Abstract
Background
Acute kidney injury (AKI) is a common condition among patients in intensive care units (ICUs), and is associated with high death. Renal replacement therapy (RRT) is a blood purification technique used to treat the most severe forms of AKI. The optimal time to initiate RRT so as to improve clinical outcomes remains uncertain.
This review complements another Cochrane review by the same authors: Intensity of continuous renal replacement therapy for acute kidney injury.
Objectives
To assess the effects of different timing (early and standard) of RRT initiation on death and recovery of kidney function in critically ill patients with AKI.
Search methods
We searched the Cochrane Kidney and Transplant’s Specialised Register to 23 August 2018 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov. We also searched LILACS to 11 September 2017.
Selection criteria
We included all randomised controlled trials (RCTs). We included all patients with AKI in ICU regardless of age, comparing early versus standard RRT initiation. For safety and cost outcomes we planned to include cohort studies and non‐RCTs.
Data collection and analysis
Data were extracted independently by two authors. The random‐effects model was used and results were reported as risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CI).
Main results
We included five studies enrolling 1084 participants. Overall, most domains were assessed as being at low or unclear risk of bias. Compared to standard treatment, early initiation may reduce the risk of death at day 30, although the 95% CI does not rule out an increased risk (5 studies, 1084 participants: RR 0.83, 95% CI 0.61 to 1.13; I2 = 52%; low certainty evidence); and probably reduces the death after 30 days post randomisation (4 studies, 1056 participants: RR 0.92, 95% CI 0.76 to 1.10; I2= 29%; moderate certainty evidence); however in both results the CIs included a reduction and an increase of death. Earlier start may reduce the risk of death or non‐recovery kidney function (5 studies, 1076 participants: RR 0.83, 95% CI 0.66 to 1.05; I2= 54%; low certainty evidence). Early strategy may increase the number of patients who were free of RRT after RRT discontinuation (5 studies, 1084 participants: RR 1.13, 95% CI 0.91 to 1.40; I2= 58%; low certainty evidence) and probably slightly increases the recovery of kidney function among survivors who discontinued RRT after day 30 (5 studies, 572 participants: RR 1.03, 95% CI 1.00 to 1.06; I2= 0%; moderate certainty evidence) compared to standard; however the lower limit of CI includes the null effect. Early RRT initiation increased the number of patients who experienced adverse events (4 studies, 899 participants: RR 1.10, 95% CI 1.03 to 1.16; I2 = 0%; high certainty evidence). Compared to standard, earlier RRT start may reduce the number of days in ICU (4 studies, 1056 participants: MD ‐1.78 days, 95% CI ‐3.70 to 0.13; I2 = 90%; low certainty evidence), but the CI included benefit and harm.
Authors' conclusions
Based mainly on low quality of evidence identified, early RRT may reduce the risk of death and may improve the recovery of kidney function in critically patients with AKI, however the 95% CI indicates that early RRT might worsen these outcomes. There was an increased risk of adverse events with early RRT. Further adequate‐powered RCTs using appropriate criteria to define the optimal time of RRT are needed to reduce the imprecision of the results.
Timing of initiation of renal replacement therapy for acute kidney injury
What is the issue?
Acute kidney injury (AKI) is very common among patients admitted to intensive care unit (ICU); it is associated with high death rates and characterised by the rapid loss of the kidney function. Patients with AKI show increased levels of serum uraemic toxins (creatinine and urea), serum potassium and metabolic acids, accumulation of water and in the most cases a reduction in urine output. In this population these chemicals and fluid overload are related to increased rates of death. Theoretically, early removal of toxins and excess water from the bloodstream might improve patient outcomes (such as death rate and recovery of kidney function).
Renal replacement therapy (RRT) is a blood purification technique that enables removal of excess water and toxins. RRT involves blood being diverted from the patient via a catheter (a hollow, flexible tube placed into a vein) through a filtering system which removes excess water and toxins; purified blood is then returned to the patient via the catheter. Early initiation of RRT improves the removal of toxins and excess water. The aim of this review was to investigate the effect of different timing of RRT initiation (early or standard) on death, recovery of kidney function, and adverse events in people with AKI who are critically ill.
What did we do?
We searched the literature up until August 2018 and identified five studies enrolling 1084 critically ill patients with AKI that were evaluated in this review.
What did we find?
Five randomised studies enrolling 1084 participants were included in our review. Compared to standard, early initiation of RRT may reduce the risk of death, may increase the recovery of kidney function, or increase the risk of adverse events in patients with AKI in intensive care units. Nevertheless, in death the early initiation of RRT showed a range of values that included benefit (decrease death), as well as harm (increase death).
Summary of findings
Summary of findings for the main comparison.
Early versus standard renal replacement therapy (RRT) initiation for acute kidney injury (AKI)
| Early versus standard RRT initiation for AKI | |||||
| Patient or population: patients with AKI Setting: ICU Intervention: early initiation of RRT Comparison: standard RRT | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with standard initiation | Risk with early initiation | ||||
| Death at day 30 | 414 per 1.000 | 344 per 1.000 (253 to 468) | RR 0.83 (0.61 to 1.13) | 1084 (5) | ⊕⊕⊝⊝ LOW 1, 2 |
| Death after 30 days | 487 per 1.000 | 448 per 1.000 (370 to 536) | RR 0.92 (0.76 to 1.10) | 1056 (4) | ⊕⊕⊕⊝ MODERATE 2 |
| Death or non recovery kidney function at day 90 | 541 per 1.000 | 449 per 1.000 (357 to 568) | RR 0.83 (0.66 to 1.05) | 1078 (5) | ⊕⊕⊝⊝ LOW 1, 2 |
| Patients free of RRT after discontinuing RRT (all patients) Follow‐up: 90 days | 469 per 1.000 | 530 per 1.000 (427 to 656) | RR 1.13 (0.91 to 1.40) | 1084 (5) | ⊕⊕⊝⊝ LOW 1, 2 |
| Number of patients with any adverse events Follow‐up: 90 days | 657 per 1.000 | 723 per 1.000 (683 to 769) | RR 1.10 (1.04 to 1.17) | 437 (3) | ⊕⊕⊕⊕ HIGH |
| ICU length of stay Follow‐up: 30 days | The mean length of stay was 14.6 days | MD 1.78 days lower (3.7 lower to 0.13 higher) | ‐ | 1056 (4) | ⊕⊕⊝⊝ LOW 1, 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; ICU: intensive care unit/s | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Serious inconsistency: due to moderate heterogeneity
2 Serious Imprecision: due to the CI crossed the threshold for clinically meaningful effects
Summary of findings 2.
Early initiation versus standard renal replacement therapy (RRT) for acute kidney injury (AKI): subgroup analysis
| Subgroup analysis: early initiation according to clinical‐biochemical parameters versus time criteria | |||||
| Patient or population: patients with AKI who need RRT Setting: ICU Intervention: early initiation Comparison: standard initiation of RRT | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with standard initiation | Risk with early initiation | ||||
| Death: early initiation according to clinical‐biochemical criteria Follow‐up: 30 days | 857 per 1.000 | 146 per 1.000 (43 to 523) | RR 0.17 (0.05 to 0.61) | 28 (1) | ⊕⊕⊝⊝ LOW 1,2 |
| Death: early initiation according to time criteria Follow‐up: 30 days | 407 per 1.000 | 371 per 1.000 (318 to 432) | RR 0.91 (0.78 to 1.06) | 1049 (4) | ⊕⊕⊕⊝ MODERATE 3 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Risk of bias: due to an incomplete outcome data (attrition bias) limiting the internal validity of the study
2 Indirectness: the population included is restricted to people who only have surgery‐acquired AKI
3 Imprecision: due to wide CI which crossed the threshold for clinically meaningful effects
Background
This review complements another Cochrane systematic review by the same authors: Intensity of continuous renal replacement therapy for acute kidney injury (Fayad 2016).
Description of the condition
Acute kidney injury (AKI) is a complex clinical entity characterised by an abrupt decline in kidney function (Mehta 2007). AKI incidence among adults admitted to intensive care units (ICUs) ranges from 5% to 20% (Joannidis 2005); in children the incidence is 10% (Schneider 2010). Despite its potential to be reversed, AKI is associated with high rates of morbidity and death (Bagshaw 2007). Renal replacement therapy (RRT) has become a form of renal support for critically ill patients with AKI (Wald 2015). Despite advances in clinical care and RRT the presence of AKI in ICU setting is associated with poor prognosis, and requires significant healthcare resources (Sutherland 2010; Uchino 2005).
Description of the intervention
RRT is an extracorporeal blood purification therapy, intended to support impaired kidney function. We included the following RRT modalities: Continuous RRT (CRRT) slowly removes fluid (Foland 2004;Gibney 2008; Goldstein 2001) and higher molecular weight solutes efficiently over prolonged periods (Brunnet 1999;Clark 1999;Liao 2003; Sieberth 1995), and confers beneficial haemodynamic stability effects. CRRT modalities are defined by their main solute clearance mechanism. These are convection (continuous venovenous haemofiltration (CVVHF)), diffusion (continuous venovenous haemodialysis (CVVHD)), or a combination of both convection and diffusion (continuous venovenous haemodiafiltration, CVVHDF)) (Palevsky 2002).The intermittent RRT (IRRT) removes fluid and lower molecular weight solutes over short period of time (sessions of 3 to 5 hours), two or three times in a week. The diffusion is the main solute clearance mechanism. These are intermittent haemodialysis (IHD), intermittent haemofiltration (IHF), intermittent haemodiafiltration (IHDF), and intermittent high‐flux dialysis (IHFD). The hybrid therapies, also known as prolonged IRRTs, such as sustained low‐efficiency dialysis (SLED) and extended‐duration dialysis (EDD); that provides RRT for an extended period of time (6 to 18 hours), at least three times per week (Edrees 2016); includes both convective (i.e. haemofiltration) and diffusive (i.e. haemodialysis) therapies, depending on the method of solute removal (Marshall 2011). Peritoneal dialysis modality was not included.
Timing of RRT initiation is generally related to "when to start renal support in critical ill patients with AKI". A number of organisations have published practice guidelines that include statements on timing of RRT initiation in ICU settings. The Kidney Disease Improving Global outcomes (KDIGO 2012), the National Institute for Health and Care Excellence (NICE 2013) and the French Intensive Care Society (Vinsonneau 2015) have published practice guidelines that include statements on timing of RRT initiation in ICU settings. There has been consensus on the standard initiation criteria: when life‐threatening changes in fluid, electrolyte, and acid‐based balance exist according to different guidelines; however none of the recommendations have been graded. Unfortunately, there has been little consensus on early begin of RRT in ICU‐patients with AKI. Some published studies have used urine output and serum creatinine (Sugahara 2004) or urine output and clearance of creatinine (Bouman 2002), as surrogate criteria of early initiation. Other authors have considered time to ICU admission (Bagshaw 2009), time to fulfilling AKI stage 2 within 8 hr (ELAIN 2016) or within 12 hr using a novel kidney damage biomarker neutrophil gelatinase‐associated lipocalin (NGAL) (STARRT‐AKI 2013), and time to fulfilling AKI stage 3 (AKIKI 2015). With poor agreement (expert opinion), NICE 2013 and Vinsonneau 2015 also published possible indicators for early renal support therapy e.g. weight "gain less than 10%, urea less than 25 mmol/litre and oliguria 0.5 ml/kg/hour or less for at least 24 hours" or "KDIGO AKI stage 2 or within 24 hr after onset of AKI of which reversibility seems unlikely, respectively". In our review, we will assign definitions given in included studies in relation to early and standard RRT initiation.
How the intervention might work
A hypothesis that timing of RRT commencement may affect survival emerged from animal and human studies over the past decade. Animal studies investigating sepsis (Mink 1995) and pancreatitis (Yekebas 2002) suggested beneficial effects on physiologic and clinical endpoints when haemofiltration was started early, simultaneously, or two hours after injury. Several observational studies investigated the effect of timing in patients with AKI; Teschan 1960 reported improved survival rates relating to RRT timing in patients commencing dialysis with low blood urea nitrogen; Gettings 1999 indicated improved survival in early haemofiltration patients with AKI related to trauma, the same was found in patients with AKI post cardiac surgery (Demirkilic 2004; Elahi 2004). Randomised controlled trials (RCTs) found in patients with pancreatitis that the survival was also significantly better in patients who received early haemofiltration (within 48 hours after onset of abdominal pain) than in the group with late haemofiltration (96 hours after onset abdominal pain) (Jiang 2005), while other RCTs failed to demonstrate these advantages (AKIKI 2015; Bouman 2002; STARRT‐AKI 2013).
Why it is important to do this review
Studies assessing RRT timing (early versus standard) have reported inconsistent results: earlier studies indicated significant improvements in survival and kidney function recovery; yet others, including RCTs and meta‐analyses, did not find these benefits. We investigated the relationship between different timing of RRT initiation and clinical outcomes for critical patients with AKI. Review evidence could have direct relevance to guide clinical practice.
Objectives
To assess the effects of different timing (early and standard) of RRT initiation on death and recovery of kidney function in critically ill patients with AKI.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs looking at RRT modalities for people with AKI in ICU settings were eligible for inclusion. For outcomes such as safety and costs, non‐RCTs and cohort studies were also to be included if sufficiently high quality, sampling was clearly described, patients characterised, proportions of patients experiencing any adverse events or who dropped out because of adverse events was adequately reported, co‐interventions were described, and at least 80% of patients included were analysed after treatment.
Types of participants
Inclusion criteria
We included all patients with AKI in ICU being treated with RRT regardless of age and gender. We assigned AKI definitions cited by the included studies.
Exclusion criteria
We excluded patients who received dialysis treatment before admission to ICU, patients admitted for drug overdose (doses exceeding therapeutic requirements), or with acute poisoning (all toxins).
Types of interventions
We compared early (intervention group) versus standard (control) initiation in CRRT and IRRT. We excluded peritoneal dialysis modality. The criteria of time were defined as published in the original publications.
Types of outcome measures
Primary outcomes
Death
Death from any cause at days 7, 15, 30, 60 and 90
Death or non‐recovery kidney function at day 90.
Recovery of kidney function
Numbers of patients free of RRT after discontinuing RRT
Numbers of patients free of RRT after discontinuing RRT at days 30, 60 and 90.
Secondary outcomes
Adverse events
Numbers of patients experiencing any adverse events
Numbers of patients who dropped out because of adverse events (technique or patient‐dependent factors)
Numbers of patients with intervention‐related complications (e.g. disequilibrium, hypokalaemia, hypophosphataemia, hypocalcaemia, bleeding, hypotension)
Numbers of patients with catheter‐related complications.
We looked for differences in overall drop‐out rates and any adverse effects by type (mild or severe). We defined adverse events severity where medical therapeutic interventions were implied in reporting. Withdrawals due to protocol violation or loss to follow‐up were not included in counts of adverse events.
Length of stay
Days in hospital
Days in ICU.
Cost
We planned to assess costs of RRT modalities including:
Type and number of dialyser filters
Use or no use of anticoagulation
Types of anticoagulation and anticoagulants
Use of replacement fluid
Number of days on RRT.
All costs were to be reported in international monetary units.
Cost per day of RRT
Length of hospital stay with RRT
Length of ICU stay with RRT.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 23 August 2018 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources. .
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP). Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register were identified through search strategies for CENTRAL, MEDLINE and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference, proceedings and current awareness alert, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms and strategies used for this review.
Searching other resources
LILACS (Latin American and Caribbean Health Sciences) (from March 1980 to September 2017)
Reference lists of review articles, relevant studies and clinical practice guidelines
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies with potential relevance to the review. Titles and abstracts were screened independently by two authors who discarded studies that were not applicable; however, studies and reviews that could include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and if necessary, the full text of these studies to determine which satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐ English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data was used. We resolved any discrepancy by discussion.
Assessment of risk of bias in included studies
The following items were independently assessed using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For normally distributed outcomes, we calculated summary estimates of treatment effects using the inverse variance method. For dichotomous outcomes (death, kidney recovery and adverse events) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (length of stay, cost) the mean difference (MD) was used or the standardised mean difference (SMD) if different scales were used. The results were interpreted taking into account the size of the effect (magnitude or importance) (see CKT 2017; EPOC 2013).
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing to corresponding author) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (e.g. last‐observation‐ carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
If possible, funnel plots were to be used to assess the potential existence of small study bias (Higgins 2011).
Data synthesis
Data were to be pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity (such as intervention, parameters to define early or standard initiation, participant and study quality). Heterogeneity among participants could relate to age, gender, fluid overload (< 10% and > 10% in body weight relative to baseline), and timing of RRT for AKI in homogenous subpopulations such as cardiac surgery or sepsis patients, effects of early initiation on severity of illness. We used appropriate scores of illness severity, such as Pediatric Risk of Mortality (PRISM), Pediatric Index of Mortality (PIM), Acute Physiology and Chronic Health Evaluation (Apache), Sequential Organ Failure Assessment (SOFA), and Cleveland Clinic ICU Acute Renal Failure (CCF). Adverse effects were tabulated and assessed using descriptive techniques. Where possible, the risk difference with 95% CI was calculated for each adverse effect, either compared with no treatment or another agent.
Sensitivity analysis
We performed sensitivity analyses to explore the influence of the following factors on effect size:
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). One summary of findings tables were created. Summary of findings table 1 summarizes the main findings for the comparison "Early versus standard initiation of RRT for acute kidney injury". We presented the following outcomes.
Death until day 30 post‐randomisation
Death after 30 days post‐randomisation
Kidney function recovery: number of patients free of RRT after discontinuing RRT (all patients)
Number of patients with adverse events
Subgroup analysis: death in patients who start RRT according to KDIGO AKI stage criteria and in patients who start RRT according to physiological (e.g. urine output) and or biochemical criteria (e.g., urea, serum creatinine).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies
Results of the search
We identified 208 reports from electronic databases (MEDLINE, EMBASE, CENTRAL, Cochrane Kidney and Transplant’s Specialised Register and LILACS) to 23 August 2018 and two records from handsearching. Of these, 5 studies (10 records) (AKIKI 2015; Bouman 2002; ELAIN 2016; STARRT‐AKI 2013; Sugahara 2004) were included and 84 studies (198 records) were excluded. One ongoing study was identified (NCT00837057), and one study has been completed but there are currently no publications (IDEAL‐ICU 2014). These studies and will be assessed in a future update of this review (Figure 1). There was no disagreement among authors regarding inclusion of studies.
Figure 1.

Flow chart showing number of reports retrieved by database searching and the number of studies included in this review
Included studies
Five included studies (AKIKI 2015; Bouman 2002; ELAIN 2016; STARRT‐AKI 2013; Sugahara 2004) enrolled a total of 1084 participants.
Study participants were all admitted to ICU. The mean age was between 62.8 and 64.5 years, and the proportion of males ranged from 59.6% to 79%. Surgery or cardio‐surgery were the primary causes of AKI in three studies (Bouman 2002; ELAIN 2016; Sugahara 2004) and mixed (medical or surgical) in the other two studies (AKIKI 2015; STARRT‐AKI 2013).
All studies were reported between 2002 and 2016. Two were single‐centre studies (ELAIN 2016; Sugahara 2004) and three were multicentre (AKIKI 2015; Bouman 2002; STARRT‐AKI 2013).
Three studies used CRRT (Bouman 2002; ELAIN 2016; Sugahara 2004) and two used combined therapy (intermittent and continuous) (AKIKI 2015; STARRT‐AKI 2013).
All the included studies assessed the effects of timing (early and standard) of RRT initiation on clinical outcomes of critical patients with AKI. In Bouman 2002, two of three arms received the same timing of RRT initiation (early) but differed only in the intensities of continuous therapy. For the purpose of the analysis we combined these two early treatment arms to create one early arm.
Sugahara 2004 did not report the treatment allocation of 8/36 participants that did not start the treatment. We assumed that they were evenly distributed among treatment arms (18 participants per arm). Similarly, we assumed that these 8 participants had a favourable evolution (none of them died and all of them recovered).
The included studies used a wide spectrum of definitions for early and standard initiation of RRT. Bouman 2002 and Sugahara 2004 defined early RRT initiation based on physiologic (urine output) and biochemical parameters (creatinine clearance/serum creatinine, respectively). The other three studies defined early as starting of RRT by time criteria e.g. within 8 and 12 hours of fulfilling KDIGO stage 2 (ELAIN 2016; STARRT‐AKI 2013) or within 6 hours of fulfilling KDIGO stage 3 (AKIKI 2015).
For full details see Characteristics of included studies.
Excluded studies
We excluded 84 studies (198 records). Two of 22 reports in RENAL 2006 assessed timing however the study design was not randomised (retrospective nested cohort). Seven studies did not mandate the presence of AKI (Durmaz 2003; HEROICS 2015; Han 2015; Koo 2006; Payen 2009) or ICU stay as an inclusion criteria in the early initiation arm (Jamale 2013; Pursnani 1997) and two studies did not assess the outcomes of interest to this review (Cole 2002; Misset 1996). The remaining 74 studies did not assessing timing of RRT (See Characteristics of excluded studies).
Risk of bias in included studies
Included studies were generally assessed at low or unclear risk of bias for most domains; one study was assessed as high risk for incomplete outcome data (Sugahara 2004). Risk of bias assessments of the included studies are summarised in Figure 2 and Figure 3.
Figure 2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Bouman 2002 did not provide detailed information on random sequence generation and allocation concealment processes. Authors were contacted and we were informed that random sequence generation was appropriate (computer‐generated) and sealed opaque envelopes were used for the allocation process. Three studies (AKIKI 2015; ELAIN 2016; STARRT‐AKI 2013) were assessed as being at low risk of selection bias due to appropriate random sequence generation (computer‐generated) and for allocation concealment process. The random sequence generation and allocation concealment processes were considered unclear in Sugahara 2004 as it provided insufficient information to enable judgment.
Blinding
All included studies were assessed at low risk of detection bias (outcome measurement was unlikely to be influenced by lack of blinding), and unclear risk of performance bias (insufficient information to enable judgment).
Incomplete outcome data
One study (Sugahara 2004) was assessed at high risk of attrition (data from > 20% of randomised patients were not available for inclusion in the analysis). Intention‐to‐treat analysis was performed in the other four studies.
Selective reporting
All five studies reported all expected outcomes and were considered to be at low risk of bias (AKIKI 2015; Bouman 2002;ELAIN 2016; STARRT‐AKI 2013; Sugahara 2004).
Other potential sources of bias
Of the five included studies, 3 received pharmaceutical industry funding (AKIKI 2015; ELAIN 2016; STARRT‐AKI 2013), which is a potential source of bias; however the sponsors had no role in the design, data collection, analysis and results, review or approval of the manuscript (low risk of bias). These data were not available in the remaining two studies (Bouman 2002;Sugahara 2004) (unclear risk of bias).
Evaluation of publication bias
We constructed a funnel plot to investigate potential publication bias. Meta‐analysis of death at day 30 was analysed. We found reasonable symmetry indicating a low risk of publication bias (Figure 4).
Figure 4.
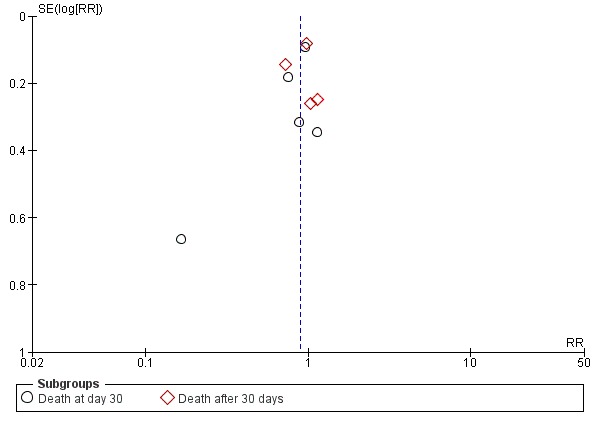
Funnel plot of comparison: 1 Early vs. late initiation, outcome: 1.1 Death.
Effects of interventions
The effects of early RRT initiation versus standard for main results and the quality of the evidence are summarised in Table 1.
Primary outcomes
Death
All five studies assessed the effect of different timing of RRT initiation on death. These studies varied in reporting timing: 90 days (ELAIN 2016; STARRT‐AKI 2013); 60 days (AKIKI 2015); 28 days after randomisation (Bouman 2002); and 14 days after coronary bypass graft surgery (Sugahara 2004).
Compared to standard, early initiation of RRT may reduce the risk of death at day 30 (Analysis 1.1.1 (5 studies, 1084 participants): RR 0.83, 95% CI 0.61 to 1.13; low certainty evidence). There was moderate heterogeneity (I2 = 52%). We assessed the certainty of evidence as low due to concerns about imprecision and heterogeneity. However, early start probably reduces the risk of death after 30 days post‐randomisation in comparison with standard initiation (Analysis 1.1.2 (4 studies, 1056 participants): RR 0.92, 95% CI 0.76 to 1.10; I2= 29%; moderate certainty evidence). We assessed the certainty of evidence as moderate due to concerns about imprecision. Nevertheless, the CIs of both outcomes included a range of plausible values with clinically important benefits as well as harms (Table 1).
Analysis 1.1.

Comparison 1 Early versus standard initiation, Outcome 1 Death.
Subgroup analysis and investigation of heterogeneity
There was evidence of moderate heterogeneity in the magnitude of the effect among the included studies that measured death at different times of randomisation. To explore heterogeneity among participants, we planned to performed pre‐specified subgroup analyses. Only data for AKI aetiology, parameters for early initiation and modalities of RRT were available.
The effect of AKI aetiology was considered using two subgroups: patients with AKI secondary to surgical causes and patients with AKI related to non‐surgical causes. Compared to standard, early RRT initiation probably reduces the risk of death in patients with non‐surgical AKI (Analysis 2.1.1 (2 studies, 719 participants): RR 0.95, 95% CI 0.79 to 1.13; I2= 0%; moderate certainty evidence), while it may reduce the risk of death in patients with AKI related to surgical causes (Analysis 2.1.2 (3 studies, 373 participants): RR 0.66, 95% CI 0.32 to 1.36; I2= 69%; low certainty evidence). There was no heterogeneity between groups (Test for subgroup differences: Chi2 = 0.90, df = 1; P = 0.34, I2 = 0%).
Analysis 2.1.

Comparison 2 Subgroup analysis: death, Outcome 1 Death by AKI aetiology.
The effect of different parameters used to define RRT initiation was assessed using two subgroups: patients starting RRT according to time to fulfilled KDIGO AKI stage 2 and 3 and patients initiating RRT according to physiological (urine output) and or biochemical parameters. Compared to standard RRT, early strategy probably reduces the risk of death in patients initiating RRT according to physiological or biochemical parameters (Analysis 2.2.1 (1 studies, 28 participants): RR 0.17; 95% CI 0.05 to 0.61; P = 0.007; low certainty evidence) and in patients starting RRT according to time criteria (Analysis 2.2.2 (4 studies, 1049 participants): RR 0.91, 95% CI 0.78 to 1.06; I2 = 0%; moderate certainty evidence). The heterogeneity observed in subgroup analyses could be explained by different criteria used for RRT initiation (Test for subgroup differences: Chi2 = 6.41; P = 0.01; I2 = 84.4%).
Analysis 2.2.

Comparison 2 Subgroup analysis: death, Outcome 2 Death by definition of early RRT initiation.
The effect of modalities of RRT was considered using two subgroups: patients with continuous renal support and patients who received mixed modalities (continuous and intermittent). Compared to standard, early RRT initiation may reduce the risk of death in patients with CRRT (Analysis 2.3.1 (3 studies, 365 participants): RR 0.65, 95% CI 0.31to 1.36); I2= 70%; low certainty evidence), while it probably slightly reduces the risk of death in patients treated with mixed modalities ((Analysis 2.3.2 (2 studies, 719 participants): RR 0.95, 95% CI 0.79 to 1.13; I2= 0%; moderate certainty evidence). Without heterogeneity between groups (test for subgroup differences: Chi2 = 0.95, df = 1; P = 0.33; I2= 0%).
Analysis 2.3.

Comparison 2 Subgroup analysis: death, Outcome 3 Death by RRT modality.
Sensitivity analysis
The sensitivity analysis was performed excluding studies by risk of bias and large studies. When the analysis was developed taking risk of bias into account we observed that Sugahara 2004 contributed to heterogeneity, and when excluded, heterogeneity was not significant (P = 0.29; I2= 20%). The reason for exclusion was incomplete outcome data (attrition bias), but the overall estimation of effect did not change, and the direction of effects remained constant. We found no changes in heterogeneity when the study with larger sample size was excluded.
Death or non‐recovery of kidney function at 90 days
This composite outcome was available from all five studies (AKIKI 2015; Bouman 2002; ELAIN 2016; STARRT‐AKI 2013; Sugahara 2004). Compared with standard initiation, early initiation may reduce the risk of death or non‐recovery kidney function (Analysis 1.2 (5 studies, 1078 participants): RR 0.83, 95% CI 0.66 to 1.05; I2= 54%; low certainty evidence). However, the CI included both clinical benefits and harms. We assessed the certainty of evidence as low due to concerns about imprecision and heterogeneity (Table 1).
Analysis 1.2.

Comparison 1 Early versus standard initiation, Outcome 2 Death or non‐recovery kidney function at day 90.
Subgroup analysis and investigation of heterogeneity
Data was not available in any of the included studies.
Sensitivity analysis
The sensitivity analysis was performed excluding studies by risk of bias and large studies. When the analysis was developed taking risk of bias into account we observed that Sugahara 2004 contributed to heterogeneity, and when excluded, heterogeneity was not significant (P = 0.53; I2= 0%). The reason for exclusion was study limitation (attrition bias), but the overall estimation of effect did not change, and the direction of effects remained constant. We found no changes in heterogeneity when the study with larger sample size was excluded.
Recovery of kidney function
Five studies reported information on recovery of kidney function (in all patients and among patients’ survivors). Studies varied in reporting of kidney recovery timing: at 90 days after randomisation (AKIKI 2015; ELAIN 2016; STARRT‐AKI 2013), 28 days or at hospital discharge (Bouman 2002), or 14 days after coronary bypass graft surgery (Sugahara 2004).
Compared to standard, early RRT initiation may increase the numbers of patients who were free of RRT after RRT discontinuation (Analysis 1.3.1 (5 studies, 1084 participants): RR 1.13, 95% CI 0.91 to 1.40; I2= 58%; low certainty evidence). There was moderate heterogeneity (I2= 58%). We assessed the certainty of evidence as low due to concerns about imprecision and heterogeneity.
Analysis 1.3.

Comparison 1 Early versus standard initiation, Outcome 3 Recovery of kidney function.
Among survivors who discontinued RRT after day 30, early initiation of RRT probably slightly increases the recovery of kidney function compared to standard (Analysis 1.3.2 (5 studies, 572 participants): RR 1.03, 95% CI 1.00 to 1.06; I2= 0%; moderate certainty evidence). We assessed the certainty of evidence as moderate due to concerns about indirectness (Table 1).
Subgroup analysis and investigation of heterogeneity
There was evidence of heterogeneity in the magnitude of the effect among the included studies that measured recovery of kidney function in all patients at different times after randomisation. To explored heterogeneity among participants we planned to performed pre‐specified subgroup analyses. Only data for AKI aetiology, parameters of early initiation and modalities of RRT were available.
The effect of AKI aetiology was considered using two subgroups: patients with AKI predominantly related to surgical causes and patients with AKI related to non‐surgical causes. Compared to standard, early RRT initiation may increase the recovery of kidney function in patients with AKI related to surgical causes (Analysis 3.1.1 (3 studies, 365 participants): RR 1.31, 95% CI 0.78 to 2.18; I2= 74%; low certainty evidence), while it probably slightly increases the recovery of kidney function in patients with non‐surgical AKI (Analysis 3.1.2 (2 studies, 719 participants): RR 1.04, 95% CI 0.90 to 1.20; I2= 0%; moderate certainty evidence). There was no heterogeneity between groups (test for subgroup differences: Chi2 = 0.71, df = 1; P = 0.40; I2= 0%).
Analysis 3.1.

Comparison 3 Subgroup analysis: recovery of kidney function, Outcome 1 Recovery of kidney function by AKI aetiology.
The effect of different parameters used to define early RRT initiation was assessed by two subgroups: patients who started RRT according to time criteria (time to fulfilled KDIGO AKI stage 2 and 3) and patients initiating RRT according to physiological (urine output) and or biochemical parameters. Compared to standard, early initiation RRT probably improves recovery of kidney function in patients starting RRT according to time criteria (Analysis 3.2.1 (4 studies, 1050 participants): RR 1.10, 95% CI 0.97 to 1.24; I2 = 7%; moderate certainty evidence), but had uncertain effect on kidney function recovery in patients initiating RRT according to physiological and or biochemical parameters (Analysis 3.2.2 (1 study, 28 participants): RR 5; 95% CI 1.33 to 18.8; very low certainty evidence). The heterogeneity between groups could be explained by different time criteria of RRT initiation (test for subgroup differences: Chi2 = 4.98, df = 1; P = 0.03; I2= 79.9%).
Analysis 3.2.

Comparison 3 Subgroup analysis: recovery of kidney function, Outcome 2 Recovery of kidney function by definition of early RRT Initiation.
The effect of modality of RRT was considered using two subgroups: patients with CRRT and patients who received mixed modalities (continuous and intermittent). Compared to standard, early RRT initiation may increase the recovery of kidney function in patients with CRRT (Analysis 3.3.1 (3 studies, 365 participants): RR 1.36, 95% CI 0.79 to 2.34; I2= 76%; low certainty evidence), and probably slightly improves kidney function in patients treated with mixed modalities (Analysis 3.3.2 (2 studies, 719 participants): RR 1.04, 95% CI 0.90 to 1.20; I2= 0%; moderate certainty evidence). There was no heterogeneity between groups (test for subgroup differences: Chi2 = 0.89, df = 1; P = 0.35; I2= 0%).
Analysis 3.3.

Comparison 3 Subgroup analysis: recovery of kidney function, Outcome 3 Recovery of kidney function by RRT modality.
Sensitivity analysis
The sensitivity analysis was performed excluding studies at high risk of bias and large studies. When the analysis was developed taking risk of bias into account we observed that Sugahara 2004 contributed to heterogeneity, and when excluded, heterogeneity was not significant (P = 0.24; I2= 29%). The reason for exclusion was study limitation (attrition bias), but the overall estimation of effect did not change, and the direction of effects remained constant. We found no changes in heterogeneity when the study with larger sample size was excluded.
Adverse events
The effects of timing of RRT initiation on adverse events were reported in four studies (AKIKI 2015; Bouman 2002; ELAIN 2016; STARRT‐AKI 2013).
Early initiation of RRT increase the number of patients who experienced adverse events compared to standard (Analysis 1.4.1 (4 studies, 899 participants): RR 1.10, 95% CI 1.03 to 1.16; I2 = 0%; high certainty evidence). However, it is uncertain whether early initiation of RRT increases or reduces the risk of bleeding (Analysis 1.4.2 (1 study, 106 participants): RR 1.71, 95% CI 0.50 to 5.84; very low certainty evidence) or the occurrence of thrombocytopenia (Analysis 1.4.3 (1 study, 106 participants): RR 1.03, 95% CI 0.20 to 5.35; very low certainty evidence) compared with standard initiation. We assessed the certainty of evidence as very low due to concerns about very serious imprecision and indirectness (Table 1)
Analysis 1.4.

Comparison 1 Early versus standard initiation, Outcome 4 Adverse events.
Length of stay
Four studies assessed the effect of timing on length of stay (AKIKI 2015; Bouman 2002; ELAIN 2016; STARRT‐AKI 2013).
Early initiation of RRT may reduce the number of days in ICU (Analysis 1.5.1 (4 studies, 1056 participants): MD ‐1.78 days, 95% CI ‐3.70 to 0.13; I2 = 90%; low certainty evidence) compared to standard. There was substantial heterogeneity among studies. We assessed the certainty of evidence as low due to concerns with imprecision and heterogeneity.
Analysis 1.5.

Comparison 1 Early versus standard initiation, Outcome 5 Length of stay.
Data on hospital length of stay (mean, SD) was not available in any of the included studies.
Subgroup analysis and investigation of heterogeneity
There was evidence of high heterogeneity in the magnitude of the effect among the included studies that measured length of stay. To assess heterogeneity among participants, we performed sensitivity analyses to explore the above listed factors on the effect size. However, no data were available to conduct any of these subgroups analyses.
Sensitivity analysis
Sensitivity analysis was performed excluding studies with high risk of bias and those with large sample sizes. When the analysis was developed taking studies with larger sample size into account, we observed that AKIKI 2015 contributed to heterogeneity. When excluded, heterogeneity was not significant (P = 0.62; I2 = 0%). The reason for exclusion was indirectness. The effect of early initiation did not change.
Cost
This outcome was not reported by any of the included studies.
Discussion
Summary of main results
Our systematic review and subsequent meta‐analysis examined the effect of different timing of initiation of RRT on death, kidney recovery function, length of stay, and adverse events among 1084 critically ill patients with AKI. Most of the included studies were assessed as low or unclear risk of bias for all domains. One study was at high risk bias, by incomplete outcome data (attrition bias).
Within the time of RRT initiation assessed, earlier start may have a beneficial effect on death at different time points post‐randomisation (day 30 or after 30 days) and kidney recovery function (in all patients) compared to standard initiation. The overall estimated effects on risk of death showed clinically important benefits (decreased death by 17% and 8%); but the CIs were sufficiently wide to include benefits and harm (imprecision), with moderate and low (I2= 51% and I2= 29%) levels of heterogeneity, respectively (inconsistency).
Earlier initiation may increase the number of patients who recovered kidney function. The magnitude of the possible benefit was clinically relevant (increased by 13%); however, CIs also included damage (imprecision), with a moderate level of heterogeneity (I2= 58%; inconsistency). In contrast, there was little or no difference to kidney recovery among survivors between treatments. However, reporting kidney recovery among survivors alone does not preserve the previously achieved randomisation. Therefore, the interpretation of this results may be misleading given death is a competing endpoint for recovery of kidney function in patients with high short‐term risk of death (indirectness).
Earlier initiation increased the number of patients who experienced adverse events compared to standard strategy (increased risk by 10%), CIs included harm between 3% to 16%, although it had uncertain effects on other adverse events (bleeding or thrombocytopenia) when compared to standard initiation.
In relation to length of stay, early initiation may reduce the number of days in ICU in patients with AKI. This result should be interpreted with caution owing to the high level of heterogeneity found (I2= 90%; inconsistency). Some studies have reported days in hospital and days in ICU, but in this population death is a competing endpoint for length of stay.
With focus on the size (magnitude and importance) of the effect estimate, we observed that early initiation may have beneficial effects on death and recovery of kidney function, while it increased the risk of adverse events; however, all results (except adverse events) were imprecise because the CIs crossed both the important effect threshold and the no difference threshold.
An important limitation of this systematic review was the moderate to high heterogeneity found in the main results, as death at day 30 (I2= 52%), death or non‐recovery of kidney function in all patients at 30 or more days (I2= 54%), recovery of kidney function in all patients (I2 = 58%), and ICU length of stay (90%). There was no heterogeneity identified for adverse events and recovery of kidney function among survivors.
We explored this heterogeneity by prespecified subgroup analyses; by aetiology of AKI; according to criteria used to define timing of RRT initiation; and by modalities of RRT. The subgroup by criteria of time of RRT initiation was identified as a source of heterogeneity in the size of the effect observed in death and recovery of kidney function (Test for subgroup differences: Chi2 = 6.41; P = 0.01; I2 = 84.4% and Chi2 = 4.98; P = 0.03; I2= 79.9%, respectively). These results should be interpreted with caution owing to the fact that only one small study reported these data.
In the subgroup of aetiology of AKI, we observed an important reduction on death rate (34%) in patients with surgery‐acquired compared to those patients with non‐surgery‐acquired AKI (5%), but there were not statistically significant differences between groups. The surgical AKI‐population should be taken into account in future researches.
We were not able to explore other subgroup analyses due to insufficient data.
Overall completeness and applicability of evidence
See Description of studies; Characteristics of included studies
Five RCTs (AKIKI 2015; Bouman 2002; ELAIN 2016; STARRT‐AKI 2013; Sugahara 2004) evaluated the effect of different times of RRT initiation on death, recovery of kidney function, length of stay, and adverse events in critically ill patients with AKI. Three studies (AKIKI 2015; Bouman 2002; STARRT‐AKI 2013) have not demonstrated beneficial effects on clinical outcomes with an earlier initiation of RRT. In contrast, two other studies (ELAIN 2016; Sugahara 2004) favoured earlier strategy. These results are consistent with those reported in our review.
Disparity and the heterogeneity found in these results among the studies probably may be explained by several factors such as differences in the methodological quality of studies, sample size, patient characteristics, illness severity, modalities, delivered dialysis dose, and heterogeneous definitions of timing of RRT initiation.
Thirty six patients with AKI following coronary bypass surgery (Sugahara 2004) were randomised to early group when the urine output remained ≤ 30 mL/h for three consecutive hours and their serum creatinine had increased by ≥ 0.5 mg/dL/d whereas in the late group RRT was started when urine output fell to < 20 mL/h for at least 2 hours. Only 28 patients were analysed (14 in each group) and effectively received protocol treatment; Of the patients treated per protocol, only 2 patients (14%) in the early group died at 2 weeks compared with 12 patients (86%) in the late arm. We observed some limitations in this study: an incomplete outcome data (attrition bias) (limiting the internal validity); a low incidence of patients with AKI‐related to sepsis; and a non‐validated short‐term outcome (limiting the external validity).
In ELAIN Study 2016, 231 critically ill patients with AKI were randomised to initiation of RRT either within 8 hours of diagnosis of KDIGO stage 2 AKI (early), or within 12 hours of reaching KDIGO stage 3 AKI, or when specific metabolic and clinical indications were present (delayed). Death at day 90 was significantly lower among patients of early initiation arm compared with those in the delayed initiation arm (from 39.3% versus 54.7%; P = 0.03). Similarly benefits were observed on recovery of kidney function in survivors (early 53.6& versus delayed 38.7%; P = 0.02); RRT duration (9 versus 25 days; P = 0.04) or hospital length of stay (51 versus 82 days P = 0.001) but not in RRT dependence or ICU length of stay. The death difference (15.4%) observed in favour of early treatment exceeds the survival benefit expect in this population and is probably related to the high incidence of patients with postsurgical AKI, limiting the external validity. It is interesting to note that only 9% of the patients randomised to the late‐arm were never treated with RRT, because they spontaneously recovered or died. These studies provide support towards earlier initiation of RRT in surgical patients with postsurgical AKI but do not completely answer the question when to the optimally initiate renal support in all ICU patients. Additionally, both studies (ELAIN 2016; Sugahara 2004) were unblinded and single‐centre (limiting the internal and external validity respectively).
In contrast, three studies did not demonstrate any effect of timing (early versus standard) of RRT initiation on survival and recovery of kidney function. Bouman 2002 conducted a small study evaluating both intensity and timing of initiation of CVVHF in 106 critical patients with AKI. There were no differences on the survival, recovery of kidney function, length of stay and adverse events for either initiation time or intensity. The survival at day 28 was greater than expected (69% to 75% in all groups) and was probably associated to low incidence of patients with AKI‐related to sepsis or it might be attributed to the closed format of the ICUs (limiting the external validity). The recovery of kidney function (based on independence of RRT) was 100% in all survivors. Of note, 6 out of 36 patients assigned to late therapy were never treated with RRT; 4 recovered kidney function and 2 died before fulfilling the criteria for late strategy. The study was underpowered to definitive detect differences due to the small sample size. STARRT‐AKI 2013 was a multicentre RCT comparing accelerated RRT initiation (12 hours or less from eligibility) with standard strategy for starting RRT based on persistent AKI and or the development of more conventional indications. They found no difference in death at 90 days, recovery of kidney function and adverse events (39.6% versus 44.2%). Only 25% of patients randomised to standard RRT strategy recovered kidney function spontaneously with no need to start RRT; therefore, an accelerated treatment is probably to expose some patients to unnecessary RRT. The study was underpowered to detect differences (non‐inferiority of delayed RRT) in clinically important outcomes due to the small sample size. Finally, AKIKI 2015 was a multicentre (31 ICUs) unblinded RCT which assessed whether a delayed strategy may improve outcomes in critically ill AKI patients. A total of 620 patients were randomised to either initiate RRT within 6 hours of fulfilling KDIGO stage 3 AKI (early) or not begin renal support unless specific clinical or metabolic indications for RRT were present (delayed). This study reported no beneficial effect on recovery of kidney function and death at 60 days with delayed strategy. It is important to note that 51% of patients in the delayed arm did not receive RRT. This cohort had a lower death rate (37.1%) when compared with patients who received either early (48.5%) or delayed (61.8%) RRT.
ELAIN 2016 and AKIKI 2015 are important contributions towards informing practice on this question; however, their contradictory results require careful interpretation. The patients enrolled in ELAIN 2016 had greater severity of illness as assessed by Sequential Organ Failure Assessment score, with lower rates of severe sepsis or sepsis shock and had AKI related to postsurgical cause, whereas patients in AKIKI 2015 were predominantly medical AKI (80%). The RRT management was per protocol only in ELAIN 2016; selection of modalities, dialysis dose were to the criteria of each site, but monitored in accordance with French guidelines. The relevant difference between ELAIN 2016 and AKIKI 2015 was their criteria for timing of RRT initiation, ELAIN 2016 commenced RRT after stage 3 AKI (delayed strategy) and while in AKIKI 2015 the threshold for early initiation was stage 3 AKI.
Although the analyses included data obtained from a comprehensive and rigorous search, we identified gaps in several areas. The majority of participants in the included studies were adults, limiting the applicability of our finding to children. In general, the incidence of AKI secondary to sepsis in ICU is high; however, in three studies it was observed that the majority of patients had post‐surgical AKI, and relatively few had sepsis or pre‐existing chronic kidney disease, limiting the applicability of our results to general ICU population. Two included studies were single‐centre and all were unblinded, limiting external and internal validity of the results.
There were large variations in the definition of timing of RRT initiation among included studies. Heterogeneous indicators such as different serum urea or serum creatinine levels, urine output, time from randomisation and time to fulfilling KDIGO AKI stage, remain widely used to measure timing of RRT; however, this approach provides an incomplete assessment of optimal timing in this population and limits the applicability of our results. The long‐term kidney outcomes after hospital discharge among survivors of AKI remain poorly characterised. The studies did not report data on death and kidney function recovery in patients with pre‐existing chronic kidney disease and with low or intermediate scores of severity of illness.
The results on length of stay (days in hospital and in the ICU) and recovery of kidney function should be interpreted with caution, especially when the death risk is taken into account in this high‐risk population.
Data on number of patients with adverse events were limited and only provided by four studies in our review. We were unable to address all of the objectives of this review due to the lack of data in the included studies.
Taking these results into account, it is important to know when early RRT initiation may be essential (to improve outcomes) or unnecessary treatment for AKI‐patients in ICU (to increase potential harms).
We included only RCTs with the purpose of reducing bias.
Quality of the evidence
We conducted this review according to the process described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Our review was based on evidence from five RCTs (1084 participants) that compared different timing of RRT initiation in critically ill patients with AKI. The certainty evidence for our main outcomes was drawn from studies assessed at low risk of bias for random sequence generation and allocation concealment processes, incomplete outcomes data, intention to treat analysis, selective outcomes reporting, performance and detection bias and other sources of bias; and unclear risk for detection bias. One study was at high risk for incomplete outcome data (attrition bias).
Data comparing the effect of early RRT initiation against standard on death at day 30 or after were obtained from five and four well‐conducted RCTs respectively, but we downgraded the certainty of evidence to low, mainly due to inconsistency (I2 value of 52%) and imprecision (CIs included a range of plausible value with clinically important benefits, but also included harm); and rate moderate by imprecision for death after 30 days. Similarly, we downgraded the certainty of evidence to low for recovery of kidney function in all patients due to imprecision and inconsistency (I2 = 58%) and rate as moderate data obtained for recovery of kidney function among survivors at 30 days by indirectness (the recovery of kidney function in this high risk group is affected when the risk of death is taken into account).
Data used to assess the impact of early versus standard initiation of CRRT on adverse events were obtained from three well‐conducted RCTs, providing treatment effects with clinically important harms; we rates this as high certainty evidence. One study provided data on number of patients with bleeding and thrombocytopenia; we downgraded the certainty of evidence to very low due to serious imprecision and indirectness.
Length of stay was reported by four well‐conducted RCTs, but we downgraded the certainty of evidence to low due to substantial inconsistency (I2 value of 90%) and indirectness (the length of stay in this high risk group is affected when the risk of death is taken into account).
Potential biases in the review process
While this review was conducted according to rigorous methods developed by the Cochrane Collaboration, some bias may be present in the review process. We searched for all relevant studies using sensitive and validated strategies in major medical databases and grey literature sources. However, it is possible that some studies (such as unpublished data and studies with negative or no effects) were not identified. An analysis for evidence to assess the risk of publication bias was not possible for all outcomes due to the small number of studies available in each meta‐analysis (Figure 4).
Several subgroups analyses were planned to explore potential sources of heterogeneity in our review; however a lack of data prevented us from doing these analyses.
Agreements and disagreements with other studies or reviews
Our systematic review in keeping with previous meta‐analysis on timing in RRT (Karvellas 2011; Liu 2014; Luo 2017; Wang 2012; Wang 2017) found that early initiation of RRT may have beneficial effects on death and kidney recovery function in critically ill patients with AKI compared to standard therapy. These results were not consistent with other two systematic reviews that included randomised and observational studies (Seabra 2008; Wierstra 2016) and recent meta‐analyses based only RCTs (Lai 2017; Mavrakanas 2017; Xu 2016).
The hypothesis that in critically AKI patients, especially those with acidaemia, fluid overload, or systemic inflammation, could benefit from an early RRT was proposed by several researchers. Our review has found that early strategy may have beneficial effect on death at day 30. This result is consistent with two individual RCTs (ELAIN 2016; Sugahara 2004), but does not agree with those reported in three multicentre RCTs (AKIKI 2015; Bouman 2002; STARRT‐AKI 2013). It is important to note that differences in death between AKIKI 2015 and ELAIN 2016 were observed (41.6% versus 30.4% of patients at day 30 respectively). These differences may be due to several factors including: different severity and aetiology of AKI e.g. prevalence of patients with AKI‐related to surgical cause in the ELAIN 2016 or septic AKI‐patients was more frequent in AKIKI 2015 (both aetiology have different pathophysiology and prognosis), and variable criteria for defining early RRT initiation (KDIGO AKI stage 3 for AKIKI 2015 and KDIGO AKI stage 2 for ELAIN 2016). See Overall completeness and applicability of evidence.
There has been increased interest in recovery of kidney function. Indeed, lack of recovery of kidney function implies the need for long‐term dialysis associated with low quality of life. Our review has found that early strategy may have beneficial effect on recovery of kidney function in all patients. This finding is consistent with two individual RCTs (ELAIN 2016; Sugahara 2004) and does not agree with other three multicentre RCTs (AKIKI 2015; Bouman 2002; STARRT‐AKI 2013). Differences on recovery of kidney function between studies may be due to the same factors related above. However, in patients with a high short‐term death risk, the interpretation of this result may be misleading given that death is a competing endpoint for recovery of kidney function (Palevsky 2005).
Patients with AKI have a longer hospital stay. In our review, earlier strategy may reduce ICU length of stay, this result is consistent with four individual RCTs (Bouman 2002; ELAIN 2016; STARRT‐AKI 2013; Sugahara 2004) and does not agree with those reported of one RCT (AKIKI 2015). However, the length of stay in this high risk population may be affected when death is taken into account.
There was an increased risk in the number of patients who have any adverse events with early initiation of RRT compared with standard. Our results were consistent with other two RCTs (AKIKI 2015; Bouman 2002). This outcome was not examined by previous systematic reviews.
Our review has an important limitation due to the significant heterogeneity observed in the main outcomes. We explored heterogeneity but found no association between effect estimate and different subgroup analysed (aetiology of AKI, modality of AKI) in agreement with other reviews (Karvellas 2011; Seabra 2008; Wierstra 2016), except for criteria of time of RRT initiation subgroup in keeping with recent review (Luo 2017). We were unable to address all of the pre‐specified subgroup analyses of this review due to the lack of data in the included studies.
Previous reviews explored the effect of time to initiation RRT in patients with AKI; however, these reviews included studies that we excluded from our review due to: different inclusion criteria applied e.g. hospitalised patients were not in an ICU setting (Pursnani 1997) or did not require AKI for enrolment in early arm (Durmaz 2003; HEROICS 2015; Jamale 2013; Koo 2006) and differences in the methodological studies design (cohort studies). Although the abundance of cohorts studies provided more power (increases the sample size) to find significant clinical differences between both treatments, these studies have an important limitations: patients between treatments group were different e.g. patients who would have been assigned to late arm treatment might have died before initiating the therapy, while others who lived enough to be assigned to the late group might have been less sick than those or with a high likelihood of recovering kidney function without RRT; and a relevant point, the patients do not have the same opportunity to receive early or standard treatment(allocation or selection bias). Consequently, to minimise the risk of bias in our review, we included only randomised studies for main outcomes.
Authors' conclusions
With available data of included RCTs, early RRT initiation may have beneficial effects on death or recovery of kidney function; although in both results the CIs included clinical benefits and harm; however, there was an increased risk of adverse events compared to standard therapy. The absence of high quality evidence of efficacy and the possibility of increased adverse events does not support the routine use of early RRT in critically ill patients with AKI.
These results do not minimise the importance of the timing in RRT of critically ill patients, but rather reinforce the need to better understand in whom and when earlier initiation translates into improved patients outcomes. Minimal standards for the initiation of RRT appear to have been identified in different guidelines (KDIGO 2012; NICE 2013; Vinsonneau 2015); however these approaches provide an incomplete assessment of optimal timing of RRT.
Until now, given the low certainty evidence observed in the main outcomes, decision regarding the optimal timing of RRT should remain based on individual patients characteristic and clinician judgment.
Given the persistently high death rate among critically ill patients with AKI, it would be important to accurately determine the effect of timing of RRT on death. In view of the inconsistencies observed in the main outcomes and the inability to assess all possible causes of heterogeneity, it would be important to perform pooled analyses of individual patient data from all completed studies to deal with heterogeneity issues. Also, the intensity of RRT during therapy needs to be rigorously evaluated.
Adequately‐powered randomised studies that included appropriate and reproducible criteria to define the optimal time of RRT allowing improvement in clinical outcomes in AKI patients are needed. At present, two on‐going RCTs (IDEAL‐ICU 2014; NCT00837057) in this area will provide definitive answers that will guide clinical practice.
Acknowledgements
The authors wish to thank the referees for their advice and feedback during the preparation of this review.
The authors would also like to thank all study authors who provided additional information about their studies and Daniel Comandé, Librarian of the Institute for Clinical Efectiveness and Health Policy (Argentina) for his help in search for the literature.
We would like to thank Cochrane Kidney and Transplant for their help with this review and especially to Marta Roque Figuls, Iberoamerican Cochrane Centre for her support and editorial advice during the reporting of this systematic review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
| LILACS |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random) |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention | |
| Unclear: Insufficient information about the sequence generation process to permit judgement | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes) |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure | |
| Unclear: Randomisation stated but no information on method used is available | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon) |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias |
Data and analyses
Comparison 1.
Early versus standard initiation
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death at day 30 | 5 | 1084 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.61, 1.13] |
| 1.2 Death after 30 days | 4 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.10] |
| 2 Death or non‐recovery kidney function at day 90 | 5 | 1078 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.66, 1.05] |
| 3 Recovery of kidney function | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Patients free of RRT after discontinuing CRRT (all patients) | 5 | 1084 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.40] |
| 3.2 Patients free of RRT after discontinuing CRRT (patients surviving more than 30 days) | 5 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [1.00, 1.06] |
| 4 Adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Number of patients with any adverse events | 4 | 899 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.03, 1.16] |
| 4.2 Number of patients with bleeding | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.50, 5.84] |
| 4.3 Number of patients with thrombocytopenia | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.20, 5.35] |
| 5 Length of stay | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Length of stay in ICU | 4 | 1056 | Mean Difference (IV, Random, 95% CI) | ‐1.78 [‐3.70, 0.13] |
Comparison 2.
Subgroup analysis: death
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by AKI aetiology | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 patients with AKI related to non‐surgical causes | 2 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.13] |
| 1.2 patients with AKI related to surgical causes | 3 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.32, 1.36] |
| 2 Death by definition of early RRT initiation | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Early initiation according clinical‐biochemical criteria | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.61] |
| 2.2 Early initiation according time criteria | 4 | 1049 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 3 Death by RRT modality | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Continuous RRT | 3 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.31, 1.36] |
| 3.2 Continuous and intermittent RRT | 2 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.13] |
Comparison 3.
Subgroup analysis: recovery of kidney function
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovery of kidney function by AKI aetiology | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Patient with AKI related to surgical causes | 3 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.78, 2.18] |
| 1.2 patients with AKI related to non‐surgical causes | 2 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.90, 1.20] |
| 2 Recovery of kidney function by definition of early RRT Initiation | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Early according time criteria | 4 | 1050 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.97, 1.24] |
| 2.2 Early initiation according clinical‐biochemical criteria | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [1.33, 18.81] |
| 3 Recovery of kidney function by RRT modality | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Continuous RRT | 3 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.79, 2.34] |
| 3.2 Continuous and intermittent RRT | 2 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.90, 1.20] |
History
Protocol first published: Issue 6, 2013 Review first published: Issue 12, 2018
| Date | Event | Description |
|---|---|---|
| 26 September 2017 | New search has been performed | Search strategies for MEDLINE, EMBASE & CENTRAL updated to reflect change in title |
Differences between protocol and review
We modified the title of our review "Timing of renal replacement therapy initiation for acute kidney injury"
Inclusion criteria: we included all patients with AKI in ICU being treated with renal replacement therapy regardless of age and gender.
Measures of treatment effect: These results were interpreted taking the size of the effect (magnitude or importance) into account (see CKT 2017; EPOC 2013).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
|
|
| Participants |
|
|
| Interventions | RRT modalities
Treatment group
Control group
Cointerventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomly assigned to one of the two treatment groups by means of a centralized, computer‐generated method |
| Allocation concealment (selection bias) | Low risk | Central allocation process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement (for kidney recovery was unclear risk but for death was low risk) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data were reported |
| Selective reporting (reporting bias) | Low risk | The study reported death, kidney function recovery and adverse events |
| Other bias | Low risk | Funding sources were reported (not for profit funding) |
| Methods |
|
|
| Participants |
|
|
| Interventions | RRT modality
Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomly assigned to one of the two treatment groups using computer‐generated method |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were kept in numbered, sealed opaque envelopes that were opened at the time of enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement (for kidney recovery was unclear risk but for death was low risk) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data were reported |
| Selective reporting (reporting bias) | Low risk | The study reported death, kidney function recovery and adverse events |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods |
|
|
| Participants |
|
|
| Interventions | RRT modality
Treatment group
Control group
Cointerventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned using computer‐generated method |
| Allocation concealment (selection bias) | Low risk | Each patient received a study identification number and treatment allocation at enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement (for kidney recovery was unclear risk but for death was low risk) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data were reported |
| Selective reporting (reporting bias) | Low risk | The study reported death, kidney function recovery and adverse events |
| Other bias | Low risk | Quote: "The study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication" |
| Methods |
|
|
| Participants |
|
|
| Interventions | RRT modality
Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomly assigned to one of two treatment using computer‐generated method |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were kept in numbered, sealed opaque envelopes that were opened in numeric sequence at the time of enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement (for kidney recovery was unclear risk but for death was low risk) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data were reported |
| Selective reporting (reporting bias) | Low risk | The study reported death, kidney function recovery and adverse events |
| Other bias | Low risk | Not for profit funding |
| Methods |
|
|
| Participants |
|
|
| Interventions | RRT modality
Treatment group
Co‐interventions
|
|
| Outcomes | Primary outcome
Secondary outcome
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; Quote: "All patients were divided randomly into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measurement was unlikely to be influenced by lack of blinding (for kidney recovery was unclear risk but for death was low risk) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 20% of included patients not reported |
| Selective reporting (reporting bias) | Low risk | Published report included survival and recovery of kidney function |
| Other bias | Unclear risk | Insufficient information to permit judgement |
AIDS ‐ acquired immune deficiency syndrome; AKI ‐ acute kidney injury; BUN ‐ blood urea nitrogen; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CRRT ‐ continuous renal replacement therapy; CVVH ‐ continuous venovenous haemofiltration; CVVHDF ‐ continuous venovenous haemodiafiltration; (e)GFR ‐ (estimated) glomerular filtration rate; GN ‐ glomerulonephritis; HF ‐ haemofiltration; ICU ‐ intensive care unit; IHD intermittent haemodialysis; M/F ‐ male/female; NGAL ‐ plasma neutrophil gelatinase‐associated lipocalin; RCT ‐ randomised controlled trial; RRT ‐ renal replacement therapy; SCr ‐ serum creatinine; SD ‐ standard deviation; UF ‐ ultrafiltration
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abe 2010c | Wrong intervention: timing of RRT initiation was not assessed |
| Albino 2014 | Wrong intervention: timing of RRT initiation was not assessed |
| Ambrós 1995 | Wrong intervention: timing of RRT initiation was not assessed |
| Andrade 1997 | Wrong intervention: timing of RRT initiation was not assessed (evaluated CAVHF) |
| ATN 2005 | Wrong intervention: timing of RRT initiation was not assessed (compared the survival and kidney recovery in critically ill patients treated with intensive versus less‐intensive RRT) |
| Augustine 2004 | Wrong intervention: timing of RRT initiation was not assessed |
| Badawy 2013 | Wrong intervention: timing of RRT initiation was not assessed (compared the efficacy of CVVHDF and EDD in patients with AKI after cardiac surgery) |
| Baldwin 2007 | Wrong intervention: timing of RRT initiation was not assessed (compared EDD with HF or CVVHF with regard to fluid removal and haemodynamics) |
| Berg 2007 | Wrong intervention: timing of RRT initiation was not assessed |
| Berger 2004 | Wrong intervention: timing of RRT initiation was not assessed |
| Boussekey 2008 | Wrong intervention: timing of RRT initiation was not assessed |
| Boyle 1995 | Wrong intervention: timing of RRT initiation was not assessed |
| Cole 2001 | Wrong intervention: timing of RRT initiation was not assessed |
| Cole 2002 | Outcomes of interest not investigated: evaluated the effect of early and CVVHF on the plasma concentrations of several humoral mediators of inflammation in septic patients |
| CRITERIA 2012 | Wrong intervention: timing of RRT initiation was not assessed |
| Daud 2006 | Wrong intervention: timing of RRT initiation was not assessed |
| Davenport 1991 | Wrong intervention: timing of RRT initiation was not assessed |
| Davenport 1993b | Wrong intervention: timing of RRT initiation was not assessed |
| Davies 2008 | Wrong intervention: timing of RRT initiation was not assessed |
| de Pont 2006 | Wrong intervention: timing of RRT initiation was not assessed |
| Durmaz 2003 | Wrong population: the presence of AKI was no obligatory condition for enrolment in the early arm |
| Gabriel 2008 | Wrong intervention: timing of RRT initiation was not assessed (compared the role of HVPD to daily HD in patients with AKI; HVPD was not included in this review) |
| Garcia‐Fernandez 2000 | Wrong intervention: timing of RRT initiation was not assessed |
| Gasparovic 2003 | Wrong intervention: timing of RRT initiation was not assessed |
| George 2011 | Wrong intervention: timing of RRT initiation was not assessed |
| Ghani 2006 | Wrong intervention: timing of RRT initiation was not assessed |
| Gillum 1986 | Wrong intervention: timing of RRT initiation was not assessed |
| Haase 2007b | Wrong intervention: timing of RRT initiation was not assessed. High‐adsorption CVVHD was not included in this review |
| Han 2015 | Wrong population: the presence of AKI was no obligatory condition for enrolment in the early arm |
| HAN‐D‐OUT 2009 | Wrong intervention: timing of RRT initiation was not assessed |
| HEROICS 2015 | Wrong population: the presence of AKI was no obligatory condition for enrolment in the early arm |
| Hoste 1995 | Wrong intervention: timing of RRT initiation was not assessed |
| Jamale 2013 | Wrong population: included patients with AKI, but ICU stay was no obligatory condition for enrolment in the early arm |
| Jeffrey 1994 | Wrong intervention: timing of RRT initiation was not assessed |
| John 2001 | Wrong intervention: timing of RRT initiation was not assessed |
| Jones 1998 | Wrong intervention: timing of RRT initiation was not assessed |
| Kellum 1998 | Wrong intervention: timing of RRT initiation was not assessed |
| Kielstein 2004 | Wrong intervention: timing of RRT initiation was not assessed |
| Kielstein 2005 | Wrong intervention: timing of RRT initiation was not assessed |
| Kierdorf 1995 | Wrong intervention: timing of RRT initiation was not assessed |
| Klouche 2007 | Wrong intervention: timing of RRT initiation was not assessed |
| Koo 2006 | Wrong population: the presence of AKI was no obligatory condition for enrolment in the early arm |
| Kumar 2004 | Wrong intervention: timing of RRT initiation was not assessed |
| Lentini 2009 | Wrong intervention: timing of RRT initiation was not assessed (compared pulse high volume HF and coupled plasma filtration adsorption in septic shock patients) |
| Manns 1996 | Wrong intervention: timing of RRT initiation was not assessed |
| Maxvold 2000 | Wrong intervention: timing of RRT initiation was not assessed |
| Mehta 2001 | Wrong intervention: timing of RRT initiation was not assessed |
| Meloni 1996 | Wrong intervention: timing of RRT initiation was not assessed |
| Misset 1996 | Outcomes of interest not assessed: evaluated the haemodynamic response to IHF and continuous HF in ICU patients with AKI |
| Morgera 2004 | Wrong intervention: timing of RRT initiation was not assessed |
| Morgera 2006 | Wrong intervention: timing of RRT initiation was not assessed |
| Noble 2006 | Wrong intervention: timing of RRT initiation was not assessed |
| OMAKI 2012 | Wrong intervention: timing of RRT initiation was not assessed |
| Oudemans‐van‐Straaten 2009a | Wrong intervention: timing of RRT initiation was not assessed |
| Paganini 1996 | Wrong intervention: timing of RRT initiation was not assessed |
| Park 2016 | Wrong intervention: timing of RRT initiation was not assessed |
| Payen 2009 | Wrong population: the presence of AKI was no obligatory condition for enrolment in the early arm |
| Pettila 2001 | Wrong intervention: timing of RRT initiation was not assessed |
| Ponce 2011 | Wrong intervention: timing of RRT initiation was not assessed |
| Ponce 2013 | Wrong intervention: timing of RRT initiation was not assessed |
| Pursnani 1997 | Wrong population: included patients with AKI, but ICU stay was no obligatory condition for enrolment in the early arm |
| Ratanarat 2012 | Wrong intervention: timing of RRT initiation was not assessed |
| RENAL 2006 | Wrong study design: 2 records of this study assessed timing of CRRT, but were not RCTs (retrospective nested cohort) |
| RESCUE 2012 | Wrong intervention: timing of RRT initiation was not assessed |
| Ricci 2006 | Wrong intervention: timing of RRT initiation was not assessed |
| Ronco 1999a | Wrong intervention: timing of RRT initiation was not assessed |
| Ronco 2000a | Wrong intervention: timing of RRT initiation was not assessed |
| Ronco 2001 | Wrong intervention: timing of RRT initiation was not assessed |
| Saudan 2006 | Wrong intervention: timing of RRT initiation was not assessed |
| Schiffl 1997 | Wrong intervention: timing of RRT initiation was not assessed |
| Schiffl 2002 | Wrong intervention: timing of RRT initiation was not assessed |
| SHARF 2009 | Wrong intervention: timing of RRT initiation was not assessed |
| Stefanidis 1995 | Wrong intervention: timing of RRT initiation was not assessed |
| Storck 1991 | Wrong intervention: timing of RRT initiation was not assessed |
| Tan 2001 | Wrong intervention: timing of RRT initiation was not assessed |
| Tolwani 2008 | Wrong intervention: timing of RRT initiation was not assessed |
| Uehlinger 2005 | Wrong intervention: timing of RRT initiation was not assessed |
| van der Voort 2005 | Wrong intervention: timing of RRT initiation was not assessed |
| Vinsonneau 2006 | Wrong intervention: timing of RRT initiation was not assessed |
| Wynckel 1998 | Wrong intervention: timing of RRT initiation was not assessed |
| Wynckel 2004 | Wrong intervention: timing of RRT initiation was not assessed |
| Zhang 2004a | Wrong intervention: timing of RRT initiation was not assessed |
| Zhao 2009a | Wrong intervention: timing of RRT initiation was not assessed |
| Zimmerman 1999 | Wrong intervention: timing of RRT initiation was not assessed |
AKI ‐ acute kidney injury; CAVHF ‐ continuous arteriovenous haemofiltration; CVVHDF ‐ continuous venovenous haemodiafiltration; EDD ‐ extended daily dialysis; HD ‐ haemodialysis; HF ‐ haemofiltration; HVPD ‐ high volume peritoneal dialysis; ICU ‐ intensive care unit; IHF ‐ intermittent haemofiltration; RRT ‐ renal replacement therapy
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes | Primary outcome
Secondary outcome
|
| Notes |
AKI ‐ acute kidney injury; ICU ‐ intensive care unit; RCT ‐ randomised controlled study; RRT ‐ renal replacement therapy
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Early continuous renal replacement therapies (CRRT) in patients with severe sepsis or septic shock with acute kidney injury |
| Methods |
|
| Participants |
|
| Interventions | Dialysis modality
Treatment group
Control group
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | February 2009 |
| Contact information | sbhong@amc.seoul.kr |
| Notes |
|
CKD ‐ chronic kidney disease; CRRT ‐ continuous renal replacement therapy; CVVHF ‐ continuous venovenous haemofiltration; ESKD ‐ end‐stage kidney disease; RCT ‐ randomised controlled trial
Contributions of authors
Draft the protocol: AF, DB, AC
Study selection: AF, DB
Extract data from studies: AF, DB
Enter data into RevMan: AF
Carry out the analysis: AF, AC
Interpret the analysis: AF, DB, AC
Draft the final review: AF, DB, AC
Disagreement resolution: AC
Update the review: AF
Sources of support
Internal sources
No sources of support supplied
External sources
-
Instituto de Efectividad Clínica y Sanitaria (Institute for Clinical Effectiveness and Health Policy) (IECS‐CONICET), Argentina.
The Institute for Clinical Effectiveness and Health Policy (IECS‐CONICET) is an independent, non‐for‐profit organization devoted to research, education and technical support (www.iecs.org.ar). Over the last few years, IECS has been a leading institution in Latin America (LA) in regards to developing HTA reports and economic evaluations (EE) to study the impact and financial implications of the adoption of technologies on health care systems.
Declarations of interest
Alicia I Fayad: none known
Daniel G Buamscha: none known
Agustín Ciapponi: none known
New
References
References to studies included in this review
- Gaudry S, Hajage D, Schortgen F, Martin‐Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal‐replacement therapy in the intensive care unit. New England Journal of Medicine 2016;375(2):122‐133. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Gaudry S, Hajage D, Schortgen F, Martin‐Lefevre L, Ricard J, Dreyfuss D. Comparison of two strategies for initiating renal replacement therapy in the ICU: Study protocol plan for a multicenter, randomized, controlled trial from the AKIKI Research Group [abstract no: P299]. Critical Care 2015;19(Suppl 1):S106. [EMBASE: 71938703] [Google Scholar]; Gaudry S, Hajage D, Schortgen F, Martin‐Lefevre L, Tubach F, Pons B, et al. Comparison of two strategies for initiating renal replacement therapy in the intensive care unit: study protocol for a randomized controlled trial (AKIKI). Trials [Electronic Resource] 2015;16:170. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Gaudry S, Hajage D, Schortgen F, Martin‐Lefevre L, Tubach F, Pons B, et al. Effect of renal replacement therapy strategies in septic‐shock patients with severe acute kidney injury: a post hoc analysis of a randomized controlled trial [abstract]. Annals of Intensive Care 2017;7(1 Suppl 1):5. [EMBASE: 614625655]28050898 [Google Scholar]
- Bouman CS, Oudemans‐Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high‐volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Critical Care Medicine 2002;30(10):2205‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zarbock A, Gers J, Aken H, Boanta A, Kellum JA, Meersch M. Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury (The ELAIN‐Trial): study protocol for a randomized controlled trial.[Erratum appears in Trials. 2016;17(1):260; PMID: 27216334]. Trials [Electronic Resource] 2016;17(1):148. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Zarbock A, Kellum JA, Schmidt C, Aken H, Wempe C, Pavenstadt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016;315(20):2190‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smith OM, Wald R, Adhikari NK, Pope K, Weir MA, Bagshaw SM. Standard versus accelerated initiation of renal replacement therapy in acute kidney injury (STARRT‐AKI): study protocol for a randomized controlled trial. Trials [Electronic Resource] 2013;14:320. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Wald R, Adhikari NK, Smith OM, Weir MA, Pope K, Cohen A, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney International 2015;88(4):897‐904. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sugahara S, Suzuki H. Early start on continuous hemodialysis therapy improves survival rate in patients with acute renal failure following coronary bypass surgery. Hemodialysis International 2004;8(4):320‐5. [EMBASE: 2005300769] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Abe M, Okada K, Suzuki M, Nagura C, Ishihara Y, Fujii Y, et al. Comparison of sustained hemodiafiltration with continuous venovenous hemodiafiltration for the treatment of critically ill patients with acute kidney injury. Artificial Organs 2010;34(4):331‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Albino BB, Balbi AL, Abrao JM, Ponce D. Dialysis complications in acute kidney injury patients treated with prolonged intermittent renal replacement therapy sessions lasting 10 versus 6 hours: results of a randomized clinical trial. Artificial Organs 2015;39(5):423‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Albino BB, Balbi AL, Ponce D. Dialysis complications in AKI patients treated with extended daily dialysis: is the duration of therapy important?. BioMed Research International 2014;2014:153626. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrós Checa A, Sánchez‐Izquierdo Riera JA, Pérez Vela JL, Alted Lopez E, Sánchez Casado M, Cobo Castelianos P. Changes in respiratory parameters in patients with severe multiple trauma with continuous haemofiltration [abstract no: 118]. Medicina Intensiva 1995;19(Suppl 1):32. [CENTRAL: CN‐00724914] [Google Scholar]; Pérez Vela JL, Sánchez‐Izquierdo Riera JA, Ortuño de Solo B, Caballero Cubedo R, Sánchez Casado M, Alted Lopez E. Depuration capacity of thromboxane A2 in continuous haemofiltration in patients with severe multiple trauma [abstract no: 345] [Capacidad de depuracion de tromboxano A2 por hemofiltracion continua en pacientes politraumatizados severos]. Medicina Intensiva 1996;20(Suppl 1):101. [CENTRAL: CN‐00740493] [Google Scholar]; Sánchez‐Izquierdo Riera JA, Pérez Vela JL, Ortuño de Solo B, Alted Lopez E, Vaquerizo Alonso C, Novillo‐Fertreil Vazquez P. Depuration capacity of leukotriene B4 in continuous haemofiltration in patients with severe multiple trauma [abstract no: 210]. Medicina Intensiva 1996;20(Suppl 1):63. [CENTRAL: CN‐00740492] [Google Scholar]
- Andrade L, Marotto MS, Marotto PC, Sztajnbok J, Seguro AC. Efficacy of continuous arterio‐venous hemodiafiltration (CAVHDF) on the outcome of acute renal failure and respiratory distress of a single etiology (leptospirosis) [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A168. [CENTRAL: CN‐00483015] [Google Scholar]
- Afshinnia F, Belanger K, Palevsky PM, Young EW. Effect of ionized serum calcium on outcomes in acute kidney injury needing renal replacement therapy: secondary analysis of the Acute Renal Failure Trial Network Study. Renal Failure 2013;35(10):1310‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Crowley S, Schein R, Dev D, Finkel K, Vijayan A, Paganini E, et al. Dialysis catheter complications in the VA/NIH ATN study [abstract no: SA‐PO557]. Journal of the American Society of Nephrology 2007;18(Abstracts):463A. [Google Scholar]; Crowley S, Schein R, Dev D, Finkel K, Vijayan A, Paganini E, et al. Lessons for successful study enrolment from the VA/NIH ATN study [abstract no: SA‐PO932]. Journal of the American Society of Nephrology 2006;17(Abstracts):770A. [CENTRAL: CN‐00601950] [Google Scholar]; Crowley ST, Chertow GM, Vitale J, O'Connor T, Zhang J, Schein RM, et al. Lessons for successful study enrollment from the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(4):955‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Delos Santos RB, Li T, Palevsky PM, Vijayan A. Intermittent hemodialysis in acute kidney injury: results from the VA/NIH ATN trial [abstract no: TH‐OR037]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):9A. [Google Scholar]; Demirjian S, Paganini EP, Zhang JH, O'Connor TZ, Vitale J, Palevsky PM. Severity of illness does not modify the effect of intensity of renal replacement therapy (RRT) on outcome in critically ill patients with AKI: results from the VA/NIH Acute Renal Failure Trial Network (ATN) Study [abstract no: SA‐PO2997]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):791A. [Google Scholar]; Demirjian S, Paginini EP, Zhang JH, O'Connor TZ, Vitale J, Palevsky PM, et al. Predictive scoring systems perform poorly in critically ill patients with AKI requiring renal replacement: data from the VA/NIH Acute Renal Failure Trial Network (ATN) Study [abstract no: SA‐PO3010]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):794A. [Google Scholar]; Ganta K, Ng Y, Davis HT, Unruh ML. Vascular access in acute kidney injury: results from the ATN study [abstract no: TH‐PO847]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):286a. [Google Scholar]; Johansen KL, Smith MW, Unruh ML, Siroka AM, O'Connor T, Palevsky PM. Predictors of health utility among 60‐day survivors of AKI in the VA/NIH Acute Renal Failure Network Study [abstract no: TH‐FC086]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):21A. [DOI] [PMC free article] [PubMed] [Google Scholar]; Johansen KL, Smith MW, Unruh ML, Siroka AM, O'Connor TZ, Palevsky PM, et al. Predictors of health utility among 60‐day survivors of acute kidney injury in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(8):1366‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Joyce VR, Smith MW, Johansen KL, Unruh ML, Siroka AM, O'Connor TZ, et al. Health‐related quality of life as a predictor of mortality among survivors of AKI. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(7):1063‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Causland FR, Asafu‐Adjei J, Betensky RA, Palevsky PM, Waikar SS. Comparison of urine output among patients treated with more intensive versus less intensive RRT: results from the Acute Renal Failure Trial Network Study. Clinical Journal of the American Society of Nephrology: CJASN 2016;11(8):1335‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; McCausland FR, Asafuadjei JK, Betensky RA, Palevsky PM, Waikar SS. Renal replacement intensity and urine volume in critically Ill patients‐results from the ATN study [abstract no: SA‐PO003]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):633A. [Google Scholar]; Murugan R, Wen X, Keener C, Pike F, Palevsky PM, Unruh M, et al. Associations between intensity of RRT, inflammatory mediators, and outcomes. Clinical Journal of the American Society of Nephrology: CJASN 2015;10(6):926‐33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Ng YH, Ganta K, Davis H, Pankratz VS, Unruh M. Vascular access site for renal replacement therapy in acute kidney injury: a post hoc analysis of the ATN Study. Frontiers in Medicine 2017;4:40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Palevsky PM, Franchini R, O'Connor TZ, Zhang JH, VA/NIH Acute Renal Failure Trial Network. Recovery of kidney function in critically ill patients with acute kidney injury (AKI) treated with intensive versus less‐intensive renal replacement therapy (RRT) [abstract no: SA‐PO2994]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):790A. [Google Scholar]; Palevsky PM, O'Connor T, Zhang JH, Star RA, Smith MW. Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. Clinical Trials 2005;2(5):423‐35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Palevsky PM, O'Connor TZ, Chertow GM, Crowley ST, Zhang JH, Kellum JA, et al. Intensity of renal replacement therapy in acute kidney injury: perspective from within the Acute Renal Failure Trial Network Study. Critical Care (London, England) 2010;13(4):310. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Palevsky PM, O'Connor TZ, Zhang JH, Star R. VA/NIH Acute Renal Failure Trial: study design [abstract no: SA‐PO970]. Journal of the American Society of Nephrology 2003;14(Program & abstracts):512A. [CENTRAL: CN‐00583741] [Google Scholar]; Palevsky PM, Overberger P, Franchini R, O'Connor TZ, Zhang JH, VA/NIH VA/NIH Acute Renal Failure Trial Network. One‐year outcomes in critically ill patients with acute kidney injury (AKI) treated with intensive versus less‐intensive renal replacement therapy (RRT) [abstract no: SA‐FC414]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):93A. [Google Scholar]; Palevsky PM, Zhang J, O'Connor T. Intensive versus non‐intensive renal replacement therapy (RRT) in critically ill patients with acute kidney injury (AKI) [abstract]. American Thoracic Society International Conference; 2008 May 16‐21; Toronto, Canada. 2008:A767. [Google Scholar]; Pesacreta M, Overberger P, Palevsky PM, VA/NIH Acute Renal Failure Trial Network. Management of renal replacement therapy in acute renal failure: a survey of practitioner prescribing practices [abstract no: SA‐PO227]. Journal of the American Society of Nephrology 2004;15(Oct):350A. [CENTRAL: CN‐00601951] [DOI] [PMC free article] [PubMed] [Google Scholar]; VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, et al. Intensity of renal support in critically ill patients with acute kidney injury.[Erratum appears in N Engl J Med. 2009 Dec 10;361(24):2391]. New England Journal of Medicine 2008;359(1):7‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang JH, O'Connor T, Palevsky PM, VA/NIH Acute Renal Failure Trial Network. Evaluation of treatment separation in the VA/NIH Acute Renal Failure Trial Network (ATN) Study [abstract]. Clinical Trials 2009;6(5):523‐4. [DOI: CN-00783257] [Google Scholar]; Zhang JH, O'Connor T, Swanson K, Palevsky PM, VA/NIH Acute Renal Failure Trial Network. Evaluation of trial safety in an ICU trial: experience from the VA/NIH Acute Renal Failure Trial Network (ATN) Study [abstract no: P71]. Clinical Trials 2009;6(5):560. [CENTRAL: CN‐00783258] [Google Scholar]; Zhang JH, Palevsky PM, Chertow GM, Hartigan J, O'Connor TZ, Guarino P, et al. Piecewise analysis of patient survival after onset of AKI. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(10):1679‐84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JJ, Sandy D, Lee J, Paganini EP. Hemodynamic stability in a prospective randomized trial of intermittent versus continuous dialysis in acute renal failure [abstract no: SU‐PO893]. Journal of the American Society of Nephrology 2003;14(Nov):731A. [CENTRAL: CN‐00583467] [Google Scholar]; Augustine JJ, Sandy D, Seifert TH, Paganini EP. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. American Journal of Kidney Diseases 2004;44(6):1000‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Sandy D, Moreno L, Lee JC, Paganini EP. A randomized, stratified, dose equivalent comparison of continuous veno‐venous hemodialysis (CVVHD) vs intermittent hemodialysis (IHD) support in ICU acute renal failure patients (ARF) [abstract no: S253]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):225A. [CENTRAL: CN‐00447576] [Google Scholar]
- Badawy SS, Hassan AR, Samir EM. A prospective randomized comparative pilot trial on extended daily dialysis versus continuous venovenous hemodiafiltration in acute kidney injury after cardiac surgery. Egyptian Journal of Cardiothoracic Anesthesia 2013;7(2):69‐73. [DOI: 10.4103/1687-9090.124035] [DOI] [Google Scholar]
- Baldwin I, Bellomo R, Naka T, Koch B, Fealy N. A pilot randomized controlled comparison of extended daily dialysis with filtration and continuous veno‐venous hemofiltration: fluid removal and hemodynamics. International Journal of Artificial Organs 2007;30(12):1083‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Baldwin I, Naka T, Koch B, Fealy N, Bellomo R. A pilot randomised controlled comparison of continuous veno‐venous haemofiltration and extended daily dialysis with filtration: effect on small solutes and acid‐base balance. Intensive Care Medicine 2007;33(5):830‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Chua HR, Baldwin I, Fealy N, Naka T, Bellomo R. Amino acid balance with extended daily diafiltration in acute kidney injury. Blood Purification 2012;33(4):292‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berg A, Norberg A, Martling CR, Gamrin L, Rooyackers O, Wernerman J. Glutamine kinetics during intravenous glutamine supplementation in ICU patients on continuous renal replacement therapy. Intensive Care Medicine 2007;33(4):660‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berger MM, Shenkin A, Revelly JP, Roberts E, Cayeux MC, Baines M, et al. Copper, selenium, zinc, and thiamine balances during continuous venovenous hemodiafiltration in critically ill patients. American Journal of Clinical Nutrition 2004;80(2):410‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boussekey N, Chiche A, Faure K, Devos P, Guery B, d'Escrivan T, et al. A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Medicine 2008;34(9):1646‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boyle M, Wyndham K, Jacobs S, Torda TA. Comparative clearance performance of two dialyser units used in the CVVHD mode. Australian Critical Care 1995;8(2):20‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cole L, Bellomo R, Baldwin I, Hayhoe M, Ronco C. The impact of lactate‐buffered high‐volume hemofiltration on acid‐base balance. Intensive Care Medicine 2003;29(7):1113‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Cole L, Bellomo R, Journois D, Davenport P, Baldwin I, Tipping P. High‐volume haemofiltration in human septic shock. Intensive Care Medicine 2001;27(6):978‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cole L, Bellomo R, Hart G, Journois D, Davenport P, Tipping P, et al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Critical Care Medicine 2002;30(1):100‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shi W. Effect of the intensity of continuous renal replacement therapy in patients with acute kidney injury. www.clinicaltrials.gov/ct2/show/NCT01560650 (first received 22 March 2012).
- Daud KM, Leong GB, Visvanathan R. Acute dialytic support for the critically ill: continuous venovenous haemodialysis versus continuous venovenous haemofiltration. International Medical Journal 2006;13(1):37‐42. [EMBASE: 2006177999] [Google Scholar]
- Davenport A, Will EJ, Davison AM. Continuous vs. intermittent forms of haemofiltration and/or dialysis in the management of acute renal failure in patients with defective cerebral autoregulation at risk of cerebral oedema. Contributions to Nephrology 1991;93:225‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Davenport A, Will EJ, Davison AM. Effect of renal replacement therapy on patients with combined acute renal and fulminant hepatic failure. Kidney International ‐ Supplement 1993;41:S245‐51. [MEDLINE: ] [PubMed] [Google Scholar]
- Davies HT, Leslie G, Pereira SM, Webb SA. A randomized comparative crossover study to assess the affect on circuit life of varying pre‐dilution volume associated with CVVH and CVVHDF. International Journal of Artificial Organs 2008;31(3):221‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pont AC, Bouman CS, Bakhtiari K, Schaap MC, Nieuwland R, Sturk A, et al. Predilution versus postdilution during continuous venovenous hemofiltration: a comparison of circuit thrombogenesis. ASAIO Journal 2006;52(4):416‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Durmaz I, Yagdi T, Calkavur T, Mahmudov R, Apaydin AZ, Posacioglu H, et al. Prophylactic dialysis in patients with renal dysfunction undergoing on‐pump coronary artery bypass surgery. Annals of Thoracic Surgery 2003;75(3):859‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gabriel DP, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury. Kidney International ‐ Supplement 2008;108:S87‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Gabriel DP, Caramori JT, Martin LC, Barretti P, Balbi AL. Continuous peritoneal dialysis compared with daily hemodialysis in patients with acute kidney injury. Peritoneal Dialysis International 2009;29:S62‐71. [MEDLINE: ] [PubMed] [Google Scholar]
- Garcia‐Fernandez N, Lavilla FJ, Rocha E, Purroy A. Haemostatic changes in systemic inflammatory response syndrome during continuous renal replacement therapy. Journal of Nephrology 2000;13(4):282‐9. [MEDLINE: ] [PubMed] [Google Scholar]; Garcia‐Fernandez N, Lavilla J, Rocha E, Purroy A. The hemostatic effects of continuous renal replacement therapy (CRRT) in acute renal failure (ARF) associated with systemic inflammatory response syndrome (SIRS) [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A224. [CENTRAL: CN‐00484046] [Google Scholar]
- Gasparovic V, Filipovic‐Grcic I, Merkler M, Pisl Z. Continuous renal replacement therapy (CRRT) or intermittent hemodialysis (IHD)‐‐what is the procedure of choice in critically ill patients?. Renal Failure 2003;25(5):855‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- George J, Pisharody R, Upendran B, Varma S, Leelakumari M, Palliyil S. Clinical trial of continuous veno venous hemodiafiltration and peritoneal dialysis in critically ill acute renal failure [abstract no: SaP218]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi305. [Google Scholar]; George J, Varma S, Kumar S, Thomas J, Gopi S, Pisharody R. Comparing continuous venovenous hemodiafiltration and peritoneal dialysis in critically ill patients with acute kidney injury: a pilot study. Peritoneal Dialysis International 2011;31(4):422‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ghani RA, Zainudin S, Ctkong N, Rahman AF, Wafa SR, Mohamad M, et al. Serum IL‐6 and IL‐1‐ra with sequential organ failure assessment scores in septic patients receiving high‐volume haemofiltration and continuous venovenous haemofiltration. Nephrology 2006;11(5):386‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Zainudin S, Ghani RA, Mohd M, Wafa SRW, Tong NKC. Stability of haemodynamic parameters during CRRT: a comparison between standard continuous venovenous haemofiltration and high‐volume haemofiltration in patients with acute renal failure and sepsis [abstract no: SP286]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv108. [CENTRAL: CN‐00583762] [Google Scholar]
- Gillum DM, Dixon BS, Yanover MJ, Kelleher SP, Shapiro MD, Benedetti RG, et al. The role of intensive dialysis in acute renal failure. Clinical Nephrology 1986;25(5):249‐55. [MEDLINE: ] [PubMed] [Google Scholar]
- Haase M, Silvester W, Uchino S, Goldsmith D, Davenport P, Tipping P, et al. A pilot study of high‐adsorption hemofiltration in human septic shock. International Journal of Artificial Organs 2007;30(2):108‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Han F, Sun R, Ni Y, Hu X, Chen X, Jiang L, et al. Early initiation of continuous renal replacement therapy improves clinical outcomes in patients with acute respiratory distress syndrome. American Journal of the Medical Sciences 2015;349(3):199‐205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Faulhaber‐Walter R, Hafer C, Jahr N, Vahlbruch J, Haller H, Fliser D, et al. The Hannover‐Dialysis‐Outcome (Hand‐Out)‐Study: comparison of standard versus intensified extended daily dialysis in treatment of patients with septic acute renal failure on the intensive‐care unit [abstract no: FO017]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi9. [CENTRAL: CN‐00716040] [DOI] [PubMed] [Google Scholar]; Faulhaber‐Walter R, Hafer C, Jahr N, Vahlbruch J, Hoy L, Haller H, et al. The Hannover Dialysis Outcome study: comparison of standard versus intensified extended dialysis for treatment of patients with acute kidney injury in the intensive care unit. Nephrology Dialysis Transplantation 2009;24(7):2179‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Faulhaber‐Walter R, Kielstein J, Vahlbruch J, Jahr N, Hoy L, Haller H, et al. The Hannover Dialysis Outcome (HAN‐D‐OUT) study: comparison of standard versus intensified dialysis in treatment of patients with acute kidney injury in the ICU [abstract no: P475]. Critical Care (London, England) 2008;12(Suppl 2):P475. [CENTRAL: CN‐00691817] [Google Scholar]
- Combes A, Brechot N, Amour J, Cozic N, Lebreton G, Guidon C, et al. Early high‐volume hemofiltration versus standard care for post‐cardiac surgery shock. the HEROICS study. American Journal of Respiratory & Critical Care Medicine 2015;192(10):1179‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoste E, Vanholder R, Claus S, Decruyenaere J, Colardyn F, Lameire N. Better survival on intermittent vs. continuous hemodialysis in acute renal failure [abstract]. Journal of the American Society of Nephrology 1995;6(3):980. [CENTRAL: CN‐00484407] [Google Scholar]
- Jamale TE, Hase NK, Kulkarni M, Pradeep KJ, Keskar V, Jawale S, et al. Earlier‐start versus usual‐start dialysis in patients with community‐acquired acute kidney injury: a randomized controlled trial. American Journal of Kidney Diseases 2013;62(6):1116‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jeffrey RF, Khan AA, Prabhu P, Todd N, Goutcher E, Will EJ, et al. A comparison of molecular clearance rates during continuous hemofiltration and hemodialysis with a novel volumetric continuous renal replacement system. Artificial Organs 1994;18(6):425‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- John S, Griesbach D, Baumgartel M, Weihprecht H, Schmieder RE, Geiger H. Effects of continuous haemofiltration vs intermittent haemodialysis on systemic haemodynamics and splanchnic regional perfusion in septic shock patients: a prospective, randomized clinical trial. Nephrology Dialysis Transplantation 2001;16(2):320‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jones CH, Goutcher E, Newstead CG, Will EJ, Dean SG, Davison AM. Hemodynamics and survival of patients with acute renal failure treated by continuous dialysis with two synthetic membranes. Artificial Organs 1998;22(8):638‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kellum JA, Johnson JP, Kramer D, Palevsky P, Brady JJ, Pinsky MR. Diffusive vs. convective therapy: effects on mediators of inflammation in patient with severe systemic inflammatory response syndrome. Critical Care Medicine 1998;26(12):1995‐2000. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Kretschmer U, Ernst T, Hafer C, Bahr MJ, Haller H, et al. Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: a randomized controlled study. American Journal of Kidney Diseases 2004;43(2):342‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Kielstein JT, Kretschmer U, Ernst T, Hafer C, Haller H, Fliser D. Extended dialysis for treatment of critically patients with renal failure in the intensive care unit [abstract]. ISN ‐ERA/EDTA World Congress of Nephrology Satellite Symposium on ARF; 2003 Jun 13‐15; Ghent, Belgium. 2003:O20. [CENTRAL: CN‐00461064] [Google Scholar]; Kielstein JT, Kretschmer U, Ernst T, Hafer C, Haller H, Fliser D. Randomized controlled study on efficacy and cardiovascular tolerability of slow low‐efficient daily dialysis (SLEDD) vs. CVVH in critically ill patients with acute renal failure. [abstract no: SU‐PO897]. Journal of the American Society of Nephrology 2003;14(Nov):732A. [CENTRAL: CN‐00653751]12595510 [Google Scholar]; Kielstein JT, Kretschmer U, Ernst T, Hafer C, Haller H, Fliser D. Slow low‐efficient daily dialysis (SLED) as renal replacement therapy for acute renal failure in the intensive care unit: combination of superior detoxification and excellent cardiovascular tolerability in severely ill patients [abstract no: W381]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):667. [CENTRAL: CN‐00446081] [Google Scholar]
- Kielstein JT, Hafer C, Reising A, Haller H, Fliser D. Extended daily dialysis (EDD) but not veno‐venous hemofiltration (CVVH) increases soluble TNF receptor (sTNFR) plasma concentration in septic patients with acute renal failure [abstract no: F‐PO946]. Journal of the American Society of Nephrology 2005;16:542A. [Google Scholar]
- Kierdorf H, Leue C, Heintz B, Riehl J, Melzer H, Sieberth HG. Continuous venovenous hemofiltration in acute renal failure: is a bicarbonate‐ or lactate‐buffered substitution better?. Contributions to Nephrology 1995;116:38‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klouche K, Amigues L, Deleuze S, Beraud JJ, Canaud B. Complications, effects on dialysis dose, and survival of tunneled femoral dialysis catheters in acute renal failure. American Journal of Kidney Diseases 2007;49(1):99‐108. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Koo J, Yoon J, Oh J, Lee Y, Kim S, Seo J, Bae J. Prospective evaluation of early continuous venovenous hemofiltration (CVVH) on the outcome in patients with severe sepsis or septic shock [abstract no: F‐FC062]. Journal of the American Society of Nephrology 2006;17(Abstract):50A. [Google Scholar]
- Kumar V, Tseng R, Depner T. Extended daily dialysis (EDD) controls azotemia in intensive care unit (ICU) patients with acute renal failure [abstract no: 42]. American Journal of Kidney Diseases 2002;39(4):A21. [CENTRAL: CN‐00401569] [Google Scholar]; Kumar VA, Yeun JY, Depner TA, Don BR. Extended daily dialysis vs. continuous hemodialysis for ICU patients with acute renal failure: a two‐year single center report. International Journal of Artificial Organs 2004;27(5):371‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lentini P, Cruz D, Nalesso F, Cal M, Bobek I, Garzotto F, et al. A pilot study comparing pulse high volume hemofiltration (pHVHF) and coupled plasma filtration adsorption (CPFA) in septic shock patients [Coupled plasma filtration adsorption (CPFA) versus emofiltrazione continua con regime intermittente di alti volumi (pHVHF) nel trattamento dello shock settico: uno studio pilota]. Giornale Italiano di Nefrologia 2009;26(6):695‐703. [MEDLINE: ] [PubMed] [Google Scholar]
- Manns M, Sigler M, Teehan B. Comparison of continuous venovenous haemodialysis and intermittent haemodialysis in patients with acute renal failure. Wiener Klinische Wochenschrift 1996;108(Suppl 1):29‐30. [CENTRAL: CN‐00251998]8835429 [Google Scholar]
- Maxvold NJ, Smoyer WE, Custer JR, Bunchman TE. Amino acid loss and nitrogen balance in critically ill children with acute renal failure: a prospective comparison between classic hemofiltration and hemofiltration with dialysis. Critical Care Medicine 2000;28(4):1161‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mehta R, McDonald B, Gabbai F, Pahl M, Farkas A, Pascual M, et al. Co‐morbidities influence outcome from acute renal failure (ARF) in the ICU: results from a randomized multicenter trial. [abstract no: A0743]. Journal of the American Society of Nephrology 1996;7(9):1394. [CENTRAL: CN‐00446714] [Google Scholar]; Mehta R, McDonald B, Gabbai F, Pahl M, Farkas A, Pascual M, et al. Continuous versus intermittent dialysis for acute renal failure (ARF) in the ICU: results from a randomized multicenter trial [abstract no: A1044]. Journal of the American Society of Nephrology 1996;7(9):1457. [CENTRAL: CN‐00446713] [Google Scholar]; Mehta R, McDonald B, Gabbai F, Pahl M, Pascual M, Farkas A, et al. Continuous versus intermittent dialysis for acute renal failure (ARF) in the ICU: efficacy of solute and fluid control [abstract no: A1022]. Journal of the American Society of Nephrology 1997;8(Program and Abstracts):221A. [CENTRAL: CN‐00626119] [Google Scholar]; Mehta R, McDonald B, Gabbai F, Pahl M, Pascual M, Farkas A, et al. Costs associated with intermittent hemodialysis (IHD) and continuous renal replacement therapy (CRRT) for treatment of acute renal failure (ARF) in the ICU: results from a randomized multicenter trial [abstract no: A0796]. Journal of the American Society of Nephrology 1998;9(Program and Abstracts):155A. [CENTRAL: CN‐00446717]9527391 [Google Scholar]; Mehta R, McDonald B, Gabbai F, Pahl M, Pascual M, Farkas A, et al. Indication for dialysis influences outcome from acute renal failure in the ICU: results from a randomized multicenter trial [abstract no: A0682]. Journal of the American Society of Nephrology 1997;8(Program and Abstracts):144A. [CENTRAL: CN‐00583931] [Google Scholar]; Mehta RL. Continuous dialysis in the ICU [abstract no: 51]. Nephrology 1997;3(Suppl 1):S20. [CENTRAL: CN‐00461292] [Google Scholar]; Mehta RL, McDonald B, Gabbai FB, Pahl M, Pascual MT, Farkas A, et al. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney International 2001;60(3):1154‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meloni C, Morosetti M, Meschini L, Palombo G, Latorre PC, Taccone‐Gallucci M, et al. Blood purification procedures for acute renal failure: convenient strategy related to clinical conditions. Blood Purification 1996;14(3):242‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Misset B, Timsit JF, Chevret S, Renaud B, Tamion F, Carlet J. A randomized cross‐over comparison of the hemodynamic response to intermittent hemodialysis and continuous hemofiltration in ICU patients with acute renal failure. Intensive Care Medicine 1996;22(8):742‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morgera S, Melzer C, Sobottke V, Vargas‐Hein O, Zuckermann‐Becker H, Bellomo R, et al. Renal replacement therapy with high cut‐off hemofilters: impact of convection and diffusive on cytokine clearances and protein status [abstract no: P151]. Critical Care 2004;8(Suppl 1):P151. [CENTRAL: CN‐00724921] [DOI] [PubMed] [Google Scholar]; Morgera S, Slowinski T, Melzer C, Sobottke V, Vargas‐Hein O, Volk T, et al. Renal replacement therapy with high‐cutoff hemofilters: impact of convection and diffusion on cytokine clearances and protein status. American Journal of Kidney Diseases 2004;43(3):444‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morgera S, Haase M, Kuss T, Vargas‐Hein O, Zuckermann‐Becker H, Melzer C, et al. Pilot study on the effects of high cutoff hemofiltration on the need for norepinephrine in septic patients with acute renal failure. Critical Care Medicine 2006;34(8):2099‐104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Morgera S, Kuss T, Neumayer H. Pilot study on the effects of high cut‐off hemofiltration on the need for norepinephrine in septic patients with acute renal failure [abstract no: SP288]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv109. [DOI] [PubMed] [Google Scholar]
- Noble JS, Simpson K, Allison ME. Long‐term quality of life and hospital mortality in patients treated with intermittent or continuous hemodialysis for acute renal and respiratory failure. Renal Failure 2006;28(4):323‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Simpson K, Allison ME. Dialysis and acute renal failure: can mortality be improved? [abstract]. Nephrology Dialysis Transplantation 1993;8(9):946. [CENTRAL: CN‐00402661] [Google Scholar]
- Wald R, Friedrich JO, Bagshaw SM, Burns KE, Garg AX, Hladunewich MA, et al. Optimal Mode of clearance in critically ill patients with Acute Kidney Injury (OMAKI) ‐ a pilot randomized controlled trial of hemofiltration versus hemodialysis: a Canadian Critical Care Trials Group project. Critical Care (London, England) 2012;16(5):R205. [EMBASE: 2012734569] [DOI] [PMC free article] [PubMed] [Google Scholar]; Wald R, Friedrich JO, Bagshaw SM, Burns KE, Garg AX, Hladunewich MA, et al. The optimal mode of renal replacement therapy in acute kidney injury (OMAKI): a pilot randomized controlled trial of CVVH vs CVVHD [abstract no: LB‐PO3173]. Journal of the American Society of Nephrology 2011;22(Abstracts):10B. [Google Scholar]
- Oudemans‐van Straaten HM, Schilfgaarde M, Molenaar PJ, Wester JP, Leyte A. Hemostasis during low molecular weight heparin anticoagulation for continuous venovenous hemofiltration: a randomized cross‐over trial comparing two hemofiltration rates. Critical Care (London, England) 2009;13(6):R193. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganini EP, Sandy D, Moreno L, Kozlowski L, Sakai K. The effect of sodium and ultrafiltration modelling on plasma volume changes and haemodynamic stability in intensive care patients receiving haemodialysis for acute renal failure: a prospective, stratified, randomized, cross‐over study. Nephrology Dialysis Transplantation 1996;11 Suppl 8:32‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Park JT, Lee H, Kee YK, Park S, Oh HJ, Han SH, et al. High‐dose versus conventional‐dose continuous venovenous hemodiafiltration and patient and kidney survival and cytokine removal in sepsis‐associated acute kidney injury: a randomized controlled trial. American Journal of Kidney Diseases 2016;68(4):599‐608. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Payen D, Mateo J, Cavaillon JM, Fraisse F, Floriot C, Vicaut E, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Critical Care Medicine 2009;37(3):803‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pettila V, Tiula E. Intermittent hemodiafiltration in acute renal failure in critically ill patients. Clinical Nephrology 2001;56(4):324‐31. [MEDLINE: ] [PubMed] [Google Scholar]
- Ponce D, Balbi AL. Different prescribed doses of high volume peritoneal dialysis and outcome of patients with acute kidney injury [abstract]. Peritoneal Dialysis International 2012;32(Suppl 3):S4. [EMBASE: 71927756] [PubMed] [Google Scholar]; Ponce D, Brito GA, Abrao JG, Balb AL. Different prescribed doses of high‐volume peritoneal dialysis and outcome of patients with acute kidney injury. Advances in Peritoneal Dialysis 2011;27(1):118‐24. [MEDLINE: ] [PubMed] [Google Scholar]
- Ponce D, Balbi AL, Abrao JM, Berbel MN, Pinto MP, Brito GA. High volume peritoneal dialysis (PD) versus sustained low efficiency dialysis: a randomized controlled trial in patients with acute kidney injury: Initial results [abstract]. Peritoneal Dialysis International 2012;32(Suppl 1):S4. [EMBASE: 71927757] [Google Scholar]; Ponce D, Berbel MN, Abrao JM, Goes CR, Balbi AL. A randomized clinical trial of high volume peritoneal dialysis versus extended daily hemodialysis for acute kidney injury patients. International Urology & Nephrology 2013;45(3):869‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pursnani ML, Hazra DK, Singh B, Pandey DN. Early haemodialysis in acute tubular necrosis. Journal of the Association of Physicians of India 1997;45(11):850‐2. [MEDLINE: ] [PubMed] [Google Scholar]
- Ratanarat R, Chaipruckmalakarn T, Laowahutanont N, Larpparisuth N, Vasuvattakul S. Efficacy and hemodynamic outcome of prolonged intermittent renal replacement therapy (PIRRT) in critically ill patients: a preliminary report. Journal of the Medical Association of Thailand 2012;95 Suppl 2:S265‐71. [MEDLINE: ] [PubMed] [Google Scholar]
- Bellomo R. Do we know the optimal dose for renal replacement therapy in the intensive care unit?. Kidney International 2006;70(7):1202‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Kim I, et al. The relationship between hypophosphataemia and outcomes during low‐intensity and high‐intensity continuous renal replacement therapy.[Erratum appears in Crit Care Resusc. 2014 Jun;16(2):139 Note: McGuiness, Shay [corrected to McGuinness, Shay]]. Critical Care & Resuscitation 2014;16(1):34‐41. [MEDLINE: ] [PubMed] [Google Scholar]; Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, et al. Calorie intake and patient outcomes in severe acute kidney injury: findings from the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy (RENAL) study trial. Critical Care (London, England) 2014;18(2):R45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, et al. Daily protein intake and patient outcomes in severe acute kidney injury: findings of the Randomized Evaluation of Normal versus Augmented Level of replacement therapy (RENAL) trial. Blood Purification 2014;37(4):325‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Bellomo R, Lipcsey M, Calzavacca P, Haase M, Haase‐Fielitz A, Licari E, et al. Early acid‐base and blood pressure effects of continuous renal replacement therapy intensity in patients with metabolic acidosis. Intensive Care Medicine 2013;39(3):429‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Bellomo R, Martensson J, Kaukonen KM, Lo S, Gallagher M, Cass A, et al. Epidemiology of RBC transfusions in patients with severe acute kidney injury: analysis from the randomized evaluation of normal versus augmented level study. Critical Care Medicine 2016;44(5):892‐900. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Finfer S, Cass A, Gallagher M, Lee J, Su S, Bellomo R, et al. The RENAL (Randomised Evaluation of Normal vs. Augmented Level of replacement therapy) study: statistical analysis plan. Critical Care & Resuscitation 2009;11(1):58‐66. [MEDLINE: ] [PubMed] [Google Scholar]; Gallagher M, Bellomo R, Cass A, Finfer S, Gattas D, Lee J, et al. Long term outcomes of severe AKI: results of the post‐RENAL study [abstract no: 071]. Nephrology 2012;17(Suppl 2):45‐6. [EMBASE: 71377309] [Google Scholar]; Gallagher M, Cass A, Bellomo R, Finfer S, Gattas D, Lee J, et al. Long‐term survival and dialysis dependency following acute kidney injury in intensive care: extended follow‐up of a randomized controlled trial. PLoS Medicine 2014;11(2):e1001601. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Jun M, Bellomo R, Cass A, Gallagher M, Lo S. Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal vs augmented level of replacement therapy trial [abstract no: 008]. Nephrology 2012;17(Suppl 2):29‐30. [EMBASE: 71377246] [Google Scholar]; Jun M, Bellomo R, Cass A, Gallagher M, Lo S, Lee J, et al. Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal versus augmented level of replacement therapy study. Critical Care Medicine 2014;42(8):1756‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Lin J, Wang Y, Bellomo R, Duan ML, Gallagher MP. Sofa coagulation score and patient outcomes in severe AKI: analysis from the Randomised Evaluation of Normal versus Augmented Level (RENAL) study [abstract no: PUB049]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):976. [Google Scholar]; RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Design and challenges of the Randomized Evaluation of Normal versus Augmented Level Replacement Therapy (RENAL) Trial: high‐dose versus standard‐dose hemofiltration in acute renal failure. [Review] [31 refs]. Blood Purification 2008;26(5):407‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Intensity of continuous renal‐replacement therapy in critically ill patients. New England Journal of Medicine 2009;361(17):1627‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Screening and study enrolment in the Randomized Evaluation of Normal vs. Augmented Level (RENAL) Replacement Therapy Trial. Blood Purification 2009;27(2):199‐205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement therapy trial. Critical Care Medicine 2012;40(6):1753‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Roberts D, Roberts M, Liu X, Roberts J, Lipman J, Bellomo R. Clearance of antibiotics by high and low intensity continuous renal replacement therapy in critically ill patients [abstract no: 232]. Nephrology 2010;15(Suppl 4):87. [EMBASE: 70467236] [Google Scholar]; Roberts DM, Liu X, Roberts JA, Nair P, Cole L, Roberts MS, et al. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Critical Care (London, England) 2015;19:84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang A, Lo S, Bellomo R, Cass A, Gallagher M. Ace inhibitor use and AKI outcomes: an analysis of the Randomised Evaluation of Normal vs Augmented Level of replacement therapy (RENAL) trial [abstract no: 104]. Nephrology 2013;18(Suppl 1):41. [EMBASE: 71357086]23252802 [Google Scholar]; Wang AY, Bellomo R, Cass A, Finfer S, Gattas D, Myburgh J, et al. Health‐related quality of life in survivors of acute kidney injury: The prolonged outcomes study of the Randomized Evaluation of Normal versus Augmented Level replacement therapy study outcomes. Nephrology 2015;20(7):492‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Wang AY, Bellomo R, Cass A, Myburgh J, Finfer S, Gatta D, et al. Predictors of health‐related quality of life in survivors following acute kidney injury: A secondary analysis of post‐renal study outcomes [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii107‐8. [EMBASE: 71491744] [Google Scholar]; Wang AY, Bellomo R, Ninomiya T, Lo S, Cass A, Jardine M, et al. Angiotensin‐converting enzyme inhibitor usage and acute kidney injury: a secondary analysis of RENAL study outcomes. Nephrology 2014;19(10):617‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Doig GS. Concerns regarding use of one‐tailed tests in the SLED‐BD vs. CVVH trial. Critical Care (London, England) 2012;16(5):448. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Schwenger V, Weigand MA, Hoffmann O, Dikow R, Kihm LP, Seckinger J, et al. Sustained low efficiency dialysis using a single‐pass batch system in acute kidney injury ‐ a randomized interventional trial: the REnal Replacement Therapy Study in Intensive Care Unit PatiEnts.[Erratum appears in Crit Care. 2012;16(5):451]. Critical Care 2012;16(4):R140. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci Z, Ronco C, Bachetoni A, D'amico G, Rossi S, Alessandri E, et al. Solute removal during continuous renal replacement therapy in critically ill patients: convection versus diffusion. Critical Care (London, England) 2006;10(2):R67. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C, Bellomo R, Brendolan A, Pinna V, Greca G. Brain density changes during renal replacement in critically ill patients with acute renal failure. Continuous hemofiltration versus intermittent hemodialysis. Journal of Nephrology 1999;12(3):173‐8. [MEDLINE: ] [PubMed] [Google Scholar]; Ronco C, Brendolan A, Bellomo R. On‐line monitoring of blood volume in continuous and intermittent renal replacement therapies. Clinical Intensive Care 1999;10(4):125‐9. [EMBASE: 1999305798] [Google Scholar]
- Ho TB, Jefferson HJ, Rhodes A. Continuous haemofiltration in acute renal failure. Lancet 2000;356(9239):1441‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, et al. Effects of different doses in continuous veno‐venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Edtna‐Erca Journal 2002;Suppl 2:7‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, et al. Effects of different doses in continuous veno‐venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000;356(9223):26‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Ronco C, Homel P, Bellomo R, Brendolan A. Prospective randomised trial on dose delivery versus outcomes of RF treated by continuous veno‐venous hemofiltration (CVVH) [abstract no: A0717]. Journal of the American Society of Nephrology 2000;11(Sept):133A. [CENTRAL: CN‐00644229] [Google Scholar]; Schiffl H. Continuous haemofiltration in acute renal failure. Lancet 2000;356(9239):1441. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Than N, Turney JH. Continuous haemofiltration in acute renal failure. Lancet 2000;356(9239):1441. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ronco C, Bellomo R, Ricci Z. Hemodynamic response to fluid withdrawal in overhydrated patients treated with intermittent ultrafiltration and slow continuous ultrafiltration: role of blood volume monitoring. Cardiology 2001;96(3‐4):196‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Saudan P, Niederberger M, Seigneux S, Romand J, Pugin J, Perneger T, et al. A prospective randomized trial comparing continuous hemodiafiltration versus hemofiltration in critically ill patients with acute renal failure [abstract no: PUB003]. Journal of the American Society of Nephrology 2004;15(Oct):763A. [CENTRAL: CN‐00724917] [Google Scholar]; Saudan P, Niederberger M, Seigneux S, Romand J, Pugin J, Perneger T, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney International 2006;70(7):1312‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Saudan P, Niederberger M, Sellweger M, Pugin J, Romand J, Perneger T, et al. Continuous hemofiltration versus continuous hemodiafiltration in critically ill patients with acute renal failure [abstract no: PUB003]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):666. [CENTRAL: CN‐00447595] [Google Scholar]; Saudan P, Ponte B, Pugin J, Martin P. Improving survival with a greater dialysis dose in patients with AKI: does it depend on early onset of treatment? [abstract no: SU121]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [Google Scholar]; Saudan P, Triverio PA, Romand JA, Pugin J, Martin PY. Long‐term prognosis in critically ill patients with acute renal failure treated by continuous renal replacement therapy [abstract no: TH‐PO822]. Journal of the American Society of Nephrology 2006;17(Abstracts):282A. [CENTRAL: CN‐00724918] [Google Scholar]; Triverio PA, Martin PY, Romand J, Pugin J, Perneger T, Saudan P. Long‐term prognosis after acute kidney injury requiring renal replacement therapy. Nephrology Dialysis Transplantation 2009;24(7):2186‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schiffl H, Lang SM, Konig A, Held E. Dose of intermittent hemodialysis (IHD) and outcome of acute renal failure (ARF): a prospective randomized study [abstract no: A1333]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):290‐1A. [CENTRAL: CN‐00447619] [Google Scholar]
- Drazen JM, Ingelfinger JR, Curfman GD. Expression of concern: Schiffl H, et Al. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 2002;346:305‐10.[Expression of Concern for N Engl J Med. 2002 Jan 31;346(5):305‐10; PMID: 11821506]. New England Journal of Medicine 2003;348(21):2137. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Drazen JM, Ingelfinger JR, Curfman GD. Removal of expression of concern: Schiffl H, et Al. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 2002;346:305‐10. New England Journal of Medicine 2003;349(20):1965. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure.[Expression of Concern in N Engl J Med. 2003 May 22;348(21):2137; PMID: 12761371]. New England Journal of Medicine 2002;346(5):305‐10. [MEDLINE: ]11821506 [Google Scholar]; Schiffl H, Lang SM, Fischer R, Gartner R, Held E. Does more haemodialysis enhance survival in acute renal failure? [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A114. [CENTRAL: CN‐00447618] [Google Scholar]
- Elseviers MM, Lins RL, Niepen P, Hoste E, Malbrain M, Damas P, et al. Baseline creatinine is an independent predictive factor for mortality in ARF: results of the SHARF 4 study [abstract no: SP307]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv115. [CENTRAL: CN‐00583400] [Google Scholar]; Elseviers MM, Lins RL, Niepen P, Hoste E, Malbrain M, Damas P, et al. Long‐term cost analysis of ARF in patients admitted to the ICU [abstract no: TH‐PO837]. Journal of the American Society of Nephrology 2006;17(Abstracts):285A. [CENTRAL: CN‐00602061]16319190 [Google Scholar]; Lins R, Elseviers M, Niepen P, Hoste E, Malbrain M, Damas P, et al. Delayed ICU admission as independent risk factor for mortality in ARF patients: results of the SHARF4 study [abstract no: F‐PO944]. Journal of the American Society of Nephrology 2005;16(Oct):542A. [CENTRAL: CN‐00583456] [Google Scholar]; Lins R, Elseviers M, Niepen P, Hoste E, Malbrain M, Damas P, et al. Influence of dialysis dose on outcome in ARF patients: results of SHARF4 study [abstract no: F‐PO945]. Journal of the American Society of Nephrology 2005;16(Oct):542A. [CENTRAL: CN‐00583424] [Google Scholar]; Lins RL, Elseviers M, Malbrain M, Niepen P, Damas P, Hoste E. Stratified comparison between different treatment modalities in acute renal failure [abstract no: SU‐PO942]. Journal of the American Society of Nephrology 2003;14(Nov):743A. [CENTRAL: CN‐00550532] [Google Scholar]; Lins RL, Elseviers M, SHARF Study Group. Short and long‐term outcome of acute renal failure with different treatment modalities [abstract no: A0909]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):174A. [CENTRAL: CN‐00446420] [Google Scholar]; Lins RL, Elseviers MM, Niepen P, Hoste E, Malbrain M, Damas P, et al. A randomised trial of different renal replacement modalities in acute renal failure: results of the SHARF study [abstract no: SO11]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v6‐7. [CENTRAL: CN‐00602116] [Google Scholar]; Lins RL, Elseviers MM, Niepen P, Hoste E, Malbrain M, Damas P, et al. Outcome of different treatment modalities in ARF: descriptive results. [abstract no: F‐PO579]. Journal of the American Society of Nephrology 2004;15(Oct):194A. [CENTRAL: CN‐00550547]14694173 [Google Scholar]; Lins RL, Elseviers MM, Niepen P, Hoste E, Malbrain M, Damas P, et al. Outcome of different treatment modalities in ARF: randomized population [abstract no: FPO580]. Journal of the American Society of Nephrology 2004;15(Oct):195A. [CENTRAL: CN‐00550540] [Google Scholar]; Lins RL, Elseviers MM, Niepen P, Hoste E, Malbrain ML, Damas P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrology Dialysis Transplantation 2009;24(2):512‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Lins RL, Elseviers MM, Niepen P, Hoste E, Malbrain M, Damas P, et al. Conservative treatment versus renal replacement therapy in patients with acute renal failure: results of the SHARF study [abstract no: SP074]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v44. [CENTRAL: CN‐00644338] [Google Scholar]; Malbrain M, Elseviers M, Niepen P, Damas P, Hoste E, Devriendt J. Interim results of the SHARF4 study: outcome of acute renal failure with different treatment modalities [abstract no: 153]. Critical Care (London, England) 2004;8(Suppl 1):15. [CENTRAL: CN‐00740490] [Google Scholar]; Berendoncks A, Elseviers MM, Lins RL. Outcome of acute renal failure with different treatment modalities: long term follow‐up [abstract no: SP299]. Nephrology Dialysis Transplantation 2006;31(Suppl 4):iv112. [CENTRAL: CN‐00690969] [Google Scholar]; Berendoncks AM, Elseviers MM, Lins RL, SHARF Study Group. Outcome of acute kidney injury with different treatment options: long‐term follow‐up. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(10):1755‐62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidis I, Hagel J, Kierdorf H, Maurin N. Influencing hemostasis during continuous venovenous hemofiltration after acute renal failure: comparison with intermittent hemodialysis. Contributions to Nephrology 1995;116:140‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Inthorn D, Storck M, Hartl WH, Zimmerer E. Improved survival rate of postoperative renal failure caused by high volume hemofiltration [Verbesserung der uberlebensrate des postoperativen nierenversagens durch hochvolumige hamofiltration]. Zentralblatt fur Chirurgie 1991;116(16):961‐8. [MEDLINE: ] [PubMed] [Google Scholar]; Storck M, Hartl WH, Zimmerer E, Inthorn D. Comparison of pump‐driven and spontaneous continuous haemofiltration in postoperative acute renal failure. Lancet 1991;337(8739):452‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tan HK, Bellomo R, M'Pis DA, Ronco C. Phosphatemic control during acute renal failure: intermittent hemodialysis versus continuous hemodiafiltration. International Journal of Artificial Organs 2001;24(4):186‐91. [MEDLINE: ] [PubMed] [Google Scholar]
- Lyndon W, Wille K, Tolwani A. Solute clearance in CRRT: comparing measured effluent volume to actual delivered dose [abstract no: 177]. American Journal of Kidney Diseases 2011;57(4):A61. [EMBASE: 70379736] [Google Scholar]; Lyndon WD, Wille KM, Tolwani AJ. Solute clearance in CRRT: comparing measured effluent volume to actual delivered dose. 16th International Conference on CRRT; 2011 Feb 22‐25; San Diego (CA). 2011:127. [Google Scholar]; Lyndon WD, Wille KM, Tolwani AJ. Solute clearance in CRRT: prescribed dose versus actual delivered dose. Nephrology Dialysis Transplantation 2012;27(3):952‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high‐dose CVVHDF for ICU‐related acute renal failure. Journal of the American Society of Nephrology 2008;19(6):1233‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Tolwani AJ, Speer R, Stofan B, Lai KR, Wille KM. A randomized prospective study comparing high dose continuous venovenous hemodiafiltration (CVVHDF) to standard CVVHDF in critically ill patients with acute renal injury [abstract no: 22]. Blood Purification 2007;25:193. [Google Scholar]
- Farese S, Jakob SM, Frey FJ, Uehlinger DE. More than 50% lower costs by IHD than by CVVHDF to treat acute renal failure in the ICU [abstract no: SA‐PO939]. Journal of the American Society of Nephrology 2006;17(Abstracts):772A. [CENTRAL: CN‐00740528] [Google Scholar]; Farese S, Jakob SM, Kalicki R, Frey FJ, Uehlinger DE. Treatment of acute renal failure in the intensive care unit: lower costs by intermittent dialysis than continuous venovenous hemodiafiltration. Artificial Organs 2009;33(8):634‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Ferrari P, Uehlinger DE, Jakob SM, Eichelberger M, Huynh‐Do U, Marti HP, et al. A comparison of continuous and intermittent renal replacement therapy for acute renal failure [abstract no: 47]. Nephrology 2003;8(Suppl 3):A66. [CENTRAL: CN‐00644145] [DOI] [PubMed] [Google Scholar]; Uehlinger DE, Jakob SM, Eichelberger M, Ferrari P, Huynh‐Do U, Marti HP, et al. A randomized, controlled single‐center study for the comparison of continuous renal replacement therapy (CVVHDF) with intermittent hemodialysis (IHD) in critically ill patients with acute renal failure [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):278A. [CENTRAL: CN‐00448092] [Google Scholar]; Uehlinger DE, Jakob SM, Ferrari P, Eichelberger M, Huynh‐Do U, Marti HP, et al. Comparison of continuous and intermittent renal replacement therapy for acute renal failure. Nephrology Dialysis Transplantation 2005;20(8):1630‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Voort PH, Gerritsen RT, Kuiper MA, Egbers PH, Kingma WP, Boerma EC. Filter run time in CVVH: pre‐ versus post‐dilution and nadroparin versus regional heparin‐protamine anticoagulation. Blood Purification 2005;23(3):175‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple‐organ dysfunction syndrome: a multicentre randomised trial. Lancet 2006;368(9533):379‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wynckel A, Wuillai A, Bene B, Leon A, Cornillet J, Chanard J. Comparison of acetate‐free continuous venovenous hemofiltration (AF‐CVVH) and conventional CVVH in acute renal failure (ARF) [abstract no: A0969]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):186A. [CENTRAL: CN‐00448431] [Google Scholar]; Wynckel A, Wullai A, Bene B, Randoux C, Leon A, Melin JP, et al. Acetate free continuous venovenous hemofiltration (AFCVVH): a new treatment of acute renal failure (ARF) allowing the separate control of uremia and acid base disturbances [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):187A. [CENTRAL: CN‐00448432] [Google Scholar]
- Wynckel A, Cornillet J, Bene B, Stolz A, Lepouse C, Paris B, et al. Improved removal of small proteins using continuous venovenous hemofiltration to treat acute renal failure. ASAIO Journal 2004;50(1):81‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zhang J, Tao LJ, Ning JP, Xu H, Ai YH, Zhao SP. Effects of high‐volume hemofiltration on serum levels of tumor necrosis factor and its receptors in patients with multiple organ dysfunction syndromes. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue [Chinese Critical Care Medicine] 2004;16(2):81‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Zhao SP, Wu J, Ai YH, Sun B, Xu DM, Guo QL. Comparison of clinical efficacy between continuous veno‐venous hemofiltration (CVVH) and CVVH combined with hemoperfusion for the treatment of multiple organ dysfunction syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue [Chinese Critical Care Medicine] 2009;21(6):373‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Zimmerman D, Cotman P, Ting R, Karanicolas S, Tobe SW. Continuous veno‐venous haemodialysis with a novel bicarbonate dialysis solution: prospective cross‐over comparison with a lactate buffered solution. Nephrology Dialysis Transplantation 1999;14(10):2387‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Barbar SD, Binquet C, Monchi M, Bruyere R, Quenot JP. Impact on mortality of the timing of renal replacement therapy in patients with severe acute kidney injury in septic shock: the IDEAL‐ICU study (initiation of dialysis early versus delayed in the intensive care unit): study protocol for a randomised controlled trial. Trials 2014;15(1):270. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- Hong S. A randomised controlled multi‐centre trial of the early application of CRRT in patients with severe sepsis or septic shock. www.clinicaltrials.gov/ct2/show/NCT00837057 (first received 5 February 2009).
Additional references
- Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Critical Care (London, England) 2007;11(3):R68. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. Journal of Critical Care 2009;24(1):129‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brunnet S, Leblanc M, Geadah D, Parent D, Courteau S, Cardinal J. Diffusive and convective solute clearances during continuous renal replacement therapy at various dialysate and ultrafiltration flow rates. American Journal of Kidney Diseases 1999;34(3):486–92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- CKT. Reporting results in CKT reviews (using material adapted from EPOC and CCCR). www.kidneyandtransplant.cochrane.org/sites/kidneyandtransplant.cochrane.org/files/public/uploads/Resources/reporting_results_in_ckt_reviews_2017.pdf (date accessed 26 November 2018).
- Clark WR, Ronco C. CRRT efficiency and efficacy in relation to solute size. Kidney International ‐ Supplement 1999;72:S3‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Demirkilic U, Kuralay E, Yenicesu M, Caglar K, Oz BS, Cingoz F, et al. Timing of replacement therapy for acute renal failure after cardiac surgery. Journal of Cardiac Surgery 2004;19(1):17–20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Edrees F, Li T, Vijayan A. Prolonged intermittent renal replacement therapy. Advances in Chronic Kidney Disease 2016;23(3):195‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Elahi MM, Lim MY, Joseph RN, Dhannapuneni RR, Spyt TJ. Early hemofiltration improves survival in post‐cardiotomy patients with acute renal failure. European Journal of Cardiothoracic Surgery 2004;26(5):1027–31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Effective Practice of Care. Interpreting statistical significance. www.epoc.cochrane.org (last accessed prior to 26 November 2018).
- Fayad AI, Buamscha DG, Ciapponi A. Intensity of continuous renal replacement therapy for acute kidney injury. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD010613] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Critical Care Medicine 2004;32(8):1771–6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gettings LG, Reynolds HN, Scalea T. Outcome in post‐traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intensive Care Medicine 1999;25(8):805–13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gibney N, Cerda J, Davenport A, Ramirez J, Singbartl K, Leblanc M, et al. Volume management by renal replacement therapy in acute kidney injury. International Journal of Artificial Organs 2008;31(2):145‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goldstein SL, Currier H, Graf JM, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 2001;107(6):1309‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HL, Xue WJ, Li DQ, Yin AP, Xin X, Li CM, et al. Influence of continuous venovenous hemofiltration on the course of acute pancreatitis. World Journal of Gastroenterology 2005;11(31):4815‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joannidis M, Metnitz PG. Epidemiology and natural history of acute renal failure in the ICU. Critical Care Clinics 2005;21(2):239–49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Karvellas CJ, Farhat MR, Sajjad I, Mogensen SS, Leung AA, Wald R, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta‐analysis. Critical Care (London, England) 2011;15(1):R72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- KDIGO. Dialysis interventions for treatment of AKI. Kidney International ‐ Supplement 2012;2(1):89‐115. [PUBMED: 25018921] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TS, Shiao CC, Wang JJ, Huang CT, Wu PC, Chueh E, et al. Earlier versus later initiation of renal replacement therapy among critically ill patients with acute kidney injury: a systematic review and meta‐analysis of randomized controlled trials. Annals of Intensive Care 2017;7(1):38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Zhang W, Hardy PA, Poh CK, Huang Z, Kraus MA, et al. Kinetic comparison of different acute dialysis therapies. Artificial Organs 2003;27(9):802–7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Liu Y, Davari‐Farid S, Arora P, Porhomayon J, Nader ND. Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury after cardiac surgery: a systematic review and meta‐analysis. Journal of Cardiothoracic & Vascular Anesthesia 2014;28(3):557–63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Luo K, Fu S, Fang W, Xu G. The optimal time of initiation of renal replacement therapy in acute kidney injury: a meta‐analysis.[Erratum appears in Oncotarget. 2018 Jan 15;9(5):6657; PMID: 29465716]. Oncotarget 2017;8(40):68795‐808. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MR, Golper TA. Low‐efficiency acute renal replacement therapy: role in acute kidney injury. Seminars in Dialysis 2011;24(2):142‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mavrakanas TA, Aurian‐Blajeni DE, Charytan DM. Early versus late initiation of renal replacement therapy in patients with acute kidney injury: a meta‐analysis of randomised clinical trials. Swiss Medical Weekly 2017;147:w14507. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care (London, England) 2007;11(2):R31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink SN, Jha P, Wang R, Yang J, Bose D, Jacobs H, et al. Effect of continuous arteriovenous hemofiltration combined with systemic vasopressor therapy on depressed left ventricular contractility and tissue oxygen delivery in canine Escherichia coli sepsis. Anesthesiology 1995;83(1):178‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- NICE: National Institute for Health and Care Excellence. Acute kidney injury: prevention, detection and management. Clinical guideline [CG169] Published date: August 2013. www.nice.org.uk/guidance/cg169 (accessed 26 November 2018).
- Palevsky PM, Bunchman T, Tetta C. The Acute Dialysis Quality Initiative‐‐part V: operational characteristics of CRRT. Advances in Renal Replacement Therapy 2002;9(4):268–72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Palevsky PM, Baldwin I, Davenport A, Goldstein S, Paganini E. Renal replacement therapy and the kidney: minimizing the impact of renal replacement therapy on recovery of acute renal failure. Current Opinion in Critical Care 2005;11(6):548‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Critical Care Medicine 2010;38(3):933‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Seabra VF, Balk EM, Liangos O, Sosa AM, Cendoroglo M, Jaber BL. Timing of renal replacement therapy initiation in acute renal failure: a meta‐analysis. American Journal of Kidney Diseases 2008;52(2):272‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sieberth HG, Stummvoll HK, Kierdoff H (editors). Continuous extracorporeal treatment in multiple organ dysfunction syndrome: 3rd International Conference on Continuous Hemofiltration. Vienna, July 8, 1994 (Contributions to Nephrology). Vol. 116, Basal: Karger, 1995. [Google Scholar]
- Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. American Journal of Kidney Diseases 2010;55(2):316–25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teschan PE, Baxter CR, O’Brien TF, Freyhof JN, Hall WH. Prophylactic hemodialysis in the treatment of acute renal failure. Annals of Internal Medicine 1960;53:992‐1016. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294(7):813‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vinsonneau C, Allian‐Launay E, Blayau C, Darmon M, Ducheyron D, Gaillot T, et al. Renal replacement therapy in adult and pediatric intensive care: Recommendations by an expert panel from the French Intensive Care Society (SRLF) with the French Society of Anesthesia Intensive Care (SFAR) French Group for Pediatric Intensive Care Emergencies (GFRUP) the French Dialysis Society (SFD). Annals of Intensive Care 2015;5(1):58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald R, McArthur E, Adhikari NK, Bagshaw SM, Burns KE, Garg AX, et al. Changing incidence and outcomes following dialysis‐requiring acute kidney injury among critically ill adults: a population‐based cohort study. American Journal of Kidney Diseases 2015;65(6):870‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang X, Jie Yuan W. Timing of initiation of renal replacement therapy in acute kidney injury: a systematic review and meta‐analysis. Renal Failure 2012;34(3):396‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang C, Lv LS, Huang H, Guan J, Ye Z, Li S, et al. Initiation time of renal replacement therapy on patients with acute kidney injury: a systematic review and meta‐analysis of 8179 participants. Nephrology 2017;22(1):7‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wierstra B, Kadri S, Alomar S, Burbano X, Barrisford G, Kao R. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Critical Care (London, England) 2016;20(1):122. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Gao J, Zheng X, Zhong B, Na Y, Wei J. Timing of initiation of renal replacement therapy for acute kidney injury: a systematic review and meta‐analysis of randomized‐controlled trials. Clinical & Experimental Nephrology 2016;21(4):552‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yekebas EF, Strate T, Zolmajd S, Eisenberger CF, Erbersdobler A, Saalmuller A, et al. Impact of different modalities of continuous venovenous hemofiltration on sepsis‐induced alterations in experimental pancreatitis. Kidney International 2002;62(5):1806‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Fayad AI, Buamscha DG, Ciapponi A. Timing of continuous renal replacement therapy initiation for acute kidney injury. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010612] [DOI] [PMC free article] [PubMed] [Google Scholar]


