Abstract
Vascular calcification is a pathologic phenomenon consisting of calcium phosphate crystal deposition in the vascular walls. Vascular calcification has been found to be a risk factor for cardio-vascular diseases, due to its correlation with cardiovascular events and mortality, and it has been as-sociated with aging, diabetes, and chronic kidney disease.
Studies of vascular calcification have focused on phosphate homeostasis, primarily on the important role of hyperphosphatemia. Moreover, vascular calcification has been associated with loss of plasma pyrophosphate, one of the main inhibitors of calcification, thus indicating the importance of the phos-phate/pyrophosphate ratio.
Extracellular pyrophosphate can be synthesized from extracellular ATP by ecto-nucleotide pyrophos-phatase/phosphodiesterase, whereas pyrophosphate is hydrolyzed to phosphate by tissue-nonspecific alkaline phosphatase, contributing to the formation of hydroxyapatite crystals.
Over the last decade, vascular calcification has been the subject of numerous reviews and studies, which have revealed new agents and activities that may aid in explaining the complex physiology of this condition. This review summarizes current knowledge about alkaline phosphatase and its role in the process of vascular calcification as a key regulator of the phosphate/pyrophosphate ratio.
Keywords: Vascular calcification, TNAP, alkaline phosphatase, pyrophosphate, hemodialysis, CKD, aging
1. INTRODUCTION
Vascular calcification is a pathologic phenomenon consisting of the deposition of calcium phosphate crystals in vascular walls [1, 2]. Vascular calcification has been found to be a risk factor for cardiovascular diseases due to its correlation with cardiovascular events and mortality, and it has been associated with aging, diabetes, and Chronic Kidney Disease (CKD) [3-5]. The clinical characteristics of vascular calcification include vessel thickening and the presence of hydroxyapatite crystals in vessel walls [6].
Physiologically, mineralization occurs in hard tissues, mainly bones and teeth, during development. Under pathological conditions, however, soft tissues such as vascular vessels may also undergo mineralization [7, 8], with calcium crystals deposited primarily in large elastic and muscular arteries [9], especially the aorta and the coronary and carotid arteries [1, 10], as well as in heart valves [11, 12], increasing the risk of cardiovascular infarction.
Pathological calcifications have been classified into two major types, both of which prevent correct vessel function, and both often coexisting [2, 13]. The first type is intimal calcification, which is associated with atherosclerotic plaque and is dependent on lipid and cholesterol accumulation [14]. The second type of calcification, known as Mönckeberg’s sclerosis, takes place on the medial layer of the aortic wall and involves the deposition of minerals within Vascular Smooth Muscle Cells (VSMCs) [8, 15, 16].
Extracellular pyrophosphate is one of the main inhibitors of calcification, preventing the formation of hydroxyapatite crystals [17]. Extracellular pyrophosphate may be synthesized from extracellular ATP by ecto-nucleotide pyrophosphatase/phosphodiesterase [18, 19] and may be subsequently hydrolyzed to phosphate by phosphatases [20], mainly by Tissue-Nonspecific Alkaline Phosphatase (TNAP), thereby promoting calcification [6, 21-23].
2. ALKALINE PHOSPHATASE TYPES
Alkaline Phosphatases (APs) (E.C. 3.1.3.1) are ubiquitous ectoenzymes that are widely distributed in nature, from bacteria to humans, and are found in nearly all living organisms, except for some plants. Despite their wide distribution, their sequences are highly conserved, with ~57.8% of amino acids conserved in all mammals, suggesting that this family of enzymes plays a key role in physiological processes throughout evolution [24]. Although the main features of these enzymes are conserved from bacteria to mammals, mammalian APs have specific characteristics that enable them to adapt to different environments, such as higher activity and Km values and lower heat stability [25].
This family of enzymes was first described in 1923 as phosphatases present in the skeleton that are responsible for generating the phosphate required for bone formation [26].
In humans, APs are encoded by four homologous genes. Three of these enzymes, Placental AP (PLAP), Germ Cell AP (GCAP), and Intestinal AP (IAP), are tissue-specific, with highly restricted expression. By contrast, the fourth isozyme, tissue non-specific AP (TNAP), is present in numerous tissues but is especially abundant in mineralizing tissues, the kidneys, and the Central Nervous System (CNS) [24].
3. SYNTHESIS
APs are synthesized as 66-kDa proteins, which are modified in the endoplasmic reticulum by the addition of carbohydrate chains through O- and N-linked sugar chains. These modified proteins are subsequently processed in the Golgi apparatus and finally localize to the outer membrane via Glycosylphosphatidyl Inositol (GPI) anchors. Post-transductional events allow these proteins to anchor to the membrane via GPI motifs, enabling movement of the enzymes in the membrane. TNAP is also detected in the systemic circulation and other biological fluids due to the action of phospholipases that cleave GPI from the membrane and release TNAP. The sugar chains of all AP isoforms differ from each other, and although all APs have the same peptide sequence, each of these isoforms has different sites of glycosylation [27].
4. STRUCTURE
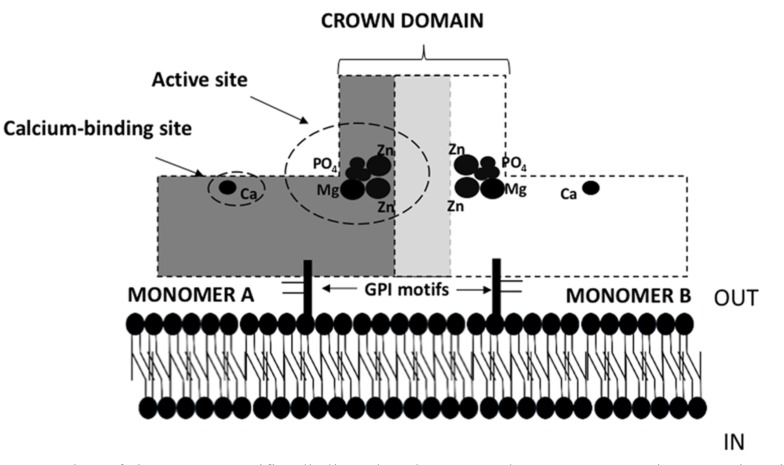
All APs are homodimers consisting of two monomers (Fig. 1), which are anchored to the cytoplasmic membrane via GPI [28]. These anchors consist of an ethanolamine phosphate, three residues of mannose, a glucosamine, and a phosphatidylinositol. Each monomer contains 484 amino acid residues, four metal atoms, one phosphate ion, and 603 water molecules. The central core is formed by an extended β-sheet flanked by α-helices [27]. The active site of each monomer consists of two Zn binding sites, an Mg2+ binding site, and a serine residue, which binds phosphate and enables monophosphate hydrolysis. The two monomers are connected by a two-fold crystallographic axis called the crown domain, which is a flexible loop formed by the insertion of a 60-residue segment from each monomer. This crown domain is formed by two small b-sheets and is surrounded by six large, flexible loops containing a short α-helix. This region is responsible for isozyme properties, such non-competitive inhibition, heat stability, and interactions with extracellular matrix proteins [27].
Fig. (1).
Schematic representation of tissue non-specific alkaline phosphatase. Each monomer contains an active site (metal binding site) where phosphorus binds, and a calcium binding site. The calcium and metal union site allow interactions with Ca, Mg or Zn, which are necessary for the enzyme to express activity. The two monomers are connected in the crowd domain by flexible sequences of each. Both monomers are anchors to the membrane via GPI motifs.
Each of these monomers contains three metal-binding sites (M1 and M2 for Zn2+ and M3 for Mg2+), one phosphate-binding site, and one calcium-binding site (M4 site), all of which are necessary for enzymatic activity (Fig. 1). Other molecules such as iron have been shown to interact with APs, decreasing its activity in a dose-dependent manner, suggesting that other molecules could interact with the metal binding sites, thereby further regulating TNAP activity [29]. Moreover, a recent study suggested that the Mg2+/Ca2+ ratio can affect TNAP activity in the aortic wall and, therefore, affect pyrophosphate hydrolysis and the process of calcification [30].
5. ENZYME REACTIONS
Among their many activities, the role played by APs in monophosphate ester hydrolysis suggests their potential influence on phosphorus-associated pathologic conditions, such as vascular calcification. APs have many substrates and participate in many metabolic and biosynthetic pathways [31], as well as being involved in diverse microbial survival mechanisms.
The main role of AP is in the hydrolysis of phosphoric monoesters, which releases inorganic phosphate. The reaction involves the attack of a serine alkoxide on a phosphorus of the substrate to form an enzyme-phosphate complex, followed by hydrolysis of the serine phosphate [32]. Table 1 shows some of the main reactions of and products produced by APs.
Table 1.
Alkaline phosphatase substrates and reactions.
| Substrate | Product |
|---|---|
| R-Phosphate + H20 | R + Phosphate (Pi) |
| Pyrophosphate | Pi+Pi |
| Nucleoside triphosphate | Nucleoside diphosphate + Pi |
| Nucleoside diphosphate | Nucleoside monophosphate + Pi |
| Pyridoxal 5´-phosphate | Pyridoxal + Pi |
| Ethylphosphate | Ethanol + Pi |
| Thiamin monophosphate | Thiamine + Pi |
| Glycerona phosphate | Glycerone + Pi |
| 4-nitrpphenyl phosphate | 4-Nitrophenol + Pi |
6. HYPOPHOSPHATASIA
One of the most common manifestations of the loss of TNAP activity is hypophosphatasia [33, 34], a systemic bone disease characterized by hypomineralization of hard tissues like teeth and structural bones. Severe forms of hypophophatasia can result in respiratory failure and death. Akp2 [35] is one of the genes encoding TNAP in mice, and knockout of this gene results in a suitable animal model of hypophosphatasia. Akp2 knockout mice transfected with a gene encoding human TNAP have also been used to assess the potential role of enzyme replacement therapy in patients with hypophophatasia [36, 37].
7. Role of alkaline phosphatase in vascular calcification
Extracellular pyrophosphate is a potent physicochemical inhibitor of hydroxyapatite crystal formation and growth [17, 38, 39]. Recent studies suggest that plasma pyrophosphate deficiency is associated with vascular calcification [40]. For example, in hemodialysis patients, plasma pyrophosphate concentration is reduced after standard hemodialysis [41, 42]. Moreover, plasma pyrophosphate concentration was 4-fold lower in a mouse model of progeria than in wild-type mice [43]. These reductions in plasmatic pyrophosphate were associated with excessive vascular calcification in the medial layer of the aortic wall. Moreover, daily injections of exogenous pyrophosphate were found to prevent vascular calcification in rats and mice [43-45].
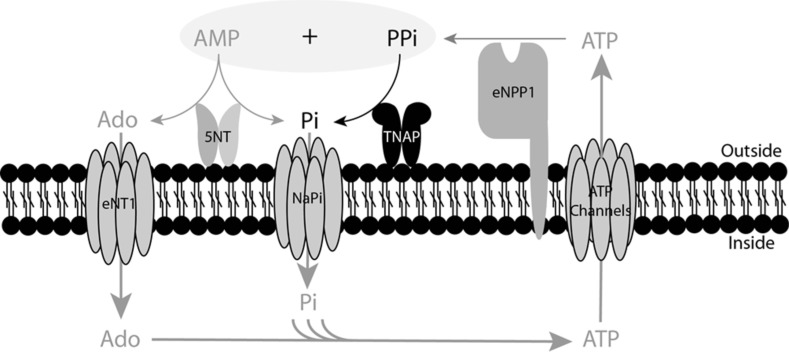
TNAP hydrolyzes pyrophosphate to phosphate (Fig. 2) in extracellular fluids [6, 21]. The addition of AP to culture media is sufficient to cause matrix calcification [46]. Moreover, over-expression of TNAP in cells is sufficient to induce medial vascular calcification in rat aortic rings ex vivo [21]. TNAP activity is increased in models of medial calcification, including uremic rats [23] and a mouse model of Hutchison-Gilford Progeria Syndrome [43]. Moreover, dialysis was recently shown to affect both plasma AP activity [42] and pyrophosphate hydrolysis [47] . Over-expression of TNAP in vivo increases skeletal mineralization [48]. However, phosphatase inhibitors have been shown to prevent vascular smooth muscle calcification in vitro [49, 50] and ablation of phosphatase function induces a loss of skeletal mineralization [51]. Finally, TNAP stimulates vascular smooth muscle cell trans-differentiation into chondrocytes through calcium deposition and BMP-2 activation [52]. Taken together, all of these findings suggest that TNAP could represent a target for the treatment of ectopic calcification in blood vessels [53, 54].
Fig. (2).
Role of TNAP in the extracellular pyrophosphate metabolism. ATP is released by cells via exocytotic mechanisms and through multiple types of membrane channels. Ectonucleotide pyrophosphatase phosphodiesterase (eNPP) hydrolyzes ATP, releasing pyrophosphate (PPi) and adenosine-5’-monophosphate (AMP). PPi is degraded to phosphate (Pi) by tissue non-specific alkaline phosphatase (TNAP). AMP is degraded to adenosine (Ado) and Pi via ecto-5´nucleotidase (5NT). Ado and Pi are recovered from the extracellular space by equilibrative nucleoside transporter 1 (ENT1) and sodium phosphate transporter (NaPi), respectively. ATP is generated in the mitochondria or through another metabolic pathway.
8. GENETICS
Human APs are encoded by four genes, each of which encodes one of the four isozymes, with the ALPL, ALPP, ALPP2, and ALPI genes encoding TNAP, placental AP, germ cell AP and intestinal AP, respectively. ALPL, the gene encoding TNAP, is located on the short arm of chromosome 1 (1p36.1-34), whereas the other genes are present on the long arm of chromosome 2 (2q34-37) [25].
The ALPL gene extends over approximately 40–50 kb of DNA [55] and consists of 12 exons. Exons 2–12 are coding exons, whereas exon 1 consists of two alternative noncoding exons and regulatory motifs in the 5’-untranslated regions, allowing two transcripts to originate from the same coding region. In contrast to ALPL, the genes encoding the tissue specific APs are very compact, occupying less than 5 kb each, and show highly homologous organization [27].
CLINICAL PERSPECTIVE AND CONCLUSION
AP activity has been associated with the occurrence of vascular calcification. The potential role of APs in calcification suggests that inhibition of APs may constitute a potential therapeutic approach to prevent calcification. Studies assessing the effects of inhibitors of AP expression and activity in diseases that manifest excessive vascular calcification are warranted.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
FUNDING
RV-B is supported by grants from the Spanish Ministerio de Economía y Competitividad (SAF-2014-60669) and Progeria Research Foundation (PRF-2016-68) from USA.
REFERENCES
- 1.Villa-Bellosta R., Egido J. Phosphate, pyrophosphate, and vascular calcification: A question of balance. Eur. Heart J. 2017;38:1801–1804. doi: 10.1093/eurheartj/ehv605. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal H.T., Lansing A.I., Wheeler P.A. Calcification of the media of the human aorta and its relation to intimal arteriosclerosis, ageing and disease. Am. J. Pathol. 1944;20:665–687. [PMC free article] [PubMed] [Google Scholar]
- 3.Nicoll R., Henein M.Y. The predictive value of arterial and valvular calcification for mortality and cardiovascular events. Int. J. Cardiol. Heart Vessels. 2014;3:1–5. doi: 10.1016/j.ijchv.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. 2012. [DOI] [PMC free article] [PubMed]
- 5.Shanahan C.M., Crouthamel M.H., Kapustin A., Giachelli C.M. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ. Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villa-Bellosta R. Synthesis of extracellular pyrophosphate increases in vascular smooth muscle cells during phosphate-induced calcification. Arterioscler. Thromb. Vasc. Biol. 2018;38:2137–2147. doi: 10.1161/ATVBAHA.118.311444. [DOI] [PubMed] [Google Scholar]
- 7.Farzaneh-Far A., Proudfoot D., Shanahan C., Weissberg P.L. Vascular and valvar calcification: Recent advances. Heart. 2001;85:13–17. doi: 10.1136/heart.85.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha-Singh K.J., Zeller T., Jaff M.R. Peripheral arterial calcification: Prevalence, mechanism, detection, and clinical implications. Catheter. Cardiovasc. Interv. 2014;83:E212–E220. doi: 10.1002/ccd.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai M.Y., Cremer P.C., Schoenhagen P. Thoracic aortic calcification: Diagnostic, prognostic, and management considerations. JACC Cardiovasc. Imaging. 2018;11:1012–1026. doi: 10.1016/j.jcmg.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Andrews J., Psaltis P.J., Bartolo B.A.D., Nicholls S.J., Puri R. Coronary arterial calcification: A review of mechanisms, promoters and imaging. Trends Cardiovasc. Med. 2018;28:491–501. doi: 10.1016/j.tcm.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Shekar C., Budoff M. Calcification of the heart: Mechanisms and therapeutic avenues. Expert Rev. Cardiovasc. Ther. 2018;16:527–536. doi: 10.1080/14779072.2018.1484282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myasoedova V.A., Ravani A.L., Frigerio B., et al. Novel pharmacological targets for calcific aortic valve disease: Prevention and treatments. Pharmacol. Res. 2018;136:74–82. doi: 10.1016/j.phrs.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Proudfoot D., Shanahan C.M. Biology of calcification in vascular cells: Intima versus media. Herz. 2001;26:245–251. doi: 10.1007/pl00002027. [DOI] [PubMed] [Google Scholar]
- 14.Panh L., Lairez O., Ruidavets J.B., Galinier M., Carrié D., Ferrières J. Coronary artery calcification: From crystal to plaque rupture. Arch. Cardiovasc. Dis. 2017;110:550–561. doi: 10.1016/j.acvd.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Ho C.Y., Shanahan C.M. Medial arterial calcification: An overlooked player in peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2016;36:1475–1482. doi: 10.1161/ATVBAHA.116.306717. [DOI] [PubMed] [Google Scholar]
- 16.Iyemere V.P., Proudfoot D., Weissberg P.L., Shanahan C.M. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J. Intern. Med. 2006;260:192–210. doi: 10.1111/j.1365-2796.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 17.Schibler D., Russell R.G., Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin. Sci. 1968;35:363–372. [PubMed] [Google Scholar]
- 18.Lee S.Y., Müller C.E. Nucleotide pyrophosphatase/phosphodieste-rase 1 (NPP1) and its inhibitors. MedChemComm. 2017;8:823–840. doi: 10.1039/c7md00015d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrakis A., Moolenaar W.H. Autotaxin: Structure-function and signaling. J. Lipid Res. 2014;55:1010–1018. doi: 10.1194/jlr.R046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobryshev Y.V., Orekhov A.N., Sobenin I., Chistiakov D.A. Role of bone-type tissue-nonspecific alkaline phosphatase and PHOSPO1 in vascular calcification. Curr. Pharm. Des. 2014;20:5821–5828. doi: 10.2174/1381612820666140212193011. [DOI] [PubMed] [Google Scholar]
- 21.Villa-Bellosta R., Wang X., Millán J.L., Dubyak G.R., O’Neill W.C. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H61–H68. doi: 10.1152/ajpheart.01020.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutsch F., Vaingankar S., Johnson K., et al. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am. J. Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lomashvili K.A., Garg P., Narisawa S., Millan J.L., O’Neill W.C. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: Potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millán J.L. Alkaline Phosphatases : Structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006;2:335–341. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma U., Pal D., Prasad R. Alkaline phosphatase: An overview. Indian J Clin Biochem IJCB. 2014;29:269–278. doi: 10.1007/s12291-013-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robison R. The possible significance of hexosephosphoric esters in ossification. Biochem. J. 1923;17:286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manes T., Glade K., Ziomek C.A., Millán J.L. Genomic structure and comparison of mouse tissue-specific alkaline phosphatase genes. Genomics. 1990;8:541–554. doi: 10.1016/0888-7543(90)90042-s. [DOI] [PubMed] [Google Scholar]
- 28.Harrison G., Shapiro I.M., Golub E.E. The phosphatidylinositol-glycolipid anchor on alkaline phosphatase facilitates mineralization initiation in vitro. J. Bone Miner. Res. 1995;10:568–573. doi: 10.1002/jbmr.5650100409. [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki K., Hagiwara H. Excess iron inhibits osteoblast metabolism. Toxicol. Lett. 2009;191:211–215. doi: 10.1016/j.toxlet.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Villa-Bellosta R. Impact of magnesium:calcium ratio on calcification of the aortic wall. PLoS One. 2017;12:e0178872. doi: 10.1371/journal.pone.0178872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchet R., Millán J.L., Magne D. Multisystemic functions of alkaline phosphatases. Methods Mol. Biol. 2013;1053:27–51. doi: 10.1007/978-1-62703-562-0_3. [DOI] [PubMed] [Google Scholar]
- 32.Holtz K.M., Kantrowitz E.R. The mechanism of the alkaline phosphatase reaction: Insights from NMR, crystallography and site-specific mutagenesis. FEBS Lett. 1999;462:7–11. doi: 10.1016/s0014-5793(99)01448-9. [DOI] [PubMed] [Google Scholar]
- 33.Conti F., Ciullini L., Pugliese G. Hypophosphatasia: Clinical manifestation and burden of disease in adult patients. Clin. Cases Miner. Bone Metab. 2017;14:230–234. doi: 10.11138/ccmbm/2017.14.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon S., Resch H., Klaushofer K., Roschger P., Zwerina J., Kocijan R. Hypophosphatasia: From diagnosis to treatment. Curr. Rheumatol. Rep. 2018;20:69. doi: 10.1007/s11926-018-0778-5. [DOI] [PubMed] [Google Scholar]
- 35.Millán J.L., Whyte M.P. Alkaline phosphatase and hypophosphatasia. Calcif. Tissue Int. 2016;98:398–416. doi: 10.1007/s00223-015-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whyte M.P., Greenberg C.R., Salman N.J., et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N. Engl. J. Med. 2012;366:904–913. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- 37.Bowden S.A., Foster B.L. Profile of asfotase alfa in the treatment of hypophosphatasia: Design, development, and place in therapy. Drug Des. Devel. Ther. 2018;12:3147–3161. doi: 10.2147/DDDT.S154922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rathan S., Yoganathan A.P., O’Neill C.W. The role of inorganic pyrophosphate in aortic valve calcification. J. Heart Valve Dis. 2014;23:387–394. [PMC free article] [PubMed] [Google Scholar]
- 39.Villa-Bellosta R., O’Neill W.C. Pyrophosphate deficiency in vascular calcification. Kidney Int. 2018;93:1293–1297. doi: 10.1016/j.kint.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 40.Lomashvili K.A., Narisawa S., Millán J.L., O’Neill W.C. Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney Int. 2014;85:1351–1356. doi: 10.1038/ki.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lomashvili K.A., Khawandi W., O’Neill W.C. Reduced plasma pyrophosphate levels in hemodialysis patients. J. Am. Soc. Nephrol. 2005;16:2495–2500. doi: 10.1681/ASN.2004080694. [DOI] [PubMed] [Google Scholar]
- 42.Villa-Bellosta R., González-Parra E., Egido J. Alkalosis and dialytic clearance of phosphate increases phosphatase activity: A hidden consequence of hemodialysis. PLoS One. 2016;11:e0159858. doi: 10.1371/journal.pone.0159858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villa-Bellosta R., Rivera-Torres J., Osorio F.G., et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation. 2013;127:2442–2451. doi: 10.1161/CIRCULATIONAHA.112.000571. [DOI] [PubMed] [Google Scholar]
- 44.Riser B.L., Barreto F.C., Rezg R., et al. Daily peritoneal administration of sodium pyrophosphate in a dialysis solution prevents the development of vascular calcification in a mouse model of uraemia. Nephrol. Dial. Transplant. 2011;26:3349–3357. doi: 10.1093/ndt/gfr039. [DOI] [PubMed] [Google Scholar]
- 45.O’Neill W.C., Lomashvili K.A., Malluche H.H., Faugere M.C., Riser B.L. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011;79:512–517. doi: 10.1038/ki.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lomashvili K.A., Cobbs S., Hennigar R.A., Hardcastle K.I., O’Neill W.C. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J. Am. Soc. Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 47.Azpiazu D., González-Parra E., Egido J., Villa-Bellosta R. Hydrolysis of Extracellular Pyrophosphate increases in post-hemodialysis plasma. Sci. Rep. 2018;8:11089. doi: 10.1038/s41598-018-29432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narisawa S., Yadav M.C., Millán J.L. In vivo overexpression of tissue-nonspecific alkaline phosphatase increases skeletal mineralization and affects the phosphorylation status of osteopontin. J. Bone Miner. Res. 2013;28:1587–1598. doi: 10.1002/jbmr.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narisawa S., Harmey D., Yadav M.C., O’Neill W.C., Hoylaerts M.F., Millán J.L. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J. Bone Miner. Res. 2007;22:1700–1710. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- 50.Kiffer-Moreira T., Yadav M.C., Zhu D., et al. Pharmacological inhibition of PHOSPHO1 suppresses vascular smooth muscle cell calcification. J. Bone Miner. Res. 2013;28:81–91. doi: 10.1002/jbmr.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadav M.C., Simão A.M.S., Narisawa S., et al. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: A unified model of the mechanisms of initiation of skeletal calcification. J. Bone Miner. Res. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fakhry M., Roszkowska M., Briolay A., et al. TNAP stimulates vascular smooth muscle cell trans-differentiation into chondrocytes through calcium deposition and BMP-2 activation: Possible implication in atherosclerotic plaque stability. Biochim. Biophys. Acta. 2017;1863:643–653. doi: 10.1016/j.bbadis.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Haarhaus M., Brandenburg V., Kalantar-Zadeh K., Stenvinkel P., Magnusson P. Alkaline phosphatase: A novel treatment target for cardiovascular disease in CKD. Nat. Rev. Nephrol. 2017;13:429–442. doi: 10.1038/nrneph.2017.60. [DOI] [PubMed] [Google Scholar]
- 54.al-Rashida M., Iqbal J. Therapeutic potentials of ecto-nucleoside triphosphate diphosphohydrolase, ecto-nucleotide pyrophosphatase/phosphodiesterase, ecto-5′-nucleotidase, and alkaline phosphatase inhibitors. Med. Res. Rev. 2014;34(4):703–743. doi: 10.1002/med.21302. [DOI] [PubMed] [Google Scholar]
- 55.Hsu H.H., Anderson H.C. The isolation and partial sequencing of human bone alkaline phosphatase gene. Int. J. Biochem. 1989;21(8):847–851. doi: 10.1016/0020-711x(89)90282-6. [DOI] [PubMed] [Google Scholar]