Abstract
Imbibitional oxidative stress of different magnitude, imposed by treatment with different titer of H2O2 (both elevated, 20 mM and low, 500 µM) to an indica rice cultivar (Oryza sativa L., Cultivar Ratna) caused formation of differential redox cues at the metabolic interface, as evident from significant alteration of ROS/antioxidant ratio, efficacy of ascorbate–glutathione cycle, radical scavenging property, modulation of total thiol content and expression of oxidative membrane protein and lipid damages as biomarkers of oxidative stress. All the redox parameters examined, substantiate the experimental outcome that treatment with elevated concentration of H2O2 caused serious loss of redox homeostasis and germination impairment, whereas low titre H2O2 treatment not only restored redox homeostasis but also improve germination and post-germinative growth. The inductive pulse of H2O2 (500 µM) exhibited significantly better performance of ascorbate–glutathione pathway, which was otherwise down-regulated significantly in 20 mM H2O2 treatment-raised seedlings. A comparison between imbibitional chilling stress-raised experimental rice seedlings with 20 mM H2O2 treated rice seedling revealed similar kind of generation of redox cues and oxidative stress response. Further, imbibitional H2O2 treatments in rice also revealed a dose-dependent regulation of expression of genes of Halliwell-Asada pathway enzymes, which is in consonance with the redox metabolic response of germinating rice seeds. In conclusion, a dose-dependent regulation of H2O2 mediated redox cues and redox regulatory properties during germination in rice are suggested, the knowledge of which may be exploited as a promising seed priming technology.
Electronic supplementary material
The online version of this article (10.1007/s12298-019-00656-6) contains supplementary material, which is available to authorized users.
Keywords: Redox cue, Germination, Hydrogen peroxide, Ascorbate–glutathione cycle, Antioxidative enzyme, Transcript abundance
Introduction
Germination of seed is one of the most significant developmental stages of plant life whose progression determines the nature of seedling establishment and subsequent development of plant (Bewley et al. 2013). While germination takes place in natural condition, seeds in hydrated state enhance respiratory activity and spur the formation of reactive oxygen species (ROS) H2O2 from mitochondrial electron transport chain and other redox systems (He et al. 2009; Wojtyla et al. 2016). Enhanced generation of H2O2 and its accumulation together with decline in efficiency of antioxidative defense is associated with oxidative damages and may affect successful completion of germination (He et al. 2009; Wojtyla et al. 2016). The precise regulation of H2O2 titer by the antioxidant system is found to be extremely crucial for the regulation of germination and subsequent seedling establishment. Therefore, the specific regulation of endogenous H2O2 concentration by antioxidative system is the determining factor to attain a balance between ROS signaling that promote germination and oxidative damage that prevent or delay germination. Apart from its signaling role, H2O2 formation during germination might sponsor nutrition mobilization through oxidative modification of metabolite, that may be recognized by the storage organs as redox signals to mobilize reserve to the rapidly growing embryo axis. In fact, the oxidized form, the storage proteins may be regarded as ROS scavengers that in turn regulate its endogenous titer (Wojtyla et al 2016). The oxidative changes of enzymes of glycolytic pathway, mitochondrial ETC components, methionine synthase, aldolase reductase, translation factors, or even molecular chaperonins may be considered as positive stimulator of germination (Wojtyla et al. 2016; Barba-Espín et al. 2011; Lariguet et al. 2013).
Apart from that, there are some strong evidence of H2O2—regulated gene expression through protein oxidation, activation and regulation in redox signaling network, redox changes in thiol group protein, oxidative modification of cysteine residues of transcription factors during germination (Bykova et al. 2011; Barba-Espín et al. 2011; Bazin et al. 2011; Lariguet et al. 2013). All these episodes are directly regulated by cellular redox state of germinating tissue, which in turn is managed by ROS-antioxidant interaction (Barba-Espín et al. 2011; Bazin et al. 2011; Lariguet et al. 2013). Further, some work also proposes coordinated regulation of germination involving H2O2 signaling through MAPK pathway and through the receptor for activated C kinase1 (Zhang et al. 2014). H2O2 also found to be involved in redox regulation for the synthesis of hydrolytic enzymes in barley (Ishibashi et al. 2012). He et al (2009) showed that seed pretreatment with H2O2 impart drought tolerance to wheat seedlings by improving photosynthesis, water use efficiency and growth of seedlings through management of oxidative stress.
Studies on phytohormone interaction with H2O2 during germination have also shown interesting results. Exogenous application of ABA found to inhibit ROS accumulation in rice (Ye et al. 2012) and sunflower (El-Maarouf-Bouteau et al. 2015). On the contrary, exogenous treatment of H2O2 does not influence endogenous level of ABA and associated signaling but has more pronounce effect on GA, resulting in lilt of endogenous titer of growth promoter/inhibitor with subsequent impact on germination (Lariguet et al. 2013). Enhanced level of ROS H2O2 and has been found in germinating Arabidopsis seeds treated with exogenous GA; whereas reduction in ROS level have been noticed in seeds treated with ABA (Lariguet et al. 2013).
An interaction between redox state of germinating tissue and phyto-hormones coordinated by H2O2 is found to be involved in the modulation of proteins associated with seed germination. An augmentation in germination performance accompanied by reduction of growth regulator ABA, SA, JA and IAA with exogenous supplementation of H2O2, strongly support the fact that H2O2 can influence hormonal signaling network associated with germination (Barba-Espín et al. 2012; El-Maarouf-Bouteau et al. 2015; Wojtyla et al. 2016).
In general, under hydrogen peroxide homeostasis, the elevated level of H2O2 is largely controlled by scavenging enzymes that include ascorbate peroxidase, catalase, superoxide dismutase and glutathione reductase (Bhattacharjee 2012; Mittler et al. 2011). Along with these antioxidative enzymes, low molecular weight antioxidant molecules, like ascorbate and glutathione provide efficient antioxidant machinery for tightly regulating the H2O2-mediated redox state of the cell (Mittler et al. 2011; Kapoor et al. 2015). However, in most of the cases, this equilibrium is lost due to imposition of environmental stresses, causing transient increase in the titre of H2O2 and change in redox state, eventually imposing oxidative threat and metabolic dysfunction. The oxidative burst of H2O2 and change in redox cues, which have been reported under most of the environmental stresses, also used by the plant to activate signalling network and ultimately the expression of redox-sensitive genes that help to cope up with environmental stresses (Desikan et al. 2001; Vanderauwera et al. 2005; Bhattacharjee 2012).
Several works emphasized the significance of ascorbate–glutathione (ASC–GSH) cycle in plant tissues against adverse changes in redox cues of the actively metabolising cells (Noctor and Foyer 1998; Smirnoff 2000; Wang et al. 2009). In this antioxidative defence system, the elevated titre of H2O2 is restricted by its reduction to H2O by ascorbate peroxidase (APOX) using ascorbic acid as co-substrate (Smirnoff 2000). In the metabolic cycling, ascorbate is regenerated by the enzyme dehydroascorbate reductase (DHAR) from dehydroascorbate (DHA) at the expense of reduced glutathione (GSH). Utilization of GSH for the reduction of DHA on the other hand yields GSSG which finally got reduced to GSH through reduction by NADPH + H+ due to the action of Glutathione reductase (GR). Therefore, the low molecular weight antioxidant ASC and GSH are linked in this ASC–GSH cycle. Efficiency of this cycle in many cases determines the fate of oxidative stressed plant cell (Wheeler et al. 1998; Moradi and Ismail 2007; Wang et al. 2009). In spite of the immense potential of this cycle, scant attention is being paid to the efficacy of this cycle and the turn-over of its key components associated with H2O2 stress tolerance, in the perspective of signalling role of H2O2during early germination. Therefore, present study is designed to critically evaluate the efficacy of ASC–GSH cycle and also to identify key components involved under different redox cues instigated by different magnitude of oxidative stress imposed by H2O2 treatment in a germinating indica rice cultivar.
A number of recent studies also have shown that priming or pre-treatment with H2O2 can modulate abiotic stress tolerance of plants (Bhattacharjee 2012; Hossain and Fujita 2013; Lin et al. 2009, Wang et al. 2014). Although H2O2 is known to act as signalling molecule, the mechanisms activating multiple defence responses in germinating tissue that reinforce resistance against environmental odds are poorly understood (Petrov and Van Breusegem 2012; Wojtyla et al. 2016). Similarly, very little is known about how the titer-specific responses of H2O2 are coordinated that ultimately regulates the physiology of H2O2 in germinating tissue. In the present work, efforts have also been made to make a clear inventory as to how the opposing roles of H2O2 are regulated at metabolic interface in an indica cultivar of rice during early germination. The role of redox-disruptive and redox-regulatory processes under different redox cues as instigated by dose-dependent treatment of H2O2 is investigated to understand the significance of redox-regulatory process, like ascorbate–glutathione pathway, under different magnitude of oxidative stress. Additionally, the role of H2O2 mediated (titre-specific) changes redox cues in the regulation of transcript abundance of genes of some important antioxidative enzymes was also assessed for corroborating the dose-dependent responses of H2O2 and understanding their influence in redox status in rice during early germination.
Materials and methods
Plant growth and imposition of imbibitional oxidative and chilling stress
Seeds of an indica cultivar of rice (Oryza sativa L. Cultivar Ratna), selected as experimental material, have been collected from Chinsurah Rice Research Institute, Government of West Bengal, India. Seeds of the experimental cultivar were washed with distilled water and were treated with 0.2% HgCl2 for 5 min and then washed thrice with sterile distilled water. Imbibitional oxidative and chilling stress was imposed based on standardized procedure of Bhattacharjee (2013). The surface sterilized seeds were imbibed in distilled water for 24 h in darkness at 25° ± 2 °C and thereafter, were sown on moist filter paper in Petri plates and were placed in standardized conditions of thermostat-controlled seed germinator cum stability chamber (Remi 82 BL, India) maintained at 8 °C temperature for duration of 16 h to impose low temperature stress. For imposing imbibitional oxidative stress of different magnitude two different 24 h water-imbibed seed lots were treated with 500 µM and 20 mM H2O2 for 24 h (30 seeds/petriplate), with intermittent change of treating solutions in petriplates (25 mL in eight hours interval). The selection of two different treating concentrations of H2O2 titer for imposing IOS (500 µM and 20 mM H2O2) was based on the initial standardization of dose-response experimentation. For untreated control set, 24 h water imbibed seeds were sown directly in petriplates and exposed at 25o±2o C. Thereafter, all the seed lots were allowed to grow at 25°±2 °C with 12 h photo period (light intensity 270 µmol m−2 s−1) and 78 ± 2% relative humidity. For all biochemical analysis 72 h old seedlings raised from aforesaid conditions were used (Supplementary Fig. 1).
Determination of early growth performances
For studying early growth performances, relative growth index (RGI), biomass accumulation and vigor index (VI) were calculated according to Rubio-Casal et al. (2003) and Bhattacharjee (2008).
RNA isolation and analysis of transcript profile by semi quantitative RT-PCR
RNA was extracted from untreated control, imbibitional oxidative and chilling stress-raised seedlings of the experimental cultivar of rice using Guanidium isothiocyanate-phenol based reagent (RNA-XPress™ reagent, HiMedia) according to manufacturer’s instructions. To ensure the comparability of the resulting band intensity, quantification of RNA was done using Nano drop Spectrophotometer (ND1000, Nanodrop technologies, USA) and confirmed by applying equal amounts of total RNA to an agarose gel using ethydium bromide staining.
Preparation of cDNA
First strand cDNA synthesis was done by using Revert Aid First Strand cDNA synthesis kit (Fermentas, Thermo Scientific) according to the manufacturer’s protocol and quality of cDNA was checked by running 2 µL of total cDNA in 1.1% agarose gel using tris-acetate-EDTA (TAE, pH 8) buffer. Quantification of cDNA was performed by Nano Drop spectrophotometer (ND1000, Nanodrop technologies, USA) at 260 nm and stored at − 80 °C for further use.
Differential accumulation of transcript using reverse-transcriptase RT-PCR
Semi-quantitative RT-PCR was performed as described by Burch-Smith et al (2006). After synthesis, cDNA was diluted 1:10 and 4 µL of cDNA was used as template for PCR amplification in a 25 µL reaction mixture. Reaction contained selected couples of the gene-specific primers for SodCc2 CatA, OsAPx2 and 18S rRNA (Hsk) (Supplementary Table 1). PCR conditions were as follow: initial denaturation of 2 min at 95 °C, followed by 30 cycles of denaturation for 30 s at 95 °C, annealing at 54 °C for 30 s, extension at 72 °C for 30 s, and a final extension of 8 min at 72 °C. The conditions of semi-quantitative RT-PCR were chosen so that none of the mRNAs analyzed reached a plateau at the end of amplification cycles, i.e they were in exponential phase of amplification, and that the two sets of primers (one set of gene specific primer and other set of 18S rRNA primers) used in the reaction did not compete with each other. Apropriate number of cycles was determined by testing different cycles of 15, 20 and 25 for both antioxidant gene transcripts and 18S rRNA (internal control) amplification. It was important to select the appropriate number of cycles so that the amplified product was clearly visible on agarose gel and can be quantified, while the amplification was in exponential range and did not reach plateau yet. Moreover, the optimal number of cycles was strictly maintained to be in the same range for specific mRNA of interest and the internal control, so that both can be measured on the same gel. Equal amounts of PCR products were the run on the same 1.2% agarose gel for comparison and quantification. Images of the RT-PCR products in ethydium bromide stained agarose gel were acquired in BIO-Rad Molecular Imager Gel Doc XR system with high resolution CCD camera and quantification of bands was performed by Bio Rad image Densitometer GS-700 using quantity one software (version-4.6.9).
Estimation of Reactive oxygen species and in situ localization of ROS
Estimation of “total ROS” generation
For estimation total ROS generation an in vivo assay was performed by placing seedling tissue (50 mg) in 8 mL 40 mM TRIS-HCl buffer (pH 7) in presence of 100 µM 2′,7′-dichloroflorescindiacetate (DCFDA, Sigma) at 30 °C. Supernatant was removed after 60 min and fluorescence was monitored in a spectroflurometer (Hitachi, Model F-4500 FL Spectrophotometer) with excitation at 488 nm and emission at 521 nm (Simontacchi et al. 1993).
Estimation of “ and H2O2” generation
Hydrogen peroxide was extracted and estimated following the procedure of MacNevin and Uron (1953) using titanic sulfate. For the determination of superoxide, the method of Chaitanya and Naithani (1994) was followed with some necessary modifications. 500 mg of tissues were homogenized in cold with 5 mL of 0.2 M sodium phosphate buffer, pH 7.2, with addition of diethyldithiolcarbomate (10−3 M) to inhibit SOD activity. The homogenates was immediately centrifuged at 2000 g at 4 °C for 1 min. In the supernatant, superoxide anion was measured by its capacity to reduce nitroblue tetrazolium (2.5 × 10−4 M). The absorbance of the end product was measured at 540 nm. Formation of superoxide was expressed as ΔA540 g−1 (d.m.) min−1.
In situ localization of and H2O2
Superoxide anion in stressed germinating seeds was detected according to He et al. (2011). The germinating seeds from different treatments (imbibitional oxidative and chilling stress) were incubated in 6 mM nitroblue tetrazolium in 10 mM tris-HCl buffer, pH 7.4 at room temperature for 1–2 h. The accumulation of superoxide anion could be detected by visualization of dark blue colour as compared to untreated control and was photographed.
For the detection of H2O2, the process of He et al (2011) was followed with little modification. Oxidative stressed and control rice seedlings were soaked in a 0.42 mM TMB solution in Tris-acetate, pH 5 buffer for 2 h. The appearance of blue color could be monitored to indicate the accumulation of H2O2.
Determination of antioxidative defense
DPPH (2, 2′-diphenyl-1-pycryl hydrazyl) free radical scavenging activity
For determination of DPPH free radical scavenging activity, the process of Mensor et al. (2001) was followed.
FRAP assay
This procedure was carried out according to Benzie and Strain (1996) with little medications. For the preparation of tissue extract, 1.5 g of dry sample (seedling tissue kept at 45 °C for two days) was extracted with 30 mL 80% methanol at 28 °C for 24 h in shaking incubator. Extracts were centrifuged at 3500 rpm for 20 min at 4o C. Supernatant was collected and filtrate was used for FRAP assay. A total of 150 µL of tissue extract with 2850 µL of freshly prepared FRAP reagent was mixed in a cuvette and kept for 30 min in dark. Finally the absorbance was taken at 593 nm. The standard curve using grades of trolox was prepared for estimating the unknown. The final result was expressed as antioxidant capacity in µM trolox equivalent g−1 dm.
ABTS decolorization assay
ABTS (2, 2′ azinobis (3-ethylbenzthiazoline)-6-sulfonic acid) free radical decolourization assay was done according to Re et al. (1999) with some necessary modifications.
Extraction and estimation of enzymatic antioxidants
For the extraction and estimation of catalase (CAT, EC 1.11.1.6), the process of Snell and Snell (1971) was followed.
The extraction and enzyme activity of superoxide dismutase (SOD, EC 1.15.1.1) was determined by measuring the photochemical reduction ability of NBT according to Giannopolities and Ries (1977).
Ascorbate peroxidase (APOX; EC 1.11.1.11) activity was determined according to Nakano and Asada (1981) using homogenates previously supplemented with 0.5 mM ascorbic acid and 0.1 mM EDTA. Parallel experiments in presence of p-chloromercuribenzoate (50 μM) were performed to rule out any interference from guaiacol peroxidases.
Glutathione reductase (GR, EC 1.6.4.2) activity was measured according to Schaedle and Bassham (1977). The reaction mixture contained 50 mMTris-HCl (pH 7.6), 0.15 mM NADPH, 0.5 mM oxidized glutathione (GSSG), 3 mM MgCl2 and 100 μL homogenate (7 mg protein mL−1). NADPH oxidation was followed at 340 nm. The enzyme activity in all cases was expressed as enzyme unit min−1 g−1 d.m. according to Fick and Qualset (1975).
Determination of indices of oxidative damage to membrane protein and lipid (membrane protein thiol level, free carbonyl content and thiobarbituric acid reactive substances)
Membrane protein thiol level
For the determination of membrane protein thiol level (MPTL), the membrane was prepared according to Singh (1997) with some necessary modifications. 1 g of plant tissue was homogenized in 10 mL ice cold buffer (0.05 M Tris-HCl, pH 7.0). The homogenate was centrifuged at 10,000×g at 4 °C for 30 min and the pellet was discarded. The membranes were then sedimented at 100,000×g at 4 °C for 3 h and the pellet containing the membrane fractions was suspended in ice cold buffer (0.05 M Tris-HCl, pH 7.0). The membrane fractions were stored under ice. The membrane associated protein bound thiol group was assayed after protein precipitation with TCA (10% mass/volume) and quantified with DTNB following the procedures of Ellman (1959) and Dekok and Kuiper (1986).
Free carbonyl content
Protein oxidation (PO) under oxidative stress was estimated as the content of carbonyl groups (RC=O) following the procedure of Jiang and Zhang (2001).
Thiobarbituric acid reactive substances
To estimate membrane lipid peroxidation (MLPO), test for thiobarbituric acid reactive substances (TBARS) was performed using the procedure of Heath and Packer (1968).
Determination of components of ascorbate–glutathione (ASC–GSH) pathway
Estimation of ascorbate content was performed according to the process of Hodges et al. (2001). Total ASC and reduced ASC contents were estimated from the standard curve of 0-40 n mol L-ASC determined by the above methods. Dehydroascorbate (DHA) contents were calculated by the subtraction of ASC from total ASC. For the estimation of total glutathione content, 1gm tissue was homogenized in 5% metaphosphoric acid and the homogenate was centrifuged at 18,000 g and the supernatant was used. 0.4 mL of the supernatant was neutralized with 0.6 mL 0.5 M phosphate buffer (pH 7.5). For total GSSG assay, the GSH was masked by adding 20 µL of 2-Vinylpyridine to the neutralized supernatant, whereas 20 µL of water was added in the aliquots utilized for total glutathione pool assay. Tubes were vortexed until emulsion was formed. Glutathione content was determined in 1 mL of assay mixture containing 0.2 mM NADPH, 100 mM phosphate buffer (pH 7.5), 5 mM EDTA, 0.6 mM 5, 5′dithiobis (2-nitrobenzoic acid), and 0.1 mL of sample. The reaction was started by adding 03 units of glutathione reductase and was monitored by measuring changes in absorbance at 412 nm. The contents of glutathione were estimated from the standard curve of 0-20 nmol glutathione (Mytilineou et al. 2002; Zhang and Kirkham 1996).
Both mono-dehydroascorbate reductase (MDHAR, EC 1.6.5.4) and dehydroascorbate reductase (DHAR, 1.8.5.1) activities were determined according to Song et al. (2005). DHAR activity was measured by monitoring the absorbance at 265 nm for 3 min in the reaction mixture that consisted of enzyme extract, 50 mM Na-phosphate buffer (pH 7.0), 0.3 mM glutathione, 0.06 mM Na2-EDTA and 0.2 mM DHA. MDHAR activity was measured by the monitoring the absorbance at 340 nm for 3 min in 50 mM Na-phosphate buffer (pH 7.6), 0.1 mM NADPH and 0.1 unit AsA oxidase (Sigma, MO, USA) and 2.5 mM AsA. Non-enzymatic reduction of DHA or MDHA in phosphate buffer was measured in a separate cuvette at the same time.
Statistical analysis
Each treatment consisted of three replicates and each experiment was carried out twice at different times. For statistical analysis of the data for significance, the two sample t-test was employed with the help of Minitab 15 package, showing the significant variations between untreated control and temperature stress-raised seedlings.
Result
Changes in endogenous redox cues in response to different magnitude of imbibitional oxidative and chilling stress in germinating rice (Oryza sativa L. cv. Ratna) seedlings
When imbibitional oxidative stress (IOS) of different magnitude were imposed in experimental indica rice cultivar (Ratna) by treatment with 500 µM and 20 mM H2O2 for 24 h, a change in internal redox cues of growing seedlings were observed in terms of accumulation of prooxidants (assessed in terms of total ROS by DCFDA oxidation and estimation of superoxide and H2O2 content, in-situ staining for and H2O2) and antioxidative defense (assessed in terms of total radical scavenging activity, activities of antioxidative enzymes and efficacy of ascorbate–glutathione cycle). Figure 1 shows the level of endogenous prooxidants in terms of accumulation of total reactive oxygen species (oxidation of DCFDA) and individual ROS content ( and H2O2) in imbibitional oxidative stress-raised (500 µm & 20 mM H2O2) seedlings. Results clearly showed a dose-dependent response of H2O2 treatment on internal redox cues in germinating tissues of experimental rice. Low titer H2O2 treatment (500 µm) caused significant reduction in accumulation of reactive oxygen species and H2O2 with concomitant enhancement of antioxidant efficiency (assessed in terms of total antioxidant capacity and H2O2 processing antioxidants). On the contrary, elevated concentration of H2O2 (20 mM) treatment exhibited a reverse trend, showing significantly higher accumulation of both individual and total ROS, reduced radical scavenging activity and efficiency of H2O2 processing antioxidative enzymes. Histochemical studies involving tissue staining for ROS, also revealed similar trend of ROS production (Fig. 2). Both staining with NBT (nitroblue tetrazolium chloride) and TMB (3,3′,5,5′-tetramethylbenzidine) revealed that and H2O2 accumulation was maximum in higher magnitude of IOS (20 mM H2O2) as compared to lower magnitude of IOS (500 µM H2O2). For seedlings raised from 20 mM H2O2 treated seeds, the intensity of staining was found to be more intense, spreading throughout the major surface area of root. On the contrary, when the seedlings were raised from lower titer of H2O2 treatment, not only the intensity of staining was faint but also found to be patchy across the whole root surface (Fig. 2).
Fig. 1.
Measurement of prooxidants (assessed in terms of total ROS by DCFDA oxidation (a) and estimation of H2O2 (b) and superoxide (c) content) of imbibitional oxidative (500 µM and 20 mM H2O2) and chilling (8 °C for 16 h) stress (ICS) raised rice (Oryza sativa L. cv. Ratna) seedlings. Results are mean of three replicates ± standard deviation.*Significant from control at 0.05 level (t test); **significant from control at 0.01 level (t test)
Fig. 2.
Visualization of superoxide (a) and hydrogen peroxide (b) production after NBT and TMB staining respectively of rice seedling raised from different magnitude of imbibitional oxidative (500 µM and 20 mM H2O2) and chilling stress (8 °C for 16 h)
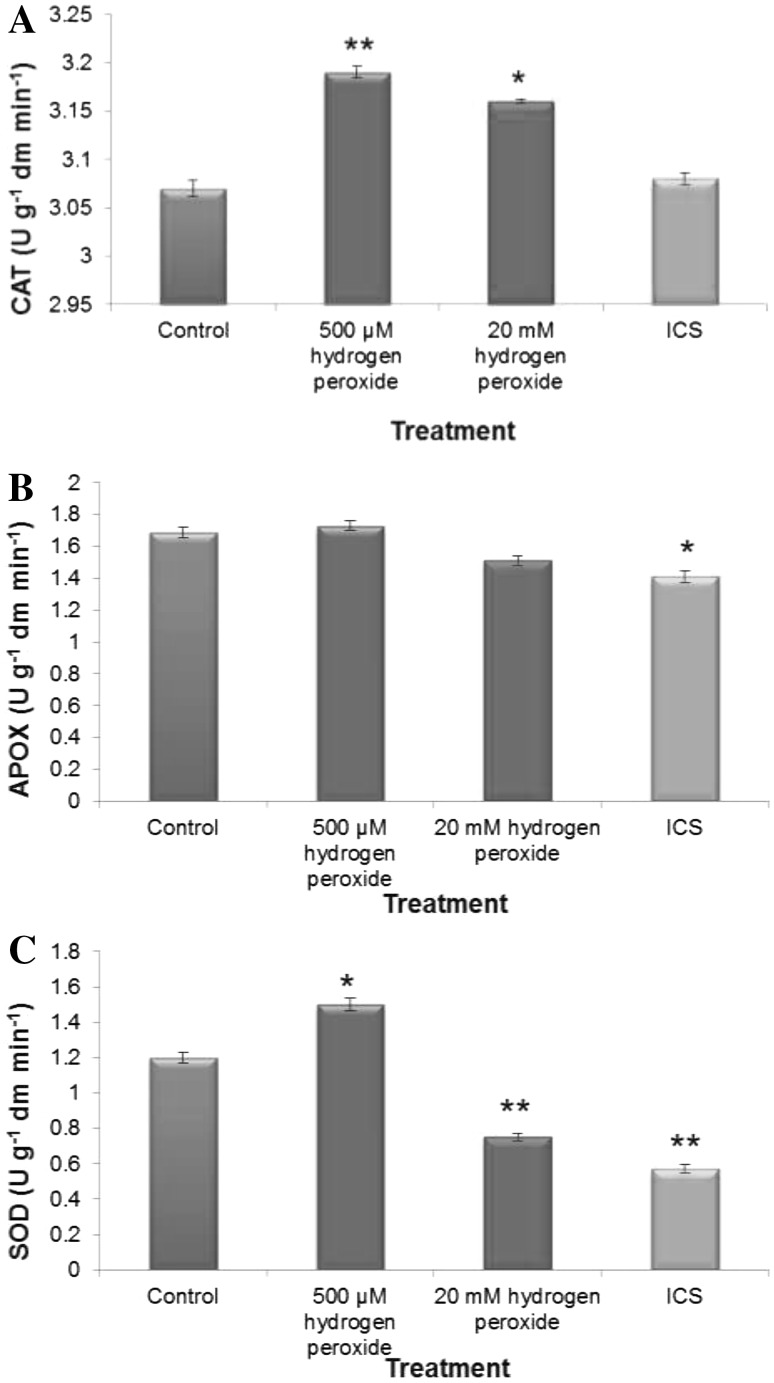
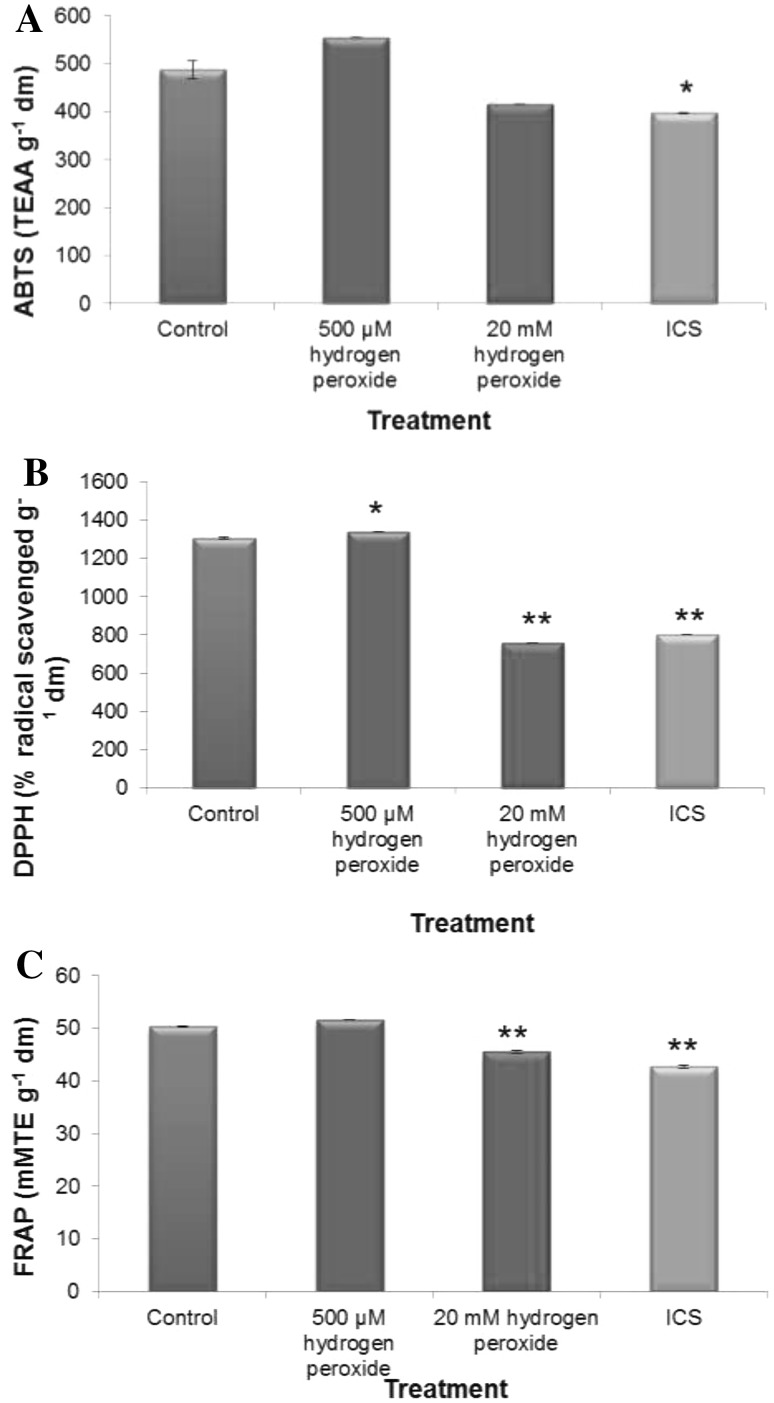
That H2O2 treatment changed internal redox cues which even continued 72 h of post treatment. This could be substantiated from all the data of dose-dependent changes in antioxidative defense (assessed in terms of activities of H2O2 processing enzymes and total antioxidant capacity Figs. 3, 4). Elevated level of H2O2 treatment during early imbibitional phase of germination reduced total radical scavenging activities (assessed in terms of ABTS, DPPH and FRAP assay, Fig. 4) and activities of superoxide dismutase and catalase, which are comparable to imbibitional chilling stress (ICS) raised germinating tissues of rice, conforming serious loss of redox homeostasis. On the contrary, low titer H2O2 treatment significantly enhanced competence of antioxidative defense system, as revealed from the data of DPPH radical scavenging activities (Fig. 4) and activities of catalase and SOD. When compared, elevated levels of H2O2 treatment during early imbibitional phase of germination found to exert similar trend of inhibition in antioxidative defense and ROS accumulation as that of ICS hinting at serious loss of redox homeostasis of germinating tissues.
Fig. 3.
Activities of enzymatic antioxidants [Catalase (a), Ascorbate peroxidise (b) and Superoxide dismutase (c)] of imbibitional oxidative (500 µM and 20 mM H2O2) and chilling (8 °C for 16 h) stress (ICS) raised rice (Oryza sativa L. cv. Ratna) seedlings. Results are mean of three replicates ± standard deviation. *Significant from control at 0.05 level (t test). **significant from control at 0.01 level (t test)
Fig. 4.
Imbibitional oxidative (500 µM and 20 mM H2O2) and chilling (8 °C for 16 h) stress (ICS) induced changes in total antioxidant capacity, radical scavenging activity [ assessed in terms of ABTS (a), DPPH (b) and FRAP (c) assay ] of indica rice cultivar (Oryza sativa L. cv. Ratna). Results are mean of three replicates ± standard deviation. *Significant from control at 0.05 level (t test). **significant from control at 0.01 level (t test)
Spectrofluorometric estimation of oxidation of DCFDA to a fluorescent compound was used for in vitro assay to determine the total ROS generation in germinating rice seedlings triggered by exogenous H2O2 treatment and imbibitional chilling stress. Both elevated level of H2O2 treatment and imbibitional chilling stress were found to enhance the oxidation of DCFDA, implying serious loss of redox homeostasis in germinating rice seeds. However, the germinating tissues of experimental rice cultivar raised from low titer H2O2 treatment (500 µm), though caused oxidation of DCFDA over untreated control, the extent of oxidation was significantly lesser implicating significant lesser accumulation of total ROS as compared to ICS and elevated H2O2-treated seedlings and found to be absolute necessary for maintenance of redox homeostasis. When assessed, both the endogenous titre of and H2O2were found to corroborate the data of total ROS, confirming the ability of germinating tissue to maintaining redox homeostasis under low titer inductive H2O2 pulse. Whereas, elevated dose of H2O2 caused a disturbance in the redox homeostasis which is quite similar to ICS raised seedlings. So, the data of redox status of germinating rice tissue (exposed to imbibitional oxidative and chilling stress) clearly revealed that higher magnitude of IOS and ICS caused change in internal redox cues by shifting redox homeostasis due to higher accumulation of prooxidants and lesser competence of antioxidative defense comprising of thiol compounds and activities of other radical scavenging compounds and important H2O2 processing antioxidative enzymes.
Efficacy of ascorbate–glutathione (ASC–GSH) cycle under varying magnitude of imbibitional oxidative and chilling stress in germinating rice seedling
500 µM H2O2 treated germinating rice seedling exhibited significant enhancement of the components of ASC–GSH cycle in general, which otherwise got significantly depleted under elevated H2O2 treatment and ICS (Table 1 and Fig. 3b). A significant difference in MDHAR (monodehydroascorbate reductase) and DHAR (dehydro ascorbate reductase) activities were observed between elevated and low dose H2O2 treatment-raised experimental rice seedlings (Table 1). Activities of MDHAR and DHAR were found to be augmented by 28.5% and 24% over untreated control in 500 µM H2O2 treated germinating tissues, whereas reduction in activities in terms of 43.4% and 40% for MDHAR and DHAR over untreated control were observed for 20 mM treated rice seedlings. When Glutathione reductase activity was assessed and compared between 500 µM and 20 mM treated rice seedlings, exhibited a similar trend with significant rise in activity for low dose H2O2 treated seedlings which could otherwise suffer a significant decline under elevated H2O2 treatment.
Table 1.
Dose-dependent effect of imbibitional oxidative stress (IOS) and chilling stress (ICS) on the efficacy of ascorbate–glutathione cycle [assessed in terms of accumulation of ascorbate, glutathione and activities of glutathione reductase (GR), monodehydroascorbatereductase (MDHAR) and dehydroascorbatereductase (DHAR)]
| Treatment | Ascorbate (µmol g−1 dm) | Glutathione (µmol g−1 dm) | Activities of ascorbate–glutathione cycle enzymes (Ug−1 dm h−1) | ||
|---|---|---|---|---|---|
| MDHAR | DHAR | GR | |||
| Untreated Control | 67.5 ± 0.17 | 6.5 ± 0.04 | 0.56 ± 0.004 | 0.38 ± 0.002 | 0.325 ± 0.002 |
| IOS (20 mM H2O2) | 48.2 ± ± 0.11** | 4.3 ± 0.05* | 0.39 ± 0.007* | 0.27 ± ± 0.004* | 0.205 ± 0.001** |
| IOS (500 µM H2O2) | 74.3 ± 0.19* | 7.9 ± 0.07** | 0.72 ± 0.004** | 0.50 ± 0.007** | 0.390 ± 0.004* |
| ICS | 46.5 ± 0.21** | 5.2 ± 0.03** | 0.41 ± 0.004* | 0.25 ± 0.001** | 0.160 ± 0.001* |
Results are mean of three replicates ± standard error. *Significant from control at 0.05 level (t test); **significant from control at 0.01 level (t test)
Low titre H2O2 treatment further enhanced the level of both ASC and GSH in the germinating rice seedlings while a remarkable reduction was observed in elevated H2O2 treated rice seedlings. When compared, ICS-raised seedlings of rice exhibited similar kind of response in terms of activity of MDHAR, DHAR, GR and accumulation of ASC and GSH, corroborating the fact that both elevated H2O2 treatment and ICS might reduce the efficiency of ASC–GSH cycle and have suffered serious changes in endogenous redox cues, whereas low titer H2O2 treatment up-regulate the efficiency of ASC–GSH cycle.
Changes in MPTL, MLPO, PO in response to varying magnitude of imbibitional oxidative and chilling stress in rice seedlings
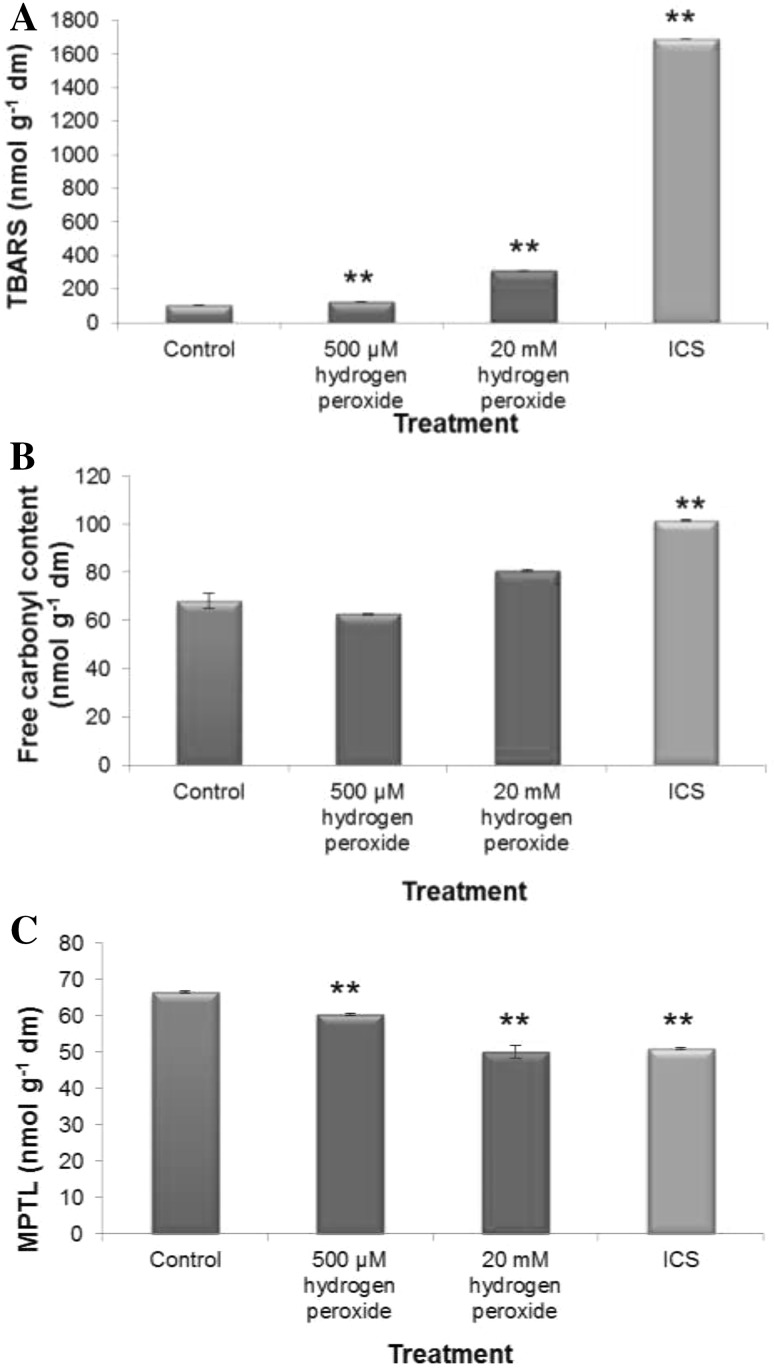
The oxidation of membrane protein and lipid, which may occur in plant cell under oxidative stress, is a reliable indicator of loss of redox homeostasis of the cell that largely conveys the status of the internal redox cues of the tissue. In order to ascertain the impact of low and high doses of H2O2 treatment and chilling stress during imbibitional phase of germination on oxidative protein and lipid damages of newly assembled membrane system of germinating experimental rice cultivar, free carbonyl content (RC=O), membrane protein thiol level (MPTL) and accumulation of thiobarbituric acid reactive substances (TBARS) were assessed and compared. Results clearly exhibited that both 20 mM and 500 µM H2O2 treatment caused just reverse effect (Fig. 5). Both elevated concentration of H2O2 treatment and imbibitional chilling stress caused enhancement of accumulation of free carbonyl content and TBARS with concomitant reduction of MPTL over untreated control (Fig. 5). On the other hand, low titer H2O2 treatment restores MPTL and significantly reduced TBARS and RC=O content over untreated control seedlings of experimental rice cultivar (Fig. 5). So the findings strongly revealed that different magnitude of exogenous H2O2 treatment has not only exerted influence on internal redox cues but could also subsequently affect oxidative damage to membrane protein and lipid.
Fig. 5.
Measurement of imbibitional oxidative (500 µM and 20 mM H2O2) and chilling (8 °C for 16 h) stress (ICS) using lipid peroxidation [assessed in terms of TBARS accumulation (a), protein oxidation (assessed in terms of free carbonyl content (b) and membrane protein thiol level (c) in seedlings of rice (Oryza sativa L. cv Ratna). Results are mean of three replicates ± standard deviation. *Significant from control at 0.05 level (t test). **significant from control at 0.01 level (t test)
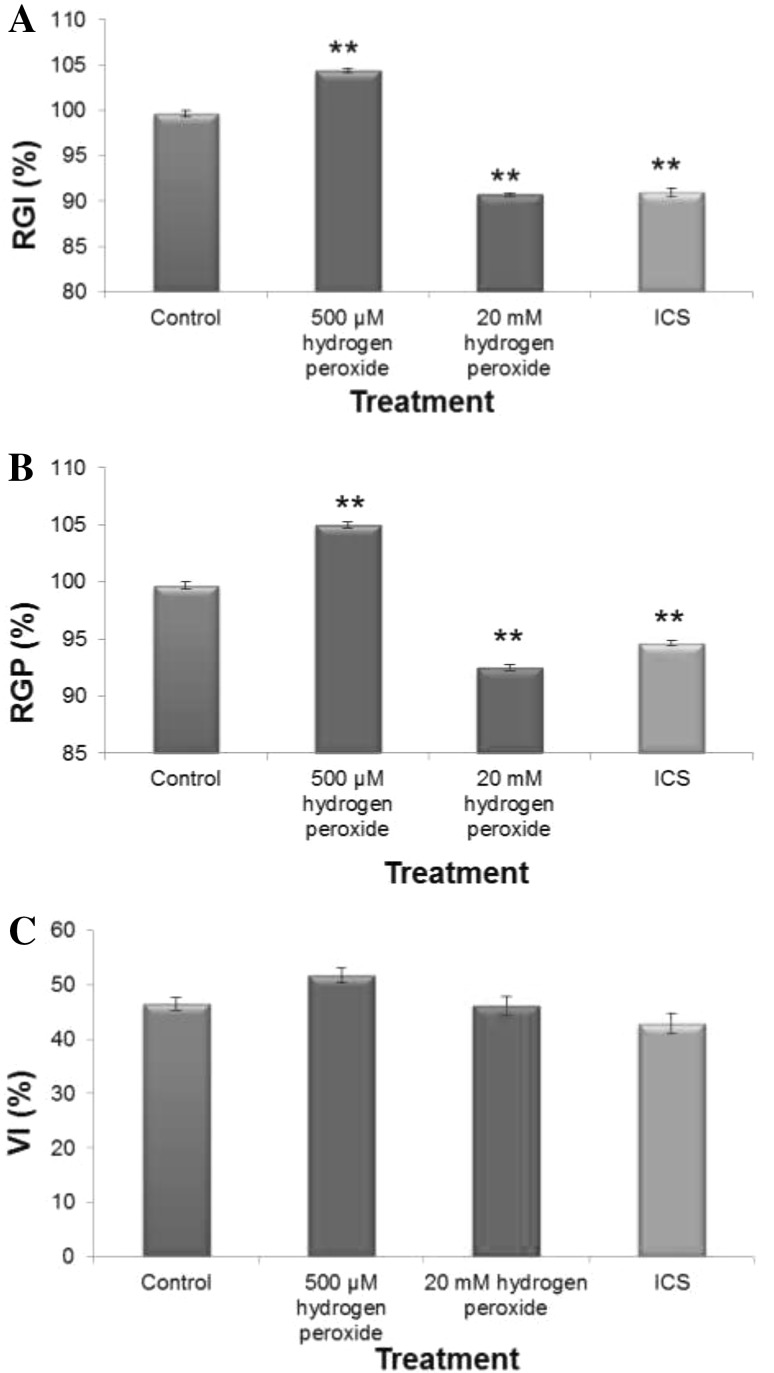
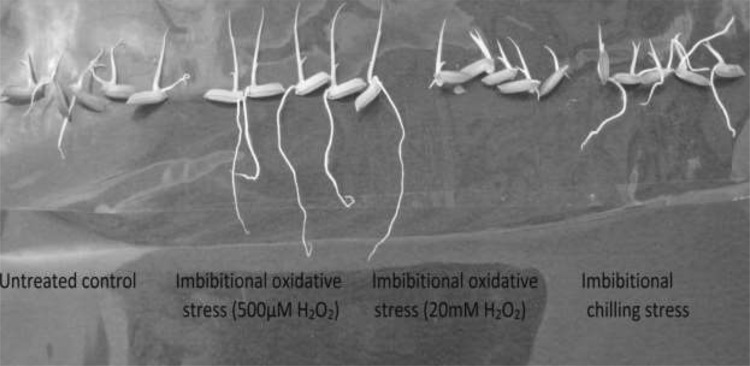
The oxidative damage to membrane protein and lipid which may occur in plant tissue suffering from changes in internal redox cues due to over accumulation of prooxidants, is a reliable indicator of loss of redox homeostasis and hence a biomarker of oxidative stress. In order to ascertain the impact of dose-dependent responses of H2O2 and imbibitional chilling stress on early growth performances in our experimental rice cultivar (Fig. 6), different early growth parameters, like relative germination performance (RGP), relative growth index (RGI) and vigor index (VI) were assessed. The RGI and VI of 20 mM H2O2 treated seedlings were found to be 90.5% and 47%, respectively as against 114.5% and 51% for 500 µM treated seedlings, hinting dose-dependent response of H2O2 treatment in which elevated titre of H2O2 exhibited inhibition of early growth performances, whereas, low titer inductive pulse of H2O2 treatment showed stimulation (Fig. 6). When compared, both elevated titer of H2O2 treated seedlings and chilling stress raised seedlings exhibited similar pattern of early growth response, strongly supporting the role of internal redox cues as the determinant of early growth performance. In fact, both the elevated titer of H2O2 treatment and chilling stress during early imbibitional phase of germination were found to exert similar trend of antioxidative defense, ROS accumulation and oxidative damages to membrane thiol protein and lipid (Figs. 1, 2, 3, 4, 5) hinting not only at serious loss of internal redox cues but also exhibited oxidative damage which were reflected in terms of poor early growth performances of the seedling (Fig. 7). On the contrary, low titre H2O2 treatment during early germination could regulate the synthesis of thiol compounds and radical scavenging molecules necessary for combating prooxidant accumulation thereby maintaining the redox cues towards homeostasis necessary for augmentation of early growth performance.
Fig. 6.
Imbibitional oxidative (500 µM and 20 mM H2O2) and chilling (8 °C for 16 h) stress (ICS) induced changes in germination and early growth performances [assessed in terms of relative growth index (RGI) relative germination performance (RGP) and vigure index (VI),] of indica rice cultivar (Oryza sativa L. cv. Ratna). Results are mean of three replicates ± standard deviation.*Significant from control at 0.05 level (t test). **significant from control at 0.01 level (t test)
Fig. 7.
Photograph of seedlings of indica rice cultivar (Oryza sativa L. cv Ratna) showing different growth impact under different magnitude of imbibitional oxidative stress (500 µM and 20 mM H2O2) and chilling (8 °C for 16 h) stress (ICS)
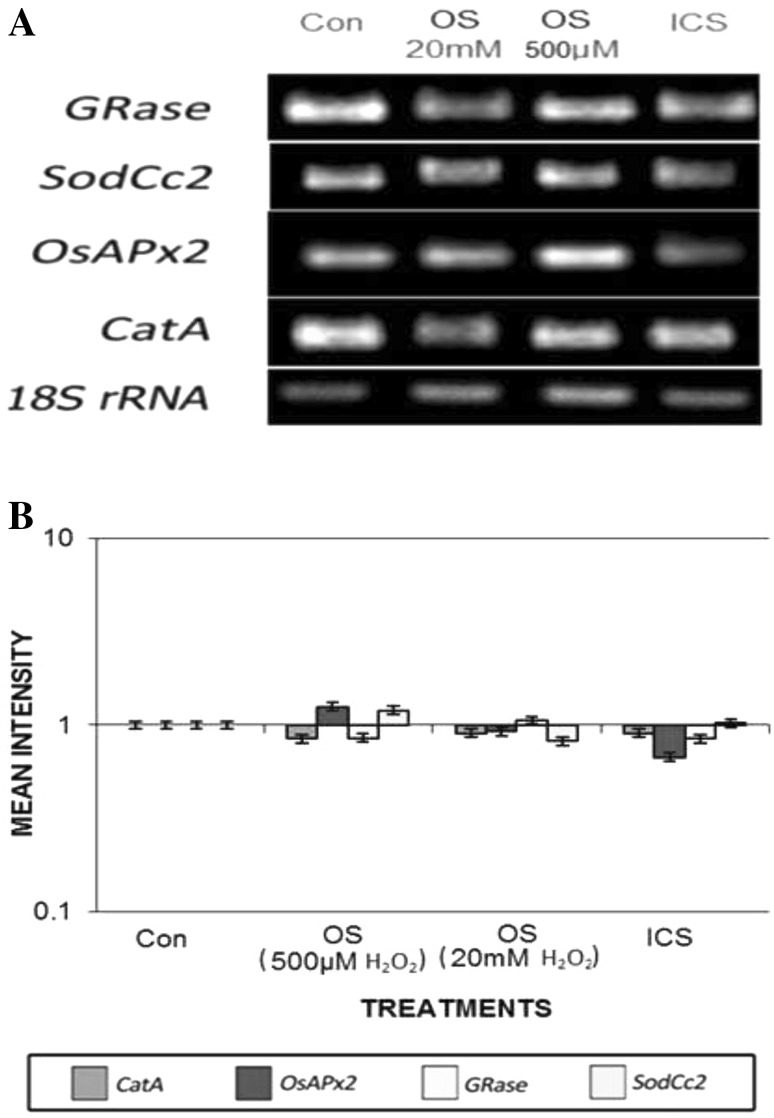
Differential transcript abundance of H2O2 processing antioxidative genes in germinating rice seedlings under different redox cues initiated by varying magnitudes of imbibitional oxidative and chilling stress
The antioxidant defence system is an important component of redox network in plant cell, which in a well-coordinated manner at the advent of oxidative stress control the subsequent episodes of uncontrolled oxidative damages by ROS removal. Here we have investigated the changes in key antioxidative system viz. Ascorbate–glutathione cycle (ASC–GSH), and other H2O2 scavenging system at both activity and gene expression levels in both elevated and low titre H2O2 treated and ICS-raised germinating rice seed. The DNA bands resulted from semi-quantitative RT-PCR provided the value of relative transcript abundance, hence demonstrating the level of gene expressions were markedly different in both high and low titre H2O2 raised rice seedlings (Fig. 8a). Hence, transcripts encoding the major antioxidative enzymes that efficiently remove ROS, particularly H2O2 including ASC–GSH cycle were determined relative to 18S rRNA using semi-quantitative RT-PCR from 20 mM and 500 µM H2O2 treated and ICS raised seedlings of experimental rice cultivar. The 18S rRNA transcripts remained more or less same in germinating tissues of both elevated and low titre treated and ICS-raised seeling of rice cultivar and hence were found suitable in gel loading control for H2O2 processing antioxidative transcript analysis (Fig. 8A).
Fig. 8.
a Transcript abundance of SodCc2, OsAPx2, GRase and CatA genes of imbibitional chilling and oxidative stress raised seedlings of rice cultivar Ratna. Semi-quantitative RT-PCR was performed as described in “Materials and methods” section. The rice 18S rRNA control reaction below each set of corresponding antioxidant gene reaction was conducted on equivalent cDNA batches to verify equivalent loading reaction volumes on the gel. Antioxidant genes were amplified for 25 cycles. b Bar diagram represents mean intensity of relative expression of genes
Therefore, to investigate the molecular mechanism of dose-dependent responses of H2O2 stress in the experimental indica rice cultivar, we used semi-quantitative RT-PCR to assess changes in transcript abundance of some major genes of H2O2 processing antioxidative enzymes. Transcript abundance of genes of SodCc2, CatA, OsAPx2 and GRase, coding respectively for cytosolic Cu/Zn SOD, CAT, APOX, GR exhibited differential relative expression under varying magnitude of H2O2 stress (Fig. 8B). Seedlings of rice cultivar raised from low titre (500 µM) inductive pulse of H2O2 exhibited enhanced transcript abundance for APOX and GRase, whereas elevated titer of H2O2 treatment caused significant decrease in transcript abundance of both the genes. A minor decrease in transcript abundance of both CAT and SOD were observed for both elevated and low dose H2O2 treatment raised rice seedlings.
The ability of maintenance of higher antioxidative enzyme activities of ASC–GSH cycle (APOX and GR) of rice seedlings raised from low titre H2O2 treatment was accompanied by increased expression of OsAPx2 and GRase (Fig. 8). On the contrary, transcript levels for SodCc2, OsAPx2, GRase genes remained significantly lower in seedlings raised from elevated titre of H2O2, endorsing well with the data of reduced enzyme activities, where lack of transcriptional regulation for H2O2 processing antioxidative enzymes appeared to hold germinating tissues incompetent to cope up with elevated level of H2O2. ICS exhibited similar pattern of changes in transcript abundance as that of higher magnitude of oxidative stress (20 mM H2O2 treatment), validating well with the data of antioxidant defence enzymes and redox status of germinating tissues.
Discussion
In the present study, we have investigated the role of redox-regulatory processes (both promotive and disruptive) under varying magnitude of imbibitional oxidative (imposed by 500 µm and 20 mM H2O2 treatment) and chilling stress (8 °C for 16 h) in a warm weathered indica rice cultivar (Oryza sativa L., Cultivar Ratna). The imposition of IOS of different magnitude and ICS changes internal redox cues due to interaction of prooxidants and antioxidants at metabolic interface. The data exhibited dose-dependent responses of H2O2 in terms of generation of internal redox cues in germinating seedlings of indica rice cultivar Ratna. Elevated titre of H2O2 (20 mM) induced serious disruption of redox homeostasis and oxidative damage to membrane protein and lipid of germinating tissue, which even continued 72 h post-treatment period. Being long lived ROS, higher dose of H2O2 treatment, elevated the level of endogenous H2O2, due to its access to the germinating rice seedling through aquaporin-mediated transport system and accelerated processes like Haber-Weiss/Fenton reactions, resulting in generation of other potent ROS OH., which disrupt the redox homeostasis and subsequently caused oxidative damage to membrane protein thiol and lipid (Bhattacharjee 2013; Hossain et al. 2015; Awasthi et al. 2015).
Since low titre inductive pulse of H2O2 treatment caused changes in internal redox cue at metabolic interface, more towards reductants, a stress acclamatory response possibly takes place by cellular adjustment through activation of antioxidative defence system, both enzymatic and non-enzymatic (Liu et al. 2010). A change in the internal redox status, followed by activation of transcription factors and up-regulation of expression of some genes of ASC–GSH cycle during early germination under low magnitude of IOS might trigger a stress acclamatory response required for better post-germination performance of the experimental rice cultivar (Yu et al 2003; Chakraborty and Bhattacharjee 2015). In fact, the activities of antioxidative defense enzymes SOD, CAT, APOX, GR, MDHAR, and DHAR are significantly augmented in seedlings developed from low magnitude inductive pulse of H2O2 as compared to the seedlings developed from higher magnitude of oxidative stress, suggesting dose dependent regulation of antioxidative defence (Hossain et al. 2015).
Generally, the ASC–GSH cycle involves an efficient way in scavenging the ROS H2O2 in plant cell (Anjum et al. 2011; Hossain et al. 2013). In this cycle four enzymes APOX, MDHAR, DHAR and GR work in tandem to detoxify H2O2 and reduce the pro-oxidant level. In our study, the activities of all four enzymes were found to be up-regulated under inductive pulse of IOS; whereas IOS of higher magnitude (20 mM treatment) and ICS caused down-regulation of the activities of ASC–GSH enzymes. Although the enzymes APOX is directly utilised for detoxification of endogenous H2O2 using ASC as electron donor but MDHAR and DHAR are utilised for regulation of reduced ascorbate. MDHAR and DHAR are the major enzymes of ASC–GSH cycle and function as important defensive enzymes to keep the ASC–GSH cycle on, required for the maintenance of redox homeostasis (Noctor and Foyer 1998; Asada 1999; Hossain et al. 2013). The cycle also utilises the endogenous reductant NADPH + H+ for regulation of reduced ascorbate (Asada 1999; Hossain et al. 2013).
In our study, low titre inductive pulse of H2O2 exhibited significantly higher transcript level of the enzyme ascorbate peroxidase and glutathione reductase (OsAPx2 and GRase), corroborating well with their significantly higher activities in germinating tissue. On the contrary, elevated titre of H2O2 treatment caused significant reduction in transcript abundance of both the genes. The experimental results clearly support the view that augmentation in the activities of two crucial enzymes (APOX, GR) of ASC–GSH pathway in seedling raised from low titre inductive pulse of H2O2 is accompanied by enhanced expression of OsAPx2 and GRase. However, the transcript Sod Cc2, OsAPx2 and GRase genes remain significantly lower in seedling raised from higher magnitude of oxidative stress (20 mM), substantiating well with the data of corresponding enzyme activities and efficiency of ASC–GSH pathway. Both APOX and GR play significant role in detoxification of H2O2, primarily through ASC–GSH cycle for the maintenance of redox homeostasis in germinating tissue raised from inductive pulse of oxidative stress (Foyer et al 1997; Foyer and Noctor 2013). Furthermore, both APOX and GR can efficiently detoxify H2O2, which is inaccessible to the enzyme calalase (CAT), due to its presence in different subcellular compartments (Mittler 2002). Therefore, the transcriptional activation of OsAPx2 and GRase under inductive pulse of H2O2 treatment in experimental rice cultivar corresponded well with the better maintenance of two crucial enzyme of ASC–GSH pathway (APOX and GR) to protect cellular components from oxidative damage, particularly the juvenile membrane components in germinating rice tissue (Yoshimura et al. 2000; Ara et al. 2013; Chakraborty and Bhattacharjee 2015).
Significantly higher radical scavenging properties in rice seedling raised from the inductive pulse of H2O2 treatment corresponded with better redox-regulatory properties or maintenance of redox homeostasis required for post-germinative growth of experimental rice cultivar (Roychoudhury et al. 2008; Basu et al. 2010; Chakraborty and Bhattacharjee 2015). The termination of free radical reaction by donation of H atom to free radical is an important parameter for assessing reducing power of a sample. The total antioxidative capacity or radical scavenging property of the tissue extracts reflects mainly their non-enzymatic antioxidative activities (Basu et al. 2010; Chakraborty and Bhattacharjee 2015). In fact, the ability of reducing power of non-enzymatic antioxidants enables them to scavenge ABTS, FRAP and DPPH radicals through formation of their reduced forms (Dat et al 1998; Sun et al 1999).The use of DPPH as a source of stable radical species in methanolic solution for screening plant samples for their radical scavenging properties by chain-breaking reactions has been widely utilized (Basu et al. 2010; Benard and Runner 2007; Chakraborty and Bhattacharjee 2015). Although the mode of action for the assay of ABTS, FRAP and DPPH are different, i.e. scavenging of ABTS and DPPH radicals and reduction of Fe3+ ions in FRAP assay, but in our experiment, the ranking of all three antioxidant assessment exhibited a similar pattern with H2O2-processing antioxidative enzymes and efficiency of ASC–GSH pathway of the experimental rice cultivar under varying magnitude of imbibitional oxidative and chilling stress.The internal redox cues assessed in terms of the ratios of cumulative ROS: total antioxidant capacity, found to be altered enormously in favour of the former under elevated magnitude of IOS and ICS of the experimental rice cultivar. On the contrary, for the low titre H2O2 treatment, the ratio has more bias towards the later, initiating oxidative stress signal. The accumulation of non-enzymatic radical scavenging compounds under low titre inductive pulse of H2O2might also protect the newly assembled membrane components by suppressing oxidation of membrane protein thiol compounds and lipid peroxidation (Novarri-izzo et al. 1994; Chakraborty and Bhattacharjee 2015).Maintenance of thiol compounds (GSH) might also restore membrane protein and enzymes (Bartoli et al. 1999). So, a major function of higher pool of GSH, radical scavenging activity of rice seedling under inductive pulse of H2O2 treatment was to keep ROS scavengers in reduced state and hence in active form by operating ASC–GSH cycle (Zhang and Kirkham 1996; Chakraborty and Bhattacharjee 2015). Another important strategy that largely contributes in the mentainance of redox homeostasis is restoration of reduced glutathione at cellular level by transcriptional activation of glutathione reductase gene (GRase) and corresponding up-regulation of the activity of the enzyme. In fact, the significantly enhanced activity of GR from lower magnitude of IOS-raised seedling (500 µM H2O2) as compared to higher magnitude of IOS (20 mM H2O2) and ICS-raised seedling might be responsible to enhance the ratio of NADP+/NADPH + H+, thereby ensuring the availability of NADP+ for carbon reduction cycle and hence the redox homeostasis.
A comparison of elevated H2O2 treatment with ICS-raised seedlings of rice exhibited similar pattern of responses in terms of formation of internal redox cues and associated oxidative damage to membrane protein thiol and lipid. The extent of loss of membrane protein thiol, accumulation of free carbonyl content, lipid peroxidation, accumulation of individual and total ROS, in situ ROS staining property, total radical scavenging activities, thiol content, activities of H2O2 processing antioxidants caused by both 20 mM H2O2 treatment and ICS not only exhibited similar pattern but also showed correlation with early growth responses (assessed in terms of RGI, VI and RGP) of the experimental rice cultivar. Although these parameters of oxidative membrane damage instigated by 20 mM H2O2 and ICS (MPTL, RC=O and TBARS) are not completely linked, in all experiments early growth performances decreases with lower MPTL and higher accumulation of TBARS and RC=O. This strongly suggests that elevated titre of H2O2 treatment might generate similar kind of adverse redox cues as that of ICS-raised seedlings, where the early growth performances are seriously impaired (Bhattacharjee 2008; He et al. 2009; Hossain et al. 2015). The extent of oxidative damage to newly assembled membrane system (assessed in terms of MPTL, RC=O and TBARS) caused by treatment of elevated titre of H2O2 (20 mM) could be correlated well with internal redox cues, i.e. ratio of pro-oxidant/antioxidant level [DCFDA oxidation, TAC (ABTS, FRAP, DPPH radical scavenging activities)] and the in situ staining properties of superoxide and hydrogen peroxide in germinating tissues (Hossain and Fujita 2013; Ashraf et al. 2014). Estimation of membrane protein thiol and protein carbonylation is more sensitive indicators of loss of redox homeostasis than lipid peroxidation, as the latter may be catabolised more rapidly than protein oxidation (Palma et al. 2002; Bhattacharjee 2012). Further, the susceptibility of newly assembled membrane system towards elevated level of H2O2 stress strongly suggests the significance of maintenance of integrity of membrane system during early post-germination growth of rice seedlings (Bhattacharjee 2013, 2012). The elevated H2O2 treatment could also cause serious damage to organelles like chloroplast and mitochondria (Mittler 2002). In fact, the nature of transient changes in endogenous redox cues at metabolic interface, triggered by exogenous H2O2 treatment of varying magnitude, determine whether signal activation of antioxidative protective mechanism or oxidative deterioration will take place or not (Neill et al. 1999; Wahid et al. 2007; Kovalchuk 2010; Bhattacharjee 2013).
Inductive pulse of H2O2 dependent promotion of germination and seedling establishment might also depend on phytochrome signalling, which initially require ROS interaction with GA (Wojtyla et al. 2016). The significant improvement of germination and early growth performance under inductive pulse of H2O2 treatment (500 µM H2O2) over higher magnitude of H2O2 treatment might be considered as advanced germination metabolism (Soeda et al. 2005), involving significant boast in antioxidant defence (Chen and Arora 2011; Bhattacharjee 2013) and altered hormonal balance (El-Araby et al. 2006), cell division, water transport, post-translational protein folding and targeted proteolysis (Chen and Arora 2013; Kubala et al. 2015). However, the augmentation of germination and early growth performance of the experimental indica rice germplasm is attributable to the maintenance of redox homeostasis through hormone signalling, induction of antioxidative system and subsequent mitigation of oxidative damage to membrane protein and lipid (Chen and Arora 2011; Wojtyla et al. 2016; Bhattacharjee 2013).
Therefore, the differential H2O2 mediated responses of the experimental rice cultivar during early germination might be due to subsequent changes in internal redox cues, which may be adverse or protective in nature. Low titre H2O2 (500 µM) might act as an inductive pulse, during early imbibitional phase of germination, which subsequently elicit an acclamatory response by cellular adjustment through induction of H2O2 processing enzymes and stimulation of ascorbate–glutathione pathway of antioxidative defence (Liu et al. 2010; Bhattacharjee 2013; Kovalchuk 2010). A change in internal redox cues followed by activation of transcription factors and up-regulation of antioxidative enzymes by inductive pulse of H2O2 during early germination to combat unfavourable environmental stresses have been suggested by Yu et al. (2003), Liu et al. (2010) and Bhattacharjee (2013).The results of the present experiments confirmed that both IOS and ICS effectively modulate redox regulatory metabolism, according to the internal redox cues developed, and control related gene expression that subsequently lead to altered germination and early growth performance (Hung et al. 2005; He et al. 2009; Wojtyla et al. 2016).
The present work also throws light on the significance and feasibility of ROS priming of rice seeds for the improvement of antioxidative defense mechanism. Though several earlier workers have highlighted the positive impact of H2O2 priming on stress tolerance (Bhattacharjee 2012; Hossain and Fujita 2013; Lin et al. 2009; Wang et al. 2014), but the role of ROS-antioxidant interaction at metabolic interface that generates internal redox cue, due to ROS pre-treatment, necessary for up-regulating defense mechanism was not explored. So, the proper priming of rice seeds directly with ROS, that have an effect on generating internal redox cue necessary for gene expression associated with antioxidative defence and management of redox homeostasis of the germinating seeds necessary of improved plant performance is a unique finding of the work.
In conclusion, the present work indicated the central role of internal redox cue, produced at metabolic interface, under varying magnitude of imbibitional oxidative and chilling stress, as determinant of oxidative stress response, whether adverse or acclamatory (Supplementary Fig. 2).The work also strongly suggests the significance of maintenance of integrity of membrane system, particularly under oxidative stress, during post-germination growth of rice seedlings (Supplementary Fig. 2). Further, the novelty of the present work is that it suggests the significance of ascorbate–glutathione pathway in redox-regulation under oxidative stress encountered by rice during early germination (Supplementary Fig. 2).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. Experimental exhibiting overall processes from preparing samples under treatment to assessment of redox-parameters, efficacy of ascorbate–glutathione cycle, gene expression and oxidative damages to membrane protein and lipid of an indica rice cultivar (Oryza sativa L. cv. Ratna). Figure 2: Molecular model hypothesizing the results of biochemical assay and differential expression of genes of antioxidant enzymes associated with differential redox-regulation, efficacy of ascorbate–glutathione cycle and oxidative membrane damage of indica rice cultivar (Ratna) towards different magnitude of imbibitional oxidative stress and chilling stress. (PDF 83 kb)
Acknowledgements
The work is supported by a Major Research Project grant from the University Grants Commission, New Delhi, India [F. No. 41-429/2012(SR) Dated 16.07.2012]. AC acknowledges UGC for research fellowship. Authors acknowledge UGC, New Delhi, for instrumentation facility of UGC-CAS (F.5-13/2012 (SAP II).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anjum BA, Xie X, Wang L, Saleem MF, Man C, Lei W. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res. 2011;6:2026–2032. [Google Scholar]
- Ara N, Nakkanong K, Wenhui LV, Yang J, Hu Z, Zhang M. Antioxidant enzymatic activitie4 s and gene expression associated with heat tolerance in the stems and roots of two cucurbit species (Cucurbita maxima and Cucurbita moschata) and their inter-specific inbreed line Maxchata. Int J Mol Sci. 2013;14(12):24008–24028. doi: 10.3390/ijms141224008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Ashraf MA, Rasheed R, Hussain I, Iqbal M, Haider MZ, Parveen S, et al. Hydrogen peroxide modulates antioxidant system and nutrient relation in maize (Zea mays L.) under water-deficit conditions. Arch Agron Soil Sci. 2014;61:507–523. [Google Scholar]
- Awasthi R, Bhandari K, Nayyar H. Temperature stress and redox homeostasis in agricultural crops. Front Environ Sci. 2015 [Google Scholar]
- Barba-Espín G, Diaz-Vivancos P, Job D, Belghazi M, Job C, Hernández JA. Understanding the role of H2O2 during pea seed germination: a combined proteomic and hormone profiling approach. Plant Cell Environ. 2011;34:1907–1919. doi: 10.1111/j.1365-3040.2011.02386.x. [DOI] [PubMed] [Google Scholar]
- Barba-Espín G, Hernández JA, Diaz-Vivancos P. Role of H2O2 in pea seed germination. Plant Signal Behav. 2012;7:193–195. doi: 10.4161/psb.18881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Simontacchi M, Tambussi E, Beltrano J, Montaldi E, Puntarulo S. Drought and watering dependent oxidative stress: effect on antioxidative content in Triticum aestivum L. leaves. J Exp Bot. 1999;50(322):375–383. [Google Scholar]
- Basu A, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140(9):1582–1587. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin J, Langlade N, Vincourt P, Arribat S, Balzergue S, El-Maarouf-Bouteau H, et al. Targeted mRNA oxidation regulates sunflower seed dormancy alleviation during dry after-ripening. Plant Cell. 2011;23:2196–2208. doi: 10.1105/tpc.111.086694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard J, Runner RTM. Constituents of Gardenia volkensii: their brine shrimp lethality and DPPH radical scavenging properties. Nat Prod Res. 2007;21(2):121–125. doi: 10.1080/14786410600905907. [DOI] [PubMed] [Google Scholar]
- Benzie IFE, Strain JJ. The ferric reducing ability of plasma (FRAP) as measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Bradford KJ, Hilhorst HMW, Nonogaki H. Seeds: physiology of development, germination and dormancy. New York: Springer; 2013. [Google Scholar]
- Bhattacharjee S. Calcium-dependent signaling pathway in heat-induced oxidative injury in Amaranthus lividus. Biol Plant. 2008;52:1137–1140. [Google Scholar]
- Bhattacharjee S. An inductive pulse of hydrogen peroxide pretreatment restores redox-homeostasis and mitigates oxidative membrane damage under extremes of temperature in two rice cultivars (Oryza sativa L., Cultivars Ratna and SR 26B) Plant Growth Regul. 2012;68:395–410. [Google Scholar]
- Bhattacharjee S. Heat and chilling induced disruption of redox homeostasis and its regulation by hydrogen peroxide in rice (Oryza sativa L., Cultivar Ratna) Physiol Mol Biol Plants. 2013;19:199–207. doi: 10.1007/s12298-012-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Schiff M, Lin D-KS. Efficient virus induced gene silencing in Arabidopsis. Plant Physiol. 2006;42:21–27. doi: 10.1104/pp.106.084624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykova NV, Hoehn B, Rampitsch C, Banks T, Stebbing JA, Fan T, et al. Redox-sensitive proteome and antioxidant strategies in wheat seed dormancy control. Proteomics. 2011;11:865–882. doi: 10.1002/pmic.200900810. [DOI] [PubMed] [Google Scholar]
- Chaitanya KSK, Naithani SC. Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability of Shorea robusta Gaertn F. New Phytol. 1994;126:623–627. [Google Scholar]
- Chakraborty A, Bhattacharjee S. Differential competence of redox-regulatory mechanism under extremes of temperature determines growth performances and cross tolerance in two indica rice cultivars. J Plant Physiol. 2015;176:65–77. doi: 10.1016/j.jplph.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Chen K, Arora R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in spinach (Spinacia oleracea) Plant Sci. 2011;180:212–220. doi: 10.1016/j.plantsci.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Chen K, Arora R. Priming memory invokes seed stress-tolerance. Environ Exp Bot. 2013;94:33–45. [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998;116:1351–1357. doi: 10.1104/pp.116.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekok LJ, Kuiper PJC. Effect of short term incubation with sulfate, chloride and selenate on glutathione content of spinach leaf disc. Physiol Plant. 1986;68:472–482. [Google Scholar]
- Desikan R, Mackerness S, Hancock JT, Neill SJ. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001;127:159–172. doi: 10.1104/pp.127.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Araby MM, Moustafa SMA, Ismail AI, Hegazi AZA. Hormone and phenol levels during germination and osmopriming of tomato seeds, and associated variations in protein patterns and anatomical seed features. Acta Agron Hung. 2006;54:441–458. [Google Scholar]
- El-Maarouf-Bouteau H, Sajjad Y, Bazin J, Langlade N, Cristescu SM, Balzergue S, et al. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ. 2015;38:364–374. doi: 10.1111/pce.12371. [DOI] [PubMed] [Google Scholar]
- Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:72–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fick NG, Qualset CD. Genetic control of plant amylase activity. Proc Natl Acad Sci USA. 1975;72:852–862. doi: 10.1073/pnas.72.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox signalling in plants. Antioxid Redox Signal. 2013;18:2081–2090. doi: 10.1089/ars.2013.5278. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delago H, Dat JF, Scott JM. Hydrogen peroxide and glutathione associated mechanism of acclamatory stress tolerance and signaling. Physiol Plant. 1997;100:241–251. [Google Scholar]
- Giannopolities CN, Ries SK. Superoxide dismutase: I. Occurrence in higher plants. Plant Physiol. 1977;59:309–319. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Gao Z, Li R. Pretreatment of seed with H2O2 enhances drought tolerance of wheat seedlings. Afr J Biotechnol. 2009;8(22):6151–6157. [Google Scholar]
- He D, Han C, Yao J, Shen S, Yang P. Constructing the metabolic and regulatory pathways in germinating rice seeds through proteomic approach. Proteomics. 2011;11:2693–2713. doi: 10.1002/pmic.201000598. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photo-oxidation in isolated chloroplasts: kinetics and stoichiometry of fatty acid oxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hodges DM, Forney CF, Wisme WV. Antioxidant responses in harvested leaves of two cultivars of spinach differing in senescence rates. J Soc Hortic Sci. 2001;126:611–617. [Google Scholar]
- Hossain MA, Fujita M. Hydrogen peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.) Plant Gene Trait. 2013;4:109–123. [Google Scholar]
- Hossain MA, Ismail MR, Uddin MK, Islam MZ, Ashrafuzzaman M. Efficacy of ascorbate–glutathione cycle for scavenging H2O2 in two contrasting rice genotypes during salinity stress. Aust J Crop Sci. 2013;7:1801–1808. [Google Scholar]
- Hossain MA, Bhattacharjee S, Armin SA, Qian P, Xin W, Li HU, Burritt DJ, Fujita M, Tran L-SP. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SH, Yu CW, Lin CH. Hydrogen peroxide functions as a stress signal in plants. Bot Bull Acad Sin. 2005;46:1–10. [Google Scholar]
- Ishibashi Y, Tawaratsumida T, Kondo K, Kasa S, Sakamoto M, Aoki N, et al. Reactive oxygen species are involved in gibberellins/abscisic acid signaling in barley aleurone cells. Plant Physiol. 2012;158:1705–1714. doi: 10.1104/pp.111.192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Effect of abscisic acid on active oxygen species, antioxidative defense system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001;42(11):1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Sharma R, Handa N, Kaur H, Rattan A, Yadav P, et al. Redox homeostasis in plants under abiotic stress: role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front Environ Sci. 2015;03:13–23. [Google Scholar]
- Kovalchuk I. Multiple roles of radicals in plants. In: Dutta Gupta S, editor. Reactive oxygen species and antioxidants in higher plants. Boca Raton: CRC Press; 2010. pp. 31–44. [Google Scholar]
- Kubala S, Garnczarska M, Wojtyla L, Clippe A, Kosmala A, Żmieńko A, et al. Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Sci. 2015;231:94–113. doi: 10.1016/j.plantsci.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Lariguet P, Ranocha P, De Meyer M, Barbier O, Penel C, Dunand C. Identification of a hydrogen peroxide signalling pathway in the control of light-dependent germination in Arabidopsis. Planta. 2013;238:381–395. doi: 10.1007/s00425-013-1901-5. [DOI] [PubMed] [Google Scholar]
- Lin F, Ding H, Wang J, Zhang H, Zhang A, Zhang Y, Tan M, Dong W, Jiang M. Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. J Exp Bot. 2009;60:3221–3238. doi: 10.1093/jxb/erp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Guo YK, Bi JG. Exogenous hydrogen peroxide change antioxidant enzyme activity and plastid ultrastructure in leaves of two cucumber ecotypes under osmotic stress. J Plant Growth Regul. 2010;29(02):171–183. [Google Scholar]
- MacNevin WM, Uron PF. Spectrum of hydrogen peroxide from organic hydroperoxides. Anal Chem. 1953;25:1760–1761. [Google Scholar]
- Mensor LI, Menezes FS, Leitao GG, Reis AS, dos Santos T, Coube CS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, et al. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Moradi F, Ismail AAM. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann Bot. 2007;99:1161–1173. doi: 10.1093/aob/mcm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8:385–387. doi: 10.1016/s1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Neill S, Desikan R, Clarke A, Hancock J. Hydrogen peroxide signaling in plant cells. In: Smallwood MF, Calvert CJ, Bowlas DJ, editors. Plant responses to environmental stress. Oxford: BIOS Scientific Publishers; 1999. pp. 59–64. [Google Scholar]
- Noctor G, Foyer CH. Ascorbsate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Novarri-Izzo F, Pinzino C, Quartacci MF, Sgherri CLM. Intracellular membranes : kinetics of superoxide production and changes in thylakoids of resurrection plants upon dehydration and rehydration. Proc R Soc Edinb Sect B. 1994;4(102B):187–191. [Google Scholar]
- Palma JM, Sandalio LM, Corpas FJ, Romero-Puertas MC, Mccarthy I, Delrio LA. Plant proteases, protein degradation and oxidative stress: role of peroxisomes. Plant Physiol Biochem. 2002;40:521–530. [Google Scholar]
- Petrov VD, Van Breusegem F. Hydrogen peroxide-a central hub for information flow in plant cells. AoB Plants. 2012 doi: 10.1093/aobpla/pls014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R, Pellegrinni N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activities applying an improved ABTS radical cationdecolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Roychoudhury A, Basu S, Sarkar S, Sengupta DN. Comparative physiological and molecular responses of a common aromatic indica rice cultivar to high salinity with non-aromatic Indicarice cultivar. Plant Cell Reprod. 2008;27:1395–1410. doi: 10.1007/s00299-008-0556-3. [DOI] [PubMed] [Google Scholar]
- Rubio-Casal AE, Castillo JM, Lucue C, Fig Ureo ME. Influence of salinity on germination and seed viability of two primary colonizers of Mediterranean salt plants. J Arid Environ. 2003;53:145–152. [Google Scholar]
- Schaedle M, Bassham JA. Chloroplast glutathione reductase. Plant Physiol. 1977;511:011–012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simontacchi M, Caro A, Fraga CG, Puntarulo S. Oxidative stress affects α-tocopherol content in soyabean embryonic axes upon imbibitions. Plant Physiol. 1993;103:943–953. doi: 10.1104/pp.103.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP. Interaction of temperature and microsomal peroxidase in aflatoxin degradation by Aspergillus flavus 102566. Curr Sci. 1997;72:529–532. [Google Scholar]
- Smirnoff N. Ascorbic acid: metabolism & functions of a multi-facetted molecule. Curr Plant Biol. 2000;3:229–235. [PubMed] [Google Scholar]
- Snell FD, Snell CT. Colorimetric methods of analysis. New York: Van Nostard Reinford Co; 1971. [Google Scholar]
- Soeda A, Nakashima T, Okumura A, Kuwata K, Shinoda J, Iwama T. Cognitive impairment after traumatic brain injury: a functional magnetic resonance imaging study using the Stroop task. Neuroradiology. 2005;47:501–506. doi: 10.1007/s00234-005-1372-x. [DOI] [PubMed] [Google Scholar]
- Song Y, Manson JE, Buring JE, et al. Association of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr. 2005;24:376–384. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- Sun CP, Zhang JZ, Duan SJ. Free radical biology. Hefei: Science and Technology University of China Press; 1999. [Google Scholar]
- Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inze D, Van Breusegem F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005;139:806–821. doi: 10.1104/pp.105.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid A, Parveen M, Gelani S, Basra SMA. Pretreatment of seeds with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol. 2007;164:283–294. doi: 10.1016/j.jplph.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xing S, Birkenbihl RP, Zachgo S. Conserved functions of Arabidopsis and rice CC-type glutaredoxins in flower development and pathogen response. Mol Plant. 2009;2:323–335. doi: 10.1093/mp/ssn078. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang J, Li JL, Ma XR. Exogenous hydrogen peroxide enhanced the thermotolerance of Festuca arundinacea and Lolium perenne by increasing the antioxidative capacity. Acta Physiol Plant. 2014;36:2915–2924. [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Wojtyla Ł, Lechowska K, Kubala S, Garnczarska M. Different modes of hydrogen peroxide action during seed germination. Front Plant Sci. 2016;7:66. doi: 10.3389/fpls.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye N, Zhu G, Liu Y, Zhang A, Li Y, Liu R, et al. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J Exp Bot. 2012;63:1809–1822. doi: 10.1093/jxb/err336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S. Expression of spinach ascorbate peroxidise isozymes in response to oxidative stresses. Plant Physiol. 2000;123:223–234. doi: 10.1104/pp.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CW, Murphy TM, Liu CH. Hydrogen peroxide induced chilling tolerance in mung bean mediated through ABA-induced glutathione accumulation. Funct Plant Biol. 2003;30:958–963. doi: 10.1071/FP03091. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kirkham MB. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996;132:361–373. doi: 10.1111/j.1469-8137.1996.tb01856.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Chen L, Li D, Lv B, Chen Y, Chen J, et al. OsRACK1 is involved in abscisic acid- and H2O2-mediated signaling to regulate seed germination in rice (Oryza sativa, L.) PLoS ONE. 2014;9:e97120. doi: 10.1371/journal.pone.0097120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Experimental exhibiting overall processes from preparing samples under treatment to assessment of redox-parameters, efficacy of ascorbate–glutathione cycle, gene expression and oxidative damages to membrane protein and lipid of an indica rice cultivar (Oryza sativa L. cv. Ratna). Figure 2: Molecular model hypothesizing the results of biochemical assay and differential expression of genes of antioxidant enzymes associated with differential redox-regulation, efficacy of ascorbate–glutathione cycle and oxidative membrane damage of indica rice cultivar (Ratna) towards different magnitude of imbibitional oxidative stress and chilling stress. (PDF 83 kb)