Abstract
Objectives
Bone and joint infections caused by Staphylococcus aureus are becoming increasingly difficult to treat due to rising antibiotic resistance, resilient biofilms and intracellular survival of S. aureus. It has been challenging to identify and develop antimicrobial agents that can be used to kill extracellular and intracellular bacteria while having limited toxicity towards host cells. In addressing this challenge, this study investigates the antimicrobial efficacy and toxicity of silver nanoparticles (AgNPs).
Methods
Intracellular bacteria were generated using a co-culture model of human osteoblast cells and S. aureus. Extracellular and intracellular S. aureus were treated with AgNPs, antibiotics and their combinations, and numbers of colonies were quantified. Toxicity of AgNPs against human osteoblast cells was determined by quantifying the number of viable cells after treatment.
Results
AgNPs demonstrated excellent antimicrobial activity against extracellular S. aureus with a 100% killing efficacy at concentrations as low as 56 μM, along with a high intracellular killing efficacy of 76% at 371 μM. AgNPs were non-toxic or slightly toxic towards human osteoblasts at the concentrations studied (up to 927 μM). Moreover, smaller-sized (40 nm) AgNPs were more efficacious in killing bacteria compared with their larger-sized (100 nm) counterparts and synergistic antimicrobial effects against extracellular bacteria were observed when AgNPs were combined with gentamicin.
Conclusions
AgNPs and their combination with antibiotics have demonstrated high extracellular and intracellular bacterial killing and presented unique aspects for potential clinical applications, especially for chronic and recurrent infections where intracellular bacteria may be the cause.
Introduction
Staphylococcus aureus is one of the most common pathogens found in bone and joint infections, with clinical manifestations such as osteomyelitis, septic arthritis and prosthetic joint infection.1–3S. aureus is also ubiquitous in many other infections such as blood, respiratory tract, skin, soft-tissue and device-related infections.4–7 The manifestations of S. aureus infections are broad, collectively leading to high morbidity, mortality and economic burden.8,9 Currently, medical and surgical treatment approaches utilizing antibiotics have been used to treat bone and joint infections; however, there are still significant treatment failures leading to chronic and recurrent infection.10 This may be attributed to many factors. One important factor is the formation of S. aureus biofilms, which are difficult to eradicate.11 Another concern is associated with antibiotic overuse, which has resulted in the emergence of antibiotic-resistant bacteria such as MRSA thereby limiting treatment options.12,13 Though these issues are widely known, an emerging factor that is of increasing concern is the presence of intracellular bacteria causing these infections. S. aureus is classically thought of as an extracellular pathogen; however, in vitro and in vivo evidence has demonstrated that it has the ability to survive intracellularly as well.14–20 This leads to chronic and recurrent infection, as intracellular S. aureus has the capability to elude host defence mechanisms and most current treatment agents by hiding intracellularly.21–23 Thus, in order to treat S. aureus infections adequately, not only must the antimicrobial agent be able to reduce biofilms and overcome antimicrobial resistance, but it must also be able to eliminate intracellular pathogens.
One promising antimicrobial agent is silver nanoparticles (AgNPs), which are ultra-fine particles of metallic silver in the nanoscale range (i.e. 1–100 nm), and are emerging as a new treatment option against a wide array of pathogens.24–30 Studies have demonstrated that AgNPs are effective against Gram-positive and Gram-negative bacteria, anaerobes, fungi and viruses. More importantly, AgNPs can be used against antibiotic-resistant bacteria, as development of resistance is less likely due to its unique and multimodal mechanisms of action.31 While AgNPs have been shown to be an effective treatment option against a wide range of extracellular bacteria, there have been few reports investigating its role as an intracellular agent and thus remains an area of further investigation.32,33 In addition, concern about the toxicity of AgNPs has limited its clinical use.34 Therefore, our objectives were to determine the antimicrobial properties of AgNPs against both extracellular and intracellular S. aureus, to compare it with conventional antibiotics, to investigate the combined efficacy of AgNPs and conventional antibiotics together, and to determine the toxicity of AgNPs towards human osteoblast cells. To accomplish these objectives, AgNPs and antibiotics at different concentrations and treatment intervals were incubated with extracellular and intracellular S. aureus and evaluated for cfu formation. In addition, the viability of human osteoblasts (uninfected) after treatment with AgNPs was determined using a trypan blue assay. We hypothesized that AgNPs and their combination with conventional antibiotics were effective in eliminating both extracellular and intracellular bacteria at concentrations that were not cytotoxic towards human cells.
Materials and methods
Reagents
A clinical isolate of S. aureus (SA1004) was obtained from Ruby Memorial Hospital (Morgantown, WV, USA) and used in this study. Antibiotic susceptibility testing was performed by the Clinical Microbiology Laboratory at West Virginia University and revealed that the isolate was resistant to ampicillin, cefoxitin and penicillin, and susceptible to antibiotics like cefazolin, clindamycin, gentamicin, rifampicin, vancomycin, ciprofloxacin, tetracycline, erythromycin, levofloxacin, moxifloxacin, linezolid, oxacillin and tigecycline. Conventional antibiotics (gentamicin, vancomycin and rifampicin) were purchased from Sigma–Aldrich (St Louis, MO, USA). AgNPs (40 and 100 nm), capped or complexed with polyvinylpyrrolidone (PVP) for stabilization, were obtained from nanoComposix, Inc. (San Diego, CA, USA). A human osteoblast cell line (CRL-11372) was purchased from ATCC (Manassas, VA, USA).
AgNP characterization
Analysis of the morphology of AgNPs was performed using a JEOL JEM-2100 transmission electron microscope (TEM). AgNP suspensions were pipetted onto amorphous carbon-coated copper grids and allowed to dry under vacuum for TEM imaging. The size distribution of the AgNPs was determined using a Malvern NanoSight NS300. The size distribution of each sample was captured in 30 s windows and performed in triplicate. The zeta potential was measured using a Malvern Zetasizer Nano Z. Each sample was measured a minimum of 10 times to determine the average zeta potential.
Extracellular antimicrobial activity of AgNPs and antibiotics
The killing efficacy of AgNPs and conventional antibiotics against extracellular S. aureus was determined by extracellular antimicrobial assays as described previously.35 Briefly, colonies of S. aureus were suspended in tryptic soy broth (TSB) and incubated for 16 h at 37°C. Afterwards, the inoculum was diluted with fresh TSB and incubated at 37°C for an additional 2.5 h to achieve log-phase growth. The inoculum was then diluted with sterile PBS to achieve a concentration of 1.0 × 105 cfu/mL. Various molar concentrations of AgNPs, vancomycin, gentamicin or combination treatment were added to the inoculum in a total mixture volume of 1 mL and incubated for 1 h at 37°C. Treatments with sterile PBS were used as controls. After treatment, the samples were diluted (10−1, 10−2 and 10−3) in sterile PBS, plated on 5% sheep blood agar plates and incubated for 24 h at 37°C.
The extracellular killing efficacy for each treatment was presented in terms of percentage killing, which was calculated by dividing the difference between the cfu of the control and the cfu of the treated samples with the cfu of the control and then multiplying by 100. All experiments were performed in triplicate and the average was reported.
Intracellular antimicrobial activity of AgNPs and antibiotics
The killing efficacy of AgNPs and conventional antibiotics against intracellular S. aureus was determined by intracellular antimicrobial assays as described previously.35 Intracellular S. aureus was obtained using our co-culture infection model of osteoblasts and S. aureus.16,23 Briefly, human osteoblasts were seeded in a 12-well plate at a cell density of 4 × 105 cells/mL and incubated at 37°C with 5% CO2 for 24 h. Following incubation, the cells were cultured with log-phase S. aureus (2 × 108 cfu/mL) and incubated for 2 h. Afterwards, lysostaphin was added and incubated for another 2 h to eliminate extracellular bacteria.16,36. Different molar concentrations of AgNPs, gentamicin, rifampicin, combination treatment or plain DMEM (control) were added. After 2 h incubation, Triton X-100 (0.1%) was added to lyse the osteoblasts. Kinetic studies were also conducted with incubation times up to 16 h. Samples were serially diluted in sterile PBS, plated on 5% sheep blood agar plates and incubated for 24 h at 37°C. The intracellular killing efficacy for each treatment was presented in terms of percentage killing at various molar concentrations. All experiments were performed in triplicate.
Osteoblast viability assay
Osteoblast cells were seeded in a 12-well plate at a cell density of 4 × 105 cells/mL and incubated with 5% CO2 at 37°C for 24 h to form a confluent monolayer. The wells were then treated with AgNPs or plain DMEM (control) and incubated at 37°C for 2 h, which was equivalent to the incubation time for the intracellular AgNP dose–response curve. After incubation, trypsin was added to each well, and the plate placed in the incubator at 37°C, to detach the cells. DMEM was added to neutralize the trypsin. To count the number of viable cells, trypan blue dye was added to the cell suspension in a 1:1 dilution and examined using a haemocytometer for the quantification of live osteoblast cells.
The viability of the osteoblasts at each molar concentration of AgNPs was presented in terms of percentage viability. The percentage viability was calculated by dividing the difference between the number of live osteoblast cells in the control and the number of live osteoblast cells in the treated samples by the number of live osteoblast cells in the control and then multiplying by 100. All experiments were performed in triplicate and the averages were reported.
Statistical analysis
All data are presented as mean ± SD. Differences in percentage killing of all treatments against extracellular and intracellular S. aureus and differences in percentage viability of osteoblasts were assessed using JMP-V9 statistical software (SAS Institute, Cary, NC, USA). Comparisons between two groups were conducted using Student’s t-test. Comparisons between three groups or more were conducted with one-way ANOVA followed by Tukey’s honestly significant difference (HSD) test. Synergistic analysis was done with a t-test-based contrast analysis. A P value <0.05 was considered statistically significant.
Results
Characterization of AgNPs
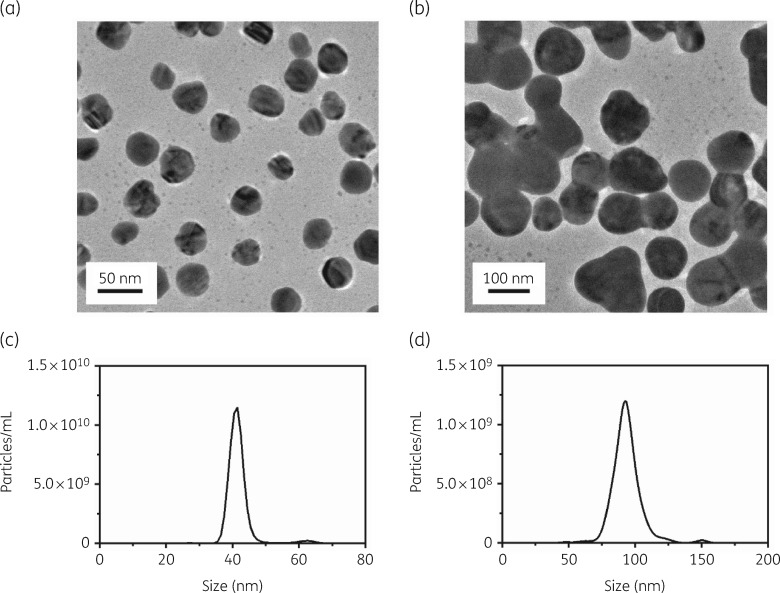
The AgNPs used in this study were analysed for their morphology, size distribution and zeta potential. The shape of the AgNPs was examined using TEM. A typical TEM micrograph demonstrated spherical AgNPs (Figure 1a and b). The size distributions for the 40 and 100 nm AgNPs were determined to be 44.1 ± 2.5 nm and 93.9 ± 0.5 nm, respectively (Figure 1c and d and Table 1). The surface zeta potential for the 40 and 100 nm AgNPs was determined to be −11.1 ± 0.7 mV and −20.0 ± 0.3 mV, respectively (Table 1).
Figure 1.
Morphology and size distribution of AgNPs. TEM images of (a) 40 nm and (b) 100 nm AgNPs and size distribution of (c) 40 nm and (d) 100 nm AgNPs.
Table 1.
Size distribution and zeta potential of 40 and 100 nm AgNPs
| Sample | Diameter (nm) | Zeta potential (mV) |
|---|---|---|
| PVP-capped AgNPs (40 nm) | 44.1 ± 2.5 | −11.1 ± 0.7 |
| PVP-capped AgNPs (100 nm) | 93.9 ± 0.5 | −20.0 ± 0.3 |
Extracellular bacterial killing efficacy
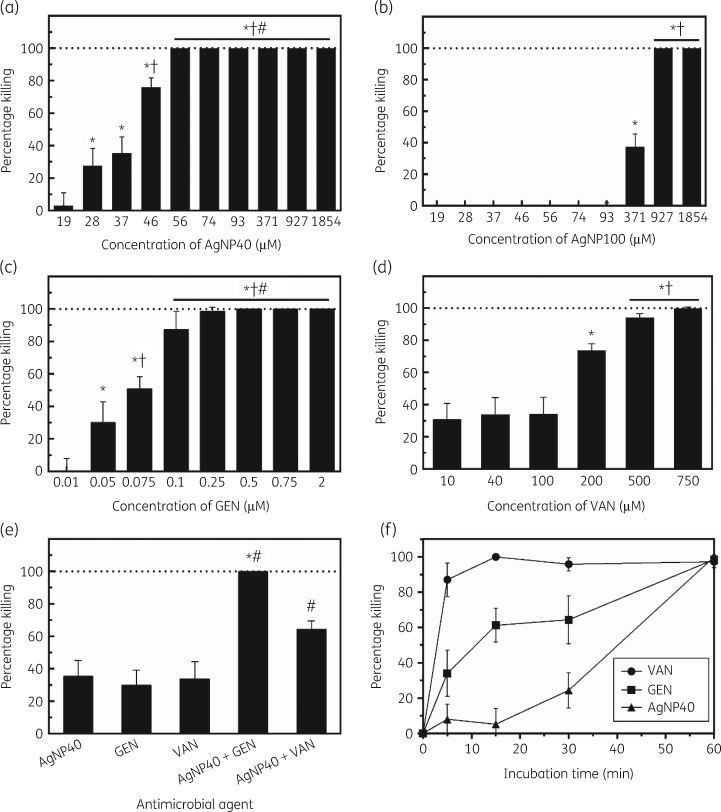
An extracellular antibacterial assay was conducted whereby log-phase extracellular S. aureus was treated with different molar concentrations of 40 and 100 nm AgNPs, gentamicin, vancomycin, 40 nm AgNPs combined with gentamicin or vancomycin, or plain PBS (control), and plated on sheep blood agar to quantify cfu (Figure 2a–d). A wide range of concentrations were investigated to generate dose–response curves; these concentration ranges were similar to what is used clinically in treating S. aureus infections. For instance, in treating periprosthetic joint infections, vancomycin has been used at a dose of 15–20 mg/kg/dose, which is roughly equivalent to 128 to 178 μM. All treatment agents displayed concentration-dependent killing efficacy of extracellular S. aureus; the bacterial killing efficacy increased with increasing concentrations of the treatment agents until reaching 100% killing. The 40 nm AgNPs exhibited strong antibacterial activity against extracellular S. aureus, displaying 76.0% killing efficacy at 46 μM and reached 100% killing efficacy at 56 μM (Figure 2a). The antibacterial activity of 100 nm AgNPs was not as potent as the 40 nm AgNPs in comparison, displaying 37.4% killing efficacy at 371 μM and requiring up to 927 μM to reach 100% killing efficacy (Figure 2b).
Figure 2.
Extracellular bacterial killing efficacy and kinetics of AgNPs, gentamicin and vancomycin. (a) 40 nm AgNP treatment for 1 h at 37°C (*P < 0.0001 compared with 19 μM, †P < 0.0001 compared with 28 and 37 μM, #P = 0.0001 compared with 46 μM). (b) 100 nm AgNP treatment for 1 h at 37°C (*P < 0.0001 compared with 93 μM, †P < 0.0001 compared with 371 μM). (c) Gentamicin treatment for 1 h at 37°C (*P < 0.0001 compared with 0.01 μM, †P < 0.001 compared with 0.05 μM, #P < 0.0001 compared with 0.075 μM). (d) Vancomycin treatment (*P < 0.0001 compared with 10, 40 and 100 μM, †P < 0.001 compared with 200 μM). (e) Combination treatments for 1 h at 37°C (*P < 0.0001 compared with AgNPs and gentamicin, #P < 0.0001 compared with AgNPs and vancomycin). (f) Kinetic profiles at 37°C. AgNP40, 40 nm AgNPs; AgNP100, 100 nm AgNPs; GEN, gentamicin; VAN, vancomycin.
Gentamicin exhibited strong antibacterial activity and displayed killing efficacy of 87.4%, 98.5% and 100% at concentrations of 0.1, 0.25 and 0.5 μM, respectively (Figure 2c). Vancomycin displayed killing efficacy of 73.6%, 93.9% and 99.6% at concentrations of 200, 500 and 750 μM, respectively (Figure 2d).
The killing efficacy against extracellular S. aureus was further investigated using 40 nm AgNPs (37 μM) in combination with gentamicin (0.05 μM) or vancomycin (40 μM) (Figure 2e). These concentrations were selected as each individual antimicrobial agent had ∼30% killing efficacy, which would allow for the delineation of potential additive or synergistic effects in the combination treatments. Individually, the killing efficacy for AgNPs, gentamicin and vancomycin was 35.4%, 30.1% and 33.7%, respectively. When used in combination, the killing efficacy increased significantly. The combination of AgNPs and gentamicin completely inhibited all bacterial growth and displayed a killing efficacy of 100%. This was found to be synergistic, based on a t-test contrast analysis (P = 0.0015). The combination of AgNPs and vancomycin displayed a combined killing efficacy of 64.4%, which was additive but not synergistic (P = 0.4519).
The killing efficacy kinetics against extracellular S. aureus was investigated using 40 nm AgNPs (56 μM), gentamicin (0.25 μM) and vancomycin (750 μM) at timepoints up to 60 min (Figure 2f). The concentration for each agent was chosen based on the previous dose–response curves where the corresponding killing efficacy was nearly 100% (Figure 2a, c and d). Vancomycin displayed relatively fast bactericidal activity and displayed an 87.0% killing efficacy within 5 min and ∼100% killing efficacy at 15 min. In comparison, 40 nm AgNPs and gentamicin had relatively slower killing efficacy kinetics, as both only had 24.4% and 64.3% killing, respectively, at 30 min and both required 60 min to achieve ∼100% killing (Figure 2f).
Intracellular bacterial killing efficacy
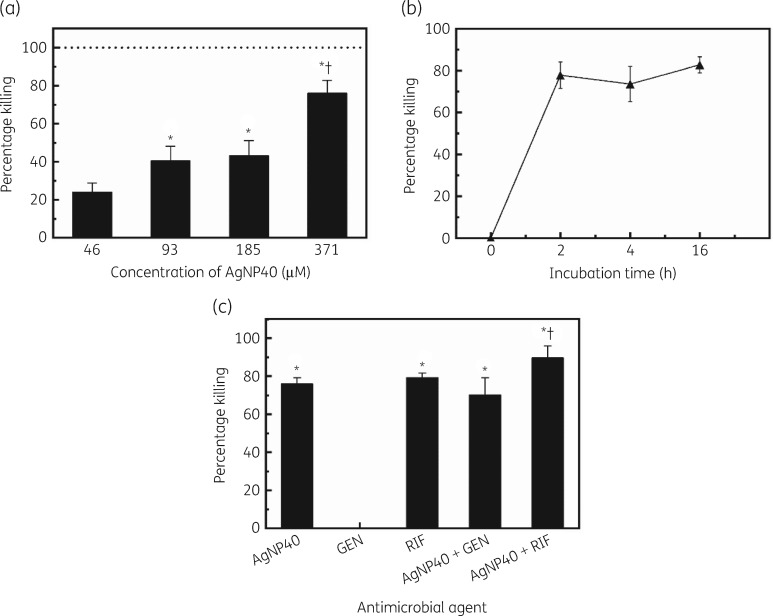
An intracellular antibacterial assay was conducted whereby human osteoblast cells and S. aureus were co-cultured to obtain intracellular S. aureus, which was subsequently treated with antimicrobial agents. After treatment, the osteoblast cells were lysed to release intracellular S. aureus and plated on sheep blood agar to quantify the number of intracellular cfu. There was a dose-dependent killing efficacy in AgNP treatments; the killing efficacy increased with increasing AgNP concentration and a high intracellular killing of 76% was obtained at 371 μM (Figure 3a). The kinetics studies showed no significant changes in intracellular bacterial killing after 2 h (Figure 3b).
Figure 3.
Intracellular bacterial killing efficacy and kinetics studies. (a) Intracellular bacterial killing efficacy of AgNPs at various concentrations (*P < 0.001 compared with 46 μM, †P < 0.0001 compared with 93 and 185 μM). (b) Kinetics of AgNPs in killing intracellular S. aureus. (c) Intracellular bacterial killing efficacy of AgNPs, gentamicin, rifampicin and their combinations (*P < 0.0001 compared with gentamicin, †P = 0.0002 compared with AgNPs, †P = 0.0056 compared with rifampicin, †P < 0.0001 compared with AgNPs + gentamicin). AgNP40, 40 nm AgNPs; GEN, gentamicin; RIF, rifampicin.
The killing efficacy against intracellular S. aureus was also investigated using AgNPs (40 nm) in combination with gentamicin or rifampicin (Figure 3c). Individually, the killing efficacy for AgNPs (371 μM), gentamicin (1000 μM) and rifampicin (1000 μM) was 76.0%, 0% and 79.3%, respectively. The combination of AgNPs and gentamicin displayed a killing efficacy of 70.1%. The combination of AgNPs and rifampicin achieved a killing efficacy of 89.6%; no synergistic effects were observed. The combination of AgNPs with rifampicin presented significantly higher killing efficacy compared with those of AgNPs, gentamicin, rifampicin and the combination of AgNPs with gentamicin.
Viability of osteoblasts
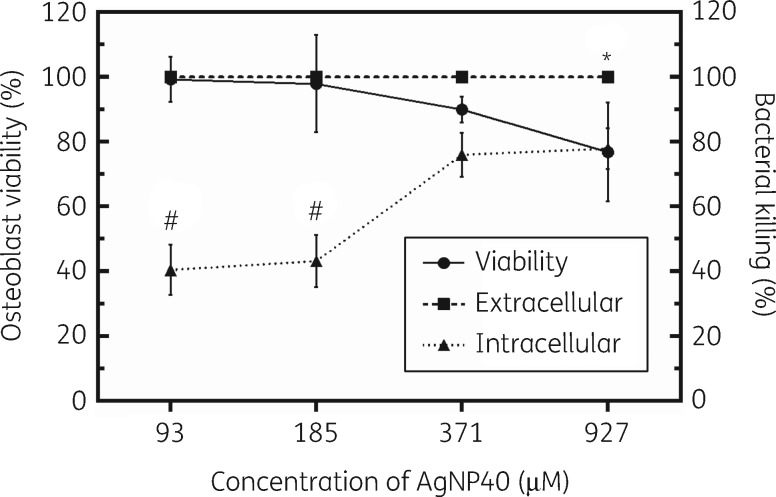
The number of viable human osteoblast cells after treating with 40 nm AgNPs was determined using the trypan blue assay. Excellent viability of osteoblasts was observed at relatively low concentrations (e.g. 93 and 185 μM) of AgNPs (Figure 4). The percentage viability of osteoblasts at AgNP concentrations of 93, 185, 371 and 927 μM was determined to be 99.3%, 98.0%, 90.1% and 76.9%, respectively (Figure 4). The viability of osteoblast cells at 927 μM was significantly lower than that at 93 μM (P = 0.044).
Figure 4.
Osteoblast viability and extracellular and intracellular bacterial killing of AgNPs. *P = 0.044 compared with viability at 93 μM. #P < 0.0001 compared with percentage killing at 371 and 927 μM. AgNP40, 40 nm AgNPs.
Discussion
Our studies indicate that AgNPs could be a unique treatment agent against extracellular bacteria. In particular, 40 nm AgNPs displayed excellent antimicrobial activity against extracellular S. aureus with complete inhibition of bacterial growth at concentrations as low as 56 μM (Figure 2a). The size of the nanoparticles plays a major role in their antimicrobial activity as our studies demonstrated that the use of 100 nm AgNPs required concentrations as high as 927 μM to achieve complete inhibition of bacterial growth (Figure 2b). The increased efficacy of smaller nanoparticles can be attributed to their increased dissolution of Ag+ ions (with the Ag+ ion being the biologically active agent) and their increased particle–surface reactions.37,38
In the treatment of bone and joint infection, gentamicin has been commonly used39 due to its broad antimicrobial spectrum and vancomycin has been widely used in cases where MRSA is suspected.40 Against extracellular bacteria, gentamicin was found to be the most effective of all of the treatments that we studied, requiring only 0.5 μM to completely inhibit bacterial growth (Figure 2c), and vancomycin required at least 750 μM to completely inhibit bacterial growth (Figure 2d). The kinetics studies indicated that vancomycin acts relatively faster upon bacteria compared with gentamicin and AgNPs, though a higher molarity of vancomycin was used. In these studies, concentrations of AgNPs, gentamicin and vancomycin were selected based on the previous dose–response curves (Figure 2a–d) that showed almost 100% killing efficacy at the lowest concentration tested. Vancomycin achieved a killing efficacy of 87.0% within 5 min and displayed complete inhibition of growth within 15 min incubation time (Figure 2f). In contrast, gentamicin and AgNPs both required at least 60 min incubation time to achieve ∼100% killing efficacy (Figure 2f).
While AgNPs were effective against extracellular bacteria, the more challenging form of bacteria that allows for recurring infection stems from the presence of biofilms and intracellular bacteria. Previous studies have reported that AgNPs might be effective against biofilms by preventing biofilm formation and killing bacteria in established biofilms.33,41,42 However, the efficacy of AgNPs against intracellular bacteria is less known, which we have investigated in this study. AgNPs could be taken up into cells via endocytosis,43,44 whereby the intracellular environment facilitates the release of Ag+ ions.45 Our study here investigated the killing efficacy of AgNPs towards intracellular bacteria and compared its efficacy with those of conventional antibiotics. The findings of our study demonstrated that 40 nm AgNPs presented a high killing efficacy of 76% at 371 μM (Figure 3a). However, increasing the incubation time from 2 h to 16 h did not make a significant difference in the percentage killing (Figure 3b). It is unclear why increasing AgNP incubation times did not lead to further increase of intracellular percentage killing. This, on the other hand, confirms the challenges in dealing with intracellular bacteria like S. aureus. The killing efficacy of AgNPs was compared with gentamicin and rifampicin, which were selected due to the excellent intracellular penetration of rifampicin and poor intracellular penetration of gentamicin.46,47 When compared with the antibiotics gentamicin and rifampicin, 40 nm AgNPs displayed a similar efficacy to rifampicin, as 1000 μM of rifampicin had a killing efficacy of 79.3% (Figure 3c). As expected, gentamicin displayed an intracellular killing efficacy of 0%, as it is unable to penetrate intracellularly (Figure 3c). Rifampicin is a commonly used antibiotic in the treatment of prosthetic joint infections, due to its excellent antimicrobial and biofilm penetration capabilities.48,49 However, the use of rifampicin has led to rapid development of resistance.50–52 Since our studies demonstrate similar efficacy for AgNPs and rifampicin, AgNPs may be a better long-term solution to avoid the drawbacks of rifampicin.
Combination treatments of AgNPs and antibiotics against extracellular and intracellular bacteria were assessed. It was found that the combination of AgNPs and gentamicin had a synergistic killing efficacy against extracellular bacteria as the combination completely inhibited bacterial growth (Figure 2e). Against intracellular bacteria, our findings indicate no synergistic activity of AgNPs with gentamicin or rifampicin (Figure 3c) and this remains an area for further research in regard to potential synergistic effect in combination with other antibiotics.
AgNPs may be a good choice for killing intracellular bacteria;53 however, they may also present relatively higher toxicity towards human cells.54–57 The potential toxicity of AgNPs towards human osteoblast cells was investigated and our findings reveal that 40 nm AgNPs were non-cytotoxic with high viability of osteoblast cells at concentrations less than 371 μM (Figure 4). Based on a cytotoxicity classification from Kong et al.,58 >90% cell viability is considered to be non-cytotoxic, 60% to 90% cell viability is slightly cytotoxic, 30% to 59% cell viability is moderately cytotoxic and <30% cell viability is severely cytotoxic. The highest concentration tested, 927 μM, still had relatively high viability with 76.9% of viable osteoblast cells (Figure 4). The determination of the potential toxicity of AgNPs is important because it gives insight towards the identification of therapeutic windows for the treatment of bacteria with AgNPs that may have limited detrimental effects against host cells. For the treatment of extracellular S. aureus with 40 nm AgNPs, our findings support the use of concentrations as low as 56 μM to eliminate all bacteria with close to 100% viability of osteoblast cells. Our study suggests that AgNPs at concentrations of 185 μM or below would be non-toxic toward osteoblasts while presenting 100% killing of extracellular S. aureus and ∼40% killing of intracellular S. aureus. AgNPs at 371 μM were non-cytotoxic or slightly toxic towards osteoblasts and presented high killing of both extracellular (100%) and intracellular (76%) S. aureus, respectively (Figure 4).
The current study demonstrates that AgNPs are a unique treatment agent against extracellular and intracellular bacteria. AgNPs were shown to be very potent in eliminating extracellular bacteria with low toxicity towards human osteoblast cells. AgNPs also exhibited improved antibacterial activity in combination with antibiotics against extracellular bacteria, particularly gentamicin, whose interaction was found to be synergistic. When compared with individual antibiotics, gentamicin was found to be more efficacious than AgNPs against extracellular bacteria. However, against intracellular bacteria, AgNPs were more efficacious, whereas gentamicin had no intracellular activity. This is important since some bacteria, such as S. aureus, have the ability to persist intracellularly and may lead to difficult-to-treat chronic and recurrent infections. Our study is one of the first to report on the intracellular efficacy of AgNPs and our findings indicate that AgNPs present a high intracellular bacterial killing efficacy of 76% with low toxicity toward osteoblasts at concentrations of 371 μM and below; in comparison, rifampicin is known to have high intracellular bacterial killing activity and a similar intracellular killing efficacy (79.3%) was observed at a much higher concentration (i.e. 1000 μM) in this study. This study has the potential to stimulate new treatments of chronic and recurrent infections of the bone and joint caused by intracellular bacteria and we have demonstrated that AgNPs can be used against these infections with relatively low toxicity towards the host cells.
Acknowledgements
We acknowledge Gerald R. Hobbs at West Virginia University for help with data analysis, Elijah Roberts for assistance with experiments and Suzanne Danley for proofreading.
Funding
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Medical Research Program, Discovery Awards under Award Number W81XWH1810203. We also acknowledge financial support from the AO Foundation, the Osteosynthesis & Trauma Care Foundation, the West Virginia National Aeronautics and Space Administration Experimental Program to Stimulate Competitive Research (WV NASA EPSCoR), WVU PSCoR and WVCTSI. In addition, we acknowledge the use of the WVU Shared Research Facilities that are supported by NIH grants 5U54GM104942-03, 5P20RR016477, U57GM104942, P30GM103488, P20GM109098 and P20GM103434.
Transparency declarations
None to declare.
Disclaimer
Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the funding agencies.
References
- 1. Kavanagh N, Ryan EJ, Widaa A. et al. Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin Microbiol Rev 2018; 31: e00084–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corrado A, Donato P, Maccari S. et al. Staphylococcus aureus-dependent septic arthritis in murine knee joints: local immune response and beneficial effects of vaccination. Sci Rep 2016; 6: 38043.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sendi P, Banderet F, Graber P. et al. Periprosthetic joint infection following Staphylococcus aureus bacteremia. J Infect 2011; 63: 17–22. [DOI] [PubMed] [Google Scholar]
- 4. Corey GR. Staphylococcus aureus bloodstream infections: definitions and treatment. Clin Infect Dis 2009; 48 Suppl 4: S254–9. [DOI] [PubMed] [Google Scholar]
- 5. Besier S, Smaczny C, von Mallinckrodt C. et al. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J Clin Microbiol 2007; 45: 168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCaig LF, McDonald LC, Mandal S. et al. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis 2006; 12: 1715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arrecubieta C, Asai T, Bayern M. et al. The role of Staphylococcus aureus adhesins in the pathogenesis of ventricular assist device-related infections. J Infect Dis 2006; 193: 1109–19. [DOI] [PubMed] [Google Scholar]
- 8. Tong SY, Davis JS, Eichenberger E. et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28: 603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noskin GA, Rubin RJ, Schentag JJ. et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med 2005; 165: 1756–61. [DOI] [PubMed] [Google Scholar]
- 10. Osmon DR, Berbari EF, Berendt AR. et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56: e1–25. [DOI] [PubMed] [Google Scholar]
- 11. Brady RA, Leid JG, Calhoun JH. et al. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol 2008; 52: 13–22. [DOI] [PubMed] [Google Scholar]
- 12. Ventola CL. The antibiotic resistance crisis. Part 1: causes and threats. P T 2015; 40: 277–83. [PMC free article] [PubMed] [Google Scholar]
- 13. Li B, Webster TJ.. Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopedic infections. J Orthop Res 2018; 36: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gresham HD, Lowrance JH, Caver TE. et al. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol 2000; 164: 3713–22. [DOI] [PubMed] [Google Scholar]
- 15. Hudson MC, Ramp WK, Nicholson NC. et al. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog 1995; 19: 409–19. [DOI] [PubMed] [Google Scholar]
- 16. Hamza T, Li B.. Differential responses of osteoblasts and macrophages upon Staphylococcus aureus infection. BMC Microbiol 2014; 14: 207.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tuchscherr L, Medina E, Hussain M. et al. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med 2011; 3: 129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Surewaard BGJ, Deniset JF, Zemp FJ. et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zautner AE, Krause M, Stropahl G. et al. Intracellular persisting Staphylococcus aureus is the major pathogen in recurrent tonsillitis. PLoS One 2010; 5: e9452.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clement S, Vaudaux P, Francois P. et al. Evidence of an intracellular reservoir in the nasal mucosa of patients with recurrent Staphylococcus aureus rhinosinusitis. J Infect Dis 2005; 192: 1023–8. [DOI] [PubMed] [Google Scholar]
- 21. Fraunholz M, Sinha B.. Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect Microbiol 2012; 2: 43.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maurin M, Raoult D.. Use of aminoglycosides in treatment of infections due to intracellular bacteria. Antimicrob Agents Chemother 2001; 45: 2977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamza T, Dietz M, Pham D. et al. Intra-cellular Staphylococcus aureus alone causes infection in vivo. Eur Cell Mater 2013; 25: 341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guzman M, Dille J, Godet S.. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomedicine 2012; 8: 37–45. [DOI] [PubMed] [Google Scholar]
- 25. Lu Z, Rong K, Li J. et al. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J Mater Sci Mater Med 2013; 24: 1465–71. [DOI] [PubMed] [Google Scholar]
- 26. Panáček A, Kolář M, Večeřová R. et al. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009; 30: 6333–40. [DOI] [PubMed] [Google Scholar]
- 27. Gaikwad S, Ingle A, Gade A. et al. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine 2013; 8: 4303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lara HH, Garza-Trevino EN, Ixtepan-Turrent L. et al. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol 2011; 9: 30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lara HH, Ayala-Núñez NV, del Carmen Ixtepan Turrent L. et al. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol 2010; 26: 615–21. [Google Scholar]
- 30. Ayala-Núñez NV, Lara Villegas HH, del Carmen Ixtepan Turrent L. et al. Silver nanoparticles toxicity and bactericidal effect against methicillin-resistant Staphylococcus aureus: nanoscale does matter. Nanobiotechnol 2009; 5: 2–9. [Google Scholar]
- 31. Franci G, Falanga A, Galdiero S. et al. Silver nanoparticles as potential antibacterial agents. Molecules 2015; 20: 8856–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jena P, Mohanty S, Mallick R. et al. Toxicity and antibacterial assessment of chitosan-coated silver nanoparticles on human pathogens and macrophage cells. Int J Nanomedicine 2012; 7: 1805–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohanty S, Mishra S, Jena P. et al. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomedicine 2012; 8: 916–24. [DOI] [PubMed] [Google Scholar]
- 34. Stensberg MC, Wei Q, McLamore ES. et al. Toxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imaging. Nanomedicine (Lond) 2011; 6: 879–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Noore J, Noore A, Li B.. Cationic antimicrobial peptide LL-37 is effective against both extra- and intracellular Staphylococcus aureus. Antimicrob Agents Chemother 2013; 57: 1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Easmon CS, Lanyon H, Cole PJ.. Use of lysostaphin to remove cell-adherent staphylococci during in vitro assays of phagocyte function. Br J Exp Pathol 1978; 59: 381–5. [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J, Sonshine DA, Shervani S. et al. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 2010; 4: 6903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sarkar S, Leo BF, Carranza C. et al. Modulation of human macrophage responses to Mycobacterium tuberculosis by silver nanoparticles of different size and surface modification. PLoS One 2015; 10: e0143077.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang Y, Tai CL, Hsieh PH. et al. Gentamicin in bone cement: a potentially more effective prophylactic measure of infection in joint arthroplasty. Bone Joint Res 2013; 2: 220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim B-N, Kim ES, Oh M-D.. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J Antimicrob Chemother 2014; 69: 309–22. [DOI] [PubMed] [Google Scholar]
- 41. Martinez-Gutierrez F, Boegli L, Agostinho A. et al. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 2013; 29: 651–60. [DOI] [PubMed] [Google Scholar]
- 42. Qin H, Cao H, Zhao Y. et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials 2014; 35: 9114–25. [DOI] [PubMed] [Google Scholar]
- 43. Gliga AR, Skoglund S, Odnevall Wallinder I. et al. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol 2014; 11: 11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greulich C, Diendorf J, Simon T. et al. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater 2011; 7: 347–54. [DOI] [PubMed] [Google Scholar]
- 45. Jiang X, Miclăuş T, Wang L. et al. Fast intracellular dissolution and persistent cellular uptake of silver nanoparticles in CHO-K1 cells: implication for cytotoxicity. Nanotoxicology 2015; 9: 181–9. [DOI] [PubMed] [Google Scholar]
- 46. Darouiche RO, Hamill RJ.. Antibiotic penetration of and bactericidal activity within endothelial cells. Antimicrob Agents Chemother 1994; 38: 1059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sandberg A, Hessler JH, Skov RL. et al. Intracellular activity of antibiotics against Staphylococcus aureus in a mouse peritonitis model. Antimicrob Agents Chemother 2009; 53: 1874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saginur R, Stdenis M, Ferris W. et al. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother 2006; 50: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dunne WM, Mason EO, Kaplan SL.. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother 1993; 37: 2522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu C, Bayer A, Cosgrove SE. et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52: e18–55. [DOI] [PubMed] [Google Scholar]
- 51. Falagas ME, Bliziotis IA, Fragoulis KN.. Oral rifampin for eradication of Staphylococcus aureus carriage from healthy and sick populations: a systematic review of the evidence from comparative trials. Am J Infect Control 2007; 35: 106–14. [DOI] [PubMed] [Google Scholar]
- 52. Achermann Y, Eigenmann K, Ledergerber B. et al. Factors associated with rifampin resistance in staphylococcal periprosthetic joint infections (PJI): a matched case-control study. Infection 2013; 41: 431–7. [DOI] [PubMed] [Google Scholar]
- 53. Armstead AL, Li B.. Nanomedicine as an emerging approach against intracellular pathogens. Int J Nanomedicine 2011; 6: 3281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Armstead AL, Li B.. Nanotoxicity: emerging concerns regarding nanomaterial safety and occupational hard metal (WC-Co) nanoparticle exposure. Int J Nanomedicine 2016; 11: 6421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Armstead AL, Arena CB, Li B.. Exploring the potential role of tungsten carbide cobalt (WC-Co) nanoparticle internalization in observed toxicity toward lung epithelial cells in vitro. Toxicol Appl Pharmacol 2014; 278: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu LZ, Ding M, Zheng JZ. et al. Tungsten carbide-cobalt nanoparticles induce reactive oxygen species, AKT, ERK, AP-1, NF-κB, VEGF, and angiogenesis. Biol Trace Elem Res 2015; 166: 57–65. [DOI] [PubMed] [Google Scholar]
- 57. Armstead AL, Simoes TA, Wang X. et al. Toxicity and oxidative stress responses induced by nano- and micro-CoCrMo particles. J Mater Chem B 2017; 5: 5648–57. [Google Scholar]
- 58. Kong N, Jiang T, Zhou Z. et al. Cytotoxicity of polymerized resin cements on human dental pulp cells in vitro. Dent Mater 2009; 25: 1371–5. [DOI] [PubMed] [Google Scholar]