Summary
The disconnect between the genomic prediction of secondary metabolite biosynthetic potential and the observed laboratory production profile of microorganisms is well-documented. While heterologous expression of biosynthetic gene clusters (BGCs) is often seen as a potential solution to bridge this gap, it is not immune to many challenges including impaired regulation, the inability to recruit essential building blocks, and transcriptional and/or translational silence of the biosynthetic genes. Here we report the discovery, cloning, refactoring, and heterologous expression of a cryptic hybrid phenazine-type BGC (spz) from the marine actinomycete Streptomyces sp. CNB-091. Overexpression of the engineered spz pathway resulted in increased production and chemical diversity of phenazine natural products belonging to the streptophenazine family, including bioactive members containing an unprecedented N-formylglycine attachment. An atypical discrete adenylation enzyme in the spz cluster is required to introduce the formylglycine moiety and represents a phylogenetically distinct class of adenylation proteins.
Bauman et al., designed and utilized promoter cassettes to transcriptionally activate the cryptic streptophenazine biosynthetic pathway from marine Streptomyces bacteria. Pathway refactoring resulted in production of over 100 compounds, including streptophenazines with a rare N-formylglycine moiety that showed enhanced antibiotic activity.
Keywords: streptophenazine, refactoring, promoter, cryptic biosynthetic gene cluster, adenylation enzyme, heterologous expression, natural product, antibiotic, marine Streptomyces
Graphical Abstract
Introduction
Small molecules provide a fundamental means by which microorganisms interact with each other and their environment, facilitating diverse yet critical functions that can include defense, cell signaling, and/or nutrient acquisition (Demain and Fang, 2000). Nature has engineered a chemically diverse array of molecules designed to interact with a variety of biological targets and thereby mediate microbial communication. The potent biological activities that define secondary metabolites have been harnessed by humankind as well, most famously as sources of and inspiration for pharmaceuticals (Koehn and Carter, 2005; Newman and Cragg, 2016). The enzymes necessary for production of these metabolites are genetically encoded, and in bacteria, are grouped together on contiguous stretches of DNA, forming biosynthetic gene clusters (BGCs) (Osbourn, 2010). The plummeting cost of high-throughput microbial DNA sequencing and resulting flood of genomic information has revealed that up to 90% of the chemical potential of microorganisms is unexplored as many BGCs are silent, particularly under laboratory conditions, and thus their molecular products have not been identified (Bentley et al., 2002; Omura et al., 2001).
Controlled heterologous expression is a powerful tool to unlock the biosynthetic potential encoded by silent and cryptic BGCs. This process can be utilized to circumvent issues with culturing and difficulties with genetic manipulation of the native producers, allowing access to the molecules produced by unculturable and symbiotic organisms (Gomez-Escribano and Bibb, 2014). However, the heterologous production of secondary metabolites can have low efficiency, and certain BGCs may remain silent even within validated heterologous expression systems (Rutledge and Challis, 2015). Additional genetic manipulations are often necessary to improve or activate production within heterologous hosts. These may include manipulating the pathway-specific regulatory genes (Olano et al., 2008), or using synthetic biology tools to rewrite native genetic elements with optimized components (e.g., promoters, ribosome binding sites (RBSs), etc.) (Freestone et al., 2017; Montiel et al., 2015; Myronovskyi and Luzhetskyy, 2016).
Phenazines are a large class of heterocyclic, nitrogen containing compounds produced by both Gram-positive and Gram-negative organisms (Laursen and Nielsen, 2004; Pierson and Pierson, 2010; Price-Whelan et al., 2006). Phenazine metabolites are derived from either phenazine-1-carboxylic acid (PCA) (1) or phenazine-1,6-dicarboxylic acid (PDC) (2), which are biosynthesized by a conserved set of five essential genes, phzBDEFG, typically encoded in a single operon (Blankenfeldt and Parsons, 2014; Mavrodi et al., 2010). While all phenazines share a dibenzo annulated pyrazine core structure, they exhibit incredible diversity in modifications of that core that result in potent and often therapeutically relevant bioactivities (Figure 1A). These activities can vary widely, including broad-spectrum antibiotic (saphenic acid, 4), antitumor (esmeraldin B, 8), and mosquito larvicidal (saphenamycin, 5) activities (Geiger et al., 1988; Laursen and Nielsen, 2004; Rui et al., 2012). Phenazines play important ecological roles as well. For instance, pyocyanin (3) is an important signaling factor for its microbial producer Pseudomonas aeruginosa and a key facilitator of microbial survival in anoxic biofilms (Dietrich et al., 2006, 2008; Ramos et al., 2010). Because of the diverse and important bioactivities of phenazines, their structural similarity to cofactors in primary metabolism, and their abilities to modify gene expression, it has been suggested that phenazine metabolites blur the line between primary and secondary metabolism, and refute the paradigm that so-called ‘secondary’ metabolites are not essential for survival (Price-Whelan et al., 2006).
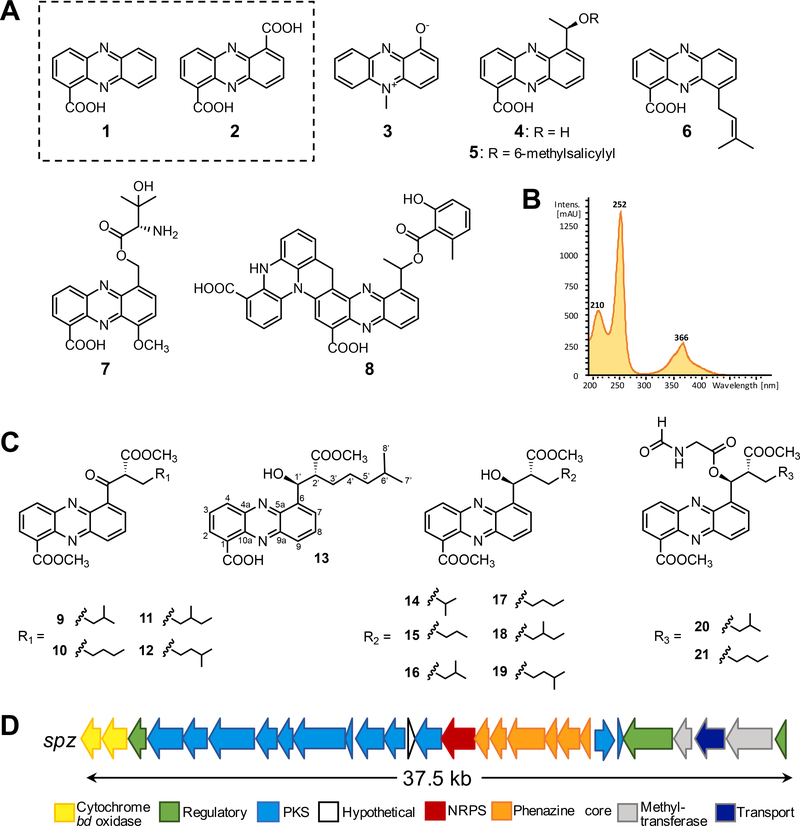
Figure 1. Chemical structures of selected phenazine metabolites and gene organization of the spz biosynthetic gene cluster from Streptomyces sp. CNB091.
A. Phenazine compounds isolated from Streptomyces and Pseudomonas bacteria: phenazine-1-carboxylic acid (PCA) (1), phenazine-1,6-dicarboxylic acid (PDC) (2), pyocyanin (3), saphenic acid (4), saphenamycin (5), endophenazine A (6), pelagiomicin A (7), esmeraldin B (8). B. Characteristic UV absorption spectrum of streptophenazines (λmax 252 nm, 366 nm). C. Chemical structures of streptophenazine metabolites associated with the spz BGC from S. sp. CNB-091. Previously reported compounds include streptophenazines A (16), B (17), C (13), D (14), F (19), G (18). Compounds isolated in this work include oxo-streptophenazines A (9), B (10), G (11), F (12), and streptophenazines P (15), Q (20), R (21). D. Gene arrangement of the spz BGC from S. sp. CNB-091. See also Figures S1, S2, S5.
Herein, we report the identification, capture, engineering, and heterologous expression of a unique cryptic phenazine-type BGC (spz) from the marine actinomycete Streptomyces sp. CNB-091. Refactoring of the spz cluster resulted in tremendous structural diversity of the streptophenazine suite of natural products, PDC-derived phenazines with variable alkyl side chains isolated from Streptomyces bacteria (Bunbamrung et al., 2014; Kunz et al., 2014; Liang et al., 2017; Mitova et al., 2008). Our refactoring efforts resulted in the production of previously unseen N-formylglycine analogues with antibacterial activity.
Results
Identification of the spz BGC
We previously sequenced the 8.2 Mb draft genome of the marine actinomycete Streptomyces sp. CNB-091 (GCA_000377965.1) and identified 41 metabolically diverse BGCs, revealing the astounding biosynthetic potential of this organism (Ray et al., 2016). Beyond the previously characterized salinamides, a class of anti-inflammatory and antibacterial depsipeptides (Degen et al., 2014; Moore et al., 1999; Trischman et al., 1994), no other natural products have been reported from this strain. To explore the metabolic capacity of this organism, we cultured it in diverse growth conditions and monitored its molecular profile by Liquid Chromatography Mass Spectrometry (LCMS). We identified a group of orange pigmented molecules that were produced sporadically and at low titers. Comprehensive analysis of UV (Figure 1B) and high resolution mass spectrometry (HRMS) revealed production of a series of streptophenazine-like compounds with accurate mass and fragmentation identical to previously characterized streptophenazines A (16), B (17), F (18), and G (19), first isolated from Streptomyces sp. HB202 (Mitova et al., 2008) (Figure 1C).
Inspection of the S. sp. CNB-091 genome revealed a single set of phenazine biosynthetic genes (spz16–21) co-localized with genes encoding the biosynthesis of polyketide extender unit(s) and polyketide synthase (PKS) extension cycle(s) (spz4–12) as well as nonribosomal peptide synthetase (NRPS) functions (spz15), suggestive of a putative streptophenazine BGC (spz) (Table 1, Figure 1D) (Weber et al., 2015). While the presence of the PKS genes in the spz cluster can be rationalized by the installation of C-6 alkyl chains in streptophenazines (Figure 1C), there have been no previously reported streptophenazine structures that incorporate amino acid(s), indicative of NRPS biochemistry. Therefore, we speculated that the spz BGC may encode the biosynthesis of streptophenazine-like molecules with different chemical scaffolds. We thus set out to interrogate the spz BGC with the intent of not only exploring its full biosynthetic potential, but also to probe issues of BGC expression that might enable the future discovery of additional metabolites from S. sp. CNB-091.
Table 1.
Open reading frames (ORFs) in the spz biosynthetic gene cluster and their proposed roles.
| Proposed function | Protein | aa | BLAST closest homolog | %Cover/ Identity | Homolog accession number |
|---|---|---|---|---|---|
| Cytochrome bd ubiquinol oxidase II | Spz1 | 329 | Streptomyces sp. ms184 | 100/98 | WP_097875779.1 |
| Cytochrome bd ubiquinol oxidase I | Spz2 | 439 | Streptomyces albovinaceus | 100/96 | WP_086670717.1 |
| LysR-type regulator | Spz3 | 293 | Streptomyces spp. | 100/97 | WP_073774781.1 |
| Acyl-CoA synthetase | Spz4 | 600 | Streptomyces albovinaceus | 100/98 | WP_086670715.1 |
| Crotonyl-CoA carboxylase/reductase | Spz5 | 416 | Streptomyces spp. | 100/99 | WP_073774779.1 |
| Type I PKS (KS*, AT) | Spz6 | 800 | Streptomyces albovinaceus | 100/97 | WP_086670713.1 |
| Ketoreductase (KR) | Spz7 | 351 | Streptomyces spp. | 100/99 | WP_073774774.1 |
| Thioesterase (TE) | Spz8 | 264 | Streptomyces sp. ms184 | 100/97 | WP_097876860.1 |
| Type I PKS (KS, ACP) | Spz9 | 914 | Streptomyces sp. ms184 | 100/98 | WP_097876858.1 |
| Acyl carrier protein (ACP) | Spz10 | 109 | Kitasatospora purpeofusca | 95/61 | WP_078880679.1 |
| Aldehyde dehydrogenase | Spz11 | 481 | Streptomyces albovinaceus | 100/97 | WP_086670706.1 |
| PDC adenylase | Spz12 | 365 | Streptomyces sp. ms184 | 100/98 | WP_097876855.1 |
| Hypothetical protein | Spz13 | 113 | Streptomyces sp. TSRI0445 | 100/96 | OKI73654.1 |
| FAD-binding oxidoreductase | Spz14 | 455 | Streptomyces spp. | 100/98 | WP_073776179.1 |
| D-alanine-poly(phosphoribitol) ligase | Spz15 | 545 | Streptomyces sp. ms184 | 100/98 | WP_097877165.1 |
| Pyridoxamine 5'-phosphate oxidase (PhzG) | Spz16 | 220 | Streptomyces sp. ms184 | 100/99 | WP_097877165.1 |
| 2,3-Dihydro-3-hydroxylanthranilate isomerase (PhzF) | Spz17 | 283 | Streptomyces spp. | 100/98 | WP_073774764.1 |
| Anthranilate synthase (PhzE) | Spz18 | 627 | Streptomyces sp. ms184 | 99/98 | WP_097877170.1 |
| Isochorismatase (PhzD) | Spz19 | 207 | Streptomyces sp. ms184 | 100/99 | WP_097877172.1 |
| Phospho-2-dehydro-3-deoxyheptonate aldolase (DAHP synthetase) (PhzC) | Spz20 | 391 | Streptomyces sp. ms184 | 100/98 | WP_097877172.1 |
| Phenazine biosynthesis protein (PhzA/B) | Spz21 | 166 | Streptomyces sp. ms184 | 100/98 | WP_097877172.1 |
| 3-Oxoacyl-ACP synthase III (FabH) | Spz22 | 344 | Streptomyces sp. ms184 | 100/99 | WP_097877172.1 |
| Aryl carrier domain protein | Spz23 | 97 | Kitasatospora purpeofusca | 92/61 | WP_030398483.1 |
| LuxR-type regulator | Spz24 | 846 | Kitasatospora purpeofusca | 96/55 | WP_030398483.1 |
| Class I SAM-dependent methyltransferase | Spz25 | 289 | Streptomyces sp. TSRI0445 | 96/99 | WP_079198635.1 |
| MFS transporter | Spz26 | 516 | Streptomyces sp. ms184 | 100/97 | WP_097874479.1 |
| HemK family methylase | Spz27 | 781 | Streptomyces kanasensis | 100/70 | WP_058941165.1 |
| TetR/AcrR regulator | Spz28 | 207 | Streptomyces griseus | 100/98 | WP_030809832.1 |
lacking catalytic cysteine and histidine residues
Transformation-associated recombination (TAR) cloning and heterologous expression of the spz BGC
To exploit the full biosynthetic potential of the spz BGC and facilitate genetic manipulations, we captured the spz pathway using TAR cloning methodology (Kouprina and Larionov, 2016; Tang et al., 2015; Yamanaka et al., 2014). Initially, a 49.5 kb DNA region covering the proposed spz BGC was captured from S. sp. CNB-091 genomic DNA (gDNA), resulting in an approximately 60.3 kb construct, pSMM. Restriction digest was used to verify correct assembly of the construct isolated from yeast (Figure S1). However, pSMM proved to be unstable upon transformation and manipulation in Escherichia coli, limiting its use for future genetic alterations. Inspection of peripheral genes of the DNA insert captured into pSMM revealed almost 12 kb of DNA sequence unlikely associated with streptophenazine biosynthesis. Therefore, the spz BGC was recaptured using a PCR-based TAR method (Shao et al., 2009) targeting strictly genes spz1–28. Eight approximately 5 kb DNA fragments covering the spz pathway were amplified and purified for further TAR cloning into the pCAP03 capture vector (Tang et al., 2015). The resulting construct, pKDB01, remained stable upon transformation into E. coli (Figure S1). We then transferred pKDB01 into E. coli ET12567 (MacNeil et al., 1992) for further conjugation into the Streptomyces coelicolor M1146 heterologous host and chromosomal integration into the ϕC31 attachment site (Gomez-Escribano and Bibb, 2011; Jones et al., 2013). The resulting strain was cultured under a variety of growth conditions, but we ultimately failed to detect any streptophenazines in its metabolic profile (Figure 2).
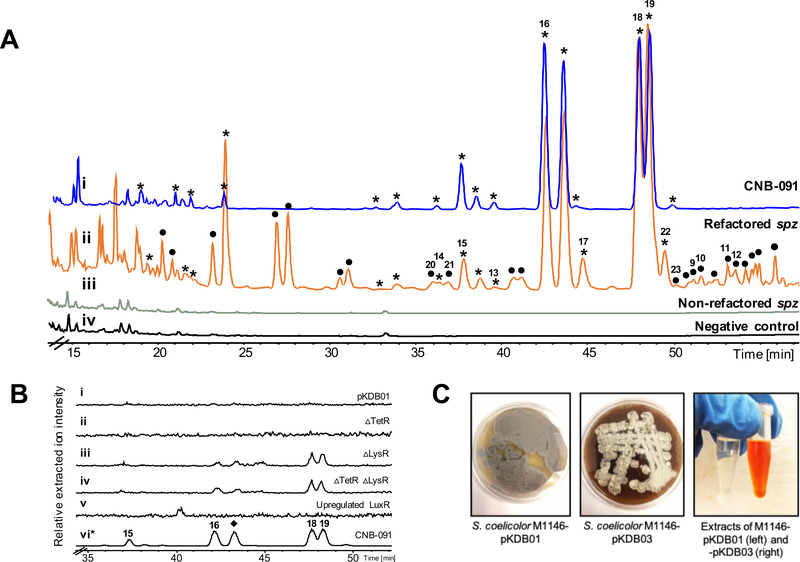
Figure 2. Analysis of the crude extracts from the engineered spz cluster in the heterologous host S. coelicolor M1146 and the wild type producer S. sp CNB-091.
A. HPLC chromatograms (254 nm) of ethyl acetate extracts of: i – CNB-091, * indicates peaks with streptophenazine UV profile; ii – M1146-pKDB03 (refactored spz), • indicates peaks with streptophenazine UV profile appearing in refactored strain; iii – M1146-pKDB01 (non-refactored spz); iv – M1146-pCAP03 (negative control). B. Extracted LCMS chromatograms (m/z 425.2, 439.2, corresponding to streptophenazines A, B, F, G) of the organic extracts from regulatory gene mutant strains: i – pKDB01 (non-refactored spz); ii – pKDB02 (non-refactored spzΔTetR); iii – pKDB01ΔLysR, iv – pKDB02ΔLysR; v – pKDB01-ermE*p-LuxR, vi – CNB-091 (*trace is minimized 100x for scale). ◆ indicates peak that was not isolated. C. Production of streptophenazines on solid media by S. coelicolor M1146-pKDB03 and corresponding ethyl acetate extracts of strains with non-refactored (pKDB01) and refactored (pKDB03) spz cluster. Bright orange color is due to the abundant production of streptophenazines. See also Figures S2 and S3.
While disappointing at first, the silence of the spz BGC within S. coelicolor M1146 presented an ideal opportunity to explore issues of expression within heterologous host systems and apply genetic tools to activate a silent pathway. The spz BGC is an excellent model system to probe these questions for several reasons: (1) phenazine metabolites are pigmented, facilitating visual detection and aiding purification processes and (2) streptophenazine-like compounds were detected in the native producer which simplifies the search for these molecules within the heterologous system. To awaken the spz cluster in the S. coelicolor M1146 host strain, we chose to harness synthetic biology tools (Kim et al., 2015) to establish genetic control over the expression and regulation of the spz biosynthetic genes. Ultimately, we predict these tools can be applied to other BGCs from S. sp. CNB-091, allowing access to the entire biosynthetic potential of this organism.
Genetic manipulation of the pathway-specific spz regulatory genes
Three putative pathway-specific regulatory genes are found within the spz BGC: spz28 and spz3, encoding TetR and LysR homologs, respectively, which are hypothesized to be repressors, and spz24, which encodes a LuxR homolog predicted to be an activator of phenazine biosynthesis. To examine the role of the TetR homolog, we created a construct, pKDB02, with the spz pathway lacking the spz28 gene (Figure S1). The cosmid was then integrated into the genome of S. coelicolor M1146 but the profile of this mutant strain did not reveal any streptophenazine metabolites (Figure 2B). Using λ-Red recombineering, we then replaced the second putative repressor gene, spz3, with an apramycin resistance cassette in both plasmids, pKDB01 and the TetR deletion mutant pKDB02 (Figure S2). The resulting strains S. coelicolor M1146–pKDB01Δspz3 and the double mutant –pKDB02Δspz3 had identical metabolite profiles, characterized by trace production of four streptophenazine compounds (Figure 2B). However, this production was inconsistent and observed at levels much lower than in the native organism. This observation suggested that the LysR-homolog (Spz3) may serve as a repressor of the spz pathway. Finally, we introduced the well characterized strong constitutive promoter ermE*p (Bibb et al., 1985) in front of spz24 (Figure S2). The resulting cosmid pKDB01-ermE*p-spz24 was then integrated into the genome of S. coelicolor M1146. LCMS analysis revealed that the upregulation of the spz LuxR homolog did not activate streptophenazine production (Figure 2B). Ultimately, genetic manipulations of the spz regulatory genes failed to achieve reliable production of streptophenazines in the heterologous host.
Refactoring the spz pathway with strong constitutive promoters
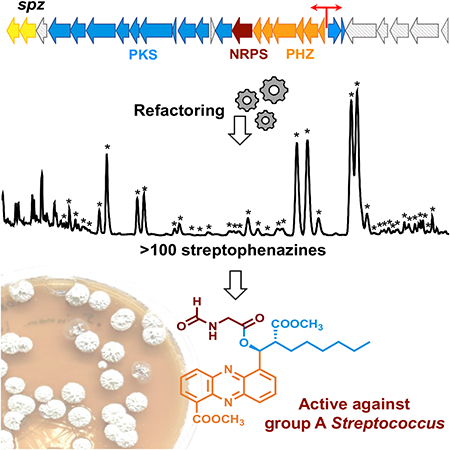
We performed reverse transcription-PCR (RT-PCR) to test expression of every gene in the cloned spz pathway integrated into the S. coelicolor M1146 genome. Results revealed that the spz cluster was not transcriptionally active in a heterologous environment (Figure 3A), which strongly suggests a malfunction with the cluster’s native promoters. Therefore, we decided to bypass the native regulation of the pathway and refactor the spz BGC by introducing characterized, constitutively-active, actinomycete-compatible promoters in four locations based on the operonic structure of the cluster (Figure 3B). We created three cassettes (two unidirectional and one bidirectional) that harbor selection markers, promoters, and RBS sequences. Importantly, all cassettes were designed to lack oriT that is present in readily available λ-Red cassettes (Gust et al., 2003, 2004), to be compatible with the pCAP series of vectors that already harbor an oriT site (Yamanaka et al., 2014).
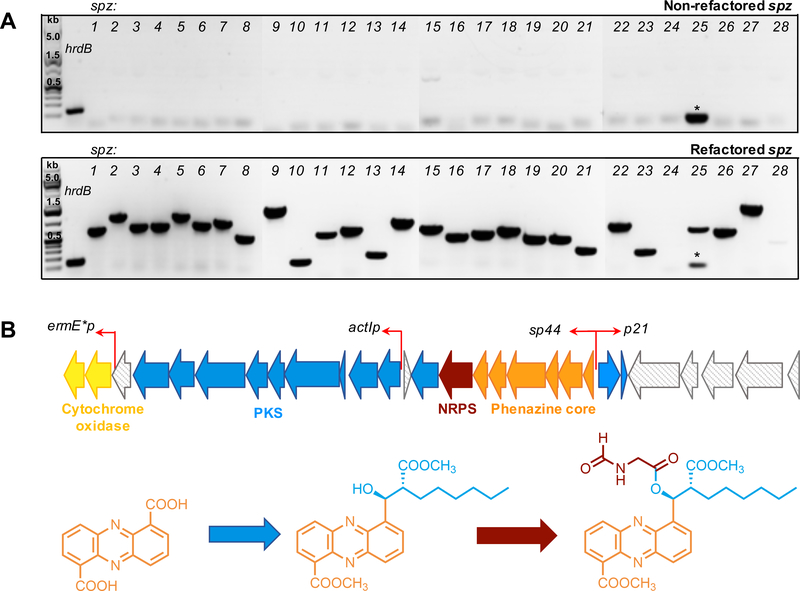
Figure 3. Refactoring the spz biosynthetic gene cluster.
A. RT-PCR analysis of expression of biosynthetic genes in the spz BGC before and after refactoring. Gene encoding a sigma-like transcription factor of S. coelicolor, hrdB, was used as a positive control. Strains S. coelicolor M1146-pKDB01 (non-refactored spz) and S. coelicolor M1146-pKDB03 (refactored spz) were used for analysis. Expected sizes (bp) of amplified fragments as follows: hrdB – 132, spz genes: spz1 – 679; 2 – 1135; 3 – 731; 4 – 750; 5 –1093; 6 – 800; 7 – 946; 8 – 544; 9 – 1134; 10 – 180; 11 – 587; 12 – 689; 13 – 268; 14 – 963; 15 – 768; 16 – 532; 17 – 611; 18 – 722; 19 – 515; 20 – 540; 21 – 328; 22 – 727; 23 – 242; 24 – 1339; 25 – 667; 26 – 623; 27 – 1499; 28 – 410. * indicates low molecular weight band corresponding to primer dimer. Expression of the gene spz24 (encodes LuxR-type regulator) was not observed for both versions of the spz cluster, refactored and non-refactored. B. Schematic illustration of the refactoring strategy indicating locations and directions of the promoters introduced (red arrows). See also Figure S2 and Table S1.
We designed the first cassette, the sp44–p21 cassette, to include two promoters: sp44 and p21, facing opposite directions. The sp44 promoter is a synthetic derivative of the kasO* promoter from the coelimycin BGC and was shown to be 20-fold stronger than the ermE* promoter, traditionally used in Streptomyces research (Bai et al., 2015). The sp44 promoter was introduced in front of the phenazine operon to allow robust production of the PDC core for further biosynthetic derivatization. The p21 promoter is a derivative of ermEp1, with 3-fold greater strength than ermE*p (Myronovskyi and Luzhetskyy, 2016; Siegl et al., 2013). This cassette also included the apramycin resistance gene aac(3)IV sandwiched between two FRT sites to allow excision of the resistance marker after a successful recombination event. Eight additional nucleotides were added between the sp44 and FRT sequences to ensure an in-frame nonpolar deletion. The second cassette, named actIp. was designed to govern transcription of the PKS genes (spz4–12). This cassette included the actI promoter, RBS, the actII-ORF4 gene required for activation of the actI promoter from the actinorhodin BGC (Fayed et al., 2015; Fernández-Moreno et al., 1991; Rowe et al., 1998), and the aac(3)IV gene to leverage apramycin resistance for selection. Finally, the third cassette, ermE*p, was used to introduce the ermE* promoter in front of the cytochrome bd oxidase-encoding genes (spz1,2). To avoid cross resistance, the ampicillin resistance gene bla was included to allow for selection in E. coli. Gibson assembly was used to stitch together functional elements as discussed in the STAR methods. Cassette sequences were confirmed by restriction digest and Sanger sequencing (Table S1). These cassettes were then used to replace putative native promoter regions within the spz cluster in the pKDB01 cosmid in a step wise manner using λ-Red recombineering (Figure S2B). Insertion of the sp44–p21 cassette followed by excision of the apramycin resistance marker resulted in construct pKDB03. The latter was used for insertion of the actIp cassette to give construct pKDB04, and subsequent insertion of the ermE*p cassette yielded pKDB05. Each version of the refactored cosmid was conjugated into S. coelicolor M1146 for comparative metabolite profiling.
We performed an analogous RT-PCR experiment, as was done prior to refactoring, on RNA isolated from S. coelicolor M1146-pKDB03 carrying the refactored spz pathway. Interestingly, insertion of the bidirectional promoter cassette alone was sufficient to activate transcription of all biosynthetic genes within the cluster (Figure 3A).
Streptophenazine production by the refactored spz BGC
S. sp. CNB-091 (positive control) and S. coelicolor M1146 with integrated pCAP03 (negative control), pKDB01 (non-refactored spz), and pKDB03, −04, and −05 (refactored versions of spz) were grown for comparative metabolomics. After two days of culturing the production media turned dark orange for the strains only carrying the refactored spz BGC, yielding bright orange organic extracts (Figure 2C). Analysis of LCMS data of these pigmented extracts from S. coelicolor M1146-pKDB03, −04, and −05 revealed intense production of at least 38 streptophenazines, as corroborated by HRMS, UV, and MS/MS fragmentation (Figures 2A, S3, and Table S2). This chemical bounty included the 16 streptophenazine compounds previously detected in S. sp CNB091 (Figure 2A), but with increased production levels, as judged by LCMS analysis. Importantly, the production profile proved to be consistent and achievable under a variety of growth conditions, which speaks to the advantage of genetic control. No changes in chemical diversity or production titers from S. coelicolor M1146-pKDB03, – 04, and –05 strains were observed (Figure S3). Therefore, it was decided to continue experiments with S. coelicolor M1146-pKDB03, the strain possessing the spz pathway with the fewest genetic alterations.
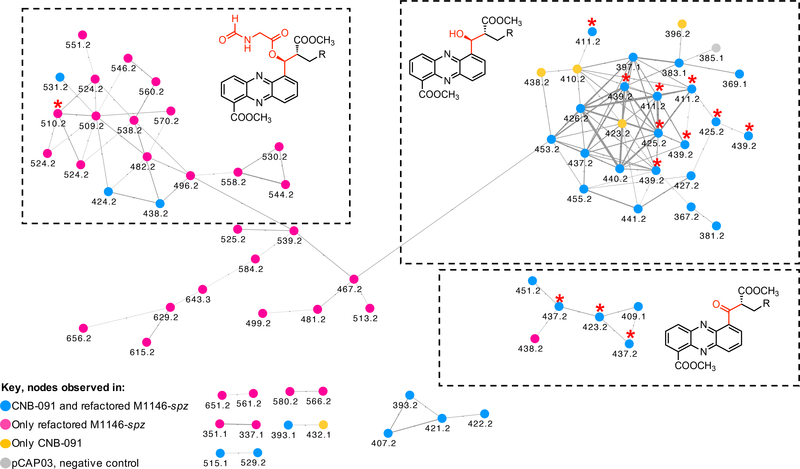
We next used molecular networking to compare S. coelicolor M1146-pKDB01, M1146-pKDB03, CNB-091, and the negative control (M1146-pCAP03) to identify analogs that might have been neglected in the initial visual analysis (Figure S4) (Wang et al., 2016). Immediately, the cluster of nodes with molecular ions corresponding to the streptophenazines stood out (Figure 4). Each node in the putative streptophenazine cluster was inspected for association with streptophenazines by manual analysis of the corresponding MS/MS spectra, UV, and accurate mass (Table S2). The network identified 72 nodes corresponding to streptophenazine-type compounds; 67 of the nodes were found within the refactored strain (pink and blue nodes), 34 of which were found in both the refactored strain and in S. sp. CNB-091 (blue nodes). Five nodes were identified only in S. sp. CNB-091 (orange nodes) and one node included in the cluster (grey) was found in the negative control and is likely to be an artifact of the algorithm. Using the molecular network as a guide, manual inspection of the LCMS data ultimately revealed 112 streptophenazines produced by the refactored spz BGC. In most cases, extracting the LCMS data for the precursor mass found in the network resulted in more peaks than nodes in the network. For example, extracting LCMS data for m/z 411.2 revealed eight peaks corresponding to eight different streptophenazines, rather than the three nodes seen in the network. Several pairs of nodes (e.g., m/z 351.1 and 337.1) appeared separately in the molecular network and did not form clusters with other nodes corresponding to streptophenazines. This finding can be explained by low abundances of these masses in the LCMS data, which resulted in MS/MS fragment spectra with additional peaks from chemical noise, causing the algorithm to create separate clusters (Caraballo-Rodríguez et al., 2017). Table S2 summarizes all streptophenazine products of the spz BGC and contains predicted molecular formulas for compounds where appropriate.
Figure 4. Streptophenazine cluster of nodes from the molecular network.
MS/MS data of extracts of CNB-091, M1146-pKDB01 (non-refactored spz), M1146-pKDB03 (refactored spz), and pCAP03 (negative control) are included and color coded as per key. All nodes are labeled with the corresponding precursor ion mass. Isolated compounds are marked with red stars. See also Figures S3 and S4 and Table S2.
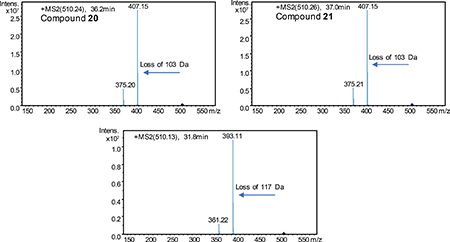
Interestingly, a number of nodes with even mass number were present in the network (e.g., m/z 510.2, 524.2, and 538.2), which suggested an additional nitrogen atom in the chemical formula. These molecular ions were present only in the sample from the refactored spz pathway. We identified three isomeric compounds with m/z 510.2. The early eluting compound (31.8 min) showed a distinctive loss of 117 Da, while the latter two isomers (36.2 and 37 min) instead had identical losses of 103 Da. These fragments suggested formylalanine and formylglycine attachments, respectively, with different methylation patterns at the C-1 carboxylic group (STAR methods). Such modifications are reasonable as the spz pathway contains the gene spz15 that encodes a putative adenylation protein with predicted specificity for alanine.
Isolation and characterization of streptophenazines
Production cultures of S. coelicolor M1146-pKDB03 were scaled up to 6 L to enable isolation and structural elucidation of observed streptophenazines (STAR methods). Ethyl acetate extraction of cultures resulted in 8 g of crude mixture which underwent multiple rounds of flash chromatography (C18 resin and silica resin). Final purification of the targeted compounds was achieved by reversed phase preparative and/or semipreparative HPLC. Ultimately, we isolated 15 streptophenazine analogs.
For tables with NMR assignments, spectra, and key COSY and HMBC correlations for all isolated compounds, see Data S1. Analysis of 1H NMR and 2D spectra revealed that isolated compounds 16, 17, 13, 14, 19, and 18 correspond to reported streptophenazines A, B, C, D, F, and G, respectively. To confirm stereochemistry, we performed circular dichroism (CD) measurements for compound 18 and compared results to the literature data for streptophenazine G with established 1’S,2’R absolute configuration (Kunz et al., 2014; Liang et al., 2017; Yang et al., 2012) (Figure S5A). Due to the proximity of the C-1’ stereocenter to the phenazine chromophore, this chiral center will affect the absorption of circularly polarized light and generate a characteristic shift in optical rotation (Cotton effect) evident in a CD spectrum. A strong negative Cotton effect at 251 nm, which mirrored that of previously characterized streptophenazine G, confirmed the S conformation of the C-1’ stereocenter of 18. 1H NMR shifts of compound 18 were identical to those reported in the literature for streptophenazine G, indicating that the absolute configuration of compound 18 was also 1’S,2’R. Based on the CD result and the fact that stereochemistry of these centers remains unchanged for all reported streptophenazines, we propose the same stereochemistry for all compounds isolated in this work. To further support the stereochemistry at C-1’, we performed bioinformatic analysis of the ketoreductase (KR) protein Spz7, which we presume catalyzes the stereospecific reduction of the β-keto intermediate to the 1’-hydroxyl group. KR domains of modular PKS enzymes define the configuration at α- and β-carbons, and conserved amino acid motifs that control the stereochemical outcome of this reduction have been defined (Caffrey, 2003; Keatinge-Clay, 2007; Smith and Tsai, 2007). While Spz7 shows relatively low amino acid sequence similarity to modular KR domains (Figure S5B), we identified the LDD amino acid motif characteristic of type B KR domains, which would generate the 1’S stereocenter in streptophenazines, consistent with the established stereochemistry.
Compounds isolated in this work included 9, 10, 11, and 12, which were individually isolated as orange powders. HRMS analysis showed that 9 and 10 share the same molecular formula C24H27N2O5 (m/z 423.1893 [M + H]+ and m/z 423.1925 [M + H]+, respectively), while compounds 11 and 12 have molecular formula C25H29N2O5 (m/z 437.2075 [M + H]+ and m/z 437.2087 [M + H]+, respectively). These four compounds appeared to be derivatives of streptophenazines A, B, G, and F, respectively, but were missing the C-1’ oxymethine low field shift (δC ~ 72) seen in previously characterized streptophenazines. Instead, HMBC correlations revealed a carbon shift indicative of a ketone carbonyl (δC ~ 198.3) that showed correlations to both the phenazine core and the alkyl side chain. We suggest the name oxostreptophenazine A for compound 9, oxo-streptophenazine B for compound 10, oxostreptophenazine G for compound 11, and oxo-streptophenazine F for compound 12.
Compound 15 (C23H27N2O5, m/z [M + H]+ 411.1912) shares a 2D structure with streptophenazine D, but terminates in a single methyl group rather than a branched chain (Figure 1D). Paralleling the established nomenclature of streptophenazines, we propose the name streptophenazine P for this compound. Additionally, compounds 22 and 23 had NMR spectra that appeared almost identical to streptophenazines G and F, however, the proton NMR signals associated with C-3’, C-2’, the C-2’ carbomethoxy group (2’-COOCH3), and C-1’ were slightly shifted (Data S1), which indicated that compounds 22 and 23 were diastereomers at the C-2’ position of streptophenazines G and F, respectively.
Two of the three detected compounds with m/z 510.2 were isolated (20 and 21), both with MS/MS fragmentation patterns suggestive of a formylglycine moiety adjoined to the streptophenazine core structure, most plausibly via the hydroxyl group at C-1’. The molecular formula C27H32N3O7 was established for compounds 20 and 21 (m/z [M + H]+ 510.2236 and 510.2234, respectively). NMR spectra of these compounds contained a signal (δH 8.24, δC 161), suspected to be the formyl group, which showed COSY correlations to two protons (δH 4.22, 4.18) with a carbon shift of δC 40.25, as well as a COSY correlation to a broad peak δH 6.15 with no corresponding carbon shift in the HSQC shift (amide proton) (Data S1). Two doublets (δH 4.22, 4.18) showed key HMBC correlations to two carbonyl groups (δC 161 and 168) and COSY correlations to the amide and formyl protons, establishing their position as the glycine α-protons, and thus fully confirming the presence of the N-formylglycine moiety. Compound 20 was found to terminate the alkyl chain in two branched methyl groups while compound 21 terminated the chain in a single methyl group (Figure 1D). We propose the names streptophenazine Q and streptophenazine R, respectively, for compounds 20 and 21.
Anti-microbial activity of streptophenazines
A series of four streptophenazines, 9, 13, 16, and 20, were tested for antibiotic activity against two Gram-positive organisms: group A Streptococcus (GAS) and methicillin-resistant Staphylococcus aureus (MRSA) TCH1516, and two Gram-negative organisms: Acinetobacter baumannii 5075 and Klebsiella pneumoniae 1100. The four test compounds were selected because they share the same alkyl chain but differ in the C-1’ substituent (keto, hydroxyl, or formylglycine) and methylation state of the C-1 carboxylic group. Results showed that compounds 9, 13, and 16 had minimum inhibitory concentration (MICs) higher than their tested concentrations, indicating weak or absent antimicrobial activity (Table S3). However, compound 20, the N-formylglycine analogue, showed significantly more potent activity than the hydroxyl or ketone containing analogs against GAS (MIC 2.5 μg/mL). Compound 20 also inhibited the growth of MRSA and the tested Gram-negative pathogens, while no such activity was observed for the other analogs. This set of structurally similar compounds allows us to infer structure-activity relationships of streptophenazines, and this data suggests that the formylglycine moiety leads to superior bioactivity.
Spz15 – a unique standalone adenylation protein required for formylglycine modification
Arguably the most interesting aspect of the spz biosynthetic pathway is the production of the N-formylglycine-containing streptophenazines Q and R (20, 21). Although common in ribosomal protein biosynthesis, N-formylation is a rare biosynthetic tailoring modification in NRPS-synthesized peptides, famously performed in linear gramicidin A by a specific formylation domain (Kessler et al., 2004; Schoenafinger et al., 2006). Bioinformatic analysis suggests that spz15 encodes an adenylation protein, and identification of the GXXGXPKG motif for a phosphate binding loop and AMP-binding Pfam domain (PF00501) reinforced this hypothesis (Figure S6) (Perego et al., 1995). However, neither a canonical condensation domain nor a formylation domain is present in the spz cluster. The lack of a traditional suite of NRPS domains suggested that perhaps Spz15 is solely responsible for the addition of the N-formylglycine moiety. To prove involvement of the Spz15 protein in the biosynthesis of the amino acid-containing compounds, we deleted the spz15 gene. The resulting mutant construct (pKDB03Δspz15) was confirmed by restriction digest (Figure S6) and integrated into the genome of the heterologous host. LCMS analysis of organic extracts of the mutant strain showed no signs of streptophenazines containing the N-formylglycine moiety. However, production of all other streptophenazines remained unchanged, ruling out polar effects on downstream genes in the spz15 deletion mutant (Figure S6).
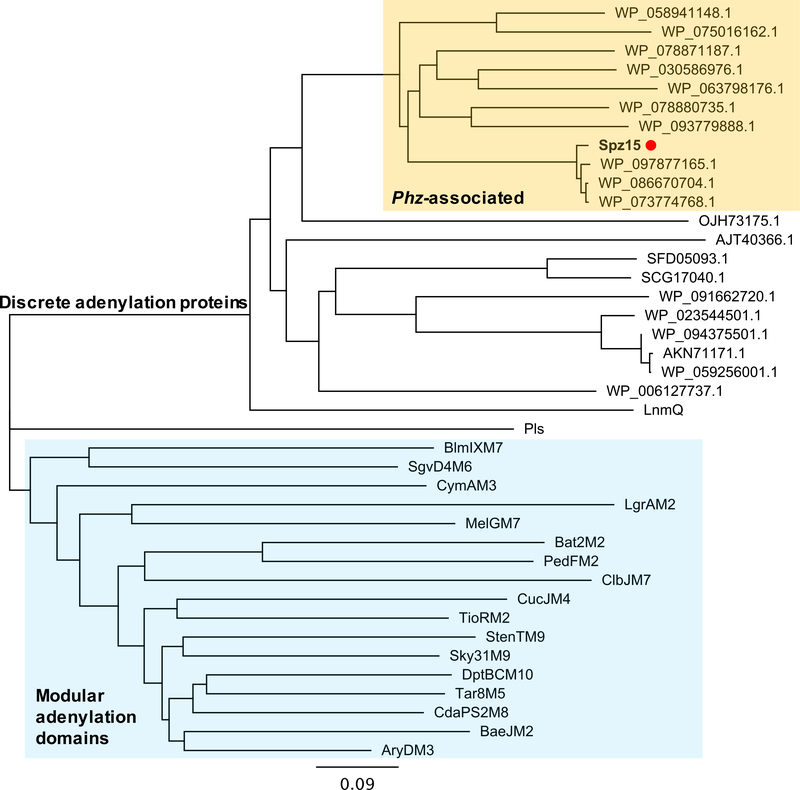
Phylogenetic analysis of Spz15 revealed that this enzyme is strikingly distinct from canonical adenylation domains and instead forms a separate evolutionary clade (Figure 5). Analysis of genomes containing Spz15-like proteins revealed that these putative adenylation proteins are all found adjacent to phenazine biosynthesis genes (Figure S7). This arrangement indicates that spz15 may represent a genetic hook into unusual hybrid phenazine-type biosynthetic pathways. Biochemical characterization and investigation of the role of Spz15 in this unusual addition of an N-formylglycine moiety to streptophenazine molecules are currently ongoing.
Figure 5. Phylogenetic analysis of Spz15 adenylation protein.
Phylogenetic tree of Spz15 with characterized adenylation domains and NCBI blastp homologs of Spz15 was constructed using Geneious software. Spz15 (marked with orange dot) forms a separate clade from canonical modular characterized adenylation domains (highlighted in blue). Proteins that clade with Spz15 (highlighted in orange) are found within putative phenazine type (phz) BGCs. The remaining protein sequences are discrete standalone adenylation enzymes. Protein abbreviations as follows: LnmQ – leinamycin, Pls – ε-poly-L-lysine, NRPS: BlmIXM7 – bleomycin module 7, SgvD4M6 – griseoviridin module 6, CymAM3 – cyclomarin module 3, LgrM2 – linear gramicidin module 2, MelGM7 – melithiazol module 7, Bat2M2 – batumin module 2, PedFM2 – pederin module 2, ClbJM7 – colibactin module 7, CucJM4 – cupriachelin module 4, TioRM2 – thiocoraline module 2, StenTM – stenothricin module 9, Sky31M9 – skyllamycin module 9, DptBCM10 – daptomycin module 10, Tar8M5 – taromycin module 5, CdaPS2M8 – calcium dependent antibiotic module 8, BaeJM2 – bacillaene module 2, AryDM3 – arylomycin module 3; all sequences were downloaded from NCBI protein database. See also Figures S6 and S7.
Discussion
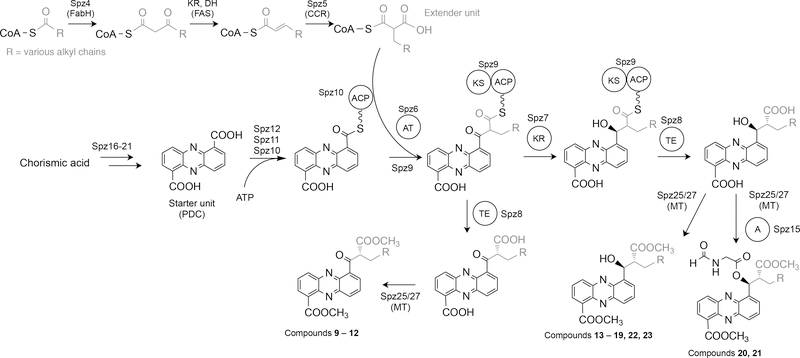
The discovery of the unique hybrid PHZ/PKS/NRPS BGC encoded in the genome of marine S. sp. CNB-091 uncovered a pathway that involves unusual biosynthetic transformations and yields a tremendous wealth of chemical products. Fundamentally, the spz BGC is arranged as a series of subclusters, each responsible for a distinct aspect of the biosynthesis of the final molecules, evoking the concept of a ‘cluster of clusters’ (Figure 3B). A set of contiguous genes (spz16–21) are responsible for the biosynthesis of the PDC core, which serves as the starter unit for a single PKS extension with an unusual long branched chain malonyl-CoA constructed by a crotonyl-CoA carboxylase (CCR)-like mechanism (Figure 6) (Wilson and Moore, 2012). To date, there is only one other example of a PKS extension of a phenazine core substrate, described in the biosynthesis of the esmeraldins and saphenamycin phenazine metabolites (Rui et al., 2012).
Figure 6. Proposed biosynthesis of streptophenazines from Streptomyces sp. CNB-091.
R denotes alkyl substituents for the side chain. A variety of polyketide extender units arise from diverse substituted malonyl-CoA derivatives from primary metabolism through catalysis by a crotonyl-CoA carboxylase/reductase (Spz5).
A phylogenetically distinct adenylation enzyme (Spz15), transcribed from the same operon as the spz16–21 genes, is unambiguously involved in the addition of an N-formylglycine moiety to streptophenazines, generating unusual hybrid molecules with potent bioactivities (Figure S6). Finally, two genes at the west bound of the cluster (spz1,2) encode cytochrome bd oxidase enzymes, which may represent a resistance mechanism for the phenazine-producing strain S. sp. CNB-091 (Voggu et al., 2006). The spz BGC represents unique biosynthetic machinery that does not fit neatly within any biosynthetic systems described, and therefore, opens the doors for future mechanistic biochemical studies (Figure 6).
Rewriting native genetic elements in the spz gene cluster with optimized promoters and RBS sequences allowed us to circumvent issues associated with the complex network of native regulation and ultimately gain mastery over this unique biosynthetic system. Interestingly, inserting just the first bidirectional promoter cassette resulted in activation of transcription of all spz biosynthetic genes (Figure 3). It is possible that production of the phenazine core (PDC) is required to trigger expression of the remaining biosynthetic genes (‘self-induction’). Certain small molecules can bind to specific DNA regions resulting in activation of transcription of the downstream genes (Chalabaev et al., 2008; Denison and Kodadek, 1998). Alternatively, the strength of the sp44 promoter could be sufficient to activate transcription of the genes beyond the phenazine-encoding operon, and there is precedent of a single promoter driving transcription of multiple genes, spanning over 10 kb (Amagai et al., 2017; Luo et al., 2015).
Ultimately, refactoring the spz pathway produced an incredible wealth of chemical diversity (112 streptophenazines) that can serve as a library of compounds, much like a combinatorial chemist might produce. This diversity-oriented biosynthesis is generally considered an evolutionary advantage (Fischbach and Clardy, 2007), as diverse analogs may have different biological targets or act synergistically as an antibiotic cocktail (Wu and Seyedsayamdost, 2017). This may explain the lack of production of the N-formylglycine analogs by the wild type strain; bioactivity testing revealed that these compounds did possess distinct bioactivity, and thus they may be produced only under certain environmental and/or physiological stress. Therefore, amino-acid containing streptophenazines are excellent starting points for further chemical derivatization with the goal of ultimately achieving superior antibiotic properties.
The problem regarding inconsistent or absent production of secondary metabolites is a common occurrence, both within native organisms and heterologous hosts, and the disconnect between the genetically encoded biosynthetic potential of an organism and the actual suite of compounds produced is immense. The issue addressed here is not just successful activation of cryptic BGCs, but exploitation of the full range of capabilities housed by these pathways. Through engineering, we were able to achieve production of broad chemical diversity, including compounds not seen in the native organism that contain an unprecedented formylglycine moiety that imparts enhanced antibiotic activity. This example suggests that even transcriptionally active clusters may benefit from refactoring.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Bradley Moore (bsmoore@ucsd.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Streptomyces sp. CNB-091 was the original streptophenazine producer and S. coelicolor M1146 was used for heterologous expression of the streptophenazine biosynthetic gene cluster. E. coli DH10B cells were used for plasmid storage and replication, E. coli ET12567 and E. coli ET12567/pUB307 were used for triparental intergeneric conjugation. E. coli BW25113 and E. coli DH5α/BT340 (Cherepanov and Wackernagel, 1995; Datsenko and Wanner, 2000) were used for λ-Red recombineering. Culture conditions followed literature procedures and are explained in Method Details.
METHOD DETAILS
General methods
Primers, plasmids and strains used in this study are summarized in Tables S5–S7.
Bacterial strains and growth conditions
Streptomyces strains were grown in TSBY liquid medium (3% tryptic soy broth, 10.3% sucrose, 0.5% yeast extract) for isolation of genomic DNA and on SFM agar plates (2% D-mannitol, 2% soya flour, 2% agar) for conjugation and strain maintenance. Liquid cultures were grown at 30 °C with shaking at 220 rpm in a rotary shaker. Solid cultures were grown at 30 °C for 10–12 days.
For streptophenazine production, seed medium (TSBY) was inoculated with S. coelicolor M1146 spore suspension and cultured for 36 hrs. A 5% inoculum of seed culture were used for fermentation medium (also TSBY medium). Fermentation was carried out at 30 °C and 220 rpm in a rotary incubation for 3 days.
E. coli strains were grown in Luria-Bertani (LB) broth or on LB agar at 37 °C with appropriate antibiotics for selection (apramycin 50 μg/mL, ampicillin 100 μg/mL, chloramphenicol 25 μg/mL, kanamycin 50 μg/mL, nalidixic acid 25 μg/mL). For conjugation purposes, E. coli was grown using 2TY medium (1.6% tryptone, 1% yeast extract, 0.5% NaCl) with appropriate antibiotic selection.
Software and tools used
The web-based bioinformatics program antiSMASH was used to analyze the whole genome sequence of S. sp. CNB-091 (Weber et al., 2015). The sequence of the spz gene cluster was further analyzed using BLAST (Altschul et al., 1990). Protein sequence alignments were made using Clustal Omega software (Sievers and Higgins, 2014). Geneious software was used for all plasmid maps, promoter cassette design, and phylogenetic tree reconstruction (Kearse et al., 2012). HRMS data were analyzed with MassHunter software (Agilent), low resolution MS data was analyzed with Compass DataAnlalysis software (Bruker Daltonic). NMR data was recorded with Bruker Topspin 2.1.6 software and analyzed with MestreNova software. GNPS (gnps.ucsd.edu) was used for molecular networking analysis (Wang et al., 2016). The spectral networks were imported into Cytoscape 3.6.0 (Shannon et al., 2003).
Preparation of genomic DNA and PCR fragments for TAR cloning
S. sp. CNB-091 gDNA was isolated following standard procedures from Practical Streptomyces Genetics (Kieser et al., 2000). Approximately 200 μg of gDNA was digested with SnaBI, in an overnight reaction at 30 °C. Digested gDNA fragments were precipitated and cleaned by isopropanol precipitation. The resulting gDNA pellet was dissolved in 50 μL 10 mM Tris buffer (pH 7).
Takara PrimeSTAR HS DNA polymerase with GC buffer was used to amplify the eight fragments of the spz cluster for TAR cloning using gDNA as a template. PCR reactions were carried out in a BioRad MyCycler with gradient option. Fragments were designed with average 200 bp overlap to neighboring fragments and the first and last fragment with 40 bp overlap to pCAP03 capture vector. For primers see Table S5. PCR conditions were as follows:
| PCR reaction: | Vol (μL) |
|---|---|
| Milli-Q Water | 20.5 |
| Primer 1 (10 μM) | 1 |
| Primer 2 (10 μM) | 1 |
| Template gDNA | 1 |
| 2X GC Buffer | 25 |
| dNTPs (10 mM) | 1 |
| Primestar polymerase | 0.5 |
| Total volume | 50 |
| PCR cycling condition (30 cycles) | ||
| Initial denaturation | 98 °C | 1 min |
| Denaturation | 98 °C | 10 s |
| Annealing | 60 °C | 10 s |
| Extension | 72 °C | 5 min |
| Final extension | 72 °C | 10 min |
| 4 °C | ∞ | |
PCR fragments were cut from agarose gels and purified using the Qiagen QIAquick Gel Extraction kit according to the manufacturer’s instructions.
TAR cloning of the spz cluster
Initially, gDNA was used as a template to directly capture the 48.5 kb region encompassing the spz BGC following general procedures described by (Tang et al., 2015; Yamanaka et al., 2014). The spz pathway-specific capture vector was constructed by introducing two PCR-amplified 1 kb homology arms corresponding to flanking regions of the targeted region into pCAP03. S. cerevisiae VL6–48N (Table S4) was grown to an OD600 of 0.7 to 1.0 in 50 mL of YPD medium (2% D-glucose, 1% yeast extract, 2% peptone) supplemented with adenine hemisulfate salt (100 mg/L) at 30 °C with shaking. Cells were harvested and washed with ice cold water and osmotically stabilized in 1M sorbitol at 4 °C overnight prior to spheroplast preparation. Preparation of spheroplast cells was carried out with zymolase at a final concentration of 0.1 mg/mL with 30–40 minutes of incubation. Spheroplast cells were mixed with approximately 0.5 μg of gDNA and 0.5 μg of linearized specific capture vector. Transformed spheroplasts were mixed with 8 mL of synthetic drop-out tryptophan top agar at 55 °C and overlaid on synthetic tryptophan drop-out (SD-Trp) top agar containing 5-fluorootic acid (5-FOA) for selection. Plates were incubated at 30 °C for 4–5 days before transformants were picked and screened by PCR for positive colonies. Plasmids were extracted and then transferred into E. coli DH10B cells by electroporation. Plasmids were then purified from antibiotic resistance clones, and the resulting constructions confirmed by restriction analysis.
PCR-based cloning (Shao et al., 2009) followed the same procedures described above and by Yamanaka et al. (2014) and Tang et al. (2015), mixing 200 ng of each PCR fragment with the linearized capture vector pCAP03.
Heterologous expression of the spz cluster
pKDB01 and its derivatives were transformed into E. coli ET12567 by heat shock at 42 °C for 55 s, and then transferred to S. coelicolor M1146 by triparental intergeneric conjugation facilitated by E. coli ET12567/pUB307 (MacNeil et al., 1992) (Table S4). Exconjugants were grown on soy flour mannitol agar containing MgCl2 (10 mM). After incubation at 30 °C for 18 h, plates were overlaid with 1 mL water containing 0.5 mg nalidixic acid followed by 1 mL water containing 1 mg apramycin and then grown until appearance of exconjugants, which were then replated for a second round of selection.
RNA extraction and RT-PCR analysis
S. coelicolor M1146-pKDB01 and -pKDB03 were grown following the methods for production of streptophenazines. After 24 h growth in production media, 1 mL of culture was sampled, pelleted and washed with RNase free water. RNA was extracted using Ambion’s PureLink RNA extraction kit following methods for bacterial cells. Briefly, cells were lysed and homogenized using a 20G needle attached to an RNase-free syringe. RNA was bound to a spin column, washed, and eluted with RNase-free water. Concentrations were analyzed using Invitrogen Qubit Fluorometric Quantitation. For primers see Table S6.
RT-PCR was performed using Qiagen’s OneStep RT-PCR kit as follows:
| PCR reaction: | Vol (μL) |
|---|---|
| RNase-free water | 23.5 |
| 5x buffer | 10 |
| 5x Q-solution | 10 |
| dNTPs (10 mM each) | 2.0 |
| Primer 1 (30 μM) | 1 |
| Primer 2 (30 μM) | 1 |
| Enzyme mix | 2.0 |
| Template RNA | 0.5 |
| Total volume | 50 |
| RT-PCR cycling condition (30 cycles) | ||
| Reverse transcription | 50 °C | 30 min |
| Initial PCR inactivation | 90 °C | 15 min |
| Denaturation | 94 °C | 1 min |
| Annealing | 67 °C | 1 min or 1.5 min |
| Extension | 72 °C | 1 min |
| Final extension | 72 °C | 10 min |
| 4 °C | ∞ | |
Promoter cassette construction
The vector pCAP03 was used as the backbone for cloning and construction of all cassettes. pCAP03 was digested with XhoI and NdeI restriction enzymes and recovered from a 0.7% agarose gel. Apramycin and ampicillin resistance genes were PCR amplified from in house plasmids (pKY01 and pETduet-1, respectively). For the bidirectional promoter cassette, the apramycin resistance gene was amplified with primers that contained flanking FRT sites added synthetically. Promoters regions were PCR amplified from in-house plasmids (not published) with primers containing appropriate homology regions for further Gibson assembly. Target fragments were recovered from a 0.7% agarose gel. Gibson assembly was used to combine the digested and purified PCR products with the vector according to manufacturer’s guidelines, followed by transformation into E. coli DH10B competent cells using heat shock at 42 °C for 55 s. Cells were transferred onto LB plates with appropriate antibiotics for selection. After incubation overnight, transformants were picked and inoculated into 10 mL LB broth containing appropriate antibiotics and incubated at 37 °C with shaking at 220 rpm for approximately 16 h. Plasmids were isolated and identity was confirmed by restriction analysis and DNA sequencing. Finally, promoter cassettes were PCR amplified using primers with 50 bp overlap to the adjacent sequence of the targeted DNA within the spz cluster (Table S5).
DNA manipulation
All genetic manipulations (gene deletions and promoter replacements) in pKDB01 and pKDB02 were carried out using the λ-Red recombination-mediated PCR-targeted gene deletion/insertion method, as described by Gust et al. (2003). Electrocompetent E. coli BW25113 carrying the pIJ790 plasmid were electroporated (Eppendorf Electroporator 2510) at 2,500 V in a 2-mm cuvette with the pKDB01 or pKDB02 cosmid. Expression of the λ-Red genes was induced with arabinose (10 mM) and the cells were then transformed with the promoter cassette described above and incubated at 37 °C. Cosmid DNA was then isolated and tested by restriction digest and Sanger sequencing to confirm correct genetic alteration, prior to conjugation into S. coelicolor M1146. For the creation of pKDB03, the pKBD01 cosmid containing the sp44-p21 promoter cassette was transformed into E. coli DH5α cells containing the temperature sensitive FLP recombination plasmid BT340. FLP production and loss of the plasmid were induced at 42 °C. Cosmid DNA was isolated and the successful excision of the resistance cassette was verified by restriction digest and Sanger sequencing prior to integration into S. coelicolor M1146.
For analytical restriction digests, 1.5 μL 10X CutSmart Buffer (NEB), 500 ng DNA, 1 unit of restriction enzyme, and MQ water to a total volume of 15 μL were incubated for 2 hrs at 37 °C and then heat inactivated at 65 °C for 20 minutes. For preparative restriction digests, 50 μL reactions including 5 μL 10X CutSmart Buffer (NEB), 1 μg DNA, 2 units of restriction enzyme, and MQ water were incubated for 5 hrs at 37 °C prior to heat inactivation and further use.
Streptophenazine metabolite analysis
Production cultures (liquid and solid) were extracted by an equal volume of ethyl acetate. Liquid cultures were adjusted to pH ~4 with formic acid prior to extraction. The organic phase was evaporated and samples were reconstituted in methanol and filtered through a 0.2 μm filter for subsequent LCMS analysis.
A 10 μL aliquot of methanol extract was injected onto a Phenomenex Luna C18 reversed-phase HPLC column (5 μm, 250 mm × 4.6 mm) and analyzed with a Bruker Amazon Ion Trap MS system coupled to an Agilent 1260 Infinity LC system. A solvent system of acetonitrile and water both containing 0.1% formic acid (v/v) was used. Samples were eluted over a 60 min method with a gradient from 10 to 45% acetonitrile over 15 minutes, 45 to 65% over the next 30 min, and then to 100% over 10 min. 100% acetonitrile was held for 1 min before concentration was dropped to 10%. Flow rate was 0.75 mL/min
Eluent was detected using electrospray ionization-mass spectrometry (ESI-MS) monitoring m/z 70–2,200 in positive mode with a speed of 32,500 m/z /s. HRMS was carried out on an Agilent 6530 Accurate-Mass Q-TOF in positive mode, low resolution data was acquired using an Agilent 1260 Infinity IonTrap in positive mode.
Molecular networking
Input data for molecular networking was generated through conversion of the LCMS/MS raw data to .mzXML data format using ProteoWizard MSConvert software. Data were submitted to the molecular networking workflow at the GNPS online platform (gnps.ucsd.edu).
The GNPS molecular network was created using parameters as follows: A molecular network was created using the online workflow at GNPS. The data was clustered with MS-Cluster with a parent mass tolerance of 0.5 Da and a MS/MS fragment ion tolerance of 0.5 Da to create consensus spectra. Further, consensus spectra that contained less than 2 spectra were discarded. A network was then created where edges were filtered to have a cosine score above 0.5 and more than 2 matched peaks. Edges between two nodes were kept in the network if each of the nodes appeared in each other’s respective top 10 most similar nodes. The spectra in the network were then searched against GNPS’ spectral libraries. The library spectra were filtered in the same manner as the input data. All matches kept between network spectra and library spectra were required to have a score above 0.7 and at least 6 matched peaks. See table below for parameters.
| Parameter | Value |
|---|---|
| Pairs_Min_Cosine | 0.5 |
| Analog_Search | 0 |
| Tolerance.PM_tolerance | 0.5 |
| Tolerance.ion_tolerance | 0.5 |
| Min_matched_peaks | 2 |
| TopK | 10 |
| Cluster_Min_Size | 2 |
| Maximum_component_size | 100 |
| Min_peak_int | 0.0 |
| Filter_Stddev_peak_int | 0.0 |
| Run_MScluster | on |
| Filter_precursor_window | 0 |
| Filter_library | 1 |
| Window_filter | 0 |
| Score_threshold | 0.7 |
| Min_matched_peaks_search | 6 |
| Max_shift_mass | 100.0 |
Molecular networks can be viewed at: https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=24d515f76b4c4d6694a934234435b9bf (Related to Figure 2, Figure 4, Figure S4 and Table S2) and https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=9a2d02c583c24422bfaa16e2f8ae9ceb (Related to Figure 2 and Figure S3)
HRMS, UV, and MS/MS data for each precursors mass in the network were inspected to confirm relationship to streptophenazines. Example of characteristic MS/MS below:
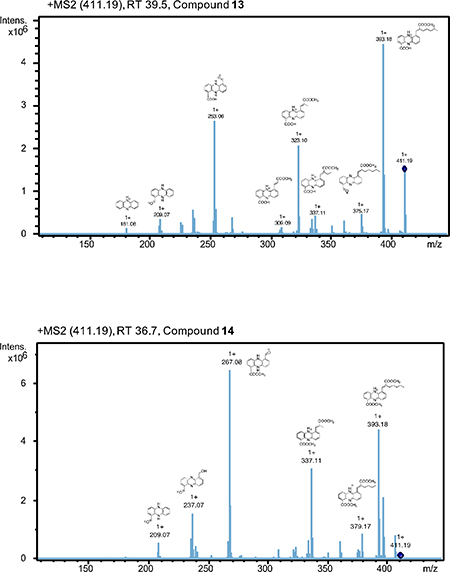
MS/MS spectra of three m/z 510.2 compounds, including the two N-formylglycine-containing compounds (20 and 21) and the proposed formylalanine-containing compound:
Purification and isolation of streptophenazines
Approximately 6 L of 14-day-old culture of S. coelicolor M1146-pKDB03 were adjusted to pH ~4 and extracted with an equal volume of ethyl acetate. Organic layers were combined and solvent was evaporated, yielding 8 g of orange solid. The extract was redissolved in MeOH and separated on a bench-packed C18 column, which was washed with increasing MeOH concentrations. Fractions containing streptophenazine metabolites were combined, and the organic solvent was evaporated under vacuum followed by lyophilization. The sample was further purified using a column of silica gel (12 nm, 70 mesh) using a mixture of hexane/ethyl acetate.
Further purification was achieved by preparative HPLC using a Phenomenex Luna C18 column (5 μm, 100 mm x 2 mm), along with an Agilent Technologies system composed of a PrepStar pump, a ProStar 410 autosampler, and a ProStar UV detect (Agilent Technologies, Inc, Santa Clara, USA). Acetonitrile and water, both with 0.1% trifluoroacetic acid (TFA) (v/v), were used as mobile phase. Samples were eluted over 60 min at a flow rate of 15 mL/min. Samples were subsequently subjected to further semi-preparative HPLC purification, using a Phenomenex Luna C18 reversed-phase HPLC column 5 μm, 250 mm × 4.6 mm) using acetonitrile and water, both containing 0.1% TFA, flow rate 2.5 mL/min. Final fractions yielded 1 mg of compounds 13, 14, 15, 22 and 23, 3 mg compounds 9–12, and over 5 mg of compounds 16–21. All stages of purification were monitored by LCMS analysis.
Structural characterization of streptophenazines
All NMR data were collected using at the UCSD Skaggs School of Pharmacy and Pharmaceutical Sciences NMR Facility on a 600 MHz Bruker NMR spectrometer (Topspin 2.1.6 software, Bruker) consisting of a Magnex 54 mm bore superconducting magnet operating at 14.1 Tesla with a 1.7 mm cryoprobe. Deuterated chloroform containing 1% (v/v) TMS standard was used as a solvent. See Data S1.
Bioactivity testing of streptophenazines and MIC determination
MIC values were determined using broth microdilution in accordance with the Clinical Laboratory Standards Institute (CLSI) guidelines using cation-adjusted Mueller Hinton Broth (MHB) with minor modifications. Briefly, bacteria were grown to mid-log phase (OD600nm = 0.4) at 37 ºC while shaking except GAS which was grown under static condition. Bacterial cells were then centrifuged, washed, and diluted in PBS to obtain 2×106 cfu/mL, and 10 μL was added to individual wells of a 96-well plate containing 170 μL MHB.
Serial dilutions of compounds 9, 13, 16 starting at 500 μg/mL and compound 20 at 400 μg/mL were made in a separate plate, 20 μL of each compound was then added to the test plate. The plates were sealed with parafilm and incubated at 37 ºC for 24 h. The turbidity of each plate was measured at OD600nm using the EnSpire Alpha plate reader. MIC was defined as the lowest concentration of the drugs that inhibited bacterial growth.
Phylogenetic analysis of Spz15
The Geneious neighbor-joining tree building method was used for phylogenetic tree reconstruction. Proteins selected for analysis included the closest uncharacterized homologs of Spz15 found using protein BLAST analysis, characterized discrete adenylation proteins, and modular NRPS-associated adenylation domains from characterized BGCs with specificity for alanine or glycine. The sequences of A domains were trimmed according to the annotation by antiSMASH and the MIBiG repository (Medema et al., 2015).
QUANTIFICATION AND STATISTICAL ANALYSIS
For bioactivity assays and MIC determination, each sample was repeated three times.
DATA AND SOFTWARE AVAILABILITY
All promoter cassette plasmids are available at Addgene: pCAP03-sp44/p21 cassette (Addgene ID 120232), pCAP03-actIp cassette (Addgene ID 120233), pCAP03ermE*p cassette (Addgene ID 120234). The public datasets used for the GNPS molecular networking are available at: ftp://massive.ucsd.edu/MSV000083081 and ftp://massive.ucsd.edu/MSV000083082. The molecular networks can be accessed at: https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=24d515f76b4c4d6694a934234435b9bf (for Figure 3, Figure S4, and Table S2) and: https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=9a2d02c583c24422bfaa16e2f8ae9ceb (for Figure S3).
Supplementary Material
Table S2, related to Figures 2, 3 and 4. High resolution MS (HRMS), retention time, and predicted chemical formula for nodes in streptophenazine cluster from molecular network. Excel file attached separately.
Highlights.
Streptophenazine biosynthetic pathway (spz) was cloned from marine Streptomyces
Refactoring the spz pathway led to tremendous chemical diversity (112 compounds)
Streptophenazines with N-formylglycine moiety show potent antibiotic activity
Discrete adenylation protein is required to produce N-formylglycine analogs
Significance.
This work presents the discovery, engineering, and heterologous expression of the hybrid streptophenazine (spz) biosynthetic gene cluster from marine Streptomyces that unites phenazine, polyketide, and nonribosomal peptide biochemistry in an unprecedented expansion on the biosynthetic and chemical logic of the phenazine class of metabolites. We developed a set of promoter cassettes compatible with the widely used pCAP series of shuttle vectors to successfully link the cryptic spz BGC to its molecular products, including N-formylglycine analogs that possess superior antibiotic activity. We identified one gene, spz15, that encodes a discrete adenylation protein as a genetic search hook into analogous systems, and propose its involvement in the attachment of the rare N-formylglycine moiety. This discovery encourages future work to reveal the mechanism behind this biosynthetic transformation. Complete understanding of the biosynthesis of diverse streptophenazines opens an opportunity for engineering to improve the bioactivities of these compounds. Ultimately, using biology to inform chemistry in this way to reliably regulate expression of BGCs is fundamental in discovering, understanding, and governing the chemistry of our natural world.
Acknowledgements
We are grateful to P.R. Jensen and W. Fenical (Scripps Institution of Oceanography) for providing Streptomyces sp. CNB-091, M. Bibb (John Innes Center) for S. coelicolor M1146, B.M. Dungan (UC San Diego) for assistance with NMR, F.A. Tezcan and R.G. Alberstein (UC San Diego) for assistance with CD, and L. Ray, S.K. McKinnie, T. de Rond, and P.A. Jordan (Scripps Institution of Oceanography) for helpful discussions. This work was supported by grants from the National Institutes of Health through grants R01-GM085770 and R01-AI117712 to B.S.M. and NIH Marine Biotechnology Training Grant Predoctoral Fellowship (T32 GM067550) to K.D.B.
Footnotes
Declaration of interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Gish W, Miller W, Myers EW, and Lipman DJ (1990). Basic local alignment search tool. J. Mol. Biol 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Amagai K, et al. (2017). Identification of a gene cluster for telomestatin biosynthesis and heterologous expression using a specific promoter in a clean host. Sci. Rep 7, 3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C, Zhang Y, Zhao X, Hu Y, Xiang S, Miao J, Lou C, and Zhang L (2015). Exploiting a precise design of universal synthetic modular regulatory elements to unlock the microbial natural products in Streptomyces. Proc. Natl. Acad. Sci. U.S.A 112, 12181–12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley SD, et al. (2002). Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417, 141–147. [DOI] [PubMed] [Google Scholar]
- Bibb MJ, Janssen GR, and Ward JM (1985). Cloning and analysis of the promoter region of the erythromycin resistance gene (ermE) of Streptomyces erythraeus. Gene 38, 215–226. [DOI] [PubMed] [Google Scholar]
- Blankenfeldt W, and Parsons JF (2014). The structural biology of phenazine biosynthesis. Curr. Opin. Struct. Biol 0, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunbamrung N, Dramae A, Srichomthong K, Supothina S, and Pittayakhajonwut P (2014). Streptophenazines I–L from Streptomyces sp. BCC21835. Phytochem. Lett 10, 91–94. [Google Scholar]
- Caffrey P (2003). Conserved amino acid residues correlating with ketoreductase stereospecificity in modular polyketide synthases. Chembiochem. 4, 654–657. [DOI] [PubMed] [Google Scholar]
- Caraballo-Rodríguez AM, Dorrestein PC, and Pupo MT (2017). Molecular interkingdom interactions of endophytes isolated from Lychnophora ericoides. Sci. Rep 7, 5373–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalabaev S, Turlin E, Bay S, Ganneau C, Brito-Fravallo E, Charles J-F, Danchin A, and Biville F (2008). Cinnamic acid, an autoinducer of its own biosynthesis, is processed via Hca enzymes in Photorhabdus luminescens. Appl. Environ. Microbiol 74, 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov PP, and Wackernagel W (1995). Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibioticresistance determinant. Gene 158, 9–14. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, and Wanner BL (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen D, et al. (2014). Transcription inhibition by the depsipeptide antibiotic salinamide A. ELife 3, e02451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain AL, and Fang A (2000). The natural functions of secondary metabolites. Adv. Biochem. Eng. Biotechnol 69, 1–39. [DOI] [PubMed] [Google Scholar]
- Denison C, and Kodadek T (1998). Small-molecule-based strategies for controlling gene expression. Chem. Biol 5, R129–R145. [DOI] [PubMed] [Google Scholar]
- Dietrich LEP, Price-Whelan A, Petersen A, Whiteley M, and Newman DK (2006). The phenazine pyocyanin is a terminal signaling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol 61, 1308–1321. [DOI] [PubMed] [Google Scholar]
- Dietrich LEP, Teal TK, Price-Whelan A, and Newman DK (2008). Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321, 1203–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed B, Ashford DA, Hashem AM, Amin MA, Gazayerly ONE, Gregory MA, and Smith MCM (2015). Multiplexed integrating plasmids for engineering of the erythromycin gene cluster for expression in Streptomyces spp. and combinatorial biosynthesis. Appl. Environ. Microbiol 81, 8402–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Moreno MA, Caballero J, Hopwood DA, and Malpartida F (1991). The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell 66, 769–780. [DOI] [PubMed] [Google Scholar]
- Fischbach MA, and Clardy J (2007). One pathway, many products. Nat. Chem. Biol 3, 353–355. [DOI] [PubMed] [Google Scholar]
- Flett F, Mersinias V, and Smith CP (1997). High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol. Lett 155, 223–229. [DOI] [PubMed] [Google Scholar]
- Freestone TS, Ju K-S, Wang B, and Zhao H (2017). Discovery of a phosphonoacetic acid derived natural product by pathway refactoring. ACS Synth. Biol 6, 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger A, Keller-Schierlein W, Brandl M, and Zähner H (1988). Metabolites of microorganisms. 247 phenazines from Streptomyces antibioticus, strain Tü 2706. J. Antibiot. (Tokyo) 41, 1542–1551. [DOI] [PubMed] [Google Scholar]
- Gomez-Escribano JP, and Bibb MJ (2011). Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb. Biotechnol 4, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Escribano JP, and Bibb MJ (2014). Heterologous expression of natural product biosynthetic gene clusters in Streptomyces coelicolor: from genome mining to manipulation of biosynthetic pathways. J. Ind. Microbiol. Biotechnol 41, 425–431. [DOI] [PubMed] [Google Scholar]
- Gust B, Challis GL, Fowler K, Kieser T, and Chater KF (2003). PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. U.S.A 100, 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust B, Chandra G, Jakimowicz D, Yuqing T, Bruton CJ, and Chater KF (2004). Lambda red-mediated genetic manipulation of antibiotic-producing Streptomyces. Adv. Appl. Microbiol 54, 107–128. [DOI] [PubMed] [Google Scholar]
- Jones AC, Gust B, Kulik A, Heide L, Buttner MJ, and Bibb MJ (2013). Phage p1-derived artificial chromosomes facilitate heterologous expression of the FK506 Gene cluster. PLoS ONE 8, e69319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28, 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge-Clay AT (2007). A tylosin ketoreductase reveals how chirality is determined in polyketides. Chem. Biol 14, 898–908. [DOI] [PubMed] [Google Scholar]
- Kessler N, Schuhmann H, Morneweg S, Linne U, and Marahiel MA (2004). The linear pentadecapeptide gramicidin is assembled by four multimodular nonribosomal peptide synthetases that comprise 16 modules with 56 catalytic domains. J. Biol. Chem 279, 7413–7419. [DOI] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater KF, and Hopwood DA (2000). Practical Streptomyces Genetics (John Innes Foundation; ). [Google Scholar]
- Kim E, Moore BS, and Yoon YJ (2015). Reinvigorating natural product combinatorial biosynthesis with synthetic biology. Nat. Chem. Biol 11, 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn FE, and Carter GT (2005). The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov 4, 206–220. [DOI] [PubMed] [Google Scholar]
- Kouprina N, and Larionov V (2016). Transformation-associated recombination (TAR) cloning for genomics studies and synthetic biology. Chromosoma 125, 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz AL, Labes A, Wiese J, Bruhn T, Bringmann G, and Imhoff JF (2014). Nature’s lab for derivatization: new and revised structures of a variety of streptophenazines produced by a sponge-derived Streptomyces strain. Mar. Drugs 12, 1699–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen JB, and Nielsen J (2004). Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chem. Rev 104, 1663–1686. [DOI] [PubMed] [Google Scholar]
- Liang Y, Chen L, Ye X, Anjum K, Lian X-Y, and Zhang Z (2017). New streptophenazines from marine Streptomyces sp. 182SMLY. Nat. Prod. Res 31, 411–417. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhang L, Barton KW, and Zhao H (2015). Systematic identification of a panel of strong constitutive promoters from Streptomyces albus. ACS Synth. Biol 4, 1001–1010. [DOI] [PubMed] [Google Scholar]
- MacNeil DJ, Gewain KM, Ruby CL, Dezeny G, Gibbons PH, and MacNeil T (1992). Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111, 61–68. [DOI] [PubMed] [Google Scholar]
- Mavrodi DV, et al. (2010). Diversity and evolution of the phenazine biosynthesis pathway. Appl. Environ. Microbiol 76, 866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema MH, et al. (2015). Minimum Information about a Biosynthetic Gene cluster. Nat. Chem. Biol 11, 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitova MI, Lang G, Wiese J, and Imhoff JF (2008). Subinhibitory concentrations of antibiotics induce phenazine production in a marine Streptomyces sp. J. Nat. Prod 71, 824–827. [DOI] [PubMed] [Google Scholar]
- Montiel D, Kang H-S, Chang F-Y, Charlop-Powers Z, and Brady SF (2015). Yeast homologous recombination-based promoter engineering for the activation of silent natural product biosynthetic gene clusters. Proc. Natl. Acad. Sci. U.S.A 112, 8953–8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BS, Trischman JA, Seng D, Kho D, Jensen PR, and Fenical W (1999). Salinamides, antiinflammatory depsipeptides from a marine streptomycete. J. Org. Chem 64, 1145–1150. [Google Scholar]
- Myronovskyi M, and Luzhetskyy A (2016). Native and engineered promoters in natural product discovery. Nat. Prod. Rep 33, 1006–1019. [DOI] [PubMed] [Google Scholar]
- Newman DJ, and Cragg GM (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod 79, 629–661. [DOI] [PubMed] [Google Scholar]
- Olano C, Lombó F, Méndez C, and Salas JA (2008). Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab. Eng 10, 281–292. [DOI] [PubMed] [Google Scholar]
- Omura S, et al. (2001). Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. U. S. A 98, 12215–12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourn A (2010). Secondary metabolic gene clusters: evolutionary toolkits for chemical innovation. Trends Genet. TIG 26, 449–457. [DOI] [PubMed] [Google Scholar]
- Perego M, Glaser P, Minutello A, Strauch MA, Leopold K, and Fischer W (1995). Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis identification of genes and regulation. J. Biol. Chem 270, 15598–15606. [DOI] [PubMed] [Google Scholar]
- Pierson LS, and Pierson EA (2010). Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol 86, 1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Whelan A, Dietrich LEP, and Newman DK (2006). Rethinking “secondary” metabolism: physiological roles for phenazine antibiotics. Nat. Chem. Biol 2, 71–78. [DOI] [PubMed] [Google Scholar]
- Ramos I, Dietrich LEP, Price-Whelan A, and Newman DK (2010). Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res. Microbiol 161, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L, Yamanaka K, and Moore BS (2016). A peptidyl-transesterifying type I thioesterase in salinamide biosynthesis. Angew. Chem. Int. Ed Engl 55, 364–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CJ, Cortés J, Gaisser S, Staunton J, and Leadlay PF (1998). Construction of new vectors for high-level expression in actinomycetes. Gene 216, 215–223. [DOI] [PubMed] [Google Scholar]
- Rui Z, Ye M, Wang S, Fujikawa K, Akerele B, Aung M, Floss HG, Zhang W, and Yu T-W (2012). Insights into a divergent phenazine biosynthetic pathway governed by a plasmid-born esmeraldin gene cluster. Chem. Biol 19, 1116–1125. [DOI] [PubMed] [Google Scholar]
- Rutledge PJ, and Challis GL (2015). Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol 13, 509–523. [DOI] [PubMed] [Google Scholar]
- Schoenafinger G, Schracke N, Linne U, and Marahiel MA (2006). Formylation domain: an essential modifying enzyme for the nonribosomal biosynthesis of linear gramicidin. J. Am. Chem. Soc 128, 7406–7407. [DOI] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, and Ideker T (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Zhao H, and Zhao H (2009). DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways. Nucleic Acids Res. 37, e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl T, Tokovenko B, Myronovskyi M, and Luzhetskyy A (2013). Design, construction and characterisation of a synthetic promoter library for fine-tuned gene expression in actinomycetes. Metab. Eng 19, 98–106. [DOI] [PubMed] [Google Scholar]
- Sievers F, and Higgins DG (2014). Clustal Omega, accurate alignment of very large numbers of sequences. Methods Mol. Biol 1079, 105–116. [DOI] [PubMed] [Google Scholar]
- Smith S, and Tsai S-C (2007). The type I fatty acid and polyketide synthases: a tale of two megasynthases. Nat. Prod. Rep 24, 1041–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Li J, Millán-Aguiñaga N, Zhang JJ, O’Neill EC, Ugalde JA, Jensen PR, Mantovani SM, and Moore BS (2015). Identification of thiotetronic acid antibiotic biosynthetic pathways by target-directed genome mining. ACS Chem. Biol 10, 2841–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trischman JA, Tapiolas DM, Jensen PR, Dwight R, Fenical W, McKee TC, Ireland CM, Stout TJ, and Clardy J (1994). Salinamides A and B: Anti-inflammatory depsipeptides from a marine streptomycete. J. Am. Chem. Soc 116, 757–758. [Google Scholar]
- Voggu L, Schlag S, Biswas R, Rosenstein R, Rausch C, and Götz F (2006). Microevolution of cytochrome bd oxidase in Staphylococci and its implication in resistance to respiratory toxins released by Pseudomonas. J. Bacteriol 188, 8079–8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, et al. (2016). Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol 34, 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, et al. (2015). antiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 43, W237–W243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, and Moore BS (2012). Beyond ethylmalonyl-CoA: the functional role of crotonyl-CoA carboxylase/reductase homologs in expanding polyketide diversity. Nat. Prod. Rep 29, 72–86. [DOI] [PubMed] [Google Scholar]
- Wu Y, and Seyedsayamdost MR (2017). Synergy and target promiscuity drive structural divergence in bacterial alkylquinolone biosynthesis. Cell Chem. Biol 24, 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Reynolds KA, Kersten RD, Ryan KS, Gonzalez DJ, Nizet V, Dorrestein PC, and Moore BS (2014). Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc. Natl. Acad. Sci. U. S. A 111, 1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Jin X, Guaciaro M, and Molino BF (2012). Asymmetric synthesis and absolute configuration of streptophenazine G. J. Org. Chem 77, 3191–3196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2, related to Figures 2, 3 and 4. High resolution MS (HRMS), retention time, and predicted chemical formula for nodes in streptophenazine cluster from molecular network. Excel file attached separately.
Data Availability Statement
All promoter cassette plasmids are available at Addgene: pCAP03-sp44/p21 cassette (Addgene ID 120232), pCAP03-actIp cassette (Addgene ID 120233), pCAP03ermE*p cassette (Addgene ID 120234). The public datasets used for the GNPS molecular networking are available at: ftp://massive.ucsd.edu/MSV000083081 and ftp://massive.ucsd.edu/MSV000083082. The molecular networks can be accessed at: https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=24d515f76b4c4d6694a934234435b9bf (for Figure 3, Figure S4, and Table S2) and: https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=9a2d02c583c24422bfaa16e2f8ae9ceb (for Figure S3).