Abstract
The lack of consensus on bone marrow (BM) and splenic immune cell profiles in preclinical mouse strains complicates comparative analysis across different studies. Although studies have documented relative distribution of immune cells from peripheral blood in mice, similar studies for BM and spleen from naïve mice are lacking. In an effort to establish strain- and gender-specific benchmarks for distribution of various immune cell sub-types in these organs, we performed immunophenotypic analysis of BM cells and splenocytes from both genders of three commonly used murine strains (C57BL/6NCr, 129/SvHsd, and BALB/cAnNCr). Total neutrophils, and splenic macrophages were significantly higher in C57BL/6NCr; whereas total B-cells were lower. Within C57BL/6NCr female mice, BM B-cells were elevated with respect to the males whereas splenic mDCs and splenic neutrophils were reduced. Within BALB/cAnNCr male mice, BM CD4+ Tregs were elevated with respect to the other strains. Furthermore, in male BALB/cAnNCr mice, NK cells were elevated with respect to the other strains in both BM and spleen. Splenic CD4+ Tregs and splenic CD8+ T cells were reduced in male BALB/c mice in comparison to female mice. Bone marrow CD4+ T cells and mDCs were significantly increased in 129/SvHsd whereas splenic CD8+ T cells were reduced. In general, males exhibited higher immature myeloid cells, macrophages and NK cells. To our knowledge, this study provides a first attempt to systematically establish organ-specific benchmarks on immune cells in studies involving these mouse strains.
Keywords: Immunophenotyping, flow-cytometry, murine strains, bone marrow, spleen, gender
INTRODUCTION
Hematopoiesis is a tightly regulated biological process 1, 2. The immune cells in hematopoietic organs undergo constant differentiation, proliferation and programmed cell death 3–5. This in turn orchestrates a finely regulated immune cell homeostasis, ensuring that leukocyte subpopulations are maintained within a constant range 3–5. In mice, bone marrow and spleen are two major hematopoietic organs 5, 6. Although various immune cell subsets from these organs are commonly evaluated in studies, there is a lack of strain- and gender-specific benchmarks for each leukocyte cell subtype for these organs in healthy mice 7–9.
In contrast to studies on peripheral blood, where the frequency of leukocyte subpopulations have been charted out in clear detail, there is a gap regarding numerical range for immune cell subtypes in adult murine hematopoietic organs 10–16. The recent success of cancer immunotherapy has fueled an explosive rise in pre-clinical translational studies to develop new treatment strategies 17. However, many such studies are designed to focus on the biology of only specific leukocyte subpopulations in response to treatments with experimental agents 7, 18–23. Based on the experimental design of the immunological study, in vivo experiments are carried out in different mouse strains and immune profiles are evaluated at different steps following treatment regimen 24. The variations in experimental variables such as mouse strain, animal physiology, age, gender, drug combinations, time-points, dose, treatment strategies, tumor sub-types and tumor inoculation methods can create infinite confounders that influence the immune parameters and need to be considered even for a study with a single agent 7, 9–15, 24–30.
For example, Petkova et al have reported marked differences in the relative proportions of leukocyte sub-populations in peripheral blood among different mouse strains 15. The SJL/J strains exhibit inverse B and T cell ratios in comparison to the C57BL/6NCr mice, highlighting the effect of strain on general immune profile of the organism 9. Gajewski et al found that mice from The Jackson Laboratory (JAX) exhibited a dramatically different immune response to implanted melanoma tumors in comparison to mice from Taconic Biosciences (TAC) 28. Similarly, gender also impacts Th1 cells, Tregs, and DCs in both intestinal and peripheral immune populations and has been suggested to be an underlying cause for susceptibility to intestinal disorders 25. However, there have been no systematic studies to analyze whether such strain- and gender-specific differences exist in murine hematopoietic organs. Hence, there is a pressing need for comprehensive studies to address strain and gender-specific characterization of the entire gamut of leukocyte subpopulations in normal mice 10, 11, 31. Since the raw flow cytometry data can be normalized differently (e.g. cell number vs. percentage, percentage leukocytes vs. percentage lymphocytes), a cross-comparison of any immune cell subtype among different studies is obscured 32–34. Therefore, a lack of specific benchmarks on basal immune cell distribution confounds comparative immunophenotypic analysis across different studies and eventually leads to discordant data. In order to determine the distribution of individual immune cell subtypes in bone marrow and splenic isolates of three commonly used mouse strains, we conducted an unbiased analysis of cells from BM and spleen of both genders.
The three major immunocompetent murine strains (C57BL/6NCr, BALB/cAnNCr, 129/SvHsd) used in the present study are broadly used in pre-clinical research 29, 35. Whereas BALB/c mice, which are recognized for their use in cancer research, readily develop tumors in response to carcinogenic stimuli, and develop spontaneous tumors at later stages of their lifespan 29, 35–37, C57BL/6 mice are multi-functional model organisms routinely used in studies involving infectious diseases, congenital anomalies and cancer 29, 35, 38. 129/Sv mice on other the hand, are frequently employed in both transgenic/knockout models and oncology studies 39–41. We evaluated a spectrum of multiple immune cell types representing both the myeloid and lymphoid lineages of hematopoietic cells in these three mouse strains and found important strain and gender-specific trends within cells of both lineages. Results of the study underscore that both innate and cell mediated immune profile varies dramatically based on mouse strain and gender.
METHODS
Reagents and Antibodies
RBC lysis buffer was purchased from Bio-Legend (Bio-Legend, San Diego, CA). FACS buffer was reconstituted by adding 3% FBS to ice cold 1X PBS. FBS was purchase from Atlanta Biologicals (Flowery Branch, GA). Antibodies were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), Miltenyi Biotec (Auburn, CA) and Thermo-Fischer (Waltham, MA). The clone number and dilution of antibodies are provided in Table 1. The conjugated fluorophores used in the study are enlisted in Table 2. UltraComp™ eBeads were purchased from eBiosciences and used for compensation. Mouse Fc Blocking Reagent was purchased from Miltenyi Biotec. Fixation/Permeabilization Solution Kit with BD Golgi-Plug™ kit and Mouse Foxp3 Buffer Set was used for intracellular staining and purchased from BD Biosciences.
Table 1.
Antibody combinations used in immunophenotyping
| iMC | CD11b Vio Blue | Gr-1 APC-eFluor780 | *Ly6b FITC | |
| Macrophages | CD11b Vio Blue | F4/80 APC | CD68 PE | |
| mDC | CD11b Vio Blue | CD11c PE | CD80 APC | CD86 FITC |
| pDC | *CD11b Vio Blue | CD11c PE | B220 Vio770 | Siglec H FITC |
| CD4 T-cells | CD3 APC-Cy7 | CD4 eFluor450 | ||
| CD4 T-regs | CD3 APC-Cy7 | CD4 eFluor450 | CD25 PE | FoxP3APC |
| CD8 T-cells | CD3 APC-Cy7 | CD8 FITC | ||
| B cells | B220 Vio770 | CD19 PE | ||
| NK cells | CD3 APC-Cy7 | NKp46 FITC | ||
| Neutrophils | CD11b Vio Blue | Gr-1 APC-eFluor780 | Ly6b FITC | *F4/80 APC |
negative tor this antibody
Table 2.
Details of antibodies used in the study
| Antibody | Fluorochrome | Vendor | Clone | Catalogue # | Antibody Concentration (in 100 pL FACS buffer) |
|---|---|---|---|---|---|
| CD3e | APC-Cy7 | Fisher Scientific | 145–2C11 | BDB557596 | 1 pL |
| CD4 | eFluor450 | eBioscience | GK1.5 | 48–0041-82 | 1 pL |
| CD8 | FITC | eBioscience | 53–6.7 | 11–0081-82 | 0.6 pL |
| CD11b | Vio Blue | Miltenyi | M1/70.15.11.5 | 130–097-336 | 3.75 pL |
| CD11c | PE | Miltenyi | N418 | 130102545 | 3.75 pL |
| CD19 | PE | Miltenyi | 6D5 | 130–102-598 | 5 pL |
| CD25 | PE | eBioscience | PC61.5 | 12–0251-81 | 0.5 pL |
| CD68 | PE | Miltenyi | FA-11 | 130–102-614 | 3.75 pL |
| CD80 | APC | Miltenyi | 16–10A1 | 130–102-584 | 3.75 pL |
| CD86 | FITC | Miltenyi | PO3.3 | 130–102-506 | 5 pL |
| B220 | APC-Vio770 | Miltenyi | RA3–6B2 | 130–102-267 | 3.75 pL |
| F4/80 | APC | Miltenyi | REA126 | 130–102-379 | 3.75 pL |
| FoxP3 | APC | eBioscience | FJK-16s | 17–5773-82 | 5 pL |
| Gr1 | APC-eFluor780 | eBioscience | RB6–8C5 | 47–5931-80 | 1.2 pL |
| Ly6b | FITC | abcam | 7/4 | ab53453 | 0.65 pL |
| NKp46 | FITC | Miltenyi | 29A1.4.9 | 130–102-300 | 3.75 pL |
| SiglecH | FITC | eBioscience | eBio440c | 11–0333-82 | 0.5 pL |
Mouse
Eight-week-old female and male pathogen-free BALB/cAnNCr, C57BL/6NCr, and 129/SvHsd mice were obtained from Envigo RMS, Inc (Indianapolis, IN). Animals were housed in accordance with established guidelines and protocols approved by the UAB Institutional Animal Care and Use Committee (UAB-IACUC).
Isolation of immune cells and flow cytometry
On the day of the analysis, mice from each strain were first anaesthetized and then sacrificed by cervical dislocation. Bone marrow cells were obtained by flushing the marrow cavities with FACS buffer (PBS with 3% FBS) using insulin syringe with 28G needle, followed by passage through a 100 mm sterile cell strainer. Splenic cells were obtained by gentle pressure-dissociation of spleen using FACS buffer, and then passed through a 100 mm sterile cell strainer. For all experiments, cells were washed with FACS buffer and pelleted. Red blood cells were lysed by addition of 5 ml RBC lysis buffer (Bio-Legend, San Diego, CA), mixed briefly to re-suspend cells and incubated for 5 minutes at room temperature, followed by FACS buffer wash and spin. Cells were re-suspended in FACS buffer and incubated with Fc block at room temperature for 15 minutes. Cells were divided into individual tubes for respective cell type analysis, suspended in 100 μl of FACS buffer and stained for the designated cell type. The data was acquired on BD LSRII (BD Biosciences, New Jersey) and analyzed using FlowJo software (FlowJo LLC, Oregon).
Gating strategies
Markers used for immune cell strategies are described in Table 1 and 2. The gating strategy for immune-cell types is shown in Supplementary Figure 1 and 2. All gating was performed off of live cell populations. Unstained controls and single color controls were utilized to establish base line gate settings for each respective antibody-fluorophore(s) combination used in individual experiments. The gating strategy for each cell-type examined is outlined below and visually represented in Supplementary Figure 1 and 2.
CD4+ T cells
P1 = live cells, P2 = CD3 APC-Cy7 and CD4 eFluor450 double positive gate
CD8+ T cells
P1 = live cells, P2 = CD3 APC-Cy7 and CD8 FITC double positive gate
CD4+ Tregs
P1 = live cells, P2 = CD3 APC-Cy7 and CD4 eFluor450 double positive gate, P3 (intracellular staining) = CD25 PE and FoxP3 APC double positive gate.
B cells
P1 = live cells, P2 = CD19 PE and B220 Vio770 double positive gate
NK cells
P1 = live cells, P2 = CD3 APC-Cy7 negative and NKp46 FITC positive gate
iMCs
P1 = live cells, P2 = CD11b VioBlue and Gr-1 APC-eFluor780 double positive gate, P3 = F/80 APC negative and Ly6b FITC negative, F4/80 APC positive and Ly6b FITC negative, F4/80 APC positive and Ly6b FITC positive gate.
Neutrophils
P1 = live cells, P2 = CD11b VioBlue and Gr-1 APC-eFluor780 double positive gate, P3 = F4/80 APC negative and Ly6b positive gate
Macrophages
P1 = live cells, P2 = CD11b VioBlue and F4/80 APC double positive gate, P3 = CD68 PE (intracellular staining) positive histogram gate
mDC
P1 = live cells, P2 = CD11b VioBlue and CD11c PE double positive gate
pDC
P1 = live cells, P2 = CD11b VioBlue negative and CD11c PE positive gate, P3 = B220 Vio770 and Siglec H FITC double positive gate
Statistical analysis
Data was analyzed with multi-variate analysis using two-way ANOVA with multiple comparisons corrected with the Tukey Test within Prism™. Results are represented as scatter plot graphs indicating each group’s Mean and SD. Differences were considered significant if p≤0.05. Sample values at or beyond +/− (1.7 × SD) were excluded from the analysis. Overall statistics describing the source of variation are listed in Supplementary Figure 3.
RESULTS
CD4+ T cells are significantly elevated in 129/SvHsd mice
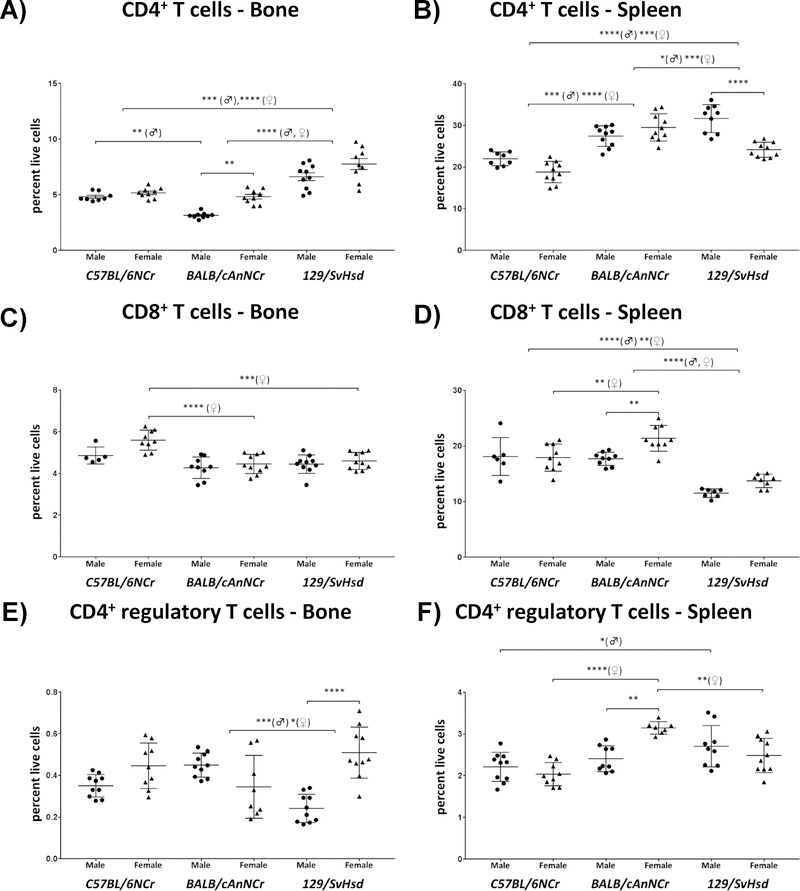
CD4+ T cells are critical players in cell-mediated and humoral immunity and differentiate into specialized CD4+ subtype based on interaction with antigen-MHC complex and resident cytokine level 42. The percentages of CD4+ T cells in BM and spleen ranged from mean values of 3.15% to 7.76% and 18.80% to 31.67%, respectively, in the three murine strains analyzed (Figures 1A, B). In all cases, CD4+ T cells were significantly higher in the spleen as compared to the BM (Figure 1). We observed many strain- and gender-specific variations in CD4+ T cells among all three strains. 129/SvHsd mice exhibited a significantly higher BM CD4+ T cell population in both genders (Figure 1A, p≤0.001♂ and p≤0.0001♀ for C57BL/6NCr; p≤0.0001 for BALB/cAnNCr). In BM, C57BL/6NCr males exhibited a significantly higher CD4+ T cell percentage in comparison to BALB/cAnNCr males (Figure 1A, p≤0.01). As observed in BM, splenic CD4+ T cells in 129/SvHsd mice were also significantly elevated with respect to C57BL/6NCr (Figure 1B, p≤0.0001♂, p≤0.001♀). Splenic CD4+ T cells in male 129/SvHsd mice were significantly higher in comparison to BALB/cAnNCr, but this trend was reversed in female mice (Figure 1B, p≤0.05♂ and p≤0.001♀). Splenic CD4+ T cells were significantly elevated in BALB/cAnNCr in comparison to C57BL/6NCr (Figure 1B, p≤0.001♂ and p≤0.0001♀).
Figure 1: Immune cell distribution of T cells in C57BL/6NCr, BALB/cAnNCr and 129/SvHsd male and female mice.
Cell suspensions from BM and spleen were isolated from 8-week old mice. The cells were stained with CD3 and CD4 antibodies for CD4+ T cells, CD3 and CD8 antibodies for CD8+ T cells, and CD3, CD4, CD25 and FoxP3 antibodies for T-regs. The cells were then subjected to flow cytometry to determine cell-type percentages. (A) CD4+ T cells in BM, (n≥8). (B) CD4+ T cells in the spleen, (n≥8). (C) CD8+ T cells in BM, (n≥5). (D) CD8+ T in spleen, (n≥6). (E) CD4+ T-regs in BM, (n≥8). (F) CD4+ T-regs in spleen, (n≥7). Results, shown as scatter plots, depict average cell percentages (percent of live cells). Error bars denote SEM. Each dot represents the value from a single mouse. (♂) and (♀) represent male and female mice, respectively.*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p≤0.0001.
Bone marrow CD4+ T cells are elevated in female mice irrespective of mouse strains
Bone marrow CD4+ T cells were significantly elevated in females of BALB/cAnNCr (Figure 1A, p≤0.01). Bone marrow CD4+ T cells were also elevated in females of other two strains, however the trend was not significant. Similarly, splenic CD4+ T cells were also higher in BALB/cAnNCr females although the trend was not statistically significant. This trend was reversed for splenic CD4+ T cells for C57BL/6NCr and 129/SvHsd, in which males had a higher percentage of CD4+ T cells in comparison to females (Figure 1B, n.s. for C57BL/6NCr; p≤0.0001 for 129/SvHsd).
CD8+ T cells are elevated in BM of C57BL/6NCr and reduced in spleens of 129/SvHsd mice
The BM represents a specialized niche for development of memory CD8+ T cells whereas spleen is a recognized organ for being abundant in all T cells 5, 6. The percentages of CD8+ T cells in BM and spleen ranged from 4.28% to 5.60% and 11.51% to 21.39% respectively in the three murine strains analyzed (Figures 1C, D). C57BL/6NCr females exhibited a significantly higher baseline BM CD8+ T cell population in comparison to BALB/cAnNCr and 129/SvHsd (Figure 1C, p≤0.0001 and p≤0.001, respectively). 129/SvHsd exhibited a significantly reduced splenic CD8+ T cell in comparison to C57BL/6NCr and BALB/cAnNCr (Figure 1D, p≤0.0001 ♂ and p≤0.01 ♀ in C57BL/6NCr and p≤0.0001 for both genders in BALB/cAnNCr). In contrast to BM, splenic CD8+ T cells in C57BL/6NCr females were significantly reduced in comparison to BALB/cAnNCr females (Figure 1D, p≤0.01). In BALB/cAnNCr, splenic CD8+ T cells were significantly elevated in female in comparison to their male counterparts (Figure 1D, p≤0.01).
Bone marrow CD4+ regulatory T cells exhibit significant gender-dependent variation in all strains
CD4+ regulatory T cells have been suggested to reside in BM sinuses and under homeostatic conditions can be found mainly in thymus, peripheral blood, lymph nodes, and spleen 5, 43. In BM, CD4+ regulatory T were present in the range of 0.24% to 0.51% across three strains (Figure 1E, F). Like CD4+ T cells, the CD4+ Tregs were also elevated in splenic tissue in comparison to BM with a range of 2.04 to 3.15% (Figure 1F). BM CD4+ Tregs were significantly increased in male BALB/cAnNCr with respect to male 129/SvHsd mice; however this trend was reversed among females (Figure 1E, p≤0.001 ♂ and p≤0.05 ♀). We also observed some similarities in strain-specific distribution within different categories of splenic T cells that were analyzed. All three classes of splenic T cells analyzed (CD4+, CD8+ and CD4+ Tregs cells) were significantly reduced in female C57BL/6NCr and 129/SvHsd mice in comparison to female BALB/cAnNCr mice (Figure 1F, p≤0.0001 for C57BL/6NCr and p≤0.01 for 129/SvHsd). Splenic CD4+ Tregs were significantly increased in male 129/SvHsd in comparison to male C57BL/6NCr, a trend that was also observed in splenic-CD4+ T cells (Figure 1B and Figure 1F, p≤0.0001 and p≤0.05 respectively).
Although there were many gender-specific variations in Treg frequency within both organ sites, unlike BM CD4+ T cells, there was no preponderance of one gender over the other (Figures 1E, F). Whereas BM CD4+ Tregs were higher in females of C57BL/6NCr and 129/SvHsd, the trend was reversed for BALB/cAnNCr (Figure 1E, p≤0.0001 for 129/SvHsd). In spleen these trends appeared to be reversed for each case, being significant in BALB/cAnNCr (Figure 1F, p≤0.01). Overall, the differences within various subsets of T cells suggest that even under normal physiological conditions, T cells exhibit significant strain- and gender-dependent variation, which could be an important factor to consider when designing in-vivo experiments. For instance, gender and strain-specific differences in CD8+ T cells might have important consequences on CTL response to either pathogenic or oncogenic stimuli. Similarly, differences in CD4+ Tregs could impact studies where immunosuppression plays a significant role.
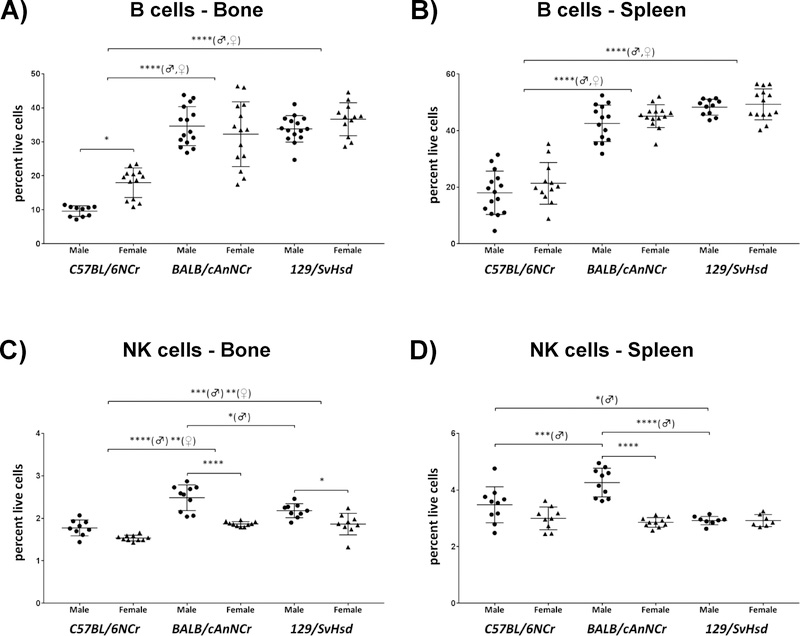
B cells are significantly reduced in C57BL/6NCr mice
B cells can function as plasma cells that secrete antibodies against different antigens; or as memory B cells, which retain immunological memory of the antigen in order to proliferate and differentiate into plasma cells under appropriate stimuli 42. Within all the cell types analyzed, B cells were the most abundant immune cell type in both bone marrow and spleen (Figures 2A, B). Among the mouse strains characterized, B cell percentages in bone marrow and spleen were between 9.59% to 36.65% and 18.01% to 49.30%, respectively (Figures 2A, B). In both organs sites, we observed a significantly reduced B cell percentage in C57BL/6NCr in comparison to the other two strains (Figures 2A, B, p≤0.0001). In BM, C57BL/6NCr cells also exhibited significant gender-specific divergence, as female mice exhibited almost twice the amount of B cells in comparison to males (Figure 2A, p≤0.05).
Figure 2: Immune cell distribution of B cells and NK cells in C57BL/6NCr, BALB/cAnNCr and 129/SvHsd for male and female mice.
Cell suspensions from BM and spleen were isolated from 8-week old mice. The cells were stained with B220 and CD19 antibodies for B cells, and CD3 and NKp46 antibodies for NK cells. The cells were then subjected to flow cytometry to determine cell-type percentages. (A) B cells in BM, (n≥10). (B) B cells in the spleen, (n ≥11). (C) NK cells in BM, (n≥9). (D) NK cells in the spleen, (n≥7). Results are shown as scatter plots depicting average cell percentages (percent of live cells). Error bars denote SEM. Each dot represents the value from a single mouse. (♂) and (♀) represent male and female mice respectively. **p ≤ 0.01, ***p ≤ 0.001, ****p≤0.0001.
NK cells are significantly elevated in male BALB/cAnNCr mice
Natural killer (NK) cells play a critical role in the anti-tumor surveillance, elimination of virally infected cells, and graft versus host disease (GVHD) 44. NK cell percentages in bone marrow and spleen were between 1.54% to 2.49% and 2.86% to 4.26%, respectively (Figures 2C, D). In the BM, NK cells were significantly reduced in C57BL/6NCr (Figure 2C, p≤0.0001♂, p≤0.01♀ for BALB/cAnNCr and p≤0.001♂, p≤0.01♀ for 129/SvHsd). BALB/cAnNCr males had higher NK numbers than 129/SvHsd in the BM (Figure 2C, p≤0.05). In the spleen, male BALB/cAnNCr mice had higher NK numbers than either C57BL/6NCr or 129/SvHsd (Figure 2D, p≤0.001 and p≤0.0001, respectively). Additionally, male C57BL/6NCr had higher splenic NK cells than male 129/SvHsd (Figure 2D, p≤0.05). Examining differences by gender, male BALB/cAnNCr and 129/SvHsd had higher NK cell numbers in the BM (Figure 2C, p≤0.0001 and p≤0.05, respectively), while only male BALB/cAnNCr had significantly higher NK cell numbers compared to female counterparts in the spleen (Figure 2D, p≤0.0001).
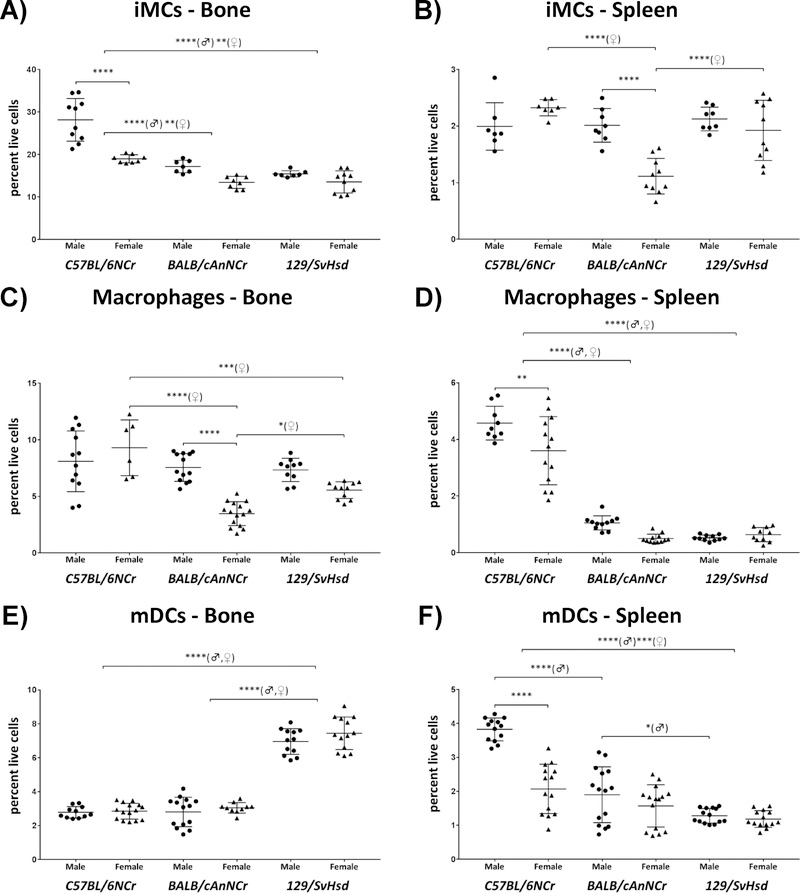
Immature myeloid cells exhibit a male-bias in BM and spleen
Immature myeloid cells (iMCs) exist in the bone marrow as precursors for dendritic cells, macrophages and neutrophils 45. In the three strains that were assessed, iMC percentages in bone marrow and spleen ranged from 13.46% to 28.17% and 1.12% to 2.33%, respectively (Figure 3). BM iMCs were significantly elevated in C57BL/6NCr (Figure 3A, p≤0.0001♂, p≤0.01♀ for both BALB/cAnNCr and 129/SvHsd). BALB/cAnNCr female mice exhibited significantly reduced splenic iMCs in comparison to female C57BL/6NCr and 129/SvHsd mice (Figure 3B, p≤0.0001). In the BM, male C57BL/6NCr mice had significantly higher iMCs than female counterparts (Figure 3A, p≤0.0001). In the spleen, male BALB/cAnNCr mice exhibited greater iMC numbers in comparison to corresponding females (Figure 3B, p≤0.0001).
Figure 3: Immune cell distribution of immature myeloid cells (iMCs), macrophages and myeloid dendritic cells (mDCs) in C57BL/6NCr, BALB/cAnNCr and 129/SvHsd for male and female mice.
Cell suspensions from BM and spleen were isolated from 8-week old mice. The cells were stained with CD11b, F4/80 and CD68 antibodies for macrophages, CD11b and Gr-1 antibodies for iMCs, and CD11b and CD11c antibodies for mDCs. Ly6B was used as negative marker for iMCs. The cells were then subjected to flow cytometry to determine cell-type percentages. (A) iMCs in BM, (n≥7). (B) iMCs in the spleen, (n≥7). (C) Macrophages in BM, (n≥ 6). (D) Macrophages in spleen, (n≥9) (E) mDCs in BM, (n ≥ 10). (F) mDCs in the spleen, (n ≥ 13). Results are shown as scatter plots depicting average cell percentages (percent of live cells). Error bars denote SEM. Each dot represents the value from a single mouse. (♂) and (♀) represent male and female mice, respectively. **p ≤ 0.01, ***p ≤ 0.001, ****p≤0.0001.
Splenic and BM macrophages in C57BL/6NCr females are significantly elevated
Macrophages are phagocytic immune cells residing in different tissues that function as antigen presenting cells (APCs) 42. Macrophages arise from monocytic lineage and differentiate in a tissue-specific manner 42. In our studies, macrophage percentages in bone marrow and spleen were between 3.48% to 9.30% and 0.50% to 4.58%, respectively, in the three murine strains analyzed (Figures 3C & D). We observed many interesting strain- and gender-dependent variations in macrophage population. Within the BM, C57BL/6NCr females had higher macrophage numbers than both BALB/cAnNCr and 129/SvHsd females (Figure 3C, p≤0.0001 and p≤0.001, respectively). BALB/cAnNCr females exhibited significantly reduced BM macrophages than 129/SvHsd females (Figure 3C, p≤0.05). Splenic macrophages were significantly elevated in C57BL/6NCr among both genders in comparison to BALB/cAnNCr and 129/SvHsd (Figure 3D, p≤0.0001). BM macrophages were significantly higher in male BALB/cAnNCr mice with respect to their female counterparts (Figure 3C, p≤0.0001), while the only gender bias noted in the spleen was among was C57BL/6NCr males which exhibited higher splenic-macrophage numbers in comparison to females of the same strain (Figure 3D, p≤0.01).
Myeloid Dendritic Cells are significantly elevated in BM of 129/SvHsd and spleen of C57BL/6NCr mice
Dendritic cells are professional APCs that play key roles in both the innate and adaptive immunity42, 46. Myeloid dendritic cells (mDCs) are specialized subsets of dendritic cells that capture, process, and present antigens on their surface to T cells42, 46. Percentages of mDCs in BM and spleen ranged from 2.78% to 7.45% and 1.19% to 3.83% respectively in the three murine strains analyzed (Figures 3E, F). 129/SvHsd mice exhibited significantly higher mDCs within BM (Figure 3E, p≤0.0001; both strains and in both genders). In contrast, splenic mDCs were significantly higher among C57BL/6NCr in comparison to 129/SvHsd (Figure 3F, p≤0.0001♂, p≤0.001♀). Male C57BL/6NCr also exhibited significantly higher splenic mDCs in comparison to BALB/cAnNCr mice, but in females this effect was not statistically significant among these two strains (Figure 3F, p≤0.0001). Splenic mDCs were significantly elevated in male BALB/cAnNCr mice in comparison to male 129/SvHsd mice, (Figure 3F, p≤0.05). The only gender specific variation noted was a higher splenic mDC population within C57BL/6NCr males (Figure 3F, p≤0.0001).
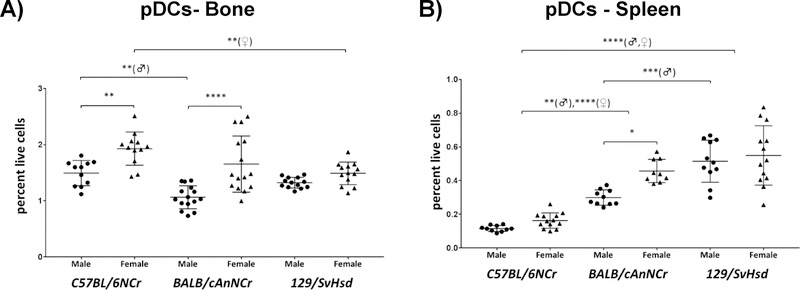
Plasmacytoid dendritic cells exhibit a strong gender difference in bone marrow of all three strains and in C57BL/6NCr splenocytes
Plasmacytoid dendritic cells (pDCs) are specialized dendritic cells that perform a critical role in both innate and adaptive immunity 42, 47. Found in circulating blood and peripheral lymphoid organs, pDCs secrete IFNs, and mediate anti-viral immunity and anti-inflammatory responses42, 47. In our studies, pDC percentages in bone marrow and spleen ranged from 1.06% to 1.93% and 0.12% to 0.55% respectively in the three murine strains analyzed (Figure 4A, B). Within the BM, C57BL/6NCr male mice exhibited a significantly higher pDC population in comparison to BALB/cAnNCr male mice (Figure 4A, p≤0.01). pDCs were significantly elevated in C57BL/6NCr female mice in comparison to 129/SvHsd female mice (Figure 4A, p≤0.01). pDCs were also significantly elevated in C57BL/6NCr male mice in comparison to BALB/cAnNCr male mice (Figure 4A, p≤0.01). On the other hand, C57BL/6NCr mice had a significantly reduced baseline percentage of pDCs within the spleen (Figure 4B, p≤0.01♂, p≤0.0001♀ for BALB/cAnNCr and p≤0.0001 in both genders for 129/SvHsd). Splenic pDCs were significantly elevated in 129/SvHsd female mice in comparison to BALB/cAnNCr female mice (Figure 4B, p≤0.001). There was clear gender-specific difference in pDCs within the BM, with females from C57BL/6NCr and BALB/cAnNCr strains exhibiting a significantly higher pDC population in comparison to the corresponding males (Figure 4A, p≤0.01 and p≤0.0001 respectively). This trend was observed to be significant in the spleen for BALB/cAnNCr, whose females were higher than male counterparts (Figure 4B, p≤0.05).
Figure 4: Immune cell distribution of plasmacytoid dendritic cells in C57BL/6NCr, BALB/cAnNCr and 129/SvHsd male and female mice.
Cell suspensions from BM and spleen were isolated from 8-week old mice. The cells were stained with CD11c, B220 and Siglec H antibodies. CD11b antibody was included as a negative marker for pDCs. The cells were then subjected to flow cytometry to determine cell-type percentages. (A) pDCs in BM, (n≥11). (B) pDCs in the spleen, (n ≥9). Results are shown as scatter plots depicting average cell percentages (percent of live cells). Error bars denote SEM. Each dot represents the value from a single mouse. (♂) and (♀) represent male and female mice respectively.*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p≤0.0001.
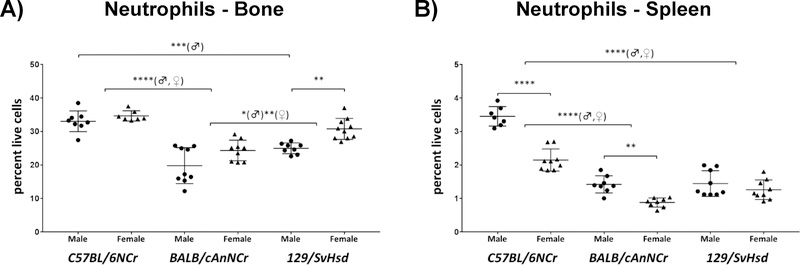
Neutrophils are elevated in C57BL/6NCr and exhibit gender-specific variation in both bone marrow and spleen
Neutrophils are the most abundant granulocytes, comprising 40% to 75% of peripheral blood immune cells42, 48. Formed within the bone-marrow, neutrophils play critical roles in phagocytosis of invading microbes42, 48. Neutrophil percentages were between 19.81% to 34.67% and 0.88% to 3.45 % in BM and spleen, respectively, in the three murine strains analyzed (Figure 5A, B). In BM, C57BL/6NCr male mice exhibited a significantly higher neutrophil population in comparison to 129/SvHsd (Figure 5A, p≤0.001). This trend was observed in both genders when comparing C57BL/6NCr and BALB/cAnNCr (Figure 5A, p≤0.0001). Similarly splenic neutrophils of C57BL/6NCr were significantly higher than both BALB/cAnNCr and 129/SvHsd (Figure 5B, p≤0.0001). 129/SvHsd exhibited a significantly higher BM neutrophil percentage in comparison to BALB/cAnNCr strain (Figure 5A, p≤0.05♂, p≤0.01♀). Within the BM, gender bias was observed among 129/SvHsd, with females higher than males (Figure 5A, p≤0.01). Within the spleen, female C57BL/6NCr and BALB/cAnNCr mice had higher neutrophils in comparison to males (Figure 5B, p≤0.0001 and p≤0.01, respectively).
Figure 5: Immune cell distribution of neutrophils in C57BL/6NCr, BALB/cAnNCr and 129/SvHsd for male and female mice.
Cell suspensions from BM and spleen were isolated from 8-week old mice. The cells were stained with CD11b, Gr-1 and Ly6b antibody. F4/80 antibody was included as a negative marker for neutrophils. The cells were then subjected to flow cytometry to determine cell-type percentages. (A) Neutrophils in BM, (n≥7). (B) Neutrophils in spleen, (n ≥7). Results are shown as scatter plots depicting average cell percentages (percent of live cells). Error bars denote SEM. Each dot represents the value from a single mouse. (♂) and (♀) represent male and female mice respectively.*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p≤0.0001.
DISCUSSION
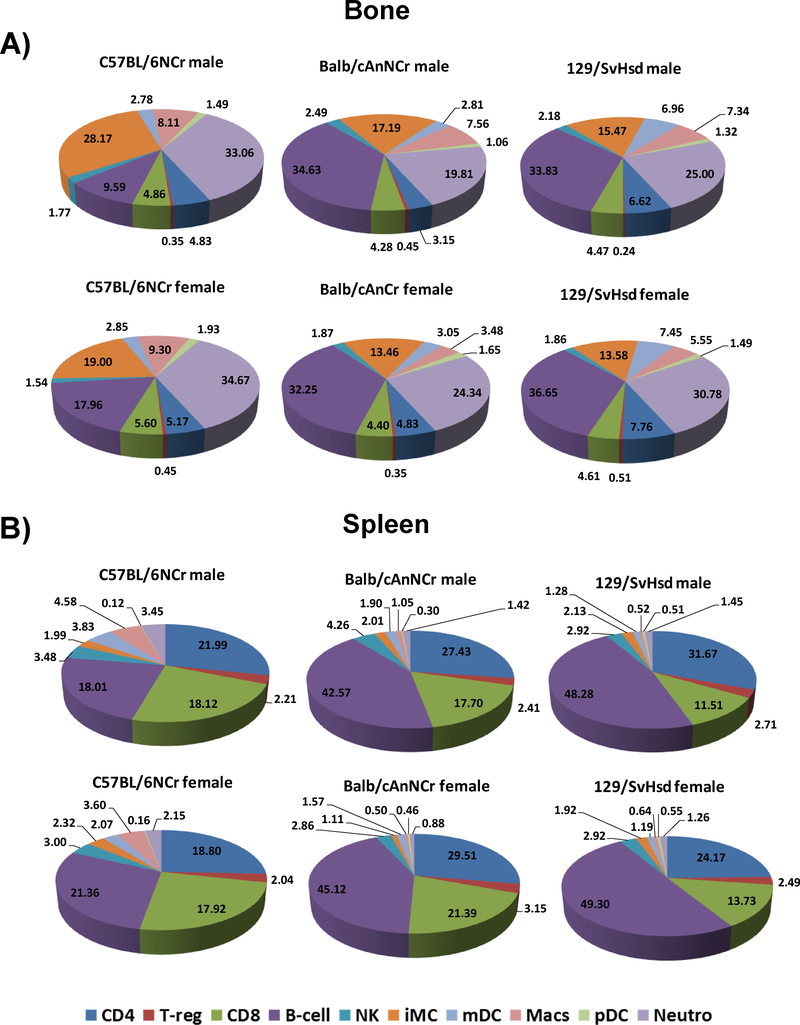
The methodology of conducting immunophenotypic analysis of different cell populations can vary considerably from one study to another based on the endpoints and the goals of the study24, 29, 31, 32. For instance, different studies may report immune cell percentages in distinct ways based on normalization strategies and gating methods (e.g. percent of total lymphocytes, percent of total T cells, percent of total CD4+ T cells, absolute T cell number etc.), which can result in substantial differences in the interpretation of the results even while evaluating the same cell type 31, 32. Importantly, although some studies have determined immune cell frequencies in peripheral blood, only minimal inference is gleaned for the expected immune profile in bone marrow and spleen from such studies12–15. Bone marrow and spleen serve as major reservoirs of immune cells in mice and maintain immune homeostasis in peripheral tissue 49. As observed in this detailed study, a summary of immune cell distribution in BM and spleen of C57BL/6NCr, BALB/cAnNCr and 129/SvHsd mice, indicates that this homeostasis varies significantly between the commonly used in vivo mouse models (Figure 6; 50, 51). In the absence of an organ-specific baseline immune profile in normal mice, a comparative analysis within different strains and/or gender following experimental stimuli can be challenging, and may impose inherent bias while extrapolating experimental data. Therefore, there is a pressing need for unified studies cataloguing baseline immune cell distribution in different strains of mice 31.
Figure 6: Strain and gender-specific immune cell distribution of critical cell types in three mouse strains commonly used in pre-clinical research.
(A) Pie charts representing the frequency of CD4+ and CD8+ T cells, Tregs, B cells, NK cells, iMCs, pDCs, mDCs, macrophages, and neutrophils in BM cells for C57BL/6NCr, BALB/cAnNCr, and 129/SvHsd mice. The upper three panels represent distribution in female mice and lower panels depict similar distribution in male mice. (B) Strain and gender-specific immune cell distribution for CD4+ and CD8+ T cells, Tregs, B cells, NK cells, iMCs, pDCs, mDCs, macrophages, and neutrophils in spleens of C57BL/6NCr, BALB/cAnNCr, and 129/SvHsd mice. The upper and lower three panels represent distribution in female and male mice, respectively.
From the results of the present study, it is encouraging to find that overall immune cell percentages observed by us in BM and spleen was in close agreement to previously published murine and human studies examining other organs 12–15, 25–27, 45, 52–55. Our comprehensive analysis reveals many interesting findings. For instance total neutrophils and splenic macrophages are significantly higher in C57BL/6NCr; whereas total B-cells are lower, compared to BALB/cAnNCr and 129/SvHsd mice. Similarly, the significantly higher number of mDCs in BM from 129/SvHsd is also apparent. There are many critical trends that become apparent upon careful examination of immune cell subtypes through the present study. The identified strain-specific variation, both in CD4+ and CD8+ T cells underscores the importance of this difference when using different strains and gender of mice in preclinical studies mimicking human diseases, including cancer, which responds differently based on the profile of the adaptive immune system 50, 51. Notably, C57BL/6NCr and BALB/cAnNCr mice, which exhibited strain- and gender-specific differences in T-cell profile, are recognized as prototypical Th1- and Th2 responders, respectively 56. These strains are known to have distinct differences in sensitivity to pathophysiological challenge and kinetics of tumor growth 56–61. Given the observed differences in T cell profile observed by us across these strains, it can be surmised that mice strains with differing baseline T cell profiles would respond differently in pre-clinical evaluation of new immunotherapeutic drugs 50, 51, 57, 60.
Many in vivo murine studies are often conducted in female mice due to ease of handling 62. Due to inherent genetic characteristics favoring tumor susceptibility, BALB/cAnNCr mice are frequently employed in cancer related studies 36, 37. We found that within BALB/cAnNCr, female mice had significantly elevated CD8+ T cells. This higher baseline of CD8+ T cells noted in female mice could skew observations related to tumor kinetics, leading to contrasting experimental results dependent on gender. We also observed considerable gender-specific differences in BM CD4+ Tregs. Th2 cytokines in bronchoalveolar lavage fluid are significantly elevated in females of certain mouse strains upon ovalbumin challenge 63. CD4+ Tregs, which are recognized as suppressors of inflammation and critical mediators in airway hyper-responsiveness, exhibited significant gender dependent difference in this study, suggesting that baseline, gender-variation in Tregs may skew the results in murine asthma studies 64. Overall, we observed both strain and gender-specific differences in baseline T cell population, both of which can skew experimental results of in-vivo pathophysiological studies and lead to discordance within clinical results61.
We identified that C57BL/6NCr exhibited a dramatically reduced B cell percentage with respect to the other two strains. Interestingly, our results demonstrate B-cells to be elevated in BALB/cAnNCr (Th2 responder) compared to C57BL/6NCr (Th1 responder). In this regard, it is noteworthy that a study documented that Th2 responders preferentially activate B cells 65. Studies have demonstrated that BALB/cAnNCr mice exhibit a higher IgG titer than C57BL/6NCr following infection with T. cruzi 66. Strain-specific benchmarking of B cell percentage in healthy organisms could prove useful for selecting appropriate murine models to study lymphomas and autoimmune diseases where the B cell population is significantly altered67. We also noticed both inter-strain and intra-strain gender differences in NK cells. In light of these observations, it is notable that different strains of inbred mice, including C57BL/6NCr and BALB/cAnNCr, exhibit marked differences in susceptibility to MCMV infection based on their NK cell profile 68, 69.
We observed distinct gender bias in BALB/cAnNCr, with males displaying a higher percentage of iMCs both in BM and spleen. In fact, within the BM, the males were found to have higher iMCs in comparison to their female counterparts. Under the influence of tumor derived trophic factors such TGF- β, IL-1β, IL-6, IL-10 and VEGF, CD11b+Gr1+ cells lose their capacity to differentiate and inhibit the function of T cells, dendritic cells, macrophages, and natural killer (NK) cells, thereby creating an immunosuppressive pro-tumorigenic environment 45. It can be inferred that a baseline difference in iMCs impacts general immune-surveillance and is a critical factor mediating differences in tumor kinetics among mouse strains 70. It was recently shown that iMCs directly contribute to skin tumor development by recruiting IL-17–producing CD4+ T cells71. Differences in baseline iMC population could in principle also impact MDSC profile upon pathological challenge and result in varying immunosuppressive characteristics based on gender and strain of the mice70–72. In fact, genetic background of C57BL/6NCr and BALB/cAnNCr defines the levels of MDSCs and subsequent immunosuppressive effect following parasitic infections72, 73. Similarly, our findings showing gender bias within the same mouse strain suggest the need to consider baseline iMCs prior to experiments that may be affected by immunosuppression.
There were also significant strain-specific differences in splenic macrophages, with C57BL/6NCr exhibiting a higher frequency when compared to the other strains. Murine macrophage polarization and activation in response to pathogen challenge varies dramatically based on strain74–76. Similarly, the strain and gender differences within myeloid dendritic cells observed by us could impact susceptibility from infectious agents such as Brucella and Paracoccidioides 77, 78. Antigen presentation by pDC is known to induce a context-dependent regulation of CD4+ T cell activation and B-cell growth and differentiation79, 80. Interestingly, we observed that splenic CD4+ T cells, pDCs and B cell populations were significantly reduced in C57BL/6NCr mice. Furthermore, BM CD4+ T cells, CD8+ T-cells and pDCs were elevated in females of all strains.
C57BL/6NCr mice exhibited significantly higher neutrophils with respect to the other two strains. Studies indicate sensitivity of C57BL/6NCr and BALB/cAnNCr strains to infections correlate with neutrophil activity81, 82. Given that these mice strains are frequently employed to assess changes in infectious burden upon experimental stimuli, a baseline evaluation of neutrophil content in these strains is critical 9. Similarly, in light of many studies that have reported strain and gender discrepancies in clinical asthma studies, considering the gender in such murine studies is also critical in evaluating neutrophil recruitment 58, 63, 83. Results of this study indicate that it is important to evaluate a baseline immune profile of mice before embarking upon on in vivo studies as frequency of immune cell types may skew results. A rational choice of mouse strain and gender, based on immune specificity profile should provide better experimental readouts. While further information can be gleaned from other strains, organ sites (lymph nodes, pulmonary sites, etc.), cytokine profiles and immune cell subtypes (Th1 vs. Th2, M1 vs. M2, etc.), this study provides an important point-of-comparison resource for studies utilizing these mice strains by highlighting critical strain- and gender-specific trends that could influence experimental results.
Supplementary Material
ACKNOWLEDGEMENT
Financial support from the National Institute of Health research grants: CA184770 and AR060948, and the Department of Defense grant: PR141945 is greatly acknowledged. We sincerely thank ENVIGO for providing mice for the study.
REFERENCES
- 1.Doulatov S, Notta F, Laurenti E, et al. Hematopoiesis: a human perspective. Cell Stem Cell 2012;10(2):120–136. [DOI] [PubMed] [Google Scholar]
- 2.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol 2008;9(2):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plas DR, Rathmell JC, Thompson CB. Homeostatic control of lymphocyte survival: potential origins and implications. Nat Immunol 2002;3(6):515–521. [DOI] [PubMed] [Google Scholar]
- 4.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 2002;109 Suppl:S97–107. [DOI] [PubMed] [Google Scholar]
- 5.Zhao E, Xu H, Wang L, et al. Bone marrow and the control of immunity. Cell Mol Immunol 2012;9(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity 2013;39(5):806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hensel JA, Khattar V, Ashton R, et al. Location of tumor affects local and distant immune cell type and number. Immun Inflamm Dis 2017;5(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juul S, Pliskin JS, Fineberg HV. Variation and information in white blood cell differential counts. Med Decis Making 1984;4(1):69–80. [DOI] [PubMed] [Google Scholar]
- 9.Sellers RS, Clifford CB, Treuting PM, et al. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol 2012;49(1):32–43. [DOI] [PubMed] [Google Scholar]
- 10.Bogue MA, Grubb SC. The Mouse Phenome Project. Genetica 2004;122(1):71–74. [DOI] [PubMed] [Google Scholar]
- 11.Grubb SC, Churchill GA, Bogue MA. A collaborative database of inbred mouse strain characteristics. Bioinformatics 2004;20(16):2857–2859. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Harrison DE. Quantitative trait loci regulating relative lymphocyte proportions in mouse peripheral blood. Blood 2002;99(2):561–566. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Flurkey K, Harrison DE. A reduced peripheral blood CD4(+) lymphocyte proportion is a consistent ageing phenotype. Mech Ageing Dev 2002;123(2–3):145–153. [DOI] [PubMed] [Google Scholar]
- 14.Peters LL, Cheever EM, Ellis HR, et al. Large-scale, high-throughput screening for coagulation and hematologic phenotypes in mice. Physiol Genomics 2002;11(3):185–193. [DOI] [PubMed] [Google Scholar]
- 15.Petkova SB, Yuan R, Tsaih SW, et al. Genetic influence on immune phenotype revealed strain-specific variations in peripheral blood lineages. Physiol Genomics 2008;34(3):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Geijn GJ, van Rees V, van Pul-Bom N, et al. Leukoflow: multiparameter extended white blood cell differentiation for routine analysis by flow cytometry. Cytometry A 2011;79(9):694–706. [DOI] [PubMed] [Google Scholar]
- 17.Zitvogel L, Pitt JM, Daillere R, et al. Mouse models in oncoimmunology. Nat Rev Cancer 2016;16(12):759–773. [DOI] [PubMed] [Google Scholar]
- 18.Sawant A, Schafer CC, Ponnazhagan S, et al. The dual targeting of immunosuppressive cells and oxidants promotes effector and memory T-cell functions against lung cancer. Oncoimmunology 2014;3(1):e27401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawant AC, Adhikari P, Narra SR, et al. Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol J 2014;21(5):500–508. [DOI] [PubMed] [Google Scholar]
- 20.Schafer CC, Wang Y, Hough KP, et al. Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment. Oncotarget 2016;7(46):75407–75424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson-Casey JL, Deshane JS, Ryan AJ, et al. Macrophage Akt1 Kinase-Mediated Mitophagy Modulates Apoptosis Resistance and Pulmonary Fibrosis. Immunity 2016;44(3):582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawant A, Deshane J, Jules J, et al. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res 2013;73(2):672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy S, Feduska JM, Sawant A, et al. Immature myeloid cells are critical for enhancing bone fracture healing through angiogenic cascade. Bone 2016;93:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidance Development Review C, Working Group for Clinical Studies of Cancer I, Working Group for Effector Cell T, et al. 2015 Guidance on cancer immunotherapy development in early-phase clinical studies. Cancer Sci 2015;106(12):1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elderman M, van Beek A, Brandsma E, et al. Sex impacts Th1 cells, Tregs, and DCs in both intestinal and systemic immunity in a mouse strain and location-dependent manner. Biol Sex Differ 2016;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 27.Pinchuk LM, Filipov NM. Differential effects of age on circulating and splenic leukocyte populations in C57BL/6 and BALB/c male mice. Immun Ageing 2008;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015;350(6264):1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li QX, Feuer G, Ouyang X, et al. Experimental animal modeling for immuno-oncology. Pharmacol Ther 2017;173:34–46. [DOI] [PubMed] [Google Scholar]
- 30.Abdullah M, Chai PS, Chong MY, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol 2012;272(2):214–219. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Huang A, Sun J, et al. Inference of immune cell composition on the expression profiles of mouse tissue. Sci Rep 2017;7:40508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahne F, Khodabakhshi AH, Bashashati A, et al. Per-channel basis normalization methods for flow cytometry data. Cytometry A 2010;77(2):121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finak G, Langweiler M, Jaimes M, et al. Standardizing Flow Cytometry Immunophenotyping Analysis from the Human ImmunoPhenotyping Consortium. Sci Rep 2016;6:20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bashashati A, Brinkman RR. A survey of flow cytometry data analysis methods. Adv Bioinformatics 2009:584603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostrand-Rosenberg S Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr Opin Immunol 2004;16(2):143–150. [DOI] [PubMed] [Google Scholar]
- 36.Clapp NK, Tyndall RL, Otten JA. Differences in tumor types and organ susceptibility in BALB-c and RF mice following dimethylnitrosamine and diethylnitrosamine. Cancer Res 1971;31(2):196–198. [PubMed] [Google Scholar]
- 37.Ullrich RL. Tumor induction in BALB/c female mice after fission neutron or gamma irradiation. Radiat Res 1983;93(3):506–515. [PubMed] [Google Scholar]
- 38.Mekada K, Abe K, Murakami A, et al. Genetic differences among C57BL/6 substrains. Exp Anim 2009;58(2):141–149. [DOI] [PubMed] [Google Scholar]
- 39.Jiang LI, Nadeau JH. 129/Sv mice--a model system for studying germ cell biology and testicular cancer. Mammalian genome : official journal of the International Mammalian Genome Society 2001;12(2):89–94. [DOI] [PubMed] [Google Scholar]
- 40.Clapcote SJ, Roder JC. Deletion polymorphism of Disc1 is common to all 129 mouse substrains: implications for gene-targeting studies of brain function. Genetics 2006;173(4):2407–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodgers RJ, Augar R, Berryman N, et al. Atypical anxiolytic-like response to naloxone in benzodiazepine-resistant 129S2/SvHsd mice: role of opioid receptor subtypes. Psychopharmacology 2006;187(3):345–355. [DOI] [PubMed] [Google Scholar]
- 42.Owen JA, Punt J, Stranford SA, et al. Kuby immunology. New York: :: W.H. Freeman, 2013. [Google Scholar]
- 43.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood 2006;108(2):426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vivier E, Tomasello E, Baratin M, et al. Functions of natural killer cells. Nat Immunol 2008;9(5):503–510. [DOI] [PubMed] [Google Scholar]
- 45.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merad M, Sathe P, Helft J, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013;31:563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol 2005;79(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13(3):159–175. [DOI] [PubMed] [Google Scholar]
- 49.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology 2005;116(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ngiow SF, Young A, Jacquelot N, et al. A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1. Cancer Res 2015;75(18):3800–3811. [DOI] [PubMed] [Google Scholar]
- 51.Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017;114(19):4993–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hackstein H, Wachtendorf A, Kranz S, et al. Heterogeneity of respiratory dendritic cell subsets and lymphocyte populations in inbred mouse strains. Respir Res 2012;13:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velasquez-Lopera MM, Correa LA, Garcia LF. Human spleen contains different subsets of dendritic cells and regulatory T lymphocytes. Clin Exp Immunol 2008;154(1):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langeveld M, Gamadia LE, ten Berge IJ. T-lymphocyte subset distribution in human spleen. Eur J Clin Invest 2006;36(4):250–256. [DOI] [PubMed] [Google Scholar]
- 55.Asselin-Paturel C, Brizard G, Pin JJ, et al. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol 2003;171(12):6466–6477. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe H, Numata K, Ito T, et al. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 2004;22(5):460–466. [DOI] [PubMed] [Google Scholar]
- 57.Rivera J, Tessarollo L. Genetic background and the dilemma of translating mouse studies to humans. Immunity 2008;28(1):1–4. [DOI] [PubMed] [Google Scholar]
- 58.Gueders MM, Paulissen G, Crahay C, et al. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res 2009;58(12):845–854. [DOI] [PubMed] [Google Scholar]
- 59.Schulte S, Sukhova GK, Libby P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am J Pathol 2008;172(6):1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lechner MG, Karimi SS, Barry-Holson K, et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother 2013;36(9):477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jovicic N, Jeftic I, Jovanovic I, et al. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PLoS One 2015;10(7):e0134089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Florez-Vargas O, Brass A, Karystianis G, et al. Bias in the reporting of sex and age in biomedical research on mouse models. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito C, Okuyama-Dobashi K, Miyasaka T, et al. CD8+ T Cells Mediate Female-Dominant IL-4 Production and Airway Inflammation in Allergic Asthma. PLoS One 2015;10(10):e0140808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity 2009;31(3):438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rothermel AL, Gilbert KM, Weigle WO. Differential abilities of Th1 and Th2 to induce polyclonal B cell proliferation. Cell Immunol 1991;135(1):1–15. [DOI] [PubMed] [Google Scholar]
- 66.Bryan MA, Guyach SE, Norris KA. Specific humoral immunity versus polyclonal B cell activation in Trypanosoma cruzi infection of susceptible and resistant mice. PLoS Negl Trop Dis 2010;4(7):e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morbach H, Eichhorn EM, Liese JG, et al. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol 2010;162(2):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pyzik M, Kielczewska A, Vidal SM. NK cell receptors and their MHC class I ligands in host response to cytomegalovirus: insights from the mouse genome. Semin Immunol 2008;20(6):331–342. [DOI] [PubMed] [Google Scholar]
- 69.Lee SH, Girard S, Macina D, et al. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat Genet 2001;28(1):42–45. [DOI] [PubMed] [Google Scholar]
- 70.Ouzounova M, Lee E, Piranlioglu R, et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun 2017;8:14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ortiz ML, Kumar V, Martner A, et al. Immature myeloid cells directly contribute to skin tumor development by recruiting IL-17-producing CD4+ T cells. J Exp Med 2015;212(3):351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmid M, Zimara N, Wege AK, et al. Myeloid-derived suppressor cell functionality and interaction with Leishmania major parasites differ in C57BL/6 and BALB/c mice. Eur J Immunol 2014;44(11):3295–3306. [DOI] [PubMed] [Google Scholar]
- 73.Van Ginderachter JA, Beschin A, De Baetselier P, et al. Myeloid-derived suppressor cells in parasitic infections. Eur J Immunol 2010;40(11):2976–2985. [DOI] [PubMed] [Google Scholar]
- 74.Santos JL, Andrade AA, Dias AA, et al. Differential sensitivity of C57BL/6 (M-1) and BALB/c (M-2) macrophages to the stimuli of IFN-gamma/LPS for the production of NO: correlation with iNOS mRNA and protein expression. J Interferon Cytokine Res 2006;26(9):682–688. [DOI] [PubMed] [Google Scholar]
- 75.Depke M, Breitbach K, Dinh Hoang Dang K, et al. Bone marrow-derived macrophages from BALB/c and C57BL/6 mice fundamentally differ in their respiratory chain complex proteins, lysosomal enzymes and components of antioxidant stress systems. J Proteomics 2014;103:72–86. [DOI] [PubMed] [Google Scholar]
- 76.Bertolini TB, de Souza AI, Gembre AF, et al. Genetic background affects the expansion of macrophage subsets in the lungs of Mycobacterium tuberculosis-infected hosts. Immunology 2016;148(1):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Billard E, Cazevieille C, Dornand J, et al. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis, B. abortus, and B. melitensis. Infect Immun 2005;73(12):8418–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pina A, de Araujo EF, Felonato M, et al. Myeloid dendritic cells (DCs) of mice susceptible to paracoccidioidomycosis suppress T cell responses whereas myeloid and plasmacytoid DCs from resistant mice induce effector and regulatory T cells. Infect Immun 2013;81(4):1064–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann N Y Acad Sci 2010;1183:89–103. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H, Gregorio JD, Iwahori T, et al. A distinct subset of plasmacytoid dendritic cells induces activation and differentiation of B and T lymphocytes. Proc Natl Acad Sci U S A 2017;114(8):1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sousa LM, Carneiro MB, Resende ME, et al. Neutrophils have a protective role during early stages of Leishmania amazonensis infection in BALB/c mice. Parasite Immunol 2014;36(1):13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen L, Watanabe T, Watanabe H, et al. Neutrophil depletion exacerbates experimental Chagas’ disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur J Immunol 2001;31(1):265–275. [DOI] [PubMed] [Google Scholar]
- 83.Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax 2012;67(7):625–631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.