Abstract
Background
Biocompatible peritoneal dialysis (PD) solutions, including neutral pH, low glucose degradation product (GDP) solutions and icodextrin, have previously been shown to favourably influence some patient‐level outcomes, albeit based on generally sub‐optimal quality studies. Several additional randomised controlled trials (RCT) evaluating biocompatible solutions in PD patients have been published recently. This is an update of a review first published in 2014.
Objectives
This review aimed to look at the benefits and harms of biocompatible PD solutions in comparison to standard PD solutions in patients receiving PD.
Search methods
The Cochrane Kidney and Transplant Specialised Register was searched up to 12 February 2018 through contact with the Information Specialist using search terms relevant to this review. Studies in the Specialised Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register Search Portal and ClinicalTrials.gov.
Selection criteria
All RCTs and quasi‐RCTs in adults and children comparing the effects of biocompatible PD solutions (neutral pH, lactate‐buffered, low GDP; neutral pH, bicarbonate(± lactate)‐buffered, low GDP; glucose polymer (icodextrin)) in PD were included. Studies of amino acid‐based solutions were excluded.
Data collection and analysis
Two authors extracted data on study quality and outcomes. Summary effect estimates were obtained using a random‐effects model, and results were expressed as risk ratios and 95% confidence intervals (CI) for categorical variables, and mean differences (MD) or standardised mean differences (SMD) and 95% CI for continuous variables.
Main results
This review update included 42 eligible studies (3262 participants), including six new studies (543 participants). Overall, 29 studies (1971 participants) compared neutral pH, low GDP PD solution with conventional PD solution, and 13 studies (1291 participants) compared icodextrin with conventional PD solution. Risk of bias was assessed as high for sequence generation in three studies, allocation concealment in three studies, attrition bias in 21 studies, and selective outcome reporting bias in 16 studies.
Neutral pH, low GDP versus conventional glucose PD solution
Use of neutral pH, low GDP PD solutions improved residual renal function (RRF) preservation (15 studies, 835 participants: SMD 0.19, 95% CI 0.05 to 0.33; high certainty evidence). This approximated to a mean difference in glomerular filtration rate of 0.54 mL/min/1.73 m2 (95% CI 0.14 to 0.93). Better preservation of RRF was evident at all follow‐up durations with progressively greater preservation observed with increasing follow up duration. Neutral pH, low GDP PD solution use also improved residual urine volume preservation (11 studies, 791 participants: MD 114.37 mL/day, 95% CI 47.09 to 181.65; high certainty evidence). In low certainty evidence, neutral pH, low GDP solutions may make little or no difference to 4‐hour peritoneal ultrafiltration (9 studies, 414 participants: SMD ‐0.42, 95% CI ‐0.74 to ‐0.10) which approximated to a mean difference in peritoneal ultrafiltration of 69.72 mL (16.60 to 122.00 mL) lower, and may increase dialysate:plasma creatinine ratio (10 studies, 746 participants: MD 0.01, 95% CI 0.00 to 0.03), technique failure or death compared with conventional PD solutions. It is uncertain whether neutral pH, low GDP PD solution use led to any differences in peritonitis occurrence, hospitalisation, adverse events (6 studies, 519 participants) or inflow pain (1 study, 58 participants: RR 0.51, 95% CI 0.24 to 1.08).
Glucose polymer (icodextrin) versus conventional glucose PD solution
In moderate certainty evidence, icodextrin probably reduced episodes of uncontrolled fluid overload (2 studies, 100 participants: RR 0.30, 95% CI 0.15 to 0.59) and augmented peritoneal ultrafiltration (4 studies, 102 participants: MD 448.54 mL/d, 95% CI 289.28 to 607.80) without compromising RRF (4 studies, 114 participants: SMD 0.12, 95% CI ‐0.26 to 0.49; low certainty evidence) which approximated to a mean creatinine clearance of 0.30 mL/min/1.73m2 higher (0.65 lower to 1.23 higher) or urine output (3 studies, 69 participants: MD ‐88.88 mL/d, 95% CI ‐356.88 to 179.12; low certainty evidence). It is uncertain whether icodextrin use led to any differences in adverse events (5 studies, 816 participants) technique failure or death.
Authors' conclusions
This updated review strengthens evidence that neutral pH, low GDP PD solution improves RRF and urine volume preservation with high certainty. These effects may be related to increased peritoneal solute transport and reduced peritoneal ultrafiltration, although the evidence for these outcomes is of low certainty due to significant heterogeneity and suboptimal methodological quality. Icodextrin prescription increased peritoneal ultrafiltration and mitigated uncontrolled fluid overload with moderate certainty. The effects of either neutral pH, low GDP solution or icodextrin on peritonitis, technique survival and patient survival remain uncertain and require further high quality, adequately powered RCTs.
Plain language summary
Biocompatible dialysis fluids for peritoneal dialysis
What is the issue?
Peritoneal dialysis is a form of dialysis therapy for people with kidney failure delivered at home. Patients are required to use peritoneal dialysis solutions to perform the dialysis by putting solution in their abdomen. Peritoneal dialysis uses the lining of the abdomen called the “peritoneal membrane” as a filter, across which toxins and fluids are removed from the body. The longevity of peritoneal dialysis can be limited by peritoneal membrane injury, which is partly as a result of biologically 'unfriendly' peritoneal dialysis solutions, which are acidic and consist of high levels of glucose and toxic glucose breakdown products. To overcome these hurdles, biocompatible peritoneal dialysis solutions (i.e. with a neutral pH and low levels of glucose breakdown products or with a glucose‐alternative like icodextrin) have been manufactured with the aim of providing patient benefit.
What did we do?
We conducted a review of the literature to examine the benefits and harms from the use of biocompatible peritoneal dialysis solutions.
What did we find?
We identified 42 studies (3262 participants) examining the effects of these solutions on patient outcomes. When compared to conventional peritoneal dialysis solutions, we found that neutral pH, low glucose breakdown product peritoneal dialysis solutions resulted in better preservation of a patient's own kidney function including urine output. Patients who received non‐glucose based (icodextrin) peritoneal dialysis solutions achieved greater fluid removal with their dialysis and were 70% less likely to experience uncontrolled episodes of fluid overload. No significant harms were identified with any of the biocompatible peritoneal dialysis solutions. Many of the studies were limited by small size, short follow‐up duration, suboptimal methodological quality, and inconsistent reporting of outcomes. Consequently, the effects of biocompatible peritoneal dialysis solutions on the length of time that a patient is able to either remain on peritoneal dialysis or stay alive are uncertain.
Conclusions Compared with peritoneal dialysis patients treated with conventional peritoneal dialysis solutions, those treated with biocompatible solutions experience important benefits including better preservation of their own kidney function and urine volume with neutral pH, low glucose breakdown product peritoneal dialysis solutions and more effective prevention of fluid overload due to increased dialysis‐related fluid removal with icodextrin. Whether these benefits help patients to stay on peritoneal dialysis longer or live longer are uncertain and require further study.
Summary of findings
Background
Description of the condition
Peritoneal dialysis (PD) is a widely utilised and highly cost‐effective method of renal replacement therapy that is practised by approximately 11% of the world’s population (Li 2017). Its annual global growth rate of 8% per annum exceeds that of haemodialysis (HD) (6% to 7% per annum) (Li 2017) and public policy changes in some parts of the world (e.g. Thailand and USA) have triggered unprecedented expansion of the therapy (Mehrotra 2016). Compared with HD, PD offers a number of advantages including better preservation of residual renal function (RRF), improved quality of life, greater treatment satisfaction, improved ability to provide incremental dialysis, deferred need for vascular access creation, reduced bacteraemia risk, reduced hepatitis transmission, less restricted diet and lifestyle, greater ability to travel, and possibly superior survival in the first few years after dialysis (Nadeau‐Fredette 2015). Additional advantages in lower income countries include reduced requirements for technical support and electricity, treatment simplicity, lesser need for trained clinical staff and lower propensity to be adversely affected by natural disasters (Li 2017). Improvements in PD patient survival have outstripped those of HD patients and progressive technical innovations have allowed patients to remain on PD for longer periods of time (Li 2017). However, the majority of PD patients ultimately transfer to HD after a few years, primarily because of infection, membrane failure, mechanical complications or patient burnout (Lan 2016).
A significant factor underpinning PD technique failure is the development of structural and functional changes of the peritoneal membrane with increasing PD duration (Williams 2003). These changes include thickening of the submesothelial compact collagenous zone and subendothelial hyalinisation of post‐capillary venules, with obliteration or narrowing of the vascular lumen (Williams 2003). The risk of encapsulating peritoneal sclerosis also increases with longer duration of PD (Brown 2009; Brown 2017; Johnson 2010; Rigby 1998). Progressive damage to the peritoneal membrane contributes to inadequate solute clearance, ultrafiltration (UF) failure, and change in peritoneal membrane transport properties, thereby affecting the ability of the membrane to function adequately. Loss of RRF also contributes to both UF failure and reduced solute clearance and is a predictor of increased death (Bargman 2001).
Both peritoneal membrane changes and loss of RRF in PD patients may be at least partly related to the use of PD solutions. Conventional PD solutions have an acidic pH and rely on hyperosmolar dextrose solutions to achieve an adequate gradient for UF across the peritoneal membrane (Szeto 2017). Their low pH and hyperosomolarity have been implicated in acute toxicity, such as inflow pain (Cho 2012; Nataatmadja 2017; Szeto 2017). Furthermore, heat sterilisation of PD solutions results in generation of glucose degradation products (GDP) (Nilsson‐Thorell 1993; Wieslander 1996),which in turn leads to formation of advanced glycation end products (AGE) (Lamb 1995; Nakayama 1997) and possibly to chronic peritoneal membrane toxicity, such as increased peritoneal solute transport rate, membrane fibrosis and neovascularisation, membrane failure and encapsulating peritoneal sclerosis (Mateijsen 1999; Pollock 2005; Szeto 2017).Damage to the peritoneal membrane may also impair host defences and predispose patients to peritonitis (Jorres 1992; Topley 1997). Finally, systemic absorption of GDPs may lead to direct nephrotoxicity and loss of RRF (Justo 2005).
Description of the intervention
Newer, biocompatible, dialysis solutions have been designed to minimise perturbation of the physiological milieu in the peritoneal cavity. The main approaches to creation of biocompatible solutions have been generation of solutions with a neutral pH and low GDP content, use of bicarbonate (± lactate) buffer, substitution of dextrose with glucose polymers (resulting in low GDP content albeit with an acidic pH), and use of amino acids as the osmotic agent.
How the intervention might work
Results of in vitro studies and small studies using surrogate end points suggest that biocompatible PD fluids may cause less damage to the peritoneal membrane than conventional fluids, and hence may improve patient outcomes (Mortier 2004; Mortier 2005). Improvement in peritoneal morphology with use of biocompatible PD solution has also been reported (Ayuzawa 2012). Furthermore, use of glucose polymer PD solution has been shown to augment peritoneal UF (Johnson 2003). Reduced exposure to GDP in biocompatible PD solutions may also lead to reduced systemic GDP absorption and ensuing nephrotoxicity (Justo 2005).
Why it is important to do this review
In comparison to conventional PD solutions, biocompatible solutions are more costly in some countries and the effect of these solutions on 'hard' (patient‐level clinical outcomes) endpoints are unclear. Furthermore, their role in clinical practice has not been established
Objectives
This review aimed to look at the benefits and harms of biocompatible PD solutions in comparison to standard PD solutions in patients receiving PD.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the effects of biocompatible dialysis solutions on patient outcomes in PD. The first period of randomised cross‐over studies was also included. When it was not possible to establish which data from cross‐over studies was from the first arm of the study, studies were excluded from the meta‐analysis.
Types of participants
Adults and children receiving any type of home‐based PD (continuous ambulatory PD (CAPD) or automated PD (APD)).
Types of interventions
Studies comparing the treatment of biocompatible PD solution to conventional PD solution were included. Groups of biocompatible PD solutions considered were:
Neutral pH, lactate‐buffered, low GDP
Neutral pH, bicarbonate (± lactate) buffered low GDP
Glucose polymer (icodextrin)
Combination regimens (e.g. PPEN)
The following types of studies were included:
Studies of neutral pH, low GDP PD solutions (lactate and bicarbonate ± lactate buffered) against conventional PD solutions
Studies of icodextrin against conventional PD solution
Studies of amino acid‐based dialysis fluids were excluded.
Types of outcome measures
Primary outcomes
Decline in RRF (changes in residual creatinine clearance (CrCl), urea clearance, Kt/V, glomerular filtration rate (GFR), and urine output)
Peritoneal UF (during peritoneal equilibration test and daily UF)
Peritonitis rate (episodes/y, episode/total patient‐months on PD) and incidence (number of events/follow‐up period)
Technique survival (number of participants remaining on PD at study completion)
Patient survival (number of participants alive at study completion)
Toxicity/adverse events (e.g. rash, uncontrolled fluid overload)
Secondary outcomes
Inflow pain
Changes in peritoneal membrane transport (four‐hour dialysate:plasma creatinine)
Dialysis adequacy (CrCl, Kt/V)
Hospitalisation (number of hospitalisation days during study follow‐up period)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 12 February 2018 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The review was undertaken by six authors. The search strategy described was used to obtain titles and abstracts of studies that have been relevant to the review. The titles and abstracts were screened independently by two authors. Studies that were not applicable were discarded. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by the same authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the most recent or most complete data set were used. Any discrepancy between published versions was highlighted. Disagreements were resolved by consultation.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
If a sufficient number of studies (greater than 10) measured the same outcome, thereby assessed to have enough power to detect asymmetry, a funnel plot was performed to evaluate for possible publication bias.
Measures of treatment effect
For dichotomous outcomes (e.g. death, inflow pain, peritonitis) results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. decline in RRF, urine volume), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used. When data were not presented in a format suitable for inclusion in meta‐analysis (e.g. median, interquartile range (IQR)), they were presented in tabulated form.
Unit of analysis issues
For cross‐over studies where data were available, only data from the first phase of studies were included.
Dealing with missing data
Any further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Two authors independently assessed the risk of reporting biases in studies using the risk of bias assessment tool (Higgins 2011).
Data synthesis
Data were summarised using the random‐effects model although the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was performed where feasible to explore possible sources of heterogeneity (e.g. study duration, participants, interventions and study quality). Heterogeneity among participants may have been related to age and renal pathology. Heterogeneity in treatments may have been related to prior agents used and the agent, dose and duration of therapy (Table 3).
1. Summary of analyses.
| Outcome | Subgroup analyses performed | ‘Other data’ tables |
| Neutral pH, low GDP PD solutions versus conventional PD solutions | ||
| Residual renal function |
|
‐‐ |
| Urine volume |
|
‐‐ |
| Anuria | ‐‐ | ‐‐ |
| 4‐hour peritoneal ultrafiltration |
|
‐‐ |
| Daily peritoneal ultrafiltration |
|
‐‐ |
| Peritoneal solute transport rate (4‐hour dialysate:plasma creatinine) |
|
‐‐ |
| Dialysis adequacy (CrCl/ Kt/V urea) | ‐‐ | Dialysis adequacy and peritoneal transport in anuric patients (median (IQR)) |
| Inflow pain | ‐‐ | ‐‐ |
| Peritonitis |
|
‐‐ |
| Technique failure | ‐‐ | ‐‐ |
| Hospitalisation | ‐‐ | ‐‐ |
| Death (all causes) | ‐‐ | ‐‐ |
| Glucose polymer (icodextrin) versus conventional PD solution | ||
| Uncontrolled fluid overload | ‐‐ | ‐‐ |
| Rash | ‐‐ | ‐‐ |
| Residual renal function | ‐‐ | ‐‐ |
| Urine volume | ‐‐ | Change in urine volume (mL) |
| Daily peritoneal ultrafiltration | Change in ultrafiltration volume/membrane transport characteristics | ‐‐ |
| Peritoneal solute transport rate (4‐hour dialysate:plasma creatinine) | ‐‐ | ‐‐ |
| Dialysis adequacy (CrCl) | Change in peritoneal CrCl/membrane transport characteristics | ‐‐ |
| Peritonitis | ‐‐ | ‐‐ |
| Technique failure | ‐‐ | ‐‐ |
| Death (all causes) | ‐‐ | ‐‐ |
CrCl ‐ creatinine clearance; GFR ‐ glomerular filtration rate; IQR ‐ interquartile range; PET ‐ peritoneal equilibration test; PD ‐ peritoneal dialysis
Separate analyses were performed for glucose polymer (icodextrin) solutions and glucose‐based biocompatible fluids due to anticipated differences in outcomes. For neutral pH low GDP PD solutions, sub‐group analyses were performed for lactate‐buffered and bicarbonate (± lactate) buffered solutions.
Sensitivity analysis
Where sufficient studies were available we investigated the following:
study duration
incident versus prevalent patients
single versus multicentre studies
parallel versus cross‐over design
PD fluid types
presence of selection bias
presence of other significant bias
weekly residual GFR in patients with baseline GFR > 2 mL/min/1.73 m2.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also included an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defined the quality of a body of evidence as the extent to which one could be confident that an estimate of effect or association was close to the true quantity of specific interest. The quality of a body of evidence involved consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Summary of findings (Table 1)
RRF
Urine volume (mL/d)
Peritoneal UF (mL/4 hours)
Peritoneal solute transport rate (4 hours dialysis:plasma creatinine)
Peritonitis rate (episodes/total patient‐months)
Technique failure (death‐censored)
Death (all causes)
Inflow pain and adverse events
Summary of findings (Table 2)
Uncontrolled fluid overload
Rash
RRF
Urine volume (mL/d)
Daily UF (mL/d)
Peritonitis
Technique failure (death‐censored)
Death (all causes) and adverse events
Results
Description of studies
Results of the search
We searched the Cochrane Kidney and Transplant Register of Studies (12 February 2018) and identified 59 new reports. After full‐text assessment 21 new studies were identified. Six new studies (six reports) were included (Cho 2013; Park 2012a; STARCH 2015; Szeto 2015; TRIO 2016; Yoo 2015), 10 (17 reports) were excluded (Chang 2016; Chow 2014; Coester 2006; EDEN 2013; Hiss 2013; IMPENDIA 2013; Lui 2012; Selby 2007a; Yehia 2014; Yoon 2014), and one ongoing study was identified (NCT01228279). Five studies are awaiting assessment (recently completed; no data available/abstract‐only publication and awaiting author; unable to access full‐text) (De Los Rios 2016; Do 2006a; Kim 2006; Kocyigit 2015; NCT01753154). We also identified 30 new reports of existing included and excluded studies.
A total of 42 studies (133 reports, 3262 participants) were included, 35 were excluded, 10 are awaiting assessment, and there is one ongoing study (Figure 1).
1.
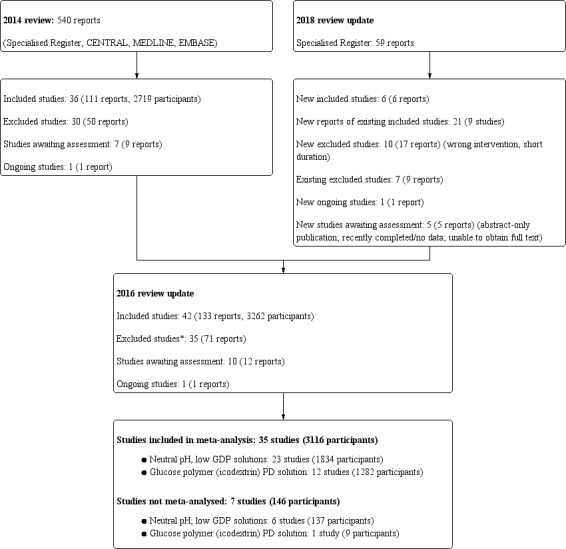
Flow diagram.
* Non‐RCTs excluded from the 2018 update
Included studies
Twenty‐nine studies (1971 participants) examined the effect of neutral PH, low GDP PD solution against conventional PD solutions (Bajo 2011; balANZ 2010; Cancarini 1998; Carrasco 2001; Choi 2008; Cnossen 2011; Coles 1997; DIUREST 2010; EURO‐BALANCE 2004; Fan 2008; Feriani 1998; Fernandez‐Perpen 2012; Fusshoeller 2004; Kim 2003; BALNET 2008; Lai 2012a; Mactier 1998; Pajek 2008; Rippe 2001; Schmitt 2002; Szeto 2007; Szeto 2015; Tranaeus 2000; TRIO 2016; Weiss 2009; Yoo 2015; Zeier 2003). Of these, 18 studies (1416 participants) examined lactate‐buffered neutral PH, low GDP PD solutions (Bajo 2011; balANZ 2010; Carrasco 2001; Cho 2013; Choi 2008; Cnossen 2011; DIUREST 2010; EURO‐BALANCE 2004; Fan 2008; Kim 2003; BALNET 2008; Lai 2012a; Park 2012a; Rippe 2001; Szeto 2007; Szeto 2015;TRIO 2016; Zeier 2003) and 11 studies (555 participants) examined bicarbonate (±lactate)‐buffered neutral PH, low GDP PD solutions (Cancarini 1998; Coles 1997; Feriani 1998; Fernandez‐Perpen 2012; Fusshoeller 2004; Mactier 1998; Pajek 2008; Schmitt 2002; Tranaeus 2000; Weiss 2009; Yoo 2015).
Thirteen studies (1291 participants) compared the clinical outcomes of once daily use of icodextrin with that of conventional PD solution (Bredie 2001; Davies 2003; di Paolo 2000; Finkelstein 2005; Konings 2003; Lin 2009a; MIDAS 1994; Paniagua 2008; Plum 2002; Posthuma 1997; STARCH 2015; Takatori 2011; Wolfson 2002). Of these, three studies examined the effect of icodextrin in patients with high or high average membrane transport characteristics (Davies 2003; Finkelstein 2005; Paniagua 2008).
Excluded studies
A total of 35 studies were excluded from the review. The reasons for exclusion included wrong intervention (e.g. amino acid‐based solutions; comparison of two regimes of the same biocompatible PD solution; comparison of two different types of biocompatible PD solution) or short duration studies.
For this update non‐RCTs have been removed.
Risk of bias in included studies
Risk of bias domains of individual studies is presented in Figure 2 and the summary of risk of bias of all the included studies is presented in Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
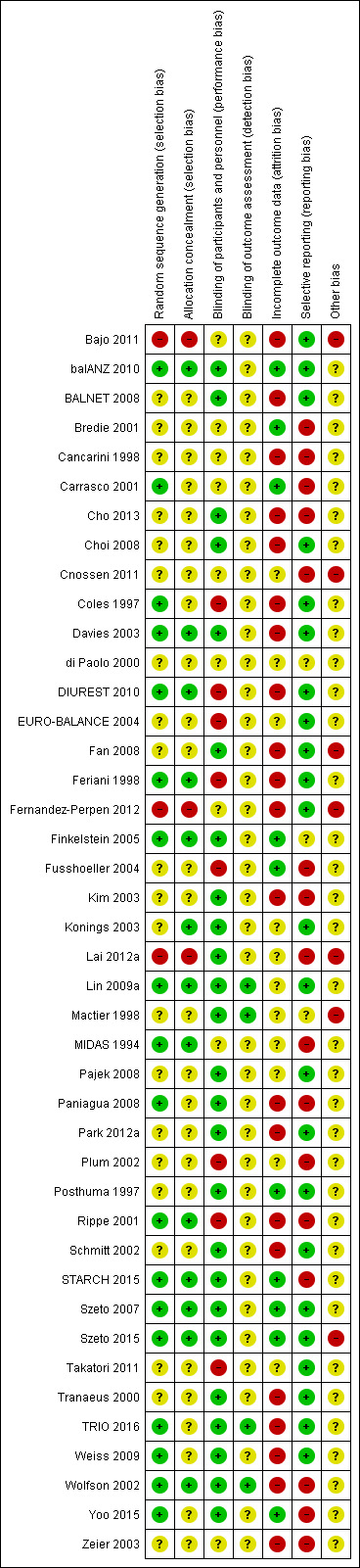
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Random sequence generation was judged to be at low risk of bias in 18 studies (balANZ 2010; Carrasco 2001; Coles 1997; Davies 2003; DIUREST 2010; Feriani 1998; Finkelstein 2005; Lin 2009a; MIDAS 1994; Paniagua 2008; Rippe 2001; STARCH 2015; Szeto 2007; Szeto 2015; TRIO 2016; Weiss 2009; Wolfson 2002; Yoo 2015) and at high risk of bias in three studies (Bajo 2011; Fernandez‐Perpen 2012; Lai 2012a). The risk of bias was unclear in 21 studies.
Allocation concealment
Allocation concealment was judged to be at low risk of bias in 13 studies (balANZ 2010; Davies 2003; DIUREST 2010; Feriani 1998; Finkelstein 2005; Konings 2003; Lin 2009a; MIDAS 1994; Rippe 2001; STARCH 2015; Szeto 2007; Szeto 2015; Wolfson 2002) and at high risk of bias in three studies (Bajo 2011; Fernandez‐Perpen 2012; Lai 2012a). The risk of bias was unclear in 26 studies.
Blinding
Performance bias (blinding of participants and investigators) was judged to be at low risk of bias in 25 studies (balANZ 2010; BALNET 2008; Cho 2013; Choi 2008; Davies 2003; Fan 2008; Finkelstein 2005; Kim 2003; Konings 2003; Lai 2012a; Lin 2009a; Mactier 1998; Pajek 2008; Paniagua 2008; Park 2012a; Posthuma 1997; Schmitt 2002; STARCH 2015; Szeto 2007; Szeto 2015; Tranaeus 2000; TRIO 2016; Weiss 2009; Wolfson 2002; Yoo 2015) and at high risk of bias in eight studies (Coles 1997; DIUREST 2010; EURO‐BALANCE 2004; Feriani 1998; Fusshoeller 2004; Plum 2002; Rippe 2001; Takatori 2011). The risk of bias was unclear in nine studies.
Detection bias (blinding of outcome assessors) was judged to be at low risk of bias in four studies (Lin 2009a; Mactier 1998; TRIO 2016; Wolfson 2002) and was unclear in 38 studies.
Incomplete outcome data
Attrition bias was judged to be at low risk of bias in 10 studies (balANZ 2010; Bredie 2001; Carrasco 2001; Finkelstein 2005; Fusshoeller 2004; Posthuma 1997; STARCH 2015; Szeto 2007; Szeto 2015; Yoo 2015) and at high risk of bias in 21 studies (Bajo 2011; BALNET 2008; Cancarini 1998; Cho 2013; Choi 2008; Coles 1997; Davies 2003; DIUREST 2010; Fan 2008; Feriani 1998; Fernandez‐Perpen 2012; Kim 2003; Paniagua 2008; Park 2012a; Rippe 2001; Schmitt 2002; Tranaeus 2000; TRIO 2016; Weiss 2009; Wolfson 2002; Zeier 2003). The risk of bias was unclear in 11 studies.
Patients lost to follow‐up ranged from 0% to 83.4%.
Selective reporting
Reporting bias was judged to be at low risk of bias in 23 studies (Bajo 2011; balANZ 2010; BALNET 2008; Choi 2008; Coles 1997; Davies 2003; DIUREST 2010; EURO‐BALANCE 2004; Fan 2008; Feriani 1998; Fernandez‐Perpen 2012; Konings 2003; Lin 2009a; Pajek 2008; Park 2012a; Posthuma 1997; Schmitt 2002; Szeto 2007; Szeto 2015; Takatori 2011; Tranaeus 2000; TRIO 2016; Weiss 2009) and at high risk of bias in 16 studies (Bredie 2001; Cancarini 1998; Carrasco 2001; Cho 2013; Cnossen 2011; Fusshoeller 2004; Kim 2003; Lai 2012a; MIDAS 1994; Paniagua 2008; Plum 2002; Rippe 2001; STARCH 2015; Wolfson 2002; Yoo 2015; Zeier 2003). The risk of bias was unclear in three studies.
Other potential sources of bias
Seven studies (17%) were identified as high risk for other potential sources of bias (Bajo 2011; Cnossen 2011; Fan 2008; Fernandez‐Perpen 2012; Lai 2012a; Mactier 1998; Szeto 2015). The potential sources of bias included mixed use of neutral pH, low GDP solutions from different manufacturers with variable levels of GDP and types of buffers in the intervention group (two studies), potential centre‐related effects (one study), different baseline characteristics between patients in intervention and control groups (three studies), unclear description of participant details (one study). The risk of bias was unclear for all other studies.
Effects of interventions
Summary of findings for the main comparison. Low GDP (all buffer types) compared to standard glucose dialysate for peritoneal dialysis (PD).
| Neutral pH, low GDP PD solutions versus standard glucose PD solutions | ||||||
| Patient or population: PD patients Setting: community Intervention: neutral, low GDP dialysate (all buffer types) Comparison: standard glucose dialysate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard glucose dialysate | Risk with low GDP dialysate (all buffer types) | |||||
|
Residual renal function (GFR; follow‐up 3 months to more than 3 years) |
The mean residual renal function (GFR) was 0.54 mL/min/1.73 m2 higher with low GDP dialysate (0.14 to 0.93 higher) compared to standard glucose dialysate | ‐ | 835 (15) | ⊕⊕⊕⊕ HIGH a | SMD 0.19 higher (0.05 to 0.33 higher) | |
|
Urine volume (follow‐up to more than 3 years) |
The mean urine volume was 114.37 mL/d higher with low GDP dialysate (47.09 to 181.65 higher) compared to standard glucose dialysate | ‐ | 791 (11) | ⊕⊕⊕⊕ HIGH b | ‐ | |
|
Peritoneal ultrafiltration: 4 hours (follow‐up to 24 months) |
The estimated mean peritoneal ultrafiltration 69.72 mL/4 hours lower with low GDP dialysate (16.60 to 122.84 lower) compared to standard glucose dialysate | ‐ | 414 (9) | ⊕⊕⊝⊝ LOW 1 | ‐ | |
|
Peritoneal solute transport rate (4‐hour dialysis:plasma creatinine) (follow‐up to more than 3 years) |
The mean peritoneal solute transport rate was 0.01 higher with low GDP dialysate (0 to 0.03 higher) compared to standard glucose dialysate | ‐ | 746 (10) | ⊕⊕⊝⊝ LOW 2 | SMD 0.42 lower (0.74 to 0.10 lower) | |
|
Peritonitis rate (episodes/total patient‐months) (up to 24 months) |
31 per 1,000 | 36 per 1,000 (26 to 51) | RR 1.18 (0.84 to 1.64) | 18,184 (10) | ⊕⊕⊝⊝ LOW 3 | ‐ |
|
Technique failure (death‐censored) (follow‐up to more than 3 years) |
74 per 1,000 | 81 per 1,000 (55 to 120) | RR 1.10 (0.75 to 1.63) | 1275 (15) | ⊕⊕⊝⊝ LOW 4 | ‐ |
|
Death (all causes) (follow‐up to more than 3 years) |
77 per 1,000 | 21 fewer per 1,000 (41 fewer to 11 more) | RR 0.73 (0.47 to 1.14) | 1229 (15 ) | ⊕⊕⊝⊝ LOW 4 | ‐ |
| In very low certainty evidence, it is uncertain whether neutral pH, low GDP solution use led to any differences in inflow pain compared with standard PD solution (1 studies, 58 participants) | ||||||
| In very low certainty evidence, it is uncertain whether neutral pH, low GDP solution use led to adverse events including exit site/tunnel infection, non‐PD related infection/general infection, inadequate dialysis, fluid overload/hypervolaemia, hypertension, hypotension, hernia, peritoneal leak, catheter blockage, malposition, gastrointestinal disorder, abdominal pain, pancreatitis, enteritis, vomiting, newly diagnosed cancer, arthritis, angina, apoplexy, hypercalcaemia, hypocalcaemia, hyperphosphataemia, and hyperglycaemia (6 studies, 519 participants). | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio GFR: glomerular filtration rate | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Benefit was evident at all time points assessed
b Benefit was greater with longer follow‐up duration (i.e. longer than 12 months)
1 Downgraded two levels for moderate level of heterogeneity which could not be explained and indirectness
2 Downgraded two levels for very serious study limitation significantly different baseline peritoneal solute transport rate in 30% of studies
3 Downgraded two levels for study limitation (high risk of attrition bias amongst studies analysed) and moderate heterogeneity observed
4 Downgraded two levels for very serious study limitation (none of the studies were adequately powered and number after combining studies remained too small to accurately assess this outcome)
Summary of findings 2. Glucose polymer (icodextrin) compared to standard glucose dialysate for peritoneal dialysis (PD).
| Glucose polymer (icodextrin) compared to standard glucose dialysate for PD | ||||||
| Patient or population: PD patients Setting: community Intervention: glucose polymer (icodextrin) Comparison: standard glucose dialysate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard glucose dialysate | Risk with glucose polymer (icodextrin) | |||||
|
Uncontrolled fluid overload (follow‐up to 24 months) |
531 per 1,000 | 159 per 1,000 (80 to 313) | RR 0.30 (0.15 to 0.59) | 100 (2) | ⊕⊕⊕⊝ MODERATE 1 | ‐ |
|
Rash (follow‐up to 12 months) |
43 per 1,000 | 109 per 1,000 (26 to 466) | RR 2.51 (0.59 to 10.72) | 755 (3) | ⊕⊕⊝⊝ LOW 2 | ‐ |
|
Residual renal function (renal CrCl) (follow‐up to 24 months) |
The mean residual renal function (renal CrCl) was 0.30 mL/min higher with icodextrin (0.65 lower to 1.23 higher) than standard glucose dialysate | ‐ | 114 (4) | ⊕⊕⊝⊝ LOW 3 | SMD 0.12 higher (0.26 lower to 0.49 higher) |
|
|
Urine volume (follow‐up to 24 months) |
The mean urine volume was 88.88 mL/d lower with icodextrin (356.88 lower to 179.12 higher) than standard glucose dialysate | ‐ | 69 (3) | ⊕⊕⊝⊝ LOW 3 | ‐ | |
|
Daily ultrafiltration (follow‐up to 24 months) |
The mean daily ultrafiltration was 448.54 mL/d higher with icodextrin (289.28 to 607.8 higher) than standard glucose dialysate | ‐ | 102 (4) | ⊕⊕⊕⊝ MODERATE 1 | ‐ | |
|
Peritonitis (follow‐up to 24 months) |
236 per 1,000 | 12 fewer per 1,000 (54 fewer to 42 more) | RR 0.95 (0.77 to 1.18) | 667 (6) | ⊕⊕⊝⊝ LOW 4 | ‐ |
|
Technique failure (death‐censored) (up to 24 months) |
107 per 1,000 | 64 per 1,000 (34 to 120) | RR 0.60 (0.32 to 1.12) | 350 (4) | ⊕⊝⊝⊝ VERY LOW 5 | ‐ |
| In very low certainty evidence, it is uncertain whether icodextrin use led to any difference in death (all causes) compared to standard glucose solution (6 studies, 816 participants). | ||||||
| In very low certainty evidence, it is uncertain whether icodextrin use led to any difference in adverse events including abdominal discomfort, anaemia, arterial emboli, cardiac failure, cerebrovascular accident, diabetic foot, dizzy, electrolyte disturbances, exit site infection, fatigue, fluid overload, headache, stroke, hyperglycaemia, hypotension, myocardial infarction, pain, pleural effusion, pneumonia, pulmonary embolism, thirsty, uncontrolled hypertension, congestion, upper respiratory tract infection, and vomiting (5 studies, 816 participants). | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio CrCl: creatinine clearance; GFR: glomerular filtration rate | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level for imprecision (small number of included studies and participants)
2 Downgraded two levels for imprecision (small number of included studies and participants) and study limitation (high attrition bias)
3 Downgraded two levels for imprecision (small number of included studies and participants) and study limitation (limited number of long‐term studies)
4 Downgraded two levels for very serious study limitation (high attrition bias , limited number of long‐term studies)
5 Downgraded three levels for imprecision (small number of included studies and participants) and very serious study limitation (limited number of long‐term studies, studies were not adequately powered to detect a difference in technique survival)
6 Studies were not adequately powered to detect a difference in technique survival
The studies were analysed using both random effects and fixed effects models and found no difference between the two models. The results presented below therefore refer to those obtained using a random‐effects model. Quantitative analyses with high levels of heterogeneity (I2 ≥ 75%) were not reported.
Neutral pH, low GDP versus conventional glucose PD solution
Residual renal function
In high certainty evidence, the use of neutral pH, low GDP solution improved the preservation of RRF (Analysis 1.1 (15 studies, 835 participants: SMD 0.19, 95% CI 0.05 to 0.33; I2 = 0%). This approximated to a mean difference in GFR of 0.54 mL/min/1.73m2 (95% CI 0.14 to 0.93). This effect was presented for all follow‐up duration categories analysed: up to 12 months (Analysis 1.2 (11 studies, 722 participants): SMD 0.18, 95% CI 0.05 to 0.32; I2 = 3%), 12 to 24 months (Analysis 1.3 (10 studies, 641 participants): SMD 0.25, 95% CI 0.10 to 0.41; I2 = 0%), and more than 24 months (Analysis 1.4 (6 studies, 343 participants): SMD 0.30, 95% CI 0.08 to 0.51; I2 = 0%). This translated into MD in GFR of 0.59 mL/min/1.73 m2 (95% CI 0.16 to 1.05), 0.71 mL/min/1.73 m2 (95% CI 0.28 to 1.16) and 0.85 mL/min/1.73 m2 (95% CI 0.23 to 1.44), respectively. Subgroup analysis was performed on PD fluid types (Analysis 1.5). This analysis was limited by the fact that the majority of studies used only one solution type (Balance®) such that no useful conclusions could be drawn. There was no evidence of publication bias Figure 4.
1.1. Analysis.
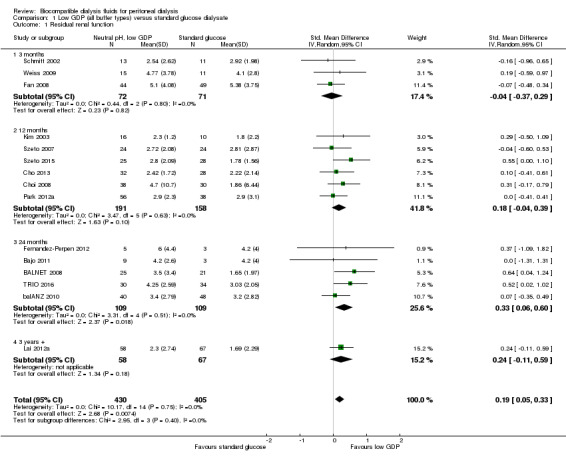
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 1 Residual renal function.
1.2. Analysis.
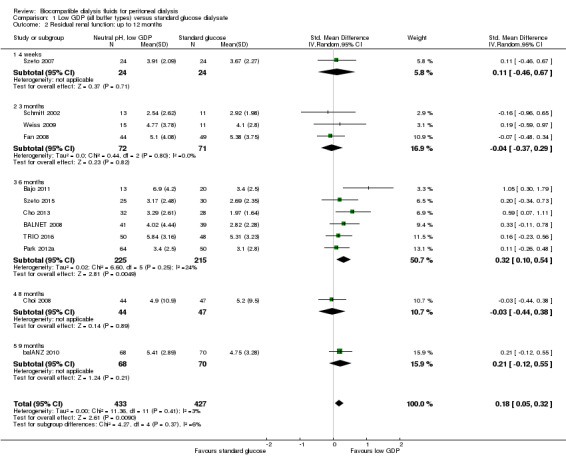
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 2 Residual renal function: up to 12 months.
1.3. Analysis.
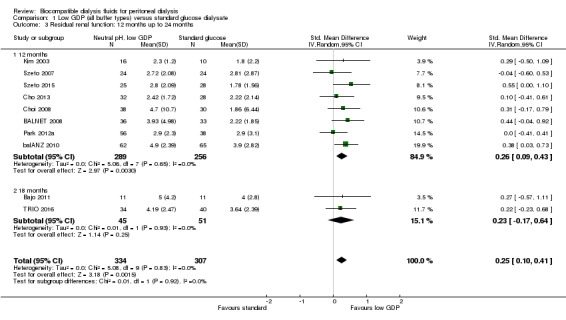
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 3 Residual renal function: 12 months up to 24 months.
1.4. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 4 Residual renal function: 24 months and beyond.
1.5. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 5 Residual renal function: PD fluid types.
4.
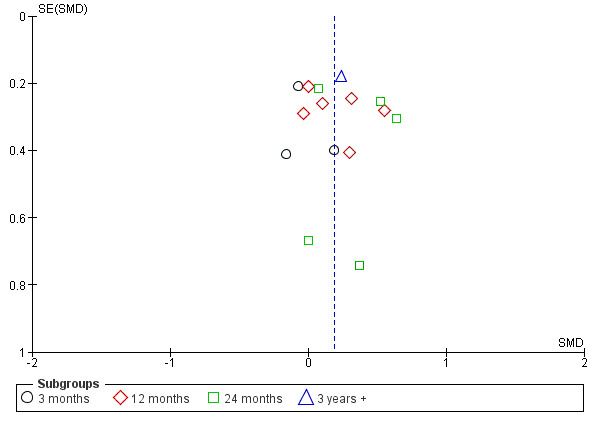
Funnel plot of comparison: 1 Low GDP (all buffer types) versus standard glucose dialysate, outcome: 1.1 Residual renal function.
Urine volume
In high certainty evidence, daily residual diuresis was higher in PD patients receiving neutral pH, low GDP PD solutions (Analysis 1.6 (11 studies, 791 participants): MD 114.37 mL/d, 95% CI 47.09 to 181.65; I2 = 3%). There was little of no difference in urine volume up to 12 months follow‐up (Analysis 1.7 (10 studies, 819 participants): MD 69.72 mL/d, 95% CI ‐55.95 to 195.40; I2 = 60%). The benefit was observed with greater than 1 year follow‐up durations: 12 months to 24 months (Analysis 1.8 (8 studies, 579 participants): MD 110.57 mL/d, 95% CI 40.81 to 180.34; I2 = 0%) and more than 24 months (Analysis 1.9 (3 studies, 279 participants): MD 169.22 mL/d, 95% CI 23.98 to 314.46; I2 = 0%). Subgroup analysis was performed on PD fluid types (Analysis 1.10). Again, this analysis was limited by the fact that the majority of trials used only one solution type (Balance®) such that no useful conclusions could be drawn. The proportion of patients who developed anuria was not different between the two groups (Analysis 1.11 (2 studies 246 participants): RR 0.56, 95% CI 0.18 to 1.75; I2 = 60%).
1.6. Analysis.
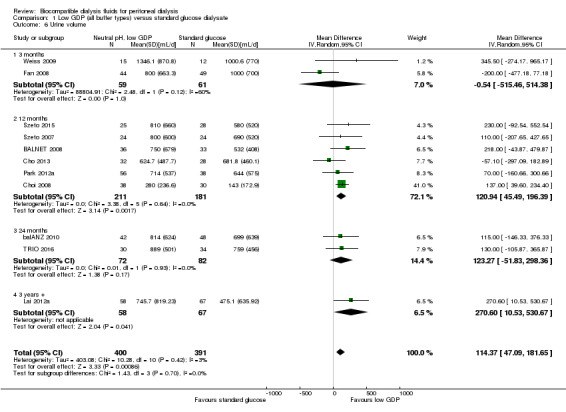
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 6 Urine volume.
1.7. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 7 Urine volume: up to 12 months.
1.8. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 8 Urine volume: 12 months up to 24 months.
1.9. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 9 Urine volume: 24 months and beyond.
1.10. Analysis.
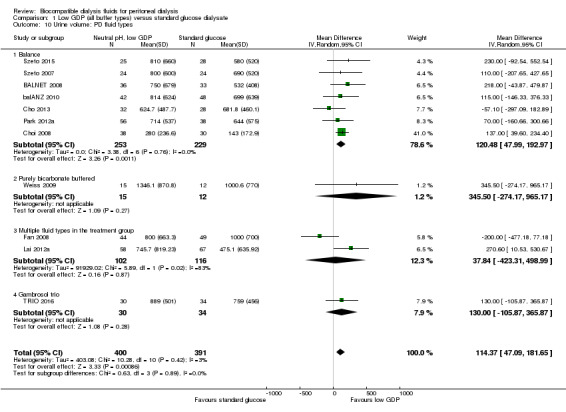
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 10 Urine volume: PD fluid types.
1.11. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 11 Anuria.
Peritoneal ultrafiltration
The four‐hour peritoneal UF measured during a peritoneal equilibration test may be lower in the neutral pH, low GDP solution group (Analysis 1.12 (9 studies, 414 participants): SMD ‐0.42, 95% CI ‐0.74 to ‐0.10; I2 = 51%; estimated MD ‐69.72 mL/4 hours, 95% CI ‐122.84 to ‐16.60; low certainty evidence). However, there was a moderate heterogeneity, which could not be explained by differences in study design, study population, or risk of bias. Outcomes from daily peritoneal UF analysis could not be reported due to high heterogeneity (I2 = 82%).
1.12. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 12 Peritoneal ultrafiltration: 4 hours.
Peritoneal solute transport rate
The four‐hour dialysate:plasma creatinine ratio (D/PCreat) measured during a peritoneal equilibration test may be higher in the neutral pH, low GDP solution group (Analysis 1.13 (10 studies, 746 participants): MD 0.01, 95% CI 0.00 to 0.03; I2 = 1%; low certainty evidence). However, subgroup analysis with patient characteristics (Analysis 1.14), fluid types (Analysis 1.15) and study design (Analysis 1.16) showed no significant difference in four‐hour D/PCreat values between the neutral pH, low GDP solution and control groups.
1.13. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 13 4‐hour dialysate:plasma creatinine (2.27%, 2.4%, or 2.5% glucose).
1.14. Analysis.
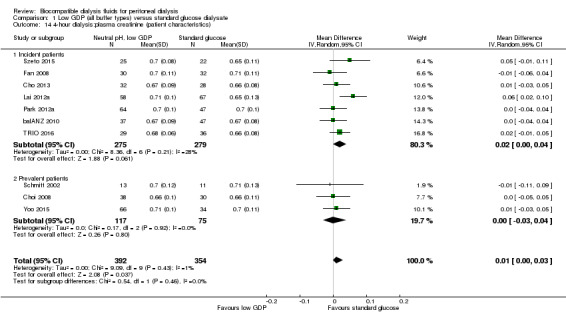
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 14 4‐hour dialysis:plasma creatinine (patient characteristics).
1.15. Analysis.
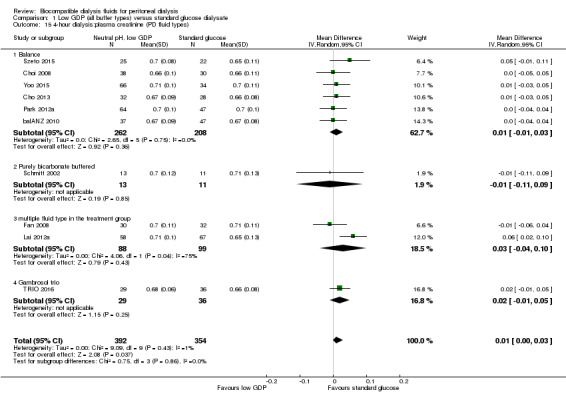
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 15 4‐hour dialysis:plasma creatinine (PD fluid types).
1.16. Analysis.
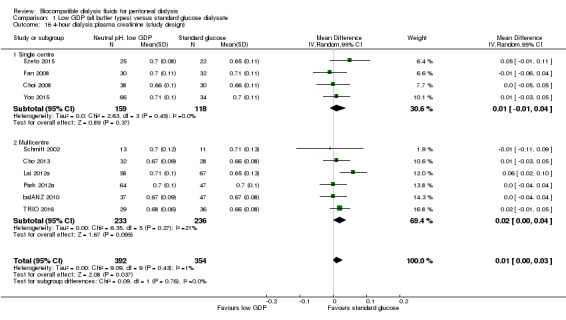
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 16 4‐hour dialysis:plasma creatinine (study design).
Peritoneal small solute clearance
Neutral pH low GDP PD solution may make little or no difference to peritoneal creatinine clearance (Analysis 1.17 (7 studies, 510 participants): MD ‐0.44 L/week/1.73 m2, 95% CI ‐2.03 to 1.15; I2 = 0%) or peritoneal urea clearance (Analysis 1.18 (6 studies, 422 participants): MD ‐0.01, 95% CI ‐0.12 to 0.09; I2 = 26%; low certainty evidence) between treatments using and conventional PD solution.
1.17. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 17 Peritoneal creatinine clearance [L/wk/1.73 m²].
1.18. Analysis.
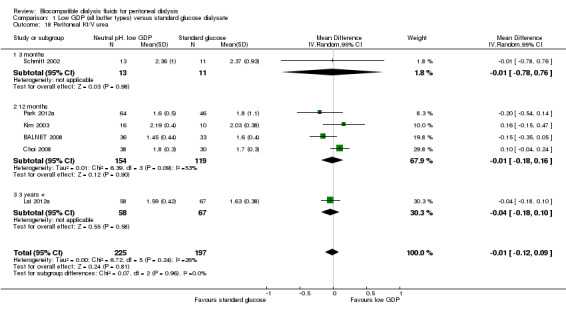
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 18 Peritoneal Kt/V urea.
Peritonitis
Neutral pH, low GDP and conventional PD solution groups may make little or no difference to the incidence of peritonitis (Analysis 1.19 (12 studies, 1055 participants): RR 1.26, 95% CI 0.92 to 1.72; I2 = 69%; low certainty evidence). Similarly, there was little or no difference to peritonitis rate (Analysis 1.20 (10 studies, 18,184 patient‐months): RR 1.18, 95% CI 0.84 to 1.64; I2 = 67%; low certainty evidence). A moderate level of heterogeneity was noted for both analyses and when studies were classified according to the risk of attrition bias, the incidence of peritonitis was lower in the neutral pH, low GDP solution group in studies with a low risk for attrition bias (Analysis 1.21 (3 studies, 359 participants): RR 0.65, 95% CI 0.47 to 0.90; I2 = 0%).
1.19. Analysis.
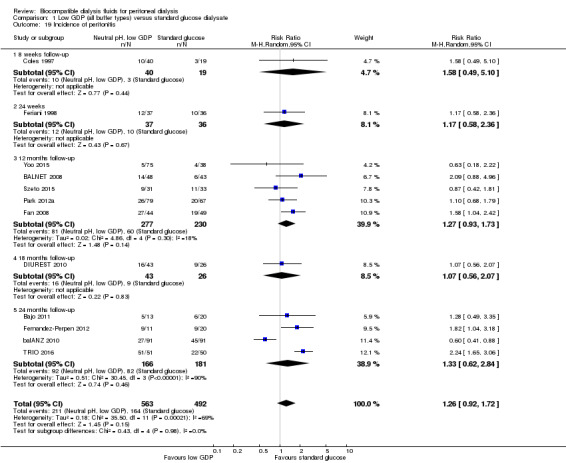
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 19 Incidence of peritonitis.
1.20. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 20 Peritonitis rate (episodes/total patient‐months).
1.21. Analysis.
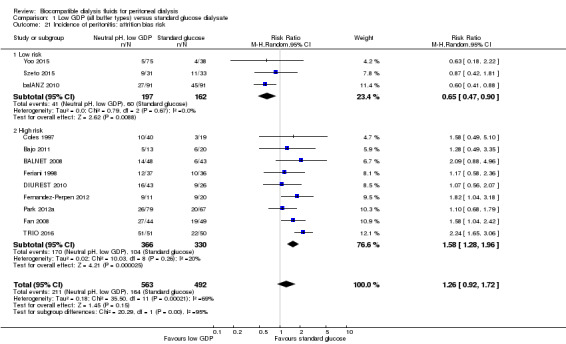
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 21 Incidence of peritonitis: attrition bias risk.
Inflow pain
In very low certainty evidence, it is uncertain whether neutral pH, low GDP solution use led to any differences in may decrease the incidence of inflow pain (Analysis 1.22 (1 study, 58 participants): RR 0.51, 95% CI 0.24 to 1.08). Two additional cross‐over RCTs reported significantly lower risk of inflow pain with its use (Fusshoeller 2004; Mactier 1998). In the study by Mactier 1998, bicarbonate/lactate‐buffered PD solution may have had a more favourable effect on inflow pain than purely bicarbonate‐buffered PD solution. These latter two cross‐over studies were excluded from meta‐analysis due to the inability to isolate data from the first arm of the study.
1.22. Analysis.
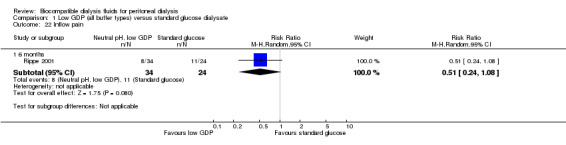
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 22 Inflow pain.
Hospitalisation
In very low certainty evidence, it is unsure whether neutral pH, low GDP PD solutions reduce the duration of hospitalisation (Analysis 1.23 (2 studies, 230 participants): MD 3.02 days, 95% CI ‐7.08 to 13.12; I2 = 45%).
1.23. Analysis.
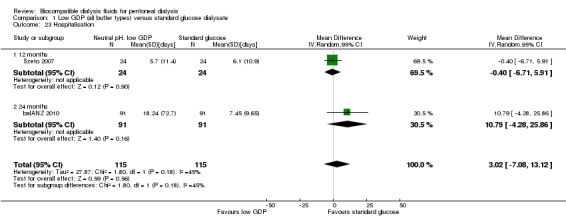
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 23 Hospitalisation.
Technique failure
In low certainty evidence neutral pH, low GDP PD solutions may make little or no difference to death‐censored technique failure, although overall participant numbers were relatively small for assessing this outcome (Analysis 1.24 (15 studies, 1275 participants): RR 1.10, 95% CI 0.75 to 1.63; I2 = 0%).
1.24. Analysis.
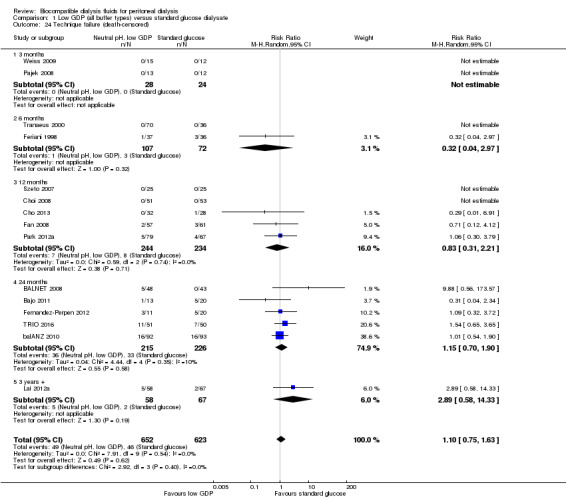
Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 24 Technique failure (death‐censored).
Patient survival
In low certainty evidence neutral pH, low GDP PD solutions may make little or no difference to death (all causes), although overall participant numbers were relatively small for assessing this outcome (Analysis 1.25 (15 studies, 1229 participants): RR 0.73, 95% CI 0.47 to 1.14; I2 =0%).
1.25. Analysis.

Comparison 1 Low GDP (all buffer types) versus standard glucose dialysate, Outcome 25 Death (all causes).
Adverse events
In very low certainty evidence, it is uncertain whether neutral pH, low GDP PD solutions use led to any differences in adverse events compared with conventional PD solutions (6 studies, 519 participants) (balANZ 2010; Coles 1997; EURO‐BALANCE 2004; Feriani 1998; Schmitt 2002; Tranaeus 2000) (Table 4).
2. Adverse effects reported in studies.
| Adverse events | Standard glucose solution | Low GDP solution | Studies reporting outcome | ||
| No. events | No. at risk | No. events | No. at risk | ||
| Neutral pH, low GDP PD solution (excluding peritonitis, death) | |||||
| Exit site infection | 6 0 1 |
91 19 36 |
4 5 3 |
91 40 37 |
balANZ 2010 Coles 1997 Feriani 1998 |
| Tunnel infection | 2 | 91 | 1 | 91 | balANZ 2010 |
| Non‐PD related infection/general infection | 20 0 |
91 19 |
4 6 |
91 40 |
balANZ 2010 Coles 1997 |
| Inadequate dialysis | 1 | 91 | 1 | 91 | balANZ 2010 |
| Fluid overload/hypervolaemia | 3 1 2 |
91 19 36 |
1 4 0 |
91 40 37 |
balANZ 2010 Coles 1997 Feriani 1998 |
| Hypertension | 2 1 |
19 36 |
3 0 |
40 37 |
Coles 1997 Feriani 1998 |
| Hypotension | 0 | 36 | 1 | 37 | Feriani 1998 |
| Hernia | 11 1 |
91 36 |
10 0 |
91 37 |
balANZ 2010 Feriani 1998 |
| Peritoneal leak | 3 | 91 | 1 | 91 | balANZ 2010 |
| Catheter blockage | 4 | 91 | 5 | 91 | balANZ 2010 |
| Malposition | 2 | 91 | 1 | 91 | balANZ 2010 |
| Gastrointestinal disorder | 6 | 91 | 14 | 91 | balANZ 2010 |
| Abdominal pain | 0 | 19 | 3 | 40 | Coles 1997 |
| Pancreatitis | 1 | 36 | 0 | 37 | Feriani 1998 |
| Enteritis | 0 | 36 | 2 | 37 | Feriani 1998 |
| Vomiting | 0 | 36 | 1 | 37 | Feriani 1998 |
| Newly diagnosed cancer | 3 | 91 | 4 | 91 | balANZ 2010 |
| Arthritis | 1 | 36 | 0 | 37 | Feriani 1998 |
| Angina | 0 | 36 | 1 | 37 | Feriani 1998 |
| Apoplexy | 1 | 36 | 1 | 37 | Feriani 1998 |
| Hypercalcaemia | 0 3 |
19 36 |
3 2 |
40 37 |
Coles 1997 Feriani 1998 |
| Hypocalcaemia | 0 | 19 | 3 | 40 | Coles 1997 |
| Hyperphosphataemia | 3 | 19 | 4 | 40 | Coles 1997 |
| Hyperglycaemia | 1 | 36 | 0 | 37 | Feriani 1998 |
| Glucose polymer (icodextrin) (excluding rash, peritonitis, death) | |||||
| Abdominal discomfort | 1 | 103 | 0 | 98 | Lin 2009a |
| Anaemia | 24 26 |
29 112 |
6 39 |
30 175 |
Paniagua 2008 Wolfson 2002 |
| Arterial emboli | 1 | 103 | 0 | 106 | MIDAS 1994 |
| Cardiac failure | 1 | 103 | 1 | 106 | MIDAS 1994 |
| Cerebrovascular accident | 0 | 103 | 2 | 106 | MIDAS 1994 |
| Diabetic foot | 5 | 29 | 1 | 30 | Paniagua 2008 |
| Dizzy | 0 | 103 | 1 | 98 | Lin 2009a |
| Electrolyte disturbances | 4 | 29 | 1 | 30 | Paniagua 2008 |
| Exit site infection | 3 24 |
27 112 |
4 28 |
33 175 |
STARCH 2015 Wolfson 2002 |
| Fatigue | 0 | 103 | 2 | 98 | Lin 2009a |
| Fluid overload | 17 17 |
132 29 |
6 5 |
136 30 |
Lin 2009a Paniagua 2008 |
| Headache | 9 | 112 | 25 | 175 | Wolfson 2002 |
| Stroke | 1 | 27 | 0 | 33 | STARCH 2015 |
| Hyperglycaemia | 27 | 29 | 8 | 30 | Paniagua 2008 |
| Hypotension | 28 | 215 | 25 | 273 |
Lin 2009a Wolfson 2002 |
| Myocardial infarction | 4 3 |
103 29 |
2 0 |
106 30 |
MIDAS 1994 Paniagua 2008 |
| Pain | 18 | 112 | 30 | 175 | Wolfson 2002 |
| Pleural effusion | 6 | 29 | 1 | 30 | Paniagua 2008 |
| Pneumonia | 0 | 103 | 1 | 106 | MIDAS 1994 |
| Pulmonary embolism | 0 | 103 | 1 | 106 | MIDAS 1994 |
| Thirsty | 0 | 103 | 1 | 98 | Lin 2009a |
| Uncontrolled hypertension | 0 21 |
103 112 |
1 40 |
106 175 |
MIDAS 1994 Wolfson 2002 |
| Hypotension | 1 | 27 | 3 | 33 | STARCH 2015 |
| Hypertension | 0 | 27 | 1 | 33 | STARCH 2015 |
| Congestion | 1 | 27 | 0 | 33 | STARCH 2015 |
| Upper respiratory tract infection | 25 | 112 | 41 | 175 | Wolfson 2002 |
| Vomiting | 1 | 103 | 0 | 98 | Lin 2009a |
GDP ‐ glucose degradation products; PD ‐ peritoneal dialysis
Glucose polymer (icodextrin) versus convention glucose PD solution
Peritoneal ultrafiltration
In moderate certainty evidence, icodextrin uniformly augmented peritoneal UF compared with glucose exchanges (Analysis 2.1 (4 studies, 102 participants): MD 448.54 mL/d, 95% CI 289.28 to 607.80; I2 = 0%). It should be noted that only one of these four studies allowed the use of hypertonic glucose PD solution (3.86%) in the control group (Finkelstein 2005). In this study of 92 APD patients with higher peritoneal solute transport rate (defined as D/PCreat > 0.7) and UF failure (defined as four‐hour net UF < 100 mL using 2.5% dextrose), icodextrin resulted in a higher net UF volumes (+373.8 ± 58.9 mL/d) compared with 4.25% dextrose (‐239.7 mL ± 151.0 mL/d) in the controls. Similarly, when the use of icodextrin was compared to 2.5% dextrose PD solution according to the peritoneal equilibration test category, Lin 2009a reported increases in UF capacities in all patients except low transporters. Patients with higher peritoneal transport characteristics derived greater UF benefit.
2.1. Analysis.

Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 1 Daily ultrafiltration.
Episodes of uncontrolled fluid overload
In moderate certainty evidence, icodextrin probably reduced reported episodes of uncontrolled fluid overload (Analysis 2.2 (2 studies, 100 participants): RR 0.30, 95% CI 0.15 to 0.59; I2 = 0%).
2.2. Analysis.
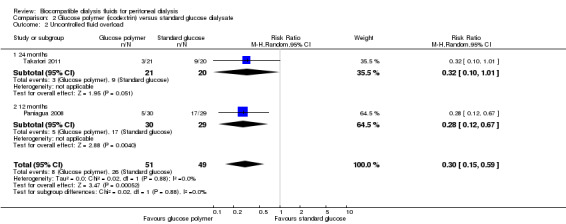
Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 2 Uncontrolled fluid overload.
Residual renal function
In low certainty evidence, icodextrin may make little or no difference to RRF (Analysis 2.3 (4 studies, 114 participants): SMD 0.12, 95% CI ‐0.26 to 0.49, P = 0.5; I2 = 0%). This approximated to a mean difference in renal CrCl of 0.30 mL/min (95% CI ‐0.65 to 1.23).
2.3. Analysis.

Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 3 Residual renal function.
Urine volume
In low certainty evidence, icodextrin‐induced increase in peritoneal UF volumes may make little or no difference to daily urine volumes (Analysis 2.4 (3 studies, 69 participants): MD ‐88.88 mL/d, 95% CI ‐356.88 to 179.12, P = 0.5; I2 = 0%). Indeed, Davies 2003 reported better maintenance of urine volume with the use of icodextrin at six months when compared to 2.27% dextrose PD solution use (Analysis 2.5).
2.4. Analysis.
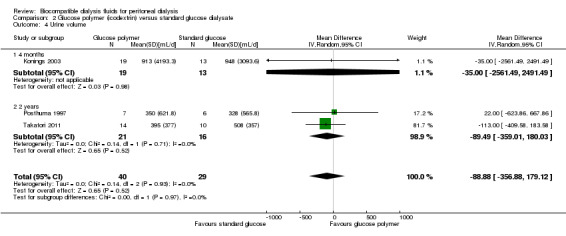
Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 4 Urine volume.
2.5. Analysis.
Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 5 Change in urine volume (mL).
| Change in urine volume (mL) | |||
|---|---|---|---|
| Study | Time point | Glucose polymer | Standard glucose solution |
| Davies 2003 | 1 month | ‐44.3 | ‐44.1 |
| Davies 2003 | 3 months | ‐34.6 | ‐56.6 |
| Davies 2003 | 6 months | ‐10.7 | ‐126.6 |
Peritoneal small solute clearance
In low certainty evidence, icodextrin may make little or no difference to peritoneal CrCl (Analysis 2.6 (3 studies, 237 participants): SMD 0.36, 95% CI ‐0.24 to 0.96; I2 = 66%; estimated MD 0.36 mL/min 95% CI 0.24 to 0.95). Moderate to high heterogeneity was observed and appeared to be related to study design variability. Two studies were open‐label in design with unclear description of the number of participants in each peritoneal equilibration test category (Plum 2002; Posthuma 1997). Similar to their findings with peritoneal UF, Lin 2009a reported greater peritoneal CrCl measurements in all participants except low transporters.
2.6. Analysis.
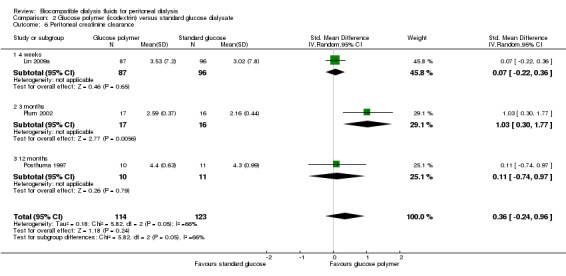
Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 6 Peritoneal creatinine clearance.
Peritonitis
In low certainty evidence, icodextrin may make little or no difference to peritonitis incidence (Analysis 2.7 (6 studies, 667 participants): RR 0.95, 95% CI 0.77 to 1.18; I2 = 0%).
2.7. Analysis.
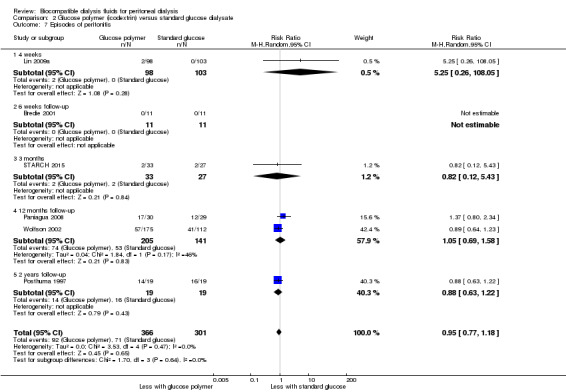
Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 7 Episodes of peritonitis.
Technique failure
The majority of studies had short follow‐up duration (less than six months) and low event numbers. . It is uncertain whether icodextrin use led to any differences in technique failure (Analysis 2.9 (4 studies, 350 participants): RR 0.60, 95% CI 0.32 to 1.12, I2 =0%; very low certainty evidence).
2.9. Analysis.

Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 9 Technique failure (death‐censored).
Patient survival
In the context of low event numbers and short follow‐up durations, it is uncertainty whether icodextrin improves patient survival (Analysis 2.10 (6 studies, 816 participants): RR 0.82, 95% CI 0.32 to 2.13; I2 = 0%; very low certainty evidence).
2.10. Analysis.
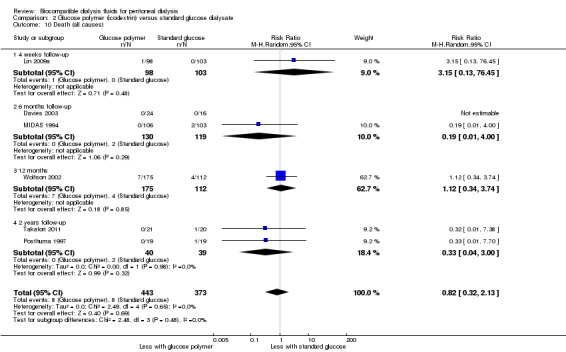
Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 10 Death (all causes).
Adverse events
In low certainty evidence, icodextrin may make little or no difference to the risk of rash compared with glucose exchanges (Analysis 2.8 (3 studies, 755 participants): RR 2.51, 95% CI 0.59 to 10.72; I2 = 38%). %). In very certainty evidence, it is uncertain whether icodextrin use led to any differences in adverse events (5 studies, 816 participants) (Lin 2009a; MIDAS 1994; Paniagua 2008; STARCH 2015; Wolfson 2002) (Table 4).
2.8. Analysis.

Comparison 2 Glucose polymer (icodextrin) versus standard glucose dialysate, Outcome 8 Rash.
Discussion
Summary of main results
This systematic review has demonstrated strong evidence that the use of neutral pH, low GDP PD solution improves preservation of RRF and urine volume compared with conventional PD solution. Its impact on inflow pain was at least comparable if not superior compared to conventional glucose PD solutions. Although the use of neutral pH, low GDP PD solution led to higher D/Pcreat creatinine and lower UF volume during peritoneal equilibration test, there was a moderate level of heterogeneity affecting analysis of UF. Moderate‐to‐high heterogeneity was also observed in the analysis examining the risk of peritonitis, such that the effect of neutral pH, low GDP PD solutions on peritonitis currently remains uncertain. However, when the analysis was repeated according to the risk of attrition bias, the incidence of peritonitis was lower using neutral pH, low GDP PD solutions in studies identified at a low risk of attrition bias. Peritoneal small solute clearance was not affected by neutral pH, low GDP PD solutions. Treatment using neutral pH, low GDP solution was not observed to affect death‐censored technique failure, duration of hospitalisation and death (all causes), although the event rates were too low to be confident that there was no effect on these outcomes. The use of icodextrin in one PD exchange daily led to increased peritoneal UF volumes and a lower risk of uncontrolled fluid overload compared with glucose PD exchanges alone. These benefits were more pronounced in patients with higher peritoneal solute transport rates and extended to individuals with identified UF failure. The augmentation of peritoneal UF was not associated with any changes in residual renal clearance or urine volume. Icodextrin did not affect peritoneal solute transport rate or peritoneal small solute clearance. It also did not affect technique survival or patient survival, although the event rates and study durations were insufficient to adequately study these outcomes.
Overall completeness and applicability of evidence
The review demonstrated that RRF and urine volume were better preserved with neutral pH, low GDP PD solution than with conventional PD solution. The benefit of RRF preservation was observed in both shorter (≤ 1 year) and longer follow‐up durations (> 2 years). The exact mechanism of neutral pH, low GDP solution on preservation of RRF and urine volume remains unclear, however, it is postulated that lower GDP exposure might result in minimisation of GDP‐induced tubular damage and apoptosis based on the previously published experimental work published by Justo 2005. Interestingly, the majority of studies analysed used one particular solution (Balance®, Fresenius Medical Care, Bad Homburg, Germany; Bajo 2011; balANZ 2010; BALNET 2008; Cho 2013; Choi 2008; EURO‐BALANCE 2004; Kim 2003; Park 2012a; Szeto 2007; Szeto 2015), which reportedly contains the lowest level of GDP amongst products available in market (Jorres 2012).
In contrast to the previous review (Cho 2014), this update demonstrated lower UF volume with treatment using neutral pH, low GDP PD solution during the four‐hour peritoneal equilibration test compared to conventional PD solution. The reduced peritoneal UF with neutral pH, low GDP PD solution could lead to body volume expansion, increased urine volume and augmented RRF (Bargman 2010). Another potential explanation could be faster decline in RRF resulting from volume depletion from higher peritoneal UF volume in the conventional PD solution group, as reported in the recent analysis of predictors of RRF based on the results from balANZ 2010 (Htay 2016). Furthermore, it is important to acknowledge the presence of moderate level of heterogeneity, which could not be explained by differences in the study design, study population or risks of bias in included studies. Such observed heterogeneity undermines confidence that neutral pH, low GDP PD solutions alter peritoneal UF.
Moreover, it is also possible that reduced UF capacity in patients receiving neutral pH, low GDP PD solutions could have been the consequence of higher peritoneal solute transport rate (defined as 4‐hour dialysate/plasma creatinine ratio), as shown here. These results should also be interpreted with caution as peritoneal solute transport rate was assessed at a single time point at the end of study rather than as longitudinal changes in peritoneal solute transport rate over the study period and so did not take baseline differences into consideration. This is relevant as three studies (30%) in the analyses reported higher baseline peritoneal solute transport rate in patients treated with neutral pH, low GDP PD solutions compared to those who received conventional PD solutions (balANZ 2010; Lai 2012a; Szeto 2015). Ideally, the effects of types of PD solutions on longitudinal peritoneal solute transport rate trends should be compared rather than comparing the values at a single time point without accounting for inter‐individual differences. For example, balANZ 2010 reported peritoneal solute transport rate stability over two years in patients who were treated with neutral pH, low GDP PD solutions as opposed to a progressively rising peritoneal solute transport rate trajectory over time in those who received conventional PD solution (Johnson 2012).
Another potential benefit of neutral pH, low GDP PD solutions was alleviation of inflow pain. This condition is reported to occur in up to 73% of PD patients receiving conventional PD solutions and has been attributed to their acidic pH (Vaamonde 1975). The trend towards benefit of neutral pH, low GDP fluids on inflow pain in this study supports the common practice of using these fluids for this clinical indication. Nevertheless, the results of the present review should be interpreted cautiously as the only study included in the meta‐analysis was not blinded (Rippe 2001). Consequently, the results may have been potentially influenced by observer and performance biases. Furthermore, one of the cross‐over design studies reported appreciable variation in the frequency of inflow pain amongst the nine participating centres, raising the possibility of confounding centre effects (Mactier 1998).
A noteworthy finding of this review was the uncertain impact of neutral pH, low GDP fluids on either the proportion of individuals experiencing peritonitis or overall peritonitis rates. This issue has become highly topical since the (balANZ 2010) reported that neutral pH, low GDP fluid use resulted in a 50% increase in time to first peritonitis episodes and a 36% reduction in overall peritonitis rates compared with conventional solution. The suggested explanation for this finding was improved peritoneal host defence mechanisms, given that there was considerable experimental evidence that neutral pH, low GDP fluids significantly improved viability and function of peritoneal mesothelial cells, leukocytes and macrophages (Boulanger 2002; Jorres 1998; Mortier 2003; Schambye 1996; Topley 1997; Witowski 2005). A similar beneficial effect of biocompatible PD solutions on peritonitis rates had also been reported following extended follow‐up in another study (Tranaeus 2000). In contrast, most investigations, which were small and underpowered, found no effect of biocompatible PD solutions on peritonitis risk (Bajo 2011; BALNET 2008; Choi 2008; Coles 1997; DIUREST 2010; Fan 2008; Feriani 1998; Fernandez‐Perpen 2012; Park 2012a; Rippe 2001; Szeto 2015; TRIO 2016; Yoo 2015). Perhaps not surprisingly, when all of these studies were combined in a meta‐analysis, significant heterogeneity was observed. When this heterogeneity was explored, the main contributing factor appeared to be risk of attrition bias (i.e. due to patient drop‐out), such that peritonitis rates were significantly reduced by neutral pH, low GDP PD solutions in studies with low risk of attrition bias due to low drop‐out rates (balANZ 2010; Szeto 2015; Yoo 2015). Future well‐designed RCTs are warranted to address this relation.
Icodextrin significantly augmented peritoneal UF in both short‐term and long‐term studies (up to 24 months) and when compared to various concentrations of glucose PD solutions, including hypertonic exchanges. For instance Finkelstein 2005 observed a net change in UF volume of 401.6 ± 79 mL/d in the icodextrin group compared to ‐6.98 ± 57.2 mL/d in the 4.25% glucose group at two weeks. Importantly, the UF benefit of icodextrin extended to patients with UF failure and was superior to 4.25% glucose PD solution use (+373.8 ± 58.9 mL/d versus ‐239.7 ± 151.0 mL/d, respectively; Finkelstein 2005). Similarly, subgroup analysis of the two studies (Posthuma 1997; Takatori 2011) with the longest follow‐up periods (24 months; Analysis 2.1) showed a MD of 510.55 mL/d (95% CI 10.10 to 1011, P = 0.05), in favour of icodextrin. The findings of this systematic review therefore support the recommendations of the International Society for Peritoneal Dialysis (ISPD) Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis that icodextrin should be used in the long dwell of patients who are identified to have high peritoneal solute transport rate or UF failure (Mujais 2000). It also supports the ISPD Cardiovascular and Metabolic Guideline recommendation that “once‐daily icodextrin be considered as an alternative to hypertonic glucose PD solutions for long dwells in PD patients experiencing difficulties maintaining euvolaemia due to insufficient peritoneal UF, taking into account the individual patient’s peritoneal transport state” (Wang 2015).
Given that manipulation of peritoneal UF via various interventions has not infrequently been reported to induce reciprocal changes in urine volume and residual renal clearance measurements (Bargman 2010; Davies 2009), these outcomes were specifically examined in the present review and found not to be compromised by icodextrin‐enhanced peritoneal UF.
Similarly, the additional fluid volume removed via the peritoneal cavity with icodextrin was not associated with increased peritoneal small solute clearance measurements. It should be noted however that moderate to high study heterogeneity was detected, primarily related to variability in the peritoneal membrane transport characteristics of patients included in each study. Indeed, enhancement of small solute clearance with icodextrin use was reported by two studies, with benefit seen only in those individuals with higher peritoneal solute transport rate (Finkelstein 2005; Lin 2009a). Further studies are therefore warranted to examine the effects of icodextrin on peritoneal small solute clearance according to peritoneal transport status.
Reassuringly, icodextrin was not found to be associated with increased harm compared with glucose exchanges alone. Skin rash was the most commonly reported adverse event, which led to cessation of icodextrin in 0% to 4.3% of patients (Finkelstein 2005; Lin 2009a; Wolfson 2002) across the identified studies. However, no study reported occurrence of rash severe enough to warrant hospitalisation or additional therapeutic interventions other than cessation of icodextrin. It is unknown whether any of these patients were subsequently re‐challenged using icodextrin. Similarly, six studies reported comparable incidence of adverse events with the use of neutral pH, low GDP PD solutions compared to conventional PD solutions (balANZ 2010; Coles 1997; EURO‐BALANCE 2004; Feriani 1998; Schmitt 2002; Tranaeus 2000).
Despite the fact that use of neutral pH, low GDP PD solution was associated with better preservation of RRF and urine volume, its use did not result in improved technique or patient survival compared with conventional PD solution. Given that the numbers of events and included patients in this meta‐analysis were relatively small, the review was inadequately powered to examine these hard end‐points.
Quality of the evidence
The quality of the present review was suboptimal as many studies failed to specify methods of randomisation, allocation concealment and blinding of outcome assessors. In addition, a considerable number of studies had high risks of attrition bias (50%) and reporting bias (38%). It was often unclear whether data were analysed based on intention‐to‐treat analysis and how the study dealt with dropouts. In general, studies were limited by small sample size, large dropout numbers, and short follow‐up durations, which reduced the strength of the review.
Potential biases in the review process
The strength of this review is that it included an up‐to‐date and comprehensive systematic review of previous publications through a thorough MEDLINE, EMBASE, and CENTRAL search and included only RCTs or quasi‐RCTs, as pre‐specified. For cross‐over RCTs, in order to minimise the carry‐over effect, only the data from the first phase of studies were included for analyses. Data extraction, data analysis, and quality assessment were performed by two independent investigators, and any differences in consensus were checked with an additional two authors. The outcome of peritonitis was examined in terms of both peritonitis rate and incidence of peritonitis to account for non‐standardised methods of reporting across studies. However, it should be noted that there is a potential for bias as one of the authors of this review (DJ) was the principal investigator of balANZ 2010 which was included in this review.
Agreements and disagreements with other studies or reviews
This review demonstrated that the use of low GDP PD solution improved preservation of RRF at all study time points. This finding is similar to that of a previous review by Yohanna 2015, who also reported that the benefit of better preservation of RRF with low GDP PD solution was observed even in the early (within six months) follow‐up period and at all various study periods. This review also drew a similar conclusion regarding RRF and urine volume preservation as that of balANZ 2010, one of the largest and highest quality RCTs examining the effects of low GDP PD solution on RRF. This study reported that the use of low GDP PD solution was associated with 27% better preservation of RRF and 37% better preservation of urine volume than conventional PD solution, after adjusting for potential confounders (Htay 2016).
The peritoneal solute transport rate (D/Pcreat) was significantly higher with the use of low GDP PD solution in the present review. The disparity of this finding compared with that of previous reviews (Cho 2014; Yohanna 2015) might relate to the fact that peritoneal solute transport rate was assessed at a single time point at the end of study, rather than as longitudinal changes in peritoneal solute transport rate over the study period and so did not take baseline differences into consideration. This finding of the review also contrasted with that of one of the largest RCTs (balANZ 2010), which compared the longitudinal changes of peritoneal solute transport rate between the two PD solutions and observed that peritoneal solute transport rate was stable in patients treated with low GDP PD solution but was increased in patients treated with conventional PD solution over a two year follow‐up period (Johnson 2012).
The present review also observed lower four‐hour peritoneal UF volume measured during a peritoneal equilibration test in patients treated with neutral pH low GDP PD solution compared with those treated with conventional PD solution. This finding was not observed in the previous review. This analysis in the present review suffered a moderate level of heterogeneity which could not be satisfactorily explained and which reduced confidence in the finding.
Authors' conclusions
Implications for practice.
In PD patients, treatments using neutral pH, low GDP PD solutions should be used to improve preservation of RRF and urine volume.
Neutral pH, low GDP PD solutions should also be considered when inflow pain is present.
Icodextrin use should be used in patients who require an increase in peritoneal UF to achieve optimal fluid status, particularly those with high or high‐average membrane transport characteristics.
The current available evidence is insufficient to accurately determine the effects of neutral pH, low GDP PD solutions or icodextrin on other clinical outcomes including peritonitis, peritoneal solute transport rate, technique survival or patient survival.
Implications for research.
Further studies are needed to adequately determine the effect of neutral pH, low GDP PD solution on patient level‐outcomes, such as peritonitis and technique survival. These studies should be adequately powered and of sufficient duration. Studies should only include one type of biocompatible PD solution in the treatment group given variable concentrations of GDP amongst available products. This is particularly relevant when examining the effect on peritonitis as different products using different connectology.
Specific outcomes to add value would include assessment of longitudinal change in peritoneal solute transport rate rather than an absolute result to assess the trend in peritoneal solute transport rate.
Future research should be conducted using standard definitions and clearly state co‐interventions used if they are likely to influence the measured outcome (e.g. icodextrin and peritoneal UF). A comprehensive list of definitions is available from the ISPD guidelines to guide designing future studies.
Future appropriately powered studies are needed to examine the effect of icodextrin on hypertension control and cardiovascular endpoints
What's new
| Date | Event | Description |
|---|---|---|
| 27 August 2018 | New citation required and conclusions have changed | New data added |
| 27 August 2018 | New search has been performed | Search strategies updated; new studies incorporated |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 18 August 2016 | Amended | Correction of table 2 results |
| 2 May 2014 | Amended | Minor edits to study names to match Renal Group's Specialised Register |
Acknowledgements
The authors gratefully acknowledge the contribution of Drs Rafael Selgas, Jiaqi Qian, Jun Wada, Kook‐Hwan Oh, Antonio Fernandez‐Perpen, Claus Peter Schmitt, Mariano Feriani, Adelheid Gauly, Cheuk‐Chun Szeto, Sun‐Hee Park, Tabo Sikaneta, Tae‐Hyun Yoo, Thyago Proenca de Moraes, who responded to our queries about their studies. David Johnson is a current recipient of a National Health and Medical Research Council Practitioner Fellowship. Yeoungjee Cho is a current recipient of a National Health and Medical Research Council Early Career Development Fellowship.
We would like to thank the referees for their comments and feedback during the preparation of this review update.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Low GDP (all buffer types) versus standard glucose dialysate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Residual renal function | 15 | 835 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.05, 0.33] |
| 1.1 3 months | 3 | 143 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.37, 0.29] |
| 1.2 12 months | 6 | 349 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.04, 0.39] |
| 1.3 24 months | 5 | 218 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.06, 0.60] |
| 1.4 3 years + | 1 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.11, 0.59] |
| 2 Residual renal function: up to 12 months | 12 | 860 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.05, 0.32] |
| 2.1 4 weeks | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.46, 0.67] |
| 2.2 3 months | 3 | 143 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.37, 0.29] |
| 2.3 6 months | 6 | 440 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [0.10, 0.54] |
| 2.4 8 months | 1 | 91 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.44, 0.38] |
| 2.5 9 months | 1 | 138 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.12, 0.55] |
| 3 Residual renal function: 12 months up to 24 months | 10 | 641 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [0.10, 0.41] |
| 3.1 12 months | 8 | 545 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.09, 0.43] |
| 3.2 18 months | 2 | 96 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.17, 0.64] |
| 4 Residual renal function: 24 months and beyond | 6 | 343 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.08, 0.51] |
| 4.1 24 months | 5 | 218 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.06, 0.60] |
| 4.2 3 years + | 1 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.11, 0.59] |
| 5 Residual renal function: PD fluid types | 15 | 835 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.05, 0.33] |
| 5.1 Balance | 9 | 495 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.02, 0.37] |
| 5.2 Purely bicarbonate buffered | 3 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.46, 0.59] |
| 5.3 Multiple fluid types in treatment group | 2 | 218 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.20, 0.41] |
| 5.4 Gambrosol trio | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.02, 1.02] |
| 6 Urine volume | 11 | 791 | Mean Difference (IV, Random, 95% CI) | 114.37 [47.09, 181.65] |
| 6.1 3 months | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐515.46, 514.38] |
| 6.2 12 months | 6 | 392 | Mean Difference (IV, Random, 95% CI) | 120.94 [45.49, 196.39] |
| 6.3 24 months | 2 | 154 | Mean Difference (IV, Random, 95% CI) | 123.27 [‐51.83, 298.36] |
| 6.4 3 years + | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 270.6 [10.53, 530.67] |
| 7 Urine volume: up to 12 months | 10 | 819 | Mean Difference (IV, Random, 95% CI) | 69.72 [‐55.95, 195.40] |
| 7.1 1 month | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐30.0 [‐399.49, 339.49] |
| 7.2 3 months | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐515.46, 514.38] |
| 7.3 6 months | 6 | 558 | Mean Difference (IV, Random, 95% CI) | 157.25 [58.48, 256.01] |
| 7.4 8 months | 1 | 91 | Mean Difference (IV, Random, 95% CI) | ‐147.2 [‐262.68, ‐31.72] |
| 8 Urine volume: 12 months up to 24 months | 8 | 579 | Mean Difference (IV, Random, 95% CI) | 110.57 [40.81, 180.34] |
| 8.1 12 months | 6 | 392 | Mean Difference (IV, Random, 95% CI) | 120.94 [45.49, 196.39] |
| 8.2 18 months | 2 | 187 | Mean Difference (IV, Random, 95% CI) | 60.94 [‐186.22, 308.10] |
| 9 Urine volume: 24 months and beyond | 3 | 279 | Mean Difference (IV, Random, 95% CI) | 169.22 [23.98, 314.46] |
| 9.1 24 months | 2 | 154 | Mean Difference (IV, Random, 95% CI) | 123.27 [‐51.83, 298.36] |
| 9.2 3 years + | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 270.6 [10.53, 530.67] |
| 10 Urine volume: PD fluid types | 11 | 791 | Mean Difference (IV, Random, 95% CI) | 114.37 [47.09, 181.65] |
| 10.1 Balance | 7 | 482 | Mean Difference (IV, Random, 95% CI) | 120.48 [47.99, 192.97] |
| 10.2 Purely bicarbonate buffered | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 345.50 [‐274.17, 965.17] |
| 10.3 Multiple fluid types in the treatment group | 2 | 218 | Mean Difference (IV, Random, 95% CI) | 37.84 [‐423.31, 498.99] |
| 10.4 Gambrosol trio | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 130.0 [‐105.87, 365.87] |
| 11 Anuria | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.18, 1.75] |
| 12 Peritoneal ultrafiltration: 4 hours | 9 | 414 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.74, ‐0.10] |
| 12.1 2.26%/2.5% glucose | 4 | 218 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.76, 0.07] |
| 12.2 3.86%/4.25% glucose | 5 | 196 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.08, 0.11] |
| 13 4‐hour dialysate:plasma creatinine (2.27%, 2.4%, or 2.5% glucose) | 10 | 746 | Mean Difference (IV, Random, 95% CI) | 0.01 [0.00, 0.03] |
| 13.1 3 months | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 13.2 12 months | 6 | 448 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 13.3 24 months | 2 | 149 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 13.4 3 years + | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 14 4‐hour dialysis:plasma creatinine (patient characteristics) | 10 | 746 | Mean Difference (IV, Random, 95% CI) | 0.01 [0.00, 0.03] |
| 14.1 Incident patients | 7 | 554 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.00, 0.04] |
| 14.2 Prevalent patients | 3 | 192 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
| 15 4‐hour dialysis:plasma creatinine (PD fluid types) | 10 | 746 | Mean Difference (IV, Random, 95% CI) | 0.01 [0.00, 0.03] |
| 15.1 Balance | 6 | 470 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 15.2 Purely bicarbonate buffered | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 15.3 multiple fluid type in the treatment group | 2 | 187 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 15.4 Gambrosol trio | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 16 4‐hour dialysis:plasma creatinine (study design) | 10 | 746 | Mean Difference (IV, Random, 95% CI) | 0.01 [0.00, 0.03] |
| 16.1 Single centre | 4 | 277 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 16.2 Multicentre | 6 | 469 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.00, 0.04] |
| 17 Peritoneal creatinine clearance [L/wk/1.73 m²] | 7 | 510 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐2.03, 1.15] |
| 17.1 3 months | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐7.46 [‐20.65, 5.73] |
| 17.2 12 months | 4 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.28, 1.66] |
| 17.3 24 months | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐6.80, 1.60] |
| 17.4 3 years + | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐2.37, 4.97] |
| 18 Peritoneal Kt/V urea | 6 | 422 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.12, 0.09] |
| 18.1 3 months | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.78, 0.76] |
| 18.2 12 months | 4 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.18, 0.16] |
| 18.3 3 years + | 1 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.18, 0.10] |
| 19 Incidence of peritonitis | 12 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.92, 1.72] |
| 19.1 8 weeks follow‐up | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.49, 5.10] |
| 19.2 24 weeks | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.58, 2.36] |
| 19.3 12 months follow‐up | 5 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.93, 1.73] |
| 19.4 18 months follow‐up | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.56, 2.07] |
| 19.5 24 months follow‐up | 4 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.62, 2.84] |
| 20 Peritonitis rate (episodes/total patient‐months) | 10 | 18184 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.84, 1.64] |
| 21 Incidence of peritonitis: attrition bias risk | 12 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.92, 1.72] |
| 21.1 Low risk | 3 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| 21.2 High risk | 9 | 696 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.28, 1.96] |
| 22 Inflow pain | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 6 months | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.24, 1.08] |
| 23 Hospitalisation | 2 | 230 | Mean Difference (IV, Random, 95% CI) | 3.02 [‐7.08, 13.12] |
| 23.1 12 months | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐6.71, 5.91] |
| 23.2 24 months | 1 | 182 | Mean Difference (IV, Random, 95% CI) | 10.79 [‐4.28, 25.86] |
| 24 Technique failure (death‐censored) | 15 | 1275 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.75, 1.63] |
| 24.1 3 months | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 6 months | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.97] |
| 24.3 12 months | 5 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.31, 2.21] |
| 24.4 24 months | 5 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.70, 1.90] |
| 24.5 3 years + | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [0.58, 14.33] |
| 25 Death (all causes) | 15 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.47, 1.14] |
| 25.1 3 months | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 3.09] |
| 25.2 12 months follow‐up | 7 | 633 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.19] |
| 25.3 18 months follow‐up | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.84] |
| 25.4 24 months follow‐up | 4 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.56, 2.51] |
| 25.5 3 years + | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.29, 2.03] |
Comparison 2. Glucose polymer (icodextrin) versus standard glucose dialysate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daily ultrafiltration | 4 | 102 | Mean Difference (IV, Random, 95% CI) | 448.54 [289.28, 607.80] |
| 1.1 3 months | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 416.0 [236.26, 595.74] |
| 1.2 4 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 607.0 [‐2164.12, 3378.12] |
| 1.3 24 months | 2 | 37 | Mean Difference (IV, Random, 95% CI) | 510.55 [10.10, 1011.00] |
| 2 Uncontrolled fluid overload | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.59] |
| 2.1 24 months | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.10, 1.01] |
| 2.2 12 months | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.12, 0.67] |
| 3 Residual renal function | 4 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.26, 0.49] |
| 3.1 Residual GFR (mL/min) | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.75, 0.66] |
| 3.2 Renal CrCl (mL/min/1.73 m²) | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.51, 1.07] |
| 3.3 Renal CrCl (mL/min) | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.05, 0.58] |
| 3.4 Residual renal function (mL/min/1.73 m²) | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.29, 1.09] |
| 4 Urine volume | 3 | 69 | Mean Difference (IV, Random, 95% CI) | ‐88.88 [‐356.88, 179.12] |
| 4.1 4 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐35.0 [‐2561.49, 2491.49] |
| 4.2 2 years | 2 | 37 | Mean Difference (IV, Random, 95% CI) | ‐89.49 [‐359.01, 180.03] |
| 5 Change in urine volume (mL) | Other data | No numeric data | ||
| 6 Peritoneal creatinine clearance | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.24, 0.96] |
| 6.1 4 weeks | 1 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.22, 0.36] |
| 6.2 3 months | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 1.03 [0.30, 1.77] |
| 6.3 12 months | 1 | 21 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.74, 0.97] |
| 7 Episodes of peritonitis | 6 | 667 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.77, 1.18] |
| 7.1 4 weeks | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 5.25 [0.26, 108.05] |
| 7.2 6 weeks follow‐up | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 3 months | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.12, 5.43] |
| 7.4 12 months follow‐up | 2 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.69, 1.58] |
| 7.5 2 years follow‐up | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.22] |
| 8 Rash | 3 | 755 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [0.59, 10.72] |
| 9 Technique failure (death‐censored) | 4 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.32, 1.12] |
| 10 Death (all causes) | 6 | 816 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.32, 2.13] |
| 10.1 4 weeks follow‐up | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.13, 76.45] |
| 10.2 6 months follow‐up | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 4.00] |
| 10.3 12 months | 1 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.34, 3.74] |
| 10.4 2 years follow‐up | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bajo 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "patients were alternately assigned to either 'balance' or standard PD fluid depending on the time point of inclusion" |
| Allocation concealment (selection bias) | High risk | Quote: "patients were alternately assigned to either 'balance' or standard PD fluid depending on the time point of inclusion" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High drop‐out rate: 21/33 (63.6%) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | High risk | Outcome parameters significantly different at baseline (e.g. urine volume, RRF) |
balANZ 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To ensure adequate concealment of allocation, randomization was performed using a central computer and web‐based link to the central database, with stratification according to centre and the presence or absence of diabetic nephropathy" |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure adequate concealment of allocation, randomization was performed using a central computer and web‐based link to the central database, with stratification according to centre and the presence or absence of diabetic nephropathy" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "An open‐label study", but unlikely to have influenced the objective outcomes measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate 3/185 (1.6%), balanced between groups |
| Selective reporting (reporting bias) | Low risk | All relevant clinical parameters reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
BALNET 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
After 4‐week run‐in phase on conventional fluid, each group started CAPD with the designated PD solution |
|
| Outcomes | Primary end point
Secondary end points
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "open‐labelled, randomised, prospective study" However, unlikely to have influenced the objective outcomes measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate, 22/91 (24.1%), 5/48 patients in low GDP group switched to HD ‐ no reason specified that may have been relevant to the therapy they had received |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Bredie 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Patients performed CAPD with both control and treatment fluids for a period of 6 weeks each |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate: 1/22 (5%) |
| Selective reporting (reporting bias) | High risk | Limited clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Cancarini 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open label, however unlikely to have affected outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate of 7/33 (21.2%). Reason for dropout not reported |
| Selective reporting (reporting bias) | High risk | Limited reporting of outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Carrasco 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (3 patients/block) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open label, however, unlikely to have affected measured outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | High risk | Limited reporting of clinical outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Cho 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | open‐label, unlikely to affect the outcomes of study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24% dropout |
| Selective reporting (reporting bias) | High risk | limited outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Choi 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label design thus no blinding of investigators or participants. However, unlikely to have impacted on objective clinical outcomes (e.g. urine volume) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of patients voluntarily changed to the low GDP PD solution (36/104 (35%)). Per protocol analysis |
| Selective reporting (reporting bias) | Low risk | All appropriate outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Cnossen 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low drop‐out rate (3/26 (11.5%)) |
| Selective reporting (reporting bias) | High risk | Limited reporting of clinical outcomes |
| Other bias | High risk | A large difference in baseline age between the treatment and control groups raise concern for inadequate randomisation |
Coles 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation in groups of 3, done separately for each centre; actual method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. Measured outcome (e.g. abdominal pain) may have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out 13/59 (22%). Unequal between three groups with peritonitis being the major cause which may have been due to the treatment received |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Davies 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Methods section of paper: "Randomised 1:1 with stratification for centre/country, dialysis modality (CAPD or APD), and presence of cardiovascular disease or LVH" |
| Allocation concealment (selection bias) | Low risk | Quote from Methods section of paper: "The treatment codes were supplied to study sites in sealed envelopes, which were checked at the end of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from Methods section of paper: "Identity of the long‐dwell solution blinded to patients, investigators and clinical monitors; specially created packaging was used to conceal which solution was which solution was which" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate 20% (10/50). Quote from Results section of paper: "Additional withdrawals from the 2.27% glucose group were for UF failure and patient preference" |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
di Paolo 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Cross‐over design, with two study periods of 3 months each, separated by a 2 week wash out period |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
DIUREST 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Methods section of paper: "Randomization was performed by means of a centrally managed list based on a table of random numbers in blocks of four and stratified for the presence of diabetes" |
| Allocation concealment (selection bias) | Low risk | Quote from Methods section of paper: "Randomization was performed by means of a centrally managed list based on a table of random numbers in blocks of four and stratified for the presence of diabetes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from Methods section of paper: "... open, parallel study". As fluid balance is one of the main outcomes assessed, risks influencing co‐intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High drop‐out rate (41/80 (51.3%)), imbalance between the number of dropouts from each arm. Per protocol analysis |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
EURO‐BALANCE 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Patients received 12 weeks of treatment with both solutions |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Specific randomisation technique not reported. Quote: "After the run‐in phase, patients were randomized (1:1) to either ..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study with subjective outcome measure such as inflow pain was one of the assessed parameters |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate: 15/86 (17%). Although per protocol analysis performed, the reasons for dropout from initial study group is balanced after excluding the reasons that are unlikely to be dialysis related (e.g. transplantation, flare‐up of vasculitis) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Fan 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of investigators or participants. However, unlikely to have impacted on objective clinical outcomes (e.g. urine volume) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate of 21.2% (25/118). Not all accounted for with many under "did not complete". Reason unclear |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | High risk | Issue of connectology and allowance of Nutrineal/icodextrin usage in patients who used Baxter System. Multiple types of PD solutions used in both intervention and control groups |
Feriani 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised centrally and separately for each study centre and block randomisation in steps of four used |
| Allocation concealment (selection bias) | Low risk | No specific information provided. However central randomisation with probable low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label, may have affected symptom assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of participants missing between two phases of study that are not accounted for in the paper: 18/123 (14.6%) for 12 weeks; 4/73 (5.5%) for 24 week study |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Fernandez‐Perpen 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "patients were randomly assigned to either BicaVera or the standard PD fluid by the doctors" |
| Allocation concealment (selection bias) | High risk | Quote: "patients were randomly assigned to either BicaVera or the standard PD fluid by the doctors" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate (23 /31 (74.2%)). Imbalance in missing data between the two groups |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | High risk | Outcome parameters significantly different at baseline (e.g. urine volume, RRF) |
Finkelstein 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally maintained randomisation list |
| Allocation concealment (selection bias) | Low risk | Centrally maintained randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind ‐ use of identical solution bags |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate: 7/92 (8%), balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes within the short time frame reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Fusshoeller 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Patients completed 6 months of APD using either control or treatment PD solution, followed by a further 6 months of APD using the alternative PD solution |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. This is particularly relevant as 'inflow pain' was reported as one of the outcomes in unblinded state |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate: 2/14 (14%) |
| Selective reporting (reporting bias) | High risk | Primary outcome of interest of this study related to peritoneal macrophages and inflammatory markers. No other clinical parameter was reported ‐ including peritonitis, survival, RRF |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Kim 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement, stratified for diabetes mellitus |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label, however unlikely to have influenced the objective outcome measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates ‐ 38/64 (59%). Missing participants not accounted for. Per protocol analysis |
| Selective reporting (reporting bias) | High risk | No report of peritonitis or survival |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Konings 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | "Randomized with the use of sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open label ‐ but unlikely to have influenced objective outcomes measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout 20% (8/40), but all accounted for. Per protocol analysis |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lai 2012a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Composite co‐primary outcomes
Also determined
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random assignments were made by the patient's training nursing officer at the individual renal centre |
| Allocation concealment (selection bias) | High risk | Random assignments were made by the patient's training nursing officer at the individual renal centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label, however unlikely to have affected the objective outcomes measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Per protocol analysis |
| Selective reporting (reporting bias) | High risk | Peritonitis not reported |
| Other bias | High risk | Although baseline characteristics were reported to be similar. The paper did not disclose the duration of PD that these patients received, so one cannot exclude that they may represent different vintage. Also, for biochemical analyses, there is no baseline value available, thus it is difficult to be certain whether differences are present truly or due to type I error Multiple types of PD solutions used in intervention and control groups. |
Lin 2009a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using a computer program that generated numbers instead of treatment assignments"; block randomisation, using a 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Quote: "Envelopes that contained the number corresponding to the dialysate regiment were held by personnel not directly involved with the study, and could be opened only in an emergency" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither doctors nor patients knew the dialysate regimen" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither doctors nor patients knew the dialysate regimen" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low dropout rate, 18/201 (8.9%) |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcome parameters are reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Mactier 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Patients evaluated during two dialysis exchanges with each test solution in random order. Thus, all patients underwent six separate study dwells, with a maximum of 2 test evaluations in 1 day, but it was required that these study exchanges were separated by a routine dwell (40 mM lactate solution) of at least 4 hours. All dwells for at least 3 hours, using 3.86% glucose solutions. Solutions used:
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1/18 (5.6%) lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Limited reporting of outcomes, but given short duration of study, not possible |
| Other bias | High risk | Large variation within the 8 participating centres in the frequency of inflow pain |
MIDAS 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "telephone from a single office (Innovata Biomed) at the first visit" |
| Allocation concealment (selection bias) | Low risk | Quote: "telephone from a single office (Innovata Biomed) at the first visit" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although mod‐high dropout rate (71/209 (34%)), all missing participants are accounted for and reasonably balanced in terms of cause |
| Selective reporting (reporting bias) | High risk | RRF not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Pajek 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement other than, quote: "patients were randomized (1:1)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "open‐label, randomized". However, unlikely to have influenced the measured objective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate ‐ 5/26 (19.2%). Per protocol analysis |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Paniagua 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Liberal use of 2.5% or 4.25% glucose was allowed in both groups in order to reach treatment goals. Dietary sodium intake prescription was 50 mmol/d for both groups |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Assignment was in a 1:1 ratio through a central randomisation centre |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. However, unlikely to have influenced the objective outcomes measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23 participants dropped out. It is also unclear if 23 were out of 59, or the 82 participants to start with. Per protocol analysis |
| Selective reporting (reporting bias) | High risk | Limited reporting of clinical outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Park 2012a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label, unlikely to affect outcomes of study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall 23% dropout |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Plum 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label, could have affected introduction of co‐intervention affecting the fluid balance measurement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Per protocol analysis; 6/39 (15.4%) lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Peritonitis not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Posthuma 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open label, unlikely to have influenced the objective outcomes measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate of 34% (13/38) at 24 months, but all accounted for and balanced |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Rippe 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation office (stratified randomisation with respect to patient age (< 55, > 55 years), diabetes (using insulin or not), and time on PD (< 9 months, > 9 months)) |
| Allocation concealment (selection bias) | Low risk | Not reported but presume low risk given central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open‐label". During pain assessment phase, no blinding took place which may have affected patient response |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although all dropouts accounted for, extremely high proportion (67/80, 83.75%) did not complete the study duration |
| Selective reporting (reporting bias) | High risk | RRF not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Schmitt 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Patients performed their usual APD regimen with either the control or treatment fluid for 12 weeks. After a 4 week washout period they completed 12 weeks of APD using the alternative fluid |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label, however, unlikely to have influenced the objective outcome measures reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate (12/28, 43%), unclear during which phase of treatment the dropouts occurred. Per protocol analysis |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
STARCH 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized randomisation schedule in coordinating centre with Random allocation software |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation using random allocation software |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label, unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% dropout rate |
| Selective reporting (reporting bias) | High risk | limited outcomes of study reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Szeto 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized by drawing sealed envelopes, which were prepared and then maintained by a third party not involved in the conduction of the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomized by drawing sealed envelopes, which were prepared and then maintained by a third party not involved in the conduction of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Open‐label", but unlikely to have influenced the objective outcomes measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported other than for Subjective global assessment ‐ trained observers were blinded from treatment group allocation and biochemical results of the patients |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate: 2/50 (4%) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Szeto 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | "This study was supported in part by the Fresenius Medical Care, and CUHK research account 6901031. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a standard randomisation table |
| Allocation concealment (selection bias) | Low risk | Randomised by third party using randomisation table |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label, unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% dropout rate |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | High risk | Different in baseline characteristic between two groups |
Takatori 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label multicenter clinical trial". Given fluid balance is one of the major outcomes assessed, unblinded nature may have influenced introduction of co‐intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High dropout rate ‐ 18/41 (43.9%). However, the majority of dropouts (12) were due to reaching the primary endpoint (i.e. fluid overload), presence of attrition bias for other outcomes cannot be excluded |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Tranaeus 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Open‐label", however, unlikely to have influenced the objective outcomes measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18/106 (17%) at 6 months; 13/57 (23%) at 12 months, dropout rate at 12 months not accounted for. Overall drop‐out rate 62/106 (58.5%) |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
TRIO 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Standard computerized algorithm |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label, unlikely to have major impact on outcomes of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to effect the outcomes of study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33% dropout rate |
| Selective reporting (reporting bias) | Low risk | All outcomes of study were reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Weiss 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A centralized randomization procedure was applied stratifying for diabetes status and time on PD" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "open‐label study", however during pain assessment phase the treatment was instituted in blinded manner. Other measured parameters are objective and unlikely to have been affected |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate, 19/53 (35.8%). Although all missing participants accounted for, but not reported at what stage. Difficult to be certain how many patients completed each phase of the study. Four patients did not participate in pain assessment phase and reasons not provided |
| Selective reporting (reporting bias) | Low risk | All relevant clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Wolfson 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Dialysate volume was either 2L or 2.5L, depending on the patient's usual prescriptions |
|
| Outcomes | Efficacy study
Safety study
During the study period, group added quality of life assessment as part of the study protocol |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program |
| Allocation concealment (selection bias) | Low risk | Central list maintained by personnel not directly involved with the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind status |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate: 118/287 (41%); efficacy (12/175, 6.9%); safety (118/287, 41%) |
| Selective reporting (reporting bias) | High risk | Limited clinical outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Yoo 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label, unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15.5% dropout |
| Selective reporting (reporting bias) | High risk | Limited outcomes are reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Zeier 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate, 6/21 (28.6%) |
| Selective reporting (reporting bias) | High risk | Limited reporting of clinical outcomes. However, the primary purpose of the study was to examine the non‐clinical effects (e.g. biomarkers) of GDP in biocompatible PD solutions |
| Other bias | Unclear risk | Insufficient information to permit judgement |
APD ‐ automated peritoneal dialysis; CAPD ‐ continuous ambulatory peritoneal dialysis; CrCl ‐ creatinine clearance; ESKD ‐ end‐stage kidney disease; GFR ‐ glomerular filtration rate; HD ‐ haemodialysis; HIV ‐ human immunodeficiency virus; M/F ‐ male/female; PET ‐ peritoneal equilibration test; PD ‐ peritoneal dialysis; RCT ‐ randomised controlled trial; RRF ‐ residual renal function; SD ‐ standard deviation; SLE ‐ systemic lupus erythromatosus; UF ‐ ultrafiltration
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| BIOKID 2004 | Wrong intervention: comparing two types of biocompatible PD solutions |
| Braide 2009 | Wrong intervention: citrate supplementation rather than use of biocompatible PD fluids |
| Chang 2016 | Wrong intervention: icodextrin versus non‐icodextrin in the background of using low GDP biocompatible solutions |
| Chow 2014 | Wrong intervention: icodextrin as part of treatment of peritonitis |
| Coester 2006 | Wrong intervention: effect of glucose sparing therapy (NEP) |
| Dallas 2004 | Wrong intervention: icodextrin versus Icodextrin/glucose combination novel fluid |
| de Fijter 1993 | Study conducted over a short period with a view to assessing macrophage function hence study duration too short to evaluate pre‐specified outcomes |
| EDEN 2013 | Wrong intervention: glucose sparing therapy (NEP) |
| Fang 2008 | Wrong intervention: neutral pH, low GDP PD solution as peritoneal equilibration test solution |
| Feriani 1993 | Wrong intervention: two solutions with differences in buffers (bicarbonate against lactate) |
| Fischbach 2004 | Study conducted over two days to examine the effect of PD solutions on intraperitoneal pressure. Duration of study was too short to evaluate pre‐specified patient level clinical outcomes |
| Hiss 2013 | Wrong intervention: normal versus reduced glucose content in the dialysis solution |
| Hwang 2006 | Wrong intervention: icodextrin as peritoneal equilibration test solution |
| IMPENDIA 2013 | Wrong intervention: glucose sparing therapy (NEP) |
| Jenkins 2003 | Wrong intervention: icodextrin versus novel combination solution composed of icodextrin and dextrose |
| John 2008 | Study conducted over one day to examine the effect of biocompatible PD solutions on baroreflex sensitivity. Duration of study was too short to evaluate pre‐specified patient level clinical outcomes |
| le Poole 2004 | Wrong intervention: amino acid‐based dialysis solution |
| Liberek 2002 | Study conducted following 2 overnight dwells (one week apart) of biocompatible and conventional PD solutions to measure inflammatory markers in the PD effluent. Duration of study was too short to evaluate pre‐specified patient level clinical outcomes |
| Lui 2012 | Wrong intervention: amino acid‐containing dialysis solution |
| Martikainen 2005 | Wrong intervention: amino acid‐containing dialysis solution |
| Parikova 2007 | Wrong intervention: neutral pH, low GDP PD solution as a peritoneal equilibration test solution |
| Pedersen 1985 | Wrong intervention: comparing buffers in PD solution |
| Peers 1997 | Wrong intervention: novel icodextrin/glucose combination fluid |
| Plum 1997 | Wrong intervention: amino acid‐containing dialysis solution |
| Rodriguez‐Carmona 2007 | Wrong intervention: amino acid‐based dialysis solution |
| Sav 2009 | Wrong intervention: twice‐daily icodextrin versus daily icodextrin |
| Sav 2010 | Wrong intervention: twice‐daily icodextrin versus daily icodextrin |
| Selby 2005 | Study conducted over two days to examine the effect of PD solutions on haemodynamic parameters. Duration of study was too short to evaluate pre‐specified patient level clinical outcomes |
| Selby 2007a | Wrong intervention: amino acid fluid |
| Smit 2000 | Wrong intervention: glycerol‐based fluid |
| Smit 2001 | Wrong intervention: different strengths of glucose solutions |
| Ueda 2000 | Wrong intervention: amino acid fluid study |
| Van Biesen 2004 | Wrong intervention: amino acid fluid study |
| Yehia 2014 | Wrong intervention: icodextrin versus exchange‐free dialysis regimen in the setting of peritonitis |
| Yoon 2014 | Wrong intervention: icodextrin versus non ‐icodextrin in the background of using biocompatible solution (physioneal) |
GDP ‐ glucose degradation products; NEP ‐ neutral endopeptidase; PD ‐ peritoneal dialysis
Characteristics of studies awaiting assessment [ordered by study ID]
Cho 2010.
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
Group 3
Group 4
|
| Outcomes |
|
| Notes |
|
Dai 2010.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
De Los Rios 2016.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
Do 2006a.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Infante 2000.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Kim 2006.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Kocyigit 2015.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
NCT01753154.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
Opatrna 2002.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
Rodriguez‐Carmona 2012.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
5 weeks in each arm then cross‐over |
| Outcomes |
|
| Notes |
|
Yang 2002b.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
APD ‐ automated peritoneal dialysis; BP ‐ blood pressure; CAPD ‐ continuous ambulatory peritoneal dialysis; CrCl ‐ creatinine clearance; CRP ‐ C‐reactive protein; GDP ‐ glucose degradation products; ECG ‐ echocardiogram; PD ‐ peritoneal dialysis; RCT ‐ randomised controlled trial; RRF ‐ residual renal function; UF ‐ ultrafiltration
Characteristics of ongoing studies [ordered by study ID]
NCT01228279.
| Trial name or title | Sympathetic Activity in Patients With End‐stage Renal Disease on Peritoneal Dialysis (SAPD) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date |
|
| Contact information | Marcel Ruzicka, MD, mruzicka@ottawahospital.on.ca |
| Notes |
BP ‐ blood pressure; CAPD ‐ continuous ambulatory peritoneal dialysis; CCPD ‐ continuous cycling peritoneal dialysis; RRF ‐ residual renal function
Differences between protocol and review
Blood pressure was not analysed as an outcome in the review due to a high degree of variability and hence difficulty in ascertaining the relationship between different types of PD solutions and the control of blood pressure. Similarly, fluid balance measurement was unable to be included as an outcome in the review, primarily due to lack of reporting of relevant information in the literature.
Contributions of authors
Screening of titles and abstracts: YC, HH
Study eligibility: YC, HH
Quality assessment, data extraction, data analysis: YC, HH, GFMS
Writing of review: HH, YC, DJ, KW, GFMS, JC, SB
Disagreements were resolved in consultation with DJ, SB, JC
Declarations of interest
Htay Htay: none known
Professor David Johnson has previously received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care unrelated to the preparation of this review.
Kathryn J Wiggins: none known
Sunil V Badve: none known
Jonathan C Craig: none known
Giovanni FM Strippoli: none known
Yeoungjee Cho: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bajo 2011 {published data only}
- Bajo MA, Priez‐Lozano ML, Albar‐Vizcaino P, Peso G, Castro MJ, Gonzalez‐Mateo G, et al. Low‐GDP peritoneal dialysis fluid ('balance') has less impact in vitro and ex vivo on epithelial‐to‐mesenchymal transition (EMT) of mesothelial cells than a standard fluid. Nephrology Dialysis Transplantation 2011;26(1):282‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
balANZ 2010 {published and unpublished data}
- Brown F, Johnson DW. A randomized controlled trial to determine whether treatment with at neutral pH, low glucose degradation product dialysate (balance) prolongs residual renal function in peritoneal dialysis patients. Peritoneal Dialysis International 2006;26(1):112‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Cho Y, Johnson DW, Vesey DA, Hawley CM, Clarke M, Topley N, et al. Utility of urinary biomarkers in predicting loss of residual renal function: the balANZ trial. Peritoneal Dialysis International 2015;35(2):159‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Johnson DW, Vesey DA, Hawley CM, Pascoe EM, Clarke M, et al. Dialysate interleukin‐6 predicts increasing peritoneal solute transport rate in incident peritoneal dialysis patients. BMC Nephrology 2014;15:8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard K, Hayes A, Cho Y, Cass A, Clarke M, Johnson DW. Economic evaluation of neutral‐pH, low‐glucose degradation product peritoneal dialysis solutions compared with standard solutions: a secondary analysis of the balANZ Trial. American Journal of Kidney Diseases 2015;65(5):773‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. Journal of the American Society of Nephrology 2012;23(6):1097‐107. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. The effect of low glucose degradation product, neutral pH versus standard peritoneal dialysis solutions on peritoneal membrane function: the balANZ trial. Nephrology Dialysis Transplantation 2012;27(12):4445‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. The effects of biocompatible compared with standard peritoneal dialysis solutions on peritonitis microbiology, treatment, and outcomes: the balANZ trial. Peritoneal Dialysis International 2012;32(5):497‐506. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Clarke M, Wilson V, Woods F, Brown FG. Rationale and design of the balANZ trial: a randomised controlled trial of low GDP, neutral pH versus standard peritoneal dialysis solution for the preservation of residual renal function. BMC Nephrology 2010;11:25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow G. A randomized controlled trial to determine whether treatment with at neutral pH, low glucose degradation product dialysate (balance) prolongs residual renal function in peritoneal dialysis patients. Peritoneal Dialysis International 2006;26(1):113‐4. [MEDLINE: ] [PubMed] [Google Scholar]
BALNET 2008 {published and unpublished data}
- Kim S, Bang K, Kim SJ, Oh J, Chung W, Joo KW, et al. Effect of low GDP fluid use on residual renal function in incident peritoneal dialysis patients over 24‐month period [abstract no: F‐PO1627]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):473A. [Google Scholar]
- Kim S, Kim SG, Oh JE, Chung W, Oh K, Kim YS, et al. Clinical benefits of a low glucose degradation products solution in patients starting peritoneal dialysis: preliminary report [abstract no: TH‐PO809]. Journal of American Society of Nephrology 2006;17(Abstracts):278A. [CENTRAL: CN‐00740544] [Google Scholar]
- Kim S, Oh J, Kim SG, Chung W, Ahn C, Kim SG, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1‐year study. Nephrology Dialysis Transplantation 2009;24(9):2899‐908. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kim S, Oh K, Kim SG, Oh JE, Chung W, Yang J, et al. Preserved residual renal function in incident peritoneal dialysis patients using low glucose degradation products solutions [abstract no: SA‐PO796]. Journal of the American Society of Nephrology 2007;18(Abstracts):518A. [Google Scholar]
- Kim S, Oh KH, Oh J, Kim SJ, Chung W, Song YR, et al. Biocompatible peritoneal dialysis solution preserves residual renal function. American Journal of Nephrology 2012;36(4):305‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim S, Hwang YH, Kim K, Oh JE, Chung W, et al. Could solutions low in glucose degradation products preserve residual renal function in incident peritoneal dialysis patients? A 1‐year multicenter prospective randomized controlled trial (Balnet study). Peritoneal Dialysis International 2008;28 Suppl 3:S117‐22. [MEDLINE: ] [PubMed] [Google Scholar]
- Kim SJ, Oh JE, Chung WK, Oh KH, Kim SG. Effect of biocompatible PD fluid on preservation of residual renal function in incident CAPD patients: two‐year extended follow‐up study [abstract no: SA418]. NDT Plus 2010;3:iii175‐iii6. [EMBASE: 70483884] [Google Scholar]
Bredie 2001 {published data only}
- Bredie SJ, Bosch F, Demacker PN, Stalenhoef AF, Leusen R. Effects of peritoneal dialysis with an overnight icodextrin dwell on parameters of glucose and lipid metabolism [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):203‐4A. [CENTRAL: CN‐00550440] [PubMed] [Google Scholar]
- Bredie SJ, Bosch FH, Demacker PN, Stalenhoef AF. Effects on peritoneal dialysis with an overnight icodextrin dwell on parameters of glucose and lipid metabolism [abstract]. Netherlands Journal of Medicine 2001;58:A11. [CENTRAL: CN‐00716064] [PubMed] [Google Scholar]
- Bredie SJ, Bosch FH, Demacker PN, Stalenhoef AF, Leusen R. Effects of peritoneal dialysis with an overnight icodextrin dwell on parameters of glucose and lipid metabolism. Peritoneal Dialysis International 2001;21(3):275‐81. [MEDLINE: ] [PubMed] [Google Scholar]
Cancarini 1998 {published data only}
- Cancarini GC, Faict D, Vos C, Guiberteau R, Tranaeus A, Minetti L, et al. Clinical evaluation of a peritoneal dialysis solution with 33 mmol/L bicarbonate. Peritoneal Dialysis International 1998;18(6):576‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Carrasco 2001 {published data only}
- Carrasco AM, Rubio MA, Sanchez Tommero JA, Fernandez Giron F, Gonzalez Rico M, Peso Gilsanz G, et al. Acidosis correction with a new 25 mmol/l bicarbonate/15 mmol/l lactate peritoneal dialysis solution. Peritoneal Dialysis International 2001;21(6):546‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Cho 2013 {published data only}
- Cho KH, Do JY, Park JW, Yoon KW, Kim YL. The effect of low‐GDP solution on ultrafiltration and solute transport in continuous ambulatory peritoneal dialysis patients.[Erratum appears in Perit Dial Int. 2013 Sep‐Oct;33(5):473‐4]. Peritoneal Dialysis International 2013;33(4):382‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2008 {published data only}
- Choi HY, Kim DK, Lee TH, Han SH, Lee SC, Lee JE, et al. The clinical impacts of novel peritoneal dialysis fluids (PDFs) with neutral pH and low glucose degradation product (GDP) concentration (Balance®) [abstract no: SP708]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv254. [CENTRAL: CN‐00653807] [Google Scholar]
- Choi HY, Kim DK, Lee TH, Moon SJ, Han SH, Lee JE, et al. The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: an open randomized prospective trial. Peritoneal Dialysis International 2008;28(2):174‐82. [MEDLINE: ] [PubMed] [Google Scholar]
- Lee HY, Choi HY, Kim JS, Han SH, Lee SC, Lee JE, et al. The clinical usefulness of peritoneal dialysis fluids (PDFs) with neutral pH and low glucose degradation product (GDP) Concentration‐Balance® [abstract no: SA‐FC138]. Journal of the American Society of Nephrology 2005;16:112A. [CENTRAL: CN‐00653808] [Google Scholar]
Cnossen 2011 {published data only}
- Cnossen TT, Gladziwa U, Kerkhof JJ, Schalkwijk CG, Scheijen J, Amersfoort J, et al. The influence of bicarbonate/lactate‐buffered PD fluids on NƐ‐(carboxyethyl)lysine and NƐ‐(carboxymethyl)lysine in peritoneal effluent. Peritoneal Dialysis International 2011;31(2):189‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coles 1997 {published data only}
- Coles GA, Gokal R, Ogg C, Jani F, O'Donoghue DT, Cancarini GC, et al. A randomized controlled trial of a bicarbonate‐ and a bicarbonate/lactate‐containing dialysis solution in CAPD.[Erratum appears in Perit Dial Int 1997 May‐Jun;17(3):223 Note: Cancarinu GC [corrected to Cancarini GC]]. Peritoneal Dialysis International 1997;17(1):48‐51. [MEDLINE: ] [PubMed] [Google Scholar]
- Coles GA, O'Donoghue DJ, Pritchard N, Ogg CS, Jani FM, Gokal R, et al. A controlled trial of two bicarbonate‐containing dialysis fluids for CAPD‐‐final report. Nephrology Dialysis Transplantation 1998;13(12):3165‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mackenzie RK, Jones S, Moseley A, Holmes CJ, Argyle R, Williams JD, et al. In vivo exposure to bicarbonate/lactate‐ and bicarbonate‐buffered peritoneal dialysis fluids improves ex vivo peritoneal macrophage function. American Journal of Kidney Diseases 2000;35(1):112‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Topley N, Mackenzie R, Williams JD, Coles GA, Tranaeus A, Faict D, et al. In vivo exposure to bicarbonate/lactate‐buffered PDF (TBL) improves ex vivo PMO function, compared to bicarbonate‐(TB) or lactate‐buffered PDF (PD4) [abstract]. Nephrology 1997;3(Suppl 1):S408. [CENTRAL: CN‐00461877] [Google Scholar]
- Williams JD, Holmes CJ, Jones S, Mackenzie R, Coles GA, Faict D, et al. Long‐term in vivo exposure to bicarbonate/lactate dialysis fluid improves ex vivo peritoneal macrophage (PMO) TNFa secretion [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):292A. [CENTRAL: CN‐00448375] [Google Scholar]
Davies 2003 {published data only (unpublished sought but not used)}
- Davies S, Plum J, Heimburger O. Increasing our understanding of the mechanism of action of icodextrin (ICO) [abstract no: F‐PO407]. Journal of the American Society of Nephrology 2004;15(Oct):156A. [CENTRAL: CN‐00583222] [Google Scholar]
- Davies S, Woodrow G, Johansson C, Williams P, Divino J. Icodextrin improves fluid balance in PD patients [abstract no: O52]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):17. [CENTRAL: CN‐00509151] [Google Scholar]
- Davies SJ, Garcia Lopez E, Woodrow G, Donovan K, Plum J, Williams P, et al. Longitudinal relationships between fluid status, inflammation, urine volume and plasma metabolites of icodextrin in patients randomized to glucose or icodextrin for the long exchange. Nephrology Dialysis Transplantation 2008;23(9):2982‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double‐blind randomized controlled trial. Journal of the American Society of Nephrology 2003;14(9):2338‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Davies SJ, Woodrow G, Plum J, Heimburger O, Donovan K, Divino J. Icodextrin improves fluid status in PD patients [abstract no: SU‐PO798]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):632A‐3A. [CENTRAL: CN‐00445007] [Google Scholar]
di Paolo 2000 {published data only}
- Paolo B, Stingone A, Vito R, Stuard S, D'Angelo B, Caravelli L, et al. Cardiovascular and hemodynamic profile related to hypotensive effect long term of icodextrine (ICO) in CAPD patients [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):206A. [CENTRAL: CN‐00550665] [Google Scholar]
DIUREST 2010 {published data only (unpublished sought but not used)}
- Haag‐Weber M, Haug U, Wieslander A, Nabut J, Deppisch R, DIUREST Study Group. Decline of residual renal function (RRF) in peritoneal dialysis (PD) patients (pts) depends on uptake of carbonyl compounds (CC) from the peritoneal cavity: first data of a prospective clinical trial [abstract]. Journal of the American Society of Nephrology 2003;14:476A‐7A. [CENTRAL: CN‐00756119] [Google Scholar]
- Haag‐Weber M, Kramer R, Haake R, Islam MS, Prischl F, Haug U, et al. Low‐GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrology Dialysis Transplantation 2010;25(7):2288‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EURO‐BALANCE 2004 {published data only (unpublished sought but not used)}
- Mackenzie RK, Craig KJ, Lage C, Williams JD, Schaub T, Passlick‐Deetjen J, et al. Treatment with low GDP solution (CAPD balance) is associated with an increase in effluent CA125 and a decrease in HA content: data from the multi centre European Balance Trial [abstract no: F‐P0685]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):199A. [CENTRAL: CN‐00446522] [Google Scholar]
- Mackenzie RM, Lage C, Craig KJ, Williams JD, Schaub T, Passlick‐Deetjen J, et al. Continuous treatment with low GDP solution (CAPD Balance®) is associated with an increase in effluent CA125 and a decrease in HA content: data from the Multicenter European Balance Trial [abstract]. Peritoneal Dialysis International 2002;22(1):101. [CENTRAL: CN‐00446523] [Google Scholar]
- Williams JD, Craig KJ, Passlick‐Deetjen J, Topley N, Lage C, Pischetsrieder M, et al. Reduced systemic AGE formation with a low GDP solution (CAPD balance): data from the multicentre European Balance Trial [abstract no: F‐PO683]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):199A. [CENTRAL: CN‐00448374] [Google Scholar]
- Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro‐Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney International 2004;66(1):408‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Witowski J, Korybalska K, Ksiazek K, Wisniewska‐Elnur J, Jorres A, Lage C, et al. Peritoneal dialysis with solutions low in glucose degradation products is associated with improved biocompatibility profile towards peritoneal mesothelial cells. Nephrology Dialysis Transplantation 2004;19(4):917‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fan 2008 {published data only (unpublished sought but not used)}
- Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney International 2008;73(2):200‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Srivastava S, Hildebrand S, Fan SL. Long‐term follow‐up of patients randomized to biocompatible or conventional peritoneal dialysis solutions show no difference in peritonitis or technique survival. Kidney International 2011;80(9):986‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feriani 1998 {published and unpublished data}
- Feriani M, Kirchgessner J, Greca G, Passlick‐Deetjen J. Randomized long‐term evaluation of bicarbonate‐buffered CAPD solution. Kidney International 1998;54(5):1731‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Feriani M, Pablick‐Deetjen J, Brown C, Buoncristiani U, Paolo N, Gahl G, et al. An open controlled randomized clinical trial with bicarbonate CAPD solutions: interim results [abstract]. Journal of the American Society of Nephrology 1994;5(3):414. [CENTRAL: CN‐00550652] [Google Scholar]
- Feriani M, Passlick‐Deetjen J, Kirchgessner J. Long term safety and efficacy of bicarbonate buffered peritoneal dialysis solutions [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A253. [CENTRAL: CN‐00261316] [Google Scholar]
- Passlick‐Deetjen J, Feriani M. Experiences with bicarbonate solutions in peritoneal dialysis [Erfahrungen mit bikarbonathaltigen peritonealdialyselosungen]. Nieren‐und Hochdruckkrankheiten 1994;23(Suppl 2):S82‐7. [EMBASE: 1995014810] [Google Scholar]
Fernandez‐Perpen 2012 {published and unpublished data}
- Fernandez Perpen A, Sanchez Tomero JA, Bajo MA, Perez Lozano ML, Peso G, Albar P, et al. Effects of bicavera (BV) dialysate for peritoneal dialysis on the epithelial‐to‐mesenchymal transition (EMT) of the mesothelial cell (MC) ex vivo [abstract]. NDT Plus 2010;3:iii477. [EMBASE: 70484718] [Google Scholar]
- Fernandez‐Perpen A, Perez‐Lozano ML, Bajo MA, Albar‐Vizcaino P, Sandovar Correa P, Peso G, et al. Influence of bicarbonate/low‐GDP peritoneal dialysis fluid (Bicavera) on in vitro and ex vivo epithelial‐to‐mesenchymal transition of mesothelial cells. Peritoneal Dialysis International 2012;32(3):292‐304. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkelstein 2005 {published data only (unpublished sought but not used)}
- Finkelstein F, Healy H, Abu‐Alfa A, Ahmad S, Brown F, Gehr T, et al. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. Journal of the American Society of Nephrology 2005;16(2):546‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fusshoeller 2004 {published data only}
- Fusshoeller A, Plail M, Ausobski G, Grabensee B, Plum J. Biocompatibility pattern of neutral bicarbonate/lactate buffered peritoneal dialysis solution in chronic APD patients ‐ a prospective; randomized; cross over study [abstract no: M672]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):214. [CENTRAL: CN‐00782432] [Google Scholar]
- Fusshoeller A, Plail M, Grabensee B, Plum J. Biocompatibility pattern of a bicarbonate/lactate‐buffered peritoneal dialysis fluid in APD: a prospective, randomized study. Nephrology Dialysis Transplantation 2004;19(8):2101‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2003 {published data only}
- Do J, Cho K, Park J, Yoon K, Cho D, Kim Y. Local and systemic effects of neutral pH, low GDP dialysate in CAPD patients [abstract no: SA‐FC202]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):41A. [CENTRAL: CN‐00445121] [Google Scholar]
- Do J, Kim Y, Kim T, Park J, Yoon K, Park S. The effect of neutral pH, low GDPs dialysis solution on fluid, solute transport and edema control in CAPD patients [abstract no: SA‐PO337]. Journal of American Society of Nephrology 2004;15(Oct):375A. [CENTRAL: CN‐00583512] [Google Scholar]
- Do JY, Kim YL, Park JW, Chang KA, Lee SH, Ryu DH, et al. The association between the vascular endothelial growth factor‐to‐cancer antigen 125 ratio in peritoneal dialysis effluent and the epithelial‐to‐mesenchymal transition in continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 2008;28 Suppl 3:S101‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Do JY, Kim YL, Park JW, Cho KH, Kim TW, Yoon KW, et al. The effect of low glucose degradation product dialysis solution on epithelial‐to‐mesenchymal transition in continuous ambulatory peritoneal dialysis patients. Peritoneal Dialysis International 2005;25 Suppl 3:S22‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Kim C, Kim Y, Park S, Do J, Yoon K, Lee E, et al. Effects of low glucose degradation products (GDPs) dialysis solution on the levels of surrogate markers of peritoneal inflammation, integrity and angiogenesis. Preliminary report [abstract no: SA‐PO822]. Journal of the American Society of Nephrology 2003;14(Nov):479A. [CENTRAL: CN‐00583514] [DOI] [PubMed] [Google Scholar]
- Kim YL, Do J, Park SH, Cho K, Park J, Yoon K, et al. Low glucose degradation products dialysis solution modulates the levels of surrogate markers of peritoneal inflammation, integrity, and angiogenesis: preliminary report. Nephrology 2003;8 Suppl:S28‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Park J, Do J, Kim Y, Kim T, Yoon K, Park S. The effect of low GDPs solution on peritoneal fibrosis markers [abstract no: SA‐PO333]. Journal of the American Society of Nephrology 2004;15(Oct):374A. [CENTRAL: CN‐00583515] [Google Scholar]
Konings 2003 {published data only}
- Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, Wall Bake AW, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney International 2003;63(4):1556‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Konings CJ, Kooman JP, Schonck M, Sande FM, Hoorntje SJ, Leunissen KM. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters [abstract no: F‐PO719]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):206A. [CENTRAL: CN‐00446147] [Google Scholar]
- Konings CJ, Schalkwijk CG, Sande FM, Leunissen KM, Kooman JP. Influence of icodextrin on plasma and dialysate levels of NƐ‐(carboxymethyl)lysine and NƐ‐(carboxyethyl)lysine. Peritoneal Dialysis International 2005;25(6):591‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Kooman JP, Schalkwijk CG, Konings CJ. The increase in plasma levels of NƐ‐(carboxymethyl)lysine during icodextrin treatment of peritoneal dialysis patients is not associated with increased plasma levels of vascular cell adhesion molecule‐1. Peritoneal Dialysis International 2006;26(3):410‐1. [MEDLINE: ] [PubMed] [Google Scholar]
Lai 2012a {published data only}
- Lai KN, Lam MF, Leung JC, Chan LY, Lam CW, Chan IH, et al. A study of the clinical and biochemical profile of peritoneal dialysis fluid low in glucose degradation products. Peritoneal Dialysis International 2012;32(3):280‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2009a {published and unpublished data}
- Lin A, Qian J, Li X, Yu X, Liu W, Sun Y, et al. Randomized controlled trial of icodextrin versus glucose containing peritoneal dialysis fluid. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(11):1799‐804. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mactier 1998 {published data only}
- Mactier RA, Sprosen TS, Gokal R, Williams PF, Lindbergh M, Naik RB, et al. Bicarbonate and bicarbonate/lactate peritoneal dialysis solutions for the treatment of infusion pain. Kidney International 1998;53(4):1061‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sprosen TS, Mactier RA, Gokal R, Lindbergh M, Tranaeus A, Faict D. Treatment of infusion pain (InP) with novel bicarbonate containing PD solutions [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):273A. [CENTRAL: CN‐00447821] [Google Scholar]
MIDAS 1994 {published data only (unpublished sought but not used)}
- Armstrong A, Sayers JA, Scrimgeour AC. Reduction in the prevalence of CAPD symptoms during 6 months treatment with icodextrin [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A240. [CENTRAL: CN‐00261303] [Google Scholar]
- Gokal R, Mistry CD, Peers EM. Peritonitis occurrence in a multicenter study of icodextrin and glucose in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Dialysis. Peritoneal Dialysis International 1995;15(6):226‐30. [MEDLINE: ] [PubMed] [Google Scholar]
- Gokal R, Moberly J, Ogrinc F, Gordon A, Peers E, MIDAS Study Group. Improvement of hyperlipidemia with icodextrin use in CAPD patients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):283A. [CENTRAL: CN‐00445499] [Google Scholar]
- Mistry CD, Gokal R, Peers E. A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney International 1994;46(2):496‐503. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pajek 2008 {published and unpublished data}
- Pajek J, Kveder R, Bren A, Gucek A, Bucar M, Skoberne A, et al. Short‐term effects of bicarbonate/lactate‐buffered and conventional lactate‐buffered dialysis solutions on peritoneal ultrafiltration: a comparative crossover study. Nephrology Dialysis Transplantation 2009;24(5):1617‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pajek J, Kveder R, Bren A, Gucek A, Ihan A, Osredkar J, et al. Short‐term effects of a new bicarbonate/lactate‐buffered and conventional peritoneal dialysis fluid on peritoneal and systemic inflammation in CAPD patients: a randomized controlled study. Peritoneal Dialysis International 2008;28(1):44‐52. [MEDLINE: ] [PubMed] [Google Scholar]
- Pajek J, Kveder R, Gucek A, Skoberne A, Bren A, Bucar M, et al. Cell‐free DNA in the peritoneal effluent of peritoneal dialysis solutions. Therapeutic Apheresis & Dialysis 2010;14(1):20‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paniagua 2008 {published data only (unpublished sought but not used)}
- Orihuela O, Jesus V, Avila‐Diaz M, Cisneros A, Vicente‐Martinez M, Furlong MD, et al. Effect of icodextrin on heart rate variability in diabetic patients on peritoneal dialysis. Peritoneal Dialysis International 2014;34(1):57‐63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniagua R, Orihuela O, Ventura MD, Avila‐Diaz M, Cisneros A, Vicente‐Martinez M, et al. Echocardiographic, electrocardiographic and blood pressure changes induced by icodextrin solution in diabetic patients on peritoneal dialysis. Kidney International ‐ Supplement 2008;73(108):S125‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paniagua R, Ventura MD, Avila‐Diaz M, Cisneros A, Vicente‐Martinez M, Furlong MD, et al. Icodextrin improves metabolic and fluid management in high and high‐average transport diabetic patients. Peritoneal Dialysis International 2009;29(4):422‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Park 2012a {published data only}
- Park SH, Do JY, Kim YH, Lee HY, Kim BS, Shin SK, et al. Effects of neutral pH and low‐glucose degradation product‐containing peritoneal dialysis fluid on systemic markers of inflammation and endothelial dysfunction: a randomized controlled 1‐year follow‐up study. Nephrology Dialysis Transplantation 2012;27(3):1191‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plum 2002 {published data only}
- Plum J, Gentile S, Verger C, Brunkhorst R, Bahner U, Faller B, et al. Efficacy and safety of a 7.5% icodextrin peritoneal dialysis solution in patients treated with automated peritoneal dialysis. American Journal of Kidney Diseases 2002;39(4):862‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Posthuma 1997 {published data only}
- Peers E. Icodextrin plus glucose combinations for use in CAPD. Peritoneal Dialysis International 1997;17 Suppl 2:S68‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Posthuma N, Verbrugh HA, Donker AJ, Dorp W, Dekker HA, Peers EM, et al. Peritoneal kinetics and mesothelial markers in CCPD using icodextrin for daytime dwell for two years. Peritoneal Dialysis International 2000;20(2):174‐80. [MEDLINE: ] [PubMed] [Google Scholar]
- Posthuma N, ter Wee P, Donker AJ, Dekker HA, Oe PL, Verbrugh HA. Peritoneal defense using icodextrin or glucose for daytime dwell in CCPD patients. Peritoneal Dialysis International 1999;19(4):334‐42. [MEDLINE: ] [PubMed] [Google Scholar]
- Posthuma N, ter Wee PM. Icodextrin (I) use in CCPD patients during peritonitis: serum disaccharide levels and ultrafiltration (UF) [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A184. [CENTRAL: CN‐00261438] [DOI] [PubMed] [Google Scholar]
- Posthuma N, ter Wee PM, Donker AJ, Dekker HAT, Oe PL, Verhoef J, et al. Ex vivo peritoneal defense characteristics and peritonitis rate in CCPD patients using glucose or icodextrin as daytime dwell [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):223A. [CENTRAL: CN‐00447263] [Google Scholar]
- Posthuma N, ter Wee PM, Donker AJ, Oe LP, Verbrugh HA, Peers E. Disaccharide ("total maltose") levels in CCPD patients using icodextrin [abstract]. Journal of the American Society of Nephrology 1995;6(3):513. [CENTRAL: CN‐00485460] [Google Scholar]
- Posthuma N, ter Wee PM, Donker AJ, Oe PL, Peers EM, Verbrugh HA. Assessment of the effectiveness, safety, and biocompatibility of icodextrin in automated peritoneal dialysis. The Dextrin in APD in Amsterdam (DIANA) Group. Peritoneal Dialysis International 2000;20 Suppl 2:S106‐13. [MEDLINE: ] [PubMed] [Google Scholar]
- Posthuma N, ter Wee PM, Donker AJ, Oe PL, Dorp W, Peers EM, et al. Serum disaccharides and osmolality in CCPD patients using icodextrin or glucose as daytime dwell. Peritoneal Dialysis International 1997;17(6):602‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Posthuma N, ter Wee PM, Donker AJ, Verbrugh HA, Oe PL, Dorp W, et al. Icodextrin (I) use in CCPD patients during peritonitis: serum disaccharide (maltose) levels and ultrafiltration (UF) [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):270A. [CENTRAL: CN‐00447266] [Google Scholar]
- Posthuma N, ter Wee PM, Donker AJM, Oe LP, Verbrugh HA, Peers E. Improved ultrafiltration in CCPD patients using icodextrin (I) instead of glucose (G) for the long daytime dwell [abstract]. Journal of the American Society of Nephrology 1995;6(3):513. [CENTRAL: CN‐00485461] [Google Scholar]
- Posthuma N, ter Wee PM, Donker AJM, Verbrugh HA, Oe PL, Dorp W. Peritoneal membrane characteristics in CCPD patients using glucose or icodextrin as daytime dwell [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):223A. [CENTRAL: CN‐00447265] [Google Scholar]
- Posthuma N, ter Wee PM, Niessen H, Donker AJ, Verbrugh HA, Schalkwijk CG. Amadori albumin and advanced glycation end‐product formation in peritoneal dialysis using icodextrin. Peritoneal Dialysis International 2001;21(1):43‐51. [MEDLINE: ] [PubMed] [Google Scholar]
- Posthuma N, ter Wee PM, Verbrugh HA, Oe PL, Peers E, Sayers J, et al. Icodextrin instead of glucose during the daytime dwell in CCPD increases ultrafiltration and 24‐h dialysate creatinine clearance. Nephrology Dialysis Transplantation 1997;12(3):550‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Posthuma N, ter Weel PM, Donker AJ, Peers EM, Oe PL, Verbrugh HA. Icodextrin use in CCPD patients during peritonitis: ultrafiltration and serum disaccharide concentrations. Nephrology Dialysis Transplantation 1998;13(9):2341‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rippe 2001 {published data only}
- Rippe B, Christensson A, Haraldsson B, Simonsen O, Stelin G, Weiss L, et al. More Ca125 in dialysate after long‐term treatment with a new, less toxic PD‐fluid (PD‐bio) [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A180. [CENTRAL: CN‐00509439] [Google Scholar]
- Rippe B, Christensson A, Haraldsson B, Simonsen O, Stelin G, Weiss L, et al. Patient dialysate CA 125 and hyaluronan (HA) after 1, 6 and 12 months of treatment with a PD‐fluid containing less cytotoxic glucose degradation products [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):182A. [CENTRAL: CN‐00447414] [Google Scholar]
- Rippe B, Simonsen O, Heimburger O, Christensson A, Haraldsson B, Stelin G, et al. Long‐term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney International 2001;59(1):348‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rippe B, Wieslander A. Biologic significance of reduced levels of glucose degradation products. Peritoneal Dialysis International 2001;21 Suppl 3:S114‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Rippe B, Wieslander A, Musi B. Long‐term results with low glucose degradation product content in peritoneal dialysis fluids. Contributions to Nephrology 2003;140:47‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Simonsen O, Rippe B, Heimburger O, Christensson A, Haraldsson B, Stelin G, et al. Long‐term clinical effects of a peritoneal dialysis fluid with less glucose degradation products [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):322A. [DOI] [PubMed] [Google Scholar]
Schmitt 2002 {published and unpublished data}
- Haas S, Schmitt CP, Arbeiter K, Bonzel KE, Fischbach M, John U, et al. Improved acidosis correction and recovery of mesothelial cell mass with neutral‐pH bicarbonate dialysis solution among children undergoing automated peritoneal dialysis. Journal of the American Society of Nephrology 2003;14(10):2632‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haas S, Schmitt CP, Schaefer F, Schaub T, Mid European Pediatric Peritoneal Dialysis Study Group (MEPPS). Randomized cross‐over evaluation of bicarbonate‐vs lactate buffered PD solutions in children on APD [abstract]. Pediatric Nephrology 2002;17(Suppl):C53. [CENTRAL: CN‐00445609] [Google Scholar]
- Haas S, Schmitt CP, Schaub T, Schaefer F. Better biocompatibility and correction of acidosis using bicarbonate vs lactate buffered PD fluid in children on APD: a randomized cross‐over clinical trial [abstract]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):202A. [CENTRAL: CN‐00445610] [Google Scholar]
- Schmitt C, Haas S, Schaub T, Schaefer F. Randomized cross‐over administration of pH‐neutral, bicarbonate buffered PD solution in children on APD [abstract no: O51]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):17. [Google Scholar]
- Schmitt CP, Doetschmann R, Kirchgessner J, Mehls O, Schaefer F. Comparison of solute and acid‐base transport kinetics in children using bicarbonate‐ vs. lactate‐buffered PD solutions [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):193A. [CENTRAL: CN‐00447630] [Google Scholar]
- Schmitt CP, Haraldsson B, Doetschmann R, Zimmering M, Greiner C, Boswald M, et al. Effects of pH‐neutral, bicarbonate‐buffered dialysis fluid on peritoneal transport kinetics in children. Kidney International 2002;61(4):1527‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schmitt CP, Heyl D, Haas S, Mehls O, Schaefer F, Mid European Pediatric PD Study Group (MEPPS). Peritoneal dialysis (PD) solution with reduced glucose degradation product (GPD) content lowers serum concentrations of advanced glycated endproducts (AGE) in children on automated PD [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):210‐1. [CENTRAL: CN‐00447631] [Google Scholar]
- Schmitt CP, Heyl D, Rieger S, Arbeiter K, Bonzel KE, Fischbach M, et al. Reduced systemic advanced glycation end products in children receiving peritoneal dialysis with low glucose degradation product content. Nephrology Dialysis Transplantation 2007;22(7):2038‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STARCH 2015 {published data only}
- Moraes TP, Andreoli MC, Canziani ME, Silva DR, Caramori JC, Ponce D, et al. Icodextrin reduces insulin resistance in non‐diabetic patients undergoing automated peritoneal dialysis: results of a randomized controlled trial (STARCH). Nephrology Dialysis Transplantation 2015;30(11):1905‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Szeto 2007 {published data only}
- Szeto C, Chow K, Leung C, Lam CWK, Kwan BCH, Chung K, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose‐degradation‐product ‐ a randomized control trial [abstract no: SA‐FC137]. Journal of the American Society of Nephrology 2005;16:112A. [Google Scholar]
- Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose‐degradation products‐‐a 1‐year randomized control trial. Nephrology Dialysis Transplantation 2007;22(2):552‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Szeto 2015 {published data only}
- Szeto CC, Kwan BC, Chow KM, Cheng PM, Kwong VW, Choy AS, et al. The effect of neutral peritoneal dialysis solution with low glucose‐degradation‐product on the fluid status and body composition‐‐a randomized control trial. PLoS ONE [Electronic Resource] 2015;10(10):e0141425. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takatori 2011 {published and unpublished data}
- Akagi S, Sugiyama H, Takatori Y, Takiue K, Inoue J, Kojo S, et al. Effect of icodextrin on the fluid status in diabetic peritoneal dialysis patients ‐ an interim report [abstract no: SA443]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
- Takatori Y, Akagi S, Sugiyama H, Inoue J, Kojo S, Morinaga H, et al. Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: a randomized controlled trial. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(6):1337‐44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tranaeus 2000 {published data only}
- Cooker LA, Luneburg P, Holmes CJ, Jones S, Topley N, Bicarbonate/Lactate Study Group. Interleukin‐6 levels decrease in effluent from patients dialyzed with bicarbonate/lactate‐based peritoneal dialysis solutions. Peritoneal Dialysis International 2001;21 Suppl 3:S102‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Holmes CJ, Jones S, Mackenzie R, Coles GA, Williams JD, Tranaeus A, et al. Bicarbonate/lactate‐buffered (TBL) peritoneal dialysis fluid (PDF) decreases pro‐collagen I levels in peritoneal dialysis effluent (PDE) [abstract no: A1214]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):264A. [CENTRAL: CN‐00445763] [Google Scholar]
- Jones S, Holmes CJ, Krediet RT, Mackenzie R, Faict D, Tranaeus A, et al. Bicarbonate/lactate‐based peritoneal dialysis solution increases cancer antigen 125 and decreases hyaluronic acid levels. Kidney International 2001;59(4):1529‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jones S, Holmes CJ, Mackenzie RK, Stead R, Coles GA, Williams JD, et al. Continuous dialysis with bicarbonate/lactate‐buffered peritoneal dialysis fluids results in a long‐term improvement in ex vivo peritoneal macrophage function. Journal of the American Society of Nephrology 2002;13(Suppl 1):S97‐S103. [PUBMED: 11792769] [PubMed] [Google Scholar]
- O'Donoghue DJ, Bicarbonate/Lactate Study Group. A long term study of a bicarbonate‐lactate based peritoneal dialysis solution [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):266A. [Google Scholar]
- Toley N, Krediet R, Jones S, Faict D, Tranaeus A, Holmes C, et al. Peritoneal dialysate ca125, hyaluronan (ha), tgfá1 and pro‐collagen i peptide (picp) in a randomized, controlled study of bicarbonate/lactate based CAPD solution [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):230A. [Google Scholar]
- Topley N, Jones S, Holmes CJ, Mackenzie R, Coles GA, Faict D, et al. In vivo exposure to bicarbonate/lactate‐buffered (TBL) peritoneal dialysis fluid (PDF) decreases pro‐collagen I and TGF‐b1 (TGF‐b1) levels in peritoneal dialysis effluent (PDE) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):290A. [CENTRAL: CN‐00448032] [Google Scholar]
- Topley N, Krediet R, Jones S, Faict D, Tranaeus A, Holmes C. Peritoneal dialysate CA125, hyaluronan (HA), TGFb1 and pro‐collagen I peptide (PICP) in a randomized, controlled study of bicarbonate/lactate based CAPD solution. [abstract no: A1169]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):230A. [CENTRAL: CN‐00583766] [Google Scholar]
- Tranaeus A. A long‐term study of a bicarbonate/lactate‐based peritoneal dialysis solution‐‐clinical benefits. The Bicarbonate/Lactate Study Group. Peritoneal Dialysis International 2000;20(5):516‐23. [MEDLINE: ] [PubMed] [Google Scholar]
TRIO 2016 {published data only}
- Sikaneta T, Wu G, Abdolell M, Ng A, Mahdavi S, Svendrovski A, et al. The Trio Trial ‐ a randomized controlled clinical trial evaluating the effect of a biocompatible peritoneal dialysis solution on residual renal function. Peritoneal Dialysis International 2016;36(5):526‐32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2009 {published and unpublished data}
- Weiss L, Stegmayr B, Malmsten G, Tejde M, Hadimeri H, Siegert CE, et al. Biocompatibility and tolerability of a purely bicarbonate‐buffered peritoneal dialysis solution. Peritoneal Dialysis International 2009;29(6):647‐55. [MEDLINE: ] [PubMed] [Google Scholar]
Wolfson 2002 {published data only (unpublished sought but not used)}
- Guo A, Just P. Quality of life of peritoneal dialysis patients on icodextrin: a longitudinal study [abstract]. Quality of Life Research 2002;11(7):667. [CENTRAL: CN‐00493774] [Google Scholar]
- Guo A, Wolfson M, Holt R. Early quality of life benefits of icodextrin in peritoneal dialysis. Kidney International ‐ Supplement 2002;81:S72‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wolfson M, Hagen T, Ogrinc F, Martis L, Icodextrin Study Group. One year results‐icodextrin vs dextrose for the long dwell in peritoneal dialysis [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):317A. [Google Scholar]
- Wolfson M, Hagen T, Ogrinc F, Martis L, Icodextrin Study Group. Effects of icodextrin on ultrafiltration (UF) and small solute clearance in continuous ambulatory dialysis patients (CAPD) [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):317A. [CENTRAL: CN‐00653810] [Google Scholar]
- Wolfson M, Piraino B, Hamburger RJ, Morton AR, Icodextrin Study Group. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. American Journal of Kidney Diseases 2002;40(5):1055‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoo 2015 {published data only}
- Yoo TH, Lee MJ, Oh HJ, Park JT, Han SH, Kang SW, et al. Is It beneficial to convert to a neutral‐pH bicarbonate/lactate‐buffered PD solution in long‐term CAPD patients? A single‐center prospective study. Peritoneal Dialysis International 2015;35(3):366‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeier 2003 {published data only}
- Zeier M, Deppisch R, Haug U, Schwenger V, Henle T, Bahner U, et al. Resorption of age‐promoting glucose degradation products (GDP) from peritoneal dialysis (PD) fluids leads to increased levels of age in plasma [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):223A. [CENTRAL: CN‐00583875] [Google Scholar]
- Zeier M, Schwenger V, Deppisch R, Haug U, Weigel K, Bahner U, et al. Glucose degradation products in PD fluids: do they disappear from the peritoneal cavity and enter the systemic circulation?. Kidney International 2003;63(1):298‐305. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
BIOKID 2004 {published data only}
- Nau B, Schmitt CP, Almeida M, Arbeiter K, Ardissino G, Bonzel KE, et al. BIOKID: randomized controlled trial comparing bicarbonate and lactate buffer in biocompatible peritoneal dialysis solutions in children [ISRCTN81137991]. BMC Nephrology 2004;5(1):14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt CP, Gemulla G, Bonzel KE, Holtta T, Testa S, Fischbach M, et al. BIOKID: randomized controlled trial comparing bicarbonate and lactate buffer in biocompatible peritoneal dialysis solutions in children [abstract no: SU‐PO519]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):696A. [Google Scholar]
- Schmitt CP, Nau B, Gemulla G, Bonzel KE, Holtta T, Testa S, et al. Effect of the dialysis fluid buffer on peritoneal membrane function in children. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(1):108‐15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braide 2009 {published data only}
- Braide M, Haraldsson B, Persson U. Citrate supplementation of PD fluid: effects on net ultrafiltration and clearance of small solutes in single dwells. Nephrology Dialysis Transplantation 2009;24(1):286‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chang 2016 {published data only}
- Chang TI, Ryu DR, Yoo TH, Kim HJ, Kang EW, Kim H, et al. Effect of icodextrin solution on the preservation of residual renal function in peritoneal dialysis patients: a randomized controlled study. Medicine 2016;95(13):e2991. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chow 2014 {published data only}
- Chow KM, Szeto CC, Kwan BC, Pang WF, Ma T, Leung CB, et al. Randomized controlled study of icodextrin on the treatment of peritoneal dialysis patients during acute peritonitis. Nephrology Dialysis Transplantation 2014;29(7):1438‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coester 2006 {published data only}
- Coester AM, Speake M, Summers AM, Parikova A, Hutchison AJ, Krediet RT. Effect of biocompatible peritoneal dialysis on transport status: the nutrineal, extraneal, physioneal (NEP) study [abstract no: TH‐PO812]. Journal of the American Society of Nephrology 2006;17(Abstracts):279A. [Google Scholar]
Dallas 2004 {published data only}
- Dallas F, Jenkins SB, Wilkie ME. Enhanced ultrafiltration using 7.5% icodextrin/1.36% glucose combination dialysate: a pilot study. Peritoneal Dialysis International 2004;24(6):542‐6. [MEDLINE: ] [PubMed] [Google Scholar]
de Fijter 1993 {published data only}
- Fijter CW, Verbrugh HA, Oe LP, Heezius E, Donker AJ, Verhoef J, et al. Biocompatibility of a glucose‐polymer‐containing peritoneal dialysis fluid. American Journal of Kidney Diseases 1993;21(4):411‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fijter CWH, Oe PL, Verbrugh HA, Peters EDJ, Meulen J, Donker AJM, et al. Glucose polymers as osmotic agent in CAPD fluids: a more favorable effect on peritoneal macrophage (PMO) function than glucose‐based solutions [abstract]. Kidney International 1991;40(5):978. [Google Scholar]
EDEN 2013 {published data only}
- Bargman JM, Culleton BF, Do JY, Gomez RA, Yu AW, Prichard SS, et al. The impact of a low glucose peritoneal dialysis solution regimen on serum lipids and lipoproteins in diabetic patients: The IMPENDIA and EDEN randomized, controlled clinical trials [abstract no: FR‐OR027]. Journal of the American Society of Nephrology 2012; Vol. 23:37A.
- Li PK, Culleton BF, Ariza A, Do JY, Johnson DW, Sanabria M, et al. Randomized, controlled trial of glucose‐sparing peritoneal dialysis in diabetic patients. Journal of the American Society of Nephrology 2013; Vol. 24:1889‐900. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
- Sniderman AD, Sloand JA, Li PK, Story K, Bargman JM. Influence of low‐glucose peritoneal dialysis on serum lipids and apolipoproteins in the IMPENDIA/EDEN trials. Journal of Clinical Lipidology 2014; Vol. 8, issue 4:441‐7. [MEDLINE: ] [DOI] [PubMed]
Fang 2008 {published data only}
- Fang W, Mullan R, Shah H, Mujais S, Bargman JM, Oreopoulos DG. Comparison between bicarbonate/lactate and standard lactate dialysis solution in peritoneal transport and ultrafiltration: a prospective, crossover single‐dwell study. Peritoneal Dialysis International 2008;28(1):35‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Feriani 1993 {published data only}
- Feriani M, Dissegna D, Greca G, Passlick‐Deetjen J. Continuous ambulatory peritoneal dialysis with bicarbonate buffer‐‐a pilot study. Peritoneal Dialysis International 1993;13 Suppl 2:S88‐91. [MEDLINE: ] [PubMed] [Google Scholar]
- Feriani M, Dissegna D, Greca G, Passlick‐Deetjen J. Short‐term clinical study with bicarbonate‐containing peritoneal dialysis solution. Peritoneal Dialysis International 1993;13(4):296‐301. [MEDLINE: ] [PubMed] [Google Scholar]
Fischbach 2004 {published data only}
- Fischbach M, Terzic J, Chauve S, Laugel V, Muller A, Haraldsson B. Effect of peritoneal dialysis fluid composition on peritoneal area available for exchange in children. Nephrology Dialysis Transplantation 2004;19(4):925‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hiss 2013 {published data only}
- Hiss M, Gerstein F, Haller H, Gueler F. Randomised prospective clinical study on outcome of peritoneal dialysis in patients using normal versus reduced glucose dialysis solution [abstract]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i437. [EMBASE: 71076352] [Google Scholar]
Hwang 2006 {published data only}
- Hwang JC, Wang HY, Wang CT, Chen HC. Comparison of peritoneal equilibrium test with icodextrin and 2.5% glucose dialysis solutions. Journal of Nephrology 2006;19(6):758‐63. [MEDLINE: ] [PubMed] [Google Scholar]
IMPENDIA 2013 {published data only}
- Bargman JM, Culleton BF, Do JY, Gomez RA, Yu AW, Prichard SS, et al. The impact of a low glucose peritoneal dialysis solution regimen on serum lipids and lipoproteins in diabetic patients: The IMPENDIA and EDEN randomized, controlled clinical trials [abstract no: FR‐OR027]. Journal of the American Society of Nephrology 2012; Vol. 23, issue Abstracts:37A.
- Li PK, Culleton BF, Ariza A, Do JY, Johnson DW, Sanabria M, et al. Randomized, controlled trial of glucose‐sparing peritoneal dialysis in diabetic patients. Journal of the American Society of Nephrology 2013; Vol. 24, issue 11:1889‐900. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
- Li PK, Dorval M, Johnson DW, Rutherford P, Shutov E, Story K, et al. The benefit of a glucose‐sparing PD therapy on glycemic control measured by serum fructosamine in diabetic patients in a randomized, controlled trial (IMPENDIA). Nephron 2015; Vol. 129, issue 4:233‐40. [MEDLINE: ] [DOI] [PubMed]
- Sniderman AD, Sloand JA, Li PK, Story K, Bargman JM. Influence of low‐glucose peritoneal dialysis on serum lipids and apolipoproteins in the IMPENDIA/EDEN trials. Journal of Clinical Lipidology 2014; Vol. 8, issue 4:441‐7. [MEDLINE: ] [DOI] [PubMed]
Jenkins 2003 {published data only}
- Jenkins SB, Tindale W, Wilkie ME. Sodium and water clearance during peritoneal dwells with a novel combination dialysate (1.36% glucose/7.5% Icodextrin) [abstract]. Peritoneal Dialysis International 2002;22(1):114. [CENTRAL: CN‐00401399] [Google Scholar]
- Jenkins SB, Wilkie ME. An exploratory study of a novel peritoneal combination dialysate (1.36% glucose/7.5% icodextrin), demonstrating improved ultrafiltration compared to either component studied alone. Peritoneal Dialysis International 2003;23(5):475‐80. [MEDLINE: ] [PubMed] [Google Scholar]
John 2008 {published data only}
- John SG, Selby NM, McIntyre CW. Effects of peritoneal dialysis fluid biocompatibility on baroreflex sensitivity. Kidney International ‐ Supplement 2008;108:S119‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
le Poole 2004 {published data only}
- Poole CY, Ittersum FJ, Valentijn RM, Ter Wee PM, Schalk C. PD regimen contributes to the blood concentration of glucose degradation products (GDP) in new CAPD patients [abstract no: SA‐PO820]. Journal of the American Society of Nephrology 2003;14(Nov):478A. [Google Scholar]
- Welten A, Poole K, ter Wee P, Ittersum F, Valentijn R, Beelen RH, et al. Biocompatibility markers of standard versus high‐glucose regimen of CAPD patients in a multi‐centered, cross‐over study [abstract no: SA‐PO812]. Journal of the American Society of Nephrology 2003;14(Abstracts):476a. [Google Scholar]
- Welten AG, Poole C, ter Wee PM, Ittersum FJ, Beelen RH, Born J. The effects of a high‐versus low‐glucose regime on cell recruitment and biomarkers of CAPD patients. A multi‐center, prospective cross‐over study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):214‐5. [CENTRAL: CN‐00448337] [Google Scholar]
- Welten AG, Poole C, Ittersum FJ, ter Wee PM, Beelen RH, Born J. Biocompatibility of high versus low glucose regime on peritoneal cells of CAPD patients in a multicenter cross‐over study [abstract]. Peritoneal Dialysis International 2002;22(1):111. [CENTRAL: CN‐00403070] [Google Scholar]
- Welten AG, Poole C, Ittersum FJ, ter Wee PM, Beelen RH, Born J. Biocompatibility of high‐ versus low‐glucose regime on peritoneal cells of CAPD patients in a multi‐centered cross‐over study [abstract no: F‐PO698]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):202A. [CENTRAL: CN‐00448338] [Google Scholar]
- Poole C, Weijmer M, Ittersum F, Valentijn R, ter Wee P. Clinical effects of a PD regimen low in glucose and GDP's (LG) compared to a standard PD regimen (SPD) in newly CAPD patients [abstract no: F‐PO688]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):200A. [CENTRAL: CN‐00446297] [Google Scholar]
- Poole C, Weijmer M, Ittersum F, Valentijn R, ter Wee P. Outcome of routine laboratory data after 30 weeks of treatment with a PD regimen low in glucose and GDPs (LG) or a standard PD regimen (SPD) in newly CAPD patients [abstract no: F‐PO689]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):200A. [CENTRAL: CN‐00446298] [Google Scholar]
- Poole CY, Schalkwijk CG, Teerlink T, Valentijn RM, ter Wee PM, Ittersum FJ. Higher plasma levels of von Willebrand factor and C‐reactive protein during a peritoneal dialysis regimen with less glucose and glucose degradation products. Peritoneal Dialysis International 2013;33(2):208‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CY, Welten AG, Weijmer MC, Valentijn RM, Ittersum FJ, ter Wee PM. Initiating CAPD with a regimen low in glucose and glucose degradation products, with icodextrin and amino acids (NEPP) is safe and efficacious. Peritoneal Dialysis International 2005;25 Suppl 3:S64‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Poole CY, Welten AG, ter Wee PM, Paauw NJ, Djorai AN, Valentijn RM, et al. A peritoneal dialysis regimen low in glucose and glucose degradation products results in increased cancer antigen 125 and peritoneal activation. Peritoneal Dialysis International 2012;32(3):305‐15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CY, Ittersum FJ, Lindholm B, Suliman ME, Valentijn RM, ter Wee PM, et al. Peritoneal dialysis regimen low in glucose and GDPs results in lower plasma pentosidine levels [abstract no: SA‐PO338]. Journal of the American Society of Nephrology 2004;15(Oct):375A. [CENTRAL: CN‐00626030] [Google Scholar]
- Poole CY, Ittersum FJ, Valentijn RM, Teerlink T, Lindholm B, ter Wee PM, et al. "NEPP" peritoneal dialysis regimen has beneficial effects on plasma CEL and 3‐DG, but not pentosidine, CML, and MGO. Peritoneal Dialysis International 2012;32(1):45‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CY, Ittersum FJ, Valentijn RM, ter Wee PM. Clinical effects of PD regimens high or low in glucose and GDP's in a one year multi‐center randomized cross‐over trial [abstract no: SA‐PO819]. Journal of the American Society of Nephrology 2003;14(Nov):478A. [CENTRAL: CN‐00626033] [Google Scholar]
- Poole CY, Ittersum FJ, Valentijn RM, ter Wee PM, Schalkwijk CG. Higher markers of endothelial activation during treatment with an alternative peritoneal dialysis solution regimen low in glucose and GDPs (NEPP) [abstract no: F‐PO423]. Journal of the American Society of Nephrology 2004;15(Oct):159A. [CENTRAL: CN‐00626031] [Google Scholar]
- Poole CY, Ittersum FJ, Valetijn RM, ter Wee PM. Metabolic effects of PD regimens high or low in glucose and GDP's in a one year multi‐center randomized cross‐over trial [abstract no: SA‐PO818]. Journal of the American Society of Nephrology 2003;14(Nov):478A. [CENTRAL: CN‐00626032] [Google Scholar]
- Poole CY, Ittersum FJ, Weijmer MC, Valentijn RM, ter Wee PM. Clinical effects of a peritoneal dialysis regimen low in glucose in new peritoneal dialysis patients: a randomized crossover study. Advances in Peritoneal Dialysis 2004;20:170‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Ittersum FJ, Welten A, Poole C, Ter Wee PM, Valentijn R, Beelen RH, et al. Low glucose/GDP dialysis regimen results in increased mesothelial regeneration and vascular permeability [abstract no: SA‐FC129]. Journal of the American Society of Nephrology 2005;16:110A. [Google Scholar]
Liberek 2002 {published data only}
- Liberek T, Lichodziejewska‐Niemierko M, Knopinska‐Posluszny W, Schaub TP, Kirchgessner J, Passlick‐Deetjen J, et al. Generation of TNFalpha and interleukin‐6 by peritoneal macrophages after overnight dwells with bicarbonate‐ or lactate‐buffered dialysis fluid. Peritoneal Dialysis International 2002;22(6):663‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Lui 2012 {published data only}
- Lui SL, Lui S, Yung S, Tang C, Ng F, Lo WK, et al. Effect of biocompatible peritoneal dialysis fluids on daily urine volume, serum adiponectin levels, small solute permeability and mesothelial cell integrity six months after conversion back to conventional peritoneal dialysis fluids [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii460. [EMBASE: 70766678] [Google Scholar]
- Lui SL, Yung S, Yim A, Wong KM, Tong KL, Wong KS, et al. A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: a randomized clinical trial. American Journal of Kidney Diseases 2012;60(6):966‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yung S, Lui SL, Ng CK, Yim A, Ma MK, Lo KY, et al. Impact of a low‐glucose peritoneal dialysis regimen on fibrosis and inflammation biomarkers. Peritoneal Dialysis International 2015;35(2):147‐58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martikainen 2005 {published data only}
- Martikainen TA, Teppo AM, Gronhagen‐Riska C, Ekstrand AV. Glucose‐free dialysis solutions: inductors of inflammation or preservers of peritoneal membrane?. Peritoneal Dialysis International 2005;25(5):453‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Parikova 2007 {published data only}
- Parikova A, Struijk DG, Zweers MM, Langedijk M, Schouten N, Berg N, et al. Does the biocompatibility of the peritoneal dialysis solution matter in assessment of peritoneal function?. Peritoneal Dialysis International 2007;27(6):691‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Pedersen 1985 {published data only}
- Pedersen FB, Ryttov N, Deleuran P. Acetate versus lactate in peritoneal dialysis solutions. Nephron 1985;39(1):55‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peers 1997 {published data only}
- Peers E. Icodextrin plus glucose combinations for use in CAPD. Peritoneal Dialysis International 1997;17 Suppl 2:S68‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Plum 1997 {published data only}
- Plum J, Erren C, Fieseler C, Kirchgessner J, Passlick‐Deetjen J, Grabensee B. An amino acid‐based peritoneal dialysis fluid buffered with bicarbonate versus glucose/bicarbonate and glucose/lactate solutions: an intraindividual randomized study. Peritoneal Dialysis International 1999;19(5):418‐28. [MEDLINE: ] [PubMed] [Google Scholar]
- Plum J, Fusholler A, Schoenicke G, Busch T, Erren C, Fieseler C, et al. In vivo and in vitro effects of amino‐acid‐based and bicarbonate‐buffered peritoneal dialysis solutions with regard to peritoneal transport and cytokines/prostanoids dialysate concentrations. Nephrology Dialysis Transplantation 1997;12(8):1652‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Plum J, Fussholler A, Schonicke G, Busch T, Erren C, Fieseler C, et al. Effects of alternative peritoneal dialysis solutions on peritoneal transport and cytokines/prostanoids dialysate concentrations. Nieren‐und Hochdruckkrankheiten 1997;26(Suppl 1):S80‐5. [EMBASE: 1997355309] [Google Scholar]
Rodriguez‐Carmona 2007 {published data only}
- Perez‐Fontan M, Rodriguez‐Carmona A, Garcia‐Lopez E, Garcia‐Falcon T, Diaz‐Cambre E. Icodextrin‐based nocturnal automated peritoneal dialysis (APD) allows sustained ultrafiltration (UF) while reducing peritoneal glucose load. A crossover study [abstract no: TH‐PO806]. Journal of the American Society of Nephrology 2006;17(Abstracts):278A. [PubMed] [Google Scholar]
- Rodriguez‐Carmona A, Perez Fontan M, Garcia Lopez E, Garcia Falcon T, Diaz Cambre H. Use of icodextrin during nocturnal automated peritoneal dialysis allows sustained ultrafiltration while reducing the peritoneal glucose load: a randomized crossover study. Peritoneal Dialysis International 2007;27(3):260‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Sav 2009 {published data only}
- Sav T, Oymak O, Inanc MT, Dogan A, Tokgoz B, Utas C. Effects of twice‐daily icodextrin administration on blood pressure and left ventricular mass in patients on continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 2009;29(4):443‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Sav T, Oymak O, Sipahioglu H, Unal A, Akcakaya M, Tokgoz B, et al. The double dose icodextrin use can decrease the risk of the coronary heart disease in patients on peritoneal dialysis [abstract no: S‐PO‐0223]. 4th World Congress of Nephrology.19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21‐25; Rio de Janeiro, Brazil. 2007:108.
- Sav T, Oymak O, Sipahioglu MH, Unal A, Akcakaya M, Tokgoz B, et al. Double dose icodextrin not affect the peritoneal and systemic inflammation [abstract no: SaP201]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi299. [CENTRAL: CN‐00774417] [Google Scholar]
Sav 2010 {published data only}
- Sav T, Inanc MT, Dogan A, Oymak O, Utas C. Two daytime icodextrin exchanges decrease brain natriuretic peptide levels and improve cardiac functions in continuous ambulatory peritoneal dialysis patients. Nephrology 2010;15(3):307‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Selby 2005 {published data only}
- Selby NM, Fluck RJ, Taal MW, McIntyre CW. Hypertonic glucose‐based peritoneal dialysate is associated with higher blood pressure and adverse haemodynamics as compared with icodextrin [abstract no: MP420]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v340. [DOI] [PubMed] [Google Scholar]
- Selby NM, Fonseca S, Hulme L, Fluck RJ, Taal MW, McIntyre CW. Hypertonic glucose‐based peritoneal dialysate is associated with higher blood pressure and adverse haemodynamics as compared with icodextrin. Nephrology Dialysis Transplantation 2005;20(9):1848‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Selby 2007a {published data only}
- Selby NM, Fialova J, Burton JO, McIntyre CW. The haemodynamic and metabolic effects of hypertonic‐glucose and amino‐acid‐based peritoneal dialysis fluids. Nephrology Dialysis Transplantation 2007;22(3):870‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smit 2000 {published data only}
- Smit W, Ho‐dac‐Pannekeet MM. Peritoneal permeability characteristics using glycerol based dialysate in CAPD [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A182. [CENTRAL: CN‐00261426] [Google Scholar]
- Smit W, Waart DR, Struijk DG, Krediet RT. Peritoneal transport characteristics with glycerol‐based dialysate in peritoneal dialysis. Peritoneal Dialysis International 2000;20(5):557‐65. [MEDLINE: ] [PubMed] [Google Scholar]
Smit 2001 {published data only}
- Smit W, Langedijk MJ, Schouten N, Berg N, Struijk DG, Krediet RT. A comparison between 1.36% and 3.86% glucose dialysis solution for the assessment of peritoneal membrane function. Peritoneal Dialysis International 2001;20(6):734‐41. [MEDLINE: ] [PubMed] [Google Scholar]
- Smit W, Struijk DG, Krediet RT. A comparison between 1.36% and 3.86% glucose dialysis solutions for the assessment of peritoneal membrane function [abstract no: A1160]. Journal of the American Society of Nephrology 2000;11(Sept):219A. [PubMed] [Google Scholar]
- Smit W, Struijk DG, Krediet RT. A comparison between 1.36% and 3.86% glucose dialysis solutions for the assessment of peritoneal membrane function [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A233. [CENTRAL: CN‐00461758] [PubMed] [Google Scholar]
Ueda 2000 {published data only}
- Ueda Y, Miyata T, Goffin E, Yoshino A, Inagi R, Ishibashi Y, et al. Effect of dwell time on carbonyl stress using icodextrin and amino acid peritoneal dialysis fluids. Kidney International 2000;58(6):2518‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Biesen 2004 {published data only}
- Biesen W, Boer W, Greve B, Dequidt C, Vijt D, Faict D, et al. A randomized clinical trial with a 0.6% amino acid/ 1.4% glycerol peritoneal dialysis solution. Peritoneal Dialysis International 2004;24(3):222‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Yehia 2014 {published data only}
- Yehia M, Muyoma G, Topley N, Collins JF. The use of exchange‐free periods alternating with daily exchanges of icodextrin in the initial treatment of peritoneal dialysis‐associated peritonitis: a safety study. Peritoneal Dialysis International 2014;34(7):810‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2014 {published data only}
- Yoon HE, Chang YK, Shin SJ, Choi BS, Kim BS, Park CW, et al. Benefits of a continuous ambulatory peritoneal dialysis (CAPD) technique with one icodextrin‐containing and two biocompatible glucose‐containing dialysates for preservation of residual renal function and biocompatibility in incident CAPD patients. Journal of Korean Medical Science 2014;29(9):1217‐25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cho 2010 {published data only}
- Cho KH, Jung SY, Do JY, Park JW, Yoon KW. The effect of low GDP solution on ultrafiltration and solute transport in CAPD patients [abstract]. NDT Plus 2010;3:iii166. [EMBASE: 70483857] [Google Scholar]
Dai 2010 {published data only}
- Dai HL, Lin AW, Qian JQ, Fang W, Ni ZH, Cao LO, et al. Icodextrin improve angiogenesis of peritoneal membrane in continuous ambulatory peritoneal dialysis patients. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2010;90(40):2843‐7. [MEDLINE: ] [PubMed] [Google Scholar]
De Los Rios 2016 {published data only}
- Rios T, Perez‐Martinez J, Portoles J, Lichodziejewska‐Niemierko M, Rivera M, Nowicki M, et al. Effect of balance solution on the peritoneal membrane in automated peritoneal dialysis. Peritoneal Dialysis International 2016;36(5):569‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Do 2006a {published data only}
- Do J, Bae D, Kim M, Lee S, Park J, Yoon K. The effect of low GDPs solution on survival in continuous ambulatory peritoneal dialysis patients and the associated factors with survival [abstract no: F‐PO347]. Journal of the American Society of Nephrology 2006;17(Abstracts):411A. [Google Scholar]
Infante 2000 {published data only}
- Gesualdo L, Infante B, Guastadisegni MC, Grandaliano G, Petrarulo F, Giannattasio M, et al. Ca125/pai‐1 ration as a marker of biocompatibility in CAPD: a prospective randomized clinical trial [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):208A. [CENTRAL: CN‐00615878] [Google Scholar]
- Infante B, Guastadisegni MC, Grandaliano G, Colucci M, Semeraro N, Petrarulo F, et al. Ca125/pai‐1 ration may be a marker of peritoneal dialysis (PD) fluids biocompatibility and membrane longevity: a clinical randomized prospective study [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A238. [CENTRAL: CN‐00460988] [Google Scholar]
Kim 2006 {published data only}
- Kim Y, Choi B, Yang C, Chang Y, Bang B. Clinical benefits of a bicarbonate/lactate‐based peritoneal dialysis solution [abstract no: SP706]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv253. [Google Scholar]
Kocyigit 2015 {published data only}
- Kocyigit I, Unal A, Gungor O, Orscelik O, Eroglu E, Dogan E, et al. Effects of dialysis solution on the cardiovascular function in peritoneal dialysis patients. Internal Medicine 2015;54(1):3‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT01753154 {published data only}
- Feriani M. The effect of balance PD solution on the peritoneal membrane in patients on automated peritoneal dialysis. www.clinicaltrials.gov/ct2/show/NCT01753154 (first received 20 December 2012).
Opatrna 2002 {published data only}
- Opatrna S, Linhartova K, Opatrny J, Senft V, Stehlik P, Sefrna F, et al. The effect of icodextrin dialysis solution on selected metabolic parameters in patients on continuous ambulatory peritoneal dialysis (CAPD) [abstract]. Kidney & Blood Pressure Research 2000:131. [CENTRAL: CN‐00509396] [Google Scholar]
- Opatrna S, Racek J, Stehlik P, Senft V, Sefrna F, Topolcan O, et al. Effect of a dialysis solution with icodextrin on ultrafiltration and selected metabolic parameters in patients treated with peritoneal dialysis [Vliv podani dialyzacniho roztoku s icodextrinem na ultrafiltraci a vybrane metabolicke parametry nemocnych lecenych peritonealni dialyzou]. Casopis Lekaru Ceskych 2002;141(9):281‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Rodriguez‐Carmona 2012 {published data only}
- Rodriguez‐Carmona A, Perez‐Fontan M, Guitian A, Peteiro J, Garcia‐Falcon T, Lopez‐Muniz A, et al. Effect of low‐GDP bicarbonate‐lactate‐buffered peritoneal dialysis solutions on plasma levels of adipokines and gut appetite‐regulatory peptides. A randomized crossover study. Nephrology Dialysis Transplantation 2012;27(1):369‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2002b {published data only}
- Yang CW, Wu CH, Yu CC, Weng SM, Fang JT, Wu MS, et al. Dependence of ultrafiltration response to icodextrin on peritoneal membrane transport type [abstract no: F‐PO684]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):199A. [CENTRAL: 00448455] [Google Scholar]
References to ongoing studies
NCT01228279 {published data only}
- Ruzicka M. Effects of non‐glucose‐based peritoneal dialysis solution "extraneal" on changes in leptin levels and sympathetic activity induced by conventional glucose‐based dialysate "dianeal" in patients on peritoneal dialysis. www.clinicaltrials.gov/ct2/show/NCT01228279 (first received 26 October 2010).
Additional references
Ayuzawa 2012
- Ayuzawa N, Ishibashi Y, Takazawa Y, Kume H, Fujita T. Peritoneal morphology after long‐term peritoneal dialysis with biocompatible fluid: recent clinical practice in Japan. Peritoneal Dialysis International 2012;32(2):159‐67. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bargman 2001
- Bargman JM, Thorpe KE, Churchill DN, CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. Journal of the American Society of Nephrology 2001;12(10):2158‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bargman 2010
- Bargman JM. Slouching towards Bethlehem: the beast of biocompatibility. Nephrology Dialysis Transplantation 2010;25(7):2050‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boulanger 2002
- Boulanger E, Wautier MP, Wautier JL, Boval B, Panis Y, Wernert N, et al. AGEs bind to mesothelial cells via RAGE and stimulate VCAM‐1 expression. Kidney International 2002;61(1):148‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 2009
- Brown EA, Biesen W, Finkelstein F, Hurst H, Johnson DW, Kawanishi H, et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Peritoneal Dialysis International 2009;29(6):595‐600. [MEDLINE: ] [PubMed] [Google Scholar]
Brown 2017
- Brown EA, Bargman J, Biesen W, Chang MY, Finkelstein FO, Hurst H, et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD: 2017 update. Peritoneal Dialysis International 2017;37(4):362‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cho 2012
- Cho Y, Badve SV, Hawley CM, Wiggins K, Johnson DW. Biocompatible peritoneal dialysis fluids: clinical outcomes. International Journal of Nephrology 2012;2012:812609. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2009
- Davies SJ. Preserving residual renal function in peritoneal dialysis: volume or biocompatibility?. Nephrology Dialysis Transplantation 2009;24(9):2620‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman A D, Akl E A, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Htay 2016
- Htay H, Cho Y, Pascoe EM, Darssan D, Hawley CM, Johnson DW, et al. Predictors of residual renal function decline in peritoneal dialysis patients: the balANZ trial. Peritoneal Dialysis International 2017;37(3):283‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 2003
- Johnson DW, Agar J, Collins J, Disney A, Harris DC, Ibels L, et al. Recommendations for the use of icodextrin in peritoneal dialysis patients. Nephrology 2003;8(1):1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 2010
- Johnson DW, Cho Y, Livingston BE, Hawley CM, McDonald SP, Brown FG, et al. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney International 2010;77(10):904‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 2012
- Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. The effect of low glucose degradation product, neutral pH versus standard peritoneal dialysis solutions on peritoneal membrane function: the balANZ trial. Nephrology Dialysis Transplantation 2012;27(12):4445‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jorres 1992
- Jorres A, Topley N, Gahl GM. Biocompatibility of peritoneal dialysis fluids. International Journal of Artificial Organs 1992;15(2):79‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Jorres 1998
- Jorres A, Bender TO, Finn A, Witowski J, Frohlich S, Gahl GM, et al. Biocompatibility and buffers: effect of bicarbonate‐buffered peritoneal dialysis fluids on peritoneal cell function. Kidney International 1998;54(6):2184‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jorres 2012
- Jörres A. Novel peritoneal dialysis solutions‐‐what are the clinical implications?.[Erratum appears in Blood Purif. 2012;34(1):17]. Blood Purification 2012;33(1‐3):153–9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Justo 2005
- Justo P, Belen Sanz A, Egido J, Ortiz A. 3,4‐Dideoxyglucosone‐3‐ene induces apoptosis in renal tubular epithelial cells. Diabetes 2005;54(8):2424‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lamb 1995
- Lamb EJ, Cattell WR, Dawnay AB. In vitro formation of advanced glycation end products in peritoneal dialysis fluid. Kidney International 1995;47(6):1768‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lan 2016
- Lan PG, Clayton PA, Johnson DW, McDonald SP, Borlace M, Badve SV, et al. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Peritoneal Dialysis International 2016;36(6):623‐30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2017
- Li P, Chow KM, Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nature Reviews Nephrology 2017;13(2):90‐103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mateijsen 1999
- Mateijsen MA, Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, et al. Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Peritoneal Dialysis International 1999;19(6):517‐25. [MEDLINE: ] [PubMed] [Google Scholar]
Mehrotra 2016
- Mehrotra R, Devuyst O, Davies SJ, Johnson DW. The current state of peritoneal dialysis. Journal of the American Society of Nephrology 2016;27(11):3238‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortier 2003
- Mortier S, Vriese AS, McLoughlin RM, Topley N, Schaub TP, Passlick‐Deetjen J, et al. Effects of conventional and new peritoneal dialysis fluids on leukocyte recruitment in the rat peritoneal membrane. Journal of the American Society of Nephrology 2003;14(5):1296‐306. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mortier 2004
- Mortier S, Faict D, Schalkwijk CG, Lameire NH, Vriese AS. Long‐term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney International 2004;66(3):1257‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mortier 2005
- Mortier S, Faict D, Lameire NH, Vriese AS. Benefits of switching from a conventional to a low‐GDP bicarbonate/lactate‐buffered dialysis solution in a rat model. Kidney International 2005;67(4):1559‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mujais 2000
- Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, et al. Evaluation and management of ultrafiltration problems in peritoneal dialysis. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Peritoneal Dialysis International 2000;20 Suppl 4:S5‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Nadeau‐Fredette 2015
- Nadeau‐Fredette AC, Chan CT, Cho Y, Hawley CM, Pascoe EM, Clayton PA, et al. Outcomes of integrated home dialysis care: a multi‐centre, multi‐national registry study. Nephrology Dialysis Transplantation 2015;30(11):1897‐904. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakayama 1997
- Nakayama M, Kawaguchi Y, Yamada K, Hasegawa T, Takazoe K, Katoh N, et al. Immunohistochemical detection of advanced glycosylation end‐products in the peritoneum and its possible pathophysiological role in CAPD. Kidney International 1997;51(1):182‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nataatmadja 2017
- Nataatmadja M, Cho Y, Johnson DW. Evidence for biocompatible peritoneal dialysis solutions. Contributions to Nephrology 2017;189:91‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nilsson‐Thorell 1993
- Nilsson‐Thorell CB, Muscalu N, Andren AH, Kjellstrand PT, Wieslander AP. Heat sterilization of fluids for peritoneal dialysis gives rise to aldehydes. Peritoneal Dialysis International 1993;13(3):208‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Pollock 2005
- Pollock C. Pathogenesis of peritoneal sclerosis. International Journal of Artificial Organs 2005;28(2):90‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rigby 1998
- Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrology Dialysis Transplantation 1998;13(1):154‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schambye 1996
- Schambye HT. Effect of different buffers on the biocompatibility of CAPD solutions. Peritoneal Dialysis International 1996;16 Suppl 1:S130‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Szeto 2017
- Szeto CC, Johnson DW. Low GDP solution and glucose sparing strategies for peritoneal dialysis. Seminars in Nephrology 2017;37(1):30‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Topley 1997
- Topley N. In vitro biocompatibility of bicarbonate‐based peritoneal dialysis solutions. Peritoneal Dialysis International 1997;17(1):42‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Vaamonde 1975
- Vaamonde CA, Michael UF, Metzger RA, Carroll KE Jr. Complications of acute peritoneal dialysis. Journal of Chronic Diseases 1975;28(11‐12):637‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2015
- Wang AY, Brimble KS, Brunier G, Holt SG, Jha V, Johnson DW, et al. ISPD cardiovascular and metabolic guidelines in adult peritoneal dialysis patients Part I ‐ assessment and management of various cardiovascular risk factors. Peritoneal Dialysis International 2015;35(4):379‐87. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wieslander 1996
- Wieslander A, Forsback G, Svensson E, Linden T. Cytotoxicity, pH, and glucose degradation products in four different brands of PD fluid. Advances in Peritoneal Dialysis 1996;12:57‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Williams 2003
- Williams JD, Craig KJ, Topley N, Williams GT. Peritoneal dialysis: changes to the structure of the peritoneal membrane and potential for biocompatible solutions. Kidney International ‐ Supplement 2003;84:S158–61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Witowski 2005
- Witowski J, Jorres A. Effects of peritoneal dialysis solutions on the peritoneal membrane: clinical consequences. Peritoneal Dialysis International 2005;25 Suppl 3:S31‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Yohanna 2015
- Yohanna S, Alkatheeri AM, Brimble SK, McCormick B, Iansavitchous A, Blake PG, et al. Effect of neutral‐pH, low‐glucose degradation product peritoneal dialysis solutions on residual renal function, urine volume, and ultrafiltration: a systematic review and meta‐analysis. Clinical Journal of The American Society of Nephrology: CJASN 2015;10(8):1380‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cho 2014
- Cho Y, Johnson DW, Craig JC, Strippoli GF, Badve SV, Wiggins KJ. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD007554.pub2] [DOI] [PubMed] [Google Scholar]
Wiggins 2009
- Wiggins KJ, Craig JC, Johnson DW, Strippoli GF. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007554] [DOI] [PubMed] [Google Scholar]


