Abstract
Study Objectives
Research indicates that sleep efficiency below 80% substantially increases mortality risk in elderly persons. The aim of this study was to identify factors that would best predict poor sleep efficiency in the elderly, and to determine whether associations between these factors and sleep efficiency were similar for men and women and for younger and older elderly persons.
Methods
A total of 2468 individuals aged 65–96 years (40.7% men) participated. They were recruited via random generation of telephone numbers according to a geographic sampling strategy. The participants agreed to have health professionals visit their home and to answer structured interview questions. Sleep efficiency was calculated based on interview responses. Descriptive statistics and logistic regressions were conducted.
Results
The factors most strongly associated with sleep efficiency below 80% were pain, nocturia, sleep medication use, and awakening from bad dreams. Some factors varied by sex: women aged 75 years and older or who had an anxiety disorder were more likely to have sleep efficiency below 80%, whereas being single or having painful illness raised the likelihood for men only. Except for sex, all the factors that showed associations with sleep efficiency affected younger and older elderly persons similarly.
Conclusions
Poor sleep efficiency is prevalent among elderly persons. The results shed new light on factors associated with poor sleep efficiency, highlighting the presence of sex differences and that certain factors make no significant contribution, such as typically proscribed sleep hygiene behaviors, mood disorders, and illness in general.
Keywords: aging, sleep efficiency, physical illness, mental disorders, pain, sleep medication, sleep hygiene
Statement of Significance.
Sleep efficiency below 80% substantially increases mortality risk in elderly persons. This population-based study of community-dwelling elderly persons is the first to simultaneously investigate a large range of factors that could best predict poor sleep efficiency and to examine interactions with age and sex. When included in the same model, many factors showed a stronger association with sleep efficiency compared with age. These findings can orient researchers and clinicians toward a deeper understanding of sleep efficiency in the elderly and the development of new ways to help them improve it. Future studies should use objective sleep measures to explore factors that can predict that a patient with good sleep efficiency will subsequently develop poor sleep efficiency.
Introduction
Sleep efficiency, defined as the ratio of total sleep time to time in bed, decreases clearly and significantly with age [1–4]. It is also one of the few sleep parameters to continue declining after age 60 [3]. Sleep efficiency drops from 85% in 39- to 49-year-olds to 81% in 60- to 69-year-olds and plummets to 76% in over 80-year-olds [4]. Assessed at 77% in a small sample of men aged 60 to 70 years, sleep efficiency fell to just 46% in men aged 85 to 105 years [5]. Some geriatric sleep specialists contend that sleep efficiency, and not sleep duration, should be the primary parameter to consider in older persons who want to preserve their health and sleep quality with the passing of years [6].
Blackwell and colleagues found that actigraphy-measured sleep efficiency below 70%, but not objectively measured sleep duration, predicts cognitive function and decline in older women [7] and in older men [8]. After adjusting for multiple potential confounders, poorer sleep efficiency (<70%) measured using actigraphy was also independently associated with higher odds of greater frailty status in a cohort of over 3,000 men aged 67 and over [9]. Furthermore, over an average follow-up period of 13 years, elderly persons with objectively measured sleep efficiency below 80% were at 1.93 times greater risk of death after accounting for age, sex, and initial health status [10]. The poor health outcomes associated with poor sleep efficiency in these studies call for investigation of this sleep parameter in elderly populations.
Although other factors besides age may be related to sleep efficiency, the research on this topic is scarce. Nevertheless, it would be reasonable to assume strong associations between sleep efficiency and certain sociodemographic factors [2, 3, 11, 12], mental [13–19] and/or physical health problems [20–22], sleep hygiene [23–28], and other sleep-related variables such as pain [29], bad dreams [30], and nocturia [29, 31]. In 2002, the International Continence Society (ICS) defined nocturia as “the complaint that the individual has to wake at night one or more times to void” [32]. In 2018, the ICS proposed removing the complaint aspect from the definition [33].
The rare studies that address sleep efficiency in elderly persons target a single variable in relation to sleep efficiency, and the results are not clearly applicable to both men and women, nor to both younger and older elderly age groups. Either the age groups were not distinguished, or else no participants aged 75 and older were included.
Against this background, the present study aimed to (1) investigate a large range of factors to identify the best predictors of sleep efficiency in the elderly after accounting for the other factors; and (2) determine whether the associations between these factors and sleep efficiency are similar for men and women and for younger and older elderly persons. In addition, sample size permitting, potentially confounding variables were controlled for.
Methods
ESA study
Data for this cross-sectional study were obtained from the longitudinal Québec Survey on Seniors’ Health (Enquête sur la santé des aînés, ESA) conducted in 2005–2006 [34]. The aim of the ESA study was to assess the physical and mental health of the French-speaking community-dwelling population aged over 65 in the province of Québec, Canada (Québec has 8.4 million inhabitants, of which 78% speak French as their first language). The researchers used a probabilistic sample of older adults from each of the 16 administrative regions. A random digit dialling generation method was used to develop the survey sampling scheme, stratified by three geographic areas (metropolitan, urban, and rural) to account for regional variations in the health service system. A random sampling method was also used to select one elder from each household. People living in remote areas (Abitibi-Témiscamingue, Côte-Nord, Gaspésie, Îles-de-la-Madeleine, and Saguenay–Lac-Saint-Jean), comprising 10% of the elderly population of Québec, were excluded from the sampling frame on feasibility grounds. Data were weighted to account for the actual proportion of older adults living in each administrative region of Québec.
Procedure
Data were collected as follows. First, a health professional contacted potential respondents to describe the study objectives and procedures, answer their questions, and ask them to participate in an in-home interview. Next, a letter describing the study was sent to reassure potential participants of the credibility of the investigation and the interviewer. Appointments were then made with those who volunteered. The in-home interviews took place within 2 weeks following contact by the health professionals. Written informed consent to conduct the interview was obtained from all the volunteers at the beginning of the interview. The ESA study obtained prior approval by the Ethics Research Board of the Sherbrooke University Institute of Geriatrics. The interview was completed only with participants who scored 22 or higher on the Mini-Mental State Examination (MMSE) [35], as the others were likely to present moderate or severe cognitive decline, and consequently would be unable to respond to the questionnaire. This cut-off was based on Crum, Anthony, Bassett, and Folstein [36] in order to avoid the categorization of cognitively intact elderly people with little education as demented.
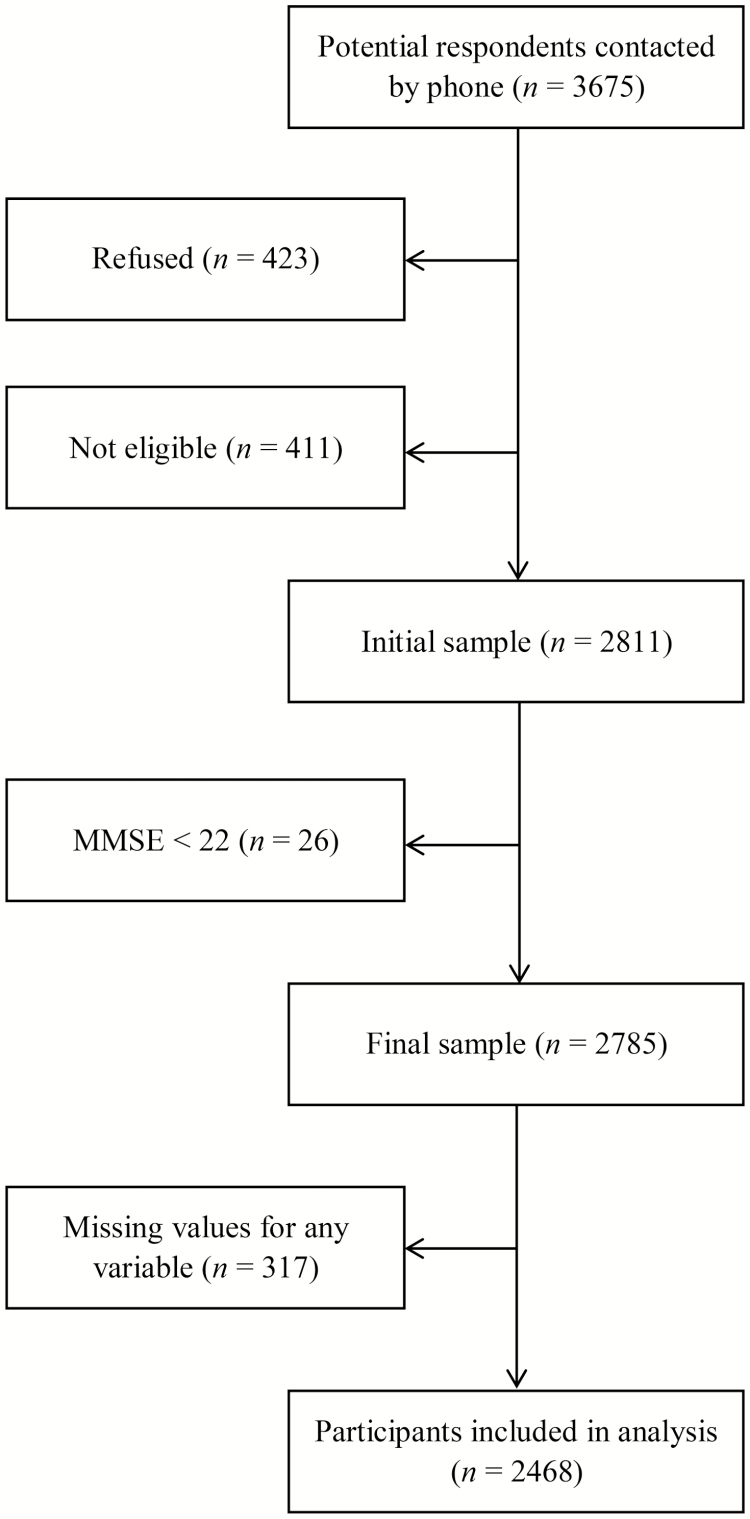
Figure 1 shows the flow chart of the study. There were 3675 people contacted. The response rate was 76.5% (n = 2811). Nearly 12% refused to take part in the study and another 12% were not eligible because they did not speak French, were confused, had significant hearing loss, or were dead. There was no difference between respondents and nonrespondents according to the available data (i.e. age, sex, and geographical area). Twenty-six participants with an MMSE score of <22 were excluded, leaving a sample of 2785 participants.
Figure 1.
Study flow chart.
Variables
Sleep efficiency
Based on self-report measures over the past month, this outcome variable was calculated with the following formula: total sleep time/time in bed × 100. Time in bed = sleep onset latency + total sleep time + time awake after initial onset but before final awakening + time attempting to sleep after final awakening [37]. Sleep efficiency was then categorized as <80% vs. ≥80%, similar to prior publication [10, 18, 38, 39]. This cut-off was selected in light of research findings that sleep efficiency below 80% substantially increases mortality risk in elderly persons [10]. Moreover, knowing that the average sleep efficiency in this population ranges from 76% to 81% [4, 40], the intention was to address a significant percentage of elderly persons rather than the most extreme cases among them.
Other sleep-related variables
Participants were asked the following questions: “Do you do any exercise two hours before you go to bed?”; “In the past month, have you taken any naps during the day?”; “Do you drink any caffeinated beverages after six o’clock in the evening?”; “In the past month, how many times have you had trouble sleeping because you had to get up to use the bathroom?”; “…because you were in pain?”; “…because you had a bad dream?”; and “In the past month, how many times did you take medication (with or without a prescription) to help you sleep?”
Mental disorders
Participants underwent a diagnostic interview based on the DSM-IV, as described elsewhere [34]. Mood disorder refers to a diagnosis of major depression, minor depression, or mania. Anxiety disorder refers to a diagnosis of generalized anxiety disorder, social phobia, specific phobia, obsessive-compulsive disorder, agoraphobia, or panic disorder.
Illness
Participants responded to the question: “To your knowledge, and in a doctor’s opinion, do you currently suffer from one of the following illnesses?” The participants responded to the following items, grouped under four main categories of illness: (1) painful illness (arthritis or rheumatism; severe spinal or back pain; functional digestive disorder or stomach, gastric, or duodenal ulcer; migraine or frequent headache), (2) cardiac or cardiovascular illness (high blood pressure, cardiac disease, and high blood cholesterol), (3) respiratory illness (asthma, emphysema, chronic bronchitis, and persistent cough), and (4) other illness (goiter or thyroid disorder, diabetes, anemia, liver disease, kidney disease, and urinary disorder).
Sociodemographic data
Participants responded to questions about their age, civil status, annual income, and highest education level completed. Geographic location was classified as rural if they lived in a settlement or community with a population of less than 1000, and urban if the population numbered 1000 or more. For purposes of this study, participants living in a metropolitan region were classified without further distinction as urban.
Statistical analysis
Participants with missing values for any variable (n = 317) were removed, leaving a final net sample of 2468 participants. Any significant differences between excluded and included participants were checked using Student t-tests for continuous variables and Pearson’s chi-square tests for categorical variables. The final net sample was then characterized with a descriptive analysis, using Pearson’s chi-square test to detect differences in the variables between men and women.
Prior to further analysis, all the variables were tested for dependency. Results showed that annual income clearly distinguished from the other variables, with a strong dependent relationship with marital status (Cramér’s V = 0.35; p < 0.001). To retain model robustness, this variable was removed from subsequent analysis. The presence of multicollinearity was assessed by tolerance and variance inflation factor (VIF). Tolerance lower than 0.10 or VIF higher than 10 indicates multicollinearity problems and requires further inspection of the data. The lowest tolerance obtained was 0.85 and the highest VIF was 1.18, clearly indicating absence of multicollinearity.
Simple logistic regressions were then conducted to examine associations between each variable and participants’ sleep efficiency (above or below 80%; crude odds ratio, OR), and a multiple logistic regression was performed to examine a model explaining sleep efficiency that included all the variables (adjusted odds ratio, AOR). Results were expressed as crude and adjusted odds ratios and 95% confidence intervals (CI). The same procedure was used to determine significant interaction terms for all the variables separately for sex and age (65–74 vs. ≥75 years).
In a secondary analysis, linear regressions were run to determine the stability of the results when sleep efficiency was analyzed as a continuous rather than a dichotomous outcome (above or below 80%). Logistic regressions were also repeated with sleep efficiency above or below 70% to assess the impact of alternative outcome definitions.
All analyses were performed using IBM SPSS Statistics Version 24 with two-sided tests and p < 0.05 considered statistically significant.
Results
Sample characteristics
In the final net sample (n = 2468), mean age was 73.7 (SD = 6.1, range 65–96), 59.3% were female, 81.0% had an annual income over CAN$15,000, 46.2% lived in a couple, and 61.0% lived in an urban area. Of the participants, 28.4% had lower than 80% sleep efficiency.
Compared with included participants, excluded participants (n = 317) were, on average, slightly older (74.7 vs. 73.7 years old, p = 0.003) and more educated (40% vs. 34% with post-secondary education, p = 0.030). They also took more sleep medication (36% vs. 28% with at least one intake in the past month, p = 0.004), woke more often due to pain (28% vs. 23% at least once in the past month, p = 0.030), and had fewer respiratory disorders (10% vs. 15%, p = 0.007). Otherwise, no significant differences were found, and notably in sleep efficiency or sex.
Table 1 presents the sample characteristics. Sex differences are observed for most variables. Women are more likely than men to be aged 75 and over, to be single, to have no post-secondary education, and to live in an urban area. Women reported more anxiety disorders, mood disorders, and painful illnesses. Compared with men, women also woke up more regularly because they had to use the bathroom, had bad dreams, or felt pain. In addition, more women than men took sleep medication. On the other hand, more men than women took naps or drank caffeinated beverages after 6:00 pm. No differences were obtained between men and women in terms of cardiac, respiratory, or “other” disease. Neither did men differ from women in terms of exercising shortly before bedtime.
Table 1.
Study sample variables by sex
| Variables | Women (%) | Men (%) | P |
|---|---|---|---|
| Age group | <0.001 | ||
| 65–74 | 54.1 | 65.2 | |
| ≥ 75 | 45.9 | 34.8 | |
| Marital status | <0.001 | ||
| Married/Com.-law | 37.5 | 58.8 | |
| Single/Sep.-Div./Widowed | 62.5 | 41.2 | |
| Education | <0.001 | ||
| Post high school | 27.5 | 44.4 | |
| None to high school | 72.5 | 55.6 | |
| Geographic location | 0.042 | ||
| Urban | 62.6 | 58.6 | |
| Rural | 37.4 | 41.4 | |
| Exercise before bedtime | 0.140 | ||
| Never | 85.0 | 82.8 | |
| Rarely to often | 15.0 | 17.2 | |
| Naps | <0.001 | ||
| Never or rarely | 47.1 | 38.2 | |
| Sometimes to very often | 52.9 | 61.8 | |
| Caffeine after 6 p.m. | <0.001 | ||
| Never | 71.5 | 61.1 | |
| Rarely to often | 28.5 | 38.9 | |
| Mood disorder | <0.001 | ||
| No | 91.7 | 95.8 | |
| Yes | 8.3 | 4.2 | |
| Anxiety disorder | 0.010 | ||
| No | 94.6 | 96.8 | |
| Yes | 5.4 | 3.2 | |
| Sleep medication | <0.001 | ||
| 0 per month | 67.6 | 78.3 | |
| ≥ once per month | 32.4 | 21.7 | |
| Nocturia | <0.001 | ||
| < 4 per month | 63.3 | 70.9 | |
| ≥ 4 per month | 36.7 | 29.1 | |
| Pain | <0.001 | ||
| 0 per month | 72.3 | 83.9 | |
| ≥ once per month | 27.7 | 16.1 | |
| Bad dreams | 0.029 | ||
| 0 per month | 85.4 | 88.4 | |
| ≥ once per month | 14.6 | 11.6 | |
| Painful illness | <0.001 | ||
| No | 38.1 | 60.0 | |
| Yes | 61.9 | 40.0 | |
| Cardiac illness | 0.122 | ||
| No | 27.3 | 30.2 | |
| Yes | 72.7 | 69.8 | |
| Respiratory illness | 0.281 | ||
| No | 83.9 | 85.5 | |
| Yes | 16.1 | 14.5 | |
| Other illness | 0.467 | ||
| No | 56.2 | 54.7 | |
| Yes | 43.8 | 45.3 |
Factors related to sleep efficiency
Table 2 presents the results of a first model without interaction, showing the associations between all the variables and sleep efficiency. In the fully adjusted model, seven factors are significantly associated with sleep efficiency. In descending order of association, they are pain (AOR = 2.01, 95% CI: 1.61, 2.50), nocturia (AOR = 1.98, 95% CI: 1.63, 2.40), sleep medication use (AOR = 1.82, 95% CI: 1.48, 2.22), bad dreams (AOR = 1.66, 95% CI: 1.28, 2.15), painful illness (AOR = 1.32, 95% CI: 1.08, 1.62), sex (AOR = 1.32, 95% CI: 1.06, 1.61), and age (AOR = 1.23, 95% CI: 1.01, 1.49). These factors are also significant in the nonadjusted model, along with five other variables: anxiety disorder (OR = 1.56, 95% CI: 1.05, 2.31), mood disorder (OR = 1.40, 95% CI: 1.00, 1.95), education (OR = 1.33, 95% CI: 1.10, 1.61), marital status (OR = 1.25, 95% CI: 1.04, 1.49), and caffeine after 6:00 pm (OR = 0.82, 95% CI: 0.68, 1.00). The variables geographic location, exercise before bedtime, naps, and illness other than pain-related show no significant associations with sleep efficiency, in both the nonadjusted and fully adjusted model.
Table 2.
Factors associated with sleep efficiency
| Crude | Fully adjusted | ||||
|---|---|---|---|---|---|
| Variables | Sleep efficiency < 80% (%) | Odds ratio (95% CI) | P | Odds ratio (95% CI) | P |
| Sex | |||||
| Men | 21.4 | 1.00 | 1.00 | ||
| Women | 33.1 | 1.82 (1.52–2.17) | <0.001 | 1.32 (1.06–1.61) | 0.010 |
| Age group | |||||
| 65–74 | 26.2 | 1.00 | 1.00 | ||
| ≥ 75 | 31.5 | 1.30 (1.09–1.55) | 0.004 | 1.23 (1.01–1.49) | 0.038 |
| Marital status | |||||
| Married/Com.-law | 26.0 | 1.00 | 1.00 | ||
| Single/Sep.-Div./Widowed | 30.4 | 1.25 (1.04–1.49) | 0.015 | 1.11 (0.92–1.35) | 0.277 |
| Education | |||||
| Post high school | 24.6 | 1.00 | 1.00 | ||
| None to high school | 30.4 | 1.33 (1.10–1.61) | 0.003 | 1.18 (0.96–1.45) | 0.118 |
| Geographic location | |||||
| Urban | 28.3 | 1.00 | 1.00 | ||
| Rural | 28.6 | 1.01 (0.85–1.21) | 0.882 | 1.03 (0.85–1.25) | 0.791 |
| Exercise before bedtime | |||||
| Never | 28.3 | 1.00 | 1.00 | ||
| Rarely to often | 29.1 | 1.04 (0.82–1.32) | 0.764 | 1.00 (0.78–1.29) | 0.990 |
| Naps | |||||
| Never or rarely | 27.8 | 1.00 | 1.00 | ||
| Sometimes to very often | 28.9 | 1.06 (0.89–1.26) | 0.539 | 0.93 (0.77–1.13) | 0.470 |
| Caffeine after 6 p.m. | |||||
| Never | 29.6 | 1.00 | 1.00 | ||
| Rarely to often | 25.8 | 0.82 (0.68–1.00) | 0.046 | 0.96 (0.78–1.18) | 0.694 |
| Mood disorder | |||||
| No | 27.9 | 1.00 | 1.00 | ||
| Yes | 35.2 | 1.40 (1.00–1.95) | 0.047 | 0.86 (0.59–1.23) | 0.402 |
| Anxiety disorder | |||||
| No | 28.0 | 1.00 | 1.00 | ||
| Yes | 37.8 | 1.56 (1.05–2.31) | 0.027 | 1.14 (0.74–1.75) | 0.558 |
| Sleep medication | |||||
| 0 per month | 23.7 | 1.00 | 1.00 | ||
| ≥ once per month | 40.3 | 2.17 (1.80–2.62) | <0.001 | 1.82 (1.48–2.22) | <0.001 |
| Nocturia | |||||
| < 4 per month | 22.5 | 1.00 | 1.00 | ||
| ≥ 4 per month | 40.0 | 2.30 (1.92–2.76) | <0.001 | 1.98 (1.63–2.40) | <0.001 |
| Pain | |||||
| 0 per month | 23.5 | 1.00 | 1.00 | ||
| ≥ once per month | 44.8 | 2.65 (2.17–3.22) | <0.001 | 2.01 (1.61–2.50) | <0.001 |
| Bad dreams | |||||
| 0 per month | 26.1 | 1.00 | 1.00 | ||
| ≥ once per month | 43.2 | 2.15 (1.70–2.73) | <0.001 | 1.66 (1.28–2.15) | <0.001 |
| Painful illness | |||||
| No | 21.9 | 1.00 | 1.00 | ||
| Yes | 34.1 | 1.84 (1.54–2.20) | <0.001 | 1.32 (1.08–1.62) | 0.006 |
| Cardiac illness | |||||
| No | 26.5 | 1.00 | 1.00 | ||
| Yes | 29.1 | 1.14 (0.93–1.38) | 0.206 | 0.95 (0.76–1.17) | 0.601 |
| Respiratory illness | |||||
| No | 28.2 | 1.00 | 1.00 | ||
| Yes | 29.5 | 1.06 (0.83–1.35) | 0.649 | 0.86 (0.67–1.12) | 0.268 |
| Other illness | |||||
| No | 27.2 | 1.00 | 1.00 | ||
| Yes | 29.9 | 1.15 (0.96–1.37) | 0.131 | 0.98 (0.81–1.19) | 0.845 |
CI = Confidence interval.
Interactions with sex and age
Sex differences were also considered. Table 3 presents the fully adjusted odds ratios for each variable separately for women and men, along with the percentage of men and women with sleep efficiency below 80% for each variable level. Four factors that were significantly associated with sleep efficiency for either men or women differ statistically between men and women: age group, marital status, anxiety disorder, and painful illness. Specifically, having an anxiety disorder (AOR = 1.69, 95% CI: 1.04, 2.75) and being 75 years old or more (AOR = 1.50, 95% CI: 1.18, 1.91) are each associated with higher odds of below 80% sleep efficiency for women only. On the other hand, having a painful illness (AOR = 1.69, 95% CI: 1.21, 2.37) and being single (AOR = 1.54, 95% CI: 1.11, 2.13) each increase the odds of sleep efficiency below 80% for men only. Figure 2 presents the factors that are significantly associated with sleep efficiency for either men or women and that differ statistically between men and women.
Table 3.
Factors associated with sleep efficiency, according to sex
| Variables | Women vs. Men | Women | Men | |||||
|---|---|---|---|---|---|---|---|---|
| Fully adjusted | Fully adjusted | |||||||
| P | Sleep efficiency < 80% (%) | Odds ratio (95% CI) | P | Sleep efficiency < 80% (%) | Odds ratio (95% CI) | P | ||
| Age group | 0.010 | |||||||
| 65–74 | 29.5 | 1.00 | 22.0 | 1.00 | ||||
| ≥ 75 | 37.3 | 1.50 (1.18–1.91) | 0.001 | 21.0 | 0.87 (0.62–1.22) | 0.415 | ||
| Marital status | 0.021 | |||||||
| Married/Com.-law | 33.7 | 1.00 | 19.4 | 1.00 | ||||
| Single/Sep.-Div./Widowed | 32.8 | 0.95 (0.75–1.22) | 0.717 | 24.9 | 1.54 (1.11–2.13) | 0.009 | ||
| Education | 0.159 | |||||||
| Post high school | 31.2 | 1.00 | 18.8 | 1.00 | ||||
| None to high school | 33.8 | 1.03 (0.79–1.35) | 0.831 | 24.0 | 1.41 (1.00–1.96) | 0.050 | ||
| Geographic location | 0.526 | |||||||
| Urban | 32.6 | 1.00 | 22.1 | 1.00 | ||||
| Rural | 34.0 | 1.06 (0.83–1.35) | 0.636 | 21.2 | 0.93 (0.66–1.30) | 0.660 | ||
| Exercise before bedtime | 0.030 | |||||||
| Never | 33.8 | 1.00 | 20.5 | 1.00 | ||||
| Rarely to often | 29.9 | 0.79 (0.57–1.11) | 0.174 | 27.4 | 1.42 (0.95–2.14) | 0.091 | ||
| Naps | 0.277 | |||||||
| Never or rarely | 32.3 | 1.00 | 19.2 | 1.00 | ||||
| Sometimes to very often | 33.8 | 0.87 (0.69–1.11) | 0.260 | 23.2 | 1.10 (0.78–1.54) | 0.588 | ||
| Caffeine after 6 pm | 0.174 | |||||||
| Never | 34.0 | 1.00 | 22.8 | 1.00 | ||||
| Rarely to often | 31.4 | 1.07 (0.82–1.39) | 0.617 | 19.9 | 0.80 (0.57–1.11) | 0.182 | ||
| Mood disorder | 0.089 | |||||||
| No | 32.2 | 1.00 | 22.0 | 1.00 | ||||
| Yes | 43.1 | 1.00 (0.66–1.52) | 0.984 | 14.0 | 0.40 (0.15–1.06) | 0.066 | ||
| Anxiety disorder | 0.012 | |||||||
| No | 32.1 | 1.00 | 22.0 | 1.00 | ||||
| Yes | 50.0 | 1.69 (1.04–2.75) | 0.034 | 9.1 | 0.33 (0.10–1.06) | 0.063 | ||
| Sleep medication | 0.543 | |||||||
| 0 per month | 27.2 | 1.00 | 19.5 | 1.00 | ||||
| ≥ once per month | 45.1 | 1.94 (1.52–2.48) | <0.001 | 29.7 | 1.69 (1.17–2.45) | 0.006 | ||
| Nocturia | 0.359 | |||||||
| < 4 per month | 26.9 | 1.00 | 17.1 | 1.00 | ||||
| ≥ 4 per month | 44.0 | 1.87 (1.47–2.37) | <0.001 | 32.6 | 2.26 (1.62–3.16) | <0.001 | ||
| Pain | 0.676 | |||||||
| 0 per month | 27.6 | 1.00 | 18.7 | 1.00 | ||||
| ≥ once per month | 47.7 | 2.03 (1.56–2.63) | <0.001 | 37.2 | 1.82 (1.21–2.76) | 0.004 | ||
| Bad dreams | 0.711 | |||||||
| 0 per month | 30.5 | 1.00 | 20.3 | 1.00 | ||||
| ≥ once per month | 48.6 | 1.63 (1.19–2.34) | 0.003 | 32.5 | 1.81 (1.14–2.88) | 0.012 | ||
| Painful illness | 0.044 | |||||||
| No | 27.9 | 1.00 | 17.3 | 1.00 | ||||
| Yes | 36.3 | 1.10 (0.85–1.42) | 0.461 | 28.3 | 1.69 (1.21–2.37) | 0.002 | ||
| Cardiac illness | 0.891 | |||||||
| No | 30.7 | 1.00 | 20.9 | 1.00 | ||||
| Yes | 34.0 | 0.96 (0.73–1.25) | 0.756 | 22.1 | 0.99 (0.69–1.42) | 0.951 | ||
| Respiratory illness | 0.070 | |||||||
| No | 32.5 | 1.00 | 22.4 | 1.00 | ||||
| Yes | 36.6 | 0.98 (0.75–1.41) | 0.848 | 17.2 | 0.60 (0.37–0.98) | 0.052 | ||
| Other illness | 0.862 | |||||||
| No | 31.2 | 1.00 | 21.0 | 1.00 | ||||
| Yes | 35.6 | 0.98 (0.78–1.25) | 0.894 | 22.5 | 0.95 (0.68–1.32) | 0.758 | ||
CI = Confidence interval.
Figure 2.
Logistic regression plot of odds ratios and 95% confidence intervals; factors significantly associated with sleep efficiency that differ statistically between men and women.
The same procedure was used to examine interactions between each variable and age (65–74 vs. ≥ 75 years old), and no significant interactions were found, except for an interaction between age and sex. Thus, for both younger and older elderly persons, the same factors are associated with sleep efficiency, with the exception that being a woman increases the likelihood for sleep efficiency below 80% in the 75 years and older group.
Sensitivity analysis
The results of the sensitivity analysis were very similar to those of the primary analysis. In this secondary analysis, when sleep efficiency was considered as a continuous variable, the factors with the strongest associations with sleep efficiency after adjusting for all the other considered factors were, in descending order, pain (β = −4.87, p < 0.001), nocturia (β = −4.71, p < 0.001), sleep medication use (β = −3.72, p < 0.001), bad dreams (β = −2.80, p < 0.001), age group (β = −2.32, p < 0.001), sex (β = −1.55, p = 0.008), and painful illness (β = −1.33, p = 0.019). When a sleep efficiency cut-off of below 70% was used, the strongest associated factors were, in descending order, pain (AOR = 2.27, 95% CI: 1.75, 2.94), sleep medication use (AOR = 1.80, 95% CI: 1.42, 2.29), nocturia (AOR = 1.67, 95% CI: 1.32, 2.12), age group (AOR = 1.48, 95% CI: 1.17, 1.88), and marital status (AOR = 1.39, 95% CI: 1.09, 1.79). Of the participants, 15.6% had lower than 70% sleep efficiency.
Discussion
This study obtained several key findings. First, in a population-based sample of community-dwelling elderly persons, a number of factors when included in the same model showed a stronger association with below 80% sleep efficiency compared with age. These factors are pain, nocturia, sleep medication use, bad dreams, painful illness, and sex. Second, psychological disorders and illness in general were not significantly associated with sleep efficiency, except for a painful illness such as arthritis, back pain, stomach ulcer, or migraine. Third, typically proscribed sleep hygiene behaviors such as exercising shortly before bedtime, drinking caffeinated beverages after 6:00 pm, and naps during the day did not increase the odds for poor sleep efficiency. Fourth, certain risk factors differed significantly according to sex. Anxiety disorder and belonging to the 75 years and older group each carried greater odds of below 80% sleep efficiency for women only, whereas a painful illness and single status were likelihood factors for men only. Fifth, persons aged 65 to 74 years had the same likelihood factors as those in the 75 years and older group, except for women: women aged 75 years and older were more likely to have poorer sleep efficiency than women in the younger age group.
Some of these results concur with the small number of population studies that identified main risk factors for sleep disturbances or insomnia. A study of 959 Korean adults aged 45 years and older found that pain and using the bathroom three or more times a night were the two most important risk factors for sleep disturbances [41]. Nocturnal micturition and regular use of hypnotics were the two greatest risk factors for insomnia in a study of 2045 noninstitutionalized older individuals aged 65 years and older residing in an urban community in Taiwan [42]. However, our results depart from a systematic literature review of risk factors for sleep disturbances in older adults [43] in which being female, depressed mood, and physical illness presented the most consistent risks for sleep disturbances. These discrepancies in the findings may be partly explained by the fact that, whereas sleep efficiency, sleep disturbances, and insomnia can overlap, they remain distinct concepts.
Although pain and sleep have a bidirectional relationship, the research to date indicates that sleep disturbances generally predict pain more than pain predicts sleep disturbances [44–46]. Nevertheless, pain has been demonstrated as a reliable predictor of poor sleep efficiency [46]. Our results support this finding, even when controlling for factors such as depression and anxiety. Moreover, in chronic pain populations, sleep fragmentation is the most common alteration found, as reflected in decreased sleep efficiency [47].
Nocturia has also been associated with polysomnography-measured sleep efficiency in the elderly [31]. In a sample of over 1,400 older persons, nocturia was the most frequently reported reason for a poor night’s sleep and was cited four times more often than the next most frequent reason, namely, pain [29]. Given that the authors of that study used self-reported reasons for sleep disturbances, some researchers suggest that the participants mistakenly blamed their awakenings on the need to urinate, whereas in fact they woke up for other reasons and then took the opportunity to use the toilet. It seems that the precise reasons for certain behaviors can be elusive. One study showed that most awakenings from sleep attributed by patients to the need to urinate were actually directly secondary to sleep apnea, snoring, or periodic leg movements in sleep [48]. Bliwise [49] justifiably proposed that this could be why bathroom trips for urination have been frequently overlooked in epidemiological studies of factors associated with poor sleep. In our study, the participants did not have to provide their own explanations for how diverse factors affected their sleep efficiency, adding weight to the idea that bathroom trips constitute a non-negligible variable in investigations of sleep efficiency. If the perceived urge to void follows nocturnal awakenings, interventions such as cognitive-behavioral treatments for insomnia would be recommended, whereas if the urge to void precedes the awakenings, bladder control exercises, and medications to treat urine production or urgency would be in order [49].
On the subject of medications, the most common treatments for sleep disorders remain pharmacological rather than psychological, and the insomnia medication industry is enjoying rapid growth [50]. Furthermore, older age is an independent predictor of psychotropic medication use [50]. However, in the present study, participants who used sleep medication were actually more likely to have poor sleep efficiency (below 80%). Although the present study did not allow establishing a cause-and-effect relationship, this result, which concurs with other studies [51, 52], suggests that sleep medications are at best useless for improving sleep efficiency and at worst actually impair it. With effective medication, sleep efficiency would not be expected to differ significantly between users and nonusers.
Awakening from bad dreams was found to be a significant risk factor for sleep efficiency. Studies on bad dreams and nightmares in the elderly are extremely rare. The prevalence of nightmares in older adults has been assessed at slightly over 4% [53]. This prevalence rises to 11.4% and 17.1% in those with symptoms of depression and anxiety, respectively [54]. In adults aged 60 and older, the frequency of bad dreams was associated with depression, anxiety, and worries [55]. Our results add to this scant knowledge by revealing that bad dreams constitute a significant risk factor for poor sleep efficiency, even after controlling for anxiety and mood disorders.
Studies increasingly support that common sleep disorders in older adults are secondary to comorbid medical and psychiatric disorders, and are not simply due to aging as such [20, 56–60]. In fact, sleep complaints are closely associated with chronic disease [20–22]. Moreover, anxiety and mood disorders have been associated with poor sleep efficiency (self-reported and actigraphy-measured) in older women and men alike [13–19]. Interestingly, the results of the present study indicate that illness other than pain-related is not a risk factor for poor sleep efficiency. Similarly, a study of sleep onset and maintenance problems in 702 nondemented participants aged 70 and older found no relationships between sleep disturbances and measures of common medical conditions [61], although a robust relationship was found between sleep disruption and symptoms of depression and anxiety. Anxiety and mood disorders were also risk factors in the nonadjusted model in our study, although the effects disappeared in the fully adjusted model. Significant interaction effects showed that anxiety disorder was associated with higher odds of poor sleep efficiency in women only, whereas painful illness constituted a risk factor for men only.
Recommended sleep hygiene practices generally include avoiding physical activity shortly before bedtime, abstaining from napping, and limiting caffeine consumption. However, a recent literature review revealed that physical activity improves sleep efficiency in the elderly, independently of activity type and intensity [23], although whether the timing of the activity is determinant remained unclear. Furthermore, contrary to the usual recommendations, napping, whether measured subjectively [24] or objectively [25], appeared to have no negative effect on sleep efficiency in the elderly. What is more, studies have found associations between napping and improved sleep efficiency, measured using actigraphy or the Pittsburgh Sleep Quality Index (PSQI) [62], in both good and poor sleepers aged 60 to 89 years [26, 27]. Finally, and this time in line with the recommendations, a study revealed that caffeine (compared with a placebo) significantly reduced laboratory-measured sleep efficiency, and that the effect grew with increasing age [28].
Our results on exercise shortly before bedtime, before-bed caffeine consumption, and naps concur with McCrae [63], who found that the effects of overall sleep hygiene behaviors did not differ among four groups of participants: noncomplainers without insomnia symptoms, complainers without insomnia symptoms, noncomplainers with insomnia symptoms, and complainers with insomnia symptoms. Moreover, in a population study of 4730 participants, caffeine was associated with insomnia symptoms, but only in nonadjusted models, and the association between insomnia and caffeine was explained by anxiety [64]. Therefore, the commonly proscribed sleep hygiene behaviors appear to be only slightly determinant for sleep efficiency, if at all. Instead, certain practices could be encouraged, such as exercise. A more recent longitudinal study using sleep polysomnography found that physical activity was associated with smaller odds of poor sleep efficiency in elderly persons [39].
One noteworthy finding of the present study is that certain factors differed according to sex. Anxiety disorder was associated with higher likelihood of poor sleep efficiency for women only. Studies have increasingly demonstrated that men and women differ in their expression of anxiety disorders. For example, post-traumatic stress disorder (PTSD) manifests as higher occurrences of intrusive thoughts [65, 66], nightmares or distressing dreams [65, 66], and sleep disturbances [65–68] for women compared with men. These sex differences in anxiety symptoms could explain our findings.
Furthermore, single status emerged as a risk factor for men only. Researchers have proposed a few explanations for why it is more difficult for men to cope with singlehood [69]. This particular generation of men grew up at a time when women did almost all the housekeeping, food shopping, and cooking. Hence, single men would be more likely to experience unintentional weight loss, weakness, and exhaustion. It has also been advanced that, compared with unmarried women, unmarried men would have fewer family and social relationships, leading to social isolation. These proposals could generate explanatory hypotheses for the sex differences we observed in the relationships between marital status and sleep efficiency.
This study contains a number of strengths. First, a large, randomly recruited sample of community-dwelling elderly persons was investigated. Second, unlike other community-based studies, cognitively impaired elderly persons were excluded using the MMSE cut-off score, thereby removing a bias. Third, instead of questionnaires, diagnostic interviews were held to establish anxiety and mood disorder diagnoses. In addition, a large number of factors were considered simultaneously.
This study also has some limitations. First, sleep efficiency was assessed by subjective reports rather than polysomnography measures. Moreover, some questions targeted a period of 1 month previously, which could have affected recall. In addition, the study design did not allow determining cause-and-effect relationships. Furthermore, the presence of sleep disorders such as apnea and restless legs syndrome was not assessed. However, a recent study in 348 participants aged 60 years and older revealed that high sleep-disordered breathing (apnea and hypopnea index ≥ 15) was not related to polysomnography-measured sleep efficiency [70]. Another study in 982 elderly men showed that actigraphy-measured sleep efficiency in 167 men having restless legs syndrome did not differ significantly from that of other participants [71].
In summary, the results shed new light on factors that increase the odds of poor sleep efficiency, and they single out certain factors that make no significant contribution. Improving sleep efficiency would be beneficial for elderly persons, even for those without sleep complaints or insomnia. These findings can orient researchers and clinicians toward a deeper understanding of sleep efficiency in the elderly and the development of new ways to help them improve it.
Funding
This study was supported by the Canadian Institutes of Health Research (200403MOP).
Conflict of interest statement. None declared.
References
- 1. Åkerstedt T, et al. The relation between polysomnography and subjective sleep and its dependence on age – poor sleep may become good sleep. J Sleep Res. 2016;25(5):565–570. [DOI] [PubMed] [Google Scholar]
- 2. McCrae CS, et al. Self-reported sleep, demographics, health, and daytime functioning in young old and old old community-dwelling seniors. Behav Sleep Med. 2008;6(2):106–126. [DOI] [PubMed] [Google Scholar]
- 3. Ohayon MM, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. [DOI] [PubMed] [Google Scholar]
- 4. Unruh ML, et al. Subjective and objective sleep quality and aging in the Sleep Heart Health Study. J Am Geriatr Soc. 2008;56(7):1218–1227. [DOI] [PubMed] [Google Scholar]
- 5. Mazzotti DR, et al. Human longevity is associated with regular sleep patterns, maintenance of slow wave sleep, and favorable lipid profile. Front Aging Neurosci. 2014;6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoch CC, et al. Protecting sleep quality in later life: a pilot study of bed restriction and sleep hygiene. J Gerontol B Psychol Sci Soc Sci. 2001;56(1):P52–P59. [DOI] [PubMed] [Google Scholar]
- 7. Blackwell T, et al. ; Study of Osteoporotic Fractures Group. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61(4):405–410. [DOI] [PubMed] [Google Scholar]
- 8. Blackwell T, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. 2014;37(4):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ensrud KE, et al. ; Osteoporotic Fractures in Men Study Group. Sleep disturbances and frailty status in older community-dwelling men. J Am Geriatr Soc. 2009;57(11):2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dew MA, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65(1):63–73. [DOI] [PubMed] [Google Scholar]
- 11. van den Berg JF, et al. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32(10):1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams LL. Factors Associated with Sleep Disruption among Comminges-Dwelling Older Adults in the Health and Retirement Study[doctoral thesis]. 2009. https://www.mhsl.uab.edu/dt/2009p/williamsl.pdf Accessed October 18, 2018. [Google Scholar]
- 13. Chang KJ, et al. Perceived sleep quality is associated with depression in a Korean elderly population. Arch Gerontol Geriatr. 2014;59(2):468–473. [DOI] [PubMed] [Google Scholar]
- 14. Leblanc MF, et al. Sleep problems in anxious and depressive older adults. Psychol Res Behav Manag. 2015;8:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maglione JE, et al. Depressive symptoms and subjective and objective sleep in community-dwelling older women. J Am Geriatr Soc. 2012;60(4):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naismith SL, et al. Sleep disturbance relates to neuropsychological functioning in late-life depression. J Affect Disord. 2011;132(1–2):139–145. [DOI] [PubMed] [Google Scholar]
- 17. Paudel ML, et al. ; Osteoporotic Fractures in Men Study Group. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56(7):1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spira AP, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sukegawa T, et al. Sleep disturbances and depression in the elderly in Japan. Psychiatry Clin Neurosci. 2003;57(3):265–270. [DOI] [PubMed] [Google Scholar]
- 20. Foley D, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. [DOI] [PubMed] [Google Scholar]
- 21. Morphy H, et al. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30(3):274–280. [PubMed] [Google Scholar]
- 22. Sarsour K, et al. Association of insomnia severity and comorbid medical and psychiatric disorders in a health plan-based sample: insomnia severity and comorbidities. Sleep Med. 2010;11(1):69–74. [DOI] [PubMed] [Google Scholar]
- 23. Dolezal BA, et al. Interrelationship between sleep and exercise: a systematic review. Adv Prev Med. 2017;2017:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Picarsic JL, et al. Self-reported napping and duration and quality of sleep in the lifestyle interventions and independence for elders pilot study. J Am Geriatr Soc. 2008;56(9):1674–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell SS, et al. Effects of a nap on nighttime sleep and waking function in older subjects. J Am Geriatr Soc. 2005;53(1):48–53. [DOI] [PubMed] [Google Scholar]
- 26. Dautovich ND, et al. Subjective and objective napping and sleep in older adults: are evening naps “bad” for nighttime sleep? J Am Geriatr Soc. 2008;56(9):1681–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lai HL. Self-reported napping and nocturnal sleep in Taiwanese elderly insomniacs. Public Health Nurs. 2005;22(3):240–247. [DOI] [PubMed] [Google Scholar]
- 28. Carrier J, et al. Effects of caffeine on daytime recovery sleep: a double challenge to the sleep-wake cycle in aging. Sleep Med. 2009;10(9):1016–1024. [DOI] [PubMed] [Google Scholar]
- 29. Bliwise DL, et al. Nocturia and disturbed sleep in the elderly. Sleep Med. 2009;10(5):540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simor P, et al. Disturbed dreaming and sleep quality: altered sleep architecture in subjects with frequent nightmares. Eur Arch Psychiatry Clin Neurosci. 2012;262(8):687–696. [DOI] [PubMed] [Google Scholar]
- 31. Martin SA, et al. Nocturia, other lower urinary tract symptoms and sleep dysfunction in a community-dwelling cohort of men. Urology. 2016;97:219–226. [DOI] [PubMed] [Google Scholar]
- 32. van Kerrebroeck P, et al. ; Standardisation Sub-committee of the International Continence Society. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):179–183. [DOI] [PubMed] [Google Scholar]
- 33. Meijlink J. ICS Committees. Standardisation Home. Terminology Discussions. Nocturia https://www.ics.org/committees/standardisation/terminologydiscussions/nocturia. Accessed October 18, 2018.
- 34. Préville M, et al. ; Scientific Committee of the ESA Study. The epidemiology of psychiatric disorders in Quebec’s older adult population. Can J Psychiatry. 2008;53(12):822–832. [DOI] [PubMed] [Google Scholar]
- 35. Folstein MF, et al. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 36. Crum RM, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- 37. Reed DL, et al. Measuring sleep efficiency: what should the denominator be? J Clin Sleep Med. 2016;12(2):263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dam TT, et al. ; Osteoporotic Fractures in Men Research Group. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56(9):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mesas AE, et al. The bidirectional association between physical activity and sleep in middle-aged and older adults: a prospective study based on polysomnography. Sleep. 2018;41(9):zsy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Redline S, et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–418. [DOI] [PubMed] [Google Scholar]
- 41. Heejung C, et al. Prevalence and risk factors of sleep disturbance in community dwelling adults in Korea. Korean J Adult Nurs. 2013;25(2):183–193. [Google Scholar]
- 42. Su TP, et al. Prevalence and risk factors of insomnia in community-dwelling Chinese elderly: a Taiwanese urban area survey. Aust N Z J Psychiatry. 2004;38(9): 706–713. [DOI] [PubMed] [Google Scholar]
- 43. Smagula SF, et al. Risk factors for sleep disturbances in older adults: evidence from prospective studies. Sleep Med Rev. 2016;25:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Finan PH, et al. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koffel E, et al. The bidirectional relationship between sleep complaints and pain: analysis of data from a randomized trial. Health Psychol. 2016;35(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tang NK, et al. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35(5):675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bjurstrom MF, et al. Polysomnographic characteristics in nonmalignant chronic pain populations: a review of controlled studies. Sleep Med Rev. 2016;26:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pressman MR, et al. Nocturia. A rarely recognized symptom of sleep apnea and other occult sleep disorders. Arch Intern Med. 1996;156(5):545–550. [DOI] [PubMed] [Google Scholar]
- 49. Bliwise DL, et al. Nocturia reported in nightly sleep diaries: common occurrence with significant implications? Health Psychol. 2014;33(11):1362–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bertisch SM, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Béland SG, et al. Benzodiazepine use and quality of sleep in the community-dwelling elderly population. Aging Ment Health. 2010;14(7):843–850. [DOI] [PubMed] [Google Scholar]
- 52. Ensrud KE, et al. ; Study of Osteoporotic Fractures Research Group. Use of selective serotonin reuptake inhibitors and sleep disturbances in community-dwelling older women. J Am Geriatr Soc. 2006;54(10):1508–1515. [DOI] [PubMed] [Google Scholar]
- 53. Salvio MA, et al. Nightmare prevalence in the healthy elderly. Psychol Aging. 1992;7(2):324–325. [DOI] [PubMed] [Google Scholar]
- 54. Mallon L, et al. Sleeping difficulties in relation to depression and anxiety in elderly adults. Nord J Psychiatry. 2000;54(5):355–360. [Google Scholar]
- 55. Nadorff MR, et al. Bad dream frequency in older adults with generalized anxiety disorder: prevalence, correlates, and effect of cognitive behavioral treatment for anxiety. Behav Sleep Med. 2014;12(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10 (Suppl 1):S7–S11. [DOI] [PubMed] [Google Scholar]
- 57. Neikrug AB, et al. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56(2):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vitiello MV, et al. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53(1):555–559. [DOI] [PubMed] [Google Scholar]
- 59. Foley DJ, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. [DOI] [PubMed] [Google Scholar]
- 60. Foley DJ, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22 (Suppl 2):S366–S372. [PubMed] [Google Scholar]
- 61. Zimmerman ME, et al. Are sleep onset/maintenance difficulties associated with medical or psychiatric comorbidities in nondemented community-dwelling older adults? J Clin Sleep Med. 2013;9(4):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 63. McCrae CS, et al. Sleep hygiene practices in two community dwelling samples of older adults. Sleep. 2006;29(12):1551–1560. [DOI] [PubMed] [Google Scholar]
- 64. Chaudhary NS, et al. Caffeine consumption, insomnia, and sleep duration: results from a nationally representative sample. Nutrition. 2016;32(11-12):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Carmassi C, et al. New DSM-5 maladaptive symptoms in PTSD: gender differences and correlations with mood spectrum symptoms in a sample of high school students following survival of an earthquake. Ann Gen Psychiatry. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hourani L, et al. Gender differences in the expression of PTSD symptoms among active duty military personnel. J Anxiety Disord. 2015;29:101–108. [DOI] [PubMed] [Google Scholar]
- 67. Carragher N, et al. Discriminant validity and gender differences in DSM-5 posttraumatic stress disorder symptoms. J Affect Disord. 2016;190:56–67. [DOI] [PubMed] [Google Scholar]
- 68. Fullerton CS, et al. Gender differences in posttraumatic stress disorder after motor vehicle accidents. Am J Psychiatry. 2001;158(9):1486–1491. [DOI] [PubMed] [Google Scholar]
- 69. Trevisan C, et al. Marital status and frailty in older people: gender differences in the Progetto Veneto Anziani longitudinal study. J Womens Health (Larchmt). 2016;25(6):630–637. [DOI] [PubMed] [Google Scholar]
- 70. Kim SH, et al. Impact of self-reported symptoms of allergic rhinitis and asthma on sleep disordered breathing and sleep disturbances in the elderly with polysomnography study. PLoS One. 2017;12(2):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Koo BB, et al. Restless legs syndrome and depression: effect mediation by disturbed sleep and periodic limb movements. Am J Geriatr Psychiatry. 2016;24(11):1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]