Abstract
Introduction:
Ivacaftor is a CFTR potentiator that improves pulmonary function in cystic fibrosis (CF) patients with at least one copy of the G551D CFTR mutation. The purpose of this study is to evaluate the impact of ivacaftor on chronic rhinosinusitis (CRS) symptoms in this population.
Methods:
The G551D Observational (GOAL) study was a multicenter prospective cohort study enrolling CF patients ≥ 6 years with at least 1 G551D mutation. Subjects were provided SNOT-20 questionnaires prior to ivacaftor therapy and at 1, 3, and 6-months (m) afterwards. The impact on rhinologic(R), psychological(P), sleep(S), and ear/facial(E) quality of life (QOL) domains was evaluated separately.
Results:
129 of 153 (84%) subjects completed all questionnaires. Typical baseline symptom burden was low (75% with scores < 1) and degree of improvement (i.e. reduced scores) was greater with higher baseline scores. SNOT-20 decreased, reflecting improvement, at all follow up intervals (1 m, −0.25(0.53), p<0.01; 3 m, −0.29(0.58), p<0.01; 6 m, −0.21(0.58), p=0.02), but less than the pre-specified minimal clinically important difference (0.8). Significant improvement was observed at 1, 3 and 6 m in R (1m −0.24,p<0.01; 3m −0.34,p<0.01; 6m −0.25,p<0.01) and P domains (1m −0.25,p<0.01; 3m −0.32,p<0.01; 6m −0.26,p<0.01), and 1 and 3 m in S domain (1m −0.35,p<0.01; 3m −0.32,p<0.01; 6m −0.18,p=0.07). There was no improvement in E domain at any time point.
Conclusion:
Ivacaftor improves R, P, and S QOL in G551D CF patients, although QOL instruments validated for CRS may not translate well to CF CRS patients since symptom burden was surprisingly low.
Keywords: Chronic sinusitis, quality of life, cystic fibrosis, paranasal sinus disease, chronic disease
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease that affects fluid and electrolyte transport in the upper and lower aerodigestive tracts. It is the most common autosomal recessive disease affecting the Caucasian population (1). The underlying pathophysiology of the disease is related to abnormal function or deficiency of the CF transmembrane conductance regulator (CFTR), an apical membrane anion transport channel (2). There are several different mechanisms by which the CFTR channel can be disrupted, including class I mutations resulting in defective synthesis, class II mutations which lead to defective processing, class III mutations that cause decreased open time of the channel, class IV mutations that interfere with conductance of the channel, class V mutations that result in unstable or insufficient channels, and class VI mutations that produce normal protein but have increased turnover at the cell membrane (3,4). The G551D mutation is a class 3 mutation that results in diminished ATP binding and hydrolysis, which effectively locks the channel in the closed position (5).
The downstream effects of dysfunctional CFTR result in decreased airway surface liquid, thickened, more viscous mucus, and decreased mucociliary transport (6). Clinically, this leads to chronic infection of the upper and lower respiratory tracts, pancreatic insufficiency, and malnutrition due to obstruction of the exocrine secretory glands (7). The sinonasal epithelium is also composed of these same exocrine glands that, when dysfunctional, lead to disruption of the normal mucociliary clearance pathway (8). Abnormal anion transport causes a disruption of the normal lining of the sinonasal epithelium, the superficial mucus layer and the periciliary low viscosity layer (9). The downstream effects of this include high viscosity secretions that obstruct normal sinus ostia leading to hypoxia, mucosal edema and bacterial overgrowth (10).
In the past, therapy for CF has focused on managing the downstream effects of the dysfunctional CFTR channel. However, recent technological advances have allowed researchers to identify the molecular basis of receptor dysfunction, thereby opening the door for targeted drug therapy. Ivacaftor is a novel therapeutic that targets the CFTR channel directly, increasing the probability of the open phase of the channel. This CFTR potentiator unlocks the closed state of the channel and permits activation of the channel via the normal cAMP/PKA signaling pathway (11). The advent of CFTR modulator therapy provides the opportunity to evaluate the effects of augmented CFTR function in patients. Initially approved for only the G551D mutation, ivacaftor imparts significant improvement in pulmonary symptoms and function (11-13). Studies have shown the rate of decline of pulmonary function is almost half that of patients managed with standard therapy (12). Extensive research has been conducted to evaluate the benefit of CFTR modulation on pulmonary function. The G551D Observational (GOAL) study reported a number of physiologic and clinical improvements in G551D CF patients treated with ivacaftor. CF related quality of life (QOL) was improved; however, the study did not focus on improvements in sinus disease (13). The objective of the current study is to evaluate the potential benefit of ivacaftor on sinonasal QOL in CF patients with at least one copy of the G551D mutation by using the patient cohort included in the GOAL study.
Methods
The GOAL study was a multicenter prospective longitudinal cohort study in 2012-2013 (12). The study comprised patients age 6 years and older with CF, at least one G551D mutation, and no previous exposure to ivacaftor. These patients were initiated on ivacaftor in 2012-2013 after FDA approval of the drug for patients with G551D mutations. As part of the study, baseline completion of the SNOT-20 questionnaire was performed prior to initiation of ivacaftor therapy (14). Participants were then presented the questionnaire again at 1, 3 and 6 months after starting the drug. SNOT-20 was utilized in this study, as opposed to the updated SNOT-22, as at the outset of this study it was the more established and published questionnaire.
Baseline composite average score per question was compared to composite average score per question at the 1, 3, and 6-month follow-ups. Paired t-test analysis of the change from baseline was used to determine the significance of improvement in the composite scores during the follow up period. The questionnaire was then divided into 4 subsets, as had been previously reported in the literature (15). The first 10 questions represent physical symptoms, and these were further subdivided into rhinologic symptoms (need to blow nose, sneezing, runny nose, postnasal drainage, and thick nasal discharge) and ear/facial symptoms (ear fullness, dizziness, ear pain, and facial pain/pressure). The second 10 questions represent QOL measures, and these were further subdivided into sleep function (difficulty falling asleep, waking up at night, and lack of a good night’s sleep) and psychological function (fatigue, reduced productivity, reduced concentration, frustration/restlessness/irritability, sadness, embarrassment). Mean scores of the 4 subsets were then analyzed. Baseline scores were compared to the 1, 3, and 6-month follow up scores using linear regression with generalized estimating equations to account for multiple observations per person.
Results
A total of 153 patients were initially enrolled in the study and 151 patients started ivacaftor. Overall, 133 patients had documentation of follow up at all study intervals, and 129 of those patients had full completion of all SNOT-20 questionnaires.
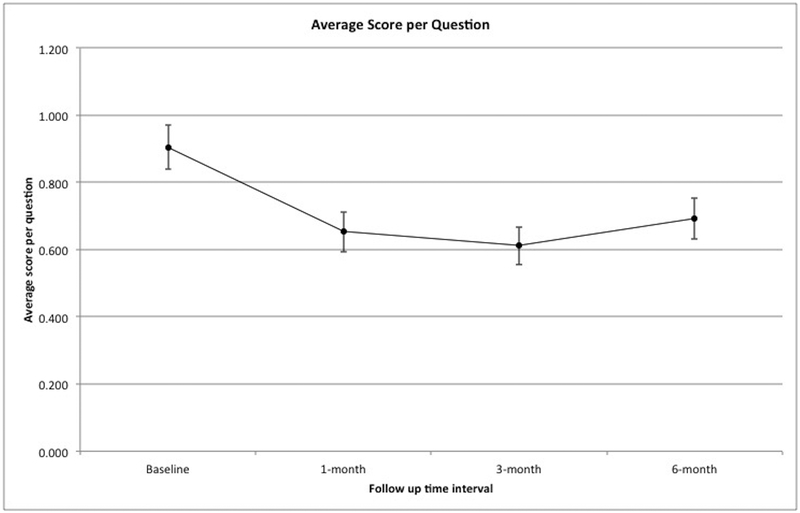
Overall the average score per question was low at baseline (mean 0.90, std dev 0.75). This correlates to an average total SNOT-20 score of 18.09, std dev 14.99. This represents an overall low symptom burden from a sinonasal standpoint. Figure 1 shows average score per question at all time intervals, as well as average change from baseline. There was significant improvement at all follow up time intervals (1 month – mean 0.65, std dev 0.68, mean change −0.25, p-value < 0.01; 3 month – mean 0.61, std dev 0.64, mean change −0.29, p value <0.01; 6 month – mean 0.69, std dev 0.70, mean change −0.21, p value = 0.02).
Figure 1:
Average score per question at all time intervals.
The subset components and individual scores of the questionnaire at all time intervals are listed in Table 1. The subset with the highest mean baseline score was the sleep QOL subset, 1.13. “Lack a good nights sleep” was the highest scoring component of the sleep subset with a score of 1.30. The lowest scoring component in the sleep subset was “Difficulty falling asleep” with an average score of 1.01. Baseline scores of the rhinology subset ranged from 0.66 to 1.34, with the mean being 1.04. The highest scoring component in the rhinology symptom subset was “Need to blow nose” at 1.34, and the lowest scoring was “Thick nasal drainage” at 0.66. The psychological QOL and ear/face symptom subsets were the lowest scoring with average baseline scores of 0.80 and 0.31, respectively.
Table 1.
Subset Components – Mean scores
| Rhinology Subset | Baseline mean (SD) |
1 month mean (SD) |
3 month mean (SD) |
6 month mean (SD) |
|---|---|---|---|---|
| Need to blow nose | 1.34(1.15) | 1.06(1.04) | 0.94(1.13) | 0.95(0.98) |
| Sneezing | 0.78(0.91) | 0.78(0.98) | 0.78(0.94) | 0.72(1.02) |
| Runny nose | 1.19(1.14) | 0.88(1.11) | 0.65(0.96) | 0.84(1.04) |
| Postnasal drainage | 1.21(1.25) | 0.78(1.06) | 0.71(1.10) | 0.91(1.19) |
| Thick nasal discharge | 0.66(1.06) | 0.50(0.99) | 0.42(0.90) | 0.50(1.00) |
| Ear/Facial Subset | Baseline mean (SD) |
1 month mean (SD) |
3 month mean (SD) |
6 month mean (SD) |
| Ear Fullness | 0.37(0.88) | 0.32(0.76) | 0.29(0.80) | 0.39(0.92) |
| Dizziness | 0.23(0.51) | 0.19(0.59) | 0.24(0.67) | 0.25(0.67) |
| Ear pain | 0.14(0.41) | 0.16(0.54) | 0.19(0.70) | 0.25(0.75) |
| Face pain/pressure | 0.50(1.12) | 0.35(0.80) | 0.36(0.81) | 0.47(0.93) |
| Sleep subset | Baseline mean (SD) |
1 month mean (SD) |
3 month mean (SD) |
6 month mean (SD) |
| Difficulty falling asleep | 1.01(1.44) | 0.70(1.28) | 0.79(1.24) | 0.94(1.38) |
| Waking up at night | 1.09(1.39) | 0.67(1.21) | 0.75(1.22) | 0.91(1.34) |
| Lack good nights sleep | 1.30(1.54) | 0.98(1.36) | 0.90(1.29) | 1.01(1.39) |
| Psychological Subset | Baseline mean (SD) |
1 month mean (SD) |
3 month mean (SD) |
6 month mean (SD) |
| Fatigue | 1.21(1.49) | 0.81(1.17) | 0.87(1.23) | 0.88(1.33) |
| Reduced productivity | 0.83(1.23) | 0.50(1.03) | 0.36(0.86) | 0.46(1.00) |
| Reduced concentration | 0.90(1.25) | 0.64(1.15) | 0.52(0.99) | 0.68(1.19) |
| Frustration/Restlessness/Irritability | 0.98(1.15) | 0.77(1.16) | 0.53(0.98) | 0.66(1.05) |
| Sadness | 0.43(0.91) | 0.34(0.83) | 0.38(0.92) | 0.36(0.88) |
| Embarrassed | 0.45(0.89) | 0.28(0.78) | 0.22(0.65) | 0.22(0.68) |
Table 2 details the average subset scores and their change from baseline. In regards to the rhinology subset, there was significant improvement in scores at all of the follow up intervals (1-month – mean change −0.24, p-value <0.01; 3-month – mean change −0.34, p-value <0.01; 6-month – mean change −0.25, p-value <0.01). Similarly, there was significant improvement at all follow up intervals in the psychological QOL subset (1-month – mean change −0.25, p-value <0.01; 3-month – mean change −0.32, p-value <0.01; 6-month −0.26, p-value <0.01). There was significant improvement in the sleep QOL subset at the 1- and 3-month follow up intervals. Sleep subset scores at the 6-month follow up were improved from baseline, though not significantly (1-month – mean change −0.35, p-value <0.01; 3-month – mean change −0.32, p-value <0.01, 6-month – mean change −0.18, p-value 0.07). The ear and facial symptom subset had overall low baseline scores, and therefore did not have significant improvement at any of the follow up intervals (1-month – mean change −0.06, p-value 0.20; 3-month – mean change −0.04, p-value 0.36; 6-month – mean change 0.03, p-value 0.61).
Table 2.
Mean subset scores and change from baseline
| Rhinology | Mean (SD) | Mean Change | p-Value |
|---|---|---|---|
| Baseline | 1.04 (0.99) | ||
| 1 Month | 0.80 (0.90) | −0.24 | <0.01 |
| 3 Month | 0.70 (0.87) | −0.34 | <0.01 |
| 6 Month | 0.78 (0.86) | −0.25 | <0.01 |
| Ear/Face | Mean (SD) | Mean Change | p-value |
| Baseline | 0.31 (0.56) | ||
| 1 month | 0.26 (0.50) | −0.06 | 0.194 |
| 3 month | 0.27 (0.53) | −0.04 | 0.355 |
| 6 month | 0.34 (0.64) | 0.03 | 0.608 |
| Sleep | Mean (SD) | Mean Change | p-value |
| Baseline | 1.13 (1.34) | ||
| 1 month | 0.78 (1.18) | −0.35 | <0.01 |
| 3 month | 0.81 (1.13) | −0.32 | <0.01 |
| 6 month | 0.95 (1.23) | −0.18 | 0.074 |
| Psych | Mean (SD) | Mean Change | p-value |
| Baseline | 0.80 (0.92) | ||
| 1 month | 0.55 (0.85) | −0.25 | <0.01 |
| 3 month | 0.48 (0.77) | −0.32 | <0.01 |
| 6 month | 0.54 (0.86) | −0.26 | <0.01 |
Discussion
In this study, the SNOT-20 questionnaire was used as an assessment of overall symptom burden and QOL related to sinus disease in patients with CF and the G551D CFTR mutation. Overall composite scores showed significant improvement after the initiation of ivacaftor at all of the follow up intervals. Based on a previous validity study of the SNOT-20 questionnaire, a mean change of 0.8 over a 6-month period was deemed to be clinically meaningful (14). It is recognized that the mean change in this study falls below that of the documented MCID. We attribute this to the overall low reported symptom burden at the onset of treatment, an effect previously well documented in the literature (16-19). Several previous studies have identified an overall low sinonasal symptom burden in the CF population with some reporting that less than 10% of CF patients report significant sinonasal symptoms, despite the presence of significant objective disease (19). Quality of life questionnaires, such as SNOT-20 and SNOT-22, have corroborated this finding, with previous studies reporting average SNOT-20/22 scores in the CF population to be around 15-20, similar to what was found in this cohort of patients (20,21). It has been suggested that this is related to these patients lack of a “normal” baseline for comparison. Others have proposed that sinonasal symptoms are reported to be low as they are overshadowed by other systemic symptoms, such as significant pulmonary and/or gastrointestinal symptoms (1, 20-23). Regardless of the exact reasoning as to why CF patients do not report a high level of sinonasal morbidity, the low symptom burden in these patients makes the assessment of response to treatment difficult.
As previously reported in the literature, the SNOT-20 questionnaire has been suggested to be more clinically useful if broken down into 4 subsets (15). When this was performed, we found that there was significant improvement in the rhinologic symptom subset, the sleep QOL subset, and the psychological QOL subset.
Previous reports have documented both in vivo and in vitro effects following administration of ivacaftor. Several case reports have reported improvement in objective CT findings in the sinuses of CF patients after treatment with ivacaftor (24-26). Chang et. al. also documented decreased viscosity of airway surface liquid in cell culture from sinus samples (25), an effect observed in ALI cultures of G551D epithelial cells (27). Furthermore, in vitro studies have shown increased anti-biofilm activity against Pseudomonas aeruginosa when ivacaftor is used in combination with I-Methionine, an amino acid with anti-biofilm activity (28). Given these previous reports, it was hypothesized that the addition of ivacaftor to the therapeutic regimen of G551D CF patients would lead to improvement in rhinologic symptoms. This study used a measure of symptom burden in order to demonstrate this improvement. The rhinology subset scores showed significant improvement at all follow up intervals.
As with the rhinology subset, there was significant improvement in the psychological QOL subset scores. Poor psychological health, which often accompanies a diagnosis of CF, has been shown to have deleterious effects on overall pulmonary function, treatment adherence, hospitalization rates, and health care costs (29). The results of this study suggest that patients have improved psychological health related QOL following treatment with ivacaftor. It is recognized that this is not attributable to improvement solely in the sinonasal realm, but likely related to systemic symptomatic improvement (30). Future studies should explore the mental health related QOL improvements in CF patients treated with ivacaftor, as mental health is a heavy contributor to morbidity in this patient population.
Interestingly in the sleep subset, there was significant improvement at the 1- and 3-month follow up. Mean scores were lower when compared to baseline at the 6-month follow up, but this was not significant. We postulate that the high baseline scores of the sleep subset and the failure to maintain significant improvement at 6-months may be related to the impact of systemic CF symptoms on sleep related QOL. In general, sleep disturbances among CF patients is multifactorial. It has been suggested that increased mucus production and reflux may cause nocturnal cough, anxiety and depression may lead to difficulty falling asleep, and chronic pain may interfere with sleep quality (31-34).
There was no significant improvement in the ear/facial symptom subset over any time interval. However, this subset had the lowest average scores at baseline ranging from 0.17 to 0.50 (mean=0.31), leaving little room for improvement. This is not surprising, as several previous studies have documented a low incidence of middle ear disease in patients with CF (35-37).
In addition to the improvements in CF related sinonasal quality of life as described here, ivacaftor has been shown to offer benefit systemically. While subjectively, patients do report improved symptoms and quality of life, objective improvements have also been documented in the literature. The GOAL study evaluated objective measures to determine systemic improvement in this patient cohort. Rowe, et al. found significant improvement in FEV1 and FVC in this patient cohort. In addition, there was significant improvement in sweat chloride assay in these patients after initiation of ivacaftor, as well as a decline in hospitalizations. Furthermore, there was significant improvement in all quality of life domains of the Cystic Fibrosis Questionnaire-Revised (CFQ-R) throughout this study. A subset of these patients also had sustained improvement in mucociliary clearance throughout the duration of the GOAL study (13).
There are several limitations to this study of note. First, while large, the study was an uncontrolled cohort design. Further, the authors recognize that SNOT-20 is a validated QOL survey for patients with non-CF chronic rhinosinusitis. While the questionnaire may be a good resource for evaluating QOL in CF patients, it has not been validated for this purpose (38). As alluded to previously, baseline SNOT-20 scores in this cohort of patients are much lower than what is typically observed in standard CRS patients, where average baseline SNOT-22 scores are generally greater than 40 (21, 39-43). This, in addition to the lack of a validated questionnaire to specifically target sinonasal QOL in the CF population, makes assessment of this patient population difficult, and highlights the importance of development of a sinus-specific quality of life instrument for use in the CF population. Finally, ivacaftor has been shown to confer benefit to other organ systems, thus improvement in SNOT-20 scores may reflect improvement in overall systemic symptoms (30). Therefore, the significant improvement observed in overall SNOT-20 scores cannot be solely attributed to improvement in sinonasal symptoms alone.
Conclusion
While CF sinus disease does cause significant morbidity and reduced QOL, the sino-nasal symptom burden at baseline was quite low. Significant benefit was observed in several subset domains of the SNOT-20, including rhinologic QOL, indicating the drug likely imparts benefit for CF sinus disease in this population.
Acknowledgements
The authors would like to acknowledge the subsites, principal investigators and research coordinators of the GOAL study.
Seth Walker (Emory); Karen Callahan, Pamela Zeitlin (Johns Hopkins); Ahmet Uluer (Children’s Hospital Boston; Carla Frederick, Jameelah Ali (Womens and Childrens Hospital Buffalo); Michael Konstan, Colette Bucur, Bobbi Ksenich (Case Western); Frank Accurso, Churee Pardee (Denver Childrens Hospital); Ibrahim Abdulhamid, Catherine Van Wagnen (Wayne State); Christopher Oermann, Nicoline Schaap (Texas Children’s Hospital); Joanne Billings, Denise Stacklie, Cynthia Williams, Patricia Grover, Brooke Noren (University of Minnesota); Ronald Rubenstein, Erin Donnelly, Christina Kubrak (Children’s Hospital of Philadelphia); Joseph Pilewski, Elizabeth Hartigan (Pittsburgh Children’s Hospital); Steven Rowe, Ginger Reeves (Universityy of Alabama at Birmingham); Philip Black, Candy Schmoll (Children’s Mercy Hospital); Nadia Shive, Scott Donaldson (University of North Carolina); Theodore Liou, Kristyn Packer (Utah Health Science Center); Susan Millard, Thomas Symington (Helen Devos Children’s Hospital); Isabel Virella-Lowell, Ashley Warden (MUSC); John Clancy, Dee Terrill (Cincinnati Children’s); Carlos Milla, Zoe Davies, Colleen Dunn (Stanford); Leonard Sicilian (Massachusetts General Hospital); Rebekah Brown, Pamela Berry (Vanderbilt); Kathryn Moffett, Sue Collins (University of West Virginia); Jennifer Taylor-Cousar, Connie St. Clair (National Jewish Hospital); Raksha Jain, Ashley Keller (University of Texas Southwestern); Douglas Conrad, Mark Pian (University of California, San Diego); Edward Naureckas, Spring Holland (University of Chicago).
Funding Support: This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-03) and National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482) to B.A.W.
Footnotes
Dr. Woodworth is a consultant for Cook Medical and Olympus
Presented at the ARS Annual Meeting in October 2018
Contributor Information
Justin McCormick, University of Alabama at Birmingham Department of Otolaryngology Head and Neck Surgery.
Do-Yeon Cho, University of Alabama at Birmingham Department of Otolaryngology Head and Neck Surgery.
Brooks Lampkin, University of South Alabama College of Medicine.
Joshua Richman, University of Alabama at Birmingham Department of Otolaryngology Head and Neck Surgery.
Heather Hathorne, University of Alabama at Birmingham.
Steven M Rowe, University of Alabama at Birmingham Department of Medicine, Director of Gregory Fleming James Cystic Fibrosis Research Center.
Bradford A. Woodworth, University of Alabama at Birmingham Department of Otolaryngology Head and Neck Surgery.
References
- 1.Savastnao V, Bertin S, Vittori T, Tripodi C, Magliugo G. Evaluation of chronic rhinosinusitis management using the SNOT-22 in adult cystic fibrosis patients. Eur Rev Med Pharmacol Sci 2014; 18(14):1985–9. [PubMed] [Google Scholar]
- 2.Collins FS. Cystic fibrosis: molecular biology and therapeutic implications. Science 1992; 256(5058):774–9. [DOI] [PubMed] [Google Scholar]
- 3.Ferril GR, Nick JA, Getz AE, Barham HP, Saavedra MT, Taylor-Cousar JL, Nichols DP, Curran-Everett D, Kingdom TT, Ramakrishnan VR. Comparison of radiographic and clinical characteristics of low-risk and high-risk cystic fibrosis genotypes. Int Forum Allergy Rhinol 2014; 4(11):915–20. [DOI] [PubMed] [Google Scholar]
- 4.Lommatzsch ST, Aris R. Genetics of cystic fibrosis. Semin Respir Crit Care Med 2009; 30(5): 531–8. [DOI] [PubMed] [Google Scholar]
- 5.Solomon GM, Marshall SG, Ramsey BW, Rowe SM. Breakthrough therapies: cystic fibrosis (CF) potentiators and correctors. Pediatr Pulmonol 2015; Suppl 40: S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho DY, Skinner D, Zhang S, Fortenberry J, Sorscher EJ, Dean NR, Woodworth BA. Cystic fibrosis transmembrane conductance regulator activation by the solvent ethanol: implications for topical drug delivery. Int Forum Allergy Rhinol 2016; 6(2): 178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkins MD, Parkins VM, Rendall JC, Elborn S. Changing epidemiology and clinical issues arising in an ageing cystic fibrosis population. Ther Adv Respir Dis 2011; 5(2): 105–19. [DOI] [PubMed] [Google Scholar]
- 8.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 2002; 109(5): 571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regnis JA, Robinson M, Bailey DL, Cook P, Hooper P, Chan HK, Gonda I, Bautovisch G, Bye PT. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am J Respir Crit Care Med 1994; 150(1): 66–71. [DOI] [PubMed] [Google Scholar]
- 10.Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy 2013; 27(5):387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durle PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 2010; 363(21): 1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawicki GS, McKone EF, Pasta DJ, Millar SJ, Wagener JS, Johnson CA, Konstan MW. Sustained benefit from Ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med 2015; 192(7): 836–42. [DOI] [PubMed] [Google Scholar]
- 13.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator Ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014; 190(2): 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccirillo JF, Merritt MG Jr, Richards ML. Psychometric and clinimetric validity of the 20-item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg 2002; 126:41–47. [DOI] [PubMed] [Google Scholar]
- 15.Browne JP, Hopkins C, Slack R, Cano SJ. The Sino-Nasal Outcome Test (SNOT): Can we make it more clinically meaningful? Otolaryngol Head Neck Surg 2007; 136(5):736–741. [DOI] [PubMed] [Google Scholar]
- 16.Boari L, de Castro Júnior NP. Diagnosis of chronic rhinosinusitis in patients with cystic fibrosis: correlation between anamnesis, nasal endoscopy and computed tomography. Braz J Otorhinolaryngol. 2005;71(6):705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freitas MR, Vasconcelos DN, Freitas AE, Maia Filho JH, Castro e Silva C. Nasal endoscopic and CT scan alterations of the paranasal sinuses as predictors of severity in patients with cystic fibrosis. Braz J Otorhinolaryngol. 2013;79(4):480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henriksson G, Westrin KM, Karpati F, Wikström AC, Stierna P, Hjelte L. Nasal polyps in cystic fibrosis: clinical endoscopic study with nasal lavage fluid analysis. Chest. 2002;121(1):40–47. [DOI] [PubMed] [Google Scholar]
- 19.Robertson JM, Friedman EM, Rubin BK. Nasal and sinus disease in cystic fibrosis. Paediatr Respir Rev 2008; 9(3): 213–9. [DOI] [PubMed] [Google Scholar]
- 20.Bock JM, Schien M, Fischer C, Naehrlich L, Kaeding M, Guntinas-Lichius O, et al. Importance to question sinonasal symptoms and to perform rhinoscopy and rhinomanometry in cystic fibrosis patients. Pediatr Pulmonol 2017; 52: 167–174. [DOI] [PubMed] [Google Scholar]
- 21.Kang SH, Meotti CD, Bombardelli K, Piltcher OB, Dalcin PR. Sinonasal characteristics and quality of life by SNOT-22 in adult patients with cystic fibrosis. Eur Arch Otorhinolaryngol 2017; 274(4): 1873–1882. [DOI] [PubMed] [Google Scholar]
- 22.Kang SH, Dalcin P de TR, Piltcher OB, Migliavacca R de O. Chronic rhinosinusitis and nasal polyposis in cystic fibrosis: update on diagnosis and treatment. Jornal Brasileiro de Pneumologia. 2015;41(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan DK, McNamara S, Park JS, Vajda J, Gibson RL, Parikh SR. Sinonasal Quality of Life in Children With Cystic Fibrosis. JAMA Otolaryngol Head Neck Surg. 2016;142(8):743–749. [DOI] [PubMed] [Google Scholar]
- 24.Hayes D, McCoy KS, Sheikh SI. Improvement of sinus disease in cystic fibrosis with Ivacaftor therapy. Am J Respir Crit Care Med 2014; 190(4): 468. [DOI] [PubMed] [Google Scholar]
- 25.Chang EH, Tang XX, Shah VS, Launspach JL, Ernst SE, Kilkin B, Karp PH, et al. Medical reversal of chronic sinusitis in a cystic fibrosis patient with Ivacaftor. Int Forum Allergy Rhinol 2015; 5(2): 178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vreede CL, Berkhout MC, Sprij AJ, Fokkens WJ, Heijerman HG. Ivacaftor and sinonasal pathology in a cystic fibrosis patient with genotype deltaF508/S1215N. J Cyst Fibros 2015; 14(3): 412–3. [DOI] [PubMed] [Google Scholar]
- 27.Birket SE, Chu KK, Houser GH, Liu L, Fernandez CM, Solomon GM, et al. Combination therapy with cystic fibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am J Physiol Lung Cell Mol Physiol 2016; 310(10): 928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho DY, Lim DJ, Mackey C, Weeks CG, Pena Garcia JA, Skinner D, Grayson JW, Hill HS, Alexander DK, Zhang S, Woodworth BA. I-Methionine anti-biofilm activity against Pseudomonas aeruginosa in enhanced by the cystic fibrosis transmembrane conductance regulator potentiator, Ivacaftor. Int Forum Allergy Rhinol 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talwalkar JS, Koff JL, Lee HB, Critto CJ, Mulenos AM, Georgiopoulos AM. Cystic fibrosis transmembrane regulator modulators: Implications for the management of depression and anxiety in cystic fibrosis. Psychosomatics 2017; 58(4): 343–54. [DOI] [PubMed] [Google Scholar]
- 30.Quittner A, Suthoff E, Rendas-Baum R, Bayliss MS, Sermet-Gaudelus I, Castiglione B, Vera-Llonch M. Effect of Ivacaftor treatment in patients with cystic fibrosis and the G551D-CFTR mutation: patient reported outcomes in the STRIVE randomized, controlled trial. Health Qual Life Outcomes 2015; 13: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandeleur M, Walter LM, Armstrong DM, Robinson P, Nixon GM, Home RSC. What keeps children with cystic fibrosis awake at night? J Cyst Fibros 2017; 16: 719–26. [DOI] [PubMed] [Google Scholar]
- 32.Vandeleur M, Walter LM, Armstrong DM, Robinson P, Nixon GM, Home RSC. Quality of life and mood in children with cystic fibrosis: Associations with sleep quality. J Cyst Fibros 2017; 17. [DOI] [PubMed] [Google Scholar]
- 33.Katz ES. Cystic fibrosis and sleep. Clin Chest Med 2014; 35(3): 495–504. [DOI] [PubMed] [Google Scholar]
- 34.Bouka A, Henning T, Liebich L, Dumitrascu R, Hecker C, Reichenberger F, et al. Quality of life in clinically stable adult cystic fibrosis outpatients: Associations with daytime sleepiness and sleep quality. Resp Med 2012; 106(9): 1244–9. [DOI] [PubMed] [Google Scholar]
- 35.Haddad J, Gonzalez C, Kurland G, Orenstein DM, CAsselbrant ML. Ear disease in children with cystic fibrosis. Arch Otolaryngol Head Neck Surg 1994; 120: 491–3. [DOI] [PubMed] [Google Scholar]
- 36.Jorrisen M, De Boeck K, Feenstra L. Middle ear disease in cystic fibrosis Int J Pediatr Otorhinolaryngol 1998; 43: 123–8. [DOI] [PubMed] [Google Scholar]
- 37.Yildirim N, Sone M, Mutlu C, Schachern PA, Paparella MM, Le CT. Histopathologic features of the temporal bone in patients with cystic fibrosis Ann Otolaryngol Head Neck Surg 2000; 126; 75–8. [DOI] [PubMed] [Google Scholar]
- 38.Virgin F Clinical chronic rhinosinusitis outcomes in pediatric patients with cystic fibrosis. Laryngoscope Investig Otolaryngol 2017; 2(5): 276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chowdhury NI, Mace JC, Bodner TE, Alt JA, Deconde AS, Levy JM, Smith TL. Does medical therapy improve sinonasal outcomes test-22 domain scores? An analysis of clinically important differences. Laryngoscope 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alt JA, Orlandi RR, Mace JC, Soler ZM, Smith TL. Does delaying endoscopic sinus surgery adversely impact quality-of-life outcomes? Laryngoscope 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tait S, Kallogjeri D, Suko J, Kukuljan S, Schneider J, Piccirillo JF. Effect of budesonide added to large-volume, low-pressure saline sinus irrigation for chronic rhinosinusitis: A randomized clinical trial. JAMA Otolaryngol Head Neck Surg 2018; 144(7): 605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soler ZM, Jones R, Le P, Rudmik L, Mattos JL, Nguyen SA, Schlosser RJ. Sino-nasal outcome test-22 outcomes after sinus surgery: A systematic review and meta-analysis. Laryngoscope 2018; 128(3): 581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy JM, Mace JC, Rudmik L, Soler ZM, Smith TL. Low 22-item sinonasal outcome test scores in chronic rhinosinusitis: Why do patients seek treatment? Laryngoscope 2017; 127(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]