Abstract
Given that the search for effective pharmacotherapies for cocaine use disorder has, thus far, been fruitless, there remains a critical need for conceptually innovative approaches toward identifying new medications to treat this disease. A better understanding of the neurocircuits and neurobiological mechanisms underlying cocaine taking and seeking may identify molecular substrates that could serve as targets for novel pharmacotherapies to treat cocaine use disorder. Recent preclinical evidence suggests that glucagon-like peptide-1 (GLP-1) receptor agonists could be re-purposed to treat cocaine craving-induced relapse. This review endeavors to comprehensively summarize the current literature investigating the efficacy of GLP-1 receptor agonists in reducing the rewarding and reinforcing effects of cocaine in animal models of cocaine use disorder. The role of central endogenous GLP-1 circuits in voluntary cocaine taking and seeking is also discussed. Behavioral, neurochemical, electrophysiological and molecular biology studies indicate that central GLP-1 receptor activation functionally modulates the mesolimbic reward system and decreases addiction-like phenotypes in rodents. Overall, an emerging preclinical literature provides compelling evidence to advance GLP-1 receptor agonists into clinical trials testing the efficacy of these medications in preventing cocaine craving-induced relapse.
Keywords: relapse, drug self-administration, reinstatement, drug discovery, mesolimbic reward system, GLP-1, exendin-4, addiction
1. Introduction
1.1. Cocaine Use Disorder
Cocaine use disorder continues to be a significant public health concern in the United States with approximately 5.9 million Americans (2.2% of the population) aged 12 years or older reporting having used cocaine in the past year [1]. Recent epidemiological data indicate that the prevalence of cocaine use is increasing [2]. For example, ~2% of all young adults (18–25 years of age) were classified as current users of cocaine in 2017, the highest rate reported for this age group in the past 10 years [1]. One hallmark of cocaine use disorder is the high rate of relapse following detoxification [3, 4]. Unfortunately, there are no FDA-approved medications to treat cocaine craving-induced relapse despite decades of focused preclinical and clinical research that have advanced our understanding of the anatomical, neurochemical and molecular bases of drug addiction [4, 5]. Thus, there is a clear need for innovative research aimed at identifying novel neurobiological mechanisms underlying cocaine addiction and new therapeutic drug targets to treat this disease.
1.2. Animal Models of Cocaine Use Disorder
Preclinical studies utilizing rodent models of addiction-like behaviors are critical towards identifying molecular substrates that could serve as targets for novel pharmacotherapies aimed at preventing or reducing relapse in human cocaine addicts. The conditioned place preference (CPP) paradigm is a popular behavioral model used to study the rewarding effects of drugs of abuse [6, 7]. Drug-induced CPP is rooted in classical Pavlovian conditioning and reflects a preference (or avoidance) for a context based on the contiguous pairing of the context with drug delivery. In the CPP paradigm, the rewarding properties of a drug serve as an unconditioned stimulus (US) that is repeatedly paired with a neutral set of environmental stimuli which acquire, during the conditioning phase, secondary rewarding properties such that these stimuli now function as conditioned stimuli (CS). In subsequent CPP tests, CS exposure in the absence of the US elicits either a preference or aversion for a previously drug-paired environment depending on whether the US produced primary motivational properties that were rewarding or aversive, respectively [6, 7]. Thus, CPP measures drug-induced learned associations that are fundamentally distinct from voluntary drug taking. However, it is important to note that CPP lacks face validity as a model of drug reward in humans [7].
Cocaine craving and relapse of drug-taking behavior in abstinent human addicts are precipitated by three major stimuli: stressful life events, re-exposure to environmental cues previously associated with drug taking, and re-exposure to the previously self-administered drug itself [8–11]. Craving-induced relapse of drug taking/seeking in humans is typically modeled in laboratory animals as follows: after a period of drug self-administration and the subsequent extinction of the drug-reinforced operant behavior, the ability of stress exposure, drug-associated stimuli, or re-exposure to the drug itself to reinstate drug-seeking behavior is assessed [12–14]. For example, after extinction of cocaine self-administration, administration of relatively low doses of cocaine reinstate operant responding in the absence of drug reinforcement in both non-human primates and rodents [8, 15–17]. As the most commonly used animal model of relapse, the reinstatement model has proven invaluable for elucidating the neural circuits and neurobiological mechanisms underlying cocaine-seeking behavior [18, 19].
1.3. Neurocircuitry Mediating the Rewarding and Reinforcing Efficacy of Cocaine
Cocaine functions, in part, as a non-selective biogenic amine transporter inhibitor that binds to and inhibits dopamine, serotonin and norepinephrine transporters [20]. Biogenic amine transporters are directly coupled to transmembrane Na+/Cl− gradients and convey neurotransmitters from the extracellular space into the presynaptic nerve terminal [21]. Indeed, the primary mechanism by which dopamine, serotonin and norepinephrine signaling is inactivated in the brain is through high-affinity transporter-mediated uptake [21]. Thus, cocaine’s main pharmacological mechanism of action in the brain is to increase extracellular concentrations of dopamine, norepinephrine and serotonin resulting in enhanced transmission of these biogenic amines.
Drugs of abuse including cocaine produce their reinforcing effects through actions in the mesolimbic reward system, a circuit of functionally and anatomically interconnected nuclei that are responsible for the influence of motivational, emotional, contextual and affective information on behavior [18, 22]. Limbic nuclei, including the amygdala, hippocampus, and medial prefrontal cortex (mPFC), send major glutamatergic projections to the nucleus accumbens, which functions as a hub integrating this information with midbrain dopamine innervation that encodes motivational information pertaining to the rewarding and reinforcing properties of drugs of abuse [23]. The nucleus accumbens is broadly divided into two main subregions, the accumbens shell and core [24]. The nucleus accumbens sends segregated efferent GABAergic projections to the ventral pallidum and ventral tegmental area (VTA)/substantia nigra [24, 25]. Both the ventral pallidum and VTA, in turn send GABAergic efferent projections to the medial dorsal thalamus. Glutamatergic projections from the medial dorsal thalamus to the mPFC close this limbic circuit [26–28]. Dopaminergic neurons in the VTA innervate the nucleus accumbens, amygdala, hippocampus, mPFC, and ventral pallidum, and changes in dopaminergic transmission play a critical role in modulating the flow of information through the mesolimbic reward system [29–32]. Thus, excitatory input from cortical and subcortical structures to the nucleus accumbens is filtered and integrated by dopamine-mediated mechanisms, thereby shaping information output to the basal ganglia [18].
Midbrain dopamine neurons exhibit distinctive firing patterns including occasional, high-frequency trains of action potentials known as bursts [33, 34]. Bursts of action potentials from midbrain dopamine neurons and their associated release of dopamine in target nuclei are collectively termed “phasic” dopamine signaling [35]. Phasic burst firing of midbrain dopamine neurons and subsequent phasic release of dopamine in the nucleus accumbens are key neurophysiological mechanisms that implicate mesolimbic dopamine signaling as critical for goal-directed behaviors including drug reinforcement [36–41]. Phasic mesolimbic dopamine signaling is thought to reinforce learned associations between predictive stimuli and primary reward [39, 42]. Based on a large literature examining reward prediction error, phasic dopamine is commonly referred to as a “teaching” signal that functionally regulates motivational aspects of behavior [35, 43, 44]. Phasic dopamine signaling is also believed to play an important role in the incentive-sensitization theory of addiction [for further review, please see 45, 46, 47].
The reinforcing effects of cocaine are primarily dependent upon activation of the mesolimbic dopamine system [22, 48, 49]. Dopaminergic projections from the VTA to limbic nuclei including the nucleus accumbens play a critical role in cocaine self-administration and the reinstatement of cocaine-seeking behavior [19, 22, 49–51]. Additionally, recent studies have begun to examine how the mesolimbic dopamine system is modulated by downstream nuclei. For example, regions such as the lateral septum, laterodorsal tegmental nucleus (LDTg), habenula, and lateral hypothalamus project to the VTA and regulate cocaine-mediated behaviors by modulating dopamine transmission in midbrain and forebrain nuclei [52–58].
In addition to regulating drug craving and relapse, the mesolimbic dopamine system also plays an important role in regulating the hedonic value of food and consequently behaviors directed towards the consumption of food [59–62]. Moreover, the neurobiological mechanisms underlying food intake and drug seeking overlap to a degree [63]. For example, neuropeptide-mediated signaling in the VTA plays a key role in modulating mesolimbic dopamine transmission and the rewarding properties of both food and drugs of abuse [61, 63]. These findings suggest that the same biochemical and molecular mechanisms that play a role in the control of food intake may also influence voluntary drug taking and seeking. Indeed, the idea that shared neural mechanisms regulating both food intake and drug seeking has informed basic science approaches toward identifying novel pharmacotherapies for drug addiction.
1.4. Metabolic Factors Regulate the Mesolimbic Reward System and Food Intake
Identifying endogenous modulators of the mesolimbic reward system may provide new targets for drug discovery programs aimed at developing novel medications to treat obesity and substance use disorders [61, 64]. Over the last two decades, it has become clear that peripheral and central homeostatic regulators of hunger, satiety and body weight interact with and influence the mesolimbic reward system [63, 65, 66]. These metabolic factors regulate the mesolimbic reward system directly by stimulating or inhibiting VTA dopamine neurons through cognate receptors or indirectly through downstream nuclei projecting to the VTA [67–71]. Indeed, metabolic factors such as insulin, leptin, glucagon-like peptide-1 (GLP-1) and amylin inhibit VTA dopamine neurons and decrease food intake [64, 72]. Furthermore, central administration of both leptin and insulin are known to decrease striatal dopamine release as well as act directly on dopamine neurons to regulate food intake [68, 73–75]. In contrast, the feeding hormone ghrelin promotes ad libitum food intake through actions on ghrelin receptors expressed in the VTA and nucleus accumbens [76, 77]. The effects of ghrelin on mesolimbic dopamine signaling and food intake, however, are complex. For example, infusions of ghrelin directly into the VTA, but not the nucleus accumbens, increase food motivated behaviors (i.e. operant responding for food) adding further complexity to how the mesoaccumbens dopamine system integrates feeding signals [71, 78, 79]. These divergent effects may be due to poly-synaptic connections between feeding-relevant nuclei as infusions of ghrelin directly into the lateral hypothalamus, but not VTA, potentiate phasic dopamine release in the nucleus accumbens in response to food reward and food-predictive cues [80, 81]. Based on their ability to modulate the mesolimbic reward system, it has been hypothesized that metabolic factors may regulate non-drug motivated behaviors including addiction-like phenotypes [82, 83].
1.5. Glucagon-like Peptide-1 (GLP-1)
Glucagon-like peptide-1 (GLP-1) is an incretin hormone and satiation factor that is released predominantly from L cells of the small intestine and neurons in the nucleus tractus solitarius (NTS) of the caudal brainstem [65, 84, 85]. GLP-1 is the primary posttranslational product of the preproglucagon (PPG) gene [86, 87]. PPG-expressing neurons in the NTS are the primary central source of GLP-1 and these neurons project to many midbrain and forebrain areas including those implicated in goal-directed behaviors (e.g., VTA and nucleus accumbens) [67, 88, 89]. Peripheral GLP-1 has a relatively short plasma half-life of ~2 minutes in both humans and rodents as it is rapidly degraded by the enzyme dipeptidyl peptidase-IV (DPP-IV), which is highly expressed in tissue compartments throughout the body including the central nervous system [90–93]. The GLP-1 receptor is a G-protein coupled receptor that is expressed on both pre- and post-synaptic sites throughout the brain [84, 88, 94]. The GLP-1 receptor couples to different G proteins, including Gs, Gq, and Gi subunits, that stimulate or inhibit intra-cellular second messenger systems [95–97]. However, most intra-cellular signaling is initiated predominately by Gs subunits [97]. Studies have shown that activation of GLP-1 receptors increases intracellular calcium levels and activity of downstream signaling molecules such as adenylate cyclase, phospholipase C (PLC), protein kinase A (PKA), protein kinase C (PKC), phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) [84, 94, 98].
GLP-1 plays an important role in energy homeostasis and food intake in both humans [99, 100] and animal models [101, 102]. Activation of GLP-1 receptors produces a number of physiological responses including increased insulin secretion from pancreatic β-cells, inhibition of gastric emptying and reduced food intake [84, 103–106]. With regard to energy homeostasis, GLP-1 receptor agonists reduce food intake, in part, by activating central GLP-1 receptors [104, 107, 108]. Based on their ability to regulate blood glucose levels, food intake and body weight, GLP-1 receptor agonists are FDA-approved for the treatment of type II diabetes mellitus [106] and obesity [109].
Of interest to the drug addiction field is a body of research showing that GLP-1 receptor activation influences the hedonic value of palatable food by direct modulation of the mesolimbic reward system [66]. GLP-1-producing neurons in the NTS send monosynaptic projections to the VTA and nucleus accumbens and direct infusions of GLP-1 receptor agonists into these brain regions have been shown to reduce consumption of palatable food [66, 67, 94, 110–112]. Interestingly, some of these studies identified intra-cranial doses of GLP-1 receptor agonists that selectively reduced intake of palatable food but not intake of normal chow [67, 110]. These findings indicate that activation of GLP-1 receptors in the mesolimbic reward system selectively modulates the hedonic value of food and not homeostatic feeding [82]. Given that the reinforcing effects of drugs of abuse, including cocaine, are regulated by neural circuits that include the VTA and nucleus accumbens, these findings suggest that central GLP-1 receptor activation may also reduce non-feeding motivated behaviors including drug taking and seeking. Indeed, an emerging body of evidence reviewed below highlights a novel role for central GLP-1 receptor-expressing circuits in regulating the reinforcing efficacy of cocaine and the behavioral mechanisms underlying cocaine taking-and seeking-behaviors.
2. GLP-1 Receptor Agonist Efficacy in Animal Models of Cocaine Use Disorder
Emerging evidence indicates that GLP-1 receptors play an important role in preclinical models of substance use disorder and that systemic administration of GLP-1 receptor agonists reduces the rewarding and reinforcing effects of drugs of abuse [83]. Indeed, a growing literature has investigated the effects of GLP-1 receptor activation on cocaine-mediated behaviors as described below and summarized in Table 1.
Table 1:
Effects of GLP-1 receptor activation on cocaine-mediated behaviors
| Study | Species/Strain/Sex | Study design | Behavior | Treatment | Route of Administration | Results |
|---|---|---|---|---|---|---|
| Graham et al., 2013 | Mice, C57BL/6J, male | Between-subjects | CPP | 10, 30, 100 μg/kg exendin-4 | i.p. | Attenuated cocaine CPP |
| Egecioglu et al., 2013 | Mice, NMRI, male | Between-subjects | Locomotor activity CPP |
2.4 μg/kg exendin-4 2.4 μg/kg exendin-4 |
i.p. i.p. |
Decreased cocaine-induced hyper-locomotion Attenuated cocaine CPP |
| Harasta et al., 2015 | Mice, Glp-1r−/−, male | Between-subjects | Locomotor activity CPP |
AAV-GFP AAV-GLP-1R-GFP AAV-GFP AAV-GLP-1R-GFP |
Intra-dLS Intra-dLS Intra-dLS Intra-dLS |
Augmented cocaine-induced hyper-locomotion Normalized cocaine-induced hyper-locomotion to wild-type control levels Augmented cocaine CPP Normalized cocaine CPP to wild-type control levels |
| Sorensen et al., 2015 | Mice, NMRI, male Mice, C57BL/6J, male |
Between-subjects Within-subjects |
Locomotor activity Self-administration (single session) Self-administration |
0.3, 3, 30 μg/kg exendin-4 30, 100 μg/kg exendin-4 10 μg/kg exendin-4 |
i.p. i.p. i.p. |
Decreased basal locomotion and cocaine-induced hyper-locomotion Reduced number of nose-pokes for cocaine infusions Attenuated infusions of various unit doses of cocaine |
| Schmidt et al., 2016 | Rats, Sprague-Dawley, male | Within-subjects | Self-administration | 0.05* μg exendin-4 | Intra-VTA | Attenuated total active lever responses |
| Hernandez et al., 2017 | Rats, Sprague-Dawley, male | Wi thin-subjects | Reinstatement of cocaine-seeking behavior | 0.005*, 0.05* μg exendin-4 0.05* μg exendin-4 |
Intra-NAc core Intra-NAc shell |
Attenuated total active lever responses Attenuated total active lever responses |
| Hernandez et al., 2018 | Rats, Sprague-Dawley, male | Within-subjects | Reinstatement of cocaine-seeking behavior | 0.1*, 0.2* μg/kg exendin-4 0.05* μg exendin-4 |
i.p. Intra-VTA |
Attenuated total active lever responses Attenuated total active lever responses |
indicates doses that are behaviorally selective for cocaine; dLS=dorsal lateral septum, VTA=ventral tegmental area, NAc=nucleus accumbens, CPP=conditioned place preference, AAV-GFP=control adeno-associated virus, AAV-GLP-1R-GFP=adeno-associated virus with GLP-1 receptor transcript
2.1. Systemic Administration of a GLP-1 Receptor Agonist Attenuates Cocaine-Mediated Behaviors
Initial studies showed that systemic administration of a GLP-1 receptor agonist attenuated psychostimulant-induced conditioned place preference (CPP) and that these effects were associated with reduced extracellular dopamine levels in the nucleus accumbens of mice [113, 114]. Cocaine-induced CPP was significantly decreased in mice pretreated with exendin-4 (10.0, 30.0 and 100.0 μg/kg, i.p.) compared to vehicle-treated controls [114]. Consistent with these effects, another study found that a lower dose of exendin-4 (2.4 μg/kg, i.p.) attenuated cocaine-induced CPP in mice [113]. Together, these results indicate that peripheral administration of a GLP-1 receptor agonist is sufficient to reduce the rewarding effects of cocaine. The efficacy of exendin-4 in reducing voluntary cocaine taking has also been examined. Systemic administration of exendin-4 (30.0 and 100.0 μg/kg, i.p.) significantly decreased tail-delivered cocaine in restrained mice oriented toward a single nose poke aperture during a single self-administration session [115]. In a repeated self-administration paradigm that used freely moving mice with jugular catheters, exendin-4 (10 μg/kg, i.p.) pretreatment shifted the cocaine dose-response curve downward by decreasing the total number of infusions earned for various unit doses of cocaine, suggesting a decrease in the reinforcing efficacy of cocaine [116]. However, caution is warranted when interpreting findings from these studies. Systemic doses of exendin-4 (2.4 – 30.0 μg/kg) have been shown to reduce the locomotor activating effects of cocaine [113, 115]. Therefore, it is possible that the effects of exendin-4 on cocaine CPP and self-administration in restrained and freely moving mice are due to suppression of locomotor activity and not specific effects on drug reward and reinforcement, respectively. Indeed, doses of exendin-4 as low as 0.3 μg/kg suppress locomotor activity in both mice and rats [116–118]. This potential limitation is further highlighted by reduced self-administration of all unit doses of cocaine on both the ascending and descending limbs of the cocaine dose-response curve in mice [116].
Another notable caveat to the above studies is the exceedingly high doses of exendin-4 tested have been shown to produce nausea and malaise-like symptoms in addition to suppressing locomotion Doses of exendin-4 as low as 0.25 μg/kg produce nausea/malaise in rodents [119]. These results clearly indicate that doses of exendin-4 greater than 0.25 μg/kg produce malaise-like adverse effects associated with high doses of peripherally administered GLP-1 receptor agonists in humans [120]. Since the doses of exendin-4 (2.4 – 100.0 μg/kg) shown to reduce cocaine CPP and self-administration are likely producing malaise-like effects in mice, it is impossible to draw firm conclusions about the exact role of GLP-1 receptors in addiction-like behaviors from these previous studies [113–115].
Administration of a GLP-1 receptor agonist during abstinence following cocaine self-administration is sufficient to reduce cocaine-seeking behavior in rats. Specifically, systemic administration of exendin-4 (0.1 and 0.2 μg/kg, i.p.) significantly attenuated the reinstatement of drug-seeking behavior elicited by both a priming injection of cocaine and re-exposure to conditioned cues previously paired with cocaine taking [121]. Importantly, these doses of exendin-4 did not affect ad libitum food intake (cumulative chow intake, meal size or meal frequency) or body weight in cocaine-experienced rats [121]. Moreover, these behaviorally-relevant doses of exendin-4 do not produce nausea/malaise-like adverse effects in rodents [119] further highlighting the selectivity of this behavioral response at these doses. This is the first study to our knowledge identifying systemic doses of a GLP-1 receptor agonist that selectively attenuate cocaine-mediated behaviors and do not produce adverse effects commonly associated with higher doses in rodents. The translational implications of these findings are profound in that they support potential therapeutic approaches toward the specific use of GLP-1 receptor agonists for the treatment of cocaine craving and relapse. These findings are also provocative in that they suggest that drug and non-drug motivated behaviors can be differentially modulated by GLP-1 receptor activation in a cocaine-experienced animal.
2.2. Potential Mechanisms Underlying the Effects of Systemic GLP-1 Receptor Agonists on Cocaine-Mediated Behaviors
The suppressive effects of systemic GLP-1 receptor agonists on cocaine-mediated behaviors are due, in part, to activation of GLP-1 receptors in the brain. Microdialysis experiments in mice have shown that peripheral infusions of exendin-4 (2.4 and 30.0 μg/kg, i.p.) significantly decrease cocaine-evoked dopamine release in the nucleus accumbens and the dorsal striatum [113, 116]. Moreover, systemic exendin-4 (30.0 μg/kg, i.p.) attenuated dopamine D1 receptor agonist-mediated hyperlocomotion, which suggests that the suppressive effects of GLP-1 receptor agonists on cocaine-mediated behaviors may be due to inhibition of dopamine D1 receptor signaling in the ventral striatum [115]. Further support for this hypothesis comes from studies showing that systemic exendin-4 blocks cocaine-induced c-fos expression in the striatum [115]. Taken together, these findings suggest that exendin-4 attenuates cocaine reward and reinforcement by reducing dopamine signaling in the striatal complex. A recent study expands these findings and showed that systemic administration of exendin-4 (1.0 and 30.0 μg/kg, i.p.) attenuated cocaine-evoked dopamine release in the lateral septum [122], a nucleus known to regulate cocaine-mediated behaviors through its connections with the VTA [123, 124]. Interestingly, lower doses of exendin-4 (1.0 μg/kg in mice and 0.01 μg/kg in rats) had the same magnitude of effect as higher doses (30.0 μg/kg in mice and 1.0 μg/kg in rats) in reducing cocaine-evoked dopamine release in the lateral septum [122]. Together with previous data identifying behaviorally-selective doses of exendin-4 that reduce cocaine seeking, these studies indicate that lower doses of exendin-4 reduce cocaine-evoked dopamine release, do not impact feeding and do not induce malaise-like effects in cocaine-experienced rats [121]. Thus, future studies should utilize behaviorally-selective doses of exendin-4 that attenuate drug-evoked dopamine release when elucidating the neural mechanisms that cause these effects.
The mechanisms by which peripheral GLP-1 receptor agonists reduce cocaine-evoked dopamine release in the brain remain unclear. A recent study showed that application of GLP-1 ex vivo upregulates dopamine transporter (DAT) surface expression and increases dopamine uptake in the lateral septum [122]. These results suggest that GLP-1 receptor activation may decrease the duration of dopamine signaling in the synapse following cocaine exposure by enhancing dopamine reuptake into presynaptic terminals [122]. These effects were associated with decreased levels of the retrograde messenger endocannabinoid 2-arachidonylglycerol (2-AG) and its cleaved form arachidonic acid in the lateral septum [122]. Since cannabinoid CB1 receptor antagonists decrease the reward effects of cocaine (analogous to reduced 2-AG levels) and arachidonic acid reduces function of the dopamine transporter [125, 126], these findings suggest that GLP-1 receptor activation enhances dopamine uptake in the lateral septum by decreasing arachidonic acid levels, which may reduce cocaine-evoked increases in lateral septum dopamine levels as well as cocaine-mediated behaviors [122]. It is important to note, however, that this mechanism may only be specific to the lateral septum due to exendin-4 treatment failing to decrease 2-AG and arachidonic acid levels in the striatum [122]. Additionally, the functional significance of reduced 2-AG and arachidonic acid levels in cocaine-mediated behaviors has not been determined. It should also be emphasized that these studies only examined the effects of GLP-1 receptor activation on an acute injection of cocaine. It remains to be determined whether similar neural mechanisms mediate the behavioral effects of GLP-1 receptor agonists in drug-dependent animals that have been stably self-administering cocaine for a prolonged period of time. Finally, although strong evidence indicates that the suppressive effects of GLP-1 receptor agonists on cocaine-mediated behaviors are due to reduced dopamine signaling in the brain, further studies are needed to elucidate the exact mechanisms by which systemic GLP-1 receptor agonists modulate neural activity.
3. Central GLP-1 Receptor Activation Attenuates Cocaine-Mediated Behaviors
GLP-1 receptors are expressed throughout the brain including nuclei known to play important roles in voluntary cocaine-taking and -seeking behaviors [19, 55, 82, 88, 127]. This section highlights the current literature examining the role of GLP-1 receptors in discrete brain regions in cocaine-mediated behaviors (see Table 1 for a comprehensive list of these studies).
3.1. Ventral Tegmental Area
The first study examining the role of central GLP-1 receptors in voluntary cocaine taking showed that activation of GLP-1 receptors in the VTA was sufficient to attenuate cocaine self-administration in rats. Intra-VTA infusions of exendin-4 dose-dependently reduced cocaine taking in rats responding on a progressive ratio (PR) schedule of reinforcement [128]. Exendin-4 pretreatment in the VTA shifted the cocaine dose-response curve downward for all unit doses of cocaine in rats responding on a PR schedule, results that indicate that activation of GLP-1 receptors in the VTA is sufficient to reduce the reinforcing efficacy of cocaine [129, 130]. The behaviorally relevant dose of intra-VTA exendin-4 (0.05 μg) that reduced cocaine self-administration did not affect sucrose self-administration, indicating that the suppressive effects of exendin-4 on cocaine taking are reinforcer-specific and not due to non-specific effects (e.g., general motor impairments).
VTA GLP-1 receptors have also been shown to play an important role in the reinstatement of cocaine-seeking behavior during abstinence following cocaine self-administration. Systemic doses of exendin-4 that selectively attenuated cocaine reinstatement crossed the blood brain barrier and bound putative GLP-1 receptors located on neurons and astrocytes throughout the brain including the VTA, which suggests that the suppressive effects of peripheral exendin-4 on cocaine seeking are due, in part, to activation of central GLP-1 receptors [121]. Pharmacological inhibition of GLP-1 receptors in the VTA was sufficient to attenuate the efficacy of systemic exendin-4 in reducing cocaine reinstatement providing further support for this hypothesis [121]. More direct evidence supporting a critical role of central GLP-1 receptors in cocaine seeking was provided by studies showing that infusions of exendin-4 (0.05 μg) directly into the VTA significantly decreased cocaine seeking [121]. Overall, these findings clearly indicate that activation of VTA GLP-1 receptors is sufficient to reduce cocaine-seeking behavior. Consistent with systemic exendin-4 dose-response studies, the dose of intra-VTA exendin-4 (0.05 μg) used in these studies reduced cocaine seeking and did not affect ad libitum chow intake or promote malaise-like effects in rats [67, 110, 131]. Moreover, intra-VTA infusion of exendin-4 (0.05 μg) did not alter the reinstatement of sucrose seeking further supporting the selectivity of lower doses to reduce cocaine seeking [121]. There is some evidence that administration of 0.05 μg exendin-4 into the VTA reduced operant responding for palatable food [131]. However, these effects were transient and did not persist with more prolonged operant sessions [128, 131]. Overall, these studies have identified both systemic and intra-cranial doses of exendin-4 that selectively reduce cocaine-taking and seeking-behaviors in rats and not produce adverse feeding or malaise-like effects.
3.2. Nucleus Accumbens
In addition to binding putative GLP-1 receptors in the VTA, doses of systemic exendin-4 that attenuate cocaine seeking also distribute to the core and shell subregions of the nucleus accumbens [132]. These functionally distinct subregions are known to play differential roles in motivated behaviors including drug addiction [133]. The nucleus accumbens shell, which is classified as part of the limbic system, is implicated in the primary rewarding effects of drugs of abuse as well as regulating instrumental responding in the presence of motivationally relevant stimuli [18]. Alternatively, the nucleus accumbens core, which is considered part of the basal ganglia, mediates the incentive value of reward-conditioned stimuli and contributes to drug-associated, cue-induced cocaine seeking [18]. Infusions of exendin-4 directly into the nucleus accumbens core and shell were sufficient to attenuate the reinstatement of drug-seeking behavior elicited by an acute priming injection of cocaine [132]. Interestingly, the potency of exendin-4 differed between subregions. Cocaine-seeking behavior was decreased in rats pretreated with 0.05 μg exendin-4 in both the accumbens core and shell [132]. In contrast, 0.005 μg exendin-4 significantly attenuated cocaine seeking when infused directly into the accumbens core, but not shell [132]. Previous studies have also demonstrated differential effects of GLP-1 receptor agonist doses in the shell and core subregions in decreasing food intake [67, 111]. Lower doses of exendin-4 reduced intake of palatable food when infused into the accumbens core compared to the accumbens shell [67]. The lowest effective dose of exendin-4 used in this study was 0.025 μg, which produced a transient effect on food intake following activation of GLP-1 receptors in the accumbens core and no effect on food intake when infused into the accumbens shell [67]. Collectively, these findings suggest that GLP-1 signaling in the accumbens core may play a more prominent role in regulating drug-seeking behavior and hedonic feeding.
In contrast to studies of intra-accumbens exendin-4 on cocaine reinstatement, studies of sucrose reinstatement found no effect of 0.05 μg exendin-4 in the nucleus accumbens core and shell on sucrose seeking. Although administration of exendin-4 directly into the accumbens shell reduced sucrose self-administration on a progressive ratio schedule of reinforcement [131], doses of exendin-4 lower than 0.1 μg when infused into the nucleus accumbens shell or core do not affect operant responding for sucrose, ad libitum chow intake, or produce adverse malaise-like effects [67, 131, 134]. Therefore, exendin-4 infusions into the nucleus accumbens core (0.05 and 0.005 μg) and shell (0.05 μg) of cocaine-experienced rats selectively reduced cocaine seeking and do not affect food intake. Together with systemic and intra-VTA studies that identified behaviorally-selective doses of exendin-4 that reduced cocaine seeking [121], these findings further strengthen evidence supporting the efficacy of lower doses of GLP-1 receptor agonists to selectively attenuate cocaine seeking versus non-drug motivated behaviors [132].
3.3. Lateral Septum
The lateral septum is a brain region that regulates cocaine seeking by relaying contextual information from the CA3 region of the dorsal hippocampus to the VTA [135]. GABA neurons in the lateral septum project onto GABA interneurons in the VTA. Activation of lateral septum GABA neurons promotes dopamine release in the brain and regulates goal-directed behaviors via disinhibition of VTA dopamine neurons [135]. In addition to regulating cocaine seeking, the lateral septum also plays an important functional role in the expression of cocaine-induced CPP [53]. GLP-1 receptors are highly enriched in the lateral septum and GLP-1 receptor-expressing neurons in the lateral septum are directly innervated by VTA dopamine neurons [122, 136]. Intrinsic excitability of GABA neurons in the lateral septum was enhanced in mutant mice lacking GLP-1 receptors compared to wild-type controls, suggesting that endogenous GLP-1 receptor signaling functions to reduce neuronal excitability of GABA neurons in the lateral septum [136]. Constitutive GLP-1 receptor knockout mice also displayed enhanced sensitization to the locomotor activating effects of cocaine as well as increased cocaine-induced CPP compared to wild-type controls [136]. These results suggest that the endogenous central GLP-1 system functions as a homeostatic regulator of cocaine reward. Further support for this functional role comes from studies that showed that virally-mediated knockdown of endogenous GLP-1 receptor expression in the VTA facilitates/augments cocaine self-administration and that increased endogenous central GLP-1 signaling may function to reduce cocaine taking and seeking [121, 128]. The absence of central GLP-1 receptors in knockout mice also caused an increase in baseline anxiety-like behaviors measured in open field and elevated plus maze paradigms [136]. These findings may confound GLP-1 receptor agonist effects on cocaine-induced locomotion and CPP as central GLP-1 signaling is known to play a role in stress and anxiety which can interfere with reward-associated behaviors [82]. To determine the role of central GLP-1 receptors in these behavioral responses, GLP-1 receptors were re-expressed selectively in the lateral septum of constitutive GLP-1 receptor knockout mice. Viral vector-mediated GLP-1 receptor gene delivery in the lateral septum of mutant mice lacking endogenous GLP-1 receptors restored cocaine-induced locomotor activity and CPP to levels similar to wild-type controls [136]. However, increased expression of GLP-1 receptors in the lateral septum had no effect on enhanced anxiety-like behaviors seen in knockout mice, suggesting that GLP-1 receptors in other nuclei mediate these effects [136]. Taken together, these findings indicate that lateral septum GLP-1 receptors mediate the locomotor activating and conditioned rewarding effects of cocaine. However, future studies should examine how GLP-1 receptor activation in the lateral septum influences downstream nuclei such as the VTA to decrease behavioral responses to cocaine.
4. Cocaine Modulates Central Endogenous GLP-1 Circuits
Previous studies have shown that acute cocaine exposure increases neuronal activity in the NTS [137–139]. A recent study expanded these findings and showed that cocaine self-administration increased c-Fos expression in GLP-1-producing neurons in the NTS [128]. Since the NTS projects to nuclei throughout the brain including the VTA and nucleus accumbens [67, 89], cocaine-induced activation of NTS GLP-1-producing neurons may result in increased GLP-1 release in midbrain and forebrain areas. Thus, increased endogenous central GLP-1 signaling following cocaine exposure may act as a homeostatic response to reduce further cocaine consumption.
The mechanisms by which cocaine activates NTS GLP-1-producing neurons are not clear but may involve increased corticosterone signaling in the hindbrain. Since cocaine taking increases plasma and central corticosterone levels [140–143] and peripheral administration of corticosterone increases activation of NTS GLP-1-producing neurons [128], it is possible and likely that cocaine self-administration increases activation of GLP-1-producing neurons in the NTS through a corticosterone-mediated mechanism of action. Indeed, administration of corticosterone directly into the 4th ventricle attenuated cocaine self-administration and these effects were blocked by pharmacological inhibition of GLP-1 receptors in the VTA [128]. These findings indicate that increased corticosterone signaling in the hindbrain reduces cocaine taking, in part, through activation of the endogenous central GLP-1 system [128]. Collectively, these data suggest that voluntary cocaine taking activates GLP-1-producing neurons in the NTS through a corticosterone-mediated mechanism of action and that increased GLP-1 receptor signaling in midbrain and forebrain areas represents a negative-feedback response that reduces the reinforcing efficacy of cocaine. Consistent with this notion, reduced expression of endogenous GLP-1 receptors in the VTA augmented cocaine self-administration [128]. Future studies using chemogenetic and optogenetic approaches are required to determine the exact role of endogenous central GLP-1 circuits in cocaine-mediated behaviors.
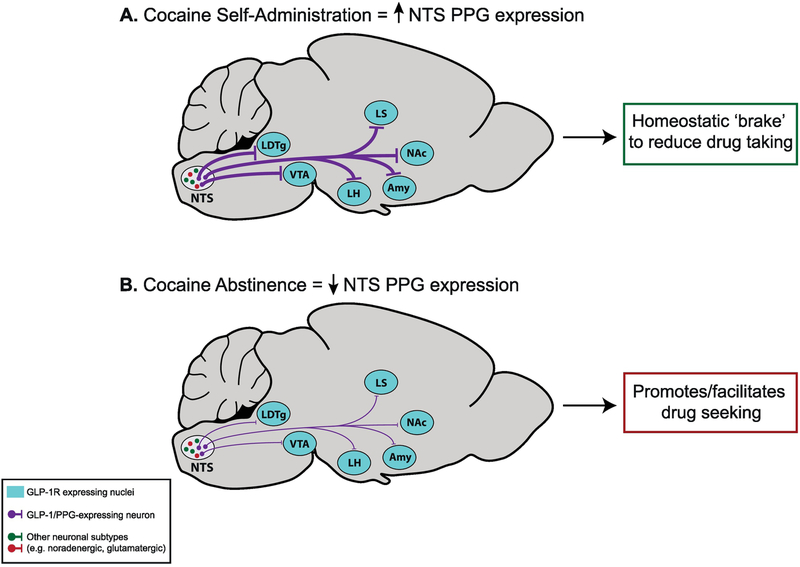
Recently published findings have found that cocaine taking and subsequent abstinence dynamically regulate expression of preproglucagon (PPG), the gene that encodes GLP-1, in the NTS (Figure 1). Specifically, cocaine self-administration increased endogenous PPG expression in the NTS [121] (Figure 1A). These data are consistent with findings that support the hypothesis that increased central GLP-1 signaling may serve as a ‘brake’ to reduce further cocaine consumption [128]. In contrast, PPG expression in the NTS was significantly decreased following seven days of abstinence [121], a time point that coincides with robust drug-seeking behavior [132, 144, 145] (Figure 1B). These results suggest that decreased endogenous PPG expression in the NTS during abstinence may facilitate cocaine craving and relapse. It is possible that that decreased NTS PPG expression may result in a ‘hunger-like’ state that promotes cocaine seeking, as hunger is a potent driver of reward seeking and motivated behaviors [35, 146]. For example, previous studies have shown that chronic food restriction results in enhanced burst firing of substantia nigra dopamine neurons, augmentation of cocaine-induced burst firing, and persistence of increased burst firing after animals are refed [147]. Consistent with these neurophysiological effects, food-restriction enhances cocaine-induced conditioned place preference indicating an important modulatory role for energy balance in drug reward [148]. Further evidence supporting the hypothesis that decreased NTS PPG expression produces a hunger-like behavioral state that promotes/facilitates drug seeking comes from a previous study showing that decreased PPG expression in the NTS produces hyperphagia and exacerbates high fat diet-induced obesity [102].
Figure 1. Cocaine taking and subsequent abstinence dynamically modulate endogenous central PPG expression.
(A) Cocaine self-administration increases PPG (GLP-1 precursor) mRNA expression in the nucleus tractus solitarius (NTS), effects that may be associated with enhanced GLP-1 tone in midbrain and forebrain nuclei known to regulate cocaine taking and seeking. (B) Abstinence following cocaine self-administration decreases PPG mRNA expression in the NTS, which may promote/facilitate cocaine seeking during withdrawal. Together, these findings reveal a novel GLP-1-focused neuroendocrine mechanism underlying cocaine taking and seeking. These results are described in more detail in the main text. PPG = preproglucagon, LDTg = laterodorsal tegmental nucleus, VTA = ventral tegmental area, LH = lateral hypothalamus, LS = lateral septum, Amy = amygdala, NAc = nucleus accumbens.
The effects of cocaine self-administration and subsequent abstinence on GLP-1 receptor mRNA expression in the VTA and nucleus accumbens have also been examined. Cocaine self-administration and extinction had no effect on VTA and nucleus accumbens GLP-1 receptor expression [121, 132]. Collectively, these results suggest that voluntary cocaine taking and subsequent abstinence may influence GLP-1 tone in the brain by modulating NTS PPG expression without producing corresponding changes in endogenous GLP-1 receptor expression. However, exendin-4 binding has been shown to regulate trafficking and surface expression of GLP-1 receptors [149]. Therefore, it is possible that cocaine taking and subsequent abstinence may alter GLP-1 receptor trafficking and/or surface expression in nuclei known to regulate drug-taking and -seeking behaviors. Future studies are required to further delineate the role of endogenous central GLP-1 signaling in cocaine-mediated behaviors, including whether cocaine affects GLP-1 receptor trafficking and/or the kinetics of ligand-mediated GLP-1 receptor trafficking. Overall, these studies suggest that the endogenous central GLP-1 system, particularly GLP-1-producing neurons in the NTS, is dynamically regulated by cocaine taking and withdrawal and highlight a novel neurobiological mechanism underlying cocaine addiction (Figure 1). However, the NTS is comprised of heterogeneous cell populations [89, 139, 150] and thus further studies investigating the exact role of each of these populations in cocaine-mediated behaviors must be investigated.
5. Neurophysiological Mechanisms Underlying the Effects of GLP-1 Receptor Agonists on Cocaine-Seeking Behavior
Initial studies showed that systemic infusions of exendin-4 reduced cocaine-evoked dopamine release in the nucleus accumbens [113, 116]. However, the relative contributions of peripheral and central GLP-1 receptors to these neurochemical changes is not clear. A recent study investigated the role of central GLP-1 receptors in attenuating cocaine-evoked dopamine release in the nucleus accumbens core and shell subregions using in vivo fast-scan cyclic voltammetry. Intracerebroventricular (ICV) infusion of exendin-4 attenuated phasic dopamine release in the accumbens core, but not the shell, of rats receiving non-contingent infusions of cocaine [151]. To investigate the mechanisms by which exendin-4 reduces cocaine-evoked dopamine release in the accumbens core, electrical stimulation of the VTA was performed in rats receiving non-contingent infusions of cocaine. Exendin-4 did not affect the ability of cocaine to attenuate dopamine uptake, which suggests that GLP-1 receptor activation exerts its suppressive effects on cocaine-induced dopamine release in the accumbens core by reducing the excitability of midbrain dopamine neurons [151]. While these findings are the first to highlight a role for central GLP-1 receptors in suppression of cocaine-evoked dopamine release in the accumbens [151], one limitation of this study is that ICV delivery of exendin-4 causes broad GLP-1 receptor activation throughout the brain. Therefore, it is difficult to delineate the exact brain regions mediating the neurochemical and neurophysiological effects of exendin-4. Also, while exendin-4 attenuates cocaine self-administration [116, 128], it is not clear whether exendin-4 suppresses conditioned phasic dopamine release aligned with cocaine-predictive cues and operant responding observed during voluntary cocaine taking [152, 153]. Thus, our understanding of how central GLP-1 receptor activation influences the mesolimbic dopamine system to reduce addiction-like behaviors would benefit from future electrophysiological studies further delineating the effects of central GLP-1 receptor activation on phasic versus tonic dopamine cell firing.
Previous studies have shown that GLP-1 receptor agonists modulate neuronal activity in the brain by both pre-synaptic effects on neurotransmitter release and regulation of post-synaptic membrane excitability [134, 154, 155]. However, there is a paucity of studies investigating the effects of GLP-1 receptor activation on neuronal activity in preclinical models of cocaine use disorder. In order to advance our knowledge of the neurophysiological mechanisms by which GLP-1 receptor agonists attenuate cocaine-mediated behaviors, ex vivo electrophysiological studies are needed to elucidate the effects of GLP-1 receptor activation on neuronal activity in a cocaine-exposed brain. A recent study characterized the effects of exendin-4 on activity of medium spiny neurons (MSNs) in the nucleus accumbens of cocaine-experienced rats [132]. Following extinction of cocaine self-administration, application of 1 μM exendin-4 increased the frequency of MSN action potential firing in the nucleus accumbens core and shell [132]. No other measures of membrane excitability (e.g., resting membrane potential, rheobase, action potential amplitude and action potential duration) were altered by GLP-1 receptor activation in ex vivo striatal slices from cocaine-experienced rats [132]. In contrast to previous studies showing presynaptic effects of GLP-1 receptor activation in the VTA and accumbens core of drug-naïve rats [110, 134], exendin-4 had no effects on spontaneous excitatory post-synaptic currents (sEPSCs) frequency and paired pulse ratio (PPR) of evoked EPSCs in nucleus accumbens core and shell MSNs from cocaine-experienced rats [132]. Taken together, these results indicate that increased activation of GLP-1 receptors in the nucleus accumbens during cocaine abstinence increases intrinsic, but not synaptic, excitability of MSNs and is sufficient to reduce the reinstatement of cocaine-seeking behavior [132]. It should be noted that these results are limited to activity exclusively within the nucleus accumbens, as these studies were conducted in accumbens slices without input from VTA cell bodies. The effects of GLP-1 receptor activation on accumbens MSN activity should be further explored in an in vivo model, as it has been shown that input from the VTA to the nucleus accumbens impacts dopamine signaling dynamics. Specifically, phasic dopamine release in the accumbens is dependent on burst firing of VTA dopamine neurons [156]. Moreover, studies utilizing amphetamine have highlighted the importance of VTA input on nucleus accumbens dopamine release patterns. While dopamine efflux is preserved in ex vivo slices treated with amphetamine [157], phasic dopamine release is only observed in in vivo studies with preserved VTA inputs to the accumbens [158]. Thus, active VTA input into the nucleus accumbens may be required for a more accurate picture of how GLP-1 receptor activation modulates nucleus accumbens MSN activity in cocaine-experienced animals.
An interesting alternative hypothesis is that VTA input may not be necessary to stimulate dopamine release in the nucleus accumbens. Recent studies have revealed that cholinergic interneurons in the accumbens are capable of stimulating dopamine release and that these effects are regulated, in part, by the endocannabinoid system and prefrontal cortical afferents to the accumbens [159–161]. Since GLP-1 receptor activation has been shown to modulate both endocannabinoid signaling and presynaptic glutamate release [122, 134], it is possible that exendin-4 may influence cholinergic interneuron signaling in the nucleus accumbens via these systems or by direct action interneurons themselves. Further support for cholinergic interneurons playing a role in modulating the effects of metabolic factors on striatal dopamine levels and reward comes from a recent study showing that insulin increases striatal dopamine release by activating cholinergic interneurons in the accumbens [162]. Thus, future electrophysiological studies are needed to determine if the effects of exendin-4 on MSN activity are due to activation of cholinergic interneurons alone or require VTA input as discussed above.
Cocaine abstinence is associated with decreased nucleus accumbens MSN membrane excitability due to changes in conductance of sodium, calcium and potassium [163–165]. Lower membrane excitability during cocaine abstinence may enhance action potential sensitivity to GLP-1 receptor activation in the nucleus accumbens [132]. Cocaine exposure is likely a critical component in this scenario, given the previous findings of minimal effects of exendin-4 on accumbens MSN firing in drug-naïve rats [134] combined with recent studies showing increased frequency of MSN action potential firing in the nucleus accumbens core and shell in cocaine-experienced rats [132]. In line with this hypothesis, a recent study showed that exendin-4 suppressed cocaine-evoked phasic release of dopamine in the accumbens core, effects not due to changes in dopamine reuptake [151]. These neurophysiological changes, however, may not be specific to the accumbens as exendin-4 administration was also associated with reduced cocaine-evoked dopamine release in the lateral septum [122, 136]. GLP-1 signaling has also been shown to regulate other neurotransmitter systems including glutamatergic [134] and GABAergic [155, 166] transmission in the brain. Understanding the mechanisms by which GLP-1 receptor activation in the nucleus accumbens reduces cocaine seeking will benefit from a comprehensive analysis of how exendin-4 regulates integration of synaptic and intrinsic MSN excitability in the context of drug priming-induced reinstatement. Thus, intrinsic GLP-1 receptor-coupled mechanisms may contribute to previous findings that a broad increase in nucleus accumbens MSN action potential output is associated with attenuation of rewarding consummatory behaviors [167, 168].
While activation of GLP-1 receptors in the VTA has been shown to attenuate cocaine self-administration and reinstatement [121, 128], the neurophysiological mechanisms underlying these behavioral responses are not clear. In drug-naïve mice, exendin-4 reduced AMPA receptor-mediated EPSCs in VTA dopamine neurons that project to the nucleus accumbens shell [72]. AMPA/NMDA EPSC ratio in the VTA was also decreased following exendin-4 application suggesting a postsynaptic modification that functions to reduce excitatory synaptic strength via removal of AMPA receptors from the postsynaptic membrane [72]. Interestingly, exendin-4 also increased amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs) in VTA dopamine neurons [72]. These results indicate that GLP-1 receptor activation modulates both excitatory and inhibitory transmission in VTA dopamine neurons that project to the nucleus accumbens. It is provocative to think that similar mechanisms (i.e., decreased excitatory synaptic strength and/or facilitation of inhibitory synaptic inputs in VTA dopamine neurons) may mediate the effects of GLP-1 receptor activation in the VTA on cocaine taking and seeking.
6. Clinical Potential of GLP-1 Receptor Agonists to Treat Cocaine Use Disorder
Although strong preclinical evidence indicates that various metabolic hormones regulate addiction-like phenotypes in animal models [83, 169], the relationship between endogenous levels of metabolic factors and substance abuse disorders in humans is not clear. A recent study investigated the effects of cocaine on plasma levels of metabolic factors [ghrelin (total and acly-ghrelin), amylin, GLP-1, insulin, leptin and peptide YY (PYY)] in human cocaine addicts [170]. Acute cocaine (25 mg) significantly decreased serum GLP-1 and PYY concentrations in cocaine users with trends towards decreased insulin and amylin concentrations [170]. However, these effects were seen in a relatively small sample size (n=8). Thus, future studies with larger sample sizes and healthy controls are needed to further validate and determine the specificity of these effects in human cocaine addicts. Additionally, future studies would benefit from correlating metabolic hormone serum concentrations with drug craving to further support the hypothesis that GLP-1, and possibly PYY, may serve as potential targets to treat cocaine craving and relapse. Consistent with these effects, other clinical studies have shown that crack cocaine use is associated with lower serum levels of leptin [171]. Preclinical studies have also assessed the correlation between circulating levels of metabolic hormones and cocaine taking and seeking in rats. Serum ghrelin levels have been shown to be positively correlated with increased reinstatement of cocaine-seeking behavior [172]. Interestingly, a recent study has shown that 14 days of cocaine self-administration in rats was associated with increased ghrelin and GLP-1 plasma levels while leptin and insulin levels were decreased [143]. While these preclinical findings appear to contrast findings from human GLP-1 studies, they are consistent with the working hypothesis that cocaine-induced increases in central GLP-1 signaling may represent a homeostatic response to reduce further cocaine consumption [128] (Figure 1B).Thus, larger clinical trials are needed to determine the exact effects of cocaine taking and withdrawal on plasma GLP-1 levels and validate findings from preclinical models quantifying drug-induced changes in circulating metabolic factors.
The mechanisms by which cocaine decreases GLP-1 serum concentrations in human cocaine addicts and the functional significance of decreased circulating GLP-1 remains to be determined. However, taken together with preclinical studies showing that increased GLP-1 receptor activation decreases cocaine taking- and seeking-behaviors, these results suggest that decreased GLP-1 may promote cocaine use. This hypothesis is consistent with preclinical studies demonstrating that cocaine taking and subsequent abstinence decreased PPG mRNA expression in the NTS, effects likely associated with reduced GLP-1 signaling in the mesolimbic reward system, which may promote cocaine seeking [121] (Figure 1A). It is also interesting to speculate that decreased serum GLP-1 levels observed in human addicts may serve as a biomarker for cocaine use disorder and/or treatment response [173]. Moreover, there are several known single nucleotide polymorphisms (SNPs) in the human GLP-1 receptor [174]. One SNP in particular, the T149M variant, displayed reduced GLP-1 binding and cAMP signaling as well as differences in the extent of reduced functional responses to various GLP-1 receptor ligands [174, 175]. It is possible that expression of the T149M variant and/or decreased baseline circulating GLP-1 levels in human cocaine addicts may serve as predictors of treatment response and/or relapse, as these phenotypes are associated with decreased endogenous GLP-1 signaling in the body. If increased endogenous GLP-1 signaling functions as a ‘brake’ to reduce cocaine consumption [128], cocaine users with the T149M variant and/or reduced blood GLP-1 levels may be more susceptible to cocaine craving and relapse. This hypothesis is supported by clinical studies showing that another GLP-1 receptor SNP variant (168Ser) is associated with alcohol use disorder and increased alcohol consumption in humans with alcohol use disorder [176]. Thus, it is provocative to think that GLP-1 receptor variants may be predictors of relapse and/or treatment response in humans with cocaine use disorders.
From a translational perspective, preclinical studies have identified systemic and intra-cranial doses of exendin-4 that attenuate cocaine taking and seeking [121, 128, 132] and do not produce notable metabolic or adverse effects associated with GLP-1 receptor agonists in humans and rodents [119, 120, 177]. Since GLP-1 analogs are currently FDA-approved for treating type II diabetes and obesity [106, 109], these pre-clinical findings suggest that GLP-1 receptor agonists could be re-purposed at low doses to treat cocaine use disorder. However, clinical studies are needed to validate the efficacy of GLP-1 receptor agonists in reducing cocaine craving-induced relapse and to determine if circulating GLP-1 could be used as a biomarker for craving and/or treatment response.
Currently, there are many different GLP-1 receptor agonists available on the market to treat type II diabetes [178]. The elimination half-lives of GLP-1 receptor agonists differ depending upon their chemical structures, that include, but are not limited to, specific amino acid sequences that are resistant to DPP-IV degradation, as well as conjugations (i.e., pegylation, acetylations, etc.) to the peptide sequence [179]. These important pharmacokinetic properties are likely to influence brain penetrance, distribution to nuclei known to regulate drug reinforcement, and the therapeutic utility of GLP-1 receptor agonists for treating cocaine use disorder. Thus, it is important for future studies to directly compare the efficacy of different GLP-1 receptor agonists in attenuating drug-mediated behaviors. It will also be important for future clinical trials to determine the efficacy of recently developed oral formulations of GLP-1 analogs in treating cocaine use disorder as the safety and pharmacokinetics of these medications may differ from GLP-1 receptor agonists administered subcutaneously [178, 180].
In addition to causing nausea and malaise in humans, GLP-1 analogs have also been shown to reduce blood glucose concentrations and food intake in healthy subjects [181, 182]. However, GLP-1 receptor agonists cause fewer hypoglycemic events compared to insulin in patients with type II diabetes and no reports of hypoglycemia or severe weight loss have been observed in healthy subjects [181–184]. Therefore, it is not likely that GLP-1 receptor agonist treatment will result in glycemic dysregulation in otherwise healthy patients [84, 185]. Moreover, there is no scientific evidence supporting development of pancreatic toxicity as initially suggested in patients treated with GLP-1 receptor agonists [185]. Thus, the benefit/risk profile of GLP-1 receptor agonists supports their clinical use and the translational relevance of preclinical drug addiction studies.
7. Potential Efficacy of GLP-1 Receptor Agonists in Treating Other Substance Use Disorders
In addition to reducing cocaine-mediated behaviors, the efficacy of GLP-1 receptor agonists in regulating behaviors associated with other drugs of abuse has been investigated. Specifically, GLP-1 receptor agonists have been shown to reduce ethanol-, amphetamine-, and nicotine-mediated behaviors in rodents [113, 186–190]. Systemic administration of exendin-4 (2.4 μg/kg) decreased ethanol-induced CPP, the locomotor-activating effects of ethanol, and ethanol self-administration [186]. These behavioral responses were associated with reduced ethanol-induced dopamine release in the striatum [186]. Other studies have replicated these findings and shown that higher doses of exendin-4 (20 μg/kg) decreased ethanol-induced CPP in mice while 0.3 and 1.0 μg/kg exendin-4 decreased voluntary ethanol intake in rats [188]. These effects are mediated, in part, by central GLP-1 receptors as infusions of exendin-4 directly into the VTA were sufficient to reduce ethanol self-administration in rats [188]. Analogous findings have been shown in studies investigating the efficacy of GLP-1 receptor agonists in reducing amphetamine-mediated behaviors. Specifically, administration of exendin-4 (2.4 μg/kg) attenuated amphetamine-induced locomotor activity, CPP and accumbal dopamine release [113]. With regard to nicotine use disorder, exendin-4 (2.4 μg/kg) pretreatment reduced nicotine-induced locomotion, sensitization, CPP and accumbal dopamine release [187]. Another study has expanded these findings and shown that nicotine activates endogenous GLP-1-producing neurons in the NTS [190]. Moreover, systemic administration of exendin-4 (10 μg/kg) and the DPP-IV inhibitor sitagliptin (10 mg/kg) decreased nicotine consumption in mice [190]. Consistent with these effects, GLP-1 receptor knockout mice self-administer more nicotine than wild-type controls [190]. To investigate the endogenous circuits mediating these behavioral responses, optogenetic studies revealed that GLP-1 excites medial habenula projections to the interpeduncular nucleus and that activation of GLP-1 receptors in this circuit is sufficient to attenuate nicotine consumption [190]. The authors conclude that GLP-1 acts as a ‘satiety sensor’ to limit nicotine intake by stimulating the medial habenula to interpeduncular nucleus circuit to promote nicotine avoidance before its aversive effects are encountered [190]. Collectively, these studies indicate that systemic administration of a GLP-1 receptor agonist decreases the reinforcing and rewarding efficacy of multiple drugs of abuse and that these effects are associated with decreased drug-evoked dopamine release in the nucleus accumbens. It is important to note, however, that relatively high doses of systemic GLP-1 receptor agonists were used in these studies and that these doses have been shown previously to produce nausea/malaise and decrease food intake and body weight in rats [119, 177]. Achieving behavioral selectivity through proper dosing of GLP-1 receptor agonists is critical for drawing firm conclusions about the efficacy of GLP-1 receptor agonists to reduce addiction-like phenotypes in preclinical models. These foundational studies do, however, provide preliminary data supporting the potential efficacy of GLP-1 receptor agonists in treating alcohol, nicotine, and amphetamine use disorders.
8. Concluding Remarks
The results summarized in this review indicate that activation of GLP-1 receptors has profound effects on cocaine-mediated behaviors in rodents. Moreover, emerging evidence indicates that cocaine and subsequent abstinence dynamically alter endogenous central GLP-1 signaling (Figure 1). However, much remains to be discovered about how central GLP-1 circuits function to reduce cocaine-mediated behaviors and the efficacy of GLP-1 receptor agonists in different behavioral paradigms. For example, future studies are needed to assess the efficacy of repeated administration of GLP-1 receptor agonists on voluntary cocaine taking and seeking as studies to date have only assessed the acute effects of exendin-4 on these behaviors (Table 1). It will be important to know if tolerance and/or adverse effects develop to repeated administration of GLP-1 receptor agonists. Furthermore, no studies to date have examined potential sex differences in the efficacy of GLP-1 receptor agonists to reduce cocaine-mediated behaviors. Recent evidence indicates that females are more sensitive to the effects of a GLP-1 receptor agonist infused directly into the brain on food intake [191]. It is provocative to think that the potency of GLP-1 receptor agonists may differ between sexes in preclinical models of addiction. In addition to exendin-4, preclinical studies should also test the efficacy of other GLP-1 system-targeted drugs with different pharmacokinetic profiles (e.g., liraglutide) and pharmacological mechanisms of action (e.g., sitagliptin) in reducing cocaine-taking and -seeking behaviors. Finally, there are significant gaps in our understanding of the central GLP-1 circuits that regulate cocaine-mediated behaviors, as well as the cellular, molecular and neurophysiological mechanisms by which GLP-1 receptor agonists attenuate these behavioral responses. Critical first steps toward characterizing central GLP-1 circuits will be phenotyping GLP-1 receptor-expressing cells in brain nuclei know to regulate drug-taking and -seeking behaviors as well as their downstream targets. In conclusion, an exciting body of preclinical evidence supports clinical studies examining the efficacy of GLP-1 receptor agonists in treating cocaine use disorder, as well as the development of pharmacotherapies aimed at increasing GLP-1 receptor signaling in the brain to prevent cocaine craving-induced relapse.
Highlights.
Central GLP-1 receptors play an important role in the reinforcing effects of cocaine
GLP-1 receptor agonists attenuate cocaine-mediated behaviors
Cocaine dynamically alters the endogenous central GLP-1 system
Central GLP-1 signaling may be a novel target to treat cocaine use disorder
9. Acknowledgements
The authors would like to thank Drs. Samantha Fortin and Matthew Hayes for their critical reading of this manuscript. H.D.S is supported by a grant from the National Institutes of Health (R01 DA037897). N.S.H is supported by a Gilliam Fellowship from the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
H.D.S. receives funding from Novo Nordisk that was not used to support these studies. The authors declare no other competing financial interests.
References
- [1].Results from the 2017 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration; 2018. [PubMed] [Google Scholar]
- [2].Hughes A, Williams MR, Lipari RN, and Van Horn S State estimates of past year cocaine use among young adults: 2014 and 2015. In: Report TC, editor. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2016. [PubMed] [Google Scholar]
- [3].Dackis CA, O’Brien CP Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat. 2001,21:111–7. [DOI] [PubMed] [Google Scholar]
- [4].O’Brien CP A range of research-based pharmacotherapies for addiction. Science. 1997,278:66–70. [DOI] [PubMed] [Google Scholar]
- [5].Pierce RC, O’Brien CP, Kenny PJ, Vanderschuren LJ Rational development of addiction pharmacotherapies: successes, failures, and prospects. Cold Spring Harb Perspect Med. 2012,2:a012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tzschentke TM Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998,56:613–72. [DOI] [PubMed] [Google Scholar]
- [7].Bardo MT, Bevins RA Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl). 2000,153:31–43. [DOI] [PubMed] [Google Scholar]
- [8].de Wit H, Stewart J Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl). 1981,75:134–43. [DOI] [PubMed] [Google Scholar]
- [9].O’Brien CP, Childress AR, McLellan AT, Ehrman R Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992,654:400–15. [DOI] [PubMed] [Google Scholar]
- [10].Sinha R, Catapano D, O’Malley S Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl). 1999,142:343–51. [DOI] [PubMed] [Google Scholar]
- [11].Jaffe JH, Cascella NG, Kumor KM, Sherer MA Cocaine-induced cocaine craving. Psychopharmacology (Berl). 1989,97:59–64. [DOI] [PubMed] [Google Scholar]
- [12].Shalev U, Grimm JW, Shaham Y Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002,54:1–42. [DOI] [PubMed] [Google Scholar]
- [13].Shaham Y, Shalev U, Lu L, De Wit H, Stewart J The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl). 2003,168:3–20. [DOI] [PubMed] [Google Scholar]
- [14].Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016,41:335–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gerber GJ, Stretch R Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacology, biochemistry, and behavior. 1975,3:1055–61. [DOI] [PubMed] [Google Scholar]
- [16].Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacology, biochemistry, and behavior. 1999,64:327–36. [DOI] [PubMed] [Google Scholar]
- [17].Anderson SM, Bari AA, Pierce RC Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl). 2003,168:132–8. [DOI] [PubMed] [Google Scholar]
- [18].Schmidt HD, Pierce RC Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010,1187:35–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005,526:65–76. [DOI] [PubMed] [Google Scholar]
- [20].Ritz MC, Cone EJ, Kuhar MJ Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990,46:635–45. [DOI] [PubMed] [Google Scholar]
- [21].Elliott JM, Beveridge TJ Psychostimulants and monoamine transporters: upsetting the balance. Curr Opin Pharmacol. 2005,5:94–100. [DOI] [PubMed] [Google Scholar]
- [22].Pierce RC, Kumaresan V The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006,30:215–38. [DOI] [PubMed] [Google Scholar]
- [23].Kelley AE Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004,44:161–79. [DOI] [PubMed] [Google Scholar]
- [24].Zahm DS Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999,877:113–28. [DOI] [PubMed] [Google Scholar]
- [25].Groenewegen HJ, Wright CI, Beijer AV, Voorn P Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999,877:49–63. [DOI] [PubMed] [Google Scholar]
- [26].Alexander GE, Crutcher MD Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990,13:266–71. [DOI] [PubMed] [Google Scholar]
- [27].Kalivas PW, Nakamura M Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999,9:223–7. [DOI] [PubMed] [Google Scholar]
- [28].Groenewegen HJ, Uylings HB The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000,126:3–28. [DOI] [PubMed] [Google Scholar]
- [29].Wise RA Brain reward circuitry: insights from unsensed incentives. Neuron. 2002,36:229–40. [DOI] [PubMed] [Google Scholar]
- [30].Feltenstein MW, See RE The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008,154:261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jay TM Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003,69:375–90. [DOI] [PubMed] [Google Scholar]
- [32].Bromberg-Martin ES, Matsumoto M, Hikosaka O Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010,68:815–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grace AA, Bunney BS The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984,4:2877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marinelli M, Rudick CN, Hu XT, White FJ Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006,5:79–97. [DOI] [PubMed] [Google Scholar]
- [35].Hsu TM, McCutcheon JE, Roitman MF Parallels and Overlap: The Integration of Homeostatic Signals by Mesolimbic Dopamine Neurons. Front Psychiatry. 2018,9:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004,24:1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007,54:237–44. [DOI] [PubMed] [Google Scholar]
- [38].Day JJ, Roitman MF, Wightman RM, Carelli RM Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007,10:1020–8. [DOI] [PubMed] [Google Scholar]
- [39].Schultz W Updating dopamine reward signals. Curr Opin Neurobiol. 2013,23:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sulzer D, Cragg SJ, Rice ME Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia. 2016,6:123–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009,324:1080–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schultz W Behavioral dopamine signals. Trends Neurosci. 2007,30:203–10. [DOI] [PubMed] [Google Scholar]
- [43].Hollerman JR, Schultz W Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998,1:304–9. [DOI] [PubMed] [Google Scholar]
- [44].Schultz W Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci. 2016,17:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Berridge KC From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012,35:1124–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Berridge KC, Robinson TE Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016,71:670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Robinson TE, Berridge KC The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993,18:247–91. [DOI] [PubMed] [Google Scholar]
- [48].Wise RA Neurobiology of addiction. Curr Opin Neurobiol. 1996,6:243–51. [DOI] [PubMed] [Google Scholar]
- [49].Schmidt HD, Pierce RC Systemic administration of a dopamine, but not a serotonin or norepinephrine, transporter inhibitor reinstates cocaine seeking in the rat. Behav Brain Res. 2006,175:189–94. [DOI] [PubMed] [Google Scholar]
- [50].Schmidt HD, Anderson SM, Pierce RC Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006,23:219–28. [DOI] [PubMed] [Google Scholar]
- [51].Schmidt HD, Pierce RC Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006,142:451–61. [DOI] [PubMed] [Google Scholar]
- [52].Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain research. 2010,1314:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sartor GC, Aston-Jones GS A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci. 2012,32:4623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sotomayor-Zarate R, Renard GM, Araya KA, Carreno P, Fuentealba JA, Andres ME, et al. Long-term loss of dopamine release mediated by CRF-1 receptors in the rat lateral septum after repeated cocaine administration. Behav Brain Res. 2013,250:206–10. [DOI] [PubMed] [Google Scholar]
- [55].Schmidt HD, Famous KR, Pierce RC The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009,30:1358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lammel S, Lim BK, Malenka RC Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014,76 Pt B:351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zapata A, Hwang EK, Lupica CR Lateral Habenula Involvement in Impulsive Cocaine Seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017,42:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lopez AJ, Jia Y, White AO, Kwapis JL, Espinoza M, Hwang P, et al. Medial habenula cholinergic signaling regulates cocaine-associated relapse-like behavior. Addiction biology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Narayanan NS, Guarnieri DJ, DiLeone RJ Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol. 2010,31:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kenny PJ Reward mechanisms in obesity: new insights and future directions. Neuron. 2011,69:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].DiLeone RJ, Taylor JR, Picciotto MR The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012,15:1330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wise RA Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006,361:1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kenny PJ Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011,12:638–51. [DOI] [PubMed] [Google Scholar]
- [64].Volkow ND, Wise RA, Baler R The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci. 2017,18:741–52. [DOI] [PubMed] [Google Scholar]
- [65].Grill HJ, Hayes MR Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell metabolism. 2012,16:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Williams KW, Elmquist JK From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012,15:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Alhadeff AL, Rupprecht LE, Hayes MR GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012,153:647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006,51:801–10. [DOI] [PubMed] [Google Scholar]
- [69].Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain research. 2003,964:107–15. [DOI] [PubMed] [Google Scholar]
- [70].Labouebe G, Liu S, Dias C, Zou H, Wong JC, Karunakaran S, et al. Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat Neurosci. 2013,16:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011,180:129–37. [DOI] [PubMed] [Google Scholar]
- [72].Wang XF, Liu JJ, Xia J, Liu J, Mirabella V, Pang ZP Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Rep. 2015,12:726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Figlewicz DP, MacDonald Naleid A, Sipols AJ Modulation of food reward by adiposity signals. Physiol Behav. 2007,91:473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Krugel U, Schraft T, Kittner H, Kiess W, Illes P Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003,482:185–7. [DOI] [PubMed] [Google Scholar]
- [75].McCaleb ML, Myers RD Striatal dopamine release is altered by glucose and insulin during push-pull perfusion of the rat’s caudate nucleus. Brain Res Bull. 1979,4:651–6. [DOI] [PubMed] [Google Scholar]
- [76].Naleid AM, Grace MK, Cummings DE, Levine AS Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005,26:2274–9. [DOI] [PubMed] [Google Scholar]
- [77].Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. The Journal of clinical investigation. 2006,116:3229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H, et al. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin’s effect on food reward but not food intake. Neuropharmacology. 2013,73:274–83. [DOI] [PubMed] [Google Scholar]
- [79].Skibicka KP, Hansson C, Egecioglu E, Dickson SL Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addiction biology. 2012,17:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cone JJ, McCutcheon JE, Roitman MF Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014,34:4905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cone JJ, Roitman JD, Roitman MF Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 2015,133:844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hayes MR, Schmidt HD GLP-1 influences food and drug reward. Curr Opin Behav Sci. 2016,9:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jerlhag E Gut-brain axis and addictive disorders: A review with focus on alcohol and drugs of abuse. Pharmacol Ther. 2018. [DOI] [PubMed] [Google Scholar]
- [84].Holst JJ The physiology of glucagon-like peptide 1. Physiol Rev. 2007,87:1409–39. [DOI] [PubMed] [Google Scholar]
- [85].Baggio LL, Drucker DJ Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007,132:2131–57. [DOI] [PubMed] [Google Scholar]
- [86].Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997,77:257–70. [DOI] [PubMed] [Google Scholar]
- [87].Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res. 1986,16:97–107. [DOI] [PubMed] [Google Scholar]
- [88].Merchenthaler I, Lane M, Shughrue P Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999,403:261–80. [DOI] [PubMed] [Google Scholar]
- [89].Rinaman L Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain research. 2010,1350:18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995,44:1126–31. [DOI] [PubMed] [Google Scholar]
- [91].Deacon CF, Johnsen AH, Holst JJ Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995,80:952–7. [DOI] [PubMed] [Google Scholar]
- [92].Kieffer TJ, McIntosh CH, Pederson RA Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995,136:3585–96. [DOI] [PubMed] [Google Scholar]
- [93].Mentlein R Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999,85:9–24. [DOI] [PubMed] [Google Scholar]
- [94].Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell metabolism. 2011,13:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, et al. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology. 1999,140:1132–40. [DOI] [PubMed] [Google Scholar]
- [96].Hallbrink M, Holmqvist T, Olsson M, Ostenson CG, Efendic S, Langel U Different domains in the third intracellular loop of the GLP-1 receptor are responsible for Galpha(s) and Galpha(i)/Galpha(o) activation. Biochim Biophys Acta. 2001,1546:79–86. [DOI] [PubMed] [Google Scholar]
- [97].Fletcher MM, Halls ML, Christopoulos A, Sexton PM, Wootten D The complexity of signalling mediated by the glucagon-like peptide-1 receptor. Biochem Soc Trans. 2016,44:582–8. [DOI] [PubMed] [Google Scholar]
- [98].Rupprecht LE, Mietlicki-Baase EG, Zimmer DJ, McGrath LE, Olivos DR, Hayes MR Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. Am J Physiol Endocrinol Metab. 2013,305:E751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999,276:R1541–4. [DOI] [PubMed] [Google Scholar]
- [100].Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999,44:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996,379:69–72. [DOI] [PubMed] [Google Scholar]
- [102].Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011,31:3904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hayes MR, De Jonghe BC, Kanoski SE Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010,100:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011,152:3103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009,150:1174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lovshin JA, Drucker DJ Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009,5:262–9. [DOI] [PubMed] [Google Scholar]
- [107].Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. The Journal of clinical investigation. 2014,124:2456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. The Journal of clinical investigation. 2014,124:4473–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Shukla AP, Buniak WI, Aronne LJ Treatment of obesity in 2015. Journal of cardiopulmonary rehabilitation and prevention. 2015,35:81–92. [DOI] [PubMed] [Google Scholar]
- [110].Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. American journal of physiology. Endocrinology and metabolism. 2013,305:E1367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dossat AM, Lilly N, Kay K, Williams DL Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011,31:14453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003,284:R1427–35. [DOI] [PubMed] [Google Scholar]
- [113].Egecioglu E, Engel JA, Jerlhag E The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013,8:e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Graham DL, Erreger K, Galli A, Stanwood GD GLP-1 analog attenuates cocaine reward. Mol Psychiatry. 2013,18:961–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sorensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, et al. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav. 2015,149:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, et al. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav. 2015,149:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005,146:3748–56. [DOI] [PubMed] [Google Scholar]
- [118].Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond). 2006,30:1332–40. [DOI] [PubMed] [Google Scholar]
- [119].Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012,62:1916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009,374:39–47. [DOI] [PubMed] [Google Scholar]
- [121].Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology. 2018,43:2000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Reddy IA, Pino JA, Weikop P, Osses N, Sorensen G, Bering T, et al. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Translational psychiatry. 2016,6:e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009,513:597–621. [DOI] [PubMed] [Google Scholar]
- [124].Mahler SV, Aston-Jones GS Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012,32:13309–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Xi ZX, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, et al. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008,33:1735–45. [DOI] [PubMed] [Google Scholar]
- [126].L’Hirondel M, Cheramy A, Godeheu G, Glowinski J Effects of arachidonic acid on dopamine synthesis, spontaneous release, and uptake in striatal synaptosomes from the rat. J Neurochem. 1995,64:1406–9. [DOI] [PubMed] [Google Scholar]
- [127].Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995,7:2294–300. [DOI] [PubMed] [Google Scholar]
- [128].Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016,41:1917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Arnold JM, Roberts DC A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacology, biochemistry, and behavior. 1997,57:441–7. [DOI] [PubMed] [Google Scholar]
- [130].Richardson NR, Roberts DC Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996,66:1–11. [DOI] [PubMed] [Google Scholar]
- [131].Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012,32:4812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hernandez NS, O’Donovan B, Ortinski PI, Schmidt HD Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats. Addiction biology. 2019,24:170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Di Chiara G Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002,137:75–114. [DOI] [PubMed] [Google Scholar]
- [134].Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, et al. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J Neurosci. 2014,34:6985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011,333:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Harasta AE, Power JM, von Jonquieres G, Karl T, Drucker DJ, Housley GD, et al. Septal Glucagon-Like Peptide 1 Receptor Expression Determines Suppression of Cocaine-Induced Behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015,40:1969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Grabus SD, Glowa JR, Riley AL Morphine- and cocaine-induced c-Fos levels in Lewis and Fischer rat strains. Brain research. 2004,998:20–8. [DOI] [PubMed] [Google Scholar]
- [138].Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S, et al. Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010,35:445–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Buffalari DM, Rinaman L Cocaine self-administration and extinction alter medullary noradrenergic and limbic forebrain cFos responses to acute, noncontingent cocaine injections in adult rats. Neuroscience. 2014,281C:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mantsch JR, Schlussman SD, Ho A, Kreek MJ Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. J Pharmacol Exp Ther. 2000,294:239–47. [PubMed] [Google Scholar]
- [141].Galici R, Pechnick RN, Poland RE, France CP Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol. 2000,387:59–62. [DOI] [PubMed] [Google Scholar]
- [142].Goeders NE, Guerin GF Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996,64:337–48. [DOI] [PubMed] [Google Scholar]
- [143].You ZB, Wang B, Gardner EL, Wise RA Cocaine and cocaine expectancy increase growth hormone, ghrelin, GLP-1, IGF-1, adiponectin, and corticosterone while decreasing leptin, insulin, GIP, and prolactin. Pharmacology, biochemistry, and behavior. 2018,176:53–6. [DOI] [PubMed] [Google Scholar]
- [144].Schmidt HD, McFarland KN, Darnell SB, Huizenga MN, Sangrey GR, Cha JH, et al. ADAR2-dependent GluA2 editing regulates cocaine seeking. Mol Psychiatry. 2015,20:1460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008,11:344–53. [DOI] [PubMed] [Google Scholar]
- [146].Maniscalco JW, Rinaman L Vagal Interoceptive Modulation of Motivated Behavior. Physiology (Bethesda). 2018,33:151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, et al. Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci. 2013,33:13861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zheng D, Cabeza de Vaca S, Carr KD Food restriction increases acquisition, persistence and drug prime-induced expression of a cocaine-conditioned place preference in rats. Pharmacology, biochemistry, and behavior. 2012,100:538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Roed SN, Wismann P, Underwood CR, Kulahin N, Iversen H, Cappelen KA, et al. Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol Cell Endocrinol. 2014,382:938–49. [DOI] [PubMed] [Google Scholar]
- [150].Zheng H, Stornetta RL, Agassandian K, Rinaman L Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Struct Funct. 2015,220:3011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Fortin SM, Roitman MF Central GLP-1 receptor activation modulates cocaine-evoked phasic dopamine signaling in the nucleus accumbens core. Physiol Behav. 2017,176:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005,30:853–63. [DOI] [PubMed] [Google Scholar]
- [153].Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM Subsecond dopamine release promotes cocaine seeking. Nature. 2003,422:614–8. [DOI] [PubMed] [Google Scholar]
- [154].Acuna-Goycolea C, van den Pol A Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004,24:8141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Korol SV, Jin Z, Babateen O, Birnir B GLP-1 and exendin-4 transiently enhance GABAA receptor-mediated synaptic and tonic currents in rat hippocampal CA3 pyramidal neurons. Diabetes. 2015,64:79–89. [DOI] [PubMed] [Google Scholar]
- [156].Sombers LA, Beyene M, Carelli RM, Wightman RM Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009,29:1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Jones SR, Gainetdinov RR, Wightman RM, Caron MG Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998,18:1979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. Eur J Neurosci. 2016,43:1661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang HL, Zhang S, et al. Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron. 2017,96:1112–26 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Cachope R, Cheer JF Local control of striatal dopamine release. Front Behav Neurosci. 2014,8:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012,2:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015,6:8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Zhang XF, Cooper DC, White FJ Repeated cocaine treatment decreases whole-cell calcium current in rat nucleus accumbens neurons. J Pharmacol Exp Ther. 2002,301:1119–25. [DOI] [PubMed] [Google Scholar]
- [164].Zhang XF, Hu XT, White FJ Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J Neurosci. 1998,18:488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Hu XT, Basu S, White FJ Repeated cocaine administration suppresses HVA-Ca2+ potentials and enhances activity of K+ channels in rat nucleus accumbens neurons. J Neurophysiol. 2004,92:1597–607. [DOI] [PubMed] [Google Scholar]
- [166].Babateen O, Korol SV, Jin Z, Bhandage AK, Ahemaiti A, Birnir B Liraglutide modulates GABAergic signaling in rat hippocampal CA3 pyramidal neurons predominantly by presynaptic mechanism. BMC Pharmacol Toxicol. 2017,18:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Nicola SM, Yun IA, Wakabayashi KT, Fields HL Firing of nucleus accumbens neurons during the consummatory phase of a discriminative stimulus task depends on previous reward predictive cues. J Neurophysiol. 2004,91:1866–82. [DOI] [PubMed] [Google Scholar]
- [168].Wheeler RA, Carelli RM Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009,56 Suppl 1:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Engel JA, Jerlhag E Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS drugs. 2014,28:875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Bouhlal S, Ellefsen KN, Sheskier MB, Singley E, Pirard S, Gorelick DA, et al. Acute effects of intravenous cocaine administration on serum concentrations of ghrelin, amylin, glucagon-like peptide-1, insulin, leptin and peptide YY and relationships with cardiorespiratory and subjective responses. Drug Alcohol Depend. 2017,180:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Escobar M, Scherer JN, Ornell F, Bristot G, Soares CM, Guimaraes LSP, et al. Leptin levels and its correlation with crack-cocaine use severity: A preliminary study. Neurosci Lett. 2018,671:56–9. [DOI] [PubMed] [Google Scholar]
- [172].Tessari M, Catalano A, Pellitteri M, Di Francesco C, Marini F, Gerrard PA, et al. Correlation between serum ghrelin levels and cocaine-seeking behaviour triggered by cocaine-associated conditioned stimuli in rats. Addiction biology. 2007,12:22–9. [DOI] [PubMed] [Google Scholar]
- [173].Bough KJ, Amur S, Lao G, Hemby SE, Tannu NS, Kampman KM, et al. Biomarkers for the development of new medications for cocaine dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014,39:202–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Beinborn M, Worrall CI, McBride EW, Kopin AS A human glucagon-like peptide-1 receptor polymorphism results in reduced agonist responsiveness. Regul Pept. 2005,130:1–6. [DOI] [PubMed] [Google Scholar]
- [175].Koole C, Wootten D, Simms J, Valant C, Miller LJ, Christopoulos A, et al. Polymorphism and ligand dependent changes in human glucagon-like peptide-1 receptor (GLP-1R) function: allosteric rescue of loss of function mutation. Mol Pharmacol. 2011,80:486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, et al. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Translational psychiatry. 2015,5:e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity. 2011,19:1342–9. [DOI] [PubMed] [Google Scholar]
- [178].Gentilella R, Pechtner V, Corcos A, Consoli A Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev. 2019,35:e3070. [DOI] [PubMed] [Google Scholar]
- [179].Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annual review of nutrition. 2014,34:237–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Granhall C, Donsmark M, Blicher TM, Golor G, Sondergaard FL, Thomsen M, et al. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of the Novel Oral Human GLP-1 Analogue, Oral Semaglutide, in Healthy Subjects and Subjects with Type 2 Diabetes. Clin Pharmacokinet. 2018. [DOI] [PubMed] [Google Scholar]
- [181].Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. American journal of physiology. Endocrinology and metabolism. 2001,281:E155–61. [DOI] [PubMed] [Google Scholar]
- [182].Thazhath SS, Marathe CS, Wu T, Chang J, Khoo J, Kuo P, et al. The Glucagon-Like Peptide 1 Receptor Agonist Exenatide Inhibits Small Intestinal Motility, Flow, Transit, and Absorption of Glucose in Healthy Subjects and Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes. 2016,65:269–75. [DOI] [PubMed] [Google Scholar]
- [183].Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Patients With Type 2 Diabetes on Metformin and Glimepiride (AWARD-2). Diabetes Care. 2015,38:2241–9. [DOI] [PubMed] [Google Scholar]
- [184].Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010,375:2234–43. [DOI] [PubMed] [Google Scholar]
- [185].Drucker DJ Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell metabolism. 2018,27:740–56. [DOI] [PubMed] [Google Scholar]
- [186].Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology. 2013,38:1259–70. [DOI] [PubMed] [Google Scholar]
- [187].Egecioglu E, Engel JA, Jerlhag E The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One. 2013,8:e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Shirazi RH, Dickson SL, Skibicka KP Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One. 2013,8:e61965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Vallof D, Maccioni P, Colombo G, Mandrapa M, Jornulf JW, Egecioglu E, et al. The glucagon-like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addiction biology. 2016,21:422–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci. 2017,20:708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Richard JE, Anderberg RH, Lopez-Ferreras L, Olandersson K, Skibicka KP Sex and estrogens alter the action of glucagon-like peptide-1 on reward. Biol Sex Differ. 2016,7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]