ABSTRACT
β-Alanine supplementation is one of the world's most commonly used sports supplements, and its use as a nutritional strategy in other populations is ever-increasing, due to evidence of pleiotropic ergogenic and therapeutic benefits. Despite its widespread use, there is only limited understanding of potential adverse effects. To address this, a systematic risk assessment and meta-analysis was undertaken. Four databases were searched using keywords and Medical Subject Headings. All human and animal studies that investigated an isolated, oral, β-alanine supplementation strategy were included. Data were extracted according to 5 main outcomes, including 1) side effects reported during longitudinal trials, 2) side effects reported during acute trials, 3) effect of supplementation on circulating health-related biomarkers, 4) effect of supplementation on skeletal muscle taurine and histidine concentration, and 5) outcomes from animal trials. Quality of evidence for outcomes was ascertained using the Grading of Recommendations Assessment Development and Evaluation (GRADE) framework, and all quantitative data were meta-analyzed using multilevel models grounded in Bayesian principles. In total, 101 human and 50 animal studies were included. Paraesthesia was the only reported side effect and had an estimated OR of 8.9 [95% credible interval (CrI): 2.2, 32.6] with supplementation relative to placebo. Participants in active treatment groups experienced similar dropout rates to those receiving the placebo treatment. β-Alanine supplementation caused a small increase in circulating alanine aminotransferase concentration (effect size, ES: 0.274, CrI: 0.04, 0.527), although mean data remained well within clinical reference ranges. Meta-analysis of human data showed no main effect of β-alanine supplementation on skeletal muscle taurine (ES: 0.156; 95% CrI: −0.38, 0.72) or histidine (ES: −0.15; 95% CrI: −0.64, 0.33) concentration. A main effect of β-alanine supplementation on taurine concentration was reported for murine models, but only when the daily dose was ≥3% β-alanine in drinking water. The results of this review indicate that β-alanine supplementation within the doses used in the available research designs, does not adversely affect those consuming it.
Keywords: carnosine, taurine, histidine, paraesthesia, safety, adverse effects
Introduction
The primary role of carnosine (β-alanine-l-histidine) in skeletal muscle metabolism is to act as an intracellular buffer (1), with additional potential actions including reduction of reactive oxygen/nitrogen species, and/or calcium regulation (2, 3). β-Alanine availability is the limiting step in intramuscular carnosine synthesis. Accordingly, supplementation increases intramuscular carnosine concentration (4, 5), the ergogenic potential of which is well established. A recent meta-analysis confirmed the efficacy of β-alanine supplementation to improve high-intensity exercise performance, with optimum benefit reported for capacity-based assessments lasting between 30 s and 10 min (6). Indeed, β-alanine is 1 of just 5 sports supplements recognized by the International Olympic Committee as having sufficient evidence of efficacy to warrant its use in specific situations (7). Additionally, therapeutic supplementation with β-alanine is gaining in popularity. Recently, the therapeutic potential of carnosine was reviewed (8) and a wide range of targets and conditions that may be improved by β-alanine or carnosine supplementation were highlighted. These included protection against the effects of senescence (9), conveying a neuroprotective influence (10, 11), inhibition of tumor growth (12), improved clinical outcomes in participants suffering from Parkinson's disease (13), enhanced glucose sensitivity (14), and accelerated recovery after acute kidney failure (15). Much of this evidence was based on animal or in vitro models, and the efficacy of β-alanine supplementation to meaningfully impact these parameters, or indeed to increase carnosine concentration in tissues other than the skeletal muscle, has yet to be ascertained. The therapeutic potential of β-alanine supplementation represents a topical and exciting progression of the current evidence base, and research in this area is likely to exponentially increase in the coming years, as ever-more targets are identified for this pleiotropic nutritional agent.
In contrast to a large, and increasing, evidence base for ergogenic and therapeutic effects of β-alanine supplementation, limited information is available on the safety of this nutritional strategy. Regular risk assessment of common nutritional supplements and ergogenic aids is essential as nutrients generally exert a biphasic dose response, whereby optimal intakes exert a stimulatory and beneficial response, whereas lower or higher intakes may be harmful or inhibitory. Theoretical concerns related to an excess intake of β-alanine include a possible reduction of tissue taurine and/or free histidine concentration. Reduced intracellular taurine may occur as elevated β-alanine availability increases competition for their shared transporter, Tau-T (16). Histidine is also required for carnosine synthesis, and if not matched by dietary intake, the free histidine pool may become depleted as a result of chronic β-alanine supplementation (17). In addition, β-alanine supplementation has been reported to cause acute paraesthesia, which has been described as an uncomfortable sensation on the surface of the skin that occurs within 10–20 min after ingestion (4). Little is known, however, about the occurrence or physiological consequences of these outcomes. The aim of the current investigation was to undertake a systematic risk assessment of β-alanine supplementation, comprising comprehensive review and analysis procedures, to synthesize and evaluate all available evidence from both human and animal trials.
Methods
This risk assessment followed recommendations from the Council for Responsible Nutrition Vitamin and Mineral Safety Handbook, third edition (18), which are commonly used to risk assess nutritional supplements (19, 20). The protocol was designed in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (21). The review was prospectively registered in an international register of systematic reviews (PROSPERO registration no. CRD42017071843).
Study selection
Study selection was guided by the Population, Intervention, Comparator, Outcomes and Study Design (PICOS) approach, and the criteria within each of these categories were as follows:
Population: Human populations were restricted to healthy individuals of any age or activity level. Animal models were considered for inclusion only if conducted on healthy, wild-type mammals.
Intervention: Original studies investigating the effects of isolated oral β-alanine supplementation interventions were considered for inclusion in the review.
Comparator: No human comparators were required, but randomized, blinded, placebo-controlled studies were assigned a higher quality rating and prioritized in the interpretation of results.
Outcomes: Human data were analyzed according to 4 outcomes, namely 1) side effects reported during longitudinal trials, 2) side effects reported during acute trials, 3) effect of supplementation on circulating health-related biomarkers, and 4) effect of supplementation on skeletal muscle taurine and histidine concentration. For the animal trials, data related to species, dosing strategy, study aims, and primary outcomes were extracted. Dosing strategy was reported as intervention length (d), the concentration of β-alanine provided in the drinking water (%) or chow, and the total dose ingested by each animal (mg β-alanine/g body weight). Daily intake (g/d) was based on the mean weight of the animals in the β-alanine group, estimated as the mean of the start and end weights reported. If not reported, weights were estimated using normative data from the same strain (22). If specific fluid intakes were not reported, these were estimated assuming an intake of 0.1 mL/g for rats, and 0.15 mL/g for mice (23).
Study Design: Only studies that used an intervention-based design were included within the review.
Search strategy
A 3-stage screening strategy (title/abstract screening, full-text screen, full-text appraisal) was independently undertaken by 2 reviewers. The search was conducted using 4 databases (Medline, Embase, Sport Discus, and Web of Science) with the terms “beta alanine” OR “carnosine” concatenated with “intervention” OR “trial” OR “supplementation” OR “health” OR “safety” OR “paraesthesia” OR “taurine” OR “side-effect” OR “adverse effect” OR “toxicity.” In addition, Medical Subject Heading searches, with the key-term beta-alanine were conducted using Medline and Embase, and with database-specific subheadings. Searches were limited to original studies in English published between 1980 and 2018. The final searches were completed in September 2018.
Quality appraisal and data extraction
All data were extracted using a pre-piloted spreadsheet, and independently verified by a second member of the review team. Quality ratings for outcomes 1 and 2 (side effects reported in all acute and longitudinal human trials) were assigned using the recommendations of the Grading of Recommendations Assessment Development and Evaluation (GRADE) working group (24). An a priori ranking of high, moderate, or low was assigned, based on whether the study was a randomized placebo-controlled trial, a nonrandomized placebo-controlled trial, or a nonrandomized and nonplacebo-controlled trial. Studies were also provided an a priori ranking of high quality if they used a matched pair allocation design. Studies were then assessed, and downgraded a level if appropriate, based on the response to 3 questions: Q.1) Were participants blinded to the treatment? Q.2) Were side effects reported in the study? Q.3) Were participants specifically instructed to report side effects? This procedure allowed the quality of evidence for each outcome to be categorized as “high,” “moderate,” “low,” or “very low.”
Data analysis
All meta-analyses were conducted within a Bayesian framework enabling studies with zero events to be included without requiring correction factors. In addition, Bayesian methods enabled log odds ratios to be modelled without assuming a normal distribution and provided an efficient means of downweighting potentially biased studies, that is, those without a control condition (25). Hierarchical Bayesian random effects models were used to meta-analyze outcome data on cases reporting paraesthesia and dropouts. Binomial specifications were used at the first level of the model to estimate probability of an event, with parameters allowed to vary across studies. Intercept terms for logit transformed probabilities were estimated for the control comparison and an additional effect term included for active supplementation. Effect terms were assumed to follow a normal distribution at the second level of the hierarchical model, with the mean representing the average log odds ratio across all studies and the variance indicating study-to-study variability. Noninformative normal and uniform priors were used for the mean and variance parameters, respectively.
The effects of β-alanine supplementation on tissue taurine and histidine concentration in human and animal populations were quantified using standardized mean difference effect sizes. Standard formulae for raw score effect sizes and associated sampling variances were used for independent groups posttest (animal studies only), single group pretest-posttest (26), and pretest-posttest-control study designs (27). Observed effect sizes were assumed to follow a normal distribution with mean identified by hyperparameters representing the average effect across all studies and variance indicating study-to-study variability. To control for potential bias in human studies featuring noncontrolled designs, a sensitivity analysis was included comprising downweighting of effect sizes through a hierarchical power prior model (28). Finally, a meta-regression controlling for daily dose (≤3%) and total cumulative dose (mg β-alanine/g body weight) was included for animal studies that measured taurine concentration post supplementation. Inferences from all models were performed on posterior samples generated by Markov Chain Monte Carlo with Bayesian 95% credible intervals (CrIs) constructed to enable probabilistic interpretations of parameter values. Models were run in OpenBUGS (version 3.2.3, MRC Biostatistics Unit) and in R (version 3.3.1 R Development Core Team) using the R2OpenBugs package.
Results
Study characteristics
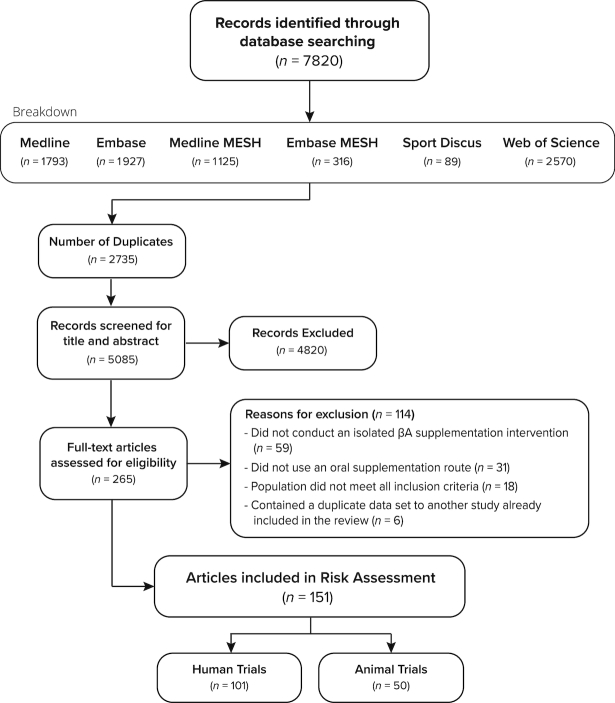
One hundred and one human intervention studies [94 longitudinal and 8 acute, with 1 study comprising both acute and longitudinal arms; (4)] and 50 animal studies were included (see Figure 1). In total 2,268 humans were included in the final analyses (1,820 men and 448 women), with 1,295 of these consuming the active β-alanine supplement. The majority of studies were conducted on healthy young adults, and participants had a median (IQR) age of 23.5 (5.5) y. Seven studies were conducted using a population with a mean age >50 y (9, 29–34) and 5 studies were conducted using adolescent populations [mean age 10–19 y (35–39)]. The majority of longitudinal studies were conducted using athletic (48%) or recreationally trained (34%) groups, with the remaining described as being untrained (8%) or undefined (10%). Further information related to the training type and status of participants is provided in Supplemental Tables 1 and 2. Three inclusions within the review were based on data sourced outside of the described search-strategy. Two of these were presented at international conferences (40, 41) and the other was a doctoral thesis, (42). The decision to include these data sets was based on their relevance to the topic area, along with the completeness of data and design information available.
FIGURE 1.
Search flow diagram.
Outcome 1: side effects reported during longitudinal trials
Ninety-four longitudinal studies, comprising 99 outcomes, were identified, and are described in Supplemental Table 1. The median (IQR: range) intervention period and daily dose was 28 (14: 7–168) d, and 6 (1.65: 1.6–12) g/d, resulting in a total cumulative dose of 179.2 (60.5: 34.3–1075.2) g. The quality of evidence for side effects reported was primarily moderate or low (23% high, 34% moderate, 33% low, 9% very low; Figure 2A). Of the 91% of studies initially allocated an a priori rating of “high quality,” most were subsequently downgraded based on the secondary nature of side effect reporting, which resulted in the provision of limited information regarding side effects experienced and the mode of assessment. Meta-analysis of withdrawal rates between participants allocated to β-alanine or placebo groups were nonsignificant (OR: 0.72; 95% CrI: 0.50, 1.05). Two additional sensitivity analyses were conducted after: 1) removing data from studies that reported very high withdrawal rates from both groups (30, 43) (OR: 0.67; 95% CrI: 0.39, 1.01) and 2) including data only from studies that specifically reported withdrawal information (OR: 0.74; 95% CrI: 0.45, 1.05). These sensitivity analyses did not alter the original findings. Analysis of incidence of paraesthesia was conducted with data from studies that were assigned a “high” quality rating only (n = 22, with 285 and 219 participants assigned to the β-alanine and placebo groups, respectively). Incidence of paraesthesia was 18.6% in the active treatment group and 5.7% in the placebo group. Meta-analysis of reported incidences of paraesthesia demonstrated a significantly increased likelihood of paraesthesia reporting with active supplementation (OR: 8.9; 95% CrI: 2.2, 32.6). Wide variation was evident in both the incidence and severity of paraesthesia symptoms. This finding, along with wide heterogeneity in study design, dosing protocols, compliance monitoring, and mode of side effect reporting precluded statistical identification of factors that moderated paraesthesia. One longitudinal study examined paraesthesia occurrence when participants were provided a fixed dose of 6 g/d of β-alanine for 28 d, in either sustained or rapid release formulations (44). The group who ingested the rapid release formulation reported a more frequent paraesthesia occurrence than those who consumed the sustained release formulation. In contrast, the sustained release group reported a similar paraesthesia occurrence to the placebo group. No differences in compliance were identified between the groups.
FIGURE 2.
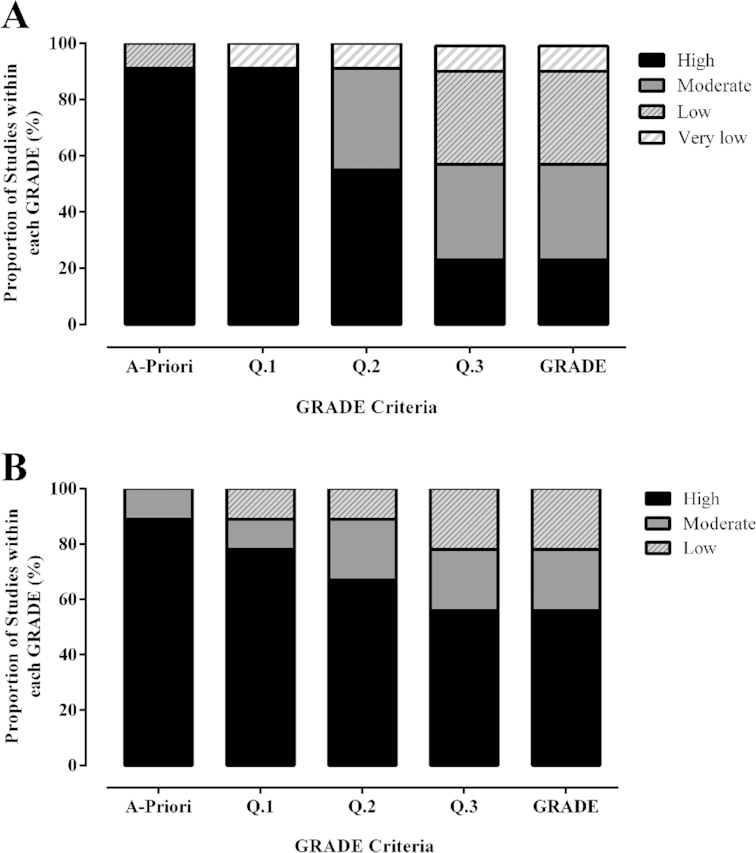
Grading of Recommendations Assessment Development and Evaluation (GRADE) quality rating of outcomes from longitudinal (A) and acute (B) trials conducted on healthy humans. Each bar represents the proportion of articles assigned a “high,” “moderate,” “low,” or “very low” quality rating, for side effects reported from 94 longitudinal (A) and 8 acute (B) trials. The x-axis represents the different stages of the GRADE process, with Q. 1–3 indicating the questions asked to determine risk of bias in each study, with the final bar representing the cumulative GRADE.
Outcome 2: side effects reported during acute trials
Eight studies, comprising 9 outcomes, that reported data related to side effects experienced during acute β-alanine supplementation were identified (4, 42, 45–51), and are described in Supplemental Table 2. The median (IQR: range) dose ingested was 1.6 (0.38: 0.8–3.2) g and the quality of evidence for this outcome measure was primarily “high” (56% high, 22% moderate, 22% low, Figure 2B). Statistical meta-analyses of outcomes were not conducted because of the small number of studies available, combined with large heterogeneity in study design and outcome measures, and so a narrative synthesis is presented. Similar to longitudinal trials, paraesthesia was the only side effect reported. The extent and time to peak blood β-alanine concentration emerged as the primary determinant of the occurrence and intensity of paraesthesia. This was first investigated by Harris et al. (4), who administered different acute β-alanine forms and doses. β-Alanine [40 mg/kg body mass (BM)] ingested in the form of carnosine and anserine contained in chicken broth did not result in paraesthesia, while an equivalent intake of β-alanine in its pure form invoked responses of tingling, itch, and irritation, representative of paraesthesia. Response occurred in a dose-related manner with 40 mg/kg BM (∼3.2 g) causing sensations that were considered unpleasant by all participants, and intolerable by 2. In contrast, lower doses (10 and 20 mg/kg BM/∼0.8 and 1.6 g) invoked similar sensations, but of milder intensities. Décombaz et al. (46), investigated response to an equivalent β-alanine intake (1.6 g), provided in slow-release capsules or in its pure form dissolved in aqueous solution. Participants completed questionnaires related to paraesthesia symptoms in parallel with blood sampling. Only β-alanine in solution produced evident sensations, with the intensity described as “pins and needles.” Sensory response anticipated and paralleled that of plasma β-alanine concentration, and paraesthesia symptoms were influenced by the extent and time to peak plasma β-alanine concentration. Stautemas et al. (51) investigated the influence of acute ingestion of a fixed (1.4 g) compared with a weight-relative (10 mg/kg BM) β-alanine dose on β-alanine pharmacokinetics. Paraesthesia was not reported by any participant consuming the weight-relative dose, whereas 2 of the 28 participants experienced paraesthesia when they consumed the fixed dose, the timing of which matched their individual maximum blood BA concentration recorded (Cmax). Some evidence exists suggesting that ethnicity, sex (48), or the individual's body size (42) may moderate the occurrence, or intensity, of paraesthesia experienced. More specifically, Asians, women, and lighter individuals (<75kg) reported stronger or more frequent experience of paraesthesia compared to Caucasians, men, and men who weighed >85 kg.
Outcome 3: effect of β-alanine supplementation on health-related biomarkers
Seven studies reported data on the influence of β-alanine supplementation on circulating health-related biomarkers (4, 9, 30, 41, 52–54), comprising 220 individuals, with 87 of these taking the active β-alanine supplement. These studies used a median (IQR) total cumulative dose of 179.2 (84) g. Studies were conducted using older male and female participants (9, 30), healthy young males (4, 41, 52), healthy young men and women (53), or trained cyclists (54). No individual study reported a significant change to any of the measured biomarkers. Meta-analyses were conducted on any marker that was measured in ≥2 studies and results are presented in Table 1. A statistically significant effect of β-alanine supplementation was obtained for alanine aminotransferase [ALT; effect size (ES): 0.274; 95% CrI: 0.04, 0.527], and trends toward significantly increased alkaline phosphatase (ALP; ES: 0.434; 95% CrI: −0.067, 0.811) and sodium (ES: 0.497; 95% CrI: −0.033, 1.063) were also observed. Additionally, Harris et al. (4) conducted a 12-lead ECG, and reported no change to cardiac function after a 4-wk β-alanine supplementation intervention.
TABLE 1.
Circulating health-related biomarker response to β-alanine supplementation in healthy humans1
| Marker (n β-alanine/n placebo) | ES (95% CrI) | Tau (50% CrI) | Marker (n β-alanine/n placebo) | ES (95% CrI) | Tau (50% CrI) |
|---|---|---|---|---|---|
| Albumin (176/137) | −0.257 (−0.642, 0.130) | 0.551 (0.420, 0.651) | MCHC (77/80) | 0.153 (−0.242, 0.512) | 0.298 (0.129, 0.407) |
| ALP (176/137) | 0.434 (−0.067, 0.811) | 0.462 (0.325, 0.574) | MCV (77/80) | 0.014 (−0.291, 0.323) | 0.189 (0.066, 0.262) |
| ALT (159/115) | 0.274 (0.04, 0.527) | 0.187 (0.08, 0.262) | Monocytes (61/54) | 0.398 (−0.685, 1.479) | 1.214 (0.765, 1.487) |
| AST (188/143) | 0.056 (−0.74, 0.283) | 0.207 (0.09, 0.292) | Neutrophils (61/54) | −0.400 (−0.820, 0.022) | 0.288 (0.104, 0.394) |
| Basophils (61/54) | 0.265 (−0.427, 0.946) | 0.670 (0.390, 0.845) | Platelets (208/163) | −0.085 (−0.266, 0.101) | 0.101 (0.040, 0.142) |
| CK (102/54) | −0.165 (−0.537, 0.206) | 0.270 (0.107, 0.372) | Potassium (57/60) | −0.513 (−1.183, 0.250) | 0.609 (0.299, 0.774) |
| Creatinine (188/143) | −0.028 (−0.226, 0.173) | 0.152 (0.057, 0.220) | RBC (77/80) | −0.043 (−0.354, 0.265) | 0.181 (0.066, 0.248) |
| Eosinophils (61/54) | −0.080 (−1.745, 1.591) | 2.357 (1.621, 2.785) | RDW(48/52) | −0.053 (−0.584, 0.469) | 0.325 (0.088. 0.398) |
| GFR (119/77) | 0.048 (−0.256, 0.346) | 0.181 (0.063, 0.252) | Sodium (57/60) | 0.497 (−0.033, 1.063) | 0.392 (0.132, 0.511) |
| Globulin (155/117) | −0.028 (−0.265, 0.196) | 0.153 (0.063, 0.214) | Bilirubin (69/66) | −0.285 (−0.800, 0.212) | 0.442 (0.232, 0.571) |
| Hematocrit (81/78) | 0.075 (−0.224, 0.375) | 0.166 (0.060, 0.227) | Protein (147/108) | 0.066 (−0.186, 0.327) | 0.209 (0.095, 0.292) |
| Hemoglobin (89/86) | 0.058 (−0.288, 0.392) | 0.180 (0.057, 0.246) | Urea (41/34) | 0.178 (−0.881, 1.193) | 0.861 (0.364, 1.064) |
| LDH (131/83) | 0.018 (−0.292, 0.335) | 0.237 (0.094, 0.330) | Uric acid (119/77) | −0.110 (−0.410, 0.205) | 0.209 (0.078, 0.291) |
| Lymphocytes (61/54) | 0.022 (−0.478, 0.507) | 0.394 (0.168, 0.530) | WBC (77/80) | −0.220 (−0.545, 0.093) | 0.199 (0.072, 0.277) |
| MCH (77/80) | 0.078 (−0.248, 0.397) | 0.205 (0.074, 0.283) | — | — | — |
1Meta-analyses conducted on any circulating health-related biomarker that was measured in ≥2 independent studies. n refers to the number of observations included in each analysis for the β-alanine or placebo groups. ES (CrI) refers to the effect size and credible interval for each outcome. Tau is a heterogeneity statistic, that estimates the extent of variation among observed effects. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate transaminase; CK, creatine kinase; CrI, credible interval; ES, effect size; GFR, glomerular filtration rate; LDH, lactate dehydrogenase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean cell volume; RBC, red blood cell; RDW, red cell distribution width; WBC, white blood cell.
Outcome 4: effect of β-alanine supplementation on skeletal muscle taurine and histidine concentration (human data)
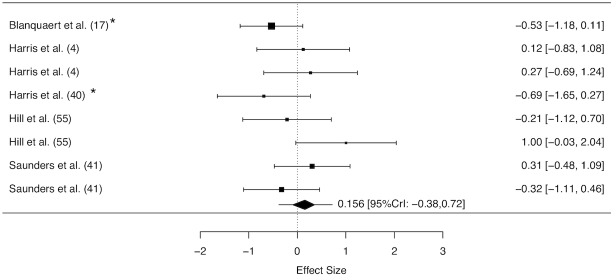
Five studies reported data on the effect of β-alanine supplementation on taurine (4, 17, 40, 41, 55) and 4 on histidine (17, 44, 53, 56) concentration. Skeletal muscle taurine concentration was measured in 63 individuals, with 45 allocated to the active β-alanine supplement, whereas histidine concentration was measured in 73 individuals, with 55 allocated to the active β-alanine supplement. Meta-analyses indicated that the β-alanine supplementation protocols employed did not exert a main effect on skeletal muscle taurine (ES: 0.156; 95% CrI: −0.38, 0.72, Figure 3) or histidine (ES: −0.15; 95% CrI: −0.64, 0.33) concentration. Sensitivity analyses conducted to control for potential bias in studies not including a placebo group did not meaningfully change results attained for any of these parameters (data not shown).
FIGURE 3.
Forest plot displaying the effect of β-alanine supplementation on skeletal muscle taurine concentration in humans. The study-specific intervals represent individual effect size estimates and sampling error. The diamond represents the pooled estimate generated with Bayesian inference along with the 95% credible interval (95% CrI). This analysis included 83 observations (63 β-alanine/18 placebo).
Outcome 5: outcomes from animal studies
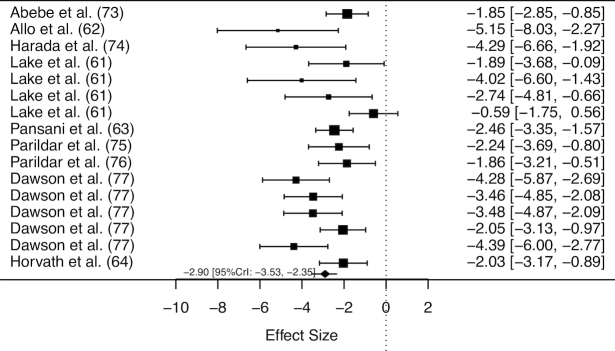
Fifty animal studies were included in the review, and an overview of these studies is provided in Supplemental Table 3. Meta-analyses of all studies including data on tissue taurine concentration in both β-alanine supplemented and pair-fed control murines indicated a main effect of β-alanine supplementation on taurine concentration (ES: −1.94; 95% CrI: −2.39, −1.52). Substantial variation existed in relation to the potential effect of moderators including daily dose, intervention duration, total cumulative dose, and tissue type. After a sequential modelling approach to account for potential moderators and reduce between-study heterogeneity, a final meta-regression was performed using data from cardiac or skeletal muscle only, and included a binary variable (daily dose: <3% or ≥3% of β-alanine in drinking water), and a covariate [total cumulative dose (TCD) mg β-alanine/g body weight]. No effect of β-alanine supplementation on taurine concentration was shown when a daily dose of <3% was ingested (ES: −0.32, 95% CrI: −0.80, 0.14, Figure 4), whereas a dose of 3% induced a significant reduction to tissue taurine concentration (ES: −2.90, 95% CrI: −3.53, −2.35, Figure 5). The difference between these doses was statistically significant (ES: −2.35; 95% CrI: −3.27, −1.48). No effect of TCD (ES: −0.007; 95% CrI: −0.030, 0.017) was obtained, nor was there an interaction between daily dose and TCD (ES: 0.021; 95% CrI: −0.010, 0.051). Only 1 study reported data on the effect of β-alanine supplementation on tissue histidine concentration (57). This study provided data on histidine concentration in 5 brain sites, and no effect of β-alanine supplementation was identified (ES: 0.57; 95% CrI: −0.24, 1.39).
FIGURE 4.
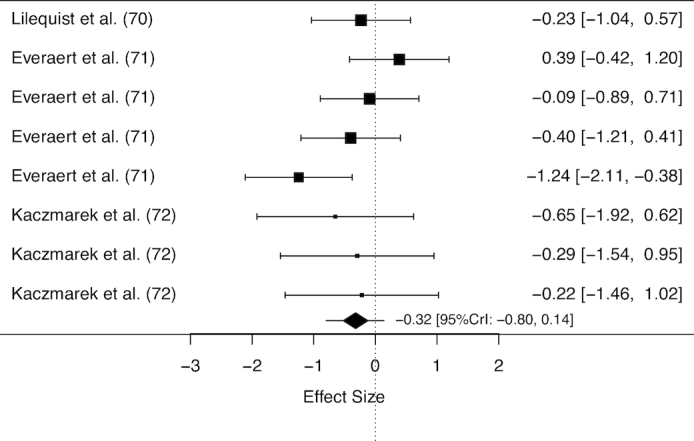
Forest plot displaying the effect of β-alanine supplementation (<3% in drinking water) on taurine concentration in murine cardiac or skeletal muscle. The study-specific intervals represent individual effect size estimates and sampling error. The diamond represents the pooled estimate generated with Bayesian inference along with the 95% credible interval (95% CrI). This analysis included 126 observations (63 β-alanine/63 placebo).
FIGURE 5.
Forest plot displaying the effect of β-alanine supplementation (≥3% in drinking water) on taurine concentration in murine cardiac or skeletal muscle. The study-specific intervals represent individual effect size estimates and sampling error. The diamond represents the pooled estimate generated with Bayesian inference along with the 95% credible interval (95% CrI). This analysis included 249 observations (127 β-alanine/122 placebo).
Discussion
No adverse effects of oral β-alanine supplementation, within the doses and intervention durations investigated, were identified within this systematic risk assessment. Meta-analysis of animal data indicates that β-alanine supplementation of ≥3% is required to reduce cardiac or skeletal muscle taurine concentration, and that this reduction is not impacted by intervention duration. Meta-analysis of human data showed no effect of β-alanine supplementation on skeletal muscle taurine or histidine concentration, which is likely to be because of the lower relative doses employed in human studies. Paraesthesia was the only side effect identified during human trials; however, no evidence exists to indicate that this is harmful, and thus it is not considered to represent an adverse event. Participants in the active treatment groups were not found to experience higher dropout rates than those consuming a placebo. Although a significant effect of β-alanine supplementation on circulating ALT concentration was identified, the effect was small, and reported values remained well within clinical reference ranges. Supplementation did not meaningfully alter any of the other health-related biomarkers reported.
Effect of β-alanine supplementation on circulating health-related biomarkers
A wide range of clinical biomarkers was investigated pre- and post-supplementation, including indicators of renal, muscle, and hepatic function, along with various clinical hematological markers. No individual study reported a significant effect of β-alanine supplementation on any of these biomarkers (4, 9, 30, 41, 52, 53). Additionally, 2 studies conducted additional analyses, to identify the proportion of individuals with values outside of normative ranges for each of the biomarkers identified, and whether this varied between the β-alanine and placebo groups (41, 52). No trends were apparent from either of these studies. Meta-analysis of any biomarker that was measured in ≥2 studies did, however, show a main effect of β-alanine supplementation on ALT concentration, along with trends toward an increase in sodium and ALP. The effect of β-alanine supplementation on ALT was “small” (ES: 0.274; CrI: 0.04, 0.527). Considering the pooled SD of all baseline data reported (11.7), this would correspond to a mean increase of ∼3.2 U/L for each participant within the β-alanine group. Mean baseline ALT concentration was 22.5 U/L, and so this small increase would still result in blood ALT concentration well within clinical reference ranges, which are typically considered to be <40 U/L, although wide variation does exist in individual laboratory reference ranges. ALT is a transaminase enzyme, primarily present in the liver. Liver damage may cause ALT to “leak” into the bloodstream, and thus elevated blood concentration can be indicative of liver dysfunction. We do not believe that this is the case within the current investigation, given the small effect identified, and the fact that all data remained well within clinical reference ranges pre- and post-supplementation. Interestingly, it has previously been reported that only a small amount of supplemented β-alanine (∼3%) is actually used for carnosine synthesis (58), with the rest being used in processes such as transamination or energy delivery (59). Given that ALT is a transaminase enzyme, it seems plausible to suggest that the small increases identified may represent increased transamination activity from elevated β-alanine availability. Alternatively, it is widely recognized that ALP and ALT are nonspecific biomarkers, impacted by a range of factors, including physical activity (60). Given that β-alanine supplementation is widely recognized to increase capacity for performance of high-intensity exercise, another potential explanation for this finding may be increased activity within the β-alanine group. These suggestions are, of course, speculative, and further research on the broader metabolic consequences of β-alanine supplementation is required to enhance understanding in this area.
Effect of β-alanine supplementation on tissue taurine and histidine concentration
The meta-analysis conducted on animal trials provides strong evidence that β-alanine supplementation can cause a reduction in skeletal or cardiac muscle taurine concentration at high doses (defined here as at least 3% solution in drinking water; ES: −2.90, 95% CrI: −3.53, −2.35), but not at lower doses (defined as <3% in drinking water, −0.32, 95% CrI: −0.80, 0.14). No evidence of an effect of β-alanine supplementation on skeletal muscle taurine concentration was identified in humans (ES: 0.156; 95% CrI: −0.38; 0.72). This is likely because of the substantially lower dose typically used in the human studies, when compared to the animal studies. The highest dose used in human studies was 6.4 g/d (41). For an 80-kg male, this is the equivalent of 80 mg/kg/d. Assuming that a typical adult male mouse or rat weighs 25 g or 400 g, respectively, and drinks 0.15 or 0.1 mL/g/d this would equate to an intake of 4,500 or 3,000 mg/kg/d for a mouse or rat who is provided 3% β-alanine in drinking water, which is ∼38–56-fold greater than the typical human dose provided. Direct murine-to-human inferences are, of course, limited because of vastly different metabolic rates along with species-specific biochemistry; however, the available evidence indicates that the daily dose typically used in human studies (∼3.2-6.4 g/d) is not of the magnitude required to measurably reduce skeletal muscle taurine concentration.
No effect of TCD, nor of an interaction between TCD and daily dose, was obtained, indicating that intervention duration does not moderate the influence of β-alanine on taurine, and that this effect does not increase over time. Lake and De Marte (61) reported that cardiac taurine concentration in rats was significantly reduced after 1, 2, and 3 wk treatment with 3% β-alanine in drinking water, although the magnitude of effect was smaller after 3 wk compared to that identified at 1 and 2 wk, whereas after 6 wk of treatment the β-alanine group was not different to the pair-fed control animals. These data suggest that not only does the influence of β-alanine not increase with time, it may in fact be reversed. Recently, a downregulation of the β-alanine/taurine transporter Tau-T was reported in humans ingesting 6.4 g/d of β-alanine for 24 wk (5), which may potentially represent a means of maintaining taurine homeostasis during periods of elevated β-alanine availability. Further research is required to elucidate the mechanistic pathways through which both carnosine and taurine are regulated during β-alanine supplementation.
Recently, it was reported that β-alanine supplementation may reduce plasma and muscle free histidine availability (17) and this was suggested as having potentially adverse consequences for muscle protein synthesis. The current meta-analysis showed no main effect of β-alanine supplementation on histidine, in either human or murine models. It is important to highlight, however, that limited animal data were available, and the only animal study available investigated the influence of 100 mg/kg β-alanine on brain histidine concentration (57). An influence of higher β-alanine doses, as was observed in the taurine meta-analysis, or an influence on other tissues, cannot therefore, be ruled out.
The animal data described in Supplemental Table 3 provided insight into potential alterations to skeletal, cardiac, hepatic, renal, and nervous function that may occur in response to the very high β-alanine doses used in these studies. Interestingly, the altered physiological processes described therein were neither exclusively positive nor negative in nature. For example, β-alanine supplementation has been reported to exert both a protective (62) and a harmful (63) influence on cardiac function in rats, with limited consensus on the factors that dictate the nature of this response. An important limitation of many of the available animal studies, was that they typically focused on the influence of β-alanine-induced taurine deficiency, and rarely considered the broader influences of β-alanine supplementation, which include increased carnosine, or the independent action of β-alanine per se. For example, Horvath et al. (64) investigated the influence of taurine supplementation, and β-alanine-induced taurine depletion, on skeletal muscle contractility and fatigue resistance in wild-type and mdx mice, and reported that both interventions had a positive effect on muscle function. β-Alanine supplementation-induced increases to muscle carnosine concentration are known to enhance skeletal muscle function and high-intensity exercise performance (2), and these results were likely because of the increased carnosine, rather than the taurine depletion that was reported. Conversely, Lu et al. (65) reported a neurotoxic influence of β-alanine supplementation in cats, a species known to have a low capacity for endogenous taurine synthesis and to have a more severe and negative reaction to chronic β-alanine supplementation. The authors identified that the neurotoxicity that occurred in their study resulted from β-alanine accumulation, rather than from taurine depletion. The finding of a neurotoxic influence of β-alanine accumulation is also evident in humans suffering from the rare genetic condition “hyper-β-alalinemia,” which results in the accumulation of β-amino acids in the body (66). β-Alanine accumulation such as this is unlikely to occur in healthy humans, particularly in response to the doses commonly employed in practice, because of processes such as transamination, energy delivery (59), or incorporation into carnosine (4), and therefore is not considered a risk of supplementation. These examples do, however, serve to highlight the importance of considering the broader influences of β-alanine supplementation (e.g., the independent influence of β-alanine per se, along with collateral effects on other elements such as taurine, histidine, and carnosine), when interpreting physiological results.
Side effects experienced from β-alanine intake in human trials
Paraesthesia was the only side effect reported during human supplementation trials. This “tingling” or “pricking” sensation of the skin occurs as a result of a histamine-independent neural pathway and is most likely induced on binding of β-alanine to the peripheral neuronal receptor MrgprD (67). This phenomenon is generally considered to be both transient and harmless and appears not to be a cause for concern. Indeed some athletes have reported the sensation of paraesthesia to improve their affective response to exercise (45), although other participants in the same study reported the sensation to be uncomfortable or unpleasant, demonstrating that the experience of paraesthesia, and whether it should be considered a beneficial side effect or adverse effect, is a subjective experience that is specific to the individual. Collectively, the literature indicates that the development of paraesthesia is dose-related, and closely matches the extent and time to peak blood β-alanine concentrations (4, 46). Large heterogeneity in dosing studies used in longitudinal studies, along with minimal reporting of side effects in many of the available studies precluded statistical identification of the most effective strategy to reduce the incidence of paraesthesia. However, acute studies indicated that the splitting of doses (4) or the use of sustained release capsules (46) may be an effective way to reduce the extent and/or time to peak blood β-alanine concentration, and thus to reduce or remove the occurrence and intensity of paraesthesia symptoms. Irrespective of dosing strategy used, there was evidence of considerable within and between participant variability in occurrence and intensity of paraesthesia development, and ongoing investigation of the individual determinants of paraesthesia determinant would be of interest.
Recommendations for research and practice
The current investigation highlights a number of limitations and gaps in the current evidence base related to theoretical risks and physiological consequences of β-alanine supplementation. Collectively, the assessment and reporting of side effects in human studies were suboptimal, thus limiting conclusions that can be drawn, and potentially causing an underestimation of lower level side effects experienced. Reliance on participant self-report is ill-advised, and it is stressed that researchers should, in future, employ predefined, systematic and objective means of side effect assessment and reporting. In addition, evidence of compliance to dosing protocols, including the spacing and timing of dosing throughout the day, is important to identify whether or not strategies to reduce the occurrence of paraesthesia are effective. Although no significant changes to any health-related biomarkers were identified in any of the individual studies that provided these data, statistical meta-analysis identified a main effect of β-alanine supplementation on ALT, along with a trend toward increased ALP, although these markers remained well within clinical reference ranges. We suggest that further research should measure these markers, thus adding to the evidence base available. It is important to acknowledge that relatively limited data related to the influence of β-alanine supplementation on taurine and histidine in humans is currently available, and it is possible that the available data set may have been insufficient to allow detection of small changes. It is recommended that measurements of taurine and histidine, in addition to carnosine, are included in future studies. In recognition of the considerable individual variability in response to most sports nutrition-based interventions, consideration of the individual response of participants to these parameters would be of interest (68). Additionally, oversimplistic interpretations of the physiological relevance of any observed changes should be avoided. Too often, minimal nutrient or biomarker changes are dichotomously interpreted as being positive, or negative, which fails to acknowledge the complexity and interaction of these processes. Changes to the tissue concentration of these elements should be interpreted within the context of measured changes to relevant clinical or functional outcomes. In the absence of this information, findings should be neutrally interpreted and nonevidence-based speculations related to physiological consequences avoided.
Dosing recommendations for healthy humans
According to the recommendations of the safety evaluation model used, if no data are available to establish adverse effects in humans, then a safe upper intake cannot be identified. This was the case within the current risk assessment, and so the highest observed limit with sufficient evidence of safety was used to guide recommendations. Recently, 2 studies have been conducted using a dosing strategy of 12 g/d for a period of 7 (69) or 14 d (53), and no adverse effects were reported. Given the short follow-up of these studies, we recommend that intakes of 12 g/d should not yet be employed in general practice, pending further research. Intakes up to 6.4 g/d were commonly used in the studies included within this review, and it is recommended that this intake should be adopted as the current highest observed limit with sufficient evidence of safety. No evidence of adverse effects have been reported when such doses are consumed for up to 24 wk (41). Importantly, much of the evidence described in the current risk assessment was conducted recently, with 95% of human studies published within the last 10 y. Should research continue at its current rate, it is likely that knowledge of the mechanistic actions and ergogenic and therapeutic potential of β-alanine supplementation will substantially expand in the coming years. We recommend that information presented herein is continually updated based on emerging evidence, ensuring that dosing recommendations are made in accordance with the best quality and most recent evidence available.
Summary and conclusions
The current comprehensive risk assessment of human and animal data revealed no adverse effects of β-alanine supplementation in healthy humans, within the doses and durations described. Paraesthesia was the only reported side effect, and no evidence exists to indicate that this phenomenon has any adverse consequences. Considerable within and between participant variability exists in relation to both the frequency and intensity of paraesthesia, although strategies to slow β-alanine absorption, thus reducing the extent and time to peak plasma β-alanine, can be used to reduce its occurrence and intensity. Although β-alanine supplementation in high doses was shown to reduce tissue taurine concentration in animal models, the available human data showed no observable effect of β-alanine supplementation on skeletal muscle taurine or histidine concentration. Collectively, the available evidence indicates that β-alanine supplementation, within the doses and durations described herein, is safe for human consumption.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ED and BG: designed the research; ED and VdSP: conducted all searches; BM, BSH, and FIS: extracted all data; PAS: undertook all statistical analysis; ED and BS: analyzed the data; ED: wrote the manuscript with ongoing critical input from GGA, BG, PAS, and BS; and all authors: read and approved the final paper.
Notes
Supported by research grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) to ED (2015/11328-2 and 2017/09635-0), BG (2013/14746-4), BS (2016/50438-0 and 2017/04973-4), GGA (2014/11948-8), and VSP (2013/04806-0).
Author disclosures: ED, PAS, VSP, BSH, BM, FIS, GGA, and BG, no conflicts of interest. BS has previously received a scholarship from Natural Alternatives International (NAI), San Marcos, California for a study unrelated to this one. NAI has also partially supported an original study conducted within our lab. This company has not had any input (financial, intellectual, or otherwise) into this review.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ALP, alkaline phosphatase; ALT, alanine aminotransferase; BM, body mass; CrI, credible interval; ES, effect size; TCD, total cumulative dose.
References
- 1. Dolan E, Saunders B, Dantas W, Murai I, Roschel H, Artioli G, Harris RC, Bicudo JEPW, Sale C, Gualano B. A comparative study of hummingbirds and chickens provides mechanistic insight into the histidine containing dipeptide role in skeletal muscle metabolism. Sci Rep. 2018;8(1):14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sale C, Artioli GG, Gualano B, Saunders B, Hobson RM, Harris RC. Carnosine: from exercise performance to health. Amino Acids. 2013;44(6):1477–91. [DOI] [PubMed] [Google Scholar]
- 3. Boldyrev A, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013;93(4):1803–45. [DOI] [PubMed] [Google Scholar]
- 4. Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA. The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006;30(3):279–89. [DOI] [PubMed] [Google Scholar]
- 5. Saunders B, De Salles Painelli V, De Oliveira LF, Da Eira Silva V, Da Silva RP, Riani L, Franchi M, Goncalves LS, Harris RC, Roschel H et al.. Twenty-four weeks of β-alanine supplementation on carnosine content, related genes, and exercise. Med Sci Sports Exerc. 2017;49(5):896–906. [DOI] [PubMed] [Google Scholar]
- 6. Saunders B, Elliott-Sale K, Artioli GG, Swinton PA, Dolan E, Roschel H, Sale C, Gualano B. β-Alanine supplementation to improve exercise capacity and performance: a systematic review and meta-analysis. Br J Sports Med. 2017;51(8):658–69. [DOI] [PubMed] [Google Scholar]
- 7. Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, Rawson ES, Walsh NP, Garthe I, Geyer H et al.. IOC consensus statement: dietary supplements and the high-performance athlete. Br J Sports Med. 2018;52(7):439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Artioli G, Sale C, Jones R. Carnosine in health and disease. Eur J Sport Sci. 2018;1–10., doi: 10.1080/17461391.2018. [DOI] [PubMed] [Google Scholar]
- 9. Del Favero S, Roschel H, Solis MY, Hayashi AP, Artioli GG, Otaduy MC, Benatti FB, Harris RC, Wise JA, Leite CC et al.. Beta-alanine (CarnosynTM) supplementation in elderly subjects (60–80 years): effects on muscle carnosine content and physical capacity. Amino Acids. 2012;43(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Marchis S, Modena C, Peretto P, Migheli A, Margolis F, Fasolo A. Carnosine-related dipeptides in neurons and glia. Biochem. 2000;65(7):824–33. [PubMed] [Google Scholar]
- 11. Dobrota D, Federova T, Stvolinksy S, Babusikova E, Likavcanova K, Drgova A, Strapkova A, Boldyrev A. Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury: after-stroke-effect. Neurochem Res. 2005;30(10):1263–38. [DOI] [PubMed] [Google Scholar]
- 12. Renner C, Zemitzsch N, Fuchs B, Geiger KD, Hermes M, Hengstler J, Gebhardt R, Meixenberger J, Gaunitz F. Carnosine retards tumor growth in vivo in an NIH3T3-HER2/neu mouse model. Mol Cancer. 2010;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boldyrev A, Fedorova T, Stepanova M, Dobrotvorskaya I, Kozlova E, Boldanova N, Bagyeva G, Ivanova-Smolenskaya I, Illarioshkin S. Carnosine increases efficiency of DOPA therapy of Parkinson's disease: a pilot study. Rejuvenation Res. 2008;11(4):821–7. [DOI] [PubMed] [Google Scholar]
- 14.de Courten B, Jakubova M, de Courten M, Kukurova I, Vallova S, Krumpolec P, Valkovic L, Kurdiova R, Garzon F, Barbaresi S et al.. Effects of carnosine supplementation on glucose metabolism: pilot clinical trial. Obesity. 2016;24(5):1027–34. [DOI] [PubMed] [Google Scholar]
- 15. Kurata H, Fujii T, Tsutsui H, Katayama T, Ohkita M, Takaoka M, Tsuruoka N, Kiso Y, Ohno Y, Fujisawa Y et al.. Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp Ther. 2006;319(2):640–7. [DOI] [PubMed] [Google Scholar]
- 16. Shaffer J, Kocsis J. Taurine mobilizing effects of beta alanine and other inhibitors of taurine transport. Life Sci. 1981;28(24):2727–36. [DOI] [PubMed] [Google Scholar]
- 17. Blancquaert L, Everaert I, Missinne M, Baguet A, Stegen S, Volkaert A, Petrovic M, Vervaet C, Achten E, De Maeyer DE et al.. Effects of histidine and β-alanine supplementation on human muscle carnosine storage. Med Sci Sport Exerc. 2017;49(3):602–9. [DOI] [PubMed] [Google Scholar]
- 18. Hathcock J. Vitamin and mineral safety. Vitam Miner Saf. 2013;3rd. ed:1–190. [Google Scholar]
- 19. Shao A, Hathcock JN. Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regul Toxicol Pharmacol. 2008;50(3):376–99. [DOI] [PubMed] [Google Scholar]
- 20. Shao A, Hathcock JN. Risk assessment for creatine monohydrate. Regul Toxicol Pharmacol. 2006;45(3):242–51. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman D; PRISMA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 22. Animal Resources Centre. Rat and Mice Weights. [Internet] [cited 28 Sep, 2018]. Available from: http://www.arc.wa.gov.au/?page_id=125. [Google Scholar]
- 23. Johns Hopkins University. Animal care and use committee: species specific information [Internet] [cited 29 Aug, 2018]. Available from: http://web.jhu.edu/animalcare/procedures/index.html.
- 24. Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ; GRADE working group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welton N, Sutton A, Cooper A, Abrams K, Ades A. Meta-analysis using Bayesian methods in evidence synthesis for decision making in healthcare. John Wiley & Sons; 2012;76–93. [Google Scholar]
- 26. Morris S, DeShon R. Combining effect size estimates in meta-analysis with repeated measures and independent-group designs. Psychol Methods. 2002;7(1):105–25. [DOI] [PubMed] [Google Scholar]
- 27. Morris S. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11(2):364–86. [Google Scholar]
- 28. Zhang J, Ko C, Nie L, Chen Y, Tiwari R. Bayesian hierarchical methods for meta-analysis combining randomized-controlled and single-arm studies. Stat Methods Med Res. 2018; doi: 10.1177/0962280218754928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stout JR, Graves BS, Smith AE, Hartman MJ, Cramer JT, Beck TW, Harris RC. The effect of beta-alanine supplementation on neuromuscular fatigue in elderly (55–92 years): a double-blind randomized study. J Int Soc Sports Nutr. 2008;5(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCormack WP, Stout JR, Emerson NS, Scanlon TC, Warren AM, Wells AJ, Gonzales AM, Mangine GT, Robinson EH, Fragala ME et al.. Oral nutritional supplement fortified with beta-alanine improves physical working capacity in older adults: a randomized, placebo-controlled study. Exp Gerontol. 2013;48(9):933–9. [DOI] [PubMed] [Google Scholar]
- 31. Glenn JM, Gray M, Stewart R, Moyen NE, Kavouras SA, Dibrezzo R, Turner R, Baum J. Incremental effects of 28 days of beta-alanine supplementation on high-intensity cycling performance and blood lactate in masters female cyclists. Amino Acids. 2015;47(12):2593–600. [DOI] [PubMed] [Google Scholar]
- 32. Allman B, Biwer A, Maitland C, DiFabio B, Coughlin E, Smith-Ryan A, Ormsbee MJ. The effect of short term beta alanine supplementation on physical performance and quality of life in Parkinson's Disease: a pilot study. J Exerc Physiol online. 2018;21(1):1–13. [Google Scholar]
- 33. Bailey CH, Signorile JF, Perry AC, Jacobs KA, Myers ND. Beta-alanine does not enhance the effects of resistance training in older adults. J Diet Suppl. 2018;15(6):860–70. [DOI] [PubMed] [Google Scholar]
- 34. Furst T, Massaro A, Miller C, Williams BT, LaMacchia ZM, Horvath PJ. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J Int Soc Sports Nutr. 2018;15(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brisola GMP, Artioli GG, Papoti M, Zagatto AM. Effects of four weeks of β-alanine supplementation on repeated sprint ability in water polo players. PLoS One. 2016;11(12):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Claus G, Redkva P, Brisola G, Malta E, de Araujo Bonetti de Poli R, Miyagi W, Zagatto AM. Beta-alanine supplementation improves throwing velocities in repeated sprint ability and 200-m swimming performance in young water polo players. Pediatr Exerc Sci. 2017;29(2):203–12. [DOI] [PubMed] [Google Scholar]
- 37. Milioni F, Redkva PE, Barbieri FA, Zagatto AM. Six weeks of β-alanine supplementation did not enhance repeated-sprint ability or technical performances in young elite basketball players. Nutr Health. 2017;23(2):111–8. [DOI] [PubMed] [Google Scholar]
- 38. De Andrade Kratz C, de Salles Painelli V, de Andrade Nemezio KM, da Silva RP, Franchini E, Zagatto AM, Gualano B, Artioli GG. Beta-alanine supplementation enhances judo-related performance in highly-trained athletes. J Sci Med Sport. 2017;20(4):403–8. [DOI] [PubMed] [Google Scholar]
- 39. Brisola G, de Souza Malta E, Santiago P, Vieira L, Zagatto A. Four weeks of β-alanine supplementation improves high-intensity game activities in water polo. Int J Physiol Perform. 2018;13(9):1–23. [DOI] [PubMed] [Google Scholar]
- 40. Harris R, Kim H, Kim C, Kendrick I, Price K, Wise J. Simultaneous changes in muscle carnosine and taurine during and following supplementation with β-alanine. Med Sci Sport Exerc. 2010;42(S5):107. [Google Scholar]
- 41. Saunders B, Franchi M, de Oliveira L, da Eira Silva V, da Silva RP, de Salles Painelli V, Riani Costa LR, Sale C, Harris RC, Roschel H et al.. Chronic (24 weeks) β-alanine supplementation does not affect muscle taurine or blood clinical chemistry (Abstract). Med Sci Sports Exerc. 2018;50:590. [Google Scholar]
- 42. Kelly V. β-Alanine: performance effects, usage and side effects (dissertation). University of Queensland: 104–19.
- 43. Chung W, Shaw G, Anderson ME, Pyne DB, Saunders PU, Bishop DJ, Burke LM. Effect of 10 week beta-alanine supplementation on competition and training performance in elite swimmers. Nutrients. 2012;4(10):1441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Varanoske AN, Hoffman JR, Church DD, Coker NA, Baker KM, Dodd SJ, Harris RC, Oliveira LP, Dawson VL, Wang R et al.. Comparison of sustained-release and rapid-release β-alanine formulations on changes in skeletal muscle carnosine and histidine content and isometric performance following a muscle-damaging protocol. Amino Acids. 2018;(0123456789):1–12., doi: 10.1007/s00726-018-2609-4. [DOI] [PubMed] [Google Scholar]
- 45. Bellinger PM, Minahan CL. Performance effects of acute β-alanine induced paresthesia in competitive cyclists. Eur J Sport Sci. 2016;16(1):88–95. [DOI] [PubMed] [Google Scholar]
- 46. Décombaz J, Beaumont M, Vuichoud J, Bouisset F, Stellingwerff T. Effect of slow-release β-alanine tablets on absorption kinetics and paresthesia. Amino Acids. 2012;43(1):67–76. [DOI] [PubMed] [Google Scholar]
- 47. Glenn J, Smith K, Moyen N, Binns A, Gray M. Effects of acute beta-alanine supplementation on anaerobic performance in trained female cyclists. J Nutr Sci Vitaminol. 2015;61(2):161–6. [DOI] [PubMed] [Google Scholar]
- 48. MacPhee S, Weaver I, Weaver D. An evaluation of interindividual responses to the orally administered neurotransmitter beta-alanine. J Amino Acids. 2013;2013::429847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bex T, Chung W, Baguet A, Stegen S, Stautemas J, Achten E, Derave W. Muscle carnosine loading by beta-alanine supplementation is more pronounced in trained vs. untrained muscles. J Appl Physiol. 2014;116(2):204–9. [DOI] [PubMed] [Google Scholar]
- 50. Mor A, Ipekoglu G. The acute effects of beta-alanine on blood gas of athletes after maximal research. Res J Pharm Biol Chem Sci. 2018;9:4. [Google Scholar]
- 51. Stautemas J, Everaert I, Lefevere FBD, Derave W. Pharmacokinetics of β-alanine using different dosing strategies. Front Nutr. 2018;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stellingwerff T, Anwander H, Egger A, Buehler T, Kreis R, Décombaz J, Boesch C. Effect of two β-alanine dosing protocols on muscle carnosine synthesis and washout. Amino Acids. 2012;42(6):2461–72. [DOI] [PubMed] [Google Scholar]
- 53. Church D, Hoffman J, Varanoske A, Wang R, Baker K, La Monica M, Beyer KS, Dodd SJ, Oliveira LP, Harris RC et al.. Comparison of two β-alanine dosing protocols on muscle carnosine elevations. J Am Coll Nutr. 2017;36(8):608–16. [DOI] [PubMed] [Google Scholar]
- 54. da Silva RP, de Oliveira LF, Saunders B, de Andrade Kratz C, de Salles Painelli V, da Eira Silva V, Marins JCB, Franchini E, Gualano B, Artioli GG. Effects of β-alanine and sodium bicarbonate supplementation on the estimated energy system contribution during high-intensity intermittent exercise. Amino Acids. 2018;(0123456789):1–14., doi: 10.1007/s00726-018-2643-2. [DOI] [PubMed] [Google Scholar]
- 55. Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA. Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids. 2007;32(2):225–33. [DOI] [PubMed] [Google Scholar]
- 56. Varanoske A, Hoffman J, Church D, Wang R, Baker K, Dodd SJ, Coker NA, Oliveira LP, Dawson VL, Fukuda DH et al.. Influence of skeletal muscle carnosine content on fatigue during repeated resistance exercise in recreationally active women. Nutrients. 2017;9(9):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hoffman JR, Zuckerman A, Ram O, Sadot O, Stout JR, Ostfeld I, Cohen H. Behavioral and inflammatory response in animals exposed to a low-pressure blast wave and supplemented with β-alanine. Amino Acids. 2017;49(5):871–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stegen S, Blancquaert L, Everaert I, Bex T, Taes Y, Calders P, Acthen E, Derave W. Meal and beta-alanine coingestion enhances muscle carnosine loading. Med Sci Sports Exerc. 2013;45(8):1478–85. [DOI] [PubMed] [Google Scholar]
- 59. Blancquaert L, Baba SP, Kwiatkowski S, Stautemas J, Stegen S, Barbaresi S, Chung W, Boakye AA, Hoetker JD, Bhatnagar A et al.. Carnosine and anserine homeostasis in skeletal muscle and heart is controlled by β-alanine transamination. J Physiol. 2016;594(17):4849–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pettersson J, Hindorff U, Persson P, Bengstsson T, Malmqvist U, Werkstrom V, Ekelund M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br J Clin Pharmacol. 2008;65(2):253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lake N, De Marte L. Effects of beta-alanine treatment on the taurine and DNA content of the rat heart and retina. Neurochem Res. 1988;13(10):1003–6. [DOI] [PubMed] [Google Scholar]
- 62. Allo S, Bagby L, Schaffer S. Taurine depletion, a novel mechanism for cardioprotection from regional ischemia. Am J Physiol. 1997;273(4):1956–61. [DOI] [PubMed] [Google Scholar]
- 63. Pansani MC, Azevedo PS, Rafacho BPM, Minicucci MF, Chiuso-Minicucci F, Zorzella-Pezavento SG, Marchini JS, Padovan GJ, Fernandes AA, Matsubara BB et al.. Atrophic cardiac remodeling induced by taurine deficiency in wistar rats. PLoS One. 2012;7(7):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Horvath DM, Murphy RM, Mollica JP, Hayes A, Goodman CA. The effect of taurine and β-alanine supplementation on taurine transporter protein and fatigue resistance in skeletal muscle from mdx mice. Amino Acids. 2016;48(11):2635–45. [DOI] [PubMed] [Google Scholar]
- 65. Lu P, Xu W, Sturman JA. Dietary β-alanine results in taurine depletion and cerebellar damage in adult cats. J Neurosci Res. 1996;43(1):112–9. [DOI] [PubMed] [Google Scholar]
- 66. Scriver C, Pueschel M, Davies E. Hyper-beta-alinemia associated with beta-aminoaciduria and y-aminobutyricaciduria, somnolence and seizures. N Engl J Med. 1966;274(12):635–43. [DOI] [PubMed] [Google Scholar]
- 67. Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by β-alanine. J Neurosci. 2012;32(42):14532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Swinton P, Stephens Hemingway B, Saunders B, Gualano B, Dolan E. A statistical framework to interpret individual response to intervention: paving the way for personalised nutrition and exercise prescription. Front Nutr. 2018;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hoffman J, Gepner Y, Hoffman M, Zelicha H, Shapira S, Ostfeld I. Effect of high-dose, short-duration β-alanine supplementation on circulating IL-10 concentrations during intense military training. J Strength Cond Res. 2018;32(10):2978–81. [DOI] [PubMed] [Google Scholar]
- 70. Lilequist R, Paasonen M, Solatunturi E. Beta-alanine and alpha-L-alanine inhibit the exploratory activity of spontaneously hypertensive rats. Experientia. 1982;38(3):379–80. [DOI] [PubMed] [Google Scholar]
- 71. Everaert I, Stegen S, Vanheel B, Taes Y, Derave W. Effect of beta-alanine and carnosine supplementation on muscle contractility in mice. Med Sci Sport Exerc. 2013;45(1):43–51. [DOI] [PubMed] [Google Scholar]
- 72. Kaczmarek D, Łochyński D, Everaert I, Pawlak M, Derave W, Celichowski J. Role of histidyl dipeptides in contractile function of fast and slow motor units in rat skeletal muscle. J Appl Physiol. 2016;121(1):164–72. [DOI] [PubMed] [Google Scholar]
- 73. Abebe W, Mozaffari MS. Effect of taurine deficiency on adenosine receptor-mediated relaxation of the rat aorta. Vascul Pharmacol. 2003;40(4):219–28. [DOI] [PubMed] [Google Scholar]
- 74. Harada H, Cusack BJ, Olson RD, Stroo W, Azuma J, Hamaguchi T, Schaffer SW. Taurine deficiency and doxorubicin: interaction with the cardiac sarcolemmal calcium pump. Biochem Pharmacol. 1990;39(4):745–51. [DOI] [PubMed] [Google Scholar]
- 75. Parildar-Karpuzoǧlu H, Doǧru-Abbasoǧlu S, Balkan J, Aykaç-Toker G, Uysal M. Decreases in taurine levels induced by β-alanine treatment did not affect the susceptibility of tissues to lipid peroxidation. Amino Acids. 2007;32(1):115–9. [DOI] [PubMed] [Google Scholar]
- 76. Parildar H, Dogru-Abbasoglu S, Mehmetçik G, Ozdemirler G, Koçak-Toker N, Uysal M. Lipid peroxidation potential and antioxidants in the heart tissue of beta-alanine- or taurine-treated old rats. J Nutr Sci Vitaminol (Tokyo). 2008;54(1):61–5. [DOI] [PubMed] [Google Scholar]
- 77. Dawson R, Biasetti M, Messina S, Dominy J. The cytoprotective role of taurine in exercise-induced muscle injury. Amino Acids. 2002;22(4):309–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.