Abstract
Background/Aims
The medial amygdala (MeA) responds to olfactory stimuli and alters reproductive physiology. However, the neuronal circuit that relays signals from the MeA to the reproductive axis remains poorly defined. This study aimed to test whether MeA kisspeptin (MeAKiss) neurons in male mice are sensitive to sexually relevant olfactory stimuli and transmit signals to alter reproductive physiology. We also investigated whether MeAKiss neurons have the capacity to elaborate glutamate and GABA neurotransmitters and potentially contribute to reproductive axis regulation.
Methods
Using female urine as a pheromone stimulus, MeAKiss neuronal activity was analysed and serum luteinizing hormone (LH) was measured in male mice. Next, using a chemogenetic approach, MeAKiss neurons were bi-directionally modulated to measure the effect on serum LH and evaluate the activation of the preoptic area. Lastly, using in situ hybridization, we identified the proportion of MeAKiss neurons that express markers for GABAergic (Vgat) and glutamatergic (Vglut2) neurotransmission.
Results
Male mice exposed to female urine showed a two-fold increase in the number of c-Fos-positive MeAKiss neurons concomitant with raised LH. Chemogenetic activation of MeAKiss neurons significantly increased LH in the absence of urine exposure, whereas inhibition of MeAKiss neurons did not alter LH. In situ hybridization revealed that MeAKiss neurons are a mixed neuronal population in which 71% express Vgat mRNA, 29% express Vglut2 mRNA, and 6% express both.
Conclusions
Our results uncover, for the first time, that MeAKiss neurons process sexually relevant olfactory signals to influence reproductive hormone levels in male mice, likely through a complex interplay of neuropeptide and neurotransmitter signalling.
Keywords: Amygdala, Kiss-1, Luteinizing hormone, Male reproduction, Neuroendocrinology
Introduction
Highly evolved sensory and behavioural strategies enable animals to identify a mate and ensure reproductive success. The medial amygdala (MeA) is associated with mediating sexual behaviour in mammals [1], including humans [2], and is known to influence reproductive hormone levels [3]. In rodents, chemical signals called pheromones carry sex- and species-specific information that elicits pre-programmed mating and endocrine responses. Pheromone signals activate sensory neurons in the vomeronasal organ, which relay information through the accessory olfactory bulb (AOB) for processing in the MeA [4]. Projections from the MeA to the preoptic area (POA) of the hypothalamus then influence neuroendocrine status and mating behaviours. The MeA appears to have both inhibitory and stimulatory influences on reproductive physiology and associated behaviours. MeA lesions advance puberty onset in rodents [5] and monkeys [6], but also disrupt sexual behaviours including the investigation and preference towards opposite-sex pheromones [7]. Exposure to opposite-sex pheromones leads to neuronal activation throughout the MeA [3, 8] and raised serum luteinizing hormone (LH) in male rodents [9, 10].
Although the MeA is a critical node for processing sexually relevant stimuli to influence mating behaviour and reproductive endocrine function, the underlying brain circuitry that mediates these activities remains poorly defined. A population of kisspeptin neurons located in the MeA (MeAKiss) are cogent candidates [11] for transmitting olfactory influences to the reproductive axis. In the hypothalamus, kisspeptin regulates gonadotropin-releasing hormone (GnRH) secretion, which is critical for mammalian reproduction. Humans and mice with inactive kisspeptin (KISS1) or its cognate receptor (KISS1R) genes display a delay in or absence of puberty, reproductive hormone deficits, lack of sexual behaviour, and infertility [12, 13]. Although hypothalamic kisspeptin neurons are well established as stimulators of GnRH, the specific role of MeAKiss neurons in the reproductive endocrine axis has not been directly demonstrated.
MeAKiss neurons are reciprocally connected to the AOB and a subset of MeAKiss neurons contact GnRH neurons in the POA [14]. Chemogenetic activation of MeAKiss neurons reduces anxiety and enhances the preference for oestrous females in male mice [15]. Intra-MeA infusions of kisspeptin impact LH levels and lead to increased numbers of ex-copula erections in male rats [16], suggesting that MeAKiss signalling regulates GnRH release and participates in sexual arousal. However, Kiss1r expression has not been identified in MeAKiss neurons, therefore it remains unclear whether MeAKiss neurons directly regulate these responses or whether another neuronal population is involved.
GnRH neurons receive both GABAergic and glutamatergic inputs [17, 18] and glutamatergic and GABAergic neurons in the MeA project to the ventromedial hypothalamus [19]. Pharmacological manipulations of GABA and glutamate receptors directly influence GnRH pulsatility and LH levels [20]. Therefore, it is important to understand whether MeAKiss neurons also have the capacity to elaborate these neurotransmitters that contribute to modulating reproductive hormone levels.
In the current study, we tested the hypothesis that MeAKiss neurons are sensitive to sexually relevant olfactory stimuli and directly contribute to reproductive endocrine responses. First, we assessed whether exposure to female mouse urine led to activation of MeAKiss neurons in male mice. Next, we employed DREADDs (designer receptors exclusively activated by designer drugs) to determine whether specific modulation of MeAKiss neurons impacts circulating LH levels. We then quantified Fos immediate-early gene expression in GnRH neurons and the POA after chemogenetic manipulations to elucidate whether MeAKiss modulation can directly or indirectly influence neuronal activity within this reproductive brain centre. Finally, because GABA and glutamate are known to influence GnRH activity and a subset of MeAKiss neurons project directly to GnRH neurons, we used multiplexed in situ hybridization/immunohistochemistry (ISH/IHC) for definitive markers of GABAergic and glutamatergic transmission to study the neurotransmitter composition of MeAKiss neurons.
Materials and Methods
Subjects
All animal procedures were conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 approved by the University of St. Andrews Ethics Committee under the UK Home Office project license No. 7007924. The genetic mouse line Kiss1CreEGFP (Kiss1tm1.1(cre/EGFP)Stei/J) was generated and characterized by the Steiner Lab (University of Washington, USA) [21] and Rosa26lox-stop-lox-tdTomato mice were generated and characterized by the Zeng Lab [22].
All breeding and genotyping were performed at the University of St. Andrews. Mice were maintained on a 12: 12 h light/dark cycle (lights on at 7: 00 a.m.) with temperature maintained at 21 ± 5°C and humidity at 55 ± 10%. Mice had ad libitum access to standard mouse chow (Special Diets Services, UK) and water. Mice were weaned at 21 days and genotyped by PCR analysis of ear clips. Male mice used for this study were group-housed (3–4 mice/cage) in individually ventilated cages (Sealsafe, Tecniplast, UK) and were kept in a single holding room.
Although Kiss1CreEGFP/wt mice express EGFP in hypothalamic Kiss neurons [21], we were unable to visualize EGFP expression in the MeA using IHC even with enhanced amplification methods. In addition, the Kiss1 mRNA signal generated from RNAscope ISH in the MeA was too low to combine with evaluating c-Fos IHC, therefore to robustly mark MeAKiss neurons with tdTomato, we generated double transgenic Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt mice by breeding Kiss1CreEGFP mice with Rosa26lox-stop-lox-tdTomato. Adult (sexually naive, 10–14 weeks old, n = 12) gonad-intact Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt males were used for pheromone exposure and neurotransmitter co-expression studies. Adult (sexually naive, 10–14 weeks old, n = 30) gonad-intact Kiss1CreEGFP/wt male mice were subjected to recombinant adeno-associated virus (rAAV) injections to enable Cre-dependent expression of DREADDs in MeAKiss neurons.
Pheromone Stimuli
Urine presented as chemosensory stimuli was pooled from sexually naive ovary-intact females primarily from proestrous and oestrous stages and stored in aliquots at −80°C. Urine exposure experiments were conducted on adult gonad-intact Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice (n = 12) between 9: 00 and 11: 30 a.m. Forty-eight hours prior to each experiment, males were housed individually in clean cages. On the morning of exposure, mice were randomly assigned to either exposure or control groups (n = 6 animals in each group). Depending on the group, 50 µL of female urine or water was pipetted onto sterile cotton pads and placed into their home cage for 15 min. A blood sample was collected from each mouse 15 min following exposure to urine. At 30 min post-exposure, males were anaesthetized with sodium pentobarbital (150 mg/kg i.p.) and transcardially perfused with 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in 0.1 M PBS. Brains were removed and post-fixed in 4% PFA/0.1 M PBS overnight at 4°C followed by cryoprotection in 30% sucrose for 72 h. Brains were embedded in OCT compound (Fisher Scientific, Cat. No. 12678086), placed in a cryomold, and flash frozen in cooled isopentane (–60°C). Frozen brains were stored at −80°C until processing.
Stereotaxic Surgery
Adult Kiss1CreEGFP/wt mice (n = 30) were anaesthetized using 1.5–2% isofluorane delivered using a Univentor 410 anaesthesia unit (Univentor Limited, Malta). Subjects were then fixed into a stereotaxic frame (Stoelting Co., Wood Dale, IL, USA) on top of a homeothermic pad (Harvard Apparatus Ltd., UK) and connected to a mouse anaesthetic mask. A small incision was made to expose the skull and a small craniotomy was drilled directly above each target site. Each DREADD vector plasmid was packaged into rAAVs (serotype 1/2) using the method described [23]. One construct (pAAV-EF1a-DIO-hM3D[Gq]-mCherry [Addgene plasmid #50460], a gift from Bryan Roth) encodes a modified G protein-coupled receptor hM3D(Gq) that, after binding to its non-endogenous ligand, clozapine N-oxide (CNO), induces neuronal burst firing [24, 25]. The other construct (pAAV-hSyn-dF-HA-hKORD-IRES-mCitrine [Addgene plasmid #65417], another gift from Bryan Roth) encodes a modified G protein-coupled receptor (hKORD) that, after binding to its non-endogenous ligand, salvinorin B (SALB), activates inward-rectifying potassium channels, resulting in hyperpolarization (neuronal silencing) [26]. These two rAAVs were mixed equally (each titre 4.2 × 1011 genome copies/mL) and delivered bilaterally with 0.4 µL of virus per site at the rate of 0.1 µL/min using calibrated pulled-glass capillary needles (Drummond Scientific company, 2-000-005) by pressure injection. Stereotaxic coordinates: Bregma −1.7 mm, ±2.2 mm lateral to the midline, and −5 mm below the skull at Bregma [27] was used to target the centre of the MeA with the aim of facilitating viral spread to the anterior and posterior MeA. The needle remained in place for 5 min before being withdrawn over 60 s. The incision was closed using Vetbond (3M Vetbond, 084-1469SB) and the mice were allowed to recover in a heated cage before being returned to their home cage. Post-surgery, subjects were given analgesic (Buprecare, 1 µg/g in palatable jelly). All DREADD modulation experiments began 3 weeks after viral injections.
Chemogenetic Modulation of Reproductive Hormone Levels
CNO was obtained from Tocris Bioscience (Cat. No. 4936) and SALB was obtained from Sigma-Aldrich (Cat. No. 75250). Both were dissolved in DMSO to prepare stock solutions (CNO 10 mg/mL and SALB 5 mg/mL). A crossover study design (3-period, 3-treatment) was used for drug treatments (DMSO, SALB, CNO), where the order of the drug treatments was randomized, and each animal received all 3 treatments, with a wash-out period of 2 weeks between treatments. The treatment regimen started 3 weeks following rAAV injections, with mice housed singly for 48 h along with handling acclimation prior to the first treatment. Depending on the treatment groups, mice were injected with either CNO (3 mg/kg i.p.), SALB (10 mg/kg s.c.), or vehicle (DMSO i.p.) diluted in saline between 9: 00 and 11: 30 a.m. These drug doses have been used previously to activate and inhibit DREADD receptors [26] and a higher dose of SALB was used because it is administered subcutaneously, whereas CNO is administered intraperitoneally. Blood samples were collected at 30 min post-treatment. During the third and final round, at 60 min after treatments, males were euthanized with an overdose of sodium pentobarbital (150 mg/kg i.p.) and transcardially perfused with 0.1 M PBS followed by 4% PFA in 0.1 M PBS.
Hormone ELISA
Blood samples were collected in Multivette blood collection tubes (SARSTEDT; 15.1670) by submandibular cheek bleed. Serum was collected by centrifugation of whole blood at 2,000 rcf for 10 min and stored at −20°C. LH levels were measured using a super-sensitive LH ELISA developed by the Chen Lab [28]. Serum samples were diluted 1/10 with assay buffer and 50 µL of each diluted sample was assayed. The o-phenylenediamine substrate (Sigma-Aldrich; P918) was developed for 45 min at room temperature and stopped by the addition of 3 M H2SO4. The absorbance in each well was then measured at 490 nm. The LH ELISA had a detection limit of 0.04 ng/mL and good reproducibility with values of 6.4 and 11.5%, respectively, for the intra- and inter-assay variation coefficients.
Histological Methods
Coronal brain sections (30 µm) were cut on a Leica cryostat and stored in 48-well plates in PBS containing 0.01% thimerosal at 4°C.
To verify that tdTomato-labelled MeA neurons in Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice expressed Kiss1 mRNA, we combined RNAscope ISH for Kiss1 mRNA with immunofluorescence labelling for tdTomato on 30-µm coronal brain sections from Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt males. The ISH was performed using the RNAscope Multiplex Fluorescent Kit v2 (Advanced Cell Diagnostics Inc.) using a mouse Kiss1 probe set (Advanced Cell Diagnostics Inc.; Cat. No. 4762921) in accordance with the manufacturer's suggested procedure. The Kiss1 probe set consists of a series of 20 target probes designed to hybridize to the 121- to 1,376-bp region of the mouse Kiss1 transcript (accession No. XM_006529679.2). Following completion of the RNAscope protocol, sections were immunolabelled using a rabbit anti-RFP polyclonal affinity purified antibody (1: 1,000; Rockland, 600-401-379) [29] and a donkey anti-rabbit Alexa Fluor 555 (1: 400; Invitrogen, A31572).
To investigate the responses of MeAKiss neurons to pheromone exposure in Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt males (n = 6 in each group), sections were immunolabelled using the rabbit anti-RFP antibody (1: 1,000; Rockland) to label tdTomato+ (MeAKiss) neurons and a goat anti-c-Fos polyclonal affinity purified antibody (1: 400; Santa Cruz Biotechnology, sc-52-G) [30] to label for c-Fos protein (a surrogate marker of neuronal activation). Negative controls for c-Fos IHC using either no primary antibody or isotype controls did not produce any significant fluorescence labelling. Sections were mounted on SuperFrost Plus slides (ThermoScientific) and dried for 1 h at 40°C in a hybridization oven. The slides were washed in PBST (PBS + 0.05% Tween-20) for 10 min followed by a 5-min wash in PBS. Sections were blocked for 1 h at room temperature in blocking buffer (3% normal donkey serum in PBS with 0.4% Triton-X100) followed by overnight incubation with primary antibodies at 4°C. Sections were then washed thrice (5 min in PBS, 10 min in PBST, and 15 min in PBS) and incubated in secondary antibodies donkey anti-rabbit Alexa Fluor 594 (1: 500; Jackson ImmunoResearch, 711-585-152) and donkey anti-goat Alexa Fluor 488 (1: 500; Life Technologies, A11055) for 1 h at 37°C. After a 10-min wash in PBST, sections were incubated in Hoechst 33258 nuclear stain (1 µg/mL in PBS; Sigma-Aldrich, 861405). Slides were then washed thrice before sections were mounted using Permafluor mountant (ThermoScientific; TA-030-FM) and coverslipped. Slides were allowed to dry in the dark overnight before image acquisition. This method was used for all the immunostaining experiments throughout the study. Sections from the AVPV and ARC were also processed using the same primary antibody combination to evaluate whether AVPVKiss or ARCKiss neurons respond to pheromone exposure. However, due to the endogenous EGFP expression in the AVPV and ARC region of Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt, the secondary antibodies used for this experiment were donkey anti-rabbit Alexa Fluor 555 (1: 500; Life Technologies, A31572) and donkey anti-goat Alexa Fluor 647 (1: 600; Jackson ImmunoResearch, 705-605-147).
To visualize the localization of DREADD injections and the co-expression of hKORD and hM3D(Gq) in Kiss1CreEGFP/wt male mice (n = 3), we localized mCitrine, the reporter that is co-expressed with hKORD in the viral transgene (pAAV-hSyn-dF-HA-hKORD-IRES-mCitrine) using a chicken anti-GFP antibody (1: 10,000; Abcam, 13970) and we localized mCherry, which is linked to hM3D(Gq) in the viral construct (pAAV-EF1a-DIO-hM3D[Gq]-mCherry) using a rat anti-mCherry antibody (1: 800, Life Technologies, M11217). The secondary antibodies used for this study were donkey anti-chicken Alexa Fluor 488 (1: 500; Jackson ImmunoResearch, 703-546-155) and donkey anti-rat Alexa Fluor 594 (1: 800).
To detect the level of DREADD-mediated modulation of hM3D(Gq)-expressing MeAKiss neurons and hKORD-expressing MeAKiss neurons in Kiss1CreEGFP/wt male mice (n = 3 in each treatment group), we again used c-Fos as a marker of neuronal activation. We immunolabelled MeA sections using anti-mCherry and anti-c-Fos antibodies with donkey anti-rat Alexa Fluor 594 and donkey anti-goat Alexa Fluor 647 (1: 600; Jackson ImmunoResearch, 705-605-147).
To interrogate how DREADD-induced modulation of MeAKiss neurons influences neuronal activity in the POA, then to analyse c-Fos specifically in GnRH neurons, immunofluorescence analysis was carried out on every 4th section from 0.74 mm anterior to Bregma to 0.46 mm posterior to Bregma using the goat anti-c-Fos antibody and a rabbit anti-GnRH polyclonal antisera (1: 1,200; GF4, a generous gift from Prof. N. Sherwood, University of Victoria). The secondary antibodies used for this experiment were donkey anti-goat Alexa Fluor 647 and donkey anti-rabbit Alexa Fluor 488 (1: 500; Invitrogen, A21206).
To identify which neurotransmitters MeAKiss neurons express, we combined multiplexed RNAscope ISH for definitive markers of GABAergic (Slc32a1 encoding the GABA vesicular transporter, Vgat; Advanced Cell Diagnostics Inc.; Cat. No. 319191) and glutamatergic transmission (Slc17a6 encoding the glutamate vesicular transporter, Vglut2; Advanced Cell Diagnostics Inc.; Cat. No. 319171) with immunofluorescence labelling of tdTomato+ MeAKiss neurons on coronal brain sections from Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt males (n = 3). The dual ISH was performed using the RNAscope Multiplex Fluorescent Kit v2 (Advanced Cell Diagnostics Inc.) with probes for Vgat mRNA (Slc32a1; this probe set was designed to target the 894- to 2,037-bp region of the mouse Slc32a1 transcript; accession No. NM_009508.2) and Vglut2 mRNA (Slc17a6: this probe set was designed to target the 1,986- to 2,998-bp region of the mouse Slc17a6 transcript; accession No. NM_080853.3) in accordance with the manufacturer's suggested procedure. Following completion of the RNAscope protocol, sections were immunolabelled using the rabbit anti-RFP antibody (1: 1,000; Rockland) and a donkey anti-rabbit Alexa Fluor 555 (1: 400; Invitrogen, A31572) antibody.
Imaging and Analysis
The multiplexed RNAscope ISH/immunofluorescence labelling experiments were imaged using a Leica TCS SP8 confocal laser scanning microscope equipped with a Leica 20× multi-immersion objective (NA 0.75). The z-stack images were acquired at 1.04-µm steps through 16 µm of tissue (1,024 × 1,024 pixels, pinhole size set at 1 Airy unit). Stacks were analysed using Leica Application Suite Advanced Fluorescence software (LAS X; Leica Microsystems, Wetzlar, Germany). To evaluate the co-expression levels of tdTomato (Kiss neurons), mRNAs for Vgat (Slc32a1) and Vglut2 (Slc17a6), z-stacks were collected using a Nyquist sampling rate and approximately 150 MeAKiss neurons were analysed in each of 3 animals over three Bregma positions. All other fluorescence immunolabelling experiments were imaged using a Leica CTR5500 fluorescence microscope. Light intensities and image acquisition settings were kept constant across all treatments and post-imaging analysis and quantification was done using Fiji software [31]. Investigators were blinded to exposure conditions/treatments and a second investigator independently confirmed all analyses.
To quantify the number of c-Fos-positive nuclei, then to quantify the number of c-Fos-positive MeAKiss neurons (tdTomato+) in Kiss1CreEGFP/wt/Rosa26lox-stop-tdTomato/wt males in response to pheromone exposure, a total of 6 sections from 1.22 to 1.82 mm posterior to Bregma were analysed. One section was representative of anterior MeAa and five sections covered both the posteroventral MeA (MeApv) and posterodorsal MeA (MeApd) from each mouse. Regions of interest were identified and selected based on the mouse brain atlas [27] by using the optic tract as a landmark. Counts were carried out manually after subtracting the background and adjusting the brightness and contrast equally for all images using Fiji software [31]. To quantify the number of c-Fos-positive AVPVKiss neurons (tdTomato+) in Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt males in response to pheromone exposure, a total of 7 sections starting at 0.50 mm anterior to Bregma to 0.22 mm posterior to Bregma were analysed from each mouse. Regions of interest were identified based on the mouse brain atlas [27] using the third ventricle and the anterior commissure as landmarks. To quantify the number of c-Fos-positive ARCKiss neurons (tdTomato+) in Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt males in response to pheromone exposure, a total of 6 sections starting at 1.34 mm posterior to Bregma to 1.94 mm posterior to Bregma were analysed from each mouse. Regions of interest were identified based on the mouse brain atlas [27] using the third ventricle and the median eminence as landmarks. Co-expression analysis was carried out manually using the ROI Manager in Fiji software [31].
Similarly, DREADD and c-Fos co-expression in MeAKiss neurons was analysed in 6 sections from 1.22 to 1.82 mm posterior to Bregma. Quantification of c-Fos in the POA was carried out on every 4th section from 0.74 mm anterior to Bregma to 0.46 mm posterior to Bregma. Regions of interest were identified and selected based on the mouse brain atlas [27] by using the third ventricle, anterior commissure, and optic tract as landmarks. Counts were carried using the “Analyze particles” plugin in Fiji software [31] using the following selection criteria – minimum pixel area size set at 2 and maximum 30 and circularity criteria between 0 and 1. Selected nuclei were confirmed visually.
Experimental Design and Statistical Analysis
Only male mice were used for these studies as our objective was to understand the role of MeAKiss neurons in mediating pheromone influences on male reproductive endocrine physiology. The sample size for each individual experiment is listed in Results. Statistical analysis was conducted using GraphPad Prism, version 7 (GraphPad Software, La Jolla, CA, USA). The number of cells per group or animals per group is indicated by n. Data were compared as dictated by distribution and experimental design; tests are specified in the results. Data were analysed using a two-tailed unpaired Student's t test or a one-way ANOVA followed by Tukey's multiple comparison test. Data are reported as mean ± SEM. Significance was set at p < 0.05 in all analyses.
Results
Female Urine Exposure Activates MeAKiss Neurons and Raises LH in Male Mice
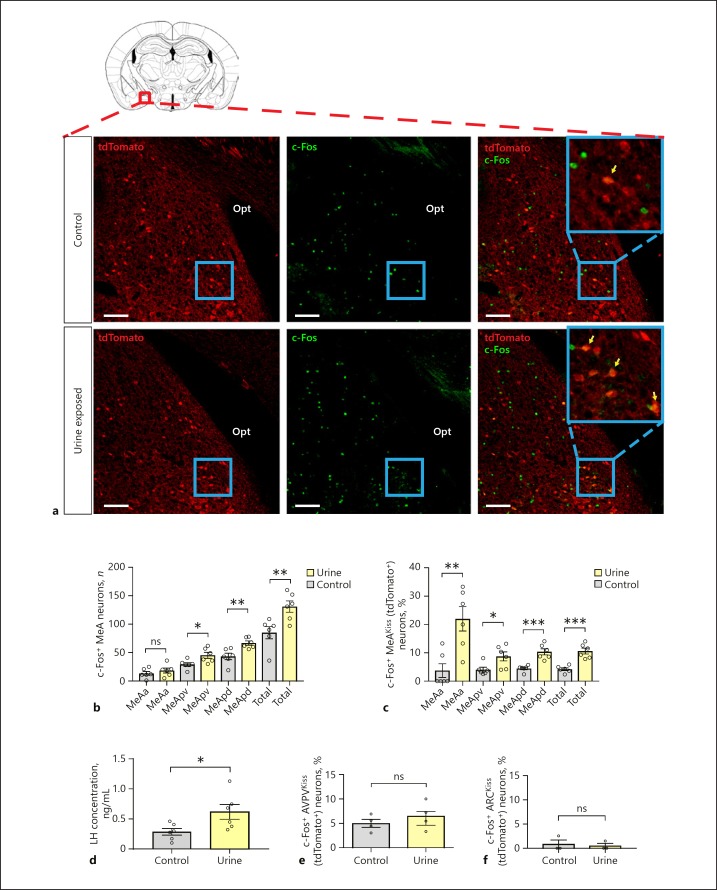
To determine whether MeAKiss neurons integrate chemosensory stimuli with reproductive endocrine status, we exposed double transgenic Kiss1CreEGFP/wt/ Rosa26lox-stop-lox-tdTomato/wt males to female urine and measured c-Fos expression in tdTomato+ MeAKiss neurons. Counting the overall number of c-Fos+ cells in each subdivision revealed that only the MeApv and MeApd subdivisions of the MeA showed significant induction of c-Fos, whereas the MeAa did not (Fig. 1a, b). Double immunofluorescence labelling (for c-Fos and tdTomato) revealed that the percentage of c-Fos immunoreactive (c-Fos-ir) MeAKiss neurons was significantly higher in the MeApd of males exposed to female urine (10.4 ± 1.1%; n = 80 ± 5 tdTomato+ neurons counted in the MeApd of 6 animals) compared to water exposure (4.5 ± 0.4%; n = 81 ± 3, total number of tdTomato+ neurons counted in the MeApd of 6 animals) (*** p < 0.001; unpaired two-tailed Student's t test; Fig. 1a, c). Similarly, a significantly higher percentage of c-Fos-ir MeAKiss neurons was observed in the MeApv of males exposed to female urine (8.7 ± 1.6%; n = 70 ± 7 tdTomato+ neurons counted in the MeApv of 6 animals) compared to water exposure (4.1 ± 0.8%; n = 69 ± 4 tdTomato+ neurons counted in the MeApv of 6 animals) (* p < 0.05; unpaired two-tailed Student's t test; Fig. 1c and online suppl. Fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000496106). Despite the lack of change in overall neuronal activity within the MeAa, a significantly increased number of c-Fos-ir MeAKiss neurons was observed in the MeAa of males exposed to female urine (21.9 ± 4.3%; n = 14 ± 1, total number of tdTomato+ neurons counted in the MeAa of 6 animals) compared to animals exposed to water (3.7 ± 2.4%; n = 14 ± 1 tdTomato+ neurons counted in the MeAa of 6 animals) (* p < 0.05; unpaired two-tailed Student's t test; Fig. 1c and online suppl. Fig. S2). To test for pheromone-induced changes in serum LH levels, blood was sampled 15 min following urine exposure. Mean serum LH levels of both groups are shown in Figure 1d. Serum LH concentrations at T = 15 were significantly higher in males exposed to female urine (0.68 ± 0.13 ng/mL, monitored in 5 animals) compared to water (0.31 ± 0.06 ng/mL, monitored in 5 animals; p < 0.05; unpaired two-tailed Student's t test), but no significant activation of AVPVKiss (tdTomato+) neurons was detected with urine exposure (6.5 ± 1.4 vs. 4.9 ± 0.8% in control; n = 6 in each group; no significant difference; unpaired two-tailed Student's t test; Fig. 1e and online suppl. Fig. S3). Similarly, no significant activation of ARCKiss (tdTomato+) neurons was detected with urine exposure (0.5 ± 0.5 vs. 0.85 ± 0.85% in control; n = 3 in each group; no significant difference; unpaired two-tailed Student's t test; Fig. 1f and online suppl. Fig. S4).
Fig. 1.
Exposure to female urine regulates the activity of MeAKiss neurons and impacts luteinizing hormone (LH) levels in Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice. a Co-localization of c-Fos (green) in MeAKiss neurons (tdTomato+; red) of male mice exposed to control (top panel) or female urine (bottom panel). Yellow arrows indicate tdTomato+ neurons that express c-Fos. Scale bars, 100 µm. b Number of c-Fos+ cells in the MeA subdivisions after exposure to control or urine (n = 6 in each group). c Percentage of MeAKiss (tdTomato+) neurons that express c-Fos in the MeA after control or urine exposure (n = 6 in each group). d Serum LH concentrations (ng/mL) in male mice 15 min post-exposure (n = 5 in each group). e Percentage of c-Fos+ AVPVKiss (tdTomato+) neurons after exposure to control or urine (n = 4 in each group). f Percentage of c-Fos+ ARCKiss (tdTomato+) neurons after exposure to control or urine (n = 3 in each group). Data are represented as mean ± SEM. Values of individual mice are plotted as circles. ns, no significant difference; * p < 0.05, ** p < 0.01, *** p < 0.001 (unpaired two-tailed Student's t test). opt, optic tract, MeAa, anterior medial amygdaloid nucleus, MeApv, posteroventral medial amygdaloid nucleus, MeApd, posterodorsal medial amygdaloid nucleus.
Verification of MeAKiss Neuronal Labelling Strategies
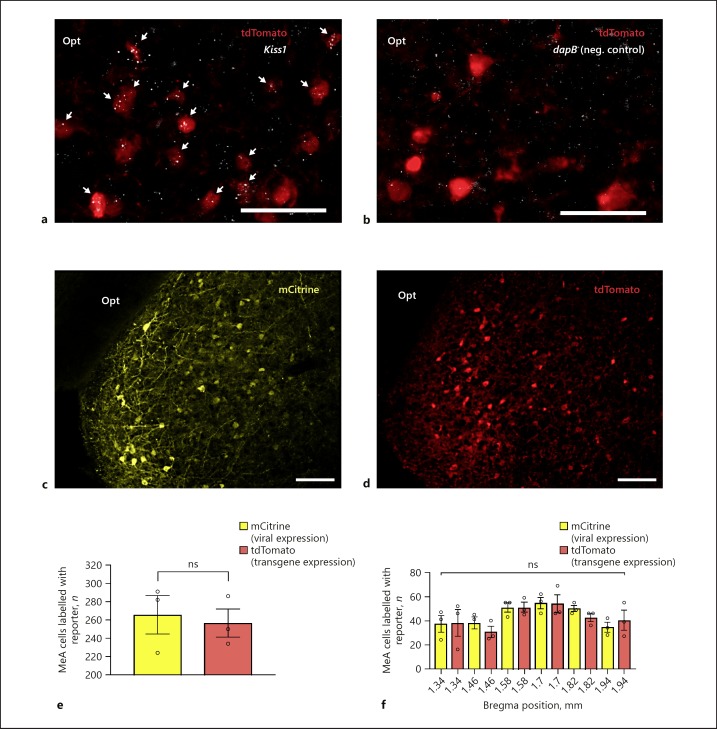
To verify that fluorescently labelled neurons in the MeA of Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt mice express Kiss1, we analysed the number of tdTomato+ neurons in the MeA that express Kiss1 mRNA by combining RNAscope ISH and IHC (Fig. 2a). The negative control RNAscope in situ probes did not display any significant signal in tdTomato+ MeA neurons in Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice (Fig. 2b). A total of 73 tdTomato+ neurons were counted in 3 MeA sections (1.22, 1.46, and 1.58 mm posterior to Bregma) of which 67 tdTomato+ neurons displayed a positive Kiss1 mRNA signal (approx. 92%). We also compared the number of fluorescently labelled (tdTomato+) neurons in adult Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt mice to fluorescently labelled (mCitrine+) neurons in Kiss1CreEGFP/wt mice infused with Cre-dependent AAVs encoding hKORD (this DREADD is linked to mCitrine). No significant difference in the number of labelled cells was observed between the two labelling approaches (Fig. 2c, d). An average of 265.7 ± 21 mCitrine+ neurons were observed in 3 Kiss1CreEGFP/wt male mice infected with hKORD and 256.7 ± 15.4 tdTomato+ neurons were observed in the MeA of 3 Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice (Fig. 2e). The distribution of fluorescently labelled cells in the MeA between 1.34 and 1.94 mm posterior to Bregma revealed no significant difference (unpaired two-tailed Student's t test; Fig. 2f).
Fig. 2.
Verification and comparison of MeA neuron targeting using Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt mice and Cre-dependent rAAV infection of Kiss1CreEGFP/wt mice. a Representative photomicrograph showing tdTomato expression (red) and Kiss1 mRNA expression (white) in the MeA of a double transgenic Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mouse. White arrows mark tdTomato+ cells that are positive for Kiss1 expression. b Representative photomicrograph showing tdTomato expression (red) and negligible RNAscope in situ signal (white) with a negative control probe (dapB; a bacterial gene) in the MeA of a double transgenic Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mouse. c Representative photomicrograph showing mCitrine (green) labelling in the MeA (Bregma 1.34 mm) of male Kiss1CreEGFP/wt mice infected with rAAVs encoding hSyn-DIO-hKORD-IRES-mCitrine. d Representative photomicrograph of the MeA (Bregma 1.34 mm) labelled with tdTomato from a double transgenic Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mouse. e Comparison between the number of mCitrine+ neurons in the MeA of Kiss1CreEGFP/wt male mice infected with AAV-hSyn-DIO-hKORD-IRES-mCitrine and tdTomato+ neurons in the Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice. f Distribution of fluorescently labelled neurons in the MeA between 1.34 and 1.94 mm posterior to Bregma in Kiss1CreEGFP/wt male mice infected with AAV-hSyn-DIO-hKORD-IRES-mCitrine (virally induced expression) and Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice (transgenic induced expression). Scale bars, 100 µm.
Localization of DREADD Infections and Co-Expression of hM3D(Gq) and hKORD
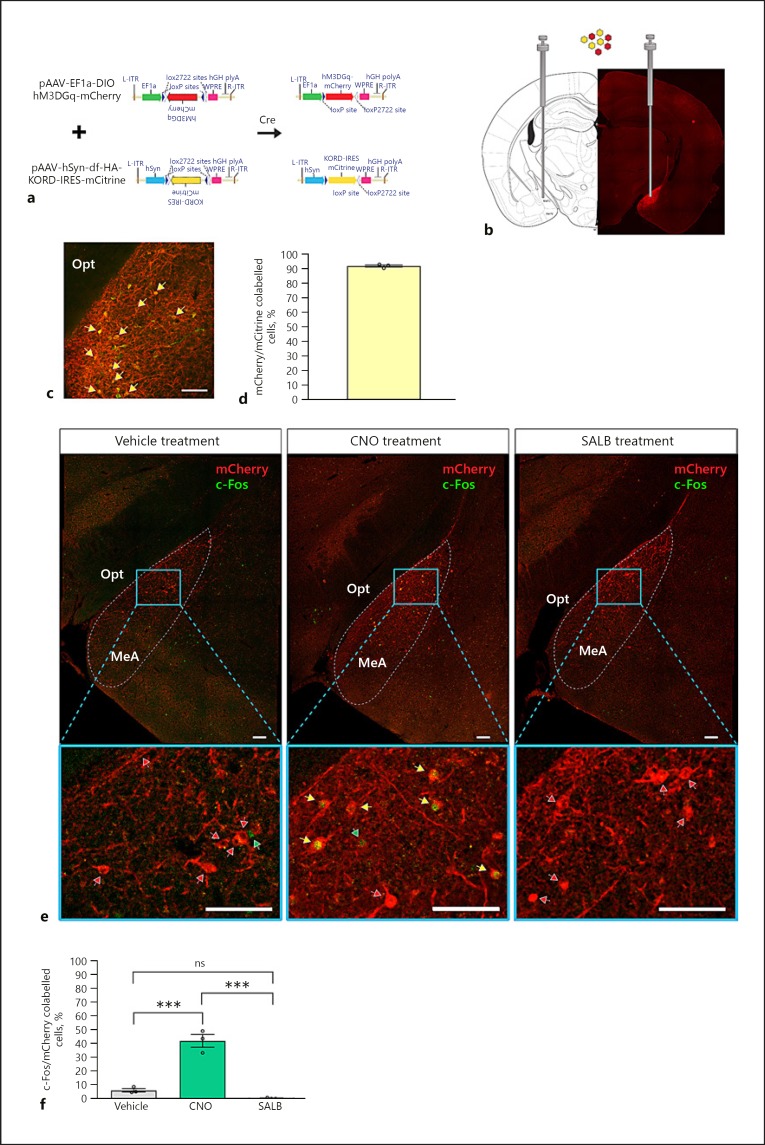
To investigate the influence of MeAKiss neurons on the reproductive endocrine axis, we reciprocally modulated MeAKiss neurons by co-infusing two Cre-dependent rAAVs encoding hM3D(Gq) (EF1α-DIO-hM3D[Gq]-mCherry) and hKORD (hSyn-DIO-hKORD-IRES-mCitrine) DREADDs into the MeA of Kiss1CreEGFP/wt male mice (Fig. 3a, b). Approximately 92% co-expression was observed between mCitrine- and mCherry-expressing neurons in the MeA (n = 145 ± 7, number of mCherry+ neurons expressing mCitrine monitored in 3 animals; Fig. 3c, d). This high degree of co-expression indicated that both fluorescent markers could be used interchangeably, and on-target injections were confirmed using mCherry immunofluorescence (Fig. 3b). Of the 30 Kiss1CreEGFP/wt male mice that received rAAV-DREADD injections, 24 mice received on-target bilateral rAAV infections. In mice that received on-target rAAV infections, CNO (the hM3D[Gq] agonist) significantly elevated c-Fos expression in MeAKiss neurons (42.0 ± 4.6%, n = 107 ± 7, number of mCherry+ neurons monitored in 3 animals) compared to the SALB (hKORD agonist)-treated group (0.3 ± 0.3%, n = 144 ± 15, mCherry+ neurons monitored in 3 animals) and vehicle (DMSO) controls (6.1 ± 1.2%, n = 115 ± 24 mCherry+ neurons monitored in 3 control animals, *** p < 0.001, F value = 66.56; one-way ANOVA followed by Tukey's multiple comparison test; Fig. 3e, f) with no significant difference observed between the SALB-treated group and controls.
Fig. 3.
Chemogenetic control of MeAKiss neurons in Kiss1CreEGFP/wt male mice. Male Kiss1CreEGFP/wt mice received bilateral MeA stereotaxic injections of an equal mixture of AAV1/2- EF1α-DIO-hM3D(Gq)-mCherry and AAV1/2-hSyn-DIO-hKORD-IRES-mCitrine. After 3 weeks, the mice were treated with CNO (3 µg/g), SALB (10 µg/g), or vehicle (DMSO). a Schematic representation of the AAV vectors encoding hM3D(Gq) and hKORD, both with a FLEX switch allowing recombination (activation) under the control of Cre-recombinase. b Location of stereotaxic viral injection to target Cre-expressing MeAKiss neurons and representative image montage that confirms mCherry expression restricted to the injection site. c Co-localization of hM3D(Gq) (mCherry) and hKORD (mCitrine) marked by yellow arrows. d Quantification of hM3D(Gq) (mCherry) and hKORD (mCitrine) virus co-expression in the MeA (n = 3). e Location of virally expressed hM3D(Gq) (mCherry) and induction of c-Fos (green) 60 min after treatments (vehicle, CNO, or SALB). Yellow arrows mark c-Fos+ virally transduced cells (mCherry), red arrows mark mCherry cells lacking c-Fos expression, and green arrows mark c-Fos+ nuclei. f Quantification of c-Fos+ cells as a percentage of mCherry cells in MeA 60 min after vehicle, CNO, or SALB treatment (n = 3 in each group). Data are represented as mean ± SEM. Circles represent the individual values for each mouse. ns, no significant difference; *** p < 0.001 (one-way ANOVA followed by Tukey's multiple comparison test). opt, optic tract, MeA, medial amygdaloid nucleus. Scale bars, 100 µm.
Chemogenetic Stimulation of MeAKiss Neurons Leads to a Significant LH Rise
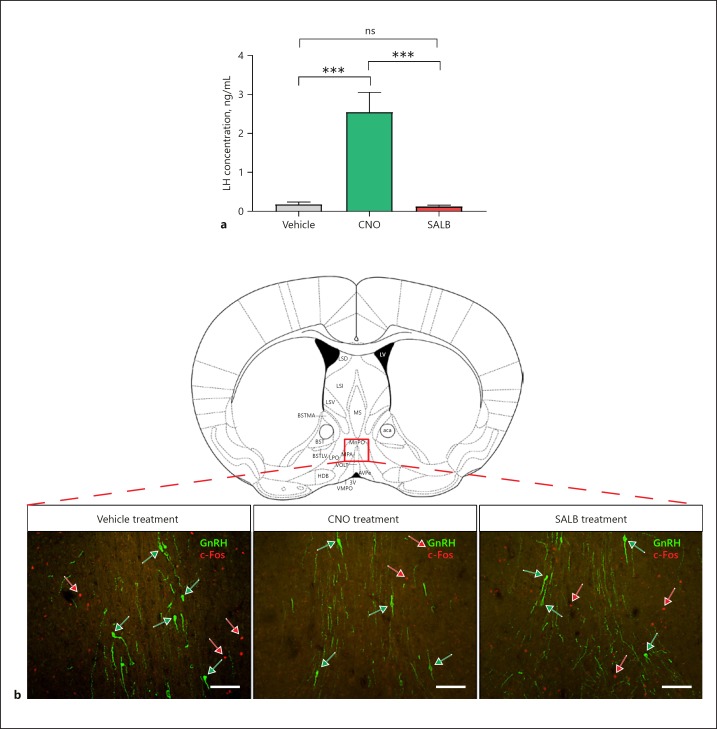
To investigate whether MeAKiss neurons can directly elicit a reproductive endocrine response, we measured serum LH concentrations after administration of chemogenetic drugs. Blood samples were collected 30 min after treating mice with CNO (3 µg/g), SALB (10 µg/g), or vehicle (DMSO). Serum LH concentrations increased significantly in mice receiving CNO compared to SALB or vehicle treatment. At 30 min post-treatment, mean serum LH was significantly elevated in the CNO group (2.40 ± 0.48 ng/mL, monitored in 24 animals) compared to either the SALB-treated group (0.11 ± 0.01 ng/mL, monitored in 24 animals) or the vehicle-treated controls (0.17 ± 0.05 ng/mL in the vehicle treated controls, monitored in 23 animals, *** p < 0.001, F value = 23.39; one-way ANOVA followed by Tukey's multiple comparison test, Fig. 4a). To check for non-specific drug-induced effects on serum LH, blood samples from mice that were administered chemogenetic drugs but received off-target rAAV injections were also analysed. No significant changes to LH were observed in these animals (online suppl. Fig. S5).
Fig. 4.
Chemogenetic activation of MeAKiss neurons in Kiss1CreEGFP/wt male mice stimulates LH release but fails to activate GnRH neurons. Male Kiss1CreEGFP/wt mice received MeA injections of rAAVs encoding EF1α-DIO-hM3D(Gq)-mCherry and hSyn-DIO-hKORD-IRES-mCitrine. After 3 weeks, the mice were treated with SALB (10 µg/g), CNO (3 µg/g), or vehicle (DMSO). a Serum LH concentration (ng/mL) in male mice 30 min following drug treatment (n = 23–24 in each group). Data are represented as mean ± SEM. ns, no significant difference; *** p < 0.001 (one-way ANOVA followed by Tukey's multiple comparison test). b Representative photomicrographs showing GnRH (green) and c-Fos (red) labelling in the POA (Bregma 0.50 mm) 60 min after vehicle, CNO, or SALB treatment. Green arrows mark GnRH cell bodies and red arrows mark c-Fos+ nuclei. Scale bars, 100 µm.
Chemogenetic Modulation of MeAKiss Neurons Decreases Neuronal Activity in the POA
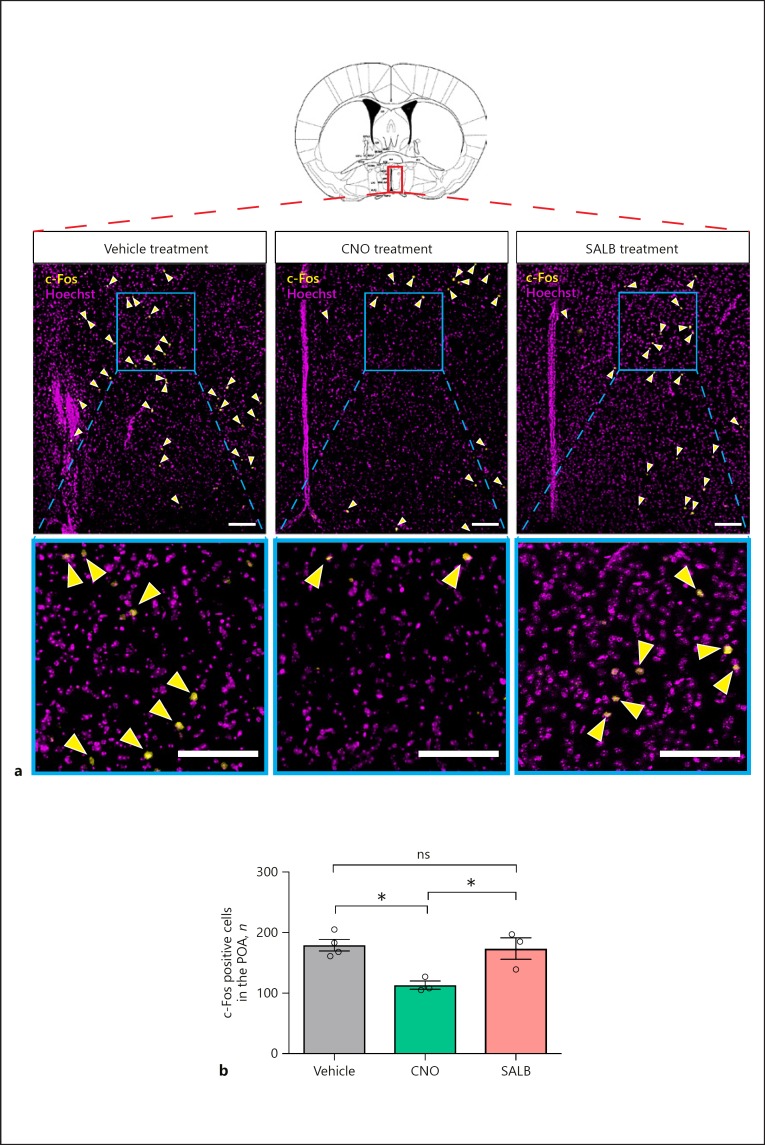
To determine whether the CNO-mediated rise in serum LH coincided with increased c-Fos-ir in GnRH neurons (Fig. 4b), we examined the level of c-Fos activity in GnRH neurons using double immunofluorescence labelling (for GnRH and c-Fos). Despite the elevated LH after CNO treatment, c-Fos-ir was undetectable in GnRH neurons in all groups (66 ± 5 GnRH neurons counted in 3 mice receiving DMSO, 60 ± 6 GnRH neurons counted in 3 mice receiving SALB, and 66 ± 9 GnRH neurons counted in 3 mice receiving CNO). Instead, CNO-mediated stimulation of hM3D(Gq)-expressing MeAKiss neurons led to reduced neuronal activity in the POA compared to controls and SALB treatment (Fig. 5a). The number of c-Fos-ir cells in the POA after CNO treatment was 113.2 ± 6.9 compared to 173.7 ± 17.7 with SALB treatment and 179.3 ± 9.7 in controls (* p < 0.05, F value = 9.055; one-way ANOVA followed by Tukey's multiple comparison test; n = 3 each, Fig. 5b).
Fig. 5.
Chemogenetic activation of MeAKiss neurons reduces neuronal activity in the POA in male mice. Male Kiss1CreEGFP/wt mice received MeA injections of rAAVs encoding EF1α-DIO-hM3D(Gq)-mCherry and hSyn-DIO-hKORD-IRES-mCitrine. After 3 weeks, the mice were treated with SALB (10 µg/g), CNO (3 µg/g), or vehicle (DMSO). a Representative photomicrographs showing Hoechst-stained nuclei (magenta) and c-Fos+ nuclei (yellow) in the POA (Bregma −0.10 mm) 60 min after treatments. Arrowheads mark c-Fos+ nuclei. Scale bars, 100 µm. b Number of c-Fos+ nuclei in the POA analysed 60 min after treatments (n = 3 in each group). Data are represented as mean ± SEM. Circles represent the individual values for each mouse. ns, no significant difference; * p < 0.05, (one-way ANOVA followed by Tukey's multiple comparison test).
A Higher Proportion of MeAKiss Neurons Express Markers for GABAergic Transmission rather than Glutamatergic Transmission
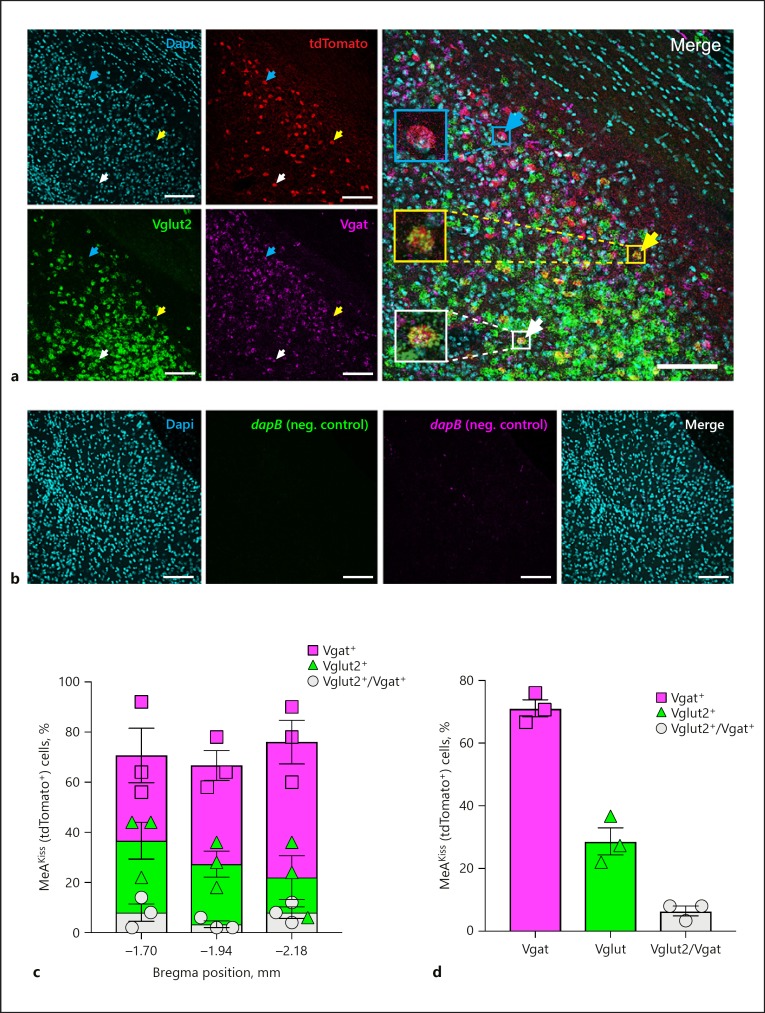
In order to determine which neurotransmitters MeAKiss neurons (tdTomato+) elaborate, we carried out multiplexed RNAscope ISH for mRNA encoding the glutamate vesicular transporter type 2 (Vglut2) or the GABA vesicular transporter (Vgat) on brain sections from Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice. Using a confocal laser scanning microscope (Leica TCS SP8), z-stack images were acquired to analyse Vglut2 and Vgat mRNA expression in MeAKiss (tdTomato+) neurons (Fig. 6a). The negative control RNAscope in situ probes designed against the bacterial dapB gene did not display any significant signal in the MeA in Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice (Fig. 6b). In MeAKiss neurons, the highest proportion were labelled for Vgat (71.1 ± 2.7%) compared to Vglut2 (28.7 ± 4.3%), and a small proportion expressed both (6.4% ± 1.6%, n = 150 tdTomato+ neurons monitored in each of 3 animals; Fig. 6c, d).
Fig. 6.
Amygdala Kiss1 neurons express mRNAs encoding Vgat and Vglut2 in male mice. a A representative confocal micrograph (16 µm thickness) displays Dapi nuclear stain (cyan), tdTomato (red), Vglut2 (green), and Vgat (magenta) expression in the MeA in Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice. A cyan arrow marks a tdTomato+ cell that expresses Vgat mRNA, a yellow arrow marks a tdTomato+ cell that expresses Vglut2 mRNA, and a white arrow marks a tdTomato+ cell that expresses both. Scale bars, 100 µm. b Representative confocal micrographs (16 µm thickness) that display negligible RNAscope in situ signals with RNAscope 3-plex negative control probes in the MeA in Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice. Dapi nuclear stain (cyan); RNAscope 3-plex negative control probe (dapB; a bacterial gene) developed in the FITC channel (green); RNAscope 3-plex negative control probe (dapB; a bacterial gene) developed in the Cy5 channel (magenta), and Merge of all three channels. Scale bars, 100 µm. c Quantification of labelled MeAKiss neurons expressing Vgat (squares), Vglut2 (triangles), or both (circles) as a percentage of tdTomato+ cells at three Bregma positions (n = 150 cells monitored in each of 3 animals). d The overall percentage of labelled MeAKiss neurons expressing Vgat (squares), Vglut2 (triangles), or both (circles) (n = 3). Data are represented as mean ± SEM with the value from each individual mouse indicated.
Discussion
In rodents, the MeA receives pheromonal information from the accessory olfactory system and signals to the hypothalamus to coordinate appropriate sexual behaviours and reproductive endocrine responses. Although activation of the MeA in response to opposite-sex chemosensory cues has been reported previously [8, 32], the specific neural circuit underlying the reproductive endocrine response remains poorly defined. Due to the recently revealed connectivity of MeAKiss neurons with the AOB and GnRH neurons [14], we hypothesized that MeAKiss neurons process sexually relevant olfactory stimuli to regulate reproductive neuroendocrine function. Male mice are preferentially attracted to ovary-intact females in proestrous and oestrous stages [33], therefore urine used as a chemosensory stimulus was collected from ovary-intact females primarily from proestrous and oestrous stages. Using double transgenic Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt male mice, we observed a significant elevation of c-Fos in both the MeApv and MeApd, but not in the MeAa after female urine exposure. These data are in line with other studies in male mice which found elevated c-Fos throughout the MeA after female pheromone exposure, but c-Fos-ir was most prominent in the MeApd [8, 32]. We then quantified c-Fos-ir specifically within MeA tdTomato+ (MeAKiss) neurons in response to urine exposure and observed raised c-Fos levels in MeAKiss neurons throughout the MeA, but this was most significant in the MeApd. Our data are supported by anatomical tracing studies that show the AOB provides dense input to all three MeA subdivisions [34], but neurons sensitive to volatile molecules which induce reproductive neuroendocrine effects (oestrous induction, puberty acceleration or delay) terminate principally in the MeApd [35, 36]. Reciprocal connections between MeApd MeAKiss neurons and the anterior AOB have been identified previously [14], suggesting that these neurons are directly targeted by pheromonal pathways but also may feedback to the AOB. When the overall number of c-Fos+ neurons in the MeA was compared to the number of c-Fos+ MeAKiss (tdTomato+) neurons (online suppl. Table 1), only a small number of c-Fos+ neurons were found to be MeAKiss neurons. This result is similar to that of a recent study using another transgenic mouse line (Kiss1-hrGFP), which found that the majority of pheromone-induced c-Fos-ir in the MeA was not in MeAKiss neurons [37]. This study detected increased c-Fos-ir in MeAKiss (hrGFP+) neurons, but the increase was not found to be statistically significant, likely due to the small number of samples analysed.
Using the double transgenic Kiss1CreEGFP/wt/Rosa 26lox-stop-lox-tdTomato/wt mouse line enabled visualization of MeAKiss neurons in mice, which has been difficult using conventional immunological methods or other transgenic approaches (e.g., Kiss1CreEGFP). Acknowledging the limitation of this approach and considering that crossing the Kiss1CreEGFP mice with Cre-dependent reporter lines may label transient Cre-expressing cells (e.g., cells that express Cre during development) [21], we first verified Kiss1 mRNA expression in MeA tdTomato+ cells and found approximately 92% of tdTomato+ cells to be positive for Kiss1 mRNA expression. We then compared adult male mice from the Kiss1CreEGFP/wt/Rosa26lox-stop-lox-tdTomato/wt line with adult male Kiss1CreEGFP/wt mice infused with Cre-dependent rAAVs 3 weeks prior, where cell labelling occurs only if viral infection coincides with Cre expression within the period post-infusion. Counting the number of fluorescently marked neurons in the MeA revealed no difference between the two labelling approaches, supporting that these two labelling strategies mark equivalent numbers of Cre-expressing MeA (MeAKiss) neurons, which indicates that a negligible number of MeA neurons are labelled during development. Our imaging studies found that fluorescently labelled MeAKiss neurons were concentrated in the MeApd but were also distributed throughout the MeAa and MeApv, whereas previous studies have located Kiss1 mRNA principally in the MeApd of mice [38]. In mice, kisspeptin protein is almost undetectable using conventional immunolabelling techniques, probably due to much lower expression or more rapid release compared to other rodents [38, 39]. However, recent labelling approaches using different transgenic mouse lines have confirmed that Kiss1 gene-driven reporters localize in the MeAa and MeApv [37, 40], supporting our data.
Sex pheromones are an important means of communication between many rodent species. However, the neuronal and molecular basis of processing pheromone signals appears to be sexually dimorphic. In female rats, male odours lead to activation of AVPVKiss neurons coincident with elevated LH and increased sexual behaviour [41]. Similarly, in mice, male urine leads to the activation of AVPVKiss neurons and increased lordosis behaviour in females [42]. These studies in female rodents contrast with our male study, which found that female urine failed to activate AVPVKiss or ARCKiss neurons in male mice (online suppl. Table 2, Fig. S3, S4), but instead activated MeAKiss neurons coincident with significantly raised LH. These data indicate that in male mice, MeAKiss neurons have a crucial role in processing opposite-sex pheromones to influence reproductive endocrine status.
In order to precisely define whether MeAKiss neurons regulate hypothalamic activity and orchestrate endocrine responses, we utilized a multiplexed chemogenetic approach, injecting a mixture of Cre-dependent AAVs encoding hM3D(Gq) (stimulatory) and hKORD (inhibitory) DREADDs to reciprocally modulate MeAKiss neurons in the absence of female urine exposure. Co-expression of hM3D(Gq) and hKORD has previously been used to remotely control neural circuits in mice [26] and our study achieved over 90% co-expression of hM3D(Gq) and hKORD DREADDs in MeAKiss neurons. Treatment with CNO depolarizes and increases the firing rate of hM3D(Gq)-expressing neurons [24], whereas SALB administration induces hyperpolarization (silencing) of hKORD-expressing neurons [26]. In our study, administration of CNO significantly elevated c-Fos-ir in hM3D(Gq)-expressing neurons and led to an impressive 15-fold higher LH level compared to controls and SALB-treated mice, whereas SALB treatments failed to reduce LH below the level in vehicle-treated controls. However, the LH levels in control animals were already very low, which may have precluded the demonstration of a decrease to near unmeasurable levels after SALB treatment. It is also plausible that MeAKiss neurons do not constitutively release an HPG-stimulatory factor (e.g., kisspeptin or glutamate) in the absence of an olfactory stimulus. Surprisingly, the large CNO-mediated induction of LH release was not accompanied by increased c-Fos-ir in GnRH neurons despite ∼40% of the MeAKiss neurons being activated. Previous studies in male mice have also failed to detect pheromone-induced c-Fos-ir in GnRH neurons accompanying LH rises [3] or after increased neuronal activation in the amygdala [8]. Previous research has shown that GnRH neurons in mice extend long dendritic projections to the median eminence and receive synaptic input along their length. The absence of GnRH soma activation may be due to the ability of these synapses to induce action potentials without activating the cell soma [43]. Interestingly, the median eminence is largely devoid of any MeAKiss projections (online suppl. Fig. S6), thus it appears unlikely that MeAKiss neurons directly influence GnRH release at GnRH nerve terminals. Therefore, the activation of the reproductive axis through MeAKiss neurons may be multi-modal where the release of GABA, glutamate, and kisspeptin may exert effects along the GnRH dendrite or through inter-neuronal pathways.
Despite the absence of c-Fos in GnRH neurons, when analysing the POA, CNO-mediated activation of MeAKiss neurons led to decreased neuronal activity compared to control and SALB-treated mice. Recent studies have identified MeAKiss neurons and the POA as regulators of sexual motivation and behaviour in male rodents [15, 16, 44]. Several studies have identified various neuronal types in the POA as key players in mediating male-typical mating and paternal behaviour including neurons expressing oestrogen receptor alpha [45], progesterone receptor [46], and galanin [47]. Therefore, future studies will be required to identify the exact cellular substrate in the POA responsive to modulation by MeAKiss neurons. A decrease in neuronal activity in the POA in association with an increase in LH secretion has been previously reported in response to peripheral administration of kisspeptin [48]. It was proposed that kisspeptin suppresses GABA neurons in the POA to reduce inhibition of GnRH neurons, which leads to increased LH secretion. Previous work had already established that kisspeptin can modulate GABA activity [49] and GABA neurons are known to inhibit GnRH activity and suppress LH secretion [50, 51]. Considering that GnRH neurons are known to be sensitive to GABA and glutamate [17, 52], we investigated whether MeAKiss neurons may modulate GnRH neurons through elaborating GABA and/or glutamate neurotransmission directly. We found that 71% of MeAKiss neurons express the GABAergic phenotypic marker Vgat and 29% express the glutamatergic marker Vglut2, whereas 6% express both. These results reveal that MeAKiss neurons consist of a heterogeneous population that may release inhibitory and/or excitatory transmitters to accompany peptidergic actions.
Several human studies have shown the influence of olfactory signals on sociosexual behaviour [53], but there is no direct evidence linking MeAKiss neurons to this pathway. Instead, visual stimuli may serve a more obvious influence on sexual motivation and consummation and this has been recently linked to amygdala activation [54]. Adult males have a larger amygdalar volume than females [55] and this is thought to correlate to higher sexual drive [2]. Sexually arousing visual stimuli leads to increased activation in the male MeA [54] and kisspeptin administration in humans enhances the activity of the left amygdala in response to sexual and couple-bonding stimuli [56]. Moreover, sexual differences in amygdala responses have been linked to the differential prevalence of psychological and sexual disorders in men and women [54] and it is worth considering whether MeAKiss neurons are involved. However, further research is required to show that MeAKiss neurons in particular do indeed contribute to any sex differences in sexual or affective disorders.
Overall, our study is the first to demonstrate that male MeAKiss neurons are sensitive to female urinary chemosensory stimuli, stimulate LH secretion, and suppress POA neuronal activity. These neurons could be using peptidergic as well as amino acidergic co-transmission to regulate the reproductive axis. Together, these results establish that MeAKiss neurons functionally link olfactory cues with the reproductive neuroendocrine axis.
Statement of Ethics
All animal procedures were conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 approved by the University of St. Andrews Ethics Committee under the UK Home Office project license No. 7007924 granted to J.A.T.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the Wellcome Trust Institutional Strategic Support Fund to the University of St. Andrews (awarded to J.A.T.), the British Society for Neuroendocrinology (Project Support Grant to S.A. and J.A.T.), and the RS MacDonald Trust Grant (awarded to S.A. and J.A.T.).
Author Contributions
S.A. designed and performed experiments, acquired, analysed, and interpreted data, and wrote the first draft of the paper. C.T. analysed and interpreted data. K.S. conducted experiments and analysed data. H.W.K. conducted experiments and analysed data. R.P.M. analysed and interpreted data and critically revised the paper. J.A.T. conceptualized the study, designed and performed experiments, acquired, analysed, and interpreted data, and wrote the paper.
Acknowledgements
We thank Dr. A. Caraty and Prof. N. Sherwood for providing us with primary antibodies and Dr. Agnes Tello for critical and technical reading of the manuscript.
References
- 1.Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980 Oct;210((4469)):557–60. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- 2.Baird AD, Wilson SJ, Bladin PF, Saling MM, Reutens DC. The amygdala and sexual drive: insights from temporal lobe epilepsy surgery. Ann Neurol. 2004 Jan;55((1)):87–96. doi: 10.1002/ana.10997. [DOI] [PubMed] [Google Scholar]
- 3.Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann N Y Acad Sci. 1998 Nov;855(1 OLFACTION AND):349–61. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- 4.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998 Aug;21((8)):323–31. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 5.Li XF, Hu MH, Hanley BP, Lin YS, Poston L, Lightman SL, et al. The Posterodorsal Medial Amygdala Regulates the Timing of Puberty Onset in Female Rats. Endocrinology. 2015 Aug 7;:en.2015–1366. doi: 10.1210/en.2015-1366. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens SB, Raper J, Bachevalier J, Wallen K. Neonatal amygdala lesions advance pubertal timing in female rhesus macaques. Psychoneuroendocrinology. 2015 Jan;51:307–17. doi: 10.1016/j.psyneuen.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo Y, Sachs BD. Disparate effects of small medial amygdala lesions on noncontact erection, copulation, and partner preference. Physiol Behav. 2002 Aug;76((4-5)):443–7. doi: 10.1016/s0031-9384(02)00682-0. [DOI] [PubMed] [Google Scholar]
- 8.Taziaux M, Bakker J. Absence of Female-Typical Pheromone-Induced Hypothalamic Neural Responses and Kisspeptin Neuronal Activity in α-Fetoprotein Knockout Female Mice. Endocrinology. 2015 Jul;156((7)):2595–607. doi: 10.1210/en.2015-1062. [DOI] [PubMed] [Google Scholar]
- 9.Coquelin A, Clancy AN, Macrides F, Noble EP, Gorski RA. Pheromonally induced release of luteinizing hormone in male mice: involvement of the vomeronasal system. J Neurosci. 1984 Sep;4((9)):2230–6. doi: 10.1523/JNEUROSCI.04-09-02230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gore AC, Wersinger SR, Rissman EF. Effects of female pheromones on gonadotropin-releasing hormone gene expression and luteinizing hormone release in male wild-type and oestrogen receptor-α knockout mice. J Neuroendocrinol. 2000 Dec;12((12)):1200–4. doi: 10.1046/j.1365-2826.2000.00578.x. [DOI] [PubMed] [Google Scholar]
- 11.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004 Sep;145((9)):4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 12.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003 Oct;349((17)):1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 13.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012 Feb;366((7)):629–35. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 14.Pineda R, Plaisier F, Millar RP, Ludwig M. Amygdala Kisspeptin Neurons: Putative Mediators of Olfactory Control of the Gonadotropic Axis. Neuroendocrinology. 2017;104((3)):223–38. doi: 10.1159/000445895. [DOI] [PubMed] [Google Scholar]
- 15.Adekunbi DA, Li XF, Lass G, Shetty K, Adegoke OA, Yeo SH, et al. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J Neuroendocrinol. 2018 Mar;30((3)):e12572. doi: 10.1111/jne.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gresham R, Li S, Adekunbi DA, Hu M, Li XF, O'Byrne KT. Kisspeptin in the medial amygdala and sexual behavior in male rats. Neurosci Lett. 2016 Aug;627:13–7. doi: 10.1016/j.neulet.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011 Jul;23((7)):557–69. doi: 10.1111/j.1365-2826.2011.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iremonger KJ, Constantin S, Liu X, Herbison AE. Glutamate regulation of GnRH neuron excitability. Brain Res. 2010 Dec;1364:35–43. doi: 10.1016/j.brainres.2010.08.071. [DOI] [PubMed] [Google Scholar]
- 19.Bian X, Yanagawa Y, Chen WR, Luo M. Cortical-like functional organization of the pheromone-processing circuits in the medial amygdala. J Neurophysiol. 2008 Jan;99((1)):77–86. doi: 10.1152/jn.00902.2007. [DOI] [PubMed] [Google Scholar]
- 20.Mahesh VB, Brann DW. Regulatory role of excitatory amino acids in reproduction. Endocrine. 2005 Dec;28((3)):271–80. doi: 10.1385/ENDO:28:3:271. [DOI] [PubMed] [Google Scholar]
- 21.Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011 Nov;152((11)):4298–309. doi: 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010 Jan;13((1)):133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClure C, Cole KL, Wulff P, Klugmann M, Murray AJ. Production and titering of recombinant adeno-associated viral vectors. J Vis Exp. 2011 Nov;((57)):e3348–3348. doi: 10.3791/3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009 Jul;63((1)):27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011 Apr;121((4)):1424–8. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vardy E, Robinson JE, Li C, Olsen RH, DiBerto JF, Giguere PM, et al. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron. 2015 May;86((4)):936–46. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: 32; 2001. [Google Scholar]
- 28.Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013 Dec;154((12)):4939–45. doi: 10.1210/en.2013-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Probst S, Daza RA, Bader N, Hummel JF, Weiß M, Tanriver Y, et al. A dual-fluorescence reporter in the Eomes locus for live imaging and medium-term lineage tracing. genesis. 2017 Aug;55:e23043. doi: 10.1002/dvg.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oka Y, Ye M, Zuker CS. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature. 2015 Apr;520((7547)):349–52. doi: 10.1038/nature14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012 Jun;9((7)):676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aste N, Honda S, Harada N. Forebrain Fos responses to reproductively related chemosensory cues in aromatase knockout mice. Brain Res Bull. 2003 May;60((3)):191–200. doi: 10.1016/s0361-9230(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 33.Ingersoll DW, Weinhold LL. Modulation of male mouse sniff, attack, and mount behaviors by estrous cycle-dependent urinary cues. Behav Neural Biol. 1987 Jul;48((1)):24–42. doi: 10.1016/s0163-1047(87)90544-9. [DOI] [PubMed] [Google Scholar]
- 34.Cádiz-Moretti B, Otero-García M, Martínez-García F, Lanuza E. Afferent projections to the different medial amygdala subdivisions: a retrograde tracing study in the mouse. Brain Struct Funct. 2016 Mar;221((2)):1033–65. doi: 10.1007/s00429-014-0954-y. [DOI] [PubMed] [Google Scholar]
- 35.Halpern M, Martínez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003 Jun;70((3)):245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 36.Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, et al. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000 Jun;405((6788)):792–6. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- 37.Lima LB, Haubenthal FT, Silveira MA, Bohlen TM, Metzger M, Donato J, Jr, et al. Conspecific odor exposure predominantly activates non-kisspeptin cells in the medial nucleus of the amygdala. Neurosci Lett. 2018 Aug;681:12–6. doi: 10.1016/j.neulet.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011 May;152((5)):2020–30. doi: 10.1210/en.2010-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, et al. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J. 2012;59((2)):161–71. doi: 10.1507/endocrj.ej11-0193. [DOI] [PubMed] [Google Scholar]
- 40.Yeo SH, Kyle V, Morris PG, Jackman S, Sinnett-Smith LC, Schacker M, et al. Visualisation of Kiss1 Neurone Distribution Using a Kiss1-CRE Transgenic Mouse. J Neuroendocrinol. 2016 Nov;28((11)):713. doi: 10.1111/jne.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe Y, Ikegami K, Ishigaki R, Ieda N, Uenoyama Y, Maeda KI, et al. Enhancement of the luteinising hormone surge by male olfactory signals is associated with anteroventral periventricular Kiss1 cell activation in female rats. J Neuroendocrinol. 2017 Aug;29((8)):e12505. doi: 10.1111/jne.12505. [DOI] [PubMed] [Google Scholar]
- 42.Hellier V, Brock O, Candlish M, Desroziers E, Aoki M, Mayer C, et al. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat Commun. 2018 Jan;9((1)):400. doi: 10.1038/s41467-017-02797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herde MK, Iremonger KJ, Constantin S, Herbison AE. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci. 2013 Jul;33((31)):12689–97. doi: 10.1523/JNEUROSCI.0579-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meisel RL. Recovery of masculine copulatory behavior from lesions of the medial preoptic area: effects of age versus hormonal state. Behav Neurosci. 1983 Oct;97((5)):785–93. doi: 10.1037//0735-7044.97.5.785. [DOI] [PubMed] [Google Scholar]
- 45.Wei YC, Wang SR, Jiao ZL, Zhang W, Lin JK, Li XY, et al. Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat Commun. 2018 Jan;9((1)):279. doi: 10.1038/s41467-017-02648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013 May;153((4)):896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014 May;509((7500)):325–30. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Comninos AN, Anastasovska J, Sahuri-Arisoylu M, Li X, Li S, Hu M, et al. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct Funct. 2016 May;221((4)):2035–47. doi: 10.1007/s00429-015-1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009 Aug;150((8)):3699–708. doi: 10.1210/en.2008-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012 Jan;153((1)):316–28. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. γ-aminobutyric acid B receptor mediated inhibition of gonadotropin-releasing hormone neurons is suppressed by kisspeptin-G protein-coupled receptor 54 signaling. Endocrinology. 2009 May;150((5)):2388–94. doi: 10.1210/en.2008-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeFazio RA, Elias CF, Moenter SM. GABAergic transmission to kisspeptin neurons is differentially regulated by time of day and estradiol in female mice. J Neurosci. 2014 Dec;34((49)):16296–308. doi: 10.1523/JNEUROSCI.3057-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grammer K, Fink B, Neave N. Human pheromones and sexual attraction. Eur J Obstet Gynecol Reprod Biol. 2005 Feb;118((2)):135–42. doi: 10.1016/j.ejogrb.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Hamann S. Sex differences in the responses of the human amygdala. Neuroscientist. 2005 Aug;11((4)):288–93. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001 Jun;11((6)):490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 56.Comninos AN, Wall MB, Demetriou L, Shah AJ, Clarke SA, Narayanaswamy S, et al. Kisspeptin modulates sexual and emotional brain processing in humans. J Clin Invest. 2017 Feb;127((2)):709–19. doi: 10.1172/JCI89519. [DOI] [PMC free article] [PubMed] [Google Scholar]