Abstract
Activin receptor-like kinases (ALKs), members of the type I activin receptor family, belong to the serine/threonine kinase receptors of the transforming growth factor-β (TGF-β) superfamily. ALKs mediate the roles of activin/TGF-β in a wide variety of physiological and pathological processes, ranging from cell differentiation and proliferation to apoptosis. For example, the activities of ALKs are associated with an advanced tumor stage in prostate cancer and the chondrogenic differentiation of mesenchymal stem cells. Therefore, potent and selective small molecule inhibitors of ALKs would not only aid in investigating the function of activin/TGF-β, but also in developing treatments for these diseases via the disruption of activin/TGF-β. In recent studies, several ALK inhibitors, including LY-2157299, SB-431542 and A-83-01, have been identified and have been confirmed to affect stem cell differentiation and tumor progression in animal models. This review discusses the therapeutic perspective of small molecule inhibitors of ALKs as drug targets in tumor and stem cells.
Keywords: activin receptor-like kinases, inhibitor, cancer, stem cells
1. Introduction
Activin receptor-like kinases (ALKs) belong to the type I activin receptor family. The activin receptor was first cloned in 1991, and subsequently named the type II activin receptor (ActRII) (1,2). In 1992, the second activin receptor was defined as the type I activin receptor (ActRI) and termed activin receptor-like kinase (ALK) (3,4). To date, seven ALKs, ALK1-7, have been identified in mammals. These ALKs are transmembrane proteins, known as serine/threonine kinase receptors belonging to the transforming growth factor-β (TGF-β) superfamily. The ALKs harbor a transmembrane domain, an extracellular binding domain and a glycine- and serine-rich sequence (GS) domain. The GS domain is a kinase site activated by the TGF-β superfamily type II receptor and can trigger downstream signal transduction. The ALKs elicit various downstream effects of activin/TGF-β, including cell differentiation, proliferation, apoptosis, migration and adhesion as critical modulators of these biological processes. ALKs are also involved in a variety of diseases that include tumorigenesis, skeletal malfunctions, hemorrhagic telangiectasia, renal and immune diseases (5–7). This review briefly discusses the therapeutic prospects of small molecule inhibitors of ALKs as drug targets in tumor and stem cells.
2. Activin signal transduction
In order to elucidate the biological actions of small molecule inhibitors of ALKs, an understating of the basic signal transduction of activin/TGF-β is important. The TGF-β superfamily includes several subfamilies, such as TGF-β, inhibin/activin, myostatin/GDF11 and bone morphogenetic protein (BMP) (8–11). As a member of the TGF-β superfamily, activin belongs to a group of multifunctional cytokines that possess numerous biological functions, including cell differentiation, proliferation and matrix formation (12–14). SMAD proteins, the Drosophila mothers against decapentaplegic gene homologs in mammals, are shared by activin and TGF-β in a classical signal transduction pathway (15–21). Members of the TGF-β superfamily bind to type II receptors that are subsequently stimulated by dimerization with their specific phosphorylated type I receptor to activate shared canonical and distinct non-canonical pathways (15–18). A total of 7 ALKs are designated as type I receptors of the TGF-β superfamily, ALK1-ALK7 (17,18). Ligands can bind multiple ALKs, albeit with different affinities. TGF-β interacts with ALK1 and ALK5 with high affinity. Activin binds to ALK2 and ALK4 with high affinity and with moderate affinity to ALK7. In addition, BMP interacts with ALK1, ALK3 and ALK6 with high affinity and with moderate affinity to ALK2. The activated ALKs phosphorylate and activate SMAD proteins and mediate intracellular signal transduction (19).
The mammalian SMAD protein family is a family of 8 members that serve as intracellular signaling mediators of the TGF-β superfamily (20,21). Smad2 and Smad3 mediate TGF-β and activin/inhibin signaling, while BMP signaling is mediated by Smad1, Smad5 and Smad8. On the other hand, Smad6 and Smad7 act as intracellular antagonists in the signaling pathway of the TGF-β superfamily. In the canonical pathway, the type I receptors (ALKs) phosphorylate Smad2 and/or Smad3, facilitating the formation of a protein complex with Smad4. The Smad2/3-Smad4 complex is translocated to the nucleus where a substantial number of genes are either transcriptionally activated or repressed. In addition, mitogen-activated protein kinase (MAPK)/ERK, PI3K/AKT, WNT and Notch are activated by the TGF-β superfamily, which in turn can transduce the signaling of the independent SMAD proteins; this cascade constitutes the non-canonical pathways (22–25). Moreover, ALK4 mediates activin signaling transduction in a SMAD-independent manner (26).
The recently identified activin receptor-interacting proteins (ARIPs) are present in the cytoplasm (27). These proteins contain the PDZ domain and mediate activin signaling in a SMAD-independent manner (27,28). PDZ proteins play a critical role in assembling the signaling molecules close to the sub-membranous regions and membranous receptors (29). ARIP1 has multiple protein-protein interacting domains that include 5 PDZ domains and 2 WW domains, and bind to ActRII through the fifth PDZ domain at the C-terminal (27,30). ARIP2 possesses only one PDZ domain that can also interact with ActRII and Ral binding protein 1 (RalBP1). The ternary complex of ARIP2, ActRII and RalBP1 is assembled near the sub-membranous regions (31). The overexpression of ARIP1 and ARIP2 suppresses the gene transcription induced by activin in a dose-dependent manner (27,31). The subsequently identified isoforms of ARIP2, ARIP2b and 2c (32), also harbor only one PDZ domain that binds specifically to ActRII. However, overexpression of ARIP2b and 2c enhances activin signaling transduction. Although structural homology is observed between ARIP2b, ARIP2c and ARIP2, the biological activities are different (31,32). Furthermore, current studies have revealed that ARIPs are not only functionally distinct but also exhibit differences in histological distribution (32–35).
3. Critical roles of activin in tumorigenesis
Activin shows pleiotropic functions in embryonic development, erythropoiesis, wound healing, inflammation, arterial pressure regulation, cancer initiation and progression (36–39). Also, it promotes the production of the extracellular matrix, which is the main factor causing liver, lung, heart and renal fibrosis (36,37). Furthermore, activin regulates the activities of macrophages, such as in promoting the activation of resting macrophages and in the polarization of M2 macrophages, while inhibiting the function of activated M1 macrophages in a dual-directional manner (38,39). Activin functions as a neuroprotective and neurotrophic factor in the survival of cultured neurons and promotes the neurite outgrowth of dorsal root ganglia neurons (40,41). Nevertheless, these studies suggest that activin plays a major role in the migration, proliferation and apoptosis of cancer cells (10,42).
Activin not only promotes the genesis and progression of certain tumors, but can also inhibit tumorigenesis, depending on the tumor type and signaling pathways involved (42,43). The overexpression of activin is associated with colorectal cancer, metastatic prostate cancer, lung cancer, hepatocellular carcinomas and pancreatic cancer (42–44), with poor patient prognosis and positive lymph node status in oral squamous cell carcinomas and lung adenocarcinoma. Additionally, a recombinant activin A promoted the proliferation of lung cancer cell lines SKLU1 and H460 and the invasion of ovarian cancer cell lines OCC1 and SKOV-3 without affecting proliferation (45). Recent evidence has implicated overactive activin signaling in breast cancer cell lines, including MCF7 and MDA-MB231, and higher levels of p-Smad2, p-Smad3 and activin A in advanced breast cancer (46,47). Activin A also promotes invasion, angiogenesis, epithelial-mesenchymal transition (EMT) and stemness in breast cancer cells (46).
A high level of activin is an independent prognostic factor for the survival of patients with cancer. In addition, it also serves as a marker for the severity of the neoplastic disease or the inflammatory process, and might be correlated with survival by effectuating the loss of skeletal muscle mass and the development of cachexia (43,48). Some studies have utilized adeno-associated viral vectors to increase the levels of circulating activin A, thereby inducing a rapid and profound body weight loss (49). Similarly, in cachectic patients the concentrations of activin A are higher than those in non-cachectic patients and are positively associated with weight loss (50,51). This phenomenon established a correlation between circulating activin A levels and anorexia/cachexia syndrome in patients with cancer.
Other studies have shown that activin also exerts a protective effect on patients with cancer. The T47D breast cancer cell model demonstrated that activin A treatment inhibits cell proliferation and induces cell cycle arrest (52). Additionally, in thyroid papillary carcinoma cells, activin exhibits an anti-proliferative mechanism, thereby regulating thyroid tumorigenesis (53). Consistently, activin A is an effective anti-angiogenic factor that inhibits the proliferation of endothelial cells and tube formation by downregulating the expression of cyclin D1 and retinoblastoma protein and enhancing the production of the cell cycle inhibitor p21 (54).
Other members of the TGF-β superfamily are also involved in tumor development (55–57). Previous studies indicated that TGF-β1 promotes cancer cell progression in a mechanism similar to that of activin, and induces EMT via the Smad-related canonical signaling pathway in various cancer cells. It has been reported that TGF-β1 suppresses the expression of E-cadherin by upregulating the expression of Twist related protein 1, zinc finger protein SNAI1 and zinc finger protein SNAI2 (Slug). Also, TGF-β1 induces EMT through non-canonical Smad signaling. Furthermore, a recent report stated that TGF-β1 acts as an inducer through TNF receptor-associated factor 6 to promote receptor cleavage of ALK5 (type 1 receptor of TGF-β) in a non-canonical signaling manner (58,59).
4. Effects of activin on stem cell differentiation
Stem cells have the ability to self-renew and generate differentiated cells that are retained in a specific tissue, and are defined as different types of embryonic stem cells (ESCs), somatic stem cells or induced pluripotent stem cells (iPSCs) (60–62). Several studies have demonstrated that activin A induces early embryonic development and plays a critical role in the differentiation of erythroblasts, including the generation and maturation of erythrocytes (63,64). Recent studies have demonstrated that activin enhances eye field formation from ESCs and promotes the generation of mature photoreceptors in primary rodent retina cultures; however, it inhibits the differentiation of pluripotent stem cells by influencing the expression of key genes, including Nanog and Oct4, in stem cells (65–68). During the embryonic development and differentiation of ESCs, high-intensity signals of Nodal/activin are required in pluripotent stem cell derivatives that give rise to the definitive endoderm (DE), compared with the posterior derivative that differentiates into the mesoderm (69,70). Furthermore, the effect of the length of stimulation with activin A plus Wnt3a on the development of hepatic and pancreatic progenitors from the DE cells derived from human pluripotent stem cells has also been investigated (71). Another study reported that activin upregulates the expression of developmental pluripotency associated 3 in early primordial germ cells (PGCs) and the tyrosine kinase receptor cKIT genes in both standard-derived and activin-derived human embryonic stem cells (hESCs), suggesting that activin induces differentiation by priming hESCs to become part of the PGC lineage (72). Interestingly, the treatment of human and mouse ESCs with high concentrations of activin A triggers stem cell differentiation via the activation of ALKs/Smads (73–75).
Additionally, marked changes in BMP and activin/Nodal signaling levels determine the specification of cardiomyocytes and the cardiac mesoderm. Human iPSC and ESC translational studies have indicated that the co-expression of platelet derived growth factor receptor A and kinase insert domain protein receptor directs the emergence of the cardiac mesoderm, and that this is dependent on the optimal levels of BMP and activin/Nodal signaling (70,76). Accumulating evidence has demonstrated that some cancer stem cells show stem cell properties, such as multipotency, self-renewal and the expression of stem cell markers in the tumor. Such cells have been identified in breast, brain and blood cancer (77–79). As the seeding cells, cancer stem cells are capable of forming a new tumor with the properties of the parental tumor. Activin/nodal signaling can control tumor metastasis and progression by influencing the differentiation and proliferation of cancer stem cells via the activin-related signaling pathway similar to that in somatic or embryonic stem cells, and activin signaling enhances the self-renewal of colorectal cancer stem cells and promotes the progression of colorectal cancer in vivo (80,81).
5. Activin receptor-like kinases as a target for anti-tumor therapy
Several ALKs exert regulatory roles in cell differentiation, proliferation, apoptosis, invasion and migration, and mutation of ALKs in various human cancer types has also been identified (5,82). The majority of tumors need a functional vascular network to maintain an environment conducive to tumor growth beyond local boundaries and to facilitate tumor metastasis; thus, a promising approach to cancer therapy is anti-angiogenic treatment. To date, a number of studies have investigated the anti-angiogenic and anti-tumor growth functions of ALK inhibitors with respect to anti-tumor therapy (5,83–85).
ALK1 has been found to exist widely in the tumor blood vessels of lymphoma and cancer of the skin, kidney, prostate, ovary, lung, thyroid, pancreas and liver (5). ALK1 has high affinity for BMP9 and is mainly expressed in endothelial cells, wherein it regulates angiogenesis (83). High ALK1 protein levels in blood vessels of tumor tissues may be a prognostic marker of tumor metastasis in patients with breast cancer. A total of two pharmacological inhibitors of ALK1, PF-03446962, a human antibody against the extracellular domain of ALK1 (84,85), and ACE-041/Dalantercept, a soluble ALK1-Fc fusion protein (84,86), have been used as anti-angiogenic drugs in clinical studies (84–86). ALK1-Fc (Dalantercept) is a chimeric protein consisting of the ligand-binding ALK1 extracellular domain fused to the Fc region of the antibody (84). The ALK1 inhibitor PF-03446962 can prevent the binding of TGF-β and BMP ligands to the extracellular domain of ALK1 (85). The inhibition of ALK1 results in a decrease in the phosphorylation of p38, a decline in the expression of EMT markers, including matrix metalloproteinase 1, vimentin, Slug and cadherin-2, and downregulation of the expression of the DNA binding 1 (ID1) and ID2 proteins. Pharmacological inhibition of ALK1 also prevents lung colonization and metastatic dissemination in endocrine pancreatic and mammary carcinoma mouse models (87).
Recent studies have reported that BMP9 induces the phosphorylation of Smad1/5 through ALK1 and ALK2, which contributes to tumorigenesis by promoting the migration and proliferation of cancer cells (88–90). Abnormal expression of ALK2 is associated with a variety of diseases. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is required for the proliferation of TF-1 cells; however, BMP9 also promotes the proliferation of cultured TF-1 cells in the absence of GM-CSF. The overexpression of ALK2 triggers the autophosphorylation of Smad1/5 in TF-1 cells, leading to proliferation of the cells (91,92). KRC203 and KRC360, two specific ALK2 inhibitors, suppressed the BMP9 and ALK2-induced migration and proliferation of cancer cells. Compared to LDN193189, these two inhibitors were more specific and effective in inhibiting ALK2 and were considered as promising drug candidates for the treatment of cancers or diseases with abnormal BMP9 or ALK2 signaling (93,94).
In adult tissues, ALK4 mediates the anti-proliferative effect of activin in pituitary tumor cells. The truncated form of ALK4 functions as a dominant negative inhibitor of activin signaling and eliminates the growth suppression induced by activin (95), while the overexpression of wild-type ALK4 restores the anti-proliferative activity of activin in human pituitary tumor cells (96). The expression of ALK4 was decreased only slightly in breast cancer tissues when compared to normal breast tissues (97). Based on the abnormal activation of TGF-β signaling in hepatocellular carcinoma, LY2157299, a small molecule inhibitor targeting the serine/threonine kinases of TGF-βRI has been designed, which is currently under clinical investigation. LY2157299 exerts anti-tumor roles in patients with hepatocellular carcinoma and glioblastoma (98). LY2157299 blocked the migration and invasion of hepatocellular carcinoma cells, inhibited the growth of hepatocellular carcinoma growth, blocked the production of connective tissue growth factor and reduced stromal reactions (99,100).
The role of ALK5 in tumorigenesis has been studied extensively. In the prostate cancer cell line LNCaP, loss of the tumor suppressive effects of TGF-β was attributed to the rearrangement of the ALK5 gene; transfection with wild-type ALK5 restored the tumor suppressive effects of TGF-β (101). Furthermore, human prostate cancer displays a decrease in the mRNA and protein levels of ALK5, and the loss of ALK5 activity has been associated with an advanced tumor stage and poor 4-year survival (102). The critical role of ALK5 in the activity of TGF-β led to the development of drugs that target ALK5 for the inhibition of TGF-β signaling. Several ALK5 inhibitors have been identified and shown to affect fibrosis and tumor progression in animal models (103). CYLD lysine 63 deubiquitinase (CYLD), a deubiquitinating enzyme, is considered to be a potent tumor inhibitor. The knockdown of CYLD stimulates TGF-β signaling by maintaining ALK5 stabilization in a cell-autonomous fashion. Moreover, the ALK5 inhibitor TGF-βRI kinase inhibitor II completely blocks the invasive phenotypes of cancer cells induced by CYLD knockdown (104).
6. Activin receptor-like kinase as a target for stem cell differentiation
The activities of ALKs are closely related to stem cell differentiation (105). Bone marrow-derived mesenchymal stem cells (BMSCs) are promising factors that regenerate the cartilage, as they are able to differentiate into chondrocytes in cartilage tissue. TGF-β is a critical inducer of chondrogenic differentiation in BMSCs. ALK1 and ALK5 are important factors for the chondrogenic differentiation of BMSCs induced by TGF-β. In addition, ALK1 and ALK5 mediate signal transduction in BMSCs (105,106).
BMP signaling is also a critical mediator of cartilage differentiation and endochondral ossification. The mutation of arginine 206 to histidine results in the abnormal activation of ALK2, and this mutation exists in 95% of patients with fibrodysplasia ossificans progressiva (FOP) (107). FOP is a rare autosomal genetic disease that results in the development of ectopic bone formation in muscle. Activation of 5′AMP activated protein kinase catalytic subunit α-2 promotes the expression of Smad6 and E3-ubiquitin protein ligase Smurf1, leading to increased interactions and inducing the proteasome-dependent degradation of ALK2 (108). Conversely, Smad6 or Smurf1 knockdown arrests metformin-induced ALK2 reduction. FOP fibroblasts were transformed into iPSCs, followed by osteogenic differentiation of iPSCs in vitro. Osteogenic differentiation of iPSCs was blocked by metformin, a pharmacological AMPK activator (108). Therefore, ALK2 acts as a potential therapeutic target during lesion-induced early chondrogenic stages to avoid the heterotopic bone formation in FOP and/or other pathological events (108,109).
4-(4-(Benzo(d)(1,3)dioxol-5-yl)-5-(pyridin-2-yl)- 1H-imidazol- 2- yl) benzamide, also known as SB-431542, is a selective and potent inhibitor of the type I receptors of ALK4, ALK5 and ALK7 in the activin/TGF-β family, specifically ALK5 (IC50=94 nM) (105,109–111). On the other hand, divergent ALK family members, such as ALK3, ALK5 or ALK6 recognize TGF-β and BMP. This neither alters the components of JNK, ERK, or p38 MAPK pathways, nor does it influence their signal transduction. SB-431542 blocks the Smad signaling pathway activated by the TGF-β superfamily, promotes the differentiation of ESCs and iPSCs, and inhibits the renewal of ESCs and iPSCs. SB-431542 enhances the reprogramming efficiency when used together with PD0325901, a MEK inhibitor (109–111).
3-(6-Methylpyridin-2-yl)-N-phenyl-4-(quinolin-4-yl)-1H- pyrazole-1-carbothioamide, also known as A-83-01, is a selective ALK inhibitor; for example, it is a strong inhibitor of ALK4 (IC50, 45 nM), ALK5 (IC50, 12 nM) and ALK7 (IC50, 7.5 nM), but only a weak inhibitor of ALK1, ALK2, ALK3 and ALK6. A-83-01 is a TGF-β/ALK inhibitor that can block TGF-β-induced EMT via the downregulation of Smad2 phosphorylation levels (112). Moreover, A-83-01 can maintain the pluripotency of rat iPSCs, leading to long-term and homogenous self-renewal and to the formation of ESC-like colonies in vitro. It also can rapidly and uniformly alter the fate of mouse embryonic stem cells from the pluripotent to neuronal state (113,114).
7. Conclusions
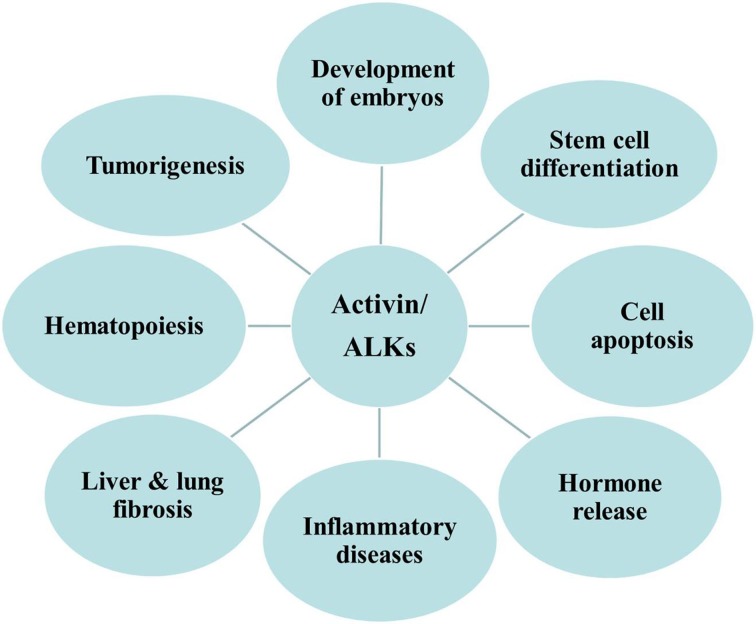
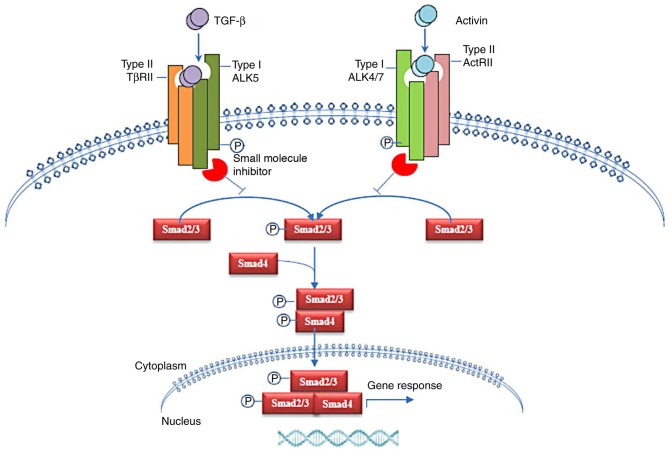
Activin and ALKs exhibit a wide range of biological activities (Fig. 1). ALKs play a central role in the activin/TGF-β signaling pathway by propagating activin/TGF-β signals to intracellular signaling molecules, such as Smads (Fig. 2). Each ALK is capable of mediating signaling pathways induced via multiple ligands to form complexes with type II receptors. ALKs are essential for lineage determination, endoderm and mesoderm formation, body axis patterning during embryogenesis and for exerting regulatory effects on cell proliferation and apoptosis in tumorigenesis. In recent studies, several ALK inhibitors have been identified in the activin/TGF-β signaling pathway (Fig. 2 and Table I). These small molecule inhibitors of ALKs can affect stem cell differentiation, fibrosis and tumor progression in animal models (110,111,115–127). For example, a small molecule inhibitor, LY2157299, targeting the serine/threonine kinases of TGF-β type I receptors has been developed, which is now under clinical studies and has exhibited anti-tumor roles in patients with hepatocellular carcinoma and glioblastoma (Table I). While these receptors represent potential targets for cancer therapies, minimizing the side effects of these receptors on the tissue distribution and activities of several types of cells and tissues is still a challenge. Thus, additional studies are required to test these receptors in the targeted treatment of various diseases.
Figure 1.
Biological functions of activin and activin receptor-like kinases. ALK, activin receptor-like kinase.
Figure 2.
Small molecule inhibitors and the ALK-Smad signaling pathway. ALK, activin receptor-like kinase; TGF-β, transforming growth factor-β; ActRII, type II activin receptor; TβRII, transforming growth factor-β receptor type II.
Table I.
Characteristics of small molecule inhibitors of activin receptor-like kinase.
| Authors, year | Name | Molecular structure | Target | Function | (Refs.) |
|---|---|---|---|---|---|
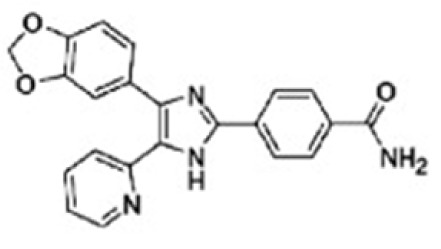
| Laping et al, 2002 Inman et al, 2002 Halder et al, 2005 Matsuyama et al, 2003 Sato et al, 2015 | SB-431542 |
|
Selective inhibitor for ALK4, 5 and 7, and weak effect on ALK3 | SB-431542 as a selective inhibitor of activin/TGF-β signaling can reduce nuclear accumulation of Smads and inhibit collagen I and fibronectin expression. Additionally, SB-431542 can also attenuate TGF-β tumor-promoting effects, EMT, cell motility, migration and invasion, and inhibit proliferation of osteosarcoma cells and lung metastasis of breast cancer. | (110,111,115–117) |
| Kim et al, 2017 Wang et al, 2018 | SB-505124 |
|
ALK4, 5, 7 | SB-505124 can offer protection of the neocortex, hippocampus, and thalamus with enhancing cerebral autophagy contributing to the decrease in the extent of progressive neuronal cell death, and up-regulate the expression of nephrin and synaptopodin, providing a novel therapeutic target for diabetic nephropathy. | (118,119) |
| Grygielko et al, 2005 | SB-525334 |
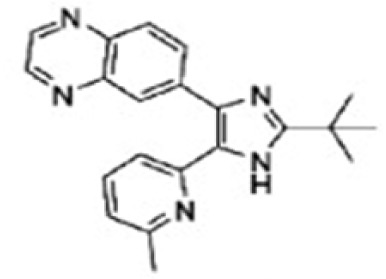
|
ALK4, 5, 7 | SB-525334 has been demonstrated to block phosphorylation and Smad2/3 nuclear translocation, and inhibits renal fibrosis by reducing procollagen and PAI-1 expression. | (120) |
| Xu et al, 2012 Gauger et al, 2012 Kimura-Kuroda et al, 2010 | LY-364947 |
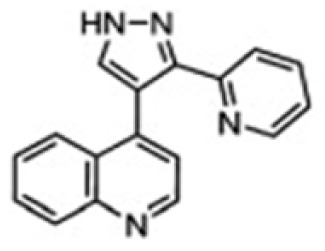
|
ALK4, 5, 7 | LY-364947 has an anti-fibrotic role by inhibiting fibroblasts proliferation and epithelium cell transdifferentiation, and prevents proliferative vitreoretinopathy and subsequent tractional retinal detachment in vivo. | (121–123) |
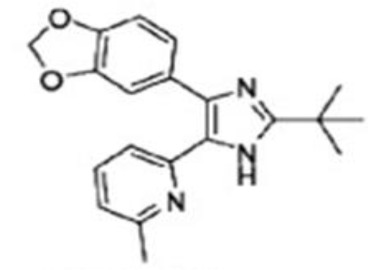
| Tojo et al, 2005 | A-83-01 |
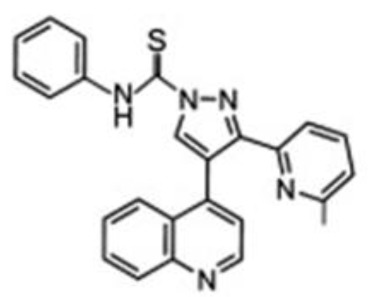
|
Selective inhibitor for ALK4, 5 and 7, and weak effect on ALK1, 2, 3 and 6 | A-83-01 can inhibit the activities of ALK4, 5 and 7. For other ALK receptors such as ALK1, 2, 3 and 6, A-83-01 showed weak inhibition. A-83-01 was proved to reduce EMT. | (112) |
| Giannelli et al, 2014 Bueno et al, 2008 | LY-2157299 |
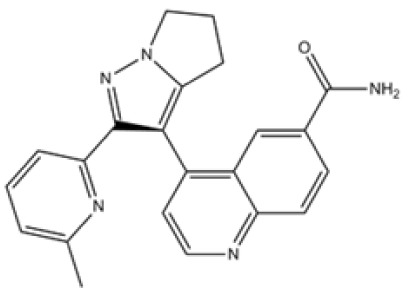
|
ALK4, 5, 7 | LY-2157299 is now under phase II clinical studies of its anti-carcinoma activities against glioblastoma and hepatocellular carcinoma, and has also been reported that it inhibits the tumor growth in human lung anaplastic carcinoma cells and breast carcinoma cells. | (124,125) |
| de Gouville et al, 2005 | GW-6604 |
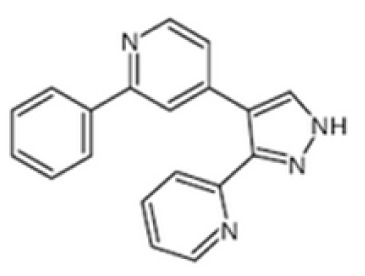
|
ALK4, 5, 7 | GW-6604 can prevent matrix deposition and promote hepatocyte regeneration as anti-cancer drug development. | (126) |
| Leung et al, 2006 | SD-208 |
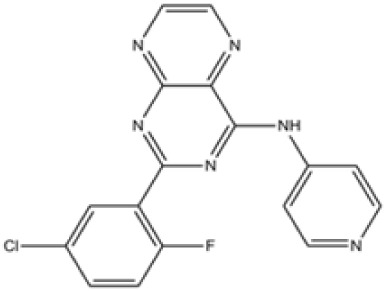
|
ALK4, 5, 7 | SD-208 inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells. | (127) |
ALK, Activin receptor like kinases; EMT, epithelial to mesenchymal transition; PAI-1, plasminogen activator inhibitor-1.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Basic Research Program of China (grant no. 2015CB943300), National Natural Science Foundation of China (grant nos. 31871510 and 31500738), and Research Projects of Science and Health of Jilin Province (grant nos. 608692, 2014Z066 and 20180414040GH).
Availability of data and materials
Data sharing is not applicable to this review article, as no datasets were generated or analyzed during the current study.
Authors' contributions
XC, YQ and ZL conceptualized and designed the study. XC, SS, XL and JZ collected, organized and drafted the information. XC and ZL wrote the manuscript. YQ and ZL revised the manuscript critically. All authors have read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mathews LS, Vale WW. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991;65:973–982. doi: 10.1016/0092-8674(91)90549-E. [DOI] [PubMed] [Google Scholar]
- 2.Mathews LS, Vale WW, Kintner CR. Cloning of a second type of activin receptor and functional characterization in Xenopus embryos. Science. 1992;255:1702–1705. doi: 10.1126/science.1313188. [DOI] [PubMed] [Google Scholar]
- 3.Attisano L, Wrana JL, Cheifetz S, Massagué J. Novel activin receptors: Distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell. 1992;68:97–108. doi: 10.1016/0092-8674(92)90209-U. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchida K, Mathews LS, Vale WW. Cloning and characterization of a transmembrane serine kinase that acts as an activin type I receptor. Proc Natl Acad Sci USA. 1993;90:11242–11246. doi: 10.1073/pnas.90.23.11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu-Lowe DD, Chen E, Zhang L, Watson KD, Mancuso P, Lappin P, Wickman G, Chen JH, Wang J, Jiang X, et al. Targeting activin receptor-like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Cancer Res. 2011;71:1362–1373. doi: 10.1158/0008-5472.CAN-10-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-Félix JM, López-Novoa JM, Martínez-Salgado C. Heterozygous disruption of activin receptor-like kinase 1 is associated with increased renal fibrosis in a mouse model of obstructive nephropathy. Kidney Int. 2014;85:319–332. doi: 10.1038/ki.2013.292. [DOI] [PubMed] [Google Scholar]
- 8.Song T, Zhao J, Jiang T, Jin X, Li Y, Liu X. Formononetin protects against balloon injury-induced neointima formation in rats by regulating proliferation and migration of vascular smooth muscle cells via the TGF β1/Smad3 signaling pathway. Int J Mol Med. 2018;42:2155–2162. doi: 10.3892/ijmm.2018.3784. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Zhong L, Yuan T, Chen S, Zhou Y, An L, Guo Y, Fan M, Li Y, Sun Y, et al. MicroRNA-155 inhibits the osteogenic differentiation of mesenchymal stem cells induced by BMP9 via downregulation of BMP signaling pathway. Int J Mol Med. 2018;41:3379–3393. doi: 10.3892/ijmm.2018.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi S, Nakasatomi M, Takei Y, Ikeuchi H, Sakairi T, Kaneko Y, Hiromura K, Nojima Y, Maeshima A. Identification of urinary activin A as a novel biomarker reflecting the severity of acute kidney injury. Sci Rep. 2018;8:5176. doi: 10.1038/s41598-018-23564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirruccello-Straub M, Jackson J, Wawersik S, Webster MT, Salta L, Long K, McConaughy W, Capili A, Boston C, Carven GJ, et al. Blocking extracellular activation of myostatin as a strategy for treating muscle wasting. Sci Rep. 2018;8:2292. doi: 10.1038/s41598-018-20524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan P, Dubey OA, Kallioinen S, Rogers KW, Muehlethaler K, Müller P, Rimoldi D, Constam DB. Paracrine activin-A signaling promotes melanoma growth and metastasis through immune evasion. J Invest Dermatol. 2017;137:2578–2587. doi: 10.1016/j.jid.2017.07.845. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Yu Y, Zhang P, Chen Y, Li C, Chen J, Wang Y, Li Y. The crucial role of activin A/ALK4 pathway in the pathogenesis of Ang-II-induced atrial fibrosis and vulnerability to atrial fibrillation. Basic Res Cardiol. 2017;112:47. doi: 10.1007/s00395-017-0634-1. [DOI] [PubMed] [Google Scholar]
- 14.Xie D, Liu Z, Wu J, Feng W, Yang K, Deng J, Tian G, Santos S, Cui X, Lin F. The effects of activin A on the migration of human breast cancer cells and neutrophils and their migratory interaction. Exp Cell Res. 2017;357:107–115. doi: 10.1016/j.yexcr.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Heldin CH, Miyazono K, Ten Dijke P. TGF-beta signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 16.Massagué J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Hawinkels LJ, Garcia de Vinuesa A, Ten Dijke P. Activin receptor-like kinase 1 as a target for anti-angiogenesis therapy. Expert Opin Investig Drugs. 2013;22:1371–1383. doi: 10.1517/13543784.2013.837884. [DOI] [PubMed] [Google Scholar]
- 18.Hinck AP, Mueller TD, Springer TA. Structural biology and evolution of the TGF-β family. Cold Spring Harb Perspect Biol. 2016;8(pii):a022103. doi: 10.1101/cshperspect.a022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu L, Cui X, Qi Y, Xie D, Wu Q, Chen X, Ge J, Liu Z. Involvement of TGF-β1/Smad3 signaling in carbon tetrachloride-induced acute liver injury in mice. PLoS One. 2016;11:e0156090. doi: 10.1371/journal.pone.0156090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afrakhte M, Morén A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin CH, Heldin NE, Ten Dijke P. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. Biochem Biophys Res Commun. 1998;249:505–511. doi: 10.1006/bbrc.1998.9170. [DOI] [PubMed] [Google Scholar]
- 21.Qi Y, Ge J, Ma C, Wu N, Cui X, Liu Z. Activin A regulates activation of mouse neutrophils by Smad3 signalling. Open Biol. 2017;7(pii):160342. doi: 10.1098/rsob.160342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kua HY, Liu H, Leong WF, Li L, Jia D, Ma G, Hu Y, Wang X, Chau JF, Chen YG, et al. c-Abl promotes osteoblast expansion by differentially regulating canonical and non-canonical BMP pathways and p16INK4a expression. Nat Cell Biol. 2012;14:727–737. doi: 10.1038/ncb2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Fei T, Zhang J, Zhu G, Wang L, Lu D, Chi X, Teng Y, Hou N, Yang X, et al. BMP4 signaling acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell. 2012;10:171–182. doi: 10.1016/j.stem.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Kobayashi T, Funatsu O, Morita A, Ikekita M. Activin A induces neuronal differentiation and survival via ALK4 in a SMAD-independent manner in a subpopulation of human neuroblastomas. Biochem Biophys Res Commun. 2010;394:639–645. doi: 10.1016/j.bbrc.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 27.Shoji H, Tsuchida K, Kishi H, Yamakawa N, Matsuzaki T, Liu Z, Nakamura T, Sugino H. Identification and characterization of a PDZ protein that interacts with activin types II receptors. J Bol Chem. 2000;275:5485–5492. doi: 10.1074/jbc.275.8.5485. [DOI] [PubMed] [Google Scholar]
- 28.Kurisaki A, Inoue I, Kurisaki K, Yamakawa N, Tsuchida K, Sugino H. Activin induces long-lasting N-methyl-D-aspartate receptor activation via scaffolding PDZ protein activin receptor interacting protein 1. Neuroscience. 2008;151:1225–1235. doi: 10.1016/j.neuroscience.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Fanning AS, Anderson JM. PDZ domains: Fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest. 1999;103:767–772. doi: 10.1172/JCI6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuchida K, Matsuzaki T, Yamakawa N, Liu ZH, Sugino H. Intracellular and extracellular control of activin function by novel regulatory molecules. Mol Cell Endocrinol. 2001;180:25–31. doi: 10.1016/S0303-7207(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzaki T, Hanai S, Kishi H, Liu Z, Bao Y, Kikuchi A, Tsuchida K, Sugino H. Regulation of endocytosisi of activin type II receptors by a novel PDZ protein through Ral/Ral-binding protein 1-dependent pathway. J Biol Chem. 2002;277:19008–19018. doi: 10.1074/jbc.M112472200. [DOI] [PubMed] [Google Scholar]
- 32.Liu ZH, Tsuchida K, Matsuzaki T, Bao YL, Kurisaki A, Sugino H. Characterization of isoforms of activin receptor-interacting protein 2 that augment activin signaling. J Endocrinol. 2006;189:409–421. doi: 10.1677/joe.1.06420. [DOI] [PubMed] [Google Scholar]
- 33.Liu HY, Chen FF, Ge JY, Wang YN, Zhang CH, Cui XL, Yu F Tai GX, Liu ZH. Expression and localization of activin receptor-interacting protein 2 in mouse tissues. Gen Comp Endocrinol. 2009;161:276–282. doi: 10.1016/j.ygcen.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Qi Y, Ge JY, Wang YN, Liu HY, Li YM, Liu ZH, Cui XL. Co-expression of activin receptor-interacting protein 1 and 2 in mouse nerve cells. Neurosci Lett. 2013;542:53–58. doi: 10.1016/j.neulet.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Liu HY, Wang YN, Ge JY, Li N, Cui XL, Liu ZH. Localization and role of activin receptor-interacting protein 1 in mouse brain. J Neuroendocrinol. 2013;25:87–95. doi: 10.1111/j.1365-2826.2012.02371.x. [DOI] [PubMed] [Google Scholar]
- 36.Manavski Y, Abel T, Hu J, Kleinlützum D, Buchholz CJ, Belz C, Augustin HG, Boon RA, Dimmeler S. Endothelial transcription factor KLF2 negatively regulates liver regeneration via induction of activin A. Proc Natl Acad Sci USA. 2017;114:3993–3998. doi: 10.1073/pnas.1613392114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Q, Wang YN, Liu HY, Yang J, Yang CY, Liu M, Liu YF, Yang P, Liu ZH. The expression and role of activin A and follistatin in heart failure rats after myocardial infarction. Int J Cardiol. 2013;168:2994–2997. doi: 10.1016/j.ijcard.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa K, Funaba M, Chen Y, Tsujimoto M. Activin A functions as a Th2 cytokine in the promotion of the alternative activation of macrophages. J Immunol. 2006;177:6787–6794. doi: 10.4049/jimmunol.177.10.6787. [DOI] [PubMed] [Google Scholar]
- 39.Li N, Cui X, Ge J, Li J, Niu L, Liu H, Qi Y, Liu Z, Wang Y. Activin A inhibits activities of lipopolysaccharide-activated macrophages via TLR4, not of TLR2. Biochem Biophys Res Commun. 2013;435:222–228. doi: 10.1016/j.bbrc.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 40.Schubert D, Kimura H, LaCorbiere M, Vaughan J, Karr D, Fische WH. Activin is a nerve cell survival molecule. Nature. 1990;344:868–870. doi: 10.1038/344868a0. [DOI] [PubMed] [Google Scholar]
- 41.Fang L, Wang YN, Cui XL, Fang SY, Ge JY, Sun Y, Liu ZH. The role and mechanism of action of activin A in neurite outgrowth of chicken embryonic dorsal root ganglia. J Cell Sci. 2012;125:1500–1507. doi: 10.1242/jcs.094151. [DOI] [PubMed] [Google Scholar]
- 42.Ottley EC, Nicholson HD, Gold EJ. Activin A regulates microRNAs and gene expression in LNCaP cells. Prostate. 2016;76:951–963. doi: 10.1002/pros.23184. [DOI] [PubMed] [Google Scholar]
- 43.Loomans HA, Andl CD. Intertwining of activin A and TGFβ signaling: Dual roles in cancer progression and cancer cell invasion. Cancers (Basel) 2014;7:70–91. doi: 10.3390/cancers7010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S, Qi Y, Niu LM, Xie DX, Cui XL, Liu ZH. Activin A as a novel biomarker for colorectal adenocarcinoma in humans. Eur Rev Med Pharmacol Sci. 2015;19:4371–4378. [PubMed] [Google Scholar]
- 45.Steller MD, Shaw TJ, Vanderhyden BC, Ethier JF. Inhibin resistance is associated with aggressive tumorigenicity of ovarian cancer cells. Mol Cancer Res. 2005;3:50–61. [PubMed] [Google Scholar]
- 46.Bashir M, Damineni S, Mukherjee G, Kondaiah P. Activin-A signaling promotes epithelial-mesenchymal transition, invasion, and metastatic growth of breast cancer. NPJ Breast Cancer. 2015;1:15007. doi: 10.1038/npjbcancer.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalli M, Mpekris F, Wong CK, Panagi M, Ozturk S, Thiagalingam S, Stylianopoulos T, Papageorgis P. Activin A signaling regulates IL13Rα2 expression to promote breast cancer metastasis. Front Oncol. 2019;9:32. doi: 10.3389/fonc.2019.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang KP, Kao HK, Liang Y, Cheng MH, Chang YL, Liu SC, Lin YC, Ko TY, Lee YS, Tsai CL, et al. Overexpression of activin a in oral squamous cell carcinoma: Association with poor prognosis and tumor progression. Ann Surg Oncol. 2010;17:1945–1956. doi: 10.1245/s10434-010-0926-2. [DOI] [PubMed] [Google Scholar]
- 49.Chen JL, Walton KL, Qian H, Colgan TD, Hagg A, WattM J, Harrison CA, Gregorevic P. Differential effects of Il6 and activin a in the development of cancer-associated cachexia. Cancer Res. 2016;76:5372–5382. doi: 10.1158/0008-5472.CAN-15-3152. [DOI] [PubMed] [Google Scholar]
- 50.Loumaye A, de Barsy M, Nachit M, Lause P, van Maanen A, Trefois P, Gruson D, Thissen JP. Circulating Activin A predicts survival in cancer patients. J Cachexia Sarcopenia Muscle. 2017;8:768–777. doi: 10.1002/jcsm.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loumaye A, de Barsy M, Nachit M, Lause P, Frateur L, van Maanen A, Trefois P, Gruson D, Thissen JP. Role of activin a and myostatin in human cancer cachexia. J Clin Endocrinol Metab. 2015;100:2030–2038. doi: 10.1210/jc.2014-4318. [DOI] [PubMed] [Google Scholar]
- 52.Burdette JE, Jeruss JS, Kurley SJ, Lee EJ, Woodruff TK. Activin A mediates growth inhibition and cell cycle arrest through Smads in human breast cancer cells. Cancer Res. 2005;65:7968–7975. doi: 10.1158/0008-5472.CAN-04-3553. [DOI] [PubMed] [Google Scholar]
- 53.Matsuo SE, Leoni SG, Colquhoun A, Kimura ET. Transforming growth factor-beta1 and activin A generate antiproliferative signaling in thyroid cancer cells. J Endocrinol. 2006;190:141–150. doi: 10.1677/joe.1.06713. [DOI] [PubMed] [Google Scholar]
- 54.Kaneda H, Arao T, Matsumoto K, De Velasco MA, Tamura D, Aomatsu K, Kudo K, Sakai K, Nagai T, Fujita Y, et al. Activin A inhibits vascular endothelial cell growth and suppresses tumour angiogenesis in gastric cancer. Br J Cancer. 2011;105:1210–1217. doi: 10.1038/bjc.2011.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zonneville J, Safina A, Truskinovsky AM, Arteaga CL, Bakin AV. TGF-β signaling promotes tumor vasculature by enhancing the pericyte-endothelium association. BMC Cancer. 2018;18:670. doi: 10.1186/s12885-018-4587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furler RL, Nixon DF, Brantner CA, Popratiloff A, Uittenbogaart CH. TGF-β sustains tumor progression through biochemical and mechanical signal transduction. Cancers (Basel) 2018;10(pii):E199. doi: 10.3390/cancers10060199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu B, An HM, Yan X, Zheng JL, Huang XW, Li M. Traditional Chinese medicine formulation Yanggan Jiedu Sanjie inhibits TGF-β1-induced epithelial-mesenchymal transition and metastatic potential in human hepatocarcinoma Bel-7402 cells. BMC Complement Altern Med. 2019;19:67. doi: 10.1186/s12906-019-2477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yi EY, Park SY, Jung SY, Jang WJ, Kim YJ. Mitochondrial dysfunction induces EMT through the TGF-β/Smad/Snail signaling pathway in Hep3B hepatocellular carcinoma cells. Int J Oncol. 2015;47:1845–1853. doi: 10.3892/ijo.2015.3154. [DOI] [PubMed] [Google Scholar]
- 60.Zou G, Liu T, Guo L, Huang Y, Feng Y, Duan T. MicroRNA-32 silences WWP2 expression to maintain the pluripotency of human amniotic epithelial stem cells and β islet like cell differentiation. Int J Mol Med. 2018;41:1983–1991. doi: 10.3892/ijmm.2018.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu L, Long J, Shi C, Zhang N, Lv Y, Feng J, Xuan A, He X, Li Q, Bai Y, et al. Effect of leukocyte inhibitory factor on neuron differentiation from human induced pluripotent stem cell-derived neural precursor cells. Int J Mol Med. 2018;41:2037–2049. doi: 10.3892/ijmm.2018.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Iessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Thomsen G, Woolf T, Whitman M, Sokol S, Vaughan J, Vale W, Melton DA. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990;63:485–493. doi: 10.1016/0092-8674(90)90445-K. [DOI] [PubMed] [Google Scholar]
- 64.Murata M, Eto Y, Shibai H, Sakai M, Muramatsu M. Erythroid differentiation factor is encoded by the same mRNA as that of the inhibin beta A chain. Proc Natl Acad Sci USA. 1988;85:2434–2438. doi: 10.1073/pnas.85.8.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertacchi M, Lupo G, Pandolfini L, Casarosa S, D'Onofrio M, Pedersen RA, Harris WA, Cremisi F. Activin/Nodal signaling supports retinal progenitor specification in a narrow time window during pluripotent stem cell neuralization. Stem Cell Reports. 2015;5:532–545. doi: 10.1016/j.stemcr.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis AA, Matzuk MM, Reh TA. Activin A promotes progenitor differentiation into photoreceptors in rodent retina. Mol Cell Neurosci. 2000;15:11–21. doi: 10.1006/mcne.1999.0806. [DOI] [PubMed] [Google Scholar]
- 67.Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MW, Cho CH, Martinez A, Rugg-Gunn P, et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang F, Wang N, Wang Y, Yu T, Wang H. Activin-SMAD signaling is required for maintenance of porcine iPS cell self-renewal through upregulation of NANOG and OCT4 expression. J Cell Physiol. 2017;232:2253–2262. doi: 10.1002/jcp.25747. [DOI] [PubMed] [Google Scholar]
- 69.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Toivonen S, Lundin K, Balboa D, Ustinov J, Tamminen K, Palgi J, Trokovic R, Tuuri T, Otonkoski T. Activin A and Wnt-dependent specification of human definitive endoderm cells. Exp Cell Res. 2013;319:2535–2544. doi: 10.1016/j.yexcr.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 72.Duggal G, Heindryckx B, Warrier S, Taelman J, Van der Jeught M, Deforce D, Chuva de Sousa Lopes S, De Sutter P. Exogenous supplementation of Activin A enhances germ cell differentiation of human embryonic stem cells. Mol Hum Reprod. 2015;21:410–423. doi: 10.1093/molehr/gav004. [DOI] [PubMed] [Google Scholar]
- 73.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 74.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 75.Lu AQ, Popova EY, Barnstable CJ. Activin signals through Smad2/3 to increase photoreceptor precursor yield during embryonic stem cell differentiation. Stem Cell Reports. 2017;9:838–852. doi: 10.1016/j.stemcr.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, et al. Human cardiovascular progenitor cells develop from a KDR+embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 77.Kim T, Echeagaray OH, Wang BJ, Casillas A, Broughton KM, Kim BH, Sussman MA. In situ transcriptome characteristics are lost following culture adaptation of adult cardiac stem cells. Sci Rep. 2018;8:12060. doi: 10.1038/s41598-018-30551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao L, Yang Y, Ye Z, Lin B, Zeng J, Li C, Liang T, Zhou K, Li J. Quercetin-3-methyl ether suppresses human breast cancer stem cell formation by inhibiting the Notch1 and PI3K/Akt signaling pathways. Int J Mol Med. 2018;42:1625–1636. doi: 10.3892/ijmm.2018.3741. [DOI] [PubMed] [Google Scholar]
- 79.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 80.Spiller CM, Feng CW, Jackson A, Gillis AJ, Rolland AD, Looijenga LH, Koopman P, Bowles J. Endogenous Nodal signaling regulates germ cell potency during mammalian testis development. Development. 2012;139:4123–4132. doi: 10.1242/dev.083006. [DOI] [PubMed] [Google Scholar]
- 81.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 82.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: Comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2017;31:509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 83.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 84.de Vinuesa AG, Bocci M, Pietras K, Ten Dijke P. Targeting tumour vasculature by inhibiting activin receptor-like kinase (ALK) 1 function. Biochem Soc Trans. 2016;44:1142–1149. doi: 10.1042/BST20160093. [DOI] [PubMed] [Google Scholar]
- 85.Goff LW, Cohen RB, Berlin JD, de Braud FG, Lyshchik A, Noberasco C, Bertolini F, Carpentieri M, Stampino CG, Abbattista A, et al. A phase I study of the anti-activin receptor-like kinase 1 (ALK-1) monoclonal antibody PF-03446962 in patients with advanced solid tumors. Clin Cancer Res. 2016;22:2146–2154. doi: 10.1158/1078-0432.CCR-15-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burger RA, Deng W, Makker V, Collins Y, Gray H, Debernardo R, Martin LP, Aghajanian C. Phase II evaluation of dalantercept in the treatment of persistent or recurrent epithelial ovarian cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2018;150:466–470. doi: 10.1016/j.ygyno.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cunha SI, Pardali E, Thorikay M, Anderberg C, Hawinkels L, Goumans MJ, Seehra J, Heldin CH, Ten Dijke P, Pietras K. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med. 2010;207:85–100. doi: 10.1084/jem.20091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herrera B, Garcia-Álvaro M, Cruz S, Walsh P, Fernández M, Roncero C, Fabregat I, Sánchez A, Inman GJ. BMP9 is a proliferative and survival factor for human hepatocellular carcinoma cells. PLoS One. 2013;8:e69535. doi: 10.1371/journal.pone.0069535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Q, Gu X, Weng H, Ghafoory S, Liu Y, Feng T, Dzieran J, Li L, Ilkavets I, Kruithof-de Julio M, et al. Bone morphogenetic protein-9 induces epithelial to mesenchymal transition in hepatocellular carcinoma cells. Cancer Sci. 2013;104:398–408. doi: 10.1111/cas.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suzuki Y, Ohga N, Morishita Y, Hida K, Miyazono K, Watabe T. BMP-9 induces proliferation of multiple typese of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123:1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 91.Machiya A, Tsukamoto S, Ohte S, Kuratani M, Fujimoto M, Kumagai K, Osawa K, Suda N, Bullock AN, Katagiri T. Effects of FKBP12 and type II BMP receptors on signal transduction by ALK2 activating mutations associated with genetic disorders. Bone. 2018;111:101–108. doi: 10.1016/j.bone.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 92.Macías-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- 93.Kim M, Choi O, Pyo S, Choi SU, Park CH. Identification of novel ALK2 inhibitors and their effect on cancer cells. Biochem Biophys Res Commun. 2017;492:121–127. doi: 10.1016/j.bbrc.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 94.Zhang L, Wang H, Yu D, Chen J, Xing C, Li J, Li J, Cai Y. The effects of mouse ovarian granulosa cell function and related gene expression by suppressing BMP/Smad signaling pathway. Anim Cells Syst (Seoul) 2018;22:317–323. doi: 10.1080/19768354.2018.1497706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y, Sun H, Danila DC, Johnson SR, Sigai DP, Zhang X, Klibanski A. Truncated activin type I receptor Alk4 isoforms are dominant negative receptors inhibiting activin signaling. Mol Endocrinol. 2000;14:2066–2075. doi: 10.1210/mend.14.12.0570. [DOI] [PubMed] [Google Scholar]
- 96.Danila DC, Zhang X, Zhou Y, Haidar JN, Klibanski A. Overexpression of wild-type activin receptor alk4-1 restores activin antiproliferative effects in human pituitary tumor cells. J Clin Endocrinol Metab. 2002;87:4741–4746. doi: 10.1210/jc.2002-020527. [DOI] [PubMed] [Google Scholar]
- 97.Jeruss JS, Sturgis CD, Rademaker AW, Woodruff TK. Down-regulation of activin, activin receptors, and smads in high-grade breast cancer. Cancer Res. 2003;63:3783–3790. [PubMed] [Google Scholar]
- 98.Rodon J, Carducci MA, Sepulveda-Sánchez JM, Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I, Cleverly AL, et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21:553–560. doi: 10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557–1566. doi: 10.1002/hep.22201. [DOI] [PubMed] [Google Scholar]
- 100.Mazzocca A, Fransvea E, Dituri F, Lupo L, Antonaci S, Giannelli GL. Down-regulation of connective tissue growth factor by inhibition of transforming growth factor beta blocks the tumor-stroma cross-talk and tumor progression in hepatocellular carcinoma. Hepatology. 2010;51:523–534. doi: 10.1002/hep.23285. [DOI] [PubMed] [Google Scholar]
- 101.Kim IY, Ahn HJ, Zelner DJ, Shaw JW, Sensibar JA, Kim JH, Kato M, Lee C. Genetic change in transforming growth factor beta (TGF-beta) receptor type I gene correlates with insensitivity to TGF-beta 1 in human prostate cancer cells. Cancer Res. 1996;56:44–48. [PubMed] [Google Scholar]
- 102.Kim IY, Ahn HJ, Lang S, Oefelein MG, Oyasu R, Kozlowski JM, Lee C. Loss of expression of transforming growth factor-beta receptors is associated with poor prognosis in prostate cancer patients. Clin Cancer Res. 1998;4:1625–1630. [PubMed] [Google Scholar]
- 103.Singh J, Ling LE, Sawyer JS, Lee WC, Zhang F, Yingling JM. Transforming the TGFbeta pathway: Convergence of distinct lead generation strategies on a novel kinase pharmacophore for TbetaRI (ALK5) Curr Opin Drug Discov Devel. 2004;7:437–445. [PubMed] [Google Scholar]
- 104.Shinriki S, Jono H, Maeshiro M, Nakamura T, Guo J, Li JD, Ueda M, Yoshida R, Shinohara M, Nakayama H, et al. Loss of CYLD promotes cell invasion via ALK5 stabilization in oral squamous cell carcinoma. J Pathol. 2018;244:367–379. doi: 10.1002/path.5019. [DOI] [PubMed] [Google Scholar]
- 105.Zeddou M, Relic B, Malaise O, Charlier E, Desoroux A, Beguin Y, de Seny D, Malaise MG. Differential signalling through ALK-1 and ALK-5 regulates leptin expression in mesenchymal stem cells. Stem Cells Dev. 2012;21:1948–1955. doi: 10.1089/scd.2011.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Kroon LM, Narcisi R, Blaney Davidson EN, Cleary MA, van Beuningen HM, Koevoet WJ, van Osch GJ, van der Kraan PM. Activin receptor-like kinase receptors ALK5 and ALK1 are both required for TGFβ-induced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2015;10:e0146124. doi: 10.1371/journal.pone.0146124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med. 2015;7:303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin H, Ying Y, Wang YY, Jiang SS, Huang D, Luo L, Chen YG, Gerstenfeld LC, Luo Z. AMPK downregulates ALK2 via increasing the interaction between Smurf1 and Smad6, leading to inhibition of osteogenic differentiation. Biochim Biophys Acta Mol Cell Res. 2017;1864:2369–2377. doi: 10.1016/j.bbamcr.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, Ding S. A chemical platform for improved induction of human iPSCs. Nature Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 111.Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 112.Tojo M, Hamashima Y, Hanyu A, Kajimoto T, Saitoh M, Miyazono K, Node M, Imamura T. The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci. 2005;96:791–800. doi: 10.1111/j.1349-7006.2005.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 114.Klincumhom N, Tharasanit T, Thongkittidilok C, Tiptanavattana N, Rungarunlert S, Dinnyés A, Techakumphu M. Selective TGF-β1/ALK inhibitor improves neuronal differentiation of mouse embryonic stem cells. Neurosci Lett. 2014;578:1–6. doi: 10.1016/j.neulet.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 115.Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–521. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matsuyama S, Iwadate M, Kondo M, Saitoh M, Hanyu A, Shimizu K, Aburatani H, Mishima HK, Imamura T, Miyazono K, Miyazawa K. SB-431542 and Gleevec inhibit transforming growth factor-beta-induced proliferation of human osteosarcoma cells. Cancer Res. 2003;63:7791–7798. [PubMed] [Google Scholar]
- 117.Sato M, Matsubara T, Adachi J, Hashimoto Y, Fukamizu K, Kishida M, Yang YA, Wakefield LM, Tomonaga T. Differential proteome analysis identifies TGF-β-related pro-metastatic proteins in a 4T1 murine breast cancer model. PLoS One. 2015;10:e0126483. doi: 10.1371/journal.pone.0126483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim BH, Guardia Clausi M, Frondelli M, Nnah IC, Saqcena C, Dobrowolski R, Levison SW. Age-dependent effects of ALK5 inhibition and mechanism of neuroprotection in neonatal hypoxic-ischemic brain injury. Dev Neurosci. 2017;39:338–351. doi: 10.1159/000477490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang XB, Zhu H, Song W, Su JH. Gremlin regulates podocyte apoptosis via transforming growth factor-β (TGF-β) pathway in diabetic nephropathy. Med Sci Monit. 2018;24:183–189. doi: 10.12659/MSM.905758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grygielko ET, Martin WM, Tweed C, Thornton P, Harling J, Brooks DP, Laping NJ. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-beta type I receptor kinase in puromycin-induced nephritis. J Pharmacol Exp Ther. 2005;313:943–951. doi: 10.1124/jpet.104.082099. [DOI] [PubMed] [Google Scholar]
- 121.Xu H, Yang F, Sun Y, Yuan Y, Cheng H, Wei Z, Li S, Cheng T, Brann D, Wang R. A new antifibrotic target of Ac-SDKP: Inhibition of myofibroblast differentiation in rat lung with silicosis. PLoS One. 2012;7:e40301. doi: 10.1371/journal.pone.0040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gauger KJ, Chenausky KL, Murray ME, Schneider SS. SFRP1 reduction results in an increased sensitivity to TGF-β signaling. BMC Cancer. 2011;11:59. doi: 10.1186/1471-2407-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kimura-Kuroda J, Teng X, Komuta Y, Yoshioka N, Sango K, Kawamura K, Raisman G, Kawano H. An in vitro model of the inhibition of axon growth in the lesion scar formed after central nervous system injury. Mol Cell Neurosci. 2010;43:177–187. doi: 10.1016/j.mcn.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 124.Giannelli G, Villa E, Lahn M. Transforming growth factor-β as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014;74:1890–1894. doi: 10.1158/0008-5472.CAN-14-0243. [DOI] [PubMed] [Google Scholar]
- 125.Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, Trocóniz IF. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44:142–150. doi: 10.1016/j.ejca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 126.de Gouville AC, Boullay V, Krysa G, Pilot J, Brusq JM, Loriolle F, Gauthier JM, Papworth SA, Laroze A, Gellibert F, Huet S. Inhibition of TGF-beta signaling by an ALK5 inhibitor protects rats from dimethylnitrosamine-induced liver fibrosis. Br J Pharmacol. 2005;145:166–177. doi: 10.1038/sj.bjp.0706172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leung SY, Niimi A, Noble A, Oates T, Williams AS, Medicherla S, Protter AA, Chung KF. Effect of transforming growth factor-beta receptor I kinase inhibitor 2,4-disubstituted pteridine (SD-208) in chronic allergic airway inflammation and remodeling. J Pharmacol Exp Ther. 2006;319:586–594. doi: 10.1124/jpet.106.109314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review article, as no datasets were generated or analyzed during the current study.