Abstract
Aims
Glucagon plays pivotal roles in systemic glucose homeostasis mainly by promoting hepatic glucose output. Using a sandwich enzyme-linked immunosorbent assay (ELISA), we evaluated fasting plasma glucagon levels in hospitalized patients with type 1 or type 2 diabetes, and assessed the relationships between glucagon levels and various clinical parameters.
Methods
We enrolled adult Japanese diabetes patients admitted to Osaka University Medical Hospital for glycemic control between July 2017 and May 2018 in this study. After patients had fasted for 12 h, blood samples were obtained and plasma glucagon levels were measured using a sandwich ELISA.
Results
Total 107 patients participated in the study. The mean fasting plasma glucagon level of patients with acute onset type 1 diabetes was significantly lower than that of patients with type 2 diabetes (p < 0.05). Plasma glucagon levels were not significantly correlated with plasma glucose levels in patients with type 1 diabetes or in patients with type 2 diabetes. Multiple regression analysis indicated that fasting glucagon levels were independently and significantly correlated with fasting serum C-peptide levels in patients with type 2 diabetes.
Conclusions
Our results suggest that insulin and glucagon secretion are balanced in the fasting state in patients with type 2 diabetes.
Keyword: Metabolism
1. Introduction
Glucagon is secreted by pancreatic alpha cells and plays pivotal roles in systemic glucose homeostasis. Glucagon promotes hepatic glucose output by enhancing glycogenolysis and gluconeogenesis, thereby contributes to a counteraction to hypoglycemia [1, 2]. In patients with type 2 diabetes, elevated fasting glucagon levels and paradoxical increases of glucagon in the hyperglycemic state have been reported [2, 3, 4, 5, 6]. In patients with type 1 diabetes, elevated plasma glucagon levels under hyperglycemia and defective responses of glucagon to hypoglycemia have also been reported [7, 8]. Moreover, glucagon is one of the important therapeutic targets for diabetes by dipeptidyl-peptidase IV (DPP-4) inhibitor and glucagon-like peptide-1 (GLP-1) analog which are currently used worldwide to treat diabetes. Consequently, the clinical evaluation of plasma glucagon levels is important for appropriate application of therapeutic methods in diabetes.
In this study, we evaluated fasting plasma glucagon levels in hospitalized patients with type 1 or type 2 diabetes by using the sandwich enzyme-linked immunosorbent assay (ELISA), and analyzed the relationships between glucagon levels and other clinical parameters to clarify the clinical significance of glucagon in diabetes.
2. Materials and methods
2.1. Participants
A total of 148 adult Japanese diabetes patients admitted to Osaka University Medical Hospital for glycemic control between July 2017 and May 2018 and whose fasting plasma glucagon levels were measured after admission were enrolled in the study. Patients receiving treatment for cancer; patients with severe renal function disorder (estimated glomerular filtration rate <30 mL/min/1.73m2); and patients with secondary diabetes caused by endocrine diseases, drugs, or pancreatic resection were excluded from the study. Type 1 diabetes patients were categorized as having acute-onset type 1 diabetes, fulminant type 1 diabetes or slowly progressive type 1 diabetes according to the diagnostic criteria and clinical history [9, 10, 11]. Type 2 diabetes patients were also diagnosed according to the diagnostic criteria [12]. The study protocol was approved by the Ethical Review Board of Osaka University Hospital (No. 16136). The study was carried out following the tenets of the Declaration of Helsinki and provided patients with the right to opt out.
2.2. Clinical evaluation
Detailed information on clinical parameters, medical history, and current treatment were obtained on patient admission. Between the second and fourth day of admission, a fasting blood sample was obtained after the patient had fasted for 12 h. Fasting plasma glucose levels were measured using the hexokinase glucose-6-phosphate dehydrogenase method. Serum C-peptide levels were measured using a chemiluminescent immunoassay. Fasting plasma glucagon levels were measured using the sandwich ELISA (Mercodia, Uppsala, Sweden).
2.3. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics Version 22 (SPSS, Chicago, IL, USA). Descriptive statistics were calculated. Differences in continuous variables between two groups were assessed using the Mann-Whitney U test. The comparison of mean values between multiple groups was performed using ANOVA. If the difference in group mean values was significant according to ANOVA, post hoc pairwise group comparisons were conducted using the Scheffé test. Simple linear regression analyses were performed between fasting plasma glucose and glucagon levels and between fasting C-peptide and glucagon levels. Because the fasting serum C-peptide level of many patients with type 1 diabetes was below the minimum detection level (<0.1 ng/mL), simple linear regression analysis was not performed between fasting C-peptide and glucagon levels in patients with type 1 diabetes. Pearson's correlation coefficients and p values were calculated to assess the associations between fasting glucagon levels and other variables. To identify independent factors associated with fasting glucagon levels of patients with type 2 diabetes, multiple linear regression analysis was performed using sex and fasting C-peptide levels as explanatory variables. Because the population size of patients with type 1 diabetes was small (n = 21), multiple linear regression analysis was not performed in this group. However, multiple linear regression analysis was performed in patients with type 2 diabetes. The results are expressed as means±standard deviations (S.D.), and p < 0.05 was considered statistically significant.
3. Results
Total 107 patients participated in the study. The clinical characteristics of the subjects are shown in Table 1. The fasting glucagon levels were 14 ± 10 pg/mL in patients with acute-onset type 1 diabetes (n = 9), 11 ± 2 pg/mL in fulminant type 1 diabetes (n = 4), and 25 ± 15 pg/mL in slowly progressive type 1 diabetes (n = 8). The glucagon level of all patients with type 1 diabetes (17 ± 12 pg/mL, n = 21) was significantly lower than that of patients with type 2 diabetes (29 ± 15 pg/mL, n = 86; p < 0.01). Among patients with acute-onset type 1 diabetes, fulminant type 1 diabetes, slowly progressive type 1 diabetes, and type 2 diabetes, the glucagon level of patients with acute-onset type 1 diabetes was significantly lower than that of patients with type 2 diabetes (p = 0.04).
Table 1.
Clinical characteristics of the subjects.
| N | Total |
Type 1 |
Type 2 |
p value∗ |
|---|---|---|---|---|
| 107 | 21 | 86 | ||
| Sex (Male/Female) | 45/64 | 7/14 | 36/50 | 0.76 |
| Age (years) | 66 ± 13 | 62 ± 14 | 67 ± 13 | 0.15 |
| Duration of Diabetes (years) | 16 ± 12 | 22 ± 15 | 15 ± 11 | 0.01 |
| Body Mass Index (kg/m2) | 25.8 ± 5.3 | 22.7 ± 4.7 | 26.5 ± 5.1 | 0.01 |
| Fasting plasma glucose (mg/dL) | 146 ± 49 | 140 ± 75 | 148 ± 42 | 0.25 |
| Fasting serum C-peptide (ng/mL) | 1.4 ± 1.0 | 0.4 ± 0.8 | 1.7 ± 0.9 | <0.01 |
| Fasting plasma glucagon (pg/mL) | 26 ± 15 | 17 ± 12 | 29 ± 15 | <0.01 |
| HbA1c (mmol/mol) | 73 ± 19 | 73 ± 21 | 75 ± 17 | 0.89 |
| Treatment of Diabetes | ||||
| Dipeptidyl-peptidase IV inhibitor | 50 | |||
| Glucagon-like peptide-1 receptor agonist | 5 | |||
| Sodium glucose cotransporter 2 inhibitor | 8 | |||
| Insulin injection | 44 | |||
| Others | 45 | |||
Significant differences between patients with type 1 diabetes and with type 2 diabetes.
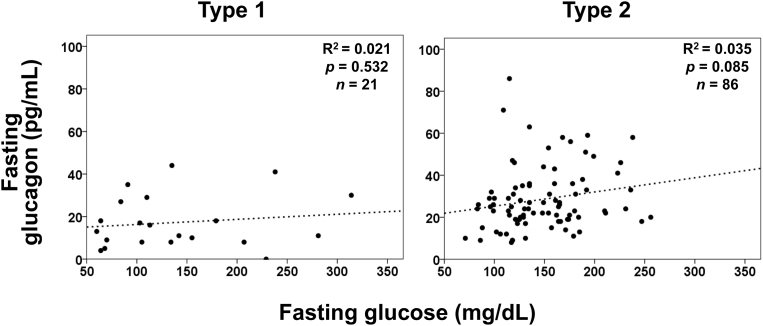
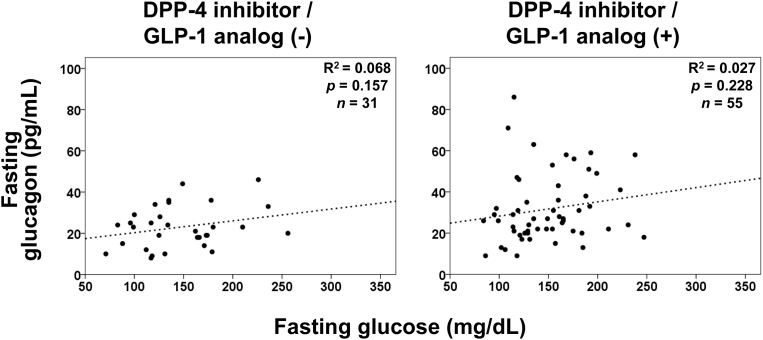
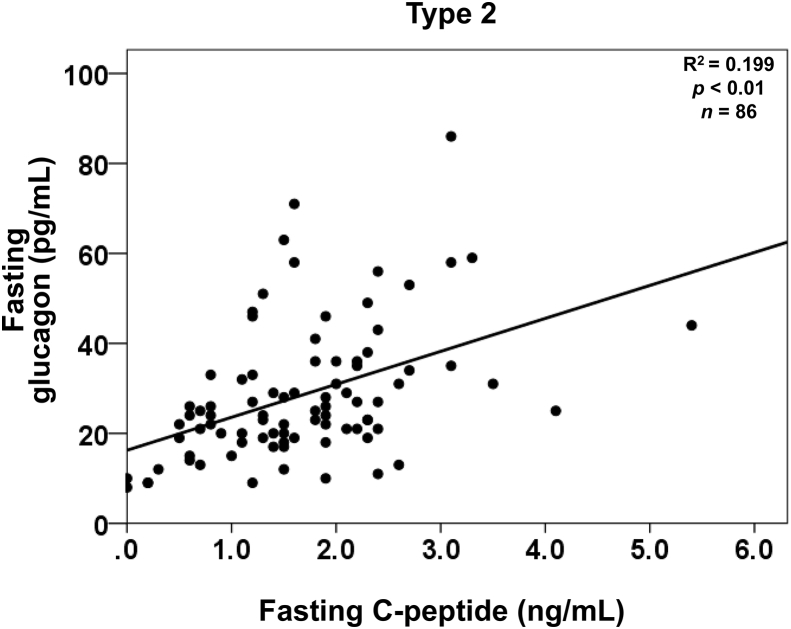
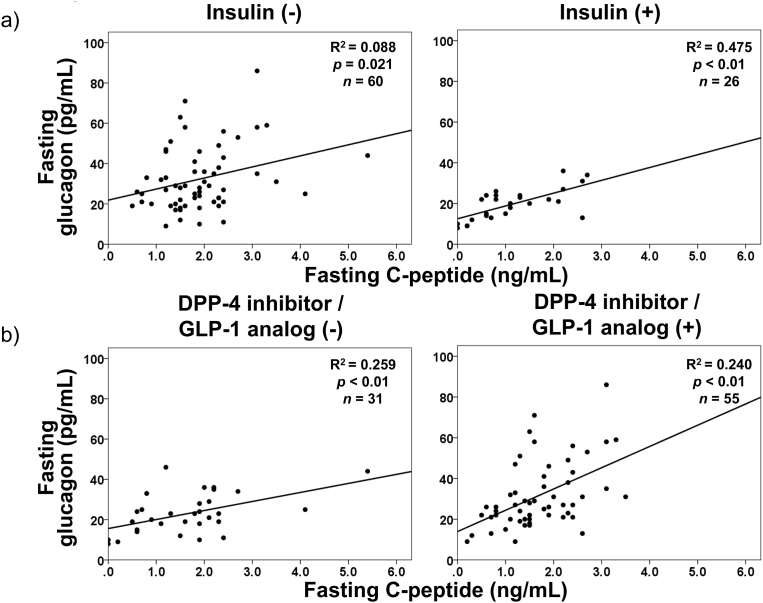
Glucagon and glucose levels were not correlated in patients with type 1 diabetes or in patients with type 2 diabetes, according to simple linear regression analyses (Fig. 1). It is widely recognized that incretin-based therapies affect both insulin secretion and glucagon suppression. However, the glucagon level was not significantly correlated with glucose regardless of treatment with DPP-4 inhibitors or GLP-1 analog in patients with type 2 diabetes (Fig. 2). In contrast, fasting plasma glucagon levels were significantly correlated with serum C-peptide levels in patients with type 2 diabetes (Fig. 3), regardless of the treatment method (Fig. 4). In simple correlation analysis, sex exhibited a significant correlation with glucagon levels as well as fasting C-peptide levels, but no other clinical parameters did (Table 2). In multiple linear regression analysis including sex and C-peptide levels, C-peptide levels were independently and significantly correlated with glucagon levels (Table 2).
Fig. 1.
Simple linear regression analyses between fasting glucose and glucagon levels in patients with type 1 diabetes and type 2 diabetes.
Fig. 2.
Simple linear regression analyses between fasting glucose and glucagon levels in patients with type 2 diabetes dividing into under treatment with or without DPP-4 inhibitor or GLP-1 analog.
Fig. 3.
Simple linear regression analyses between fasting C-peptide and glucagon levels in patients with type 2 diabetes.
Fig. 4.
Simple linear regression analyses between fasting C-peptide and glucagon levels in patients with type 2 diabetes dividing into under treatment with or without insulin (a) and into under treatment with or without DPP-4 inhibitor or GLP-1 analog (b).
Table 2.
Simple correlation and multiple linear regression analyses between fasting glucagon levels and other variables in patients with type 2 diabetes.
| Simple correlation |
Multiple linear regression analysis |
|
|---|---|---|
| R (p value) | beta (p value) | |
| Sex | - 0.246 (0.02) | |
| Age | 0.139 (0.20) | |
| Duration of Diabetes | - 0.164 (0.13) | |
| Body Mass Index | 0.187 (0.09) | |
| Fasting glucose | 0.187 (0.09) | |
| Fasting C-peptide | 0.446 (<0.01) | 0.446 (<0.01) |
| HbA1c | 0.037 (0.74) |
4. Discussion
In this study, we showed that the mean fasting glucagon level of patients with acute onset type 1 diabetes was significantly lower than that of patients with type 2 diabetes. This is the first report, to our knowledge, to have evaluated and compared the fasting plasma glucagon levels of patients with type 1 and type 2 diabetes using the sandwich ELISA.
Plasma glucagon levels have long been measured using a radioimmunoassay (RIA) employing polyclonal antibodies against the glucagon C-terminus. However, the assessment of glucagon dynamics is required to be revised using recent advanced ELISA system [13]. This system uses monoclonal antibodies against both the glucagon N-terminus and C-terminus and exhibits improved accuracy [14, 15]. This sandwich ELISA assay system is expected to contribute to the progress in understanding of the pathophysiology of glucagon in diabetes. However, clinical data in patients with diabetes evaluated using this sandwich ELISA remain insufficient. We evaluated fasting plasma glucagon levels in hospitalized patients with type 1 or type 2 diabetes by using the sandwich ELISA and revealed the following new insight.
The glucagon levels in acute onset type 1 diabetes patients is significantly lower than that of type 2 diabetes patients. Currently, the underlying mechanism of this difference remains unclear. Physiological glucagon secretion is regulated by various inputs, including the autonomic nervous system and the paracrine effect of neighboring beta cells [16]. For autonomic nervous system, the glucagon secretion might not be appropriately stimulated, even in the fasting state, due to defective function of this system related to diabetic neuropathy. For paracrine effect of neighboring beta cells, glucagon secretion might be impaired due to lack of paracrine control resulting from the destruction or severe impairment of beta cells in acute onset type 1 diabetes. Indeed, the glucagon level tended to be higher in patients with slowly progressive type 1 diabetes in which beta cells and insulin secretion are relatively remaining. Further studies are necessary to clarify the underlying mechanism, but our finding of significantly lower fasting glucagon levels in acute onset type 1 diabetes could, at least partly, explain the fragility to hypoglycemia in acute onset type 1 diabetes patients compared with type 2 diabetes.
No correlation was found between glucagon and glucose levels in this study. This finding suggests that glucagon levels were not regulated by plasma glucose levels in patients with diabetes. Especially, the glucagon level was not suppressed when the fasting glucose level was high and was not elevated when the glucose level was low in patients with type 1 and type 2 diabetes. In previous reports, fasting glucagon levels measured by RIA were elevated despite hyperglycemic states in patients with type 2 diabetes [3], suggesting its involvement in the fasting hyperglycemia of diabetes. The clinical data of glucagon levels evaluated by the conventional RIA method now need to be confirmed or revised using other methods with higher accuracy. Our current study using sandwich ELISA confirmed the presence of dysregulated glucagon secretion in diabetes patients. Our findings also support the previously proposed concept of the impact of dysregulated glucagon upon both hyper- and hypo-glycemia in diabetes. Indeed, another study evaluating fasting plasma glucagon levels reported no significant correlation between plasma glucagon and glucose levels, further validating our results [17]. In addition to our results for fasting patients, we recently reported no correlation of glucagon and glucose levels in type 1 diabetes patients at any time, thereby highlighting totally dysregulated glucagon secretion in diabetes [18]. These results further emphasize the pathophysiological impact of dysregulated glucagon on glycemic control in diabetes.
In our study, fasting glucagon levels were significantly and positively correlated with C-peptide levels in patients with type 2 diabetes. Previously, Sharma et al. reported a weak correlation in 310 non-diabetic subjects [19]. Færch et al. suggested that fasting glucagon levels increased together with insulin levels in individuals with normal glucose tolerance, individuals with impaired glucose tolerance, and patients with type 2 diabetes [20]. Because both studies evaluated plasma glucagon levels using RIA, it is necessary to re-measure glucagon levels using other methods with improved accuracy. Our results were obtained by ELISA, and are therefore likely more accurate and reliable than those in previous reports. The data in Figs. 3 and 4 showing significant associations between C-peptide and glucagon suggest that the secretion of insulin and glucagon are certainly balanced by their interaction. The primary target of both insulin and glucagon is the liver, and this organ is responsible for maintaining the systemic energy status, especially in the fasting state. Glucagon enhances hepatic glucose output and amino acid metabolism, thereby providing energy for the whole body. Insulin enhances systemic glucose uptake to provide energy to organs. Consequently, well-balanced coordination of these two important hormones would be necessary to efficiently control the whole-body energy turnover, especially in the fasting state, which can be considered an energy-deprived state [21]. Further investigations are necessary to clarify the underlying mechanism of this balance and its physiological significance.
The current study has some limitations. The sample size is small, especially for type 1 diabetes patients. The plasma glucagon levels of individuals with normal glucose tolerance were not evaluated. Glucagon was measured once in the fasting state only; it was not measured at other times, e.g. after the intake of a test meal or 75-g glucose. We did not evaluate serum immunoreactive insulin and other clinical parameters such as homeostatic model assessment (HOMA) insulin resistance and HOMA-beta that are calculated using the immunoreactive insulin level, because some patients were treated with basal insulin. However, our study has certain merits that plasma glucagon levels were measured in standardized hospitalized conditions after patients had fasted for 12 h, thereby eliminating the influence of food intake and exercise. Moreover, we evaluated plasma glucagon levels using the sandwich ELISA, which has higher accuracy than conventional RIAs. Studies involving a large number of participants and additional measurement points might further strengthen our current findings and contribute to progress in understanding the pathophysiological role of glucagon in diabetes.
5. Conclusions
In the current study, we found a difference in the fasting glucagon levels between patients with acute onset type 1 diabetes and patients with type 2 diabetes by using a sandwich ELISA for the first time. Interestingly, fasting glucagon levels were significantly correlated with serum C-peptide levels in patients with type 2 diabetes. Our results can be a clue to find the suitable therapeutic methods for diabetes patients whose condition widely varies. Previous reports indicate that patients with type 2 diabetes effective of DPP-4 inhibitor or GLP-1 analog exhibit significantly higher fasting serum C-peptide levels [22, 23, 24]. In other words, these higher serum C-peptide levels correspond to higher plasma glucagon levels according to our current results. Consequently, it is possible that these incretin-based therapies have a significant suppressive effect on higher glucagon secretion, as well as enhancing insulin secretion, thereby leading to better glycemic control. Additional studies are necessary to confirm and further clarify the clinical implications of fasting plasma glucagon levels.
Declarations
Author contribution statement
Yoshiya Hosokawa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Junji Kozawa, Dan Kawamori, Hiromi Iwahashi: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hitoshi Nishizawa, Norikazu Maeda, Michio Otsuki, Taka-aki Matsuoka, Iichiro Shimomura: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank David Smallbones, BSc, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1.Ramnanan C.J., Edgerton D.S., Kraft G., Cherrington A.D. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes. Metab. 2011;13:118–125. doi: 10.1111/j.1463-1326.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unger R.H., Cherrington A.D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J. Clin. Investig. 2012;122:4–12. doi: 10.1172/JCI60016. https://www.jci.org/articles/view/60016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knop F.K., Aaboe K., Vilsbøll T., Vølund A., Holst J.J., Krarup T., Madsbad S. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes. Metab. 2012;14:500–510. doi: 10.1111/j.1463-1326.2011.01549.x. [DOI] [PubMed] [Google Scholar]
- 4.Müller W.A., Faloona G.R., Aguilar-Parada E., Unger R.H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N. Engl. J. Med. 1970;283:109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- 5.Kawai K., Murayama Y., Okuda Y., Yamashita K. Postprandial glucose, insulin and glucagon responses to meals with different nutrient compositions in non-insulin-dependent diabetes mellitus. Endocrinol. Jpn. 1987;34:745–753. doi: 10.1507/endocrj1954.34.745. https://www.jstage.jst.go.jp/article/endocrj1954/34/5/34_5_745/_article [DOI] [PubMed] [Google Scholar]
- 6.Kozawa J., Okita K., Iwahashi H., Yamagata K., Imagawa A., Shimomura I. Early postprandial glucagon surge affects postprandial glucose levels in obese and non-obese patients with type 2 diabetes. Endocr. J. 2013;60:813–818. doi: 10.1507/endocrj.ej13-0018. https://www.jstage.jst.go.jp/article/endocrj/60/6/60_EJ13-0018/_article [DOI] [PubMed] [Google Scholar]
- 7.Sherr J., Xing D., Ruedy K.J., Beck R.W., Kollman C., Buckingham B., White N.H., Fox L., Tsalikian E., Weinzimer S., Arbelaez A.M., Tamborlane W.V. Diabetes in Children Network. Lack of association between residual insulin production and glucagon response to hypoglycemia in youth with short duration of type 1 diabetes. Diabetes Care. 2013;36:1470–1476. doi: 10.2337/dc12-1697. http://care.diabetesjournals.org/content/36/6/1470.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherr J., Tsalikian E., Fox L., Buckingham B., Weinzimer S., Tamborlane W.V., White N.H., Arbelaez A.M., Kollman C., Ruedy K.J., Cheng P., Beck R.W. Diabetes Research in Children Network. Evolution of abnormal plasma glucagon responses to mixed-meal feedings in youth with type 1 diabetes during the first 2 years after diagnosis. Diabetes Care. 2014;37:1741–1744. doi: 10.2337/dc13-2612. http://care.diabetesjournals.org/content/37/6/1741.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawasaki E., Maruyama T., Imagawa A., Awata T., Ikegami H., Uchigata Y., Osawa H., Kawabata Y., Kobayashi T., Shimada A., Shimizu I., Takahashi K., Nagata M., Makino H., Hanafusa T. Diagnostic criteria for acute-onset type 1 diabetes mellitus (2012): report of the committee of Japan diabetes society on the research of fulminant and acute-onset type 1 diabetes mellitus. J. Diabetes Investig. 2014;5:115–118. doi: 10.1111/jdi.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imagawa A., Hanafusa T., Awata T., Ikegami H., Uchigata Y., Osawa H., Kawasaki E., Kawabata Y., Kobayashi T., Shimada A., Shimizu I., Takahashi K., Nagata M., Makino H., Maruyama T. Report of the committee of the Japan diabetes society on the research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012) J. Diabetes Investig. 2012;3:536–539. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka S., Ohmori M., Awata T., Shimada A., Murao S., Maruyama T., Kamoi K., Kawasaki E., Nakanishi K., Nagata M., Fujii S., Ikegami H., Imagawa A., Uchigata Y., Okubo M., Osawa H., Kajio H., Kawaguchi A., Kawabata Y., Satoh J., Shimizu I., Takahashi K., Makino H., Iwahashi H., Miura J., Yasuda K., Hanafusa T., Kobayashi T. Committee on type 1 diabetes. Diagnostic criteria for slowly progressive insulin-dependent (type 1) diabetes mellitus (SPIDDM) (2012): report by the committee on slowly progressive insulin-dependent (type 1) diabetes mellitus of the Japan diabetes society. Diabetol. Int. 2015;6:1–7. https://link.springer.com/article/10.1007/s13340-014-0199-2 [Google Scholar]
- 12.Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Seino Y., Nanjo K., Tajima N., Kadowaki T., Kashiwagi A., Araki E., Ito C., Inagaki N., Iwamoto Y., Kasuga M., Hanafusa T., Haneda M., Ueki K. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bak M.J., Albrechtsen N.W., Pedersen J., Hartmann B., Christensen M., Vilsbøll T., Knop F.K., Deacon C.F., Dragsted L.O., Holst J.J. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur. J. Endocrinol. 2014;170:529–538. doi: 10.1530/EJE-13-0941. https://eje.bioscientifica.com/view/journals/eje/170/4/529.x [DOI] [PubMed] [Google Scholar]
- 14.Wewer Albrechtsen N.J., Hartmann B., Veedfald S., Windeløv J.A., Plamboeck A., Bojsen-Møller K.N., Idorn T., Feldt-Rasmussen B., Knop F.K., Vilsbøll T., Madsbad S., Deacon C.F., Holst J.J. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014;57:1919–1926. doi: 10.1007/s00125-014-3283-z. https://link.springer.com/article/10.1007%2Fs00125-014-3283-z [DOI] [PubMed] [Google Scholar]
- 15.Miyachi A., Kobayashi M., Mieno E., Goto M., Furusawa K., Inagaki T., Kitamura T. Accurate analytical method for human plasma glucagon levels using liquid chromatography-high resolution mass spectrometry: comparison with commercially available immunoassays. Anal. Bioanal. Chem. 2017;409:5911–5918. doi: 10.1007/s00216-017-0534-0. https://link.springer.com/article/10.1007%2Fs00216-017-0534-0 [DOI] [PubMed] [Google Scholar]
- 16.Kawamori D., Welters H.J., Kulkarni R.N. Molecular pathways underlying the pathogenesis of pancreatic alpha-cell dysfunction. Adv. Exp. Med. Biol. 2010;654:421–445. doi: 10.1007/978-90-481-3271-3_18. [DOI] [PubMed] [Google Scholar]
- 17.Niwano F., Hiromine Y., Noso S., Babaya N., Ito H., Yasutake S., Matsumoto I., Takeyama Y., Kawabata Y., Ikegami H. Insulin deficiency with and without glucagon: a comparative study between total pancreatectomy and type 1 diabetes. J. Diabetes Investig. 2018;9:1084–1090. doi: 10.1111/jdi.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamori D., Katakami N., Takahara M., Miyashita K., Sakamoto F., Yasuda T., Matsuoka T.A., Shimomura I. Dysregulated plasma glucagon levels in Japanese young-adult type 1 diabetes patients. J. Diabetes Investig. 2018 doi: 10.1111/jdi.12862. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma A., Varghese R.T., Shah M., Man C.D., Cobeli C., Rizza R.A., Bailey K.R., Vella A. Impaired insulin action is associated with increased glucagon concentrations in nondiabetic humans. J. Clin. Endocrinol. Metab. 2018;103:314–319. doi: 10.1210/jc.2017-01197. https://academic.oup.com/jcem/article/103/1/314/4590235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Færch K., Vistisen D., Pacini G., Torekov S.S., Johansen N.B., Witte D.R., Jonsson A., Pedersen O., Hansen T., Lauritzen T., Jørgensen M.E., Ahrén B., Holst J.J. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes. 2016;65:3473–3481. doi: 10.2337/db16-0240. http://diabetes.diabetesjournals.org/cgi/pmidlookup?view=long&pmid=27504013 [DOI] [PubMed] [Google Scholar]
- 21.Campbell J.E., Drucker D.J. Islet α cells and glucagon—critical regulators of energy homeostasis. Nat. Rev. Endocrinol. 2015;11:329–338. doi: 10.1038/nrendo.2015.51. https://www.nature.com/articles/nrendo.2015.51 [DOI] [PubMed] [Google Scholar]
- 22.Kozawa J., Inoue K., IwamotoR, Kurashiki Y., Okauchi Y., Kashine S., Kitamura T., Maeda N., Okita K., Iwahashi H., Funahashi T., Imagawa A., Shimomura I. Liraglutide is effective in type 2 diabetic patients with sustained endogenous insulin-secreting capacity. J. Diabetes Investig. 2012;3:294–297. doi: 10.1111/j.2040-1124.2011.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozawa J., Kitamura T., Nishizawa H., Yasuda T., Maeda N., Otsuki M., Okita K., Iwahashi H., Kaneto H., Funahashi T., Imagawa A., Shimomura I. Dipeptidyl peptidase-4 inhibitors are effective in Japanese type 2 diabetic patients with sustained endogenous insulin-secreting capacity, a higher body mass index and insulin resistance. J. Diabetes Investig. 2013;4:190–194. doi: 10.1111/jdi.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usui R., Sakuramachi Y., Seino Y., Murotani K., Kuwata H., Tatsuoka H., Hamamoto Y., Kurose T., Seino Y., Yabe D. Retrospective analysis of liraglutide and basal insulin combination therapy in Japanese type 2 diabetes patients: the association between remaining β-cell function and the achievement of the glycated hemoglobin target 1 year after initiation. J. Diabetes Investig. 2018;9:822–830. doi: 10.1111/jdi.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]