Abstract
Aim of the present study was to evaluate the effect of different drying techniques on chemical composition, color and antioxidant activity of kinnow peel. Fresh peel was dehydrated by three different techniques (tray, vacuum and freeze) and regardless of the method results showed significant decreases in moisture, total phenols, flavonoids, antioxidant activity and color when compared to the fresh sample. Freeze drying was found superior in retention of polyphenolic characteristics and color attributes when compared with other drying techniques. The fresh and freeze-dried citrus peel extract was analyzed for individual phenolics and flavonoids with the help of HPLC indicating highest concentrations of ferulic acid and hesperidin.
Keywords: Kinnow peel, Drying techniques, Freeze-drying, Phytochemicals analysis
Introduction
Kinnow mandarin (Citrus reticulata), a hybrid of King and Willow leaf is grown in northern states of India, mainly Punjab. In the process of juice extraction from kinnow fruit, 30–34% of peel is generated from a number of fruit processing industries and fruit vendors which has been recognized as the richest source of bioactive compounds with comparatively higher polyphenol content compared to other fractions of fruit (Lim et al. 2007). Health promoting properties of citrus peel have prompted researchers to explore this waste as a value added ingredient in many food processing operations as a potential source of antioxidants (Babbar et al. 2011; Rafiq et al. 2018).
Several drying techniques are available as commercial options for dehydration, including freeze drying, convective drying and vacuum drying. Research has been conducted on effect of different drying techniques on various quality aspects of fruits and vegetables (Huang and Zhang 2015; Hamid and Abdel Nour 2018; Izli and Polat 2018; Morais et al. 2015; Eren and Kaymak-Ertekin 2007; Mudgal and Pandey 2009; Shalini et al. 2009).
Literature regarding effect of different drying techniques on particularly kinnow peel in comparison to other fruits and vegetables is very scarce. Therefore, the purpose of this research was to explore the effect of drying techniques (tray, vacuum and freeze drying) on the proximate composition, physicochemical and phytochemical properties of kinnow peel. The outcome of this research may open new prospects for the valorization of kinnow peel as a functional ingredient. Moreover, statistical analysis was done to (1) identify correlation between the color coordinates (simple linear correlation) and (2) explain the effect of different drying techniques on phytochemical properties of the kinnow peel (cluster analysis and principal component analysis).
Materials and methods
Sample preparation
Kinnow (Citrus reticulata Blanco) were obtained from pomology fields of Sher-e-Kashmir University of Agricultural Sciences and Technology, Jammu, (India). The defective and injured fruits were sorted-out and the best suited were retained for further processing. Fruits were peeled manually and cutted into strips of uniform width (2–3 mm).
Drying of kinnow peel
Kinnow peel strips were spread on trays in single layer for drying in different (freeze, oven and vacuum) dryers. Drying conditions were adopted from Izli and Polat (2018). Three different drying (freeze i.e. − 50 °C, oven i.e. 60 °C and vacuum at 500 mm Hg) techniques were used to dry kinnow peel strips until their weight became constant. Fresh peel stored in deep freezer at − 20 °C was used as a control sample to determine the outcome of drying techniques on sample quality. The dried peel samples from all three methods were ground, packed in laminated pouches and stored at ambient temperature until analyzed.
Proximate analysis
Proximate analysis of fresh kinnow peel was carried out according to AOAC (2000). Moisture content was performed by drying sample to a constant weight at 105 °C. Ash content was performed at 550 °C using muffle furnace (AOAC method 923.03). Micro Kjeldahl procedure (AOAC method 960.52) was used for the determination of crude protein content. Crude fat content was determined using petroleum ether as solvent in a Soxhlet apparatus. Crude fibre was estimated by subjecting sample to digestion (acid) and then distillation (alkaline) (AOAC method 962.09).
Total mineral content was quantified in dry ash samples at 550 °C according to AOAC (2002). Mineral contents individually were determined using an atomic absorption spectrophotometer (Model AA670 Shimadzu, Kyoto, Japan).
Colour analysis
The color parameter (L*, a*, b*) of the fresh and dried sample were determined by following Grabowski et al. (2006) using a Hunter Lab colourimeter (Labscan XE) coupled with EasyMatch QC software. Numerical whiteness index (WI) and browning index (BI) were calculated using the equations adopted from Gat et al. (2015a).
Extraction of phytochemicals
Extraction of polyphenols was done according to Franke et al. (2004) and Chun et al. (2003). Extraction yield of polyphenols was defined as the amount of product (g) obtained from 100 gm of kinnow peel dry weight was estimated using equation adopted by Dhanani et al. (2015).
Phytochemical analysis
Total phenolic and flavonoid content of the extracts was estimated using colorimetric assay adapted from Gat et al. (2015b). Gallic acid standard curve was used as calibration curve and the values were reported as mg/gallic acid equivalent/gram dry weight (g dw). For determination of total flavonoid content, standard used was quercetin and experimental values were presented as mg of quercetin equivalent (QE) per gram of dry powder. Ascorbic acid was quantified using 2,6,dichlorophenol indophenol dye by following procedure of Sogi et al. (2013).
The antiradical activity was determined by different methods described as follows. Scavenging activity (SA) was measured as suggested by Wang et al. (2016). The antiradical activity was also determined as IC50 μg/mL (concentration for 50% DPPH inhibition) for the peel extract. Reducing power was estimated as described by Jayanthi and Lalitha (2011). Trolox equivalent antioxidant capacity (TEAC) was determined using method adapted from Wang et al. (2016) using trolox as standard standard. β-Carotene bleaching test was adopted from Koleva et al. (2002) to evaluate the capacity of methanolic peel extract to inhibit the β-carotene bleaching and standard used was BHT.
Individual polyphenolic compounds analysis by HPLC
Individual polyphenolic compounds analysis were conducted for fresh and freeze dried kinnow peel by reversed-phase HPLC system (1200 Series, Agilent Technologies, Germany), with a quaternary pump, a degasser, manual injector with a fixed volume of 20 µL, UV–Vis diode array detector (DAD) and C18 column (100 mm × 4.6 mm) thermostatically controlled at 35 °C. The flow rate was kept constant at 1 mL/min. The mobile phase solvents consisted of water, acetonitrile, orthophosphoric acid and methanol. Wavelength used for detection and quantification was 280 nm. Quantification of phenolic compounds was done by comparison of their retention time with standards (Gallic acid, Vanillic acid, p-Coumaric acid, Ferulic acid, p-Hydroxybenzoic acid, Caffeic acid, Neo-hesperidin, Naringin, Hesperidin, Catechin Narenginin and Rutin) previously injected.
Statistical analysis
One-way ANOVA followed by Duncan’s LSD test (p ≤ 0.05) was performed for analysis of experimental values using SPSS 16.0. Principal component analysis (PCA) and cluster analysis were conducted to get a distinctive comprehensive overview of phytochemical properties of dried kinnow peel and the output obtained was plotted as a dendrogram. All experiments were performed in triplicate, and values were reported as the mean ± standard deviation (SD). Pearson correlation coefficient among means of color parameters was determined with the help of statistical tool (SPSS 16.0).
Results and discussion
Effect of drying techniques on proximate composition of kinnow peel
The effect of different drying techniques on the proximate composition of fresh kinnow peel is shown in Table 1. Moisture, crude fat, protein, fiber and ash of fresh kinnow peel was found to be 77.6, 1.59, 0.67, 0.64 and 0.55% respectively. Effect of drying techniques (tray, vacuum and freeze) on proximate composition showed significant differences (p ≤ 0.05) for moisture. These findings were found parallel with Garau et al. (2007), Malla et al. (2015) and Barros et al. (2012) for fresh orange (citrus aurantium), different cultivars of citrus and kinnow mandarin waste respectively. Among all techniques, freeze dried peel had highest moisture content of 7.38%. Drying also resulted in slight non-significant (p ≤ 0.05) decrease in fat and protein content and the most affected sample was tray dried followed by vacuum dried which might be due to exudation of fat with moisture evaporation. The findings in the present investigation were comparable to those reported by Sogi et al. (2013) and Chukwu and Shaba (2009). Decrease in protein content might be because of Maillard reaction as it causes changes in food composition due to the reaction between carbohydrates and proteins. Lee-Hoon Ho et al. (2016) also reported decrease in protein content and attributed this change to the formation of complexes between anti-nutritional components and proteins in the presence of heat which subsequently reduces protein availability (Enomfon-Akpan and Umoh 2004).
Table 1.
Effect of drying (tray, vacuum, and freeze) on proximate composition of kinnow peel
| Fresh peel | Tray dried peel | Vacuum dried peel | Freeze dried peel | |
|---|---|---|---|---|
| Moisture (%) | 77.47 ± 0.11a | 4.93 ± 0.09c | 3.03 ± 0.06d | 7.38 ± 0.07b |
| Ash (%) | 0.49 ± 0.03b | 0.57 ± 0.04ab | 0.57 ± 0.06ab | 0.59 ± 0.04a |
| Crude protein (%) | 0.67 ± 0.02a | 0.63 ± 0.06a | 0.65 ± 0.04a | 0.58 ± 0.08a |
| Crude fat (%) | 1.55 ± 0.04a | 1.22 ± 0.02b | 1.54 ± 0.03a | 1.58 ± 0.03a |
| Crude fiber (%) | 0.64 ± 0.02a | 0.59 ± 0.07a | 0.55 ± 0.05a | 0.63 ± 0.06a |
| Potassium (µg/100 g) | 152.23 ± 2.05b | 157.32 ± 3.16a | 154.21 ± 1.10ab | 154.05 ± 2.07ab |
| Magnesium (µg/100 g) | 108.45 ± 1.00b | 114.13 ± 1.40a | 109.31 ± 0.25b | 109.15 ± 0.60b |
| Calcium (µg/100 g) | 85.30 ± 0.60c | 89.75 ± 0.75a | 87.89 ± 1.05b | 84.94 ± 0.54c |
| Sodium (µg/100 g) | 176.30 ± 0.53d | 189.00 ± 0.60a | 187.23 ± 0.65b | 182.06 ± 0.46c |
| Iron (µg/100 g) | 8.52 ± 0.46d | 10.01 ± 0.55a | 9.88 ± 0.81b | 9.58 ± 0.11c |
| Zinc (µg/100 g) | 4.44 ± 0.42a | 4.93 ± 0.05a | 4.58 ± 0.51a | 4.46 ± 0.05a |
| Copper (µg/100 g) | 0.34 ± 0.11a | 0.31 ± 0.02a | 0.37 ± 0.02a | 0.37 ± 0.02a |
All values are mean ± standard deviation of triplicate analysis and values in the same row bearing different superscript lowercase letters are significant difference (p ≤ 0.05) by Duncan’s test
The mineral contents of fresh, tray, vacuum and freeze dried kinnow peel are given in Table 1. As compare to fresh sample, the mineral profile of dried kinnow peels was higher because of increasing dry matter content. Potassium, calcium and iron content of fresh peel were found 152.2, 85.3 and 324.67 µg/100 g respectively. Parallel findings were reported by Khalid et al. (2012) and Ghanem et al. (2012). The differences between Mg, Zn and Cu contents of fresh, tray and vacuum dried peels were not statistically significant (p ≤ 0.05). The remaining minerals were higher in tray dried samples than the fresh samples. The tray drying method led to the highest increase in mineral values due to convective heat transfer mechanism in tray drying which could cause more increase in the solubility of the minerals (Arslan and Ozcan 2008).
Effect of drying techniques on color properties of kinnow peel
Drying techniques exert a significant (p ≤ 0.05) effect on the colour values of kinnow peel. L*, a* and b* values of the fresh samples were found to be 57.75, 16.31 and 65.18 respectively (Table 2), similar to those reported by Aggarwal and Michael (2014) while studying the effect of osmo-dehydration on kinnow peel. After drying the L* value of kinnow peel decreased significantly (p ≤ 0.05) while a* value increased significantly (p ≤ 0.05) in dried samples (tray, vacuum and freeze), indicating darker sheds of dried peel which might be due to caramelization of the sugar or a Maillard reaction causing browning during drying. On the other hand b* value of fresh peel was higher than dried peel powder, indicating degradation of the carotene pigment during drying. The hue and chroma of dried powder was found significantly (p ≤ 0.05) lower, indicating decrease in color intensity and saturation. It can also be observed that drying (tray and vacuum) led to increase in browning index and decrease in whitening index which might be due to formation of brown products. Freeze drying dehydrates product by sublimation which is carried out at very low temperature, so prevents browning reactions and results in more stable colour coordinates. The overall high b* value in freeze dried samples may be indicative of high β-carotene retention. Abonyi et al. (2002) and Caparino et al. (2012) observed more retention of carotenoid pigments in freeze dried samples than drum dried ones. Maskan (2001), Goncalves et al. (2007), Guine and Barroca (2012), Vega-Galvez et al. (2012) also supports similar findings. Statistical analysis revealed that L* value was negatively correlated with b* value, hue, chroma and browning index. Whitening index was negatively correlated with browning index. a* value was found positively correlated with b* value and negatively correlated with whitening index (R2 = 0.997, p < 0.05 and R2 = −0.965, p < 0.05, respectively) (Table 3).
Table 2.
Effect of drying (tray, vacuum and freeze) on color and phytochemical analysis of kinnow peel
| Fresh peel | Tray dried peel | Vacuum dried peel | Freeze dried peel | |
|---|---|---|---|---|
| L* | 57.75 ± 0.21a | 37.14 ± 0.90b | 57.64 ± 0.30a | 59.75 ± 0.05a |
| a* | 16.31 ± 0.04b | 19.63 ± 0.20a | 15.63 ± 0.10c | 15.31 ± 0.06d |
| b* | 65.18 ± 0.10a | 54.05 ± 0.56d | 56.21 ± 0.21c | 59.18 ± 0.11b |
| Hue | 75.95 ± 1.0a | 69.86 ± 0.20b | 74.41 ± 0.06a | 75.65 ± 0.16a |
| Chroma | 67.15 ± 0.15a | 58.13 ± 0.26b | 58.14 ± 0.10b | 64.393 ± 1.89a |
| Browning index | 290.59 ± 2.21a | 265.47 ± 2.57b | 222.00 ± 1.59c | 216.76 ± 2.07d |
| Whitening index | 14.63 ± 0.28b | 20.18 ± 0.04ab | 26.81 ± 0.12a | 27.9 ± 0.40a |
| Total phenolic content (mg GAE/g) | 24.51 ± 0.08a | 16.84 ± 0.05d | 17.94 ± 0.03c | 21.96 ± 0.12b |
| Total flavonoid content (mg QE/g) | 19.12 ± 0.09a | 11.11 ± 0.09d | 13.36 ± 0.08c | 15.32 ± 0.05b |
| DPPH assay (IC50 value) μg/mL | 66.5 ± 0.50d | 73.15 ± 0.06a | 72.18 ± 0.10b | 68.15 ± 0.09c |
| Scavenging activity (%) | 86.55 ± 0.53b | 83.28 ± 0.04d | 89.07 ± 0.06a | 84.77 ± 0.60c |
| TEAC µmolTE/g | 51.77 ± 0.05b | 44.17 ± 0.10d | 47.2 ± 0.12c | 51.21 ± 0.05a |
| Β-carotene bleaching assay (%) | 33.88 ± 0.07a | 23.19 ± 0.09d | 24.12 ± 0.10c | 29.05 ± 0.03b |
| Ascorbic acid (mg/100 g) | 46.87 ± 0.10a | 29.92 ± 0.07d | 31.08 ± 0.08c | 41.24 ± 0.20b |
| Extraction yield (%) | 5.19 ± 0.20a | 14.95 ± 0.01d | 15.56 ± 0.07c | 22.04 ± 0.16b |
All values are mean ± standard deviation of triplicate analysis and values in the same row bearing different superscript lowercase letters are significant difference (p ≤ 0.05) by Duncan’s test
Table 3.
Correlation coefficients among color parameters of kinnow peel
| L | a | b | Hue | Chroma | WI | BI | |
|---|---|---|---|---|---|---|---|
| L | 1 | − 0.485 | − 0.551 | − 0.558 | − 0.674 | 0.698 | − 0.507 |
| a | 1 | 0.997* | 0.996 | 0.973 | − 0.965 | 1.000* | |
| b | 1 | 0.998** | 0.988 | − 0.982 | 0.999* | ||
| Hue | 1 | 0.989 | − 0.984 | 0.998* | |||
| Chroma | 1 | − 0.999 | 0.979 | ||||
| WI | 1 | − 0.971 | |||||
| B I | 1 |
*Correlation is significant at 0.05 level
**Correlation is significant at 0.01 level
Effect of drying techniques on extraction yield of polyphenols
Dying techniques (tray, vacuum and freeze) affected the extraction yield (p ≤ 0.05) significantly and in all cases yield per cent obtained was higher than fresh kinnow peel sample (Table 2) indicating that drying may impart a positive effect on the extraction of polyphenolic compounds. Extraction yield was highest in freeze dried samples (22.04 gm/100 gm) followed by vacuum (15.56 gm/100 gm) and tray dried (14.95 gm/100 gm) and lowest in fresh sample (5.19 gm/100 gm). Our results were comparable to the yield percentage of lime (15.80 gm/100 gm) but lower than the orange variety (37.27 gm/100 gm), grape fruit (50.13 gm/100 gm) and lemon (44.68 gm/100 gm) evaluated by Guimaraes et al. (2010). Variation in yield might be due to the diversity in natural matrix as well as many parameters related to the extraction method adopted (temperature, time contact, solvent to solid ratio, solvent type etc.).
Effect of drying techniques on phytochemical properties of kinnow peel
The total phenolic and flavonoid content of kinnow fresh peel was found to be 24.51 mgGAE/g and 19.12 mgQE/gm respectively (Table 2). Our results revealed higher concentration of TPC than reported by Babbar et al. (2011). The difference in concentrations might be due to differences in cultivar, geographical origin, harvesting time and methods adopted for extraction and drying of extract. Drying techniques seemed to have (p ≤ 0.05) significant effect on total phenolic and flavonoid content. The reduced level of polyphenolic compounds in dried peels might be due to chemical, enzymatic or thermal decomposition of phenolic acids and favonoids.
The content of ascorbic acid ranged from 46.87 to 29.92 mg/100 gm. Piga et al. (2003) and Piga et al. (2009) also reported reduction of ascorbic acid during drying at temperatures between 55 and 85 °C in plum and peach, which may be attributed to oxidative and thermal degradation. The high content of ascorbic acid was found in peel powder of freeze dried followed by vacuum dried and lower values were observed in tray dried peel powders but when compared with the fresh peel, overall decline in ascorbic acid content was recorded which could be due to thermo- sensitive nature of ascorbic acid in the tray and vacuum dried peel powder and oxidation in the freeze dried peel powder.
Fresh kinnow peel extract showed higher IC50 value (66.5 μg/mL) than that of the standard antioxidant i.e., BHT (IC50 = 25 μg/mL). Muthiah et al. (2012) reported a communsurate antiradical activity when evaluating the extract from Indian Citrus aurantium peel (IC50 = 86.83 μg/mL). Kinnow peel methanolic extract exhibit higher reducing power ability than BHT. These results coincide with the Babbar et al. (2011) who carried out a study on different fruit residues and reported kinnow peel exhibits higher reducing power ability than BHT. Results of present study recommended a positive linear relationship between reducing power. Trolox equivalent antioxidant capacity (TEAC) activity determined was 51.77 µmolTE/g db. This result implied comparatively higher ABTS scavenging activity in comparison to the study conducted by Ramful et al. (2010). Our results revealed kinnow peel extract exhibits 33.88% inhibition. After drying by different techniques the values observed for antioxidant capacity as radical scavenging activity (%), IC50, Trolox equivalent and beta-carotene bleaching assay were within the range of 89.07–83.28%, 68.15–73.15, 44.17–53.21 and 23.19–29.05 respectively (Table 2). Significant (p ≤ 0.05) decrease in Trolox equivalent and beta-carotene bleaching assay was more predominant in tray and vacuum dried samples, might be due to the detrimental effect of heat on antioxidant components. Scavenging activity of the dried kinnow peel increased significantly (p ≤ 0.05) might be due to the breakage of complex polyphenols into low-molecular-weight antioxidant activity containing compounds and formation of melanoidin like pigments during mallard’s reaction which are well known for their antioxidant activity. Our experimental results are in parallel with the findings of Gat and Ananthanarayan (2016) and Dewanto et al. (2002). Changes in the structure of flavonoids during drying would result in the formation of low molecular weight phenolic compounds which might affect the antioxidative activities. Similar findings were presented by Jeong et al. (2004) and Chen et al. (2011).
Identification and quantification of polyphenolic compounds by HPLC
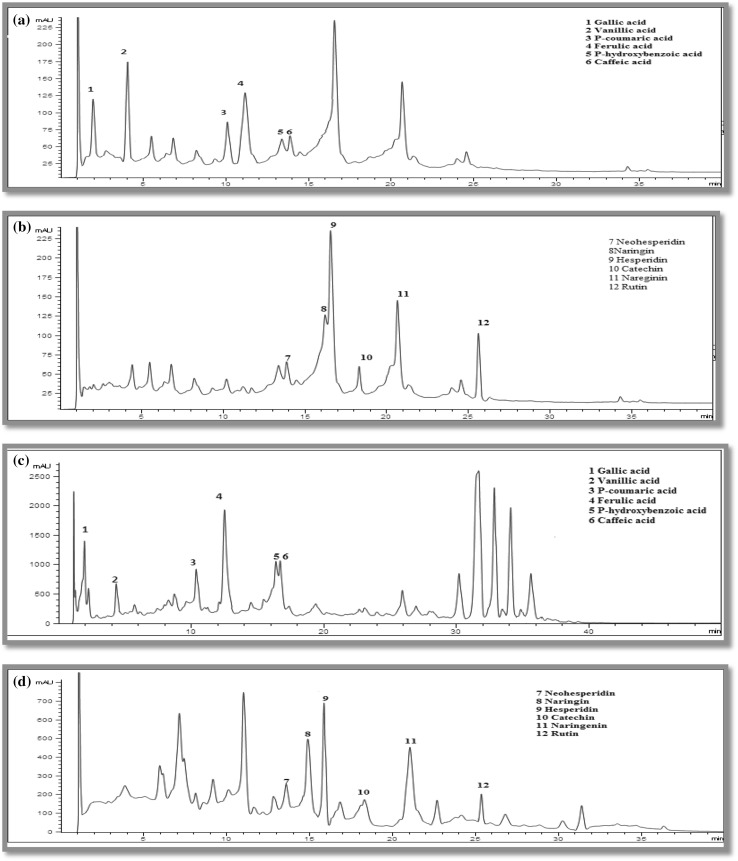
The fresh citrus peel extract and freeze dried peel was analyzed for individual phenolics and flavonoids with the help of HPLC. HPLC chromatogram of fresh peel shown in Fig. 1a, b detected Gallic acid (111.6 µg/g dry matter), Vanillic acid (191.4 µg/g dry matter), p-Coumaric acid (301.4 µg/g dry matter), Ferulic acid (441.7 µg/g dry matter), p-Hydroxybenzoic acid (29.0 µg/g dry matter), Caffeic acid (19.3 µg/g dry matter) and six flavonoids as; Neo-hesperidin (1.8 µg/g dry matter), Naringin (39.9 µg/g dry matter), Hesperidin (2795.8 µg/g dry matter), Catechin (33.1 µg/g dry matter), Narenginin (512.3 µg/g dry matter) and Rutin (163.4 µg/g dry matter) and chromatograms of freeze dried citrus peel (Fig. 1c, d) revealed the presence of Gallic acid, p-hydroxybenzoic acid, Vanillic acid p-Coumaric acid, Ferulic acid, caffeic acid in the quantity of 119.4, 26.4, 187.4, 294.2, 421.6 and 19.1 µg/g dry matter and flavonoids profile revealed the presence of Catechin, Naringin, Narenginin, Neo-hesperidin, Hesperidin and Rutin in the quantity of 31.1, 38.7, 492.2, 2715.8, 153.1 and 1.5 identified by using UV–Vis spectra and chromatographic comparisons with their standards. Our results were well supported with study reported by Ramful et al. (2010) who examined flavedo extracts of twenty-one varieties of citrus fruits and reported that Hesperidin was present at the highest concentrations in all flavedo extracts. Khan et al. (2010), Safdar et al. (2016) also reported highest phenolic compound concentrations (gallic, chlorogenic acid, Hesperidin, Naringin) in peel of C. sinensis and C. reticulate through HPLC.
Fig. 1.
HPLC chromatogram of a phenolic acids in fresh kinnow peel; b flavonoids present in fresh kinnow peel c phenolic acids in freeze dried kinnow peel and d flavonoids present in freeze dried kinnow peel
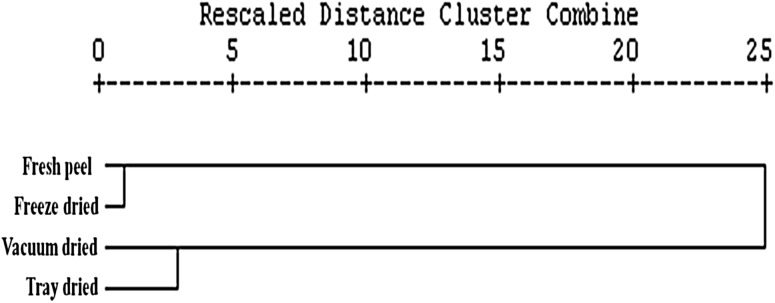
Principle component and cluster analysis
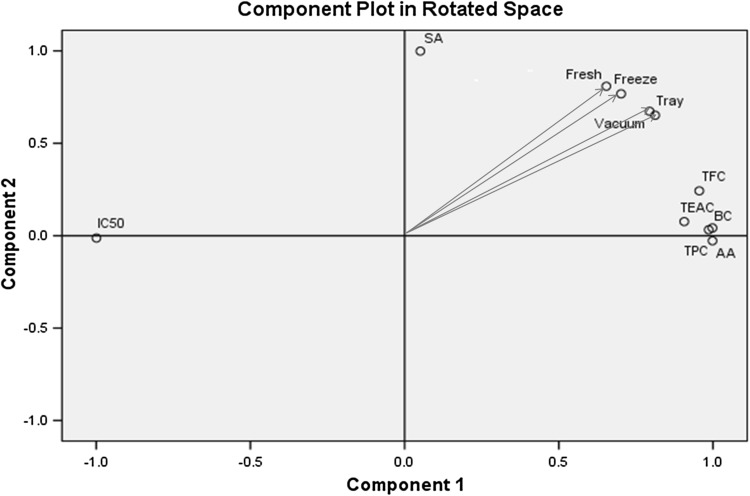
Multivariate analysis was conducted to ensure the effect of different drying techniques on the phytochemical components of the kinnow peel. In cluster analysis (CA) output of data plotted as a dendrogram (Fig. 2) two distinct clusters can be visualized. The first cluster comprises the fresh and freeze dried peel whereas, the second cluster includes vacuum and tray dried sample, indicating all the drying techniques had a significant (p ≤ 0.05) effect on the different phytochemical characteristics of kinnow peel. As CA provides mere information on the similarity of the different samples, Principal Component Analysis (PCA) explains variables which accounts for most of the variability in the data. It is an appropriate statistical technique used for reduction of the original variables (total phenolics, total flavonoids, DPPH scavenging activity, IC50 value, TEAC, ascorbic acid and bleaching assay) to a smaller number of elemental variables (Principal Component) which affirms the interrelationships between the different variables and to extract the optimum number of Principal Components. Maximum variation is explained by the first PC followed successively by second one which shows minor parts of original variance. This means that variables (correlated) are explained by the same PC and less correlated variables by different PC. The results of our plot obtained from PCA indicated that samples (fresh, tray, vacuum and freeze dried) were grouped together in the same quadrant, which represent fairly high levels of six attributes (total phenolic content, total flavonoid content, TEAC, β-Carotene bleaching assay, scavenging activity and ascorbic acid) (Fig. 3).
Fig. 2.
Cluster analysis of the different drying methods of kinnow peel
Fig. 3.
Component plot of the antioxidant activity of different drying techniques. Where, antioxidant activity was expressed as: IC 50 half maximal inhibitory concentration, SA scavenging activity, AA ascorbic acid, TEAC trolox equivalent antioxidant capacity, TPC total phenolic content, TFC total flavonoid content, BC β-carotene content
Conclusion
This research presents an overview of the richness of polyphenols in kinnow peel and loss of phytochemicals during drying by different methods. Results suggest that both fresh and dehydrated kinnow peel obtained in fruit processing industries could be the best suitable sources of bioactive compounds. Twelve phenolic compounds including six phenolic acids and six flavonoids, were identified and quantified by HPLC. Peel obtained as bio-waste in fruit processing industries can be safe-guarded by dehydration process. Freeze drying followed by vacuum drying was shown to be very effective in preserving the thermo-sensitive polyphenolic components and color of kinnow peel. We conclude that freeze and vacuum dried peels can be capitalized in food sector due to their richness in bioactive components that wield antioxidant properties. In future, point of convergence should be about incorporation of such powders and suitability of host product.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abonyi B, Feng H, Tang J, et al. Quality retention in strawberry and carrot purees dried with refractance window™ system. J Food Sci. 2002;67:1051–1056. doi: 10.1111/j.1365-2621.2002.tb09452.x. [DOI] [Google Scholar]
- Aggarwal P, Michael M. Effect of replacing sucrose with fructose on the physicochemical sensory characteristics of kinnow candy. Czech J Food Sci. 2014;32:158–163. doi: 10.17221/221/2013-CJFS. [DOI] [Google Scholar]
- AOAC . Official method of analysis of AOAC International, vol II. 17. Washington: Association of Analytical Chemists; 2000. [Google Scholar]
- Arslan D, Ozcan MM. Evaluation of drying methods with respect to drying kinetics, mineral content & color characteristics of rosemary leaves. Energ Convers Manage. 2008;49:1258–1264. doi: 10.1016/j.enconman.2007.08.005. [DOI] [Google Scholar]
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int. 2011;44:391–396. doi: 10.1016/j.foodres.2010.10.001. [DOI] [Google Scholar]
- Barros HRM, Ferreira TAP, Genovese MI. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012;134:1892–1898. doi: 10.1016/j.foodchem.2012.03.090. [DOI] [PubMed] [Google Scholar]
- Caparino OA, Tang J, Nindo CI, Sablani SS, Powers JR, Fellman JK. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’ var.) powder. J Food Eng. 2012;111:135–148. doi: 10.1016/j.jfoodeng.2012.01.010. [DOI] [Google Scholar]
- Chen ML, Yang DJ, Liu SC. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int J Food Sci Technol. 2011;46:1179–1185. doi: 10.1111/j.1365-2621.2011.02605.x. [DOI] [Google Scholar]
- Chukwu O, Shaba I. Effects of drying methods on proximate compositions of catfish (Clarias gariepinus) WJAS. 2009;5:114–116. [Google Scholar]
- Chun OK, Kim DO, Moon HY, Kang HG, Lee CY. Contribution of individual polyphenolics to total antioxidant capacity of plums. J Agric Food Chem. 2003;51:7240–7245. doi: 10.1021/jf0343579. [DOI] [PubMed] [Google Scholar]
- Dewanto V, Wu XZ, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Dhanani T, Shah S, Kumar S. A validated high-performance liquid chromatography method for determination of tannin-related marker constituent’s gallic acid, corilagin, chebulagic acid, ellagic acid and chebulinic Acid in four Terminalia species from India. J Chromatogr Sci. 2015;53(4):625–632. doi: 10.1093/chromsci/bmu096. [DOI] [PubMed] [Google Scholar]
- Enomfon-Akpan J, Umoh IB. Effect of heat and tetracycline treatments on the food quality and acridity factors in cocoyam (Xanthosoma sagittifolium (L.) Schott) Pak J Nutr. 2004;3:240–243. doi: 10.3923/pjn.2004.240.243. [DOI] [Google Scholar]
- Eren I, Kaymak-Ertekin F. Optimization of osmotic dehydration of potato using response surface methodology. J Food Eng. 2007;79:344–352. doi: 10.1016/j.jfoodeng.2006.01.069. [DOI] [Google Scholar]
- Franke AA, Custer LJ, Arakak IC, Murphy SP. Vitamin C and flavonoid levels of fruits and vegetables consumed in Hawaii. J Food Compos Anal. 2004;17:1–35. doi: 10.1016/S0889-1575(03)00066-8. [DOI] [Google Scholar]
- Garau MC, Simal S, Rossello C, Femenia A. Effect of air-drying temperature on physicochemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007;104:1014–1024. doi: 10.1016/j.foodchem.2007.01.009. [DOI] [Google Scholar]
- Gat Y, Ananthanarayan L. Effect of extrusion process parameters and pregelatinized rice flour on physicochemical properties of ready-to-eat expanded snacks. J Food Sci Technol. 2015;52:2634–264. doi: 10.1007/s13197-014-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat Y, Ananthanarayan L. Physicochemical, phytochemical and nutritional impact of fortified cereal-based extrudate snacks. Nutrafoods. 2015;14(3):141–149. doi: 10.1007/s13749-015-0036-7. [DOI] [Google Scholar]
- Gat Y, Ananthanarayan L. Use of paprika oily extract as pre-extrusion coloring of rice extrudates: impact of processing and storage on color stability. J Food Sci Technol. 2016;53(6):2887–2894. doi: 10.1007/s13197-016-2271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem N, Mihoubib D, Mihoubic KN, Mihoubi NB. Microwave dehydration of three citrus peel cultivars: effect on water and oil retention capacities, color, shrinkage and total phenols content. Ind Crops Prod. 2012;40:167–177. doi: 10.1016/j.indcrop.2012.03.009. [DOI] [Google Scholar]
- Goncalves EM, Pinheiro J, Abreu M, Brao TRS, Silva CLM. Modelling the kinetics of peroxidase inactivation, color and texture changes of pumpkin (Cucurbita maxima L.) during blanching. J Food Eng. 2007;81:693–701. doi: 10.1016/j.jfoodeng.2007.01.011. [DOI] [Google Scholar]
- Grabowski JA, Daubert Truong VD, Daubert CR. Spray-drying of amylase hydrolyzed sweet potato puree and physicochemical properties of powder. J Food Sci. 2006;71:209–217. doi: 10.1111/j.1750-3841.2006.00036.x. [DOI] [Google Scholar]
- Guimaraes R, Barros L, Barreira JCM, Sousa MJ, Carvalho AM, Ferreira ICFR. Targeting excessive free radicals with peels and juices of citrus fruits: Grapefruit, lemon, lime and orange. Food Chem Toxicol. 2010;48:99–106. doi: 10.1016/j.fct.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Guine RPF, Barroca MJ. Effect of drying treatments on texture and color of vegetables (pumpkin and green pepper) Food Bioprod Process. 2012;90:58–63. doi: 10.1016/j.fbp.2011.01.003. [DOI] [Google Scholar]
- Hamid MG, Abdel Nour AAM. Effect of different drying methods on quality attributes of beetroot (Beta vulgaris) slices. WJSTSD. 2018 [Google Scholar]
- Huang J, Zhang M. Effect of three drying methods on the drying characteristics and quality of okra. Dry Technol. 2015 [Google Scholar]
- Izli N, Polat A. Freeze and convective drying of quince (Cydonia oblonga Miller.): effects on drying kinetics and quality attributes. Heat Mass Transf. 2018 [Google Scholar]
- Jayanthi P, Lalitha P. Reducing power of the solvent extracts of Echhornia crassipes (mart.) Solms. Int J Pharm Pharm Sci. 2011;3:126–128. [Google Scholar]
- Khalid S, Malik AU, Saleem BA, Khan AS, Khalid MS, Amin M. Tree age and canopy position affect rind quality, fruit quality and rind nutrientcontent of ‘Kinnow’ m&arin (Citrus nobilis Lour × Citrus deliciosa Tenora) Sci Hortic. 2012;135:137–144. doi: 10.1016/j.scienta.2011.12.010. [DOI] [Google Scholar]
- Khan MK, Abert-Vian M, Fabiano-Tixier AS, Dangles O, Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119:851–858. doi: 10.1016/j.foodchem.2009.08.046. [DOI] [Google Scholar]
- Koleva TA, Jozef PH, Linssen AG, Lyuba NE. Screening of plant extracts for antioxidant activity. A comparative study on three testing methods. Phytochem Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Lee-Hoon H, Suhaimi MA, Ismail I, Mustafa KA. Effect of different drying conditions on proximate compositions of Red- and Yellow-Fleshed watermelon rind powders. J Agric Biotechnol. 2016;7:1–12. doi: 10.1016/j.bcab.2016.04.006. [DOI] [Google Scholar]
- Lim YY, Lim TT, Tee JJ. Antioxidant properties of several tropical fruits: a comparative study. Food Chem. 2007;103:1003–1008. doi: 10.1016/j.foodchem.2006.08.038. [DOI] [Google Scholar]
- Malla BA, Rastogi A, Sharma RK, Ishfaq A, Farooq J. Kinnow madarin (Citrus nobilis lour × Citrus deliciosa tenora) fruit waste silage as potential feed for small ruminants. Vet World. 2015;8(1):19–23. doi: 10.14202/vetworld.2015.19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskan M. Kinetics of color change of kiwifruits during hot air and microwave drying. J Food Eng. 2001;48(2):169–175. doi: 10.1016/S0260-8774(00)00154-0. [DOI] [Google Scholar]
- Morais DR, Rotta EM, Sargi SC, Schmidt EM, Bonafe EG, Eberlin MN, Sawaya ACHF, Visentainer JV. Antioxidant activity, phenolics and UPLC–ESI–MS of extracts from different tropical fruits parts and processed peels. Food Res Int. 2015;77:392–399. doi: 10.1016/j.foodres.2015.08.036. [DOI] [Google Scholar]
- Mudgal VD, Pandey VK. Thin layer drying kinetics of bittergourd. J Food Sci Technol. 2009;46(3):236–239. [Google Scholar]
- Muthiah P, Umamaheswari M, Asokkumar K. In vitro antioxidant activities of leaves, fruits and peel extracts of Citrus. Int J Phytopharm. 2012;2:13–20. [Google Scholar]
- Piga A, Del Caro A, Corda G. From plums to prunes: Influence of drying parameters on polyphenols and antioxidant activity. J Agric Food Chem. 2003;51(12):3675–3681. doi: 10.1021/jf021207+. [DOI] [PubMed] [Google Scholar]
- Piga A, Romeo FV, Poiana M, Del Caro A, Sanguinetti AM, Piscopo A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur Food Res Technol. 2009;228(3):441–448. doi: 10.1007/s00217-008-0951-6. [DOI] [Google Scholar]
- Rafiq S, Kaul R, Sofi SA, Bashir N, Nazir F, Nayik GA. Citrus peel as a source of functional ingredient: a review. J Saudi Soc Agric Sci. 2018;17:351–358. [Google Scholar]
- Ramful D, Bahorunb T, Bourdonc E, Tarnusc E, Aruoma OI. Bioactive phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: potential prophylactic ingredients for functional foods application. Toxicology. 2010;278:75–87. doi: 10.1016/j.tox.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Safdar MN, et al. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J Food Drug Anal. 2016 doi: 10.1016/j.jfda.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalini R, Gupta DK, Singh A. Drying kinetics of apple pomace cake. J Food Sci Technol. 2009;46(5):477–479. [Google Scholar]
- Sogi DS, Siddiq M, Greiby I, Dolan KD. Total phenolics, antioxidant activity and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013;141:2649–2655. doi: 10.1016/j.foodchem.2013.05.053. [DOI] [PubMed] [Google Scholar]
- Vega-Galvez A, Ah-Hen K, Chacana M. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, color, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012;132:51–59. doi: 10.1016/j.foodchem.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu YH, Cao JG, Wang QX, Xiao JB. Flavonoids, antioxidant potential, and acetyl cholinesterase inhibition activity of the extracts from the gametophyte and archegoniophore of Marchantia polymorpha L. Molecules. 2016;21:360. doi: 10.3390/molecules21030360. [DOI] [PMC free article] [PubMed] [Google Scholar]