Abstract
Importance
In China diabetes prevalence has increased substantially in recent decades, but there are no reliable estimates of the excess mortality currently associated with diabetes.
Objective
To assess the proportional excess mortality associated with diabetes, and to estimate the diabetes-related absolute excess mortality in rural and urban China.
Design, setting, and participants
A 7-year nationwide prospective study of 512,869 adults aged 30-79 years from 10 (5 rural, 5 urban) localities across China, recruited from 6/2004 to 7/2008 and followed until 1/2014.
Exposure
Diabetes (previously diagnosed or screen-detected) recorded at baseline.
Main outcome measures
All-cause and cause-specific mortality, collected through established death registries. Cox regression was used to estimate adjusted mortality rate ratios (RRs) comparing those with versus without diabetes at baseline.
Results
Overall, the mean (SD) age was 51.5 (10.7) years, 59% (n=302,618) were women, and 5.9% (n=30,280) had diabetes (rural 4.1%, urban 8.1%, men 5.8%, women 6.1%, previously diagnosed 3.1%, screen-detected 2.8%). During 3.64 million person-years of follow-up, there were 24,909 deaths, including 3,384 among individuals with diabetes. Compared to adults without diabetes, individuals with diabetes had a significantly increased risk of all-cause mortality (1373 vs 646 deaths per 100,000; adjusted RR, 2.00 [95%CI, 1.93 to 2.08]), which was higher in rural than urban areas (rural RR, 2.17 [95%CI 2.07 to 2.29]; urban RR, 1.83 [95%CI, 1.73 to 1.94]). Presence of diabetes was associated with increased mortality from ischaemic heart disease (3287 deaths; RR, 2.40 [95%CI, 2.19 to 2.63]), stroke (4444 deaths; RR, 1.98 [95%CI, 1.81 to 2.17]), chronic liver disease (481 deaths; RR, 2.32 [95%CI, 1.76 to 3.06]), infections (425 deaths; RR, 2.29 [95%CI, 1.76 to 2.99]), and cancer of the liver (1325 deaths; RR, 1.54 [95%CI 1.28 to1.86]), pancreas (357 deaths; RR, 1.84 [95%CI, 1.35 to 2.51]), female breast (217 deaths; RR, 1.84 [95%CI, 1.24 to 2.74]), and female reproductive system (210 deaths; RR, 1.81 [95%CI, 1.20 to 2.74]). For chronic kidney disease (365 deaths), the RR was higher in rural than urban areas (18.69 [95%CI, 14.22 to 24.57] versus 6.83 [95%CI, 4.73 to 9.88]). Among those with diabetes, 10% of all deaths (rural 16%, urban 4%) were due to definite or probable diabetic ketoacidosis or coma (408 deaths).
Conclusions and relevance
Among adults in China, diabetes was associated with increased mortality from a range of cardiovascular and non-cardiovascular diseases. Although diabetes was more common in urban areas, it was associated with a greater excess mortality in rural areas.
Introduction
The prevalence of diabetes in China has more than quadrupled in recent decades, with an estimated 110 million adults having diabetes in 2010, and 490 million adults estimated to have “pre-diabetes”.1–4 A previous study estimated that diabetes accounted for 5-7% of overall adult mortality or disability-adjusted life-years (DALYs) in China in 2010.5 Such estimates were, however, derived mainly from extrapolation of relative risk estimates from studies in high-income countries where many patients with diabetes have reasonably good control of blood glucose and take cardiovascular-protective medications.6–9 Previous studies of diabetes and mortality in China have been limited by small sample size, enrollment of participants many decades ago (when the prevalence of diabetes was relatively low), or restriction to local occupational or urban cohorts.10–12
In China many cases of diabetes are undiagnosed,1,3,4 and among persons diagnosed with diabetes, many are not adequately managed,4 particularly in rural areas, thereby increasing the risk of premature death. Because the increase in diabetes prevalence in China is recent the full effect on mortality and morbidity is unknown. Moreover, the main adult disease patterns in China differ appreciably from those in the West (e.g. more people die from stroke than from ischaemic heart disease [IHD] in China) and also vary greatly between different regions.13 Hence, reliable estimates of the emerging epidemic of mortality associated with diabetes are needed nationally and regionally to plan prevention and treatment programs. The present nationwide prospective study examines the association of diabetes with cause-specific mortality in rural and urban China.
Methods
Study population
Details of the China Kadoorie Biobank (CKB) design, methods and participants have been reported previously.14,15 Briefly, the 2004-8 baseline survey took place in 10 (5 urban, 5 rural) localities across China, chosen from China’s nationally representative Disease Surveillance Points to retain geographic and social diversity. All 1,801,200 registered residents thought to be aged 35-74 years in study areas were identified through local residential records and invited by door-to-door delivery of letters and information leaflets to attend study clinics, and 512,869 participated, including 12,665 just outside this age range (making the actual baseline age range from 30-79 years). As a substantial minority of registered residents would be disabled or have been living elsewhere, it was estimated that about a third of the non-disabled invitees actually living in the study areas participated. Prior to commencement of the study, international, national and local ethics approval was obtained, and all participants provided written informed consent.
Data collection
Trained health workers administered laptop-based questionnaires at local study clinics on socio-demographic factors, smoking, alcohol consumption, diet, physical activity and medical history, and measured height, weight, waist and hip circumference, lung function, blood pressure and heart rate. A non-fasting venous blood sample was collected (recording the time since last food) for storage and on-site random plasma glucose (RPG) testing using the SureStep Plus system (LifeScan, Milipitas, CA, USA). Participants with no prior diabetes and an on-site RPG level of 140-200 mg/dL (7.8-11.0 mmol/L) were invited for fasting plasma glucose (FPG) testing the following day.16
Assessment of diabetes status
Previously diagnosed diabetes was defined by a “yes” response to the question “Has a doctor ever told you that you had diabetes?”. Participants who responded yes provided additional information about age at first diagnosis and current use of certain medications for diabetes (e.g. insulin and metformin), which were used to differentiate between type 1 and 2 diabetes (which was not asked specifically). Respondents also provided information on medications for cardiovascular disease (e.g. aspirin, lipid and blood pressure lowering agents). Among those without previously diagnosed diabetes, screen-detected diabetes was defined as RPG ≥126 mg/dL (7.0 mmol/L) with time since last food ≥8 hours, or ≥200 mg/dL (11.1 mmol/L) with time since last food <8 hours, or FPG ≥126 mg/dL (7.0 mmol/L) on subsequent testing.
Mortality follow-up
Cause-specific mortality was monitored through China’s Disease Surveillance Points system17 and electronic health insurance records, with annual active confirmation of survival through local residential and administrative records. In each area, the Disease Surveillance Points system provides reasonably complete and reliable death registration, in which almost all adult deaths were medically certified. For the few (<5%) without medical attention prior to death, standardized procedures were used to determine probable causes from symptoms or signs described by relevant informants (usually family members).18
The trained Disease Surveillance Points staff coded all diseases on the death certificates and assigned underlying causes using ICD-10. For deceased participants, the information entered into the study follow-up system (including scanned images of original death certificates) was reviewed centrally by study clinicians, who were unaware of baseline information, who classified diabetes as the underlying cause only for deaths from diabetic ketoacidosis or coma or from diabetes with no other (e.g. vascular or renal) antecedent cause on the death certificates (eTable 1).
Statistical analysis
Mean values and prevalences of baseline variables by diabetes status were standardized for 5-year age groups, sex and study area, as were mortality rates, using the total CKB study population as the standard. Cox proportional hazard models related baseline diabetes to cause-specific mortality, yielding mortality rate ratios (RRs) and 95% confidence intervals (CIs), adjusted for baseline covariates (education, smoking, alcohol, physical activity and BMI) and stratified by location (10 areas), age-at-risk (5-year groups) and sex.
In analyses of mortality by duration of diabetes, the RR for each category of duration (0 [screen-detected diabetes], <5, 5 to <10, 10 to <15 and ≥15 years) was accompanied by a CI derived only from the variance of the log risk in that one category. Hence, each RR, including that for the reference group, is associated with a group-specific CI that reflects the amount of data in only that one category.19 The 95% group-specific CI for RR is (RR/T, RR×T), where T=exp(1.96√v) and v is the variance of the log risk, and RR-1 gives the proportional excess risk.
Comparison of RRs for the first four and subsequent years of follow-up revealed no evidence of departure from the proportional hazards assumption for all-cause mortality. Adjusted RRs were compared across strata of other covariates, and chi-squared tests for trend and heterogeneity were applied to the log RRs and their standard errors. The population-attributable fraction (PAF) was calculated using P(RR-1)/(1+P[RR-1])20 where P is the prevalence of diabetes in this study. Two-sided P-values were used and P<0.05 denotes statistical significance; no correction for multiple testing was made. All analyses used SAS version 9.3.
Results
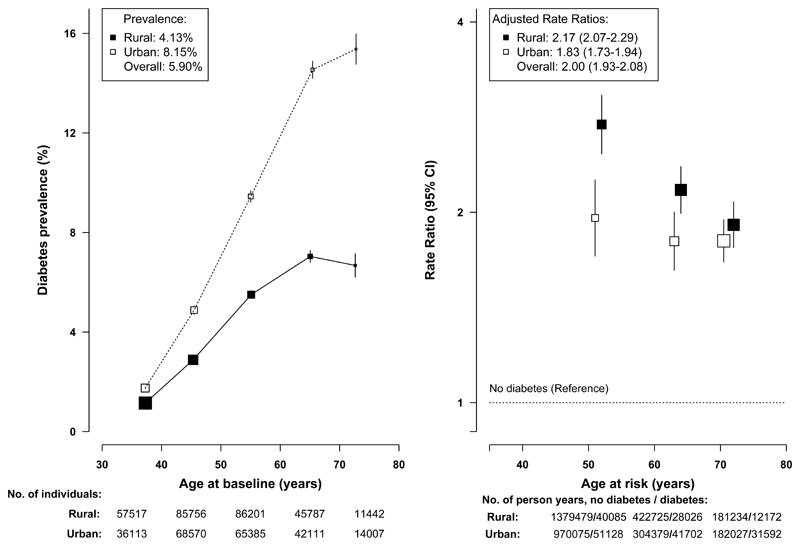
Of the 512,869 participants (mean age 51.5), 5.9% (3.1% previously diagnosed, 2.8% screen-detected) had diabetes at baseline and the prevalence was higher in urban than in rural areas (8.1% versus 4.1%). Individuals with diabetes were older and better educated, especially in urban areas, and after adjustment for age they were less physically active and had higher levels of BMI, waist circumference and blood pressure (Table 1). They were also more likely to have a prior history of hypertension, cardiovascular, chronic kidney and chronic liver diseases and to have a family history of diabetes. Based on age at diagnosis (<30 years) and insulin use, <1% of cases were likely to have been type 1 diabetes and they were included in the analyses. Diabetes prevalence increased with age (from 1.3% at 30-39 to 11.4% at 70-79 years, Figure 1).
Table 1. Baseline characteristics by diabetic status in rural and urban areas a.
| Characteristic b | Rural |
Urban |
All |
|||
|---|---|---|---|---|---|---|
| No diabetes (n=274,838) |
Diabetes (n=11,854) |
No diabetes (n=207,751) |
Diabetes (n=18,426) |
No diabetes (n=482,589) |
Diabetes (n=30,280) |
|
| Age and Socioeconomic factors | ||||||
| Mean age (SD), years | 50.7 (10.4) | 56.3 (9.4) | 51.8 (10.7) | 58.5 (9.8) | 51.2 (10.6) | 57.2 (10.1) |
| Female, % | 58.5 | 58.5 | 59.6 | 59.6 | 59.0 | 59.0 |
| ≥ 6 years of education, % | 34.3 | 33.7 | 67.7 | 70.7 | 48.7 | 54.8 |
| Lifestyle factors | ||||||
| Ever regular smoker, % | 65.7 | 66.8 | 70.0 | 68.9 | 67.6 | 68.1 |
| Ever regular alcohol drinker, % | 84.5 | 84.6 | 81.8 | 81.9 | 83.3 | 82.8 |
| Mean physical activity (SD), MET-h/day | 23.3 (12.4) | 20.6 (16.6) | 18.6 (10.5) | 16.7 (17.0) | 21.2 (11.6) | 18.9 (17.6) |
| Anthropometry and blood pressure | ||||||
| Mean standing height (SD), m | 1.58 (0.05) | 1.58 (0.07) | 1.60 (0.05) | 1.60 (0.08) | 1.59 (0.05) | 1.59 (0.08) |
| Mean BMI (SD), kg/m2 | 23.1 (3.1) | 24.5 (4.8) | 24.2 (3.3) | 25.4 (5.2) | 23.6 (3.2) | 24.9 (5.2) |
| Mean waist circumference (SD), cm | 78.7 (9.0) | 84.0 (13.0) | 81.7 (8.9) | 85.9 (12.8) | 80.0 (9.0) | 84.8 (13.7) |
| Mean waist-to-hip ratio (SD) | 0.88 (0.06) | 0.92 (0.09) | 0.87 (0.06) | 0.91 (0.11) | 0.88 (0.06) | 0.92 (0.10) |
| Mean SBP (SD), mmHg | 132.2 (19.7) | 139.3 (25.7) | 128.7 (19.1) | 136.9 (26.8) | 130.6 (19.5) | 138.3 (27.5) |
| Mean DBP (SD), mmHg | 78.0 (10.9) | 80.8 (14.6) | 77.2 (10.7) | 80.0 (15.7) | 77.7 (10.8) | 80.5 (15.8) |
| Mean RPG (SD), mg/dl | 100.8 (19.8) | 234.0 (133.2) | 104.4 (19.8) | 216.0 (142.2) | 102.6 (19.8) | 226.8 (144.0) |
| Medical history and medications | ||||||
| Prior diseases | ||||||
| Hypertension, % | 9.4 | 20.6 | 12.7 | 24.0 | 10.8 | 22.4 |
| CVD, % | 1.6 | 3.3 | 4.2 | 7.9 | 2.7 | 6.0 |
| Chronic renal, % | 1.4 | 1.7 | 1.6 | 2.3 | 1.4 | 2.1 |
| Chronic liver, % | 1.2 | 1.5 | 1.2 | 1.3 | 1.2 | 1.4 |
| CVD medications c | ||||||
| Statin, % | 3.2 | 2.6 | 1.1 | 0.6 | 2.1 | 1.3 |
| Aspirin, % | 12.0 | 5.1 | 7.7 | 4.6 | 9.8 | 4.7 |
| Blood pressure lowering d, % | 29.7 | 16.1 | 28.0 | 17.4 | 28.8 | 16.8 |
| Anti-diabetic medication | ||||||
| Chlorpropamide or metformin, % | - | 75.2 | - | 59.8 | - | 65.1 |
| Insulin, % | - | 7.4 | - | 18.2 | - | 14.5 |
| Both, % | - | 3.5 | - | 3.7 | - | 3.6 |
| Any, % | - | 79.9 | - | 75.3 | - | 76.9 |
| Family history of diabetes, % | 4.1 | 15.3 | 10.0 | 26.8 | 6.7 | 21.9 |
Participants (n=22) with missing or implausible values for key variables (e.g. blood pressure, anthropometric measures and duration of diabetes) were excluded, leaving 512,869 for the present analyses
Adjusted for age, gender and region as appropriate
Among participants with hypertension, CVD or diabetes at baseline (N=88,738)
Blood pressure lowering drugs: ACE-I, β-blocker, diuretics, calcium antagonist
BMI=body mass index, SBP=systolic blood pressure, DBP=diastolic blood pressure, RPG=random plasma glucose, CVD=cardiovascular disease. All comparisons between diabetes and non-diabetes were significant at P<0.01, except for smoking, alcohol drinking, standing height, waist to hip ratio and prior history of chronic liver disease.
Figure 1. Prevalence of total diabetes at baseline and adjusted all-cause mortality RRs by age and area.
Left panel shows age-specific prevalence and the percentages in the key represent the overall age- and gender-adjusted prevalence for urban and rural regions. The size of each box is proportional to the number of participants with diabetes and the error bars indicate the 95%CI. Right panel shows adjusted all-cause mortality rate ratios (RRs) by age-at-risk in three groups (35-59, 60-69, 70-79) and area and the values in the key represent the overall, urban and rural RRs comparing those with versus without diabetes at baseline, adjusted for age, geographic area (5 within each of rural and urban region), sex, education, smoking, alcohol drinking, physical activity and BMI. Age at risk was calculated according to baseline age and length of follow-up, with censoring date by 1.1.2014 or age of death if earlier. Each RR has a CI that reflects the variance of the log risk in that one group, taking into account the variance of the log risk in the non-diabetic reference group (shown with a dotted line, with shading indicating 95% group-specific CI) and has a vertical solid line that represents the 95%CI. Mortality RRs are plotted on a floating absolute scale. Each box has an area inversely proportional to the effective variance of the log RR. The analyses were restricted to those who died at age-at-risk 35-79 years, excluding 5 deaths at age-at-risk <35 and 1014 deaths at age-at-risk ≥80 years. The point estimates on the x-axis for both panels represent the mean of each age groups, with number of individuals (left panel) and number of person-years (right panel) shown underneath the x-axis. To avoid overlap of 95%CI lines, the boxes and their 95% CIs for rural and urban areas in right panel were moved apart slightly from the actual positions.
Among those with previously diagnosed diabetes (n=16,142; rural n=5617, urban n=10,525), median age at diagnosis was 53 years and median time since diagnosis was 6 years. Overall, 77% of those with previously diagnosed diabetes reported use of anti-diabetic medications (65% oral, 15% insulin and 4% both). Use of oral agents was higher in rural than urban areas (75% vs 60%), whereas the opposite was true for insulin (7% vs 18%). Despite widespread use of anti-diabetic treatments, their mean plasma glucose levels remained significantly elevated (eFigure 1). However, at the time of the baseline survey, few of those with diabetes, either previously diagnosed or diagnosed based upon screening, were using statin or anti-hypertensive medications (Table 1), particularly in those with previously diagnosed diabetes (1.1% and 14.5% respectively) (eTable 2).
During 3.64 million person-years of follow-up (until 1.1.2014), 24,909 (4.9%) participants died (3384 with diabetes and 21,525 with no diabetes) at age-at-risk 35-79 years and 2204 (0.4%) were lost to follow-up. Overall, individuals with diabetes had a significantly elevated all-cause mortality (adjusted RR, 2.00 [95%CI, 1.93 to 2.08]). Compared to persons without diabetes, all-cause mortality for persons with diabetes increased with age, with absolute mortality rates of 716 vs 253 per 100,000 at age-at-risk 35-59 (adjusted RR, 2.41 [95%CI 2.22 to 2.62]), 1666 vs 916 per 100,000 at age-at-risk 60-69 (RR, 2.01 [95%CI 1.88 to 2.14]) and 3760 vs 2435 per 100,000 at age-at-risk 70-79 years (RR, 1.84 [95%CI 1.75 to 1.95]). As shown in Figure 1, the adjusted RRs comparing those with diabetes to those without was greater in rural than urban areas, both overall (rural RR, 2.17 [95%CI, 2.07 to 2.29] vs urban RR, 1.83 [95%CI, 1.73 to 1.94]) and at each specific age group (Figure 1), as were the absolute excess mortality rates among those with diabetes (age 35-59: rural 737 vs urban 290; age 60-69: rural 1295 vs urban 545; age 70-79: rural 2443 vs urban 1317 per 100,000). The adjusted RRs were greater in women than men after age 60 years (eFigure 2). The excess mortality associated with diabetes accounted for 4.7% of the male deaths (absolute death rates for men with diabetes vs no diabetes were 2043 vs 930 per 100,000) and 6.9% of the female deaths (absolute death rates for women with diabetes vs no diabetes were 1416 vs 418 per 100,000). Moreover, among those without baseline diabetes the RPG was associated positively with all-cause mortality (RR, 1.11 [95% CI 1.10-1.12] per 18 mg/dL [1 mmol/L] higher usual RPG).
Diabetes was associated with a RR of 2.13 (95%CI, 2.01 to 2.26) for death from cardiovascular disease (Table 2), including IHD (2.40 [95%CI: 2.19 to 2.63]), stroke (1.98 [95%CI, 1.81 to 2.17]) (~75% of stroke deaths were due to intracerebral haemorrhage, RR, 1.87 [95%CI, 1.67 to 2.09]) and other vascular diseases (1.96 [95%CI, 1.71 to 2.26]). The RRs for vascular mortality were greater at younger than older ages (age 35-59 RR, 2.62 [95%CI 2.28 to 3.02] vs age 70-79 RR, 1.98 [95%CI 1.83 to 2.15]) and in women than men (women RR, 2.36 [95%CI 2.18 to 2.56] vs men RR, 1.93 [95%CI 1.77 to 2.10]), but did not differ significantly between rural and urban areas (eFigures 3-5). Likewise, diabetes was associated with an increased RR for mortality from chronic liver disease (2.32 [95%CI, 1.76 to 3.06]), infections (2.29 [95%CI, 1.76 to 2.99]), cancer of the liver (1.54 [95%CI, 1.28 to 1.86]), pancreas (1.84 [95%CI, 1.35 to 2.51]), female breast and reproductive system (RR 1.84 [95%CI, 1.24 to 2.74] and 1.81 [95%CI 1.20-2.74], respectively). Diabetes was not associated with increased mortality from cancers of lung, stomach, oesophagus and intestine. For chronic respiratory disease, mainly COPD, the RR was 1.29 ([95%CI, 1.10 to 1.51]). For deaths from external (i.e. accident, suicide and violence) and other medical causes, diabetes was associated with significant excess risk.
Table 2. Number of deaths, standardised mortality rates (per 100,000) and adjusted rate ratios for cause-specific mortality by diabetic status at baseline.
| Cause of deaths | Diabetes (n=30208) |
No diabetes (n=482589) |
Rate ratiob (95% CI) | ||
|---|---|---|---|---|---|
| No. of deaths | Ratea (95% CI) | No. of deaths | Ratea (95% CI) | ||
| Diabetic ketoacidosis or coma | 345 | 185.07 (159.37-210.77) | 63 | 1.90 (1.43-2.37) | 99.59 (75.13-132.01) |
| Definite | 128 | 75.06 (57.19-92.93) | 15 | 0.45 (0.22-0.68) | 181.85 (103.95-318.14) |
| Probable | 217 | 110.01 (91.54-128.49) | 48 | 1.45 (1.04-1.87) | 75.96 (54.68-105.52) |
| Chronic renal disease | 177 | 82.81 (66.90-98.71) | 188 | 5.64 (4.83-6.44) | 13.10 (10.45-16.42) |
| Cardiovascular disease | 1461 | 538.42 (504.14-572.69) | 7804 | 235.61 (230.37-240.85) | 2.13 (2.01-2.26) |
| IHD | 634 | 207.94 (187.13-228.74) | 2653 | 81.06 (77.96-84.15) | 2.40 (2.19-2.63) |
| Stroke | 580 | 246.45 (222.98-269.92) | 3864 | 115.41 (111.76-119.06) | 1.98 (1.81-2.17) |
| Other cardiovascular disease | 247 | 84.03 (70.21-97.85) | 1287 | 39.14 (37.00-41.29) | 1.96 (1.71-2.26) |
| Respiratory disease | 167 | 76.53 (62.50-90.56) | 1943 | 58.30 (55.71-60.90) | 1.29 (1.10-1.51) |
| COPD | 145 | 68.00 (54.79-81.21) | 1796 | 53.80 (51.31-56.30) | 1.26 (1.06-1.50) |
| Other respiratory disease | 88 | 31.72 (21.81-41.62) | 425 | 13.12 (11.87-14.37) | 2.00 (1.58-2.54) |
| Cancer | 790 | 300.39 (274.04-326.73) | 7789 | 234.31 (229.09-239.53) | 1.27 (1.18-1.37) |
| Lung | 198 | 64.82 (54.05-75.59) | 1897 | 57.77 (55.16-60.38) | 1.20 (1.03-1.39) |
| Liver | 133 | 61.96 (48.35-75.56) | 1192 | 35.44 (33.42-37.46) | 1.54 (1.28-1.86) |
| Pancreas | 50 | 14.57 (9.65-19.48) | 307 | 9.33 (8.29-10.38) | 1.84 (1.35-2.51) |
| Oesophagus | 51 | 22.52 (15.31-29.72) | 936 | 27.83 (26.04-29.61) | 0.92 (0.69-1.23) |
| Stomach | 98 | 36.14 (27.62-44.65) | 1105 | 33.25 (31.29-35.22) | 1.16 (0.94-1.44) |
| Colorectal | 57 | 18.83 (12.81-24.85) | 540 | 16.39 (15.00-17.78) | 1.11 (0.84-1.46) |
| Female breast | 31 | 10.35 (6.05-14.65) | 186 | 5.55 (4.75-6.35) | 1.84 (1.24-2.74) |
| Female reproductive system | 28 | 10.74 (5.47-16.01) | 182 | 5.44 (4.65-6.23) | 1.81 (1.20-2.74) |
| Other cancers | 144 | 60.47 (47.75-73.20) | 1444 | 43.31 (41.07-45.55) | 1.21 (1.01-1.44) |
| Chronic liver disease | 63 | 34.25 (23.71-44.78) | 418 | 12.33 (11.14-13.51) | 2.32 (1.76-3.06) |
| Liver cirrhosis | 33 | 16.44 (9.34-23.54) | 189 | 5.63 (4.82-6.43) | 2.36 (1.61-3.46) |
| Viral hepatitis | 21 | 12.16 (5.93-18.39) | 169 | 4.95 (4.20-5.70) | 2.10 (1.32-3.35) |
| Other chronic liver disease | 9 | 5.65 (0.99-10.32) | 60 | 1.75 (1.31-2.20) | 2.89 (1.39-6.00) |
| Infection | 72 | 22.56 (16.35-28.78) | 353 | 10.82 (9.69-11.95) | 2.29 (1.76-2.99) |
| Pneumonia | 55 | 15.15 (10.37-19.94) | 190 | 5.98 (5.12-6.83) | 2.47 (1.80-3.38) |
| Infection excluding pneumonia | 17 | 7.41 (3.44-11.38) | 163 | 4.84 (4.10-5.59) | 1.83 (1.09-3.05) |
| External | 139 | 75.29 (59.79-90.79) | 1760 | 51.07 (48.68-53.47) | 1.55 (1.30-1.85) |
| Other medical cause | 170 | 57.64 (46.42-68.87) | 1207 | 36.37 (34.31-38.43) | 1.66 (1.41-1.96) |
| All-cause mortalityc | 3384 | 1372.96 (1313.84-1432.08) | 21525 | 646.35 (637.70-655.01) | 2.00 (1.93-2.08) |
Standardized to age, sex and study area structure of CKB population
Stratified by age sex and study area and adjusted for education, smoking alcohol, physical activity and BMI
The analyses were restricted to those who died at age-at-risk 35-79 years, excluding 5 deaths at age <35 and 1014 deaths at age ≥80 years. Overall a total of 248 deaths at age 35-79 were attributed to unknown cause and the adjusted RR associated with diabetes was 1.53 (1.10-2.23).
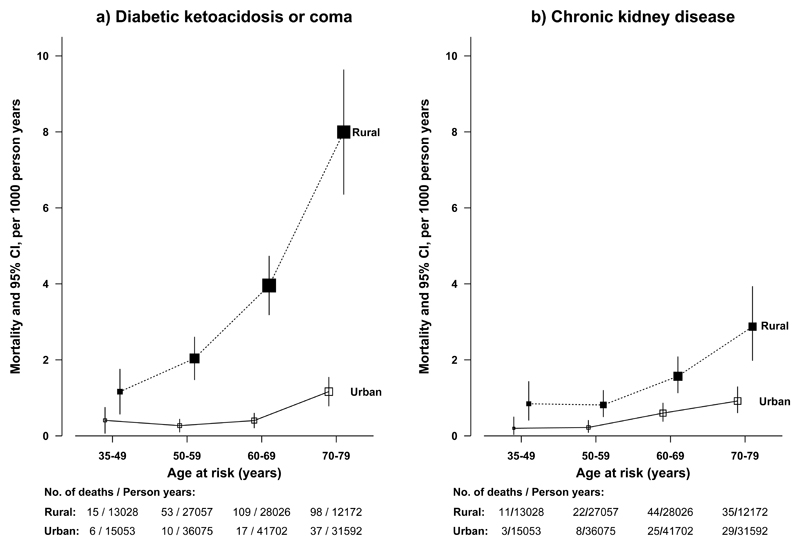
Among individuals with diabetes at baseline, definite diabetic ketoacidosis or coma accounted for 3.8% (128 of 3384) (rural 6.3% [109 of 5617], urban 1.1% [19 of 10525]) of the deaths compared with 0.07% (15 of 21525) of the deaths among those without diabetes at baseline (as some developed diabetes during follow-up) (RR of 181.85 [95%CI, 103.95 to 318.14]). A further 6.4% (217 of 3384) (rural 9.6% [166 of 5617], urban 3.1% [51 of 10525]) of deaths were due to probable diabetic ketoacidosis or coma (i.e. unspecified diabetic deaths), with a RR of 75.96 (95%CI, 54.68 to 105.52). The RR for mortality from any diabetic ketoacidosis or coma was greater in rural than urban areas (RR comparing individuals with diabetes to those without: rural 115.29 [95%CI, 84.31 to 157.65] vs urban 47.43 [95%CI, 25.19 to 89.32]) (eFigure 3). Similarly, the absolute death rate from diabetic ketoacidosis or coma was higher in rural areas (rural 3.49, urban 0.56 per 1000) and increased with age (Figure 2).
Figure 2. Rural and urban mortality rates of diabetic ketoacidosis or coma (definite or probable) and chronic kidney disease among people with diabetes by age at risk.
The death rates by four age-at-risk groups (35-49, 50-59, 60-69 and 70-79) were standardized for sex, using the total diabetic population in CKB as the standard. The age at risk was calculated according to baseline age and length of follow-up, with censoring date by 1.1.2014 or age of death if earlier. The analyses were restricted to those who died at age-at-risk 35-79 years, excluding 0 deaths at age-at-risk <35 and 5 and 8 deaths at age-at-risk ≥80 years for diabetic ketoacidosis or coma and chronic kidney disease, respectively. The point estimates on the x-axis for both panels represent the rates for each age category, with number of deaths and person-years shown underneath the x-axis. The size of each box is proportional to the number of deaths in each group and the error bars indicate the 95%CI. To avoid overlap of 95%CI lines, the boxes and their 95% CIs for rural and urban areas were moved apart slightly from the actual positions.
Individuals with diabetes had a significantly elevated (RR, 13.10 [95%CI, 10.45 to 16.42]) mortality from chronic kidney disease (CKD), mainly diabetes-related CKD (RR, 83.29, [95%CI, 53.15 to 130.51]) rather than other or unspecified kidney disease (RR, 1.72 [95%CI, 1.13 to 2.60]). The RR of CKD was greater in rural than urban areas (18.69 [95%CI, 14.22 to 24.57] vs 6.83 [95%CI, 4.73 to 9.88]) (eFigure 3), as were absolute death rates from CKD among those with diabetes, both overall (rural 1.2, urban 0.4 per 1000) and at each age group (Figure 2).
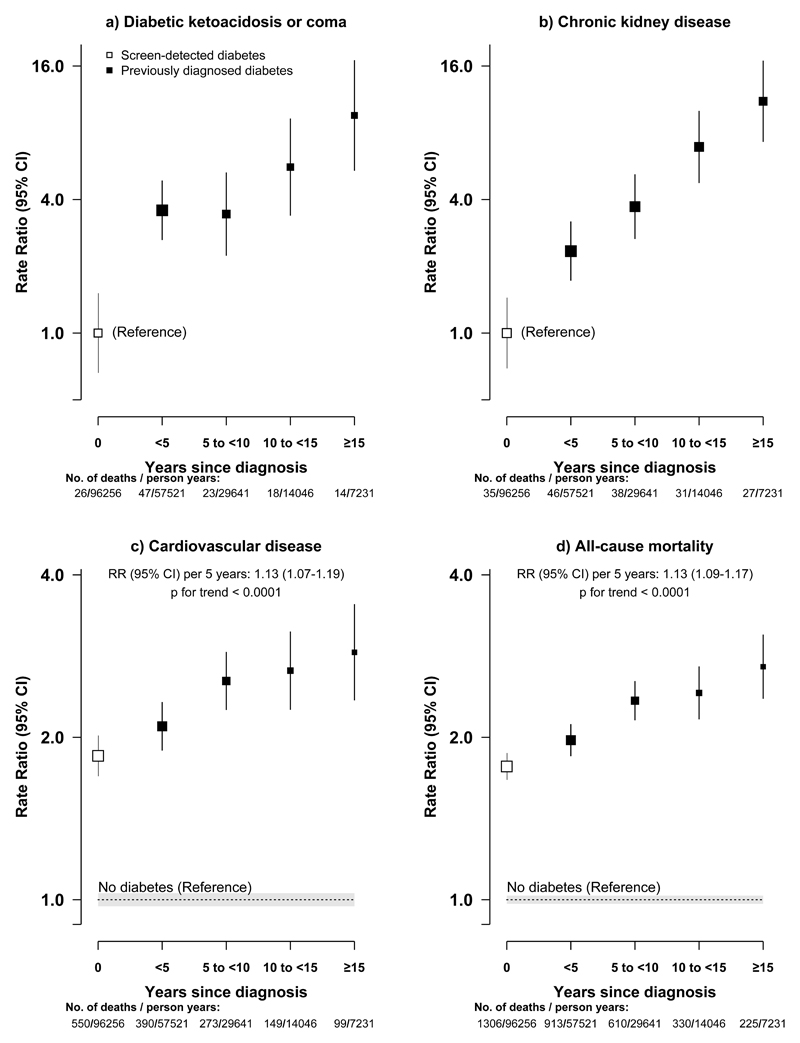
The RRs were higher with previously diagnosed than with screen-detected diabetes for all-cause mortality (2.20 [95%CI, 2.11 to 2.30] vs 1.76 [95%CI, 1.67 to 1.86]; eTable 3) and for mortality from several specific diseases, including diabetic ketoacidosis or coma (164.35, [95%CI, 143.02 to 188.86] vs 46.33 [95%CI, 36.99 to 58.03]), CKD (18.88 [95%CI, 15.78 to 22.59] vs 6.31 [95%CI, 4.54 to 8.78]), IHD (2.76 [95%CI, 2.51 to 3.05] vs 1.91 [95%CI, 1.67 to 2.18]), stroke (2.16 [95%CI, 1.93 to 2.41] vs 1.79 [95%CI, 1.58 to 2.03]), and infection (2.88 [95%CI, 2.19 to 3.79] vs 1.45 [95%CI, 0.91 to 2.30]). Among those with diabetes, the risk increased with time since first diagnosis, with each 5-year increase associated with 13% (RR, 1.13 [95%CI, 1.09 to 1.17]; p for trend <0.0001) higher overall mortality (Figure 3). This trend was driven mainly by diabetic ketoacidosis or coma, CKD and cardiovascular mortality, especially in rural areas (eFigure 6).
Figure 3. Adjusted rate ratios for all-cause mortality and selected disease-specific mortality by duration since diagnosis at baseline.
a) Diabetic ketoacidosis or coma, b) Chronic kidney disease, c) Cardiovascular disease, d) All-cause mortality. The adjusted RRs are relative to screen-detected diabetes (for diabetic ketoacidosis or coma and chronic kidney disease) or to those without diabetes (for cardiovascular disease and all-cause mortality). The point estimates on the x-axis are placed by each equally-spaced diabetes duration category, with number of deaths and person-years shown underneath the x-axis for those with diabetes. The dotted line indicates the RR for the reference group with shading indicating 95% group-specific CI. Other conventions for symbols same as in Figure 1.
The all-cause mortality RRs also varied by several additional baseline risk factors (eFigure 7), especially among those with previously diagnosed diabetes. Among those with screen-detected diabetes the RRs also varied by area, BMI (mainly for non-vascular mortality, eFigure 8) and SBP (mainly for vascular mortality, eFigure 9), but not by sex. Apart from the rural versus urban difference, the RRs did not differ significantly across 10 geographic regions (eFigure 10) and were largely unaffected by additional adjustment for blood pressure and several dietary factors (e.g. fresh fruit, vegetable, meat), by exclusion of individuals with major prior diseases (e.g. CVD, cancer, COPD and chronic liver diseases) at baseline (2.03, [95%CI, 1.93 to 2.14]), or exclusion of the first 3 years of follow-up (1.92 [95%CI, 1.84 to 2.02]) or those with new onset of diabetes during follow-up (1.93 [95%CI, 1.85 to 2.03]). Additional adjustment for use of medications also had little statistical effect on all-cause mortality RR (1.83 [95%CI, 1.75 to 1.93]).
Discussion
This large prospective study of adults from rural and urban China showed that diabetes was associated with significantly increased mortality from a wide range of diseases, with the greatest proportional excess mortality from diabetic ketoacidosis or coma and CKD, followed by IHD, stroke, other vascular, chronic liver disease, infection, certain cancers (mainly liver, pancreatic, female breast and endometrial cancers) and external causes. While the prevalence of diabetes was higher in urban areas, diabetes was associated with greater excess mortality in rural regions. Several large prospective studies, and meta-analyses of such studies, have provided reliable evidence about the relevance of diabetes for total and certain cause-specific mortality.7–9 However, most of these previous studies were conducted in high-income countries where people with diabetes were generally well-managed and mainly assessed the effects of previously diagnosed diabetes. Overall the all-cause mortality RRs associated with previously diagnosed diabetes were more modest in these studies7–9 than those observed in the present study, however, differences in study characteristics could partially account for the differences. The low use of cardiovascular-protective medications (e.g. statin) in the CKB diabetes population would be expected to yield even greater excess cardiovascular mortality than those reported in high-income countries, but this may have been offset by relatively short duration of diabetes. The present study also showed that the main causes of death associated with diabetes differed between China and elsewhere. In many Western populations, diabetes is associated with more deaths from IHD than from stroke, whereas in China the opposite is true, even though the mortality RRs for IHD and stroke in the present study were similar to those reported previously.7–9 Moreover, existing evidence relating haemorrhagic stroke to diabetes is more limited. In a meta-analysis of >100 prospective studies with 1200 haemorrhagic strokes, individuals with diabetes had ~50% excess risk.6 This study included more deaths (>3200) from haemorrhagic stroke than in the previous meta-analysis and provided reliable evidence of positive associations of diabetes with death from haemorrhagic stroke. For several major non-vascular conditions examined, the risk estimates also appeared to be similar in magnitude to previous reports, including cancer, infection, chronic liver diseases and deaths from external causes.7,9 However, for deaths from diabetic ketoacidosis or coma and CKD, the excess risks in the present study, particularly in rural areas, were much greater than those reported in high-income countries.
Few previous prospective studies provided information about deaths from diabetic ketoacidosis or coma, perhaps reflecting the rarity of such deaths. Available population-based registry data suggested that in the US <1% of deaths among people with diabetes were due to diabetic ketoacidosis or coma.21 In the rural population, although a high proportion of diabetes cases was treated with anti-diabetic medications, ~16% of all deaths among them were due to definite or probable diabetic ketoacidosis or coma, with the absolute death rate being almost 10 times as high as in urban areas, although the absolute number of deaths remains low. A recent nationwide survey in China, which had a similar treatment rate with antidiabetic medications as in the present study, reported that only about one-third of the treated diabetes cases had achieved adequate glycaemic control,4 as opposed to three-quarters in USA.22 Similarly, for CKD mortality, the observed RR in the present study was about 4 times as high as those reported in previous studies,8,9 reflecting poor management of diabetes and its complications, particularly in rural areas where both the relative risk and absolute rates were almost 3 times as great as in urban areas. Consistent with the present study findings, the mortality from diabetes-related CKD in China has more than doubled since 1990.13 By contrast, the proportional all-cause excess mortality risk among individuals with type 2 diabetes declined significantly in most Western populations in that period, for example to only about 15% in Sweden (i.e. RR=1.15),23 attributed largely to better glycaemic control and routine use of cardio-protective agents (e.g. aspirin, statins and anti-hypertensive treatment).
As in many previous studies,7–9,24,25 greater all-cause and cardiovascular mortality RRs were seen among women than men, especially after age 60. The differences were seen mainly in previously diagnosed, rather than in screen-detected, diabetes, suggesting that the sex difference in excess risk associated with diabetes was probably driven mainly by factors related to detection and management of diabetes, which few previous studies were able to investigate fully.
The probability of death associated with diabetes in the general population could be estimated by combining the age-specific all-cause mortality RRs in this study with 2010 age-specific mortality rates from China,26 while taking into consideration effects of diabetes duration. We estimated that at the 2010 Chinese death rates the 25-year probability of death was 69% among those diagnosed with diabetes at age 50 and 38% among those who remained free of diabetes at age 75 years, corresponding to loss of a median of 9 (rural 10, urban 8) years of life for individuals with diabetes diagnosed at age 50 (eFigure 11), assuming the excess mortality is largely causal.
This study has several strengths. Although not nationally representative with a relatively low participation rate at baseline, the large sample size, diversity of areas covered and broadly consistent findings across study population subgroups means that the present relative risk estimates are likely not biased and can be generalizable to the population at large. Moreover, the study has several other strengths, including standardised approaches and stringent quality control for data collection, availability of information on previously diagnosed and screen-detected diabetes along with duration and management of diabetes, central review of death certificates and completeness of follow-up.
However, the study also has several limitations. First, the prevalence of diabetes in this study was only about half of that reported in a 2010 nationally representative survey in China, arising mainly from a difference in the prevalence of screen-detected (2.8% CKB vs 8.1% national survey), rather than previously diagnosed (3.1% CKB vs 3.5% national survey), diabetes.4 Apart from difference in sampling methods and effects of temporal trends in diabetes prevalence, the 2010 China national survey used three different tests (i.e. HbA1c, fasting and post-load blood glucose) to identify screen-detected diabetes, whereas the present study used RPG and FPG. However, prevalence estimates in the present study were similar to those reported in other contemporaneous, representative Chinese surveys during the 2000s that used similar approaches,2,27 and the 2009-10 China survey of CKD that reported a prevalence of 7.0% in urban and 4.3% in rural areas.28 Nevertheless, it is likely that a proportion of diabetes cases in the present study were undetected at baseline, which could result in underestimation of diabetes associated risk, even though exclusion of those who had new onset of diabetes during follow-up did not alter the proportional risk estimates. In addition, it was not possible to determine the prevalence of type 1 diabetes. However, based on age at diagnosis (<30 years) and insulin use, <1% of cases were likely to have been type 1 diabetes. Future studies are also needed to confirm whether diabetes detected by different approaches would have similar mortality risk, which may affect the reliability of our estimates on absolute mortality associated with diabetes in China. Second, it was not possible to adjust for lipid and other blood-related factors, so residual confounding may still persist. Third, no detailed information was available about severity and complications of diabetes, which may modify mortality risk estimates.
China’s 2030 Sustainable Development Goals include reducing non-communicable disease mortality by one-third, and monitoring the changes over time. In China, the under-70 overall adult mortality rates are decreasing due to many dietary, social, occupational and health-care changes, and declined by about 15% during 2000-10.5,29 This decreasing trend may be slowed or even halted by increasing tobacco-attributed mortality in men,30 and the increasing prevalence of diabetes in both sexes. Moreover, among people of a given age the risk of death is strongly associated with the duration of diabetes, so the lifetime hazards will be even greater for people who develop diabetes in early adult life than for those who do so after age 50 years. As the prevalence of diabetes in young adults increases and the adult population grows,31 the annual number of deaths related to diabetes is likely to continue to increase, unless there is substantial improvement in prevention and management.
Conclusions
Among adults in China, diabetes was associated with significantly increased risks of death from a range of cardiovascular and non-cardiovascular diseases. Although diabetes was more common in urban areas, it was associated with greater excess mortality in rural areas.
Supplementary Material
Key Points.
Question: To assess the excess mortality associated with diabetes in rural and urban China.
Findings: In this 7-year nationwide prospective study of 512,869 adults, diabetes was more common in urban than rural areas (8.1% vs 4.1%) and individuals with diabetes had significantly increased risk of mortality from all-causes and from a range of cardiovascular and non-cardiovascular diseases.
Meaning: In China, diabetes is more common in urban than rural areas, and is associated with increased mortality. With an increasing adult population and rising prevalence of diabetes in young adults, the burden of diabetes-associated mortality will increase further.
Acknowledgments
The chief acknowledgment is to the participants, the project staff, and the China National Centre for Disease Control and Prevention (CDC) and its regional offices for access to death and disease registries. The Chinese National Health Insurance scheme provides electronic linkage to all hospital admission data. Fiona Bragg acknowledges support from the BHF Centre of Research Excellence, Oxford. We thank Jonathan R Emberson in CTSU, Oxford, for helpful advice on reviewing and coding of diabetes-related deaths.
Funding: Baseline survey: Kadoorie Charitable Foundation, Hong Kong. Long-term continuation: UK Wellcome Trust (088158/Z/09/Z, 104085/Z/14/Z), Chinese Ministry of Science and Technology (2011BAI09B01, 2012-14), Chinese National Natural Science Foundation (81390541). The British Heart Foundation, UK Medical Research Council and Cancer Research UK provide core funding to the Oxford CTSU.
Footnotes
Access to data: FB, AI, LL and ZC had full access to the data in the study and take responsibility for the integrity of the data.
Data analysis: AI, FB, MH and ZC conducted and are responsible for the data analysis and accuracy of the results.
Contributors: All authors were involved in study design, conduct, long-term follow-up, review and coding of death certificates, analysis of data, interpretation, or writing the report.
Conflicts of interest: We declare that we have no conflict of interest.
References
- 1.Pan XR, Yang WY, Li GW, Liu J. Prevalence of diabetes and its risk factors in China, 1994. Diabetes Care. 1997;20:1664–9. doi: 10.2337/diacare.20.11.1664. [DOI] [PubMed] [Google Scholar]
- 2.Li LM, Rao KQ, Kong LZ, et al. A description on the Chinese national nutrition and health survey in 2002. Chin Epidemiol J. 2005;26:478–84. [PubMed] [Google Scholar]
- 3.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 5.Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2010;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35:1835–44. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodward M, Zhang X, Barzi F, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care. 2003;26:360–6. doi: 10.2337/diacare.26.2.360. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Grundy SM, Wang W, et al. Ten-year risk of cardiovascular incidence related to diabetes, prediabetes, and the metabolic syndrome. Am Heart J. 2007;153:552–8. doi: 10.1016/j.ahj.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Shen C, Schooling CM, Chan WM, Lee SY, Leung GM, Lam TH. Self-reported diabetes and mortality in a prospective Chinese elderly cohort study in Hong Kong. Prev Med. 2014;64:20–6. doi: 10.1016/j.ypmed.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 12.An Y, Zhang P, Wang J, et al. Cardiovascular and all-cause mortality over a 23-year period among Chinese with newly diagnosed diabetes in the Da Qing IGT and Diabetes Study. Diabetes Care. 2015;38:1365–71. doi: 10.2337/dc14-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 387:251–72. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Lee L, Chen J, et al. Cohort profile: The Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–9. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Chen J, Collins R, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–66. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bragg F, Li L, Smith M, et al. Associations of blood glucose and prevalent diabetes with risk of cardiovascular disease in 500 000 adult Chinese: the China Kadoorie Biobank. Diabet Med. 2014;31:540–51. doi: 10.1111/dme.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang GH, Stroup DF, Thacker SB. National public health surveillance in China: implications for public health in China and the United States. Biomed Environ Sci. 1997;10:1–13. [PubMed] [Google Scholar]
- 18.Yang G, Rao C, Ma J, et al. Validation of verbal autopsy procedures for adult deaths in China. Int J Epidemiol. 2006;35:741–8. doi: 10.1093/ije/dyi181. [DOI] [PubMed] [Google Scholar]
- 19.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–35. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 20.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes Public Health Resource: Diabetes Complications. [Accessed 10 March, 2016];2016 http://www.cdc.gov/diabetes/statistics/mortalitydka.
- 22.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368:1613–24. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 23.Tancredi M, Rosengren A, Svensson A-M, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–32. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 24.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973–80. doi: 10.1016/S0140-6736(14)60040-4. [DOI] [PubMed] [Google Scholar]
- 25.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–51. doi: 10.1007/s00125-014-3260-6. [DOI] [PubMed] [Google Scholar]
- 26.Chinese Ministry of Health. China Statistical Yearbook of Health. Beijing: Publishing House of Peking Union Medical College; 2011. [Google Scholar]
- 27.Gu D, Reynolds K, Duan X, et al. Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International Collaborative Study of Cardiovascular Disease in Asia (InterASIA) Diabetologia. 2003;46:1190–8. doi: 10.1007/s00125-003-1167-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 379:815–22. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 29.Norheim OF, Jha P, Admasu K, et al. Avoiding 40% of the premature deaths in each country, 2010-30: review of national mortality trends to help quantify the UN Sustainable Development Goal for health. Lancet. 385:239–52. doi: 10.1016/S0140-6736(14)61591-9. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Peto R, Zhou M, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. 386:1447–56. doi: 10.1016/S0140-6736(15)00340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UN Population Division. World population prospects: The 2012 Revision. New York: United Nations; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.