Abstract
Reduced glutathione (GSH) is the most prevalent non-protein thiol in animal cells. Its de novo and salvage synthesis serves to maintain a reduced cellular environment and the tripeptide is a co-factor for many cytoplasmic enzymes and may also act as an important post-translational modification in a number of cellular proteins. The cysteine thiol acts as a nucleophile in reactions with both exogenous and endogenous electrophilic species. As a consequence, reactive oxygen species (ROS) are frequently targeted by GSH in both spontaneous and catalytic reactions. Since ROS have defined roles in cell signaling events as well as in human disease pathologies, an imbalance in expression of GSH and associated enzymes has been implicated in a variety of circumstances. Cause and effect links between GSH metabolism and diseases such as cancer, neurodegenerative diseases, cystic fibrosis (CF), HIV, and aging have been shown. Polymorphic expression of enzymes involved in GSH homeostasis influences susceptibility and progression of these conditions. This review provides an overview of the biological importance of GSH at the level of the cell and organism.
Keywords: Glutathione (GSH), Glutathione transferases (GST), Reactive oxygen species (ROS), Human diseases
1. Introduction
Glutathione (GSH) is a water-soluble tripeptide composed of the amino acids glutamine, cysteine, and glycine. The thiol group is a potent reducing agent, rendering GSH the most abundant intracellular small molecule thiol, reaching millimolar concentrations in some tissues. As an important antioxidant, GSH plays a role in the detoxification of a variety of electrophilic compounds and peroxides via catalysis by glutathione S-transferases (GST) and glutathione peroxidases (GPx). The importance of GSH is evident by the widespread utility in plants, mammals, fungi and some prokaryotic organisms [1]. In addition to detoxification, GSH plays a role in other cellular reactions, including, the glyoxalase system, reduction of ribonucleotides to deoxyribonucleotides, regulation of protein and gene expression via thiol:disulfide exchange reactions [2].
The tripeptide can exist intracellularly in either an oxidized (GSSG) or reduced (GSH) state. Maintaining optimal GSH:GSSG ratios in the cell is critical to survival, hence, tight regulation of the system is imperative. A deficiency of GSH puts the cell at risk for oxidative damage. It is not surprising that an imbalance of GSH is observed in a wide range of pathologies, including, cancer, neurodegenerative disorders, cystic fibrosis (CF), HIV and aging. The role of GSH in these disorders will be discussed in this review.
2. Glutathione synthesis
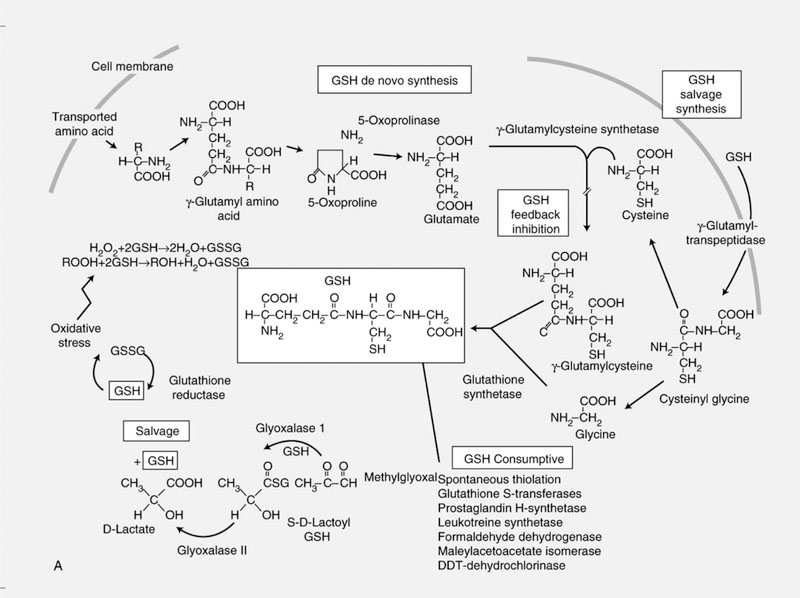
GSH is synthesized de novo from the amino acids glycine, cysteine and glutamic acid. Synthesis of GSH requires the consecutive action of two enzymes, γ-glutamylcysteine synthetase (γ-GCS) and GSH synthetase [3], (Fig. 1). γ-GCS is a heterodimer composed of a catalytically active heavy subunit γ-GCS-HS (73 kDa) and a regulatory subunit, γ-GCS-LS (30 kDa) [4,5]. The regulation of γ-GCS is complex. Induction of γ-GCS expression has been demonstrated in response to diverse stimuli in a cell specific manner. The bioavailability of cysteine is rate limiting for the synthesis of GSH. Cysteine and the oxidized form of the amino acid, cystine, are transported into the cell via sodium dependent and independent transporters, respectively [6]. Oxidants (including hyperoxide, H2O2 and electrophilic compounds) promote cystine uptake and a concomitant increase in expression of γ-GCS. The γ-GCS promotor region contains a putative AP-1 binding site, an antioxidant response element (ARE), and an electrophile responsive element [7–9]. The AP-1 site is critical to constitutive expression of the γ-GCS-HS subunit [7]. Post-translational modifications of γ-GCS also influence GSH synthesis [10,11]. Specifically, phosphorylation of γ-GCS leads to the inhibition of GSH synthesis. GSH itself regulates the activity of γ-GCS via a negative feedback mechanism [3]. Hence, GSH depletion increases the rate of GSH synthesis.
Fig. 1.
Homeostasis of glutathione is maintained intracellularly through a de novo and salvage synthesis pathway. Tight regulation of the GSH:GSSG ratio is maintained by glutathione reductase.
3. GSH redox cycle
The formation of excessive amounts of reactive O2 species (ROS), including peroxide (H2O2) and superoxide anions (O2−•) is toxic to the cell. Hence, metabolizing and scavenging systems to remove them are functionally critical and tightly controlled in the cell. GSH peroxidase (GPx) in concert with catalase and superoxide dismutase (SOD) function to protect the cell from damage due to ROS. GPx detoxifies peroxides with GSH acting as an electron donor in the reduction reaction, producing GSSG as an end product. The reduction of GSSG is catalyzed by GSH reductase (GR) in a process that requires NADPH. GR is a member of the flavoprotein disulfide oxidoreductase family and exists as a dimer [2]. Under conditions of oxidative stress, GR is regulated at the level of transcription as well as by posttranslational modifications. Alterations in GR expression and activity have been implicated in cancer and aging [2].
GPx is an 80 kDa protein that is composed of four identical subunits. Five distinct GPx isozymes have been characterized in mammals, (Table 1) [12]. While GPx’s are ubiquitously expressed, individual isoforms are tissue specific. GPx expression is induced by oxidative stress and aberrant expression of GPx’s has been associated with a wide variety of pathologies, including hepatitis [13], HIV [14], and a wide variety of cancers, including skin [15], kidney [16], bowel [17] and breast [18].
Table 1.
Allelic variation in GPx and corresponding tissue distribution
| Allele | Tissue distribution |
|---|---|
| GPX1 | Erythrocytes, kidney, liver |
| GPX2 | GI tract |
| GPX3 | Kidney |
| GPX4 | Ubiquitous, highest in renal epithelium and testis |
| GPX5 | Ubiquitous |
Adapted from reference [12].
4. Redox balance and glutathionylation in regulation pathways
Many drugs and chemicals can produce ROS as direct or indirect by-products. An interpretation of data from some cell survival assays suggests that low levels of ROS can have growth stimulatory effects. For example, Adriamycin under-goes redox cycling to produce quinone intermediates that provide a potent source of ROS. Using standard colony formation assays, low concentrations of Adriamycin (low nM) can actually stimulate proliferation resulting in survival above 100%. As concentrations increase, a normal exponential decline in survival occurs. How ROS causes this proliferative effect is best addressed by examining the cellular protective mechanisms that deal with ROS, returning focus to GSH and thiol regulatory pathways. Redox status has dual effects on upstream signaling systems and downstream transcription factors. Oxidants can stimulate many upstream kinases in signaling pathway cascades, and yet inhibit transcription factors AP-1 and NFκB. Reductants can have opposite effects producing AP-1 and NFκB activation [19].
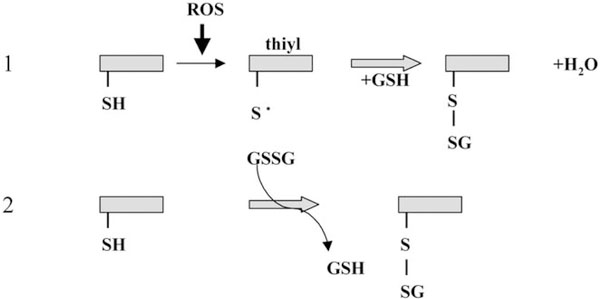
GSH homeostasis and GST expression in non-mammalian phyla can also influence proliferative signaling pathways. Tobacco seedlings transgenically overexpressing both GST and GPx cDNAs grew significantly faster than control seedlings when exposed to low temperatures or salt stress [20]. During stress periods, GSSG levels were significantly higher in the transgenic seedlings when compared to control. In addition, growth of the control seedlings was accelerated by GSSG treatment and decreased by GSH or other sulfhydryl reducing agents. These authors concluded that oxidation of the GSH pool was stimulatory to the proliferative process. While there was no specific explanation for the underlying mechanism, S-thiolation of growth regulatory proteins could be implied. Site-specific reactive cysteines are found in many proteins and these may be targets for thiol:disulfide exchange reactions. Experimentally, glutathionylation of these cysteines may be studied by using a number of different techniques including radiolabeled GSSG that acts as the GS-donor (Fig. 2). This post-translational modification adds the tripeptide glycine–cysteine–glutamic acid to the acceptor cysteine with a subsequent change in redox state, charge and three dimensional structure. By masking the thiol, the protein will be altered in its capacity to form disulfide bonds and to fold into different functional (perhaps active) conformations. Glutathionylation has been shown to occur on a number of proteins (Table 2) and in some cases, to alter their biological activity [21].
Fig. 2.
Table 2.
Proteins characterized as susceptible to glutathionylation
| Actin |
| Vimentin |
| Myosin |
| Tropomyosin |
| Cofilin |
| Profilin |
| Pyruvate kinaseglyceraldehyde-3 phosphate dehydrogenase |
| Enolase |
| Aldolase |
| Adenylate kinase |
| Phosphoglycerate kinase |
| 6-Phosphogluconolactonase |
| Ubiquitin conjugating enzyme |
| Triosephosphate isomerase |
| Pyrophosphatase |
| Thioredoxin |
| Protein disulfide isomerase |
| Cytochrome c oxidase |
| Peroxiredoxin 1 |
| Cyclophilin |
| HSP60 |
| HSP70 |
| Nucleophosmin |
| Transgelin |
| Galectin |
| Fatty acid binding protein |
Adapted from reference [35].
In many cases, alteration of the ratio of GSH:GSSG can have a direct effect upon signaling pathways. For example, a shift in ratio towards the oxidized pool has been shown to induce glutathionylation of the cysteine located in the DNA binding site of c-jun as well as produce a disulfide bond between cysteines proximal to the leucine zipper motif. The impact of these changes is the capacity to induce a direct and reversible control of transcriptional regulation through these stress kinase pathways [22]. The transcription factor Nrf2 has been shown to be a regulator of some members of the phase II detoxification enzyme family (including GSTs) which are controlled by the ARE. A negative regulatory protein, Keap1, has been shown under non-stressed conditions to keep Nrf2 in a silent state [23]. Exposure to ROS dissociated Nrf2 from Keap1 and allowed relocation of Nrf2 to the nucleus, where ARE responsive genes became actively transcribed. It has been suggested that the Keap1 protein may contain critical cysteine residues, which upon oxidation alter the conformation of the protein releasing any associated protein such as Nrf2 [24]. In addition, GSH was shown to exert negative regulation on cellular PKC isozymes which have cysteine residues at, or near, the active site of the enzyme and this control was removed when GSH pools were depleted by exposure to ROS [25]. Manipulation of intracellular GSH pools by supplementation with GSH precursors was found to block induction of JNK activity by methylating agents [26] Similarly p53 function has been reported to be redox sensitive, primarily through in vitro modification of its cysteine residues [27]. At the cellular level, thiol antioxidants induced apoptosis in transformed cell lines but not in normal cells, suggesting that direct redox control of p53 may be influential in determining cell fate under stress conditions [28].
There are also indications that GSH and associated enzymes play a role in cellular immunity. For example, GSH levels and antigen-presenting cells determine whether a Th1 or Th2 pattern of response predominates [25]. The Th1 response is characterized by production of interleukin-12 (IL-12) and interferon a and the enhancement of delayed hypersensitivity response; Th2 by IL-4 and IL-10 production and up-regulation of a number of antibody responses. The molecular basis for this difference is not known, but it is also significant that HIV patients receiving N-acetyl cysteine (NAC), have longer survival times than untreated controls. In diseases such as HIV, Th2 predominance is a critical component of immune response and thus, GSH levels in antigen presenting cells may play an integral role in determining disease progression [29]. A possible link between JNK and immune response/myeloproliferation has been suggested. To this end, T cells from a JNK1 knockout mouse hyperproliferated, exhibited decreased activation and induced cell death and preferentially differentiated into Th2 cells [30]. Despite the redundancy accorded to this system by the continued production of JNK2, it appears reasonable to conclude that the JNK1 signaling pathway may be playing a role in T-cell receptor initiated Th cell proliferation, differentiation or apoptosis.
Other thiol rich proteins can also have a direct influence on regulatory pathways. For example, thioredoxins are a family of redox active proteins of approximately 12 kDa responsible for mediating numerous cytoplasmic functions influenced by available cysteine residues in the monomeric protein. Dimerization at some of these sites mitigates many of the redox dependent functions of the protein. Recent data implicate a secreted form of thioredoxin in control of cell growth, where the redox function is essential for growth stimulation [31]. Tumor cells transfected with thioredoxin demonstrate increased growth and decreased sensitivity to drug induced apoptosis. Thioredoxin has also been shown to bind to ASK1 to inhibit its activity as a kinase [32]. This inhibition is attenuated by ROS that can lead to dimerization of thioredoxin [33]. Thioredoxin regulation of the glucocorticoid receptor through association at the DNA binding domain has also been reported [34] and this too is influenced by ROS. More recently, thioredoxin itself has been shown to be subject to reversible glutathionylation [35]. The glutathionylation site was identified as Cys-72 and this residue could be modified either by GSSG or S-nitrosoglutathione. Modification of the site abolished enzymatic activity of thioredoxin. However, activity spontaneously recovered suggesting that thioredoxin was able to de-glutathionylate itself. Such results support the concept of a co-ordination of regulatory pathways through cross-talk between thioredoxin and GSH homeostasis. They also provide an example analogous to the GSTπ/JNK association and imply a broad ranging role for thiol:disulfide balance in the regulation of cellular functions and proliferation.
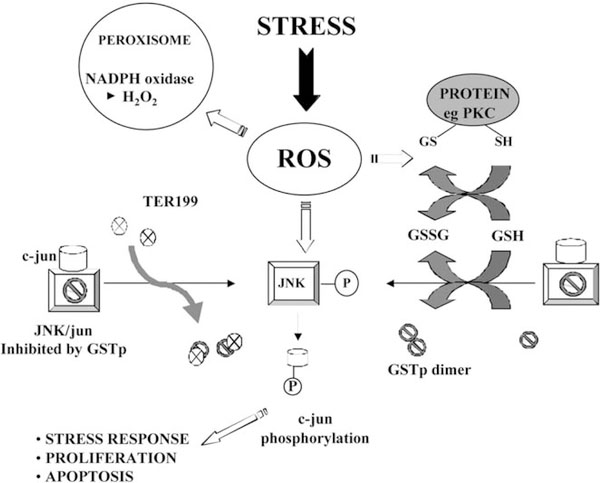
In summation, alterations in redox balance by exposure to ROS cause dose dependent changes in GSH:GSSG ratios, which can potentially influence a number of target proteins by causing oxidation and disulfide exchange reactions at specific cysteine residues. For GSTπ, the conversion from monomer to dimer (or multimers) causes a dissociation from JNK with resultant activation of the kinase. With subsequent phosphorylation of c-Jun and enhanced transcription of AP-1 responsive genes, the stage is set for signal transduction for stress response, proliferation or apoptosis pathways. Cysteine residues of GSTπ have been shown to be sensitive to oxidation by H2O2 [36]. The model shown in Fig. 3 provides the means by which oxidative stress can be transmitted through a GST “switch” tying into the kinase cascade of pathways. Perhaps future reports of drug induced perturbations in GSH/GST levels should consider whether the biological implications of drug treatment extend further than an enhanced rate of GST mediated catalytic thioether product formation.
Fig. 3.
5. Heterogeneity of GSH metabolizing enzymes and associated pathologies
5.1. Polymorphism of γ-GCS
Alterations in GSH levels are associated with a wide variety of pathologies, including cancer, HIV, lung disease and Parkinson’s disease (PD). Hence polymorphisms in the genes governing GSH levels may contribute to the etiology of these disorders. Polymorphisms within the γ-GCS gene have been identified within the heavy subunit, (Table 3) [37]. The gene, located on chromosome 1, encompasses 22 kb and contains seven exons and six introns. Three alleles of the γ-GCS-HS subunit have been identified that differ by the number of GAG trinucleotide repeats in the 5' coding and non-coding region. The contribution of these alleles toward GSH homeostasis pathologies remains unclear. However, there are examples of altered γ-GCS activity associated with a variety of diseases. The essential role of γ-GCS and GSH synthesis has been demonstrated in knockout mice that were incompetent to form the heavy chain of γ-GCS [38]. The mutation was shown to be embryonic lethal, however, cell lines were derived from the mutants when either GSH or NAC was supplemented in the culture medium. In humans, γ-GCS activity is diminished in patients with haemolytic anemia [39]. These patients were shown to carry an A→T mutation at nucleotide 1109 that resulted in the substitution of histidine with leucine at amino acid 370. Two additional alterations were identified in an intron (+206) and a CGC repeat in the 3' untranslated region [39]. Some patients with malignant mesothelioma have a deletion on chromosome 1 in the region that encompasses the γ-GCS gene [40]. This deletion can lead to γ-GCS deficiency and it was proposed that this could contribute to the malignant phenotype of this disease.
Table 3.
Allelic variation in γ-GCS due to GAG trinucleotide repeat
Adapted from reference [37].
5.2. Glutathione S-transferases
GSTs are a family of Phase II detoxification enzymes that have co-evolved with GSH and are abundant throughout most life forms. GSTs catalyze the conjugation of GSH to a wide variety of endogenous and exogenous electrophilic compounds (Fig. 1). Human GSTs are divided into two distinct super family members; the membrane bound microsomal and cytosolic family members. Microsomal GSTs play a key role in the endogenous metabolism of leukotrienes and prostaglandins [41]. Cytosolic GSTs are divided into six classes: α, μ, ω, π, θ and ζ (Table 4).
Table 4.
Cytosolic GST’s
| Class | Gene | Chromosome location |
|---|---|---|
| Alpha (α) | GSTA1–2 | 6 |
| Mu (μ) | GSTM1–5 | 1 |
| Omega (ω) | GSTO1 | 10 |
| Pi (π) | GSTP1 | 11 |
| Theta (θ) | GSTT1–2 | 22 |
| Zeta (ζ) | GSTZ1 | 14 |
GSTs can be induced by structurally unrelated compounds known to result in chemical stress and carcinogenesis including, phenobarbital, planar aromatic compounds, ethoxyquin, BHA, and trans-stilbene oxide [42]. Some of the compounds known to induce GSTs are themselves substrates of the enzyme, suggesting that induction is an adaptive response mechanism. Many clinical useful drugs are also potential substrates for GST and development of drug resistance can frequently be a key element in treatment failure. Not surprisingly, therefore, GSTs have been linked with the development of resistance toward chemotherapy agents, insecticides, herbicides, and microbial antibiotics [43–46].
In addition to the transferase function, GSTs have been shown to form protein:protein interactions with members of the mitogen activated protein (MAP) kinase pathway thereby serving a regulatory role in the balance between cell survival and apoptosis. By interacting directly with MAP kinases, including c-Jun N-terminal kinase 1 (JNK1) and apoptosis signal-regulating kinase 1 (ASK1), GSTs function to sequester the ligand in a complex, preventing interactions with their downstream targets [32,47–49]. Many anticancer agents induce apoptosis via activation of MAP kinase pathways, in particular those involving JNK and p38 [50,51]. This novel, non-enzymatic role for GST’s has direct relevance to the GST over-expressing phenotypes of many drug resistant tumors. As an endogenous switch for the control of signaling cascade pathways, elevated expression of GST can alter the balance of regulation of kinase pathways during drug treatment, thereby, conferring a potential selective advantage. This process can also provide a plausible explanation for the numerous examples of drug resistance linking GST overexpression with agents that are not substrates for these enzymes.
5.3. Polymorphism of GSTs
While polymorphisms have been identified within each class of GSTs, only the heterogeneity that may contribute to variations in pathology or clinical response will be addressed in this review. The μ and θ class of GST have a null phenotype (GSTM*0 and GSTT*0) whereby individuals do not express catalytically active protein. The lack of enzyme activity, and therefore, an inability to detoxify carcinogens is associated with an increased risk toward a variety of cancers. The GSTM1*0 allele is observed in ~50% of the Caucasian population [52] and is associated with an increased risk of lung, colon and bladder cancer and is a risk factor for pulmonary asbestosis [53,54]. The GSTT*0 phenotype varies between ethnic groups and is found to be highest in Chinese (65%) and lowest in Mexican American (9%) populations [55]. The GSTT*0 phenotype is associated with an increased risk of tumors of the head and neck, oral cavity, pharynx, and larynx [56,57].
The μ class of GSTs has five genes (GSTM1–5) [58] that are found in a gene cluster on chromosome 1 [59]. The GSTM1 gene contains four alleles and has been the most widely studied (Table 5). GSTM1*A has been associated with a decreased risk of bladder cancer and has an allele frequency of 20% [52]. Neurodegenerative diseases such as PD and schizophrenia are characterized by the degeneration of dopaminergic neurons. GSTM2–2 has been shown to catalyze the conjugation of GSH to aminochrome, a ROS generated in the redox cycling of orthoquinones within dopaminergic neurons [60]. Hence, GSTM2–2 has been proposed to play a protective role against neurodegenerative diseases.
Table 5.
GSTM1 polymorphism
| Allele | Frequency in Caucasian populations | Cancer risks |
|---|---|---|
| M1*A | 0.2 | Decreased risk of bladder and breast cancer |
| M1*B | 0.2 | Decreased risk ofpituitary adenomas |
| M1*0 | 0.59 | Increased risk of lung, colon, bladder, and post-menopausal breast cancer |
A single gene spanning ~3 kb located on chromosome 11 encodes for proteins designated in the π class of GSTs [61]. Polymorphisms at the GSTP1 locus result in four alleles, GSTP1*A–D, that differ structurally and functionally [62]. The promoter region contains a TATA box, two SP1 sites, an insulin response element and an anti-oxidant response element within an AP1 site [62]. GSTP1*A plays a role in the acquisition of resistance to cisplatin by enhancing the capacity of the cell to form platinum–GSH conjugates [63]. GSTP1*B is an allele in which a single nucleotide (A→G) substitution at position 313 substantially diminishes catalytic activity [64]. Homozygocity for GSTP1*B is favorable in the treatment of cancer patients because they have a diminished capacity to detoxify platinum based anticancer agents [65]. GSTP1*C is an allelic variant that is more predominant in malignant glioma cells and differs from other GSTP1 variants by two transitions resulting in Ile104Val and Ala113Val [62]. No major functional property has yet been assigned to this polymorphism.
6. Diseases associated with altered glutathione metabolism
6.1. Defects in enzymes of the γ-glutamyl cycle
To date, hereditary defects have been described in four of the major enzymes that mediate GSH metabolism through the γ-glutamyl cycle [66]. As mentioned previously, polymorphic variants of γ-GCS have been linked to specific diseases. However, where mutant enzyme expression occurs, the most prevalent syndromes include hemolytic anemia, either with or without hepatosplenomegaly. Hereditary defects in GSH synthetase are autosomal recessive and can lead to mental retardation and neuropsychiatric dysfunction in approximately 50% of patients, while this deficiency is routinely accompanied by metabolic acidosis and hemolytic anemia. The cycle intermediate 5-oxoproline is found in excess in the bloodstream of these patients, presumably because of the lack of feedback inhibition by GSH on γ-GCS. Where GSH synthetase deficiency is restricted to erythrocytes, the hemolytic anemia is not accompanied by a generalized oxoprolinuria [67]. Glutathionemia (excess GSH in the blood) occurs with aberrant expression of γ-glutamyl-transpeptidase. This can lead to an imbalance in glutamic acid homeostasis, classifying the disease as an inherited disorder of dicarboxylic acid catabolism. Symptoms can include mental retardation, but it is not clear whether there is a straightforward inheritance pattern for this genetic abnormality.
6.2. Parkinson’s disease
While GSH is found in millimolar concentrations in the brain [68] this organ is more susceptible to oxidative damage than other tissues [69]. Hence an alteration in GSH homeostasis that may lead to oxidative stress has been associated with neurodegenerative diseases, such as PD. PD, affecting nearly 1% of individuals over the age of 65, is a progressive neurodegenerative disorder that results in impaired motor and cognitive functions [70]. The underlying cause of the disease stems from the destruction of dopaminergic neurons in the substantia nigra pars compacta (SNpc) region of the midbrain [71]. These cells are involved in the metabolism of dopamine, hence PD is characterized by a dopamine deficiency. During normal endogenous dopamine metabolism, ROS, such as H2O2, may be generated and its removal by GSH serves to protect the SNpc. The progression of PD is associated with a depletion of GSH levels and an increase in ROS within the SNpc [72,73]. Using a murine model, Andersen et al. [74] showed that BSO treatment resulted in selective damage to the neurons within the SNpc. Given that GSH depletion may lead to progression of the disease, then in principle, restoration of GSH levels may halt further damage. In a clinical study, improvements in patients with PD were observed following administration of reduced GSH [75]. Whether prolonged, systemic treatments with reductive agents that cross the blood brain barrier would prove to be an effective preventive treatment for patients at high risk remains to be established.
6.3. HIV
Nearly 21.8 million deaths associated with HIV/AIDS were reported worldwide between 1981 and 2000. Prior to clinical manifestations of the disease, the immune system is compromised. As stated in previous sections, GSH levels have an impact on many immune functions, including activation of lymphocytes. Consequently, it was postulated that GSH deficiency could lead to the progression of immune dysfunction, a hallmark of AIDS. GSH levels are depleted in plasma, epithelial lining fluid (ELF), peripheral blood mononuclear cells and monocytes in asymptomatic HIV-infected individuals and in AIDS patients [76]. Systemic GSH deficiency has also been reported in symptom-free HIV seropositive individuals [76]. Clinical studies have shown that GSH deficiency is correlated with morbidity [29]. It seems reasonable to conclude that a generally impaired antioxidant system is an obvious contributory factor that may contribute to these clinical findings, however, the precise importance of GSH deficiency presents a more complex scenario. Decreased GSH levels have been shown to activate NFκB, leading to a series of downstream signal transduction events that allow HIV expression [77]. The long terminal repeat of HIV (HIV LTR) contains an NFκB site. In vitro studies have shown that NFκB binds to and activates genes controlled by the HIV LTR [78]. NAC supplementation blocked HIV LTR gene expression, thereby confirming the importance of thiol status in HIV positive cells [77].
Depletion of the CD4+ T-cell lymphocytes accompanies the etiology of HIV progression. Decreased GSH levels are known to be one contributory factor in the induction of apoptosis in CD4+ T lymphocytes [79]. Oxidative stress indices were measured in blood samples from 85 HIV/AIDS patients and compared to 40 healthy individuals [80]. These studies showed that reduced GSH levels were accompanied by an increase of DNA fragmentation in lymphocytes, indicative of apoptosis [80].
These factors combined suggest that maintenance or restoration of GSH levels is a potential therapeutic approach in HIV patients. In fact, several studies have shown that NAC restores GSH levels, prevents the activation of NFκB and replication of HIV [81]. Interestingly, treatment with NAC has provided beneficial effects for HIV-infected individuals, even though GSH levels in lymphocytes are not altered [81]. In separate studies, plasma GSH levels in HIV-infected individuals were increased to 89% of the uninfected controls following an 8-week treatment with oral NAC [82]. One dietary manipulation, which proves to be effective is supplementation with cysteine-rich whey proteins, where short term increases in plasma GSH levels have been observed [83]. In concordance with other dietary protocols, GSH precursor amino acid supplements seem, at least on the surface, to be a beneficial additive.
6.4. Liver disease
High intracellular content of GSH in liver are congruous with the detoxification functions of this organ. Inherited disorders in GSH synthesis and metabolism can significantly disrupt liver function and in some instances can be conditionally lethal. In humans, regular dietary intake of precursor sulfur containing amino acids will maintain hepatic intracellular GSH levels in the 5–10 mM range. Alterations in liver GSH are either the cause or effect of a number of pathologies. For example, in alcoholics, pools of mitochondrial GSH are depleted with the concomitant result that ROS damage can be exacerbated producing cell death and contributing to cirrhosis. Interestingly, the defect that leads to reduced levels of mitochondrial GSH involves a partial inactivation of a specific mitochondrial membrane transport protein [84]. Thus while a build up of cytosolic GSH occurs, the inability to transport this into mitochondria is caused by physicochemical alterations to the inner mitochondrial membrane caused by long term alcohol exposure. The selective depletion of GSH in this organelle can sensitize hepatocytes to the oxidative effects of cytokines such as tumor necrosis factor (TNF) [85]. In patients with chronic hepatitis C infections, GSH levels were severely depleted in hepatic and plasma fractions and also in peripheral blood mononuclear cells. These conditions were more pronounced in patients who had a concomitant HIV infection [86].
6.5. Cystic fibrosis
CF is a genetic disorder affecting nearly 250 000 children world-wide per year. The severe lung dysfunction that characterizes the disease is due to an alteration in an ion transport protein, CF transmembrane conductance regulator (CFTR). The recessive mutation renders the channel dysfunctional or absent. CFTR is an organic anion efflux channel with functional properties that are redundant to MRP, a related class of ATP binding cassette transporters. CFTR maintains a cellular homeostatic balance of ions, including sodium, chloride and GSH. Normal levels of GSH in the ELF of the lung are 150 times higher than other tissues [87] where it serves as an essential antioxidant that protects the tissue from inhaled toxins. However, the presence of GSH in the ELF also provides a sensor system for maintaining surfactant production, as well as a trigger for inflammation. CF is characterized by systemic GSH deficiency that progresses over time [88]. Cellular GSH deficiency has been associated with an increase in transcription of NFκB, which participates in the regulation of the inflammatory cytokines [87]. Low levels of GSH lead to inflammation, a hallmark of CF, and oxidative stress that can lead to damage to cell membranes, cellular proteins and DNA. In support of the causative influence of ROS in CF, these patients frequently have higher levels of lipid peroxidation byproducts [89,90].
Adding to the complexity of disease progression is the expression pattern of GSTs (specifically GSTM) in CF patients. GSTM1 plays a role in the detoxification of hydroperoxides. Additionally, GSTM1 is a negative regulator of ASK1, a kinase involved in the apoptotic pathway [49]. CF patients with the null phenotype for GSTM1 (GSTM*0) have a poorer prognosis than individuals with other GSTM1 alleles [91]. At this time, the impact of allelic variation on the regulation of kinase signaling is unknown. We do know, however, that CF patients have a diminished immune response that is attributed to decreased GSH levels leading to premature apoptosis in macrophages and neutrophils recruited to the lung [87].
Some therapeutic strategies for CF are aimed at restoring GSH levels, particularly in the ELF. Cysteine supplementation has been undertaken in other disorders associated with GSH depletion, including PD. Because GSH deficiency is due to an aberration in efflux rather than synthesis, augmentation with GSH is desirable. Thus far three clinical strategies to supplement GSH have been attempted including intravenous and oral administration or inhalation [92]. Due to stability and uptake, inhalation methods appear more promising. Recent studies in seven CF patients treated with 600 mg of a GSH aerosol administered twice daily for 3 d showed an increase in ELF GSH levels and should provide a platform for future clinical trials [93].
6.6. Aging
Much has been written concerning the plausible inverse correlation between the generation of free radicals and subsequent ROS and the longevity of an organism. Intuitively, one might assume that natural selection would provide an adaptive force to secure cellular protective mechanisms that permit more efficient protection against such stresses. However, a number of issues cloud this principle and influence the selective advantage of efficient ROS detoxification systems. Specifically, protection of organisms early in life to permit the attainment of reproduction will serve to propagate the species and provide selective advantage. Thus, when diseases of pre-puberty result in production of ROS through, for example, a protective inflammatory response, the consequence would be survival to reach a reproductive age. To achieve this, unavoidable cumulative collateral damage may adversely influence the organism in the later stages of life. This outcome would have little consequence to the success of the individual in terms of selective advantage. In other words, the biological advantage of protecting somatic cells in old age will not have a major impact on survival of the species, whereas energy expended at younger ages will be a strong selective advantage. Therefore, there is significant advantage to counteracting childhood infections with macrophage mediated ROS defenses. Notwithstanding the age related relevance of the cellular defense mechanisms, the pleiotropic and redundant array of protective enzyme systems that counteract ROS include many that utilize GSH directly. The type and complement of these protective systems can vary significantly, both between tissues and between organisms. In the blood and or tissues of numerous organisms any or all of the following can contribute to protection against ROS: water soluble radical scavengers including GSH, ascorbate or urate; lipid soluble scavengers, α-tocopherol, γ-tocopherol, flavonoids, carotenoids, ubiquinol; enzymatic scavengers such as SOD, catalase and GPx and some GSTs; small molecule thiol-rich anti-oxidants such as thioredoxin and metallothionein; the enzymes that maintain small molecule anti-oxidants in a reduced state, thioredoxin reductase, GR, dehydroascorbate reductase, the glyoxalase system; the complement of enzymes that maintain a reduced cellular environment including glucose-6-phosphate dehydrogenase, in part responsible for maintaining levels of NADPH. The functional redundancy and cooperative interactions between this collection of defense pathways illustrates just how critical protection against ROS is to survival.
Numerous reports have correlated age related induction of oxidized or glycated proteins with a decreased ratio of GSH:GSSG in both invertebrates and vertebrates. A number of important metabolic enzymes, including aconitase III can be inactivated by oxidation [94]. This enzyme participates in the citric acid cycle and possesses an active site iron–sulfur cluster which is sensitive to inactivation by superoxide (O2−•) [95]. Carbonic anhydrase III has two reactive sulfhydryls that are subject to conversion to cysteine sulfinic acid or cysteic acid in the presence of H2O2, peroxy radicals or hypochlorous acid (HOCl). These reactions are competitively inhibited by GSH, perhaps as a consequence of glutathionylation of the effected cysteine residues [96]. These authors reported that in menadione treated rats, the extent of cysteine sulfinic acid damage to aconitase III was higher in older animals compared to young ones. In light of the potential widespread occurrence of protein glutathionylation (vide supra), it does not seem unreasonable to propose that this mechanism may prove to be functionally protective for a number of critical cellular proteins.
Some credence has been given to the relevance of ROS induced cell membrane damage as a causative event in cell aging and senescence. In this context, oxygen toxicity can be promoted by metals (such as iron and copper) that catalyze the cleavage of ROOH groups. This Fenton reaction generates hydroxyl radicals (OH•) which can abstract protons to initiate lipid peroxidation reactions. In healthy individuals, the potential catalytic activity of these metals is negatively regulated through binding to proteins such as ferritin and transferrin [69]. In aging humans the total body content of iron increases with age (in women after menopause). It was proposed that this increase would promote the occurrence of oxidative damage during the aging process [97] and that perhaps lipid peroxide induced membrane damage could be crucial to the onset of geriatric disease. Interestingly, a further indication that lipid peroxidation may be linked to aging is provided by studies in Drosophila [98]. By disrupting a microsomal glutathione S-transferase (mGST) like gene these authors showed that the mutant flies had a significantly reduced life-span compared to controls. One of the characteristic properties of mGST’s is their efficacy in detoxifying lipid peroxidation, particularly in the membrane compartments of cells.
A recent small scale study in healthy humans aged 19–85 measured the ratios of cysteine:cystine and GSH:GSSG in plasma [99]. For the former, a linear oxidation rate was observed throughout life. For GSH:GSSG ratios, there was no alteration in the redox balance until age 45, after which there was an enhanced oxidation at a nearly linear rate. While the correlation between low GSH levels or low GSH:GSSG ratios and aging seems clear, the precise reasons for the age related change in the content of GSH is less well characterized. One recent report has shown that a down-regulation of the regulatory subunit of γ-GCS occurs in the rat brain during aging [100]. As the combination of catalytic and regulatory subunits of GCS contributes to the de novo synthesis of GSH, this age related alteration in expression of this enzyme subunit may be a significant contributory factor in leading to the lower GSH levels.
Support for the free radical theory of aging has also been forthcoming from clinical studies in accelerated aging diseases such as Down’s syndrome, progeria—both adult (Werner’s syndrome) and childhood (Hutchison–Gilford). In each case a significantly shortened life-span is accompanied by evidence of increased oxidative stress and disturbance in the redox balance of host cells [101].
As discussed earlier, the immune response with respect to balance of Th1 and Th2 production can also be influenced by redox conditions. For example, thioredoxin transgenic mice had a longer life expectancy than their wild type counterparts. In these mice, peritoneal resident macrophages showed a higher ratio of GSH:GSSG compared to age-matched wild type animals [102]. These so-called reductive macrophages predominated and were associated with sustained maintenance of Th1 prevalence during aging until 2 years in the transgenic mice, while wild type littermates showed a rapid polarization to Th2 at 8 months. Cytokine production was different in these animals suggesting that altered redox balance, particularly as a consequence of GSH changes could influence immune response.
Dietary supplements frequently claim to be enriched in anti-oxidants and free radical scavengers. Are such claims of value to prevention of aging and the diseases associated with age? In the absence of carefully controlled (and possibly long term) clinical studies, this is a difficult question to answer. Inclusion of GSH in over-the-counter supplements is of limited value, since the reduced state will not be maintained when exposed to normal atmospheric conditions and room temperature. Perhaps the product will provide a supply of the constituent amino acids, where, in particular, cysteine may be useful in stimulating gastrointestinal synthesis of GSH. There is sufficient evidence that thiol containing compounds can rescue patients from acute exposure to oxidative or electrophilic stress (for example, NAC in acetaminophen overdose). It is also a general principle that cells prefer and thrive in a mildly reduced environment and that mild oxidative stress can stimulate growth in a manner not conducive to benign cellular homeostasis. As such, there would seem to be no harm in supplementing a diet with reducing equivalents. However, whether this will prove to be the elixir of longevity remains a contentious and difficult issue to answer accurately.
References
- [1].Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 1998;111–2:1–14. [DOI] [PubMed] [Google Scholar]
- [2].Mullineaux P, Creissen GP. Glutathione reductase: regulation and role in oxidative stress Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- [3].Meister MAA. Glutathione. Annu Rev Biochem 1983;52:711–60. [DOI] [PubMed] [Google Scholar]
- [4].Sierra-Rivera ESM, Dasouki M, Krishnamani MR, Phillips JA, Freeman ML. Assignment of the gene (GLCLC) that encodes the heavy subunit of y-glutamylcysteine synthetase to human chromosome 6. Cytogenet Cell Genet 1995;70:278–9. [DOI] [PubMed] [Google Scholar]
- [5].Sierra-Rivera EDM, Summar ML, et al. Assignment of the human gene (GLCLR) that encodes the regulatory subunit of y-glutamylcysteine synthetase to chromosome 1p21. Cytogenet Cell Genet 1996;72:252–4. [DOI] [PubMed] [Google Scholar]
- [6].Bannai S, Christensen HN, Vadgama JV, Ellory JC, Englesberg E, Guidotti GG, et al. Amino acid transport systems. Nature 1984;311:308. [DOI] [PubMed] [Google Scholar]
- [7].Moinova HR, Mulcahy RT. An electrophilic responsive element (EpRE) regulates beta-naphthoflavone induction of the human gamma-glutamylcysteine synthetase regulatory subunit gene. Constitutive expression is mediated by an adjacent AP-1 site. J Biol Chem 1998;273:14683–9. [DOI] [PubMed] [Google Scholar]
- [8].Wild A, Gipp JJ, Mulcahy T. Overlapping antioxidant response element and PMA response element sequences mediate basal and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase catalytic subunit gene. Biochem J 1998; 332(Pt 2):373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rahman ISC, Antonicelli F, MacNee W. Characterisation of y-glutamylcysteine-heavy subunit gene promoter:critical role for AP-1. FEBS Lett 1998;427:129–33. [DOI] [PubMed] [Google Scholar]
- [10].Bella DL, Hosokawa Y, Stipanuk MH. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am J Physiol 1999;276:E326–35. [DOI] [PubMed] [Google Scholar]
- [11].Gomi AMT, Ishikawa T, Kuo MT. Posttranscriptional regulation of MRP/GS-X pump and y-glutamylcysteine synthetase expression by 1(4-amino-2-methyl-5-pyrimidinyl)-methyl-3-(2-chloroethyl)-3-nitrosourea and by cycloheximide in human glioma cells. Biochem Biophys Res Commun 1997;239:51–6. [DOI] [PubMed] [Google Scholar]
- [12].Brigelius-Flohe R Tissue-specific functions of individual glutathione peroxidases. Free Radical Biol Med 1999;27:951–65. [DOI] [PubMed] [Google Scholar]
- [13].Downey JS, Bingle CD, Cottrell S, Ward N, Churchman D, Dobrota M, et al. The LEC rat possesses reduced hepatic selenium, contributing to the severity of spontaneous hepatitis and sensitivity to carcinogenesis. Biochem Biophys Res Commun 1998;244:463–7. [DOI] [PubMed] [Google Scholar]
- [14].Banki K, Hutter E, Gonchoroff NJ, Perl A. Molecular ordering in HIV-induced apoptosis. Oxidative stress, activation of caspases, and cell survival are regulated by transaldolase. J Biol Chem 1998;273:11944–53. [DOI] [PubMed] [Google Scholar]
- [15].Shisler JL, Senkevich TG, Berry MJ, Moss B. Ultraviolet-induced cell death blocked by a selenoprotein from a human dermatotropic poxvirus. Science 1998;279:102–5. [DOI] [PubMed] [Google Scholar]
- [16].Okamoto K, Toyokuni S, Uchida K, Ogawa O, Takenewa J, Kakehi Y, et al. Formation of 8-hydroxy-2'-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer 1994;58:825–9. [DOI] [PubMed] [Google Scholar]
- [17].Chinery R, Beauchamp RD, Shyr Y, Kirkland SC, Coffey RJ, Morrow JD. Antioxidants reduce cyclooxygenase-2 expression, prostag-landin production, and proliferation in colorectal cancer cells. Cancer Res 1998;58:2323–7. [PubMed] [Google Scholar]
- [18].Lee YJ, Galoforo SS, Berns CM, Chen JC, Davis BH, Sim JE, et al. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J Biol Chem 1998; 273:5294–9. [DOI] [PubMed] [Google Scholar]
- [19].Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal 1999;11:1–14. [DOI] [PubMed] [Google Scholar]
- [20].Roxas VP, Smith RK, Allen ER, Allen RD. Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat Biotechnol 1997;15:988–91. [DOI] [PubMed] [Google Scholar]
- [21].Lind C, Gerdes R, Schuppe-Koistinen I, Cotgreave IA. Studies on the mechanism of oxidative modification of human glyceraldehyde-3 phosphate dehydrogenase by glutathione: catalysis by glutaredoxin. Biochem Biophys Res Commun 1998;247:481–6. [DOI] [PubMed] [Google Scholar]
- [22].Klatt P, Pineda-Molina E, Garcia de Lacoba M, Padilla A, Martinez-Galisteo E, Barcena JA, et al. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J 1999;13:1481–90. [DOI] [PubMed] [Google Scholar]
- [23].Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 1999;13:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alam J, Camhi S, Choi AMK. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and induce dependent transcriptional enhancer. J Biol Chem 1995;270:11977–84. [DOI] [PubMed] [Google Scholar]
- [25].Ward NE, Pierce DS, Chung SE, Gravitt KR, O’Brian CA. Irreversible inactivation of protein kinase C by glutathione. J Biol Chem 1998; 273:12558–66. [DOI] [PubMed] [Google Scholar]
- [26].Wilhelm D, Bender K, Knebeland AP. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol 1997;17:4792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rainwater R, Parks D, Anderson ME, Tegtmeyer P, Mann K. Role of cysteine residues in regulation of p53 function. Mol Cell Biol 1995; 15:3892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu M, Pelling JC, Ju J, Chu E, Brash DE. Antioxidant action via p53-mediated apoptosis. Cancer Res 1998;58:1723–9. [PubMed] [Google Scholar]
- [29].Herzenberg LA, DeRosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, et al. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci USA 1997;94:1967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T-cell differentiation in the absence of JNK1. Science 1998; 281:2092–5. [DOI] [PubMed] [Google Scholar]
- [31].Powis G, Kirkpatrick DL, Angulo M, Baker A. Thioredoxin redox control of cell growth and death and the effects of inhibitors. Chem Biol Interact 1998;111–112:23–4. [DOI] [PubMed] [Google Scholar]
- [32].Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 1998;17:2596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gotoh Y, Cooper JA. Reactive oxygen species and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem 1998;273:17477–82. [DOI] [PubMed] [Google Scholar]
- [34].Makino Y, Yoshikawa N, Okamoto K, Hirota K, Yodoi J, Makino I, et al. Direct Association with thioredoxin allows redox regulation of glucocorticoid receptor function. J Biol Chem 1999;274:3182–8. [DOI] [PubMed] [Google Scholar]
- [35].Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, et al. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc Natl Acad Sci USA 2002;99:9745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shen H, Tsuchida S, Tamai K, Sato K. Identification of cysteine residues involved in disulfide formation in the inactivation of glutathione transferase P-form by hydrogen peroxide. Arch Biochem Biophys 1993;300:137–41. [DOI] [PubMed] [Google Scholar]
- [37].Walsh AC, Rosen DR, Lawrence DA. Genetic mapping of GLCLC, the human gene encoding the catalytic subunit of y-glutamylcysteine synthetase, to chromosome band 6pl 12 and characterisation of a polymorphic trinucleotide repeat within its 5'-untranslated region. Cytogenet Cell Genet 1996;75:114–6. [DOI] [PubMed] [Google Scholar]
- [38].Shi ZZ, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, et al. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci USA 2000;97:5101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Beutler EGT, Kondo T, Matsunaga AT. The molecular basis of a case of y-glutamylcysteine synthetase deficiency. Blood 1999;94:2890–4. [PubMed] [Google Scholar]
- [40].Rozet JM, Perrault I, et al. Structure and refinement of the physical mapping of the y-glutamylcysteine ligase regulatory subunit (GLCLR) gene to chromosome 1p22.1 within the critically deleted region of human malignant mesothelioma. Cytogenet Cell Genet 1998;82:91–4. [DOI] [PubMed] [Google Scholar]
- [41].Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci USA 1999;96:7220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mannervik B The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol 1985;57:357–417. [DOI] [PubMed] [Google Scholar]
- [43].Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res 1994;54:4313–20. [PubMed] [Google Scholar]
- [44].McLellan LI, Wolf CR. Glutathione and glutathione-dependent enzymes in cancer drug resistance. Drug Resist Update 1999;2:153–64. [DOI] [PubMed] [Google Scholar]
- [45].Tang AH, Tu CP. Biochemical characterization of Drosophila glutathione S-transferases D1 and D21. J Biol Chem 1994;269:27876–84. [PubMed] [Google Scholar]
- [46].Ranson H, Prapanthadara L, Hemingway J. Cloning and characterization of two glutathione S-transferases from a DDT-resistant strain of Anopheles gambiae. Biochem J 1997;324(Pt 1):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Adler V, Yin Z, Fuchs SY, Benerza M, Rosario L, Tew KD, et al. Regulation of JNK signaling by GSTp. EMBO J 1999;18:1321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yin Z, Ivanov V, Habelhah H, Tew KD, Ronai Z. Glutathione S-transferase p elicits protection against hydrogen peroxide-induced cell death via coordinated regulation of stress kinases. Cancer Res (Adv. In Brief) 2000;60:4053–7. [PubMed] [Google Scholar]
- [49].Cho SG, Lee YH, Park HS, Ryoo K, Kang KW, Park J, et al. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem 2001; 276:12749–55. [DOI] [PubMed] [Google Scholar]
- [50].Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000;103:239–52. [DOI] [PubMed] [Google Scholar]
- [51].Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal 2000;12:1–13. [DOI] [PubMed] [Google Scholar]
- [52].Smith G, Stanley LA, Sim E, Strange RC, Wolf CR. Metabolic polymorphisms and cancer susceptibility. Cancer Surv 1995;25:27–65. [PubMed] [Google Scholar]
- [53].Strange RC, Jones PW, Fryer AA. Glutathione S-transferase: genetics and role in toxicology. Toxicol Lett 2000;112–3:357–63. [DOI] [PubMed] [Google Scholar]
- [54].Smith CM, Kelsey KT, Wiencke JK, Leyden K, Levin S, Christiani DC. Inherited glutathione-S-transferase deficiency is a risk factor for pulmonary asbestosis. Cancer Epidemiol Biomarkers Prev 1994; 3:471–7. [PubMed] [Google Scholar]
- [55].Nelson HH, Wiencke JK, Christiani DC, Cheng TJ, Zuo ZF, Schwartz BS, et al. Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis 1995;16:1243–5. [DOI] [PubMed] [Google Scholar]
- [56].Strange RC, Fryer AA. The glutathione S-transferases: influence of polymorphism on cancer susceptibility. IARC Sci Publ 1999:231–49 [Chapter 19]. [PubMed] [Google Scholar]
- [57].Chenevix-Trench G, Young J, Coggan M, Board P. Glutathione S-transferase M1 and T1 polymorphisms: susceptibility to colon cancer and age of onset. Carcinogenesis 1995;16:1655–7. [DOI] [PubMed] [Google Scholar]
- [58].Pearson WR, Vorachek WR, Xu SJ, Berger R, Hart I, Vannais D, et al. Identification of class-mu glutathione transferase genes GSTM1-GSTM5 on human chromosome 1p13. Am J Hum Genet 1993;53:220–33. [PMC free article] [PubMed] [Google Scholar]
- [59].DeJong JL, Mohandas T, Tu CP. The human Hb (mu) class glutathione S-transferases are encoded by a dispersed gene family. Biochem Biophys Res Commun 1991;180:15–22. [DOI] [PubMed] [Google Scholar]
- [60].Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J 1997;324(Pt 1):25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cowell IG, Dixon KH, Pemble SE, Ketterer B, Taylor JB. The structure of the human glutathione S-transferase pi gene. Biochem J 1988; 255:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lo HW, Ali-Osman F. Structure of the human allelic glutathione S-transferase-pi gene variant, hGSTP1 C, cloned from a glioblastoma multiforme cell line. Chem Biol Interact 1998;111–2:91–102. [DOI] [PubMed] [Google Scholar]
- [63].Goto S, Iida T, Cho S, Oka M, Kohno S, Kondo T. Overexpression of glutathione S-transferase pi enhances the adduct formation of cisplatin with glutathione in human cancer cells. Free Radical Res 1999; 31:549–58. [DOI] [PubMed] [Google Scholar]
- [64].Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis 1998;19:275–80. [DOI] [PubMed] [Google Scholar]
- [65].Stoehlmacher J, Park DJ, Zhang W, Groshen S, Tsao-Wei DD, Yu MC, et al. Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst 2002;94:936–42. [DOI] [PubMed] [Google Scholar]
- [66].Ristoff E, Larsson A. Patients with genetic defects in the gamma-glutamyl cycle. Chem Biol Interact 1998;111–2:113–21. [DOI] [PubMed] [Google Scholar]
- [67].Shi ZZ, Habib GM, Rhead WJ, Gahl WA, He X, Sazer S, et al. Mutations in the glutathione synthetase gene cause 5-oxoprolinuria. Nat Genet 1996;14:361–5. [DOI] [PubMed] [Google Scholar]
- [68].Dringen RGJ, Hirlinger J. Glutathione metabolism in the brain. Eur J Biochem 2000;267:4912–6. [DOI] [PubMed] [Google Scholar]
- [69].Halliwell B, Gutteridge JM. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys 1986;246:501–14. [DOI] [PubMed] [Google Scholar]
- [70].Youdim MB. Understanding Parkinson’s disease. Sci Am 1997;276:52–9. [DOI] [PubMed] [Google Scholar]
- [71].Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 1996;55:259–72. [DOI] [PubMed] [Google Scholar]
- [72].Sofic ELK, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantial nigra of patients with Parkinson’s disease. Neurosci Lett 1992;142:128–30. [DOI] [PubMed] [Google Scholar]
- [73].Adams JD Jr, Klaidman LK. Parkinson’s disease—redox mechanisms. Curr Med Chem 2001;8:809–14. [DOI] [PubMed] [Google Scholar]
- [74].Andersen JK, Hom DG, Lee FY, Harnish P, Hamill RW, McNeil TH. Effect of buthionine sulfoximine, a synthesis inhibitor of the antioxidant glutathione, on the murine nigrostriatal neurons. J Neurochem 1996;67:2164–71. [DOI] [PubMed] [Google Scholar]
- [75].Sechi GDM, Bua G, Satta WM, Deiana GA, Pes GM, Rosati G. Reduced intravenous glutathione in the treatment of early Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 1996;20:1159–70. [DOI] [PubMed] [Google Scholar]
- [76].Buhl R, Jaffe HA, Holroyd KJ, Wells FB, Mastrangeli A, Saltini C, et al. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet 1989;2:1294–8. [DOI] [PubMed] [Google Scholar]
- [77].Staal F, Roederer M, Herzenberg LA, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kB and transcription of human immunodeficiency virus. Proc Natl Acad Sci USA 1990;87:9943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Duh E, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci USA 1989;86:5674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Suthanthiran M, Anderson ME, Sharma VK, Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci USA 1990;87:3343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gil L, Martinez G, Gonzalez I, Tarinas A, Alvarez A, Giuliani A, et al. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res 2003;47:217–24. [DOI] [PubMed] [Google Scholar]
- [81].Nakamura HMH, Yodoi J. Redox imbalance and its control in HIV infection. Antioxid Redox Signal 2002;4:455–64. [DOI] [PubMed] [Google Scholar]
- [82].DeRosa SC, Zaretsky MD, Dubs JG, Roederer M, Anderson M, Green A, et al. N-acetylcysteine replenishes glutathione in HIV infection. Eur J Clin Invest 2000;30(10):915–29. [DOI] [PubMed] [Google Scholar]
- [83].Micke PBK, Schlaak JF, Buhl R. Oral supplementation with whey proteins increases plasma glutathione levels of HIV-infected patients. Eur J Clin Invest 2001;31:171–8. [DOI] [PubMed] [Google Scholar]
- [84].Fernandez-Checa JC, Colell A, Garcia-Ruiz C. S-adenosyl-L-methionine and mitochondrial reduced glutathione depletion in alcoholic liver disease. Alcohol 2002;27(3):179–83. [DOI] [PubMed] [Google Scholar]
- [85].Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis 1998;18(4):389–401. [DOI] [PubMed] [Google Scholar]
- [86].Barbaro G, DiLorenzo G, Soldini M, Parrotto S, Bellomo G, Belloni G, et al. Hepatic glutathione deficiency in chronic hepatitis C: quantitative evaluation in patients who are HIV positive and HIV negative and correlations with plasmatic and lymphocytic concentrations and with the activity of the liver disease. Am J Gastroenterol 1996;91(12):2569–73. [PubMed] [Google Scholar]
- [87].Hudson V Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radical Biol Med 2001;30:1440–61. [DOI] [PubMed] [Google Scholar]
- [88].Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol 1993;75:2419–24. [DOI] [PubMed] [Google Scholar]
- [89].Brown RK, ScBurney A, Lunec J, Kelly FJ. Oxidative damage to DNA in patients with cystic fibrosis. Free Radical Biol Med 1995;234:137–46. [DOI] [PubMed] [Google Scholar]
- [90].Brown RK, Wyatt H, Price JF, Kelly FJ. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur Respir J 1996; 9:334–9. [DOI] [PubMed] [Google Scholar]
- [91].Hull J, Thomson AH. Contribution of genetic factors other than CFTR to disease severity in cystic fibrosis. Thorax 1998;53:1018–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Buhl R, Vogelmeier C, Critenden M, Hubbard RC, Hoyt RF Jr, Wilson EM, et al. Augmentation of glutathione in the fluid lining the epithelium of the lower respiratory tract by directly administering glutathione aerosol. Proc Natl Acad Sci USA 1990;87:4063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Roum JH, Borok Z, McElvaney NG, Grimes GJ, Bokser AD, Buhl R, et al. Glutathione aerosol suppresses lung epithelial surface inflammatory cell-derived oxidants in cystic fibrosis. J Appl Physiol 1999;87:438–43. [DOI] [PubMed] [Google Scholar]
- [94].Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe–S cluster containing hydro-lyases by superoxide. J Biol Chem 1993; 268:22369–76. [PubMed] [Google Scholar]
- [95].Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem 1991;266:19328–33. [PubMed] [Google Scholar]
- [96].Mallis RJ, Hamann MJ, Zhao W, Zhang T, Hendrich S, Thomas JA. Irreversible thiol oxidation in carbonic anhydrase III: protection by S-glutathiolation and detection in aging rats. Biol Chem 2002;383:649–62. [DOI] [PubMed] [Google Scholar]
- [97].Koster JF, Sluiter W. Is increased tissue ferritin a risk factor for atherosclerosis and ischaemic heart disease? Br Heart 1995;73:208–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Toba G, Aigaki T. Disruption of the microsomal glutathione S-transferase-like gene reduces life span of Drosophila melanogaster. Gene 2000;8:179–87. [DOI] [PubMed] [Google Scholar]
- [99].Jones DP, Mody VC Jr, Carlson JL, Lynn MJ, Sternberg P Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radical Biol Med 2002;33:1290–300. [DOI] [PubMed] [Google Scholar]
- [100].Liu RM. Down-regulation of gamma-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J Neurosci Res 2002;68:344–51. [DOI] [PubMed] [Google Scholar]
- [101].Knight JA. The biochemistry of aging. Adv Clin Chem 2000;35:1–62. [DOI] [PubMed] [Google Scholar]
- [102].Murata Y, Amao M, Yoneda J, Hamuro J. Intracellular thiol redox status of macrophages directs the Th1 skewing in thioredoxin transgenic mice during aging. Mol Immunol 2002;38:747–57. [DOI] [PubMed] [Google Scholar]