Abstract
Functional differences in the anterior and posterior hippocampus during episodic memory processing have not been examined in human electrophysiological data. This is in spite of strong evidence for such differences in rodent data, including greater place cell specificity in the dorsal hippocampus, greater sensitivity to the aversive or motivational content of memories in ventral regions, connectivity analyses identifying preferential ventral hippocampal connections with the amygdala, and gene expression analyses identifying a dorsal–ventral gradient. We asked if memory–related oscillatory patterns observed in human hippocampal recordings, including the gamma band and slow–theta (2.5–5 Hz) subsequent memory effects, would exhibit differences along the longitudinal axis and between hemispheres. We took advantage of a new dataset of stereo electroencephalography patients with simultaneous, robotically targeted anterior and posterior hippocampal electrodes to directly compare oscillatory subsequent memory effects during item encoding. This same data set allowed us to examine left–right connectivity and hemispheric differences in hippocampal oscillatory patterns. Our data suggest that a power increase during successful item encoding in the 2.5–5 Hz slow–theta frequency range preferentially occurs in the posterior hippocampus during the first 1000 msec after item presentation, while a gamma band power increase is stronger in the dominant hemisphere. This dominant–non dominant pattern in the gamma range appears to reverse during item retrieval, however. Intra–hippocampal phase coherence was found to be stronger during successful item encoding. Our phase coherence data are also consistent with existing reports of a traveling wave for theta oscillations propagating along the septo–temporal (longitudinal) axis of the human hippocampus. We examine how our findings fit with theories of functional specialization along the hippocampal axis.
Introduction
Rodent hippocampal theta oscillations have demonstrated a reliable relationship to memory encoding in investigations involving multiple memory modalities and behavioral tasks, but in humans the data have been mixed. There is strong evidence of a gamma band power increase during successful encoding (a positive subsequent memory effect, SME), but a commensurate theta–range positive effect has not been consistently observed in human hippocampal electrodes (Burke et al., 2014; Sederberg et al., 2007; Jacobs, 2014; Watrous, Fried, and Ekstrom, 2011). However, there is evidence that a subset of hippocampal contacts exhibit significant positive effects in the 2–5 Hz frequency range, that is, at the lower edge of the traditional 3–8 Hz theta band (Lega, Jacobs, and Kahana, 2011; Watrous, Fried, and Ekstrom, 2011). Previous studies, using hippocampal depth electrodes inserted principally via open craniotomy, have focused on the anterior portion of the hippocampus, with relatively few contacts in the posterior body and tail. There is considerable evidence in the rodent literature for functional and structural differences between the dorsal (posterior in humans) and ventral (anterior in humans) portions of the hippocampus (Moser and Moser, 1998; Strange et al., 2014; Fanselow and Dong, 2010; Poppenk et al., 2013). Differences in spatial processing (dorsal), contextual memory (dorsal), and emotional/aversive/motivational content of memories (ventral) have been observed along the septo–temporal axis (analogous to the anterior–posterior longitudinal axis in humans) (Maggio and Segal, 2009; Henke, 1990; Fanselow and Dong, 2010; Moser, Moser, and Andersen, 1993; Matus-Amat et al., 2004). There has been equivocal support for longitudinal specialization in human hippocampal function, with studies that report an anterior–posterior transition of activation for encoding versus retrieval (Greicius et al., 2003) and for spatial versus non–spatial processing (Ryan et al., 2010), although conflicting data across memory tasks have spawned more nuanced theories of longitudinal specialization positing differences in the “scale” of information processing (Poppenk et al., 2013). However, there have as yet been no direct brain recordings in humans to provide evidence in support of these views or to directly link human data with results in animals. Most human hippocampal data has been collected from the anterior hippocampus, which shows consistent gamma band power elevations during successful encoding of episodic memories and during spatial navigation (Sederberg et al., 2007; Ekstrom et al., 2009). There is also evidence of a strong low–frequency power decrease most evident in the 5–9 Hz frequency band (a negative SME) for these recordings (Ekstrom et al., 2005).
Hemispheric differences in hippocampal activation during mnemonic processing have been observed most strongly in spatial navigation, which is associated with stronger activation effects in the non–dominant hemisphere in non–invasive (Abrahams et al., 1997; Bohbot et al., 1998; Pu et al., 2016) and invasive studies in humans (Jacobs et al., 2010). Strong lateralization for episodic memory has been less consistently observed in fMRI (Fletcher, Frith, and Rugg, 1997), although structural data (Ezzati et al., 2016) suggest verbal memory stimuli are encoded more strongly in the left hemisphere. Human electrophysiological data examining inter–hemispheric connectivity have been rare due to the implantation arrays in traditional datasets.
Stereo electroencephalography has gained increasing popularity as a complement to grid electrode studies for intracranial seizure monitoring because it is less–invasive and it offers the ability to sample from multiple subcortical structures in both hemispheres (Gonzalez-Martinez et al., 2013). With robotic assistance, the accuracy of electrode placement is sub–millimeter and this permits the use of laterally–inserted depth electrodes into the anterior and posterior hippocampus, with dorsal hippocampal contacts located posterior to where the hippocampus begins to curve medially around the thalamus. Existing iEEG datasets that have been collected as subjects performed episodic memory tasks, with electrodes placed via open craniotomy, have almost exclusively included laterally–inserted anterior hippocampal electrode contacts (Lega, Jacobs, and Kahana, 2011; Watrous, Fried, and Ekstrom, 2011).
We took advantage of a unique dataset of 23 participants implanted with both anterior and posterior laterally inserted hippocampal depth electrodes to examine oscillatory differences between the anterior and posterior human hippocampus. All of these patients had electrodes in both locations simultaneously, permitting us to examine phase relationships for the oscillations that exhibited mnemonically–relevant properties. 12 of these patients had bilaterally inserted (anterior) hippocampal electrodes, permitting us also to examine left–right connectivity during memory encoding, revealing left–right synchrony that predicts successful item encoding 600–1000 msec after the onset of item presentation. We provide evidence that the slow–theta power increase previously reported in a minority of hippocampal recordings (Lega, Jacobs, and Kahana, 2011; Watrous, Fried, and Ekstrom, 2011) is strongest in the posterior hippocampus (in both hemispheres), while the gamma band subsequent memory effect does not differ along the longitudinal axis but is stronger in the language dominant hemisphere during encoding but not item retrieval. We place our findings into the context of existing animal and human electrophysiological and imaging data and examine our results as they pertain to novel theories of human hippocampal processing.
Materials and Methods
Participants
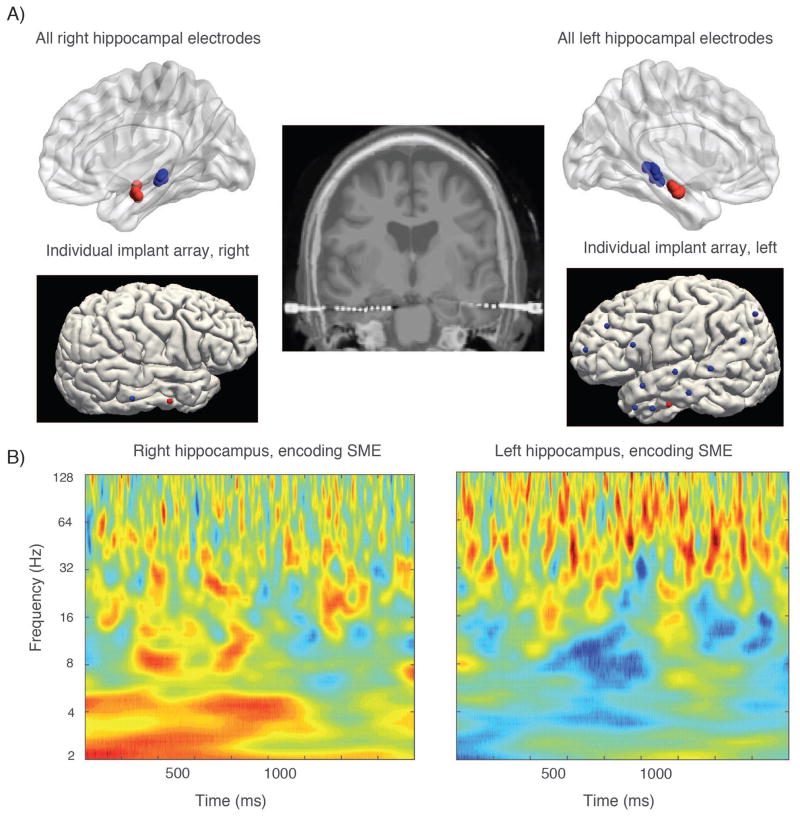
23 subjects with medication-resistant epilepsy who underwent stereoelectroencephalography surgery with the goal of identifying their ictal onset region(s) participated in the study during their monitoring period. Participants came from the UT Southwestern epilepsy surgery program over a period of two years. All participants with at least one anterior–posterior hippocampal electrode pair that was not the site of seizure onset were included in the study. Subjects had up to 15 intracranial depth electrode implants at locations specified by the neurology team, two of which included targeting the anterior and posterior segment of the hippocampus using a lateral approach with robotic assistance for sub–millimeter accuracy. Each electrode contained ten contacts spaced 3 to 5 mm apart. Final electrode localization determination as in or out of the hippocampus was made by expert neuroradiology review of the electrode contact locations. In planning, demarcation along the hippocampal axis was made using the junction of the tectum and tegmentum in the coronal plane; this is slightly posterior to segmental demaracation at the uncal apex described in Poppenk et al. (2013). Figure 1 shows MRI localization of hippocampal electrodes and contacts for one subject. Overall, there were 71 anterior and 70 posterior hippocampal electrodes included in the dataset. These were also broken down as 65 right sided and 76 left sided electrodes, though only 31 of these electrodes were from the 12 patients with bilateral contacts. Hemisphere of language dominance was determined by pre–operative Wada or fMRI testing prior to implantation, as part of routine clinical practice.
Figure 1. Example of power subsequent memory effect in left and right sided contacts.
A) (top row) shows location of left and right hippocampal contacts across all subjects in the dataset using normalized electrode coordinates. Panels beneath are from a single patient showing electrode locations from a stereo EEG evaluation for a patient contributing anterior and posterior hippocampal data on a surface rendering (electrode entry locations); middle image shows these electrodes in coronal cross section. B) shows the subsequent memory effect in the right and left hippocampus, respectively from the same patient whose electrodes are shown in A. Gamma subsequent memory effect is stronger in the left sided contact for this patient, a pattern that persists across subjects. The right–sided SME also is stronger in the slow–theta range for this subject.
Behavioral task
The participants performed a verbal free recall task, in which visually presented words from a predetermined pool of common nouns were presented on a laptop monitor one at a time for 1.6 second each followed by a blank screen of 4 seconds with 100 msec of random jitter for a total of 15 memory items in a single list. Each list was followed by a 30 second period of simple math distractors in the form of A+B+C= ?? to limit rehearsal. Participants were instructed to recall as many words as possible from the list just presented to them in no particular order within a 30 second recall period (memory retrieval). This was done 25 times per session and each participant completed from one to three sessions. Further details of the task are described in Sederberg et al. (2003).
Subsequent Memory Effect Analysis
We sought to compare oscillatory power from successfully encoded memory items to that of unsuccessfully encoded items to identify a relationship between differences in oscillatory power and likelihood of subsequent recall. Signal was sampled at 1 kHz on a Nihon–Koden platform. Line noise was notch filtered, and we excluded activity from electrodes that were the site of seizure onset locations or frequent inter–ictal activity (total of 5 electrodes from two patients). Power analyses were conducted using a Laplacian reference scheme for consistency with other studies and to take advantage of favorable signal–to–noise characteristics (Lega, Jacobs, and Kahana, 2011; Sederberg et al., 2007; Zhang and Jacobs, 2015). For connectivity/phase coherence analysis we used a bipolar re–reference for hippocampal signal in which hippocampal contacts were referenced to adjacent white matter. We used a kurtosis algorithm (with threshold of 4) to exclude abnormal events and inter–ictal activity. The power values were extracted from 1800 msec time windows following the appearance of the study item using Morlet wavelets with width of six at 49 log–spaced frequencies centered at 2(n/8), n = 8 : 64 (Tallon-Baudry et al., 1997). Power values across the time series derived from recalled events were then compared to values from non recalled events via a Wilcoxon rank-sum test at each time-frequency point. Within the rank-sum test, we incorporated a permutation procedure (1000 iterations) to generate an unbiased estimate of the type 1 error rate (Sederberg et al., 2003). We generated a p value by identifying the position of the true ranksum statistic from the test applied to real data with 1000 ranksum values generated from shuffling recalled and non–recalled event labels. We then applied normal inverse transformation to the p values matrices of each electrode to convert them to Z values to combine across electrodes and subjects.
Our a priori hypotheses were that we would observe a difference between anterior and posterior hippocampal activity and left versus right hippocampal activity in gamma and slow–theta activity; we therefore statistically compared the SME for different hippocampal locations using a t–test applied to the distribution of Z values from each time–frequency step for electrodes within each hippocampal region. To correct for multiple comparisons across these six frequency bands, we used a Bonferroni correction after averaging power within each band. We also applied a clustering requirement by which we required that significant effects persisted for at least one continuous half cycle of a given oscillation. Reported significant results for these power analyses are from a random effects model, in which power values were averaged for all electrodes within each subject before comparing distributions of language dominant/non dominant or ventral/dorsal contacts. In general, we used non–parametric statistical tests when comparing distributions of oscillatory power and parametric tests when comparing distributions of test statistics (Z values).
Phase Analysis
The oscillatory phase for each time–frequency pixel was extracted using the same method as described for power using Morlet wavelets. The resulting phase values were used to determine oscillatory synchrony between anterior hippocampal contacts and posterior hippocampal contacts. Calculation of synchrony was implemented with Rayleigh tests including a shuffle procedure for randomization (boostrapping), generating a phase locking statistic (PLS) as described previously (Lachaux et al., 1999). A separate distribution of PLS values for recalled and non–recalled events was obtained for all dorsal–ventral and dominant–non dominant hippocampal pairs for each subject (total of 137 left–right pairs, 222 dorsal–ventral pairs). A subsequent memory effect in dominant–non dominant hippocampal synchrony was analyzed by running a t–test between distributions Z values of recalled events and of non-recalled events for all contact pairs from all subjects. This resulted in a p value at every time-frequency step. Within the time windows that revealed significant phase coherence, we calculated the mean phase difference for each electrode pair across trials. Phase reset analysis was performed by testing the uniformity of the distribution of phase values across trials at each time step within a given electrode, then comparing the distributions of Z values from recalled and non–recalled trials across electrodes (Rizzuto et al., 2006). We calculated the speed of propagation for a presumed traveling wave centered at 4 Hz in the hippocampus (Zhang and Jacobs, 2015) by calculating the mean phase difference (for time points exhibiting significant phase coherence, p < 0.05) between anterior and posterior hippocampal contacts. Using the Euclidean distance between the two electrode locations calculated via Talairaich coordinates for the electrodes, the frequency of the oscillation, and this phase offset, we were able to calculate the speed of propagation.
Analysis during item retrieval period
To analyze brain oscillatory activity during retrieval, one must create a comparison condition for the period immediately prior to item retrieval during which the representation of a memory item is being reinstated. Because false recall events were rare, we employed a previously published method (Burke et al., 2014) by which retrieval events were filtered to ensure there were not any other retrieval events within 1500 msec before or 500 msec afterward in the time series, and then statistically comparing a distribution of these 1400 msec epochs to a control distribution of 1 second time segments drawn from within the retrieval epoch. The results we present are from correct retrieval events only.
Results
Anterior–posterior differences in subsequent memory effects
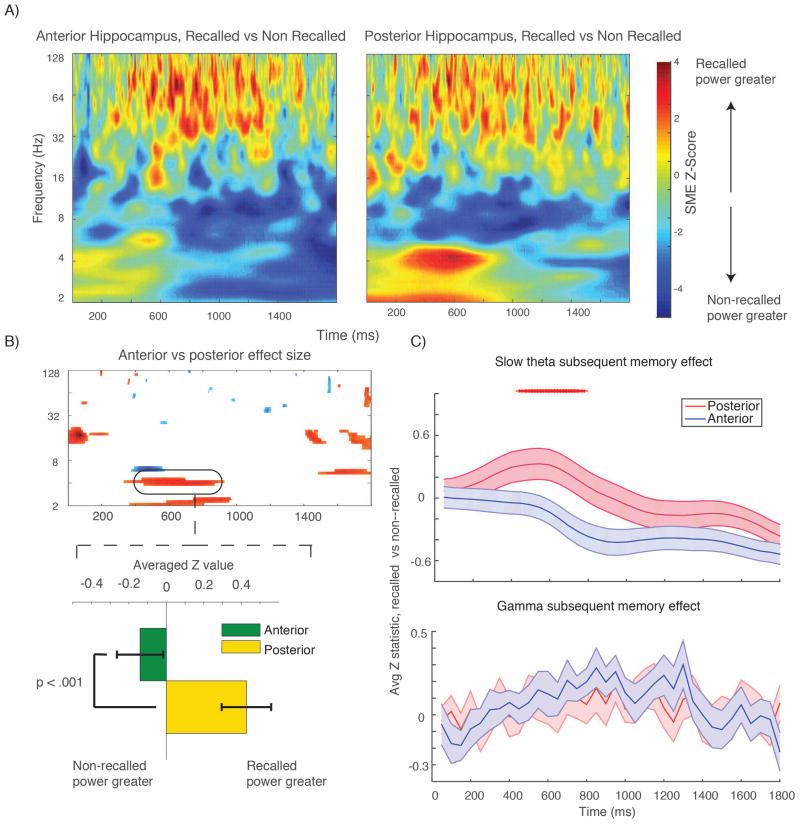
We analyzed 23 subjects with stereo EEG electrodes inserted into the anterior and posterior hippocampus. The average distance between anterior and posterior electrodes was 22.4 mm (range, 18–31 mm). We quantified the subsequent memory effect for the 71 anterior and 70 posterior electrodes using a ranksum test with shuffle procedure; the aggregate SME from each subregion (average Z value from this shuffle test) is shown in Figure 2, top panel. We directly compared the SME for anterior and posterior electrodes at the subject level by averaging Z values for all electrodes in each subject and then comparing the resulting distributions using a t–test. Results of this anterior–posterior comparison in time–frequency space are shown in the lower left panel in Figure 2 (filtered for p < 0.05 for one continuous half cycle of the oscillation); they suggest there is a positive SME in the 2.5–5 Hz slow–theta range that is significantly greater in the posterior than anterior hippocampus. We further tested this observation by averaging the Z values within each frequency band before performing the t–test between regions (Figure 2, lower right plot) and then Bonferroni correcting the resultant p values (t(22) > 2.9 significance threshold for corrected values, paired t–test). The slow–theta positive SME is significantly greater for a continuous half–cycle for the posterior hippocampus centered at 500 msec after presentation of the memory items. In the anterior hippocampus, there is a negative subsequent memory effect that is strongest in the second half of the time series, though the comparison with the posterior hippocampus does not survive correction at the subject level (corrected p > 0.05). These data suggest that sampling from the anterior hippocampus exclusively will under–estimate a positive slow–theta effect during item encoding, and that there may be human analogues of functional differences observed along the dorsal–ventral axis in rodent hippocampi. The gamma band subsequent memory effect is not significantly different between anterior and posterior contacts, however (see Figure 2, lower right).
Figure 2. SME in anterior versus posterior hippocampus.
A shows aggregate subsequent memory effect across all electrodes (effect size) in anterior (left panel) and posterior (right panel) time–frequency plots. B shows time–frequency plot shows pixels across all subjects (averaging signal from all electrodes in each subject’s hippocampus) that survive correction (p < 0.05 for one continuous half cycle of the oscillation, t–test across 23 subjects SME Z values). Red colors indicate posterior > anterior effect size; blue is anterior > posterior. Bar plot below show the mean slow–theta SME for anterior and posterior contacts for the highlighted time–frequency segment (SME Z values averaged in time–frequency window highlighted, t–test across 23 subjects). This plot shows that the observed SME difference is driven by a slightly negative effect in the anterior hippocampus versus a positive effect in the posterior hippocampus in the first 1000 msec after item presentation. C, upper plot shows the average effect in the slow–theta band across the time series. Red tick marks indicate time points at which SME difference survives Bonferroni correction (p < 0.008) for one continuous half cycle. There are no significant differences in the gamma band shown in the lower panel.
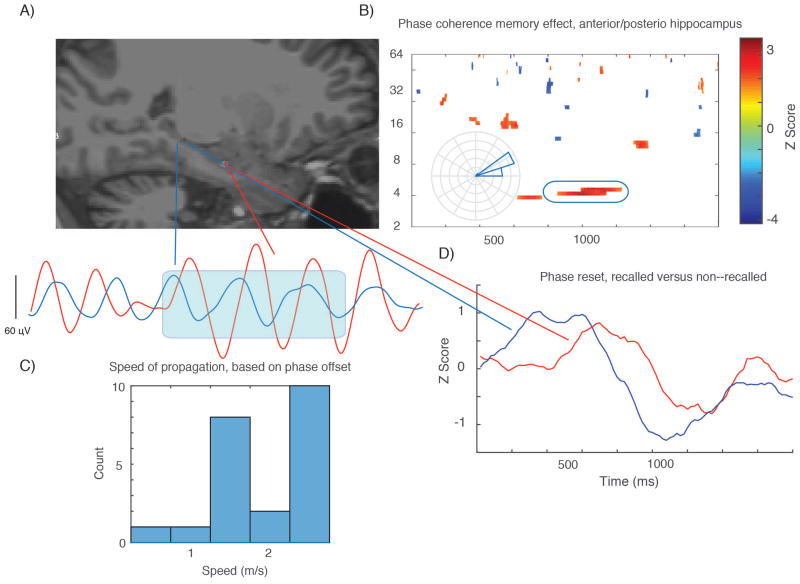
We further studied the properties of this slow–theta oscillation by examining anterior–posterior connectivity during successful encoding. We quantified connectivity using the phase locking statistic (PLS) between anterior and posterior contacts, taking advantage of the unique properties of our stereo EEG dataset to capture information from both locations simultaneously with good confidence about the anatomical origin of the signal. We compared PLS values during successful versus unsuccessful item encoding to identify time–frequency points for which there was a connectivity subsequent memory effect. For the 2.5–5 Hz slow theta oscillation, there was increased anterior–posterior connectivity centered at 1000 msec after item presentation for nearly one full cycle of the oscillation, indicating there is greater connectivity at this frequency within the hippocampus during successful encoding (p < 0.05, paired t–test between recalled and non–recalled PLS distributions, 222 electrode pairs). The average phase difference between these locations for successful encoding events (φanterior − φposterior) was +9.2 +/− 12.3 degrees. The observed phase offset may be consistent with existing data suggesting oscillations at this frequency are best modeled as a traveling wave propagating along the longitudinal axis of the hippocampus (Zhang and Jacobs, 2015). For each subject with anterior–posterior electrode pairs, we calculated a speed of propagation for such a putative traveling wave using the phase difference between the locations in this same frequency range (during the coherent segments of the time series) and the distance between the contacts (average was 24 mm). The subject–level histogram for this calculation is shown in Figure 3, lower left. The mean speed for such a wave across subjects is 1.62+/− 0.23 m/sec, consistent with values in the 3–5 Hz range previously reported in humans (Zhang and Jacobs, 2015).
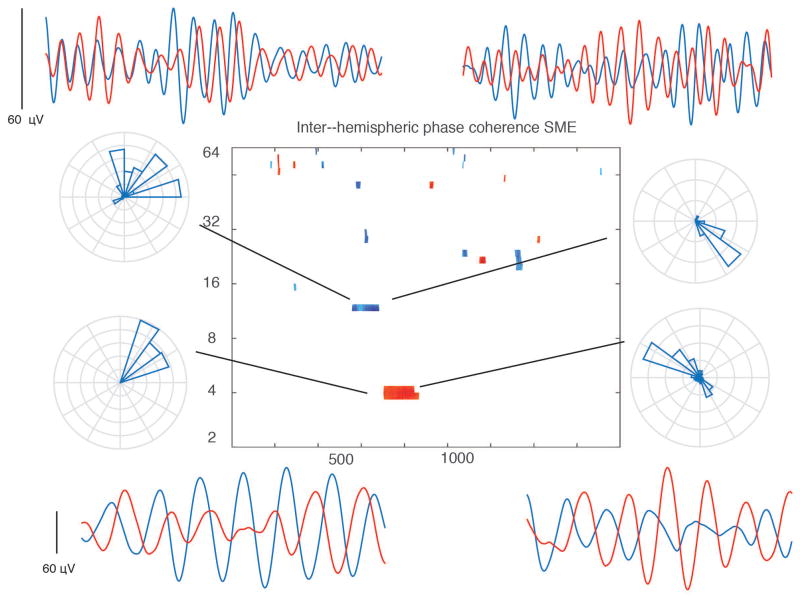
Figure 3. Phase coherence along dorsal–ventral hippocampal axis during item encoding.
A shows electrode locations in dorsal and ventral hippocampus in an experimental subject with single trial slow–theta wave from this same patient plotted below (1800 msec time window). B shows a significant connectivity subsequent memory effect (t–test across 23 subjects, comparing connectivity in recalled versus non–recalled events), with time–frequency points with p < 0.05 for one continuous half cycle of an oscillation). Red color indicates greater connectivity during successful encoding. Inset rose plot shows the mean phase difference for the selected time–frequency window (the blue oval) for an individual electrode pair, consistent with existing data and the example given in panel A. Phase difference of +15 degrees indicates the ventral contacts are ahead of dorsal contacts, consistent with dorsal–ventral direction of propagation. C shows the mean speed of propagation (histogram) for each subject. D shows results of a phase reset SME analysis, comparing phase dispersion during recalled and non–recalled events. SME Z value (t–test across subjects comparing phase reset in recalled versus non–recalled events) is plotted. The SME magnitude is not strong, but the difference in the timing of the maximum phase resent between the two locations is also consistent with dorsal–ventral propagation of a slow theta wave.
To further characterize the properties of this oscillation, we examined phase reset in the anterior and posterior hippocampus. Phase reset involves quantifying the phase dispersion across events at each time sample following the onset of a study item; this is not a measure of connectivity as phase dispersion is quantified (using the Rayleigh test) within a brain location (in this case, separately for anterior and posterior electrodes). Results are shown in Figure 3, lower right panel. In the first half of the time series, there is greater reset of phase for successfully encoded items (Z value greater than zero) for both anterior and posterior hippocampal electrodes, but reset of phase occurs earlier in the time series in the posterior contacts. The timing of this phase reset SME is consistent with a model of a posterior–anterior traveling wave in that reset occurs earlier in the posterior hippocampus. However, these analyses cannot rule out the possibility that theta oscillations with separate anterior and posterior (or some third location) generators respond to memory stimuli sequentially in time, a point we address directly in the Discussion.
Hemispheric differences in subsequent memory effects
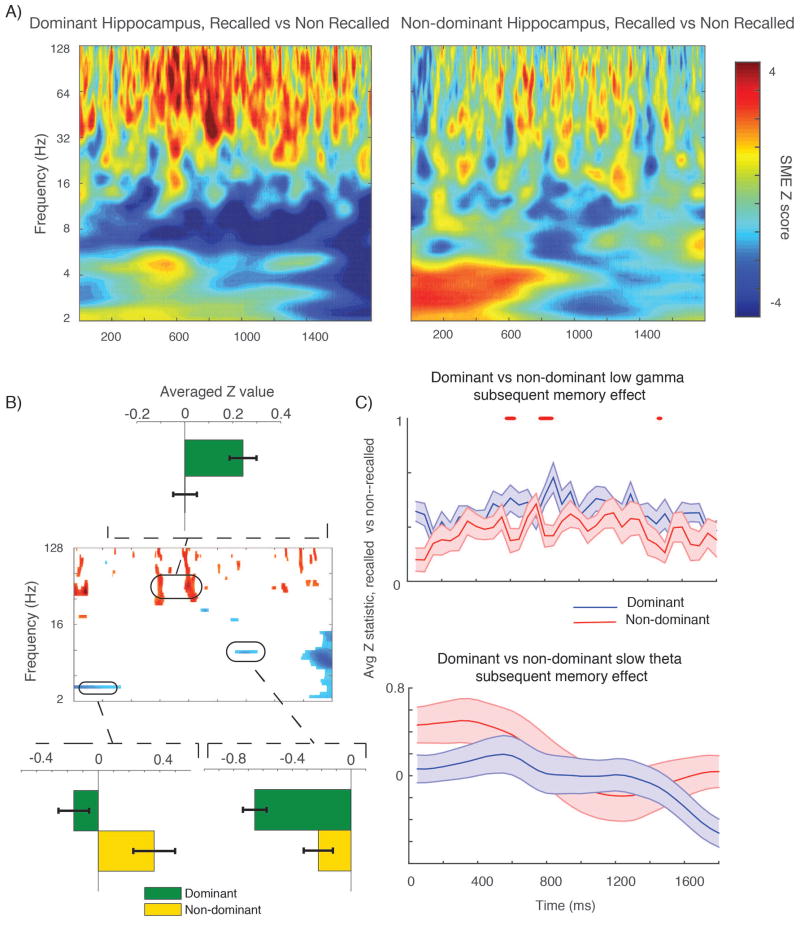
We compared the subsequent memory effect for dominant (76) and non–dominant (65) electrodes by first quantifying a subsequent memory effect (recalled versus non–recalled events, Z values from shuffle procedure) for electrodes from each hemisphere, and then contrasted these distributions directly using a t–test across subjects (analogous to the anterior–posterior comparison described above, t(33) > 1.7 for one half continuous oscillatory cycle). Results are shown in Figure 4. There is a significantly greater positive gamma band SME especially in the middle of the time series in the dominant hippocampus; this survives Bonferroni correction for a continuous half cycle (Figure 4, right side) at several points (p < 0.05, corrected across 6 frequency bands). For the 2.5–5 Hz slow–theta oscillation by contrast, there is a positive SME in the first half of the time series in the non-dominant hippocampal electrodes, although this effect does not survive Bonferroni correction across subjects for a continuous half cycle of the oscillation.
Figure 4. Dominant versus non–dominant hemisphere SME.
A shows aggregate subsequent memory effect across all electrodes (effect size) in dominant (left panel) and non–dominant (right panel) time–frequency plots. B, center shows time–frequency pixels across all subjects (t–test across 18 subjects contributing dominant and 17 subjects contributing non–dominant electrodes) that meet requirement of p < 0.05 for one continuous half cycle of the oscillation. Inset bar plot above shows average gamma band SME Z value in the highlighted window, showing a larger effect in the dominant hemisphere. Lower inset plots show a large slow–theta effect for the non– dominant hemisphere, although this does not survive Bonferroni correction (shown in C). Negative SME observed at 9 Hz is stronger in the dominant hemisphere. C shows SME Z values between dominant and non–dominant electrodes (averaged within subject) across the time series for the gamma band (top) and slow theta band (bottom). Red tick marks indicate time points at which SME difference survives Bonferroni correction for one continuous half cycle of the oscillation. Although there is a difference at the beginning of the time series in the slow–theta range, it does not survive correction.
We followed up on this analysis by examining dominant–non dominant phase synchrony during successful and unsuccessful memory encoding; this analysis was restricted to subjects who had both left and right sided electrodes (12), which were exclusively in the ventral hippocampus based on clinical practice. We compared the distribution of PLS values for recalled versus non–recalled events. The results are shown in Figure 5. A significant difference in PLS values between the distributions is found between 600 and 900 msec after item presentation for oscillations centered both at 4 Hz and at 9–10 Hz. These effects go in opposite directions: the 4 Hz oscillation exhibited an increase in phase coherence (greater coupling) during successful encoding, while the 9 Hz oscillation exhibits a decrease. Phase coherence during recalled events for this window yielded an average phase difference of +88 +/− 20.3 degrees φdominant − φnon——dominant for dominant and non dominant hippocampi, although there was significant heterogeneity among electrode pairs (ranging from 35 to 285 degrees) for both oscillations. This is reflected in the examples from individual electrode pairs surrounding the central plot in Figure 5, and it implies that while many subjects exhibit differential connectivity during successful encoding between left and right hippocampi, the preferred phase of inter–hippocampal coupling is quite variable. This finding, along with the more complex inter–hemispheric circuitry (as compared to the within–hippocampus analysis described above) makes it difficult to hypothesize if one hemisphere is “leading” the other using this analysis.
Figure 5. Inter–hippocampal connectivity during item encoding.
Time–frequency plot at center shows pixels across 12 subjects (t–test between recalled and non–recalled PLS distributions) that exhibit a significant SME (p < 0.05 for one continuous half cycle of oscillation), indicating greater connectivity during successful item encoding between left and right hippocampi at 4 Hz. There is decreased connectivity at ~10 Hz. Red colors indicate greater connectivity during successful encoding, blue colors indicate less connectivity. Surrounding plots are individual examples of filtered waves (single trials from individual electrodes, one from each hemisphere, 1800 msec time series) and rose plot histograms indicate there is significant heterogeneity in the phase offset across electrodes for each frequency range, precluding any inferences regarding left–right directionality from these data.
Retrieval power analysis
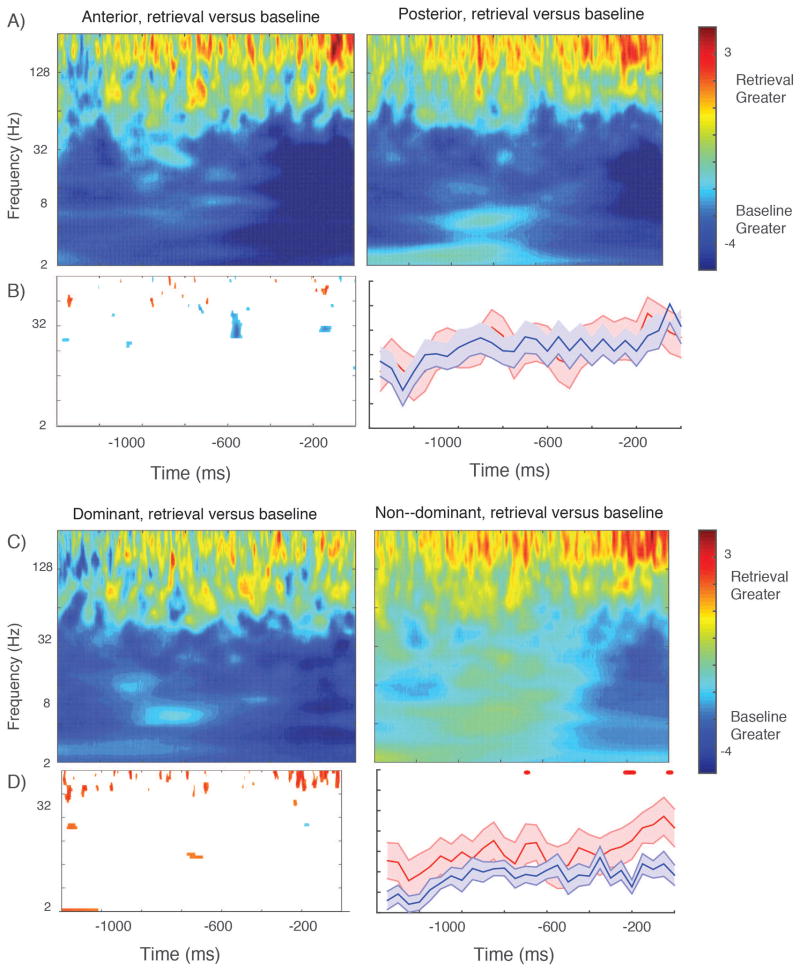
We repeated the dominant–non dominant and anterior–posterior power comparisons for the retrieval phase of the free recall task. For this analysis, correct retrieval events (excluding list intrusions) were compared to randomly selected one second time epochs sampled from within the retrieval period that were temporally distant from a recall event (see Methods). The average effects across anterior and posterior electrode contacts are illustrated in Figure 6, panel A. A broad low–frequency power decrease is visible along with a gamma band power increase that is most pronounced in the immediate 200 msec prior to vocalization of a retrieved memory item. Anterior–posterior differences that survive correction at the subject level are limited to rare time–points in the gamma and beta band; these do not survive correction. The same broad gamma band power increase is visible in the dominant and non–dominant hippocampi, but it is of greater magnitude (greater gamma power increase before item vocalization) in the non–dominant hemisphere, especially at the end of the time series. We tested for hemispheric differences in this gamma band retrieval effect using methods analogous to those described above (t–test at the subject level across the distributions of Z values). The difference survives Bonferroni correction for multiple cycles within the time series in the high–gamma range. The low frequency power decrease is stronger and more broad in the dominant hemisphere (Figure 6, panel B) but this difference is significant only for sporadic time points and it does not survive Bonferroni correction. Overall, the dominant–non dominant comparison is a reversal of the effect during item encoding, for which the gamma band subsequent memory effect is stronger in the dominant hemisphere. Since the retrieval effects were calculated relative to a baseline period, we wanted to make sure this apparently greater incrase in gamma power in the non–dominant hemisphere was not due to lower baseline gamma power during this period (artificially inflating the gamma effect we observed relative to the dominant hemisphere). We tested for this possibility by randomly selecting baseline power values, normalizing them and then comparing these directly (separately for low gamma and high gamma) between the dominant and non–dominant hemispheres. Baseline gamma power in both bands was higher for non–dominant hemisphere, consistent with an elevation of power in this frequency range that increases immediately before item vocalization but occurs throughout the retrieval period. It indicates that baseline differences do not account for the greater gamma power elevation in the non–dominant hemisphere observed in the event–locked data shown in Figure 6. If anything, our method of comparing to a baseline condition might slightly understate the magnitude of the hemispheric differences in the 1000 msec preceding vocalization of a retrieved memory item.
Figure 6. Oscillatory power during item retrieval.
Panel A shows the results of t–test comparing retrieval effect size across all electrodes between anterior and posterior electrode groups. B shows time–frequency points across all electrodes that exceed p < 0.05 for one continuous cycle of an oscillation; this is limited brief differences between anterior and posterior hippocampi in the beta frequency range, related to a greater power decrease relative to baseline in the posterior electrodes. Right panel in B shows results for gamma frequency range across 23 subjects; no points survive Bonferroni correction for one continuous half cycle. C shows the comparison between the dominant and non–dominant hemisphere across all electrodes. The low frequency power decrease occurs immediately prior to item retrieval in the non–dominant hemisphere versus throughout the time series in the dominant hemisphere, visible in the direct comparison between electrode sets in D, left plot. The most striking difference is in the high gamma range, for which a power increase relative to baseline is stronger in the non–dominant hemisphere. This difference survives Bonferroni correction at the subject level (D, right panel.)
Discussion
Anterior–posterior differences in hippocampal function
Our examination of anterior versus posterior hippocampal oscillatory activity showed that while the gamma band subsequent memory effect is similar between both locations, there is a positive slow–theta SME exclusively in the posterior hippocampus. Explicating this anatomical difference in oscillatory activity provides human evidence of dorsal–ventral differences in hippocampal function observed in rodent data. Results in rodents include the observation that place cells in the dorsal hippocampus have more precise spatial fields than those found in the ventral hippocampus; this difference in place fields is preserved in non–human primates (Strange et al., 2014; Killiany et al., 2002). Classical horseradish peroxidase studies suggest a difference in the anatomical segregation of hippocampal efferent fibers between dorsal and ventral regions including preferential projection of the ventral hippocampus to the amygdala (Meibach and Siegel, 1977a; Meibach and Siegel, 1977b; Strange et al., 2014; Pitkänen et al., 2000), and whole exome sequencing has identified sharp demarcations in gene expression pattern along the hippocampal axis. These structural and functional differences, preserved across species, motivated our hypothesis that we would observe differences in anterior versus posterior slow theta and gamma activity during memory encoding.
One might reasonably ask if the dorsal–ventral differences in the rodent hippocampus can be expected to apply to humans; in humans the ventral region (adjacent to the amygdala) has undergone relatively greater expansion compared to the dorsal portions of the hippocampus (for a review, see Box 1 in Strange et al. (2014)). Functional imaging and lesion data has suggested the posterior hippocampus in humans shows preferential activity during spatial navigation in the non–dominant hemisphere (Pu et al., 2016; Abrahams et al., 1997), although this has not reliably been demonstrated for verbal memory tasks in the dominant hemisphere or with intracranial EEG, in which aggregated hippocampal electrodes have been shown to exhibit theta power decreases and gamma band power increases during memory encoding (Sederberg et al., 2007). However, to our knowledge, there has not been a systematic attempt to differentiate anterior and posterior hippocampal electrodes and directly compare oscillatory effects. Generally, our data support the hypothesis that memory encoding–related neural activity in the human hippocampus differs along the longitudinal axis, in that we observed a robust difference in the modulation of slow–theta oscillatory activity between the anterior and posterior hippocampus. However, we did not observe an anterior–posterior difference in the gamma band positive subsequent memory effect or the ~9 Hz negative SME that have been previously described for the hippocampus (Sederberg et al., 2007).
Several models of differential hippocampal function along the ventral–dorsal (anterior–posterior in primates) have been proposed. A model proposed by Moser and Moser (1998) and elaborated by Fanselow and Dong (2010) proposes that the ventral region exhibits sensitivity to emotional content of successfully retrieved memory items, while the dorsal hippocampus is implicated preferentially in encoding the non–emotional content or context for an individual item. Our results do not directly address this proposal, insofar as the affective content of the memory items was not manipulated, although our observation that the anterior hippocampus exhibits a robust gamma range SME on par with the posterior hippocampus suggests that both regions are involved in representing features of the memory items. Without manipulating affective content directly, it is not possible to discern whether the anterior gamma SME is related exclusively to such a feature of the memory items, while posterior gamma is related to non–affective features of the items. Fanselow and Dong (2010) also discuss a more general theory of hippocampal specialization, by which the anterior hippocampus supports pattern completion while the posterior hippocampus performs pattern separation. A related view articulated by Poppenk et al. (2013) posits that there is a change in the “gain of representation” along the dorsal–ventral axis, with more general information represented in anterior hippocampus and more specific contextual information represented in the posterior hippocampus. This theory is most easily understood in terms of spatial navigation, as the anterior hippocampus would represent more general location information while the posterior hippocampus would represent precise information of a specific location. The authors propose that, for episodic memory, this would entail greater representation of contextual details in the posterior hippocampus, although human fMRI data have not observed stronger posterior activation (Rugg and Vilberg, 2013). This theory can account for many animal observations including place field specificity and connectivity–related differences along the hippocampal axis. Without a definitive assessment of the relative contribution of slow–theta versus gamma oscillations in supporting episodic memory, it is difficult argue if our data directly support this view. A memory task that manipulates the specificity of memories during retrieval, perhaps incorporating both item and category information, may help address this question..
Slow–theta oscillations in humans and rodents
Our data add to the growing literature examining subtleties in low frequency activity in the human mesial temporal lobe during the encoding of episodic memories. Previous work has suggested that there is a broad decrease in power in the hippocampus during successful memory encoding, but that a select subpopulation of electrodes exhibits power increases in a band spanning the traditional delta–theta range, from 2.5–5 Hz (Lega, Jacobs, and Kahana, 2011). The results we present here suggest that when SME data is aggregated across many anterior and posterior hippocampal contacts (and the time series is collapsed after item presentation) the overall pattern is a negative SME across the entire 2–11 Hz frequency range (Sederberg et al., 2007; Lega, Jacobs, and Kahana, 2011), but that segregating electrodes according to position along the long axis and preserving the time series information reveals a positive effect in the posterior contacts in the slow–theta range. Connectivity data has suggested brain regions are coupled via activity in this frequency range immediately prior to memory items that are successfully encoded (Haque et al., 2015), and also that parietal–hippocampal interaction at this frequency range (though also at other frequencies) is increased during successful item retrieval (Watrous et al., 2013). The evidence for slow–theta power increases during mnemonic processing has been mixed, however: a recent non–invasive study using iEEG and MEG failed to show power increases in the hippocampus during spatial navigation (Crespo-García et al., 2016) for example. The data we present here is drawn from patients undergoing stereo EEG; this technique is better–suited to investigating anterior–posterior differences in hippocampal oscillatory activity because it permits precise targeting separately to the head and tail of the hippocampus. Depth electrodes with contacts along the hippocampal axis have previously revealed evidence of activity in the 2.5–5 Hz range in both the anterior and posterior region, though this has not been linked to differences in memory encoding (Zhang and Jacobs, 2015), while a study with similar methods identified differences in adjacent–pair signal coherence in the ventral–dorsal transition (Staresina et al., 2012).
Evidence for a theta gradient along the dorsal–ventral axis in the rodent literature links greater spike–field coherence for CA3 cells in the dorsal hippocampus to greater place cell specificity (for a summary of rodent results, see discussion in Royer et al. (2010)). Royer et al. (2010) also identified relatively less power for theta oscillations in the ventral versus dorsal hippocampus during spatial navigation, though as with our study there was strong coherence in these oscillations between dorsal and ventral locations. The authors hypothesized that the paucity of human evidence for strong theta activity may reflect the expansion of the ventral hippocampus relative to dorsal sections in primate evolution (diminishing the theta source). We would add that the pattern of electrode implantation typically employed in seizure localization efforts likely also contributes to this disparity between the human and animal literature.
Data comparing activity in human CA1 versus CA3 may be relevant to our results: in humans, CA1 is relatively expanded in the anterior hippocampus, and fMRI experiments suggest greater CA3 activation for pattern separation (identifying lures) versus pattern completion (hits) when CA1 activity is greater (Bakker et al., 2008). These data however did not suggest a significant difference in BOLD activation along the longitudinal axis. If CA3 is more directly responsible for generating the slow theta positive SME as suggested by rodent data (Buzsáki, 2002), its relatively greater representation (relative to the size of CA1) in the posterior hippocampus might explain our results. We made a preliminary attempt at resolving differences in slow-theta activity among hippocampal subfields that is presented in the supplemental material section, but our data set is not well-suited to this question. Resolving the issue of the contribution of different hippocampal subfields to the slow–theta SME will likely require microelectrode recordings with more precise intra–hippocampal targeting. Ideally, comparisons of anterior ? posterior slow-theta activity would be performed within the same subfield. Our dataset also suffers a related limitation, namely an inability to resolve differences among the three hippocampal sectors (anterior, middle, and posterior) rather than two (anterior versus posterior) (Strange et al., 2014).
Traveling wave properties of human hippocampal slow theta oscillations
A previous study directly examined theta oscillations along the longitudinal axis in the human hippocampus using occipital depth electrodes; its results were thought to be most consistent with theta oscillations acting as traveling waves along this axis (Zhang and Jacobs, 2015). The reported propagation speed (approximately 1.2–2 m/s) and magnitude of the phase change along the hippocampal axis matches what we calculated using our own data. The estimated speed of propagation of rodent hippocampal oscillations for the predominant 8 Hz oscillation is 0.16 m/s, although this seemed to vary with behavior (Patel et al., 2012). It is important to note that in our data, the phase coherence between dorsal and ventral hippocampus was significantly stronger during the encoding of later recalled than later forgotten study items, which raises the possibility that coherent traveling theta waves arise in the hippocampus preferentially during periods of successful episodic encoding. This observation suggests that intra–hippocampal integration in these lower frequency ranges supports mnemonic processing.
There are of course competing explanations for our observations. The consistent phase offset between dorsal and ventral hippocampal locations could be due to a common theta generator propagating to both locations; our dataset is not capable of resolving this question which requires recordings from electrode contacts placed continuously along the anterior–posterior axis. But a model of multiple theta “repeaters” along the dorsal–ventral axis with a theta source in the septum fits well with the phase reset data we present (Lubenov and Siapas, 2009), and the relatively narrow phase difference between locations is more likely due to a traveling wave than two independent theta oscillations (one in each location) that exhibit phase coherence. A lower phase shift per unit distance for the relevant theta frequency in the human hippocampus compared to rodents would theoretically permit greater integration of activity along the longitudinal axis (Zhang and Jacobs, 2015), especially if the phase difference along the full axis is less than a half cycle of the oscillation (facilitating simultaneous potentiation of membrane effects). In rodents, variability of the speed of propagation has also been observed for a hippocampal traveling wave (Lubenov and Siapas, 2009), similar to our results illustrated in the histogram in Figure 3.
Hemispheric differences in hippocampal oscillatory properties
There is an extensive literature describing the memory deficits associated with seizure onset or lesions in either dominant or non–dominant hemisphere (Bonelli et al., 2010; Abrahams et al., 1997; Pu et al., 2016). Commensurate with these clinical observations, right lateralized hippocampal oscillations have been observed in humans in spatial memory tasks (Cornwell et al., 2008; Ekstrom et al., 2005; Jacobs et al., 2010). However, a matching difference in the gamma band SME for episodic memory processing has not been observed in previous studies (Sederberg et al., 2007). Our data demonstrate a stronger gamma band positive SME in the dominant hemisphere (during encoding). Insofar as the hippocampus represents or indexes cortical representations of individual memory items, this left–lateralized signal may be an oscillatory correlate of the representation of semantic features of the study words (Evans and Federmeier, 2007). The left–right difference in the 9 Hz power decrease matched the gamma band effect. Left–right connectivity has not been extensively studied in human intracranial EEG, owing to the relative rarity of strong bi–hemispheric sampling from subjects performing episodic memory tasks. Our results implicate a transient elevation in connectivity during successful encoding in the slow–theta range. Inter–hippocampal functional connectivity has been linked to memory performance in young adults (Wang et al., 2010) and victims of traumatic brain injury (de la Plata et al., 2011). This connectivity may reflect the integrity of the dorsal hippocampal commissure (Gloor et al., 1993), although other pathways including the uncinate fasciculus may also contribute (Diehl et al., 2008).
During successful recall, we observed a gamma band power increase that preceded vocalization of the retrieved memory item; this effect was stronger in the non–dominant hemisphere, in opposition to the pattern we observed during encoding. This finding is hard to reconcile with the previously reported recapitulation of encoding–related oscillatory patterns during item retrieval (Manning et al., 2011), although this may reflect a failure to distinguish between hemispheres when quantifying hippocamapal activity at retrieval. It is interesting that the present hemispheric difference occurs in the high gamma range principally, while the asymmetry in the encoding gamma related effect occurs across the entire gamma spectrum. It will be informative to observe how electrical stimulation of one hippocampus alters oscillatory patterns in the other during mnemonic processing, and how more subtle item–context manipulations affect the hemispheric gamma band differences in gamma band power changes during retrieval. We note in passing that the present findings that encoding-related gamma effects were left lateralized, while retrieval-related effects were more prominent on the right, are reminiscent of the ‘HERA’ principle held at one time to apply to the prefrontal cortex (Habib, Nyberg, and Tulving, 2003; Babiloni et al., 2006). While we are unaware of any prior evidence suggesting that this principle applies to the hippocampus, it is possible that our findings are indicative of a division of labor along these lines.
Conclusion
Using electrodes precisely targeted to the anterior and posterior hippocampus, we observed a difference in an SME in the slow–theta (3.5–5 Hz) frequency range indicating that the posterior but not anterior hippocampus exhibits a power increase during successful item encoding. Connectivity analyses suggested that intra–hippocampal phase coherence at this frequency also predicts successful item encoding, and that oscillations at this frequency propagate along the longitudinal axis possibly as a traveling wave. Differences in slow–theta activity during encoding along the axis of the hippocampus may be consistent with postulated differences in the functional specialization of the anterior versus posterior hippocampus, including more detailed representation of temporal or spatial context information in the posterior hippocampus. While the gamma band positive SME was not significantly different in anterior and posterior hippocampi, we did observe differences in the SME at this frequency band between the dominant and non–dominant hemispheres. This hemispheric asymmetry may reflect greater activity related to the representation of the semantic content of encoded memory items in the dominant hemisphere, although the reversal of this pattern during item retrieval makes this result more difficult to interpret.
Supplementary Material
Acknowledgments
Funding in part via UT Brain Seed Grant #366582, NIH R21 NS095094 and DARPA Restoring Active Memory project. We thank Blackrock Microsystems for providing neural recording and stimulation equipment. This work was supported by the DARPA Restoring Active Memory (RAM) program (Cooperative Agreement N66001-14-2-4032). The views, opinions, and/or findings contained in this material are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government. The authors declare no competing conflicts of interest.
References
- Abrahams S, Pickering Alan, Polkey CE, Morris RG. Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia. 1997;35(1):11–24. doi: 10.1016/s0028-3932(96)00051-6. [DOI] [PubMed] [Google Scholar]
- Babiloni Claudio, Vecchio Fabrizio, Cappa Stefano, Pasqualetti Patrizio, Rossi Simone, Miniussi Carlo, Rossini Paolo Maria. Functional frontoparietal connectivity during encoding and retrieval processes follows hera model: A high-resolution study. Brain research bulletin. 2006;68(4):203–212. doi: 10.1016/j.brainresbull.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Bakker Arnold, Brock Kirwan C, Miller Michael, Stark Craig EL. Pattern separation in the human hippocampal ca3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot Veronique D, Kalina Miroslav, Stepankova Katerina, Spackova Natasa, Petrides Michael, Nadel LYNN. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36(11):1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Bonelli Silvia B, Powell Robert HW, Yogarajah Mahinda, Samson Rebecca S, Symms Mark R, Thompson Pamela J, Koepp Matthias J, Duncan John S. Imaging memory in temporal lobe epilepsy: predicting the effects of temporal lobe resection. Brain. 2010:awq006. doi: 10.1093/brain/awq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke John F, Sharan Ashwini D, Sperling Michael R, Ramayya Ashwin G, Evans James J, Karl Healey M, Beck Erin N, Davis Kathryn A, Lucas Timothy H, Kahana Michael J. Theta and high-frequency activity mark spontaneous recall of episodic memories. The Journal of Neuroscience. 2014;34(34):11355–11365. doi: 10.1523/JNEUROSCI.2654-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki György. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. Journal of Neuroscience. 2008;28(23):5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-García Maité, Zeiller Monika, Leupold Claudia, Kreiselmeyer Gernot, Rampp Stefan, Hamer Hajo M, Dalal Sarang S. Slow-theta power decreases during item-place encoding predict spatial accuracy of subsequent context recall. NeuroImage. 2016;142:533–543. doi: 10.1016/j.neuroimage.2016.08.021. [DOI] [PubMed] [Google Scholar]
- de la Plata, Marquez Carlos D, Garces Juanita, Kojori Ehsan Shokri, Grinnan Jack, Krishnan Kamini, Pidikiti Rajesh, Spence Jeffrey, Devous Michael D, Moore Carol, McColl Rodderick, et al. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Archives of neurology. 2011;68(1):74–84. doi: 10.1001/archneurol.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl Beate, Busch Robyn M, Duncan John S, Piao Zhe, Tkach Jean, Lüders Hans O. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49(8):1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Ekstrom Arne, Suthana Nanthia, Millett David, Fried Itzhak, Bookheimer Susan. Correlation between bold fmri and theta-band local field potentials in the human hippocampal area. Journal of neurophysiology. 2009;101(5):2668–2678. doi: 10.1152/jn.91252.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans Karen M, Federmeier Kara D. The memory that’s right and the memory that’s left: Event-related potentials reveal hemispheric asymmetries in the encoding and retention of verbal information. Neuropsychologia. 2007;45(8):1777–1790. doi: 10.1016/j.neuropsychologia.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati Ali, Katz Mindy J, Zammit Andrea R, Lipton Michael L, Zimmerman Molly E, Sliwinski Martin J, Lipton Richard B. Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia. 2016;93:380–385. doi: 10.1016/j.neuropsychologia.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow Michael S, Dong Hong-Wei. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Rugg MD. The functional neuroanatomy of episodic memory. Trends in neurosciences. 1997;20(5):213–218. doi: 10.1016/s0166-2236(96)01013-2. [DOI] [PubMed] [Google Scholar]
- Gloor P, Salanova Vicenta, Olivier A, Quesney LF. The human dorsal hippocampal commissure. Brain. 1993;116(5):1249–1273. doi: 10.1093/brain/116.5.1249. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez Jorge, Bulacio Juan, Alexopoulos Andreas, Jehi Lara, Bingaman William, Najm Imad. Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: early experience from a north american epilepsy center. Epilepsia. 2013;54(2):323–330. doi: 10.1111/j.1528-1167.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- Greicius Michael D, Krasnow Ben, Boyett-Anderson Jesse M, Eliez Stephan, Schatzberg Alan F, Reiss Allan L, Menon Vinod. Regional analysis of hippocampal activation during memory encoding and retrieval: fmri study. Hippocampus. 2003;13(1):164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Habib Reza, Nyberg Lars, Tulving Endel. Hemispheric asymmetries of memory: the hera model revisited. Trends in cognitive sciences. 2003;7(6):241–245. doi: 10.1016/s1364-6613(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Haque Rafi U, Wittig John H, Damera Srikanth R, Inati Sara K, Zaghloul Kareem A. Cortical low-frequency power and progressive phase synchrony precede successful memory encoding. The Journal of Neuroscience. 2015;35(40):13577–13586. doi: 10.1523/JNEUROSCI.0687-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke Peter G. Granule cell potentials in the dentate gyrus of the hippocampus: coping behavior and stress ulcers in rats. Behavioural brain research. 1990;36(1–2):97–103. doi: 10.1016/0166-4328(90)90164-a. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Korolev IO, Caplan JB, Ekstrom AD, Litt B, Baltuch G, Fried I, Schulze-Bonhage A, Madsen JR, Kahana MJ. Right-lateralized brain oscillations in human spatial navigation. Journal of Cognitive Neuroscience. 2010;22(5):824–836. doi: 10.1162/jocn.2009.21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs Joshua. Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2014;369(1635):20130304. doi: 10.1098/rstb.2013.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS, et al. Mri measures of entorhinal cortex vs hippocampus in preclinical ad. Neurology. 2002;58(8):1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human Brain Mapping. 1999;8(4):194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lega BC, Jacobs J, Kahana MJ. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2011;22(4):748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- Lubenov Evgueniy V, Siapas Athanassios G. Hippocampal theta oscillations are travelling waves. Nature. 2009;459(7246):534–539. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- Maggio Nicola, Segal Menahem. Differential modulation of long-term depression by acute stress in the rat dorsal and ventral hippocampus. The Journal of Neuroscience. 2009;29(27):8633–8638. doi: 10.1523/JNEUROSCI.1901-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Polyn SM, Baltuch G, Litt B, Kahana MJ. Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. Proceedings of the National Academy of Sciences, USA. 2011;108(31):12893– 12897. doi: 10.1073/pnas.1015174108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat Patricia, Higgins Emily A, Barrientos Ruth M, Rudy Jerry W. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. The Journal of Neuroscience. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibach Richard C, Siegel Allan. Efferent connections of the hippocampal formation in the rat. Brain Research. 1977a;124(2):197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- Meibach Richard C, Siegel Allan. Efferent connections of the septal area in the rat: an analysis utilizing retrograde and anterograde transport methods. Brain research. 1977b;119(1):1–20. doi: 10.1016/0006-8993(77)90088-9. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. Journal of Neuroscience. 1993;13(9):3916. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Patel Jagdish, Fujisawa Shigeyoshi, Berényi Antal, Royer Sébastien, Buzsáki György. Traveling theta waves along the entire septotemporal axis of the hippocampus. Neuron. 2012;75(3):410–417. doi: 10.1016/j.neuron.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen Asla, Pikkarainen Maria, Nurminen Nina, Ylinen Aarne. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: a review. Annals of the New York Academy of Sciences. 2000;911(1):369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Poppenk Jordan, Evensmoen Hallvard R, Moscovitch Morris, Nadel Lynn. Long-axis specialization of the human hippocampus. Trends in cognitive sciences. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Pu Yi, Cornwell Brian R, Cheyne Douglas, Johnson Blake W. The functional role of human right hippocampal/parahippocampal theta rhythm in environmental encoding during virtual spatial navigation. Human Brain Mapping. 2016 doi: 10.1002/hbm.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Kahana MJ. Human neocortical oscillations exhibit theta phase differences between encoding and retrieval. NeuroImage. 2006;31(3):1352–1358. doi: 10.1016/j.neuroimage.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Royer Sébastien, Sirota Anton, Patel Jagdish, Buzsáki György. Distinct representations and theta dynamics in dorsal and ventral hippocampus. The Journal of Neuroscience. 2010;30(5):1777–1787. doi: 10.1523/JNEUROSCI.4681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg Michael D, Vilberg Kaia L. Brain networks underlying episodic memory retrieval. Current opinion in neurobiology. 2013;23(2):255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan Lee, Lin Chun-Yu, Ketcham Katie, Nadel Lynn. The role of medial temporal lobe in retrieving spatial and nonspatial relations from episodic and semantic memory. Hippocampus. 2010;20(1):11–18. doi: 10.1002/hipo.20607. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cerebral Cortex. 2007;17(5):1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Sederberg Per B, Kahana Michael J, Howard Marc W, Donner Elizabeth J, Madsen Joseph R. Theta and gamma oscillations during encoding predict subsequent recall. The Journal of Neuroscience. 2003;23(34):10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina Bernhard P, Henson Richard NA, Kriegeskorte Nikolaus, Alink Arjen. Episodic reinstatement in the medial temporal lobe. The Journal of Neuroscience. 2012;32(50):18150–18156. doi: 10.1523/JNEUROSCI.4156-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange Bryan A, Witter Menno P, Lein Ed S, Moser Edvard I. Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience. 2014;15(10):655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry Catherine, Bertrand Olivier, Delpuech Claude, Pernier Jacques. Oscillatory γ-band (30–70 hz) activity induced by a visual search task in humans. The Journal of neuroscience. 1997;17(2):722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Liang, Negreira Alyson, LaViolette Peter, Bakkour Akram, Sperling Reisa A, Dickerson Bradford C. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010;20(3):345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous AJ, Fried I, Ekstrom AD. Behavioral correlates of human hippocampal delta and theta oscillations during navigation. Journal of Neurophysiology. 2011;105(4):1747–1755. doi: 10.1152/jn.00921.2010. [DOI] [PubMed] [Google Scholar]
- Watrous Andrew J, Lee Darrin J, Izadi Ali, Gurkoff Gene G, Shahlaie Kiarash, Ekstrom Arne D. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus. 2013;23(8):656–661. doi: 10.1002/hipo.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Honghui, Jacobs Joshua. Traveling theta waves in the human hippocampus. The Journal of Neuroscience. 2015;35(36):12477–12487. doi: 10.1523/JNEUROSCI.5102-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.