Abstract
Objective:
New pharmacological measures assessing medication adherence, including longitudinal drug levels in hair, are emerging. Little is known, however, about how best to present results from such measures to patients and clinicians in comprehensive, easy-to-understand, acceptable formats. We, therefore, developed three graphical display prototypes of hypothetical daily drug concentrations measured in hair, and assessed their acceptability among participants.
Methods:
We interviewed 30 HIV-positive patients and 29 clinicians to examine perceived acceptability for each graphical display prototype.
Results:
Patients and clinicians generally found the prototypes acceptable for facilitating understanding of patient adherence; however, areas for optimization were identified. For patients with lower health literacy, prototypes did not provide sufficient understanding of the link between medication-taking and drug concentrations in hair. These patients also preferred pictographs over bar or line graphs. Clinicians largely preferred daily drug concentration data in bar graphs with information included about the measure’s accuracy. Participants questioned the utility of showing drug concentrations above a therapeutic range, though they found color-coding results acceptable.
Conclusions:
Assessing prototype versions of graphical displays of hypothetical longitudinal adherence data indicated ways to optimize their acceptability.
Practice Implications:
Acceptable prototype-tested graphical displays of longitudinal patient-specific drug concentrations may enhance adherence monitoring in clinical settings.
Keywords: medication adherence, data visualization, health communication
1. INTRODUCTION
Suboptimal adherence is a critical problem for individuals receiving long-term, daily therapies for chronic health problems [1,2], including HIV-positive individuals taking antiretroviral therapy (ART) [3]. Treatment adherence is important for both the health of individuals with the disease, and to reduce forward transmission [4].
Several factors make it challenging for providers to monitor and clinically support patients’ medication-taking behavior, including reliance on adherence assessment via patient self-report [5]. For many patients, difficulty remembering and/or social-desirability bias may lead to inaccurate adherence reporting [6], particularly when recalling specific days or times of missed doses. Researchers and clinicians have worked to improve self-reported adherence validity by using visual analogue scales and daily medication diaries [7], but these approaches do not always overcome the problem of over-estimating adherence. Innovative technologies are increasingly being used to provide more objective, real-time, and longitudinal measures of medication adherence [8–11]. These include: electronic notification of opening pill bottles or filling prescriptions [5]; ingestible sensors [12,13]; pharmacological measures such as the use of dried blood spots; [14] and monitoring drug concentrations in patient hair [15–17].
The longitudinal nature of these new technologies necessitates more complex reporting than the previous single value adherence scores, and such complexities may be difficult for some patients and clinicians to comprehend and implement in clinical practice. As researchers work to further refine adherence monitoring technologies, it is important to consider how such data should be presented to patients and clinicians.
Visual depiction of health information has gained increasing attention as a means for comprehensibly presenting complex data to patients. Accordingly, visualization of personal health data, including medication adherence, is a growing research area [13,18–24]. Carefully developed graphical displays are particularly helpful to overcome low levels of literacy and numeracy that may initially impede patient comprehension of health data [25]. Moreover, presenting health data in understandable formats can help clinicians more effectively communicate with and counsel patients [26]. This is important as research has demonstrated the quality of patient-clinician communication affects medication adherence [27–29]. Yet, evidence-based guidance on how best to present quantitative, longitudinal health data is lacking. To inform the development of a new longitudinal ART adherence measure, we sought to address this gap by qualitatively assessing factors that impact patient and clinician acceptability of graphical display prototypes depicting hypothetical adherence data.
2. METHODS
Study Context and Design
Establishing Novel Antiretroviral Imaging for Hair to Elucidate Non-adherence (ENLIGHTEN) is an on-going NIAID study (R01AI122319) seeking to enhance adherence monitoring via infra-red matrix-assisted laser desorption electrospray ionization (IR-MALDESI) technology for mass spectrometry imaging (MSI) [17]. Once validated, this technology will reveal daily ART drug concentrations, representing weeks to months of retrospective drug ingestion. It will require about 4–8 strands of a patient’s hair and approximately 2 hours processing time. In a future study, we will pilot test the use of IR-MALDESI MSI for generating real-time graphical displays of longitudinal adherence data to provide objective feedback regarding medication-taking in a clinic setting. In preparation for the future pilot study, we conducted this cross-sectional, formative study, which included the development and assessment of graphical display prototypes.
Development of Graphical Display Prototypes
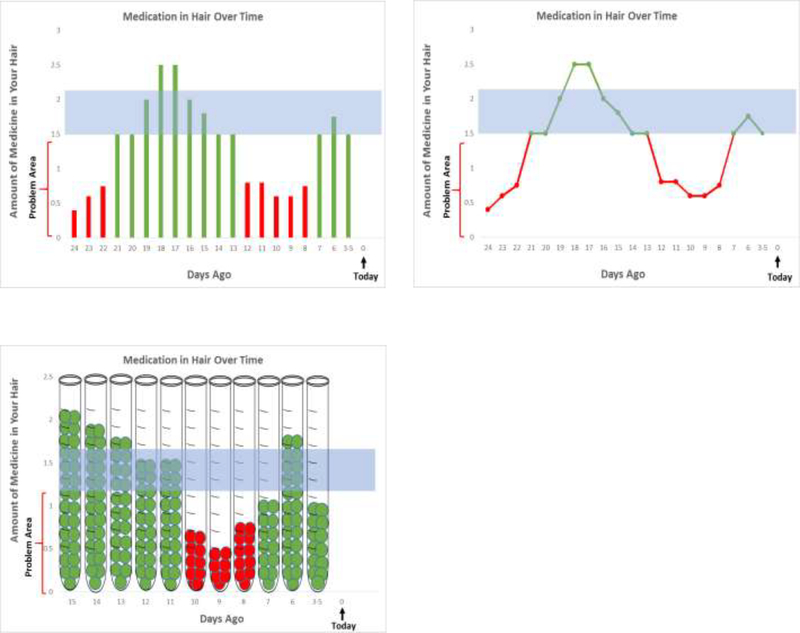
We used a multipronged approach to develop graphical display prototypes of hypothetical data. First, we based prototypes on initial proof-of-concept for Efavirenz response in hair. Second, we consulted empirical and theoretical literature on health care decision making, risk communication, and health literacy [25,30,31]. Third we met with experts, including: health communication experts; infectious diseases clinicians; and patients from the Community Advisory Board of the AIDS Clinical Trials Group. Based on input from these steps, we developed initial prototypes which were then reviewed, discussed, refined, and approved by the full study team. The three final versions of the prototypes included a bar graph, a line graph, and a test-tube pictograph. All three presented “number of days ago” on the x-axis, with “today” represented on the far right and the maximum number of days IR-MALDESI MSI detected drug concentrations displayed on the far left. The y-axis depicted the drug concentration. A problem area, shown in red, revealed sub-optimal medication concentrations, while a horizontal shaded area represented an optimal range of concentrations (Figure 1).
Figure 1:
Prototype Graphical Displays (Bar, Line and Pictograph) of Hypothetical Personal Adherence Data Tested During Formative Interviews
Participant Recruitment and Enrollment
Patients and clinicians were recruited at a university-based infectious disease (ID) clinic. Patients were recruited on a convenience basis by a clinic screener and were eligible if they were HIV-positive, 18 years or older, had been prescribed ART for ≥3 months, had ≥1 centimeter of scalp hair, and had a recorded viral load test at the ID clinic within the last 12 months. We purposefully recruited patients to: A) achieve proportional recruitment across three viral load (HIV RNA) strata: 1) all HIV viral loads <50 copies(c)/mL within the past 12 months; 2) at least one between 50–1,000 c/mL; 3) at least one HIV viral load >1,000 c/mL; and B) reflect the sex and racial/ethnic demographics of the HIV-positive patient population at the clinic. As this was a small formative study, we did not track refusal rates. We enrolled a total of 30 eligible patients.
Clinicians (physicians, advanced practice clinicians, pharmacists, nurses and social workers) at the ID clinic were recruited by email and informational flyers. We enrolled all 29 clinicians who provided patient care in the ID clinic at the time of the study.
Data Collection
Acceptability is an implementation science outcome that can be assessed by asking participants’ their opinions of the content or complexity of a particular “treatment, service, practice, or innovation” [32]. We developed semi-structured interview guides (separately for patients and providers), based on implementation science theory, to explore participant acceptability of our graphical display prototypes [32]. Specifically, the guides explored participants’: 1) opinions of and preferences for the prototypes; 2) feedback on how well they understood them; and 3) suggestions for improvement. Qualitative researchers used these guides to conduct in-depth (45–90 minute) interviews with HIV-positive patients and ID clinicians. At the conclusion of each interview, patients and clinicians completed brief demographic questionnaires; patients also completed the Newest Vital Sign, a validated measure assessing health literacy, as health literacy affects how individuals make decisions about their care and treatment [33,34]. All interviews were digitally recorded and transcribed (except one for which the interviewer took detailed notes at the participant’s request).
Analysis
To conduct this analysis, we largely used a deductive approach within the Framework Method [35]. We began by reading transcripts for familiarity and content, writing memos and summaries of the interviews. We then used our interview guides to develop a list of a priori codes for patient and clinician transcripts, separately. We piloted these codebooks with a subset of initial interviews, iteratively editing the codebooks to refine codes and code definitions, and to facilitate their consistent application. Once final codebooks were established, each transcript was coded twice (once each by two separate authors) using ATLAS.ti Version 8.0 software. Two authors coded patient transcripts while two others coded clinician transcripts. Differences in coding were reconciled between coding pairs for each transcript until agreement was achieved. Once coding was completed, we created detailed matrices for patients and clinicians, separately, in Excel. Each row represented a single participant, while each column represented a separate code. We summarized content relevant to each code by and across participants [35,36]. Because the goal of this analysis was to examine prototype acceptability, we were specifically interested in the a priori topics outlined in our interview guides: participants’: 1) opinions of and preferences for the prototypes; 2) feedback on how well they understood them; and 3) suggestions for improvement. Emphasis was also given to identifying whether opinions on these topics differed by patient health literacy levels.
Ethical Approval
This study was conducted in accordance with ICH Good Clinical Practice standards and with the Declaration of Helsinki. It is registered at ClinicalTrials.gov (clinicaltrials.gov NCT03218592) and ethical approval was granted by the Institutional Review Board at the University of North Carolina at Chapel Hill (protocol no. 15–2933). All participants provided written informed consent prior to being interviewed, and all were compensated for their time.
3. RESULTS
We have organized the results by based on our theory-driven research questions. First, we present results regarding participants’ (patients’ and clinicians’) opinions of, and preferences for, the three graphical displays of hypothetical adherence data. This included participants’ overall impressions of the graphs and whether they preferred the bar, line, or pictograph. Second, we highlight factors participants noted to be integral to comprehension of the graphs and we identify suggestions made by patients and clinicians regarding ways to maximize comprehension for both patients and clinicians. Table 1 summarizes participant demographics.
Table 1.
Participant Demographic Information
| Demographic Information | Patient Number (%) | Clinician Number (%) | |
|---|---|---|---|
| Race/ Ethnicity | |||
| Black/ African American | 16 (53%) | 2 (7%) | |
| Black and Indian | 1 (3%) | 4 (14%) | |
| Latino/ Hispanic | 3 (10%) | 2 (7%) | |
| White | 10 (33%) | 21 (72%) | |
| Sex | |||
| Female | 9 (30%) | 18 (62%) | |
| Male | 21 (70%) | 10 (34%) | |
| Patient Viral Load | Clinician Type | Number (%) | |
| <50c/mL | 12 (40%) | Nurse | 3 (10%) |
| 50–1000c/mL | 8 (27%) | Nurse Practitioner/Physician Assistant | 3 (10%) |
| >1000c/ml | 10 (33%) | Pharmacist | 1 (4%) |
| Patient Health Literacy | Physician | 19 (66%) | |
| Low literacy | 6 (20%) | Social Worker | 3 (10%) |
| Possibly low literacy | 11 (37%) | ||
| Adequate literacy | 13 (43%) | ||
Overall Opinions and Preferences for Graphical Displays
1. Opinions
Participants generally found the prototypes acceptable. In particular, most participants noted that the visual depiction of longitudinal personal adherence data was appealing. Specifically, patients of varying health literacy levels found the image of the pictograph appealing. One patient stated: “If you had shown it to somebody who couldn’t read, or spoke a different language, that picture is the most explanatory, I think…” (Female patient, VL 50–100c/mL, adequate literacy). Another noted: “Data is hard, math is hard but if they see this, they could just stop and say, “What does that mean?” (Male patient, VL <50, low literacy). Clinicians also liked the idea of having a visual depiction of the information, regardless of the type of graph: “Showing people and having a visual, or having objective data, I think is very useful as an adjunct to the counseling piece.” (ID clinician)
Participants also generally liked the longitudinal aspect of the graphical displays, noting that it might help identify adherence patterns and challenges. One patient explained: “It’s cool that it can tell you that far back… I think it would give you a better idea of how good you do at taking your medicine and help you keep it in check.” (Male patient, VL >1000c/mL, adequate literacy). And another questioned: “I would be curious to know like on the 18th and the 17th, what did I do? Was it a day that I took my medication at night and then I tried to get back on track and take it that morning? Is that why it’s high?” (Male patient, VL <50c/mL, adequate literacy). Clinicians perceived the longitudinal nature of the prototypes as a potentially useful adherence reinforcement tool they could use during adherence counseling sessions with patients:
“I think it’s probably an incredibly nice way to have it so that you could show it to your patient. I think this is something that a patient can understand… [You] could be like, “What was going on the week of such and such and such?” or, “What was going on here?” (ID clinician).
2. Preferences
Among all patients, a slight majority preferred the bar graph. However, when we examined preferences by health literacy level, there were clear differences. Nearly all patients with low literacy preferred the pictograph, whereas patients with possibly low literacy had mixed preferences, and patients with adequate literacy largely preferred the line or bar graphs.
Most patient preferences seemed tied to the type of graph they could best “relate to” from previous experiences. For example, one patient noted her preference for the line graph by stating: “I guess I just remember this one more from school. I can understand it a little better.” (Female patient, VL >1000c/mL, adequate literacy). Likewise, another explained his preference for the bar graph by stating: “I just always preferred a bar graph over anything.” (Male patient, VL <50c/mL, adequate literacy). Patients explained preferences for the pictograph in a similar manner: “It just reminds me of the tube that [they use] when they draw the blood,” (Male patient, VL 50–1000 c/mL, low literacy).
In contrast, clinicians almost exclusively preferred the bar or line graph to the pictograph. Yet, clinicians too based their preferences largely on familiarity, finding similarities between the more traditional graphs and those used in clinical practice. In discussing the line graph, for example, one clinician described:
“In a way, that’s a little bit how we’ve represented viral load and CD4 count tracking across time to patients… This line graph is what we use in the back of our patient’s summary reports in clinic, and how we present our viral load and CD4 data, so I think that would be a match to that. I’d prefer this one.” (ID clinician).
When asked what type of graph their patients might prefer, many clinicians suggested the pictograph: “It’s hard for me to answer about what I think someone else would like better, but it’s possible that the patient might like the test tube better because they might be able to say, “Oh, there wasn’t much in these samples at this time.” (ID clinician). A few clinicians thought “lower-educated patients” might prefer the pictograph because it’s “catchy” and potentially “more immediately obvious.” Moreover, these clinicians perceived some patients with low literacy might find the bar and line graphs challenging to interpret.
Features Affecting Comprehension and Suggestions for Optimization
We identified four key areas affecting comprehensibility of the prototypes: 1) insufficient background and orientation for some patients; 2) insufficient detail for clinicians; 3) depiction of the therapeutic range; and 4) the use of colors. Details and suggestions for optimization are described below.
1. Insufficent background and orientation for some patients
Some patients, particularly those with lower health literacy levels, had difficulty understanding the way that medication is processed in the body--that oral medication, once ingested, can be similarly detected in the body and in hair-- and, thus, struggled to interpret the graph. For example, one patient stated: “That’s just in my hair. That doesn’t mean in my body that it’s that way. It just may be in my hair, because you know, of course, you would like to think that when I take the medication, that it starts—it works on the body. The hair really isn’t important.” (Male patient, VL >1000c/mL, possibly low literacy). Researchers spent extra time with these patients, ensuring they understood the link between medication-taking and drug concentrations in hair, before discussing the prototypes in more detail.
When discussing the prototypes, patients across health literacy levels, questioned why drug concentrations might fluctuate over time, specifically asking why the drug concentration is “higher, then it goes lower, then lower, then higher” (Male patient, VL <50c/mL, possibly low literacy). Another wondered: “Why would there be more on one day than another? Does it build up over time?” (Female patient, VL 50–1000c/mL, adequate literacy). These patients appeared to expect drug concentrations to remain stable over time.
Some patients also perceived the display of “number of days ago” going right to left as time “going backwards”. One patient explained: “Probably I can comprehend this. When you showed me this is today, I thought it should be going that way instead of backwards.” (Male patient, VL <50, adequate literacy). Patients who noted this as a potential source of confusion suggested the addition of dates might help them better identify the timing of missed doses: “You could have the dates on here…if you could put whatever date day 24 was on this end, and put today’s date over here, and then—yeah.” (Male patient, VL 50–1000, adequate literacy). This patient thought dates would be helpful for understanding longitudinal results.
Several patients offered further suggestions, such as including detailed legends or narrative summaries. One patient proposed: “If you give them some kind of explanation, like… an actual written explanation of… what they’re reading, I think they will understand it from that point” (Male patient, VL <50c/mL, possibly low literacy). A few clinicians echoed this recommendation as a way to help patients more fully understand the graphs.
2. Insufficient Detail for Clinicians
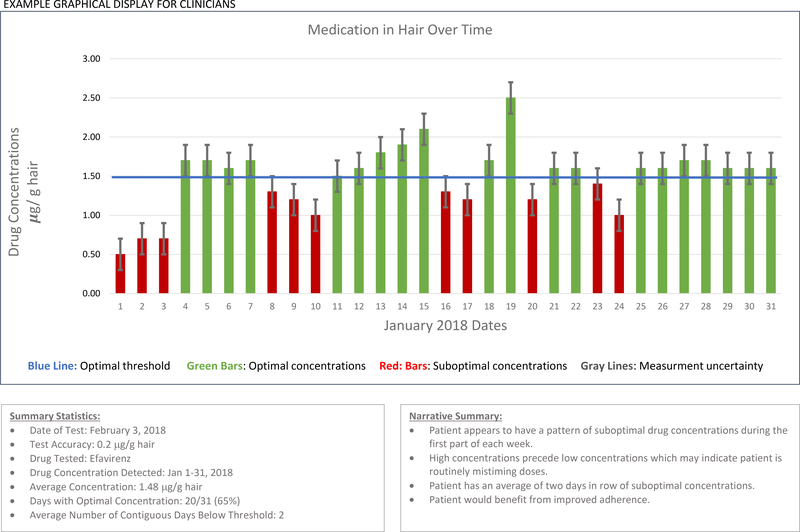
Some clinicians also suggested they would like to see more detail than what was depicted on the prototypes. For example, in addition to expressing interest in seeing units and dates on the graphs, some clinicians asked about the accuracy of the test. For example:
“Well, I think one thing that will definitely come up is how confident are you in these things? If you’re going to challenge a patient, they’re telling you that they’re taking their meds, and then you see this, and you tell them, “I’m not sure you are.” Then they’re going to be like, “Well, how do you know?” Of course, I would want to be super confident. When you get this report, is there anything that tells you about the confidence or the quality of the data?” (ID clinician)
This clinician insinuated the test would likely only be used in clinical practice if the accuracy was high and the graphical display contained sufficient detail to inform adherence counseling.
3. Depiction of the Therapeutic Range
On each of the graphs, a horizontal shaded region represented the range of drug concentrations sufficient to reach viral suppression. Some data points were depicted above this range, and many participants questioned the utility of this. Patients across health literacy levels wondered what might contribute to such high concentrations, for example: “Did the person just take too much?” (Male patient, VL <50c/mL, adequate literacy). Clinicians similarly thought: “Patients might ask: ‘Well, is this bad to have that much medicine in my system?’” (ID clinician). Some clinicians also anticipated their patients might conclude: “Oh, I’m taking too much…” (ID clinician). These clinicians perceived patients’ reactions to such information had the potential to adversely affect their future adherence. As one clinician insinuated, this could be justification for taking fewer doses: “I have some patients that are convinced they don’t need medicine every day. If they see that ‘… wait, I’m above it’…” (ID clinician). A few other clinicians questioned the general utility of showing results above the therapeutic range unless there is potential for those results to reveal concentrations of drug “toxicity”: “Unless you knew for sure that these levels above “X” were actually dangerous, I wouldn’t—you could almost argue that if you’re above a line, you don’t even need the second line.” (ID clinician). This clinician, along with a couple of others, suggested not displaying an upper limit for sufficient drug concentration.
4. The Use of Colors
The colors (green for optimal drug concentrations and red for sub-optimal concentrations) were considered comprehensible by most patients of varying literacy levels. For example, a patient with low literacy noted: “In this case red, for me, is appropriate because this means that there is a problem. Green, that means that you are good.” (Male patient, VL <50, low literacy). A patient with adequate literacy likewise explained: “I guess it’s pretty basic, where it’s almost like green-go, and red-stop, or red-danger.” (Female patient, VL 50–1000, adequate literacy). A few clinicians made remarkably similar comments regarding use of colors: “Generally, I think, most patients just want see green is go, red is stop, and that’s it” (ID clinician). These clinicians thought color-coded dichotomous results (illustrating optimal or sub-optimal adherence) might be preferred by patients.
4.1. DISCUSSION
Our formative study of reactions to graphical display prototypes using hypothetical longitudinal adherence data from drug concentrations in hair suggests patients and clinicians found these visual depictions of adherence data generally acceptable, comprehensible, and potentially useful. Our findings are consistent with other studies showing data presented visually rather than numerically may improve health understanding among patients [37]. Though, overall, patients did not express a clear preference for graph type, a slight majority preferred the bar graph, as did nearly all clinicians. Nayak et al. (2016) similarly found patients preferred bar graphs to a line graph or a tabular format of longitudinal health data [38], although the authors did not assess preferences for pictographs. Our results suggest lower health literacy patients may prefer pictographs over bar graphs. These findings are consistent with work by Kalichman et al., demonstrating the validity of pictographic scales to assess self-efficacy among populations with low literacy [39]. This preference, coupled with research demonstrating a negative relationship between low health literacy and adherence [40], suggest the need for further examining the usefulness of pictographs for patients with low health literacy [26].
Moreover, as previous data visualization and health communication research indicates, it is important to assess how well patients understand graphical representations of personal health risk, regardless of what type of graph they prefer [38,41]. As noted above, researchers in our study needed to provide additional background to some patients, particularly those with lower health literacy. Therefore, it may be useful to first ensure patients understand the concept underlying the graphical display, as this will affect their ability to understand it in more detail. This could be done through additional counseling and/ or a short educational video. Moreover, for clinicians operating within time constraints, graphical displays should have sufficient detail to quickly interpret and explain to patients.
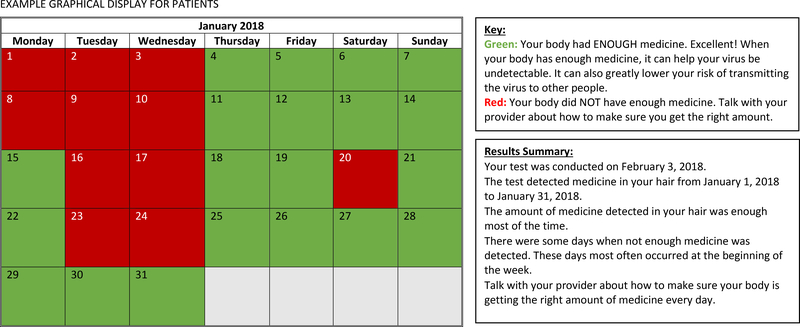
Results from this analysis also suggest a number of ways in which graphical displays of longitudinal pharmacological adherence measures can be optimized. In Figure 2, we show examples of alternative displays that could be used in the future. First it may be helpful to have two separate versions: a simple, color-coded dichotomized calendar version (optimal/ sub-optimal adherence) for patients, such as that provided by MEMS® Caps (AARDEX Group), and a more detailed version depicting specific drug concentrations over time and test accuracy information for clinicians. Both versions should: 1) include legends detailing key components of the graphs; 2) avoid including a ceiling for the therapeutic range of drugs that are not toxic at high concentrations; and, 3) if possible, they should include dates to facilitate recall and improve understanding of adherence patterns.
Figure 2:
Examples (Based on Formative Interview Results) of Alternative Graphical Displays That Could Be Used in the Future
A calendar with daily, color-coded dichotomized results would potentially address several of the concerns and opinions patients expressed. It would avoid depicting variation in drug concentrations over time and counting “backwards in time,” both of which were questioned by some patients. It would also leverage patients’ seemingly intuitive recognition that “green is good and red is bad”. Finally, as other studies have suggested, a short narrative explaining the results may also enhance comprehensibility among patients [42]. Our results suggest that these features would enhance acceptability of graphical displays for patients across health literacy levels.
For clinicians, our results suggest optimal graphs might look similar to a bar graph, or possibly a line graph, with the addition of drug-specific units and information about the pharmacological measure’s accuracy to detect a missing dose.
Limitations
This study has several limitations. First, prototypes depicted hypothetical data which were difficult for some patients to comment on. If actual patient data are not available, future efforts to prototype-test graphical displays of adherence data should consider including vignettes to help patients understand the context behind hypothetical data, as these have successfully been used to elicit patients’ and clinicians’ views on a variety of health topics [43]. Also, an order effect may have contributed to perceived preferences for the bar graph, as has occured in previous data display studies [41]. Moreover, because members of our research team are currently validating the accuracy of IR-MALDESI-MSI to detect missing doses, this information was not presented to participants. Finally, results may not be generalizable to other clinic populations or patients on other chronic medication regimens as this study was conducted in a single clinic and among patients living with HIV and taking ART.
4.2. CONCLUSION
New pharmacological measures are in development for objectively quantifying longitudinal medication adherence, including measures evaluating drug concentrations in hair [17]. Assessing patient and clinician reactions to graphical display prototypes of ART data generated by IR-MALDESI MSI provided an opportunity to identify how such data should be communicated to patients and clinicians. The prototypes were generally acceptable, though we identified methods for optimization.
4.3. PRACTICE IMPLICATIONS
Key considerations to enhance acceptability of longitudinal graphical displays for medication adherence include: avoiding the depiction of drug concentrations above therapeutic ranges for nontoxic drugs; depicting color-coded dichotomous results in a calendar format for patients; and providing detailed bar or line graphs for clinicians with information about the accuracy of the lab assay. Though ENLIGHTEN focuses on ART adherence, lessons learned may also apply to longitudinal displays of adherence data for other medications and disease states.
HIGHLIGHTS.
Participants generally found longitudinal graphical display prototypes acceptable.
Pictographs with narratives may enhance acceptability among patients.
More detail and test accuracy data may enhance acceptability among clinicians.
ACKNOWLEDGEMENTS
We would like to thank the Infectious Disease Clinic at the University of North Carolina at Chapel Hill for their ongoing support of this study. We would also like to thank the participants, without whom this research would not have been possible.
FUNDING
The ENLIGHTEN project is generously supported by NIH/ National Institute of Allergy and Infectious Diseases (NIAID) grant #:R01 AI122319–01 and the University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410). Dr. Golin’s salary was also partially supported by the by Eunice Kennedy Shriver National Institute of Child Health and Human Development (K24 HD069204). Dr. Gandhi’s salary was partially supported by NIH/NIAID grant 2AI098472. Dr. Hill’s salary was supported by a National Research Service Award Post-Doctoral Traineeship from the Agency for Healthcare Research and Quality sponsored by The Cecil G. Sheps Center for Health Services Research, The University of North Carolina at Chapel Hill, Grant No. T32-HS000032. Ms. Wallace was partially supported by a National Research Service Award Predoctoral Traineeship from the Agency for Healthcare Research and Quality (T32-HS000032).
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sabaté E (2003) Adherence to long-term therapies: Evidence for action. Geneva, Switzerland: World Health Organization (WHO). [PubMed] [Google Scholar]
- 2.Neiman AB, Ruppar T, Ho M, Garber L, Weidle PJ, et al. (2017) CDC Grand Rounds: Improving Medication Adherence for Chronic Disease Management - Innovations and Opportunities. MMWR Morb Mortal Wkly Rep 66: 1248–1251. doi: 10.15585/mmwr.mm6645a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer L, Skarbinski J (2014) Adherence to antiretroviral therapy among HIV-infected adults in the United States. AIDS Educ Prev 26: 521–537. doi: 10.1521/aeap.2014.26.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. (2016) Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med 375: 830–839. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam WY, Fresco P (2015) Medication adherence measures: an overview. Biomed Res Int 2015: 217047. doi: 10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, et al. (2014) Interventions for enhancing medication adherence. Cochrane Database Syst Rev: CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalichman SC, Amaral CM, Swetzes C, Jones M, Macy R, et al. (2009) A simple single-item rating scale to measure medication adherence: further evidence for convergent validity. J Int Assoc Physicians AIDS Care (Chic) 8: 367–374. doi: 10.1177/1545109709352884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS (2014) Electronic medication packaging devices and medication adherence: a systematic review. JAMA 312: 1237–1247. doi: 10.1001/jama.2014.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amico KR (2015) Evidence for Technology Interventions to Promote ART Adherence in Adult Populations: a Review of the Literature 2012–2015. Curr HIV/AIDS Rep 12: 441–450. doi: 10.1007/s11904-015-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy A, Huseman TL, Canamucio A, Marcus SC, Asch DA, et al. (2017) Patient and partner feedback reports to improve statin medication adherence: A randomized control trial. J Gen Intern Med 32: 256–261. doi: 10.1007/s11606-016-3858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E, et al. (2013) Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs 73: 545–562.doi: 10.1007/s40265-013-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson D, Mackay T, Matthews M, Edwards J, Peters N, et al. (2017) Direct Adherence Measurement Using an Ingestible Sensor Compared With Self-Reporting in High-Risk Cardiovascular Disease Patients Who Knew They Were Being Measured: A Prospective Intervention. JMIR Mhealth Uhealth 5: e76.doi: 10.2196/mhealth.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browne SH, Behzadi Y, Littlewort G (2015) Let Visuals Tell the Story: Medication Adherence in Patients with Type II Diabetes Captured by a Novel Ingestion Sensor Platform. JMIR Mhealth Uhealth 3: e108.doi: 10.2196/mhealth.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo-Mancilla J, Seifert S, Campbell K, Coleman S, McAllister K, et al. (2016) Emtricitabine-Triphosphate in Dried Blood Spots as a Marker of Recent Dosing. Antimicrob Agents Chemother 60: 6692–6697. doi: 10.1128/AAC.01017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, et al. (2011) Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 52: 1267–1275.doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxi SM, Liu A, Bacchetti P, Mutua G, Sanders EJ, et al. (2015) Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr 68: 13–20. doi: 10.1097/QAI.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen EP, Thompson CG, Bokhart MT, Prince HMA, Sykes C, et al. (2016) Analysis of Antiretrovirals in Single Hair Strands for Evaluation of Drug Adherence with Infrared-Matrix-Assisted Laser Desorption Electrospray Ionization Mass Spectrometry Imaging. Anal Chem 88: 1336–1344.doi: 10.1021/acs.analchem.5b03794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West VL, Borland D, Hammond WE (2015) Innovative information visualization of electronic health record data: a systematic review. J Am Med Inform Assoc 22: 330–339. doi: 10.1136/amiajnl-2014-002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner K, Klaman SL, Shea CM (2016) Personal health records for people living with HIV: a review. AIDS Care 28: 1181–1187. doi: 10.1080/09540121.2016.1153594. [DOI] [PubMed] [Google Scholar]
- 20.Sieben A, van Onzenoort HA, van Laarhoven KJ, Bredie SJ (2016) A Multifaceted Nurse- and Web-Based Intervention for Improving Adherence to Treatment in Patients With Cardiovascular Disease: Rationale and Design of the MIRROR Trial. JMIR Res Protoc 5: e187. doi: 10.2196/resprot.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Miedany Y, El Gaafary M, Palmer D (2012) Assessment of the utility of visual feedback in the treatment of early rheumatoid arthritis patients: a pilot study. Rheumatol Int 32: 3061–3068.doi: 10.1007/s00296-011-2098-1. [DOI] [PubMed] [Google Scholar]
- 22.Tufte ER, Graves-Morris PR (1983) The visual display of quantitative information. Cheshire, CT: Graphics Press. [Google Scholar]
- 23.Moore JO, Boyer EW, Safren S, Robbins GK, Boudreaux ED, et al. (2011) Designing interventions to overcome poor numeracy and improve medication adherence in chronic illness, including HIV/AIDS. J Med Toxicol 7: 133–138. doi: 10.1007/s13181-011-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons A, Bilker WB, Hines J, Gross R (2016) Effect of format on comprehension of adherence data in chronic disease: A cross-sectional study in HIV. Patient Educ Couns 99: 154–159.doi: 10.1016/j.pec.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters E, Hibbard J, Slovic P, Dieckmann N (2007) Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff (Millwood) 26: 741–748.doi: 10.1377/hlthaff.26.3.741. [DOI] [PubMed] [Google Scholar]
- 26.Kalichman SC, Cherry C, Kalichman MO, Amaral C, White D, et al. (2013) Randomized clinical trial of HIV treatment adherence counseling interventions for people living with HIV and limited health literacy. J Acquir Immune Defic Syndr 63: 42–50. doi: 10.1097/QAI.0b013e318286ce49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zullig LL, Shaw RJ, Shah BR, Peterson ED, Lindquist JH, et al. (2015) Patient-provider communication, self-reported medication adherence, and race in a postmyocardial infarction population. Patient Prefer Adherence 9: 311–318. doi: 10.2147/PPA.S75393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bultman DC, Svarstad BL (2000) Effects of physician communication style on client medication beliefs and adherence with antidepressant treatment. Patient Educ Couns 40: 173–185. [DOI] [PubMed] [Google Scholar]
- 29.Beach MC, Keruly J, Moore RD (2006) Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med 21: 661–665.doi: 10.1111/j.1525-1497.2006.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCaffery KJ, Holmes-Rovner M, Smith SK, Rovner D, Nutbeam D, et al. (2013) Addressing health literacy in patient decision aids. BMC Med Inform Decis Mak 13 Suppl 2: S10. doi: 10.1186/1472-6947-13-S2-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trevena LJ, Zikmund-Fisher BJ, Edwards A, Gaissmaier W, Galesic M, et al. (2013) Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak 13 Suppl 2: S7. doi: 10.1186/1472-6947-13-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, et al. (2011) Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 38: 65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, et al. (2005) Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 3: 514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Newest Vital Sign: A Health Literacy Assessment Tool (2011).
- 35.Gale NK, Heath G, Cameron E, Rashid S, Redwood S (2013) Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 13: 117.doi: 10.1186/1471-2288-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolley EE, Ulin PR, Mack N, Robinson ET, Succop SM (2016) Qualitative methods in public health: A field guide for applied research. Second edition. San Francisco, CA: Wiley. [Google Scholar]
- 37.Lipkus IM (2007) Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making 27: 696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 38.Nayak JG, Hartzler AL, Macleod LC, Izard JP, Dalkin BM, et al. (2016) Relevance of graph literacy in the development of patient-centered communication tools. Patient Educ Couns 99: 448–454.doi: 10.1016/j.pec.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Kalichman SC, Cain D, Fuhrel A, Eaton L, Di Fonzo K, et al. (2005) Assessing medication adherence self-efficacy among low-literacy patients: development of a pictographic visual analogue scale. Health Educ Res 20: 24–35. doi: 10.1093/her/cyg106. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen-Bohlman LT, Panzer AM, Hamlin B, Kindig DA (2004) Health Literacy: A Prescription to End Confusion Committeeon Health Literacy, Board on Neuroscience and Behavioral Health. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 41.Brown SM, Culver JO, Osann KE, MacDonald DJ, Sand S, et al. (2011) Health literacy, numeracy, and interpretation of graphical breast cancer risk estimates. Patient Educ Couns 83: 92–98.doi: 10.1016/j.pec.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogardus ST, Holmboe E, Jekel JF (1999) Perils, pitfalls, and possibilities in talking about medical risk. JAMA 281: 1037–1041. doi: 10.1001/jama.281.11.1037. [DOI] [PubMed] [Google Scholar]
- 43.Michael N, O’Callaghan C, Clayton JM (2016) Exploring the utility of the vignette technique in promoting advance care planning discussions with cancer patients and caregivers. Patient Educ Couns 99: 1406–1412. doi: 10.1016/j.pec.2016.03.021. [DOI] [PubMed] [Google Scholar]