Abstract
Background and objective:
Obesity produces restrictive effects on lung function. We previously reported that obese patients with asthma exhibit a propensity toward small airway closure during methacholine challenge which improved with weight loss. We hypothesized that increased abdominal adiposity, a key contributor to the restrictive effects of obesity on the lung, mediates this response. This study investigates the effect of body mass index (BMI) versus waist circumference (WC) on spirometric lung function, sensitivity to airway narrowing and closure, and airway closure during bronchoconstriction in patients with asthma.
Methods:
Participants underwent spirometry and methacholine challenge. Sensitivity to airway closure and narrowing were assessed from the dose response slopes of the forced vital capacity (FVC), and the ratio of forced expiratory volume in one second (FEV1) to FVC, respectively. Airway closure during bronchoconstriction (closing index) was computed as the percent reduction in FVC divided by the percent reduction in FEV1 at maximal bronchoconstriction.
Results:
A total of 116 asthmatic patients (56 obese) underwent methacholine challenge. Spirometric lung function was inversely related to WC (p <0.05), rather than BMI. Closing index increased significantly during bronchoconstriction in obese patients and was related to increasing BMI (p =0.01), but not WC. Sensitivity to airway closure and narrowing were not associated with BMI or WC.
Conclusion:
Although WC is associated with restrictive effects on baseline lung function, increased BMI, rather than WC, predisposes to airway closure during bronchoconstriction. These findings suggest that obesity predisposes to airway closure during bronchoconstriction through mechanisms other than simple mass loading.
Keywords: Airway closure, airway hyperresponsiveness, asthma, obesity, spirometry
Summary at a Glance:
The effect of BMI and waist circumference (WC) on spirometric lung function and airway closure during induced bronchoconstriction was assessed in patients with asthma. WC, but not BMI, was associated with restrictive effects on baseline spirometry. However, during bronchoconstriction, airway closure was associated with increased BMI, rather than WC.
INTRODUCTION
Obesity and asthma have both increased in incidence during the past three decades. Although not conventionally thought of as sharing a common pathophysiology, evidence is mounting that they are associated in some way. A meta-analysis of several prospective studies revealed that a high body mass index (BMI) is associated with increased odds of incident asthma.1 Furthermore, obese individuals with asthma typically present with more severe symptoms than their lean counterparts,2 and do not respond as well to controller medication.3–6 While the epidemiology is striking, the mechanisms linking obesity and asthma are less clear.
One plausible explanation for the relationship between these two conditions is mechanical compression of the respiratory system by excess adipose tissue. Excessive accumulation of fat in the thorax and abdomen substantially alters the mechanics of the lungs and chest wall.7–10 Consequently, the operating volume of the lungs is reduced;11 this leads to airway narrowing and closure,12–14 and airway hyperresponsiveness (AHR).15
Although BMI is a simple and convenient method for classifying the severity of obesity, it does not distinguish between central (abdominal) and peripheral (hips and thighs) adiposity. It also does not account for the pattern of distribution of visceral versus subcutaneous fat. This distinction is important because central obesity—which can be assessed by waist circumference (WC)16—likely has a direct mechanical effect on pulmonary function. Indeed, central obesity is a marker of visceral adipose tissue,17 and is a critical determinant of a restrictive (rather than obstructive) spirometric pattern.16, 18, 19 On the other hand, general obesity is typically associated with a chronic state of low-grade inflammation,20 and this likely contributes to increased airway wall thickness,21 increased baseline airway tone,22 and increased vascular congestion of the airway wall.23
We previously reported that obese individuals with asthma demonstrate an increased tendency toward small airway closure during bronchial challenge which improved with weight loss, particularly in patients with late-onset disease.12, 24 We hypothesized that this was related to the restrictive effects of obesity on the lung. Here, we investigate the effect of BMI versus WC on baseline spirometric lung function, sensitivity to airway closure and narrowing, and airway closure during induced bronchoconstriction in a diverse population of adults with asthma. We hypothesized that if obesity reduced lung function due to mass loading, then increased WC would be associated with increased sensitivity to airway closure and greater degree of airway closure during bronchoconstriction.
METHODS
Study design
The current study involves data from the Study of Asthma and Nasal Steroids (STAN), a multicenter, randomized, placebo-controlled, double-blind trial conducted by the American Lung Association—Airways Clinical Research Centers Network (NCT01118312 at ClinicalTrials.gov).25 Full details of the STAN trial have been described elsewhere.25 Briefly, this 24-week trial investigated whether treatment of chronic sinonasal disease with intranasal corticosteroids improved asthma control in poorly controlled adults and children with asthma. As part of the initial screening for the study, participants underwent an extensive evaluation of the severity of their asthma symptoms and control. Assessments of spirometric lung function and airway hyperresponsiveness to methacholine (AHR) were performed. The study was approved by the institutional review board at each of the 19 centers, and written informed consent obtained from all participants.
Inclusion and exclusion criteria
The current study was limited to adults aged 18 years and older with evidence of AHR. All participants had a physician diagnosis of asthma. Other inclusion criteria included the presence of chronic sinonasal disease defined by a mean score ≥ 1 on the Sinonasal Questionnaire (SNQ),26 and poorly controlled asthma defined by a score of ≤ 19 on the Asthma Control Test (ACT).27 Participants with > 10 pack year smoking history were excluded from the study. Also, patients using systemic or nasal steroids, and patients with other significant co-morbidities were excluded.
Questionnaires
Participants were asked to complete the ACT,27 and the Asthma Symptom Utility Index28 for assessment of their asthma control, and the severity of their asthma symptoms, respectively. Participants were also asked to complete the SNQ to confirm the presence of chronic sinonasal disease.26
Anthropometric measurements
Weight and height were measured with a calibrated scale and stadiometer and BMI calculated. WC was measured as the circumferential distance around the midpoint between the lowest rib and the iliac crest. Hip circumference was measured at the level of the greater trochanters and used to calculate waist-to-hip ratio (WHR). BMI was taken as a global measure of adiposity, while WC was taken as an index of central obesity.29, 30
Assessment of lung function and airway hyperresponsiveness
Spirometric evaluation of lung function and AHR to methacholine were conducted (Koko, Ferraris Respiratory Inc, Louisville, CO) according to the American Thoracic Society guidelines.31, 32 AHR was calculated using the dose-response slope (DRS) for forced expiratory volume in one second (FEV1), as the percent reduction in FEV1 at the end of the challenge divided by the cumulative methacholine dose in μmol.33, 34 A reduction > 4.4% in the FEV1/μmol ratio (20% decrease in FEV1 at < 16 mg/mL) was considered a positive response and used as evidence of AHR.12
Sensitivity to airway narrowing was computed as the percent reduction in the ratio of FEV1 to forced vital capacity (FVC) at the end of the challenge divided by the highest dose of methacholine, which is the DRS for FEV1/FVC ratio. Sensitivity to airway closure was computed using the DRS for FVC, as the percent reduction in FVC at the end of the challenge divided by the highest dose of methacholine. The closing index, a measure of the overall change in FEV1 attributed to airway closure, was computed as the percent reduction in FVC divided by the percent reduction in FEV1 at maximal bronchoconstriction.
Statistical analysis
Participants were divided into BMI quartiles as follows: lean (BMI 18.5–24.9kg/m2), overweight (BMI 25–29.9kg/m2), obese (BMI 30 −39.9kg/m2) and severely obese (BMI > 40kg/m2). Participants were also grouped into 4 quartiles according to their WC. Demographic data were summarized with descriptive statistics. Continuous variables were compared using analysis of variance, with log transformation for non-normally distributed variables, while proportions were compared using χ2 analysis. Data from the obese group were adjusted for age of asthma onset. We used multiple regression analyses to determine the effects of both BMI and WC on lung function parameters while controlling for race, gender and age. All analyses were performed using STATA 13.0 (StataCorp LLC, College Station TX). P-values < 0.05 were considered statistically significant.
RESULTS
Demographic data and baseline characteristics of study participants
The current study includes 116 adult participants who completed methacholine challenge testing. Many participants in the main clinical trial did not perform methacholine challenge because their FEV1 was lower than 70% predicted. Thirty-one percent of overweight and 34% of obese participants had an FEV1 < 70%, whereas only 19% of lean participants had a low FEV1 (χ2 p = 0.04).
Table 1 presents baseline demographics of the study participants by BMI class. There was a significant difference in race distribution, with a lower proportion of Caucasians in the higher BMI categories. Spirometric indices were not significantly different between the BMI groups, but peak flow rates appeared to be lower in those with BMI > 40 kg/m2. The provocative concentration of methacholine that induced a 20% fall in FEV1, as well as asthma symptoms and asthma control, were similar across BMI categories. There were no significant gender differences in BMI, age of asthma onset, asthma control, or asthma severity (not shown).
Table 1:
Baseline Demographics of Study Population by BMI group
| BMI (kg/m2) | < 25 | 25–29.9 | 30–39.9 | > 40 | p |
|---|---|---|---|---|---|
| n (female) | 37 (24) | 23 (14) | 39 (29) | 17 (13) | 0.58 |
| Age (years) | 37.4±15.5 | 36.8±11.8 | 40.1±14.2 | 39.6±12.4 | 0.76 |
| Race | 0.002 | ||||
| Caucasian | 31 | 12 | 16 | 10 | |
| Black | 3 | 9 | 22 | 5 | |
| Other | 3 | 2 | 1 | 2 | |
| Age of Asthma Onset (years) | 15.9±16.2 | 12.8±11.6 | 13.9±13.2 | 18.8±14.0 | 0.54 |
| Waist Circumference (cm) | 84.7±14.4 | 91.4±15.3 | 99.8±13.9 | 109.5±24.0 | < 0.001 |
| FEV1 (% pred) | 88.2±13.2 | 91.2±15.0 | 88.5±17.1 | 86.9±12.1 | 0.82 |
| FVC (% pred) | 96.8±13.3 | 103.5±15.8 | 97.6±15.5 | 94.9±12.1 | 0.22 |
| FEV1/FVC (% pred) | 91.4±9.2 | 88.3±8.7 | 90.5±8.7 | 91.9±8.7 | 0.52 |
| Peak flow (% pred) | 85.9±15.3 | 90.4±18.9 | 89.5±16.8 | 77.7±18.1 | 0.08 |
| PC20 (mg/ml) | 4.23±5.37 | 3.50±5.46 | 4.26±4.79 | 3.84±6.08 | 0.44‡ |
| ASUI (range 0–1)↑ | 0.71±0.20 | 0.73±0.15 | 0.73±0.19 | 0.67±0.19 | 0.70 |
| ACT (range 5–25)↑ | 15.7±2.9 | 16.1±3.1 | 15.8±3.2 | 14.3±3.6 | 0.33 |
BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEV1/FVC, ratio of FEV1 to FVC; PC20, the provocative concentration of methacholine that induced a 20% fall in FEV1; ASUI, asthma symptom utility index; ACT, asthma control test
Values shown are mean ± SD,
higher score indicative of better health
p value shown for Kruskal Wallis test
Baseline demographics and lung function of study participants were also compared across WC quartiles: AHR, asthma symptoms, and asthma control were not significantly different among WC quartiles, whereas baseline FEV1 and FVC were significantly lower in participants with higher WC (Table 2). Similar results were observed with WHR (Supplementary Table S1).
Table 2:
Baseline Demographics of Study Population by waist circumference quartile
| Waist Circumference Quartile |
1 | 2 | 3 | 4 | p |
|---|---|---|---|---|---|
| Waist Circumference (cm) | 74.9±7.0 | 88.1±2.5 | 98.7±3.8 | 119.6±13.5 | |
| n (female)† | 30 (27) | 27 (14) | 28 (20) | 26 (17) | 0.02 |
| Age (years) | 35.3±15.5 | 35.9±12.1 | 42.4±13.8 | 42.7±12.5 | 0.07 |
| Race | 0.60 | ||||
| Caucasian | 20 | 18 | 16 | 12 | |
| Black | 8 | 7 | 10 | 13 | |
| Other | 2 | 2 | 2 | 1 | |
| Age Asthma Onset | 15.8±15.7 | 12.8±12.8 | 16.0±14.4 | 15.9±13.8 | 0.81 |
| BMI | 25.0±4.3 | 30.6±7.5 | 30.6±7.0 | 36.3±9.6 | < 0.001 |
| FEV1 (% pred) | 94.2±13.5 | 85.8±12.2 | 91.6±14.1 | 83.8±16.0 | 0.02 |
| FVC (% pred) | 104.3±14.4 | 97.6±9.2 | 99.7±15.3 | 91.7±15.5 | 0.01 |
| FEV1/FVC (% pred) | 90.8±9.8 | 87.8±8.8 | 92.2±7.2 | 91.3±8.1 | 0.26 |
| Peak flow (% pred) | 85.5±15.0 | 85.2±19.6 | 94.3±11.2 | 85.5±20.1 | 0.08‡ |
| PC20 (mg/ml) | 3.05±4.17 | 3.67±5.21 | 6.86±7.15 | 2.83±3.05 | 0.27‡ |
| ASUI (range 0–1)↑ | 0.72±0.19 | 0.67±0.16 | 0.72±0.17 | 0.73±0.20 | 0.62 |
| ACT (range 5–25)↑ | 16.2±2.9 | 14.6±3.4 | 16.0±3.0 | 15.4±3.2 | 0.24 |
BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEV1/FVC, ratio of FEV1 to FVC; PC20, the provocative concentration of methacholine that induced a 20% fall in FEV1; ASUI, asthma symptom utility index; ACT, asthma control test
Values shown are mean ± SD,
higher score indicative of better health
111 participants had measurements of waist circumference
p value shown for Kruskal Wallis test
Effect of BMI on spirometric lung function, sensitivity to airway narrowing and closure, and closing index
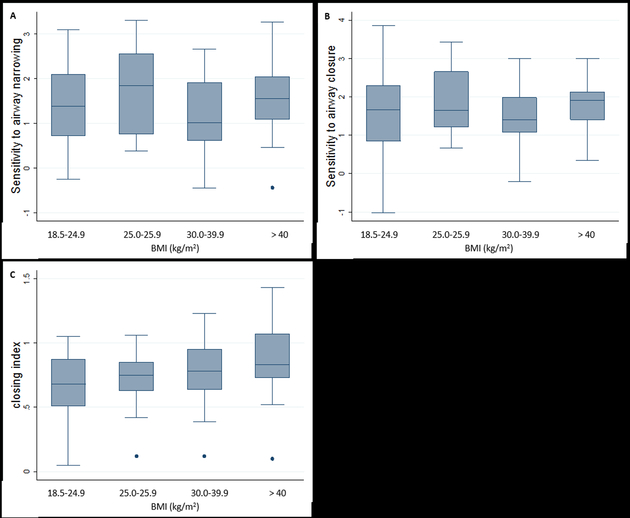
To determine the effect of global obesity on spirometric lung function, sensitivity to airway narrowing and closure, and the closing index, we assessed the relationship of these parameters to BMI category. BMI was not significantly related to spirometric lung function (Table 1). Sensitivity to airway narrowing and closure were not significantly associated with BMI (Figures 1A and 1B). However, the closing index was significantly different across BMI categories, with significantly higher indices recorded in patients with higher BMI (Figure 1C).
Figure 1:
Relationship between sensitivity to airway narrowing and closure, and closing index with BMI group: 1A relationship between sensitivity to airway narrowing and BMI category, p =0.72, by trend test; 1B relationship between sensitivity to airway closure and BMI category, p =0.94, by trend test; 1C relationship between closing index and BMI category, p < 0.01 by trend test.
Effect of WC on spirometric lung function, sensitivity to airway narrowing and closure, and closing index
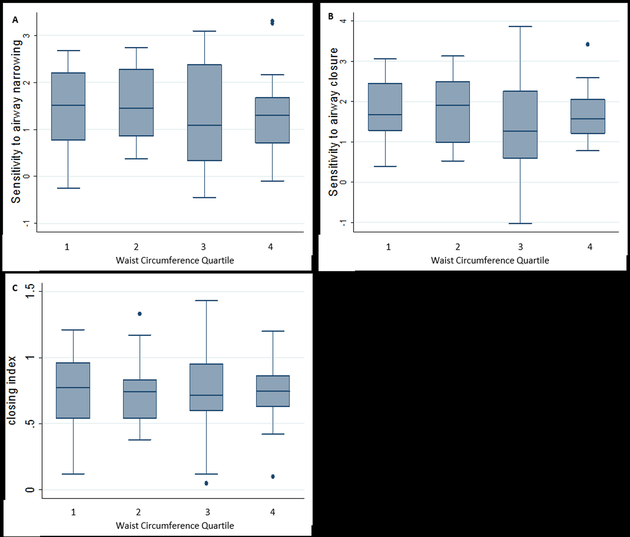
To determine the effect of central obesity on spirometric lung function, sensitivity to airway narrowing and closure, and the closing index, we assessed the relationship of these parameters to WC. WC was inversely related to baseline FEV1 and FVC (Table 2) but not to sensitivity to airway narrowing or airway closure (Figures 2A and 2B), or the closing index (Figure 2C).
Figure 2:
Relationship between sensitivity to airway narrowing and closure, and closing index with waist circumference quartile: 2A relationship between sensitivity to airway narrowing and WC, p =0.95, by trend test; 2B relationship between sensitivity to airway closure and waist circumference quartile, p =0.17, by trend test; 2C relationship between closing index and waist circumference quartile, p =0.85 by trend test.
Multi-variate analysis of combined effects of WC and BMI on spirometric lung function, sensitivity to airway narrowing and closure, and closing index
We investigated the combined effects of BMI and WC on spirometric lung function. When BMI and WC were included in the same model, BMI was associated with increased FVC, whereas WC was associated with decreased FEV1 and FVC (Table 3).
Table 3:
Multi-Variate Regression Analysis of relationship between anthropomorphic measures and lung function
| Waist circumference* | BMI* | ||||
|---|---|---|---|---|---|
| Coef (SE) | p | coef (SE) | p | R2 | |
| FEV1 % predicted | −22.9 (8.8) | 0.01 | 9.41 (6.40) | 0.15 | 0.04 |
| FVC % predicted | −32.4 (8.5) | <0.001 | 13.7 (6.2) | 0.03 | 0.11 |
| FEV1/FVC % pred | 5.49 (5.41) | 0.31 | −3.10 (3.94) | 0.43 | 0.00 |
| DRS FEV1/FVC* | −0.09 (0.61) | 0.89 | −0.38 (0.45) | 0.40 | 0.00 |
| DRS FVC* | −0.56 (0.52) | 0.29 | 0.05 (0.90) | 0.72 | 0.00 |
| Closing Index* | −0.31 (0.30) | 0.30 | 0.56 (0.22) | 0.01 | 0.06 |
BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEV1/FVC, ratio of FEV1 to FVC; DRS, dose response slope
log transformed for regression analysis. WC and BMI were not subject to collinearity.
Sensitivity to airway narrowing and closure were not related to either BMI or WC in univariate analysis, or multi-variate analysis (Table 3). In multi-variate analysis, the closing index was significantly related to BMI but was not related to either WC (Table 3) or WHR (Table S2).
DISCUSSION
We investigated the effect of general obesity (BMI) and central adiposity (WC) on baseline spirometry, sensitivity to airway narrowing and closure, and airway closure during induced bronchoconstriction in individuals with asthma. The purpose of this study was to distinguish the restrictive effects of obesity on lung function from other factors related to obesity. Our principal findings were that (i) WC was the main determinant of decreased spirometric lung function, (ii) sensitivity to airway narrowing and closure were unaffected by BMI or WC, (iii) increased BMI predisposed patients with asthma to airway closure, independent of WC.
The relationship between obesity and asthma has been well documented,1 but there is a paucity of data on the physiological effects of BMI versus WC on some distinguishing features of asthma. There is a growing appreciation that the term obesity includes many pathophysiological entities which variably affect baseline lung function and asthma.35 Indeed, it is generally thought that obesity simply leads to restrictive effects on lung physiology, but this relationship may not be so straightforward, as illustrated by the current study.
In terms of the effects of obesity on spirometric lung function, we found that baseline FEV1 and FVC were inversely related to WC but not BMI (Tables 1–2), suggesting that these parameters were more directly related to the restrictive aspects of obesity. This finding is consistent with a large cross-sectional study that found WC predicted FEV1 and FVC independent of BMI.18 Unexpected in the current study was that BMI was associated with increased FVC (Table 3). Although BMI and FVC initially appears to be unrelated (Table 1), FVC becomes positively related to BMI in the study population after controlling for both WC and BMI (Table 3). We do not know the reason for this, but studies in children provide some insights—obesity is associated with accelerated lung growth, and FVC is increased out of proportion with FEV1, producing airway dysanapsis.36 In longitudinal and cross-sectional studies of young adults, increased body weight is associated with moderate increases in FVC.37 This is attributed to the “muscularity” effect and is dependent on age and height. We do not have longitudinal data, but it is possible that differential effects of BMI on lung growth, and WC on lung restriction may both be involved in the effects of obesity on spirometric lung function in adults.
Prior studies have suggested that peripheral airway dysfunction in response to methacholine occurs in obese non-asthmatics,22, 38 and we have reported that weight loss improves sensitivity to airway closure in late onset non-allergic asthmatics.39 However, to the best of our knowledge, the present study is the first to investigate whether increased adiposity or body-fat distribution increases sensitivity to airway closure during methacholine challenge. Neither WC nor BMI have a significant effect on sensitivity to narrowing or closure, consistent with the fact that not all studies have found a relationship between BMI and AHR.40, 41 In fact, some studies have reported a relationship between BMI and AHR in non-asthmatics but not in asthmatics.14, 42
BMI but not WC contributed significantly to the closing index, a measure of the degree of airway closure during bronchoconstriction. This is in agreement with a recent study by Burgess and colleagues that reported that airway closure – measured by percent fall in FVC rather than the closing index – explained nearly half the increased airway reactivity associated with increased BMI, and this relationship was not altered by including WC in the model.14 These data suggest that the tendency towards airway closure during bronchoconstriction is not simply a function of the restrictive effects of obesity, but rather is related to factors that track more closely with BMI than WC. Perhaps, this could be related to lung growth, as discussed above. Alternatively, it may relate to factors more closely associated with subcutaneous versus visceral adipose tissue. Circulating leptin is one factor more strongly related to subcutaneous than visceral adipose tissue.43, 44 Leptin has several functions that could impact AHR such as involvement in surfactant production, lung development and bronchial tone.40, 45, 46
An important limitation of this study is that it is only comprised of patients with poorly controlled asthma and chronic sinonasal disease who demonstrated FEV1 > 70%. This resulted in the exclusion of a substantial proportion of overweight and obese participants. It is therefore unclear whether the findings of this study are generalizable to patients with more severe airflow limitation who do not present with upper airway disease. However, since a greater proportion of obese study participants had baseline FEV1 lower than 70%, we speculate that the effect of increased BMI on airway narrowing and airway closure would be heightened in these subjects.
In summary, we have shown that WC is associated with restrictive effects on baseline spirometry. However, increased BMI, rather than WC, predisposes to airway closure during bronchoconstriction. Taken together, these findings suggest that obesity predisposes to airway closure during bronchoconstriction through mechanisms other than simple restriction. This could be clinically important because increased airway closure during bronchoconstriction in obese subjects could limit deposition of inhaled medications and reduce the efficacy of standard therapeutic approaches.
Supplementary Material
Acknowledgements
This work was supported by grants from the American Lung Association – Airways Clinical Research Center Award, and the NIH (UO1HL089464 & U01 HL089510).
Abbreviations:
- ACT
asthma control test
- AHR
airway hyperresponsiveness
- ASUI
asthma symptom utility index
- BMI
body mass index
- DRS
dose-response slope
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- FEV1/FVC
ratio of FEV1 to FVC
- PC20
the provocative concentration of methacholine that induced a 20% fall in FEV1
- SNQ
Sinonasal Questionnaire
- STAN
Study of Asthma and Nasal Steroids
- WC
waist circumference
Footnotes
Data availability statement:
Research data and the statistical analysis code that underlie the results reported in this article are available upon request after de-identification (text, tables, and figures) between 3 months and 5 years after publication. Proposals for data sharing can be sent to the corresponding author; data requestors will be asked to sign a data access agreement.
Disclosure statement
This study was previously presented at the American Thoracic Society International Conference in 2016.
REFERENCES
- 1.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. American journal of respiratory and critical care medicine. 2007; 175: 661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, Haselkorn T. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. The Journal of allergy and clinical immunology. 2014; 133: 1549–56. [DOI] [PubMed] [Google Scholar]
- 3.Mosen DM, Schatz M, Magid DJ, Camargo CA, Jr. The relationship between obesity and asthma severity and control in adults. The Journal of allergy and clinical immunology. 2008; 122: 507–11 e6. [DOI] [PubMed] [Google Scholar]
- 4.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER, National Heart Ln, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181: 315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. American journal of respiratory and critical care medicine. 2008; 178: 682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon A The treatment of asthma in obesity. Expert review of respiratory medicine. 2012; 6: 331–40. [DOI] [PubMed] [Google Scholar]
- 7.Watson RA, Pride NB, Thomas EL, Fitzpatrick J, Durighel G, McCarthy J, Morin SX, Ind PW, Bell JD. Reduction of total lung capacity in obese men: comparison of total intrathoracic and gas volumes. J Appl Physiol (1985). 2010; 108: 1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedenstierna G, Santesson J. Breathing mechanics, dead space and gas exchange in the extremely obese, breathing spontaneously and during anaesthesia with intermittent positive pressure ventilation. Acta Anaesthesiol Scand. 1976; 20: 248–54. [DOI] [PubMed] [Google Scholar]
- 9.NAIMARK A, CHERNIACK RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960; 15: 377–82. [DOI] [PubMed] [Google Scholar]
- 10.SHARP JT, HENRY JP, SWEANY SK, MEADOWS WR, PIETRAS RJ. THE TOTAL WORK OF BREATHING IN NORMAL AND OBESE MEN. J Clin Invest. 1964; 43: 728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006; 130: 827–33. [DOI] [PubMed] [Google Scholar]
- 12.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014; 19: 1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman DG, Berend N, King GG, Salome CM. Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J. 2008; 32: 1563–9. [DOI] [PubMed] [Google Scholar]
- 14.Burgess JA, Matheson MC, Diao F, Johns DP, Erbas B, Lowe AJ, Gurrin LC, Lodge CJ, Thomas PS, Morrison S, Thompson BR, Feather I, Perret JL, Abramson MJ, Giles GG, Hopper JL, Dharmage SC, Walters EH. Bronchial hyperresponsiveness and obesity in middle age: insights from an Australian cohort. Eur Respir J. 2017; 50. [DOI] [PubMed] [Google Scholar]
- 15.Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the Normative Aging Study. Thorax. 2002; 57: 581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wehrmeister FC, Menezes AM, Muniz LC, Martínez-Mesa J, Domingues MR, Horta BL. Waist circumference and pulmonary function: a systematic review and meta-analysis. Syst Rev. 2012; 1: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994; 73: 460–8. [DOI] [PubMed] [Google Scholar]
- 18.Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009; 179: 509–16. [DOI] [PubMed] [Google Scholar]
- 19.Sorlí-Aguilar M, Martín-Luján F, Flores-Mateo G, Jardí-Piñana C, Aparicio-Llopis E, Basora-Gallisà J, Solà-Alberich R, investigators ESG. Adiposity markers and lung function in smokers: a cross-sectional study in a Mediterranean population. BMC Pulm Med. 2016; 16: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012; 186: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton JH, Ireland A, Fitzpatrick M, Kessinger C, Camp D, Weinman R, McMahon D, Leader JK, Holguin F, Wenzel SE, Morris A, Gingo MR. Adiposity influences airway wall thickness and the asthma phenotype of HIV-associated obstructive lung disease: a cross-sectional study. BMC pulmonary medicine. 2016; 16: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai AG, Togias A, Schechter C, Fisher B, Parow A, Skloot G. Peripheral airways dysfunction in obesity reflects increased bronchomotor tone. The Journal of allergy and clinical immunology. 2015; 135: 820–2. [DOI] [PubMed] [Google Scholar]
- 23.Oppenheimer BW, Berger KI, Ali S, Segal LN, Donnino R, Katz S, Parikh M, Goldring RM. Pulmonary Vascular Congestion: A Mechanism for Distal Lung Unit Dysfunction in Obesity. PloS one. 2016; 11: e0152769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Alwan A, Bates JH, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, Dixon AE. The nonallergic asthma of obesity. A matter of distal lung compliance. American journal of respiratory and critical care medicine. 2014; 189: 1494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Lung Association-Asthma Clinical Research Centers’ Writing C, Dixon AE, Castro M, Cohen RI, Gerald LB, Holbrook JT, Irvin CG, Mohapatra S, Peters SP, Rayapudi S, Sugar EA, Wise RA. Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma. The Journal of allergy and clinical immunology. 2015; 135: 701–9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon AE, Sugar EA, Zinreich SJ, Slavin RG, Corren J, Naclerio RM, Ishii M, Cohen RI, Brown ED, Wise RA, Irvin CG, American Lung Association-Asthma Clinical Research C. Criteria to screen for chronic sinonasal disease. Chest. 2009; 136: 1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Kosinski M, Pendergraft TB, Jhingran P. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. The Journal of allergy and clinical immunology. 2006; 117: 549–56. [DOI] [PubMed] [Google Scholar]
- 28.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 1998; 114: 998–1007. [DOI] [PubMed] [Google Scholar]
- 29.Valsamakis G, Chetty R, Anwar A, Banerjee AK, Barnett A, Kumar S. Association of simple anthropometric measures of obesity with visceral fat and the metabolic syndrome in male Caucasian and Indo-Asian subjects. Diabet Med. 2004; 21: 1339–45. [DOI] [PubMed] [Google Scholar]
- 30.Alberti KG, Zimmet P, Shaw J, Group IETFC. The metabolic syndrome--a new worldwide definition. Lancet. 2005; 366: 1059–62. [DOI] [PubMed] [Google Scholar]
- 31.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. American journal of respiratory and critical care medicine. 2000; 161: 309–29. [DOI] [PubMed] [Google Scholar]
- 32.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. The European respiratory journal. 2005; 26: 319–38. [DOI] [PubMed] [Google Scholar]
- 33.Peat JK, Salome CM, Berry G, Woolcock AJ. Relation of dose-response slope to respiratory symptoms and lung function in a population study of adults living in Busselton, Western Australia. Am Rev Respir Dis. 1992; 146: 860–5. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor G, Sparrow D, Taylor D, Segal M, Weiss S. Analysis of dose-response curves to methacholine. An approach suitable for population studies. Am Rev Respir Dis. 1987; 136: 1412–7. [DOI] [PubMed] [Google Scholar]
- 35.Peters U, Suratt BT, Bates JHT, Dixon AE. Beyond BMI: Obesity and Lung Disease. Chest. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forno E, Weiner DJ, Mullen J, Sawicki G, Kurland G, Han YY, Cloutier MM, Canino G, Weiss ST, Litonjua AA, Celedon JC. Obesity and Airway Dysanapsis in Children with and without Asthma. American journal of respiratory and critical care medicine. 2017; 195: 314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bande J, Clément J, Van de Woestijne KP. The influence of smoking habits and body weight on vital capacity and FEV1 in male Air Force personnel: a longitudinal and cross-sectional analysis. Am Rev Respir Dis. 1980; 122: 781–90. [DOI] [PubMed] [Google Scholar]
- 38.Skloot G, Schechter C, Desai A, Togias A. Impaired response to deep inspiration in obesity. Journal of applied physiology. 2011; 111: 726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Blasio MJ, Boije M, Kempster SL, Smith GC, Charnock-Jones DS, Denyer A, Hughes A, Wooding FB, Blache D, Fowden AL, Forhead AJ. Leptin Matures Aspects of Lung Structure and Function in the Ovine Fetus. Endocrinology. 2016; 157: 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001; 56: 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sood A, Verhulst SJ, Varma A, Eagleton LE, Henkle JQ, Hopkins-Price P. Association of excess weight and degree of airway responsiveness in asthmatics and non-asthmatics. The Journal of asthma : official journal of the Association for the Care of Asthma. 2006; 43: 447–52. [DOI] [PubMed] [Google Scholar]
- 43.Gepner Y, Shelef I, Schwarzfuchs D, Zelicha H, Tene L, Yaskolka Meir A, Tsaban G, Cohen N, Bril N, Rein M, Serfaty D, Kenigsbuch S, Komy O, Wolak A, Chassidim Y, Golan R, Avni-Hassid H, Bilitzky A, Sarusi B, Goshen E, Shemesh E, Henkin Y, Stumvoll M, Bluher M, Thiery J, Ceglarek U, Rudich A, Stampfer MJ, Shai I. Effect of Distinct Lifestyle Interventions on Mobilization of Fat Storage Pools: The CENTRAL MRI Randomized Controlled Trial. Circulation. 2017. [DOI] [PubMed] [Google Scholar]
- 44.Tinggaard J, Hagen CP, Christensen AN, Mouritsen A, Mieritz MG, Wohlfahrt-Veje C, Helge JW, Beck TN, Fallentin E, Larsen R, Jensen RB, Juul A, Main KM. Anthropometry, DXA, and leptin reflect subcutaneous but not visceral abdominal adipose tissue on MRI in 197 healthy adolescents. Pediatr Res. 2017; 82: 620–8. [DOI] [PubMed] [Google Scholar]
- 45.Torday JS, Powell FL, Farmer CG, Orgeig S, Nielsen HC, Hall AJ. Leptin integrates vertebrate evolution: from oxygen to the blood-gas barrier. Respir Physiol Neurobiol. 2010; 173 Suppl: S37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arteaga-Solis E, Zee T, Emala CW, Vinson C, Wess J, Karsenty G. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell metabolism. 2013; 17: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.