Abstract
5-hydroxymethyl-2-furaldehyde (5-HMF) is an important substance that affect quality of honey and shows toxicity for humans and honey bees. The pathway of 5-HMF formation in honey is still unknown. In this study, we tested the effect of thermal treatment (at 90 °C for 4 h) on the formulation of 5-HMF formulation in rapeseed with varied honey composition. 5-HMF content of honey increased at higher water content, Ca2+ and Mg2+ content and lower pH. However, the formation of 5-HMF was not significantly influenced by glucose, fructose, Na+, or K+ contents. Furthermore, different content of proline, the most abundant amino acid in honey (a substance in Maillard reaction), had no effect on 5-HMF formation. Free acids in honey can catalyze fructose and glucose to form 5-HMF. These results suggest that dehydration of glucose or fructose, instead of the Maillard reaction, is the main pathway of 5-HMF formation in honey. This study gives new insights for the mechanisms of 5-HMF formation and provides method for reducing 5-HMF formation during honey processing.
Keywords: 5-hydroxymethyl-2-furaldehyde (5-HMF), Formation pathway, Thermal treatment, Hexose dehydration, Maillard reaction
Introduction
Honey is a natural sweet substance, transformed from nectar or honeydew via dehydration and breakdown of sucrose to simple sugars by enzymatic reactions (Ball 2007). Honey has been used as a traditional medicine for its antioxidant and antimicrobial activity, and for immunity-improving and antitumor activity (Fukuda et al. 2010). From a chemical point of view, honey contains about 80% sugars (glucose, fructose, sucrose, maltose and higher sugars), 19% water (Majtan 2014) and 1% other components. pH values of honey ranges from 3.4 to 6.1. In honey, the most abundant amino acid is proline, counting for around 70% of the total amino acids (Rückriemen et al. 2015). The total amino acid content accounts for about 1/1000 of the dry matter (Pätzold and Brückner 2006). Furthermore, various flavor compounds and pigments are also present (da Silva et al. 2016).
A well-known heterocyclic compound in honey, 5-hydroxymethyl-2-furaldehyde (5-HMF), is usually formed during long time storage or after exposing high temperature or both. Formation of 5-HMF has been proven as one of the most important factors that reduce the quality of honey and produce the precursor of polymer such as pigment (Aslanova et al. 2010). 5-HMF formation in honey was thought to be due to the Maillard reaction whereby acids catalyze degradation of reducing sugars (Capuano and Fogliano 2011). This was inferred from 5-HMF formation in sugar solution with organic or amino acids. But there is no data in honey directly, so the exact formation pathway of 5-HMF in honey is probably still unknown.
5-hydroxymethyl-2-furaldehyde is a potential toxin, mutagen, and carcinogen for humans (Michail et al. 2007) and is highly toxic to honey bees (LeBlanc et al. 2009). 5-HMF increases the incidence of aberrant crypt foci in rat colon, skin papillomas in mice, lipomatous tumors in rat kidney (Capuano and Fogliano 2011), small intestine adenomas in mice (Svendsen et al. 2009), hepatocellular adenomas in female mice (NTP 2010), and has mutagenic effects on S. typhimurium (Lee et al. 1995). 5-HMF can be catalyzed by sulfotransferase to 5-sulphoxymethylfurfural (SMF), which is genotoxic (Severin et al. 2010), and nephrotoxic (Bakhiya et al. 2009). SMF also increases small intestine adenomas potential in mice (Svendsen et al. 2009) and regeneration and atypical hyperplasia of tubules and hepatotoxic effects and serositis of peritoneal tissues (Bauer-Marinovic et al. 2012). Humans may be more sensitive to 5-HMF because sulfotransferase, which can transform 5-HMF into SMF, is expressed in extrahepatic tissues at higher levels than rodents (Teubner et al. 2007). The estimated daily intake of 5-HMF for humans is approximately 2.5 mg/kg body weight as 5-HMF is also present in dried fruits, coffee, cereals, and baked products (Capuano and Fogliano 2011). It is thus important to reduce 5-HMF levels in honey so that total intake of 5-HMF can be reduced for humans.
5-hydroxymethyl-2-furaldehyde formation is influenced by processing temperature and duration, types of honey, storage condition and other factors. 5-HMF contents in honey was less than 24.87 mg/kg after 6 months storage but increased to 1131.6 mg/kg after 24 months storage (Khalil et al. 2010). 5-HMF contents in different types of honey varied widely after thermal treatment at the same temperature and duration (Lu et al. 2006; Singh and Bath 1997, 1998), which was correlated with their chemical characteristics such as: pH, free acids, total acidity and lactones (Fallico et al. 2004) and metal cations (Fe, Mg, Mn and Zn or mixture) (Anam and Dart 1995). However, for the same type of honey, how do different components of honey affect 5-HMF formation has not yet been investigated. It is unknown that which one of the Maillard Reaction and acids catalyze degradation of reducing sugars is the dominant pathway of 5-HMF formation during thermal treatment or longtime storage. In order to investigate the primary and secondary factors affecting 5-HMF formation and the dominant pathway of 5-HMF formation, in the present study how could pH, and amount of water, glucose, fructose, proline, initial 5-HMF, K+, Na+, Ca2+ and Mg2+ contents differently affect 5-HMF formation under the same thermal treatment, and then investigated the possible mechanism of the formation of 5-HMF in honey.
Materials and methods
Reagents
Standards for 5-HMF, glucose and fructose (analytical reagent, AR grade) were purchased from Sigma-Aldrich Com. HPLC grade methanol and acetonitrile and other chemicals (AR grade) were purchased from SCRC (Sinopharm Chemical Reagent Co., Ltd, China).
Honey samples
Rapeseed honey (Brassica napus L.) was bought from an apiary in Yunnan province, China. Fresh honey samples were packaged in different plastic bottles (2L) and placed at 4 °C before component modification and thermal treatment. Pre-treatment was carried out in a water bath at 50 °C until crystallization was liquefied. All measurements were carried out in triplicates (n = 3). Sample analyses were carried out within the later four months.
Measurements of pH and moisture, glucose, fructose, proline, 5-HMF, K+, Na+, Ca2+, and Mg2+ contents in honey samples
pH
Honey samples (10 g) were diluted to 75 mL distilled water to measure pH at 20 °C using a pH-meter (PHS-3C, INESA Scientific Instrument Co., Ltd) (Terrab et al. 2004).
Water content
Water content of honey was measured by an Abbe Refractometer (WYA-2 W, INESA Scientific Instrument Co., Ltd, China) set at 40 °C with water tube connected to a water bath (de Almeida-Muradian et al. 2013).
Glucose, fructose and proline contents
Glucose and fructose contents were determined by HPLC (LC-20A, Shimadzu, Japan) with an InertsilNH2 column (5 μm, 4.6 × 250 mm, Shimadzu-GL) and RID-10A detector (Shimadzu) after it was filtered through a Millex-HN nylon clarification kit (0.45 μm pore size, Tianjin Jinteng Experiment Equipment Co., Ltd, China) (de Almeida-Muradian et al. 2013).
The proline content of honey was determined by using a color comparison according to a method using proline standard curve with a UV Spectrophotometer (T6, Beijing Purkinje General Instrument Co., Ltd, China) (Meda et al. 2005).
Contents of potassium, sodium, calcium and magnesium cations
Honey samples (500 mg) were dissolved with 20 times of reverse osmosis (RO) water (w:w) and filtered by a 0.22 μm filter (Tianjin Jinteng Experiment Equipment Co., Ltd, China). The content of potassium (K+), sodium (Na+), calcium (Ca2+) and magnesium (Mg2+) was determined by the standard curve method with ion chromatography (Dionex ICS-2100; Thermo Scientific) (de Caland et al. 2012).
HPLC analysis of 5-HMF
Honey samples (0.1 g) were dissolved in 10 mL water. The solution (10 g/L) was filtered with a Millex-HN nylon clarification kit (0.45 μm pore size) for analysis by an HPLC–PDA system (LC-20A, Shimadzu, Japan) with a WondaSilC18-WR column (5 μm, 4.6 × 250 mm, Shimadzu-GL) and PDA detector (Shimadzu). The injection volume was 20 μL. The mobile phase was 100% water for 0–10 min, then a linear gradient of 100% water to 100% methanol, from 10 to 55 min, and 100% methanol from 55 to 65 min at a flow rate of 0.5 mL/min. Column temperature was set at 25 °C. Spectral data from all peaks were accumulated in the range of 200–800 nm, and chromatograms were recorded at 284 nm for 5-HMF (Ajlouni and Sujirapinyokul 2010).
Adjustments of honey compositions and thermal treatment
The moisture contents in honey samples were modified to 21.12%, 23.12%, 25.12% and 27.12% using RO water. Na-citrate or citric acid solution (1 M) was added to modify sample pH to 3.44, 3.95, 4.36, 4.87 and 5.48. Glucose powder was added to change its contents in honey to 35.05, 36.05, 37.05, 38.05 and 39.05%. Fructose contents was also adjusted to 38.40, 39.40, 40.40, 41.40 and 42.40% (w/v).
Mental cations contents were modified by adding KCl, NaCl, CaCl2 and MgCl2 to increase the content of K+, Na+, Ca2+ and Mg2+ for 10, 20, 40 and 80 ppm of each cation in the honey.
Proline solution (0.1 g/ml) and RO water were added in order to adjust the final proline contents in honey as 254.3 to 654.3 mg/kg at 100 mg increments.
Initial 5-HMF content was obtained by adding 5-HMF standard to honey, with the final contents as 15 ± 0.31, 30 ± 0.14, 45 ± 0.21 and 60 ± 0.26 mg/kg.
After these adjustments, honey samples were heated at 90 °C for 4 h. These heated honey samples (10 g) were taken out and cooled by ice-water. After that the samples were stored at 4 °C before further tests within 72 h. 5-HMF contents in heated honey were then determined by HPLC.
Statistical analysis
The results are reported as mean ± standard error (± SE). The relationships between 5-HMF content and water, fructose, glucose, proline, initial 5-HMF, K+, Na+, Ca2+ and Mg2+ contents and pH were determined by regression. All analyses were done using StatView for Windows (Version 5.0.1, SAS Institute Inc. 1992–1998, NC, USA).
Results and discussion
Honey composition
Rapeseed honey (N = 3 determinations for all parameters) pH was 3.95 ± 0.02 and contents of water, glucose (retention time, RT = 11.3 min), fructose (RT = 9.2 min) and proline in rapeseed honey sample were 19.12 ± 0.01%, 35.05 ± 0.03%, 38.40 ± 0.02% and 0.025 ± 0.001%, respectively. The water, proline, glucose and fructose contents in samples were close to a previous report of rapeseed honey in the same province (Chen et al. 2010).
During the HPLC analysis to measure 5-HMF contents, the linear range was 0.73–14.6 µg/mL (RT: 14.15 min). 5-HMF was not detected in pre-treated honey (N = 3) with a detection limit of 0.64–14.67 mg/kg, which means there were no effect of pretreatment on 5-HMF in samples.
The contents of K+, Na+, Ca2+ and Mg2+, which RT were 5.83, 4.19, 11.58 and 9.37 min, in pre-treated honey samples were 514.12 ± 22.49, 72.80 ± 2.10, 111.91 ± 2.77 and 29.87 ± 0.88 mg/L, respectively (N = 3 per measurement). The contents of Mg2+ in rapeseed honey sample were close to coffee honey in South Yunnan province, China (Wei et al. 2016). Other iron contents were higher than other species honey South Yunnan province (Wei et al. 2016), which may due to different nectar plant (Nanda et al. 2003) and geographical origin (Tuzen et al. 2007).
5-HMF contents in heated honey
5-HMF content in honey with different moisture contents
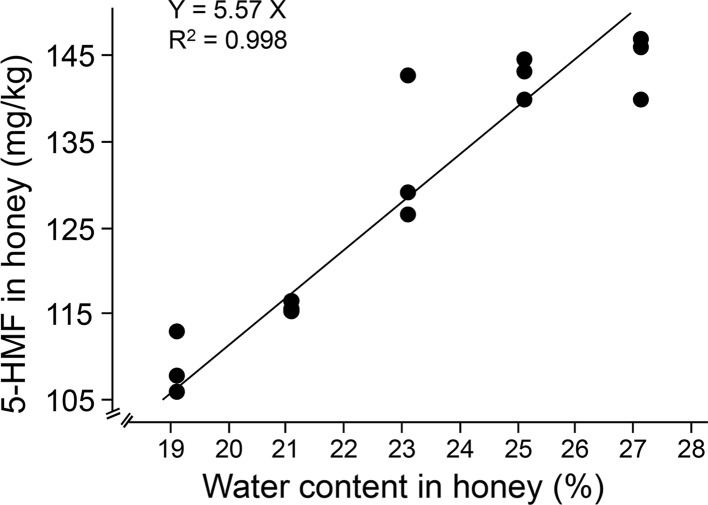
5-hydroxymethyl-2-furaldehyde formation was significantly influenced by water content. Water content and 5-HMF concentration showed a direct and linear relationship (Fig. 1; F1, 14 = 93.91, P < 0.0001). The regression equation between water content (X) and 5-HMF yield (Y) is Y = 5.565X (R2 = 0.998). The R2 decreased to 0.88 (Y = 4.862X + 16.489) if a “zero” intercept was not chosen, the SE of intercept is 11.72 (not significantly different from 0, P = 0.183). Previous studies yielded conflicting results, but those studies did not use honey directly (Sun et al. 2011; Kong et al. 2013; Li and Yang 2014; Cao et al. 2015). Higher moisture content caused higher 5-HMF content in a tetrahydrofuran solution system (Sun et al. 2011; Kong et al. 2013; Cao et al. 2015). 5-HMF yields increased with increasing moisture contents from 60 to 90% (Sun et al. 2011; Kong et al. 2013) in reaction systems of acid and fructose, which is in line with present results (Fig. 1). However, in another study, water content had a negative effect on the reaction system of fructose and sucrose to 5-HMF with proline-derived ionic liquids under thermal conditions at 90 °C for 60 min (Li and Yang 2014). The 5-HMF formation in honey is in line with 5-HMF in reaction systems of acid and fructose. So we can conclude that 5-HMF is transformed from the dehydration of fructose and glucose in honey.
Fig. 1.
The correlation between initial water contents (19.12, 21.12, 23.12, 25.12 and 27.12%) and 5-HMF formation in honey heated at 90 °C for 4 h. Each data point was based on one independent experiment
5-HMF content of honey at varied pH
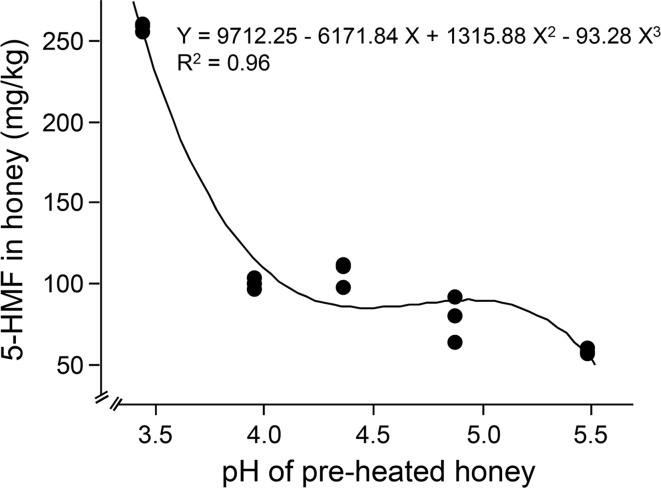
5-hydroxymethyl-2-furaldehydecontents were remarkably affected by pH of honey. There was a multiple regression relationship between 5-HMF content and pH (Fig. 2; F2, 12 = 42.98, P < 0.0001). The regression equation between pH (X) and 5-HMF yield (Y) is Y = 9712.248 − 6171.84 X + 1315.882 X2 − 93.279 X3 (R2 = 0.96), the SE of intercept is 1653.848 (significantly different from 0, P = 0.0001). 5-HMF content was significantly higher when honey pH was at 3.44 in this work. This result was in line with previous report (Singh and Bath 1997), which showed that 5-HMF formation in the Trifolium honey (pH 4.1) was higher than other two types of honey (pH 4.76 or 4.25) under the same thermal treatment condition even though these later honeys have higher water content. 5-HMF was also related to pH of four types of honey (Fallico et al. 2004). There was the same results in other reaction systems of acid and fructose solutions. In AlCl3-H2O/THF biphasic medium, the introduction of HCl, formic, acetic or lactic acids enhanced the reaction rate of fructose dehydration to 5-HMF (de Souza et al. 2012). Low pH enhanced 5-HMF formation in HCl-catalyzed fructose solution (27 wt%) treated with microwave at 130 °C for 5 min (Hansen et al. 2009). Higher 5-HMF development was also measured in lower pH in aqueous solutions ranging from 7 to 1.5 (de Souza et al. 2012). There are organic acids (such as: gluconic, formic, butyric, malic, succinic, lactic and pyroglutamic acids) (Stinson and Subers 1960; Suarez-Luque et al. 2002) and phenolic acids (such as: ellagic, p-hydroxybenzoic, syringic, o-coumaric and gallic acids) (Andrade et al. 1997) which can enhance the reaction rate of fructose dehydration to 5-HMF. But there is no effect of pH on 5-HMF formation in a solution with glucose and amino acids (Ajandouz and Puigserver 1999). So we can conclude that 5-HMF formation pathway was the dehydration of fructose and glucose but glucose and amino acids model system (Maillard reaction).
Fig. 2.
The correlation between initial pH (3.44, 3.95, 4.36, 4.87 and 5.48) and 5-HMF formation in honey heated at 90 °C for 4 h. Each data point was based on one independent experiment
5-HMF content in honey with different glucose and fructose contents
5-hydroxymethyl-2-furaldehyde contents were not influenced by the initial glucose (F1, 13 = 3.32, P = 0.09) and fructose (F1, 13 = 0.56, P = 0.47) contents. The regression equation between glucose content (X) and 5-HMF yield (Y) is Y = 320.575 − 5.259X (R2 = 0.203), the SE of intercept is 320.575 (significantly different from 0, P = 0.0103). The regression equation between fructose content (X) and 5-HMF yield (Y) is Y = 199.013 − 1.672X (R2 = 0.041), the SE of intercept is 199.013 (significantly different from 0, P = 0.0469). Previous studies generated two views about 5-HMF formation in other reaction systems. These results are puzzling. On one hand, the formation of 5-HMF from fructose was 31.2 times higher than from glucose over 22 days when their concentrations were 0.5 M in 0.05 M citric acid at pH 3.5 (Kuster 1990). On the other hand, enolization of hexose into enediol is the rate-limiting step for 5-HMF formation (Kuster 1990). Enolization rate of glucose is lower than that of fructose because glucose can form a very stable ring structure. We can deduce that higher fructose content in pre-treated honey should result in higher 5-HMF content. But present results showed that there was no effect of fructose content on 5-HMF formation. The results in this work seemed to be against 5-HMF formation via the dehydration of fructose and glucose, which may be due to enough fructose (more than 30.9% in honey) (Ball 2007) to take part in the dehydration and enolization reaction for 5-HMF development.
5-HMF development in honey with different initial contents of Mg2+, Ca2+, K+ and Na+ cations
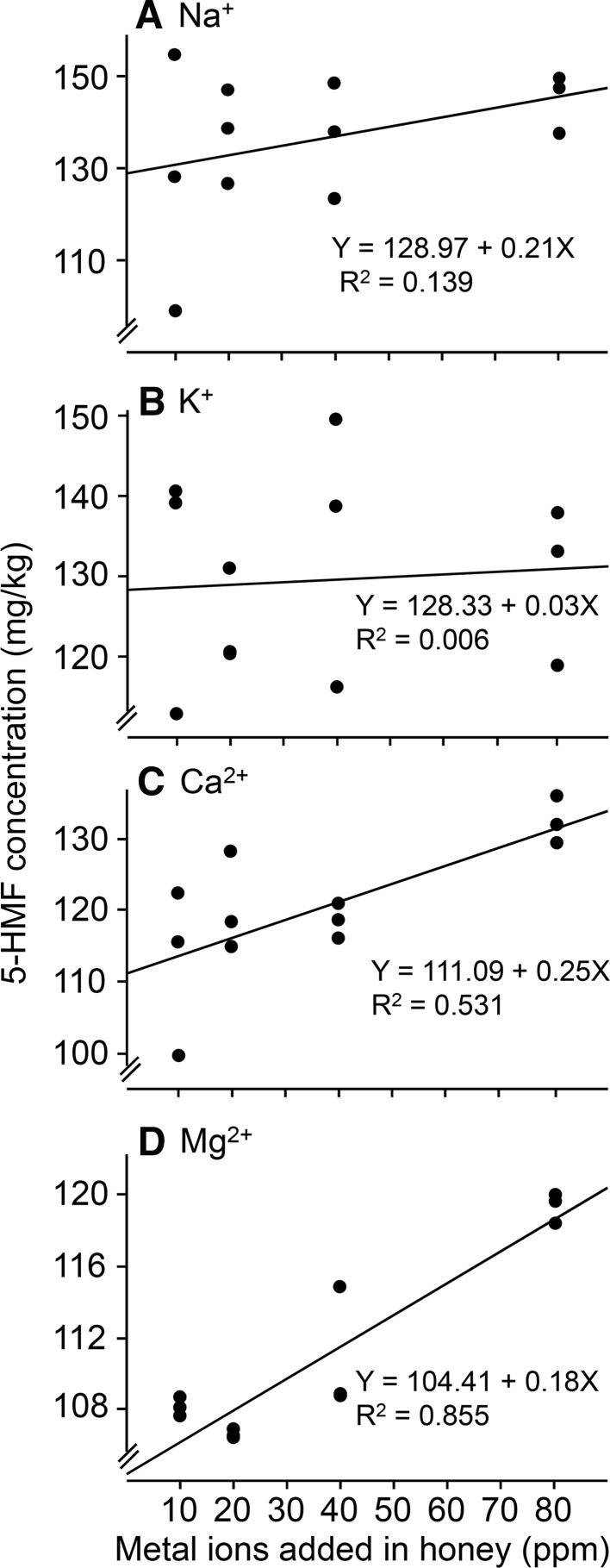
5-hydroxymethyl-2-furaldehyde formation was enhanced by increasing the initial contents of Ca2+ (Fig. 3; F1, 11 = 11.32, P = 0.0072, R2 = 0.53, Y = 111.09 + 0.25X, X denotes Ca2+ content and Y 5-HMF yield, the SE of intercept is 3.46 (significantly different from 0, P < 0.0001)) and Mg2+ (Fig. 3; F1, 11 = 59.13, P < 0.0001, R2 = 0.855; Y = 104.41 + 0.18X, X presents Mg2+ content and Y 5-HMF yield, the SE of intercept is 1.06 (significantly different from 0, P < 0.0001)). But 5-HMF development were not affected by different initial contents of K+ (Fig. 3; F1, 11 = 0.06, P = 0.81, R2 = 0.006, Y = 128.33 + 0.03X, X denotes K+ content and Y 5-HMF yield, the SE of intercept is 6.09 (significantly different from 0, P < 0.0001)) and Na+ (Fig. 3; F1, 11 = 1.61, P = 0.23, R2 = 0.14, Y = 128.97 + 0.21X, X means Na+ content and Y 5-HMF yield, the SE of intercept is 7.47 (significantly different from 0, P < 0.0001)). Previous reports showed that 5-HMF content was significantly increased as the higher amount of cations, especially Ca2+ and Mg2+ in glucose or fructose solution (Gökmen and Şenyuva 2007). Sodium chloride accelerated the formation of 5-HMF in the processing of model biscuits (Fiore et al. 2012) and thermal treatment of fructose solution (Gomes et al. 2015). This suggests that Ca2+ and Mg2+ function similarly in both honey and sugar solutions in enhancing 5-HMF production, but the effect of Na+ varies depending on the reaction system. This may be due to the higher catalytic reaction rate and stronger interaction between hexose and metal cations due to the surface charge increases and the ionic radius decreases (Seri et al. 2001; Marcus 1994; Anam and Dart 1995) in aqueous solutions.
Fig. 3.
The relationship between 5-HMF formation and different initial amounts of metal cations Na+ (a), K+ (b), Ca2+ (c) and Mg2+ (d) on in honey heated at 90 °C for 4 h. Each data point was based on one independent experiment
5-HMF development in honey with different initial proline contents
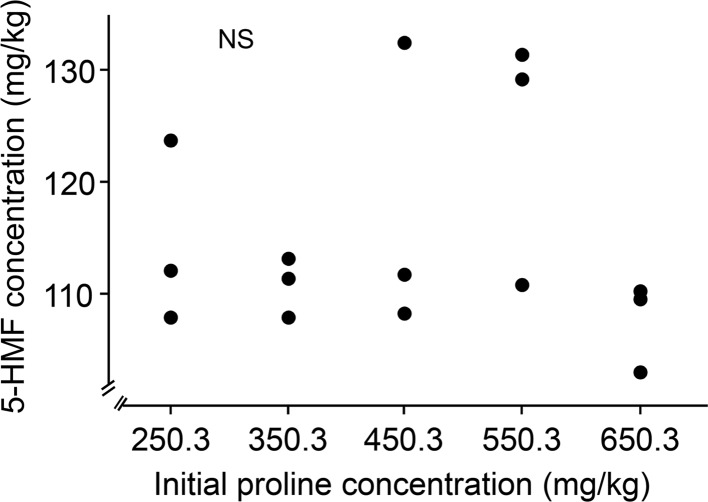
Initial proline content had no influence on the formation of 5-HMF in heated honey (Fig. 4; F1, 14 = 0.003, P = 0.96, R2 = 0.0002, Y = 115.23 − 0.001X (X denotes proline content and Y 5-HMF yield, the SE of intercept is 4.40 (significantly different from 0, P < 0.0001))). It has been reported that glucose and amino acids were combined to form 5-HMF in the Maillard reaction (Fiore et al. 2012). Proline is the predominant amino acid in honey, which accounts for 70% of the total free amino acids (Rückriemen et al. 2015). Given that proline content did not enhance 5-HMF production, present results do not support that glucose-proline model system (Maillard reaction) is the main pathway of 5-HMF formation in honey.
Fig. 4.
No correlation between initial proline contents (254.3, 354.3, 454.3, 554.3 and 654.3 mg/kg) and 5-HMF formation in honey heated at 90 °C for 4 h. Each data point was based on one independent experiment
5-HMF development in honey with different initial 5-HMF contents
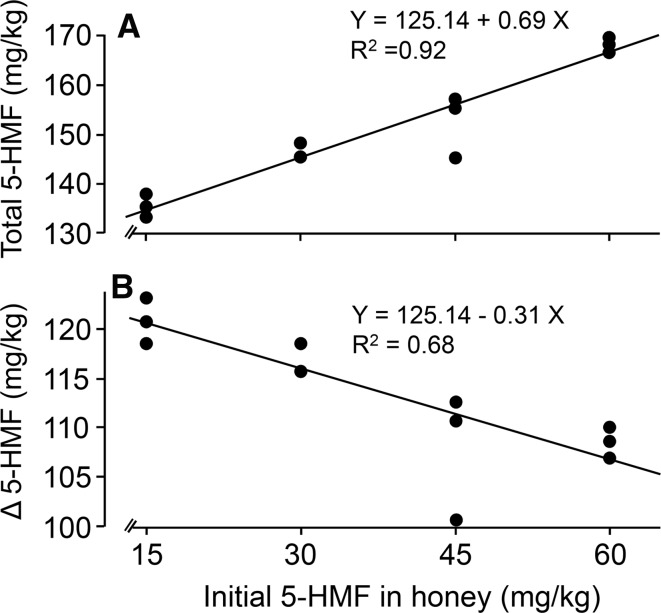
The total 5-HMF contents was increased with the increase in the initial 5-HMF contents after 4 h treatment at 90 °C (Fig. 5 A; F1, 11 = 107.57, P < 0.0001, R2 = 0.92, Y = 125.14 + 0.694X, X denotes initial 5-HMF content and Y 5-HMF yield, the SE of intercept is 2.75 (significantly different from 0, P < 0.0001)).However, if we only consider the net 5-HMF formation (subtracting the initial 5-HMF from the total), the latter would decrease with increasing the initial 5-HMF contents (Fig. 5 B; F1, 11 = 20.97, P = 0.001, R2 = 0.68, Y = 125.14 − 0.306X, X denotes initial 5-HMF content and Y net 5-HMF formation, the SE of intercept is 2.75 (P < 0.0001)). More initial 5-HMF may enhance its transformation to other compounds such as: levulinic acid (LA), formic acid (FA) and humins in acidic solution (Girisuta et al. 2006). Because the color of honey became darker than pretreated honey, which is also observed and due to the increase of certain polyphenols or pigments (Karabagias et al. 2018).
Fig. 5.
The correlation between different initial 5-HMF amounts (15, 30, 45 and 60 mg/kg) and final 5-HMF content (a) or 5-HMF formation (b) in honey heated at 90 °C for 4 h. Each data point was based on one independent experiment
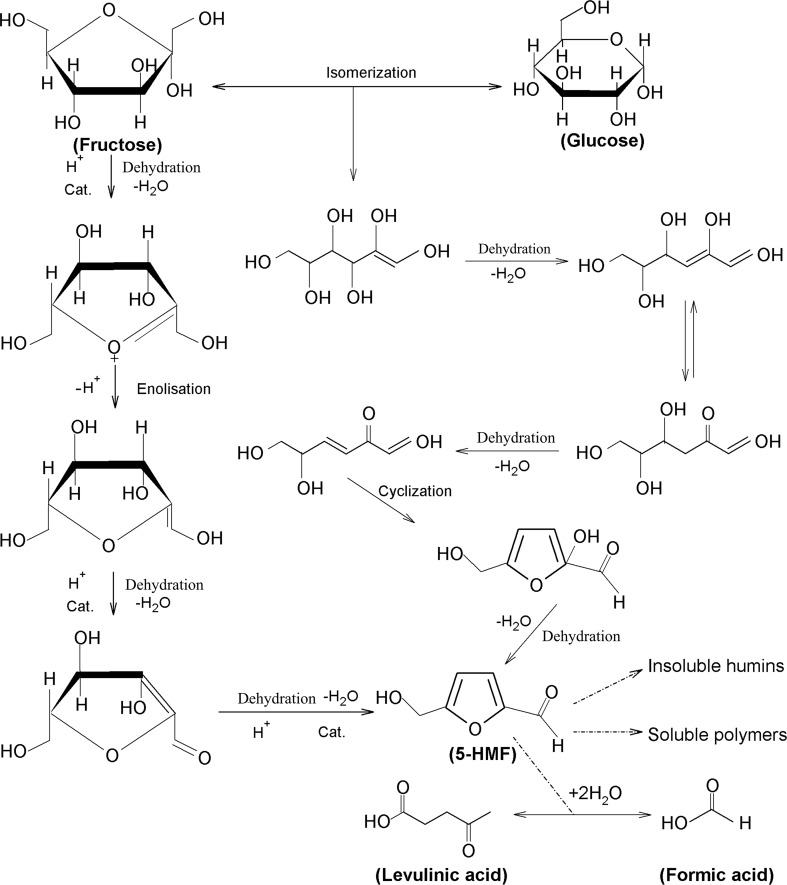
In conclusion, a significant increase of 5-HMF content was observed in honey with higher water content, lower pH and higher Ca2+ and Mg2+contents. Initial 5-HMF content decreased its formation. Fructose, glucose, K+, Na+ and proline contents had no effect on 5-HMF formation. Higher water content may provide more activity of reactant and accelerates the formation of 5-HMF in honey (present study). pH of honey ranged from 3.4 to 6.1 due to gluconic, formic, butyric, malic, succinic, lactic and pyroglutamic acids (Stinson and Subers 1960; Suarez-Luque et al. 2002; Andrade et al. 1997). These acids can catalyze fructose and glucose to form 5-HMF. 5-HMF formation rate from fructose was 31.2 times higher than glucose (Kuster 1990). However, different amounts of proline, a substance in Maillard reaction, had no effect on 5-HMF formation. We therefore conclude that dehydration of fructose or glucose, especially fructose, is the main pathway of 5-HMF formation in honey (Fig. 6, the mechanism of dehydration of fructose or glucose to 5-HMF; Adapted from Li and Yang 2014; Capuano and Fogliano 2011; Perez Locas and Yaylayan 2008.), while it was traditionally thought that 5-HMF formation in honey is due to the Maillard reaction pathway via the rearrangement of Amadori compounds. In order to liquefy crystallized honey, delay crystallization, destroy the microorganisms in honey or facilitate processing, thermal treatment condition should be considered to control 5-HMF according to pH, water content and metal ions contents in honey.
Fig. 6.
Proposed mechanism for catalytic dehydration of fructose and glucose to 5-HMF and its degradation products
(Adapted from Li and Yang 2014; Capuano and Fogliano 2011; Perez Locas and Yaylayan 2008). Solid arrows indicate formation pathway of 5-HMF and broken arrows indicate its degradation
Acknowledgements
We thank Hailong Li, Yinghua Wang, Yiru Yin, Qishuang Li, Xinsheng Zhang for their help performing some experiments. This study was supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-45-KXJ19) and Education and Scientific Research Project of Middle and Youth Teachers in Fujian (JA15151).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zachary Yong Huang, Phone: +1-517-353-8136, Email: bees@msu.edu.
Xiaoqing Miao, Phone: +86-591-83789426, Email: fjbees@126.com.
References
- Ajandouz EH, Puigserver A. Nonenzymatic browning reaction of essential amino acids: effect of pH on caramelization and Maillard reaction kinetics. J Agric Food Chem. 1999;47:1786–1793. doi: 10.1021/jf980928z. [DOI] [PubMed] [Google Scholar]
- Ajlouni S, Sujirapinyokul P. Hydroxymethylfurfuraldehyde and amylase contents in Australian honey. Food Chem. 2010;119:1000–1005. doi: 10.1016/j.foodchem.2009.07.057. [DOI] [Google Scholar]
- Anam OO, Dart RK. Influence of metal ions on hydroxymethylfurfural formation in honey. Anal Proc. 1995;32:515–517. doi: 10.1039/ai9953200515. [DOI] [Google Scholar]
- Andrade P, Ferreres F, Amaral MT. Analysis of honey phenolic acids by HPLC, its application to honey botanical characterization. J Liq Chromatogr Relat Technol. 1997;20:2281–2288. doi: 10.1080/10826079708006563. [DOI] [Google Scholar]
- Aslanova D, Bakkalbasi E, Artik N. Effect of storage on 5-hydroxymethylfurfural (HMF) formation and color change in jams. Int J Food Prop. 2010;13:904–912. doi: 10.1080/10942910902908896. [DOI] [Google Scholar]
- Bakhiya N, Monien B, Frank H, Seidel A, Glatt H. Renal organic anion transporters OAT1 and OAT3 mediate the cellular accumulation of 5-sulfooxymethylfurfural, a reactive, nephrotoxic metabolite of the Maillard product 5-hydroxymethylfurfural. Biochem Pharmacol. 2009;78:414–419. doi: 10.1016/j.bcp.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Ball DW. The chemical composition of honey. J Chem Educ. 2007;84:1643. doi: 10.1021/ed084p1643. [DOI] [Google Scholar]
- Bauer-Marinovic M, Taugner F, Florian S, Glatt H. Toxicity studies with 5-hydroxymethylfurfural and its metabolite 5-sulphooxymethylfurfural in wild-type mice and transgenic mice expressing human sulphotransferases 1A1 and 1A2. Arch Toxicol. 2012;86:701. doi: 10.1007/s00204-012-0807-5. [DOI] [PubMed] [Google Scholar]
- Cao F, Schwartz TJ, McClelland DJ, Krishna SH, Dumesic JA, Huber GW. Dehydration of cellulose to levoglucosenone using polar aprotic solvents. Energy Environ Sci. 2015;8:1808–1815. doi: 10.1039/C5EE00353A. [DOI] [Google Scholar]
- Capuano E, Fogliano V. Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT- Food Sci Technol. 2011;44:793–810. doi: 10.1016/j.lwt.2010.11.002. [DOI] [Google Scholar]
- Chen J, Yang Y, Hu Y, Xu R, Chen N, Cao W. Determination of proline content in rape honey and jujube honey. Apic China. 2010;10:11–13. [Google Scholar]
- da Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R. Honey: chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- de Almeida-Muradian LB, Stramm KM, Horita A, Barth OM, da Silva de Freitas A, Estevinho LM. Comparative study of the physicochemical and palynological characteristics of honey from Melipona subnitida and Apis mellifera. Int J Food Sci Technol. 2013;48:1698–1706. doi: 10.1111/ijfs.12140. [DOI] [Google Scholar]
- de Caland LB, Silveira ELC, Tubino M. Determination of sodium, potassium, calcium and magnesium cations in biodiesel by ion chromatography. Anal Chim Acta. 2012;718:116–120. doi: 10.1016/j.aca.2011.12.062. [DOI] [PubMed] [Google Scholar]
- de Souza RL, Yu H, Rataboul F, Essayem N. 5-Hydroxymethylfurfural (5-HMF) production from hexoses: limits of heterogeneous catalysis in hydrothermal conditions and potential of concentrated aqueous organic acids as reactive solvent system. Challenges. 2012;3:212–232. doi: 10.3390/challe3020212. [DOI] [Google Scholar]
- Fallico B, Zappala M, Arena E, Verzera A. Effects of conditioning on HMF content in unifloral honeys. Food Chem. 2004;85:305–313. doi: 10.1016/j.foodchem.2003.07.010. [DOI] [Google Scholar]
- Fiore A, Troise AD, Ataç Mogol B, Roullier V, Gourdon A, El Mafadi Jian S, Hamzalioglu BA, Fogliano V. Controlling the Maillard reaction by reactant encapsulation: sodium chloride in cookies. J Agric Food Chem. 2012;60:10808–10814. doi: 10.1021/jf3026953. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kobayashi K, Hirono Y, Miyagawa M, Ishida T, Ejiogu EC, Sawai M, Pinkerton KE, Takeuchi M (2010) Jungle honey enhances immune function and antitumor activity. Evidence-Based Complementary Alternative Medicine 2011. 10.1093/ecam/nen086 [DOI] [PMC free article] [PubMed]
- Girisuta B, Janssen LPBM, Heeres HJ. A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid. Green Chem. 2006;8:701–709. doi: 10.1039/b518176c. [DOI] [Google Scholar]
- Gökmen V, Şenyuva HZ. Effects of some cations on the formation of acrylamide and furfurals in glucose–asparagine model system. Eur Food Res Technol. 2007;225:815–820. doi: 10.1007/s00217-006-0486-7. [DOI] [Google Scholar]
- Gomes FNDC, Pereira LR, Ribeiro NFP, Souza MMVM. Production of 5-hydroxymethylfurfural (HMF) via fructose dehydration: effect of solvent and salting-out. Braz J Chem Eng. 2015;32:119–126. doi: 10.1590/0104-6632.20150321s00002914. [DOI] [Google Scholar]
- Hansen TS, Woodley JM, Riisager A. Efficient microwave-assisted synthesis of 5-hydroxymethylfurfural from concentrated aqueous fructose. Carbohydr Res. 2009;344:2568–2572. doi: 10.1016/j.carres.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Karabagias VK, Karabagias IK, Gatzias I. The impact of different heating temperatures on physicochemical, color attributes, and antioxidant activity parameters of Greek honeys. J Food Process Eng. 2018;41:e12668. doi: 10.1111/jfpe.12668. [DOI] [Google Scholar]
- Khalil M, Sulaiman S, Gan S. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem Toxicol. 2010;48:2388–2392. doi: 10.1016/j.fct.2010.05.076. [DOI] [PubMed] [Google Scholar]
- Kong S, Liu S, Li L, Yu S. Preparation of 5-hydroxymethylfurfural by dehydration of glucose. Sci Technol Chem. 2013;34:73–76. [Google Scholar]
- Kuster BFM. 5Hydroxymethylfurfural (HMF). A review focusing on its manufacture. StarchStärke. 1990;42:314–321. doi: 10.1002/star.19900420808. [DOI] [Google Scholar]
- LeBlanc BW, Eggleston G, Sammataro D, Cornett C, Dufault R, Deeby T, St Cyr E. Formation of hydroxymethylfurfural in domestic high-fructose corn syrup and its toxicity to the honey bee (Apis mellifera) J Agric Food Chem. 2009;57:7369–7376. doi: 10.1021/jf9014526. [DOI] [PubMed] [Google Scholar]
- Lee YC, Shlyankevich M, Jeong HK, Douglas JS, Surh YJ. Bioactivation of 5-hydroxymethyl-2-furaldehyde to an electrophilic and mutagenic allylic sulfuric acid ester. Biochem Bioph Res Co. 1995;209:996–1002. doi: 10.1006/bbrc.1995.1596. [DOI] [PubMed] [Google Scholar]
- Li H, Yang S. Catalytic transformation of fructose and sucrose to HMF with proline-derived ionic liquids under mild conditions. Int J Chem Eng. 2014;2014:e978708. [Google Scholar]
- Lu K, Cao W, Zheng J. Effect of the thermal treatment on 5- Hydroxymethyl-2-furfural (HMF) contents in different floral honey. J Northwest Univ (Nat Sci Edition) 2006;36:253–256. [Google Scholar]
- Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22:187–192. doi: 10.1111/wrr.12117. [DOI] [PubMed] [Google Scholar]
- Marcus Y. A simple empirical model describing the thermodynamics of hydration of ions of widely varying charges, sizes, and shapes. Biophys Chem. 1994;51:111–127. doi: 10.1016/0301-4622(94)00051-4. [DOI] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Michail K, Matzi V, Maier A, Herwig R, Greilberger J, Juan H, Kunert O, Wintersteiger R. Hydroxymethylfurfural: an enemy or a friendly xenobiotic? A bioanalytical approach. Anal Bioanal Chem. 2007;387:2801–2814. doi: 10.1007/s00216-007-1121-6. [DOI] [PubMed] [Google Scholar]
- Nanda V, Sarkar BC, Sharma HK, Bawa AS. Physico-chemical properties and estimation of mineral content in honey produced from different plants in Northern India. J Food Compos Anal. 2003;16:613–619. doi: 10.1016/S0889-1575(03)00062-0. [DOI] [Google Scholar]
- National Toxicology Program (2010) NTP toxicology and carcinogenesis studies of 5-(Hydroxymethyl)-2-furfural (CAS No. 67-47-0) in F344/N rats and B6C3F1 mice (gavage studies). Natl Toxicol Program Tech Rep Ser 554: 7. PMID: 20725154 [PubMed]
- Pätzold R, Brückner H. Gas chromatographic detection of D-amino acids in natural and thermally treated bee honeys and studies on the mechanism of their formation as result of the Maillard reaction. Eur Food Res Technol. 2006;223:347–354. doi: 10.1007/s00217-005-0211-y. [DOI] [Google Scholar]
- Perez Locas C, Yaylayan VA. Isotope labeling studies on the formation of 5-(hydroxymethyl)-2-furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. J Agric Food Chem. 2008;56:6717–6723. doi: 10.1021/jf8010245. [DOI] [PubMed] [Google Scholar]
- Rückriemen J, Schwarzenbolz U, Adam S, Henle T. Identification and quantitation of 2-Acetyl-1-pyrroline in Manuka Honey (Leptospermum scoparium) J Agric Food Chem. 2015;63:8488–8492. doi: 10.1021/acs.jafc.5b03042. [DOI] [PubMed] [Google Scholar]
- Seri KI, Inoue Y, Ishida H. Catalytic activity of lanthanide (III) ions for the dehydration of hexose to 5-hydroxymethyl-2-furaldehyde in water. Bull Chem Soc Jpn. 2001;74:1145–1150. doi: 10.1246/bcsj.74.1145. [DOI] [Google Scholar]
- Severin I, Dumont C, Jondeau-Cabaton A, Graillot V. Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicol Lett. 2010;192:189–194. doi: 10.1016/j.toxlet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Singh N, Bath PK. Quality evaluation of different types of Indian honey. Food Chem. 1997;58:129–133. doi: 10.1016/S0308-8146(96)00231-2. [DOI] [Google Scholar]
- Singh N, Bath PK. Relationship between heating and HMF formation in different honey types. J Food Sci Technol. 1998;35:154–156. [Google Scholar]
- Stinson EE, Subers MH. The composition of honey. V. Separation and identification to the organic acids. Arch Biochem Btophys. 1960;89:6–12. doi: 10.1016/0003-9861(60)90003-5. [DOI] [PubMed] [Google Scholar]
- Suarez-Luque S, Mato I, Huidobro JF, Simal-Lozano J, Sancho MT. Rapid determination of minority organic acids in honey by high-performance liquid chromatography. J Chromatogr A. 2002;955:207–214. doi: 10.1016/S0021-9673(02)00248-0. [DOI] [PubMed] [Google Scholar]
- Sun C, Liu S, Li L, Xie C. Dehydration of fructose to prepare 5-hydroxymethylfurfural catalyzed by alkaline acidic ionic liquids. J Qingdao Uni Sci Technol Nat Sci Ed. 2011;32:331–334. [Google Scholar]
- Svendsen C, Husøy T, Glatt H, Paulsen JE, Alexander J. 5-Hydroxymethylfurfural and 5-sulfooxymethylfurfural increase adenoma and flat ACF number in the intestine of Min/+ mice. Anticancer Res. 2009;29:1921–1926. [PubMed] [Google Scholar]
- Terrab A, Recamales AF, Hernanz D, Heredia FJ. Characterisation of Spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chem. 2004;88:537–542. doi: 10.1016/j.foodchem.2004.01.068. [DOI] [Google Scholar]
- Teubner W, Meinl W, Florian S, Kretzschmar M, Glatt H. Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem J. 2007;404:207–215. doi: 10.1042/BJ20061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzen M, Silici S, Mendil D, Soylak M. Trace element levels in honeys from different regions of Turkey. Food Chem. 2007;103:325–330. doi: 10.1016/j.foodchem.2006.07.053. [DOI] [Google Scholar]
- Wei Y, Chen F, Wang Y, Chen LZ, Zhang XW, Wang YH, Wu LM, Zhou Q. Application of ICP-MS to identify the botanic source of characteristic honey in South Yunnan. Guang pu. 2016;36:262–267. [PubMed] [Google Scholar]