Abstract
Vocal fold (VF) mucosal fibrosis results in substantial voice impairment and is recalcitrant to current treatments. To reverse this chronic disorder, anti-fibrotic therapies should target the molecular pathology of aberrant collagen accumulation in the extracellular matrix. We investigated the therapeutic potential of siRNA against Serpinh1, a collagen-specific chaperone that enables cotranslational folding and assembly of procollagens in the endoplasmic reticulum. We implemented a previously validated siRNA construct, conducted transfection experiments using in vitro and in vivo rat models, and measured knockdown efficiency, dose responses, delivery strategies, and therapeutic outcomes. Liposome-mediated delivery of Serpinh1-siRNA downregulated collagen production in naive and scar VF fibroblasts as well as naive VF mucosa; moreover, sustained Serpinh1 knockdown in fibrotic VF mucosa reversed scar-associated collagen accumulation within 4 weeks. Analysis of therapeutic effects at the transcriptome level showed evidence of cell cycle upregulation, catabolism, matrix disassembly, and morphogenesis. These findings indicate that Serpinh1-siRNA holds potential as a molecular therapy for chronic VF mucosal fibrosis.
Keywords: anti-fibrotic, extracellular matrix, fibroblast, gp46, hsp47, larynx, chronic scar
Graphical Abstract

Introduction
Vocal fold (VF) mucosal fibrosis can cause an intractable dysphonia that leads to substantial communication difficulty, occupational disadvantage, and social isolation.1, 2, 3, 4 The primary driver of this fibrosis is the VF fibroblast (VFF). Quiescent VFFs regulate extracellular matrix (ECM) turnover, helping to maintain the VF lamina propria’s biomechanical capacity for voice production;5 however, when activated by sustained inflammatory and profibrotic stimuli, VFFs differentiate into pathologic myofibroblasts.6, 7, 8, 9 These fibrogenic and contractile cells produce an abundance of disorganized fibrous proteins, most notably collagen, that negatively affect tissue viscoelasticity, biomechanics, and physiologic function.10, 11, 12, 13 Effective treatment of this ECM impairment is challenging. Current approaches include scar excision, biomaterial injection or implantation, steroid injection, and laser irradiation;14, 15, 16, 17, 18, 19, 20 candidate therapies, under investigation in early-phase clinical trials, include basic fibroblast growth factor,21 hepatocyte growth factor,22 and autologous cells isolated from the adipose tissue-derived stromal vascular fraction.23 Although beneficial in select cases, surgical manipulation carries a risk of further iatrogenic injury, whereas current and emerging pharmacologic, biologic, and cell therapies fail to specifically target the molecular pathology of the disordered ECM and its hallmark feature of aberrant collagen accumulation. Consequently, no treatment is uniformly effective.14, 24, 25
Mature collagen synthesis requires cotranslational folding and assembly of hydroxylated and glycosylated procollagen chains within the endoplasmic reticulum (ER). Serpin peptidase inhibitor clade H, member 1 (SERPINH1, also known as collagen-binding protein 1 [colligin], glycoprotein 46 [gp46], and heat shock protein 47 [HSP47]) is a collagen-specific chaperone protein that associates with procollagen in the ER, facilitates folding and triple helix formation, and dissociates by the time the procollagen reaches the cis-Golgi.26, 27, 28 SERPINH1 synthesis is tightly aligned with that of collagen in normal and fibrotic tissues.29, 30, 31 Serpinh1−/− mice (lethal by embryonic day 11.5 [E11.5]) and fibroblasts produce misfolded, fragile collagen helices that are easily digested;32 further, targeted interruption of Serpinh1 expression has been shown to reverse pathologic collagen accumulation and improve organ function in multiple in vivo fibrosis models.33, 34, 35, 36 These mechanistic and preclinical data suggest that SERPINH1 is a promising molecular target for ameliorating fibrosis and its associated morbidity.
Here we investigated the feasibility and therapeutic potential of small interfering RNA (siRNA)-based Serpinh1 interruption for treating chronic VF mucosal fibrosis. We implemented a previously validated siRNA construct,33, 34, 35, 36 conducted transfection experiments using robust in vitro and in vivo rat models,6, 37, 38 and measured knockdown efficiency, dose responses, delivery strategies, and therapeutic outcomes. Additionally, we surveyed the transcriptomic response to Serpinh1 interruption to identify new downstream gene targets and develop a more complete understanding of this siRNA’s mechanism of action as an anti-fibrotic therapy.
Results
Liposome-Mediated Delivery of Serpinh1-siRNA Causes Dose-Dependent Knockdown of Serpinh1 Transcription in VFFs
Prior work has shown successful siRNA uptake by human VFFs when supported by lipofection.39 To determine whether rat VFFs are amenable to transfection with siRNA targeting Serpinh1, we incubated naive cells with 50 nM Serpinh1-siRNA for 24 h with and without a liposomal vector. Serpinh1-siRNA significantly downregulated Serpinh1 expression when delivered in a liposome but had no effect when delivered as a naked construct (Figure 1A). We therefore used a liposome vector for subsequent experiments. Serpinh1-siRNA exhibited dose-dependent inhibition of Serpinh1 expression in the 1- to 100-nM range, with ∼70% knockdown at 100 nM; however, delivery of negative control siRNA containing a scramble sequence (scr-siRNA) resulted in nonspecific inhibition at this dose (Figure 1B). We therefore used a 50-nM dose (for which there was no effect of scr-siRNA on Serpinh1 expression) for subsequent in vitro experiments. Next we labeled Serpinh1-siRNA-liposome complexes with the fluorescent dye 6-carboxyfluorescein (FAM) and assessed transfection efficiency in both naive VFFs and early-passage scar VFFs that exhibit a myofibroblast phenotype.6 After a 1-h incubation period, flow cytometry revealed signal uptake by more than 75% of cells (Figure 1C), and fluorescence microscopy confirmed intracellular (with some perinuclear) distribution (Figure 1D).
Figure 1.
Liposome-Mediated Delivery of Serpinh1-siRNA Causes Dose-Dependent Knockdown of Serpinh1 Transcription in VFFs
(A) Effect of Serpinh1-siRNA transfection on Serpinh1 expression in naive VFFs when delivered as a naked construct (lip−) or via a liposomal vector (lip+). (B) Dose-dependent responses to Serpinh1-siRNA and scr-siRNA transfection in naive VFFs. (C) Uptake of 6-carboxyfluorescein-labeled siRNA (Serpinh1-siRNA-FAM) by VFFs. The histograms are representative; the bar chart summarizes data from both naive and scar VFFs. (D) Representative intracellular distribution of Serpinh1-siRNA-FAM (white arrows) in VFFs. Comparable images were obtained from naive and scar VFFs. Scale bar, 30 μm. Quantitative data are presented as mean ± SEM; n = 3–10 biological replicates per condition; **p < 0.01 versus non-transfected (NT) control. The siRNA dose was 50 nM in (A), (C), and (D). Cells for qRT-PCR were harvested 24 h following transfection. Flow cytometry and microscopy were performed immediately following transfection.
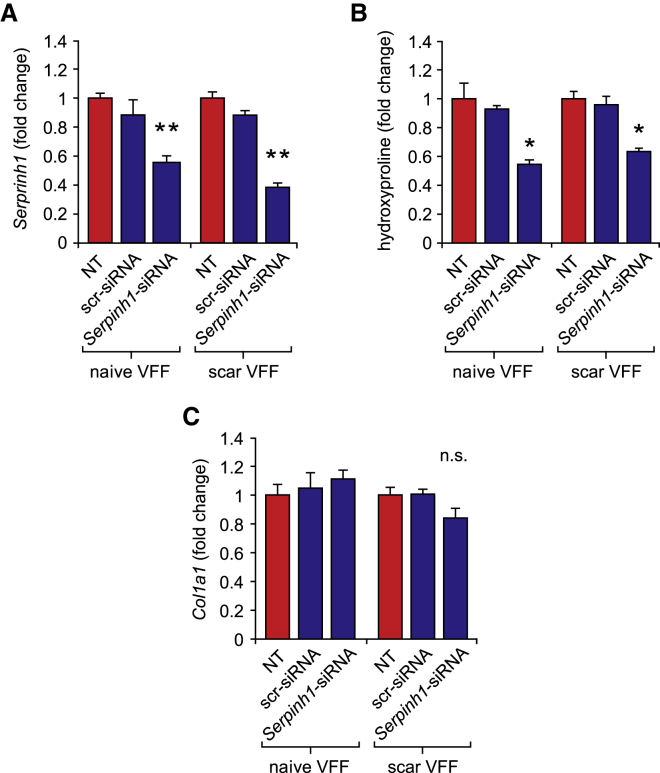
Serpinh1 Knockdown Suppresses Collagen Synthesis but Not Col1a1 Expression in Naive and Scar VFFs
Next, to assess the effect of on Serpinh1 inhibition on collagen production in naive and scar VFFs, we transfected cells with 50 nM Serpinh1 siRNA and assayed gene transcription at 24 h and protein secretion at 48 h. Mature collagen secretion was inferred from hydroxyproline abundance in the culture supernatant.40 In both naive and scar VFFs, Serpinh1 knockdown (Figure 2A) corresponded to reduced hydroxyproline abundance (Figure 2B) but had no effect on Col1a1 expression (Figure 2C), consistent with the proposed mechanism of SERPINH1 as a molecular chaperone that interacts with procollagen cotranslationally.26
Figure 2.
Serpinh1 Knockdown Suppresses Collagen Synthesis but Not Col1a1 Transcription in Naive and Scar VFFs
(A–C) Effect of Serpinh1-siRNA and scr-siRNA transfection on (A) Serpinh1 expression, (B) secreted hydroxyproline abundance, and (C) Col1a1 expression in naive and scar VFFs. Data are presented as mean ± SEM; n = 3–4 biological replicates per condition; **p < 0.01 versus NT control; *p < 0.05 versus NT control; n.s., non-significant versus NT control. The siRNA dose was 50 nM; cells for qRT-PCR were harvested 24 h following transfection. Cells for hydroxyproline analysis were collected 48 h following transfection.
Serpinh1-siRNA Causes Transient Serpinh1 Knockdown and Reduced Collagen Abundance in Naive VF Mucosa
Given the collagen suppression effect of Serpinh1-siRNA in both naive and scar VFF models in vitro, we explored parameters for local delivery of siRNA to the bilateral VF mucosae in vivo. We used a rat model with transoral laryngeal exposure because the rat is a well-established preclinical model in VF scar biology11, 37, 38 and because transoral exposure is minimally invasive and directly translatable to human patients.41 First, we injected a 5-μL bolus containing 50 ng Serpinh1-siRNA, 50 ng scr-siRNA, or PBS to each medial VF mucosa. Injection of PBS or scr-siRNA upregulated Serpinh1 expression at 48 h, whereas Serpinh1-siRNA had no effect compared with naive tissue control (Figure 3A). We reasoned that this increase in Serpinh1 expression under both negative control conditions might be caused by an acute wound response to the injection needle and that such wound-induced transcription was neutralized by Serpinh1-siRNA. We therefore adjusted our technique and performed a lateral injection of 50 ng Serpinh1-siRNA or scr-siRNA to each posterior ventricular fold, avoiding direct injury of the VF mucosa while allowing diffusion of liposome-siRNA complexes to the target region.42 Lateral injection of Serpinh1-siRNA downregulated Serpinh1 in the VF mucosa by ∼40%, whereas scr-siRNA had no effect (Figure 3A). We therefore used a lateral injection technique for subsequent in vivo experiments.
Figure 3.
Effect of Serpinh1-siRNA Delivery to Naive VF Mucosae
(A) Comparison of Serpinh1 expression following medial or lateral injection of Serpinh1-siRNA, scr-siRNA, or PBS to naive rat VF mucosae. The schematic illustrates needle placement; all injections were performed bilaterally. (B) Effect of Serpinh1-siRNA dose escalation on Serpinh1 expression. (C) Time course of Serpinh1 expression following Serpinh1-siRNA or scr-siRNA transfection. (D) Time course of hydroxyproline abundance following Serpinh1-siRNA or scr-siRNA transfection. Data are presented as mean ± SEM; n = 3–6 biological replicates per condition; **p < 0.01 versus naive (NT) control; *p < 0.05 versus naive (NT) control or as otherwise indicated in (B). The siRNA dose was 50 ng in (A), (C), and (D). A lateral injection site was used in (B)–(D). Cells for qRT-PCR were harvested 48 h following transfection in (A) and (B).
We conducted additional experiments to determine the optimal dose and frequency of Serpinh1-siRNA delivery to achieve sustained Serpinh1 knockdown. Serpinh1-siRNA doses ranging from 50–200 ng had a comparable inhibitory effect on Serpinh1 expression at 48 h (Figure 3B); we therefore used a 50-ng dose for subsequent in vivo work. At the 50-ng dose, Serpinh1 expression was most depressed at 48 h and recovered to basal levels by 6 days (Figure 3C). Parallel analysis of hydroxyproline abundance showed no effect at 48 h, followed by significant inhibition at 4 and 6 days (Figure 3D). These data highlight a delay between Serpinh1 knockdown and its downstream effect on collagen synthesis in vivo and, additionally, suggest that sustained Serpinh1 knockdown in VF mucosae requires Serpinh1-siRNA delivery every 3–4 days.
Sustained Serpinh1 Knockdown Reverses Scar-Associated Collagen Accumulation in VF Mucosae
Based on these findings in naive tissue, we attempted sustained Serpinh1 knockdown in scarred VF mucosae via serial Serpinh1-siRNA delivery and assessed its effect on collagen abundance and tissue architecture. First, we performed bilateral VF mucosal injuries in rats and waited 8 weeks for scar maturation (Figure 4A).37 We then delivered 50 ng Serpinh1-siRNA or scr-siRNA to each mucosa twice per week for 4 weeks; naive and untreated (scarred) VF mucosae served as additional controls. The 4-week treatment period was based on demonstrated efficacy of the same siRNA construct in multiple rat models of liver cirrhosis.33 Serpinh1-siRNA treatment resulted in a significant reduction in hydroxyproline abundance compared with scr-siRNA-treated and untreated mucosae; we observed no difference between Serpinh1-siRNA-treated and naive mucosae (Figure 4B). Similar data were obtained for Col1a1 expression (Figure 4C), suggesting that, unlike in the in vitro system (Figure 2C), cotranslational collagen suppression via Serpinh1 interruption additionally resets transcriptional activity in vivo. Histological assessment revealed improved VF mucosal morphology following Serpinh1-siRNA treatment, characterized by less muscle fiber invasion of the lamina propria and reduced buckling of the epithelial contour (Figure 4D). Masson’s trichrome staining as well as COL1 and COL3 immunostaining corroborated the hydroxyproline data (Figures 4D–4F) and confirmed that sustained Serpinh1 knockdown reverses scar-associated collagen accumulation in VF mucosae.
Figure 4.
Sustained Serpinh1 Knockdown Reverses Scar-Associated Collagen Accumulation in VF Mucosae
(A) Schematic illustrating the injury, scar formation, and siRNA delivery schedule. Tissue harvest occurred 4 days following the final siRNA dose. (B) Hydroxyproline abundance. (C) Col1a1 expression. (D) Representative Masson’s trichrome-stained coronal VF sections and corresponding analysis of total collagen abundance. (E) Representative COL1-immunostained coronal VF sections and corresponding analysis of COL1 abundance. (F) Representative COL3-immunostained coronal VF sections and corresponding analysis of COL3 abundance. Quantitative data are presented as mean ± SEM; n = 5 biological replicates per condition; **p < 0.01 versus naive (NT) control; *p < 0.05 versus naive (NT) control. A lateral injection site was used. Image analysis data represent the lamina propria region. Scale bars, 200 μm (D–F).
Serpinh1 Knockdown Induces a Transcriptome-Level Shift in Cell Phenotype
Given that Serpinh1-siRNA reversed the histologic and collagen deposition features of VF mucosal fibrosis, a multifaceted ECM disorder, we questioned whether Serpinh1 knockdown affects additional downstream targets beyond collagen. Existing data indicate that this siRNA does not induce an interferon-α-mediated immune response or other off-target effects;33 in addition to its targeted effect on de novo collagen assembly, its therapeutic mechanism is hypothesized to include sustained activity by matrix mellatoproteinase (MMP) enzymes and apoptosis of collagen-synthesizing cells.33, 34, 36, 43 To identify additional gene sets and pathways that are downstream of Serpinh1 interruption, we returned to the in vitro model, used expression microarrays to profile the transcriptomes of Serpinh1-siRNA- and scr-siRNA-treated scar VFFs, and compared these with previously reported data from naive and untreated scar VFFs.6 We used an empirical Bayes approach to evaluate equivalent and differential gene expression (equivalent expression [EE] and differential expression [DE], respectively)44 and built two statistical models to identify therapeutically relevant gene sets of interest. First, using three-way comparisons, we identified genes with an expression pattern indicative of an siRNA-induced therapeutic shift from the scar VFF phenotype: DE between Serpinh1-siRNA and both scr-siRNA and untreated scar VFF conditions as well as EE between scr-siRNA and untreated scar VFF conditions (Figure 5A). A total of 551 oligonucleotide probes corresponding to 388 unique genes fit this expression pattern. Enrichment analysis of this gene set, conducted using Enrichr45, 46 and Gene Ontology annotations,47 highlighted clusters of biological process terms associated with mitosis and cell division, metabolism and catabolism, ECM disassembly, development and morphogenesis, and a non-specific stimulus response; cellular component terms primarily associated with the nucleus and ECM; and molecular function terms associated with ATP and microtubule binding as well as DNA binding and transcriptional regulation.
Figure 5.
Serpinh1 Knockdown Induces a Transcriptome-Level Shift in Cell Phenotype
(A) Analysis of Serpinh1-induced therapeutic shift in the scar VFF transcriptome. The experimental schematic (top left) represents the three-way expression pattern of interest, defined as a therapeutic shift from the scar VFF phenotype. EE, equivalent expression; DE, differential expression. The remainder summarizes the enrichment analysis of 388 genes that fit this therapeutic shift pattern. Enriched Gene Ontology terms are depicted as nodes; node and label font size are proportional to the generality of the term in the underlying ontology. Cellular component terms are shown in green; molecular function terms are shown in red; biological process terms are shown in blue. Node color intensity represents the −log10-adjusted p value associated with term enrichment. Nodes representing semantically similar terms are connected by gray lines and grouped within gray ovals. (B) Analysis of Serpinh1-induced therapeutic normalization in the scar VFF transcriptome. The experimental (top) schematic represents the four-way expression pattern of interest, defined as a shift from the scar VFF to naive VFF phenotype. The 33 genes that fit this therapeutic normalization pattern are listed according to upregulation (n = 14) or downregulation (n = 19) under both Serpinh1-siRNA and naive VFF conditions. Enrichment analysis (lower region) was performed using the entire gene set and exclusively identified terms in the cellular component domain. Analyses were performed with 3–4 biological replicates per condition. The siRNA dose was 50 nM. Cells were harvested 24 h following transfection.
Next, using four-way comparisons that included the naive VFF condition, we identified genes with an expression pattern indicative of therapeutic normalization, defined as a shift from the scar VFF to naive VFF phenotype: EE between Serpinh1-siRNA and naive VFF conditions and between scr-siRNA and untreated scar VFF conditions; DE for comparisons between all other conditions (Figure 5B). These criteria yielded 39 probes associated with 33 unique genes. Fourteen of these genes were upregulated in the Serpinh1-siRNA and naive VFF conditions, including the cell cycle-regulatory genes Ccna2, Mki67, and S100b as well as the mesoderm differentiation and pattern formation gene Tbx4. The remaining 19 genes were downregulated, including the ECM-related genes Acan, Tnn, and Tgfbi; the immunoregulatory genes C3, C1qtnf3, and Ccl11; and the cytoskeleton-associated genes Acta1, Actg2, Cnn1, Epb41l4a, Myh11, Myh2, and Sgcg. Enrichment analysis of the entire gene set identified cellular component terms associated with the extracellular region; we identified no enrichment of terms in the biological process or molecular function domains.
Discussion
VF mucosal fibrosis is associated with substantial morbidity;1, 2, 3, 4 however, to date, most preclinical treatment research has focused on scar prophylaxis via manipulation of early wound healing events.48, 49, 50, 51, 52 Here, we directly tackled the molecular pathology of chronic VF mucosal fibrosis by targeting collagen assembly and accumulation in the disordered ECM. Liposome-mediated delivery of siRNA against the collagen-specific chaperone Serpinh1 downregulated collagen production in naive and scar VFFs as well as naive rat VF mucosae; further, sustained Serpinh1 knockdown in rat VF mucosae reversed scar-associated collagen accumulation within a 4-week period. These findings show that Serpinh1-siRNA holds potential as a molecular therapy for chronic VF mucosal fibrosis.
Prior work suggests that interruption of Serpinh1 expression ameliorates fibrosis through several mechanisms. Misfolded procollagen molecules accumulate in the ER of transfected cells, overwhelm their autophagy machinery, and drive stress-mediated apoptosis.43 A repository of ECM-associated MMPs remains available to digest pre-existing (stable) and any newly synthesized (fragile) collagen, which may drive further apoptosis by anoikis.33, 34, 36 Of note, this apoptosis of collagen-producing cells may indirectly account for the reduction in Col1a1 transcription observed in our in vivo data; a similar effect was reported following Serpinh1-siRNA treatment of cirrhotic liver.33 Our transcriptomic data corroborated these suggested mechanisms by identifying Serpinh1-siRNA-induced expression patterns indicative of ECM disassembly, catabolism, and cell cycle regulation, for which many regulatory genes and components of the nuclear machinery play a role in both apoptosis and proliferation.53 We further discovered DE genes and gene sets associated with immunoregulation as well as morphogenesis and pattern formation, indicating that Serpinh1 interruption may achieve its therapeutic effect via multiple downstream pathways and regenerative functions.
siRNA transfection is inherently vulnerable to off-target effects and stimulation of innate immune responses, making careful oligonucleotide design and the inclusion of suitable controls imperative to appropriate data interpretation. We utilized a previously validated Serpinh1-siRNA construct33 comprised of a 27-nt RNA duplex (designed to optimize potency)54 containing a 2-nt 3′ overhang and no 5′ triphosphate terminus (designed to mitigate interferon induction).55 Our scr-siRNA data confirmed the absence of nonspecific effects at therapeutic doses during in vitro and in vivo experiments; a prior report, focused on hepatic stellate cells and cirrhotic liver, validated the specificity of the Serpinh1-siRNA construct and absence of a bystander effect by showing equivalent knockdown and reversal of fibrosis with two other siRNAs against the same mRNA target.33 This prior work additionally showed no evidence of a Serpinh1-siRNA-triggered innate immune response in vivo. Despite these encouraging findings, future preclinical studies should carefully assess off-target or other adverse effects of local Serpinh1-siRNA delivery to the larynx. In addition, because age and sex influence VF biology56, 57, 58, 59 (and our data were generated using 4- to 7-month-old male rats), future studies should evaluate the effect of these biological variables on Serpinh1 knockdown and therapeutic response.
With a view to clinical translation, we used transoral injection to non-traumatically deliver Serpinh1-siRNA to the VF mucosae via diffusion and further identified the optimal dose and delivery frequency required for sustained Serpinh1 knockdown in vivo. Other work targeting Serpinh1 has used vitamin A-conjugated liposomes to traffic Serpinh1-siRNA to hepatic and pancreatic stellate cells33, 36 as well as lung and skin myofibroblasts.34, 35 Such an approach harnesses physiologic transport via plasma retinol-binding protein to facilitate intravenous delivery to target cells. We did not explore this approach in the current study because, unlike in other organs, vitamin A-storing VF stellate cells are clustered in distinct anatomic niches60 and do not appear to be the primary cells responsible for fibrosis.38, 61, 62 Further improvement in Serpinh1-siRNA delivery to fibroblasts throughout the VF mucosae might be achieved with a controlled-release system,63 increasing the duration of Serpinh1 knockdown and, as a result, the interval between injections.
Materials and Methods
Animals
Fischer 344 inbred male rats (Charles River Laboratories) were used for all experiments; all procedures were approved by the Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health. Four-month-old rats received bilateral VF mucosal injuries as described previously,37, 38 followed by a 2-month scar maturation period. These rats were then used for scar VFF isolation and primary culture or reserved for in vivo siRNA experiments. Uninjured age-matched rats were used for naive VFF isolation and primary culture (6 months old) and as naive controls in the in vivo siRNA experiments (7 months old).
Primary Cell Isolation and Culture
Primary VFF cultures were conducted using an explant culture technique.6 Briefly, bilateral VF mucosae were microdissected from freshly harvested rat larynges, minced in a 10-cm culture dish, and immersed in DMEM supplemented with 10% fetal bovine serum (FBS), L-glutamine, antibiotics, and antimycotics (all reagents from Sigma-Aldrich). Cells were cultured at 37°C in 5% CO2; medium was exchanged twice per week. Outgrown primary cells were trypsinized and passaged 14–21 days after initial explant plating.
siRNA Preparation
We used previously reported 27-nt RNA duplexes with 2-nt 3′ overhangs (Hokkaido System Science) designed to maximize silencing efficacy and minimize immune activation.33 The sense and anti-sense strands were as follows: Serpinh1, beginning at nucleotide 757, 5′-GUUCCACCAUAAGAUGGUAGACAACAG-3′ (sense), 5′-GUUGUCUACCAUCUUAUGGUGGAACAU-3′ (antisense); scramble, 5′-CGAUUCGCUAGACCGGCUUCAUUGCAG-3′ (sense), 5′-GCAAUGAAGCCGGUCUAGCGAAUCGAU-3′ (antisense). In experiments designed to confirm siRNA uptake via flow cytometry and fluorescence microscopy, Serpinh1-siRNA was prepared with FAM coupled to the 5′ end of the sense strand.
siRNA Transfection of VFFs
All in vitro experiments were performed at passage 1 (P1), based on data showing that the scar and naive VFF phenotypes coalesce at later passages.6 Cells were seeded in 6-well plates or 2-well chamber slides at a density of 2 × 104 cell/well and cultured until 80% confluent. Immediately prior to transfection, the culture medium was aspirated, and cells were rinsed in PBS.
Transfection was performed using the Lipotrust delivery system (Hokkaido System Science) according to the manufacturer’s protocol. Each 2-mL transfection preparation contained 1.8 mL Opti-MEM (Invitrogen) supplemented with 10% FBS and 200 μL siRNA-liposome complex comprised of 2–200 pmol siRNA (corresponding to a 1- to 100-nM final concentration) and 40 nmol cationic lipid. In one experiment, where indicated, transfection was performed without the liposomal vector. Cells were incubated with the siRNA preparation at 37°C for 1 h, rinsed in PBS, and either harvested for immediate flow cytometry and fluorescence microscopy or maintained in culture for a further 24 or 48 h.
siRNA Delivery to Rat VF Mucosae
Rats were anesthetized using an intraperitoneal cocktail of 90 mg/kg ketamine hydrochloride and 9 mg/kg xylazine hydrochloride; 0.05 mg/kg atropine sulfate was used to reduce secretions in the laryngeal lumen. The larynx was visualized via a 1.9-mm diameter, 25° rigid endoscope (Richard Wolf), and 5 μL siRNA-liposome complex (containing 50–200 ng siRNA) or PBS was injected to the medial or lateral VF mucosae bilaterally. Injections were performed using a microsyringe and 33G needle (Hamilton). Rats in the pilot experiment received one pair of bilateral injections, followed by larynx harvest at 2, 4, or 6 days; rats in the therapeutic trial received serial bilateral injections twice per week for 4 weeks. Larynges were harvested 4 days following the final injection.
RNA Isolation and qRT-PCR
Total RNA was isolated from pelleted cells and VF mucosa using the RNeasy Mini kit (QIAGEN) according to the manufacturer’s protocol. Samples were treated with DNase I to eliminate contamination by genomic DNA (Ambion); protein was acetone-precipitated from the column flowthrough and reserved for hydroxyproline measurement. RNA yield and purity were evaluated with a NanoDrop ND-1000 spectrophotometer. cDNA was generated by reverse transcription using TaqMan reagents (Applied Biosystems).
qRT-PCR amplification was performed using rat-specific commercial primers (QuantiTect, QIAGEN): QT00370622 (Col1a1), QT00195958 (Sdha), and QT01081010 (Serpinh1). Reactions were performed on a 7500 Fast Real-Time PCR system (Applied Biosystems) using the QuantiTect SYBR Green PCR kit (QIAGEN). Each 25-μL total volume reaction contained 12.5 μL 2 × QuantiTect master mix, 2.5 μL 10 × QuantiTect primer assay, and 10 μL cDNA template diluted in nuclease-free H2O. The cycling program was as follows: initial activation at 95°C for 15 min, followed by 40 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s. All PCR reactions were performed in technical duplicates. Relative mRNA expression was calculated using the 2−ΔΔCT method;64 data were normalized against the reference gene Sdha.65
Microarrays
Biotinylated antisense cRNA was prepared by single-round in vitro amplification of 0.9 μg input RNA using the MessageAmp II-Biotin Enhanced aRNA kit (Ambion) according to the manufacturer’s instructions (the in vitro transcription reaction was performed at 37°C for 14 h). Polyadenylated RNA controls (Affymetrix) were spiked into each reaction. Fragmented cRNA sample quality was confirmed using 2% agarose gel electrophoresis and Agilent 2100 Bioanalyzer analysis. Samples were hybridized to Affymetrix GeneChip Rat Genome 230 2.0 arrays at 45°C for 16 h. Post-processing was performing using the GeneChip Fluidics Station 450, arrays were scanned using the GC3000 G7 scanner, and fluorescent intensity data were background-corrected and extracted using Expression Console software (Affymetrix). All hybridization, post-processing, and scanning procedures were performed according to Affymetrix protocols; all control parameters were within the manufacturer’s guidelines. Microarray data are available from the Gene Expression Omnibus (GEO): GSE62204 (https://www.ncbi.nlm.nih.gov/geo/).
Flow Cytometry
Serpinh1-siRNA-FAM-treated and naive VFFs were harvested, washed, pelleted, and resuspended in PBS containing 1% BSA. Flow cytometry was performing using a FACSCalibur instrument (BD Biosciences); data were analyzed in FlowJo 8.7.1 (Tree Star).
Hydroxyproline Assay
Hydroxyproline concentration in culture supernatant (100 μL) and VF mucosae (10 μg tissue-isolated protein) was assayed using the Hydroxyproline Colorimetric Assay kit (BioVision) according to the manufacturer’s protocol. Acid hydrolysis was performed for 3 h at 120°C. Absorbance was measured at 560 nm using a FlexStation 3 microplate reader (Molecular Devices).
Cytochemistry, Histology, and Immunohistochemistry
Serpinh1-siRNA-FAM-treated and naive VFFs, cultured on chamber slides, were fixed using 4% paraformaldehyde (PFA) and counterstained with DAPI (MP Biomedicals) for 20 min prior to imaging.
Explanted larynges were dehydrated in PBS containing 25% sucrose at 4°C for 24 h, embedded in optimal cutting temperature compound (Tissue-Tek), and snap-frozen using acetone and dry ice. Serial 8-μm coronal sections were prepared using a cryostat (CM-3050 S, Leica Microsystems), air-dried, and stored at –80°C prior to staining.
Masson’s trichrome staining was performed using a commercial kit (Newcomer Supply) according to the manufacturer’s protocol. Sections intended for immunostaining were fixed with 4% PFA for 4 min and permeabilized with 0.5% Triton X-100 (Sigma-Aldrich) for 15 min. Image-iT FX signal enhancer (Invitrogen) was applied for 30 min, followed by blocking with Block Ace (AbD Serotec) for 10 min. Sections were sequentially incubated with a primary antibody for 1 h and a relevant secondary antibody for 1 h and counterstained with DAPI (MP Biomedicals) for 20 min. All procedures were performed at room temperature; slides were washed 3 times, 5 min per wash, between each step.
The primary antibodies used were goat anti-collagen type I (1:20; 1310-01, Southern Biotechnology) and rabbit anti-collagen type III (1:50; 600-401-105, Rockland Immunochemicals). The secondary antibodies used were Alexa Fluor 594 goat anti-rabbit or donkey anti-goat immunoglobulin G (IgG; 1:400, A-11012 or A-11058, Invitrogen). Negative controls, exposed to the secondary antibody in the absence of the primary antibody, revealed no immunostain.
Microscopy and Image Analysis
Light and fluorescence microscopy were performed using a Nikon E-600 microscope and Olympus DP-71 camera; images were captured with consistent exposure settings. For all collagen stains, MetaMorph 7.5 (Molecular Devices) was used to quantify the positively stained area within the lamina propria (defined as the region between the basement membrane and thyroarytenoid muscle) of each VF coronal section. The threshold value for determining a positive signal was set according to each stain’s background signal strength and corresponding negative control; this value was applied consistently across all samples. The number of positive pixels was normalized to the lamina propria cross-sectional area and expressed as a percentage.
Statistical Analysis
All experiments were performed with 3–10 independent biological replicates per condition; sample sizes for each experiment are reported in the corresponding figure legends. With the exception of microarray data, analyses were performed using SAS 9.2 (SAS Institute). Raw data were initially evaluated for normality and equality of variance using visual inspection of plots and Levene’s test; data were rank- or log-transformed where indicated to meet assumptions for parametric testing. qRT-PCR, hydroxyproline, and histologic or immunohistologic stain density data were analyzed using one-way ANOVA; flow cytometry data were analyzed using a paired t test. In the ANOVA models, when the omnibus F test revealed a significant difference, planned pairwise comparisons were performed using Fisher’s protected least significant difference method. A type I error rate of 0.05 was used; p values were two-sided.
Microarray data were analyzed within R.66 Probe-level data were preprocessed using robust multiarray analysis,67 based on evidence of improved precision over default Affymetrix algorithms.68 Probes without a corresponding gene symbol were purged from all gene-level analyses. In cases where multiple probes corresponded to a single gene symbol, we calculated the average expression for each probe across arrays and selected the probe with the median average expression. In the case of an even number of matched probes, we selected the larger of the two median probe intensities. The resulting normalized data were clustered to check for consistency prior to formal analysis.
Expression analysis was performed using an empirical Bayes approach as implemented in the R package EBarrays.44 A lognormal-normal (LNN) model was fit to the data. Parameter estimates were obtained via 20 iterations of an expectation-maximization algorithm; in all cases, convergence was achieved after 10 iterations. Diagnostic testing of the LNN assumption in EBarrays was performed using quantile-quantile (QQ) plots of log intensity data versus a standard normal distribution. We further used QQ plots to evaluate the assumption of a scaled inverse χ2 prior on the gene-specific variances used in the LNN model. The diagnostics showed no violations of model assumptions.
Using the output from EBarrays, we compared expression levels under Serpinh1-siRNA and scr-siRNA conditions at P1 with previously reported microarray data from naive and scar rat VFFs at P1.6 These previously reported data were generated in parallel to the siRNA transfection data using an identical cell isolation and culture protocol as well as identical RNA extraction, amplification, hybridization, and post-processing protocols. For all comparisons, thresholding was performed using more than 0.95 posterior probability of DE, corresponding to a 5% false discovery rate.
Tests of enrichment via overrepresentation were conducted using Enrichr,45, 46 the Gene Ontology dataset,47 and genes that fit the expression patterns of interest. Overrepresented Gene Ontology terms required at least 10 distinct DE genes and a Benjamini-Hochberg adjusted p value of less than 0.01 and were further processed using the REViGO semantic similarity and term redundancy algorithm69 followed by Cytoscape 2.8.2.70
Author Contributions
Y.K. designed all experiments, conducted in vitro and in vivo experiments, analyzed data, and helped write the manuscript. M.Y. conceived the study and performed in vitro pilot testing. A.W. and Y.T. assisted with in vivo experiments and analyzed data. S.Y. analyzed all microarray data under the direction of C.K. N.V.W. helped to develop the study, obtained funding, assisted with design and implementation of in vivo and in vitro experiments, analyzed data, and helped write the manuscript. All authors reviewed and approved the final version.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We gratefully acknowledge consultation provided by Yoshiro Niitsu (Department of Molecular Target Exploration, Sapporo Medical University, Sapporo, Japan) and histology services provided by Toshi Kinoshita (Department of Pathology, University of Wisconsin School of Medicine and Public Heath, Madison, WI). This research was supported by grants R01 DC004428 and R01 DC010777 from the National Institute on Deafness and other Communicative Disorders and grant UL1 RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources.
References
- 1.Welham N.V., Dailey S.H., Ford C.N., Bless D.M. Voice handicap evaluation of patients with pathologic sulcus vocalis. Ann. Otol. Rhinol. Laryngol. 2007;116:411–417. doi: 10.1177/000348940711600604. [DOI] [PubMed] [Google Scholar]; Welham, N.V., Dailey, S.H., Ford, C.N., and Bless, D.M. (2007). Voice handicap evaluation of patients with pathologic sulcus vocalis. Ann. Otol. Rhinol. Laryngol. 116, 411-417. [DOI] [PubMed]
- 2.Rosen C.A., Lee A.S., Osborne J., Zullo T., Murry T. Development and validation of the voice handicap index-10. Laryngoscope. 2004;114:1549–1556. doi: 10.1097/00005537-200409000-00009. [DOI] [PubMed] [Google Scholar]; Rosen, C.A., Lee, A.S., Osborne, J., Zullo, T., and Murry, T. (2004). Development and validation of the voice handicap index-10. Laryngoscope 114, 1549-1556. [DOI] [PubMed]
- 3.Cohen S.M., Kim J., Roy N., Asche C., Courey M. Direct health care costs of laryngeal diseases and disorders. Laryngoscope. 2012;122:1582–1588. doi: 10.1002/lary.23189. [DOI] [PubMed] [Google Scholar]; Cohen, S.M., Kim, J., Roy, N., Asche, C., and Courey, M. (2012). Direct health care costs of laryngeal diseases and disorders. Laryngoscope 122, 1582-1588. [DOI] [PubMed]
- 4.Cohen S.M., Dupont W.D., Courey M.S. Quality-of-life impact of non-neoplastic voice disorders: a meta-analysis. Ann. Otol. Rhinol. Laryngol. 2006;115:128–134. doi: 10.1177/000348940611500209. [DOI] [PubMed] [Google Scholar]; Cohen, S.M., Dupont, W.D., and Courey, M.S. (2006). Quality-of-life impact of non-neoplastic voice disorders: a meta-analysis. Ann. Otol. Rhinol. Laryngol. 115, 128-134. [DOI] [PubMed]
- 5.Gray S.D. Cellular physiology of the vocal folds. Otolaryngol. Clin. North Am. 2000;33:679–698. doi: 10.1016/s0030-6665(05)70237-1. [DOI] [PubMed] [Google Scholar]; Gray, S.D. (2000). Cellular physiology of the vocal folds. Otolaryngol. Clin. North Am. 33, 679-698. [DOI] [PubMed]
- 6.Kishimoto Y., Kishimoto A.O., Ye S., Kendziorski C., Welham N.V. Modeling fibrosis using fibroblasts isolated from scarred rat vocal folds. Lab. Invest. 2016;96:807–816. doi: 10.1038/labinvest.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kishimoto, Y., Kishimoto, A.O., Ye, S., Kendziorski, C., and Welham, N.V. (2016). Modeling fibrosis using fibroblasts isolated from scarred rat vocal folds. Lab. Invest. 96, 807-816. [DOI] [PMC free article] [PubMed]
- 7.Jetté M.E., Hayer S.D., Thibeault S.L. Characterization of human vocal fold fibroblasts derived from chronic scar. Laryngoscope. 2013;123:738–745. doi: 10.1002/lary.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jette, M.E., Hayer, S.D., and Thibeault, S.L. (2013). Characterization of human vocal fold fibroblasts derived from chronic scar. Laryngoscope 123, 738-745. [DOI] [PMC free article] [PubMed]
- 8.Vyas B., Ishikawa K., Duflo S., Chen X., Thibeault S.L. Inhibitory effects of hepatocyte growth factor and interleukin-6 on transforming growth factor-β1 mediated vocal fold fibroblast-myofibroblast differentiation. Ann. Otol. Rhinol. Laryngol. 2010;119:350–357. doi: 10.1177/000348941011900513. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vyas, B., Ishikawa, K., Duflo, S., Chen, X., and Thibeault, S.L. (2010). Inhibitory effects of hepatocyte growth factor and interleukin-6 on transforming growth factor-β1 mediated vocal fold fibroblast-myofibroblast differentiation. Ann. Otol. Rhinol. Laryngol. 119, 350-357. [DOI] [PMC free article] [PubMed]
- 9.Branco A., Bartley S.M., King S.N., Jetté M.E., Thibeault S.L. Vocal fold myofibroblast profile of scarring. Laryngoscope. 2016;126:E110–E117. doi: 10.1002/lary.25581. [DOI] [PMC free article] [PubMed] [Google Scholar]; Branco, A., Bartley, S.M., King, S.N., Jette, M.E., and Thibeault, S.L. (2016). Vocal fold myofibroblast profile of scarring. Laryngoscope 126, E110-E117. [DOI] [PMC free article] [PubMed]
- 10.Hirano S., Minamiguchi S., Yamashita M., Ohno T., Kanemaru S., Kitamura M. Histologic characterization of human scarred vocal folds. J. Voice. 2009;23:399–407. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]; Hirano, S., Minamiguchi, S., Yamashita, M., Ohno, T., Kanemaru, S., and Kitamura, M. (2009). Histologic characterization of human scarred vocal folds. J. Voice 23, 399-407. [DOI] [PubMed]
- 11.Welham N.V., Montequin D.W., Tateya I., Tateya T., Choi S.H., Bless D.M. A rat excised larynx model of vocal fold scar. J. Speech Lang. Hear. Res. 2009;52:1008–1020. doi: 10.1044/1092-4388(2009/08-0049). [DOI] [PMC free article] [PubMed] [Google Scholar]; Welham, N.V., Montequin, D.W., Tateya, I., Tateya, T., Choi, S.H., and Bless, D.M. (2009). A rat excised larynx model of vocal fold scar. J. Speech Lang. Hear. Res. 52, 1008-1020. [DOI] [PMC free article] [PubMed]
- 12.Rousseau B., Hirano S., Scheidt T.D., Welham N.V., Thibeault S.L., Chan R.W., Bless D.M. Characterization of vocal fold scarring in a canine model. Laryngoscope. 2003;113:620–627. doi: 10.1097/00005537-200304000-00007. [DOI] [PubMed] [Google Scholar]; Rousseau, B., Hirano, S., Scheidt, T.D., Welham, N.V., Thibeault, S.L., Chan, R.W., and Bless, D.M. (2003). Characterization of vocal fold scarring in a canine model. Laryngoscope 113, 620-627. [DOI] [PubMed]
- 13.Thibeault S.L., Gray S.D., Bless D.M., Chan R.W., Ford C.N. Histologic and rheologic characterization of vocal fold scarring. J. Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]; Thibeault, S.L., Gray, S.D., Bless, D.M., Chan, R.W., and Ford, C.N. (2002). Histologic and rheologic characterization of vocal fold scarring. J. Voice 16, 96-104. [DOI] [PubMed]
- 14.Friedrich G., Dikkers F.G., Arens C., Remacle M., Hess M., Giovanni A., Duflo S., Hantzakos A., Bachy V., Gugatschka M., European Laryngological Society. Phonosurgery Committee Vocal fold scars: current concepts and future directions. Consensus report of the Phonosurgery Committee of the European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2013;270:2491–2507. doi: 10.1007/s00405-013-2498-9. [DOI] [PubMed] [Google Scholar]; Friedrich, G., Dikkers, F.G., Arens, C., Remacle, M., Hess, M., Giovanni, A., Duflo, S., Hantzakos, A., Bachy, V., and Gugatschka, M.; European Laryngological Society. Phonosurgery Committee (2013). Vocal fold scars: current concepts and future directions. Consensus report of the Phonosurgery Committee of the European Laryngological Society. Eur. Arch. Otorhinolaryngol. 270, 2491-2507. [DOI] [PubMed]
- 15.Mortensen M.M., Woo P., Ivey C., Thompson C., Carroll L., Altman K. The use of the pulse dye laser in the treatment of vocal fold scar: a preliminary study. Laryngoscope. 2008;118:1884–1888. doi: 10.1097/MLG.0b013e31817d7546. [DOI] [PubMed] [Google Scholar]; Mortensen, M.M., Woo, P., Ivey, C., Thompson, C., Carroll, L., and Altman, K. (2008). The use of the pulse dye laser in the treatment of vocal fold scar: a preliminary study. Laryngoscope 118, 1884-1888. [DOI] [PubMed]
- 16.Mortensen M., Woo P. Office steroid injections of the larynx. Laryngoscope. 2006;116:1735–1739. doi: 10.1097/01.mlg.0000231455.19183.8c. [DOI] [PubMed] [Google Scholar]; Mortensen, M., and Woo, P. (2006). Office steroid injections of the larynx. Laryngoscope 116, 1735-1739. [DOI] [PubMed]
- 17.Young W.G., Hoffman M.R., Koszewski I.J., Whited C.W., Ruel B.N., Dailey S.H. Voice outcomes following a single office-based steroid injection for vocal fold scar. Otolaryngol. Head Neck Surg. 2016;155:820–828. doi: 10.1177/0194599816654899. [DOI] [PubMed] [Google Scholar]; Young, W.G., Hoffman, M.R., Koszewski, I.J., Whited, C.W., Ruel, B.N., and Dailey, S.H. (2016). Voice outcomes following a single office-based steroid injection for vocal fold scar. Otolaryngol. Head Neck Surg. 155, 820-828. [DOI] [PubMed]
- 18.Kishimoto Y., Hirano S., Kojima T., Kanemaru S., Ito J. Implantation of an atelocollagen sheet for the treatment of vocal fold scarring and sulcus vocalis. Ann. Otol. Rhinol. Laryngol. 2009;118:613–620. doi: 10.1177/000348940911800902. [DOI] [PubMed] [Google Scholar]; Kishimoto, Y., Hirano, S., Kojima, T., Kanemaru, S., and Ito, J. (2009). Implantation of an atelocollagen sheet for the treatment of vocal fold scarring and sulcus vocalis. Ann. Otol. Rhinol. Laryngol. 118, 613-620. [DOI] [PubMed]
- 19.Sataloff R.T., Spiegel J.R., Hawkshaw M., Rosen D.C., Heuer R.J. Autologous fat implantation for vocal fold scar: a preliminary report. J. Voice. 1997;11:238–246. [PubMed] [Google Scholar]; Sataloff, R.T., Spiegel, J.R., Hawkshaw, M., Rosen, D.C., and Heuer, R.J. (1997). Autologous fat implantation for vocal fold scar: a preliminary report. J. Voice 11, 238-246. [PubMed]
- 20.Molteni G., Bergamini G., Ricci-Maccarini A., Marchese C., Ghidini A., Alicandri-Ciufelli M., Luppi M.P., Presutti L. Auto-crosslinked hyaluronan gel injections in phonosurgery. Otolaryngol. Head Neck Surg. 2010;142:547–553. doi: 10.1016/j.otohns.2009.12.035. [DOI] [PubMed] [Google Scholar]; Molteni, G., Bergamini, G., Ricci-Maccarini, A., Marchese, C., Ghidini, A., Alicandri-Ciufelli, M., Luppi, M.P., and Presutti, L. (2010). Auto-crosslinked hyaluronan gel injections in phonosurgery. Otolaryngol. Head Neck Surg. 142, 547-553. [DOI] [PubMed]
- 21.Kanazawa T., Komazawa D., Indo K., Akagi Y., Lee Y., Nakamura K., Matsushima K., Kunieda C., Misawa K., Nishino H., Watanabe Y. Single injection of basic fibroblast growth factor to treat severe vocal fold lesions and vocal fold paralysis. Laryngoscope. 2015;125:E338–E344. doi: 10.1002/lary.25315. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kanazawa, T., Komazawa, D., Indo, K., Akagi, Y., Lee, Y., Nakamura, K., Matsushima, K., Kunieda, C., Misawa, K., Nishino, H., and Watanabe, Y. (2015). Single injection of basic fibroblast growth factor to treat severe vocal fold lesions and vocal fold paralysis. Laryngoscope 125, E338-E344. [DOI] [PMC free article] [PubMed]
- 22.Hirano S., Kawamoto A., Tateya I., Mizuta M., Kishimoto Y., Hiwatashi N., Kawai Y., Tsuji T., Suzuki R., Kaneko M. A phase I/II exploratory clinical trial for intracordal injection of recombinant hepatocyte growth factor for vocal fold scar and sulcus. J. Tissue Eng. Regen. Med. 2018;12:1031–1038. doi: 10.1002/term.2603. [DOI] [PubMed] [Google Scholar]; Hirano, S., Kawamoto, A., Tateya, I., Mizuta, M., Kishimoto, Y., Hiwatashi, N., Kawai, Y., Tsuji, T., Suzuki, R., Kaneko, M., et al. (2018). A phase I/II exploratory clinical trial for intracordal injection of recombinant hepatocyte growth factor for vocal fold scar and sulcus. J. Tissue Eng. Regen. Med. 12, 1031-1038. [DOI] [PubMed]
- 23.Mattei A., Magalon J., Bertrand B., Grimaud F., Revis J., Velier M., Veran J., Dessi P., Sabatier F., Giovanni A. Autologous adipose-derived stromal vascular fraction and scarred vocal folds: first clinical case report. Stem Cell Res. Ther. 2018;9:202. doi: 10.1186/s13287-018-0842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mattei, A., Magalon, J., Bertrand, B., Grimaud, F., Revis, J., Velier, M., Veran, J., Dessi, P., Sabatier, F., and Giovanni, A. (2018). Autologous adipose-derived stromal vascular fraction and scarred vocal folds: first clinical case report. Stem Cell Res. Ther. 9, 202. [DOI] [PMC free article] [PubMed]
- 24.Welham N.V., Choi S.H., Dailey S.H., Ford C.N., Jiang J.J., Bless D.M. Prospective multi-arm evaluation of surgical treatments for vocal fold scar and pathologic sulcus vocalis. Laryngoscope. 2011;121:1252–1260. doi: 10.1002/lary.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]; Welham, N.V., Choi, S.H., Dailey, S.H., Ford, C.N., Jiang, J.J., and Bless, D.M. (2011). Prospective multi-arm evaluation of surgical treatments for vocal fold scar and pathologic sulcus vocalis. Laryngoscope 121, 1252-1260. [DOI] [PMC free article] [PubMed]
- 25.Dailey S.H., Ford C.N. Surgical management of sulcus vocalis and vocal fold scarring. Otolaryngol. Clin. North Am. 2006;39:23–42. doi: 10.1016/j.otc.2005.10.012. [DOI] [PubMed] [Google Scholar]; Dailey, S.H., and Ford, C.N. (2006). Surgical management of sulcus vocalis and vocal fold scarring. Otolaryngol. Clin. North Am. 39, 23-42. [DOI] [PubMed]
- 26.Ishida Y., Nagata K. Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol. 2011;499:167–182. doi: 10.1016/B978-0-12-386471-0.00009-2. [DOI] [PubMed] [Google Scholar]; Ishida, Y., and Nagata, K. (2011). Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol. 499, 167-182. [DOI] [PubMed]
- 27.Ono T., Miyazaki T., Ishida Y., Uehata M., Nagata K. Direct in vitro and in vivo evidence for interaction between Hsp47 protein and collagen triple helix. J. Biol. Chem. 2012;287:6810–6818. doi: 10.1074/jbc.M111.280248. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ono, T., Miyazaki, T., Ishida, Y., Uehata, M., and Nagata, K. (2012). Direct in vitro and in vivo evidence for interaction between Hsp47 protein and collagen triple helix. J. Biol. Chem. 287, 6810-6818. [DOI] [PMC free article] [PubMed]
- 28.Widmer C., Gebauer J.M., Brunstein E., Rosenbaum S., Zaucke F., Drögemüller C., Leeb T., Baumann U. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. USA. 2012;109:13243–13247. doi: 10.1073/pnas.1208072109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Widmer, C., Gebauer, J.M., Brunstein, E., Rosenbaum, S., Zaucke, F., Drogemuller, C., Leeb, T., and Baumann, U. (2012). Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. USA 109, 13243-13247. [DOI] [PMC free article] [PubMed]
- 29.Sunamoto M., Kuze K., Iehara N., Takeoka H., Nagata K., Kita T., Doi T. Expression of heat shock protein 47 is increased in remnant kidney and correlates with disease progression. Int. J. Exp. Pathol. 1998;79:133–140. doi: 10.1046/j.1365-2613.1998.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sunamoto, M., Kuze, K., Iehara, N., Takeoka, H., Nagata, K., Kita, T., and Doi, T. (1998). Expression of heat shock protein 47 is increased in remnant kidney and correlates with disease progression. Int. J. Exp. Pathol. 79, 133-140. [DOI] [PMC free article] [PubMed]
- 30.Brown K.E., Broadhurst K.A., Mathahs M.M., Brunt E.M., Schmidt W.N. Expression of HSP47, a collagen-specific chaperone, in normal and diseased human liver. Lab. Invest. 2005;85:789–797. doi: 10.1038/labinvest.3700271. [DOI] [PubMed] [Google Scholar]; Brown, K.E., Broadhurst, K.A., Mathahs, M.M., Brunt, E.M., and Schmidt, W.N. (2005). Expression of HSP47, a collagen-specific chaperone, in normal and diseased human liver. Lab. Invest. 85, 789-797. [DOI] [PubMed]
- 31.Chen J.-J., Zhao S., Cen Y., Liu X.X., Yu R., Wu D.M. Effect of heat shock protein 47 on collagen accumulation in keloid fibroblast cells. Br. J. Dermatol. 2007;156:1188–1195. doi: 10.1111/j.1365-2133.2007.07898.x. [DOI] [PubMed] [Google Scholar]; Chen, J.-J., Zhao, S., Cen, Y., Liu, X.X., Yu, R., and Wu, D.M. (2007). Effect of heat shock protein 47 on collagen accumulation in keloid fibroblast cells. Br. J. Dermatol. 156, 1188-1195. [DOI] [PubMed]
- 32.Nagai N., Hosokawa M., Itohara S., Adachi E., Matsushita T., Hosokawa N., Nagata K. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell Biol. 2000;150:1499–1506. doi: 10.1083/jcb.150.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nagai, N., Hosokawa, M., Itohara, S., Adachi, E., Matsushita, T., Hosokawa, N., and Nagata, K. (2000). Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell Biol. 150, 1499-1506. [DOI] [PMC free article] [PubMed]
- 33.Sato Y., Murase K., Kato J., Kobune M., Sato T., Kawano Y., Takimoto R., Takada K., Miyanishi K., Matsunaga T. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]; Sato, Y., Murase, K., Kato, J., Kobune, M., Sato, T., Kawano, Y., Takimoto, R., Takada, K., Miyanishi, K., Matsunaga, T., et al. (2008). Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 26, 431-442. [DOI] [PubMed]
- 34.Otsuka M., Shiratori M., Chiba H., Kuronuma K., Sato Y., Niitsu Y., Takahashi H. Treatment of pulmonary fibrosis with siRNA against a collagen-specific chaperone HSP47 in vitamin A-coupled liposomes. Exp. Lung Res. 2017;43:271–282. doi: 10.1080/01902148.2017.1354946. [DOI] [PubMed] [Google Scholar]; Otsuka, M., Shiratori, M., Chiba, H., Kuronuma, K., Sato, Y., Niitsu, Y., and Takahashi, H. (2017). Treatment of pulmonary fibrosis with siRNA against a collagen-specific chaperone HSP47 in vitamin A-coupled liposomes. Exp. Lung Res. 43, 271-282. [DOI] [PubMed]
- 35.Yamakawa T., Ohigashi H., Hashimoto D., Hayase E., Takahashi S., Miyazaki M., Minomi K., Onozawa M., Niitsu Y., Teshima T. Vitamin A-coupled liposomes containing siRNA against HSP47 ameliorate skin fibrosis in chronic graft-versus-host disease. Blood. 2018;131:1476–1485. doi: 10.1182/blood-2017-04-779934. [DOI] [PubMed] [Google Scholar]; Yamakawa, T., Ohigashi, H., Hashimoto, D., Hayase, E., Takahashi, S., Miyazaki, M., Minomi, K., Onozawa, M., Niitsu, Y., and Teshima, T. (2018). Vitamin A-coupled liposomes containing siRNA against HSP47 ameliorate skin fibrosis in chronic graft-versus-host disease. Blood 131, 1476-1485. [DOI] [PubMed]
- 36.Ishiwatari H., Sato Y., Murase K., Yoneda A., Fujita R., Nishita H., Birukawa N.K., Hayashi T., Sato T., Miyanishi K. Treatment of pancreatic fibrosis with siRNA against a collagen-specific chaperone in vitamin A-coupled liposomes. Gut. 2013;62:1328–1339. doi: 10.1136/gutjnl-2011-301746. [DOI] [PubMed] [Google Scholar]; Ishiwatari, H., Sato, Y., Murase, K., Yoneda, A., Fujita, R., Nishita, H., Birukawa, N.K., Hayashi, T., Sato, T., Miyanishi, K., et al. (2013). Treatment of pancreatic fibrosis with siRNA against a collagen-specific chaperone in vitamin A-coupled liposomes. Gut 62, 1328-1339. [DOI] [PubMed]
- 37.Tateya T., Tateya I., Sohn J.H., Bless D.M. Histologic characterization of rat vocal fold scarring. Ann. Otol. Rhinol. Laryngol. 2005;114:183–191. doi: 10.1177/000348940511400303. [DOI] [PubMed] [Google Scholar]; Tateya, T., Tateya, I., Sohn, J.H., and Bless, D.M. (2005). Histologic characterization of rat vocal fold scarring. Ann. Otol. Rhinol. Laryngol. 114, 183-191. [DOI] [PubMed]
- 38.Ling C., Yamashita M., Waselchuk E.A., Raasch J.L., Bless D.M., Welham N.V. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 2010;18:89–97. doi: 10.1111/j.1524-475X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ling, C., Yamashita, M., Waselchuk, E.A., Raasch, J.L., Bless, D.M., and Welham, N.V. (2010). Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 18, 89-97. [DOI] [PMC free article] [PubMed]
- 39.Paul B.C., Rafii B.Y., Gandonu S., Bing R., Born H., Amin M.R., Branski R.C. Smad3: an emerging target for vocal fold fibrosis. Laryngoscope. 2014;124:2327–2331. doi: 10.1002/lary.24723. [DOI] [PubMed] [Google Scholar]; Paul, B.C., Rafii, B.Y., Gandonu, S., Bing, R., Born, H., Amin, M.R., and Branski, R.C. (2014). Smad3: an emerging target for vocal fold fibrosis. Laryngoscope 124, 2327-2331. [DOI] [PubMed]
- 40.Neuman R.E., Logan M.A. The determination of hydroxyproline. J. Biol. Chem. 1950;184:299–306. [PubMed] [Google Scholar]; Neuman, R.E., and Logan, M.A. (1950). The determination of hydroxyproline. J. Biol. Chem. 184, 299-306. [PubMed]
- 41.Ford C.N., Roy N., Sandage M., Bless D.M. Rigid endoscopy for monitoring indirect vocal fold injection. Laryngoscope. 1998;108:1584–1586. doi: 10.1097/00005537-199810000-00030. [DOI] [PubMed] [Google Scholar]; Ford, C.N., Roy, N., Sandage, M., and Bless, D.M. (1998). Rigid endoscopy for monitoring indirect vocal fold injection. Laryngoscope 108, 1584-1586. [DOI] [PubMed]
- 42.Inagi K., Connor N.P., Ford C.N., Schultz E., Rodriquez A.A., Bless D.M., Pasic T., Heisey D.M. Physiologic assessment of botulinum toxin effects in the rat larynx. Laryngoscope. 1998;108:1048–1054. doi: 10.1097/00005537-199807000-00018. [DOI] [PubMed] [Google Scholar]; Inagi, K., Connor, N.P., Ford, C.N., Schultz, E., Rodriquez, A.A., Bless, D.M., Pasic, T., and Heisey, D.M. (1998). Physiologic assessment of botulinum toxin effects in the rat larynx. Laryngoscope 108, 1048-1054. [DOI] [PubMed]
- 43.Kawasaki K., Ushioda R., Ito S., Ikeda K., Masago Y., Nagata K. Deletion of the collagen-specific molecular chaperone Hsp47 causes endoplasmic reticulum stress-mediated apoptosis of hepatic stellate cells. J. Biol. Chem. 2015;290:3639–3646. doi: 10.1074/jbc.M114.592139. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kawasaki, K., Ushioda, R., Ito, S., Ikeda, K., Masago, Y., and Nagata, K. (2015). Deletion of the collagen-specific molecular chaperone Hsp47 causes endoplasmic reticulum stress-mediated apoptosis of hepatic stellate cells. J. Biol. Chem. 290, 3639-3646. [DOI] [PMC free article] [PubMed]
- 44.Kendziorski C.M., Newton M.A., Lan H., Gould M.N. On parametric empirical Bayes methods for comparing multiple groups using replicated gene expression profiles. Stat. Med. 2003;22:3899–3914. doi: 10.1002/sim.1548. [DOI] [PubMed] [Google Scholar]; Kendziorski, C.M., Newton, M.A., Lan, H., and Gould, M.N. (2003). On parametric empirical Bayes methods for comparing multiple groups using replicated gene expression profiles. Stat. Med. 22, 3899-3914. [DOI] [PubMed]
- 45.Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q., Wang Z., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90-7. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kuleshov, M.V., Jones, M.R., Rouillard, A.D., Fernandez, N.F., Duan, Q., Wang, Z., Koplev, S., Jenkins, S.L., Jagodnik, K.M., Lachmann, A., et al. (2016). Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44 (W1), W90-7. [DOI] [PMC free article] [PubMed]
- 46.Chen E.Y., Tan C.M., Kou Y., Duan Q., Wang Z., Meirelles G.V., Clark N.R., Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen, E.Y., Tan, C.M., Kou, Y., Duan, Q., Wang, Z., Meirelles, G.V., Clark, N.R., and Ma’ayan, A. (2013). Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128. [DOI] [PMC free article] [PubMed]
- 47.Blake J.A., Dolan M., Drabkin H., Hill D.P., Li N., Sitnikov D., Bridges S., Burgess S., Buza T., McCarthy F., Gene Ontology Consortium Gene Ontology annotations and resources. Nucleic Acids Res. 2013;41:D530–D535. doi: 10.1093/nar/gks1050. [DOI] [PMC free article] [PubMed] [Google Scholar]; Blake, J.A., Dolan, M., Drabkin, H., Hill, D.P., Li, N., Sitnikov, D., Bridges, S., Burgess, S., Buza, T., McCarthy, F., et al.; Gene Ontology Consortium (2013). Gene Ontology annotations and resources. Nucleic Acids Res. 41, D530-D535. [DOI] [PMC free article] [PubMed]
- 48.Wingstrand V.L., Grønhøj Larsen C., Jensen D.H., Bork K., Sebbesen L., Balle J., Fischer-Nielsen A., von Buchwald C. Mesenchymal stem cell therapy for the treatment of vocal fold scarring: a systematic review of preclinical studies. PLoS ONE. 2016;11:e0162349. doi: 10.1371/journal.pone.0162349. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wingstrand, V.L., Gronhoj Larsen, C., Jensen, D.H., Bork, K., Sebbesen, L., Balle, J., Fischer-Nielsen, A., and von Buchwald, C. (2016). Mesenchymal stem cell therapy for the treatment of vocal fold scarring: a systematic review of preclinical studies. PLoS ONE 11, e0162349. [DOI] [PMC free article] [PubMed]
- 49.Bless D.M., Welham N.V. Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models. Curr. Opin. Otolaryngol. Head Neck Surg. 2010;18:481–486. doi: 10.1097/MOO.0b013e3283407d87. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bless, D.M., and Welham, N.V. (2010). Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models. Curr. Opin. Otolaryngol. Head Neck Surg. 18, 481-486. [DOI] [PMC free article] [PubMed]
- 50.Hiwatashi N., Kraja I., Benedict P.A., Dion G.R., Bing R., Rousseau B., Amin M.R., Nalband D.M., Kirshenbaum K., Branski R.C. Nanoparticle delivery of RNA-based therapeutics to alter the vocal fold tissue response to injury. Laryngoscope. 2018;128:E178–E183. doi: 10.1002/lary.27047. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hiwatashi, N., Kraja, I., Benedict, P.A., Dion, G.R., Bing, R., Rousseau, B., Amin, M.R., Nalband, D.M., Kirshenbaum, K., and Branski, R.C. (2018). Nanoparticle delivery of RNA-based therapeutics to alter the vocal fold tissue response to injury. Laryngoscope 128, E178-E183. [DOI] [PMC free article] [PubMed]
- 51.Chang Z., Kishimoto Y., Hasan A., Welham N.V. TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis. Model. Mech. 2014;7:83–91. doi: 10.1242/dmm.013326. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chang, Z., Kishimoto, Y., Hasan, A., and Welham, N.V. (2014). TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis. Model. Mech. 7, 83-91. [DOI] [PMC free article] [PubMed]
- 52.Hall J.E., Suehiro A., Branski R.C., Garrett C.G., Rousseau B. Modulation of inflammatory and profibrotic signaling in a rabbit model of acute phonotrauma using triamcinolone. Otolaryngol. Head Neck Surg. 2012;147:302–307. doi: 10.1177/0194599812440419. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hall, J.E., Suehiro, A., Branski, R.C., Garrett, C.G., and Rousseau, B. (2012). Modulation of inflammatory and profibrotic signaling in a rabbit model of acute phonotrauma using triamcinolone. Otolaryngol. Head Neck Surg. 147, 302-307. [DOI] [PMC free article] [PubMed]
- 53.Pucci B., Kasten M., Giordano A. Cell cycle and apoptosis. Neoplasia. 2000;2:291–299. doi: 10.1038/sj.neo.7900101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pucci, B., Kasten, M., and Giordano, A. (2000). Cell cycle and apoptosis. Neoplasia 2, 291-299. [DOI] [PMC free article] [PubMed]
- 54.Kim D.-H., Behlke M.A., Rose S.D., Chang M.-S., Choi S., Rossi J.J. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat. Biotechnol. 2005;23:222–226. doi: 10.1038/nbt1051. [DOI] [PubMed] [Google Scholar]; Kim, D.-H., Behlke, M.A., Rose, S.D., Chang, M.-S., Choi, S., and Rossi, J.J. (2005). Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat. Biotechnol. 23, 222-226. [DOI] [PubMed]
- 55.Kim D.-H., Longo M., Han Y., Lundberg P., Cantin E., Rossi J.J. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat. Biotechnol. 2004;22:321–325. doi: 10.1038/nbt940. [DOI] [PubMed] [Google Scholar]; Kim, D.-H., Longo, M., Han, Y., Lundberg, P., Cantin, E., and Rossi, J.J. (2004). Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat. Biotechnol. 22, 321-325. [DOI] [PubMed]
- 56.Gugatschka M., Ainödhofer H., Gruber H.J., Graupp M., Kieslinger P., Kiesler K., Saxena A., Hirano S., Friedrich G. Age effects on extracellular matrix production of vocal fold scar fibroblasts in rats. Eur. Arch. Otorhinolaryngol. 2014;271:1107–1112. doi: 10.1007/s00405-013-2722-7. [DOI] [PubMed] [Google Scholar]; Gugatschka, M., Ainodhofer, H., Gruber, H.J., Graupp, M., Kieslinger, P., Kiesler, K., Saxena, A., Hirano, S. and Friedrich, G. (2014). Age effects on extracellular matrix production of vocal fold scar fibroblasts in rats. Eur. Arch. Otorhinolaryngol. 271, 1107-1112. [DOI] [PubMed]
- 57.Graupp M., Kiesler K., Friedrich G., Ainödhofer H., Gruber H.-J., Kieslinger P., Saxena A., Hirano S., Gugatschka M. Vocal fold fibroblast response to growth factor treatment is age dependent: results from an in vitro study. J. Voice. 2014;28:420–423. doi: 10.1016/j.jvoice.2013.11.005. [DOI] [PubMed] [Google Scholar]; Graupp, M., Kiesler, K., Friedrich, G., Ainodhofer, H., Gruber, H.-J., Kieslinger, P., Saxena, A., Hirano, S., and Gugatschka, M. (2014). Vocal fold fibroblast response to growth factor treatment is age dependent: results from an in vitro study. J. Voice 28, 420-423. [DOI] [PubMed]
- 58.Gray S.D., Titze I.R., Alipour F., Hammond T.H. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann. Otol. Rhinol. Laryngol. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]; Gray, S.D., Titze, I.R., Alipour, F., and Hammond, T.H. (2000). Biomechanical and histologic observations of vocal fold fibrous proteins. Ann. Otol. Rhinol. Laryngol. 109, 77-85. [DOI] [PubMed]
- 59.Gray S.D., Titze I.R., Chan R., Hammond T.H. Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope. 1999;109:845–854. doi: 10.1097/00005537-199906000-00001. [DOI] [PubMed] [Google Scholar]; Gray, S.D., Titze, I.R., Chan, R., and Hammond, T.H. (1999). Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope 109, 845-854. [DOI] [PubMed]
- 60.Toya Y., Riabroy N., Davis C.R., Kishimoto Y., Tanumihardjo S.A., Bless D.M., Welham N.V. Interspecies comparison of stellate cell-containing macula flavae and vitamin A storage in vocal fold mucosa. J. Anat. 2014;225:298–305. doi: 10.1111/joa.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]; Toya, Y., Riabroy, N., Davis, C.R., Kishimoto, Y., Tanumihardjo, S.A., Bless, D.M., and Welham, N.V. (2014). Interspecies comparison of stellate cell-containing macula flavae and vitamin A storage in vocal fold mucosa. J. Anat. 225, 298-305. [DOI] [PMC free article] [PubMed]
- 61.Ling C., Yamashita M., Zhang J., Bless D.M., Welham N.V. Reactive response of fibrocytes to vocal fold mucosal injury in rat. Wound Repair Regen. 2010;18:514–523. doi: 10.1111/j.1524-475X.2010.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ling, C., Yamashita, M., Zhang, J., Bless, D.M., and Welham, N.V. (2010). Reactive response of fibrocytes to vocal fold mucosal injury in rat. Wound Repair Regen. 18, 514-523. [DOI] [PMC free article] [PubMed]
- 62.Tateya I., Tateya T., Lim X., Sohn J.H., Bless D.M. Cell production in injured vocal folds: a rat study. Ann. Otol. Rhinol. Laryngol. 2006;115:135–143. doi: 10.1177/000348940611500210. [DOI] [PubMed] [Google Scholar]; Tateya, I., Tateya, T., Lim, X., Sohn, J.H., and Bless, D.M. (2006). Cell production in injured vocal folds: a rat study. Ann. Otol. Rhinol. Laryngol. 115, 135-143. [DOI] [PubMed]
- 63.Obata Y., Nishino T., Kushibiki T., Tomoshige R., Xia Z., Miyazaki M., Abe K., Koji T., Tabata Y., Kohno S. HSP47 siRNA conjugated with cationized gelatin microspheres suppresses peritoneal fibrosis in mice. Acta Biomater. 2012;8:2688–2696. doi: 10.1016/j.actbio.2012.03.050. [DOI] [PubMed] [Google Scholar]; Obata, Y., Nishino, T., Kushibiki, T., Tomoshige, R., Xia, Z., Miyazaki, M., Abe, K., Koji, T., Tabata, Y., and Kohno, S. (2012). HSP47 siRNA conjugated with cationized gelatin microspheres suppresses peritoneal fibrosis in mice. Acta Biomater. 8, 2688-2696. [DOI] [PubMed]
- 64.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]; Livak, K.J., and Schmittgen, T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods 25, 402-408. [DOI] [PubMed]
- 65.Chang Z., Ling C., Yamashita M., Welham N.V. Microarray-driven validation of reference genes for quantitative real-time polymerase chain reaction in a rat vocal fold model of mucosal injury. Anal. Biochem. 2010;406:214–221. doi: 10.1016/j.ab.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chang, Z., Ling, C., Yamashita, M., and Welham, N.V. (2010). Microarray-driven validation of reference genes for quantitative real-time polymerase chain reaction in a rat vocal fold model of mucosal injury. Anal. Biochem. 406, 214-221. [DOI] [PMC free article] [PubMed]
- 66.R Development Core Team . R Foundation for Statistical Computing; Vienna: 2007. R: a language and environment for statistical computing. [Google Scholar]; R Development Core Team (2007). R: a language and environment for statistical computing (Vienna: R Foundation for Statistical Computing).
- 67.Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]; Irizarry, R.A., Hobbs, B., Collin, F., Beazer-Barclay, Y.D., Antonellis, K.J., Scherf, U., and Speed, T.P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249-264. [DOI] [PubMed]
- 68.Wu Z., Irizarry R.A. Preprocessing of oligonucleotide array data. Nat. Biotechnol. 2004;22:656–658, author reply 658. doi: 10.1038/nbt0604-656b. [DOI] [PubMed] [Google Scholar]; Wu, Z., and Irizarry, R.A. (2004). Preprocessing of oligonucleotide array data. Nat. Biotechnol. 22, 656-658, author reply 658. [DOI] [PubMed]
- 69.Supek F., Bošnjak M., Škunca N., Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]; Supek, F., Bošnjak, M., Škunca, N., and Šmuc, T. (2011). REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6, e21800. [DOI] [PMC free article] [PubMed]
- 70.Cline M.S., Smoot M., Cerami E., Kuchinsky A., Landys N., Workman C., Christmas R., Avila-Campilo I., Creech M., Gross B. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2007;2:2366–2382. doi: 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cline, M.S., Smoot, M., Cerami, E., Kuchinsky, A., Landys, N., Workman, C., Christmas, R., Avila-Campilo, I., Creech, M., Gross, B., et al. (2007). Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2, 2366-2382. [DOI] [PMC free article] [PubMed]