Abstract
Background
The intensive care unit (ICU) stay has been linked with a number of physical and psychological sequelae, known collectively as post‐intensive care syndrome (PICS). Specific ICU follow‐up services are relatively recent developments in health systems, and may have the potential to address PICS through targeting unmet health needs arising from the experience of the ICU stay. There is currently no single accepted model of follow‐up service and current aftercare programmes encompass a variety of interventions and materials. There is uncertain evidence about whether follow‐up services effectively address PICS, and this review assesses this.
Objectives
Our main objective was to assess the effectiveness of follow‐up services for ICU survivors that aim to identify and address unmet health needs related to the ICU period. We aimed to assess effectiveness in relation to health‐related quality of life (HRQoL), mortality, depression and anxiety, post‐traumatic stress disorder (PTSD), physical function, cognitive function, ability to return to work or education and adverse effects.
Our secondary objectives were to examine different models of follow‐up services. We aimed to explore: the effectiveness of service organisation (physician‐ versus nurse‐led, face‐to‐face versus remote, timing of follow‐up service); differences related to country (high‐income versus low‐ and middle‐income countries); and effect of delirium, which can subsequently affect cognitive function, and the effect of follow‐up services may differ for these participants.
Search methods
We searched CENTRAL, MEDLINE, Embase and CINAHL on 7 November 2017. We searched clinical trials registers for ongoing studies, and conducted backward and forward citation searching of relevant articles.
Selection criteria
We included randomised and non‐randomised studies with adult participants, who had been discharged from hospital following an ICU stay. We included studies that compared an ICU follow‐up service using a structured programme and co‐ordinated by a healthcare professional versus no follow‐up service or standard care.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data, assessed risk of bias, and synthesised findings. We used the GRADE approach to assess the certainty of the evidence.
Main results
We included five studies (four randomised studies; one non‐randomised study), for a total of 1707 participants who were ICU survivors with a range of illness severities and conditions. Follow‐up services were led by nurses in four studies or a multidisciplinary team in one study. They included face‐to‐face consultations at home or in a clinic, or telephone consultations or both. Each study included at least one consultation (weekly, monthly, or six‐monthly), and two studies had up to eight consultations. Although the design of follow‐up service consultations differed in each study, we noted that each service included assessment of participants' needs with referrals to specialist support if required.
It was not feasible to blind healthcare professionals or participants to the intervention and we did not know whether this may have introduced performance bias. We noted baseline differences (two studies), and services included additional resources (two studies), which may have influenced results, and one non‐randomised study had high risk of selection bias.
We did not combine data from randomised studies with data from one non‐randomised study. Follow‐up services for improving long‐term outcomes in ICU survivors may make little or no difference to HRQoL at 12 months (standardised mean difference (SMD) ‐0.0, 95% confidence interval (CI) ‐0.1 to 0.1; 1 study; 286 participants; low‐certainty evidence). We found moderate‐certainty evidence from five studies that they probably also make little or no difference to all‐cause mortality up to 12 months after ICU discharge (RR 0.96, 95% CI 0.76 to 1.22; 4 studies; 1289 participants; and in one non‐randomised study 79/259 deaths in the intervention group, and 46/151 in the control group) and low‐certainty evidence from four studies that they may make little or no difference to PTSD (SMD ‐0.05, 95% CI ‐0.19 to 0.10, 703 participants, 3 studies; and one non‐randomised study reported less chance of PTSD when a follow‐up service was used).
It is uncertain whether using a follow‐up service reduces depression and anxiety (3 studies; 843 participants), physical function (4 studies; 1297 participants), cognitive function (4 studies; 1297 participants), or increases the ability to return to work or education (1 study; 386 participants), because the certainty of this evidence is very low. No studies measured adverse effects.
We could not assess our secondary objectives because we found insufficient studies to justify subgroup analysis.
Authors' conclusions
We found insufficient evidence, from a limited number of studies, to determine whether ICU follow‐up services are effective in identifying and addressing the unmet health needs of ICU survivors. We found five ongoing studies which are not included in this review; these ongoing studies may increase our certainty in the effect in future updates. Because of limited data, we were unable to explore whether one design of follow‐up service is preferable to another, or whether a service is more effective for some people than others, and we anticipate that future studies may also vary in design. We propose that future studies are designed with robust methods (for example randomised studies are preferable) and consider only one variable (the follow‐up service) compared to standard care; this would increase confidence that the effect is due to the follow‐up service rather than concomitant therapies.
Plain language summary
Follow‐up services to improve the long‐term after‐effects of a stay in the intensive care unit
What is the aim of this review
More people survive the intensive care unit (ICU), but are prone to suffering from physical and psychological consequences that may affect their quality of life. Follow‐up services are a relatively new development in healthcare. These services, which include consultations with healthcare professionals, are intended to identify and address these after‐effects more effectively than standard care (which does not use follow‐up services). The aim of this Cochrane Review was to find out if follow‐up services for people after they have been in the ICU are effective. We collected and analysed all relevant studies to answer this question and found five studies.
Key messages
Overall, we found few studies, each of which used a different design of a follow‐up service, and so our confidence in deciding whether ICU follow‐up services are effective was limited. We found no evidence of whether using a follow‐up service after a stay in the ICU improves a person's health‐related quality of life, anxiety and depression, post‐traumatic stress disorder (PTSD), or physical and mental function. We found no evidence of whether using a follow‐up service reduces the number of people who die or the number of people who return to work 12 months after ICU discharge.
During our search of the literature, we found five ongoing studies. These are not included in this review, but including them in future updates may increase the certainty of the evidence and our confidence in deciding whether ICU follow‐up services are effective.
What was studied in the review
We studied some of the physical and psychological consequences that people may suffer after they have been in the ICU, which may affect their quality of life, for example, anxiety and depression, or PTSD. We assessed whether these consequences were improved if a follow‐up service was used.
What are the main results of the review
We found four randomised studies with 1297 participants and one non‐randomised study with 410 participants. These studies were conducted in Denmark, Germany, Sweden, UK and USA. Participants had a range of conditions in the ICU, and varied in severity of these conditions. One study included only participants who had sepsis.
We included studies that compared a follow‐up service provided after a stay in the ICU with standard care (which provided no follow‐up service). Follow‐up services were led by nurses in four studies, and by a multidisciplinary team (nurses, doctors, and physiotherapists) in the fifth study. Consultations were given face‐to‐face at home or in a clinic, or were made on the telephone, or both. Participants had more than one consultation as part of the service, and in two studies participants had up to eight consultations. Although the design of follow‐up service consultations differed in each study, we noted that each service included assessment of participants' needs with referrals to specialist support if required.
We found that follow‐up services may make little or no difference to people's health‐related quality of life 12 months after their stay in the ICU (1 study; 286 participants; low‐certainty evidence), and probably make little or no difference to the number of deaths after 12 months (5 studies; 1707 participants; moderate‐certainty evidence). Follow‐up services may make little or no difference to PTSD (3 studies; 703 participants; low‐certainty evidence).
We are not confident in the evidence of whether using a follow‐up service reduces depression and anxiety (3 studies; 843 participants), physical function (4 studies; 1297 participants), cognitive function (4 studies; 1297 participants), or increases the ability to return to work or education (1 study; 386 participants); we assessed this evidence as very low certainty. No studies measured adverse effects.
We had hoped to look at differences between types of ICU follow‐up service and between people who may or may not have experienced delirium, to give us more information about whether certain styles of service are better, or whether these services are more useful for people with different conditions. However, we found insufficient studies to be able to look at these differences.
How up to date is this review
We searched for studies that had been published up to November 2017.
Summary of findings
Summary of findings for the main comparison. ICU follow‐up services compared with standard care or no follow‐up service for survivors of critical illness.
| ICU follow‐up services compared with standard care or no follow‐up service for survivors of critical illness | ||||
|
Patient or population: adult survivors of the ICU, excluding those already in an existing follow‐up or rehabilitation programme Settings: clinics in a hospital or in the participant's home (via telephone) in: Denmark, Germany, Sweden, UK and USA Intervention: ICU follow‐up service Comparison: standard care or no follow‐up service | ||||
| Outcomes | Effects of follow‐up services for adult survivors of the ICU | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Health‐related quality of life Scoring tool: EQ‐5D Direction of scale: lower scores indicate better HRQoL Time point of measurement: 12 months |
Using a follow‐up service after ICU discharge may make little or no difference to HRQoL of survivors of critical illness SMD ‐0.0, 95% CI ‐0.1 to 0.1a | 286 participants (1 study) | ⊕⊕⊝⊝ Lowb |
|
|
All‐cause mortality Time point of measurement: 2 months in 1 randomised study; 12 months in 3 randomised studies; 14 months in 1 non‐randomised study |
From 5 studies, we found that using a follow‐up service probably makes little or no difference to the number of people who die after ICU discharge. We pooled data from 4 studies(RR 0.96, 95% CI 0.76 to 1.22) |
1289 participants (4 studies) | ⊕⊕⊕⊝ Moderatec |
We did not include data from one non‐randomised study in meta‐analysis. Study authors reported number of deaths in the intervention group: 79/259; and in the control group: 46/151 |
|
Depression and anxiety Scoring tool: HADS‐D and HADS‐A Direction of scale: lower scores indicate less depression and less anxiety Time point of measurement: 12 months in 2 randomised studies; 14 months in 1 non‐randomised study |
It is uncertain whether using a follow‐up service reduces depression. Estimates from 2 randomised studies were SMD ‐0.1, 95% CI ‐1.2 to 1.0a; and absolute risk reduction (usual care vs intervention) ‐0.20, 95% CI ‐1.12 to 0.72a; and 1 non‐randomised study reported little or no difference in scores (women: P = 0.09; men: P = 0.47)a It is uncertain whether using a follow‐up service reduces anxiety. Estimates from 2 randomised studies were SMD ‐0.8, 95% CI ‐1.9 to 0.4a; and absolute risk reduction (usual care vs intervention) ‐0.21, 95% CI ‐1.22 to 0.80a; and 1 non‐randomised study reported no difference in scores (women: P = 0.14; men: P = 0.78)a |
1082 participants (3 studies) | ⊕⊝⊝⊝ Very lowd |
|
|
Post‐traumatic stress disorder (PTSD) Scoring tools: DVT, HTQ‐IV, IES, and PTSS‐10 Direction of scales: lower scores indicate less distressing symptoms of PTSD Time point of measurement: 12 months in 2 randomised studies; 14 months in 1 non‐randomised study |
From 4 studies, it is uncertain whether using a follow‐up service reduces PTSD. Estimates showed little or no difference in PTSD in 3 randomised studies (SMD ‐0.05, 95% CI ‐0.19 to 0.10; 702 participants) |
703 participants (3 studies) |
⊕⊕⊝⊝ Lowe |
We did not include data from one non‐randomised study in meta‐analysis. Study authors reported lower IES scores (indicating less chance of PTSD) in women who received a follow‐up service (P = 0.01) |
|
Physical function Scoring tool: PCS Direction of scales: higher scores indicate improved physical function Time point of measurement: at 12 months in 3 randomised studies (using SF‐36), and at 2 months in 1 randomised study (using SF‐8) |
From 4 studies, it is uncertain whether using a follow‐up service improves physical function at 12 months. Estimates showed little or no difference in physical function at 12 months in 2 studies (MD 1.31, 95% CI ‐0.86 to 3.49) |
422 participants (2 studies) | ⊕⊝⊝⊝ Very lowg |
We did not included data from 2 studies in meta‐analysis. One of these studies reported improved physical function at 2 months in participants who received a follow‐up service (P = 0.02)f, and one reported little or no difference in physical function at 12 months (P > 0.05) |
|
Cognitive function Scoring tools: MCS of SF‐36 and SF‐8 Direction of scales: higher scores indicate improved cognitive function Time point of measurement: at 12 months in 2 randomised studies and at 6 months in 1 randomised study (using SF‐36), and at 2 months in 1 randomised study (using SF‐8) |
From 4 studies, it is uncertain whether using a follow‐up service improves cognitive function at 12 months. Estimates showed little or no difference in cognitive function at 6 and 12 months in 3 studies (MD 1.44, 95% CI ‐0.51 to 3.39) |
622 participants (3 studies) |
⊕⊝⊝⊝ Very lowg |
We did not include data from 1 study in meta‐analysis. Study authors reported little or no difference in cognitive function at 2 monthsf |
|
Ability to return to work or education (reported at 12 months) |
It is uncertain whether using a follow‐up service increases the number of participants who are able to return to work at 12 months (OR 1.06, 95% CI 0.35 to 3.21)a | 386 participants (1 study) |
⊕⊝⊝⊝ Very lowh |
|
| Adverse effects | Not measured | ‐ | ‐ | |
| CI: Confidence interval;DTS: Davidson Trauma Scale; EQ‐5D: Euroqol‐5D; HADS‐A: Hospital Anxiety and Depression scale for anxiety; HADS‐D: Hospital Anxiety and Depression scale for depression; HTQ‐IV: Harvard Trauma Questionnaire Part IV; IES: Impact of Events scale; MCS: mental component score of SF‐36; MD: mean difference; OR: odds ratio; PCS: physical component of SF‐36; PTSD: post‐traumatic stress disorder; PTSS‐10: Post Traumatic Symptom Scale; RR: risk ratio; SF‐36: 36‐item Short Form Survey; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially differenti is moderate Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially differenti is high Very low: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially differenti is very high | ||||
aeffect estimate or P values as reported by study authors. bIntervention group received additional therapy (manual‐based physiotherapy) which may have influenced results; downgraded by one level for study limitations. One study with few participants; downgraded by one level for imprecision. cAnalysis was at different time points, and we noted some potential differences between studies in baseline characteristics between studies; downgraded by one level for inconsistency. dIntervention group in one study received an additional therapy (manual‐based physiotherapy programme), and one non‐randomised study had a high risk of selection bias; we downgraded by one level for study limitations. Outcomes were measured at different time points, and we noted some baseline differences between studies; downgraded by one level for inconsistency. Evidence was from few studies; downgraded one level for imprecision. eIntervention group in one study received an additional therapy (manual‐based physiotherapy programme), and one non‐randomised study had a high risk of selection bias; downgraded by one level for study limitations. We noted differences at baseline in one non‐randomised study (more women in control group had a previous history of psychological problems) which may have influenced results for this outcome, and we noted inconsistent results between three combined randomised studies and one non‐randomised study; we downgraded one level for inconsistency. fdata re‐analysed by study authors accounting for death. gIntervention group in one study received an additional therapy (manual‐based physiotherapy programme), and in another study intervention group were also involved in preparation of a discharge summary plan; downgraded one level for study limitations. Outcomes were measured at different time points, we noted some baseline differences between studies, and we noted a wide confidence interval in analysed data; downgraded by two levels for inconsistency. hIntervention group received additional therapy (manual‐based physiotherapy) which may have influenced results; downgraded by one level for study limitations. One study with few participants and we noted a wide confidence interval; downgraded by two levels for imprecision. isubstantially different = a large enough difference that it might affect a decision.
Background
In 2014 to 2015, approximately 150,000 patients were admitted to adult intensive care units (ICUs) or high‐dependency units (HDUs) in England, Wales and Northern Ireland, and approximately 45,000 patients in Scotland, a large percentage of whom survived (ICNARC 2016; SICSAG). An ever‐increasing number of people, in the UK and globally, are surviving the ICU, and short‐term mortality for critical illnesses is decreasing in general (Needham 2012). Despite this progress, ICU stay has been linked with a number of physical and psychological sequelae that afflict these survivors, potentially for years after critical illness. ICU follow‐up services are relatively recent developments in healthcare systems, the purposes of which are to help address this wide variety of impairments by identifying and addressing patients' health needs directly or by providing access to additional healthcare services.
Description of the condition
Critical illness, and the ICU stay itself, can be traumatic experiences, which have been known to cause physical and psychological distress that can extend far beyond the initial illness and any short‐term treatment. The long‐term problems arising from the ICU, known as 'post‐intensive care syndrome' (PICS), (Needham 2012), include mortality, post‐traumatic stress disorder (PTSD), anxiety, depression and physical impairments, and can also include sexual dysfunction, amnesia of the ICU period, and various related social problems (Griffiths 2007; Oeyen 2010). PICS not only affects ICU survivors, but also amplifies the burden for their families and dramatically increases costs for healthcare systems (Jones 1998; Needham 2011).
Mortality figures at one year after discharge range from 26% to 63%, and those for five years after discharge are reported to be between 40% and 58% (Williams 2005).
The quality‐of‐life scores of ICU survivors are lower than average (for an age‐ and gender‐matched population), and while research shows that quality of life and basic functionality does begin to slowly improve, this disparity compared with the general population tends to remain for at least five years after discharge (Cuthbertson 2005; Cuthbertson 2010; Eddleston 2000; Oeyen 2010), and may never fully return to pre‐admittance levels (Van der Schaaf 2009).
Additionally, between 19% to 22% of ICU survivors are affected by PTSD up to 10 years after critical illness, and for survivors of acute respiratory distress syndrome (ARDS) this figure could be as high as 44% (Davydow 2008a; Davydow 2008b). Anxiety may affect 23% to 48% of ARDS survivors up to 28 months after illness. The incidence of depression in the same group ranges from 17% to 43%, and this incidence may affect 8% to 57% of the general ICU population at 14 months (Davydow 2008b; Davydow 2008c).
Even with this research, there exist significant gaps in our knowledge of post‐ICU cognitive morbidities, and more attention may need to be paid in particular to the impact of delirium and prior health status, for example to include frailty (Bagshaw 2015; Cuthbertson 2009; Needham 2012; NICE 2009; Pandharipande 2013).
Description of the intervention
For this review we define an ICU follow‐up strategy as any service set up to address specifically the various health needs of ICU survivors, to prevent the development of physical, psychological and social problems over the long term. There is, however, no one accepted model for such services (Rattray 2007). The UK has been at the centre of research into critical care follow‐up (Lasiter 2016; Williams 2008), and there has been substantial investment in ICU follow‐up services, leading to a doubling of their number between 2002 and 2006 (Cuthbertson 2003; Griffiths 2006). Though the first follow‐up clinic in the UK was set up in 1985 (Griffiths 2006), and following official recommendations coming from the King’s Fund Panel in 1989 (King's Fund 1989), and the ‘Critical to Success’ audit commission in 1999 (Audit Commission 1999), the development of ICU follow‐up clinics has been an ad hoc, experimental process, not a systematic one (Angus 2003; Jensen 2015). Today, still, there is no standardisation of such services across National Health Service (NHS) trusts or other healthcare systems globally.
Indeed, on a global level, ICU follow‐up programmes have seen mixed levels of attention and implementation. Recent initiatives by the Institute of Medicine in the USA have resulted in greater attention being paid to this important aspect of post‐critical care (Lasiter 2016), with systems such as the Indiana University School of Medicine's Critical Care Recovery Center (CCRC) being set up (Khan 2015) and the THRIVE Peer Support Collaborative (Society of Critical Care Medicine). In Scandinavian countries (Norway, Denmark and Sweden), there is evidence of local initiatives dating back to the early 1990s. While UK services have emphasised physical rehabilitation (NICE 2009), the programmes in the Scandinavian countries have tended to focus on patient‐led initiatives, including diaries and dialogue (Egerod 2013; Jensen 2015). There appears to be a lack of available data from other countries, which is perhaps no surprise given the slow implementation even in more developed healthcare systems.
Types of services that may be offered to ICU survivors range from informal interviews to more organised sessions. They may be patient‐led and focus around the sharing of experiences, or led by healthcare personnel with the purpose of providing information to the patient; equally, they may be focused around physical rehabilitation, or around addressing cognitive dysfunction (NICE 2009). Guidelines published by the National Institute for Health and Care Excellence (NICE) recommended both that preventative measures should be started in the ICU setting and that multidisciplinary functional assessments should be conducted by appropriately trained personnel two to three months after ICU discharge (NICE 2009). Importantly however, these guidelines acknowledge the limitations of the current consensus surrounding ICU follow‐up (NICE 2009).
How the intervention might work
The general aims of a follow‐up service in this review are to: provide a forum in which to identify and address any unmet health needs; and to identify possible PICS, and allow for their further management within or without the hospital setting. How such a service might achieve these aims can vary widely, however. Follow‐up services may take the form of informal meetings that facilitate a patient‐led sharing of experiences that can provide reassurance to the ICU survivor and potentially reduce depression or anxiety, or they may involve access to standard general practitioner (GP) services.
More organised sessions, which may either be nurse‐ or physician‐led, might involve discussion of specific physical or psychological conditions and subsequent referral to appropriate health providers to manage these conditions. A follow‐up service might be conducted face‐to‐face or by remote access. It might be assessed using locally derived questionnaires, or through standardised questionnaires using validated scales. For complex interventions such as this one, a preferred model may be one that is tailored to local circumstances rather than being completely standardised (Craig 2008). Equally, the inherent heterogeneity of the patient population within any single ICU might further complicate any standardisation of follow‐up services. It has been suggested, for example, that patients who have had a longer ICU stay, or who have had incidents of delirium, may react to follow‐up services differently. So while it might be beneficial for clinics to target their resources at those most likely to benefit (Aitken 2015; Cuthbertson 2009; Jensen 2015), the lack of a thorough epidemiological study base for these differences makes conclusions in this area speculative (Needham 2012).
Globally, ICUs treat people with a large range of diseases and general afflictions, and varying severities of conditions, patient backgrounds and socioeconomic factors. It is feasible that follow‐up services may be more beneficial to particular patient groups. For example, the socioeconomic conditions of an individual can affect quality of life, cause or exacerbate anxiety and depression, and affect physical function, and, in lower‐income countries, mortality. Another important consideration, and one that has been overlooked in much of the literature (Williams 2008), is that of ICU access. Access to hospital‐based follow‐up services, which may be relatively simple for UK‐based patients, has the potential to be extremely difficult for those living in very large tertiary care catchment areas. This means that conclusions reached about these services may not be relevant for clinicians and patients in rural areas around the world.
Why it is important to do this review
Though there is a growing civil, scholarly, and governmental desire for information on the role that ICU follow‐up services might play within an integrated recovery process, which starts in the ICU and continues long afterwards, there has been, and still is, a lack of medical consensus (Angus 2003; NICE 2009). In the UK, the USA and around the world, ICU follow‐up initiatives have not received as much dedicated funding or widespread implementation as those of oncology care, spinal injury care, or military veterans' care (Needham 2012). ICU follow‐up services appear intuitively beneficial (Cuthbertson 2003; Rattray 2007), but it is still important that they are grounded in the principles of evidence‐based medicine.
To date, there has been no Cochrane Review to assess the effectiveness of ICU follow‐up services as a general system of care. We have identified a number of reviews dedicated to this subject (Jensen 2015; Niven 2014; Williams 2008). These reviews, among other differences, either require updating (Williams 2008), or have different emphases (Jensen 2015; Niven 2014). Niven 2014, for example, focuses on ICU transition services and the risk of readmission, whereas Jensen 2015 has subtle differences regarding inclusion criteria for studies. Jensen and colleagues only included randomised studies. Our emphasis in this review will be on both randomised and non‐randomised studies and will be directed towards services that are both delivered by a healthcare professional and address unmet health needs related to the ICU period. This is an area of clinical importance that warrants a systematic approach.
Objectives
Our main objective was to assess the effectiveness of follow‐up services for ICU survivors that aim to identify and address unmet health needs related to the ICU period. We aimed to assess effectiveness in relation to health‐related quality of life (HRQoL), mortality, depression and anxiety, post‐traumatic stress disorder (PTSD), physical function, cognitive function, ability to return to work or education and adverse effects.
Our secondary objectives were to examine different models of follow‐up services. We aimed to explore: the effectiveness of service organisation (physician‐ versus nurse‐led, face‐to‐face versus remote, timing of follow‐up service); differences related to country (high‐income versus low‐ and middle‐income countries); and effect of delirium, which can subsequently affect cognitive function, and the effect of follow‐up services may differ for these participants.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and non‐randomised studies. We did not find any controlled before‐after studies (defined as those in which observations are made before and after the implementation of an intervention) or interrupted time series studies (studies that use observations at multiple time points before and after an intervention in order to detect significant change over time). We included full‐text studies; none were conference abstracts or unpublished data from grey literature searches. We did not exclude studies based on outcomes or methods of analysis.
Types of participants
We included adults who had been discharged from hospital following a stay in an ICU that required level 3 care. We did not exclude participants based on the reason they were admitted to the ICU, so long as they were subject to level 3 care. We defined level 3 care, or the equivalent grade in other healthcare systems, as requiring advanced respiratory support, or care that required the artificial support of at least two organs (Intensive Care Society 2009). We included participants who had been admitted to any ICU, and planned to include admission to high‐dependency or critical care units or other hospital wards specifically designed to cater for patients who were critically ill.
We excluded participants who were in any existing rehabilitation programme, for example those associated with traumatic brain injury, spinal cord injury, military trauma and cancer or cardiac care. We did not exclude otherwise eligible patients based on location, geographical dispersion, gender, or any other factor.
Types of interventions
We included studies that assessed a follow‐up service (intervention) attended by ICU survivors on at least one occasion compared to either no follow‐up service or standard care (control). We defined a follow‐up service as any consultation delivered by a healthcare professional (such as a nurse or doctor) or an appropriately trained other person, which sought to specifically identify or address unmet health needs directly related to the ICU period. We included studies in which the service was conducted either face‐to‐face or remotely (e.g. through email or telephone contact), and at an appropriate location, such as a clinic or home visit. We included services that started at any time within six months of discharge from hospital. We included studies in which the follow‐up service sought to address needs through immediate support or subsequent referrals.
We excluded studies that offered a follow‐up service that only provided general (non‐ICU related) information or educational materials to the participant, and we excluded studies that were not delivered by a healthcare professional or appropriately trained other person. We excluded studies of specialist services designed to manage physical or psychological conditions, such as rehabilitation services. Although these services may address conditions related to the ICU stay, for the purpose of this review we treated a rehabilitation service as distinct from a follow‐up service, in which a consultation‐style service aims to identify any type of unmet need; participants may be referred to these specialist rehabilitation services during a follow‐up consultation. We excluded studies of use of diaries kept during the ICU stay, which are given to participants at or after ICU discharge; this is reviewed elsewhere (Ullman 2014).
Standard care (control group), which may also be described by study authors as usual care, included general practitioner (GP) visits and care related to ongoing known medical conditions that were not targeted at identifying and addressing unmet needs related to the period spent in the ICU. For the purpose of this review, we referred to 'usual care' as 'standard care'. We anticipated that standard care may differ in each study because of differences in institution protocols and primary care services; for example, diagnosis of some ICU‐related symptoms (such as PTSD or anxiety) may also be made during scheduled or unscheduled GP appointments. We reported descriptions of standard care in each study during data extraction and management.
Types of outcome measures
We assessed the effectiveness of follow‐up services by measuring differences in physical and psychological outcomes for study participants. Our main outcome was an overall assessment of health‐related quality of life (HRQoL). We collected data from studies that used a validated tool to assess HRQoL (Euroqol‐5D (EQ‐5D)), and reported an overall mean value for study participants from the validated tools; the EQ‐5D scale assesses mobility, self‐care, main activity, family/leisure activity, pain/discomfort, anxiety and depression (RAND). We collected data on the number of deaths from any cause up to 12 months post‐ICU. We reported psychological outcomes in terms of anxiety or depression or both, and collected these data from components of the above scales or other validated tools, such as the Hospital Anxiety and Depression Scale for anxiety and depression (HADS‐A and HADS‐D)(Zigmond 1983).
For post‐traumatic stress disorder (PTSD), we used validated scales reported by study authors: Davidson Trauma Scale (DTS) (Davidson 2002); Harvard Trauma Questionnaire (HTQ) (Mollica 1992); 10‐item Post Traumatic Symptom Scale (PTSS‐10) (Raphael 1989) and Impact of Events Scale (IES) (Weiss 1996). These assessment scales use self‐report measurements. We reported physical function and cognitive function using the 36 item Short Form Survey (SF‐36), or a simpler version of this tool (SF‐8). The SF‐36 scale assesses the following: physical functioning, social functioning, role limitations, pain, mental health, vitality, and general health perceptions (Brazier 1993). It has two components (physical component (PCS), and mental component (MCS), which are appropriate to measure physical and cognitive functioning. Data for the ability of participants to return to work was collected as the percentage of people who have returned to work at the follow‐up time point.
We planned to collect data for adverse events. Examples of adverse events included increased or continued dependency on medical services rather than a transition into activities of daily living; potential exacerbation of symptoms, for example because of formalised recollection of ICU experiences; or duplication or fragmentation of medical services as noted by study investigators, for example because the participant is offered access to an ICU physician‐led follow‐up service alongside other rehabilitation services.
We collected data for all outcomes at the final time point measured by study authors.
In summary, we collected data for the following outcomes:
Primary outcomes
Health‐related quality of life (HRQoL)
All cause mortality
Depression and anxiety
Secondary outcomes
Post‐traumatic stress disorder (PTSD)
Physical function
Cognitive function
Ability to return to work or education
Adverse effects
We included studies regardless of whether they reported data for our review outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE) for primary studies included in related systematic reviews.
We searched the following databases on 7 November 2017:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 11), in the Cochrane Library
MEDLINE Ovid (1985 to 7 November 2017)
Embase Ovid (1985 to 7 November 2017)
CINAHL EBSCO (1985 to 7 November 2017)
The Effective Practice and Organisation of Care (EPOC) Information Specialist (IS) in consultation with the review authors developed the search strategies. Search strategies are comprised of keywords and controlled vocabulary terms. We applied no language or time limits. We searched all databases from database start to date of search. See Appendix 1 for search strategies. We used a PRISMA study flow diagram to report results of the search (Figure 1).
1.
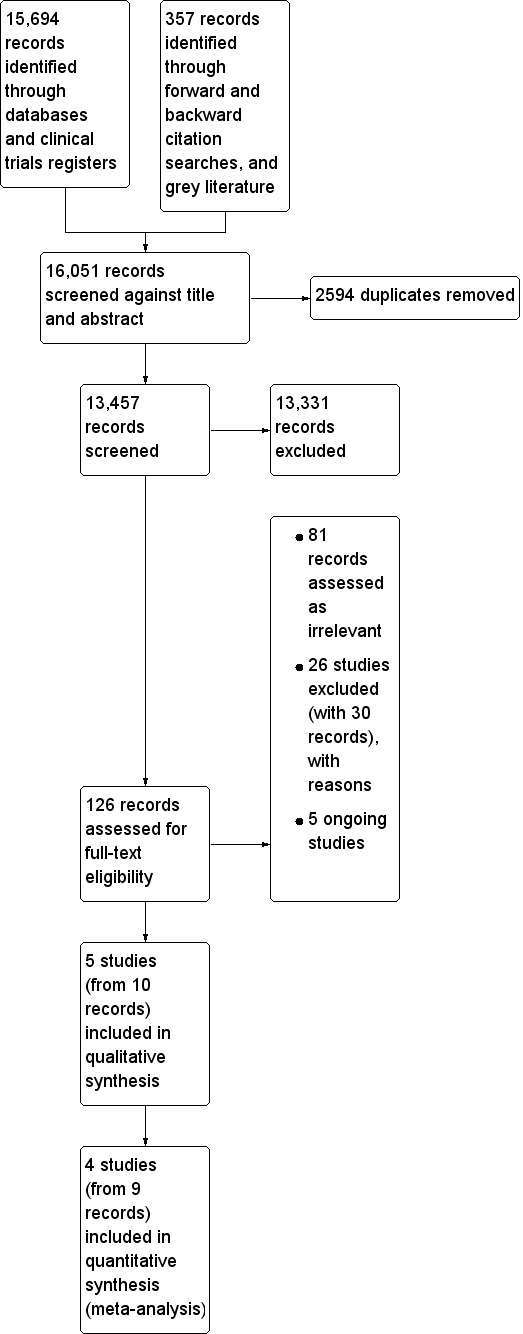
Study flow diagram
Searching other resources
Trials registries
We searched the following trials registers on 22 August 2017.
WHO ICTRP (World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp)
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (www.clinicaltrials.gov)
Grey literature
We conducted a grey literature search to identify studies not indexed in the databases listed above. We searched the following sources on 30 October 2017.
National Institute for Health and Clinical Excellence (NICE) (www.evidence.nhs.uk)
OpenGrey (www.opengrey.eu)
We also reviewed reference lists of all included studies and relevant systematic reviews (Jensen 2015; Lasiter 2016; Mehlhorn 2014; Svenningsen 2017; Williams 2008), for additional, potentially eligible primary studies. We conducted forward citation reference searches for all included studies in ISI Web of Science (Web of Science Core Collection).
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database and removed duplicates. Oliver Schofield‐Robinson (OSR) and Sharon Lewis (SL) independently screened all titles and abstracts and removed studies that were very unlikely to be eligible. If no abstract was available but the title was possibly relevant, we obtained the full text of the article. We independently reviewed the full text of potentially relevant titles using the criteria for studies (Criteria for considering studies for this review). We resolved any disagreement through discussion and by consultation with a third review author, Phil Alderson (PA). We collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We used Covidence software (Covidence) to manage selection of studies.
Data extraction and management
For data extraction and management for all study designs, we used Covidence software (Covidence). We created a template in Covidence using an adapted standard EPOC data collection form (EPOC 2013a), for study characteristics and outcome data; we piloted this form on one included study. Two review authors (OSR and SL) independently extracted the following study characteristics from the included studies.
Methods: study design, number of study centres and location, study setting, date of study
Participants: number, mean age, age range, ethnicity, gender, socioeconomic descriptions (e.g. economic status, education and employment status), APACHE II score, presence of ARDS, reason for ICU stay, episodes of delirium whilst in the ICU (CAM‐ICU score; Ely 2001), withdrawals, diagnostic criteria, length of stay in the ICU, duration of sedation, inclusion criteria, exclusion criteria, other relevant characteristics
Interventions: intervention components, comparison (control group: standard care or no follow‐up service) components, direct or remote clinic, materials involved, time point of intervention, time point of follow‐up, physician‐ or nurse‐led, number of attended clinics, number of participants per clinic
Outcomes: main and other outcomes specified and collected, time points reported
Notes: funding for study, notable conflicts of interest of study authors, ethical approval
We resolved disagreements by consensus or by consultation with a third review author (PA).
Assessment of risk of bias in included studies
Two review authors (SL and OSR) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), and guidance from Cochrane EPOC. For randomised and non‐randomised studies we assessed the following criteria (EPOC 2009).
Was the allocation sequence adequately generated?
Was the allocation adequately concealed?
Were baseline outcome measurements similar?
Were baseline characteristics similar?
Were incomplete outcome data adequately addressed?
Was knowledge of the allocated interventions adequately prevented during the study?
Was the study adequately protected against contamination?
Was the study free from selective outcome reporting?
Was the study free from other risks of bias?
We judged each potential source of bias as high, low, or unclear and provided a justification for our judgment in the 'Risk of bias' table. We summarised 'Risk of bias' judgements across different studies for each of the domains listed.
We did not exclude studies on the grounds of their risk of bias. We used the EPOC 'Risk of bias' guidance information to help reach our judgements (EPOC 2009). We used Covidence software (Covidence), to record 'Risk of bias' decisions; see Appendix 2 for a draft of the 'Risk of bias' table that we modified for use in Covidence.
Assessment of bias in conducting the systematic review
We conducted the review according to our published protocol (Schofield‐Robinson 2017), and have reported any deviations from it in Differences between protocol and review.
Measures of treatment effect
For randomised and non‐randomised studies, we collected continuous data from validated scales (for: HRQoL, depression and anxiety, PTSD, physical function, cognitive function), as reported by study authors at the end of follow‐up time point. We collected these data as mean scores; if mean scores were not available we collected effect estimates reported by study authors (which were: standardised mean difference (SMD), and absolute risk reductions), or median scores. We collected dichotomous data for mortality and the number of participants who were able to return to work at the end of follow‐up.
None of the included studies presented data in graphs or figures, so we did not need to reanalyse any data. We did not include studies in meta‐analysis in which data were not suitable for pooling.
Unit of analysis issues
We noted no unit of analysis issues in any studies.
Dealing with missing data
We did not contact investigators to verify missing study characteristics; we used data as presented in each published version of the studies. We used available data published by study authors, using intention‐to‐treat data when reported. We did not impute missing data with replacement values in this review.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by consideration of study design, participants and how the follow‐up clinics were conducted. Differences, for example, in the socioeconomic background of the participants, has the potential to influence outcome data, and substantial heterogeneity warranted decisions not to pool data. We assessed statistical heterogeneity using the Chi² statistic and related P value, or the I² statistic with associated percentage values (Higgins 2003), for outcomes in which it was possible to combine study data. We used the following cut‐offs as a guide to interpretation: I² statistic at 0% to 40% is not considered important, 30% to 60% suggests moderate heterogeneity, 50% to 90% suggests substantial heterogeneity, and 75% to 100% is considerable heterogeneity (Deeks 2017). If we identified substantial clinical, methodological or statistical heterogeneity we planned to explore it by prespecified subgroup analysis.
We expected heterogeneity in our included study designs to derive from:
type of follow‐up clinic used (e.g. nurse‐led or physician‐led; face‐to‐face or remote);
time points of clinics;
time points of outcome assessment;
potential risk of developing long‐term symptoms relating to the ICU stay; and
socioeconomic conditions of participant.
Certain conditions may increase the likelihood of long‐term psychological symptoms for ICU survivors, for example, people with acute respiratory distress syndrome (ARDS) who survive the ICU may be at a higher risk of developing depression, anxiety and PTSD (Davydow 2008b). We assessed heterogeneity by consideration of differences in baseline data between studies, for example in: presence of ARDS, length of ICU stay, length of sedation, and APACHE II and SAPS II scores.
Assessment of reporting biases
We used data as presented in each published version of the studies; we did not contact investigators to verify missing outcome data. We assessed the risk of reporting bias using the Cochrane 'Risk of bias' tool; we searched for prospective clinical trials registration documents for included studies to use in our assessment of risk of reporting bias. We were unable to explore the risk of publication bias through examination of funnel plots (Sterne 2011), because we identified fewer than 10 studies in the review (Sterne 2017).
Data synthesis
We conducted meta‐analysis only where this was meaningful, that is, if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense. We noted scales used to measure continuous outcomes. We combined data if scales were the same and data were suitable for pooling. If scales were different but were sufficiently similar (and direction of effect was the same), we combined data using generic inverse variance to account for anticipated differences in the scales, study populations, and interventions (Deeks 2017). When study authors reported measurement scales, we presented direction of the effect for these scales in order to make meaningful interpretation of differences between groups. A common way that investigators indicate when they have skewed data is by reporting medians and interquartile ranges. When we encountered this, we noted that the data may be skewed.
For dichotomous data, we used risk ratios (RR) with 95% CI, using Mantel‐Haenszel. We used a random‐effects model for meta‐analysis, which accounts for possible differences between studies in which participant conditions may vary and type of follow‐up service design may vary. We conducted meta‐analysis using the Review Manager 5 (RevMan 5) calculator (Review Manager 2014).
If it was not possible to meta‐analyse the data we summarised the results in the text.
We reported in the Characteristics of included studies whether study authors had used adjusted or unadjusted data in analysis of effect estimates, including factors that they had adjusted for. If we did not combine mean scores in analysis, we reported adjusted effect estimates of single studies in an additional table.
GRADE and 'Summary of findings' table
We summarised the findings of the main intervention comparison for all the outcomes (HRQoL, mortality, depression and anxiety, PTSD, physical and cognitive function, time (ability) to return to work or education, and adverse effects) in a 'Summary of findings' table. This table enabled us to draw conclusions about the certainty of the evidence within the text of the review. Two review authors (OSR and SL) independently assessed the certainty of the evidence (high, moderate, low, and very low), using the five GRADE considerations (study design, consistency of effect, imprecision, indirectness, and publication bias; Guyatt 2008). We used methods and recommendations described in Section 8.5 (Higgins 2017), and Chapters 11 (Schünemann 2017), of the Cochrane Handbook for Systematic Reviews of Intervention, the EPOC worksheets (EPOC 2013b), and GRADEpro software (GRADEpro GDT 2015). We resolved disagreements on certainty ratings by discussion and provided justification for decisions to downgrade the certainty of the evidence using footnotes in the table. We made comments to aid readers' understanding of the review where necessary. We used plain language statements to report these findings in the review.
Subgroup analysis and investigation of heterogeneity
We did not conduct statistical subgroup analyses because we had insufficient studies (we did not have more than 10 studies; Deeks 2017). We described differences between studies using two distinct categories (particular patient groups, and style of service), for subgroups that we defined a priori, as follows.
Physician‐led clinic versus nurse‐led clinic
Face‐to‐face clinic versus remote clinic
Participants from low‐ and middle‐income countries versus participants from high‐income countries (according to World Development Index (WDI), (World Bank 2016))
Intervention conducted earlier than three months post‐ICU versus three to six months
Experienced ICU delirium versus no delirium
Subgroup analysis aimed to assess whether certain follow‐up services have disproportionate benefit for different groups. Organisation, style and timing of follow‐up services between studies may introduce heterogeneity (Williams 2008), and some of these differences may be explained by socioeconomic factors according to the country of the study or inequity in access to healthcare services, or both. For example, current UK guidelines recommend face‐to‐face ICU follow‐up at two to three months post‐ICU discharge (NICE 2009), which may be achievable in a developed health economy but not in a low‐ or middle‐income country. An important socioeconomic consideration is the influence specifically of a nation's status as a low‐income or high‐income economy, which can impinge upon its citizens' access to healthcare services. To this end, we will assess country of study according to the WDI (World Bank 2016). Delirium in the ICU and resultant cognitive dysfunction, which has been shown to be a prevalent affliction among the ICU survivor population and can affect quality of life (Gordon 2004), also have the potential to contribute to clinical heterogeneity. Such subgroup analyses might aid more precise targeting of resources in future studies.
We collected data during the Data extraction and management stage of the review to decide the subgroup for each study.
Sensitivity analysis
We did not perform sensitivity analyses because of the nature of included studies in this review. We did not include unpublished studies; no studies were at low risk of bias, and we did not use imputed data.
Results
Description of studies
Results of the search
We screened 13,457 titles and abstracts from database searches, clinical trials register searches, grey literature, and forward and backward citation searches. We carried out full‐text review of 126 records, and reported details of 36 studies (with 45 records). We identified five eligible studies (with 10 records), and five ongoing studies. See Figure 1.
Included studies
We included five studies (with 10 records) with 1707 participants (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schandl 2012; Schmidt 2016). Four studies were randomised studies (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schmidt 2016) and one was a non‐randomised study, with a before‐after study design (Schandl 2012). All five studies employed a parallel‐study design. See Characteristics of included studies.
Study population and setting
All studies were in countries with advanced industrial economies. Two were single‐centre studies (Douglas 2007; Schandl 2012) and three were multicentre studies (three centres: Cuthbertson 2009; 10 centres: Jensen 2016; nine centres: Schmidt 2016).
Included studies enrolled adult participants who were admitted to and were expected to survive the intensive care unit (ICU); one study enrolled participants who were at least 16 years of age but we determined from the mean age at baseline that most participants in this study were likely to be more than 18 years of age (Schandl 2012). Conditions of participants were varied but typical of ICU admission, and included participants with either medical, surgical and infective conditions, or injuries related to trauma.
Three studies used the Acute Physiology and Chronic Health Evaluation II scoring system (APACHE II) to report baseline severity of participant illness (Cuthbertson 2009; Jensen 2016; Schandl 2012), and one study used APACHE III for this purpose (Douglas 2007). This scoring system can be used to predict patient mortality (Knaus 1985), and whilst we noted some variation in the range of scores between Jensen 2016 and those in Cuthbertson 2009 and Schandl 2012, in general we found that these scores were in a typical range for people in the ICU.
Although we acknowledge that length of stay may not be a direct indicator of illness severity, for example some institutions may have capacity to move patients more swiftly from the ICU to an alternative high‐dependency unit, we noted wide differences in mean or median lengths of stay between studies. Schmidt 2016 reported the longest stay in the ICU amongst included studies, with a mean stay in the control group of 35.2 (standard deviation (SD) ± 26.7) days, whilst Cuthbertson 2009 reported the shortest length of stay amongst included studies with median stays of 2.9 (interquartile range 1.7 to 9.5) days in the intervention group and 3.1 (interquartile range 1.2 to 7.5) days in the control group.
Interventions and comparators
Follow‐up services were led by nurses or multidisciplinary teams and included face‐to‐face consultations, telephone consultations or both. Each study included at least one consultation (weekly, monthly, or six‐monthly) and two studies had up to eight consultations.
Follow‐up services were led by nurses in four studies (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schmidt 2016), and in one study by a multi‐disciplinary team, which included nurses, physicians, and physiotherapists (Schandl 2012). Participants attended a clinic in two studies (on two occasions: Cuthbertson 2009; on one occasion: Jensen 2016), and from the description in a third study we assumed that the follow‐up service was also in a clinic setting (on three occasions: Schandl 2012). In Jensen 2016, participants received two subsequent telephone consultations. One study assessed a follow‐up service with a minimum of eight visits to the participant's home or the extended care facility at which the participant was staying (Douglas 2007), and in one study participants received monthly telephone consultations (Schmidt 2016).
Although each study described a different process by which the follow‐up service was conducted, in each study we noted that healthcare personnel carried out reviews and discussions with participants that included assessments and monitoring of participants' needs. All studies referred participants to other specialist support if necessary. One study involved construction of an illness narrative, with dialogue aided by photographs and use of reflective sheets, which required completion of pre‐set sentences (e.g. "What I want most is...") (Jensen 2016).
Comparison groups in each study received standard care as directed by each institution; standard care did not involve a follow‐up service.
Reported outcomes
All included studies reported review outcomes, which were: health‐related quality of life (HRQoL), (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schmidt 2016); mortality (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schandl 2012; Schmidt 2016); depression and anxiety (Cuthbertson 2009; Jensen 2016; Schandl 2012); post‐traumatic stress disorder (PTSD), (Cuthbertson 2009; Jensen 2016; Schandl 2012); physical and cognitive function (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schmidt 2016); and ability to return to work (Cuthbertson 2009). No studies reported adverse effects.
Times of assessments were: at six and 12 months post‐ICU discharge (Cuthbertson 2009); at two months post‐ICU discharge (Douglas 2007) at three and 12 months post‐ICU discharge (Jensen 2016); at 14 months post‐ICU discharge (Schandl 2012); and at six and 12 months post‐ICU discharge (Schmidt 2016). We reported outcome data at the final time point in each study.
Funding sources
All studies received independent or department funding, which we believed represented no apparent source of conflict in study preparation and interpretation of results.
Excluded studies
We assessed 126 records for full‐text eligibility. We excluded 81 of these because they did not meet our review criteria; we have not included details of these in the review.
We excluded 20 studies (with 24 records) that compared an intervention that did not meet our definition of a follow‐up clinic: seven studies provided educational materials to ICU patients (Alberto 2011; IRCT201110197844N1; Jones 2003; NCT00976807; NCT02415634; Shaw 2012; Strahan 2003); two studies compared a rehabilitation service (Jackson 2012; Walsh 2015); seven studies compared use of a diary given to participants after an ICU stay (Backman 2010; Garrouste‐Orgeas 2010; Huynh 2017; Jones 2010; Knowles 2009; NCT02067559; Robson 2008); three studies compared a psychotherapy intervention (Cox 2014; Holmes 2007; ISRCTN97280643); and one study provided training to participants (Cox 2017). We excluded two studies that did not recruit ICU patients (ward‐based participants: Ball 2003; coronary care unit participants: Farazmand 2017). Following unsuccessful attempts to contact study authors, we excluded four studies that were published only as abstracts (Bourseau 2016; Cave 2016; Davidson 2015; Ramnarain 2015); we will include these in future review updates pending publication of full texts and assessment of eligibility. See Characteristics of excluded studies.
Ongoing studies
We identified five eligible ongoing studies; four of which were identified through clinical trials database searching (ACTRN12616000206426; NCT01796509; NCT02077244; NCT03124342), and one through primary database searching (Paratz 2014). All are randomised studies and aim to recruit adult participants who have been in the intensive care unit. Two studies specifically aim to recruit participants with diabetes mellitis (ACTRN12616000206426) and with sepsis (Paratz 2014). Ongoing studies aim to recruit 1684 participants. See Characteristics of ongoing studies.
Risk of bias in included studies
See Characteristics of included studies and see 'Risk of bias' summary and 'Risk of bias' graph (Figure 2; Figure 3).
2.
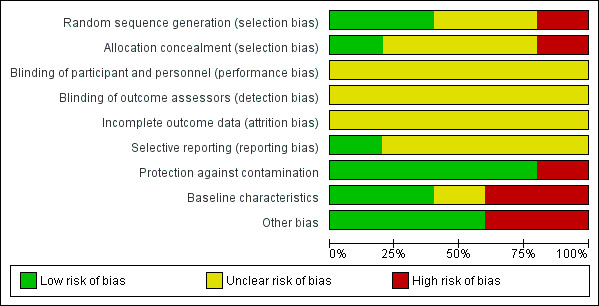
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
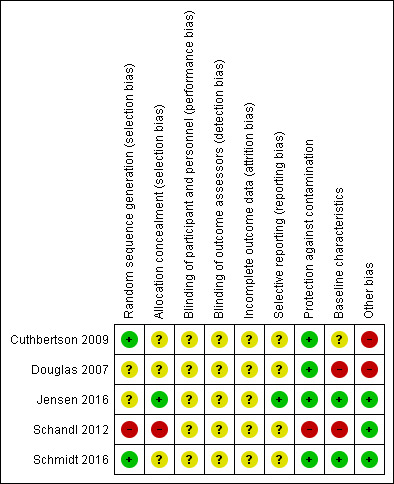
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Four studies reported that participants were randomised (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schmidt 2016). Two studies provided sufficient detail of randomisation methods and we judged these studies to have a low risk of bias for sequence generation (Cuthbertson 2009; Schmidt 2016). We judged two studies to have unclear risk of sequence generation bias because information on randomisation methods was insufficient (Douglas 2007; Jensen 2016).
Three studies reported no methods for allocation concealment and we judged these to have an unclear risk of selection bias (Cuthbertson 2009; Douglas 2007; Schmidt 2016). One study described sealed, opaque envelopes in which to conceal the allocation, and we judged this to have low risk of selection bias (Jensen 2016).
One study was a non‐randomised study (Schandl 2012). This study design introduces a high risk of bias because participants are not divided into groups using a random method, and personnel would have known the allocation.
Blinding
This intervention precluded the possibility of blinding of participants and personnel. We could not be certain whether performance may have been influenced by knowledge of the intervention (i.e. those that were receiving a follow‐up service); we judged all studies to have an unclear risk of performance bias.
Each study measured outcomes using participant self‐assessments (e.g. completion of questionnaires) and, although questionnaires were validated and appropriate for their purpose, we could not be certain whether knowledge of receiving the intervention would influence self‐assessments. We judged all studies to have an unclear risk of detection bias.
Incomplete outcome data
All studies reported a high number of participant losses, in excess of 10% of the patient populations. However, a high number of participant losses are expected in studies with long follow‐up periods (Cuthbertson 2005; Oeyen 2010; Williams 2011). Loss of participants in each study was balanced between groups and we judged all studies to have an unclear risk of attrition bias.
Selective reporting
Three studies reported registration with clinical trials registers (Cuthbertson 2009; Jensen 2016; Schmidt 2016). Registration was retrospective in Cuthbertson 2009 and Schmidt 2016, and it was not feasible to use these documents to assess the risk of selective outcome reporting. Jensen 2016 reported prospective registration, and using these documents we judged this study to have a low risk of selective outcome reporting bias. Two studies did not report registration with clinical trials registers and we judged these studies to have unclear risk of selective outcome reporting bias (Douglas 2007; Schandl 2012).
Protection against contamination
In all studies, a procedure for the follow‐up service was adhered to, and healthcare professionals were used to carry out the intervention. We judged the risk of contamination of the control group to be low across randomised studies (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schmidt 2016). Because of the time difference between the control group and the intervention group in the non‐randomised study, we could not be certain that other variables in service delivery were equivalent over time and we judged this study to have high risk of bias for this domain (Schandl 2012).
Baseline characteristics
We judged the baseline characteristics between groups to be comparable in two studies and we judged these to have a low risk of bias for baseline characteristics (Jensen 2016; Schmidt 2016). Because of a possible reporting error in Cuthbertson 2009, we judged this study to have an unclear risk of bias for baseline characteristics; we could not be certain whether the range of ages was equivalent between groups.
We judged two studies to have high risk of bias for baseline characteristics (Douglas 2007; Schandl 2012). In one study, we noted an imbalance in severity of illness scores and HRQoL (Douglas 2007). The non‐randomised study only reported baseline characteristics for participants who received a questionnaire at 14 months (losses up to this stage could mostly be explained by participant death), and we could not ascertain whether baseline characteristics were equivalent for all participants included in the study (Schandl 2012). Also in Schandl 2012, we noted differences in these baseline characteristics; more women in the control had had previous psychological problems and we noted differences in length of ICU stay, duration of sedation and types of diagnoses.
Other potential sources of bias
We noted no additional sources of bias in three studies (Jensen 2016; Schandl 2012; Schmidt 2016).
We judged two studies to have an additional high risk of bias (Cuthbertson 2009; Douglas 2007). In Cuthbertson 2009, participants in the intervention group also received a manual‐based physiotherapy programme and it is possible that this programme could have influenced the outcome data rather than subsequent attendance at follow‐up clinics. In Douglas 2007, we noted that participants and family members in the intervention group were involved in preparation of a discharge summary plan, and it is possible that preparing a discharge summary plan could have influenced outcome data rather than subsequent attendance at follow‐up clinics.
Effects of interventions
See: Table 1
See Table 1, and Appendix 3.
Primary outcomes
1. Health‐related quality of life (HRQoL)
Results from one study (286 randomised participants; Cuthbertson 2009) suggest that a follow‐up service may make little or no difference to HRQoL at 12 months. This study reported HRQoL as a composite measure using Euroqol‐5D (EQ‐5D); lower scores on this scale indicate better HRQoL. Study authors reported little or no difference in quality of life scores at 12 months (standardised mean difference (SMD) ‐0.0, 95% confidence interval (CI) ‐0.1 to 0.1; P = 0.57; low‐certainty evidence; downgraded by one level for study limitations and one level for imprecision). We have reported mean scores as reported by study authors in Table 2.
1. Additional data.
| Study | Measurement tool and time point | Intervention group dataa | Control group dataa | Effect sizea | P valuea |
| Outcome: HRQoL | |||||
| Cuthbertson 2009 | EQ‐5D at 12 months | Mean (SD): 0.58 (± 0.37); n = 108 | Mean (SD) 0.60 (± 0.30); n = 113 | SMD ‐0.0, 95% CI ‐0.1 to 0.1 | 0.57 |
| Outcome: depression and anxiety | |||||
| Cuthbertson 2009 | HADS‐D at 12 months | Mean/median not reported; n = 92 | Mean/median not reported; n = 100 | SMD ‐0.1, 95% CI ‐1.2 to 1.0 | 0.86 |
| Jensen 2016 | HADS‐D at 12 months | Mean/median data not reported; n = 130 | Mean/median data not reported; n = 130 | Absolute risk reduction (SC vs intervention) ‐0.20, 95% CI ‐1.12 to 0.72 | 0.67 |
| Schandl 2012 | HADS‐D at 14 months | Women: median (range not reported) 3 ; n = 31; Men: median (range not reported) 4; n = 67 |
Women: median (range not reported) 7; n = 27; Men: median (range not reported) 4; n = 46 |
Difference between control and follow‐up groups (negative values indicate lower values in follow‐up group); 25th to 75th percentiles: Women: 1.7 to ‐5.4 Men: ‐0.2 to ‐1.0 |
Women: 0.09; Men: 0.47 |
| Cuthbertson 2009 | HADS‐A at 12 months | Mean (SD) 5.5 (± 4.6); n = 92 | Mean (SD) 6.4 (± 4.4); n = 100 | SMD ‐0.8, 95% CI ‐1.9 to 0.4 | 0.18 |
| Jensen 2016 | HADS‐A at 12 months | Mean/median data not reported; n = 131 | Mean/median data not reported; n = 130 | Absolute risk reduction (SC vs intervention) ‐0.21, 95% CI ‐1.22 to 0.80 | 0.68 |
| Schandl 2012 | HADS‐A at 14 months | Women ‐ median (range not reported): 3; n = 31; Men ‐ median (range not reported): 4; n = 67 |
Women ‐ median (range not reported): 6; n = 27; Men ‐ median (range not reported): 3; n = 46 |
Difference between control and follow‐up groups (negative values indicate lower values in follow‐up group); 25th to 75th percentiles: Women: ‐1.8 to ‐3.2 Men: ‐0.5 to ‐0.8 |
Women: 0.14; Men: 0.78 |
| Outcome: PTSD | |||||
| Schandl 2012 | IES at 14 months | Women ‐ median (range not reported): 20; n = 31; Men ‐ median (range not reported): 16; n = 67 |
Women ‐ median (range not reported): 31; n = 27; Men ‐ median (range not reported): 10; n = 46 |
Difference between control and follow‐up groups (negative values indicate lower values in follow‐up group); 25th to 75th percentiles: Women: ‐6.6 to ‐17.6 Men: 1.9 to 4.4 |
Women: 0.01; Men: 0.27 |
| Outcome: ability to return to work | |||||
| Cuthbertson 2009 | at 12 months | 18 participants returned to work; n = 32 | 17 participants returned to work; n = 31 | OR 1.06, 95% CI 0.35 to 3.21 | Not reported |
aas reported by study authors CI: confidence interval; EQ‐5D: Euroqol 5D; HRQoL: health‐related quality of life; HTQ‐IV: Harvard Trauma Questionnaire part IV;IES: Impact of events scale; n: number of analysed participants; OR: odds ratio; PTSD: post‐traumatic stress disorder; SC: standard care; SD: standard deviation; SMD: standardised mean difference
2. All‐cause mortality
Five studies (1707 participants) reported data for mortality (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schandl 2012; Schmidt 2016). We combined four randomised studies (1297 randomised participants) for mortality at end of follow‐up (2 months in: Douglas 2007; and 12 months in: Cuthbertson 2009; Jensen 2016; Schmidt 2016). Using a follow‐up clinic probably makes little or no difference to mortality up to 12 months after ICU discharge (risk ratio (RR) 0.96, 95% confidence interval (CI) 0.76 to 1.22; 1289 analysed participants; moderate‐certainty evidence; downgraded one level for inconsistency). See Analysis 1.1.
1.1. Analysis.
Comparison 1 Follow‐up service vs control, Outcome 1 All‐cause mortality.
One non‐randomised study reported number of participants who died before study follow‐up at 14 months as part of the study flow diagram (Schandl 2012). Study authors did not report analysis of this data, and reported 79 deaths in the intervention (of 259 participants) and 46 deaths in the control group (of 151 participants).
3. Depression and Anxiety
Three studies (1082 participants) reported data for depression and anxiety using the Hospital Anxiety and Depression scale (HADS) (Cuthbertson 2009; Jensen 2016; Schandl 2012); lower scores indicate less depression and less anxiety on each scale.
We were unable to combine data for two randomised studies (672 randomised participants; Cuthbertson 2009; Jensen 2016), because study authors in Jensen 2016 did not report data in a format suitable for pooling. Both study authors reported little or no difference in HADS scores for depression (HADS‐D) at 12 months between participants who received a follow‐up service after ICU discharge and those who received no follow‐up service (SMD ‐0.1, 95% CI ‐1.2 to 1.0, P = 0.86 in Cuthbertson 2009; absolute risk reduction (usual care vs intervention) ‐0.20, 95% CI ‐1.12 to 0.72, P = 0.67 in Jensen 2016). One non‐randomised study (410 participants) reported little or no difference in HADS‐D scores between participants who received a follow‐up service after ICU discharge and participants who received no follow‐up service (women: P = 0.09; men: P = 0.47). We have included data reported by study authors in Table 2, and we noted that Schandl 2012 reported median scores, which suggests that data may be skewed.
Study authors also reported little or no difference in HADS scores for anxiety (HADS‐A) at 12 months between participants who received a follow‐up service after ICU discharge and participants who received no follow‐up service (SMD ‐0.8, 95% CI ‐1.9 to 0.4, P = 0.18 in Cuthbertson 2009; absolute risk reduction (usual care vs intervention) ‐0.21, 95% CI ‐1.22 to 0.80, P = 0.68 in Jensen 2016). We have included data as reported by study authors in Table 2. One non‐randomised study (410 participants) reported little or no difference in HADS‐A scores (women: P = 0.14; men: P = 0.78) (Schandl 2012). We have included data reported by study authors in Table 2, and we noted that Schandl 2012 reported median scores, which suggests that data may be skewed.
It is uncertain whether using a follow‐up service reduces depression and anxiety because the certainty of this evidence is very low (we downgraded by one level for study limitations, one level for inconsistency, and one level for imprecision).
Secondary outcomes
1. Post‐traumatic stress disorder (PTSD)
Four studies (1082 participants) reported PTSD (Cuthbertson 2009; Jensen 2016; Schandl 2012; Schmidt 2016). Scales used were the Davidson Trauma Scale (DTS) (Cuthbertson 2009), the Harvard Trauma Questionnaire Part IV (HTQ‐IV) (Jensen 2016), Impact of Events Scale (IES) (Schandl 2012), and the 10‐item Post Traumatic Symptom Scale (PTSS‐10) (Schmidt 2016).
We combined data at 12 months in Cuthbertson 2009, Jensen 2016, and Schmidt 2016 using inverse variance to account for differences in measurement tools. We found little or no difference in PTSD between those who received a follow‐up service and those who did not (SMD ‐0.05, 95% CI ‐0.19 to 0.10; 703 participants; 3 studies; low‐certainty evidence; downgraded one level for study limitations and one level for inconsistency). See Analysis 1.4.
1.4. Analysis.
Comparison 1 Follow‐up service vs control, Outcome 4 PTSD.
Schandl 2012 used the Impact of Events scale (IES) at 14 months; lower scores indicate less chance of PTSD. Study authors reported that female participants who received a follow‐up service had a lower score (P = 0.01), which indicated a reduced chance of having PTSD; study authors reported no difference in scores between groups for male participants (P = 0.27). We have included data as reported by study authors in Table 2.
2. Physical function
Four randomised studies (1297 participants) reported physical functioning using the physical component score (PCS) of SF‐36 (Cuthbertson 2009; Jensen 2016; Schmidt 2016), and SF‐8 (Douglas 2007); higher scores indicate less impairment.
Jensen 2016 reported mean and mean difference scores, and we used the calculator in Review Manager 2014 to calculate SDs for each group. We combined data for two randomised studies and found little or no difference in physical function scores between participants who received a follow‐up service after ICU discharge and those who received no follow‐up service (MD 1.31, 95% CI ‐0.86 to 3.49; 422 participants). See Analysis 1.2.
1.2. Analysis.
Comparison 1 Follow‐up service vs control, Outcome 2 Physical function.
We could not combine data for Douglas 2007 and Schmidt 2016 because study authors did not report data as mean (SD) and we could not calculate this from the data in the study reports.
In Douglas 2007, study authors reported little or no difference in physical scores at two months after ICU discharge once baseline scores and APACHE III scores were controlled for (P = 0.40). However, study authors also reported re‐analysis of these results, accounting for loss of participants because of death. In this analysis, study authors reported that more participants who received a follow‐up service had improved physical HRQoL (P = 0.02).
In Schmidt 2016, study authors reported little or no difference in physical HRQoL at 12 months between participants who received a follow‐up service after ICU discharge and those who received no follow‐up service (P > 0.05).
It is uncertain whether using a follow‐up service improves physical function because the certainty of this evidence is very low. We downgraded by one level for study limitations and by two levels for inconsistency.
3. Cognitive function
Four randomised studies (1297 participants) reported cognitive functioning using the mental component score (MCS) of SF‐36 (in: Cuthbertson 2009; Jensen 2016; Schmidt 2016) and SF‐8 (Douglas 2007); higher scores indicate less impairment.
Jensen 2016 and Schmidt 2016 reported mean and mean difference scores, and we used the calculator in Review Manager 2014 to calculate SDs for each group in each study. We found some differences in calculations that may be explained by study authors who reported that, "due to rounding, change scores may not add up precisely". We combined data for three studies and found little or no difference in MCS scores between participants who received a follow‐up service after ICU discharge and those who received no follow‐up service (MD 1.44, 95% CI ‐0.51 to 3.39; 622 analysed participants). See Analysis 1.3.
1.3. Analysis.
Comparison 1 Follow‐up service vs control, Outcome 3 Cognitive function.
We did not include data for Douglas 2007 in analysis because study authors did not report data as mean (SD) and we could not calculate this from the data in study reports. Study authors reported re‐analysis of results accounting for loss of participants because of death; in this analysis study authors reported no difference in cognitive function scores at two months between participants who received a follow‐up service after ICU discharge and those who received no follow‐up service (study authors did not report P values).
It is uncertain whether using a follow‐up service improves physical function because the certainty of this evidence is very low. We downgraded by one level for study limitations and by two levels for inconsistency.
4. Ability to return to work
One randomised study reported number of participants who returned to work at 12 months (Cuthbertson 2009; 286 participants). Study authors reported little or no difference between participants who received a follow‐up service after ICU discharge and those who received no follow‐up service in the number of participants who returned to work. We included data reported by study authors in Table 2.
It is uncertain whether using a follow‐up service improves the ability to return to work because the certainty of this evidence is very low. We downgraded by one level for study limitations and by two levels for imprecision.
5. Adverse effects
No studies reported adverse events.
Subgroup analysis
We found insufficient studies for subgroup analyses. We narratively reported differences between studies following our planned subgroups.
Physician‐led clinic versus nurse‐led clinic: four studies used a follow‐up service that was nurse‐led (Cuthbertson 2009; Douglas 2007; Jensen 2016; Schmidt 2016). One study included a multi‐disciplinary team, which included nurses, physicians, and physiotherapists (Schandl 2012).
Face‐to‐face clinic versus remote clinic: three studies used a face‐to‐face clinic (Cuthbertson 2009; Douglas 2007; Schandl 2012). Jensen 2016 incorporated both face‐to‐face and telephone contact with participants, and Schmidt 2016 used telephone contact with participants.
Participants from low‐ and middle‐income countries versus participants from high‐income countries (according to World Development Index (WDI) (World Bank 2016)): all included studies took place in high‐income countries and we could not perform subgroup analysis for this.
Intervention conducted earlier than three months post‐ICU versus three to six months: one study conducted a follow‐up service only within three months of ICU discharge (Douglas 2007). Two studies conducted follow‐up services that began at three months post‐ICU discharge (Cuthbertson 2009; Schandl 2012). Two studies conducted follow‐up services that began earlier than three months post‐ICU discharge and continued after three months post‐ICU discharge (Jensen 2016; Schmidt 2016).
Experience of ICU delirium versus no delirium: three studies did not report whether participants experienced delirium (Cuthbertson 2009; Douglas 2007; Schandl 2012). One study excluded participants with cognitive deficits and we assumed that included participants in this study did not have delirium (Schmidt 2016). One study reported median number of days of delirium at baseline for participants who had been assessed for delirium (Jensen 2016).
Sensitivity analysis
Restricting the analysis to published studies: we used only data from published studies and could not perform sensitivity analysis for this.
Restricting the analysis to studies with a low risk of selection bias: we found no studies that we judged to have a low risk of selection bias for both random sequence generation and allocation concealment, and therefore we could not perform sensitivity analysis for this.
Using available case data or using imputed data (from last observation carried forward) where studies had missing data: we used data reported by study authors, and when available we used intention‐to‐treat analysis as reported by study authors. We did not impute any study data in this review.
Discussion
Summary of main results
We included five studies comparing a follow‐up service provided to survivors of the intensive care unit (ICU) versus standard care, which had no follow‐up service; four studies were randomised studies and one was a non‐randomised study. We also identified five ongoing studies.
In summary, we found little or no difference for each of our outcomes between participants who received a follow‐up service and participants who received standard care. We found low‐certainty evidence from one randomised study that a follow‐up service may make little or no difference to HRQoL at 12 months after ICU discharge and moderate‐certainty evidence from meta‐analysis of four randomised studies that a follow‐up service may make little or no difference in the number of participants who die up to 12 months after ICU discharge (one non‐randomised study reported mortality in each group but we did not analyse this). Evidence for depression and anxiety from two randomised studies and one non‐randomised study was very low‐certainty.
We found that a follow‐up service may make little or no difference to PTSD (low‐certainty evidence from three randomised studies); one non‐randomised study reported that women had less chance of having PTSD. Our evidence for physical and cognitive function was from four randomised studies, and for ability to return to work was from one study; we could not be certain whether follow‐up services had an effect on these outcomes because evidence was very low certainty. No study reported adverse effects.
Overall completeness and applicability of evidence
We identified five studies including 1707 participants who survived their stay in the ICU after having been admitted for a variety of reasons.
We noted differences between studies in participant diagnoses, ranges of prognostic scores (using APACHE II and APACHE III), and durations of ICU stay, and one study included only participants who had severe sepsis or septic shock. However, all studies included participants that had conditions typical of the general ICU population, and whilst we noted the same conditions in some studies (e.g. cardiovascular or neurological conditions), we were unable to clarify whether all conditions were comparable between all studies. We noted that three studies included some participants who had injuries related to trauma and it is possible that these participants may have had additional psychological difficulties related to their injury (for example PTSD), rather than the ICU stay (Cuthbertson 2009; Jensen 2016; Schandl 2012). In this review we did not explore whether outcome data may be affected by type of condition that ICU survivors had experienced. Included studies were conducted between 2001 and 2015, and were likely to represent more recent ICU patient management.
All studies were conducted in high‐income countries and any results are applicable only to these countries, in which healthcare resources are more likely to be comparable.
We anticipated a variety of types of follow‐up services and this was evident from our included studies. All studies provided a follow‐up service with a nurse and only one study included other healthcare professionals. However, types of service (face‐to‐face or via telephone; in a clinic setting or at home) differed between studies and participants received a different number of consultations (up to eight consultations in total) and the time between consultations also differed (weekly, monthly, or up to six months apart).
We were unable to conduct subgroup analysis because we found insufficient studies, and therefore it was not possible to apply our results to any single design of follow‐up service.
Certainty of the evidence
Few studies reported sufficient methods for random sequence generation and only one study reported methods of allocation concealment.
Attrition was high, which may be explained by study population, types of assessment (e.g. completion and return of questionnaires) or length of follow‐up at 12 months or longer. Only one study reported prospective clinical trials registration and was at low risk of selective outcome reporting bias.
We noted differences in two studies in which participants in the intervention group received resources in addition to follow‐up consultations, which may have influenced results, and we noted differences in baseline characteristics (e.g. length of ICU stay) within and between studies.
We included few randomised studies and evidence from one non‐randomised study, which we believed to have high risk of bias because of its study design. We did not combine data from these different types of study design. Overall, we had limited data for each outcome, and meta‐analysis included very few studies.
We considered study limitations identified from 'Risk of bias' assessments, differences between studies (in terms of time points of measurement) and limited number of studies as reasons to downgrade the certainty of evidence for each of our outcomes. We judged evidence for HRQoL and PTSD to be low certainty, for mortality to be moderate certainty, and for all other outcomes to be very low certainty.
Potential biases in the review process
We conducted a thorough search, using two review authors to assess eligibility, extract data, and assess risk of bias according to the published protocol (Schofield‐Robinson 2017). During the peer review process, a referee identified one potentially relevant study that our searches did not find (Jónasdóttir 2018). Consequently, we have noted this study for future consideration and plan to re‐evaluate the search strategy for the next review update.
We did not contact authors of included studies during the review process, and our reporting of data is limited to the information in published reports. However, outcome data were sufficiently reported in all studies, and we did not downgrade evidence during GRADE assessments based on information that was missing (for example, details of selection procedures), in the published report.
We edited the intervention criteria to include follow‐up services that were started within six months but may have continued beyond six months after ICU discharge. Four included studies had follow‐up services that occurred beyond six months and we believed that these were an appropriate design. Also, we extended the time point at which end of follow‐up data were collected beyond 12 months because we found studies that continued follow‐up services up to 12 months after ICU discharge, and it was important to include data assessed after these final follow‐up consultations. We believed that these edits did not introduce bias and increased the generalisability of the evidence to a wide range of follow‐up services.
Agreements and disagreements with other studies or reviews
Our review findings are broadly consistent with the findings of a recent review by Jensen and colleagues, who concluded that, while follow‐up clinics might cause a minor decrease in post‐traumatic stress, there is no evidence of further effects (Jensen 2015). We noted that Jensen and colleagues included studies of diary interventions, which were not included in this review, and which contributed to the result for PTSD in Jensen 2015. Another review, by Williams and colleagues in 2008, suggested that there was no evidence of an effect of follow‐up clinics, using similar outcomes to the present review (Williams 2008).
Authors' conclusions
Implications for practice.
Whilst we found little or no difference in outcomes between participants who received a follow‐up service and those who received standard care, this review presented insufficient evidence to determine whether ICU follow‐up services are effective. We included only four randomised studies, and one non‐randomised study, with relatively few participants. In addition, this review concentrated on outcomes agreed during preparation of the protocol (Schofield‐Robinson 2017), and as such we have only attempted to measure the effectiveness of an ICU follow‐up service using these outcomes. For example, we did not explore the number of subsequent referrals to specialist services or participant satisfaction with an ICU follow‐up service versus standard care, and we did not perform a cost‐benefit analysis of ICU follow‐up services.
As yet no consensus exists to quantify all the components of an ICU follow‐up service and subsequently evidence for this review was from a wide‐ranging definition of such a service. Because of insufficient studies, we could not perform subgroup analysis; this subgroup analysis sought to establish differences between models of follow‐up services. In addition, we could not determine that control groups in studies (in which participants received standard care) were comparable; healthcare resources and existing services after people leave the ICU may vary widely between hospital institutions and primary care services.
ICU follow‐up continues to be a topical issue in global healthcare, and we are encouraged by the identification of five ongoing studies. Whilst effectiveness has not been demonstrated in this review, neither have we concluded that ICU follow‐up services are not effective, and we anticipate that follow‐up services will continue to be developed in line with national policies (for example, following the recommendation of multidisciplinary functional assessment after ICU discharge; NICE 2009). Inclusion of ongoing studies may influence the results of this review in future updates.
Implications for research.
Further evidence is required to establish whether ICU follow‐up services are effective in addressing physical and psychological consequences of an ICU stay. Because of insufficient studies, we were unable to examine through subgroup analysis whether one design of follow‐up service was more effective than another, and it is therefore not appropriate to propose one single design of follow‐up service to test in an interventional study. We expect that future studies are likely at this stage to present different models of follow‐up service. However to reduce the risk of bias, we propose that the follow‐up service is the only variable between study groups (i.e. the follow‐up service does not include additional resources that may confound data). We would encourage study authors to report clear descriptions of standard care services. Randomised studies of interventions are a more robust study design and would increase certainty of an effect.
This review included studies only from high‐income countries, in which healthcare resources may be greater. We encourage additional research in low‐ and middle‐income countries, which would allow for an assessment of the effectiveness of an ICU follow‐up service in a wider variety of resource settings.
Acknowledgements
We acknowledge the help and support of Cochrane Effective Practice and Organisation of Care (EPOC). We also thank the following editors and peer referees who provided comments to improve the protocol: Julia Worswick, Mary Ann O’Brien, Jemma Hudson, Bernard Burnand, Daniela Goncalves Bradley, Paul Miller, Daniel Niven and Janet Jensen; and to Megan Prictor for copy editing the protocol. We would like to thank the following editors and peer referees who provided comments to improve the review: Julia Worswick, Mary Ann O’Brien, Paul Miller, Sofia Massa, Jennifer Cove, Teresa Williams, Bernard Burnand, Daniel Niven and Janet Jensen.
We acknowledge the support of the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to EPOC. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
We thank Dr David Evans for his initial work in conceiving the idea for the review.
Appendices
Appendix 1. Search strategies
CENTRAL: the Cochrane Library (Wiley)
| 1 | [mh aftercare] | 17505 |
| 2 | [mh counseling] | 4768 |
| 3 | [mh "long‐term care"] | 1243 |
| 4 | MeSH descriptor: [Patient Discharge] explode all trees | 1442 |
| 5 | MeSH descriptor: [Disease Management] explode all trees | 3662 |
| 6 | MeSH descriptor: [Case Management] explode all trees | 784 |
| 7 | (aftercare or after next care or after next treatment):ti,ab | 27468 |
| 8 | (diary or diaries):ti,ab | 7973 |
| 9 | counsel*:ti,ab | 10771 |
| 10 | email?:ti,ab | 164 |
| 11 | telephone*:ti,ab | 9482 |
| 12 | phone*:ti,ab | 4122 |
| 13 | ((follow* next up or discharge) near/2 (appointment* or consultation* or clinic* or program* or strateg* or service?)):ti,ab | 3733 |
| 14 | (recover* near/2 (appointment* or consultation* or clinic* or program* or strateg* or service?)):ti,ab | 909 |
| 15 | ((care or case or disease) near management):ti,ab | 7581 |
| 16 | patient discharge:ti,ab | 9326 |
| 17 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 | 94461 |
| 18 | [mh "intensive care units"] | 3462 |
| 19 | [mh "multiple trauma"] | 216 |
| 20 | [mh shock] | 1615 |
| 21 | [mh sepsis] | 3631 |
| 22 | [mh "critical illness"] | 1604 |
| 23 | [mh "critical care"] | 2193 |
| 24 | (after or post or discharge? or surviv* or follow* next up):ti,ab | 522685 |
| 25 | #18 or #19 or #20 or #21 or #22 or #23 | 10168 |
| 26 | #24 and #25 | 5056 |
| 27 | ((after or post or discharge or surviv* or follow* next up) near/5 (trauma or level 3 or level three)):ti,ab | 1514 |
| 28 | ((after or post or discharge? or surviv* or follow* next up) near/5 (critical* next (care or ill*))):ti,ab | 300 |
| 29 | ((after or post or discharge? or surviv* or follow* next up) near/5 (intensive next care or intensive next therapy or intensive next treatment or icu)):ti,ab | 1564 |
| 30 | ((after or post or discharge? or surviv* or follow* next up) near/5 (sepsis or septicaemi? or septicemi? or bacteremi? or bacteraemi? or fungaemi? or fungemi? or septic shock or pyaemi? or pyemi? or pyohemi? or blood next poison*)):ti,ab | 677 |
| 31 | ((after or post or discharge? or surviv* or follow* next up) near/5 (serious* next injur*)):ti,ab | 6 |
| 32 | ((after or post or discharge? or surviv* or follow* next up) near/5 (multiple next organ* next failure* or multiple next organ* next dysfunction)):ti,ab | 32 |
| 33 | ((after or post or discharge? or surviv* or follow* next up) near/5 (major next shock)):ti,ab | 0 |
| 34 | ((after or post or discharge? or surviv* or follow* next up) near/5 (multiple next (trauma or injur* or wound? or fracture?))):ti,ab | 31 |
| 35 | ((after or post or discharge? or surviv* or follow* next up) near/5 polytrauma):ti,ab | 10 |
| 36 | #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 | 8130 |
| 37 | #17 and #36 | 1131 |
| 38 | In trials | 1096 |
MEDLINE (Ovid) including Epub ahead of print, In‐process & Other non‐indexed citations and MEDLINE <1946 to present>
| 1 | aftercare/ | 8001 |
| 2 | exp counseling/ | 42071 |
| 3 | long‐term care/ | 25149 |
| 4 | patient discharge/ | 25827 |
| 5 | case management/ | 9904 |
| 6 | disease management/ | 31134 |
| 7 | (aftercare or after care or after treatment).ti,ab. | 162005 |
| 8 | (diary or diaries).ti,ab. | 21651 |
| 9 | counsel?ing.ti,ab. | 81832 |
| 10 | email?.ti,ab. | 5001 |
| 11 | telephone*.ti,ab. | 54395 |
| 12 | phone*.ti,ab. | 30298 |
| 13 | ((follow up or discharge) adj2 (appointment* or consultation* or clinic* or program* or strateg* or service?)).ti,ab. | 32135 |
| 14 | (recover* adj2 (appointment* or consultation* or clinic* or program* or strateg* or service?)).ti,ab. | 7977 |
| 15 | ((care or case or disease) adj management).ti,ab. | 28675 |
| 16 | patient discharge.ti,ab. | 1207 |
| 17 | or/1‐16 | 514825 |
| 18 | exp intensive care units/ | 75549 |
| 19 | exp multiple trauma/ | 12812 |
| 20 | exp shock/ | 72960 |
| 21 | exp sepsis/ | 116251 |
| 22 | exp critical illness/ | 24950 |
| 23 | exp critical care/ | 54690 |
| 24 | (after or post or discharge? or surviv* or follow* up).ti,ab. | 6050571 |
| 25 | or/18‐23 | 294460 |
| 26 | 24 and 25 | 95420 |
| 27 | ((after or post or discharge or surviv* or follow* up) adj5 (trauma or level 3 or level three)).ti,ab. | 30714 |
| 28 | ((after or post or discharge? or surviv* or follow* up) adj5 (critical* adj (care or ill*))).ti,ab. | 2818 |
| 29 | ((after or post or discharge? or surviv* or follow* up) adj5 (intensive care or intensive therapy or intensive treatment or icu)).ti,ab. | 14421 |
| 30 | ((after or post or discharge? or surviv* or follow* up) adj5 (sepsis or septicaemi? or septicemi? or bacteremi? or bacteraemi? or fungaemi? or fungemi? or septic shock or pyaemi? or pyemi? or pyohemi? or blood poison*)).ti,ab. | 12160 |
| 31 | ((after or post or discharge? or surviv* or follow* up) adj5 (serious* adj injur*)).ti,ab. | 170 |
| 32 | ((after or post or discharge? or surviv* or follow* up) adj5 (multiple organ* failure* or multiple organ* dysfunction)).ti,ab. | 864 |
| 33 | ((after or post or discharge? or surviv* or follow* up) adj5 (major adj shock)).ti,ab. | 0 |
| 34 | ((after or post or discharge? or surviv* or follow* up) adj5 (multiple adj (trauma or injur* or wound? or fracture?))).ti,ab. | 571 |
| 35 | ((after or post or discharge? or surviv* or follow* up) adj5 polytrauma).ti,ab. | 280 |
| 36 | or/26‐35 | 135021 |
| 37 | 17 and 36 | 6733 |
| 38 | randomized controlled trial.pt. | 498494 |
| 39 | controlled clinical trial.pt. | 99301 |
| 40 | multicenter study.pt. | 250271 |
| 41 | pragmatic clinical trial.pt. | 744 |
| 42 | (randomis* or randomiz* or randomly).ti,ab. | 808337 |
| 43 | groups.ab. | 1851829 |
| 44 | (trial or multicenter or multi center or multicentre or multi centre).ti. | 231013 |
| 45 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 8719683 |
| 46 | non‐randomized controlled trials as topic/ | 259 |
| 47 | interrupted time series analysis/ | 379 |
| 48 | controlled before‐after studies/ | 301 |
| 49 | or/38‐48 | 9740756 |
| 50 | exp animals/ | 22541187 |
| 51 | humans/ | 17855892 |
| 52 | 50 not (50 and 51) | 4685295 |
| 53 | review.pt. | 2450539 |
| 54 | meta analysis.pt. | 92508 |
| 55 | news.pt. | 189293 |
| 56 | comment.pt. | 726576 |
| 57 | editorial.pt. | 465444 |
| 58 | cochrane database of systematic reviews.jn. | 14562 |
| 59 | comment on.cm. | 726574 |
| 60 | (systematic review or literature review).ti. | 109746 |
| 61 | or/52‐60 | 8203801 |
| 62 | 49 not 61 | 6805800 |
| 63 | 37 and 62 | 3659 |
Embase (Ovid) <1974 to present>
| 1 | *aftercare/ | 2496 |
| 2 | *follow up/ | 30453 |
| 3 | *long term care/ | 19115 |
| 4 | *hospital discharge/ | 10717 |
| 5 | *disease management/ | 5347 |
| 6 | *case management/ | 4762 |
| 7 | (aftercare or after care or after treatment).ti,ab. | 209467 |
| 8 | (diary or diaries).ti,ab. | 30708 |
| 9 | counsel?ing.ti,ab. | 107800 |
| 10 | email?.ti,ab. | 11229 |
| 11 | telephone*.ti,ab. | 69032 |
| 12 | phone*.ti,ab. | 41772 |
| 13 | ((follow up or discharge) adj2 (appointment* or consultation* or clinic* or program* or strateg* or service?)).ti,ab. | 48699 |
| 14 | (recover* adj2 (appointment* or consultation* or clinic* or program* or strateg* or service?)).ti,ab. | 10327 |
| 15 | ((care or case or disease) adj management).ti,ab. | 36310 |
| 16 | patient discharge.ti,ab. | 1790 |
| 17 | or/1‐16 | 603291 |
| 18 | exp *intensive care unit/ | 34901 |
| 19 | *multiple trauma/ | 6633 |
| 20 | exp *shock/ | 51084 |
| 21 | exp *sepsis/ | 88888 |
| 22 | *multiple organ failure/ | 5263 |
| 23 | *critical illness/ | 10557 |
| 24 | exp *intensive care/ | 236979 |
| 25 | (after or post or discharge? or surviv* or follow* up).ti,ab. | 7611229 |
| 26 | or/18‐24 | 397085 |
| 27 | 25 and 26 | 142251 |
| 28 | ((after or post or discharge or surviv* or follow* up) adj5 (trauma or level 3 or level three)).ti,ab. | 36940 |
| 29 | ((after or post or discharge? or surviv* or follow* up) adj5 (critical* adj (care or ill*))).ti,ab. | 4356 |
| 30 | ((after or post or discharge? or surviv* or follow* up) adj5 (intensive care or intensive therapy or intensive treatment or icu)).ti,ab. | 23658 |
| 31 | ((after or post or discharge? or surviv* or follow* up) adj5 (sepsis or septicaemi? or septicemi? or bacteremi? or bacteraemi? or fungaemi? or fungemi? or septic shock or pyaemi? or pyemi? or pyohemi? or blood poison*)).ti,ab. | 17614 |
| 32 | ((after or post or discharge? or surviv* or follow* up) adj5 (serious* adj injur*)).ti,ab. | 205 |
| 33 | ((after or post or discharge? or surviv* or follow* up) adj5 (multiple organ* failure* or multiple organ* dysfunction)).ti,ab. | 1102 |
| 34 | ((after or post or discharge? or surviv* or follow* up) adj5 (major adj shock)).ti,ab. | 1 |
| 35 | ((after or post or discharge? or surviv* or follow* up) adj5 (multiple adj (trauma or injur* or wound? or fracture?))).ti,ab. | 669 |
| 36 | ((after or post or discharge? or surviv* or follow* up) adj5 polytrauma).ti,ab. | 374 |
| 37 | or/27‐36 | 198309 |
| 38 | 17 and 37 | 9022 |
| 39 | randomized controlled trial/ | 480672 |
| 40 | controlled clinical trial/ | 452801 |
| 41 | quasi experimental study/ | 4143 |
| 42 | pretest posttest control group design/ | 332 |
| 43 | time series analysis/ | 20419 |
| 44 | experimental design/ | 15081 |
| 45 | multicenter study/ | 170777 |
| 46 | (randomis* or randomiz* or randomly).ti,ab. | 1029216 |
| 47 | groups.ab. | 2373186 |
| 48 | (trial or multicentre or multicenter or multi centre or multi center).ti. | 288372 |
| 49 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 10527848 |
| 50 | or/39‐49 | 11742553 |
| 51 | (systematic review or literature review).ti. | 123907 |
| 52 | "cochrane database of systematic reviews".jn. | 6726 |
| 53 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 25514585 |
| 54 | human/ or normal human/ or human cell/ | 19214784 |
| 55 | 48 not (48 and 49) | 80230 |
| 56 | 51 or 52 or 55 | 210760 |
| 57 | 50 not 56 | 11574665 |
| 58 | 38 and 57 | 6156 |
CINAHL (Ebsco)
| S1 | (MH "After Care") | 9256 |
| S2 | (MH "Counseling+") | 28127 |
| S3 | (MH "Long Term Care") | 21775 |
| S4 | aftercare or after care or after treatment or diary or diaries or counsel* or email? or telephone* or phone* | 362086 |
| S5 | ((follow up or discharge) N2 (appointment* or consultation* or clinic* or program* or strateg* or service?)) | 9978 |
| S6 | (MH "patient discharge") | 12419 |
| S7 | (MH "case management") | 14758 |
| S8 | (MH "disease management") | 12642 |
| S9 | recover* N2 (appointment* or consultation* or clinic* or program* or strateg* or service?) | 2486 |
| S10 | ((care or case or disease) N0 management) | 35925 |
| S11 | patient discharge | 43812 |
| S12 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 | 449635 |
| S13 | (MH "Intensive Care Units+") | 44024 |
| S14 | (MH "Multiple Trauma") | 2653 |
| S15 | (MH "Multiple Organ Dysfunction Syndrome+") | 11182 |
| S16 | (MH "Sepsis+") | 19307 |
| S17 | (MH "Critical Illness") | 8606 |
| S18 | (MH "Critical Care+") | 21658 |
| S19 | S13 OR S14 OR S15 OR S16 OR S17 OR S18 | 86758 |
| S20 | after or post or discharge? or surviv* or follow* up | 857022 |
| S21 | S19 AND S20 | 23314 |
| S22 | ((after or post or discharge or surviv* or follow* up) N5 (trauma or level 3 or level three)) | 28498 |
| S23 | ((after or post or discharge? or surviv* or follow* up) N5 (critical* N0 (care or ill*))) | 1448 |
| S24 | ((after or post or discharge? or surviv* or follow* up) N5 (intensive care or intensive therapy or intensive treatment or icu)) | 9008 |
| S25 | ((after or post or discharge? or surviv* or follow* up) N5 (sepsis or septicaemi? or septicemi? or bacteremi? or bacteraemi? or fungaemi? or fungemi? or septic shock or pyaemi? or pyemi? or pyohemi? or blood poison*)) | 2391 |
| S26 | ((after or post or discharge? or surviv* or follow* up) N5 (serious* N0 injur*)) | 64 |
| S27 | ((after or post or discharge? or surviv* or follow* up) N5 (multiple organ* failure* or multiple organ* dysfunction)) | 439 |
| S28 | ((after or post or discharge? or surviv* or follow* up) N5 (major N0 shock)) | 0 |
| S29 | ((after or post or discharge? or surviv* or follow* up) N5 (major N0 shock)) | 0 |
| S30 | ((after or post or discharge? or surviv* or follow* up) N5 (multiple N0 (trauma or injur* or wound? or fracture?))) | 151 |
| S31 | ((after or post or discharge? or surviv* or follow* up) N5 polytrauma) | 59 |
| S32 | S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 | 56366 |
| S33 | S12 AND S32 | 29851 |
| S34 | PT randomized controlled trial | 57777 |
| S35 | PT clinical trial | 80067 |
| S36 | PT research | 1534160 |
| S77 | (MH "Randomized Controlled Trials") | 59401 |
| S38 | (MH "Clinical Trials") | 133382 |
| S39 | (MH "Intervention Trials") | 7169 |
| S40 | (MH "Nonrandomized Trials") | 254 |
| S41 | (MH "Experimental Studies") | 19334 |
| S42 | (MH "Pretest‐Posttest Design+") | 34009 |
| S43 | (MH "Quasi‐Experimental Studies+") | 10642 |
| S44 | (MH "Multicenter Studies") | 61668 |
| S45 | (MH "Health Services Research") | 11674 |
| S46 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 202670 |
| S47 | TI (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) OR AB (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 1372528 |
| S48 | S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 | 2258084 |
| S49 | S33 AND S48 | 25958 |
Appendix 2. Modified 'Risk of bias' tool
| Domain | Description | Review authors' judgement |
| Sequence generation | ||
| Allocation concealment | ||
| Blinding of participants and personnel | ||
| Blinding of outcome assessors | ||
| Incomplete outcome data | ||
| Selective reporting | ||
| Other sources of bias | ||
| Baseline outcomes | ||
| Contamination | ||
| Baseline characteristics | ||
| Intervention independent? (ITS) | ||
| Appropriate analysis? (ITS) | ||
| Shape of effect prespecified? (ITS) | ||
| Effect on data collection? (ITS) | ||
| Blinding (ITS) | ||
| Incomplete outcome data (ITS) | ||
| Selective reporting (ITS) | ||
| Other sources of bias (ITS) |
ITS: interrupted time series
Appendix 3. GRADE evidence profile
| ICU follow‐up services compared with standard care or no follow‐up service for survivors of critical illness | ||||||
| Quality assessment | Effect | |||||
| Number of studies and design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |
| Health‐related quality of life (assessed using EQ‐5D; reported at 12 months) | ||||||
| 1 randomised study | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | SMD ‐0.0, 95% CI ‐0.1 to 0.1 |
| All‐cause mortality (assessed at 2 months in 1 randomised study, at 12 months in 3 randomised studies, and at 14 months in 1 non‐randomised study) | ||||||
| 4 randomised studies 1 non‐randomised study |
No serious risk of bias | Seriousc | No serious indirectness | No serious imprecision | None | RR 0.96, 95% CI 0.76 to 1.22; 4 randomised studies;1289 analysed participants. In 1 non‐randomised study, number of deaths in the intervention group were: 79/259; and in the control group were: 46/151 |
| Depression and anxiety (assessed using HADS‐D and HADS‐A; at 12 months in 2 randomised studies, and 12 months in 1 non‐randomised study) | ||||||
| 2 randomised studies 1 non‐randomised study |
Seriousd | Seriouse | No serious indirectness | Seriousf | None |
For depression: SMD ‐0.1, 95% CI ‐1.2 to 1.0; and absolute risk reduction (usual care vs intervention) ‐0.20, 95% CI ‐1.12 to 0.72; 2 randomised studies. No difference in scores for depression (women: P = 0.09; men: P = 0.47) in 1 non‐randomised study For anxiety: SMD ‐0.8, 95% CI ‐1.9 to 0.4; and absolute risk reduction (usual care vs intervention) ‐0.21, 95% CI ‐1.22 to 0.80; 2 randomised trials. No difference in scores for anxiety (women: P = 0.14; men: P = 0.78) in 1 non‐randomised trial |
| Post‐traumatic stress disorder (PTSD) (assessed using DTS; HTQ‐IV, PTSS‐10 at 12 months in 3 randomised studies, and IES at 12 months in 1 non‐randomised study) | ||||||
| 3 randomised studies 1 non‐randomised study |
Seriousg | Serioush | No serious indirectness | No serious imprecision | None | SMD ‐0.05, 95% CI ‐0.19 to 0.10; 703 participants; 3 randomised studies. In 1 non‐randomised study, women who had received a follow‐up service had lower IES scores (indicating less chance of PTSD), (P = 0.01) |
| Physical function (assessed using PCS of SF‐36 at 12 months in 2 randomised studies, and at 2 months in 1 randomised study, and using SF‐8 at 6 months in 1 randomised study) | ||||||
| 4 randomised studies | Seriousi | Very seriousj | No serious indirectness | No serious imprecision | None | MD 1.31, 95% CI ‐0.86 to 3.49; 2 non‐randomised studies. Little or no difference in physical function at 12 months (P > 0.05) in 1 randomised study. Improved physical function at 2 months in participants who had received a follow‐up service (P = 0.02) in 1 randomised study |
| Cognitive function (assessed using MCS of SF‐36 at 12 months in 2 randomised studies, and at 2 months in 1 randomised study, and using SF‐8 at 6 months in 1 randomised study) | ||||||
| 4 randomised studies | Seriousi | Very seriousj | No serious indirectness | No serious imprecision | None | MD 1.44, 95% CI ‐0.51 to 3.39; 3 randomised studies. No difference in cognitive function at 2 months in 1 randomised study |
| Ability to return to work (at 12 months) | ||||||
| 1 randomised study | Seriousa | No serious inconsistency | No serious indirectness | Very seriousk | None | OR 1.06, 95% CI 0.35 to 3.21 |
| Adverse effects | ||||||
| Not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
aIntervention group received an additional therapy (manual‐based physiotherapy programme) which may have influenced outcome data. bOne study with few participants. cAnalysis was at different time points, and we noted some potential differences between studies in baseline characteristics between studies. dIntervention group in one study received an additional therapy (manual‐based physiotherapy programme), and one non‐randomised study had a high risk of selection bias. eOutcomes were measured at different time points, and we noted some baseline differences between studies. fEvidence from few studies. gIntervention group in one study received an additional therapy (manual‐based physiotherapy programme), and one non‐randomised study had a high risk of selection bias. hWe noted differences at baseline in one non‐randomised study (more women in control group had a previous history of psychological problems) which may have influenced results for this outcome. We noted inconsistent results between three combined randomised studies and one non‐randomised study. iIntervention group in one study received an additional therapy (manual‐based physiotherapy programme), and in another study intervention group were also involved in preparation of a discharge summary plan. One non‐randomised study had a high risk of selection bias. jOutcomes were measured at different time points, we noted some baseline differences between studies, and we noted a wide confidence interval in analysed data. kOne study with few participants and we noted a wide confidence interval.
Data and analyses
Comparison 1. Follow‐up service vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | 1289 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.22] |
| 2 Physical function | 2 | 422 | Mean Difference (IV, Fixed, 95% CI) | 1.31 [‐0.86, 3.49] |
| 3 Cognitive function | 3 | 622 | Mean Difference (IV, Fixed, 95% CI) | 1.44 [‐0.51, 3.39] |
| 4 PTSD | 3 | 703 | Std. Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.19, 0.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cuthbertson 2009.
| Methods | Randomised study Multicentre (3 centres: 2 teaching hospitals and 1 district general hospital; in the UK: high‐income country) Parallel design Participant as the unit of allocation |
|
| Participants | Total number of randomised patients: 286 Inclusion criteria: all patients receiving level 3 dependency (ICU) care at any time during their hospital stay and who survived until hospital discharge Exclusion criteria: patients < 18 years of age, not expected to survive to leave hospital, unable to complete questionnaires or attend clinics, and who did not consent to participate Baseline characteristics Follow‐up service group Age, median (IQR): 59 (46‐49) years (as reported by study authors, we assumed that there was a typo in these data) Gender, male (%): 86 (60) APACHE II, median IQR: 19 (15‐24) Reason for ICU admission: respiratory 48, cardiovascular 43, neurological 5, gastrointestinal 27, renal 5, metabolic/endocrine 2, haematological 0, trauma 13 HADS‐A, median (IQR): 7 (3‐10) HADS‐D, median (IQR): 6 (3‐9) SF‐36 mental, mean (SD): 40.9 (± 15.2) SF‐36 physical, mean (SD): 33.4 (± 10.0) EQ‐5D, median (IQR): 0.52 (0.26‐0.73) Length of ICU stay, median (IQR): 2.9 (1.7‐9.5) days Control group Age median (IQR): 60 (46‐71) years Gender male (%): 86 (60) APACHE II median (IQR): 19 (15‐24) Reason for ICU admission: respiratory 42, cardiovascular 42, neurological 11, gastrointestinal 27, renal 3, metabolic/endocrine 2, haematological 1, trauma 15 HADS‐A median (IQR): 7 (4‐10) HADS‐D median (IQR): 5 (3‐9) SF‐36 mental mean (SD): 41.4 (± 14.2) SF‐36 physical mean (SD): 32.6 (± 9.9) EQ‐5D, median (IQR): 0.49 (0.19‐0.69) Length of ICU stay, median (IQR): 3.1 (1.2‐7.5) days |
|
| Interventions |
Follow‐up service group Randomised participants = 143, analysed participants at 6 months = 105; analysed participants at 12 months = 92 Number of losses with reasons: 18 died; 6 formally withdrew; 16 lost to follow‐up. 6 did not complete questionnaire at 6 months but completed it at 12 months. Then at 12 months, 18 died, 11 formal withdrawal; 22 lost‐to follow‐up Description of service: participants were given a manual‐based, self‐directed, physical rehabilitation programme developed by a physiotherapist and introduced by a study nurse. Participants were formally reviewed at a face‐to‐face clinic, which included structured case review, discussion of experiences of the ICU, formal assessment of requirement for specialist medical referral, screening for psychological morbidity relating to admission to the ICU. Number and timing of follow‐up clinics: 2 clinics (1 at 3 months and 1 at 9 months after ICU discharge) Co‐ordinator of service: nurse‐led Number of participants in clinic attendance: 104 at 3 months; 94 at 9 months Number of carers or family members in clinic attendance at 3 months: 46; and at 9 months: 31 Subsequent referrals to other services: referrals made if required Control group Randomised participants = 143, analysed participants at 6 months = 115; analysed participants at 12 months = 100 Number of losses with reasons: 7 died; 15 lost to follow‐up; 6 did not complete questionnaire at 6 months but completed it at 12 months. Then at 12 months, 14 died; 2 formal withdrawal; 27 lost to follow‐up Description of service: follow‐up in accordance with standard clinical practice with no ICU follow‐up after hospital discharge. Participants followed up by GP and primary hospital specialty |
|
| Outcomes |
All outcomes measured by postal questionnaire |
|
| Notes |
Funding/declarations of interest: "the study is supported by a research grant from the Chief Scientist Office of the Scottish Government Health Directorates. The Health Services Research Unit is also funded by the Chief Scientist Office of the Scottish Government Health Directorates. The researchers are completely independent of the funders, and the views expressed are those of the authors alone. The study sponsor was the University of Aberdeen, which had no role in the study design; collection, analysis, and interpretation of data; writing of the article; or the decision to submit it for publication. The researchers are completely independent of the sponsors in their research activities." Study dates: September 2006‐October 2007 Note: study authors reported effect estimates that were adjusted for minimisation covariates (age, sex, HADS score, APACHE II score, ICE score and study centre. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised telephone randomisation service |
| Allocation concealment (selection bias) | Unclear risk | No evidence of attempts to conceal allocation |
| Blinding of participant and personnel (performance bias) | Unclear risk | Not feasible to blind personnel and participants to study. It is unclear whether this may have influenced performance |
| Blinding of outcome assessors (detection bias) | Unclear risk | Self‐reported outcome collection through completion of questionnaires. It is possible that this may have influenced outcome data because participants were aware of intervention. Researchers handling outcome data from questionnaires were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | High loss of participants, but this loss may be explained by illness severity of participants. Also, we noted some discrepancies with denominator data in outcome tables, and the number of analysed participants differed for each outcome |
| Selective reporting (reporting bias) | Unclear risk | Retrospective registration with clinical trials register: ISRCT24294750. Not feasible to judge risk of selective outcome reporting |
| Protection against contamination | Low risk | Standard NHS pathway, rigorously applied to ensure standardisation |
| Baseline characteristics | Unclear risk | Randomisation service incorporated baseline minimisation. We could not be certain whether ages were balanced between groups because we noted median age of participants in the intervention group included an error. |
| Other bias | High risk | Participants in the intervention group also received a manual‐based physiotherapy programme, which required participants to monitor their own compliance. Participants in the control group did not receive this. It is possible that this programme could have influenced results, rather than the clinic appointment |
Douglas 2007.
| Methods | Randomised study Single‐centre (950‐bed tertiary care facility; University Hospitals of Cleveland, USA; a high‐income country) Parallel design Participant as the unit of allocation |
|
| Participants | Total number of randomised patients: 334 Inclusion criteria: patients who required mechanical ventilation for > 72 h, at high risk for death or prolonged hospitalisation with multi‐organ dysfunction and continuing care needs after discharge from the hospital. No ventilator dependency before the index hospitalisations, and discharge location within 80 miles of the study site Exclusion criteria: hospice patients and patients who had received organ transplants and case management from the transplant team Baseline characteristics Follow‐up service group Age, mean (SD): 60.7 (± 16.6) years Gender, male (%): 100 (43.3) Ethnicity, n (%): 146 white (63.5) APACHE III, mean (SD): 56.6 (± 26.3) Reason for ICU admission: pulmonary disease 51, coronary artery disease 54, neurological abnormalities 46, other 80 SF‐8 mental, mean (SD): 41.9 (± 12.8) SF‐8 physical, mean (SD): 30.6 (± 8.7) Length of ICU stay, mean (SD): 17.3 (± 12.9) days Control group Age, mean (SD): 61.4 (± 16.1) years Gender, male (%): 47 (45.6) Ethnicity, n (%): 60 white (58.3) APACHE III, mean (SD): 63.8 (± 24.3) Reason for ICU admission: pulmonary disease 31, coronary artery disease 19, neurological abnormalities 13, other 40 SF‐8 mental, mean (SD): 42.9 (± 13.3) SF‐8 physical mean (SD): 35.8 (± 10.5) Length of ICU stay, mean (SD): 16.9 (± 14.9) days Pretreatment: note differences in APACHE III scores between groups. Also, HRQoL mean physical score at discharge is higher for the control group. |
|
| Interventions |
Follow‐up service group Randomised participants = 231, analysed participants = 180 Number of losses with reasons: died 43, dropped out 6, lost to follow‐up 2 Description of service: most participants received face‐to‐face follow‐up. Some participants received telephone follow‐up (52/231, 22.5%). Service was verbal. Number and timing of follow‐up clinics: meeting with participant and family before hospital discharge. Nurse completed a discharge summary plan, which was sent to all relevant out‐of‐hospital healthcare providers. Then participants received a visit within 48 h, and another visit within the first week, then at least weekly for next 3 weeks, and at least every other week for 4 weeks with minimum of 8 visits. Visits took place at participant's home or extended care facility and included case management activities relevant to the participant's condition and needs. Participants/carers had access to pager 24 h/day Co‐ordinator of service: nurse‐led (advance practice nurse) Number of participants who received follow‐up service: 180 Carers or family members were included in follow‐up service Subsequent referrals to other services were made Control group Randomised participants = 103, analysed participants = 67 Number of losses with reasons: died 20, dropped out 9, lost to follow‐up 7 Description of service: no contact with advanced practice nurse. Interviewed by study nurses within 2 weeks of discharge for completion of study instruments, then at 2 months after discharge for data collection. If advice was needed, participants were referred to their primary care provider, staff at extended care facility or home care agency |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: this study was funded by grant RO1‐NR0‐0527 from the National Institute of Nursing Research Study dates: March 2001‐December 2003 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation in ratio of 2:1, which was later changed to 4:1. No additional details |
| Allocation concealment (selection bias) | Unclear risk | No evidence of attempts to conceal allocation |
| Blinding of participant and personnel (performance bias) | Unclear risk | Not feasible to blind participants or nurses to intervention. It is unclear whether this may have influenced performance |
| Blinding of outcome assessors (detection bias) | Unclear risk | Self‐reported assessment at baseline. Study authors do not describe who assessed outcomes at 2 months, but we assumed outcomes were self‐reported |
| Incomplete outcome data (attrition bias) | Unclear risk | High number of participant loss, with more losses in the control group |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to assess risk of selective outcome reporting |
| Protection against contamination | Low risk | Follow‐up service was operated by trained nurses, and it is unlikely that the control group received the intervention |
| Baseline characteristics | High risk | Study authors acknowledge that baseline characteristics (APACHE III and HRQoL physical function) are unequal. |
| Other bias | High risk | Intervention began before participants were discharged from hospital. Participants/families in the intervention group were involved in discharge summary plan, which was circulated to all out‐of‐hospital teams. This may have influenced outcome data relative to other studies in which follow‐up started after discharge. |
Jensen 2016.
| Methods | Randomised study Multicentre (10 ICUs; in Denmark; a high‐income country) Parallel design Participant as the unit of allocation |
|
| Participants | Total number of randomised patients: 386 Inclusion criteria: Danish‐speaking adults (≥ 18 years of age) who had been mechanically ventilated ≥ 48 h and who did not meet criteria for baseline dementia. Exclusion criteria: participants, who were not oriented in personal data according to the verbal response in GCS, with detected delirium using CAM‐ICU at randomisation, or enrolled in other follow‐up studies Baseline characteristics Follow‐up service group Age, median (IQR): 66 (57.75‐73.5) years Gender, male (%): 112 (58.9) APACHE II, median (IQR): 25 (19.0‐30.3), SAPS II median (IQR): 44.5 (35.0‐54.3) Duration of sedation, median (IQR): 159.1 (83.5‐384.7) h Reason for ICU admission: neurological 12, respiratory 70, cardiovascular 26, gastrointestinal 21, renal 1, haematological 1, metabolic/endocrine 0, sepsis 56, trauma/intoxications 3 Days of delirium, median (IQR): 0 (1‐2) Length of ICU stay, median (IQR): 10 (5‐20) days Control group Age, median (IQR): 67.5 (58‐75) years Gender, male (%): 117 (59.7%) APACHE II, median (IQR): 24.5 (20.0‐30.0), SAPS II, median (IQR): 48.5 (39.3‐60.0) Reason for ICU admission: neurological 12, respiratory 70, cardiovascular 26, gastrointestinal 21, renal 1, haematological 1, metabolic/endocrine 0, sepsis 56, trauma/intoxications 3 Days of delirium, median (IQR): 0 (0‐1) Length of ICU stay, median (IQR): 9 (16‐18) days |
|
| Interventions |
Follow‐up service group Randomised participant = 190, analysed participants = 116 Number of losses with reasons: did not fulfil inclusion criteria 2, did not receive intervention 54, invalid questionnaire 20, died 53, did not respond for other reasons 64 Description of service: participants received an information pamphlet 'Life after ICU'. First, consultation at clinic with participant and close relative at 1‐3 months post‐ICU. Intention was to construct an illness narrative; dialogue was aided by using photographs of the participant taken by ICU nurses during participant recovery. Second and third consultations were at 5 and 10 months post‐ICU, by telephone; prior to these telephone calls participants completed a reflective sheet by finishing pre‐set sentences (e.g. "What I want most is...") Number and timing of follow‐up clinics: 3 clinics (1 face‐to face clinic at 3 months. Telephone calls at 5 and 10 months) Co‐ordinator of service: nurse‐led Number of participants in clinic attendance: 1st session: 136/190; 2nd session: 120/190, 3rd session: 110/190 Carers or family members were invited to attend clinic Subsequent referrals to other services were made Control group Randomised participants = 196, analysed participants = 119 Number of losses with reasons: did not fulfil inclusion criteria 5, did not receive intervention 3, invalid questionnaire 18, died 85, did not respond for other reasons 64 Description of service: ICU discharge without follow‐up |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: the study was supported by grants from the Danish Nursing Organization, The Novo Nordisk Foundation and Nordsjællands Hospital, University of Copenhagen, Denmark. None of these had any influence on the design or conduct of the study; data collection, data management, analysis, and interpretation of the data; or findings Study dates: December 2012‐December 2015 Note: study authors reported effect estimates that adjusted for study centres |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation, but no additional details |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed, opaque envelopes |
| Blinding of participant and personnel (performance bias) | Unclear risk | Not feasible to blind participants or personnel. It is unclear whether this may have influenced performance |
| Blinding of outcome assessors (detection bias) | Unclear risk | Unclear as to whether outcome assessors were blind, and some outcomes were self‐reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Large loss of participant data |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration NCT01721239. Outcomes are reported according to prepublished documents |
| Protection against contamination | Low risk | Limited risk of contamination based on details of intervention and professional delivery |
| Baseline characteristics | Low risk | Well‐balanced groups |
| Other bias | Low risk | No additional sources of bias identified |
Schandl 2012.
| Methods | Non‐randomised study (using a before‐after design) Single‐centre (general ICU; in Sweden; a high‐income country) Parallel design Participant as the unit of allocation |
|
| Participants | Total number of randomised patients: 410 Inclusion criteria: patients ≥ 16 years of age, treated for > 96 h in the general ICU Exclusion criteria: patients that did not speak Swedish and patients with no address Baseline characteristics (for those who received the questionnaire) Follow‐up service group Age, mean (SD): men 53 (± 17) years; women 52 (± 18) years Gender, male (%): 102 (65) APACHE II, mean (SD): men 23 (± 9); women 21 (± 8) Reason for ICU admission: participants categorised in terms of trauma, surgical, medical, infection Length of ICU stay, mean (SD): men: 11 (± 7) days; women: 10 (± 7) days Duration of sedation, median (IQR): men 3 (1‐6) h; women 3 (1‐5) h Control group Age, mean (SD): men: 52 (± 17) years; women: 54 (± 20.5) years Gender, male (%): 64 (63) APACHE II, mean (SD): men 21 (± 8); women 19 (± 10) Reason for ICU admission: participants categorised in terms of trauma, surgical, medical, infection Length of ICU stay, mean (SD): men 9 (± 7) days; women 9 (± 8) days Duration of sedation, median (IQR): men 2 (0‐4) h; women 2 (0‐4) h |
|
| Interventions |
Follow‐up service group Randomised participants = 259, analysed participants = 102 men and 54 women received questionnaire at 14 months, of which 98 participants responded Number of losses with reasons: 103 excluded or lost to follow‐up, only 98 responded to the questionnaire Description of service: face‐to‐face. Multidisciplinary follow‐up consultations in which participants met a nurse, physician, and physiotherapist from the general ICU. Location of consultation is not reported in the study report, but we assumed that these were in a hospital clinic setting. Number and timing of follow‐up clinics: within 1 week from ICU discharge, nurse visited participant on the ward. Then offered multidisciplinary follow‐up consultations at 3, 6, and 12 months after ICU Co‐ordinator of service: nurse and physician‐led Materials involved: the consultation involved re‐stating ICU care and treatment. Memories, delusions and/or nightmares identified with the ICU‐Memory‐Tool were discussed, functional status also assessed. Subsequent referrals to other services were made. Control group Number randomised: 151. Number analysed: receiving questionnaire at 14 months: 64 men, 38 women. 73 participants responded Number of losses with reasons: 49 lost to follow‐up and then only 73 responded to questionnaire Description of service: no ICU follow‐up was available. Participants were called for routine surgical or medical follow‐up consultations |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: grants from Lena and Per Sjöberg Research Foundation and the Karolinska University Hospital and Karolinska Institutet Committé of Strategic Research Study dates: January‐December 2006 for the control group, January 2007‐September 2008 for the intervention group Note: study aim was to compare psychological morbidity and treatment effects between men and women and all study results are reported by gender. Study authors reported median scores, with percentiles, which were unadjusted and adjusted (for age, length of ICU stay, and previous psychological problems); we reported adjusted percentile differences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised study with a before‐after study design |
| Allocation concealment (selection bias) | High risk | No randomisation process, therefore no group allocation concealment |
| Blinding of participant and personnel (performance bias) | Unclear risk | Not feasible to blind participants or personnel. It is unclear whether this may have influenced performance |
| Blinding of outcome assessors (detection bias) | Unclear risk | No evidence of blinding of outcome assessors. Self‐reported outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | High participant losses, adequately explained |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to assess risk of reporting bias |
| Protection against contamination | High risk | Risk of contamination high because of time period difference in control and intervention groups, during which other variables in service delivery may have changed |
| Baseline characteristics | High risk | More women had a previous psychological problem in the control group. We noted that ICU length of stay was longer in intervention group, and we noted some differences in types of diagnoses, and median duration of sedation. Also, we noted that baseline characteristics were only reported for those who received a questionnaire at 14 months post‐ICU discharge |
| Other bias | Low risk | We identified no other sources of bias |
Schmidt 2016.
| Methods | Randomised study Multicentre (9 ICUs; in Germany; a high‐income country) Parallel design Participant as the unit of allocation |
|
| Participants | Total number of randomised patients: 291 Inclusion criteria: adult (≥ 18 years of age) survivors of severe sepsis or septic shock, and were fluent in German Exclusion criteria: cognitive impairment as determined by a telephone interview of cognitive status Baseline characteristics Follow‐up service group Age, mean (SD): 62.1 (± 14.1) years Gender, male (%): 105 (70.9) Reason for ICU admission: sepsis SF‐36 mental, mean (SD): 48.8 (± 12.5) SF‐36 physical mean (SD): 25.9 (± 9.4) Length of ICU stay, mean (SD): 31.5 (± 27.7) days Control group Age, mean (SD): 61.2 (± 14.9) years Gender, male (%): 87 (61.3) Reason for ICU admission: sepsis SF‐36 mental mean (SD): 49.2 (± 12.6) SF‐36 physical mean (SD): 24.7 (± 8.0) Length of ICU stay, mean (SD): 35.2 (± 26.7) days |
|
| Interventions |
Follow‐up service group Randomised participants = 148, analysed participants at 6 months = 104 Number of losses with reasons: 32 withdrew from study, 4 missed the 6‐month follow‐up, 8 were excluded for missing data Description of service: structured, nurse‐led intervention post‐discharge aimed at identifying and dealing with likely sequelae of critical illness. Nurses were trained to identify sepsis sequelae, and monitored participants' symptoms using validated screening tools; problems were escalated with referrals if necessary. This was a primary care‐based intervention, involving training of participants and primary care providers, telephone monitoring. Number and timing of follow‐up clinics: initial training on sepsis sequelae 8 days post‐ICU discharge, then monthly telephone follow‐up for 6 months, then every 3 months for the subsequent 6 months Co‐ordinator of service: nurses Carer or family member were not invited to attend clinic because this was a telephone‐based service. Subsequent referrals to other services were made Control group Randomised participants = 143, analysed participants at 6 months = 96 Number of losses with reasons: 34 withdrew from study, 1 missed the 6‐month follow‐up, 11 were excluded for missing data Description of service: usual care by primary care provider |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: the study was supported by the CSCC, funded by the German Federal Ministry of Education and Research and the German Sepsis Society Study dates: February 2011‐December 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permutated blocks were used |
| Allocation concealment (selection bias) | Unclear risk | No evidence of attempts to conceal allocation |
| Blinding of participant and personnel (performance bias) | Unclear risk | Not feasible to blind participants or personnel. It is unclear whether this may have influenced performance |
| Blinding of outcome assessors (detection bias) | Unclear risk | Some outcomes were self‐reported. Not clear whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Moderate levels of patient attrition, but explained adequately |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trials registration; therefore, unclear whether bias has been introduced |
| Protection against contamination | Low risk | Limited risk of contamination based on details of intervention and professional delivery |
| Baseline characteristics | Low risk | No evidence of major baseline characteristics differences |
| Other bias | Low risk | No evidence of additional bias |
ADL: activities in daily living; APACHE II (or APACHE III): Acute Physiology and Chronic Health Evaluation II (or III); CAM‐ICU: Confusion Assessment Method for the intensive care unit; CSCC: Center for Sepsis Control and Care; DTS: Davidson Trauma Scale; EQ‐5D: Euroqol 5D; GCS: Glasgow Coma Score; GP: general practitioner; h: hour(s); HADS: Hospital Anxiety and Depression score; HADS‐A: Hospital Anxiety and Depression score for anxiety; HADS‐D: Hospital Anxiety and Depression score for depression; HRQoL: health‐related quality of life; HTQ‐IV: Harvard Trauma Questionnaire Part IV; ICE: intensive care experience; IES: Impact of Event Scale; ICU: intensive care unit; IQR: interquartile range; MCS: mental component score; n: number of participants; PCS: physical component score; PTSD: post‐traumatic stress disorder; PTSS‐10: Post Traumatic Symptom Scale; QALYs: quality of life years; SAPS: Simplified Acute Physiology Score; SD: standard deviation; SF‐36: Short Form‐36; SF‐8: Short Form‐8; SOC: Sense of Coherence
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alberto 2011 | Wrong intervention: liaison nurse providing education rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Backman 2010 | Wrong intervention: ICU diary study rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Ball 2003 | Wrong patient population: ward‐based patients, not ICU patients |
| Bourseau 2016 | Adult ICU patients (> 18 years of age), mechanically ventilated for ≥ 5 days. Participants were examined 1 month after ICU discharge by a multidisciplinary team. Study published as an abstract only, which contains insufficient information to justify inclusion. We attempted to contact the study authors by email (on 1 occasion), which was unsuccessful. We will reassess eligibility if this study is published in full, and if it is eligible, we will incorporate the study results in a future review update. |
| Cave 2016 | Adult patients, discharged from the ICU. Intervention includes an ICU follow‐up day clinic programme. Study published as an abstract only, which contains insufficient information to justify inclusion. We attempted to contact the study authors by email (on 1 occasion), which was unsuccessful. We will re‐assess eligibility if this study is published in full, and if it is eligible, we will incorporate the study results in a future review update. |
| Cox 2014 | Wrong intervention: specific psychotherapy intervention rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Cox 2017 | Wrong intervention: training programme rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Davidson 2015 | Adult ICU survivors with ARDS or septic shock, mechanically ventilated for > 24 h. Participants attended a structured clinic with a medication review consultation and an assessment of physical function and evaluation of ongoing issues related to their illness. Study published as an abstract only, which contains insufficient information to justify inclusion. We attempted to contact the study authors by email (on 1 occasion), which was unsuccessful. We will re‐assess eligibility if this study is published in full, and if it is eligible, we will incorporate the study results in a future review update. |
| Farazmand 2017 | Wrong patient population; CCU patients, and were exposed to level 2 care, instead of the level 3 care our review required. |
| Garrouste‐Orgeas 2010 | Wrong intervention: diary study rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Holmes 2007 | Wrong intervention: specific form of psychotherapy rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Huynh 2017 | Wrong intervention: diary study rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| IRCT201110197844N1 | Wrong intervention: educational package rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| ISRCTN97280643 | Wrong intervention: cognitive behavioural therapy rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Jackson 2012 | Wrong intervention: cognitive rehabilitation rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Jones 2003 | Wrong intervention: both groups received follow‐up service. Intervention group received self‐help manual rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Jones 2010 | Wrong intervention: ICU diary study rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Knowles 2009 | Wrong intervention: ICU diary study rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| NCT00976807 | Wrong intervention: education and physical rehabilitation programme rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| NCT02067559 | Wrong intervention: ICU diary and psychoeducation programme rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| NCT02415634 | Wrong intervention: education and rehabilitation programme rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Ramnarain 2015 | Patients who were treated in an ICU for > 5 days. Participants attended a post‐ICU aftercare clinic. Study published as an abstract only, which contains insufficient information to justify inclusion. We attempted to contact the study authors by email (on 1 occasion), which was unsuccessful. We will re‐assess eligibility if this study is published in full, and if it is eligible, we will incorporate the study results in a future review update. |
| Robson 2008 | Wrong intervention: ICU diary study rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Shaw 2012 | Wrong intervention: education and psychological support programme rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Strahan 2003 | Wrong intervention: education programme rather than a follow‐up service used to assess unmet health needs related to the ICU period |
| Walsh 2015 | Wrong intervention: rehabilitation programme rather than a follow‐up service used to assess unmet health needs related to the ICU period |
ARDS: acute respiratory distress syndrome; CCU: coronary care unit;ICU: intensive care unit
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000206426.
| Trial name or title | Survivors of intensive care with type two diabetes and the effect of shared care follow‐up clinics: the SWEET‐AS feasibility study |
| Methods | Randomised study, parallel design |
| Participants | Target number of participants: 80 Inclusion criteria: 18‐85 years of age, established pre‐admission diagnosis of type 2 diabetes mellitus, discharged from ICU after ≥ 5 days of ICU care. Exclusion criteria: distance from hospital to home < 50 kilometres, > 85 years of age, major psychiatric illness, anticipated to die within six months of ICU discharge, pregnancy |
| Interventions | All patients in the intervention group will receive a 10‐min telephone call from a research co‐ordinator or 1 of the investigators 2 weeks after hospital discharge as a reminder of the upcoming clinic appointment. During this telephone call, inquiries about significant hypoglycaemic (blood glucose level < 4 mmol/L) or hyperglycaemic (blood glucose level > 13 mmol/L) blood concentrations will be made. If necessary, changes in treatment will be instituted by the study diabetologist and recorded for each participant. Attendance at a shared care follow‐up clinic will occur 1 month after hospital discharge (+/‐ 14 days). Participants will be assessed by both an intensivist and a diabetologist at the clinic (2 separate 45‐min appointments with each staff member at a single clinic visit) |
| Outcomes | Study feasibility, anthropometric measurements, glycaemic control, distal peripheral neuropathy, cardiovascular autonomic neuropathy, nephropathy, HRQoL (using EQ‐5D and SF‐36), employment status, healthcare utilisation |
| Starting date | 14 February 2016 |
| Contact information | Dr Yasmine Ali Abdelhamid (yasmine.aliabdelhamid@sa.gov.au) |
| Notes | Feasibility study |
NCT01796509.
| Trial name or title | Multicenter randomised, controlled trial of a intensive care follow‐up programme in improving long‐term outcomes of ICU survivors |
| Methods | Randomised study, parallel design |
| Participants | Target number of participants: 600 Inclusion criteria: > 18 years of age, living in an area near the hospital, hospitalised in the ICU medical surgical hospitals in this study, required mechanical ventilation > 3 days, life expectancy > 1 year, having a GP identified, affiliated to a social health care, informed consent Exclusion criteria: patients hospitalised in ICU in the previous year, patients followed for a pre‐existing myopathy, burn patients, patients with brain injury (GCS < 8) or trauma, patients hospitalised for suicide or self‐induced poisoning, patients with psychiatric disorders, patients with dementia, pregnant women, patients who do not speak fluent French, patients with guardianship, homeless patients, having no GP identified |
| Interventions | In the intervention group, medical, psychological and social consultation will be planned within the first 7 days after inclusion, and then at 3, 6, and 12 months. During medical consultation a general examination will be performed, and muscle strength, cognitive function, and functional disabilities will be assessed. |
| Outcomes | Quality of life, anxiety and depression, social re‐insertion, economic healthcare costs |
| Starting date | December 2012 |
| Contact information | ‐ |
| Notes |
NCT02077244.
| Trial name or title | A randomised controlled trial to evaluate the effect of nurse led follow up after being a patient in the intensive care unit |
| Methods | Randomised study, parallel design |
| Participants | Target number of participants: 250 Inclusion criteria: adult patients with an ICU stay ≥ 24 h who speak and understand Norwegian and who are conscious and cognitively oriented at the time of inclusion Exclusion criteria: severe psychiatric disorder |
| Interventions | Nurse‐led follow‐up talks on the ward, and at 1 and 2 months later |
| Outcomes | Change from baselines measures for: PTSD, pain, HRQoL, sense of coherence, work participation |
| Starting date | March 2014 |
| Contact information | Kirsti Tøien (kirsti.toien@ous‐hf.no) |
| Notes |
NCT03124342.
| Trial name or title | Vanderbilt ICU recovery program pilot trial |
| Methods | Randomised study, parallel design |
| Participants | Target number of participants: 550 Inclusion criteria: patients > 18 years of age, admitted to the MICU at Vanderbilt University Medical Center for ≥ 48 h, who had an estimated risk of 30‐day same‐hospital readmission > 15%, and who were not previously enrolled on the study Exclusion criteria: long‐term residence at a skilled nursing facility, long‐term mechanical ventilation prior to admission, solid organ or stem cell transplantation, recorded primary residency > 200 miles from Vanderbilt, comfort care only |
| Interventions | 10‐component ICU recovery programme intervention, including: nurse practitioner in‐person visit at the time of transfer from the ICU; provision of an ICU recovery programme pamphlet describing post‐intensive care syndrome and providing online resources; performance of formal medication reconciliation at the time of transfer from the ICU, access to a dedicated 24‐h/day, 7‐day/week contact line; ICU recovery clinic visit medical examination, ICU recovery clinic medication reconciliation and counselling; ICU recovery clinic cognitive/mental health assessment and psychoeducation. A brief session of psychotherapy conducted by a clinical psychologist; ICU recovery clinic case management. A brief case management consultation; ICU recovery clinic patient‐centred consultation. A final consultation with patients and families by a physician; directed subspecialty referrals |
| Outcomes | Number of components of the ICU recovery programme received, same‐hospital readmission in the 30 days after hospital discharge, readmission‐free days, death or readmission in the 30 days after hospital discharge, number of same‐hospital emergency department visits in the 30 days after hospital discharge, number of same‐hospital outpatient clinic visits in the 30 days after hospital discharge, number of referrals to specialty providers |
| Starting date | 1 May 2017 |
| Contact information | Matthew W Semler (matthew.w.semler@vanderbilt.edu) |
| Notes |
Paratz 2014.
| Trial name or title | IMPOSE (improving outcomes after sepsis) ‐ the effect of a multidisciplinary follow‐up service on health‐related quality of life in patients postsepsis syndromes ‐ a double‐blinded randomised controlled trial: protocol |
| Methods | Randomised study, parallel design |
| Participants | Target number of participants: 204 Inclusion criteria: participants will be recruited from among patients being discharged from a quaternary university‐affiliated ICU at Royal Brisbane and Women's Hospital, Brisbane, Australia. Patients > 18 years of age, with a documented episode of sepsis, plus proven or strongly suspected infection, severe sepsis defined as sepsis plus organ failure, septic shock (defined as severe sepsis not responding to management) and requiring respiratory support for > 48 h Exclusion criteria: neurological injuries, spinal injuries and burns. Patients with haematological conditions or requiring palliative care post‐ICU. Patients with psychiatric and/or mental disabilities that preclude them from understanding the questionnaires, and non‐English speaking patients |
| Interventions | Participants in the intervention group will attend a follow‐up clinic twice a month for up to 6 months after discharge from the hospital. Screening instruments will be utilised on the first visit and appropriate management and referral provided. Following the results of the screening and team discussion, participants and/or carer will be referred to appropriate agencies. |
| Outcomes | HRQoL (using SF‐36), participants’ readmission rates to hospital (medical record data), mortality at 12 months and economics and healthcare resource use |
| Starting date | 3 June 2013 |
| Contact information | Dr Jennifer Paratz (j.paratz@uq.edu.au) |
| Notes |
EQ‐5D: Euroqol‐5D; GCS: Glasgow Coma Score; GP: general practitioner; HRQoL: health‐related quality of life;ICU: intensive care unit; MICU: medical intensive care unit; PTSD: post‐traumatic stress disorder; SF‐36: short form‐36
Differences between protocol and review
We made the following changes to the published protocol (Schofield‐Robinson 2017).
Methods (throughout): we planned to include interrupted time‐series studies and controlled before‐after studies. Because we did not find these study designs during our search, we removed plans of managing these studies from the review. If future updates include these study designs, we will incorporate methods published in the protocol.
Types of intervention: we altered the timing of the intervention for clarity. The published protocol stated that the service, "occurs at any time within six months of discharge" and we changed this to state that the service, "started" within six months. We found that included studies had follow‐up services that were ongoing up to 12 months after ICU discharge and it was not appropriate to exclude these studies as the design was appropriate for this review. We added extra exclusions (exclusion of rehabilitation services, and exclusion of assessment of diaries); these were not follow‐up services that included a consultation to identify and address unmet needs but, because these were offered to ICU survivors, we sought to clarify in this section that these studies were distinct from an ICU follow‐up service and were excluded from the review.
Types of outcomes: we altered the time point at which data were collected. The published protocol stated that we would "collect data for all outcomes at time points measured by study authors up to 12 months post‐ICU discharge". We changed this to collect data "at the final time point" reported by study authors.
Search methods: we did not search the following grey literature sources because we did not have access to these databases: Healthcare Management Information Consortium (HMIC); National Technical Information Service (NTIS); or Agency for Healthcare Research and Quality (AHRQ). We did not contact researchers with expertise in the field, and we did not conduct handsearching of journals and conference proceedings.
Data collection and analysis: despite our best efforts, we were unable to contact study authors of included studies for additional information. All data reported in the review were from published records. We did not perform sensitivity analysis, because of the nature of included studies. Planned sensitivity analyses were: restricting the analysis to published studies; restricting the analysis to studies with a low risk of selection bias; using available case data or using imputed data (from last observation carried forward) where studies have missing data.
Contributions of authors
Conceiving the review: PA
Designing the review: OSR
Co‐ordinating the review: OSR
Undertaking manual searches: PM, OSR, SL
Screening search results: OSR, SL
Organising retrieval of papers: OSR, SL
Screening retrieved papers against inclusion criteria: OSR, SL
Appraising quality of papers: OSR, SL
Abstracting data from papers: OSR, SL
Managing data for the review: OSR, SL
Entering data into Review Manager 5: OSR, SL
Analysing statistical data: OSR, SL
Interpreting data: all
Writing the review: OSR, SL
Providing general advice on the review: PA, AS, JM
Securing funding for the review: AS
Serving as guarantor for the review: AS
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant 13/89/16 ’Back to normal’: speed and quality of recovery after surgery, major injury and critical care
Declarations of interest
Oliver Schofield‐Robinson: no conflicts of interest
Sharon Lewis: no conflicts of interest
Andrew F Smith: no conflicts of interest
Joanne McPeake is the chief investigator on a Health Foundation study on rehabilitation after ICU stay. The study is funded by the Health Foundation, under the grant number 7672, and is run through NHS Greater Glasgow and Clyde. There are no additional interests to declare.
Phil Alderson: no conflicts of interest
New
References
References to studies included in this review
Cuthbertson 2009 {published data only}
- Cuthbertson BH, Rattray J, Campbell MK, Gager M, Roughton S, Smith A, et al. The PRaCTICaL study of nurse led, intensive care follow‐up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ 2009;339:b3723. [PUBMED: 19837741] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson BH, Rattray J, Johnston M, Wildsmith JA, Wilson E, Hernendez R, et al. A pragmatic randomised, controlled trial of intensive care follow up programmes in improving longer‐term outcomes from critical illness. The PRACTICAL study. BMC Health Services Research 2007;7:116. [PUBMED: 17645791] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RA, Jenkinson D, Vale L, Cuthbertson BH. Economic evaluation of nurse‐led intensive care follow‐up programmes compared with standard care: the PRaCTICaL trial. European Journal of Health Economics 2014;15(3):243‐52. [PUBMED: 23535984] [DOI] [PubMed] [Google Scholar]
- ISRCTN24294750. A pragmatic randomised, controlled trial of intensive care post‐discharge review clinics in improving longer‐term outcomes from critical illness. isrctn.com/ISRCTN24294750 (first received 21 December 2006).
Douglas 2007 {published data only}
- Douglas SL, Daly BJ, Kelley CG, O'Toole E, Montenegro H. Chronically critically ill patients: health‐related quality of life and resource use after a disease management intervention. American Journal of Critical Care 2007;16(5):447‐57. [PUBMED: 17724242] [PMC free article] [PubMed] [Google Scholar]
Jensen 2016 {published data only}
- Jensen JF, Egerod I, Bestle MH, Christensen DF, Elklit A, Hansen RL, et al. A recovery program to improve quality of life, sense of coherence and psychological health in ICU survivors: a multicenter randomized controlled trial, the RAPIT study. Intensive Care Medicine 2016;42(11):1733‐43. [PUBMED: 27695894] [DOI] [PubMed] [Google Scholar]
- Jensen JF, Overgaard D, Bestle MH, Christensen DF, Egerod I. Towards a new orientation: a qualitative longitudinal study of an intensive care recovery programme. Journal of Clinical Nursing 2017;26(1‐2):77‐90. [PUBMED: 27667681] [DOI] [PubMed] [Google Scholar]
- NCT01721239. Recovery and aftercare in post intensive care therapy patients ‐ RAPIT study [Recovery and aftercare in post intensive care therapy patients ‐ the RAPIT study. The effectiveness and experiences of a follow‐up program for Danish mechanically ventilated ICU patients: a pragmatic randomized controlled multicentre trial]. clinicaltrials.gov/ct2/show/NCT01721239 (first received 5 November 2012).
Schandl 2012 {published data only}
- Schandl A, Bottai M, Hellgren E, Sundin Ö, Sackey P. Gender differences in psychological morbidity and treatment in intensive care survivors ‐ a cohort study. Critical Care 2012;16:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2016 {published data only}
- Schmidt K, Worrack S, Korff M, Davydow D, Brunkhorst F, Ehlert U, et al. Effect of a primary care management intervention on mental health‐related quality of life among survivors of sepsis: a randomized clinical trial. JAMA 2016;315(24):2703‐11. [PUBMED: 27367877] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alberto 2011 {published data only}
- Alberto L, Canete A, Zotarez H, Baca Niklas JE, Martinez MC. Liaison nurse role development: intensive care unit discharged patients follow up during the start up period of an acute care facility. Intensive Care Medicine 2011;37:S267. [Google Scholar]
Backman 2010 {published data only}
- Backman CG, Orwelius L, Sjoberg F, Fredrikson M, Walther SM. Long‐term effect of the ICU‐diary concept on quality of life after critical illness. Acta Anaesthesiologica Scandinavica 2010;54(6):736‐43. [PUBMED: 20236095] [DOI] [PubMed] [Google Scholar]
Ball 2003 {published data only}
- Ball C, Kirkby M, Williams S. Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ 2003;327:1014. [PUBMED: 14593033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bourseau 2016 {published data only}
- Bourseau T, Fremondiere F, Dubus V, Gohier B, Gal D, Cave F, et al. Evaluation of disabilities and rehabilitation needs after critical illness: impact of an intensive care unit follow‐up clinic in the University Hospital of Angers. Annals of Physical and Rehabilitation Medicine 2016;59S:e152. [Google Scholar]
Cave 2016 {published data only}
- Cave F, Mercat A, Lerolle N. Implementation of an intensive care follow‐up clinic. Annals of Intensive Care. Conference: French Intensive Care Society, International Congress Reanimation. 2016; Vol. 6:no pagination.
Cox 2014 {published data only}
- Cox CE, Porter LS, Buck PJ, Hoffa M, Jones D, Walton B, et al. Development and preliminary evaluation of a telephone‐based mindfulness training intervention for survivors of critical illness. Annals of the American Thoracic Society 2014;11:173‐81. [PUBMED: 24303911] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2017 {published data only}
- Cox CE, Carson SS, Hough CL, Kahn J, White DB, Olsen MK, et al. Coping skills training to improve psychological distress among critical illness survivors: a randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2017;195:A6732. [DOI: ] [DOI] [PubMed] [Google Scholar]
Davidson 2015 {published data only}
- Davidson J, Files DC, Bakhru RN, Griffin K, Morris PE. The design and implementation of a MICU survivors' clinic: a fellow's journey starting from square one. American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS. 2015; Vol. 191:no pagination.
Farazmand 2017 {published data only}
- Farazmand J, Nasiripour AA, Raeissi P. The effect of telephone follow‐up programs after hospital discharge on hope and the quality of life in patients admitted to the coronary care unit (CCU). Journal of Babol University of Medical Sciences 2017;19:41‐6. [Google Scholar]
Garrouste‐Orgeas 2010 {published data only}
- Garrouste‐Orgeas M, Coquet I, Perier A, Timsit JF, Pochard F, Philippart F, et al. Impact of an ICU diary on family and patient's psychological symptoms after an ICU stay. Intensive Care Medicine 2010;36:S154. [PUBMED: 22584757]22584757 [Google Scholar]
Holmes 2007 {published data only}
- Holmes A, Hodgins G, Adey S, Menzel S, Danne P, Kossmann T, et al. Trial of interpersonal counselling after major physical trauma. Australian and New Zealand Journal of Psychiatry 2007;41:926‐33. [PUBMED: 17924246] [DOI] [PubMed] [Google Scholar]
Huynh 2017 {published data only}
- Huynh TG, Covalesky M, Sinclair S, Gunter H, Norton T, Chen A, et al. Measuring outcomes of an intensive care unit family diary program. AACN Advanced Critical Care 2017;28:179‐90. [PUBMED: 28592478] [DOI] [PubMed] [Google Scholar]
IRCT201110197844N1 {published data only}
- IRCT201110197844N1. Post intensive care unit patient's recovery [Assessing the impact of using a self help rehabilitation program on post ICU patient's health status in university hospitals of Kerman University of Medical Sciences]. apps.who.int/trialsearch/Trial3.aspx?trialid=IRCT201110197844N1 (first received 11 June 2014).
ISRCTN97280643 {published data only}
- ISRCTN97280643. Cognitive behavioural therapy (CBT) for the treatment of post‐traumatic stress disorder (PTSD) in intensive care unit (ICU) survivors [A randomised controlled pilot study of the effectiveness of recreating a coherent narrative of events using novel developments in cognitive and behaviour therapy for the treatment of post‐traumatic stress disorder in survivors of intensive care treatment]. www.isrctn.com/ISRCTN97280643 (first received 21 May 2010).
Jackson 2012 {published data only}
- Hoenig H, Morey M, Jackson J, Siebert C, Williams N, Clune J, et al. The RETURN trial: a pilot study of in‐home rehabilitation for ICU survivors. Journal of the American Geriatrics Society 2010;58:S8. [Google Scholar]
- Jackson JC, Ely EW, Morey MC, Anderson VM, Denne LB, Clune J, et al. Cognitive and physical rehabilitation of intensive care unit survivors: results of the RETURN randomized controlled pilot investigation. Critical care medicine 2012;40:1088‐97. [PUBMED: 22080631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2003 {published data only}
- Jones C, Skirrow P, Griffiths RD, Humphris GH, Ingleby S, Eddleston J, et al. Rehabilitation after critical illness: a randomized, controlled trial. Critical Care Medicine 2003;31:2456‐61. [PUBMED: 14530751] [DOI] [PubMed] [Google Scholar]
Jones 2010 {published data only}
- Jones C, Backman C, Capuzo M, Egerod I, Flaatten H, Granja C, et al. ICU diaries reduce posttraumatic stress disorder after critical illness: a randomised, controlled trial. Intensive Care Medicine 2009;35:S115. [Google Scholar]
- Jones C, Backman C, Capuzzo M, Egerod I, Flaatten H, Granja C, et al. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Critical Care 2010;14:R168. [PUBMED: 20843344] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knowles 2009 {published data only}
- Knowles RE, Tarrier N. Evaluation of the effect of prospective patient diaries on emotional well‐being in intensive care unit survivors: a randomized controlled trial. Critical Care Medicine 2009;37:184‐91. [PUBMED: 19050634] [DOI] [PubMed] [Google Scholar]
NCT00976807 {published data only}
- NCT00976807. Rehabilitation following critical illness [A randomised controlled trial of rehabilitation following critical illness: a short term feasibility and follow‐up pilot study]. clinicaltrials.gov/ct2/show/record/NCT00976807 (first received 11 September 2009).
NCT02067559 {published data only}
- NCT02067559. Preventing post‐traumatic stress in ICU survivors: a pilot randomized controlled trial of ICU diaries. clinicaltrials.gov/ct2/show/record/NCT02067559 (first received 18 February 2014).
NCT02415634 {published data only}
- NCT02415634. Rehabilitation after critical illness assisted discharge pack (RECAP) [Investigation of physiotherapy led ICU discharge facilitation using the rehabilitation after critical illness assisted discharge pack (RECAP) model; a pilot randomized controlled trial]. clinicaltrials.gov/ct2/show/record/NCT02415634 (first received 6 April 2015).
Ramnarain 2015 {published data only}
- Ramnarain D, Slobbe C, Schapendonk W, Gorp J, Gnirrip I, Voermans S, et al. Hospital anxiety and depression after ICU survival: results of a post‐ICU aftercare program. Critical Care 2015;19:S192. [PUBMED: 4470454]4470454 [Google Scholar]
Robson 2008 {published data only}
- Robson WP. An evaluation of patient diaries in intensive care. CONNECT: The World of Critical Care Nursing 2008;6:34‐7. [Google Scholar]
Shaw 2012 {published data only}
- Shaw RJ, Harvey J, Soar J, Gregory S, Morris M, Gunary R, et al. Posttraumatic stress symptoms in the intensive care unit: longitudinal course and effects of treatment intervention. American Journal of Respiratory and Critical Care Medicine 2012;185:A1079. [Google Scholar]
Strahan 2003 {published data only}
- Strahan E, McCormick J, Uprichard E, Nixon S, Lavery G. Immediate follow‐up after ICU discharge: establishment of a service and initial experiences. Nurse Critical Care 2003;8:49‐55. [PUBMED: 12737188] [DOI] [PubMed] [Google Scholar]
Walsh 2015 {published data only}
- ISRCTN09412438. The RECOVER study: rehabilitation after intensive care [Evaluation of a rehabilitation complex intervention for patients following intensive care discharge. The RECOVER study]. www.isrctn.com/ISRCTN09412438 (first received 24 August 2010).
- Walsh TS, Salisbury LG, Boyd J, Ramsay P, Merriweather J, Huby G, et al. A randomised controlled trial evaluating a rehabilitation complex intervention for patients following intensive care discharge: the RECOVER study. BMJ Open 2012;2(4):pii: e001475. [PUBMED: 22761291] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TS, Salisbury LG, Merriweather JL, Boyd JA, Griffith DM, Huby G, et al. Increased hospital‐based physical rehabilitation and information provision after intensive care unit discharge: the RECOVER randomized clinical trial. JAMA internal medicine 2015;175:901‐10. [PUBMED: 25867659] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616000206426 {published data only}
- ACTRN12616000206426. Survivors of intensive care with type 2 diabetes and the effect of shared care follow‐up clinics: the SWEET‐AS feasibility study [The effect of shared care follow‐up clinics on survivors of intensive care with type 2 diabetes: a randomised controlled feasibility study]. anzctr.org.au/Trial/Registration/TrialReview (first received 16 February 2016).
NCT01796509 {published data only}
- NCT01796509. Study in intensive care follow‐up programme in improving long‐term outcomes of ICU survivors [Multicenter randomised, controlled trial of a intensive care follow‐up programme in improving long‐term outcomes of ICU survivors]. clinicaltrials.gov/ct2/show/NCT01796509 (first received 21 February 2013).
NCT02077244 {published data only}
- NCT02077244. Follow up after intensive care. The FUT study [Randomized controlled trial to evaluate the effect of nurse led follow up after being a patient in the intensive care unit]. clinicaltrials.gov/ct2/show/NCT02077244 (first received 4 March 2014).
NCT03124342 {published data only}
- NCT03124342. Vanderbilt ICU recovery program pilot trial. clinicaltrials.gov/ct2/show/NCT03124342 (first received 21 April 2017).
Paratz 2014 {published data only}
- Paratz JD, Kenardy J, Mitchell G, Comans T, Coyer F, Thomas P, et al. IMPOSE (IMProving Outcomes after Sepsis)‐the effect of a multidisciplinary follow‐up service on health‐related quality of life in patients postsepsis syndromes‐a double‐blinded randomised controlled trial: protocol. BMJ Open 2014;4(5):e004966. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aitken 2015
- Aitken LM, Marshall AP. Monitoring and optimising outcomes of survivors of critical illness. Intensive & Critical Care Nursing 2015;31(1):1‐9. [PUBMED: 25466983] [DOI] [PubMed] [Google Scholar]
Angus 2003
Audit Commission 1999
- Audit Commission. Critical to success: the place of efficient and effective critical care services within the acute hospital. www.wales.nhs.uk/sites3/Documents/768/CriticalToSuccess.pdf 1999 (accessed prior to 10 June 2017).
Bagshaw 2015
- Bagshaw SM, Stelfox HT, Johnson JA, McDermid RC, Rolfson DB, Tsuyuki RT, et al. Long‐term association between frailty and health‐related quality of life among survivors of critical illness: a prospective multicenter cohort study. Critical Care Medicine 2015;43(5):973‐82. [PUBMED: 25668751] [DOI] [PubMed] [Google Scholar]
Brazier 1993
- Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF‐36 Health Survey Questionnaire. Quality of Life Research 1993;2(3):169‐80. [PUBMED: 8401453] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Ltd. Covidence. Melbourne: Veritas Health Innovation Ltd, 2017.
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ (Clinical Research Ed.) 2008;337:a1655. [PUBMED: 18824488] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuthbertson 2003
Cuthbertson 2005
- Cuthbertson BH, Scott J, Strachan M, Kilonzo M, Vale L. Quality of life before and after intensive care. Anaesthesia 2005;60(4):332‐9. [PUBMED: 15766335] [DOI] [PubMed] [Google Scholar]
Cuthbertson 2010
- Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Critical Care 2010;14(1):R6. [PUBMED: 20089197] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 2002
- Davidson JR, Tharwani HM, Connor KM. Davidson Trauma Scale (DTS): normative scores in the general population and effect sizes in placebo‐controlled SSRI trials. Depression and anxiety 2002;15(2):75‐8. [PUBMED: 11891997] [DOI] [PubMed] [Google Scholar]
Davydow 2008a
- Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. General Hospital Psychiatry 2008;30(5):421‐34. [PUBMED: 18774425] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davydow 2008b
- Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosomatic Medicine 2008;70:512‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davydow 2008c
- Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Medicine 2009;35:796‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Eddleston 2000
- Eddleston JM, White P, Guthrie E. Survival, morbidity, and quality of life after discharge from intensive care. Critical Care Medicine 2000;28(7):2293‐9. [PUBMED: 10921555] [DOI] [PubMed] [Google Scholar]
Egerod 2013
- Egerod I, Risom SS, Thomsen T, Storli SL, Eskerud RS, Holme AN, et al. ICU‐recovery in Scandinavia: a comparative study of intensive care follow‐up in Denmark, Norway and Sweden. Intensive and Critical Care Nursing 2013;29:103‐11. [DOI] [PubMed] [Google Scholar]
Ely 2001
- Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM‐ICU). Critical Care Medicine 2001;29:1370‐8. [DOI] [PubMed] [Google Scholar]
EPOC 2009
- Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for Authors. Oslo: Norwegian Knowledge Centre for the Health Services 2009 Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2013a
- Effective Practice, Organisation of Care (EPOC). Data collection form. EPOC Resources for Authors. Oslo: Norwegian Knowledge Centre for the Health Services 2013 Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2013b
- Effective Practice, Organisation of Care (EPOC). EPOC worksheets. EPOC Resources for Review Authors. Oslo: Norwegian Knowledge Centre for the Health Services 2013. Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
Gordon 2004
- Gordon SM, Jackson JC, Ely EW, Burger C, Hopkins RO. Clinical identification of cognitive impairment in ICU survivors: insights for intensivists. Intensive Care Medicine 2004;30:1997‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org. GRADEpro Guideline Development Tool. Hamilton: McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org, 2015.
Griffiths 2006
- Griffiths JA, Barber VS, Cuthbertson BH, Young JD. A national survey of intensive care follow‐up clinics. Anaesthesia 2006;61(10):950‐5. [PUBMED: 16978309] [DOI] [PubMed] [Google Scholar]
Griffiths 2007
- Griffiths RD, Jones C. Seven lessons from 20 years of follow‐up of intensive care unit survivors. Current Opinion in Critical Care 2007;13:508‐13. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
ICNARC 2016
- Intensive Care National Audit and Research Centre. Key statistics from the Case Mix Programme ‐ adult, general critical care units. www.icnarc.org/Our‐Audit/Audits/Cmp/Reports/Summary‐Statistics 2016 (accessed prior to 10 June 2017).
Intensive Care Society 2009
- Intensive Care Society. Levels of critical care for adult patients. www2.rcn.org.uk/__data/assets/pdf_file/0005/435587/ICS_Levels_of_Critical_Care_for_Adult_Patients_2009.pdf 2009 (accessed prior to 10 June 2017).
Jensen 2015
- Jensen JF, Thomsen T, Overgaard D, Bestle MH, Christensen D, Egerod I. Impact of follow‐up consultations for ICU survivors on post‐ICU syndrome: a systematic review and meta‐analysis. Intensive Care Medicine 2015;41(5):763‐75. [PUBMED: 25731633] [DOI] [PubMed] [Google Scholar]
Jones 1998
- Jones C, Humphris GM, Griffiths RD. Psychological morbidity following critical illness ‐ the rationale for care after intensive care. Clinical Intensive Care 1998;9:199‐205. [Google Scholar]
Jónasdóttir 2018
- Jónasdóttir RJ, Jónsdóttir H, Gudmundsdottir BJ, Sigurdsson GH. Psychological recovery after intensive care: outcomes of a long‐term quasi‐experimental study of structured nurse‐led follow‐up. Intensive and Critical Care Nursing 2018;4:59‐66. [DOI] [PubMed] [Google Scholar]
Khan 2015
- Khan BA, Lasiter S, Boustani MA. Critical Care Recovery Center: an innovative collaborative care model for ICU survivors. American Journal of Nursing 2015;115:24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
King's Fund 1989
- King's Fund Panel. Intensive care in the United Kingdom: report from the King's Fund panel. Anaesthesia 1989;44(5):428‐31. [PUBMED: 2500864] [DOI] [PubMed] [Google Scholar]
Knaus 1985
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical Care Medicine 1985;13(10):818‐29. [PUBMED: 3928249] [PubMed] [Google Scholar]
Lasiter 2016
- Lasiter S, Oles SK, Mundell J, London S, Khan B. Critical care follow‐up clinics: a scoping review of interventions and outcomes. Clinical Nurse Specialist 2016;30:227‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehlhorn 2014
- Mehlhorn J, Freytag A, Schmidt K, Brunkhorst FM, Graf J, Troitzsch U, et al. Rehabilitation interventions for postintensive care syndrome: a systematic review. Critical Care Medicine 2014;42(5):1263‐71. [PUBMED: 24413580] [DOI] [PubMed] [Google Scholar]
Mollica 1992
- Mollica RF, Caspi‐Yavin Y, Bollini P, Truong T, Tor S, Lavelle J. The Harvard Trauma Questionnaire. Validating a cross‐cultural instrument for measuring torture, trauma, and posttraumatic stress disorder in Indochinese refugees. Journal of Nervous and Mental Disease 1992;180(2):111‐6. [PUBMED: 1737972] [PubMed] [Google Scholar]
Needham 2011
Needham 2012
- Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Critical Care Medicine 2012;40(2):502‐9. [PUBMED: 21946660] [DOI] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Clinical Excellence. Rehabilitation after critical illness in adults. NICE Clinical Guideline (CG83) 2009. [www.nice.org.uk/guidance/cg83] [PubMed]
Niven 2014
- Niven DJ, Bastos JF, Stelfox HT. Critical care transition programs and the risk of readmission or death after discharge from an ICU: a systematic review and meta‐analysis. Critical Care Medicine 2014;42(1):179‐87. [PUBMED: 23989177] [DOI] [PubMed] [Google Scholar]
Oeyen 2010
- Oeyen SG, Vandijck DM, Benoit DD, Annemans L, Decruyenaere JM. Quality of life after intensive care: a systematic review of the literature. Critical Care Medicine 2010;38(12):2386‐400. [PUBMED: 20838335] [DOI] [PubMed] [Google Scholar]
Pandharipande 2013
- Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long‐term cognitive impairment after critical illness. New England Journal of Medicine 2013;369(14):1306‐16. [PUBMED: 24088092] [DOI] [PMC free article] [PubMed] [Google Scholar]
RAND
- RAND. 36‐Item Short Form Survey (SF‐36), Medical Outcomes Study (MOS). www.rand.org/health/surveys_tools/mos/36‐item‐short‐form.html (accessed prior to 10 June 2017).
Raphael 1989
- Raphael B, Lundin T, Weisaeth L. A research method for the study of psychological and psychiatric aspects of disaster. Acta Psychiatrica Scandinavica. Supplementum 1989;353:1‐75. [PUBMED: 2816476] [DOI] [PubMed] [Google Scholar]
Rattray 2007
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
SICSAG
- The Scottish Intensive Care Society Audit Group. Audit of critical care in Scotland 2015, reporting on 2014. www.icnarc.org/Our‐Audit/Audits/Cmp/Reports/Summary‐Statistics 2016 (accessed 22 December 2017).
Society of Critical Care Medicine
- Society of Critical Care Medicine. THRIVE Peer Support Collaborative. www.sccm.org/Research/Quality/thrive/Pages/default.aspx (accessed 27 December 2017).
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI: 10.1136/bmj.d4002] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Svenningsen 2017
- Svenningsen H, Langhorn L, Agard AS, Dreyer P. Post‐ICU symptoms, consequences, and follow‐up: an integrative review. Nursing in Critical Care 2017;22(4):212‐20. [PUBMED: 25688675] [DOI] [PubMed] [Google Scholar]
Ullman 2014
- Ullman AJ, Aitken LM, Rattray J, Kenardy J, Brocque R, MacGillivray S, et al. Diaries for recovery from critical illness. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD010468.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Schaaf 2009
- Schaaf M, Beelen A, Dongelmans DA, Vroom MB, Nollet F. Functional status after intensive care: a challenge for rehabilitation professionals to improve outcome. Journal of Rehabilitation Medicine 2009;41(5):360‐6. [PUBMED: 19363570] [DOI] [PubMed] [Google Scholar]
Weiss 1996
- Weiss DS, Marmar CR. The impact of event scale ‐ revised. In: Wilson J, Keane TM editor(s). Assessing psychological trauma and PTSD. New York: Guilford, 1996:399‐411. [Google Scholar]
Williams 2005
- Williams TA, Dobb GJ, Finn JC, Webb SA. Long‐term survival from intensive care: a review. Intensive Care Medicine 2005;31(10):1306‐15. [PUBMED: 16132895] [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams TA, Leslie GD. Beyond the walls: a review of ICU clinics and their impact on patient outcomes after leaving hospital. Australian Critical Care 2008;21(1):6‐17. [PUBMED: 18206381] [DOI] [PubMed] [Google Scholar]
Williams 2011
- Williams TA, Leslie GD. Challenges and possible solutions for long‐term follow‐up of patients surviving critical illness. Australian Critical Care 2011;24(3):175‐85. [PUBMED: 21514838] [DOI] [PubMed] [Google Scholar]
World Bank 2016
- World Bank. World Development Indicators. data.worldbank.org/data‐catalog/world‐development‐indicators 2016 (accessed prior to 10 June 2017).
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(3):361‐70. [PUBMED: 6880820] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schofield‐Robinson 2017
- Schofield‐Robinson OJ, Lewis SR, Smith AF, McPeake J, Alderson P. Follow‐up services for improving long‐term outcomes in intensive care unit (ICU) survivors. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD012701] [DOI] [PMC free article] [PubMed] [Google Scholar]


