Summary
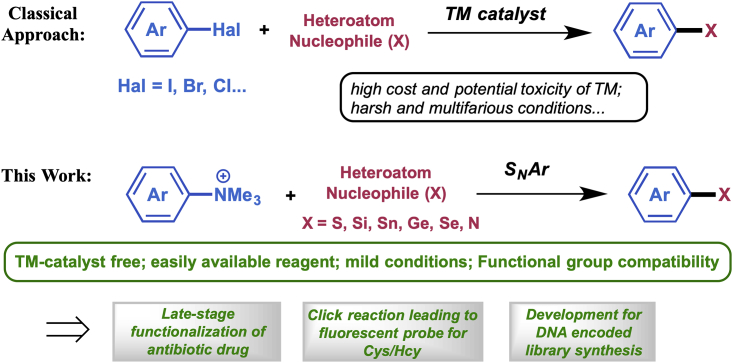
Aryl–heteroatom (C–X) bonds ubiquitously exist in organic, medicinal, and material chemistry, but a universal method to construct diverse C–X bonds is lacking. Here we report our discovery of a convenient and efficient approach to construct various C–X bonds using arylammonium salts as the substrate via an SNAr process. This strategy features mild reaction condition, no request of transition metal catalyst, and easy formation of various C–X bonds (C–S, C–Si, C–Sn, C–Ge, C–Se, C–N). The method was successfully applied to a late-stage functionalization of an existing antibiotic drug, to a Clickable reaction of NBD-based ammonium salt as turn-on fluorescent probe to recognize L-cysteine and homocysteine, and to the synthesis of a DNA encoded library (DEL) bearing different C–X bonds.
Subject Areas: Organic Chemistry, Organic Synthesis, Chemical Reaction Engineering
Graphical Abstract
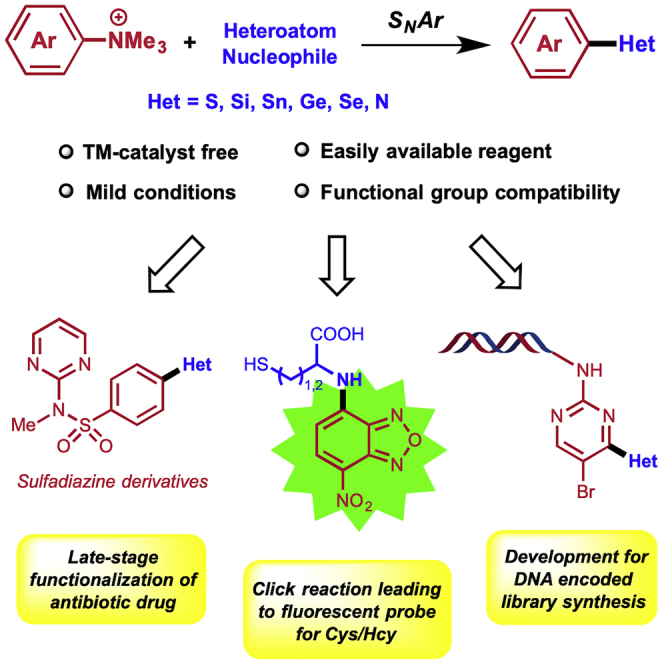
Highlights
-
•
An efficient approach to construct various C–heteroatom bonds
-
•
Readily accessible ammonium salts as substrates
-
•
No request of transition metal catalyst and broad functional group compatibility
-
•
Great applicability in late-stage functionalization, fluorescent probe, and DEL
Organic Chemistry; Organic Synthesis; Chemical Reaction Engineering
Introduction
Aryl carbon-heteroatom (C–X) bonds are prevalent in natural and unnatural organic molecules with ample capacities. Some common C–X bonds such as C–O, C–S (Feng et al., 2016), C–N (Ruiz-Castillo and Buchwald, 2016), and C–Si (Franz and Wilson, 2013) bonds are widely embedded in many organic intermediates and therapeutic drugs, whereas the relatively less common C–X bonds such as C–Se (Mugesh et al., 2001), C–Sn (Cordovilla et al., 2015), and C–Ge (Nakamura et al., 2002) bonds generally serve as synthetic precursors for many pharmaceuticals, organic materials, and polymers. In recent years, numerous synthetic methods for C–X bond formations have been developed (Jones et al., 2018, Shen et al., 2014, Liu et al., 2017, Wang et al., 2018a, Hartwig, 2008, Surry and Buchwald, 2008, Bariwal and Van der Eycken, 2013, Cheng and Hartwig, 2015, Zarate and Martin, 2014, Taniguchi and Onami, 2004, Shu et al., 2016, Yoshida, 2016, Gu and Martín, 2017, Komami et al., 2018). Normally, these aryl C–X bonds were formed starting from the same substrate aryl (pseudo)halides through cross-couplings with appropriate heteroatom nucleophiles under transition-metal (TM) catalysis (Scheme 1). However, these methods suffer from limited applications because of several drawbacks, including the additional steps necessary to presynthesize aryl halides, harsh reaction conditions, costive metal catalysts that are either toxic or difficult to remove, especially in the pharmaceutical industry (Wu et al., 2012, Chan et al., 2013). Therefore, it is highly desired to invent a universal method to construct diverse C–X bonds starting from a same substrate (Li et al., 2017, Xu et al., 2016). Ideally, this method should feature (1) using a ubiquitous readily available substrate; (2) wide scope to form various C–X bonds; (3) no TM catalyst needed.
Scheme 1.
Strategies for Diverse Aryl-heteroatom Bond Formation
Anilines are one of the most prevalent naturally abundant or readily synthetic accessible reagents in organic synthesis. Therefore, use of anilines, instead of aryl halides, as substrates to undergo the cross-coupling has long been a synthetic aspiration, but only with limited success (Ouyang et al., 2015, Li et al., 2014, Xu et al., 2017), owing to the high inertness of the C–N bond and the high reactivity of the NH2 group itself. Fortunately, conversion of anilines to the arylammonium species has been reported to enable the TM-catalyzed cross-couplings with appropriate partners (Wenkert et al., 1988, Blakey and MacMillan, 2003, Xie and Wang, 2011, Wang et al., 2016, Zhang et al., 2015, Zhang and Wang, 2014). However, transformations of arylammonium salts through an SNAr mechanism in the absence of TM catalyst are rare. Limited examples include the fluorination of ammonium salts (Irie et al., 1982) to radiolabel bio-active molecules for positron-emission tomography imaging and the formation of aryl ethers via C–N bond cleavage in the absence of TM catalyst (Wang et al., 2018b). Inspired by these work, we herein describe a universal method to access diverse aryl–heteroatom, especially those uncommon C–Sn/C–Ge/C–Se bonds using aryl ammonium salts as the ubiquitous substrate under mild reaction conditions.
Results and Discussion
C–X Bond Formation
TM-catalyzed thiolation of aryl halides typically requires the strong base to generate thiolates from thiols and a high temperature or specially designed ligands to avoid the deactivation of TM catalyst by the strong coordination of the thiolates (Feng et al., 2016, Hartwig, 2008). Herein, we report the first example of conversion of aryltrimethylammonium salts to aryl thioethers in the presence of a weak base under room temperature, without the need for any TM catalyst and ligand. In the beginning, we chose the reaction of 4-cyanophenyltrimethylammonium salt with 1-dodecanethiol as the mode reaction and optimized the substitution conditions in the absence of TM catalyst (for details, see Table S1). It was found that the best result was obtained by using TfO– as the counter anion of 1 in DMF with K2CO3 as base at room temperature in 3 h without the need of argon protection. We next focused on the synthetic scope and functional group compatibility of the reaction (Scheme 2A). Thiols bearing an ester (2a), free amino (2b), hydroxyl (2c, i), silyl (2d), allyl (2e), furyl (2f), halogen (2g, h), or carboxylic (2j) group, as well as the sterically congested tertiary thiol (2m), were found to proceed the reaction without difficulty, generating the desired thioetheric products in 71%–90% isolated yields. To further evaluate the synthetic applicability of this protocol, we examined reactions of 1a with several biologically active molecules or their derivatives containing an -SH moiety (2k−o). All the reactions occurred smoothly affording corresponding C–S bond-containing products 3ak−3ao in 75%–88% yields. To illustrate the scalable potential of this method, we conducted the reaction of Tiopronin (antidote) derivative 2l with 1a on a gram scale, and the product 3al was obtained in 83% yield after a simple workup procedure.
Scheme 2.
Substrate Scope Investigation
(A) Thioesterification reactions. K2CO3: 1.5 eq. (for 2i, 2j, 2l, 2n, 2o: 3.0 eq.). aFor 3ir, a mixture of DMF and H2O was used as the solvent.
(B) Silylation reactions; (C) Stannylation reactions; (D) Germylation reactions; (E) Selenation reactions; (F) Amination reactions. For 11d, 11e, 11i and 11j, Cl– was used as the counter anion of 1. Yields were calculated based on NMR (bold) and isolation (in parentheses).
See also Figures S1–S6.
Meanwhile, the functional group tolerance on the aryl trimethylammonium triflate was also investigated (Scheme 2A). It was found that aryl ammonium salts 1 bearing acyl (1b), formyl (1c), sulfonyl (1d), cyano (1f, 1g), halogen (1h), ester (1k), and heterocycle (1e) group reacted without difficulty, providing corresponding C–S bond products in up to 92% yields. It is worth highlighting that ammonium salts 1j–l derived from the antibiotic drug Avlosulfon, sunscreen lotion Padimate A, and Methyl Yellow, respectively, took part in the C–S bond formation as well to afford the thioetheric products, suggesting the potential use of this reaction in the late-stage functionalization of existing drugs. Interestingly, the tricyclic substrate 1i prepared from naphthalimide, a well-known fluorophore, was found to readily react with glutathione, providing the corresponding C–S bond product 3ir in 70% yield. In view of the strong internal charge transfer character of naphthalimides (Zhou et al., 2016) and involvement of glutathione in many biological processes, the current C–S bonding formation process might be used as a biomarker in vivo.
Since the C–Si/Sn/Ge bonds belong to the same group in the periodic table of elements, they are widely used in drug design as a bioisosteric replacement of C–C bond or as a precursor for further transformation. Traditional methods to generate Ar–Si/Ge/Sn involved the reaction of Si/Ge/Sn electrophiles with air-sensitive organometallic reagents, alternatively TM-catalyzed C–Si/Ge/Sn coupling reactions at high temperature (McNeill et al., 2007, Komami et al., 2018, Corcoran et al., 2012). We, herein, report an unprecedented silylation, germylation, and stannylation of various ammonium salts via the SNAr process with stable substrates at low temperature without the need of TM-catalyst. First, we examined the feasibility of C–Si/Sn/Ge bond formation by simply treating arylammonium salts with appropriate heteroatom nucleophiles. A quick survey of the reaction conditions (for details, see Tables S2 and S3) suggested that the optimum conditions for C–Si/Sn/Ge bond formation are to use CsF as the base, NMP/DMF as the solvent, at room temperature (r.t.) within 8 h. With this result in hand, a small series of arylammonium salts were used to explore the substrate scope. As shown in Scheme 2B–2D, arylammonium salts bearing an ester (5a, 5e, 7f, 7g, 9e), acetylene (5d), acyl (5f, 7e), pyridyl (5g, 7j), sulfonyl (7h, 7i), or cyano (5c, 7a-d) substituent went through the reaction very well providing the corresponding C–Si/Sn/Ge bond products in moderate to high yields. Interestingly, 1-naphthyl ammonium salt lacking an electron-withdrawing substituent also participated in the reaction nicely yielding compound 5h in 75% yield. Padimate A derivatives (5b, 7k, 7l), Sulfonamides derivatives (9a−9c), and the dual functionalized derivatives of the antibiotic drug Avlosulfon (5i, 7m, 9d) were also easily prepared in moderate to high yields.
Organoselenium compounds have gained more and more interests recently; however, methods to construct C–Se bond are rather limited. To explore the potential of our current method using arylammonium salts to build up C–Se bond, we used RSeSeR (R = Ph, Bn, Me, Et) as the selenation source and KBH4 as the base (for optimization details, see Table S4). As shown in Scheme 2E, arylammonium salts containing a nitro, acyl, formyl, cyano, sulfonyl substituent and purine/pyrimidine derivatives were tolerant in the reaction conditions yielding corresponding C–Se products 11a−j in up to 96% yields. Meanwhile, the cyclic ammonium salts 1q prepared from indoline derivative was also suitable substrate, affording the ring-opened product 11k in 92% yield.
Finally, we decided to explore the feasibility of constructing C–N bond under our SNAr substitution protocol without the TM-involved catalysis. With this optimized reaction condition in hand (for details, Table S5), we tested the substrate scope by using various arylammonium salts and differently substituted anilines. As shown in Scheme 2F, arylammonium salts with an aminosulfonyl, nitro, or cyano substituent were well tolerant, and the corresponding diaryl amines were obtained in moderate to high yields. Anilines bearing either electron-donating or electron-withdrawing substituents are suitable nucleophiles.
Late-Stage Functionalization of Biologically Active Compounds
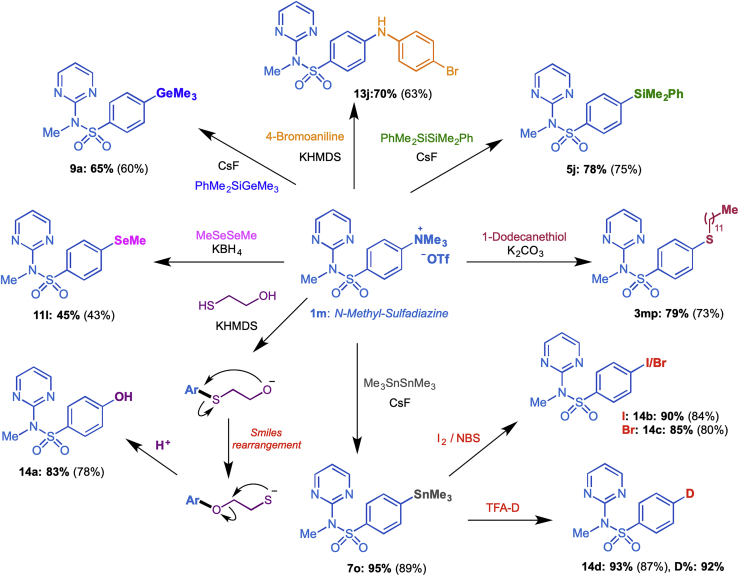
To evaluate the utility of our current protocol in the construction of diverse C–X bonds, we conducted a late-stage diversification of the antibiotic drug Sulfadiazine. As shown in Scheme 3, Sulfadiazine was first converted to the ammonium salt 1m, which was then subjected to the corresponding C–X bond formation reactions. A small library of sulfadiazine analogues bearing a new C–S (3mp), C–Se (11h), C–Sn (7o), C–Si (5j), C–Ge (9a), and C–N (13h) was conveniently established in moderate to good yields. Interestingly, phenol 14a bearing a new C–O(H) bond was obtained by treating 1m with 2-mercaptoethan-1-ol in the presence of KHMDS as the base. Likely, a Smiles rearrangement of the initially formed aryl thioether to the aryl ether, followed by elimination of thiirane was involved in the transformation (Boschi et al., 2001). All these products can be used as key intermediates for further functional group transformation. For example, treatment of aryltrimethyltin derivative 7o with iodine, NBS, or deuterated trifluoroacetic acid provided corresponding iodo-, bromo-, or deuterated derivatives 14b-d in 80%–87% yields.
Scheme 3.
Late-stage Functionalization of the Antibiotic Drug Sulfadiazine
Clickable Synthesis of Fluorescent Probes from NBD-Ammonium Salt with Biological Thiols
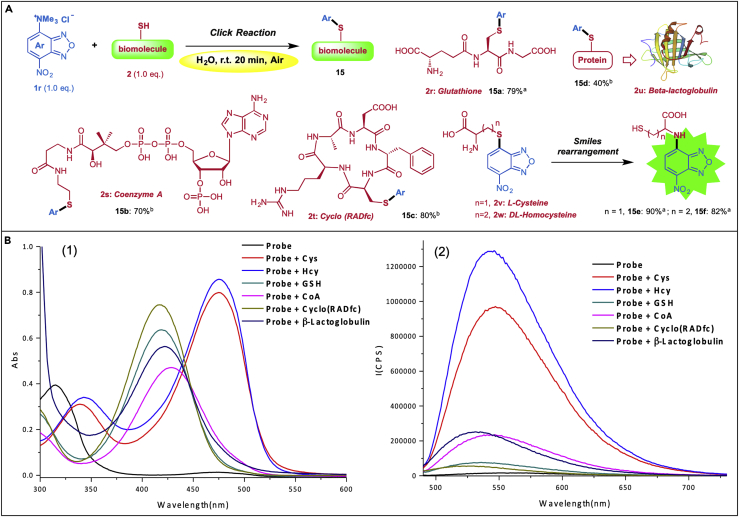
7-Nitrobenz-2-oxa-1,3-diazole (NBD) moiety has been widely used as a fluorophore in many fluorescent chemosensors because of its emission at longer wavelengths and good cell permeability (Uchiyama et al., 2001). To explore the application of our C–X bond formation protocol, we first prepared C4-ammonium NBD 1r and tested its sensitivity to various biological thiols, including small-molecule L-cysteine (Cys, 2v), homocysteine (Hcy, 2w), glutathione (2r), coenzyme A (2s), cyclopeptide (2t), as well as biomacromolecule antibody β-Lactoglobulin (2u). As shown in Scheme 4A, all the reactions proceeded very well just by “Clicking” the ammonium salt 1r with an appropriate thiol in water at r.t. for 20 min, providing corresponding products in moderate to high yields. Notably, the reactions with complex thiols 2r-2u afforded the expected C–S bond products, whereas reactions with simple thiols 2v-w gave products bearing a C–N bond. The production of 15e and 15f is likely formed through Smiles rearrangement that converted the initial C–S bond products to the N-substituted NBDs. This rearrangement has been further verified by spectroscopic comparison (Wood et al., 2003) (see Figures S7 and S8). In general, the N-substituted NBDs have longer-wavelength absorption and stronger fluorescence than the corresponding S-substituted ones (Chen et al., 2016); we then tested the fluorescent properties of these NBD compounds. The absorption and fluorescence emission responses (Scheme 4B) showed that probe 1r was non-fluorescent, whereas treatment of 1r with simple thiols Cys/Hcy induced a dramatic increase of fluorescence intensity at 550 nm. Treatment of 1r with complex thiols 2r, 2s, or 2t showed an absorption maximum at 419, 427, and 417 nm, respectively, and no significant fluorescence response was observed. These results indicated that our NBD probe 1r could specifically recognize and discriminate Cys/Hcy over other more complex bioactive thiols. Since Cys/Hcy play critically important roles in maintaining redox homeostasis in physiological processes (Wood et al., 2003), our NBD-ammonium salt probe and the readily clickable reaction conditions may find use in monitoring biological process in vivo.
Scheme 4.
Clickable Synthesis of Turn-on Fluorescent Probe
(A) Clickable thioetherification of NBD-ammonium salts with various biological thiols. aNMR yield; bliquid chromatography-mass spectrometry conversion.
(B) Absorption (1) and fluorescence (2) spectra of probe 1r (50 μM) before (black curve) and after addition of 250 μM Cys (red), Hcy (blue), GSH (green), Coenzyme A (pink), cyclopeptide (brown), and β-Lactoglobulin (purple), respectively, incubated for 30 min in H2O at 25°C. Excitation wavelength: 480nm.
See also Figure S7.
On-DNA Reaction Development for DNA Encoded Library Synthesis
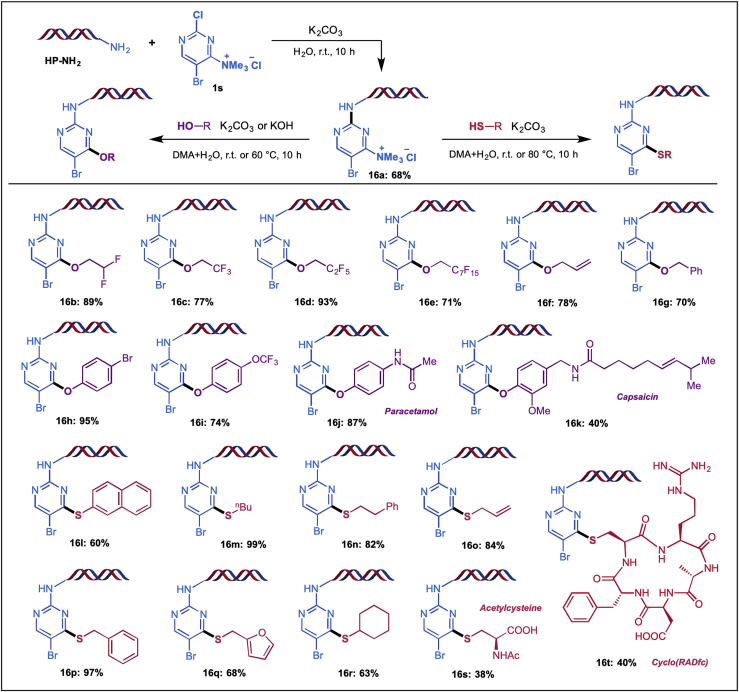
DNA-encoded library (DEL) is a powerful tool for hit identification in small molecule drug discovery (Goodnow et al., 2017, Mullard, 2016, Buller et al., 2010), and several compounds derived from their original DEL hits have progressed to clinical development (Belyanskaya et al., 2017). The chemical diversity of DEL library is the key to successful discovery of drug-like molecules but is often limited to DNA compatible synthetic reactions (Wang et al., 2014, Satz et al., 2015, Li et al., 2016). Since our C–X bond formation protocol proceeded in mild reaction conditions, it is ideal to be used for the DEL synthesis. 2,4-Dichloropyrimidine derivatives, a widely used core structure of kinase inhibitors, could be used as valuable building blocks (BB) in the DNA encoded library synthesis (Ding et al., 2016). SNAr reaction of DNA headpiece (HP, Figure S9) with excessive 2,4-dichloropyrimidine derivatives routinely provides C-4 DNA-conjugated pyrimidine in high regioselectivity (Satz et al., 2015).
In contrast, we developed a novel ammonium building block (1s), which could be reacted with HP to give C-2 DNA-conjugated pyrimidine (16a) selectively (Scheme 5). By following our protocol, the corresponding C–O/S bond formations occurred smoothly in dilute conditions and provided DNA-conjugated pyrimidines 16b-t bearing various functional groups (Scheme 5). Several biologically active molecules as well as fluorine-containing functional groups could also be introduced. Alcohol BBs are quite challenging for on-DNA reactions, but our new on-DNA reaction could tolerate a series of alcohol BBs, which significantly increases the diversity of DEL. It is noteworthy that the Br moiety in pyrimidine scaffold might be used for further functionalization through cross-coupling reactions (Ding and Clark, 2015) to provide a more complex compound library with ample chemical diversity. Such novel chemical selectivity brought by 1s enables the design and synthesis of different types of DNA encoded pyrimidine library that is currently under development for library synthesis.
Scheme 5.
On-DNA Reaction Development.
Reactions were carried out in dilute conditions (1 mM). Conversion determined by liquid chromatography-mass spectrometry. See also Figure S10.
Conclusion
In summary, we have established a convenient and efficient approach to construct diverse aryl–heteroatom bonds using arylammonium salts as the common substrates via a SNAr process. This strategy features mild reaction condition, no request of transition metal catalyst, and wide scope for various C-X bonds, especially those uncommon C–Sn/C–Ge/C–Se formation. The application of this method was exemplified by a late-stage functionalization of an existing antibiotic drug and by a Clickable reaction using NBD-based ammonium salt as a turn-on fluorescent probe for Cys and Hcy. Meanwhile, on-DNA reaction development for DEL was also successfully realized starting from a pyrimidinylammonium salt.
Limitation of Study
Ammonium salts derived from electron-rich anilines (e.g., p-R-C6H4-NMe3+, R = Me, MeO) showed poor or no reactivity toward heteroatom nucleophiles. Silylation product with acetyl group could not be obtained probably due to the strong basicity of silyl anions.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by grants (to A. Zhang, X.-J. Lu, and D.-Y. Wang.) from the National Natural Science Foundation of China (81773565, 21702216, 21877117, 81430080), the National Program on Key Basic Research Project (973 Program) of China (2015CB910603), the International Cooperative Program (GJHZ1622) and the Key Program of Frontier Science (160621) of the Chinese Academy of Sciences, the Shanghai Commission of Science and Technology (16XD1404600, 14431905300, 14431900400, 18431907100). X.-J. Lu and D.-Y. Wang. gratefully acknowledges the support of National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” China (No. 2018ZX09711002-005, No. 2018ZX09711002-006-003), Sanofi-SIBS 2017 Post-doctoral Fellowship, and China Postdoctoral Science Foundation (2017M621566, 2018T110416). This work was also supported by JSPS Grant-in-Aid for Scientific Research, Japan: No. 17H05430 (to M. Uchiyama), No. 17H06173 (to M. Uchiyama), and No. 18K06544 (to C. Wang).
Author Contributions
D.-Y.W. planned, carried out most of experiments, analyzed, and summarized the experiments. X.W. performed the DEL experiment. J.-N.Z., C.-D.X., and C.-Y.D. participated in the experiments or discussions. Q.M. and H.Z. performed biological thiols analysis. C.W. and M.U. conceived and designed the project. X.-J.L. directed the study on DNA encoded library synthesis. A.Z. supervised the whole research and wrote the manuscript with feedback from all authors.
Declaration of Interests
The authors declare no competing interests.
Published: May 31, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.04.038.
Contributor Information
Xiao-Jie Lu, Email: xjlu@simm.ac.cn.
Ao Zhang, Email: aozhang@simm.ac.cn.
Supplemental Information
References
- Bariwal J., Van der Eycken E. C-N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 2013;42:9283. doi: 10.1039/c3cs60228a. [DOI] [PubMed] [Google Scholar]; Bariwal, J., and Van der Eycken, E. (2013). C-N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 42, 9283. [DOI] [PubMed]
- Belyanskaya S.L., Ding Y., Callahan J.F., Lazaar A.L., Israel D.I. Discovering drugs with DNA-encoded library technology: from concept to clinic with an inhibitor of soluble epoxide hydrolase. ChemBioChem. 2017;18:837–842. doi: 10.1002/cbic.201700014. [DOI] [PubMed] [Google Scholar]; Belyanskaya, S.L., Ding, Y.., Callahan, J.F., Lazaar, A.L., and Israel, D.I. (2017). Discovering drugs with DNA-encoded library technology: from concept to clinic with an inhibitor of soluble epoxide hydrolase. ChemBioChem 18, 837−842. [DOI] [PubMed]
- Blakey S.B., MacMillan D.W.C. The first Suzuki cross-couplings of aryltrimethylammonium salts. J. Am. Chem. Soc. 2003;125:6046–6047. doi: 10.1021/ja034908b. [DOI] [PubMed] [Google Scholar]; Blakey, S.B., and MacMillan, D.W.C. (2003). The first Suzuki cross-couplings of aryltrimethylammonium salts. J. Am. Chem. Soc. 125, 6046-6047. [DOI] [PubMed]
- Boschi D., Sorba G., Bertinaria M., Fruttero R., Calvino R., Gasco A. Unsymmetrically substituted furoxans. Part 18.1 Smiles rearrangement in furoxan systems and in related furazans. J. Chem. Soc. Perkin Trans. 2001;1:1751–1757. [Google Scholar]; Boschi, D., Sorba, G., Bertinaria, M., Fruttero, R., Calvino, R., and Gasco, A. (2001). Unsymmetrically substituted furoxans. Part 18.1 Smiles rearrangement in furoxan systems and in related furazans. J. Chem. Soc. Perkin Trans. 1 1751-1757.
- Buller F., Mannocci L., Scheuermann J., Neri D. Drug discovery with DNA-encoded chemical libraries. Bioconjug. Chem. 2010;21:1571–1580. doi: 10.1021/bc1001483. [DOI] [PubMed] [Google Scholar]; Buller, F., Mannocci, L., Scheuermann, J., and Neri, D. (2010). Drug discovery with DNA-encoded chemical libraries. Bioconjug. Chem. 21, 1571−1580. [DOI] [PubMed]
- Chan T.L., Wu Y., Choy P.Y., Kwong F.Y. A radical process towards the development of transition-metal-free aromatic carbon–carbon bond-forming reactions. Chem. Eur. J. 2013;19:15802–15814. doi: 10.1002/chem.201301583. [DOI] [PubMed] [Google Scholar]; Chan, T.L., Wu, Y., Choy, P.Y., and Kwong, F.Y. (2013). A radical process towards the development of transition-metal-free aromatic carbon-carbon bond-forming reactions. Chem. Eur. J. 19, 15802-15814. [DOI] [PubMed]
- Chen W., Luo H., Liu X., Foley J., Song X. Broadly applicable strategy for the fluorescence based detection and differentiation of glutathione and cysteine/homocysteine: demonstration in vitro and in vivo. Anal. Chem. 2016;88:3638–3646. doi: 10.1021/acs.analchem.5b04333. [DOI] [PubMed] [Google Scholar]; Chen, W., Luo, H., Liu, X., Foley, J., and Song, X. (2016). Broadly applicable strategy for the fluorescence based detection and differentiation of glutathione and cysteine/homocysteine: demonstration in vitro and in vivo. Anal. Chem. 88, 3638-3646. [DOI] [PubMed]
- Cheng C., Hartwig J.F. Catalytic silylation of unactivated C−H bonds. Chem. Rev. 2015;115:8946–8975. doi: 10.1021/cr5006414. [DOI] [PubMed] [Google Scholar]; Cheng, C., and Hartwig, J.F. (2015). Catalytic silylation of unactivated C−H bonds. Chem. Rev. 115, 8946−8975. [DOI] [PubMed]
- Corcoran E.B., Williams A.B., Hanson R.N. A synthetic method for palladium-catalyzed stannylation at the 5- and 6‑benzo positions of indoles. Org. Lett. 2012;14:4630–4633. doi: 10.1021/ol302076d. [DOI] [PubMed] [Google Scholar]; Corcoran, E.B., Williams, A.B. and Hanson, R.N. A synthetic method for palladium-catalyzed stannylation at the 5- and 6-benzo positions of indoles, Org. Lett. 14, 2012, 4630-4633. [DOI] [PubMed]
- Cordovilla C., Bartolomé C., Martínez-Ilarduya J.M., Espinet P. The Stille reaction, 38 years later. ACS Catal. 2015;5:3040–3053. [Google Scholar]; Cordovilla, C., Bartolome, C., Martinez-Ilarduya, J.M., and Espinet, P. (2015). The Stille reaction, 38 years later. ACS Catal. 5, 3040-3053.
- Ding Y., Clark M.A. Robust Suzuki–Miyaura cross-coupling on DNA-linked substrates. ACS Comb. Sci. 2015;17:1–4. doi: 10.1021/co5001037. [DOI] [PubMed] [Google Scholar]; Ding, Y., and Clark, M.A. (2015). Robust Suzuki-Miyaura cross-coupling on DNA-linked substrates. ACS Comb. Sci., 17, 1-4. [DOI] [PubMed]
- Ding Y., DeLorey J.L., Clark M.A. Novel catalyst system for Suzuki-Miyaura coupling of challenging DNA-linked aryl chlorides. Bioconjug. Chem. 2016;27:2597–2600. doi: 10.1021/acs.bioconjchem.6b00541. [DOI] [PubMed] [Google Scholar]; Ding, Y., DeLorey, J.L., and Clark, M.A. (2016). Novel catalyst system for Suzuki-Miyaura coupling of challenging DNA-linked aryl chlorides. Bioconjug. Chem. 27, 2597−2600. [DOI] [PubMed]
- Feng M., Tang B., Liang S., Jiang X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016;16:1200–1216. doi: 10.2174/1568026615666150915111741. [DOI] [PMC free article] [PubMed] [Google Scholar]; Feng, M., Tang, B., Liang, S., and Jiang, X. (2016). Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 16, 1200-1216. [DOI] [PMC free article] [PubMed]
- Franz A.K., Wilson S.O. Organosilicon molecules with medicinal applications. J. Med. Chem. 2013;56:388–405. doi: 10.1021/jm3010114. [DOI] [PubMed] [Google Scholar]; Franz, A.K., and Wilson, S.O. (2013). Organosilicon molecules with medicinal applications. J. Med. Chem. 56, 388−405. [DOI] [PubMed]
- Goodnow R.A., Jr., Dumelin C.E., Keefe A.D. DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat. Rev. Drug Discov. 2017;16:131–147. doi: 10.1038/nrd.2016.213. [DOI] [PubMed] [Google Scholar]; Goodnow, R.A. Jr., Dumelin, C.E., and Keefe, A.D. (2017). DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat. Rev. Drug Discov. 16, 131−147. [DOI] [PubMed]
- Gu Y., Martín R. Ni-catalyzed stannylation of aryl esters via C–O Bond cleavage. Angew. Chem. Int. Ed. 2017;56:3187–3190. doi: 10.1002/anie.201611720. [DOI] [PubMed] [Google Scholar]; Gu, Y.., and Martin, R. (2017). Ni-catalyzed stannylation of aryl esters via C-O Bond cleavage. Angew. Chem. Int. Ed. 56, 3187−3190. [DOI] [PubMed]
- Hartwig J.F. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 2008;41:1534–1544. doi: 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hartwig, J.F. (2008). Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 41, 1534-1544. [DOI] [PMC free article] [PubMed]
- Irie T., Fukushi K., Inoue O., Yamasaki T., Ido T., Nozaki T. Preparation of 18F-labeled 6- and 2-fluoro-9- benzylpurine as a potential brain-scanning agent. Int. J. Appl. Radiat. Isot. 1982;33:633–636. doi: 10.1016/0020-708x(82)90061-8. [DOI] [PubMed] [Google Scholar]; Irie, T., Fukushi, K., Inoue, O., Yamasaki, T., Ido, T., and Nozaki, T. (1982). Preparation of 18F-labeled 6- and 2-fluoro-9- benzylpurine as a potential brain-scanning agent. Int. J. Appl. Radiat. Isot. 33, 633-636. [DOI] [PubMed]
- Jones K.D., Power D.J., Bierer D., Gericke K.M., Stewart S.G. Nickel phosphite/phosphine-catalyzed C–S cross-coupling of aryl chlorides and thiols. Org. Lett. 2018;20:208–211. doi: 10.1021/acs.orglett.7b03560. [DOI] [PubMed] [Google Scholar]; Jones, K.D., Power, D.J., Bierer, D., Gericke, K.M., and Stewart, S.G. (2018). Nickel phosphite/phosphine-catalyzed C-S cross-coupling of aryl chlorides and thiols. Org. Lett. 20, 208−211. [DOI] [PubMed]
- Komami N., Matsuoka K., Yoshino T., Matsunaga S. Palladium-catalyzed germylation of aryl bromides and aryl triflates using hexamethyldigermane. Synthesis. 2018;50:2067–2075. [Google Scholar]; Komami, N., Matsuoka, K., Yoshino, T., and Matsunaga, S. (2018). Palladium-catalyzed germylation of aryl bromides and aryl triflates using hexamethyldigermane. Synthesis 50, 2067-2075.
- Li Q., Zhang S.-Y., He G., Ai Z., Nack W.A., Chen G. Copper-catalyzed carboxamide-directed ortho amination of anilines with alkylamines at room temperature. Org. Lett. 2014;16:1764–1767. doi: 10.1021/ol500464x. [DOI] [PubMed] [Google Scholar]; Li, Q., Zhang, S.-Y., He, G., Ai, Z., Nack, W.A., and Chen, G. (2014). Copper-catalyzed carboxamide-directed ortho amination of anilines with alkylamines at room temperature. Org. Lett. 16, 1764−1767. [DOI] [PubMed]
- Li Y., Gabriele E., Samain F., Favalli N., Sladojevich F., Scheuermann J., Neri D. Optimized reaction conditions for amide bond formation in DNA-encoded combinatorial libraries. ACS Comb. Sci. 2016;18:438–443. doi: 10.1021/acscombsci.6b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li, Y., Gabriele, E., Samain, F., Favalli, N., Sladojevich, F., Scheuermann, J., and Neri, D. (2016). Optimized reaction conditions for amide bond formation in DNA-encoded combinatorial libraries. ACS Comb. Sci. 18, 438−443. [DOI] [PMC free article] [PubMed]
- Li J.-M., Wang Y.-H., Yu Y., Wu R.-B., Weng J., Lu G. Copper-catalyzed remote C–H functionalizations of naphthylamides through a coordinating activation strategy and single-electron-transfer (SET) mechanism. ACS Catal. 2017;7:2661–2667. [Google Scholar]; Li, J.-M., Wang, Y.-H., Yu, Y., Wu, R.-B., Weng, J., and Lu, G. (2017). Copper-catalyzed remote C-H functionalizations of naphthylamides through a coordinating activation strategy and single-electron-transfer (SET) mechanism. ACS Catal. 7, 2661−2667.
- Liu B., Lim C., Miyake G.M. Visible-light-promoted C−S cross-coupling via intermolecular charge transfer. J. Am. Chem. Soc. 2017;139:13616–13619. doi: 10.1021/jacs.7b07390. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, B., Lim, C., and Miyake, G.M. (2017). Visible-light-promoted C−S cross-coupling via intermolecular charge transfer. J. Am. Chem. Soc. 139, 13616-13619. [DOI] [PMC free article] [PubMed]
- McNeill E., Barder T.E., Buchwald S.L. Palladium-catalyzed silylation of aryl chlorides with hexamethyldisilane. Org. Lett. 2007;9:3785–3788. doi: 10.1021/ol701518f. [DOI] [PubMed] [Google Scholar]; McNeill, E., Barder, T.E. and Buchwald, S.L. Palladium-catalyzed silylation of aryl chlorides with hexamethyldisilane, Org. Lett. 9, 2007, 3785-3788. [DOI] [PubMed]
- Mugesh G., du Mont W.-W., Sies H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001;101:2125–2180. doi: 10.1021/cr000426w. [DOI] [PubMed] [Google Scholar]; Mugesh, G., du Mont, W.-W., and Sies, H. (2001). Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 101, 2125−2180. [DOI] [PubMed]
- Mullard A. DNA-encoded drug libraries come of age. Nat. Biotechnol. 2016;34:450–451. doi: 10.1038/nbt0516-450b. [DOI] [PubMed] [Google Scholar]; Mullard, A. (2016). DNA-encoded drug libraries come of age. Nat. Biotechnol. 34, 450−451. [DOI] [PubMed]
- Nakamura T., Kinoshita H., Shinokubo H., Oshima K. Biaryl synthesis from two different aryl halides with tri(2-furyl)germane. Org. Lett. 2002;4:3165–3167. doi: 10.1021/ol026613t. [DOI] [PubMed] [Google Scholar]; Nakamura, T., Kinoshita, H., Shinokubo, H., and Oshima, K. (2002). Biaryl synthesis from two different aryl halides with tri(2-furyl)germane. Org. Lett. 4, 3165−3167. [DOI] [PubMed]
- Ouyang K., Hao W., Zhang W., Xi Z. Transition-metal-catalyzed cleavage of C−N single bonds. Chem. Rev. 2015;115:12045–12090. doi: 10.1021/acs.chemrev.5b00386. [DOI] [PubMed] [Google Scholar]; Ouyang, K., Hao, W., Zhang, W., and Xi, Z. (2015). Transition-metal-catalyzed cleavage of C−N single bonds. Chem. Rev. 115, 12045-12090. [DOI] [PubMed]
- Ruiz-Castillo P., Buchwald S.L. Transition-metal-catalyzed C–S, C–Se, and C–Te bond formation via cross-coupling and atom-economic addition reactions. Chem. Rev. 2016;116:12564–12649. [Google Scholar]; Ruiz-Castillo, P., and Buchwald, S.L. (2016). Transition-metal-catalyzed C-S, C-Se, and C-Te bond formation via cross-coupling and atom-economic addition reactions. Chem. Rev. 116, 12564-12649. [DOI] [PubMed]
- Satz A.L., Cai J., Chen Y., Goodnow R., Gruber F., Kowalczyk A., Petersen A., Naderi-Oboodi G., Orzechowski L., Strebel Q. DNA compatible multistep synthesis and applications to DNA encoded libraries. Bioconjug. Chem. 2015;26:1623–1632. doi: 10.1021/acs.bioconjchem.5b00239. [DOI] [PubMed] [Google Scholar]; Satz, A.L., Cai, J., Chen, Y., Goodnow, R., Gruber, F., Kowalczyk, A., Petersen, A., Naderi-Oboodi, G., Orzechowski, L., and Strebel, Q. (2015). DNA compatible multistep synthesis and applications to DNA encoded libraries. Bioconjug. Chem. 26, 1623−1632. [DOI] [PubMed]
- Shen C., Xu J., Yu W., Zhang P. A highly active and easily recoverable chitosan@copper catalyst for the C–S coupling and its application in the synthesis of zolimidine. Green. Chem. 2014;16:3007–3012. [Google Scholar]; Shen, C., Xu, J., Yu, W., and Zhang, P. (2014). A highly active and easily recoverable chitosan@copper catalyst for the C-S coupling and its application in the synthesis of zolimidine. Green. Chem. 16, 3007−3012.
- Shu S., Fan Z., Yao Q., Zhang A. Ru(II)-catalyzed direct C(sp2)−H activation/selenylation of arenes with selenyl chlorides. J. Org. Chem. 2016;81:5263–5269. doi: 10.1021/acs.joc.6b00634. [DOI] [PubMed] [Google Scholar]; Shu, S., Fan, Z., Yao, Q., and Zhang, A. (2016). Ru(II)-catalyzed direct C(sp2)−H activation/selenylation of arenes with selenyl chlorides. J. Org. Chem. 81, 5263−5269. [DOI] [PubMed]
- Surry D.S., Buchwald S.L. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 2008;47:6338–6361. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]; Surry, D.S., and Buchwald, S.L. (2008). Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 47, 6338-6361. [DOI] [PMC free article] [PubMed]
- Taniguchi N., Onami T. Magnesium-induced copper-catalyzed synthesis of unsymmetrical diaryl chalcogenide compounds from aryl iodide via cleavage of the Se−Se or S−S bond. J. Org. Chem. 2004;69:915–920. doi: 10.1021/jo030300+. [DOI] [PubMed] [Google Scholar]; Taniguchi, N., and Onami, T. (2004). Magnesium-induced copper-catalyzed synthesis of unsymmetrical diaryl chalcogenide compounds from aryl iodide via cleavage of the Se−Se or S−S bond. J. Org. Chem. 69, 915-920. [DOI] [PubMed]
- Uchiyama S., Santa T., Okiyama N., Fukushima T., Imai K. Fluorogenic and fluorescent labeling reagents with a benzofurazan skeleton. Biomed. Chromatogr. 2001;15:295–318. doi: 10.1002/bmc.75. [DOI] [PubMed] [Google Scholar]; Uchiyama, S., Santa, T., Okiyama, N., Fukushima, T., and Imai, K. (2001). Fluorogenic and fluorescent labeling reagents with a benzofurazan skeleton. Biomed. Chromatogr. 15, 295-318. [DOI] [PubMed]
- Wang X., Sun H., Liu J., Dai D., Zhang M., Zhou H., Zhong W., Lu X. Ruthenium-promoted C−H activation reactions between DNA-conjugated acrylamide and aromatic acids. Org. Lett. 2014;16:1968–1971. doi: 10.1021/acs.orglett.8b01837. [DOI] [PubMed] [Google Scholar]; Wang, X., Sun, H., Liu, J., Dai, D., Zhang, M., Zhou, H., Zhong, W., and Lu, X. (2014). Ruthenium-promoted C−H activation reactions between DNA-conjugated acrylamide and aromatic acids. Org. Lett. 16, 1968-1971. [DOI] [PubMed]
- Wang D.-Y., Kawahata M., Yang Z.-K., Miyamoto K., Komagawa S., Yamaguchi K., Wang C., Uchiyama M. Stille coupling via C–N bond cleavage. Nat. Commun. 2016;7:12937. doi: 10.1038/ncomms12937. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang, D.-Y., Kawahata, M., Yang, Z.-K., Miyamoto, K., Komagawa, S., Yamaguchi, K., Wang, C., and Uchiyama, M. (2016). Stille coupling via C-N bond cleavage. Nat. Commun. 7, 12937. [DOI] [PMC free article] [PubMed]
- Wang M., Qiao Z., Zhao J., Jiang X. Palladium-catalyzed thiomethylation via a three-component cross-coupling strategy. Org. Lett. 2018;20:6193–6197. doi: 10.1021/acs.orglett.8b02677. [DOI] [PubMed] [Google Scholar]; Wang, M., Qiao, Z., Zhao, J., and Jiang, X. (2018a). Palladium-catalyzed thiomethylation via a three-component cross-coupling strategy. Org. Lett. 20, 6193−6197. [DOI] [PubMed]
- Wang D.-Y., Yang Z.-K., Wang C., Zhang A., Uchiyama M. From anilines to aryl ethers: a facile, efficient, and versatile synthetic method employing mild conditions. Angew. Chem. Int. Ed. 2018;57:3641–3645. doi: 10.1002/anie.201712618. [DOI] [PubMed] [Google Scholar]; Wang, D.-Y., Yang, Z.-K., Wang, C., Zhang, A., and Uchiyama, M. (2018b). From anilines to aryl ethers: a facile, efficient, and versatile synthetic method employing mild conditions. Angew. Chem. Int. Ed. 57, 3641-3645. [DOI] [PubMed]
- Wenkert E., Han A.-L., Jenny C.-J. Nickel-induced conversion of carbon–nitrogen into carbon–carbon bonds. One-step transformations of aryl, quaternary ammonium salts into alkylarenes and biaryls. J. Chem. Soc. Chem. Commun. 1988:975–976. [Google Scholar]; Wenkert, E., Han, A.-L., and Jenny, C.-J. (1988). Nickel-induced conversion of carbon-nitrogen into carbon-carbon bonds. One-step transformations of aryl, quaternary ammonium salts into alkylarenes and biaryls. J. Chem. Soc. Chem. Commun. 975-976.
- Wood Z.A., Schroder E., Harris R.J., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]; Wood, Z.A., Schroder, E., Harris, R.J., and Poole, L.B. (2003). Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28, 32-40. [DOI] [PubMed]
- Wu Y., Wong S.M., Mao F., Chan T.L., Kwong F.Y. Intramolecular direct C–H bond arylation from aryl chlorides: a transition-metal-free approach for facile access of Phenanthridines. Org. Lett. 2012;14:5306–5309. doi: 10.1021/ol302489n. [DOI] [PubMed] [Google Scholar]; Wu, Y., Wong, S.M., Mao, F., Chan, T.L., and Kwong, F.Y. (2012). Intramolecular direct C-H bond arylation from aryl chlorides: a transition-metal-free approach for facile access of Phenanthridines. Org. Lett. 14, 5306−5309. [DOI] [PubMed]
- Xie L., Wang Z. Nickel-catalyzed cross-coupling of aryltrimethylammonium iodides with organozinc reagents. Angew. Chem. Int. Ed. 2011;50:4901–4904. doi: 10.1002/anie.201100683. [DOI] [PubMed] [Google Scholar]; Xie, L., and Wang, Z. (2011). Nickel-catalyzed cross-coupling of aryltrimethylammonium iodides with organozinc reagents. Angew. Chem. Int. Ed. 50, 4901-4904. [DOI] [PubMed]
- Xu J., Shen C., Zhu X., Zhang P., Ajitha M.J., Huang K., An Z., Liu X. Remote C−H activation of quinolines through copper-catalyzed radical cross-coupling. Chem. Asian J. 2016;11:882–892. doi: 10.1002/asia.201501407. [DOI] [PubMed] [Google Scholar]; Xu, J., Shen, C., Zhu, X., Zhang, P., Ajitha, M.J., Huang, K., An, Z., and Liu, X. (2016). Remote C−H activation of quinolines through copper-catalyzed radical cross-coupling. Chem. Asian J. 11, 882−892. [DOI] [PubMed]
- Xu J., Qiao L., Shen J., Chai K., Shen C., Zhang P. Nickel(II)-catalyzed site-selective C–H bond trifluoromethylation of arylamine in water through a coordinating activation strategy. Org. Lett. 2017;19:5661–5664. doi: 10.1021/acs.orglett.7b02823. [DOI] [PubMed] [Google Scholar]; Xu, J., Qiao, L., Shen, J., Chai, K., Shen, C., and Zhang, P. (2017). Nickel(II)-catalyzed site-selective C-H bond trifluoromethylation of arylamine in water through a coordinating activation strategy. Org. Lett. 19, 5661−5664. [DOI] [PubMed]
- Yoshida H. Stannylation reactions under base metal catalysis: some recent. Synthesis. 2016;48:2540–2552. [Google Scholar]; Yoshida, H. (2016). Stannylation reactions under base metal catalysis: some recent. Synthesis 48, 2540-2552.
- Zarate C., Martin R. A mild Ni/Cu-catalyzed silylation via C−O cleavage. J. Am. Chem. Soc. 2014;136:2236–2239. doi: 10.1021/ja412107b. [DOI] [PubMed] [Google Scholar]; Zarate, C., and Martin, R. (2014). A mild Ni/Cu-catalyzed silylation via C−O cleavage. J. Am. Chem. Soc. 136, 2236-2239. [DOI] [PubMed]
- Zhang X.-Q., Wang Z.-X. Nickel-catalyzed cross-coupling of aryltrimethylammonium triflates and amines. Org. Biomol. Chem. 2014;12:1448–1453. doi: 10.1039/c3ob41989d. [DOI] [PubMed] [Google Scholar]; Zhang, X.-Q., and Wang, Z.-X. (2014). Nickel-catalyzed cross-coupling of aryltrimethylammonium triflates and amines. Org. Biomol. Chem. 12, 1448-1453. [DOI] [PubMed]
- Zhang H., Hagihara S., Itami K. Making dimethylamino a transformable directing group by nickel-catalyzed C–N borylation. Chem. Eur. J. 2015;2015:16796–16800. doi: 10.1002/chem.201503596. [DOI] [PubMed] [Google Scholar]; Zhang, H., Hagihara, S., and Itami, K. (2015). Making dimethylamino a transformable directing group by nickel-catalyzed C-N borylation. Chem. Eur. J. 2015, 16796-16800. [DOI] [PubMed]
- Zhou P., Yao J., Hu G., Fang J. Naphthalimide scaffold provides versatile platform for selective. ACS Chem. Biol. 2016;11:1098–1105. doi: 10.1021/acschembio.5b00856. [DOI] [PubMed] [Google Scholar]; Zhou, P., Yao, J., Hu, G., and Fang, J. (2016). Naphthalimide scaffold provides versatile platform for selective. ACS Chem. Biol. 11, 1098−1105. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.