Abstract
Background
Chronic kidney disease (CKD) is defined as reduced function of the kidneys present for 3 months or longer with adverse implications for health and survival. For several decades low protein diets have been proposed for participants with CKD with the aim of slowing the progression to end‐stage kidney disease (ESKD) and delaying the onset of renal replacement therapy. However the relative benefits and harms of dietary protein restriction for preventing progression of CKD have not been resolved. This is an update of a systematic review first published in 2000 and updated in 2006 and 2009.
Objectives
To determine the efficacy of low protein diets in preventing the natural progression of CKD towards ESKD and in delaying the need for commencing dialysis treatment in non‐diabetic adults.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 2 March 2018 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
We included randomised controlled trials (RCTs) or quasi RCTs in which adults with non‐diabetic chronic kidney disease (stages 3 to 5) not on dialysis were randomised to receive a very low protein intake (0.3 to 0.4 g/kg/d) compared with a low protein intake (0.5 to 0.6 g/kg/d) or a low protein intake compared with a normal protein intake (≥ 0.8 g/kg/d) for 12 months or more.
Data collection and analysis
Two authors independently selected studies and extracted data. For dichotomous outcomes (death, all causes), requirement for dialysis, adverse effects) the risk ratios (RR) with 95% confidence intervals (CI) were calculated and summary statistics estimated using the random effects model. Where continuous scales of measurement were used (glomerular filtration rate (GFR), weight), these data were analysed as the mean difference (MD) or standardised mean difference (SMD) if different scales had been used. The certainty of the evidence was assessed using GRADE.
Main results
We identified an additional six studies to include 17 studies with 2996 analysed participants (range 19 to 840). Four larger multicentre studies were subdivided according to interventions so that the review included 21 separate data sets. Mean duration of participant follow‐up ranged from 12 to 50 months.
Random sequence generation and allocation concealment were considered at low risk of bias in eleven and nine studies respectively. All studies were considered at high risk for performance bias as they were open‐label studies. We assessed detection bias for outcome assessment for GFR and ESKD separately. As GFR measurement was a laboratory outcome all studies were assessed at low risk of detection bias. For ESKD, nine studies were at low risk of detection bias as the need to commence dialysis was determined by personnel independent of the study investigators. Five studies were assessed at high risk of attrition bias with eleven studies at low risk. Ten studies were at high risk for reporting bias as they did not include data which could be included in a meta‐analysis. Eight studies reported funding from government bodies while the remainder did not report on funding.
Ten studies compared a low protein diet with a normal protein diet in participants with CKD categories 3a and b (9 studies) or 4 (one study). There was probably little or no difference in the numbers of participants who died (5 studies 1680 participants: RR 0.77, 95% CI 0.51 to 1.18; 13 fewer deaths per 1000; moderate certainty evidence). A low protein diet may make little or no difference in the number of participants who reached ESKD compared with a normal protein diet (6 studies, 1814 participants: RR 1.05, 95% CI 0.73 to 1.53; 7 more per 1000 reached ESKD; low certainty evidence). It remains uncertain whether a low protein diet compared with a normal protein intake impacts on the outcome of final or change in GFR (8 studies, 1680 participants: SMD ‐0.18, 95% CI ‐0.75 to 0.38; very low certainty evidence).
Eight studies compared a very low protein diet with a low protein diet and two studies compared a very low protein diet with a normal protein diet. A very low protein intake compared with a low protein intake probably made little or no difference to death (6 studies, 681 participants: RR 1.26, 95% CI 0.62 to 2.54; 10 more deaths per 1000; moderate certainty evidence). However it probably reduces the number who reach ESKD (10 studies, 1010 participants: RR 0.65, 95% CI 0.49 to 0.85; 165 per 1000 fewer reached ESKD; moderate certainty evidence). It remains uncertain whether a very low protein diet compared with a low or normal protein intake influences the final or change in GFR (6 studies, 456 participants: SMD 0.12, 95% CI ‐0.27 to 0.52; very low certainty evidence).
Final body weight was reported in only three studies. It is uncertain whether the intervention alters final body weight (3 studies, 89 participants: MD ‐0.40 kg, 95% CI ‐6.33 to 5.52; very low certainty evidence).Twelve studies reported no evidence of protein energy wasting (malnutrition) in their study participants while three studies reported small numbers of participants in each group with protein energy wasting. Most studies reported that adherence to diet was satisfactory. Quality of life was not formally assessed in any studies.
Authors' conclusions
This review found that very low protein diets probably reduce the number of people with CKD 4 or 5, who progress to ESKD. In contrast low protein diets may make little difference to the number of people who progress to ESKD. Low or very low protein diets probably do not influence death. However there are limited data on adverse effects such as weight differences and protein energy wasting. There are no data on whether quality of life is impacted by difficulties in adhering to protein restriction. Studies evaluating the adverse effects and the impact on quality of life of dietary protein restriction are required before these dietary approaches can be recommended for widespread use.
Plain language summary
Low protein diets for non‐diabetic adults with chronic kidney disease
What is the issue?
Various forms of kidney disease can lead to kidney failure with affected people ultimately requiring dialysis treatment. A diet low in protein may be recommended to try to slow the progress of kidney disease to kidney failure. We still do not know whether low protein diets can slow the progress of kidney disease and delay the need to start dialysis.
What did we do?
We searched the Cochrane Kidney and Transplant Specialised Register up to 2 March 2018 for randomised controlled trials (RCT), which enrolled non‐diabetic adult patients with chronic kidney disease, not yet requiring dialysis, and which compared different dietary protein intakes, including very low (0.3 to 0.4 g/kg/d), low (0.5 to 0.6 g/kg/d) or normal protein intakes (≥ 0.8 g/kg/d) for 12 months or more.
What did we find?
We examined the evidence from 17 studies (21 data sets) with 2996 people with reduced kidney function. We found that very low protein diets compared with low or normal protein intakes probably reduce the number of people with advanced kidney failure, who progress to dialysis. When low protein diets were compared with normal protein diets, there was little of no difference in the number of people with less severe kidney failure, who progressed to dialysis. Side effects of low protein diets such as weight loss were uncommon but many studies did not report on side effects.
Conclusions
In people with advanced kidney failure, a very low protein intake probably slows the progress to kidney failure. However we need more information on the side effects of low protein diets and on whether quality of life is reduced because of difficulties in keeping to such a diet.
Summary of findings
Background
Description of the condition
Chronic kidney disease (CKD) is defined as abnormalities of the structure or function of the kidneys present for 3 months or more with adverse implications for health (KDIGO 2012). It is classified based on the cause, the severity of reduced kidney function as measured by the glomerular filtration rate (GFR) and the severity of albuminuria. KDIGO 2012 defined a GFR of less than 60 mL/min/1.73 m2 as indicating reduced kidney function (normal GFR in young healthy adults is approximately 125 mL/min/1.73 m2). CKD is associated with a range of complications leading to adverse health outcomes. Death (all causes) and cardiovascular death increase in individuals with GFR < 60 mL/min/1.73 m2 (Matshushita 2010). The rate of deterioration in kidney function is variable and depends on the underlying cause of CKD and is associated among other factors with elevated blood pressure, increasing levels of proteinuria and diabetes mellitus. In many people, though not all, with CKD, kidney function deteriorates progressively with people developing symptoms of uraemia. Eventually people require treatment with haemodialysis or peritoneal dialysis with some receiving kidney transplants.
Description of the intervention
The World Health Organisation (WHO) recommends that healthy adults should receive a daily protein intake of 0.8 g/kg/d. Most healthy adults in developed countries consume a diet with a protein intake exceeding 1 g/kg/d. In CKD with reduced GFR, nephrologists and dietitians have prescribed low (0.5 to 0.6 g/kg/d) or very low (0.3 to 0.4 g/kg/d) high biologic‐value protein diets aiming to reduce the rate at which GFR deteriorates and to alleviate some of the complications of advanced CKD including metabolic acidosis, bone disease and uraemic symptoms and thus delay the onset of end‐stage kidney disease (ESKD), which leads to significant reduction in quality of life. To achieve very low protein intakes, some centres prescribe vegetarian diets (Garneata 2013). Very low protein diets are frequently supplemented with essential amino acids and nitrogen free keto‐analogues of amino acids to reduce the risk of malnutrition. If sufficient calories are ingested, keto‐analogues can be converted to amino acids via urea recycling. Extensive nutritional counselling is required to ensure that participants understand how to maintain a low or very low protein diet with an adequate calorie intake (30‐35 Kcal/kg ideal body weight/day). Compliance with a reduced protein diet is frequently assessed with measurement of the urinary urea nitrogen in 24‐hour urine collections and calculation of protein intake using the Maroni formula (6.25 X [urinary urea nitrogen x 0.03 body weight in kg]) (Maroni 1985).
How the intervention might work
Experimental studies in rats have shown that loss of nephrons leads to increased glomerular filtration in the remaining nephrons. The compensatory hyperfiltration results from increased plasma flow rates and increasing hydraulic pressure in the remaining nephrons. Eventually these haemodynamic changes lead to increased glomerular permeability with proteinuria and the development of progressive glomerulosclerosis. Long‐term studies in rats have demonstrated that compared with rats with CKD on a high protein diet, rats with CKD on a low protein diet had fewer sclerotic glomeruli and less proteinuria (Hostetter 1986). These experimental data supported the view that protein restriction in people with CKD could protect glomeruli from progressive glomerulosclerosis, slow the deterioration in kidney function and delay the onset of ESKD. In addition, protein restriction reduces uraemic symptoms associated with metabolic acidosis, CKD‐metabolic bone disease, hypertension, and fluid overload which could also delay the onset of ESKD even if the rate of kidney function deterioration measured by GFR does not change (Kasiske 1998).
Why it is important to do this review
There remains considerable controversy as to whether protein restriction does slow the rate of deterioration in kidney function in people with non‐diabetic CKD with proponents providing data to support or refute the benefit of protein restriction (Johnson 2006; Mandayam 2006). KDIGO 2012 concluded that dietary protein intake < 0.8 g/kg/d did not offer any advantage over 0.8 g/kg/d and suggested that protein restriction to 0.8 g/kg/d be limited to adults with GFR < 30 mL/min/1.73 m2. KDIGO 2012 also advised that people on any dietary protein restriction required careful monitoring of clinical and biochemical markers to avoid nutritional deficiencies.
Most of the clinical studies (both randomised controlled trials (RCTs) and observational studies) were designed to test the efficacy of reducing protein intake on surrogate kidney function outcomes, such as decline in creatinine clearance (CrCl) or changes in the reciprocal of creatinine over time. Unfortunately, changing protein intake modifies creatinine markers because reducing protein intake decreases creatinine production and changes kidney function (glomerular filtration as well as CrCl) by unidentified mechanisms. Although a few studies used methods to measure GFR using non‐creatinine measures such as 51CrEDTA clearance and I‐125 Iothalamate clearance, the results from these studies have been conflicting.
Two non‐Cochrane systematic reviews (Kasiske 1998; Pedrini 1996) have evaluated the efficacy of reduced protein diets. Pedrini 1996 reported that a low‐protein diet significantly reduced the risk of kidney failure or death. In contrast Kasiske 1998 found that dietary protein restriction reduced the rate of decline in estimated GFR by only 0.53 mL/min/y. Because the decision to commence dialysis is not based only on declining GFR but also on the presence of uraemic symptoms and nephrologists vary in their criteria for commencing dialysis, it is quite possible that those on a higher protein intake will have more uraemic symptoms and be considered for dialysis earlier than those on lower protein intakes with an equivalent rate of GFR decline but fewer uraemic symptoms. In this publication we update a Cochrane systematic review first published in 2000 (Fouque 2000b) and updated in 2006 (Fouque 2006) and 2009 (Fouque 2009). The 2009 update (Fouque 2009) reported the composite outcome of death and ESKD (dialysis initiation or renal transplantation) as the primary outcome. Overall fewer events (deaths, ESKD) were observed with very low or low protein intake compared with those occurring with low or normal protein intake suggesting that a reduced protein intake reduces the number of people who die or reach ESKD. We aimed to determine whether the addition of further RCTs would further clarify whether low or very low protein diets benefit adults with non‐diabetic CKD by delaying the onset of ESKD and/or slowing the rate of GFR decline without adverse effects on nutritional status.
Objectives
To determine the efficacy of low protein diets in preventing the natural progression of CKD towards ESKD and in delaying the need for commencing dialysis treatment in non‐diabetic adults.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) in which those in the experimental group received a reduced protein intake for 12 months or more while those in the control group received a higher or normal protein intake. Cross‐over studies were to be considered if the starting period of the intervention was randomly allocated and each intervention was in place for at least 12 months.
Types of participants
Adults suffering from moderate to severe CKD, as estimated by either serum creatinine (SCr), CrCl or GFR measurement but excluding participants on peritoneal dialysis, haemodialysis or following a kidney transplant.
Because of the difficulty to control for confounding factors, studies of diabetic participants or children with CKD were excluded from the review though studies including small numbers of diabetic participants were included.
Types of interventions
Studies comparing a normal protein intake (≥ 0.8 g/kg/d) with a low protein intake (0.5 to 0.6 g/kg/d) or very low protein intake (0.3 to 0.4 g/kg/d) for 12 months or more
Studies comparing a low protein intake (0.5 to 0.6 g/kg/d) with a very low protein intake (0.3 to 0.4 g/kg/d) for 12 months or more
Studies in which participants received supplements of essential amino acids, keto‐analogues or both were included provided that the total nitrogen intake differed between treatment groups.
Types of outcome measures
Primary outcomes
Death (all causes)
ESKD as defined by the need to commence dialysis during follow up or to receive a kidney transplant during follow‐up.
Secondary outcomes
End of study or change in GFR
End of study body weight
End of study body mass index (BMI)
Development of protein energy wasting (malnutrition) as defined by the study authors
Quality of life.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 2 March 2018 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The initial review and updates to 2009 was undertaken by four authors using the search strategy described. The titles and abstracts were screened by two authors, based on the defined inclusion criteria. They discarded studies that were not relevant (i.e. studies of lipid lowering agents) although studies and reviews that could have included relevant data or information on studies was retained initially. Disagreements were resolved by discussion.
This update was undertaken by three authors (DH, EH, DF). Potentially relevant studies were initially determined by two authors from titles and abstracts. Full text articles of potentially eligible articles were reviewed for eligibility by two authors to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction and assessment of risk of bias were performed independently by two authors using standardised data extraction forms. Studies in languages other than English were translated before data extraction. Where more than one report of a study was identified, data were extracted from the most complete report but the remaining reports were checked for additional information. Where there were discrepancies between reports, data from the primary source were used. Any further information required from the original authors was requested by written correspondence and any relevant information obtained in this manner was included in the review. Any disagreements were resolved in consultation with the third author.
Assessment of risk of bias in included studies
The following items were assessed independently by two authors using the risk of bias assessment tool (Higgins 2011) (Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (death (all causes), requirement for dialysis, adverse effects) the risk ratios (RR) with 95% confidence intervals (CI) for individual studies were calculated and summary statistics estimated using the random effects model. Where continuous scales of measurement were used to assess the effects of treatment (GFR, weight, BMI), these data were analysed as the mean difference (MD) or standardised mean difference (SMD) if different scales had been used. Either final levels or change in levels were included in meta‐analyses of continuous scales of measurement. When both measures were provided in a study, final levels were included. Where standard deviations (SD) for changes in levels or final levels were missing and not available from triallists, these were imputed (Higgins 2011).
Unit of analysis issues
Data from cross‐over studies were to be included in the meta‐analyses if separate data for the first part of the study were available. No cross‐over studies were identified.
Dealing with missing data
We aimed to analyse available data in meta‐analyses using intention‐to‐treat (ITT) data. However, where ITT data were not provided, or additional information could not be obtained from authors, available published data were used in the analyses.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. Heterogeneity was then analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). A guide to the interpretation of I2 values was as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
Assessment of reporting biases
The search strategy used aimed to reduce publication bias related to failure to publish negative results. Where there were multiple publications from the same study, the primary publications and additional reports were reviewed to identify all outcomes to reduce the risk of selective outcome reporting bias.
Data synthesis
Data were combined using random‐effects model for dichotomous and continuous data.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to assess differences in results possibly related to population groups, different ways of measuring decline in GFR and to risk of bias assessment but there were too few studies in each analysis to allow meaningful subgroup analyses.
Sensitivity analysis
Where a single study differed considerably from the other studies in the meta‐analysis, this study was temporarily excluded to determine whether its removal altered the results of the meta‐analysis.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Death in low protein versus normal protein diet groups
ESKD in low protein versus normal protein diet groups
End or change in GFR low protein versus normal protein diet
Death in very low protein versus higher protein diet groups
ESKD in very low versus low protein diet groups
End or change in GFR in very low protein diet versus higher protein diet
Adverse effects ‐ weight loss in very low protein diet versus higher protein diets
Results
Description of studies
Results of the search
The first version of this review included five studies (six reports; 1494 participants) (Fouque 2000b). Subsequent updates of the review published in 2006 and 2009 included eight studies (18 reports; 1524 participants) (Fouque 2006) and 10 studies (30 reports; 2000 participants) (Fouque 2009) respectively.
For this latest update of our search (to 2 March 2018) we identified seven new studies with 24 reports (Anonymous Study 1 1990; Anonymous Study 2 1990; Bergstrom 1986; Garneata 2013; MDRD Feasibility Study A 1989; MDRD Feasibility Study B 1989; Meloni 2004; Milovanov 2009; Teplan 1998) and 109 additional reports of eight previously included studies. We excluded 34 reports. Therefore 17 studies (21 data sets; 163 reports; 3100 randomised participants, 2996 analysed particpants) were included in the update of this review (Figure 1).
1.
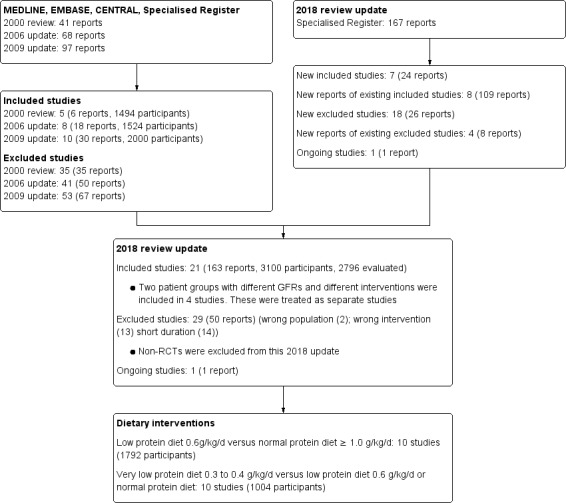
Study flow diagram
One ongoing study was identified and will be assessed in a future update (NCT01418508).
Included studies
Two previously included studies (MDRD Study 1 1989; MDRD Study 2 1989; Rosman Study 1 1984; Rosman Study 2 1984) and two newly identified studies (Anonymous Study 1 1990; Anonymous Study 2 1990; MDRD Feasibility Study A 1989; MDRD Feasibility Study B 1989) were divided into two studies each because they included two groups of participants with different mean GFRs, who received different protein intakes (low protein intake versus normal protein intake or very low protein intake versus low protein intake). Teplan 1998 (published only as an abstract) was a three‐arm study comparing very low (0.4 g/kd/d), low (0.6 g/kg/d) and a restricted diet (0.8 to 1.0 g/kg/d); data could not be extracted and was not included in any of the meta‐analyses. Therefore in this systematic review we considered there to be 21 separate studies.
Nine studies (Anonymous Study 1 1990; Bergstrom 1986; Locatelli 1989; MDRD Feasibility Study A 1989; MDRD Study 1 1989; Meloni 2004; Milovanov 2009; Rosman Study 1 1984; Williams 1991), in which most participants had CKD category 3a or 3b (KDIGO 2012), compared a prescribed low protein diet (0.5 to 0.6 g/kg/d) with a normal protein diet (≥ 1 g/kg/d). A tenth study (Cianciaruso 2008a), which included participants with CKD category 4 and randomised participants to a low protein diet (0.55 g/kg/d) or to the WHO recommended normal protein intake (0.8 g/kg/d), was included in the meta‐analyses with studies comparing low with normal protein intake. Protein intake was estimated from urinary urea nitrogen measurements (Maroni 1985). The mean calculated protein intake was 0.68 g/kg/d (range 0.49 to 0.85 g/kg/d) for the low protein intervention and 1.0 g/kg/d (range 0.61 to 1.54 g/kg/d) for the normal or free protein diet group. No data on calculated protein intake were available for Rosman Study 1 1984 since data on urea excretion were only provided graphically.
Eight studies (Anonymous Study 2 1990; Di Iorio 2003; Garneata 2013; Chauveau 1986; Malvy 1999; MDRD Feasibility Study B 1989; MDRD Study 2 1989; Mircescu 2007), in which participants had CKD stage 4/5 compared prescribed very low protein diets (0.3 to 0.4 g/kg/d with keto‐analogues) with low protein diets (0.58 to 0.65 g/kg/d). The mean calculated protein intake for the participants who received a very low protein diet was 0.4 g/kg/d (range 0.29 to 0.5 g/kg/d) and it was 0.64 g/kg/d (range 0.56 to 0.79 g/kg/d) for participants receiving the low protein diet. No data on calculated protein intake were available for Chauveau 1986 since data on urea excretion were not provided. Ihle 1989 and Rosman Study 2 1984, in which participants had CKD category 4 and which compared very low protein diets (0.4 g/kg/d) with normal protein diets, were included with the eight studies comparing very low with low protein intakes. Actual protein intake could not be calculated for these two studies.
Seven studies were multicentre studies (Anonymous Study 1 1990; Anonymous Study 2 1990; Locatelli 1989; MDRD Feasibility Study A 1989; MDRD Feasibility Study B 1989; MDRD Study 1 1989; MDRD Study 2 1989), two studies involved two sites (Malvy 1999; Williams 1991) and the remainder were single centre studies. Participant numbers ranged from 19 to 840 with an age range of 15 to 75 years. Glomerulopathies accounted for CKD in between 23% (Williams 1991) and 60% (Meloni 2004) of participants; the types of kidney disease included were not reported in Bergstrom 1986, while Milovanov 2009 included only participants with lupus nephritis or other vasculitides. Six diabetic nephropathy participants (three in each group) were included in Di Iorio 2003. Anonymous Study 1 1990 and Anonymous Study 2 1990 reported that fewer than 10% of diabetic participants were included among the 554 participants assessed for inclusion in the study though it was unclear whether any diabetic participants were included in the 336 randomised participants. No diabetic participants were included in the remaining studies.
We chose to include Anonymous Study 1 1990 and Anonymous Study 2 1990 although there were known to be some participants from one centre, who were included in these studies and in Locatelli 1989. We were unable to obtain an exact number of participants included in both studies. However it appeared that only about 30 participants were included in both studies, which would be only 8.9% of 336 randomised participants in Anonymous Study 1 1990 and Anonymous Study 2 1990 and 6.6% of 456 randomised participants in Locatelli 1989 (personal communication from Professor Norbert Gretz).
Mean duration of follow up ranged from 12 to 50 months.
Excluded studies
In the 2009 update, there were 53 excluded studies (67 reports). In the 2016 update we identified a total of 71 excluded studies (101 reports). Based on the Cochrane recommendations for dealing with excluded studies, we limited the excluded studies to randomised controlled trials (RCT) and removed all non‐randomised studies from excluded studies. Therefore in this update we have excluded 29 studies. Of these 13 studies investigated ineligible interventions for this review, two studies included an ineligible population such as dialysis participants or participants with diabetes mellitus and in 14 studies the duration of follow‐up was less than one year.
Risk of bias in included studies
2.
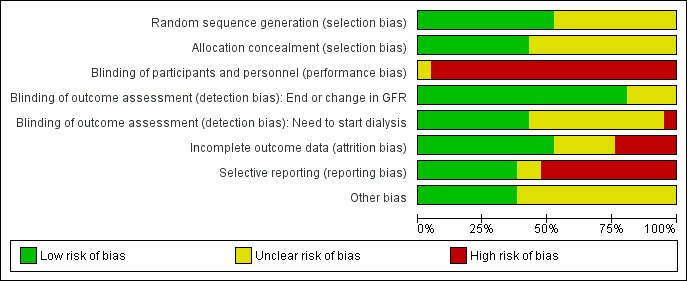
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
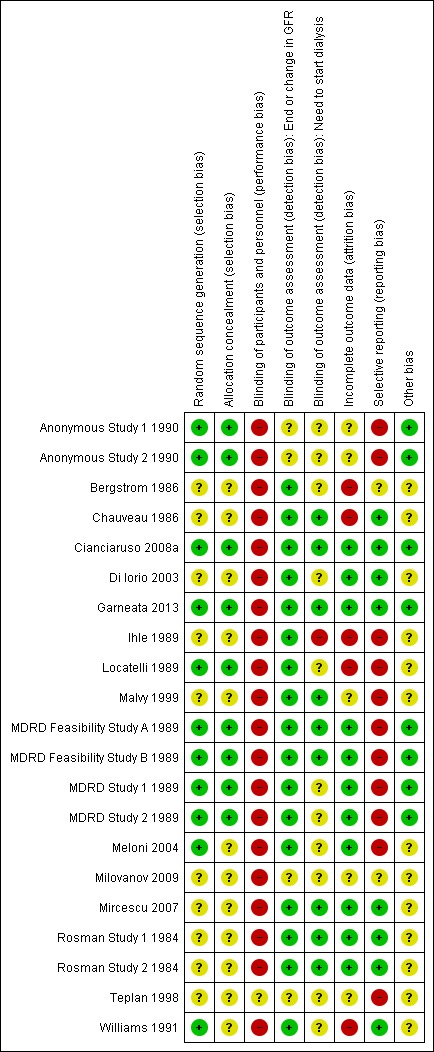
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was considered at low risk of bias in 11 studies (Anonymous Study 1 1990; Anonymous Study 2 1990; Cianciaruso 2008a; Garneata 2013; Locatelli 1989; MDRD Feasibility Study A 1989; MDRD Feasibility Study B 1989; MDRD Study 1 1989; MDRD Study 2 1989; Meloni 2004; Williams 1991) and unclear in the remaining studies.
Allocation concealment was considered to be at low risk of bias in nine studies (Anonymous Study 1 1990; Anonymous Study 2 1990; Cianciaruso 2008a; Garneata 2013; Locatelli 1989; MDRD Feasibility Study A 1989; MDRD Feasibility Study B 1989; MDRD Study 1 1989; MDRD Study 2 1989) and assessed as unclear in the remaining studies with insufficient information available to permit judgement.
Blinding
All 20 studies were open‐label studies and so were considered at high risk for performance bias.
Detection bias (outcome assessment) was recorded separately for GFR and for the need to commence dialysis. All studies which reported this outcome were assessed to be at low risk for detection bias for GFR measurement as the outcomes were laboratory‐based and unlikely to be influenced by lack of blinding. Nine studies were assessed as at low risk of bias for need to commence dialysis as they provided information to indicate that the onset of ESKD with the start of dialysis treatment was assessed independently of the study investigators (Chauveau 1986; Cianciaruso 2008a; Garneata 2013; Malvy 1999; MDRD Feasibility Study A 1989; MDRD Feasibility Study B 1989; Mircescu 2007; Rosman Study 1 1984; Rosman Study 2 1984). One study was at high risk (Ihle 1989) and the remaining studies did not provide any information on how the onset of ESKD was assessed.
Incomplete outcome data
Five studies were considered at high risk of attrition bias as more than 10% of participants were lost to follow up or excluded from the analysis (Bergstrom 1986; Chauveau 1986; Ihle 1989; Locatelli 1989; Williams 1991). Eleven studies (Cianciaruso 2008a; Di Iorio 2003; Garneata 2013; MDRD Feasibility Study A 1989; MDRD Feasibility Study B 1989; MDRD Study 1 1989; MDRD Study 2 1989; Meloni 2004; Mircescu 2007; Rosman Study 1 1984; Rosman Study 2 1984) were assessed as at low risk of detection bias and in the remaining four studies, it was unclear how many participants were lost to follow‐up.
Selective reporting
Studies were considered to be at high risk of bias if data were provided in a format, which could not be entered into the meta‐analyses or if the study did not provide data on death, requirement for dialysis or the nutritional status of the participants. We assessed 11 studies at high risk of selective reporting (Anonymous Study 1 1990; Anonymous Study 2 1990; Ihle 1989; Locatelli 1989; Malvy 1999; MDRD Feasibility Study A 1989; MDRD Feasibility Study B 1989; MDRD Study 1 1989; MDRD Study 2 1989; Meloni 2004; Teplan 1998). Eight studies were assessed as low risk of selective reporting (Cianciaruso 2008a; Chauveau 1986; Di Iorio 2003; Garneata 2013; Mircescu 2007; Rosman Study 1 1984; Rosman Study 2 1984; Williams 1991) and two studies were judged as unclear.
Other potential sources of bias
We assessed eight studies (Anonymous Study 1 1990; Anonymous Study 2 1990; Cianciaruso 2008a; Garneata 2013; MDRD Feasibility Study A 1989; MDRD Feasibility Study B 1989; MDRD Study 1 1989; MDRD Study 2 1989) to be at low risk of potential bias as they were funded by educational or philanthropic organisations and the remaining fourteen studies were considered as unclear as there was insufficient information to permit judgement regarding funding sources.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Low protein diet versus normal protein diet for non‐diabetic adults with chronic kidney disease (CKD).
| Low protein diet versus normal protein diet for non‐diabetic adults with CKD | |||||
| Patient or population: non‐diabetic adults with CKD Setting: all settings Intervention: low protein diet Comparison: normal protein diet | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with normal protein diet | Risk with low protein diet | ||||
| Death (all causes) | 55 per 1,000 | 42 per 1,000 (28 to 65) | RR 0.77 (0.51 to 1.18) | 1680 (5) | ⊕⊕⊕⊝ MODERATE 1 |
| ESKD | 144 per 1,000 | 151 per 1,000 (105 to 220) | RR 1.05 (0.73 to 1.53) | 1814 (6) | ⊕⊕⊝⊝ LOW 1 2 |
| End or change in GFR | The SMD for end or change in GFR was 0.18 lower (0.75 lower to 0.38 higher) with low protein diet compared to normal protein diet | ‐ | 1680 (8) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; ESKD: end‐stage kidney disease; GFR: glomerular filtration rate; SMD ‐ standardised mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 The confidence intervals include potential for important benefits and harms
2 Important and unexplained heterogeneity present
3 The outcome reported is a surrogate outcome
Summary of findings 2. Very low protein diet versus low or normal protein diet for non‐diabetic adults with chronic kidney disease (CKD).
| Very low protein diet versus low or normal protein diet for non‐diabetic adults with CKD | |||||
| Patient or population: non‐diabetic adults with CKD Setting: all settings Intervention: Very low protein diet Comparison: low or normal protein diet | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with low or normal protein diet | Risk with very low protein diet | ||||
| Death (all causes) | 39 per 1,000 | 49 per 1,000 (24 to 99) | RR 1.26 (0.62 to 2.54) | 681 (6) | ⊕⊕⊕⊝ MODERATE 1 |
| ESKD | 458 per 1,000 | 293 per 1,000 (225 to 389) | RR 0.64 (0.49 to 0.85) | 1010 (10) | ⊕⊕⊕⊝ MODERATE 2 |
| End or change in GFR | The SMD for end or change in GFR was 0.12 (0.27 lower to 0.52 higher) with very low protein diet compared to low or normal protein diet | ‐ | 456 (6) | ⊕⊕⊝⊝ LOW 1 2 3 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; ESKD: end‐stage kidney disease; GFR: glomerular filtration rate; SMD ‐ standardised mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 The confidence intervals are wide and include potential for important benefits and harms
2 Serious unexplained heterogeneity
3 Outcome is a surrogate outcome
4 Unclear allocation concealment in 4 studies contributing information to analysis
Summary of findings 3. Nutritional measures for non‐diabetic adults with chronic kidney disease (CKD).
| Nutritional measures for non‐diabetic adults with CKD | |||||
| Patient or population: non‐diabetic adults with CKD Setting: all settings Intervention: very low of low protein diet Comparison: normal or low protein diet | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with normal or low protein diet | Risk with very low or low protein diet | ||||
| Final body weight: low protein versus normal protein diet | The mean final body weight 3.09 kg lower (5.02 to 1.16 lower) with low protein diet compared to normal protein diet | ‐ | 223 (2) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| Final body weight: very low protein diet versus low protein diet | The mean final body weight 1.4 kg higher (3.40 lower to 6.21 higher) with very low protein diet compared to low protein diet | ‐ | 291 (4) | ⊕⊝⊝⊝ VERY LOW 3 4 | |
| Protein energy wasting (malnutrition) | 4 per 1,000 | 6 per 1,000 (2 to 17) | RR 1.31 (0.42 to 4.13) | 2373 (15) | ⊕⊕⊝⊝ LOW 2 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Increased risk of bias related to incomplete outcome data and selective reporting
2 Small studies and wide confidence intervals and include potential for important benefits and harms
3 3/4 studies are unclear for allocation concealment and random sequence generation
4 Serious unexplained heterogeneity
Low protein diets versus normal or free protein diet
Eleven studies compared low protein diets (0.55 to 0.6 g/kg/d) with a normal or free protein diet (0.8 to 1 g/kg/d). Nine studies enrolled participants with category 3a and 3b CKD while one study (Cianciaruso 2008a) enrolled participants with CKD category 4 and 5.
Death (all causes)
Five of 10 studies reported data on death (all causes), which could be included in a meta‐analysis. The certainty of the evidence was considered as moderate (Table 1) because of imprecision. Thus a low protein intake probably leads to little or no difference in death between participants, who received a lower protein diet, and those receiving a normal or free protein diet (Analysis 1.1 (5 studies, 1680 participants): RR 0.77, 95% CI 0.51 to 1.18; I2 = 0%). There were 13 fewer deaths per 1000 in the low protein group (27 fewer to 10 more deaths). Five studies did not report this outcome.
1.1. Analysis.

Comparison 1 Low protein diet versus normal protein diet, Outcome 1 Death (all causes).
End‐stage kidney disease
Six of 10 studies reported data on this outcome, which could be included in a meta‐analysis. The certainty of the evidence was considered as low (Table 1) because of imprecision and moderate heterogeneity. A low protein diet may make little or no difference in the number of participants who reached ESKD compared with a normal protein diet (Analysis 1.2 (6 studies, 1814 participants): RR 1.05, 95% CI 0.73 to 1.53; I2 = 62%). There were 7 participants more per 1000 reaching ESKD in the low protein group (39 fewer to 76 more) compared with the normal protein group. Removal of Cianciaruso 2008a, which included participants with CKD category 4, did not influence the heterogeneity. Exclusion of Anonymous Study 1 1990 reduced this heterogeneity (I2 = 26%). Anonymous Study 1 1990 did not report information which allowed us to determine whether there was detection bias or selective reporting in this study so it is possible that increased risk of bias in these domains in this study contributed to the heterogeneity. Four studies (Bergstrom 1986, MDRD Feasibility Study A 1989; Meloni 2004; Milovanov 2009) did not report on this outcome.
1.2. Analysis.
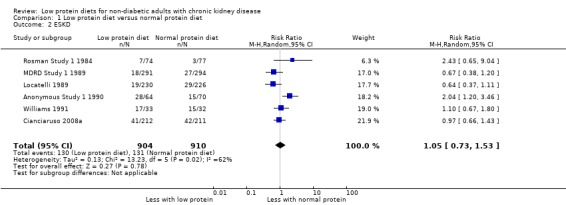
Comparison 1 Low protein diet versus normal protein diet, Outcome 2 ESKD.
End or change in GFR
Eight of 10 studies reported data on this outcome, which could be included in a meta‐analysis. Studies used different methods to express the final GFR or the change in GFR. Because of imprecision, use of a surrogate outcome and substantial heterogeneity (Summary of findings table 1), the certainty of the evidence was considered to be very low. It therefore remains uncertain whether a low protein diet influences the final or change in GFR compared with a normal protein diet (Analysis 1.3; 8 studies, 1680 participants): SMD ‐0.18, 95% CI ‐0.75 to 0.38; I2 = 96%). Heterogeneity was reduced by exclusion of the studies by Locatelli 1989 and Meloni 2004 but it was not clear why these studies should provide data that differed from other studies. Anonymous Study 1 1990 did not report this outcome while Rosman Study 1 1984 only reported the data as medians, which could not be included in the meta‐analysis.
1.3. Analysis.
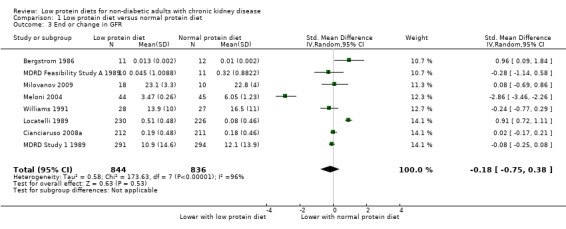
Comparison 1 Low protein diet versus normal protein diet, Outcome 3 End or change in GFR.
Very low protein diet versus low protein diet
Eight studies compared a very low protein diet (0.3 to 0.4 g/kg/d) with a low protein diet (0.58 to 0.65 g/kg/d) while two studies compared a very low protein diet with a normal protein diet. All studies enrolled participants with CKD category 4.
Death (all causes)
Six of 10 studies reported data for this outcome, which could be included in a meta‐analysis. Because of imprecision in the results, the certainty of the evidence was considered to be moderate (Table 2). Thus a very low protein intake probably leads to little or no difference in death between participants, who received a lower protein diet, and those receiving a normal or free protein diet (Analysis 2.1 (6 studies, 681 participants): RR 1.26, 95% CI 0.62 to 2.54; I2 = 0%). There were 10 more deaths per 1000 in the very low protein group (15 fewer to 60 more) compared with the normal or low protein group. Anonymous Study 2 1990; Di Iorio 2003; Ihle 1989; MDRD Feasibility Study B 1989 did not report data for this outcome.
2.1. Analysis.
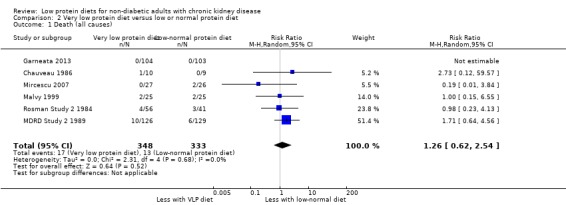
Comparison 2 Very low protein diet versus low or normal protein diet, Outcome 1 Death (all causes).
End‐stage kidney disease
All ten studies reported data on this outcome, which could be included in a meta‐analysis. Because of moderate heterogeneity, the certainty of the evidence was considered to be moderate (Table 2). Thus a very low protein diet probably reduces the number of participants, who reach ESKD, compared with a low or normal protein intake (Analysis 2.2 (10 studies, 1010 participants): RR 0.64, 95% CI 0.49 to 0.85; I2 = 56%). There were 165 fewer participants per 1000, who reached ESKD with a very low protein diet compared with a low or normal protein diet (69 to 233 fewer). Exclusion of MDRD Feasibility Study B 1989 reduced heterogeneity slightly though it remains unclear why this study's results differed from those of the other studies. Exclusion of Ihle 1989 and Rosman Study 2 1984, which compared a very low protein intake with a normal protein intake, did not influence the degree of heterogeneity.
2.2. Analysis.
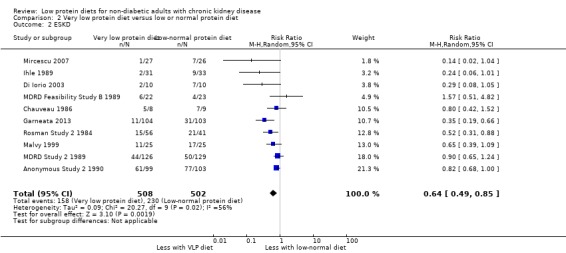
Comparison 2 Very low protein diet versus low or normal protein diet, Outcome 2 ESKD.
End or change in GFR
Six of 10 studies reported data on this outcome, which could be included in a meta‐analysis. Because of substantial heterogeneity, use of a surrogate outcome and a high risk of bias in some included studies, the certainty of the evidence was considered to be very low (Table 2). It therefore remains uncertain whether a low protein diet influences the final or change in GFR compared with a normal protein diet (Analysis 2.3 (6 studies, 456 participants): SMD 0.12, 95% CI ‐0.27 to 0.52; I2 = 68%). Heterogeneity was reduced with the exclusion of MDRD Study 2 1989 and MDRD Feasibility Study B 1989. Both of these studies were at low risk of bias for selection bias unlike the other studies included in the analysis but the reason for the heterogeneity remains unclear. Garneata 2013 reported the data on GFR as medians with 95% confidence intervals and these data could not be included in the meta‐analysis. The study found a significantly higher GFR in the very low protein group compared with the low protein group after 15 months (P < 0.01). Three studies did not report the outcome (Anonymous Study 2 1990; Malvy 1999; Rosman Study 2 1984).
2.3. Analysis.
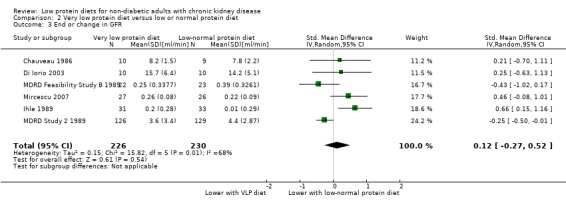
Comparison 2 Very low protein diet versus low or normal protein diet, Outcome 3 End or change in GFR.
Other outcomes
Most studies did not discuss adverse events. However all the studies reported that body weight, BMI and mid arm circumferences were measured though most studies did not provide numerical data that could be included in meta‐analyses.
Most studies reported on dietary adherence and measured this at one to three monthly intervals using urine nitrogen excretion to calculate protein intake and/or by dietary recall or interviews, facilitated by dietitians. The differences between prescribed protein intakes and actual protein intakes are shown in Table 7 and Table 8. While most studies reported that adherence to diet was satisfactory, studies of participants with CKD 3a and 3b tended to have larger differences between actual protein intakes and prescribed protein intakes (Table 7). Anonymous Study 1 1990 and Anonymous Study 2 1990 reported large SD for actual protein intakes because of the wide variation among participants in adherence to diet. Two studies (Chauveau 1986; Locatelli 1989) specifically reported difficulty in maintaining dietary adherence with the low protein diet. Garneata 2013 excluded people who were not able to agree to adhere to protein restriction. No study formally assessed quality of life.
1. Prescribed versus calculated differences in protein intake in studies comparing low with normal or free protein diets.
| Study | Difference in prescribed protein intake | Difference in actual protein intake | Difference between prescribed and actual protein intake |
| Anonymous Study 1 1990 | 0.4 g/kg/d | 0.12 g/kg/d | 0.28 g/kg/d |
| Bergstrom 1986 | 0.45 g/kg/d | 0.21 g/kg/d | 0.24 g/kg/d |
| Cianciaruso 2008a | 0.25 g/kg/d | 0.17 g/kg/d | 0.08 g/kg/d |
| Chauveau 1986 | 0.2 g/kg/d | 0.2 g/kg/d | 0 g/kg/d |
| Locatelli 1989 | 0.4 g/kg/d | 0.18 g/kg/d | 0.22 g/kg/d |
| MDRD Feasibility Study A 1989 | 0.625 g/kg/d | 0.19 g/kg/d | 0.435 g/kg/d |
| MDRD Study 1 1989 | 0.72 g/kg/d | 0.4 g/kg/d | 0.32 g/kg/d |
| Meloni 2004 | 0.4 g/kg/d | 0.87 g/kg/d | 0.47 g/kg/d |
| Williams 1991 | 0.4 g/kg/d | 0.45 g/kg/d | 0.05 g/kg/d |
2. Prescribed versus calculated differences in protein intake in studies comparing very low with low protein diets.
| Study | Difference in prescribed protein intake | Difference in actual protein intake |
| Anonymous Study 2 1990 | 0.3 g/kg/d | 0.21 g/kg/d |
| Di Iorio 2003 | 0.3 g/kg/d | 0.29 g/kg/d |
| Garneata 2013 | 0.3 g/kg/d | 0.29 g/kg/d |
| Malvy 1999 | 0.3 g/kg/d | 0.21 g/kg/d |
| MDRD Feasibility Study B 1989 | 0.295 g/kg/d | 0.22 g/kg/d |
| MDRD Study 2 1989 | 0.3 g/kg/d | 0.3 g/kg/d |
| Mircescu 2007 | 0.3 g/kg/d | 0.27 g/kg/d |
Body weight
Seven studies in total reported data on end of study body weight. The data were subgrouped according to protein intakes. Because of small numbers, imprecision and a high risk of bias, the certainty of the evidence was considered to be very low (Table 3).
In two studies which compared low protein diets with normal protein diets, the certainty of the evidence was considered very low because of increased risk of bias and imprecision, and therefore it is uncertain whether a low protein diet reduces the final body weight (Analysis 3.1.1 (2 studies, 223 participants): MD ‐3.09 kg, 95% CI ‐5.02 to ‐1.16; I2 = 0%). Cianciaruso 2008a reported final body weights as a percentage of the baseline; actual weights could not be calculated as baseline weights were provided separately for men and women. At 12 months, the final body weights were 99.8% or more of baseline weights.
3.1. Analysis.
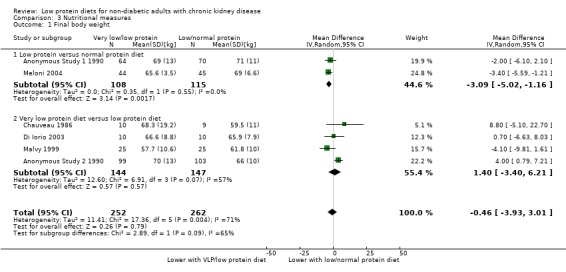
Comparison 3 Nutritional measures, Outcome 1 Final body weight.
In four studies which compared very low protein diets with low protein diets, the certainty of the evidence was considered very low because of increased risk of bias and imprecision, and therefore it is uncertain whether a low protein diet reduces the final body weight (Analysis 3.1.2 (4 studies, 291 participants): MD 1.4 kg, 95% CI 3.40 to 6.21; I2 = 56%).
Thus it is uncertain whether the intervention alters final body weight. The data for MDRD Study 1 1989; MDRD Study 2 1989 are shown in Table 9 as data were reported separately for men and women so could not be added to the meta‐analysis. Three studies reported that body weight dropped during the first few months of commencing a low protein diet but that subsequently weight stabilised (Ihle 1989; Malvy 1999; Meloni 2004).
3. Final body weight in participants in MDRD studies 1 and 2.
| MDRD Study 1 | MDRD Study 2 | |||||||
| Usual protein diet | Low protein diet | Low protein diet | Very low protein diet | |||||
| Final body weight (kg) | N | Final body weight (kg) | N | Final body weight (kg) | N | Final body weight (kg) | N | |
| Men | 88.5 ± 14.6 | 179‐183 | 83.2 ± 12.8 | 165‐170 | 79.6 ± 11.5 | 74‐77 | 79.3 ± 10.9 | 69‐71 |
| Women | 72.2 ± 14.9 | 98‐105 | 69.3 ± 13.7 | 107‐115 | 65.9 ± 11.9 | 49‐51 | 65.0 ± 14.3 | 49‐52 |
Protein energy wasting (malnutrition)
Fifteen studies made reference to protein energy wasting. Of these 12 studies reported no evidence of malnutrition while three studies reported small numbers of participants with protein energy wasting in both groups (Analysis 3.2 (15 studies, 2373 participants): RR 1.31, 95% CI 0.42 to 4.13; I2 = 0%; low certainty evidence).
3.2. Analysis.

Comparison 3 Nutritional measures, Outcome 2 Protein energy wasting.
Body mass index
Four studies reported on this outcome. Two studies (Anonymous Study 1 1990; Meloni 2004) comparing a low protein with a normal protein diet, found no difference in BMI between groups. Two studies (Anonymous Study 2 1990; Garneata 2013) comparing very low protein with a low protein diet, also demonstrated no difference between diet groups. Studies were not combined in a meta‐analysis as three studies (Anonymous Study 1 1990; Anonymous Study 2 1990; Garneata 2013) provided the data as medians and ranges.
Discussion
Summary of main results
For this update we identified six additional studies to provide a total of 17 studies (21 data sets) with 3100 participants (2996 analysed participants) so we were able to report separately on the outcomes of death (all causes), numbers with ESKD, and final or change in GFR. We could also report data separately for studies which compared low with normal protein intakes and those which compared very low with low protein intakes. We sub‐divided four studies which compared different protein intakes in participants with different stages of CKD so that 20 studies were included in this review.
Ten studies, mainly evaluating participants with CKD categories 3a and 3b, compared a low prescribed protein diet (0.55 to 0.6 g/kg/d) with a normal protein diet (0.8 to ≥ 1.0 g/kg/d). The difference in calculated protein intake was 0.32 g/kg/d between the intervention groups. A low protein diet compared with a normal protein diet probably makes little or no difference in the numbers of participants who died (moderate certainty evidence) and may make little or no difference in the number of participants who progressed to ESKD (low certainty evidence). It remains uncertain whether a low protein diet compared with a normal protein intake impacts on the final or change in GFR (very low certainty evidence) (Table 1).
Ten studies, which evaluated participants with CKD 4 or 5, compared a prescribed very low protein diet (0.3 to 0.4 g/kg/d) with a low protein diet (0.58 to 0.65 g/kg/d) (eight studies) or with a normal protein diet (two studies). The difference in calculated protein intake was 0.25 g/kg/d between the intervention groups. A very low protein intake compared with a low protein intake probably makes little or no difference to death but it probably reduces the number of participants, who reach ESKD (moderate certainty evidence). It remains uncertain whether a very low protein diet compared with a low or normal protein intake influences the final or change in GFR (very low certainty evidence) (Table 2).
Fifteen studies reported on the numbers of participants with protein energy wasting (malnutrition); 12 studies had no participants with protein energy wasting while three studies reported small numbers in both treatment groups. Only eight studies provided numerical data for body weight although most studies reported that weight was measured. Only three of the 15 studies reported any evidence of protein energy wasting. No study formally assessed quality of life in the participants. Most studies reported that adherence to diet was satisfactory though studies of participants with CKD 3a and 3b tended to have smaller differences between actual protein intakes, as measured by urinary nitrogen excretion, and prescribed protein intakes.
Overall completeness and applicability of evidence
For this review we identified 16 studies (reported as 20 studies) which evaluated the efficacy and safety of low protein diets in non‐diabetic CKD. Several studies, particularly the older and smaller studies, were of low methodological quality. The primary outcomes of this review (death and ESKD) were not reported in 10 and 4 studies respectively. Numerical data on weight difference and protein energy wasting were provided in few studies though all the studies reported that participants' body weight, BMI and mid arm circumference were measured. Adherence was reported in all the studies and was measured at one to three monthly intervals utilising urine nitrogen excretion for calculated protein intake or dietary recall. While most studies reported satisfactory adherence, difficulty in maintaining adherence was reported in two studies. No studies reported any assessment of quality of life although one study commented that quality of life would be improved if participants were not restricted in their dietary protein intake. Quality of life is significantly reduced in patients who require dialysis. Quality of life should be assessed with dietary interventions, aimed at delaying the onset of ESKD, to confirm that any impact of diet on quality of life is minimal compared with the impact of dialysis treatment.
Although Anonymous Study 1 1990, Anonymous Study 2 1990 and Bergstrom 1986 were reported as full text journal articles, these articles only provided preliminary results and our updated search and contact with authors did not identify a publication of the full results for the studies. Most studies were small with only five studies enrolling more than 100 participants in each treatment group. Although we identified six studies not previously included in this review, only Garneata 2013 was a large, well reported and high quality study. Only two new studies (Garneata 2013; Milovanov 2009) and the full text publication of Cianciaruso 2008a were published since the 2009 update. The other four new studies identified for this update had been published before 2009.
Quality of the evidence
For all the studies included in the review, full length journal articles were available. However included studies were commonly reported incompletely and were of poor methodological quality and this may reflect pre‐2001 CONSORT (Consolidated Standards of Reporting Trials) practices in the older studies (www.consort‐statement.org). Random sequence generation and allocation concealment were considered at low risk of bias in 11 and nine studies respectively. All studies were considered at high risk for performance bias as they were open‐label studies. We assessed bias for outcome assessment (detection bias) for GFR and ESKD separately. As the outcome measurement for GFR measurement was a laboratory outcome all studies were assessed at low risk of detection bias. We felt it important to include a separate assessment of bias for outcome assessment of ESKD as this outcome is more likely to be at risk of detection bias. Eight of 16 studies, reporting data on this outcome, were at unclear or high risk for detection bias; the other eight studies were at low risk of bias since the need to commence dialysis was determined by personnel independent of the trial investigators. Where outcome assessment for the need to commence dialysis is not blinded, the time of dialysis commencement may be influenced by the physicians' knowledge of the treatment groups (Kasiske 1998). Five studies were assessed at high risk of attrition bias with eleven studies at low risk. Eleven studies were at high risk for reporting bias as they did not include data which could be included in a meta‐analysis.
The overall certainty of the evidence using GRADE (GRADE 2011a; GRADE 2011b) was assessed as moderate, low or very low for different outcomes (Table 1; Table 2). The certainty of the evidence for death was assessed as moderate for studies comparing low with normal protein diets and for studies comparing very low with low or normal protein diets. The certainty of the evidence for ESKD was low for studies comparing low with normal protein diets and moderate for studies comparing very low with low or normal protein intake. The certainty of the evidence for end or change in GFR and body weight was assessed as very low.
Potential biases in the review process
A comprehensive search of the Cochrane Kidney and Transplant’s Specialised Register was performed for this review thus reducing the possibility that potential eligible studies were omitted from the review. Eligible studies published after the last search date or published in conference proceedings not routinely searched could have been missed. The review was completed independently by at least two authors who participated in all steps of the review, which limited the risk of errors in determining study eligibility, in data extraction, in risk of bias assessment and in data synthesis.
Agreements and disagreements with other studies or reviews
The 2009 update of this review found that reduced protein intake in CKD participants reduced the number of participants, who died or reached ESKD (Fouque 2009). The benefit was primarily seen in the subgroup of studies comparing very low protein diets with low or normal protein diets. In this update with additional studies, we were able to report separately on death and ESKD. We confirmed that the reduction in the number of participants reaching ESKD was limited to studies comparing very low protein diet with low or normal protein diets (moderate certainty evidence).
Two other systematic reviews have evaluated the efficacy of low protein diets in participants with CKD. Five RCTs, including 1413 participants with non‐diabetic CKD, were reviewed by Pedrini 1996. Dietary protein restriction compared with a normal protein intake reduced the risk of the combined outcome of death and ESKD (RR 0.67, 95% CI 0.50 to 0.89). All five RCTs are included in our updated review and include two large studies (Locatelli 1989; MDRD Study 1 1989; MDRD Study 2 1989). This study evaluated the number of participants reaching ESKD but did not evaluate change in GFR.
Kasiske 1998 evaluated 13 RCTs (1919 participants) including four studies of patients with diabetic CKD. As in this systematic review, the Modification of Diet in Renal Disease study (MDRD Study 1 1989; MDRD Study 2 1989) was treated as two studies. The difference in dietary protein intake between the intervention and control groups was 0.33 ± 0.26 g/kg/d. In the pooled results, the authors found that dietary protein restriction reduced the rate of decline in estimated GFR by only 0.53 mL/min/y (95% CI 0.08 to 0.98). They concluded that though there was a decline in GFR with protein restriction, the magnitude of this effect was relatively weak. This review evaluated changes in GFR but not the number of participants, who died or reached ESKD. The authors pointed out that their results were in keeping with the major findings of the MDRD study, which showed little benefit of protein restriction on the number of participants reaching ESKD or on GFR in participants with GFR below 30 mL/min/1.73 m2 (MDRD Study 2 1989).
The use of low protein diets in participants with CKD varies between countries and within countries. Few nephrologists in the USA or Canada prescribe dietary therapy for participants with CKD (Kalantar‐ Zadeh 2016) following the negative results of the MDRD study (MDRD Study 1 1989; MDRD Study 2 1989) while low protein diets are more commonly prescribed in Europe (Bellizzi 2016). International (KDIGO 2012) and national guidelines (Wright 2011) now recommend protein intakes of 0.75 to 0.8 g/kg/d for adults with GFR ≤ 30 mL/min/ 1.73 m2. These recommendations are in line with the recommended daily intake (RDI) for the general population. The average protein intake in adults in developed countries is approximately twice the RDI so guidelines suggest that participants with excess protein intakes reduce their intake to RDI levels since a high protein intake may accelerate the decline of kidney function in CKD (KDIGO 2012; Johnson 2013).
Authors' conclusions
Implications for practice.
Available data from RCTs outlined in this systematic review found that very low protein diets (0.3 to 0.4 g/kg/d with supplements of essential amino acids and keto‐analogues) compared with low or normal protein intakes probably reduce the number of participants with CKD 4 or 5, who progress to ESKD (moderate certainty evidence). Compared with normal protein diets (0.8 to ≥ 1.0 g/kg/d), low protein diets (0.5 to 0.6 g/kg/d) in participants with CKD 3 may make little or no difference to the number progressing to ESKD (low certainty evidence). However there were very limited data available on adverse effects ‐ in particular weight differences and protein energy malnutrition ‐ and on participants' quality of life, which could be affected by difficulties in maintaining dietary adherence. In this systematic review, we found a very small difference in GFR between very low and low protein intakes although the ESKD was lower in the participants receiving a very low protein intake compared with a low protein intake. This suggests that the benefit of protein restriction in participants with advanced CKD is not due to a direct effect on kidney function but via its role in maintaining nutrition and health in participants with CKD, particularly in correcting metabolic acidosis and reducing adverse effects associated with phosphate and sodium retention (Mitch 2016;Kalantar‐Zadeh 2017).
Implications for research.
Additional studies in CKD participants are required to evaluate the effects of nutritional interventions, including reduced protein intake, on slowing the progression to ESKD. These include strategies to control metabolic acidosis, to reduce phosphate retention leading to lower parathyroid hormone levels and less metabolic bone disease and to reduce sodium intake, which would enhance the efficacy of angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists or other antiproteinuric medications in slowing the progression to ESKD (Mitch 2016; Bellizzi 2016). Further information on the role of the different rates of CKD progression prior to the intervention and of adherence to reduced protein diets on CKD outcome are required to understand the contribution of dietary restrictions to slowing the progression to ESKD. Whether a reduced protein intake, achieved with satisfactory adherence to the diet and without interfering with quality of life, could achieve these outcomes needs to be further evaluated (Piccoli 2016).
What's new
| Date | Event | Description |
|---|---|---|
| 7 September 2018 | New citation required and conclusions have changed | New studies added; SOF tables included |
| 7 September 2018 | New search has been performed | Six new studies added. Conclusions changed. |
| 7 February 2012 | Amended | Search strategies & search methods updated |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 12 May 2009 | New citation required but conclusions have not changed | Author list updated |
| 31 March 2009 | Amended | Two new studies added, no change to conclusions |
| 13 October 2008 | Amended | Converted to new review format. |
| 30 November 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful to Prof Jean‐Pierre Boissel and Professor Maurice Laville, who contributed to the earlier versions of this review (Fouque 2000b; Fouque 2006), contributing to the design, quality assessment, data collection, entry, analysis and interpretation, and writing.
We thank Dr Garneata and Professor Norbert Gretz for providing additional information on new studies included in this review. We thank the staff of Cochrane Kidney and Transplant (Fiona Russell, Gail Higgins and Narelle Willis) for their assistance with this review and are particularly indebted to Michel Cucherat MD PhD, Margaret Haugh PhD and the Centre Cochrane Français for statistical and methodological assistance.
Prof Bert Kasiske and Prof Teut Risler should be acknowledged for critically reviewing the manuscript.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE (OVID SP) |
|
| EMBASE (OVID SP) |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement. | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Low protein diet versus normal protein diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (all causes) | 5 | 1680 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.18] |
| 2 ESKD | 6 | 1814 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.73, 1.53] |
| 3 End or change in GFR | 8 | 1680 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.75, 0.38] |
Comparison 2. Very low protein diet versus low or normal protein diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (all causes) | 6 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.62, 2.54] |
| 2 ESKD | 10 | 1010 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.49, 0.85] |
| 3 End or change in GFR | 6 | 456 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.27, 0.52] |
Comparison 3. Nutritional measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final body weight | 6 | 514 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐3.93, 3.01] |
| 1.1 Low protein versus normal protein diet | 2 | 223 | Mean Difference (IV, Random, 95% CI) | ‐3.09 [‐5.02, ‐1.16] |
| 1.2 Very low protein diet versus low protein diet | 4 | 291 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐3.40, 6.21] |
| 2 Protein energy wasting | 15 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.42, 4.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anonymous Study 1 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet group
Normal protein diet group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation scheme for each centre |
| Allocation concealment (selection bias) | Low risk | Sealed and numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Unclear risk | No information provided. Outcome not reported in this study |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | Unclear how endpoint of onset of ESKD and the need for dialysis was determined |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear whether data on all participants were reported |
| Selective reporting (reporting bias) | High risk | No report of death or GFR; medians of BMI only available |
| Other bias | Low risk | Grant from Bundesministerium für Forschung und Technologie, FRG (No. 0704743) |
Anonymous Study 2 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet group
Low protein diet group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation scheme for each centre |
| Allocation concealment (selection bias) | Low risk | Sealed and numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Unclear risk | No information provided. Outcome not reported in this study |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | Unclear how end point of the onset of ESKD and the need for dialysis was determined |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear whether data on all participants were reported |
| Selective reporting (reporting bias) | High risk | No report of death or GFR; medians of BMI only available |
| Other bias | Low risk | Grant from Bundesministerium für Forschung und Technologie, FRG (No. 0704743) |
Bergstrom 1986.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet group
Normal protein diet group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Laboratory measurement of GFR and laboratory measure unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | No information provided. Need to start dialysis was not recorded as a study outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30% (7/23) excluded from analysis or lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Only outcome reported was GFR and unclear what other outcomes planned |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Chauveau 1986.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet group
Low protein diet group
Both groups had calorie intake of 35 to 40 Kcal/kg/d Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Primary outcome (1/Cr or CrCl) was laboratory based and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Low risk | Decision to commence dialysis made by dialysis staff independently of study investigators |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26% (5/19) excluded from analysis of 1/Cr but information on dialysis available for all |
| Selective reporting (reporting bias) | Low risk | Reported on dialysis, death, body weight, GFR measure reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Cianciaruso 2008a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet group
Normal protein diet group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomised list, which was concealed from investigators |
| Allocation concealment (selection bias) | Low risk | Numbered opaque sealed envelopes opened in sequence by administration staff personnel not involved in patient care |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Laboratory measure & calculation (eGFR measured by MDRD formula) unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Low risk | Single centre study with same criteria for commencing dialysis (eGFR = 6 mL/min/1.73 m2, hyperkalaemia, fluid overload, malnutrition) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for; 9/423 (2%) lost to follow‐up at mean 13 months; 15/423 (3.5%) lost to follow‐up by 4 years |
| Selective reporting (reporting bias) | Low risk | All expected outcomes (death, commencement of dialysis, GFR, protein‐calorie malnutrition) reported |
| Other bias | Low risk | Investigator driven. Partially funded by grant from Italian Ministry of University & Scientific Research (PRIN‐2001; Grant 061427) |
Di Iorio 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet group
Low protein diet group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Outcome was based on laboratory outcome and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | No information provided on criteria used to commence dialysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included participants accounted for |
| Selective reporting (reporting bias) | Low risk | Reported on expected outcomes (death, dialysis, GFR, body weight) |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Garneata 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet group
Low protein diet group
Both groups received 30 kcal/d Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using computer generated numbers |
| Allocation concealment (selection bias) | Low risk | Allocation utilising opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | End point of eGFR (calculated from serum creatinine by MDRD formula) is a laboratory measure and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Low risk | End point of the onset of ESKD determined by committee without knowledge of treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.3% lost to follow up/discontinued diet but all participants included in analyses |
| Selective reporting (reporting bias) | Low risk | Expected outcomes (ESKD, death (all causes), GFR, BMI) reported |
| Other bias | Low risk | No funding support received |
Ihle 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet group
Normal protein diet group
Both groups received 35 to 40 kcal/kg/d Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Primary outcome was laboratory measurement of GFR by EDTA clearance and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | High risk | Decision to commence dialysis depending on uraemic symptoms and/or SCr > 1300 µmol/L |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/72 (11%) excluded from analyses (3 withdrew; 5 excluded for non‐compliance with diet) |
| Selective reporting (reporting bias) | High risk | Report on number reaching ESKD & GFR. Body weight only reported graphically. Deaths not reported but there appear to be no deaths |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Locatelli 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet group
Normal protein diet group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomisation‐ blocks of 4, 1/1 ratio performed at study headquarters " |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Laboratory measurement and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31.7% lost by final follow up |
| Selective reporting (reporting bias) | High risk | Not all reviews pre‐specified outcomes mentioned. No weights, no adverse events |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Malvy 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet group
Low protein diet group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Primary outcome of CrCl based on laboratory outcome so unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Low risk | GFR < 5 mL/min/1.73 m2 estimated from (CrCl + urea clearance)/2 or uraemic symptoms requiring dialysis as determined by 2 nephrologists |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether all participants included in outcome measurement |
| Selective reporting (reporting bias) | High risk | Numbers reaching GFR endpoint only available graphically |
| Other bias | Unclear risk | Insufficient information to permit judgement |
MDRD Feasibility Study A 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet
Normal protein diet
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random permutated blocks to ensure equal balance of participants assigned to each treatment combination" |
| Allocation concealment (selection bias) | Low risk | "Centrally administered at data co‐ordination centre through telephone contact" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Laboratory measurement (iothalamate clearance) and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Low risk | Onset of ESKD endpoint reviewed by Clinical Committee without knowledge of dietary assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for; 4 patients lost to follow‐up |
| Selective reporting (reporting bias) | High risk | No report of numbers in each group death (combined data only). No information on final weights provided |
| Other bias | Low risk | National Institute of Diabetes, Digestive and Kidney Disease and Health Care Finance Administration, NIH, USA |
MDRD Feasibility Study B 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet
Low protein diet
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random permutated blocks to ensure equal balance of participants assigned to each treatment combination" |
| Allocation concealment (selection bias) | Low risk | "Centrally administered at data co‐ordination centre through telephone contact" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Laboratory measurement (iothalamate clearance) and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Low risk | Onset of ESKD endpoint reviewed by Clinical Committee without knowledge of dietary assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for; 1 patient lost to follow‐up |
| Selective reporting (reporting bias) | High risk | No report of numbers in each group on death (combined data only). No information on final weights provided |
| Other bias | Low risk | National Institute of Diabetes, Digestive and Kidney Disease and Health Care Finance Administration, NIH, USA |
MDRD Study 1 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet
Normal protein diet
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random permutated blocks to ensure equal balance of participants assigned to each treatment combination" Patients stratified before randomisation according to blood pressure & rate of progression of kidney disease during 3 month baseline period |
| Allocation concealment (selection bias) | Low risk | "Centrally administered at data co‐ordination centre through telephone contact" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Laboratory measurement (iothalamate clearance) and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | No information on criteria for starting dialysis provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 lost to follow‐up from low protein diet group and 3 lost to follow‐up from normal protein diet group |
| Selective reporting (reporting bias) | High risk | No report of numbers in each group reaching ESKD or death (combined data only); did report weight & GFR measure |
| Other bias | Low risk | National Institute of Diabetes, Digestive and Kidney Disease and Health Care Finance Administration, NIH, USA |
MDRD Study 2 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet group
Low protein diet group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random permutated blocks to ensure equal balance of participants assigned to each treatment combination" Patients stratified before randomisation according to blood pressure & rate of progression of kidney disease during 3 month baseline period" |
| Allocation concealment (selection bias) | Low risk | "Centrally administered at data co‐ordination centre through telephone contact" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Laboratory measurement (iothalamate clearance) and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | No information on criteria for starting dialysis provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 1.2% (3/255) in study 2 |
| Selective reporting (reporting bias) | High risk | No report of numbers in each group reaching ESKD or death (combined data only). Did report weight & GFR measure |
| Other bias | Low risk | National Institute of Diabetes, Digestive and Kidney Disease and Health Care Finance Administration, NIH, USA |
Meloni 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet group
Normal protein diet group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Simple randomisation using dedicated software generating casual numbers to assign participants to treatment groups and remaining participants were placed in control group" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Primary outcome of GFR was measured by EDTA clearance and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | No information provided as "need to start dialysis" was not a reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appear to have completed follow up |
| Selective reporting (reporting bias) | High risk | Did not report on deaths or ESKD |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Milovanov 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet group
Normal diet group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Unclear risk | Method of GFR measurement unclear |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Mircescu 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet
Low protein diet
Total recommended energy intake: 30 kcal/kg/d Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | eGFR calculated from SCr using MDRD formula. Based on laboratory measure and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Low risk | "RRT initiation was decided by the Ethical Committee of the Hospital considering the clinical and biochemical status of the patient (...). Members of the Committee were unaware of which arm the patient had been assigned to" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All reviews prespecified outcomes mentioned |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Rosman Study 1 1984.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet group (group B)
Normal protein diet groups (Groups A1, A2)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | End point of CrCl is a laboratory outcome and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Low risk | Need to start dialysis determined by CrCl < 4 mL/min/1.73 m2 Also included in analysis of ESKD were participants who received pre‐emptive transplants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% (10/248) excluded from analysis (lost to follow‐up 9, withdrawn 1) |
| Selective reporting (reporting bias) | Low risk | Reported ESKD, GFR, death and weight but weight and GFR data not able to be included in meta‐analyses |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Rosman Study 2 1984.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet group (Group C)
Normal protein diet group (Group A2)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | End point of CrCl is a laboratory outcome and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Low risk | Need to start dialysis determined by CrCl < 4 mL/min/1.73 m2 Also included in analysis of ESKD were participants who received pre‐emptive transplants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% (10/248) excluded from analysis (lost to follow up 9, withdrawn 1) |
| Selective reporting (reporting bias) | Low risk | Reported ESKD, GFR, death and weight but weight and GFR data not able to be included in meta‐analyses |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Teplan 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Very low protein diet
Low protein diet
Restricted protein diet
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | No extractable data; no full‐text publication identified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Williams 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Low protein diet group
Normal protein diet group
Daily energy intake: 30 kcal/kg/d Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pack of numbered cards and random number tables |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence patient management |
| Blinding of outcome assessment (detection bias): End or change in GFR End of change in GFR | Low risk | Primary outcome (change in CrCl) was a laboratory measure and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): Need to start dialysis Need to start dialysis | Unclear risk | No information provided on criteria for starting dialysis |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15% (10/65) excluded from calculation of primary outcome of change in CrCl |
| Selective reporting (reporting bias) | Low risk | Reported on number requiring dialysis, deaths, change in creatinine and weight |
| Other bias | Unclear risk | Insufficient information to permit judgement |
ACEi ‐ angiotensin‐converting enzyme inhibitors; BMI ‐ body mass index; BP ‐ blood pressure; CCB ‐ calcium channel blockers; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CRP ‐ C‐reactive protein; DM ‐ diabetes mellitus; ESA ‐ erythrocyte stimulating agent; ESKD ‐ end‐stage kidney disease; EPO ‐ erythropoietin; GI ‐ gastrointestinal; (e)GFR ‐ (estimated) glomerular filtration rate; Hb ‐ haemoglobin; MAP ‐ mean arterial pressure; M/F ‐ male/female; MDRD ‐ Modification of Diet in Renal Disease; NSAID ‐ nonsteroidal anti‐inflammatory drugs; RPGN ‐ rapidly progressive glomerulonephritis; SCr ‐ serum creatinine; SD ‐ standard deviation; SGA ‐ Subjective Global Assessment; UPE ‐ urinary protein excretion
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bernard 1996 | Wrong intervention: RCT comparing keto acid supplements with no supplements in participants on the same protein intake; patients only treated for three months |
| Bernhard 2001 | Wrong intervention: RCT, randomised to keto acid supplements with participants maintaining the same protein diet |
| Choi 2012a | Wrong intervention: RCT comparing keto acid supplementation with no supplements in participants on the same low protein diets |
| Coresh 1994 | Wrong intervention: RCT, participants randomised to keto acid supplements |
| Di Iorio 2009a | Wrong duration of follow‐up: cross‐over study comparing very low protein diet supplemented with keto acids with low protein diet; follow‐up for only 6 months in each part of study |
| Di Iorio 2012a | Wrong duration of follow‐up: cross‐over study comparing very low protein diet supplemented with keto acids with low protein diet; each intervention for one week only |
| DODE Study 2000 | Wrong intervention: RCT, participants randomised to diet or dialysis |
| El Nahas 1987 | Wrong intervention: RCT comparing different distribution through day of protein intake; protein intake in both groups the same |
| ERIKA Study 2007 | Wrong population. included 35% diabetic patients |
| Garibotti 2018 | Wrong duration: 24 weeks duration only |
| Garini 1992 | Wrong intervention: RCT comparing very low protein intake (0.4 g/kg/d) + keto acids/essential amino acids (0.2 g/kg/d) with low protein intake (0.6 g/kg/d) but nitrogen intake did not differ between groups |
| Hecking 1980 | Wrong duration: six weeks duration only |
| Herselman 1995 | Wrong duration: RCT comparing very low protein diet with low protein diet; follow‐up only for 9 months |
| Ideura 2003 | Wrong duration: RCT comparing different protein intakes but duration of follow up is uncertain |
| IRCCA Study 1990 | Wrong intervention: RCT, randomised to keto acid supplements |
| Kopple 1982 | Wrong duration: RCT comparing low with very low protein diet but follow up averaged only 12 weeks |
| Laville 1994 | Wrong duration: RCT comparing low protein with normal protein diet but follow‐up for only 6 months |
| Lim 2000 | Wrong duration: six months duration only |
| Maksic 2004 | Wrong duration: RCT, six months duration |
| Prakash 2004 | Wrong duration: less than 12 months duration; compared low protein with very low protein diet supplemented with keto‐analogues |
| Ren 2002 | Wrong intervention: RCT comparing same low protein diet (0.5 to 0.6 g/kg/d) in both groups with keto acids added in one group |
| Rosenberg 1987 | Wrong duration: cross‐over study with participants only studied for 11 days in each phase |
| Sanchez 2010 | Wrong intervention and duration: RCT comparing low protein diet with diet in which some foods were replaced by low protein foods; follow‐up only 6 months |
| Teplan 2003 | Wrong intervention: RCT comparing additional keto acids with no addition in participants on a low protein diet and EPO |
| Teplan 2006 | Wrong intervention: RCT comparing low protein diet (0.6 g/kg/d) + keto‐analogues with low protein diet + placebo in obese participants with CKD |
| Ursea 2002 | Wrong duration: RCT comparing 0.3 g/kg of protein (with keto‐analogues) versus 0.6 g/kg but follow up only to 6 months |
| Vujic 1987 | Wrong intervention: RCT but no separation in reported nitrogen intake as low protein diet supplemented by amino acids equivalent to 0.2 g/kg/d of protein |
| Zhang 2015 | Wrong duration: RCT comparing low protein with normal protein diet but outcome data only available to 6 months |
| Zhang 2016b | Wrong population: patients with steroid resistant nephrotic syndrome and minor reduction in GFR |
CKD ‐ chronic kidney disease; EPO ‐ erythropoietin; GFR ‐ glomerular filtration rate; RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01418508.
| Trial name or title | Effects of Low Protein Diet Supplemented Keto‐/Amino Acid in Preventing the Progression of Chronic Kidney Disease (CKD) (ELPD Study) |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | August 2011 |
| Contact information | Xuemei Li, M.D.& Ph.D. 8610‐65295058 0605.mei@gmail.com, Limeng Chen, M.D.& Ph.D. 8610‐65295351 climeng2000@yahoo.com.c |
| Notes | Investigators contacted but no reply received |
CKD ‐ chronic kidney disease; DM ‐ diabetes mellitus; GFR ‐ glomerular filtration rate; NYHA ‐ New York Heart Association; RCT ‐ randomised controlled trial
Contributions of authors
DH and EH selected additional studies for inclusion in the 2018 update, undertook data analysis and risk of bias assessment and wrote the review update.
DF reviewed the data and contributed to writing the review.
Sources of support
Internal sources
Hospices Civils de Lyon, France.
University Claude Bernard Lyon 1, France.
External sources
No sources of support supplied
Declarations of interest
Professor Denis Fouque has received lecture fees for nutrition in renal disease.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Anonymous Study 1 1990 {published data only}
- European Study Group for the Conservative Management of Chronic Renal Failure. Dietary compliance in the trial of the European Study Group. A preliminary analysis. European Study Group for the Conservative Management of Chronic Renal Failure. Contributions to Nephrology 1990;81:61‐70. [MEDLINE: ] [PubMed] [Google Scholar]
- European Study Group for the Conservative Management of Chronic Renal Failure. Dietary compliance in the trial of the European Study Group. An interim analysis. European Study Group for the Conservative Management of Chronic Renal Failure. Contributions to Nephrology 1992;98:133‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Anonymous Study 2 1990 {published data only}
- European Study Group for the Conservative Management of Chronic Renal Failure. Dietary compliance in the trial of the European Study Group. A preliminary analysis. European Study Group for the Conservative Management of Chronic Renal Failure. Contributions to Nephrology 1990;81:61‐70. [MEDLINE: ] [PubMed] [Google Scholar]
- European Study Group for the Conservative Management of Chronic Renal Failure. Dietary compliance in the trial of the European Study Group. An interim analysis. European Study Group for the Conservative Management of Chronic Renal Failure. Contributions to Nephrology 1992;98:133‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Bergstrom 1986 {published data only}
- Alvestrand A, Bucht H, Gutierrez A, Bergström J. Progression of chronic renal failure in man as influenced by frequency and quality of clinical follow‐ups [abstract]. Kidney International 1985;27(1):240. [Google Scholar]
- Bergstrom J, Alvestrand A, Bucht H, Gutierrez A. Progression of chronic renal failure in man is retarded with more frequent clinical follow‐ups and better blood pressure control. Clinical Nephrology 1986;25(1):1‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Bergstrom J, Alvestrand A, Bucht H, Gutierrez A. Stockholm clinical study on progression of chronic renal failure‐‐an interim report. Kidney International ‐ Supplement 1989;36(Suppl 27):S110‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Bergstrom J, Alvestrand A, Bucht H, Gutierrez A. What is the role of controls in an outpatient department on progression of renal disease?. Blood Purification 1988;6(6):336‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chauveau 1986 {published data only}
- Chauveau P, Lebkiri B, Ployard F, Ciancioni C, Man NK, Jungers P. Effect of keto analogs of essential amino acids on the progress of advanced renal insufficiency: controlled prospective study [Effet des cetoanalogues des acides amines essentiels sur la progression de l'insuffisance renale chronique avancee: etude prospective controlee]. Nephrologie 1986;7(5):137‐42. [MEDLINE: ] [PubMed] [Google Scholar]
- Jungers P, Chauveau P, Ployard F, Lebkiri B, Ciancioni C, Man NK. Comparison of ketoacids and low protein diet on advanced chronic renal failure progression. Kidney International ‐ Supplement 1987;22:S67‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Cianciaruso 2008a {published data only}
- Cianciaruso B, Pota A, Bellizzi V, Giuseppe D, Micco L, Minutolo R, et al. Effect of a low‐ versus moderate‐protein diet on progression of CKD: follow‐up of a randomized controlled trial. American Journal of Kidney Diseases 2009;54(6):1052‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cianciaruso B, Pota A, Pisani A, Torraca S, Annecchini R, Lombardi P, et al. Metabolic effects of two low protein diets in chronic kidney disease stage 4‐5‐‐a randomized controlled trial. Nephrology Dialysis Transplantation 2008;23(2):636‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cianciaruso B, Pota A, Torraca S, Somma G, Nazzaro P, Annecchini R, et al. Comparison of two different protein intakes (0.3‐0.6 vs 0.7‐0.9 g/kg/day) on the metabolic control of advanced renal failure [abstract no: MP197]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:295.
- Pota A, Pisani A, Annecchini R, Giuseppe D, Cuccurullo P, Caputo DL, et al. Treatment of chronic renal failure (CRF) with two different low protein diets. Effects on the metabolic control and on the cardiovascular risk factors [abstract no: MP306]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv401. [Google Scholar]
- Pota A, Pisani A, Ravani P, Micco L, Giuseppe D, Sabbatini M, et al. Impact of two different low protein diets on survival of CKD patients (stage4‐5) [abstract no: TH‐PO880]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):308A. [Google Scholar]
Di Iorio 2003 {published data only}
- Iorio BR, Bellizzi V, Minutolo R, Nicola L, Iodice C, Conte G. Supplemented very low‐protein diet in advanced CRF: is it money saving?. Kidney International 2004;65(2):742. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Iorio BR, Minutolo R, Nicola L, Bellizzi V, Catapano F, Iodice C, et al. Supplemented very low protein diet ameliorates responsiveness to erythropoietin in chronic renal failure. Kidney International 2003;64(5):1822‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garneata 2013 {published data only}
- Garneata L, Mircescu G. Effect of low‐protein diet supplemented with keto acids on progression of chronic kidney disease. Journal of Renal Nutrition 2013;23(3):210‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Garneata L, Stancu A, Dragomir D, Mircescu G. Effect of very low protein diet supplemented with ketoanalogues of the essential amino acids on the progression of chronic kidney disease [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii39. [EMBASE: 71491557] [Google Scholar]
- Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G. Ketoanalogue‐supplemented vegetarian very low‐protein diet and CKD progression. Journal of the American Society of Nephrology 2016;27(7):2164‐76. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ihle 1989 {published data only}
- Becker GJ, Whitworth JA, Ihle BU, Shahinfar S, Kincaid‐Smith PS. Prevention of progression in non‐diabetic chronic renal failure. Kidney International ‐ Supplement 1994;45(Suppl 45):S167‐70. [MEDLINE: ] [PubMed] [Google Scholar]
- Ihle BU, Becker GJ, Whithworth JA, Charlwood RA, Kincaid‐Smith PS. The effect of protein restriction on the progression of renal insufficiency. New England Journal of Medicine 1989;321(26):1773‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locatelli 1989 {published data only}
- D'Amico G, Gentile MG, Fellin G, Manna G, Cofano F. Effect of dietary protein restriction on the progression of renal failure: a prospective randomized trial. Nephrology Dialysis Transplantation 1994;9(11):1590‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Locatelli F. Controlled study of protein‐restricted diet in chronic renal failure. Contributions to Nephrology 1989;75:141‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F. Dietary compliance in patients with chronic renal failure: experience in a northern Italy trial. Contributions to Nephrology 1990;81:102‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Alberti D, Gentile MG, Graziani G, Buccianti G, Cosci P, et al. Effects of two different protein prescriptions on chronic renal failure progression. Cooperative study. [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:20.
- Locatelli F, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A. Prospective, randomised, multicentre trial of effect of protein restriction on progression of chronic renal insufficiency. Northern Italian Cooperative Study Group. Lancet 1991;337(8753):1299‐304. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A, et al. Blood pressure and chronic renal failure progression: results from a multicentre trial [abstract]. Nephrology Dialysis Transplantation 1992;7(7):712. [Google Scholar]
- Locatelli F, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A, et al. Factors affecting chronic renal failure progression: results from a multicentre trial. The Northern Italian Cooperative Study Group. Mineral & Electrolyte Metabolism 1992;18(2‐5):295‐302. [MEDLINE: ] [PubMed] [Google Scholar]
- Locatelli F, Vecchio L, Andrulli S, Marai P, Tentori F. The role of underlying nephropathy in the progression of renal disease. Kidney International ‐ Supplement 2000;75:S49‐55. [MEDLINE: ] [PubMed] [Google Scholar]
- Locatelli F, Marcelli D, Tentori F, Marai P. Hypertension and progression of chronic renal failure (CRF): a prospective study [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:37A.
- Locatelli F, Marcelli D, et al. Factors affecting the progression of chronic renal failure: a multivariate analysis [abstract]. Nephrology Dialysis Transplantation 1989;4(5):457. [Google Scholar]
- Tentori F, Marai P. Monocentric prospective randomised controlled study on the progression rate of chronic renal failure with two different protein intakes [abstract]. Nephrology Dialysis Transplantation 1987;2(5):400‐1. [Google Scholar]
- Tentori F, Marai P, Marcelli D, Ponti R, Locatelli F. Monocentric prospective randomized controlled study on the progression rate of chronic renal failure (CRF) with two different protein intakes [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:20.
Malvy 1999 {published data only}
- Malvy D, Maingourd C, Pengloan J, Bagros P, Nivet H. Effects of severe protein restriction with ketoanalogues in advanced renal failure. Journal of the American College of Nutrition 1999;18(5):481‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nivet H, Maingourd C, Malvy D, Pengloan J, Bagros PH. Effects of severe protein‐restriction with ketoanalogues in advanced renal failure [abstract]. Journal of the American Society of Nephrology 1992;3(3):286. [DOI] [PubMed] [Google Scholar]
MDRD Feasibility Study A 1989 {published data only}
- The Modification of Diet in Renal Disease Study: design, methods, and results from the feasibility study. American Journal of Kidney Diseases 1992;20(1):18‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coggins CH, Dwyer JT, Greene T, Petot G, Snetselaar LG, Lente F, et al. Serum lipid changes associated with modified protein diets: results from the feasibility phase of the Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases 1994;23(4):514‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klahr S, Levey AS, Sandberg AM, Williams GW, MDRD Study Group. Major results of the feasibility study of the Modification of Diet in Renal Disease (MDRD) study [abstract]. Kidney International 1989;35(1):195. [Google Scholar]
- Kopple JD, Berg R, Houser H, Steinman TI, Teschan P. Nutritional status of patients with different levels of chronic renal insufficiency. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney International ‐ Supplement 1989;27:S184‐94. [MEDLINE: ] [PubMed] [Google Scholar]
- Laidlaw SA, Berg RL, Kopple JD, Naito H, Walker WG, Walser M. Patterns of fasting plasma amino acid levels in chronic renal insufficiency: results from the feasibility phase of the Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases 1994;23(4):504‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Berg RL, Gassman JJ, Hall PM, Walker WG. Creatinine filtration, secretion and excretion during progressive renal disease. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney International ‐ Supplement 1989;27:S73‐80. [MEDLINE: ] [PubMed] [Google Scholar]
- Levey AS, Gassman JJ, Hall PM, Walker WG. Assessing the progression of renal disease in clinical studies: effects of duration of follow‐up and regression to the mean. Modification of Diet in Renal Disease (MDRD) Study Group. Journal of the American Society of Nephrology 1991;1(9):1087‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Gassman JJ, Hall PM, Walker WG, MDRD Study Group. Poor correlation of rates of change of creatinine clearance, reciprocal serum creatinine and GFR [abstract]. Kidney International 1989;35(1):197. [Google Scholar]
- MDRD Study Group, Mitch ME, Steinman TI. Objectives and design of the cooperative study, Modification of Diet in Renal Disease [abstract]. Kidney International 1987;31(1):210. [Google Scholar]
- Perrone R, Steinman T, Royal H, Lawlor M, Hunsicker L, Modification of Diet in Renal Disease Study Group. Markers of GFR: comparison of 99TC‐DTPA (TC), 169YB‐DTPA (YB) and 125I‐Iothalamate (IO) to inulin (IN) [abstract]. Kidney International 1987;31(1):213. [Google Scholar]
- Perrone RD, Steinman TI, Beck GJ, Skibinski CI, Royal HD, Lawlor M, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I‐iothalamate, 169Yb‐DTPA, 99mTc‐DTPA, and inulin. The Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases 1990;16(3):224‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teschan PE, Beck GJ, Dwyer JT, Greene T, Klahr S, Levy AS, et al. Effect of a ketoacid‐aminoacid‐supplemented very low protein diet on the progression of advanced renal disease: a reanalysis of the MDRD feasibility study. Clinical Nephrology 1998;50(5):273‐83. [MEDLINE: ] [PubMed] [Google Scholar]
MDRD Feasibility Study B 1989 {published data only}
- The Modification of Diet in Renal Disease Study: design, methods, and results from the feasibility study. American Journal of Kidney Diseases 1992;20(1):18‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coggins CH, Dwyer JT, Greene T, Petot G, Snetselaar LG, Lente F, et al. Serum lipid changes associated with modified protein diets: results from the feasibility phase of the Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases 1994;23(4):514‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klahr S, Levey AS, Sandberg AM, Williams GW, MDRD Study Group. Major results of the feasibility study of the Modification of Diet in Renal Disease (MDRD) study [abstract]. Kidney International 1989;35(1):195. [Google Scholar]
- Kopple JD, Berg R, Houser H, Steinman TI, Teschan P. Nutritional status of patients with different levels of chronic renal insufficiency. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney International ‐ Supplement 1989;27:S184‐94. [MEDLINE: ] [PubMed] [Google Scholar]
- Laidlaw SA, Berg RL, Kopple JD, Naito H, Walker WG, Walser M. Patterns of fasting plasma amino acid levels in chronic renal insufficiency: results from the feasibility phase of the Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases 1994;23(4):504‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Berg RL, Gassman JJ, Hall PM, Walker WG. Creatinine filtration, secretion and excretion during progressive renal disease. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney International ‐ Supplement 1989;27:S73‐80. [MEDLINE: ] [PubMed] [Google Scholar]
- Levey AS, Gassman JJ, Hall PM, Walker WG. Assessing the progression of renal disease in clinical studies: effects of duration of follow‐up and regression to the mean. Modification of Diet in Renal Disease (MDRD) Study Group. Journal of the American Society of Nephrology 1991;1(9):1087‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Gassman JJ, Hall PM, Walker WG, Modification of Diet in Renal Disease Study. Poor correlation of rates of change of creatinine clearance, reciprocal serum creatinine and GFR [abstract]. Kidney International 1989;35(1):197. [Google Scholar]
- MDRD Study Group, Mitch ME, Steinman TI. Objectives and design of the cooperative study, Modification of Diet in Renal Disease [abstract]. Kidney International 1987;31(1):210. [Google Scholar]
- Perrone R, Steinman T, Royal H, Lawlor M, Hunsicker L, Modification of Diet in Renal Disease Study Group. Markers of GFR: comparison of 99TC‐DTPA (TC), 169YB‐DTPA (YB) and 125I‐Iothalamate (IO) to inulin (IN) [abstract]. Kidney International 1987;31(1):213. [Google Scholar]
- Perrone RD, Steinman TI, Beck GJ, Skibinski CI, Royal HD, Lawlor M, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I‐iothalamate, 169Yb‐DTPA, 99mTc‐DTPA, and inulin. The Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases 1990;16(3):224‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teschan PE, Beck GJ, Dwyer JT, Greene T, Klahr S, Levy AS, et al. Effect of a ketoacid‐aminoacid‐supplemented very low protein diet on the progression of advanced renal disease: a reanalysis of the MDRD feasibility study. Clinical Nephrology 1998;50(5):273‐83. [MEDLINE: ] [PubMed] [Google Scholar]
MDRD Study 1 1989 {published data only}
- Effects of diet and antihypertensive therapy on creatinine clearance and serum creatinine concentration in the Modification of Diet in Renal Disease Study.[Erratum appears in J Am Soc Nephrol 1997 Aug;8(8):1354]. Journal of the American Society of Nephrology 1996;7(4):556‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Effects of dietary protein restriction on the progression of moderate renal disease in the Modification of Diet in Renal Disease Study.[Erratum appears in J Am Soc Nephrol 1997 Mar;8(3):493]. Journal of the American Society of Nephrology 1996;7(12):2616‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Short‐term effects of protein intake, blood pressure, and antihypertensive therapy on glomerular filtration rate in the Modification of Diet in Renal Disease Study. Journal of the American Society of Nephrology 1996;7(10):2097‐109. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- The Modification of Diet in Renal Disease Study: design, methods, and results from the feasibility study. American Journal of Kidney Diseases 1992;20(1):18‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beck GJ, Berg RL, Coggins CH, Gassman JJ, Hunsicker LG, Schluchter MD, et al. Design and statistical issues of the Modification of Diet in Renal Disease Trial. The Modification of Diet in Renal Disease Study Group. Controlled Clinical Trials 1991;12(5):566‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Buckalew VM Jr, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. American Journal of Kidney Diseases 1996;28(6):811‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chawla V, Wang X, Greene J, Kusek JW, Collins AJ, Levey AS, et al. Dyslipidemia and mortality in chronic kidney disease [abstract no: TH‐PO895]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):311A‐2A. [Google Scholar]
- Chen JL, Lerner D, Ruthazer R, Castaneda‐Sceppa C, Levey AS. Association of physical activity with mortality in chronic kidney disease. Journal of Nephrology 2008;21(2):243‐52. [MEDLINE: ] [PubMed] [Google Scholar]
- Chen JL, Sceppa C, Ruthazer R, Levey AS. Leisure time physical activity status and mortality in chronic kidney disease: preliminary findings from the MDRD study [abstract no: 21]. American Journal of Kidney Diseases 2006;47(4):A24. [Google Scholar]
- Coggins CH, Breyer Lewis J, Caggiula AW, Castaldo LS, Klahr S, Wang SR. Differences between women and men with chronic renal disease. Nephrology Dialysis Transplantation 1998;13(6):1430‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coyne T, Olson M, Bradham K, Garcon M, Gregory P, Scherch L. Dietary satisfaction correlated with adherence in the Modification of Diet in Renal Disease Study. Journal of the American Dietetic Association 1995;95(11):1301‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dennis VW. Decoding the Modification of Diet in Renal Disease Study. Cleveland Clinic Journal of Medicine 1994;61(4):254‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dolecek TA, Olson MB, Caggiula AW, Dwyer JT, Milas NC, Gillis BP, et al. Registered dietitian time requirements in the Modification of Diet in Renal Disease Study. Journal of the American Dietetic Association 1995;95(11):1307‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gassman JJ, Drabik MJ, Leatherman JR, Fatica KJ, McPherson JA, Modification of Diet in Renal Disease Study Group. Development and use of distributed data entry and electronic communication in a multi‐center study of progressive renal disease [abstract]. Kidney International 1989;35(1):226. [Google Scholar]
- Gennari FJ, Hood VL, Greene T, Wang X, Levey AS. Effect of Dietary Protein Intake on Serum Total CO2 Concentration in Chronic Kidney Disease: Modification of Diet in Renal Disease Study Findings. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(1):52‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gillis BP, Averbach FM, Caggiula AW, Jones FL, Naujelis J, Maurer E, et al. Features of the nutrient database and analysis system for the Modification of Diet in Renal Disease Study.[Erratum appears in Control Clin Trials 1994 Aug;15(4):326]. Controlled Clinical Trials 1994;15(1):44‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gillis BP, Caggiula AW, Chiavacci AT, Coyne T, Doroshenko L, Milas NC, et al. Nutrition intervention program of the Modification of Diet in Renal Disease Study: a self‐management approach. Journal of the American Dietetic Association 1995;95(11):1288‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grams ME, Coresh J, Segev DL, Kucirka LM, Tighiouart H, Sarnak MJ. Vascular disease, ESRD, and death: interpreting competing risk analyses. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(10):1606‐14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grams ME, Tin A, Rebholz CM, Shafi T, Kottgen A, Perrone RD, et al. Metabolomic alterations associated with cause of CKD. Clinical Journal of The American Society of Nephrology: CJASN 2017;12(11):1787‐94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene T, Beck G, Wang S, Kusek J, Levey A, Modification of Diet in Renal Disease Study Group. The effect of the low protein diet in the MDRD study A is dependent on the underlying rate of disease progression [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):165A. [DOI] [PubMed] [Google Scholar]
- Greene T, Bourgoignie JJ, Habwe V, Kusek JW, Snetselaar LG, Soucie JM, et al. Baseline characteristics in the Modification of Diet in Renal Disease Study.[Republished from J Am Soc Nephrol 1993 May;3(11):1819‐34]. Journal of the American Society of Nephrology 1993;4(5):1221‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hebert LA, Greene T, Hunsicker LG, Levey AS, Wang S, MDRD Study Group. Relationship of urine volume (uv) and urine osmolality (Uosm) to the progression of renal disease in the Modification of Diet in Renal Disease (MDRD) study [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):165A‐6A. [Google Scholar]
- Hebert LA, Kusek JW, Greene T, Agodoa LY, Jones CA, Levey AS, et al. Effects of blood pressure control on progressive renal disease in blacks and whites. Modification of Diet in Renal Disease Study Group. Hypertension 1997;30(3 (Pt 1)):428‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney International 1997;51(6):1908‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hunsicker LG, MDRD Study Group. Secondary analysis of the Modification of Diet in Renal Disease (MDRD) study [abstract]. 13th International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:64.
- Ix JH, Shlipak MG, Sarnak MJ, Beck GJ, Greene T, Wang X, et al. Fetuin‐A is not associated with all‐cause or cardiovascular mortality in chronic kidney disease: data from the Modification of Diet in Renal Disease (MDRD) study [abstract no: SU‐PO711]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):741A. [Google Scholar]
- Klahr S. Primary and secondary results of the modification of diet in renal disease study. Mineral & Electrolyte Metabolism 1996;22(1‐3):138‐42. [MEDLINE: ] [PubMed] [Google Scholar]
- Klahr S, Breyer JA, Beck GJ, Dennis VW, Hartman JA, Roth D, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group.[Erratum appears in J Am Soc Nephrol 1995 Oct;6(4):1318]. Journal of the American Society of Nephrology 1995;5(12):2037‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. New England Journal of Medicine 1994;330(13):877‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klahr S, Modification of Diet in Renal Disease Study. The Modification of Diet in Renal Disease Study. New England Journal of Medicine 1989;320(13):864‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kopple JD, Greene T, Chumlea WC, Hollinger D, Maroni BJ, Merrill D, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney International 2000;57(4):1688‐703. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kopple JD, Levey AS, Greene T, Chumlea WC, Gassman JJ, Hollinger DL, et al. Effect of dietary protein restriction on nutritional status in the Modification of Diet in Renal Disease Study. Kidney International 1997;52(3):778‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ku E, Glidden DV, Johansen KL, Sarnak M, Tighiouart H, Grimes B, et al. Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end‐stage renal disease. Kidney International 2015;87(5):1055‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusek JW, Coyne T, Velasco A, Drabik MJ, Finlay RA, Gassman JJ, et al. Recruitment experience in the full‐scale phase of the Modification of Diet in Renal Disease Study. Controlled Clinical Trials 1993;14(6):538‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kusek JW, Coyne T, deVelasco A, Gassman J, Kiefer S, Powers S, et al. Recruitment experience in the full scale phase of the Modification of Diet in Renal Disease (MDRD) Study [abstract]. Controlled Clinical Trials 1991;12:678. [DOI] [PubMed] [Google Scholar]
- Kwong YT, Stevens LA, Selvin E, Zhang YL, Greene T, Lente F, et al. Imprecision of urinary iothalamate clearance as a gold‐standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. American Journal of Kidney Diseases 2010;56(1):39‐49. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JM, Bourgoignie JJ, Buckalew VM, Greene T, Levey AS, Milas NC, et al. Achievement and safety of a low blood pressure goal in chronic renal disease. The Modification of Diet in Renal Disease Study Group. Hypertension 1997;29(2):641‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leonberg‐Yoo AK, Tighiouart H, Levey AS, Beck GJ, Sarnak MJ. Urine potassium excretion, kidney failure, and mortality in CKD. American Journal of Kidney Diseases 2017;69(3):341‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Adler S, Caggiula AW, England BK, Greene T, Hunsicker LG, et al. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases 1996;27(5):652‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of Internal Medicine 1999;130(6):461‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate.[Erratum appears in Ann Intern Med. 2008 Oct 7;149(7):519]. Annals of Internal Medicine 2006;145(4):247‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Greene T, Beck GJ, Caggiula AW, Kusek JW, Hunsicker LG, et al. Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study Group. Journal of the American Society of Nephrology 1999;10(11):2426‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Greene T, Sarnak M, Wang X, Beck GJ, Kusek JW, et al. Effect of low protein diet on progression of kidney disease in the Modification of Diet in Renal Disease study A: long‐term follow‐up [abstract no: SU‐PO247]. Journal of the American Society of Nephrology 2004;15(Oct):586A‐7A. [Google Scholar]
- Levey AS, Greene T, Sarnak MJ, Wang X, Beck GJ, Kusek JW, et al. Effect of dietary protein restriction on the progression of kidney disease: long‐term follow‐up of the Modification of Diet in Renal Disease (MDRD) Study. American Journal of Kidney Diseases 2006;48(6):879‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Greene T, Sarnak MJ, Wang X, Beck GJ, Kusek JW, et al. The effect of very low protein diet on progression of kidney disease and mortality in Modification of Diet in Renal Disease Study B [abstract no: SU‐PO246]. Journal of the American Society of Nephrology 2004;15(Oct):586A. [Google Scholar]
- Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, et al. Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. Journal of the American Society of Nephrology 1993;4(5):1159‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- MDRD Study Group. The modification of diet in renal disease study: results from the full‐scale trial [abstract]. 12th International Congress of Nephrology; 1993 Jun 13‐18; Jerusalem, Israel. 1993:593.
- MDRD Study Group, Chumlea WC, Gassman JJ, Hollinger DL, Merrill D, Scherch LK, et al. Nutritional response to diet prescription in the MDRD study [abstract]. Journal of the American Society of Nephrology 1994;5(3):335. [Google Scholar]
- MDRD Study Group, Hunsicker LG, Adler S, Caggiula AW, England B, Greene T, et al. Relationship among baseline proteinuria (p), mean arterial blood pressure (map) during follow‐up, and decline in glomerular filtration rate (aGFR) in the Modification of Diet in Renal Disease study [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):254. [Google Scholar]
- MDRD Study Group, Kopple JD, Chumlea WC, Gassman JJ, Hollinger DL, Maroni BJ, et al. Relationship between FGR and nutritional status ‐ results from the MDRD study [abstract]. Journal of the American Society of Nephrology 1994;5(3):335. [Google Scholar]
- MDRD Study Group, Kusek JW, Agodoa L, Greene T, Jones C. Comparison of decline of GFR in blacks versus non‐blacks in the MDRD study [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):253. [Google Scholar]
- MDRD Study Group, Levey AS, Beck GJ. Trends toward a beneficial effect of a low protein diet during additional follow‐up in the modification of diet in renal disease study. MDRD Study Group. [abstract]. Nephrology Dialysis Transplantation 1995;10(6):985. [Google Scholar]
- MDRD Study Group, Levey AS, Beck GJ, Bosch JP, Caggiula AW, Greene T, et al. Short‐term effects of protein intake, blood pressure, and antihypertensive therapy on GFR in the MDRD study. Journal of the American Society of Nephrology 1995;6(3):395. [Google Scholar]
- MDRD Study Group, Levey AS, Beck GJ, Caggiula AW, Greene T, Hunsicker LG, et al. A hypothesis for the results of the Modification of Diet in Renal Disease (MDRD) study. [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):253. [DOI] [PubMed] [Google Scholar]
- MDRD Study Group, Levey AS, Beck GJ, Caggiula AW, Greene T, Kusek JW, et al. Trends toward a beneficial effect of a low protein diet during additional follow‐up in the Modification of Diet in Renal Disease study [abstract]. Journal of the American Society of Nephrology 1994;5(3):336. [Google Scholar]
- MDRD Study Group, Levey AS, Bosch JP, Coggins CH, Greene T, Mitch WE, et al. Effects of diet and blood pressure on creatinine clearance (CCr) and serum creatinine (PCr) in the MDRD study [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):253. [Google Scholar]
- MDRD Study Group, Peterson JC, Burkart J, Greene T, Hebert L, King A, et al. The effect of blood pressure control on progression of renal disease depends on level of proteinuria (p) at baseline evaluation [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):254. [Google Scholar]
- MDRD Study Group, Peterson JC, Greene T, Klahr S, Burkart JM, Hebert L, et al. Effect of reducing proteinuria (PR) on subsequent glomerular filtration rate (GFR) decline in patients with chronic renal failure (CRF) [abstract]. Journal of the American Society of Nephrology 1994;5(3):339. [Google Scholar]
- MDRD Study Group, Porush JG, Lazarus JM, Bourgoignie JJ, Buckalew VM, Greene T, et al. Efficacy of anti‐hypertensive interventions in reducing blood pressure in the MDRD study [abstract]. Journal of the American Society of Nephrology 1995;6(3):400. [Google Scholar]
- MDRD Study Group, Powers SN, Kurtzman DA, Petot GJ, Raizman DJ, Wetstein L. Promoting dietary compliance through computer‐assisted education, assessment, and counseling. [abstract]. Kidney International 1988;36(Suppl 27):S306. [Google Scholar]
- MDRD Study Group, Rocco MV, Coyne T, Eastin S, Faubert J, Gassman JJ, et al. Patient symptoms and quality of life in the MDRD study at enrollment‐correlation with GFR [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):254. [Google Scholar]
- Madero M, Sarnak M, Wang X, Greene T, Collins A, Beck G, et al. Uric acid and development of kidney failure in chronic kidney disease [abstract no: F‐PO1932]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):547A. [Google Scholar]
- Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, et al. Uric acid and long‐term outcomes in CKD. American Journal of Kidney Diseases 2009;53(5):796‐803. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madero M, Sarnak MJ, Wang X, Sceppa CC, Greene T, Beck GJ, et al. Body mass index and mortality in CKD. American Journal of Kidney Diseases 2007;50(3):404‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Madero M, Wang X, Green T, Hunsicker LG, Beck GJ, Kusek JW, et al. Effect of trial participation on outcomes in the randomized and non‐randomized cohorts of the Modification of Diet in Renal Disease study [abstract no: F‐PO037]. Journal of the American Society of Nephrology 2006;17(Abstracts):344A. [Google Scholar]
- Menon V, Green T, Pereira A, Xuelei W, Beck G, Kusek J, et al. C‐reactive protein (CRP) as a predictor of all‐cause and cardiovascular (CVD) mortality in patients with chronic kidney disease (CKD) [abstract no: F‐P0275]. Journal of the American Society of Nephrology 2004;15(Oct):125A‐6A. [Google Scholar]
- Menon V, Greene T, Pereira A, Wang X, Beck G, Kusek J, et al. Hemoglobin A1c and mortality in chronic kidney disease [abstract no: F‐FC149]. Journal of the American Society of Nephrology 2005;16:71A. [DOI] [PubMed] [Google Scholar]
- Menon V, Greene T, Pereira A, Wang X, Beck G, Kusek J, et al. Insulin resistance as a predictor of all‐cause and cardiovascular mortality (CVD) in chronic kidney disease (CKD) [abstract no: F‐P0284]. Journal of the American Society of Nephrology 2004;15(Oct):128A. [Google Scholar]
- Menon V, Greene T, Pereira AA, Wang X, Beck GJ, Kusek JW, et al. Glycosylated hemoglobin and mortality in patients with nondiabetic chronic kidney disease. Journal of the American Society of Nephrology 2005;16(11):3411‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Greene T, Pereira AA, Wang X, Beck GJ, Kusek JW, et al. Relationship of phosphorus and calcium‐phosphorus product with mortality in CKD. American Journal of Kidney Diseases 2005;46(3):455‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, et al. C‐reactive protein and albumin as predictors of all‐cause and cardiovascular mortality in chronic kidney disease. Kidney International 2005;68(2):766‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Kopple JD, Wang X, Beck GJ, Collins AJ, Kusek JW, et al. Effect of a very low‐protein diet on outcomes: long‐term follow‐up of the Modification of Diet in Renal Disease (MDRD) Study. American Journal of Kidney Diseases 2009;53(2):208‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Li L, Greene T, Wang X, Chandra P, Balakrishnan V, et al. Adiponectin and outcomes in chronic kidney disease [abstract no: TH‐PO430]. Journal of the American Society of Nephrology 2006;17(Abstracts):199A. [Google Scholar]
- Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, et al. Adiponectin and mortality in patients with chronic kidney disease. Journal of the American Society of Nephrology 2006;17(9):2599‐606. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Sarnak MJ, Greene T, Wang X, Pereira AA, Beck GJ, et al. Relationship between homocysteine and mortality in chronic kidney disease. Circulation 2006;113(12):1572‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Annals of Internal Medicine 2007;147(1):19‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Tighiouart H, Vaughn NS, Beck GJ, Kusek JW, Collins AJ, et al. Serum bicarbonate and long‐term outcomes in CKD. American Journal of Kidney Diseases 2010;56(5):907‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Wang X, Greene T, Beck GJ, Kusek JW, Marcovina SM, et al. Relationship between C‐reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. American Journal of Kidney Diseases 2003;42(1):44‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Wang X, Greene T, Beck GJ, Kusek JW, Selhub J, et al. Homocysteine in chronic kidney disease: effect of low protein diet and repletion with B vitamins. Kidney International 2005;67(4):1539‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menon V, Wang X, Sarnak MJ, Hunsicker LH, Madero M, Beck GJ, et al. Long‐term outcomes in nondiabetic chronic kidney disease. Kidney International 2008;73(11):1310‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Milas NC, Nowalk MP, Akpele L, Castaldo L, Coyne T, Doroshenko L, et al. Factors associated with adherence to the dietary protein intervention in the Modification of Diet in Renal Disease Study. Journal of the American Dietetic Association 1995;95(11):1295‐300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Newsome B, Ix JH, Tighiouart H, Sarnak MJ, Levey AS, Beck GJ, et al. Effect of protein restriction on serum and urine phosphate in the modification of diet in renal disease (MDRD) study. American Journal of Kidney Diseases 2013;61(6):1045‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Olson M, Coyne T, Caggiula A. Social factors influence dietary satisfaction in the Modification of Diet in Renal Disease Study [abstract]. Controlled Clinical Trials 1996;17(2 Suppl):133S. [Google Scholar]
- Olson M, Coyne T, Caggiula A, Gregory P. Patient satisfaction with a dietary intervention: the Modification of Diet in Renal Disease Study [abstract]. Controlled Clinical Trials 1995;16(3 Suppl):107S. [Google Scholar]
- Olson M, Dolecek T, Caggiula A, Dwyer J. Time required for the protein intervention in the Modification of Diet in Renal Disease Study [abstract]. Controlled Clinical Trials 1995;16(3 Suppl):129S‐30S. [Google Scholar]
- Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Annals of Internal Medicine 1995;123(10):754‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Piccoli M, Codognotto M. Missing stratification by polycistic kidney disease (PKD) flaws risk estimates of GFR decline. Lesson from the MDRD Study [abstract no: F‐PO175]. Journal of the American Society of Nephrology 2004;15(Oct):103A. [Google Scholar]
- Rocco MV, Gassman JJ, Wang SR, Kaplan RM. Cross‐sectional study of quality of life and symptoms in chronic renal disease patients: the Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases 1997;29(6):888‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, et al. The effect of a lower target blood pressure on the progression of kidney disease: long‐term follow‐up of the Modification of Diet in Renal Disease study. Annals of Internal Medicine 2005;142(5):342‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Wang SR, Beck GJ, Kusek JW, Selhub J, Greene T, et al. Homocysteine, cysteine, and B vitamins as predictors of kidney disease progression. American Journal of Kidney Diseases 2002;40(5):932‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Wang X, Greene T, Gerald J, Beck J, Kusek JW, et al. Effect of strict blood pressure control on long‐term outcome in the Modification of Diet in Renal Disease (MDRD) study [abstract no: F‐P0599]. Journal of the American Society of Nephrology 2003;14(Nov):192A. [Google Scholar]
- Schluchter MD. Estimating correlation between alternative measures of disease progression in a longitudinal study. Modification of Diet in Renal Disease Study. Statistics in Medicine 1990;9(10):1175‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stollar C, Scherch L, Adler S, Kusek J, Caggiula A. Patterns of patient dietary compliance in the Modification of Diet in Renal Disease (MDRD) study, phase III. [abstract]. 12th International Congress of Nephrology; 1993 Jun 13‐18; Jerusalem, Israel. 1993:609.
- Tangri N, Stevens LA, Schmid CH, Zhang YL, Beck GJ, Greene T, et al. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney International 2011;79(4):471‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto ME, Olson MB, Stollar C, MDRD Study Group. Effects of weight and Na+ change on blood pressures of hypertensive MDRD study patients [abstract]. Journal of the American Society of Nephrology 1995;6(3):408. [Google Scholar]
- Young JA, Terrin N, Greene T, Wang X, Beck G, Kusek J, et al. Asymmetric dimethylarginine and prevalent cardiovascular disease in patients with chronic kidney disease [abstract no: F‐PO935]. Journal of the American Society of Nephrology 2007;18(Abstracts):308A‐9A. [Google Scholar]
- Young JA, Terrin N, Wang X, Green T, Beck G, Kusek J, et al. ADMA and mortality in stage 3‐4 chronic kidney disease [abstract no: TH‐PO894]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):311A. [Google Scholar]
MDRD Study 2 1989 {published data only}
- Klahr S. The modification of diet in renal disease study. New England Journal of Medicine 1989;320(13):864‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Adler S, Caggiula AW, England BK, Greene T, Hunsicker LG, et al. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases 1996;27(5):652‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levey AS, Greene T, Beck GJ, Caggiula AW, Kusek JW, Hunsicker LG, et al. Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. Journal of the American Society of Nephrology 1999;10(11):2426‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meloni 2004 {published data only}
- Meloni C, Tatangelo P, Cipriani S, Rossi V, Suraci C, Tozzo C, et al. Adequate protein dietary restriction in diabetic and nondiabetic patients with chronic renal failure. Journal of Renal Nutrition 2004;14(4):208‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Milovanov 2009 {published data only}
- Milovanov IuS, Lysenko LV, Milovanova LI, Dobrosmyslov IA. The role of balanced low‐protein diet in inhibition of predialysis chronic kidney disease progression in patients with systemic diseases. Terapevticheskii Arkhiv 2009;81(8):52‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Mircescu 2007 {published data only}
- Mircescu G, Garneata L, Stancu SH, Capusa C. Effects of a supplemented hypoproteic diet in chronic kidney disease. Journal of Renal Nutrition 2007;17(3):179‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rosman Study 1 1984 {published data only}
- Bock HA, Brunner FP. Dietary protein restriction in chronic renal failure. Lancet 1985;1(8426):465‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Donker AJ, Piers‐Becht TP. Four‐years follow‐up of patients with chronic renal insufficiency randomly allocated to a protein‐restricted diet and a control group [abstract]. Nephrology Dialysis Transplantation 1989;4(5):455. [Google Scholar]
- Donker AJ, Rosman JB, Piers‐Becht TP, Sluiter WJ. Effect of protein restriction in chronic renal insufficiency: a prospective randomized trial [abstract]. Kidney International 1985;27:137. [Google Scholar]
- Rosman JB, Donker AJ. The response to a low‐protein diet to retard the progression of renal failure is sex‐dependent [abstract]. Nephrology Dialysis Transplantation 1989;4(5):457. [Google Scholar]
- Rosman JB, Donker AJ, Meijer S, Sluiter WJ, Piers‐Becht TP, Hem GK. Two years' experience with protein restriction in chronic renal failure. Contributions to Nephrology 1986;53:109‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosman JB, Langer K, Brandl M, Piers‐Becht TP, Hem GK, ter Wee PM, et al. Protein‐restricted diets in chronic renal failure: a four year follow‐up shows limited indications. Kidney International ‐ Supplement 1989;27:S96‐S102. [MEDLINE: ] [PubMed] [Google Scholar]
- Rosman JB, Ter Wee PM, Piers‐Becht GP, Sluiter WJ, Woude F, Meijer S, et al. Early protein restriction in chronic renal failure [abstract]. Kidney International 1984;26(4):510. [Google Scholar]
- Rosman JB, Ter Wee PM, Sluiter WJ, Meijer S, Hem GK, Donker AJ. Four years experience with dietary protein restriction in chronic renal failure [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:28.
- Rosman JB, ter Wee PM. Relationship between proteinuria and response to low protein diets early in chronic renal failure. Blood Purification 1989;7(1):52‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosman JB, ter Wee PM, Meijer S, Piers‐Becht TP, Sluiter WJ, Donker AJ. Prospective randomised trial of early dietary protein restriction in chronic renal failure. Lancet 1984;324(8415):1291‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosman JB, ter Wee PM, Piers‐Becht GP, Sluiter WJ, Woude FJ, Meijer S, et al. Early protein restriction in chronic renal failure. Proceedings of the European Dialysis & Transplant Association ‐ European Renal Association 1985;21:567‐73. [MEDLINE: ] [PubMed] [Google Scholar]
Rosman Study 2 1984 {published data only}
- Bock HA, Brunner FP. Dietary protein restriction in chronic renal failure. Lancet 1985;1(8426):465‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Donker AJ, Piers‐Becht TP. Four‐years follow‐up of patients with chronic renal insufficiency randomly allocated to a protein‐restricted diet and a control group [abstract]. Nephrology Dialysis Transplantation 1989;4(5):455. [Google Scholar]
- Donker AJ, Rosman JB, Piers‐Becht TP, Sluiter WJ. Effect of protein restriction in chronic renal insufficiency: a prospective randomized trial [abstract]. Kidney International 1985;27:137. [Google Scholar]
- Rosman JB, Donker AJ. The response to a low‐protein diet to retard the progression of renal failure is sex‐dependent [abstract]. Nephrology Dialysis Transplantation 1989;4(5):457. [Google Scholar]
- Rosman JB, Donker AJ, Meijer S, Sluiter WJ, Piers‐Becht TP, Hem GK. Two years' experience with protein restriction in chronic renal failure. Contributions to Nephrology 1986;53:109‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosman JB, Langer K, Brandl M, Piers‐Becht TP, Hem GK, ter Wee PM, et al. Protein‐restricted diets in chronic renal failure: a four year follow‐up shows limited indications. Kidney International ‐ Supplement 1989;27:S96‐102. [MEDLINE: ] [PubMed] [Google Scholar]
- Rosman JB, Ter Wee PM, Piers‐Becht GP, Sluiter WJ, Woude F, Meijer S, et al. Early protein restriction in chronic renal failure [abstract]. Kidney International 1984;26(4):510. [Google Scholar]
- Rosman JB, Ter Wee PM, Sluiter WJ, Meijer S, Hem GK, Donker AJ. Four years experience with dietary protein restriction in chronic renal failure. [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:28.
- Rosman JB, ter Wee PM. Relationship between proteinuria and response to low protein diets early in chronic renal failure. Blood Purification 1989;7(1):52‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosman JB, ter Wee PM, Meijer S, Piers‐Becht TP, Sluiter WJ, Donker AJ. Prospective randomised trial of early dietary protein restriction in chronic renal failure. Lancet 1984;324(8415):1291‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosman JB, ter Wee PM, Piers‐Becht GP, Sluiter WJ, Woude FJ, Meijer S, et al. Early protein restriction in chronic renal failure. Proceedings of the European Dialysis & Transplant Association ‐ European Renal Association 1985;21:567‐73. [MEDLINE: ] [PubMed] [Google Scholar]
Teplan 1998 {published data only}
- Teplan V, Schuck O, Bubenicek P, Mengerova O, Tesarova Z, Hajny J, et al. The effect of long‐term administration of a low‐protein diet on the metabolic status and progression of chronic renal failure: a multicentre study [abstract]. Nephrology Dialysis Transplantation 1998;13:820. [CENTRAL: CN‐00301551] [Google Scholar]
Williams 1991 {published data only}
- Williams P, Stevens ME, Fass G, Bone JM. A randomised trial of the effect of protein and phosphate restriction on the progression of chronic renal failure [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:38.
- Williams PS, Stevens M, Fass G, Bone JM. A randomised trial of the effect of protein and phosphate restriction on the progression of chronic renal failure [abstract]. Nephrology Dialysis Transplantation 1987;2(4):285. [Google Scholar]
- Williams PS, Stevens M, Irons L, Fass G, Bone JM. Failure of dietary protein/phosphate restriction to slow the progression of chronic renal failure; a prospective randomised controlled trial [abstract]. Nephrology Dialysis Transplantation 1990;5(8):674. [Google Scholar]
- Williams PS, Stevens ME. A randomised trial of the effect of protein and phosphate restriction on the progression of chronic renal failure [abstract]. Nephrology Dialysis Transplantation 1987;2(5):401. [Google Scholar]
- Williams PS, Stevens ME, Fass G, Irons L, Bone JM. Failure of dietary protein and phosphate restriction to retard the rate of progression of chronic renal failure: a prospective, randomized, controlled trial. Quarterly Journal of Medicine 1991;81(294):837‐55. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Bernard 1996 {published data only}
- Bernard S, Fouque D, Laville M, Zech P. Effects of low‐protein diet supplemented with ketoacids on plasma lipids in adult chronic renal failure. Mineral & Electrolyte Metabolism 1996;22(1‐3):143‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Bernhard 2001 {published data only}
- Bernhard J, Beaufrere B, Laville M, Fouque D. Adaptive response to a low‐protein diet in predialysis chronic renal failure patients. Journal of the American Society of Nephrology 2001;12(6):1249‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Choi 2012a {published data only}
- Choi HY, Lee YK, Kim BS, Park HC, Shin SK, Ha SK, et al. Effects of a keto/amino acid supplemented low protein diet on the delay of progressieve renal failure in chronic kidney disease [abstract no: 37]. Kidney Research & Clinical Practice 2012;31(2):A25. [EMBASE: 70814746] [Google Scholar]
Coresh 1994 {published data only}
- Coresh J, Walser M, Hill S. Long‐term outcome of treatment of chronic renal failure with a supplemented low protein diet [abstract]. Journal of the American Society of Nephrology 1994;5(3):489. [DOI] [PubMed] [Google Scholar]
Di Iorio 2009a {published data only}
- Iorio BR, Cucciniello E, Martino R, Frallicciardi A, Tortoriello R, Struzziero G. Acute and persistent antiproteinuric effect of a low‐protein diet in chronic kidney disease [Acuto e persistente effetto antiproteinurico della dieta ipoproteica artificiale nella malattia renale cronica]. Giornale Italiano di Nefrologia 2009;26(5):608‐15. [MEDLINE: ] [PubMed] [Google Scholar]
Di Iorio 2012a {published data only}
- Iorio B, Micco L, Torraca S, Sirico ML, Russo L, Pota A, et al. Acute effects of very‐low‐protein diet on FGF23 levels: a randomized study.[Erratum appears in Clin J Am Soc Nephrol. 2012 Aug;7(8):1369]. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(4):581‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marzocco S, Dal Piaz F, Micco L, Torraca S, Sirico ML, Tartaglia D, et al. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purification 2013;35(1‐3):196‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DODE Study 2000 {published data only}
- Brunori G, Viola BF, Parrinello G, De Biase, Como G, Franco V, et al. Efficacy and safety of a very‐low‐protein diet when postponing dialysis in the elderly: a prospective randomized multicenter controlled study. American Journal of Kidney Diseases 2007;49(5):569‐80. [DOI] [PubMed] [Google Scholar]
- Brunori G, Viola F, Zubani R, Biase V, Franco V, Cuoma D, et al. The DODE Study (diet or dialysis in elderly): interim analysis [abstract no: T501]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):440. [Google Scholar]
- Maiorca R, Brunori G, Viola BF, Zubani R, Cancarini G, Parrinello G, et al. Diet or dialysis in the elderly? The DODE study: a prospective randomized multicenter trial. Journal of Nephrology 2000;13(4):267‐70. [MEDLINE: ] [PubMed] [Google Scholar]
- Scalone L, Borghetti F, Brunori G, Viola BF, Brancati B, Sottini L, et al. Cost‐benefit analysis of supplemented very low‐protein diet versus dialysis in elderly CKD5 patients. Nephrology Dialysis Transplantation 2010;25(3):907‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
El Nahas 1987 {published data only}
- Nahas AM, Meadows JH, Smith WG, Coles GA. The importance of timing of protein intake in chronic renal failure (CRF): a pilot study [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:10.
ERIKA Study 2007 {published data only}
- Bellizzi V, Chiodini P, Nicola L, Minutolo R, Conte G, Iorio B. Long‐term effectiveness of very low protein diet in CKD: a pilot study [abstract no: SU‐PO1045]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):817A. [Google Scholar]
- Bellizzi V, Chiodini P, Nicola L, Minutolo R, Conte G, Iorio B. Long‐term outcome of very low protein diet in CKD: a pilot study [abstract no: 180]. Journal of Renal Nutrition 2008;18(3 Suppl 1):S46. [Google Scholar]
- Bellizzi V, Signoriello S, Minutolo R, Iorio B, Nazzaro P, Conte G, et al. Effect of very low‐protein diet versus standard low‐protein diet on renal death in patients with chronic kidney disease: A pragmatic, randomized, controlled, multicenter trial [abstract]. Nephrology Dialysis Transplantation 2015;30:iii476. [Google Scholar]
Garibotti 2018 {published data only}
- Garibotto G, Sofia A, Parodi EL, Ansaldo F, Bonanni A, Picciotto D. Effects of low protein, and supplemented very low–protein diets, on muscle protein turnover in patients with CKD. KI Reports 2018 Jan 11:[epub ahead of print]. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garini 1992 {published data only}
- Garini G, Arisi L, Borrini A, Fiaccadori E, Guariglia A, Magnati G, et al. Adherence to dietetic treatment, the nutritional metabolic status and the progression of chronic kidney failure [Aderenza al trattamento dietetico, stato metabolico nutrizionale e progressione dell'insufficienza renale cronica]. Annali Italiani di Medicina Interna 1992;7(2):71‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Hecking 1980 {published data only}
- Hecking E, Andrzejewski L, Prellwitz W, Opferkuch W, Muller D. Double‐blind cross‐over study with oral alpha‐ketoacids in patients with chronic renal failure. American Journal of Clinical Nutrition 1980;33(7):1678‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herselman 1995 {published data only}
- Herselman MG, Albertse EC, Lombard CJ, Swanepoel CR, Hough FS. Supplemented low‐protein diets‐‐are they superior in chronic renal failure?. South African Medical Journal 1995;85(5):361‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Ideura 2003 {published data only}
- Ideura T, Shimazui M, Tayama H, Inoue Y, Koiwa F, Morita H, et al. Protein intake more than 0.5g/kg body weight (BW)/Day is not effective in retarding the progression of chronic renal failure [abstract no: PUB062]. Journal of the American Society 2003;14(Nov):786A. [Google Scholar]
IRCCA Study 1990 {published data only}
- Forget D, Caranhac G, Quillot MJ. French multicentric trial (IRCCA) for testing a new ketoanalog and essential amino acid mixture in patients with chronic renal failure [abstract]. Kidney International 1988;36(Suppl 27):S300. [Google Scholar]
- Forget D, Caranhac G, Quillot MJ, Besnier MO. Compliance with very low protein diet and ketoanalogues in chronic renal failure. The French Multicentric Trial IRCCA. Contributions to Nephrology 1990;81:79‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kopple 1982 {published data only}
- Kopple JD, Roberts CE, Grodstein GP, Shah GM, Winer RL, Davidson WP, et al. Adherence to low protein diets by chronically uremic patients [abstract]. Kidney International 1982;21(1):171. [Google Scholar]
- Roberts C, Kopple J, Grodstein G, Shah G, Winer R, Davidson W, et al. Acceptance of protein and amino acid diets by patients with chronic renal failure [abstract]. Kidney International 1983;24(Suppl 16):S‐350. [Google Scholar]
Laville 1994 {published data only}
- Fouque D, Bouc Y, Laville M, Zech P. Abnormal serum ingf‐binding proteins profile before and after a low‐protein diet (LPD) in CRF patients [abstract]. Journal of the American Society of Nephrology 1994;5(3):328. [Google Scholar]
- Laville M, Fouque D, Durozard D, Combarnous F, Baverel G, Zech P. Muscle energetics in renal failure: effects of a low‐protein diet (LPD) supplemented with ketoacids (KA) [abstract]. Journal of the American Society of Nephrology 1994;5(3):336. [Google Scholar]
Lim 2000 {published data only}
- Lim CS, Norashikin AB, Noor Aini MY, Zahara AM, Angl BB, Aparicio M, et al. The effect of very low protein supplemented by ketoamino acid versus low protein diet on progression of chronic renal failure [abstract]. 13th Asian Colloquium in Nephrology; 2000 Nov 23‐25; Bali, Indonesia. 2000:68.
- Lim CS, Tan SY, Aparicio M. A prospective study on the impact of very low protein diet supplemented by keto/amino acids vs low protein diet on rate of progression of chronic renal failure [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):221A. [Google Scholar]
- Noor Aini MY, Zahara AM, Norashikin AB, Lim CS, Ang BB, Aparicio M, et al. Dietary changes and nutritional status of chronic renal failure patients on very low protein diet supplemented by ketoamino acid versus low protein diet [abstract]. 13th Asian Colloquium in Nephrology; 2000 Nov 23‐25; Bali, Indonesia. 2000:140.
Maksic 2004 {published data only}
- Maksic D, Pavlovic G, Kostic‐Milosavljevic M, Mijuskovic Z, Radjen S, Bokonjic D, et al. Comparison of effects of low protein diet (LPD) and very low protein diet (VLPD) supplemented with essential and ketoanalogue amino acids on the progression of predialysis chronic renal failure [abstract]. ERA ‐ EDTA Congress; 2004; May 15‐18; Lisbon, Portugal. 2004:296.
Prakash 2004 {published data only}
- Prakash S, Pande D, Suresh K, Kulkarni H. Randomized double blind placebo controlled trial of ketoanalogues in retardation of chronic renal failure in tropics [abstract no: M416]. Nephrology Dialysis Transplantation 2003;18 Suppl(4):130. [CENTRAL: CN‐00447278] [Google Scholar]
- Prakash S, Pande DP, Sharma S, Sharma D, Bal CS, Kulkarni H. Randomized, double‐blind, placebo‐controlled trial to evaluate efficacy of ketodiet in predialytic chronic renal failure. Journal of Renal Nutrition 2004;14(2):89‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ren 2002 {published data only}
- Ren H, Chen N, Zao Q, Zhang D. Evaluation of the effect of low‐protein diet and combination diet with (alpha)‐ketoacid therapy in chronic renal failure. Shanghai Medical Journal 2002;25(11):671‐4. [CENTRAL: CN‐00461589] [Google Scholar]
- Ren H, Chen N, Zhao Q, Zhang D, Chen X, Zhang W. Evaluation of the effect of low‐protein diet and with a‐keto acid therapy in chronic renal failure [abstract no: PUB030]. Journal of the American Society of Nephrology 2003;14(Nov):779A. [Google Scholar]
Rosenberg 1987 {published data only}
- Rosenberg ME, Howe RB, Zanjani ED, Hostetter TH. The response of erythropoietin to dietary protein in human renal disease. Journal of Laboratory & Clinical Medicine 1989;113(6):735‐42. [MEDLINE: ] [PubMed] [Google Scholar]
- Rosenberg ME, Swanson JE, Thomas BL, Hostetter TH. Glomerular and hormonal responses to dietary protein intake in human renal disease. American Journal of Physiology 1987;253(6 (Pt 2)):F1083‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosenberg ME, Thomas BL, Swanson JE, Hostetter TH. Hormonal and glomerular responses to dietary protein intake in human renal disease [abstract]. Kidney International 1987;31(1):215. [DOI] [PubMed] [Google Scholar]
Sanchez 2010 {published data only}
- Sanchez C, Aranda P, Planells E, Galindo P, Perez de la Cruz A, Larrubia M, et al. Influence of low‐protein dietetic foods consumption on quality of life and levels of B vitamins and homocysteine in patients with chronic renal failure. Nutricion Hospitalaria 2010;25(2):238‐44. [MEDLINE: ] [PubMed] [Google Scholar]
Teplan 2003 {published data only}
- Teplan V, Schuck O, Knotek A, Hajny J, Horackova M. Ketoacids and recombinant human erythro‐poietin may influence progression of chronic renal insufficiency: Czech multicentre study [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A96. [Google Scholar]
- Teplan V, Schuck O, Knotek A, Hajny J, Horackova M, Kvapil M, et al. Enhanced metabolic effect of erythropoietin and keto acids in CRF patients on low‐protein diet: Czech multicenter study. American Journal of Kidney Diseases 2003;41(3 Suppl 1):S26‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teplan V, Schuck O, Knotek A, Hajny J, Surel S. Metabolic effect of erythropoietin and keto acids in CRF: Czech multicentre study [abstract]. Nutrition 2003;19(4):399. [DOI] [PubMed] [Google Scholar]
- Teplan V, Schuck O, Poledne R, Mengerova O. The influence of erythropoietin (r‐Hu EPO) and keto amino acids (KA) on lipid metabolism and renal function tests in chronic renal failure (CRF) [abstract no: A3094]. Journal of the American Society of Nephrology 1996;7(9):1865. [Google Scholar]
- Teplan V, Schuck O, et al. Erythropoietin (r‐Hu EPO) and keto amino acids (KA): an effect on lipid metabolism in predialysis [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A263. [Google Scholar]
Teplan 2006 {published data only}
- Teplan V, Mareckova O, Vyhnankova I, Valkovsky I, Lopatnik M, Racek J, et al. Asymmetric dimethylarginine and pentosidin in patients with chronic kidney disease and obesity: a randomized controlled trial [Asymetricky dimethylarginin a pentosidin u nemocnych s chronickym onemocnenim ledvin a obezitou: Randomizovana kontrolovana studie]. Aktuality v Nefrologii 2008;14(4):185‐90. [EMBASE: 2009017142] [Google Scholar]
- Teplan V, Schuck O, Hanzal V, Hajny J, Horackova M, Ryba M, et al. Obesity and progression of chronic renal insufficiency: a Czech long term prospective double‐blind randomised multicentre study [Obezita a progrese chronicke renalni insuficience: ceska dlouhodoba prospektivni randomizovana dvojite slepa multicentricka studie]. Vnitrni Lekarstvi 2006;52(6):571‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Teplan V, Schuck O, Racek J, Mareckova O, Stollova M, Hanzal V, et al. Reduction of plasma asymmetric dimethylarginine in obese patients with chronic kidney disease after three years of a low‐protein diet supplemented with keto‐amino acids: a randomized controlled trial. Wiener Klinische Wochenschrift 2008;120(15‐16):478‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ursea 2002 {published data only}
- Ursea N, Garneata L, Hildegard Stancu S. Effects of a hypoproteic diet supplemented with ketoanalogues in chronic renal failure [abstract no: T173]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):237. [Google Scholar]
Vujic 1987 {published data only}
- Vujic D, Djukanovic L. The effect of low protein diet on the progression of chronic renal failure [abstract]. 24th Annual Congress. EDTA‐ERA; 1987 Oct 25‐29; West Berlin, West Germany. 1987:69.
Zhang 2015 {published data only}
- Zhang MM, Zhao Y, Zhu YL. Effect of individualized low‐protein diet intervention on renal function of patients with chronic kidney disease. Chung‐Kuo i Hsueh Ko Hsueh Yuan Hsueh Pao Acta Academiae Medicinae Sinicae 2015;37(4):384‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2016b {published data only}
- Zhang J, Xie H, Fang M, Wang K, Chen J, Sun W, et al. Keto‐supplemented low protein diet: a valid therapeutic approach for patients with steroid‐resistant proteinuria during early‐stage chronic kidney disease. Journal of Nutrition, Health & Aging 2016;20(4):420‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01418508 {published data only}
- Li X, Chen L. Effects of low protein diet supplemented keto‐/amino acid in preventing the progression of chronic kidney disease(CKD)‐ ELPD study (ELPD‐CKD). www.clinicaltrials.gov/ct2/show/NCT01418508 (first received 15 August 2011).
Additional references
Bellizzi 2016
- Bellizzi V, Cupisti A, Locatelli F, Bolasco P, Brunori G, Cancarini G, et al. Low‐protein diets for chronic kidney disease patients: the Italian experience. BMC Nephrology 2016;17(1):77. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, BrozekJ, et al. GRADE Guidelines:1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2011b
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE Guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hostetter 1986
- Hostetter TH, Meyer TW, Rennke HG, Brenner BM. Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney International 1986;30(4):509‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 2006
- Johnson DW. Dietary protein restriction as a treatment for slowing chronic kidney disease progression: the case against. Nephrology 2006;11(1):58‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 2013
- Johnson DW, Atai E, Chan M, Poon RK, Scott C, Toussaint ND, et al. KHA‐CARI Guideline: Early chronic kidney disease: Detection, prevention and management. Nephrology 2013;18(5):340‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kalantar‐ Zadeh 2016
- Kalantar‐Zadeh K, Moore LW, Tortorici AR, Chou JA, St‐ Jules DE, Aoun A, et al. North American Experience with low protein diet for non dialysis dependent chronic kidney disease. BMC Nephrology 2016;17(1):90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalantar‐Zadeh 2017
- Kalantar‐Zadeh K, Fouque D. Nutritional management of chronic kidney disease. New England Journal of Medicine 2017;377(18):1765‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kasiske 1998
- Kasiske BL, Lakatua JD, Ma JZ, Louis TA. A meta‐analysis of the effects of dietary protein restriction on the rate of decline in renal function. American Journal of Kidney Diseases 1998;31(6):954‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2012
- Levin A, Stevens PE, Bilous RW, Coresh J, Francisco AL, Jong PE, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney international ‐ Supplement 2013;3(1):1‐150. [EMBASE: 369856107] [Google Scholar]
Mandayam 2006
- Mandayam S, Mitch WE. Dietary protein restriction benefits patients with chronic kidney disease. Nephrology 2006;11(1):53‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maroni 1985
- Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney International 1985;27(1):58‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matshushita 2010
- Chronic Kidney Disease Prognosis Consortium, Matshushita K, Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet 2010;375(9731):2073‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitch 2016
- Mitch WE, Remuzzi G. Diets for patients with chronic kidney disease, should we reconsider?. BMC Nephrology 2016;17(1):80. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedrini 1996
- Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta‐analysis. Annals of Internal Medicine 1996;124(7):627‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piccoli 2016
- Piccoli GB, Capizzi I, Vigotti FN, Leone F, D'Alessandro C, Giuffrida D, et al. Low protein diets in patients with chronic kidney disease: a bridge between mainstream and complementary‐alternative medicines?. BMC Nephrology 2016;17(1):76. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Wright 2011
- Wright M, Jones C. Renal Association clinical practice guideline on nutrition in CKD. Nephron 2011;118 Suppl 1:153‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fouque 2000a
- Fouque D, Wang P, Laville M, Boissel JP. Low protein diets delay end‐stage renal disease in non‐diabetic adults with chronic renal failure. Nephrology Dialysis Transplantation 2000;15(12):1986‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fouque 2000b
- Fouque D, Wang PH, Laville M, Boissel JP. Low protein diets for chronic renal failure in non diabetic adults. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD001892] [DOI] [PubMed] [Google Scholar]
Fouque 2006
- Fouque D, Laville M, Boissel J‐P. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD001892.pub2] [DOI] [PubMed] [Google Scholar]
Fouque 2009
- Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001892.pub3] [DOI] [PubMed] [Google Scholar]


