramide versus active control.
CI: confidence interval.
Comparison of 5‐HT3 blockers versus active control
Five studies, involving 583 participants, compared 5‐HT3 blockers against an active control (Barrett 2011; Braude 2008; Chae 2011; Egerton‐Warburton 2014; Patka 2011). Four trials evaluated ondansetron (Barrett 2011; Braude 2008; Egerton‐Warburton 2014; Patka 2011), and one trial evaluated tropisetron (Chae 2011).
Primary outcomes
Severity of nausea
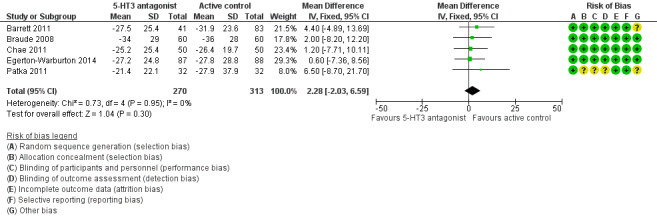
All five trials reported the primary outcome of mean VAS rating change for nausea severity from baseline to 30 minutes (Barrett 2011; Braude 2008; Chae 2011; Egerton‐Warburton 2014; Patka 2011). From pooled results, the difference in mean VAS rating change (MD) at 30 minutes between 5‐HT3 blockers and any active control was 2.88 (95% CI ‐2.03 to 6.59) (Figure 5). The results were not affected by exclusion of the study with high risk of bias (Patka 2011), or by including studies only evaluating ondansetron (Analysis 7.1).
5.
Forest plot of comparison: 3 5HT‐3 Antagonists versus active control, outcome: 6.1 Change in nausea severity at 30 minutes.
7.1. Analysis.
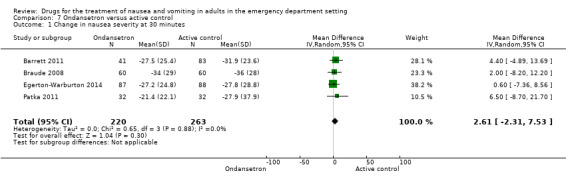
Comparison 7 Ondansetron versus active control, Outcome 1 Change in nausea severity at 30 minutes.
Three trials, involving 356 participants, compared 5‐HT3 blockers with metoclopramide (Barrett 2011; Chae 2011; Egerton‐Warburton 2014). From pooled results, the difference in mean VAS rating change (MD) at 30 minutes between metoclopramide and 5‐HT3 antagonist was ‐1.74 (95% CI ‐6.88 to 3.40) (Analysis 4.1). Separately, two trials, involving 256 participants, compared ondansetron with metoclopramide (Barrett 2011; Egerton‐Warburton 2014), while the other trial, involving 100 participants, compared tropisetron with metoclopramide (Chae 2011). From pooled results, the difference in mean VAS rating change (MD) at 30 minutes between metoclopramide and ondansetron was ‐2.00 (95% CI ‐8.30 to 4.29) (Analysis 5.1), while for tropisetron it was ‐1.20 (95% CI ‐10.11 to 7.71) (Table 6).
Two trials, involving 204 participants, compared ondansetron with promethazine (Barrett 2011; Braude 2008). From pooled results, the difference in mean VAS rating change (MD) at 30 minutes was 3.16 (95% CI ‐4.29 to 10.60) (Analysis 8.1).
8.1. Analysis.
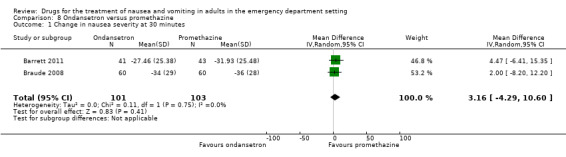
Comparison 8 Ondansetron versus promethazine, Outcome 1 Change in nausea severity at 30 minutes.
One trial, involving 64 participants, compared ondansetron with prochlorperazine (Patka 2011). The difference in mean VAS rating change (MD) at 30 minutes between ondansetron and prochlorperazine was 6.50 (95% CI ‐8.70 to 21.70) (Table 11). We deemed this trial at high risk of bias (Risk of bias in included studies).
10. Ondansetron versus prochlorperazine.
| Outcome | Participants | Statistical methods | Effect estimate |
| Change in nausea severity at 30 minutes | 64 | Mean Difference (IV, Random, 95% CI [mm]) | 6.50 [‐8.70, 21.70] |
| Proportion of participants requiring rescue medication | 64 | Odds Ratio (M‐H, Random, 95% CI) | 5.74 [0.63, 52.23] |
| Proportion of participants who required hospital admission | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.00 [0.97, 25.77] |
Data from single trial comparing ondansetron versus prochlorperazine (Patka 2011).
CI: confidence interval.
Number of vomiting episodes
Three of the five trials evaluating 5‐HT3 antagonists did not report the number of vomiting episodes within the time frame of interest to this review (Barrett 2011; Braude 2008; Chae 2011). The related findings from Egerton‐Warburton 2014 have been previously described (see 'Comparison of drug versus placebo: Primary outcomes: Number of vomiting episodes'). Patka 2011 reported the proportion of participants vomiting from 0 to 30 minutes and 31 to 60 minutes. This was low for both ondansetron and prochlorperazine (0 to 30 minutes: 2/32 (6%) with ondansetron and 0/32 (0%) with prochlorperazine; 31 to 60 minutes: 0/32 (0%) with ondansetron and 1/32 (3%) with prochlorperazine).
Adverse reactions
All five trials reported adverse events (Barrett 2011; Braude 2008; Chae 2011; Egerton‐Warburton 2014; Patka 2011); however, variability in reporting precluded meaningful pooling of results.
Adverse events from the trials of Barrett 2011; Chae 2011; Egerton‐Warburton 2014 were described in preceding sections (see 'Comparison of drug versus placebo: Primary outcomes: Adverse reactions'). Braude 2008 reported mean change in anxiety and sedation on a VAS from baseline to 30 minutes. For anxiety, the mean changes for ondansetron and promethazine were ‐13 (SD 27) with ondansetron and ‐14 (SD 26) with promethazine (MD ‐1, 95% CI ‐10 to 10). For sedation, the mean changes were less for ondansetron compared with promethazine (5 (SD 25) with ondansetron versus 19 (SD 30) with promethazine; MD 14, 95% CI 5 to 24). Patka 2011 reported no difference in akathisia rates between ondansetron and prochlorperazine (1/32 (3%) with ondansetron versus 3/32 (9%) with prochlorperazine). Sedation scores were also reported to be similar between groups (no details given), while headache scores were reported to be "significantly lower" (P value < 0.05) for prochlorperazine at all time points, but no data were provided.
The result favouring ondansetron over active control was a lower rate of sedation (Braude 2008). The result favouring an active control over ondansetron was a lower headache score for prochlorperazine (Patka 2011).
Secondary outcomes
Proportion of participants requiring rescue medication
Five trials, involving 582 participants, reported the proportion of participants requiring rescue medication (Barrett 2011; Braude 2008; Chae 2011; Egerton‐Warburton 2014; Patka 2011). From pooled results, there was no difference in requirement for rescue medication between 5‐HT3 blockers and any active control (OR 1.47, 95% CI 0.72 to 3.01) (Analysis 6.2).
6.2. Analysis.
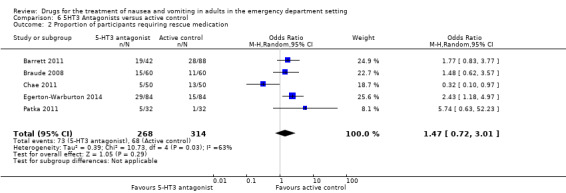
Comparison 6 5HT3 Antagonists versus active control, Outcome 2 Proportion of participants requiring rescue medication.
From the four trials, involving 482 participants, which evaluated ondansetron against any active control, the pooled analysis found a higher requirement for rescue medication for ondansetron than for active control (OR 2.00, 95% CI 1.29 to 3.09) (Analysis 7.2) (Barrett 2011; Braude 2008; Egerton‐Warburton 2014; Patka 2011). This result did not change with exclusion of the study at high risk of bias (Patka 2011).
7.2. Analysis.
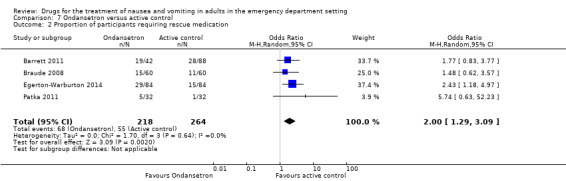
Comparison 7 Ondansetron versus active control, Outcome 2 Proportion of participants requiring rescue medication.
Three of the trials, involving 353 participants, compared 5‐HT3 blockers with metoclopramide (Barrett 2011; Chae 2011; Egerton‐Warburton 2014). From pooled results, there was no difference in requirement for rescue medication between metoclopramide and 5‐HT3 blockers (OR 0.71, 95% CI 0.20 to 2.50) (Analysis 4.2). Two of these trials, involving 254 participants, compared ondansetron with metoclopramide (Barrett 2011; Egerton‐Warburton 2014). Pooled results showed that more participants in the ondansetron group required rescue medication (OR 0.39, 95% CI 0.22 to 0.68) (Analysis 5.2).
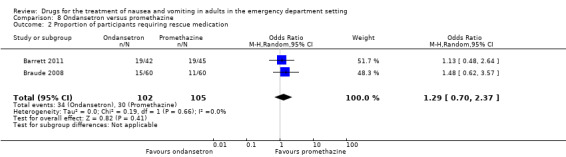
Two trials, involving 207 participants, compared ondansetron with promethazine (Barrett 2011; Braude 2008). Pooled results showed no difference in requirement for rescue medication (OR 1.29, 95% CI 0.70 to 2.37) (Analysis 8.2).
8.2. Analysis.
Comparison 8 Ondansetron versus promethazine, Outcome 2 Proportion of participants requiring rescue medication.
One trial, involving 64 participants, which compared ondansetron with prochlorperazine, reported no difference in requirement for rescue medication (OR 5.74, 95% CI 0.63 to 52.23) (Table 11) (Patka 2011).
Pooled results favoured any active control over ondansetron for requirement for rescue medication (Analysis 7.2). For individual drugs, the only significant result was that favouring metoclopramide over ondansetron (Analysis 5.2).
Proportion of participants who required hospital admission
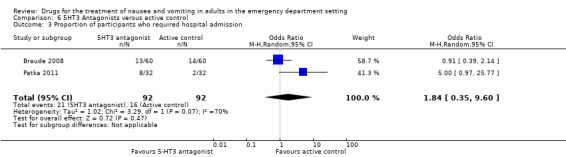
Two trials, involving 184 participants, compared need for admission between 5‐HT3 blockers and active control (Braude 2008; Patka 2011). Pooled results showed no difference between 5‐HT3 blockers and active control (OR 1.84, 95% CI 0.35 to 9.60) (Analysis 6.3). The result did not change with the exclusion of the trial at high risk of bias (Patka 2011). Separately, Braude 2008 reported the admission rates to be 13/60 (22%) with ondansetron versus 14/60 (23%) with promethazine (OR 0.91, 95% CI 0.39 to 2.14) (Table 12), while Patka 2011 reported admission rates to be 8/32 (25%) with ondansetron versus 2/32 (6%) with prochlorperazine (OR 5.00, 95% CI 0.97 to 25.77) (Table 11).
6.3. Analysis.
Comparison 6 5HT3 Antagonists versus active control, Outcome 3 Proportion of participants who required hospital admission.
11. Ondansetron versus promethazine.
| Outcome | Participants | Statistical methods | Effect estimate |
| Proportion of participants who required hospital admission | 120 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.39, 2.14] |
| Participant satisfaction | 92 | Odds Ratio (M‐H, Random, 95% CI) | 2.63 [0.76, 9.11] |
Data from single trials comparing ondansetron versus active control.
CI: confidence interval.
Mean or median emergency department length of stay
None of the trials reported the mean or median ED length of stay (Barrett 2011; Braude 2008; Chae 2011; Egerton‐Warburton 2014; Patka 2011).
Participant satisfaction with intervention
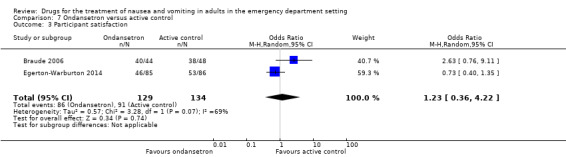
Two trials, involving 263 participants, reported participant satisfaction with intervention (Braude 2008; Egerton‐Warburton 2014). Pooled results showed no difference in satisfaction between ondansetron and any active control (OR 1.23, 95% CI 0.36 to 4.22) (Analysis 7.3). Separately, Braude 2008 reported satisfaction to be 40/44 (91%) with ondansetron versus 38/48 (79%) with promethazine (OR 2.63, 95% CI 0.76 to 9.11) (Table 12), while Egerton‐Warburton 2014 reported satisfaction to be 53/86 (61.6%) with metoclopramide versus 46/85 (54.1%) with ondansetron (OR 1.36, 95% CI 0.74 to 2.50) (Table 10).
7.3. Analysis.
Comparison 7 Ondansetron versus active control, Outcome 3 Participant satisfaction.
Comparison of prochlorperazine versus active control
Three trials, involving 219 participants, evaluated prochlorperazine against an active control (Braude 2006; Ernst 2000; Patka 2011).
Primary outcomes
Severity of nausea
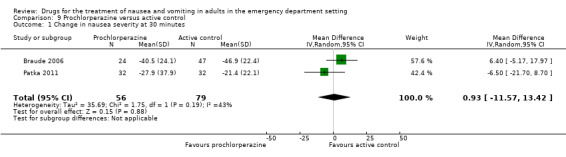
Two trials, involving 135 participants, reported the primary outcome of mean VAS rating change for nausea severity from baseline to 30 minutes (Braude 2006; Patka 2011). From pooled results, the difference in mean VAS rating change (MD) at 30 minutes between prochlorperazine and active control was 0.93 (95% CI ‐11.57 to 13.42) (Analysis 9.1).
9.1. Analysis.
Comparison 9 Prochlorperazine versus active control, Outcome 1 Change in nausea severity at 30 minutes.
One trial, involving 84 participants, reported median VAS rating change at 30 and 60 minutes (Ernst 2000). These were 45 with prochlorperazine and 27 with promethazine at 30 minutes, and 60.5 with prochlorperazine and 47 with promethazine at 60 minutes. No variances were reported, but the difference was reported to be statistically significant in favour of prochlorperazine (P value = 0.004 at 30 minutes, and P value < 0.001 at 60 minutes).
One trial compared prochlorperazine with droperidol (46 participants), and metoclopramide (49 participants) (Braude 2006). Results favoured droperidol over prochlorperazine, with a difference in mean VAS rating change (MD) at 30 minutes of 14.00 (95% CI 1.67 to 26.33) (Table 13), but there was no difference between metoclopramide and prochlorperazine (MD 0.30, 95% CI ‐13.12 to 13.72) (Table 8).
12. Prochlorperazine versus droperidol.
| Outcome | Participants | Statistical methods | Effect estimate |
| Change in nausea severity at 30 minutes | 46 | Mean Difference (IV, Random, 95% CI [mm]) | 14.00 [1.67, 26.33] |
| Proportion of participants requiring rescue medication | 46 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [0.16, 22.66] |
| Participant satisfaction | 46 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.08, 3.05] |
Data from single trials comparing prochlorperazine versus droperidol.
CI: confidence interval.
One trial, involving 64 participants, compared prochlorperazine with ondansetron (Patka 2011). The difference in mean VAS rating change (MD) at 30 minutes was 6.50 (95% CI ‐8.70 to 21.70) (Table 11).
One result favoured prochlorperazine over promethazine (Ernst 2000). One result favoured droperidol over prochlorperazine (Table 13) (Braude 2006).
Number of vomiting episodes
None of the trials evaluating prochlorperazine reported the number of vomiting episodes. The related findings from Patka 2011 have been previously described (see 'Comparison of 5‐HT3 blockers versus active control: Primary outcomes: Number of vomiting episodes').
Adverse reactions
All three trials reported adverse events (Braude 2006; Ernst 2000; Patka 2011); however, variations in reporting precluded pooling of data. There were no serious adverse events in any of the trials.
Adverse events from Braude 2006 and Patka 2011 have been described in previous sections (see 'Comparison of 5‐HT3 blockers versus active control: Primary outcomes: Adverse reactions'). Ernst 2000 reported identical akathisia rates at 6/42 (14%) with prochlorperazine and promethazine, and drowsiness at 38% with prochlorperazine and 71% with promethazine (difference 33%, 95% CI 13% to 53%; P value = 0.02).
The significant result was of a lower rate of drowsiness for prochlorperazine compared with promethazine (Ernst 2000).
Secondary outcomes
Proportion of participants requiring rescue medication
All three trials, involving 219 participants, reported proportion of participants requiring rescue medication (Braude 2006; Ernst 2000; Patka 2011). From pooled results, there was no difference between prochlorperazine and active control (OR 0.77, 95% CI 0.07 to 8.74) (Analysis 9.2). Exclusion of results from the trial at high risk of bias did not change the result (Patka 2011).
9.2. Analysis.
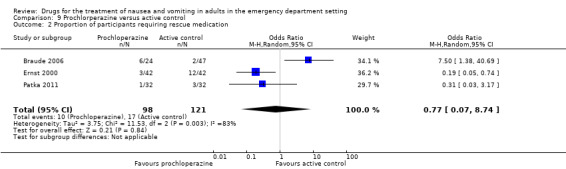
Comparison 9 Prochlorperazine versus active control, Outcome 2 Proportion of participants requiring rescue medication.
Braude 2006 reported requirement for rescue medication in 1/25 (4%) with metoclopramide compared with 6/24 (25%) with prochlorperazine (OR 0.13, 95% CI 0.01, 1.13) (Table 8), and 1/22 (4%) with droperidol (OR 1.91, 95% CI 0.16 to 22.66) (Table 13). Patka 2011 reported requirement for rescue medication in 5/32 (16%) with ondansetron and 1/32 (3%) with prochlorperazine (OR 5.74, 95% CI 0.63 to 52.23) (Table 11). Ernst 2000 reported requirement for rescue medication in 3/42 (7%) with prochlorperazine and 12/42 (29%) with promethazine (OR 0.19, 95% CI 0.05 to 0.74) (Table 14).
13. Prochlorperazine versus promethazine.
| Outcome | Participants | Statistical method | Effect estimate |
| Proportion of participants requiring rescue medication | 84 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.05, 0.74] |
| Proportion of participants who required hospital admission | 84 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.22] |
Data from single trials comparing prochlorperazine versus promethazine.
CI: confidence interval.
The only significant result was that fewer participants required rescue medication with prochlorperazine compared with promethazine (Table 14) (Ernst 2000).
Proportion of participants who required hospital admission
Two trials, involving 148 participants, reported proportion of participants who required hospital admission (Ernst 2000; Patka 2011). From pooled results, the difference favoured prochlorperazine versus active control (OR 0.22, 95% CI 0.05 to 0.95) (Analysis 9.3). Exclusion of the trial at high risk of bias did change the result (Patka 2011), since Ernst 2000 reported the difference in proportions requiring admission as OR 0.33 (95% CI 0.01 to 8.22) (Table 14).
9.3. Analysis.
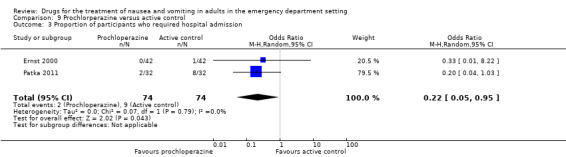
Comparison 9 Prochlorperazine versus active control, Outcome 3 Proportion of participants who required hospital admission.
Mean or median emergency department length of stay
None of the trials evaluating prochlorperazine reported mean or median ED length of stay (Braude 2006; Ernst 2000; Patka 2011).
Participant satisfaction with intervention
Only one trial reported participant satisfaction (Braude 2006). There was no difference between the groups (20/24 (83%) with prochlorperazine versus 41/47 (87%) with active control; OR 0.73, 95% CI 0.19 to 2.89) (Table 15), or separately between prochlorperazine and droperidol (20/24 (83%) with prochlorperazine versus 20/22 (95%) with droperidol; OR 0.50, 95% CI 0.08 to 3.05) (Table 13), or prochlorperazine and metoclopramide (20/24 (83%) with prochlorperazine versus 21/25 (84%) with metoclopramide; OR 1.05, 95% CI 0.23 to 4.78) (Table 8).
14. Prochlorperazine versus active control.
| Outcome | Participants | Statistical method | Effect estimate |
| Participant satisfaction | 71 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.19, 2.89] |
Data from single trials comparing prochlorperazine versus active control.
CI: confidence interval.
Comparison of promethazine versus active control
Three trials, involving 328 participants, evaluated promethazine versus active control (Barrett 2011; Braude 2008; Ernst 2000).
Primary outcomes
Severity of nausea
Two trials, involving 244 participants, reported the primary outcome of mean VAS rating change for nausea severity from baseline to 30 minutes (Barrett 2011; Braude 2008). From pooled results, the difference in mean VAS rating change (MD) at 30 minutes between promethazine and active control was ‐2.17 (95% CI ‐8.99 to 4.66) (Analysis 10.1).
10.1. Analysis.
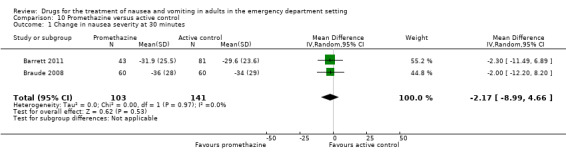
Comparison 10 Promethazine versus active control, Outcome 1 Change in nausea severity at 30 minutes.
One trial, involving 84 participants, reported median VAS rating change at 30 and 60 minutes (Ernst 2000). These were 45 mm with prochlorperazine and 27 mm with promethazine at 30 minutes, and 60.5 mm with prochlorperazine and 47 mm with promethazine at 60 minutes. No variances were reported, but the difference was reported as being statistically significant in favour of prochlorperazine (P value = 0.004 at 30 minutes, and P value < 0.001 at 60 minutes).
Two trials, involving 204 participants, compared promethazine with ondansetron (Barrett 2011; Braude 2008). From pooled results, difference in mean VAS rating change (MD) to 30 minutes between ondansetron and promethazine was 3.16 (95% CI ‐4.29 to 10.60) (Analysis 8.1).
One trial, involving 83 participants, compared promethazine with metoclopramide (Barrett 2011). The difference in mean VAS rating change (MD) at 30 minutes was 0.10 (95% CI ‐10.06 to 10.26) (Table 7).
The only significant result was that favouring prochlorperazine over promethazine (Ernst 2000).
Number of vomiting episodes
None of the trials reported number of vomiting episodes.
Adverse reactions
All three trials reported adverse events, but variable reporting precluded pooling of results (Barrett 2011; Braude 2008; Ernst 2000). These have been described in detail in previous sections (see 'Comparison of drug versus placebo: Primary outcomes: Adverse reactions' and 'Comparison of prochlorperazine versus active control: Primary outcomes: Adverse reactions'). In brief, Ernst 2000 reported more drowsiness for promethazine versus prochlorperazine (71% with promethazine versus 38% with prochlorperazine; difference 33%, 95% CI 13% to 53%; P value = 0.02), while rates of akathisia were similar at 14% in both groups. Braude 2008 reported more sedation for promethazine versus ondansetron (difference in mean VAS rating at 30 minutes 14, 95% CI 5 to 24). Barrett 2011 reported no difference in sedation at 30 minutes between promethazine and any active control (OR 1.58, 95% CI 0.74 to 3.34).
Secondary outcomes
Proportion of participants requiring rescue medication
Three trials, involving 334 participants, reported proportion of participants requiring rescue medication (Barrett 2011; Braude 2008; Ernst 2000). From pooled results, there was no difference in need for rescue medication between promethazine and active control (OR 1.55, 95% CI 0.58 to 4.14) (Analysis 10.2).
10.2. Analysis.
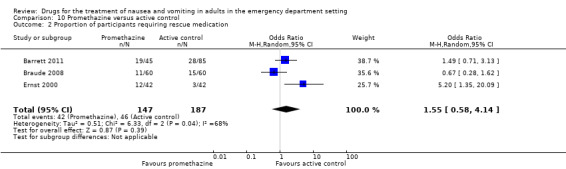
Comparison 10 Promethazine versus active control, Outcome 2 Proportion of participants requiring rescue medication.
Two trials, involving 207 participants, found no difference between ondansetron and promethazine (OR 1.29, 95% CI 0.70 to 2.37) (Analysis 8.2) (Barrett 2011; Braude 2008).
One trial, involving 88 participants, reported less need for rescue medication with metoclopramide versus promethazine (19/43 (22%) with metoclopramide versus 9/45 (44%) with promethazine (OR 0.36, 95% CI 0.14 to 0.93) (Table 7) (Barrett 2011).
One trial, involving 84 participants, reported less need for rescue medication with prochlorperazine versus promethazine (3/42 (7%) with prochlorperazine versus 12/42 (29%) with promethazine; OR 0.19, 95% CI 0.05 to 0.74) (Table 14) (Ernst 2000).
There was a greater requirement for rescue medication for promethazine in comparison with both metoclopramide (Table 7) (Barrett 2011) and prochlorperazine (Table 14) (Ernst 2000).
Proportion of participants who required hospital admission
Two trials, involving 204 participants, reported proportion of participants who required hospital admission (Braude 2008; Ernst 2000). From pooled results, there was no difference in admission requirement between promethazine and active control (OR 1.18, 95% CI 0.51 to 2.70) (Analysis 10.3). One trial, involving 120 participants, reported no difference in admission requirement between ondansetron and promethazine (13/60 (22%) with ondansetron versus 14/60 (23%) with promethazine; OR 0.91, 95% CI 0.39 to 2.14) (Table 12) (Braude 2008). One trial, involving 84 participants, reported no difference in admission requirement between prochlorperazine and promethazine (0/42 (0%) with prochlorperazine versus 1/42 (2.4%) with promethazine; OR 0.33, 95% CI 0.01 to 8.22) (Table 14) (Ernst 2000).
10.3. Analysis.
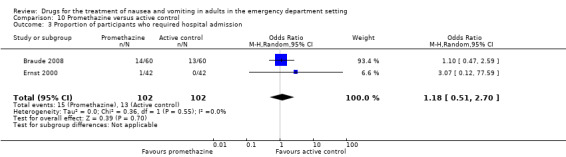
Comparison 10 Promethazine versus active control, Outcome 3 Proportion of participants who required hospital admission.
Mean or median emergency department length of stay
None of the three trials reported mean or median ED length of stay.
Participant satisfaction with intervention
One trial, involving 92 participants, reported participant satisfaction with intervention (Braude 2008). There was no difference between ondansetron and promethazine (40/44 (91%) with ondansetron versus 38/48 (79%) with promethazine; OR 2.63, 95% CI 0.76 to 9.11) (Table 12).
Comparison of droperidol versus active control
One trial, involving 71 participants, evaluated droperidol against active control (Braude 2006).
Primary outcomes
Severity of nausea
Braude 2006 reported the primary outcome of mean VAS rating change for nausea severity from baseline to 30 minutes. From pooled results, the difference in mean VAS rating change (MD) at 30 minutes between droperidol and active control was ‐14.10 (95% CI ‐24.26 to ‐3.94) (Table 16). Separately, the differences in mean VAS rating changes at 30 minutes also favoured droperidol in comparison with metoclopramide (MD 14.30, 95% CI 2.21 to 26.39) (Table 9), and with prochlorperazine (MD 14.00, 95% CI 1.67 to 26.33) (Table 13).
15. Droperidol versus active control.
| Outcome | Participants | Statistical method | Effect estimate |
| Change in nausea severity at 30 minutes | 71 | Mean Difference (IV, Random, 95% CI [mm]) | ‐14.10 [‐24.26, ‐3.94] |
| Proportion of participants requiring rescue medication | 71 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.48] |
| Participant satisfaction | 69 | Odds Ratio (M‐H, Random, 95% CI) | 3.41 [0.39, 29.68] |
Data from single trial by Braude 2006.
CI: confidence interval.
Number of vomiting episodes
The trial did not evaluated number of vomiting episodes.
Adverse reactions
Adverse events for this trial have been previously described (see 'Comparison of drug versus placebo: Primary outcomes: Adverse reactions') (Braude 2006). There were no serious adverse events reported. In brief, the mean changes for anxiety ratings were droperidol ‐25.9 (SD 30.2), metoclopramide ‐25.4 (SD 24.3) and prochlorperazine ‐21.9 (SD 38.8); and for sedation were droperidol 13.5 (SD 32.2), metoclopramide 0.4 (SD 30.1) and prochlorperazine 5.1 (SD 26.5).
Secondary outcomes
Proportion of participants requiring rescue medication
Braude 2006 reported requirement for rescue medication, which was similar for droperidol and active control (1/22 (4.5%) with droperidol versus 7/49 (14%) with active control; OR 0.29, 95% CI 0.03 to 2.48) (Table 16). Separately, 1/22 (4.5%) with droperidol was compared with the 1/25 (4%) with metoclopramide (OR 0.88, 95% CI 0.05 to 14.87) (Table 9) and 6/24 (25%) with prochlorperazine (OR 1.91, 95% CI 0.16 to 22.66) (Table 13).
Proportion of participants who required hospital admission
The trial did not report proportion of participants who required hospital admission.
Mean or median emergency department length of stay
The trial did not report mean or median ED length of stay.
Participant satisfaction with intervention
The trial reported participant satisfaction with intervention (Braude 2006). From pooled results, this was similar between droperidol and active control (20/21 (95%) with droperidol versus 41/48 (85%) with active control; OR 3.41, 95% CI 0.39 to 29.68) (Table 16). Separately, the 20/21 (95%) with droperidol was compared with 21/25 (84%) with metoclopramide (OR 0.53, 95% CI 0.09 to 3.19) (Table 9) and 20/24 (83%) with prochlorperazine (OR 0.50, 95% CI 0.08 to 3.05) (Table 13).
Discussion
Summary of main results
Nausea and vomiting are frequently present in people in the ED with many different conditions. Early antiemetic drug use is common, regardless of the underlying cause, due to the distressing nature of the symptoms, and the potential for secondary complications. However, despite the frequency of the clinical problem, the limited number of studies eligible for inclusion in this systematic review was surprising. This limited the potential for pooling of results and consideration of potential confounding factors, such as primary diagnostic groups or amount of intravenous fluid administered was not possible.
Accepting these limitations, this Cochrane review found no convincing evidence that any one drug had a more clinically significant effect than any other drug, or that any one of a number of drugs was superior to placebo. The three trials with a placebo arm, both individually and with pooling of results where possible, found that there was no significant difference in the VAS reductions between placebo and metoclopramide, ondansetron, prochlorperazine or promethazine (Barrett 2011; Braude 2006; Egerton‐Warburton 2014). For individual drugs versus any other drug (active control), where results could be pooled from three trials, together with the other four studies that compared different antiemetic drugs (Braude 2008; Chae 2011; Ernst 2000; Patka 2011), differences in VAS reductions between groups were not significant.
Only two trials made conclusions of superiority for a particular drug (Braude 2006; Ernst 2000). Braude 2006 reported that the VAS reduction for droperidol was significantly greater than that for each of metoclopramide, prochlorperazine and placebo, and Ernst 2000 concluded that the VAS reduction for prochlorperazine was significantly greater than that for promethazine, but since the reductions in all groups exceeded the MCSD, the clinical significance of this superiority is uncertain. Similarly, two of the trials that included a placebo arm reported a statistically non‐significant trend towards superiority for ondansetron, metoclopramide and promethazine in comparison with placebo (Barrett 2011; Egerton‐Warburton 2014), but in both these trials the lower limit of the 95% CI of the VAS reduction for placebo still exceeded the MCSD, so again, the clinical significance of these statistical trends is also doubtful. Although it may seem intuitive that statistically greater reductions would equate with greater clinical benefits, there is no literature to date that supports this notion.
Reduction in number of post‐treatment vomiting episodes is used in other settings (Carlisle 2006), as a primary outcome measure, and so we included it in this review. It proved not be a useful measure, as the majority of participants included in the ED‐based trials had nausea only, and in the three trials that reported number of vomits (Chae 2011; Egerton‐Warburton 2014; Patka 2011), frequency was so low that demonstration of a significant reduction within a 30‐minute period was impossible.
The final primary outcome measure of adverse events showed variable results, but overall these were fairly mild and did not require specific therapies. There were no serious adverse events in any of the included trials. Promethazine was associated with more sedation or drowsiness in two trials (Braude 2008; Ernst 2000), ondansetron with headaches (Patka 2011), and metoclopramide and prochlorperazine with some akathisia (Chae 2011; Egerton‐Warburton 2014; Patka 2011), although these effects were relatively unusual and mild. One large systematic review on drugs for preventing postoperative nausea and vomiting reported on adverse effects from 380 trials finding droperidol increased the risk of drowsiness, while decreasing the risk of headache, and ondansetron increased the risk of headache but found no evidence for a difference in risk in other adverse effects (Carlisle 2006).
Of the secondary outcome measures under consideration, only dispensing of additional rescue medication was included in all trials. The small number of trials, along with variable and inconsistent results, meant that this was of limited utility. This may stem from a lack of definition as to what constituted a need for rescue medication, as in all studies this was at the discretion of the treating ED doctor. Of note, treatment with promethazine was associated with higher requirement for rescue medication, and interestingly participants treated with ondansetron were more likely to require rescue medication compared to either active control or metoclopramide. Few trials reported participant satisfaction or hospital admission rates, with hospital admission rates not being included in any of the trials with placebo control. Participant satisfaction was similar with all drugs included in the review. None of the trials reported ED lengths of stay. It is noteworthy that we did not demonstrate the superiority of 5HT3 antagonists compared to other classes for any of the outcomes assessed, and perhaps contrary to the common anecdotal perception of effectiveness.
Overall completeness and applicability of evidence
Overall, there was a paucity of clinical trials assessing the effectiveness of antiemetic medications for nausea and vomiting in the ED setting. In total, fewer than 1000 participants have been evaluated in this setting. This is somewhat surprising, given the frequency of the symptom in EDs, and although treatment of the condition with antiemetics in clinical practice is very common, there is little consensus on the most appropriate treatment. Interpretation of the available evidence is hampered by clinical heterogeneity, specifically the variety of different drugs evaluated in studies to date, difference in baseline severity and inclusion criteria, and the wide variety of underlying illnesses leading to the symptom of nausea in the ED setting. Most of the trials included in this Cochrane review did have fairly broad inclusion criteria, so while general conclusions could be drawn, the applicability of the findings to all people with nausea in the ED, or to particular subsets of them, remains uncertain. Given the relative paucity of trials in the ED setting, in certain circumstances it may be appropriate to extrapolate evidence from systematic reviews in other settings (e.g. nausea and vomiting in early pregnancy) (Mathews 2010). The drugs evaluated in the eight included trials mirrored common practice, with metoclopramide being included in five trials, 5‐HT3 antagonists in four, promethazine in three, prochlorperazine in two and droperidol in one, but there are other agents and the use of drugs in combination was not studied.
The use of change in the VAS, on which the conclusions of this review are primarily based, could also be debated. The VAS, for measurement and monitoring of change in nausea severity, has been validated. High correlation between adjectival descriptors of severity and VAS measurement ranges has been demonstrated, and the MCSD has been defined as the mean VAS change when people report symptom severity as being "a little less". Research on the MCSD is somewhat limited, however, but it appears that the MCSD is greater for people whose baseline nausea is severe, than for people with moderate or mild severity. Hence, reported figures have ranged between 12 and 30 mm, seemingly dependent on the severity mix of the particular population. In this review, we noted that while differences in VAS reductions between groups were similar, the VAS reductions reported for the same drugs in different studies varied quite widely. It was generally the case that when baseline VAS ratings were higher, the reported post‐treatment reductions, including for placebo where included, were greater. This finding seems consistent with reports of variability in the MCSD for different severity subgroups, and highlights the difficulty of pre‐defining a single MCSD for multiple populations. We did nominate a mid‐range MCSD of 15 mm for use in this review, which is obviously problematic, but since VAS reductions for all treatments in all studies comfortably exceeded this figure, it seems reasonable to conclude that the reported levels of participant improvement were clinically significant.
Quality of the evidence
The methodology of the trials included in the review appeared to be adequate overall, and are reported further in the Assessment of risk of bias in included studies section. We judged two trials to have a high risk of bias because they were inadequately blinded or did not report adequately on certain domains that we were unable to clarify with authors (Cham 2004; Patka 2011). We judged the remaining included trials to be low risk of bias overall, although some minor methodological issues remained.
Potential biases in the review process
Potential biases were minimized by performing a comprehensive search for potentially eligible studies. We were unable to obtain trial reports, or sufficient data on four unpublished studies identified through searching clinical trial registries, which may introduce some possibility of bias (Friedland 2008; Haensel 2007; Thacker 2003; Thacker 2004).
Clinical heterogeneity between trials made pooling of data for meta‐analysis difficult for some outcomes. The clinical heterogeneity consisted of included trials evaluating different agents, different doses, and using different active control groups and only three trials including a placebo control arm (Barrett 2011; Braude 2006; Egerton‐Warburton 2014). Results and analysis of random‐effects model analysis were presented for outcomes comparing both individual drugs and combined with active controls. Comprehensive data comparing various doses of drugs included in the review were lacking, and we thought this unlikely to affect the results substantially. We believe the comparisons presented here to be valid and informative.
Two authors of this review were also authors of one of the included studies (Egerton‐Warburton 2014). We minimized bias as data extraction and assessment of quality was conducted by an author not involved with the study (JF). There were no disagreements with this trial in assessment of quality or data extraction, and as such did not require arbitration with an independent person.
Agreements and disagreements with other studies or reviews
We are not aware of any previous systematic reviews on the treatment of nausea and vomiting in the ED setting.
Authors' conclusions
Implications for practice.
The findings of this review suggest that in an emergency department (ED) population, nausea severity tends to decrease by a similar and apparently clinically significant amount over a period of 30 minutes, regardless of whether an antiemetic drug or saline placebo is given. Presumably this initial improvement is due to whatever specific therapies are provided for the person's underlying condition, probably including the provision of intravenous fluids.
This review found no definite evidence to support the superiority of any one drug for the treatment of ongoing nausea, so choice of drug should be dictated by other considerations such as a person's preference, adverse‐effect profile and cost.
Implications for research.
Evidence supports that any future ED‐based antiemetic studies should include a placebo control arm. Despite some likely variability in the minimum clinically significant difference (MCSD) for different populations, change in severity on the visual analogue scale (VAS) appears to be the most useful outcome measure in this setting, but the clinical importance of reductions greater than the MCSD warrants exploration. The change in number of vomiting episodes does not appear useful, and researchers should look to define need for rescue medication more tightly and what is contributing to a person's decision on satisfaction.
Research to date has almost exclusively compared the effect on self reported nausea severity of a single dose of one drug over a time period of 30 minutes, with the clinical significance of the severity reduction at this time point also being based on somewhat limited literature. Further investigation of the MCSD for both global ED populations and in different initial severity subgroups would be useful. The effect of initial concurrent administration of different drugs on early reduction of symptoms, as often occurs in the oncology setting, could also be explored and compared to placebo. The longer‐term effect, still within the ED, of repeat doses of either the same or different drugs for persistent nausea might also be useful.
Further research would also be useful in focusing on specific individual diagnostic groups within the ED population (e.g. presumed, uncomplicated gastroenteritis), which may demonstrate more consistent results from antiemetic drug administration, than the more heterogeneous undifferentiated ED population with nausea and vomiting. A further consideration in future trials would be to control the amount of intravenous fluid administered accurately, or to evaluate the antiemetic effects of intravenous fluid alone.
Other participant‐related outcomes, still confined to the ED episode of care, such as change in severity by time of disposition, ED length of stay and need for hospital admission should be considered in future studies.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Karen Hovhannisyan and Bronia Renison for assistance with database search strategies, John Carlisle and Ronan O'Sullivan (content editors), Nathan Pace (statistical editor), Scott Strassels, Gilwan Kim, Julieta Scalo, David Sohl, Leo Eberhart, Bita Mesgarpour (peer reviewers), Anne Lyddiatt and Ann E Fonfa (consumer editor/referee) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. MEDLINE Ovid search strategy
1. antiemetics/ or antiemesis.ti,ab. or antiemetic*.ti,ab. or antiemetogenic.ti,ab. or dopamine antagonists/ or (dopamine$ adj2 antagonists).ti,ab. or (chlorpromazine or droperidol or domperidone or metoclopramide or haloperidol or prochlorperazine or promethazine or alizapride).ti,ab. or serotonin antagonists/ or (serotonin adj2 antagonist$).ti,ab. or (dolasetron or granisetron or ondansetron or tropisetron or palonosetron).ti,ab. or cholinergic antagonists/ or (anticholinergic agents or scopolamine or hyoscine).ti,ab. or histamine H1 antagonists/ or antihistamines.ti,ab. or buclizine.ti,ab. or cyclizine.ti,ab. or dimenhydrinate.ti,ab. or trimethobenzamide.ti,ab. or meclizine.ti,ab. or pheniramine.ti,ab. or piphenhydramine.ti,ab. or benzodiazepines/ or lorazepam.ti,ab. or diazepam.ti,ab. or adrenal cortex hormones/ or corticosteroids.ti,ab. or dexamethasone.ti,ab. or methylprednisolone.ti,ab. or betamethasone.ti,ab. or cannabinoids/ or cannabinoid$.ti,ab. or marijuana.ti,ab. or marinol.ti,ab. or dronabinol.ti,ab. 2. nausea/ or vomiting/ or nausea*.ti,ab. or vomit*.ti,ab. or emesis.ti,ab. or emet*.ti,ab. or emergency service, hospital/ or emergency medical services/ or (emergency adj3 (medic* or servic* or ward*)).ti,ab. or (intensive adj3 care).ti,ab. 3. 1 and 2 4. (randomized controlled trial.pt. or randomized.ab. or random*.ti.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 2. Search strategy for EMBASE (OvidSP)
1. exp antiemetic agent/ or exp dopamine receptor blocking agent/ or exp serotonin antagonist/ or exp cholinergic receptor blocking agent/ or exp histamine H1 receptor antagonist/ or exp benzodiazepine derivative/ or exp corticosteroid/ or exp cannabinoid/ or anti?eme*.mp. or ((serotonin or dopamine* or cholinergic) adj3 antagonist*).ti,ab. or (chlorpromazine or droperidol or domperidone or metoclopramide or haloperidol or prochlorperazine or promethazine or alizapride or dolasetron or granisetron or ondansetron or tropisetron or palonosetron or anticholinergic agent* or scopolamine or hyoscine or antihistamin* or buclizine or cyclizine or dimenhydrinate or trimethobenzamide or meclizine or pheniramine or piphenhydramine or lorazepam or diazepam or corticosteroid* or dexamethasone or methylprednisolone or betamethasone or cannabinoid* or marijuana or marinol or dronabinol).mp. 2. exp nausea/ or exp vomiting/ or exp "nausea and vomiting"/ or (nausea* or vomit* or emesis or emet*).mp. 3. exp emergency health service/ or exp emergency medicine/ or exp emergency care/ or exp emergency ward/ or exp evidence based emergency medicine/ or exp intensive care/ or (emergency adj3 (medic* or servic* or ward*)).mp. or (intensive adj3 care).mp. 4. 1 and 2 and 3 5. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 6. 4 and 5
Appendix 3. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Antiemetics explode all trees #2 MeSH descriptor Dopamine Antagonists explode all trees #3 MeSH descriptor Serotonin Antagonists explode all trees #4 MeSH descriptor Cholinergic Antagonists explode all trees #5 MeSH descriptor Histamine H1 Antagonists explode all trees #6 MeSH descriptor Adrenal Cortex Hormones explode all trees #7 MeSH descriptor Benzodiazepines explode all trees #8 MeSH descriptor Cannabinoids explode all trees #9 anti?eme* or chlorpromazine or droperidol or domperidone or metoclopramide or haloperidol or prochlorperazine or promethazine or alizapride or dolasetron or granisetron or ondansetron or tropisetron or palonosetron or anticholinergic agent* or scopolamine or hyoscine or antihistamin* or buclizine or cyclizine or dimenhydrinate or trimethobenzamide or meclizine or pheniramine or piphenhydramine or lorazepam or diazepam or corticosteroid* or dexamethasone or methylprednisolone or betamethasone or cannabinoid* or marijuana or marinol or dronabinol #10 (serotonin or dopamine* or cholinergic) near antagonist* #11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 MeSH descriptor Nausea explode all trees #13 MeSH descriptor Vomiting explode all trees #14 nausea* or vomit* or emesis or emet* #15 (#12 OR #13 OR #14) #16 MeSH descriptor Emergency Medical Services explode all trees #17 MeSH descriptor Intensive Care explode all trees #18 emergency near (medic* or servic* or ward*) #19 intensive near care #20 (#16 OR #17 OR #18 OR #19) #21 (#11 AND #15 AND #20)
Appendix 4. ISI Web of Science search strategy
#1 TI=antieme* or TS=(dopamine antagonist* or chlorpromazine or droperidol or domperidone or metoclopramide or haloperidol or prochlorperazine or promethazine or alizapride or serotonin antagonist* or dolasetron or granisetron or ondansetron or tropisetron or palonosetron or cholinergic antagonist*or anticholinergic agent* or scopolamine or hyoscine or histamine H1 antagonist* or antihistamin*or buclizine or cyclizine or dimenhydrinate or trimethobenzamide or meclizine or pheniramine or piphenhydramine or benzodiazepine* or lorazepam or diazepam or adrenal cortex hormone* or corticosteroid* or dexamethasone or methylprednisolone or betamethasone or cannabinoid* or marijuana or marinol or dronabinol) #2 TI=(nausea or vomiting or emesis or emet*) or TS=(emergency SAME (medic* or servic* or ward*)) or TS=(intensive SAME care) #3 TS=(random* or ((control*or clinical) SAME trial)) not TS=(animal* not (human* and animals*)) #4 #3 AND #2 AND #1
Appendix 5. Data Collection Form
Review author initials (completing form):
Study ID (surname of author and year):
Report ID (created by review author):
Citation and contact details:
First Author:
Journal/Conference proceeding etc:
Date:
Contact details:
NOTES:
Eligibility Verification
1. Identified as Randomized Controlled trial: [ ] Yes [ ] No [ ] Unclear
2. Emergency Department setting: [ ] Yes [ ] No [ ] Unclear
3. Adults (> 80% Participants ≥16 years): [ ] Yes [ ] No [ ] Unclear
4. Undifferentiated Nausea and Vomiting: [ ] Yes [ ] No [ ] Unclear
5. Pharmacological agent and appropriate comparator: [ ] Yes [ ] No [ ] Unclear
(Appropriate comparators are placebo, no treatment or active control)
6. Relevant outcomes: [ ] Yes [ ] No [ ] Unclear
(Nausea any scale and/or vomiting episodes)
Note: If answered no to any of the above questions the study should not be included in the review. If study is to be included in “excluded studies” section of the review, record information to be inserted into “Table of excluded studies”
EXCLUDED STUDY DETAILS:
Participant characteristics
| Details | |
| Age (mean, median, range, etc.) | |
| Sex of participants (numbers/%) | |
| Other |
Trial Characteristics
| Further details | |
| Single centre / Multicentre | |
| Country / Countries | |
| Setting (e.g. Emergency Department) | |
| How was participant eligibility defined? | |
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Comparator | |
| Dose / frequency of administration | |
| Duration of treatment | |
| Median (range) length of follow‐up reported in this paper | |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in RevMan | |
| Trial design | |
| Outcomes measured (units) | |
| Other |
Risk of Bias Assessment
Selection Bias
| Domain | Random sequence generation |
| Review authors' judgement: | [ ] Low risk [ ] High risk [ ] Unclear risk |
| Support for judgement: | |
| Domain | Allocation concealment |
| Review authors' judgement: | [ ] Low risk [ ] High risk [ ] Unclear risk |
| Support for judgement: |
|
Performance Bias
| Domain | Blinding of participants and personnel |
| Review authors' judgement: | [ ] Low risk [ ] High risk [ ] Unclear risk |
| Support for judgement: |
|
Detection Bias
| Domain | Blinding of outcome assessment |
| Review authors' judgement: | [ ] Low risk [ ] High risk [ ] Unclear risk |
| Support for judgement: |
|
Attrition Bias
| Domain | Incomplete outcome data |
| Review authors' judgement: | [ ] Low risk [ ] High risk [ ] Unclear risk |
| Support for judgement: |
|
Reporting Bias
| Domain | Selective reporting |
| Review authors' judgement: | [ ] Low risk [ ] High risk [ ] Unclear risk |
| Support for judgement: |
|
Other Bias
| Domain | Other sources of Bias |
| Review authors' judgement: | [ ] Low risk [ ] High risk [ ] Unclear risk |
| Support for judgement: |
|
Results
|
Outcomes relevant to review | |
| Reported in paper (circle) | |
| Primary outcome ‐ Nausea severity | Yes / No |
| Primary outcome ‐ Complete resolution of nausea | Yes/No |
| Primary outcome ‐ Number of vomits | Yes / No |
| Primary outcome ‐ Adverse events | Yes / No |
| Secondary outcomes | |
| Outcome 1 ‐ Requiring rescue medication | Yes / No |
| Outcome 2 – Proportion requiring admission | Yes / No |
| Outcome 3 – Emergency length of stay | Yes / No |
| Outcome 4 ‐ Patient satisfaction | Yes / No |
| For Continuous data | |||||||
| Code of paper |
Outcomes |
Unit of measurement |
Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| Primary Outcome – Nausea severity | |||||||
| Primary outcome – Number of vomits | |||||||
| Secondary outcome 3 – Emergency department LOS | |||||||
| For Dichotomous data | |||
| Code of paper | Outcomes | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| Primary outcome – Adverse events reported | |||
| Primary outcome ‐ Complete resolution of nausea | |||
| Secondary outcome 1 – requiring rescue medication | |||
| Secondary outcome 2 – requiring admission | |||
| Secondary outcome 4 – Patient satisfaction | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
| |
| Freehand space for writing actions such as contact with study authors and changes |
Additional information.
| Funding Source | |
| Key conclusions of study authors | |
| Clarifications with study authors |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal / Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| | ||
Data and analyses
Comparison 1. Metoclopramide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 3 | 301 | Mean Difference (IV, Random, 95% CI) | ‐5.27 [‐11.33, 0.80] |
| 2 Proportion of participants requiring rescue medication | 3 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.53] |
| 3 Participant satisfaction | 2 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.60, 1.91] |
1.1. Analysis.
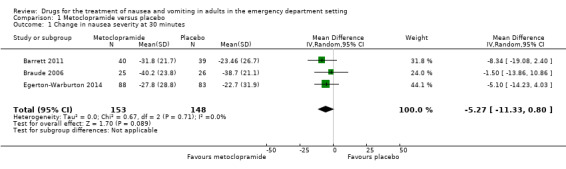
Comparison 1 Metoclopramide versus placebo, Outcome 1 Change in nausea severity at 30 minutes.
Comparison 2. Ondansetron versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 2 | 250 | Mean Difference (IV, Random, 95% CI) | ‐4.32 [‐11.20, 2.56] |
| 2 Proportion of participants requiring rescue medication | 2 | 247 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.49, 1.37] |
Comparison 3. Metoclopramide versus active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 4 | 470 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐4.50, 4.49] |
| 2 Proportion of participants requiring rescue medication | 4 | 469 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.73] |
| 3 Participant satisfaction | 2 | 242 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.71, 2.17] |
Comparison 4. Metoclopramide versus 5HT3 antagonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 3 | 356 | Mean Difference (IV, Random, 95% CI) | ‐1.74 [‐6.88, 3.40] |
| 2 Proportion of participants requiring rescue medication | 3 | 353 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.20, 2.50] |
Comparison 5. Metoclopramide versus ondansetron.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 2 | 256 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐8.30, 4.29] |
| 2 Proportion of participants requiring rescue medication | 2 | 253 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.22, 0.68] |
Comparison 6. 5HT3 Antagonists versus active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 5 | 583 | Mean Difference (IV, Fixed, 95% CI) | 2.28 [‐2.03, 6.59] |
| 2 Proportion of participants requiring rescue medication | 5 | 582 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.72, 3.01] |
| 3 Proportion of participants who required hospital admission | 2 | 184 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.35, 9.60] |
6.1. Analysis.
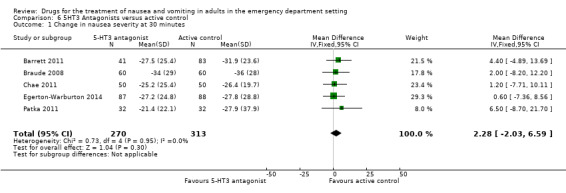
Comparison 6 5HT3 Antagonists versus active control, Outcome 1 Change in nausea severity at 30 minutes.
Comparison 7. Ondansetron versus active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 4 | 483 | Mean Difference (IV, Random, 95% CI) | 2.61 [‐2.31, 7.53] |
| 2 Proportion of participants requiring rescue medication | 4 | 482 | Odds Ratio (M‐H, Random, 95% CI) | 2.00 [1.29, 3.09] |
| 3 Participant satisfaction | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.36, 4.22] |
Comparison 8. Ondansetron versus promethazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 2 | 204 | Mean Difference (IV, Random, 95% CI) | 3.16 [‐4.29, 10.60] |
| 2 Proportion of participants requiring rescue medication | 2 | 207 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.70, 2.37] |
Comparison 9. Prochlorperazine versus active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 2 | 135 | Mean Difference (IV, Random, 95% CI) | 0.93 [‐11.57, 13.42] |
| 2 Proportion of participants requiring rescue medication | 3 | 219 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.07, 8.74] |
| 3 Proportion of participants who required hospital admission | 2 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.95] |
Comparison 10. Promethazine versus active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nausea severity at 30 minutes | 2 | 244 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐8.99, 4.66] |
| 2 Proportion of participants requiring rescue medication | 3 | 334 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.58, 4.14] |
| 3 Proportion of participants who required hospital admission | 2 | 204 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.51, 2.70] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrett 2011.
| Methods | Study design: randomized, double‐blind, placebo‐controlled trial Study duration: not stated Follow‐up: 30 min |
|
| Participants | Country: USA Setting: single urban, university affiliated ED Annual census: 54,000 Inclusion criteria: aged ≥ 18 years, nausea and vomiting of any cause Exclusion criteria: received antiemetic drug in prior 24 hours, nausea VAS severity < 40 mm, hypotension, allergy Number: total allocated 171; treatment group 1: 42; treatment group 2: 43; treatment group 3: 45; control group: 41 Number: total analysed (primary outcome) 163; treatment group 1: 41; treatment group 2: 40; treatment group 3: 43; treatment group 4: 39 Median age (IQR) (years): treatment group 1: 34 (27‐47); treatment group 2: 37 (24‐52); treatment group 3: 28 (23‐46); control group: 32 (22‐44) Sex (M/W): treatment group 1: 15/27; treatment group 2: 13/30; treatment group 3: 14/31; control group: 14/27 |
|
| Interventions | Treatment group 1: ondansetron 4 mg IV Treatment group 2: metoclopramide 10 mg IV Treatment group 3: promethazine 12.5 mg IV Control group: placebo All groups received IV fluid over the 30‐min study period (median overall 500 mL) |
|
| Outcomes | Primary outcome: change in nausea severity score at 30 min Secondary outcomes: proportion of participants requiring rescue medication, adverse reactions (including akathisia, headache, pain at injection site and sedation) |
|
| Notes | All treatment groups received a median of 500 mL of isotonic IV fluid, control group received a median of 450 mL Additional data provided by author ‐ means and SD for treatment groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Well described, randomization statistical software in blocks of 24 |
| Allocation concealment (selection bias) | Low risk | Well described, prepared by study pharmacist and syringes sent by pneumatic tube |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All drugs presented as 2 mL of clear fluid in syringe labelled "antiemetic study medication". Poor agreement in kappa with doctors guessing study drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding seemed adequate. Primary outcome self reported VAS by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis "modified intention to treat" as 3 people did not get their study drug, and a further person did not get 30 min nausea score. Minimal likely effect on results |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | An unplanned interim analysis and post hoc power calculation and an amended sample size may have introduced some bias |
Braude 2006.
| Methods | Study design: randomized, double‐blind, controlled trial Study duration: December 1998 to December 1999 Follow‐up: 30 min |
|
| Participants | Country: USA Setting: single urban teaching hospital ED Annual census: 55,000 Inclusion criteria: aged 18‐65 years, primary or secondary complaint of nausea or vomiting, or both Exclusion criteria: received antiemetic drug in prior 24 hours, nausea VAS severity < 40 mm, hypotension, known CCF or pregnancy, given > 1000‐mL IV fluid prior to enrolment Number: total 97; treatment 1 (22); treatment group 2 (25); treatment group 3 (24); control group (26) Mean age ± SD (years): treatment group 1: 36.6 ± 12.6; treatment group 2: 38.9 ± 11.5; treatment group 3: 36.3 ± 11.0; control group: 38.2 ± 12.5 Sex (M/W): treatment group 1: 7/15; treatment group 2: 8/17; treatment group 3: 17/7; control group: 10/16 |
|
| Interventions | Treatment group 1: droperidol 1.25 mg IV Treatment group 2: metoclopramide 10 mg IV Treatment group 3: prochlorperazine 10 mg IV Control group: placebo All interventions were administered as a single push All groups received IV fluid over the 30‐min study period |
|
| Outcomes | Primary outcome: change in nausea severity score at 30 min Secondary outcomes: proportion of participants requiring rescue medication, participant satisfaction, adverse reactions (akathisia and sedation) |
|
| Notes | All groups received IV fluid with a mean (± SD) 739 ± 445 mL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Low risk | Drug supplied by pharmacy, allocation known only to them |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs appeared identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Appeared to be adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/100 participants failed to provide 30‐min rating. Unlikely to influence results |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
| Other bias | Low risk | No other issues identified |
Braude 2008.
| Methods | Study design: randomized, double‐blind, non‐inferiority trial Study duration: August 2003 to November 2005 Follow‐up: 30 min |
|
| Participants | Country: USA Setting: single urban university teaching hospital ED Annual census: 75,000 Inclusion criteria: aged ≥ 18 years, chief or secondary complaint of nausea or vomiting Exclusion criteria: aged < 18 or > 65 years, unable to provide informed consent, received antiemetic drug in prior 24 hours, nausea VAS severity < 40 mm, known or suspected pregnancy, given > 1000 mL IV fluid prior to enrolment Number: total 120; treatment 1: 60; treatment group 2: 60 Mean age ± SD (years): treatment group 1: 36 ± 11.2; treatment group 2: 39 ± 14.2 Sex (M/W): treatment group 1: 24/36; treatment group 2: 14/46 |
|
| Interventions | Treatment group 1: ondansetron 4 mg IV Treatment group 2: promethazine 25 mg IV Both interventions diluted to 10 mL, and administered as a single push over 2 min Both groups received similar amounts of IV fluid |
|
| Outcomes | Primary outcome: change in nausea severity score at 30 min Secondary outcomes: proportion of participants requiring rescue medication, adverse reactions (change in self reported anxiety and sedation) |
|
| Notes | At 30 min, both groups received a similar amount of IV fluid mean (± SD): promethazine 497 ± 360 mL and ondansetron 460 ± 356 mL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized in blocks of 10" but method of sequence generation not described in published report, clarified with authors as computer generated |
| Allocation concealment (selection bias) | Low risk | Identical appearing vials, prepared by pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "study drugs appeared identical" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clearly stated that allocations were not revealed until after all analyses were complete |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcome. Some attrition for 24‐hour follow‐up (equal in groups) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | Convenience sample, and slow recruitment. Effect of potential bias low. Funded by GlaxoSmithKline (GSK), makers of ondansetron. Stated that GSK not involved in study design, data collection, analysis, writing the manuscript or approval of final manuscript |
Chae 2011.
| Methods | Study design: randomized, double‐blind superiority trial Study duration: October 2009 to March 2010 Follow‐up: 180 min |
|
| Participants | Country: Australia Setting: single centre, urban teaching hospital ED Annual census: 70,000 Inclusion criteria: aged ≥ 18 years, with nausea or vomiting and ED doctor recommended antiemetic Exclusion criteria: received antiemetic drug in prior 6 hours, unable to provide informed consent, allergy, symptoms associated with migraine Number: total 100; treatment group 1: 50; treatment group 2: 50 Mean age ± SD (years): treatment group 1: 53 ± 21.0; treatment group 2: 56.7 ± 19.2 Sex (M/W): treatment group 1: 21/29; treatment group 2: 21/29 |
|
| Interventions | Treatment group 1: tropisetron 5 mg IV Treatment group 2: metoclopramide 10 mg IV Both interventions administered as a single bolus |
|
| Outcomes | Primary outcome: number of vomiting episodes (vomits per person‐hours) Secondary outcomes: change in nausea severity score at 30 min, proportion of participants requiring rescue medication, adverse reactions (akathisia score ‐ modified Prince Henry's scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated by an independent pharmacist |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "double‐blind". Drugs were prepared and administered from usual ward stock by "independent" nurses, with the participant and doctor blinded. Although there is the potential for this process to be compromised, we thought this was unlikely to have occurred, and hence judged the study domain to be low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above, while there was potential for the blinding to be compromised, we determined it was unlikely and hence judged the domain to be low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data of relevance to this review well reported. Some minor inaccuracies in reporting exact numbers at each time point, but unlikely to lead to systematic bias |
| Selective reporting (reporting bias) | Low risk | Change in nausea VAS listed as primary outcome on clinical trial registry, secondary outcome in report. Time point 240 min (not relevant to this review) listed on Clinical Trial Registry but not reported in published paper (these are unlikely to substantially affect the review) |
| Other bias | Low risk | Convenience sample, not likely to have impact on results |
Cham 2004.
| Methods | Study design: prospective, single‐blind, randomized trial Study duration: October 2001 to July 2003 Follow‐up: 30 min |
|
| Participants | Country: Australia Setting: 2 urban teaching hospital EDs Annual census: 75,000 (combined) Inclusion criteria: aged ≥ 18 year, who required treatment for nausea or vomiting, or both Exclusion criteria: known allergy; previous dystonic reaction; suspected gastrointestinal obstruction; gastrointestinal haemorrhage; having received any antiemetic, narcotic or phenothiazine in the last 24 hours; treatment with chemotherapy; pregnancy and a history of epilepsy Number: total 58; treatment 1 (24); treatment group 2 (34) Median age, range (years): treatment group 1 (42, 21‐83); treatment group 2 (34, 18‐76) Sex (M/W): treatment group 1 (8/16); treatment group 2 (9/25) |
|
| Interventions | Treatment group 1: metoclopramide 0.4 mg/kg to a maximum of 32 mg Treatment group 2: metoclopramide 10 mg IV Both interventions administered as a single bolus |
|
| Outcomes | Primary outcome: change in nausea severity score at 30 min Secondary outcomes: proportion of participants requiring rescue medication, adverse reactions |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by random number allocation (note groups were unevenly balanced) |
| Allocation concealment (selection bias) | Low risk | Dose regimen contained in numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported as single blind, but no attempt made to blind clinical staff. Not elaborated further |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were self reported; however, staff aware of treatment allocation may have led to bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | Other reporting issues included: no planned sample size presented, a very low recruitment rate, unknown if this sample is representative and the groups were unbalanced. Unlikely to have led to systematic bias |
Egerton‐Warburton 2014.
| Methods | Study design: double‐blind, randomized controlled trial Study duration: September 2009 to April 2010 Follow‐up: 30 min |
|
| Participants | Country: Australia Setting: 2 EDs, 1 urban district and 1 tertiary referral Annual census: 57,000 (urban district) and 59,000 (tertiary referral) Inclusion criteria: aged ≥ 18 years, and nausea or vomiting (or both) during their ED episode of care for which the attending doctor recommended IV Exclusion criteria: haemodynamic instability or primary diagnosis requiring time critical intervention; pregnancy or lactation, Parkinson's disease or restless leg syndrome; use of any antiemetic drug in the previous 8 hours or prior IV fluid in ED, ED nausea or vomiting that was motion related or associated with vertigo; currently undergoing chemotherapy or radiotherapy; inability to understand study explanation of outcome measures; known allergy or previous adverse reaction to study drugs Number: total 258; treatment group 1 (87); treatment group 2 (88); control group (83) Median age, IQR (years): treatment group 1 (42, 27‐61); treatment group 2 (42, 27‐67); control group (42, 28‐62) Sex (M/W): treatment group 1 (31/56); treatment group 2 (30/58); control group (28/55) |
|
| Interventions | Treatment group 1: ondansetron 4 mg IV Treatment group 2: metoclopramide 10 mg IV Control group: placebo All interventions were administered as a single push over 2 min All groups received IV fluid over the 30‐min study period |
|
| Outcomes | Primary outcome: change in nausea severity score at 30 min Secondary outcomes: proportion of participants requiring rescue medication, adverse reactions, participant satisfaction |
|
| Notes | Median (IQR) IV fluid received: group 1 180 (125‐250); group 2 200 (125‐300); control group 200 (125‐250) Additional data provided by author ‐ means and SD for treatment groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence in blocks of 6 by independent trial pharmacist |
| Allocation concealment (selection bias) | Low risk | Study drug prepared and packed in sequentially numbered packs by independent pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All 3 study drugs prepared to look identical as 2 x 2 mL syringes of clear fluid, labelled only as study medications |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Code not broken until after all data entry and analysis complete |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data for 12/270 participants. Small likelihood of bias |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
| Other bias | Low risk | No other biases |
Ernst 2000.
| Methods | Study design: randomized, double‐blind parallel trial Study duration: not stated Follow‐up: 60 min |
|
| Participants | Country: USA Setting: 2 university Hospital EDs Annual census: not stated Inclusion criteria: uncomplicated gastritis or gastroenteritis, aged ≥ 18 years, reported inability to drink fluids without recurrence of nausea or vomiting and required IV hydration and administration of antiemetic Exclusion criteria: another possible source of the nausea, vomiting and diarrhoea; significant abdominal pain in association with other causes; any underlying serious illness such as diabetes or renal failure, or altered sensorium; people who had received prior antiemetics; inability to understand English; drug or alcohol use; pregnancy; refusal to participate and inability to perform VAS ratings Number: total 84; treatment group 1 (42); treatment group 2 (42) Mean age ± SD (years): treatment group 1 (29 ± 11); treatment group 2 (30 ± 14) Sex (M/W): treatment group 1 (14/28); treatment group 2 (11/31) |
|
| Interventions | Treatment group 1: prochlorperazine 10 mg IV Treatment group 2: promethazine 25 mg IV All interventions were administered as a stat dose Both groups received IV fluid administration |
|
| Outcomes | Primary outcome: change in nausea severity score at 30 and 60 min Secondary outcomes: proportion of participants requiring rescue medication, proportion of participants who required hospital admission, adverse effects |
|
| Notes | Mean ± SD of IV fluid: group 1 1300 ± 700 mL; group 2 1100 ± 600 mL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | 1 investigator mixed solutions according to the randomization table, but did not participate in obtaining VAS data or administration. Together with "convenience sampling" the process has the potential to compromise allocation concealment; however, we thought that this was unlikely to have occurred to the extent to systematically bias results, and judged the domain to be low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Efforts made to blind participants and investigators. 2 medications were prepared to appear identical as 10 mL of clear fluid in a syringe |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efforts made to blind participants and investigators. Another investigator, blinded to the allocation and not involved in the data collection, performed the data entry and analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported attrition |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other source of bias |
Patka 2011.
| Methods | Study design: prospective, randomized, active controlled, double‐blinded study Study duration: not stated Follow‐up: 120 min |
|
| Participants | Country: not stated Setting: not stated Annual census: not stated Inclusion criteria: if admitted to ED with nausea or vomiting, or both Exclusion criteria: previous treatment in the ED with antiemetics; missed last menstrual period or pregnancy; aged < 18 years; conditions with impaired gastrointestinal tract function (i.e. irritable bowel syndrome); impaired mental status; treatment with antineoplastic agents within 7 days prior to randomization; people unable to read English language; people leaving the ED against medical advice Number: total 64; treatment group 1 (32); treatment group 2 (32) Mean age (years) (SD not reported): treatment group 1 (41); treatment group 2 (40) Sex (M/W): treatment group 1 (15/17); treatment group 2 (14/18) |
|
| Interventions | Treatment group 1: prochlorperazine 10 mg IV Treatment group 2: ondansetron 4 mg IV Prochlorperazine administered as single pushed dose over 2 min and ondansetron administered pushed over 2‐5 min |
|
| Outcomes | Primary outcome: number of vomiting episodes. Secondary outcomes: change in nausea severity score at 30 (60 and 120 min), proportion of participants requiring rescue medication, adverse effects (sedation, headache, akathisia) |
|
| Notes | Additional data provided by author ‐ means and SD for treatment groups, and clarifications about methodology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment using a 1 : 1 random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Mechanism for allocation concealment not elucidated in report |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Mechanism for blinding not reported. Inconsistency with "blinding" in reporting, e.g. reported as double blind, however reported interventions differed in their administration times 2 vs. 2‐5 min |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes were self reported; however' mechanism for blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data for some outcomes, but unlikely to have substantial effect on results |
| Selective reporting (reporting bias) | Low risk | Primary outcome of review reported satisfactorily. Secondary outcomes incompletely reported, e.g. VAS scores for headache and sedation reported divided into quartiles only. Unlikely to substantially influence results |
| Other bias | Unclear risk | Generally poor reporting, no reasons given for non‐recruitment substantial numbers screened |
CCF: congestive cardiac failure; ED: emergency department; IQR: interquartile range; IV: intravenous; M: men; min: minute; SD: standard deviation; VAS: visual analogue scale; W: women.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agorastos 1981 | Setting not clearly identified as ED |
| Cohen 1999 | Not identified as ED study, "outpatient setting". Exclusion criteria included requirement for intravenous treatment |
| Ordog 1984 | Not a randomized trial, no comparator group |
| Roy 1991 | Setting not ED (general practice), outcomes measured beyond the time frame considered for this review |
| Sussman 1999 | Setting not clearly identified as ED (quote: "many of the centres conducting the study in an ED"), outcomes reported only at 24 hours, therefore not relevant to this review |
ED: emergency department.
Characteristics of studies awaiting assessment [ordered by study ID]
Friedland 2008.
| Methods | Study design: prospective, randomized, double‐blind, controlled clinical trial Study duration: December 2004 to April 2006 Follow‐up: 60 min |
| Participants | Country: not stated Setting: single urban ED Annual census: 80,000 Inclusion criteria: aged ≥ 18 years, acute nausea or vomiting Exclusion criteria: hepatic disease, head trauma, small bowel obstruction, fever > 100.2 °F (37.2 °C), severe abdominal pain, gastrointestinal bleed, hernia Number: treatment group 1: 32; treatment group 2: 35 Median age, IQR (years): combined both groups 33 (IQR not reported) Sex (M/W): combined both groups 72% women |
| Interventions | Treatment group 1: granisetron 0.1 mg IV Treatment group 2: prochlorperazine 10 mg IV |
| Outcomes | Main outcomes: change in nausea VAS at 30 and 60 min, rescue medication use |
| Notes | Data reported in abstract only Results: change in nausea VAS at 30 min was group 1 ‐34.6 mm (95% CI ‐43.2 to ‐26.1), group 2 ‐35.5 mm (95% CI ‐44.3 to ‐26.7), at 60 min was ‐47.6 mm (95% CI ‐57.7 to ‐37.4) and ‐45.6 mm (95% CI ‐54.5 to ‐36.8). "No difference in rescue medication use" |
Haensel 2007.
| Methods | Study design: prospective, randomized, double‐blind study Study duration: 12 months in 2005‐2006 Follow‐up: 30 min |
| Participants | Country: not stated Setting: adult ED Annual census: not stated Inclusion criteria: nausea and at least 1 episode of vomiting within 12 hours of presentation Exclusion criteria: not stated Number: total 132 participants (not reported separately) Mean age, SD (years): not reported Sex (M/W): not reported |
| Interventions | Treatment group 1: ondansetron 4 mg Treatment group 2: ondansetron 2 mg Treatment group 3: metoclopramide 10 mg At least 500 mL of IV saline |
| Outcomes | Primary outcome: nausea VAS at 30 min Secondary outcomes: complete relief of nausea, subjective change in nausea, adverse effects |
| Notes | Reported in abstract only Results: "all 3 groups had significant and similar reduction in VAS" from 66.4 mm to 33.6 mm (not reported separately. No reported adverse events |
Thacker 2003.
| Methods | Study design: prospective, randomized study Study duration: not stated Follow‐up: 60 min |
| Participants | Country: not stated Setting: not stated Annual census: not stated Inclusion criteria: adults undergoing treatment for nausea Exclusion criteria: pregnancy Number: treatment group 1: 16; treatment group 2: 12 Mean age (years): total 33.8 (95% CI 29.3 to 38.4), not reported separately Sex (M/W): 63% women, not reported separately |
| Interventions | Treatment group 1: droperidol 1.25 mg IV Treatment group 2: metoclopramide 10 mg IV |
| Outcomes | Outcomes: nausea VAS, somnolence VAS, adverse effects, QTc |
| Notes | Abstract only available Results: mean change in nausea VAS: group 1 (‐46.9 mm, 95% CI ‐55.3 to ‐38.5); group 2 (‐45.2 mm, 95% CI ‐62.6 to ‐27.9). Akathisia reported in 2 (12.5%) participants in group 1 and 1 (9.1%) participants in group 2 |
Thacker 2004.
| Methods | Study design: prospective, randomized, study Study duration: not stated Follow‐up: 60 min |
| Participants | Country: not stated Setting: not stated Annual census: 100,000 Inclusion criteria: adults presenting with nausea and vomiting who were to receive an IV antiemetic Exclusion criteria: pregnancy, mental disabilities, and QTc > 440 m seconds Number: treatment group 1: 6; treatment group 2: 15; treatment group 3: 5 Mean age, SD (years): not reported Sex (M/W): not reported |
| Interventions | Treatment group 1: droperidol 0.625 mg IV Treatment group 2: droperidol 1.25 mg IV Treatment group 3: droperidol 2.5 mg IV |
| Outcomes | Outcomes: nausea and somnolence VAS at 0 and 60 min, and any extrapyramidal reactions and dysrhythmias |
| Notes | Abstract only available Results: mean change in nausea VAS, for group 1: ‐44.2 mm, 95% CI 9.9 to 78.4; group 2: ‐30.4 mm, 95% CI 19.0 to 41.7; group 3: ‐45.0 mm, 95% CI 20.2 to 69.8. Mean change in somnolence VAS for group 1: 0.0 mm, 95% CI ‐26.5 to 26.5; group 2: 4.8 mm, 95% CI ‐12.6 to 22.5; group 3: 20.0 mm, 95% CI ‐56.5 to 60.5. No significant difference in QTc or adverse events |
CI: confidence interval; ED: emergency department; IQR: interquartile range; IV: intravenous; M: men; min: minute; SD: standard deviation; VAS: visual analogue scale; W: women.
Differences between protocol and review
The search strategy of the review differed from the published protocol with the omission of the LILACS database (Furyk 2012).
The search identified trials with multiple intervention groups, therefore, we combined groups to create single pair‐wise comparisons as outlined in Section 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For dichotomous outcomes, we summed both the sample sizes and the numbers of people with events across groups, and for continuous outcomes, we combined means and standard deviations using methods described in Section 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The protocol stipulated use of risk ratios to measure treatment effect of dichotomous outcomes, whereas in the review we have reported results as odds ratios.
Missing data for the primary outcome of change in nausea severity was not an issue, therefore, there was no requirement to use imputation methods for 'worst‐case', 'best‐case' and 'average‐case' scenarios or to perform sensitivity analyses.
In addition to assessing for statistical heterogeneity as described in the protocol (Furyk 2012), we assessed for clinical heterogeneity, with consideration to the characteristics of included studies regarding participants, interventions and outcome measures.
Planned subgroup analysis on nausea and vomiting associated with pregnancy, opiate administration and chemotherapy was not possible due to no data.
For our 'Summary of findings' table, we presented the data for the comparison of metoclopramide versus placebo, as it was the most commonly evaluated drug. We could not include outcomes of time to treatment success, intravenous fluid volume and admission rate as stated in the protocol due to a lack of data (Furyk 2012). We also included outcomes of the requirement for rescue medication and participant satisfaction in the 'Summary of findings' table.
Contributions of authors
Jeremy S Furyk (JF), Robert A Meek (RM), Diana Egerton‐Warburton (DEW).
Conceiving the review: JF.
Co‐ordinating the review: JF.
Undertaking manual searches: JF, RM.
Screening search results: JF, RM.
Organizing retrieval of papers: JF.
Screening retrieved papers against inclusion criteria: JF, RM.
Appraising quality of papers: JF, RM.
Abstracting data from papers: JF, RM.
Writing to authors of papers for additional information: JF.
Providing additional data about papers: JF.
Obtaining and screening data on unpublished studies: JF.
Data management for the review: JF.
Entering data into Review Manager (RevMan 2014): JF.
RevMan statistical data: JF.
Other statistical analysis not using RevMan: JF.
Interpretation of data: JF, RM, DEW.
Statistical inferences: JF, RM, DEW.
Writing the review: JF, RM, DEW.
Securing funding for the review: JF.
Performing previous work that was the foundation of the present study: JF, RM, DEW.
Guarantor for the review (one author): JF.
Person responsible for reading and checking review before submission: JF.
Sources of support
Internal sources
The Townsville Hospital, Emergency Department and James Cook University, School of Medicine and Dentistry & School of Public Health and Rehabilitation Sciences, Australia.
External sources
Queensland Emergency Medicine Research Foundation (QEMRF), Australia.
Declarations of interest
Jeremy S Furyk: none known.
Robert Meek and Diana Egerton‐Warburton are authors of one of the trials included in this review (Egerton‐Warburton 2014).
JF and RM independently appraised the study for inclusion, risk of bias and data extraction.
There were no disagreements in this process, or need for independent arbiter.
Edited (no change to conclusions)
References
References to studies included in this review
Barrett 2011 {published and unpublished data}
- Barrett T, Dipersio D, Jenkins C, Jack M, McCoin N, Storrow A. A randomized, placebo‐controlled trial of ondansetron, metoclopramide, and promethazine in adults. American Journal of Emergency Medicine 2011;29(3):247‐55. [PUBMED: 20825792] [DOI] [PubMed] [Google Scholar]
Braude 2006 {published data only}
- Braude D, Soliz T, Crandall C, Hendey G, Andrews J, Weichenthal L. Antiemetics in the ED: a randomized controlled trial comparing 3 common agents. American Journal of Emergency Medicine 2006;24(2):177‐82. [PUBMED: 16490647] [DOI] [PubMed] [Google Scholar]
Braude 2008 {published data only}
- Braude D, Crandall C. Ondansetron versus promethazine to treat acute undifferentiated nausea in the emergency department: a randomized, double‐blind, noninferiority trial. Academic Emergency Medicine 2008;15(3):209‐15. [PUBMED: 18304050] [DOI] [PubMed] [Google Scholar]
Chae 2011 {published data only}
- Chae J, Taylor DM, Frauman AG. Tropisetron versus metoclopramide for the treatment of nausea and vomiting in the emergency department: a randomized, double‐blind, clinical trial. Emergency Medicine Australasia 2011;23(5):554‐61. [PUBMED: 21995469] [DOI] [PubMed] [Google Scholar]
Cham 2004 {published data only}
- Cham S, Basire M, Kelly A‐M. Intermediate dose metoclopramide is not more effective than standard dose metoclopramide for patients who present to the emergency department with nausea and vomiting: a pilot study. Emergency Medicine Australasia 2004;16(3):208‐11. [PUBMED: 15228463] [DOI] [PubMed] [Google Scholar]
Egerton‐Warburton 2014 {published and unpublished data}
- Egerton‐Warburton D, Meek R, Mee MJ, Braitberg G. Antiemetic use for nausea and vomiting in adult emergency department patients: randomized controlled trial comparing ondansetron, metoclopramide and placebo. Annals of Emergency Medicine 2014;64(5):526‐32. [DOI: 10.1016/j.annemergmed.2014.03.017; PUBMED: 24818542] [DOI] [PubMed] [Google Scholar]
Ernst 2000 {published data only}
- Ernst A, Weiss S, Park S, Takakuwa K, Diercks D. Prochlorperazine versus promethazine for uncomplicated nausea and vomiting in the emergency department: a randomized, double‐blind clinical trial. Annals of Emergency Medicine 2000;36(2):89‐94. [PUBMED: 10918098] [DOI] [PubMed] [Google Scholar]
Patka 2011 {published and unpublished data}
- Patka J, Wu DT, Abraham P, Sobel R. Randomized controlled trial of ondansetron vs prochlorperazine in adults in the emergency department. Western Journal of Emergency Medicine 2011;12(1):1‐5. [PUBMED: 21691464] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Agorastos 1981 {published data only}
- Agorastos I, Zissis N, Kaprinis I, Goulis G. Double‐blind evaluation of domperidone in acute vomiting and dyspeptic disorders. Journal of International Medical Research 1981;9(2):143‐7. [PUBMED: 7014284] [DOI] [PubMed] [Google Scholar]
Cohen 1999 {published data only}
- Cohen N, Alon I, Almoznino‐Sarfian D, Gorelik O, Chachasvili S, Litvinjuk V, et al. Sulpiride versus metoclopramide in nononcologic patients with vomiting or nausea. Journal of Clinical Gastroenterology 1999;21(1):59‐62. [PUBMED: 10405234] [DOI] [PubMed] [Google Scholar]
Ordog 1984 {published data only}
- Ordog G, Vann P, Owashi N, Wasserberger J, Herman LS, Balasubramaniam S. Intravenous prochlorperazine for the rapid control of vomiting in the emergency department. Annals of Emergency Medicine 1984;13:253‐8. [PUBMED: 6703431] [DOI] [PubMed] [Google Scholar]
Roy 1991 {published data only}
- Roy P, Patel N, Miller A. A comparison of controlled release metoclopramide and domperidone in the treatment of nausea and vomiting. British Journal of Clinical Practice 1991;45(4):247‐51. [PUBMED: 1810356] [PubMed] [Google Scholar]
Sussman 1999 {published data only}
- Sussman G, Shurman J, Creed M, Larsen L, Ferrer‐Brechner T, Noll D, et al. Intravenous ondansetron for the control of opioid‐induced nausea and vomiting. Clinical Therapeutics 1999;21(7):1216‐27. [PUBMED: 10463519] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Friedland 2008 {published data only (unpublished sought but not used)}
- Friedland H, David S, Renae B, Amit S, Latisse M, Monica S, et al. A randomized trial comparing the efficacy of low dose iv granisetron (0.1mg) versus iv prochlorperazine (10mg) in the treatment of acute nausea and vomiting. Proceedings of the Annual Meeting of the Society for Academic Emergency Medicine (SAEM); 2008 May 29‐June 1; Washington. Washington: Academic Emergency Medicine, 2008.
Haensel 2007 {published data only (unpublished sought but not used)}
- Haensel C, Smith D, Pollack M, Owens L. Ondansetron versus metoclopramide for nausea and vomiting in the emergency department. Proceedings of the Annual Meeting of the Society for Academic Emergency Medicine; 2007 May 16‐19; Chicago. Chicago: Academic Emergency Medicine, 2007.
Thacker 2003 {published data only (unpublished sought but not used)}
- Thacker J, Miner J, Biros M. Droperidol versus metoclopramide for the treatment of nausea. Proceedings of the American College of Emergency Physicians; 2003 Oct 12‐13; Boston. Boston: American College of Emergency Physicians, 2003.
Thacker 2004 {published data only (unpublished sought but not used)}
- Thacker J, Miner J. Droperidol dosing for nausea and vomiting. Proceedings of the American College of Emergency Physicians; 2004 Oct 16‐18; San Francisco. San Francisco: American College of Emergency Physicians, 2004.
Additional references
AGA 2001
- AGA. American Gastroenterological Association medical position statement: nausea and vomiting. Gastroenterology 2001;120(1):261‐2. [DOI: ] [DOI] [PubMed] [Google Scholar]
Billio 2010
- Billio A, Morello E, Clarke MJ. Serotonin receptor antagonists for highly emetogenic chemotherapy in adults. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006272.pub2] [DOI] [PubMed] [Google Scholar]
Bork 2011
- Bork B, Ditkoff J, Hang BS. Nausea and vomiting. In: Tintinalli JE editor(s). Tintinalli's Emergency Medicine: a Comprehensive Study Guide. 7th Edition. New York: McGraw Hill Medical, 2011. [ISBN: 0071484809] [Google Scholar]
Carlisle 2006
- Carlisle J, Stevenson CA. Drugs for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004125.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 1990
- Carpenter DO. Neural mechanisms of emesis. Canadian Journal of Physiology and Pharmacology 1990;68:230‐6. [PUBMED: 2178747] [DOI] [PubMed] [Google Scholar]
Dorman 2010
- Dorman, S, Perkins, P. Droperidol for treatment of nausea and vomiting in palliative care patients. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD006938.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315(71091):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ezzo 2006
- Ezzo J, Richardson MA, Vickers A, Allen C, Dibble S, Issell BF, et al. Acupuncture‐point stimulation for chemotherapy‐induced nausea or vomiting. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD002285.pub2] [DOI] [PubMed] [Google Scholar]
Fedorowicz 2011
- Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD005506.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendey 2005
- Hendey GW, Donner NF, Fuller K. Clinically significant changes in nausea as measured on a visual analog scale. Annals of Emergency Medicine 2005;45(1):77‐81. [DOI: 10.1016/j.annemergmed.2004.07.446; PUBMED: 15635314 ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jordan 2007
- Jordan K, Sippel C, Schmoll H. Guidelines for antiemetic treatment of chemotherapy‐induced nausea and vomiting: past, present, and future recommendations. Oncologist 2007;12:1143‐50. [PUBMED: 17914084] [DOI] [PubMed] [Google Scholar]
Kris 2006
- American Society of Clinical Oncology, Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark‐Snow R, Koeller JM, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update. Journal of Clinical Oncology 2006;24(18):2932‐47. [PUBMED: 16717289] [DOI] [PubMed] [Google Scholar]
LaValley 2003
- LaValley J, Crandall C, Braude D. Nausea and vomiting management in US emergency departments (abstract). Academic Emergency Medicine 2003;10:461. [Google Scholar]
Lee 2009
- Lee A, Fan LTY. Stimulation of the wrist acupuncture point P6 for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003281.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maranzano 2005
- Maranzano E, Feyer P, Molassiotis A, Rossi R, Clark‐Snow R, Olver I, et al. Evidence‐based recommendations for the use of antiemetics in radiotherapy. Radiotherapy & Oncology 2005;76(3):227‐33. [PUBMED: 16150504] [DOI] [PubMed] [Google Scholar]
Mathews 2010
- Mathews A, Dowswell T, Hass DM, Doyle M, O'Mathuna DP. Interventions for nausea and vomiting in early pregnancy. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007575.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mee 2011
- Mee MJ, Egerton‐Warburton D, Meek R. Treatment and assessment of emergency department nausea and vomiting in Australasia: a survey of anti‐emetic management. Emergency Medicine Australasia 2011;23:162‐8. [DOI: 10.1111/j.1742-6723.2011.01386.x; PUBMED: 21489163] [DOI] [PubMed] [Google Scholar]
Perkins 2009
- Perkins P, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006271.pub2] [DOI] [PubMed] [Google Scholar]
Quigley 2001
- Quigley EMM, Hasler WL, Parkman HP. AGA technical review on nausea and vomiting. Gastroenterology 2001;120(1):263‐86. [DOI: 10.1053/gast.2001.20516; PUBMED: 11208736] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Zun 2010
- Zun LS, Singh A. Nausea and vomiting. In: Marx JA editor(s). Rosen's Emergency Medicine: Concepts and Clinical Practice. Philadelphia: Mosby Elsevier, 2010. [ISBN: 978‐0‐323‐05472‐0] [Google Scholar]
References to other published versions of this review
Furyk 2012
- Furyk JS, Egerton‐Warburton D, Meek RA. Drugs for the treatment of nausea and vomiting in adult patients in the emergency department setting. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010106] [DOI] [PMC free article] [PubMed] [Google Scholar]


