Abstract
Background
Following introduction of 7-valent pneumococcal conjugate vaccine (PCV7) in the United States, epidemiology of pneumococcal disease shifted such that disease incidence in the elderly exceeded that in children. We evaluated the impact of replacing PCV7 with PCV13 on disease burden in adults and identified age/risk-specific subgroups with the highest remaining disease burden.
Methods
A retrospective design and data from two US healthcare claims repositories were used. Study population included adults aged ≥18 years and was stratified by age (18–49, 50–64, 65–74, ≥75) and risk profile (healthy, at-risk, high-risk). Rate ratios comparing invasive pneumococcal disease (IPD), all-cause hospitalized pneumonia (ACHP), and pneumococcal pneumonia requiring hospitalization among at-risk and high-risk adults vs healthy counterparts were estimated for 2007–2010 (pre-PCV13), 2011–2012 (peri-PCV13), and 2013–2015 (post-PCV13).
Results
Across study periods, IPD and ACHP rates increased with age (2–27 times higher in persons ≥75 vs 18–49) and comorbidity (4–20 times higher in high-risk vs healthy). From pre- to post-PCV13 period, IPD rates declined 5%–48% and ACHP rates declined 4%–19% across age and risk groups (ACHP did not decline in persons ≥75). Decline in IPD and ACHP was attenuated among older adults and those with comorbidities. Accordingly, rate ratios among at-risk and high-risk persons (vs healthy counterparts) increased during the peri- and post-PCV13 periods compared with the pre-PCV13 period.
Conclusions
The switch to PCV13 was associated with large declines in pneumococcal disease among US adults. However, the decline was attenuated with increasing age (and, for ACHP, was absent in persons ≥75) and in those with comorbidities.
Keywords: Streptococcus pneumonia, pneumococcal infections, pneumonia, immunocompromised, comorbidity
Declining incidence of invasive pneumococcal disease and all-cause pneumonia following introduction of 13-valent pneumococcal conjugate vaccine for children was observed in adults aged ≥18 years. However, decline varied by age and comorbidity profile, with smaller reductions in older individuals and those with comorbidity.
The epidemiology of pneumococcal disease dramatically shifted in the United States following the introduction of 7-valent pneumococcal conjugate vaccine (PCV7). Prior to the introduction of PCV7 in 2000, the highest incidence rates of invasive pneumococcal disease (IPD) were found in children aged <2 years. Universal PCV7 immunization of birth cohorts beginning in 2000 dramatically reduced the incidence of pneumococcal disease in children such that disease incidence in adults aged ≥65 years now exceeds that in children [1, 2].
The decline in pneumococcal disease in children results primarily from direct protection elicited by universal immunization with pneumococcal conjugate vaccines. The widespread use of PCV7 among young children in the United States also resulted—via a herd effect—in reduced levels of IPD among older children and adults [3–6]. However, by 2010, increasing numbers of cases of IPD due to nonvaccine serotypes began to erode the reductions in disease in both adults and children.
In part as a response to increasing replacement disease, PCV13 was introduced in the United States for use in all children aged <2 years and as catch-up dosing for children between the ages of 2 and 5 years in 2010 [7]. Two years later, in 2012, PCV13 was recommended for adults aged ≥18 years with immunocompromising conditions. In 2014, the recommendation was expanded to include all adults aged ≥65 years. The incidence of IPD in children declined soon after the introduction of PCV13, reflecting the direct protection achieved [2]. Subsequently, the IPD incidence in adults declined as well [1, 2].
Prior research conducted by our group focused on estimating the magnitude of increased risk of pneumococcal disease among children and adults with “at-risk” (immunocompetent with at least 1 chronic medical condition identified as an indication for pneumococcal vaccination) and “high-risk” (immunocompromised or with a cochlear implant) conditions in the era of universal childhood PCV7 immunization [8, 9]. The results of this research demonstrated that the increased risk of pneumococcal disease associated with specific comorbid conditions persisted among both children and adults during the PCV7 era and that the magnitude of excess risk varied with age and comorbid condition. Investigators have reported similar findings from the United Kingdom and the United States, although rates and relative rates have varied across studies [10–12]. Our research also demonstrated that individuals with comorbid conditions suffer a disproportionate burden of pneumococcal disease. For example, 66% of pneumococcal pneumonia was found to occur among 24% of the adult population with at-risk and high-risk conditions, and immunocompetent adults with 2 or more at-risk conditions had rates of pneumococcal disease that were as high (or higher than) those among adults with immunodeficiency.
Recognizing that differences in the incidence of pneumococcal disease in adults are related to age and comorbidity status, we hypothesized that the reported decline in pneumococcal disease burden in adults [2, 13] following introduction of PCV13 in children was likely to vary across the adult population. To compare the burden of pneumococcal disease in adults before and after introduction of PCV13 with a specific focus on age and comorbidity, we used 2 large healthcare claims repositories to identify the burden of disease by age and comorbidity profile and to measure the temporal change in disease burden across age- and risk-specific adult subgroups. While lacking certain elements of clinical richness, the repositories that were used provide access to the health profile and healthcare experience—across the continuum of care settings—for tens of millions of persons over a multiyear period and thus contain information on large numbers of persons with and without specific medical conditions.
METHODS
We used a retrospective design and data from 2 US private healthcare claims repositories—the Truven Health Analytics MarketScan Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits Databases and the Optum Clinformatics Claims Database. For this study, data spanned the period from January 2005 through December 2015. A detailed description of the data sources may be found in the Supplementary Appendix A.
Study cohorts were identified as of the beginning of each calendar quarter of observation from January 2007 to December 2015 and were characterized in terms of their age and risk profile. Patient age was determined assuming a birth date of 30 June in their birth year. Patient risk profile was determined based on the presence of medical conditions that are current indications for pneumococcal vaccination in recommendations set forth by the US Advisory Committee on Immunization Practices (ACIP); operational algorithms and codes used to identify these conditions are set forth in the Supplementary Appendix B [10, 14]. High-risk adults included those who were immunocompromised or had a cochlear implant. At-risk adults included those who were immunocompetent with 1 or more chronic medical conditions. Healthy adults included those without evidence of any high-risk or at-risk condition. Risk profiles were characterized based on information recorded during the 2-year period prior to the beginning of the corresponding calendar quarter. To ensure that risk profiles were characterized comprehensively and consistently across study cohorts, patients without healthcare claims data for 2 or more years prior to the first day of the calendar quarter were excluded from consideration. Patients who met criteria for inclusion in multiple calendar quarters contributed a corresponding number of observations to analyses.
For each of these cohorts, episodes of IPD, all-cause pneumonia, and pneumococcal pneumonia requiring hospitalization were ascertained over the 3-month (“follow-up”) period beginning on the first day of the corresponding calendar quarter and ending on the last day of the same calendar quarter (or the date of loss to follow-up, if earlier). Hospitalizations for IPD were identified based on a diagnosis of bacteremia, meningitis, or selected other invasive manifestations, and a diagnosis of pneumococcus, with 1 such diagnosis in the first-listed position. Hospitalizations for pneumonia were identified based on a first-listed diagnosis of pneumonia or on a first-listed diagnosis of bacteremia or respiratory failure and a second-listed diagnosis of pneumonia, based on the study by Lindenauer and colleagues [15]. Hospitalizations for pneumococcal pneumonia also required a diagnosis of pneumococcus. Operational algorithms and codes for the outcomes of interest are set forth in the Supplementary Appendix C. Episodes of pneumococcal disease that were separated by 90 or more days were considered as separate events for each patient.
Crude incidence rates of pneumococcal disease among healthy, at-risk, and high-risk persons, respectively, were calculated by calendar quarter for each age group. Rates were calculated by dividing the number of disease episodes within the age/risk-specific subgroup in a given quarter by the total number of person-years in the corresponding age/risk-specific subgroup in that quarter. Rates were expressed per 100 000 person-years, and confidence intervals (CIs) were computed using the Wilson score interval [16]. Rate ratios for disease episodes among persons with at-risk and high-risk conditions vs their age-stratified healthy counterparts were estimated for the pre- (2007–2010), peri- (2011–2012), and post-PCV13 (2013–2015) periods using Poisson regression.
RESULTS
A total of 56.6 million persons contributed 158.1 million person-years of observation from January 2007 through December 2015 and thus were included in the study population. The number of person-years and the distribution of person-years by age and risk profile were largely comparable between the pre-PCV13 period (2007–2010), the peri-PCV13 period (2011–2012), and the post-PCV13 period (2013–2015) (Supplementary Table 1).
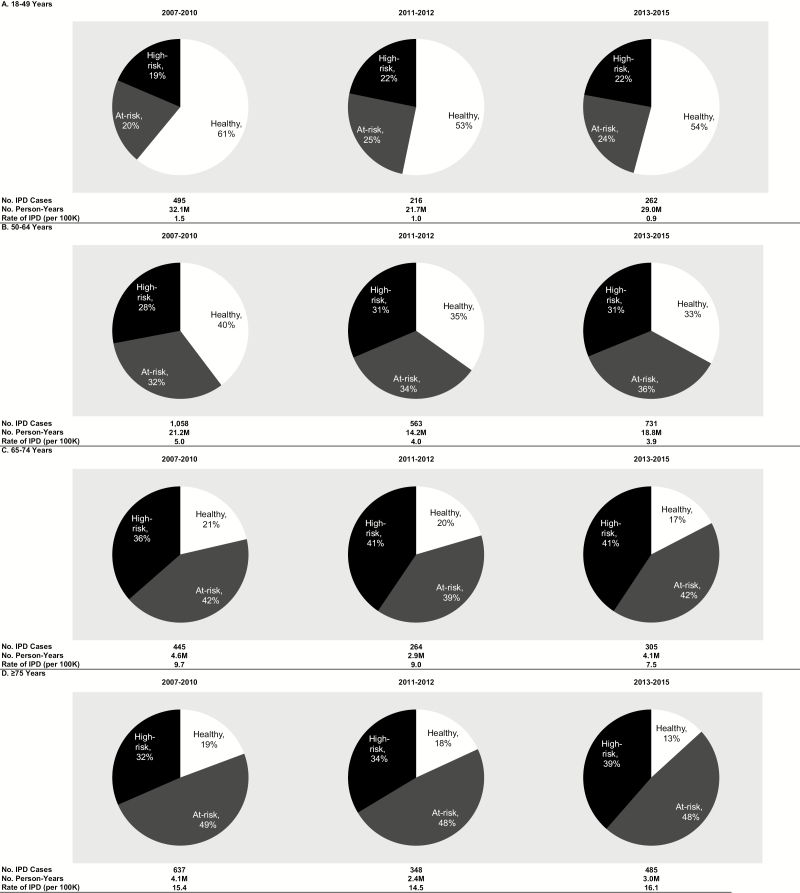
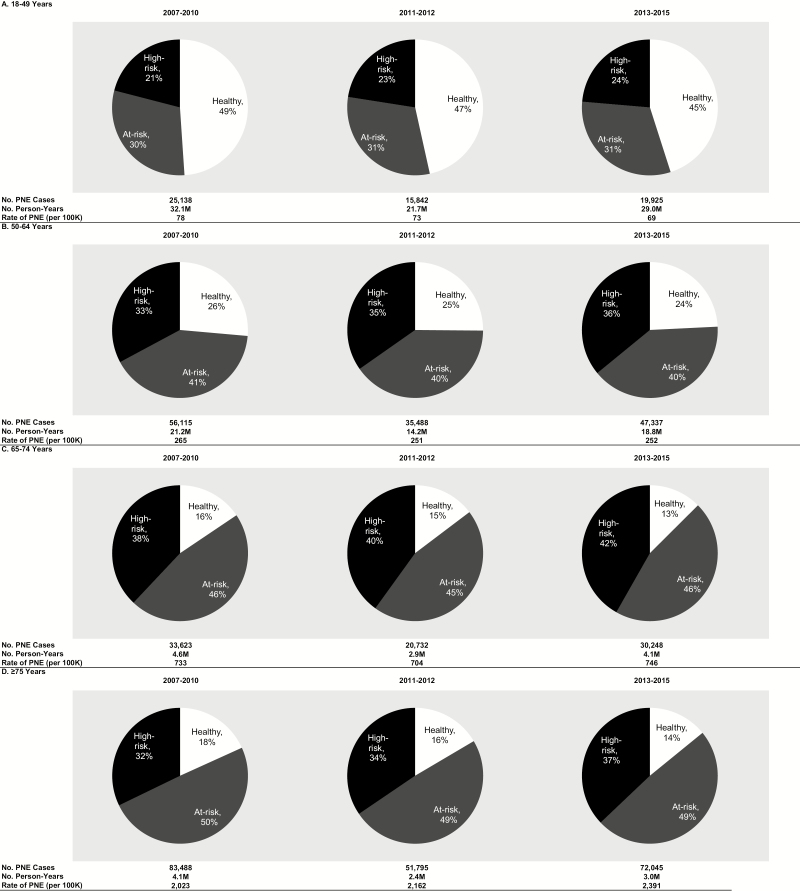
In all 3 periods, incidence rates for IPD, all-cause pneumonia, and pneumococcal pneumonia increased monotonically with age and risk profile (Table 1, Supplementary Table 2, Supplementary Figures 1–3). For example, in the post-PCV13 period (2013–2015), the rate of all-cause pneumonia among adults aged ≥75 years was 6–27 times (depending on risk profile) the rate in those aged 18–49 years, and the rates among at-risk and high-risk adults were 3–6 and 4–20 times (depending on age), respectively, the rate in healthy persons.
Table 1.
Rates and Rate Ratios for Invasive Pneumococcal Disease and All-cause Pneumonia Requiring Hospitalization, by Age and Risk Profile
| Rate per 100 000 Person-years (95% CI) | Percent Decline in Rate (95% CI) From 2007–2010 to | Rate Ratios (95% CI) vs Healthy | ||||||
|---|---|---|---|---|---|---|---|---|
| 2007–2010 | 2011–2012 | 2013–2015 | 2011–2012 | 2013–2015 | 2007–2010 | 2011–2012 | 2013–2015 | |
| Invasive pneumococcal disease | ||||||||
| Age/risk profile | ||||||||
| 18–49 years | ||||||||
| Healthy | 1.1 (1.0, 1.2) | 0.6 (0.5, 0.7) | 0.6 (0.5, 0.7) | 43 (30, 54) | 47 (36, 57) | ... | ... | ... |
| At-risk | 3.2 (2.6, 3.9) | 2.4 (1.8, 3.1) | 2.1 (1.6, 2.6) | 24 (–5, 46) | 35 (11, 53) | 3.0 (2.4, 3.7) | 4.0 (2.9, 5.5) | 3.7 (2.7, 4.9) |
| High-risk | 12.7 (10.4, 15.6) | 9.3 (7.0, 12.3) | 8.6 (6.7, 11.2) | 27 (–3, 49) | 32 (6, 51) | 11.9 (9.4, 15.0) | 15.3 (10.9, 21.5) | 15.4 (11.3, 20.9) |
| 50–64 years | ||||||||
| Healthy | 2.8 (2.6, 3.1) | 2.0 (1.7, 2.3) | 1.9 (1.6, 2.1) | 29 (16, 40) | 34 (23, 44) | ... | ... | ... |
| At-risk | 7.0 (6.3, 7.8) | 5.8 (5.0, 6.7) | 6.0 (5.3, 6.7) | 18 (2, 31) | 15 (1, 28) | 2.5 (2.2, 2.9) | 2.9 (2.4, 3.6) | 3.2 (2.7, 3.8) |
| High-risk | 20.5 (18.3, 23.0) | 17.3 (15.0, 20.1) | 16.4 (14.4, 18.7) | 16 (–2, 30) | 20 (5, 33) | 7.3 (6.3, 8.5) | 8.7 (7.1, 10.7) | 8.8 (7.4, 10.6) |
| 65–74 years | ||||||||
| Healthy | 4.0 (3.3, 4.9) | 3.6 (2.8, 4.7) | 2.7 (2.0, 3.5) | 10 (–26, 35) | 33 (7, 52) | ... | ... | ... |
| At-risk | 11.8 (10.2, 13.6) | 9.9 (8.2, 12.0) | 8.7 (7.3, 10.4) | 16 (–7, 34) | 26 (7, 41) | 3.0 (2.3, 3.8) | 2.7 (2.0, 3.8) | 3.3 (2.4, 4.5) |
| High-risk | 26.4 (22.6, 30.8) | 25.9 (21.4, 31.2) | 20.8 (17.5, 24.9) | 2 (–25, 23) | 21 (0, 38) | 6.6 (5.1, 8.5) | 7.2 (5.2, 9.9) | 7.8 (5.7, 10.8) |
| ≥75 years | ||||||||
| Healthy | 7.1 (6.0, 8.5) | 6.8 (5.3, 8.7) | 6.0 (4.7, 7.7) | 5 (–29, 30) | 15 (–14, 37) | ... | ... | ... |
| At-risk | 19.2 (17.2, 21.4) | 17.1 (14.7, 19.9) | 18.3 (16.1, 20.8) | 11 (–8, 26) | 5 (–13, 20) | 2.7 (2.2, 3.3) | 2.5 (1.9, 3.4) | 3.0 (2.3, 4.0) |
| High-risk | 26.3 (22.9, 30.2) | 24.1 (20.1, 28.9) | 28.0 (24.2, 32.3) | 8 (–15, 27) | –6 (–30, 13) | 3.7 (3.0, 4.6) | 3.6 (2.6, 4.8) | 4.6 (3.5, 6.2) |
| All-cause pneumonia | ||||||||
| Age/risk profile | ||||||||
| 18–49 years | ||||||||
| Healthy | 44 (43, 44) | 39 (38, 40) | 35 (35, 36) | 11 (8, 13) | 19 (16, 21) | ... | ... | ... |
| At-risk | 238 (233, 243) | 218 (212, 224) | 207 (202, 212) | 9 (5, 12) | 13 (10, 16) | 5.5 (5.3, 5.6) | 5.6 (5.4, 5.8) | 5.8 (5.6, 6.0) |
| High-risk | 730 (710, 750) | 704 (681, 727) | 701 (681, 721) | 4 (–1, 8) | 4 (0, 8) | 16.8 (16.2, 17.3) | 18.1 (17.4, 18.8) | 19.7 (19.1, 20.5) |
| 50–64 years | ||||||||
| Healthy | 99 (98, 101) | 90 (89, 92) | 88 (87, 90) | 9 (6, 11) | 11 (9, 13) | ... | ... | ... |
| At-risk | 471 (465, 478) | 434 (427, 442) | 429 (423, 435) | 8 (6, 10) | 9 (7, 11) | 4.7 (4.7, 4.8) | 4.8 (4.7, 4.9) | 4.9 (4.7, 5.0) |
| High-risk | 1278 (1260, 1297) | 1208 (1187, 1229) | 1226 (1207, 1244) | 6 (3, 8) | 4 (2, 6) | 12.9 (12.6, 13.2) | 13.3 (13.0, 13.7) | 13.9 (13.5, 14.2) |
| 65–74 years | ||||||||
| Healthy | 220 (215, 226) | 204 (197, 211) | 191 (185, 197) | 8 (3, 12) | 13 (10, 17) | ... | ... | ... |
| At-risk | 979 (964, 995) | 905 (887, 923) | 941 (925, 957) | 8 (5, 10) | 4 (2, 6) | 4.4 (4.3, 4.6) | 4.4 (4.3, 4.6) | 4.9 (4.8, 5.1) |
| High-risk | 2081 (2046, 2118) | 2005 (1962, 2049) | 2124 (2087, 2162) | 4 (1, 6) | –2 (–5, 0) | 9.4 (9.1, 9.8) | 9.8 (9.4, 10.3) | 11.1 (10.7, 11.5) |
| ≥75 years | ||||||||
| Healthy | 880 (867, 895) | 918 (899, 938) | 957 (938, 975) | –4 (–7, –2) | –9 (–11, –6) | ... | ... | ... |
| At-risk | 2536 (2512, 2561) | 2592 (2560, 2624) | 2745 (2717, 2774) | –2 (–4, –1) | –8 (–10, –7) | 2.9 (2.8, 2.9) | 2.8 (2.8, 2.9) | 2.9 (2.8, 2.9) |
| High-risk | 3511 (3470, 3554) | 3676 (3623, 3730) | 3992 (3944, 4040) | –5 (–7, –3) | –14 (–16, –12) | 4.0 (3.9, 4.1) | 4.0 (3.9, 4.1) | 4.2 (4.1, 4.3) |
Abbreviation: CI, confidence interval.
Pneumococcal disease rates generally declined from the pre-PCV13 period to the peri-PCV13 period to the post-PCV13 period for adults aged <75 years and across risk groups. However, the reduction in disease incidence was generally attenuated with increasing age and those with comorbidities. For example, between the pre-PCV13 and post-PCV13 periods, the rate of all-cause hospitalized pneumonia declined by 19% among healthy adults aged 18–49 years, declined by 13% among healthy adults aged 65–74 years, and increased by 2% among high-risk adults aged 65–74 years. Among adults aged ≥75 years, incidence of all-cause pneumonia requiring hospitalization increased from the pre-PCV13 period to the post-PCV13 period. We also calculated absolute reductions in IPD and all-cause pneumonia hospitalizations by age and risk profile (Table 2). The greatest reduction in disease burden was observed in those aged <65 years who were healthy or had an at-risk (nonimmunocompromising) condition. In those with the greatest incidence of disease, older adults with high-risk (immunocompromising) conditions, little, if any, reduction in the numbers of IPD or all-cause pneumonia events was observed.
Table 2.
Absolute Reduction in Number of Cases of Invasive Pneumococcal Disease and All-cause Pneumonia Requiring Hospitalization per Year, by Age and Risk Profile
| Number of Person-years (in Millions) in 2015 | Decline in Rate per 100 000 Person-years | Absolute Reduction in Cases per Year | ||
|---|---|---|---|---|
| Percent | Absolute | |||
| Invasive pneumococcal disease | ||||
| Age/risk profile | ||||
| 18–49 yearsa | 8.6 | 46 | 0.7 | 57 |
| Healthy | 7.5 | 47 | 0.5 | 38 |
| At-risk | 0.9 | 35 | 1.1 | 10 |
| High-risk | 0.2 | 32 | 4.1 | 8 |
| 50–64 yearsa | 5.6 | 28 | 1.2 | 69 |
| Healthy | 3.8 | 34 | 1.0 | 37 |
| At-risk | 1.3 | 15 | 1.1 | 15 |
| High-risk | 0.4 | 20 | 4.1 | 17 |
| 65–74 yearsa | 1.1 | 29 | 2.6 | 29 |
| Healthy | 0.5 | 33 | 1.3 | 7 |
| At-risk | 0.4 | 26 | 3.1 | 12 |
| High-risk | 0.2 | 21 | 5.6 | 9 |
| ≥75 yearsa | 0.8 | 6 | 0.4 | 3 |
| Healthy | 0.3 | 15 | 1.1 | 3 |
| At-risk | 0.4 | 5 | 0.9 | 3 |
| High-risk | 0.2 | –6 | –1.7 | –3 |
| All-cause pneumonia | ||||
| Age/risk profile | ||||
| 18–49 yearsa | 8.6 | 18 | 11.0 | 952 |
| Healthy | 7.5 | 19 | 8.1 | 605 |
| At-risk | 0.9 | 13 | 31.2 | 288 |
| High-risk | 0.2 | 4 | 29.0 | 59 |
| 50–64 yearsa | 5.6 | 10 | 21.5 | 1,205 |
| Healthy | 3.8 | 11 | 10.8 | 415 |
| At-risk | 1.3 | 9 | 42.2 | 567 |
| High-risk | 0.4 | 4 | 52.7 | 222 |
| 65–74 yearsa | 1.1 | 8 | 22.1 | 244 |
| Healthy | 0.5 | 13 | 29.6 | 157 |
| At-risk | 0.4 | 4 | 38.7 | 157 |
| High-risk | 0.2 | –2 | –42.7 | –70 |
| ≥75 yearsa | 0.8 | –10 | –227.7 | –1,878 |
| Healthy | 0.3 | –9 | –76.2 | –210 |
| At-risk | 0.4 | –8 | –209.2 | –750 |
| High-risk | 0.2 | –14 | –480.6 | –918 |
aFor each age group, absolute reductions in cases per year were calculated by multiplying risk-specific absolute declines in rates and risk-specific numbers of person-years in 2015 and summing products across risk groups.
Because the reduction in pneumococcal disease rates from the pre-PCV13 period to the post-PCV13 period was disproportionately lower (or the increase was disproportionately higher) among adults with at-risk or high-risk conditions, corresponding rate ratios comparing disease incidence among at-risk and high-risk persons to healthy persons generally increased from the pre-PCV13 period to the post-PCV13 period. For example, among adults aged 65–74 years with high-risk conditions, the relative rate of all-cause pneumonia was 9.4 (95% CI, 9.1–9.8) in the pre-PCV13 period vs 11.1 (95% CI, 10.7–11.5) in the post-PCV13 period. Among adults aged 65–74 years with at-risk conditions, corresponding rate ratios for all-cause pneumonia were 4.4 (95% CI, 4.3–4.6) and 4.9 (95% CI, 4.8–5.1).
Although pneumococcal disease incidence decreased over time in virtually all age groups and among those with at-risk and high-risk conditions, the proportion of disease occurring in persons with at-risk and high-risk conditions increased (Figures 1 and 2, Supplementary Figure 4). For example, among persons aged 65–74 years, the proportion of all-cause pneumonia in persons with high-risk conditions increased from 38% to 42% during the study period; among persons aged ≥75 years, it increased from 32% to 37%. Over time, an increasing proportion of IPD occurred in persons with comorbid conditions regardless of age, resulting in increasing rate ratios comparing IPD rates in at-risk and high-risk persons to healthy persons from the pre- to post-PCV13 periods. The shifting burden of disease to older individuals and those with specified at-risk and high-risk conditions was comparable for IPD and pneumococcal pneumonia, although, in many cases, only nominal differences in rate ratios were observed.
Figure 1.
Distribution of episodes of invasive pneumococcal disease requiring hospitalization by risk profile in 2007–2010, 2011–2012, and 2013–2015, by age. Abbreviation: IPD, invasive pneumococcal disease.
Figure 2.
Distribution of episodes of all-cause pneumonia requiring hospitalization by risk profile in 2007–2010, 2011–2012, and 2013–2015, by age. Abbreviation: PNE, all-cause pneumonia.
DISCUSSION
Using a retrospective design and 2 large healthcare claims repositories, we evaluated changes in rates of pneumococcal disease among US adults from 2007–2015 by age and risk profile to better understand the impact of PCV13. We detailed incidence rates of IPD, all-cause pneumonia, and pneumococcal pneumonia requiring hospitalization over a 9-year period. We evaluated IPD with the recognition that it represents the most objective endpoint in a claims database as it required a microbiologic confirmation and was therefore least susceptible to physician coding variations. We also evaluated hospitalized pneumonia, both all-cause and pneumococcal, as they represent the most frequent clinical syndrome due to the pneumococcus. We limited our analysis to hospitalized patients with pneumonia to focus attention on cases that are more likely due to pneumococcal disease. Our results were largely consistent across all 3 clinical syndromes.
Pneumococcal disease rates demonstrated seasonal patterns with greatest incidence in winter months. Disease incidence declined following the replacement of PCV7 with PCV13 in 2010, and the effect increased with time (and vaccine uptake in the pediatric population) as incidence rates were further reduced (generally) in 2013–2015 compared to 2011–2012. Pneumococcal disease burden was higher among older vs younger adults and was higher for adults with “at-risk” and “high-risk” conditions compared to those without these conditions. Moreover, and most importantly, the decline in pneumococcal disease in adults varied by demographic and clinical profile and was attenuated among older adults and those with comorbidities. Similar observations with regard to age were reported by Simonsen (pneumonia) [13] and Bruce (IPD) [17] where significant indirect benefits were only observed in younger adults but not in older adults; however, an analysis by comorbid conditions was not performed. Thorrington observed a discord between IPD and hospitalized pneumonia where IPD declined across all age groups but less so in those aged ≥65 years; hospitalized pneumonia (organism unspecified) declined only in those aged <15 years and increased in older age groups. The increases were observed mainly in those in clinical risk groups, providing support for the differential effect we observed both by age and comorbidity status [18].
Despite recommendations for PCV13 immunization in adults with immunocompromising conditions (2012) and adults aged ≥65 years (2014), uptake in adults was still relatively small by the end of our study period (December 2015). The Centers for Medicare and Medicaid Services reported that only 22.9% of adults aged ≥65 years had received PCV13 [19]. With relatively low vaccine penetration in adults, the major influence on the declining adult disease burden most likely reflects an indirect effect following the universal immunization of children, including catch-up dosing through age 5 years. Our results demonstrate that the decline in pneumococcal disease was lower in older persons and those with comorbidity. This finding is consistent with our prior published data that individuals aged ≥65 years and those with comorbid conditions are at increased risk of disease compared to younger and otherwise healthy adults. The greater disease burden observed in older individuals and those with comorbid conditions is likely the result of waning host defense mechanisms. Despite a 95% decline in vaccine-type IPD in children and an 87% decline in adults following universal immunization of children [1], the overall reduction in pneumococcal disease in adults aged ≥65 years was only 58% [1] by 2015 (compared to 2000) compared to a 90% reduction in children aged <5 years.
The discordant reductions may be a result of replacement disease, secular trends in nonvaccine serotypes in adults, or changing epidemiology of nonpneumococcal pathogens. McLaughlin and colleagues found that hospitalized pneumococcal pneumonia (as identified by Binax NOW and PCV13 serotype-specific urinary antigen detection) in adults aged ≥65 years in the post-PCV13 era was more likely to be due to a nonvaccine serotype than to a vaccine serotype, suggesting a dominant role for nonvaccine serotypes in this age cohort [20]. Since PCV13 uptake in adults during the time span covering our analysis was quite limited (approximately 23%), we do not believe that uptake could explain the decline in IPD and ACHP observed; therefore, the most plausible explanation is the indirect effect [19]. This is consistent with reports from Public Health England where replacement disease has been substantial in older adults (the United Kingdom does not vaccinate adults aged >65 years with PCV13) [21]. Further support for the strong indirect effect of PCV13 can be derived from the observation that the largest proportional and absolute reduction in IPD and ACHP events occurred in younger and healthier groups who do not fall within the ACIP recommendations for PCV13 and therefore do not achieve direct protection. Similarly in the United Kingdom, initial declines in overall IPD rates observed in adults aged ≥65 years since PCV13 introduction in children [22] have been reversed with no net change in overall IPD rates due to increased incidence of nonvaccine serotypes [21].
While healthcare claims databases provide information on large numbers of patients with specific diagnoses who receive care for specific conditions, several limitations from the use of such databases should be noted. Use of operational algorithms will undoubtedly result in misclassification of some patients who actually have the underlying conditions (ie, they will be designated as not having at-risk/high-risk conditions) as well as some patients who actually do not have the underlying conditions (ie, they will be designated as having at-risk/high-risk conditions) [23, 24]. Similarly, episodes of IPD and pneumonia may be misclassified somewhat due to the less than perfect sensitivity and specificity of our case-ascertainment algorithms. Episodes of pneumonia were identified using International Classification of Diseases, Ninth Edition, Clinical Modification diagnostic codes (which do not require X-ray or any other confirmation) and included cases without any X-ray confirmation as well as those with radiographic infiltrates of noninfectious origin. Unfortunately, it is not possible to undertake a formal evaluation, for example, via chart review or use of additional data sources (eg, electronic medical records), of the accuracy of these algorithms within the context of this study. Analyses may not have been adequately powered to compare rates of pneumococcal disease within age- and risk-specific subgroups, which may have resulted in only nominal differences. Another limitation of the study is the lack of knowledge about PCV13 and 23-valent pneumococcal polysaccharide vaccine (PPV23) uptake among healthy, at-risk, and high-risk individuals. Although potentially differential rates of uptake could impact disease reduction, published data show that uptake of PCV13 was low among Medicare eligible individuals and individuals with high-risk conditions, which suggests that direct protection in these cohorts does not significantly reduce disease during the study period [19]. Finally, adults with public health insurance and adults without health insurance are not represented in the study databases; caution should be used when generalizing study results to other populations and settings.
In summary, the findings of this large observational study suggest that the switch to PCV13 was associated with large declines in IPD among all US adults irrespective of age and risk profile and with substantial decreases in ACHP in US adults except those aged ≥75 years. Moreover, we observed that older adults and those with comorbidities have the highest incidence of disease and appear to have benefited to a lesser extent, if at all, compared to their healthy counterparts. In the long term, we need new strategies such as expanded valency vaccines or modulation of the chronic inflammatory state associated with chronic disease and aging. In the short term, pneumococcal and influenza vaccinations are recommended for prevention, and uptake of both is below recommended levels for adults.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The study sponsor, Pfizer Inc., reviewed the study research plan and study manuscript; data management, processing, and analyses were conducted by Policy Analysis Inc. (PAI). All final analytic decisions and the decision to submit for publication were made solely by the study investigators.
Author contributions. Authorship was designated based on guidelines promulgated by the International Committee of Medical Journal Editors (2004). All persons who met criteria for authorship were listed as authors on the title page. The contribution of each of these persons to this study is as follows: (1) conception and design (all authors), acquisition of data (Bornheimer, Sato, Weycker), analysis or interpretation of data (all authors); and (2) preparation of manuscript (Pelton, Weycker), critical review of manuscript (all authors). All authors have read and approved the final version of the manuscript.
Acknowledgment. We thank Shaina Hastings for her assistance with manuscript preparation.
Financial support. This work was supported by Pfizer Inc.
Potential conflicts of interest K. M. S. and S. I. P. are employed by Boston University Schools of Medicine and Public Health and received financial support from Pfizer Inc. for their participation in study design, data analysis, and data interpretation. K. M. S. has received an investigator-initiated research grant from Pfizer Inc. S. I. P. has served as an advisory board member for and received investigator-initiated research grants from, Pfizer Inc. and other vaccine manufacturers. R. B., R. D., and D. W. are employees of PAI, which received financial support from Pfizer Inc. for this study (including manuscript preparation). R. S. is employed by and owns stock in Pfizer Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Pneumococcal disease: surveillance and reporting Available at: https://www.cdc.gov/pneumococcal/surveillance.html. Accessed September 6th, 2017.
- 2. Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet 2015;15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369:1179–86. [DOI] [PubMed] [Google Scholar]
- 4. Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis 2007; 196:1346–54. [DOI] [PubMed] [Google Scholar]
- 5. Lexau CA, Lynfield R, Danila R, et al. ; Active Bacterial Core Surveillance Team Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005; 294:2043–51. [DOI] [PubMed] [Google Scholar]
- 6. Pilishvili T, Lexau C, Farley MM, et al. ; Active Bacterial Core Surveillance/Emerging Infections Program Network Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 7. Cortese MM, Parashar UD; Centers for Disease Control and Prevention Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58:1–25. [PubMed] [Google Scholar]
- 8. Pelton SI, Weycker D, Farkouh RA, Strutton DR, Shea KM, Edelsberg J. Risk of pneumococcal disease in children with chronic medical conditions in the era of pneumococcal conjugate vaccine. Clin Infect Dis 2014; 59:615–23. [DOI] [PubMed] [Google Scholar]
- 9. Shea KM, Edelsberg J, Weycker D, Farkouh RA, Strutton DR, Pelton SI. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis 2014; 1:ofu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect 2012; 65:17–24. [DOI] [PubMed] [Google Scholar]
- 11. Muhammad RD, Oza-Frank R, Zell E, et al. Epidemiology of invasive pneumococcal disease among high-risk adults since the introduction of pneumococcal conjugate vaccine for children. Clin Infect Dis 2013; 56:e59–67. [DOI] [PubMed] [Google Scholar]
- 12. Kyaw MH, Rose CE Jr, Fry AM, et al. ; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005; 192:377–86. [DOI] [PubMed] [Google Scholar]
- 13. Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med 2014; 2:387–94. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among children aged 19–35 months—United States, 2007. MMWR Morb Mortal Wkly Rep 2008; 57;961–66. [PubMed] [Google Scholar]
- 15. Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA 2012; 307:1405–13. [DOI] [PubMed] [Google Scholar]
- 16. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927; 22:209–12. [Google Scholar]
- 17. Bruce MG, Singleton R, Bulkow L, et al. Impact of the 13-valent pneumococcal conjugate vaccine (pcv13) on invasive pneumococcal disease and carriage in Alaska. Vaccine 2015; 33:4813–9. [DOI] [PubMed] [Google Scholar]
- 18. Thorrington D, Andrews N, Stowe J, Miller E, van Hoek AJ. Elucidating the impact of the pneumococcal conjugate vaccine programme on pneumonia, sepsis and otitis media hospital admissions in England using a composite control. BMC Med 2018; 16:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Black CL, Williams WW, Warnock R, Pilishvili T, Kim D, Kelman JA. Pneumococcal vaccination among Medicare beneficiaries occurring after the Advisory Committee on Immunization Practices recommendation for routine use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults aged ≥65 years. MMWR Morb Mortal Wkly Rep 2017; 66:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McLaughlin JM, Jiang Q, Isturiz RE, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis, ciy312, doi: 10.1093/cid/ciy312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis 2018; 18:441–51. [DOI] [PubMed] [Google Scholar]
- 22. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015; 15:535–43. [DOI] [PubMed] [Google Scholar]
- 23. Saczynski JS, Andrade SE, Harrold LR, et al. Mini-sentinel systematic evaluation of health outcome of interest definitions for studies using administrative and claims data: heart failure. Pharmacoepidem Drug Saf 2012; 21. doi: 10.1002/pds.2313 [Google Scholar]
- 24. Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD 2009; 6:388–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.