Significance
RIPK1 plays a critical role in mediating deleterious responses downstream of TNFR1. RIPK1 inhibitors have been progressed successfully past human phase I clinical studies. This paper discusses why RIPK1 inhibitors present an opportunity for developing oral drugs for a range of human degenerative and inflammatory diseases, especially CNS pathologies, including ALS, Alzheimer’s disease, Parkinson’s disease, traumatic brain injury, stroke, and lysosomal storage diseases.
Keywords: RIPK1, necroptosis, apoptosis, inflammation, neurodegeneration
Abstract
RIPK1 kinase has emerged as a promising therapeutic target for the treatment of a wide range of human neurodegenerative, autoimmune, and inflammatory diseases. This was supported by extensive studies which demonstrated that RIPK1 is a key mediator of apoptotic and necrotic cell death as well as inflammatory pathways. Furthermore, human genetic evidence has linked the dysregulation of RIPK1 to the pathogenesis of ALS as well as other inflammatory and neurodegenerative diseases. Importantly, unique allosteric small-molecule inhibitors of RIPK1 that offer high selectivity have been developed. These molecules can penetrate the blood–brain barrier, thus offering the possibility to target neuroinflammation and cell death which drive various neurologic conditions including Alzheimer’s disease, ALS, and multiple sclerosis as well as acute neurological diseases such as stroke and traumatic brain injuries. We discuss the current understanding of RIPK1 regulatory mechanisms and emerging evidence for the pathological roles of RIPK1 in human diseases, especially in the context of the central nervous systems.
Cell death is a fundamental process that controls organismal development and homeostasis by regulating cell numbers and eliminating damaged or infected cells. The discovery of apoptosis as a regulated cell death mechanism suggested the possibility of developing therapeutics to block pathologic cell death, common in human degenerative diseases. Caspases, a family of homologous mammalian cysteine proteases that are key mediators of apoptosis, emerged as attractive targets in preventing pathologic cell death (1). A major drug discovery effort targeting caspases ensued in the mid-1990s (2). However, these efforts largely failed to deliver new therapies. The reasons for this failure include the presence of multiple members of caspases with somewhat redundant functions, the involvement of caspases in important physiological functions, the activation of alternative cell death mechanisms upon inhibition of apoptosis and intrinsic toxicity-related challenges in inhibiting this class of cysteine proteases. As a result, it remained unclear whether blocking cell death could be an effective strategy for the treatment of degenerative and inflammatory human diseases.
Unexpectedly, apoptosis is not the only contributor to pathology in human diseases; other forms of regulated cell death also play critical roles. In particular, necrosis, defined morphologically by cell lysis with the release of intracellular contents into the extracellular space and traditionally believed to represent passive cell death, has attracted significant attention. Necrosis can be induced by diverse events that disrupt normal cellular physiological processes. However, a key question emerged whether there may also be a dedicated regulated mechanism that mediates the execution of necrosis, similar to that of caspases in mediating apoptosis. The first established example of such a pathway is necroptosis, a regulated necrotic cell death mechanism that can be activated under apoptosis-deficient conditions and is controlled by the kinase activity of RIPK1 and its downstream mediators: RIPK3 kinase and the pseudokinase MLKL (3, 4). Interestingly, while mice carrying mutations in different caspases display significant developmental defects (5), necroptosis-resistant mutant mice carrying different RIPK1 kinase dead knock-in mutations, including D138N, K45A, K584R, and ΔG26F27, as well as RIPK3 or MLKL knockout mutations, show no abnormality in development or in the adult animals (6–10). Thus, necroptosis might be predominantly activated under pathological conditions, which makes inhibiting this pathway an attractive option for the treatment of chronic human diseases.
Necroptosis was first defined by a series of small-molecule inhibitors (necrostatins), including Nec-1/Nec-1s, Nec-3, Nec-4, and Nec-5, which blocked TNF-α–induced necrotic cell death (11). Subsequent studies of necrostatins revealed a key role of RIPK1 as an important pharmacological target for inhibiting necroptosis (12). Importantly, the kinase activity of RIPK1 plays a central role in mediating multiple deleterious inflammatory cell death mechanisms upon the activation of TNFR1 by TNF-α, which is known to be involved in the pathogenesis of various human diseases (13). Anti-TNF agents have achieved major clinical success for the treatment of human inflammatory diseases in the periphery, such as rheumatoid arthritis, colitis, and psoriasis. However, anti-TNF strategy is not safe for the treatment of CNS diseases due to the involvement of TNFR2 in mediating neural regeneration (14). The specificity of RIPK1 in mediating TNFR1 signaling provides the possibility to safely ameliorate the deleterious TNF responses in the CNS without affecting TNFR2.
RIPK1 has become an important drug target in the pharmaceutical industry not only due to its key roles in TNF signaling responses but also because the kinase structure of RIPK1 is highly amenable for developing specific pharmacological small-molecule inhibitors. Nec-1/Nec-1s have been widely used to define the role of necroptosis and RIPK1 in human diseases using animal models. This included studies examining the role of this kinase in both acute injuries like ischemia as well as chronic neurodegenerative conditions such as multiple sclerosis (MS), ALS, and Alzheimer’s disease (AD) (13, 15, 16). To date, two different RIPK1 kinase inhibitor programs have progressed through human phase I safety trials.
Regulation of Necroptosis by RIPK1 Kinase in Response to TNF-α
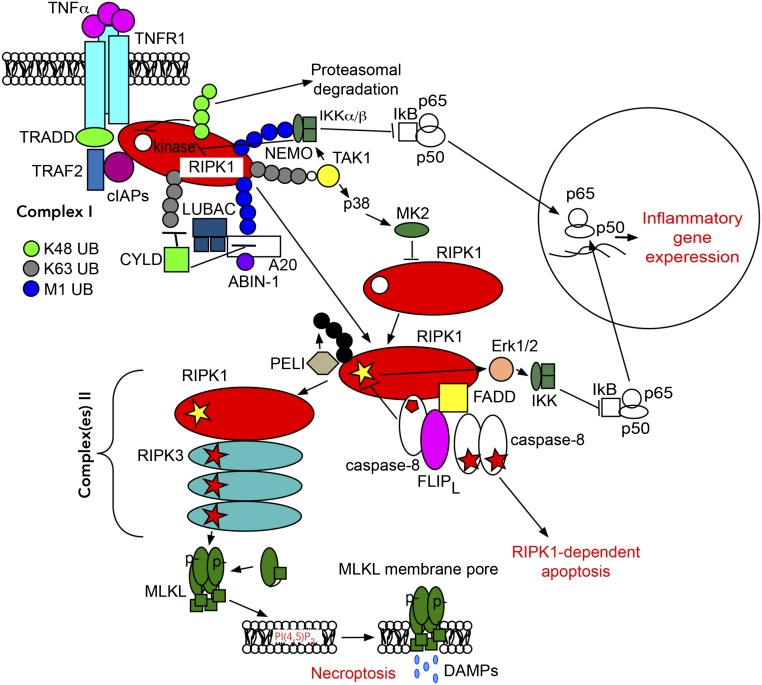
RIPK1 is a multidomain protein comprising an N-terminal kinase domain, an intermediate domain, and a C-terminal death domain (DD). The intermediate domain of RIPK1 contains an RHIM [receptor interacting protein (rip) homotypic interaction motif] domain which is important for interacting with other RHIM-containing proteins such as RIPK3, TRIF, and ZBP1. The C-terminal DD mediates its recruitment by interacting with other DD-containing proteins, such as TNFR1 and FADD, and its homodimerization to promote the activation of the N-terminal kinase domain (10). In the case of TNF-α signaling, ligand-induced TNFR1 trimerization leads to the assembly of a large receptor-bound signaling complex, termed Complex I, which includes multiple adaptors (TRADD, TRAF2, and RIPK1), and E3 ubiquitin ligases (cIAP1/2, LUBAC complex) (15, 17) (Fig. 1).
Fig. 1.
Schematic presentation of the RIPK1-dependent signaling events in response to TNF-α. Signaling bifurcates into poly-ubiquitin-dependent NF-κB activation mediated by receptor-bound Complex I through TAK1 and IKK kinase complexes. Alternatively, RIPK1 kinase activation and deubiquitination/reubiquitinatiuon promotes formation of secondary cytosolic Complex IIa and IIb. Depending on the activity of caspase-8 and RIPK3, signaling by Complexes II may lead to apoptosis, necroptosis, and increased inflammatory gene expression. The activating events are shown by arrows; however, it is not meant to indicate direct connections between signaling nodes. In many cases, mechanistic details remain to be uncovered.
RIPK1 is regulated by multiple posttranslational modifications, but one of the most critical regulatory mechanisms is via ubiquitination. The E3 ubiquitin ligases cIAP1/2 are recruited into Complex I with the help of TRAF2 to mediate RIPK1 K63 ubiquitination. K63 ubiquitination of RIPK1 by cIAP1/2 promotes the recruitment and activation of TAK1 kinase through the polyubiquitin binding adaptors TAB2/TAB3 (18). K63 ubiquitination also facilitates the recruitment of the LUBAC complex, which in turn performs M1- type ubiquitination of RIPK1 and TNFR1. M1 ubiquitination of Complex I is important for the recruitment of the trimeric IκB kinase complex (IKK) through a polyubuiquitin-binding adaptor subunit IKKγ/NEMO (15, 17). The activation of RIPK1 is inhibited by direct phosphorylation by TAK1, IKKα/β, MK2, and TBK1 (13). cIAP1 was also found to mediate K48 ubiquitination of RIPK1, inhibiting its catalytic activity and promoting degradation (19).
When cells are defective in activating the apical apoptotic mediator caspase-8, TNF-α stimulation promotes activation of a secondary cytosolic amyloid-like “necrosome” complex, also known as Complex IIb. This complex contains a hetero-oligomer of RIPK1 and RIPK3 which interact through their cognate RHIM domains (20). The activation of RIPK1 kinase precedes and is essential for the formation of the necrosome. Activated RIPK1 undergoes autophosphorylation on multiple residues including Ser14/15, Ser20, and Ser161/166 in the activation segment (12). Ser166 phosphorylation has emerged as a biomarker for RIPK1 activation (12, 21, 22). RIPK3 is phosphorylated in necrosomes on Ser227, and RIPK3 homo-oligomers eventually phosphorylate MLKL on the activation segment residues Thr357/Ser358 (23). The ensuing conformational change in MLKL leads to the formation of disulfide bond dependent amyloid-like polymers (24, 25), which cause rapid plasma membrane lysis—a hallmark of necrotic cell death.
It should also be noted that RIPK1 is not absolutely required for necroptosis beyond the ligands in the TNF family. For example, RHIM-domain-dependent activators TRIF and ZBP1 have been shown to engage and activate RIPK3 directly upon TLR3/4 engagement and viral infection (26, 27). Thus, RIPK1 inhibitors may provide specificity toward sterile inflammation mediated by TNF involved in human pathology, without affecting host defense mechanisms activated by TLR pathways upon pathogen invasion.
Activation of RIPK1-Dependent Apoptosis
Treatment with TNF-α when combined with a protein synthesis inhibition was originally found to induce RIPK1-independent apoptosis (RIA) (28). Since inhibition of protein synthesis does not occur in vivo, RIA might represent a largely artificial cell death paradigm that only occurs in vitro. However, when one of the checkpoints in complex I fails, such as the loss of cIAP1/2 and LUBAC ubiquitin ligases or TAK1, TBK1, IKK, and MK2 kinases, TNF-α stimulation leads to RIPK1-dependent apoptosis (RDA). RDA is characterized by the early activation of RIPK1 in Complex I within minutes of TNF-α stimulation (29). Activated RIPK1 in Complex I is then transited into a highly insoluble form, known as “iuRIPK1.” Activation of RDA is mediated by a secondary cytosolic Complex IIa which includes RIPK1, FADD, and caspase-8 to promote the activation of caspase-8 (Fig. 1). Activation of RDA has been implicated in ALS: in patients with haploid loss of TBK1, this pathway is more readily activated in aging human brains to mediate the pathogenesis of the disease (30).
Ubiquitination of Complex I recruits TAK1, which in turn mediates a critical inhibitory mechanism by the phosphorylation of multiple residues in the intermediate domain of RIPK1, including Ser321 residue in murine RIPK1 and Ser320 in human RIPK1 (31). In addition, TAK1-mediated IKK complex phosphorylates and inhibits RIPK1 in Complex I through a yet-to-be-identified site (32). The activation of TAK1 also suppresses cytosolic RIPK1 by promoting the activation of p38 and its target MAPKAPK2 (MK2), which phosphorylate Ser321/320 of RIPK1 (33).
While the kinase activity of RIPK1 mediates the activation of caspase-8 and RIPK3 to promote cell death when appropriate signals are present, RIPK1 also serves as a signaling scaffold to prevent the activation of caspase-8 and RIPK3 in a kinase-independent manner. This explains the neonatal death of mice deficient in RIPK1; this lethality is reversed by the combined deletion of both caspase-8 and RIPK3 in RIPK1-knockout mice (34, 35). The signals leading to activation of RIPK3/caspase-8 in the absence of RIPK1 may originate from the microbiome (36). RIPK1 was also shown to prevent skin inflammation by inhibiting RIPK3-MLKL–mediated necroptosis in a manner dependent upon Z-DNA binding protein 1 (ZBP1, also known as DAI/DLM1). ZBP1 deficiency inhibits keratinocyte necroptosis and skin inflammation in epidermis-specific RIPK1 knockout mice (37, 38). This is consistent with recent patient data where rare biallelic mutations in Ripk1 are associated with severe immune deficiency, arthritis and intestinal inflammation (39, 40). It is still not completely clear, however, why RIPK1 knockout promotes the activation of RIPK3 and MLKL. In any case, the scaffold function of RIPK1 is entirely different from that of its kinase activity as the inhibition of RIPK1 kinase prevents the activation of both caspase-8 (in RDA) and RIPK3 (in necroptosis).
Control of RIPK1 Kinase-Mediated Cell Death Decisions Regulates Necroptosis and RDA
The control of RIPK1 activation in the TNFR1 pathway occurs at two distinct checkpoints which regulate the activation of RDA and necroptosis (Fig. 1). As we discussed above, the first checkpoint controls the activation of RIPK1 in Complex I. Specific kinases, such as TAK1, TBK1, and IKKs, and ubiquitin ligases, such as cIAP1/2 and LUBAC, play critical roles in suppressing the activation of RIPK1 in Complex I. The failure of any of these kinases and ubiquitin ligases promotes early activation of RIPK1 in Complex I and consequently RDA when cells are stimulated by TNF-α. Since a subset of aging human brains are characterized by the reduced expression of TAK1 (30), laxed suppression of RIPK1 in aging human brains may provide an important mechanism that makes this tissue susceptible to RIPK1 activation and may point to an underlying mechanism that leads to progressive neurodegeneration.
Activation of caspase-8 mediated by complex IIa provides the second checkpoint to suppress the activation of RIPK1 downstream from Complex I. When apoptosis-competent cells are stimulated by TNF-α, RIPK1 is rapidly cleaved by caspase-8, which separates the N-terminal kinase domain from the intermediate domain and DD required for mediating the activation of the kinase activity of RIPK1 (41). Caspase-8 function is regulated by c-FLIPL/S, the inactive homolog of caspase-8. While caspase-8 in complex with the FLIPL isoform is partially active and this heterodimer can inhibit necroptosis (42), elevated levels of FLIPS may suppress the activation of caspase-8 to promote necroptosis (22). Thus, timing and mechanism of caspase-8 activation plays an important role in cell death. In addition to cleaving RIPK1, caspase-8 also mediates the cleavage of CYLD, a deubiquitinating enzyme that promotes necroptosis (43, 44).
A distinct ubiquitination code on RIPK1 may dictate different downstream events. While K63 ubiquitination of RIPK1 mediated by cIAP1/2 suppresses the activation of RIPK1 (45), K63 ubiquitination of RIPK1 by E3 ligase PELI on Lys115 residue promotes the activation of the kinase activity of RIPK1 (46). This step is likely proceeded by the removal of Complex I K63/M1 ubiquitin chains mediated by CYLD, which is recruited into the complex through LUBAC component HOIP (47, 48). These events are opposed by the ABIN-1/A20 complex, which interacts with M1 chains and prevents their removal (49). Regulation of RIPK1 by ubiquitination may reflect a very delicate balance as both reduced and increased levels of A20 may promote the activation of RIPK1 to mediate RDA and necroptosis (31, 50). The temporal aspect of RIPK1 activation is also critical for its regulation: While a transient phosphorylation event on Ser321 negatively regulates the activation of RIPK1 in Complex I, sustained TAK1 activation-mediated phosphorylation of RIPK1 on Ser321 promotes RDA and necroptosis (31). Thus, the intricate interplay of multiple ubiquitination and phosphorylation events on RIPK1 controls its activation to modulate cell death and inflammation.
RIPK1 Kinase Is a Key Mediator of Inflammatory Gene Expression
Necrotic cells are known to release damage-associated molecular patterns (DAMPs) which can activate an inflammatory response by various inflammasomes such as the NLRP3 complex (51). While apoptotic cells are effectively and rapidly removed by engulfment (52), the mechanism by which dying necroptotic cells are removed is still unclear. If necroptotic cells cannot be removed effectively before cell lysis, the release of DAMPs would contribute significantly to an inflammatory response. Activation of MLKL in necroptosis leads to its oligomerization, disruption of the integrity of plasma membrane, and leakage of intracellular contents (53) (Fig. 1). In macrophages upon inhibition of TAK1 either by Yersinia pseudotuberculosis YopJ or by the TAK1 inhibitor 5z-7-oxozeaenol, the activation of caspase-8 in RDA can promote the cleavage of Gasdermin D (GSDMD), which is known to mediate pyroptosis, another form of a regulated and highly inflammatory necrotic death (54–59). Similar to the cleavage of GSDMD by caspase-1/4/5/11 which promotes pore formation in pyroptosis (59, 60), RDA may also promote the release of DAMPs via pores formed by GSDMD after its cleavage by caspase-8.
Besides inflammation mediated by DAMPs, the activation of RIPK1 in necroptosis and RDA can also rapidly mediate the expression of inflammatory genes to promote inflammation independently from cell lysis (61, 62). In particular, activation of RIPK1 in the cells of myeloid lineage (e.g., microglia and macrophages) promotes the expression of inflammatory genes and the release of proinflammatory cytokines (e.g., TNF-α) independently from cell death (63–65). Furthermore, autocrine signaling by the released TNF family members, produced upon RIPK1 activation, is an important component of tissue injury. This has been shown in mice deficient in proteins that regulate RIPK1 activation, such as NEMO and Sharpin, the latter a component of the LUBAC complex (21, 66, 67).
Recent data delineated cell-death-independent signaling activities of RIPK1 (sometimes along with RIPK3) leading to transcriptional up-regulation of a wide range of inflammatory molecules (61–63, 65). Regulation of inflammatory gene expression involves a range of MAPKs downstream from RIPK1, including p38 and Erk1/2, as well as TBK1/IKKε complex. These kinase circuits further activate the transcription factors AP1, Sp1, NF-κB, and IRF3/7. In the context of necroptosis, this may provide a burst of inflammatory signals in addition to the responses elicited by DAMPs released from dead cells. However, there is also growing evidence that RIPK1-dependent proinflammatory responses may occur independent from necroptosis in vivo. For example, RIPK1 kinase activity is required for inflammatory gene expression in myeloid cells after mouse challenge with a sublethal dose of LPS (61). Similarly, RIPK1 exacerbates chronic inflammation during embryonic development when both branches of cell death, controlled by caspase-8 and RIPK3, are absent (68). Pathologically, RIPK1-dependent inflammatory gene expression has been linked to neutrophilic dermatitis in Ptpn6spin mice (69), clearance of West Nile virus in mice (70), and neuroinflammation in human diseases that will be discussed below.
Necrostatins and Other RIPK1 Kinase Inhibitors
Deciphering the necroptosis pathway followed an unusual route as it involved the extensive use of small-molecule tools to understand the relevance and mechanistic underpinnings of this process. A first set of selective necroptosis inhibitors, which we termed necrostatins, was identified using a cell-based screen of TNF-induced necroptosis in a human U937 monocytic cell line (11). Necrostatin-1 completely prevented the activation of necroptosis across a range of cellular models where TNF/FasL-induced activation of necrosis-like death had been observed (11). Importantly, Nec-1 was also shown to reduce oxygen/glucose deprivation-induced death in primary neurons and to decrease the infarction size after transient middle cerebral artery occlusion (MCAO), a mouse model of ischemic stroke, providing the first indication for the pathologic relevance of necroptosis. Since then, necroptosis has been firmly established as a component of many ischemia-reperfusions injuries in the brain, retina, heart, kidneys, and liver (71–75). Overall, these early findings using necrostatins were significant because they demonstrated the existence of a common mechanism of necrotic death across a range of cell types in response to TNF and under pathologic conditions in vivo.
Subsequently, we identified RIPK1 as the direct target of Nec-1 and other structurally distinct necrostatins (12). Importantly, we found that a mutation of Ser161 in the activation segment of RIPK1 offered resistance to inhibition by Nec-1 and other necrostatins. These data confirmed that RIPK1 is the sole target of necrostatins in necroptosis. Furthermore, the fact that all the hits in an unbiased cell-based screen were all RIPK1 inhibitors highlighted a unique significance of this kinase for the effective and selective inhibition of necroptosis. To date, Nec-1, Nec-1s, and other recently developed RIPK1 inhibitors have been instrumental in establishing the important contribution of RIPK1 to a wide range of human pathologies, representing areas of major unmet medical need, including brain trauma, sepsis, and neurodegenerative and autoimmune disorders (13, 16).
Subsequent analyses revealed several remarkable features of necrostatins. All necrostatins isolated in an unbiased cell-based screen display almost exclusive selectivity for RIPK1 (63), which is very unusual for kinase inhibitors. The structural basis of inhibition by Necs was revealed by the crystal structure of RIPK1 in complex with three different necrostatins—Nec-1s, Nec-3, and Nec-4—reported by Shi and coworkers (76). All three necrostatins belong to a type III class of allosteric kinase inhibitors (77). Furthermore, all three Necs bind to the exact same allosteric site on RIPK1 formed behind the active center. This pocket becomes accessible when Mg-binding DLG motif assumes an inactive DLG-out conformation and αC helix is outwardly displaced to the Glu-out conformation with a characteristic loss of Glu-Lys ionic pair critical for catalysis (Fig. 2A). While inhibitors targeting either DFG-out (type II inhibitors) or Glu-out (αC helix displacing inhibitors) conformations have been described previously, RIPK1 is the only kinase for which doubly inactive DLG-out/Glu-out conformation has been described to date.
Fig. 2.
Mode of inhibition of RIPK1 by necrostatins and other type III inhibitors. (A) Rendering of the crystal structure of Nec-1s with RIPK1 (85). Nec-1s is occupying the back pocket localized behind the ATP binding center. This pocket is created due to the outward movement of αC-helix, resulting in the loss of ionic pair between catalytic Lys45 and Glu63 of αC-helix. The other side of the pocket is formed by the DLG motif (shown in green) in the inactive DLG-in conformation (catalytic Asp146 facing away from the active center) and the activation segment, which immediately follows the DLG motif (shown in red). Ser161 residue of the activation segment, which forms a critical hydrogen bond with the indole of Nec-1s, is also shown. (B) A number of additional type III inhibitors have been recently developed by GlaxoSmithKline and Takeda, including clinical candidate GSK′772 (88–91). These molecules (rendered in red, based on published crystal structures) occupy the same back pocket as Nec-1s (shown in green) in the same Glu-out/DLG-out conformation but extend into the ATP binding pocket, which may contribute to the increased affinity.
This unique allosteric pocket of RIPK1 is in part due to an increased flexibility of the DLG motif in RIPK1, compared with a more rigid DFG motif found in most kinases (78). However, to engage this pocket necrostatins still need to fit into the allosteric site very precisely and tightly, resulting in a high degree of specificity. Overall, the unusual dynamics of RIPK1 and the unique binding mode of necrostatins most likely collectively dictate an unprecedented selectivity of these molecules. Validating this conclusion, GlaxoSmithKline and Takeda also successfully developed inhibitors targeting the same allosteric pocket/inactive conformation of RIPK1 (Fig. 2B) and these molecules also display exclusive selectivity toward RIPK1 (79–82). Conversely, more typical type I inhibitors, targeting the ATP pocket (VX-680 and Pazopanib), and type II inhibitors, engaging residues in the ATP pocket and back pocket in Glu-in/DLG-out conformation (Ponatinib and Rebastinib), were also found in screening of kinase inhibitor collections, but these molecules are not specific for RIPK1 (78, 83, 84).
Development of type III RIPK1 inhibitors offers hope that inhibition of RIPK1 may prove a successful strategy in many chronic degenerative and autoinflammatory diseases, where inhibitor selectivity is paramount to ensure safety. One of these molecules, a clinical candidate compound GSK2982772 developed by GlaxoSmithKline, has successfully completed phase I safety trials (85). Another molecule DNL747, developed by Denali Therapeutics, also successfully completed a phase I trial.
Developing RIPK1 Inhibitors for the Treatment of Human Diseases
The identification of RIPK1 kinase as a critical mediator of both cell death and inflammation presents an exciting new opportunity for developing therapeutics for the treatment of human diseases. This is particularly of interest in the context of neurodegenerative diseases because the pathology in many of these diseases is characterized by necrosis (86). New drug targets are needed in the field as previous trials of potential therapies for neurodegenerative diseases such as AD, ALS, and Parkinson’s disease (PD) have largely failed or demonstrated minimal efficacy. We have recently reviewed the mechanism of RIPK1 and its role in mediating CNS diseases in general (13). Our discussion here will focus on developing RIPK1 inhibitors for the treatment of specific human diseases with an emphasis on neurodegenerative diseases. While RIPK1 inhibitors are expected to be also effective for the treatment of peripheral human inflammatory diseases, the possibility to develop highly specific RIPK1 inhibitors that can pass the blood–brain barrier (BBB) provides a special opportunity for the treatment of CNS diseases.
RIPK1 in Acute Neuronal Injury.
The involvement of necroptosis in acute neurological injuries (e.g., ischemic brain injury) was first demonstrated with Nec-1 before its targeting mechanism was revealed (11). Nec-1, but not inactive Nec-1i derivative, was shown to attenuate infarct volume in the MCAO model of stroke in a dose-dependent manner. It remained active when administered both prophylactically and therapeutically after the onset of ischemic insult with a significant time window. The identification of RIPK1 as a specific target of Nec-1 revealed this kinase as a new mediator of acute ischemic neurological insult. Notably, RIPK1 also contributes to ischemic injuries of other organs including eye, heart, and kidney (71, 87–91). An increase in RIPK1 and RIPK3 and the formation of RIPK1/RIPK3 complex were found following permanent MCAO (92). RIPK1 activation also occurs after traumatic brain injury (TBI); similar to the MCAO model, both RIPK1 and RIPK3 are increased in the brains of animals in the fluid percussion brain injury model in rats (93). Interestingly, posttraumatic hypothermia (33 °C), which is known to reduce brain injury following stroke and TBI, led to decreases in the levels of RIPK1, RIPK3, and MLKL. These data suggest that deleterious RIPK1-dependent signaling may play a causal role in neuronal loss following TBI. Consistent with this notion, mice administered Nec-1 before controlled cortical impact (CCI) had reduced neuronal death and improved motor and spatial memory outcomes. Furthermore, improved spatial memory was observed even when necrostatin-1 was administered 15 min after CCI (94). These data implicate RIPK1 as a central mediator of neurodegeneration following acute neuronal injury.
RIPK1 in MS.
There is growing evidence that RIPK1 mediates deleterious processes in chronic neurodegeneration. A key similarity between acute injury and chronic neurodegeneration is the presence of neuroinflammation. MS is an inflammatory disease of the CNS that leads to chronic neurodegeneration. TNF-α signaling has been strongly linked to the pathophysiology of MS (95, 96). The role of RIPK1 in mediating a deleterious response downstream of TNFR1 suggests the role of RIPK1 in this disease. In CNS lesions of both brain samples from MS patients and in mouse models of MS, RIPK1 is relocalized to the insoluble fraction (22), which occurs when RIPK1 is activated (97). Inhibition of RIPK1 by Nec-1s ameliorated disease pathology, improved animal behavior, and attenuated experimental allergic encephalomyelitis (EAE)-induced cytokine increase and recruitment of immune cells (22). The role of RIPK1 in EAE was also validated by the use of another RIPK1 inhibitor developed by Takeda (79). Mechanistically, RIPK1 is highly expressed in macrophages and microglia in the EAE lesions, and Nec-1s may dampen the innate immune response in these cells. Consistently, blocking RIPK1 activity modulates the inflammatory and cell death responses in microglia cells (62, 98).
While RIPK1 and its downstream binding partners (RIPK3, caspase-8, and MLKL) are highly expressed in immune cells they are also present in other cell types of the CNS. In particular we identified a RIPK1-deleterious pathway in primary oligodendrocyte lineage cells, which are specifically killed by a toxin in the cuprizone model of demyelinating disease (22). Previous studies had shown that oligodendrocytes are sensitive to TNF-α stimulation, in particular in the presence of other CNS cells (99). Our study showed that this TNF-α–induced oligodendrocyte cell death could be inhibited by either pharmacological inhibition of RIPK1 with Nec-1s or genetic deletion of RIPK3. Consistent with this, RIPK3–MLKL interaction and necroptosis were shown to be critical for oligodendrocyte necroptosis following oxygen–glucose deprivation as well as in MCAO (71, 100).
The deleterious role of both RIPK1-dependent inflammatory signaling and necroptosis in MS is further supported by the robust finding that RIPK1, RIPK3, and MLKL are activated in tissues from postmortem pathological samples from MS patients (22). The beneficial effect of inhibiting RIPK1 is likely attributable to both modulation of inflammation as well as blocking necroptosis in susceptible oligodendrocytes and neurons (Fig. 3). RIPK1 may act in a cell-autonmous as well as non-cell-autonomous manners to induce necroptosis susceptibility in oligodendrocytes.
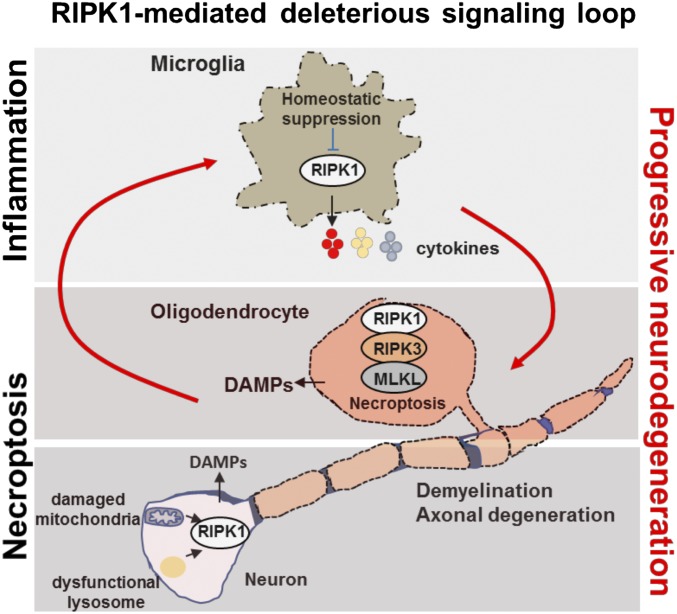
Fig. 3.
Bimodal RIPK1 activation in neurological disease leads to progressive deleterious signaling loop to promote neuroinflammation and cell death. Activation of RIPK1 in either microglia oligodendrocytes or neurons can initiate a degenerative signaling loop. This cascade relies on microglial-driven deleterious inflammation and necrotic cell death in the CNS. Microglial. RIPK1 regulates a degenerative neuroinflammatory milieu in the CNS that can lead to necroptosis of oligodendrocytes and axonal degeneration. Neurons with damaged mitochondria and lysosomes may undergo necroptosis. In turn, necroptosis of either oligodendrocytes or neurons promotes inflammation by driving the cell-autonomous expression of proinflammatory cytokines in microglia as well as by releasing of the cellular content from necrotic cells (including DAMPs) into the CNS. This deleterious axis creates a progressive inflammatory and degenerative environment in the brain to promote the progression of neurodegenerative disease.
RIPK1 and ALS.
ALS is a progressive neurodegenerative disease that leads to degeneration of motor neurons and paralysis. Activation of RIPK1-mediated neuroinflammation and cell death is directly linked with the genetics of ALS in humans. Mutations in the genes that lead to familial ALS in humans, including Optineurin and TBK1, have been shown to promote the onset of RIPK1-dependent necroptosis and RDA in the CNS (30, 101). Optn encodes a ubiquitin-binding protein that regulates RIPK1 ubiquitination and degradation. Optn knockout mice developed neuropathology resembling ALS, including Wallerian-like axonal pathology and dysmyelination (101). The loss of optineurin in both oligodendrocytes and microglia, but not motor neurons or astrocytes, is sufficient to promote neuropathology. Inhibition of RIPK1 also protects against the degeneration of oligodendrocytes in SODG93A transgenic mice, which is known to occur before the onset of motor dysfunction (101, 102). These results suggest that RIPK1 might promote axonal degeneration and neuroinflammation noncell autonomously in ALS.
While Optn regulates the ubiquitination of RIPK1, TBK1 regulates RIPK1 through direct phosphorylation on multiple sites including Thr189 to suppress RIPK1 kinase activity by blocking the interaction with its substrates (30, 103). TBK1 mutations are a major genetic cause of patients with ALS/frontotemporal dementia (FTD) comorbidity (10.8%) and to a lesser extent causal in ALS alone (0.5%) (104, 105). Reduced expression of TBK1 by mutant alleles associated with ALS/FTD has led to the proposal that haploinsufficiency of TBK1 is a pathological mechanism. TBK1 knockout mice are embryonically lethal, and this lethality is blocked in TBK1−/−; RIPK1D138N kinase-dead knock-in mutant mice, which provides a genetic verification for the critical function of TBK1 in regulating the activation of RIPK1 (30). This is consistent with in vitro observations that TBK1 deficiency or pharmacological inhibition sensitizes cells to RIPK1-dependent cell death induced by TNF-α (30, 103). Interestingly, while TBK1+/− mice are normal, the haploid loss of TAK1, another RIPK1 suppressor, in the myeloid lineage of TBK1+/− mice leads to many of the hallmarks of ALS/FTD, including dysmyelination, axonal degeneration, neuronal loss and cytoplasmic TDP-43 aggregates in the CNS (30). Thus, the activation of RIPK1 might be involved in mediating ALS pathology in multiple lineages and CNS cell types (Fig. 3). Furthermore, reduced expression of TAK1 in the CNS of aging human brains may provide a common mechanism to promote not only ALS, but also other age-dependent neurodegenerative diseases including AD and PD.
RIPK1 in AD.
AD is the most common age-related neurodegenerative disorder. AD is characterized by cognitive impairment with loss of memory and neuropathological features such as the accumulation of amyloid plaque deposits composed of amyloid-β (Aβ), hyperphosphorylation of microtubule-associated protein tau, neuroinflammation, cerebrovascular pathology, and neuronal loss. The amyloid hypothesis proposes that the accumulation of Aβ and formation of amyloid plaques is the main cause of AD pathogenesis. The amyloid hypothesis is supported by the early onset familial Alzheimer’s disease (FAD) mutations in the amyloid precursor protein (APP) and the presenilin genes that drive amyloidosis (106). However, the poor performance of Aβ-directed therapies in clinical trials has forced rethinking of additional factors that can contribute to AD pathogenesis. A hallmark of AD is chronic brain inflammation marked by increases in the levels of proinflammatory cytokines (107). Neuroinflammation characterized by the overactivated dystrophic microglia is prevalent in late stage AD (LOAD) and may contribute significantly to dementia (108). The role of neuroinflammation has also been supported by the genome-wide association studies on LOAD which have identified variants in microglia-associated genes, such as the genes encoding triggering receptor expressed on myeloid cells 2 (TREM2) and the microglial surface receptor CD33, that confer higher risks for developing LOAD.
Under physiological conditions, microglia participate in regulating synaptic pruning and play a surveillance role in maintaining homeostasis by removing cellular debris, dying cells, or misfolded proteins. During AD pathogenesis, microglia activation may play a different role in early (preclinical) and late (clinical) AD stages. Acutely activated microglia can promote the degradation of Aβ by phagocytosis, but sustained chronically activated microglia may be deficient in degradation of Aβ and instead contribute to neurotoxicity and synapse loss by triggering proinflammatory cascades. Microglial activation triggered by the focal aggregation of extracellular Aβ into insoluble amyloid may occur late during preclinical stages of AD and set the stage for the onset of dementia (109). Activated microglia secrete proinflammatory cytokines such as TNF-α, IL-1β, and IL-6, as well as chemokines that recruit and promote further activation of glial cells and neuronal damage. A genome-wide association study of LOAD risk factors identified polymorphisms in the TNF promoter that are linked to increased TNF-α production (110).
RIPK1 has a well-recognized role in mediating transcription of neuroinflammatory genes in microglia (13, 101). RIPK1 is highly expressed in microglia in mouse and human brain samples (111). The levels of RIPK1, at both the mRNA and protein levels, in postmortem samples from individuals with LOAD are increased compared with controls and positively correlate with the reduction in brain weights and Braak stages (111, 112). RIPK1, MLKL, and pMLKL levels were significantly higher in the brains of 11-mo-old 5xFAD mice compared with nontransgenic littermates, suggesting the involvement of necroptosis in AD (112). RIPK1 may be involved in regulating a transcriptional response in AD as the set of genes regulated by RIPK1 overlapped significantly with multiple independent AD transcriptomic signatures, including multiple genes linked with the expression of AD-risk variant genes and other CNS diseases (111, 112) (Fig. 3). Inhibition of RIPK1 kinase activity has also been shown to promote the ability of microglia to degrade Aβ (111). The levels of amyloid plaques in APP/PS1 mice are reduced upon pharmacological inhibition of RIPK1 using Nec-1s. Thus, inhibition of RIPK1 may be able to reduce inflammatory microglia and restore the phagocytic ability of microglia.
The RIPK1-mediated gene expression signature in microglia provides clues as to how RIPK1 activation may mediate inflammation. Activation of RIPK1 in microglia regulates the expression of important genes involved in mediating innate immune response. In particular, Ch25h, Cst7, Clec7a, and Csf1 proteins are elevated in microglia of multiple mouse models of AD and ALS, for example 5xFAD mice and SOD1G93A mice, and in aging microglia (111). Among the genes regulated by RIPK1, the Ch25h gene encodes cholesterol 25-hydroxylase, which mediates the production of 25-hydroxycholesterol, a potent corepressor of SREBP (sterol regulatory element binding protein) and an important regulator of the gene expression involved in lipid metabolism. The expression of the Ch25h gene can be induced both by infection and inflammation. Increased expression of Ch25h is found in the vulnerable brain regions of AD brain samples and correlates with Braak (NFT) staging of disease progression (113). The potential role of RIPK1 in regulating the expression of Ch25h in AD suggests the possibility of lipid changes serving as a biomarker for RIPK1 activity. In addition to Ch25h, RIPK1 has also been shown to regulate the expression of Cst7, Csf1, and Clec7a, which have been shown recently as the biomarkers of a microglial class known as disease-associated microglia (DAM) (111, 114). It is believed that during AD pathogenesis, DAM evolve from homeostatic microglia by progressively acquiring a unique transcriptional profile. Up-regulated Cst7, which encodes an endosomal/lysosomal cathepsin inhibitor known as cystatin F, is a biomarker for DAM at advanced stages of AD (114) and is promoted by activation of RIPK1 (111).
Elevated levels of Cst7 were found in microglia around Aβ deposits in the APP/PS1 AD mouse model, whereas dispersed microglia were generally negative for Cst7. Thus, increased expression of Cst7 might lead to lysosomal dysfunction in microglia and contribute to reduced proteostasis in AD. Since inhibition of RIPK1 reduces the microglial expression of CST7 in APP/PS1 mice and promotes the degradation of Aβ by microglia (111), the deleterious effect of RIPK1 activation in AD is likely due at least in part to reducing the phagocytic activity of DAM. These results predict that inhibition of RIPK1 kinase activity in human AD patients should lead to reduced levels of Aβ accumulation and neuroinflammation, two hallmarks of AD.
Notably, microglial CST7 expression is strongly induced across the range of animal models of neurodegenerative diseases, including SOD1G93A AD transgenic mice, during demyelination, in a prion disease model, and with aging (115–117). Therefore, RIPK1 may be broadly involved in modulating DAM activity in neurodegenerative diseases.
RIPK1 in Lysosomal Storage Diseases and PD.
The lysosomal storage diseases (LSDs) are a group of about 50 rare genetic disorders that are characterized by lysosomal defects and an accumulation of waste products in the lysosomes. Other than these typical LSDs, aging and neurodegenerative diseases are also characterized by defects in degradative mechanisms, including autophagy and lysosomal function, and the buildup of misfolded proteins. A recent study demonstrated an increase in the formation of insoluble, lipofuscin-like lysosomal inclusions in microglia, leading to lysosomal storage and immune dysfunctions during aging (118). In microglia, RIPK1 can be activated in response to both proteasomal and lysosomal inhibition (111). An enticing possibility is that progressive alterations in the cellular degradative machinery may contribute to RIPK1 activation in both aging neurodegenerative diseases and LSD. We already discussed the former, and in the case of the latter, inhibition of RIPK1 both pharmacologically with the RIPK1 inhibitor GSK′547 and genetically in the RIPK1 knock-down mice improves survival of a mouse model of Neimann–Pick disease, a lysosomal storage disorder (119). NPC1 is a membrane protein that regulates cholesterol trafficking, and it is remarkable that inhibition of RIPK1 is sufficient to alter the progression of disease. Interestingly, combination of RIPK1 inhibition and 2-hydroxypropyl-β-cyclodextrin cholesterol-chelating therapy, which has been shown to slow neurological disease progression in NPC1 mice, cats, and patients, had additive effects (119). Surprisingly, RIPK3 knockout animals did not show an improved survival in the NPC model (119). This dichotomy may point to a clear role for RIPK1 either as a nonnecroptotic effector or as a neuroinflammatory mediator.
These findings are in contrast to a study a mouse model of Gaucher’s disease (GD). GD is the most common LSD caused by mutations in the glucocerebrosidase gene (GBA). Loss-of-function mutation in GBA leads to glucosylceramide and glucosylsphingosine accumulation in the brain and neuronal loss in patients and in animal models of this disease. In the GBA knockout mouse model, RIPK3 deficiency substantially improved the clinical course of GD, with increased survival and motor coordination and salutary effects on cerebral as well as hepatic injury (120). Since RIPK1 mediates the activation of RIPK3, the role of RIPK1 should be tested in GD.
While the complete loss of function in GBA leads to GD, a partial loss of function is associated with PD (121). Similar to the role of necroptosis observed in a mouse model of GD, the increases in the levels of RIPK1, RIPK3, and MLKL were observed in the postmortem substantia nigra of individuals with PD compared with controls. Furthermore, in a systematic RNAi screen for regulators of RDA, leucine-rich repeat kinase 2 (LRRK2), the gene which is often mutated in PD, was shown to promote the activation of Complex I-associated RIPK1 (29). In addition, Optic atrophy 1 (OPA1), a GTPase that regulates mitochondrial fission and fusion, is mutated in some PD patients. Neural cells differentiated from induced pluripotent stem cells from OPA1-mutant PD patients displayed severe mitochondrial dysfunction and cell death that was inhibited by Nec-1s (122). These results suggest that dysfunction in lysosome and mitochondria may promote the activation of RIPK1 and sensitize cells to the activation of necroptosis and inhibiting the kinase activity of this enzyme can change the disease course in LSDs and PD (Fig. 3).
Concluding Remarks
RIPK1 has emerged as a promising target for a spectrum of human CNS and peripheral pathologies. RIPK1 activity is tightly controlled by multiple layers of regulatory factors that we have summarized as checkpoint I in Complex I and checkpoint II in Complex II. Disabling any one of these control points promotes the activation of RIPK1-dependent cell death and inflammation and thus may provide a common pathological mechanism of activation of RIPK1 in human diseases. This is exemplified by the loss of activity or haploinsufficiency in TAK1 and TBK1 that promote the development of ALS/FTD and ALS in aging (30, 101). Similarly, down-regulation of caspase-8 was observed in the white matter lesions of MS patients (22). Insufficient caspase-8 activity may also contribute to RIPK1 activity in some cases of AD (123). Conditions of acute energy loss and oxidative stress may inhibit caspase activation, promoting necroptosis under acute neurologic stress conditions (124). On other side of the equation, overproduction of TNF-α and the loss of lysosomal and nonlysosomal degradative functions may promote RIPK1 activation in both LSDs and aging-related neurodegenerative conditions. While we still do not fully understand the positive and negative regulators of RIPK1 that operate in human diseases, many critical controls on RIPK1 activity have been uncovered in the last few years, and this presents putative mechanisms that need to be examined in human diseases in the future.
The activation of RIPK1 kinase mediates the majority of the deleterious response downstream of TNFR1 (13). Just like anti-TNF has been proven to provide transformative therapies for the treatment of peripheral inflammatory diseases, safe and BBB-permeable small-molecule RIPK1 inhibitors may provide an unique opportunity to develop oral drugs for a range of degenerative CNS pathologies, including ALS, AD, PD, TBI, stroke, and LSDs as well as peripheral inflammatory diseases in a cost-effective manner. The proof-of-concept clinical trials for some of these indications are expected to commence in the near future. Of course, there is a major stigma associated with the use of kinase inhibitors in chronic CNS diseases, mainly due to their generally limited selectivity and, therefore, safety. As we discussed above, the unusual conformation of the back pocket is uniquely suited for small-molecule inhibitors that, thus far, has only been described for RIPK1. Resulting inhibitors display exceptional specificity for RIPK1, significantly derisking targeting RIPK1 for the treatment of chronical human diseases. Consistently, two phase I trials of RIPK1 inhibitors were completed and did not show any significant adverse events.
RIPK1 kinase was initially considered a specific activator of necroptosis. This view is rapidly changing with the discovery of RDA and the inflammatory gene expression networks controlled by RIPK1. At this point, however, there is still a limited understanding of the specific modalities of RIPK1 involvement in different diseases. In a few cases the answer can be deduced based on the additional analyses of the functional roles or the lack thereof of different downstream effectors, especially caspase-8, RIPK3, and MLKL. For example, recent data suggest that in age-related neurodegeneration RDA may be a primary mode of action (30), while in acute neurologic injuries necroptosis was proposed to be important (11). In yet another set of pathologies, exemplified by MS, AD, or Niemann–Pick disease, cell-death-independent regulation of inflammatory gene expression or other non-cell-death responses may be important contributors (22, 111, 119). Understanding the different modalities of the regulation is important for uncovering pathophysiology of human diseases and development of the specific predictive biomarkers of the drug responses. At the same time, the major power of targeting RIPK1 is the promise of simultaneously targeting all of these modalities with oral drugs in a sustained and safe manner.
Acknowledgments
Studies in the authors’ laboratories are supported by NIH Grants 1R01AG047231, RF1AG055521, and R21AG059073 (to J.Y.); and 1R01CA190542, R21AI124049, and 1R56AG058642 (to A.D.).
Footnotes
Conflict of interest statement: J.Y. is a consultant for Denali Therapeutics. D.O. is an employee of Sanofi.
This article is a PNAS Direct Submission.
References
- 1.Degterev A, Boyce M, Yuan J (2003) A decade of caspases. Oncogene 22:8543–8567. [DOI] [PubMed] [Google Scholar]
- 2.Callus BA, Vaux DL (2007) Caspase inhibitors: Viral, cellular and chemical. Cell Death Differ 14:73–78. [DOI] [PubMed] [Google Scholar]
- 3.Christofferson DE, Yuan J (2010) Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallach D, Kang TB, Dillon CP, Green DR (2016) Programmed necrosis in inflammation: Toward identification of the effector molecules. Science 352:aaf2154. [DOI] [PubMed] [Google Scholar]
- 5.Zheng TS, Flavell RA (2000) Divinations and surprises: Genetic analysis of caspase function in mice. Exp Cell Res 256:67–73. [DOI] [PubMed] [Google Scholar]
- 6.Shutinoski B, et al. (2016) K45A mutation of RIPK1 results in poor necroptosis and cytokine signaling in macrophages, which impacts inflammatory responses in vivo. Cell Death Differ 23:1628–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duprez L, et al. (2011) RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35:908–918. [DOI] [PubMed] [Google Scholar]
- 8.Polykratis A, et al. (2014) Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol 193:1539–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, et al. (2017) RIP1 kinase activity-dependent roles in embryonic development of Fadd-deficient mice. Cell Death Differ 24:1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng H, et al. (2018) Death-domain dimerization-mediated activation of RIPK1 controls necroptosis and RIPK1-dependent apoptosis. Proc Natl Acad Sci USA 115:E2001–E2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degterev A, et al. (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119. [DOI] [PubMed] [Google Scholar]
- 12.Degterev A, et al. (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan J, Amin P, Ofengeim D (2019) Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci 20:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett HA, et al. (2001) TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4:1116–1122. [DOI] [PubMed] [Google Scholar]
- 15.Ofengeim D, Yuan J (2013) Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol 14:727–736. [DOI] [PubMed] [Google Scholar]
- 16.Zhou W, Yuan J (2014) Necroptosis in health and diseases. Semin Cell Dev Biol 35:14–23. [DOI] [PubMed] [Google Scholar]
- 17.Peltzer N, Darding M, Walczak H (2016) Holding RIPK1 on the ubiquitin leash in TNFR1 signaling. Trends Cell Biol 26:445–461. [DOI] [PubMed] [Google Scholar]
- 18.Kanayama A, et al. (2004) TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell 15:535–548. [DOI] [PubMed] [Google Scholar]
- 19.Annibaldi A, et al. (2018) Ubiquitin-mediated regulation of RIPK1 kinase activity independent of IKK and MK2. Mol Cell 69:566–580.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, et al. (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger SB, et al. (2014) Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol 192:5476–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ofengeim D, et al. (2015) Activation of necroptosis in multiple sclerosis. Cell Rep 10:1836–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, et al. (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227. [DOI] [PubMed] [Google Scholar]
- 24.Petrie EJ, Czabotar PE, Murphy JM (2019) The structural basis of necroptotic cell death signaling. Trends Biochem Sci 44:53–63. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, et al. (2017) MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc Natl Acad Sci USA 114:E7450–E7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser WJ, et al. (2013) Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288:31268–31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upton JW, Kaiser WJ, Mocarski ES (2012) DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J (2001) NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 21:5299–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amin P, et al. (2018) Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFα-mediated apoptosis. Proc Natl Acad Sci USA 115:E5944–E5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu D, et al. (2018) TBK1 suppresses RIPK1-driven apoptosis and inflammation during development and in aging. Cell 174:1477–1491.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng J, et al. (2017) Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun 8:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dondelinger Y, et al. (2015) NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell 60:63–76. [DOI] [PubMed] [Google Scholar]
- 33.Jaco I, et al. (2017) MK2 phosphorylates RIPK1 to prevent TNF-induced cell death. Mol Cell 66:698–710.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rickard JA, et al. (2014) RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157:1175–1188. [DOI] [PubMed] [Google Scholar]
- 35.Dillon CP, et al. (2014) RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 157:1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaiser WJ, et al. (2014) RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci USA 111:7753–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin J, et al. (2016) RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton K, et al. (2016) RIPK1 inhibits ZBP1-driven necroptosis during development. Nature 540:129–133. [DOI] [PubMed] [Google Scholar]
- 39.Cuchet-Lourenço D, et al. (2018) Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science 361:810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, et al. (2019) Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc Natl Acad Sci USA 116:970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Y, Devin A, Rodriguez Y, Liu ZG (1999) Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev 13:2514–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberst A, et al. (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471:363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Donnell MA, et al. (2011) Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol 13:1437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hitomi J, et al. (2008) Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135:1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertrand MJ, et al. (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 30:689–700. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, et al. (2017) PELI1 functions as a dual modulator of necroptosis and apoptosis by regulating ubiquitination of RIPK1 and mRNA levels of c-FLIP. Proc Natl Acad Sci USA 114:11944–11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elliott PR, et al. (2016) SPATA2 links CYLD to LUBAC, activates CYLD, and controls LUBAC signaling. Mol Cell 63:990–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kupka S, et al. (2016) SPATA2-mediated binding of CYLD to HOIP enables CYLD recruitment to signaling complexes. Cell Rep 16:2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dziedzic SA, et al. (2018) ABIN-1 regulates RIPK1 activation by linking Met1 ubiquitylation with Lys63 deubiquitylation in TNF-RSC. Nat Cell Biol 20:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Carbonell R, et al. (2018) Elevated A20 promotes TNF-induced and RIPK1-dependent intestinal epithelial cell death. Proc Natl Acad Sci USA 115:E9192–E9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saïd-Sadier N, Ojcius DM (2012) Alarmins, inflammasomes and immunity. Biomed J 35:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platt N, da Silva RP, Gordon S (1998) Recognizing death: The phagocytosis of apoptotic cells. Trends Cell Biol 8:365–372. [DOI] [PubMed] [Google Scholar]
- 53.Dondelinger Y, et al. (2014) MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep 7:971–981. [DOI] [PubMed] [Google Scholar]
- 54.Frank D, Vince JE (2019) Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ 26:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Man SM, Karki R, Kanneganti TD (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi J, Gao W, Shao F (2017) Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42:245–254. [DOI] [PubMed] [Google Scholar]
- 57.Orning P, et al. (2018) Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarhan J, et al. (2018) Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci USA 115:E10888–E10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kayagaki N, et al. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671. [DOI] [PubMed] [Google Scholar]
- 60.Shi J, et al. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. [DOI] [PubMed] [Google Scholar]
- 61.Najjar M, et al. (2016) RIPK1 and RIPK3 kinases promote cell-death-independent inflammation by Toll-like receptor 4. Immunity 45:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu K, et al. (2018) Necroptosis promotes cell-autonomous activation of proinflammatory cytokine gene expression. Cell Death Dis 9:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christofferson DE, et al. (2012) A novel role for RIP1 kinase in mediating TNFα production. Cell Death Dis 3:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNamara CR, et al. (2013) Akt regulates TNFα synthesis downstream of RIP1 kinase activation during necroptosis. PLoS One 8:e56576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saleh D, et al. (2017) Kinase activities of RIPK1 and RIPK3 can direct IFN-β synthesis induced by lipopolysaccharide. J Immunol 198:4435–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taraborrelli L, et al. (2018) LUBAC prevents lethal dermatitis by inhibiting cell death induced by TNF, TRAIL and CD95L. Nat Commun 9:3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerlach B, et al. (2011) Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471:591–596. [DOI] [PubMed] [Google Scholar]
- 68.Kang TB, Jeong JS, Yang SH, Kovalenko A, Wallach D (2018) Caspase-8 deficiency in mouse embryos triggers chronic RIPK1-dependent activation of inflammatory genes, independently of RIPK3. Cell Death Differ 25:1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lukens JR, et al. (2013) RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature 498:224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daniels BP, et al. (2017) RIPK3 restricts viral pathogenesis via cell death-independent neuroinflammation. Cell 169:301–313.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y, et al. (2018) Necrostatin-1 improves long-term functional recovery through protecting oligodendrocyte precursor cells after transient focal cerebral ischemia in mice. Neuroscience 371:229–241. [DOI] [PubMed] [Google Scholar]
- 72.Hong JM, Kim SJ, Lee SM (2016) Role of necroptosis in autophagy signaling during hepatic ischemia and reperfusion. Toxicol Appl Pharmacol 308:1–10. [DOI] [PubMed] [Google Scholar]
- 73.Zhe-Wei S, Li-Sha G, Yue-Chun L (2018) The role of necroptosis in cardiovascular disease. Front Pharmacol 9:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenbaum DM, et al. (2010) Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res 88:1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Mässenhausen A, et al. (2018) Phenytoin inhibits necroptosis. Cell Death Dis 9:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie T, et al. (2013) Structural basis of RIP1 inhibition by necrostatins. Structure 21:493–499. [DOI] [PubMed] [Google Scholar]
- 77.Roskoski R., Jr (2016) Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacol Res 103:26–48. [DOI] [PubMed] [Google Scholar]
- 78.Najjar M, et al. (2015) Structure guided design of potent and selective ponatinib-based hybrid inhibitors for RIPK1. Cell Rep 10:1850–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshikawa M, et al. (2018) Discovery of 7-oxo-2,4,5,7-tetrahydro-6 H-pyrazolo[3,4- c]pyridine derivatives as potent, orally available, and brain-penetrating receptor interacting protein 1 (RIP1) kinase inhibitors: Analysis of structure-kinetic relationships. J Med Chem 61:2384–2409. [DOI] [PubMed] [Google Scholar]
- 80.Harris PA, et al. (2016) DNA-encoded library screening identifies benzo[b][1,4]oxazepin-4-ones as highly potent and monoselective receptor interacting protein 1 kinase inhibitors. J Med Chem 59:2163–2178. [DOI] [PubMed] [Google Scholar]
- 81.Harris PA, et al. (2017) Discovery of a first-in-class receptor interacting protein 1 (RIP1) kinase specific clinical candidate (GSK2982772) for the treatment of inflammatory diseases. J Med Chem 60:1247–1261. [DOI] [PubMed] [Google Scholar]
- 82.Wang W, et al. (2018) RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell 34:757–774.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fauster A, et al. (2015) A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis 6:e1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martens S, et al. (2018) RIPK1-dependent cell death: A novel target of the Aurora kinase inhibitor Tozasertib (VX-680). Cell Death Dis 9:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weisel K, et al. (2017) Randomized clinical study of safety, pharmacokinetics, and pharmacodynamics of RIPK1 inhibitor GSK2982772 in healthy volunteers. Pharmacol Res Perspect 5:e00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nicotera P, Leist M, Manzo L (1999) Neuronal cell death: A demise with different shapes. Trends Pharmacol Sci 20:46–51. [DOI] [PubMed] [Google Scholar]
- 87.Dvoriantchikova G, Degterev A, Ivanov D (2014) Retinal ganglion cell (RGC) programmed necrosis contributes to ischemia-reperfusion-induced retinal damage. Exp Eye Res 123:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim CR, Kim JH, Park HL, Park CK (2017) Ischemia reperfusion injury triggers TNFα induced-necroptosis in rat retina. Curr Eye Res 42:771–779. [DOI] [PubMed] [Google Scholar]
- 89.Smith CC, et al. (2007) Necrostatin: A potentially novel cardioprotective agent? Cardiovasc Drugs Ther 21:227–233. [DOI] [PubMed] [Google Scholar]
- 90.Zhang S, et al. (2016) Necrostatin-1 attenuates inflammatory response and improves cognitive function in chronic ischemic stroke mice. Medicines (Basel) 3:E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newton K, et al. (2016) RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ 23:1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ni Y, et al. (2018) RIP1K contributes to neuronal and astrocytic cell death in ischemic stroke via activating autophagic-lysosomal pathway. Neuroscience 371:60–74. [DOI] [PubMed] [Google Scholar]
- 93.Liu T, et al. (2016) Therapeutic hypothermia attenuates tissue damage and cytokine expression after traumatic brain injury by inhibiting necroptosis in the rat. Sci Rep 6:24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.You Z, et al. (2008) Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab 28:1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pegoretti V, Baron W, Laman JD, Eisel ULM (2018) Selective modulation of TNF-TNFRs signaling: Insights for multiple sclerosis treatment. Front Immunol 9:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gregory AP, et al. (2012) TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 488:508–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moquin DM, McQuade T, Chan FK (2013) CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One 8:e76841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim SJ, Li J (2013) Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis 4:e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Selmaj KW, Raine CS (1988) Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol 23:339–346. [DOI] [PubMed] [Google Scholar]
- 100.Fan H, et al. (2016) Reactive astrocytes undergo M1 microglia/macrohpages-induced necroptosis in spinal cord injury. Mol Neurodegener 11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ito Y, et al. (2016) RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 353:603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kang SH, et al. (2013) Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 16:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lafont E, et al. (2018) TBK1 and IKKε prevent TNF-induced cell death by RIPK1 phosphorylation. Nat Cell Biol 20:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cirulli ET, et al. ; FALS Sequencing Consortium (2015) Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347:1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Freischmidt A, et al. (2015) Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci 18:631–636. [DOI] [PubMed] [Google Scholar]
- 106.Walsh DM, Selkoe DJ (2004) Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 44:181–193. [DOI] [PubMed] [Google Scholar]
- 107.Mandrekar-Colucci S, Landreth GE (2010) Microglia and inflammation in Alzheimer’s disease. CNS Neurol Disord Drug Targets 9:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sarlus H, Heneka MT (2017) Microglia in Alzheimer’s disease. J Clin Invest 127:3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Streit WJ, et al. (2018) Microglial activation occurs late during preclinical Alzheimer’s disease. Glia 66:2550–2562. [DOI] [PubMed] [Google Scholar]
- 110.Collins JS, et al. (2000) Association of a haplotype for tumor necrosis factor in siblings with late-onset Alzheimer disease: The NIMH Alzheimer Disease Genetics Initiative. Am J Med Genet 96:823–830. [DOI] [PubMed] [Google Scholar]
- 111.Ofengeim D, et al. (2017) RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc Natl Acad Sci USA 114:E8788–E8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caccamo A, et al. (2017) Necroptosis activation in Alzheimer’s disease. Nat Neurosci 20:1236–1246. [DOI] [PubMed] [Google Scholar]
- 113.Papassotiropoulos A, et al. (2005) Cholesterol 25-hydroxylase on chromosome 10q is a susceptibility gene for sporadic Alzheimer’s disease. Neurodegener Dis 2:233–241. [DOI] [PubMed] [Google Scholar]
- 114.Keren-Shaul H, et al. (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169:1276–1290.e17. [DOI] [PubMed] [Google Scholar]
- 115.Chiu IM, et al. (2009) Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc Natl Acad Sci USA 106:20960–20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma J, et al. (2011) Microglial cystatin F expression is a sensitive indicator for ongoing demyelination with concurrent remyelination. J Neurosci Res 89:639–649. [DOI] [PubMed] [Google Scholar]
- 117.Nuvolone M, et al. (2017) Cystatin F is a biomarker of prion pathogenesis in mice. PLoS One 12:e0171923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Safaiyan S, et al. (2016) Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 19:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cougnoux A, et al. (2018) Necroptosis inhibition as a therapy for Niemann-Pick disease, type C1: Inhibition of RIP kinases and combination therapy with 2-hydroxypropyl-β-cyclodextrin. Mol Genet Metab 125:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vitner EB, et al. (2014) RIPK3 as a potential therapeutic target for Gaucher’s disease. Nat Med 20:204–208. [DOI] [PubMed] [Google Scholar]
- 121.Cullen V, et al. (2011) Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann Neurol 69:940–953. [DOI] [PubMed] [Google Scholar]
- 122.Iannielli A, et al. (2018) Pharmacological inhibition of necroptosis protects from dopaminergic neuronal cell death in Parkinson’s disease models. Cell Rep 22:2066–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rehker J, et al. (2017) Caspase-8, association with Alzheimer’s disease and functional analysis of rare variants. PLoS One 12:e0185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chandra J, Samali A, Orrenius S (2000) Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 29:323–333. [DOI] [PubMed] [Google Scholar]