Abstract
The complement system plays an essential role in both innate and adaptive immunity. The traditional understanding of this system comes from studies investigating complement proteins produced by the liver and present in plasma to “complement” the immune cell-mediated response to invading pathogens. Recently, it has been reported that immune cells including, but not limited to, T-cells and monocytes, express complement proteins. This complement is referred to as intracellular (IC) and implicated in the regulation of T-cell activation. The mechanisms and the structure-activity relationship between nanomaterials and IC, however, are currently unknown. Herein, we describe a structure-activity relationship study demonstrating that under in vitro conditions, only polymeric materials with cationic surfaces activate IC in T-cells. The effect also depends on particle size and occurs through a mechanism involving membrane damage, thereby IC on the cell surface serves as a self-opsonization marker in response to the nanoparticle-triggered danger affecting the cell integrity.
Keywords: complement, IC, hypersensitivity, infusion reactions, anaphylatoxins, CARPA, nanoparticles, in vitro, preclinical, T-cells
Background
The complement system known widely to the scientific community is a group of approximately 30 proteins produced by the liver and present in plasma. These proteins are proteases, which upon activation, trigger a proteolytic cascade generating anaphylatoxins and opsonins, and culminating with the formation of the membrane attack or terminal complex aimed at destructing the invading pathogen. Depending on the sensing mechanism, the proteolytic cascade-type activation of the plasma complement can occur through three pathways: classical, alternative, and lectin. The long-standing observation that several coagulation proteins are capable of cleaving C3 and C5 components of the complement suggests the existence of the fourth complement activation pathway often referred to as the “extrinsic protease pathway”1–3. Individual arms of the complement activation converge on the C3 component of the complement, which continues the common pathway and eventually leads to the assembly of the terminal complex. The short complement cleavage products with cytokine-like activity are called anaphylatoxins. They include C3a, C4a, and C5a peptides which have a wide range of biological activities including but not limited to smooth muscle contraction, mast cell degranulation and histamine release, vasodilation, enhanced vascular permeability, inflammation, activation of chemotaxis, and cytotoxic oxygen radical generation. When these biological reactions occur in response to a drug product, they lead to the IgE-independent immediate hypersensitivity reactions also known as complement activation-related pseudoallergy (CARPA). Interaction of the C3a and C5a with their cognate receptors present on mast cells, basophils, platelets, monocytes, neutrophils triggers degranulation of the cells and release of mediators responsible for CARPA symptoms and induction of pro-inflammatory cytokines4. The larger cleavage products of the complement components generated during complement cleavage are known as opsonins (e.g., C3b, C4b, C5b). These proteins attach to the membrane of an invading pathogen (or a drug product) to accelerate its clearance and destruction by the phagocytic cells.
Over the past decade, this traditional understanding of the complement system and its role in the innate immunity has been expanded to include the contribution of complement proteins to the adaptive immune response. The complement proteins were shown to participate in B- and T-cells activation, antibody generation, cytokine secretion and dendritic cell maturation5–8. In addition, the complement proteins were shown to participate in the elimination of cellular debris of apoptotic and necrotic cells9, 10. The physiological significance of this function of the complement comes from studies of patients with complement deficiencies. In such individuals, the elimination of cellular debris does not occur properly and leads to the development of autoimmune disorders11, 12.
Recently, this expanded model of the complement system and its role in the innate and adaptive immunity was further challenged by the discovery of so-called intracellular complement (IC)13. Unlike the plasma complement produced by the liver, the IC is either constitutively expressed by the immune cells, or triggered by the activation stimuli13. Despite the detection of the IC in a variety of cells (e.g., monocytes, neutrophils, T-and B-lymphocytes, epithelial cells, endothelial, fibroblasts, adipocytes, and even cancer cells)13–15, the function of IC is poorly understood. The IC produced by cancer cells was shown to facilitate tumor growth through engagement of the pro-survival PI3K/Akt pathway15; that produced by dendritic cells - to trigger T-cell activation16; and the IC of the T-cell origin - to play a critical role in Th1 differentiation and metabolic reprogramming of T-lymphocytes14, 17.
Our team is interested in the complement system from the standpoint of safety of nanotechnology-formulated drugs. Knowing the importance of the plasma complement for innate and adaptive immunity, we and others routinely analyze nanoformulations intended for systemic administrations to understand their ability to activate plasma complement. The consequences of the interaction between nanoparticles and the plasma complement system vary from rapid clearance of the administrated nanoparticles to the development of life-threatening infusion reactions, among which the CARPA represents the best-understood mechanism18, 19. While various types of nanomaterials were shown to trigger CARPA, PEGylated liposomes are among the most “CARPAgenic” agents4. For example, 10% and 30% of patients develop CARPA in response to systemic administration of liposomal doxorubicin (Doxil) and amphotericin (Ambisome), respectively20. The research of CARPA and the development of the immediate type hypersensitivity reactions is focused on detection of C3a, C5a or MAC in the plasma of animals or human patients treated with nanoformulations, as well as in vitro. The role of the IC in CARPA and other drug-mediated infusion reactions is currently unknown.
Unlike the interaction between nanoparticles and the plasma complement, the effects of nanotechnology carriers commonly used in drug delivery on the IC are currently unknown. Understanding the propensity of nanomaterials to activate IC, therefore, represents an important area of research. Such importance is emphasized by the current knowledge of the role of the IC in the delayed-type hypersensitivity (DTH) reactions and autoimmunity5, 7, 14, 17, 21. Since both of these immunotoxicities result from functional changes in the immune system and require a longer time to manifest than the immediate type reactions (ITR) triggered by the plasma complement, understanding the interaction between nanomaterials and IC may aid in establishing a long-term safety profile of nanomaterials. Additionally, getting an insight into a potential contribution of the IC to the ITRs, is needed to improve the understanding of the complement function.
Herein, we present an in vitro study utilizing human peripheral blood mononuclear cells and a model T-cell line, Jurkat, to investigate the structure-activity relationship between nanoparticles and the IC, and understand the mechanism of the activation.
Materials
Cells and Reagents
The assessment of IC activation was conducted using peripheral blood mononuclear cells. A T-cell line Jurkat (ATCC) was used for some experiments. All cells were cultured in complete RPMI-1640 containing 10% FBS, 1% L-Glutamine and 1% penicillin/streptomycin. RPMI 1640, Fetal Bovine Serum, Penicillin-Streptavidin, DPBS, L-glutamine and Ficoll-Paque Premium were obtained from GE Healthcare (Chicago, IL). Bovine Serum Albumin, Polymyxin B, Poly-L-Lysine, and Cremophor were from Sigma (St.Louis, MO). Plasma membrane protein extraction kit (ab65400) was from Abcam (Cambridge, UK). Monosodium urate NanoSiO2 and Ultrapure E.coli K12 LPS were from Invivogen (San Diego, CA). Min-U-Sil5 (cat# 15061710) was from US Silica (Frederick, MD). Silicon powder and zinc oxide nanoparticles were from Strem Chemicals (Newburyport, MA). Doxil was from Avanti Polar Lipids (Alabaster, AL). Ambisome and Propofol were obtained from NIH Pharmacy. Colloidal gold nanoparticles were from TedPella (Redding, CA). Colloidal silver nanoparticles were from Nanocomposix (San Diego, CA). PAMAM dendrimers were from Dendritech (Midland, MI). Physicochemical characterization of these particles was described in details elsewhere22–26. Particles from the same batches as those described by us in the earlier studies22–26 were used in this study. Synthesis and characterization of triazine dendrimers were also described earlier27–29. The complete list of reagents and their sources is available in the supplementary materials.
PBMC isolation and treatment.
PBMCs were isolated from heparinized whole blood using Ficoll Paque Premium reagent according to a manufacturer’s protocol. Isolated PBMC were reconstituted in full culture media (RPMI-1640, 10% FBS, penicillin-streptavidin, 2mM L-glutamine). PBMC or Jurkat cells were seeded at 1×106 cells per sample into 24 well plates and treated with different reagents for 1hour at 37°C, 5%CO2. For experiments with protease inhibitors, cells were pre-treated with different protease inhibitors for 1 hour before treatments with PAMAM dendrimers. For positive control, PBMC were treated with human anti-CD3 OKT antibodies at concentration 0.5 μg/mL for 1 hour at 37°C and 5% CO2. At the end of the incubation time, PBMC were washed twice with DPBS.
Flow cytometry
The detection of IC and cytokines was performed by flow cytometry according to the previously published protocol14 and with minor adjustments described in the supplementary materials and methods.
Plasma Membrane Fraction Isolation and Western Blot were performed using commercial kits and reagents according to the manufacturers’ specified protocols. The details are provided in the supplementary section.
LC-MS-based proteomics
Membrane proteins from the experimental and control sample were solubilized and digested using trypsin (Promega, Madison, WI) as previously described30. Peptide digests were prepared and fractionated prior to LC-MS analysis employing off-line strong cation exchange (SCX) chromatography (LC), as previously described31. Based on the SCX-LC profile, peptides were pooled into 10 fractions and analyzed in duplicates using an Orbitrap Elite mass spectrometer (ThermoElectron, San Jose, CA) coupled to a nano-flow reversed phase (RP) LC (Agilent 1100, Sanat Clara, CA) as previously described31. Previously described subtractive proteomic analysis32 was used to reveal a non-redundant list of proteins identified solely in dendrimer-treated cells. The details are provided in the supplementary information.
Results
IC activation depends on particle type
To screen nanoparticles for their ability to activate IC we choose several commercially available materials. We organized these materials into three categories based on the current knowledge regarding their immunological properties in the context of CARPA, hypersensitivity or immunogenicity, and effects on the integrity of cellular organelles (Table 1). The first group included silver colloids, gold colloids, nickel and zinc oxide nanoparticles. These materials were reported to cause hypersensitivity reactions in vivo33–36. CARPAgenic nanoparticles included Doxil, Cremophor EL, Ambisome, Feraheme, and Propofol, and constituted the second group19, 37. Since the original study describing the involvement of cellular protease cathepsin L in the generation of intracellular C3a fragment, we tested nanoparticles which could trigger protease release from intracellular compartments in the third group14, 38. This group included silica, nano silica, silicone, and PAMAM dendrimers. Whenever available, each type of test material included particles with either different size or charge. Physicochemical characterization of these materials was conducted by our group and published in several earlier studies22–26, 39.
Table 1: Nanomaterials tested in this study.
Before analysis in biological assays, all particles were analyzed by dynamic light scattering or, where applicable, by transmission electron microscopy to determine particle size. Zeta-potential was determined for all materials studied by DLS. The details of characterization were published before and shown in the supplementary section. Representative flow cytometry images in support of the data summarized in the column “complement on the cell surface” are shown in Supplementary Figure 1.
| Nanoparticles | Size, nm | Complement on the cell surface | |
|---|---|---|---|
| Nanoparticles with demonstrated ability to contribute to protein immunogenicity and cause delayed-type hypersensitivity reactions | Au | 5, 30, 80, 250 | Not detected |
| Ag (PVP and citrate stabilized) | 20, 110 | Not detected | |
| Ni | 200–400, with aggregates up to 700 | Not detected | |
| Zinc Oxide | 30 with aggregates up to 1400 | Not detected | |
| Nanoparticles With demonstrated ability to activate plasma complement and cause CARPA in sensitive individuals | PEGylated liposomes | 89 | Not detected |
| Ambisome | 110–120 | Not detected | |
| Propofol | 208 | Not detected | |
| Feraheme | ~25 | Not detected | |
| Cremophor | 15 | Not detected | |
| Nanoparticles with a known ability to disrupt or perturb cellular organelles | Silica | more than 1 μm | Not detected |
| Silicon | more than 1 μm | Not detected | |
| Nanosilica | 180, with aggregate up more than 1 μm | Not detected | |
| G5 PAMAM amine- and guanidine terminated dendrimers | 6.5 | Detected | |
| G5 triazine dendrimers | 8.0 | Detected |
The original study describing the presence of intracellularly activated complement on the surface of T-cells demonstrated that the cleavage of C3 protein expressed in these cells occurs after cross-linking of the CD3 receptor by the CD3-specific antibodies (clone OKT-3)14. We tested anti-CD3 (OKT-3 clone) from different vendors and only Abcam antibodies (product code ab86883) of specific lots (#GR52307–4 and #GR197169–1) induced the expression of the complement detectable by the C3adesArg antibody clone 2991 on the surface of lymphocytes (Figure 1A and B). Therefore, we stocked these antibodies and used them throughout the entire project.
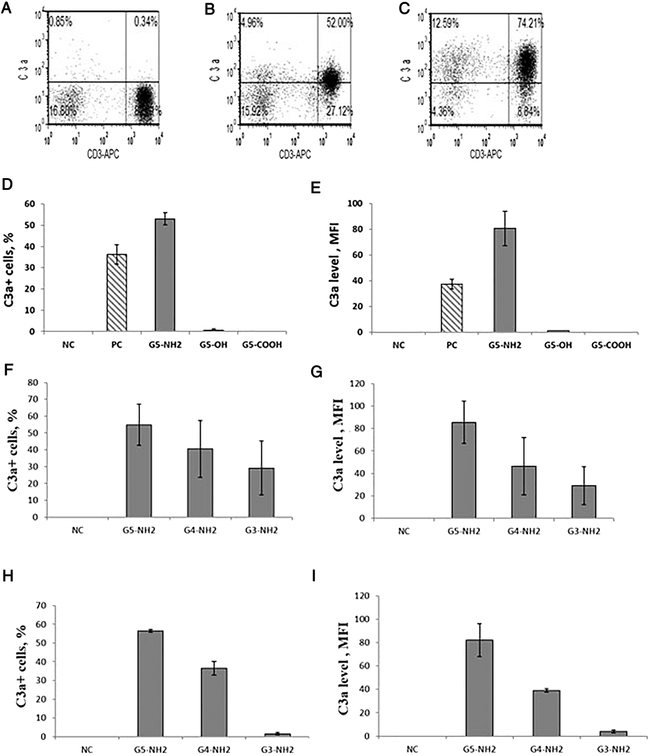
Figure 1. Detection of IC on the surface of α-CD3 and dendrimer activated T-cells.
PBMC from at least three healthy donor volunteers were either untreated or treated with an anti-CD3 antibody or PAMAM dendrimers for 1 h. Analysis of IC expression was performed by flow cytometry as described in materials and methods. (A) Negative control (untreated cells); (B) cells treated with α–CD3 (OKT) antibodies; (C) cells treated with G5 amine-terminated PAMAM dendrimers. (D, E) Cells were treated with G5 PAMAM dendrimers with different surface functional groups. G5-NH2 are cationic, amine-terminated dendrimers, G5-OH are aminoethanol-terminated, neutral dendrimers, and G5-COOH are succinamic acid-terminated, anionic dendrimers). (F, G) Amine-terminated PAMAM dendrimers of various generations were analyzed at equivalent mass (20μg/mL). (H, I) Amine-terminated PAMAM dendrimers of various generations were analyzed at equivalent molar (0.7 μM) concentration. (D, F, H) Percentage of lymphocytes stained positive with C3adesArg antibody (C3a+). (E, G, I) Level of IC expression on individual cells (C3a level, MFI). Each sample from each donor was analyzed in duplicate. Shown is the mean response from three donors and standard deviation (N=3). MFI – mean fluorescence intensity.
All tested nanoparticles, except for the amine-terminated PAMAM dendrimers, did not induce the detectable complement on the cell surface (Figure 1 C and Supplementary Figure 1). The IC activation by cationic dendrimers was more pronounced in G5-NH2 dendrimer-treated cells. Therefore, all subsequent studies focused on these particles.
Physicochemical properties determine the induction of IC
To understand the role of surface functional groups in the IC activation, we treated PBMC with succinamic acid-, aminoethanol- and amine-terminated PAMAM dendrimers of the same generation (G5). Only amine-terminated PAMAM dendrimers induced the expression of the complement on lymphocyte surface (Figure 1 D and E).
To understand the role of particle size, we studied amine-terminated PAMAM dendrimers of various generations (G3, G4, and G5)22. We tested these particles at an equivalent mass concentration (μg/mL) to estimate the role of particle size while keeping the total number of surface amines the same22. The equimolar concentrations were analyzed to understand the role of surface group density22. The expression of the complement on the lymphocytes surface increased proportionally to both the particle size (Figure 1 F and G) and the density of surface amines (Figure 1 H and I).
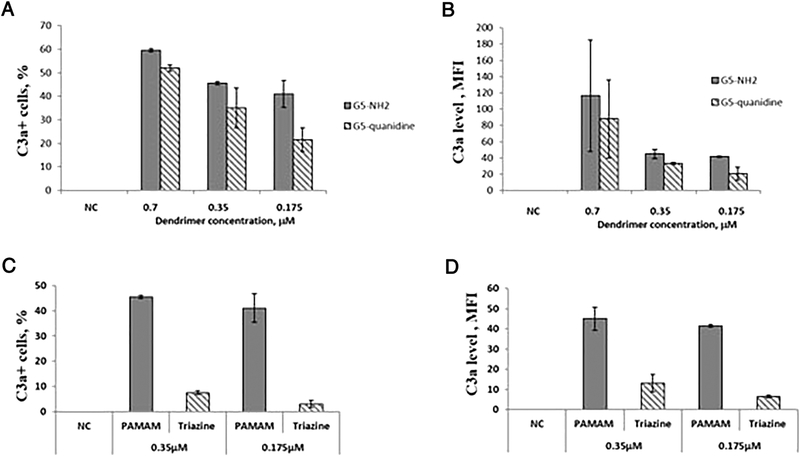
To further understand the structural characteristics of cationic PAMAM dendrimers that trigger IC activation, we studied dendrimers with amine- and guanidine-terminal groups. The expression of the complement on lymphocytes was comparable between amine- and guanidine-terminated dendrimers (Figure 2 A and B). Only at the lowest tested concentration, the amine-terminated dendrimers were more potent inducers of the IC than their guanidine-terminated counterparts (p < 0.05) (Figure 2 A and B).
Figure 2. Dendrimer composition and architecture, but not the type of cationic surface groups, influence the activation of IC.
PBMC from at least three healthy donor volunteers were treated with either PBS, negative control (NC) or dendrimers for 1 h. (A, B) Surface functionality responsible for the cationic charge of PAMAM dendrimers was either amine or guanidine. The filled bars show the data generated with amine-terminated G5 PAMAM dendrimers and hatched bars show the results obtained with guanidine-terminated G5 PAMAM dendrimers. (C,D) Dendrimers with different composition and architecture, PAMAM and triazine, were studied. The filled bars show the data generated with amine-terminated G5 PAMAM dendrimers and hatched bars show the results obtained with triazine dendrimers. Analysis of IC expression on the surface of cells was performed by flow cytometry as described in materials and methods. (A, C) The percent of lymphocytes positive for surface staining with C3adesArg antibody (C3a+ cells, %). (B, D) The level of IC expression detectable by the C3adesArg antibody on individual cells (C3a level, MFI). Each bar shows the mean response of three donors and standard deviation (N=3). A statistically significant difference (p < 0.05) was observed between amine- and guanidine-terminated dendrimers at the lowest tested concentration of 0.175 μM as well as between PAMAM and triazine dendrimers. MFI – mean fluorescence intensity.
Dendrimer composition and architecture influence IC activation
To study the role of the composition and architecture, we tested cationic G5 triazine dendrimers. These particles also have cationic surface functionality but differ from G5 PAMAM dendrimers by composition and architecture40, 41. Triazine dendrimers treatment increased both the percentage of complement-expressing cells and the expression level on individual cells (Figure 2 C and D). However, unlike the PAMAM dendrimers of the same generation, the effect of triazine dendrimers was significantly lower (Figure 2C and D, compare filled bars and hatched bars). To further verify the role of architecture, we treated cells with the linear cationic polymer (poly-L-lysine, PLL) and a cationic peptide (polymixin B, PMB) at an equivalent molar concentration to that of G5-amine-terminated PAMAM dendrimers. The induction of IC was observed only on cells treated with PLL but not with PMB (Supplementary Figure 1).
Membrane damage is involved in the IC activation
To understand the mechanism of the IC activation by cationic PAMAM dendrimers, we tested a comprehensive set of proteases with various specificity (Supplementary Table 1). The inhibition of Cathepsin L, previously described as a key trigger of the IC14, did not affect both the G5-NH2 dendrimer- and OKT3-induced IC (Supplementary Figure 2). Next, we assessed the role of other cellular enzymes, previously reported to process plasma complement21. We tested both cell-permeable and extracellular protease inhibitors. The use of a variety of protease inhibitors was equally unsuccessful (data not shown).
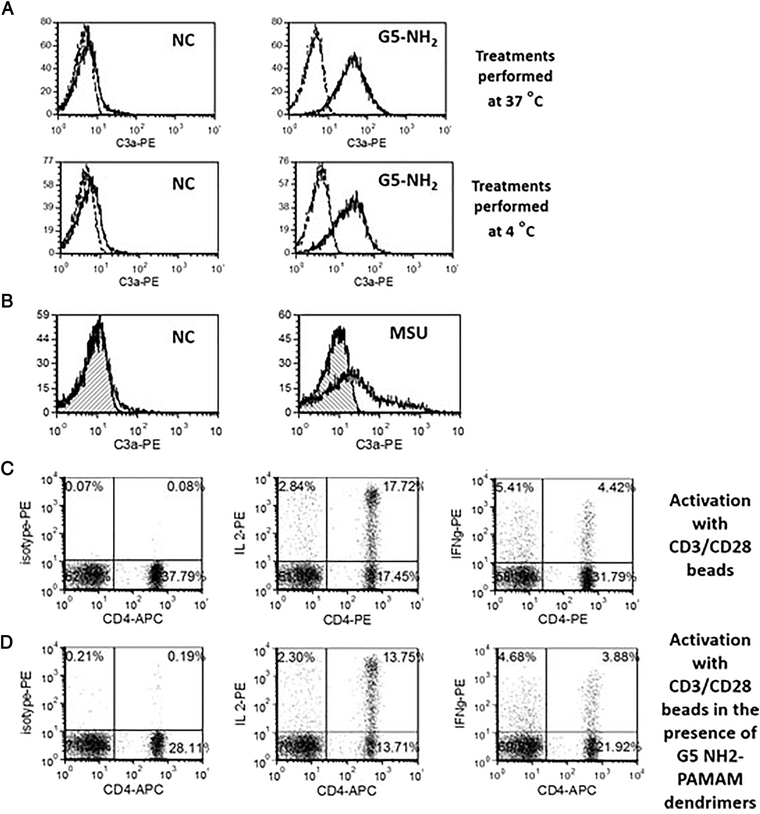
Since cationic dendrimers are known for their ability to poke holes in cellular membranes42, 43, we hypothesized that the processing of IC occurs inside the cell, and then small peptides (e.g., C3a) leak through the holes onto the membrane. Therefore, we treated cells with G5 amine-terminated dendrimers at 37 °C and on ice. The IC was equally expressed at both temperature conditions (Figure 3 A). This finding was further confirmed by the leakage of fluorescein from the fluorescein diacetate-loaded cells after the treatment with dendrimers (Supplementary Figure 3). We also performed a western blot and ELISA analysis of total cellular lysates to confirm that low levels of C3 split products are already present in the untreated cells (Supplementary Figures 4A and 5).
Figure 3. Membrane permeability underlies the mechanism of IC activation by cationic PAMAM dendrimers.
PBMC from at least three healthy donor volunteers were either untreated or treated G5 cationic PAMAM for 1 h. Percent of lymphocytes positive for the surface expression of C3a were analyzed by flow cytometry. (A) The experiment was performed under 37 °C or 4 °C. Dotted line – isotype; solid line – C3adesArg (B) Monosodium urate crystals (MSU) at concentration 0.1 mg/mL were tested to verify the role of other danger signals with membrane permeabilizing activity on the IC activation. Hatched histogram – isotype control; clear histogram – C3adesArg (C) T-cell activation with CD3/CD28 beads results in expression of intracellular cytokines IL-2 and IFNγ (D) The presence of C3a inducing G5 amine-terminated PAMAM dendrimers during activation with CD3/CD28 beads did not interfere with T-cell functionality. Shown are representative images from one donor. Total of at least three donors was used in each experiment, and all treatments in individual donors were tested in duplicate.
Since these data supported the membrane leakage mechanism, we next hypothesized that danger signals known to increase the permeability of a cellular membrane might have the same effect on the IC. The monosodium urate crystals (MSU) induced complement expression on lymphocytes (Figure 3 B). To rule out a potential cell death contribution, we induced apoptosis in T-cells using CD95 antibody. While the cell death was verified, it was not accompanied by the appearance of the IC on lymphocytes’ surface (Supplementary Figure 6). Moreover, we demonstrated that T-cells retain their functionality. We activated T-cells with CD3/CD28 beads either alone or in the presence of G5-PAMAM dendrimers. IC expression (data not shown), and activation markers (intracellular cytokines IL-2 and IFNγ) were measured. Despite the IC induction, dendrimers did not affect the lymphocyte activation by CD3/CD28 cross-linking (Figure 3 C and D). Weak inhibition of IL-7 stimulated proliferation was observed. However neither its physiological consequence nor the role of IC in this phenomenon are fully understood (Supplementary Figure 7).
Proteomics verified IC presence on dendrimer-treated lymphocytes
During this study, we noticed that only one clone (2991) of C3adesArg specific antibodies detects IC activation by both dendrimers and CD3/CD28 beads. The same clone detected C3a in plasma treated with cobra venom factor (CVF), a known activator of the plasma complement (Supplementary Figure 4B). Two other clones (M81625 and K13/16) did not work in the flow cytometry assay. The authors of the original study14 describing the IC activation by CD3/CD28-cross-linking confirmed the same observation (personal communication). Both M81625 and K13/16 clone detected C3 split product(s) when CVF-treated plasma samples were tested by western blot analysis (Supplementary Figure 5). Therefore, even though we used the isotype control to rule-out false-positive results, we conducted additional studies to verify the flow cytometry data by mass spectrometry.
Since the proteomics analysis required cellular membrane fractions from the large (2 × 108) number of cells, we had to use a model cell line. In preliminary experiments, we demonstrated that Jurkat cells replicate the data observed with primary human PBMC. Therefore, for this study, we treated Jurkat cells with G5 amine-terminated dendrimers or PBS (as a negative control) and split each sample into two halves. One half was analyzed by flow cytometry to confirm the IC induction (data not shown). The second half was used to isolate the membrane fraction for mass spectrometry.
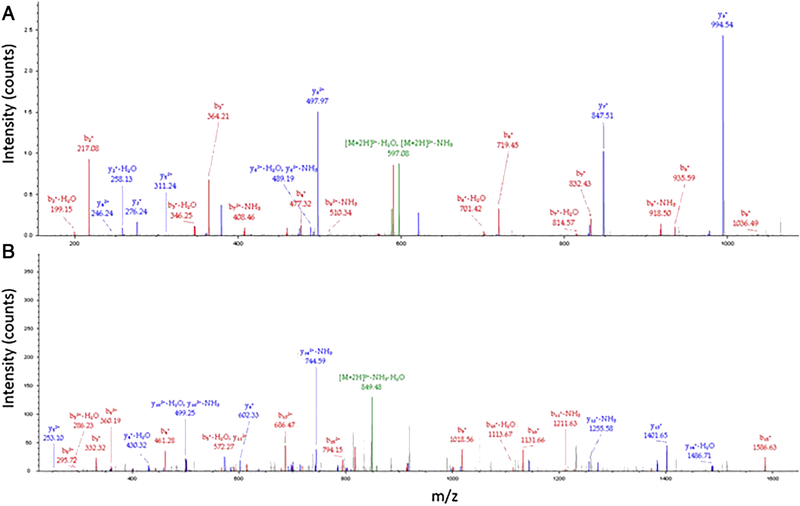
The subtractive shot-gun membrane proteomics44 identified 13,164 tryptic peptides that corresponded to detection of 2.903 protein groups in the dendrimer-treated cells whereas the analysis of the negative control Jurkat cells yielded the identification of a total of 16,275 tryptic peptides that corresponded to detection of 3,350 protein groups. The subsequent analysis identified 270 proteins, including C3 complement, present solely in the membrane fraction of dendrimer-treated cells. The analyses of annotated MS2 spectra and the output of the BLAST analysis (Supplementary Figure 8) against the bovine complement C3 unambiguously confirmed the identification of the human C3 peptides C3d and C3c (Figure 4 and Supplementary Figure 9).
Figure 4. Mass Spectrometry identified complement peptides C3c and C3d in membrane fractions of Jurkat cells treated with amine-terminated PAMAM dendrimers.
Jurkat cells were either untreated or treated with 10 mg/mL of amine-terminated PAMAM dendrimers for one hour. The membrane fractions were isolated and analyzed by mass spectrometry as described in materials and methods. (A) Identification of the tryptic peptide NTMILEICTR corresponding to the human C3d complement split product. (B) Identification of the tryptic peptide DICEEQVNSLPGSITK corresponding to the human C3c complement split product. Each peak represents a corresponding fragment ion.
Since the antibody used for flow cytometry is distributed commercially as that specific to C3adesArg and the mass spectrometry identified C3d and C3c fragments, we attempted to verify a cross-reactivity between the commercial C3adesArg antibody and recombinant C3c protein. To do so, we prepared total cell lysates of control and dendrimer-treated Jurkat cells and analyzed them side-by-side with plasma-derived and recombinant E.coli expressed human C3c proteins. The analysis was performed by Western blot using C3adesArg-specific antibody clone 2991 (used in flow cytometry experiments), C3adesArg-specific antibody clone K13/16 (claimed to be suitable for western blotting), and C3c-specific antibody. A band with molecular weight, expected for the C3c component of the complement, was detected in cell lysates by both C3adesArg-specific antibodies (Supplementary Figure 5). However, only clone K13/16 also reacted with recombinant C3c. The clone 2991 did not produce a band in lanes with recombinant C3c (Supplementary Figure 5). The C3c-specific antibody detected a specific band with molecular weight expected for C3c in both the recombinant protein and cell lysate lanes (Supplementary Figure 5). Consistent with the mass spectrometry findings, when used in flow cytometry, the C3c antibody detected C3c-positive Jurkat cells after the treatment with PAMAM-dendrimers, but not in the control sample (Supplementary Figure 10). The analysis of C3d in lysates and on cells was not performed due to the lack of commercially available reagents suitable for this analysis.
Discussion
The induction of IC was observed in one out of the seventeen studied nanoparticles (Table 1). The cationic dendrimers induced IC expression in a size and surface group density-dependent manner (Figure 1). These data emphasize the importance of the chemical composition, surface functionality, size and the density of surface groups, and are in agreement with our earlier studies of the dendrimer hematocompatibility22, 23, 26. The source of the cationic charge did not appear as a key contributor for the IC activation as both amine- and guanidin-terminated dendrimers triggered the IC expression on the surface of lymphocytes and weak, but statistically significant difference between two surface types, was observed only at the lowest tested concentration (Figure 2 A and B). Since PAMAM-dendrimers were more potent IC inducers than triazine dendrimers of the same generation (Figure 2), the data highlight the role of the particle composition and architecture. The induction of IC was observed only on cells treated with a cationic linear polymer (PLL) but not with a polycationic peptide (PMB) (Supplementary Figure 1), further suggesting that the architecture is an important factor in that polymeric and globular materials have the propensity of poking holes in the cell membrane. The size of these holes is sufficient to deliver the danger signal and result-in self-opsonization through the leakage of IC, yet insufficient to reduce cell viability. The leakage hypothesis was also confirmed using fluorescein diacetate loaded T-cells (Supplementary Figure 3).
We attributed the mechanism of the IC activation by dendrimers to their membrane-hole poking activity (Figure 3) and not due to cell death (data not shown). The cell viability and functionality, as assessed by the production of intracellular cytokines (Figure 3) and proliferation (data not shown) in response to CD3/CD28 activation, were confirmed. This data correlated with the results obtained using a known danger signal, MSU (Figure 3), suggesting that lymphocytes sense cationic dendrimers as danger signals. The IC expression on the cell surface, therefore, serves as the self-opsonization mechanism to identify damaged cells and, possibly, mediate their clearance. However, the functional consequence of this mechanism requires further investigation. We speculate that our in vitro models are insensitive to such functional changes. Interestingly, Cathepsin L inhibitors from different suppliers (Supplementary Table 1) did not inhibit the IC induction by dendrimers (Supplementary Figure 2), suggesting that the cleavage of C3 complement occurs constitutively in our test-model. Both western blot and ELISA analysis (Supplementary Figures 4 and 5) confirmed this finding. The equal inefficiency of Cathepsin L inhibitors to suppress OKT3-triggered IC activation, observed in our study (Supplementary Figure 2), is in contrast to the original report by Liszewski M et al.,14.
We demonstrated that commercial monoclonal antibodies with claimed specificity to the neo-epitope of cleaved C3a component of the complement (C3adesArg) have different specificity and may cross-react with other C3 split products. We verified the presence of C3 split products C3c and C3d on the surface of dendrimer-treated lymphocytes by mass spectrometry (Figure 4). While this data was subsequently verified by cross-qualification experiments of C3adesArg and C3c antibodies using both western blot and flow cytometry, our data emphasize the importance of the thorough antibody characterization and special care in interpreting and reporting the IC activation data generated using commercial monoclonal antibodies.
In summary, we demonstrated that the nanoparticle physicochemical properties (size, charge, surface functionalities and architecture) determine the activation of IC through the mechanism involving membrane damage and without substantial changes of cytokine production and proliferative responses of these cells in vitro. Functional consequences of such IC activation by nanomaterials remain unknown. One potential role of the IC could be a process of self-opsonization, thereby the damaged cells communicate the presence of the danger to other cells in their microenvironment and are cleared from the body. It would also be interesting to see if the activation of IC in T-cells in vivo is linked to the DTH reactions developed by a human subject exposed to dendrimers in the occupational settings and reported earlier45 or to other types of immunotoxicity experienced by patients treated with nanomedicines. Therefore, further research, including both in vivo and clinical studies, is warranted to understand the functional significance of the IC activation by nanomaterials, and the IC contribution to immunotoxicity observed in some patients undergoing nanomedicine-based therapies.
Supplementary Material
Intracellular complement is recently discovered and not studied in the context of nanomaterial toxicity to the immune cells. The figure contrasts intracellular complement to the plasma complement. In our study we investigated structure activity relationship and performed mechanistic study to understand nanoparticle-mediated activation of the complement produced by human T-cells in vitro
Acknowledgment
The study was supported in part (A.N.I., A.S., J.B., and M.A.D) by federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The study was also funded in part (A.E.E., E.E.S.) by the Robert A. Welch Foundation (P-0008) and Department of Defense (W81XWH-12-1-0338). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a servicse to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest
References
- 1.Lee H, Whitfeld PL and Mackay CR, Receptors for complement C5a. The importance of C5aR and the enigmatic role of C5L2. Immunol Cell Biol. 2008;86:153–60 [DOI] [PubMed] [Google Scholar]
- 2.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. , Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–7 [DOI] [PubMed] [Google Scholar]
- 3.Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, et al. , Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szebeni J, Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. 2014;61:163–73 [DOI] [PubMed] [Google Scholar]
- 5.Heeger PS and Kemper C, Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217:216–24 [DOI] [PubMed] [Google Scholar]
- 6.Reis ES, Barbuto JA, Kohl J and Isaac L, Impaired dendritic cell differentiation and maturation in the absence of C3. Mol Immunol. 2008;45:1952–62 [DOI] [PubMed] [Google Scholar]
- 7.Ghannam A, Fauquert JL, Thomas C, Kemper C and Drouet C, Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Mol Immunol. 2014;58:98–107 [DOI] [PubMed] [Google Scholar]
- 8.Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Kohl J, et al. , C3a modulates IL-1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122:3473–81 [DOI] [PubMed] [Google Scholar]
- 9.Ricklin D, Hajishengallis G, Yang K and Lambris JD, Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baudino L, Sardini A, Ruseva MM, Fossati-Jimack L, Cook HT, Scott D, et al. , C3 opsonization regulates endocytic handling of apoptotic cells resulting in enhanced T-cell responses to cargo-derived antigens. Proc Natl Acad Sci U S A. 2014;111:1503–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botto M and Walport MJ, C1q, autoimmunity and apoptosis. Immunobiology. 2002;205:395–406 [DOI] [PubMed] [Google Scholar]
- 12.Pickering MC, Botto M, Taylor PR, Lachmann PJ and Walport MJ, Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324 [DOI] [PubMed] [Google Scholar]
- 13.Morgan BP and Gasque P, Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol. 1997;107:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, et al. , Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho MS, Vasquez HG, Rupaimoole R, Pradeep S, Wu S, Zand B, et al. , Autocrine effects of tumor-derived complement. Cell Rep. 2014;6:1085–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Q, Li K, Patel H, Sacks SH and Zhou W, Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol. 2006;176:3330–41 [DOI] [PubMed] [Google Scholar]
- 17.Kolev M, Dimeloe S, Le Friec G, Navarini A, Arbore G, Povoleri GA, et al. , Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity. 2015;42:1033–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szebeni J, Muggia F, Gabizon A and Barenholz Y, Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention. Adv Drug Deliv Rev. 2011;63:1020–30 [DOI] [PubMed] [Google Scholar]
- 19.Szebeni J, Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–21 [DOI] [PubMed] [Google Scholar]
- 20.Szebeni J, Bedocs P, Rozsnyay Z, Weiszhar Z, Urbanics R, Rosivall L, et al. , Liposome-induced complement activation and related cardiopulmonary distress in pigs: factors promoting reactogenicity of Doxil and AmBisome. Nanomedicine. 2012;8:176–84 [DOI] [PubMed] [Google Scholar]
- 21.Kolev M, Le Friec G and Kemper C, Complement--tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–20 [DOI] [PubMed] [Google Scholar]
- 22.Dobrovolskaia MA, Patri AK, Potter TM, Rodriguez JC, Hall JB and McNeil SE, Dendrimer-induced leukocyte procoagulant activity depends on particle size and surface charge. Nanomedicine (Lond). 2012;7:245–56 [DOI] [PubMed] [Google Scholar]
- 23.Dobrovolskaia MA, Patri AK, Simak J, Hall JB, Semberova J, De Paoli Lacerda SH, et al. , Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol Pharm. 2012;9:382–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobrovolskaia MA, Neun BW, Clogston JD, Grossman JH and McNeil SE, Choice of method for endotoxin detection depends on nanoformulation. Nanomedicine (Lond). 2014;9:1847–56 [DOI] [PubMed] [Google Scholar]
- 25.Enciso AE, Neun B, Rodriguez J, Ranjan AP, Dobrovolskaia MA and Simanek EE, Nanoparticle Effects on Human Platelets in Vitro: A Comparison between PAMAM and Triazine Dendrimers. Molecules (Basel, Switzerland). 2016;21:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilinskaya AN, Man S, Patri AK, Clogston JD, Crist RM, Cachau RE, et al. , Inhibition of phosphoinositol 3 kinase contributes to nanoparticle-mediated exaggeration of endotoxin-induced leukocyte procoagulant activity. Nanomedicine (Lond). 2014;9:1311–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim J, Kostiainen M, Maly J, da Costa VC, Annunziata O, Pavan GM, et al. , Synthesis of large dendrimers with the dimensions of small viruses. Journal of the American Chemical Society. 2013;135:4660–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enciso AE, Abid ZM and Simanek EE, Rapid, semi-automated convergent synthesis of low generation triazine dendrimers using microwave assisted reactions. Polymer Chemistry. 2014;5 [Google Scholar]
- 29.Raut S, Enciso AE, Pavan GM, Lee C, Yepremyan A, Tomalia DA, et al. , Intrinsic fluorescence of triazine dendrimers provides a new approach to study dendrimer structure and conformational dynamics. J. Phys. Chem. C 2017;121:6946 [Google Scholar]
- 30.Ye X, Johann DJ Jr., Hakami RM, Xiao Z, Meng Z, Ulrich RG, et al. , Optimization of protein solubilization for the analysis of the CD14 human monocyte membrane proteome using LC-MS/MS. Journal of proteomics. 2009;73:112–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blonder J, Chan KC, Issaq HJ and Veenstra TD, Identification of membrane proteins from mammalian cell/tissue using methanol-facilitated solubilization and tryptic digestion coupled with 2D-LC-MS/MS. Nature protocols. 2006;1:2784–90 [DOI] [PubMed] [Google Scholar]
- 32.Johann DJ Jr., Wei BR, Prieto DA, Chan KC, Ye X, Valera VA, et al. , Combined blood/tissue analysis for cancer biomarker discovery: application to renal cell carcinoma. Analytical chemistry. 2010;82:1584–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budinger L and Hertl M, Immunologic mechanisms in hypersensitivity reactions to metal ions: an overview. Allergy. 2000;55:108–15 [DOI] [PubMed] [Google Scholar]
- 34.Lanone S and Boczkowski J, Titanium and gold nanoparticles in asthma: the bad and the ugly. Eur Respir J. 2011;37:225–7 [DOI] [PubMed] [Google Scholar]
- 35.Hussain S, Vanoirbeek JA, Luyts K, De Vooght V, Verbeken E, Thomassen LC, et al. , Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. Eur Respir J. 2011;37:299–309 [DOI] [PubMed] [Google Scholar]
- 36.Ilves M, Palomaki J, Vippola M, Lehto M, Savolainen K, Savinko T, et al. , Topically applied ZnO nanoparticles suppress allergen induced skin inflammation but induce vigorous IgE production in the atopic dermatitis mouse model. Part Fibre Toxicol. 2014;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unterweger H, Janko C, Schwarz M, Dezsi L, Urbanics R, Matuszak J, et al. , Nonimmunogenic dextran-coated superparamagnetic iron oxide nanoparticles: a biocompatible, size-tunable contrast agent for magnetic resonance imaging. International journal of nanomedicine. 2017;12:5223–5238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stern ST, Adiseshaiah PP and Crist RM, Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah A, Mankus CI, Vermilya AM, Soheilian F, Clogston JD and Dobrovolskaia MA, Feraheme(R) suppresses immune function of human T lymphocytes through mitochondrial damage and mitoROS production. Toxicology and applied pharmacology. 2018;350:52–63 [DOI] [PubMed] [Google Scholar]
- 40.Simanek EE and Enciso AE, 2014. Cationic Triazine Dendrimers: Synthesis, Characterization and Biological Applications in Samal SK and Dubruel P (eds) Cationic Polymers in Regenerative Medicine: Methods and Applications, RSC Polymer Chemistry, Cambridge, UK [Google Scholar]
- 41.Simanek EE, Abdou H, Lalwani S, Lim J, Mintzer M, Venditto VJ, et al. , The eight year thicket of triazine dendrimers: strategies, targets and applications. Proc. Royal Soc 2010;466:1445 [Google Scholar]
- 42.Hong S, Bielinska AU, Mecke A, Keszler B, Beals JL, Shi X, et al. , Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: hole formation and the relation to transport. Bioconjugate chemistry. 2004;15:774–82 [DOI] [PubMed] [Google Scholar]
- 43.Hong S, Leroueil PR, Janus EK, Peters JL, Kober MM, Islam MT, et al. , Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjugate chemistry. 2006;17:728–34 [DOI] [PubMed] [Google Scholar]
- 44.Schirmer EC, Florens L, Guan T, Yates JR 3rd and Gerace L, Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science (New York, N.Y.). 2003;301:1380–2 [DOI] [PubMed] [Google Scholar]
- 45.Toyama T, Matsuda H, Ishida I, Tani M, Kitaba S, Sano S, et al. , A case of toxic epidermal necrolysis-like dermatitis evolving from contact dermititis of the hands associated with exposure to dendrimers. Contact Dermatitis. 2008;59:122–123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.