Abstract
Nʹ-Nitrosonornicotine (NNN), a carcinogenic tobacco-specific Nʹ-nitrosamine (TSNA), is on the FDA list of harmful and potentially harmful constituents (HPHCs). Nornicotine, a product of the demethylation of nicotine, is the immediate alkaloid precursor for NNN formation. Nicotine, nornicotine and NNN are optically active. The accumulation of the isomers of nicotine, nornicotine, and NNN impacts their biological activity. In this paper, we report the determination of tobacco alkaloid enantiomers (including nicotine, nornicotine, anabasine, and anatabine) in samples of different tobacco lines using a reversed phase ultra-performance liquid chromatography-tandem mass spectrometer (UPLC/MS/MS) method. Current method demonstates excellent detection capability for all alkaloid enantiomers, with correlation coefficients (r2) > 0.996 within their linear dynamic ranges. The limit of detection (LOD) and limit of quantitation (LOQ) of all analytes are less than 10 ng/mL and 30 ng/mL, respectively. In addition, their recovery and coefficient of variation (CV%) are within 100–115% and 0.2–3.7%, respectively. The method validated in this paper is simple, fast, and sensitive for the quantification of alkaloid enantiomers in tobacco leaf and has been applied to investigations of tobacco alkaloid enantiomer ratios in different tobacco lines and tobacco products.
Keywords: Agriculture, Analytical chemistry
1. Introduction
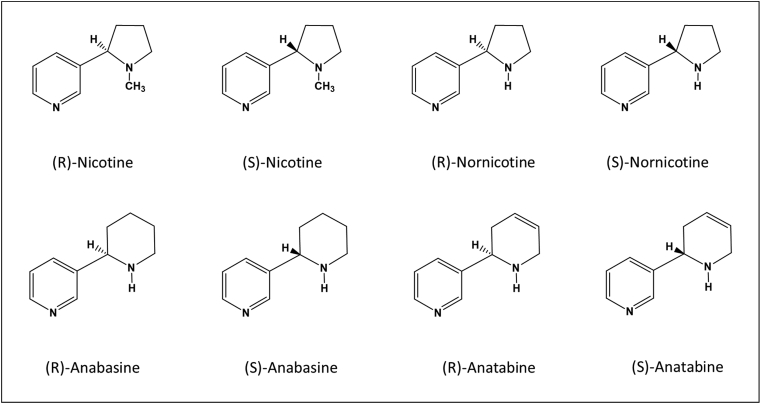
Smoking damages many body organs and causes diseases to smokers. Cigarette smoking is the leading preventable cause of death in the United States. Worldwide, smoking causes approximately 7 million deaths each year [1]. In June 2009, the law authorized U.S. Food and Drug Administration (FDA) to regulate the manufacture, marketing, and distribution of tobacco and tobacco products. In March 2012, FDA established a list of harmful and potentially harmful constituents (HPHCs) in tobacco products and tobacco smoke [2]. Nʹ-Nitrosonornicotine (NNN) is listed as a carcinogenic compound on the FDA HPHCs list. Nornicotine, the result of nicotine demethylation, is the immediate alkaloid precursor for NNN formation. Both nicotine and nornicotine exist as (R)- and (S)- enantiomeric isoforms that differ at the 2′-C position of the pyrrolidine ring (Fig. 1). The different alkaloid enantiomers have different pharmacological activities. It has been reported that (S)-nicotine is more pharmacologically potent than (R)-nicotine [3, 4] while (S)-NNN is more carcinogenic than the (R)-isoform [5]. Although (R)-nicotine shares many physicochemical properties with (S)-nicotine, it has been found that (S)-nicotine has a greater level of toxicity. LD50s for intravenous administration of (R)-nicotine in several species of animals have been approximately 18 times higher than that of (S)-nicotine [4].
Fig. 1.
Structures of tobacco alkaloid enantiomers.
The carcinogenicity of (S)-NNN has been postulated to be greater than (R)-NNN in rat esophagus [6, 7], and this was confirmed by a recent rat feeding assay [5]. Since nornicotine is the major metabolite of nicotine and the precursor of NNN, and it has been verified that (S)-NNN is produced only from (S)-nornicotine [8], we may reduce the harmful effects of cigarettes by adjusting the enantiomeric ratio of nornicotine, and hence the enantiomeric ratio of NNN. More of the (R) form would be desirable. The enantiomeric composition of NNN is dependent on the enantiomeric composition of its alkaloid precursor, nornicotine [8]. Quantitative analysis of the enantiomers of nicotine, nornicotine, and NNN is crucial to understand the metabolomic mechanism and compositional changes in the enantiomers of nicotine and nornicotine, and thus the enantiomer composition changes of NNN and potentially its biological activity.
A few papers have reported the determination of alkaloid enantiomers in tobacco. Alkaloid enantiomer analyses have been performed using normal phase high-performance liquid chromatography (HPLC) coupled with a diode-array or UV detector, multi-dimensional gas chromatography with mass spectrometry, or combined HPLC-UV detector and GC/MS [9, 10, 11, 12, 13]. However, these methods have limitations for routine analysis. The GC method involves the derivatization of alkaloids and has a long analysis time. Normal phase HPLC uses toxic and strong organic solvents, thereby requiring instrumentation to have greater tolerance for organic solvents. In this paper, a novel reverse-phase UPLC/MS/MS method for determination of alkaloid enantiomers in tobacco is presented. The advantages of this method include simple sample preparation with the extraction of alkaloids using methanol and water, running HPLC analysis with regular mobile phases, as well as higher sensitivity comparing with existing methods. This method has been successfully applied to alkaloid enantiomers analysis in different tobacco lines and tobacco products.
2. Experimental
2.1. Samples
Reference ground tobacco products (burley, flue-cured, oriental, dark fire-cured, and dark air-cured tobacco leaf) were purchased from the Center for Tobacco Reference Products (University of Kentucky, KY). CORESTA smokeless tobacco reference products were obtained from North Carolina State University, Raleigh, NC. Plants of different tobacco lines were grown at the University of Kentucky Agricultural Spindletop Farm. Lines included (1) a high nicotine demethylation line of TN90, (2) low nicotine demethylation lines of TN86 and TN90, and (3) double and triple mutant lines of TN86 and TN90 for decreased nicotine demethylation. The triple mutant lines carry knockouts of genes CYP82E4, CYP82E5, and CYP82E10 for nicotine demethylation and the double mutant line carries knockouts of CYP82E4 and CYP82E5 [8]. The transgenic plants were RNAi knockouts of all nicotine dementylation genes in the TN90 line [14]. All tobacco plants were harvested and air-cured in a traditional air-curing barn. After curing, the fourth leaf from the top of each plant was removed, the lamina and midrib were separated, and then five leaves from different plants were combined, freeze-dried, and ground as one sample. There were four replicates for each sample.
2.2. Reagents and materials
The alkaloid analytical standards (racemic R, S-nicotine (purity ≥99%), racemic R, S-nornicotine (purity ≥98%), and racemic R, S-anabasine (purity ≥97%)), and ammonium formate (LC/MS grade, putrity ≥99.0%) were purchased from Sigma-Aldrich Corporation (St. Louis., MO). Racemic R, S-anatabine (purity ≥97%) was from Matrix Scientific (Columbia, SC). (R)-nicotine (purity ≥98%), (S)-nornicotine (purity ≥96%), and isotopically labeled racemic R, S-nicotine-d4 (purity ≥98%) were obtained from Toronto Research Chemicals (Toronto, Canada). Labeled racemic R, S-nornicotine-d4 (purity ≥98%) was from CDN Isotopes Inc. (Quebec, Canada). Nicotine-d4 and nornicotine-d4 isomers were used for internal standards. LC/MS grade acetonitrile and methanol were from Fisher Scientific (Hampton, NH). Tomato leaf (NIST 1573a) standard reference material was purchased from the National Institute for Standards and Technology (NIST) (Gaithersburg, MD). The tomato leaves were used as the matrix during the experiment because tomato and tobacco are members of the same plant family, Solanaceae [15], but tomato leaves do not contain detectable levels of tobacco alkaloids.
2.3. Instrumentation and apparatus
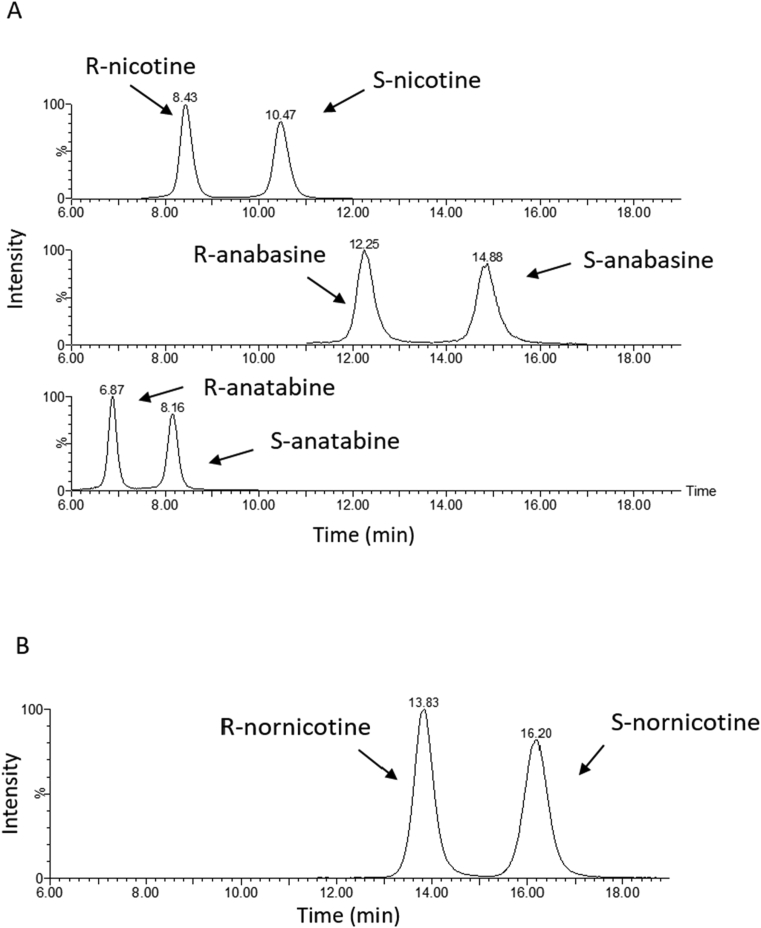
All analyses were done on a Waters ACQUITY UPLC H-Class System equipped with Xevo TQD Triple Quadrupole Mass Spectrometry (Waters Corporation; Milford, MA). The CHIRALPAK AGP column (150 × 4 mm column with 5 μm particle size) and LUX-Cellulose-2 column (150 × 2 mm column with 3 μm particle size) were used for nicotine and nornicotine enantiomer analysis, respectively. The enantiomers of anabasine and anatabine can be separated on both columns. Only the CHIRALPAK AGP column was used for analysis of anabasine and anatabine enantiomers in this paper. Separation of the enantiomers of nicotine, anabasine, and anatabine was achieved using an isocratic mobile phase program consisting of 90:10 (v/v) of 30 mM ammonium formate with 0.3% NH4OH and methanol with a flow of 0.4 mL/min (Fig. 2). For nornicotine enantiomer separation, an isocratic program of 90:10 (v/v) of 20 mM ammonium formate with 0.2% NH4OH:acetonitrile was used, and the flow rate was 0.2 mL/min (Fig. 2). The Waters Xevo TQD was operated in the electrospray ionization (ESI) in the positive mode with Multiple Reaction Monitoring (MRM) (Table 1). Source and desolvation temperatures were 150 and 500 °C, respectively. The desolvation gas flow was 800 L/hr. The capillary voltage was 0.38 kV.
Fig. 2.
Separation of nicotine, anatabine, and anabasine enantiomers from racemic alkaloid standards on the CHIRALPAK AGP column (A). R-nicotine and S-anatabine were distinguished and quantified by the differences in quantitation and confirmation transitions (m/z) for each (see Table 1). Separation of nornicotine enantiomers from the racemic nornicotine standard on the LUX Cellulose-2 column (B).
Table 1.
Mass spectrometric parameters for the quantification and confirmation of alkaloids in the multiple reaction monitoring (MRM) mode.
| Compound | Quantitation Transition (m/z) | Collision (V) | Confirmation Transition (m/z) | Collision (V) |
|---|---|---|---|---|
| (R)-Nicotine | 163 > 132 | 16 | 163 > 117 | 26 |
| (S)-Nicotine | 163 > 80 | 14 | 163 > 117 | 26 |
| (R,S)-Nicotine-d4 | 167 > 136 | 14 | 167 > 121 | 24 |
| (R,S)-Nornicotine | 149 > 117 | 20 | 149 > 80 | 18 |
| (R,S)-Nornicotine-d4 | 153 > 121 | 22 | 153 > 96 | 24 |
| (R,S)-Anabasine | 163 > 92 | 20 | 163 > 80 | 18 |
| (R,S)-Anatabine | 161 > 107 | 12 | 161 > 80 | 28 |
2.4. Sample preparation and analysis procedure
Tobacco plant samples were freeze-dried and ground to pass through a 1 mm screen and mixed well to ensure homogeneity. 200 mg of ground tobacco samples were placed in 20 mL glass vials. Isotopes of racemic R, S nicotine-d4 and racemic R, S-nornicotine-d4 as internal standards were spiked into the same vial prior to the addition of 200 μL of 5N NaOH pre-treatment solution, and the samples were allowed to stand for 10 minutes. After that, 10 mL 70% methanol was added and the mixture was placed on a horizontal shaker to shake for one hour. The extracts were filtered through a 0.22 μm PTFE filter to remove the tobacco powder and then diluted 20-fold with 70% methanol for alkaloid measurement. A 2 μL aliquot of the filtered extract solution was injected into the UPLC/MS/MS to determine the alkaloid enantiomers in each tobacco sample. For each tobacco sample, only one extraction needs to be done, but two injections onto the appropriate column were required to obtain the enantiomer results for all of the alkaloids.
3. Results and discussion
3.1. Column selection
There are many types of chiral columns on the market at present. The first step in method development was column screening. The CHIRALPAK AGP and CBH columns, LUX Cellulose-1, LUX Cellulose-2, LUX Cellulose-3, LUX Cellulose-4 and LUX Cellulose-5 columns were tested. Preliminary results indicated that the enantiomers of nicotine, anabasine, and anatabine had full baseline separation when the CHIRALPAK AGP column was used. However, the enantiomers of nornicotine only reached 85% baseline separation with this column. To obtain high resolution separation of nornicotine isomers, the LUX Cellulose-2 column was used (Fig. 2). Both columns separated anatabine and anabasine enantiomers very well, the resolution value is greater than 1.5.
3.2. Optimization of mobile phase
Different buffer solutions, including ammonium acetate, ammonium bicarbonate, and ammonium formate, were investigated for an appropriate mobile phase. The optimum result was achieved with ammonium formate. It was found that the pH value was critical for separation of the alkaloid enantiomers (data not shown). pH affects peak separation as well as the retention time. Ammonium hydroxide was used to adjust the pH to 9.5 for alkaloid enantiomer separation. Different organic solvents, such as acetonitrile, methanol, 2-propanal, and t-butanol were tested as mobile phase modifiers to improve the enantiomer separation. Our results indicate that methanol and acetonitrile are good mobile phase modifiers for the separation of nicotine and nornicotine enantiomers, respectively.
3.3. Optimization of extraction method
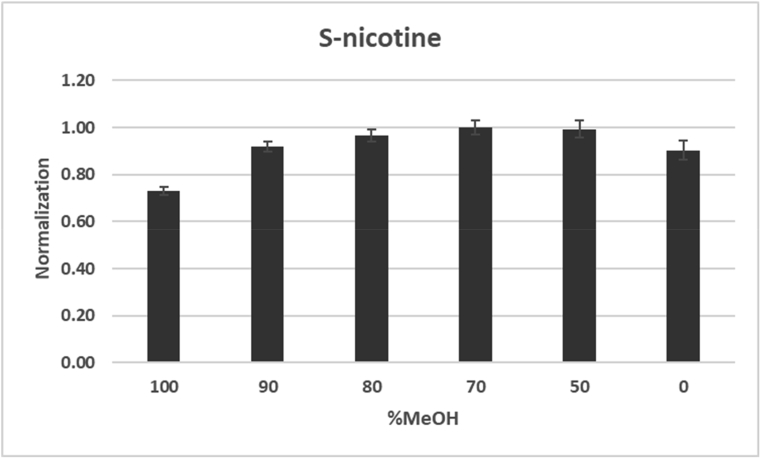
To optimize the extraction of alkaloids from ground tobacco leaf, the samples were extracted by shaking for one hour in different percentages of methanol and water. Mixtures consisting of 100%, 90%, 80%, 70%, 50%, and 0% methanol in water were evaluated. Five replicates were performed for each condition. The 70% and 50% methanol/water mixtures were found to have similar extraction efficiency for all target analytes. The S-nicotine extraction efficiency as an example was shown in Fig. 3. However, 50% methanol, which has greater water content, is capable of removing more salt from the tobacco samples and could potentially cause salt precipitation issues later. Therefore, 70% methanol was chosen as the extraction solution in these studies.
Fig. 3.
Extraction efficiency of S-nicotine from ground tobacc in different concentrations of methanol (n = 5). Data were normalized to 70% MeOH extraction.
3.4. Method validation
The standard calibration curves of the alkaloid enantiomers were established by injecting a series of individual alkaloid enantiomer standard solutions of known concentrations into the UPLC/MS/MS. The standard calibration solutions of these alkaloid enantiomers were made by spiking a known amount of alkaloid enantiomers and internal standards (isotope racemic R, S nicotine-d4 and racemic R, S nornicotine-d4) in the tomato leaf matrix before following the sample extraction procedure mentioned above in the Experimental Section. A 2 μL aliquot of each filtered extract solution was injected into the UPLC/MS/MS. Masslynx software was used to collect and process the data. The ratios of the peak area of each enantiomer to their corresponding internal standard were calculated. The graphs of the peak area ratio versus the concentration ratio of each alkaloid to its appropriate internal standard were plotted. The calibration type is linear with 1/x weighting, and the regression lines are not forced through the origin. All alkaloid enantiomers showed an excellent linear response (>0.996) (Table 2).
Table 2.
Summary of the limits of detection (LODs), the limits of quantitation (LOQs), and calibration curve range/linearity (n = 5).
| LOD (ng/mL) | LOQ (ng/mL) | Calibration Range (ng/mL) | Slope (average) | Intercept (average) | Linearity, R2 (average) | |
|---|---|---|---|---|---|---|
| (R)-nicotine | 8.4 | 28 | 100–4200 | 1.0651 | -5.8554 | 0.9987 |
| (S)-nicotine | 5.4 | 18 | 350–14000 | 0.1437 | -4.2977 | 0.9982 |
| (R)-nornicotine | 6.0 | 20 | 50–2100 | 1.1244 | -5.3211 | 0.9988 |
| (S)-nornicotine | 6.0 | 20 | 50–2100 | 1.1177 | -5.6773 | 0.9988 |
| (R)-anatabine | 4.8 | 16 | 50–2110 | 0.5545 | -1.1916 | 0.9967 |
| (S)-anatabine | 5.7 | 19 | 50–2110 | 0.5630 | -3.0739 | 0.9975 |
| (R)-anabasine | 1.2 | 4 | 15–530 | 0.8571 | -0.8130 | 0.9984 |
| (S)-anabasine | 4.2 | 14 | 15–530 | 0.8223 | 0.3679 | 0.9991 |
The method was validated for precision and accuracy of each analyte at different concentrations. The low, medium, and high levels of the alkaloid isomers with internal standards were spiked onto the tomato leaf matrixes. Three replicates of each concentration level were tested. The blank tomato leaf with internal standards only was used as the control. The recovery and coefficient of variation (CV%) of each enantiomer in the extraction procedure was 100–115% and 0.2–3.7%, respectively (Table 3), which indicates that our method has good accuracy and precision. The limit of detection (LOD) and the limit of quantitation (LOQ) were estimated by injecting a series of known-concentration alkaloid enantiomer standards into the UPLC/MS/MS 5 times. The standard deviations of the concentration from the five injections versus the concentration of each alkaloid were plotted. The value of the y-intercept of a linear regression (s0) is the estimation of standard deviation when the analyte is zero. The LOD and LOQ were estimated as 3s0 and 10s0, respectively [16]. The LOD and LOQ results are presented in Table 2.
Table 3.
Method accuracy with spiked alkaloids into the tomato leaf matrix (n = 3).
| Analyte | Spiked Concentration |
Recovery |
CV |
|---|---|---|---|
| (ng/mL) | % | % | |
| (R)-nicotine | 944.6 | 104.6 | 2.4 |
| 1889.2 | 107.4 | 1.3 | |
| 3778.4 | 103.9 | 0.5 | |
| (S)-nicotine | 944.6 | 108.8 | 3.3 |
| 1889.2 | 106.2 | 0.7 | |
| 3778.4 | 104.9 | 1.9 | |
| (R)-nornicotine | 428.8 | 114.5 | 3.7 |
| 857.6 | 115.7 | 0.2 | |
| 1715.2 | 113.2 | 2.6 | |
| (S)-nornicotine | 428.8 | 112.8 | 2.0 |
| 857.6 | 113.4 | 1.1 | |
| 1715.2 | 109.0 | 1.1 | |
| (R)-anatabine | 422.0 | 103.4 | 2.9 |
| 844.0 | 104.8 | 1.4 | |
| 1688.0 | 102.7 | 0.4 | |
| (S)-anatabine | 422.0 | 102.6 | 1.7 |
| 844.0 | 103.4 | 1.3 | |
| 1688.0 | 101.9 | 0.7 | |
| (R)-anabasine | 213.6 | 101.4 | 3.6 |
| 427.2 | 104.0 | 1.0 | |
| 854.4 | 102.9 | 1.8 | |
| (S)-anabasine | 213.6 | 107.2 | 3.5 |
| 427.2 | 108.9 | 1.0 | |
| 854.4 | 107.3 | 1.8 |
3.5. Detection of enantiomers in tobacco leaf and tobacco products with current method
TSNAs are carcinogenic compounds present in tobacco leaf, and (S)-NNN has more toxicity than (R)-NNN [5]. In order to reduce the harmful effects of cigarette smoking, scientists have recently expended considerable effort to change the accumulation of nornicotine, the precursor of NNN, especially in burley tobacco. These efforts include the screening of parental seed source plants, the release of low nicotine demethylation (LC seed) varieties [17], and GMO tobacco lines [8]. Depending on the tobacco line, the percentage of R-nornicotine in total nornicotine varies from 4 to 75% [18]. Alkaloid enantiomer analysis can help elucidate the composition of these enantiomers in tobacco. Our reverse phase UPLC/MS/MS method has been used to identify and quantify the alkaloid enantiomers in different tobacco lines including low nicotine demethylation (low conversion, LC) and high nicotine demethylation tobacco samples, as well as double- and triple-gene mutants for lower nicotine demethylation, and RNAi transgenic plants. In these samples, the triple mutant and the RNAi transgenic tobacco samples have ultra-low levels of nicotine demethylation. Results from ultra-low nicotine demethylation tobaccos indicate that the ratio of nornicotine isomers is altered in the leaf. The ultra-low nicotine demethylation activity decreased the percentage of (R)-nornicotine in the leaf (Table 4). This is consistent with the previous results of Cai and Bush (2012) [18]. Our method was also used to measure the enantiomers of tobacco alkaloids in different kinds of tobacco samples, such as cigar filler, reference cigarette filler, CORESTA smokeless tobacco reference products, as well as different tobacco market types, including burley, oriental, dark air-cured, dark fire-cured, and flue-cured. Our results are consistent with other published data [10, 11, 12]. In these tobacco samples, the major enantiomer of nicotine is (S)-nicotine (>99%) and most of them have a higher percentage of (S)-nornicotine than (R)-nornicotine. The range of the percentage of (R)-nornicotine was 15–50%. The ratio of (R)- and (S)-anabasine was consistent in the different tobacco samples, and the levels of (S) anabasine were slightly higher than R-anabasine. The percentage of (R)-anatabine was also consistent in the different tobacoo samples, approximately 15% (Table 5).
Table 4.
Percentage of R-alkaloids and demethylation of nicotine in the different tobacco lines (n = 4).
| Sample | Type | Nicotine |
Nornicotine |
Anatabine |
Anabasine |
Nicotine |
|---|---|---|---|---|---|---|
| R/total |
R/total |
R/total |
R/total |
Demethylation |
||
| % | % | % | % | % | ||
| TN90 | transgenic | 4.11 | 16.93 | 9.13 | 33.95 | 0.58 |
| triple mutant | 3.81 | 17.20 | 9.57 | 40.08 | 0.57 | |
| double mutant | 0.32 | 75.89 | 10.15 | 42.40 | 2.02 | |
| Low Conversion | 0.09 | 57.86 | 9.35 | 40.37 | 2.43 | |
| TN86 | triple mutant | 4.32 | 15.02 | 9.88 | 40.19 | 0.66 |
| Low Conversion | 0.11 | 53.72 | 10.28 | 42.78 | 2.61 | |
| TN90 | High Conversion | 0.11 | 7.21 | 10.69 | 48.63 | 38.84 |
Table 5.
Percentage of R-alkaloids found in individual tobacco types, cigarette and cigar filler, and smokeless reference products (n = 3).
| Sample | Type | Nicotine |
Nornicotine |
Anatabine |
Anabasine |
|---|---|---|---|---|---|
| R/total |
R/total |
R/total |
R/total |
||
| % | % | % | % | ||
| Tobacco leaf | RT2 (flue cured) | 0.10 | 33.22 | 15.13 | 37.74 |
| RT3 (oriental) | 0.38 | 14.94 | 15.72 | 41.97 | |
| RT4 (Burley) | 0.07 | 32.41 | 13.84 | 37.58 | |
| RTDAC (dark air) | 0.06 | 48.75 | 13.28 | 40.05 | |
| RTDFC (dark fire) | 0.40 | 40.14 | 13.42 | 40.76 | |
| Filler | RT6 (cigar filler) | 0.27 | 16.24 | 13.43 | 38.67 |
| RT1 (1R6F filler) | 0.13 | 30.32 | 14.65 | 38.60 | |
| Smokeless tobacco | CRP1.1 (Snus) | 0.32 | 31.42 | 15.57 | 42.75 |
| CRP2.1 (Moist Snuff) | 0.40 | 25.97 | 15.08 | 41.99 | |
| CRP3.1 (Dry Snuff) | 0.44 | 25.91 | 14.37 | 39.68 | |
| CRP4.1 (Loose- leaf Chewing) | 0.21 | 17.53 | 13.72 | 41.19 |
4. Conclusions
We have developed and validated a simple, rapid, and sensitive UPLC/MS/MS method for the quantification of alkaloid enantiomers in tobacco samples. This reverse phase LC/MS/MS method has been successfully applied to the determination of tobacco alkaloid enantiomers in tobacco leaves as well as a wide range of tobacco products.
Declarations
Author contribution statement
Huihua Ji: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ying Wu: Performed the experiments; Analyzed and interpreted the data.
Franklin Fannin: Contributed reagents, materials, analysis tools or data.
Lowell Bush: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by the University of Kentucky, Kentucky Tobacco Research and Development Center Summit Grant and in part, by the U.S. Food and Drug Administration through grant RFA-FD-14-001 and RFA-FD-15-026. The views expressed in this paper do not necessarily reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.WHO Report on the Global Tobacco Epidemic. 2017. http://apps.who.int/iris/bitstream/handle/10665/255874/9789241512824-eng.pdf;jsessionid=2C22C4F8E9201354D0A22AF1950AD744?sequence=1 [Google Scholar]
- 2.Reporting Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke under Section 904(a)(3) of the Federal Food, Drug, and Cosmetic Act. 2012. https://www.fda.gov/downloads/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm297828.pdf [Google Scholar]
- 3.Matta S.G., Balfour D.J., Benowitz N.L., Boyd R.T., Buccafusco J.J., Caggiula A.R., Craig C.R., Collins A.C., Damaj M.I., Donny E.C., Gardiner P.S., Grady S.R., Heberlein U., Leonard S.S., Levin E.D., Lukas R.J., Markou A., Marks M.J., McCallum S.E., Parameswaran N., Perkins K.A., Picciotto M.R., Quik M., Rose J.E., Rothenfluh A., Schafer W.R., Stolerman I.P., Tyndale R.F., Wehner J., Zirger J.M. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 4.Pogocki D., Ruman T., Danilczuk M., Celuch M., Walajtys-Rode E. Application of nicotine enantiomers, derivatives and analogues in therapy of neurodegenerative disorders. Eur. J. Pharmacol. 2007;563:18–39. doi: 10.1016/j.ejphar.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 5.Balbo S., James-Yi S., Johnson C.S., O’Sullivan M.G., Stepanov I., Wang M., Bandyopadhyay D., Kassie F., Carmella S., Upadhyaya P., Hecht S.S. (S)-N'-Nitrosonornicotine, a constituent of smokeless tobacco, is a powerful oral cavity carcinogen in rats. Carcinogenesis. 2013;34:2178–2183. doi: 10.1093/carcin/bgt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntee E.J., Hecht S.S. Metabolism of N‘-Nitrosonornicotine enantiomers by cultured rat esophagus and in vivo in rats. Chem. Res. Toxicol. 2000;13:192–199. doi: 10.1021/tx990171l. [DOI] [PubMed] [Google Scholar]
- 7.Lao Y., Yu N., Kassie F., Villalta P.W., Hecht S.S. Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-N'-nitrosonornicotine. Chem. Res. Toxicol. 2007;20:246–256. doi: 10.1021/tx060208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai B., Ji H., Fannin F.F., Bush L.P. Contribution of nicotine and nornicotine toward the production of N'-Nitrosonornicotine in air-cured tobacco (Nicotiana tabacum) J. Nat. Prod. 2016;79:754–759. doi: 10.1021/acs.jnatprod.5b00678. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y., Zielinski L.W., Bigott M.H. Separation of nicotine and nornicotine enantiomers via normal phase HPLC on derivatized cellulose chiral stationary phases. Chirality. 1998;10:364–369. [Google Scholar]
- 10.Liu B., Chen C., Wu D., Su Q. Enantiomeric analysis of anatabine, nornicotine and anabasine in commercial tobacco by multi-dimensional gas chromatography and mass spectrometry. J. Chromatogr. B. 2008;865:13–17. doi: 10.1016/j.jchromb.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong D., Wang X., Lee J., Liu Y. Enantiomeric composition of nornicotine, anatabine, and anabasine in tobacco. Chirality. 1999;11:82–84. [Google Scholar]
- 12.Moldoveanu S. Evaluation of several minor alkaloid levels in tobacco with separation of R- and S-nornicotine. Contrib. Tob. Res. 2013;25:649–659. [Google Scholar]
- 13.Moldoveanu S., Dull G.M., Oden R.J., Scott W.A. Analysis of (S)- and (R)-nicotine in various nicotine samples and in e-liquids, 70th TSRC. Tob. Sci. Res. Conf. 2017;71 abstract. 086 https://www.coresta.org/abstracts/analysis-s-and-r-nicotine-various-nicotine-samples-and-e-liquids-30936.html. [Google Scholar]
- 14.Lewis R., Bowen S., Keogh M., Dewey R. Three nicotine demethylase genes mediate nornicotine biosynthesis in Nicotiana tabacum L Functional characterization of the CYP82E10 gene. Photochemistry. 2010;71:1988–1998. doi: 10.1016/j.phytochem.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Gebhardt C. The historical role of species from the Solanaceae plant family in genetic research. Theor. Appl. Genet. 2016;129:2281–2294. doi: 10.1007/s00122-016-2804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor J.K. Lewis Publishers; 1987. Quality Assurance of Chemical Measurement. [Google Scholar]
- 17.Jack, A. and Bush, L.P. https://www.uky.edu/Ag/Tobacco/Pdf/LC-Protocol.pdf.
- 18.Cai B., Bush L.P. Variable nornicotine enantiomeric composition caused by nicotine demethylase CYP82E4 in tobacco leaf. J. Agric. Food Chem. 2012;60:11586–11591. doi: 10.1021/jf303681u. [DOI] [PubMed] [Google Scholar]