Abstract Abstract
A combined ecological, morphological, and molecular approach was used to examine 26 herbarium specimens and eight strains of Moesziomyces. The phylogenetic analysis resolved eight well-supported clades, of which three contained type specimens of known species of Moesziomyces. One clade contained two specimens that produced a teleomorph in the flowers of Echinochloakimberleyensis in Australia. The name Moesziomyceskimberleyensis is proposed for this smut fungus. Another clade contained specimens that produced sori in the flowers of Leersiahexandra. The name Thecaphoraglobuligera (now Moesziomycesglobuligerus) is available for this species, which is lectotypified. The teleomorph of Moesziomycesantarcticus, previously known only from Japan, is found for the first time in China, on Echinochloacrus-galli.
Keywords: Ecology, plant pathogens, phylogeny, Ustilaginaceae , Ustilaginomycotina
Introduction
The genus Moesziomyces (Ustilaginales, Ustilaginaceae) was established by Vánky (1977) for smut fungi that produce sori in the ovaries of grasses, lack a columella, and have spores with irregular meshes and wings on the surface, bound in firmly agglutinated spore balls. Vánky (1977) recognized four species, M.bullatus, M.evernius, M.globuligerus, and M.penicillariae. Vánky (1986, 2012, 2013) later synonymised these names with the oldest available name, M.bullatus, and considered Moesziomyces as monotypic. Species of Moesziomyces are known to produce both free-living saprobic anamorphs (yeast-like) and plant pathogenic teleomorphs (smuts) (Wang et al. 2015; Kruse et al. 2017). The anamorphs of Moesziomyces are readily culturable on artificial media and have been isolated from a range of substrates, while the teleomorphs are formed in ovaries of seven genera of grasses (Poaceae). Wang et al. (2015) recombined four species known only by their anamorphs (Pseudozymaantarctica, P.aphidis, P.parantarctica, and P.rugulosa) into Moesziomyces, based on a molecular phylogenetic analysis. Subsequently, Tanaka et al. (2019) showed that one of these species, M.antarcticus, produced a teleomorph on Echinochloacrus-galli in Japan. A further five species, M.bullatus, M.eriocauli, M.evernius, M.penicillariae, and M.verrucosus, have been characterized from teleomorphs (Vánky 2012; Wang et al. 2015; Kruse et al. 2017). Kruse et al. (2017) recognized six species of Moesziomyces based on phylogenetic analysis, and treated M.aphidis and M.rugulosus as synonyms of M.bullatus.
The teleomorphs of Ustilaginaceae are mostly host specific (Stoll et al. 2003, 2005; Skibbe et al. 2010; McTaggart et al. 2012; Li et al. 2017a, 2017b). Given that species of Moesziomyces have been reported from seven different genera of grasses (Echinochloa, Leersia, Panicum, Paspalum, Pennisetum, Polytrias, and Uranthoecium), it is likely that additional species remain to be discovered. The aim of this study was to build on the work of Kruse et al. (2017) by examining specimens of Moesziomyces held in herbaria BRIP (Queensland Plant Pathology Herbarium), HMAS (Herbarium Mycologicum Academiae Sinicae), and HUV (Herbarium Ustilaginales Vánky, now deposited in BRIP), as well as eight yeast strains deposited in LC Culture Collection (personal culture collection held in the laboratory of Dr Lei Cai).
Materials and methods
Specimen examination
Specimens borrowed from several herbaria were examined by light microscopy (Table 1) by mounting the spores in lactic acid (100% v/v). Teliospore measurements were expressed as ranges (min–) mean-standard deviation-mean + standard deviation (–max) (n = 50). Images were captured by using a Nikon DS-Fi1 camera attached to a Nikon Eclipse 80i microscope with Nomarski differential interference contrast. Helicon Focus ver. 4.46.1 (Helicon Soft Ltd) was used to combine images to increase depth of field. Nomenclatural novelties and descriptions were registered in MycoBank (http://www.MycoBank.org).
Table 1.
Collection details for Moesziomyces specimens newly sequenced in this study.
| Species | Specimen/strain no.1 | Host | Source | Location | Year of collection | ITS GenBank accession number2 |
|---|---|---|---|---|---|---|
| Moesziomyces antarcticus | HMAS 248025 | Echinochloa crus-galli | Sorus | China | 2017 | MK027038 |
| M. antarcticus | HMAS 248026 | E. crus-galli | Sorus | China | 2017 | MK027039 |
| M. antarcticus | HMAS 60130 | E. crus-galli | Sorus | China | 1989 | MK027043 |
| M. bullatus | HMAS 146471 | E. crus-galli | Sorus | China | 2003 | MK027040 |
| M. bullatus | HMAS 50052 | E. crus-galli | Sorus | China | 1985 | MK027041 |
| M. bullatus | LC-CLS58-3-2 | Setaria faberii | Leaf surface | China | 2017 | MK024201 |
| M. bullatus | LC-CLS58-3-21 | S. faberii | Leaf surface | China | 2017 | MK024202 |
| M. bullatus | LC-CLS58-3-22 | S. faberii | Leaf surface | China | 2017 | MK024203 |
| M. bullatus | LC-CLS60-2-22 | Pennisetum sp. | Leaf surface | China | 2017 | MK024204 |
| M. bullatus | LC-CLS60-2-4 | Pennisetum sp. | Leaf surface | China | 2017 | MK024205 |
| M. bullatus | LC-SY1-2-11 | Digitaria sp. | Leaf surface | China | 2017 | MK024206 |
| M. bullatus | LC-SY1-2-21 | Digitaria sp. | Leaf surface | China | 2017 | MK024207 |
| M. bullatus | LC-SY1-2-22 | Digitaria sp. | Leaf surface | China | 2017 | MK024208 |
| M. bullatus | HMAS 50454 | E. crus-galli | Sorus | Japan | 1985 | MK027042 |
| M. bullatus | HMAS 70876 | E. crus-galli | Sorus | China | 1991 | MK027045 |
| M. bullatus | HMAS 73871 | E. crus-galli | Sorus | China | 1996 | MK027046 |
| M. bullatus | HUV 2442* | E. crus-galli | Sorus | Poland | 1869 | MK027047 |
| M. bullatus | HUV 305 | E. crus-galli | Sorus | Germany | 1905 | MK027050 |
| M. globuligerus | BRIP 27384 | Leersia hexandra | Sorus | Australia | 1998 | MK027025 |
| M. globuligerus | BRIP 44301 | L. hexandra | Sorus | Australia | 2004 | MK027029 |
| M. globuligerus | BRIP 44569 | L. hexandra | Sorus | Australia | 2004 | MK027030 |
| M. globuligerus | BRIP 47767 | L. hexandra | Sorus | Thailand | 2005 | MK027031 |
| M. globuligerus | BRIP 47768 | L. hexandra | Sorus | Thailand | 2005 | MK027032 |
| M. globuligerus | BRIP 51872 | L. hexandra | Sorus | Australia | 2008 | MK027035 |
| M. globuligerus | HMAS 248027 | L. hexandra | Sorus | China | 2017 | MK027037 |
| M. kimberleyensis | BRIP 51843* | E. kimberleyensis | Sorus | Australia | 2008 | MK027034 |
| M. kimberleyensis | BRIP 52498 | E. kimberleyensis | Sorus | Australia | 2009 | MK027036 |
| M. penicillariae | HUV 2487 | Pe. glaucum | Sorus | Gambia | 1973 | MK027048 |
| M. penicillariae | HUV 2488 | Pe. glaucum | Sorus | India | 1912 | MK027049 |
| M. verrucosus | BRIP 39886 | Paspalum distichum | Sorus | Australia | 2003 | MK027026 |
| M. verrucosus | BRIP 43727 | Pa. distichum | Sorus | Australia | 2004 | MK027027 |
| M. verrucosus | BRIP 43735 | Pa. distichum | Sorus | Australia | 2004 | MK027028 |
| M. verrucosus | BRIP 51772 | Pa. distichum | Sorus | India | 1992 | MK027033 |
| M. verrucosus | HMAS 66437 | Pa. distichum | Sorus | India | 1992 | MK027044 |
1BRIP: Queensland Plant Pathology Herbarium, Brisbane, Australia; HMAS: Herbarium Mycologicum Academiae Sinicae; HUV: Herbarium Ustilaginales Vánky (located at BRIP). 2GenBank accessions derived from this study are shown in bold. * Type specimens.
DNA extraction, PCR amplification and sequencing
Sori were carefully removed from herbarium specimens, up to 149 years old, with a fine needle, sterilized by dipping in 75% ethanol for 30 s, air-dried on sterilized filter paper, and deposited in cell lysis solution (CTAB). Pure yeast colonies grown on yeast extract peptone dextrose (YPD) plates were transferred to cell lysis solution directly. Genomic DNA was extracted following the protocol of Cubero et al. (1999). Fragments of internal transcribed spacer rDNA were amplified by PCR with primers M-ITS1/ITS4 (White et al. 1990; Stoll et al. 2003).
PCR amplifications were carried out in 25 μl reactions containing 1 μl of genomic DNA template, 9.5 μl distilled water, 12.5 μl of 2 X Taq Plus Master Mix (Nanjing Vazyme Biotech Co. Ltd, Nanjing, China) and 1 μl of each primer (10 μM). Amplification reactions were run as follows: initial denaturation of 95 °C for 5 min followed by 35 cycles at 95 °C for 30 s, 45 s at 58 °C (annealing temperature) and 1 min at 72 °C with a final extension of 10 min at 72 °C. PCR products were sent to Tianyihuiyuan (Beijing, China) for sequencing with the forward and reverse primers indicated above. AB1 sequence traces were assembled with Sequencher version 5 (Genecodes, Ann Arbor, USA).
Phylogenetic analyses
The sequences included in this study (Tables 1, 2) were aligned online with MAFFT (https://mafft.cbrc.jp/alignment/server/index.html) using auto strategy, and observed in MEGA 5 (Katoh and Toh 2008). Phylogenetic analyses were based on both maximum likelihood (ML) and Bayesian Inference (BI). RAxML (Stamatakis 2006) and PhyML 3.0 (Guindon et al. 2010) were used for ML analyses. GTRGAMMA was specified as the model of evolution in both programs. The RAxML analyses were run with a rapid Bootstrap analysis (command -f a) using a random starting tree and 1 000 ML bootstrap replicates. The PhyML analyses were implemented using the ATGC bioinformatics platform (available at: http://www.atgcmontpellier.fr/phyml/), with six substitution type and SPR tree improvement, and support obtained from an approximate likelihood ratio test (Anisimova et al. 2011).
Table 2.
List of Moesziomyces, Triodiomyces, and Ustilago sequences taken from GenBank and used in the phylogenetic analysis.
| Species | Source | ITS GenBank accession number | Reference |
|---|---|---|---|
| Moesziomyces antarcticus | – | JX094775 | Gujjari et al. (unpubl.) |
| – | JN942669 | An (unpubl.) | |
| unpolished Japanese rice | AB089360 | Sugita et al. 2003 | |
| Antarctica sediment | AF294698 | Avis et al. 2001 | |
| Albiziajulibrissin flower | AY641557 | Wei et al. 2005 | |
| lake sediment | AB089358 | Sugita et al. 2003 | |
| tomato rhizosphere | KF493994 | Johnston-Monje et al. (unpubl.) | |
| Echinochloacrus-galli sorus | LC368624 | Tanaka et al. 2019 | |
| Echinochloacrus-galli sorus | LC368624 | Tanaka et al. 2019 | |
| Echinochloacrus-galli sorus | LC368624 | Tanaka et al. 2019 | |
| Echinochloacrus-galli sorus | LC368624 | Tanaka et al. 2019 | |
| Echinochloacrus-galli sorus | LC368624 | Tanaka et al. 2019 | |
| Echinochloacrus-galli sorus | LC368624 | Tanaka et al. 2019 | |
| Echinochloacrus-galli sorus | LC368625 | Tanaka et al. 2019 | |
| Moesziomyces bullatus | human preterm low birth weight infant | KF926673 | Okolo et al. 2015 |
| – | DQ831013 | Matheny et al. 2006 | |
| Japanese pear fruit | AB204896 | Yasuda et al. 2007 | |
| Saccharum officinarum | AB704889 | Morita et al. 2012 | |
| Leucaena glauca | HQ662536 | Wei et al. 2011 | |
| human | EU105207 | Lin et al. 2008 | |
| human blood | AB089362 | Sugita et al. 2003 | |
| human | HQ848933 | Xie et al. unpubl. | |
| Fallopia japonica | KC282385 | Wang & Liu (unpubl.) | |
| human blood | KM610219 | Bosco-Borgeat & Taverna (unpubl.) | |
| Leucaena glauca | HQ647299 | Wei et al. 2011 | |
| Saccharum officinarum | AB704890 | Morita et al. 2012 | |
| poplar leaf | KM268868 | Sun & Yan (unpubl.) | |
| Forcipomyia taiwana | KM555221 | Chen (unpubl.) | |
| seaweed | KP269028 | Wang et al. (unpubl.) | |
| aphid secretion | AF294699 | Avis et al. 2001 | |
| Neoreglia cruenta | FN424100 | Garcia et al. (unpubl.) | |
| Saccharum officinarum | AB704878 | Morita et al. 2012 | |
| giant panda secretion | KF973199 | Li et al. (unpubl.) | |
| Camelliasinensis leaf lesions | HQ832804 | Li et al. (unpubl.) | |
| Echinochloa crus-galli | GU390690 | Hamayun & Ahmad (unpubl.) | |
| aphid secretion on Solanumpseudocapsicum | JN942666 | An (unpubl.) | |
| Citrus leaf | JQ425372 | Soliman (unpubl.) | |
| – | JN942667 | An (unpubl.) | |
| mouldy Zeamays leaf | AB089370 | Sugita et al. 2003 | |
| plant leaf | HE650886 | Han et al. 2012 | |
| ex-leaf of corn | AF294697 | Avis et al. 2001 | |
| Hyoscyamus muticus | AB500693 | Abdel-Motaal & Itu (unpubl.) | |
| Coffea arabica | EU002890 | Vega et al. (unpubl.) | |
| Coffea arabica | DQ778919 | Vega et al. 2008 | |
| Saccharumofficinarum leaf | LC053989 | Surussawadee & Limtong (unpubl.) | |
| marine environment | DQ178645 | Chang et al. 2008 | |
| Helicoverpaarmigera larva gut | AM160637 | Molnar & Prillinger (unpubl.) | |
| Moesziomyces bullatus | marine sediment | KC834821 | Qu et al. (unpubl.) |
| – | KR047769 | Wang et al. (unpubl.) | |
| pharmaceutical effluent | KF922220 | Selvi & Das (unpubl.) | |
| barley kernels and leaf | HG532070 | Korhola et al. 2014 | |
| Ericaceae roots | HQ260042 | Walker et al. 2011 | |
| cleaned rice | AB235999 | Ikeda et al. 2007 | |
| Arabidopsisthaliana infected with Albugolaibachii | KY930224 | Kruse et al. 2017 | |
| Echinochloa crus-galli | KY424439 | Kruse et al. 2017 | |
| Echinochloa crus-galli | KY424428 | Kruse et al. 2017 | |
| Echinochloa crus-galli | KY424429 | Kruse et al. 2017 | |
| Echinochloa muricata | KY424430 | Kruse et al. 2017 | |
| Echinochloa muricata | KY424431 | Kruse et al. 2017 | |
| Echinochloa muricata | KY424432 | Kruse et al. 2017 | |
| Echinochloa muricata | KY424433 | Kruse et al. 2017 | |
| Echinochloa crus-galli | KY424434 | Kruse et al. 2017 | |
| Echinochloa crus-galli | KY424435 | Kruse et al. 2017 | |
| Echinochloa crus-galli | KY424436 | Kruse et al. 2017 | |
| Echinochloa crus-galli | KY424437 | Kruse et al. 2017 | |
| Echinochloa crus-galli | KY424427 | Kruse et al. 2017 | |
| Echinochloa crus-galli | KY424438 | Kruse et al. 2017 | |
| shoot of tip pepper | GU975792 | Sim et al. (unpubl.) | |
| Moesziomyces eriocauli | Eriocaulon cinereum | AY740041 | Stoll et al. 2005 |
| Moesziomyces parantarcticus | – | KP132543 | Irinyi et al. 2015 |
| human blood | AB089356 | Sugita et al. 2003 | |
| – | NR130693 | An (unpubl.) | |
| – | JN544036 | Chen (unpubl.) | |
| yam tuber steep water | KF619567 | Babajide et al. 2015 | |
| Axonopuscompressus soil | HQ436080 | Kee & Chia (unpubl.) | |
| Moesziomyces penicillariae | Pennisetum glaucum | KY424440 | Kruse et al. 2017 |
| Moesziomyces verrucosus | Paspalum distichum | AY740153 | Stoll et al. 2005 |
| Triodiomyces altilis | Triodia pungens | AY740166 | Stoll et al. 2005 |
| Ustilago echinata | Phalaris arundinacea | AY345001 | Stoll et al. 2003 |
For BI, MrBayes was used with a Markov Chain Monte Carlo algorithm incorporating four runs, each consisting of four chains, until the standard deviation of split frequencies was reached. The cold chain was heated at a temperature of 0.25. Substitution model parameters were sampled every 50 generations and trees were saved every 5000 generations. Convergence of the Bayesian analysis was confirmed using AWTY (Nylander et al. 2008) (available at: http://ceb.csit.fsu.edu/awty/). A user-defined tree obtained from the PhyML analyses was used as a starting point for all the Bayesian analyses, which helped to improve convergence of the four runs.
Results
The ITS dataset comprised the newly sequenced Moesziomyces specimens and strains (Table 1) together with the reference sequences of Moesziomyces from Kruse et al. (2017) and Tanaka et al. (2019) (Table 2) and Triodiomycesaltilis and Ustilagoechinata as the outgroup based on the phylogenetic analyses of Wang et al. (2015). The topology of the ML and BI analyses (Fig. 1) were congruent. The phylogenetic analyses revealed eight distinct groups with high support values, including six clades consistent with those recovered by Kruse et al. (2017). The largest clade included specimens of M.bullatus on Echinochloacrus-galli (the host for the type specimen of M.bullatus) and E.muricata from Europe, related yeast strains as well as strains formerly assigned to the synonymous species names Pseudozymaaphidis and P.rugulosa (Kruse et al. 2017). Four well-supported clades comprised teleomorphic specimens on Echinochloakimberleyensis, Leersiahexandra, Paspalumdistichum, and Pennisetumglaucum (the latter with related yeast strains). One well-supported clade comprised yeast strains assigned to M.parantarcticus. One moderately supported clade comprised teleomorphic specimens on E.crus-galli from China and Japan and related yeast strains, assigned to M.antarcticus. The remaining single-sequence lineage was formed by Moesziomyceseriocauli on Eriocauloncinereum (Eriocaulaceae).
Figure 1.
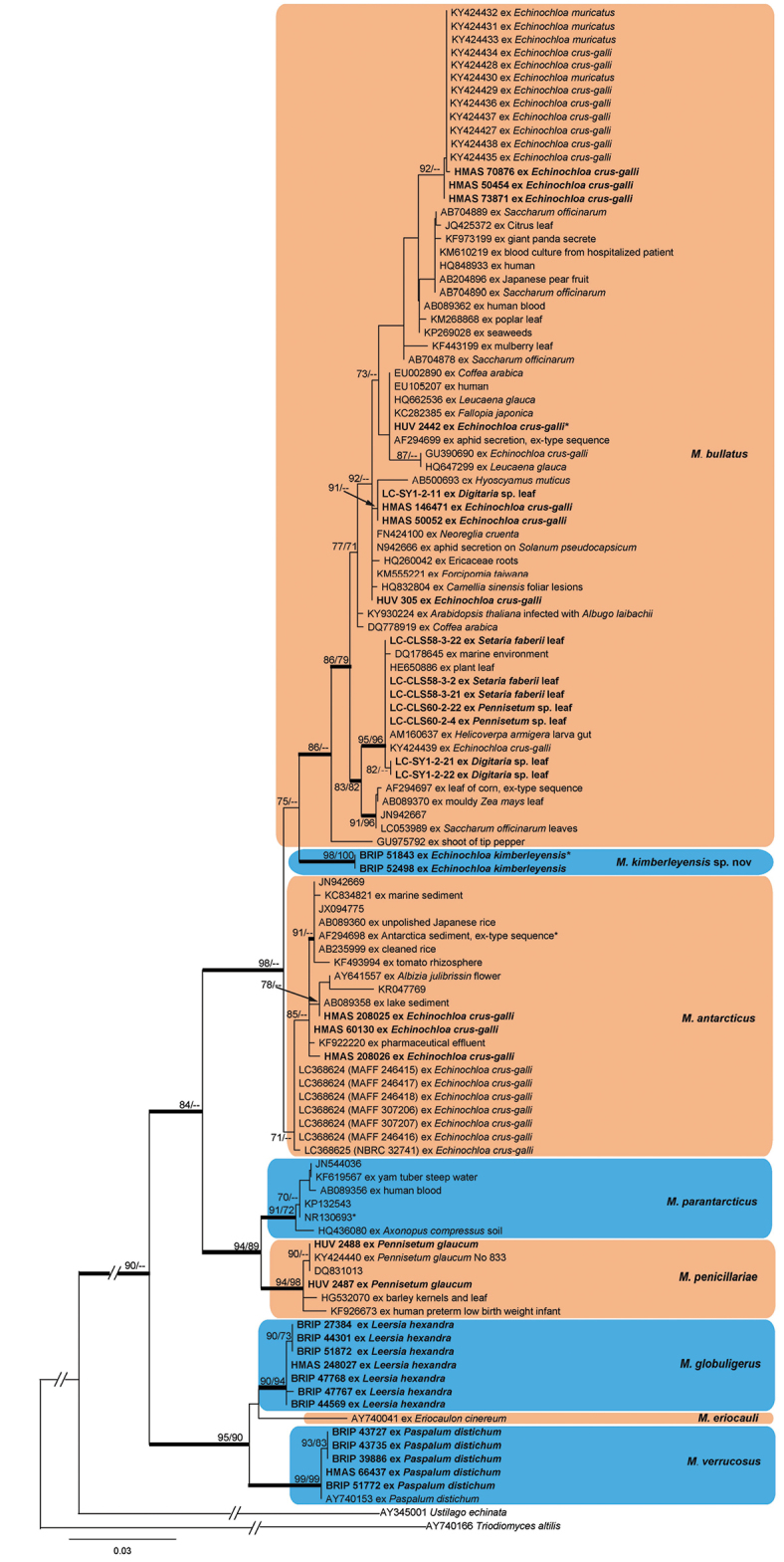
Phylogram obtained from a ML analysis based on the ITS sequence alignment. Values above the branches represent ML bootstrap values (> 70%) from RaxML and PhyML analysis respectively. Thickened branches represent Bayesian posterior probabilities (> 0.95). The scale bar indicates 0.03 expected substitutions per site. * indicates type specimens or type strains.
Taxonomy
Based on the phylogenetic analysis and the hosts of the teleomorphs, a new species of Moesziomyces is described and another species resurrected. Additionally, the teleomorph of M.antarcticus is reported for the first time from China.
Moesziomyces antarcticus
(Goto, Sugiyama & Iizuka) Q.M. Wang, Begerow, F.Y. Bai & Boekhout, Stud. Mycol. 81: 81 (2015)
Figure 2.
a–dMoesziomyceskimberleyensis (holotype BRIP 51843) e–gMoesziomycesglobuligerus (BRIP 27384) h–kMoesziomycesantarcticus (HMAS 60130). a, b: sori. c, d, f, j, k: spores under LM. g: spores under SEM. Scale bars: 1 mm (b, i); 10 µm (c, d, f, g, j, k).
Sporobolomyces antarcticus Goto, Sugiyama & Iizuka, Mycologia 61: 759 (1969). [Basionym]
Candida antarctica (Goto, Sugiyama & Iizuka) Kurtzman et al. Yeasts: 86 (1983).
Vanrija antarctica (Goto, Sugiyama & Iizuka) R.T. Moore, Bibltheca Mycol. 108: 167 (1987).
Pseudozyma antarctica (Goto, Sugiyama & Iizuka) Boekhout, J. Gen. Appl. Microbiol. 41: 364 (1995).
Trichosporon oryzae H. Ito, Iizuka & T. Sato, Agric. Biol. Chem. 38: 1599 (1974). (synonymy by Q.M. Wang, Begerow, F.Y. Bai and Boekhout).
Description.
Sori in scattered ovaries, sometimes deciduous, globose to ovoid, 2–3 mm in length, covered by a smooth green membrane of host tissue origin that becomes brown and ruptures irregularly to expose a granular, black to dark brown mass of spore balls; columella absent. Spore balls variable in shape and size, globose, subglobose, ovoid, elongate to irregular, 130–200 μm in diameter, dark brown, composed of up to several hundred spores, united firmly by fungal sterile cells and spore meshes and wings. Spore globose, ovoid to irregular, slightly polyhedral, (8–) 8.5–9.5 (–10) × (6–) 7–9 (–10) μm, usually with well-developed meshes and wings, subhyaline to pale yellowish-brown; wall 0.5 μm thick, smooth. Some of the sterile cells empty at maturity, thin-walled, with irregular meshes or wings on the spore surface when the spores separates; other sterile cells, globose, ovoid to irregular, slightly polyhedral, (8–) 8.5–9.5 (–10) × (6–) 7–9 (–10) μm, subhyaline to pale yellowish brown; wall 1–1.5 μm thick, smooth.
Specimens examined.
CHINA, Sichuan, Chengdu, on Echinochloacrus-galli, 15 Sept. 1989, L. Guo leg., HMAS 60130; Guangxi, on E.crus-galli, Oct. 2017, R.G. Shivas, M.D.E. Shivas & Y.-M. Li leg., HMAS 208025; Guangxi, on E.crus-galli, Oct. 2017, R.G. Shivas, M.D.E. Shivas & Y.-M. Li leg., HMAS 208026.
Notes.
The teleomorph of Moesziomycesantarcticus was previously reported from Japan, on Echinochloacrus-galli (Tanaka et al. 2019). The current report from China, also on E.crus-galli, suggests that this smut fungus may be common in the teleomorphic stage, at least in East Asia.
Moesziomyces globuligerus
(Berk. & Broome) Vánky, Bot. Not. 130: 135 (1977)
Thecaphora globuligera Berk. & Broome, Trans. Linn. Soc. London, Bot., Ser. 2, 1: 407 (1880). — Type: AUSTRALIA, Queensland, Brisbane, on Leersiahexandra, F.M. Bailey, No. 86 (K(M) 252436, lectotype designated here, MBT 385180, not seen; K(M) 252437, syntype). [Basionym]
Tolyposporium globuligerum (Berk. & Broome) Ricker, J. Mycol. 11:112 (1905).
Testicularia leersiae Cornu, Ann. Sci. Nat. Bot., Sér. 6, 15: 275 (1883).
Description.
Sori in some of the ovaries, often deciduous, ellipsoidal to oval, 2.5–4 × 1.5–3 mm, green at first, later brown, smooth, ruptures irregularly to reveal a granular, dark brown mass of spore balls; columella absent. Spore balls subglobose, ellipsoidal or irregular, 75–150 µm in diameter, yellowish brown, composed of up to several hundred spores that separate by moderate pressure. Spores subglobose, ovoid to irregularly polyhedral, (8–) 8.5–11 (–13) × (6–) 7–9 (–10) μm (xˉ = 9.6 ± 1.2 × 7.9 ± 0.9 μm, n = 50), subhyaline to pale yellowish brown, attached together by multiple narrow cylindrical protuberances about 2 μm wide and 1–2 μm long; wall with irregular meshes and wings, less than 0.5 μm thick, smooth. (Based on specimen BRIP 27384).
Specimens examined.
AUSTRALIA, Queensland, Willowbank, on Leersiahexandra, 9 Mar. 1998, C. Vánky & K. Vánky leg., BRIP 27384; Queensland, Mareeba, on L.hexandra, 1 May 2004, M.D.E. Shivas & R.G. Shivas leg., BRIP 44301; Queensland, Mt Garnet, on L.hexandra, 5 May 2005, T.S. Marney & R.G. Shivas leg., BRIP 44569; Northern Territory, Darwin, on L.hexandra, 15 Apr. 2008, J. Ray, A.A. Mitchell, A.R. McTaggart & R.G. Shivas leg., BRIP 51872. CHINA, Guangxi province, on L.hexandra, Oct. 2017, R.G. Shivas, M.D.E. Shivas, Y.-M. Li, P. Zhao & X.-H. Qi leg., HMAS 248027. THAILAND, Kanchanaburi, on L.hexandra, 16 Dec. 2005, R.G. Shivas & M.D.E. Shivas leg., BRIP 47767; Chiang Mai, on L.hexandra, 26 Dec. 2005, R.G. Shivas & M.D.E. Shivas leg., BRIP 47768.
Notes.
Vánky (1986) considered that M.globuligerus was a synonym of M.bullatus based on their similar morphologies. Phylogenetic analyses support M.globuligerus as a distinct species (Fig. 1), with a teleomorph specific to the pantropical grass Leersiahexandra (Berkeley and Broome 1880). The name Testicularialeersiae (Cornu 1883), described from infected Leersiahexandra in Algeria, is likely a heterotypic synonym of M.globuligerus, but this has not been checked by molecular phylogenetic analysis. The type material of Thecaphoraglobuligera was collected circa 1878 from near the Brisbane River, Queensland, Australia by the botanist F. M. Bailey (Berkeley and Broome 1880). Original material of this specimen (F.M. Bailey, No. 86) could not be found in the Australian herbaria BRI and BRIP, where most of F.M. Bailey’s specimens are held. Two syntypes were located in K(M), of which K(M) 252436 ex C.E. Broome herbarium (BM) was selected as lectotype of T.globuligera (now M.globuligerus). The material in the second specimen, K(M) 252437 from the Berkeley herbarium, was scant (Dr Begoña Aguirre-Hudson pers. comm).
Moesziomyces kimberleyensis
Y.M. Li, L. Cai & R.G. Shivas sp. nov.
827986
Type.
AUSTRALIA, Western Australia, Kununurra, Mulligan’s Lagoon Road, on Echinochloakimberleyensis, 9 Apr. 2008, A.R. McTaggart, V.L. Challinor, A.D.W. Geering, M.D.E. Shivas & R.G. Shivas leg. (holotype: BRIP 51843).
Etymology.
Named after the Kimberley region of northern Western Australia from where it was collected.
Description.
Sori in some of the ovaries, often deciduous, globose to ovoid, 3–6 × 2–4 mm, green at first, later brown, smooth, ruptures irregularly to reveal a granular, dark brown mass of spore balls; columella absent. Spore balls subglobose, ovoid, elongate or irregular, 275–100 µm diam, dark brown, composed of up to several hundred spores, separated by moderate pressure. Spore globose, ovoid to irregular, slightly polyhedral, (9–) 9.5–12 (–14.5) × (8–) 8.5–9.5 (–10) μm (xˉ = 10.5 ± 1.2 × 8.9 ± 0.7 μm, n = 50), subhyaline to yellowish brown, attached together by multiple narrow cylindrical protuberances about 2 μm wide and 1–2 μm long; wall with irregular meshes and wings, 0.5 μm thick, smooth.
Additional specimen examined.
AUSTRALIA, Western Australia, Kununurra, Mulligan’s Lagoon Road, on E.kimberleyensis, 7 May 2009, A.R. McTaggart, M.J. Ryley, M.D.E. Shivas & R.G. Shivas leg. (BRIP 52498).
Notes.
Moesziomyceskimberleyensis was shown in the phylogenetic analysis to reside in a well-supported clade sister to M.bullatus. Moesziomyceskimberleyensis is only known from the teleomorph, which forms sori in flowers of E.kimberleyensis, and thereby differs from M.bullatus by host association. Moesziomyceskimberleyensis is only known from one location in Western Australia on E.kimberleyensis, which is an endemic grass in the tropical and subtropical woodlands of northern Australia.
Discussion
The phylogenetic analyses in this study supported the host specificity of the teleomorphic stage of six species of Moesziomyces, specifically, M.antarcticus on Echinochloacrus-galli, M.bullatus on E.crus-galli and E.muricata, M.globuligerus on Leersiahexandra, M.kimberleyensis on E.kimberleyensis, M.penicillariae on Pennisetumglaucum, and M.verrucosus on Paspalumdistichum. The teleomorph of M.eriocauli may be specific to Eriocaulon spp., although this cannot be ascertained from the sequence data of one specimen. Specimens that have been assigned to M.bullatus were not well resolved and formed a number of smaller clades with varying degrees of support (Fig. 1). The M.bullatus clade contained several anamorphic yeasts isolated from diverse habitats (Wang et al. 2015; Kruse et al. 2017), including leaves of Digitaria sp., Pennisetum sp., and Setariafaberii. This shows that the anamorphs of Moesziomyces are widespread in the environment as saprobes.
The anamorphs of Moesziomyces, together with most members of the Ustilaginales, have a dimorphic lifecycle comprised of a parasitic dikaryotic phase characterized by teliospores, together with a saprobic yeast-like haploid phase (Brefeld 1883; de Bary 1884; Sampson 1939; Begerow et al. 2014). The teliospores are generally thick-walled and darkened, which protects against desiccation and UV radiation, thereby facilitating survival and long-distance dispersal (Piepenbring et al. 1998). The basidiospores are usually thin-walled, hyaline, and survive as free-living saprobic yeasts that may occur on a vast diversity of substrates (Wang et al. 2015; Kruse et al. 2017; Tanaka et al. 2019). There is genomic evidence that some saprobic ustilaginalean yeasts, e.g. M.antarcticus, Kalmanozymabrasiliensis (= P.brasiliensis), Pseudozymahubeiensis, and the yeast stage of M.bullatus (= P.aphidis), have retained the capacity to produce effector proteins, which hints at the possibility that undiscovered plant pathogenic stages may exist for these fungi (Sharma et al. 2018). Indeed, a teleomorph for M.antarcticus (=P.antarctica) was recently reported for the first time on Echinochloacrus-galli (Tanaka et al. 2019). Further collections are needed to resolve the ecological relationships and elucidate the life cycles of the ustilaginalean fungi and their hosts.
Supplementary Material
Acknowledgements
We thank Dr Begoña Aguirre-Hudson (Royal Botanic Gardens, Kew) for providing information about the syntypes of Thecaphoraglobuligera. We are also grateful to Dr Julia Kruse (University of Southern Queensland) for helpful comments about the manuscript. Marjan Shivas, Peng Zhao, Fang Liu, and Xiao-Hua Qi are thanked for assistance with specimen collection. This study was financially supported by CAS-QYZDB-SSW-SMC044 and CAAS-ASTIP-IVFCAAS.
Citation
Li Y-M, Shivas RG, Li B-J, Cai L (2019) Diversity of Moesziomyces (Ustilaginales, Ustilaginomycotina) on Echinochloa and Leersia (Poaceae). MycoKeys 52: 1–16. https://doi.org/10.3897/mycokeys.52.30461
Funding Statement
This study was financially supported by CAS-QYZDB-SSW-SMC044 and CAAS-ASTIP-IVFCAAS.
References
- Anisimova M, Gil M, Dufayard J-F, Dessimoz C, Gascuel O. (2011) Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Systematic Biology 60: 685−699. 10.1093/sysbio/syr041 [DOI] [PMC free article] [PubMed]
- Ariyawansa HA, Hawksworth DL, Hyde KD, Jones EBG, Maharachchikumbura SSN, Manamgoda DS, Thambugala KM, Udayanga D, Camporesi E, Daranagama A, Jayawardena R, Liu JK, McKenzie EHC, Phookamsak R, Shivas RG. (2014) Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Diversity 69: 57–91. 10.1007/s13225-014-0315-4 [DOI] [Google Scholar]
- Avis TJ, Caron SJ, Boekhout T, Hamelin RC, Bélanger RR. (2001) Molecular and physiological analysis of the powdery mildew antagonist Pseudozymaflocculosa and related fungi. Phytopathology 91: 249–254. 10.1094/PHYTO.2001.91.3.249 [DOI] [PubMed] [Google Scholar]
- Babajide JM, Maina S, Kiawa B, Skilton R. (2015) Identification of fungal isolates from steeped yam (Gbodo): Predominance of Meyerozymaguilliermondii. Food Science & Biotechnology 24: 1041–1047. 10.1007/s10068-015-0133-9 [DOI] [Google Scholar]
- Barnett JA, Payne RW, Yarrow D. (1983) Yeasts: Characteristics & Identification. Cambridge University Press, New York, 86 pp. [Google Scholar]
- Begerow D, Schafer AM, Kellner R, Yurkov A, Kemler M, Oberwinkler F, Bauer R. (2014) Ustilaginomycotina. In: McLaughlin DJ, Spatafora JW. (Eds) The Mycota VII: Systematics & Evolution Part A.2nd edition. Springer-Verlag, Berlin, 295–329.
- Berkeley MJ, Broome CE. (1880) . XXII. List of fungi from Brisbane, Queensland; with descriptions of new species. Transactions of the Linnean Society London, Botany 1: 399−407. 10.1111/j.1095-8339.1879.tb00140.x [DOI]
- Boekhout T. (1995) Pseudozyma Bandoni emend. Boekhout, a genus for yeast-like anamorphs of Ustilaginales. J Gen Appl Microbiol 41: 359–366. 10.2323/jgam.41.359 [DOI] [Google Scholar]
- Brefeld O. (1883) Botanische Untersuchungen uber Hefepilze. 5. Die Brandpilze I (Ustilagineen). A. Felix, Leipzig, Germany, 220 pp. [Google Scholar]
- Chang MH, Kim HJ, Jahng KY, Hong SC. (2008) The isolation and characterization of Pseudozyma sp. JCC 207, a novel producer of squalene. Applied Microbiology & Biotechnology 78: 963. 10.1007/s00253-008-1395-4 [DOI] [PubMed]
- Cornu M. (1883) Sur quelques Ustilaginees nouvelles ou peu connues. Ann Sci Nat Bot, Ser 6 15: 269−296.
- Cubero OF, Crespo A, Fatehi J, Bridge PD. (1999) DNA extraction and PCR amplification method suitable for fresh, herbarium-stored, lichenized, and other fungi. Plant Systematics & Evolution 216: 243−249. 10.1007/BF01084401 [DOI]
- De Bary A. (1884) Vergleichende Morphologie und Biologie der Pilze, Mycetozoen und Bacterien. Verlag W. Engelmann, Leipzig, Germany, 558 pp 10.5962/bhl.title.42380 [DOI] [Google Scholar]
- Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59: 307−321. 10.1093/sysbio/syq010 [DOI] [PubMed]
- Goto S, Sugiyama J, Iizuka H. (1969) A taxonomic study of Antarctic yeasts. Mycologia 61: 748–774. 10.2307/3757466 [DOI] [PubMed] [Google Scholar]
- Han PJ, Qiu JZ, Wang QM, Bai FY. (2012) Udeniomyceskanasensis sp. nov., a ballistoconidium-forming yeast species in the Cystofilobasidiales. Antonie van Leeuwenhoek 102: 45–51. 10.1007/s10482-012-9711-5 [DOI] [PubMed] [Google Scholar]
- Ikeda S, Fuji SI, Sato T, Furuya H, Naito H, Ytow N, Ezura H, Minamisawa K, Fujimura T. (2007) Microbial diversity in milled rice as revealed by ribosomal intergenic spacer analysis. Microbes & Environments 22: 165–174. 10.1264/jsme2.22.165 [DOI] [Google Scholar]
- Irinyi L, Serena C, Garcia-Hermoso D, Arabatzis M, Desnos-Ollivier M, Vu D, Cardinali G, Arthur I, Normand A-C, Giraldo A, da Cunha KC, Sandoval-Denis M, Hendrickx M, Nishikaku AS, de Azevedo Melo AS, Merseguel KB, Khan1 A, Rocha11 JAP, Sampaio P, da Silva Briones MR, Ferreira RC, de Medeiros Muniz M, Castanon-Olivares LR, Estrada-Barcenas D, Cassagne C, Mary C, Duan SY, Kong FR, Sun AY, Zeng XY, Zhao Z, Gantois N, Bottere F, Robbertse B, Schoch D, Gams W, Ellis D, Halliday C, Chen S, Sorrell TC, Piarroux R, Colombo AL, Pais C, de Hoog S, Zancope-Oliveira RM, Taylor ML, Toriello C, de Almeida Soares CM, Delhaes L, Stubbe D, Dromer F, Ranque S, Guarro J, Cano-Lira JF, Robert V, Velegraki A, Meyer W. (2015) International Society of Human & Animal Mycology (ISHAM)-ITS reference DNA barcoding database — the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Medical Mycology 53: 313–317. 10.1093/mmy/myv008 [DOI] [PubMed] [Google Scholar]
- Katoh K, Toh H. (2008) Recent developments in the MAFFT multiple sequence alignment program. Briefings in bioinformatics 9 (4): 286−298. 10.1093/bib/bbn013 [DOI] [PubMed]
- Korhola M, Hakonen R, Juuti K, Edelmann M, Kariluoto S, Nyström L, Sontag-Strohm T, Piironen V. (2014) Production of folate in oat bran fermentation by yeasts isolated from barley and diverse foods. Journal of Applied Microbiology 117: 679–689. 10.1111/jam.12564 [DOI] [PubMed] [Google Scholar]
- Kruse J, Doehlemann G, Kemen E, Thines M. (2017) Asexual and sexual morphs of Moesziomyces revisited. IMA Fungus 8: 117–129. 10.5598/imafungus.2017.08.01.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-M, Shivas RG, Cai L. (2017a) Cryptic diversity in Tranzscheliella spp. (Ustilaginales) is driven by host switches. Scientific Reports 7: 43549. 10.1038/srep43549 [DOI] [PMC free article] [PubMed]
- Li Y-M, Shivas RG, McTaggart AR, Zhao P, Cai L. (2017b) Ten new species of Macalpinomyces on Eriachne in northern Australia. Mycologia 109: 408–421. 10.1080/00275514.2017.1330026 [DOI] [PubMed] [Google Scholar]
- Lin SS, Pranikoff T, Smith SF, Brandt ME, Gilbert K, Palavecino EL, Shetty AK. (2008) Central venous catheter infection associated with Pseudozymaaphidis in a child with short gut syndrome. Journal of Medical Microbiology 57: 516–518. 10.1099/jmm.0.47563-0 [DOI] [PubMed] [Google Scholar]
- Matheny PB, Gossmann JA, Zalar P, Kumar TA, Hibbett DS. (2006) Resolving the phylogenetic position of the Wallemiomycetes: an enigmatic major lineage of Basidiomycota. Botany: 84: 1794–1805. 10.1139/b06-128 [DOI] [Google Scholar]
- McTaggart AR, Shivas RG, Geering AD, Callaghan B, Vanky K, Scharaschkin T. (2012) Soral synapomorphies are significant for the systematics of the Ustilago-Sporisorium-Macalpinomyces complex (Ustilaginaceae). Persoonia 29: 63−77. 10.3767/003158512X660562 [DOI] [PMC free article] [PubMed]
- Morita T, Fukuoka T, Imura T, Hirose N, Kitamoto D. (2012) Isolation and screening of glycolipid biosurfactant producers from sugarcane. Bioscience, Biotechnology, and Biochemistry 76: 1788–1791. 10.1271/bbb.120251 [DOI] [PubMed] [Google Scholar]
- Moore RT. (1987) Additions to the genus Vanrija. Bibliotheca Mycologica 108: 167–173. [Google Scholar]
- Nylander JA, Wilgenbusch JC, Warren DL, Swofford DL. (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24: 581−583. 10.1093/bioinformatics/btm388 [DOI] [PubMed]
- Okolo OM, Van Diepeningen AD, Toma B, Nnadi NE, Ayanbimpe MG, Ikenna KO, Mohammed ZS, Benle EB, Marizeth G, Fabio S, Zanyul DE, Giuseppe C, Orazio R. (2015) First report of neonatal sepsis due to Moesziomycesbullatus in a preterm low-birth-weight infant. JMM Case Reports 2(2). 10.1099/jmmcr.0.000011 [DOI]
- Piepenbring M, Hagedorn G, Oberwinkler F. (1998) Spore liberation and dispersal in smut fungi. Botanica Acta 111: 444−460. 10.1111/j.1438-8677.1998.tb00732.x [DOI]
- Sampson K. (1939) Life cycles of smut fungi. Transactions of the British Mycological Society 23: 1–23. 10.1016/S0007-1536(39)80012-2 [DOI] [Google Scholar]
- Sharma R, Oekmen B, Doehlemann G, Thines M. (2018) Pseudozyma saprotrophic yeasts have retained a large effector arsenal, including functional Pep1 orthologs. bioRxiv, 489690. 10.1101/489690 [DOI]
- Skibbe DS, Doehlemann G, Fernandes J, Walbot V. (2010) Maize tumors caused by Ustilagomaydis require organ-specific genes in host and pathogen. Science 328: 89−92. 10.1126/science.1185775 [DOI] [PubMed]
- Stamatakis A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688−2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed]
- Stoll M, Begerow D, Oberwinkler F. (2005) Molecular phylogeny of Ustilago, Sporisorium, and related taxa based on combined analyses of rDNA sequences. Mycological Research 109: 342−356. 10.1017/S0953756204002229 [DOI] [PubMed]
- Stoll M, Piepenbring M, Begerow D, Oberwinkler F. (2003) Molecular phylogeny of Ustilago & Sporisorium species (Basidiomycota, Ustilaginales) based on internal transcribed spacer (ITS) sequences. Canadian Journal of Botany 81: 976−984. 10.1139/b03-094 [DOI]
- Sugita T, Masako T, Natteewan P, Nanthawan M, Kaewjai M, Benjaporn T, Soem P, Pakeenee L, Toshiaki K. (2003) The first isolation of ustilaginomycetous anamorphic yeasts, Pseudozyma species, from patients’ blood and a description of two new species: P.parantarctica and P.thailandica. Microbiology & Immunology 47: 183–190. 10.1111/j.1348-0421.2003.tb03385.x [DOI] [PubMed] [Google Scholar]
- Tanaka E, Koitabashi M, Kitamoto H. (2019) A teleomorph of the ustilaginalean yeast Moesziomycesantarcticus on barnyardgrass in Japan provides bioresources that degrade biodegradable plastics. Antonie van Leeuwenhoek 112: 599–561. 10.1007/s10482-018-1190-x [DOI] [PubMed] [Google Scholar]
- Vánky K. (1977) Moesziomyces, a new genus of Ustilaginales. Botaniska Notiser 130: 131–135.
- Vánky K. (1986) The genus Moesziomyces (Ustilaginales). Nordic Journal of Botany 6: 67–73. 10.1111/j.1756-1051.1986.tb00860.x [DOI] [Google Scholar]
- Vánky K. (2012) Smut Fungi of the World. St. Paul, Minnesota: APS Press, 418–420.
- Vánky K. (2013) Illustrated Genera of Smut Fungi, 3rd edition. St Paul, MN, USA, APS Press, 418–420 pp.
- Vega FE, Posada F, Aime MC, Peterson SW, Rehner SA. (2008) Fungal endophytes in green coffee seeds. Mycosystema 27: 75–84. [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238−4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed]
- Walker JF, Aldrich-Wolfe L, Riffel A, Barbare H, Simpson NB, Trowbridge J, Jumpponen A. (2011) Diverse Helotiales associated with the roots of three species of Arctic Ericaceae provide no evidence for host specificity. New Phytologist 191: 515–527. 10.1111/j.1469-8137.2011.03703.x [DOI] [PubMed] [Google Scholar]
- Wang Q-M, Begerow D, Groenewald M, Liu X-Z, Theelen B, Bai F-Y, Boekhout T. (2015) Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina Studies in Mycology 81: 55−83. 10.1016/j.simyco.2015.10.004 [DOI] [PMC free article] [PubMed]
- Wei YH, Lee F-L, Hsu W-H, Chen S-R, Chen C-C, Wen C-Y, Lin S-J, Chu W-S, Yuan G-F, Liou G-Y. (2005) Pseudozymaantarctica in Taiwan: a description based on morphological, physiological and molecular characteristics. Botanical Bulletin of Academia Sinica 46: 223–229. 10.1016/j.aquabot.2005.02.006 [DOI] [Google Scholar]
- Wei YH, Liou GY, Lee FL. (2011) Pseudozymaaphidis, a new record of ustilaginomycetous anamorphic fungi in Taiwan. Fungal Science 26: 1–5. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (Eds) PCR Protocols: a Guide to Methods & Applications. Academic Press, Inc., San Diego, 315−322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Yasuda F, Yamagishi D, Izawa H, Kodama M, Otani H. (2007) Fruit stain of Japanese pear caused by basidiomycetous, yeastlike fungi Meirageulakonigii & Pseudozymaaphidis. Japanese Journal of Phytopathology 73: 166–171. 10.3186/jjphytopath.73.166 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



