Abstract
Recently, molecular hydrogen (H2) has become known as a new class of antioxidants and redox-modulating interventions. Effects of H2 have been documented for many acute and chronic pathological conditions. The present study was aimed at determining the effect of hydrogen on the physiology and longevity of Drosophila. The flies were given a patented food supplement consisting of a mixture of inert salts with metallic magnesium, which reacted with acidic aqueous solutions, thereby releasing hydrogen gas. The supplementation with hydrogen-rich food prolonged the life span of the wild-type strain. To gain insights into the effect of hydrogen, we used previously generated mutant under-expressing redox-regulating enzymes, peroxiredoxins, in mitochondria. The hydrogen-releasing material lessened the severe shortening of life span of the mutant. Hydrogen also delayed the development of intestinal dysfunction caused by under-expression of peroxiredoxins in the intestinal epithelium. Hydrogen also averted a significant decrease in the mobility of mutant flies that under-expressed peroxiredoxins globally or in specific tissues. Together, the results showed that the introduction of hydrogen to aging or short-lived flies could increase their survival, delay the development of the intestinal barrier dysfunction and significantly improve physical activity.
Keywords: Molecular biology, Genetics, Physiology
Abbreviations: ORP, oxidation-reduction potential; ROS, reactive oxygen species; Prx, peroxiredoxin; DM, double mutant
1. Introduction
Over the last two decades, previously considered as an inert gas, molecular hydrogen (H2) has emerged as a novel agent with profound biological effects on cell function and physiology. The effects of hydrogen on the treatment of various pathological conditions and diseases have been demonstrated in numerous studies with animal models and clinical studies (reviewed in [1, 2, 3]). The beneficial effects of hydrogen are attributed to its ability to function as a selective antioxidant, which unlike other antioxidants is highly effective in scavenging strictly harmful reactive oxygen species (ROS), such as hydroxyl radicals and peroxynitrite [4], while retaining the activity of functionally important ROS, such as H2O2 and NO [4, 5]. H2 can also act as a signaling molecule and induce various defense responses [6, 7, 8]. The most pronounced therapeutic effects of H2 have been documented in diseases associated with oxidative stress [1, 5, 9, 10].
Another advantage of molecular hydrogen as a therapeutic agent is its mobility and permeability. A small uncharged H2 molecule can easily diffuse through cell membranes and penetrate into organelles, such as mitochondria, one of the main source of ROS production, and protect this organelle from damage by excessive concentrations of ROS, thereby preventing the development of diseases associated with mitochondrial dysfunction and oxidative stress.
There are many obstacles that impede the development of H2-based therapy. The introduction of hydrogen by inhalation, intake or injection of H2-rich solutions suffers from many drawbacks, such as safety problems or difficulties in controlling the dosage [11]. An alternative is to produce hydrogen in the reaction of metals with acidic solutions.
In the presented study, we used magnesium (Mg)-containing food supplement, MagH2 and tested its biological effects on fruit flies. Although molecular hydrogen is now recognized as a promising therapeutic option for the treatment of various diseases associated with oxidative stress and mitochondrial dysfunction, studies showing hydrogen influence on aging are virtually absent. Here we used the Drosophila model, well known in the aging studies, to determine the effect of H2 on aging and physiology at the organismal level.
2. Materials and methods
2.1. Fly material
The y w reference strain has been maintained in this laboratory for >23 years. The Da-GAL4 and D42-GAL4 driver lines were kindly supplied by Blanka Rogina (University of Connecticut Health Science Center), and NP1-GAL4 driver line was a kind gift of Heinrich Jasper (The Buck Institute for Research on Aging). The dprx3,dprx5 double mutant (DM) under-expressing both mitochondrial peroxiredoxins (Prxs) is described in previous publications [12, 13, 14]. Briefly, global or tissue-specific under-expression of Prxs has been achieved by expressing the RNAi-dPrx3 hairpin construct, using the global Da-GAL4 or tissue-specific D42-GAL4 (motor neurons) and NP1-GAL4 (midgut) drivers in the dprx5 −/− mutant background.
2.2. Preparation and evaluation of hydrogen-enriched fly food
The yeast-sugar broth was made of the 0.5% yeast extract and 5% sugar and methylparaben added to the final concentration 0.19%. A mix of propionic, acetic acid and o-phosphoric acid (31.5%, 10% and 3.5% respectively) was used to adjust the pH to 4.5–4.8. The broth has been applied to cotton balls or sponges inserted in glass vials.
To prepare hydrogen-rich broth, we used a patented composition named MagH2, kindly provided by Dr. Miljkovic (Nano H2 Minus, Inc., US patents 8,852,660 B2 and 9,144,581 B2). The composition contains metallic magnesium microparticles mixed with basic magnesium carbonate and is able to release molecular hydrogen when added to acidic aqueous solutions. MagH2 was added to the acidic yeast-sugar broth at different concentrations immediately before experiments. pH of the solution has been adjusted to 4.5–4.8 using the same mix of acids used for the broth preparation. Under these conditions, the metallic magnesium instantly produces molecular hydrogen in a reaction: Mg + 2H+ Mg+2 + H2.
To determine potential toxicity of the MagH2 components and to exclude the possibility that the effects of MagH2 were exclusively due to molecular hydrogen and not associated with other components in the mixture, MagH2 solutions were boiled for three times to remove the generated hydrogen gas. The anti-oxidant capacity of fly food has been evaluated by measuring the oxidation-reduction potential (ORP) using Beckman - 350 pH Temp/mV meter (Beckman Coulter).
2.3. Procedures
For experimental studies, male and female flies were collected within 1–2 days after hatching and reared on a standard sucrose-cornmeal medium at 25 °C or transferred to food containing MagH2. Survivorship studies were performed as described in previous publications [12]. In each experiment, approximately 50–60 flies were used for each fly line.
Negative geotaxis was evaluated as described [15, 16] with some modifications. Briefly, a number of flies that are able to climb or jump ∼4 cm distance and to reach the top of a vial was counted at different time intervals. The flies were gently tapped down to the bottom of the vial and allowed to climb for 30 seconds. The assay was repeat for the same group three times, allowing for 10 minute rest period between each trial. The geotaxis was expressed as a number of climbers/jumpers to the total number of flies with observations performed for 30 seconds for each vial.
Intestinal barrier dysfunction was tested using the “smurf” phenotype assay [17]. Briefly, flies were transferred to vials with food containing 2.5% (wt./vol.) of Blue dye no. 1. Flies with normal intestinal function had the blue stain restricted to the intestinal lumen. When integrity of the intestinal epithelial barrier was impaired, the blue dye produced a broader staining throughout the body, which was documented by microscopy examination.
2.4. Statistical analysis
All statistics were calculated using Excel and Prism for Macintosh version 6.0b software (GraphPad Software, Inc. San Diego, CA). Differences in negative geotaxis were compared between groups by analysis of variance. The mean survivorship time and statistical significance of differences between survival curves were assessed by the log-rank test. Differences in survivorship at 10% mortality and the development of the “smurf” phenotype were determined by two-way analysis of variance.
3. Results
3.1. MagH2 reduced the oxidation-reduction potentia
Previous in vitro studies have shown that when dissolved in water, MagH2 forms H2 nanobubbles, thereby maintaining high amounts of dissolved H2 over a relatively long period of time [18]. It also led to a stable negative oxidation-reduction potential at relatively high pH values [18]. Here we have determined that MagH2 is also capable of increasing negative ORP values in fly food under acidic conditions that are comparable to those in the gastric space, which approximates the conditions to those in vivo.
Addition of different concentrations of MagH2 to the yeast-sugar broth (pH 4.4–4.8) caused a significant reduction in the oxidation–reduction potential. Thus, ORP rapidly dropped from +185 mV (regular food) to (–160) – (–166) mV (0.1 mg/ml MagH2), (–320) – (–330) mV (0.3 mg/ml MagH2), and to (–410) – (–470) mV (1 and 3 mg/ml MagH2) and lasted at negative values for at least 4 h. Thus, the observed effects of MagH2 on fly food were comparable to those observed in water solutions [18], suggesting the existence of molecular hydrogen in the form of nanobubbles and relative stability of its concentrations in the fly food. We also determined that 5 minutes of boiling was sufficient to remove hydrogen gas from solutions, since the ORP values ranged from +180 to +190 mV and were comparable to the ORP values in a regular fly food.
3.2. MagH2 extended life span
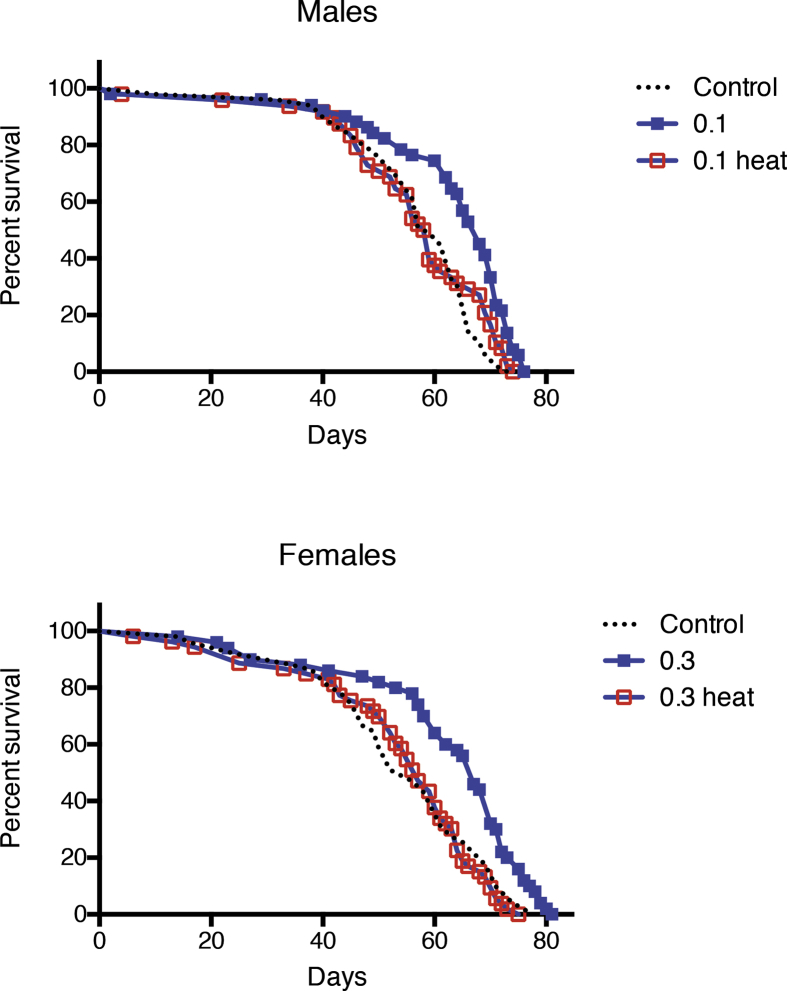
To determine the effects of MagH2 on longevity, male and female flies of the long-lived y w strain of Drosophila received food mixed with MagH2 in the range of concentrations of 0.1, 0.3, 1, 3 and 10 mg/ml. MagH2 has been given starting at 42 days of age, or at ∼ 50% of their respective life span and just before the onset of rapid increase in mortality, to avoid potential adverse effects in young healthy organisms. The beneficial effects were observed when males were fed food with 0.1–0.3 mg/ml of MagH2 and females with 0.3–1 mg/ml, while higher concentrations, 3 and 10 mg/ml were toxic and decreased survivorship (Fig. 1 and unpublished data). The addition of MagH2 in the optimal range of concentrations led to an increase in median (50% death) by ∼ 15% in males and ∼25% in females and mean (average) age, but a small increase in maximum (90% death) age (Fig. 1). Thus, MagH2 only moderately prolonged the longevity, but significantly improved the age conditions, or life span of flies. The removal of H2 by heating solutions prior to the introduction into flies completely abolished the positive effects of the optimal doses of MagH2. On the other hand, the observed toxicity of high MagH2 content (3–10 mg/ml) was due to other components of MagH2, rather than to H2, since the longevity of flies was significantly reduced, regardless of the removal of H2 (unpublished observations).
Fig. 1.
Effects of MagH2 on fly life spans. The y w flies were used in the experiment. Flies were reared on a regular fly food followed by transfer to food containing MagH2 on day 42. MagH2 was given at concentrations 0.1 mg/ml (0.1) and 0.3 mg/ml (0.3). Control – flies maintained on a regular food. Heat – solutions of MagH2 boiled before addition to fly food in order to remove hydrogen gas. Shown are representative data of two independent biological experiments. There was a significant difference (p < 0.05) in survivorship between controls and flies fed MagH2, as determined by the log rank test. Similar results were obtained in the biological replicate experiment. The data are summarized in Table 1 A.
3.3. MagH2 delayed premature aging in mutants underexpressing mitochondrial peroxiredoxins
To get insights into the mechanisms of H2 effects, we used flies with impaired mitochondrial function. The importance of mitochondria in the regulation of aging is well recognized [19]. Normal mitochondrial function and integrity is maintained by many redox-related factors, including thiol-related peroxidases, peroxiredoxins, which are known to play an important role in longevity [12, 13]. Previously, we showed that the deficit in activity of mitochondria-localized Prxs, dPrx3 and dPrx5, resulted in a 80% decrease in mean life span, accompanied by age-associated increase in tissue-specific apoptosis, changes in cellular redox, as well as altered transcriptional profiling of the genes involved in stress responses and mitochondrial maintenance [12, 13].
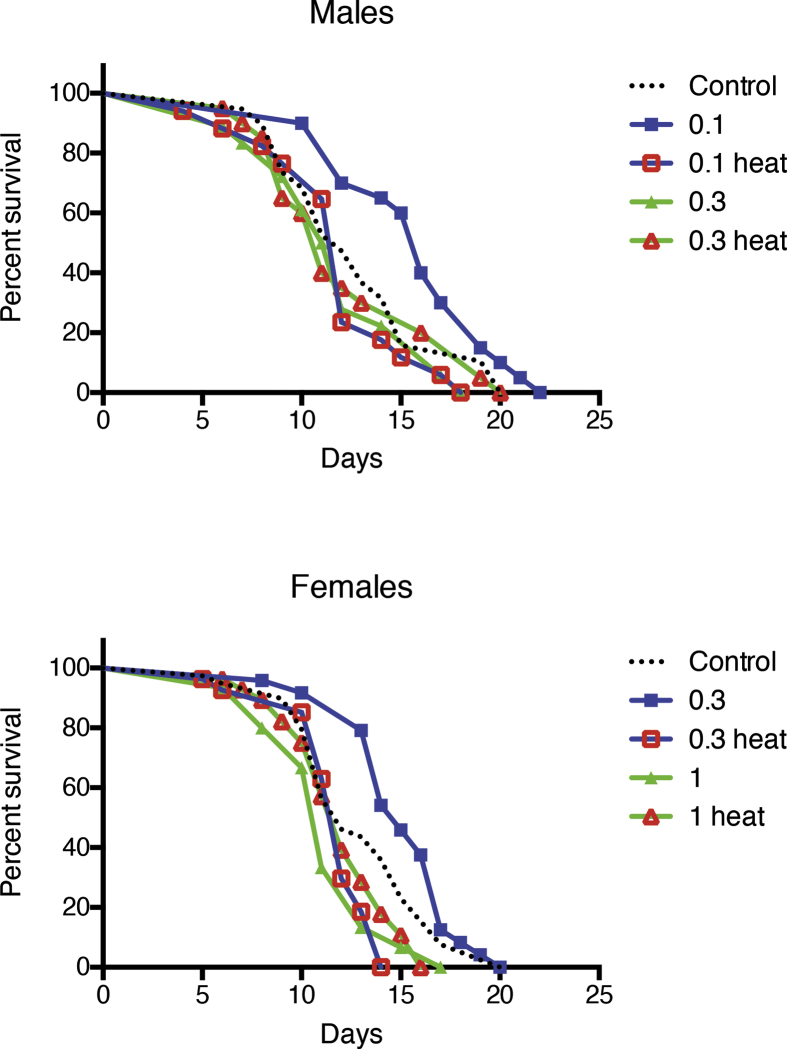
Although the normal life span of the mutant underexpressing Prxs globally with Da-GAL4 driver was not completely restored, the intake of MagH2 positively influenced the survival of these flies. Like in the old flies, the effects were dose-dependent with optimal concentrations of MagH2 0.1 mg/ml for males and 0.3 mg/ml for females (Fig. 2). Similarly to control y w flies, concentrations of MagH2 three times higher than optimal were toxic and led to increased mortality of the mutant, irrespective of removal of H2 by heating (data not shown).
Fig. 2.
Effects of MagH2 on mortality of the double mutant. The DM flies were obtained by underexpressing both mitochondrial Prxs globally with the Da-GAL4 driver. Flies were transferred to the MagH2-containing food on day 5, or just before the onset of a rapid death. There was a significant difference (p < 0.05) in survivorship between controls and flies fed MagH2 at optimal concentrations, 0.1 mg/ml for males and 0.3 mg/ml for females. Shown are representative data of two independent biological cohorts. Similar results were obtained in the biological replicate experiment. The data are summarized in Table 1 B.
3.4. MagH2 has positive effects on the intestinal barrier function
It is reported that the intake of hydrogen can ameliorate intestinal inflammatory disorders by reducing injuries and breakdown of the epithelial barrier (reviewed in [8]). Normal functioning of the gastrointestinal tract is essential for a healthy aging and longevity. Decline in intestinal barrier function is a characteristic of old flies and can be evaluated using the “smurf” assay (Material and Methods), a marker for loss of intestinal integrity [17].
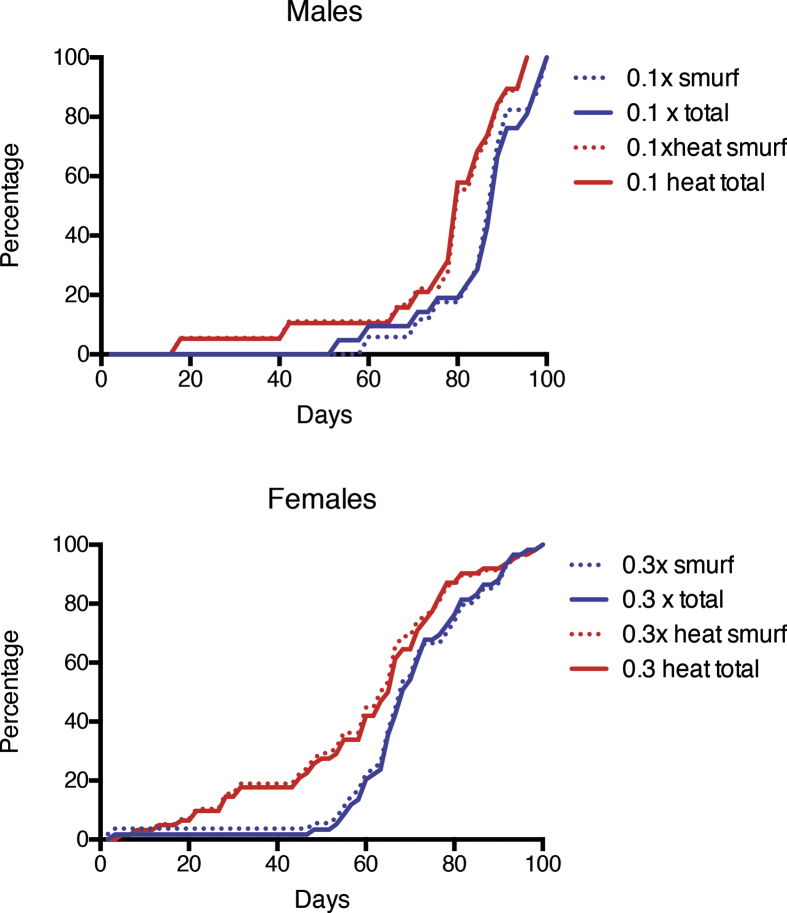
Effects of MagH2 on the intestinal barrier function have been investigated in flies under-expressing Prxs in the midgut tissue with the NP1-GAL4 driver, using the “smurf” assay. Microscopy examination has shown, that like in old flies ([17] and unpublished observations), deaths of flies with decreased mitochondrial Prx activity in the midgut epithelia was closely associated with the “smurf” phenotype (Fig. 3). Thus, almost all flies died after losing the integrity of the intestinal epithelium. Supplementation with MagH2 delayed mortality and development of the “smurf” phenotype, but did not alter the correlation between mortality and intestinal integrity loss, indicating that it improved the survival of flies via maintaining the integrity of the intestinal epithelium (see Table 1).
Fig. 3.
Effects of MagH2 on survivorship and development of the “smurf” phenotype in flies under-expressing mitochondrial peroxiredoxins in the midgut. Underexpression of mitochondrial Prxs in the midgut epithelia was achieved by the NP1-GAL4 driver. A number of flies with the “smurf” phenotype was counted after feeding the flies with food containing the Blue dye added to food supplemented with optimal for males (0.1 mg/ml) and females (0.3 mg/ml) (see Figs. 1 and 2) concentrations of MagH2 or MagH2 heated controls. Percentage of the dead and ‘smurf’ flies is shown on the Y axis. Since the largest difference in mortality and the development of the “smurf” phenotype was observed in young flies, a statistical analysis was performed at the time point when 10% of the flies died or developed the “smurf” phenotype. Statistical analysis showed significant difference (p < 0.05) in age, which corresponded to 10% mortality or the appearance of “smurf” flies between heated controls (heat) and flies that received unheated hydrogen-producing MagH2. Similar results were obtained in the biological replicate experiment. The data are summarized in Table 1 C.
Table 1.
Life spans of flies under different treatments. A and B: The experiments were conducted as described in the Fig. 1 (A) and Fig. 2 (B) legends. Values for median age obtained in two independent experiments are listed in columns 1 and 3. Columns 2 and 4 indicate the percentage of median age changes between experimental flies and flies maintained on a regular food (Control). Statistically significant differences between control and flies treated with MagH2, determined by the log-rank test (*P < 0.05), are indicated by asterisks. C: The experiments were conducted as described in the Fig. 3 legend. The age of the flies at 10% of mortality and the “smurf” phenotype are shown in columns 1 and 3. Values are obtained in two independent experiments. Columns 2 and 4 show the percentage change between flies fed MagH2 and flies fed heated MagH2 solutions that served as a hydrogen-free control. Statistically significant differences between flies fed MagH2 and flies fed heated MagH2 solutions, determined by two-way ANOVA (*P < 0.05), are indicated by asterisks and bold. There were no statistically significant differences between mortality and the development of the “smurf” phenotype.
| A |
Males |
Females |
||
|---|---|---|---|---|
| Treatment | Median age (days) 1 |
% vs. Control 2 |
Median age (days) 3 |
% vs. Control 4 |
| Control | 59; 56 | 54; 51 | ||
| MagH2 | 68; 64 | 115*; 114* | 67; 65 | 124*; 127* |
| MagH2 heat | 58.5; 57 | 99; 102 | 57; 52 | 106; 102 |
| B |
Males |
Females |
||
|---|---|---|---|---|
| Treatment | Median age (days) 1 |
% vs. Control 2 |
Median age (days) 3 |
% vs. Control 4 |
| Control | 12; 8 | 12; 13 | ||
| MagH2 0.1 | 16; 11 | 133*; 137.5* | ||
| MagH2 0.1 heat | 12; 8.5 | 100; 106 | ||
| MagH2 0.3 | 11.5; 10 | 96; 125 | 15; 15 | 125*; 115* |
| MagH2 0.3 heat | 11; 10 | 92; 125* | 12; 12 | 100; 92 |
| MagH2 1 | 11; 13 | 92; 100 | ||
| MagH2 1 heat | 12; 11 | 100; 85 | ||
| C |
Males |
Females |
||
|---|---|---|---|---|
| Treatment | Age (days) at 10% mortality/10%”smurf” 1 |
% MagH2 0.1 vs. MagH2 0.1 heat 2 |
Age (days) at 10% mortality/10% ”smurf” 3 |
% MagH2 0.3 vs. MagH2 0.3 heat 4 |
| MagH2 0.1 | 27; 31/32; 31 | 142*; 129*/168*; 124* | ||
| MagH2 0.1 heat | 19; 24/19; 25 | |||
| MagH2 0.3 | 34; 33/33; 32 | 261*; 220*/236*; 200* | ||
| MagH2 0.3 heat | 13; 15/14; 16 | |||
3.5. MagH2 significantly improves physical activity of mitochondrial peroxiredoxin mutants
Although the effects of MagH2 on fly survivorship were relatively moderate, the supplement significantly improved activity of flies, as determined by the negative geotaxis test (Fig. 4). Negative geotaxis (startle-induced vertical locomotion) is frequently used to measure locomotor ability and track the age-related locomotor impairment in Drosophila [20].
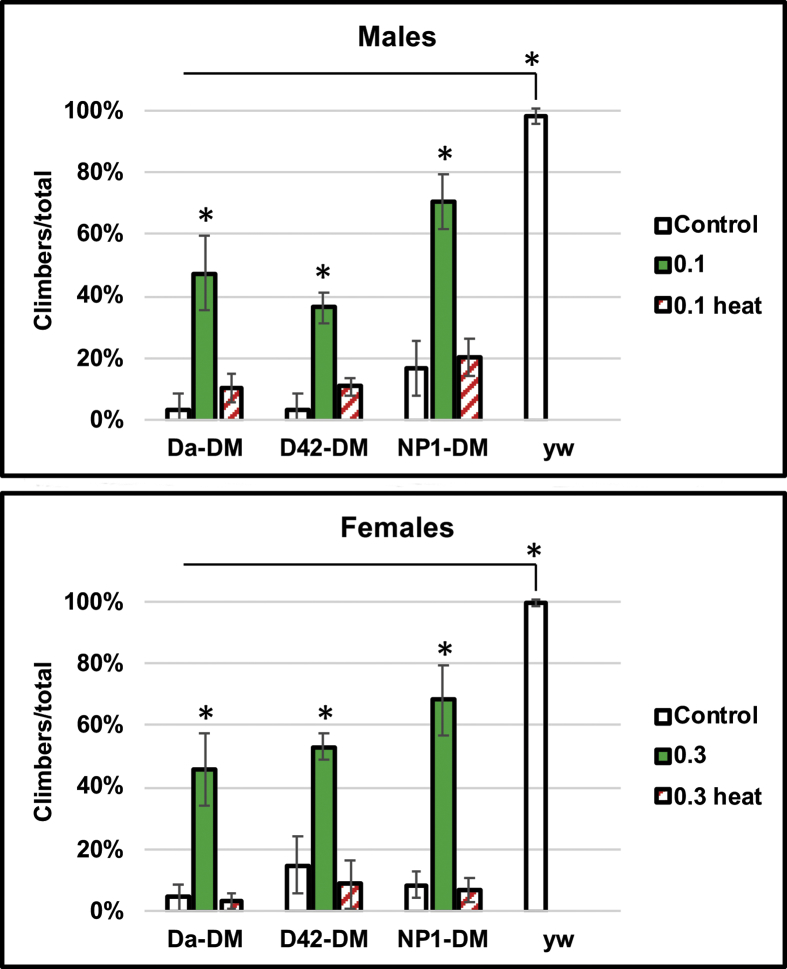
Fig. 4.
Effects of MagH2 on negative geotaxis. Shown are fly lines under-expressing mitochondrial Prxs globally (Da-DM), in motor neurons (D42-DM) and in the midgut (NP1-DM), as well as y w control. All flies tested for geotaxis were one week old, or at the age when their mortality did not exceed 10%. Flies were fed hydrogen-producing food starting from day 1–2 after eclosion, or 5–6 days prior to the geotaxis experiments. Assays were performed as indicated in Materials and Methods and geotaxis was expressed as a number of climbers/jumpers to the total number of flies. The activity of approximately 50 flies for each fly line was measured. Analysis was conducted in triplicate for each of two biological replicates. Shown are means ± SEM (n = 6). Asterisks denote statistically significant differences (*P < 0.05).
To extend the studies and pinpoint critical tissues for which MagH2 might have a particularly beneficial effects, we under-expressed peroxiredoxins in motor neurons using the D42-GAL4 driver. The flies fed MagH2 showed a more than 50-fold increase in negative geotaxis (Fig. 4). Surprisingly, flies under-expressing Prx in other tissues with the NP1-GAL4 driver or globally (Da-GAL4) also showed a dramatic improvement in fitness when feeding MagH2 (Fig. 4), suggesting a global positive effect of hydrogen, regardless of in which tissues Prxs were underexpressed. Although not fully restored, the activity of mutants treated with MagH2 was comparable to the activity levels of y w controls of the same chronological age (Fig. 4). Comparable improvements in activity due to MagH2 feeding were also observed in old flies (data not shown). Thus, the results suggest that MagH2 can be particularly beneficial for maintaining proper motor activity in older population, as well as in diseased flies with impaired motorneuronal and/or neuromuscular function.
4. Discussion
Aging is a complex process due to the involvement of many factors, such as chronic inflammation of the intestinal epithelium, oxidative stress, slowing of mobility and dysfunction of mitochondria. The main finding of this study is that the introduction of molecular hydrogen by feeding fruit flies with a H2-producing supplement, MagH2, extends the life span of Drosophila, and also favorably affects the physiology of mutants with impaired mitochondrial function. To our knowledge, this study is the first attempt to investigate the effect of hydrogen on longevity of wild-type animals. So far, the only report related to the effect of hydrogen on life span was on a partial restoration of the life span of mice shortened by a high-fat diet [21].
The studies conducted on the mutant with impaired redox and mitochondrial dysfunction also showed beneficial effects of hydrogen on survivorship (Figs. 2 and 3). Mitochondria is a major source of production of ROS, which tends to increase during aging. Mitochondrial dysfunction can induce oxidative stress; conversely, oxidative stress may worsen mitochondria-related pathological conditions. Since conventional antioxidants have limited therapeutic effects because they are not effectively taken up by mitochondria, hydrogen can be particularly useful for controlling the mitochondrial disorders. A recent insight into the mechanisms of exogenous H2 has revealed prominent effects on mitochondria, as was determined in cultured neuroblastoma cells [22]. Mitochondrial function and physiological parameters were improved in patients taking H2-generating minerals [23]. Metabolism- and mitochondrial function-related lactic acidosis have been reduced in the treatment of a patient by drinking H2 water [24]. Since the phenotype characteristics of the DM [12] resembled those of mitochondrial disease models, including mitochondrial myopathy and neuromuscular disease [25], positive effects of H2 could be due to improved mitochondrial function.
Aging and mitochondrial diseases compromise many physiological functions including mobility. The most remarkable finding of this study is that molecular hydrogen has had a dramatic positive effect on the physiology of flies by maintaining physical vigor. Documented as negative geotaxis (Fig. 4), the motor capability was significantly improved in flies fed H2-enriched food. One possibility is that hydrogen may restore the neuromuscular functional deficits observed in the DM by counteracting oxidative damages and changes in the cellular redox [13]. For instance, administration of an H2-rich saline solution reduced oxidative stress and apoptosis, which led to improved locomotor function [26]. Another possibility is that H2 could act by increasing energy production and consumption of O2 by mitochondria.
Alternatively, the effects of hydrogen in maintaining physical vigor could be due to a shift to more alkalizing conditions caused by MagH2, thus counteracting potential metabolic acidosis in the DM. The metabolic changes observed in the DM [12] can lead to accumulation of acidic metabolites and alterations in pH homeostasis, similarly to those observed in the mutants that affects regulation of intracellular sugar/mitochondrial metabolism or alter mitochondrial function [27, 28]. Studies conducted in humans have shown that the exercise-induced metabolic disturbance and acidosis due to lactate accumulation were mitigated by the administration of alkalizing H2-rich water [29, 30].
We also found the protective effect of H2 on the integrity of the intestinal epithelium, usually affected by aging and pro-inflammatory conditions. In the DM with a deficiency in mitochondrial function in the midgut, the development of the “smurf” phenotype appears to have occurred just before the onset of mortality, since almost all dead flies had a “smurf” phenotype. This means that their death coincided with the loss of intestinal integrity.
Our data demonstrate that hydrogen has the ability to delay the development of the “smurf” phenotype and, therefore, has the potential for improving the survivorship of the mutants with impaired mitochondrial function in the guts. The positive effects of H2-rich solutions by reducing oxidative damage and inflammation have also been reported in other intestinal injury models [31, 32], and gaseous hydrogen released by intestinal bacteria has been linked to a decrease in the symptoms of inflammatory bowel disease [33]. In another study, H2 administration down-regulated inflammatory mediators in the intestinal tissue and prevented intestinal barrier dysfunction [34].
Our study also revealed the dose-dependent effects of MagH2. As stated in studies conducted on human and animal models, the introduction of hydrogen by inhalation or consumption of H2-rich water usually does not adversely affects the cellular function and physiology of the organism (reviewed in [2]), although there are limited data on the hydrogen-associated toxicity [35, 36, 37, 38]. However, the likelihood of adverse effects of hydrogen is indeed low, although such an assumption was made on the grounds that hydrogen, acting as an antioxidant, does not react with functionally important ROS as H2O2 [4]. However, the toxicity of the high doses of MagH2 observed in our study was most likely due to high concentrations of magnesium that are harmful to Drosophila, rather than hydrogen itself.
Taken together, the data showed that the introduction of molecular hydrogen orally by consuming H2-rich food leads to the preservation of intestinal integrity and improved physical activity in flies with impaired mitochondrial function. Consumption of H2-rich food also extended healthy aging.
Declarations
Author contribution statement
Vladimir I. Klichko: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Vladimir L. Safonov: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Marina Yu. Safonov: Contributed reagents, materials, analysis tools or data.
Svetlana N. Radyuk: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the National Institute on Aging/National Institutes of Health, USA (grant number RO1 AG20715).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ohno K., Ito M., Ichihara M., Ito M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxid. Med. Cell Longev. 2012;2012:353152. doi: 10.1155/2012/353152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015;555:289–317. doi: 10.1016/bs.mie.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 3.Ge L., Yang M., Yang N.N., Yin X.X., Song W.G. Molecular hydrogen: a preventive and therapeutic medical gas for various diseases. Oncotarget. 2017;8:102653–102673. doi: 10.18632/oncotarget.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Katsura K., Katayama Y., Asoh S., Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 5.Hong Y., Chen S., Zhang J.M. Hydrogen as a selective antioxidant: a review of clinical and experimental studies. J. Int. Med. Res. 2010;38:1893–1903. doi: 10.1177/147323001003800602. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki H., Guan J., Tamama K. Hydrogen gas treatment prolongs replicative lifespan of bone marrow multipotential stromal cells in vitro while preserving differentiation and paracrine potentials. Biochem. Biophys. Res. Commun. 2010;397:608–613. doi: 10.1016/j.bbrc.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Itoh T., Fujita Y., Ito M., Masuda A., Ohno K., Ichihara M., Kojima T., Nozawa Y., Ito M. Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochem. Biophys. Res. Commun. 2009;389:651–656. doi: 10.1016/j.bbrc.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 8.Huang C.S., Kawamura T., Toyoda Y., Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic. Res. 2010;44:971–982. doi: 10.3109/10715762.2010.500328. [DOI] [PubMed] [Google Scholar]
- 9.Ono H., Nishijima Y., Adachi N., Tachibana S., Chitoku S., Mukaihara S., Sakamoto M., Kudo Y., Nakazawa J., Kaneko K., Nawashiro H. Improved brain MRI indices in the acute brain stem infarct sites treated with hydroxyl radical scavengers, Edaravone and hydrogen, as compared to Edaravone alone. A non-controlled study. Med. Gas Res. 2011;1:12. doi: 10.1186/2045-9912-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han A.L., Park S.H., Park M.S. Hydrogen treatment protects against cell death and senescence induced by oxidative damage. J. Microbiol. Biotechnol. 2017;27:365–371. doi: 10.4014/jmb.1608.08011. [DOI] [PubMed] [Google Scholar]
- 11.Ohta S. Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharmaceut. Des. 2011;17:2241–2252. doi: 10.2174/138161211797052664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odnokoz O., Nakatsuka K., Klichko V.I., Nguyen J., Solis L.C., Ostling K., Badinloo M., Orr W.C., Radyuk S.N. Mitochondrial peroxiredoxins are essential in regulating the relationship between Drosophila immunity and aging. Biochim. Biophys. Acta. 2016 doi: 10.1016/j.bbadis.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radyuk S.N., Rebrin I., Klichko V.I., Sohal B.H., Michalak K., Benes J., Sohal R.S., Orr W.C. Mitochondrial peroxiredoxins are critical for the maintenance of redox state and the survival of adult Drosophila. Free Radic. Biol. Med. 2010;49:1892–1902. doi: 10.1016/j.freeradbiomed.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radyuk S.N., Michalak K., Klichko V.I., Benes J., Rebrin I., Sohal R.S., Orr W.C. Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem. J. 2009 doi: 10.1042/BJ20082003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pendleton R.G., Parvez F., Sayed M., Hillman R. Effects of pharmacological agents upon a transgenic model of Parkinson's disease in Drosophila melanogaster. J. Pharmacol. Exp. Ther. 2002;300:91–96. doi: 10.1124/jpet.300.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Ali Y.O., Escala W., Ruan K., Zhai R.G. Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. J. Vis. Exp. 2011 doi: 10.3791/2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rera M., Clark R.I., Walker D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safonov V.L., K Khitrin A. Hydrogen nanobubbles in a water solution of dietary supplement. Colloid. Surf. Physicochem. Eng. Asp. 2013;436:333–336. [Google Scholar]
- 19.Hill S., Van Remmen H. Mitochondrial stress signaling in longevity: a new role for mitochondrial function in aging. Redox Biol. 2014;2:936–944. doi: 10.1016/j.redox.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grotewiel M.S., Martin I., Bhandari P., Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 2005;4:372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Kamimura N., Ichimiya H., Iuchi K., Ohta S. Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1alpha to enhance fatty acid metabolism. NPJ Aging Mech. Dis. 2016;2:16008. doi: 10.1038/npjamd.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami Y., Ito M., Ohsawa I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korovljev D., Trivic T., Drid P., Ostojic S.M. Molecular hydrogen affects body composition, metabolic profiles, and mitochondrial function in middle-aged overweight women. Ir. J. Med. Sci. 2018;187:85–89. doi: 10.1007/s11845-017-1638-4. [DOI] [PubMed] [Google Scholar]
- 24.Ohta S., Nakao A., Ohno K. The 2011 medical molecular hydrogen symposium: an inaugural symposium of the journal medical gas research. Med. Gas Res. 2011;1:10. doi: 10.1186/2045-9912-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Ayala D.J., Chen S., Kemppainen E., O'Dell K.M., Jacobs H.T. Gene expression in a Drosophila model of mitochondrial disease. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C., Chen Q., Mao Y., Xu S., Xia C., Shi X., Zhang J.H., Yuan H., Sun X. Hydrogen-rich saline protects against spinal cord injury in rats. Neurochem. Res. 2010;35:1111–1118. doi: 10.1007/s11064-010-0162-y. [DOI] [PubMed] [Google Scholar]
- 27.Bricker D.K., Taylor E.B., Schell J.C., Orsak T., Boutron A., Chen Y.C., Cox J.E., Cardon C.M., Van Vranken J.G., Dephoure N., Redin C., Boudina S., Gygi S.P., Brivet M., Thummel C.S., Rutter J. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh A.C., O'Connor M.B. Systemic Activin signaling independently regulates sugar homeostasis, cellular metabolism, and pH balance in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 2014;111:5729–5734. doi: 10.1073/pnas.1319116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostojic S.M., Stojanovic M.D. Hydrogen-rich water affected blood alkalinity in physically active men. Res. Sports Med. 2014;22:49–60. doi: 10.1080/15438627.2013.852092. [DOI] [PubMed] [Google Scholar]
- 30.Aoki K., Nakao A., Adachi T., Matsui Y., Miyakawa S. Pilot study: effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med. Gas Res. 2012;2:12. doi: 10.1186/2045-9912-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng X., Mao Y., Cai J., Li Y., Liu W., Sun P., Zhang J.H., Sun X., Yuan H. Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic. Res. 2009;43:478–484. doi: 10.1080/10715760902870603. [DOI] [PubMed] [Google Scholar]
- 32.Buchholz B.M., Kaczorowski D.J., Sugimoto R., Yang R., Wang Y., Billiar T.R., McCurry K.R., Bauer A.J., Nakao A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am. J. Transplant. 2008;8:2015–2024. doi: 10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 33.Pimentel M., Chow E.J., Lin H.C. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am. J. Gastroenterol. 2000;95:3503–3506. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda M., Shimizu K., Ogura H., Kurakawa T., Umemoto E., Motooka D., Nakamura S., Ichimaru N., Takeda K., Takahara S., Hirano S.I., Shimazu T. Hydrogen-rich saline regulates intestinal barrier dysfunction, dysbiosis and bacterial translocation in a murine model of sepsis. Shock. 2017 doi: 10.1097/SHK.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 35.Nakao A., Toyoda Y., Sharma P., Evans M., Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J. Clin. Biochem. Nutr. 2010;46:140–149. doi: 10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostojic S.M., Vukomanovic B., Calleja-Gonzalez J., Hoffman J.R. Effectiveness of oral and topical hydrogen for sports-related soft tissue injuries. Postgrad. Med. 2014;126:187–195. doi: 10.3810/pgm.2014.09.2813. [DOI] [PubMed] [Google Scholar]
- 37.Ito M., Ibi T., Sahashi K., Ichihara M., Ito M., Ohno K. Open-label trial and randomized, double-blind, placebo-controlled, crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Med. Gas Res. 2011;1:24. doi: 10.1186/2045-9912-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishibashi T., Sato B., Rikitake M., Seo T., Kurokawa R., Hara Y., Naritomi Y., Hara H., Nagao T. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med. Gas Res. 2012;2:27. doi: 10.1186/2045-9912-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]