SUMMARY
Background:
Cutaneous viral infections and immune suppression are risk factors for some forms of non-melanoma skin cancer (NMSC), however, their interrelationship is poorly understood.
Objective:
To examine cross-sectional associations between cutaneous viral infections and circulating forkhead-box P3 (FOXP3) expressing T regulatory (Treg) cells, suppressive cells that dampen effective anti-tumour immunity.
Methods:
Blood, eyebrow hair (EBH) and skin swab samples (SSW) were collected from 352 skin screening patients 60 years and older without prevalent skin cancer participating in an ongoing prospective cohort study of cutaneous viral infections and skin cancer. DNA corresponding to 98 cutaneous human papillomavirus (HPV) types and five polyomaviruses (HPyV) was assessed in EBH and SSW. Distinct classes of circulating Treg cell subpopulations were defined by flow cytometry including cutaneous lymphocyte antigen (CLA) and CCR4high Treg cells, both previously associated with cutaneous diseases. Age- and gender-adjusted associations between circulating T-cell populations and infection were estimated using logistic regression.
Results:
Total Treg cell proportion in peripheral blood was not associated with beta HPV or HPyV infection. However, the proportion of circulating CLA+ Treg cells was inversely associated with gamma HPV EB infection (OR= 0.54, 95% CI=0.35–0.84). Interestingly, circulating Treg cells expressing markers indicative of antigen activation (CD27−CD45RA−FOXP3+CD4+) were also inversely associated with gamma HPV infection in SS (OR=0.55, 95% CI=0.30–0.99) and EB (OR=0.56, 95% CI=0.36–0.86).
Conclusions:
Inverse associations between circulating Treg cells and gamma HPV infection suggest that localised viral infection may promote immunosuppressive cell migration into skin.
INTRODUCTION
Cutaneous human papillomavirus (HPV) and human polyomavirus (HPyV) infections are highly prevalent in the general population1, 2, and have been linked to increased risks of cutaneous squamous cell carcinoma (cuSCC) and Merkel cell carcinoma (MCC), respectively3–6. In a previous case-control study conducted in immunocompetent individuals, we observed keratinocyte carcinomas (KC), including cuSCC, to be positively associated with cutaneous HPV or HPyV infection measured in eyebrow hairs (EBH)7, as well as by serology4, 8–10. While these findings are compelling, it is unclear whether these infections are playing a role in carcinogenesis, either directly or indirectly, or whether they are simply surrogate markers for underlying immune dysregulation associated with cuSCC.
Immunosuppression is an established risk factor for cuSCC. Organ transplant recipients have an over 100-fold increased risk of cuSCC 11–13 and a 103-fold increased risk of MCC compared to the general population14. While the pathogenesis of cuSCC is multifactorial15, incidence among transplant recipients increases with the magnitude and duration of immunosuppressive therapies12, 16, 17. While lifelong immunosuppressive treatments to prevent organ transplantation rejection entails significant suppression of the immune system, evidence suggests that lower levels of immunosuppression may also influence cuSCC risk. For example, long-term use of oral glucocorticoids was associated with cuSCC in a population of otherwise “immunocompetent” individuals18. Therefore, biomarkers of immune status should be incorporated into epidemiologic studies of cutaneous viral infections to better understand this interplay in relation to the development of cuSCC.
Treg cells, a subset of immunosuppressive FOXP3+CD4+ T lymphocytes19, are key mediators of immune homeostasis in healthy individuals, including suppression of immune response against self-antigens20, 21. Increased prevalence of circulating Treg cells has been observed in patients with various cancers compared to controls22–25. Higher proportions of Treg cells have been observed in cuSCC tumours compared to normal skin26, 27 and peripheral blood27, suggesting potential underlying immune dysregulation in cuSCC cases. Similarly, higher frequencies of circulating Treg cells have been observed in cervical cancer patients compared to controls28, with circulating Treg cells shown to suppress immune response against HPV 16 in cervical cancer patients, in vitro28. By analogy, it is plausible that our previously observed associations between cutaneous HPV infections and KC4, 7, 8 may be mediated by Treg cell-related suppression of immune response against these infections, KC or both. Thus, inter-individual variation in Treg cell-mediated immune surveillance against cutaneous HPV or HPyV infections may affect one’s ability to suppress anti-viral immunity and promote viral replication and proliferation. If these infections, in turn, promote skin carcinogenesis by inhibiting apoptosis of cells with ultraviolet radiation-induced DNA damage29, 30, then individuals with an immunosuppressive profile, marked by higher numbers of circulating Treg cells or display a phenotype associated with more suppressive behaviour or homing to skin, may be at a greater risk of infection with cutaneous HPV or HPyV, and ultimately, virus-associated KC.
To examine the complex interplay of cutaneous viral infections, immune response and subsequent KC risk, we are conducting an ongoing prospective cohort study of viruses and skin cancer, the VIRUSCAN Study, among patients undergoing skin cancer screening exams. Using cross-sectional data from patients enrolled in the first year of the VIRUSCAN Study, we examined the association between circulating Treg cell phenotypes and infection with beta and gamma HPV and HPyV in EBH and skin swabs (SSW). We hypothesized that circulating Treg cells would be associated with cutaneous HPV and HPyV infections.
MATERIALS AND METHODS
Study population:
The VIRUSCAN Study is an ongoing, prospective cohort study being conducted at Moffitt Cancer Center and the University of South Florida (USF), in Tampa, Florida, to examine the association between cutaneous viral infections and KC. Patients attending the USF Dermatology Clinic for routine skin cancer screening exams were eligible for the study if they were ages 60 years or older, and had not had both a cuSCC and a basal cell carcinoma prior to or at the time of study enrolment. At study enrolment, patients underwent a full body skin cancer screening exam as part of their clinical visit, and any suspicious skin lesions detected were biopsied as part of routine clinical care. Patients completed an electronic questionnaire on skin cancer risk factors, and several biospecimens were collected, as described below.
A total of 917 patients were approached to participate in year 1 of the study (July 15, 2014 – July 14, 2015), of whom 448 (49%) were enrolled. Sex and age did not significantly differ between participants and non-participants. Peripheral blood mononuclear cells (PBMC) samples were cryopreserved at baseline for 390 patients, of which 378 had sufficient numbers of viable cells for flow cytometry. Based on pathology results of baseline biopsies, 23 patients were diagnosed with KC and excluded from this analysis. Of the remaining patients, baseline SSW and EBH were available for 323 and 344 patients, respectively, and were included in the present analysis. All study methods were approved by the USF Institutional Review Board, and all patients provided written informed consent.
Cell isolation
PBMCs were isolated by Ficoll-Hypaque gradient centrifugation using 10–20 ml of Lymphocyte Separation Media (Ficoll) according to manufacturer’s recommended methods (Amersham Pharmacia Biotech, Piscataway, NJ). Two cryovials of PBMCs were viably frozen using freezing medium (10% DMSO, 90% FBS), step-frozen in −80 C freezer and transferred to LN boxes for storage. Two additional vials of PMBCs were centrifuged to create cell pellets and snap frozen for future DNA extraction.
Eyebrow hair and skin swab collection:
3–4 EBH were plucked from each brow using disposable tweezers. EBH with attached follicles were snap frozen in liquid nitrogen and stored at −80°C until further processing. An area of the top of the sun exposed forearm, approximately 5×5 cm, was sprayed with 0.9% saline solution. A cotton-tipped Dacron swab (Digene, Gaithersburg, MD, USA) was then rubbed back and forth 10 times to collect exfoliated skin cells. Individual swabs were placed in a separate vial, preserved in Digene Standard Transport Medium, and stored at 4°C until further processing. EBH and SSW were subsequently shipped to the International Agency for Research on Cancer (IARC) for viral DNA extraction and genotyping.
Characterisation of Treg cells:
Viably frozen PBMCs were used to assess proportions of circulating Treg cells from peripheral blood samples using antibodies shown in Figure S1 (BD Biosciences; Table S1) and staining methods described previously31. Proportions of the following circulating T-cells were derived as follows: 1) CD4+ and CD8+ T-cells (CD3+CD4+ and CD3+CD4− among total T-cells), 2) total Treg cells and non-Treg CD4+ T-cells (CD3+CD4+CD25+ FOXP3+ and CD3+CD4+CD25−FOXP3−, respectively, among CD3+CD4+ T-cells), 3) Treg cells with naïve and post-activation markers including CD45RA+/CD27+ naïve, CD45RA−/CD27+ long-term memory, CD45RA+/CD27− exhausted, and CD45RA−/CD27− activated “effector” Tregs cells, as defined previously31, 32, 4) “skin homing” CLA+ Treg cells (% of CLA+ cells among Treg cells) and 5) “skin homing” CCR4high Treg cells (% of CCR4+ cells among Treg cells) (Fig. S1; see Supporting Information).
Viral DNA genotyping:
DNA extraction was performed using the Qiagen BioRobot EZ1 with the EZ1 DNA tissue kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). Briefly, the swabs were incubated overnight in proteinase K and buffer G2 (Qiagen, Hilden, Germany) at 56°C. An EZ1 DNA Forensic protocol was used to extract the DNA from EBH per the manufacturer’s instructions. Viral DNA was detected by bead-based multiplex PCR-Luminex assay, as described in detail previously33, using approximately 100 ng of total DNA and specific primers amplifying parts of the E7 and large T-antigen genes for HPV and polyomaviruses, respectively. Two primers for the amplification of β-globin primers were used as a positive control for assessment of template DNA quality. PCRs were performed with the QIAGEN multiplex PCR kit according to the instructions of the manufacturer. This multiplex PCR protocol is highly sensitive33 and reproducible34, being able to detect only 10 copies of the viral genome. Using four multiplex-assays (one beta HPV panel, two gamma HPV panels and a polyomavirus panel), DNA corresponding to the following viruses was measured from SSW and EBH samples: 1) HPV types in genus beta (n=46): species 1 (5, 8, 12, 14, 19, 20, 21, 24, 25, 36, 47, 93, 98, 99,105, 118, 124, 143, 152), species 2 (9, 15, 17, 22, 23, 37, 38, 80, 100, 104, 107, 110, 111, 113, 120, 122, 145, 151, 159, 174), species 3 (49, 75, 76, 115), species 4 (92) and species 5 (96, 150); 2) HPV types in genus gamma (n=52): using panel 1 (4, 48, 50, 60, 65, 88, 95, 101, 103, 108, 109, 112, 116, 119, 121, 123, 126, 127, 128, 129, 130, 131, 132, 133, 134, 148, 149, 56, SD2) and panel 2 (161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 175, 178, 179, 180, 184, 197, 199, 200, 201 and 202); 3) cutaneous polyomaviruses: HPyV6, HPyV7, HPyV9, Merkel cell polyomavirus and trichodysplasia spinulosa-associated polyomavirus. Viral DNA results from a given SSW or EBH sample were only included in the analysis if the sample was positive for the human β-globin gene on at least two of the four genotyping panels.
Statistical analyses:
Wilcoxon rank-sum test and Spearman’s correlation coefficient were used to test for differences in Tregs by gender and age, respectively. Correlations between circulating Treg cell phenotypes were described using Spearman’s correlation coefficient. Circulating Treg cell phenotypes were dichotomised using the median proportion of phenotype-specific Treg cells. Genus- (or species-) level HPV infection in SSW and EBH was defined as presence of at least one type of HPV corresponding to a given genus or species. The associations between each of the circulating Treg cell phenotypes with HPV and HPyV infection were estimated with odds ratios (ORs) and 95% confidence interval (CIs), calculated using logistic regression, adjusted for age and gender. Correlations between circulating Treg cell phenotypes and number of HPV types detected in SSW and EBH were examined using Spearman’s correlation coefficients. False discovery rate corrected (FDR) p-values were calculated and two sided p-values <0.05 were considered significant. All analyses were performed using R, version 3.3.2 (R Foundation for Statistical Computing, Vienna Austria).
RESULTS
As described in Table 1, females comprised 54% of the study population, the mean age was 69 years, and a majority of patients were White (97%) and non-Hispanic (94%). Total circulating Treg cells were not associated with age or gender (Table 2). However, circulating CD27−/CD45RA−, CLA+, and CCR4hi Treg cell populations were significantly greater in males than females and positively correlated with age (Table 2).
Table 1.
Baseline characteristics of 352 skin cancer screening patients enrolled in the first year of the Viruses in Skin Cancer (VIRUSCAN) Study, Tampa FL, July 2014-July 2015 who screened negative for skin cancer at the time of study enrollment
| Patient Characteristics | n | % |
|---|---|---|
| Age in years | ||
| Mean (standard deviation) | 69.37 | 6.2 |
| Gender | ||
| Female | 189 | 53.7 |
| Male | 163 | 46.3 |
| Race | ||
| White | 342 | 97.2 |
| Others | 10 | 2.8 |
| Ethnicity | ||
| Non-Hispanic | 331 | 94.0 |
| Hispanic or Latino | 19 | 5.4 |
| Unknown | 2 | 0.6 |
| Ever taken oral steroids for >30 days | ||
| No | 214 | 61.9 |
| Yes | 132 | 38.2 |
| Ever used topical cortisone or steroid cream | ||
| No | 170 | 49.3 |
| Yes | 175 | 50.7 |
| Ever had an organ transplant | ||
| No | 344 | 99.4 |
| Yes | 2 | 0.6 |
| Ever smoked 100 cigarettes | ||
| No | 176 | 50.7 |
| Yes | 171 | 49.3 |
| History of keratinocyte carcinoma at study enrolment | ||
| No known keratinocyte carcinoma | 222 | 63.1 |
| Squamous cell carcinoma only | 58 | 16.5 |
| Basal cell carcinoma only | 59 | 16.8 |
| Single non-melanoma skin cancer of unspecified type | 13 | 3.7 |
Table 2.
Baseline circulatory T (Treg) cell phenotypes by age and gender among 352 skin cancer screening patients enrolled in the first year of the Viruses in Skin Cancer (VIRUSCAN) Study who screened negative for skin cancer at the time of study enrollment
| All (n=352) |
Female (n=189) |
Male (n=163) |
Age (range 60–89) |
||||
|---|---|---|---|---|---|---|---|
| Circulating Treg cell phenotypes1 | mean (SD2) |
median | mean (SD) |
mean (SD) |
p-value3 | r | p-value4 |
| Lymphocyte populations | |||||||
| Total Treg | 4.3 (2.2) | 4.0 | 4.2 (2.0) | 4.4 (2.4) | 0.28 | 0.03 | 0.52 |
| Treg cell subpopulations | |||||||
| CD27−/CD45RA− (activated) Treg | 13.7 (6.9) | 12.2 | 12.9 (6.9) | 14.5 (6.8) | 0.01* | 0.11 | 0.05* |
| CD27+/CD45RA− Treg | 75.2 (9.8) | 77.1 | 75.4 (10.1) | 74.9 (9.5) | 0.36 | 0.04 | 0.42 |
| CD27−/CD45RA+ Treg | 0.8 (1.6) | 0.4 | 0.9 (1.5) | 0.8 (1.7) | 0.10 | -0.05 | 0.34 |
| CD27+CD45RA+ Treg | 10.3 (8.6) | 8.3 | 10.8 (9.0) | 9.8 (8.2) | 0.08 | -0.15 | 0.01* |
| CLA+ Treg | 57.6 (11.2) | 57.8 | 56.1 (11.4) | 59.3 (10.7) | 0.01* | 0.09 | 0.09 |
| CCR4hi Treg | 82.8 (8.6) | 84.2 | 81.3 (8.5) | 84.5 (8.4) | <0.01* | 0.13 | 0.01* |
| PD1 Treg | 20.2 (8.4) | 19.1 | 19.8 (7.9) | 20.7 (9.0) | 0.46 | 0.00 | 0.95 |
Total Treg: Percent of FOXP3+CD25+ cells among CD4 T-cellsCD27−CD45RA− Treg: Percent of CD27−CD45RA− cells among Treg cellsCD27+/CD45RA− Treg: Percent of CD27+/CD45RA- among Treg cellsCD27−/CD45RA+ Treg: Percent CD27−/CD45RA+ among Treg cellsCD27+/CD45RA+ Treg: Percent of CD27+/CD45RA+ among Treg cellsCLA+ Treg: Percent of CLA+ cells among Treg cellsCCR4hi Treg: Percentage of Treg cells with high CCR4 expressionPD-1 Treg: Percent of PD-1+ cells among Treg Cells
SD: standard deviation
Calculated by Wilcoxon rank-sum test
Calculated by Spearman’s rank correlation
The total circulating Treg cells was not associated with any beta HPV, any gamma HPV or any HPyV in SSW (Table 3a) or EBH (Table 3b). However, higher proportions of circulating activated ‘effector’ CD27−/CD45RA− Treg cells, were significantly and inversely associated with any gamma HPV infection in both SSW (Table 3a, OR=0.55, 95% CI=0.30–0.99) and EBH (Table 3b, OR=0.56, 95% CI=0.36–0.86), the latter of which remained statistically significant after FDR adjustment (p=0.036). Interestingly, circulating skin homing CLA+ Treg cells were also significantly inversely associated with any gamma HPV infection in EBH (Table 3b, OR=0.54, 95% CI=0.35–0.84; FDR adjusted p-value = 0.036. None of the other circulating Treg cell phenotypes was significantly associated with HPV/HPyV infections in either SSW or EBH. No significant associations with circulating effector Treg cells were observed for specific beta HPV species in SSW or EBH (Table S2a and S2b, respectively).
Table 3-a.
Baseline circulating regulatory T (Treg) cell phenotypes and cutaneous viral infections in skin swabs among 323 skin cancer screening patients in the VIRUSCAN Study who screened negative for skin cancer at study enrollment
| Circulating | Any beta HPV infection | Any gamma HPV infection | Any HPyV infection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treg cell phenotypes1 | HPV− n (%) | HPV+ n (%) | OR (95%CI)2 | p-value | HPV− n (%) | HPV+ n (%) | OR (95%CI) | p-value | HPV− n (%) | HPV+ n (%) | OR (95%CI) | p-value |
| Treg cells | ||||||||||||
| (<=4.05) | 13 (50.0) | 147 (49.5) | 1.0 (ref.) | 0.99 | 34 (58.6) | 126 (47.5) | 1.0 (ref.) | 0.18 | 49 (55.1) | 111 (47.4) | 1.0 (ref.) | 0.34 |
| (>4.05) | 13 (50.0) | 150 (50.5) | 1.00 (0.44–2.25) | 24 (41.4) | 139 (52.5) | 1.49 (0.84–2.68) | 40 (44.9) | 123 (52.6) | 1.27 (0.77–2.10) | |||
| CD27−/CD45RA− (activated) Treg (Effector Treg) cells | ||||||||||||
| (<=12.2) | 11 (42.3) | 158 (53.2) | 1.0 (ref.) | 0.23 | 25 (43.1) | 144 (54.3) | 1.0 (ref.) | 0.053 | 49 (55.1) | 120 (51.3) | 1.0 (ref.) | 0.98 |
| (>12.2) | 15 (57.7) | 139 (46.8) | 0.60 (0.26–1.36) | 33 (56.9) | 121 (45.7) | 0.55 (0.30–0.99)* | 40 (44.9) | 114 (48.7) | 1.01 (0.61–1.67) | |||
| CD27+/CD45RA− Treg (CM Treg) cells | ||||||||||||
| (<=77.1) | 15 (57.7) | 148 (49.8) | 1.0 (ref.) | 0.42 | 33 (56.9) | 130 (49.1) | 1.0 (ref.) | 0.29 | 43 (48.3) | 120 (51.3) | 1.0 (ref.) | 0.64 |
| (>77.1) | 11 (42.3) | 149 (50.2) | 1.40 (0.62–3.24) | 25 (43.1) | 135 (50.9) | 1.37 (0.77–2.46) | 46 (51.7) | 114 (48.7) | 0.89 (0.54–1.46) | |||
| CD27−/CD45RA+ Treg (TEM Treg) cells | ||||||||||||
| (<=0.43) | 17 (65.4) | 149 (50.2) | 1.0 (ref.) | 0.13 | 32 (55.2) | 134 (50.6) | 1.0 (ref.) | 0.48 | 52 (58.4) | 114 (48.7) | 1.0 (ref.) | 0.09 |
| (>0.43) | 9 (34.6) | 148 (49.8) | 1.92 (0.84–4.64) | 26 (44.8) | 131 (49.4) | 1.23 (0.69–2.20) | 37 (41.6) | 120 (51.3) | 1.54 (0.94–2.56) | |||
| CD27+CD45RA+ Treg (Naïve Treg) cells | ||||||||||||
| (<=8.34) | 12 (46.2) | 147 (49.5) | 1.0 (ref.) | 0.76 | 30 (51.7) | 129 (48.7) | 1.0 (ref.) | 0.52 | 46 (51.7) | 113 (48.3) | 1.0 (ref.) | 0.39 |
| (>8.34) | 14 (53.8) | 150 (50.5) | 0.88 (0.38–1.99) | 28 (48.3) | 136 (51.3) | 1.21 (0.68–2.17) | 43 (48.3) | 121 (51.7) | 1.25 (0.76–2.07) | |||
| CLA+ Treg cells | ||||||||||||
| (<=57.8) | 15 (57.7) | 152 (51.2) | 1.0 (ref.) | 0.63 | 32 (55.2) | 135 (50.9) | 1.0 (ref.) | 0.78 | 50 (56.2) | 117 (50) | 1.0 (ref.) | 0.64 |
| (>57.8) | 11 (42.3) | 145 (48.8) | 1.22 (0.54–2.86) | 26 (44.8) | 130 (49.1) | 1.09 (0.61–1.96) | 39 (43.8) | 117 (50) | 1.13 (0.68–1.87) | |||
| CCR4hi Treg cells | ||||||||||||
| (<=84.2) | 14 (53.8) | 154 (51.9) | 1.0 (ref.) | 0.98 | 31 (53.4) | 137 (51.7) | 1.0 (ref.) | 0.82 | 48 (53.9) | 120 (51.3) | 1.0 (ref.) | 0.74 |
| (>84.2) | 12 (46.2) | 143 (48.1) | 1.01 (0.44–2.35) | 27 (46.6) | 128 (48.3) | 0.93 (0.51–1.69) | 41 (46.1) | 114 (48.7) | 0.92 (0.55–1.53) | |||
| PD1+ Treg cells | ||||||||||||
| (<=19.1) | 11 (42.3) | 154 (51.9) | 1.0 (ref.) | 0.35 | 28 (48.3) | 137 (51.7) | 1.0 (ref.) | 0.61 | 40 (44.9) | 125 (53.4) | 1.0 (ref.) | 0.15 |
| (>19.1) | 15 (57.7) | 143 (48.1) | 0.68 (0.29–1.52) | 30 (51.7) | 128 (48.3) | 0.86 (0.49–1.53) | 49 (55.1) | 109 (46.6) | 0.70 (0.42–1.14) | |||
T cells were dichotomized using median cut-offs as follows: Treg cell subpopulations: 4.045, CD27−/CD45RA− Treg: 12.2, CD27+/CD45RA− Treg: 77.1, CD27−/CD45RA+ Treg: 0.43, CD27+CD45RA+ Treg: 8.34, CLA+ Treg: 57.8, CCR4hiTreg: 84.2, PD1+ Treg: 19.1;
Odds ratios (OR) and 95% confidence intervals (CI) calculated using logistic regression, adjusted for age and gender;
P-value adjusted for false discovery rate, p-value = 0.39;
statistically significant at p<0.05
Table 3-b.
Baseline circulating regulatory T (Treg) cell phenotypes and cutaneous viral infections in eyebrow hairs among 344 skin cancer screening patients in the VIRUSCAN Study who screened negative for skin cancer at study enrollment
| Circulating | Any beta HPV infection | Any gamma HPV infection | Any HPyV infection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treg cell phenotypes1 | HPV− n (%) | HPV+ n (%) | OR (95%CI)2 | p-value | HPV− n (%) | HPV+ n (%) | OR (95%CI) | p-value | HPV− n (%) | HPV+ n (%) | OR (95%CI) | p-value |
| Treg cells | ||||||||||||
| (<=4.05) | 45 (47.9) | 127 (50.8) | 1.0 (ref.) | 0.60 | 98 (50.3) | 74 (49.7) | 1.0 (ref.) | 0.85 | 120 (49.2) | 52 (52.0) | 1.0 (ref.) | 0.54 |
| (>4.05) | 49 (52.1) | 123 (49.2) | 0.88 (0.55–1.42) | 97 (49.7) | 75 (50.3) | 1.04 (0.68–1.60) | 124 (50.8) | 48 (48.0) | 0.86 (0.54–1.38) | |||
| CD27−/CD45RA− (activated) Treg (Effector Treg) cells | ||||||||||||
| (<=12.2) | 44 (46.8) | 128 (51.2) | 1.0 (ref.) | 0.43 | 85 (43.6) | 87 (58.4) | 1.0 (ref.) | 0.013a | 124 (50.8) | 48 (48.0) | 1.0 (ref.) | 0.81 |
| (>12.2) | 50 (53.2) | 122 (48.8) | 0.82 (0.51–1.34) | 110 (56.4) | 62 (41.6) | 0.56 (0.36–0.86)* | 120 (49.2) | 52 (52.0) | 1.06 (0.66–1.71) | |||
| CD27+/CD45RA− Treg (CM Treg) cells | ||||||||||||
| (<=77.1) | 47 (50.0) | 126 (50.4) | 1.0 (ref.) | 0.91 | 104 (53.3) | 69 (46.3) | 1.0 (ref.) | 0.19 | 121 (49.6) | 52 (52.0) | 1.0 (ref.) | 0.69 |
| (>77.1) | 47 (50.0) | 124 (49.6) | 0.97 (0.60–1.57) | 91 (46.7) | 80 (53.7) | 1.33 (0.87–2.05) | 123 (50.4) | 48 (48.0) | 0.91 (0.57–1.45) | |||
| CD27−/CD45RA+ Treg (TEM Treg) cells | ||||||||||||
| (<=0.43) | 48 (51.1) | 125 (50) | 1.0 (ref.) | 0.87 | 98 (50.3) | 75 (50.3) | 1.0 (ref.) | 0.97 | 119 (48.8) | 54 (54.0) | 1.0 (ref.) | 0.41 |
| (>0.43) | 46 (48.9) | 125 (50) | 1.04 (0.65–1.68) | 97 (49.7) | 74 (49.7) | 0.99 (0.65–1.52) | 125 (51.2) | 46 (46.0) | 0.82 (0.51–1.31) | |||
| CD27+CD45RA+ Treg (Naïve Treg) cells | ||||||||||||
| (<=8.34) | 47 (50.0) | 125 (50) | 1.0 (ref.) | 0.96 | 106 (54.4) | 66 (44.3) | 1.0 (ref.) | 0.08 | 121 (49.6) | 51 (51.0) | 1.0 (ref.) | 0.91 |
| (>8.34) | 47 (50.0) | 125 (50) | 1.01 (0.63–1.64) | 89 (45.6) | 83 (55.7) | 1.47 (0.96–2.27) | 123 (50.4) | 49 (49.0) | 0.97 (0.61–1.56) | |||
| CLA+ Treg cells | ||||||||||||
| (<=57.8) | 46 (48.9) | 125 (50.0) | 1.0 (ref.) | 0.88 | 84 (43.1) | 87 (58.4) | 1.0 (ref.) | 0.013b | 126 (51.6) | 45 (45.0) | 1.0 (ref.) | 0.37 |
| (>57.8) | 48 (51.1) | 125 (50.0) | 0.96 (0.60–1.56) | 111 (56.9) | 62 (41.6) | 0.54 (0.35–0.84)* | 118 (48.4) | 55 (55.0) | 1.24 (0.78–2.00) | |||
| CCR4hi Treg cells | ||||||||||||
| (<=84.2) | 40 (42.6) | 132 (52.8) | 1.0 (ref.) | 0.08 | 94 (48.2) | 78 (52.3) | 1.0 (ref.) | 0.55 | 129 (52.9) | 43 (43.0) | 1.0 (ref.) | 0.17 |
| (>84.2) | 54 (57.4) | 118 (47.2) | 0.64 (0.39–1.05) | 101 (51.8) | 71 (47.7) | 0.87 (0.56–1.35) | 115 (47.1) | 57 (57.0) | 1.40 (0.86–2.26) | |||
| PD1+ Treg cells | ||||||||||||
| (<=19.1) | 49 (52.1) | 125 (50.0) | 1.0 (ref.) | 0.75 | 96 (49.2) | 78 (52.3) | 1.0 (ref.) | 0.59 | 126 (51.6) | 48 (48.0) | 1.0 (ref.) | 0.57 |
| (>19.1) | 45 (47.9) | 125 (50.0) | 1.08 (0.67–1.74) | 99 (50.8) | 71 (47.7) | 0.89 (0.58–1.37) | 118 (48.4) | 52 (52.0) | 1.15 (0.72–1.83) | |||
T cells were dichotomized using median cut-offs as follows: Treg: 4.045, CD27−/CD45RA− Treg: 12.2, CD27+/CD45RA− Treg: 77.1, CD27−/CD45RA+ Treg: 0.43, CD27+CD45RA+ Treg: 8.34, CLA+ Treg: 57.8, CCR4hiTreg: 84.2, PD1+ Treg: 19.1;
Odds ratios (OR) and 95% confidence intervals (CI) calculated using logistic regression, adjusted for age and gender; P-value adjusted for false discovery rate,
p-value =0.036,
p-value = 0.036;
statistically significant at p<0.05
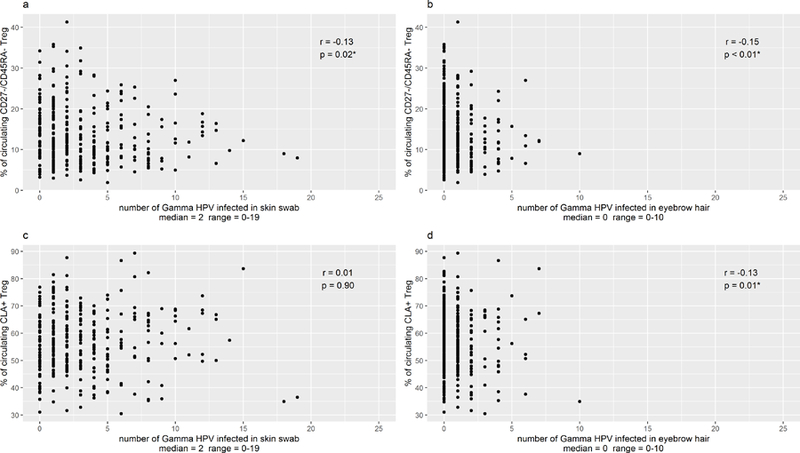
Circulating total Treg cells were not correlated with circulating activated effector Treg cells or skin homing Treg cells (Table 4). However, significant, positive correlations were observed between circulating activated effector Treg cells and the circulating skin homing CLA+ and CCR4hi Treg cells (Table 4). A statistically significant, inverse correlation was observed between proportion of circulating activated effector CD27−/CD45RA− Treg cells and number of gamma HPV types detected in both SSW (Fig. 1, median=2, range 0–19) and EBH (median=0, range =0–10), with the number of gamma HPV types decreasing with increasing proportion of circulating CD27−/CD45RA− Treg cells. An inverse correlation was also observed between proportion of circulating CLA+ Treg cells and number of gamma HPV types in EBH (p=0.01). Of interest, the numbers of beta HPV types were not correlated with proportion of circulating CD27−/CD45RA− or circulating CLA+ Treg cells (data not shown).
Table 4.
Spearman rank correlation matrix for baseline circulating T regulatory (Treg) cell phenotypes among 352 skin cancer screening patients enrolled in the first year of the Viruses in Skin Cancer (VIRUSCAN) Study who screened negative for skin cancer at study enrollment
| Circulating | CD27−/CD45RA− Treg | CLA+ Treg | CCR4hi Treg | |||
|---|---|---|---|---|---|---|
| T Regulatory Cells | r | p-value | r | p-value | r | p-value |
| Total Treg cells | -0.09 | 0.09 | 0.03 | 0.55 | 0.09 | 0.10 |
| CD27−/CD45RA− Treg cells | 0.32 | <0.01 | 0.38 | <0.01 | ||
| CLA+ Treg cells | 0.64 | <0.01 | ||||
Figure1.
Flow cytometry gating strategy for T regulatory (T reg) cell populations. Cells were first gated on CD3 expression and divided into CD4+ and CD4- populations. The CD4- population was assumed to be CD8+ T cells. CD4+ populations were further determined to be Foxp3+CD25+ (regulatory T cells, Treg cells) or Foxp3-CD25- (Th CD4). CD8, ThCD4 and Treg cell populations were analysed for CD45RA and CD27 memory populations as well as CLA, PD-1 and CCR4 expression.
DISCUSSION
Total circulating Treg cells from peripheral blood were not associated with cutaneous HPV or HPyV infections, while higher proportions of circulating activated effector CD27−/CD45RA− Treg cells were significantly inversely associated with the presence of gamma HPV infection in both SSW and EBH, the latter of which remained significant after correction for multiple comparisons. Circulating skin homing CLA+ Treg cells were also significantly inversely associated with gamma HPV infection in EBH, and both CD27−/CD45RA− and CLA+ Treg cells were inversely associated with the number of gamma HPV types with which an individual was infected, evidence of a dose-response relationship.
Treg cells play a key role in maintaining balanced host immunity against foreign pathogens while limiting tissue damage caused by excessive immune response35–37. These cells are generated through thymic selection (ie, natural Treg cells) or through phenotypic conversion from effector cells in the peripheral lymphoid compartments (ie, inducible Treg cells)36. Germaine to this study, Treg cell subpopulations with functional relevance to the skin were delineated based on unique expression of surface markers38. As reported previously, increased circulating CLA+ Treg cells in patients undergoing stem cell transplant were inversely associated with cutaneous graft-versus-host disease, suggesting a role of CLA+ Tregs in prevention of skin-tissue specific pathology37. Moreover, CCR4 is not expressed on thymic Treg cells but is exceptionally high among FOXP3+ T-cells in the skin39.
Interestingly, mouse studies have demonstrated the exodus of activated Treg cells from peripheral circulation into tissues, especially the skin, whereby they can induce local immune homeostasis as tissue resident cells40. This recirculation function has been reported during chronic inflammation and in viral infections41. Treg cells, similar to conventional T-cells, modify their CD45RA and CD27 cell surface marker expression based on their history or repeated antigen exposure, such as in the instances described above32. This phenotype conversion is similar to that observed in conventional T-cell memory cells.
It should be noted that the role of Treg cells in viral infections is complex. For some viral infections like hepatitis C virus (HCV), higher Treg cells in hepatic tissue42 and peripheral blood43 have been found to correlate with chronic HCV infection and HCV associated skin lesions, respectively. In contrast, lower circulating Treg cells have been observed in patients with progressive HIV disease compared to HIV-negative controls and HIV-positive patients with controlled infection35. Given the high prevalence of HPV/HPyV in the asymptomatic general population, these infections may not trigger strong immune response involving circulating Treg cells, which may explain a lack of positive association in our study. Alternatively, HPV tissue infection may lead to activation and recruitment of circulating Treg cells to the relevant sites resulting in a corresponding decrease in peripheral circulation. Peripheral blood Treg cells are decreased in HIV infected individuals, perhaps as a result of recruitment of Treg cells to sites of infection with HIV35. Finally, a chance finding in our study cannot be ruled out. However, our observation of similar inverse association between two distinct subpopulations of circulating Treg cells and gamma HPV measured by two different tissue locations (SSW and EBH), overall and with number of HPV types, warrants further research44.
To our knowledge, this study is the first to report associations between subpopulations of circulating Treg cells and cutaneous viral infections. There is a dearth of epidemiologic data on the associations between circulating Treg cells and both KC and cutaneous HPV, particularly among immunocompetent individuals. Thus, our study has the unique strength of examining circulating Treg cells in preserved blood samples available from the parent VIRUSCAN prospective study. The parent study will facilitate further examination of circulating Treg cells measured at baseline with both incident HPV infections and KC diagnosed throughout the study follow-up period. Another study strength is the large panel of HPV and HPyV types examined using two different biomarkers of viral infection. Of note, EBH follicles are enriched for slow-cycling, epithelial stem cells, whereas SSW of the skin’s surface represent a mixture of sloughed stem cells and surface epithelial cells. Therefore, while type-specific infections are correlated across the two sites44, the relative recency of infection and rate of viral replication may vary across sites. Finally, several different subpopulations of circulating Treg cells were investigated, including activated and skin-homing Treg cells, which may be more relevant to skin-associated viral infections. Thus, the present study provides a comprehensive examination of the role of circulating Treg cells in cutaneous viral infections.
Some limitations of the study are noted. A number of host and environmental factors, including age, medical conditions and ultraviolet radiation, have been associated with circulating Treg cells31, 45. While all analyses were adjusted for age and gender, it is possible that circulating activated effector Treg cells are a surrogate marker for an underlying condition or exposures that impact gamma HPV infection. Future experimental studies should investigate the biological underpinning of the observed associations between circulating effector Treg cells and gamma HPV infection. Due to the cross-sectional design of this study, temporality of association between circulating Treg cells and infection cannot be established. However, examination of the associations between circulating Treg cells measured at baseline and subsequent incident cutaneous infections, as well as incident KC, is planned once follow-up is completed for the ongoing, prospective VIRUSCAN Study. While the results are generalizable only to individuals over the age of 60, this is the relevant age range for people at increased risk for KC.
In conclusion, in this large cohort of skin-cancer free individuals, circulating CD27−/CD45RA− and CLA+ Treg cell subpopulations were significantly and inversely associated with cutaneous gamma HPV infection in both SSW and EBH. Examination of the association between baseline Treg cells and incident KC, as well as the association between baseline HPV infection and incident KC, within the context of the ongoing VIRUSCAN study, will provide further insight into the complex interplay between host immune response, cutaneous HPV infection and KC.
Supplementary Material
What’s already known about this topic?
Cutaneous viral infections such as the human papillomavirus (HPV) and polyomavirus (HPyV) may play a role in the development of some non-melanoma skin cancer types, including cutaneous squamous cell carcinoma (cuSCC) and Merkel cell carcinoma (MCC).
Immunosuppression is an established risk factor for cuSCC and MCC.
The relationship between cutaneous viral infections, immunosuppression and the development of cuSCC and MCC is not well understood.
What does this study add?
Higher proportions of circulating antigen activated CD27-/CD45RA- Treg cells were inversely associated with gamma HPV infection in skin swabs and eyebrow hairs, whereas no associations were observed for beta HPV or polyomavirus infections.
Circulating skin homing CLA+ Treg cells were inversely associated with gamma HPV eyebrow hair infection.
Gamma HPV infections may recruit immunosuppressive lymphocytes into the skin, perhaps contributing to cutaneous malignancy development.
Acknowledgments
Funding sources:
This work was supported by the National Cancer Institute at the National Institutes of Health [RO1-CA177586] awarded to DE Rollison and a Team Science grant awarded by the Moffitt Cancer Center to DE Rollison and PK Epling-Burnette. R Hesterberg was partially supported by the Moffitt Skin Cancer SPORE grant [P50-CA168536]. This work was also supported in part by the Flow Cytometry, Survey Methods and Tissue Cores at the H. Lee Moffitt Cancer Center and Research Institute, a comprehensive cancer center designated by the National Cancer Institute and funded in part by Moffitt’s Cancer Center Support Grant [P30-CA076292]. The funders were not involved in the study design, data collection/ analysis or manuscript preparation.
Footnotes
Conflict of interest:
Dr. Anna Giuliano reports being a Merck & Co, Inc. grant recipient and advisory board member. The remaining authors state no conflict of interest.
REFERENCES
- 1.Hampras SS, Giuliano AR, Lin HY, Fisher KJ, Abrahamsen ME, Sirak BA, et al. Natural history of cutaneous human papillomavirus (HPV) infection in men: the HIM study. PLoS One 2014;9(9):e104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampras SS, Giuliano AR, Lin HY, Fisher KJ, Abrahamsen ME, McKay-Chopin S, et al. Natural history of polyomaviruses in men: the HPV infection in men (HIM) study. J Infect Dis 2015;211(9):1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farzan SF, Waterboer T, Gui J, Nelson HH, Li Z, Michael KM, et al. Cutaneous alpha, beta and gamma human papillomaviruses in relation to squamous cell carcinoma of the skin: A population-based study. Int J Cancer 2013;28(10):28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iannacone MR, Gheit T, Waterboer T, Giuliano AR, Messina JL, Fenske NA, et al. Case-control study of cutaneous human papillomaviruses in squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev 2012;21(8):1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson K, Michael KM, Luostarinen T, Waterboer T, Gislefoss R, Hakulinen T, et al. Prospective study of human papillomavirus seropositivity and risk of nonmelanoma skin cancer. Am J Epidemiol 2012;175(7):685–95. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, MacDonald M, You J. Merkel cell polyomavirus infection and Merkel cell carcinoma. Current opinion in virology 2016;20:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iannacone MR, Gheit T, Pfister H, Giuliano AR, Messina JL, Fenske NA, et al. Case-control study of genus-beta human papillomaviruses in plucked eyebrow hairs and cutaneous squamous cell carcinoma. Int J Cancer 2014;134(9):2231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iannacone MR, Gheit T, Waterboer T, Giuliano AR, Messina JL, Fenske NA, et al. Case-control study of cutaneous human papillomavirus infection in Basal cell carcinoma of the skin. The Journal of investigative dermatology 2013;133(6):1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollison DE, Giuliano AR, Messina JL, Fenske NA, Cherpelis BS, Sondak VK, et al. Case-control study of Merkel cell polyomavirus infection and cutaneous squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2012;21(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampras SS, Michel A, Schmitt M, Waterboer T, Kranz L, Gheit T, et al. Merkel cell polyomavirus (MCV) T-antigen seroreactivity, MCV DNA in eyebrow hairs, and squamous cell carcinoma. Infectious agents and cancer 2015;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moloney FJ, Comber H, O’Lorcain P, O’Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. The British journal of dermatology 2006;154(3):498–504. [DOI] [PubMed] [Google Scholar]
- 12.Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. Journal of the American Academy of Dermatology 1999;40(2 Pt 1):177–86. [DOI] [PubMed] [Google Scholar]
- 13.Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. The British journal of dermatology 2000;143(3):513–9. [PubMed] [Google Scholar]
- 14.Ma JE, Brewer JD. Merkel Cell Carcinoma in Immunosuppressed Patients. Cancers 2014;6(3):1328–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. The New England journal of medicine 2003;348(17):1681–91. [DOI] [PubMed] [Google Scholar]
- 16.Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet (London, England) 1998;351(9103):623–8. [DOI] [PubMed] [Google Scholar]
- 17.Frezza EE, Fung JJ, van Thiel DH. Non-lymphoid cancer after liver transplantation. Hepato-gastroenterology 1997;44(16):1172–81. [PubMed] [Google Scholar]
- 18.Karagas MR, Cushing GL, Greenberg ER, Mott LA, Spencer SK, Nierenberg DW. Non-melanoma skin cancers and glucocorticoid therapy. British Journal of Cancer 2001;85(5):683–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005;22(3):329–41. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. The Journal of experimental medicine 1982;156(6):1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol 1998;160(3):1212–8. [PubMed] [Google Scholar]
- 22.Liyanage UK, Moore TT, Joo H-G, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002;169(5):2756–61. [DOI] [PubMed] [Google Scholar]
- 23.Perez SA, Karamouzis MV, Skarlos DV, Ardavanis A, Sotiriadou NN, Iliopoulou EG, et al. CD4+CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2007;13(9):2714–21. [DOI] [PubMed] [Google Scholar]
- 24.Chikamatsu K, Sakakura K, Whiteside TL, Furuya N. Relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head Neck 2007;29(2):120–7. [DOI] [PubMed] [Google Scholar]
- 25.Karagoz B, Bilgi O, Gumus M, Erikci AA, Sayan O, Turken O, et al. CD8+CD28- cells and CD4+CD25+ regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Med Oncol 2010;27(1):29–33. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Fujita H, Mitsui H, Yanofsky VR, Fuentes-Duculan J, Pettersen JS, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One 2013;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai C, August S, Behar R, Polak M, Ardern-Jones M, Theaker J, et al. Characteristics of immunosuppressive regulatory T cells in cutaneous squamous cell carcinomas and role in metastasis. Lancet (London, England) 2015;385 Suppl 1:S59. [DOI] [PubMed] [Google Scholar]
- 28.Visser J, Nijman HW, Hoogenboom BN, Jager P, van Baarle D, Schuuring E, et al. Frequencies and role of regulatory T cells in patients with (pre)malignant cervical neoplasia. Clin Exp Immunol 2007;150(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson S, Storey A. E6 proteins from diverse cutaneous HPV types inhibit apoptosis in response to UV damage. Oncogene 2000;19(4):592–8. [DOI] [PubMed] [Google Scholar]
- 30.Muschik D, Braspenning-Wesch I, Stockfleth E, Rösl F, Hofmann TG, Nindl I. Cutaneous HPV23 E6 Prevents p53 Phosphorylation through Interaction with HIPK2. PLoS ONE 2011;6(11):e27655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hesterberg R, Amorrortu RP, Zhao Y, Hampras S, Akuffo AA, Fenske N, et al. T-regulatory subpopulations associated with recent ultraviolet radiation exposure in a skin cancer screening cohort. J Immunol [In press]. [DOI] [PMC free article] [PubMed]
- 32.Mailloux AW, Sugimori C, Komrokji RS, Yang L, Maciejewski JP, Sekeres MA, et al. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J Immunol 2012;189(6):3198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gheit T, Billoud G, de Koning MN, Gemignani F, Forslund O, Sylla BS, et al. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect Betapapillomavirus types. Journal of clinical microbiology 2007;45(8):2537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moscicki A-B, Ma Y, Gheit T, McKay-Chopin S, Farhat S, Widdice LE, et al. Prevalence and Transmission of Beta and Gamma Human Papillomavirus in Heterosexual Couples. Open Forum Infectious Diseases 2017;4(1):ofw216-ofw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keynan Y, Card CM, McLaren PJ, Dawood MR, Kasper K, Fowke KR. The role of regulatory T cells in chronic and acute viral infections. Clin Infect Dis 2008;46(7):1046–52. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nature immunology 2006;7(4):401–10. [DOI] [PubMed] [Google Scholar]
- 37.Engelhardt BG, Jagasia M, Savani BN, Bratcher NL, Greer JP, Jiang A, et al. Regulatory T cell expression of CLA or α(4)β(7) and skin or gut acute GVHD outcomes. Bone marrow transplantation 2011;46(3):436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nature immunology 2009;10(7):689–95. [DOI] [PubMed] [Google Scholar]
- 39.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. The Journal of experimental medicine 2007;204(6):1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomura M, Honda T, Tanizaki H, Otsuka A, Egawa G, Tokura Y, et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. The Journal of clinical investigation 2010;120(3):883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham JB, Da Costa A, Lund JM. Regulatory T cells shape the resident memory T cell response to virus infection in the tissues. J Immunol 2014;192(2):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward SM, Fox BC, Brown PJ, Worthington J, Fox SB, Chapman RW, et al. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol 2007;47(3):316–24. [DOI] [PubMed] [Google Scholar]
- 43.Farid C, Sheikh WE, Swelem R, El-Ghitany E. Frequency of FOXP3+ Regulatory T-cells in the Blood of Chronic Hepatitis C Patients with Immune Mediated Skin Manifestations; Relationship to Hepatic Condition and Viral Load. Clin Lab 2016;62(12):2339–48. [DOI] [PubMed] [Google Scholar]
- 44.Rollison DE, Schell MJ, Fenske NA, Cherpelis B, Messina JL, Giuliano AR, et al. Cutaneous viral infections across two anatomic sites among a cohort of skin cancer screening patients. J Infect Dis 2018. [DOI] [PMC free article] [PubMed]
- 45.Hampras SS, Nesline M, Wallace PK, Odunsi K, Furlani N, Davis W, et al. Predictors of immunosuppressive regulatory T lymphocytes in healthy women. Journal of cancer epidemiology 2012;2012:191090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


