Abstract
Francisella tularensis, the causative agent of the zoonotic disease tularemia, is characterized by high morbidity and mortality rates in over 190 different mammalian species, including humans. Based on its low infectious dose, multiple routes of infection, and ability to induce rapid and lethal disease, F. tularensis has been recognized as a severe public health threat—being designated as a NIH Category A Priority Pathogen and a CDC Tier 1 Select Agent. Despite concerns over its use as a bioweapon, most U.S. tularemia cases are tick-mediated and ticks are believed to be the major environmental reservoir for F. tularensis in the U.S. The American dog tick (Dermacentor variabilis) has been reported to be the primary tick vector for F. tularensis, but the lone star tick (Amblyomma americanum) and other tick species also have been shown to harbor F. tularensis. This review highlights what is known, not known, and is debated, about the roles of different tick species as environmental reservoirs and transmission vectors for a variety of F. tularensis genotypes/strains.
Keywords: tularemia, tick, Francisella tularensis, vector-borne disease, Dermacentor, Amblyomma
Introduction
Francisella tularensis (Ft), the causative agent of the zoonotic disease tularemia, can infect and cause lethal disease in over 300 species, including humans (Dennis et al., 2001; Keim et al., 2007). This Gram-negative coccobacillus is divided into three subspecies: subsp. tularensis (Type A), subsp. holarctica (Type B), and subsp. mediasiatica. However, only subsp. tularensis and subsp. holarctica are virulent for humans. A separate species, F. novicida, is associated with rare disease in immunocompromised humans and is sometimes used as a surrogate to study Ft pathogenesis (Oyston and Quarry, 2005; Kingry and Petersen, 2014). Type A strains, found solely in North America, are the most virulent for humans with a low infectious dose (<10 organisms) and high mortality rates (up to 60% mortality if untreated) (Ellis et al., 2002). Type B strains, although less virulent, still cause debilitating illness and are distributed throughout the northern hemisphere (Ellis et al., 2002; Oyston and Quarry, 2005). Type A strains can be further divided into three subpopulations: A1a, A1b, and A2, with A1b causing the most serious infections (Kugeler et al., 2009). Interest in tularemia research has increased over the past two decades due to the classification of this organism as a Tier 1 select agent by the U.S. Centers for Disease Control, highlighting the high morbidity and mortality, ease of aerosolization, and low infectious dose of this pathogen (Petersen and Schriefer, 2005). Aside from aerosolization, Ft can be transmitted to humans via the handling of infected animal carcasses, ingestion of contaminated food or water, or by bites by infected arthropods (Petersen et al., 2009).
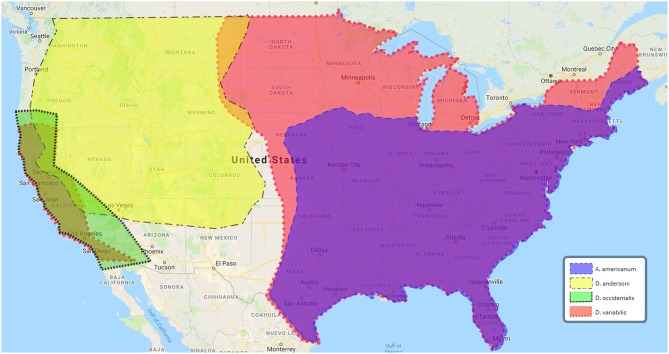
In the U.S. alone, tick-borne disease (TBD) cases have nearly doubled between 2004 and 2016, with nearly 50,000 TBD reported in 2016. TBD include Lyme disease, anaplasmosis/ehrlichiosis, spotted fever, babesiosis, Powassan virus, and tularemia (Rosenberg et al., 2018). Ticks initially were discovered as a vector of tularemia in 1923 (Parker et al., 1924). In the 1960's, 85% of all tularemia cases in the south-central U.S. were reported to be associated with tick exposure (Brooks and Buchanan, 1970). More recently, approximately half of U.S. tularemia infections are tick-associated (Eisen, 2007; Rosenberg et al., 2018). Ulceroglandular tularemia, the most common presentation of the disease in the U.S., typically is attributed to bites by infected arthropods (Ellis et al., 2002). In the U.S., the most commonly reported tularemia tick vectors include Amblyomma americanum, Dermacentor andersoni, D. occidentalis, and Dermacentor variabilis (Figure 1 and Table 1). In Europe, D. reticulatus and Ixodes ricinus are most frequently associated with Ft (Table 1). These ticks are members of the family Ixodidae (hard ticks) but variations in their host preference, geographic distribution, and habitat likely influence their ability to transmit Ft (Table 1). Despite evidence that ticks are important for both the environmental persistence and transmission of Ft (Goethert and Telford, 2010), major questions remain about which tick species allow Ft replication and persistence, transmit Ft to naïve hosts, or prime Ft for mammalian infection. A cursory review of published literature indicates that despite over 1,300 reports of Ft infections in humans and animals, <10% (n = 141) of those examined the role of ticks—highlighting that Ft-tick studies are understudied. This review will highlight what is known, and not known, about Ft prevalence in different ticks, Ft transmission by infected ticks, Ft-tick interactions, and areas for future research.
Figure 1.
U.S. geographic distribution of ticks associated with human tularemia. Data adapted from the Centers for Disease Control and Prevention, https://www.cdc.gov/ticks/geographic__distribution.html.
Table 1.
Ticks Associated with Human Tularemia.
| Tick species | Host preferencea | Preferred habitat | Transmit Ft?b | Ft subspeciesC | Transovarial transmission?C | Transstadial transmission?C | References |
|---|---|---|---|---|---|---|---|
| Amblyomma americanum (lone star tick) | Humans (L,N,A); small&large animals (N&L); large animals (A) | Woodlands | Yes (Exp&Nat) | Type B | No | Yes | (Calhoun, 1954; Mani et al., 2015; Sonenshine, 2018; Raghavan et al., 2019) |
| Dermacentor andersoni (Rocky Mountain wood tick) | Rodents, rarely humans (L&N), large mammals, humans (A) | Shrubs, tall grasses, and lightly wooded areas | Yes (Exp&Nat) | Type A | Yes | Yes | (Parker et al., 1924); (Mather, 2005; Sonenshine, 2018) |
| Dermacentor occidentalis (Pacific coast tick) | Small rodents and mammals (L&N), humans (N&A); large animals (A) | Shrubs | Yes (Nat) | Unk | Unk | Unk | (Parker et al., 1929); (Mather, 2005) |
| Dermacentor reticulatus (ornate cow tick) | Small mammals (L&N); medium mammals, sometimes humans (N); medium-large mammals, humans (A) | River basins, vegetation | Yes (Nat) | Type B | No | Unk | (Genchi et al., 2015; Földvári et al., 2016) |
| Dermacentor variabilis (American dog tick) | Host-specific (N&L); small mammals (L&N); small-medium mammals, humans (A) | Vegetation, tall grasses | Yes (Exp&Nat) | Type A&B | No | Yes | (Goethert et al., 2004; Mather, 2005; Mani et al., 2012; Sonenshine, 2018) |
| Ixodes ricinus (castor bean tick) | Humans (L,N,A); small-medium animals, mostly rodents (L); mostly birds and rodents (N); large animals (A) | Shrubs, tall grasses, deciduous woodlands | Yes (Nat) | Type B | No | Unk | (Genchi et al., 2015; Sonenshine, 2018; Sprong et al., 2018; Wilson and Elston, 2018) |
L, larvae; N, nymph; A, adult.
Exp, experimental; Nat, natural.
Unk, unknown.
Tularemia-Associated Tick Species, Tick Infection Rates, and Geographic Locations
From 2004 to 2016, 2,102 tick-borne tularemia cases were reported to the U.S. National Notifiable Disease Surveillance System (Rosenberg et al., 2018), with the majority of infections occurring in Missouri and Arkansas (Eisen, 2007). D. variabilis (American dog tick) and A. americanum (lone star tick), arguably the two most important tick vectors of U.S. human tularemia, both are found in Missouri and Arkansas (Figure 1) (Petersen et al., 2009). Seasonal peaks of tularemia, April–August, correlate with the active period for both tick species (Eisen, 2007). D. variabilis has the widest geographic range, being found in nearly every state east of the Rocky Mountains and most of California (Figure 1). By comparison, A. americanum is confined mainly to the south-east U.S. (Figure 1). Although both D. variabilis and A. americanum are naturally infected with Ft (Calhoun, 1954; Goethert et al., 2004), percentages of ticks infected by Type A or Type B Ft are unknown. At least three studies have demonstrated that A. americanum and D. variabilis can maintain Ft infections over winter (or for >4 months), supporting their role as environmental reservoirs (Hopla, 1953, 1960;Mani et al., 2015).
Ticks are responsible for the majority of U.S. tularemia cases, yet Ft-tick prevalence studies indicate wide variations of infected ticks in the environment: < 0.1% of Minnesota D. variabilis ticks (n = 2,000) were Ft-infected (Green, 1931); 17% of South Dakota D. variabilis ticks were Ft-infected (Markowitz et al., 1985); no Mississippi A. americanum ticks (n = 191) were Ft-infected (Castellaw et al., 2010); finally, 2% of Arkansas A. americanum ticks (n = 12,845) were Ft-infected but no Arkansas D. variabilis (n = 2,201) Haemaphysalis leporispalustris (rabbit tick; n = 1,494) or Ixodes scapularis (deer/blacklegged tick; vector for Lyme disease and many other pathogens; n = 142) were Ft-infected (Calhoun, 1954).
Martha's Vineyard, Massachusetts is an important site in the epidemiology of U.S. tularemia, as two major outbreaks have been reported: one in 1978 affecting 15 people and a second in 2000 affecting 15 people (Goethert et al., 2004). Although the cause of each outbreak remains unknown, four of the cases were linked to bites from D. variabilis ticks (Goethert et al., 2004). Analysis of >4,200 Martha's Vineyard D. variabilis ticks following the 2000 outbreak revealed that 0.7% were infected with Type A Ft but no other ticks (Ixodes dammini deer ticks; >600 tested) were infected (Goethert et al., 2004). Although sequence analyses of fopA (outer membrane protein) and PPI-helicase from these Ft strains indicated that they were nearly identical to the Type A reference strain SchuS4, multiple tandem-repeat analysis of two loci identified 10 unique genotypes, indicating that the degree of Ft genetic diversity on Martha's Vineyard is as great as the diversity found in Ft strains across North America and that Martha's Vineyard has a long history of enzootic Ft transmission (Goethert et al., 2004). Between 2004 and 2007, Ft DNA was detected in 2.7–4.3% of Martha's Vineyard D. variabilis ticks (>7,000 ticks tested), with 13 different Ft genotypes being identified by multiple tandem-repeat analysis (Goethert and Telford, 2009). Importantly, Ft numbers in Martha's Vineyard infected ticks were found to range from 0 to 1011 Ft genome equivalents (ge)/tick, with half of ticks harboring 108-109 Ft ge/tick (Goethert and Telford, 2010).
Dogs have been implicated to bring infected ticks into contact with humans. Early studies reported that Ft was detected in 0.4% of A. americanum ticks collected from Arkansas dogs (Calhoun, 1954). From 2006 to 2016, 1,814 U.S. human tularemia cases were reported, 735 (40%) of which had records indicating how exposure might have occurred (Kwit et al., 2018). Of those, 24 (3.3%) were dog-related and four (0.5%) were due to tick exposure from dogs (Kwit et al., 2018). In 1984, a tick-borne tularemia outbreak in twenty people from South Dakota Indian reservations was linked to dog exposures, with 17% of D. variabilis ticks from dogs found to harbor either Type A (12.5%) or Type B (87.5%) Ft (Markowitz et al., 1985). Unfortunately, clinical isolates were not collected from those patients so correlations between transmission of Type A and Type B Ft from infected ticks could not be determined.
Rabbit and lagomorph infections likely have contributed to the perpetuation of tularemia in the environment and to humans. The rabbit tick, H. leporispalustris, which is distributed across North America, likely is important for transmitting Ft to rabbits (Hopla, 1960; Goethert and Telford, 2010) and has been found to be naturally infected with Ft. Those findings are in contrast to the previously referenced study that did not detect Ft in Arkansas H. leporispalustris ticks (Calhoun, 1954). Although H. leporispalustris was reported to transovarially transmit Ft to its offspring and serve as a reservoir for F. tularensis (Parker, 1934), H. leporispalustris has not been associated with human tularemia, questioning the relevance of H. leporispalustris to human disease. Ft also has been reported to naturally infect other ticks, including Dermacentor andersoni (Rocky Mountain wood tick; Figure 1) (Parker et al., 1924), Dermacentor occidentalis (Pacific coast tick; Figure 1) (Parker et al., 1929), and Haemaphysalis cinnabarina (bird tick) (Parker et al., 1932), but transmission of Ft from these ticks to humans needs further study.
Ft-infected ticks are not unique to the U.S., as Ft Type B has been found in several European tick vectors. Between 0 and 2.3% of Dermacentor reticulatus (ornate cow tick; n = 5,131; Table 1) in Austria, Czech Republic, Germany, Poland, and Slovakia were found to be infected with Ft Type B (Gurycová et al., 1995). Ft was not detected in Ixodes ricinus (castor bean tick; n = 8,994) in France, Denmark, Italy, the Netherlands, Norway, or Poland (Mancini et al., 2014; Michelet et al., 2014; Quarsten et al., 2015; Stensvold et al., 2015; Wójcik-Fatla et al., 2015). However, other studies noted that 0.02–3.8% (n = 123,761) of I. ricinus were Ft Type B infected in France, Germany, Poland, Serbia, Slovakia, and Switzerland (Table 1) (Gurycová et al., 1995; Milutinovic et al., 2008; Reis et al., 2011; Gehringer et al., 2013; Wójcik-Fatla et al., 2015; Tomaso et al., 2018; Wittwer et al., 2018). Finally, in Slovakia, 2.8% of Haemaphysalis concinna (bush tick; n = 35) were infected with Ft Type B (Gurycová et al., 1995). In summary, more information is needed about tick infection rates and infected tick species in the U.S., primarily in states with high tularemia rates (e.g., Arkansas, Colorado, Kansas, Missouri, Oklahoma, South Dakota). In addition, although more Ft-tick prevalence studies have been performed in Europe and more tularemia cases occur yearly in Europe (relative to the U.S.) (Hestvik et al., 2015), it still is unclear what tick species transmits Ft Type B in Europe or if differences in tick species and Ft genotypes between Europe and the U.S. correlate with differences in tularemia disease severity.
Francisella-Like Endosymbionts
As noted above, ticks harbor and transmit several human pathogens but they also are colonized with endosymbionts that are closely related to pathogenic bacteria, offer fitness advantages to host ticks, and appear to promote pathogen acquisition/transmission (Bonnet et al., 2017). Francisella-like endosymbionts (FLEs) share 16s rDNA similarity to Ft, are widely distributed in many different ticks, replicate intracellularly, can be transmitted transovarially, and appear to have evolved from pathogenic Ft strains (Gerhart et al., 2016, 2018; Liu et al., 2016). However, unlike virulent Ft, FLEs do not grow in cell-free media and their transmission to and virulence in humans is unknown (Ivanov et al., 2011; Wójcik-Fatla et al., 2015). FLEs have been found in various Dermacentor sp., as well as Hyalomma marginatum, Hyalomma aegyptium (tortoise tick), and Rhipicephalus sanguineus (brown dog tick), among others (Ivanov et al., 2011; Wójcik-Fatla et al., 2015). Importantly, one U.S. study reported that up to 60% of ticks colonized with FLEs were falsely identified as Ft-positive when using 16S rRNA PCR only (Kugeler et al., 2005). However, additional testing of the same ticks using a Ft multitarget TaqMan assay, specifically amplifying the insertion sequence ISFtu2, outer membrane lipoprotein tul4, and intracellular growth locus iglC, revealed that the ticks actually were not Ft-infected (Kugeler et al., 2005). The wide distribution of FLEs in different tick species is further highlighted by studies finding that >94% of D. andersoni, D. variabilis, and D. occidentalis ticks from the western U.S. were positive for FLEs (Niebylski et al., 1997; Rounds et al., 2012). A Canadian study reported that 86–93% of D. variabilis and D. andersoni ticks were colonized with FLEs (Dergousoff and Chilton, 2012). Further afield, 50% (n = 530) of Polish D. reticulatus ticks (Wójcik-Fatla et al., 2015), 84–100% (n = 257) of Israeli Haemaphysalis sp. ticks (Kreizinger et al., 2013; Azagi et al., 2017), and 3% (n = 361) of Hungarian D. reticulatus ticks have been found to contain FLEs (Kreizinger et al., 2013). FLEs are not the only microbe in ticks and, interestingly, FLEs were found to comprise up to 41% of the microbiome of California D. occidentalis ticks (no ticks were positive for Ft) (Gurfield et al., 2017). Another study reported that Ft and FLEs accounted for ~80% (20% Ft, 60% FLE) of the midgut microbiome of D. andersoni ticks collected in Oregon and Montana (Gall et al., 2016). In summary, because of genetic similarity to virulent Ft, FLEs may have artificially inflated Ft infection rates in some of the above referenced Ft-tick prevalence studies. In addition, although it is clear that FLEs are present in many ticks that transmit Ft, much more work is needed to determine if FLEs interact with Ft, determine if FLEs aid in Ft infection of ticks, and examine if FLEs play important roles in Ft transmission to naïve hosts.
Transstadial Transmission of F. tularensis in Ticks
The tick lifecycle is complex, spanning up to 3 years, requiring a blood meal to transition from one life stage to the next (larva-nymph-adult), and requiring a final blood meal before mating and/or egg laying (Petersen et al., 2009). The frequency and length of tick blood meals depends on the type of tick (soft vs. hard) and on the tick species. Important for Ft, hard ticks (e.g., A. americanum and D. variabilis) feed for up to 11 days, taking two-thirds of the total blood volume in the last 24–48 h (Sojka et al., 2013). Female hard ticks feed once per life stage and die several days after oviposition. Because of this complex life cycle, there are questions about whether Ft can be transstadially-transmitted from one life stage to the next, if all tick life stages can transmit Ft to naïve hosts, or if infected female adult ticks can transovarially transmit Ft to their eggs.
Ft-infected D. andersoni and D. variabilis ticks have been shown to molt from larvae to nymphs and from nymphs to adults, demonstrating that transstadial transmission of Ft can occur at all life stages. Importantly, all tick life stages also were shown to transmit Ft to naive guinea pigs, hares, or rabbits (Parker et al., 1924; Philip and Jellison, 1934). More recent studies demonstrated the Ft Type B attenuated live vaccine strain (LVS) was transstadially-transmitted in D. variabilis larvae to nymphs and nymphs to adults, noting that bacterial numbers decreased before each molt, then increased 3–4 logs after each molt (Mani et al., 2012). However, only 22% of nymphs maintained LVS infection through day 28 post-infection (close to molting), 25% of those infected nymphs survived molting, and only 25% of LVS-infected adult ticks maintained LVS through day 165 post-infection. Because D. variabilis in those studies were artificially fed using capillary tubes, it is difficult to determine if most Ft infections are cleared in naturally-infected ticks or if natural Ft infections negatively impact molting (Mani et al., 2012). The authors of that study also capillary-fed A. americanum with LVS, observing transstadial transmission between all life stages, LVS decreases before molting, LVS increases after molting, and low maintenance of LVS over time (Mani et al., 2015). By comparison, one study noted very high transstadial transmission rates of virulent Ft strains from D. variabilis larvae to nymph (fed on infected mice): Type A1b (93.3%), Type A2 (96.7%), and Type B (100%) (Reese et al., 2010).
Although older studies detected Ft in the eggs of infected adult female D. variabilis ticks and noted that oviposition was unimpaired by infected ticks (Bell, 1945), neither study examined if Ft was present in hatched larvae. More recently, transovarial transmission from capillary-infected A. americanum or D. variabilis ticks was not observed (Mani et al., 2012, 2015). Additionally, while Ft Type B was detected in oocytes of infected adult female D. reticulatus and I. ricinus ticks fed on infected guinea pigs, transovarial transmission was not observed (Genchi et al., 2015). Taken together, it appears that Ft can be transstadially-transmitted between all tick life stages and all tick life stages have been reported to transmit Ft to naïve hosts. However, transovarial transmission of virulent Ft should be examined, more studies are needed to understand if naturally-infected ticks clear Ft over time, and additional studies are needed to examine transmission of virulent Ft by infected ticks to naive hosts.
F. tularensis-Tick Interactions
Questions about potential negative impacts of Ft infections on ticks and whether ticks restrict Ft replication/persistence have been examined in a number of studies in D. variabilis. Whereas environmentally-collected ticks have a number of limitations (e.g., low infection rate), Ft-tick infection experiments in the laboratory have their own limitations, including targeting biologically-relevant Ft numbers in ticks, selecting the appropriate tick life stage to infect, and selecting the tick infection model (e.g., infected mouse vs. capillary feeding). These limitations are further confounded by ticks requiring 3–7 days to feed to repletion and mice succumbing to virulent Ft infection within 4–5 days (Coburn et al., 2015). Although blood meal feeding mimics natural infection cues, bacterial numbers can be highly variable (Hopla, 1953; Coburn et al., 2015) and some ticks die while feeding on an infected host, suggesting that Ft has negative impacts on ticks. Uninfected D. variabilis nymphs have been reported to survive significantly better (58.5% survival) than nymphs infected with Ft Type A2 (11.6% survival) or Type B (29.8% survival). Interestingly, no significant difference in survival of uninfected and Type A1b-infected D. variabilis was observed (Reese et al., 2010). In contrast, another study noted that A1b-infected adult D. variabilis ticks had significantly lower survival rates (82% survival) than uninfected (92% survival), A2-infected (95% survival), or Type B-infected ticks (90% survival) (Reese et al., 2011). Ft Type A1a also appears to negatively impact tick survival, as only 11% of A1a-infected D. variabilis collected from Martha's Vineyard survived 6 months, compared with 52% survival for uninfected ticks (Goethert and Telford, 2011). By comparison, an older study found no significant difference in mortality rates between uninfected and Ft-infected D. variabilis (Bell, 1945). Some evidence indicates that high bacterial numbers or rapid bacterial replication (2- to 5-log increases in Ft Type A2 over 65 days) in ticks correlated with tick mortality (Reese et al., 2010). In contrast, another study found virtually no difference in survival rates for D. variabilis that were either uninfected (65% survival) of capillary-infected with Ft LVS (63% survival) (Mani et al., 2012). Considering the wide variations in reported survival rates for both uninfected (52–92%) and Ft-infected ticks (11% to 95% survival), it is difficult to conclude if Ft infections negatively impact ticks or if these results hold true for other tick species, including A. americanum.
With respect to Ft numbers and replication in ticks, two studies reported that Ft LVS numbers decline in capillary-tube fed D. variabilis or A. americanum ticks (Mani et al., 2012, 2015). For naturally-infected ticks, it has been speculated that anti-Ft antibodies from the mammalian host may limit bacterial replication/survival in ticks, as D. variabilis ticks fed on an immune host cleared Ft infections (Bell, 1945). Conversely, it also has been reported that Ft-infected A. americanum nymphs fed on hyperimmune dogs, rabbits, or rats retained Ft infections (Hopla, 1953, 1960). A fairly recent study reported reproducible tick infections by placing D. variabilis nymphs onto uninfected mice for approx. 77 h, retro-orbitally infecting those mice with 106−108 CFU of Ft LVS, and harvesting ticks 24 h later. In that study, mouse blood CFU/ml directly correlated with CFU/tick, Ft numbers increased over time in D. variabilis (after an initial decrease), and Ft doses <106 CFU resulted in less efficient infection of and maintenance in ticks through molting to adult, indicating that a threshold of F. tularensis is needed to infect D. variabilis (Coburn et al., 2015). Similarly, another study noted that ticks must feed on an infected host during peak bacteremia to become infected (Bell, 1945). Finally, another study concluded that, as compared to direct injections of Ft, natural infections of ticks (feeding on an infected host) are necessary for proper colonization and bacterial dissemination (Genchi et al., 2015).
In theory, capillary tube feeding or direct injection of Ft into ticks can produce more consistent, standardized infections, but these methods lack natural infection cues (Mani et al., 2012, 2015). In one capillary feeding study, D. variabilis nymphs were capillary fed 107 CFU/ml Ft LVS. One day later, only 30% of nymphs were infected and, of those, bacterial numbers were 4-logs less than the infectious dose (Mani et al., 2012). In direct injection studies, < 2 CFU Ft LVS delivered into the hemocoel of D. variabilis adults resulted in ~40% infection rate, whereas similar A. americanum adult injections did not establish infections (Mani et al., 2012, 2015).
Compared to Borrelia burgdorferi, which is found exclusively in I. scapularis midguts (De Silva and Fikrig, 1995), Ft has been reported to quickly (<24 h) disseminate from the gut to hemolymph and salivary glands of capillary-fed A. americanum ticks (Mani et al., 2015). Ft dissemination is further supported by one study noting that Ft migrated to the salivary glands of D. reticulatus and I. ricinus 6 days after ticks were removed from infected guinea pigs (Genchi et al., 2015) and another study noting that capillary-fed D. variabilis maintained Ft in their guts for up to 21 days before the bacteria spread to hemolymph and salivary glands (Mani et al., 2012). Conversely, a separate study noted that Ft did not disseminate to D. variabilis salivary glands (Coburn et al., 2015).
Transmission efficiency of Ft from infected ticks to hosts appears to be dependent on many factors, including the Ft strain, tick species, tick attachment efficiency, and feeding time. Results from one study suggested that Ft infection decreases tick attachment rates to naïve mice, with 96% attachment for uninfected D. variabilis adults, 86% attachment for A1b-infected, 58% attachment for A2-infected, and 52% attachment for Type B-infected ticks. In addition, Ft infection appeared to limit tick feeding, with 46% of uninfected ticks feeding to repletion, and only 23% of A1b-infected ticks feeding to repletion (Reese et al., 2011). In another study, 55% of D. variabilis ticks on uninfected mice fed to repletion, compared with only 3.7% of ticks feeding to repletion on A2-infected mice, and most of the ticks dying while feeding on A1b- and A2-infected mice (Reese et al., 2010). Although those results indicate that Ft infections alter tick feeding behaviors, other variables could account for these findings, including the reported preference of adult D. variabilis ticks for larger hosts (Sonenshine, 2018). Interestingly, one study noted that different Ft genotypes may be transmitted to naïve hosts at different frequencies (using infected D. variabilis): Type A1b transmitted to 67% of mice; Type A2 transmitted to 89% of mice; and Type B transmitted to 58% of mice (Reese et al., 2011). Differences in transmission could not be correlated to differences in bacterial numbers in ticks, as bacterial burdens in A1b-infected ticks (>109 CFU) were significantly higher than bacterial burdens in A2- or Type B-infected ticks (~108 CFU) (Reese et al., 2011). Given these conflicting findings, more studies are needed to better understand if Ft infections negatively impact different tick species, if Ft infections alter tick feeding behaviors, and if Ft genotypes are transmitted to naïve hosts at different frequencies.
Conclusions
A large number of complex studies have been performed to understand which tick vectors are infected with Ft, which ticks are most likely to transmit Ft, which Ft genotypes are most likely to be tick-transmitted, what tick life stage is the most infectious, or if Ft infections have impacts on ticks. The majority of Ft-tick studies have focused on D. variabilis which, in the U.S., has the widest geographic range (Figure 1) and is most often associated with human tularemia. The second major tick vector for U.S. tularemia appears to be A. americanum. However, a number of other ticks, including those that feed primarily on small mammals, likely play important roles in Ft environmental persistence (Table 1). FLEs are a relatively new research field and much remains to be learned about how they interact with virulent Ft, if they provide metabolites/nutrients that support Ft persistence/replication in ticks, or if they contribute to transmission and disease. All tick life stages appear to support Ft and Ft can be transstadially transmitted from larva-nymph-adult. However, more studies are needed to understand if naturally-infected ticks can control or restrict Ft persistence/replication or if Ft infections have negative consequences on infected ticks. Finally, although it is clear that Ft is transmitted from infected ticks to naïve hosts, detailed studies are needed to understand if Ft genotypes are transmitted at different efficiencies.
In many cases, it is difficult to directly compare the highlighted studies because of differences in tick infection techniques (e.g., feeding on infected animals, capillary tube feeding, intrahemocoelic injection), environmental vs. laboratory infections, animals that transmitted Ft to ticks (e.g., mice, guinea pigs, rabbits, dogs), tick life stage used (larvae, nymph, adult), Ft infectious dose, and Ft genotypes/strains used (A1a, A1b, A2, B, LVS). Given these differences, future studies should directly compare bacterial replication in different ticks over time, transstadial transmission efficiency in different ticks, survival rates of different infected ticks, and Ft transmission to naïve hosts for D. variabilis and A. americanum, as well as other relevant ticks.
Finally, very little is known about Ft genes/proteins required for tick infection, persistence/replication in ticks, and transmission to naïve hosts. To our knowledge, only one study investigated the ability of a Ft mutant, a ΔpurMCD strain, to infect and replicate in ticks (Coburn et al., 2015). Although ΔpurMCD is avirulent in mice, it successfully colonized D. variabilis but was unable to persist in these ticks through the molt to the adult stage (Coburn et al., 2015). This finding indicated that, similar to biosynthetic pathways required for mammalian infections, the ability of Ft to synthesize purines is essential for replication in ticks. Studies to identify Ft genes/proteins required for persistence/replication in ticks, or the development of small molecule inhibitors that block Ft persistence/replication in ticks, could be important for reducing bacterial numbers in the environment, limiting enzootic episodes, and reducing human tularemia infections.
Author Contributions
BZ and JH both read and reviewed all referenced papers and wrote this review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. JH is supported by start-up and bridge funding from the University of Toledo College of Medicine and Life Sciences.
References
- Azagi T., Klement E., Perlman G., Lustig Y., Mumcuoglu K. Y., Apanaskevich D. A., et al. (2017). Francisella-like endosymbionts and rickettsia species in local and imported hyalomma ticks. Appl. Environ. Microbiol. 83, 1302–1317 10.1128/AEM.01302-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. F. (1945). The infection of Ticks (Dermacentor variabilis) with Pasteurella tularensis. J. Infect. Dis. 76, 83–95. 10.1093/infdis/76.2.83 [DOI] [Google Scholar]
- Bonnet S. I., Binetruy F., Hernández-Jarguín A. M., Duron O. (2017). The tick microbiome: why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front. Cell. Infect. Microbiol. 7:236. 10.3389/fcimb.2017.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G. F., Buchanan T. M. (1970). Tularemia in the United States: epidemiologic aspects in the 1960s and follow-up of the outbreak of tularemia in Vermont. J. Infect. Dis. 121, 357–359. 10.1093/infdis/121.3.357 [DOI] [PubMed] [Google Scholar]
- Calhoun E. L. (1954). Natural occurrence of tularemia in the lone star tick, Amblyomma americanus (Linn.), and in dogs in Arkansas. Am. J. Trop. Med. Hyg. 3, 360–366. 10.4269/ajtmh.1954.3.360 [DOI] [PubMed] [Google Scholar]
- Castellaw A. H., Showers J., Goddard J., Chenney E. F., Varela-Stokes A. S. (2010). SDetection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J. Med. Entomol. 47, 473–476. 10.1093/jmedent/47.3.473 [DOI] [PubMed] [Google Scholar]
- Coburn J., Maier T., Casey M., Padmore L., Sato H., Frank D. W. (2015). Reproducible and quantitative model of infection of Dermacentor variabilis with the live vaccine strain of Francisella tularensis. Appl. Environ. Microbiol. 81, 386–395. 10.1128/AEM.02917-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva A. M., Fikrig E. (1995). Growth and migration of borrelia-burgdorferi in ixodes ticks during blood-feeding. Am. J. Trop. Med. Hygiene 53, 397–404. 10.4269/ajtmh.1995.53.397 [DOI] [PubMed] [Google Scholar]
- Dennis D. T, Inglesby T. V, Henderson D. A, Bartlett J. G, Ascher M. S, Eitzen E., Tonat, et al. (2001). Tularemia as a biological weapon: medical and public health management. JAMA 285, 2763–2773. 10.1001/jama.285.21.2763 [DOI] [PubMed] [Google Scholar]
- Dergousoff S. J., Chilton N. B. (2012). Association of different genetic types of Francisella-like organisms with the rocky mountain wood tick (Dermacentor andersoni) and the American dog tick (Dermacentor variabilis) in localities near their northern distributional limits. Appl. Environ. Microbiol. 78, 965–971. 10.1128/AEM.05762-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L. (2007). A call for renewed research on tick-borne Francisella tularensis in the Arkansas-Missouri primary national focus of tularemia in humans. J. Med. Entomol. 44, 389–397. 10.1093/jmedent/44.3.389 [DOI] [PubMed] [Google Scholar]
- Ellis J., Oyston P. C., Green M., Titball R. W. (2002). Tularemia. Clin. Microbiol. Rev. 15, 631–646. 10.1128/CMR.15.4.631-646.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Földvári G., Široký P., Szekeres S., Majoros G., Sprong H. (2016). Dermacentor reticulatus: a vector on the rise. Parasit. Vectors 9:314. 10.1186/s13071-016-1599-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall C. A., Reif K. E., Scoles G. A., Mason K. L., Mousel M., Noh S. M., et al. (2016). The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME J. 10, 1846–1855. 10.1038/ismej.2015.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehringer H., Schacht E., Maylaender N., Zeman E., Kaysser P., Oehme R., et al. (2013). Presence of an emerging subclone of Francisella tularensis holarctica in Ixodes ricinus ticks from south-western Germany. Ticks Tick Borne Dis. 4, 93–100. 10.1016/j.ttbdis.2012.09.001 [DOI] [PubMed] [Google Scholar]
- Genchi M., Prati P., Vicari N., Manfredini A., Sacchi L., Clementi E., et al. (2015). Francisella tularensis: no evidence for transovarial transmission in the tularemia tick vectors dermacentor reticulatus and Ixodes ricinus. PLoS ONE 10:e0133593. 10.1371/journal.pone.0133593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. G., Auguste Dutcher H., Brenner A. E., Moses A. S., Grubhoffer L., Raghavan R. (2018). Multiple acquisitions of pathogen-derived francisella endosymbionts in soft ticks. Genome Biol. Evol. 10, 607–615. 10.1093/gbe/evy021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. G., Moses A. S., Raghavan R. (2016). A Francisella-like endosymbiont in the Gulf Coast tick evolved from a mammalian pathogen. Sci. Rep. 6:33670. 10.1038/srep33670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethert H. K., Shani I., Telford S. R., 3rd (2004). Genotypic diversity of Francisella tularensis infecting Dermacentor variabilis ticks on Martha's Vineyard, Massachusetts. J. Clin. Microbiol. 42, 4968–4973. 10.1128/JCM.42.11.4968-4973.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethert H. K., Telford S. R., III (2009). Nonrandom distribution of vector ticks (Dermacentor variabilis) infected by Francisella tularensis. PLoS Pathog. 5:e1000319. 10.1371/journal.ppat.1000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethert H. K., Telford S. R., III (2010). Quantum of infection of Francisella tularensis tularensis in host-seeking Dermacentor variabilis. Ticks Tick Borne Dis. 1, 66–68. 10.1016/j.ttbdis.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethert H. K., Telford S. R., III (2011). Differential mortality of dog tick vectors due to infection by diverse Francisella tularensis tularensis genotypes. Vector Borne Zoonotic Dis. 11, 1263–1268. 10.1089/vbz.2010.0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. G. (1931). The Occurence of Bact. Tularense in the Eastern Wood Tick, Dermacentor variabilis. Am. J. Epidemiol. 14, 600–613. 10.1093/oxfordjournals.aje.a117793 [DOI] [Google Scholar]
- Gurfield N., Grewal S., Cua L. S., Torres P. J., Kelley S. T. (2017). Endosymbiont interference and microbial diversity of the Pacific coast tick, Dermacentor occidentalis, in San Diego County, California. PeerJ 5:e3202. 10.7717/peerj.3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurycová D., Kocianová E., Výrosteková V., Rehácek J. (1995). Prevalence of ticks infected with Francisella tularensis in natural foci of tularemia in western Slovakia. Eur. J. Epidemiol. 11, 469–474. 10.1007/BF01721235 [DOI] [PubMed] [Google Scholar]
- Hestvik G., Warns-Petit E., Smith L. A., Fox N. J., Uhlhorn H., Artois M., et al. (2015). The status of tularemia in Europe in a one-health context: a review. Epidemiol. Infect. 143, 2137–2160. 10.1017/S0950268814002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopla C. E. (1953). Experimental studies on tick transmission of tularemia organisms. Am. J. Hygeine 58, 101–118. 10.1093/oxfordjournals.aje.a119585 [DOI] [PubMed] [Google Scholar]
- Hopla C. E. (1960). The transmission of tularemia organisms by ticks in the southern states. South. Med. J. 53, 92–97. 10.1097/00007611-196001000-00020 [DOI] [PubMed] [Google Scholar]
- Ivanov I. N., Mitkova N., Reye A. L., Hübschen J. M., Vatcheva-Dobrevska R. S., Dobreva E. G., et al. (2011). Detection of new francisella-like tick endosymbionts in Hyalomma spp. and Rhipicephalus spp. (Acari: Ixodidae) from Bulgaria. Appl. Environ. Microbiol. 77, 5562–5565. 10.1128/AEM.02934-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim P., Johansson A., Wagner D. M. (2007). Molecular epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. 1105, 30–66. 10.1196/annals.1409.011 [DOI] [PubMed] [Google Scholar]
- Kingry L. C., Petersen J. M. (2014). Comparative review of Francisella tularensis and Francisella novicida. Front. Cell. Infect. Microbiol. 4:35. 10.3389/fcimb.2014.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreizinger Z., Hornok S., Dán A., Hresko S., Makrai L., Magyar T., et al. (2013). Prevalence of Francisella tularensis and Francisella-like endosymbionts in the tick population of Hungary and the genetic variability of Francisella-like agents. Vector Borne Zoonotic Dis. 13, 160–163. 10.1089/vbz.2012.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler K. J., Gurfield N., Creek J. G., Mahoney K. S., Versage J. L., Petersen J. M. (2005). Discrimination between Francisella tularensis and Francisella-like endosymbionts when screening ticks by PCR. Appl. Environ. Microbiol. 71, 7594–7597. 10.1128/AEM.71.11.7594-7597.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler K. J., Mead P. S., Janusz A. M., Staples J. E., Kubota K. A., Chalcraft L. G., et al. (2009). Molecular Epidemiology of Francisella tularensis in the United States. Clin. Infect. Dis. 48, 863–870. 10.1086/597261 [DOI] [PubMed] [Google Scholar]
- Kwit N. A., Schwartz A., Kugeler K. J., Mead P. S., Nelson C. A. (2018). Human tularaemia associated with exposure to domestic dogs-United States, 2006-2016. Zoonoses Public Health. 66, 417–421. 10.1111/zph.12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. N., Yu Z. J., Liu L. M., Li N. X., Wang R. R., Zhang C. M., et al. (2016). Identification, distribution and population dynamics of francisella-like endosymbiont in haemaphysalis doenitzi (Acari: Ixodidae). Sci. Rep. 6:35178. 10.1038/srep35178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini F., Di Luca M., Toma L., Vescio F., Bianchi R., Khoury C., et al. (2014). Prevalence of tick-borne pathogens in an urban park in Rome, Italy. Ann. Agric. Environ. Med. 21, 723–727. 10.5604/12321966.1129922 [DOI] [PubMed] [Google Scholar]
- Mani R. J., Metcalf J. A., Clinkenbeard K. D. (2015). Amblyomma americanum as a Bridging Vector for Human Infection with Francisella tularensis. PLoS ONE 10:e0130513. 10.1371/journal.pone.0130513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani R. J., Reichard M. V., Morton R. J., Kocan K. M., Clinkenbeard K. D. (2012). Biology of Francisella tularensis subspecies holarctica live vaccine strain in the tick vector Dermacentor variabilis. PLoS ONE 7:e35441. 10.1371/journal.pone.0035441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz L. E., Hynes N. A., de la Cruz P., Campos E., Barbaree J. M., Plikaytis B. D., et al. (1985). Tick-borne tularemia. An outbreak of lymphadenopathy in children. JAMA 254, 2922–2925. 10.1001/jama.1985.03360200074030 [DOI] [PubMed] [Google Scholar]
- Mather T. (2005). TickEncounter Resource Center. Kingston, RI: The University of Rhode Island. [Google Scholar]
- Michelet L., Delannoy S., Devillers E., Umhang G., Aspan A., Juremalm M., et al. (2014). High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 4:103. 10.3389/fcimb.2014.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinovic M., Masuzawa T., Tomanovic S., Radulovic Z., Fukui T., Okamoto Y. (2008). Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Francisella tularensis and their co-infections in host-seeking Ixodes ricinus ticks collected in Serbia. Exp. Appl. Acarol. 45, 171–183. 10.1007/s10493-008-9166-6 [DOI] [PubMed] [Google Scholar]
- Niebylski M. L., Peacock M. G., Fischer E. R., Porcella S. F., Schwan T. G. (1997). Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Appl. Environ. Microbiol. 63, 3933–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyston P. C., Quarry J. E. (2005). Tularemia vaccine: past, present and future. Antonie Van Leeuwenhoek 87, 277–281. 10.1007/s10482-004-6251-7 [DOI] [PubMed] [Google Scholar]
- Parker R., Spencer R., Francis E. (1924). Tularmia: XI. Tularmia Infection in Ticks of the Species Dermacentor andersoni Stiles in the Bitterroot Valley, Mont. Public Health Rep. (1896-1970) 39, 1057–1073. 10.2307/457715119314929 [DOI] [Google Scholar]
- Parker R. R. (1934). Recent studies of tick borne diseases made at the U.S. public health service laboratories at Hamilton in Proceedings of 5th Pacific Science Conference 5, (Hamilton, MT: ) 3367–3374. [Google Scholar]
- Parker R. R., Brooks C. S., Marsh H. (1929). The occurrence of Bacterium tularense in the wood tick (Dermacentor occidentalis) in California. Public Health Rep. 44, 1299–1300. 10.2307/457926519315193 [DOI] [Google Scholar]
- Parker R. R., Philip C. B., Davis G. E. (1932). Tularaemia: Occurrence in the sage hen, Centrocercus urophasianus. Public Health Rep. 47, 479–487. 10.2307/458036019315336 [DOI] [Google Scholar]
- Petersen J. M., Mead P. S., Schriefer M. E. (2009). Francisella tularensis: an arthropod-borne pathogen. Vet. Res. 40:7. 10.1051/vetres:2008045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. M., Schriefer M. E. (2005). Tularemia: emergence/re-emergence. Vet. Res. 36, 455–467. 10.1051/vetres:2005006 [DOI] [PubMed] [Google Scholar]
- Philip C. B., Jellison W. L. (1934). The american dog tick, dermacentor variabilis, as a host of Bacterium Tularense. Public Health Rep. 49, 1896–1970. 10.2307/4581119 [DOI] [Google Scholar]
- Quarsten H., Skarpaas T., Fajs L., Noraas S., Kjelland V. (2015). Tick-borne bacteria in Ixodes ricinus collected in southern Norway evaluated by a commercial kit and established real-time PCR protocols. Ticks Tick Borne Dis. 6, 538–544. 10.1016/j.ttbdis.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Raghavan R. K., Peterson A. T., Cobos M. E., Ganta R., Foley D. (2019). Current and future distribution of the lone star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 14:e0209082. 10.1371/journal.pone.0209082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese S. M., Dietrich G., Dolan M. C., Sheldon S. W., Piesman J., Petersen J. M., et al. (2010). Transmission dynamics of Francisella tularensis subspecies and clades by nymphal Dermacentor variabilis (Acari: Ixodidae). Am. J. Trop. Med. Hyg. 83, 645–652. 10.4269/ajtmh.2010.10-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese S. M., Petersen J. M., Sheldon S. W., Dolan M. C., Dietrich G., Piesman J., et al. (2011). Transmission efficiency of Francisella tularensis by adult american dog ticks (Acari: Ixodidae). J. Med. Entomol. 48, 884–890. 10.1603/ME11005 [DOI] [PubMed] [Google Scholar]
- Reis C., Cote M., Paul R. E., Bonnet S. (2011). Questing ticks in suburban forest are infected by at least six tick-borne pathogens. Vector Borne Zoonotic Dis. 11, 907–916. 10.1089/vbz.2010.0103 [DOI] [PubMed] [Google Scholar]
- Rosenberg R., Lindsey N. P., Fischer M., Gregory C. J., Hinckley A. F., Mead P. S., et al. (2018). Vital Signs: trends in reported vectorborne disease cases - United States and Territories, 2004-2016. MMWR Morb. Mortal. Wkly. Rep. 67, 496–501. 10.15585/mmwr.mm6717e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds M. A., Crowder C. D., Matthews H. E., Philipson C. A., Scoles G. A., Ecker D. J., et al. (2012). Identification of endosymbionts in ticks by broad-range polymerase chain reaction and electrospray ionization mass spectrometry. J. Med. Entomol. 49, 843–850. 10.1603/ME12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka D., Franta Z., Horn M., Caffrey C. R., Mareš M., Kopacek P. (2013). New insights into the machinery of blood digestion by ticks. Trends Parasitol. 29, 276–285. 10.1016/j.pt.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Sonenshine D. E. (2018). Range expansion of tick disease vectors in north america: implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 15:E478. 10.3390/ijerph15030478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong H., Azagi T., Hoornstra D., Nijhof A. M., Knorr S., Baarsma M. E., et al. (2018). Control of Lyme borreliosis and other Ixodes ricinus-borne diseases. Parasit. Vectors 11:145. 10.1186/s13071-018-2744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold C. R., Al Marai D., Andersen L. O., Krogfelt K. A., Jensen J. S., Larsen K. S., et al. (2015). Babesia spp. and other pathogens in ticks recovered from domestic dogs in Denmark. Parasit Vectors 8, 262. 10.1186/s13071-015-0843-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaso H., Otto P., Peters M., Suss J., Karger A., Schamoni H., et al. (2018). Francisella tularensis and other bacteria in hares and ticks in North Rhine-Westphalia (Germany). Ticks Tick-Borne Dis 9, 325–329. 10.1016/j.ttbdis.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Wilson K. D., Elston D. M. (2018). What's eating you? Ixodes tick and related diseases, part 3: coinfection and tick-bite prevention. Cutis 101, 328–330. [PubMed] [Google Scholar]
- Wittwer M., Altpeter E., Pilo P., Gygli S. M., Beuret C., Foucault F., et al. (2018). Population genomics of Francisella tularensis subsp. holarctica and its implication on the eco-epidemiology of tularemia in Switzerland. Front. Cell Infect. Microbiol. 8:89. 10.3389/fcimb.2018.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcik-Fatla A., Zajac V., Sawczyn A., Cisak E., Sroka J., Dutkiewicz J. (2015). Occurrence of Francisella spp. in Dermacentor reticulatus and Ixodes ricinus ticks collected in eastern Poland. Ticks Tick Borne Dis. 6, 253–257. 10.1016/j.ttbdis.2015.01.005 [DOI] [PubMed] [Google Scholar]