Abstract
Three-dimensional printing (3DP) has enabled the fabrication of tissue engineering scaffolds that recapitulate the physical, architectural, and biochemical cues of native tissue matrix more effectively than ever before. One key component of biomimetic scaffold fabrication is the patterning of growth factors, whose spatial distribution and temporal release profile should ideally match that seen in native tissue development. Tissue engineers have made significant progress in improving the degree of spatiotemporal control over which growth factors are presented within 3DP scaffolds. However, significant limitations remain in terms in pattern resolution, the fabrication of true gradients, temporal control of growth factor release, the maintenance of growth factor distributions against diffusion, and more. This review summarizes several key areas for advancement of the field in terms of improving spatiotemporal control over growth factor presentation, and additionally highlights several major tissues of interest that have been targeted by 3DP growth factor patterning strategies.
Keywords: spatiotemporal, growth factor, pattern, gradient, bioprinting, printing, fabrication
1. Introduction
Three-dimensional printing (3DP) has emerged as a powerful collection of techniques for scaffold fabrication. These methods are particularly attractive for tissue engineering and regenerative medicine, as they can create biologically-inspired constructs of a variety of clinically-relevant sizes, generate complex external and internal architectures, deposit material in highly specific locations within a construct, and incorporate a growing number of synthetic and natural materials either independently or as composite mixtures. With these capabilities in mind, tissue engineers seek to leverage 3D printing for the fabrication of templates that a) mirror complex tissue architectures found in native tissue, b) deposit spatially and temporally specific biochemical cues for different tissue types, and c) ultimately promote new tissue formation, to solve current clinical challenges. A variety of 3DP techniques have already been adapted for biomedical research, and these strategies have been extensively reviewed.(1–8)
3D bioprinting stands as the next progression of these techniques for medical applications. In bioprinting, biological and composite materials are similarly deposited layer-by-layer to create three-dimensional structures. However, in contrast to acellular and/or biologically inert scaffolds, these techniques specifically focus on the incorporation of living cells and bioactive molecules directly into the printed solutions without loss of function.(1,9) The precise spatiotemporal control offered by 3DP allows tissue engineers and clinicians to create replacement tissues and tissue templates that more effective mimic the biochemical and physical microenvironment of the native tissue.(9)
Of particular interest in bioprinting is the ability to spatially pattern different growth factors (GFs) and other bioactive proteins, and furthermore to create concentration gradients of these molecules, in order to mimic the complex developmental profiles of different native tissues. Proper incorporation of these chemical cues within tissue scaffolds directly impacts new tissue formation, and it has been shown extensively that several different GFs often operate synergistically to facilitate or inhibit tissue growth.(10–14) Additionally, correctly controlling the release and temporal presentation of growth factors is critical to ensure that functional tissues are developed with the desired phenotype. In order to progress from simple, thin tissues to highly heterogeneous tissues, interfacial regions, and whole organs, both spatial and temporal concentration profiles and gradients must be well understood.(4) In particular, musculoskeletal tissues, nervous tissue, and vasculature are comprised of a heterogeneous collection of tissues with coordinated functions.(1) For example, the osteochondral unit contains bone, cartilage, and transitional layers with gradated mechanical and biological properties, and it has been demonstrated that synergistic interactions of bone morphogenetic proteins and transforming growth factor-β (TGF-β) directly result in the proper or improper formation of all three sections. (1,15–17) Similarly, proper formation of nervous tissue is highly dependent upon accurate directional gradients of several different growth factors, including nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF).(18)
Despite the potential offered by 3D bioprinting, there remains a number of significant limitations with these techniques. Techniques for growth factor deposition are largely limited to inkjet printing, laser-assisted printing, and low-temperature extrusion bioprinting. Other techniques such as selective laser sintering and soft/stereolithography are often excluded due to their use of high temperatures, harsh solvents, or extended use of UV light, which may inhibit the activity of the bioactive molecules unless they are encapsulated within a protective shell or delivery vehicle.(9,19–21) Proper selection of a shear-thinning polymer solution must also be considered, as the shear stresses applied at the nozzle tip may impact this bioactivity. Additionally, achieving the proper resolution necessary for truly biomimetic tissue templates continues to pose a challenge. As Murphy and Atala note, the primary challenge is adequately reproducing the complexity of the ECM microenvironment so as to restore biological function.(9) Creating accurate true biochemical gradients within scaffolds also remains out of reach, as most approaches in the literature have developed bi/tri phasic scaffolds with discrete layers rather than true gradients. Finally, improvements must be made in the temporal control of growth factor presentation after deposition, as the current standard for controlled release in these scaffolds is largely passive diffusion from hydrolyzed microparticles and hydrogels.(20,21) Ultimately, it is clear that functional tissue regeneration is dependent on the accurate coordination of multiple bioactive factors, both in terms of spatial localization as well as release kinetics.(20)
In this review, we discuss the current state of growth factor patterning within 3D bioprinted scaffolds, with a specific focus on achieving spatiotemporal control of these cues and its importance to the creation of functional tissue templates. Specifically, we describe recent advances in growth factor patterning and recurrent challenges associated with these methods, including limitations in printing resolution, difficulties in maintaining controlled release profiles of growth factors, and more. Finally, we highlight studies from recent literature describing bioprinting approaches towards several target tissues and provide a summary of future work that remains in order to progress these techniques towards the end goal of generating fully functional tissues and organs.
2. Fabricating Spatiotemporal Growth Factor Patterns
Spatiotemporal growth factor patterns and gradients are critical for tissue development, especially for heterogeneous tissues such as the osteochondral unit and directionally oriented tissues such as nerves and blood vessels.(1) By fabricating scaffolds with directional gradients of one or more growth factors, one can mimic the guided migration of cells and tissue morphogenesis seen in native tissue development – e.g., during embryonic tissue development.(22) In extrusion and inkjet printing techniques, the generation of patterns and gradients is often achieved by the printing and blending of multiple “inks” containing different growth factor formulations.(1,23) In light-mediated techniques such as stereolithography (SLA), multilayer scaffolds with gradient-like presentation of growth factors can be generated by using multiple resin solutions, which must be washed out or interchanged between the processing of successive layers.(23,24) Extrusion and inkjet methods present a more facile process for growth factor gradient printing by simply switching and/or blending bioinks between subsequent layers, provided that the printing system is equipped to handle multiple printheads or cartridges.(25) The level of granularity that can be achieved in growth factor patterning is thus highly dependent on both the number of bioinks that a printing system can handle as well as the ability of the print system to blend or rapidly switch between these bioinks during printing. Furthermore, the resolution at which 3DP systems can deposit or crosslink their inks/resins of choice will, accordingly, determine the resolution at which encapsulated growth factors can be patterned. The temporal control of spatial gradients after printing is another critical issue that is dependent on the kinetics of both growth factor release and subsequent diffusion.(26) Ideally, the rate of release for a particular growth factor included in a bioprinting approach should match that of the native tissue during embryonic development.(26) Additionally, growth factor release should be tuned to produce spatial patterns that are maintained in the presence of aqueous diffusion.(27) Finally, the printing of ceramic materials and other biochemical cues in gradients can be used to complement growth factor gradients, creating more highly biomimetic environments for tissue development.(23,28)
The following discussion highlights key advances, challenges, and areas for improvement related to the spatiotemporal control of growth factor patterning – namely, printing of multiple bioinks, improving resolution of patterning, achieving temporal control of growth factor release, maintaining growth factor patterns against diffusion, co-printing biochemical gradients, and directly conjugating growth factors to scaffolds.
2.1. Printing of Multiple Bioinks
The ability to handle and print multiple bioinks is a critical prerequisite for growth factor patterning. While 3DP systems have traditionally allowed for the deposition of just a single ink or material, recent developments in extrusion and inkjet based systems have now enabled the printing of multiple inks in tandem using both custom and commercially available printers.(29) If multiple bioinks differ only in their growth factor composition, then a single printhead/nozzle may be used in combination with multiple cartridges.(25) If the bioinks differ in material composition, however – e.g., to generate physical or mechanical gradients accompanying the growth factor gradient – then multiple printheads/nozzles may be necessary to generate appropriate conditions such as temperature, pressure, etc. for each material.(1) One primary advantage of a single printhead/nozzle setup is, of course, the ability to switch rapidly between bioinks rather than having to pause in the range of 4–20 seconds for printhead/nozzle switching.(25,30) In addition to reducing the duration of printing, this allows for deposition to more closely resemble that of single, continuous flow, which helps reduce defect formation and furthermore, helps prevent errors traditionally caused by poor start/stoppage of flow or inconsistent nozzle alignment that are often seen with multi-printhead systems.(29,31) Liu et al. created one exemplary single printhead system which afforded near instantaneous switching of up to seven bioink cartridges, and furthermore allowed for coaxial fiber deposition of multiple bioink formulations.(25) In their setup, seven discrete channels, each connected to its own bioink cartridge, were fed at programmable proportions of pneumatic flow into a single dispensing printhead channel to generate fibers with coaxial distributions of several materials.(25) Excitingly, they patterned both multiple cell types and inks of varying hydroxyapatite concentration, which in the future can be used in tandem with growth factor patterning to generate complex, heterogeneous constructs for multi-unit tissues such as the osteochondral unit, which presents a gradient of both mineral composition and cell phenotype.(25) Hardin et al. developed another microfluidic printing system that enabled direct mixing of two extruded bioinks during the well-defined and tunable transition period between each ink.(31) However, their mixing of inks was achieved using polydimethylsiloxane inks with similar composition and rheological properties, and the mixing of inks of dissimilar polymer composition or behavior under flow could likely be more challenging.(31) The ability to seamlessly mix bioinks during printing is powerful because it enables the creation of true horizontal and vertical gradients by continuously adjusting the proportion of multiple bioinks, rather than simply creating pseudogradients composed of discrete layers. Tarafder et al. showcased another multi-cartridge printing approach towards temporomandibular joint (TMJ) disc regeneration in which alternating strands of poly(ε-caprolactone) (PCL) were printed with poly(lactic-co-glycolic acid) (PLGA) microspheres containing either connective tissue growth factor (CTGF) or TGF-β3.(32) Other extrusion and inkjet techniques have utilized multi-printhead systems to great success for printing bioinks with distinct temperature, pneumatic pressure, and nozzle size requirements, such as the integrated tissue-organ printer system developed by Kang et al.(33) Ultimately, single-printhead systems may be most suitable for the printing of bioinks with highly similar material compositions, while multi-printhead systems on the other hand will benefit from improvements to speed of printhead switching and the ability to maintain continuous printing when switching between bioinks with print conditions.
2.2. Improving Resolution of Growth Factor Patterning
High resolution printing is critical to the development of functional growth factor templates for tissue development – as Zhu et al., among others, have noted, printing resolution “must still be developed down to the submicron scale for effective tissue fabrication”.(34) Furthermore, distributing growth factors at the precise spatial locations where they are needed can help reduce the required concentration of growth factor loading, reducing the risks of off-site diffusion and ectopic tissue growth associated with delivering supraphysiological growth factor dosages.(35,36) As the resolution of 3DP techniques increases, so does the ability to print highly granular growth factor patterns and gradients. In general, SLA and other light-based techniques allow for the highest resolution of printing,(1) though for the aforementioned reasons, extrusion and inkjet printing are still generally preferred for their ease of patterning multiple bioink formulations. In extrusion based techniques, the resolution is limited to around 200 μm and is generally improved by using smaller diameter needles/nozzles.(25,37,38) Smaller diameter needles, in turn, require lower bioink viscosity for effective extrusion, and the viscosity of an ink formulation varies based on parameters related to the print material itself – such as molecular weight and chemical composition – to printing conditions such as temperature and pneumatic pressure.(37) Improving the resolution of printing and by extension, of growth factor patterning in extrusion-based systems, thus requires experimental determination of the optimal print conditions for one’s bioink of choice. For inkjet systems, the resolution of printing is instead determined by droplet size instead, which is presently limited to about 1 picoliter or approximately 12 μm in diameter due to physical factors of droplet generation.(39) Compared to extrusion based systems, inkjet systems typically present an improved resolution of around 20–100 μm, though the precision of ink patterning can be compromised by the inherent heterogeneity of droplet size.(38)
Advancements in the field of additive manufacturing may pave the way for higher resolution bioprinting systems. For instance, Gunduz et al. discovered that the application of ultrasonic vibrations to the nozzle of an extrusion printer could significantly reduce wall friction and flow stress, enabling the printing of high viscosity materials.(40) One could imagine this approach being used to print bioinks of a given viscosity through increasingly small-diameter needles, thereby improving the resolution with which the bioinks can be deposited. Thus, fine spatial control over growth factor patterning will benefit from novel techniques for higher resolution 3D printing.
2.3. Temporal Control of Growth Factor Release Patterns
As discussed previously, the rate of growth factor release in 3DP scaffolds should match that seen in native tissue development, meaning that each area of a growth factor gradient or pattern should have differential release kinetics. Given that growth factors are typically delivered by encapsulation in intermediate vessels such as polymeric microparticles or secondary hydrogels, the release kinetics can be tuned by utilizing different vessels for each factor or by adjusting physical and chemical parameters of the vessel materials such as their surface area and hydrolytic degradability.(26) Lee et al. demonstrated one approach in which CTGF and TGF-β3 were encapsulated in microspheres of 50:50 and 75:25 poly(lactic acid):poly(glycolic acid), respectively, and delivered in separate locations of a construct to produce a spatially and temporally distinct growth factor release profile.(41) This approach took advantage of the greater hydrolytic degradability of poly(glycolic acid) to effect more rapid release of CTGF.(41) This approach is exemplary of many similar growth factor delivery strategies that utilize carriers with differential release kinetics, and many existing reviews have summarized the advances and ongoing pitfalls associated with such strategies.(26,42,43) Ultimately, combining the multibioink systems discussed previously with differentially degradable growth factor vessels can effect a combination of spatial and temporal control over growth factor release.
However the degradation of these secondary vessels is highly dependent on interactions with the in vivo microenvironment, and in vitro experiments cannot fully model the complex biochemical and physical factors present at the site of a tissue defect.(26) It can thus be beneficial to modulate growth factor release post-implantation using external stimuli. The usage of external physical stimuli to modulate behavior of tissue engineering scaffolds has gained popularity in recent years,(44–46) though few approaches have applied this methodology so far to 3DP scaffolds. One very interesting approach by Gupta et al. utilized 3D-printed PLGA shells doped with plasmonic gold nanorods (AuNRs) to achieve photothermally mediated rupture of the shells and subsequent release of a payload from the aqueous core (Figure 1).(47) By utilizing AuNRs of different length, they could print shells with different wavelength-specificity,(47) which, in a tissue engineering context, could be utilized for the selective spatiotemporal release of multiple growth factors.
Fig 1:
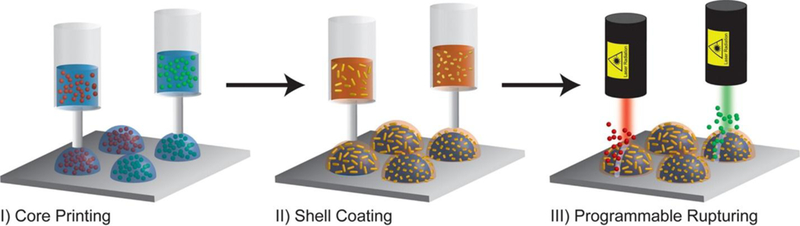
Printing of drug-loaded aqueous cores, followed by deposition of AuNR-doped PLGA shells, and then wavelength-specific rupturing of shells to effect selective release of payloads. Reproduced with permission from (47).
The fine spatial control afforded by using a laser stimulus of specific wavelength and the high degree of temporal control afforded by external control shows the promise of this method to create user-defined spatiotemporal growth factor gradients in situ, with exciting clinical implications. Drawbacks, however, include the low penetration depth of lasers and other external physical stimuli, as well as the potential cytotoxicity of reactions such as the photothermal response.(47) While some studies have been performed on the 3D printing of light-responsive additives such as AuNRs and graphene oxide or magnetically responsive additives such as iron oxide nanoparticles, further research is needed to elucidate how the patterning of these materials can be used to for external spatiotemporal control of growth factor release.(47–49)
2.4. Controlling for the Effects of Growth Factor Diffusion
One major concern in growth factor patterning is the maintenance of fabricated growth factor patterns against diffusion. Growth factor gradients are particularly susceptible to losing granularity as released growth factors diffuse and disrupt any finely tuned gradient that may have been present upon scaffold fabrication.(27) A number of factors affect the aqueous diffusion of growth factors, including the molecular weight of the growth factor, the porous architecture of the scaffold, the degradation of scaffold materials, and more. As growth factor molecular weight decreases or scaffold pore size increases, for instance, growth factors will diffuse more readily.(26) Thinner constructs, especially those below about 100–200 μm in overall thickness as a rule of thumb, experience relatively greater diffusion of growth factors and other biomolecules, which can produce ectopic effects in the surrounding tissue.(1,33,50) Furthermore, sharper gradients are likely to at least retain some bioactive effects compared to shallower growth factor gradients, which may completely lose their gradient distribution as a result of diffusion.(23) Given that a multitude of biomolecule and scaffold-specific factors affect the survival/loss of printed growth factor gradients, it is important to optimize these parameters experimentally for any scaffold by measuring the effective growth factor concentrations produced in vitro.
Some approaches, however, have utilized diffusion as a means of actually producing growth factor gradients in scaffolds that target axially oriented tissues such as nerves and blood vessels.(18,51) Johnson et al. printed two hydrogels – one loaded with nerve growth factor and the other with glial cell line-derived neurotrophic factor – at different spatial distributions along the sensory and motor pathways of a bifurcated silicone conduit and allowed diffusion to form continuous gradients of these two growth factors along the construct.(18) Interestingly, they used a drug release model for thin film hydrogel systems to predict the formation of diffusive gradients in their printed constructs.(18) Akar et al. similarly modeled platelet-derived growth factor BB (PDGF-BB) diffusion and encapsulated PDGF-BB in PLGA microspheres at one end of a poly(ethylene glycol) (PEG) construct, enabling its subsequent burst release to gradually create a PDGF-BB gradient along the entire construct when implanted in vivo (Figure 2).(51)
Fig 2:
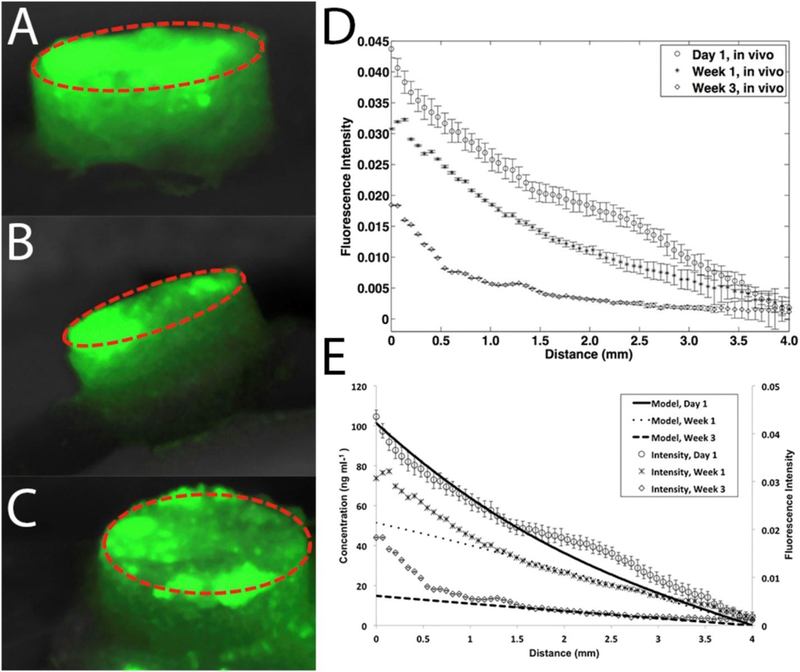
In vivo gradient of fluorescently-labeled PDGF-BB created by diffusion of the growth factor from its original area of burst release (red circle). Fluorescent images were taken at day 1 (A), day 7 (B), and day 21 (C). Also shown are the spatial distribution of growth factor fluorescence (D) as well as a comparison of the experimental growth factor distribution to the predicted distribution from mathematical modeling (E). Reproduced with permission from (51).
Mathematical models of protein diffusion can thus be utilized to produce post-fabrication growth factor gradients in 3DP scaffolds, though one must be mindful that theoretical and even in vitro models cannot fully account for the complex physical factors that may be influence aqueous diffusion in vivo. Methods that utilize diffusion to generate spatial gradients thus present a higher degree of uncertainty and potentially less applicability to in vivo scenarios compared to more conventional growth factor patterning methods that utilize spatially defined printing to directly generate the patterns.
2.5. Co-Printing of Biochemical Gradients
Growth factor gradients can be printed in tandem with other biochemical gradients to generate microenvironments that more closely resemble the composition of native tissue.(52) Calcium phosphates such as hydroxyapatite, for instance, can be printed alongside osteogenic growth factor patterns to mimic the biochemical distribution of these cues in native bone matrix.(53,54) Several examples exist in the literature where calcium phosphates have been printed in a vertical gradient with camphene,(55) poly(propylene fumarate),(56) and other scaffold materials(23) to generate biochemical distributions and physical architectures that mimic features of natural bone tissue – e.g., the transition from cortical to cancellous bone.(57) Literature is much sparser, however, on the creation of calcium phosphate gradients in combination with growth factor gradients. Ahlfeld et al. showed one case in which a two-channel plotting system was used to fabricate biphasic scaffolds containing a calcium phosphate cement (CPC) component and a vascular endothelial growth factor (VEGF)-loaded hydrogel component.(28) A gradient-like distribution was created in their scaffold by varying the proportion of CPC strands and VEGF-loaded hydrogel strands printed throughout the scaffold (Figure 3).(28) The co-printing of biochemical gradients in coordination with growth factors patterning is thus a highly unexplored approach towards biomimetic scaffold fabrication, and future studies could investigate the co-patterning of growth factors with other highly bioactive extracellular components such as glycosaminoglycans.(58)
Fig 3:
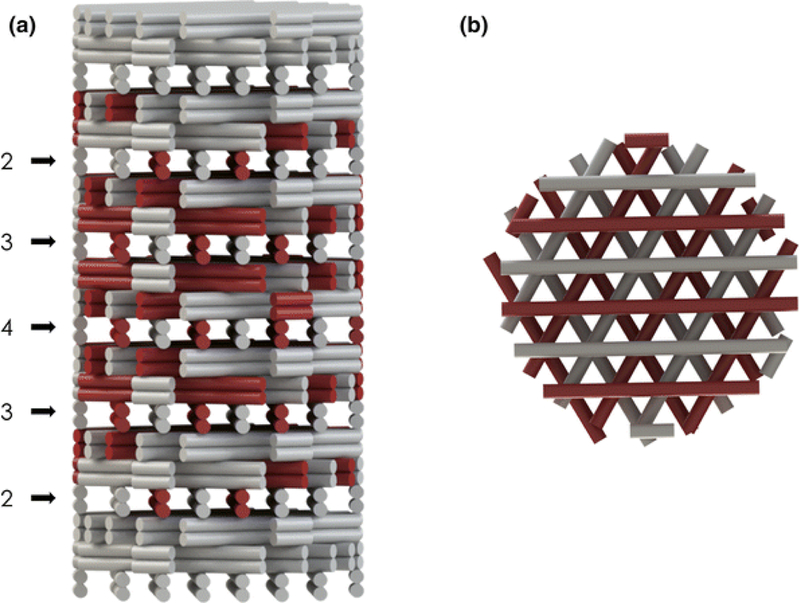
Gradient-like distribution of CPC strands (white) and VEGF-loaded alginate-gellan hydrogel strands (red) generated by a two-channel plotting system. The 3D printed scaffold is shown from side (a) and top (b) views. Reproduced with permission from (28).
2.6. Printing of Covalently Conjugated Growth Factor Patterns
An alternative approach to physical encapsulation is to covalently conjugate growth factors or bioactive peptide sequences directly to the scaffold materials, avoiding the aforementioned issues related to diffusion of soluble growth factors and effecting more constant presentation of patterned growth factors. Existing reviews have summarized the considerations related to bioconjugation of biomolecules to tissue engineering scaffolds,(59,60) and these strategies have occasionally been applied to 3DP scaffolds.(59,61,62) Gao et al., for instance, conjugated acrylated peptides to acrylated PEG during inkjet printing to generate crosslinked hydrogels with bioactive peptide presentation through the scaffold.(61) Lee et al., on the other hand, performed post-fabrication conjugation of biomolecules by using mussel-inspired adhesive chemistry to adhere bone morphogenetic protein-2 (BMP-2) to the surface of a 3D printed PCL scaffold.(62) While relatively few examples exist of biomolecule conjugation to 3DP scaffolds, the authors of this review could not find literature in which biomolecule patterns or gradients were generated on 3DP scaffolds by bioconjugation strategies. Future work in bioprinting could thus apply bioconjugation strategies such as the usage of “click” and activated ester chemistry to produce biofunctionalized inks for subsequent gradient and pattern printing.(60,63) One notable drawback of direct bioconjugation strategies, however, is the inability to temporally control growth factor presentation after scaffold fabrication, as biomolecules will be bound to the matrix until the scaffold itself degrades.(26) The relative importance of controlling growth factor release kinetics vs. maintaining constant growth factor presentation via bioconjugation should thus be considered for any tissue engineering application.
3. Target Tissues and Applications
Spatiotemporal growth factor patterning and gradient design can be used to create physiologically-inspired template scaffolds that present complex and/or sequential biochemical stimuli for the development of heterogeneous tissues.(1,10,64–67) Effective growth factor patterning requires an understanding of not only what growth factors are relevant to tissue formation and ingrowth and in what concentrations for each tissue, but also how factors interact to synergistically enhance or inhibit tissue formation. For example, it is well-understood that bone morphogenetic proteins (BMP-2, BMP-3, BMP-7) play a critical role in bone formation and remodeling. However, it is also important to note that, depending on the type of bone and location relative to other tissues, a variety of additional factors may need to be present for tissue growth, including transforming growth factors (TGF-β1, TGF-β2, TGF-β3), VEGF, and insulin-like growth factor (IGF-1) among others.(68) The specific BMP molecules involved in bone formation also vary temporally; BMP-2 is often the osteogenic signal molecule early in bone formation, while other BMPs may drive bone development at later stages.(69) Similarly, Miller et al. presented a study in which BMP-2 and IGF-2 were printed in various amounts to determine the effects of relative concentration between the two factors on cell phenotype, and the authors observed that BMP-2-induced osteogenesis was increasingly inhibited by IGF-2. (70) Fibroblast growth factor 2 (FGF-2) can also induce partially differentiated cells to upregulate other factors such as BMP-2 and VEGF, but the level of induced upregulation is highly dependent on the extent of differentiation.(71)
This section thus serves to highlight recent literature in which growth factor patterning has been applied to different target tissues. Specifically, highly heterogeneous tissues such as cartilaginous and osteochondral tissues and highly directional tissues such as nerves are considered here.
3.1. Cartilaginous and Osteochondral Tissues
As a major recurrent clinical challenge, cartilage and the osteochondral unit are target tissues of importance in bioprinting, and these tissues have been studied extensively using a number of different growth factor-based approaches over the last several years. A variety of previous reports using non-3DP hydrogels have demonstrated that bilayered composites with growth factor gradients can significantly improve cartilage and bone healing and tissue formation. (16,17,27,64,67,72–77) These types of experiments, which created primarily bi- and triphasic constructs with discrete layers, can be considered precursors to the growth factor patterning techniques discussed here, and the incorporation of 3DP techniques to target these tissues is compelling when considering the ability to much more specifically design growth factor gradients. For example, Kundu et al. used a multi-head extrusion-based printing system to pattern TGF-β within chondrocyte-encapsulated polycaprolactone/alginate.(78) After 4 weeks in vivo in a nude mouse model, it was shown that chondrocyte-containing constructs and chondrocytes+TGF-β-containing constructs had improved collagen II formation compared to positive controls, and in particular the patterning of TGF-β led to more mature cartilage formation.(78) In a different experiment, Castro et al. attempted to mimic the organization of bone and cartilage layers within the osteochondral unit by delivering chondrogenic TGF-β1 in a gradient with osteogenic hydroxyapatite.(79) In their experiment, a PEG-diacrylate solution was used as the bulk phase, with 20, 10, and 0 wt% HA in the subchondral, interface, and chondral layers respectively and TGF-β1 deposited only in the chondral layer, and it was demonstrated that the triphasic gradient scaffolds yielded improved adhesion, proliferation, and chondrogenic and osteogenic differentiation of seeded hMSCs as observed by significantly increased GAG production, type II collagen synthesis, and extracellular calcium deposition compared to controls.(79)
Focusing on the TMJ, Tarafder et al. in several studies demonstrated the ability to pattern CTGF and TGF-β3 into distinct regions within PCL scaffolds after encapsulation within PLGA microspheres.(80,81) The authors observed that despite printing at high temperatures, PLGA microsphere-encapsulated growth factors maintained their bioactivity and a consistent release over 42 days, and that rabbit TMJ scaffolds created with CTGF and TGF-β3 microspheres led to heterogeneous fibrocartilage formation after 6 weeks in culture. When applied in vivo, 4-week TMJ disc explant samples displayed recovery of the defect site, being mechanically sound with a highly-organized fibrocartilage structure mirroring native tissue.(80) In a separate study, the authors also demonstrated that in addition to heterogeneous tissue formation after CTGF and TGF-β3 release, gene upregulation was highly dependent on the dose of growth factor used, with the 100mg microsphere/g scaffold dose yielded significantly increased collagen I and II and aggrecan expression than the 50mg/g dose.(81)
3.2. Bone and Bone-Interfacial Tissues
Bone tissues are of great interest from a bioprinting perspective, as there is still a high demand for treatment of critical size bone defects in the clinic. Akkineni et al. improved a cold-plotting 3DP technique for the fabrication of biphasic, VEGF-patterned CPC scaffolds, such that the minimum feature size for printed fibers was under 200μm in diameter.(28,54) 3D plotting remains a relatively new technique. Like high temperature extrusion 3DP, plotting deposits fibers through a print nozzle corresponding to xyz instruction sets; however, plotting can be conducted at mild conditions, as the CPC paste bulk phase is a biocompatible oil and hardens upon contact with water. For this reason, bioactive molecules and cells can be incorporated without loss of activity. The authors demonstrated that the activity of incorporated VEGF was not impacted by plotting within CPC pastes, leading to increased cell density of dermal microvascular endothelial cells on printed scaffolds, and that plotted scaffolds could be set in a humid environment and maintain structural stability while avoiding microfracture defects occasionally observed when immersing in water due to swelling.(54)
In a follow-experiment, Ahlfeld et al. achieving the mentioned reduced feature size as well as printing a variety of layer-by-layer patterns, and selectively designed a biphasic scaffold with a gradient of VEGF concentration radially from the center of the construct.(28) Fahimipour et al. similarly used a room temperature printing technique to generate gelatin/alginate/β-tricalcium phosphate scaffolds with PLGA microparticle-encapsulated VEGF for craniofacial defect applications.(82) Their technique was also demonstrated to maintain growth factor activity, as osteoblast proliferation was significantly increased between 7 and 14 days for VEGF-containing scaffolds compared to positive and negative controls, and ALP activity for seeded cells was higher than either control at both timepoints.(82) Despite a hydrogel bulk phase, these scaffolds also demonstrated desired mechanical properties; determined by uniaxial compression, the composite scaffolds had a compressive modulus of 98 MPa, which is in the range of cancellous bone.
Several other studies have focused on the coordination of multiple growth factors for bone bioprinting. Ker et al., for example, selectively oriented polystyrene fibers and patterned BMP-2 and FGF-2 using an inkjet printer with desired use for tendon-bone interface engineering.(83) The authors observed that seeded populations of C2C12 and C3H10T1/2 cells each demonstrated directed differentiation based on their location; scaffold regions with no printed growth factor yielded myocyte formation, while cells seeded on BMP-2 and FGF-2 regions differentiated down osteoblast and tenocyte pathways respectively.(83) Finally, Gurkan et al. and Park et al. each investigated the effects of BMP-2/TGF-β1 and BMP-2/VEGF gradient delivery on cell fate.(84,85) In the latter study, the authors developed a biphasic scaffold using a PCL backbone with a 2% collagen/BMP-2 exterior and 10% alginate/10% gelatin/VEGF interior, with encapsulated dental pulp stem cells (DPSCs) throughout, as shown in Figure 4.(85) Four weeks after in vivo implantation in mice, it was observed that compared to the DPSC/collagen and DPSC/collagen/BMP-2 constructs, new vasculature had formed in the center of the biphasic scaffold in addition to the periphery, and that vessel formation at the periphery was significantly increased in this group as well. Finally, VE-Cadherin, VEGFR-2, ALP, and Runx2 were also significantly upregulated in the biphasic group, indicating synergistic osteogenic and vasculogenic effects.(85) .
Fig 4:
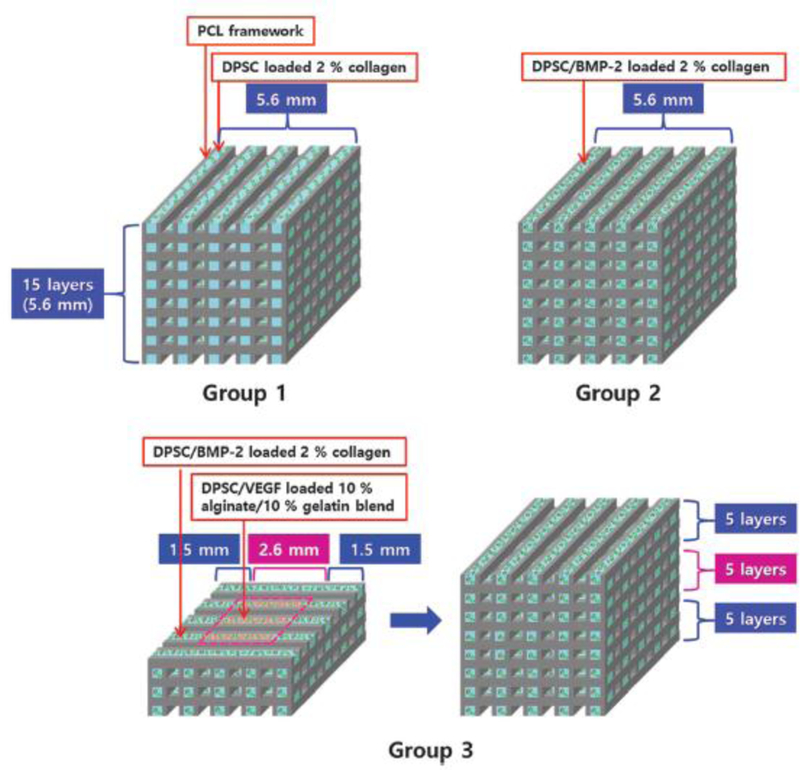
3D printed scaffold scheme, displaying uniform PCL/DPSC/collagen (Group 1), PCL/DPSC/BMP-2/collagen (Group 2), and biphasic PCL/DPSC/collagen/BMP-2 exterior PCL/DPSC/gelatin/alginate/VEGF interior (Group 3) scaffolds used by Park et al. Reproduced with permission from (85).
3.3. Nervous Tissues
Nervous tissues are a unique target tissue in that a primary challenge is achieving directional tissue development. This also makes them prime targets for spatiotemporal growth factor patterning. In addition to an accurate coordination of chemical factors, these tissues require higher printing resolution to ensure accurate macro- and micro-/submicroscopic architecture. An early study by Ilkhanizadeh et al. demonstrated that seeded neural stem cells could be controllably differentiated via growth factor delivery.(86) The authors seeded NSCs onto HydroGel™ scaffolds with inkjet bioprinted patterns of FGF-2, ciliary neurotrophic factor (CTNF), and fetal bovine serum (FBS), and it was observed that cells grown on CTNF and FBS patterned areas developed into glial and smooth muscle cells respectively, while those seeded on FGF-2 patterned areas displayed no obvious differentiation.(86) In a related study, Johnson et al. designed dual growth factor gradient scaffolds for nerve cell regeneration, using 3DP silicon casts of the sciatic nerve bifurcation, as shown in Figure 5.(18) The authors designed the bifurcation with both spatial and temporal gradients of NGF and GDNF, such that both factors were concentrated at the inlet and then deposited in progressively further locations up to the bifurcation, after which the two factors were separately printed in the proximal and distal ends to mimic sensory and motor neuron branching.(18) Prolonged release of both factors was observed out to three weeks, and in vitro, it was observed that NGF and GDNF provided directional cues to axons and Schwann cells respectively. Finally, after three months implantation in vivo in a 10mm nerve gap injury in rats, it was observed that the 3DP scaffold facilitated nerve regeneration as well as Schwann cell population in the regenerated tissue, and that rats implanted with the dual-gradient scaffolds demonstrated significantly restored function via gait analysis compared to those with control treatments.(18)
Fig 5:
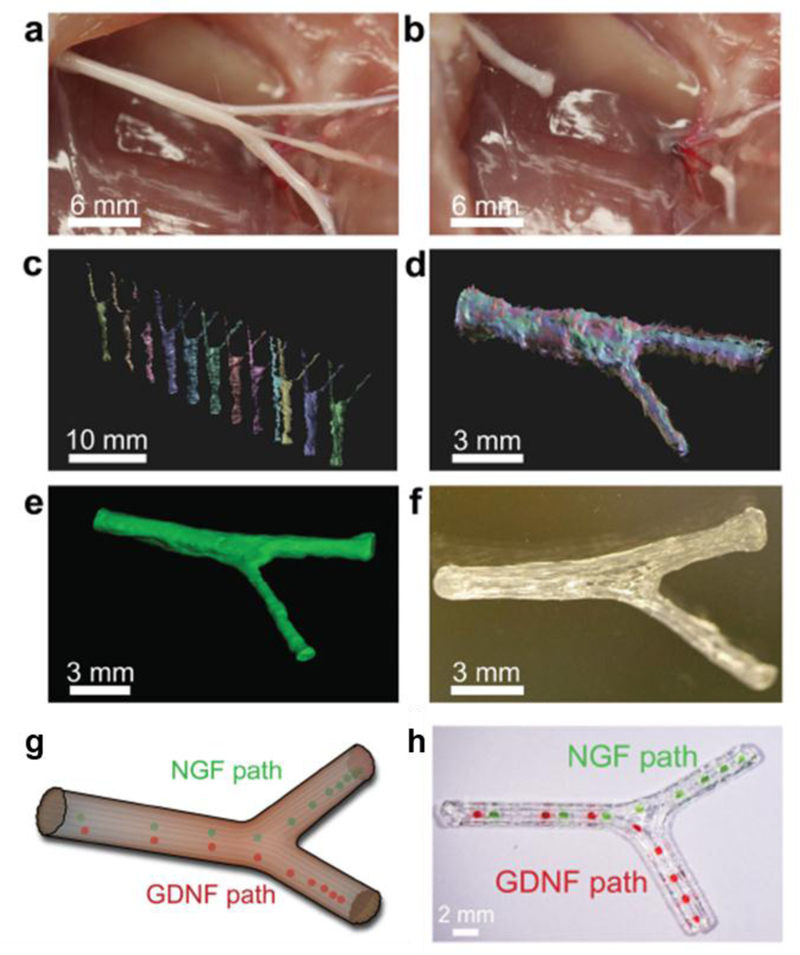
3D printed bifurcating sciatic nerve developed from computed tomography scanning used by Johnson et al. a) Sciatic nerve with branching sensory and motor nerves. b) Sciatic nerve pathway was transected for modelling. c,d) Several scans of the geometry are taken and aligned into a 3D model of the nerve pathway. e) The aligned scans are reconstructed into a full 3D template. f) Using the reconstructed images, the scaffold is printed into a model mirroring the original sciatic nerve pathway. g) Schematic of designed model with GF placement, and h) 3D bioprinted scaffolds with NGF and GDNF spatiotemporal patterns (green and red dyes used to indicate hydrogel droplet locations). Reproduced with permission from (18).
Finally, Lee et al. demonstrated the combination of coaxial electrospraying with stereolithography printing, developing bovine serum albumin (BSA) or NGF-encapsulating core-shell PLGA nanoparticles via electrospraying and then mixed into PEG/PEG-diacrylate hydrogel print solutions.(87) The authors observed that, in addition to the effects of varying the scaffold geometry, the encapsulation and delivery of NGF significantly improved neurite outgrowth compared to the blank and BSA controls.(87) Additionally, the directed extension of PC-12 cells was increased in NGF-patterned samples compared to blank scaffolds and those sprayed with non-localized NGF.(87)
Conclusions
The field of bioprinting has thus far led to the creation of highly biomimetic scaffolds that replicate the physical, biochemical, and architectural features of native tissue with a higher degree of fidelity than previously achievable. To recapitulate the dynamic array of biochemical cues seen during native tissue development, however, tissue engineers will need to achieve greater spatiotemporal control over growth factor presentation in these printed scaffolds. While significant progress has been made in the fabrication of spatially defined growth factor patterns and even growth factor gradients, notable challenges remain in several areas. The practice of growth factor patterning will greatly benefit, for instance, from improvements to the ability of print systems to handle and mix multiple bioink formulations and furthermore, to print and spatially pattern these inks at higher resolutions. Additionally, achieving true temporal control over growth factor presentation will require the development of controlled release mechanisms for multiple growth factors that can maintain these spatiotemporal distributions against the force of aqueous diffusion. Guiding growth factor release with stimuli-responsive materials may thus provide a means for greater spatiotemporal control, by using external physical cues such as light or magnetism. Other avenues which have yet to be fully explored include the co-printing of growth factor patterns with other biochemical cues such as calcium phosphates, as well as the covalent conjugation of growth factors to scaffold materials to generate biofunctionalized inks for patterned printing. By optimizing spatiotemporal control of growth factor patterning in these areas, more highly tissue-specific scaffolds can be created for heterogeneous and directionally oriented tissues such as the osteochondral unit, blood vessels, and nerve tissue. Thus, complex tissues which may have been out of reach with conventional scaffold fabrication techniques are now prime targets for repair by 3D printed scaffolds with spatiotemporally patterned growth factors.
Acknowledgements
We acknowledge support by the National Institutes of Health (P41 EB023833 and R01 AR068073) and the RegenMed Development Organization (2017-601-002) in the preparation of this work. SMB also acknowledges support from the National Science Foundation Graduate Research Fellowship Program.
Abbreviations:
- 3DP
Three-dimensional printing
- AuNRs
Plasmonic gold nanorods
- BMP
Bone morphogenetic protein
- BSA
Bovine serum albumin
- CPC
Calcium phosphate cement
- CTGF
Connective tissue growth factor
- CTNF
Ciliary neurotrophic factor
- DPSC
Dental pulp stem cell
- FBS
Fetal bovine serum
- FGF-2
Fibroblast growth factor 2
- GDNF
Glial cell line-derived neurotrophic factor
- GF
Growth factor
- IGF-1
Insulin-like growth factor 1
- NGF
Nerve growth factor
- PCL
Poly(ε-caprolactone)
- PDGF-BB
Platelet-derived growth factor BB
- PEG
Poly(ethylene glycol)
- PLGA
Poly(lactic-co-glycolic acid)
- SLA
Stereolithography
- TGF-β
Transforming growth factor β
- TMJ
Temporomandibular joint
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bittner SM, Guo JL, Melchiorri A, Mikos AG. Three-dimensional printing of multilayered tissue engineering scaffolds. Mater Today [Internet] 2018. March 20 [cited 2018 Aug 4]; Available from: http://www.sciencedirect.com/science/article/pii/S1369702117306946 [DOI] [PMC free article] [PubMed]
- 2.Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today 2013. December;16(12):496–504. [Google Scholar]
- 3.Bracaglia LG, Smith BT, Watson E, Arumugasaamy N, Mikos AG, Fisher JP. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater 2017. July 1;56:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao G, Cui X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol Lett 2016. February 1;38(2):203–11. [DOI] [PubMed] [Google Scholar]
- 5.Jakus AE, Rutz AL, Shah RN. Advancing the field of 3D biomaterial printing. Biomed Mater 2016;11(1):014102. [DOI] [PubMed] [Google Scholar]
- 6.Mandrycky C, Wang Z, Kim K, Kim D-H. 3D bioprinting for engineering complex tissues. Biotechnol Adv 2016. July;34(4):422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016. Jan;76:321–43. [DOI] [PubMed] [Google Scholar]
- 8.Sears NA, Seshadri DR, Dhavalikar PS, Cosgriff-Hernandez E. A Review of Three-Dimensional Printing in Tissue Engineering. Tissue Eng Part B Rev 2016. February 9;22(4):298–310. [DOI] [PubMed] [Google Scholar]
- 9.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32(8):773–85. [DOI] [PubMed] [Google Scholar]
- 10.Lam J, Lu S, Meretoja VV, Tabata Y, Mikos AG, Kasper FK. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater 2014. March 1;10(3):1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous Gradients of Material Composition and Growth Factors for Effective Regeneration of the Osteochondral Interface. Tissue Eng Part A 2011. August 2;17(21–22):2845–55. [DOI] [PubMed] [Google Scholar]
- 12.Duan B, Wang M. Customized Ca–P/PHBV nanocomposite scaffolds for bone tissue engineering: design, fabrication, surface modification and sustained release of growth factor. J R Soc Interface 2010. May 26;rsif20100127. [DOI] [PMC free article] [PubMed]
- 13.Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P. Microenvironments Engineered by Inkjet Bioprinting Spatially Direct Adult Stem Cells Toward Muscle- and Bone-Like Subpopulations. STEM CELLS 26(1):127–34. [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Wang J, Liu C. Biomaterials Act as Enhancers of Growth Factors in Bone Regeneration. Adv Funct Mater 26(48):8810–23. [Google Scholar]
- 15.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J Biomech 2007;40(4):750–65. [DOI] [PubMed] [Google Scholar]
- 16.Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken JJJP, et al. Technical Report: Correlation Between the Repair of Cartilage and Subchondral Bone in an Osteochondral Defect Using Bilayered, Biodegradable Hydrogel Composites. Tissue Eng Part C Methods 2015. July 15;21(12):1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam J, Lu S, Kasper FK, Mikos AG. Strategies for controlled delivery of biologics for cartilage repair. Adv Drug Deliv Rev 2015. April 1;84:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, et al. 3D Printed Anatomical Nerve Regeneration Pathways. Adv Funct Mater 25(39):6205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim JY, Kim NA, Lim DG, Kim KH, Jeong SH. Effects of thermal and mechanical stress on the physical stability of human growth hormone and epidermal growth factor. Arch Pharm Res 2015;38:1488–98. [DOI] [PubMed] [Google Scholar]
- 20.Bayer EA, Gottardi R, Fedorchak MV, Little SR. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J Controlled Release 2015. December 10;219:129–40. [DOI] [PubMed] [Google Scholar]
- 21.Nyberg EL, holmes C, Witham T, Grayson WL. Growth factor-eluting technologies for bone tissue engineering. Drug Deliv Transl Res 2016;6:184–94. [DOI] [PubMed] [Google Scholar]
- 22.Ilkhanizadeh S, Teixeira AI, Hermanson O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 2007. September 1;28(27):3936–43. [DOI] [PubMed] [Google Scholar]
- 23.Bracaglia LG, Smith BT, Watson E, Arumugasaamy N, Mikos AG, Fisher JP. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater 2017; [DOI] [PMC free article] [PubMed]
- 24.Chan V, Collens MB, Jeong JH, Park K, Kong H, Bashir R. Directed cell growth and alignment on protein-patterned 3D hydrogels with stereolithography: In this tissue engineering application, PEGbased hydrogels with different fibronectin patterns were fabricated and the influence of the protein patterns on shape and direction of seeded cells was studied. Virtual Phys Prototyp 2012;7(3):219–28. [Google Scholar]
- 25.Liu W, Zhang YS, Heinrich MA, De Ferrari F, Jang HL, Bakht SM, et al. Rapid continuous multimaterial extrusion bioprinting. Adv Mater 2017;29(3):1604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 2011;8(55):153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Controlled Release 2009;134(2):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahlfeld T, Akkineni AR, Förster Y, Köhler T, Knaack S, Gelinsky M, et al. Design and Fabrication of Complex Scaffolds for Bone Defect Healing: Combined 3D Plotting of a Calcium Phosphate Cement and a Growth Factor-Loaded Hydrogel. Ann Biomed Eng 2017. January 1;45(1):224–36. [DOI] [PubMed] [Google Scholar]
- 29.Jose RR, Rodriguez MJ, Dixon TA, Omenetto F, Kaplan DL. Evolution of bioinks and additive manufacturing technologies for 3D bioprinting. ACS Biomater Sci Eng 2016;2(10):1662–78. [DOI] [PubMed] [Google Scholar]
- 30.Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. Expert Opin Biol Ther 2007;7(8):1123–7. [DOI] [PubMed] [Google Scholar]
- 31.Hardin JO, Ober TJ, Valentine AD, Lewis JA. Microfluidic Printheads for Multimaterial 3D Printing of Viscoelastic Inks. Adv Mater Deerfield Beach Fla 2015. June 3;27(21):3279–84. [DOI] [PubMed] [Google Scholar]
- 32.Tarafder S, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication 2016;8(2):025003. [DOI] [PubMed] [Google Scholar]
- 33.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 2016;34(3):312–9. [DOI] [PubMed] [Google Scholar]
- 34.Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol 2016. August 1;40:103–12. [DOI] [PubMed] [Google Scholar]
- 35.Bayer EA, Gottardi R, Fedorchak MV, Little SR. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J Controlled Release 2015. December 10;219:129–40. [DOI] [PubMed] [Google Scholar]
- 36.Samorezov JE, Alsberg E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv Drug Deliv Rev 2015. April 1;84:45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo T, Holzberg TR, Lim CG, Gao F, Gargava A, Trachtenberg JE, et al. 3D printing PLGA: a quantitative examination of the effects of polymer composition and printing parameters on print resolution. Biofabrication 2017. April 12;9(2):024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med 2016. Sep 20;14(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derby B Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu Rev Mater Res 2010;40:395–414. [Google Scholar]
- 40.Gunduz IE, McClain MS, Cattani P, Chiu GT-C, Rhoads JF, Son SF. 3D printing of extremely viscous materials using ultrasonic vibrations. Addit Manuf 2018. August 1;22:98–103. [Google Scholar]
- 41.Lee CH, Rodeo SA, Fortier LA, Lu C, Erisken C, Mao JJ. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med 2014. December 10;6(266):266ra171–266ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park U, Kim K. Multiple growth factor delivery for skin tissue engineering applications. Biotechnol Bioprocess Eng 2017. November 1;22(6):659–70. [Google Scholar]
- 43.Prabhath A, Vernekar VN, Sanchez E, Laurencin CT. Growth factor delivery strategies for rotator cuff repair and regeneration. Int J Pharm 2018. June 15;544(2):358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai Q, Shi Y, Shan D, Jia W, Duan S, Deng X, et al. Osteogenic differentiation of MC3T3-E1 cells on poly(l-lactide)/Fe3O4 nanofibers with static magnetic field exposure. Mater Sci Eng C 2015. October 1;55:166–73. [DOI] [PubMed] [Google Scholar]
- 45.Xu H-Y, Gu N. Magnetic responsive scaffolds and magnetic fields in bone repair and regeneration. Front Mater Sci 2014. March 1;8(1):20–31. [Google Scholar]
- 46.Zhang J, Li M, Kang E-T, Neoh KG. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater 2016. March 1;32:46–56. [DOI] [PubMed] [Google Scholar]
- 47.Gupta MK, Meng F, Johnson BN, Kong YL, Tian L, Yeh Y-W, et al. 3D Printed Programmable Release Capsules. Nano Lett 2015. August 12;15(8):5321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jakus AE, Shah RN. Multi and mixed 3D-printing of graphene-hydroxyapatite hybrid materials for complex tissue engineering. J Biomed Mater Res A 2017;105(1):274–83. [DOI] [PubMed] [Google Scholar]
- 49.Martin JJ, Fiore BE, Erb RM. Designing bioinspired composite reinforcement architectures via 3D magnetic printing. Nat Commun 2015. October 23;6:8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci 2016;113(12):3179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akar B, Jiang B, Somo SI, Appel AA, Larson JC, Tichauer KM, et al. Biomaterials with persistent growth factor gradients in vivo accelerate vascularized tissue formation. Biomaterials 2015. December 1;72:61–73. [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Lipner J, Xie J, Manning CN, Thomopoulos S, Xia Y. Nanofiber scaffolds with gradients in mineral content for spatial control of osteogenesis. ACS Appl Mater Interfaces 2014;6(4):2842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li C, Jiang C, Deng Y, Li T, Li N, Peng M, et al. RhBMP-2 loaded 3D-printed mesoporous silica/calcium phosphate cement porous scaffolds with enhanced vascularization and osteogenesis properties. Sci Rep 2017. January 27;7:41331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akkineni AR, Luo Y, Schumacher M, Nies B, Lode A, Gelinsky M. 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Acta Biomater 2015. November 1;27:264–74. [DOI] [PubMed] [Google Scholar]
- 55.Ahn M-K, Moon Y-W, Maeng W-Y, Koh Y-H, Kim H-E. Design and Production of Continuously Gradient Macro/Microporous Calcium Phosphate (CaP) Scaffolds Using Ceramic/Camphene-Based 3D Extrusion. Materials 2017. June 28;10(7):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trachtenberg JE, Placone JK, Smith BT, Fisher JP, Mikos AG. Extrusion-based 3D printing of poly(propylene fumarate) scaffolds with hydroxyapatite gradients. J Biomater Sci Polym Ed 2017. April 13;28(6):532–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almela T, Brook IM, Khoshroo K, Rasoulianboroujeni M, Fahimipour F, Tahriri M, et al. Simulation of cortico-cancellous bone structure by 3D printing of bilayer calcium phosphate-based scaffolds. Bioprinting 2017. June 1;6:1–7. [Google Scholar]
- 58.Kirchmajer DM, Gorkin Iii R. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J Mater Chem B 2015;3(20):4105–17. [DOI] [PubMed] [Google Scholar]
- 59.Hajimiri M, Shahverdi S, Kamalinia G, Dinarvand R. Growth factor conjugation: Strategies and applications. J Biomed Mater Res A 2015. February 1;103(2):819–38. [DOI] [PubMed] [Google Scholar]
- 60.Ahadian S, Sadeghian RB, Salehi S, Ostrovidov S, Bae H, Ramalingam M, et al. Bioconjugated Hydrogels for Tissue Engineering and Regenerative Medicine. Bioconjug Chem 2015. October 21;26(10):1984–2001. [DOI] [PubMed] [Google Scholar]
- 61.Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol J 2015. October 1;10(10):1568–77. [DOI] [PubMed] [Google Scholar]
- 62.Lee SJ, Lee D, Yoon TR, Kim HK, Jo HH, Park JS, et al. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater 2016. August 1;40:182–91. [DOI] [PubMed] [Google Scholar]
- 63.Jiang Y, Chen J, Deng C, Suuronen EJ, Zhong Z. Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials 2014;35(18):4969–85. [DOI] [PubMed] [Google Scholar]
- 64.Guo X, Park H, Liu G, Liu W, Cao Y, Tabata Y, et al. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials 2009. May 1;30(14):2741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo X, Park H, Young S, Kretlow JD, van den Beucken JJ, Baggett LS, et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater 2010. January 1;6(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lam J, Lu S, Lee EJ, Trachtenberg JE, Meretoja VV, Dahlin RL, et al. Osteochondral defect repair using bilayered hydrogels encapsulating both chondrogenically and osteogenically predifferentiated mesenchymal stem cells in a rabbit model. Osteoarthritis Cartilage 2014. September 1;22(9):1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lam J, Clark EC, Fong ELS, Lee EJ, Lu S, Tabata Y, et al. Evaluation of cell-laden polyelectrolyte hydrogels incorporating poly(l-Lysine) for applications in cartilage tissue engineering. Biomaterials 2016. March 1;83:332–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samorezov JE, Alsberg E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv Drug Deliv Rev 2015. April 1;84:45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho T-J, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J Bone Miner Res 2002;17(3):513–20. [DOI] [PubMed] [Google Scholar]
- 70.Miller ED, Phillippi JA, Fisher GW, Campbell PG, Walker LM, Weiss LE. Inkjet printing of growth factor concentration gradients and combinatorial arrays immobilized on biologically-relevant substrates. Comb Chem High Throughput Screen 2009;12(6):604–18. [DOI] [PubMed] [Google Scholar]
- 71.Farhadi J, Jaquiery C, Barbero A, Jakob M, Schaeren S, Pierer G, et al. Differentiation-Dependent Up-Regulation of BMP-2, TGF-β1, and VEGF Expression by FGF-2 in Human Bone Marrow Stromal Cells. Plast Reconstr Surg 2005. October;116(5):1379. [DOI] [PubMed] [Google Scholar]
- 72.Guo X, Liao J, Park H, Saraf A, Raphael RM, Tabata Y, et al. Effects of TGF-β3 and preculture period of osteogenic cells on the chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in a bilayered hydrogel composite. Acta Biomater 2010. August 1;6(8):2920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 2016. March;34(3):312–9. [DOI] [PubMed] [Google Scholar]
- 74.Kim K, Lam J, Lu S, Spicer PP, Lueckgen A, Tabata Y, et al. Osteochondral tissue regeneration using a bilayered composite hydrogel with modulating dual growth factor release kinetics in a rabbit model. J Controlled Release 2013. June 10;168(2):166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken JJJP, et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 2014. October 1;35(31):8829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodrigues MT, Gomes ME, Reis RL. Current strategies for osteochondral regeneration: from stem cells to pre-clinical approaches. Curr Opin Biotechnol 2011. October;22(5):726–33. [DOI] [PubMed] [Google Scholar]
- 77.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng 2003. August;9(4):597–611. [DOI] [PubMed] [Google Scholar]
- 78.Kundu J, Shim J-H, Jang J, Kim S-W, Cho D-W. An additive manufacturing-based PCL–alginate– chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med 2015. November 1;9(11):1286–97. [DOI] [PubMed] [Google Scholar]
- 79.Castro NJ, O’Brien J, Zhang LG. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale 2015;7(33):14010–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tarafder S, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication 2016;8(2):025003. [DOI] [PubMed] [Google Scholar]
- 81.Legemate K, Tarafder S, Jun Y, Lee CH. Engineering Human TMJ Discs with Protein-Releasing 3DPrinted Scaffolds. J Dent Res 2016. July 1;95(7):800–7. [DOI] [PubMed] [Google Scholar]
- 82.Fahimipour F, Rasoulianboroujeni M, Dashtimoghadam E, Khoshroo K, Tahriri M, Bastami F, et al. 3D printed TCP-based scaffold incorporating VEGF-loaded PLGA microspheres for craniofacial tissue engineering. Dent Mater 2017. November 1;33(11):1205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ker ED, Nain AS, Weiss LE, Wang J, Suhan J, Amon CH, et al. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials 2011;32(32):8097–107. [DOI] [PubMed] [Google Scholar]
- 84.Gurkan UA, El Assal R, Yildiz SE, Sung Y, Trachtenberg AJ, Kuo WP, et al. Engineering Anisotropic Biomimetic Fibrocartilage Microenvironment by Bioprinting Mesenchymal Stem Cells in Nanoliter Gel Droplets. Mol Pharm 2014. July 7;11(7):2151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Young Park J, Shim J-H, Choi S-A, Jang J, Kim M, Hwa Lee S, et al. 3D printing technolgy to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J Mater Chem B 2015;3(27):5415–25. [DOI] [PubMed] [Google Scholar]
- 86.Ilkhanizadeh S, Teixeira AI, Hermanson O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 2007. September 1;28(27):3936–43. [DOI] [PubMed] [Google Scholar]
- 87.Lee S, Zhu W, Heyburn L, Nowicki M, Harris B, Zhang LG. Development of Novel 3-D Printed Scaffolds With Core-Shell Nanoparticles for Nerve Regeneration. IEEE Trans Biomed Eng 2017. February;64(2):408–18. [DOI] [PubMed] [Google Scholar]


